- Department of Biological Science, Division of Earth, Life, and Molecular Sciences, Graduate School of Natural Science and Technology, Okayama University, Okayama, Japan
Circadian rhythms in organisms are involved in many aspects of metabolism, physiology, and behavior. In many animals, these rhythms are produced by the circadian system consisting of a central clock located in the brain and peripheral clocks in various peripheral tissues. The oscillatory machinery and entrainment mechanism of peripheral clocks vary between different tissues and organs. The relationship between the central and peripheral clocks is also tissue-dependent. Here we review the heterogeneous nature of peripheral circadian clocks in the fruit fly Drosophila melanogaster and their dependence on the central clock, and discuss their significance in the temporal organization of physiology in peripheral tissues/organs.
Circadian rhythms are often observed in animal behavior, physiology, and gene expression (Aguilar-Roblero et al., 2015). In the fruit fly, Drosophila melanogaster; one of the most useful model organisms for the study of the circadian system, the central clock consists of about 150 clock gene expressing neurons in the brain (Taghert and Shafer, 2006; Shafer and Yao, 2014). The central clock regulates behavioral rhythms including locomotor behavior and sleep-wake cycles and is clustered in two neural groups, lateral neurons (LNs) and dorsal neurons (DNs). LNs are subdivided into three clusters, small ventral LNs (s-LNv), large ventral LNs (l-LNv), and dorsal LNs (LNd). DNs are subdivided into three clusters, DN1, DN2, and DN3(reviewed by Helfrich-Förster, 2005; Hermann-Luibl and Helfrich-Förster, 2015). The s-LNv are necessary and sufficient for sustained locomotor rhythm in constant darkness (DD) (Helfrich-Förster, 1998; Renn et al., 1999) and are the most important cluster in the central circadian network (Grima et al., 2004; Stoleru et al., 2004, 2005). The dynamic neuronal network between the clock neurons is regulated by various neurotransmitters including pigment dispersing factor (PDF), which is expressed in the s- and l-LNvs (Shafer and Yao, 2014; Yao and Shafer, 2014; Hermann-Luibl and Helfrich-Förster, 2015) and fine-tunes the circadian rhythm to adapt to the environmental cycles (Miyasako et al., 2007; Yao and Shafer, 2014).
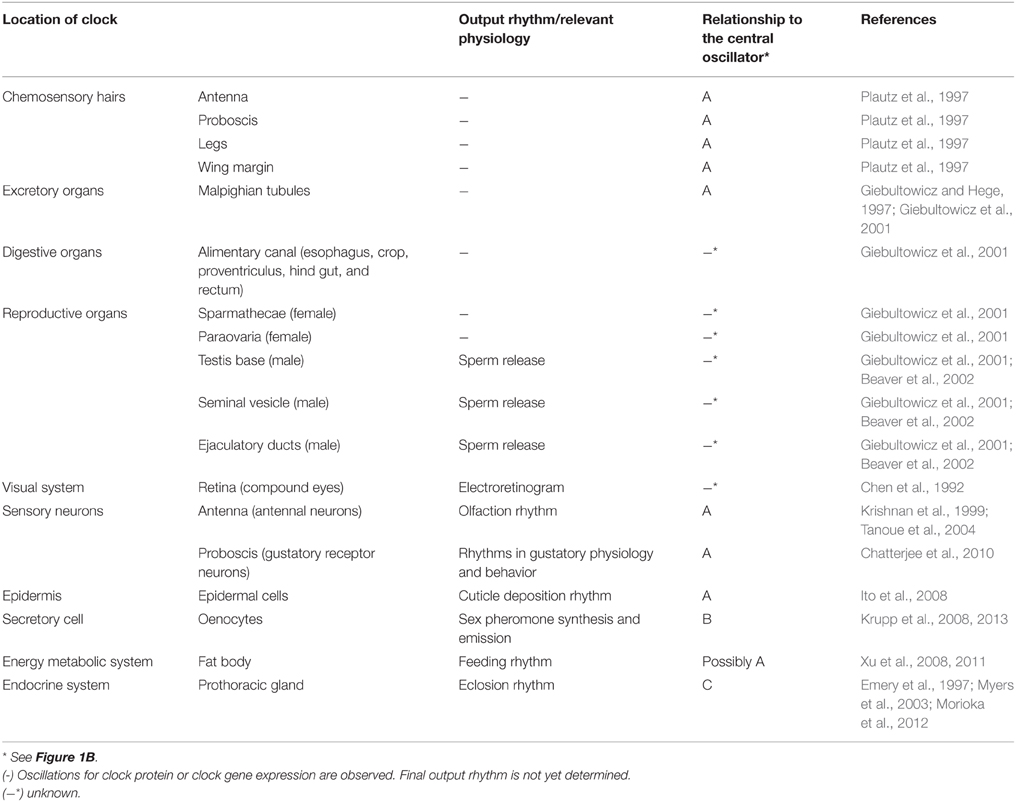
In addition to the central clock, peripheral clocks reside in various organs and tissues and likely regulate rhythms in organ/tissue specific functions (Table 1). Some peripheral clocks have been characterized only by immunohistochemistry to detect the oscillation of clock proteins or by reporter assays with luciferase expression driven by clock gene promotors (Giebultowicz and Hege, 1997; Plautz et al., 1997). Molecular studies revealed that the peripheral clocks are based on cell-autonomous molecular oscillation and that most directly respond to light when kept in culture conditions (Plautz et al., 1997). Further detailed studies focusing on molecular oscillations in the periphery and their output rhythms have revealed diversity in the circadian organization among the peripheral circadian systems (e.g., Myers et al., 2003; Ito et al., 2008; Krupp et al., 2013). In the present review, we summarize and discuss the features of peripheral oscillators, their heterogeneity, and their relationship to the central clock.
Heterogeneity in Molecular Machinery: The Function of CRY in Peripheral Clocks
The circadian oscillation in central clock neurons is based on transcriptional/translational feedback loops that involve the transcription factors CLOCK (CLK) and CYCLE (CYC) (Tataroglu and Emery, 2015). CLK and CYC dimerize and induce transcription of period (per) and timeless (tim) genes. The translated products, PER and TIM, form a heterodimer that suppresses the activity of CLK-CYC to produce rhythmic expression of per and tim with a period of about 24 h. Light input causes degradation of TIM via the blue light receptor, CRYPTOCHROME (CRY), causing the circadian clock to be reset (Stanewsky et al., 1998; Suri et al., 1998; Ceriani et al., 1999; Busza et al., 2004). In addition to CRY, the visual system, including compound eyes, ocelli, and Hofbauer-Buchner eyelets, are involved in photic entrainment of the central clock (Stanewsky et al., 1998; Helfrich-Förster et al., 2001).
The oscillatory mechanism is shared by the central and the peripheral oscillators (Hardin et al., 2003); however, the function of CRY varies between peripheral oscillators: two different roles for CRY in the periphery have been suggested. (1) CRY functions as a photoreceptor and a core clock component (Stanewsky et al., 1998; Ivanchenko et al., 2001; Collins et al., 2006), and (2) CRY serves only as a photoreceptor (Ito et al., 2008) (Figure 1A). The following two sections provide examples for tissues/organs within which CRY has different roles.
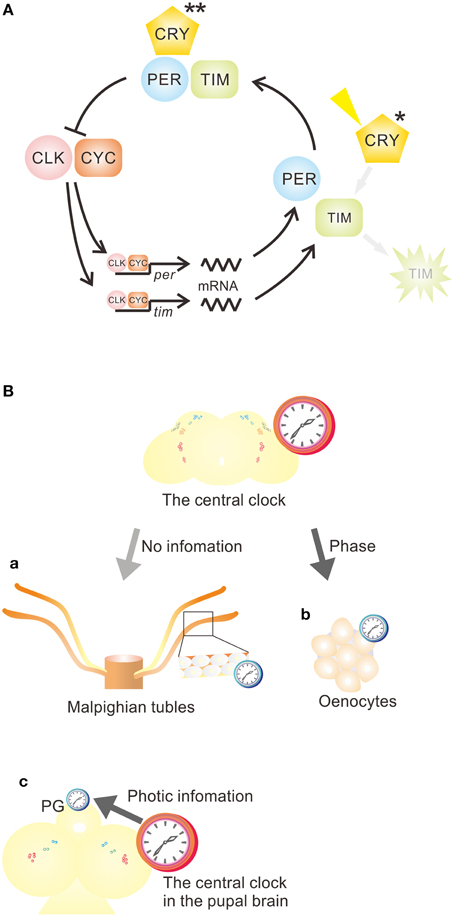
Figure 1. Function of CRY in peripheral circadian clocks and the relationship between central and peripheral clocks in Drosophila melanogaster. (A) The functions of CRY vary in peripheral circadian clocks. In most peripheral circadian systems, CRY functions as a photoreceptor (*) and a core component (**) of the clock. However, CRY acts as a photoreceptor (*), but not as a core component of the clock, in the epidermis, which controls cuticle deposition rhythm, and in the prothoracic gland (PG). CLK, CLOCK; CRY, CRYPTOCHROME; CYC, CYCLE; per, period; tim, timeless. (B) Various relationships between central and peripheral clocks. (a) Most peripheral oscillators are independent of the central clock. (b) Some peripheral oscillators, such as oenocyte oscillators, are a slave to the central clock, receiving phase information to maintain an appropriate phase relationship to the central clock. (c) Some peripheral oscillators, such as those in PG, receive light and temporal signals from the central clock to drive oscillation and coordinate molecular oscillation. See Table 1 for more examples.
CRY Serves as both a Photoreceptor and a Clock Component
The dual roles of CRY as a photoreceptor and a clock component are well-demonstrated in Malpighian tubules (MT) (Stanewsky et al., 1998; Ivanchenko et al., 2001). In this organ, a light pulse given during the subjective night induces the degradation of TIM, resulting in a reset of the phase of circadian oscillation. Light-induced TIM degradation was eliminated in cryb, hypomorphic mutants lacking functional CRY. In addition, the PER and TIM oscillations in the MT disappeared in cryb mutants (Stanewsky et al., 1998; Ivanchenko et al., 2001). These studies suggest that CRY has roles both in light entrainment and in the molecular oscillatory machinery of the clock. Another example is the antenna. Its response to odorants, as measured by an electroantennogram (EAG), increases at night and decreases during the day under light-dark (LD) cycles (Krishnan et al., 1999). The olfactory EAG rhythm is driven by a peripheral circadian clock in antennal olfactory sensory neurons, persisting under constant darkness (DD) (Krishnan et al., 1999; Tanoue et al., 2004). The rhythm is based on the molecular oscillatory mechanism and is abolished in per and tim null mutants (Krishnan et al., 1999). The circadian rhythms in olfactory EAG responses and clock gene expressions were both eliminated in cryb mutant flies (Stanewsky et al., 1998; Krishnan et al., 2001). One might argue that the loss of rhythm is derived from desynchronization among constituent clock cells caused by a loss of photic entrainability. However, it is more likely that CRY serves as an essential component for the oscillatory machinery (Krishnan et al., 2001; Levine et al., 2002; Collins et al., 2006). CRY may function as a transcriptional repressor together with PER, because overexpression of cry and per repressed CLK-CYC activity in the compound eyes. The function of CRY as a transcriptional repressor was also confirmed in cultured cells (Collins et al., 2006). Thus, CRY's repressor activity explains why most peripheral clocks lose their oscillation in cryb mutants (Levine et al., 2002).
Cry Serves only as a Photoreceptor
In some peripheral rhythms, CRY seems to serve only as a photoreceptor for photic entrainment of the clock. One good example is cuticle deposition rhythms. In Drosophila, cuticle deposition rhythmically occurs in the endocuticle of the furca, an apodemata in the thorax, and the rhythm is controlled by a peripheral circadian clock residing in epidermal cells (Ito et al., 2008). The cuticle deposition rhythm in furca was entrained to LD cycles even when the thorax was cultured in vitro, suggesting that the photic entrainment system is independent of the brain and resides in the thorax. In cryb mutants, the rhythm was not entrained to LD cycles. The entrainability was rescued by the overexpression of cry throughout whole body of cryb mutants. Interestingly, not only cryb, but also cryOUT knockout mutants completely lacking CRY, exhibit the free-running rhythm of cuticle deposition (Ito et al., 2008). These results suggest that CRY only functions as a photoreceptor and not as a component of the clock machinery in the cuticle deposition rhythm.
A similar function of CRY is seen in the prothoracic gland (PG). Fruit fly adults emerge from the pupal case around dawn (Konopka and Benzer, 1971), when high humidity is thought to protect newly emerged flies from desiccation (Pittendrigh, 1954). This eclosion behavior is a once-in-a-lifetime event, but it occurs rhythmically in a population of flies at different developmental stages. The timing of eclosion is set by the circadian system, which consists of two oscillators: one in the LNv and another in the PG (Myers et al., 2003). Myers et al. (2003) suggested that CRY plays an important role as a core component of oscillatory machinery in the PG because the eclosion rhythm was abolished in cryb mutants. However, subsequent studies yielded completely opposite results (Mealey-Ferrara et al., 2003; Dolezelova et al., 2007): the rhythmic eclosion was observed even in cryb and cry0 (knockout of cry) flies both in LD cycles and DD. Therefore, as for the cuticle deposition rhythm in furca, CRY seems not to be involved in the oscillatory mechanism of the clock in the PG. This has been confirmed by molecular studies (Emery et al., 1997; Morioka et al., 2012). The per transcript rhythm persisted in the PG under DD when the central nervous system (CNS)-ring gland (RG) complex containing the PG was cultured. The per transcript rhythm and oscillation of TIM were both intact in cultured CNS-RG from cryb mutant flies. However, the TIM oscillation seemed to free-run in PG cells isolated from a cryb mutant kept under LD cycles (Morioka et al., 2012). Therefore, in the PG, CRY may play a role in the entrainment of TIM oscillation to LD cycles. Interestingly, cry expressed in the PG does not affect per oscillation. The per transcript levels in the PG increased when the cultured CNS-RG was exposed to 12 h of light. This response of per depended on light input from CRY via the CNS, because the response was eliminated by cryb mutation, isolation of PG from the CNS, and blocking synaptic inputs from the CNS. The expression of cry transcripts was quite low in the PG compared with the brain and MT (Morioka et al., 2012). Taken together, it is likely that only a small amount of CRY is expressed in the PG and plays a role in the photic entrainment of TIM oscillation, but is not involved in the oscillatory machinery or the photic entrainment of per oscillation in the PG.
Heterogeneity in Circadian Organization in the Periphery
Although most peripheral oscillators can maintain their oscillation under in vitro culture conditions, the oscillations may not perfectly represent those in vivo where they may be influenced by various factors including those from the central clock. This issue has been addressed by several studies to date (Krishnan et al., 1999; Giebultowicz, 2000; Ivanchenko et al., 2001; Ito et al., 2008; Krupp et al., 2008, 2013; Morioka et al., 2012), and various relationships between the central and peripheral oscillators have been reported (Figure 1B).
Peripheral Clocks Are Independent of the Central Clock
The Malpighian tubules contain a peripheral clock (Giebultowicz and Hege, 1997) (Figure 1Ba). The peripheral oscillator in MT is cell-autonomous and entrained to environmental light without any cue from the central clock (Hege et al., 1997). Independency of the MT oscillator from the central clock was clearly revealed by Giebultowicz et al. (2000). They showed that the original phase of TIM oscillation of MT is maintained in DD when the MT is transplanted into the abdomen of flies previously entrained to antiphase LD cycles.
The antennal circadian clock is also independent from the central clock. The olfactory rhythm driven by the antennal clock was intact even when LNvs, the central clock neurons, were ablated or disrupted (Krishnan et al., 1999; Tanoue et al., 2004). However, the rhythm was lost in flies where the clock oscillation was disrupted only in olfactory sensory neurons (Tanoue et al., 2004). Thus, the peripheral clock in antennae is completely independent from the central clock.
The clock in the fat body of Drosophila is another example. It can be entrained independently of the central clock via a restricted feeding schedule (Xu et al., 2011). Interestingly, it has effects on fly metabolism that oppose the effect of the central clock: flies with a genetically disrupted fat body clock show increased food consumption, reduced levels of energy storage, and a higher sensitivity to starvation, whereas opposite responses are observed in energy storage and starvation when the central clock is disrupted (Xu et al., 2008).
Peripheral Oscillator Is a Slave to the Central Clock
Krupp et al. (2008) demonstrated that the circadian clock phase in oenocytes, which regulate pheromone production, is regulated by the central clock. The core clock genes, per, tim, Clk, and cyc, showed cyclic expression in LD cycles and DD in oenocytes. These cyclic expressions are abolished in per0 mutants in DD and in per7.2:2 transgenic flies, which only have PER expression in a subset of clock neurons in the brain, but not in peripheral tissues. The results suggest that oenocytes contain a per-dependent peripheral clock. The phase of clock gene expression is affected by PDF signaling: it is altered when PDF signaling is disrupted in mutants lacking PDF or a PDF receptor. In those mutants, however, clock gene expression is robustly rhythmic as in wild-type flies and the phase relationship among clock genes is maintained as normal (Krupp et al., 2013). This suggests that the peripheral oscillator in oenocytes is a slave oscillator and its phase is modulated by the central clock, although oscillation itself is maintained independent of the central oscillator (Figure 1Bb).
Peripheral Oscillator Is Driven By the Central Clock
The eclosion timing is controlled by a circadian system that consists of two hierarchically organized oscillators located in LNvs and PG, respectively (Myers et al., 2003; Morioka et al., 2012) (Figure 1Bc). The targeted disruption of either of these two circadian oscillators using tim overexpression renders the eclosion arrhythmic. The eclosion rhythm and molecular oscillation of TIM in PG are also diminished when LNvs are ablated (Myers et al., 2003). These results suggested that both LNv and PG clocks are necessary for eclosion rhythm and that the PG clock is a slave oscillator driven by the LNv clock. To further dissect this relationship, Morioka et al. (2012) observed the clock gene transcript rhythm and post-transcriptional rhythm in PG in vitro and found that the PER oscillation of PG clock receives light information from the central clock, but TIM oscillation does not. Interestingly, TIM maintains its oscillation, but PER does not in PG under DD, although both molecular oscillations are robust under LD cycles (Myers et al., 2003; Morioka et al., 2012). The control from the CNS may contribute to maintaining the robust coordinated oscillations of PER and TIM, which otherwise are dissociated from each other. Thus, the oscillator in Drosophila PG is governed by the central clock to a large extent.
Conclusion and Future Directions
In early studies, the peripheral oscillators were suggested to be cell autonomous, directly light entrainable, and independent of the central oscillator (Plautz et al., 1997; Giebultowicz, 2000). As described above, new lines of evidence have clearly demonstrated that the oscillatory machinery and degree of independence from the central clock vary among the peripheral clocks. Why and how the peripheral circadian systems have diversified within a species is an open question. Unfortunately, no good answer to this question is currently available. In particular, the function of CRY as a repressor is the most challenging issue in this field. Mammals contain two CRYs, mCRY1 and mCRY2, both of which function as core clock components (Okamura et al., 1999; van der Horst et al., 1999). Many insects also contain two CRYs including Drosophila type dCRY and mammalian type mCRY. The former is suggested to be a blue light photoreceptor and the latter to be a transcriptional repressor (Zhu et al., 2005, 2008). Drosophila have only one CRY (dCRY) in the genome, which functions as a photoreceptor in the central circadian system (Stanewsky et al., 1998; Helfrich-Förster et al., 2001), but as a transcriptional repressor in the core clock machinery of some peripheral circadian systems (Collins et al., 2006). How the same molecule acts differently in the central and peripheral clocks should be elucidated in future studies. Interestingly, hymenopteran species, including honeybees, only contain mCRY in their genome (Rubin et al., 2006; Zhan et al., 2011). Because hymenopteran species lack tim gene in their genome, mCRY is suggested to function as a partner of PER (Rubin et al., 2006). The lack of dCRY might be related to a loss of TIM, because dCRY mediates light-dependent TIM degradation that is required for clock entrainment. It is thus likely that an ancestral insect had both dCRY and mCRY, and that during the course of evolution, some species retained both, while others lost either dCRY or mCRY (Yuan et al., 2007).
The difference between central and peripheral clocks can be also characterized by cell-to-cell communication. The central clock consists of a network of neurons communicating through peptidergic neurotransmitters and maintains its oscillation in various environmental conditions (Yao and Shafer, 2014), whereas communication in the peripheral clock may be variable in organs/tissues and the synchrony among cells is rapidly lost under constant conditions (Plautz et al., 1997; Morioka et al., 2012). Thus, in the latter, synchrony is largely maintained by environmental factors (Plautz et al., 1997; Giebultowicz et al., 2000) or by neuronal or humoral signals from the central clock (Morioka et al., 2012). Detailed molecular studies are necessary to understand the variability and importance of cellular communication in peripheral clock tissue.
Another important issue is the relationship between central and the peripheral clocks, which may be required to temporally optimize local physiology in a tissue-dependent manner. As discussed above, peripheral oscillators can be classified into three types in Drosophila: independent of, slave to, and driven by the central clock. Independent peripheral oscillators maintain their own phase, whereas the latter two need to be modulated by the central clock to temporally coordinate physiological and behavioral activity. Therefore, if control from the central clock is through humoral factors, such as ecdysteroids and PDF (Krupp et al., 2013; Uryu et al., 2015), it may be not be uniform, which might be attributable to different oscillatory mechanisms of peripheral clocks. Future studies are needed to examine the possible feedback from the peripheral oscillator to the central clock, which has already been shown in mammalian clock systems (Mohawk et al., 2012).
Although most of the studies on phase control of peripheral circadian clocks have focused on the role of light and the central oscillator, we should pay attention to environmental cues other than light. Obviously the peripheral oscillators utilize temperature changes as time cue (Zeitgeber) in addition to light. Interestingly, the temperature entrainment ability of the peripheral clock is eliminated in flies lacking no receptor potential A (norpA) encoding phospholipase C (Ito et al., 2011). Although norpA mediates thermosensitive splicing of per in the central clock (Majercak et al., 2004), its role in peripheral clocks remains to be elucidated. Another important Zeitgeber may be feeding (Xu et al., 2011): the detailed mechanism by which feeding entrains the peripheral oscillator should also be elucidated. Because the precise coordination of amplitude and phase of all clocks is essential for the well-being of animals (Hastings et al., 2003), it remains challenging to explore how multiple circadian clocks in the body are coordinated through entrainment by Zeitgebers and central-peripheral interactions.
Author Contributions
CI and KT developed the concept for this mini-review and CI prepared the early draft, including the figure and table. CI researched the literature for the key papers used in this mini-review. KT developed the early draft prepared by CI.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Grant-in-Aid for JSPS Fellows (13J07838) for CI and by Grant-in-Aid (15H04400) from JSPS to KT.
Abbreviations
CLK, CLOCK; Clk, Clock; CNS, central nervous system; CRY, CRYPTOCHROME; cwo, clockwork orange; CYC, CYCLE; cyc, cycle; DD, constant darkness; DN, dorsal neurons; EAG, electroantennogram; LD, light dark; LN, lateral neurons; LNv, ventral lateral neurons; s-LNv, small lateral neurons; l-LNv, large lateral neurons; LNd, dorsal lateral neurons; LPN, lateral posterior neurons; MT, Malpighian tubules; norpA, no receptor potential A; PDF, pigment dispersing factor; PER, PERIOD; per, period; PG, prothoracic gland; RG, ring gland; TIM, TIMELESS; tim, timeless.
References
Aguilar-Roblero, R., Díaz-Muñoz, M., and Fanjul-Moles, M. (2015). Mechanisms of Circadian Systems in Animals and Their Clinical Relevance. Cham: Springer International Publishing.
Beaver, L. M., Gvakharia, B. O., Vollintine, T. S., Hege, D. M., Stanewsky, R., and Giebultowicz, J. M. (2002). Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 99, 2134–2139. doi: 10.1073/pnas.032426699
Busza, A., Emery-Le, M., Rosbash, M., and Emery, P. (2004). Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science 304, 1503–1506. doi: 10.1126/science.1096973
Ceriani, M. F., Darlington, T. K., Staknis, D., Más, P., Petti, A. A., Weitz, C. J., et al. (1999). Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science 285, 553–556. doi: 10.1126/science.285.5427.553
Chatterjee, A., Tanoue, S., Houl, J. H., and Hardin, P. E. (2010). Regulation of gustatory physiology and appetitive behavior by the Drosophila circadian clock. Curr. Biol. 20, 300–309. doi: 10.1016/j.cub.2009.12.055
Chen, D. M., Christianson, J. S., Sapp, R. J., and Stark, W. S. (1992). Visual receptor cycle in normal and period mutant Drosohpila: microspectrophotometry, electrophysiology, and ultrastructual morphometry. Vis. Neurosci. 9, 125–135. doi: 10.1017/S0952523800009585
Collins, B., Mazzoni, E. O., Stanewsky, R., and Blau, J. (2006). Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr. Biol. 16, 441–449. doi: 10.1016/j.cub.2006.01.034
Dolezelova, E., Dolezel, D., and Hall, J. C. (2007). Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genetics 177, 329–345. doi: 10.1534/genetics.107.076513
Emery, I. F., Noveral, J. M., Jamison, C. F., and Siwicki, K. K. (1997). Rhythms of Drosophila period gene expression in culture. Proc. Natl. Acad. Sci. U.S.A. 94, 4092–4096. doi: 10.1073/pnas.94.8.4092
Giebultowicz, J. M. (2000). Molecular mechanism and cellular distribution of insect circadian clocks. Annu. Rev. Entomol. 45, 769–793. doi: 10.1146/annurev.ento.45.1.769
Giebultowicz, J. M., and Hege, D. M. (1997). Circadian clock in Malpighian tubules. Nature 386, 664. doi: 10.1038/386664a0
Giebultowicz, J. M., Ivanchenko, M., and Vollintine, T. (2001). “Organization of the insect circadian system: spatial and developmental expression of clock genes in peripheral tissues of Drosophila melanogaster,” in Insect Timing: Circadian Rhythmicity to Seasonality, 1st Edn., eds D. L. Denlinger, J. Giebultowicz, and D. S. Saunders (Amsterdam: Elsevier Science B. V.), 31–42. doi: 10.1016/b978-044450608-5/50035-0
Giebultowicz, J. M., Stanewsky, R., Hall, J. C., and Hege, D. M. (2000). Transplanted Drosophila excretory tubules maintain circadian clock cycling out of phase with the host. Curr. Biol. 10, 107–110. doi: 10.1016/S0960-9822(00)00299-2
Grima, B., Chélot, E., Xia, R., and Rouyer, F. (2004). Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 431, 869–873. doi: 10.1038/nature02935
Hardin, P. E., Krishnan, B., Houl, J. H., Zheng, H., Ng, F. S., Dryer, S. E., et al. (2003). Central and peripheral circadian oscillators in Drosophila. Novartis Found Symp. 253, 140–150. discussion: 150–160. doi: 10.1002/0470090839.ch11
Hastings, M. H., Reddy, A. B., and Maywood, E. S. (2003). A clockwork web: circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 4, 649–661. doi: 10.1038/nrn1177
Hege, D. M., Stanewsky, R., Hall, J. C., and Giebultowicz, J. M. (1997). Rhythmic expression of a PER-reporter in the malpighian tubules of decapitated Drosophila: evidence for a brain-independent circadian clock. J. Biol. Rhythms 12, 300–308. doi: 10.1177/074873049701200402
Helfrich-Förster, C. (1998). Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J. Comp. Physiol. A 182, 435–453. doi: 10.1007/s003590050192
Helfrich-Förster, C. (2005). Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 4, 65–76. doi: 10.1111/j.1601-183X.2004.00092.x
Helfrich-Förster, C., Winter, C., Hofbauer, A., Hall, J. C., and Stanewsky, R. (2001). The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 30, 249–261. doi: 10.1016/S0896-6273(01)00277-X
Hermann-Luibl, C., and Helfrich-Förster, C. (2015). Clock network in Drosophila. Curr. Opin. Insect Sci. 7, 65–70. doi: 10.1016/j.cois.2014.11.003
Ito, C., Goto, S. G., Shiga, S., Tomioka, K., and Numata, H. (2008). Peripheral circadian clock for the cuticle deposition rhythm in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 105, 8446–8451. doi: 10.1073/pnas.0800145105
Ito, C., Goto, S. G., Tomioka, K., and Numata, H. (2011). Temperature entrainment of the circadian cuticle deposition rhythm in Drosophila melanogaster. J. Biol. Rhythms 26, 14–23. doi: 10.1177/0748730410391640
Ivanchenko, M., Stanewsky, R., and Giebultowicz, J. M. (2001). Circadian photoreception in Drosophila: functions of cryptochrome in peripheral and central clocks. J. Biol. Rhythms 16, 205–215. doi: 10.1177/074873040101600303
Konopka, R. J., and Benzer, S. (1971). Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 68, 2112–2116. doi: 10.1073/pnas.68.9.2112
Krishnan, B., Dryer, S. E., and Hardin, P. E. (1999). Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400, 375–378. doi: 10.1038/22566
Krishnan, B., Levine, J. D., Lynch, M. K., Dowse, H. B., Funes, P., Hall, J. C., et al. (2001). A new role for cryptochrome in a Drosophila circadian oscillator. Nature 411, 313–317. doi: 10.1038/35077094
Krupp, J. J., Billeter, J. C., Wong, A., Choi, C., Nitabach, M. N., and Levine, J. D. (2013). Pigment-dispersing factor modulates pheromone production in clock cells that influence mating in Drosophila. Neuron 79, 54–68. doi: 10.1016/j.neuron.2013.05.019
Krupp, J. J., Kent, C., Billeter, J. C., Azanchi, R., So, A. K., Schonfeld, J. A., et al. (2008). Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr. Biol. 18, 1373–1383. doi: 10.1016/j.cub.2008.07.089
Levine, J. D., Funes, P., Dowse, H. B., and Hall, J. C. (2002). Advanced analysis of a cryptochrome mutation's effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 3:5. doi: 10.1186/1471-2202-3-5
Majercak, J., Chen, W. F., and Edery, I. (2004). Splicing of the period gene 3′-terminal intron is regulated by light, circadian clock factors, and phospholipase C. Mol. Cell. Biol. 24, 3359–3372. doi: 10.1128/MCB.24.8.3359-3372.2004
Mealey-Ferrara, M. L., Montalvo, A. G., and Hall, J. C. (2003). Effects of combining a cryptochrome mutation with other visual-system variants on entrainment of locomotor and adult-emergence rhythms in Drosophila. J. Neurogenet. 17, 171–221. doi: 10.1080/714970274
Miyasako, Y., Umezaki, Y., and Tomioka, K. (2007). Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J. Biol. Rhythms 22, 115–126. doi: 10.1177/0748730407299344
Mohawk, J. A., Green, C. B., and Takahashi, J. S. (2012). Central and peripheral circadian clocks in mammals. Ann. Rev. Neurosci. 35, 445–462. doi: 10.1146/annurev-neuro-060909-153128
Morioka, E., Matsumoto, A., and Ikeda, M. (2012). Neuronal influence on peripheral circadian oscillators in pupal Drosophila prothoracic glands. Nat. Commun. 3, 909. doi: 10.1038/ncomms1922
Myers, E. M., Yu, J., and Sehgal, A. (2003). Circadian control of eclosion: interaction between a central and peripheral clock in Drosophila melanogaster. Curr. Biol. 13, 526–533. doi: 10.1016/S0960-9822(03)00167-2
Okamura, H., Miyake, S., Sumi, Y., Yamaguchi, S., Yasui, A., Muijtjens, M., et al. (1999). Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science 286, 2531–2534. doi: 10.1126/science.286.5449.2531
Pittendrigh, C. S. (1954). On temperature independence in the clock system controlling emergence time in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 40, 1018–1029. doi: 10.1073/pnas.40.10.1018
Plautz, J. D., Kaneko, M., Hall, J. C., and Kay, S. A. (1997). Independent photoreceptive circadian clocks throughout Drosophila. Science 278, 1632–1635. doi: 10.1126/science.278.5343.1632
Renn, S. C., Park, J. H., Rosbash, M., Hall, J. C., and Taghert, P. H. (1999). A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802. doi: 10.1016/S0092-8674(00)81676-1
Rubin, E. B., Shemesh, Y., Cohen, M., Elgavish, S., Robertson, H. M., and Bloch, G. (2006). Molecular and phylogenetic analyses reveal mammalian-like clockwork in the honey bee (Apis mellifera) and shed new light on the molecular evolution of the circadian clock. Genome. Res. 16, 1352–1365. doi: 10.1101/gr.5094806
Shafer, O. T., and Yao, Z. (2014). Pigment-dispersing factor signaling and circadian rhythms in insect locomotor activity. Curr. Opin. Insect Sci. 1, 73–80. doi: 10.1016/j.cois.2014.05.002
Stanewsky, R., Kaneko, M., Emery, P., Beretta, B., Wager-Smith, K., Kay, S. A., et al. (1998). The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95, 681–692. doi: 10.1016/S0092-8674(00)81638-4
Stoleru, D., Peng, Y., Agosto, J., and Rosbash, M. (2004). Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868. doi: 10.1038/nature02926
Stoleru, D., Peng, Y., Nawathean, P., and Rosbash, M. (2005). A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature 438, 238–242. doi: 10.1038/nature04192
Suri, V., Qian, Z., Hall, J. C., and Rosbash, M. (1998). Evidence that the TIM light response is relevant to light-induced phase shifts in Drosophila melanogaster. Neuron 21, 225–234. doi: 10.1016/S0896-6273(00)80529-2
Taghert, P. H., and Shafer, O. T. (2006). Mechanisms of clock output in the Drosophila circadian pacemaker system. J. Biol. Rhythms 21, 445–457. doi: 10.1177/0748730406293910
Tanoue, S., Krishnan, P., Krishnan, B., Dryer, S. E., and Hardin, P. E. (2004). Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr. Biol. 14, 638–649. doi: 10.1016/j.cub.2004.04.009
Tataroglu, O., and Emery, P. (2015). The molecular ticks of the Drosophila circadian clock. Curr. Opin. Insect Sci. 7, 51–57. doi: 10.1016/j.cois.2015.01.002
Uryu, O., Ameku, T., and Niwa, R. (2015). Recent progress in understanding the role of ecdysterioids in adult insects: germline development and circadian clock in the fruit fly Drosophila melanogaster. Zool. Lett. 1:32. doi: 10.1186/s40851-015-0031-2
van der Horst, G. T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., et al. (1999). Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398, 627–630. doi: 10.1038/19323
Xu, K., DiAngelo, J. R., Hughes, M. E., Hogenesch, J. B., and Sehgal, A. (2011). The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell. Metab. 13, 639–654. doi: 10.1016/j.cmet.2011.05.001
Xu, K., Zheng, X., and Sehgal, A. (2008). Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell. Metab. 8, 289–300. doi: 10.1016/j.cmet.2008.09.006
Yao, Z., and Shafer, O. T. (2014). The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science 343, 1516–1520. doi: 10.1126/science.1251285
Yuan, Q., Metterville, D., Briscoe, A. D., and Reppert, S. M. (2007). Insect cryptochromes: gene duplication and loss define diverse ways to construct insect circadian clocks. Mol. Biol. Evol. 24, 948–955. doi: 10.1093/molbev/msm011
Zhan, S., Merlin, C., Boore, J. L., and Reppert, S. M. (2011). The monarch butterfly genome yields insights into long-distance migration. Cell 147, 1171–1185. doi: 10.1016/j.cell.2011.09.052
Zhu, H., Sauman, I., Yuan, Q., Casselman, A., Emery-Le, M., Emery, P., et al. (2008). Cryptochromes define a novel circadian clock mechanism in monarch butterflies that may underlie sun compass navigation. PLoS Biol. 6:e4. doi: 10.1371/journal.pbio.0060004
Keywords: circadian rhythm, circadian clock, cryptochrome, Drosophila, peripheral oscillator, physiological rhythms, molecular oscillatory mechanism
Citation: Ito C and Tomioka K (2016) Heterogeneity of the Peripheral Circadian Systems in Drosophila melanogaster: A Review. Front. Physiol. 7:8. doi: 10.3389/fphys.2016.00008
Received: 25 September 2015; Accepted: 11 January 2016;
Published: 29 January 2016.
Edited by:
Petros Damos, Aristotle University of Thessaloniki, GreeceReviewed by:
Norio K. Ishida, National Institute of Advanced Industrial Science and Technology, JapanEran Tauber, University of Leicester, UK
Copyright © 2016 Ito and Tomioka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenji Tomioka, dG9taW9rYUBjYy5va2F5YW1hLXUuYWMuanA=
 Chihiro Ito
Chihiro Ito Kenji Tomioka
Kenji Tomioka