- Department of Pharmacological and Physiological Science, St. Louis University School of Medicine, St. Louis, MO, USA
The mammalian diving response is a powerful autonomic adjustment to underwater submersion greatly affecting heart rate, arterial blood pressure, and ventilation. The bradycardia is mediated by the parasympathetic nervous system, arterial blood pressure is mediated via the sympathetic system and still other circuits mediate the respiratory changes. In the present study we investigate the cardiorespiratory responses and the brainstem neurons activated by voluntary diving of trained rats, and, compare them to control and swimming animals which did not dive. We show that the bradycardia and increase in arterial blood pressure induced by diving were significantly different than that induced by swimming. Neuronal activation was calculated after immunohistochemical processing of brainstem sections for Fos protein. Labeled neurons were counted in the caudal pressor area, the medullary dorsal horn, subnuclei of the nucleus tractus solitarii (NTS), the nucleus raphe pallidus (RPa), the rostroventrolateral medulla, the A5 area, the nucleus locus coeruleus, the Kölliker–Fuse area, and the external lateral and superior lateral subnuclei of the parabrachial nucleus. All these areas showed significant increases in Fos labeling when data from voluntary diving rats were compared to control rats and all but the commissural subnucleus of the NTS, A5 area, and RPa were significantly different from swimming rats. These data provide a substrate for more precise experiments to determine the role of these nuclei in the reflex circuits driving the diving response.
Introduction
It is an honor to pay tribute to Per Scholander, a man of immeasurable enthusiasm for science who with Laurence Irving made the original observations of the physiological changes in blood pressure to underwater submersion (Irving et al., 1942) in numerous species (Scholander and Elsner, 1968). He is considered by most the pioneer cardiovascular physiologist who initiated decades of studies on the remarkable phenomenon we call today the diving response. The work of our laboratory for the past 20 years extends his legacy by offering our observations on the neural control of the diving response.
The mammalian diving response is a powerful autonomic response comprising at least three simpler reflexes that activate the parasympathetic, sympathetic, and respiratory systems (Kooyman et al., 1981; Butler and Jones, 1982, 1997; Blix and Folkow, 1983; Elsner and Gooden, 1983; de Burgh Daly, 1984; Kooyman and Ponganis, 1998; Ferretti, 2001; Davis et al., 2004; Foster and Sheel, 2005). These simple reflexes independently can be dissociated peripherally to block the different systems associated with the response. For example, the profound bradycardia of diving induced via activation of a vagally mediated parasympathetic circuit can be eliminated with systemic administration of atropine while the sympathetic and respiratory responses persist (Lin, 1974; Nakamura and Hayashida, 1992; Yavari et al., 1996; McCulloch et al., 1997). Also, the selective peripheral vasoconstriction seen in diving can be blocked with sympatholytics while the bradycardia and apnea is maintained (Lin, 1974; Yavari et al., 1996).
These simple reflexes are not dependent on suprabulbar neurons since the diving response is still elicited in mammals after transecting the brainstem through the thalamus (Drummond and Jones, 1979), colliculi (Huxley, 1913; Martner et al., 1977; Panneton et al., 2010b), or pons (Panneton et al., 2012). Thus we speculate that the circuits driving these behaviors are intrinsic to the medulla and spinal cord, but may be modulated by higher order neurons. Indeed, it has been suggested that these reflexes can be modulated by suprabulbar neurons (Blix, 1988). Seals often show either little bradycardia when diving voluntarily (Kooyman and Campbell, 1972) or may reduce heart rate (HR) in anticipation of underwater submersion (Casson and Ronald, 1975), suggesting that suprabulbar influences may indeed impinge on these medullary reflex circuits. Similarly, the hemodynamic responses to “forced” submersions when mammals are involuntarily “dunked” underwater are subtly dissimilar to the hemodynamics of voluntary diving (Drummond and Jones, 1979; Kooyman, 1989; McCulloch and Jones, 1990; Jobsis et al., 2001; Panneton et al., 2010b).
Eliciting these reflexes of the diving response apparently is dependent on innervation of the nares, since either submersion or wetting the snout induces the diving response in muskrats (Koppányi and Dooley, 1929; Drummond and Jones, 1979), rats (Lin, 1974; Panneton et al., 2010b), cats (Martner et al., 1977), pigs (Schagatay and Van Kampen, 1995), and rabbits, sheep, and lambs (Tchobroutsky et al., 1969), while numbing either the nares or the nasal mucosa inhibits these responses (Dykes, 1974; Drummond and Jones, 1979; McCulloch et al., 1995; Yavari et al., 1996; Kratschmer, 2001). In this regard we investigated the anterior ethmoidal nerve (AEN), since its receptive fields surround the nares and innervate anterior parts of the nasal mucosa (see Panneton et al., 2006, for review). This nerve projects densely into areas of the medullary dorsal horn (MDH; Panneton, 1991a; Panneton et al., 2006) where both neurons are activated (McCulloch and Panneton, 1997; McCulloch, 2005) and the bradycardia and apneic responses due to nasal stimulation are inhibited (Panneton, 1991b; Panneton and Yavari, 1995). Neuroanatomical tracing techniques show that neurons in similar parts of the MDH project to numerous nuclei in the brainstem (Panneton et al., 2000, 2006), but these tracing techniques provide no information whether these nuclei are activated by underwater diving.
The present investigation expands those of others studying the distribution of neurons labeled with the immediate early gene Fos activated either by nasal stimulation (Anton et al., 1991; Gieroba et al., 1994; Dutschmann and Herbert, 1997; McCulloch and Panneton, 1997; Dutschmann et al., 1998; Rybka and McCulloch, 2006) or by underwater diving of awake rats (McCulloch and Panneton, 2003; McCulloch, 2005). However, the nasal mucosa either was stimulated with irritating vapors or underwater submergence between 12 and 120 times over periods up to 2 h in all of these studies; excessive stimulation is known to induce spurious labeling in the Fos technique (Bullitt et al., 1992) yet a single stimulus trial can still activate neurons (Panneton et al., 2010a). In addition, the experimental animals were anesthetized in most of these reports using nasal stimulation, and anesthesia itself induces much confounding activation of neurons (Takayama et al., 1994).
Hemodynamic data in the present study are obtained from a single voluntary trial of either swimming or submersion in awake, previously trained rats instrumented with telemetric transmitters. The cardiorespiratory responses either to diving or swimming are reported herein, as well as the quantity of neurons c-Fos-labeled in various brainstem nuclei. These experiments make progress toward our long-term goal to establish the neural circuits in the brainstem driving the reflexes comprising the diving response. Much of this data has been presented previously in abstract from Panneton et al. (2009).
Materials and Methods
Cardiovascular Data
Six initially immature (70–90 g) and 10 adult (∼275–325 g) Sprague-Dawley male rats were obtained commercially (Harlan, Indianapolis, IN, USA) and used in this study. All protocols were approved by the Animal Care Committee of Saint Louis University and followed the guidelines of the National Institutes of Health Guide for Care and Handling of Laboratory Animals.
Six immature rats were trained 5 days/week for 5–6 weeks and learned to swim or dive underwater through a maze (McCulloch and Panneton, 2003; Panneton et al., 2010b). Six mature rats were trained for about 2 weeks only to swim through the maze. Once these rats reached 270–290 g they were anesthetized with ketamine/xylazine (60/40 mg/kg; IP) and the catheter of a biotelemetric transmitter (Model PA-C40; Data Sciences International, DSI; St. Paul, MN, USA) inserted into their femoral arteries while the transmitter itself was implanted in their abdominal cavities. The rats healed for 5–7 days without training, but they did not forget their willingness either to swim or submerge underwater. Cardiovascular data were obtained from these rats prior to their water tasks; this served as control data. The trained rats voluntarily either swam or dove underwater and hemodynamic data were recorded for a single trial.
The transmitter’s broadcast was received with a radio receiver (Model RLA3000; DSI), relayed to a Calibrated Pressure Analog Adaptor (Model R11CPA; DSI), and transferred through an A–D interface (1401 plus; Cambridge Electronic Design, CED; Cambridge, UK), stored in the computer, and analyzed using Spike 2 software (CED). Systolic, diastolic, and mean arterial blood pressure (MABP) were calculated from traces and HR was determined by counting peaks of systolic pressure. We assumed the rats made no attempt to breathe while underwater since they showed no difficulty in breathing after their experience and none drowned during submergence.
Neuroanatomical Data
Four untrained adult rats remained isolated in their home cage prior to perfusion and served as controls for the Fos counts. These rats, and all of the experimental rats 2 h after diving or swimming, were deeply anesthetized (Sleepaway, 0.1 ml/100 g; IP) and perfused through the heart with a peristaltic pump first with a saline–procaine solution, followed immediately by a fixative of 4% paraformaldehyde and 3% sucrose in 0.1 M sodium phosphate buffer (PB; pH 7.3). Brains and spinal cords were removed and refrigerated in the fixative with 20% sucrose at 4°C. The brains were blocked in the transverse plane using a precision brain slicer prior to cutting frozen transverse sections (40 μm) with a microtome.
Every third section was processed immunohistochemically overnight with antibodies against Fos (rabbit polyclonal IgG for c-fos p62; 1:20,000; Santa Cruz Biotechnology, Inc.) mixed in 0.1 M PB with 0.3% Triton. On the following day, the sections were washed, incubated for 1 h in goat anti-rabbit biotinylated secondary IgG (1:500; Vector Labs), washed again, and then incubated in an ABC complex (Vectastain Elite; Vector Labs) for another hour. The Fos antigen was visualized in the brainstem with the chromogen diaminobenzidine (DAB) enhanced with nickel ammonium sulfate. Sections were mounted serially on gelatin-coated slides, counter-stained with Neutral Red, dehydrated in alcohol, defatted in xylene, and coverslipped with Permount. Fos-positive neurons appeared as cells with black-labeled nuclei and were visualized with bright field optics (Nikon E800).
Fos-positive neurons in 12 nuclei/subnuclei of the brainstem were photographed digitally (MicroImager II) with Northern Eclipse Software (Empix, Inc.). Six to eight samples from sections on either side were chosen through the nucleus in all cases except for the midline commissural subnucleus of the nucleus tractus solitarii (NTS) and raphe pallidus (RPa), where three sections were chosen/case, respectively. Sections photographed bilaterally through the caudal pressor area (CPA; six photomicrographs) were at levels of, and immediately adjacent to, the caudal pole of the lateral reticular nucleus as defined previously (Sun and Panneton, 2002, 2005). Those through the commissural subnucleus of the NTS (comNTS; three photomicrographs) were taken of both sides from three sections immediately caudal to the calamus scriptorius while those for the dorsal lateral (dlNTS; eight photomicrographs) and medial (medNTS; eight photomicrographs) subnuclei of the NTS were photographed immediately caudal to the obex, overlapping the area postrema. The MDH was photographed bilaterally in the four sections (eight photomicrographs) rostral to the calamus scriptorius, with rostral sections overlapping the caudal pole of the subnucleus interpolaris. The nucleus RPa (three photomicrographs) was analyzed just rostral to the rostral pole of the inferior olivary complex, and coincided with the caudal pole of the facial motor nucleus. The rostroventrolateral medulla (RVLM; eight photomicrographs) was photographed bilaterally in four sections immediately caudal to the facial motor nucleus, the A5 area bilaterally (six photomicrographs) when juxtaposed medially to the emerging roots of the facial nerve, and the locus coeruleus (LC; six photomicrographs) in its entirety bilaterally. The Kölliker–Fuse area (KF; six photomicrographs), and external lateral (PBel; six photomicrographs) and superior lateral (PBsl; six photomicrographs) subnuclei of the peribrachial complex were photographed bilaterally in their entirety.
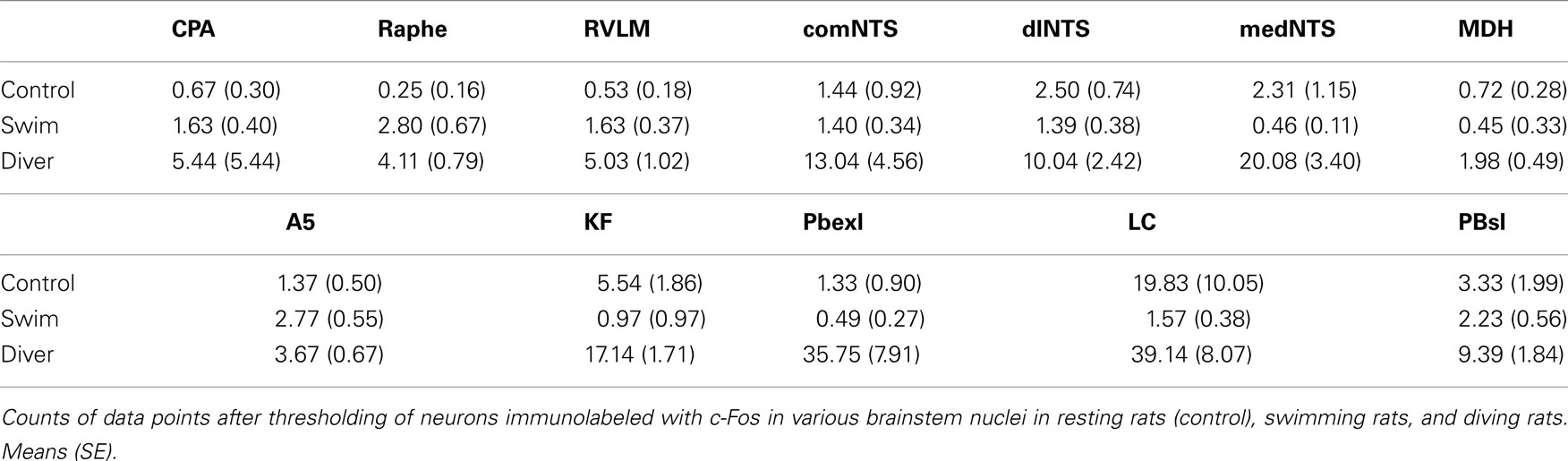
Nuclear areas were outlined in Northern Eclipse software; representative outlines are shown in Figure 7. Neurons were considered Fos-positive if visualized microscopically with nuclei containing a black immunoprecipitate, but the nuclei were stained to various degrees making consistent counts problematic. Thus, labeled profiles were discriminated and quantified with a threshold function of the Northern Eclipse software (Figure 1). Similar parameters (threshold color: red range 0, 0; green range 8, 49; blue range 14, 67) were maintained for analyses to prevent biased cell counts. This procedure negated counts of lighter, presumably less optimally labeled neurons (Bullitt et al., 1992) and presumably eliminated investigator bias. Sections were drawn with a Nikon E600 microscope and Neurolucida software (MicroBrightField, Inc.). The photomicrographs were standardized using levels, brightness and contrast in Adobe Photoshop software (v.7) and aligned in Adobe Illustrator software (v.11) for figures. All nomenclature and abbreviations are from a stereotaxic rat atlas (Paxinos and Watson, 1998) except for designations of some subnuclei.
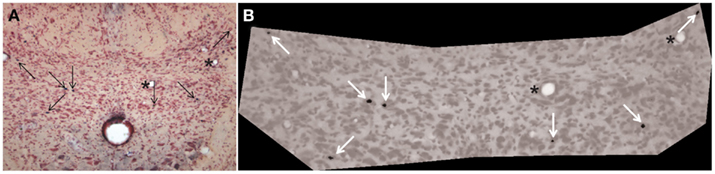
Figure 1. Photomicrographs illustrating threshold determination for quantization of neurons counted as Fos-positive. The comNTS in (A) showed seven neurons with black precipitate in their nuclei (arrows). All seven of these neurons seen visually were counted after software discrimination [(B) white arrows] in this case, but this did not always happen. Neurons sometimes were excluded if they were too lightly stained or too small (<11 pixels). Asterisks show similar blood vessels in either photomicrograph.
Data Analysis
Means and SEs (M ± SE) were determined for experimental and control groups. HR and MABP during swimming and diving were compared to data taken just prior to submersion in all experimental rats and compared for significance (SPSS software; v. 13) using the Independent Samples T-test. Counts of discriminated data points of swimming and diving groups were compared also using the Independent Samples T-test. Data are presented as M ± SE and significance was calculated as p < 0.05.
Results
Cardiovascular Data
The diving rats voluntarily dove underwater and traversed the maze in an average of 16 s while swimming rats averaged 20 s. All voluntary diving rats (n = 6) showed a marked drop in HR and an increase in MABP to diving (Figure 2), but no changes were seen during swimming (n = 6). HR dropped significantly (p < 0.001) from 434 ± 11 to 101 ± 7 bpm, a bradycardia of 77%, during underwater submersion (Figure 3) while MABP rose significantly (p < 0.05), rising from 112 ± 3 to 130 ± 2 mmHg, a 14% rise (Figure 3).
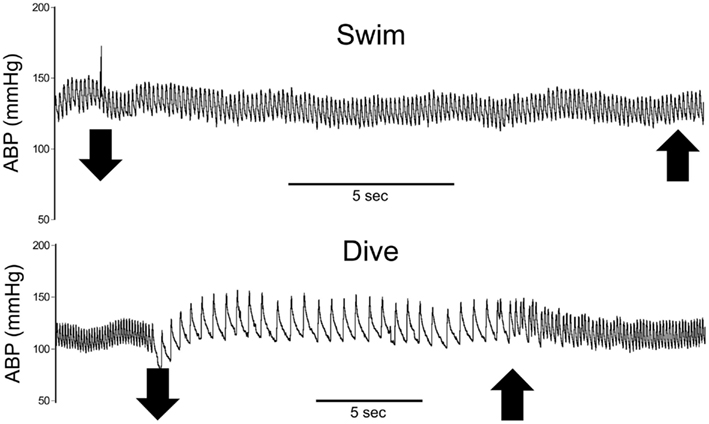
Figure 2. Traces of representative cardiovascular responses of rats to either swimming on the surface or underwater diving. Note the marked bradycardia and slight increase in arterial blood pressure while rats were underwater but no change while rats swam on the surface. Large down arrows indicate when the rat entered or submerged underwater while large up arrows indicate emersion from the water. ABP, arterial blood pressure; HR, heart rate.
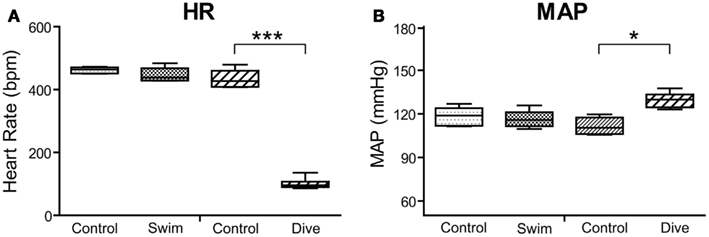
Figure 3. Box plots illustrating changes in heart rate (A) and MAP (B) in rats to either swimming or underwater diving. Note that only diving behavior induced significant bradycardia and increases in mean arterial blood pressure. ***p < 0.001; *p < 0.05.
Neuroanatomical Data
Fos labeling
Neurons labeled with Fos were visualized throughout the brainstem after swimming and underwater diving (Figure 4). Some areas were labeled sparsely in all rats, even control animals which just sat in their cages, and these areas were not analyzed. These nuclei included sporadic labeling in the parvocellular lateral reticular nucleus, the basal pontine nuclei, dorsal parts of the ventral cochlear nucleus, the dorsal tegmental nucleus, the central gray (pars alpha), neurons surrounding the trigeminal motor root, and a few close to the paracochlear glial substance. It appeared qualitatively that Fos-labeled neurons in some of these areas were increased subtly in diving rats over control and swimming rats in the parvocellular lateral reticular nucleus, near the trigeminal motor root, and the paracochlear glial substance, but these areas were not quantified. In addition, diving rats showed inconsistent, sporadic labeling in laminae III–IV of the MDH, along the ventral surface of the medulla, the lateral medulla, and the superior salivatory nucleus.
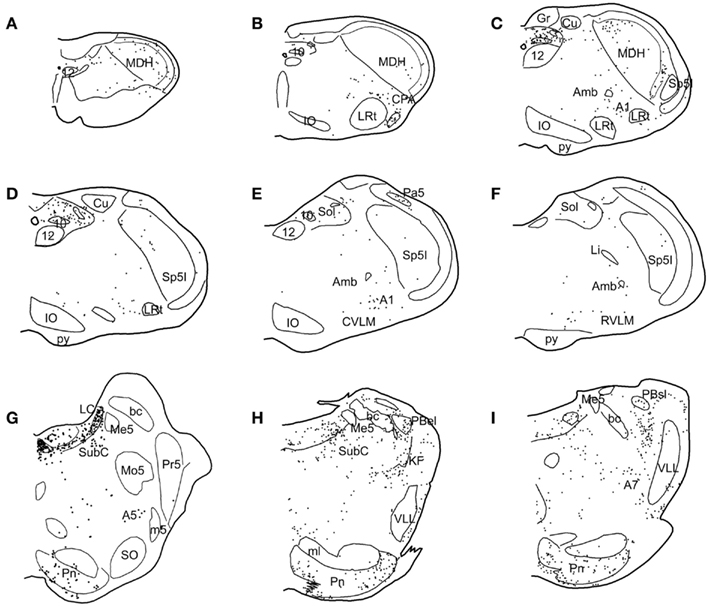
Figure 4. Line drawings illustrating the distribution of neurons immunolabeled with Fos in the brainstem after underwater diving. Each dot represents a single, non-thresholded neuron observable through the microscope. Immunolabeling for Fos was discriminated by computer in twelve nuclei/subnuclei through the medulla (A–F) and pons (G–I) from caudal (A) to rostral (I) and quantified; the results are seen in Table 1. Abbreviations: A1, A5, A7, catecholamine groups; Amb, nucleus ambiguus; Cu, cuneate nucleus; CVLM, caudal ventrolateral medulla; DT, dorsal tegmental nucleus; Gr, gracile nucleus; IO, inferior olivary nucleus; KF, Kölliker–Fuse nucleus; LC, nucleus locus coeruleus; LI, nucleus linearis; LRt, lateral reticular nucleus; MDH, medullary dorsal horn; Me5, mesencephalic trigeminal nucleus; Mo5, motor trigeminal nucleus; Pa5, paratrigeminal nucleus; PBel, external lateral subnucleus of the peribrachial complex; PBsl, superior lateral subnucleus of the peribrachial complex; Pn, pontine nucleus; Pr5, principal sensory trigeminal nucleus; SO, superior olivary nucleus; Sol, nucleus tractus solitarii; Sp5I, Spinal 5 nucleus–subnucleus interpolaris; SubC, nucleus subcoeruleus; VLL, ventral nucleus of lateral lemniscus; 10, dorsal nucleus of the vagus nerve; 12, hypoglossal nucleus; bc, brachium conjunctivum; m5, motor root of the trigeminal nerve; py, pyramidal tract; 7n, facial nerve.
Neurons in 12 brainstem nuclei/subnuclei in control (n = 4), swimming (n = 5), and diving (n = 6) groups were then counted after they met threshold functions of the computer (Table 1). These nuclei were selected since they were considered important either in the neural circuitry of the diving response or have been implicated in cardiorespiratory control. These nuclei included the comNTS, dlNTS (note: the dorsomedial subnucleus of Paxinos and Watson, 1998), and medNTS (note: the central subnucleus of Paxinos and Watson, 1998) subnuclei of the NTS, the CPA, the MDH, the RVLM, the RPa, the A5 area, the LC, the KF, and the PBel and PBsl subnuclei of the parabrachial nucleus.
Data points discriminated from all these nuclei of control rats were significantly less than those discriminated for diving rats. However, when comparing discriminated data points between swimming and diving rats (Table 1), those in the commNTS, the RPa, and the A5 group were not significantly different (Figure 5A). Discriminated data points in the dlNTS, MDH, CPA, and RVLM however were significantly different to p < 0.05 between swimmers and divers (Table 1; Figures 5B–F and 6), to p < 0.01 in the medNTS, LC, PBel, and PBsl (Figures 5G–L and 6I,K,L), and to p < 0.001 in the KF (Figure 6J).
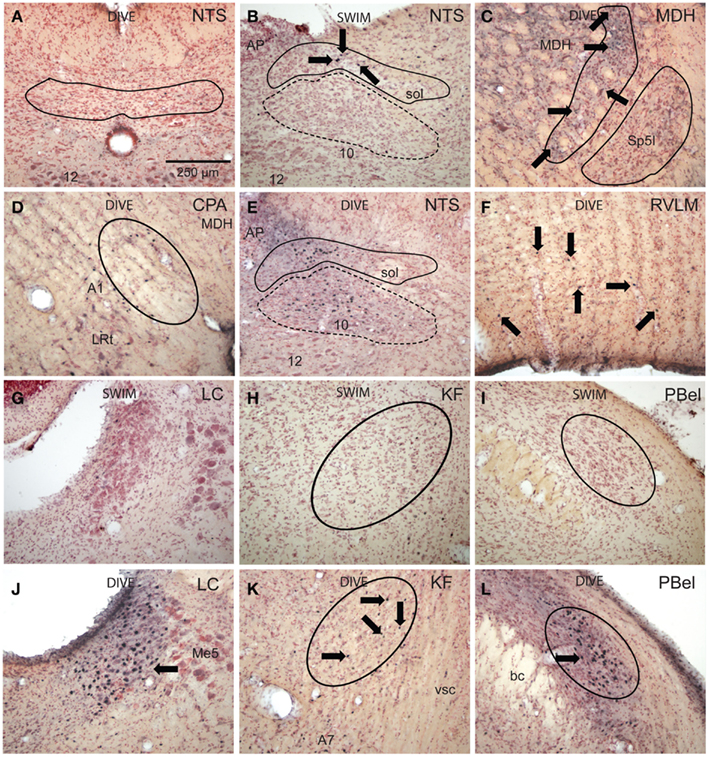
Figure 5. Bright field photomicrographs of sections showing immunolabeling of Fos in selected brainstem nuclei. Diving behavior activated few neurons in subnucleus commissuralis of the nucleus tractus solitarii [(A) NTS, outline], but numerous neurons near the levels of the obex in its dorsolateral [(B,E) solid outline] and medial subnuclei [(B,E) dashed outline] compared to swimming rats. Voluntary diving behavior in rats induced relatively few, but significant numbers of Fos-labeled neurons in the medullary dorsal horn [(C) MDH, projection field of the anterior ethmoidal nerve is outlined] over swimming rats. Note these small labeled neurons (arrows) are in the substantia gelatinosa, presumably lamina II, displaced dorsally and medially by the caudal pole of the subnucleus interpolaris (Sp5I, outlined). Neurons labeled with Fos were noted in the caudal pressure area [(D) CPA, circled] as well as presumptive noradrenergic neurons in the juxtaposed A1 area after diving. There was a significant increase of Fos labeling (arrows) in the RVLM after diving (F) compared to swimming behavior. Little label was seen in the locus coeruleus (G), Kölliker–Fuse area (H), or external lateral subnuclei (I) of the parabrachial complex after swimming, but these areas were significantly labeled after diving behavior [(J,K,L) respectively]. Arrows point to Fos-labeled neurons; all figures at the same magnification.
Discussion
Activation of brainstem neurons of rats were compared after three qualitatively different behaviors, i.e., resting in a cage, surface swimming through a maze, and navigating the same maze while underwater. We show that the cardiovascular parameters of HR and MABP were significantly different after exercise in diving rats versus swimming rats. This study also shows for the first time activation of brainstem neurons of rats after either swimming or diving behaviors. Moreover, significantly more profiles immunoreactive to Fos antigen were found after diving in several brainstem nuclei known to be important in cardiorespiratory behavior.
Since the independent parasympathetic, sympathetic, and respiratory components of the diving response apparently are all activated by the same peripheral stimulation, e.g., underwater submersion, the term diving response has replaced the former term the diving reflex. Nevertheless, these independent autonomic reflexes of diving still must have a peripheral sensory neuron, usually one or several central interneurons, and selective motoneurons to drive the bradycardia, peripheral vasoconstriction, and expiratory apnea. The neural circuitry driving these responses is intrinsic to the brainstem since the responses persist in neurally truncated mammals (Martner et al., 1977; Panneton et al., 2010b, 2012). Thus the pathways comprising the diving response are like many other reflexes with complete circuits within the spinal cord or brainstem. It has been a goal of our laboratory to establish the brainstem circuitry utilized by the three reflexes comprising the diving response. The present report adds to the considerable progress toward this goal.
Technical Considerations
The Fos technique potentially can identify the sensory, intermediate, and motor limbs of a reflex pathway, but this technique has limitations. The synthesis of Fos protein generally follows the excitation of neurons. However, Fos protein generation may be masked in continuously firing neurons (Dragunow and Faull, 1989). Also, neurons which are inhibited in a reflex circuit will not generate Fos protein, while other neurons may not express Fos regardless of the stimulus used (Dragunow and Faull, 1989). In a previous study utilizing the Fos technique in the muskrat (McCulloch and Panneton, 1997), we could not determine significance in most nuclei from control animals, perhaps since these animals were anesthetized and had experienced prior surgery, both of which greatly cloud interpretation of data (Takayama et al., 1994; Hoskin and Goadsby, 1999). These animals also were subjected to excessive stimulation, which can activate confounding neurons (Bullitt et al., 1992). These caveats were not problematic in the present study, however, since all nuclei quantified in our awake behaving animals showed significant increases in labeling when voluntary diving rats were compared to control values. Moreover, significant differences were determined in several brainstem areas when data points from nuclei immunolabeled after swimming behavior were compared to diving behavior.
Quantifying neurons labeled with Fos is difficult since not all activated nuclei are stained equally in intensity, making counting lightly stained nuclei subjective. It is unknown if faint staining is due either to their relative activation or to differences in immunohistochemical processing. Thus Fos-labeled neurons in the present study were discriminated by computer similar to Graham et al. (1995) in an effort to reduce this bias. An example of such discrimination is provided (Figure 1) of a section through the commissural subnucleus of the NTS of a diving rat showing a typical analysis. Note that all labeled nuclei in this section (Figure 1A; arrows) were discriminated and counted by the computer (Figure 1B; arrows).
Cardiovascular Data
There was no change in hemodynamics during swimming behavior in the present study, confirming data of others (Baker and Horvath, 1964; Whishaw and Schallert, 1977; McCulloch et al., 2010). The hemodynamic adjustments seen in the present study confirm our previous report in voluntary diving rats (Panneton et al., 2010b), but the increase in MABP was considerably less than that seen in another study (McCulloch et al., 2010). Numerous reports on diving mammals have shown similar discrepancies (Elsner and Gooden, 1983; de Burgh Daly, 1984; Butler and Jones, 1997) and may be related to the balance between the initiation of the parasympathetically mediated bradycardia and sympathetically mediated vasoconstriction. It also should be noted five trials/animal were used in our previous study (Panneton et al., 2010b) while only a single trial/animal was used in the present study. While there is no standard for activating Fos in neurons, we feel fewer stimulations induce less confounding data points as well as minimize stress in awake behaving animals.
The Nucleus Tractus Solitarii
The NTS, the primary relay for visceral afferent fibers, may contain interneurons of the reflex circuits driving the diving response. While the three subnuclei of the NTS quantified herein were labeled significantly with Fos in the diving versus control rats, only the dlNTS and medNTS were significantly different when compared to swimming rats. The comNTS receives numerous primary afferent fibers from neurons monitoring blood gases and is especially sensitive to hypoxia in peripheral blood (see Panneton et al., 2010b, for review). Apparently the short swimming and diving behaviors utilized in the present study were not sufficient to change partial gas pressures enough to activate many comNTS neurons. However 4/6 diving rats showed much higher activation in these neurons (Figure 6A), perhaps an introduction to the massive immunolabeling of comNTS seen when rats are submerged beyond their aerobic dive limit (Panneton et al., 2010a).
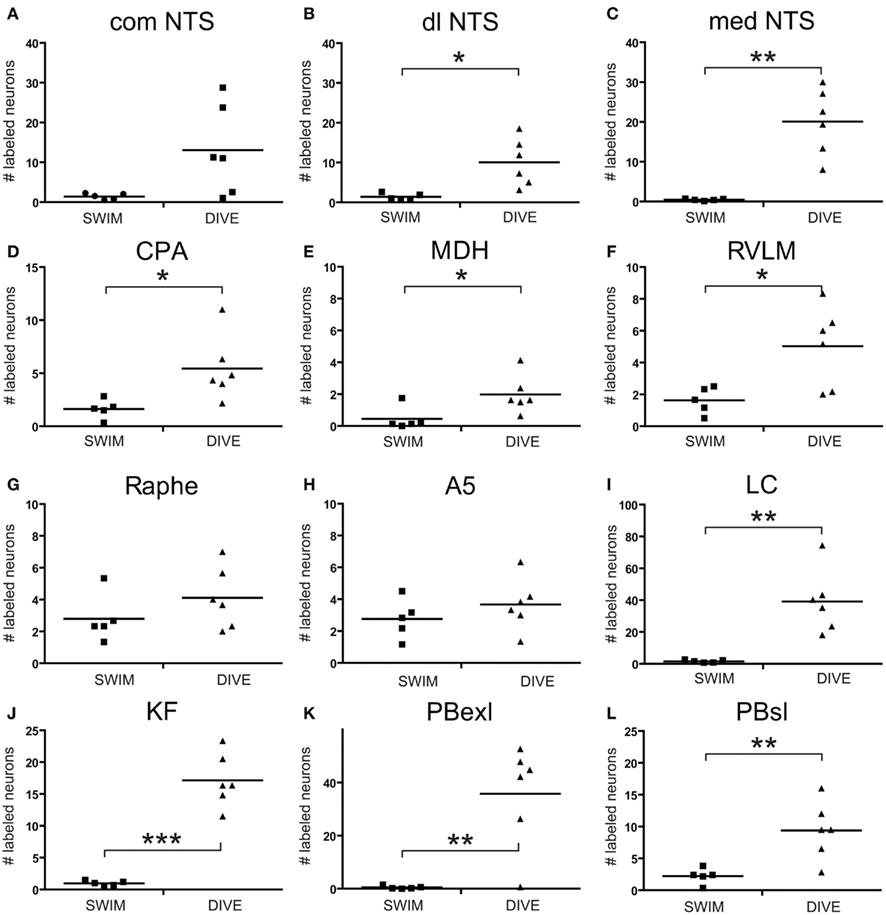
Figure 6. Scatter plots comparing the number of discriminated data points determined with thresholding in rats after swimming behavior versus those that voluntarily dove underwater. Data points were discriminated from photomicrographs with software and represent neurons labeled with Fos which met color, intensity and size determinants. Note that there was a significant increase after underwater submersion of discriminated data points in all nuclei but the comNTS, raphe, and A5 area. Square symbols mark data from individual swimming rats while triangles mark that from individual diving rats. Horizontal bars represent the mean. Independent Samples T-test; *p < 0.05, **p < 0.01, ***p < 0.001.
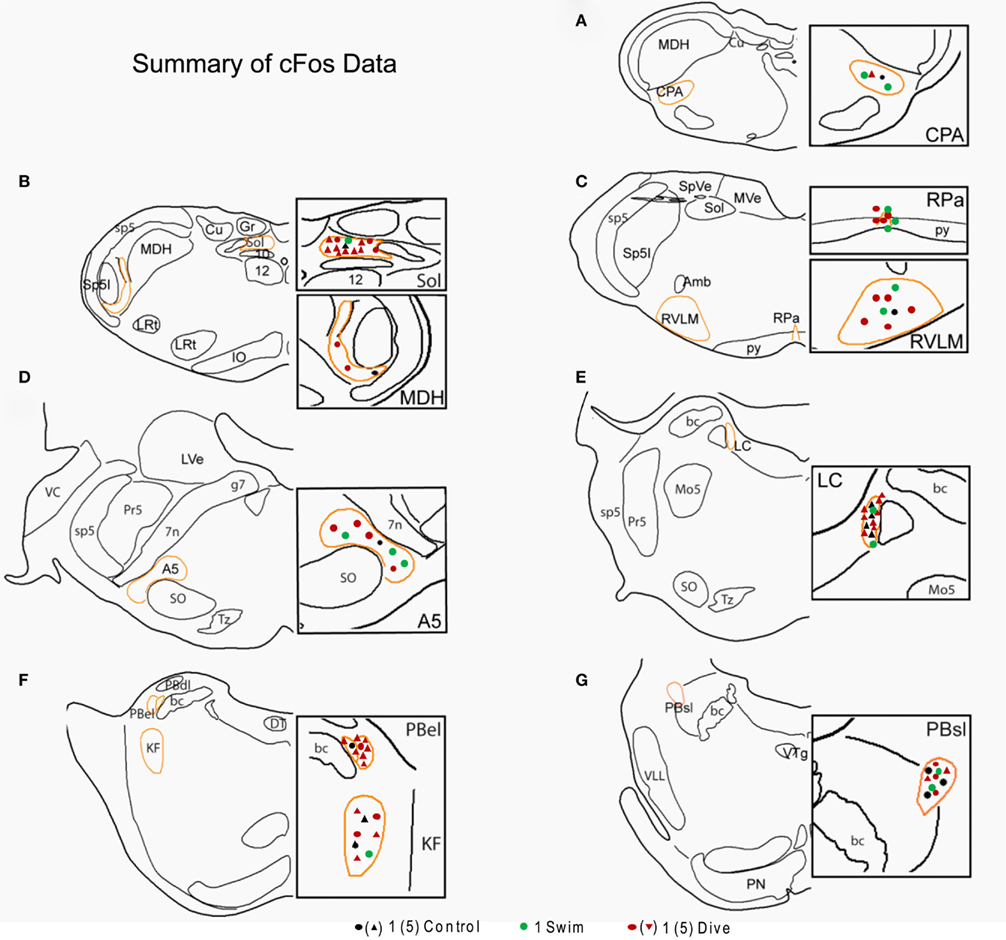
Figure 7. Line drawings comparing the mean number of data points in brainstem nuclei in control, swimming, and diving rats. Sections are drawn from the caudal medulla (A) thru the rostral pons (G). Note that underwater diving increased the number of data points in all nuclei compared to control or swimming animals. Areas circumscribed in orange outlines the area demarcated for threshold determination; these areas are magnified in the boxes on the right of the drawing. Each dot represents a single neuron while triangles represent five neurons. See Figure 4 for abbreviations.
Most labeled neurons were found in the dlNTS and medNTS and both are implicated in cardiovascular control. The dlNTS receives numerous primary afferent fibers from the carotid sinus and aortic depressor nerves (Panneton and Loewy, 1980; Housley et al., 1987; Chan and Sawchenko, 1998; Blessing et al., 1999) and contains neurons responsive to hypertensive stimuli detected neurophysiologically (Donoghue et al., 1978, 1984) as well as by activation of Fos (Chan and Sawchenko, 1994, 1998; Graham et al., 1995). Although there are but few catecholamine neurons in the dlNTS, those present are rarely double-labeled after increases of arterial blood pressure (Chan and Sawchenko, 1994, 1998). Thus these catecholamine neurons are probably not influenced by hypertensive stimuli. However, it is well known that injections of kynurenate into the NTS, which pharmacologically antagonizes excitatory amino acid receptors non-selectively, blocks the bradycardia and decrease in arterial blood pressure induced by activation of the baroreceptor reflex (Guyenet et al., 1987; Leone and Gordon, 1989); the bradycardia is mediated by both NMDA and non-NMDA receptors (Zhang and Mifflin, 1993, 1995; Frigero et al., 2000; Machado et al., 2000). Nevertheless, injections of kynurenate into dlNTS failed to block the increase in arterial blood pressure or sympathetic nerve activity induced by nasal stimulation (McCulloch et al., 1999b). Indeed, studies have shown that the baroreceptor reflex is inhibited by nasal stimulation (McCulloch et al., 1999b) and the pressor responses to nasal stimulation persist despite cutting baroreceptor nerves (Nakamura and Hayashida, 1992), suggesting the pressor response to diving may not utilize this reflex pathway during diving. Moreover, it has been shown that the cardiorespiratory components of other trigeminoautonomic reflexes also are relayed through areas other than the NTS (Kumada et al., 1977; Allen and Pronych, 1997). These data collectively argue that NTS neurons are not part of the circuit of neurons integral to the diving reflex. However, the projections originating in the ventral MDH to the dlNTS (Panneton et al., 2000, 2006) potentially could modulate these barosensitive neurons. Thus, we speculate that the significant Fos labeling in the dlNTS seen in the diving rats versus either control or swimmers possibly was due to activation of non-aminergic barosensitive neurons responding to the increase in arterial blood pressure seen with underwater submergence, but these neurons are not part of the circuit of neurons driving the diving response.
Neurons in the medNTS were significantly labeled with Fos in the diving rats and may be involved in both baro- and chemoreceptor reflexes. These neurons receive primary afferent fibers from the carotid body, carotid sinus, and aortic depressor nerves (Panneton and Loewy, 1980; Finley and Katz, 1992; Chan and Sawchenko, 1998; Blessing et al., 1999), respond electrophysiologically to chemoreceptor stimuli (Donoghue et al., 1978), and are robustly activated by hypotensive stimuli (Chan and Sawchenko, 1994, 1998; Graham et al., 1995; Dampney and Horiuchi, 2003). Moreover, blocking studies using microinjections of cobalt chloride suggest the increase in arterial blood pressure induced by electrical stimulation of the AEN is mediated by neurons in the medNTS (Dutschmann and Herbert, 1998). The medNTS contains the A2 catecholamine group and ∼70% of the A2 neurons are double-labeled with Fos in rats after underwater submersion (McCulloch and Panneton, 2003). However, few A2 neurons respond to hypertensive stimuli (Moore and Guyenet, 1985; Chan and Sawchenko, 1994; Mayne et al., 1998) and chemical depletion of amines in the NTS with 6-OHDA effects neither basal HR nor arterial pressure, nor does such depletion affect the baroreceptor reflex (Itoh et al., 1992). Nevertheless, since our diving rats experienced no hypotension, the collective data suggests medNTS is not directly part of the diving circuit per se, but agree with others (Rinaman, 2011) that A2 neurons modulate diverse roles, and speculate that many of them activated by diving reflects the organism’s response to the acute physiological stress to underwater submersion.
The MDH and CPA
The MDH serves as a primary relay for sensory fibers innervating the head and may be where the first central interneuron of a medullary reflex circuit is located. Stimulation of the skin and mucosa near the nares has been shown to initiate the diving response (Koppányi and Dooley, 1929; Tchobroutsky et al., 1969; Lin, 1974; Martner et al., 1977; Drummond and Jones, 1979; Schagatay and Van Kampen, 1995), an area innervated in part by the AEN (Williams and Warwick, 1980). Moreover, stimulating the AEN electrically induces a bradycardia, an increase in MABP and apnea, responses similar to those of diving (Dutschmann and Herbert, 1997; McCulloch et al., 1999a), and is important for the bradycardia and apnea during stimulation of the nasal mucosa (Nakamura and Hayashida, 1992; Rybka and McCulloch, 2006). Primary afferent fibers carried in the AEN of the rat project to areas of the MDH (Panneton et al., 2006) where discriminated data points representing Fos-labeled neurons were found in the present study, confirming data by others (Rybka and McCulloch, 2006; Panneton et al., 2010a). When similar areas in the MDH are blocked by injections of lidocaine or kynurenate (Panneton, 1991b; Panneton and Yavari, 1995), or after the AEN is transected (Rybka and McCulloch, 2006), the bradycardic and apneic responses to either nasal or diving stimulation are inhibited. Although the data points in the MDH of diving rats were significantly higher than those of swimmers, there were relatively few neurons immunolabeled after our single trial. The reason for this is unknown, but suggests that these neurons invoke a powerful influence over cardiorespiratory behavior with little modulation.
A significant number of CPA neurons were activated and immunolabeled with Fos after underwater submersion compared to control and swimming rats in the present study. Neurons in and around the CPA have been implicated in cardiovascular regulation, in the processing of noxious information, as well as important for the exercise pressure response (Sun and Panneton, 2002, 2005; Panneton et al., 2008, 2011; Marques-Lopes et al., 2009; Takakura et al., 2011a,b). Although we have shown previously that the area of the MDH activated by voluntary diving projects to the CPA (Panneton et al., 2006), it plays no role in the cardiorespiratory adjustments seen with nasal stimulation (Panneton et al., 2008). Thus the role played by neurons in the CPA, whether integration of sensory pathways or in autonomic behavior, must still be determined.
The RPa and RVLM
The experimental animals in this study voluntarily dove underwater for approximately 15–20 s, and were apneic during this time. This may have induced activation of central chemoreceptors, which adjust ventilation to meet metabolic needs. Neurons in the nucleus RPa abut branches of the basilar artery and are speculated by some to be chemoreceptors monitoring ventilation (Richerson, 2004). However, injections of kynurenate into the RPa had no effect on resting arterial blood pressure, sympathetic outflow, or the responses to activation of a nasotrigeminal reflex (McCulloch et al., 1999b).
It is well known that the RVLM is an important brainstem locus for controlling arterial blood pressure. Neurons in the RVLM are activated when cardiorespiratory responses similar to diving are induced by nasal stimulation, despite the increases in arterial pressure (McCulloch et al., 1999b); this contrasts their silence after similar increases in arterial blood pressure when the baroreceptor reflex is activated. Indeed, present data shows a significant increase in data points in the RVLM of diving rats when compared to both control and swimming animals. We suspect this activation induces peripheral vasoconstriction via the sympathetic system in non-essential vascular beds (see Nakamura and Hayashida, 1992), similar to that seen in aquatic species. Many RVLM neurons contain catecholamines of the C1 group, specifically adrenaline, but only 29% of C1 neurons were double-labeled with Fos after diving underwater (McCulloch and Panneton, 2003). This percent is comparable to that seen in animals with induced hypertension (Chan and Sawchenko, 1994, 1998; Erickson and Millhorn, 1994; Dampney et al., 2003) but differs dramatically from that seen in hypotensive states, where more aminergic neurons are labeled. Indeed, recent reports document separate outflows from both C1 and non-aminergic RVLM neurons to the spinal cord and activation of sympathetic outflow (Burke et al., 2011). Thus we suggest that most of the Fos-labeled RVLM neurons of diving rats are non-aminergic, and may be the faster conducting barosensitive bulbospinal RVLM neurons activated by nasal stimulation (see McCulloch et al., 1999b, for discussion).
Pontine Nuclei
The A5 area contains sympathoexcitatory neurons implicated in cardiovascular function (Loewy et al., 1979; Neil and Loewy, 1982; Byrum et al., 1984; Guyenet, 1984; Hara et al., 1997; Maiorov et al., 1999, 2000) as well as those modulating respiration (Hilaire et al., 2004; Viemari et al., 2004). The data presented herein showed the number of A5 neurons labeled with Fos after diving underwater was insignificantly different when compared to swimmers but was different than control rats. The absolute number of labeled neurons in the A5 in the present study was small, however, and reduced greatly from that seen after repeated voluntary diving stimulations reported previously (McCulloch and Panneton, 2003). However, immunolabeling of neurons after underwater diving in the LC was significantly elevated compared to control and swimming. Since neither the A5 area nor LC are activated by hypertension (Graham et al., 1995), nor is the LC a necessary component of the diving circuit (Panneton et al., 2012), their activation may be in response to the physical stress of underwater submergence.
The peribrachial complex in the dorsolateral pons, especially the PBel and PBsl subnuclei of the parabrachial nucleus and the Kölliker–Fuse nucleus, are considered important in modulation of visceral activity. The PBel, PBsl, and KF all showed significant increased labeling with Fos after underwater submersion. Lateral parabrachial neurons are involved in the baroreceptor reflex (Hayward and Felder, 1998; Saleh and Connell, 1998; Len and Chan, 2001) as well as the chemoreceptor reflex (Koshiya and Guyenet, 1994; Haibara et al., 2002). The PBel neurons induce cardiovascular changes when stimulated (Miura and Takayama, 1991; Chamberlin and Saper, 1992) and induce Fos production (Chan and Sawchenko, 1994; Potts et al., 1997; Mayne et al., 1998) when activated by changes in arterial pressure. Neurons in the lateral peribrachial complex, including the PBsl, PBel, and KF, also are important in respiratory control (Jodkowski et al., 1994; Mizusawa et al., 1995) and respiratory rates are modulated with stimulation here (Takayama and Miura, 1993; Chamberlin and Saper, 1994). Several studies have shown increased Fos labeling in the lateral parabrachial nucleus, especially the presumptive outer portion of the PBel, after changing arterial blood pressure or activating the chemoreceptor reflex (Erickson and Millhorn, 1994; Graham et al., 1995; Potts et al., 1997), but also after nasal stimulation in anesthetized animals (Dutschmann and Herbert, 1997; McCulloch and Panneton, 1997). Moreover, it has been reported that the bradycardia and apnea induced by electrical stimulation of the AEN is mediated by neurons of the Kölliker–Fuse area (Dutschmann and Herbert, 1996) and that the KF is important in trigeminoautonomic reflexes (Dutschmann and Herbert, 1998). We have shown previously that the presumptive outer portion of the PBel as well as parts of the KF area receives primary afferent fibers from the AEN (Panneton, 1991a; Panneton et al., 2006). These derelict extratrigeminal projections recently have been confirmed with staining of the PBel nucleus in genetically manipulated mice; this study (Cavanaugh et al., 2011) shows a strong TRPV1 receptor presence in the PBel, a receptor only found on primary afferent neurons. However, these pontine nuclei must not be part of the basic reflex circuit driving the diving response, since the cardiorespiratory changes induced by nasal stimulation persist after pontine transection (Panneton et al., 2012).
Perspectives
Most studies on the diving response have been done on aquatic mammals which submerge frequently. However, we (McCulloch and Panneton, 2003; Panneton et al., 2010a,b) and others (McCulloch, 2005; McCulloch et al., 2010; Fahlman et al., 2011) have shown that the diving response is brisk even in the common laboratory rat. Can the responses seen in aquatic mammals be compared legitimately to those of non-aquatic, land-bound rats? Behaviors which serve basic vegetative functions are usually less complex and more uniform across species; this offers support for studies of autonomic functions in different species. If the diving response can be considered such a basic vegetative function, it is more easily studied in a laboratory animal rather than a large aquatic mammal. The substrate for “simple” reflex behaviors are thought to be circuits located within the brainstem and the spinal cord, and we have shown the nasotrigeminal reflex IS contained in the caudal neuraxis (Panneton et al., 2012).
We have documented neurons in several brainstem nuclei which may act as interneurons in the reflex pathways integral for the parasympathetically mediated bradycardia, the sympathetically mediated vasoconstriction, and the apnea induced in the diving response. We feel our considerable effort toward studying those circuits which are the simplest, the most organized, and the most automatic is both logical and certainly worthwhile. Moreover, the cardiorespiratory responses of voluntarily diving rats are invariable at least in our hands. We already have documented the diving response inhibits basic homeostatic reflexes such as the baroreceptor and chemoreceptor reflexes; we consider the cardiorespiratory reflexes comprising the diving response collectively make it the most powerful autonomic response known. Moreover, the complexity of an animal’s behavior increases according to its place in phylogeny and is paralleled by the complexity of the neural systems driving behavior. Since neurons in the brain both coordinate and control peripheral function, including those activated during diving behavior, our laboratory has taken considerable effort toward deciphering the circuits of neurons driving the changes in respiration, HR, and peripheral vasoconstriction seen with underwater submersion as first described by Scholander and Irving. Perhaps the larger diving aquatic mammals, especially those noted for their intelligence, can use their forebrains to control this simple, highly organized autonomic reflex via suprabulbar circuits. Future studies will include investigations as to how suprabulbar neurons modulate these invariable brainstem reflexes.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jaclyn Palkert and Prashant Kasinadhuni for help in early phases of the analysis of this data. This work was supported by NIH grant HL64772 to W. Michael Panneton and monies from the Saint Louis University School of Medicine.
References
Allen, G. V., and Pronych, S. P. (1997). Trigeminal autonomic pathways involved in nociception-induced reflex cardiovascular responses. Brain Res. 754, 269–278.
Anton, F., Herdegen, T., Peppel, P., and Leah, J. D. (1991). c-FOS-like immunoreactivity in rat brainstem neurons following noxious chemical stimulation of the nasal mucosa. Neuroscience 41, 629–641.
Baker, M. A., and Horvath, S. M. (1964). Influence of water temperature on heart rate and rectal temperature of swimming rats. Am. J. Physiol. 207, 1073–1076.
Blessing, W. W., Yu, Y. H., and Nalivaiko, E. (1999). Medullary projections of rabbit carotid sinus nerve. Brain Res. 816, 405–410.
Blix, A. S., and Folkow, B. (1983). “Cardiovascular adjustments to diving in mammals and birds,” in Handbook of Physiology – The Cardiovascular System, eds J. T. Sheperd and F. M. Abboud (Bethesda, MD: American Physiological Society), 917–945.
Bullitt, E., Lee, C. L., Light, A. R., and Willcockson, H. (1992). The effect of stimulus duration on noxious-stimulus induced c-fos expression in the rodent spinal cord. Brain Res. 580, 172–179.
Burke, P. G. R., Neale, J. K. W. S., and McMullan, S. G. A. K. (2011). Patterning of somatosympathetic reflexes reveals nonuniform organization of presympathetic drive from C1 and non-C1 RVLM neurons. Am. J. Physiol. 301, R1112–R1122.
Butler, P. J., and Jones, D. R. (1982). The comparative physiology of diving in vertebrates. Adv. Comp. Physiol. Biochem. 8, 179–364.
Butler, P. J., and Jones, D. R. (1997). Physiology of diving of birds and mammals. Physiol. Rev. 77, 837–899.
Byrum, C. E., Stornetta, R., and Guyenet, P. G. (1984). Electrophysiological properties of spinally-projecting A5 noradrenergic neurons. Brain Res. 303, 15–29.
Casson, D. M., and Ronald, K. (1975). The harp seal, Pagophilus groenlandicus. XIV. Cardiac arrhthmias. Comp. Biochem. Physiol. A Comp. Physiol. 50, 307–314.
Cavanaugh, D. J., Chesler, A. T., Bráz, J. M., Shah, N. M., Julius, D., and Basbaum, A. I. (2011). Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in non-peptidergic neurons. J. Neurosci. 31, 10119–10127.
Chamberlin, N. L., and Saper, C. B. (1992). Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J. Comp. Neurol. 326, 245–262.
Chamberlin, N. L., and Saper, C. B. (1994). Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J. Neurosci. 14, 6500–6510.
Chan, R. K. W., and Sawchenko, P. E. (1994). Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J. Comp. Neurol. 348, 433–460.
Chan, R. K. W., and Sawchenko, P. E. (1998). Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J. Neurosci. 18, 371–387.
Dampney, R. A. L., and Horiuchi, J. (2003). Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Prog. Neurobiol. 71, 359–384.
Dampney, R. A. L., Polson, J. W., Potts, P. D., Hirooka, Y., and Horiuchi, J. (2003). Functional organization of brain pathways subserving the baroreceptor reflex: studies in conscious animals using immediate early gene expression. Cell. Mol. Neurobiol. 23, 597–616.
Davis, R. W., Polasek, L., Watson, R., Fuson, A., Williams, T. M., and Kanatous, S. B. (2004). The diving paradox: new insights into the role of the dive response in air-breathing vertebrates. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 138, 263–268.
de Burgh Daly, M. (1984). “Breath-hold diving: mechanisms of cardiovascular adjustments in the mammal,” in Recent Advance in Physiology, ed. P. F. Baker (Edinburgh: Churchill Livingstone), 201–245.
Donoghue, S., Felder, R. B., Jordan, D., and Spyer, K. M. (1984). The central projections of carotid baroreceptors and chemoreceptors in the cat: a neurophysiological study. J. Physiol. (Lond.) 347, 397–409.
Donoghue, S., Kidd, C., and McWilliam, P. N. (1978). The distribution of neurones in the brain stem of the cat activated by A and C fibres of the aortic nerve. J. Physiol. 285, 56–57.
Dragunow, M., and Faull, R. (1989). The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29, 261–265.
Drummond, P. C., and Jones, D. R. (1979). The initiation and maintenance of bradycardia in a diving mammal, the muskrat, Ondatra zibethica. J. Physiol. (Lond.) 290, 253–271.
Dutschmann, M., Guthmann, A., and Herbert, H. (1998). NMDA receptor subunit NR1-immunoreactivity in the rat pons and brainstem and colocalization with Fos induced by nasal stimulation. Brain Res. 809, 221–230.
Dutschmann, M., and Herbert, H. (1996). The Kölliker-Fuse nucleus mediates the trigeminally induced apnoea in the rat. Neuroreport 7, 1432–1436.
Dutschmann, M., and Herbert, H. (1997). Fos expression in the rat parabrachial and Kölliker-Fuse nuclei after electrical stimulation of the trigeminal ethmoidal nerve and water stimulation of the nasal mucosa. Exp. Brain Res. 117, 97–110.
Dutschmann, M., and Herbert, H. (1998). The medial nucleus of the solitary tract mediates the trigeminally evoked pressor response. Neuroreport 9, 1053–1057.
Dykes, R. W. (1974). Factors related to the dive reflex in harbor seals: sensory contributions from the trigeminal region. Can. J. Physiol. Pharmacol. 52, 259–265.
Elsner, R., and Gooden, B. (1983). Diving and Asphyxia: A Comparative Study of Animals and Man. New York: Cambridge University Press, 1–168.
Erickson, J. T., and Millhorn, D. E. (1994). Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J. Comp. Neurol. 348, 161–182.
Fahlman, A., Bostrom, B. L., Dillon, K. H., and Jones, D. R. (2011). The genetic component of the forced diving bradycardia response in mammals. Front. Physiol. 2:63. doi:10.3389/fphys.2011.00063
Finley, J. C. W., and Katz, D. M. (1992). The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 572, 108–116.
Foster, G. E., and Sheel, A. W. (2005) The human diving response, its function, and its control. Scand. J. Med. Sci. Sports 15, 3–12.
Frigero, A., Bonagamba, L. G. H., and Machado, B. H. (2000). The gain of the baroreflex bradycardia is reduced by microinjection of NMDA receptor antagonists into the nucleus tractus solitarii of awake rats. J. Auton. Nerv. Syst. 79, 28–33.
Gieroba, Z. J., Yu, Y.-H., and Blessing, W. W. (1994). Vasoconstriction induced by inhalation of irritant vapour is associated with appearance of Fos protein in C1 catecholamine neurons in rabbit medulla oblongata. Brain Res. 636, 157–161.
Graham, J. C., Hoffman, G. E., and Sved, A. F. (1995). c-fos expression in brain in response to hypotension and hypertension in conscious rats. J. Auton. Nerv. Syst. 55, 92–104.
Guyenet, P. G. (1984). Baroreceptor-mediated inhibition of A5 noradrenergic neurons. Brain Res. 303, 31–40.
Guyenet, P. G., Filtz, T. M., and Donaldson, S. R. (1987). Role of excitatory amino acids in rat vagal and sympathetic baroreflexes. Brain Res. 407, 272–284.
Haibara, A. S., Tamashiro, E., Olivan, M. V., Bonagamba, L. G. H., and Machado, B. H. (2002). Involvement of the parabrachial nucleus in the pressor response to chemoreflex activation in awake rats. Auton. Neurosci. 101, 60–67.
Hara, K., Miyawaki, T., Minson, J., Arnolda, L., Llewellyn-Smith, I., Chalmers, J., and Pilowsky, P. (1997). Role of spinal GABA receptors in depressor responses to chemical stimulation of the A5 area in normal and hypertensive rats. J. Auton. Nerv. Syst. 66, 53–61.
Hayward, L. F., and Felder, R. B. (1998). Lateral parabrachial nucleus modulates baroreflex regulation of sympathetic nerve activity. Am. J. Physiol. 274, R1274–R1282.
Hilaire, G., Viemari, J.-C., Coulon, P., Simmonneau, M., and Bévengut, M. (2004). Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Resp. Physiol. Neurobiol. 279, 187–197.
Hoskin, K. L., and Goadsby, P. J. (1999). Exposure and isolation of the superior sagittal sinus elicits Fos in the trigeminal nucleus caudalis and dorsal horn of the cervical spinal cord: how long should you wait? Brain Res. 824, 133–135.
Housley, G. D., Martin Body, R. L., Dawson, N. J., and Sinclair, J. D. (1987). Brain stem projections of the glossopharyngeal nerve and its carotid sinus branch in the rat. Neuroscience 22, 237–250.
Huxley, F. M. (1913). On the nature of apnoea in the duck in diving: I. The nature of submersion apnoea. J. Physiol. (Lond.) 6, 147–157.
Irving, L., Scholander, P. F., and Grinnell, S. W. (1942). The regulation of arterial blood pressure in the seal during diving. Am. J. Physiol. 135, 557–566.
Itoh, H., Alper, R. H., and Bunag, R. D. (1992). Baroreflex changes produced by serotonergic or catecholaminergic lesions in the rat nucleus tractus solitarius. J. Pharmacol. Exp. Ther. 261, 225–233.
Jobsis, P. D., Ponganis, P. J., and Kooyman, G. L. (2001). Effects of training on forced submersion responses in harbor seals. J. Exp. Biol. 204, 3877–3885.
Jodkowski, J. S., Coles, S. K., and Dick, T. E. (1994). A “pneumotaxic centre” in rats. Neurosci. Lett. 172, 67–72.
Kooyman, G. L., and Campbell, W. B. (1972). Heart rates in freely diving Weddell seals. Comp. Biochem. Physiol. 43A, 31–36.
Kooyman, G. L., Castellini, M. A., and Davis, R. W. (1981). Physiology of diving in marine mammals. Annu. Rev. Physiol. 43, 343–356.
Kooyman, G. L., and Ponganis, P. J. (1998). The physiological basis of diving to depth: birds and mammals. Annu. Rev. Physiol. 60, 19–32.
Koppányi, T., and Dooley, M. S. (1929). Submergence and postural apnea in the muskrat. Am. J. Physiol. 88, 592–595.
Koshiya, N., and Guyenet, P. G. (1994). Role of the pons in the carotid sympathetic chemoreflex. Am. J. Physiol. 267, R508–R518.
Kratschmer, F. (2001). On reflexes from the nasal mucous membrane on respiration and circulation. Respir. Physiol. 127, 93–104.
Kumada, M., Dampney, R. A. L., and Reis, D. J. (1977). The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res. 119, 305–326.
Len, W. B., and Chan, J. Y. H. (2001). GABAergic neurotransmission at the nucleus tractus solitarii in the suppression of reflex bradycardia by parabrachial nucleus. Synapse 42, 27–39.
Leone, C., and Gordon, F. J. (1989). Is L-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius. J. Pharmacol. Exp. Ther. 250, 953–962.
Lin, Y. C. (1974). Autonomic nervous control of cardiovascular response during diving in the rat. Am. J. Physiol. 227, 601–605.
Loewy, A. D., Gregorie, E. M., McKellar, S., and Baker, R. P. (1979). Electrophysiological evidence that the A5 catecholamine cell group is a vasomotor center. Brain Res. 178, 196–200.
Machado, B. H., Castania, J. A., Bonagamba, L. G. H., and Salgado, L. C. (2000). Neurotransmission of autonomic components of aortic baroreceptor afferents in the NTS of awake rats. Am. J. Physiol. Heart Circ. Physiol. 279, H67–H75.
Maiorov, D. N., Malpas, S. C., and Head, G. A. (2000). Influence of pontine A5 region on renal sympathetic nerve activity in conscious rabbits. Am. J. Physiol. 278, R311–R319.
Maiorov, D. N., Wilton, E. R., Badoer, E., Petrie, D., Head, G. A., and Malpas, S. C. (1999). Sympathetic response to stimulation of the pontine A5 region in conscious rabbits. Brain Res. 815, 227–236.
Marques-Lopes, J., Pinto, M., Pinho, D., Morato, M., Patinha, D., Albino-Teixeira, A., and Tavares, I. (2009). Microinjection of angiotensin II in the caudal ventrolateral medulla induces hyperalgesia. Neuroscience 158, 1301–1310.
Martner, J., Wadenvik, H., and Lisander, B. (1977). Apnoea and bradycardia from submersion in “chronically” decerebrated cats. Acta Physiol. Scand. 101, 476–480.
Mayne, R. G., Armstrong, W. E., Crowley, W. R., and Bealer, S. L. (1998). Cytoarchitectonic analysis of Fos-immunoreactivity in brainstem neurones following visceral stimuli in conscious rats. J. Neuroendocrinol. 10, 839–847.
McCulloch, P. F. (2005). Activation of the trigeminal medullary dorsal horn during voluntary diving in rats. Brain Res. 1051, 194–198.
McCulloch, P. F., Dinovo, K. M., and Connolly, T. M. (2010). The cardiovascular and endocrine responses to voluntary and forced diving in trained and untrained rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R224–R234.
McCulloch, P. F., Faber, K. M., and Panneton, W. M. (1999a). Electrical stimulation of the anterior ethmoidal nerve produces the diving response. Brain Res. 830, 24–31.
McCulloch, P. F., Panneton, W. M., and Guyenet, P. G. (1999b). The rostral ventrolateral medulla mediates the sympathoactivation produced by chemical stimulation of the nasal mucosa. J. Physiol. (Lond.) 516, 471–484.
McCulloch, P. F., and Jones, D. R. (1990). Cortical influences on diving bradycardia in muskrats (Ondatra zibethicus). Physiol. Zool. 63, 1098–1117.
McCulloch, P. F., Ollenberger, G. P., Bekar, L. K., and West, N. H. (1997). Trigeminal and chemoreceptor contributions to bradycardia during voluntary dives in rats. Am. J. Physiol. 273, R814–R822.
McCulloch, P. F., and Panneton, W. M. (1997). Fos immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience 78, 913–925.
McCulloch, P. F., and Panneton, W. M. (2003). Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res. 984, 42–53.
McCulloch, P. F., Paterson, I. A., and West, N. H. (1995). An intact glutamatergic trigeminal pathway is essential for the cardiac response to simulated diving. Am. J. Physiol. 269, R669–R677.
Miura, M., and Takayama, K. (1991). Circulatory and respiratory responses to glutamate stimulation of the lateral parabrachial nucleus of the cat. J. Auton. Nerv. Syst. 32, 121–134.
Mizusawa, A., Ogawa, H., Kikuchi, Y., Hida, W., and Shirato, K. (1995). Role of the parabrachial nucleus in ventilatory responses of awake rats. J. Physiol. (Lond.) 489(Pt 3), 877–884.
Moore, S. D., and Guyenet, P. G. (1985). Effect of blood pressure on A2 noradrenergic neurons. Brain Res. 338, 169–172.
Nakamura, T., and Hayashida, Y. (1992). Autonomic cardiovascular responses to smoke exposure in conscious rats. Am. J. Physiol. 262, R738–R745.
Neil, J. J., and Loewy, A. D. (1982). Decreases in blood pressure in response to L-glutamate microinjections into the A5 catecholamine cell group. Brain Res. 241, 271–278.
Panneton, W. M. (1991a). Primary afferent projections from the upper respiratory tract in the muskrat. J. Comp. Neurol. 308, 51–65.
Panneton, W. M. (1991b). Trigeminal mediation of the diving response in the muskrat. Brain Res. 560, 321–325.
Panneton, W. M., Gan, Q., Clerc, P., Palkert, J., Kasinadhuni, P., and Lemon, C. (2009). Activation of brainstem neurons by underwater submersion. Abstr. Soc. Neurosci. 34.
Panneton, W. M., Gan, Q., and Dahms, T. E. (2010a). Cardiorespiratory and neural consequences of rats brought past their aerobic dive limit. J. Appl. Physiol. 109, 1256–1269.
Panneton, W. M., Gan, Q., and Juric, R. (2010b). The rat: a laboratory model for studies of the diving response. J. Appl. Physiol. 108, 811–820.
Panneton, W. M., Gan, Q., and Juric, R. (2006). Brainstem projections from recipient zones of the anterior ethmoidal nerve in the medullary dorsal horn. Neuroscience 141, 889–906.
Panneton, W. M., Gan, Q., and Livergood, R. (2011). A trigeminoreticular pathway: implications in pain. PLoS ONE 6, e24499. doi:10.1371/journal.pone.0024499
Panneton, W. M., Gan, Q., and Sun, D. W. (2012). Persistence of the nasotrigeminal reflex after pontomedullary transection. Respir. Physiol. Neurobiol. 180, 230–236.
Panneton, W. M., Gan, Q., and Sun, W. (2008). Pressor responses to nasal stimulation are unaltered after disrupting the caudalmost ventrolateral medulla. Auton. Neurosci. 144, 13–21.
Panneton, W. M., and Loewy, A. D. (1980). Projections of the carotid sinus nerve to the nucleus of the solitary tract in the cat. Brain Res. 191, 239–244.
Panneton, W. M., McCulloch, P. F., and Sun, W. (2000). Trigemino-autonomic connections in the muskrat: the neural substrate for the diving response. Brain Res. 874, 48–65.
Panneton, W. M., and Yavari, P. (1995). A medullary dorsal horn relay for the cardiorespiratory responses evoked by stimulation of the nasal mucosa in the muskrat, Ondatra zibethicus: evidence for excitatory amino acid transmission. Brain Res. 691, 37–45.
Paxinos, G., and Watson, C. (1998). The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press.
Potts, P. D., Polson, J. W., Hirooka, Y., and Dampney, R. A. L. (1997). Effects of sinoaortic denervation on fos expression in the brain evoked by hypertension and hypotension in conscious rabbits. Neuroscience 77, 503–520.
Richerson, G. B. (2004). Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat. Rev. Neurosci. 5, 449–461.
Rinaman, L. (2011). Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R222–R235.
Rybka, E. J., and McCulloch, P. F. (2006). The anterior ethmoidal nerve is necessary for the initiation of the nasopharyngeal response in the rat. Brain Res. 1075, 122–132.
Saleh, T. M., and Connell, B. J. (1998). The parabrachial nucleus mediates the decreased cardiac baroreflex sensitivity observed following short-term visceral afferent activation. Neuroscience 87, 135–146.
Schagatay, E., and Van Kampen, M. (1995). Apneic snout immersion in trained pigs elicits a “diving response.” Adv. Exp. Med. Biol. 393, 73–76.
Scholander, P. F., and Elsner, R. W. (1968). A comparative view of cardiovascular defense against acute asphyxia. Acta Anaesthesiol. Scand. Suppl. 29, 15–33.
Sun, W., and Panneton, W. M. (2002). The caudal pressor area of the rat: its precise location and projections to the ventrolateral medulla. Am. J. Physiol. 283, R768–R778.
Sun, W., and Panneton, W. M. (2005). Defining projections from the caudal pressor area of the caudal ventrolateral medulla. J. Comp. Neurol. 482, 273–293.
Takakura, A. C., Colombari, E., Menani, J. V., and Moreira, T. S. (2011a). Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R501–R510.
Takakura, A. C., Moreira, T. S., Menani, J. V., and Colombari, E. (2011b). Inhibition of the caudal pressor area reduces cardiorespiratory chemoreflex responses. Neuroscience 177, 84–92.
Takayama, K., and Miura, M. (1993). Respiratory responses to microinjection of excitatory amino acid agonists in ventrolateral regions of the lateral parabrachial nucleus in the cat. Brain Res. 604, 217–223.
Takayama, K., Suzuki, T., and Miura, M. (1994). The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci. Lett. 176, 59–62.
Tchobroutsky, C., Merlet, C., and Rey, P. (1969). The diving reflex in rabbit, sheep and newborn lamb and its afferent pathways. Respir. Physiol. 8, 108–117.
Viemari, J. C., Bevengut, M., Coulon, P., and Hilaire, G. (2004). Nasal trigeminal inputs release the A5 inhibition received by the respiratory rhythm generator of the mouse neonate. J. Neurophysiol. 91, 746–758.
Whishaw, I. Q., and Schallert, T. (1977). Hippocampal RSA (theta), apnea, bradycardia, and effects of atropine during underwater swimming in the rat. Electroencephalogr. Clin. Neurophysiol. 42, 389–396.
Yavari, P., McCulloch, P. F., and Panneton, W. M. (1996). Trigeminally-mediated alteration of cardiorespiratory rhythms during nasal application of carbon dioxide in the rat. J. Auton. Nerv. Syst. 61, 195–200.
Zhang, W., and Mifflin, S. W. (1993). Excitatory amino acid receptors within NTS mediate arterial chemoreceptor reflexes in rats. Am. J. Physiol. Heart Circ. Physiol. 265, H770–H773.
Keywords: diving response, c-Fos, medullary dorsal horn, parabrachial nucleus, nucleus tractus solitarii, rostroventrolateral medulla, neural circuits, swimming behavior
Citation: Panneton WM, Gan Q, Le J, Livergood RS, Clerc P and Juric R (2012) Activation of brainstem neurons by underwater diving in the rat. Front. Physio. 3:111. doi: 10.3389/fphys.2012.00111
Received: 02 March 2012; Paper pending published: 21 March 2012;
Accepted: 04 April 2012; Published online: 03 May 2012.
Edited by:
Andreas Fahlman, Texas A&M University – Corpus Christi, USAReviewed by:
Michael Fine, Virginia Commonwealth University, USAManuela Gardner, Texas A&M University – Corpus Christi, USA
Copyright: © 2012 Panneton, Gan, Le, Livergood, Clerc and Juric. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: W. Michael Panneton, Department of Pharmacological and Physiological Science, St. Louis University School of Medicine, 1402 S. Grand Blvd., St. Louis, MO 63104-1004, USA. e-mail:cGFubmV0d21Ac2x1LmVkdQ==

 Qi Gan
Qi Gan