
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Phys., 08 October 2021
Sec. Optics and Photonics
Volume 9 - 2021 | https://doi.org/10.3389/fphy.2021.737866
This article is part of the Research TopicLabel Free Polarization Resolved Optical Microscopy for Biomedical ApplicationsView all 5 articles
 Benjamin T. Hogan1
Benjamin T. Hogan1 Volodimyr A. Ushenko2
Volodimyr A. Ushenko2 Anastasia-Vira Syvokorovskaya3
Anastasia-Vira Syvokorovskaya3 Alexander V. Dubolazov2
Alexander V. Dubolazov2 Oleg Ya. Vanchulyak3
Oleg Ya. Vanchulyak3 Alexander G. Ushenko2,4
Alexander G. Ushenko2,4 Yuriy A. Ushenko2
Yuriy A. Ushenko2 Mykhailo P. Gorsky3
Mykhailo P. Gorsky3 Yuriy Tomka2
Yuriy Tomka2 Sergey L. Kuznetsov5
Sergey L. Kuznetsov5 Alexander Bykov1
Alexander Bykov1 Igor Meglinski1,5,6,7,8,9*
Igor Meglinski1,5,6,7,8,9*Diseases affecting myocardial tissues are currently a leading cause of death in developed nations. Fast and reliable techniques for analysing and understanding how tissues are affected by disease and respond to treatment are fundamental to combating the effects of heart disease. A 3D Mueller matrix method that reconstructs the linear and circular birefringence and dichroism parameters has been developed to image the biological structures in myocardial tissues. The required optical data is gathered using a Stokes polarimeter and then processed mathematically to recover the individual optical anisotropy parameters, expanding on existing 2D Mueller matrix implementations by combining with a digital holography approach. Changes in the different optical anisotropy parameters are rationalised with reference to the general tissue structure, such that the structures can be identified from the anisotropy distributions. The first to fourth order statistical moments characterising the distribution of the parameters of the optical anisotropy of the polycrystalline structure of the partially depolarising layer of tissues in different phase sections of their volumes are investigated and analysed. The third and fourth order statistical moments are found to be the most sensitive to changes in the phase and amplitude anisotropy. The possibility of forensic medical differentiation of death in cases of acute coronary insufficiency (ACI) and coronary heart disease (CHD) is considered as a diagnostic application. The optimal phase plane (
Myocardial tissues form the muscles in the heart and are hence critical to human life [1–3]; diseases affecting myocardial tissues are currently the leading cause of death in developed nations [4]. Myocardial tissue sections provide an excellent platform for wider understanding of cardiac function [5], and hence imaging methods that can be applied to myocardial tissues are of significant interest. As exemplified by the recent rapid spread of COVID-19, new threats with potential to affect the heart are continually emerging [6]. To keep up, fast and reliable techniques for analysing and understanding how tissues are affected by disease, and respond to treatment, are fundamental necessities. 3D imaging of structures within tissues can provide such insights. Computed tomography scanning, magnetic resonance imaging, and X-rays are widely used. However, 3D imaging techniques can be limited by relatively low sample throughput, and high cost [7–9]. Furthermore, as medical diagnostics move away from human analysis to more automated, AI-driven approaches, different techniques for looking at tissues become more viable. In particular, statistical analysis of tissue properties can give rapid results with high degrees of accuracy [10, 11]. One possibility of emerging interest is to look at the optical anisotropy of the tissue, from which one can then infer the tissue structure and other properties [12–15].
Optical anisotropy is a result of a material interacting differently with different polarisations of incident light, such that the different polarisations are absorbed, transmitted, reflected and refracted with different intensities [16]. There are four optical anisotropy properties that can be considered: linear birefringence, linear dichroism, circular birefringence, and circular dichroism. The birefringence and dichroism of a material can be determined by measuring changes in the polarisation of light passing through the material, by so-called polarimetry measurements [16–19]. Polarimetry is a relatively easy technique to implement - at its most simple, requiring only: a light source (laser), polarising filters (and quarter wave plate for circular birefringence and dichroism measurements), and a detector [20, 21]. To achieve greater sensitivity of measurements, one can employ interferometric techniques.
Looking at the optical anisotropy of myocardial tissues is promising for two reasons: firstly, the tissues contain spatially ordered protein fibrils which should demonstrate clear linear anisotropy; secondly, the tissues are partially depolarising. After polarimetric measurements of the four anisotropy properties, it is necessary to correlate the experimental data with the physical sample under investigation. Mueller Matrix Polarimetric (MMP) diagnostics are the tool of choice for this purpose, with many distinct directions being considered, including: the investigation of scattering matrices [1, 22–25]; Mueller matrix polarimetry [17, 26–29]; polar decomposition of Mueller matrices [30, 31]; and two-dimensional Mueller matrix mapping [18, 19, 32, 33]. A Mueller matrix is a 4 × 4 matrix representing the effect of a specific optical element on the polarisation of light. The multiplication of the initial Stokes vector of light passing through the element by the Mueller matrix of the element gives the Stokes vector of the output light. MMP methods and tools have evolved around two limiting approximations. The first is the search for relationships between the angular indicatrices (1D), the coordinate Mueller matrix distributions (2D), and the structure of diffuse (depolarising) layers [1, 24–27]. The second is the MMP of optically thin, non-depolarising layers [17, 28, 29] with subsequent reconstruction of the distributions of the phase and amplitude anisotropy parameters [10, 11, 34–41].
However, while such 2D methods maybe useful for imaging and characterising surfaces or materials which are isotropic in at least one direction, they are of limited use for more complex systems. It is generally desirable to understand variations across a structure fully in three dimensions. Hence, there is a need to expand MMP diagnostic techniques to three dimensions. Additionally, most biological objects are partially depolarising. They have spatially inhomogeneous, optically anisotropic structures. Therefore, it is necessary to further develop and generalise existing MMP techniques to consider such partially depolarising structures. The theoretical basis of this direction can be established from the synthesis of methods of differential Mueller matrix [12, 13, 42–45] and holographic mapping of phase-inhomogeneous layers [46, 47]. Here, we develop and experimentally demonstrate a technique for the 3D Mueller matrix reconstruction of the phase and amplitude anisotropy parameters. We first outline the method for Mueller matrix analysis in two dimensions, before demonstrating the further development to three dimensions. We then consider the functional possibility of 3D reconstruction of each of the four parameters of the optical anisotropy of a myocardial tissue layer. Finally, the approbation of the approach is carried out for definitive diagnosis of myocardium tissue death as a result of acute coronary insufficiency (ACI) and coronary heart disease (CHD).
The 2D Mueller matrix reconstruction of the distributions of linear and circular birefringence and dichroism within biological layers has previously been considered in detail [48]. The matrix operator
It contains six distinct, non-zero parameters:
•
•
•
These phase and amplitude anisotropy parameters are in turn determined by the following identities:
Here:
By combining Eqs 2–7 with Eq. 8, we obtain algorithms determining the birefringence and dichroism from the elements of the Mueller matrix:
These analytical expressions are the basis of Mueller matrix reconstruction of mean values of the optical anisotropy parameters of the layer. Without reducing the completeness of the analysis [42], we can reduce the system to consider only the generalised parameters of linear birefringence
Also noting the identities Eq 4, 7, we now have a complete set of the four anisotropy parameters
The key principle for the determination of a three-dimensional layered series of distributions of Mueller matrix elements
where
where
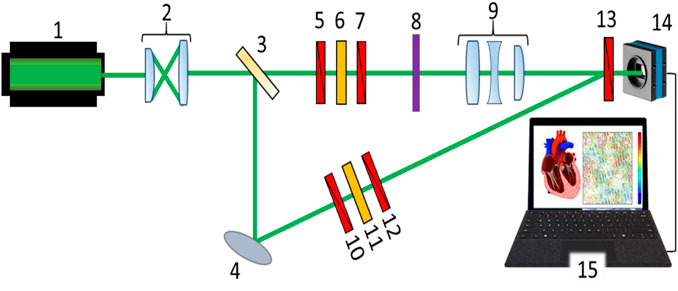
FIGURE 1. Schematic presentation of the 3D Mueller matrix-based polarimetry system: 1 – HeNe laser; 2 – collimator; 3 – beam splitter; 4 – rotary mirror; 5,7,10,12,13 – polarisation filters; 6 – quarter wave plate; 8 – sample under investigation; 9 – strain-free objective; 14 – CCD camera; and 15 – PC.
Therefore, a distribution of complex amplitudes:
is obtained for each state of polarization {Zond–Ref} in different phase planes
In single scattering approximation the phase of the fields of complex amplitudes Eq. 15 relates to physical depth
While in multiple scattering the physical or effective depth
The corresponding parameters of Stokes vector and polarization parameters of the object field for each phase plane
Based on relations Eq 14, 15, 18, the elements of Mueller matrix
Finally, the layer-by-layer distributions of the mean values of linear and circular birefringence and dichroism
Thus, such a polarization-interference-based cultivation Eqs 13–15, 18, 19 of elements of the first-order differential matrix Eqs 1–10 provides layer-by-layer maps of linear and circular birefringence and dichroism of the myocardial fibrillary networks. This approach extends significantly the functionality of the 3D Mueller-matrix imaging technique for depolarization mapping of diffuse biological layers [48, 49], that presently utilized only diagonal elements of the resulting Mueller matrix Eq. 19.
Figure 1 shows the optical arrangement of the 3D Mueller matrix-based polarimetry system developed in-house. The parallel beam (
A polarisation-inhomogeneous image of the object 8 is projected into the plane of the digital camera 14 (The Imaging Source DMK 41AU02.AS, monochrome 1/2 " CCD, Sony ICX205AL (progressive scan); resolution–1280 × 960; sensor area - 7600 × 6200 μm; sensitivity - 0.05 lx; dynamic range - 8 bit; signal-to-noise ratio - 9 bit) by the lens 9 (Nikon CFI Achromat P, focal distance - 30mm, numerical aperture - 0.1, magnification - ×4). The reference beam is guided, by the mirror 4, through the polarisation filters 10–12 into the plane of the polarisation-inhomogeneous image of the object 9. As a result, an interference pattern is formed which is recorded by the digital camera 14. The formation of the required polarisation states of the irradiating and reference beams is carried out using polarisation filters 5–7 and 10–12, each of which contains two linear polarisers (B + W Kaesemann XS-Pro Polariser MRC Nano) and a quarter-wave plate (Achromatic True Zero-Order Waveplate).
The layer-by-layer 3D Muller-matrix polarimetry system was calibrated using quarter and half wave-plates, demonstrating an accuracy of measuring the magnitude of the elements of Mueller matrix:
The layer-by-layer assessment of optical anisotropy
where
Three groups of myocardial histological sections were utilized in the study. Control group–Group 1 with the myocardial tissues that have no relation to myocardial diseases, whereas Group 2 and Group 3 with the structural malformations in myocardial tissues caused by ACI and CHD, respectively. All the groups consisted of equal number of histological sections:
Figure 2 shows the original images of the histological sections of the myocardium from all three groups. The coordinate intensity distribution
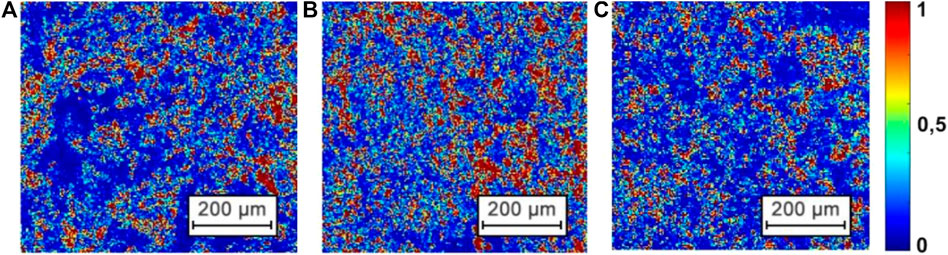
FIGURE 2. The examples of original CCD detected images of samples of histological sections of the myocardium from Group 1 (A), Group 2 (B), and Group 3 (C).
The obtained images (see Figure 2) show for all groups a fibrillar morphology structure consisting of a network of protein fibers, formed by optically active molecules of myosin and by optically isotropic molecules of actin [1].
Qualitative (visual) and quantitative (statistical) analysis of given microscopic images did not reveal significant differences between the three groups of myocardium histological samples. Table 1 presents the optical, geometrical and statistical parameters of the myocardium histological samples from each of the groups.
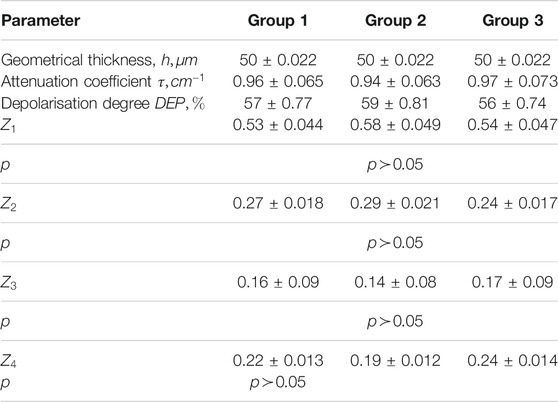
TABLE 1. Optical, geometrical and statistical parameters of the myocardium histological sections samples.
The attenuation (extinction) coefficient (
The studies conducted in accordance with the principles of the Declaration of Helsinki, and in compliance with the International Conference on Harmonization-Good Clinical Practice and local regulatory requirements. Ethical approval was obtained from the Ethics Committee of the Bureau of Forensic Medicine of the Chernivtsi National University and the Bukovinian State Medical University (Chernivtsi, Ukraine), and written informed consent was obtained from all subjects prior to study initiation.
The spatial order of fibrillary network that formed polycrystalline structure of myocardium, is optically discernible as linear birefringences and dichroism, whereas the optical activity of molecular domains of myosin forms a circular birefringence and dichroism [1–6, 17, 25]. The most optically expressed such These phenomena are seeing more clearly at the low level of depolarization background that is formed due to multiple scattering of light within the sample of histological sections of myocardium.
Therefore, polarization-based layer-by-layer detection of optical anisotropy and its variations provides a new quantitative approach of the evaluation of myocardial samples morphological structure and its pathological changes.
A key feature of the morphological structure of myocardial tissue is the presence of a spatially ordered network of protein fibrils. These fibrils are formed by optically active molecules of myosin and isotropic actin proteins [1]. In terms of optics such a network has two types of anisotropy, namely the structural anisotropy leading to linear birefringence (
3D Mueller matrix-based polarimetry approach (see Figure 1) is utilized for preconstruction of the phase and amplitude anisotropy parameters to the functional diagnostic imaging of myocardial tissues in vitro. In particular functional capabilities of 3D Mueller-matrix layer-by-layer reconstruction of optical anisotropy parameters were investigated utilizing sample of myocardium histological section from Group 1. Figures 3, 4 show structural optical anisotropy obtained, respectively, for linear and circular birefringence and linear and circular dichroism for the different physical depths Eq. 16, and, correspondingly, Figures 5, 6 demonstrate structural optical anisotropy for the effective depths Eq. 17.
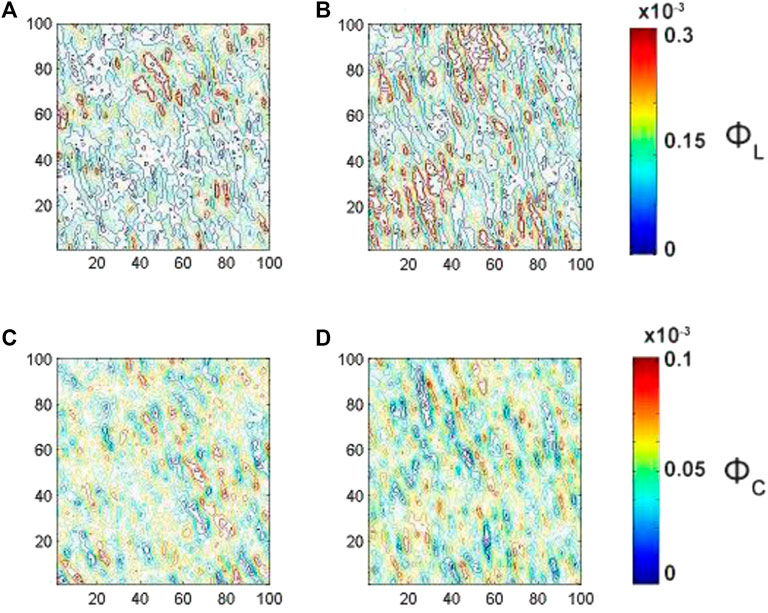
FIGURE 3. Embossed topographic maps of linear (A)
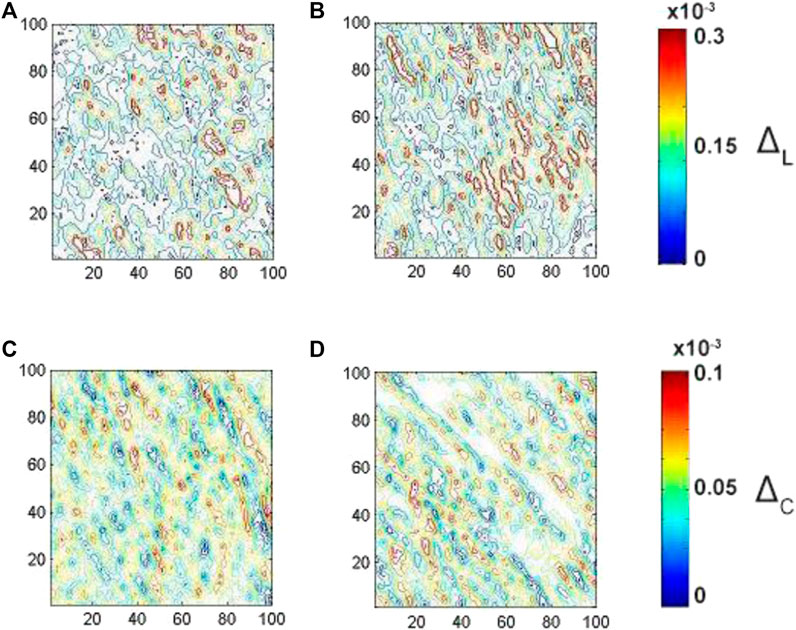
FIGURE 4. Embossed topographic maps of linear (A)
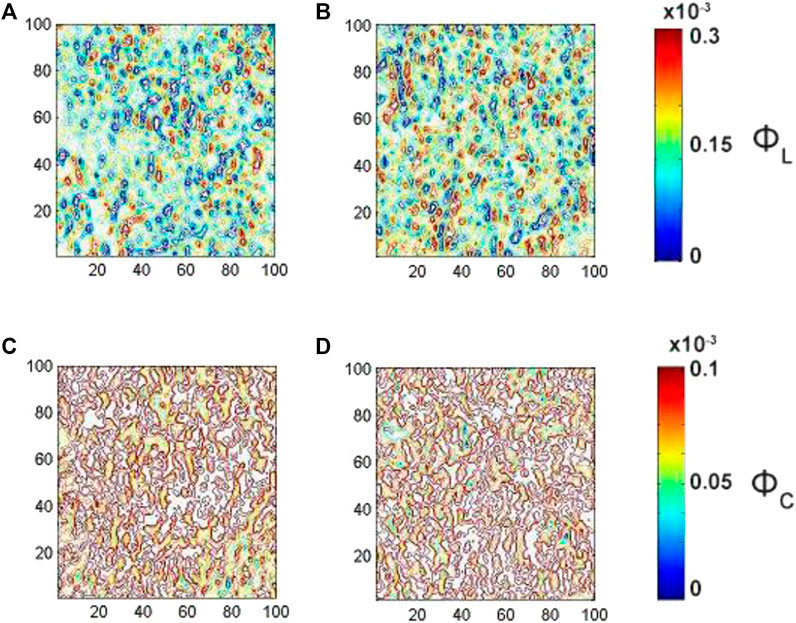
FIGURE 5. Embossed topographic maps of linear (A)
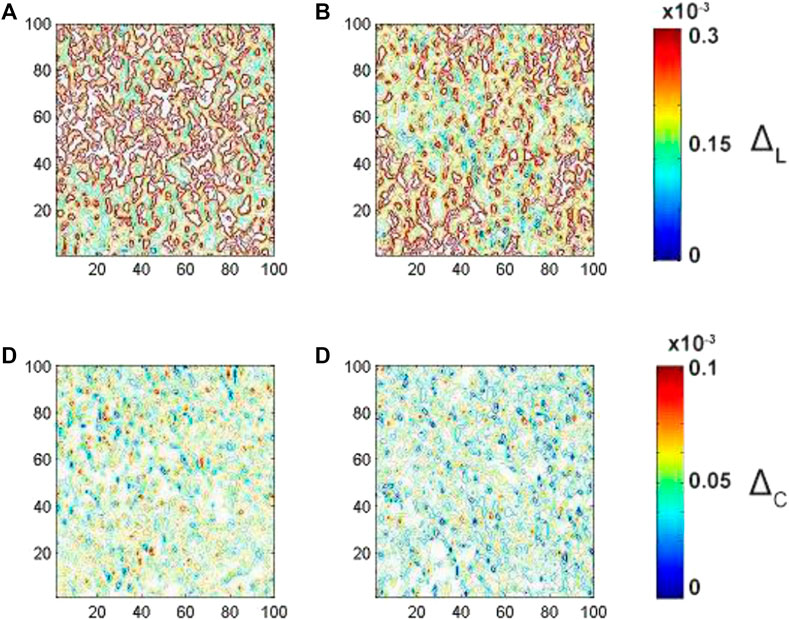
FIGURE 6. Embossed topographic maps of (A) linear
The next stage is devoted to demarcation of the samples of myocardium histological sections from Group 2 and Group 3 according the most effective phase plan Eq. 15 and the corresponding depth Eq 16, 17, guided with the following parameters:
A single pass of laser light through the histological section of the myocardium corresponds to the value θ(K = 1) ≈ 0.75 rad ⇔ z(K = 1) ≈ 50 µm double θ(K = 2) ≈ 1.5 rad ⇔ z(K = 2) ≈ 50 µm etc. In other words, the phase shifts correspond to the predominantly single scattering or low order of multiplicity of scattering. Whereas for
The analysis of the embossed topographic maps of myocardial histological section with distinctive single scattering shows a peculiar spatial distribution of linear and circular birefringence (see Figure 3) and dichroism (see Figure 4) at the variety of physical depths (Zi), defined by Eq. 16. The large-scale domains, the structure of which correlates with the size and directions of folding of protein fibrillar fibers, are seeing clearly both at the embossed topographic maps of circularly birefringence
The analysis of the embossed topographic maps of myocardial histological section at multiple scattering shows the significantly lower alterations in the structure of the spatial distributions of linear and circular birefringence (see Figure 5) and dichroism (see Figure 6) at the variety of physical depths (Zi*), defined by Eq. 17. The scale of domain structure of the embossed topographic maps of linear birefringence
From a physical point of view, this can be related to the peculiarities of the morphological structure of the myocardial tissue. Each partial fibril has a long-range geometric order that determines the structural anisotropy
Figure 7 shows the series of dependences of the statistical moments orders (
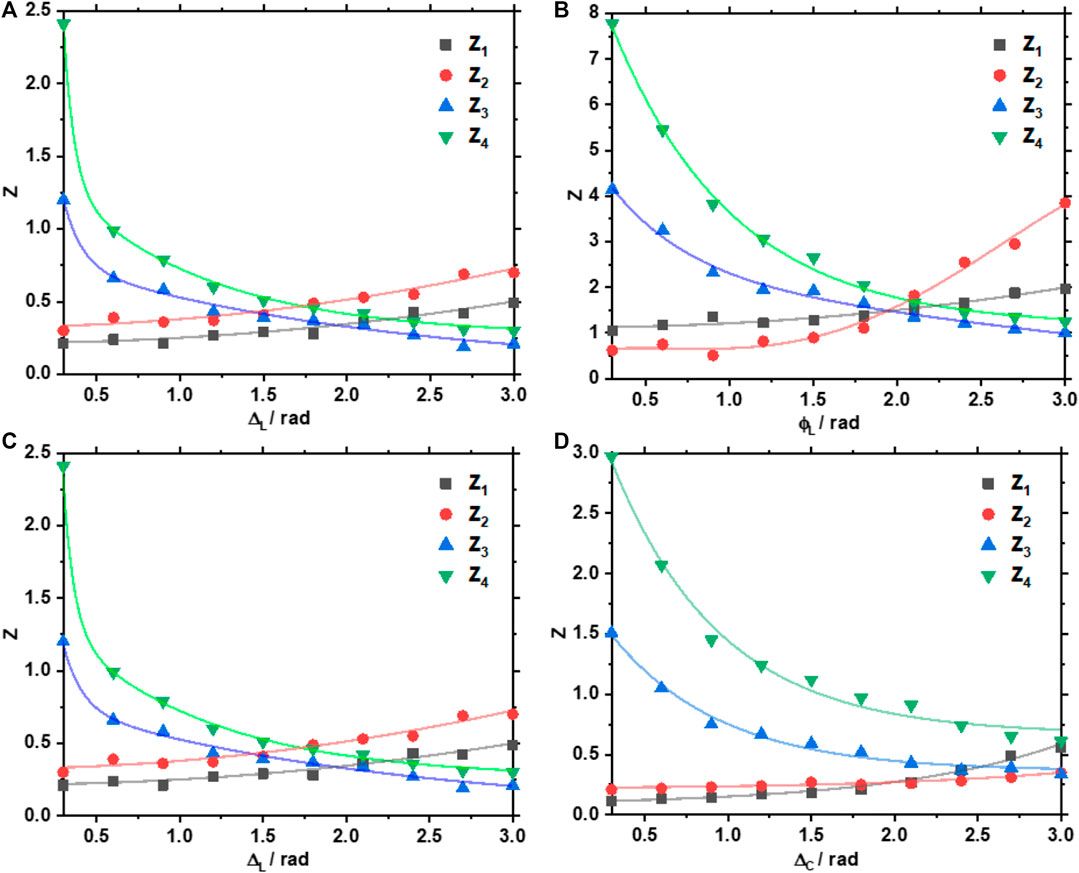
FIGURE 7. Phase dependences of the magnitude of the first to fourth statistical moments order characterising the distributions of (A)
Analysis of the obtained data revealed the following trends, which characterises the changes in the distributions of the parameters of the optical anisotropy of the myocardium layer:
The observed regularities can be related to the fact that as the multiplicity of light scattering in the volume of the myocardium increases, in the limit of small
Finally, for the definitive diagnosis of ACI and CHD by the layer-by-layer Mueller-matrix approach described above the following protocol of reconstruction of myocardium optical anisotropy parameters has been developed. 1) As soon as the position of the phase plane most sensitive to pathological changes in the parameters of the optical anisotropy structure is determined
The sensitivity (
The experimental results of 3D layer-by-layer Mueller-matrix reconstruction are presented as embossed topographic maps [48] of linear and circular birefringence (Figure 8) and dichroism (Figure 9) of ACI and CHD myocardial histological tissue samples.
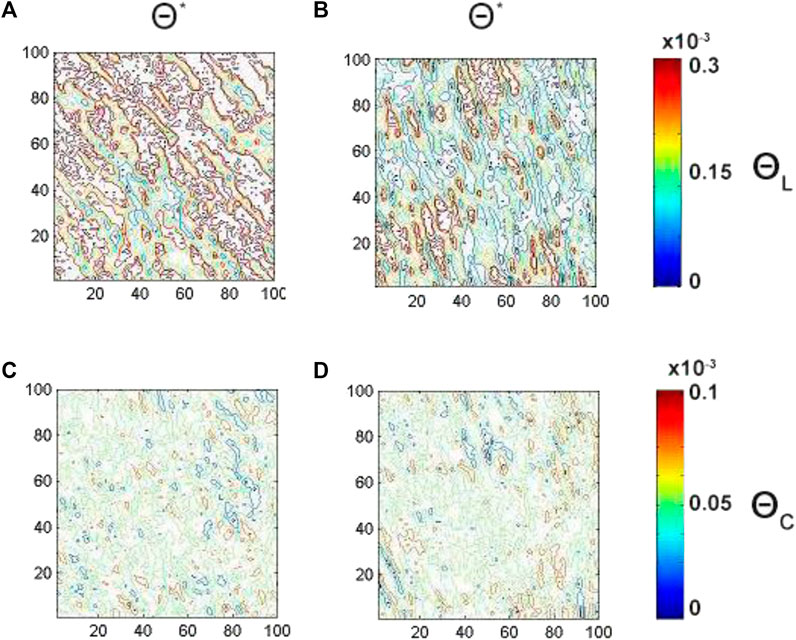
FIGURE 8. Embossed topographic maps of linear (A, B) and circular (C, D) birefringence of ACI (left column) and CHD (right column) myocardial histological sections. The determined optimal phase planes are
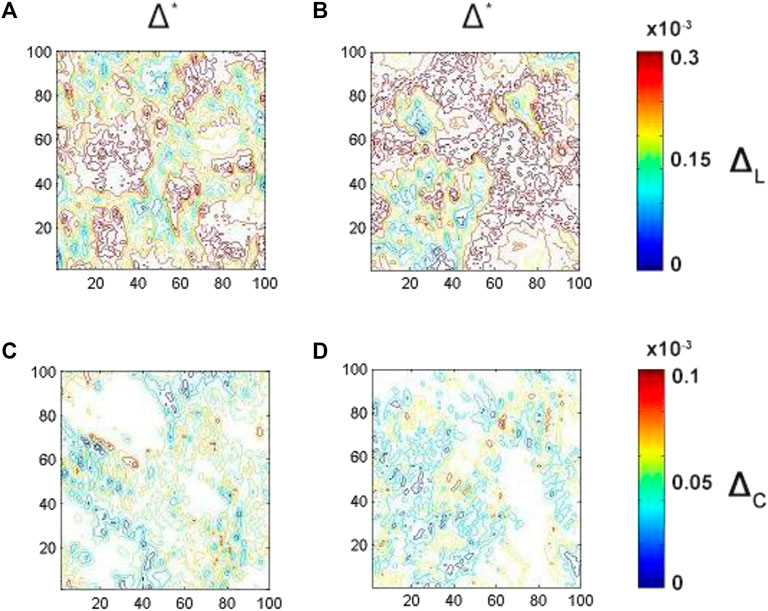
FIGURE 9. Embossed topographic maps of linear (A, B) and circular (C, D) dichroism of ACI (left column) and CHD (right column) myocardial histological sections. The determined optimal phase planes are
As one can see the average magnitude of linear birefringence and dichroism (see Figures 8A,B, 9A,B) is prevail up to 3 times compare to the magnitude of circular (see Figures 8C,D, 9C,D) birefringence and dichroism for both ACI and CHD myocardial histological samples. A decrease (
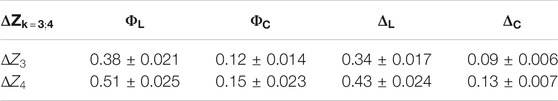
TABLE 2. Intergroup difference of high order statistical moments characterizing embossed maps of linear and circular birefringence and dichroism in optimal phase sections of myocardial histological samples. θ* = 0.7 rad.
4th order statistical moment (
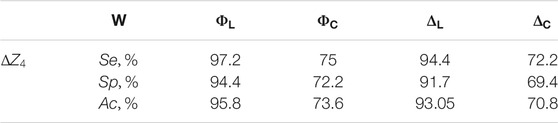
TABLE 3. Operational characteristics of diagnostic performance of myocardial histological sections by 3D Mueller-matrix reconstruction of optical anisotropy.
In comparison Table 4 presents the operational characteristics of diagnostic performance of the 3D Mueller-matrix reconstruction method of optical anisotropy of myocardial histological sections in other phase planes
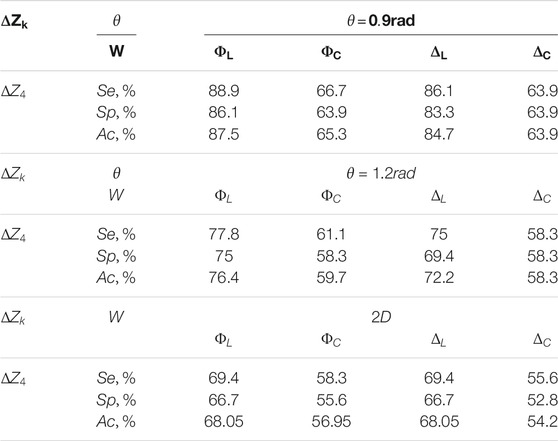
TABLE 4. Operational characteristics of the diagnostic performance of the 3D Mueller-matrix reconstruction method of optical anisotropy of myocardial histological sections.
The results show an excellent level of accuracy in differentiation of ACI and CHD:
A method for 3D Mueller matrix reconstruction of layer-by-layer distributions of the linear and circular birefringence and dichroism has been, for the first time to the best of our knowledge, introduced theoretically in line with the protocol of experimental measurements. The proposed approach has been tested for the case of partially depolarising layers of myocardial tissues. The dynamics of the change in the magnitude of the statistical moments of the first to fourth orders that characterise the distribution of the parameters of the optical anisotropy of the polycrystalline structure of the partially depolarising layer (
The developed approach has a high potential in various applications in optical histopathology and biopsy, including: early cancer detection, monitoring of stage of cancer aggressiveness by means of reconstruction of the polycrystalline structure of human fluids (blood, saliva, etc.); definitive diagnosis of histological sections of biopsy of benign and malignant tumours of human organs (prostate, uterus, breast), aseptic and septic conditions of human joints by means of reconstruction of the polycrystalline structure of the synovial fluid; and importantly in forensic pathology determining the time of death by means of temporary monitoring of necrotic changes in the polycrystalline structure of tissues and organ fluids of the deceased.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Current research supported by the ATTRACT project funded by the EC under Grant Agreement 777222, Academy of Finland (grants 314639 and 325097), National Research Foundation of Ukraine (Project 2020.02/0061) and INFOTECH strategic funding, and with the support of a grant under the Decree of the Government of the Russian Federation No. 220 of 09 April 2010 (Agreement No. 075-15-2021-615 of 04 June 2021). This work was also financed by the Ministry of Science and Higher Education of the Russian Federation (Agreement no. 075-02-2021-1748).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tuchin VV. Tissue Optics and Photonics: Biological Tissue Structures. Jbpe (2015) 1:3–21. doi:10.18287/JBPE-2015-1-1-3
2. Camici PG, Tschöpe C, Di Carli MF, Rimoldi O, Van Linthout S. Coronary Microvascular Dysfunction in Hypertrophy and Heart Failure. Cardiovasc Res (2020) 116:806–16. doi:10.1093/cvr/cvaa023
3. Meli AC, de Tombe P. Scientists on the Spot: Myocardium and Myofilaments. Cardiovasc Res (2020) 116:e96–e97. doi:10.1093/cvr/cvz317
4. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: a Systematic Analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2095–128. doi:10.1016/S0140-6736(12)61728-0
5. Pitoulis FG, Watson SA, Perbellini F, Terracciano CM. Myocardial Slices Come to Age: an Intermediate Complexity In Vitro Cardiac Model for Translational Research. Cardiovasc Res (2020) 116:1275–87. doi:10.1093/cvr/cvz341
6. Bermejo-Martin JF, Almansa R, Torres A, González-Rivera M, Kelvin DJ. COVID-19 as a Cardiovascular Disease: the Potential Role of Chronic Endothelial Dysfunction. Cardiovasc Res (2020) 116:e132–e133. doi:10.1093/cvr/cvaa140
7. Bykov A, Hautala T, Kinnunen M, Popov A, Karhula S, Saarakkala S, et al. Imaging of Subchondral Bone by Optical Coherence Tomography upon Optical Clearing of Articular Cartilage. J Biophoton (2016) 9(3):270–5. doi:10.1002/jbio.201500130
8. Farahani N, Braun A, Jutt D, Huffman T, Reder N, Liu Z, et al. Three-dimensional Imaging and Scanning: Current and Future Applications for Pathology. J Pathol Inform (2017) 8:36. doi:10.4103/jpi.jpi_32_17
9. Azaripour A, Lagerweij T, Scharfbillig C, Jadczak AE, Willershausen B, Van Noorden CJF. A Survey of Clearing Techniques for 3D Imaging of Tissues with Special Reference to Connective Tissue. Prog Histochem Cytochem (2016) 51:9–23. doi:10.1016/j.proghi.2016.04.001
10. Borovkova M, Peyvasteh M, Dubolazov O, Ushenko Y, Ushenko V, Bykov A, et al. Complementary Analysis of Mueller-Matrix Images of Optically Anisotropic Highly Scattering Biological Tissues. J Eur Opt Soc-Rapid Publ (2018) 14:20. doi:10.1186/s41476-018-0085-9
11. Das NK, Dey R, Chakraborty S, Panigrahi PK, Meglinski I, Ghosh N. Quantitative Assessment of Submicron Scale Anisotropy in Tissue Multifractality by Scattering Mueller Matrix in the Framework of Born Approximation. Opt Commun (2018) 413:172–8. doi:10.1016/j.optcom.2017.11.082
12. Devlaminck V. Physical Model of Differential Mueller Matrix for Depolarizing Uniform media. J Opt Soc Am A (2013) 30:2196. doi:10.1364/JOSAA.30.002196
13. Ossikovski R, Arteaga O. Statistical Meaning of the Differential Mueller Matrix of Depolarizing Homogeneous media. Opt Lett (2014) 39:4470. doi:10.1364/OL.39.004470
14. Peyvasteh M, Dubolazov A, Popov A, Ushenko A, Ushenko Y, Meglinski I. Two-point Stokes Vector Diagnostic Approach for Characterization of Optically Anisotropic Biological Tissues. J Phys D Appl Phys (2020) 53(39):395401. doi:10.1088/1361-6463/ab9571
15. Trifonyuk L, Sdobnov A, Baranowski W, Ushenko V, Olar O, Dubolazov A, et al. Differential Mueller Matrix Imaging of Partially Depolarizing Optically Anisotropic Biological Tissues. Lasers Med Sci (2020) 35(4):877–91. doi:10.1007/s10103-019-02878-2
16. Rodger A, Norden B. Circular Dichroism and Linear Dichroism. Oxford: Oxford University Press (1997).
17. Ushenko AG, Pishak VP. Laser Polarimetry of Biological Tissue: Principles and Applications. In: VVV̆ V Tuchin, editor. Handbook Of Coherent-Domain Optical Methods : Biomedical Diagnosis, Environmental Monitoring, and Materials Science. Springer. 2004, p. 93–138.
18. Buscemi IC, Guyot S. Near Real-Time Polarimetric Imaging System. J Biomed Opt (2013) 18:1. doi:10.1117/1.JBO.18.11.116002
19. Manhas S, Vizet J, Deby S, Vanel J-C, Boito P, Verdier M, et al. Demonstration of Full 4×4 Mueller Polarimetry through an Optical Fiber for Endoscopic Applications. Opt Express (2015) 23:3047. doi:10.1364/OE.23.003047
20. Negara C, Li Z, Längle T, Beyerer J. Simplified Stokes Polarimeter Based on Division-Of-Amplitude. In: B Zagar, P Mazurek, M Rosenberger, and P-G Dittrich, editors. Photonics and Education in Measurement Science 2019 (2019) p. 44. doi:10.1117/12.2532399
21. Lin Z, Rusch L, Chen Y, Shi W. Chip-scale, Full-Stokes Polarimeter. Opt Express (2019) 27:4867. doi:10.1364/oe.27.004867
22. Mishchenko MI, Travis LD, Lacis AA. Scattering, Absorption, and Emission of Light by Small Particles. Cambridge University Press (2002).
23. Swami MK, Patel HS, Gupta PK. Conversion of 3×3 Mueller Matrix to 4×4 Mueller Matrix for Non-depolarizing Samples. Opt Commun (2013) 286:18–22. doi:10.1016/j.optcom.2012.08.094
24. Izotova VF, Maksimova IL, Nefedov IS, Romanov SV. Investigation of Mueller Matrices of Anisotropic Nonhomogeneous Layers in Application to an Optical Model of the Cornea. Appl Opt (1997) 36:164. doi:10.1364/AO.36.000164
25. Tuchin VV. Tissue Optics and Photonics: Light-Tissue Interaction. Jbpe (2015) 1:98–134. doi:10.18287/JBPE-2015-1-2-98
26. Manhas S, Swami MK, Buddhiwant P, Ghosh N, Gupta PK, Singh J. Mueller Matrix Approach for Determination of Optical Rotation in Chiral Turbid media in Backscattering Geometry. Opt Express (2006) 14:190. doi:10.1364/OPEX.14.000190
27. Deng Y, Zeng S, Lu Q, Zhu D, Luo Q. Characterization of Backscattering Mueller Matrix Patterns of Highly Scattering media with Triple Scattering assumption. Opt Express (2007) 15:9672. doi:10.1364/OE.15.009672
28. Angelsky O, Ushenko A, Ushenko Y, Pishak V, Peresunko A. Statistical, Correlation, and Topological Approaches in Diagnostics of the Structure and Physiological State of Birefringent Biological Tissues. In: VV Tuchin, editor. Handbook Of Photonics For Biomedical Science. Boca Raton: CRC Press (2010) p. 283–322. doi:10.1201/9781439806296-c10
29. Ushenko YA, Boychuk TM, Bachynsky VT, Mincer OP. Diagnostics of Structure and Physiological State of Birefringent Biological Tissues: Statistical, Correlation and Topological Approaches. In: VV(V̆ V Tuchin, editor. Handbook Of Coherent-Domain Optical Methods. New York, NY: Springer New York (2013) p. 107–48. doi:10.1007/978-1-4614-5176-1_3
30. Lu S-Y, Chipman RA. Interpretation of Mueller Matrices Based on Polar Decomposition. J Opt Soc Am A (1996) 13:1106. doi:10.1364/JOSAA.13.001106
31. Guo Y, Zeng N, He H, Yun T, Du E, Liao R, et al. A Study on Forward Scattering Mueller Matrix Decomposition in Anisotropic Medium. Opt Express (2013) 21:18361. doi:10.1364/OE.21.018361
32. DeBoo B, Sasian J, Chipman R. Degree of Polarization Surfaces and Maps for Analysis of Depolarization. Opt Express (2004) 12:4941. doi:10.1364/OPEX.12.004941
33. Pierangelo A, Manhas S, Benali A, Fallet C, Totobenazara J-L, Antonelli M-R, et al. Multispectral Mueller Polarimetric Imaging Detecting Residual Cancer and Cancer Regression after Neoadjuvant Treatment for Colorectal Carcinomas. J Biomed Opt (2013) 18:046014. doi:10.1117/1.JBO.18.4.046014
34. Ushenko VA, Dubolazov AV, Pidkamin LY, Sakchnovsky MY, Bodnar AB, Ushenko YA, et al. Mapping of Polycrystalline Films of Biological Fluids Utilizing the Jones-matrix Formalism. Laser Phys (2018) 28(2):025602. doi:10.1088/1555-6611/aa8cd9
35. Ushenko V, Sdobnov A, Syvokorovskaya A, Dubolazov A, Vanchulyak O, Ushenko A, et al. 3D Mueller-Matrix Diffusive Tomography of Polycrystalline Blood Films for Cancer Diagnosis. Photonics (2018) 5:54. doi:10.3390/photonics5040054
36. Ushenko A, Sdobnov A, Dubolazov A, Grytsiuk M, Ushenko Y, Bykov A, et al. Stokes-Correlometry Analysis of Biological Tissues with Polycrystalline Structure. IEEE J Select Top Quan Electron (2019) 25(1):1–12. doi:10.1109/JSTQE.2018.2865443
37. Borovkova M, Trifonyuk L, Ushenko V, Dubolazov O, Vanchulyak O, Bodnar G, et al. Mueller-matrix-based Polarization Imaging and Quantitative Assessment of Optically Anisotropic Polycrystalline Networks. PLOS One (2019) 14(5):e0214494. doi:10.1371/journal.pone.0214494
38. Ushenko VA, Sdobnov AY, Mishalov WD, Dubolazov AV, Olar OV, Bachinskyi VT, et al. Biomedical Applications of Jones-matrix Tomography to Polycrystalline Films of Biological Fluids. J Innov Opt Health Sci (2019) 12(6):1950017. doi:10.1142/S1793545819500172
39. Ushenko VA, Pavlyukovich ND, Trifonyuk L. Spatial-Frequency Azimuthally Stable Cartography of Biological Polycrystalline Networks. Int J Opt (2013) 2013:1–7. doi:10.1155/2013/683174
40. Ushenko VA, Dubolazov OV, Karachevtsev AO. Two Wavelength Mueller Matrix Reconstruction of Blood Plasma Films Polycrystalline Structure in Diagnostics of Breast Cancer. Appl Opt (2014) 53:B128. doi:10.1364/AO.53.00B128
41. Prysyazhnyuk VP, Ushenko YA, Dubolazov AV, Ushenko AG, Ushenko VA. Polarization-dependent Laser Autofluorescence of the Polycrystalline Networks of Blood Plasma Films in the Task of Liver Pathology Differentiation. Appl Opt (2016) 55:B126. doi:10.1364/AO.55.00B126
42. Ortega-Quijano N, Arce-Diego JL. Depolarizing Differential Mueller Matrices. Opt Lett (2011) 36:2429. doi:10.1364/OL.36.002429
43. Ortega-Quijano N, Arce-Diego JL. Mueller Matrix Differential Decomposition. Opt Lett (2011) 36:1942. doi:10.1364/OL.36.001942
44. Ossikovski R, Devlaminck V. General Criterion for the Physical Realizability of the Differential Mueller Matrix. Opt Lett (2014) 39:1216. doi:10.1364/OL.39.001216
45. Devlaminck V, Ossikovski R. Uniqueness of the Differential Mueller Matrix of Uniform Homogeneous media. Opt Lett (2014) 39:3149. doi:10.1364/OL.39.003149
46. Yasuno Y, Ju M-J, Hong Y-J, Makita S, Miura M. (2013), In Vivo Three-Dimensional Investigation of Tissue Birefringence by Jones Matrix Tomography, Conf Lasers Electro-Optics Pacific Rim, 30 June-4 July 2013, Kyoto, Japan, IEEE.
47. Kobata T, Nomura T. Digital Holographic Three-Dimensional Mueller Matrix Imaging. Appl Opt (2015) 54:5591. doi:10.1364/AO.54.005591
48. Ushenko VA, Hogan BT, Dubolazov A, Grechina AV, Boronikhina TV, Gorsky M, et al. Embossed Topographic Depolarisation Maps of Biological Tissues with Different Morphological Structures. Sci Rep (2021) 11:3871. doi:10.1038/s41598-021-83017-2
49. Ushenko VA, Hogan BT, Dubolazov A, Piavchenko G, Kuznetsov SL, Ushenko AG, et al. 3D Mueller Matrix Mapping of Layered Distributions of Depolarisation Degree for Analysis of Prostate Adenoma and Carcinoma Diffuse Tissues. Sci Rep (2021) 11:5162. doi:10.1038/s41598-021-83986-4
50. Cassidy LD. Basic Concepts of Statistical Analysis for Surgical Research. J Surg Res (2005) 128:199–206. doi:10.1016/j.jss.2005.07.005
51. Davis CS. Statistical Methods for the Analysis of Repeated Measurements. New York, NY: Springer New York (2003) doi:10.1007/b97287
Keywords: Mueller matrix, optical anisotropy, histopathology, polarized light, optical diagnosis
Citation: Hogan BT, Ushenko VA, Syvokorovskaya A-V, Dubolazov AV, Vanchulyak OY, Ushenko AG, Ushenko YA, Gorsky MP, Tomka Y, Kuznetsov SL, Bykov A and Meglinski I (2021) 3D Mueller Matrix Reconstruction of the Optical Anisotropy Parameters of Myocardial Histopathology Tissue Samples. Front. Phys. 9:737866. doi: 10.3389/fphy.2021.737866
Received: 07 July 2021; Accepted: 13 September 2021;
Published: 08 October 2021.
Edited by:
Alex Vitkin, University of Toronto, CanadaReviewed by:
Nirmal Mazumder, Manipal Academy of Higher Education, IndiaCopyright © 2021 Hogan, Ushenko, Syvokorovskaya, Dubolazov, Vanchulyak, Ushenko, Ushenko, Gorsky, Tomka, Kuznetsov, Bykov and Meglinski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Igor Meglinski, aS5tZWdsaW5za2lAYXN0b24uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.