- V.M. Bekhterev Saint Petersburg Psychoneurological Research Institute, Saint Petersburg, Russia
Currently, schizophrenia is considered a multifactorial disease. Over the past 50 years, many investigators have considered the role of toxic free radicals in the etiology of schizophrenia. This is an area of active research which is still evolving. Here, we review the recent data and current concepts on the roles of nitric oxide (NO) and related molecules in the pathogenesis of schizophrenia. NO is involved in storage, uptake and release of mediators and neurotransmitters, including glutamate, acetylcholine, noradrenaline, GABA, taurine and glycine. In addition, NO diffuses across cell membranes and activates its own extrasynaptic receptors. Further, NO is involved in peroxidation and reactive oxidative stress. Investigations reveal significant disturbances in NO levels in the brain structures (cerebellum, hypothalamus, hippocampus, striatum) and fluids of subjects with schizophrenia. Given the roles of NO in central nervous system development, these changes may result in neurodevelopmental changes associated with schizophrenia. We describe here the recent literature on NOS gene polymorphisms on schizophrenia, which all point to consistent results. We also discuss how NO may be a new target for the therapy of mental disorders. Currently there have been 2 randomized double-blind placebo-controlled trials of L-lysine as an NOS inhibitor in the CNS.
Introduction
The pathogenesis of schizophrenia. Basic theories of development of the disease
Schizophrenia is a severe mental disease with a chronic course, mainly manifesting at a young age (Tien and Eaton, 1992). According to various data, about 0.3–0.7% of the world's population suffer from schizophrenia (McGrath et al., 2008; Tandon et al., 2008). Aetiopathogenetic mechanisms of this disease are still unclear (Eaton et al., 1995; Keshavan et al., 2013). There is also a generality of symptoms between schizophrenia proper, schizoaffective disorder, bipolar affective disorder (Rimol et al., 2010; Keshavan et al., 2013). In the present circumstances, there is a need for a more precise answer to the question of what underlies the pathogenesis of schizophrenia and defines its uniqueness.
Currently, schizophrenia is considered a multifactorial disease. Up to 80% of cases of schizophrenia are associated in one way or another with genetic factors (Sullivan et al., 2003; Käkelä et al., 2014). Recent GWAS-analyses have revealed genes associated with schizophrenia (for example, NRGN, TCF4, and TSNARE1). The functions of some genes are still unknown (Stefansson et al., 2009; Sleiman et al., 2013). Therefore, the known genetic risk cannot yet predict the cause of the disease and what underlies its pathogenesis. There are several basic hypotheses regarding the development of schizophrenia. The earliest hypothesis postulates that the cause of the disease consists of dopamine metabolism disturbance, namely in the increase in its synthesis (van Rossum, 1966; Creese et al., 1976). The second version of the same hypothesis argues that dopamine metabolism is disturbed in two directions—the level of the neuromediator increases in subcortical (striatum) and decreases in prefrontal structures of the brain (Scatton et al., 1982). In particular, the hypothesis attempted to associate negative symptoms with low-dopaminergiñ state (Davis et al., 1991). Finally, the dopaminergic theory was reduced to the concept of “a final general way” that is interpreted as an influence of various factors, including exogenous factors, on presynaptic dopamine neurotransmission in striatum and that leads to development of the disease (Howes and Kapur, 2009). It is possible to consider the recognition of the role of glutamate (NMDA-receptors) and gamma-aminobutyric acid (GABA) as an addition to the dopaminergic theory of schizophrenia in its pathogenesis (Timms et al., 2013; Hu et al., 2014). Another hypothesis is associated with detection of development disturbances of some brain areas in schizophrenics (Pino et al., 2014). The latter hypothesis is backed by a lot of evidence (Howes et al., 2011; Jenkins, 2013), especially in the aspect of neurocognitive impairment (Dickson et al., 2012). A related point of view is a neurodegenerative theory of the disease. The essence of the theory is that schizophrenia is not a consequence of the improper development, but of gradual progressive disintegration of the nervous tissue in the brain (Hardy and Gwinn-Hardy, 1998). In the latter two theories, the important role is attributed to exogenous factors (Jenkins, 2013). Taking into account available evidence, it is possible to assume that etiopathogenesis of schizophrenia involves influence of the environment on the organism both during the process of development and after its completion. Of major significance are alterations in gray matter, occurring in the critical periods of development—during childbirth, at adolescence (Clarke et al., 2011; Hart et al., 2013). But pathogenic mechanisms which lead to described disturbances are the subjects of discussions. Publications of recent years state the undoubted role of inflammation and oxidative stress in pathogenesis of mental diseases, especially schizophrenia (Song et al., 2013; Bergink et al., 2014; Haller et al., 2014). In numerous investigations, associations between existence of disease and activation of immune markers in the patient have been found (Song et al., 2013; Bergink et al., 2014; de Witte et al., 2014). Some authors consider infectious agents as etiologic factors (Brown and Derkits, 2010). But, irrespective of its cause, the inflammatory process is associated with oxidative stress which leads to alterations of the tissues, some of which are irreversible (Bitanihirwe and Woo, 2011).
Role of Oxidative Stress of CNS in Schizophrenia Development
Role of toxic free radicals in the etiology of schizophrenia was first investigated more than 50 years ago (Hoffer et al., 1954). As of now, markers of oxidative stress are revealed in patients with schizophrenia, reliably more often than in the general population. One of the latest papers is a meta-analysis by Flatow et al. (2013), where 44 investigations are considered and a conclusion about reliability of association of markers of oxidative stress with psychosis and schizophrenia existence in patients is drawn (Flatow et al., 2013). Let us consider in more detail what is included in concept of oxidative stress and what its potential role in schizophrenia development is.
The Structure of Oxidative Stress
Free Radicals
Oxidative stress is a state during which the balance between the system of antioxidants and level of free radicals, able to damage tissues, is disturbed (Kohen and Nyska, 2002). Synthesis of free radicals occurs during physiological oxidation-reduction processes (Berg et al., 2004). Free radicals are as follows: active forms of oxygen (reactive oxygen species—ROS), active forms of nitrogen—(RNitrogenS—RNS), carbon-centered radicals and sulfur-centered radicals (Miller et al., 1990). ROS is the most significant system of free radicals, because it shows the double role of oxygen in the organism (oxygen paradox)—this element is involved almost in all vital processes, and at the same time its active forms, leading to damage of tissues, are formed (Davies, 1995). Hydrogen peroxide (H2O2), superoxide-radical (O2·−) and hydroxide-radical (OH·) belong to ROS. Nitrogen-containing factors of oxidation: nitrogen oxide (NO), peroxynitrite (ONOO·) and nitrogen dioxide (N2O2). Nitric oxide is able to form hydroxyl radicals (at the expense of the unpaired electron) and to turn into nitrogen dioxide (Wu et al., 2013). During maintenance of balance, free radicals carry out important functions in the organism: they protect cells against infectious and foreign agents, serve as secondary mediators in regulation of activity of cardiovascular system, are involved in processes such as maintenance of intracellular level of calcium, phosphorylation/dephosphorylation of proteins and activation of transcription factors (Halliwell, 2007; Valko et al., 2007; Ataya et al., 2011; Wu et al., 2013). Nitric oxide possesses properties of both pro- and antioxidant. So, it is able to stimulate peroxidation of lipids and mediate antioxidant reactions in cellular membranes at the same time (Radi et al., 1991). NO is able to bind with peroxyl radicals, interrupting the oxidative process circuit. However, free nitric oxide can bind with superoxide, forming highly toxic peroxynitrite (Bitanihirwe and Woo, 2011). The imbalance of antioxidants and free radicals leads to damage of cellular structures: proteins, lipids, DNA (Kohen and Nyska, 2002).
Antioxidants
The antioxidants, serving for maintenance of oxidation-reduction balance, are as follows: superoxide dismutase (SOD), catalase (CAT) and glutation peroxidase (GSH-Px) (Yao and Keshavan, 2011). The three enzymes act in cooperation, blocking formation of free radicals at different stages of their metabolism. Hydrogen peroxide formed by SOD is decomposed into water and oxygen by catalase. Breakage of this mechanism of protection leads to activation of peroxidation of lipids. GSH-Px is able to stop the process at this stage with the use of the transfer of toxic hydroperoxides into less active forms. Disturbance of activity of any of three enzymes results in increased vulnerability of membranes of cells for damage by free radicals (Smith, 1992). Albumin, vitamin E (α-tocopherol), uric acid, bilirubin, ascorbic acid, and thioredoxin belong traditionally to nonenzyme antioxidants (Yao et al., 2000). Nonenzyme antioxidants from plasma of blood make the basic contribution to counteraction to oxidative stress (Yao et al., 2000; Reddy et al., 2003; Reddy, 2011).
Oxidative Stress and Schizophrenia
The role of oxidative stress in the development of mental diseases is still debated despite decades of investigations in this direction. The different approaches of researchers appear to be a likely reason for these debates. Free radicals dissociate very quickly and measuring their number in live organisms is difficult. Thus, indirect methods such as measurement of activity of basic antioxidant enzymes as well as the quantitative identification of peroxidation products in biological mediums are more widespread (Wu et al., 2013). The role of oxidative stress is actively studied in the terms of neuropsychiatric diseases, such as depression (Moylan et al., 2014; Rodrigues et al., 2014), bipolar disorder (Bauer et al., 2014), Alzheimer's disease (Rodrigues et al., 2014), and Huntington's chorea (Ciancarelli et al., 2014). In this review we will go into detail on schizophrenia, without mentioning the investigations, covering other diseases.
Indicators of Activity of Antioxidant Enzymes in Schizophrenia
As mentioned above, the measurement of antioxidant enzymes is complicated due to their properties. However, numerous studies have been carried out in this field. Unfortunately, an unequivocal answer still has not been obtained. Studies of SOD activity in patients with schizophrenia are met in literature more often than that of other enzymes. Many authors have concluded that activity of this enzyme is reduced in patients with schizophrenia (Ben Othmen et al., 2008; Dadheech et al., 2008; Raffa et al., 2009, 2012a; Gonzalez-Liencres et al., 2014; Wu et al., 2014). However, there are results for both hyperactivity of superoxide dismutase in this disease (Wu et al., 2012) and unaltered activity as compared to controls (Raffa et al., 2011; Srivastava et al., 2001). Results regarding measuring levels of glutation peroxidase (Raffa et al., 2009, 2012b; Dietrich-Muszalska and Kwiatkowska, 2014) and catalase (Ben Othmen et al., 2008; Raffa et al., 2011, 2012b) are contradictory. This is covered in more detail in relevant literature reviews (Yao and Keshavan, 2011; Wu et al., 2013). To present, 2 meta-analyses of research of activity of antioxidants in patients with schizophrenia have been carried out. Zhang et al. (2010) concluded that oxidative stress in schizophrenia really takes place. However, decrease in SOD activity in this disease has been recognized as reliable, levels of CAT and GSH-Px have not shown sufficient statistical effect (effect size). Authors indicate that considerable heterogeneity of the carried-out analysis means an influence of unaccounted factors on the obtained result (Zhang et al., 2010). Meta-analysis by Flatow et al. (2013), mentioned above, has confirmed decrease in SOD activity in patients with schizophrenia according to cross-sectional investigations in the first psychotic episode, in remission and in the state of exacerbation. But considerable additions to results of the previous systematic review have been made: activity of catalase was reliably decreased in patients with initial episode (first episode psychosis) (p < 0.01) and reliably increased in patients in remission (p < 0.01) as compared with controls. Authors have assumed that the total antioxidant level is a situationally conditioned marker, labile under the influence of the mental state of the patient. But enzyme SOD, which has shown, irrespective of disease's stage, decreased as compared with healthy controls activity level, can be considered as a typical trait of patients with schizophrenia (Flatow et al., 2013).
Peroxidation products were also studied as markers of oxidative stress. The basic objective of these investigations—malondialdehyde (MDA), identified with use of measuring of the content of thiobarbituric acid reactive substance (TBARS) (Vasankari et al., 1995). Investigations are so far not plentiful. Gubert et al. (2013) have found a reliable increase in the level of TBARS in patients with schizophrenia as compared with controls (Gubert et al., 2013). This result coincides with previously obtained results (Zhang et al., 2006; Dietrich-Muszalska and Kontek, 2010), including meta-analysis by Zhang et al. (2010). In addition, seasonal dynamics of level of metabolites in plasma of patients with schizophrenia have been followed up: in summer, contents of MDA in serum exceeds winter indices by 33,9% (Morera et al., 2009). Besides MDA, isoprenes, in particular 8-isoPGF2 alpha, were considered as biomarkers of peroxidation (Dietrich-Muszalska and Olas, 2009). However, there is still not enough of an evidence base for association of 8-isoPGF2 alpha with schizophrenia.
Indices of Activity of Plasma Antioxidants in Schizophrenia
As mentioned above, plasma antioxidants make the basic (>85%) contribution to the fight against oxidative stress (Wayner et al., 1987; Reddy et al., 2003; Reddy, 2011). For patients with schizophrenia, it is shown that in the first psychotic episode, plasma levels of α-tocopherol (Dadheech et al., 2008), ascorbic acid decrease (Suboticanec et al., 1990; Dadheech et al., 2008), level of thioredoxin increases (Zhang et al., 2009; Owe-Larsson et al., 2011). In opinion of Zhang et al. (2009), in patients with schizophrenia, the level of thioredoxin is close to that of healthy controls (Zhang et al., 2009). The same authors, in 2013, have confirmed that the plasma level of thioredoxin in patients with schizophrenia does not exceed reliably healthy controls and is associated with the level of cognitive abilities. It is shown that the level of thioredoxin is reliably decreased in patients with attention deficit disorder (Zhang et al., 2013). Bilirubin, uric acid and albumin have also been measured in patients with schizophrenia. Decreased plasma levels in the first psychotic episode as compared with controls have been noted (Yao et al., 1998, 2000; Reddy et al., 2003; Pae et al., 2004). It is interesting to note that attempts to associate separate symptoms of schizophrenia with levels of plasma antioxidants and the use of these symptoms as a diagnostic and predictive marker have taken place (Yao et al., 2012). Indeed, the total antioxidative status (TAS) is reliably low in the first psychotic episode, negatively correlating with negative symptoms according to PANSS (Positive and Negative Syndrome Scale) (Pazvantoglu et al., 2009; Li et al., 2011). The assumption has been made that oxidative stress arises during onset of the disease and exerts essential impact on the further course of schizophrenia, especially on the formation of negative symptoms. Preliminary results of the investigation by Albayrak et al. (2013) have revealed that patients with persistent negative symptoms (deficit schizophrenia) have a decreased total antioxidative potential and, at the same time, their index of oxidative stress is increased as compared with healthy controls and patients with non-deficit schizophrenia (Albayrak et al., 2013).
Influence of oxidative stress on the course of schizophrenia is confirmed by studies which show that treatment with antipsychotics leads to considerable alterations of the oxidation-reduction balance. However, results indicate otherwise. There is a point of view that antipsychotic therapy decreases the level of oxidants in the organism (Tsai et al., 2013). At the same time, pro-oxidant activity is attributed to Haloperidol in vitro as compared with Quetiapine and Olanzapine (Dietrich-Muszalska, 2011). Clozapine has contradictory properties regarding influence on oxidative stress (Miljevic et al., 2010; Gilca et al., 2014). But despite all contradictions, an undoubted effect of treatment with antipsychotics on indicators of oxidative stress is confirmed.
It is clear from the above paragraphs that there is a sufficient number of markers of oxidative stress, and this is an almost unlimited field for research. However, in this review we would like to consider the system of nitric oxide and related molecules within the pathogenesis of schizophrenia in more detail.
Nitric Oxide in Pathogenesis of Schizophrenia
Biochemistry of Nitric Oxide in the Organism and CNS. The Interactions of NO with other Factors of Oxidative Stress
Before considering the works devoted to the role of nitric oxide in development of schizophrenia, it is necessary to understand its biochemistry, biological parameters and functions.
Nitric oxide is involved in many processes occurring in the brain: regulation of synaptic plasticity (Hölscher and Rose, 1992), release of mediators (Lonart et al., 1992), and development of nervous tissue (Gibbs, 2003). Below we describe the main points of NO's biochemistry and its role in CNS.
Biochemistry of NO
Nitric oxide (NO) is released during conversion of L- arginine into L-citrulline under influence of nitric oxide synthase (NOS) (Ghafourifar and Cadenas, 2005). The reaction proceeds in the presence of oxygen, NADPH-containing flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), heme, thiol and tetrahydrobioprotein as cofactors (Akyol et al., 2004; Giraldi-Guimarães, 2004). NO has a very short half-life period—several seconds in water medium. However, placed under anaerobic conditions, this radical does not decompose for more than 15 s. In mixed water and lipid medium, nitric oxide is easily able to penetrate cytoplasmic membranes (Chiueh, 1999; Valko et al., 2007; Babicová et al., 2013). Free radical NO at the expense of unpaired electron possesses toxic potential. So, in high concentrations, nitric oxide reacts with superoxide-radical, forming peroxynitrite (ONOO) (Ridnour et al., 2004; Blaise et al., 2005; Wu et al., 2013), a powerful oxidant at physiological pH (Noack et al., 1999). Peroxynitrite is able to trigger lipid peroxidation and destruction of proteins, amino acids, nucleic acids; this leads to suppression of activity of enzymes and autoxidation of dopamine. This mechanism has been studied in the context of development of neurodegenerative diseases, in particular, Parkinson's disease (Tohgi et al., 1998). However, peroxynitrite quickly turns into stable nitrate NO3- (Tohgi et al., 1998; Antunes et al., 2005). Toxicity of NO increases during low concentrations of L- arginine, as in this case superoxide-radical is generated by NOS (Xia et al., 1998; Akyol et al., 2004). The state in which formation of reactive compounds of nitrogen (RNS) exceeds their neutralization is called nitrosative stress (Ridnour et al., 2004; Valko et al., 2007). It is worth mentioning that most physiological effects of NO are mediated by a radical that quickly binds with complexes: Fe2+-Haem. The reaction product is {Fe2+-NO·}. This compound allows avoiding reactions of oxidative stress (Archer, 1993; Valko et al., 2007).
NO-Synthase
The enzyme generating nitric oxide is represented in the organism in the kind of three isoforms: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS).
Nitric oxide synthesized by endothelial NO synthase renders vasodilatating, anti-inflammatory, antithrombotic and antiproliferative effects. Endothelial NO synthase itself is located in the cells of endothelium (Furchgott and Zawadzki, 1980; Endres et al., 2004). Synthesis of nitric oxide by endothelial NOS is regulated by several factors: calcium-dependent calmodulin, caveolin-1 and 3, B2-receptors of bradykinin, A1-receptors of angiotensin, steroid hormones (Fleming and Busse, 2003; Gwathmey et al., 2012; Kypreos et al., 2014). It has been revealed that disturbance of synthesis and release of nitric oxide (eNOS is responsible for this) leads to increased aggregation of thrombocytes, inflammation of endothelium and as a result—to the disturbance of cerebral blood flow (Diodati et al., 1998; Toda et al., 2009; Austin et al., 2013). The importance of this enzyme is generally considered in the context of neurodegenerative diseases (Alzheimer's disease etc.) (Toda, 2012). However, below we will introduce recent investigations about possible role of eNOS in pathogenesis of schizophrenia.
iNOS enzyme plays an essential role in inflammatory processes during damage or infectious impairment of tissues (Lerouet et al., 2002). In the brain tissue, this type of NO synthase is located in microglia, endothelium of brain vessels, infiltrating macrophages and T-lymphocytes. Unlike eNOS and nNOS, this isoform is calcium-independent (Possel et al., 2000; Bernstein et al., 2005a). Microglia produces neuro-toxic nitric oxide (Lazzaro et al., 2014). Regulation of iNOS gene transcription depends on cAMP concentration. When level of cAMP increases, cellular reactions of inflammation are blocked (Markovic et al., 2003; Valko et al., 2007). NO, generated by inducible NOS, is involved in pathogenesis of Alzheimer's diseases, septic shock, multiple sclerosis and brain ischemia (Wass et al., 2008; Ghasemi and Fatemi, 2014).
Neuronal NOS (nNOS) generates up to 90% of nitric oxide in the brain of mammals (Hara et al., 1996). Besides neurons, nNOS is found in skeletal muscles, myocardium and smooth muscle tissue (McConell et al., 2007; Seddon et al., 2007). This enzyme is linked with NMDA receptors by means of the specific domain. This allows for activation the NO synthesis in response to influx of calcium ions into the cell after excitement of receptors (Girouard et al., 2009). Synthesis of this enzyme in the neuron is determined by the function carried out by the cell. It has been shown that nNOS influences the development of neuronal structures (Chen et al., 2004). nNOS distribution in neurons depends on their localisation. The highest concentrations of the enzyme are found in substantia innominata, septum, cortex cerebelli, hypothalamus, subthalamus. The lowest concentrations are found in corpus callosum, thalamus, occipital cortex, dentate nucleus (Blum-Degen et al., 1999). In addition to neurons, nNOS can be present in the cells of glia and blood vessels of the brain (Kawakami et al., 1998). Sufficiently detailed review of metabolism of nNOS has been published by Zhou and Zhu (2009).
Role of Nitric Oxide in CNS
NO is the second mediator of activation of NMDA receptors (subtype of glutamatergic receptors). In norm, during activation of these receptors by glutamate, calcium influx into the cell is stimulated, and NO synthase forms nitric oxide that intermediately activates guanylate cyclase and causes increased cGMP synthesis (Szabadits et al., 2011). It has been identified that the concentration of NO reflects activity of glutamatergic neurotransmission (Akyol et al., 2004). However, other receptors of glutamate (for example, AMPA) also are able to generate NO (Zhou et al., 2006). This pathway modulates the release of glutamate and dopamine (Hoque et al., 2010; Szabadits et al., 2011). In addition to glutamate, NO is involved in storage, uptake and release of mediators, such as acetylcholine, noradrenaline, GABA, taurine and a glycine (Kraus and Prast, 2001; Boehning and Snyder, 2003). In addition, nitric oxide is a mediator able to activate its own extrasynaptic receptors, located some distance from the place of NO synthesis (Kiss and Vizi, 2001). It is known that nitric oxide is involved in the process of development of the nervous system (Lonart et al., 1992; Gibbs, 2003; Bernstein et al., 2011). Recent experimental studies have increased the evidence base of this statement. So, it has been established that nNOS-containing neurons actively participate in the rostral path of migration of neuroblasts, creating new synaptic connections and considerably influencing the neurogenesis (Blasko et al., 2013). Migration of astrocytes is also determined by release of NO under action of iNOS, according to an investigation by Wang et al. (2011). Nitric oxide is recognized also as an important factor of the formation of synapses and the growth of nervous fibers (Duan et al., 2012; Cooke et al., 2013; Upreti et al., 2013). It is worth repeating that NO influences the development of nervous tissue by regulation of cerebral blood flow (Furchgott and Zawadzki, 1980; Endres et al., 2004; Virarkar et al., 2013).
Role of Nitric Oxide and Related Molecules in Schizophrenia Development
As has been mentioned above, nitric oxide can also act as a free radical. To this end, it is worth considering the participation of NO in the pathogenesis of schizophrenia.
For the first time, Russian scientists Averbukh et al. (1966) and Bul'ba et al. (1968) have suspected that there is an association between NO and the onset of schizophrenia (Averbukh et al., 1966; Bul'ba et al., 1968). However, this molecule has been most actively investigated in the aspect of mental diseases during the last 25 years. Identification of activity of nitric oxide in tissues and biological media can be carried out by means of detection of NO-synthase, because the NO molecule has a very short period of existence (Babicová et al., 2013).
Post-Mortem Studies
Researchers who have found an increased level of nitric oxide in post-mortem brain tissue (Yao et al., 2004; Lauer et al., 2005; Cui et al., 2010) and in plasma (Zhang et al., 2010; Flatow et al., 2013) of schizophrenics also support the existence of an association between NO synthase activity and schizophrenia. Actually, the level of nNOS differs in patients with schizophrenia and healthy controls (Akyol et al., 2004). However, this issue is disputed. In the cortex of the cerebellum, the level of nNOS did not differ between patients with schizophrenia and healthy controls in the investigation by Doyle and Slater (1995). However, there is also other data—increased NO synthase activity has been detected in Purkinje cells and dentate nucleus of patients with schizophrenia but not with depression (Bernstein et al., 2001). With regard to the neocortex, data about presence of NO synthase are inconsistent. There is data about increasing of expression of nNOS in prefrontal cortex in schizophrenia (Baba et al., 2004), but a disproof of these findings was also published (Xing et al., 2002). Interesting data were obtained during investigations of neurons of hypothalamus. So, the decreasing of nNOS-containing neurons has been revealed in periventricular nucleus of patients with schizophrenia and affective disorders (Bernstein et al., 1998). Decreased number of neurons with nNOS in suprachiasmatic nucleus has been also described (Bernstein et al., 2005b, 2010). At the same time, the expression level of enzyme was not disturbed (Bernstein et al., 2000). It is known that NO in the hypothalamus regulates synthesis and release of hormones which regulate hypothalamic-pituitary-adrenal system (HPAS): oxytocin, vasopressin, corticoliberin (Bernstein et al., 1998). Disturbed production and release of these peptides leads to HPAS hyperactivation in patients with schizophrenia (Ryan et al., 2004). The findings indicate that the disturbed NO synthesis by means of influence on hormonal balance can lead to the development of mental diseases. This statement is also confirmed by the fact that improvement of the course of depression and other mental diseases after electroconvulsive therapy is conditioned by the effect of hypothalamic NO. Rosen et al. (2003) have shown that nitric oxide during electroconvulsive therapy exerts an essential influence on cerebral blood flow, long-term potentiation of NMDA receptors, and activity of HPAS (Rosen et al., 2003). In addition to hypothalamic structures, there are data about the change of NO activity in striopallidar system (Lauer et al., 2005), nucleus caudatus, and hippocampus in mental disorders. In one instance, based on study of 15 postmortem samples of the brain of patients with schizophrenia, the decrease in the number of nNOS-containing neurons in lateral part of the lenticular nucleus has been found (Fritzen et al., 2007). The increased activity of nitric oxide in neurons of nucleus caudatus has been also shown for patients with schizophrenia (Yao et al., 2004). Research of the hippocampus indicated differences between healthy controls from schizophrenics. It was revealed that NO-synthase been activated mainly in the right hippocampus of schizophrenics (Kristofiková et al., 2008). Although currently there are less post-mortem studies, experimental studies on modeling of schizophrenia confirm increase in the concentration of NO-synthase and activation of microglial inflammation in hypothalamus (Jing et al., 2013; Ribeiro et al., 2013). Researchers who used phencyclidine for modeling of symptoms of schizophrenia in animals confirm the alteration of metabolism of L-arginine and as a result—high concentration of NO in brain structures. An association between altered content of nitric oxide and disturbances of behavior and cognition was found (Pålsson et al., 2010; Finnerty et al., 2013; Knox et al., 2014).
NO Levels in Biological Fluids
Measurement of NO level in biological fluids of schizophrenics has also been carried out. Level of NOS and NO metabolites in the blood of patients with schizophrenia and depression has been found by various investigators, both as increased (Yilmaz et al., 2007; Akpinar et al., 2013) and as decreased (Srivastava et al., 2001). In the meta-analysis by Maia-de-Oliveira et al. (2012) it has been shown that the level of nitric oxide does not differ significantly between patients with schizophrenia and healthy controls. However, a reliably higher level of NO was found in patients treated with antipsychotics, which suggests the influence of these drugs on the metabolism of NO synthase (Maia-de-Oliveira et al., 2012). In addition to blood, attention of the investigators was drawn by cerebrospinal fluid. But articles on this topic are scarce, and results are not consistent. According to the investigation by Ramirez et al. (2004), in cerebrospinal fluid of patients with schizophrenia, the level of nitric oxide is decreased (Ramirez et al., 2004). Concentration of substances in cerebrospinal fluid reflects their synthesis by brain tissues. It is therefore possible to assume a decrease in NO synthesis by cells of the brain in schizophrenia. However, at this stage it is not yet possible to confirm this hypothesis. The evidence base is too small. The other known factors influencing the level of nitric oxide in tissues and fluids of the organism are as follows: smoking (Vleeming et al., 2002) and treatment by antipsychotics (Ramirez et al., 2004).
NO and Neurodevelopment
In view of the possible association of schizophrenia and metabolism of nitric oxide in the nervous tissue, the process of neurodevelopment is of interest. There is an opinion that disturbances of the defined localisation of NOS-containing neurons in the process of development of the brain are associated with vulnerability to schizophrenia (Eastwood and Harrison, 2003). Nitric oxide was detected in some neurons from the moment of their formation, even before migration to the point of final differentiation. From the very beginning, NO plays a key role in formation of synapses and establishment of local neurotransmitter links (Gibbs, 2003). Thus, movement of such neurons to an inappropriate place leads to multiple morphological and neurochemical alterations, which is typical of schizophrenia (Lewis et al., 2005; Connor et al., 2011). In addition, the influence of overproduction of nitric oxide in prenatal and perinatal periods is also considered. It has been shown (but only in single study) that viral infection can cause abundant formation of nitric oxide in rat's brain (Fatemi et al., 2000). The increased NO synthesis in white matter leads to damage of oligodendrocytes, demyelination of fibers (this was observed in schizophrenics) (Uranova et al., 2004). Increased activity of nNOS was also described at the postnatal stage. It also leads to specific disturbances. There was an interrelationship between NO production and NMDA receptors, which are known as low-activity in patients with schizophrenia (Northoff et al., 2005). The increased formation of NO, however, does not seem to be caused by NMDA receptors. Involvement of AMPAR receptors has been considered as the most probable, especially because their high expression in schizophrenics has been found (Dracheva et al., 2005; Tanda et al., 2009). Continuous stimulation of the release of nitric oxide results in disturbed synaptogenesis, synaptic remodeling, and alterations of synaptic membranes (Sunico et al., 2005). One should not underestimate the role of nitric oxide as a free radical. Continuous stimulation of NO synthesis results in damage of membranes of cells and mitochondrions, which has been found in schizophrenia (Ben-Shachar and Laifenfeld, 2004). However, the role of overproduction of nitric oxide in development of mental does not have a sufficient evidence base as it does in neurologic pathology (multiple sclerosis, Alzheimer's disease) and should be confirmed by further research.
The decreased synthesis of nitric oxide in the brain is also discussed as a pathogenic factor of mental disorders. It has been shown that nNOS deficiency in experiments results in cognitive and behavioral disturbances, pathognomonic for schizophrenia (Kirchner et al., 2004; Dec et al., 2014). Therefore, even the migration of neurons described above may result in NO deficit in some areas and in cognitive deficiency.
The described disturbances of synthesis and release of nitric oxide were actively studied in the aspect of the hypothesis according to which schizophrenia is conditioned by disturbed neurogenesis. A paper by Oldreive et al. (2012) has shown that an increased level of nNOS and nitric oxide deteriorates the survival rate of Purkinje cells of the cerebellum in vitro (Oldreive et al., 2012). We have previously mentioned that the increased activity of nNOS was noticed in Purkinje cells in patients with schizophrenia (Bernstein et al., 2001). It is interesting that the harmful influence of nitric oxide on cerebellar neurons depends on stage of their development: maturation of cells makes them less sensitive to NO (Oldreive et al., 2008). The influence of nitric oxide on the development of the hippocampus is in detail discussed in recently published thematic reviews (Gray and Cheung, 2014; Hu and Zhu, 2014). As additional evidence of the participation of nitric oxide in the process of neurodevelopment, experiments serve with axotomy of motoneurons. After axotomy, the neuron starts the reparation program that includes a dephenotyping, creation of new synaptic afferent and efferent links. One of the key factors in this process is NO (González-Forero and Moreno-López, 2014). Studies have revealed that nitric oxide can induce a proliferation of neuronal stem cells in vitro, and at early stages—through system of ERK/MAP-kinase, and at late stages—through guanylyl cyclase-cyclic GMP-protein kinase G (Carreira et al., 2013). Research in this direction continues and promises to shed light on features of neurogenesis and its key participants.
Nitric oxide also participates in the interrelationships between neurons and gliacytes. It was shown that increased synthesis of NO by microglia can lead to damage of neurons (Graber et al., 2012). iNOS is responsible for synthesis of nitric oxide in microglia (Contestabile et al., 2012). In vitro studies indicated NO-dependent microglial reactions in the form of death of neurons, especially under conditions of inflammation (Graber et al., 2012). Nevertheless, neurons are able to secrete substances causing apoptosis of glia for prevention of excessive synthesis of NO (Polazzi and Contestabile, 2006). Against the background of introduced results, conclusions that iNOS activates neurogenesis in dentate gyrus of hippocampus are interesting (Luo et al., 2007). These data contradict to findings of in vitro research, but at the same time supplement evidence of the participation of NO-synthase in processes of neurogenesis. There are still not enough findings about the interrelationships between the activity of iNOS and schizophrenia development. However, this enzyme is important for understanding the role of microglia and inflammation in disturbances of development of the central nervous system.
Genetic Aspects of Metabolism of NO within Schizophrenia (Table 1)
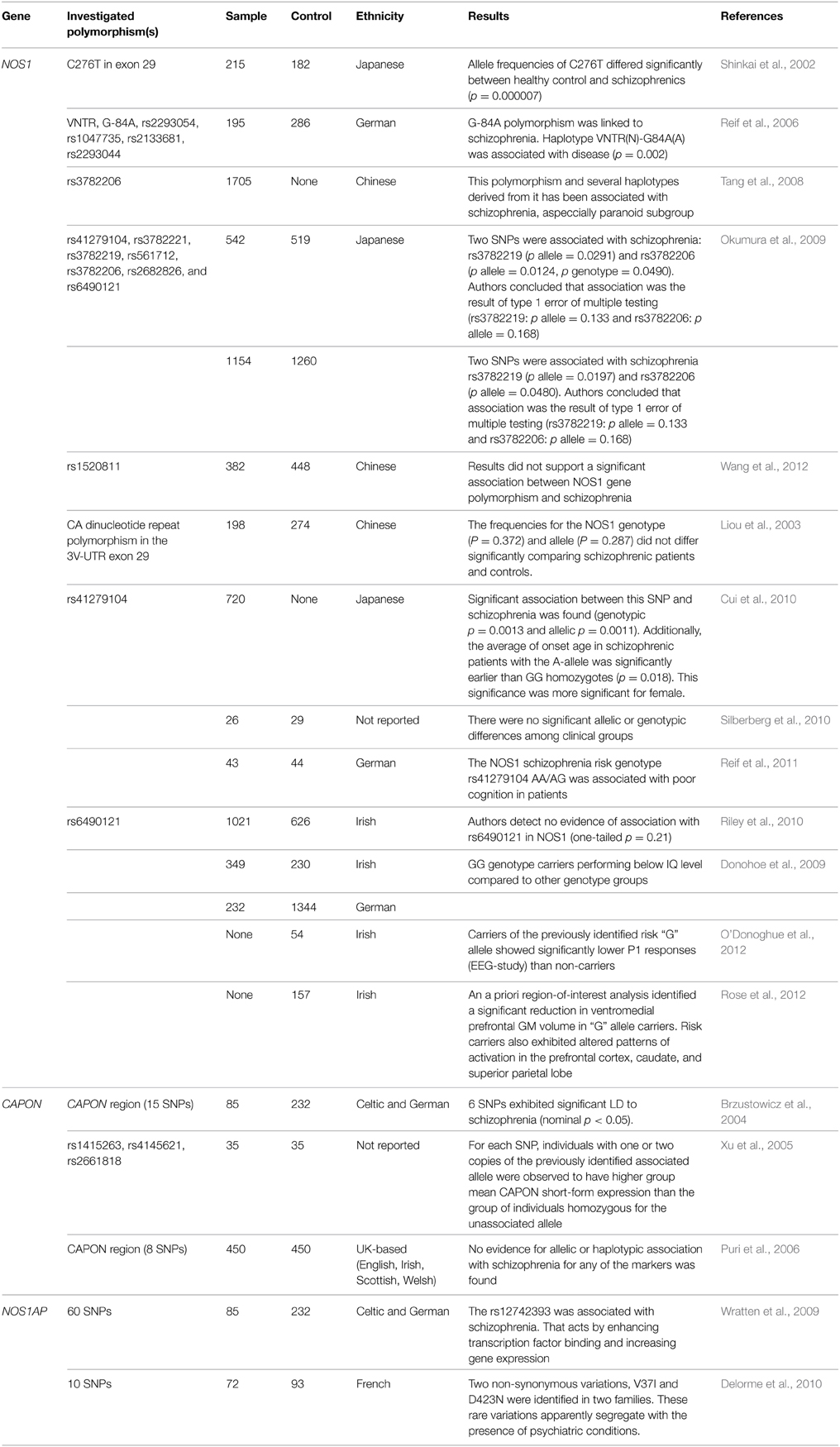
As of right now, there are associative investigations of polymorphisms of gene of neuronal NO-synthase carried out in patients with schizophrenia (is coded by NOS1 gene). In one of the earliest papers (Shinkai et al., 2002), a reliable association between the disease and the C276T polymorphism carriage was revealed (p = 0.000007) (Shinkai et al., 2002). Further research included several other points: Reif et al. (2006) have established a reliable association of regulatory exon 1c promoter polymorphism as well as nNOS-mini haplotype with the development of schizophrenia and prefrontal functioning (Reif et al., 2006). One of the papers by Chinese scientists where 11 polymorphisms of NOS1 gene are considered in 1705 patients has confirmed an association of polymorphism of rs3782206 and some haplotypes from 5′ flank region of the gene with schizophrenia (Tang et al., 2008). It is worth mentioning that there are two papers with negative results. Okumura et al. (2009) have carried out the analysis of seven polymorphisms of NOS1 gene (rs41279104, rs3782221, rs3782219, rs561712, rs3782206, rs2682826, and rs6490121), but found that reliable associations have been recognized as type I mistake in multiple calculations, and are consequently untenable (Okumura et al., 2009). A similar situation is described for polymorphism rs1520811 in the Chinese population (Wang et al., 2012). Earlier Liou et al. (2003) have also published data on the absence of an association between CA repeat polymorphism and schizophrenia (Liou et al., 2003). Positive results for the Japanese population have been presented in analysis by Cui et al. (2010), who have revealed an association of polymorphism rs41279104 with schizophrenia development. Further, it is established that carriers of allele A have reliably earlier age of manifestation of the disease as compared with GG-homozygotes. In women, the influence of genotype on tendency to disease was expressed more strongly (Cui et al., 2010). Polymorphism rs6490121 has shown a possible association with schizophrenia onset in GWAS, which was confirmed by Riley et al. (2010). Functional polymorphisms “NOS1_1d” and “NOS1_1f” have also been studied, but on small samples of assays of brain tissue, having shown a reliable increase in expression in schizophrenia (Silberberg et al., 2010). NOS1 gene polymorphisms were combined with disturbance of cognitive functions both in patients with schizophrenia (Donohoe et al., 2009; Reif et al., 2011) and in healthy persons (Donohoe et al., 2009; O'Donoghue et al., 2012; Rose et al., 2012). Moreover, an essential association between the carriage of G-allele of rs6490121 and reduction of thickness of gray matter of the ventromedial prefrontal cortex has been noted (Rose et al., 2012). Three investigations studied the association between carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase (CAPON) gene and the onset of schizophrenia across different populations. The role of this gene consists in a disturbance of functioning of NMDA-receptors (hypofunction) that keeps within one of the theories of development of schizophrenia. Two investigations have confirmed the existence of reliable linkage disequilibrium between schizophrenia and polymorphisms of this gene (Brzustowicz et al., 2004; Xu et al., 2005). However, the British scientists could not replicate previous results (Puri et al., 2006). For association of polymorphisms of gene encoding carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase (NOS1AP) with schizophrenia, few investigations have been carried out. The assumption of the possible role in disease development (Wratten et al., 2009; Delorme et al., 2010) was the only produced result. In summary, it is worth mentioning the meta-analysis carried out by Weber et al. (2014). The authors studied the role of polymorphisms of genes NOS1 and NOS1AP in schizophrenia development. Polymorphism (SNP) rs41279104 is reliably associated with disease. The carriage of the defective allele leads to a decrease of nNOS in prefrontal cortex. The bioinformatic analysis has also confirmed an interaction between NOS1 and NOS1AP in increase of the tendency to the disease (Weber et al., 2014).
Nitric Oxide as Potential Target for Development of Schizophrenia Treatment
Influence of Antipsychotics on Metabolism of NO
It has been shown that antipsychotics alter the metabolism of NO in the brain. Haloperidol is able to suppress activity of nNOS (Nel and Harvey, 2003; Zhang et al., 2012). However, long-term administration of drugs results in hyperactivity of this enzyme in striatum of rats (Lau et al., 2003). Authors of this study have assumed that late alterations of nNOS activity in neostriatum during treatment with an antipsychotic participate in pathogenesis of late dyskinesia (tardive dyskinesia) (Lau et al., 2003). It is worth repeating that in plasma of schizophrenics receiving antipsychotics, the activity of nNOS was higher than in healthy controls (Maia-de-Oliveira et al., 2012). These arguments call into question the existence of the uniform concept of influence of antipsychotics on the activity of nitric oxide. It is noted that atypical antipsychotics practically do not influence the activity of nNOS in brain structures of patients with schizophrenia (Tarazi et al., 2002). Besides the neuronal isoform, antipsychotics were studied in terms of influence on other NOS isoforms. Clozapine is able to inhibit activity of iNOS and to decrease the level of nitric oxide in the brain and microglial inflammation (Bringas et al., 2012; Ribeiro et al., 2013). The influence of antipsychotics on metabolism of nitric oxide leads to restoration of the normal function of NMDA-receptors. However, it is not the basic link of the mechanism of action of the medication.
Metabolism of Nitric Oxide as a Target for Therapy of Schizophrenia
Because the considerable evidence base showing that alterations of activity of NO are active in pathogenesis of schizophrenia, recent attempts have been undertaken to develop the therapy correcting disturbances of the synthesis and release of nitric oxide. Minocycline, semisynthetic tetracycline of the second generation, inhibiting enzyme iNOS and preventing development of microglial inflammatory process has been quite well studied. In double blind placebo-controlled investigations, minocycline has shown efficiency regarding negative symptoms of schizophrenia when added to antipsychotic therapy (Ghanizadeh et al., 2014; Khodaie-Ardakani et al., 2014; Liu et al., 2014). Reports about severe side effects in the carried-out protocols have not been registered. Besides, the meta-analysis of four randomized controlled investigations was published. In the meta-analysis, it has been confirmed that the use of minocycline is essentially more effective than placebo and reduces the severity of negative symptoms, but does not influence the change of values of scales of positive symptoms of PANSS (Oya et al., 2014). It is worth considering that minocycline have been studied relatively recently, and it is too early to speak to the delayed effects of medication. Therefore, it is necessary to wait for the long-term prospective investigations to conclude.
L-arginine was considered as one of the perspective targets. The carrier of L-arginine is cationic amino-acid transporter (CAT), which can also bind with L-lysine on the competitive base (White et al., 1982). Thus, L-lysine is considered as NO synthesis inhibitor in CNS. Currently, there are two publications confirming the positive role of L-lysine as an additional preparation in treatment of schizophrenia. Results of 8-week randomized double blind placebo-controlled investigation by Zeinoddini et al. (2014) have been recently published, in which Risperidone was supplemented by L-lysine. The authors noted that the medication improves indices on the following scales of PANSS: negative symptoms (p < 0.001), total score (p < 0.001), general psychopathology (p < 0.001). For the scale of positive symptoms, a difference was not noticed (p = 0.61). However, long-term effects of L-lysine were not revealed and the sample of patients in the investigation was also insignificant for final conclusions (Zeinoddini et al., 2014). Besides work by Zeinoddini et al. earlier results of a pilot investigation by Wass et al. (2011) with similar conclusions have been published (Wass et al., 2011).
Conclusion
In the presented review of literature, recent achievements in the study of the role of nitric oxide in pathogenesis of schizophrenia and its treatment have been considered. Genetics and pharmacogenetics of NO-synthase as well as of other genes involved in the metabolism of NO are still insufficiently investigated. For achievement of results of high evidence level, carrying out research on increased samples with account for ethnic origin is necessary. Certainly, it is impossible not to take into account the exogenous factors. Possibly, their role in the development of mental disorders will remain incomplete for a long time. However, taking into account the influence of the environment should be an integral part of each investigation. The epigenetic methods, which allow for the reflection of the influence of various factors on expression of genes, look promising. Of no doubt, it is worth including epigenetic methods in complex designs for receipt of multi-sided conclusions regarding oxidative stress in pathogenesis of schizophrenia.
Disclosure of molecular-genetic mechanisms of development of schizophrenia becomes the first step in development of pathogenetic therapy. The attempts, undertaken in the field of impact on factors of oxidative stress for the purpose of improvement of the course of the disease, clearly show positive prospects of this approach. But without a sufficient fundamental basis, achievement of significant results of clinical investigations will be a failure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Akpinar, A., Yaman, G. B., Demirdas, A., and Onal, S. (2013). Possible role of adrenomedullin and nitric oxide in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 120–125. doi: 10.1016/j.pnpbp.2013.07.003
Akyol, O., Zoroglu, S. S., Armutcu, F., Sahin, S., and Gurel, A. (2004). Nitric oxide as a physiopathological factor in neuropsychiatric disorders. In Vivo 18, 377–390.
Albayrak, Y., Ünsal, C., Beyazyüz, M., Ünal, A., and Kuloğlu, M. (2013). Reduced total antioxidant level and increased oxidative stress in patients with deficit schizophrenia: a preliminary study. Prog. Neuropsychopharmacol. Biol. Psychiatry 45, 144–149. doi: 10.1016/j.pnpbp.2013.04.020
Antunes, F., Nunes, C., Laranjinha, J., and Cadenas, E. (2005). Redox interactions of nitric oxide with dopamine and its derivatives. Toxicology 208, 207–212. doi: 10.1016/j.tox.2004.11.033
Ataya, B., Tzeng, E., and Zuckerbraun, B. S. (2011). Nitrite-generated nitric oxide to protect against intimal hyperplasia formation. Trends Cardiovasc. Med. 21, 157–162. doi: 10.1016/j.tcm.2012.05.002
Austin, S. A., Santhanam, A. V., Hinton, D. J., Choi, D. S., and Katusic, Z. S. (2013). Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J. Neurochem. 127, 691–700. doi: 10.1111/jnc.12334
Averbukh, M. L., Kas'ko, A. F., Nikolenko, E. S., and Rybas, I. I. (1966). On the diagnostic significance of Black's reaction in psychiatric patients. Lab. Delo 5, 289–291.
Baba, H., Suzuki, T., Arai, H., and Emson, P. C. (2004). Expression of nNOS and soluble guanylate cyclase in schizophrenic brain. Neuroreport 15, 677–680. doi: 10.1097/00001756-200403220-00020
Babicová, A., Havlínová, Z., Hroch, M., Rezáèová, M., Pejchal, J., Vávrová, J., et al. (2013). In vivo study of radioprotective effect of NO-synthase inhibitors and acetyl-L-carnitine. Physiol. Res. 62, 701–710.
Bauer, I. E., Pascoe, M. C., Wollenhaupt-Aguiar, B., Kapczinski, F., and Soares, J. C. (2014). Inflammatory mediators of cognitive impairment in bipolar disorder. J. Psychiatr. Res. 56, 18–27. doi: 10.1016/j.jpsychires.2014.04.017
Ben Othmen, L., Mechri, A., Fendri, C., Bost, M., Chazot, G., Gaha, L., et al. (2008). Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 155–159. doi: 10.1016/j.pnpbp.2007.08.003
Ben-Shachar, D., and Laifenfeld, D. (2004). Mitochondria, synaptic plasticity, and schizophrenia. Int. Rev. Neurobiol. 59, 273–296. doi: 10.1016/S0074-7742(04)59011-6
Berg, D., Youdim, M. B., and Riederer, P. (2004). Redox imbalance. Cell Tissue Res. 318, 201–213. doi: 10.1007/s00441-004-0976-5
Bergink, V., Gibney, S. M., and Drexhage, H. A. (2014). Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol. Psychiatry 75, 324–331. doi: 10.1016/j.biopsych.2013.09.037
Bernstein, H. G., Bogerts, B., and Keilhoff, G. (2005a). The many faces of nitric oxide in schizophrenia. A review. Schizophr. Res. 78, 69–86 doi: 10.1016/j.schres.2005.05.019
Bernstein, H. G., Heinemann, A., Krell, D., Dobrowolny, H., Bielau, H., Keilhoff, G., et al. (2005b). Hypothalamic nitric oxide synthase in affective disorder: focus on the suprachiasmatic nucleus. Cell. Mol. Biol. 51, 279–284.
Bernstein, H. G., Heinemann, A., Steiner, J., and Bogerts, B. (2010). Schizophrenia, sleep disturbances and the suprachiasmatic nucleus: reduced nitric oxide synthase may matter. Med. Hypotheses 74, 397–398. doi: 10.1016/j.mehy.2009.08.026
Bernstein, H. G., Jirikowski, G. F., Heinemann, A., Baumann, B., Hornstein, C., Danos, P., et al. (2000). Low and infrequent expression of nitric oxide synthase/NADPH-diaphorase in neurons of the human supraoptic nucleus: a histochemical study. J. Chem. Neuroanat. 20, 177–183. doi: 10.1016/S0891-0618(00)00087-9
Bernstein, H. G., Keilhoff, G., Steiner, J., Dobrowolny, H., and Bogerts, B. (2011). Nitric oxide and schizophrenia: present knowledge and emerging concepts of therapy. CNS Neurol. Disord. Drug Targets 10, 792–807. doi: 10.2174/187152711798072392
Bernstein, H. G., Krell, D., Braunewell, K. H., Baumann, B., Gundelfinger, E. D., Diekmann, S., et al. (2001). Increased number of nitric oxide synthase immunoreactive Purkinje cells and dentate nucleus neurons in schizophrenia. J. Neurocytol. 30, 661–670. doi: 10.1023/A:1016520932139
Bernstein, H. G., Stanarius, A., Baumann, B., Henning, H., Krell, D., Danos, P., et al. (1998). Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 83, 867–875. doi: 10.1016/S0306-4522(97)00461-2
Bitanihirwe, B. K., and Woo, T. U. (2011). Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav. Rev. 35, 878–893. doi: 10.1016/j.neubiorev.2010.10.008
Blaise, G. A., Gauvin, D., Gangal, M., and Authier, S. (2005). Nitric oxide, cell signaling and cell death. Toxicology 208, 177–192. doi: 10.1016/j.tox.2004.11.032
Blasko, J., Fabianova, K., Martoncikova, M., Sopkova, D., and Racekova, E. (2013). Immunohistochemical evidence for the presence of synaptic connections of nitrergic neurons in the rat rostral migratory stream. Cell. Mol. Neurobiol. 33, 753–757. doi: 10.1007/s10571-013-9956-1
Blum-Degen, D., Heinemann, T., Lan, J., Pedersen, V., Leblhuber, F., Paulus, W., et al. (1999). Characterization and regional distribution of nitric oxide synthase in the human brain during normal ageing. Brain Res. 834, 128–135. doi: 10.1016/S0006-8993(99)01444-4
Boehning, D., and Snyder, S. H. (2003). Novel neural modulators. Annu. Rev. Neurosci. 26, 105–131. doi: 10.1146/annurev.neuro.26.041002.131047
Bringas, M. E., Morales-Medina, J. C., Flores-Vivaldo, Y., Negrete-Diaz, J. V., Aguilar-Alonso, P., León-Chávez, B. A., et al. (2012). Clozapine administration reverses behavioral, neuronal, and nitric oxide disturbances in the neonatal ventral hippocampus rat. Neuropharmacology 62, 1848–1857. doi: 10.1016/j.neuropharm.2011.12.008
Brown, A. S., and Derkits, E. J. (2010). Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am. J. Psychiatry 167, 261–280. doi: 10.1176/appi.ajp.2009.09030361
Brzustowicz, L. M., Simone, J., Mohseni, P., Hayter, J. E., Hodgkinson, K. A., Chow, E. W., et al. (2004). Linkage disequilibrium mapping of schizophrenia susceptibility to the CAPON region of chromosome 1q22. Am. J. Hum. Genet. 74, 1057–1063. doi: 10.1086/420774
Bul'ba, P. A., Kas'ko, A. F., Averbukh, M. L., and Nikolenko, E. S. (1968). Peculiarities of the Black reaction and white phenomenon in mental patients. 2. Lab. Delo 6, 350–352.
Carreira, B. P., Morte, M. I., Lourenço, A. S., Santos, A. I., Inácio, A., Ambrósio, A. F., et al. (2013). Differential contribution of the guanylyl cyclase-cyclic GMP-protein kinase G pathway to the proliferation of neural stem cells stimulated by nitric oxide. Neurosignals 21, 1–13. doi: 10.1159/000332811
Chen, J., Tu, Y., Moon, C., Matarazzo, V., Palmer, A. M., and Ronnett, G. V. (2004). The localization of neuronal nitric oxide synthase may influence its role in neuronal precursor proliferation and synaptic maintenance. Dev. Biol. 269, 165–182. doi: 10.1016/j.ydbio.2004.01.024
Chiueh, C. C. (1999). Neuroprotective properties of nitric oxide. Ann. N.Y. Acad. Sci. 890, 301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x
Ciancarelli, I., De Amicis, D., Di Massimo, C., Di Scanno, C., Pistarini, C., D'Orazio, N., et al. (2014). Peripheral biomarkers of oxidative stress and their limited potential in evaluation of clinical features of Huntington's patients. Biomarkers 19, 452–456. doi: 10.3109/1354750X.2014.935955
Clarke, M. C., Tanskanen, A., Huttunen, M., Leon, D. A., Murray, R. M., Jones, P. B., et al. (2011). Increased risk of schizophrenia from additive interaction between infant motor developmental delay and obstetric complications: evidence from a population-based longitudinal study. Am. J. Psychiatry 168, 1295–1302. doi: 10.1176/appi.ajp.2011.11010011
Connor, C. M., Crawford, B. C., and Akbarian, S. (2011). White matter neuron alterations in schizophrenia and related disorders. Int. J. Dev. Neurosci. 29, 325–334. doi: 10.1016/j.ijdevneu.2010.07.236
Contestabile, A., Monti, B., and Polazzi, E. (2012). Neuronal-glial interactions define the role of nitric oxide in neural functional processes. Curr. Neuropharmacol. 10, 303–310. doi: 10.2174/157015912804499465
Cooke, R. M., Mistry, R., Challiss, R. A., and Straub, V. A. (2013). Nitric oxide synthesis and cGMP production is important for neurite growth and synapse remodeling after axotomy. J. Neurosci. 33, 5626–5637. doi: 10.1523/JNEUROSCI.3659-12.2013
Creese, I., Burt, D. R., and Snyder, S. H. (1976). Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192, 481–483. doi: 10.1126/science.3854
Cui, H., Nishiguchi, N., Yanagi, M., Fukutake, M., Mouri, K., Kitamura, N., et al. (2010). A putative cis-acting polymorphism in the NOS1 gene is associated with schizophrenia and NOS1 immunoreactivity in the postmortem brain. Schizophr. Res. 121, 172–178. doi: 10.1016/j.schres.2010.05.003
Dadheech, G., Mishra, S., Gautam, S., and Sharma, P. (2008). Evaluation of antioxidant deficit in schizophrenia. Indian J. Psychiatry 50, 16–20. doi: 10.4103/0019-5545.39753
Davis, K. L., Kahn, R. S., Ko, G., and Davidson, M. (1991). Dopamine in schizophrenia: a review and reconceptualization. Am. J. Psychiatry 148, 1474–1486. doi: 10.1176/ajp.148.11.1474
Dec, A. M., Kohlhaas, K. L., Nelson, C. L., Hoque, K. E., Leilabadi, S. N., Folk, J., et al. (2014). Impact of neonatal NOS-1 inhibitor exposure on neurobehavioural measures and prefrontal-temporolimbic integration in the rat nucleus accumbens. Int. J. Neuropsychopharmacol. 17, 275–287. doi: 10.1017/S1461145713000990
Delorme, R., Betancur, C., Scheid, I., Anckarsäter, H., Chaste, P., Jamain, S., et al. (2010). Mutation screening of NOS1AP gene in a large sample of psychiatric patients and controls. BMC Med. Genet. 11:108. doi: 10.1186/1471-2350-11-108
de Witte, L., Tomasik, J., Schwarz, E., Guest, P. C., Rahmoune, H., Kahn, R. S., et al. (2014). Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr. Res. 154, 23–29. doi: 10.1016/j.schres.2014.02.005
Dickson, H., Laurens, K. R., Cullen, A. E., and Hodgins, S. (2012). Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol. Med. 42, 743–755. doi: 10.1017/S0033291711001693
Dietrich-Muszalska, A., and Kontek, B. (2010). Lipid peroxidation in patients with schizophrenia. Psychiatry Clin. Neurosci. 64, 469–475. doi: 10.1111/j.1440-1819.2010.02132.x
Dietrich-Muszalska, A., Kontek, B., and Rabe-Jabłñska, J. (2011). Quetiapine, olanzapine and haloperidol affect human plasma lipid peroxidation in vitro. Neuropsychobiology 63, 197–201. doi: 10.1159/000321623
Dietrich-Muszalska, A., and Kwiatkowska, A. (2014). Generation of superoxide anion radicals and platelet glutathione peroxidase activity in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 10, 703–709. doi: 10.2147/NDT.S60034
Dietrich-Muszalska, A., and Olas, B. (2009). Isoprostenes as indicators of oxidative stress in schizophrenia. World J. Biol. Psychiatry 10, 27–33. doi: 10.1080/15622970701361263
Diodati, J. G., Dakak, N., Gilligan, D. M., and Quyyumi, A. A. (1998). Effect of atherosclerosis on endothelium-dependent inhibition of platelet activation in humans. Circulation 98, 17–24. doi: 10.1161/01.CIR.98.1.17
Donohoe, G., Walters, J., Morris, D. W., Quinn, E. M., Judge, R., Norton, N., et al. (2009). Influence of NOS1 on verbal intelligence and working memory in both patients with schizophrenia and healthy control subjects. Arch. Gen. Psychiatry 66, 1045–1054. doi: 10.1001/archgenpsychiatry.2009.139
Doyle, C. A., and Slater, P. (1995). Application of [3H]L-N(G)-nitro-arginine labelling to measure cerebellar nitric oxide synthase in patients with schizophrenia. Neurosci. Lett. 202, 49–52. doi: 10.1016/0304-3940(95)12196-X
Dracheva, S., McGurk, S. R., and Haroutunian, V. (2005). mRNA expression of AMPA receptors and AMPA receptor binding proteins in the cerebral cortex of elderly schizophrenics. J. Neurosci. Res. 79, 868–878. doi: 10.1002/jnr.20423
Duan, J., Li, W., Yuan, D., Sah, B., Yan, Y., and Gu, H. (2012). Nitric oxide signaling modulates cholinergic synaptic input to projection neurons in Drosophila antennal lobes. Neuroscience 219, 1–9. doi: 10.1016/j.neuroscience.2012.05.068
Eastwood, S. L., and Harrison, P. J. (2003). Interstitial white matter neurons express less reelin and are abnormally distributed in schizophrenia: towards an integration of molecular and morphologic aspects of the neurodevelopmental hypothesis. Mol. Psychiatry 8, 769, 821–831. doi: 10.1038/sj.mp.4001371
Eaton, W. W., Thara, R., Federman, B., Melton, B., and Liang, K. Y. (1995). Structure and course of positive and negative symptoms in schizophrenia. Arch. Gen. Psychiatry 52, 127–134. doi: 10.1001/archpsyc.1995.03950140045005
Endres, M., Laufs, U., Liao, J. K., and Moskowitz, M. A. (2004). Targeting eNOS for stroke protection. Trends Neurosci. 27, 283–289. doi: 10.1016/j.tins.2004.03.009
Fatemi, S. H., Cuadra, A. E., El-Fakahany, E. E., Sidwell, R. W., and Thuras, P. (2000). Prenatal viral infection causes alterations in nNOS expression in developing mouse brains. Neuroreport 11, 1493–1496. doi: 10.1097/00001756-200005150-00026
Finnerty, N. J., Bolger, F. B., Pålsson, E., and Lowry, J. P. (2013). An investigation of hypofrontality in an animal model of schizophrenia using real-time microelectrochemical sensors for glucose, oxygen, and nitric oxide. ACS Chem. Neurosci. 4, 825–831. doi: 10.1021/cn4000567
Flatow, J., Buckley, P., and Miller, B. J. (2013). Meta-analysis of oxidative stress in schizophrenia. Biol. Psychiatry 74, 400–409. doi: 10.1016/j.biopsych.2013.03.018
Fleming, I., and Busse, R. (2003). Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R1–R12. doi: 10.1152/ajpregu.00323.2002
Fritzen, S., Lauer, M., Schmitt, A., Töpner, T., Strobel, A., Lesch, K. P., et al. (2007). NO synthase-positive striatal interneurons are decreased in schizophrenia. Eur. Neuropsychopharmacol. 17, 595–599. doi: 10.1016/j.euroneuro.2006.12.004
Furchgott, R. F., and Zawadzki, J. V. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288, 373–376. doi: 10.1038/288373a0
Ghafourifar, P., and Cadenas, E. (2005). Mitochondrial nitric oxide synthase. Trends Pharmacol. Sci. 26, 190–195. doi: 10.1016/j.tips.2005.02.005
Ghanizadeh, A., Dehbozorgi, S., Sigaroodi, M. O., and Rezaei, Z. (2014). Minocycline as add-on treatment decreases the negative symptoms of schizophrenia; a randomized placebo-controlled clinical trial. Recent Pat. Inflamm. Allergy Drug Discov. 8, 211–215. doi: 10.2174/1872213X08666141029123524
Ghasemi, M., and Fatemi, A. (2014). Pathologic role of glial nitric oxide in adult and pediatric neuroinflammatory diseases. Neurosci. Biobehav. Rev. 45, 168–182. doi: 10.1016/j.neubiorev.2014.06.002
Gibbs, S. M. (2003). Regulation of neuronal proliferation and differentiation by nitric oxide. Mol. Neurobiol. 27, 107–120. doi: 10.1385/MN:27:2:107
Gilca, M., Piriu, G., Gaman, L., Delia, C., Iosif, L., Atanasiu, V., et al. (2014). A study of antioxidant activity in patients with schizophrenia taking atypical antipsychotics. Psychopharmacology. doi: 10.1007/s00213-014-3624-0
Giraldi-Guimarães, A., Bittencourt-Navarrete, R. E., and Mendez-Otero, R. (2004). Expression of neuronal nitric oxide synthase in the developing superficial layers of the rat superior colliculus. Braz. J. Med. Biol. Res. 37, 869–877. doi: 10.1590/S0100-879X2004000600013
Girouard, H., Wang, G., Gallo, E. F., Anrather, J., Zhou, P., Pickel, V. M., et al. (2009). NMDA receptor activation increases free radical production through nitric oxide and NOX2. J. Neurosci. 29, 2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009
González-Forero, D., and Moreno-López, B. (2014). Retrograde response in axotomized motoneurons: nitric oxide as a key player in triggering reversion toward a dedifferentiated phenotype. Neuroscience 283, 138–165. doi: 10.1016/j.neuroscience.2014.08.021
Gonzalez-Liencres, C., Tas, C., Brown, E. C., Erdin, S., Onur, E., Cubukcoglu, Z., et al. (2014). Oxidative stress in schizophrenia: a case¿control study on the effects on social cognition and neurocognition. BMC Psychiatry 14:268. doi: 10.1186/s12888-014-0268-x
Graber, D. J., Snyder-Keller, A., Lawrence, D. A., and Turner, J. N. (2012). Neurodegeneration by activated microglia across a nanofiltration membrane. J. Biochem. Mol. Toxicol. 26, 45–53. doi: 10.1002/jbt.20384
Gray, W. P., and Cheung, A. (2014). Nitric oxide regulation of adult neurogenesis. Vitam. Horm. 96, 59–77. doi: 10.1016/B978-0-12-800254-4.00004-0
Gubert, C., Stertz, L., Pfaffenseller, B., Panizzutti, B. S., Rezin, G. T., Massuda, R., et al. (2013). Mitochondrial activity and oxidative stress markers in peripheral blood mononuclear cells of patients with bipolar disorder, schizophrenia, and healthy subjects. J. Psychiatr. Res. 47, 1396–1402. doi: 10.1016/j.jpsychires.2013.06.018
Gwathmey, T. M., Alzayadneh, E. M., Pendergrass, K. D., and Chappell, M. C. (2012). Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R518–R530. doi: 10.1152/ajpregu.00525.2011
Haller, C. S., Padmanabhan, J. L., Lizano, P., Torous, J., and Keshavan, M. (2014). Recent advances in understanding schizophrenia. F1000Prime Rep. 6:57 doi: 10.12703/P6-57
Halliwell, B. (2007). Biochemistry of oxidative stress. Biochem. Soc. Trans. 35(Pt 5), 1147–1150. doi: 10.1042/BST0351147
Hara, H., Waeber, C., Huang, P. L., Fujii, M., Fishman, M. C., and Moskowitz, M. A. (1996). Brain distribution of nitric oxide synthase in neuronal or endothelial nitric oxide synthase mutant mice using [3H] L-NG-nitro-arginine autoradiography. Neuroscience 75, 881–890. doi: 10.1016/0306-4522(96)00313-2
Hardy, J., and Gwinn-Hardy, K. (1998). Genetic classification of primary neurodegenerative disease. Science 282, 1075–1079. doi: 10.1126/science.282.5391.1075
Hart, S. J., Bizzell, J., McMahon, M. A., Gu, H., Perkins, D. O., and Belger, A. (2013). Altered fronto-limbic activity in children and adolescents with familial high risk for schizophrenia. Psychiatry Res. 212, 19–27. doi: 10.1016/j.pscychresns.2012.12.003
Hoffer, A., Osmond, H., and Smythies, J. (1954). Schizophrenia; a new approach. II. Result of a year's research. J. Ment. Sci. 100, 29–45. doi: 10.1192/bjp.100.418.29
Hölscher, C., and Rose, S. P. (1992). An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci. Lett. 145, 165–167. doi: 10.1016/0304-3940(92)90012-V
Hoque, K. E., Indorkar, R. P., Sammut, S., and West, A. R. (2010). Impact of dopamine-glutamate interactions on striatal neuronal nitric oxide synthase activity. Psychopharmacology 207, 571–581. doi: 10.1007/s00213-009-1687-0
Howes, O., Bose, S., Turkheimer, F., Valli, I., Egerton, A., Stahl, D., et al. (2011). Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol. Psychiatry 16, 885–886. doi: 10.1038/mp.2011.20
Howes, O. D., and Kapur, S. (2009). The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr. Bull. 35, 549–562. doi: 10.1093/schbul/sbp006
Hu, W., MacDonald, M. L., Elswick, D. E., and Sweet, R. A. (2014). The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann. N.Y. Acad. Sci. 1338, 38–57. doi: 10.1111/nyas.12547
Hu, Y., and Zhu, D. Y. (2014). Hippocampus and nitric oxide. Vitam. Horm. 96, 127–160. doi: 10.1016/B978-0-12-800254-4.00006-4
Jenkins, T. A. (2013). Perinatal complications and schizophrenia: involvement of the immune system. Front. Neurosci. 7:110. doi: 10.3389/fnins.2013.00110
Jing, Y., Zhang, H., Wolff, A. R., Bilkey, D. K., and Liu, P. (2013). Altered arginine metabolism in the hippocampus and prefrontal cortex of maternal immune activation rat offspring. Schizophr. Res. 148, 151–156. doi: 10.1016/j.schres.2013.06.001
Käkelä, J., Panula, J., Oinas, E., Hirvonen, N., Jääskeläinen, E., and Miettunen, J. (2014). Family history of psychosis and social, occupational and global outcome in schizophrenia: a meta-analysis. Acta Psychiatr. Scand. 130, 269–278. doi: 10.1111/acps.12317
Kawakami, S., Ichikawa, M., Yokosuka, M., Tsukamura, H., and Maeda, K. (1998). Glial and neuronal localization of neuronal nitric oxide synthase immunoreactivity in the median eminence of female rats. Brain Res. 789, 322–326. doi: 10.1016/S0006-8993(97)01561-8
Keshavan, M. S., Clementz, B. A., Pearlson, G. D., Sweeney, J. A., and Tamminga, C. A. (2013). Reimagining psychoses: an agnostic approach to diagnosis. Schizophr. Res. 146, 10–16. doi: 10.1016/j.schres.2013.02.022
Khodaie-Ardakani, M. R., Mirshafiee, O., Farokhnia, M., Tajdini, M., Hosseini, S. M., Modabbernia, A., et al. (2014). Minocycline add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: randomized double-blind placebo-controlled study. Psychiatry Res. 215, 540–546. doi: 10.1016/j.psychres.2013.12.051
Kirchner, L., Weitzdoerfer, R., Hoeger, H., Url, A., Schmidt, P., Engelmann, M., et al. (2004). Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric Oxide 11, 316–330. doi: 10.1016/j.niox.2004.10.005
Kiss, J. P., and Vizi, E. S. (2001). Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 24, 211–215. doi: 10.1016/S0166-2236(00)01745-8
Knox, L. T., Jing, Y., Collie, N. D., Zhang, H., and Liu, P. (2014). Effects of acute phencyclidine administration on arginine metabolism in the hippocampus and prefrontal cortex in rats. Neuropharmacology 81, 195–205. doi: 10.1016/j.neuropharm.2014.02.004
Kohen, R., and Nyska, A. (2002). Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 30, 620–650. doi: 10.1080/01926230290166724
Kraus, M. M., and Prast, H. (2001). The nitric oxide system modulates the in vivo release of acetylcholine in the nucleus accumbens induced by stimulation of the hippocampal fornix/fimbria-projection. Eur. J. Neurosci. 14, 1105–1112. doi: 10.1046/j.0953-816x.2001.01735.x
Kristofiková, Z., Kozmiková, I., Hovorková, P., Rícný, J., Zach, P., Majer, E., et al. (2008). Lateralization of hippocampal nitric oxide mediator system in people with Alzheimer disease, multi-infarct dementia and schizophrenia. Neurochem. Int. 53, 118–125. doi: 10.1016/j.neuint.2008.06.009
Kypreos, K. E., Zafirovic, S., Petropoulou, P. I., Bjelogrlic, P., Resanovic, I., Traish, A., et al. (2014). Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J. Cardiovasc. Pharmacol. Ther. 19, 256–268. doi: 10.1177/1074248413513499
Lau, Y. S., Petroske, E., Meredith, G. E., and Wang, J. Q. (2003). Elevated neuronal nitric oxide synthase expression in chronic haloperidol-treated rats. Neuropharmacology 45, 986–994. doi: 10.1016/S0028-3908(03)00314-9
Lauer, M., Johannes, S., Fritzen, S., Senitz, D., Riederer, P., and Reif, A. (2005). Morphological abnormalities in nitric-oxide-synthase-positive striatal interneurons of schizophrenic patients. Neuropsychobiology 52, 111–117. doi: 10.1159/000087555
Lazzaro, M., Bettegazzi, B., Barbariga, M., Codazzi, F., Zacchetti, D., and Alessio, M. (2014). Ceruloplasmin potentiates nitric oxide synthase activity and cytokine secretion in activated microglia. J. Neuroinflammation 11, 164. doi: 10.1186/s12974-014-0164-9
Lerouet, D., Beray-Berthat, V., Palmier, B., Plotkine, M., and Margaill, I. (2002). Changes in oxidative stress, iNOS activity and neutrophil infiltration in severe transient focal cerebral ischemia in rats. Brain Res. 958, 166–175. doi: 10.1016/S0006-8993(02)03685-5
Lewis, D. A., Hashimoto, T., and Volk, D. W. (2005). Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 6, 312–324. doi: 10.1038/nrn1648
Li, X. F., Zheng, Y. L., Xiu, M. H., Chen da, C., Kosten, T. R., and Zhang, X. Y. (2011). Reduced plasma total antioxidant status in first-episode drug-naive patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1064–1067. doi: 10.1016/j.pnpbp.2011.03.001
Liou, Y. J., Tsai, S. J., Hong, C. J., and Liao, D. L. (2003). Association analysis for the CA repeat polymorphism of the neuronal nitric oxide synthase (NOS1) gene and schizophrenia. Schizophr. Res. 65, 57–59. doi: 10.1016/S0920-9964(02)00532-7
Liu, F., Guo, X., Wu, R., Ou, J., Zheng, Y., Zhang, B., et al. (2014). Minocycline supplementation for treatment of negative symptoms in early-phase schizophrenia: a double blind, randomized, controlled trial. Schizophr. Res. 153, 169–176. doi: 10.1016/j.schres.2014.01.011
Lonart, G., Wang, J., and Johnson, K. M. (1992). Nitric oxide induces neurotransmitter release from hippocampal slices. Eur. J. Pharmacol. 220, 271–272. doi: 10.1016/0014-2999(92)90759-W
Luo, C. X., Zhu, X. J., Zhou, Q. G., Wang, B., Wang, W., Cai, H. H., et al. (2007). Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J. Neurochem. 103, 1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x
Maia-de-Oliveira, J. P., Trzesniak, C., Oliveira, I. R., Kempton, M. J., Rezende, T. M., Iego, S., et al. (2012). Nitric oxide plasma/serum levels in patients with schizophrenia: a systematic review and meta-analysis. Rev. Bras. Psiquiatr. 34(Suppl. 2), S149–S155. doi: 10.1016/j.rbp.2012.07.001
Markovic, M., Miljkovic, D. J., and Trajkovic, V. (2003). Regulation of inducible nitric oxide synthase by cAMP-elevating phospho-diesterase inhibitors. Curr. Drug Targets Inflamm. Allergy 2, 63–79. doi: 10.2174/1568010033344471
McConell, G. K., Bradley, S. J., Stephens, T. J., Canny, B. J., Kingwell, B. A., and Lee-Young, R. S. (2007). Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R821–R828. doi: 10.1152/ajpregu.00796.2006
McGrath, J., Saha, S., Chant, D., and Welham, J. (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 30, 67–76. doi: 10.1093/epirev/mxn001
Miljevic, C., Nikolic, M., Nikolic-Kokic, A., Jones, D. R., Niketic, V., Lecic-Tosevski, D., et al. (2010). Lipid status, anti-oxidant enzyme defence and haemoglobin content in the blood of long-term clozapine-treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 303–307. doi: 10.1016/j.pnpbp.2009.11.024
Miller, D. M., Buettner, G. R., and Aust, S. D. (1990). Transition metals as catalysts of “autoxidation” reactions. Free Radic. Biol. Med. 8, 95–108. doi: 10.1016/0891-5849(90)90148-C
Morera, A. L., Intxausti, A., and Abreu-Gonzalez, P. (2009). Winter/summer seasonal changes in malondialdehyde formation as a source of variance in oxidative stress schizophrenia research. World J. Biol. Psychiatry 10(4 Pt 2), 576–580. doi: 10.1080/15622970801901802
Moylan, S., Berk, M., Dean, O. M., Samuni, Y., Williams, L. J., O'Neil, A., et al. (2014). Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev. 45, 46–62. doi: 10.1016/j.neubiorev.2014.05.007
Nel, A., and Harvey, B. H. (2003). Haloperidol-induced dyskinesia is associated with striatal NO synthase suppression: reversal with olanzapine. Behav. Pharmacol. 14, 251–255. doi: 10.1097/00008877-200305000-00010
Noack, H., Possel, H., Rethfeldt, C., Keilhoff, G., and Wolf, G. (1999). Peroxynitrite mediated damage and lowered superoxide tolerance in primary cortical glial cultures after induction of the inducible isoform of NOS. Glia 28, 13–24.
Northoff, G., Richter, A., Bermpohl, F., Grimm, S., Martin, E., Marcar, V. L., et al. (2005). NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr. Res. 72, 235–248. doi: 10.1016/j.schres.2004.04.009
O'Donoghue, T., Morris, D. W., Fahey, C., Da Costa, A., Foxe, J. J., Hoerold, D., et al. (2012). A NOS1 variant implicated in cognitive performance influences evoked neural responses during a high density EEG study of early visual perception. Hum. Brain Mapp. 33, 1202–1211. doi: 10.1002/hbm.21281
Okumura, T., Okochi, T., Kishi, T., Ikeda, M., Kitajima, T., Yamanouchi, Y., et al. (2009). No association between polymorphisms of neuronal oxide synthase 1 gene (NOS1) and schizophrenia in a Japanese population. Neuromolecular Med. 11, 123–127. doi: 10.1007/s12017-009-8068-z
Oldreive, C. E., Gaynor, S., and Doherty, G. H. (2008). Developmental changes in the response of murine cerebellar granule cells to nitric oxide. Neurochem. Int. 52, 1394–1401. doi: 10.1016/j.neuint.2008.02.010
Oldreive, C. E., Gaynor, S., and Doherty, G. H. (2012). Effects of nitric oxide on the survival and neuritogenesis of cerebellar Purkinje neurons. J. Mol. Neurosci. 46, 336–342. doi: 10.1007/s12031-011-9590-7
Owe-Larsson, B., Ekdahl, K., Edbom, T., Osby, U., Karlsson, H., Lundberg, C., et al. (2011). Increased plasma levels of thioredoxin-1 in patients with first episode psychosis and long-term schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1117–1121. doi: 10.1016/j.pnpbp.2011.03.012
Oya, K., Kishi, T., and Iwata, N. (2014). Efficacy and tolerability of minocycline augmentation therapy in schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Hum. Psychopharmacol. 29, 483–491. doi: 10.1002/hup.2426
Pae, C. U., Paik, I. H., Lee, C., Lee, S. J., Kim, J. J., and Lee, C. U. (2004). Decreased plasma antioxidants in schizophrenia. Neuropsychobiology 50, 54–56. doi: 10.1159/000077942
Pålsson, E., Lowry, J., and Klamer, D. (2010). Information processing deficits and nitric oxide signalling in the phencyclidine model of schizophrenia. Psychopharmacology 212, 643–651. doi: 10.1007/s00213-010-1992-7
Pazvantoglu, O., Selek, S., Okay, I. T., Sengul, C., Karabekiroglu, K., Dilbaz, N., et al. (2009). Oxidative mechanisms in schizophrenia and their relationship with illness subtype and symptom profile. Psychiatry Clin. Neurosci. 63, 693–700. doi: 10.1111/j.1440-1819.2009.02015.x
Pino, O., Guilera, G., Gómez-Benito, J., Najas-García, A., Rufián, S., and Rojo, E. (2014). Neurodevelopment or neurodegeneration: review of theories of schizophrenia. Actas Esp. Psiquiatr. 42, 185–195.
Polazzi, E., and Contestabile, A. (2006). Overactivation of LPS-stimulated microglial cells by co-cultured neurons or neuron-conditioned medium. J. Neuroimmunol. 172, 104–111. doi: 10.1016/j.jneuroim.2005.11.005
Possel, H., Noack, H., Putzke, J., Wolf, G., and Sies, H. (2000). Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia 32, 51–59. doi: 10.1002/1098-1136(200010)32:13.0.CO;2-4
Puri, V., McQuillin, A., Thirumalai, S., Lawrence, J., Krasucki, R., Choudhury, K., et al. (2006). Failure to confirm allelic association between markers at the CAPON gene locus and schizophrenia in a British sample. Biol. Psychiatry 59, 195–197. doi: 10.1016/j.biopsych.2005.08.015
Radi, R., Beckman, J. S., Bush, K. M., and Freeman, B. A. (1991). Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 288, 481–487. doi: 10.1016/0003-9861(91)90224-7
Raffa, M., Atig, F., Mhalla, A., Kerkeni, A., and Mechri, A. (2011). Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry 11:124. doi: 10.1186/1471-244X-11-124
Raffa, M., Barhoumi, S., Atig, F., Fendri, C., Kerkeni, A., and Mechri, A. (2012a). Reduced antioxidant defense systems in schizophrenia and bipolar I disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 371–375. doi: 10.1016/j.pnpbp.2012.07.013
Raffa, M., Fendri, C., Ben Othmen, L., Slama, H., Amri, M., Kerkeni, A., et al. (2012b). The reduction of superoxide dismutase activity is associated with the severity of neurological soft signs in patients with schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 52–56. doi: 10.1016/j.pnpbp.2012.05.005
Raffa, M., Mechri, A., Othman, L. B., Fendri, C., Gaha, L., and Kerkeni, A. (2009). Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1178–1183. doi: 10.1016/j.pnpbp.2009.06.018
Ramirez, J., Garnica, R., Boll, M. C., Montes, S., and Rios, C. (2004). Low concentration of nitrite and nitrate in the cerebrospinal fluid from schizophrenic patients: a pilot study. Schizophr. Res. 68, 357–361. doi: 10.1016/S0920-9964(03)00070-7
Reddy, R. (2011). Antioxidant therapeutics for schizophrenia. Antioxid. Redox Signal. 15, 2047–2055. doi: 10.1089/ars.2010.3571
Reddy, R., Keshavan, M., and Yao, J. K. (2003). Reduced plasma antioxidants in first-episode patients with schizophrenia. Schizophr. Res. 62, 205–212. doi: 10.1016/S0920-9964(02)00407-3
Reif, A., Herterich, S., Strobel, A., Ehlis, A. C., Saur, D., Jacob, C. P., et al. (2006). A neuronal nitric oxide synthase (NOS-I) haplotype associated with schizophrenia modifies prefrontal cortex function. Mol. Psychiatry 11, 286–300. doi: 10.1038/sj.mp.4001779
Reif, A., Schecklmann, M., Eirich, E., Jacob, C. P., Jarczok, T. A., Kittel-Schneider, S., et al. (2011). A functional promoter polymorphism of neuronal nitric oxide synthase moderates prefrontal functioning in schizophrenia. Int. J. Neuropsychopharmacol. 14, 887–897. doi: 10.1017/S1461145710001677
Ribeiro, B. M., do Carmo, M. R., Freire, R. S., Rocha, N. F., Borella, V. C., de Menezes, A. T., et al. (2013). Evidences for a progressive microglial activation and increase in iNOS expression in rats submitted to a neurodevelopmental model of schizophrenia: reversal by clozapine. Schizophr. Res. 151, 12–19. doi: 10.1016/j.schres.2013.10.040
Ridnour, L. A., Thomas, D. D., Mancardi, D., Espey, M. G., Miranda, K. M., Paolocci, N., et al. (2004). The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 385, 1–10. doi: 10.1515/BC.2004.001
Riley, B., Thiselton, D., Maher, B. S., Bigdeli, T., Wormley, B., McMichael, G. O., et al. (2010). Replication of association between schizophrenia and ZNF804A in the Irish Case-Control Study of Schizophrenia sample. Mol. Psychiatry 15, 29–37. doi: 10.1038/mp.2009.109
Rimol, L. M., Hartberg, C. B., Nesvåg, R., Fennema-Notestine, C., Hagler, D. J. Jr. Pung, C. J., et al. (2010). Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol. Psychiatry 68, 41–50. doi: 10.1016/j.biopsych.2010.03.036
Rodrigues, R., Petersen, R. B., and Perry, G. (2014). Parallels between major depressive disorder and Alzheimer's disease: role of oxidative stress and genetic vulnerability. Cell. Mol. Neurobiol. 34, 925–949. doi: 10.1007/s10571-014-0074-5
Rose, E. J., Greene, C., Kelly, S., Morris, D. W., Robertson, I. H., Fahey, C., et al. (2012). The NOS1 variant rs6490121 is associated with variation in prefrontal function and grey matter density in healthy individuals. Neuroimage 60, 614–622. doi: 10.1016/j.neuroimage.2011.12.054
Rosen, Y., Reznik, I., Sluvis, A., Kaplan, D., and Mester, R. (2003). The significance of the nitric oxide in electro-convulsive therapy: a proposed neurophysiological mechanism. Med. Hypotheses 60, 424–429. doi: 10.1016/S0306-9877(02)00419-X
Ryan, M. C., Sharifi, N., Condren, R., and Thakore, J. H. (2004). Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology 29, 1065–1070. doi: 10.1016/j.psyneuen.2003.08.011
Scatton, B., Worms, P., Lloyd, K. G., and Bartholini, G. (1982). Cortical modulation of striatal function. Brain Res. 232, 331–343. doi: 10.1016/0006-8993(82)90277-3
Seddon, M., Shah, A. M., and Casadei, B. (2007). Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc. Res. 75, 315–326. doi: 10.1016/j.cardiores.2007.04.031
Shinkai, T., Ohmori, O., Hori, H., and Nakamura, J. (2002). Allelic association of the neuronal nitric oxide synthase (NOS1) gene with schizophrenia. Mol. Psychiatry 7, 560–563. doi: 10.1038/sj.mp.4001041
Silberberg, G., Ben-Shachar, D., and Navon, R. (2010). Genetic analysis of nitric oxide synthase 1 variants in schizophrenia and bipolar disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1318–1328. doi: 10.1002/ajmg.b.31112
Sleiman, P., Wang, D., Glessner, J., Hadley, D., Gur, R. E., Cohen, N., et al. (2013). GWAS meta analysis identifies TSNARE1 as a novel Schizophrenia / Bipolar susceptibility locus. Sci. Rep. 3:3075. doi: 10.1038/srep03075
Smith, C. V. (1992). “Free radical mechanisms of tissue injury” in Free Radical Mechanisms of Tissue Injury, eds M. T. Moslen and C. V. Smith (Boca Raton, FL: CRC Press), 1–22.
Song, X., Fan, X., Song, X., Zhang, J., Zhang, W., Li, X., et al. (2013). Elevated levels of adiponectin and other cytokines in drug naïve, first episode schizophrenia patients with normal weight. Schizophr. Res. 150, 269–273. doi: 10.1016/j.schres.2013.07.044
Srivastava, N., Barthwal, M. K., Dalal, P. K., Agarwal, A. K., Nag, D., Srimal, R. C., et al. (2001). Nitrite content and antioxidant enzyme levels in the blood of schizophrenia patients. Psychopharmacology (Berl.) 158, 140–145. doi: 10.1007/s002130100860
Stefansson, H., Ophoff, R. A., Steinberg, S., Andreassen, O. A., Cichon, S., Rujescu, D., et al. (2009). Common variants conferring risk of schizophrenia. Nature 460, 744–747. doi: 10.1038/nature08186
Suboticanec, K., Folnegoviæ-Smalc, V., Korbar, M., Mestroviæ, B., and Buzina, R. (1990). Vitamin C status in chronic schizophrenia. Biol. Psychiatry 28, 959–966. doi: 10.1016/0006-3223(90)90061-6
Sullivan, P. F., Kendler, K. S., and Neale, M. C. (2003). Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 60, 1187–1192. doi: 10.1001/archpsyc.60.12.1187
Sunico, C. R., Portillo, F., González-Forero, D., and Moreno-López, B. (2005). Nitric-oxide-directed synaptic remodeling in the adult mammal CNS. J. Neurosci. 25, 1448–1458. doi: 10.1523/JNEUROSCI.4600-04.2005
Szabadits, E., Cserép, C., Szonyi, A., Fukazawa, Y., Shigemoto, R., Watanabe, M., et al. (2011). NMDA receptors in hippocampal GABAergic synapses and their role in nitric oxide signaling. J. Neurosci. 31, 5893–5904 doi: 10.1523/JNEUROSCI.5938-10.2011
Tanda, K., Nishi, A., Matsuo, N., Nakanishi, K., Yamasaki, N., Sugimoto, T., et al. (2009). Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol. Brain 2:19. doi: 10.1186/1756-6606-2-19
Tandon, R., Keshavan, M. S., and Nasrallah, H. A. (2008). Schizophrenia, “just the facts”: what we know in part 1: overview. Schizophr. Res. 100, 4–19. doi: 10.1016/j.schres.2008.01.022
Tang, W., Huang, K., Tang, R., Zhou, G., Fang, C., Zhang, J., et al. (2008). Evidence for association between the 5' flank of the NOS1 gene and schizophrenia in the Chinese population. Int. J. Neuropsychopharmacol. 11, 1063–1071. doi: 10.1017/S1461145708008924
Tarazi, F. I., Zhang, K., and Baldessarini, R. J. (2002). Long-term effects of newer antipsychotic drugs on neuronal nitric oxide synthase in rat brain. Nitric Oxide 7, 297–300. doi: 10.1016/S1089-8603(02)00126-X
Tien, A. Y., and Eaton, W. W. (1992). Psychopathologic precursors and sociodemographic risk factors for the schizophrenia syndrome. Arch. Gen. Psychiatry 49, 37–46. doi: 10.1001/archpsyc.1992.01820010037005
Timms, A. E., Dorschner, M. O., Wechsler, J., Choi, K. Y., Kirkwood, R., Girirajan, S., et al. (2013). Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry 70, 582–590. doi: 10.1001/jamapsychiatry.2013.1195
Toda, N. (2012). Age-related changes in endothelial function and blood flow regulation. Pharmacol. Ther. 133, 159–176. doi: 10.1016/j.pharmthera.2011.10.004
Toda, N., Ayajiki, K., and Okamura, T. (2009). Cerebral blood flow regulation by nitric oxide in neurological disorders. Can. J. Physiol. Pharmacol. 87, 581–594. doi: 10.1139/Y09-048
Tohgi, H., Abe, T., Yamazaki, K., Murata, T., Isobe, C., and Ishizaki, E. (1998). The cerebrospinal fluid oxidized NO metabolites, nitrite and nitrate, in Alzheimer's disease and vascular dementia of Binswanger type and multiple small infarct type. J. Neural Transm. 105, 1283–1291. doi: 10.1007/s007020050131
Tsai, M. C., Liou, C. W., Lin, T. K., Lin, I. M., and Huang, T. L. (2013). Changes in oxidative stress markers in patients with schizophrenia: the effect of antipsychotic drugs. Psychiatry Res. 209, 284–290. doi: 10.1016/j.psychres.2013.01.023
Upreti, C., Zhang, X. L., Alford, S., and Stanton, P. K. (2013). Role of presynaptic metabotropic glutamate receptors in the induction of long-term synaptic plasticity of vesicular release. Neuropharmacology 66, 31–39. doi: 10.1016/j.neuropharm.2012.05.004
Uranova, N. A., Vostrikov, V. M., Orlovskaya, D. D., and Rachmanova, V. I. (2004). Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr. Res. 67, 269–275. doi: 10.1016/S0920-9964(03)00181-6
Valko, M., Leibfritz, D., Moncol, J., Cronin, M. T., Mazur, M., and Telser, J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84. doi: 10.1016/j.biocel.2006.07.001
van Rossum, J. M. (1966). The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch. Int. Pharmacodyn. Ther. 160, 492–494.
Vasankari, T., Kujala, U., Heinonen, O., Kapanen, J., and Ahotupa, M. (1995). Measurement of serum lipid peroxidation during exercise using three different methods: diene conjugation, thiobarbituric acid reactive material and fluorescent chromolipids. Clin. Chim. Acta 234, 63–69. doi: 10.1016/0009-8981(94)05976-Y
Virarkar, M., Alappat, L., Bradford, P. G., and Awad, A. B. (2013). L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 53, 1157–1167. doi: 10.1080/10408398.2011.573885
Vleeming, W., Rambali, B., and Opperhuizen, A. (2002). The role of nitric oxide in cigarette smoking and nicotine addiction. Nicotine Tob. Res. 4, 341–348. doi: 10.1080/14622200210142724
Wang, H. H., Hsieh, H. L., and Yang, C. M. (2011). Nitric oxide production by endothelin-1 enhances astrocytic migration via the tyrosine nitration of matrix metalloproteinase-9. J. Cell. Physiol. 226, 2244–2256. doi: 10.1002/jcp.22560
Wang, J., Ma, X. H., Xiang, B., Wu, J. Y., Wang, Y. C., Deng, W., et al. (2012). [Association study of NOS1 gene polymorphisms and schizophrenia]. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 29, 459–463. doi: 10.3760/cma.j.issn.1003-9406.2012.04.018
Wass, C., Klamer, D., Katsarogiannis, E., Pålsson, E., Svensson, L., Fejgin, K., et al. (2011). L-lysine as adjunctive treatment in patients with schizophrenia: a single-blinded, randomized, cross-over pilot study. BMC Med. 9:40. doi: 10.1186/1741-7015-9-40
Wass, C., Svensson, L., Fejgin, K., Pålsson, E., Archer, T., Engel, J. A., et al. (2008). Nitric oxide synthase inhibition attenuates phencyclidine-induced disruption of cognitive flexibility. Pharmacol. Biochem. Behav. 89, 352–359. doi: 10.1016/j.pbb.2008.01.011
Wayner, D. D., Burton, G. W., Ingold, K. U., Barclay, L. R., and Locke, S. J. (1987). The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim. Biophys. Acta 924, 408–419. doi: 10.1016/0304-4165(87)90155-3
Weber, H., Klamer, D., Freudenberg, F., Kittel-Schneider, S., Rivero, O., Scholz, C. J., et al. (2014). The genetic contribution of the NO system at the glutamatergic post-synapse to schizophrenia: further evidence and meta-analysis. Eur. Neuropsychopharmacol. 24, 65–85. doi: 10.1016/j.euroneuro.2013.09.005
White, M. F., Gazzola, G. C., and Christensen, H. N. (1982). Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J. Biol. Chem. 257, 4443–4449.
Wratten, N. S., Memoli, H., Huang, Y., Dulencin, A. M., Matteson, P. G., Cornacchia, M. A., et al. (2009). Identification of a schizophrenia-associated functional noncoding variant in NOS1AP. Am. J. Psychiatry 166, 434–441. doi: 10.1176/appi.ajp.2008.08081266
Wu, J. Q., Chen da, C., Tan, Y. L., Tan, S., Wang, Z., Yang, F., et al. (2014). Association of altered CuZn superoxide dismutase and cognitive impairment in schizophrenia patients with tardive dyskinesia. J. Psychiatr. Res. 58, 167–174. doi: 10.1016/j.jpsychires.2014.07.028
Wu, J. Q., Kosten, T. R., and Zhang, X. Y. (2013). Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 200–206. doi: 10.1016/j.pnpbp.2013.02.015
Wu, Z., Zhang, X. Y., Wang, H., Tang, W., Xia, Y., Zhang, F., et al. (2012). Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: inverse association with positive symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 34–38. doi: 10.1016/j.pnpbp.2011.08.018
Xia, Y., Tsai, A. L., Berka, V., and Zweier, J. L. (1998). Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J. Biol. Chem. 273, 25804–25808. doi: 10.1074/jbc.273.40.25804
Xing, G., Chavko, M., Zhang, L. X., Yang, S., and Post, R. M. (2002). Decreased calcium-dependent constitutive nitric oxide synthase (cNOS) activity in prefrontal cortex in schizophrenia and depression. Schizophr. Res. 58, 21–30. doi: 10.1016/S0920-9964(01)00388-7
Xu, B., Wratten, N., Charych, E. I., Buyske, S., Firestein, B. L., and Brzustowicz, L. M. (2005). Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2:e263. doi: 10.1371/journal.pmed.0020263
Yao, J. K., Condray, R., Dougherty, G. G. Jr. Keshavan, M. S., Montrose, D. M., Matson, W. R., et al. (2012). Associations between purine metabolites and clinical symptoms in schizophrenia. PLoS ONE 7:e42165. doi: 10.1371/journal.pone.0042165
Yao, J. K., and Keshavan, M. S. (2011). Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid. Redox Signal. 15, 2011–2035. doi: 10.1089/ars.2010.3603
Yao, J. K., Leonard, S., and Reddy, R. D. (2004). Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr. Bull. 30, 923–934. doi: 10.1093/oxfordjournals.schbul.a007142
Yao, J. K., Reddy, R., and van Kammen, D. P. (1998). Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res. 80, 29–39. doi: 10.1016/S0165-1781(98)00051-1
Yao, J. K., Reddy, R., and van Kammen, D. P. (2000). Abnormal age-related changes of plasma antioxidant proteins in schizophrenia. Psychiatry Res. 97, 137–151. doi: 10.1016/S0165-1781(00)00230-4
Yilmaz, N., Herken, H., Cicek, H. K., Celik, A., Yürekli, M., and Akyol, O. (2007). Increased levels of nitric oxide, cortisol and adrenomedullin in patients with chronic schizophrenia. Med. Princ. Pract. 16, 137–141. doi: 10.1159/000098367
Zeinoddini, A., Ahadi, M., Farokhnia, M., Rezaei, F., Tabrizi, M., and Akhondzadeh, S. (2014). L-lysine as an adjunct to risperidone in patients with chronic schizophrenia: a double-blind, placebo-controlled, randomized trial. J. Psychiatr. Res. 59, 125–131. doi: 10.1016/j.jpsychires.2014.08.016
Zhang, M., Zhao, Z., He, L., and Wan, C. (2010). A meta-analysis of oxidative stress markers in schizophrenia. Sci. China Life Sci. 53, 112–124. doi: 10.1007/s11427-010-0013-8
Zhang, X. R., Wang, Y. X., Zhang, Z. J., Li, L., and Reynolds, G. P. (2012). The effect of chronic antipsychotic drug on hypothalamic expression of neural nitric oxide synthase and dopamine D2 receptor in the male rat. PLoS ONE 7:e33247. doi: 10.1371/journal.pone.0033247
Zhang, X. Y., Chen da, C., Xiu, M. H., Wang, F., Qi, L. Y., Sun, H. Q., et al. (2009). The novel oxidative stress marker thioredoxin is increased in first-episode schizophrenic patients. Schizophr. Res. 113, 151–157. doi: 10.1016/j.schres.2009.05.016
Zhang, X. Y., Chen da, C., Xiu, M. H., Yang, F. D., Tan, Y. L., He, S., et al. (2013). Thioredoxin, a novel oxidative stress marker and cognitive performance in chronic and medicated schizophrenia versus healthy controls. Schizophr. Res. 143, 301–306. doi: 10.1016/j.schres.2012.11.017
Zhang, X. Y., Tan, Y. L., Cao, L. Y., Wu, G. Y., Xu, Q., Shen, Y., et al. (2006). Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 81, 291–300. doi: 10.1016/j.schres.2005.10.011
Zhou, L., and Zhu, D. Y. (2009). Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 20, 223–230. doi: 10.1016/j.niox.2009.03.001
Keywords: schizophrenia, nitric oxide, nitric oxide synthase, oxidative stress, NO, NOS, genetics, pathogenesis
Citation: Nasyrova RF, Ivashchenko DV, Ivanov MV and Neznanov NG (2015) Role of nitric oxide and related molecules in schizophrenia pathogenesis: biochemical, genetic and clinical aspects. Front. Physiol. 6:139. doi: 10.3389/fphys.2015.00139
Received: 01 October 2014; Accepted: 18 April 2015;
Published: 11 May 2015.
Edited by:
Dmitriy N. Atochin, Massachusetts General Hospital, USAReviewed by:
Nazareno Paolocci, Johns Hopkins University, USAElena Dolmatova, Massachusetts General Hospital, USA
Copyright © 2015 Nasyrova, Ivashchenko, Ivanov and Neznanov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Regina F. Nasyrova, V.M. Bekhterev Saint Petersburg Psychoneurological Research Institute, 3 Bekhterev Street, Saint Petersburg 192019, Russia,cmVnaW5hZkBiZWtodGVyZXYucnU=
 Regina F. Nasyrova
Regina F. Nasyrova Dmitriy V. Ivashchenko
Dmitriy V. Ivashchenko Mikhail V. Ivanov
Mikhail V. Ivanov Nikolay G. Neznanov
Nikolay G. Neznanov