- 1School of Mathematics and Statistics, Central South University, Changsha, China
- 2Deparment of Mathematics, Shah Abdul Latif University, Khairpur, Pakistan
- 3Deparment of Mathematics, Dambi Dollo University, Dambi Dollo, Ethiopia
- 4Dawood University of Engineering and Technology Karachi, Karachi, Pakistan
Topological indices are mathematical descriptors of the structure of a molecule that can be used to predict its properties. They are derived from the graph theory, which describes the topology of a molecule and its connectivity. The main objective is mathematical modeling and topological properties of ϒ-graphyne. Current research focuses on two structures made from hexagonal honeycomb graphite lattices named triangular ϒ-graphyne and triangular ϒ-graphyne chains. The authors have simultaneously computed the first and second Reverse Zagreb indices, reverse hyper-Zagreb indices, and their polynomials. This research also derives mathematical closed-form formulas for some of its fundamental degree-based molecular descriptors. Researchers have been trying to synthesize a novel carbon form called Graphyne. For over a decade but with no success. Recently, some researchers have made a breakthrough in generating Carbons elusive allotrope and solved a long-standing problem in carbon materials. This wonder material is created to rival the conductivity of graphene but with control. These results opened new ways of research in the fields of semiconductors, electronics and optics. Furthermore, graphical and tabular results will help to investigate the structure-property relationships in γ-graphyne.
1 Introduction
Graph theory has been utilized extensively in the modeling of chemical structures, and their mathematical modeling is referred to as chemical graph theory. This notion has a significant impact on the advancement of chemical science. The study of topological indices that can describe and predict organic molecules’ physicochemical and pharmacological properties is novel and active research in chemical graph theory. The topological index calculates the properties of interesting compounds, and it remains invariant. In cheminformatics, if we want to study compounds’ properties and chemical bioactivity, we must utilize the Quantitative structure-property relationships (QSPR) together with topological indices. The selection of topological indices is based on previous validations by researchers, where these indices were correlated with certain physico-chemical properties in molecules; hence, they are applied to model the considered structures. Numerous applications of the topological indices are found in the Quantitative structure-activity relationship (QSAR), which is used to forecast the physio-chemical characteristics of chemical compounds [1–7]. Topological indices are the numbers that belong to a chemical’s structure and are supposed to show how it relates to other structures. The study of molecular descriptors assists in compensating for the lack of chemical experiments by giving a theoretical base for making chemical materials [8–14]. In the structure-activity relationship, the topological architecture of the chemical structure and the simple connectivity between neighboring atoms are more important than the nature of chemical bonds. This is because the nature of chemical bonds is not seen as a significant factor in the biological activity of the chemical compound [12, 15–19]. Harold Wiener, a chemist, used a topological index for the first time in 1947 when chemists and mathematicians defined 100 topological indices and investigated many chemical structural features [19, 20]. Gutman described Zagreb indices as degree-based topological indices more than 40 years ago [21]. Topological indices like these were proposed to quantify the degree to which the carbon atom skeleton is branched [22]. A molecule’s topological index is a non-empirical numerical value [23]. Topological indices are molecular graph invariants that can be employed to create QSPR/QSAR as numerical descriptors [24]. These can be shaped with a distance matrix and the chemical graph (hydrogen-suppressed graph). Notations and terminology not defined here can be found in refs [25–30] except if otherwise stated.
The Weiner index [31]. and the Gutman defined and formulated The first and second Zagreb indices as in 1972 [32].
Harold et al. [33] examined the connection between the Zagreb indices and the overall energy of π-electrons, as indicated by references [34–37] for more results related to Zagreb indices. After Gutman, Shirdel et al [38] first and second hyper-Zagreb indices such as
More see Reference [39–41]. Chemical graph theory has applications in predicting molecular properties, such as boiling points and solubilities. It also aids in understanding chemical reactions and exploring molecular databases. Overall, chemical graph theory provides a foundation for the analysis and prediction of chemical structures and properties using graph theory principles [42–47].
Graph Theory is widely used in real-world phenomena. For communication networks, computational devices, and data organization, a graph is extensively used in computer science and the study of the properties of molecules in chemistry, physics, and biology.
1.1 γ-graphyne
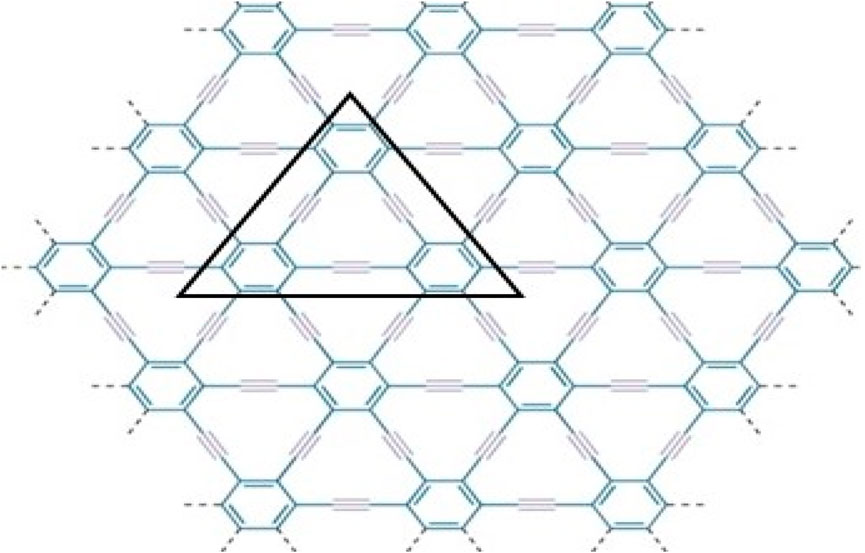
γ-graphyne is a two-dimensional carbon allotrope that belongs to the graphyne family. It is a highly intriguing material because of having unique structure and exceptional properties. Gamma graphyne has shown promising potential as a catalyst for various chemical reactions, demonstrating its usefulness in energy conversion and storage. Researchers actively explore its properties, synthesizing methods, and applications to harness its full potential. The importance of (2D) derivatives of graphite structures like graphyne is rising due to their promising properties like band tunable gaps, charge carrier mobilities, etc. Hexagons and sp2 hybridized (the mixing of one s and two p atomic orbitals) carbon atoms make up the two-dimensional sheet known as graphene. Its significance and novelty are due to its remarkable physical, mechanical, chemical, and electrical properties [48]. Recently, increasing interest in graphene-based material achieved the Nobel Prize in Physics in 2010 for groundbreaking experiments in 2D substance graphene [49]. The states of sp2 atoms remain similar when carbyne chains [50]. Acetylenic connections are added to a honeycomb structure made of carbon (C) atoms that have undergone sp hybridization to create graphyne, two-dimensional materials developed from graphene. Due to acetylene groups, these structures exhibit a diverse spectrum of electrical, optical, and mechanical properties [51]. Various graphene ganoscroll (GN) derivatives are under study, and their crystal structures are shown in [52]. Because of their high heat storage capacity on adsorption and negligible change in computed activation barriers, graphyne has been suggested by Swathi et al. for use as substrates with graphene in practical devices [53]. M. Kando et al. verified from vibrational investigations that practically all oligomer model planar configurations are at least local energy minima [54]. The fascinating and distinctive mechanical and electron-conducting properties of graphyne were predicted [55–58] optical characteristics. In particular, like in graphene, the electron conduction in graphynes would be incredibly rapid. Unlike graphene’s multidirectional conduction, some graphynes’ electron conduction can be regulated in a specific direction because triple bonds can bend Dirac cones [55, 56]. Although the topological descriptors for the structures of the graphene nanoscroll (GNs) have not yet been investigated, due to the importance in applications of structures, the study of the topological indices of these distinct networks to compare and contrast the complexity of these structures is necessary aspect [59–61]. Molecular descriptors for the structure of γ-graphyne have not investigated before, as given in Figures 1, 2. Consequently, the authors in [62] have shown its significance. The reverse vertex degree, which focuses on less-connected nodes, can illuminate various physical and chemical phenomena. For example, in catalysis, active sites often occur at low-degree vertices where unique reactions happen. Similarly, in material science, defects and irregularities frequently reside at these less-connected nodes, impacting properties like conductivity and strength. In biological networks, reverse vertex degree analysis reveals crucial nodes in protein interactions or metabolic pathways, often associated with rare but significant interactions. By examining these less-connected vertices, we uncover insights into reactivity, stability, and functionality, demonstrating the practical significance of topological indices in real-world applications.
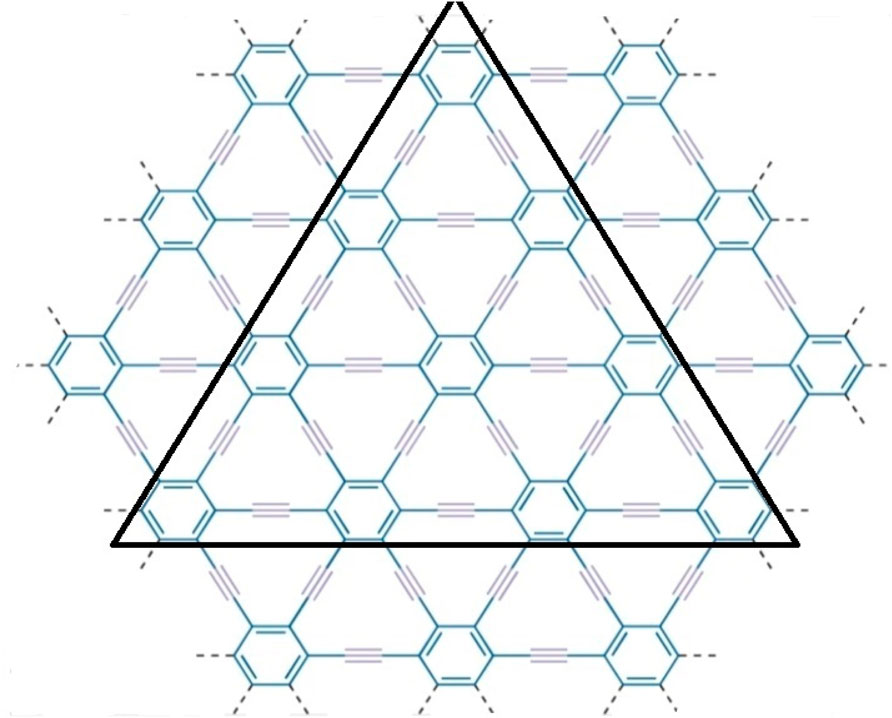
Figure 1. Molecular graph
ϒ-Graphyne, with its tunable band gap and high electron mobility, is promising for electronics, semiconductors, and energy storage devices. It enhances supercapacitors, batteries, and hydrogen storage. Additionally, it is valuable in sensors, catalysis, optoelectronics, and composite materials due to its unique structure and properties.
2 Calculation method
A molecular graph is a simple graph in which vertices and edges reflect atoms and bonds [63–65]. The number of edges that a vertex is associated with determines its degree. A graph’s maximum vertex degree is represented by the symbol (G). Kulli introduces the concept of reverse vertex degree with the formula as
Where Cv is denoted by the reverse degree,
Gutman (1972) defined and formulated the first and second Zagreb indices [32]. We defined the
Ivan Gutman introduced the first and second hyper-Zagreb indice [32, 38, 67]. We defined the
The first and second Zagreb polynomials were introduced by Ivan Gutman [11]. We defined the
The same paper first and second hyper-Zagreb indices [38]. We defined the
Similarly, many other indices are essential in relationship QSPR/QSAR analysis. Review topological indices and further information in an in-depth review of references [68–74].
3 Results and discussion
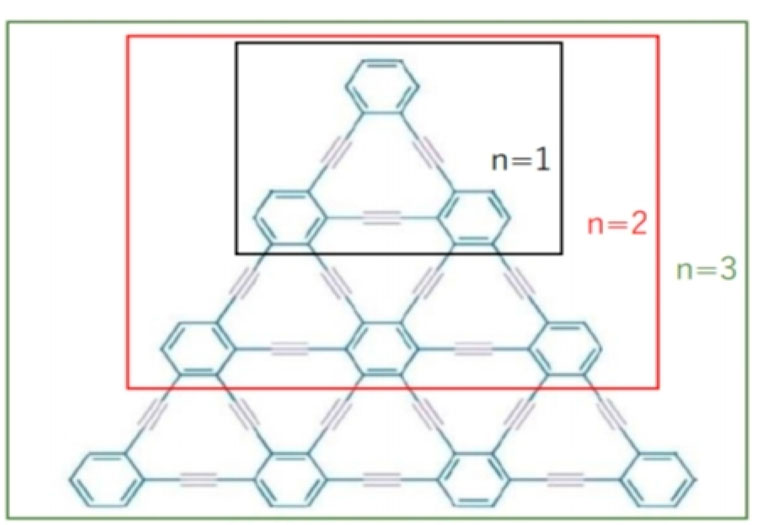
3.1 Triangular γ-graphyne
We have discussed molecular structure, edge partitioning technique, and robust computational results. In this study, as the focus is on the mathematical modeling of graphyne, we deliberately treat the π bonds within the benzene rings and the acetylene bonds bridging the benzene rings as equivalent despite their believed different chemical characteristics. We applied topological indices on the triangular γ-graphyne structure and obtained novel and new findings. Using the edge partitioning technique and topological indices, each row of the system given in Figure 3 contains
Similarly, 'd23' refers to an edge connecting two vertices, where one vertex has two edges, and the other has three. Lastly, 'd33' indicates an edge connecting vertices that each have three edges. These notations are crucial for understanding the edge-vertex relationships and their degrees in our graph-theoretical framework.
Where
Clearly. We have
We know that there are three different types of reverse edges.
Theorem 3.1.1. If G is a graph of the Triangular
i.
ii.
iii.
iv.
Proof: our results using the reverse edge partition of the triangular -
Theorem 3.1.2. If G is a graph of the Triangular
I.
II.
III.
IV.
Proof: our results using the reverse edge partition of the triangular - γ-graphyne;
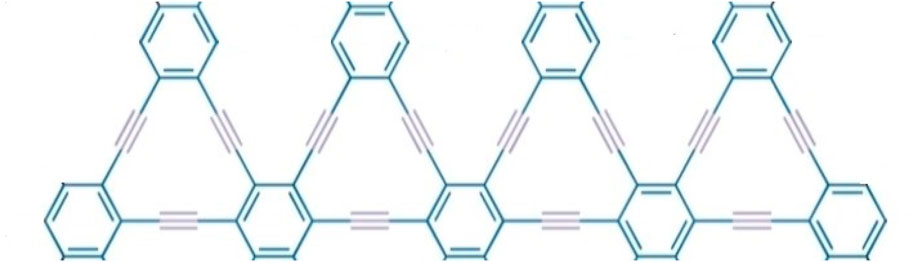
3.2 Triangular chain γ-graphyne
We have discussed molecular structure, edge partitioning technique, and robust computational results. We applied topological indices on the triangular γ-graphyne chain structure and obtained novel and new findings from Figure 4. Using the edge partitioning technique and topological indices, each column of the system given in Figure 4 having
Clearly. We have
We know that there are three different types of reverse edges.
Theorem 3.2.1. If G is a graph of the triangular chain γ-graphyne chain. Then, we have the
i.
ii.
iii.
iv.
Proof: our results using the reverse edge partition triangular chain γ-graphyne;
Theorem 3.2.2. If G is a graph of the triangular chain γ-graphyne chain. Then, we have the
i.
ii.
iii.
iv.
4 Numerical and graphical results and discussions
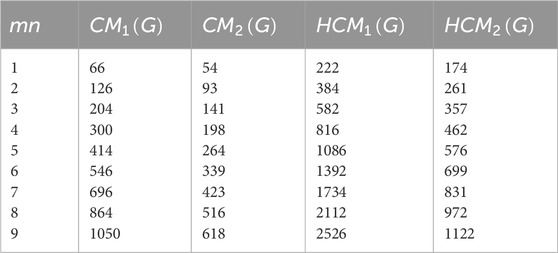
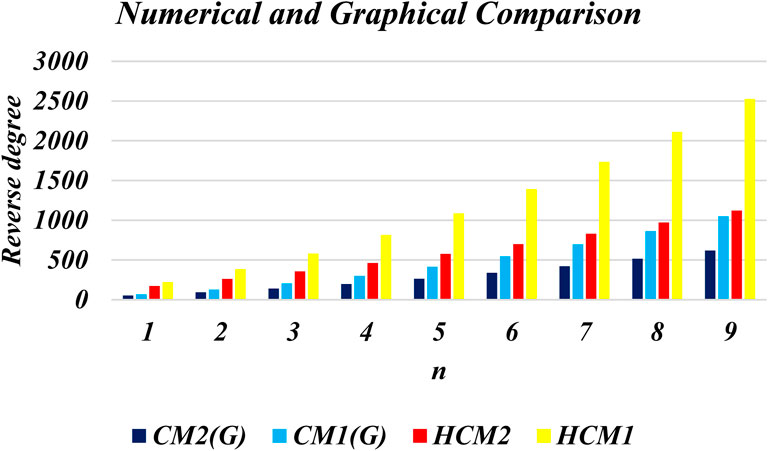
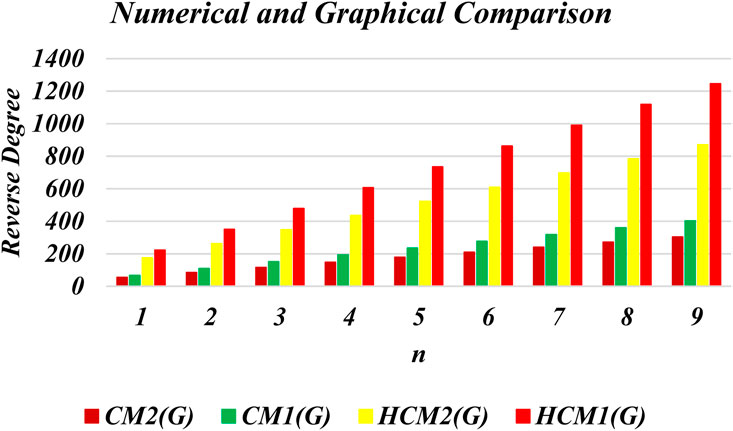
This section presents numerical and graphical results for the triangular γ-graphyne and triangular chain γ-graphyne via reverse degree-based topological indices. We compute numerical values for the first and second reverse Zagreb indices, as well as the first and second hyper-reverse Zagreb indices at different values of n, as seen in Tables 1, 2. Furthermore, we have plotted bar graphs to investigate the behavior of these topological indices at different n values, as given in Figures 5, 6. Additionally, we observed that the reverse first hyper Zagreb index has the highest value while the reverse second Zagreb index has the lowest value.
5 Conclusion
An edge partitioning technique based on graph theory is used on the molecular topology of γ–graphyne. Mathematical closed-form formulas are derived for several of its significant degree-based molecular descriptors. The results show that the reverse first hyper Zagreb index has the highest value. It is observed that the reverse first hyper Zagreb index has high predictive performance among all the other computed molecular descriptors. These numerical values signify various physicochemical properties of triangular γ-graphyne and chain triangular γ-graphyne. The results obtained in this study will help to investigate the structure-property relationships in γ-graphyne. In future research, we plan to calculate entropy, Mpolynomial indices, Zagreb connections, and distancebased topological indices to further characterise the molecular structure of Triangular γ–Graphyne and Triangular γ–Graphyne chain. Such analysis will provide additional insight into the properties and behaviour of these materials, and may contribute to their potential applications in various fields.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AH: Conceptualization, Investigation, Software, Writing–original draft, Writing–review and editing. NK: Data curation, Methodology, Supervision, Writing–original draft. HS: Formal Analysis, Project administration, Validation, Writing–original draft. FT: Funding acquisition, Resources, Writing–review and editing. OA: Methodology, Validation, Visualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Balaban AT, Devillers J. Topological indices and related descriptors in QSAR and QSPAR. CRC Press (2014).
2. Dobrynin AA, Kochetova AA. Degree distance of a graph: a degree analog of the Wiener index. J Chem Inf Computer Sci (1994) 34(5):1082–6. doi:10.1021/ci00021a008
3. Schultz HP. Topological organic chemistry. 1. Graph theory and topological indices of alkanes. J Chem Inf Computer Sci (1989) 29(3):227–8. doi:10.1021/ci00063a012
4. Mueller WR, Szymanski K, Knop JV, Trinajstic N. Molecular topological index. J Chem Inf Comput Sci (1990) 30(2):160–3. doi:10.1021/ci00066a011
5. Klein DJ, Mihalic Z, Plavsic D, Trinajstic N. Molecular topological index: a relation with the Wiener index. J Chem Inf Comput Sci (1992) 32(4):304–5. doi:10.1021/ci00008a008
6. Singh Y, Raza H, Sharma SK, Bhat VK. Computing basis and dimension of chloroquine and hydroxychloroquine by using chemical graph theory. Polycyclic Aromatic Compounds (2023) 43(5):4131–47. doi:10.1080/10406638.2022.2086269
7. Zhang X, Awais HM, Javaid M, Siddiqui MK. Multiplicative Zagreb indices of molecular graphs. J Chem (2019) 2019:1–19. doi:10.1155/2019/5294198
8. Jurić A, Gagro M, Nikolić S, Trinajstić N. Molecular topological index: an application in the QSAR study of toxicity of alcohols. J Math Chem (1992) 11(1):179–86. doi:10.1007/bf01164202
9. Plavšić D, et al. Relation between the Wiener index and the Schultz index for several classes of chemical graphs. Croatica Chemica Acta (1993) 66(2):345–53.
10. Nikolić S, Trinajstić N, Mihalid Z. Molecular topological index: an extension to heterosystems. J Math Chem (1993) 12:251–64. doi:10.1007/bf01164639
11. Gutman I. Selected properties of the Schultz molecular topological index. J Chem Inf Comput Sci (1994) 34(5):1087–9. doi:10.1021/ci00021a009
12. Klavžar S, Gutman I. A comparison of the Schultz molecular topological index with the Wiener index. J Chem Inf Comput Sci (1996) 36(5):1001–3. doi:10.1021/ci9603689
13. Hosoya H. Topological index. A newly proposed quantity characterizing the topological nature of structural isomers of saturated hydrocarbons. Bull Chem Soc Jpn (1971) 44(9):2332–9. doi:10.1246/bcsj.44.2332
14. Estrada E. Edge adjacency relationships and a novel topological index related to molecular volume. J Chem Inf Computer Sci (1995) 35(1):31–3. doi:10.1021/ci00023a004
15. Indices T. Related descriptors in QSAR and QSPR. In: Topological indices and related descriptors in QSAR and QSPR. Amsterdam: Gordon and Breach (1999).
17. Okazawa K, Tsuji Y, Yoshizawa K. Graph-theoretical exploration of the relation between conductivity and connectivity in heteroatom-containing single-molecule junctions. J Chem Phys (2022) 156(9):091102. doi:10.1063/5.0083486
18. Hakeem A, Ullah A, Zaman S. Computation of some important degree-based topological indices for γ-graphyne and Zigzag graphyne nanoribbon. Mol Phys (2023) 121:e2211403. doi:10.1080/00268976.2023.2211403
19. Hakeem A, Katbar NM, Muhammad F, Ahmed N. On the molecular structure modelling of gamma graphyne and armchair graphyne nanoribbon via reverse degree-based topological indices. Mol Phys (2023) 122:e2259510. doi:10.1080/00268976.2023.2259510
20. Wiener H. Structural determination of paraffin boiling points. J Am Chem Soc (1947) 69(1):17–20. doi:10.1021/ja01193a005
21. Gutman I, Trinajstić N. Graph theory and molecular orbitals. In: New concepts II. Springer (2005). p. 49–93.
22. Gutman I, Ruscić B, Trinajstić N, Wilcox CF Graph theory and molecular orbitals. XII. Acyclic polyenes. J Chem Phys (1975) 62(9):3399–405. doi:10.1063/1.430994
23. Gao W, Wang W, Farahani MR. Topological indices study of molecular structure in anticancer drugs. J Chem (2016) 2016:1–8. doi:10.1155/2016/3216327
24. Gutman I. Degree-based topological indices. Croatica chemica acta (2013) 86(4):351–61. doi:10.5562/cca2294
25. Dinar J, Hussain Z, Zaman S, Ur Rehman S. Wiener index for an intuitionistic fuzzy graph and its application in water pipeline network. Ain Shams Eng J (2023) 14(1):101826. doi:10.1016/j.asej.2022.101826
26. Zaman S, N. A. Koam A, Al Khabyah A, Ahmad A. The kemeny's constant and spanning trees of hexagonal ring network. Comput Mater Continua (2022) 73(3):6347–65. doi:10.32604/cmc.2022.031958
27. Zaman S, Jalani M, Ullah A, Saeedi G. Structural analysis and topological characterization of sudoku nanosheet. J Math (2022) 2022. doi:10.1155/2022/5915740
28. Khabyah AA, Zaman S, Koam ANA, Ahmad A, Ullah A. Minimum zagreb eccentricity indices of two-mode network with applications in boiling point and benzenoid hydrocarbons. Mathematics (2022) 10(9):1393. doi:10.3390/math10091393
29. Zaman S, He X. Relation between the inertia indices of a complex unit gain graph and those of its underlying graph. Linear Multilinear Algebra (2022) 70(5):843–77. doi:10.1080/03081087.2020.1749224
30. Yu X, Zaman S, Ullah A, Saeedi G, Zhang X. Matrix analysis of hexagonal model and its applications in global mean-first-passage time of random walks. IEEE Access (2023) 11:10045–52. doi:10.1109/access.2023.3240468
31. Dobrynin AA, Entringer R, Gutman I. Wiener index of trees: theory and applications. Acta Applicandae Mathematica (2001) 66:211–49.
32. Gutman I, Das KC. The first Zagreb index 30 years after. MATCH Commun Math Comput Chem (2004) 50(1):83–92.
33. Ghorbani M, Hosseinzadeh MA. A new version of Zagreb indices. Filomat (2012) 26(1):93–100. doi:10.2298/fil1201093g
34. Horoldagva B, Das KC, Selenge T-A. Complete characterization of graphs for direct comparing Zagreb indices. Discrete Appl Math (2016) 215:146–54. doi:10.1016/j.dam.2016.07.008
35. Das KC. On comparing Zagreb indices of graphs. MATCH Commun Math Comput Chem (2010) 63(2):433–40.
36. Gutman I, Milovanović E, Milovanović I. Beyond the Zagreb indices. AKCE International Journal of Graphs and Combinatorics (2018).
37. Gentile C, Stecca G, Ventura P. Graphs and combinatorial optimization: from theory to applications. CTW2020 Proc 5.
40. Raza Z, Akhter S, Shang Y. Expected value of first Zagreb connection index in random cyclooctatetraene chain, random polyphenyls chain, and random chain network. Front Chem (2023) 10:1067874. doi:10.3389/fchem.2022.1067874
39. Noureen S, Ali A, Bhatti AA, Alanazi AM, Shang Y. Predicting enthalpy of formation of benzenoid hydrocarbons and ordering molecular trees using general multiplicative Zagreb indices. Heliyon (2024) 10(10):e30913. doi:10.1016/j.heliyon.2024.e30913
41. Shang Y. Sombor index and degree-related properties of simplicial networks. Appl Math Comput (2022) 419:126881. doi:10.1016/j.amc.2021.126881
42. Estrada E. Generalization of topological indices. Chem Phys Lett (2001) 336(3-4):248–52. doi:10.1016/s0009-2614(01)00127-0
43. Das KC, Gutman I, Furtula B. On atom-bond connectivity index. Chem Phys Lett (2011) 511(4-6):452–4. doi:10.1016/j.cplett.2011.06.049
45. Das KC, Çevik AS, Cangul IN, Shang Y. On sombor index. Symmetry (2021) 13(1):140. doi:10.3390/sym13010140
46. Nadeem I, Siddique S, Shang Y. Some inequalities between general randić-type graph invariants. J Math (2024) 2024(1):1–7. doi:10.1155/2024/8204742
48. Habib MR, Liang T, Yu X, Pi X, Liu Y, Xu M. A review of theoretical study of graphene chemical vapor deposition synthesis on metals: nucleation, growth, and the role of hydrogen and oxygen. Rep Prog Phys (2018) 81(3):036501. doi:10.1088/1361-6633/aa9bbf
49. Coroş M, Pogăcean F, Măgeruşan L, Socaci C, Pruneanu S. A brief overview on synthesis and applications of graphene and graphene-based nanomaterials. Front Mater Sci (2019) 13:23–32. doi:10.1007/s11706-019-0452-5
50. Belenkova T, Chernov V, Mavrinskii V. Structures and electronic properties of graphyne layers. In: Materials science forum. Trans Tech Publ (2016).
51. Majidi R. Structural and electronic properties of α 2-graphyne nanotubes: a density functional theory study. J Electron Mater (2018) 47(5):2890–6. doi:10.1007/s11664-018-6156-2
52. Rahul M, Clement J, Singh Junias J, Arockiaraj M, Balasubramanian K. Degree-based entropies of graphene, graphyne and graphdiyne using Shannon’s approach. J Mol Struct (2022) 1260:132797. doi:10.1016/j.molstruc.2022.132797
53. Shekar SC, Swathi RS. Molecular switching on graphyne and graphdiyne: realizing functional carbon networks in synergy with graphene. Carbon (2018) 126:489–99.
54. Kondo M, Nozaki D, Tachibana M, Yumura T, Yoshizawa K. Electronic structures and band gaps of chains and sheets based on phenylacetylene units. Chem Phys (2005) 312(1-3):289–97. doi:10.1016/j.chemphys.2004.11.029
55. Malko D, Neiss C, Viñes F, Görling A. Competition for graphene: graphynes with direction-dependent Dirac cones. Phys Rev Lett (2012) 108(8):086804. doi:10.1103/physrevlett.108.086804
56. Peng Q, Ji W, De S. Mechanical properties of graphyne monolayers: a first-principles study. Phys Chem Chem Phys (2012) 14(38):13385–91. doi:10.1039/c2cp42387a
57. Kosar Z, Zaman S, Siddiqui MK. Structural characterization and spectral properties of hexagonal phenylene chain network. Eur Phys J Plus (2023) 138(5):415. doi:10.1140/epjp/s13360-023-03997-0
58. Zaman S, Yaqoob HSA, Ullah A, Sheikh M. QSPR analysis of some novel drugs used in blood cancer treatment via degree based topological indices and regression models. Polycyclic Aromatic Compounds (2023) 44:2458–74. doi:10.1080/10406638.2023.2217990
59. Madurani KA, Suprapto S, Machrita NI, Bahar SL, Illiya W, Kurniawan F. Progress in graphene synthesis and its application: history, challenge and the future outlook for research and industry. ECS J Solid State Sci Technol (2020) 9(9):093013. doi:10.1149/2162-8777/abbb6f
60. Zaman S, Salman M, Ullah A, Ahmad S, Abdelgader Abas MS. Three-dimensional structural modelling and characterization of sodalite material network concerning the irregularity topological indices. J Math (2023) 2023:1–9. doi:10.1155/2023/5441426
61. Ullah A, Zaman S, Hussain A, Jabeen A, Belay MB. Derivation of mathematical closed form expressions for certain irregular topological indices of 2D nanotubes. Scientific Rep (2023) 13(1):11187. doi:10.1038/s41598-023-38386-1
62. Hu Y, Wu C, Pan Q, Jin Y, Lyu R, Martinez V, et al. Synthesis of γ-graphyne using dynamic covalent chemistry. Nat Synth (2022) 1(6):449–54. doi:10.1038/s44160-022-00068-7
63. Ullah A, Shamsudin A, Zaman S, Hamraz A, Saeedi G. Network-based modeling of the molecular topology of fuchsine acid dye with respect to some irregular molecular descriptors. J Chem (2022) 2022:1–8. doi:10.1155/2022/8131276
64. Zaman S, Jalani M, Ullah A, Ali M, Shahzadi T. On the topological descriptors and structural analysis of cerium oxide nanostructures. Chem Pap (2023) 77(5):2917–22. doi:10.1007/s11696-023-02675-w
65. Zaman S, Ullah A. Kemeny's constant and global mean first passage time of random walks on octagonal cell network. Math Methods Appl Sci (2023) 46(8):9177–86. doi:10.1002/mma.9046
66. Kulli V. Reverse Zagreb and reverse hyper-Zagreb indices and their polynomials of rhombus silicate networks. Ann Pure Appl Math (2018) 16(1):47–51. doi:10.22457/apam.v16n1a6
67. Hakeem A, Muhammad Katbar N, Muhammad F, Ahmed N. QSPR analysis of some important drugs used in heart attack treatment via degree-based topological indices and regression models. Polycyclic Aromatic Compounds (2023) 1–10. doi:10.1080/10406638.2023.2262697
68. Gao W, Younas M, Farooq A, Virk AR, Nazeer W. Some reverse degree-based topological indices and polynomials of dendrimers. Mathematics (2018) 6(10):214. doi:10.3390/math6100214
69. Kwun YC, Virk AUR, Rafaqat M, Rehman MU, Nazeer W. Some reversed degree-based topological indices for graphene. J Discrete Math Sci Cryptography (2019) 22(7):1305–14. doi:10.1080/09720529.2019.1691329
70. Ullah A, Zaman S, Hamraz A. Zagreb connection topological descriptors and structural property of the triangular chain structures. Physica Scripta (2023) 98(2):025009. doi:10.1088/1402-4896/acb327
71. Zaman S, Jalani M, Ullah A, Ahmad W, Saeedi G. Mathematical analysis and molecular descriptors of two novel metal–organic models with chemical applications. Scientific Rep (2023) 13(1):5314. doi:10.1038/s41598-023-32347-4
72. Yan T, Kosar Z, Aslam A, Zaman S, Ullah A. Spectral techniques and mathematical aspects of K 4 chain graph. Physica Scripta (2023) 98(4):045222. doi:10.1088/1402-4896/acc4f0
73. Zhang X, Siddiqui MK, Javed S, Sherin L, Kausar F, Muhammad MH. Physical analysis of heat for formation and entropy of Ceria Oxide using topological indices. Comb Chem High Throughput Screen (2022) 25(3):441–50. doi:10.2174/1386207323999201001210832
Keywords: reverse indices, chemical graph theory, triangular ϒ-graphyne, triangular ϒ-graphyne chain, graphyne
Citation: Hakeem A, Katbar NM, Shaikh H, Tolasa FT and Abro OA (2024) Reverse degree-based topological indices study of molecular structure in triangular ϒ-graphyne and triangular ϒ-graphyne chain. Front. Phys. 12:1422098. doi: 10.3389/fphy.2024.1422098
Received: 23 April 2024; Accepted: 26 July 2024;
Published: 14 August 2024.
Edited by:
Micheal Arockiaraj, Loyola College, Chennai, IndiaReviewed by:
Yilun Shang, Northumbria University, United KingdomPaul D., Sri Sairam Institute of Technology, India
Copyright © 2024 Hakeem, Katbar, Shaikh, Tolasa and Abro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdul Hakeem, aGFrZWVtcGh1bHBvdG9AeWFob28uY29t
 Abdul Hakeem
Abdul Hakeem Nek Muhammad Katbar1
Nek Muhammad Katbar1 Fikadu Tesgera Tolasa
Fikadu Tesgera Tolasa