
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys. , 05 December 2023
Sec. Optics and Photonics
Volume 11 - 2023 | https://doi.org/10.3389/fphy.2023.1288935
 Alexander Ushenko1,2*
Alexander Ushenko1,2* Jun Zheng1*
Jun Zheng1* Mykhaylo Gorsky2
Mykhaylo Gorsky2 Alexander Dubolazov2
Alexander Dubolazov2 Yuriy Ushenko3
Yuriy Ushenko3 Iryna Soltys2
Iryna Soltys2 Ivan Mikirin2
Ivan Mikirin2 Zhebo Chen1
Zhebo Chen1 Oleh Wanchuliak4
Oleh Wanchuliak4 Ivan Gordey3
Ivan Gordey3 Cai Jingxian1
Cai Jingxian1The experimental validation of methods for the digital holographic 3D layer-by-layer reconstruction of complex amplitude distributions in different phase planes of diffuse biological tissue histological section microscopic images with a subsequent reconstruction of azimuth and ellipticity polarization maps is presented. Polarization dependencies of the integral and layer-by-layer vector structure of the laser object field azimuth and ellipticity distributions in two types of biological layer samples—the fibrous myocardium and parenchymal liver—have been investigated. Scenarios and dynamics of changes in the values of statistical moments of the first to fourth orders, which characterize the integral and layer-by-layer polarization maps of diffuse biological tissue histological section microscopic images with different morphological structures, have been determined. Criteria for selecting the polarization components of the object field that are formed by acts of scattering of different multiplicities have been identified. An example of biomedical application in cancer detection of biological tissues is provided.
Optical methods for diagnosing various biological tissues and visualizing their optically anisotropic structure (fibrillar networks and optically active molecular domains) occupy a prominent place due to their high informativeness and the possibility of multifunctional monitoring of the investigated environment (photometric, spectral, polarization, and correlation monitoring) [1–5]. However, currently, there is a lack of a unified methodological approach to diagnose such structures.
Polarimetric research [6] has emerged as a distinct direction in the field of optical analysis of biological tissues. Analyzing the polarization characteristics of scattered radiation allows obtaining qualitatively new insights into the morphological and physiological states of biological tissues, including cataracts in the lens, glucose concentration in tissues of diabetic patients, and malignant changes [7–12].
A significant advancement in the field of biomedical polarimetric diagnostics has been the development of Mueller matrix techniques, leading to the establishment of Mueller matrix microscopy (MMM) as a separate branch [13–18].
This direction of optical diagnostics is based on the synthesis of instrumental Mueller matrix imaging polarimetry with various theoretical models and methods of analysis (regression model of optical anisotropy [14], logarithmic Mueller matrix decomposition [15–18], Monte Carlo simulation of polarized radiation conversion [17], and statistical analysis of Mueller matrix images and optical anisotropy maps [14, 18]).
The fundamental results obtained within MMM [18] of biological tissues are also promising for applied clinical and preclinical studies of the polycrystalline structure of biological tissues. In particular, on account of the possibility of obtaining quantitative optical metrics to characterize the evolution of gastric tissues from a healthy state through inflammation to cancer by Mueller microscopy of gastric biopsies, a regression model of optical anisotropy and statistical analysis of the obtained images has been demonstrated [14].
Parallel to this, methods and systems of laser polarimetry for biological tissue and liquid samples have been intensively developed, utilizing a wide range of analytical algorithms (statistical, correlation, fractal, wavelet, and Fourier) for quantitative data processing [19–29].
The main informational limitation to polarimetric mapping has been the dependence of experimental data on the degree of depolarization of the laser radiation, which not only integrates the polarization distributions but also compromises the unambiguous relationships between polarization-inhomogeneous object fields and parameters of polycrystalline soft matter layers [7–18]. Therefore, the creation of new, well-reproducible, and more accurate laser polarimetry methods remains highly relevant.
A significant achievement in this field was the successful integration of polarimetric and interferometric mapping methods, which led to the development of various directions of 3D polarimetry of biological objects and environments.
Polarization-sensitive optical coherence tomography (PSOCT) techniques have become promising in this direction. The basic principles and achievements of PSOCT of biological objects over the past 25 years have been consistently set out in a series of review articles [30–32]. This laser polarization–interference technique makes it possible not only to obtain layered images from small depths (up to 2 mm) of biological tissues [33, 34] but also provides the possibility of obtaining coordinate distributions of elements of the Jones and Mueller matrices and anisotropy maps. This was demonstrated most effectively in studies of transparent eye tissues [35–37].
Studies of layered polarization and matrix images of scattering (depolarizing) biological tissues of various morphological structures and physiological states have become an extension of the functionality of PSOCT [33]. In particular, it was possible to accurately diagnose fibrosis and differentiate tumor sites with low fibrosis [38]. However, the sensitivity of such systems was limited by the distorting effect on the contrast of polarizing images of layers of such tissues of a high level of depolarized laser speckle background. In addition, PSOCT systems are expensive, have insignificant (8 microns–10 microns) resolution, and do not provide the possibility of quantitative analysis of optical parameters of biological tissues, including benign and malignant tumors [30–38].
At the same time, these studies have demonstrated new possibilities for three-dimensional polarimetric biomedical differential diagnosis of diffuse samples of benign and malignant tumors in human organs.
Nevertheless, the results obtained still remain somewhat empirical. Currently, there is no information about the possibilities of this method for tissue samples with different optical thicknesses, morphological structures, and various pathologies. From a physical point of view, the task is to establish scientific significance—to establish algorithmic relationships between the structure of polycrystalline grids in biological tissues and the layered distribution of polarization parameters (azimuth and ellipticity) of the scattered object field.
On account of this, a promising task in polarimetric biomedical optics is the development of a universal polarimetric description of scenarios for the formation of optical fields in anisotropic polycrystalline biological layers. This description should cover a more general case—the detection and selection of polarimetric components of the laser field of an object with varying degrees of light scattering by digital holographic reconstruction and high-resolution phase scanning [39–42]. It is expected that the combination of polarimetric and interferometric methods will allow a new look at the morphological and optically anisotropic structure of diffuse layers in biological tissues with various architectural anisotropic polycrystalline components.
Our work is focused on the development and experimental verification of methods for three-dimensional polarization–interference digital holographic polarimetry of repeatedly scattered object fields of biological tissues with two boundary types of morphological architecture—spatially organized fibrous networks of the myocardium and disordered structures of the liver parenchyma. The aim is to investigate the potential of layered phase selection of holographically reconstructed variously scattered components of the polarization-inhomogeneous field of a diffuse biological layer.
In this section of the article, we will briefly consider (without compromising the completeness of the analysis) the main theoretical relationships within the framework of the phase anisotropy approximation (linear
The diffuse layer of the biological tissue is illuminated with a linearly polarized beam with an azimuth angle
To each (
where
The process of local (
Here,
As a result, a partial laser wave is formed with the following azimuth
Here,
Thus, the singly scattered component of the object field of a diffuse biological layer is a polarization distribution of azimuths
For a set of acts with different multiplicities
Here,
This optical scenario leads to the formation of the following random values of azimuth
As a result, a polarimetrically inhomogeneous component of the diffuse field is formed with different distributions of azimuth values
Thus, the polarization structure of the multiply scattered laser field by a diffuse biological object can be represented as a next superposition.
The obtained expressions (1)–(10) are widely used within the framework of traditional polarimetry (or Stokes polarimetry) of the intensities of the biological objects' field.
On the other hand, the coherence of laser radiation enables a different (amplitude) analytical description of the processes forming the polarization structure of the object field in a diffuse biological layer.
For coherent laser fields, there is a direct relationship between the parameters of the Stokes vector and the orthogonal components (
Based on this, the previously obtained expressions (4) and (5) for the polarization parameters can be rewritten as follows:
Here,
In parallel with this scenario, another process occurs—the interference addition of differently polarized partial coherent waves, leading to the formation of another polarimetrically inhomogeneous component in the diffuse object field.
For the orthogonal components
Here,
For the process of forming the orthogonal components of amplitudes
The interference addition of two orthogonal components
with the following “interference” (
The probability of polarization parameters
As we can see, the secondary interference of differently polarized coherent partial waves also leads to the formation of a polarimetrically inhomogeneous component [expressed in relationships (16), (17)] of the object field in the diffuse biological layer
Thus, the object laser field in the biological tissues diffuse layers can be considered a superposition of the following polarimetric components:
A generalization of the polarization interferometry scheme [39, 40] is the Stokes-polarimetric mapping scheme on the base of the Mach–Zehnder interferometer, which is shown in Figure 1.
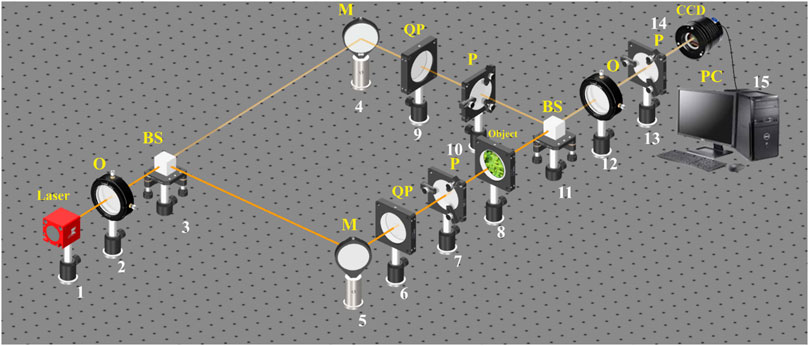
FIGURE 1. Optical scheme for polarization–interference mapping of the Stokes vector parameters. 1—He–Ne laser; 2—collimator—“O”; 3,11—beam splitters—“BS”; 4,5—mirrors—“M”; 6,9—quarter-wave plates—“QP”; 7,10,13—polarizers—“P”; 8—object; 12—polarization objective—“O”; 14—digital camera—“CCD”; 15—personal computer—“PC”.
The parallel (
The “object” beam with the help of a rotating mirror 5 is directed through the polarizing filters 6–7 in the direction of the biological layer 8 sample. The biological tissue histological Section 8 polarization-inhomogeneous image is projected by strain-free objective 12 into the plane of digital camera 14.
The “reference” beam is directed by mirror 4 through the polarization filters 9–10 into the biological tissue histological Section 8 polarization-inhomogeneous image plane.
As a result, an interference pattern is formed, the coordinate intensity distribution of which is recorded by a digital camera 14 through a polarizer 13.
To study the dependence of the diffuse biological tissue object field polarization structure on the incident radiation polarization state, plane-polarized illuminating and reference beams with different azimuths
Before carrying out measurements of biological tissues, the experimental device passed metrological certification with the introduction of model objects (“clean air,” “linear polarizer,” “phase plates
The methodology for determining the layer-by-layer measurement of the object field Stokes vector parameter (polarization maps
1. Three states of linear polarization are sequentially formed in the “irradiating” (
2. For each of the polarization states (
3. Analytical processing of microscopic interference images of biological samples was carried out using the digital Fourier transform
where
4. The results of the digital Fourier transform [relations (19)] are used to obtain distributions of complex amplitudes, according to the following algorithms:
5. By means of stepwise (
6. Distributions (20) and (21) are layered distributions of complex amplitudes and corresponding polarization maps
The morphological structure of optically anisotropic architectonics of various types of biological tissues is statistical and rather complex. This complexity is due to the presence in the volume of biological tissue of diverse scattering optical centers (cells, nuclei, and fibrils). The different spatial organization of such centers causes the formation of no less complex distributions of birefringence parameters and dichroism networks of biological crystals [1–12].
As a result of the passage of optical radiation through such spatially inhomogeneous structures, polarization maps with statistically distributed values of azimuth and ellipticity of polarization are formed [20, 21]. One of the most common quantitative methods for evaluating such maps is the calculation of a set of central statistical moments of first to fourth orders, which most fully characterize the histograms of polarization distributions.
Each of these moments provides different statistical information about the distributions (or changes) of the optical anisotropy parameters of the architectonics of biological tissues and the corresponding polarization maps. In other words, the first statistical moment characterizes the mean of the statistically distributed ensemble of random variables of birefringence and dichroism of the architectonics of biological tissue, as well as the azimuth and ellipticity of the polarization of the object field. The second central statistical moment determines the magnitude of the variance of fluctuations in the parameters of optical anisotropy and polarization states. Higher-order statistical moments characterize the asymmetry and kurtosis of probability distributions and turn out to be the most sensitive to “morphological” and “pathological” variations of optically anisotropic architectonics and polarization-inhomogeneous object fields [23–28].
This approach is universal and objective for assessing the probability distributions of optical and polarization distributions for a wide range of biological tissues, regardless of their morphological structure and physiological state.
The resulting set of polarization maps
where
Our choice of research objects is based on the generalization of the following analytical and applied aspects that have been achieved in polarimetric studies of biological tissues:
• The optical properties of a layer of any biological tissue can be represented as a two-component “amorphous polycrystalline” matrix [2–7].
• The polarization properties of the architectonics of the polycrystalline component of biological tissue are formed by structural (dendritic, spatially supramolecular fibrillary networks of proteins) and chiral (clusters of optically active molecular domains) anisotropies.
The architectonics of real biological tissue simultaneously possess both structural (linear birefringence and dichroism) and chiral (circular birefringence and dichroism) anisotropies.
The processes of interaction of optical radiation with such ensembles of biological crystals and the formation of a set of polarization maps of the object field are described most fully and universally within the framework of the Mueller matrix formalism [8–10, 23–28].
Statistical parameters of azimuth and ellipticity maps of polarization are interrelated with the features of structural and chiral anisotropies of biological tissues.
Therefore, both analytical and experimental detailing of such interrelations by means of polarization study of some “boundary structures” of optically anisotropic architectonics of biological crystals turn out to be actual fundamental and applied tasks.
From a fundamental point of view, among the variety of architectonics of biological tissues, two “marginal” cases of polarization manifestations of structural and chiral anisotropies can be distinguished:
• Structural anisotropy of fibrillar tissues—spatially ordered networks of myocardial myosin fibrils.
• Chiral anisotropy of parenchymal tissues—clusters of optically active parenchymal molecules of biological tissues (in our case, the liver).
The obtained statistical characteristics of the corresponding polarization maps can also be used in applied detection studies within the framework of the universal Mueller matrix formalism of pathological changes in optical anisotropy of the widest class of biological tissues of human organs.
Morphologically, the myocardium is formed by well-organized myosin fibrous networks, while the liver’s structure consists of spatially disordered island-like clusters (Langerhans islets).
Optically, these biological tissues possess both common and distinct properties.
Common features—each layer of the mentioned biological tissues represents a two-component isotropic–anisotropic matrix [6].
The anisotropic component performs phase modulation
Distinct features—fibrous networks create a so-called structural anisotropy, resulting in the formation of linear birefringence (
Histological sections were prepared using the conventional technique on a microtome with rapid freezing [6].
The optical geometric parameters of the histological section samples are presented in Table 1.
The extinction coefficient (
The value of the integral degree of depolarization (
This part of the article contains a sequential presentation of research materials on the integral and layered statistical and polarization-inhomogeneous structure of diffuse biological tissue object field azimuths
Figure 2 shows a series of fragments depicting integral maps of polarization azimuth [(1)–(3)] and histograms [(4)–(6)] representing the distributions of polarization azimuth values in microscopic images of the myocardial sample. Multi-channel irradiation was utilized with linearly polarized laser beams
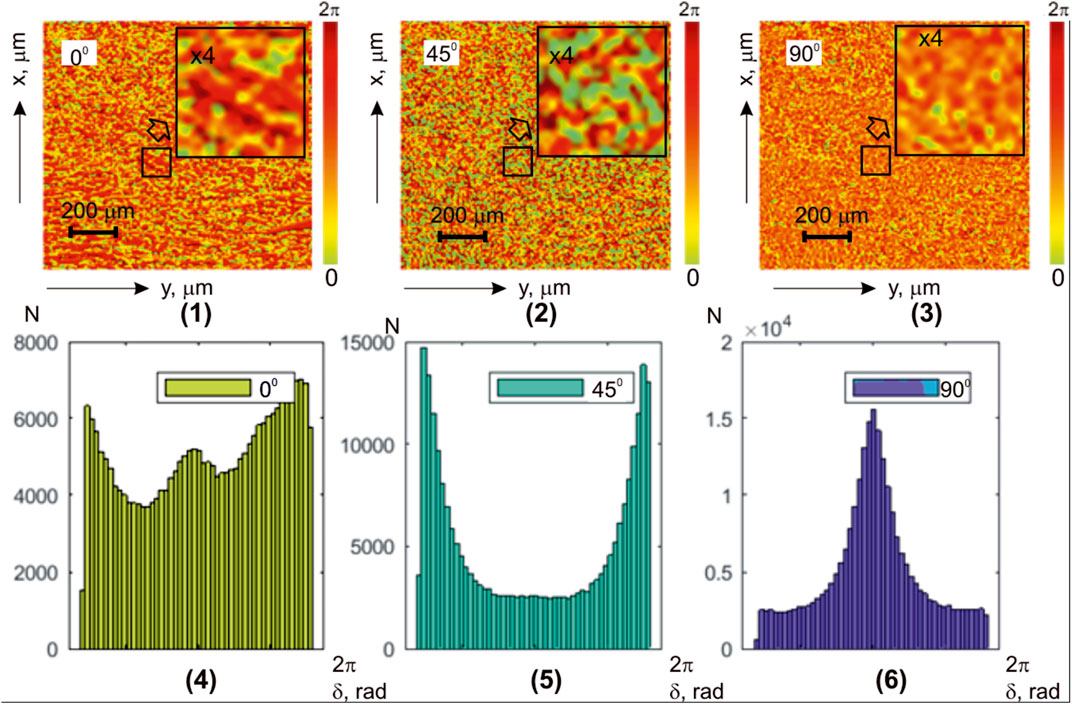
FIGURE 2. Coordinate (
Experimentally measured histograms
The quantitative statistical structure of the polarization maps
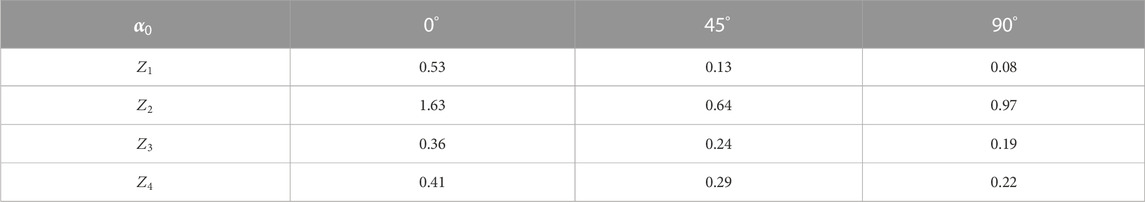
TABLE 2. Statistical parameters of the myocardium histological section microscopic image polarization azimuth maps.
The comparison of the results from the statistical analysis of the integral azimuth polarization maps
From a physical point of view, the obtained results can be attributed to the simultaneous influence of the following two factors:
1. Object-related: It arises due to the complex orientation (
2. Field-related: It is caused by the secondary interference of differently polarized partial laser waves, formed by optically anisotropic fibrous networks. As a consequence, an additional polarization-inhomogeneous “diffuse” component of the object’s laser radiation is formed (Eqs 11–16).
One of the challenges in solving inverse problems in diagnosing the polycrystalline structure of diffuse biological layers is to achieve conditions where the contribution of multiple scattering events to the formation of the vector structure of the object field is minimized.
One of the methods developed for this purpose is the phase scanning technique of holographically reconstructed distributions of complex amplitudes of laser radiation (Eqs 19–21).
The results of phase scanning and reconstruction of layered maps
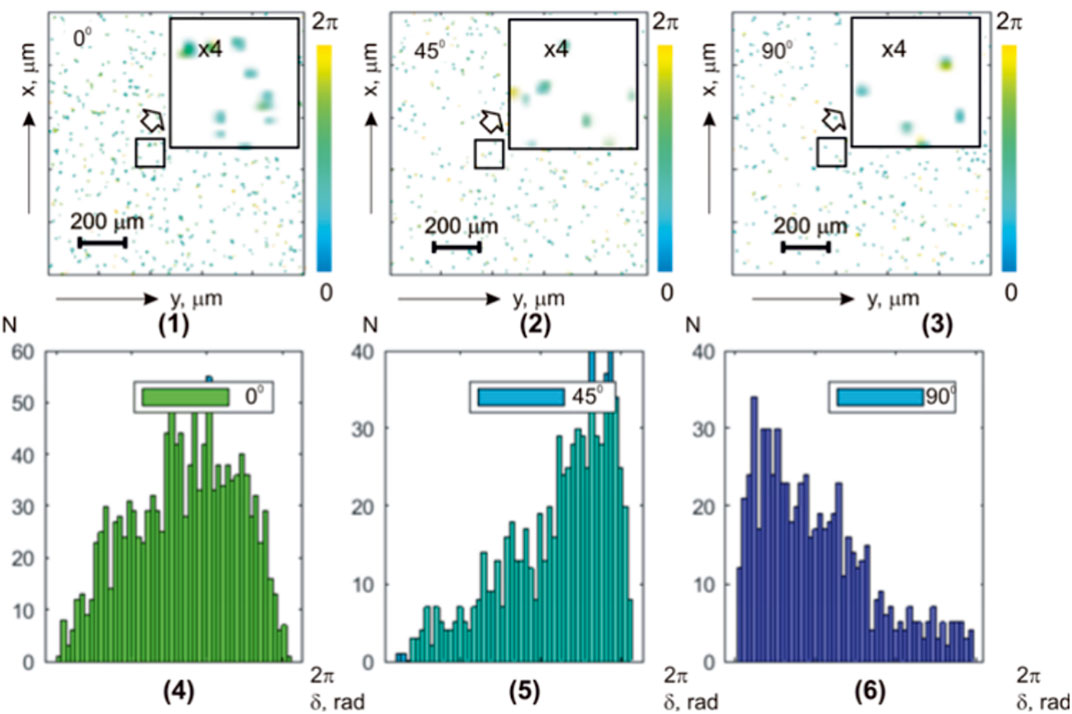
FIGURE 3. Coordinate (
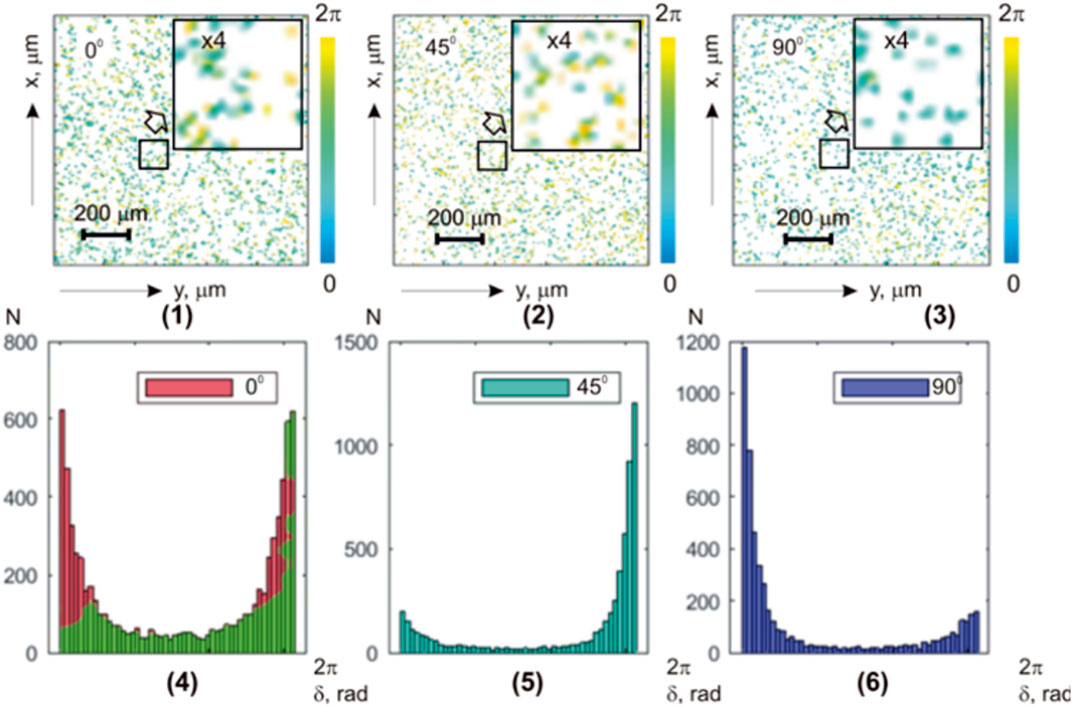
FIGURE 4. Coordinate (
Table 3 presents the data on the statistical analysis of the layered maps of the azimuth of polarization in different phase planes of the myocardium histological section microscopic images.
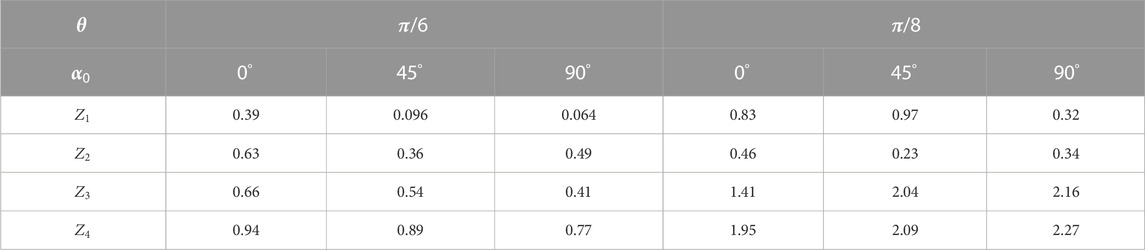
TABLE 3. Statistical parameters of the myocardium histological section microscopic image polarization azimuth maps.
It has been found that with a decrease in the value of
On the contrary, higher-order statistical moments that characterize the asymmetry
Further reduction of the parameter
Thus, the application of the phase scanning method with digital holographic reconstruction of the microscopic image phase of polarization azimuth maps of the optically thick fibrous layer allows for the experimental selection of components of different multiplicities—isolating the “object” practically single-scattered component against the background of the overall “diffuse” background, which is formed by high-order interactions.
Figure 5 shows a series of experimentally measured integral azimuth polarization maps [(1)–(3)] and histograms [(4)–(6)] of the liver histological section microscopic images.
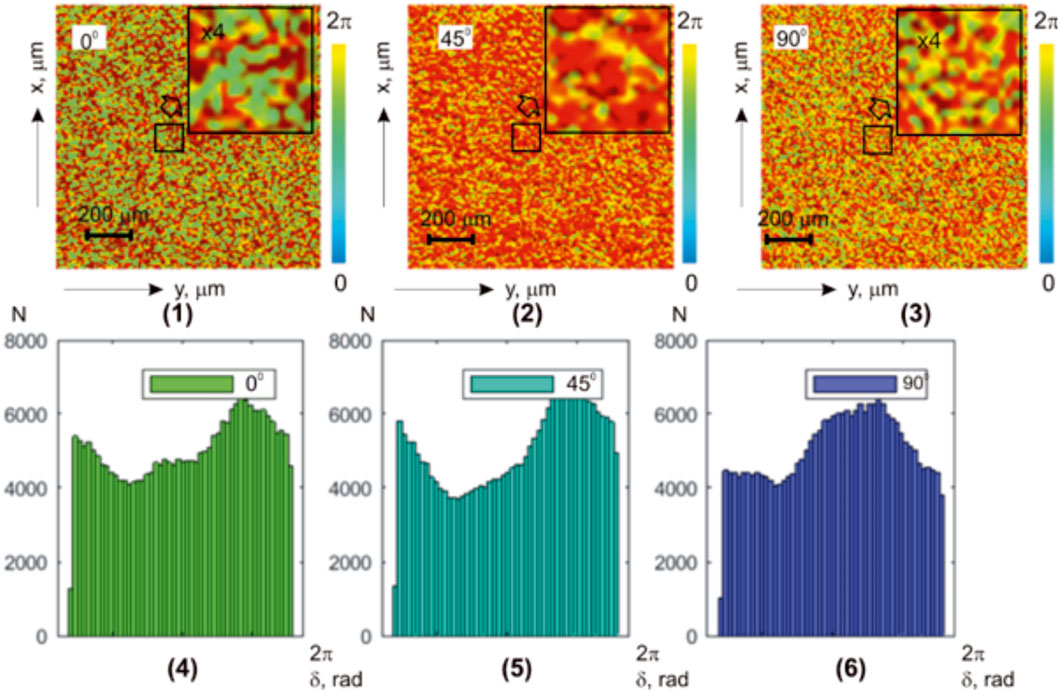
FIGURE 5. Coordinate (
Both coordinate and individual heterogeneities of the experimentally measured azimuth polarization maps
The histograms
Quantitatively, the coordinate statistical structure of the parenchymal liver layer images maps
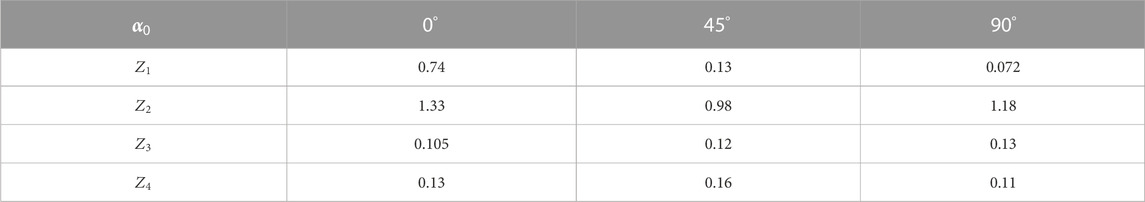
TABLE 4. Statistical parameters of the liver histological section microscopic image polarization azimuth maps.
Statistical analysis of partially depolarizing hepatic parenchyma layer microscopic images integral polarization azimuth maps revealed a significant (up to three times) decrease in the higher-order statistical moments
From a physical point of view, the obtained results can be attributed to the predominant influence of the object factor—circular birefringence (
Additionally, the spatial ordering of optically anisotropic domains (azimuthal symmetry) in the morphological structure of the liver is revealed in a relatively weak dependence of the distributions
The results of phase scanning and reconstruction of layered maps
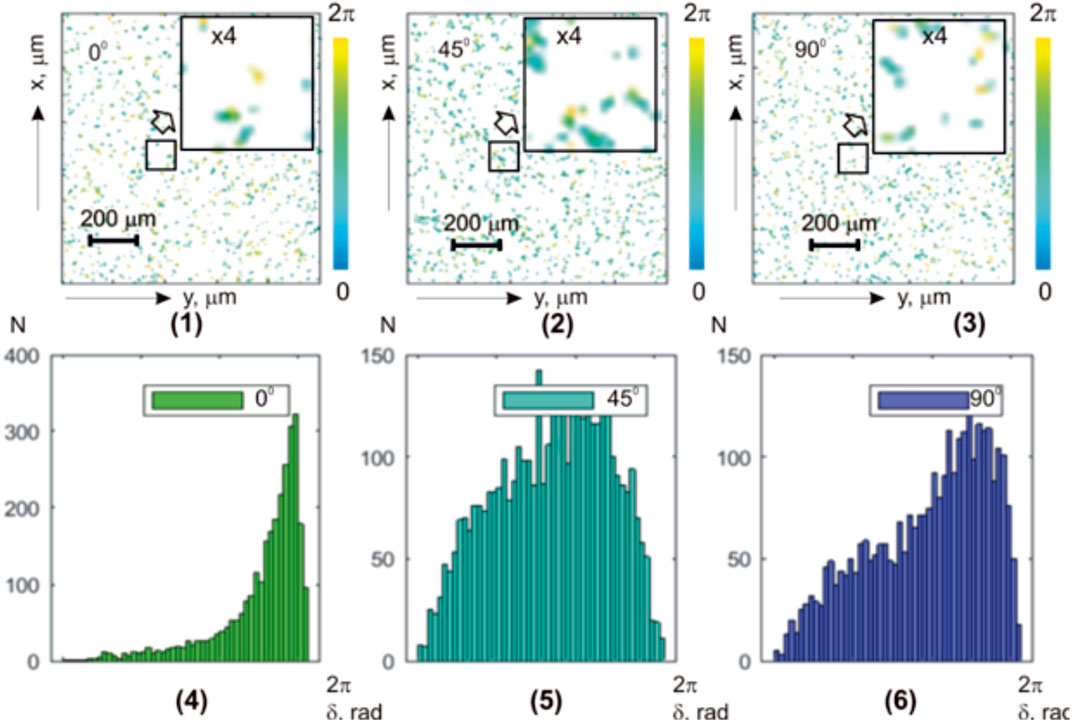
FIGURE 6. Coordinate (
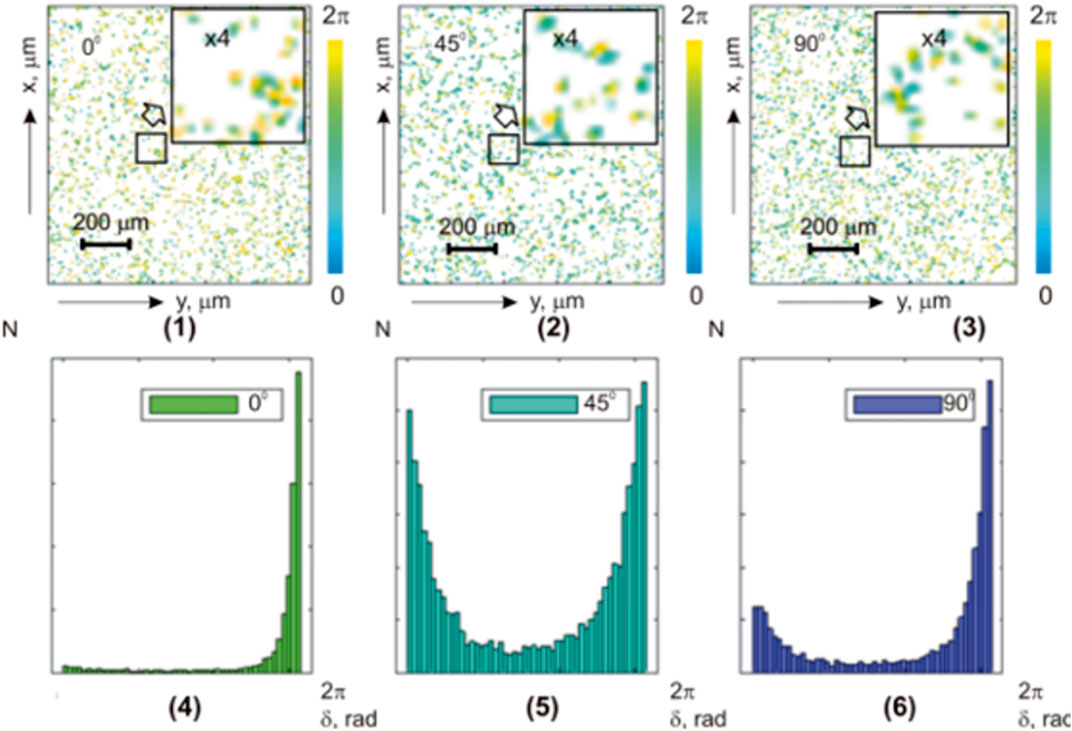
FIGURE 7. Coordinate (
The data from the statistical analysis of the optically thick liver histological section microscopic images layered polarization azimuth maps are presented in Table 5.
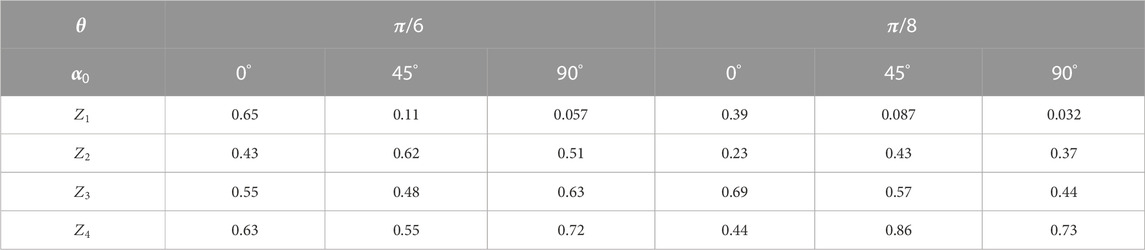
TABLE 5. Statistical parameters of the liver histological section microscopic image polarization azimuth maps.
Comparative analysis of a series of polarization azimuth
This follows from the fact that when the phase parameter
In addition, the differences between the calculated values of
A series of fragments shown in Figure 8 presents integral maps
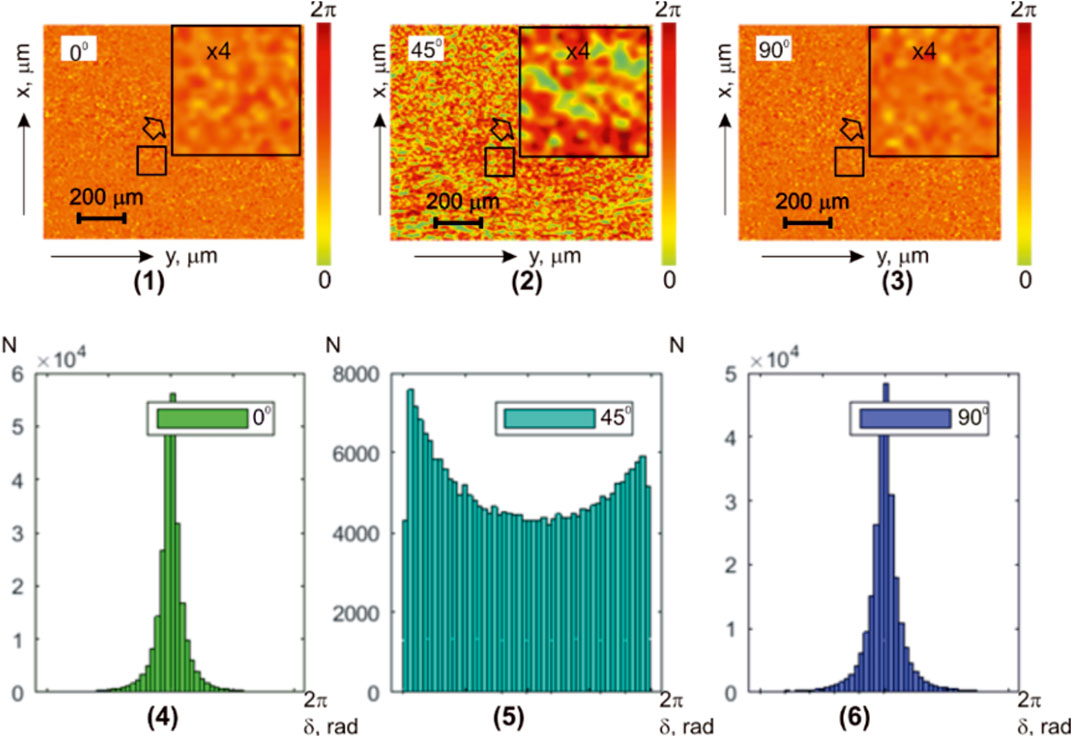
FIGURE 8. Coordinate (
The obtained results (Figure 8) revealed the individual coordinate and statistical structure of the second object field polarization map parameter—polarization ellipticity
From a physical point of view, the obtained results of polarimetry of
Possibilities of phase selection of components of the object field with different scattering multiplicities are illustrated by layer-by-layer maps
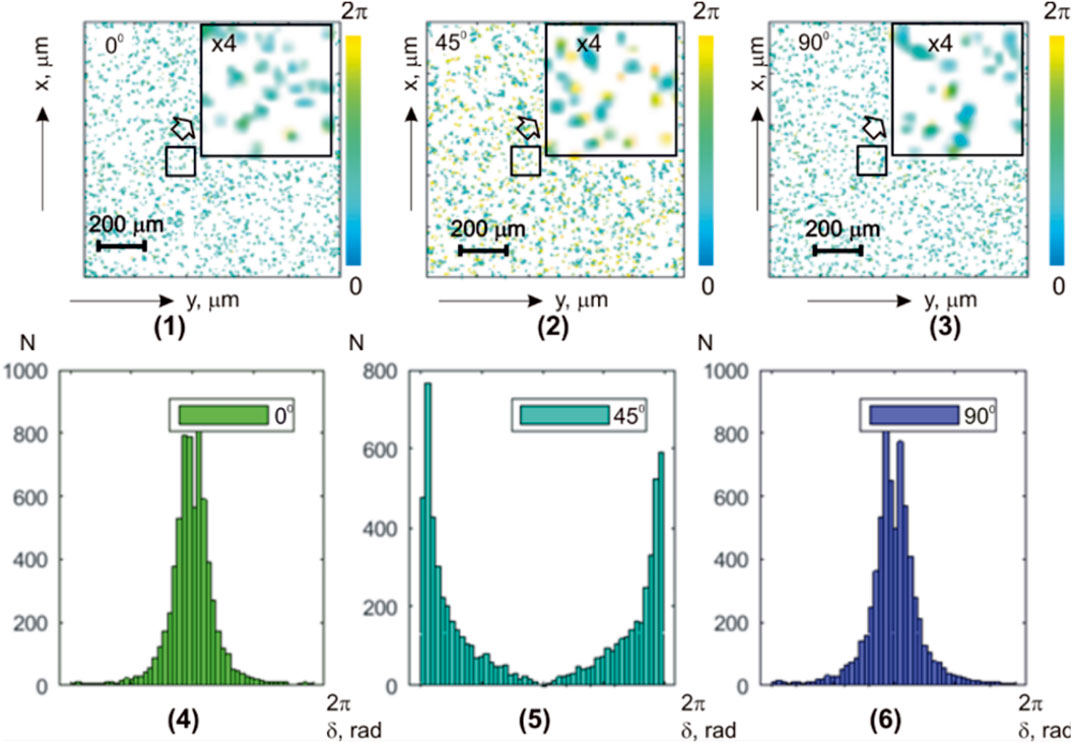
FIGURE 9. Coordinate (
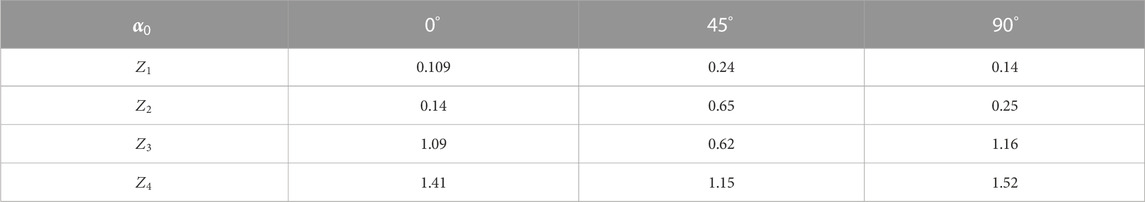
TABLE 7. Statistical parameters of the myocardium histological section microscopic image polarization ellipticity maps in the phase section
The analysis of the layer-by-layer transformation in the statistical structure
Figure 10 presents integral maps [(1)–(3)] and histograms [(4)–(6)] of liver histological section microscopic image polarization ellipticity
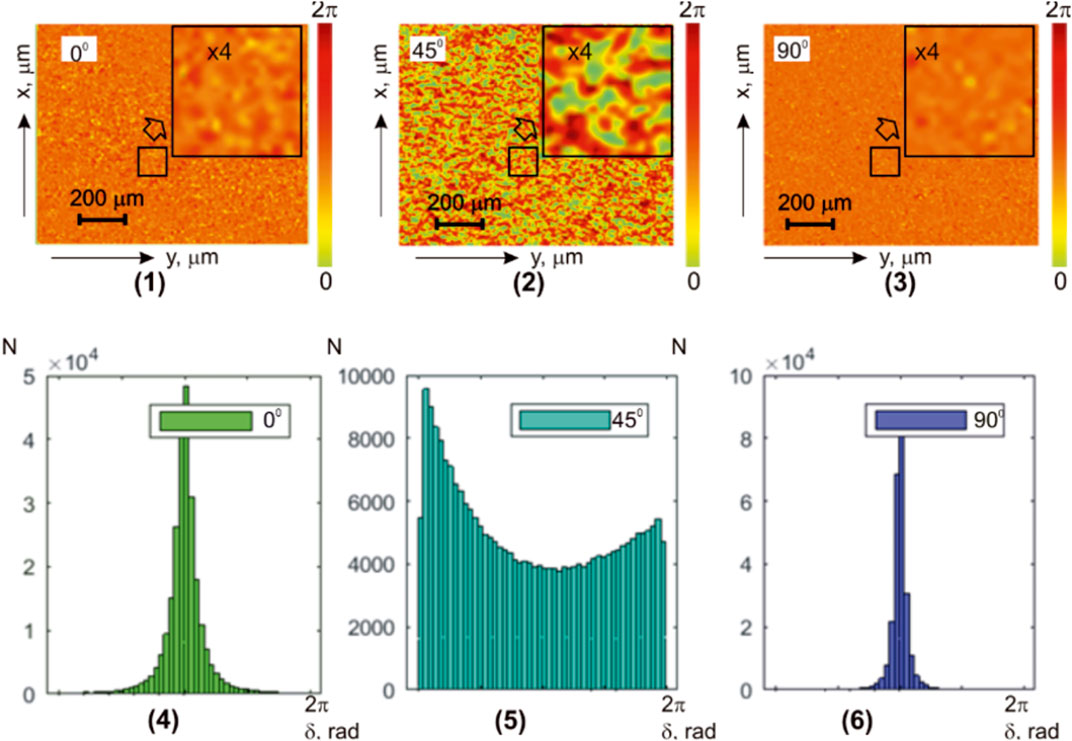
FIGURE 10. Coordinate (
Quantitative statistical characteristics of distributions
Statistical analysis of integrated topographic microscopic image polarization ellipticity maps of the parenchymal liver layer revealed significantly (by 8–10 times) smaller values of dispersion of random values
The established fact can be associated with the predominant influence of the liver parenchyma circular birefringence,
An even more clearly revealed feature of the formation of the object field linearly polarized states’ distributions illustrated by the results of phase scanning and holographic reproduction of layer-by-layer maps
FIGURE 11. Coordinate (
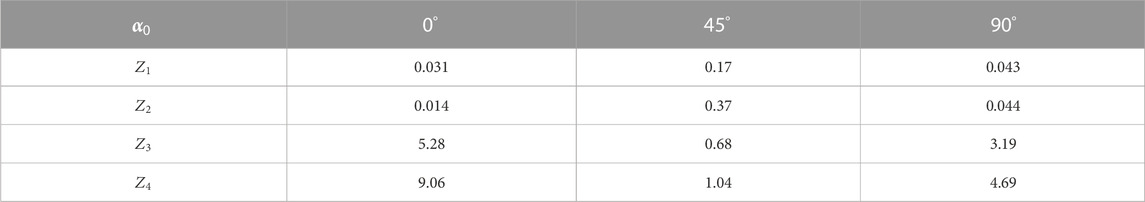
TABLE 9. Statistical parameters of the liver histological section microscopic image polarization ellipticity maps in the phase section
On the basis of a comparative analysis of a holographic reproduction of the liver diffuse histological section microscopic image set of layer-by-layer elliptical polarization maps, it was found that
In addition, the differences between the calculated values of
Here, we will briefly give one example of the developed technique clinical application.
The information analysis of the results obtained from the methods of 3D polarization–interference-layered phase scanning of object fields of two groups' research on biological tissue samples involves the use of operational characteristics from evidence-based medicine [46]:
• Sensitivity (
• Specificity (
• Accuracy (
If (
Two groups of samples of the myocardium histological sections were formed:
• Coronary heart disease (CHD)—group 1 (12 samples).
• Ischemic heart disease (IHD)—group 2 (12 samples).
The optical geometric parameters of the samples are given in Table 10.
From a morphological point of view, cases of CHD and IHD lead to various necrotic changes in myosin fibers and their spatial networks.
CHD—myosin fibers become thinner, and the spatial orderliness of fibrillar networks decreases.
IHD—myosin fibers in some areas are torn, while the spatial order of the myosin network remains unchanged.
Figure 12 presents the results of the myocardium polarization-inhomogeneous object field investigation of those who died as a result of CHD and IHD:
• integrated polarization ellipticity maps
• layer-by-layer polarization of the myocardium histological section microscopic image phase polarization ellipticity
• histograms of the magnitude [fragments (3), (4)] of polarization ellipticity
• histograms of layer-by-layer polarization ellipticity value
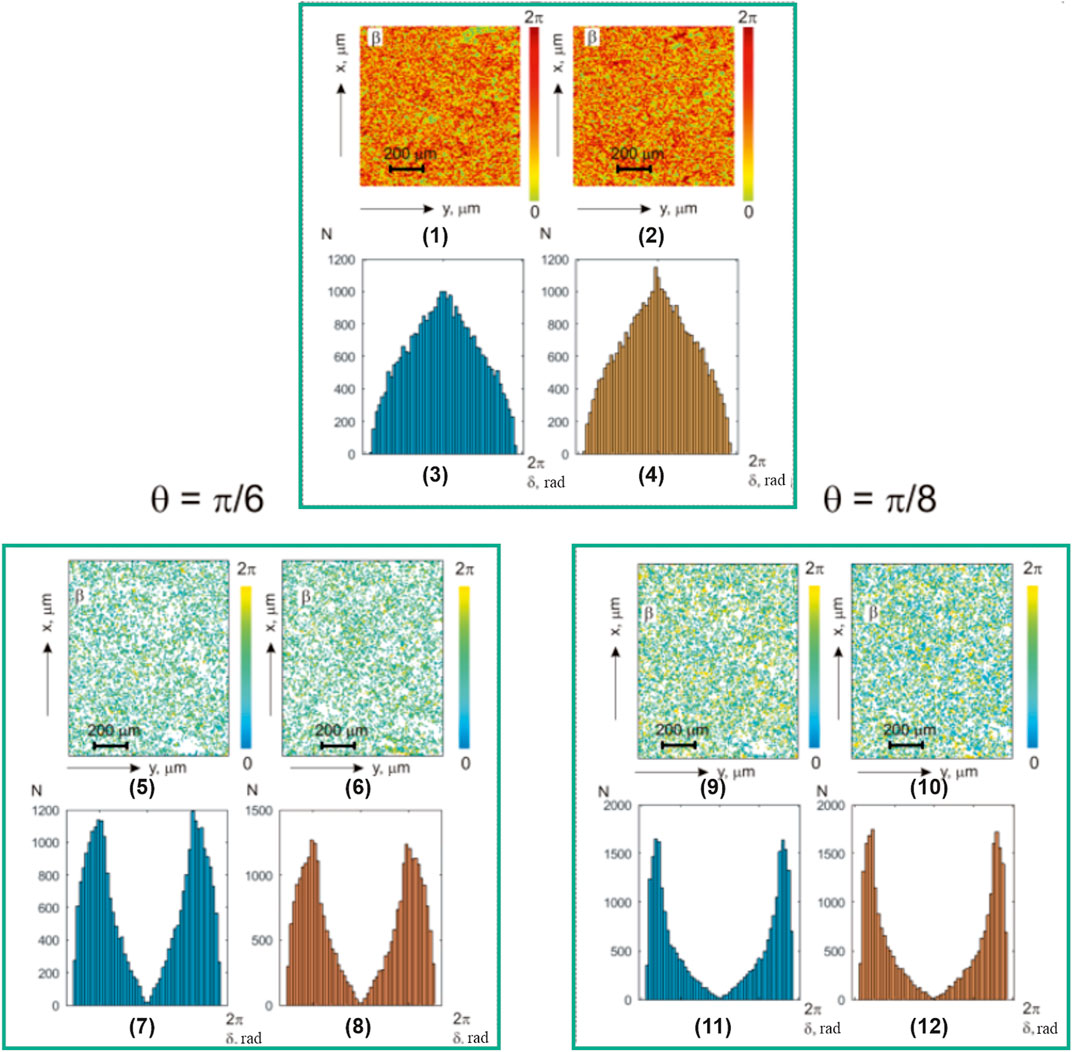
FIGURE 12. Coordinate (
Table 11 illustrates the results of the experimentally determined polarization ellipticity maps
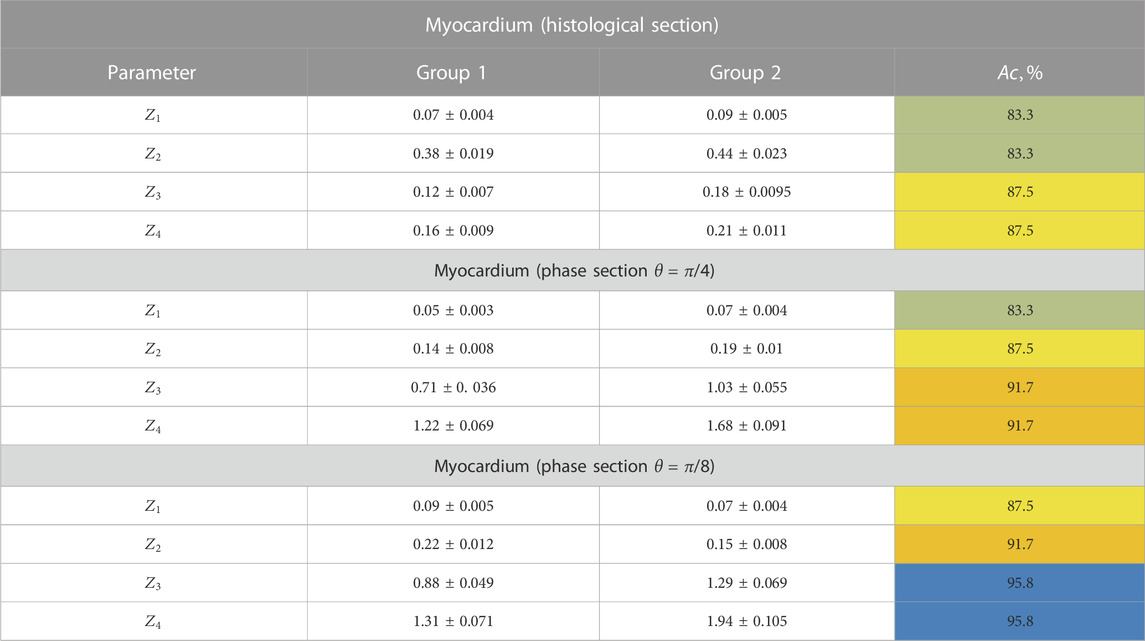
TABLE 11. Statistical moments of the first to fourth orders, which characterize the layer-by-layer polarization ellipticity maps.
The obtained results of integral and phase mapping can be associated with necrotic transformations of birefringent fibrous networks.
For the case of IHD, as a result of reducing the range of variations in the orientation of optical axes
In the case of CHD, the fast-paced morphological structures of myocardial fibrous networks due to acute coronary insufficiency show weak changes in the orientational and phase structures of optically anisotropic crystal networks
Therefore, the following statistical relationships are realized for such objects
It should be noted that the phenomenon of birefringence in optically uniaxial biological crystals predominantly generates various elliptically polarized states [6]. The probability of forming linearly polarized states in the object field points is insignificant.
Therefore, due to the advanced statistics of elliptically polarized states in the myocardial fibrous layer object field, the sensitivity of polarization ellipticity distributions to changes in the orientation and phase polycrystalline structure of the myocardium is significantly higher than when detecting histological section microscopic image polarization azimuth maps from samples in groups 1 and 2.
The given fact is supported by the results of the informational analysis, which revealed the following levels of balanced accuracy
• integral maps of polarization ellipticity–a good level with
• layered polarization ellipticity phase maps–a good level with
1. The proposed comprehensive Stokes polarimetric and interferometric analytical description allows for the formation of polarization-inhomogeneous components with different scattering multiplicities in the object laser field of diffuse layers in biological tissues with various types of optically anisotropic fibrous and parenchymal structures.
2. The methods of direct Stokes polarimetry and digital holographic 3D layered reconstruction of complex amplitudes in different phase planes of microscopic images of diffuse histological sections of biological tissues have been experimentally validated. This includes subsequent reconstruction of polarization azimuth and ellipticity maps.
3. The comparison of the results of statistical analysis of integral maps of polarization azimuth
• difference of all coordinate polarization distributions from normal or Gaussian—all statistical moments
• variations in the magnitude (from two to seven times) of all statistical moments
4. The comparative analysis of layered maps of polarization azimuth and ellipticity, reconstructed using the phase scanning method with a digital holographic reconstruction of layered distributions of complex amplitude fields, revealed that with a decrease in the magnitude of
5. It has been established that starting from a certain value of the phase parameter
6. Biomedical validation of the method revealed an excellent level (
Therefore, we have demonstrated that the application of the 3D polarization–interference method by phase scanning with digital holographic reconstruction of biological diffuse tissue object field layered polarization azimuth and ellipticity maps with different architectural optical anisotropic components allows for the experimental selection of components with different scattering multiplicities. This enables the isolation of the “object” practically singly scattered component against the background of the overall “diffuse” background, which is formed by interactions with high multiplicities.
The significant differences (from two to four times) observed in the magnitudes of the statistical moments
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Bukovinian State Medical University, 3 Teatralna Sqr. Chernivtsi, Ukraine, 58005. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because samples of histological sections of tissues of the myocardium and liver of the deceased were obtained from the Bureau of Forensic Medicine.
AU: conceptualization, data curation, resources, validation, and writing–original manuscript. JZ: validation, writing–original manuscript, investigation, and supervision. MG: writing–review and editing. AD: writing–review and editing, methodology, and software. YU: investigation, project administration, and writing–review and editing. IS: resources, visualization, and writing–review and editing. IM: data curation and writing–review and editing. ZC: formal analysis, methodology, and writing–review and editing. OW: resources and writing–review and editing. IG: formal analysis and writing–review and editing. CJ: visualization, investigation and writing–review and editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. Authors acknowledge the support from the National Research Foundation of Ukraine, Project 2022.01/0034 and Scholarship of the Verkhovna Rada of Ukraine for Young Scientists-Doctors of Science 2023.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tuchin VV. Tissue optics and Photonics: light-tissue interaction. J Biomed Photon Eng (2015) 1(2):98–134. doi:10.18287/jbpe-2015-1-2-98
3. Boas DA, Pitris C, Ramanujam N. Handbook of biomedical optics. Florida, United States: CRC Press (2016).
4. Tuchin VV. Tissue optics: light scattering methods and instruments for medical diagnosis. In: Society of photo-optical instrumentation engineers (SPIE) eBooks. Bellingham, Washington: Society of Photo-Optical Instrumentation Engineers SPIE (2015).
6. Ushenko AG, Pishak VP. Laser polarimetry of biological tissues: principles and applications. In: VV Tuchin, editor. Handbook of coherent domain optical methods. Cham: Springer US (2004). p. 93–138.
7. Ghosh N Tissue polarimetry: concepts, challenges, applications, and outlook. J Biomed Opt (2011) 16(11):110801. doi:10.1117/1.3652896
8. Jacques SL. Polarized light imaging of biological tissues. In: D Boas, C Pitris, and N Ramanujam, editors. Handbook of biomedical optics 2. Boca Raton: CRC Press (2011). p. 649–69.
9. Ghosh N, Wood M, Vitkin A. Polarized light assessment of complex turbid media such as biological tissues using mueller matrix decomposition. In: VV Tuchin, editor. Handbook of Photonics for biomedical science. Boca Raton: CRC Press (2010). p. 253–82.
10. Layden D, Ghosh N, Vitkin IA. Quantitative polarimetry for tissue characterization and diagnosis. In: RK Wang, and VV Tuchin, editors. Advanced biophotonics: tissue optical sectioning. Boca Raton: CRC Press (2013). p. 73–108.
11. Vitkin A, Ghosh N, de Martino A. Tissue polarimetry. In: DL Andrews, editor. Photonics: scientific foundations, technology and applications. John Wiley & Sons, Ltd (2015). p. 239–321.
12. Tuchin VV. Tissue optics and Photonics: biological tissue structures. J Biomed Photon Eng (2015) 1(1):3–21. doi:10.18287/jbpe-2015-1-1-3
13. Bai X, Liang Z, Zhu Z, Schwing AG, Forsyth D, Gruev V. Polarization-based underwater geolocalization with deep learning. eLight (2023) 3(15):15. doi:10.1186/s43593-023-00050-6
14. Lee HR, Lotz C, Groeber Becker FK, Dembski S, Novikova T. Digital histology of tissue with Mueller microscopy and FastDBSCAN. Appl Opt (2022) 61(32):9616. doi:10.1364/ao.473095
15. Novikova T, Kim M, Lee HR, Ossikovski R, Malfait-Jobart A, Lamarque D. Optical diagnosis of gastric tissue biopsies with Mueller microscopy and statistical analysis. J Eur Opt Soc Rapid Publications (2022) 18(2):10. doi:10.1051/jeos/2022011
16. Lee HR, Li P, Sang T, Lotz C, Groeber-Becker F, Dembski S, et al. Digital histology with Mueller microscopy: how to mitigate an impact of tissue cut thickness fluctuations. J Biomed Opt (2019) 24(07):1–11. doi:10.1117/1.jbo.24.7.076004
17. Li P, Lee HR, Chandel S, Lotz C, Groeber-Becker FK, Dembski S, et al. Analysis of tissue microstructure with Mueller microscopy: logarithmic decomposition and Monte Carlo modeling. J Biomed Opt (2020) 25(01):1. doi:10.1117/1.jbo.25.1.015002
18. Lee HR, Sang T, Li P, Lotz C, Groeber-Becker F, Dembski S, et al. Mueller microscopy of anisotropic scattering media: theory and experiments. India: HAL Le Centre pour la Communication Scientifique Directe (2018).
19. Ma H, He H, Ramella-Roman JC. Mueller matrix microscopy. In: T Novikova, and JC Ramella-Roman, editors. Polarized light in biomedical imaging and sensing. Cham: Springer (2023). p. 281–321.
20. Zabolotna N, Pavlov S, Ushenko A, Karachevtsev A, Savich V, Sobko O, et al. System of the phase tomography of optically anisotropic polycrystalline films of biological fluids. Proc SPIE (2014)(916616) 9166. doi:10.1117/12.2061116
21. Ushenko O, Dubolazov A, Balanetska V, Karachevtsev A, Sydor M. Wavelet analysis for polarization inhomogeneous laser images of blood plasma. Proc SPIE (2011) 8338. 83381H. doi:10.1117/12.920169
22. Angelsky O. Optical measurements: polarization and coherence of light fields. In: L Cocco, editor. Polarization and coherence of light fields. London, UK: INTECH Open Access Publisher (2012). p. 5–16.
23. Garazdyuk M, Bachinskyi V, Vanchulyak O, Ushenko A, Dubolazov A, Gorsky M. Polarization-phase images of liquor polycrystalline films in determining time of death. Appl Opt (2016) 55(12):B67–71. doi:10.1364/ao.55.000b67
24. Ushenko Y, Sdobnov A, Dubolazov A, Grytsiuk M, Bykov A, Meglinski I, et al. Stokes-correlometry analysis of biological tissues with polycrystalline structure. IEEE J Selected Top Quan Elect (2019) 25(1):1–12. doi:10.1109/jstqe.2018.2865443
25. Ushenko V, Sdobnov A, Mishalov W, Dubolazov A, Olar O, Bachinskyi V, et al. Biomedical applications of Jones-matrix tomography to polycrystalline films of biological fluids. J Innovative Opt Health Sci (2019) 12(06):12. doi:10.1142/s1793545819500172
26. Ushenko AG, Dubolazov OV, Ushenko VA, Oyu N, Olar OV. Fourier polarimetry of human skin in the tasks of differentiation of benign and malignant formations. Appl Opt (2016) 55(12):B56–60. doi:10.1364/ao.55.000b56
27. Peyvasteh M, Dubolazov A, Popov A, Ushenko A, Meglinski I. Two-point Stokes vector diagnostic approach for characterization of optically anisotropic biological tissues. J Phys D (2020) 53(39):395401–1. doi:10.1088/1361-6463/ab9571
28. Ushenko A, Angelsky P, Sidor M, Marchuk Y, Andreychuk D, Pashkovska N. Spatial-frequency selection of complex degree of coherence of laser images of blood plasma in diagnostics and differentiation of pathological states of human organism of various nosology. Appl Opt (2014) 53(10):B172–80. doi:10.1364/ao.53.00b172
29. Ushenko V, Dubolazov A, Pidkamin L, Sakchnovsky M, Bodnar A, Ushenko Y, et al. Mapping of polycrystalline films of biological fluids utilizing the Jones-matrix formalism. Laser Phys (2018) 28(2):025602. doi:10.1088/1555-6611/aa8cd9
30. de Boer JF, Milner TE. Review of polarization sensitive optical coherence tomography and Stokes vector determination. J Biomed Opt (2002) 7(3):359. doi:10.1117/1.1483879
31. Spandana KU, Krishna KM, Mazumder N. Polarization-resolved Stokes-Mueller imaging: a review of technology and applications. Lasers Med Sci (2019) 34(7):1283–93. doi:10.1007/s10103-019-02752-1
32. He Y, Li K, Li W, Qiu Y, Li D, Wang C, et al. Polarization coherency matrix tomography. J Biophotonics (2023) 16(9):e202300093. doi:10.1002/jbio.202300093
33. Yang D, Yuan Z, Hu M, Liang Y. Zebrafish brain and skull imaging based on polarization-sensitive optical coherence tomography. J Biophotonics (2022) 15(12):e202200112. doi:10.1002/jbio.202200112
34. Park JE, Zhou X, Kwon DY, Kim SW, Lee H, Jung MJ, et al. Application of polarization sensitive-optical coherence tomography to the assessment of phase retardation in subpleural cancer in rabbits. Tissue Eng Regenerative Med (2021) 18(1):61–9. doi:10.1007/s13770-020-00318-9
35. Willemse J, Maximilian Grafe GO, Verbraak FD, De Boer J. In vivo 3D determination of peripapillary scleral and retinal layer architecture using polarization-sensitive optical coherence tomography. Translational Vis Sci Tech (2020) 9(11):21–1. doi:10.1167/tvst.9.11.21
36. Baumann B, Augustin M, Lichtenegger A, Harper DJ, Muck M, Eugui P, et al. Polarization-sensitive optical coherence tomography imaging of the anterior mouse eye. J Biomed Opt (2018) 23(08):1–12. doi:10.1117/1.jbo.23.8.086005
37. Hariri LP, Adams DC, Applegate MB, Miller AJ, Roop BW, Villiger M, et al. Distinguishing tumor from associated fibrosis to increase diagnostic biopsy yield with polarization-sensitive optical coherence tomography. Clin Cancer Res (2019) 25(17):5242–9. doi:10.1158/1078-0432.ccr-19-0566
38. Tao K, Sun K, Ding Z, Ma Y, Kuang H, Zhao H, et al. Catheter-based polarization sensitive optical coherence tomography using similar mueller matrix method. IEEE Trans Biomed Eng (2020) 67(1):60–8. doi:10.1109/tbme.2019.2908031
39. Ushenko V, Sdobnov A, Syvokorovskaya A, Dubolazov A, Vanchulyak O, Ushenko A, et al. 3D mueller-matrix diffusive tomography of polycrystalline blood films for cancer diagnosis. Photonics (2018) 5(4):54. doi:10.3390/photonics5040054
40. Peyvasteh M, Tryfonyuk L, Ushenko V, Syvokorovskaya A, Dubolazov A, Vanchulyak O, et al. 3D Mueller-matrix-based azimuthal invariant tomography of polycrystalline structure within benign and malignant soft-tissue tumours. Laser Phys Lett (2020) 17(11):115606. doi:10.1088/1612-202x/abbee0
41. Ushenko A, Dubolazov A, Litvinenko O, Bachinskiy V, Lin B, Guo B, et al. 3D polarization correlometry of object fields of networks of biological crystals. Proc SPIE (2020):11369. 113691M. doi:10.1117/12.2553942
42. Bodnar A, Dubolazov A, Pavlyukovich A, Pavlyukovich N, Ushenko A, Motrich A, et al. 3D Stokes correlometry of the polycrystalline structure of biological tissues. Proc SPIE (2020):11509. 115090V. doi:10.1117/12.2568451
43. Ushenko VA, Hogan BT, Dubolazov A, Grechina AV, Boronikhina TV, Gorsky M, et al. Embossed topographic depolarisation maps of biological tissues with different morphological structures. Scientific Rep (2021) 11(1):3871. doi:10.1038/s41598-021-83017-2
44. Marchesini R, Bertoni A, Andreola S, Melloni E, Sichirollo AE. Extinction and absorption coefficients and scattering phase functions of human tissues in vitro. Appl Opt (1989) 28(12):2318–24. doi:10.1364/ao.28.002318
45. Edwards DK, Gier JT, Nelson KE, Roddick RD. Integrating sphere for imperfectly diffuse samples. J Opt Soc America (1961) 51(11):1279–88. doi:10.1364/josa.51.001279
Keywords: polarization, interference, holography, microscopic image, optical anisotropy, biological tissue, statistical moments
Citation: Ushenko A, Zheng J, Gorsky M, Dubolazov A, Ushenko Y, Soltys I, Mikirin I, Chen Z, Wanchuliak O, Gordey I and Jingxian C (2023) 3D digital holographic polarimetry of diffuse optically anisotropic biological tissue object fields. Front. Phys. 11:1288935. doi: 10.3389/fphy.2023.1288935
Received: 05 September 2023; Accepted: 06 November 2023;
Published: 05 December 2023.
Edited by:
Jingjiang Xu, Foshan University, ChinaReviewed by:
Shaozhen Song, Microsoft, United StatesCopyright © 2023 Ushenko, Zheng, Gorsky, Dubolazov, Ushenko, Soltys, Mikirin, Chen, Wanchuliak, Gordey and Jingxian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zheng, ZGJ6akBuZXRlYXNlLmNvbQ==; Alexander Ushenko, by51c2hlbmtvQGNobnUuZWR1LnVh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.