- 1Departamento de Neurología, Escuela de Medicina, Pontificia Universidad Católica de Chile, Santiago, Chile
- 2Laboratorio de Neurobiología, Centro de Investigaciones Médicas, Facultad de Ciencias Biológicas and Facultad de Medicina, Universidad Andrés Bello, Santiago, Chile
The role of astrocytes in brain function has evolved over the last decade, from support cells to active participants in the neuronal synapse through the release of “gliotransmitters.”Astrocytes express receptors for most neurotransmitters and respond to them through Ca2+ intracellular oscillations and propagation of intercellular Ca2+ waves. While such waves are able to propagate among neighboring astrocytes through gap junctions, thereby activating several astrocytes simultaneously, they can also trigger the release of gliotransmitters, including glutamate, d-serine, glycine, ATP, adenosine, or GABA. There are several mechanisms by which gliotransmitter release occurs, including functional hemichannels. These gliotransmitters can activate neighboring astrocytes and participate in the propagation of intercellular Ca2+ waves, or activate pre- and post-synaptic receptors, including NMDA, AMPA, and purinergic receptors. In consequence, hemichannels could play a pivotal role in astrocyte-to-astrocyte communication and astrocyte-to-neuron cross-talk. Recent evidence suggests that astroglial hemichannels are involved in higher brain functions including memory and glucose sensing. The present review will focus on the role of hemichannels in astrocyte-to-astrocyte and astrocyte-to neuron communication and in brain physiology.
Astrocytes: General Background
Astrocytes are spongiform-shaped glial cells (Bushong et al., 2002 and Ogata and Kosaka, 2002) that, contrary to common belief, are the most abundant cell type in the brain. They are divided into two major types based on their morphology, biochemistry, development, and location within the central nervous system (CNS): protoplasmic and fibrous (Miller and Raff, 1984). Given their numerous functions, several studies have tried to differentiate subpopulations of astrocytes (Lerea and McCarthy, 1989). However, such attempts have been unsuccessful due to the extraordinary capacity of astrocytes to adapt to their surrounding environment by changing the expression of a vast number of proteins. This is particularly evident in primary cultures, where they show fast changes in the expression of several receptors for neuro- and gliotransmitters (Shao and McCarthy, 1993; Shao et al., 1994). There is a remarkable heterogeneity in astrocyte populations, between different species, brain regions and within a brain region, in terms of their receptor expression, gap junction coupling, membrane currents, and their morphology (Matyash and Kettenmann, 2010; Zhang and Barres, 2010; Theis and Giaume, 2012).
Astrocytes have pivotal roles in brain function, including the maintenance of osmotic balance and optimal ionic conditions for neurons (Kimelberg, 2005), K+ clearance from the extracellular space (Wallraff et al., 2006; Sibille et al., 2013), glucose and lactate metabolism (Allaman et al., 2011), neurotransmitter recycling of the two most abundant neurotransmitters in the brain, glutamate and GABA (Simard and Nedergaard, 2004), and immune responses (Dong and Benveniste, 2001; Farina et al., 2007). Moreover, astrocytes have end-feet that cover blood vessels and release vasoactive substances to regulate cerebral microcirculation (Anderson and Nedergaard, 2003; Zonta et al., 2003; Takano et al., 2006) and blood brain barrier (BBB) permeability (Alvarez et al., 2013). In fact, their end-feet physically constitute part of the BBB. Finally, astrocytes communicate with neurons through transmitters which are released into neighboring synapses, now called “gliotransmitters.” It is not within the scope of the present review to comment on the above functions, for which we have cited very comprehensive reviews, which are highly recommended. The present review will focus on the possible role of hemichannels in astroglial function and brain physiology.
Astrocytes Respond to Synaptic Neurotransmitters
Astrocytes express membrane receptors for almost all major neurotransmitters and neuromodulators, and possess ion channels and intracellular signaling cascades that allow them to respond within milliseconds to neuronal activity and neurotransmitters released at synapses. These fast responses occur mainly as changes in intracellular free Ca2+ concentration ([Ca2+]i) (MacVicar and Tse, 1988; Marrero et al., 1989; Usowic et al., 1989; Barres et al., 1990; Salm and McCarthy, 1990; McCarthy and Salm, 1991). The mechanism by which astroglial activation occurs is believed to start when neurotransmitters released from neurons at the synapse activate receptors at the astroglial membrane, inducing activation of phospholipase C (PLC) and the concomitant production of IP3. The latter then triggers the release of intracellular Ca2+ stored at the endoplasmatic reticulum (Sheppard et al., 1997; Golovina and Blaustein, 2000; Scemes, 2000), which opens hemichannels (De Vuyst et al., 2009) and activates other Ca2+ dependent gliotransmitter release mechanisms including exocytosis. Hemichannels are hexameric plasma membrane channels formed by two different families of membrane proteins: connexins (Cx) and pannexins (Panx). Although these proteins do not share a relevant homologous primary structure, they have similar secondary and tertiary structures with four α-helical transmembrane domains, connected by one cytoplasmic and two extracellular loops, and intracellular N- and C-termini. Importantly, hemichannel opening allows the release of glutamate (Ye et al., 2003; Takeuchi et al., 2006; Kang et al., 2008; Jiang et al., 2011; Orellana et al., 2011a,b), ATP (Stout et al., 2002; Iglesias et al., 2009; Orellana et al., 2011a,b; Torres et al., 2012) and other gliotransmitters into the extracellular space. Given that astrocytes express NMDA receptors insensitive to blocking by extracellular Mg2+, and are activated following physiological synaptic transmission (Verkhratsky and Kirchhoff, 2007) and through purinergic receptor channels (Idestrup and Salter, 1998; Zhu and Kimelberg, 2004; Lalo et al., 2008; Illes et al., 2012), ATP and glutamate released via hemichannels onto the extracellular space can activate purinergic or NMDA receptor channels located in the same astrocyte or in neighboring astrocytes, inducing changes in [Ca2+]i (Zanotti and Charles, 1997; Guthrie et al., 1999). Moreover, because astrocytes envelope synapses, the release of glutamate, ATP, and other gliotransmitters also activates neighboring pre- and post-synaptic neurons, modulating synaptic activity (Dani et al., 1992; Nedergaard, 1994; Parpura et al., 1994; Kang et al., 1998; Parri et al., 2001). In fact, astrocytes stimulated by amyloid β-peptide (Aβ) release ATP and glutamate via connexin 43 (Cx43) hemichannels (Orellana et al., 2011a). Importantly, both of these gliotransmitters released by astrocytes have been shown to increase Panx1 hemichannel activity in neurons by activating P2X7 and NMDA receptors, resulting in further neuronal death. Given that high [Ca2+]i enhances Panx1 hemichannel activity (Locovei et al., 2006), it is likely that purinergic and glutamatergic receptor activation leads to Panx1 hemichannel opening by inducing Ca2+ influx or by releasing Ca2+ from intracellular stores via activation of IP3 receptors (Zanotti and Charles, 1997; Guthrie et al., 1999; Stout et al., 2002; Suadicani et al., 2006).
As reported by Cornell-Bell et al. (1990), both the initial increase, and sustained oscillation of [Ca2+]i induced by glutamate in astrocytes, depend on the concentration of the latter. Indeed, under low glutamate concentrations (>1 μM), [Ca2+]i oscillations in single astrocytes appear locally, asynchronously and are short-lasting, whereas concentrations above 100 μM generate astrocyte-to-astrocyte propagating Ca2+ waves which last up to 30 min (Cornell-Bell et al., 1990). These intercellular Ca2+ waves can be propagated among adjacent astrocytes through Cx43 and Cx30 gap junction channels (GJCs) (Cornell-Bell et al., 1990; Charles et al., 1991; Enkvist and McCarthy, 1992; Finkbeiner, 1992; Venance et al., 1995; Leybaert et al., 1998; Scemes et al., 1998; Blomstrand et al., 1999; Suadicani et al., 2006) or by the Ca2+-dependent release of ATP and glutamate and further activation of purinergic or glutamate receptors in neighboring astrocytes (Zanotti and Charles, 1997; Guthrie et al., 1999; reviewed in Bennett et al., 2003; Leybaert and Sanderson, 2012). GJCs are intercellular channels formed by docking of two hemichannels, one provided by each adjacent cell (Sáez et al., 2003). These channels connect the cytoplasmic compartments of adjacent cells, favoring the intercellular exchange of metabolites (e.g., ADP, ATP, glucose and glutathione), second messengers (e.g., cAMP and IP3) and ions (e.g., Ca2+, K+ and Na+).
To obtain a Ca2+ wave, the released Ca2+ needs to be significantly amplified. This amplification is mediated at least in part by the capacity of Ca2+ itself to activate both IP3 receptors (Finch and Turner, 1991; Bezprozvanny and Ehrlich, 1995) and phospholipase C (Berridge, 1993; Venance et al., 1997) as well as through other mechanisms reviewed in Leybaert and Sanderson, 2012. It has been reported that such Ca2+ waves originate from a localized area of the cell (Shao et al., 1994) and spread throughout the cell and into other cells in a non-decremented manner (Shao et al., 1994). Moreover, it has been suggested that astroglial Ca2+ responses occur once a “threshold” is reached and in an “all-or-none” manner reminiscent of neuronal action potentials (Shao and McCarthy, 1993; Shao et al., 1994). In consequence, the formation of Ca2+ waves is intriguing, as it must include a mechanism that will set this threshold, which may depend on the isoform of the IP3 receptor and on the concentration of IP3 and Ca2+. In neurons, during the generation of an action potential this threshold is defined by the membrane potential required to activate voltage-dependent Na+ channels, which are densely located at the axon hillock and along the axon. The mechanism by which Ca2+ oscillations or fluctuations are integrated into a threshold that determines the triggering of a Ca2+ wave remains unclear, although some hypotheses have been postulated (see Leybaert and Sanderson, 2012). Nonetheless, the idea of a Ca2+ wave being a distinct phenomenon rather than just the consequence of a larger increase in [Ca2+]i is also supported by other studies. In a study by McCarthy and Salm (1991), primary astrocytes were exposed to different neurotransmitter agonists and showed different Ca2+ responses to different neurotransmitters in distinct subpopulations. Interestingly, they found that astrocytes respond to neurotransmitter agonists by either a Ca2+ wave or [Ca2+]i oscillations (McCarthy and Salm, 1991), that is, if a cell population responded to a given agonist with a Ca2+ wave, it may respond to another agonist with [Ca2+]i oscillations and vice versa (McCarthy and Salm, 1991). This suggests, that in a manner similar to neuronal summation of post-synaptic evoked potentials, fluctuations in [Ca2+]i may be integrated additively to generate propagating Ca2+ waves that can activate entire astroglial networks.
In cultured astrocytes, [Ca2+]i oscillations can occur spontaneously in the absence of neuronal activation (Aguado et al., 2002; Perea and Araque, 2005), but can be regulated by neuronal activation and transmitter release. Ca2+ waves, on the other hand, appear in response to neurotransmitters, but given that astrocytes so far have been studied in vitro, it has been argued that Ca2+ waves may appear only in non-physiological conditions or in pathology (Scemes and Giaume, 2006). In vivo it is difficult to differentiate Ca2+ waves from [Ca2+]i oscillations due to technical difficulties. However, [Ca2+]i oscillations have been observed in vivo using imaging techniques under physiological conditions. These [Ca2+]i oscillations in astrocytes were found to be correlated to neuronal discharges (Hirase et al., 2004), and appear in response to sensory stimulation (Cirillo et al., 2012; Lind et al., 2013), electrical stimulation of afferent fibers (Johannssen and Helmchen, 2010) or ATP (Ding, 2012) and at speeds sufficiently fast to occur concomitantly with neuronal activity and hemodynamic changes (Lind et al., 2013). A very recent study has reported that whisker stimulation in awake, behaving mice induces very large Ca2+ astroglial responses spread over a large portion of cortex and which are modulated by subcortical noradrenergic input, but not by intracortical glutamate (Ding et al., 2013).
Functional Hemichannels in Astrocytes
Although the principal connexin in astrocytes is Cx43 (Dermietzel et al., 1989), they also express Cx30 GJCs (Nagy et al., 1999) and Pannexin 1 (Panx1; Iglesias et al., 2009) and Panx2 (Zappalá et al., 2007). Some studies, however, have also reported low levels of Cx26, Cx40, and Cx45 (Dermietzel et al., 1989, 2000; Nagy et al., 1997, 1999). Yet, despite the observations of these latter studies, astrocytes from Cx43/Cx30 double knockout mice fail to show gap junction-mediated communication (Wallraff et al., 2006; Rouach et al., 2008) indicating that Cx43 and Cx30 are the main functional connexins in astrocytes.
Cx43 hemichannels have mostly been studied in vitro using transfected and primary cells, as well as from acute slice experiments (Ye et al., 2003; Orellana et al., 2011a; Chen et al., 2012; Torres et al., 2012). The conditions found in vitro that favor Cx43 hemichannel opening seemed non-physiological at first, leading to a debate on its functionality under physiological conditions. This stems from an earlier belief that hemichannels opened at only highly depolarized membrane potentials (around 60 mV), making their opening virtually impossible in non-excitable cells like astrocytes, which show no large changes in membrane potential. However, recent studies have shown hemichannel opening also at negative membrane potentials (Retamal et al., 2007; Orellana et al., 2011a,b). Indeed, hemichannel-mediated uptake of several dyes (e.g., ethidium, propidium, TOPRO, YOPRO) occurs at resting membrane potentials (Contreras et al., 2003), suggesting that hemichannel opening may also be present at resting membrane conditions.
High levels of intracellular [Ca2+]i and low extracellular Ca2+ ([Ca2+]o) increase opening probability of Cx43 hemichannels (Stout and Charles, 2003; Bao et al., 2004; Wang et al., 2012) whereas normal extracellular [Ca2+]o closes them (Stout and Charles, 2003). Cx43 hemichannels have been reported to mediate the release of gliotransmitters (glutamate, ATP, glutathione) from astrocytes and glioma cells (Stout et al., 2002; Ye et al., 2003). Ye et al. (2003) demonstrated that low extracellular [Ca2+]o induces glutamate release from astrocytes through Cx43 hemichannels in an exocytosis-independent manner and involves neither large pore anion channels, purinergic receptors, nor reversal of the glutamate transporter (Ye et al., 2003). This idea was further supported by reports showing ATP release from glioma cells overexpressing Cx43 and exposed to zero extracellular [Ca2+]o (Ye et al., 2003; Contreras et al., 2004; Retamal et al., 2006). Other studies, however, have reported ATP release from astrocytes also mediated by the P2X7 receptor, Panx1 hemichannels, and exocytosis (Parpura et al., 1994; Coco et al., 2003; Bezzi et al., 2004; Mothet et al., 2005; Pascual et al., 2005; Garré et al., 2010). This suggests that ATP is released by astrocytes through different mechanisms. In a study by Garré et al. (2010), it was reported that pharmacological blockade of vesicles inhibited only early ATP release from astrocytes, while later release was reported to be mediated by P2X7 receptor activation as well by Panx1 and Cx43 hemichannel opening, suggesting that each release mechanism may occur at different periods.
Role of Astroglial Connexin and Pannexin Hemichannels in Gliotransmitter Release at the Synapse
Astrocytes release gliotransmitters into neuronal synapses, giving rise to what is now known as the tripartite synapse (Araque et al., 1998), implying a synapse between a pre- and post-synaptic neuron and their bidirectional communication with one astrocyte. Glutamate is the most important and abundant excitatory neurotransmitter of the CNS and one the most ubiquitous gliotransmitters released by astrocytes (Navarrete et al., 2012). Multiple mechanisms have been proposed to explain the release of glutamate from astrocytes, including hemichannels (Ye et al., 2003), anion channels (Wang et al., 2013), and exocytosis (Parpura et al., 1994; Coco et al., 2003; Bezzi et al., 2004; Mothet et al., 2005; Pascual et al., 2005 but see Wang et al., 2013). The other major neurotransmitter is GABA, the principal inhibitory neurotransmitter of the CNS. Although GABA is abundantly released by interneurons, it is also released by astrocytes (Lee et al., 2011).
Perhaps some of the best known gliotransmitters are D-serine and glycine, which are required for NMDAR activation of post-synaptic neurons and necessary for glutamate-mediated synaptic plasticity (Panatier et al., 2006; Henneberger et al., 2010; Hogerton and Bowser, 2013; Kang et al., 2013). D-serine has been reported to be released from astrocytes via large vesicles (Kang et al., 2013) and exocytosis (Parpura et al., 1994; Coco et al., 2003; Bezzi et al., 2004; Mothet et al., 2005; Pascual et al., 2005). It must be noted that a recent report has suggested that neurons may also release D-serine and glycine (Balu et al., 2014 and Ehmsen et al., 2013) both involved in regulating synaptic plasticity (Rosenberg et al., 2013). Until now, there has been no evidence indicating that astroglial hemichannels can release either D-serine or glycine.
As stated earlier, ATP both activates astrocytes and is also released by them. Additionally, it appears to suppress glutamatergic synapses (Zhang et al., 2003; Cao et al., 2013a) and can be turned into adenosine, which decreases excitatory transmission (Dunwiddie and Diao, 1994; Dunwiddie et al., 1997, but see Fujita et al., 2012). ATP has been shown to be released through hemichannels (Stout et al., 2002; Kang et al., 2008), P2X7 channels (Suadicani et al., 2006) and exocytosis (Parpura et al., 1994; Coco et al., 2003; Bezzi et al., 2004; Mothet et al., 2005; Pascual et al., 2005). Another gliotransmitter, glutathione, is released in response to extracellular glutamate (Frade et al., 2008) through connexin hemichannels (Rana and Dringen, 2007). Other well-known gliotransmitters include BDNF (Parpura and Zorec, 2010) and taurine (Choe et al., 2012). Although, previous studies have shown that taurine could be released via astroglial hemichannels (Stridh 2006/2008), further studies are necessary to elucidate whether BDNF could be released by the same pathway.
Astrocytic Hemichannels in Brain Function
Given that astrocytes participate in the tripartite synapse, their contribution to brain function is as wide as that of neurons; taking into account their other functions (microcirculation, BBB formation), perhaps even more so (for a schematic of main astrocytic signaling cascades see Figure 1). Hemichannels contribute to the release of glutamate which is necessary for NMDAR-dependent synaptic plasticity (Henneberger et al., 2010; Navarrete et al., 2012). In fact, recently it was shown that blockade of Cx43 hemichannels in the basolateral amygdala by microinjection of mimetic peptides impairs memory consolidation but not short-term memory (Stehberg et al., 2012). Given that this study was performed using rodent fear conditioning, which is the most accepted model of post-traumatic stress disorder in animals, it would be plausible to suggest that Cx43 hemichannels may have a role in the establishment of memories in general and traumatic memories in particular.
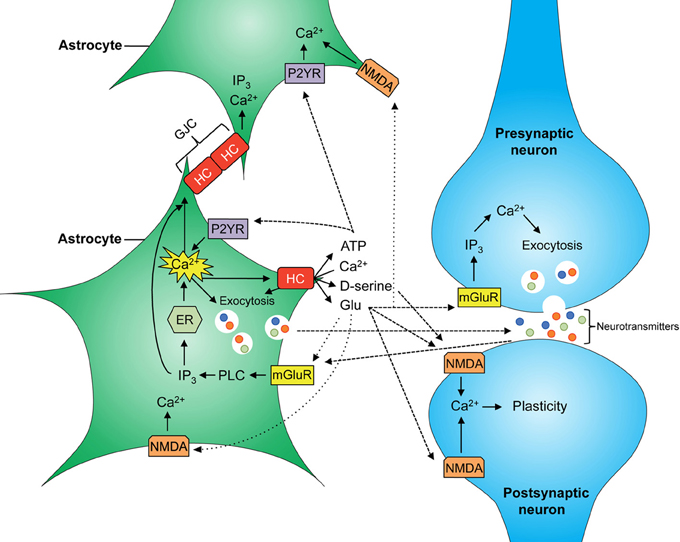
Figure 1. Scheme of major astrocyte signaling associated to gliotransmitter release. Increased intracellular free Ca2+ concentration ([Ca2+])i allows the release of gliotransmitters into the synaptic cleft through vesicles and hemichannels (HCs). D-serine, glycine, and glutamate released by astrocytes can activate NMDA receptors at the post-synaptic neuron and modulate neuronal plasticity. Astroglial glutamate also binds to mGluR at the presynaptic neuron increasing neuronal release of glutamate into the synapse. In astrocytes, increasing ([Ca2+])i allows Ca2+ wave propagation between astrocytes, mediated by gap junction channels (GJCs) and by release of glutamate and ATP, resulting in further activation of NMDA and P2YR receptors at neighboring astrocytes, respectively.
Potential Role of Astroglial Connexin and Pannexin Hemichannels in Psychiatric Diseases
There is to-date no direct evidence linking astrocytic hemichannels and psychiatric disorders, so one can only speculate what their role might be. Studies have shown abnormal expression of glial fibrillary acid protein (GFAP)—a marker for astrocytes—in the post-mortem brain of patients with major depression (Bowley et al., 2002; Altshuler et al., 2010; Rajkowska and Stockmeier, 2013), while other studies have shown reduced density of astrocytes from clinical (Ongur et al., 1998; Cotter et al., 2001; Bremner et al., 2002), post-mortem (Cotter et al., 2001), and preclinical (Banasr and Duman, 2008; Banasr et al., 2010) studies, suggesting that the density and reactivity of astrocytes are reduced in this mood disorder.
Moreover, accumulating evidence suggests that antidepressants act on astrocytes (for reviews see Czéh and Di Benedetto, 2013; Etiévant et al., 2013). These express a variety of receptors including monoaminergic transporters and receptors, leading to the possibility that antidepressants exert their effects at least in part through modifying astroglial function (Peng and Huang, 2012; Quesseveur et al., 2013a). In this sense, it's been demonstrated that application of antidepressants on rodent primary astrocyte cultures may elicit Ca2+ waves, Ca2+ oscillations, release of gliotransmitters, glucose metabolites, and neurotrophic factors (Hisaoka et al., 2011), whereas studies in post-mortem human brain tissue suggest that antidepressants may reverse major depression associated glial reductions in the amygdala (Bowley et al., 2002). Interestingly, many transmitters released by astrocytes have antidepressant or anxiolytic effects. To this effect, acute D-serine treatment (800–2700 mg/Kg) produces antidepressant-like effects in rodents (Malkesman et al., 2012), astroglial release of ATP has been shown to modulate depressive-like behaviors (Cao et al., 2013b), GABA agonists are well known to have anxiolytic effects (the mechanism of action for benzodiazepines), while overexpression of astrocytic BDNF produces anxiolytic effects (Quesseveur et al., 2013b). Given that the release of all the above could be mediated at least in part by hemichannels, it is probable that mood depends on hemichannel activity.
Studies have also shown abnormal expression of GFAP in the post-mortem brain of patients with schizophrenia (Toro et al., 2006; Feresten et al., 2013). In fact, Khan and colleagues (Khan et al., 2001) found by electron microscopy that roughly 1/3 of D2 dopamine receptors in the cortex are expressed in astrocytes, and that D2 receptor agonist quinpirole increases astroglial intracellular [Ca2+]i, suggesting that astrocytes may be a target for antipsychotics.
Finally, current evidence also suggests that astrocytes could be involved in drug abuse (Miguel-Hidalgo, 2009). All in all, given the pivotal role astrocytes play in brain function, and their active release of gliotransmitters into synapses, it is highly probable that they will become a target in the treatment of psychiatric diseases. In this respect, hemichannels constitute an attractive candidate for such treatment as they mediate gliotransmitter release at the synapse of glutamate, activating NMDA, and non NMDA-dependent mechanisms critical for synaptic plasticity and the release of ATP and adenosine which may decrease neuronal network excitation. Moreover, antidepressants and antipsychotics may act, at least in part, through various astroglial monoamine receptors and transporters to modulate cytoplasmic Ca2+ that controls hemichannel activity.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aguado, F., Espinosa-Parrilla, J. F., Carmona, M. A., and Soriano, E. (2002). Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 22, 9430–9444.
Allaman, I., Bélanger, M., and Magistretti, P. J. (2011). Astrocyte-neuron metabolic relationships: for better and for worse. Trends Neurosci. 34, 76–87. doi: 10.1016/j.tins.2010.12.001
Altshuler, L. L., Abulseoud, O. A., Foland-Ross, L., Bartzokis, G., Chang, S., Mintz, J., et al. (2010). Amygdala astrocyte reduction in subjects with major depressive disorder but not bipolar disorder. Bipolar Disord. 12, 541–549. doi: 10.1111/j.1399-5618.2010.00838.x
Alvarez, J. I., Katayama, T., and Prat, A. (2013). Glial influence on the blood brain barrier. Glia 6, 1939–1958. doi: 10.1002/glia.22575
Anderson, C., and Nedergaard, M. (2003). Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 26, 340–344. doi: 10.1016/S0166-2236(03)00141-3
Araque, A., Sanzgiri, R. P., Parpura, V., and Haydon, P. G. (1998). Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents. J. Neurosci. 18, 6822–6829.
Balu, D. T., Takagi, S., Puhl, M. D., Benneyworth, M. A., and Coyle, J. T. (2014). D-serine and serine racemase are localized to neurons in the adult mouse and human forebrain. Cell Mol. Neurobiol. 34, 419–435. doi: 10.1007/s10571-014-0027-z
Banasr, M., Chowdhury, G. M., Terwilliger, R., Newton, S. S., Duman, R. S., Behar, K. L., et al. (2010). Glial pathology in an animal model of depression: reversal of stress-induced cellular, metabolic and behavioral deficits by the glutamate modulating drug riluzole. Mol. Psychiatry 15, 501–511. doi: 10.1038/mp.2008.106
Banasr, M., and Duman, R. S. (2008). Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry 64, 863–870. doi: 10.1016/j.biopsych.2008.06.008
Bao, X., Altenberg, G. A., and Reuss, L. (2004). Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am. J. Physiol. Cell Physiol. 286, C647–C654. doi: 10.1152/ajpcell.00295.2003
Barres, B. A., Koroshetz, W. J., Chun, L. L. Y., and Corey, D. P. (1990). Ion channel expression by white matter glia: Type-1 astrocyte. Neuron 5, 527–544. doi: 10.1016/0896-6273(90)90091-S
Bennett, M. V., Contreras, J. E., Bukauskas, F. F., and Sáez, J. C. (2003). New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 26, 610–617. doi: 10.1016/j.tins.2003.09.008
Bezprozvanny, I., and Ehrlich, B. E. (1995). The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 145, 205–216.
Bezzi, P., Gundersen, V., Galbete, J. L., Seifert, G., Steinhauser, C., Pilati, E., et al. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7, 613–620. doi: 10.1038/nn1246
Blomstrand, F., Aberg, N. D., Eriksson, P. S., Hansson, E., and Ronnback, L. (1999). Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin43 expression in astrocytes in primary cultures from four brain regions. Neuroscience 92, 255–265. doi: 10.1016/S0306-4522(98)00738-6
Bowley, M. P., Drevets, W. C., Ongür, D., and Price, J. L. (2002). Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry 52, 404–412. doi: 10.1016/S0006-3223(02)01404-X
Bremner, J. D., Vythilingam, M., Vermetten, E., Nazeer, A., Adil, J., Khan, S., et al. (2002). Reduced volume of orbitofrontal cortex in major depression. Biol. Psychiatry 51, 273–279. doi: 10.1016/S0006-3223(01)01336-1
Bushong, E. A., Martone, M. E., Jones, Y. Z., and Ellisman, M. H. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22, 183–192.
Cao, X., Li, L. P., Qin, X. H., Li, S. J., Zhang, M., Wang, Q., et al. (2013a). Astrocytic adenosine 5'-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 31, 1633–1643. doi: 10.1002/stem.1408
Cao, X., Li, L. P., Wang, Q., Wu, Q., Hu, H. H., Zhang, M., et al. (2013b). Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 19, 773–777. doi: 10.1038/nm.3162
Charles, A. C., Merrill, J. E., Dirksen, E. R., and Sanderson, M. J. (1991). Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992. doi: 10.1016/0896-6273(91)90238-U
Chen, M. J., Kress, B., Han, X., Moll, K., Peng, W., Ji, R. R., et al. (2012). Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia 60, 1660–1670. doi: 10.1002/glia.22384
Choe, K. Y., Olson, J. E., and Bourque, C. W. (2012). Taurine release by astrocytes modulates osmosensitive glycine receptor tone and excitability in the adult supraoptic nucleus. J. Neurosci. 32, 12518–12527. doi: 10.1523/JNEUROSCI.1380-12.2012
Cirillo, G., De Luca, D., and Papa, M. (2012). Calcium imaging of living astrocytes in the mouse spinal cord following sensory stimulation. Neural Plast. 2012:425818. doi: 10.1155/2012/425818
Coco, S., Calegari, F., Pravettoni, E., Pozzi, D., Taverna, E., Rosa, P., et al. (2003). Storage and release of ATP from astrocytes in culture. J. Biol. Chem. 278, 1354–1362. doi: 10.1074/jbc.M209454200
Contreras, J. E., Sáez, J. C., Bukauskas, F. F., and Bennett, M. V. (2003). Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U.S.A. 100, 11388–11393. doi: 10.1073/pnas.1434298100
Contreras, J. E., Sánchez, H. A., Véliz, L. P., Bukauskas, F. F., Bennett, M. V., and Sáez, J. C. (2004). Role of connexin-based gap junction channels and hemichannels in ischemia-induced cell death in nervous tissue. Brain Res. Brain Res. Rev. 47, 290–303. doi: 10.1016/j.brainresrev.2004.08.002
Cornell-Bell, A. H., Finkbeiner, S. M., Cooper, M. S., and Smith, S. J. (1990). Glutamateinduces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473. doi: 10.1126/science.1967852
Cotter, D., Mackay, D., Landau, S., Kerwin, R., and Everall, I. (2001). Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 58, 545–553. doi: 10.1001/archpsyc.58.6.545
Czéh, B., and Di Benedetto, B. (2013). Antidepressants act directly on astrocytes: evidences and functional consequences. Eur. Neuropsychopharmacol. 23, 171–185. doi: 10.1016/j.euroneuro.2012.04.017
Dani, J. W., Chernjavsky, A., and Smith, S. J. (1992). Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 8, 429–440. doi: 10.1016/0896-6273(92)90271-E
Dermietzel, R., Gao, Y., Scemes, E., Vieira, D., Urban, M., Kremer, M., et al. (2000). Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res. Brain Res. Rev. 32, 45–56. doi: 10.1016/S0165-0173(99)00067-3
Dermietzel, R., Traub, O., Hwang, T. K., Beyer, E., Bennett, M. V., Spray, D. C., et al. (1989). Differential expression of three gap junction proteins in developing and mature brain tissues. Proc. Natl. Acad. Sci. U.S.A. 86, 10148–10152. doi: 10.1073/pnas.86.24.10148
De Vuyst, E., Wang, N., Decrock, E., De Bock, M., Vinken, M., Van Moorhem, M., et al. (2009). Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46, 176–187. doi: 10.1016/j.ceca.2009.07.002
Ding, F., O'Donnell, J., Thrane, A. S., Zeppenfeld, D., Kang, H., Xie, L., et al. (2013). α1-Adrenergic receptors mediate coordinated Ca(2+) signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54, 387–394. doi: 10.1016/j.ceca.2013.09.001
Ding, S. (2012). In vivo imaging of Ca2 signaling in astrocytes using two-photon laser scanning fluorescent microscopy. Methods Mol. Biol.814, 545–554. doi: 10.1007/978-1-61779-452-0_36
Dong, Y., and Benveniste, E. N. (2001). Immune function of astrocytes. Glia 36, 180–190. doi: 10.1002/glia.1107
Dunwiddie, T. V., and Diao, L. (1994). Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J. Pharmacol. Exp. Ther. 268, 537–545.
Dunwiddie, T. V., Diao, L., and Proctor, W. R. (1997). Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J. Neurosci. 17, 7673–7682.
Ehmsen, J. T., Ma, T. M., Sason, H., Rosenberg, D., Ogo, T., Furuya, S., et al. (2013). D-serine in glia and neurons derives from 3-phosphoglycerate dehydrogenase. J. Neurosci. 33, 12464–12469. doi: 10.1523/JNEUROSCI.4914-12.2013
Enkvist, M. O., and McCarthy, K. D. (1992). Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J. Neurochem. 59, 519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x
Etiévant, A., Lambás-Señas, L., Scarna, H., Lucas, G., and Haddjeri, N. (2013). Astrocytes and gliotransmitters: new players in the treatment of major depression? Curr. Drug Targets 14, 1295–1307. doi: 10.2174/13894501113149990197
Farina, C., Aloisi, F., and Meinl, E. (2007). Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145. doi: 10.1016/j.it.2007.01.005
Feresten, A. H., Barakauskas, V., Ypsilanti, A., Barr, A. M., and Beasley, C. L. (2013). Increased expression of glial fibrillary acidic protein in prefrontal cortex in psychotic illness. Schizophr. Res. 150, 252–257. doi: 10.1016/j.schres.2013.07.024
Finch, E. A., and Turner, T. J. (1991). Goldin SM Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252, 443–446.
Finkbeiner, S. (1992). Calcium waves in astrocytes-filling in the gaps. Neuron 8, 1101–1108. doi: 10.1016/0896-6273(92)90131-V
Frade, J., Pope, S., Schmidt, M., Dringen, R., Barbosa, R., Pocock, J., et al. (2008). Glutamate induces release of glutathione from cultured rat astrocytes–a possible neuroprotective mechanism? J. Neurochem. 105, 1144–1152. doi: 10.1111/j.1471-4159.2008.05216.x
Fujita, T., Williams, E. K., Jensen, T. K., Smith, N. A., Takano, T., Tieu, K., et al. (2012). Cultured astrocytes do not release adenosine during hypoxic conditions. J. Cereb. Blood Flow Metab. 32, e1–e7. doi: 10.1038/jcbfm.2011.142
Garré, J. M., Retamal, M. A., Cassina, P., Barbeito, L., Bukauskas, F. F., Sáez, J. C., et al. (2010). FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc. Natl. Acad. Sci. U.S.A. 107, 22659–22664. doi: 10.1073/pnas.1013793107
Golovina, V. A., and Blaustein, M. P. (2000). Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca(2+) stores in astrocytes. Glia 31, 15–28. doi: 10.1002/(SICI)1098-1136(200007)31:1<15::AID-GLIA20>3.0.CO;2-H
Guthrie, P. B., Knappenberger, J., Segal, M., Bennett, M. V., Charles, A. C., and Kater, S. B. (1999). ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 19, 520–528.
Henneberger, C., Papouin, T., Oliet, S. H., and Rusakov, D. A. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature 463, 232–236. doi: 10.1038/nature08673
Hirase, H., Qian, L., Bartho, P., and Buzsaki, G. (2004). Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2:e96. doi: 10.1371/journal.pbio.0020096
Hisaoka, K., Tsuchioka, M., Yano, R., Maeda, N., Kajitani, N., Morioka, N., et al. (2011). Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J. Biol. Chem. 286, 21118–21128. doi: 10.1074/jbc.M111.224683
Hogerton, A. L., and Bowser, M. T. (2013). Monitoring neurochemical release from astrocytes using in vitro microdialysis coupled with high-speed capillary electrophoresis. Anal. Chem. 85, 9070–9077. doi: 10.1021/ac401631k
Idestrup, C. P., and Salter, M. W. (1998). P2Y and P2U receptors differentially release intracellular Ca+2via the phospholipase c/inositol 1,4,5-triphosphatepathway in astrocytes from the dorsal spinal cord. Neuroscience 86, 913–923. doi: 10.1016/S0306-4522(98)00128-6
Iglesias, R., Dahl, G., Qiu, F., Spray, D. C., and Scemes, E. (2009). Pannexin 1: the molecular substrate of astrocyte “hemichannels.” J. Neurosci. 29, 7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009
Illes, P., Verkhratsky, A., Burnstock, G., and Franke, H. (2012). P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neuroscientist 18, 422–438. doi: 10.1177/1073858411418524
Jiang, S., Yuan, H., Duan, L., Cao, R., Gao, B., Xiong, Y. F., et al. (2011). Glutamate release through connexin 43 by cultured astrocytes in a stimulated hypertonicity model. Brain Res. 1392, 8–15. doi: 10.1016/j.brainres.2011.03.056
Johannssen, H. C., and Helmchen, F. (2010). In vivo Ca2+ imaging of dorsal horn neuronal populations in mouse spinal cord. J. Physiol. 588(Pt 18), 3397–3402. doi: 10.1113/jphysiol.2010.191833
Kang, J., Jiang, L., Goldman, S. A., and Nedergaard, M. (1998). Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat. Neurosci. 1, 683–692. doi: 10.1038/3684
Kang, J., Kang, N., Lovatt, D., Torres, A., Zhao, Z., Lin, J., et al. (2008). Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 28, 4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008
Kang, N., Peng, H., Yu, Y., Stanton, P. K., Guilarte, T. R., and Kang, J. (2013). Astrocytes release D-serine by a large vesicle. Neuroscience 240, 243–257. doi: 10.1016/j.neuroscience.2013.02.029
Khan, Z. U., Koulen, P., Rubinstein, M., Grandy, D. K., and Goldman-Rakic, P. S. (2001). An astroglia-linked dopamine D2-receptor action in prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 98, 1964–1969. doi: 10.1073/pnas.98.4.1964
Kimelberg, H. K. (2005). Astrocytic swelling in cerebral ischemia as a possible cause of injury and target for therapy. Glia 50, 389–397. doi: 10.1002/glia.20174
Lalo, U., Pankratov, Y., Wichert, S. P., Rossner, M. J., North, R. A., Kirchhoff, F., et al. (2008). P2X1 and P2X5 subunits form the functional P2X receptor in mouse cortical astrocytes. J. Neurosci. 28, 5473–5480. doi: 10.1523/JNEUROSCI.1149-08.2008
Lee, M., McGeer, E. G., and McGeer, P. L. (2011). Mechanisms of GABA release from human astrocytes. Glia 59, 1600–1611. doi: 10.1002/glia.21202
Lerea, L. S., and McCarthy, K. D. (1989). Astroglial cells in vitro are heterogeneous with respect to expression of the alpha 1-adrenergic receptor. Glia 2, 135–147. doi: 10.1002/glia.440020302
Leybaert, L., Paemeleire, K., Strahonja, A., and Sanderson, M. J. (1998). Inositol trisphosphate- dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia 24, 398–407. doi: 10.1002/(SICI)1098-1136(199812)24:4%3C398::AID-GLIA5%3E3.3.CO;2-I
Leybaert, L., and Sanderson, M. J. (2012). Intercellular Ca(2+) waves: mechanisms and function. Physiol. Rev. 92, 1359–1392. doi: 10.1152/physrev.00029.2011
Lind, B. L., Brazhe, A. R., Jessen, S. B., Tan, F. C., and Lauritzen, M. J. (2013). Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, E4678–E4687. doi: 10.1073/pnas.1310065110
Locovei, S., Wang, J., and Dahl, G. (2006). Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 580, 239–244. doi: 10.1016/j.febslet.2005.12.004
MacVicar, B. A., and Tse, R. W. Y. (1988). Norepinephrine and cyclic adenosine 30,50-cyclic monophosphate enhance a nifedipine-sensitive calcium current in cultured rat astrocytes. Glia 1, 359–365. doi: 10.1002/glia.440010602
Malkesman, O., Austin, D. R., Tragon, T., Wang, G., Rompala, G., Hamidi, A. B., et al. (2012). Acute D-serine treatment produces antidepressant-like effects in rodents. Int. J. Neuropsychopharmacol. 15, 1135–1148. doi: 10.1017/S1461145711001386
Marrero, H., Astion, M. L., Coles, A., and Orkland, R. K. (1989). Facilitation of voltage-gated ion channels in frog neuroglia by nerve impulses. Nature 339, 378–380. doi: 10.1038/339378a0
Matyash, V., and Kettenmann, H. (2010). Heterogeneity in astrocyte morphology and physiology. Brain Res. Rev. 63, 2–10. doi: 10.1016/j.brainresrev.2009.12.001
McCarthy, K. D., and Salm, A. K. (1991). Pharmacologically-distinct subsets of astroglia can be identified by their calcium response to neuroligands. Neuroscience 41, 325–333. doi: 10.1016/0306-4522(91)90330-Q
Miguel-Hidalgo, J. J. (2009). The role of glial cells in drug abuse. Curr. Drug Abuse Rev. 2, 72–82. doi: 10.2174/1874473710902010076
Miller, R. H., and Raff, M. C. (1984). Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J. Neurosci. 4, 585–592.
Mothet, J. P., Pollegioni, L., Ouanounou, G., Matineau, M., Fossier, P., and Baux, G. (2005). Glutamate receptor activation triggers a calcium-dependent andSNARE protein-dependent release of the gliotransmitter D-serine. Proc. Natl. Acad. Sci. U.S.A. 102, 5606–5611. doi: 10.1073/pnas.0408483102
Nagy, J. I., Ochalski, P. A., Li, J., and Hertzberg, E. L. (1997). Evidence for the co-localization of another connexin with connexin-43 at astrocytic gap junctions in rat brain. Neuroscience 78, 533–548. doi: 10.1016/S0306-4522(96)00584-2
Nagy, J. I., Patel, D., Ochalski, P. A., and Stelmack, G. L. (1999). Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88, 447–468. doi: 10.1016/S0306-4522(98)00191-2
Navarrete, M., Perea, G., Fernandez de Sevilla, D., Gómez-Gonzalo, M., Núñez, A., Martín, E. D., et al. (2012). Astrocytesmediate in vivo cholinergic-inducedsynapticplasticity. PLoS Biol. 10:e1001259. doi: 10.1371/journal.pbio.1001259
Nedergaard, M. (1994). Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263, 1768–1771. doi: 10.1126/science.8134839
Ogata, K., and Kosaka, T. (2002). Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113, 221–233. doi: 10.1016/S0306-4522(02)00041-6
Ongur, D., Drevets, W. C., and Price, J. L. (1998). Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc. Nati. Acad. Sci. U.S.A. 95, 13290–13295. doi: 10.1073/pnas.95.22.13290
Orellana, J. A., Froger, N., Ezan, P., Jiang, J. X., Bennett, M. V., Naus, C. C., et al. (2011b). ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 118, 826–840. doi: 10.1111/j.1471-4159.2011.07210.x
Orellana, J. A., Shoji, K. F., Abudara, V., Ezan, P., Amigou, E., Sáez, P. J., et al. (2011a). Amyloid β-induced death in neurons involves glial and neuronal hemichannels. J. Neurosci. 31, 4962–4977. doi: 10.1523/JNEUROSCI.6417-10.201
Panatier, A., Theodosis, D. T., Mothet, J. P., Touquet, B., Pollegioni, L., Poulain, D. A., et al. (2006). Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell 125, 775–784. doi: 10.1016/j.cell.2006.02.051
Parpura, V., Basarsky, T. A., Liu, F., Jeftinija, K., Jeftinija, S., and Haydon, P. G. (1994). Glutamate-mediated astrocyte-neuron signalling. Nature 369, 744–747. doi: 10.1038/369744a0
Parpura, V., and Zorec, R. (2010). Gliotransmission: exocytotic release from astrocytes. Brain Res. Rev. 63, 83–92. doi: 10.1016/j.brainresrev.2009.11.008
Parri, H. R., Gould, T. M., and Crunelli, V. (2001). Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat. Neurosci. 4, 803–812. doi: 10.1038/90507
Pascual, O., Casper, K. B., Kubera, C., Zhang, J., Revilla-Sanchez, R., Sul, J.-Y., et al. (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. doi: 10.1126/science.1116916
Peng, L., and Huang, J. (2012). Astrocytic 5-HT(2B) receptor as in vitro and in vivo target of SSRIs. Recent Pat. CNS Drug Discov. 7, 243–253. doi: 10.2174/157488912803252078
Perea, G., and Araque, A. (2005). Glial calcium signalling and neurongliacommunication. Cell Calcium 38, 375–382 doi: 10.1016/j.ceca.2005.06.015
Quesseveur, G., David, D. J., Gaillard, M. C., Pla, P., Wu, M. V., Nguyen, H. T., et al. (2013b). BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl. Psychiatry 3, e253. doi: 10.1038/tp.2013.30
Quesseveur, G., Gardier, A. M., and Guiard, B. P. (2013a). The monoaminergic tripartite synapse: a putative target for currently available antidepressant drugs. Curr. Drug Targets 14, 1277–1294. doi: 10.2174/13894501113149990209
Rajkowska, G., and Stockmeier, C. A. (2013). Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr. Drug Targets 14, 1225–1236. doi: 10.2174/13894501113149990156
Rana, S., and Dringen, R. (2007). Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci. Lett. 415, 45–48. doi: 10.1016/j.neulet.2006.12.043
Retamal, M. A., Cortés, C. J., Reuss, L., Bennett, M. V., and Sáez, J. C. (2006). S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. U.S.A. 103, 4475–4480. doi: 10.1073/pnas.0511118103
Retamal, M. A., Froger, N., Palacios-Prado, N., Ezan, P., Sáez, P. J., Sáez, J. C., et al. (2007). Dec Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 27, 13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007
Rosenberg, D., Artoul, S., Segal, A. C., Kolodney, G., Radzishevsky, I., Dikopoltsev, E., et al. (2013). Neuronal D-serine and glycine release via the Asc-1 transporter regulates NMDA receptor-dependent synaptic activity. J. Neurosci. 33, 3533–3544. doi: 10.1523/JNEUROSCI.3836-12.2013
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K., and Giaume, C. (2008). Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555. doi: 10.1126/science.1164022
Sáez, J. C., Contreras, J. E., Bukauskas, F. F., Retamal, M. A., and Bennett, M. V. (2003). Gap junction hemichannels in astrocytes of the CNS. Acta Physiol. Scand. 179, 9–22. doi: 10.1046/j.1365-201X.2003.01196.x
Salm, A. K., and McCarthy, K. D. (1990). Norepinephrine-evoked calcium transients in cultured cerebral type 1 astroglia. Glia 3, 529–538. doi: 10.1002/glia.440030612
Scemes, E. (2000). Components of astrocytic intercellular calcium signaling. Mol. Neurobiol. 22, 167–179. doi: 10.1385/MN:22:1-3:167
Scemes, E., Dermietzel, R., and Spray, D. C. (1998). Calcium waves between astrocytes from Cx43 knockout mice. Glia 24, 65–73. doi: 10.1002/(SICI)1098-1136(199809)24:1%3C65::AID-GLIA7%3E3.0.CO;2-%23
Scemes, E., and Giaume, C. (2006). Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725 doi: 10.1002/glia.20374
Shao, Y., and McCarthy, K. D. (1993). Regulation of astroglial responsiveness to neuroligands in primary culture. Neuroscience 55, 991–1001. doi: 10.1016/0306-4522(93)90313-5
Shao, Y., Porter, J. T., and McCarthy, K. D. (1994). Neuroligand receptor heterogeneity among astroglia. Perspect. Dev. Neurobiol. 2, 205–215.
Sheppard, C. A., Simpson, P. B., Sharp, A. H., Nucifora, F. C., Ross, C. A., Lange, G. D., et al. (1997). Comparison of type 2 inositol 1,4,5-trisphosphate receptor distribution and subcellular Ca21 release sites that support Ca21 waves in cultured astrocytes. J. Neurochem. 68, 2317–2327. doi: 10.1046/j.1471-4159.1997.68062317.x
Sibille, J., Pannasch, U., and Rouach, N. (2013). Astroglial potassium clearance contributes to short-term plasticity of synaptically-evoked currents at the tripartite synapse. J. Physiol. 592(Pt 1), 87–102. doi: 10.1113/jphysiol.2013.261735
Simard, M., and Nedergaard, M. (2004). The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129, 877–896. doi: 10.1016/j.neuroscience.2004.09.053
Stehberg, J., Moraga-Amaro, R., Salazar, C., Becerra, A., Echeverría, C., Orellana, J. A., et al. (2012). Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 26, 3649–3657. doi: 10.1096/fj.11-198416
Stout, C., and Charles, A. (2003). Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia 43, 265–273. doi: 10.1002/glia.10257
Stout, C. E., Costantin, J. L., Naus, C. C., and Charles, A. C. (2002). Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 277, 10482–10488. doi: 10.1074/jbc.M109902200
Suadicani, S. O., Brosnan, C. F., and Scemes, E. (2006). P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 26, 1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006
Takano, T., Tian, G. F., Peng, W., Lou, N., Libionka, W., Han, X., et al. (2006). Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 9, 260–267. doi: 10.1038/nn1623
Takeuchi, H., Jin, S., Wang, J., Zhang, G., Kawanokuchi, J., Kuno, R., et al. (2006). Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 281, 21362–21368. doi: 10.1074/jbc.M600504200
Theis, M., and Giaume, C. (2012). Connexin-based intercellular communication and astrocyte heterogeneity. Brain Res. 1487, 88–98. doi: 10.1016/j.brainres.2012.06.045
Toro, C. T., Hallak, J. E., Dunham, J. S., and Deakin, J. F. (2006). Glial fibrillary acidic protein and glutamine synthetase in subregions of prefrontal cortex in schizophrenia and mood disorder. Neurosci. Lett.404, 276–281. doi: 10.1016/j.neulet.2006.05.067
Torres, A., Wang, F., Xu, Q., Fujita, T., Dobrowolski, R., Willecke, K., et al. (2012). Extracellular Ca2 acts as a mediator of communication from neurons to glia. Sci. Signal. 5, ra8. doi: 10.1126/scisignal.2002160
Usowic, M. M., Gallo, V., and Cull-Candy, S. G. (1989). Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature 339, 380–383. doi: 10.1038/339380a0
Venance, L., Piomelli, D., Glowinski, J., and Giaume, C. (1995). Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature 376, 590–594. doi: 10.1038/376590a0
Venance, L., Stella, N., Glowinski, J., and Giaume, C. (1997). Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J. Neurosci. 17, 1981–1992.
Verkhratsky, A., and Kirchhoff, F. (2007). NMDA Receptors in glia. Neuroscientist 13, 28–37. doi: 10.1177/1073858406294270
Wallraff, A., Köhling, R., Heinemann, U., Theis, M., Willecke, K., and Steinhäuser, C. (2006). The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J. Neurosci. 26, 5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006
Wang, F., Smith, N. A., Xu, Q., Goldman, S., Peng, W., Huang, J. H., et al. (2013). Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. 33, 17404–17412. doi: 10.1523/JNEUROSCI.2178-13.2013
Wang, N., De Bock, M., Antoons, G., Gadicherla, A. K., Bol, M., Decrock, E., et al. (2012). Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res. Cardiol. 107, 304–321. doi: 10.1007/s00395-012-0304-2
Ye, Z. C., Wyeth, M. S., Baltan-Tekkok, S., and Ransom, B. R. (2003). Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 23, 3588–3596.
Zanotti, S., and Charles, A. (1997). Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release andintercellular signaling. J. Neurochem. 69, 594–602. doi: 10.1046/j.1471-4159.1997.69020594.x
Zappalá, A., Li Volti, G., Serapide, M. F., Pellitteri, R., Falchi, M., La Delia, F., et al. (2007). Expression of pannexin 2 protein in Healthy and ischemized brain of adult rats. Neuroscience 148, 653–667. doi: 10.1016/j.neuroscience.2007.06.028
Zhang, J. M., Wang, H. K., Ye, C. Q., Ge, W., Chen, Y., Jiang, Z. L., et al. (2003). ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40, 971–982. doi: 10.1016/S0896-6273(03)00717-7
Zhang, Y., and Barres, B. A. (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594. doi: 10.1016/j.conb.2010.06.005
Zhu, Y., and Kimelberg, H. K. (2004). Cellular expression of P2Y and beta-AR receptor mRNAs and proteins in freshly isolated astrocytes and tissue sections from the CA1 region of P8-12 rat hippocampus. Brain Res. Dev. Brain Res.148, 77–87. doi: 10.1016/j.devbrainres.2003.10.014
Keywords: astrocytes, hemichannel, calcium waves, tripartite synapse, connexins, brain functions
Citation: Orellana JA and Stehberg J (2014) Hemichannels: new roles in astroglial function. Front. Physiol. 5:193. doi: 10.3389/fphys.2014.00193
Received: 28 January 2014; Accepted: 07 May 2014;
Published online: 17 June 2014.
Edited by:
Mauricio Antonio Retamal, Universidad del Desarrollo, ChileReviewed by:
Christian Giaume, Collège de France, FranceMichael V. L. Bennett, Albert Einstein College of Medicine, USA
Copyright © 2014 Orellana and Stehberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jimmy Stehberg, Laboratorio de Neurobiología, Centro de Investigaciones Médicas, Facultad de Ciencias Biológicas and Facultad de Medicina, Universidad Andrés Bello, República 217, Santiago 8370146, Chile e-mail:anN0ZWhiZXJnQHVuYWIuY2w=
 Juan A. Orellana
Juan A. Orellana Jimmy Stehberg
Jimmy Stehberg