
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys., 14 March 2023
Sec. Medical Physics and Imaging
Volume 11 - 2023 | https://doi.org/10.3389/fphy.2023.1003874
This article is part of the Research TopicArtificial Intelligence-based Medical Image Automatic Diagnosis and Prognosis PredictionView all 10 articles
Introduction: The accurate cerebral cortex surface reconstruction is crucial for the study of neurodegenerative diseases. Existing voxelwise segmentation-based approaches like FreeSurfer and FastSurfer are limited by the partial volume effect, meaning that reconstruction details highly rely on the resolution of the input volume. In the computer version area, the signed distance function has become an efficient method for 3D shape representation, the inherent continuous nature makes it easy to capture the fine details of the target object at an arbitrary resolution. Additionally, as one of the most valuable breakthroughs in deep learning research, attention is a powerful mechanism developed to enhance the performance of the encoder-decoder architecture.
Methods: To further improve the reconstruction accuracy of the cortical surface, we proposed ResAttn-Recon, a residual self-attention based encoder-decoder framework. In this framework, we also developed a lightweight decoder network with skip connections. Furthermore, a truncated and weighted L1 loss function are proposed to accelerate network convergence, compared to simply applying the L1 loss function.
Results: The intersection over union curve in the training process achieved a steeper slope and a higher peak (0.948 vs. 0.920) with a truncated L1 loss. Thus, the average symmetric surface distance (AD) for the inner and outer surfaces is 0.253 ± 0.051 and the average Hausdorff distance (HD) is 0.629 ± 0.186, which is lower than that of DeepCSR, whose absolute distance equals 0.283 ± 0.059 and Hausdorff distance equals 0.746 ± 0.245.
Discussion: In conclusion, the proposed residual self-attention-based framework can be a promising approach for improving the cortical surface reconstruction performance.
In neural image processing, the brain cortical surface reconstruction plays an essential role in the study of neurodegenerative diseases [1] and psychological disorders [2]. Specifically, the cortical surface reconstruction aims to extract two surface meshes from brain magnetic resonance imaging (MRI). The inner white matter surface separates the white matter and the gray matter tissues, and the outer pial surface separates the gray matter tissue and the cerebrospinal fluid [3]. Considering the highly curved and folded intrinsic folding pattern of the cortical surface [4], it is challenging to extract anatomically plausible and topologically correct cortical surfaces in practice.
To address this, traditional approaches use a series of lengthy and computationally intensive processing algorithms, with manual intervention for hyperparameter fine-turning [5–11]. For instance, the widely used and reliable [9] toolkit usually takes hours to process a MRI volume data. In recent years, several deep learning approaches have emerged to overcome this shortage, and according to the data format being processed, these approaches can be categorized as voxel-based, mesh-based, and implicit surface representation-based. Voxel-based approaches first obtain the brain white matter tissue segmentation based on 3D-CNN [12] or 3D-Unet-like [13] architecture. Then the triangular mesh of the inner surface is extracted by applying mesh tessellation to the segmentation masks, with surface mesh smoothing and topology correction [14]. The outer pial surface mesh can then be derived from inflating the white matter surface mesh [15]. Leonie et al. [15] proposed FastSurfer to accelerate the FreeSurfer pipeline by replacing the traditional white matter segmentation algorithm with a 3D-CNN network. [16] proposed the SegRecon framework for cortical surface reconstruction and segmentation. Due to the partial volume effect (PVE) [17], voxel-based approaches have inherent limitations in capturing fine details at high resolution. Mesh-based approaches are mainly implemented by deforming the initial surface mesh to the target surface mesh, with a geometric deep-learning model. For instance, [18] proposed PialNN to reconstruct the pial surface from the white matter surface handled by the FreeSurfer pipeline. [19] proposed Voxel2Mesh to deform predefined sphere template meshes to cortical surfaces. [20] Proposed Vox2Cortex that leverages convolutional and graph convolutional neural networks to deform the template mesh to densely folded target cortical surface. Despite fast processing, theoretical guarantees are to be further developed to prevent self-intersections of the surface mesh. Implicit surface representation-based methods reformulate cortical surface reconstruction as the prediction of the implicit surface representation [21]. Typically, [3] proposed the DeepCSR network to learn an implicit surface function in a continuous coordinate system, with topology correction algorithm to ensure the geometric accuracy of the target surface.
Given brain MRI volume, existing deep learning approaches spend less time reconstructing cortical surface compared to traditional pipeline, with high reliability. Most of these approaches require voxelwise or vertexwise features extracted from the input MRI volume, however, none of them considered the long range feature dependencies, which plays an important role in model performance improvement. For instance, DeepCSR [3] directly concatenates the local and global features from the encoder feature maps, combined with the location coordinates of the query point as the input of the decoder network, PialNN [18] combined the norm and the location coordinate of the initial mesh vertex with the volumetric features extracted from local convolution, to predict the deformation displacement in the inflating process. Both approaches ignored the relationship between query points or mesh vertices. In this work, to efficiently model the long range feature dependencies in cortical surface reconstruction, the concept of the self-attention mechanism is introduced from neural language processing [22–24] and computer version [25–27] area. For pioneer works that apply self-attention to vision tasks [28], proposed Vision Transformer (ViT) for image recognition [29], proposed Tokens-To-Token Vision Transformer (T2T-ViT) to improve classification accuracy [30], proposed Swin Transformer, a general framework for image classification and segmentation, all the above works are discussed around 2D images. In this work, firstly, the input MRI volume is registered to a standard brain space, such as MNI105. Secondly, after the 3D Convolution block, the residual connected multi-head self-attention block and global flatten block, multi-scale feature maps and global feature vector are prepared to obtain volumetric features of sampling points at any given resolution. Then combined with location coordinates in standard space, the signed distance values of sampling points toward four cortical surfaces are predicted, Thirdly, after topology correction and iso-surface extraction operation, the inner and outer surface of the left and right hemispheres are reconstructed in parallel.
In this paper, we proposed ResAttn-Recon, a novel implicit surface representation approach based framework for inner and outer cortical surface reconstruction. In this work, we propose employing the concept of the self-attention mechanism and residual connection trick to the 3D convolutional neural network (3D CNN) encoder, 1 × 1 convolution is embedded into a multi-head self-attention block to fit the 3D feature map input. The proposed framework is able to reconstruct the cortical surface at an arbitrary resolution and benefit from the theoretical support of the implicit surface representation approach. The experimental performance has been substantially improved compared to the DeepCSR and the simple encoder-decoder framework without the attention block. The main contributions of this paper are as follows.
1) To the best of our knowledge, this is the first exploration in employing the residual self-attention mechanism in 3D cortical surface reconstruction.
2) A Commit2 from Review4 with skip connections is developed as an improvement over the DeepCSR decoder network, to simplify the network structure without losing performance.
3) The prior constraints are imposed on the network training with the proposed truncated L1 loss and Gaussian decay weighted L1 loss, as a new strategy for model performance improvement.
The rest of this paper is organized as follows. Section 2 introduces the basic theories and the proposed framework, as well as the dataset enrolled in this work, In Section 3, the details of the experimental evaluation and analysis are given. Section 4 and Section 5 provide a discussion and conclude this paper.
In this section, we introduced ResAttn-Recon, a residual self-attention-based cortical surface reconstruction network. Simultaneously, the lightweight decoder networks and the loss function with prior constrains are also explored to improve the reconstruction performance. As shown in Figure 1, the proposed framework consists of four main parts: 1) data preprocessing, including data acquisition from FreeSurfer toolkit and MRI volume registration; 2) feature extraction; 3) implicit surface representation performed with the proposed ResAttn-Recon network; and 4) post-processing to extract the target inner and outer cortical surfaces from the predicted signed distance function representation. The residual self-attention block is embedded in the encoder network following the intermediate feature map.
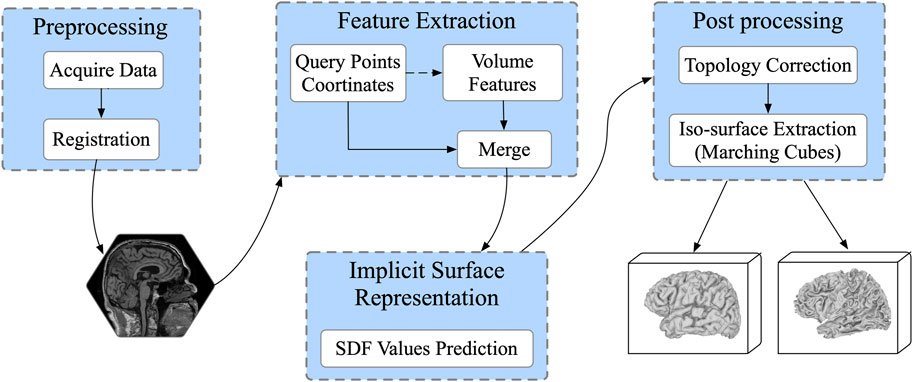
FIGURE 1. The workflow for cortical surface reconstruction. Input the 3D MRI volume, given enough sampling points, it predicts 4 mesh surfaces of arbitrary resolution in parallel.
In this paper, we used the publicly available dataset from Alzheimer’s Disease Neuroimaging Initiative (ADNI) [31], MRI scans of 560 T1 original images are enrolled in this work, 470 images for training, 30 images for validation and the remaining 60 images for testing. Ground-truth of inner and outer surfaces from the left and right hemispheres are extracted by the FreeSurfer pipeline. Images are normalized to the size of 182 × 218 × 182, with voxel spacing to [1, 1, 1]. To unify the coordinate systems of the input MRI scans, affine registration is first performed to the MNI105 brain template [32].
The signed distance function (SDF) is a continuous function to represent the surface distribution, and has been widely employed in 3D shape representation.
The SDF function can be defined as follows:
Here,
With the SDF values of given spatial points, the target surface can be expressed as a set consisting of all points satisfying the following:
Then, after Gaussian smoothing and topology correction processing, the target surface is extracted with a zero iso-surface extraction algorithm, such as marching cubes [33].
In this work, the continuous SDF in Ω space is approximated by the deep learning model. Given query point, the well-trained network predicts its SDF value to the target surface directly. This provides theoretical support for reconstructing surfaces of arbitrary resolutions. In detail, the approximator is implemented by a decoder network parameterized by θ, which is further described below.
The network architecture is illustrated in Figure 2, and the raw brain MRI need to be firstly registered to MNI105 space before being sent to the network. The feature extraction encoder module consists of three subblocks, the 3D Convolution (Conv3D) block, the Residual Self-Attention block, and the Global Flatten block. The Conv3D block consists of five Conv3D layers, each of which is followed by a Rectified Linear Units (ReLU) activation, with the 3D max pooling operation before the fourth convolution. The number of convolution output channels is sequentially increased to
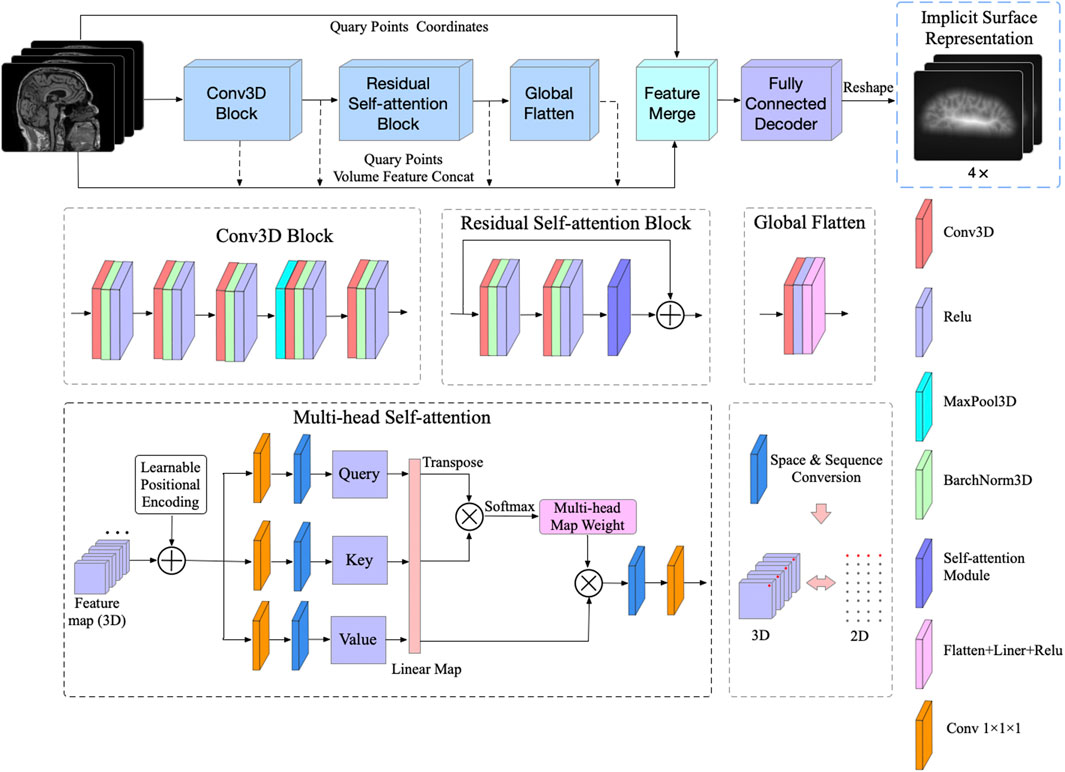
FIGURE 2. The proposed ResAttn-recon architecture for cortical surface reconstruction. The concept of the multi-head self-attention mechanism was introduced to our residual self-attention block.
To better aggregate the feature representation of the sampled points, the widely used self attention mechanism is introduced to our proposed encoder-decoder network architecture, where the detail operation can be described as follows:
Where
In this work, the multi-head self-attention mechanism [22] is applied to improve the expression ability of the attention module. For this 3D reconstruction work, as illustrated in Figure 2, the residual connection in the Residual Self-attention Block indicates that this block does not change the dimension of the input feature map, which equals
Here,
It is worth noting that to fully take advantage of the spatial sequence information extracted by sampling, the absolute positional coding strategy is applied in this work, where the positional coding is directly added to the input of the self attention module. Specifically, one raw MRI input is firstly normalized and reshaped to
For the residual self-attention block, before the self-attention module, two 3D convolution operations are performed, each followed by 3D batch normalization and ReLU activation. Notably, we added the residual connection between the Conv3D block output and the self-attention module output to help improve the learning.
To further simplify the decoder network without losing performance, the feed-forward network is composed of six fully connected layers, and feature vectors extracted from feature maps and corresponding coordinates of sampling points are concatenated as the input of the decoder network. Since position coordinates are critical for 3D shape representation [21], we introduced skip-connection to maintain the proportion of location information. As shown in Figure 3, the input feature vector is concatenated with the intermediate output of the following four fully connected layers. The output vector of the decoder network representing the SDF values of four corresponding cortical surfaces for each voxel is a vector with length 4.
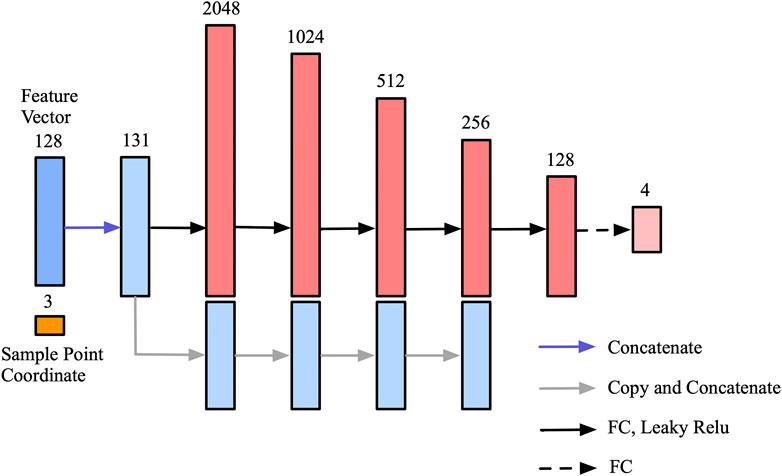
FIGURE 3. Decoder network for SDF values prediction. The skip-connection mechanism is introduced to make full use of the location information of the sampling points.
In 3D object reconstruction, L1 loss is the most frequently used loss function, and the basic form of L1 loss for one cortical surface can be written as follows, where
And for parallel training with four cortical surfaces, the formula becomes:
For cortical surface representation with signed distance function values, sampling points close to the cortical surface are critical for reconstruction details, while sampling points away from the cortical surface contribute less to the reconstruction process. To help the training network capture more detailed information around the surface, the truncate interval
Furthermore, we also propose exploring the potential of the L1 loss function with a Gaussian decay coefficient. The Gaussian function in this case is of the following form:
where A is the amplitude coefficient. Due to the smooth decay characteristic of the Gaussian function, as the distance between the sampling point and the ground-truth surface increases, the loss weight of the corresponding sampling point decreases. We hope this design can perform dynamic loss constraint during network training, and help the network to better converge to the optimal solution. The Gaussian decay L1 loss can be described as follows:
The amplitude coefficient A,
For the cortical surface extraction pipeline, firstly, the input MRI volume is registered into the MNI105 space; secondly, given arbitrary reconstruction resolution, i.e.,
To prevent grid self-intersection, and to ensure that the predicted signed distance function values is homeomorphic to a sphere, in this work, we apply a topology propagation algorithm using a fast marching technique proposed by bazin. et.al [35], that enforces the network prediction result to a desired topology.
Algorithm 1:. Marching cubes pseudocode
Data: the SDF value of the
Result: Extracted surface mesh vertices coordinates
1 Mvertices←[];
2
3 While
4 if
5
6
7
8 else
9 continue
10 end
11 end
Then, the marching cubes algorithm proposed by [33] is further employed to cortical surface extraction, followed by Laplacian smoothing. The core idea of the marching cubes algorithm can be summarized as Algorithm 1 For vertices
where
To verify the effectiveness of our method, we first designed a series of ablation studies to explore the importance of prior constraints and the skip connections mechanism, after which we evaluated the performance of three loss function. Finally, the precision analysis is performed compared with DeepCSR for challenging pial surface reconstruction.
As shown in Table 1, the ablation experiment is conducted to measure the importance of the proposed components.
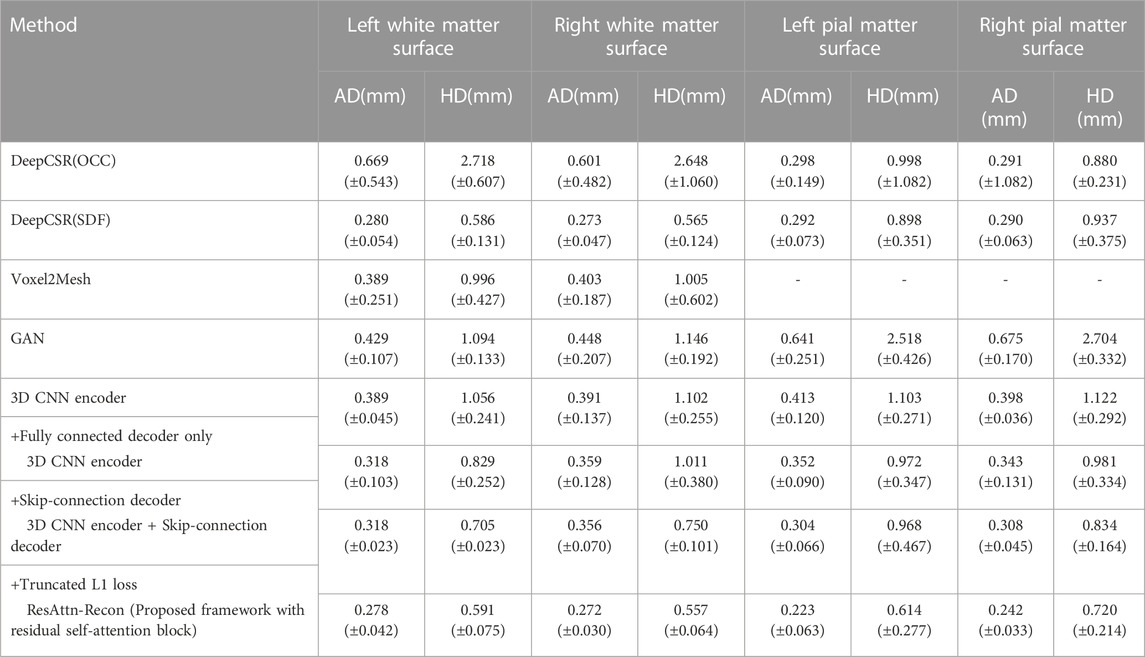
TABLE 1. Results of comparison analysis with DeepCSR and the ablation study on the proposed ResAttn-Recon framework for cortical surface reconstruction. Including the white matter surface and the pial matter surface, where AD = Average symmetric surface distance, HD = Hausdorff distance.
The average absolute distance (AD) and the Hausdorff distance (HD) [36,37] are employed as the surface evaluation metrics, and the lower the values, the better the reconstruction results.
In this experiment we explored the expressive capability of fully connected layers in the decoder network architecture with the skip connections mechanism. As shown in Table 1, row 4 records the baseline encoder-decoder network where the decoder network is the fully connected layers without skip connections mechanism, and the feature vectors and location coordinates are simply concatenated from the decoder input. It was found that the decoder with skip-connection (row 4) shows lower AD and HD indicators than that without skip connections (row 5). The results indicate that the location information of the sampling points make a considerable contribution to reconstruction work. Nevertheless, there is still much room for improvement in the reconstruction indicators, compared with the DeepCSR framework (row 3).
In order to further validate the performance of the proposed lightweight decoder network, different indicators are used to compare with the decoder network in DeepCSR framework. As shown in Table 2, the proposed decoder network outperforms DeepCSR’s decoder network in the number of parameters (2636527 vs. 3549188), parameters size (10.05 MB vs. 13.53 MB), and the average inference speed (0.47 ± 0.02 vs. 6.34 ± 0.02) for testing dataset. The average L1 Loss (computational cost shown in formula 5) of the proposed framework is 1,291.89 ± 10.50, lower than that of the DeepCSR framework (1,452.85 ± 28.98). By the way, the computational cost looks larger, because the distance space is not normalized to the [-0.5,0.5] interval.
The performance of the proposed framework between employing prior constraints (trained with a truncated L1 loss or Gaussian decay L1 loss) and without prior constraints (trained with a basic L1 loss) are compared, as shown in row five and row six of Table 1, Additionally, the reconstruction results improved with the help of prior constraints imposed on the loss function from the perspective of AD and HD indicators. For fairness, the training process of the DeepCSR-SDF network also employs the L1 loss and its variants, and take the optimal result for performance comparison and analysis.
For further comparison and analysis, besides the DeepCSR framework, Voxel2Mesh and GAN [38] were added to the experiments. Since the Voxel2Mesh framework cannot reconstruct the inner and outer surfaces in parallel, analysis of white matter surface reconstruction performance is considered for convenience.
For GAN model, the 3D-UNet is employed as the backbone of the generator network. The generator predicts the SDF representation (output channel equals 4) relevant to four cortical surfaces. The discriminator takes the MRI volume and the ground truth/predicted SDF representation as input, and discriminate true or false label, where cross entropy loss is used as the discriminator loss function. As shown in Table 3, the AD and HD values derived from Voxel2Mesh and GAN model are significantly higher than that from the proposed framework. The GAN model shows the worst performance for AD and HD indicators.

TABLE 3. The maximum IOU values with different loss functions: the L1 loss, Gaussian decay L1 loss and Truncate L1 loss functions.
From another persperctive, according to the data format being processed, the GAN model reconstruct the cortical surface based on MRI voxels, 1) Due to the partial volume effect, the reconstruction accuracy is limited by the resolution of MRI volume, 2) The idea of generative adversarial network is to infer whether each voxel is inside or outside the surface of the cerebral cortex by training the generator, and then give a true or false judgment through the decision network. Each voxel has only binary information (inside or outside), while ignoring the distance information to the surface. Mesh based Voxel2Mesh model deform predefined sphere template meshes to cortical surfaces. 1) Despite fast processing, theoretical guarantees are lack to prevent self-intersections of the surface mesh. 2) Besides, a Voxel2Mesh model only able to reconstruct a single surface once (outer or inner surface). The proposed framework reconstruct the surface based on sampling points, and therefore has the theoretical support to reconstruct cortical surfaces of arbitrary resolution.
As illustrated in Figure 4, the reconstruction of the pial surface is more challenging than that of the white matter surface. The reconstructed pial surface of the left hemisphere is shown in the first row. The proposed ResAttn-Recon framework achieves the best reconstruction result, and the reconstruction result with DeepCSR has obvious surface bumps in the red rectangle area. The encoder-decoder architecture without the attention module (no attention version) has several reconstruction defects. The upper rectangular frame area shows severe reconstruction noise near the surface, and the lower rectangular frame area has multiple grid self-intersections. For the white matter reconstruction surface of the left hemisphere shown in the second row, there is no major visual difference between the three network structures in this case.

FIGURE 4. Visual assessment of cerebral cortical reconstruction results. The first row corresponds to the pial matter surface of the left hemisphere, and the second row corresponds to the white surface of the left hemisphere.
It is also worth noting that the ground-truth surfaces handled by FreeSurfer look rougher than the actual physiological surface. To address this, both the proposed framework and the DeepCSR framework have moderately smoothed the predicted surface through a post-processing algorithm. To further visualize the robustness of the proposed model, another eight examples of cortical surfaces (prediction and corresponding ground-truth) were illustrated in Figure 5.
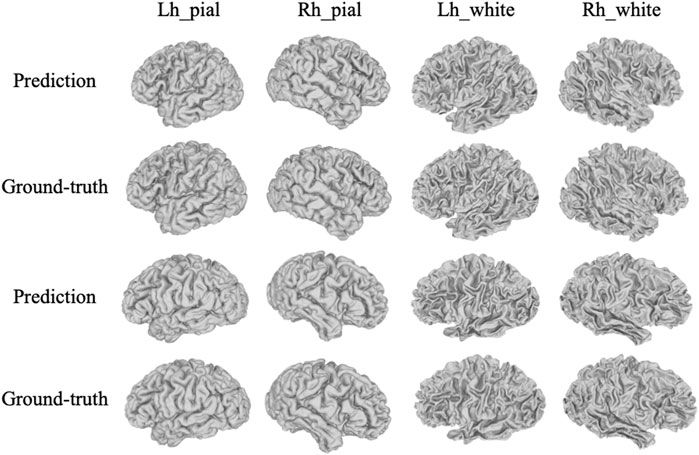
FIGURE 5. More visualization examples of the prediction results by the proposed method with corresponding ground-truth, including pial and white matter surfaces of left and right hemispheres.
As seen in Figure 6, we compared the convergence curve of the intersection over union (IOU) based on the three loss functions mentioned above. By the way, for IOU, the SDF representation of the cortical surface could be further converted to the binarized SDF mask, 0 for inner surface points and one for outer surface points, then the binarized SDF mask based IOU could be further calculated between the ground-truth SDF and the predicted SDF. It is clear that the IOU curve based on the truncated L1 loss is distributed over the other two curves throughout the training process. For the other two curves, before 10 k training steps, the slope of the IOU curve based on the basic L1 loss is larger than that based on the Gaussian decay L1 loss, after which the IOU curve of the latter surpassed that of the former.
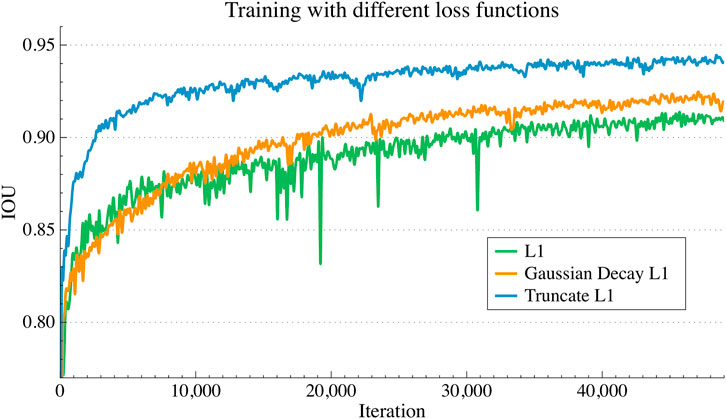
FIGURE 6. IOU convergence curves during training process with different loss functions. L1 loss, the truncated L1 loss and the Gaussian decay L1 loss were compared for analysis.
As shown in Table 3, the maximum IOU value of the trained with truncated L1 loss is 0.948 after convergence, followed by the loss function based on the Gaussian decay coefficient with a maximum IOU equals 0.935. The maximum IOU value based on the basic L1 loss is 0.92, which is considerably lower than the former. Also, thecorresponding convergence trend of the training curve can be confirmedfrom Figure 6.
The experimental results show that training with the truncated L1 loss and Gaussian decay L1 loss outperform the basic L1 loss, and among them, the truncated L1 is more effective. For the Gaussian decay L1 loss, the loss weighting coefficients tend to zero for sampling points away from the ground-truth surface, which leads to these points making less of a contribution for network training.
To measure the precision of the reconstructed cortical surface, the average absolute distance (AD) is employed in millimeters (mm). Mesh vertices with an AD greater than 1 mm, 2mm and 5 mm as a percentage of the total number of mesh vertices are calculated.
Due to the highly folded and curved geometry, pial surface reconstruction is much more challenging than white surface reconstruction. Moreover, 60 ADNI test datasets with pial surface ground-truth are enrolled for precision analysis. As shown in Table 4, compared with DeepCSR, the AD percentage greater than 1 mm (3.616 vs 5.582 for left pial), 2 mm (1.467 vs 2.432 for left pial) and 5 mm (0.131 vs 0.249 for the left pial) are lower than those of the DeepCSR framework, indicating that the proposed method has a good robustness of the overall reconstruction.
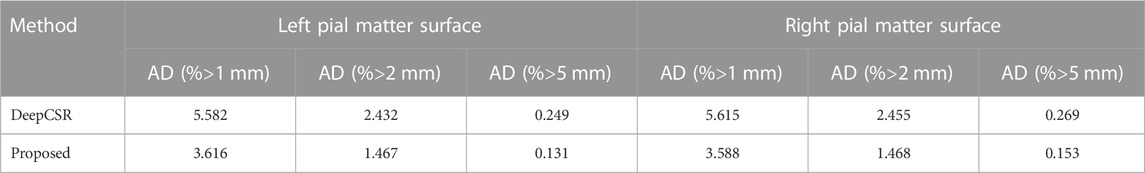
TABLE 4. Precision analysis for left pial matter surface and right pial matter surface: with range greater than 1mm, 2mm and 5 mm.
We successfully developed a residual self-attention-based architecture to reconstruct the inner and outer surface of the left and right hemispheres in parallel. In the current work of cortical surface reconstruction, including the voxel-based, mesh-based, and the implicit surfaces representation-based approaches, none of them considered the importance of capturing long range feature dependencies, during voxelwise or mesh vertex wise or sampling pointwise feature extraction, which is essential for 3D geometric surfaces representation is not considered. Given enough sampling points, the implicit surface representation-based approach has the theoretical support to reconstruct cortical surfaces of arbitrary resolution, our study is developed based on this direction and compared with the typical DeepCSR architecture. To adapt the residual connected multi-head self-attention to the 3D shape representation task, the
Ablation studies are undertaken to evaluate the impact of the residual self-attention module, the skip connections trick in decoder network, and the loss function with prior constraints. Experiments show that these components are effective in improving the reconstruction performance. We proposed the truncated L1 loss and the Gaussian decay weighted L1 loss to explore the effect of loss function on model expression potential. The truncated L1 loss achieves optimal results compared to simply applying the basic L1 loss function, and the training process achieved a higher IOU value (0.948 vs. 0.920) with the proposed truncated L1 loss. The average symmetric surface distance (AD) for the inner and outer surfaces is 0.253 ± 0.051, the average Hausdorff distance (HD) is 0.629 ± 0.186, which is lower than that of DeepCSR, whose AD equals 0.283 ± 0.059, and HD equals 0.746 ± 0.245. In addition, to measure the robustness of the overall reconstruction process, the AD greater than 1 mm, 2 mm and 5 mm as a percentage of the total number of mesh vertices are calculated, and we evaluated the challenging pial cortical surface result compared with DeepCSR, in 1 mm (3.616 vs. 5.582) and 2 mm (1.467 vs. 2.432) and 5 mm (0.131 vs. 0.249). From the perspective of visual analysis, the proposed ResAttn-Recon outperforms DeepCSR and the simple encoder-decoder architecture without the attention module. From Figure 5, it was found that the SDFs of pial matter surfaces are harder to approximate than the white matter surfaces during network training in parallel. Our proposed framework can better capture the surface details in a limited data size. Thus, the proposed residual self-attention-based framework can be a promising approach for improving the cortical surface reconstruction performance.
Our study has one main limitation: due to the lengthy processing time by the FreeSurfer pipeline, only a total of 560 T1 weighted images are enrolled in our dataset. In the future, more MRI volumes will be retrieved to expand the training dataset. Furthermore, it is desirable to enroll a larger pool of multicenter data to demonstrate the clinical value of this framework. Since topology correction algorithm during post processing usually takes a few minutes to enforce the prediction result to a desired topology, we will also pay more attention to the optimization of the post processing algorithm to further shorten the time of cortical surface reconstruction pipeline.
In this paper, we proposed ResAttn-Recon for challenging cerebral cortical surface reconstruction tasks. Firstly, we explored the concept of residual self-attention to the encoder-decoder architecture. Secondly, a lightweight decoder network with skip connection is developed to simplify the network without losing performance. In addition, experiments show that the proposed truncated L1 loss and Gaussian decay weighted L1 loss function contribute to the network training and performance improvement. The superior performance is achieved by the proposed framework compared with DeepCSR and a simple encoder-decoder framework without an attention block. The proposed framework can be a promising approach for improving the cortical surface reconstruction performance. We hope our work can inspire insights and show new directions toward cortical surface reconstruction study.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of all of the participating institutions. Informed written consent was obtained from all participants at each site. The patients/participants provided their written informed consent to participate in this study.
MA performed all the experiments and wrote the manuscript, ZL analyzed and optimized the proposed algorithm. JC collected and cleaned the raw data. YC and KT conceived and supervised the project. JW, CW, and KZ reviewed the content of the manuscript and revised the grammar, they also provided valuable suggestions for modification of figures and tables. All authors contributed to the critical reading and writing of the manuscript.
The authors express special thanks to the Alzheimer’s Disease Neuroimaging Initiative for available data providing, and all the medical students for assisting with the collection of the data.
Author KZ was employed by the company Inspur Electronic Information Industry Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphy.2023.1003874/full#supplementary-material
1. Du A-T, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, et al. ‘Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia’. Brain (2006) 130(4):1159–66. doi:10.1093/brain/awm016
2. Rimol LM, Nesvag R, Hagler DJ, Bergmann O, Fennema-Notestine C, Hartberg CB, et al. ‘Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder’. Biol Psychiatry (2012) 71(6):552–60. doi:10.1016/j.biopsych.2011.11.026
3. Cruz RS, Lebrat L, Bourgeat P, Fookes C, Fripp J, Salvado O. DeepCSR: A 3D deep learning approach for cortical surface reconstruction, In: 2021 IEEE Winter Conference on Applications of Computer Vision (WACV); January 5-9, 2021; Waikoloa, HI, USA (2021). 806–15. doi:10.1109/WACV48630.2021.00085
4. Duan D, Xia S, Rekik I, Meng Y, Wu Z, Wang L, et al. ‘Exploring folding patterns of infant cerebral cortex based on multi-view curvature features: Methods and applications’. NeuroImage (2019) 185:575–92. doi:10.1016/j.neuroimage.2018.08.041
5. MacDonald D, Kabani N, Avis D, Evans AC. ‘Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI’. NeuroImage (2000) 12(3):340–56. doi:10.1006/nimg.1999.0534
6. Kriegeskorte N, Goebel R. ‘An efficient algorithm for topologically correct segmentation of the cortical sheet in anatomical MR volumes’. NeuroImage (2001) 14(2):329–46. doi:10.1006/nimg.2001.0831
7. Shattuck DW, Leahy RM. ‘BrainSuite: An automated cortical surface identification tool’. Med Image Anal (2002) 6:129. doi:10.1016/s1361-8415(02)00054-3
8. Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, et al. ‘Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification’. NeuroImage (2005) 27(1):210–21. doi:10.1016/j.neuroimage.2005.03.036
10. Dahnke R, Yotter RA, Gaser C. ‘Cortical thickness and central surface estimation’. NeuroImage (2013) 65:336–48. doi:10.1016/j.neuroimage.2012.09.050
11. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. Neuroimage (2012) 9:197. doi:10.1006/nimg.1998.0395
12. Rao C, Liu Y. ‘Three-dimensional convolutional neural network (3D-CNN) for heterogeneous material homogenization’. Comput Mater Sci (2020) 184:109850. doi:10.1016/j.commatsci.2020.109850
13. Çiçek Ö, Abdulkadir A, Lienkamp SS, Brox T, Ronneberger O. (2016). 3d u-net: learning dense volumetric segmentation from sparse annotation. In International conference on medical image computing and computer-assisted intervention (Springer), 424–432.
14. Segonne F, Pacheco J, Fischl B. ‘Geometrically accurate topology-correction of cortical surfaces using nonseparating loops’. IEEE Trans Med Imaging (2007) 26(4):518–29. doi:10.1109/tmi.2006.887364
15. Henschel L, Conjeti S, Estrada S, Diers K, Fischl B, Reuter M. ‘FastSurfer - a fast and accurate deep learning based neuroimaging pipeline’. NeuroImage (2020) 219:117012. doi:10.1016/j.neuroimage.2020.117012
16. Gopinath K, Desrosiers C, Lombaert H. ‘SegRecon: Learning joint brain surface reconstruction and segmentation from images’. In: Medical image computing and computer assisted intervention – miccai 2021. Cham: Springer International Publishing (2021). p. 650–9.
17. Gonzalezballester M. ‘Estimation of the partial volume effect in MRI’. Med Image Anal (2002) 6(4):389–405. doi:10.1016/s1361-8415(02)00061-0
18. Ma Q, Robison EC, Kainz B, Rueckert D, Alansary A. (2021). Pialnn: A fast deep learning framework for cortical pial surfacereconstruction. In International Workshop on Machine Learning in ClinicalNeuroimaging (Springer), 73–81.
19. Wickramasinghe U, Remmelli E, Knott G, Fua P. (2020). Voxel2mesh: 3d mesh model generation from volumetric data. InInternational Conference on Medical Image Computing and Computer-Assisted Intervention (Springer), 299–308.
20. Bongratz F, Rickman AM, Polserl S, Wachinger C. Vox2Cortex: Fast explicit reconstruction of cortical surfaces from 3D MRI scans with geometric deep neural networks. In: 2022 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR); June 18 2022 to June 24 2022; New Orleans, LA, USA (2022). 20741–51. doi:10.1109/CVPR52688.2022.02011
21. Park JJ, Florence P, Straub J, Newcombe R, Lovergrove S. (2019). Deepsdf: Learning continuous signed distance functions for shape representation. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition, 165–174.
22. Vaswani A, Shazeer N, Parmar N, Uszkoreit J, Jones L, Gomez AN, et al. (2017) Attention is all you need. Advances in neural information processing systems, 30.
23. Shi H, Gao J, Ren X, Xu H, Liang X, Li Z, et al. (2021) Sparsebert: Rethinking the importance analysis in self-attention. In International Conference on Machine Learning (PMLR), 9547–9557.
24. Shen T, Zhou T, Long G, Jiang J, Pan S, Zhang C. (2018) DiSAN: Directional self-attention network for RNN/CNN-free language understanding, arXiv.
25. Ramachandran P, Parmar N, Vaswani A, Bello I, Levskaya A, Shlens J, et al. (2019) Stand-alone self-attention in vision models. Advances in Neural Information Processing Systems, 32.
26. Zhao H, Jia J, Koltun V. Exploring self-attention for image recognition. In: 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR); June 13 2020 to June 19 2020; Seattle, WA, USA (2020). 10073–82. doi:10.1109/CVPR42600.2020.01009
27. Vaswani A, Ramachandran P, Srinivas A, Parmar N, Hetchman B, Shlens J. Scaling local self-attention for parameter efficient visual backbones. In: 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR); June 20 2021 to June 25 2021; Nashville, TN, USA (2021). p. 12889–99. doi:10.1109/CVPR46437.2021.01270
28. Dosovitskiy A, Beyer L, Kolesnikov A, Weissenborn D, Zhai X, Unterthiner T, et al. (2020) An image is worth 16x16 words: Transformers for image recognition at scale. arXiv preprint arXiv:2010.11929.
29. Yuan L, Chen Y, Wang Y, Yu W, Shi Y, Jiang Z, et al. Tokens-to-Token ViT: Training vision transformers from scratch on ImageNet. In: 2021 IEEE/CVF International Conference on Computer Vision (ICCV); Oct. 11 2021 to Oct. 17 2021; Montreal, QC, Canada (2021). p. 538–47. doi:10.1109/ICCV48922.2021.00060
30. Liu Z, Lin Y, Cao Y, Hu H, Wei Y, Zhang Z, et al. (2021) Swin transformer: Hierarchical vision transformer using shifted windows. In Proceedings of the IEEE/CVF international conference on computer vision, 10012–10022.
31. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. ‘Alzheimer’s disease neuroimaging initiative (ADNI): Clinical characterization’. Neurology (2010) 74(3):201–9. doi:10.1212/wnl.0b013e3181cb3e25
32. Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. ‘A probabilistic atlas of the human brain: Theory and rationale for its development’. Neuroimage (1995) 2(2):89–101. doi:10.1006/nimg.1995.1012
34. Gehring J, Auli M, Grangier D, Yarats D, Dauphin YN. (2017) ‘Convolutional sequence to sequence learning’. arXiv. doi:10.48550/arXiv.1705.03122
35. Bazin P-L, Pham DL. ‘Topology correction of segmented medical images using a fast marching algorithm’. Comp Methods Programs Biomed (2007) 88(2):182–90. doi:10.1016/j.cmpb.2007.08.006
36. Tosun D, Rettmann ME, Naiman DQ, Resnick SM, Kraut MA, Prince JL. ‘Cortical reconstruction using implicit surface evolution: Accuracy and precision analysis’. NeuroImage (2006) 29(3):838–52. doi:10.1016/j.neuroimage.2005.08.061
37. Taha AA, Hanbury A. ‘Metrics for evaluating 3D medical image segmentation: Analysis, selection, and tool’. BMC Med Imaging (2015) 15(1):29. doi:10.1186/s12880-015-0068-x
38. Ning Z, Zhang Y, Pan Y, Zhong T, Liu M, Shen D. (2020). “Ldgan: Longitudinal-diagnostic generative adversarial network for disease progression prediction with missing structural MRI,” in M Liu. (eds) Machine learning in medical imaging. Cham: Springer International Publishing(Lecture Notes in Computer Science), 170–9. doi:10.1007/978-3-030-59861-7_18
Keywords: cortical surface reconstruction, residual self-attention mechanism, signed distance function, deep learning, MRI volume
Citation: An M, Chen J, Cao Y, Tao K, Wang J, Wang C, Zhao K and Liu Z (2023) ResAttn-recon: Residual self-attention based cortical surface reconstruction. Front. Phys. 11:1003874. doi: 10.3389/fphy.2023.1003874
Received: 01 August 2022; Accepted: 22 February 2023;
Published: 14 March 2023.
Edited by:
Yukun Lai, Cardiff University, United KingdomReviewed by:
Haoran Dou, University of Leeds, United KingdomCopyright © 2023 An, Chen, Cao, Tao, Wang, Wang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun Wang, Y2h1bndhbmdAc2R1LmVkdS5jbg==; Kun Zhao, emhhb2t1bmJqQGluc3B1ci5jb20=; Zhi Liu, bGl1emhpQHNkdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.