
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys. , 03 February 2023
Sec. Radiation Detectors and Imaging
Volume 11 - 2023 | https://doi.org/10.3389/fphy.2023.1136093
This article is part of the Research Topic Multi-Sensor Imaging and Fusion: Methods, Evaluations, and Applications View all 18 articles
Background: Anterior communicating artery (AcomA) aneurysm is the most common intracranial aneurysm (IA) and has the highest rupture rate. Previously, the preferred surgical treatment for intracranial aneurysms was microsurgery clipping (MC). With the gradual maturation of endovascular treatment (EVT), an increasing number of patients are inclined to treat IA with EVT. In recent years, an increasing number of scholars have suggested that the preferred treatment for wide-necked aneurysms is stent-assisted coiling (SAC). Currently, there are few studies on comparative analyses of the procedural results of SAC in AcomA aneurysms.
Methods: We retrospectively reviewed all consecutively treated patients who received SAC for AcomA aneurysms between 12 February 2013, and 20 January 2021. The primary procedural outcome was the occlusion rate evaluated with the Raymond–Roy occlusion classification (RROC) assessed on DSA at follow-up. Safety assessment included 1) ischemic complications (asymptomatic ischemia; intrastent thrombosis; coils falling off plug; arterial dissection); 2) bleeding complications (SAH; ICH); and 3) death. Univariate and multivariate logistic regression analyses were performed to determine patient baseline and aneurysm characteristics associated with total aneurysm occlusion at follow-up. Hemodynamic analysis was performed in one representative case each of the four stents, and six hemodynamic parameters were chosen, including wall shear stress (WSS), cavity blood flow velocity (CBFV), residual blood in the aneurysm (RBA), neck blood flow velocity (NBFV), blood flow inflow (BFI); and inflow concentration index (ICI).
Results: A total of 154 patients who underwent EVT via SAC were enrolled for comparative analysis of procedural outcomes. The median age was 55 years, and 56.49% (87) were female. At the first (6–10 months), second (12–15 months) and last (24–48 months) follow-up, complete aneurysm occlusion was observed in 94.8%, 94.8%and 94.2% of patients, respectively. There were no differences regarding the occlusion rates stratified by stent. Each stent showed a variable decrease in all hemodynamic parameters.
Conclusion: Hemodynamic parameters all decreased significantly after SAC with all four different stents, and the effect of laser-cut stents on the hemodynamic decline of aneurysms appeared to be more significant than that of woven stents. No significant difference was observed in the follow-up RROC grade among the four stents.
Intracranial aneurysm (IA) is a common cerebrovascular disease, and the anterior communicating artery (AcomA) is the most common site [1], accounting for the highest mortality rate of ruptured aneurysms [2].
AcomA aneurysms are usually clipped, but an increasing number of studies have shown that endovascular treatment (EVT) is effective [3]. Stent-assisted coiling (SAC) is commonly used to treat wide-necked aneurysms, which are defined as aneurysms with neck ≥4 mm or dome-to-neck ratio (DNR) < 2 [4]. Previous researches believe that SAC has a similar complication rate and lower recurrence rate than coiling alone [5]. In our therapeutic centre, we used a total of four stents for SAC, which were Solitaire AB stents, Enterprise stents, LVIS(JR) stents and LEO + (baby) stents. In general, the treatment effect was acceptable, and the complication rate and recurrence rate were low.
The creation and rupture of aneurysms are associated with hemodynamic factors [6, 7]. Currently, there are relatively few studies that simultaneously investigate the effects of these four different stents on hemodynamics. In addition, no study on the simultaneous effect of these four stents on the occlusion rate has been reported.
In this study, we deeply investigated the effect of multiple factors, including patient baseline characteristics, anatomical aneurysm details, and hemodynamic changes before and after stent placement on aneurysm recurrence to guide the use of the stents in the subsequent treatments.
We retrospectively analyzed the data of all consecutive patients with AcomA wide-necked aneurysms who were diagnosed and treated at the Neurosurgery of the First Affiliated Hospital of Chongqing Medical University between 12 February 2013, and 20 January 2021. All patients receiving SAC treatment with the four stents (Solitaire™, Enterprise™, LVIS(JR)™, LEO(Baby)™) were selected and analyzed.
The main inclusion criteria for all cases were 1) the diagnosis of an AcomA wide-necked aneurysm, regardless of rupture or not, 2) SAC in an elective setting, and 3) Solitaire™, Enterprise™, LVIS(JR)™ or LEO (baby)™ stents, regardless of previous treatments (EVT or MC). The main exclusion criterion was that the image display was unclear.
For all selected patients, baseline and procedural characteristics were collected from medical records and imaging studies. We selected only specific and measurable measures, for patient baseline, such as age, gender, etc., whereas height, weight, etc., were not recorded because of failure to measure precisely or variations in data. In terms of previous history, we selected arterial hypertension or not, history of previous treatment of aneurysms, other factors such as medical history which may cause difficulty in recording details due to patient forgetting or deliberate concealment, etc. so were not included as parameters.
Many reports suggest that irregular administration of clopidogrel and aspirin may result in high thromboembolic event rates [8]; therefore, we followed regular and scientific antiplatelet therapy before treatment. All patients without previous antiplatelet therapy received loading dose dual antiplatelet therapy primarily with aspirin (300 mg st) and clopidogrel (300 mg st) before SAC. If the standard dual antiplatelet dose (aspirin 100 mg qd and clopidogrel 75 mg qd) had been taken for ≥3 days, the same standard dual antiplatelet dose was given once before treatment. At the beginning of the intervention, weight-adaptive heparin was administered intra-arterially, 2/3 mg/kg for the first time. If the procedure was still not finished after 1 h, half of the first dose was injected as a bolus, and the dose was tapered sequentially. After surgery, some patients received intravenous micropumps of tirofiban for several hours, overlapping with dual antiplatelet therapy for 4 h before deactivation. For the remaining patients, coagulation profiles were reviewed at 4 h postoperatively, and low molecular weight heparin (LMWH) was administered subcutaneously if no significant abnormalities were observed, bridging dual antiplatelet therapy until the second postoperative day. All patients after SAC took aspirin for at least 3 months and clopidogrel for at least 6 weeks every day. The dosage was adjusted according to the thromboelastography (TEG) and clopidogrel genotype (CYP2C19 enzyme). Genetic polymorphisms of the CYP2C19 enzyme result in different efficacy of clopidogrel, which is usually divided into four phenotypes clinically according to the different ability to metabolize clopidogrel: ultra fast metabolism (UM), fast metabolism (FM), medium metabolism (MM) and slow metabolism (SM). When a patient’s clopidogrel genotype was expressed as UM or FM, we gave clopidogrel 75 mg qd. When clopidogrel genotype was expressed as MM, we gave clopidogrel 100 mg qd. When clopidogrel genotype was expressed as SM, we replaced clopidogrel with ticagrelor at an oral dose of 90 mg qd.
EVT is an effective treatment modality for AcomA aneurysms [9], and previous studies have shown that SAC may improve the results of embolization by allowing more complete initial coiling. The success rate of SAC is higher than that of coiling alone, especially in the treatment of wide-necked aneurysms [1, 10]. In our cases, all operations were performed under general anesthesia using a SIEMENS™ biplane angiography system. Cerebral vessel access was usually established using a 6F Chaperon Guiding Catheter System. Subsequently, a 3-D rotational angiography was performed to plan the operation. In all cases, we used the jailing technique, which could reduce navigation time and result in a lower incidence of thromboembolic events [8]: 1) a microcatheter was used to navigate to the aneurysm and fill in coils; 2) another microcatheter was used to navigate to the far end of the parent artery; and 3) the stent was partially released while coiling the IA was continued; and 4) the stent was completely released when the IA was completely coiled.
The use of each stent depends on the diameter of the parent artery, the condition of vascular tortuosity and the specific parameters of the IA, such as the location and DNR. Each stent has its specific diameter-length ratio, and the choice of stent diameter is determined according to the diameter of the parent artery, the length of the stent is determined according to the width of the aneurysm neck, both ends of the aneurysm neck need to be covered with a sufficiently long stent, and at the same time the stent cannot be too long to block branch arteries.
Solitaire™ is a self-expanding nitinol laser-cut stent with a closed-cell design and fully open structure on one side, combining the advantages of open-cell and closed-cell designs. It can be completely recovered twice, and the stent hole is adjustable. The advantages of this stent are mainly a higher success rate for SAC of small-size aneurysms and a lower risk of postoperative thrombosis [11].
Enterprise™ is also a self-expanding nitinol laser-cut stent with a closed-cell design. The conveying system provides excellent navigation and positioning, making it easier to transport and deploy, with only a 1.3% inaccurate deployment rate. Stents are deployed on the parent artery 5 mm above both sides of the aneurysm neck, with more intensive filling of the aneurysm sac and neck [8]. Our case contained a large number of ruptured aneurysms, and previous studies have shown that the Enterprise stent is safe and effective in SAC of ruptured wide-necked aneurysms [8].
LVIS™ is a self-expanding nitinol woven stent system with a closed-cell design. It has higher metal coverage, which may cause a greater reduction in velocity at the neck plane, and higher packing density, which may cause a greater reduction in velocity and WSS at the aneurysm. The greater hemodynamic alterations may cause lower recanalization in medium-sized aneurysms [12].
LEO™ is a self-expanding nitinol woven stent system with a closed-cell design. With two radio opaque standards along its full length, delivery systems that allow easier navigation and precise placement and potential for stent repositioning make it very useful for the treatment of complex cerebral aneurysms [13], in addition to lower perioperative morbidity [14]. Some scholars even believe that SAC with LEO stents may be considered the first-line treatment for wide-necked aneurysms [15].
A comparison of the technical parameters of the individual stents is detailed in Table 1.
Hemodynamics are as important as morphology in the development of aneurysms [7]. Previous studies have confirmed that 53% of patients with AcomA aneurysms have variant anatomy of the vessels surrounding the AcomA [2], and the presence of a hypoplastic A1 segment is the only parameter independently associated with the presence of AcomA aneurysms in addition to aneurysm size [16]. Research has shown that the growth of aneurysms may be associated with high wall shear stress (WSS) [17], and IAs with large maximum widths and small neck diameters, which have a greater range of low WSS areas, may be prone to rupture [18]. As aneurysms are studied more intensively, the influence of hemodynamics should not be ignored.
In this study, four patients with four different stents were randomly selected, focusing on the following six parameters: 1) wall shear stress (WSS); 2) cavity blood flow velocity (CBFV); 3) residual blood in the aneurysm (RBA); 4) neck blood flow velocity (NBFV); 5) blood flow inflow (BFI); and 6) inflow concentration index (ICI).
Aneurysm details were analyzed, including the size of the aneurysms, the DNR, ruptured or not, the number of coils filled and the diameters of the parent arteries. The angiographic results were evaluated after intervention and angiography by DSA using the Raymond–Roy Occlusion Classification (RROC) [19], which defined Class I as complete obliteration, Class II as residual neck, and Class III as residual aneurysm. RROC I was defined as the primary angiographic outcome endpoint, and all occlusion rates were compared by the stent used.
The above factors in all follow-up patients were analyzed for significant associations with complete aneurysm occlusion. We recommend that the patients be reviewed for the first time 6 months after surgery. If negative (Class I), patients should be reviewed again 1 year later. If still negative, they should be reviewed 2–3 years later. SAC was performed whenever the review was positive (Class II or Class III). The reason for this is that after aneurysm clipping, 25% of Raymond II patients will have gradually increasing aneurysms; if Raymond III, 75% of aneurysms will increase [20]. Although no relevant reports have been found after SAC, overall, more than 20% of aneurysms recurred after EVT [21].
Perioperative complications that need attention mostly include 1) ischemic complications (asymptomatic ischemia; intrastent thrombosis; coils falling off plug; arterial dissection); 2) bleeding complications (SAH; ICH); and 3) death.
All data were analyzed using statistical methods, standard descriptive statistics were used for all data endpoints, the mean and median were used to represent the degree of centralization, and the standard deviation (SD) was used to represent the distribution. Categorical variables were compared using the chi-square test or Fisher’s exact test. Continuous variables were assessed with the Mann‒Whitney U test (non-normally distributed data). Univariate and multivariate logistic regression analyses were used to identify variables associated with total aneurysm occlusion. The odds ratio (OR) and adjusted OR (AOR) had a 95% confidence interval. p < 0.05 was considered statistically significant. Regarding the hemodynamics section, we provided the original data mentioned above and technical support provided by ArteryFLOW Science and Technology Co., Ltd.
A total of 154 patients were selected; 82.47% (127) of patients were found due to subarachnoid hemorrhage, and 17.53% (27) of patients were found accidentally due to health checkup or radiological examinations owing to symptoms such as dizziness or headache. The median age was 55 years, and 56.46% (87) were female. The most common risk factor associated with IA was arterial hypertension, with 51.95% (80) detected. The mean dome diameter, the mean neck diameter and the mean DNR was 5.4 mm, 3.6 mm, and 1.5, respectively. As the AcomA is the most difficult to observe by angiography among the arteries of the circle of Willis, the mean diameter of the parent artery can only be estimated roughly, which was approximately 1.62 mm. The median number of implanted coils was 3. Table 2 shows an overview of patient characteristics and anatomical aneurysm details stratified by stent models.
Perioperative ischemic complications (11.69%, 18) were more common than hemorrhagic complications (5.19%, 8). There were no deaths in our cases during hospitalization and follow-up.In total, 6.49% (10) developed asymptomatic ischemia; the incidence was 9.09% 4) for the Solitaire stent, 2.86% 1) for the Enterprise stents, 5.88% 2) for the LVIS stents, and 7.32% 3) for the LEO stents (Table 3).
According to the Raymond-Roy Classification, 94.81% (146) of patients had total occlusion (RROC Ⅰ) at the first follow-up. The incidence of RROC I was 86.36% (38) after Solitaire stenting, 97.14% (34) after Enterprise, 100% (34) after LVIS, and 97.56% (40) after LEO. At the second follow-up, 94.81% (146) of patients had total occlusion (RROC Ⅰ). The incidence of RROC I was 97.73% (43) after Solitaire stenting, 94.29% (33) after Enterprise, 94.12% (32) after LVIS, and 92.68% (38) after LEO. At the third follow-up, 94.16% (145) of patients observed total occlusion (RROC Ⅰ). The incidence of RROC I was 93.18% (41) after Solitaire stenting, 97.14% (34) after Enterprise stenting, 94.12% (32) after LVIS stenting, and 92.68% (38) after LEO stenting (Figure 1).
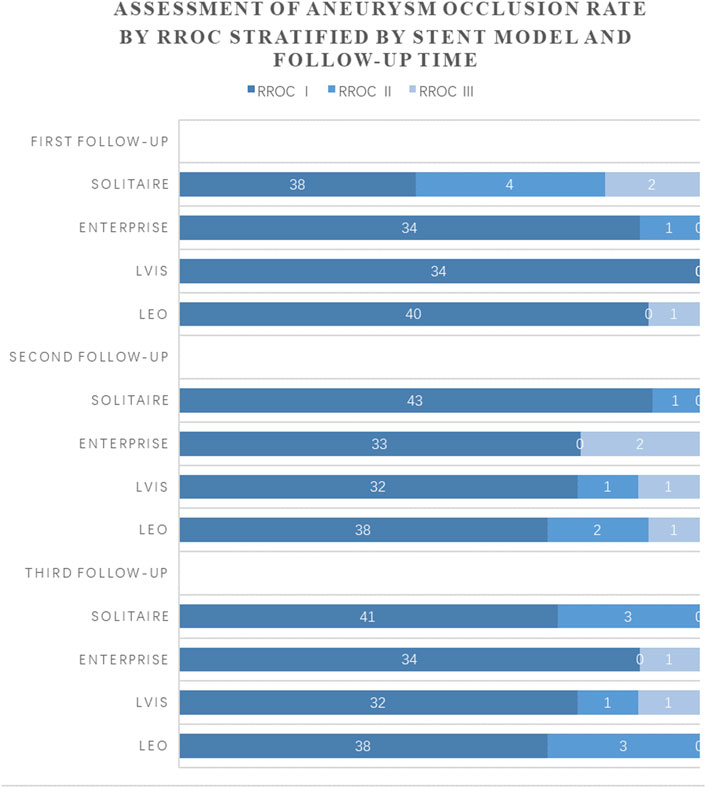
FIGURE 1. Comparison of occlusion rates at 3 follow-up visits in patients using four stents.Legend: Time of first follow-up: 6–10 months after SAC. Time of second follow-up: 12–15 months after SAC. Time of third follow-up: 24–48 months after SAC.
As shown in Table 4, most data for patients using different stents did not show significant differences. Univariate logistic regression was then used to analyze the influence of different factors on the occlusion rate at three follow-up reviews. The results showed that the influence of stents on the occlusion rate was not statistically significant; however, increasing dome size and increasing DNR were factors related to aneurysm occlusion at all three follow-ups; for details, see Table 5. Multivariable logistic analysis confirmed increasing DNR (adjusted odds ratio (aOR), 0.020; 95% CI, 0.001–0.583; p = 0.023) as an independent factor associated with complete aneurysm occlusion at the third follow-up.
As no significant difference in aneurysm complete occlusion rates by different stents has been demonstrated above, we focused on hemodynamic aspects. Figure 2 demonstrates an angiogram before and after placement of four stents and a schematic representation of stents within blood vessels. In Picture A of Figure 3, we can see that at the bifurcations and corners of the arteries, flow streamlines and WSS were elevated. High flow velocity territory (V > 0.1 m/s) was observed throughout the arterial vessel, including the aneurysmal body. In particular, from the section view, the flow velocity in the region of the dome was significantly lower than that elsewhere in the aneurysm before SAC, which may be because it was difficult for blood flow to reach the top due to the large dome diameter. After the aneurysm was coiled with a stent, it could be seen from the velocity magnitude contour at the cutting plane and isovagine surface (V > 0.1 m/s) that the blood flow in the aneurysm cavity was almost stagnant; at the same time, WSS was also significantly reduced. From the streamlines with color-coded velocity magnitude, we could see that there was no obvious change in the blood flow at stent placement; that is, it did not affect the blood flow in normal blood vessels. All stent models showed similar properties in Figure 3; in particular, Pictures A and D show slower flow velocities at the aneurysm dome site before SAC as a result of a larger DNR, whereas Picture C shows a non-significant decrease in aneurysm WSS after SAC.
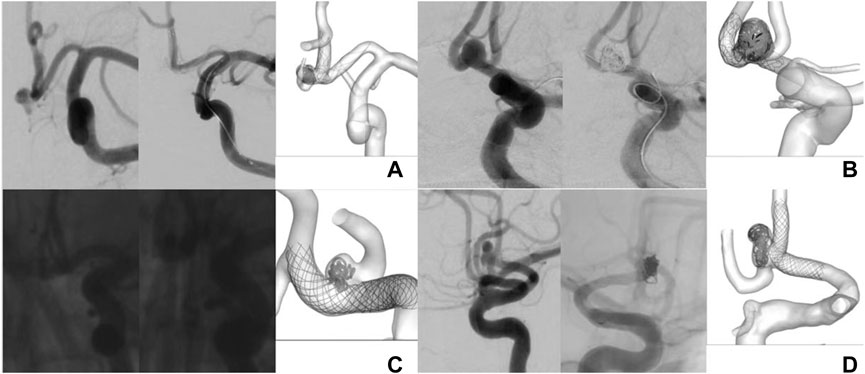
FIGURE 2. Angiogram before and after placement of four stents and schematic representation of stents within blood vessels. Picture (A) represents Solitaire stent; Picture (B) represents Enterprise stent; Picture (C) represents LVIS stent;Picture (D) represents LEO stent.
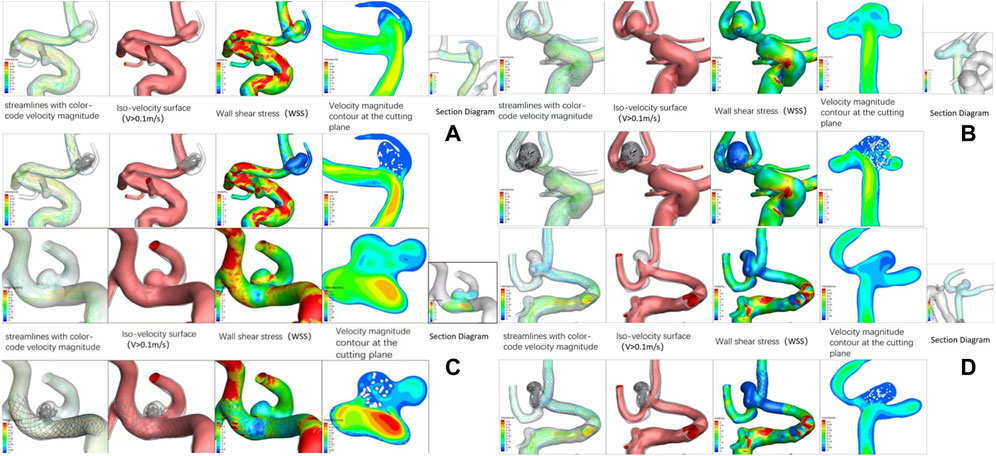
FIGURE 3. Hemodynamic patterns of four stents. Picture (A) represents Solitaire stent; Picture (B) represents Enterprise stent; Picture (C) represents LVIS stent; Picture (D) represents LEO stent.
As shown in Table 6, all indices had different decreasing trends in each stent. The decrease rate in LVIS was minimal for almost all parameters, and the Solitaire stent was optimal in four of all six indices. The decline rate of WSS was 43.74% compared with 83.27% for the LEO stent, 79.88% for the Enterprise stent and 79.76% for the Solitaire stent. The decline rate of the BFI was 79.07% compared with 92.65% for the Solitaire stent, 83.79% for the Enterprise stent and 82.39% for the LEO stent. The decline rate of the RBA was 93.10% compared with 95.36% for the Enterprise stent and 94.62% for the Solitaire stent. The decline rate of the CBFV was 68.93% compared with 86.96% for the Solitaire stent, 80.19% for the Enterprise stent and 79.17% for the LEO stent. The decline rate of the NBFV was 68.42% compared with 88.13% for the Solitaire stent, 75.93% for the Enterprise stent and 74.07% for the LEO stent. The decline rate of the ICI was 74.48% compared with 93.48% for the Solitaire stent, 84.41% for the Enterprise stent and 82.59% for the LEO stent.
The data of the present study suggest that the effect of laser-cut stents on the hemodynamic decline of aneurysms appears to be more significant than that of woven stents. The mean decline rate of WSS was 79.82% for laser-cut stents compared with 63.51% for woven stents, of BFI was 88.22% compared with 80.73% for woven stents, of RBA was 94.99% compared with 87.66% for woven stents, of CBFV was 83.58% compared with 74.05% for woven stents, of NBFV was 82.03% compared with 71.25% for woven stents, and of ICI was 88.95% compared with 78.54% for woven stents.
This study revealed several findings: 1) no significant difference was observed in follow-up RROC grade among the four stents; 2) ischemic complications were more frequent than hemorrhagic complications during our treatment; 3) hemodynamic parameters all decreased significantly after SAC with four different stents, and the effect of laser-cut stents on the hemodynamic decline of aneurysms appeared to be more significant than that of woven stents; and 4) increasing DNR was perhaps an independent factor associated with complete aneurysm occlusion.
It was previously thought that aneurysms first arise in high WSS areas, and subsequently, the growing areas change to areas of low WSS and eventually rupture there [17]. The latter is probably because low WSS (<1 Pa) is not sufficient for the self-healing process of the arterial wall, and the normal remodeling process of the arterial wall slows down, thereby leading to faster aneurysm growth and eventual rupture [22]. The WSS of the LEO stent decreased the most, which may be related to its characteristics as a woven stent with higher metal coverage or higher filling density. However, the LVIS stent, also as a woven stent, was slightly less effective in reducing WSS, suggesting that the fabrication process of the woven stent was perhaps not related to its function in reducing WSS; validation awaits further studies. Currently, the impact of WSS on aneurysm generation, growth, and rupture is recognized in two different ways by the academic community. These two schools of thought differ in the mechanisms leading to wall weakening. One view is that high WSS causes endothelial damage, which initiates chamber wall remodeling and potentially degeneration, leading to an imbalance between blood pressure and inner wall stress and resulting in local dilatation of the arterial wall. The resulting abnormal blood WSS fields lead to further aneurysm geometry development. An alternative view is that the arrest of flow locally against the wall within the dome at low WSS leads to endothelial dysfunction, with the aggregation and adhesion of platelets and leukocytes along the intimal surface, thereby causing intimal injury, inflammation, and subsequent degeneration of the canal wall. The aneurysmal wall will gradually become thinner, which may eventually lead to tearing of the tissue [23].
Stents were initially used in EVT to prevent the coils from slipping out but were found in clinical trials to straighten the parent artery and cause a corresponding change in the aneurysm sac, thereby altering aneurysm hemodynamics [24]. In sidewall aneurysms, the use of stents has previously been shown to block the inflow of flow and thereby reduce the corresponding hemodynamics [25], but in bifurcation aneurysms, it has been argued that stents may produce a greater inertial force resulting from narrowed inflow jet, which enhances flow into the aneurysms [26]. AcomA aneurysms are bifurcation aneurysms; however, in our study, the BFI (blood flow inflow) of each case was decreased, and the RBA (residual blood in the aneurysm) was decreased as well. Previous research suggests that aneurysm recurrence at >1 year after coiling is associated with higher intra-aneurysmal flow before and after coiling [27], which perhaps indicates the role of the four stents in improving the occlusion rate, and the performance of the Solitaire stent in reducing blood flow was especially excellent among the four stents. The most significant decline in RBA was in the case of LEO stents, perhaps related to the size of the stent hole and the density of coil embolization; thus far, no relevant studies have been reported.
Previous studies suggest that stents with lower porosity will cause a greater decrease in blood flow velocity [25], which was proven in our study in that the Solitaire™ stent had the largest decline rate in CBFV (cavity blood flow velocity) and NBFV (neck blood flow velocity) among the four stents (Solitaire™ possessed the largest pore size and the lowest porosity of the four stents), and the decrease in blood flow velocity is crucial to prevent recanalization after EVT [25], which proved to be one of the advantages of this kind of stent.
ICI (inflow concentration index) refers to the degree of concentration of blood flow into the aneurysm. It is defined as the percentage of the flow rate of the parent artery which enters the aneurysm divided by the percentage of the aneurysm ostium area corresponding to positive inflow velocity [23]. Studies have found that ruptured aneurysms are more likely to have a concentrated inflow jet than unruptured aneurysms [23, 28]. All four stents were effective in reducing ICI and may be effective in reducing the incidence of aneurysm rupture during and after SAC. The ability of the Solitaire stent to reduce ICI is best, perhaps because of its greater stiffness as a laser-cut stent that is less pliable.
This paper is the first to study four types of stents (Solitaire™, Enterprise™, LVIS(JR)™, LEO+(Baby)™) simultaneously. Previous studies on the effects of Atlas ™, Enterprise ™, and LEO ™ stents on angiographic outcomes after SAC of wide-neck aneurysms showed a high incidence of total occlusion at long-term follow-up [29]. No significant difference was similarly observed among different stents, and the results of this research coincided with this finding. The four stents all showed varying degrees of reduction in terms of hemodynamics. In general, the reduction in hemodynamic factors by LVIS appeared to be minimal with all four stents; however, the final complete occlusion rate was not significantly different. The effect of laser-cut stents on the hemodynamic decline of aneurysms appears to be more significant than that of woven stents, which is probably caused by the different radial strengths of the stents due to the different fabrication processes. The present study demonstrated that increasing DNR (dome-neck ratio) is perhaps an independent factor associated with complete aneurysm occlusion. Other parameters related to aneurysm size and morphology, such as LWR (length-width ratio), size ratio (height-diameter of parent artery ratio), and HNR (height-neck ratio), and whether they correlate with aneurysm occlusion rates, require further study.
It was previously believed that it was unnecessary to treat small aneurysms found incidentally [30]. However, in this study, 45% (57) of the ruptured aneurysm domes were less than 5 mm. Advances in neuroradiological techniques have significantly improved the detection rate of small unruptured aneurysms, but whether to treat microaneurysms remains to be discussed.
Low-dose aspirin alone has previously been shown to reduce the risk of aneurysm rupture and is not associated with a risk of ICH compared with no therapy, but clopidogrel does have an increased risk of SAH and ICH [31]. Vigilance is necessary when administering dual antiplatelet therapy preoperatively and postoperatively, and TEG assays may be performed if necessary. In addition, previous studies suggested that antiplatelet drugs might change blood viscosity [32], which may affect the study of hemodynamics, which is not discussed in this study. Further studies are available later.
The number of cases collected by different stents is different. Individual data values cannot be very accurate due to blurred images or personal capabilities [33]. Due to measurement factors, there may be some errors between aneurysm size and vessel diameter and the real situation. The stents are all closed-cell designs, with no involvement of open-cell stents. There were too few cases for hemodynamic studies, and the OSI (oscillatory shear index) was not studied.
Hemodynamic parameters all decreased significantly after SAC with four different stents, and the effect of laser-cut stents on the hemodynamic decline of aneurysms appeared to be more significant than that of woven stents. Although the reduction in hemodynamic factors by LVIS appeared to be minimal with all four stents, no significant difference was observed in follow-up RROC grade among the four stents. Ischemic complications were more frequent than hemorrhagic complications during our treatment, perhaps related to the operator’s maneuvers and antiplatelet and anticoagulation therapy. Increasing DNR is perhaps an independent factor associated with complete aneurysm occlusion.
Most recurrences occur within the first year after treatment; however, there is no universally agreed-upon timetable for imaging and clinical follow-up of treated aneurysms. Some scholars believe that longer follow-up should be considered for some types of high-risk aneurysms [34]. We sincerely hope that more multicenter studies on more stents with more long-term follow-up will be performed in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethical Committee of the First Affiliated Hospital of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
XZ and RX conceived the idea of the study; YQ and SP collected and analysed the data; LJ and JZ interpreted the results; YQ wrote the paper; all authors discussed the results.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yarahmadi P, Kabiri A, Bavandipour A, Jabbour P, Yousefi O. Intra-procedural complications, success rate, and need for retreatment of endovascular treatments in anterior communicating artery aneurysms: A systematic review and meta-analysis. Neurosurg Rev (2022) 45(5):3157–70. doi:10.1007/s10143-022-01853-w
2. Luckrajh Jeshika S, Harrichandparsad R, Satyapal KS, Lazarus L. A clinical investigation of the anatomy of the proximal anterior cerebral artery and its association with anterior communicating artery aneurysm. Translational Res Anat (2022) 27:100200. doi:10.1016/J.TRIA.2022.100200
3. Yao L, Wu Q, Yuan B, Wen L, Yi R, Zhou X, et al. Correlation between vascular geometry changes and long-term outcomes after Enterprise stent deployment for intracranial aneurysms located on small arteries. World Neurosurg (2021) 153:e96–e104. doi:10.1016/J.WNEU.2021.06.038
4. Bsat S, Bsat A, Tamim H, Chanbour H, Alomari SO, Houshiemy MNE, et al. Safety of stent-assisted coiling for the treatment of wide-necked ruptured aneurysm: A systematic literature review and meta-analysis of prevalence. Interv Neurora.: J Peritherapeutic Neurora. Surg Procedures Relat Neurosci. (2020) 26:547–56. doi:10.1177/1591019920945059
5. Yang H, Sun Y, Jiang Y, Lv X, Zhao Y, Li Y, et al. Comparison of stent-assisted coiling vs coiling alone in 563 intracranial aneurysms: Safety and efficacy at a high-volume center. Neurosurgery (2015) 77:241–7. doi:10.1227/NEU.0000000000000765
6. Gao B-L, Hao WL, Ren CF, Wang JW, Liu JF. Greater hemodynamic stresses initiated the anterior communicating artery aneurysm on the vascular bifurcation apex. J Clin Neurosci (2022) 96:25–32. doi:10.1016/J.JOCN.2021.12.005
7. Xiang J, Natarajan SK, Tremmel M, Ma D, Mocco J, Hopkins LN, et al. Hemodynamic-morphologic discriminants for intracranial aneurysm rupture. Stroke (2011) 42:144–52. doi:10.1161/STROKEAHA.110.592923
8. Liu A, Peng T, Qian Z, Li Y, Jiang C, Wu Z, et al. Enterprise stent-assisted coiling for wide-necked intracranial aneurysms during ultra-early (48hours) subarachnoid hemorrhage: A single-center experience in 59 consecutive patients. J Neurora. (2015) 42:298–303. doi:10.1016/j.neurad.2014.11.005
9. Hur CW, Choi CH, Cha SH, Lee TH, Jeong HW, Lee JI. Eleven year's single center experience of endovascular treatment of anterior communicating artery aneurysms: Focused on digital subtraction angiography follow-up results. J Korean Neurosurg Soc (2015) 58(3):184–91. doi:10.3340/jkns.2015.58.3.184
10. Raymond J, Darsaut TE, Bing F, Makoyeva A, Kotowski M, Gevry G, et al. Stent-assisted coiling of bifurcation aneurysms may improve endovascular treatment: A critical evaluation in an experimental model. AJNR Am J Neuroradiol (2013) 34(3):570–6. doi:10.3174/ajnr.A3231
11. Zhang J, Wang D, Li X. Solitaire AB stent-assisted coiling embolization for the treatment of ruptured very small intracranial aneurysms. Exp Ther Med (2015) 10(6):2239–44. doi:10.3892/etm.2015.2826
12. Li W, Wang Y, Zhang Y, Wang K, Zhang Y, Tian Z, et al. Efficacy of LVIS vs. Enterprise stent for endovascular treatment of medium-sized intracranial aneurysms: A hemodynamic comparison study. Front Neurol (2019) 10:522. doi:10.3389/fneur.2019.00522
13. Lv X, Li Y, Jiang C, Yang X, Wu Z. Potential advantages and limitations of the Leo stent in endovascular treatment of complex cerebral aneurysms. Eur J Radiol (2011) 79(2):317–22. doi:10.1016/j.ejrad.2010.06.021
14. Luo J, Lv X, Jiang C, Wu Z. Preliminary use of the Leo stent in the endovascular treatment of wide-necked cerebral aneurysms. World Neurosurg (2010) 73(4):379–84. doi:10.1016/j.wneu.2010.01.019
15. Lubicz B, Kadou A, Morais R, Mine B. Leo stent for endovascular treatment of intracranial aneurysms: Very long-term results in 50 patients with 52 aneurysms and literature review. Neurora. (2017) 59(3):271–6. doi:10.1007/s00234-017-1805-3
16. Zhang J, Can A, Lai PMR, Mukundan S, Castro VM, Dligach D, et al. Vascular geometry associated with anterior communicating artery aneurysm formation. World Neurosurg (2021) 146:e1318–25. doi:10.1016/j.wneu.2020.11.160
17. Wang Y, Leng X, Zhou X, Li W, Siddiqui AH, Xiang J. Hemodynamics in a middle cerebral artery aneurysm before its growth and fatal rupture: Case study and review of the literature. World Neurosurg (2018) 119:e395–e402. doi:10.1016/j.wneu.2018.07.174
18. Wan H, Ge L, Huang L, Jiang Y, Leng X, Feng X, et al. Sidewall aneurysm geometry as a predictor of rupture risk due to associated abnormal hemodynamics. Front Neurol (2019) 10:841. doi:10.3389/fneur.2019.00841
19. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke (2001) 32(9):1998–2004. doi:10.1161/hs0901.095600
20. Spiessberger A, Vogt DR, Fandino J, Marbacher S. Formation of intracranial de novo aneurysms and recurrence after neck clipping: A systematic review and meta-analysis. J Neurosurg (2019) 132(2):456–64. doi:10.3171/2018.10.jns181281
21. Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: A systematic review on initial occlusion and reopening and retreatment rates. Stroke (2009) 40(8):e523–9. doi:10.1161/STROKEAHA.109.553099
22. Farhan M, Didarul IM, Tarik AM. A study on the computational hemodynamic and mechanical parameters for understanding intracranial aneurysms of patients with hypertension and atrial fibrillation. Inform Med Unlocked (2022) 32:101031. doi:10.1016/J.IMU.2022.101031
23. Cebral JR, Mut F, Weir J, Putman C. Quantitative characterization of the hemodynamic environment in ruptured and unruptured brain aneurysms. AJNR Am J Neuroradiol (2011) 32(1):145–51. doi:10.3174/ajnr.A2419
24. Leng X, Wan H, Li G, Jiang Y, Huang L, Siddiqui AH, et al. Hemodynamic effects of intracranial aneurysms from stent-induced straightening of parent vessels by stent-assisted coiling embolization. Interv Neuroradiol (2021) 27(2):181–90. doi:10.1177/1591019921995334
25. Kono K, Shintani A, Terada T. Hemodynamic effects of stent struts versus straightening of vessels in stent-assisted coil embolization for sidewall cerebral aneurysms. PLoS One (2014) 9(9):e108033. doi:10.1371/journal.pone.0108033
26. Jeong W, Han MH, Rhee K. The hemodynamic alterations induced by the vascular angular deformation in stent-assisted coiling of bifurcation aneurysms. Comput Biol Med (2014) 53:1–8. doi:10.1016/j.compbiomed.2014.07.006
27. Damiano RJ, Tutino VM, Paliwal N, Patel TR, Waqas M, Levy EI, et al. Aneurysm characteristics, coil packing, and post-coiling hemodynamics affect long-term treatment outcome. J Neurointerv Surg (2020) 12(7):706–13. doi:10.1136/neurintsurg-2019-015422
28. Chung BJ, Doddasomayajula R, Mut F, Detmer F, Pritz MB, Hamzei-Sichani F, et al. Angioarchitectures and hemodynamic characteristics of posterior communicating artery aneurysms and their association with rupture status. AJNR Am J Neuroradiol (2017) 38(11):2111–8. doi:10.3174/ajnr.A5358
29. Strittmatter C, Meyer L, Broocks G, Alexandrou M, Politi M, Boutchakova M, et al. Procedural outcome following stent-assisted coiling for wide-necked aneurysms using three different stent models: A single-center experience. J Clin Med (2022) 11(12):3469. doi:10.3390/jcm11123469
30. Sato K, Yoshimoto Y. Risk profile of intracranial aneurysms: Rupture rate is not constant after formation. Stroke (2011) 42(12):3376–81. doi:10.1161/STROKEAHA.111.625871
31. García-Rodríguez LA, Gaist D, Morton J, Cookson C, González-Pérez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology (2013) 81(6):566–74. doi:10.1212/WNL.0b013e31829e6ffa
32. Lee CH, Jung KH, Cho DJ, Jeong SK. Effect of warfarin versus aspirin on blood viscosity in cardioembolic stroke with atrial fibrillation: A prospective clinical trial. BMC Neurol (2019) 19(1):82. doi:10.1186/s12883-019-1315-5
33. Zhu Z, He X, Qi G, Li Y, Cong B, Liu Y. Brain tumor segmentation based on the fusion of deep semantics and edge information in multimodal MRI. Inf Fusion (2023) 91:376–87. doi:10.1016/j.inffus.2022.10.022
Keywords: AcomA aneurysms, wide-necked aneurysms, SAC, hemodynamic, imaging analysis
Citation: Qiu Y, Jiang L, Peng S, Zhu J, Zhang X and Xu R (2023) Procedural outcome following and Hemodynamic imaging analysis for anterior communicating artery wide-necked aneurysms by four different stents assisted coil embolization. Front. Phys. 11:1136093. doi: 10.3389/fphy.2023.1136093
Received: 02 January 2023; Accepted: 23 January 2023;
Published: 03 February 2023.
Edited by:
Guanqiu Qi, Buffalo State College, United StatesReviewed by:
Gang Hu, Buffalo State College, United StatesCopyright © 2023 Qiu, Jiang, Peng, Zhu, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Zhang, ZG9jdG9yenhkQGhvc3BpdGFsLmNxbXUuZWR1LmNu; Rui Xu, eHVydWkyMDMzODlAaG9zcGl0YWwuY3FtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.