
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Physiol. , 07 October 2015
Sec. Cardiac Electrophysiology
Volume 6 - 2015 | https://doi.org/10.3389/fphys.2015.00271
Heart failure (HF) is a costly, challenging and highly prevalent medical condition. Hospitalization for acute decompensation is associated with high morbidity and mortality. Despite application of evidence-based medical therapies and technologies, HF remains a formidable challenge for virtually all healthcare systems. Repeat hospitalizations for acute decompensated HF (ADHF) can have major financial impact on institutions and resources. Early and accurate identification of impending ADHF is of paramount importance yet there is limited high quality evidence or infrastructure to guide management in the outpatient setting. Historically, ADHF was identified by physical exam findings or invasive hemodynamic monitoring during a hospital admission; however, advances in medical microelectronics and the advent of device-based diagnostics have enabled long-term ambulatory monitoring of HF patients in the outpatient setting. These monitors have evolved from piggybacking on cardiac implantable electrophysiologic devices to standalone implantable hemodynamic monitors that transduce left atrial or pulmonary artery pressures as surrogate measures of left ventricular filling pressure. As technology evolves, devices will likely continue to miniaturize while their capabilities grow. An important, persistent challenge that remains is developing systems to translate the large volumes of real-time data, particularly data trends, into actionable information that leads to appropriate, safe and timely interventions without overwhelming outpatient cardiology and general medical practices. Future directions for implantable hemodynamic monitors beyond their utility in heart failure may include management of other major chronic diseases such as pulmonary hypertension, end stage renal disease and portal hypertension.
Cardiovascular disease remains the leading cause of death in the United States and worldwide (Lim et al., 2012; Santulli, 2013). Heart failure (HF) is a costly, challenging and highly prevalent medical condition with major public health concerns given the associated significant morbidity and mortality (Ramani et al., 2010). Hospitalization for acute decompensated heart failure (ADHF) is a sentinel event that signifies disease progression and an inability of the heart to maintain adequate hemodynamics for perfusion and function of vital organs. Nearly half of these patients are readmitted within 6 months (Jong et al., 2002) and/or deceased by 1 year (Adams et al., 2005). The lifetime risk of developing HF for Americans age 40 years or older is approximately 20% (Yancy et al., 2013) The incidence of new HF cases exceeds 650,000 annually (Yancy et al., 2013). Over one million hospitalizations are attributed to HF annually with an estimated cost of ~$20 billion for the United States health care system (Yancy et al., 2013; Sharma et al., 2015).
To contain costs and standardize management of patients hospitalized for ADHF, the Centers for Medicare and Medicaid Services have recently introduced regulations to withhold or reduce payments for unnecessary hospitalizations for HF (http://CMS.gov)1. Increasingly scrutinized are readmission rates and other performance metrics (e.g., length of hospital stay, medical regimen at discharge) that are fuelling efforts to reduce HF readmissions. Evidence based therapies, including optimal medical therapy with neurohormonal antagonists and implantable devices (e.g., cardiac resynchronization therapy, defibrillators), are well outlined by major cardiovascular and electrophysiological societies including the American College of Cardiology, American Heart Association, Heart Failure Society of America, European Society of Cardiology, European Heart Rhythm Association, and Heart Rhythm Society (Heart Failure Society of America et al., 2010; McMurray et al., 2012; Brignole et al., 2013; Russo et al., 2013; Yancy et al., 2013; Kusumoto et al., 2014). Despite application of these treatments and technologies, HF remains a formidable challenge for virtually all healthcare systems. Moreover, the quality of evidence for care, support and monitoring systems as well as the infrastructure to support HF patients, particularly in the outpatient setting, are lacking (Yancy et al., 2013).
The recent development and clinical trials of implantable hemodynamic monitoring devices hold promise to reduce HF hospitalizations, with the potential to improve patient outcomes. The limited but emerging supportive evidence is encouraging further efforts to improve the patient experience with this clinically challenging medical condition. Leading experts in this field have acknowledged the difficulty of conducting clinical trials using cardiac monitoring embedded with therapeutic management to effect “hard” clinical outcomes and endpoints (Abraham et al., 2014). They have also underscored the importance of careful clinical trial design, endpoint selection, outcome assessment, management of actionable results, and other ethical issues (Abraham et al., 2014). This review focuses on implantable hemodynamic monitors that evolved from electrophysiologic (EP) devices with extended functionality to dedicated standalone pulmonary artery pressure monitors (e.g., CardioMEMS™)2 for guiding medical management of hemodynamic and volume status in outpatients, with demonstrable effects on reducing hospital readmission.
Early and accurate identification of impending and active ADHF is of paramount importance. Daily weight monitoring is a low-cost, easily accessible method of monitoring HF patients both in and out of the hospital. Unfortunately, weight as a reference value is easily confounded by changes in diet and muscle mass that are not related to intravascular volume status or filling pressures (Wolfel, 2007) and previous studies have found that the estimated positive predictive value for these findings are generally poor (Lewin et al., 2005; Zhang et al., 2009; Abraham et al., 2011a,b). Furthermore, while telemonitoring and collaborative multidisciplinary outpatient care teams appear to be practical effective measures to improve the management of HF, randomized studies have yet to confirm this (Chaudhry et al., 2010; Bekelman et al., 2015). The clinical symptoms of dyspnea, orthopnea, weight gain, and leg edema are often late indicators of congestion and volume overload that may already warrant hospitalization. Physical examination maneuvers such as inspection of the jugular venous pressure waveform, hepatojugular reflux and the square wave sign are useful surrogate measures of cardiac filling pressures, however, inter- and intra-observer variability, inconsistent manifestations, and the need for the patient to present for a physical examination, limit their applicability to identify early decompensated heart failure in the outpatient setting (Drazner et al., 1999, 2008). In addition, the sensitivity and specificity of these signs and symptoms vary widely, depending on the clinical study (Stevenson and Perloff, 1989; McCullough et al., 2002).
A gold standard measure of congestion in HF is not overall volume status (Verbrugge et al., 2014), but rather the pulmonary artery occlusion pressure, also known as the pulmonary capillary wedge pressure (PCWP). The PCWP reflects left sided cardiac filling pressures and is measured by a pressure transducer on the end of a pulmonary artery catheter. An inflatable balloon on the distal portion of the catheter allows placement of the catheter into a sub-selected pulmonary artery (PA) branch. An elevated PCWP, exceeding 18–22 mmHg, indicates pulmonary edema and congestion. Given the potential dangers of an indwelling PA catheter for invasive hemodynamic monitoring, the patient is by convention required to stay in the intensive care unit.
Advancement in medical microelectronics and the advent of device-based diagnostics have been developed to enable monitoring of ambulatory HF patients (Table 1). These devices transmit and report objective, quantitative data via remote monitoring systems. The premise of monitoring physiologic parameters is to enable clinicians to use these surrogate markers to optimize the patients' medical therapy in the ambulatory setting, before the onset of acute hemodynamic decompensation. This concept of remote device monitoring is also referred to as telemonitoring (Sousa et al., 2014). Several of the currently available telemonitoring systems measure various cardiac pressures and tailoring of medical therapy based upon these pressures is therefore called “pressure guided therapy.” The basis of pressure guided therapy involves the observation that most patients with HF require hospitalization because of excessive fluid accumulation. Accumulation of fluid occurs over several weeks and eventually reaches a “threshold” that requires hospitalization (Zile et al., 2008). Knowledge of these pressure increases can thus allow adjustment of medications to avoid reaching this “threshold” (Figure 1).
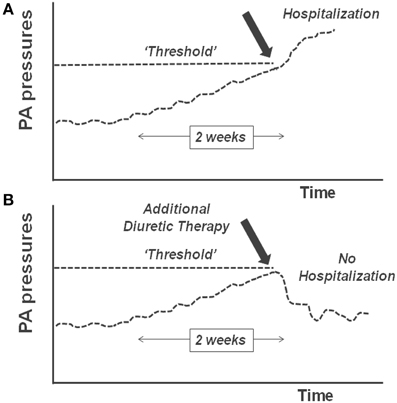
Figure 1. (A) Pulmonary artery (PA) pressures rise over time and cross a “threshold”; this results in decompensated heart failure and hospitalization. (B) When the rise in PA pressure is identified and additional diuretic therapy is given, the threshold is not crossed and hospitalization is avoided.
Early investigational, implantable heart function monitoring devices piggybacked on the existing implantable cardioverter defibrillator (ICD) technology which had already established the safety of right ventricular pacing leads and was being used in the target population. Early devices used innovative transvenous lead technology to provide mixed venous oxygen saturation and pressures in the right ventricle (RV) (Ohlsson et al., 1996, 1998). The correlation between RV end diastolic pressure and PA end diastolic pressure was demonstrated. In addition, these devices also could measure additional physiologic parameters such as heart rate variability, body temperature, and other surrogates of patient activity levels (Raina et al., 2015). Information management varied and early models initially only provided real-time data during interrogations in the office. Later designs gained the capacity to store data and to transfer it securely and remotely. These devices culminated in the development of the Chronicle® IHM (IHM-2; Model 9520) (Figure 2A). The IHM-1 and IHM-2 devices have demonstrated significant changes in RV pressures associated with changes in diuretic therapy (Braunschweig et al., 2002), β-adrenergic receptor blockers (Ishikawa et al., 2009), biventricular pacing (Bruns et al., 2005), and inhaled therapies for pulmonary hypertension patients (Fruhwald et al., 2003; Karamanoglu et al., 2007).
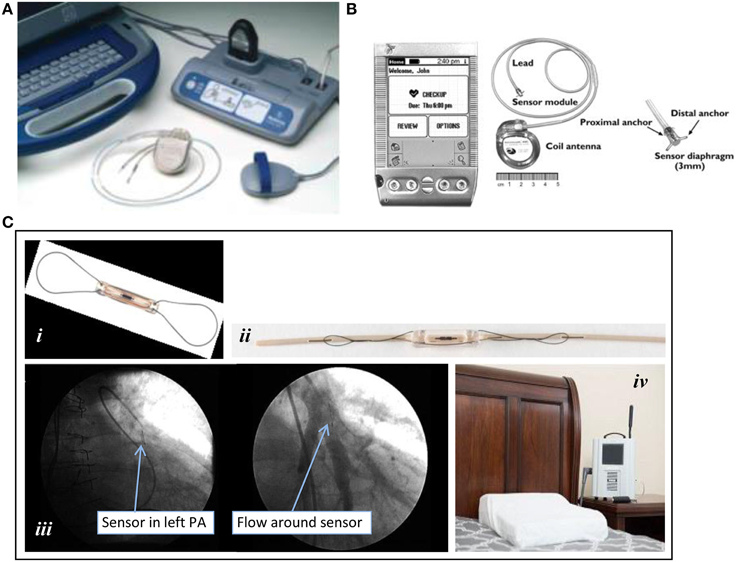
Figure 2. Implantable ambulatory heart failure hemodynamic monitors. (A) Chronicle®: (top left to bottom right) Implantable RV lead and ICD; lateral and posterior-anterior chest radiographs after device placement. (B) HeartPOD®. Implantable left atrial device and distal anchor. (C) CardioMEMS™ HF System: (i) pulmonary artery (PA) sensor; (ii) delivery catheter with preloaded PA sensor; (iii) pulmonary arteriograms showing a radiopaque PA sensor in a segmental branch of the left pulmonary artery (PA) before (left) and after (right) contrast dye injection; (iv) patient electronics system for transmission of data. All images were adapted with permission from Medtronic, Inc., St. Jude Medical, Inc., and Elsevier Inc.
Intrathoracic impedance monitoring was also evaluated as an adjunct to monitoring heart failure patients with an indication for an ICD or cardiac resynchronization therapy defibrillator (CRT-D) (Braunschweig et al., 2004; Yu et al., 2005). The OptiVol® function, an exclusive technology of Medtronic (Minneapolis, MN, USA) received the United States Food and Drug Administration (FDA) approval in 20043. ICD devices with OptiVol can longitudinally monitor the conductance of a microelectrical current between the RV defibrillating coil and device case. When the fluid index sharply rises above the baseline in conjunction with a decrease in thoracic impedance, intrathoracic fluid accumulation such as pulmonary congestion is suggested. Elevated left ventricular (LV) filling pressure is associated with increased intrathoracic fluid (i.e., lung water), which in turn is associated with increased conductance and decreased impedance as this current travels across lung tissue. Other concomitant device data including heart rate variability, resting night heart rate, patient activity level, and the burden of atrial tachycardia or fibrillation noted around the time of changes in Optivol fluid index and thoracic impedance trends may help to improve confidence in interpretation of potentially actionable data. Initial trials demonstrated increased sensitivity for early detection of HF exacerbations with decreased unexplained alarms in comparison to the traditional weight based monitoring protocol (Abraham et al., 2011b).
Ambulatory monitoring of intrathoracic impedance has not had the clinical impact that was initially anticipated, with statistically non-significant results from several contemporary trials (Conraads et al., 2011; Yang et al., 2013) Contributing factors likely include the difficulty determining the difference between appropriate detection of pre-clinical events and false alarms, as well as the need for third party involvements to monitor trends and effect appropriate therapeutic changes. Threshold changes in impedance can also occur from non-cardiac etiologies (e.g., pneumonia, pneumothorax, positive pressure ventilation), pointing to the importance of interpreting those data in association with other concomitant device data, as well as clinical data from a (phone) discussion with the patient. Furthermore, monitoring of electrophysiology (EP) devices is traditionally far removed from those empowered to make changes in a patient's HF medications and arrange for appropriate follow up. A recent retrospective review of the data from the Fluid Accumulation Status Trial (FAST) and Program to Access and Review Trending Information and Evaluate Correlation to Symptoms in Patients With Heart Failure (PARTNERS-HF) trials by Abraham and colleagues suggested a novel scheme to stratify patients at risk for a HF related hospitalization using diagnostic physiologic monitoring parameters germane to most modern ICD devices (Sharma et al., 2015). Increasing numbers of device observations correlated with an increased risk of a HF hospitalization. However, as demonstrated in prior studies, rates of HF hospitalizations associated with alerts were low, around 14% for ≤ 3 observations.
In contrast to prior efforts that combined HF monitoring therapies with therapeutic EP devices, the left atrial pressure (LAP), and pulmonary artery pressure (PAP) ambulatory heart failure monitoring implantable devices were developed as purely diagnostic devices (Figures 2B,C). LAP monitoring was being explored around the same time that intrathoracic impedance was in the early post-marketing surveillance period (Ritzema et al., 2007). The LAP monitoring device incorporates direct left atrial pressure monitoring via a pressure transducer secured to the interatrial septum. Transvenous access and a transseptal puncture are required for implantation (see Table 2). Similar to early EP devices, LAP monitors (HeartPOD®, St. Jude Medical Inc, Sylmar, CA, USA) can transfer data through radiofrequency wireless transmissions done by direct interrogation of the coil antenna using the handheld patient advisory module (PAM®) (Figure 1B). The Hemodynamically Guided Home Self-Therapy in Severe Heart Failure Patients (HOMEOSTASIS) trial was published in 2011 and reported the safety, feasibility, accuracy, and reliability of LAP monitoring (Troughton et al., 2011). The Left Atrial Pressure Monitoring to Optimize Heart Failure Therapy Study (LAPTOP-HF) trial was designed to determine the safety and clinical effectiveness of a physician-directed, patient self-management therapeutic strategy, and has recently been completed; results are eagerly awaited (Maurer et al., 2015).
Monitoring of PAP has been used for decades by cardiologists to detect early signs of HF in the intensive care setting (Rutherford et al., 1971). For ambulatory PAP monitoring, CardioMEMS (St. Jude Medical Inc, Sylmar, CA) was developed to directly measure systolic, diastolic and mean PAPs using a miniaturized wireless electromechanical sensor implanted in conjunction with a right heart catheterization procedure via transvenous access (Figure 1C). As the sole FDA-approved standalone device for outpatient HF monitoring, CardioMEMS was tested and proven to significantly reduce admissions for patients with New York Heart Association functional class III HF, regardless of left ventricular ejection fraction (LVEF) (Abraham et al., 2011a; Adamson et al., 2014), even in those with HF with preserved LVEF as opposed to those with reduced LVEF. A large post-approval trial is already recruiting (goal N = 1200) to verify the robustness, safety and usefulness of CardioMEMS in the complex real-world setting, particularly in reducing the rate of HF hospital readmissions and in improving patients' quality of life (clinicaltrials.gov).
The Chronicle features a programmable device that bears resemblance to a pacemaker pulse generator, which is implanted to process and store information from the pressure sensor near the tip of the transvenous lead (Bourge et al., 2008). The device continuously records data such as heart rate, body temperature, estimated patient activity level, RV systolic and diastolic pressure, RV pressure changes, and estimated PA diastolic pressure. It is only programmed to store a smaller dataset based on programmed intervals, recording the median 6th and 94th percentile levels over that period. In the COMPASS-HF trial, patients were asked to use a handheld radio frequency device to transmit readings at least once weekly using a telephone line. Information was stored on a secure server that clinicians could access through a secure web site.
The device characteristics and key aspects of deployment of the HeartPOD and CardioMEMS are summarized in Table 2. The HeartPOD system consists of a microelectronic sensor and diaphragm housed in a cylindrical titanium casing (approximately 3 mm × 7 mm) equipped with deployment anchors, and linked with an implantable sensor lead and a coil antenna within a can that resembles a pacemaker (Figure 1B). After gaining femoral venous access, a Brockenbrough needle and transseptal sheath are advanced, and puncture of the interatrial septum is performed. Thereafter, a guidewire is introduced via a subclavian vein to secure the transseptal location and a delivery sheath is placed to allow for placement of the sensor lead and LA sensor. Once the correct position is confirmed with intracardiac or transesophageal echocardiography, the LA sensor with the implantable sensor lead is deployed. The sensor is oriented to the LA and is buttressed and immobilized permanently with proximal and distal nitinol anchors on the respective right and left atrial sides of the interatrial septum upon deployment. The electrode is then transferred from the femoral location to the infraclavicular position via an exchange catheter and attached to communication module. The metal alloy can, referred to as the implantable communications module or ICM, containing the coil antenna and microelectronics is implanted in the same manner as for a pacemaker. A prospective open-label observational study of 84 patients found that freedom from device failure was 95% at 2 years and 88% at 4 years (Troughton et al., 2011).
CardioMEMS is a battery-free, leadless sensor (15 mm × 3 mm) consisting of a coil and capacitor encased in silicone, with a nitinol wire loop at each end of the sensor (Figure 2Ci). The CardioMEMS device is preloaded on a delivery catheter with a tether release system (Figure 2Cii). The design of the system is based on microelectromechanical principles of resonance whereby an external antenna wand emitting radiofrequency energy can cause varying degrees of oscillations in the sensor depending on the ambient pressure. The implanting procedure requires a transfemoral venous approach for accommodation of a 12-French introducer sheath for the CardioMEMS delivery catheter (Table 2). PA catheterization is performed to document right sided pressures before and after device implantation. After identifying a posterior segmental branch of the left PA by selective pulmonary arteriography (Figure 2Ciii), the sensor is liberated as the tether release wire is pulled and withdrawn while the nitinol loops uncoil from the delivery catheter to maintain device position in the PA branch. Interrogation of PAP requires the patient to be in a supine position with the supplied pillow-like wand placed underneath the patient (Figure 2Civ). After approximately 20 s, systolic, diastolic and mean PAPs are measured and transmitted via wireless cellular network to the CardioMEMS data center. In the landmark CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial, the device- or system-related complication rate was only 1.4%, with an overall pressure-sensor failure rate of 0% (Abraham et al., 2011a).
Patients enrolled in the CHAMPION trial were asked to make daily measurements of their PAPs using their portable electronic unit and a special pillow containing an antenna to take daily sensor readings which are transmitted through a modem or cellphone to a secure patient database (Adamson et al., 2011). By requesting patients to lay on the special pillow, measurements should be more consistent reproducible and ideally leveled. In the LAPTOP-HF trial, LAP, body temperature, and intracardiac electrogram were measured. Subjects were able to power and interrogate their devices with radiofrequency wireless transmissions from their patient activator module or PAM (Ritzema et al., 2007), with capacity to store up to 3 months' data if 6 waveforms are acquired daily.
Both the HeartPOD and CardioMEMS systems use a physician-guided self-management model that is intuitive and conceptually sound. However, experts in the field have universally acknowledged the challenges in conducting implantable monitoring trials to demonstrate impacts on clinical outcomes, particularly with how the interrogated physiologic data are handled (Abraham et al., 2014). Monitoring of device data requires patient compliance, “physician compliance” and a structured action plan or algorithm in order to execute a successful program. Achieving the goal of reducing patient hospitalization and readmission for HF requires a team effort involving the patient, caretaker, primary care physician, cardiologist, nurse and/or support staff. As a team, they will need sufficient resources and training to appropriately interpret data trends. Standard easy to use protocols would lead to more uniform management and optimize the ability to study IHMs. These protocols will need to have some flexibility so health care providers can customize treatment plans to each individual as necessary. In monitoring data, it has been emphasized that data trends are more crucial to successful management than acting on individual abnormal data points. Potential harm could also be introduced with injudicious remote monitoring when treatments such as diuretic therapy and vasodilators are administered without careful consideration, understanding and interpretation of abnormal data. Establishing a good line of communication with the patient, and exercising clinical judgment (e.g., focused history taking to gather clinical cues, scheduling an outpatient visit when required, and assessing renal function and/or electrolytes after adjustment of medical therapy such as diuretics), may help to clarify abnormal and outlying data trends. An online secure website (https://cardiomemshf.com/user/sign_in) is accessible to healthcare professionals to view the interrogated data. The HeartPOD, CardioMEMS and other non-EP (i.e., without pacemaking or defibrillator functions) implantable monitoring systems have built-in, untapped features including cardiac output, heart rate variability, and electrocardiographic monitoring. If officially approved by the FDA, these additional monitoring parameters will likely improve characterization of hemodynamic derangements, and potentially reduce false-positive results, and associated resource utilization.
When using surrogate measures to direct therapy, it is crucial to understand exactly what is being measured. While mean PAP and LAP are both considered adequate surrogates for filling pressures, they are two different measurements and neither is the gold standard measurement (LVEDP). The PCWP is often considered to reflect left ventricular preload and pulmonary capillary hydrostatic pressure, however, there is ongoing debate about the validity of this assumption in the setting of various conditions including chronic pulmonary disease, mechanical ventilation and pulmonary venous scarring. In principal, the LAP would be a more accurate measurement as it is physically and physiologically closer to the gold standard, LVEDP, however it is more invasive to measure. A brief literature review did not reveal any studies comparing the three measurements simultaneously, however there are a few studies comparing LAP and PCWP. In 1962, the PAP, PCWP, and LAP measurements of 11 patients with either clinically normal hearts or suspected mitral valve disease were studied with right heart catheterization in a control state, during a norepinephrine infusion, and during positive and negative intraalveolar pressures (Luchsinger et al., 1962). This study demonstrated a strong linear relationship (r = 0.95) between PCWP and LAP in all settings with the PCWP being consistently 35% higher than the LAP. A more contemporary study of lightly sedated dogs reported that the mean PCWP accurately reflected LAP (Chaliki et al., 2002). In this study, mean PCWP again was highly correlated with LAP (r = 0.99; slope = 0.99; intercept = −0.46 mmHg). However, a study of 43 dogs and 30 patients in severe hemorrhagic, traumatic or septic shock noted that a dangerous rise in PAP was not reflected by PCWP or even central venous pressure (Hardaway, 1982). This discrepancy was attributed to suspected partial obstruction of the pulmonary microcirculation due to disseminated intravascular coagulation in the pulmonary venules. Central venous pressure should only rise due to high pulmonary pressures if there is RV failure.
With IHM, it is not only the sites from which data are collected but the manner in which they are recorded, stored and reported. In the HOMEOSTASIS trial, subjects were requested to make two LAP measurements a day with additional measurements during symptoms (Troughton et al., 2011). In the CHAMPION trial, continuous PAP measurements are recorded (Abraham et al., 2011a). Clearly, there are tradeoffs between the challenge of requiring patients in the real world to make multiple daily recordings using a separate handheld device and voluminous amounts of data that require no input from patients to collect.
As already seen with implantable cardiovascular devices, there is a wealth of data that can be harnessed through minimally invasive means and transmitted to a secure data repository via remote wireless technology. Newer implantable cardiac monitoring devices for HF offer the ability to provide individualized data trends and ideally predict clinical events before they occur. However, isolated device alerts need to be used in conjunction with other clinical data to avoid overutilization of health care resources and increased hospitalizations. Successful translation of remote device based monitoring into successful clinical management of these patients will require simple prospectively validated algorithms that indicate how to use raw data from individual devices to make timely and appropriate changes in clinical management without overburdening staff. At this time, despite a wealth of smaller studies evaluating these devices (Table 3), larger clinical radnomized trials are still necessary to demonstrate that implantable device based hemodynamic sensors beneficially impact morbidity and mortality in HF patients.
Future directions for remote implantable PAP and LAP devices are broad. In cardiac patients, one can easily imagine the role for these devices in better understanding exercise physiology. They could also aid in clarifying the hemodynamics in particularly challenging outpatients such as those with difficult to assess pulmonary pressures by echocardiography (e.g., rheumatic mitral valve disease, severe pulmonary hypertension, morbidly obese patients.) Furthermore, in advanced HF patients with known arrhythmias, there can be a role to assess the clinical impact of supraventricular arrhythmias such as atrial fibrillation and ventricular arrhythmias, as well as addressing the question of whether these rhythm disturbances are causal or secondary to ADHF. Additionally, with the pressure to avoid indwelling lines, invasive procedures and overburdening intensive care units, pre-existing internal devices that monitor filling pressures could facilitate the management of these particularly high risk patients when admitted for both cardiac and non-cardiac issues, including perioperative hemodynamic and fluid management.
In advanced HF patients with left ventricular assist devices (LVAD) who are recurrently admitted with symptoms of congestion and fluid overload, LAP and PAP monitors may potentially help to discern elevated left sided filling pressures from other causes of dyspnea, and volume overload (e.g., chronic kidney disease progression, hypoalbuminemia, protein-losing enteropathy). However, further clinical review and evaluation still may be necessary to exclude a failing right ventricle in response to LVAD placement and manage other non-cardiac etiologies for recurrent hospitalizations. These devices may also be able to detect low filling pressures in patients with LVADs who urgently need increased intravascular volume in order for optimal device function and cardiac output. It remains to be seen whether regulatory agencies and transplantation societies will endorse the use of implantable LAP or PAP monitors as an alternative to indwelling PA catheters in the pre-heart transplant setting, with the intent to obviate the need for hospitalization in the intensive care unit and periodic replacement of PA catheters that are associated with procedural and other risks including line infection, sepsis, and thromboembolism.
There are also innumerable non-cardiac scenarios in which continuous assessment of cardiac hemodynamics and filling pressures would be invaluable. A recently published substudy of the CHAMPION trial found that of the 217 patients who did not meet criteria for pulmonary hypertension during the implantation right heart catheterization, 48.8% (N = 16) met criteria based on continuously observed PAP over the first week post-implantation. This implies that an implantable heart monitor may assist with improved diagnosis of pulmonary hypertension and perhaps better guide future trials targeting pulmonary hypertension (Frantz et al., 2008). It is foreseeable that future clinical investigations using these hemodynamic monitors may extend to non-HF patients, especially in efforts to improve management of volume status in the outpatient setting, improve patients' quality of life, and reduce rates of hospital readmission for hypo- or hypervolemia. Patients with end stage renal disease, primary pulmonary hypertension or portal hypertension are patient populations with similar high healthcare utilization. Renal replacement and diuretic therapies usually target a patient's known “dry weight,” which, as discussed above, is often not an accurate or reliable measure of true volume status. Furthermore, implantable hemodynamic monitors can detect other clinically significant events, such as poorly tolerated arrhythmias or hemodynamic shifts, that may be affecting patients and were previously unappreciated (Braunschweig et al., 2006).
The review was initially conceived of and manuscript outlined by all authors. DM prepared the first draft in collaboration with EF. RD and DS provided substantial revisions and contributions. DS contributed Figure 1. The final version was reviewed and approved by all authors.
Rahul N. Doshi has served as a consultant for St. Jude Medical, Inc. David M. Shavelle is a consultant and received research support from St Jude Medical, Inc. Deirdre M. Mooney and Erik Fung declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. ^CMS.gov. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Last accessed May 14, 2015.
2. ^CardioMEMS HF System Post-Approval Study. NCT02279888. https://clinicaltrials.gov/ct2/show/study/NCT02279888?term=cardiomems&rank=1.
3. ^U.S. Food and Drug Administration announcement, recently-approved devices. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm400550.htm. Last accessed: March 30, 2015.
Abraham, W. T., Adamson, P. B., Bourge, R. C., Aaron, M. F., Costanzo, M. R., Stevenson, L. W., et al. (2011a). Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377, 658–666. doi: 10.1016/S0140-6736(11)60101-3
Abraham, W. T., Compton, S., Haas, G., Foreman, B., Canby, R. C., Fishel, R., et al. (2011b). Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail. 17, 51–55. doi: 10.1111/j.1751-7133.2011.00220.x
Abraham, W. T., Stough, W. G., Pina, I. L., Linde, C., Borer, J. S., De Ferrari, G. M., et al. (2014). Trials of implantable monitoring devices in heart failure: which design is optimal? Nat. Rev. Cardiol. 11, 576–585. doi: 10.1038/nrcardio.2014.114
Adams, K. F. Jr., Fonarow, G. C., Emerman, C. L., LeJemtel, T. H., Costanzo, M. R., Abraham, W. T., et al. (2005). Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure Registry (ADHERE). Am. Heart J. 149, 209–216. doi: 10.1016/j.ahj.2004.08.005
Adamson, P. B., Abraham, W. T., Bourge, R. C., Costanzo, M. R., Hasan, A., Yadav, C., et al. (2014). Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 7, 935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229
Adamson, P. B., Conti, J. B., Smith, A. L., Abraham, W. T., Aaron, M. F., Aranda, J. M., et al. (2007). Reducing events in patients with chronic heart failure (REDUCEhf)study design: continuous hemodynamic monitoring with an implantable defibrillator. Clin. Cardiol. 30, 567–575. doi: 10.1002/clc.20250
Adamson, P. B., Gold, M. R., Bennett, T., Bourge, R. C., Stevenson, L. W., Trupp, R., et al. (2011). Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of the reducing decompensation events utilizing intracardiac pressures in patients with chronic heart failure (REDUCEhf) trial. Congest Heart Fail. 17, 248–254. doi: 10.1111/j.1751-7133.2011.00247.x
Bekelman, D. B., Plomondon, M. E., Carey, E. P., Sullivan, M. D., Nelson, K. M., Hattler, B., et al. (2015). Primary results of the patient-centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Intern. Med. 175, 725–732. doi: 10.1001/jamainternmed.2015.0315
Bourge, R. C., Abraham, W. T., Adamson, P. B., Aaron, M. F., Aranda, J. M. Jr., Magalski, A., et al. (2008). Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J. Am. Coll. Cardiol. 51, 1073–1079. doi: 10.1016/j.jacc.2007.10.061
Braunschweig, F., Kjellström, B., Gadler, F., and Linde, C. (2004). Optimization of cardiac resynchronization therapy by continuous hemodynamic monitoring. J. Cardiovasc Electrophysiol. 15, 94–96. doi: 10.1046/j.1540-8167.2004.03208.x
Braunschweig, F., Kjellstrom, B., Soderhall, M., Clyne, N., and Linde, C. (2006). Dynamic changes in right ventricular pressures during haemodialysis recorded with an implantable haemodynamic monitor. Neprhol. Dial Transplant. 21, 176–183. doi: 10.1093/ndt/gfi145
Braunschweig, F., Linde, C., Eriksson, M. J., and Hoffman-Bang, C. (2002). Continuous haemodynamic monitoring during withdrawal of diuretics in patients with congestive heart failure. Eur Heart J. 23, 59–69. doi: 10.1053/euhj.2001.2690
Brignole, M., Auricchio, A., Baron-Esquivias, G., Bordachar, P., Boriani, G., Breithardt, O. A., et al. (2013). 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 34, 2281–2329. doi: 10.1093/eurheartj/eht150
Bruns, H. J., Braunschweig, F., Ersgard, D., Stalberg, M., Reiters, P., Grandjean, P. A., et al. (2005). Opportunities for optimization of biventricular pacing using an implanted hemodynamic monitor. Comput. Cardiol. 32, 121–124. doi: 10.1109/cic.2005.1588049
Chaliki, H. P., Hurrell, D. G., Nishimura, R. A., Reinke, R. A., and Appleton, C. P. (2002). Pulmonary venous pressure: relationship to pulmonary artery, pulmonary wedge, and left atrial pressure in normal, lightly sedated dogs. Catheter Cardiovasc Interv. 56, 432–438. doi: 10.1002/ccd.10203
Chaudhry, S. I., Mattera, J. A., Curtis, J. P., Spertus, J. A., Herrin, J., Lin, Z., et al. (2010). Telemonitoring in patients with heart failure. N. Engl. J. Med. 363, 2301–2309. doi: 10.1056/NEJMoa1010029
Conraads, V. M., Tavazzi, L., Santini, M., Oliva, F., Gerritse, B., Yu, C. M., et al. (2011). Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 32, 2266–2273. doi: 10.1093/eurheartj/ehr050
Drazner, M. H., Hamilton, M. A., Fonarow, G., Creaser, J., Flavell, C., and Stevenson, L. W. (1999). Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J. Heart Lung. Transplant. 18, 1126–1132. doi: 10.1016/S1053-2498(99)00070-4
Drazner, M. H., Hellkamp, A. S., Leier, C. V., Shah, M. R., Miller, L. W., Russell, S. D., et al. (2008). Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE Trial. Circulation 1, 170–177. doi: 10.1161/circheartfailure.108.769778
Frantz, R., Kjellstrom, B., and McGoon, M. (2008). Ambulatory hemodynamic monitoring in pulmonary arterial hypertension. Adv. PH. 7.. Available online at: http://www.phaonlineuniv.org/Journal/Article.cfm?ItemNumber=741 (Last accessed May 14, 2015).
Fruhwald, F. M., Kjellström, B., Perthold, W., Watzinger, N., Maier, R., Grandjean, P. A., et al. (2003). Continuous hemodynamic monitoring in pulmonary hypertensive patients treated with inhaled iloprost. Chest 124, 351–359. doi: 10.1378/chest.124.1.351
Hardaway, R. M. III. (1982). Pulmonary artery pressure versus pulmonary capillary wedge pressure and central venous pressure in shock. Resuscitation 10, 47–56. doi: 10.1016/0300-9572(82)90008-9
Heart Failure Society of America, Lindenfeld, J., Albert, N. M., Boehmer, J. P., Collins, S. P., Ezekowitz, J. A., et al. (2010). HFSA 2010 comprehensive heart failure practice guideline. J. Card. Fail. 16, e1–194. doi: 10.1016/j.cardfail.2010.04.004
Ishikawa, M., Sato, N., Asai, K., Takano, T., and Mizuno, K. (2009). Effects of a pure alpha/beta-adrenergic receptor blocker on monocrotaline-induced pulmonary arterial hypertension with right ventricular hypertrophy in rats. Circ. J. 73, 2337–2341. doi: 10.1253/circj.CJ-09-0213
Jong, P., Vowinckel, E., Liu, P. P., Gong, Y., and Tu, J. V. (2002). Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch. Intern. Med. 162, 1689–1694. doi: 10.1001/archinte.162.15.1689
Karamanoglu, M., McGoon, M., Frantz, R. P., Benza, R. L., Bourge, R. C., Barst, R. J., et al. (2007). Right ventricular pressure waverform and wave reflection analysis in patients with pulmonary arterial hypertension. Chest 132, 37–43. doi: 10.1378/chest.06-2690
Kusumoto, F. M., Calkins, H., Boehmer, J., Buxton, A. E., Chung, M. K., Gold, M. R., et al. (2014). HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. J. Am. Coll. Cardiol. 64, 1143–1177. doi: 10.1016/j.jacc.2014.04.008
Lewin, J., Ledwidge, M., O'Loughlin, C., McNally, C., and McDonald, K. (2005). Clinical deterioration in established heart failure: what is the value of BNP and weight gain in aiding diagnosis? Eur. J. Heart Fail. 7, 953–957. doi: 10.1016/j.ejheart.2005.06.003
Lim, S. S., Vos, T., Flaxman, A. D., Danaei, G., Shibuya, K., Adair-Rohani, H., et al. (2012). A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260. doi: 10.1016/S0140-6736(12)61766-8
Luchsinger, P. C., Seipp, H. W. Jr., and Patel, D. J. (1962). Relationship of pulmonary artery-wedge pressure to left atrial pressure in man. Circ. Res. 11, 315–318. doi: 10.1161/01.RES.11.2.315
Maurer, M. S., Adamson, P. B., Costanzo, M. R., Eigler, N., Gilbert, J., Gold, M. R., et al. (2015). Rationale and design of the left atrial pressure monitoring to optimize heart failure therapy study (LAPTOP-HF). J. Card. Fail. 21, 479–488. doi: 10.1016/j.cardfail.2015.04.012
McCullough, P. A., Nowak, R. M., McCord, J., Hollander, J. E., Herrmann, H. C., Steg, P. G., et al. (2002). B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation 106, 416–422. doi: 10.1161/01.CIR.0000025242.79963.4C
McMurray, J. J., Adamopoulos, S., Anker, S. D., Auricchio, A., Böhm, M., Dickstein, K., et al. (2012). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the european society of cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 14, 803–869. doi: 10.1093/eurjhf/hfs105
Ohlsson, A., Bennett, T., Ottenhoff, F., Bitkover, C., Kjellström, B., and Nordlander, R. (1996). Long-term recording of cardiac output via an implantable haemodynamic monitoring device. Eur. Heart J. 17, 1902–1910. doi: 10.1093/oxfordjournals.eurheartj.a014810
Ohlsson, A., Nordlander, R., Bennett, T., Bitkover, C., Kjellstrom, B., Lee, B., et al. (1998). Continuous ambulatory haemodynamic monitoring with an implantable system. The feasibility of a new technique. Eur. Heart J. 19, 174–184. doi: 10.1053/euhj.1997.0563
Raina, A., Abraham, W. T., Adamson, P. B., Bauman, J., and Benza, R. L. (2015). Limitations of right heart catheterization in the diagnosis and risk stratification of patients with pulmonary hypertension related to left heart disease: insights from a wireless pulmonary artery pressure monitoring system. J. Heart Lung. Transplant. 34, 438–447. doi: 10.1016/j.healun.2015.01.983
Ramani, G. V., Uber, P. A., and Mehra, M. R. (2010). Chronic heart failure: contemporary diagnosis and management. Mayo Clin. Proc. 85, 180–195. doi: 10.4065/mcp.2009.0494
Ritzema, J., Melton, I. C., Richards, M., Crozier, I. G., Frampton, C., Doughty, R. N., et al. (2007). Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 116, 2952–2959. doi: 10.1161/CIRCULATIONAHA.107.702191
Russo, A. M., Stainback, R. F., Bailey, S. R., Epstein, A. E., Heidenreich, P. A., Jessup, M., et al. (2013). ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 10, e11–e58. doi: 10.1016/j.hrthm.2013.01.008
Rutherford, B. D., McCann, W. D., and O'Donovan, T. P. (1971). The value of monitoring pulmonary artery pressure for early detection of left ventricular failure following myocardial infarction. Circulation 43, 655–666. doi: 10.1161/01.CIR.43.5.655
Santulli, G. (2013). Epidemiology of cardiovascular disease in the 21st centruy: updated numbers and updated facts. J. Cardiovas. Disease 1, 1–2. Available online at: http://researchpub.org/journal/jcvd/archives_vol1_no1.html
Sharma, V., Rathman, L. D., Small, R. S., Whellan, D. J., Koehler, J., Warman, E., et al. (2015). Stratifying patients at the risk of heart failure hospitalization using existing device diagnostic thresholds. Heart Lung. 44, 129–136. doi: 10.1016/j.hrtlng.2014.07.007
Sousa, C., Leite, S., Lagido, R., Ferreira, L., Silva-Cardoso, J., and Maciel, M. J. (2014). Telemonitoring in heart failure: a state-of-the-art review. Rev. Port Cardiol. 33, 229–239. doi: 10.1016/j.repc.2013.10.013
Stevenson, L. W., and Perloff, J. K. (1989). The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA 261, 884–888. doi: 10.1001/jama.1989.03420060100040
Troughton, R. W., Ritzema, J., Eigler, N. L., Melton, I. C., Krum, H., Adamson, P. B., et al. (2011). Direct left atrial pressure monitoring in severe heart failure: long-term sensor performance. J. Cardiovasc. Transl. Res. 4, 3–13. doi: 10.1007/s12265-010-9229-z
Van Veldhuisen, D. J., Braunschweig, F., Conraads, V., Ford, I., Cowie, M. R., Jondeau, G., et al. (2011). Intrathoracic impedance monitoring, audible patient alerts and outcome in patients with heart failure. Circulation 124, 1719–1726. doi: 10.1161/CIRCULATIONAHA.111.043042
Verbrugge, F. H., Grieten, L., and Mullens, W. (2014). Management of the cardiorenal syndrome in decompensated heart failure. Cardiorenal Med. 4, 176–188. doi: 10.1159/000366168
Verdejo, H. E., Castro, P. F., Concepción, R., Ferrada, M. A., Alfaro, M. A., Alcaíno, M. E., et al. (2007). Comparison of a radiofrequency-based wireless pressure sensor to swan-ganz catheter and echocardiography for ambulatory assessment of pulmonary artery pressure in heart failure. J. Am. Coll. Cardiol. 50, 2375–2382. doi: 10.1016/j.jacc.2007.06.061
Whellan, D. J., Ousdigian, K. T., Al-Khatib, S. M., Pu, W., Sarkar, S., Porter, C. B., et al. (2010). Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF (program to access and review trending information and evaluate correlation to symptoms in patients with heart failure) study. J. Am. Coll. Cardiol. 55, 1803–1810. doi: 10.1016/j.jacc.2009.11.089
Wolfel, E. E. (2007). Can we predict and prevent the onset of acute decompensated heart failure? Circulation 116, 1526–1529. doi: 10.1161/CIRCULATIONAHA.107.729608
Yancy, C. W., Jessup, M., Bozkurt, B., Butler, J., Casey, D. E. Jr., Drazner, M. H., et al. (2013). 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 62, e147–239. doi: 10.1161/cir.0b013e31829e8807
Yang, X. W., Hua, W., Ding, L. G., Wang, J., Zheng, L. H., Li, C. Q., et al. (2013). OptiVol fluid index predicts acute decompensation of heart failure with a high rate of unexplained events. J. Geriatr. Cardiol. 10, 253–257. doi: 10.3969/j.issn.1671-5411.2013.03.012
Yu, C. M., Wang, L., Chau, E., Chan, R. H., Kong, S. L., Tang, M. O., et al. (2005). Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 112, 841–848. doi: 10.1161/CIRCULATIONAHA.104.492207
Zhang, J., Goode, K. M., Cuddihy, P. E., and Cleland, J. G., and TEN-HMS Investigators. (2009). Predicting hospitalization due to worsening heart failure using daily weight measurement: analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur. J. Heart Fail. 11, 420–427. doi: 10.1093/eurjhf/hfp033
Zile, M. R., Bennett, T. D., St John Sutton, M., Cho, Y. K., Adamson, P. B., Aaron, M. F., et al. (2008). Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 118, 1433–1441. doi: 10.1161/CIRCULATIONAHA.108.783910
Keywords: heart failure, implantable hemodynamic monitor, thoracic impedance, left atrial pressure monitor, pulmonary artery pressure monitor, LAPTOP trial, CHAMPION trial
Citation: Mooney DM, Fung E, Doshi RN and Shavelle DM (2015) Evolution from electrophysiologic to hemodynamic monitoring: the story of left atrial and pulmonary artery pressure monitors. Front. Physiol. 6:271. doi: 10.3389/fphys.2015.00271
Received: 25 June 2015; Accepted: 14 September 2015;
Published: 07 October 2015.
Edited by:
Gaetano Santulli, University of Naples Federico II, ItalyReviewed by:
Alessandro Capucci, Università Politecnica delle Marche, ItalyCopyright © 2015 Mooney, Fung, Doshi and Shavelle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deirdre M. Mooney, Cardiovascular Institute, Maine Medical Center, Richards 8, 22 Bramhall Street, Portland, ME 04102, USA,ZGVpcmRyZS5tb29uZXlAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.