- Department of Physiology, Midwestern University, Downers Grove, IL, USA
Pioneering studies by Per Scholander indicated that the diving response consists of reflexly induced apnea, bradycardia and an alteration of blood flow that maintains perfusion of the heart and brain. More recently field physiological studies have shown that many marine animals can adjust cardiorespiratory aspects of their diving response depending upon the behavioral situation. This could suggest that the very labile heart rate during diving is under direct cortical control. However, the final control of autonomic nervous system functioning resides within the brainstem and not the cortex. Many physiologists regard the brain as a “black box” where important neuronal functioning occurs, but the complexity of such functioning leaves systematic investigation a daunting task. As a consequence the central control of the diving response has been under-investigated. Thus, to further advance the field of diving physiology by understanding its central neuronal control, it would be first necessary to understand the reflex circuitry that exists within the brainstem of diving animals. To do this will require an appropriate animal model. In this review, two animals, the muskrat and rat, will be offered as animal models to investigate the central aspects of the diving response. Firstly, although these rodents are not marine animals, natural histories indicate that both animals can and do exploit aquatic environments. Secondly, physiological recordings during natural and simulated diving indicate that both animals possess the same basic physiological responses to underwater submersion that occur in marine animals. Thirdly, the size and ease of housing of both animals makes them attractive laboratory research animals. Finally, the enormous amount of scientific literature regarding rodent brainstem autonomic control mechanisms, and the availability of brain atlases, makes these animals ideal choices to study the central control of the mammalian diving response.
Introduction
The physiological and behavioral adaptations of marine animals are amazing and allow these animals to survive and thrive in their aquatic environment. The pioneering work by Per Scholander and colleagues such as Laurence Irving, revolutionized the field of diving physiology in the 1930s and 1940s (Irving, 1934, 1939; Scholander, 1940). In these studies the basic physiological responses to underwater submersion were investigated primarily by studying diving animals in a laboratory setting. Scholander and his colleagues described how the diving response consists of reflexly induced apnea, bradycardia and an alteration in blood flow that limits flow to non-exercising muscles while maintaining flow to the heart and brain.
The field of diving physiology was revolutionized again in the 1970s and 1980s, this time by Gerald Kooyman and his colleagues, who took physiology to the field by using time-depth recorders (TDRs), data loggers, and, more recently, critter-cams. What has became exceedingly apparent from these studies is that many marine animals, especially when they are in their natural environment, can exert a cortical control over their autonomic nervous system (ANS) and can adjust the cardiorespiratory aspects of their diving response, depending upon the situation and their behavioral response (Kooyman, 1989; Butler and Jones, 1997). Diving heart rate can include both anticipatory submersion bradycardia and anticipatory re-emersion tachycardia (Jones et al., 1973; Thompson and Fedak, 1993). This could suggest that marine mammals have direct suprabulbar control over their cardiovascular system (Ramirez et al., 2007). However, while it is true that cortical and sub-cortical regions of the brain can provide a modulatory afferent input, the final control of ANS functioning resides within the brainstem and not the cortex.
Many physiologists regard the brain as a “black box,” where important neuronal functioning occurs but the complexity of such functioning leaves systematic investigation a daunting task. As a consequence the brainstem control of the diving response has been under-investigated. Thus, to further advance the field of diving physiology, and to really understand how diving animals have such a labile diving response, it would be first necessary to understand the neuronal circuitry that exists within the brainstem of diving animals. Once the brainstem circuitry has been described, then it will be possible to determine how cortical afferent signals in marine animals can modify the basic autonomic reflex. However, to do this will require an appropriate animal model.
A difficulty in studying the brainstem control of the diving response has a lot to do with the animals being studied. It is obvious that conducting neurophysiological studies in and under the open ocean has numerous logistical problems. Thus it makes sense to utilize an appropriate animal model for the problem being investigated, as some animals could facilitate the study of central control of cardiorespiratory functioning while others would make it more difficult. Indeed, Krogh’s(1929) principle has been a recurrent theme in comparative physiology: for every research question there will be some animal of choice on which the problem can be most conveniently studied. Scholander himself used a variety of animals in his investigations that best suited his research question.
Semi-aquatic species that spend part of their lives in and around water and part of their lives on land are amazing animals as they exploit two separate environments: the underwater world and terrestrial world. Just because these animals spend only part of their lives in an aquatic environment should not exclude them from being considered “diving animals.” Thus, to engage in land, and laboratory, based investigations of the central control of the diving response, semi-aquatic animals could be ideal animal models. In this review, two animals, the muskrat and rat, will be offered as animal models to investigate the central aspects of the diving response. Although these rodents certainly cannot be considered marine animals, natural histories indicate that both the muskrat and rat can and do exploit aquatic environments. Additionally, both the muskrat and rat possess the same basic physiological responses to underwater submersion that occur in marine animals. Indeed, in his classic 1940 monograph, Scholander used rats to show that during diving blood flow to non-exercising muscles is decreased (Scholander, 1940). To illustrate that the muskrat and rat are ideal models for investigating the central control of the diving response, the physiological responses to natural and simulated diving, as well as responses from anesthetized animals used in physiological investigations, will be reviewed.
Muskrats
Natural Diving History
Muskrats (Ondatra zibethicus), the only species in the genus Ondatra, are small (approximately 1 kg) semi-aquatic rodents that are common in marshes and other wet-land areas from the tropics to the Arctic. They are native to North America, but have been introduced to parts of Europe, Asia, and South America. They spend much of their time in the water, are good swimmers (Dagg and Windsor, 1972; Fish, 1983, 1984), and can remain submerged for 12 min (Irving, 1939; MacArthur, 1984b; Signore and Jones, 1995). Thus on a per weight basis, muskrats can be included among the best of the breath-hold divers. Since muskrats do not store food for the winter, their foraging activity continues year-round, often under frozen ponds and lakes (MacArthur, 1978, 1979b, 1980; MacArthur and Aleksiuk, 1979). The muskrat once was an important fur-bearing animal, but is considered by many to be a pest because its burrowing causes damages to dikes and levees. Because they are non-hibernating, muskrats are readily available in their natural environment throughout the entire year, which can be an advantage for scientific research studies (Aleksiuk and Frolinger, 1971).
Field studies in Manitoba Canada using radiotracking devices indicate that muskrats have seasonal activity patterns (MacArthur, 1979b, 1980). In the summer there is a bimodal activity pattern, with major activity peaks occurring between sunset and sunrise. This nocturnal activity may correlate with cooler nighttime temperatures that could prevent potential heat stress during very warm daytime temperatures (MacArthur, 1979b, 1980). Additionally, in summer months muskrats tend to live in burrows or open nests that provide a cool microclimate (MacArthur and Aleksiuk, 1979). In the winter activity is more diurnal, with activity occurring in the late afternoon and early evening when daily temperatures reach their peak (MacArthur, 1979b, 1980). In winter months muskrats tend to live in lodges that provide a microclimate that may be 20°C warmer than external air temperature (MacArthur and Aleksiuk, 1979). Nest chambers in these frozen winter lodges may house up to five adult muskrats (MacArthur and Aleksiuk, 1979), and considering that muskrats may spend 13–14 h/day resting in a winter lodge (MacArthur, 1980), accumulation of CO2 and depletion of O2 could pose potential respiratory problems. Indeed, microenvironment gas measurements indicate that winter lodge CO2 levels can reach as high as 10% and O2 levels can reach as low as 18% (MacArthur, 1984b). Laboratory studies have shown that muskrats are generally quite tolerant of increased CO2 levels (Irving, 1938; MacArthur, 1984b, 1986b), although breathing 10% CO2 can significantly depress oxygen consumption (MacArthur, 1986b,c). However, whether muskrats have decreased CO2 chemoreceptor sensitivity as an adaptation to diving or to communal living in burrows and lodges is still unknown (MacArthur, 1984b).
Field studies indicate there is a 41.7% increase in oxygen storage capacity in winter-acclimatized muskrats compared with summer-acclimatized muskrats (MacArthur, 1990). The increased oxygen storage capacity of 35.7 ml O2/kg is accompanied by a 17 s increase in aerobic dive limit (ADL), to 57.9 s, which allows muskrats to dive up to 13 m further in their wintertime ice-covered environment (MacArthur, 1990). Winter adaptations also include significant increases in hematocrit, hemoglobin concentration, blood volume, blood oxygen capacity, and skeletal muscle myoglobin content (Aleksiuk and Frolinger, 1971; MacArthur, 1984c, 1990). In muskrats, blood comprises the major oxygen storage compartment, accounting for 57 and 65% of total oxygen stores in summer and winter, respectively (MacArthur, 1990). The average under-ice swimming speed of muskrats is 0.76 ± 0.04 m/s, although they can reach peak-burst speeds of 1.27 m/s during escape dives in response to human disturbance (MacArthur, 1992). In comparison, muskrats diving through a maze in the laboratory have an underwater swimming speed of 0.45 m/s (MacArthur, 1992). Field observations indicate that 86.5% of dives are within the ADL of muskrats (MacArthur, 1992), but under-ice transit dives lasting up to 96 s and escape dives lasting 91 ± 8 s (range 64–184 s) have been recorded (MacArthur and Karpan, 1989).
The diving patterns of muskrats in their natural environment have not yet been recorded. However TDRs were recently used with another small semi-aquatic mammal, the American mink, diving in shallow rivers in England (Hays et al., 2007). This study shows that the diving behavior of small free-living animals that dive only a few centimeters below the surface can be monitored, and provides the feasibility of using a TDR with species such as the muskrat. Muskrats spend the majority of their time in or near lodges and feeding shelters, usually forage within 5–10 m of a lodge or push-up, and rarely move further than 150 m from their primary dwelling lodge (MacArthur, 1978, 1980; MacArthur and Aleksiuk, 1979). This restricted geographical range could facilitate the use of biologging to gather information regarding the diving behavior and physiology of free-ranging undisturbed muskrats in their natural environment (Rutz and Hays, 2009).
Suitability as Laboratory Research Animals
Within the controlled environment of the lab, detailed physiological investigations can more easily be accomplished than during field studies. Since muskrats are expert divers, their physiological adaptations to underwater submergence are of inherent interest. It is therefore fortuitous that muskrats can be easily maintained in conventional laboratory animal facilities (Nagel and Kemble, 1974; Doyle et al., 1988), and that they adjust easily to captivity (MacArthur, 1979a). Additionally, muskrats can be easily live-trapped and transported to the lab. It is advisable, however, to obtain prior authorization from conservations agencies before trapping operations begin (Nagel and Kemble, 1974). Although muskrats can be housed in standard stainless steel cages (Doyle et al., 1988), they can also be housed in simulated pond microhabitats complete with natural foliage similar to that of a marsh microhabitat (Fish, 1983; MacArthur, 1986c; MacArthur and Karpan, 1989). From a practical standpoint, the small size of muskrats eliminates the need for extensive and/or expensive housing facilities, like those required for marine mammals, and diving tanks for muskrats can be constructed using fiberglass-lined plywood. Muskrats housed in this controlled laboratory environment have been used extensively in experiments investigating the cardiovascular, respiratory, metabolic, and behavioral responses during simulated diving. Additionally, the brains of muskrats are of a relatively uniform size and possess cytoarchitectural features comparable to other mammals (Doyle et al., 1988). Thus the preparation of a brainstem atlas allows accurate stereotaxic targeting of brainstem structures in the muskrat (Panneton and Watson, 1991).
Physiological Responses during Simulated Diving in the Laboratory
Diving response
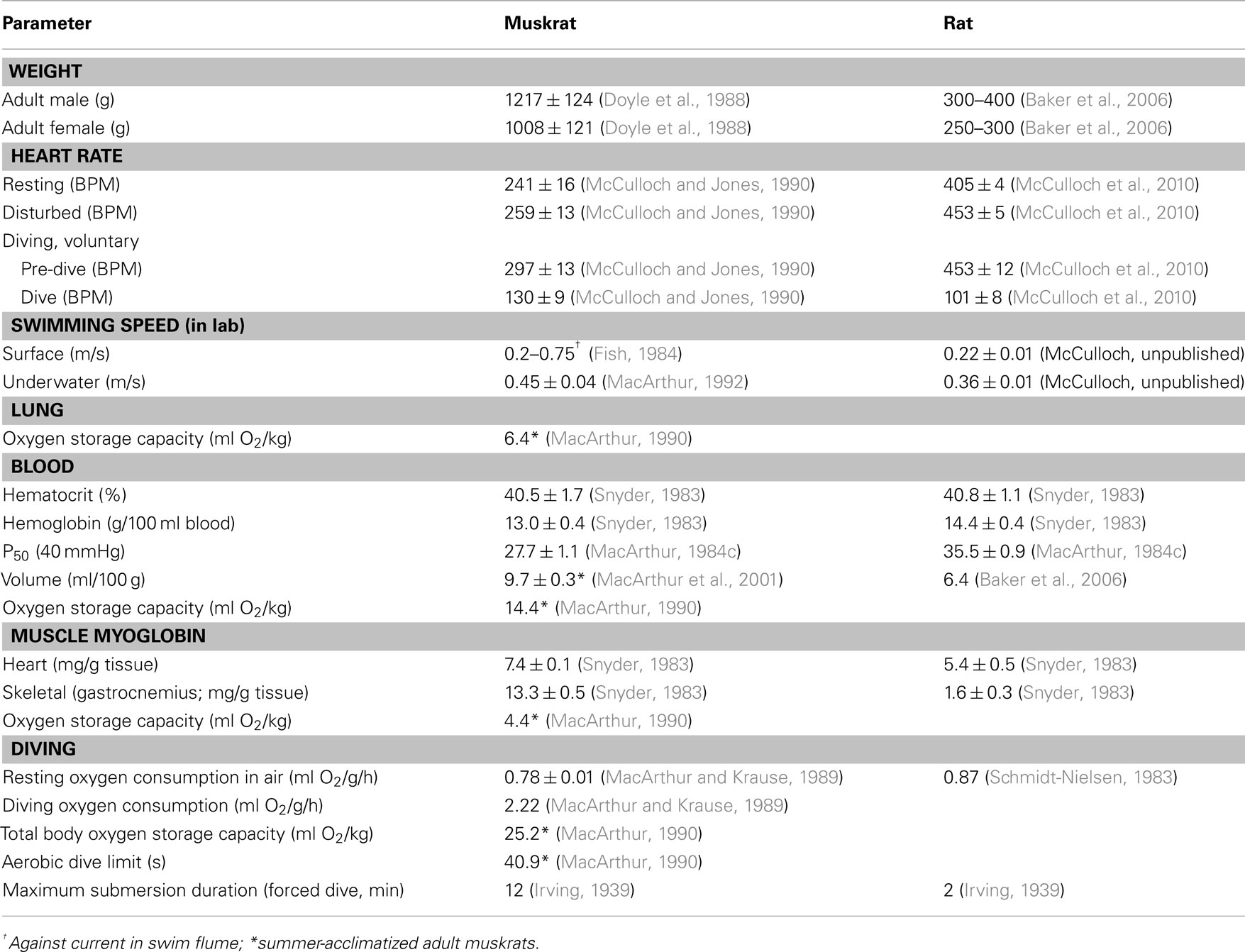
Several researchers have recorded the cardiac response of muskrats during simulated voluntary diving in laboratory facilities. In studies using ECG electrodes with trailing wire connections, the heart rate of unrestrained voluntarily diving muskrats decreases from approximately 315 to 50 BPM within 1–2 s of submergence, and then falls to approximately 30 BPM after 20–40 s (Drummond and Jones, 1979; Jones et al., 1982). The first cardiac interval after submersion is usually the longest, and upon resurfacing heart rate increases to the pre-dive level within 5 s (Drummond and Jones, 1979). Qualitatively similar results have also been found using implantable heart rate transmitters (Gilbert and Gofton, 1982; MacArthur and Karpan, 1989; McCulloch and Jones, 1990; Signore and Jones, 1995, 1996; Hindle et al., 2006; Shereshkov et al., 2006; Figure 1). However, although all data indicate that an immediate and substantial bradycardia accompanies every voluntary dive in muskrats, the extent of the bradycardia can vary with the nature of the dive.
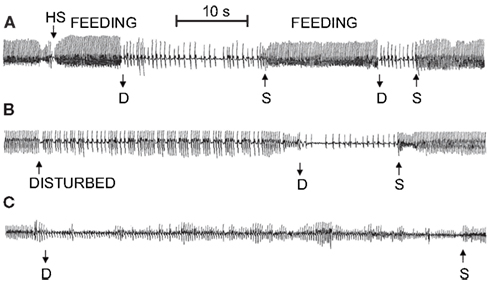
Figure 1. Sample ECG recordings for free diving muskrats in the microhabitat simulation during (A) foraging activity, (B) following disturbance by the investigator, and (C) 30 min following intramuscular injection of atropine sulfate. In (B), animal was disturbed by tapping on the side of the lodge. D, dive; S, surface; HS, head submersion while floating on the surface; feeding, period when animal was consuming aquatic vegetation while floating in push-up chamber (From MacArthur and Karpan, 1989).
Under a range of simulated field conditions in the laboratory, there is a progressively greater decrease in heart rate with foraging, exploratory, escape, and forced dives (MacArthur and Karpan, 1989; McCulloch and Jones, 1990; Figure 2). Presumably this progression is associated with increasing degrees of stress experienced by the muskrats. Dive heart rate is highest during voluntary dives (130 ± 9 BPM; 44% of pre-dive heart rate), is lower during escape dives precipitated by human investigators (95 ± 18 BPM; 35% of pre-dive heart rate), and is lowest during forced dives (74 ± 7 BPM; 27% of pre-dive heart rate; McCulloch and Jones, 1990). In studies using implantable heart rate transmitters (MacArthur and Karpan, 1989; McCulloch and Jones, 1990), and in contrast to studies using trailing ECG wires (Drummond and Jones, 1979; Jones et al., 1982), the bradycardia for any given dive is relatively stable and does not intensify toward the end of long duration dives. It is possible that in the latter studies the presence of the researchers, who need to be present to prevent entanglement of the trailing ECG wires, affect both the muskrats’ behavior and cardiac responses to diving. In animals trained to swim an underwater maze of varying length (1–16 m), telemetered dive heart rate decreases with increasing underwater swimming distances (MacArthur and Karpan, 1989). Additionally, escape dive heart rate decreases as submersion duration increases (MacArthur and Karpan, 1989). These data suggest that not only do the cardiac responses to submersion vary with the nature of the dive, but that additional stresses imposed upon muskrats can cause a cortical potentiation of the diving response. Indeed, in decorticate muskrats heart rate in escape and forced dives are similar to those seen in voluntary dives (McCulloch and Jones, 1990). However, muskrats typically do not show post-dive tachycardia or significant anticipatory changes in heart rate prior to onset or termination of spontaneous dives (Drummond and Jones, 1979; Jones et al., 1982; MacArthur and Karpan, 1989). So, although nasal receptor stimulation may be the most important factor involved in initiating reflex submersion bradycardia in muskrats (Drummond and Jones, 1979), there is a certain lability associated with the cardiac response to diving that depends, presumably, upon cortical perception of the type or condition of the dive.
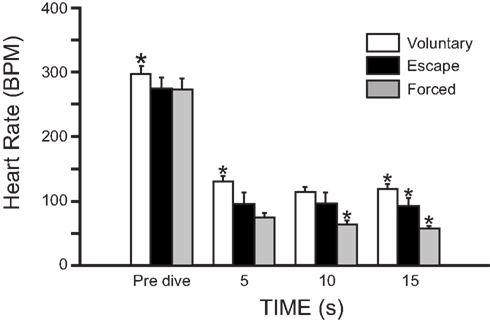
Figure 2. Mean heart rate (±SEM) of muskrats during voluntary, escape, and forced dives. In all three dives there was a substantial bradycardia on submersion. Heart rates from the three dives showed separation from each other throughout their duration, but only after 15 s into the dive were all three significantly different from each other. *Indicates that heart rate is significantly different from other two heart rates at that time (Modified from McCulloch and Jones, 1990).
Pharmacological studies indicate that the rapidly developing bradycardia that occurs during voluntary diving in muskrats is due to activation of the parasympathetic nervous system rather than sympathetic withdrawal. Injection of the muscarinic antagonist atropine eliminates diving bradycardia (MacArthur and Karpan, 1989; Signore and Jones, 1995, 1996; Shereshkov et al., 2006), while injection of β-adrenergic antagonists (nadolol or propranolol) do not (Signore and Jones, 1995, 1996; Shereshkov et al., 2006; Figure 3). However, β-adrenergic activation causes an increase in heart rate prior to voluntary dives, and when recovering from voluntary dives, the sympathetic nervous system helps return heart rate to pre-dive values within the first 5 s after resurfacing (Signore and Jones, 1995; Shereshkov et al., 2006). With regards to the control of heart rate during diving in muskrats, there is an accentuated antagonism between the two limbs of the ANS, such that the effects of the parasympathetic nervous system predominate over the effects of the sympathetic nervous system (Signore and Jones, 1995, 1996). Even injection of the β-adrenergic agonist isoproterenol does not cause an increase in heart rate during voluntary dives (Signore and Jones, 1996). Additionally, an increase in heart rate during underwater exercise in muskrats is due to a reduction in parasympathetic tone, whereas the increase in heart rate during exercise in air is due mainly to an increase in sympathetic tone (Signore and Jones, 1996).
Figure 3. Effect of injected drugs and submergence on mean heart rate of muskrats (±SEM; N = 5) in the free diving tank. Pre-dive heart rate, diving heart rate, and post-dive heart rate are shown for saline-, atropine-, propranolol-, nadolol-, and phentolamine-treated muskrats. *Indicates that diving heart rate differs significantly from diving heart rate in saline-treated animals (Adapted with permission from Signore and Jones, 1995).
During diving, bradycardia occurs in association with a peripheral vasoconstriction that is effected by sympathetic α-adrenergic control. However, diving bradycardia is unaffected by injection of the α-adrenergic antagonist phentolamine (Signore and Jones, 1995; Shereshkov et al., 2006), and thus development of diving bradycardia is independent from peripheral vasoconstriction (Figure 3). Both bradycardia and peripheral vasoconstriction are necessary for maximum underwater endurance (Signore and Jones, 1995). In control muskrats during forcible submergence, maximum underwater duration is 12.0 ± 1.1 min, which is reduced to 7.7 ± 0.1, 5.2 ± 0.4, and 5.2 ± 0.5 min after atropine, phentolamine, and a mixture of atropine and phentolamine, respectively (Signore and Jones, 1995). This suggests that both peripheral vasoconstriction and bradycardia greatly improve underwater duration, and that peripheral vasoconstriction is the more important of the two mechanisms. Curiously, muskrats lacking a diving response (the atropine and phentolamine group) are able to remain submerged for periods approaching their estimated ADL during voluntary dives (approximately 50 s; MacArthur, 1990), and are able to survive forcible submergence for more than 5 min (Signore and Jones, 1995). This suggests that anaerobic metabolism can play an important role during diving in muskrats (Signore and Jones, 1995).
Underwater endurance
The underwater endurance of freely diving air-breathing animals, including muskrats, depends upon the rate of oxygen usage, and the capacity to utilize oxygen stored in respiratory organs, blood, and muscles (Butler and Jones, 1997; Butler, 2004). The resting oxygen consumption of muskrats in air ranges between 0.78 and 0.85 ml O2/g/h (MacArthur and Krause, 1989; MacArthur and Campbell, 1994; MacArthur et al., 2003), while resting oxygen consumption in thermoneutral water (29–30°C; MacArthur, 1984a) is 0.77 ± 0.04 ml O2/g/h (Fish, 1983). In a laboratory study of recently captured animals, the allometric relationship between mass (M) and basal metabolic rate (BMR) of field acclimatized adult muskrats is BMR = 700M0.68 (Campbell and MacArthur, 1998). In muskrats underwater exercise is accompanied by an increase in energy expenditure which approaches that of surface swimming (Fish, 1983; MacArthur and Krause, 1989). In thermoneutral water estimated oxygen consumption of diving muskrats ranges between 2.05 and 2.49 ml O2/g/h (MacArthur and Krause, 1989; MacArthur et al., 2003; Hindle et al., 2006), and the proportionality coefficient for diving metabolic rate (DMR) is 2.73 times that of BMR (DMR = 1908.8M0.74; MacArthur et al., 2001). The relationship between mass and total body oxygen stores (which combines lung, blood, and muscles oxygen stores) is 33.7M1.09, giving a calculated ADL of 61.4M0.37 (MacArthur et al., 2001). The total body oxygen stores of muskrats are estimated to be 29.7 ml O2/kg (Snyder and Binkley, 1985), and using an oxygen consumption during diving of 2.22 ml O2/g/h (MacArthur and Krause, 1989), this gives an ADL of 48.2 s. In simulated diving environments in the lab, most dives are of relatively short duration and well within the aerobic limit (MacArthur and Krause, 1989; MacArthur et al., 2001), and only 4–6% of all dives by adult muskrats exceed their calculated ADLs (MacArthur et al., 2001).
A number of other factors can affect underwater endurance in muskrats, such as seasonal variations, ontological development and dive training, and temperature (both body and water). Winter-acclimatized muskrats have a 31% increase in BMR compared with summer-acclimatized muskrats (Campbell and MacArthur, 1998). However, compared with summer-acclimatized muskrats, winter-acclimatized adult muskrats have a 29–42% increase in total body oxygen stores, with a gain in blood oxygen accounting for most of this seasonal increase (MacArthur, 1984c, 1990; MacArthur et al., 2001). Winter-acclimatized muskrats appear to be superior divers, exhibiting greater cumulative and average dive durations and longer dive:pause ratios (MacArthur et al., 2001). Additionally, winter-acclimatized adult muskrats have an 8.6% increase in diving oxygen consumption and a 12.1% increase in calculated ADL, although these parameters are not significantly different from summer-acclimatized muskrats (MacArthur et al., 2001).
Contrary to allometric predictions, the diving abilities of muskrats do not increase with age or body size, and 1- to 2-month-old juvenile muskrats exhibit similar dive durations compared with adults (MacArthur et al., 2001). Younger muskrats likely are more dependent than adults on anaerobic metabolism during diving, as there is a greater tendency for smaller and younger muskrats to exceed their ADLs (MacArthur et al., 2001). During ontological development in muskrats there is a concurrent increase in diving experience, and so the effect of dive training was specifically investigated in a laboratory study that used a 9- to 11-week dive training protocol (MacArthur et al., 2003). MacArthur et al. (2003) found that dive training produces a 26% increase in blood oxygen stores, due mainly to increases in hematocrit and hemoglobin concentration, and a 13.5% increase in mean total body oxygen stores compared with muskrats restricted to surface swimming. However, the diving oxygen consumption of dive-trained muskrats (2.22 ml O2/g/h) is 14.4% greater than for swim-trained muskrats (1.94 ml O2/g/h), and consequently the calculated ADL is indistinguishable between the two groups (61.3 s for divers and 61.8 s for swimmers; MacArthur et al., 2003). Thus the relative importance of diving experience in predicting diving proficiency in muskrats is debatable.
Although muskrats actively dive beneath ice throughout winter (MacArthur, 1978, 1979b, 1980), a limitation to their wintertime diving activity may be related to thermoregulatory costs, rather than to apneic tolerance (MacArthur, 1984a). Muskrats exhibit a decrease in core body temperature when either swimming or diving in water less than 30°C (MacArthur, 1979a,b, 1984a). The extent of the decrease in body temperature and increase in post-dive oxygen consumption and recovery time is dependent upon both decreasing water temperature and increasing underwater duration (MacArthur, 1984a). Additionally, with each additional minute that a muskrat remains submerged in 3°C water, the cost of diving increases by 99 ml O2/kg (MacArthur, 1984a). In free-ranging muskrats during winter, body temperature increases prior to foraging excursions (MacArthur, 1979b), and the metabolic heat generated during feeding (HIF – the heat increment of feeding) prior to immersion could provide a thermoregulatory benefit to muskrats by retarding the development of excessive hypothermia while diving in cold water (MacArthur, 1979b; MacArthur and Campbell, 1994). Indeed, active non-shivering thermogenesis does not occur during cold water dives in muskrats, and the primary role of interscapular brown adipose tissue (BAT) in muskrats may be to mediate rapid rewarming following repetitive foraging dives (MacArthur, 1986a). Surprisingly, muskrats do not actively exploit the body heat of nest mates by huddling to attenuate decreases in body temperature or to extend foraging time in cold water (MacArthur et al., 1997). Decreasing water temperature also produces an intensification of bradycardia during exploratory and foraging dives (MacArthur and Karpan, 1989) as well as during forced submergence (Thornton et al., 1978). Diving heart rate also decreases as core body temperature decreases (MacArthur and Karpan, 1989; Hindle et al., 2006), which may be related to a more intense abdominal vasoconstriction (MacArthur and Krause, 1989). Adult muskrats are rather tolerant of hypothermia, but do not use hypothermia to maximize underwater submergence during routine diving through a depression of metabolic rate (Hindle et al., 2006). Indeed, muskrats may defer thermoregulatory costs associated with cold water diving until the post-dive recovery period (MacArthur, 1986a; Hindle et al., 2006). However, the ability of muskrats to restore deep body temperature and acid-base balance was reduced when breathing 5–10% CO2 during recovery from a cold water dive (MacArthur, 1986b,c).
The respiratory properties of muskrat blood generally do not differ from similarly sized mammals in terms of hematocrit, hemoglobin concentration, red blood cell count, and buffering capacity (MacArthur, 1984c; Rothstein et al., 1984; Snyder and Binkley, 1985). However, muskrat blood does have an increase in both oxygen affinity and Bohr effect, which may provide better oxygen uptake from the lung and oxygen delivery at the tissue, respectively (MacArthur, 1984c; Rothstein et al., 1984; Snyder and Binkley, 1985). Many respiratory properties of muskrat blood are enhanced during winter, which may be adaptive to the hypoxia encountered during diving and burrowing (MacArthur, 1984c). The increases in hematocrit and hemoglobin concentration in dive-conditioned muskrats could be due to the intermittent hypoxia experienced by these muskrats during their underwater swimming (MacArthur et al., 2003).
Muskrats have significant increases in myoglobin concentration in heart, gastrocnemius, and diaphragm muscle compared with the rat (Snyder and Binkley, 1985). Skeletal muscle myoglobin concentration varies with age and is mass dependent from 250 to 600 g (Mb = 27.7M1.63), while over 600 g muscle myoglobin concentration is independent of body size (MacArthur et al., 2001). Skeletal muscle myoglobin levels are not significantly different between muskrats trained to dive through a 16 m underwater channel and muskrats restricted to surface swimming (MacArthur et al., 2003).
Glycogen concentrations and pyruvate kinase activities in heart, brain, and gastrocnemius muscle are similar to those obtained from terrestrial animals, suggesting that muskrats tolerate submersion by adaptations primarily associated with aerobic, rather than anaerobic, metabolism (Snyder and Binkley, 1985). However enhanced buffering capacity of the hind limb swimming muscles of winter-caught muskrats implies a greater tolerance to lactic acidemia and perhaps an increased dependence on anaerobic pathways in this tissue (MacArthur et al., 2001). Isolated perfused muskrat hearts are adapted to hypoxic conditions (McKean and Landon, 1982; McKean, 1984), and have a high potential for anaerobic glycolysis (McKean et al., 1986). Also, during reoxygenation after an hypoxic insult, isolated muskrat hearts experience reduced myocardial damage compared with guinea pig hearts (McKean and Landon, 1982). Additionally, mitochondria from the left ventricle of muskrats have an increased ability to sequester calcium and maintain calcium homeostasis, which may help recovery from hypoxia, ischemia, or acidosis (McKean, 1991).
Neurophysiological Control of Diving Response
Submerging muskrats surgically anesthetized with urethane causes heart rate to immediately decrease from approximately 290 to about 80 BPM, similar to that seen in unanesthetized restrained muskrats (Drummond and Jones, 1979; Jones et al., 1982). Additionally, diving bradycardia is relatively unaffected by decerebration (Drummond and Jones, 1979; Jones et al., 1982). These experiments indicate that the cardiac responses to submersion in the muskrat are mediated within the brainstem and not at cortical levels.
The full cardiac response to submersion in anesthetized muskrats involves expression of afferent input from three groups of receptors: nasal, lung, and carotid chemoreceptors (Drummond and Jones, 1979). Pouring water on the external nares during maintained artificial ventilation causes a substantial bradycardia within 1 s, with heart rate decreasing from 292 ± 6 to 76 ± 12 BPM (Drummond and Jones, 1979). The bradycardia is eliminated if the nasal region is coated in petroleum jelly (Drummond and Jones, 1979). Bradycardia and expiratory apnea can also be caused by flowing water (Drummond and Jones, 1979; Douse and Jones, 1988; Doyle et al., 1988; Panneton, 1990; McCulloch and Panneton, 1997; Figure 4A) or drawing ammonia vapors (Doyle et al., 1988; Panneton, 1990, 1991b; Panneton and Yavari, 1995; McCulloch and Panneton, 1997; Figure 4B) through the internal nasal passages. Using saline, rather than water, attenuates the depth of the bradycardia, and anesthetizing the nasal passages with local anesthetic completely eliminates the cardiac and respiratory responses to nasal water flow (Drummond and Jones, 1979). The nasal passages are innervated by the anterior ethmoidal nerve (AEN), a branch of the ophthalmic division of the trigeminal nerve, and electrical stimulation of this nerve produces immediate and sustained bradycardia (McCulloch et al., 1999a). However, Drummond and Jones (1979) found that bilateral section of the maxillary division of the trigeminal nerve abolishes the cardiac and respiratory response to nasal water flow, whereas bilateral section of the ophthalmic division has little effect. The AEN of the muskrat contains a high concentration of small diameter fibers, 62% being unmyelinated C fibers and 27% being slightly myelinated Aδ fibers (McCulloch et al., 1999a). These small diameter fibers likely carry, from the nasal passages to the brainstem, the afferent information necessary for initiating the immediate cardiorespiratory responses seen after nasal stimulation (McCulloch et al., 1999a). In spontaneously breathing anesthetized muskrats, water stimulation of the nasal region causes apnea, with the lungs collapsing to the expiratory position (Koppanyi and Dooley, 1929; Drummond and Jones, 1979). Withdrawal of lung stretch receptor input through lung deflation enhances the cardiac response to nasal stimulation, although the response to nasal water flow is intact even when the nasal stimulation is applied during a period of maintained lung inflation (Drummond and Jones, 1979). Maintaining artificial ventilation during the period of nasal water flow reduces the magnitude of the bradycardia, whereas lung deafferentation eliminates any direct effect of artificial ventilation on heart rate during nasal stimulation (Drummond and Jones, 1979). Stimulation of peripheral chemoreceptors by decreasing oxygen tensions could contribute to and intensify the bradycardia toward the end of a long duration dive, but chemoreceptor afferents in the muskrat are likely not involved in producing the initial bradycardia seen upon submersion (Drummond and Jones, 1979; Douse and Jones, 1988). Additionally, pre-dive exposure to 5–10% CO2 has no effect on the establishment of the bradycardia in conscious muskrats during forced diving (MacArthur, 1986b).
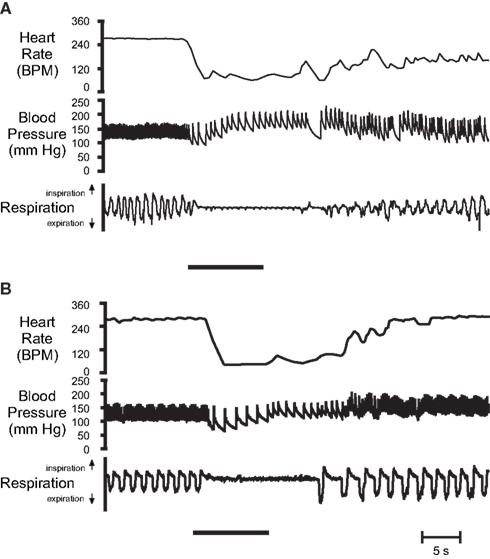
Figure 4. Original cardiovascular responses of α-chloralose-urethane anesthetized muskrats after stimulation of their nasal passages with (A) nasal water flow, and (B) 50% ammonia vapors. In both cases nasal stimulation (indicated by solid bar) produced a substantial and sustained bradycardia, an increase in arterial pressure, and an apnea that lasted longer than the stimulus duration. From top: Heart rate, arterial blood pressure, and respiration (up, inspiration; down, expiration; From McCulloch and Panneton, 1997).
Nasal water flow causes an initial decrease in mean arterial blood pressure that accompanies the onset of the bradycardia (Drummond and Jones, 1979; Douse and Jones, 1988; McCulloch and Panneton, 1997), but blood pressure usually increases to pre-stimulation values or above within 10–20 s (Drummond and Jones, 1979; Douse and Jones, 1988). Similar blood pressure responses are found in unanesthetized, anesthetized, and decerebrated muskrats during restrained dives (Jones et al., 1982). However, any afferent input by the arterial baroreceptors toward intensifying the bradycardia that develops due to nasal stimulation appears to be minimal (Drummond and Jones, 1979; Douse and Jones, 1988). Since arterial blood pressure is maintained or elevated during nasal stimulation in the muskrat, this suggests an increase in total peripheral resistance greater than the decrease in cardiac output caused by the bradycardia. This also suggests a peripheral vasoconstriction that redistributes blood flow to oxygen-dependent tissue (Scholander, 1940). Indeed, during submergence in muskrats anesthetized with pentobarbital, the proportion of cardiac output going to the brain and heart increases by factors of 15 and 5, respectively, and the proportion of cardiac output decreases to all other tissue, especially the stomach and intestines (Jones et al., 1982).
A detailed review of the central pathway of the diving response is beyond the scope of this review, and readers are instead referred to Panneton’s contribution in this issue. However, a brief synopsis of studies in which muskrats have been used for this purpose will be presented. The primary afferent projections of the nerves that innervate the upper respiratory tract of the muskrat were determined by transganglionic transport of horseradish peroxidase conjugated to wheat-germ agglutinin (HRP-WGA; Panneton, 1991a). The central projections of the AEN, the nerve that innervates the nasal passages, are found within discrete areas of the ipsilateral trigeminal sensory complex, especially the ventrolateral part of superficial laminae of the spinal trigeminal nucleus caudalis (also known as the medullary dorsal horn, MDH; Panneton, 1991a). Injections of anterograde tracers [either biotinylated dextran amine (BDA), or HRP-WGA] into this specific MDH location identified brainstem projections of the secondary afferent neurons located within the MDH (Panneton et al., 1994, 2000). Additionally, transport of a virus that crosses synapses and transports in the anterograde direction [herpes simplex virus (HSV-1), strain 129] was used to follow the primary, secondary, and tertiary brainstem relays of the AEN (Panneton et al., 2000). These studies provide an anatomical base for the potential brainstem circuit of the diving response in the muskrat, especially the afferent portion of this circuit. Functionally, the MDH is an important part of the brainstem circuitry, as reversible blockade of this location with either the local anesthetic lidocaine or the glutamate receptor antagonist kynurenate abolishes the cardiorespiratory responses to nasal stimulation (Panneton, 1991b; Panneton and Yavari, 1995). Additionally, Fos, a marker of activated neurons, is immunohistologically detected within MDH secondary neurons after repetitive nasal stimulation with either nasal water flow or ammonia vapors (McCulloch and Panneton, 1997). Significant increases in Fos are also found in the dorsal reticular formation and in the area of A5 catecholaminergic group (McCulloch and Panneton, 1997). Concerning the efferent limb of the central circuitry of the diving response that would be involved in the production of the parasympathetically mediated bradycardia, cell bodies of cardiac preganglionic motoneurons of muskrats are primarily located in the external formation of the nucleus ambiguus after WGA-HRP is injected into the cardiac plexi located near the fat pads at the base of the heart (Panneton et al., 1996). However, there is sparse anatomical information that currently links the afferent limb of this circuit in the MDH to the efferent parasympathetic limb of the circuit within the external formation of the nucleus ambiguus.
Rats
Natural Diving History
The common albino rat is a strain of Rattus norvegicus, or Norway rat, which is indicated by the fact that the two interbreed freely (Donaldson, 1912; Richter, 1954). The Norway rat, also known as the brown or sewer rat, was the first animal to have become domesticated strictly for scientific purposes (Richter, 1954). R. norvegicus originated in eastern Asia, and slowly migrated westward, reaching major European cities by the 1720s, and North America by 1775 (Donaldson, 1912; Robinson, 1965; Hanney, 1975; Walker, 1975; Lindsey et al., 2006). R. norvegicus live in nearly all parts of the world and in practically all land habitats, especially in close association with man (Jackson, 1982; Lindsey et al., 2006). Natural history of R. norvegicus indicates that they live in watery areas, such as sewers, ditches, and marshes (Walker, 1975; Jackson, 1982). While R. rattus, or black rat, usually live in the roofs of buildings, R. norvegicus usually live in the cellar or basement of buildings (Hanney, 1975; Jackson, 1982). R. norvegicus are excellent swimmers (Cottam, 1948; Dagg and Windsor, 1972; Jackson, 1982) even as neonates (Schapiro et al., 1970; Dagg and Windsor, 1972; Clarac et al., 1998), and can island-hop by swimming 400 m across open ocean (Russell et al., 2005). Feral rats will dive for food intended for fish, and prey on young fish, in a fish hatchery (Cottam, 1948). Field observations indicate that many members of some colonies of wild rats (R. norvegicus) dive for and feed on snails and mussels inhabiting the bottom of the Po River in Italy (Gandolfi and Parisi, 1973; Parisi and Gandolfi, 1974). Observational studies of wild rats in a semi-natural environment indicate that rats can dive underwater and exhibit intense predation on freshwater mollusks (Nieder et al., 1982; Nieder, 1985). Additionally, rats living in a Chilean intertidal zone prey upon 40 different types of intertidal species, including mobile organisms from the mid to very low intertidal zone, which suggests rats dive to capture prey (Navarrete and Castilla, 1993). Collectively these studies indicate that wild R. norvegicus exploit semi-aquatic environments, and will often dive underwater while foraging for food. Currently there is sparse information available regarding physiological characteristics of the natural diving performance in small-bodied mammalian divers such as the rat. However, the dive performance, oxygen storage capacity, partitioning of body oxygen stores and ADL has been investigated in the star-nosed mole, one of the world’s smallest mammalian divers (McIntyre et al., 2002). This suggests that similar information could soon be available for the rat.
Suitability as Laboratory Research Animals
Although rat fanciers in Japan bred rats for unique coat colors in the 1700s (Jacob, 2010), it is quite likely that the domestication of albino R. norvegicus involved the sport of “rat-baiting” (Robinson, 1965; Hanney, 1975; Lindsey et al., 2006). In Europe in the eighteenth century, a popular arena betting sport involved training dogs to kill as many rats as possible in as short a time as possible, and is probably the origin of the term “rat race.” A champion dog, “Billy,” was able to kill 100 rats in 5 min 30 s (Hanney, 1975)! To ensure availability of rats for this sport, promoters would collect and hoard hundreds of rats. Often, when naturally occurring albino rats were discovered, these rats would be retained and kept for show purposes and/or breeding. It is likely that these albino show rats, or their descendants, were the first to be used in scientific experiments, especially if they were semi-tamed by frequent handling from birth (Richter, 1954; Robinson, 1965). The first published paper using albino rats in the laboratory (by Philipeaux, 1856) was on the effects of adrenalectomy (Richter, 1954; Robinson, 1965).
Systematic development of breeding colonies of albino rats was started in the late 1800s and early 1900s (Lindsey et al., 2006), and Henry H. Donaldson of the Wistar Institute in Philadelphia is credited with popularizing the use of Norway rats for scientific purposes (Richter, 1954; Lindsey et al., 2006). Standardization of the albino rat at the Wistar Institute through selective breeding gave a broad foundation for the use of the rat in nutrition, biochemistry, endocrinology, genetic, behavioral, psychobiology and neuroscientific research (Lindsey et al., 2006). While working at the Wistar Institute, Eunice Chace Greene published the all-time classic “Anatomy of the Rat” (Greene, 1963), and Helen Dean King was instrumental in the inbreeding experiments that helped establish many different strains of albino rats (Lindsey et al., 2006). The Wistar Institute was also instrumental in developing modern rat husbandry standards, as clean and healthy rats are essential for accurate research (Lindsey et al., 2006). Most present-day domesticated R. norvegicus are albino, and their clean white appearance undoubtedly enhances their popularity (Richter, 1954). Additionally, defects in vision due to lack of pigmentation tends to make them less likely to escape and therefore easier to handle (Richter, 1954). There are many strains of albino and hooded R. norvegicus, including PA, Lewis, Buffalo, Long–Evans, Fischer, Sprague-Dawley, Holtzman, Albany, PAR/Lou, and germ-free (gnotobiotic), many of which can trace their origins to the original Wistar strain (Lindsey et al., 2006).
Scientific investigation with the albino rat often receives criticism about the perceived artificiality of the domestic rodent (Boice, 1971, 1981). Consistent with this doubt is the assumption that the albino rat is degenerate and dull compared with wild rats (Robinson, 1965). Albinism occurs naturally in R. norvegicus, and appears in wild Norway rats bred in captivity (Donaldson, 1912; Hatai, 1912; Richter, 1954). Albinism is due to a recessive gene, c, and rats homozygous for c are totally depigmented, having white pelage and pink eyes (Robinson, 1965). Although there are differences between wild and albino rats in terms of their endocrine system and behavior, these differences have not been attributed to the albino allele (Robinson, 1965). Albino rats are more docile and less nervous than wild rats, making them easier to handle (Richter, 1954; Hulin and Quinn, 2006). Additionally, albino rats have smaller adrenal glands, making them less capable of dealing with conditions of stress, and have larger gonads (Richter, 1954). Except for mutant forms of the laboratory rat specifically bred for biomedical investigations (Altman and Katz, 1979), the differences in the endocrine system appear to be the main physiological difference between laboratory rats and feral rats. Albino rats can thrive in feral conditions (Price and Huck, 1976; Boice and Adams, 1980), and a colony of wild albino rats estimated at 2000 individuals survived several winters (with temperatures reaching −25°F) at the dump in Missoula Montana (Minckler and Pease, 1938). Thus, although domestication has produced some anatomical, physiological, and behavioral changes in laboratory rats (Richter, 1954), research has demonstrated that behaviorally laboratory rats are representative of wild R. norvegicus (Boice, 1981) and that domestication is not necessarily equivalent to degeneracy (Boice, 1973). However, genotypically there are differences between wild R. norvegicus and various strains of inbred rats (van den Brandt et al., 2000; Kloting et al., 2003; Hulin and Quinn, 2006).
The considerable background work in physiology and biochemistry has made the rat an ideal experimental animal in many areas of biological research (Gill, 1985; Gill et al., 1989; Jacob, 2010). Indeed the rat has been used extensively in the fields of neuroscience, basic genetics, cancer, immunology, reproduction, behavior, aging, toxicology, and transplantation (Gill, 1985; Jacob, 2010). More is known about the rat than any non-human species (Richter, 1954; Gill, 1985; Gill et al., 1989; Jacob, 2010), and it is sometimes forgotten that the phenomenal progress of biomedical research would have been impossible without the use of rodents as experimental animals (Hanney, 1975; Gill, 1985). Among many of the advantages the rat has for its use in scientific research is its size: the rat is large enough to be handled easily and to allow surgical manipulations, and is small enough to be conveniently and economically housed in large numbers in animal facilities (Richter, 1954; Gill et al., 1989). The rat is a particularly useful experimental animal for cardiorespiratory research, including the diving response, because the physiology of the rat is well characterized across all major organ systems, its anatomy [both gross (Greene, 1963) and neuroanatomical (Paxinos and Watson, 1998; Paxinos et al., 1999; Swanson, 2004)] is well known, and the whole animal is ideal for work relating to systems biology (Jacob, 2010). Additionally, the recent publication of the rat genome database should facilitate an explosive growth in the use of the rat as a biomedical model in the near future (Jacob, 2010).
Physiological Responses during Simulated Diving in the Laboratory
Voluntarily diving rats have a substantial diving response that is qualitatively similar to that of muskrats. Comparable results in rats are found whether heart rate and blood pressure are recorded using trailing arterial cannulae (McCulloch et al., 1997; Ollenberger and West, 1998b) or implanted transmitters (McCulloch et al., 2010; Panneton et al., 2010b). In rats trained to dive 3 m through an underwater maze, heart rate, and mean arterial blood pressure decrease immediately upon submersion, heart rate by 78% (from 453 ± 12 to 101 ± 8 BPM) and mean arterial blood pressure by 25% (from 143 ± 1 to 107 ± 5 mmHg; McCulloch et al., 2010; Figure 5B). After its initial decrease, mean arterial blood pressure then increases, reaching a maximum of 174 ± 3 mmHg 4–5 s after submersion (McCulloch et al., 2010). Pre-existing chemoreceptor drive, achieved by altering arterial PO2 and/or PCO2, does not have any effect on the cardiovascular responses to voluntary diving (McCulloch et al., 1997). Additionally, during long duration (approximately 100 s) forced dives, rats do not attempt to breathe even though there are radical changes in arterial PO2, PCO2, and pH (Panneton et al., 2010a). Together these studies suggest that the chemoreceptor reflex in rats is not important in initiating the cardiovascular response to diving and is actually suppressed during diving. However, bilateral section of the carotid sinus nerve or destruction of the carotid body chemoreceptors attenuates the bradycardia response during forced submersion in conscious rats (Huang and Peng, 1976). Pretreatment with the muscarinic antagonist atropine eliminates the bradycardia associated with voluntary diving, and, even with the decrease in cardiac output due to the bradycardia, mean arterial blood pressure increases to 202 ± 5 mmHg during the dive (McCulloch et al., 1997). These results suggest that during voluntary diving in the rat there is both a parasympathetically mediated bradycardia and a sympathetically mediated peripheral vasoconstriction (McCulloch et al., 1997, 2010). Blood corticosterone levels indicate that rats not trained in the diving protocol find voluntary diving stressful, whereas repetitive daily training in rats decreases the stressfulness associated with voluntary diving (McCulloch et al., 2010). Trained rats find diving no more stressful than being handled daily by a human (McCulloch et al., 2010). However dive training has no effect on diving heart rate or mean arterial blood pressure, as quantitatively similar heart rate and blood pressure responses are found in both trained and untrained rats during voluntary diving (McCulloch et al., 2010).
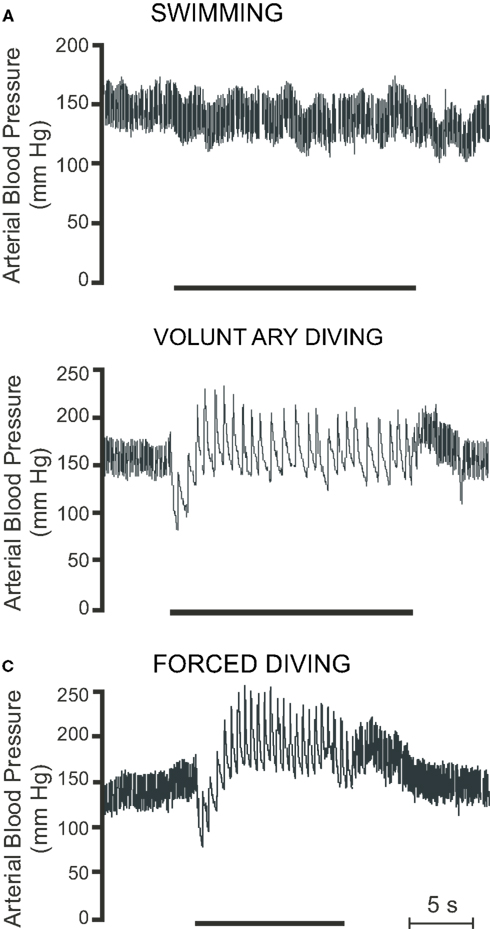
Figure 5. Raw traces showing pulsatile arterial blood pressure during (A) swimming, (B) voluntary diving, and (C) forced diving in rats trained to dive. Period of swimming or diving is indicated by the bar underneath the trace. Breaks in trace indicate periods where the radiotelemetric signal was lost (Modified from McCulloch et al., 2010).
Forced submersion in conscious rats either trained to dive or naïve to diving also produces a substantial bradycardia, with heart rate decreasing from approximately 460 to 90 BPM when using implanted transmitters (McCulloch et al., 2010; Panneton et al., 2010a,b), from approximately 400 to 105 BPM when using trailing ECG leads (Fahlman et al., 2011), and from approximately 400 to 140 BPM when using trailing arterial cannulae (Lin, 1974; Lin and Baker, 1975; Huang and Peng, 1976). During forced diving in rats trained to dive, mean arterial pressure decreases immediately upon submersion, from 135 ± 2 to 119 ± 5 mmHg, but then increases to 189 ± 4 mmHg 4–5 s into the dive (McCulloch et al., 2010; Figure 5C). During forced submersion cardiac output decreases by approximately 70% (Lin, 1974; Lin and Baker, 1975), and blood flow is maintained to the coronary, bronchial, and cerebral circulations but decreases to the intestine, spleen, kidney, tail, and skin (Lin and Baker, 1975). There is a great reduction in blood flow to muscle during long duration forced dives in rats, as blood lactate levels due to muscle anaerobic glycolysis increase during recovery and not during the dive (Scholander, 1940). During forced submersion in conscious rats, pretreatment with the muscaric antagonist atropine greatly attenuates, but does not eliminate, the bradycardia (Lin, 1974). This surprising finding may be related to the intraperitoneal, rather than intravenous, route of atropine injection. Still, total peripheral resistance increases by a factor of 4 during forced submersion, and pretreatment with intraperitoneal atropine produces a 37% increase in arterial blood pressure (Lin, 1974). These results thus suggest that forced submersion, like voluntary submersion, also causes both parasympathetically mediated bradycardia and sympathetically mediated peripheral vasoconstriction. Changes in cardiac output distribution similar to those seen during forced diving were later found in voluntarily diving rats, as there is a 69% decrease in cardiac output and a 438% increase in peripheral resistance (Ollenberger and West, 1998b). Blood flow is largely restricted to the head and thorax (Ollenberger et al., 1998), and there is a 21% decrease in cerebrovascular resistance that results in a 168% increase in cerebrovascular blood flow (Ollenberger and West, 1998b). Blood corticosterone levels indicate that forced diving is stressful to both trained and untrained rats (McCulloch et al., 2010). The magnitude of bradycardia is similar during both voluntary and forced diving (McCulloch et al., 2010; Panneton et al., 2010b), while the increase in blood pressure is greater during forced diving (McCulloch et al., 2010). Therefore rats appear to be different from muskrats, as muskrats show an intensification of the bradycardia during forced dives compared with voluntary dives (MacArthur and Karpan, 1989; McCulloch and Jones, 1990). Also rats have a maximal forced dive underwater endurance of 2 min, which is much less than the 12 min of muskrats (Irving, 1939; Scholander, 1940). Rats, like muskrats, have an accentuated antagonism between the parasympathetic and sympathetic limbs of the ANS in regards to the control of heart rate, so that during diving parasympathetic activity overpowers sympathetic activity (McCulloch et al., 2010). Due to the variability of the responses and the differences of the responses compared with voluntary diving, it is suggested that the use of forced submergence in naïve rats not trained to dive should avoided when investigating the hemodynamic responses to diving (Panneton et al., 2010b).
There may also be a genetic component to the magnitude of the bradycardic response to forced diving in rats (Fahlman et al., 2011). The responses of two inbred strains of rats (Fischer and Buffalo) were compared to the responses from an outbred strain of rats (Wistar). Despite similar pre-dive heart rates (approximately 400 BPM), dive heart rate is 121 ± 14 BPM in Fischer rats, 103 ± 31 BPM in Wistar rats, and 93 ± 13 BPM in Buffalo rats (Fahlman et al., 2011). Thus genetically distinct populations of rats demonstrate divergent cardiac responses to diving, which suggests that a portion of the mammalian diving response may be a heritable trait.
In addition to the physiological investigation of the diving response in rats, psychologists have used diving-for-food situations in rats to show the influence of spatial environment on social organization (Grasmuck and Desor, 2002) and investigate the importance of social learning in the acquisition of behavior (Galef, 1980, 1982). Additionally, psychologists have found during the investigation of learned helplessness that rats will often dive underwater during the exploratory phase of the forced swim test (Binik et al., 1979; Hawkins, 1987; Abel, 1994; Kelliher et al., 2000; Linthorst et al., 2002; Campbell et al., 2003).
Neurophysiological Control of Diving Response
Submerging rats anesthetized with urethane causes a 51% decrease in heart rate and a 23% increase in mean arterial blood pressure (Huang et al., 1991). Nasal water flow plus apnea in rats anesthetized with Innovar (a combination of droperidol and fentanyl) produces a 77% decrease in HR and a 41% decrease in mean arterial blood pressure (McCulloch and West, 1992; McCulloch et al., 1995; Ollenberger and West, 1998a; Figure 6A). Nasal stimulation with ammonia vapors in rats anesthetized with urethane causes a 43% decrease in heart rate and an 11% increase in mean arterial blood pressure (Rybka and McCulloch, 2006; Hollandsworth et al., 2009; Panneton et al., 2010b; Figure 6B). Nasal stimulation with 100% CO2 in rats anesthetized with a combination of α-chloralose and urethane causes a 47% decrease in heart rate and a 28% increase in mean arterial blood pressure (Yavari et al., 1996; Figure 6C). Collectively these studies indicate that anesthetized rats exhibit cardiovascular responses during nasal stimulation similar to those observed during conscious voluntary diving, although the magnitude of the responses are variable and dependent upon the anesthetic and method of nasal stimulation used in each preparation. However, although the physiological responses of nasopharyngeal stimulation in anesthetized animals resemble those seen in conscious diving animals, it is uncertain if the same neural circuits are used in eliciting this response (Panneton et al., 2010b). Conversely, these table preparations enable extremely detailed physiological investigations in a more controlled laboratory setting. In a comparison of various techniques that could be used to investigate the central control of the diving response, it was suggested that decerebrated, rather than anesthetized, rats receiving nasal stimulation with ammonia vapors be used, as decerebrated rats also show cardiovascular responses similar to those seen during voluntary diving (Panneton et al., 2010b). Additionally, because these responses are seen in decerebrated animals, this suggests that the neural circuits for the diving response are intrinsic to the brainstem (Panneton et al., 2010b).
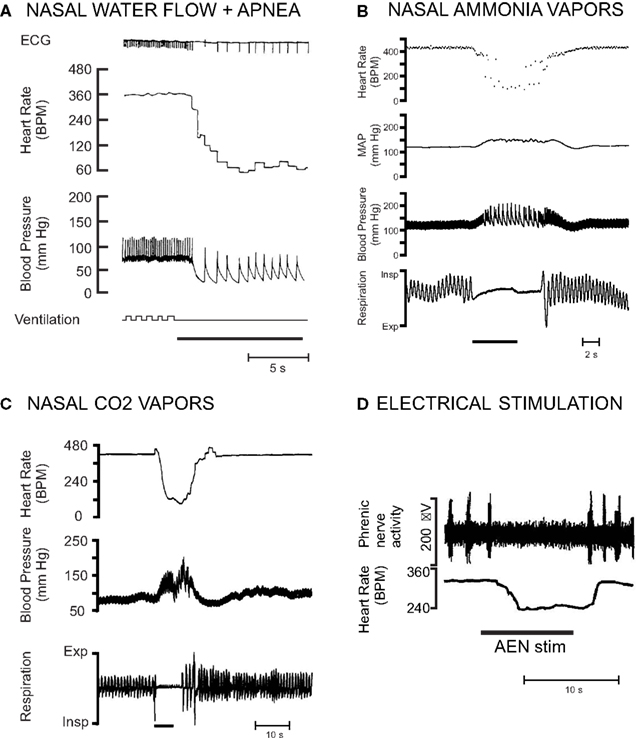
Figure 6. Examples of different ways that the diving response has been in initiated in rat preparations. The magnitude of the responses are variable and dependent upon the anesthetic and method of nasal stimulation used. (A) Nasal water flow plus concurrent apnea in a paralyzed and artificially ventilated rat anesthetized with Innovar (From McCulloch and West, 1992). (B) Nasal stimulation with ammonia vapors in a rat anesthetized with urethane (From Hollandsworth et al., 2009). (C) Nasal stimulation with 100% carbon dioxide in a rat anesthetized with a mixture of α-chloralose and urethane (From Yavari et al., 1996). (D) Electrical stimulation of the anterior ethmoidal nerve in a neonatal rat in situ unanesthetized decerebrate arterially perfused working heart brainstem preparation (Modified from Dutschmann et al., 2004).
Stimulation of the trigeminal receptors innervating the nose and nasal passages is thought to provide the most important afferent input for the initiation of the cardiovascular responses to diving. The cardiovascular changes associated with diving are initiated with immersion of the rats’ nares in the water, as opposed to just swimming on the surface of the water (McCulloch et al., 2010; Panneton et al., 2010b; Figure 5A). Infusing the nasal passages of the rat with local anesthetic eliminates the cardiovascular responses to nasal stimulation (McCulloch et al., 1995; Yavari et al., 1996). Bilateral sectioning of the trigeminal nerve, especially the AEN, also virtually eliminates the heart rate response to nasal simulation (McCulloch et al., 1995; Rybka and McCulloch, 2006). However, after unilateral sectioning of one AEN, the remaining AEN can still provide sufficient afferent input to initiate the cardiorespiratory changes consistent with the nasopharyngeal response (McCulloch et al., 1995; Rybka and McCulloch, 2006). Electrical stimulation of the rat AEN produces bradycardia, a slight hypertension, and apnea, both in anesthetized whole animal preparations (Dutschmann and Herbert, 1996, 1997, 1998b), and unanesthetized decerebrate working heart brainstem preparations (Dutschmann and Paton, 2002a,b; Dutschmann et al., 2004; Rozloznik et al., 2009; Figure 6D). It is likely that small unmyelinated fibers within the AEN of the rat are responsible for carrying the afferent information that initiates the cardiorespiratory responses to nasal simulation to secondary neurons located within the MDH (Hollandsworth et al., 2009).
Although activation of receptors in the nasal passages is important for initiating the cardiovascular responses in the anesthetized rat, lung deflation during the nasal stimulation is necessary to sustain the cardiovascular changes (McCulloch and West, 1992). Nasal water flow plus expiratory apnea results in an immediate and sustained bradycardia and hypotension, and is more effective in inducing cardiovascular changes than when either of the two stimuli are applied individually (McCulloch and West, 1992). Stimulation of afferents within the recurrent laryngeal nerve or superior laryngeal nerve is not required for elicitation of the cardiovascular responses to nasal water flow, as the responses are unaltered after sectioning of these nerves (McCulloch and West, 1992). Chemoreceptor stimulation is unnecessary for the initiation of the cardiovascular responses to nasal water flow, as reduction of chemoreceptor stimulation, or chemoreceptor stimulation through pre-existing hypoxia or hypercapnia, does not alter the cardiovascular responses to nasal stimulation (McCulloch and West, 1992). However, a potent facilitation of bradycardia is elicited by simultaneous submaximal activation of AEN afferents and peripheral chemoreceptors in a rat working heart brainstem preparation, and this could be the basis of the intensification of bradycardia during the late states of a dive when the afferent drive from peripheral chemoreceptors is increased (Rozloznik et al., 2009). Also, during long duration (50 s) simulated dives in anesthetized rats, increasing arterial CO2 produces a decrease in cerebrovascular resistance and an increase in cerebral blood flow (Ollenberger and West, 1998a).
As stated previously, a detailed review of the central pathway of the diving response is beyond the scope of this review, and again readers are instead referred to Panneton’s contribution in this issue. However, a brief synopsis of studies in which rats have been used for this purpose will be presented. The central projections of the AEN were followed using transganglionic tracers (Panneton et al., 2006; Hollandsworth et al., 2009). After injecting WGA-HRP into the AEN, terminal projections are seen throughout the trigeminal sensory complex, but primarily within laminae I and II of the ventral tip of the ipsilateral MDH at the level of the area postrema (Panneton et al., 2006; Hollandsworth et al., 2009). Most of the AEN projections to the MDH likely are small unmyelinated fibers (Hollandsworth et al., 2009). Other non-trigeminal AEN projections are to the ventrolateral medulla and parabrachial complex (Panneton et al., 2006; Hollandsworth et al., 2009). After repetitive voluntary diving (McCulloch, 2005) or nasal stimulation (Dutschmann et al., 1998; Rybka and McCulloch, 2006; Hollandsworth et al., 2009), neurons within the MDH of rats express the protein Fos, a marker of neuronal activation. Injection of anterograde tracers into the MDH identified the connections of these secondary neurons to important autonomic locations within the brainstem, including the nucleus tractus solitarius (NTS), ventrolateral medulla, A5, Kölliker-Fuse, and parabrachial complex (Feil and Herbert, 1995; Panneton et al., 2006). Injection of the retrograde tracer Fluorogold into these tertiary brainstem locations confirmed the projections of the MDH neurons (Feil and Herbert, 1995; Panneton et al., 2006). These anatomical connections may potentially identify the neural circuits of the diving response in the rat (Panneton et al., 2006).
The NTS may play a role in modulation of the diving response, as lesions of the NTS attenuates the diving bradycardia (Huang et al., 1991), and microinjection of a 5-HT3 agonist into the commissural NTS potentiates the bradycardia (Rozloznik et al., 2009). Additionally, injection of the calcium channel blocker cobalt chloride into the medial NTS blocks the pressor response elicited by electrical stimulation of the AEN (Dutschmann and Herbert, 1998a). However these results may be debatable, as injection of the glutamate receptor antagonist kynurenate into the NTS does not reduce the increase in splanchnic sympathetic nerve discharge seen after nasal stimulation with ammonia vapors (McCulloch et al., 1999b). Selective groups of catecholaminergic neurons within the brainstem, specifically the A1, C1, A2, A5, sub-coeruleus areas (McCulloch and Panneton, 2003), and globosa neurons within the lateral A7 area (McCulloch, 2003), express Fos after repetitive voluntary diving. A majority of bulbospinal sympathoexcitatory neurons within the rostral C1 area of the rostral ventrolateral medulla are activated by nasal stimulation (McCulloch et al., 1999b), and may be responsible for the increase in sympathetic tone that occurs during diving. Nasal stimulation also affects the firing patterns of neurons within the ventrolateral medulla that have respiratory-related activity (McCulloch et al., 1999b; Dutschmann and Paton, 2002a,b). Nasotrigeminal stimulation causes inspiratory neurons to cease firing and hyperpolarize, and postinspiratory neurons to depolarize and discharged persistently (Dutschmann and Paton, 2002a). After long duration forced dives, Fos-positive neurons are found in brainstem areas that contain chemosensitive neurons, such as the ventral surface of the medulla, the midline raphe, the parapyramidal nucleus, and retrotrapezoid nucleus, as well as in the commissural NTS that receives primary afferent projections from peripheral chemoreceptors (Panneton et al., 2010a). Additionally, after injections of the anterograde tracer BDA into the MDH, labeled fibers are located along the ventral surface of the medulla where presumptive chemosensitive Fos-positive neurons are located (Panneton et al., 2010a). It may be these connections that inhibit the chemoreceptor reflex during diving (Panneton et al., 2010a). More rostrally within the brainstem, neurons within the Kölliker-Fuse nucleus may mediate the apnea that is induced after trigeminal stimulation in the rat (Dutschmann and Herbert, 1996, 1997, 1998b, 1999; Dutschmann et al., 1998, 2004).
Summary
Investigation of the central nervous integration of the cardiorespiratory responses to diving is important for a number of reasons. The diving response enables animals to remain submerged underwater for extended periods of time, and an understanding of how this occurs is of inherent interest. The diving response demonstrates one of the most powerful patterns of autonomic reflexes observed in animals, and represents a radical functional reorganization of brainstem homeostatic control. This also is of inherent interest. The diving response may also be important clinically as part of the trigemino-cardiac reflex, nasopharyngeal reflex, and/or sudden infant death syndrome. Finally, an understanding of the neuronal circuitry that exists within the brainstem of animals like muskrats and rats will help determine how cortical afferent signals in marine animals can modify the basic autonomic reflex.
From preceding sections, it is obvious that both muskrats and rats have a very robust diving response similar to that of many marine species. In this regard, both these animals are ideal choices for investigation of the physiological responses to diving (Table 1). Additionally, if a species is to be used in neurophysiological or neuroanatomical studies, then the brains from adults of that species need to be of a relatively uniform size. This uniformity is necessary to enable stereotaxic targeting of brain and brainstem structures. Additionally, the organization of that species’ brain should not deviate significantly from the typical mammalian scheme. This is necessary to facilitate comparisons of the functions and anatomical connections of homologous structures across species. The brains of muskrats and rats fulfill these criteria, and atlases of the brainstem of muskrats and rats are available. These atlases have facilitated the investigation of the central pathways of the diving response. In contrast, investigations elucidating the neural control of the diving response in marine mammals are much more difficult, because of their native aquatic environment, their relatively large and non-uniformly sized brains, and the paucity of information about those brains. Additionally, the cost associated with building and maintaining the facilities necessary to house marine animals may make such investigations prohibitive.
Both the muskrat and rat have advantages and disadvantages in their use as models for investigating the central control of the diving response. One advantage for using muskrats is that they have a true semi-aquatic lifestyle in their natural habitat. Many aspects of the behavior and physiology of muskrats in both field and simulated diving situations have been investigated and well characterized. In comparison, rats are primarily terrestrial animals, although feral rats can and do dive in their natural habitat. Other advantages for using muskrats are their size, availability, and modest housing requirements, at least in comparison with marine animals. Rats have the same advantage of size. Additionally, rats are very easily available from many commercial vendors, and with genetic standardization, many inbred and outbred strains are available. Muskrats are not as freely available as rats, and the use of professional trappers and the cooperation of local Wildlife agencies may be necessary to secure a reliable source of muskrats. Rats have the advantage in that many existing animal facilities are designed for housing rodents such as rats and mice. Although muskrats can be easily housed in such facilities, regulatory hurdles to do so may be prohibitive. Legitimate concerns over the entry of zoonotic diseases and parasites, and the safety of facility personnel, may be raised. A big advantage for the use of rats is that the brains of rats are much more thoroughly characterized, both anatomically and functionally. Neuroscientific data obtained from rats are much more comparable with those from the literature, as rats are used extensively in many areas of biomedical research. Many of these other disciplines can also lay the groundwork for investigation of specific aspects of the rat diving response. In comparison, muskrats have only been used in a handful of neuroscientific studies.
Another potential disadvantage of using the rat is the “stigma” of using a domesticated animal. However, for every research question there is an appropriate animal model on which it can be most conveniently studied. Rats are often regarded as strictly terrestrial animals, and for laboratory animals this is usually true. However, if the central circuitry involved in integration of the diving response is to be characterized, it will have to be done in an animal in which the basic cardiovascular and respiratory circuitry is well characterized. The rat is such an animal. In the wild R. norvegicus is used to, and adept at, swimming and diving underwater. The laboratory rat is the domesticated albino version of the wild rat. This suggests that the laboratory rat should not be regarded merely as a terrestrial animal, but rather as an animal that rarely gets the opportunity to swim and dive because of how it is housed. The laboratory rat is descended from a semi-aquatic feral form, having been domesticated within the last 150 years, and in this sense is a good choice for studies of the diving response. Because so much is known about the physiology and central anatomy of the laboratory rat, it should make an excellent animal model for investigating the central control of the mammalian diving response.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADL, aerobic dive limit; AEN, anterior ethmoidal nerve; ANS, autonomic nervous system; BAT, brown adipose tissue; BDA, biotinylated dextran amine; BMR, basal metabolic rate; BPM, beats per minute; DMR, diving metabolic rate; HIF, heat increment of feeding; HRP-WGA, horseradish peroxidase conjugated to wheat-germ agglutinin; M, mass; MDH, medullary dorsal horn; NTS, nucleus tractus solitarius; TDR, time-depth recorders.
References
Abel, E. L. (1994). Behavioral and physiological effects of different water depths in the forced swim test. Physiol. Behav. 56, 411–414.
Aleksiuk, M., and Frolinger, A. (1971). Seasonal metabolic organization in the muskrat (Ondatra zibethica) I. Changes in growth, thyroid activity, brown adipose tissue, and organ weights in nature. Can. J. Zool. 49, 1143–1154.
Altman, P. L., and Katz, D. D. (1979). Inbred and Genetically Defined Strains of Laboratory Animals: Mouse and Rat.Bethesda, MD: Federation of American Societies for Experimental Biology.
Baker, H. J., Lindsey, J. R., and Weisbroth, S. H. (2006). “Selected normative data,” in The Laboratory Rat, eds M. A. Suckow, S. H. Weisbroth, and C. L. Franklin (Burlington, MA: Elsevier Academic Press), 883–884.
Binik, Y. M., Deikel, S. M., Theriault, G., Shustack, B., and Balthazard, C. (1979). Sudden swimming deaths: cardiac function, experimental anoxia, and learned helplessness. Psychophysiology 16, 381–391.
Boice, R. (1971). Laboratorizing wild rat (Rattus norvegicus). Behav. Res. Methods Instrum. 3, 177–182.
Boice, R. (1981). Behavioral comparability of wild and domesticated rats. Behav. Genet. 11, 545–553.
Boice, R., and Adams, N. (1980). Outdoor enclosures for feralizing rats and mice. Behav. Res. Methods Instrum. 12, 577–582.
Butler, P. J. (2004). Metabolic regulation in diving birds and mammals. Respir. Physiol. Neurobiol. 141, 297–315.
Butler, P. J., and Jones, D. R. (1997). Physiology of diving birds and mammals. Physiol. Rev. 77, 837–899.
Campbell, K. L., and MacArthur, R. A. (1998). Nutrition and the energetic tactics of muskrats (Ondatra zibethicus): morphological and metabolic adjustments to seasonal shifts in diet quality. Can. J. Zool. 76, 163–174.
Campbell, T., Lin, S., Devries, C., and Lambert, K. (2003). Coping strategies in male and female rats exposed to multiple stressors. Physiol. Behav. 78, 495–504.
Clarac, F., Vinay, L., Cazalets, J. R., Fady, J. C., and Jamon, M. (1998). Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Res. Rev. 28, 35–43.
Dagg, A., and Windsor, D. (1972). Swimming in northern terrestrial mammals. Can. J. Zool. 50, 117–130.
Donaldson, H. H. (1912). The history and zoological position of the albino rat. J. Acad. Nat. Sci. Philadelphia 15, 365–369.
Douse, M. A., and Jones, D. R. (1988). Baroreceptor activity in muskrats (Ondatra zibethicus) during nasal stimulation. Can. J. Zool. 66, 2520–2527.
Doyle, R. E., Panneton, W. M., Vogler, G. A., Romero, J. P., Watson, B. J., and Higgins, B. (1988). The muskrat in biomedical research. Lab. Anim. Sci. 38, 667–674.
Drummond, P. C., and Jones, D. R. (1979). The initiation and maintenance of bradycardia in a diving mammal, the muskrat (Ondatra zibethica). J. Physiol. 290, 253–271.
Dutschmann, M., Guthmann, A., and Herbert, H. (1998). NMDA receptor subunit NR1-immunoreactivity in the rat pons and brainstem and colocalization with Fos induced by nasal stimulation. Brain Res. 809, 221–230.
Dutschmann, M., and Herbert, H. (1996). The Kölliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport 7, 1432–1436.
Dutschmann, M., and Herbert, H. (1997). Fos expression in the rat parabrachial and Kölliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Exp. Brain Res. 117, 97–110.
Dutschmann, M., and Herbert, H. (1998a). The medial nucleus of the solitary tract mediates the trigeminally evoked pressor response. Neuroreport 9, 1053–1057.
Dutschmann, M., and Herbert, H. (1998b). NMDA and GABAA receptors in the rat Kolliker-Fuse area control cardiorespiratory responses evoked by trigeminal ethmoidal nerve stimulation. J. Physiol. 510, 793–804.
Dutschmann, M., and Herbert, H. (1999). Pontine cholinergic mechanisms enhance trigeminally evoked respiratory suppression in the anesthetized rat. J. Appl. Physiol. 1059–1065.
Dutschmann, M., Morschel, M., Kron, M., and Herbert, H. (2004). Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Respir. Physiol. Neurobiol. 143, 155–165.
Dutschmann, M., and Paton, J. F. (2002a). Influence of nasotrigeminal afferents on medullary respiratory neurones and upper airway patency in the rat. Pflugers Arch. 444, 227–235.
Dutschmann, M., and Paton, J. F. (2002b). Trigeminal reflex regulation of the glottis depends on central glycinergic inhibition in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R999–R1005.
Fahlman, A., Bostrom, B. L., Dillon, K. H., and Jones, D. R. (2011). The genetic component of the forced diving bradycardia response in mammals. Frontiers in Physiology 2:63. doi:10.3389/fphys.2011.00063
Feil, K., and Herbert, H. (1995). Topographical organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J. Comp. Neurol. 353, 506–528.
Fish, F. E. (1983). Metabolic effects of swimming velocity and water temperature in the muskrat (Ondatra zibethicus). Comp. Biochem. Physiol. A Physiol. 75, 397–400.
Fish, F. E. (1984). Mechanics, power output and efficiency of the swimming muskrat. J. Exp. Biol. 110, 183–201.
Galef, B. G. Jr. (1982). Studies of social learning in Norway rats: a brief review. Dev. Psychobiol. 15, 279–295.
Galef, B. G. (1980). Diving for food – analysis of a possible case of social-learning in wild rats (Rattus norvegicus). J. Comp. Physiol. Psychol. 94, 416–425.
Gandolfi, G., and Parisi, V. (1973). Ethological aspects of predation by rats, Rattus norvergicus (Berkenhout), on bivalves Unio pictorum L. and Cerastoderma Lamarcki (Reeve). Ital. J. Zool. 40, 69–74.
Gilbert, F. F., and Gofton, N. (1982). Heart rate values for beaver, mink, and muskrat. Comp. Biochem. Physiol. 73A, 249–251.
Gill, T. J. III, Smith, G. J., Wissler, R. W., and Kunz, H. W. (1989). The rat as an experimental animal. Science 245, 269–276.
Grasmuck, V., and Desor, D. (2002). Behavioural differentiation of rats confronted to a complex diving-for-food situation. Behav. Processes 58, 67–77.
Hatai, S. (1912). On the appearance of albino mutants in litters of the common norway rat, Mus norvegicus. Science 35, 875.
Hawkins, J. (1987). Rat strain comparisons on drug and sleep sensitive behaviors. Physiol. Behav. 40, 725–729.
Hays, G. C., Forman, D. W., Harrington, L. A., Harrington, A. L., Macdonald, D. W., and Righton, D. (2007). Recording the free-living behaviour of small-bodied, shallow-diving animals with data loggers. J. Anim. Ecol. 76, 183–190.
Hindle, A. G., Senkiw, R. W., and MacArthur, R. A. (2006). Body cooling and the diving capabilities of muskrats (Ondatra zibethicus): a test of the adaptive hypothermia hypothesis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 144, 232–241.
Hollandsworth, M. P., Dinovo, K. M., and McCulloch, P. F. (2009). Unmyelinated fibers of the anterior ethmoidal nerve in the rat co-localize with neurons in the medullary dorsal horn and ventrolateral medulla activated by nasal stimulation. Brain Res. 131–144.
Huang, T. F., and Peng, Y. I. (1976). Role of the chemoreceptors in diving bradycardia in the rat. Jpn. J. Physiol. 26, 395–401.
Huang, T. F., Peng, Y. I., and Huang, L. L. (1991). The effect of microinjection of amino acids into the nucleus tractus solitarius on the diving bradycardia in the rat. Chin. J. Physiol. 34, 167–177.
Hulin, M. S., and Quinn, R. (2006). “Wild and black rats,” in The Laboratory Rat, eds M. A. Suckow, S. H. Weisbroth, and C. L. Franklin (Burlington, MA: Elsevier Academic Press), 865–882.
Irving, L. (1934). On the ability of warm-blooded animals to survive without breathing. Scientific Mon. 38, 422–428.
Jones, D. R., Fisher, H. D., McTaggart, S., and West, N. H. (1973). Heart rate during breath holding and diving in unrestrained harbor seal (Phoca vitulina richardsi). Can. J. Zool. 51, 671–680.
Jones, D. R., West, N. H., Bamford, O. S., Drummond, P. C., and Lord, R. A. (1982). The effect of the stress of forcible submergence on the diving response in muskrats (Ondatra zibethica). Can. J. Zool. 60, 187–193.
Kelliher, P., Connor, T. J., Harkin, A., Sanchez, C., Kelly, J. P., and Leonard, B. E. (2000). Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiol. Behav. 69, 531–539.
Kloting, I., Nitschke, C., and Brandt, J. (2003). Impact of genetic profiles on experimental studies: outbred versus wild rats. Toxicol. Appl. Pharmacol. 189, 68–71.
Koppanyi, T., and Dooley, M. S. (1929). Submergence and postural apnea in the muskrat. Am. J. Physiol. 88, 592–595.
Lin, Y. C. (1974). Autonomic nervous control of cardiovascular responses during diving in the rat. Am. J. Physiol. 227, 601–605.
Lin, Y. C., and Baker, D. G. (1975). Cardiac output and its distribution during diving in the rat. Am. J. Physiol. 228, 733–737.
Lindsey, J. R., Baker, H. J., and Weisbroth, S. H. (2006). “Historical foundations,” in The Laboratory Rat, eds M. A. Suckow, S. H. Weisbroth, and C. L. Franklin (Burlington, MA: Elsevier Academic Press), 1–52.
Linthorst, A. C., Penalva, R. G., Flachskamm, C., Holsboer, F., and Reul, J. M. (2002). Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur. J. Neurosci. 16, 2441–2452.
MacArthur, R. A. (1978). Winter movements and home ranges of the muskrat. Can. Field Nat. 92, 345–349.
MacArthur, R. A. (1979a). Dynamics of body cooling in acclimatized muskrats (Ondatra zibethicus). J. Therm. Biol. 4, 273–276.
MacArthur, R. A. (1979b). Seasonal patterns of body temperature and activity in free-ranging muskrats (Ondatra zibethicus). Can. J. Zool. 57, 25–33.
MacArthur, R. A. (1980). Daily and seasonal activity patterns of the muskrat (Ondatra zibethicus) as revealed by radiotelemetry. Holarctic Ecol. 3, 1–9.
MacArthur, R. A. (1984a). Aquatic thermoregulation in the muskrat (Ondatra zibethicus): energy demands of swimming and diving. Can. J. Zool. 62, 241–248.
MacArthur, R. A. (1984b). Microenvironment gas concentrations and tolerance to hypercapnia in the muskrat Ondatra zibethicus. Physiol. Zool. 57, 85–98.
MacArthur, R. A. (1984c). Seasonal changes in hematological and respiratory properties of muskrat (Ondatra zibethicus) blood. Can. J. Zool. 62, 537–545.
MacArthur, R. A. (1986a). Brown fat and aquatic temperature regulation in muskrats, Ondatra zibethicus. Physiol. Zool. 306–317.
MacArthur, R. A. (1986b). Effects of CO2 inhalation on acid-base balance and thermal recovery following cold water dives by the muskrat (Ondatra zibethicus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 156, 339–346.
MacArthur, R. A. (1986c). Metabolic and behavioral responses of muskrat to elevated CO2 in a simulated winter microhabitat. Can. J. Zool. 64, 738–743.
MacArthur, R. A. (1990). Seasonal changes in the oxygen storage capacity and aerobic dive limits of the muskrat (Ondatra zibethicus). J. Comp. Physiol. B 160, 593–599.
MacArthur, R. A. (1992). Foraging range and aerobic endurance of muskrats diving under ice. J. Mammal. 73, 565–569.
MacArthur, R. A., and Aleksiuk, M. (1979). Seasonal microenvironments of the muskrat (Ondatra zibethicus) in a northern marsh. J. Mammal. 60, 146–154.
MacArthur, R. A., and Campbell, K. L. (1994). Heat increment of feeding and its thermoregulatory benefit in the muskrat (Ondatra zibethicus). J. Comp. Physiol. B, Biochem. Syst. Environ. Physiol. 164, 141–146.
MacArthur, R. A., Humphries, M. M., Fines, G. A., and Campbell, K. L. (2001). Body oxygen stores, aerobic dive limits, and the diving abilities of juvenile and adult muskrats (Ondatra zibethicus). Physiol. Biochem. Zool. 74, 178–190.
MacArthur, R. A., Humphries, M. M., and Jeske, D. (1997). Huddling behavior and the foraging efficiency of muskrats. J. Mammal. 78, 850–858.
MacArthur, R. A., and Karpan, C. M. (1989). Heart rates of muskrats diving under simulated field conditions: persistence of the bradycardia response and factors modifying its expression. Can. J. Zool. 67, 1783–1792.
MacArthur, R. A., and Krause, R. E. (1989). Energy requirements of freely diving muskrats (Ondatra zibethicus). Can. J. Zool. 67, 2194–2200.
MacArthur, R. A., Weseen, G. L., and Campbell, K. L. (2003). Diving experience and the aerobic dive capacity of muskrats: does training produce a better diver? J. Exp. Biol. 206, 1153–1161.
McCulloch, P. F. (2003). Globosa neurons: a distinct subgroup of noradrenergic neurons in the caudal pons of rats. Brain Res. 964, 164–167.
McCulloch, P. F. (2005). Activation of the trigeminal medullary dorsal horn during voluntary diving in rats. Brain Res. 1051, 194–198.
McCulloch, P. F., Dinovo, K. M., and Connolly, T. M. (2010). The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R224–R234.
McCulloch, P. F., Faber, K. M., and Panneton, W. M. (1999a). Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res. 830, 24–31.
McCulloch, P. F., Panneton, W. M., and Guyenet, P. G. (1999b). The rostral ventrolateral medulla mediates the sympathoexcitation produced by chemical stimulation of the rat nasal mucosa. J. Physiol. (Lond.) 516, 471–484.
McCulloch, P. F., and Jones, D. R. (1990). Cortical influences on diving bradycardia in muskrats (Ondatra zibethicus). Physiol. Zool. 63, 1098–1117.
McCulloch, P. F., Ollenberger, G. P., Bekar, L. K., and West, N. H. (1997). Trigeminal and chemoreceptor contributions to bradycardia during voluntary dives in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 273, R814–R822.
McCulloch, P. F., and Panneton, W. M. (1997). FOS immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience 78, 913–925.
McCulloch, P. F., and Panneton, W. M. (2003). Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res. 984, 42–53.
McCulloch, P. F., Paterson, I. A., and West, N. H. (1995). An intact glutamatergic trigeminal pathway is essential for the cardiac response to simulated diving. Am. J. Physiol. Regul. Integr. Comp. Physiol. 269, R669–R677.
McCulloch, P. F., and West, N. H. (1992). Cardiovascular responses to nasal water flow in rats are unaffected by chemoreceptor drive. Am. J. Physiol. Regul. Integr. Comp. Physiol. 263, R1049–R1056.
McIntyre, I. W., Campbell, K. L., and MacArthur, R. A. (2002). Body oxygen stores, aerobic dive limits and diving behaviour of the star-nosed mole (Condylura cristata) and comparisons with non-aquatic talpids. J. Exp. Biol. 205, 45–54.
McKean, T., and Landon, R. (1982). Comparison of the response of muskrat, rabbit, and guinea pig heart muscle to hypoxia. Am. J. Physiol. 243, R245–R250.
McKean, T., Schmidt, D., Tingey, J. M., Wingerson, D., and Seeb, L. W. (1986). The use of glucose, lactate, pyruvate, and palmitic acid by muskrat and guinea pig hearts. Physiol. Zool. 59, 283–292.
McKean, T. A. (1984). Response of isolated muskrat and guinea pig hearts to hypoxia. Physiol. Zool. 57, 557–562.
McKean, T. A. (1991). Calcium uptake by mitochondria isolated from muskrat and guinea pig hearts. J. Exp. Biol. 157, 133.
Minckler, J., and Pease, F. D. (1938). A colony of albino rats existing under feral conditions. Science 87, 460–461.
Nagel, J. A., and Kemble, E. D. (1974). The muskrat (Ondatra zibethicus) as a laboratory animal. Behav. Res. Methods Instrum. 6, 37–39.
Navarrete, S. A., and Castilla, J. C. (1993). Predation by Norway rats in the intertidal zone of central Chile. Mar. Ecol. Prog. Ser. 92, 187–199.
Nieder, L., Cagnin, M., and Parisi, V. (1982). Burrowing and feeding behaviour in the rat. Anim. Behav. 30, 837–844.
Ollenberger, G. P., Matte, G., Wilkinson, A. A., and West, N. H. (1998). Relative distribution of blood flow in rats during surface and submerged swimming. Comp. Biochem. Physiol. A Physiol. 119A, 271–277.
Ollenberger, G. P., and West, N. H. (1998a). Contribution of hypercapnia and trigeminal stimulation to cerebrovascular dilation during simulated diving. Am. J. Physiol. Regul. Integr. Comp. Physiol. 274, R921–R930.
Ollenberger, G. P., and West, N. H. (1998b). Distribution of regional cerebral blood flow in voluntarily diving rats. J. Exp. Biol. 201, 549–558.
Panneton, W. M. (1990). Controlled bradycardia induced by nasal stimulation in the muskrat, Ondatra zibethicus. J. Auton. Nerv. Syst. 30, 253–264.
Panneton, W. M. (1991a). Primary afferent projections from the upper respiratory tract in the muskrat. J. Comp. Neurol. 308, 51–65.
Panneton, W. M. (1991b). Trigeminal mediation of the diving response in the muskrat. Brain Res. 560, 321–325.
Panneton, W. M., Gan, Q., and Dahms, T. E. (2010a). Cardiorespiratory and neural consequences of rats brought past their aerobic dive limit. J. Appl. Physiol. 109, 1256–1269.
Panneton, W. M., Gan, Q., and Juric, R. (2010b). The rat: a laboratory model for studies of the diving response. J. Appl. Physiol. 108, 811–820.
Panneton, W. M., Gan, Q., and Juric, R. (2006). Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience 141, 889–906.
Panneton, W. M., Johnson, S. N., and Christensen, N. D. (1994). Trigeminal projections to the peribrachial region in the muskrat. Neuroscience 58, 605–625.
Panneton, W. M., McCulloch, P. F., and Sun, W. (2000). Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res. 874, 48–65.
Panneton, W. M., McCulloch, P. F., Tan, Y., Tan, Y., and Yavari, P. (1996). Brainstem origin of preganglionic cardiac motoneurons in the muskrat. Brain Res. 738, 342–346.
Panneton, W. M., and Watson, B. J. (1991). Stereotaxic atlas of the brainstem of the muskrat, Ondatra zibethicus. Brain Res. Bull. 26, 479–509.
Panneton, W. M., and Yavari, P. (1995). A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat Ondatra zibethicus: evidence for excitatory amino acid transmission. Brain Res. 691, 37–45.
Parisi, V., and Gandolfi, G. (1974). Further aspects of the predation by rats on various mollusc species. Boll. Zool. 41, 87–106.
Paxinos, G., Carrive, P., Wang, H., and Wang, P. Y. (1999). Chemoarchitectonic Atlas of the Rat Brainstem. San Diego, CA: Academic Press.
Paxinos, G., and Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates. New York: Academic Press.
Philipeaux, M. (1856). Note sur l’exstirpation des capsules surrenales chez les rats albinos (Mus rattus). Compt. Rend. Hebd. Seances Acad. Sci. 43, 904–906.
Price, E. O., and Huck, U. W. (1976). Open-field behavior of wild and domestic Norway rats. Anim. Learn. Behav. 4, 125–130.
Ramirez, J. M., Folkow, L. P., and Blix, A. S. (2007). Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 69, 113–143.
Richter, C. P. (1954). The effects of domestication and selection on the behavior of the Norway rat. J. Natl. Cancer Inst. 15, 727–738.
Rothstein, S., Jurgens, K. D., Bartels, H., and Baumann, R. (1984). Oxygen and carbon dioxide transport in the blood of the muskrat (Ondatra zibethica). Respir. Physiol. 57, 15–22.
Rozloznik, M., Paton, J. F. R., and Dutschmann, M. (2009). Repetitive paired stimulation of nasotrigeminal and peripheral chemoreceptor afferents cause progressive potentiation of the diving bradycardia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R80–R87.
Russell, J. C., Towns, D. R., Anderson, S. H., and Clout, M. N. (2005). Intercepting the first rat ashore. Nature 437, 1107.
Rybka, E. J., and McCulloch, P. F. (2006). The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res. 1075, 122–132.
Schapiro, S., Salas, M., and Vokovich, K. (1970). Hormonal effects on ontogeny of swimming ability in the rat: assessment of central nervous system development. Science 168, 147–152.
Schmidt-Nielsen, K. (1983). Animal Physiology: Adaptation and Environment. New York: Cambridge University Press.
Scholander, P. F. (1940). Experimental investigations on the respiratory function in diving animals and birds. Hvalradets Skrifter 22, 1–131.
Shereshkov, V. I., Shumilova, T. E., Kuzmin, D. A., Yanvareva, I. N., and Nozdrachev, A. D. (2006). Effects of hypoxic factors on cardiac chronotropic reactions in the muskrat Ondatra zibethicus in free behavior. J. Evol. Biochem. Physiol. 42, 461–468.
Signore, P. E., and Jones, D. R. (1995). Effect of pharmacological blockade on cardiovascular responses to voluntary and forced diving in muskrats. J. Exp. Biol. 198, 2307–2315.
Signore, P. E., and Jones, D. R. (1996). Autonomic nervous control of heart rate in muskrats during exercise in air and under water. J. Exp. Biol. 199, 1563–1568.
Snyder, G. K., and Binkley, E. L. (1985). Oxygen transport, tissue glycogen stores, and tissue pyruvate kinase activity in muskrats. Can. J. Zool. 63, 1440–1444.
Thompson, D., and Fedak, M. A. (1993). Cardiac responses of grey seals during diving at sea. J. Exp. Biol. 174, 139–154.
Thornton, R., Gordon, C., and Ferguson, J. H. (1978). Role of thermal stimuli in the diving response of the muskrat (Ondatra zibethica). Comp. Biochem. Physiol. 61A, 369–370.
van den Brandt, J., Kovacs, P., and Kloting, I. (2000). Metabolic variability among disease resistant inbred rat strains and in comparison with wild rats (Rattus norvegicus). Clin. Exp. Pharmacol. Physiol. 27, 793–795.
Keywords: muskrat, Ondatra zibethicus rat, Rattus norvegicus, diving response, autonomic control
Citation: McCulloch PF (2012) Animal models for investigating the central control of the mammalian diving response. Front. Physio. 3:169. doi: 10.3389/fphys.2012.00169
Received: 30 January 2012; Accepted: 09 May 2012;
Published online: 29 May 2012.
Edited by:
Andreas Fahlman, Texas A&M University, USAReviewed by:
Tobias Wang, University of Aarhus, DenmarkRussel D. Andrews, University of Alaska Fairbanks, USA
Riccardo Mozzachiodi, Texas A&M University, USA
Copyright: © 2012 McCulloch. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Paul Frederick McCulloch, Department of Physiology, Midwestern University, 555 31st Street, Downers Grove, IL 60515, USA. e-mail:cG1jY3VsQG1pZHdlc3Rlcm4uZWR1