- 1 Millennium Nucleus for Regenerative Biology, P. Catholic University of Chile, Santiago, Chile
- 2 NeuroUnion Biomedical Foundation, Santiago, Chile
Schwann cells (SCs) are the glial component of the peripheral nervous system, with essential roles during development and maintenance of axons, as well as during regenerative processes after nerve injury. SCs increase conduction velocities by myelinating axons, regulate synaptic activity at presynaptic nerve terminals and are a source of trophic factors to neurons. Thus, development and maintenance of peripheral nerves are crucially dependent on local signaling between SCs and axons. In addition to the classic mechanisms of intercellular signaling, the possibility of communication through secreted vesicles has been poorly explored to date. Interesting recent findings suggest the occurrence of lateral transfer mediated by vesicles from glial cells to axons that could have important roles in axonal growth and axonal regeneration. Here, we review the role of vesicular transfer from SCs to axons and propose the advantages of this means in supporting neuronal and axonal maintenance and regeneration after nerve damage.
Introduction
Originally, glial cells were considered as a sort of glue that filled the space between neurons, which is largely a passive role. In time, this view changed substantially. In the peripheral nervous system (PNS), Schwann cells (SCs) were recognized to regulate a wide variety of ongoing functions of axons (Mirsky and Jessen, 1999). It is well known that the myelin sheath, by increasing the operational resistance of the axolemma, greatly increases the velocity of the nerve impulse, which is a passive effect (Hartline and Colman, 2007).
A number of observations indicate that the cellular biology of axons is regulated by SCs. In the axolemma of unmyelinated fibers, sodium and potassium channels exist side by side (Garrido et al., 2003) but in axons surrounded by myelin, the axolemma under the sheath is poor in sodium and rich in potassium channels while the converse occurs at the nodal axolemma, where sodium channels accumulate (Salzer et al., 2008; Feinberg et al., 2010). This indicates that SCs regulate at a molecular scale the local organization of axons, thus regulating the axonal phenotype.
Nerve injury and its ensuing repair illustrate the mutual regulation of axons and SCs. Waller (1850) established that nerve section is followed by degeneration of the distal domain while SCs evolve to a dedifferentiated state. Nerve repair re-establishes the original condition (Jessen and Mirsky, 2008). After nerve injury, elongation of axons was shown to be prevented by SCs as long as they remained differentiated distal to injury (Tapia et al., 1995; Court and Alvarez, 2000), which strongly suggests that nerve repair proceeds in close interaction with the SC and not commanded by the cell body (Bray and Aguayo, 1974; Court and Alvarez, 2005). When SCs in a segment of an intact nerve are treated with a protease inhibitor, which has been shown to induce SC dedifferentiation (Alvarez et al., 1992, 2000; Tapia et al., 1995), the associated axon extends sprouts in that segment in spite of being surrounded by SCs, i.e., branches arise in an uninterrupted axon. This indicates that the axon has a growth program repressed by the differentiated SC (Court and Alvarez, 2005). Together, these phenomena illustrate that the SC locally affects the underlying axon, from its passive electrical properties, to organization of the axolemma, and even complex cellular programs embodied in the axoplasm.
We will consider now the first step of regulatory mechanisms between cells that operate on a local basis. Adhesion molecules are an important and well characterized mechanism that allows contact-mediated signaling between cells. Another mechanism involves extracellular free ligands that are produced by a cell and operate on a very short range, from its site of release to its receptor in the target cell. These two mechanisms share an important feature, namely, the machinery that produces the response belongs entirely to the target cell. A third regulatory mechanism has emerged in which a cell produces vesicles that are taken up by the target cell and the cargo is incorporated into the recipient cytoplasm (Simons and Raposo, 2009). This mechanism opens a new dimension to the intercellular interaction in that the recipient cytoplasm may contain an incomplete machinery that is completed by molecules of the donor cell upon their release from the vesicle. Our review focuses on the regulation of axons by SCs mediated by secreted vesicles and proposes the advantages of this means of communication in supporting neuronal and axonal maintenance and regeneration after nerve damage.
Evidences for Vesicular Transfer between SCs and Axons
That proteins may enter the cytoplasm from the outside is an old notion. About fifty years ago, it was established that some proteins of the oocyte yolk of the mosquito Aedes aegypti were synthesized in the gut, moved to the ovary, and were taken up from the extracellular space via pinocytic vesicles to be stored essentially as a reservoir of amino acids for the embryo (Roth and Porter, 1964). In the nervous system, glia-to-axon transfer of protein was proposed about forty years ago. The giant axon of the squid was incubated with radiolabeled amino acids and labeled proteins were recovered from its axoplasm (Lasek et al., 1974). However, the notion of transcellular transfer emerged under the assumption that axons were unable to synthesize proteins, but since this assumption was wrong as axons do synthesize protein (Koenig and Giuditta, 1999; Alvarez et al., 2000; Donnelly et al., 2010; Gumy et al., 2010), the notion of glia-to-axon transfer of protein awaited further experimental support.
Around the 1980s, the groups of Stahl and Johnstone provided evidence to support that vesicles can mediate the release of proteins during reticulocytes maturation (Harding et al., 1983; Pan et al., 1985). These vesicles named exosomes were contained within multivesicular endosomes whose fusion with the plasma membrane was followed by exosome secretion (Johnstone et al., 1987; Simons and Raposo, 2009; Thery et al., 2009). In turn, vesicles originated after the evagination of the plasma membrane were named microvesicles (Cocucci et al., 2009; Thery et al., 2009). That was the beginning of a new era in cell communication, the release of membrane vesicles.
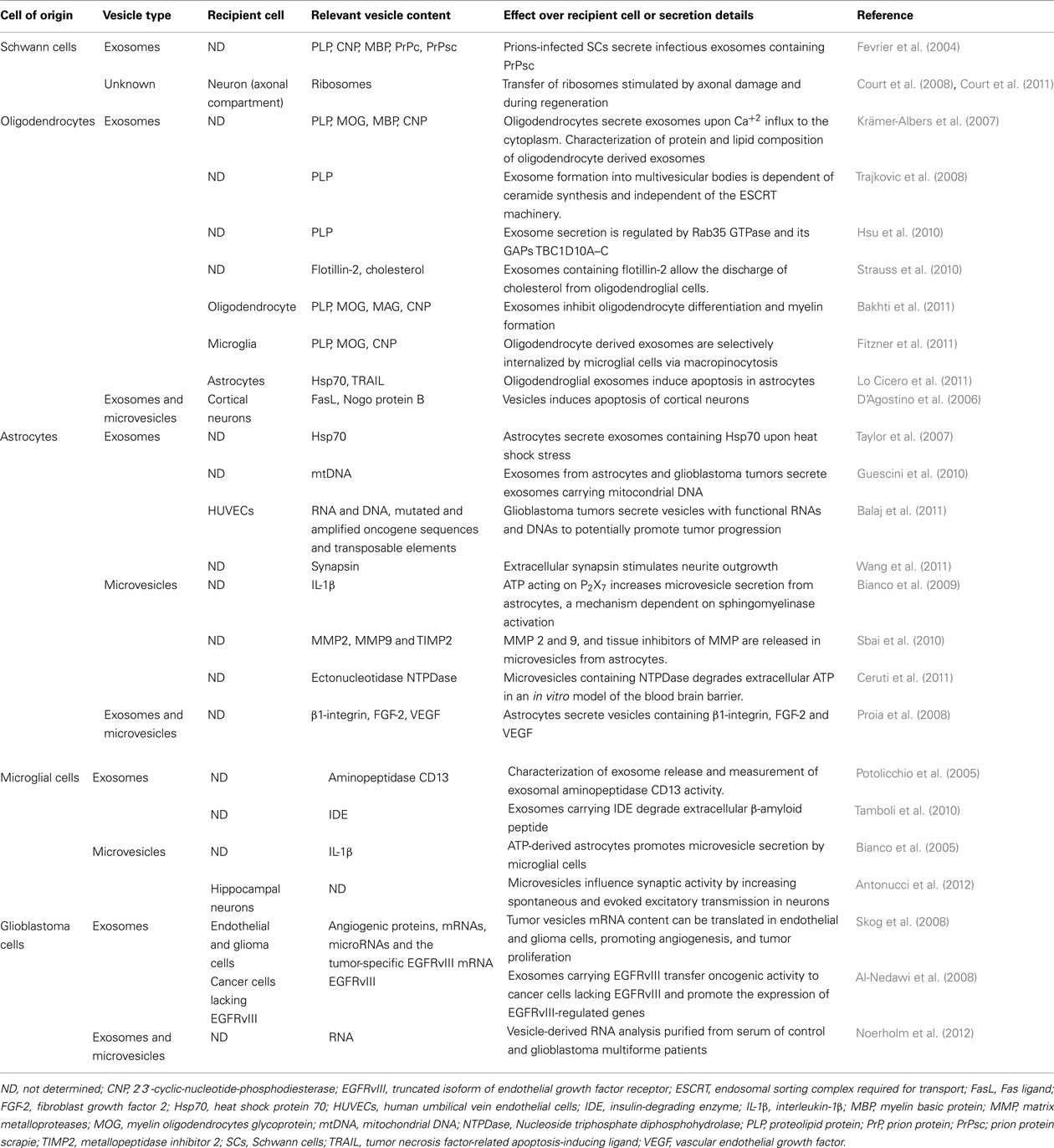
Based on these antecedents, transfer of macromolecules from SC to axons was reconsidered, this time mediated by vesicles. Thus, Buchheit and Tytell (1992) described transfer of fluorescently labeled vesicles from SCs to squid giant axons. They proposed that these vesicles carried the proteins previously thought to be transferred directly from the SC to the axon, such as heat shock protein (Hsp) 70 (Tytell et al., 1986) – a protein also carried in exosomes secreted by reticulocytes (Davis et al., 1986) – but they did neither confirmed these possibilities nor their functional significance. Nowadays, exosomes and microvesicles have been described in glial cells from the central nervous system (CNS, see Table 1), although in the PNS the evidence is scarce. Hsp70 is present in exosomes secreted from a SC cell line (Fevrier et al., 2004), SC primary cultures (Lopez-Verrilli M. A. and Court F. A., unpublished results) and in exosomes secreted by glial cells from the CNS, including astrocytes, oligodendrocytes, and microglia (Potolicchio et al., 2005; Krämer-Albers et al., 2007; Taylor et al., 2007). It remains to be investigated whether vesicular transfer of Hsp70 to axons confers neuroprotection to stress stimuli and neurodegenerative disorders.
Schwann cells are essential during regenerative processes after nerve injury, not only by secreting growth factors (Madduri and Gander, 2010; Quintes et al., 2010) but also by supplying components of the protein synthesis machinery to axons. Court et al. (2008, 2011) demonstrated in vivo the transfer of ribosomes from SCs to axons after axonal damage as well as during axonal regeneration. Electron microscope images showed ribosomes in the axoplasm but also within vesicles surrounded by two or multiple membranes. Interestingly, even multimembrane vesicles open to the axoplasm were still partially loaded with ribosomes and abundant free ribosomes seemingly discharged in the vicinity, suggesting that SC-derived vesicles were secreted and internalized in axons by endocytosis. Nevertheless, the molecular mechanism for ribosomal transfer after axonal damage and during axonal regeneration has not been disclosed yet.
Considering that SC exosomes diameter varies between 50 and 120 nm (Lopez-Verrilli M. A., and Court F. A., unpublished results), only a small amount of ribosomes could be transported within each exosome. On the other hand, microvesicles are larger vesicles (up to 1 μm; Cocucci et al., 2009) and might even transport polyribosomes. Since mRNAs can be stored in a dormant state in the distal axon until needed (Yoo et al., 2010), the transfer of mRNA-containing ribosomes from SC to axon could supply transcript for storage and translation in response to acute stimuli (e.g., nerve damage) or the transfer be triggered by the stimuli itself. In addition, vesicular transfer from SCs would accelerate the arrival of ribosomes to the axon, compared to ribosomes derived from the neuronal cell body (Twiss and Fainzilber, 2009).
In the dark side of vesicular transfer, SCs have been shown to secrete exosomes containing pathogenic prions upon cell infection in vitro, therefore prion secretion via SC-derived exosomes may spread these pathogenic proteins from the PNS to the CNS (Fevrier et al., 2004). Prions are misfolded proteins that act as infectious agents and cause neurodegenerative diseases (Weissmann et al., 2011). Furthermore, pathological cell–cell communication by endogenous vesicular vectors could be one of the mechanistic explanations for non-cell autonomous processes playing critical roles in neurodegenerative diseases (Garden and La Spada, 2012).
Summing up, SCs might provide by means of secreted vesicles, an efficient, specific, and highly localized support for axons. Vesicles interact specifically with the target cell (Rana and Zoller, 2011) supplying many copies and many kinds of macromolecules, which might allows SCs to locally regulate axonal functions without direct involvement of the neuronal cell body. Together, the evidence suggests that machineries of a given cytoplasm may be incomplete requiring the contribution of a neighboring cell to become operative (Court et al., 2008, 2011). From another point of view, the phenotype of a cell may require the contribution of an external genome to supply the missing messenger RNAs. SCs contain mRNAs coding for neurofilament proteins, which they barely translate (Roberson et al., 1992), and its transfer within vesicles could be instrumental for protein homeostasis in axons. In brief, the transfer of vesicles and their cargo of protein and RNAs open a novel mode for intercellular interaction, and a broad avenue of research.
Potential Roles and Functions of Secreted Vesicles in the PNS
Vesicle secretion as a means to supply components to a target cell offers a number of advantages considering the SC and axon dynamics, e.g., during myelination and regeneration conditions.
In the myelinated nerve fiber (Figure 1A), vesicles could be released from microvilli domains to the node of Ranvier and transfer scaffolding proteins required for the proper node formation, such as actin, tubulin, cofilin, and ankyrin-G (Krämer-Albers et al., 2007; Valadi et al., 2007). Vesicles could be secreted along the Schmidt-Lantermann incisures cytoplasm channels across the myelin sheath or paranodal domains directly to the axoplasm, providing macromolecules in a temporally and spatially regulated fashion. Both exosomes and microvesicles deliver not only proteins but also microRNAs and mRNAs that can be translated into recipient cells (Ratajczak et al., 2006; Valadi et al., 2007). In fact, elongation factors needed for mRNA translation have been found in exosomes from oligodendrocytes and microglial cells (Potolicchio et al., 2005; Krämer-Albers et al., 2007).
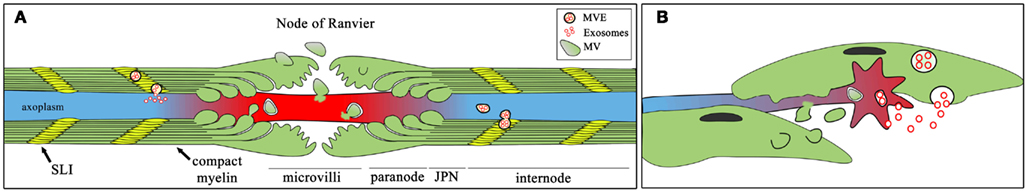
Figure 1. Possible routes and conditions for SC to axons transfer of vesicles. Schematic representation of possible routes of exosomal (red circles) and microvesicle (larger green ovoids) transfer between SCs and axons in a myelinated fiber (A) and in an axonal growth cones during axonal regeneration (B). Exosomes are contained within multivesicular endosomes (MVE) in the secreting cell, and then can move to the axon through cytoplasmic-rich region in SCs, including Schmidt-Lantermann incisures [SLI, yellow regions in (A)] or paranodal domains of myelinating fibers (A) or can be released close to the growth cone by dedifferentiated SC (B). Microvesicles, in turn, are generated from the evagination of SC plasma membrane and they can fuse or be internalized by axons.
Exosomes and microvesicles may actively participate in processes activated after nerve damage, when SCs dedifferentiate and begin to proliferate (Figure 1B). This scenario is quite complex since axons degenerate distal to the injury, while in the proximal stump, regeneration takes place (Coleman, 2005; Twiss and van Minnen, 2006). In these conditions, SCs could secrete vesicles containing mRNAs and microRNAs to negatively regulate myelination and stimulate proliferation, as observed for glial tumor cells (Skog et al., 2008). In addition, SCs-secreted vesicles could sustain protein synthesis in regenerating axons (Court et al., 2011), independently from axonal mRNA synthesized in the neuronal nuclei, which needs to be transported a long way before translation, or even provide guiding clues to regenerating axons, such as the axonal guidance protein Wnt, which has been detected in exosomes from motor neurons (Zou, 2004; Korkut et al., 2009).
If SC-derived vesicles are demonstrated to have functional roles in axonal regeneration, SC differentiation, or other processes crucial for neural tissue regeneration, these vesicles can be used for therapeutic purposes by using their endogenous potentials or by loading them with specific transcript or proteins by modifying glial cells, which can be easily manipulated in vitro (Schmitte et al., 2010; Zhang et al., 2010). It has been demonstrated that neuronal-targeted exosomes obtained from genetically modified dendritic cells in vitro can be electroporated with specific siRNA, and after intravenous injection, they specifically knock-down their target gene in brain neurons (Alvarez-Erviti et al., 2011). Vesicle-mediated drug delivery promises to overcome important challenges, such as delivery of drugs across impermeable biological barriers and using patient-derived cells to obtain tolerogenic vesicles.
Concluding Remarks
In this review we presented evidence for SC to axon communication via secreted vesicles and highlighted the functional role this process may have in the maintenance of peripheral axons and during regeneration. Increasing evidence is suggesting that axons have the ability to respond to a challenge autonomously from the cell body albeit under SCs regulation (Alvarez et al., 2000; Court and Alvarez, 2005). Since axons are of any length up to several meters, this anatomy clearly poses logistic problems. The evolutionary solution may be that SC packs the requisite components in a vesicle to convey its cargo to the axoplasm. We propose that vesicles secreted by SCs and transferred to axons are a major mechanism by which SCs locally support axonal maintenance and regeneration after nerve damage. We hope that the study of the processes will enrich our understanding of the cellular biology of the nervous system.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jaime Alvarez for critical reading of the manuscript. This work was supported by grants from FONDECYT no. 1110987 and no. 3110014 and Millenium Nucleus no. P-07-011-F.
References
Al-Nedawi, K., Meehan, B., Micallef, J., Lhotak, V., May, L., Guha, A., and Rak, J. (2008). Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10, 619–624.
Alvarez, J., Giuditta, A., and Koenig, E. (2000). Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog. Neurobiol. 62, 1–62.
Alvarez, J., Moreno, R. D., Llanos, O., Inestrosa, N. C., Brandan, E., Colby, T., and Esch, F. S. (1992). Axonal sprouting induced in the sciatic nerve by the amyloid precursor protein (APP) and other antiproteases. Neurosci. Lett. 144, 130–134.
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. (2011). Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345.
Antonucci, F., Turola, E., Riganti, L., Caleo, M., Gabrielli, M., Perrotta, C., Novellino, L., Clementi, E., Giussani, P., Viani, P., Matteoli, M., and Verderio, C. (2012). Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240.
Bakhti, M., Winter, C., and Simons, M. (2011). Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286, 787–796.
Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O., and Skog, J. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2, 180.
Bianco, F., Perrotta, C., Novellino, L., Francolini, M., Riganti, L., Menna, E., Saglietti, L., Schuchman, E. H., Furlan, R., Clementi, E., Matteoli, M., and Verderio, C. (2009). Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 28, 1043–1054.
Bianco, F., Pravettoni, E., Colombo, A., Schenk, U., Moller, T., Matteoli, M., and Verderio, C. (2005). Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 174, 7268–7277.
Bray, G. M., and Aguayo, A. J. (1974). Regeneration of peripheral unmyelinated nerves. Fate of the axonal sprouts which develop after injury. J. Anat. 117, 517–529.
Buchheit, T. E., and Tytell, M. (1992). Transfer of molecules from glia to axon in the squid may be mediated by glial vesicles. J. Neurobiol. 23, 217–230.
Ceruti, S., Colombo, L., Magni, G., Vigano, F., Boccazzi, M., Deli, M. A., Sperlagh, B., Abbracchio, M. P., and Kittel, A. (2011). Oxygen-glucose deprivation increases the enzymatic activity and the microvesicle-mediated release of ectonucleotidases in the cells composing the blood-brain barrier. Neurochem. Int. 59, 259–271.
Cocucci, E., Racchetti, G., and Meldolesi, J. (2009). Shedding microvesicles: artefacts no more. Trends Cell Biol. 19, 43–51.
Coleman, M. (2005). Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 6, 889–898.
Court, F., and Alvarez, J. (2000). Nerve regeneration in Wld(s) mice is normalized by actinomycin D. Brain Res. 867, 1–8.
Court, F. A., and Alvarez, J. (2005). Local regulation of the axonal phenotype, a case of merotrophism. Biol. Res. 38, 365–374.
Court, F. A., Hendriks, W. T., Macgillavry, H. D., Alvarez, J., and Van Minnen, J. (2008). Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 28, 11024–11029.
Court, F. A., Midha, R., Cisterna, B. A., Grochmal, J., Shakhbazau, A., Hendriks, W. T., and Van Minnen, J. (2011). Morphological evidence for a transport of ribosomes from Schwann cells to regenerating axons. Glia 59, 1529–1539.
D’agostino, S., Salamone, M., Di Liegro, I., and Vittorelli, M. L. (2006). Membrane vesicles shed by oligodendroglioma cells induce neuronal apoptosis. Int. J. Oncol. 29, 1075–1085.
Davis, J. Q., Dansereau, D., Johnstone, R. M., and Bennett, V. (1986). Selective externalization of an ATP-binding protein structurally related to the clathrin-uncoating ATPase/heat shock protein in vesicles containing terminal transferrin receptors during reticulocyte maturation. J. Biol. Chem. 261, 15368–15371.
Donnelly, C. J., Fainzilber, M., and Twiss, J. L. (2010). Subcellular communication through RNA transport and localized protein synthesis. Traffic 11, 1498–1505.
Feinberg, K., Eshed-Eisenbach, Y., Frechter, S., Amor, V., Salomon, D., Sabanay, H., Dupree, J. L., Grumet, M., Brophy, P. J., Shrager, P., and Peles, E. (2010). A glial signal consisting of gliomedin and NrCAM clusters axonal Na+ channels during the formation of nodes of Ranvier. Neuron 65, 490–502.
Fevrier, B., Vilette, D., Archer, F., Loew, D., Faigle, W., Vidal, M., Laude, H., and Raposo, G. (2004). Cells release prions in association with exosomes. Proc. Natl. Acad. Sci. U.S.A. 101, 9683–9688.
Fitzner, D., Schnaars, M., Van Rossum, D., Krishnamoorthy, G., Dibaj, P., Bakhti, M., Regen, T., Hanisch, U. K., and Simons, M. (2011). Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458.
Garden, G. A., and La Spada, A. R. (2012). Intercellular (mis)communication in neurodegenerative disease. Neuron 73, 886–901.
Garrido, J. J., Fernandes, F., Moussif, A., Fache, M. P., Giraud, P., and Dargent, B. (2003). Dynamic compartmentalization of the voltage-gated sodium channels in axons. Biol. Cell 95, 437–445.
Guescini, M., Genedani, S., Stocchi, V., and Agnati, L. F. (2010). Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 117, 1–4.
Gumy, L. F., Tan, C. L., and Fawcett, J. W. (2010). The role of local protein synthesis and degradation in axon regeneration. Exp. Neurol. 223, 28–37.
Harding, C., Heuser, J., and Stahl, P. (1983). Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 97, 329–339.
Hartline, D. K., and Colman, D. R. (2007). Rapid conduction and the evolution of giant axons and myelinated fibers. Curr. Biol. 17, R29–R35.
Hsu, C., Morohashi, Y., Yoshimura, S., Manrique-Hoyos, N., Jung, S., Lauterbach, M. A., Bakhti, M., Gronborg, M., Mobius, W., Rhee, J., Barr, F. A., and Simons, M. (2010). Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232.
Jessen, K. R., and Mirsky, R. (2008). Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia 56, 1552–1565.
Johnstone, R. M., Adam, M., Hammond, J. R., Orr, L., and Turbide, C. (1987). Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420.
Koenig, E., and Giuditta, A. (1999). Protein-synthesizing machinery in the axon compartment. Neuroscience 89, 5–15.
Korkut, C., Ataman, B., Ramachandran, P., Ashley, J., Barria, R., Gherbesi, N., and Budnik, V. (2009). Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404.
Krämer-Albers, E. M., Bretz, N., Tenzer, S., Winterstein, C., Mobius, W., Berger, H., Nave, K. A., Schild, H., and Trotter, J. (2007). Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics. Clin. Appl. 1, 1446–1461.
Lasek, R. J., Gainer, H., and Przybylski, R. J. (1974). Transfer of newly synthesized proteins from Schwann cells to the squid giant axon. Proc. Natl. Acad. Sci. U.S.A. 71, 1188–1192.
Lo Cicero, A., Schiera, G., Proia, P., Saladino, P., Savettieri, G., Di Liegro, C. M., and Di Liegro, I. (2011). Oligodendroglioma cells shed microvesicles which contain TRAIL as well as molecular chaperones and induce cell death in astrocytes. Int. J. Oncol. 39, 1353–1357.
Madduri, S., and Gander, B. (2010). Schwann cell delivery of neurotrophic factors for peripheral nerve regeneration. J. Peripher. Nerv. Syst. 15, 93–103.
Noerholm, M., Balaj, L., Limperg, T., Salehi, A., Zhu, L. D., Hochberg, F. H., Breakefield, X. O., Carter, B. S., and Skog, J. (2012). RNA expression patterns in serum microvesicles from patients with glioblastoma multiforme and controls. BMC Cancer 12:22. doi: 10.1186/1471-2407-12-22
Pan, B. T., Teng, K., Wu, C., Adam, M., and Johnstone, R. M. (1985). Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101, 942–948.
Potolicchio, I., Carven, G. J., Xu, X., Stipp, C., Riese, R. J., Stern, L. J., and Santambrogio, L. (2005). Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 175, 2237–2243.
Proia, P., Schiera, G., Mineo, M., Ingrassia, A. M., Santoro, G., Savettieri, G., and Di Liegro, I. (2008). Astrocytes shed extracellular vesicles that contain fibroblast growth factor-2 and vascular endothelial growth factor. Int. J. Mol. Med. 21, 63–67.
Quintes, S., Goebbels, S., Saher, G., Schwab, M. H., and Nave, K. A. (2010). Neuron-glia signaling and the protection of axon function by Schwann cells. J. Peripher. Nerv. Syst. 15, 10–16.
Rana, S., and Zoller, M. (2011). Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem. Soc. Trans. 39, 559–562.
Ratajczak, J., Miekus, K., Kucia, M., Zhang, J., Reca, R., Dvorak, P., and Ratajczak, M. Z. (2006). Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 20, 847–856.
Roberson, M. D., Toews, A. D., Goodrum, J. F., and Morell, P. (1992). Neurofilament and tubulin mRNA expression in Schwann cells. J. Neurosci. Res. 33, 156–162.
Roth, T. F., and Porter, K. R. (1964). Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. L. J. Cell Biol. 20, 313–332.
Salzer, J. L., Brophy, P. J., and Peles, E. (2008). Molecular domains of myelinated axons in the peripheral nervous system. Glia 56, 1532–1540.
Sbai, O., Ould-Yahoui, A., Ferhat, L., Gueye, Y., Bernard, A., Charrat, E., Mehanna, A., Risso, J. J., Chauvin, J. P., Fenouillet, E., Rivera, S., and Khrestchatisky, M. (2010). Differential vesicular distribution and trafficking of MMP-2, MMP-9, and their inhibitors in astrocytes. Glia 58, 344–366.
Schmitte, R., Tipold, A., Stein, V. M., Schenk, H., Flieshardt, C., Grothe, C., and Haastert, K. (2010). Genetically modified canine Schwann cells – in vitro and in vivo evaluation of their suitability for peripheral nerve tissue engineering. J. Neurosci. Methods 186, 202–208.
Simons, M., and Raposo, G. (2009). Exosomes – vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581.
Skog, J., Wurdinger, T., Van Rijn, S., Meijer, D. H., Gainche, L., Sena-Esteves, M., Curry, W. T. Jr., Carter, B. S., Krichevsky, A. M., and Breakefield, X. O. (2008). Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476.
Strauss, K., Goebel, C., Runz, H., Mobius, W., Weiss, S., Feussner, I., Simons, M., and Schneider, A. (2010). Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J. Biol. Chem. 285, 26279–26288.
Tamboli, I.Y., Barth, E., Christian, L., Siepmann, M., Kumar, S., Singh, S., Tolksdorf, K., Heneka, M.T., Lutjohann, D., Wunderlich, P., and Walter, J. (2010). Statins promote the degradation of extracellular amyloid β-peptide by microglia via stimulation of exosome-associated insulin-degrading enzyme (IDE) secretion. J. Biol. Chem. 285, 37405–37414.
Tapia, M., Inestrosa, N. C., and Alvarez, J. (1995). Early axonal regeneration: repression by Schwann cells and a protease? Exp. Neurol. 131, 124–132.
Taylor, A. R., Robinson, M. B., Gifondorwa, D. J., Tytell, M., and Milligan, C. E. (2007). Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev. Neurobiol. 67, 1815–1829.
Thery, C., Ostrowski, M., and Segura, E. (2009). Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593.
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., Schwille, P., Brugger, B., and Simons, M. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247.
Twiss, J. L., and Fainzilber, M. (2009). Ribosomes in axons – scrounging from the neighbors? Trends Cell Biol. 19, 236–243.
Twiss, J. L., and van Minnen, J. (2006). New insights into neuronal regeneration: the role of axonal protein synthesis in pathfinding and axonal extension. J. Neurotrauma 23, 295–308.
Tytell, M., Greenberg, S. G., and Lasek, R. J. (1986). Heat shock-like protein is transferred from glia to axon. Brain Res. 363, 161–164.
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659.
Waller, A. (1850). Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. 140, 423–429.
Wang, S., Cesca, F., Loers, G., Schweizer, M., Buck, F., Benfenati, F., Schachner, M., and Kleene, R. (2011). Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia- derived exosomes. J. Neurosci. 31, 7275–7290.
Weissmann, C., Li, J., Mahal, S. P., and Browning, S. (2011). Prions on the move. EMBO Rep. 12, 1109–1117.
Yoo, S., Van Niekerk, E. A., Merianda, T. T., and Twiss, J. L. (2010). Dynamics of axonal mRNA transport and implications for peripheral nerve regeneration. Exp. Neurol. 223, 19–27.
Zhang, J., Zhao, F., Wu, G., Li, Y., and Jin, X. (2010). Functional and histological improvement of the injured spinal cord following transplantation of Schwann cells transfected with NRG1 gene. Anat. Rec. (Hoboken) 293, 1933–1946.
Keywords: Schwann cell, axon, vesicular transfer, exosomes, microvesicles, axonal regeneration
Citation: Lopez-Verrilli MA and Court FA (2012) Transfer of vesicles from Schwann cells to axons: a novel mechanism of communication in the peripheral nervous system. Front. Physio. 3:205. doi: 10.3389/fphys.2012.00205
Received: 28 March 2012; Accepted: 23 May 2012;
Published online: 13 June 2012.
Edited by:
Claudia Verderio, Institute of Neuroscience, ItalyReviewed by:
Dandan Sun, University of Pittsburgh, USACarla Taveggia, San Raffaele Scientific Institute, Italy
Copyright: © 2012 Lopez-Verrilli and Court. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: M. Alejandra Lopez-Verrilli and Felipe A. Court, Department of Physiology, Faculty of Biology, Pontificia Universidad Católica de Chile, Av. B. O’Higgins 340/Casilla 114-D, Santiago 8331150, Chile. e-mail:ZmNvdXJ0QGJpby5wdWMuY2w=;YWxlamFuZGRyYUBnbWFpbC5jb20=