Abstract
Light plays a crucial role in ecological dynamics, both as a consumable resource and as an environmental factor. Prokaryotic and eukaryotic photoautotrophs use light as an energy source for photosynthesis, which forms the basis of food chains and determines the flow of energy and matter in ecosystems. Light availability and quality can influence resource complementarity and species coexistence, as well as the stoichiometry of primary producers and the transfer efficiency of food webs. In addition, light serves as an important source of information for organisms, influencing their activities and interactions with the environment. Light shapes biotic interactions, including competition, predator-prey relationships, and mutualistic and antagonistic relationships between photoautotrophs and heterotrophs. Anthropogenic activities affect these photoecological processes, with largely unknown consequences. Hence, understanding the ecological role and control of light is essential for understanding the functioning of ecosystems and biogeochemical cycles.
Introduction
While photosynthesis was not an initial metabolic pathway of early life on Earth and light is not a prerequisite for life per se (Shih, 2015; Ralser et al., 2021), the perception of light is already a vital trait of a vast number of prokaryotes (Williams, 2016). It is necessary for the evolution of pathways to obtain energy for metabolic processes from light. As either a consumable resource and/or an environmental factor structuring life, light is part of the ecological (Hutchinsonian) niche (Hutchinson, 1978) of most species on Earth. Niche requirements for resources and environmental factors shape the interactions of organisms with their environment and thus ecological dynamics and biogeochemical cycles. Consequently, light is one of the key drivers of such processes (Figure 1): light as a resource underlies the global ecological and biogeochemical consequences of carbon fixation by photoautotrophs since the invention of photosynthesis (Ozaki et al., 2018). Moreover, light as an environmental factor can trigger the behaviour of organisms on a global scale, such as the diel vertical migration (DVM) of marine zooplankton moving between the surface and deeper water layers (Bandara et al., 2021).
FIGURE 1
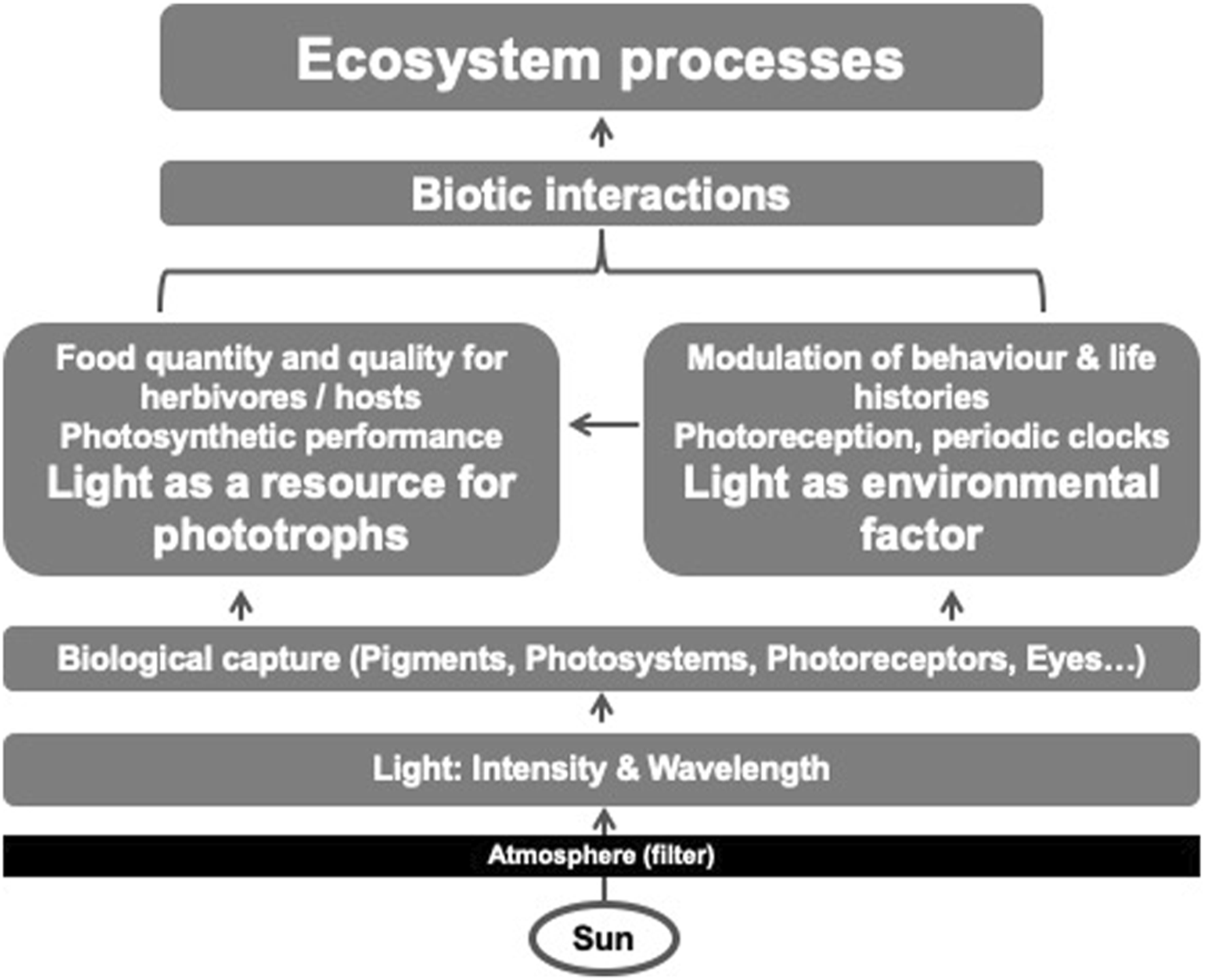
Simplified schematic of the different ways in which light affects ecosystem processes.
Light affecting ecological dynamics as a consumable resource for photoautotrophs
Prokaryotic and eukaryotic photoautotrophs use light as an energy source to split molecules, most commonly H2O, to obtain electrons to reduce carbon dioxide to organic molecules (Messinger and Shevela, 2011). These processes are typically at the base of so-called food chains or food webs - man-made maps of interactions between organisms - that determine the flow of energy and matter to higher trophic levels (May, 1974; Pimm et al., 1991). In a broader sense, the ecology of food webs is therefore largely influenced by being driven by photo-ecological processes. Even the voracious energy demands of modern human populations are largely met by light-driven biotic processes. Fossil fuels such as petroleum are largely the result of photosynthetically produced organic biomolecules that are one hundred or more million years old (Ourisson et al., 1984) and which have not yet been recycled back to carbon dioxide.
Consumable resources are necessary for the survival, growth and reproduction of individuals. Consumption of a resource by one individual precludes its availability to another individual, leading to intra- and interspecific competition for resources and evolutionary adaptations (Begon et al., 2006). As a resource, light can be considered both quantitatively (high light, low light) and qualitatively (bundles of different wavelengths). This holds true especially in aquatic environments. Phytoplankton moving up and down the water column experience a much greater variation in background colour than land-based plants. As a result, differential sensitivity to different wavelengths has evolved (Stomp et al., 2007a; Kehoe, 2010; Falkowski and Raven, 2013).
Photosynthetic active radiation (PAR) consists of wavelengths from approximately 400–700 nm (McCree, 1972) and the selective use of certain wavelengths within the resource bundle by phytoplankton can allow resource complementation and hence species coexistence (Stomp et al., 2007b). In addition, resource complementarity may also be a mechanism underlying relationships between biodiversity and ecosystem functioning, such as higher resource use efficiency of highly diverse phytoplankton communities that use the PAR spectrum more efficiently (Striebel et al., 2009).
Too much of the resource light can have detrimental effects, while too little does not allow for a positive net energy balance through photosynthesis. Therefore, a variety of responses are known to optimise light use related traits (Poorter et al., 2019). In addition to such ecophysiological processes at the individual level, there are also food web effects of varying light levels. Light can affect the stoichiometry of primary producers, mainly their carbon to nutrient ratio (Elser et al., 2000). This can theoretically lead to the so-called “energy enrichment paradox”, where an increase in light resources can have a negative effect on the transfer efficiency of the food web, as described for plankton food webs (Diehl, 2007). The mechanistic basis of this paradox is that an increase in light, and hence photosynthesis, can lead to an increase in the carbon to nutrient ratio of phytoplankton biomass (Dickmann et al., 2006), which affects food quality for higher trophic levels (Urabe et al., 2002). Higher trophic levels may experience a shift from carbon to nutrient limitation. Such a shift can result in lower production at higher trophic levels despite an enrichment of resources (more light) at the base of the food web (Urabe and Sterner, 1996).
In addition to the energy enrichment paradox mentioned above, light-driven shifts in the biomass stoichiometry of flexible primary producers can have a variety of other implications for ecological dynamics on land and underwater (Güsewell, 2004; Elser et al., 2010). Spatial and temporal light distribution and environmental light-nutrient ratios also influence terrestrial vegetation successional patterns, driving vegetation structure and biodiversity along successional dynamics (Tilman, 1985; Matsuo et al., 2021). Early successional communities are often characterized by thriving at high light to nutrient ratios, whereas the opposite is true for late successional species. Such light-dependent dynamics have far-reaching consequences, as the composition and hence functional characteristics of vegetation determine to some extent the functional and species composition of higher trophic levels (Russo et al., 2023).
Light as a resource shapes biotic interaction between photoautotrophs (mainly through competition) and between photoautotrophs and heterotrophs, either in the form of predator-prey relationships (e.g., by affecting the quantity and quality of food for herbivores as described above) or in complex mutualistic and antagonistic relationships. Examples of mutualistic relationships include spatially intimate phototroph-bacteria (Cirri and Pohnert, 2019), phototroph-ciliate (Dziallas et al., 2012), phototroph-fungi (Ahmadjian, 1993) or phototroph-animal relationships (Venn et al., 2008), where partners provide resources to each other in both directions. For example, algae can provide photosynthetic products in the form of dissolved organic carbon sources to associated bacteria, which in turn provide essential nutrients to the algae, such as fixed nitrogen or vitamin B12 (Croft et al., 2005; Thompson et al., 2012). The importance of the photosynthetic processes of coral-associated dinoflagellates for tropical reefs and their impact on biogeochemical cycles is undisputed and well documented (Roth, 2014; van Oppen and Medina, 2020). Light as a resource can also strongly regulate the important symbiotic nitrogen fixation of endosymbiotic bacteria of terrestrial plants (Taylor and Menge, 2018; Ottinger et al., 2023), with far-reaching consequences for food web processes and biogeochemical cycles. Resource availability can influence such mutualistic interactions (Hom and Murray, 2014), which are often better characterised by functioning along a mutualistic-antagonistic transition gradient (Sachs and Wilcox, 2006; Drew et al., 2021).
Combinations of phototrophic and heterotrophic nutritional pathways are not restricted to combinations of different phototrophic and heterotrophic species forming a holobiont. Both pathways can also occur within a single species, for example, carnivorous plants obtaining additional nutrients by feeding on animals (Tĕšitel et al., 2018), or algae combining photosynthesis with heterotrophic pathways, either by using organic carbon sources in addition to CO2, either dissolved or specific (Flynn et al., 2013). In addition, heterotrophic feeding can also serve as a source of nutrients from particulate matter. Aquatic environments are mostly light-limited and highly nutrient-depleted. As a result, a large number of marine and freshwater phytoplankton species are so-called mixotrophic species, operating along a gradient from predominantly autotrophic to predominantly heterotrophic (Flynn et al., 2013; Stoecker et al., 2017). Light as a resource can influence the proportion that each of the two pathways contributes to the overall carbon budget (Ptacnik et al., 2016). Assuming that approximately 50% of global carbon fixation in aquatic environments is by phytoplankton (Field et al., 1998), it is clearly important to understand the ecological role and control of mixotrophic nutrition (Mitra et al., 2014). In addition to large-scale carbon cycling, the abundance of mixotrophs within phytoplankton and their individual level of mixotrophy can also influence biomass stoichiometry and food quality, and thus transfer efficiencies within aquatic food webs (Katechakis et al., 2005; Vad et al., 2020).
Light as an environmental factor shaping biotic interactions
Light can be converted into thermal energy and is an important source of heat for poikilotherms, which depend on external heat supply to determine their activities and thus their interactions with their biotic and abiotic environment. A large number of poikilothermic organisms have evolved mechanisms to efficiently absorb light, such as specific colouration (Clusella-Trullas et al., 2007), which is often flexible in response to temperature (Zhang et al., 2020). Absorbed light can also be re-emitted at longer wavelengths by a living organism, a common phenomenon known as biofluorescence, which is known from aquatic and terrestrial habitats (Lamb and Davis, 2020). Potential functions of biofluorescence include processes such as communication (Marshall and Johnsen, 2017) or sexual selection (Hausmann et al., 2003), which affect biotic interactions. Blue light-induced fluorescence of phytoplankton chlorophyll can convey information about population density at the microscale level and regulate resource uptake (iron) in diatoms (Liu et al., 2021).
Most obviously, however, light contains rich and complex information that is very useful for exploring the environment through photosensitive morphological structures (photosensing), allowing a more or less complex picture of the visual environment to be formed and enables appropriate responses to it (photoregulation, Nilsson et al., 2022). These processes allow ecological interactions such as the identification and evaluation of other organisms for intra- or interspecific interactions such as interference competition, sexual reproduction, determining suitable prey, escaping predators, shaping predator behaviour, synchronising the timing of reproduction, etc. A famous example of such a light-induced behavioural process operating on a large scale is the Diel Vertical Migration (DVM). It is the world’s largest synchronised movement of organisms (plankton) and occurs in the open water (pelagial) of marine and freshwater systems (Bandara et al., 2021). Organisms such as zooplankton migrate into deep and therefore dark water layers during the day and return to surface waters at night. Light is either an ultimate or proximate factor controlling DVM. It can act as a proxy for a dangerous environment where visual predators such as large numbers of fish are active, resulting in a high mortality risk for their food, the zooplankton. Light can also act as an ultimate cause of DVM, such as in high altitude lakes where high UV levels in the upper water layers force zooplankton to perform a diel vertical migration to escape the risk of UV damage (Rhode et al., 2001). Light and light-driven cycles control the behaviour and activity patterns of a wide range of organisms, with major consequences for the dynamics of biotic interactions such as predation or competition. Such light-driven processes can operate even during the polar night (Berge et al., 2020), modulated by lunar and auroral components at very low light levels (Cohen et al., 2021). At the base of aquatic and terrestrial food webs, photosynthetic dynamics (such as photosynthetic light absorption) are regulated by photosensing required to detect light direction or diurnal/seasonal irradiance cycles, such that light as an environmental factor regulates the uptake of the resource light (Haeder et al., 2009; Forbes-Stovall et al., 2014; Jaubert et al., 2017; Schuback and Tortell, 2019). In an antagonistic bacteria-algae relationship, bacteria can destroy the primitive visual system of the green alga Chlamydomonas, preventing phototactic behaviour important for resource uptake (Hotter et al., 2021). Prokaryotes can also link light perception to control of motility use, thereby optimising light use efficiency for photosynthesis (Wilde and Mullineaux, 2017). For example, in non-phototrophic prokaryotes, light can regulate virulence (Bonomi et al., 2016) and thus interactions with their host organisms, which affect population and food web dynamics.
In environments lacking sufficient sunlight for visual orientation, such as the deep sea or nocturnal habitats, a large number of dark-active organisms have evolved the ability to actively produce light, known as bioluminescence (Widder, 1999; Haddock et al., 2010). Such biogenic light production can be used to attract prey, mates for reproduction, intraspecific synchronisation or to confuse predators (Widder, 2010; Ellis and Oakley, 2016; Verdes and Gruber, 2017; Wainwright and Longo, 2017). Bioluminescence is far from being an exotic phenomenon, as shown by a study that examined a marine water column from the surface to the deep sea, where bioluminescence was observed in a large number of organisms across a large variety of phylogenetic groups. Bioluminescence is considered to be a common and important ecological trait in the dark (Martini and Haddock, 2017).
Anthropogenic impacts on photoecology
In addition to the large diversity of organisms mentioned above that are able to produce their own light to attract mates or prey, or to confuse predators, humans have also developed increasingly complex cultural skills to produce light. This has allowed humans to become independent of natural day-night cycles and to extend activities that rely on vision to 24 h a day. However, the use of artificial light sources has not only allowed humans to extend vision and related activities to any time of day in any place on Earth; it is also visible to other organisms, both mobile and sessile, disrupting their natural light cycles (Rodrigues-Comino et al., 2023). Darkness, usually defined as light below a critical and individual-specific threshold, can play a similarly important role for ecological traits (feeding, reproduction) as light itself (Gerrish et al., 2009). These disturbances of the dark period can affect a wide range of ecological processes (Gaston et al., 2013; Sanders et al., 2021) (Figure 2). Light pollution can have far-reaching indirect effects on wildlife through its interactions with other environmental factors (Bennie et al., 2016). For example, artificial light at night (ALAN) can attract insects, disrupting their natural behaviour and leading to changes in insect populations. This in turn can affect the foraging behaviour of insectivorous birds and bats, leading to potential declines in their populations (Longcore and Rich, 2004). Similar far reaching impact can be seen in aquatic primary producer systems, where ALAN decreases biomass and alters community composition (Grubisic et al., 2017). Such processes affect biodiversity and associated ecosystem services (Hölker et al., 2021).
FIGURE 2
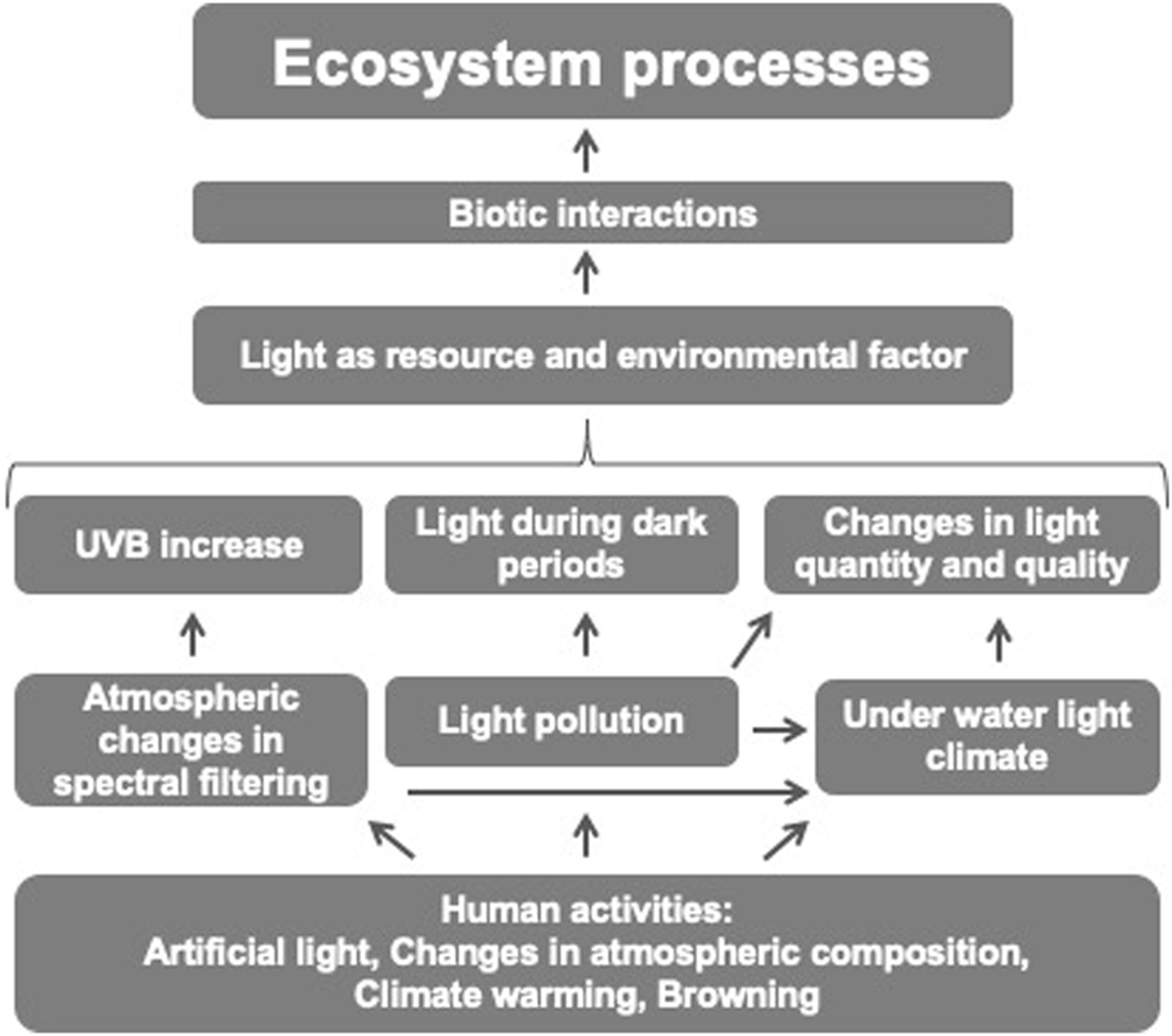
Simplified schematic of anthropogenic impacts on photoecology.
Human activities not only increase the spatial and temporal availability of light, disrupting a wide range of ecological processes, but also cause shifts in atmospheric chemistry. These shifts affect the atmospheric filtering of electromagnetic radiation from the Sun and thus the spectral composition of light at the Earth’s surface (Figure 2). A well-known example is the anthropogenic depletion of atmospheric ozone, which can result in a lower retention efficiency of the atmosphere for ultraviolet radiation (UVB) and hence higher levels of UVB reaching the Earth’s surface (Solomon, 1999). UVB radiation is known to have adverse effects on terrestrial (Caldwell et al., 1998) and aquatic organisms (Bancroft et al., 2007), with important implications for population and community dynamics and far-reaching consequences for ecosystems (Mostajir et al., 2000). Although the Montreal Protocol (1987) regulating the production and consumption of ozone depleting substances has led to an improvement of the ozone layer (Barnes et al., 2021), global climate change may cause it to deteriorate again. For example, aerosols originating from increasing wildfires have the potential to negatively impact the ozone layer (Solomon et al., 2023). In addition smoke from wildfires can result in direct changes of light quantity and light quality, for example, smoke selectively absorbs UV radiation, which can for example, have consequences for DVM in lakes (Williamson et al., 2016).
However, beside human-induced shifts in atmospheric composition, human-induced shifts in the spectral composition of underwater light also affect ecological dynamics (Figure 2). Increased transport of terrestrial carbon into aquatic systems due to global change leads to browning of water, resulting in shifts in light quantity and quality (Blanchet et al., 2022). As a result, browning can alter phytoplankton dynamics in terms of production and community composition, with far-reaching consequences for higher trophic levels (Soulié et al., 2022).
Furthermore, global change is affecting stratification and mixing of water columns. There is no clear direction of this shifts on a global scale. Warming of surface waters due to global change can lead to earlier and stronger stratification of water columns, characterised by decreasing mixing depths of upper water layers (Woolway et al., 2021; Sugimoto, 2022). In temperate seasonal regions, stratification in lakes also starts earlier, which can lead to earlier development of phytoplankton blooms in spring (Peeters et al., 2007), potentially leading to so-called match-mismatch scenarios between phototrophs and their herbivore consumers, where the timing of food availability to consumers is no longer synchronized with their seasonal dynamics (Winder and Schindler, 2004). However, climate change can also result in wind driven intensification of upper-ocean turbulence (Young and Ribal, 2019; Li et al., 2020; Sallee et al., 2021). Independent in which direction stratification and mixing depth shifts, phytoplankton suspended in the mixed layer of oceans and lakes thereby experience changes in both light quantity and quality. For example, a decrease in mixing depth results in a higher proportion of red light within the available PAR in the mixed layer, favoring cyanobacteria. Such shifts can potentially disrupt food web transfers to higher trophic levels due to the potential toxicity or poor food quality of large numbers of cyanobacteria (Stockenreiter et al., 2021).
Another aspect of global change that affects light properties for ecological dynamics is shorter ice seasons in temperate and polar regions, which shifts light availability for ecosystems, potentially leading to earlier and longer growing seasons for phototrophs, with the potential for light-driven tipping points. For example, earlier ice loss in polar regions is likely to cause extensive regime shifts, with endemic shallow-water invertebrate communities being replaced by algae, reducing coastal biodiversity and fundamentally altering ecosystem functioning (Clark et al., 2013).
Conclusion
In summary, the few selected examples described above, illustrate just how many ways light shapes the interaction between organisms and their environment; from being a resource for metabolism that influences growth, life history, and population and community dynamics, to being an environmental factor necessary to create a visual environment, or acting as a cue to structure daily and seasonal routines (Figure 1). Common to all is that light influences interactions between individuals and their biotic and abiotic environment, and thus ecological dynamics and biogeochemical cycles. Directed and undirected human-induced changes in light dynamics, as part of recent global changes, therefore affect a wide range of ecological processes (Figure 2). Modern tools in the toolbox of ecologists and environmental biologists allow the study of such processes at unprecedented resolution, providing mechanistic insights through, for example, new molecular approaches and increased causality through modern experimental capabilities. Thus, photoecology and environmental photobiology (Haeder, 2004) are core research areas that contribute to the mechanistic understanding and conservation of the unique dynamics of life on Earth.
Statements
Author contributions
HS: Conceptualization, Writing–original draft, Writing–review and editing. MS: Conceptualization, Visualization, Writing–review and editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Ahmadjian V. (1993). The lichen symbiosis. New York: John Wiley & Sons.
2
Bancroft B. A. Baker N. J. Blaustein A. R. (2007). Effects of UVB radiation on marine and freshwater organisms: a synthesis through meta-analysis. Ecol. Lett.10, 332–345. 10.1111/j.1461-0248.2007.01022.x
3
Bandara K. Varpe Ø. Wijewardene L. Tverberg V. Eiane K. (2021). Two hundred years of zooplankton vertical migration research. Biol. Rev. Camb. Philos. Soc.96, 1547–1589. 10.1111/brv.12715
4
Barnes P. W. Bornman J. F. Pandey K. K. Bernhard G. H. Bais A. F. Neale R. E. et al (2021). The success of the Montreal Protocol in mitigating interactive effects of stratospheric ozone depletion and climate change on the environment. Glob. Change Biol.27 (22), 5681–5683. 10.1111/gcb.15841
5
Begon M. Townsend C. R. Harper J. L. (2006). Ecology: from individuals to ecosystems. 4th Edn. Oxford: Blackwell Publishing.
6
Bennie J. Davies T. Cruse D. Gaston K. (2016). Ecological effects of artificial light at night on wild plants. J. Ecol.104, 611–620. 10.1111/1365-2745.12551
7
Berge J. Johnsen G. Cohen J. (2020). Polar night: life and light in the dead of the night. 1st Edn. Cham, Germany: Springer International Publishing.
8
Blanchet C. C. Arzel C. Davranche A. Kahilainen K. K. Secondi J. Taipale S. et al (2022). Ecology and extent of freshwater browning-What we know and what should be studied next in the context of global change. Sci. Total Env.812, 152420. 10.1016/j.scitotenv.2021.152420
9
Bonomi H. R. Toum L. Sycz G. Sieira R. Toscani A. M. Gudesblat G. E. et al (2016). Xanthomonas campestris attenuates virulence by sensing light through a bacteriophytochrome photoreceptor. EMBO Rep.17, 1565–1577. 10.15252/embr.201541691
10
Caldwell M. M. Bjorn L. O. Bornman J. F. Flint S. D. Kulandaivelu G. Teramura A. H. et al (1998). Effects of increased solar ultraviolet radiation on terrestrial ecosystems. J. Photochem. Photobiol. B46, 40–52. 10.1016/S1011-1344(98)00184-5
11
Cirri G. P. Pohnert G. (2019). Algae-bacteria interactions that balance the planktonic microbiome. New Phytol.223, 100–106. 10.1111/nph.15765
12
Clark G. F. Stark J. S. Johnston E. L. Runcie J. W. Goldsworthy P. M. Raymond B. et al (2013). Light-driven tipping points in polar ecosystems. Glob. Change Biol.19, 3749–3761. 10.1111/gcb.12337
13
Clusella-Trullas S. van Wyk J. H. Spotila J. R. (2007). Thermal melanism in ectotherms. J. Therm. Biol.32, 235–245. 10.1016/j.jtherbio.2007.01.013
14
Cohen J. H. Last K. S. Charpentier C. L. Cottier F. Daase M. Hobbs L. et al (2021). Photophysiological cycles in Arctic krill are entrained by weak midday twilight during the Polar Night. PLoS Biol.19, e3001413. 10.1371/journal.pbio.3001413
15
Croft M. T. Lawrence A. D. Raux-Deery E. Warren M. J. Smith A. G. (2005). Algae acquire vitamin B12 through a symbiotic relationship with bacteria. Nature438, 90–93. 10.1038/nature04056
16
Dickman E. M. Vanni M. J. Horgan M. J. (2006). Interactive effects of light and nutrients on phytoplankton stoichiometry. Oecologia149, 676–689. 10.1007/s00442-006-0473-5
17
Diehl S. (2007). Paradoxes of enrichment: effects of increased light versus nutrient supply on pelagic producer-grazer systems. Am. Nat.169, 173–191. 10.1086/516655
18
Drew G. C. Stevens E. J. King K. C. (2021). Microbial evolution and transitions along the parasite-mutualist continuum. Nat. Rev. Microbiol.19, 623–638. 10.1038/s41579-021-00550-7
19
Dziallas C. Allgaier M. Monaghan M. Grossart H. P. (2012). Act together—implications of symbioses in aquatic ciliates. Front. Microbiol.3, 288. 10.3389/fmicb.2012.00288
20
Ellis E. A. Oakley T. H. (2016). High rates of species accumulation in animals with bioluminescent courtship displays. Curr. Biol.26, 1916–1921. 10.1016/j.cub.2016.05.043
21
Elser J. J. Fagan W. F. Kerkhoff A. J. Swenson N. G. Enquist B. J. (2010). Biological stoichiometry of plant production: metabolism, scaling and ecological response to global change. New Phytol.186, 593–608. 10.1111/j.1469-8137.2010.03214.x
22
Elser J. J. Sterner R. W. Gorokhova E. Fagan W. F. Markow T. A. Cotner J. B. et al (2000). Biological stoichiometry from genes to ecosystems. Ecol. Lett.3, 540–550. 10.1111/j.1461-0248.2000.00185.x
23
Falkowski P. G. Raven J. A. (2013). Aquatic photosynthesis. Princeton, NJ: Princeton University Press.
24
Field C. B. Behrenfeld M. J. Randerson J. T. Falkowski P. (1998). Primary production of the biosphere: integrating terrestrial and oceanic components. Science281, 237–240. 10.1126/science.281.5374.237
25
Flynn K. J. Stoecker D. K. Mitra A. Raven J. Gilbert P. Hansen P. J. et al (2013). Misuse of the phytoplankton-zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J. Plankton Res.35, 3–11. 10.1093/plankt/fbs062
26
Forbes-Stovall J. Howton J. Young M. Davis G. Chandler T. Kessler B. et al (2014). Chlamydomonas reinhardtii strain CC-124 is highly sensitive to blue light in addition to green and red light in resetting its circadian clock, with the blue-light photoreceptor plant cryptochrome likely acting as negative modulator. Plant Physiol. biochem.75, 14–23. 10.1016/j.plaphy.2013.12.002
27
Gaston K. Bennie J. Davies T. Hopkins J. (2013). The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol. Rev.88, 912–927. 10.1111/brv.12036
28
Gerrish G. A. Morin J. G. Rivers T. J. Patrawala Z. (2009). Darkness as an ecological resource: the role of light in partitioning the nocturnal niche. Oecologia160, 525–536. 10.1007/s00442-009-1327-8
29
Grubisic M. Singer G. Bruno M. C. van Grunsven R. H. Manfrin A. Monaghan M. T. et al (2017). Artificial light at night decreases biomass and alters community composition of benthic primary producers in a sub‐alpine stream. Limnol. Oceanogr.62 (6), 2799–2810. 10.1002/lno.10607
30
Güsewell S. (2004). N: P ratios in terrestrial plants: variation and functional significance. New Phytol.2, 243–266. 10.1111/j.1469-8137.2004.01192.x
31
Haddock S. H. D. Moline M. A. Case J. F. (2010). Bioluminescence in the sea. Annu. Rev. Mar. Sci.2, 443–493. 10.1146/annurev-marine-120308-081028
32
Haeder D. P. (2004). Photoecology and environmental photobiology. Boca Raton, FL: CRS Press. 10.1201/b12252-47
33
Haeder D. P. Lebert M. Häder M. (2009). Photoorientation in photosynthetic flagellates. Methods Mol. Biol.571, 51–65. 10.1007/978-1-60761-198-1_3
34
Hausmann F. Arnold K. E. Marshall N. J. Owens I. P. F. (2003). Ultraviolet signals in birds are special. Proc. R. Soc. B720, 61–67. 10.1098/rspb.2002.2200
35
Hölker F. Bolliger J. Davies T. W. Giavi S. Jechow A. Kalinkat G. et al (2021). 11 pressing research questions on how light pollution affects biodiversity. Front. Ecol. Evol.9. 10.3389/fevo.2021.767177
36
Hom E. F. Y. Murray A. W. (2014). Plant-fungal ecology. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science345, 94–98. 10.1126/science.1253320
37
Hotter V. Zopf D. Kim H. J. Silge A. Schmitt M. Aiyar P. et al (2021). A polyyne toxin produced by an antagonistic bacterium blinds and lyses a Chlamydomonad alga. Proc. Natl. Acad. Sci. U.S.A.118, e2107695118. 10.1073/pnas.2107695118
38
Hutchinson G. E. (1978). An introduction to population biology. New Haven, CT: Yale University Press.
39
Jaubert M. Bouly J. P. Ribera d'Alcalà M. Falciatore A. (2017). Light sensing and responses in marine microalgae. Curr. Opin. Plant Biol.37, 70–77. 10.1016/j.pbi.2017.03.005
40
Katechakis A. Haseneder T. Kling R. Stibor H. (2005). Mixotrophic versus photoautotrophic specialist algae as food for zooplankton: the light: nutrient hypothesis might not hold for mixotrophs. Limnol. Oceanogr.50, 1290–1299. 10.4319/lo.2005.50.4.1290
41
Kehoe D. M. (2010). Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc. Natl. Acad. Sci. U.S.A.107, 9029–9030. 10.1073/pnas.1004510107
42
Lamb J. Y. Davis M. P. (2020). Salamanders and other amphibians are aglow with biofluorescence. Sci. Rep.10, 2821. 10.1038/s41598-020-59528-9
43
Lancore T. Rich C. (2004). Ecological light pollution. Front. Ecol. Environ.2, 191–198. 10.1890/1540-9295(2004)002[0191:ELP]2.0.CO;2
44
Li G. Cheng L. Zhu J. Trenberth K. E. Mann M. E. Abraham J. P. (2020). Increasing ocean stratification over the past half-century. Nat. Clim. Chang.10, 1116–1123. 10.1038/s41558-020-00918-2
45
Liu X. Xie X. Gao S. Wang L. Zhou L. Liu Y. et al (2021). Chlorophyll fluorescence as a light signal enhances iron uptake by the marine diatom Phaeodactylum tricornutum under high-cell density conditions. BMC Biol.19, 249. 10.1186/s12915-021-01177-z
46
Marshall J. Johnsen S. (2017). Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. B372, 20160335. 10.1098/rstb.2016.0335
47
Martini S. Haddock S. H. (2017). Quantification of bioluminescence from the surface to the deep sea demonstrates its predominance as an ecological trait. Sci. Rep.7, 45750. 10.1038/srep45750
48
Matsuo T. Martínez-Ramos M. Bongers F. van der Sande M. T. Poorter L. (2021). Forest structure drives changes in light heterogeneity during tropical secondary forest succession. J. Ecol.00, 2871–2884. 10.1111/1365-2745.13680
49
May R. M. (1974). Stability and complexity in model ecosystems. Princeton, NJ: Princeton University Press.
50
McCree K. J. (1972). The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol.9, 191–216. 10.1016/0002-1571(71)90022-7
51
Messinger J. Shevela D. (2011). “Principles of photosynthesis,” in Fundamentals of materials for energy and environmental sustainability. Editors GinleyD.CahenD. (Cambridge, NY: Cambridge University Press), 302–314. 10.1017/CBO9780511718786.028
52
Mitra A. Flynn K. J. Burkholder J. M. Berge T. Calbet A. Raven J. A. et al (2014). The role of mixotrophic protists in the biological carbon pump. Biogeosciences11, 995–1005. 10.5194/bg-11-995-2014
53
Mostajir B. J. Demers S. de Mora S. J. Bukata R. P. Jerome J. H. (2000). “Implications of UV radiation for the food web structure and consequences on the carbon flow,” in The effects of UV radiation in the marine environment. Editors de MoraS.DemersS.VernetM. (Cambridge, NY: Cambridge University Press), 310–320.
54
Nilsson D. E. Smolka J. Bok M. (2022). The vertical light-gradient and its potential impact on animal distribution and behavior. Front. Ecol. Evol.10, 951328. 10.3389/fevo.2022.951328
55
Ottinger S. L. Miniat C. F. Wurzburger N. (2023). Nitrogen and light regulate symbiotic nitrogen fixation by a temperate forest tree. Oecologia201, 565–574. 10.1007/s00442-023-05313-0
56
Ourisson G. Albrecht P. Rohmer M. (1984). The microbial origin of fossil fuels. Sci. Am.251, 44–51. 10.1038/scientificamerican0884-44
57
Ozaki K. Tajika E. Hong P. K. Nakagawa Y. Reinhard C. T. (2018). Effects of primitive photosynthesis on Earth’s early climate system. Nat. Geosci.11, 55–59. 10.1038/s41561-017-0031-2
58
Peeters F. Straile D. Lorke A. Ollinger D. D. A. (2007). Turbulent mixing and phytoplankton spring bloom development in a deep lake. Limnol. Oceanogr.52, 286–298. 10.4319/lo.2007.52.1.0286
59
Pimm S. L. Lawton J. H. Cohen J. E. (1991). Food web patterns and their consequences. Nature350, 669–674. 10.1038/350669a0
60
Poorter H. Niinemets Ü. Ntagkas N. Siebenkäs A. Mäenpää M. Matsubara S. et al (2019). A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol.223, 1073–1105. 10.1111/nph.15754
61
Ptacnik R. Gomes A. Royer S. J. Nerger S. A. Calbet A. Nejstgaard J. C. et al (2016). A light-induced shortcut in the planktonic microbial loop. Sci. Rep.6, 29286. 10.1038/srep29286
62
Ralser M. Varma S. Notebaart R. (2021). The evolution of the metabolic network over long timelines. Curr. Opin. Syst. Biol.28, 100402. 10.1016/j.coisb.2021.100402
63
Rhode S. C. Pawlowski M. Tollrian R. (2001). The impact of ultraviolet radiation on the vertical distribution of zooplankton of the genus Daphnia. Nature412, 69–72. 10.1038/35083567
64
Rodrigues-Comino J. Seeling S. Seeger M. K. Ries J. B. (2023). Light pollution: a review of the scientific literature. Anthr. Rev.10, 367–392. 10.1177/20530196211051209
65
Roth M. (2014). The engine of the reef: photobiology of the coral-algal symbiosis. Front. Microbiol.5, 422. 10.3389/fmicb.2014.00422
66
Russo N. J. Davies A. B. Blakey R. V. Ordway E. M. Smith T. B. (2023). Feedback loops between 3D vegetation structure and ecological functions of animals. Ecol. Lett.00, 1597–1613. 10.1111/ele.14272
67
Sachs J. L. Wilcox T. P. (2006). A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc. R. Soc. B273, 425–429. 10.1098/rspb.2005.3346
68
Sallée J. B. Pellichero V. Akhoudas C. Pauthenet E. Vignes L. Schmidtko S. et al (2021). Summertime increases in upper-ocean stratification and mixed-layer depth. Nature591 (7851), 592–598. 10.1038/s41586-021-03303-x
69
Sanders D. Frago E. Kehoe R. Patterson C. Gaston K. J. (2021). A meta-analysis of biological impacts of artificial light at night. Nat. Ecol. Evol.5, 74–81. 10.1038/s41559-020-01322-x
70
Schuback N. Tortell P. D. (2019). Diurnal regulation of photosynthetic light absorption, electron transport and carbon fixation in two contrasting oceanic environments. Biogeosciences16 (7), 1381–1399. 10.5194/bg-16-1381-2019
71
Shih P. M. (2015). Photosynthesis and early Earth. Curr. Biol.25, R855–R859. 10.1016/j.cub.2015.04.046
72
Solomon S. (1999). Stratospheric ozone depletion: a review of concepts and history. Rev. Geophys.37, 275–316. 10.1029/1999RG900008
73
Solomon S. Stone K. Yu P. Murphy D. M. Kinnison D. Ravishankara A. R. et al (2023). Chlorine activation and enhanced ozone depletion induced by wildfire aerosol. Nature615 (7951), 259–264. 10.1038/s41586-022-05683-0
74
Soulié T. Stibor H. Mas S. Braun B. Knechtel J. Nejstgaard J. C. et al (2022). Brownification reduces oxygen gross primary production and community respiration and changes the phytoplankton community composition: an in situ mesocosm experiment with high-frequency sensor measurements in a North Atlantic bay. Limnol. Oceanogr.66, 874–887. 10.1002/lno.12041
75
Sterner R. W. Elser J. J. (2002). Ecological stoichiometry. The biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press.
76
Stockenreiter M. Isanta Navarro J. Buchberger F. Stibor H. (2021). Community shifts from eukaryote to cyanobacteria dominated phytoplankton: the role of mixing depth and light quality. Freshw. Biol.66, 2145–2157. 10.1111/fwb.13822
77
Stoecker D. K. Hansen P. J. Caron D. A. Mitra A. (2017). Mixotrophy in the marine plankton. Annu. Rev. Mar. Sci.9, 311–335. 10.1146/annurev-marine-010816-060617
78
Stomp M. Huisman J. Stal L. Matthijs H. C. P. (2007a). Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. ISME J.1, 271–282. 10.1038/ismej.2007.59
79
Stomp M. Huisman J. Vörös L. Pick F. R. Laamanen M. Haverkamp T. et al (2007b). Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecol. Lett.10, 290–298. 10.1111/j.1461-0248.2007.01026.x
80
Striebel M. Behl S. Diehl S. Stibor H. (2009). Spectral niche complementarity and carbon dynamics in pelagic ecosystems. Am. Nat.174, 141–147. 10.1086/599294
81
Sugimoto S. (2022). Decreasing wintertime mixed‐layer depth in the northwestern north pacific subtropical gyre. Geophys. Res. Lett.49 (2), e2021GL095091. 10.1029/2021GL095091
82
Taylor B. N. Menge D. N. L. (2018). Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants4, 655–661. 10.1038/s41477-018-0231-9
83
Tĕšitel J. Těšitelová T. Minasiewicz J. Selosse M. A. (2018). Mixotrophy in land plants: why to stay green?Trends Plant Sci.23, 656–659. 10.1016/j.tplants.2018.05.010
84
Thompson A. W. Foster R. A. Krupke A. Carter B. J. Musat N. Vaulot D. et al (2012). Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science337, 1546–1550. 10.1126/science.1222700
85
Tilman D. (1985). The resource-ratio hypothesis of plant succession. Am. Nat.125, 827–852. 10.1086/284382
86
Urabe J. Kyle M. Makino W. Yoshida T. Anderson T. Elser J. J. (2002). Reduced light increases herbivore production due to stoichiometric effects of light/nutrient balance. Ecology83, 619–627. 10.1890/0012-9658(2002)083[0619:rlihpd]2.0.co;2
87
Urabe J. Sterner R. W. (1996). Regulation of herbivore growth by the balance of light and nutrients. Proc. Natl. Acad. Sci. U. S. A.93, 8465–8469. 10.1073/pnas.93.16.8465
88
Vad C. F. Schneider C. Lukic D. Horvath Z. Kainz M. Stibor H. et al (2020). Grazing resistance and poor food quality of a widespread mixotroph impair zooplankton secondary production. Oecologia193, 489–502. 10.1007/s00442-020-04677-x
89
van Oppen M. J. H. Medina M. (2020). Coral evolutionary responses to microbial symbioses. Philos. Trans. R. Soc. B375, 20190591. 10.1098/rstb.2019.0591
90
Venn A. A. Loram J. E. Douglas A. E. (2008). Photosynthetic symbioses in animals. J. Exp. Bot.59, 1069–1080. 10.1093/jxb/erm328
91
Verdes A. Gruber D. F. (2017). Glowing worms: biological, chemical, and functional diversity of bioluminescent annelids. Integr. Comp. Biol.57, 18–32. 10.1093/icb/icx017
92
Wainwright P. C. Longo S. J. (2017). Functional innovations and the conquest of the oceans by acanthomorph fishes. Curr. Biol.27, R550–R557. 10.1016/j.cub.2017.03.044
93
Widder E. A. (1999). “Bioluminescence,” in Adaptive mechanisms in the ecology of vision. Editors ArcherS. N.DjamgozM. B. A.LoewE. R.PartridgeJ. C.VallergaS. (Dordrecht: Springer), 4269. 10.1007/978-94-017-0619-3_19
94
Widder E. A. (2010). Bioluminescence in the ocean: origins of biological, chemical, and ecological diversity. Science328, 704–708. 10.1126/science.1174269
95
Wilde A. Mullineaux C. W. (2017). Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev.41, 900–922. 10.1093/femsre/fux045
96
Williams D. L. (2016). Light and the evolution of vision. Eye30, 173–178. 10.1038/eye.2015.220
97
Williamson C. E. Overholt E. P. Brentrup J. A. Pilla R. M. Leach T. H. Schladow S. G. et al (2016). Sentinel responses to droughts, wildfires, and floods: effects of UV radiation on lakes and their ecosystem services. Front. Ecol. Environ.14 (2), 102–109. 10.1002/fee.1228
98
Winder M. Schindler D. E. (2004). Climate change uncouples trophic interactions in an aquatic ecosystem. Ecology85, 2100–2106. 10.1890/04-0151
99
Woolway R. I. Sharma S. Weyhenmeyer G. A. Debolskiy A. Golub M. Mercado-Bettín D. et al (2021). Phenological shifts in lake stratification under climate change. Nat. Commun.12, 2318. 10.1038/s41467-021-22657-4
100
Young I. R. Ribal A. (2019). Multiplatform evaluation of global trends in wind speed and wave height. Science364 (6440), 548–552. 10.1126/science.aav9527
101
Zhang Y. Wang X. X. Feng Z. J. Cong H. S. Chen Z. S. Li Y. D. et al (2020). Superficially similar adaptation within one species exhibits similar morphological specialization but different physiological regulations and origins. Front. Cell. Dev. Biol.8, 300. 10.3389/fcell.2020.00300
Summary
Keywords
light, ecological niche, ecological stoichiometry, light induced behavior, light cycles, light pollution
Citation
Stibor H and Stockenreiter M (2023) Let there be light to interact. Front. Photobiol. 1:1284620. doi: 10.3389/fphbi.2023.1284620
Received
28 August 2023
Accepted
24 October 2023
Published
07 November 2023
Volume
1 - 2023
Edited by
Geir Johnsen, Norwegian University of Science and Technology, Norway
Reviewed by
Stephen Grant, Norwegian University of Science and Technology, Norway
Updates
Copyright
© 2023 Stibor and Stockenreiter.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herwig Stibor, stibor@bio.lmu.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.