
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Physiol. , 20 December 2013
Sec. Membrane Physiology and Membrane Biophysics
Volume 4 - 2013 | https://doi.org/10.3389/fphys.2013.00380
This article is part of the Research Topic Acid-base sensing and regulation: molecular mechanisms and functional implications in health and disease View all 27 articles
Along the gastrointestinal tract a number of epithelia contribute with acid or basic secretions in order to aid digestive processes. The stomach and pancreas are the most extreme examples of acid (H+) and base (HCO−3) transporters, respectively. Nevertheless, they share the same challenges of transporting acid and bases across epithelia and effectively regulating their intracellular pH. In this review, we will make use of comparative physiology to enlighten the cellular mechanisms of pancreatic HCO−3 and fluid secretion, which is still challenging physiologists. Some of the novel transporters to consider in pancreas are the proton pumps (H+-K+-ATPases), as well as the calcium-activated K+ and Cl− channels, such as KCa3.1 and TMEM16A/ANO1. Local regulators, such as purinergic signaling, fine-tune, and coordinate pancreatic secretion. Lastly, we speculate whether dys-regulation of acid-base transport contributes to pancreatic diseases including cystic fibrosis, pancreatitis, and cancer.
In multicellular organisms the digestive system exhibits marked acid/base segmentation and gradients across the epithelia. The most extreme examples of the acid/base transporters are the stomach and the pancreas, which conduct a vectorial transport of acid/base to one side and base/acid to the other side of the epithelium (Figure 1). In the stomach, the parietal cells of the pyloric glands secrete H+ toward lumen (HCl), leaving HCO−3 to be transported into the interstitium and blood. Thus, the phenomenon of the alkaline tide, i.e., higher blood pH in connection with digestion, is well known as part of the post-prandial gastric phase secretion, which in humans is relatively small compared to animals that ingest large amounts of food at one time (Rune and Lassen, 1968; Wang et al., 2001; Niv and Fraser, 2002). In the intestinal phase of digestion, pancreatic ducts secrete HCO−3-rich fluid that contributes to alkalinization of acid chyme in duodenum. The acid generated is then transported toward the interstitium, and one would expect an acid tide, depending on ingested food and passage through the stomach (Rune and Lassen, 1968; Ashley et al., 1994).
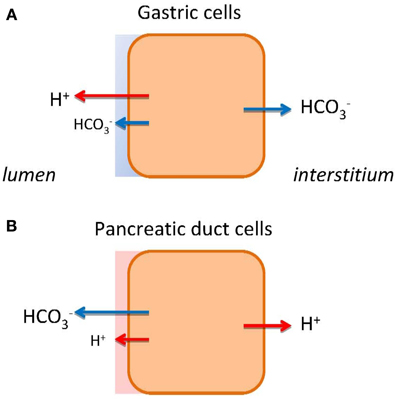
Figure 1. HCO−3 and H+ transport in gastric cells (A) and pancreatic duct cells (B). The models show schematically different types of epithelia as single cells. The transport of H+ or HCO−3 to the bulk luminal fluid is shown with large arrows. The small arrows on luminal side indicate HCO−3 and H+ secretions to the mucosal buffer zone. Flux of HCO−3 and H+ to the interstititum/blood side indicates expected alkaline or acid tides.
From these simple considerations several questions arise. Do the stomach and pancreas epithelia have some transport mechanisms in common, or do they solve the task of acid-base transport in different ways?
The molecular mechanism and regulation of stomach acid secretion is well established. In short, it involves gastric H+-K+-ATPases comprising of α1 and β subunits coded by ATP4A and ATP4B genes. These pumps are present in tubulovesicles of parietal cells and delivered to the luminal membranes in conjunction with specific K+ (KCNQ1, KCNJ15, KCNJ10) and Cl− channels (CFTR, CLIC-6, SCL26A9), and thereby resulting in HCl secretion (Sachs et al., 2007; Forte and Zhu, 2010; Chu and Schubert, 2012). Gastric acid secretion is regulated by neural, hormonal, paracrine and chemical stimuli, e.g., acetylcholine, gastrin, ghrelin, histamine. As a protection against strong acid and pepsins, the surface epithelium secretes HCO−3, mucus and other factors, forming gastric diffusion barrier (Figure 1A). The validity of the model is confirmed by well-used drugs, including proton pump inhibitors and H2-histamine receptor blockers, to curb the peptic and duodenal ulcers and reflux diseases (Sachs et al., 2010). In contrast, we do not understand the mechanism behind pancreatic alkaline (HCO−3) secretion fully. Therefore, therapeutic intervention is not possible, e.g., for cystic fibrosis patients.
Pancreas is composed of two main types of epithelia—secretory acini and excretory ducts. Acini have relatively uniform morphology. They secrete digestive enzymes, NaCl-rich fluid and various factors that contribute to signaling in down-stream ducts. Studies on normal human and rodent pancreas, stimulated by predominantly acinar agonists, e.g., cholecytokinin (CCK), result in neutral or weakly alkaline pancreatic juice (Sewell and Young, 1975; You et al., 1983; Case and Argent, 1993). However, a recent study using acinar preparation and bioimaging techniques shows that acinar secretion is acidic due to acidic zymogen granules (ZG) (Behrendorff et al., 2010), although acidity of mature ZG has been discussed (Haanes and Novak, 2010; Chu and Schubert, 2012). Nevertheless, a potential acid load from acini challenging proximal ducts has been considered (Hegyi et al., 2011a). One possible defense mechanism could be activation of ducts by acinar agonist; generally this seems not to be the case. Alternatively, paracrine agonists such as ATP released by acini could stimulate ducts by purinergic signaling (Sørensen and Novak, 2001; Novak, 2008). Lastly, pancreatic ducts might have ability to sense and react to acid/base locally. There are a number of acid/base sensors at the single cell and whole organ level (Tresguerres et al., 2010; Brown and Wagner, 2012; DeCoursey, 2013). These include acid sensitive ASIC and TRP channels, HCO−3 sensitive adenylate cyclase, pH-sensitive K+ channels, and P2X receptors. Except for the latter two, which are expressed in pancreas (see below), other candidates remain to be explored.
Pancreatic ducts comprise 5–20% of the tissue mass, depending on the species; morphologically they are different - progressing from flat centroacinar cells, cuboidal cells in intercalated, and small intralobular ducts to columnar heterogenous cells lining larger distal ducts (Kodama, 1983; Ashizawa et al., 1997; Bouwens and Pipeleers, 1998). At large, it is accepted that pancreatic ducts secrete isotonic NaHCO3 rich fluid. However, the concentration of HCO−3 is not constant; it decreases with secretory rates—a pattern that is mirrored by Cl−. The HCO−3 excretory patterns are remarkably similar between various species, providing that secretory rates are corrected for the duct mass (Figure 2A). In early studies (Bro-Rasmussen et al., 1956), it was proposed that pancreatic secretion and ionic composition is a two stage process—primary secretion and ductal modification, the so called admixture hypothesis. Another, the exchange theory, also named the salvage mechanism, states that at lower secretory rates ductal transporters are presumably not saturated and therefore, are capable of exchanging luminal HCO−3 for interstitial Cl−. This exchange phenomenon was first demonstrated on the main cat duct (Case et al., 1969). The third explanation, regarding varying HCO−3 concentrations, pertains H+ secretion from acini (see above) or ducts (see below).
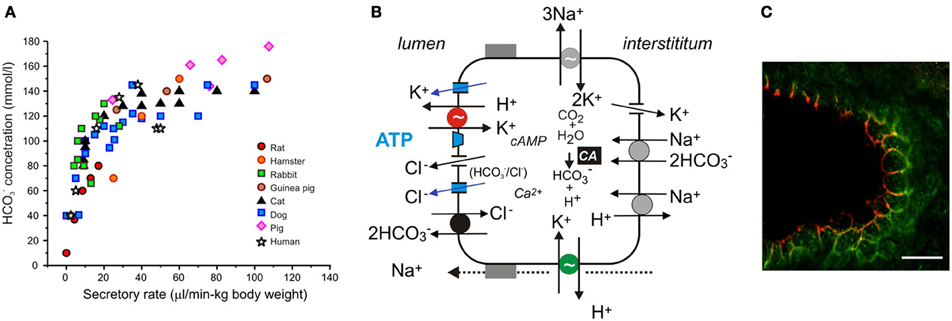
Figure 2. Acid/base transport in pancreas. (A) The relation between secretory rates and HCO−3 concentrations in pancreatic juice of various species. Secretions were stimulated by secretin and secretory rates were corrected for body weights. (B) The model of ion transport in a secreting pancreatic duct cell with novel transporters, channels and luminal purinergic signaling and receptors indicated in color and discussed in the review. Intracellular HCO−3 is derived from CO2 through the action of carbonic anhydrase (CA) and from HCO−3 uptake via the electrogenic Na+–HCO−3 cotransporter (pNBC, NBCe1). H+ is extruded at basolateral membrane by the Na+/H+ exchanger (NHE1). HCO−3 efflux across the luminal membrane is mediated by the electrogenic Cl−/HCO−3 exchanger (SLC26A6), and under certain conditions, through Cl− channels. The luminal Cl− channels are CFTR and TMEM16A (see text). There are a number of K+ channels expressed on the luminal and basolateral membranes, e.g., KCa3.1, KCa1.1, KCNQ1 (see text). The luminal and basolateral H+-K+-ATPases are indicated in red and green, and supposedly contribute to the luminal buffer zone and the H+ efflux to intersititum, respectively. Other ion channels and transporters, such as NHE3, SLC26A3, NBC3, NKCC1, and aquaporins have a differential distribution in the duct tree and for simplicity are not included in the model. (C) Immunolocalization of the gastric (red) and non-gastric (green) H+-K+ pumps in rat pancreatic duct. The bar is 20 μm. Modified from Novak et al., 2011.
The ion transport models for pancreatic ducts have been described in several recent reviews (Steward et al., 2005; Steward and Ishiguro, 2009; Lee et al., 2012; Wilschanski and Novak, 2013). The outline of the model is given in Figure 2B. The following sections will focus on novel additions to the model.
Ion channels and transporters proposed in the classical model for HCO−3 secretion rely on gradients created by the Na+/K+-ATPase (Figure 2B). However, we cannot explain formation of high HCO−3 concentrations and the fact that inhibitors of NHE1, NBC (and NKCC1), and CA are relatively ineffective in blocking secretion (Grotmol et al., 1986; Fernandez-Salazar et al., 2004). One solution is that a primary pump could be involved, such as the vacuolar type H+-ATPase (V-H+-pump), to pump H+ out to interstitium and leave HCO−3 for the luminal transport. In one study, such vacuolar H+ pump on the basolateral membrane was proposed (Villanger et al., 1995) and detected immunohistochemically (Roussa et al., 2001). Several functional studies gave contradictory findings (Zhao et al., 1994; Ishiguro et al., 1996; de Ondarza and Hootman, 1997). Taking an inspiration from gastric glands, the colon and kidney distal tubules, we considered whether pancreatic ducts express H+-K+-ATPases. Indeed, we found that rodent ducts express both the gastric and non-gastric (colonic) types H+-K+-ATPases (Novak et al., 2011). Inhibition of these with proton pump inhibitors reduced pHi recovery in response to acid loads; more importantly, they reduced secretion in isolated pancreatic ducts. Thus, these functional studies support the theory that pancreatic ducts resemble gastric glands—just working in reverse, expelling H+ toward the blood side and leaving HCO−3 for the luminal transport (Figure 1B). The immunohistochemical study showed that the H+-K+-ATPases (mainly colonic type) are localized to the basolateral membrane (Figure 2C).
However, H+-K+-ATPases, especially the gastric form, are also localized at or close to the luminal membrane (Figure 2C) (Novak et al., 2011). It seems counterintuitive to place H+ pumps on the HCO−3 secreting luminal membrane. Nevertheless, there are epithelia that are high HCO−3 secretors and yet express H+ pumps on the luminal membranes. For example, insect midgut and marine fish intestine have functional V-H+-ATPase on the luminal membranes (Wieczorek et al., 2009; Wood et al., 2010; Guffey et al., 2011). Also other epithelia, which are not high HCO−3 secretors (HCO−3 <25 mM), express various H+ pumps on the luminal membranes. For example, airway epithelia transport both base and acid, and the airway fluid layer is slightly acidic (Fischer and Widdicombe, 2006). Some studies provide evidence for the presence of bafilomycin A sensitive V-H+ pump (Inglis et al., 2003; Fischer and Widdicombe, 2006; Shan et al., 2012); other studies show that transport is sensitive to SCH28080, an inhibitor of gastric (and also non-gastric) H+-K+ pumps (Smith and Welsh, 1993; Poulsen and Machen, 1996). The non-gastric, ouabain-sensitive H+-K+-pumps were also demonstrated in some studies (Coakley et al., 2003; Krouse et al., 2004; Shan et al., 2012).
Coming back to the pancreatic luminal H+-K+ pumps, let us speculate what their function may be. They could help to defend the cell against intracellular acidification, although there is a redundancy of acid/base transporters including several NHEs, NBCs, and Cl−/HCO−3 exchangers (Figure 2B). Our proposal is—these luminal pumps are safeguarding luminal cell surface with acid secretions to protect against the bulk alkaline secretions, which at pH >8 would be caustic to cells. Thus, pancreatic ducts would have protective buffer (and mucus) zone, which is reminiscent to the buffer zone in the stomach, though achieved by H+ rather than HCO−3 secretion (Figures 1A,B). In addition, the luminal H+-K+ pumps would recirculate K+ extruded by the luminal K+ channels (Figure 2B). Lastly, luminal H+-K+ pumps in distal ducts would by virtue of H+ secretion have more impact on pancreatic juice composition at low flow rates and minor at high flow rates, thus, explaining excretory curves for HCO−3 (Figure 2A).
In addition to CFTR-dependent secretion, a number of studies showed that agonists acting via Ca2+-signaling stimulate Ca2+-activated Cl− channels (CaCC) and thus, could support duct secretion (Gray et al., 1989; Pahl and Novak, 1993; Winpenny et al., 1998; Szalmay et al., 2001; Pascua et al., 2009) (Figure 2B). The molecular identity of CaCC channels has been difficult to pinpoint [see (Duran et al., 2010)]. After suggestions of CCl-2 and bestrophins, the TMEM16/ANO family was discovered (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008), and especially TMEM16A/ANO1 became a CaCC favorite. Recent studies show that human duct cell lines express TMEM16A, which re-localizes from cytosol to the luminal membrane upon purinergic stimulation and gives rise to secretory potentials (Wang and Novak, 2013; Wang et al., 2013). In human pancreatic samples immunohistochemistry shows TMEM16A in centro-acinar and small ducts cells (Bergmann et al., 2011).
It is relevant to ask whether TMEM16A and/or Ca2+ signaling pathways lead to HCO−3 secretion. There are a few studies in support of this notion. For example, Ca2+ signaling via IRBIT stimulates NBCe1 (Shirakabe et al., 2006; Yang et al., 2009). A recent study on TMEM16A anion permeability shows that in HEK293 cell expression system and mouse salivary acinar cells the channel is directly modulated by calmodulin, which increases its HCO−3 permeability (Jung et al., 2013). This is supported by a study on ex vivo salivary glands stimulated with acetycholine, which induced production of HCO−3 rich pancreatic-like secretion when Cl− transport was inhibited (Novak and Young, 1986). Nevertheless, it cannot be excluded that there are other molecular candidates for CaCC, or that CFTR can convey part of the Ca2+-activated Cl− currents. The latter mechanism could involve Ca2+ sensitive adenylate cyclases and tyrosine kinases (Src2/Pyk complex), both of which could alter activity of CFTR, as shown for other epithelia (Billet and Hanrahan, 2013; Billet et al., 2013). Another effect at the CFTR level could be priming of some PKC isoforms that enhance CFTR activity [see (Billet and Hanrahan, 2013)]. Lastly, it is highly unlikely that Ca2+ mediated signaling stands alone, rather the two major signaling pathways of Ca2+ and cAMP/PKA act synergistically in pancreatic ducts, e.g., via IRBIT regulation of CFTR and SLC26A6 (Park et al., 2013).
The driving force for Cl− or HCO−3 exit is maintained by hyperpolarizing membrane potential created by opening of K+ channels, and GK is both present on the basolateral and luminal membranes (Novak and Greger, 1988, 1991). Equivalent-circuit analysis has shown that stimulation of luminal K+ channels contributes with at least with 10% to the total conductance. Modeling in salivary glands confirms that such ratio of luminal to basolateral K+ channels would optimize secretion without destroying the transepithelial potential and transport (Cook and Young, 1989; Almassy et al., 2012). Furthermore, luminal K+ channels could contribute to secreted K+, as pancreatic juice contains 4–8 mM K+ (Sewell and Young, 1975; Caflisch et al., 1979; Seow et al., 1991). The molecular identity of some K+ channels in pancreatic ducts is known, however, the exact localization and function remains to be verified [see (Hayashi and Novak, 2013)]. The KCa1.1 channels (maxi-K, BK, coded by KCNMA1) are present in pancreatic ducts (Hede et al., 2005; Venglovecz et al., 2011). The latter study proposes that these channels are expressed on the luminal membrane and activated by low concentrations of bile acids. However, earlier patch-clamp studies indicated that these channels were also located basolaterally (Gray et al., 1990; Hede et al., 1999). The KCa3.1 channel (IK, SK4, coded by KCNN4) was demonstrated in pancreatic ducts (Hede et al., 2005; Jung et al., 2006; Hayashi et al., 2012). Immunolocalization indicates that KCa3.1 is expressed on both membranes, though stronger on the luminal one (Figure 2B). Interestingly, the channel activator EBIO enhanced secretion potentials (Hayashi et al., 2012; Wang et al., 2013). Recent studies on pancreatic ducts offers molecular identities of several K+ channels, including KVLQT1, HERG, EAG2; Slick, and Slack (Hayashi et al., 2012), and interestingly the pH sensor TASK-2 (Fong et al., 2003). However, the function and regulation of these channels in pancreas physiology needs to be explored.
Pancreatic secretion regulated by hormonal and neural systems is well documented (Lee et al., 2012; Wilschanski and Novak, 2013). Paracrine regulation is less explored, but it is highly relevant as it allows regulation within the gland and integration of acinar and ductal responses. Pancreatic ducts can be regulated by acinar factors (trypsin, guanylin, ATP) as well as retrograde factors (bile acids) (Kulaksiz et al., 2001; Alvarez et al., 2004; Venglovecz et al., 2008; Pallagi et al., 2011; Wang and Novak, 2013). Here we concentrate on purinergic signaling and present evidence that this signaling could fine-tune and coordinate pancreatic secretion on several fronts. Pancreatic ducts express several types of purinergic receptors including members from the G-protein coupled receptor families (adenosine, P2Y) and ligand-gated ion channels (P2X receptor) families (Novak, 2008, 2011) that can potentially stimulate a variety of intracellular signaling pathways (Burnstock, 2007; Surprenant and North, 2009; Lenertz et al., 2011; Wiley et al., 2011; Bilbao et al., 2012). These receptors regulate pancreatic duct ion transport, mucin secretion, and survival of fibrogenic pancreatic stellate cells (Jung et al., 2004; Haanes et al., 2012). ATP originates from ZG where it is accumulated by the vesicular nucleotide transporter VNUT (Haanes and Novak, 2010), and in addition ATP is presumably released by nerves and ductal epithelium (Bodin and Burnstock, 2001; Novak, 2011; Burnstock and Novak, 2012). Various ecto-nucleotidases are expressed and secreted, and potentially ATP/ADP and adenosine are effective regulators of ductal functions (Sørensen et al., 2003; Kittel et al., 2004; Yegutkin et al., 2006; Burnstock and Novak, 2012).
ATP and UTP via P2 receptors have effects on intracellular Ca2+, intracellular pH, and transepithelial transport in both isolated ducts and in vivo pancreas (Ishiguro et al., 1999; Novak et al., 2010). The physiological response to nucleotides is side specific. Basolateral UTP inhibits secretion, most likely due to inhibition of KCa1.1 channels, presumably to prevent overextension of ducts. In contrast, luminal UTP/ATP application causes duct secretion and activation and Cl− and K+ channels (Hede et al., 1999; Ishiguro et al., 1999; Wang et al., 2013). In particular KCa3.1 channel activation potentiates secretion (see above). It is well documented that purinergic receptor stimulation activates CFTR, Cl−/HCO−3 exchangers and TMEM16A on the luminal membrane (Namkung et al., 2003; Wang et al., 2013). Furthermore, P2 receptors activate CaCC and CFTR interdependently and synergistically, though exact receptors and signaling pathways remain to be elucidated (see above). In addition, some effects can be due to stimulation of A2A and A2B receptors, which stimulate CFTR (Novak et al., 2008).
A number of processes in purinergic signaling are pH sensitive, and this must be of relevance in pancreatic duct lumen. For example, nucleotidase activities, CD39 and CD73 types, are stimulated at alkaline pH 8–9 (Leal et al., 2005; Rucker et al., 2008), thus, favoring conversion of ATP to adenosine in duct lumen. Furthermore, purinergic receptors are also pH sensitive. From other preparations we know that extracellular acidification enhanced the potency of UTP up to 10 fold on the rat P2Y4 but not P2Y2 receptors (Wildman et al., 2003), and the P2X2 receptors was activated by acid pH (King et al., 1996). Extracellular alkalinization enhances activity the P2X4 and P2X7 receptors (Clarke et al., 2000; Liu et al., 2009). Several types of these receptors are expressed in duct lumen including the P2Y2 and P2X7 receptors, and these enhance pancreatic secretion and integrate acini-to-duct signaling (Novak, 2008; Novak et al., 2010).
The original cellular model for pancreatic HCO−3 secretion has been supplemented with molecular identities for many ion transporters/channels. The present review challenges present concepts by including active H+ pumps in the model, and by comparing basic processes in pancreas and stomach. Furthermore, we present new additions to the model—Ca2+-activated Cl− and K+ channels, and propose that they work in synergy to regulate secretion. On the organ level, acini, and ducts integrate their function in acid/base transport and regulation, the latter exemplified by purinergic signaling. Further challenges lay in understanding dys-regulation of acid-base transport in pancreas pathophysiology. In CF patients and animal models, pancreatic juice pH decreases from values >8.1 to <6.6, and pancreas contributes to duodenal hyperacidity (Freedman et al., 2001; Uc et al., 2011) [see (Wilschanski and Novak, 2013)]. It is not clear whether the problem relates to ductal and/or acinar secretion. In acute pancreatitis, which has complex etiologies, it is now appreciated that defective pancreatic duct secretion can be the initiating factor (Lee and Muallem, 2008; Hegyi et al., 2011b). Finally, in several cancer types, various acid-base transporters and associated ion channels, such as NHE1, NBCn1, CAIX, TMEM16A, Kv10.1, and KCa3.1, change expression or function [see (Pedersen et al., 2013)]. Our knowledge about the role of acid-base transporters in pancreatic ductal adenocarcinoma clearly needs to be expanded, in order to provide potential diagnostic and therapeutic approaches.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Research projects founding basis for this review were supported by The Danish Council for Independent Research |Natural Sciences, The Lundbeck Foundation, The Novo Nordisk Foundation and The Carlsberg Foundation.
BK, big conductance K+ channel, also named KCa1.1 and maxi-K+, coded by KCNMA1; CaCC, Ca2+-activated Cl− channel, e.g., TMEM16A also known as ANO1; CA, carbonic anhydrase; CCK, cholecystokinin, CF, cystic fibrosis; [Ca2+]i, intracellular Ca2+ activity; CFTR, the cystic fibrosis transmembrane conductace regulator; EBIO, 1-ethyl-2-benzimidazolinone; GK, conductance for K+; H+-K+-ATPases or pumps, colonic type (coded by ATP12A) and gastric types (coded by ATP4A and ATP4B); IK, intermediate conductance K+ channel, also named KCa3.1; IRBIT, inositol 1,4,5-triphosphate (InsP3) receptor-binding protein released with InsP3; NBCe1 or pNBC, electrogenic Na+-HCO−3 transporter; NBCn1, electroneutral Na+-HCO3- transporter; NHE, Na+/H+ exchanger; NKCC1, Na+-K+-2Cl− cotransporter; PKA, protein kinase A; PKC, proteins kinase C; SLC26A6, electrogenic Cl−-/2HCO−3- exchanger; VNUT, vesicular nucleotide transporter, SLC17A9; V-H+-pump, vacuolar type H+-ATPase; ZG, zymogen granules.
Almassy, J., Won, J. H., Begenisich, T. B., and Yule, D. I. (2012). Apical Ca2+-activated potassium channels in mouse parotid acinar cells. J. Gen. Physiol. 139, 121–133. doi: 10.1085/jgp.201110718
Alvarez, C., Regan, J. P., Merianos, D., and Bass, B. L. (2004). Protease-activated receptor-2 regulates bicarbonate secretion by pancreatic duct cells in vitro. Surgery 136, 669–676. doi: 10.1016/j.surg.2004.01.018
Ashizawa, N., Endoh, H., Hidaka, K., Watanabe, M., and Fukumoto, S. (1997). Three-dimensional structure of the rat pancreatic duct in normal and inflammated pancreas. Microsc. Res. Tech. 37, 543–556. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<543::AID-JEMT15>3.3.CO;2-Y
Ashley, S. W., Schwarz, M., Alvarez, C., Nguyen, T. N., Vdovenko, A., and Reber, H. A. (1994). Pancreatic interstitial pH regulation: effects of secretory stimulation. Surgery 115, 503–509.
Behrendorff, N., Floetenmeyer, M., Schwiening, C., and Thorn, P. (2010). Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology 139, 1711-20, 1720.e1-5. doi: 10.1053/j.gastro.2010.07.051
Bergmann, F., Andrulis, M., Hartwig, W., Penzel, R., Gaida, M. M., Herpel, E., et al. (2011). Discovered on gastrointestinal stromal tumor 1 (DOG1) is expressed in pancreatic centroacinar cells and in solid-pseudopapillary neoplasms–novel evidence for a histogenetic relationship. Hum. Pathol. 42, 817–823. doi: 10.1016/j.humpath.2010.10.005
Bilbao, P. S., Katz, S., and Boland, R. (2012). Interaction of purinergic receptors with GPCRs, ion channels, tyrosine kinase and steroid hormone receptors orchestrates cell function. Purinergic Signal. 8, 91–103. doi: 10.1007/s11302-011-9260-9
Billet, A., and Hanrahan, J. W. (2013). The secret life of CFTR as a calcium-activated chloride channel. J. Physiol. 591, 5273–5278. doi: 10.1113/jphysiol.2013.261909
Billet, A., Luo, Y., Balghi, H., and Hanrahan, J. W. (2013). Role of tyrosine phosphorylation in the muscarinic activation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR). J. Biol. Chem. 288, 21815–21823. doi: 10.1074/jbc.M113.479360
Bodin, P., and Burnstock, G. (2001). Purinergic signaling: ATP release. Neurochem. Res. 26, 959–969. doi: 10.1023/A:1012388618693
Bouwens, L., and Pipeleers, D. G. (1998). Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 41, 629–633. doi: 10.1007/s001250050960
Bro-Rasmussen, F., Killmann, S. A., and Thaysen, J. H. (1956). The composition of pancreatic juice as compared to sweat, parotid saliva and tears. Acta Physiol. Scand. 37, 97–113. doi: 10.1111/j.1748-1716.1956.tb01346.x
Brown, D., and Wagner, C. A. (2012). Molecular mechanisms of acid-base sensing by the kidney. J. Am. Soc. Nephrol. 23, 774–780. doi: 10.1681/ASN.2012010029
Burnstock, G. (2007). Purine and pyrimidine receptors. Cell Mol. Life Sci. 64, 1471–1483. doi: 10.1007/s00018-007-6497-0
Burnstock, G., and Novak, I. (2012). Purinergic signaling in the pancreas in health and disease. J. Endocrinol. 213, 123–141. doi: 10.1530/JOE-11-0434
Caflisch, C. R., Solomon, S., and Galey, W. R. (1979). Exocrine ductal pCO2 in the rabbit pancreas. Pflugers Arch. 380, 121–125. doi: 10.1007/BF00582146
Caputo, A., Caci, E., Ferrera, L., Pedemonte, N., Barsanti, C., Sondo, E., et al. (2008). TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322, 590–594. doi: 10.1126/science.1163518
Case, R. M., and Argent, B. E. (1993). “Pancreatic duct cell secretion: control and mechanims of transport,” in The Pancreas. Biology, Pathobiology, and Diseases, eds V. L. W. Go, E. P. DiMagno, J. D. Gardner, E. Lebenthal, H. A. Reber, and G. A. Scheele (New York, NY: Raven Press), 301–350.
Case, R. M., Harper, A. A., and Scratcherd, T. (1969). The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J. Physiol. (Lond.) 201, 335–348.
Chu, S., and Schubert, M. L. (2012). Gastric secretion. Curr. Opin. Gastroenterol. 28, 587–593. doi: 10.1097/MOG.0b013e328358e5cc
Clarke, C. E., Benham, C. D., Bridges, A., George, A. R., and Meadows, H. J. (2000). Mutation of histidine 286 of the human P2X4 purinoceptor removes extracellular pH sensitivity. J. Physiol 523 (pt 3), 697–703. doi: 10.1111/j.1469-7793.2000.00697.x
Coakley, R. D., Grubb, B. R., Paradiso, A. M., Gatzy, J. T., Johnson, L. G., Kreda, S. M., et al. (2003). Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc. Natl. Acad. Sci. U.S.A. 100, 16083–16088. doi: 10.1073/pnas.2634339100
Cook, D. I., and Young, J. A. (1989). Effect of K+ channels in the apical plasma membrane on epithelial secretion based on secondary active Cl− transport. J. Membr. Biol. 110, 139–146. doi: 10.1007/BF01869469
DeCoursey, T. E. (2013). Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family. Physiol. Rev. 93, 599–652. doi: 10.1152/physrev.00011.2012
de Ondarza, J., and Hootman, S. R. (1997). Confocal microscopic analysis of intracellular pH regulation in isolated guinea pig pancreatic ducts. Am. J. Physiol. 272, G124–G134.
Duran, C., Thompson, C. H., Xiao, Q., and Hartzell, H. C. (2010). Chloride channels: often enigmatic, rarely predictable. Annu. Rev. Physiol. 72, 95–121. doi: 10.1146/annurev-physiol-021909-135811
Fernandez-Salazar, M. P., Pascua, P., Calvo, J. J., Lopez, M. A., Case, R. M., Steward, M. C., et al. (2004). Basolateral anion transport mechanisms underlying fluid secretion by mouse, rat and guinea-pig pancreatic ducts. J. Physiol. (Lond.) 556, 415–428. doi: 10.1113/jphysiol.2004.061762
Fischer, H., and Widdicombe, J. H. (2006). Mechanisms of acid and base secretion by the airway epithelium. J. Membr. Biol. 211, 139–150. doi: 10.1007/s00232-006-0861-0
Fong, P., Argent, B. E., Guggino, W. B., and Gray, M. A. (2003). Characterization of vectorial chloride transport pathways in the human pancreatic duct adenocarcinoma cell line, HPAF. Am. J. Physiol. Cell Physiol. 285, C433–C445. doi: 10.1152/ajpcell.00509.2002
Forte, J. G., and Zhu, L. (2010). Apical recycling of the gastric parietal cell H,K-ATPase. Annu. Rev. Physiol. 72, 273–296. doi: 10.1146/annurev-physiol-021909-135744
Freedman, S. D., Kern, H. F., and Scheele, G. A. (2001). Pancreatic acinar cell dysfunction in CFTR(-/-) mice is associated with impairments in luminal pH and endocytosis. Gastroenterology 121, 950–957. doi: 10.1053/gast.2001.27992
Gray, M. A., Greenwell, J. R., Garton, A. J., and Argent, B. E. (1990). Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J. Membr. Biol. 115, 203–215. doi: 10.1007/BF01868636
Gray, M. A., Harris, A., Coleman, L., Greenwell, J. R., and Argent, B. E. (1989). Two types of chloride channel on duct cells cultured from human fetal pancreas. Am. J. Physiol. 257, C240–C251.
Grotmol, T., Buanes, T., Bros, O., and Raeder, M. G. (1986). Lack of effect of amiloride, furosemide, bumetanide and triamterene on pancreatic NaHCO3 secretion in pigs. Acta Physiol. Scand. 126, 593–600. doi: 10.1111/j.1748-1716.1986.tb07860.x
Guffey, S., Esbaugh, A., and Grosell, M. (2011). Regulation of apical H+-ATPase activity and intestinal HCO−3 secretion in marine fish osmoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1682–R1691. doi: 10.1152/ajpregu.00059.2011
Haanes, K. A., and Novak, I. (2010). ATP storage and uptake by isolated pancreatic zymogen granules. Biochem. J. 429, 303–311. doi: 10.1042/BJ20091337
Haanes, K. A., Schwab, A., and Novak, I. (2012). The P2X7 receptor supports both life and death in fibrogenic pancreatic stellate cells. PLoS ONE 7:e51164. doi: 10.1371/journal.pone.0051164
Hayashi, M., and Novak, I. (2013). Molecular basis of potassium channels in pancreatic duct epithelial cells. Channels (Austin) 7, 1–10. doi: 10.4161/chan.26100
Hayashi, M., Wang, J., Hede, S. E., and Novak, I. (2012). An intermediate-conductance Ca2+-activated K+ channel is important for secretion in pancreatic duct cells. Am. J. Physiol. Cell Physiol. 303, C151–C159. doi: 10.1152/ajpcell.00089.2012
Hede, S. E., Amstrup, J., Christoffersen, B. C., and Novak, I. (1999). Purinoceptors evoke different electrophysiological responses in pancreatic ducts. P2Y inhibits K+ conductance, and P2X stimulates cation conductance. J. Biol. Chem. 274, 31784–31791. doi: 10.1074/jbc.274.45.31784
Hede, S. E., Amstrup, J., Klaerke, D. A., and Novak, I. (2005). P2Y2 and P2Y4 receptors regulate pancreatic Ca2+-activated K+ channels differently. Pflugers Arch. 450, 429–436. doi: 10.1007/s00424-005-1433-3
Hegyi, P., Maleth, J., Venglovecz, V., and Rakonczay, Z. Jr. (2011a). Pancreatic ductal bicarbonate secretion: challenge of the acinar Acid load. Front. Physiol. 2:36. doi: 10.3389/fphys.2011.00036
Hegyi, P., Pandol, S., Venglovecz, V., and Rakonczay, Z. Jr. (2011b). The acinar-ductal tango in the pathogenesis of acute pancreatitis. Gut 60, 544–552. doi: 10.1136/gut.2010.218461
Inglis, S. K., Wilson, S. M., and Olver, R. E. (2003). Secretion of acid and base equivalents by intact distal airways. Am. J. Physiol. Lung. Cell Mol. Physiol. 284, L855–L862. doi: 10.1152/ajplung.00348.2002
Ishiguro, H., Naruse, S., Kitagawa, M., Hayakawa, T., Case, R. M., and Steward, M. C. (1999). Luminal ATP stimulates fluid and HCO−3 secretion in guinea-pig pancreatic duct. J. Physiol. (Lond) 519, 551–558. doi: 10.1111/j.1469-7793.1999.0551m.x
Ishiguro, H., Steward, M. C., Wilson, R. W., and Case, R. M. (1996). Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J. Physiol. (Lond.) 495 (pt 1), 179–191.
Jung, J., Nam, J. H., Park, H. W., Oh, U., Yoon, J. H., and Lee, M. G. (2013). Dynamic modulation of ANO1/TMEM16A. Proc. Natl. Acad. Sci. U.S.A. 110, 360–365. doi: 10.1073/pnas.1211594110
Jung, S. R., Kim, K., Hille, B., Nguyen, T. D., and Koh, D. S. (2006). Pattern of Ca2+ increase determines the type of secretory mechanism activated in dog pancreatic duct epithelial cells. J. Physiol. 576, 163–178. doi: 10.1113/jphysiol.2006.114876
Jung, S. R., Kim, M. H., Hille, B., Nguyen, T. D., and Koh, D. S. (2004). Regulation of exocytosis by purinergic receptors in pancreatic duct epithelial cells. Am. J. Physiol. Cell Physiol. 286, C573–C579. doi: 10.1152/ajpcell.00350.2003
King, B. F., Ziganshina, L. E., Pintor, J., and Burnstock, G. (1996). Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br. J. Pharmacol. 117, 1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x
Kittel, A., Pelletier, J., Bigonnesse, F., Guckelberger, O., Kordas, K., Braun, N., et al. (2004). Localization of Nucleoside Triphosphate Diphosphohydrolase-1 (NTPDase1) and NTPDase2 in Pancreas and Salivary Gland. J. Histochem. Cytochem. 52, 861–871. doi: 10.1369/jhc.3A6167.2004
Kodama, T. (1983). A light and electron microscopic study on the pancreatic ductal system. Acta Pathol. Jpn. 33, 297–321.
Krouse, M. E., Talbott, J. F., Lee, M. M., Joo, N. S., and Wine, J. J. (2004). Acid and base secretion in the Calu-3 model of human serous cells. Am. J. Physiol. Lung. Cell Mol. Physiol. 287, L1274–L1283. doi: 10.1152/ajplung.00036.2004
Kulaksiz, H., Schmid, A., Honscheid, M., Eissele, R., Klempnauer, J., and Cetin, Y. (2001). Guanylin in the human pancreas: a novel luminocrine regulatory pathway of electrolyte secretion via cGMP and CFTR in the ductal system. Histochem. Cell Biol. 115, 131–145. doi: 10.1007/s004180000244
Leal, D. B., Streher, C. A., Neu, T. N., Bittencourt, F. P., Leal, C. A., da Silva, J. E., et al. (2005). Characterization of NTPDase (NTPDase1; ecto-apyrase; ecto-diphosphohydrolase; CD39; EC 3.6.1.5) activity in human lymphocytes. Biochim. Biophys. Acta 1721, 9–15. doi: 10.1016/j.bbagen.2004.09.006
Lee, M. G., and Muallem, S. (2008). Pancreatitis: the neglected duct. Gut 57, 1037–1039. doi: 10.1136/gut.2008.150961
Lee, M. G., Ohana, E., Park, H. W., Yang, D., and Muallem, S. (2012). Molecular mechanism of pancreatic and salivary gland fluid and HCO−3 secretion. Physiol. Rev. 92, 39–74. doi: 10.1152/physrev.00011.2011
Lenertz, L. Y., Gavala, M. L., Zhu, Y., and Bertics, P. J. (2011). Transcriptional control mechanisms associated with the nucleotide receptor P2X7, a critical regulator of immunologic, osteogenic, and neurologic functions. Immunol. Res. 50, 22–38. doi: 10.1007/s12026-011-8203-4
Liu, X., Ma, W., Surprenant, A., and Jiang, L. H. (2009). Identification of the amino acid residues in the extracellular domain of rat P2X(7) receptor involved in functional inhibition by acidic pH. Br. J. Pharmacol. 156, 135–142. doi: 10.1111/j.1476-5381.2008.00002.x
Namkung, W., Lee, J. A., Ahn, W., Han, W., Kwon, S. W., Ahn, D. S., et al. (2003). Ca2+ activates cystic fibrosis transmembrane conductance regulator- and Cl− -dependent HCO3 transport in pancreatic duct cells. J. Biol. Chem. 278, 200–207. doi: 10.1074/jbc.M207199200
Niv, Y., and Fraser, G. M. (2002). The alkaline tide phenomenon. J. Clin. Gastroenterol. 35, 5–8. doi: 10.1097/00004836-200207000-00003
Novak, I. (2008). Purinergic receptors in the endocrine and exocrine pancreas. Purinergic Signal. 4, 237–253. doi: 10.1007/s11302-007-9087-6
Novak, I. (2011). Purinergic signaling in epithelial ion transport—regulation of secretion and absorption. Acta Physiologica 202, 501–522. doi: 10.1111/j.1748-1716.2010.02225.x
Novak, I., and Greger, R. (1988). Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflügers Arch. 411, 58–68. doi: 10.1007/BF00581647
Novak, I., and Greger, R. (1991). Effect of bicarbonate on potassium conductance of isolated perfused rat pancreatic ducts. Pflügers Arch. 419, 76–83. doi: 10.1007/BF00373750
Novak, I., Hede, S. E., and Hansen, M. R. (2008). Adenosine receptors in rat and human pancreatic ducts stimulate chloride transport. Pflugers Arch. 456, 437–447. doi: 10.1007/s00424-007-0403-3
Novak, I., Jans, I. M., and Wohlfahrt, L. (2010). Effect of P2X7 receptor knockout on exocrine secretion of pancreas, salivary glands and lacrimal glands. J. Physiol. (Lond) 588(pt 18), 3615–3627. doi: 10.1113/jphysiol.2010.190017
Novak, I., Wang, J., Henriksen, K. L., Haanes, K. A., Krabbe, S., Nitschke, R., et al. (2011). Pancreatic bicarbonate secretion involves two proton pumps. J. Biol. Chem. 286, 280–289. doi: 10.1074/jbc.M110.136382
Novak, I., and Young, J. A. (1986). Two independent anion transport systems in rabbit mandibular salivary glands. Pflugers Arch. 407, 649–656. doi: 10.1007/BF00582647
Pahl, C., and Novak, I. (1993). Effect of vasoactive intestinal peptide, carbachol and other agonists on cell membrane voltage of pancreatic duct cells. Pflügers Arch. 424, 315–320. doi: 10.1007/BF00384358
Pallagi, P., Venglovecz, V., Rakonczay, Z. Jr., Borka, K., Korompay, A., Ozsvari, B., et al. (2011). Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl channels and luminal anion exchangers. Gastroenterology 141, 2228–2239. doi: 10.1053/j.gastro.2011.08.039
Park, S., Shcheynikov, N., Hong, J. H., Zheng, C., Suh, S. H., Kawaai, K., et al. (2013). Irbit mediates synergy between Ca2+ and cAMP signaling pathways during epithelial transport in mice. Gastroenterology 145, 232–241. doi: 10.1053/j.gastro.2013.03.047
Pascua, P., Garcia, M., Fernandez-Salazar, M. P., Hernandez-Lorenzo, M. P., Calvo, J. J., Colledge, W. H., et al. (2009). Ducts isolated from the pancreas of CFTR-null mice secrete fluid. Pflugers Arch. 459, 203–214. doi: 10.1007/s00424-009-0704-9
Pedersen, S. F., Hoffmann, E. K., and Novak, I. (2013). Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 4, 233. doi: 10.3389/fphys.2013.00233
Poulsen, J. H., and Machen, T. E. (1996). HCO3-dependent pHi regulation in tracheal epithelial cells. Pflugers Arch. 432, 546–554. doi: 10.1007/s004240050168
Roussa, E., Alper, S. L., and Thevenod, F. (2001). Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J. Histochem. Cytochem. 49, 463–474. doi: 10.1177/002215540104900406
Rucker, B., Almeida, M. E., Libermann, T. A., Zerbini, L. F., Wink, M. R., and Sarkis, J. J. (2008). E-NTPDases and ecto-5'-nucleotidase expression profile in rat heart left ventricle and the extracellular nucleotide hydrolysis by their nerve terminal endings. Life Sci. 82, 477–486. doi: 10.1016/j.lfs.2007.12.003
Rune, S. J., and Lassen, N. A. (1968). Diurnal variations in the acid-base balance of blood. Scand. J. Clin. Lab. Invest. 22, 151–156. doi: 10.3109/00365516809160961
Sachs, G., Shin, J. M., and Hunt, R. (2010). Novel approaches to inhibition of gastric acid secretion. Curr. Gastroenterol. Rep. 12, 437–447. doi: 10.1007/s11894-010-0149-5
Sachs, G., Shin, J. M., Vagin, O., Lambrecht, N., Yakubov, I., and Munson, K. (2007). The gastric H,K ATPase as a drug target: past, present, and future. J. Clin. Gastroenterol. 41 (Suppl. 2), S226–S242. doi: 10.1097/MCG.0b013e31803233b7
Schroeder, B. C., Cheng, T., Jan, Y. N., and Jan, L. Y. (2008). Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134, 1019–1029. doi: 10.1016/j.cell.2008.09.003
Seow, K. T. F. P., Case, R. M., and Young, J. A. (1991). Pancreatic secretion by the anaesthetized rabbit in response to secretin, cholecystokinin, and carbachol. Pancreas 6, 385–391. doi: 10.1097/00006676-199107000-00002
Sewell, W. A., and Young, J. A. (1975). Secretion of electrolytes by the pancreas of the anaesthetized rat. J. Physiol. (Lond.) 252, 379–396.
Shan, J., Liao, J., Huang, J., Robert, R., Palmer, M. L., Fahrenkrug, S. C., et al. (2012). Bicarbonate-dependent chloride transport drives fluid secretion by the human airway epithelial cell line Calu-3. J. Physiol. 590, 5273–5297. doi: 10.1113/jphysiol.2012.236893
Shirakabe, K., Priori, G., Yamada, H., Ando, H., Horita, S., Fujita, T., et al. (2006). IRBIT, an inositol 1,4,5-trisphosphate receptor-binding protein, specifically binds to and activates pancreas-type Na+/HCO−3 cotransporter 1 (pNBC1). Proc. Natl. Acad. Sci. U.S.A. 103, 9542–9547. doi: 10.1073/pnas.0602250103
Smith, J. J., and Welsh, M. J. (1993). Fluid and electrolyte transport by cultured human airway epithelia. J. Clin. Invest. 91, 1590–1597. doi: 10.1172/JCI116365
Sørensen, C. E., Amstrup, J., Rasmussen, H. N., Ankorina-Stark, I., and Novak, I. (2003). Rat pancreas secretes particulate ecto-nucleotidase CD39.J. Physiol. (Lond.) 551, 881–892. doi: 10.1113/jphysiol.2003.049411
Sørensen, C. E., and Novak, I. (2001). Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J. Biol. Chem. 276, 32925–32932. doi: 10.1074/jbc.M103313200
Steward, M. C., and Ishiguro, H. (2009). Molecular and cellular regulation of pancreatic duct cell function. Curr. Opin. Gastroenterol. 25, 447–453. doi: 10.1097/MOG.0b013e32832e06ce
Steward, M. C., Ishiguro, H., and Case, R. M. (2005). Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol. 67, 377–409. doi: 10.1146/annurev.physiol.67.031103.153247
Surprenant, A., and North, R. A. (2009). Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359. doi: 10.1146/annurev.physiol.70.113006.100630
Szalmay, G., Varga, G., Kajiyama, F., Yang, X. S., Lang, T. F., Case, R. M., et al. (2001). Bicarbonate and fluid secretion evoked by cholecystokinin, bombesin and acetylcholine in isolated guinea-pig pancreatic ducts. J. Physiol. (Lond.) 535, 795–807. doi: 10.1111/j.1469-7793.2001.00795.x
Tresguerres, M., Buck, J., and Levin, L. R. (2010). Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch. 460, 953–964. doi: 10.1007/s00424-010-0865-6
Uc, A., Stoltz, D. A., Ludwig, P., Pezzulo, A., Griffin, M., bu-El-Haija, M., et al. (2011). Pancreatic and biliary secretion differ in cystic fibrosis and wild-type pigs. J. Cystic Fibrosis 10, S69. doi: 10.1016/S1569-1993(11)60285-3
Venglovecz, V., Hegyi, P., Rakonczay, Z. Jr., Tiszlavicz, L., Nardi, A., Grunnet, M., et al. (2011). Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut 60, 361–369. doi: 10.1136/gut.2010.214213
Venglovecz, V., Rakonczay, Z. Jr., Ozsvari, B., Takacs, T., Lonovics, J., Varro, A., et al. (2008). Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut 57, 1102–1112. doi: 10.1136/gut.2007.134361
Villanger, O., Veel, T., and Raeder, M. G. (1995). Secretin causes H+/HCO−3 secretion from pig pancreatic ductules by vacuolar-type H+-adenosine triphosphatase. Gastroenterology 108, 850–859. doi: 10.1016/0016-5085(95)90460-3
Wang, J., Haanes, K. A., and Novak, I. (2013). Purinergic regulation of CFTR and Ca2+-activated Cl− channels and K+ channels in human pancreatic duct epithelium. Am. J. Physiol. Cell Physiol. 304, C673–C684. doi: 10.1152/ajpcell.00196.2012
Wang, J., and Novak, I. (2013). Ion transport in human pancreatic duct epithelium, Capan-1 cells, is regulated by secretin, VIP, acetylcholine, and purinergic receptors. Pancreas 42, 452–460. doi: 10.1097/MPA.0b013e318264c302
Wang, T., Busk, M., and Overgaard, J. (2001). The respiratory consequences of feeding in amphibians and reptiles. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 128, 535–549. doi: 10.1016/S1095-6433(00)00334-2
Wieczorek, H., Beyenbach, K. W., Huss, M., and Vitavska, O. (2009). Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 212, 1611–1619. doi: 10.1242/jeb.030007
Wildman, S. S., Unwin, R. J., and King, B. F. (2003). Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br. J. Pharmacol. 140, 1177–1186. doi: 10.1038/sj.bjp.0705544
Wiley, J. S., Sluyter, R., Gu, B. J., Stokes, L., and Fuller, S. J. (2011). The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78, 321–332. doi: 10.1111/j.1399-0039.2011.01780.x
Wilschanski, M., and Novak, I. (2013). The cystic fibrosis of exocrine pancreas. Cold Spring Harb. Perspect. Med. 3, a009746. doi: 10.1101/cshperspect.a009746
Winpenny, J. P., Harris, A., Hollingsworth, M. A., Argent, B. E., and Gray, M. A. (1998). Calcium-activated chloride conductance in a pancreatic adenocarcinoma cell line of ductal origin (HPAF) and in freshly isolated human pancreatic duct cells. Pflugers Arch. 435, 796–803. doi: 10.1007/s004240050586
Wood, C. M., Bucking, C., and Grosell, M. (2010). Acid-base responses to feeding and intestinal Cl− uptake in freshwater- and seawater-acclimated killifish, Fundulus heteroclitus, an agastric euryhaline teleost. J. Exp. Biol. 213, 2681–2692. doi: 10.1242/jeb.039164
Yang, D., Shcheynikov, N., Zeng, W., Ohana, E., So, I., Ando, H., et al. (2009). IRBIT coordinates epithelial fluid and HCO−3 secretion by stimulating the transporters pNBC1 and CFTR in the murine pancreatic duct. J. Clin. Invest. 119, 193–202. doi: 10.1172/JCI36983
Yang, Y. D., Cho, H., Koo, J. Y., Tak, M. H., Cho, Y., Shim, W. S., et al. (2008). TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215. doi: 10.1038/nature07313
Yegutkin, G. G., Samburski, S. S., Jalkalen, S., and Novak, I. (2006). ATP-consuming and ATP-generating enzymes secreted by pancreas. J. Biol. Chem. 281, 29441–29447. doi: 10.1074/jbc.M602480200
You, C. H., Rominger, J. M., and Chey, W. Y. (1983). Potentiation effect of cholecystokinin-octapeptide on pancreatic bicarbonate secretion stimulated by a physiologic dose of secretin in humans. Gastroenterology 85, 40–45.
Keywords: bicarbonate transport, proton transport, H+-K+-ATPase, KCa3.1, IK, TMEM16A, ANO1, pancreatic duct
Citation: Novak I, Haanes KA and Wang J (2013) Acid-base transport in pancreas—new challenges. Front. Physiol. 4:380. doi: 10.3389/fphys.2013.00380
Received: 07 October 2013; Paper pending published: 23 October 2013;
Accepted: 04 December 2013; Published online: 20 December 2013.
Edited by:
Ebbe Boedtkjer, Aarhus University, DenmarkReviewed by:
Martin Diener, University Giessen, GermanyCopyright © 2013 Novak, Haanes and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivana Novak, Molecular Integrative Physiology, Department of Biology, University of Copenhagen, August Krogh Building, Universitetsparken 13, Copenhagen Ø, DK 2100, Denmark e-mail:aW5vdmFrQGJpby5rdS5kaw==
†Present address: Kristian A. Haanes, Department of Clinical Experimental Research, Glostrup Research Institute, Copenhagen University Hospital, Glostrup, Denmark;
Jing Wang, National Institute for Viral Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.