
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Physiol. , 15 February 2012
Sec. Aquatic Physiology
Volume 3 - 2012 | https://doi.org/10.3389/fphys.2012.00021
This article is part of the Research Topic Aquaporins in fish - expression, localization and functional dynamics. View all 10 articles
The role of aquaporin water channels such as aquaporin 4 (Aqp4) in elasmobranchs such as the dogfish Squalus acanthias is completely unknown. This investigation set out to determine the expression and cellular and sub-cellular localization of Aqp4 protein in dogfish tissues. Two polyclonal antibodies were generated (AQP4/1 and AQP4/2) and these showed somewhat different characteristics in Western blotting and immunohistochemistry. Western blots using the AQP4/1 antibody showed two bands (35.5 and 49.5 kDa) in most tissues in a similar fashion to mammals. Liver had an additional band of 57 kDa and rectal gland two further faint bands of 37.5 and 38.5 kDa. However, unlike in mammals, Aqp4 protein was ubiquitously expressed in all tissues including gill and liver. The AQP4/2 antibody appeared much less specific in Western blots. Both antibodies were used in immunohistochemistry and showed similar cellular localizations, although the AQP4/2 antibody had a more restricted sub-cellular distribution compared to AQP4/1 and therefore appeared to be more specific for Aqp4. In kidney a sub-set of tubules were stained which may represent intermediate tubule segments (In-III–In-VI). AQP4/1 and AQP4/2 antibodies localized to the same tubules segments in serial sections although the intensity and sub-cellular distribution were different. AQP4/2 showed a basal or basolateral membrane distribution whereas AQP4/1 was often distributed throughout the whole cell including the nuclear region. In rectal gland and cardiac stomach Aqp4 was localized to secretory tubules but again AQP/1 and AQP/2 exhibited different sub-cellular distributions. In gill, both antibodies stained large cells in the primary filament and secondary lamellae. Again AQP4/1 antibody stained most or all the cell including the nucleus, whereas AQP4/2 had a plasma membrane or plasma membrane and cytoplasmic distribution. Two types of large mitochondrial rich transport cells are known to exist in elasmobranchs, that express either Na, K-ATPase, or V-type ATPase ion transporters. Using Na, K-ATPase, and V-type ATPase antibodies, Aqp4 was colocalized with these proteins using the AQP4/1 antibody. Results show Aqp4 is expressed in both (and all) branchial Na, K-ATPase, and V-type ATPase expressing cells.
Aquaporin 4 (AQP4) is a member of the water-selective sub-group of aquaporin water channel cell-membrane proteins found in all organisms so far investigated. This sub-group in mammals also includes other aquaporins such as AQP0, AQP1, AQP2, AQP5, and AQP6 (Ishibashi et al., 2009; Zelanina, 2010). Other subgroups such as aquaglyceroporins (AQP3, AQP7, AQP9, and AQP10) have additional transport properties (e.g., transport of urea and glycerol, etc.). In mammals, AQP4 is widely expressed in a variety of tissues including the brain (Zelanina, 2010), retina (Hirrlinger et al., 2011), salivary gland (Delporte and Steinfeld, 2006), trachea (Borok and Verkman, 2002), heart and muscle (Butler et al., 2006; Wakayama, 2010), gastrointestinal tract (Ma and Verkman, 1999; Xu et al., 2009), and kidney (Nejsum, 2005), but is not expressed in the lung itself or liver tissues (Ishibashi et al., 2009). While aquaporins such as AQP4 have been studied in a wide variety of (mostly) higher vertebrates, no complete studies have yet been published on the role of aquaporins in elasmobranch fish such as the dogfish shark (Cutler et al., 2005; Cutler, 2006, 2007). A companion paper (Cutler et al., 2012) has characterized aqp4 mRNA expression in dogfish tissues, this article gives the first information on the Aqp4 protein and its localization in tissues. In mammals such as the rat, AQP4 appears as two bands on Western blots, a non-glycosylated form of 30–32 kDa (sometimes splicoforms with different N-terminal ends exist) and a putative 50 kDa glycosylated form (Terris et al., 1995; Nicchia et al., 2008). Immunohistochemical staining of mammalian epithelial cells generally yields a basolateral localization for AQ4 proteins, however, AQP4 staining both within the cell and in the apical membrane is thought to occur in some cells (Terris et al., 1995; Nejsum, 2005; Mobasheri et al., 2011). As indicated in the companion paper (Cutler et al., 2012), dogfish aqp4 mRNA expression in tissues is largely ubiquitous. The questions this article sets out to address are, whether Aqp4 protein expression is similarly ubiquitous and if so, where within the tissues that are important for the control of water balance in sharks (gills, kidney, rectal gland, etc.), is the Aqp4 protein located?
All animal experiments were performed in accordance with IACUC regulations and had IACUC approval from both MDIBL and Georgia Southern University. Animals for experiments were held in a stock tank with running seawater at ambient temperature and were sacrificed by decapitation followed by immediate pithing of the spinal cord. Various tissues were then removed from the animal by dissection for further processing in Western blotting and immunohistochemistry experiments.
Custom-made polyclonal antibodies were produced commercially against peptides whose amino acid sequence was derived from the dogfish aqp4 nucleotide sequence. The first of these AQP4/1 (produced by ProSci, San Diego, CA, USA) was located at the C-terminal end of the protein (at positions 329–346 of the amino acid sequence) and had the sequence NH2–CGGNEEKKEKDATKELLSSV–COOH. As part of that sequence, the two glycine amino acids were added as spacers at the N-terminal end. The second antibody AQP4/2 (produced by Genscript, Piscataway, NJ, USA), was produced much more recently and was located a little further in from the C-terminal end than the first peptide (at positions 290–308 of the amino acid sequence), and had the sequence NH2–CKSTQPSGDKYAEGEDNRSQ–COOH. Peptides for both antibodies had an N-terminal cysteine amino acid added for coupling to the protein carrier. The Aqp4 peptides were coupled to keyhole limpet hemocyanin (KLH) prior to injections of the antigens into different pairs of rabbits. The resulting anti-sera were affinity purified using the same peptide (that was used for immunization), attached to a purification column.
Dogfish tissues for Western blotting experiments were kept briefly on ice and then homogenized in Tris (25 mM), sucrose (0.25 M) buffer, also containing 78 mg/ml dithiotheritol (DTT), and either protease inhibitor cocktail I (Research Product International, Mount Prospect, IL, USA) or Halt protease inhibitor cocktail (Pierce), was used according to manufacturers instructions. Hard tissues such as muscle, kidney, liver, and rectal gland, etc., were homogenized using a polytron homogenizer (Kinematica, Luzern Switzerland). Soft tissues such as brain or scraped epithelia were homogenized using a syringe and 16 gage needle. Epithelia were scraped from gill arches using a single sided razor blade and from intestine and esophagus/cardiac stomach using a glass microscope slide.
Homogenized samples were then sieved through several layers of cotton gauze. The filtrate was then centrifuged in a SS-34 (Sorvall, Asheville, NC, USA) rotor at approximately 50,000 g max for 1 h at 4°C. The resulting crude membrane pellet was then resuspended in the same buffer as previously used and measured for protein content using a Bradford’s protein assay (Boston Bioproducts, Ashland, MA, USA). Crude membrane homogenates were stored frozen at −20 or −80°C prior to use in Western blotting experiments.
Crude protein homogenates (300 μg protein/lane) were separated based on their size, on 10% Laemmli SDS-polyacrylamide gels (Laemmli, 1970) using a Protean II gel apparatus (Biorad). The gel was then transferred to a methanol-activated high protein capacity sequi-blot PVDF filter (Biorad) using a trans-blot cell electroblotter (Biorad, Taunton, MA, USA), at 30 V overnight. The resulting filters were then cut into strips for each experiment. Filter strips were incubated in TNT buffer [10 mM tris (pH 8.0), 150 mM NaCl, 0.05% Tween 20], containing 5% Blotto (fat-free dry milk powder) for 30 min room temperature. They were then washed four times in TNT buffer and primary antibody added (in TNT buffer) at 1 in 400 dilution (or 1 in 4000 dilution for peptide-blocking experiments; used because there can be a problem blocking high antibody concentrations sometimes due to the limits of peptide antigen solubility. Lower antibody concentrations allows the same result to be obtained with less peptide for 1 h at room temperature. The filters were then washed four times in TNT buffer and incubated in 1 in 4000 dilution of alkaline phosphatase enzyme cross linked – highly cross-absorbed – donkey anti-rabbit IgG secondary antibody for 1 h at room temperature. Filters were washed again twice in TNT buffer and twice in 10 mM tris (pH 8.0), 150 mM NaCl, and finally incubated in NBT/BCIP (nitro blue tetrazolium chloride/5-bromo-4-chloro-3′-indoly phosphate p-toluidine salt) alkaline phosphatase enzyme substrate containing 1 mM levamisole endogenous alkaline phosphatase inhibitor. The presence of the bound secondary antibody/alkaline phosphatase enzyme yielding a purple/blue colored product.
Dissected tissues were fixed in filtered standard phosphate buffered saline (PBS; Oxoid, Lenexa, KS, USA) containing 4% paraformaldehyde, for 1 h at room temperature. Tissues were then cut into segments to fit in standard histological cassettes. The cassettes were rinsed twice in PBS and then dehydrated through a series of alcohols (50, 70, 85, 95, and 100% ethanol), 1 h in each. Subsequently, cassettes were placed twice in histochoice clearing medium (Amresco, Solon, OH, USA) and then into molten paraffin wax three times (Paraplast) held between 56 and 58°C in an oven, 1 h each wax solution. The tissue pieces were then placed in stainless steel molds, which were filled with molten wax and were finally mounted with the back of the cassette placed on top. Once the wax had cooled and set, the molds were removed, revealing the wax-embedded tissue blocks for section cutting. Five micron thick sections were cut using a microtome (Leica, Buffalo Grove, IL, USA), these were placed on the surface of a warm waterbath (37°C) and floated onto glass microscope slides (Superfrost plus). The slides were heated at 37°C for 1 h to adhere tissue sections to the positively charged surface of the slide. Slides for experiments were then taken back through two incubations (5 min each) in histochoice clearing agent to remove the wax and through a descending series of alcohol concentrations (5 min each; 100, 95, 85, 70, 50 ethanol) to re-hydrate the tissue. Finally slides were incubated in PBS.
Slides were then placed horizontally in a slide box that had moist tissue in the bottom for humidification. The tissue on the slides was ringed using a hydrophobic barrier pen (to retain subsequent solutions on the tissue) and a solution of PBS with sodium chloride (17.5 g/l) and 0.02% Tween 20 detergent was added for 10 min to permeabilize the tissue. The slides were washed twice with PBS and then incubated for 5 min in PBS containing (2.68 g/l) ammonium chloride, to block any free aldehyde groups of the fixative. The slides were washed again twice in PBS and then incubated in Image-iT FX blocking solution (Invitrogen, Grand Island, NE, USA) for 30 min. The slides were washed again twice in PBS and then incubated in a second blocking solution of PBS containing (10 g/l) bovine serum albumin (BSA; Promega, Madison, WI, USA) and (1 g/l) gelatin (Boston Bioproducts) for 10 min. The slides were washed again twice in PBS and then incubated in 1 in 100 dilution of primary antibody in PBS for 1 h, room temperature. The slides were then washed four times in PBS and then incubated in 1 in 1000 dilution of secondary antibody (Alexa 488-, Dylight 549-, or Alexa 555-labeled, highly cross-absorbed-anti-rabbit) in PBS for 1 h, room temperature. From this point onward slide boxes were kept closed in a draw between manipulations, to reduce light exposure. The slides were then washed four times in PBS and then were mounted in Prolong Gold mounting medium containing the nuclear counterstain, DAPI (Invitrogen). The slides were then covered with a coverslip ready for microscope viewing.
Four-color immunohistochemical co-localization studies, were carried out as above with the following modifications. A rabbit anti-sculpin V-type ATPase antibody (a gift from Dr. J. B. Claiborne) was used initially and detected using a highly cross-absorbed Alexa 488 (green) fluorescently labeled anti-rabbit secondary antibody. The secondary antibody was then blocked using normal rabbit serum (sections incubated for 1 h at room temperature). The rabbit anti-dogfish AQP4/1 antibody was directly labeled with a (red) Dylight 633 fluorescent dye (using a Pierce microscale antibody labeling kit) and then used on sections. Subsequently, a mouse anti-Na, K-ATPase a5 antibody (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) was used and detected using a highly cross-absorbed (orange) Alexa 555 anti-mouse secondary antibody. A DAPI nuclear counterstain (blue) was also utilized as before.
Western blotting of crude membrane protein extracts from a variety of different dogfish tissues and using the AQP4/1 antibody showed that Aqp4 is expressed ubiquitously in all the tissues studied (Figure 1A). In most tissues, there were two protein bands on the blot with estimated molecular weights of 35.5 and 49.5 kDa respectively, and this tissue distribution was similar to that of aqp4 mRNA (see companion paper; Cutler et al. (2012)). Additionally visible in the sample from rectal gland, there were two other faint bands of 37.5 and 38.5 kDa (see also Figure 3). Also in liver there was another strongly staining band at 57 kDa. The 35.5 kDa band common to most tissues is similar in size to the estimated molecular weight of the protein (37.2 kDa) based on the amino acid sequence derived from the gene sequence. The larger 49.5 kDa band was considerably more abundant in the rectal gland and appeared to be absent in the brain. The second AQP4/2 antibody was also used in Western blotting of tissue crude membrane extracts (Figure 1B). This blot showed a lot more bands than when using AQP4/1 antibody, although the 49.5 kDa was present in each tissue similar to the AQP4/1 antibody blot, except that the 49.5 kDa protein band was also present in the brain. There was also some indication of the 35.5 kDa band on the AQP4/2 blot, but the intensity of this was variable between tissues. There were additionally some higher molecular weight bands of 99 kDa and around 140 kDa suggesting the presence of dimers or Aqp4 aggregates. There was no indication of 37.5 and 38.5 kDa bands in the rectal gland sample as seen with the AQP4/1 antibody.
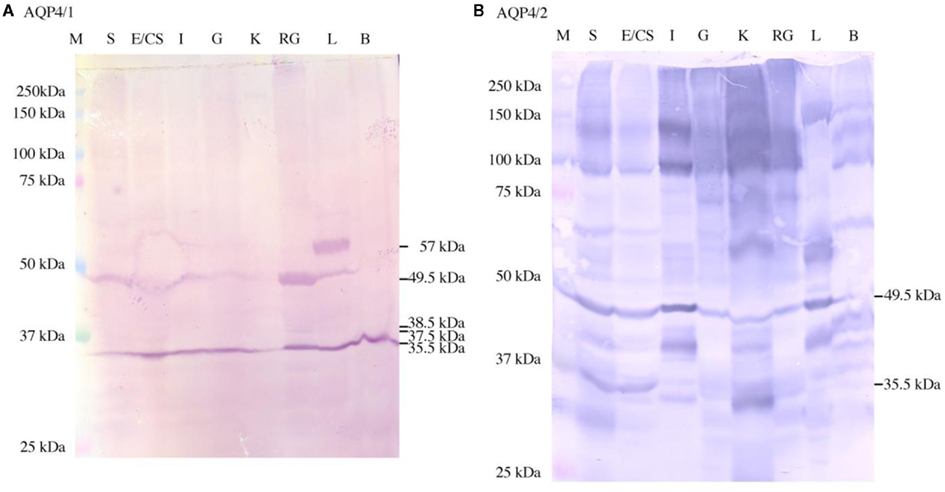
Figure 1. Western blots showing the staining in dogfish tissues using the AQP4/1 (A) or AQP4/2 (B) antibodies. Three hundred micrograms of each tissue homogenate were used including stomach (S), esophagus/cardiac stomach (E/CS), intestine (I), gill (G), kidney (K), rectal gland (RG), liver (L), and brain (B). Bands were somewhat uneven, but this is normal when using relatively large amounts of protein per lane for detection purposes. Sizes were determined using kaleidoscope pre-stained molecular weight marker proteins (M).
To test whether the antibody staining was specific, various control blots were performed (Figure 2). While the staining appeared as in the tissue blot (Figure 1) when using the AQP4/1 or AQP4/2 antibody alone, when the antibody was pre-blocked using its peptide antigen, staining was almost entirely abolished in the case of either antibody. Additionally, there was no similar staining when using either serum taken from the rabbit prior to immunization with the Aqp4 antigens (pre-immune serum) or from the secondary antibody used on its own (no primary Aqp4 antibody).
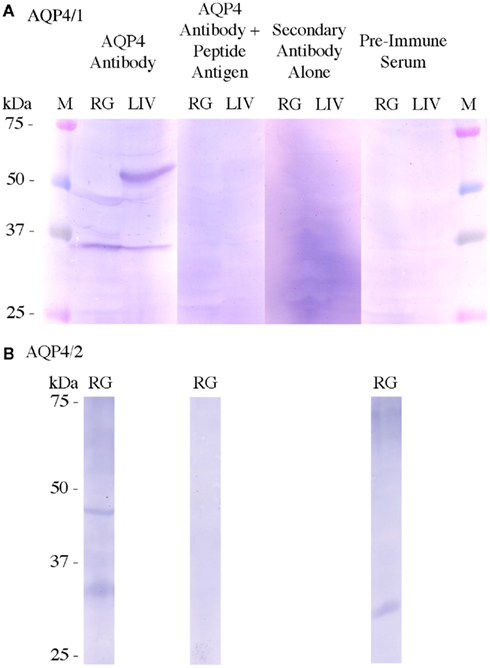
Figure 2. Control Western blots using tissue homogenates (300 μg) from rectal gland (RG) and/or liver (LIV). Normal Western blots used either AQP4/1 (A) or AQP4/2 (B) primary antibodies (Aqp4 Antibody). Control Western blots used either the Aqp4 antibody pre-blocked with the peptide antigen used to raise it (Aqp4 Antibody + peptide antigen), no primary antibody, i.e., only TNT buffer (secondary antibody alone) or serum taken from the rabbit before immunization with dogfish Aqp4 antigen (pre-immune serum). Control blots used the same primary or secondary antibody concentrations as in the normal blots. All blots were incubated 1 min in NBT/BCIP substrate. Sizes were determined using kaleidoscope pre-stained molecular weight marker proteins (M).
As the exact nature of the 49.5 and 57 kDa bands on the AQP4/1Western blot was worth further investigation, it was decided that, heat denaturation of the samples might cause some kind of aggregation (of the 35.5 kDa protein) to occur. Rectal gland crude membrane homogenate protein samples were therefore produced and blotted, that were either heated (as normal) and not heated (ambient temperature incubation, Figure 3). The lack of heat denaturation in comparison to normal had no effect on the 35.5 or 49.5 kDa bands, but the minor 37.5 and 38.5 kDa bands were absent in the un-heated protein sample lane and another diffuse band of around 32 kDa appeared instead. To further test whether any of the protein bands identified with the AQP4/1 antibody were glycosylated forms, crude membrane protein homogenates were treated with the enzyme PNGase F (New England Biolabs), which removes core N-glycosylated moieties from glycoproteins reducing their apparent molecular weight (Figure 4). However in either rectal gland or liver samples, PNGase F had no effect on the mobility of proteins identified by the AQP4/1 antibody, in comparison to similarly incubated control samples (no enzyme). This suggests that none of the protein bands represent glycosylated forms of Aqp4.
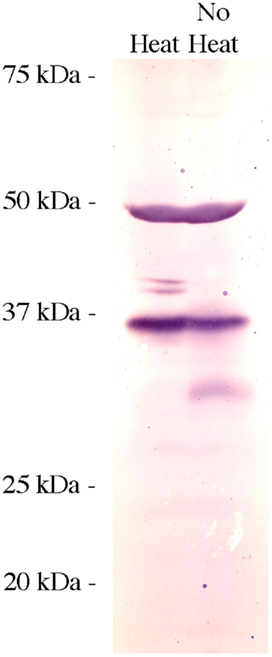
Figure 3. Western blot using rectal gland tissue homogenates (300 μg/lane) with samples either heated for 5 min at 100°C prior to running on the gel (heat) or without being heat treated (no heat). The blot was incubated with the AQP4/1 primary antibody and otherwise processed as usual.
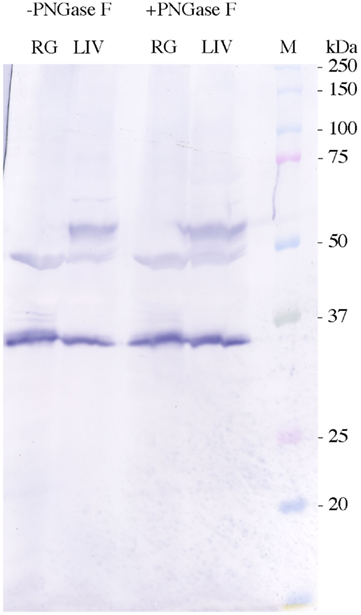
Figure 4. Western blots performed using AQP4/1 antibody and tissue homogenates (300 μg) from rectal gland (RG) or liver (LIV), incubated either with (+) or without (−) the enzyme PNGase F (5500 units) for 1 h at 37°C prior to electrophoresis. Sizes were determined using kaleidoscope pre-stained molecular weight marker proteins (M).
When the AQP4/1 and AQP4/2 antibodies were used for immunohistochemical staining of tissues, somewhat similar images were obtained with both antibodies but there were some differences, with the AQP4/2 antibody appearing to be more specific. In kidney (Figure 5), both antibodies labeled a sub-set of renal tubules both in the packed areas of the lateral bundle zone (Figures 5A–C) and in the sinus zone (Figure 5D). However, the AQP4/1 antibody (as is typical for other tissues also) stained the whole of the cell cytoplasm including in the region of the DAPI stained cell nucleus. Staining in renal tubule cells also was somewhat more intense toward the apical pole of the cell but was otherwise uniform throughout the cell. With the AQP4/2 antibody, fluorescence localized to the cytoplasm excluding the nuclear area stained by DAPI. Also there appeared to be no staining to the apical side of the nucleus in many cells. Serial sections stained with either AQP4/1 (Figure 5E) or AQP4/2 (Figure 5F) showed that the two antibodies stain the same segments of the similar renal tubule, although the type and intensity of staining was sometimes different between the two antibodies. With either antibody there appeared to be a sub-set of renal tubules (approximately 1–10% of the total stained) that showed basolateral membrane staining but with little or no cytoplasmic staining (Figure 6). In Figure 6B, a tubule has been cut through in longitudinal section but due to the depth of the section (5 μm), the bottom of the tubule is also visible. Interestingly, the membranes of an apparent stellate-shaped tubule cell have also been stained by the AQP4/1 antibody.
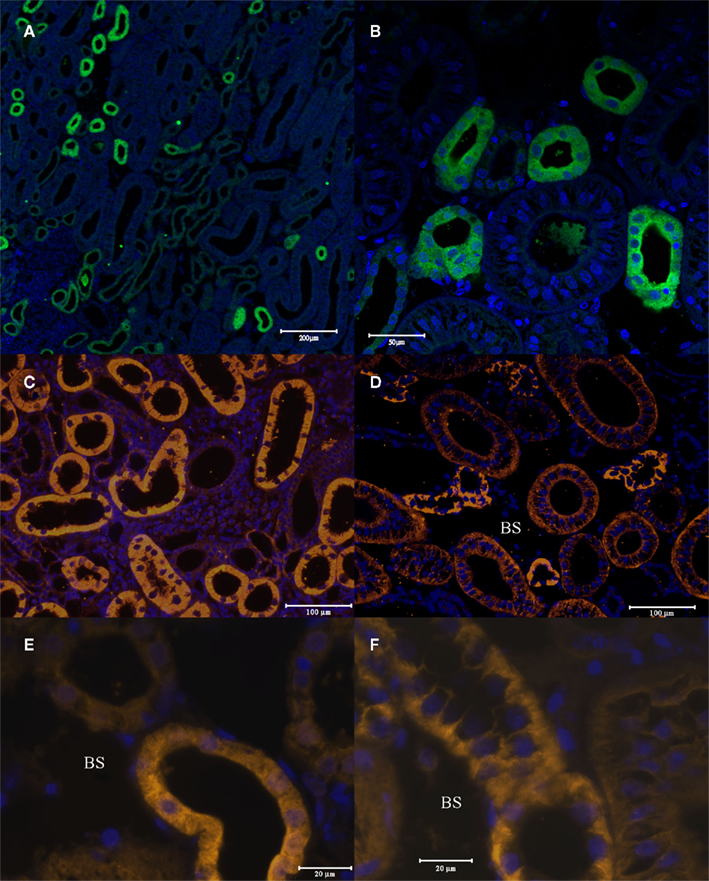
Figure 5. Immunohistochemistry of 5 μm cross-sections through the dogfish kidney. These were incubated with the AQP4/1 antibody, an Alexa 488 anti-rabbit secondary antibody (green) and viewed with a Zeiss 510 Meta confocal microscope (A,B). Or incubated with AQP4/2 antibody, an Alexa 555 anti-rabbit secondary antibody (orange) and viewed with a Zeiss Axiovert microscope (C,D,F). Or incubated with AQP4/1 antibody, an Alexa 555 anti-rabbit secondary antibody and viewed with a Zeiss Axiovert microscope (E). Nuclear counterstain, DAPI (blue). See scale bars for magnification. BS, blood sinus.
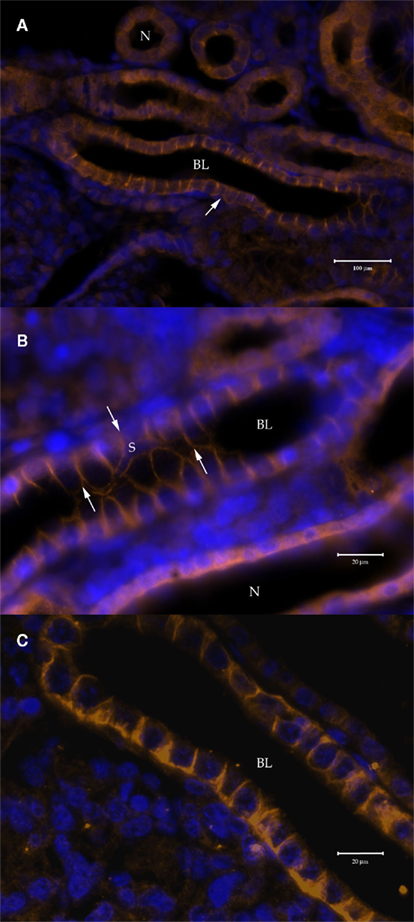
Figure 6. Immunohistochemistry of 5 μm cross-sections through the dogfish kidney showing basolateral (BL) plasma membrane staining in around 1–10% of stained tubule segments, viewed with a Zeiss Axiovert microscope. (A,B) Stained with the AQP4/1 primary and a Dylight 549 anti-rabbit secondary antibody (orange). (C) Stained with the AQP4/2 primary and a Alexa 555 anti-rabbit secondary antibody (orange). Nuclear counterstain, DAPI (blue). N = Aqp4 staining throughout the cells of the tubule. Arrows indicate the extent of the cell processes of a stellate cell (S).
Similar to the situation in renal tubule cells, in the rectal gland both antibodies fluorescently labeled all the secretory tubules of the gland. As in renal tubules AQP4/1 antibody stains the whole cell including the DAPI stained nuclear region with more intense labeling near the apical pole in many tubule cells (Figures 7A,B). The AQP4/2 antibody also stains the cytoplasm of tubule cells but with more intense staining toward the basal and apical poles of the cell and with lower intensity in the vicinity of the nucleus (Figures 7C,D). Major staining was also seen in tubule-like structures in dogfish cardiac stomach (Figure 8). Again both antibodies stained these tubules strongly although there was also some less intense staining in cells underlying the epithelium (e.g., see Figures 8A,C). In these putative cardiac stomach secretory tubules, again the AQP1/4 antibody stained the whole of the cell with more intense staining toward the apical pole. Staining with the AQP4/2 antibody was somewhat more patchy and diffuse in the cytoplasm (than with the AQP1/4 antibody) but was of lower intensity in the nuclear region.
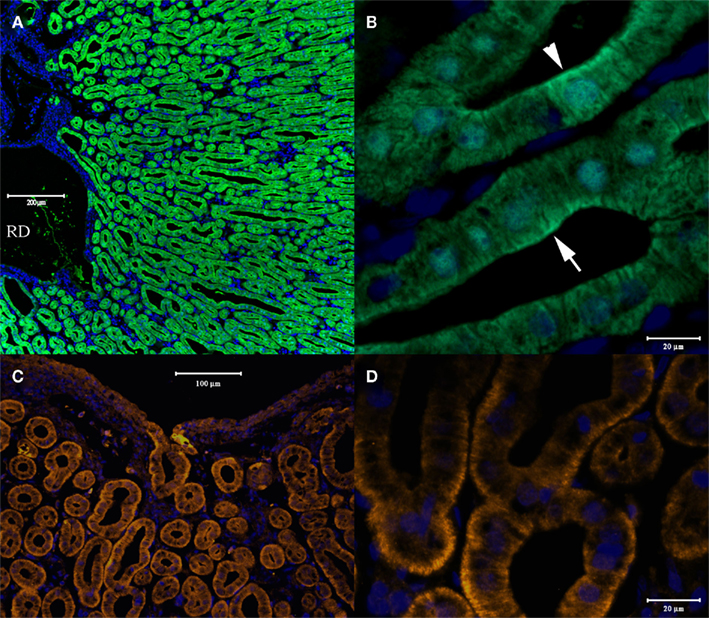
Figure 7. Cross-sections through dogfish rectal gland stained with AQP4/1 (A,B) or AQP4/2 (C,D). A, lower magnification image acquired with a Zeiss 510 Meta confocal microscope showing tubule staining and the gland’s central duct (RD). (B) Higher magnification image using a Zeiss Axiovert microscope, showing AQP/1 – Alexa 488 secondary antibody (green) staining throughout the cytoplasm of tubule cells with higher intensity in the apical pole (arrows). (C,D) Images showing AQP4/2 – Alexa 555 secondary antibody (orange) staining using a Zeiss Axiovert microscope, and showing stronger staining toward the basal pole of cells. Nuclear counterstain, DAPI (blue).
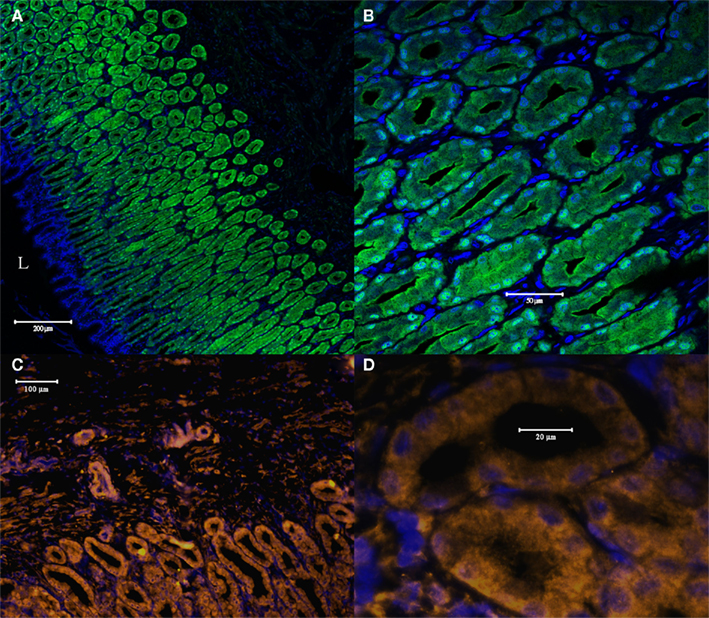
Figure 8. Cross-sections through dogfish cardiac stomach stained with AQP4/1 (A,B) and acquired with a Zeiss 510 Meta confocal microscope or with AQP4/2 (C,D) and acquired with a Zeiss Axiovert microscope. (A) Lower magnification image showing tubule staining and the cardiac stomach lumen (L). (B) Higher magnification image, showing AQP4/1 – Alexa 488 secondary antibody (green) staining throughout the cytoplasm of tubule cells with higher intensity in the apical pole. (C,D) Images showing AQP4/2 – Alexa 555 secondary antibody staining (orange), and showing patchy cytoplasmic staining but with less staining in the vicinity of the nucleus than in the case of the AQP4/1 antibody. Nuclear counterstain, DAPI (blue).
Lastly, strong staining was seen in large “chloride cell”-like cells of both the filament epithelium and the lamellae of the gill (Figure 9). The AQP4/1 antibody gave uniform cytoplasmic staining in many of these cells, and staining localized more to the plasma membrane and in the nuclear region in a minority of the other stained cells (Figures 9C,D). The AQP4/2 antibody also stained large cells in the primary filament epithelium and the secondary lamellae of the gill (Figures 9E,F) but here the staining in many of these cells was localized entirely in the region of the plasma membrane, while in others there was also some cytoplasmic staining.
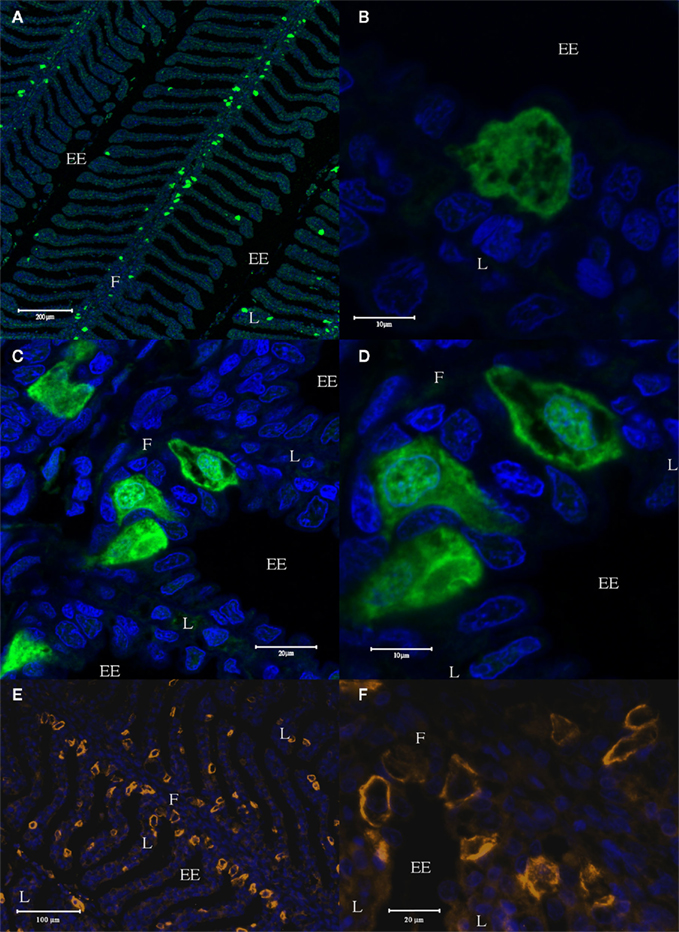
Figure 9. Longitudinal sections cut through the dogfish gill, stained with the AQP4/1 antibody and an Alexa 488 secondary antibody (green), and viewed with a Zeiss 510 Meta confocal microscope (A–D). Further sections were stained with the AQP4/2 antibody and an Alexa 555 secondary antibody (orange), and viewed with a Zeiss Axiovert microscope (E,F). Nuclear counterstain, DAPI (blue). Where F, filament, L, lamellae, and EE, external environment.
Previously, Piermarini and Evans (2001), and Wilson et al. (2002), showed that there are different large mitochondria-rich (MR) or “chloride cell”-like cells present in elasmobranch gill, that stained either for the ion transport enzyme Na+, K+-ATPase, or V-type ATPase. Thus a four-color localization study was performed to determine whether the cells staining with the Aqp4 antibodies co-localize with either transport enzyme (Figures 10 and 11). Initial studies using AQP4/1, Na+, K+-ATPase, and V-type ATPase antibodies on serial dogfish gill sections suggested some co-localization of the three antibodies (data not shown). However, four-color staining with all the antibodies on the same section clarified the situation. Although it is not clear from the wide-field image of the gill (Figure 10), essentially all of the cells staining with the AQP1/4 antibody (red) co-localize with either Na+, K+-ATPase (orange), or V-type ATPase (green) fluorescence. Only one cell was seen that appeared to express Aqp4 alone (and this one may have occurred as a consequence of the sectioning technique used). An example higher magnification image (Figure 11), clearly shows the co-localization of AQP4/1 fluorescence with the Na+, K+-ATPase, or V-type ATPase staining. Additionally this study also showed that most of the V-type ATPase expressing cells were localized predominantly in the primary filament epithelium, with only a few cells on the secondary lamellae, whereas the majority of the Na+, K+-ATPase expressing cells were on the secondary lamellae with only a few cells in the primary filament epithelium.
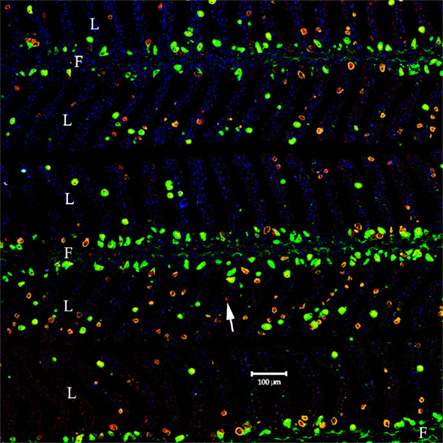
Figure 10. Wide-field view of a longitudinal section cut through the dogfish gill, stained with the AQP4/1 antibody directly labeled with Dylight 633 (red), a rabbit anti-sculpin V-ATPase antibody detected with highly cross-absorbed Alexa 488 secondary antibody (green), a mouse Na, K-ATPase monoclonal antibody detected with highly cross-absorbed anti-mouse Alexa 555 secondary antibody (orange) and nuclear counterstain, DAPI (blue). Image viewed with a Zeiss 510 Meta confocal microscope, where PF = primary filament and SL = secondary lamellae. The arrow indicates the only cell staining with AQP4 but not V-ATPase or Na, K-ATPase antibodies.
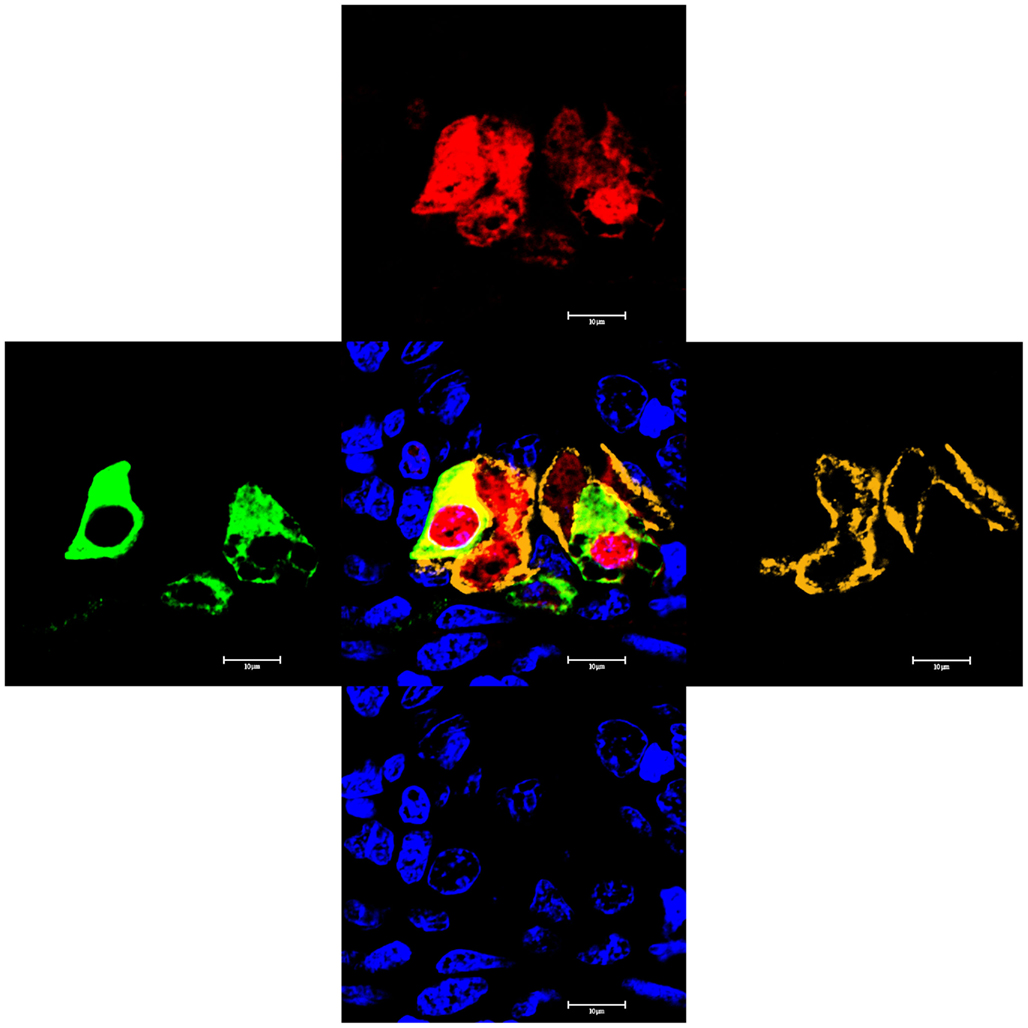
Figure 11. High magnification image of a longitudinal section cut through the dogfish gill primary filament epithelium, stained with the AQP4/1 antibody directly labeled with Dylight 633 (red), a rabbit anti-sculpin V-ATPase antibody detected with highly cross-absorbed Alexa 488 secondary antibody (green), a mouse Na, K-ATPase monoclonal antibody detected with highly cross-absorbed anti-mouse Alexa 555 secondary antibody (orange) and DAPI nuclear stain (blue). Image viewed with a Zeiss 510 Meta confocal microscope.
This study utilized two polyclonal antibodies that were raised against peptide antigens whose sequences were located in different non-overlapping regions of the dogfish Aqp4 derived amino acid sequence. Work with custom polyclonal antibodies is not always straightforward as they are often are more specific in immunohistochemistry than Western blotting or vice versa, or sometimes only work in one of those techniques. In this case, a second independent antibody was raised (AQP4/2) because unlike with the Western blot results with the AQP4/1 antibody, the immunohistochemical sub-cellular localizations, being largely cytoplasmic but also showing a nuclear location, were highly unusual for AQP4, which is normally a plasma membrane protein. To test the veracity of these results, AQP4/2 antibody was made and while it produces a larger array of bands in Western blotting (see Figure 1), as far as can be determined in this study, it produces staining in the same cells as the AQP4/1 antibody but shows a much more restricted sub-cellular localization, in particular showing either no or greatly reduced nuclear staining. It is possible to propose hypotheses that would explain the differences in results between the two antibodies (for example, it may be that only versions of Aqp4 with the C-terminal end removed (i.e., the region used to raise AQP4/1) can associate to form higher molecular weight dimers, trimers, and tetramers, hence explaining the high molecular weight banding seen with AQP4/2 (see Figure 1). However, the most parsimonious explanation is that the AQP4/1 binding is less specific in immunohistochemistry than AQP4/2, but that the opposite is true in Western blotting.
As with the tissue distribution of dogfish aqp4 mRNA expression (see Cutler et al., 2012), dogfish Aqp4 protein expression was ubiquitous and consequently dogfish Aqp4 is more widely expressed than is the case in mammals (Ishibashi et al., 2009). In particular, unlike in mammals, Aqp4 is expressed in dogfish gill and liver.
The AQP4/1 antibody produced two bands (49.5 and 35.5 kDa) in Western blots, which are essentially similar to the two bands obtained with mammalian AQP4 (Terris et al., 1995). The abundance of the 35.5 kDa protein band was similar between tissues but there was somewhat more Aqp4 protein in rectal gland, liver and brain, and with lower levels in (pyloric) stomach and kidney. In the case of mammalian AQP4, the larger band was suggested to be a glycosylated form of AQP4. While the Aqp4 amino acid sequence from dogfish as well as other species, possess putative consensus N-glycosylation sites, often glycoproteins on gels/blots yield broad diffuse bands and the 49.5 kDa band here and the 50 kDa AQP4 band in mammals are both discrete bands. It would also be expected that if the 49.5 kDa proteins were glycosylated, these bands would have their molecular weights reduced by the enzyme PNGase F, and this was not the case in this study (see Figure 4). There is some evidence from unpublished work from this laboratory concerning eel aquaporins expressed in Xenopus oocytes (i.e., the presence of dimers, trimers and tetramers), that standard Laemmli SDS-reducing gels do not abolish all interactions between proteins and also that some proteins appear to run apparently smaller than their molecular weight, suggesting incomplete unfolding (Lignot et al., 2002). Consequently it would seem likely that the 49.5 kDa band either represents Aqp4 with an accessory protein still attached to it, or an Aqp4 dimer that has run much smaller than its expected size (74.4 kDa). Lastly the 49.5 kDa band could represent a dimer that has undergone partial protease digestion. Similarly the 57 kDa protein seen in liver homogenates is likewise likely to occur due to one of the three aforementioned options. Finally it is not clear why the 37.5 and 38.5 kDa bands were absent from the un-heated rectal gland crude membrane homogenates (see Figure 3), but its likely the diffuse 32 kDa band present instead, represents some form of folded Aqp4 protein that when heat denatured runs at a larger more accurate molecular weight. It is also not clear why the 37.5 and 38.5 kDa bands were absent when using the AQP4/2 antibody on blots of rectal gland crude homogenates, although the region of Aqp4 used as an antigen to raise the AQP4/2 antibody, contains predicted serine and tyrosine kinase phosphorylation sites (NetPhos 2.0; Blom et al., 1999) and if 37.5 and 38.5 kDa represent phosphorylated Aqp4 proteins, then the AQP4/2 antibody might not be able to bind them.
Clearly from the immunohistochemistry performed with either antibody, dogfish Aqp4 is expressed in a sub-set or particular parts of renal tubules. Marine elasmobranch renal tubules are complex with two loops [with various neck (I–II), proximal (I–IV), intermediate (I–VI), distal (I–II), and collecting duct (I–II) segments] compared to the single loop of Henle found in mammals (Lacy and Reale, 1995). There are also lateral bundle zones with tightly packed tubules and sinus zones with blood sinuses seen as open areas. Based on the work of Lacy and Reale (1995), very tentative localizations for dogfish renal Aqp4 staining can be made. The majority of tubules segments staining are reminiscent of intermediate tubule segments, such as In-IV and In-V (or possibly In-II though In-IV) of the second renal tubule loop. This is because the Aqp4 antibodies stain open tubules without any apparent brush border (found in proximal segments) and these stained tubules are largely found in the lateral bundle zone. The In-IV segment is known as the “diluting segment” due to the occurrence of sodium chloride re-absorption in this region of the nephron (Friedman and Hebert, 1990). The presence of Aqp4 in this region would be curious as its thought to have low water permeability (Friedman and Hebert, 1990). If Aqp4 was trafficked into the basolateral membrane as occurs with some AQPs in mammals, provided their was no apical water conduit this would be consistent with low tubule water permeability. Additionally this would suggest Aqp4 may be involved in cell volume regulation in this segment. Because some tubules showing Aqp4 staining are also found in the sinus zone this suggests that these regions are likely to be other intermediate segments (i.e., the first part of In-VI or less likely In-I). The presence of Aqp4 in the In-VI would make sense, as this segment is thought to have high water permeability and may be involved in osmotic equilibration due to water egress from the renal tubule (Friedman and Hebert, 1990). However Aqp4 would have to reside in the plasma membrane for this to be the case and its currently not clear that that is the case. Again regulated trafficking of Aqp4 to the plasma membrane might also be an explanation for that as mention above. Additionally, the very occasional tubule showing Aqp4 staining, did appear to have some brush border material present, this suggests Aqp4 may also stain a small part of the proximal tubule. Lastly, the tubule segments showing basolateral staining probably represent a different part of the intermediate segment (In-III?). However the localization of Aqp4 to particular tubule segments currently remains difficult. Different renal tubule segments have been identified using various lectins (Althoff et al., 2006) but the results of this study suggest it would not be trivial to reliably localize Aqp4 (or other proteins) to particular tubule segments using their methods, and that is therefore beyond the scope of the current article.
The fact that Aqp4 was expressed in the dogfish rectal gland is also of interest as the gland has been a major model tissue historically for studies involving fluid secretion (Burger and Hess, 1960; Bonting, 1966; Hayslett et al., 1974; Epstein et al., 1983; Greger et al., 1988; Riordan et al., 1994; Forest, 1996; Silva et al., 1996; Evans, 2010). Despite the many studies concerning ion transport, the only study to look at water permeability of the gland suggested that it probably did not express aquaporins due to low apparent membrane water permeability (Zeidel et al., 2005). However, a series of illuminating studies by Solomon et al. (1984a,b, 1985) showed that ion secretion by the rectal gland was not stimulated when animals were perfused with hypertonic shark ringer solution (plasma salinity was raised without changing body fluid volume), but was stimulated when body fluid volume was increased using isotonic shark ringer. This strongly suggests that the principle function of the rectal gland is actually to remove excess water but due to the fact that water transport is passive, ions have to be transported to allow the water to follow by osmosis. Additionally, almost every example of secretory tissues/cells investigated has shown that the cells involved (in fluid secretion) invariably express some kind of aquaporin isoform. So it might be expected that elasmobranch rectal gland secretory tubule cells would express aquaporins. However, in the case of the staining with either of the AQP4/1 or AQP4/2 antibodies, with the respective apical or basal staining, in neither case does staining appear be present in the plasma membrane itself to any great extent, and this may explain why the study of Zeidel et al. (2005), found no significant water permeability associated with rectal gland plasma membranes. However, the question would be, why have Aqp4 then? The dogfish used in this study were normal unfed animals whose rectal glands are unlikely to have been particular active. The answer to the question therefore may be that a significant amount of Aqp4 may not reside in the plasma membranes of tubule cells until the gland is stimulated to secrete, whereupon Aqp4 may be inserted into the plasma membrane. Regulated insertion of aquaporins has been shown to occur in mammals and is a particularly important mechanism for renal AQP2 (Nejsum, 2005). The possibility of regulated trafficking of dogfish rectal gland Aqp4 may be tested by further experiments in the future.
During a screen of different dogfish tissues to see where Aqp4 was expressed, particularly strong staining was found in the cardiac stomach, which is an extension of the esophagus (anterior to the pyloric stomach) that has a totally distinct morphology in comparison to the esophagus itself (smooth brown epithelium rather than a surface covered with white cartilaginous conical structures). The Aqp4 staining appears to be localized particularly to secretory tubule structures that are likely to represent acid secreting gastric glands. The cardiac stomach of dogfish has been shown to have an acidic lumen with a pH in the range of 2–4 (Wood et al., 2007). Elasmobranchs have also been shown to express the H+, K+-ATPase enzyme in proximal stomach, which is associated with stomach acid secretions in mammals (Smolka et al., 1994; Choe et al., 2004; Shin et al. (2009)). As often occurs with ion secretions, they usually represent fluid secretions and consequently water is also secreted. This may move via the paracellular pathway, but a transcellular route via aquaporin water channels is more easily controlled. In dogfish cardiac stomach gastric glands, the immunohistochemical results in this study suggest fluid secretion may well involve Aqp4, but again there is no particular staining clearly associated with the gland cell plasma membranes although the AQP4/1 antibody shows staining toward the apical pole of cells. As mentioned previously, the dogfish in this study were unfed and it may be that Aqp4 is only inserted into the plasma membranes after secretion is stimulated by feeding. This is therefore another avenue for further study.
Another location showing strong Aqp4 staining was the epithelial cells of the gill. Studies in the even more ancient Agnathan hagfish show staining for Aqp4 only in the gill but not other tissues, suggesting this may be the original cell localization for Aqp4 in vertebrates (Nishimoto et al. (2007)). In these cyclostomes, however, Aqp4 was found only in the pavement cells of lamellae. In this study, Aqp4 staining was found in both the filament epithelium and the in lamellae, in large “chloride cell”-like or “MR”-like cells. In particular with the AQP4/2 antibody (but also to some extent the AQP4/1 antibody) there were two different staining patterns in these large cells, staining either located exclusively in the plasma membrane, or in the plasma membrane and interior cytoplasmic regions of the cell. Previous work has shown that there are two different types of “chloride cell”-like or “MR”-like cells which express either of the ion transporting enzymes, Na+, K+-ATPase, or V-type H+-ATPase (Piermarini and Evans, 2001; Wilson et al., 2002). To determine whether either of these cell types corresponded to the cells expressing Aqp4, a four-color localization study was undertaken and this showed that Aqp4 staining localizes to both the Na+, K+-ATPase, and the V-type H+-ATPase expressing cells. While the function of these cells has still not been determined, a similar situation exists in the gill of freshwater teleosts, where the V-type H+-ATPase expressing (HR) cells are thought to be concerned with acid–base balance, whereas the Na+, K+-ATPase (NaR) expressing cells are involved in calcium transport (Hwang, 2009). Whether the Aqp4-expressing dogfish gill cells have the same functions and transport properties as these teleost gill cells remains to be determined.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
For this work, Christopher P. Cutler was supported by four Salisbury Cove summer fellowships from the Mount Desert Island Biological Laboratory (2004–2007). S. Harmon and K. Burch were supported by NSF REU scholarships (2008). J. Walsh was supported by a NSF ASPIRES scholarship (2009). The mouse Na, K-ATPase alpha subunit a5 monoclonal antibody developed by D. M. Fambrough, was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Althoff, T., Hentshel, H., Luig, J., Schultz, H., Kasch, M., and Kinne, R. K.-H. (2006). Na+-D-glucose cotransporter in the kidney of Squalus acanthias: molecular identification and intrarenal distribution. Am. J. Physiol. 290, R1094–R1104.
Blom, N., Gammeltoft, S., and Brunak, S. (1999). Sequence- and structure-based prediction of eukaryote protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362.
Bonting, S. L. (1966). Studies on sodium–potassium-activated adenosinetriphosphatase. XV. The rectal gland of the elasmobranchs. Comp. Biochem. Physiol. 17, 953–966.
Borok, Z., and Verkman, A. S. (2002). Lung edema clearance: 20 years of progress. Invited review: role of aquaporin water channels in fluid transport in lung and airways. J. Appl. Physiol. 93, 2199–2206.
Burger, J. W., and Hess, W. N. (1960). Function of the rectal gland of the spiny dogfish. Science 131, 670–671.
Butler, T. L., Au, C. G., Yang, B., Egan, J. R., Tan, Y. M., Hardeman, E. C., North, K. N., Verkman, A. S., and Winlaw, D. S. (2006). Cardiac aquaporin expression in humans rats and mice. Am. J. Physiol. 291, H705–H713.
Choe, K. P., Verlander, J. W., Wingo, C. S., and Evans, D. H. (2004). A putative H+-K+-ATPase in the Atlantic stingray, Dasyatis sabina: primary sequence and expression in gills. Am. J. Physiol. 287, R981–R991.
Cutler, C. P. (2006). Cloning of aquaporin 1e gene homologues in the dogfish shark (Squalus acanthias) and hagfish (Myxine glutinosa). Bull. Mt. Desert Isl. Biol. Lab. Salisb. Cove Maine 45, 40–41.
Cutler, C. P. (2007). Cloning and identification of four aquaporin genes in the dogfish shark (Squalus acanthias). Bull. Mt. Desert Isl. Biol. Lab. Salisb. Cove Maine 46, 19–20.
Cutler, C. P., MacIver, B., Cramb, G., and Zeidel, M. L. (2012). Aquaporin 4 is a ubiquitously expressed isoform in the dogfish (Squalus acanthias) shark. Front. Physiol. 2:107. doi:10.3389/fphys.2011.00107
Cutler, C. P., Meischke, L., and Cramb, G. (2005). Evolutionary and comparative analysis of aquaporin water channel genes in fish. Bull. Mt. Desert Isl. Biol. Lab. Salisb. Cove Maine 44, 55.
Delporte, C., and Steinfeld, S. (2006). Distribution and roles of aquaporins in salivary glands. Biochim. Biophys. Acta 1758, 1061–1070.
Epstein, F. H., Stoff, J. S., and Silva, P. (1983). Mechanism and control of hyperosmotic NaCl-rich secretion by the rectal gland of Squalus acanthias. J. Exp. Biol. 106, 25–41.
Evans, D. H. (2010). A brief history of the study of fish osmoregulation: the central role of the Mt. desert island biological laboratory. Front. Physiol. 1:13. doi:10.3389/fphys.2010.00013
Forest, J. N. Jr. (1996). Cellular and molecular biology of chloride secretion in the shark rectal gland: regulation by adenosine receptors. Kidney Int. 49, 1557–1562.
Friedman, P. A., and Hebert, S. C. (1990). Diluting segment in kidney of dogfish shark. I. Localization and characterization of chloride absorption. Am. J. Physiol. 258, R398–R408.
Greger, R., Gogelein, H., and Schlatter, E. (1988). Stimulation of NaCl secretion in the rectal gland of the dogfish Squlaus acanthias. Comp. Biochem. Physiol. 90, 733–737.
Hayslett, J. P., Schon, D. A., Epstein, M., and Hogben, C. A. (1974). In vitro perfusion of the dogfish rectal gland. Am. J. Physiol. 226, 1188–1192.
Hirrlinger, P. G., Pannicke, T., Winkler, U., Claudepierre, T., Varshney, S., Schulze, C., Reichbach, A., Brunken, W. J., and Hirrlinger, J. (2011). Genetic deletion of laminin isoforms b2 and c3 induces a reduction in Kir4.1 and aquaporin-4 expression and function in the retina. PLoS ONE 6, e16106. doi:10.1371/journal.pone.0016106
Hwang, P-P. (2009). Ion uptake and acid secretion in zebrafish (Danio rerio). J. Exp. Biol. 212, 1745–1752.
Ishibashi, K., Hara, S., and Kondo, S. (2009). Aquaporin water channels in mammals. Clin. Exp. Nephrol. 13, 107–117.
Lacy, E. R., and Reale, E. (1995). “Functional morphology of the elasmobranch nephron and retention of urea,” in Cellular and Molecular Approaches to Fish Ionic Regulation, eds C. M. Wood and T. J. Shuttleworth (New York: Academic Press), 107–146.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteiophage T4. Nature 227, 680–685.
Lignot, J.-H., Cutler, C. P., Hazon, N., and Cramb, G. (2002). Immunolocalization of aquaporin 3 in the gill and the gastrointestinal tract of the European eel (Anguilla anguilla L.). J. Exp. Biol. 205, 2653–2663.
Ma, T., and Verkman, A. S. (1999). Aquaporin water channels in gastrointestinal physiology. J. Physiol. 517, 317–326.
Mobasheri, A., Kendall, B. H., Maxwell, J. E. J., Sawran, A. V., German, AJ., Marples, D., Luck, M. R., and Royal, M. D. (2011). Cellular localization of aquaporins along the secretory pathway of the lactating bovine mammary gland: an immunohistochemical study. Acta Histochem. 113, 137–149.
Nejsum, L. N. (2005). The renal plumbing system: aquaporin water channels. Cell. Mol. Life Sci. 62, 1692–1706.
Nicchia, G. P., Cogotzi, L., Rossi, A., Basco, D., Brancaccio, A., Svelto, M., and Frigeri, A. (2008). Expression of multiple AQP4 pools in the plasma membrane and their association with the dystrophin complex. J. Neurochem. 105, 2156–2165.
Nishimoto, G., Sasaki, G., Yaoita, E., Nameta, M., Li, H., Furuse, K., Fujinaka, H., Yoshida, Y., Mitsudome, A., and Yamamoto, T. (2007). Molecular characterization of water-selective AQP (EbAQP4) in hagfish: insight into ancestral origin of AQP4. Am. J. Physiol. 292, R644–R651.
Piermarini, P. M., and Evans, D. H. (2001). Immunochemical analysis of the vacuolar proton-ATPase B-subunit in the gills of a euryhaline stingray (Dasyatis sabina): effects of salinity and relation to Na+/K+-ATPase. J. Exp. Biol. 204, 3251–3259.
Riordan, J. R., Forbush, B. III, and Hanrahan, J. W. (1994). The molecular basis of chloride transport in shark rectal gland. J. Exp. Biol. 196, 405–418.
Shin, J. M., Munson, K., Vagin, O., and Sachs, G. (2009). The gastric HK-ATPase: structure, function and inhibition. Pflugers Arch. 457, 609–622.
Silva, P., Solomon, R. J., and Epstein, F. H. (1996). The rectal gland of Squalus acanthias: a model for the transport of chloride. Kidney Int. 49, 1552–1556.
Smolka, A. J., Lacy, E. R., Luciano, L., and Reale, E. (1994). Identification of gastric H, K-ATPase in an early vertebrate, the Atlantic stingray Dasyatis sabina. J. Histochem. Cytochem. 42, 1323–1332.
Solomon, R., Taylor, M., Sheth, S., Silva, P., and Epstein, F. H. (1985). Primary role of volume expansion in stimulation of the rectal gland function. Am. J. Physiol. 248, R638–R640.
Solomon, R., Taylor, M., Stoff, J. S., Silva, P., and Epstein, F. H. (1984a). In vivo effect of volume expansion on rectal gland function. I. Humoral factors. Am. J. Physiol. 246, R63–R66.
Solomon, R., Taylor, M., Rosa, R., Silva, P., and Epstein, F. H. (1984b). In vivo effect of volume expansion on rectal gland function. II. Hemodynamic changes. Am. J. Physiol. 246, R67–R71.
Terris, J., Ecelbarger, C. A., Marples, D., Knepper, M. A., and Nielsen, S. (1995). Distribution of aquaporin-4 water channel expression within rat kidney. Am. J. Physiol. 269, F775–F785.
Wakayama, Y. (2010). Aquaporin expression in normal and pathological skeletal muscles: a brief review with focus on AQP4. J. Biomed. Biotechnol. 2010, 1–9.
Wilson, J. M., Morgan, J. D., Vogl, A. W., and Randall, D. J. (2002). Branchial mitochondria-rich cells in the dogfish Squalus acanthias. Comp. Biochem. Physiol. 132, 365–374.
Wood, C. M., Kajimura, M., Bucking, C., and Walsh, P. J. (2007). Osmoregulation, ionoregulation and acid-base regulation by the gastrointestinal tract after feeding in the elasmobranch (Squalus acanthias). J. Exp. Biol. 210, 1335–1349.
Xu, H., Zhang, W., Wei, W., Shen, L., and Wu, W. (2009). Differential expression of aquaporin-4 in human gastric normal and cancer tissues. Gastroenterol. Clin. Biol. 33, 72–76.
Zeidel, J. D., Mathei, J. C., Campbell, J. D., Ruiz, W. G., Apodaca, G. L., Riordan, J., and Zeidel, M. L. (2005). Selective permeability barrier to urea in shark rectal gland. Am. J. Physiol. 289, F83–F89.
Keywords: aquaporin 4, rectal gland, gill, stomach, kidney, dogfish
Citation: Cutler CP, Harmon S, Walsh J and Burch K (2012) Characterization of aquaporin 4 protein expression and localization in tissues of the dogfish (Squalus acanthias). Front. Physio. 3:21. doi: 10.3389/fphys.2012.00021
Received: 16 September 2011;
Accepted: 29 January 2012;
Published online: 15 February 2012.
Edited by:
Steffen Madsen, University of Southern Denmark, DenmarkReviewed by:
Paul Yancey, Whitman College, USACopyright: © 2012 Cutler, Harmon, Walsh and Burch. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Christopher P. Cutler, Department of Biology, Georgia Southern University, 69 Georgia Avenue, Building 202, Statesboro, GA 30460-8042, USA. e-mail:Y2N1dGxlckBnZW9yZ2lhc291dGhlcm4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.