
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol., 19 December 2017
Sec. Membrane Physiology and Membrane Biophysics
Volume 8 - 2017 | https://doi.org/10.3389/fphys.2017.01076
This article is part of the Research TopicThe red cell life-cycle from erythropoiesis to clearanceView all 22 articles
Erythropoiesis occurs mostly in bone marrow and ends in blood stream. Mature red blood cells are generated from multipotent hematopoietic stem cells, through a complex maturation process involving several morphological changes to produce a highly functional specialized cells. In mammals, terminal steps involved expulsion of the nucleus from erythroblasts that leads to the formation of reticulocytes. In order to produce mature biconcave red blood cells, organelles and ribosomes are selectively eliminated from reticulocytes as well as the plasma membrane undergoes remodeling. The mechanisms involved in these last maturation steps are still under investigation. Enucleation involves dramatic chromatin condensation and establishment of the nuclear polarity, which is driven by a rearrangement of actin cytoskeleton and the clathrin-dependent generation of vacuoles at the nuclear-cytoplasmic junction. This process is favored by interaction between the erythroblasts and macrophages at the erythroblastic island. Mitochondria are eliminated by mitophagy. This is a macroautophagy pathway consisting in the engulfment of mitochondria into a double-membrane structure called autophagosome before degradation. Several mice knock-out models were developed to identify mitophagy-involved proteins during erythropoiesis, but whole mechanisms are not completely determined. Less is known concerning the clearance of other organelles, such as smooth and rough ER, Golgi apparatus and ribosomes. Understanding the modulators of organelles clearance in erythropoiesis may elucidate the pathogenesis of different dyserythropoietic diseases such as myelodysplastic syndrome, leukemia and anemia.
Mature red blood cells (RBCs) result from a finely regulated process called erythropoiesis that produces 2 million RBCs every second in healthy human adults (Palis, 2014). The standard model of erythropoiesis starts with hematopoietic stem cells (HSCs) in the bone marrow (BM), giving rise to multipotent progenitors that go on to erythroid-committed precursors to mature RBC. This hierarchical relationship is, however, challenged, showing a greater plasticity for the cell's potential fates, with several studies in mice (Adolfsson et al., 2005) and recent new data in human (Notta et al., 2016).
Maturation from erythroid-committed precursors is called terminal erythropoiesis and occurs in the BM within erythroblastic islands, which consist of a central macrophage surrounded by erythroblasts, and ends in the blood stream where reticulocytes complete their maturation within 1–2 days. During this phase, proerythroblasts (Pro-E) undergo morphological changes, such as cell size reduction and chromatin condensation, produce specific proteins, such as hemoglobin, and exhibit a reduced proliferative capacity to give rise to basophilic (Baso-E), polychromatophilic (Poly-E) and orthochromatophilic (Ortho-E) erythroblasts, successively. Even though several growth factors are known to regulate erythropoiesis, Epo is the main regulator of erythropoiesis driving RBC precursor proliferation and differentiation, preventing erythroblast apoptosis (Koury and Bondurant, 1990; Ji et al., 2011). The macrophage-erythroblast interaction in the BM is essential since macrophages facilitate proliferation and differentiation and provide iron to the erythroblasts (de Back et al., 2014).
At the end of the terminal maturation, mammalian erythroblasts expel their nuclei and lose all their organelles, such as the Golgi apparatus, endoplasmic reticulum (ER), mitochondria and ribosomes. After expelling its nucleus, the reticulocyte maturation continues, losing 20–30% of the cell surface (Waugh et al., 1997; Da Costa et al., 2001) and eliminating any remaining membrane-bound cytosolic organelles through an autophagy/exosome-combined pathway (Blanc et al., 2005).
While extensive literature is done concerning the general mechanisms of erythropoiesis (Palis, 2014), this review focuses on the mechanisms and molecular actors involved during organelle clearance and membrane remodeling in order to produce fully functional biconcave mature RBCs. Figure 1 summarizes the best characterized steps of organelle clearance throughout erythroblast terminal differentiation.
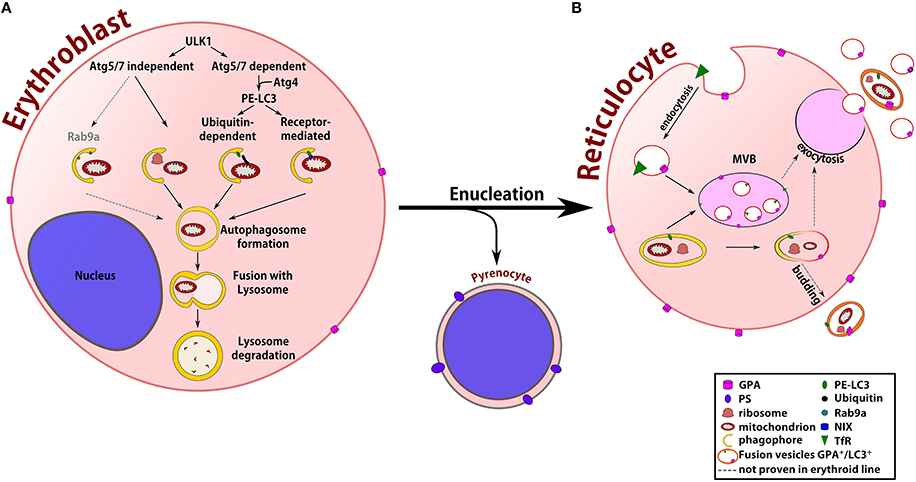
Figure 1. Terminal maturation of erythroblasts. (A) At the erythroblast stage, two Ulk1-mediated autophagic pathways are activated to allow organelle clearance: the Atg5/7-dependent pathway with the proteolytic Atg4-dependent activation of MAPLC3, microtubule-associated protein 1 light channel 3 (LC3) and the Atg5/7-independent pathway, which is not related to the LC3 protein. LC3 activation allows its insertion into the phagophore membrane, starting the engulfment of organelles through the recognition of an ubiquitin signal or by the direct binding of specialized receptors at the organelle membrane. In non-erythroid cells, Rab9a is important for the formation of the phagophore during the Atg5/7-independent autophagic pathway. After the formation of the autophagosome, its fusion with the lysosome permits the degradation of organelles by hydrolytic enzymes. The enucleation process gives rise to the pyrenocyte and the reticulocyte, which still contains some organelles that must be eliminated for the final maturation into erythrocyte. (B) During this stage, unwanted membrane proteins, such as transferrin receptor (TfR), are internalized by endocytosis and expelled by exocytosis from multi-vesicular body structures. Glycophorin A (GPA)/LC3 double-positive vesicles containing organelle remnants are also found in reticulocytes, suggesting cooperation between the endocytosis (GPA+) and autophagy (LC3+) pathways to eliminate organelles. How autophagosomes interact with multivesicular bodies (MVBs) following the same pathway of membrane protein recycling or budding directly from the plasma membrane after fusion with endocytic vesicles, however, remains unknown.
The most spectacular aspect of mammalian erythropoiesis is the generation of enucleated cells. Enucleation occurs in orthochromatic erythroblasts producing two kinds of cells, the reticulocyte and the pyrenocyte [the nucleus surrounded by a tiny layer of cytoplasm and the plasma membrane (PM)]. Pyrenocytes are rapidly eliminated by the macrophages of the erythroblastic island, where phosphatidylserine exposure acts as an “eat me” signal (Yoshida et al., 2005).
Among the changes occurring during terminal differentiation, cell cycle arrest, chromatin and nuclear condensation and nuclear polarization are important for enucleation. In addition, nucleus expulsion is believed to be dependent on adhesion protein reorganization across the PM and macrophage interactions (Lee et al., 2004; Soni et al., 2006). The transcription factor KFL1 is required for enucleation (Parkins et al., 1995; Magor et al., 2015), regulating the expression of cell cycle proteins, deacetylases, caspases, and nuclear membrane proteins (Gnanapragasam et al., 2016; Gnanapragasam and Bieker, 2017).
Nuclear and chromatin condensation is essential for enucleation (Popova et al., 2009; Ji et al., 2010) and is dependent on the acetylation status of histones H3 and H4 under the control of histone acetyl transferases (HATs) and histone deacetylases (HDACs). Accordingly, Gcn5, an HAT protein, is down-regulated, and H3K9 and H4K5 histone acetylation decreases during mouse fetal erythropoiesis. In addition, Gcn5 is up regulated by c-Myc, which is known to decrease during the late phase of the erythropoiesis (Jayapal et al., 2010). With the same model, the role of HDAC2 protein was shown to be essential not only for chromatin condensation but also for the formation of the contractile actin ring (CAR), which is involved in nuclear pyknosis (Ji et al., 2010). Moreover, it was recently shown that major histones are released through a nuclear opening that is induced by caspase 3 activity-dependent lamin B cleavage and chromatin condensation (Gnanapragasam and Bieker, 2017).
Many studies demonstrate the cell cycle dependence of enucleation (Gnanapragasam and Bieker, 2017). Interestingly, the cyclin-induced E2F-2 transcription factor, which is a direct target of KLF1 during terminal erythropoiesis, appears to play a role in enucleation by inducing the expression of CRIK (Citron Rho-interacting kinase). Away from its regular targets related to microtubule organization and cytokinesis, CRIK participates in nuclear condensation (Swartz et al., 2017).
Cytoskeletal elements play an important role in erythroblast enucleation, acting in a similar manner to cytokinesis but in an asymmetric way. Specifically, as observed by electron and immunofluorescence microscopy, actin filaments (F-actin) condensate behind the extruding nucleus to form the CAR. The use of cytochalasin D, an F-actin inhibitor, causes the complete blockage of enucleation (Koury et al., 1989). Furthermore, the formation of the CAR is dependent on Rac1 GTPase and on mDia2, a Rho GTPase downstream effector, since down-regulating these two proteins disrupts the CAR formation and blocks erythroblast enucleation (Ji et al., 2008).
Regarding other cytoskeleton elements, the pharmacological inhibition of vimentin does not affect enucleation, which is in agreement with its decrease during human erythropoiesis (Dellagi et al., 1983). However, the deregulation of microtubules diminishes the enucleation rate. Microtubules form a basket around the nucleus (Koury et al., 1989), which is displaced near the PM at the late erythroblast stages, suggesting that this network must be essential for the polarization of the nucleus. Recently, the importance of the molecular motor dynein, which mediates unidirectional movement toward the minus end of the microtubules, was shown. Furthermore, PI3K activity is induced by microtubule polymers, improves the polarization efficiency and promotes nuclear movement. However, PI3K inhibition does not block, but only delays, mice enucleation (Wang et al., 2012).
In 2010, Crispino's group observed, by electron microscopy, the formation of vesicles close to the nuclear extrusion site in both primary murine and human erythroblasts, suggesting that another mechanism contributes to enucleation. Additionally, as shown by genetic invalidation, clathrin is needed for the vesicle formation (Keerthivasan et al., 2010). More recently, it was shown that survivin is required for erythroblast enucleation, but instead of acting on cytokinesis via the chromosome passenger complex, survivin contributes to enucleation through an interaction with EPS15 and clathrin (Keerthivasan et al., 2012).
Clearly, we are still at the beginning of unraveling the molecular players involved in the enucleation process. Moreover, as shown in Table 1, most of the molecular players were identified in mice, and we are still lacking a demonstration that these players are also involved in human erythropoiesis.
The main mechanism for mitochondrial clearance is mitophagy, a selective type of autophagy that allows the degradation of damaged mitochondria. The importance of this process is highlighted by knowing that an impairment in mitochondrial function triggers an increase in reactive oxygen species production, which can in turn cause damage to cellular components (proteins, nucleic acid, and lipids) and trigger cell death (Lee et al., 2012).
During regular autophagy processes, stress or nutrient deprivation activates APM-activated protein kinase (AMPK), triggering two ubiquitin-dependent pathways (Figure 1A). One of these allows the assembly of the phagophore and involves several autophagy-related proteins (Atg), such as Atg5 and Atg7. The other aims to activate and lipidate LC3 (MAPLC3, microtubule-associated protein 1 light channel 3) by Atg4, a redox regulated protein. Atg4 and Atg7 cooperate to conjugate LC3 onto phosphatidylethanolamine in the lipid bilayer of the membrane originated from the ER-mitochondria contact site (Tooze and Yoshimori, 2010; Hamasaki et al., 2013). The elongated phagophore is then recruited to engulf targets via adaptor proteins, containing an LC3-interacting region (LIR) that forms a double-membrane autophagosome, which will fuse with a lysosome, initiating the degradation of the autophagosome components.
Upon mitochondria damage or depolarization, the mitochondrial membrane proteins are exposed and act as a beacon to recruit the phagophore membranes (Liu et al., 2014). An example is the PINK1 (P-TEN-induced kinase 1)-dependent recruitment of Parkin. Upon mitochondria depolarization, PINK1 accumulates at the OMM (outer mitochondrial membrane) and induces the mitochondrial translocation of Parkin, an RBR (ring-in-between)-type E3 ubiquitin ligase by direct phosphorylation (Kim et al., 2008; Narendra et al., 2010). The stabilization of Parkin at the OMM leads to the poly-ubiquitination of many proteins, inducing mitochondria fission and mobility stop and the phagophore recruitment by interacting with p62/SQSTM1, a LIR containing protein (Geisler et al., 2010). Unlike regular mitophagy induction, targeted mitochondria, during erythroblast maturation, are fully functional. BNIP3L/NIX, a BH3-only integral OMM protein first identified in mouse reticulocytes, appears to be the major mitochondrial protein involved during terminal differentiation (Schweers et al., 2007; Sandoval et al., 2008). This protein is upregulated during erythropoiesis and induces mitochondrial membrane depolarization and membrane conjugated LC3 recruitment to the mitochondria (Aerbajinai et al., 2003; Novak et al., 2010). Nix action is not mediated by its BH3 domain but rather seems to be due to a cytoplasmic short linear motif, acting as a cellular signal to recruit other proteins (Zhang et al., 2012). However, whether Nix-induced mitochondrial depolarization activates the Parkin-dependent pathway is still unknown (Yuan et al., 2017).
Recently, other mitochondrial receptors were found to participate in mitophagy, such as FUNDC1, induced by MARCH5, an E3 ubiquitin ligase acting in hypoxic condition (Chen et al., 2017), Bcl2-L-13 (Murakawa et al., 2015), optineurin (Wong and Holzbaur, 2014), and Prohibitin 2 (Wei et al., 2017). It remains unknown whether they play a role in erythroid maturation.
Canonical Atg proteins also participate in terminal maturation. In human erythropoiesis, LC3 cleavage is under the control of the endopeptidase Atg4 and is needed for autophagosome maturation (Betin et al., 2013). In mice, Ulk1 (Atg1) expression correlates with terminal differentiation and participates in mitochondria and ribosome elimination (Chan et al., 2007; Kundu et al., 2008). The ubiquitination-dependent pathway also plays a role in reticulocyte maturation but is not essential. Indeed, in Atg7−/− reticulocytes, mitochondrial clearance is only partially affected (Zhang and Ney, 2009; Zhang et al., 2009). However, Nix and Ulk1 activation appears to be essential (Mortensen et al., 2010; Honda et al., 2014), suggesting the coexistence of both Atg5/Atg7-dependent and independent pathways during terminal differentiation.
Some studies suggest that the Atg5/7-independent degradation of mitochondria involves endosomal trafficking regulatory Rab proteins. Autophagosomes, formed in a Ulk1-dependent pathway, fuse with Golgi-derived vesicles and late endosomes in a Rab9a-dependent manner before they are targeted to the lysosomes (Wang et al., 2016). Interestingly, Rab proteins were also recently shown to be involved in mitochondria removal in a complete autophagy-independent pathway. Depolarized mitochondria appear to be engulfed in Rab5-positive endosomes that mature into Rab7-positive late endosomes and then fuse with lysosomes (Hammerling et al., 2017a,b). Unlike canonical autophagy, which involves the surrounding of a ubiquitin-decorated target by a double membrane structure, the entire mitochondria appears to be engulfed by an early endosome membrane invagination through the ESCRT machinery. Whether this might also occur in maturing erythroblasts is not known.
Mitophagy also appears to be transcriptionally regulated. Indeed, hemin-dependent differentiation of an erythroid cell line shows features of mitophagy (Fader et al., 2016). The NF-E2 transcription factor involved in globin gene expression also regulates mitophagy through the regulation of Nix and Ulk1 genes (Gothwal et al., 2016; Lupo et al., 2016). Another key regulator is the KRAB/KAP1-miRNA regulatory cascade, which acts as an indirect repressor of mitophagy genes in mice as well as in human cells, probably by the down and up regulation of a series of miRNAs, such as miR-351 that targets Nix (Barde et al., 2013).
In parallel to the autophagic pathway, cytosolic degradation seems to occur during reticulocyte maturation. 15-lipoxygenase (15-LOX), an enzyme that catalyzes the dioxygenation of polyunsaturated fatty acids, is translationally inhibited until the reticulocyte stage and acts to permeabilize organelle membranes, allowing proteasome access and degradation. Interestingly, only mitochondria elimination is affected, while ribosome clearance remains efficient when using a lipoxygenase inhibitor (Grüllich et al., 2001). This mechanism is still controversial, as 15-LOX might also act in the autophagy pathway as an OMM pH gradient disruptor that can induce mitophagy (Vijayvergiya et al., 2004), and on the oxidation of phospholipids conjugating with LC3 during the autophagosome formation; even so, these features, as shown in Table 1 were not demonstrated in erythroid cells yet (Morgan et al., 2015).
In general, autophagy plays an essential role in the elimination of other organelles, such as lysosomes, peroxisomes and ER. However, the literature presents only very few studies in erythroid cells (Table 1).
While Nix is required for mitochondria removal, Ulk1 is involved in ribosome and mitochondria degradation (Schweers et al., 2007; Kundu et al., 2008; Sandoval et al., 2008). Similarly, an efficient clearance of ribosomes and ER and the inhibition of mitophagy was observed in Atg7−/− mice (Mortensen et al., 2010). These data suggest that non-autophagic or Atg7-independent autophagic pathways might exist for the elimination of other organelles (Figure 1A).
In non-erythroid cells from mammals, it was proposed that peroxisomes are eliminated by three different pathways: macroautophagy (Iwata, 2006), 15-LOX mediated (Yokota et al., 2001) and the peroxisomal Lon proteases (Yokota et al., 2008). Furthermore, the autophagic degradation of lysosomes (lysophagy) was recently identified in HeLa cells where it is mediated by ubiquitination and involves p62 protein (Hung et al., 2013). The similarities between pexophagy/lysophagy and mitophagy in non-erythroid cells suggest that autophagy pathways might also be involved in erythroblast terminal maturation.
After enucleation, reticulocytes mature in the bone marrow (R1) and then exit in the blood stream (R2) to complete the process. While the degradation of organelles starts at the time of enucleation, the elimination of mRNA occurs in the blood stream and is mediated by ribonucleases, generating nucleotides that are degraded by the erythroid pyrimidine nucleotidase. This elimination is crucial, as the deficiency in this enzyme causes hemolytic anemia (Valentine et al., 1974). mRNAs in R2 reticulocytes mainly belong to three overlapping categories: transport, metabolic and signal transduction (Lee et al., 2014), and their presence is essential to reach the mature RBC stage. This supports the importance of the exosome pathway for the final maturation into RBCs with an active elimination of other subcellular components.
Exosomes are small vesicles that are secreted into the extracellular medium from various kind of cells. PM invaginations form early endosomes that engulf various targets forming multivesicular bodies (MVB, late endosomes) that eventually fuse with the PM and release exosomes. In reticulocytes, this pathway is thought to be involved in cell volume and membrane remodeling to reduce volume and remove unwanted membrane proteins. This was first discovered in sheep reticulocytes where transferrin receptor (TfR) is first internalized into small vesicles of 100–200 nm before being engulfed into the MVBs (Pan et al., 1985; Johnstone et al., 1989). The internalization step is clathrin-dependent, and the degradation is lysosome-independent and occurs by exocytosis after the fusion of the MVBs with the PM as shown in Figure 1B (Killisch et al., 1992). This process is required for the final elimination of other membrane proteins that are essential for the reticulocyte but are absent in the mature cell. Proteins such as aquaporin-1 (AQP1) (Blanc et al., 2009), α4β1 integrin (Rieu et al., 2000), glucose transporter and acetylcholinestarase (Johnstone et al., 1987) are found in glycophorine-A (GPA) positive endosomes while cytoskeletal proteins, such as actin or spectrin have never been found in these endosomes (Liu et al., 2010).
While plenty of evidence notes the role of autophagy in removing organelles during terminal maturation, the degradation step itself shows discrepancies with canonical proteolysis involving lysosomal proteins because of the disappearance of the lysosomal compartment during the maturation and removal of LAMP2 by exocytosis (Barres et al., 2010). Recently, GPA-positive endosomes were found to express LC3 at the endosome membrane, suggesting the cooperation of both autophagy and exocytosis in the removal of remnant organelles in R2 reticulocytes. These hybrid vesicles contain mitochondria, Golgi and lysosomes might be formed by the fusion of the outer-membrane of the autophagosome and the PM derived endosome (Griffiths et al., 2012). The exocytosis of this vesicle might be favored by the spleen, as splenectomized patients present large vacuoles inside reticulocytes (Holroyde and Gardner, 1970).
It should be pointed out the importance of lipids domain such as cholesterol and sphingomyelin-enriched domains in the PM remodeling, as they were find both in membrane vesiculation specific sites (Leonard et al., 2017).
Even if all the animal models used to identify the molecular players involved during terminal differentiation exhibit maturation defects and anemia, links between organelle clearance and human hematological diseases are still mostly unknown. Erythroid disorders, such as β-thalassemia and myelodysplastic syndrome (MDS), are characterized by ineffective hematopoiesis, anemia, dissociation between proliferation and differentiation of progenitor cells and the inefficient elimination of aggregated protein (Arber et al., 2016; Taher et al., 2017). Indeed, defects in reticulocyte maturation and autophagy are identified in HbE/β-thalassemia patients (Lithanatudom et al., 2011; Khandros et al., 2012; Butthep et al., 2015), and enucleation defects are found in MDS patients (Garderet et al., 2010; Park et al., 2016). Impaired autophagy is involved in cytosolic toxic Lyn accumulation and mitochondria and lysosome degradation delay in chorea-acanthocytosis (Lupo et al., 2016). The use of autophagy modulators is beneficial in the case of SCD or β-thalassemia (Franco et al., 2014; Jagadeeswaran et al., 2017). Moreover, anemia in Pearson's syndrome was recently linked to incomplete mitochondrial clearance from reticulocytes (Palis, 2014) and an asynchronization of iron loading (Ahlqvist et al., 2015), while sickle cells patients showed an accumulation of proteins in their erythrocytes suggesting a defect in exosomal pathway (De Franceschi, 2009; Carayon et al., 2011).
Unraveling the molecular mechanisms and interplays ruling erythroblast terminal maturation would be priceless in hematological disease therapy. However, much of our knowledge regarding human erythropoiesis is based on animal models and/or ex vivo cultured human progenitor cells (Table 1). Great care should be applied when interpreting results, considering the important differences between mouse and human erythropoiesis as well as the in vivo and in vitro environments, as highlighted in the extensive transcriptome analysis across a terminal erythroid differentiation study (An et al., 2014).
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This study was supported by grants from Laboratory of Excellence GR-Ex, reference ANR-11-LABX-0051. The labex GR-Ex is funded by the program “Investissements d'avenir” of the French National Research Agency, reference ANR-11-IDEX-0005-02.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
MM is funded by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 665850. We thank I. Marginedas-Freixa and C. Hattab for helpful discussions.
Adolfsson, J., Månsson, R., Buza-Vidas, N., Hultquist, A., Liuba, K., Jensen, C. T., et al. (2005). Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential. Cell 121, 295–306. doi: 10.1016/j.cell.2005.02.013
Aerbajinai, W., Giattina, M., Lee, T. Y., Raffield, M., and Miller, J. L. (2003). The proapoptotic factor Nix is coexpressed with Bcl-xL during terminal erythroid differentiation. Blood 102, 712–717. doi: 10.1182/blood-2002-11-3324
Ahlqvist, K. J., Leoncini, S., Pecorelli, A., Wortmann, S. B., Ahola, S., Forsström, S., et al. (2015). MtDNA mutagenesis impairs elimination of mitochondria during erythroid maturation leading to enhanced erythrocyte destruction. Nat. Commun. 6:6494. doi: 10.1038/ncomms7494
An, X., Schulz, V. P., Li, J., Wu, K., Liu, J., Xue, F., et al. (2014). Global transcriptome analyses of human and murine terminal erythroid differentiation. Blood 123, 3466–3477. doi: 10.1182/blood-2014-01-548305
Arber, D. A., Orazi, A., Hasserjian, R., Thiele, J., Borowitz, M. J., Le Beau, M. M., et al. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405. doi: 10.1182/blood-2016-03-643544
Barde, I., Rauwel, B., Marin-Florez, R. M., Corsinotti, A., Laurenti, E., Verp, S., et al. (2013). A KRAB/KAP1-miRNA cascade regulates erythropoiesis through stage-specific control of mitophagy. Science 340, 350–353. doi: 10.1126/science.1232398
Barres, C., Blanc, L., Bette-Bobillo, P., Andre, S., Mamoun, R., Gabius, H.-J., et al. (2010). Galectin-5 is bound onto the surface of rat reticulocyte exosomes and modulates vesicle uptake by macrophages. Blood 115, 696–705. doi: 10.1182/blood-2009-07-231449
Betin, V. M. S., Singleton, B. K., Parsons, S. F., Anstee, D. J., and Lane, J. D. (2013). Autophagy facilitates organelle clearance during differentiation of human erythroblasts: evidence for a role for ATG4 paralogs during autophagosome maturation. Autophagy 9, 881–893. doi: 10.4161/auto.24172
Blanc, L., De Gassart, A., Géminard, C., Bette-Bobillo, P., and Vidal, M. (2005). Exosome release by reticulocytes—an integral part of the red blood cell differentiation system. Blood Cells. Mol. Dis. 35, 21–26. doi: 10.1016/j.bcmd.2005.04.008
Blanc, L., Liu, J., Vidal, M., Chasis, J. A., An, X., and Mohandas, N. (2009). The water channel aquaporin-1 partitions into exosomes during reticulocyte maturation: implication for the regulation of cell volume. Blood 114, 3928–3934. doi: 10.1182/blood-2009-06-230086
Butthep, P., Wisedpanichkij, R., Jindadamrongwech, S., and Fucharoen, S. (2015). Elevated erythropoietin and cytokines levels are related to impaired reticulocyte maturation in thalassemic patients. Blood Cells. Mol. Dis. 54, 170–176. doi: 10.1016/j.bcmd.2014.11.007
Carayon, K., Chaoui, K., Ronzier, E., Lazar, I., Bertrand-Michel, J., Roques, V., et al. (2011). Proteolipidic composition of exosomes changes during reticulocyte maturation. J. Biol. Chem. 286, 34426–34439. doi: 10.1074/jbc.M111.257444
Chan, E. Y. W., Kir, S., and Tooze, S. A. (2007). siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 282, 25464–25474. doi: 10.1074/jbc.M703663200
Chen, Z., Siraj, S., Liu, L., and Chen, Q. (2017). MARCH5-FUNDC1 axis fine-tunes hypoxia induced mitophagy. Autophagy 13, 1244–1245. doi: 10.1080/15548627.2017.1310789
Da Costa, L., Mohandas, N., Sorette, M., Grange, M.-J., Tchernia, G., and Cynober, T. (2001). Temporal differences in membrane loss lead to distinct reticulocyte features in hereditary spherocytosis and in immune hemolytic anemia. Blood 98, 2894–2899. doi: 10.1182/blood.V98.10.2894
de Back, D. Z., Kostova, E. B., van Kraaij, M., van den Berg, T. K., and van Bruggen, R. (2014). Of macrophages and red blood cells; a complex love story. Front. Physiol. 5:9. doi: 10.3389/fphys.2014.00009
De Franceschi, L. (2009). Pathophisiology of sickle cell disease and new drugs for the treatment. Mediterr. J. Hematol. Infect. Dis. 1:e2009024. doi: 10.4084/MJHID.2009.024
Dellagi, K., Vainchenker, W., Vinci, G., Paulin, D., and Brouet, J. C. (1983). Alteration of vimentin intermediate filament expression during differentiation of human hemopoietic cells. EMBO J. 2, 1509–1514.
Fader, C. M., Salassa, B. N., Grosso, R. A., Vergara, A. N., and Colombo, M. I. (2016). Hemin induces mitophagy in a leukemic erythroblast cell line: hemin induces mitophagy in K562 cells. Biol. Cell 108, 77–95. doi: 10.1111/boc.201500058
Franco, S. S., De Falco, L., Ghaffari, S., Brugnara, C., Sinclair, D. A., Matté, A., et al. (2014). Resveratrol accelerates erythroid maturation by activation of FoxO3 and ameliorates anemia in beta-thalassemic mice. Haematologica 99, 267–275. doi: 10.3324/haematol.2013.090076
Garderet, L., Kobari, L., Mazurier, C., De Witte, C., Giarratana, M.-C., Perot, C., et al. (2010). Unimpaired terminal erythroid differentiation and preserved enucleation capacity in myelodysplastic 5q(del) clones: a single cell study. Haematologica 95, 398–405. doi: 10.3324/haematol.2009.012773
Geisler, S., Holmström, K. M., Skujat, D., Fiesel, F. C., Rothfuss, O. C., Kahle, P. J., et al. (2010). PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 12, 119–131. doi: 10.1038/ncb2012
Gnanapragasam, M. N., and Bieker, J. J. (2017). Orchestration of late events in erythropoiesis by KLF1/EKLF. Curr. Opin. Hematol. 24, 183–190. doi: 10.1097/MOH.0000000000000327
Gnanapragasam, M. N., McGrath, K. E., Catherman, S., Xue, L., Palis, J., and Bieker, J. J. (2016). EKLF/KLF1-regulated cell cycle exit is essential for erythroblast enucleation. Blood 128, 1631–1641. doi: 10.1182/blood-2016-03-706671
Gothwal, M., Wehrle, J., Aumann, K., Zimmermann, V., Grunder, A., and Pahl, H. L. (2016). A novel role for nuclear factor-erythroid 2 in erythroid maturation by modulation of mitochondrial autophagy. Haematologica 101, 1054–1064. doi: 10.3324/haematol.2015.132589
Griffiths, R. E., Kupzig, S., Cogan, N., Mankelow, T. J., Betin, V. M. S., Trakarnsanga, K., et al. (2012). Maturing reticulocytes internalize plasma membrane in glycophorin A-containing vesicles that fuse with autophagosomes before exocytosis. Blood 119, 6296–6306. doi: 10.1182/blood-2011-09-376475
Grüllich, C., Duvoisin, R. M., Wiedmann, M., and Van Leyen, K. (2001). Inhibition of 15-lipoxygenase leads to delayed organelle degradation in the reticulocyte. FEBS Lett. 489, 51–54. doi: 10.1016/S0014-5793(01)02080-4
Hamasaki, M., Furuta, N., Matsuda, A., Nezu, A., Yamamoto, A., Fujita, N., et al. (2013). Autophagosomes form at ER–mitochondria contact sites. Nature 495, 389–393. doi: 10.1038/nature11910
Hammerling, B. C., Najor, R. H., Cortez, M. Q., Shires, S. E., Leon, L. J., Gonzalez, E. R., et al. (2017a). A Rab5 endosomal pathway mediates Parkin-dependent mitochondrial clearance. Nat. Commun. 8:14050. doi: 10.1038/ncomms14050
Hammerling, B. C., Shires, S. E., Leon, L. J., Cortez, M. Q., and Gustafsson, Å. B. (2017b). Isolation of Rab5-positive endosomes reveals a new mitochondrial degradation pathway utilized by BNIP3 and Parkin. Small GTPases 11, 1–8. doi: 10.1080/21541248.2017.1342749
Holroyde, C. P., and Gardner, F. H. (1970). Acquisition of autophagic vacuoles by human erythrocytes physiological role of the spleen. Blood 36, 566–575.
Honda, S., Arakawa, S., Nishida, Y., Yamaguchi, H., Ishii, E., and Shimizu, S. (2014). Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat. Commun. 5:4004. doi: 10.1038/ncomms5004
Hung, Y.-H., Chen, L. M.-W., Yang, J.-Y., and Yuan Yang, W. (2013). Spatiotemporally controlled induction of autophagy-mediated lysosome turnover. Nat. Commun. 4:2111. doi: 10.1038/ncomms3111
Iwata, J. (2006). Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem. 281, 4035–4041. doi: 10.1074/jbc.M512283200
Jagadeeswaran, R., Vazquez, B. A., Thiruppathi, M., Ganesh, B. B., Ibanez, V., Cui, S., et al. (2017). Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp. Hematol. 50, 46–52. doi: 10.1016/j.exphem.2017.02.003
Jayapal, S. R., Lee, K. L., Ji, P., Kaldis, P., Lim, B., and Lodish, H. F. (2010). Down-regulation of Myc is essential for terminal erythroid maturation. J. Biol. Chem. 285, 40252–40265. doi: 10.1074/jbc.M110.181073
Ji, P., Jayapal, S. R., and Lodish, H. F. (2008). Enucleation of cultured mouse fetal erythroblasts requires Rac GTPases and mDia2. Nat. Cell Biol. 10, 314–321. doi: 10.1038/ncb1693
Ji, P., Yeh, V., Ramirez, T., Murata-Hori, M., and Lodish, H. F. (2010). Histone deacetylase 2 is required for chromatin condensation and subsequent enucleation of cultured mouse fetal erythroblasts. Haematologica 95, 2013–2021. doi: 10.3324/haematol.2010.029827
Ji, Y. Q., Zhang, Y. Q., Li, M. Q., Du, M. R., Wei, W. W., and Li, D. J. (2011). EPO improves the proliferation and inhibits apoptosis of trophoblast and decidual stromal cells through activating STAT-5 and inactivating p38 signal in human early pregnancy. Int. J. Clin. Exp. Pathol. 4, 765–774.
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L., and Turbide, C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420.
Johnstone, R. M., Bianchini, A., and Teng, K. (1989). Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood 19, 1844–1851.
Keerthivasan, G., Liu, H., Gump, J. M., Dowdy, S. F., Wickrema, A., and Crispino, J. D. (2012). A novel role for survivin in erythroblast enucleation. Haematologica 97, 1471–1479. doi: 10.3324/haematol.2011.061093
Keerthivasan, G., Small, S., Liu, H., Wickrema, A., and Crispino, J. D. (2010). Vesicle trafficking plays a novel role in erythroblast enucleation. Blood 116, 3331–3340. doi: 10.1182/blood-2010-03-277426
Khandros, E., Thom, C. S., D'Souza, J., and Weiss, M. J. (2012). Integrated protein quality-control pathways regulate free α-globin in murine β-thalassemia. Blood 119, 5265–5275. doi: 10.1182/blood-2011-12-397729
Killisch, I., Steinlein, P., Romisch, K., Hollinshead, R., Beug, H., and Griffiths, G. (1992). Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J. Cell Sci. 103, 211–232.
Kim, Y., Park, J., Kim, S., Song, S., Kwon, S.-K., Lee, S.-H., et al. (2008). PINK1 controls mitochondrial localization of Parkin through direct phosphorylation. Biochem. Biophys. Res. Commun. 377, 975–980. doi: 10.1016/j.bbrc.2008.10.104
Kobayashi, I., Ubukawa, K., Sugawara, K., Asanuma, K., Guo, Y.-M., Yamashita, J., et al. (2016). Erythroblast enucleation is a dynein-dependent process. Exp. Hematol. 44, 247–256.e12. doi: 10.1016/j.exphem.2015.12.003
Koury, S. T., Koury, M. J., and Bondurant, M. C. (1989). Cytoskeletal distribution and function during the maturation and enucleation of 13 mammalian erythroblasts. J. Cell Biol. 109, 3005–3013.
Koury, M. J. (2005). In vitro maturation of nascent reticulocytes to erythrocytes. Blood 105, 2168–2174. doi: 10.1182/blood-2004-02-0616
Koury, M. J., and Bondurant, M. C. (1990). Control of red cell production: the roles of programmed cell death (apoptosis) and erythropoietin. Transfusion 30, 673–674. doi: 10.1046/j.1537-2995.1990.30891020321.x
Krauss, S. W. (2005). Nuclear substructure reorganization during late-stage erythropoiesis is selective and does not involve caspase cleavage of major nuclear substructural proteins. Blood 106, 2200–2205. doi: 10.1182/blood-2005-04-1357
Kühn, H., Belkner, J., and Wiesner, R. (1990). Subcellular distribution of lipoxygenase products in rabbit reticulocyte membranes. FEBS J. 191, 221–227. doi: 10.1111/j.1432-1033.1990.tb19113.x
Kundu, M., Lindsten, T., Yang, C.-Y., Wu, J., Zhao, F., Zhang, J., et al. (2008). Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112, 1493–1502. doi: 10.1182/blood-2008-02-137398
Lee, E., Choi, H. S., Hwang, J. H., Hoh, J. K., Cho, Y.-H., and Baek, E. J. (2014). The RNA in reticulocytes is not just debris: it is necessary for the final stages of erythrocyte formation. Blood Cells. Mol. Dis. 53, 1–10. doi: 10.1016/j.bcmd.2014.02.009
Lee, J. C.-M., Gimm, J. A., Lo, A. J., Koury, M. J., Krauss, S. W., Mohandas, N., et al. (2004). Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood 103, 1912–1919. doi: 10.1182/blood-2003-03-0928
Lee, J., Giordano, S., and Zhang, J. (2012). Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 441, 523–540. doi: 10.1042/BJ20111451
Leonard, C., Conrard, L., Guthmann, M., Pollet, H., Carquin, M., Vermylen, C., et al. (2017). Contribution of plasma membrane lipid domains to red blood cell (re)shaping. Sci. Rep. 7:4264. doi: 10.1038/s41598-017-04388-z
Lithanatudom, P., Wannatung, T., Leecharoenkiat, A., Svasti, S., Fucharoen, S., and Smith, D. R. (2011). Enhanced activation of autophagy in β-thalassemia/Hb E erythroblasts during erythropoiesis. Ann. Hematol. 90, 747–758. doi: 10.1007/s00277-010-1152-5
Liu, J., Guo, X., Mohandas, N., Chasis, J. A., and An, X. (2010). Membrane remodeling during reticulocyte maturation. Blood 115, 2021–2027. doi: 10.1182/blood-2009-08-241182
Liu, L., Sakakibara, K., Chen, Q., and Okamoto, K. (2014). Receptor-mediated mitophagy in yeast and mammalian systems. Cell Res. 24, 787–795. doi: 10.1038/cr.2014.75
Lupo, F., Tibaldi, E., Matte, A., Sharma, A. K., Brunati, A. M., Alper, S. L., et al. (2016). A new molecular link between defective autophagy and erythroid abnormalities in chorea-acanthocytosis. Blood 128, 2976–2987. doi: 10.1182/blood-2016-07-727321
Magor, G. W., Tallack, M. R., Gillinder, K. R., Bell, C. C., McCallum, N., Williams, B., et al. (2015). KLF1-null neonates display hydrops fetalis and a deranged erythroid transcriptome. Blood 125, 2405–2417. doi: 10.1182/blood-2014-08-590968
Morgan, A. H., Hammond, V. J., Sakoh-Nakatogawa, M., Ohsumi, Y., Thomas, C. P., Blanchet, F., et al. (2015). A novel role for 12/15-lipoxygenase in regulating autophagy. Redox Biol. 4, 40–47. doi: 10.1016/j.redox.2014.11.005
Mortensen, M., Ferguson, D. J. P., Edelmann, M., Kessler, B., Morten, K. J., Komatsu, M., et al. (2010). Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc. Natl. Acad. Sci.U.S.A. 107, 832–837. doi: 10.1073/pnas.0913170107
Murakawa, T., Yamaguchi, O., Hashimoto, A., Hikoso, S., Takeda, T., Oka, T., et al. (2015). Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6:7527. doi: 10.1038/ncomms8527
Narendra, D. P., Jin, S. M., Tanaka, A., Suen, D.-F., Gautier, C. A., Shen, J., et al. (2010). PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8:e1000298. doi: 10.1371/journal.pbio.1000298
Notta, F., Zandi, S., Takayama, N., Dobson, S., Gan, O. I., Wilson, G., et al. (2016). Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351:aab2116. doi: 10.1126/science.aab2116
Novak, I., Kirkin, V., McEwan, D. G., Zhang, J., Wild, P., Rozenknop, A., et al. (2010). Nix is a selective autophagy receptor for mitochondrial clearance. EMBO Rep. 11, 45–51. doi: 10.1038/embor.2009.256
Palis, J. (2014). Primitive and definitive erythropoiesis in mammals. Front. Physiol. 5:3. doi: 10.3389/fphys.2014.00003
Pan, B.-T., Teng, K., Wu, C., Adam, M., and Johnstone, R. M. (1985). Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101, 942–948. doi: 10.1083/jcb.101.3.942
Pankiv, S., Clausen, T. H., Lamark, T., Brech, A., Bruun, J.-A., Outzen, H., et al. (2007). p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145. doi: 10.1074/jbc.M702824200
Park, S. M., Ou, J., Chamberlain, L., Simone, T. M., Yang, H., Virbasius, C.-M., et al. (2016). U2AF35(S34F) promotes transformation by directing aberrant aTG7 pre-mRNA 3′ end formation. Mol. Cell 62, 479–490. doi: 10.1016/j.molcel.2016.04.011
Parkins, A. C., Sharpe, A. H., and Orkin, S. H. (1995). Lethal β-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature 375, 318–322. doi: 10.1038/375318a0
Popova, E. Y., Krauss, S. W., Short, S. A., Lee, G., Villalobos, J., Etzell, J., et al. (2009). Chromatin condensation in terminally differentiating mouse erythroblasts does not involve special architectural proteins but depends on histone deacetylation. Chromosome Res. 17, 47–64. doi: 10.1007/s10577-008-9005-y
Rieu, S., Géminard, C., Rabesandratana, H., Sainte-Marie, J., and Vidal, M. (2000). Exosomes released during reticulocyte maturation bind to fibronectin via integrin α4β1. Eur. J. Biochem. 267, 583–590. doi: 10.1046/j.1432-1327.2000.01036.x
Sandoval, H., Thiagarajan, P., Dasgupta, S. K., Schumacher, A., Prchal, J. T., Chen, M., et al. (2008). Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235. doi: 10.1038/nature07006
Schweers, R. L., Zhang, J., Randall, M. S., Loyd, M. R., Li, W., Dorsey, F. C., et al. (2007). NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc. Natl. Acad. Sci. U.S.A. 104, 19500–19505. doi: 10.1073/pnas.0708818104
Soni, S., Bala, S., Gwynn, B., Sahr, K. E., Peters, L. L., and Hanspal, M. (2006). Absence of erythroblast macrophage protein (Emp) leads to failure of erythroblast nuclear extrusion. J. Biol. Chem. 281, 20181–20189. doi: 10.1074/jbc.M603226200
Swartz, K. L., Wood, S. N., Murthy, T., Ramirez, O., Qin, G., Pillai, M. M., et al. (2017). E2F-2 promotes nuclear condensation and enucleation of terminally differentiated erythroblasts. Mol. Cell. Biol. 37:e00274–e00216. doi: 10.1128/MCB.00274-16
Taher, A. T., Weatherall, D. J., and Cappellini, M. D. (2017). Thalassaemia. Lancet. doi: 10.1016/S0140-6736(17)31822-6. [Epub ahead of print].
Tooze, S. A., and Yoshimori, T. (2010). The origin of the autophagosomal membrane. Nat. Cell Biol. 12, 831–835. doi: 10.1038/ncb0910-831
Valentine, W. N., Fink, K., Paglia, D. E., Harris, S. R., and Adams, W. S. (1974). Hereditary hemolytic anemia with human erythrocyte pyrimidine 5′-nucleotidase deficiency. J. Clin. Invest. 54, 866–879. doi: 10.1172/JCI107826
Vijayvergiya, C., De Angelis, D., Walther, M., Kühn, H., Duvoisin, R. M., Smith, D. H., et al. (2004). High-level expression of rabbit 15-lipoxygenase induces collapse of the mitochondrial pH gradient in cell culture. Biochemistry 43, 15296–15302. doi: 10.1021/bi048745v
Wang, J., Fang, Y., Yan, L., Yuan, N., Zhang, S., Xu, L., et al. (2016). Erythroleukemia cells acquire an alternative mitophagy capability. Sci. Rep. 6:24641. doi: 10.1038/srep24641
Wang, J., Ramirez, T., Ji, P., Jayapal, S. R., Lodish, H. F., and Murata-Hori, M. (2012). Mammalian erythroblast enucleation requires PI3K-dependent cell polarization. J. Cell Sci. 125, 340–349. doi: 10.1242/jcs.088286
Waugh, R. E., McKenney, J. B., Bauserman, R. G., Brooks, D. M., Valeri, C. R., and Snyder, L. M. (1997). Surface area and volume changes during maturation of reticulocytes in the circulation of the baboon. J. Lab. Clin. Med. 129, 527–535. doi: 10.1016/S0022-2143(97)90007-X
Wei, Y., Chiang, W.-C., Sumpter, R., Mishra, P., and Levine, B. (2017). Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell 168, 224–238.e10. doi: 10.1016/j.cell.2016.11.042
Weil, M., Raff, M. C., and Braga, V. M. (1999). Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr. Biol. 9, 361–365. doi: 10.1016/S0960-9822(99)80162-6
Wong, Y. C., and Holzbaur, E. L. (2014). Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc. Natl. Acad. Sci. U.S.A. 111, E4439–E4448. doi: 10.1073/pnas.1405752111
Yokota, S., Haraguchi, C. M., and Oda, T. (2008). Induction of peroxisomal lon protease in rat liver after di-(2-ethylhexyl)phthalate treatment. Histochem. Cell Biol. 129, 73–83. doi: 10.1007/s00418-007-0328-0
Yokota, S., Oda, T., and Fahimi, H. D. (2001). The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J. Histochem. Cytochem. 49, 613–621. doi: 10.1177/002215540104900508
Yoshida, H., Kawane, K., Koike, M., Mori, Y., Uchiyama, Y., and Nagata, S. (2005). Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 437, 754–758. doi: 10.1038/nature03964
Yuan, Y., Zheng, Y., Zhang, X., Chen, Y., Wu, X., Wu, J., et al. (2017). BNIP3L/NIX-mediated mitophagy protects against ischemic brain injury independent of PARK2. Autophagy 13, 1754–1766. doi: 10.1080/15548627.2017.1357792
Zhang, J., Loyd, M. R., Randall, M. S., Waddell, M. B., Kriwacki, R. W., and Ney, P. A. (2012). A short linear motif in BNIP3L (NIX) mediates mitochondrial clearance in reticulocytes. Autophagy 8, 1325–1332. doi: 10.4161/auto.20764
Zhang, J., and Ney, P. (2009). Autophagy-dependent and-independent mechanisms of mitochondrial clearance during reticulocyte maturation. Autophagy 5, 1064–1065. doi: 10.4161/auto.5.7.9749
Keywords: erythropoiesis, mitophagy, organelle clearance, reticulocytes, enucleation, erythroblast maturation
Citation: Moras M, Lefevre SD and Ostuni MA (2017) From Erythroblasts to Mature Red Blood Cells: Organelle Clearance in Mammals. Front. Physiol. 8:1076. doi: 10.3389/fphys.2017.01076
Received: 11 October 2017; Accepted: 06 December 2017;
Published: 19 December 2017.
Edited by:
Lars Kaestner, Saarland University, GermanyReviewed by:
Pablo Martín-Vasallo, Universidad de La Laguna, SpainCopyright © 2017 Moras, Lefevre and Ostuni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariano A. Ostuni, bWFyaWFuby5vc3R1bmlAaW5zZXJtLmZy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.