- 1Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Center for Research in Myology, Sorbonne Universités Université Pierre et Marie Curie University Paris 06, Paris, France
- 2Institute of Chemistry and Biochemistry, Freie Universität Berlin, Berlin, Germany
- 3Berlin-Brandenburg School for Regenerative Therapies, Charité-Universitätsmedizin Berlin, Berlin, Germany
Skeletal muscle is not only translating chemical energy into mechanical work, it is also a highly adaptive and regenerative tissue whose architecture and functionality is determined by its mechanical and physical environment. Processing intra- and extracellular mechanical signaling cues contributes to the regulation of cell growth, survival, migration and differentiation. Yes-associated Protein (YAP), a transcriptional coactivator downstream of the Hippo pathway and its paralog, the transcriptional co-activator with PDZ-binding motif (TAZ), were recently found to play a key role in mechanotransduction in various tissues including skeletal muscle. Furthermore, YAP/TAZ modulate myogenesis and muscle regeneration and abnormal YAP activity has been reported in muscular dystrophy and rhabdomyosarcoma. Here, we summarize the current knowledge of mechanosensing and -signaling in striated muscle. We highlight the role of YAP signaling and discuss the different routes and hypotheses of its regulation in the context of mechanotransduction.
Introduction
Mechanotransduction refers to the conversion of mechanical inputs to intracellular biochemical and biophysical signals (Wang et al., 2009). Based on the observation that muscle grows in response to exercise and degrades when underutilized, studies on mechanotransduction have been performed already decades ago in skeletal muscle (Goldberg, 1968; Vandenburgh and Kaufman, 1979).
Current studies on mechanotransduction consider different models. The first relies on the initiation of mechanosignaling via stimulation of “mechanosensors.” These are thought to be adhesive, structural or transmembrane proteins, which could react with conformational changes to applied forces, transmitted by the extracellular matrix (ECM) or neighboring cells. These mechanical stimulations are then integrated into signaling pathways induced by soluble factors and consequently regulate transcriptional changes. In an alternative model, the cell itself is considered a compartmentalized mechanical body with given physical properties such as its viscosity, elasticity or stiffness. Here, the cellular mechanics are mainly defined through the actin, tubulin or septin cytoskeleton, intermediate filaments and the nuclear envelope and nuclear skeleton. These intracellular networks are connected to the ECM through adhesion complexes so that the cellular mechanics are in a permanent coordination with the extracellular constraints. According to this model, mechanical changes are not translated into one specialized mechanosensing pathway but into a simultaneous change in various cell processes, which are regulated by cytoskeletal dynamics, including the activation of signaling pathways.
The IGF-1-Akt-mTOR (Insulin-like growth factor I – protein kinase B/Akt - mammalian target of Rapamycin) pathway has emerged to be the main positive regulator of muscle mass (Sandri, 2008; Miyazaki et al., 2011; Schiaffino et al., 2013) and Myostatin-Smad3 has been identified as the main negative regulator of muscle mass (Wackerhage and Ratkevicius, 2008; Rodriguez et al., 2014). Furthermore, Yes-associated Protein (YAP), a transcriptional coactivator downstream of the Hippo pathway, has been shown to be involved in myogenesis, muscle homeostasis and muscle disorders. In parallel, YAP emerged as a key player in mechanotransduction (Dupont et al., 2011; Wackerhage et al., 2014) and several crosstalks between Akt/mTOR or TGFß/SMAD and the Hippo/Yap pathways have been identified which point to a role of YAP in regulating muscle mass through mechanical cues (Jang et al., 2007; Alarcón et al., 2009; Tumaneng et al., 2012; Grannas et al., 2015).
YAP
The transcriptional co-activator YAP was first described in 1994 as a 65 kDa binding partner of the Yes protein-tyrosine kinase (Sudol et al., 1995). YAP contains a transcription activation domain (TAD) located at the carboxy-terminal half (Yagi et al., 1999), while the amino-terminal half contains one or two WW domains (Sudol, 1996). These WW domains mediate interactions with proteins containing PPxY motifs. A plethora of different proteins bind to the WW domains of YAP including LATS1/2 (Oka et al., 2008), angiomotin (AMOT) (Zhao et al., 2011) and Smad7 (Ferrigno et al., 2002). In its active state, YAP localizes to the nucleus and regulates the activity of several transcription factors including RUNX, SMAD, p73 and ErbB4 and most importantly TEAD family transcription factors, since YAP and TEAD occupy about 80% of the same genomic loci (Zhao et al., 2008). Prominent target genes of YAP include CTGF, Cyclin D1, AREG, Birc5, and myogenic transcription factor Myf5 (Dong et al., 2007; Zhao et al., 2008; Zhang et al., 2009; Watt et al., 2010).
Together with its paralog TAZ (Transcriptional co-activator with PDZ-binding motif), YAP controls a wide range of cellular functions. During embryogenesis in mice YAP is expressed at all stages from blastocyst to perinatal stage. Homozygous disruption of the YAP allele in mice results in embryonic lethality and causes developmental arrest at E8.5. In contrast, TAZ shows a later onset and is not yet expressed at blastocyst stage (Morin-Kensicki et al., 2006). This indicates a unique role of YAP in embryonic development, for which TAZ does not compensate.
Nuclear YAP activity controls the transcription of genes involved in cell cycle control, typically driving proliferation and survival and inhibiting apoptosis (Dong et al., 2007). Thus, by balancing cell proliferation and death, YAP mediates cell contact inhibition in vitro (Zhao et al., 2007) and regulates organ size in vivo (Camargo et al., 2007; Dong et al., 2007; Xin et al., 2011). As a regulator of cell-cycle control, YAP misregulation can also lead to tumorigenesis. In several tissues YAP is known to function as an oncogene, while its upstream negative regulators and their adaptors have tumor suppressor function. Furthermore, YAP expression in MCF10A cells induces epithelial to mesenchymal transition (EMT) (Overholtzer et al., 2006). In summary, dysregulated YAP activity has been implicated in a wide range of tumor types including intestinal stem cells, hepatocellular, pancreatic, renal, colorectal, breast, and skeletal muscle cancer (Dong et al., 2007; Tremblay et al., 2014; Patel et al., 2015) which has been further reviewed by S. Plouffe (Plouffe et al., 2015).
Furthermore, YAP is involved in cell fate decisions and serves as a “stemness” factor in different progenitor cell pools of the body. Examples include progenitors in the intestinal crypt, neural progenitor cells in the neural tube, epidermal stem cells and satellite cells of skeletal muscle, where YAP activity promotes proliferation and blocks differentiation (Camargo et al., 2007; Cao et al., 2008; Watt et al., 2010; Schlegelmilch et al., 2011).
YAP also influences cell migration, which is also critical for muscle development and regeneration as activated satellite cells need to migrate out of their niche and along the basal lamina of the myofiber. It was shown, that YAP overexpression in MCF10A or HEK293 cells leads to increased migration and YAP knockdown abolishes migration in T47D cells and renal carcinoma cell lines (Haskins et al., 2014; Schütte et al., 2014; Sorrentino et al., 2014; Moroishi et al., 2015).
YAP capacity to balance proliferation, apoptosis and migration also makes it a regulator of regenerative processes in different tissues including intestine and heart muscle tissue as YAP knockdown severely impairs their regenerative capacity (Cai et al., 2010; Xin et al., 2013). In zebrafish, YAP activity in fin regeneration is based on cell density differences along the regenerating tissue, which leads to a graded control of tissue growth (Mateus et al., 2015).
In brief, YAP is a regulator of the cell cycle and cell fate decisions and consequently of development, organ size and tumorigenesis.
The Hippo Pathway and YAP Regulation
YAP activity is regulated very tightly by the Hippo pathway and a great number of crosstalks, whose interplays are not completely uncovered today (Figure 1). Originally identified by genetic studies in Drosophila, the Hippo signaling cascade functions as a highly conserved canonical upstream regulator of YAP activity (Harvey et al., 2003; Wu et al., 2003). At the core of the mammalian pathway is a kinase cassette containing Mammalian Ste20-like 1/2 kinase (MST1/2) and large tumor suppressor 1/2 kinase (LATS1/2) with their adaptors Salvador and MOBKL1A/1B (Mps 1 binder kinase activator-like 1A and 1B) (Figure 1). YAP activity is regulated by phosphorylation, predominantly through five different phosphorylation sites, which are located in HXRXXS consensus motifs for LATS1/2 kinases. The most intensely studied LATS mediated phosphorylation is at serine 127, which leads to binding of 14-3-3 proteins, sequestration of YAP in the cytoplasm and consequently termination of its nuclear activity (Zhao et al., 2007). Phosphorylation at Serine 381 by LATS1/2 however, primes YAP for phosphorylation by casein kinases CK1δ or CK1ε and subsequent ubiquitination via SCFβTRCP E3 ubiquitin ligase and proteasomal degradation (Zhao et al., 2010). LATS1/2 kinases are activated by phosphorylation of activated MST1/2 kinases. The activity of MST1/2 and LATS1/2 kinases are further regulated via different trans- and autophophorylation sites. For further reading see the reviews of Visser and Yang (2010) and Rawat and Chernoff (2015).
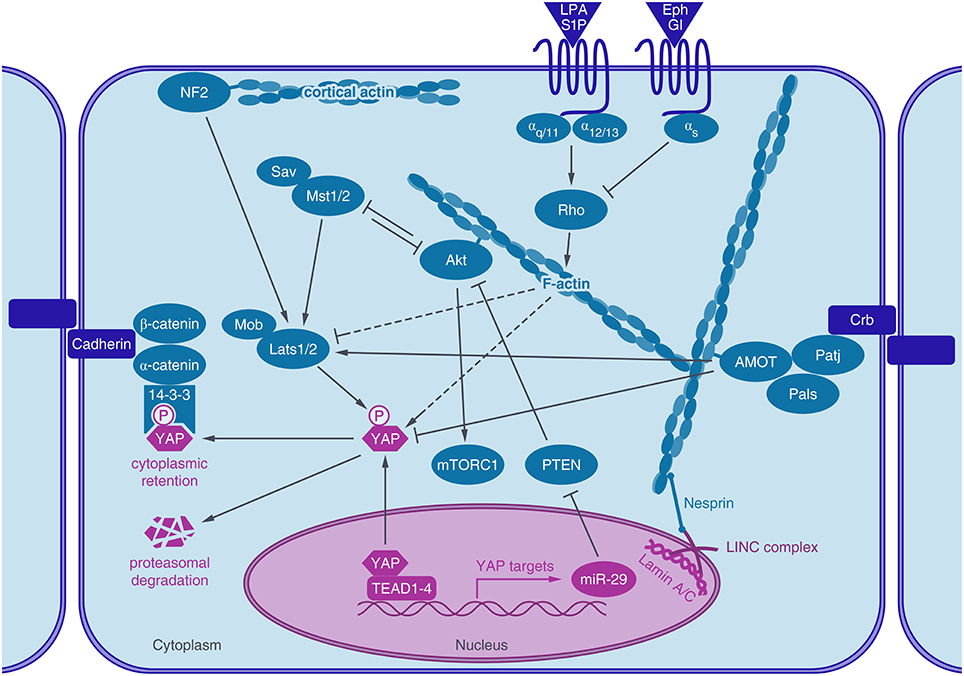
Figure 1. Actin associated proteins regulate YAP activity. The transcriptional coactivator YAP shuttles into the nucleus, where it activates TEAD mediated gene expression. After phosphorylation by LATS1/2 kinase, YAP binds to 14-3-3 proteins, leading to its cytoplasmic retention and degradation. YAP activity is regulated by the actin cytoskeleton. Actin stress fibers connect to the lamin meshwork in the nucleus via the LINC-complex. Rho GPTases are regulated by GPCR signaling which in turn regulates actin dynamics and YAP activity (dashed lines). Actin-binding proteins, like angiomotin (AMOT) or neurofibromin 2 (NF2/Merlin) are also known to regulate YAP activity, either through LATS or by direct interaction with YAP. Akt, a key regulator of the IGF-1- mTor pathway also binds to actin stress fibers, crosstalks to the Hippo pathway by interacting with MST1/2 and by YAP induced expression of a microRNA (miR-29) which inhibits the inhibition of Akt by targeting PTEN.
Moreover, YAP activity is balanced through a negative feedback loop. YAP-TEAD activity induces LATS2 kinase expression and activation of LATS1/2 kinases through Merlin/Neurofibromin 2 (NF2), leading to phosphorylation and inactivation of YAP (Moroishi et al., 2015). Thus, overshooting YAP activity including its tumorigenic potential can be counteracted by this intrinsic regulatory mechanism.
The regulation of YAP by canonical Hippo signaling in mammals was revealed in the context of contact inhibition of proliferation (CIP). Cells grown at low density show nuclear YAP and an inactive Hippo cascade, while at high cell density, the Hippo pathway is switched on and YAP is inactivated via LATS1/2 mediated phosphorylation (Zhao et al., 2007). A specific Hippo receptor as the primary trigger of the Hippo signaling cascade has not been identified yet and the dependence of YAP regulation on Hippo signaling in other contexts has been questioned (Aragona et al., 2013). The main upstream elements regulating YAP activity are discussed in the following paragraphs and are summarized in Figure 1. For further informations see the following publications (Schroeder and Halder, 2012; Johnson and Halder, 2014; Hansen et al., 2015).
Apicobasal Cell Polarity, Tight Junctions, and Adherens Junctions
YAP/TAZ proteins interact with several components of the Crumbs polarity complex and disrupting the Crumbs complex increases nuclear YAP (Varelas et al., 2010). Furthermore, AMOT proteins, which also localize to the Crumbs complex regulate YAP activity (Paramasivam et al., 2011; Zhao et al., 2011). Also, cadherin-catenin complexes were shown to regulate YAP localization and activity (Schlegelmilch et al., 2011; Silvis et al., 2011). Expression of E-cadherins as well as their association with α- and β-catenin are required for density dependent nuclear exclusion of YAP (Kim et al., 2011). Non-receptor tyrosine phosphatase PTPN14, which plays a role in regulating phosphorylation of β-catenin in adherens junctions (Wadham et al., 2003) was also shown to inhibit YAP activity by promoting its cytoplasmic localization, independently of its phosphatase activity (Michaloglou et al., 2013).
Also Merlin/NF2, another membrane-associated protein, which links cytoskeletal components with proteins in the cell membrane, regulates YAP. The tumor suppressor function of Merlin/NF2, inactivated in Neurofibromatosis type II, acts through the activation of the Hippo cascade most likely by binding and recruiting LATS to the plasma membrane, which in turn promotes LATS phosphorylation by MST (Yin et al., 2013).
Soluble Cues and Receptor Signaling
YAP can also be regulated through G-protein coupled receptors (GPCRs) and serum starvation inhibits YAP activity via reduced GPCR signaling. G12∕13-, Gq∕11-, and Gi∕o-coupled receptor agonists (e.g., Lysophosphatidic acid (LPA), sphingosine-1-phosphate (S1P)) activate YAP/TAZ while Epinephrine and Glucagon inhibit YAP/TAZ via Gs-coupled GPCR signaling (Yu et al., 2012). Recently, the G12∕13 subunit was also identified to be activated by the Wnt-FZD/ROR receptor, which would represent an alternative WNT signaling pathway acting through YAP (Park et al., 2015). The regulation of YAP by G-proteins has been shown to be either mediated by the Rho family of GTPases, actin dynamics and LATS (Yu et al., 2012) or PI3-kinase (PI3K) and phosphoinositide-dependent kinase (PDK1) (Gumbiner and Kim, 2014). In addition, epidermal growth factor receptor (EGFR) signaling activates YAP through activation of PI3-kinase (PI3K) and phosphoinositide-dependent kinase (PDK1), which binds to the Hippo core kinases complex (Fan et al., 2013). The neuregulin 1 (NRG1) activated receptor ERBB4, another member of the EGFR family interacts with YAP through its PPxY domain and activates YAP-mediated transcription (Haskins et al., 2014).
YAP: a Key Regulator in Mechanotransduction
There is increasing evidence that YAP is a key regulator of mechanotransduction. Pioneering work of Piccolo and co-workers showed that mechanical forces can serve as inputs for the regulation of YAP. By analyzing YAP localization and transcriptional response, they showed YAP activity to be regulated by ECM stiffness, cell-spreading or substrate rigidity (Dupont et al., 2011; Figures 2A–C). Along with these findings, the group of Sasaki proposed a model where cell morphology alone modulates YAP activity. By the use of a microdomain culture system the cell area of a single cell was defined preventing cell-cell contact (Wada et al., 2011). Guan and co-workers further excluded the requirement of focal adhesion sites for the regulation of YAP through cell morphology, by seeding epithelial cells on poly-lysine and attaching them via electrostatic forces (Zhao et al., 2012).
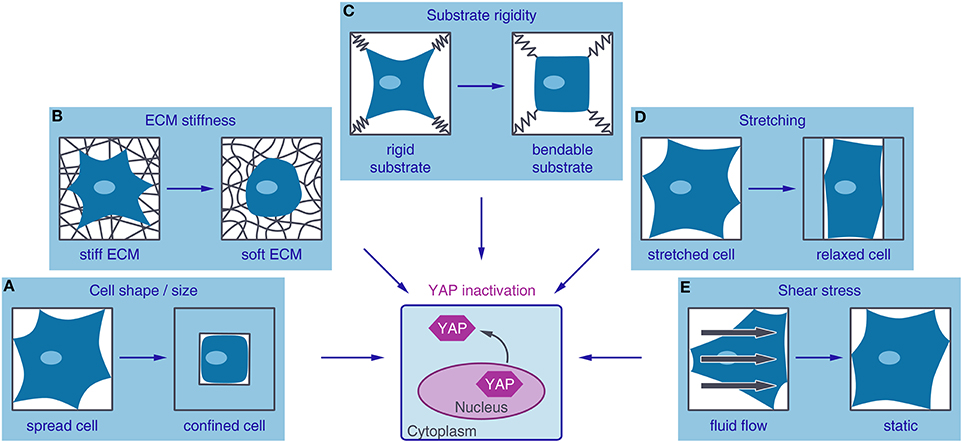
Figure 2. Different mechanical inputs regulate YAP activity. YAP (Yes-associated protein) is localized to the nucleus and active under mechanical conditions that lead to high intracellular tension such as a large adhesive area (A), stiff extracellular matrix (ECM) (B), non-bendable substrates (C), cell stretching (D), or fluid shear stress (E). Conditions favoring low contractile forces in the cell, such as small adhesive areas, soft ECM, bendable substrates, relaxation of stretching forces or culture in static media, lead to YAP inactivation by nuclear exclusion.
In addition, Piccolo and colleagues showed that YAP can be reactivated in postconfluent culture conditions by stretching the cells, while preventing that these cells lose cell-cell contact (Aragona et al., 2013). The reactivation of YAP by cyclic stretching has also been confirmed on soft surfaces together with an increase in cell spreading, stress fiber formation and proliferation (Cui et al., 2015; Figure 2D). The regulation of YAP by shear stress has only been rarely characterized so far and more research is needed to prove a shear stress-dependent YAP regulation. However, YAP was shown to be activated by fluid shear stress in osteoblasts (Kaneko et al., 2014). Furthermore, increased YAP expression, triggered by fluid shear stress, increased osteogenesis and decreased adipogenesis of hMSCs and initiated dedifferentiation of chondrocytes (Zhong et al., 2013; Figure 2E).
In addition, a mechanical memory was claimed. Long term culture of hMSCs on supraphysiologically stiff substrates can persistently activate YAP and transfer to soft hydrogels cannot inactivate YAP anymore (Yang et al., 2014).
YAP Regulation by Intracellular Stress
Cell shape and size, ECM stiffness and forces like traction or shear stress all reflect on the cytoskeleton. Published work provides compelling evidence for a critical role of actin dynamics in the regulation of YAP through mechanical cues. Particularly by correlating the activity of YAP with actin stress fiber formation and showing YAP inactivation by the use of F-actin or Rho inhibitors, but not by inhibiting microtubules or Rac1-GEFs (Dupont et al., 2011; Halder et al., 2012; Zhao et al., 2012). Also in vivo experiments on Drosophila reveal increased actin stress fiber assembly to correlate with YAP nuclear localization and overgrowth of the wing disc (Fernández et al., 2011; Sansores-Garcia et al., 2011). However, the specificity of this effect and the mechanism linking stress fiber formation to YAP activity is controversial and the focus of ongoing research.
Data providing deeper insight into how YAP is regulated by cell morphology and in coordination with cell-contact inhibition in epithelial cells was further published by the Piccolo lab. They found mechanical forces to be the overarching regulators of YAP in a multicellular context. The actin-capping and -severing proteins Cofilin, GapZ, and Gelsolin were identified as gatekeepers, limiting YAP activity in cells which experience low mechanical stress. By depleting actin-capping/severing proteins they showed that increased actin stress fiber formation can restore YAP activity in dense monolayers (Aragona et al., 2013). Assuming the cytoskeleton as the key transducer of mechanical cues into YAP signaling, the role of adaptor proteins like AMOT or other cytoskeletal structures like the tubulin or septin network remain to be further investigated. In regard to skeletal muscle, the role of the structural proteins of the sarcomere needs to be considered as well and their effects on YAP regulation remain to be examined. In the same way, the impact of the polynucleated organization of myofibers in mechanotransduction remain to be investigated.
In addition to changes in cytoskeletal structures, extracellular forces are also transmitted to the nucleus as the cytoskeleton is coupled to the nuclear envelope. The LINC-complex (Linker of Nucleoskeleton and Cytoskeleton), consisting of Nesprin and SUN proteins, thereby connects actin stress fibers to the nucleoskeleton (for further reading, see Lombardi and Lammerding, 2011). Recently, also YAP nuclear translocation was found to be dependent on the force transition to the nucleus through the LINC-complex. By the use of traction force maps, the transfer of the strain to the nucleus was considered essential for YAP localization and activity. Moreover, YAP nuclear relocalization after strain can be prevented by knocking down Nesprin, a protein of the LINC-complex (Driscoll et al., 2015). A-type lamin, an intermediate filament located at the inner nuclear membrane, binds to SUN proteins (Haque et al., 2006) and accumulates at the LINC-complex after applied tension (Guilluy et al., 2014). Consistently, satellite cell-derived myoblasts carrying a mutation in A-type lamin are unable to reactivate YAP after cyclic stretch (Bertrand et al., 2014). Mutations in nucleoskeletal proteins like A-type lamin or Emerin can cause several forms of muscular disorders whose pathophysiology is still not understood.
Transcription factors whose activity is regulated by actin dynamics are already known. For example, megakaryoblastic leukemia 1 (MKL1) binds to G-actin and is released when G-actin polymerizes to form F-actin (Miralles et al., 2003). Moreover MKL-1, together with YAP, has already been implicated in mechanosensing defects of LMNA mutant myoblasts (Bertrand et al., 2014), presumably through modulation of actin dynamics (Ho et al., 2013).
Role of LATS in Mechanotransduction
The dependence of mechanically induced YAP activation on the Hippo core kinases has been challenged. The Guan and Sasaki lab claimed YAP to be regulated by LATS, and LATS to be regulated by stress fibers (Wada et al., 2011; Zhao et al., 2012). By contrast, the Piccolo group found LATS phosphorylation not to be the primary mediator of YAP activity through mechanical cues. YAP and TAZ activity could not be rescued by knockdown of LATS1 and LATS2 after inhibition of actin polymerization (Aragona et al., 2013). Also for the highly discussed adaptor protein AMOT it is not sufficiently clarified if AMOT regulates YAP by either direct binding or via interaction with LATS2 protein (Paramasivam et al., 2011; Zhao et al., 2011). Nevertheless, if actin stress fibers inactivate LATS or sequester a LATS-independent inhibitor of YAP, or both, remains to be clarified.
YAP Crosstalks with Other Mechanosensitive Pathways
YAP interacts with components of other signaling pathways, which play a role in mechanotransduction as well. Canonical TGFβ/BMP signaling acts through SMADs and has been shown to be sensitive to mechanical inputs into the cell (Maeda et al., 2011; Kopf et al., 2014). YAP was shown to bind to activated SMAD1 proteins and to enhance their BMP induced transcriptional activity (Alarcón et al., 2009). YAP also interacts with SMAD2/3 in a TGFβ and cell density dependent manner (Grannas et al., 2015). In addition, YAP nuclear exclusion sequesters SMAD2/3 proteins to the cytoplasm and therefore suppresses TGFβ signaling (Varelas et al., 2010; Narimatsu et al., 2015). Furthermore, YAP interacts with the TGFβ signaling inhibitor SMAD7 (Ferrigno et al., 2002).
Wnt/β-catenin has been implicated in mechanotransduction as well (Huang and Ogawa, 2010; Kang and Robling, 2014). Cytoplasmic YAP inhibits Wnt/β-catenin activity by sequestering β-catenin in the cytoplasm (Imajo et al., 2012) and by interacting with its regulators disheveled and SHP2 (Barry et al., 2013; Tsutsumi et al., 2013). Overexpression of active YAP in mouse cardiomyocytes leads to increased β-catenin activity via the IGF-PI3K-AkT-GSK3β axis (Xin et al., 2011). Furthermore, Protein Kinase C zeta can phosphorylate both YAP and β-catenin to inhibit their nuclear activity (Llado et al., 2015). However, the impact of the crosstalk between YAP and TGFβ/BMP or Wnt/β-catenin signaling on mechanotransduction and its relevance in skeletal muscle homeostasis remain to be elucidated.
Mechanosensing and -Signaling in Skeletal Muscle
Muscle activity is known to be a major regulator of skeletal muscle mass, with an increase in mechanical loading resulting in muscle hypertrophy, and a decrease in mechanical loading resulting in muscle atrophy (Goldberg et al., 1975). This activity-induced muscle growth has been extensively studied (for recent review see Schiaffino et al., 2013; Piccirillo et al., 2014). Numerous signaling molecules have been identified to be involved and robust literature supports the role of the IGF-1-PI3K-Akt-mTOR pathway as a positive regulator and Myostatin-Smad3 as a negative regulator of muscle mass (Sandri, 2008; Wackerhage and Ratkevicius, 2008; Miyazaki and Esser, 2009; Rodriguez et al., 2014). Also YAP was identified to contribute to the regulation of muscle mass, as overexpression of YAP is sufficient to induce skeletal muscle hypertrophy and the amount of YAP protein is increased in skeletal muscle cells after mechanical overload (Goodman et al., 2015).
Mechanosensitive calcium channels and the kinase domain of titin, a structural protein of the sarcomere, have so far been identified as mechanosensors in skeletal muscle (Lange, 2005; Benavides Damm and Egli, 2014; Bogomolovas et al., 2014). They undergo conformational changes in response to mechanical load and thereby initiate signaling pathways, which regulate muscle mass. However, the detailed mechanisms and involved signaling pathways remain controversial.
IGF-1-PI3K-Akt-mTOR
Upon contraction, Insulin-like growth factor I (IGF-I) is released by the muscles and acts as an autocrine muscle hormone leading to muscle growth. The importance of the IGF-I-PI3K-Akt-mTOR pathway signaling has been largely confirmed and the multi-protein complex mTORC1 emerged to play a fundamental role in the regulation of skeletal muscle mass by regulating protein synthesis and cell size (Sandri, 2008; Frost and Lang, 2012). The activation of the serine/threonine-specific protein kinase Akt appears to be the crucial determinant of the cellular signaling processes and the transition point between atrophy and hypertrophy (Brooks and Myburgh, 2014). Nevertheless, the regulation of Akt/mTOR, especially its dependence on autocrine IGF-1 stimulation, has been an ongoing discussion point (Hornberger and Esser, 2004; Spangenburg, 2009). Clear evidence has been provided that mechanical loading is sufficient for Akt activation (Nader and Esser, 2001; Bolster et al., 2003; Sakamoto et al., 2003) albeit mTOR signaling can also be mechanically activated in the absence of Akt in the mouse model (Miyazaki et al., 2011). Interestingly, it has been shown that Akt binding to the cytoskeleton is dependent on mechanical stretch (Sawada and Sheetz, 2002). However, Hornberger and colleagues report that the inhibition of actin polymerization did not prevent Akt activation after mechanical strain (Hornberger et al., 2005). However, the mechanisms responsible for the mechanical activation of mTORC1 signaling are not yet fully elucidated and have recently been reviewed by Goodman (2014).
Crosstalk between YAP and the mTOR/Akt Signaling
Since mTOR regulates organ size through cell growth by regulating protein synthesis and the Hippo pathway regulates organ size by regulating proliferation it seems apparent that these pathways are coordinately regulated (Csibi and Blenis, 2012).
Akt has been reported to interact with the Hippo pathway via several routes. Akt has been shown to regulate YAP phosphorylation (Basu et al., 2003) but not by phosphorylating YAP directly (Zhao et al., 2007) but presumably through its interaction with MST1/2. In Drosophila, PI3K mediated Akt activity was shown to regulate the phosphorylation of yorkie (Yki), the YAP ortholog, most likely via activation of the MST1/2 ortholog hippo (hpo) or even upstream of hpo (Straßburger et al., 2012; Figure 1). In mammals, MST1/2 was already shown to be a binding partner of Akt and to reduce Akt activity (Cinar et al., 2007). Vice versa, MST1/2 was also found to be inhibited by the interaction with Akt (Jang et al., 2007). Finally, data demonstrate that the phosphorylation of MST1/2 can be induced by the mTOR signaling pathway and restrict MST1/2 function to inhibit cell growth in prostate cancer cells (Collak et al., 2012).
Besides protein-protein interactions, transcriptional crosstalks have been identified. Hippo pathway activity negatively regulates Akt transcription in Drosophilia (Straßburger et al., 2012; Ye et al., 2012). In human cell lines, the Hippo pathway was shown to regulate mTOR activity via the microRNA-29 (miR-29). YAP activity leads to the expression of miR-29 which inhibits the translation of PTEN, a phosphatase, which in its active state inhibits Akt (Figure 1). Consistently, YAP overexpression or Lats1/2 knockdown increase mTOR activity in skin sections (Tumaneng et al., 2012). However, YAP overexpression induced muscle hypertrophy was recently shown to act through an mTORC1-independent mechanism (Goodman et al., 2015).
YAP in Skeletal Muscle Physiology and Diseases
The role of YAP in cardiac muscle and its regeneration has emerged as a promising field of research (Papizan and Olson, 2014; Wackerhage et al., 2014; Zhou et al., 2015), but knowledge about YAP function in skeletal muscle is still limited. Interestingly, even before the discovery of YAP protein itself, evidence of its importance in muscle was supported by the identification of the muscle promoter elements MCAT, which are regulated by TEAD family transcription factors and are found in promotors of genes coding for contractile proteins (e.g., β-myosin heavy chain, skeletal α-actin) and regulators of myogenic differentiation (Myf5, Mrf4, myogenin; Mar and Ordahl, 1988; Yoshida, 2008; Ribas et al., 2011; Benhaddou et al., 2012). Furthermore, transgenic overexpression of TEAD-1 in mouse muscle leads to a change in myosin heavy chain isoform expression and therefore to a transition from fast to slow oxidative fiber phenotypes (Tsika et al., 2008). This indicates, that YAP activity regulates the transcription of genes important for muscle development, homeostasis and plasticity.
YAP in Skeletal Muscle Myogenesis and Regeneration
For muscle growth and regeneration, activated satellite cells expand, differentiate and then fuse with existing myofibers (Zhang and McLennan, 1994). In their quiescent state, Pax7 expressing muscle stem cells are located between the basal lamina and plasma membrane of the myofiber (Lepper and Fan, 2010). Upon activation, satellite cells start expressing Myf5 and MyoD and proliferate via asymmetric division. Part of this expanded satellite cell pool then undergoes differentiation, marked by myogenin expression and complete downregulation of Pax7. Finally, activated myoblasts fuse with existing myofibers, while the remaining pool of satellite cells self-renews and returns to quiescence (Tedesco et al., 2010; Figure 3).
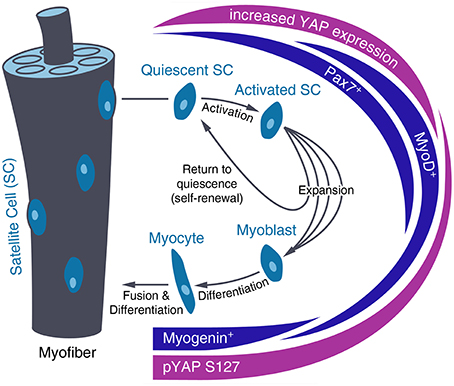
Figure 3. Regulation of YAP level and activity during satellite cell differentiation. After activation of quiescent satellite cells (SC), SCs divide and differentiate into myotubes that fuse with existing myofibers or self-renew and return to quiescence. During SC activation, YAP expression increases until this fate decision has been made. In differentiating SCs, YAP is inactivated by increased phosphorylation at Serine 127 (pYAP S127).
In this process, high YAP activity promotes proliferation of activated Pax7+ and MyoD+ muscle progenitor cells while YAP inactivation is needed for myogenic differentiation (Figure 3). Changes in YAP activity during satellite cell maturation have been shown in vitro and ex vivo on murine myoblasts. They show predominantly nuclear YAP during culture and YAP cytoplasmic translocation after myogenic differentiation along with decreased Yap mRNA and protein levels and increased YAP phosphorylation (Watt et al., 2010; Judson et al., 2012). Moreover, YAP knockdown reduces proliferation of satellite cell-derived myoblasts but has no impact on progression of their differentiation (Nagata et al., 2006). Further, evidence for the inhibition of skeletal muscle differentiation by YAP activity has been found in vivo on Xenopus laevis embryos, as YAP overexpression leads to inhibition of MyoD expression (Gee et al., 2011) and on mouse skeletal myofibers, which show reduced YAP levels during postnatal maturation (Watt et al., 2015). In vitro overexpression of constitutively active YAP in myoblast precursors results in increased Cyclin D1 and Myf5 expression as well as decreased myogenin, Mef2c and p21 expression, which inhibits terminal myogenic differentiation (Ishibashi et al., 2005; De Falco and De Luca, 2006; Watt et al., 2010).
Regarding YAP regulation, MST1 is activated during myoblast differentiation by caspase3 and active MST1 is needed for proper myoblast differentiation (Fernando et al., 2002). Furthermore, YAP has also been claimed to be involved in the activation of satellite cells by sphingosine-1-phosphate (S1P) mediated YAP activation (Nagata et al., 2006; Yu et al., 2012; Figure 1).
Also, culture conditions show evidence for a YAP dependent regulation of satellite cell differentiation. Established protocols optimized for myogenic differentiation share similarities with those for inactivation of YAP as they recommend high cell density, reduced serum conditions and substrates softer than standard cell culture plastic (Yaffe and Saxel, 1977; Kaushik and Engler, 2014).
YAP in Skeletal Muscle Homeostasis and Disease
In adult skeletal muscle, major Hippo pathway components including YAP are expressed in fast and slow muscle (Watt et al., 2010). In healthy muscle sections, YAP staining is weak and predominantly cytoplasmic, suggesting that YAP does not play a transcriptional role in the function of adult muscle (Crose et al., 2014). However, there are conflicting data on the role of YAP in muscle homeostasis and organ size, including atrophy and hypertrophy. Judson and colleagues report that high levels of a constitutively active YAP mutant drive degeneration, atrophy and necrosis in skeletal muscle fibers by use of a skeletal muscle fiber but not satellite cell specific knock-in mouse model (Judson et al., 2013). Gene expression profiling of these mice show similarities to muscles from mdx mice, a model for Duchenne muscular dystrophy (Hoffman et al., 1987). Interestingly, this muscle wasting phenotype is largely reversible as inactivation of the transgene rescues the degenerative phenotype.
Watt and colleagues on the other hand found YAP as a positive regulator of skeletal muscle size through a TEAD-dependent but mTOR-independent regulation of protein synthesis after knockdown or overexpression of YAP. Furthermore, they report YAP to limit neurogenic atrophy following muscle denervation, since YAP knock out prior to denervation dramatically increased atrophy of muscles (Watt et al., 2015). Goodman and colleagues support the hypertrophic role of YAP in muscle. The chronic mechanical overload model in mouse, which leads to a progressive increase in muscle mass, shows increased YAP expression and phosphorylation. Vice versa, overexpression of YAP in the mouse tibialis anterior leads to hypertrophy (Goodman et al., 2015). Furthermore, increasing muscle mass by blocking myostatin and activin signaling in mice in vivo leads to increased total YAP and YAP phosphorylation. Finally, physical exercises also increase YAP phosphorylation levels in mouse limb muscles (Hulmi et al., 2013).
A possible explanations for the contrasting results on the role of YAP in muscle might be the different time points, which have been analyzed, or the use of different YAP mutants, as the constitutive active YAP S127A mutant, in contrast to wild type YAP, cannot be subject to negative feedback regulation.
The upstream regulation of YAP during muscle homeostasis remains poorly characterized so far. By examining neurogenic atrophy, MST1 expression was found to be upregulated in fast- but not slow-dominant muscle and knockout of MST1 attenuated fast-dominant skeletal muscle wasting. Whether YAP phosphorylation and activity are affected here, has not been determined (Wei et al., 2013).
YAP signaling is also implicated in skeletal muscle diseases. Rhabdomyosarcomas are cancers of skeletal muscle tissue that are divided into different subtypes, the two main ones being embryonal rhabdomyosarcoma (eRMS) and alveolar rhabdomyosarcoma (aRMS). Levels of YAP phosphorylation show high variability between different RMS cell lines. Total YAP protein levels, however, are elevated in RMS cells and histological RMS tumor sections show increased nuclear YAP stainings (Crose et al., 2014). Overexpression of constitutively active YAP in activated but not quiescent satellite cells leads to muscle tumors similar to eRMS. In vitro and in vivo YAP knockdown experiments revealed that lowering YAP expression in human eRMS can rescue tumorigenicity (Tremblay et al., 2014). aRMS is characterized by expression of the paired box 3-forkhead box protein O1 (PAX3-FOXO1) (Galili et al., 1993; Shapiro et al., 1993). PAX3-FOXO1 directly upregulates RASSF4 in aRMS cells and tumors, which associates with MST1 and inhibits its tumor suppressor function, leading to tumorgenesis (Crose et al., 2014).
Furthermore, muscles of mdx mice show elevated levels of phosphorylated and total YAP protein (Judson et al., 2013). Finally, the first evidence of an involvement of YAP-mediated mechanosensing defects in patients with LMNA-related congenital muscular dystrophy has been reported recently (Bertrand et al., 2014). Along with several defects in the organization of the cytoskeleton, YAP was found not to respond to the changing mechanical properties of their environment. While YAP is excluded from the nucleus in soft environment in healthy control cells, patient derived cells maintain nuclear YAP localization in soft environment.
Concluding Remarks and Perspectives
YAP has emerged as an important player in mechanotransduction, transmitting mechanical cues into a transcriptional cell response. At the same time, YAP has been shown to be involved in skeletal muscle development and regeneration, as YAP contributes to the regulation of activation, proliferation and differentiation of satellite cells. Beyond that, YAP signaling is also important in adult skeletal muscle homeostasis as misregulation can lead to atrophy or hypertrophy and aberrant YAP activities have been observed in disease states, including skeletal muscle dystrophies. Adult muscle homeostasis again is mainly regulated by muscle activity, which is a mechanical cue itself. Akt-mTOR signaling is widely accepted as the main regulatory pathway defining muscle mass, but the autocrine activation of this pathway by IGF-1 is controversial and several crosstalks to YAP have been identified. Precise mechanisms by which YAP is regulated by mechanical cues are still unknown. Cytoskeletal and presumably also nucleoskeletal tension, in particular actin dynamics and Rho signaling, have been identified as important players of mechanotransduction on YAP, but the detailed mechanism still remains to be elucidated. Uncovering this mechanism, also in regard to the specially organized cytoskeleton of postmitotic myofibers, could reveal important insights into our understanding of muscle homeostasis and subsequently into the physiopathology of muscle diseases.
Author Contributions
MF conceived and wrote the article, PR contributed to the writing and designed the figures. PK and CC revised the article. All authors approved the work for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work has been supported by DFG funding and the Sorbonne Universités UPMC Univ Paris through Myograd (fellowship to MF); by the DFG through the Berlin-Brandenburg School for Regenerative Therapies GSC 203 (fellowship to PR) and by the DFG through the Research Unit RU2165 “Regeneration in Aged Individuals” (to PK). We also thank Prof. Dr. Hans-Georg Simon for helpful discussions.
References
Alarcón, C., Zaromytidou, A.-I., Xi, Q., Gao, S., Yu, J., Fujisawa, S., et al. (2009). Nuclear CDKs Drive Smad Transcriptional Activation and Turnover in BMP and TGF-β Pathways. Cell 139, 757–769. doi: 10.1016/j.cell.2009.09.035
Aragona, M., Panciera, T., Manfrin, A., Giulitti, S., Michielin, F., Elvassore, N., et al. (2013). A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154, 1047–1059. doi: 10.1016/j.cell.2013.07.042
Barry, E. R., Morikawa, T., Butler, B. L., Shrestha, K., De La Rosa, R., Yan, K. S., et al. (2013). Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493, 106–110. doi: 10.1038/nature11693
Basu, S., Totty, N. F., Irwin, M. S., Sudol, M., and Downward, J. (2003). Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Mol Cell 11, 11–23. doi: 10.1016/S1097-2765(02)00776-1
Benavides Damm, T., and Egli, M. (2014). Calcium's role in mechanotransduction during muscle development. Cell Physiol. Biochem. 33, 249–272. doi: 10.1159/000356667
Benhaddou, A., Keime, C., Ye, T., Morlon, A., Michel, I., Jost, B., et al. (2012). Transcription factor TEAD4 regulates expression of myogenin and the unfolded protein response genes during C2C12 cell differentiation. Cell Death Differ. 19, 220–231. doi: 10.1038/cdd.2011.87
Bertrand, A. T., Ziaei, S., Ehret, C., Duchemin, H., Mamchaoui, K., Bigot, A., et al. (2014). Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J. Cell. Sci. 127, 2873–2884. doi: 10.1242/jcs.144907
Bogomolovas, J., Gasch, A., Simkovic, F., Rigden, D. J., Labeit, S., and Mayans, O. (2014). Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line. Open Biol. 4:140041. doi: 10.1098/rsob.140041
Bolster, D. R., Kubica, N., Crozier, S. J., Williamson, D. L., Farrell, P. A., Kimball, S. R., et al. (2003). Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J. Physiol. 553, 213–220. doi: 10.1113/jphysiol.2003.047019
Brooks, N. E., and Myburgh, K. H. (2014). Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front. Physiol. 5:99. doi: 10.3389/fphys.2014.00099
Cai, J., Zhang, N., Zheng, Y., de Wilde, R. F., Maitra, A., and Pan, D. (2010). The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 24, 2383–2388. doi: 10.1101/gad.1978810
Camargo, F. D., Gokhale, S., Johnnidis, J. B., Fu, D., Bell, G. W., Jaenisch, R., et al. (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060. doi: 10.1016/j.cub.2007.10.039
Cao, X., Pfaff, S. L., and Gage, F. H. (2008). YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 22, 3320–3334. doi: 10.1101/gad.1726608
Cinar, B., Fang, P. K., Lutchman, M., Di Vizio, D., Adam, R. M., Pavlova, N., et al. (2007). The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J. 26, 4523–4534. doi: 10.1038/sj.emboj.7601872
Collak, F. K., Yagiz, K., Luthringer, D. J., Erkaya, B., and Cinar, B. (2012). Threonine-120 phosphorylation regulated by phosphoinositide-3-Kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-Like Kinase 1. J. Biol. Chem. 287, 23698–23709. doi: 10.1074/jbc.M112.358713
Crose, L. E., Galindo, K. A., Kephart, J. G., Chen, C., Fitamant, J., Bardeesy, N., et al. (2014). Alveolar rhabdomyosarcoma-associated PAX3-FOXO1 promotes tumorigenesis via Hippo pathway suppression. J. Clin. Invest. 124, 285–296. doi: 10.1172/JCI67087
Csibi, A., and Blenis, J. (2012). Hippo-YAP and mTOR pathways collaborate to regulate organ size. Nat. Cell Biol. 14, 1244–1245. doi: 10.1038/ncb2634
Cui, Y., Hameed, F. M., Yang, B., Lee, K., Pan, C. Q., Park, S., et al. (2015). Cyclic stretching of soft substrates induces spreading and growth. Nat. Commun. 6, 6333. doi: 10.1038/ncomms7333
De Falco, M., and De Luca, A. (2006). Involvement of cdks and cyclins in muscle differentiation. Eur. J. Histochem. 50, 19–23.
Dong, J., Feldmann, G., Huang, J., Wu, S., Zhang, N., Comerford, S. A., et al. (2007). Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130, 1120–1133. doi: 10.1016/j.cell.2007.07.019
Driscoll, T. P., Cosgrove, B. D., Heo, S. J., Shurden, Z. E., and Mauck, R. L. (2015). Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 108, 2783–2793. doi: 10.1016/j.bpj.2015.05.010
Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183. doi: 10.1038/nature10137
Fan, R., Kim, N. G., and Gumbiner, B. M. (2013). Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. U.S.A. 110, 2569–2574. doi: 10.1073/pnas.1216462110
Fernández, B. G., Gaspar, P., Brás-Pereira, C., Jezowska, B., Rebelo, S. R., and Janody, F. (2011). Actin-capping protein and the hippo pathway regulate F-actin and tissue growth in Drosophila. Development 138, 2337–2346. doi: 10.1242/dev.063545
Fernando, P., Kelly, J. F., Balazsi, K., Slack, R. S., and Megeney, L. A. (2002). Caspase 3 activity is required for skeletal muscle differentiation. Proc. Natl. Acad. Sci. U.S.A. 99, 11025–11030. doi: 10.1073/pnas.162172899
Ferrigno, O., Lallemand, F., Verrecchia, F., L'hoste, S., Camonis, J., Atfi, A., et al. (2002). Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-β/Smad signaling. Oncogene 21, 4879–4884. doi: 10.1038/sj.onc.1205623
Frost, R. A., and Lang, C. H. (2012). Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol. Metab. Clin. North. Am. 41, 297–322, vi. doi: 10.1016/j.ecl.2012.04.012
Galili, N., Davis, R. J., Fredericks, W. J., Mukhopadhyay, S., Rauscher, F. J. III., Emanuel, B. S., et al. (1993). Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat. Genet. 5, 230–235. doi: 10.1038/ng1193-230
Gee, S. T., Milgram, S. L., Kramer, K. L., Conlon, F. L., and Moody, S. A. (2011). Yes-associated protein 65 (YAP) expands neural progenitors and regulates Pax3 expression in the neural plate border zone. PLoS ONE 6:e20309. doi: 10.1371/journal.pone.0020309
Goldberg, A. (1968). Protein synthiesis during work-induced growth of skeletal muscle. J. Cell Biol. 36, 653–658. doi: 10.1083/jcb.36.3.653
Goldberg, A. L., Etlinger, J. D., Goldspink, D. F., and Jablecki, C. (1975). Mechanism of work-induced hypertrophy of skeletal muscle. Med. Sci. Sports 7, 185–198.
Goodman, C. A. (2014). The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 166, 43–95. doi: 10.1007/112_2013_17
Goodman, C. A., Dietz, J. M., Jacobs, B. L., McNally, R. M., You, J.-S., and Hornberger, T. A. (2015). Yes-associated protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Lett. 589, 1491–1497. doi: 10.1016/j.febslet.2015.04.047
Grannas, K., Arngården, L., Lönn, P., Mazurkiewicz, M., Blokzijl, A., Zieba, A., et al. (2015). Crosstalk between Hippo and TGFβ: subcellular localization of YAP/TAZ/Smad complexes. J. Mol. Biol. 427, 3407–3415. doi: 10.1016/j.jmb.2015.04.015
Guilluy, C., Osborne, L. D., Van Landeghem, L., Sharek, L., Superfine, R., Garcia-Mata, R., et al. (2014). Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 16, 376–381. doi: 10.1038/ncb2927
Gumbiner, B. M., and Kim, N. G. (2014). The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 127, 709–717. doi: 10.1242/jcs.140103
Halder, G., Dupont, S., and Piccolo, S. (2012). Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol. 13, 591–600. doi: 10.1038/nrm3416
Hansen, C. G., Moroishi, T., and Guan, K. L. (2015). YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513. doi: 10.1016/j.tcb.2015.05.002
Haque, F., Lloyd, D. J., Smallwood, D. T., Dent, C. L., Shanahan, C. M., Fry, A. M., et al. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006
Harvey, K. F., Pfleger, C. M., and Hariharan, I. K. (2003). The drosophila mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114, 457–467. doi: 10.1016/S0092-8674(03)00557-9
Haskins, J. W., Nguyen, D. X., and Stern, D. F. (2014). Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal 7, ra116. doi: 10.1126/scisignal.2005770
Ho, C. Y., Jaalouk, D. E., Vartiainen, M. K., and Lammerding, J. (2013). Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature 497, 507–511. doi: 10.1038/nature12105
Hoffman, E. P., Brown, R. H. Jr., and Kunkel, L. M. (1987). Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928. doi: 10.1016/0092-8674(87)90579-4
Hornberger, T. A., Armstrong, D. D., Koh, T. J., Burkholder, T. J., and Esser, K. A. (2005). Intracellular signaling specificity in response to uniaxial vs. multiaxial stretch: implications for mechanotransduction. Am. J. Physiol., Cell Physiol. 288, C185–C194. doi: 10.1152/ajpcell.00207.2004
Hornberger, T. A., and Esser, K. A. (2004). Mechanotransduction and the regulation of protein synthesis in skeletal muscle. Proc. Nutr. Soc. 63, 331–335. doi: 10.1079/PNS2004357
Huang, C., and Ogawa, R. (2010). Mechanotransduction in bone repair and regeneration. FASEB J. 24, 3625–3632. doi: 10.1096/fj.10-157370
Hulmi, J. J., Oliveira, B. M., Silvennoinen, M., Hoogaars, W. M., Ma, H., Pierre, P., et al. (2013). Muscle protein synthesis, mTORC1/MAPK/Hippo signaling, and capillary density are altered by blocking of myostatin and activins. Am. J. Physiol. Endocrinol. Metab. 304, E41–E50. doi: 10.1152/ajpendo.00389.2012
Imajo, M., Miyatake, K., Iimura, A., Miyamoto, A., and Nishida, E. (2012). A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 31, 1109–1122. doi: 10.1038/emboj.2011.487
Ishibashi, J., Perry, R. L., Asakura, A., and Rudnicki, M. A. (2005). MyoD induces myogenic differentiation through cooperation of its NH2- and COOH-terminal regions. J. Cell Biol. 171, 471–482. doi: 10.1083/jcb.200502101
Jang, S. W., Yang, S. J., Srinivasan, S., and Ye, K. (2007). Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J. Biol. Chem. 282, 30836–30844. doi: 10.1074/jbc.M704542200
Johnson, R., and Halder, G. (2014). The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79. doi: 10.1038/nrd4161
Judson, R. N., Gray, S. R., Walker, C., Carroll, A. M., Itzstein, C., Lionikas, A., et al. (2013). Constitutive expression of Yes-associated protein (Yap) in adult skeletal muscle fibres induces muscle atrophy and myopathy. PLoS ONE 8:e59622. doi: 10.1371/journal.pone.0059622
Judson, R. N., Tremblay, A. M., Knopp, P., White, R. B., Urcia, R., De Bari, C., et al. (2012). The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J. Cell Sci. 125, 6009–6019. doi: 10.1242/jcs.109546
Kaneko, K., Ito, M., Naoe, Y., Lacy-Hulbert, A., and Ikeda, K. (2014). Integrin αv in the mechanical response of osteoblast lineage cells. Biochem. Biophys. Res. Commun. 447, 352–357. doi: 10.1016/j.bbrc.2014.04.006
Kang, K. S., and Robling, A. G. (2014). New insights into Wnt-Lrp5/6-β-catenin signaling in mechanotransduction. Front. Endocrinol. (Lausanne). 5:246. doi: 10.3389/fendo.2014.00246
Kaushik, G., and Engler, A. J. (2014). From stem cells to cardiomyocytes: the role of forces in cardiac maturation, aging, and disease. Prog. Mol. Biol. Transl. Sci. 126, 219–242. doi: 10.1016/B978-0-12-394624-9.00009-9
Kim, N. G., Koh, E., Chen, X., and Gumbiner, B. M. (2011). E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. U.S.A. 108, 11930–11935. doi: 10.1073/pnas.1103345108
Kopf, J., Paarmann, P., Hiepen, C., Horbelt, D., and Knaus, P. (2014). BMP growth factor signaling in a biomechanical context. Biofactors 40, 171–187. doi: 10.1002/biof.1137
Lange, S. (2005). The kinase domain of titin controls muscle gene expression and protein turnover. Science 308, 1599–1603. doi: 10.1126/science.1110463
Lepper, C., and Fan, C. M. (2010). Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436. doi: 10.1002/dvg.20630
Llado, V., Nakanishi, Y., Duran, A., Reina-Campos, M., Shelton, P. M., Linares, J. F., et al. (2015). Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of β-Catenin and Yap by PKCζ. Cell Rep. 10, 740–754. doi: 10.1016/j.celrep.2015.01.007
Lombardi, M. L., and Lammerding, J. (2011). Keeping the LINC: the importance of nucleocytoskeletal coupling in intracellular force transmission and cellular function. Biochem. Soc. Trans. 39, 1729–1734. doi: 10.1042/BST20110686
Maeda, T., Sakabe, T., Sunaga, A., Sakai, K., Rivera, A. L., Keene, D. R., et al. (2011). Conversion of mechanical force into TGF-β-mediated biochemical signals. Curr. Biol. 21, 933–941. doi: 10.1016/j.cub.2011.04.007
Mar, J. H., and Ordahl, C. P. (1988). A conserved CATTCCT motif is required for skeletal muscle-specific activity of the cardiac troponin T gene promoter. Proc. Natl. Acad. Sci. U.S.A. 85, 6404–6408. doi: 10.1073/pnas.85.17.6404
Mateus, R., Lourenço, R., Fang, Y., Brito, G., Farinho, A., Valério, F., et al. (2015). Control of tissue growth by Yap relies on cell density and F-actin in zebrafish fin regeneration. Development 142, 2752–2763. doi: 10.1242/dev.119701
Michaloglou, C., Lehmann, W., Martin, T., Delaunay, C., Hueber, A., Barys, L., et al. (2013). The tyrosine phosphatase PTPN14 is a negative regulator of YAP activity. PLoS ONE 8:e61916. doi: 10.1371/journal.pone.0061916
Miralles, F., Posern, G., Zaromytidou, A. I., and Treisman, R. (2003). Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 113, 329–342. doi: 10.1016/S0092-8674(03)00278-2
Miyazaki, M., and Esser, K. A. (2009). Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J. Appl. Physiol. (1985) 106, 1367–1373. doi: 10.1152/japplphysiol.91355.2008
Miyazaki, M., McCarthy, J. J., Fedele, M. J., and Esser, K. A. (2011). Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J. Physiol. 589, 1831–1846. doi: 10.1113/jphysiol.2011.205658
Morin-Kensicki, E. M., Boone, B. N., Howell, M., Stonebraker, J. R., Teed, J., Alb, J. G., et al. (2006). Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 26, 77–87. doi: 10.1128/MCB.26.1.77-87.2006
Moroishi, T., Park, H. W., Qin, B., Chen, Q., Meng, Z., Plouffe, S. W., et al. (2015). A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 29, 1271–1284. doi: 10.1101/gad.262816.115
Nader, G. A., and Esser, K. A. (2001). Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J. Appl. Physiol. (1985) 90, 1936–1942.
Nagata, Y., Partridge, T. A., Matsuda, R., and Zammit, P. S. (2006). Entry of muscle satellite cells into the cell cycle requires sphingolipid signaling. J. Cell Biol. 174, 245–253. doi: 10.1083/jcb.200605028
Narimatsu, M., Samavarchi-Tehrani, P., Varelas, X., and Wrana, J. L. (2015). Distinct polarity cues direct Taz/Yap and TGFβ receptor localization to differentially control TGFβ-induced Smad signaling. Dev. Cell 32, 652–656. doi: 10.1016/j.devcel.2015.02.019
Oka, T., Mazack, V., and Sudol, M. (2008). Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J. Biol. Chem. 283, 27534–27546. doi: 10.1074/jbc.M804380200
Overholtzer, M., Zhang, J., Smolen, G. A., Muir, B., Li, W., Sgroi, D. C., et al. (2006). Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. U.S.A. 103, 12405–12410. doi: 10.1073/pnas.0605579103
Papizan, J. B., and Olson, E. N. (2014). Hippo in the path to heart repair. Circ. Res. 115, 332–334. doi: 10.1161/CIRCRESAHA.114.304389
Paramasivam, M., Sarkeshik, A., Yates, J. R. III., Fernandes, M. J., and Mccollum, D. (2011). Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol. Biol. Cell 22, 3725–3733. doi: 10.1091/mbc.E11-04-0300
Park, H. W., Kim, Y. C., Yu, B., Moroishi, T., Mo, J. S., Plouffe, S. W., et al. (2015). Alternative Wnt signaling activates YAP/TAZ. Cell 162, 780–794. doi: 10.1016/j.cell.2015.07.013
Patel, P. H., Dutta, D., and Edgar, B. A. (2015). Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 17, 1182–1192. doi: 10.1038/ncb3214
Piccirillo, R., Demontis, F., Perrimon, N., and Goldberg, A. L. (2014). Mechanisms of muscle growth and atrophy in mammals and Drosophila. Dev. Dyn. 243, 201–215. doi: 10.1002/dvdy.24036
Plouffe, S. W., Hong, A. W., and Guan, K. L. (2015). Disease implications of the Hippo/YAP pathway. Trends Mol. Med. 21, 212–222. doi: 10.1016/j.molmed.2015.01.003
Rawat, S. J., and Chernoff, J. (2015). Regulation of mammalian Ste20 (Mst) kinases. Trends Biochem. Sci. 40, 149–156. doi: 10.1016/j.tibs.2015.01.001
Ribas, R., Moncaut, N., Siligan, C., Taylor, K., Cross, J. W., Rigby, P. W., et al. (2011). Members of the TEAD family of transcription factors regulate the expression of Myf5 in ventral somitic compartments. Dev. Biol. 355, 372–380. doi: 10.1016/j.ydbio.2011.04.005
Rodriguez, J., Vernus, B., Chelh, I., Cassar-Malek, I., Gabillard, J. C., Hadj Sassi, A., et al. (2014). Myostatin and the skeletal muscle atrophy and hypertrophy signaling pathways. Cell. Mol. Life Sci. 71, 4361–4371. doi: 10.1007/s00018-014-1689-x
Sakamoto, K., Aschenbach, W. G., Hirshman, M. F., and Goodyear, L. J. (2003). Akt signaling in skeletal muscle: regulation by exercise and passive stretch. Am. J. Physiol. Endocrinol. Metab. 285, E1081–E1088. doi: 10.1152/ajpendo.00228.2003
Sandri, M. (2008). Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda). 23, 160–170. doi: 10.1152/physiol.00041.2007
Sansores-Garcia, L., Bossuyt, W., Wada, K., Yonemura, S., Tao, C., Sasaki, H., et al. (2011). Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 30, 2325–2335. doi: 10.1038/emboj.2011.157
Sawada, Y., and Sheetz, M. P. (2002). Force transduction by Triton cytoskeletons. J. Cell Biol. 156, 609–615. doi: 10.1083/jcb.200110068
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B., and Sandri, M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 280, 4294–4314. doi: 10.1111/febs.12253
Schlegelmilch, K., Mohseni, M., Kirak, O., Pruszak, J., Rodriguez, J. R., Zhou, D., et al. (2011). Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795. doi: 10.1016/j.cell.2011.02.031
Schroeder, M. C., and Halder, G. (2012). Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin. Cell Dev. Biol. 23, 803–811. doi: 10.1016/j.semcdb.2012.06.001
Schütte, U., Bisht, S., Heukamp, L. C., Kebschull, M., Florin, A., Haarmann, J., et al. (2014). Hippo signaling mediates proliferation, invasiveness, and metastatic potential of clear cell renal cell carcinoma. Transl. Oncol. 7, 309–321. doi: 10.1016/j.tranon.2014.02.005
Shapiro, D. N., Sublett, J. E., Li, B., Downing, J. R., and Naeve, C. W. (1993). Fusion of PAX3 to a member of the forkhead family of transcription factors in human alveolar rhabdomyosarcoma. Cancer Res. 53, 5108–5112.
Silvis, M. R., Kreger, B. T., Lien, W. H., Klezovitch, O., Rudakova, G. M., Camargo, F. D., et al. (2011). α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4, ra33. doi: 10.1126/scisignal.2001823
Sorrentino, G., Ruggeri, N., Specchia, V., Cordenonsi, M., Mano, M., Dupont, S., et al. (2014). Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366. doi: 10.1038/ncb2936
Spangenburg, E. E. (2009). Changes in muscle mass with mechanical load: possible cellular mechanisms. Appl. Physiol. Nutr. Metab. 34, 328–335. doi: 10.1139/H09-010
Straßburger, K., Tiebe, M., Pinna, F., Breuhahn, K., and Teleman, A. A. (2012). Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev. Biol. 367, 187–196. doi: 10.1016/j.ydbio.2012.05.008
Sudol, M. (1996). Structure and function of the WW domain. Prog. Biophys. Mol. Biol. 65, 113–132. doi: 10.1016/S0079-6107(96)00008-9
Sudol, M., Bork, P., Einbond, A., Kastury, K., Druck, T., Negrini, M., et al. (1995). Characterization of the Mammalian YAP (Yes-associated Protein) gene and its role in defining a novel protein module, the WW Domain. J. Biol. Chem. 270, 14733–14741. doi: 10.1074/jbc.270.24.14733
Tedesco, F. S., Dellavalle, A., Diaz-Manera, J., Messina, G., and Cossu, G. (2010). Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 120, 11–19. doi: 10.1172/JCI40373
Tremblay, A. M., Missiaglia, E., Galli, G. G., Hettmer, S., Urcia, R., Carrara, M., et al. (2014). The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell 26, 273–287. doi: 10.1016/j.ccr.2014.05.029
Tsika, R. W., Schramm, C., Simmer, G., Fitzsimons, D. P., Moss, R. L., and Ji, J. (2008). Overexpression of TEAD-1 in transgenic mouse striated muscles produces a slower skeletal muscle contractile phenotype. J. Biol. Chem. 283, 36154–36167. doi: 10.1074/jbc.M807461200
Tsutsumi, R., Masoudi, M., Takahashi, A., Fujii, Y., Hayashi, T., Kikuchi, I., et al. (2013). YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev. Cell 26, 658–665. doi: 10.1016/j.devcel.2013.08.013
Tumaneng, K., Schlegelmilch, K., Russell, R. C., Yimlamai, D., Basnet, H., Mahadevan, N., et al. (2012). YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat. Cell Biol. 14, 1322–1329. doi: 10.1038/ncb2615
Vandenburgh, H., and Kaufman, S. (1979). In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203, 265–268. doi: 10.1126/science.569901
Varelas, X., Samavarchi-Tehrani, P., Narimatsu, M., Weiss, A., Cockburn, K., Larsen, B. G., et al. (2010). The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev. Cell 19, 831–844. doi: 10.1016/j.devcel.2010.11.012
Visser, S., and Yang, X. (2010). LATS tumor suppressor: a new governor of cellular homeostasis. Cell Cycle 9, 3892–3903. doi: 10.4161/cc.9.19.13386
Wackerhage, H., Del Re, D. P., Judson, R. N., Sudol, M., and Sadoshima, J. (2014). The Hippo signal transduction network in skeletal and cardiac muscle. Sci. Signal. 7, re4-re4. doi: 10.1126/scisignal.2005096
Wackerhage, H., and Ratkevicius, A. (2008). Signal transduction pathways that regulate muscle growth. Essays Biochem. 44, 99–108. doi: 10.1042/bse0440099
Wada, K., Itoga, K., Okano, T., Yonemura, S., and Sasaki, H. (2011). Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914. doi: 10.1242/dev.070987
Wadham, C., Gamble, J. R., Vadas, M. A., and Khew-Goodall, Y. (2003). The protein tyrosine phosphatase Pez is a major phosphatase of adherens junctions and dephosphorylates β-catenin. Mol. Biol. Cell 14, 2520–2529. doi: 10.1091/mbc.E02-09-0577
Wang, N., Tytell, J. D., and Ingber, D. E. (2009). Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75. doi: 10.1038/nrm2594
Watt, K. I., Judson, R., Medlow, P., Reid, K., Kurth, T. B., Burniston, J. G., et al. (2010). Yap is a novel regulator of C2C12 myogenesis. Biochem. Biophys. Res. Commun. 393, 619–624. doi: 10.1016/j.bbrc.2010.02.034
Watt, K. I., Turner, B. J., Hagg, A., Zhang, X., Davey, J. R., Qian, H., et al. (2015). The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat. Commun. 6, 6048. doi: 10.1038/ncomms7048
Wei, B., Dui, W., Liu, D., Xing, Y., Yuan, Z., and Ji, G. (2013). MST1, a key player, in enhancing fast skeletal muscle atrophy. BMC Biol. 11:12. doi: 10.1186/1741-7007-11-12
Wu, S., Huang, J., Dong, J., and Pan, D. (2003). hippo Encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456. doi: 10.1016/S0092-8674(03)00549-X
Xin, M., Kim, Y., Sutherland, L. B., Murakami, M., Qi, X., Mcanally, J., et al. (2013). Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. U.S.A. 110, 13839–13844. doi: 10.1073/pnas.1313192110
Xin, M., Kim, Y., Sutherland, L. B., Qi, X., Mcanally, J., Schwartz, R. J., et al. (2011). Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal 4, ra70. doi: 10.1126/scisignal.2002278
Yaffe, D., and Saxel, O. (1977). Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725–727. doi: 10.1038/270725a0
Yagi, R., Chen, L. F., Shigesada, K., Murakami, Y., and Ito, Y. (1999). A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 18, 2551–2562. doi: 10.1093/emboj/18.9.2551
Yang, C., Tibbitt, M. W., Basta, L., and Anseth, K. S. (2014). Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652. doi: 10.1038/nmat3889
Ye, X., Deng, Y., and Lai, Z.-C. (2012). Akt is negatively regulated by Hippo signaling for growth inhibition in Drosophila. Dev. Biol. 369, 115–123. doi: 10.1016/j.ydbio.2012.06.014
Yin, F., Yu, J., Zheng, Y., Chen, Q., Zhang, N., and Pan, D. (2013). Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154, 1342–1355. doi: 10.1016/j.cell.2013.08.025
Yoshida, T. (2008). MCAT elements and the TEF-1 family of transcription factors in muscle development and disease. Arterioscler. Thromb. Vasc. Biol. 28, 8–17. doi: 10.1161/ATVBAHA.107.155788
Yu, F. X., Zhao, B., Panupinthu, N., Jewell, J. L., Lian, I., Wang, L. H., et al. (2012). Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791. doi: 10.1016/j.cell.2012.06.037
Zhang, J., Ji, J. Y., Yu, M., Overholtzer, M., Smolen, G. A., Wang, R., et al. (2009). YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat. Cell Biol. 11, 1444–1450. doi: 10.1038/ncb1993
Zhang, M., and McLennan, I. S. (1994). Use of antibodies to identify satellite cells with a light microscope. Muscle Nerve 17, 987–994. doi: 10.1002/mus.880170905
Zhao, B., Li, L., Lu, Q., Wang, L. H., Liu, C. Y., Lei, Q., et al. (2011). Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25, 51–63. doi: 10.1101/gad.2000111
Zhao, B., Li, L., Tumaneng, K., Wang, C. Y., and Guan, K. L. (2010). A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β-TRCP). Genes Dev. 24, 72–85. doi: 10.1101/gad.1843810
Zhao, B., Li, L., Wang, L., Wang, C. Y., Yu, J., and Guan, K. L. (2012). Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 26, 54–68. doi: 10.1101/gad.173435.111
Zhao, B., Wei, X., Li, W., Udan, R. S., Yang, Q., Kim, J., et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761. doi: 10.1101/gad.1602907
Zhao, B., Ye, X., Yu, J., Li, L., Li, W., Li, S., et al. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22, 1962–1971. doi: 10.1101/gad.1664408
Zhong, W., Tian, K., Zheng, X., Li, L., Zhang, W., Wang, S., et al. (2013). Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 22, 2083–2093. doi: 10.1089/scd.2012.0685
Keywords: YAP, mechanotransduction, skeletal muscle, hippo pathway, muscle homeostasis
Citation: Fischer M, Rikeit P, Knaus P and Coirault C (2016) YAP-Mediated Mechanotransduction in Skeletal Muscle. Front. Physiol. 7:41. doi: 10.3389/fphys.2016.00041
Received: 30 October 2015; Accepted: 29 January 2016;
Published: 16 February 2016.
Edited by:
Lars Larsson, Uppsala University Hospital, SwedenReviewed by:
Martina Krüger, Heinrich Heine University Düsseldorf, GermanyJohn Joseph McCarthy, University of Kentucky, USA
Roberta Sartori, University of Padova, Italy
Copyright © 2016 Fischer, Rikeit, Knaus and Coirault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Coirault, Yy5jb2lyYXVsdEBpbnN0aXR1dC1teW9sb2dpZS5vcmc=
 Martina Fischer
Martina Fischer Paul Rikeit2,3
Paul Rikeit2,3 Petra Knaus
Petra Knaus Catherine Coirault
Catherine Coirault