
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 28 March 2025
Sec. Pharmacology of Anti-Cancer Drugs
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1562167
This article is part of the Research TopicExploring Novel Resistance Mechanisms in Cancer Treatments through High-Throughput Screening and Multi-Omics AnalysisView all articles
Inhibitors of Apoptosis Proteins (IAPs) are a family of anti-apoptotic proteins that play a pivotal role in apoptosis in general but also as oncoproteins in cancer progression and, more importantly, drug resistance. IAPs enable cancer cells to evade programmed cell death and adapt to therapeutic stress by inhibiting pro-apoptotic caspase activity as well as modulating pivotal survival pathways. Recent advancements in targeting IAPs, particularly through the use of SMAC (second mitochondria-derived activator of caspase) mimetics and other small-molecule antagonists or inhibitors, have opened new avenues for overcoming drug resistance in cancers. The current review attempted to summarize the status quo of IAPs’ role in promoting chemotherapeutic drug resistance in various cancer treatments and discuss the most recent development of IAP-targeting therapies, particularly small-molecule inhibitors including their combinational strategies to enhance the sensitivity or achieve synergism to existing therapeutics. Additionally, we also outline the challenges and offer future perspectives for optimizing IAP-targeted approaches to improve clinical outcomes.
Currently, most malignant cancers and tumors have been managed to be controllable and are sometimes regarded as chronic diseases if diagnosed at an early stage and treated in time and effectively. However, cancer therapy often faces a formidable challenge of drug resistance, which leads to treatment failure and poor prognosis for patients (Zhang et al., 2023; Cui et al., 2022). More strikingly, drug resistance may account for roughly 90% of cancer-related deaths, rendering it an urgent issue to address. (Pluchino et al., 2012). Drug resistance usually arises from the ability of cancer cells to evade apoptosis, survive in adverse conditions, and proliferate despite the presence of cytotoxic chemotherapeutic agents or targeted therapies, including those cutting-edge immunotherapies (Narayanan et al., 2020; Su, 2022; Perez-Ruiz et al., 2020). The reasons conferring drug resistance are often complicated and multifaceted, including inactive metabolites by chromosomes, enhanced anti-apoptotic ability, decreased drug concentration inside cancer cells by active membrane transporters, mutation of the target, shielding of the drug to reach the target, inability to completely eliminate cancer cells, including cancer stem cells, etc. (Assaraf et al., 2019; Sajid et al., 2023; Sancho et al., 2016). Of note, one primary factor can often cause resistance to multiple drugs, termed multidrug resistance (MDR). Likewise, multiple factors may also contribute to one drug’s resistance, rendering it a conundrum to tackle.
Among the key players in these processes are the Inhibitors of Apoptosis Proteins (IAPs), which are overwhelmingly overexpressed in almost all cancer types (Ramakrishnan et al., 2014; Shahar and Larisch, 2020; Rathore et al., 2017). Recently, there has been growing interest in targeting IAPs as therapeutic strategies to overcome drug resistance. Theoretically, by inhibiting the proteins that suppress apoptosis, researchers aim to restore the natural cell death processes and sensitize tumors to those existing treatments. This review highlights the information on IAPs in inducing drug resistance in cancers and discusses the potential of targeting IAPs as an effective approach to tackling drug resistance.
The human IAP family comprises eight members, including neuronal apoptosis inhibitory protein (NAIP), cellular IAP 1 (c-IAP1), cellular IAP 2 (c-IAP2), X-linked IAP (XIAP), survivin, Baculovirus IAP Repeat (BIR)-containing ubiquitin-conjugating enzyme (BRUCE or Apollon), melanoma IAP (ML-IAP or Livin), and IAP-like Protein 2 (ILP2) (Table 1), which are functioning to apoptosis regulation (Cui et al., 2023; Cetraro et al., 2022). Generally, these proteins inhibit caspase activity, thereby preventing the execution of apoptosis (Cetraro et al., 2022). However, it should be noted that not all eight members have been clearly and fully elucidated in terms of their enzymatic/interactive functions in cancer cells, except XIAP, c-IAP1/2, survivin, and BRUCE, which will be discussed briefly below.
XIAP is the most extensively studied member of IAPs in apoptosis and in cancer, and it can exert its function through direct and indirect mechanisms. XIAP appears to be the only one that potently inhibits the enzymatic activity of the proapoptotic caspases (Kashkar, 2010). Briefly, through a direct interaction, XIAP may bind to and inhibit caspases-3, -7, and -9, thereby inhibiting the initiation and/or the cascade of apoptotic event (Chai et al., 2001; Riedl et al., 2001; Shiozaki et al., 2003). In indirect ways, XIAP may 1) undermine mitochondria-mediated apoptosis, delaying the release of cytochrome c, apoptotic protease activating factor 1 (Apaf-1), and second mitochondria-derived activator of caspase (SMAC) with the involvement of Bcl-2 family proteins (Chen et al., 2018; Flanagan et al., 2010); 2) facilitate the ubiquitination and the subsequent degradation of proapoptotic SMAC (Qin et al., 2016; Macfarlane et al., 2002); And 3) interacting with other players, such as microRNA (Xie et al., 2013), HS1-associated protein X1 (HAX-1) (Kang et al., 2010), etc.
c-IAP1 and c-IAP2 are critical regulators of apoptosis, primarily acting through their E3 ubiquitin ligase activity instead of directly inhibiting caspases (Bertrand et al., 2008). c-IAP1/2 modulate key signaling pathways, particularly those involving the tumor necrosis factor receptor 1 (TNFR1) and NF-κB, to maintain cellular homeostasis (Varfolomeev et al., 2008). In the canonical TNFR1 signaling pathway, c-IAP1/2 ubiquitinate receptor-interacting protein kinase 1 (RIPK1) at the TNFR1 signaling complex, promoting the activation of NF-κB, which is a transcription factor that drives the expression of pro-survival and anti-apoptotic genes (Varfolomeev et al., 2008). This ubiquitination prevents the formation of the pro-apoptotic complex IIb, which includes RIPK1, Fas-Associated protein with Death Domain (FADD), and caspase-8, thereby suppressing apoptosis (Varfolomeev et al., 2008; Dynek et al., 2010). In addition to regulating the canonical NF-κB pathway, c-IAP1/2 play a crucial role in the non-canonical NF-κB pathway by targeting NF-κB-inducing kinase (NIK) for continuous ubiquitination and proteasomal degradation (Zarnegar et al., 2008). This regulation keeps NIK levels low under basal conditions, preventing unwanted activation of non-canonical NF-κB signaling (Zarnegar et al., 2008). The genetic depletion or pharmacological inhibition of c-IAP1/2 disrupts their ubiquitin ligase function, leading to the accumulation of RIPK1 in its non-ubiquitinated form (Darding and Meier, 2012). This allows RIPK1 to switch roles, forming pro-apoptotic or pro-necroptotic complexes, depending on the cellular context (Schorn et al., 2023; Darding and Meier, 2012).
One intricate property of IAPs is that the different members may form a complex, e.g., the formation of a survivin-XIAP complex could promote increased XIAP stability against its ubiquitination and proteasomal destruction/degradation and, thus, lead to synergistic inhibition of apoptosis (Dohi et al., 2004). Survivin has been suggested to bind directly to caspases-3 and -7, and then inhibit their activation in proapoptosis (Shin et al., 2001). However, other studies have shown the opposite results since the chemically synthesized survivin failed to inhibit caspase 3 activity (Li et al., 2008), and a previous study also suggested that mouse and human survivin did not target and suppress caspase 3 (Banks et al., 2000). Likewise, survivin was predicted to bind to caspase 9 and inhibit its activation (O'Connor et al., 2000); however, a following study validated that survivin worked cooperatively with hepatitis B X-interacting protein (HBXIP) to bind to pro-caspase 9 and thereby, inhibiting its activation and suppressing apoptosis (Marusawa et al., 2003). While existing naturally as a dimer, monomer survivin also exerts anti-apoptotic effects via directly interacting with SMAC/DIABLO and XIAP (Pavlyukov et al., 2011). As aforementioned, survivin majorly inhibits apoptosis through the formation of a complex with other IAPs or other players in regulating apoptosis pathways. Survivin directly interacts with SMAC/DIABLO in the mitochondria, preventing its release into the cytosol and subsequent activation of apoptosis (Ceballos-Cancino et al., 2007).
BRUCE acts as both a suppressor of apoptosis and an E3 ubiquitin ligase, influencing apoptotic pathways through several mechanisms, including 1) inhibition of caspase activation. BRUCE binds to and inhibits pro-apoptotic SMAC/DIABLO and HtrA2/Omi, which are released from mitochondria during apoptosis, through which BRUCE prevents them from neutralizing XIAP, thereby indirectly suppressing caspase activation (Qiu and Goldberg, 2005). 2) ubiquitination of pro-apoptotic proteins. BRUCE ubiquitinates and targets apoptotic proteins, e.g., caspase 9, for proteasomal degradation, maintaining a balance between pro-survival and pro-death signals (Hao et al., 2004); 3) regulating mitochondrial integrity. BRUCE contributes to maintaining mitochondrial integrity, which is critical for preventing the release of apoptogenic factors such as cytochrome c and SMAC/DIABLO (Ren et al., 2005); and 4) regulating the extrinsic apoptotic pathway. BRUCE modulates the extrinsic pathway by controlling the turnover of death receptor-associated proteins, thereby influencing cell death triggered by certain extracellular signals (Liu et al., 2024).
In addition, other IAPs members have also been validated to suppress apoptosis through various mechanisms. NAIP directly inhibits caspases −3 and −7 (Maier et al., 2002), and it could inhibit procaspase-9 (Davoodi et al., 2010; Karimpour et al., 2011). Similar to NAIP, Livin also interacts with caspases-3, -7 and -9 (Kasof and Gomes, 2001). Finally, ILP2 appears to be unstable to successfully inhibit the activation of caspases (Shin et al., 2005).
IAPs are frequently overexpressed in tumors, correlating with poor prognosis, enhanced tumor aggressiveness, and increased resistance to various therapies (Liang et al., 2020). IAPs contribute to drug resistance through several interconnected mechanisms that are similar and consistent with their general biological role, such as 1) apoptosis inhibition. IAPs may bind or interact with caspases, rendering them inactive and halting the apoptotic cascade in response to cytotoxic therapies (Shiozaki et al., 2003; Maier et al., 2002); 2) activation of survival pathways. c-IAP1/2 can activate NF-κB, promoting transcription of genes involved in cell proliferation, and anti-apoptotic processes (Varfolomeev et al., 2008); 3) disruption of SMAC/DIABLO activity. SMAC/DIABLO, an endogenous antagonist of IAPs, is often inactivated or sequestered, modulating the balance toward apoptosis inhibition, which can be reversed by IAPs (Ceballos-Cancino et al., 2007); and 4) coordination with other critical signal pathways to suppress cell death as we discussed above (Figure 1).
In a word, IAPs suppress apoptosis irrespective of the underlying causes, thereby contributing to MDR across a wide range of chemotherapeutic agents. This broad-spectrum resistance poses significant challenges in cancer treatment, necessitating the development of targeted strategies to overcome IAP-mediated MDR and restore the efficacy of conventional therapies.
In Table 1, we summarized the eight members and their associated drug resistance (no study has been published regarding NAIP or ILP2 as the leading/main role in causing drug resistance in cancers). Particularly, we would like to briefly discuss the drug resistance caused by XIAP and survivin, which are the two most studied and play a leading role in conferring drug resistance in cancers (Li et al., 2000).
As shown in Table 1, XIAP contributes to resistance against Taxol in prostate, lung, pancreatic, and breast cancers, emphasizing its involvement in protecting cancer cells from apoptosis induced by this commonly used microtubule-targeting agent (Nomura et al., 2003; Lin et al., 2004; Lopes et al., 2007). XIAP mediates resistance to Docetaxel in prostate, ovarian, and pancreatic cancers (Zhang et al., 2021; Lopes et al., 2007; Sapi et al., 2004). Beyond taxanes, XIAP has been shown to confer resistance to Doxorubicin in breast cancer (Delbue et al., 2020), and to cisplatin in colon, ovarian, lung, and pancreatic cancers (Xiong et al., 2017; Xu et al., 2017; Ma et al., 2009; Lopes et al., 2007). Additionally, XIAP is implicated in resistance to Gemcitabine in pancreatic cancer (Shrikhande et al., 2006), and to Carboplatin in ovarian cancer (Thibault et al., 2018). XIAP also contributes to resistance against Vincristine in neuroblastoma (Frommann et al., 2018). Furthermore, XIAP induces resistance to tyrosine kinase inhibitors such as Lapatinib in breast cancer (Aird et al., 2010), and Imatinib in acute myeloid leukemia (AML) (Silva et al., 2013). By directly inhibiting caspases and blocking apoptosis, XIAP allows cancer cells to evade cell death mechanisms triggered by these drugs, leading to treatment failure and disease progression.
Survivin, as summarized in Table 1, is associated with resistance to Sorafenib and Doxorubicin in hepatocellular carcinoma (HCC) (Sun et al., 2021; Liu et al., 2023). It also contributes to resistance to Doxorubicin in breast cancer and AML (Wang et al., 2003), as well as Vincristine in lung cancer, acute lymphoblastic leukemia (ALL), and myeloma (Zhou J. et al., 2018; Park et al., 2011; Tsubaki et al., 2015). Furthermore, survivin induces resistance to taxanes such as Docetaxel in lung cancer and Taxol in ovarian and lung cancers (Zaffaroni et al., 2002). Resistance to DNA-damaging agents is also evident, with survivin implicated in resistance to Cisplatin in bladder (Krafft et al., 2019), gastric (Dong et al., 2014; Sun et al., 2014), and lung cancers (Hu et al., 2016), and Cytarabine in AML (Stroopinsky et al., 2018). It also mediates resistance to Gemcitabine in pancreatic cancer (Fuller et al., 2022) and Daunorubicin in ALL (Wu et al., 2008). Additionally, survivin reduces the effectiveness of targeted therapies, such as Imatinib in chronic myeloid leukemia (CML) (Speletas et al., 2011; Carter et al., 2006) and Gefitinib in lung cancer (Zhou et al., 2018).
By interfering with apoptosis and promoting treatment resistance, XIAP and survivin represent two critical therapeutic targets for overcoming drug resistance and improving cancer treatment outcomes, as discussed in Section 3. Furthermore, IAPs also contribute to resistance to immunotherapies (Evans et al., 2016; Straszewski-Chavez et al., 2004; Sugihara et al., 2020), radiation (Holcik et al., 2000; Sun et al., 2011), TRAIL (tumor necrosis factor-related apoptosis-inducing ligand) (Gillissen et al., 2013; Ndozangue-Touriguine et al., 2008), suggesting that their pivotal role and they may serve as a vulnerability to reverse drug resistance.
Therapeutic strategies targeting IAPs focus on antagonizing their antiapoptotic activity, restoring caspase function, and sensitizing cancer cells to treatment. In this review, we took a spot on small-molecule inhibitors. To clarify, non-specific inhibitors, such as many compounds derived from natural products, are excluded from this review. These small-molecule inhibitors (listed in Table 2; Figure 2), including 1) c-IAP1/2 inhibitors such as ASTX660, birinapant, and DEBIO 1143, which are all under clinical trials; 2) XIAP inhibitors, such as drug candidate SM-164, and LCL161; and 3) survivin inhibitors which disrupt its role in apoptosis inhibition and mitosis regulation. Examples include drug candidates YM155, FL118, and Terameprocol, etc. Other compounds in laboratory or preclinical studies are also included. It should be noted that, since IAPs share similar 3D motifs in their structures, thus, many of these small-molecule inhibitors may be able to target and bind to several IAPs. Furthermore, due to similar reasons, those IAPs inhibitors have demonstrated overlapping yet slightly different mechanisms, i.e., reversing IAPs-mediated caspases activation, thereby inducing apoptosis of intrinsic or extrinsic when combined with other conventional or targeted therapies.
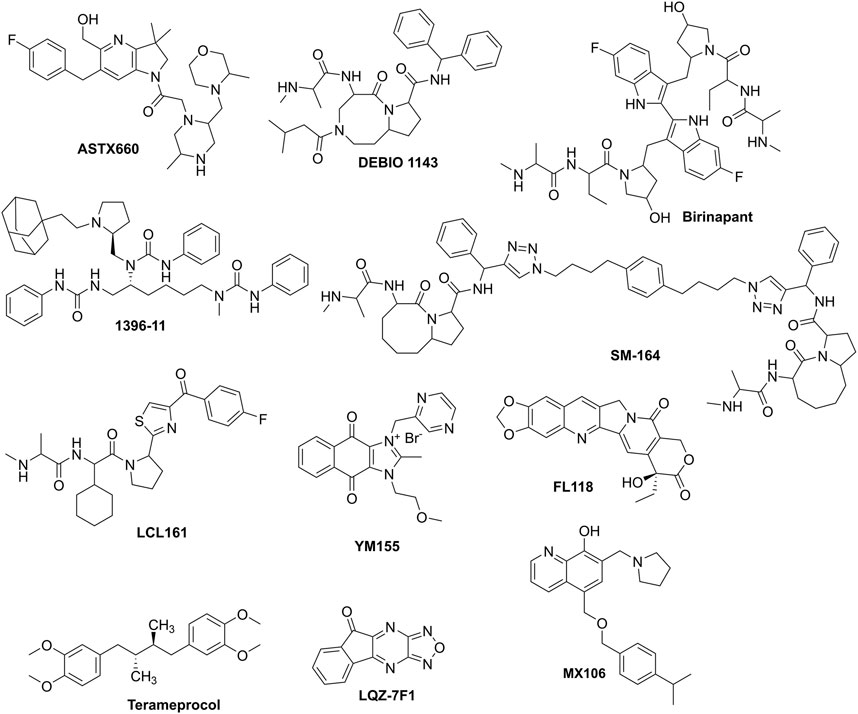
Figure 2. The prominent IAPs that have been used for combination to combat drug resistance in cancers.
DEBIO 1143 is an inhibitor used in combination with other drugs to overcome drug resistance in cancers, as summarized in Table 2. Specifically, DEBIO 1143 is combined with Carboplatin for the treatment of ovarian cancer (Thibault et al., 2018). Additionally, it is used with Cisplatin for head and neck cancer in phase 1 and 2 clinical trials, which showed promising effects among high-risk locoregionally advanced patients (Sun et al., 2020; Le Tourneau et al., 2020).
Birinapant is an IAP inhibitor used in various combinations to combat drug resistance in different cancers, as detailed in Table 2. It is combined with Venetoclax (Perimenis et al., 2016) and Bazedoxifene for colorectal cancer (Dmello et al., 2024). For triple-negative breast cancer (TNBC), Birinapant is used with Gemcitabine (Xie et al., 2021) and Taxol (Shu et al., 2019). It is also paired with Ralimetinib for non-small cell lung cancer (NSCLC) (Colombo et al., 2020), and with Bortezomib for myeloma (Zhou et al., 2019). Additionally, Birinapant is combined with Carboplatin for ovarian cancer (Singh et al., 2022), 5-Azacytidine for AML (Carter et al., 2014), Dacarbazine for melanoma (Vetma et al., 2017). This showcases Birinapant’s versatility in enhancing treatment efficacy across multiple cancer types when used in combination therapies. While extensive clinical trials have been conducted, it has been inactive recently for cancer treatment.
SM-164 is an IAP inhibitor that has been studied in combination with various drugs to overcome drug resistance in different cancers, as shown in Table 2. It is combined with Doxorubicin to treat osteosarcoma (Chen et al., 2019) and HCC (Zhang et al., 2012). Additionally, SM-164 is used with Gemcitabine for pancreatic cancer (Zhou et al., 2013). Currently, no active clinical trials have been scheduled.
LCL161, another IAP inhibitor, is utilized in combination therapies for various cancers. It is combined with Vincristine for neuroblastoma (Langemann et al., 2017; Frommann et al., 2018), Auranofin for ALL (Hass et al., 2016), and Panobinostat for myeloma (Zhou et al., 2021). LCL161 is also combined with Taxol for NSCLC (Yang et al., 2016), HCC (Tian et al., 2014), and TNBC (Bardia et al., 2018), and with Gemcitabine/cisplatin for cholangiocarcinoma (Prasopporn et al., 2022). Furthermore, it is used with Navitoclax for breast cancer (Lee et al., 2020), Vincristine/cisplatin for medulloblastoma (Chen et al., 2016), and 5-Azacytidine for AML (Gerges et al., 2016), demonstrating its wide application in improving treatment outcomes across diverse cancer types. LCL161 has been extensively tested in clinical trials for cancers in combination; however, it has not been approved yet.
YM155, a drug candidate, is a small-molecule inhibitor specifically designed to target the transcription factor survivin, thereby promoting cell survival and inhibiting apoptosis. YM155 works by suppressing the expression of survivin, which can potentially enhance the sensitivity of chemotherapeutics. In clinical and preclinical studies, YM155 has been combined with various chemotherapeutic agents to enhance treatment efficacy across various cancers. YM155 has been combined with Cisplatin, showing promising results in reducing tumor growth in malignant rhabdoid tumor, an aggressive pediatric cancer (Coyle et al., 2022). Combined with Cisplatin, YM155 has demonstrated synergistic effects in reducing cancer cell viability of head and neck cancers (Kumar et al., 2012). Two studies have highlighted the use of YM155 with Doxorubicin. One study focused on the combination’s effect on reducing tumor burden of osteosarcoma (Gao et al., 2015), while another explored its role in inducing apoptosis in osteosarcoma cells (Zhang et al., 2015). When combined with Cabazitaxel, YM155 has shown to enhance the cytotoxic effects of the chemotherapy, potentially offering a more effective treatment for advanced prostate cancer (Miyao et al., 2020). YM155 has been paired with Erlotinib, a targeted therapy for lung cancer, showing improved outcomes by targeting different pathways of cancer cell survival (Okamoto et al., 2012; Shimizu et al., 2020). Additionally, Taxol has been combined with YM155, indicating potential benefits in NSCLC treatment (Baspinar et al., 2019). For NSCLC specifically, the combination of Taxol and Carboplatin with YM155 has been researched, with some evidence of increased efficacy (Kelly et al., 2013). Docetaxel combined with YM155 has been studied for TNBC, showing potential in reducing cancer cell proliferation (Kaneko et al., 2013). Decitabine, a DNA hypomethylating agent, has been combined with YM155 for AML, aiming to increase the sensitivity of leukemia cells to treatment by altering gene expression patterns (Yao et al., 2024). Gemcitabine, a standard chemotherapy for pancreatic cancer, when used with YM155, has shown to potentially improve patient outcomes by targeting different aspects of cancer cell survival mechanisms (Yoon et al., 2012). Two different combinations have been explored for renal cancer; Rapamycin (Koike et al., 2014), which inhibits mTOR, and ABT-737 (Woo et al., 2017), a BH3 mimetic, both when used with YM155, have shown synergistic effects in promoting cancer cell death. Rituximab, a monoclonal antibody, has been combined with YM155 to target B-cell lymphomas, enhancing the immunological and apoptotic effects on lymphoma cells (Papadopoulos et al., 2016). However, no active clinical trials for YM155 are now undergoing. Interestingly, research showed that c-IAP1 overexpression could induce resistance to YM155 (Jung et al., 2015), suggesting that the members of IAPs have compensatory effects.
The above information has validated that targeting IAPs may have high potential in overcoming MDR in cancer. However, challenges remain. Here, we attempted to predict the potential future directions.
(1) The development of specific IAP inhibitors, including proteolysis targeting chimeras (PROTACs). Inhibitors that specifically target IAPs are crucial for drug development. Traditional inhibitors might lack precision, leading to side effects or resistance. A novel approach involves using PROTACs, which degrade IAPs rather than just blocking them, potentially offering a more definitive way to eliminate these proteins from cancer cells.
(2) Exploring more combination therapies. Combining IAP-targeted treatments with other cancer therapies like immunotherapy, targeted treatments could enhance their effectiveness. Such combinations could disrupt multiple survival pathways in cancer cells, reducing their ability to adapt and survive. Exploring more effective combinations may potentially lead to customized treatment strategies based on the unique characteristics of each patient.
(3) Biomarker discovery. Identifying biomarkers that can predict patient response to IAP-targeted treatments is essential for personalized medicine. This would involve looking for signs of IAP activity or related pathways within tumors. Advanced technologies for analyzing genetics, proteins, and even tumor metabolism are key here. Discovering these biomarkers would help in selecting the right patients for clinical trials, improving the chances of successful outcomes.
(4) Extensive clinical trials. To move IAP-targeted therapies from bench-side to clinical use, comprehensive trials are needed. These should evaluate not just their safety and effectiveness but also the best ways to administer them, including dosage and combination with other treatments. Long-term studies are also important to monitor sustained effectiveness, potential long-term side effects, and resistance development.
IAPs have been validated in inducing drug resistance and MDR in various cancers. Growing studies have shown that targeting IAPs is a feasible approach to overcome drug resistance in cancers. By restoring apoptotic pathways and disrupting survival mechanisms, IAPs inhibitors have the potential to improve outcomes for patients with drug-resistant cancers significantly.
QY: Conceptualization, Funding acquisition, Writing–original draft. X-ZZ: Conceptualization, Data curation, Formal Analysis, Writing–original draft. JL: Conceptualization, Methodology, Project administration, Writing–review and editing. XZ: Investigation, Project administration, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (Grant number: 82160735) and Hainan Province Clinical Medical Center.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aird, K. M., Ghanayem, R. B., Peplinski, S., Lyerly, H. K., and Devi, G. R. (2010). X-linked inhibitor of apoptosis protein inhibits apoptosis in inflammatory breast cancer cells with acquired resistance to an ErbB1/2 tyrosine kinase inhibitor. Mol. Cancer Ther. 9 (5), 1432–1442. doi:10.1158/1535-7163.MCT-10-0160
Assaraf, Y. G., Brozovic, A., Goncalves, A. C., Jurkovicova, D., Line, A., Machuqueiro, M., et al. (2019). The multi-factorial nature of clinical multidrug resistance in cancer. Drug resist. Update 46, 100645. doi:10.1016/j.drup.2019.100645
Banks, D. P., Plescia, J., Altieri, D. C., Chen, J., Rosenberg, S. H., Zhang, H., et al. (2000). Survivin does not inhibit caspase-3 activity. Blood 96 (12), 4002–4003. doi:10.1182/blood.v96.12.4002
Bardia, A., Parton, M., Kummel, S., Estevez, L. G., Huang, C. S., Cortes, J., et al. (2018). Paclitaxel with inhibitor of apoptosis antagonist, LCL161, for localized triple-negative breast cancer, prospectively stratified by gene signature in a biomarker-driven neoadjuvant trial. J. Clin. Oncol. 36, 3126–3133. doi:10.1200/JCO.2017.74.8392
Baspinar, Y., Erel-Akbaba, G., Kotmakci, M., and Akbaba, H. (2019). Development and characterization of nanobubbles containing paclitaxel and survivin inhibitor YM155 against lung cancer. Int. J. Pharm. 566, 149–156. doi:10.1016/j.ijpharm.2019.05.039
Bertrand, M. J., Milutinovic, S., Dickson, K. M., Ho, W. C., Boudreault, A., Durkin, J., et al. (2008). cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 30 (6), 689–700. doi:10.1016/j.molcel.2008.05.014
Carter, B. Z., Mak, D. H., Schober, W. D., Cabreira-Hansen, M., Beran, M., Mcqueen, T., et al. (2006). Regulation of survivin expression through Bcr-Abl/MAPK cascade: targeting survivin overcomes imatinib resistance and increases imatinib sensitivity in imatinib-responsive CML cells. Blood 107 (4), 1555–1563. doi:10.1182/blood-2004-12-4704
Carter, B. Z., Mak, P. Y., Mak, D. H., Shi, Y., Qiu, Y., Bogenberger, J. M., et al. (2014). Synergistic targeting of AML stem/progenitor cells with IAP antagonist birinapant and demethylating agents. J. Natl. Cancer. Inst. 106 (2), djt440. doi:10.1093/jnci/djt440
Ceballos-Cancino, G., Espinosa, M., Maldonado, V., and Melendez-Zajgla, J. (2007). Regulation of mitochondrial Smac/DIABLO-selective release by survivin. Oncogene 26 (54), 7569–7575. doi:10.1038/sj.onc.1210560
Cetraro, P., Plaza-Diaz, J., Mackenzie, A., and Abadia-Molina, F. (2022). A review of the current impact of inhibitors of apoptosis proteins and their repression in cancer. Cancers 14 (7), 1671. doi:10.3390/cancers14071671
Chai, J., Shiozaki, E., Srinivasula, S. M., Wu, Q., Datta, P., Alnemri, E. S., et al. (2001). Structural basis of caspase-7 inhibition by XIAP. Cell 104 (5), 769–780. doi:10.1016/s0092-8674(01)00272-0
Chao, A., Lin, C. Y., Wu, R. C., Lee, Y. S., Lee, L. Y., Tsai, C. L., et al. (2018). The combination of everolimus and terameprocol exerts synergistic antiproliferative effects in endometrial cancer: molecular role of insulin-like growth factor binding protein 2. J. Mol. Med. 96 (11), 1251–1266. doi:10.1007/s00109-018-1699-5
Chen, C., Liu, T. S., Zhao, S. C., Yang, W. Z., Chen, Z. P., and Yan, Y. (2018). XIAP impairs mitochondrial function during apoptosis by regulating the Bcl-2 family in renal cell carcinoma. Exp. Ther. Med. 15 (5), 4587–4593. doi:10.3892/etm.2018.5974
Chen, J., Chen, X., Chen, X., Sun, H., and Yang, D. (2019). SM-164 enhances the antitumor activity of adriamycin in human U2-OS cells via downregulation of X-linked inhibitor of apoptosis protein. Mol. Med. Rep. 19 (6), 5079–5086. doi:10.3892/mmr.2019.10181
Chen, J. R., Jia, X. H., Wang, H., Yi, Y. J., and Li, Y. J. (2017). With no interaction, knockdown of Apollon and MDR1 reverse the multidrug resistance of human chronic myelogenous leukemia K562/ADM cells. Oncol. Rep. 37 (5), 2735–2742. doi:10.3892/or.2017.5535
Chen, S. M., Li, Y. Y., Tu, C. H., Salazar, N., Tseng, Y. Y., Huang, S. F., et al. (2016). Blockade of inhibitors of apoptosis proteins in combination with conventional chemotherapy leads to synergistic antitumor activity in medulloblastoma and cancer stem-like cells. PLoS One 11 (8), e0161299. doi:10.1371/journal.pone.0161299
Colombo, M., Marabese, M., Vargiu, G., Broggini, M., and Caiola, E. (2020). Activity of birinapant, a SMAC mimetic compound, alone or in combination in NSCLCs with different mutations. Front. Oncol. 10, 532292. doi:10.3389/fonc.2020.532292
Coyle, R., O'Sullivan, M. J., and Zisterer, D. M. (2022). Targeting inhibitor of apoptosis proteins (IAPs) with IAP inhibitors sensitises malignant rhabdoid tumour cells to cisplatin. Cancer Treat. Res. Commun. 32, 100579. doi:10.1016/j.ctarc.2022.100579
Cui, Q., Huang, C., Liu, J. Y., and Zhang, J. T. (2023). Small molecule inhibitors targeting the “undruggable” survivin: the past, present, and future from a medicinal chemist's perspective. J. Med. Chem. 66 (24), 16515–16545. doi:10.1021/acs.jmedchem.3c01130
Cui, Q., Wang, C., Zeng, L., Zhou, Q. X., and Fan, Y. F. (2022). Editorial: novel small-molecule agents in overcoming multidrug resistance in cancers. Front. Chem. 10, 921985. doi:10.3389/fchem.2022.921985
Darding, M., and Meier, P. (2012). IAPs: guardians of RIPK1. Cell Death Differ. 19 (1), 58–66. doi:10.1038/cdd.2011.163
Davoodi, J., Ghahremani, M. H., Es-Haghi, A., Mohammad-Gholi, A., and Mackenzie, A. (2010). Neuronal apoptosis inhibitory protein, NAIP, is an inhibitor of procaspase-9. Int. J. Biochem. Cell Biol. 42 (6), 958–964. doi:10.1016/j.biocel.2010.02.008
Dean, E. J., Ward, T., Pinilla, C., Houghten, R., Welsh, K., Makin, G., et al. (2010). A small molecule inhibitor of XIAP induces apoptosis and synergises with vinorelbine and cisplatin in NSCLC. Br. J. Cancer. 102 (1), 97–103. doi:10.1038/sj.bjc.6605418
Delbue, D., Mendonca, B. S., Robaina, M. C., Lemos, L., Lucena, P. I., Viola, J., et al. (2020). Expression of nuclear XIAP associates with cell growth and drug resistance and confers poor prognosis in breast cancer. Biochim. Biophys. Acta-Mol. Cell Res. 1867 (10), 118761. doi:10.1016/j.bbamcr.2020.118761
Ding, Z. Y., Liu, G. H., Olsson, B., and Sun, X. F. (2013). Upregulation of the antiapoptotic factor Livin contributes to cisplatin resistance in colon cancer cells. Tumour Biol. 34 (2), 683–693. doi:10.1007/s13277-012-0596-8
Dmello, R. S., Palmieri, M., Thilakasiri, P. S., Doughty, L., Nero, T. L., Poh, A. R., et al. (2024). Combination of bazedoxifene with chemotherapy and SMAC-mimetics for the treatment of colorectal cancer. Cell Death Dis. 15 (4), 255. doi:10.1038/s41419-024-06631-8
Dohi, T., Okada, K., Xia, F., Wilford, C. E., Samuel, T., Welsh, K., et al. (2004). An IAP-IAP complex inhibits apoptosis. J. Biol. Chem. 279 (33), 34087–34090. doi:10.1074/jbc.C400236200
Dong, H., Liu, G., Jiang, B., Guo, J., Tao, G., Yiu, W., et al. (2014). Overexpression of the Survivin gene in SGC7901 cell resistance to cisplatin. Oncol. Lett. 8 (5), 1953–1956. doi:10.3892/ol.2014.2463
Dynek, J. N., Goncharov, T., Dueber, E. C., Fedorova, A. V., Izrael-Tomasevic, A., Phu, L., et al. (2010). c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling. EMBO. J. 29 (24), 4198–4209. doi:10.1038/emboj.2010.300
Evans, M. K., Sauer, S. J., Nath, S., Robinson, T. J., Morse, M. A., and Devi, G. R. (2016). X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity. Cell Death Dis. 7 (1), e2073. doi:10.1038/cddis.2015.412
Faversani, A., Vaira, V., Moro, G. P., Tosi, D., Lopergolo, A., Schultz, D. C., et al. (2014). Survivin family proteins as novel molecular determinants of doxorubicin resistance in organotypic human breast tumors. Breast Cancer Res. 16 (3), R55. doi:10.1186/bcr3666
Flanagan, L., Sebastia, J., Tuffy, L. P., Spring, A., Lichawska, A., Devocelle, M., et al. (2010). XIAP impairs Smac release from the mitochondria during apoptosis. Cell Death Dis. 1 (6), e49. doi:10.1038/cddis.2010.26
Frommann, K., Appl, B., Hundsdoerfer, P., Reinshagen, K., and Eschenburg, G. (2018). Vincristine resistance in relapsed neuroblastoma can be efficiently overcome by Smac mimetic LCL161 treatment. J. Pediatr. Surg. 53 (10), 2059–2064. doi:10.1016/j.jpedsurg.2018.01.012
Fuller, R. N., Kabagwira, J., Vallejos, P. A., Folkerts, A. D., and Wall, N. R. (2022). Survivin splice variant 2β enhances pancreatic ductal adenocarcinoma resistance to gemcitabine. OncoTargets Ther. 15, 1147–1160. doi:10.2147/OTT.S341720
Gao, J. Z., Chen, F. H., Wang, L., Wei, H., and Meng, S. L. (2015). YM155 inhibits tumor growth and enhances chemosensitivity to cisplatin in osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 19 (11), 2062–2069.
Gerges, S., Rohde, K., and Fulda, S. (2016). Cotreatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells. Cancer Lett. 375 (1), 127–132. doi:10.1016/j.canlet.2016.02.040
Gillissen, B., Richter, A., Richter, A., Overkamp, T., Essmann, F., Hemmati, P. G., et al. (2013). Targeted therapy of the XIAP/proteasome pathway overcomes TRAIL-resistance in carcinoma by switching apoptosis signaling to a Bax/Bak-independent 'type I' mode. Cell Death Dis. 4 (5), e643. doi:10.1038/cddis.2013.67
Hao, Y., Sekine, K., Kawabata, A., Nakamura, H., Ishioka, T., Ohata, H., et al. (2004). Apollon ubiquitinates SMAC and caspase-9, and has an essential cytoprotection function. Nat. Cell Biol. 6 (9), 849–860. doi:10.1038/ncb1159
Hass, C., Belz, K., Schoeneberger, H., and Fulda, S. (2016). Sensitization of acute lymphoblastic leukemia cells for LCL161-induced cell death by targeting redox homeostasis. Biochem. Pharmacol. 105, 14–22. doi:10.1016/j.bcp.2016.01.004
Holcik, M., Yeh, C., Korneluk, R. G., and Chow, T. (2000). Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene 19 (36), 4174–4177. doi:10.1038/sj.onc.1203765
Hoskin, P., Lee, M., Dunkley, D., Danh, M., Wickens, R., Saunders, G., et al. (2024). Dose escalation of tolinapant (ASTX660) in combination with standard radical chemoradiotherapy in cervical cancer: a study protocol for a phase 1b TiTE-CRM clinical trial (CRAIN) in UK secondary care centres. BMC Cancer 24 (1), 702. doi:10.1186/s12885-024-12310-w
Hu, W., Jin, P., and Liu, W. (2016). Periostin contributes to cisplatin resistance in human non-small cell lung cancer A549 cells via activation of Stat3 and akt and upregulation of survivin. Cell Physiol. Biochem. 38 (3), 1199–1208. doi:10.1159/000443068
Jung, S. A., Park, Y. M., Hong, S. W., Moon, J. H., Shin, J. S., Lee, H. R., et al. (2015). Cellular inhibitor of apoptosis protein 1 (cIAP1) stability contributes to YM155 resistance in human gastric cancer cells. J. Biol. Chem. 290 (16), 9974–9985. doi:10.1074/jbc.M114.600874
Kaneko, N., Yamanaka, K., Kita, A., Tabata, K., Akabane, T., and Mori, M. (2013). Synergistic antitumor activities of sepantronium bromide (YM155), a survivin suppressant, in combination with microtubule-targeting agents in triple-negative breast cancer cells. Biol. Pharm. Bull. 36 (12), 1921–1927. doi:10.1248/bpb.b13-00515
Kang, Y. J., Jang, M., Park, Y. K., Kang, S., Bae, K. H., Cho, S., et al. (2010). Molecular interaction between HAX-1 and XIAP inhibits apoptosis. Biochem. Biophys. Res. Commun. 393 (4), 794–799. doi:10.1016/j.bbrc.2010.02.084
Karimpour, S., Davoodi, J., and Ghahremani, M. H. (2011). Integrity of ATP binding site is essential for effective inhibition of the intrinsic apoptosis pathway by NAIP. Biochem. Biophys. Res. Commun. 407 (1), 158–162. doi:10.1016/j.bbrc.2011.02.130
Kashkar, H. (2010). X-linked inhibitor of apoptosis: a chemoresistance factor or a hollow promise. Clin. Cancer Res. 16 (18), 4496–4502. doi:10.1158/1078-0432.CCR-10-1664
Kasof, G. M., and Gomes, B. C. (2001). Livin, a novel inhibitor of apoptosis protein family member. J. Biol. Chem. 276 (5), 3238–3246. doi:10.1074/jbc.M003670200
Kelly, R. J., Thomas, A., Rajan, A., Chun, G., Lopez-Chavez, A., Szabo, E., et al. (2013). A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 24 (10), 2601–2606. doi:10.1093/annonc/mdt249
Kim, J., Jeong, Y., Shin, Y. M., Kim, S. E., and Shin, S. J. (2024). FL118 enhances therapeutic efficacy in colorectal cancer by inhibiting the homologous recombination repair pathway through survivin-RAD51 downregulation. Cancers 16 (19), 3385. doi:10.3390/cancers16193385
Kimura, K., Chun, J. H., Lin, Y. L., Liang, Y. C., Jackson, T., and Huang, R. (2023). Tetra-O-methyl-nordihydroguaiaretic acid inhibits energy metabolism and synergistically induces anticancer effects with temozolomide on LN229 glioblastoma tumors implanted in mice while preventing obesity in normal mice that consume high-fat diets. PLoS One 18 (5), e0285536. doi:10.1371/journal.pone.0285536
Koike, H., Nitta, T., Sekine, Y., Arai, S., Furuya, Y., Nomura, M., et al. (2014). YM155 reverses rapamycin resistance in renal cancer by decreasing survivin. J. Cancer. Res. Clin. Oncol. 140 (10), 1705–1713. doi:10.1007/s00432-014-1734-z
Krafft, U., Tschirdewahn, S., Hess, J., Harke, N. N., Hadaschik, B., Olah, C., et al. (2019). Validation of survivin and HMGA2 as biomarkers for cisplatin resistance in bladder cancer. Urol. Oncol. 37 (11), 810.e7–810. doi:10.1016/j.urolonc.2019.04.015
Kumar, B., Yadav, A., Lang, J. C., Cipolla, M. J., Schmitt, A. C., Arradaza, N., et al. (2012). YM155 reverses cisplatin resistance in head and neck cancer by decreasing cytoplasmic survivin levels. Mol. Cancer Ther. 11 (9), 1988–1998. doi:10.1158/1535-7163.MCT-12-0167
Langdon, C. G., Wiedemann, N., Held, M. A., Mamillapalli, R., Iyidogan, P., Theodosakis, N., et al. (2015). SMAC mimetic Debio 1143 synergizes with taxanes, topoisomerase inhibitors and bromodomain inhibitors to impede growth of lung adenocarcinoma cells. Oncotarget 6 (35), 37410–37425. doi:10.18632/oncotarget.6138
Langemann, D., Trochimiuk, M., Appl, B., Hundsdoerfer, P., Reinshagen, K., and Eschenburg, G. (2017). Sensitization of neuroblastoma for vincristine-induced apoptosis by Smac mimetic LCL161 is attended by G2 cell cycle arrest but is independent of NFκB, RIP1 and TNF-α. Oncotarget 8 (50), 87763–87772. doi:10.18632/oncotarget.21193
Lee, K. M., Lee, H., Han, D., Moon, W. K., Kim, K., Oh, H. J., et al. (2020). Combined the SMAC mimetic and BCL2 inhibitor sensitizes neoadjuvant chemotherapy by targeting necrosome complexes in tyrosine aminoacyl-tRNA synthase-positive breast cancer. Breast Cancer Res. 22 (1), 130. doi:10.1186/s13058-020-01367-7
Le Tourneau, C., Tao, Y., Gomez-Roca, C., Cristina, V., Borcoman, E., Deutsch, E., et al. (2020). Phase I trial of debio 1143, an antagonist of inhibitor of apoptosis proteins, combined with cisplatin chemoradiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. Clin. Cancer Res. 26 (24), 6429–6436. doi:10.1158/1078-0432.CCR-20-0425
Li, C., Wu, Z., Liu, M., Pazgier, M., and Lu, W. (2008). Chemically synthesized human survivin does not inhibit caspase-3. Sci 17 (9), 1624–1629. doi:10.1110/ps.036145.108
Li, J., Sasaki, H., Sheng, Y. L., Schneiderman, D., Xiao, C. W., Kotsuji, F., et al. (2000). Apoptosis and chemoresistance in human ovarian cancer: is Xiap a determinant? Biol. Signals Recept 9 (2), 122–130. doi:10.1159/000014631
Li, Y., He, W., Gao, X., Lu, X., Xie, F., Um, S. W., et al. (2023). Cullin7 induces docetaxel resistance by regulating the protein level of the antiapoptotic protein Survivin in lung adenocarcinoma cells. J. Thorac. Dis. 15 (9), 5006–5019. doi:10.21037/jtd-23-1110
Liang, J., Zhao, W., Tong, P., Li, P., Zhao, Y., Li, H., et al. (2020). Comprehensive molecular characterization of inhibitors of apoptosis proteins (IAPs) for therapeutic targeting in cancer. BMC Med. Genomics. 13 (1), 7. doi:10.1186/s12920-020-0661-x
Lin, M. T., Chang, C. C., Chen, S. T., Chang, H. L., Su, J. L., Chau, Y. P., et al. (2004). Cyr61 expression confers resistance to apoptosis in breast cancer MCF-7 cells by a mechanism of NF-kappaB-dependent XIAP up-regulation. J. Biol. Chem. 279 (23), 24015–24023. doi:10.1074/jbc.M402305200
Ling, X., Liu, X., Zhong, K., Smith, N., Prey, J., and Li, F. (2015). FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am. J. Transl. Res. 7 (10), 1765–1781.
Ling, X., Wu, W., Fan, C., Xu, C., Liao, J., Rich, L. J., et al. (2018). An ABCG2 non-substrate anticancer agent FL118 targets drug-resistant cancer stem-like cells and overcomes treatment resistance of human pancreatic cancer. J. Exp. Clin. Cancer Res. 37 (1), 240. doi:10.1186/s13046-018-0899-8
Liu, F., Xie, Z. H., Cai, G. P., and Jiang, Y. Y. (2007). The effect of survivin on multidrug resistance mediated by P-glycoprotein in MCF-7 and its adriamycin resistant cells. Biol. Pharm. Bull. 30 (12), 2279–2283. doi:10.1248/bpb.30.2279
Liu, J., Zhang, Q., Wang, C., Yang, J., Yang, S., Wang, T., et al. (2023). Knockdown of BAP31 overcomes hepatocellular carcinoma doxorubicin resistance through downregulation of survivin. Int. J. Mol. Sci. 24 (8), 7622. doi:10.3390/ijms24087622
Liu, S., Jiang, T., Bu, F., Zhao, J., Wang, G., Yang, G., et al. (2024). Molecular mechanisms underlying the BIRC6-mediated regulation of apoptosis and autophagy. Nat. Commun. 15 (1), 891. doi:10.1038/s41467-024-45222-1
Liu, Y., Wu, X., Sun, Y., and Chen, F. (2011). Silencing of X-linked inhibitor of apoptosis decreases resistance to cisplatin and paclitaxel but not gemcitabine in non-small cell lung cancer. J. Int. Med. Res. 39 (5), 1682–1692. doi:10.1177/147323001103900510
Lopes, R. B., Gangeswaran, R., Mcneish, I. A., Wang, Y., and Lemoine, N. R. (2007). Expression of the IAP protein family is dysregulated in pancreatic cancer cells and is important for resistance to chemotherapy. Int. J. Cancer. 120 (11), 2344–2352. doi:10.1002/ijc.22554
Ma, J. J., Chen, B. L., and Xin, X. Y. (2009). XIAP gene downregulation by small interfering RNA inhibits proliferation, induces apoptosis, and reverses the cisplatin resistance of ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 146 (2), 222–226. doi:10.1016/j.ejogrb.2009.06.011
Macfarlane, M., Merrison, W., Bratton, S. B., and Cohen, G. M. (2002). Proteasome-mediated degradation of Smac during apoptosis: XIAP promotes Smac ubiquitination in vitro. J. Biol. Chem. 277 (39), 36611–36616. doi:10.1074/jbc.M200317200
Maier, J. K., Lahoua, Z., Gendron, N. H., Fetni, R., Johnston, A., Davoodi, J., et al. (2002). The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J. Neurosci. 22 (6), 2035–2043. doi:10.1523/JNEUROSCI.22-06-02035.2002
Marusawa, H., Matsuzawa, S., Welsh, K., Zou, H., Armstrong, R., Tamm, I., et al. (2003). HBXIP functions as a cofactor of survivin in apoptosis suppression. EMBO. J. 22 (11), 2729–2740. doi:10.1093/emboj/cdg263
Miyao, T., Koike, H., Sekine, Y., Ohtsu, A., Oka, D., and Suzuki, K. (2020). YM155 reverses Cabazitaxel resistance in castration-resistant prostate cancer by reducing survivin expression. Anticancer Res. 40 (9), 5091–5095. doi:10.21873/anticanres.14512
Narayanan, S., Cai, C. Y., Assaraf, Y. G., Guo, H. Q., Cui, Q., Wei, L., et al. (2020). Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug resist. Update 48, 100663. doi:10.1016/j.drup.2019.100663
Ndozangue-Touriguine, O., Sebbagh, M., Merino, D., Micheau, O., Bertoglio, J., and Breard, J. (2008). A mitochondrial block and expression of XIAP lead to resistance to TRAIL-induced apoptosis during progression to metastasis of a colon carcinoma. Oncogene 27 (46), 6012–6022. doi:10.1038/onc.2008.197
Nomura, T., Mimata, H., Takeuchi, Y., Yamamoto, H., Miyamoto, E., and Nomura, Y. (2003). The X-linked inhibitor of apoptosis protein inhibits taxol-induced apoptosis in LNCaP cells. Urol. Res. 31 (1), 37–44. doi:10.1007/s00240-003-0300-y
O'Connor, D. S., Grossman, D., Plescia, J., Li, F., Zhang, H., Villa, A., et al. (2000). Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc. Natl. Acad. Sci. U. S. A. 97 (24), 13103–13107. doi:10.1073/pnas.240390697
Okamoto, K., Okamoto, I., Hatashita, E., Kuwata, K., Yamaguchi, H., Kita, A., et al. (2012). Overcoming erlotinib resistance in EGFR mutation-positive non-small cell lung cancer cells by targeting survivin. Mol. Cancer Ther. 11 (1), 204–213. doi:10.1158/1535-7163.MCT-11-0638
Okumu, D. O., East, M. P., Levine, M., Herring, L. E., Zhang, R., Gilbert, T., et al. (2017). BIRC6 mediates imatinib resistance independently of Mcl-1. PLoS One 12 (5), e0177871. doi:10.1371/journal.pone.0177871
Papadopoulos, K. P., Lopez-Jimenez, J., Smith, S. E., Steinberg, J., Keating, A., Sasse, C., et al. (2016). A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk. Lymphoma 57 (8), 1848–1855. doi:10.3109/10428194.2015.1113275
Park, E., Gang, E. J., Hsieh, Y. T., Schaefer, P., Chae, S., Klemm, L., et al. (2011). Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood 118 (8), 2191–2199. doi:10.1182/blood-2011-04-351239
Pavlyukov, M. S., Antipova, N. V., Balashova, M. V., Vinogradova, T. V., Kopantzev, E. P., and Shakhparonov, M. I. (2011). Survivin monomer plays an essential role in apoptosis regulation. J. Biol. Chem. 286 (26), 23296–23307. doi:10.1074/jbc.M111.237586
Peery, R., Cui, Q., Kyei-Baffour, K., Josephraj, S., Huang, C., Dong, Z., et al. (2022). A novel survivin dimerization inhibitor without a labile hydrazone linker induces spontaneous apoptosis and synergizes with docetaxel in prostate cancer cells. Bioorg. Med. Chem. 65, 116761. doi:10.1016/j.bmc.2022.116761
Perez-Ruiz, E., Melero, I., Kopecka, J., Sarmento-Ribeiro, A. B., Garcia-Aranda, M., and De Las, R. J. (2020). Cancer immunotherapy resistance based on immune checkpoints inhibitors: targets, biomarkers, and remedies. Drug resist. Update 53, 100718. doi:10.1016/j.drup.2020.100718
Perimenis, P., Galaris, A., Voulgari, A., Prassa, M., and Pintzas, A. (2016). IAP antagonists Birinapant and AT-406 efficiently synergise with either TRAIL, BRAF, or BCL-2 inhibitors to sensitise BRAFV600E colorectal tumour cells to apoptosis. BMC Cancer 16, 624. doi:10.1186/s12885-016-2606-5
Pluchino, K. M., Hall, M. D., Goldsborough, A. S., Callaghan, R., and Gottesman, M. M. (2012). Collateral sensitivity as a strategy against cancer multidrug resistance. Drug resist. Update 15 (1-2), 98–105. doi:10.1016/j.drup.2012.03.002
Prasopporn, S., Suppramote, O., Ponvilawan, B., Jamyuang, C., Chanthercrob, J., Chaiboonchoe, A., et al. (2022). Combining the SMAC mimetic LCL161 with Gemcitabine plus Cisplatin therapy inhibits and prevents the emergence of multidrug resistance in cholangiocarcinoma. Front. Oncol. 12, 1021632. doi:10.3389/fonc.2022.1021632
Qin, S., Yang, C., Zhang, B., Li, X., Sun, X., Li, G., et al. (2016). XIAP inhibits mature Smac-induced apoptosis by degrading it through ubiquitination in NSCLC. Int. J. Oncol. 49 (4), 1289–1296. doi:10.3892/ijo.2016.3634
Qiu, X. B., and Goldberg, A. L. (2005). The membrane-associated inhibitor of apoptosis protein, BRUCE/Apollon, antagonizes both the precursor and mature forms of Smac and caspase-9. J. Biol. Chem. 280 (1), 174–182. doi:10.1074/jbc.M411430200
Rada, M., Nallanthighal, S., Cha, J., Ryan, K., Sage, J., Eldred, C., et al. (2018). Inhibitor of apoptosis proteins (IAPs) mediate collagen type XI alpha 1-driven cisplatin resistance in ovarian cancer. Oncogene 37 (35), 4809–4820. doi:10.1038/s41388-018-0297-x
Ramakrishnan, V., Painuly, U., Kimlinger, T., Haug, J., Rajkumar, S. V., and Kumar, S. (2014). Inhibitor of apoptosis proteins as therapeutic targets in multiple myeloma. Leukemia 28 (7), 1519–1528. doi:10.1038/leu.2014.2
Rathore, R., Mccallum, J. E., Varghese, E., Florea, A. M., and Busselberg, D. (2017). Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 22 (7), 898–919. doi:10.1007/s10495-017-1375-1
Ren, J., Shi, M., Liu, R., Yang, Q. H., Johnson, T., Skarnes, W. C., et al. (2005). The Birc6 (Bruce) gene regulates p53 and the mitochondrial pathway of apoptosis and is essential for mouse embryonic development. Proc. Natl. Acad. Sci. U. S. A. 102 (3), 565–570. doi:10.1073/pnas.0408744102
Riedl, S. J., Renatus, M., Schwarzenbacher, R., Zhou, Q., Sun, C., Fesik, S. W., et al. (2001). Structural basis for the inhibition of caspase-3 by XIAP. Cell 104 (5), 791–800. doi:10.1016/s0092-8674(01)00274-4
Sajid, A., Rahman, H., and Ambudkar, S. V. (2023). Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer. 23, 762–779. doi:10.1038/s41568-023-00612-3
Sancho, P., Barneda, D., and Heeschen, C. (2016). Hallmarks of cancer stem cell metabolism. Br. J. Cancer. 114 (12), 1305–1312. doi:10.1038/bjc.2016.152
Sapi, E., Alvero, A. B., Chen, W., O'Malley, D., Hao, X. Y., Dwipoyono, B., et al. (2004). Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol. Res. 14 (11-12), 567–578. doi:10.3727/0965040042707943
Schorn, F., Werthenbach, J. P., Hoffmann, M., Daoud, M., Stachelscheid, J., Schiffmann, L. M., et al. (2023). cIAPs control RIPK1 kinase activity-dependent and -independent cell death and tissue inflammation. EMBO. J. 42 (22), e113614. doi:10.15252/embj.2023113614
Shahar, N., and Larisch, S. (2020). Inhibiting the inhibitors: targeting anti-apoptotic proteins in cancer and therapy resistance. Drug resist. Update 52, 100712. doi:10.1016/j.drup.2020.100712
Shen, J., Yin, Q., Chen, L., Zhang, Z., and Li, Y. (2012). Co-delivery of paclitaxel and survivin shRNA by pluronic P85-PEI/TPGS complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials 33 (33), 8613–8624. doi:10.1016/j.biomaterials.2012.08.007
Shimizu, T., Nishio, K., Sakai, K., Okamoto, I., Okamoto, K., Takeda, M., et al. (2020). Phase I safety and pharmacokinetic study of YM155, a potent selective survivin inhibitor, in combination with erlotinib in patients with EGFR TKI refractory advanced non-small cell lung cancer. Cancer. Chemother. Pharmacol. 86, 211–219. doi:10.1007/s00280-020-04112-1
Shin, H., Renatus, M., Eckelman, B. P., Nunes, V. A., Sampaio, C. A., and Salvesen, G. S. (2005). The BIR domain of IAP-like protein 2 is conformationally unstable: implications for caspase inhibition. Biochem. J. 385 (Pt 1), 1–10. doi:10.1042/BJ20041107
Shin, S., Sung, B. J., Cho, Y. S., Kim, H. J., Ha, N. C., Hwang, J. I., et al. (2001). An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 40 (4), 1117–1123. doi:10.1021/bi001603q
Shiozaki, E. N., Chai, J., Rigotti, D. J., Riedl, S. J., Li, P., Srinivasula, S. M., et al. (2003). Mechanism of XIAP-mediated inhibition of caspase-9. Mol. Cell. 11 (2), 519–527. doi:10.1016/s1097-2765(03)00054-6
Shrikhande, S. V., Kleeff, J., Kayed, H., Keleg, S., Reiser, C., Giese, T., et al. (2006). Silencing of X-linked inhibitor of apoptosis (XIAP) decreases gemcitabine resistance of pancreatic cancer cells. Anticancer Res. 26 (5A), 3265–3273.
Shu, X., Zhu, Z., Cao, D., Zheng, L., Wang, F., Pei, H., et al. (2019). PEG-derivatized birinapant as a nanomicellar carrier of paclitaxel delivery for cancer therapy. Colloid Surf. B-Biointerfaces 182, 110356. doi:10.1016/j.colsurfb.2019.110356
Silva, K. L., de Souza, P. S., Nestal, D. M. G., Moellmann-Coelho, A., Vasconcelos, F. C., and Maia, R. C. (2013). XIAP and P-glycoprotein co-expression is related to imatinib resistance in chronic myeloid leukemia cells. Leuk. Res. 37 (10), 1350–1358. doi:10.1016/j.leukres.2013.06.014
Singh, T., Neal, A., Dibernardo, G., Raheseparian, N., Moatamed, N. A., and Memarzadeh, S. (2022). Efficacy of birinapant in combination with carboplatin in targeting platinum-resistant epithelial ovarian cancers. Int. J. Oncol. 60 (3), 35. doi:10.3892/ijo.2022.5325
Speletas, M., Argentou, N., Karanikas, V., Gramoustianou, E. S., Mandala, E., Braimi, M., et al. (2011). Survivin isoform expression patterns in CML patients correlate with resistance to imatinib and progression, but do not trigger cytolytic responses. Immunol 139 (2), 155–163. doi:10.1016/j.clim.2011.01.010
Straszewski-Chavez, S. L., Abrahams, V. M., Funai, E. F., and Mor, G. (2004). X-linked inhibitor of apoptosis (XIAP) confers human trophoblast cell resistance to Fas-mediated apoptosis. Mol. Hum. Reprod. 10 (1), 33–41. doi:10.1093/molehr/gah001
Stroopinsky, D., Rajabi, H., Nahas, M., Rosenblatt, J., Rahimian, M., Pyzer, A., et al. (2018). MUC1-C drives myeloid leukaemogenesis and resistance to treatment by a survivin-mediated mechanism. J. Cell. Mol. Med. 22 (8), 3887–3898. doi:10.1111/jcmm.13662
Su, C. (2022). Emerging insights to lung cancer drug resistance. Cancer Drug Resist 5 (3), 534–540. doi:10.20517/cdr.2022.61
Sugihara, E., Hashimoto, N., Osuka, S., Shimizu, T., Ueno, S., Okazaki, S., et al. (2020). The inhibitor of apoptosis protein Livin confers resistance to fas-mediated immune cytotoxicity in refractory lymphoma. Cancer Res. 80 (20), 4439–4450. doi:10.1158/0008-5472.CAN-19-3993
Sun, J. G., Liao, R. X., Zhang, S. X., Duan, Y. Z., Zhuo, W. L., Wang, X. X., et al. (2011). Role of inhibitor of apoptosis protein Livin in radiation resistance in nonsmall cell lung cancer. Cancer biother. Radiopharm. 26 (5), 585–592. doi:10.1089/cbr.2011.0962
Sun, T., Mao, W., Peng, H., Wang, Q., and Jiao, L. (2021). YAP promotes sorafenib resistance in hepatocellular carcinoma by upregulating survivin. Cell. Oncol. 44 (3), 689–699. doi:10.1007/s13402-021-00595-z
Sun, X. P., Dong, X., Lin, L., Jiang, X., Wei, Z., Zhai, B., et al. (2014). Up-regulation of survivin by AKT and hypoxia-inducible factor 1α contributes to cisplatin resistance in gastric cancer. FEBS J. 281 (1), 115–128. doi:10.1111/febs.12577
Sun, X. S., Tao, Y., Le Tourneau, C., Pointreau, Y., Sire, C., Kaminsky, M. C., et al. (2020). Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: a double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 21 (9), 1173–1187. doi:10.1016/S1470-2045(20)30327-2
Takacs, F., Mikala, G., Nagy, N., Reszegi, A., Czeti, A., Szaloki, G., et al. (2021). Identification of a novel resistance mechanism in venetoclax treatment and its prediction in chronic lymphocytic leukemia. Acta. Oncol. 60 (4), 528–530. doi:10.1080/0284186X.2021.1878388
Tassi, E., Zanon, M., Vegetti, C., Molla, A., Bersani, I., Perotti, V., et al. (2012). Role of Apollon in human melanoma resistance to antitumor agents that activate the intrinsic or the extrinsic apoptosis pathways. Clin. Cancer Res. 18 (12), 3316–3327. doi:10.1158/1078-0432.CCR-11-2232
Thibault, B., Genre, L., Le Naour, A., Broca, C., Mery, E., Vuagniaux, G., et al. (2018). DEBIO 1143, an IAP inhibitor, reverses carboplatin resistance in ovarian cancer cells and triggers apoptotic or necroptotic cell death. Sci. Rep. 8 (1), 17862. doi:10.1038/s41598-018-35860-z
Tian, A., Wilson, G. S., Lie, S., Wu, G., Hu, Z., Hebbard, L., et al. (2014). Synergistic effects of IAP inhibitor LCL161 and paclitaxel on hepatocellular carcinoma cells. Cancer Lett. 351 (2), 232–241. doi:10.1016/j.canlet.2014.06.006
Tsubaki, M., Takeda, T., Ogawa, N., Sakamoto, K., Shimaoka, H., Fujita, A., et al. (2015). Overexpression of survivin via activation of ERK1/2, Akt, and NF-κB plays a central role in vincristine resistance in multiple myeloma cells. Leuk. Res. 39 (4), 445–452. doi:10.1016/j.leukres.2015.01.016
Varfolomeev, E., Goncharov, T., Fedorova, A. V., Dynek, J. N., Zobel, K., Deshayes, K., et al. (2008). c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 283 (36), 24295–24299. doi:10.1074/jbc.C800128200
Vaziri, S. A., Grabowski, D. R., Tabata, M., Holmes, K. A., Sterk, J., Takigawa, N., et al. (2003). c-IAP1 is overexpressed in HL-60 cells selected for doxorubicin resistance: effects on etoposide-induced apoptosis. Anticancer Res. 23 (5A), 3657–3661.
Vetma, V., Rozanc, J., Charles, E. M., Hellwig, C. T., Alexopoulos, L. G., and Rehm, M. (2017). Examining the in vitro efficacy of the IAP antagonist birinapant as a single agent or in combination with dacarbazine to induce melanoma cell death. Oncol. Res. 25 (9), 1489–1494. doi:10.3727/096504017X14897145996933
Wang, L., Zhang, G. M., and Feng, Z. H. (2003). Down-regulation of survivin expression reversed multidrug resistance in adriamycin-resistant HL-60/ADR cell line. Acta Pharmacol. Sin. 24 (12), 1235–1240.
Wang, W., Zhang, B., Mani, A. M., Wu, Z., Fan, Y., Li, W., et al. (2018). Survivin inhibitors mitigate chemotherapeutic resistance in breast cancer cells by suppressing genotoxic nuclear factor-κb activation. J. Pharmacol. Exp. Ther. 366 (1), 184–193. doi:10.1124/jpet.118.249151
Wang, X., Niu, J., Li, J., Shen, X., Shen, S., Straubinger, R. M., et al. (2018). Temporal effects of combined birinapant and paclitaxel on pancreatic cancer cells investigated via large-scale, ion-current-based quantitative proteomics (IonStar). Mol. Cell. Proteomics. 17 (4), 655–671. doi:10.1074/mcp.RA117.000519
Wang, X., Xu, J., Ju, S., Ni, H., Zhu, J., and Wang, H. (2010). Livin gene plays a role in drug resistance of colon cancer cells. Clin. Biochem. 43 (7-8), 655–660. doi:10.1016/j.clinbiochem.2010.02.004
Woo, S. M., Min, K. J., Seo, B. R., Seo, Y. H., Jeong, Y. J., and Kwon, T. K. (2017). YM155 enhances ABT-737-mediated apoptosis through Mcl-1 downregulation in Mcl-1-overexpressed cancer cells. Mol. Cell. Biochem. 429 (1-2), 91–102. doi:10.1007/s11010-016-2938-0
Wu, Y. H., You, Y., Chen, Z. C., and Zou, P. (2008). Reversal of drug resistance by silencing Survivin gene expression in acute myeloid leukemia cells. Acta Biochim. Pol. 55 (4), 673–680. doi:10.18388/abp.2008_3026
Xie, X., Lee, J., Liu, H., Pearson, T., Lu, A. Y., Tripathy, D., et al. (2021). Birinapant enhances gemcitabine's antitumor efficacy in triple-negative breast cancer by inducing intrinsic pathway-dependent apoptosis. Mol. Cancer Ther. 20 (2), 296–306. doi:10.1158/1535-7163.MCT-19-1160
Xie, Y., Tobin, L. A., Camps, J., Wangsa, D., Yang, J., Rao, M., et al. (2013). MicroRNA-24 regulates XIAP to reduce the apoptosis threshold in cancer cells. Oncogene 32 (19), 2442–2451. doi:10.1038/onc.2012.258
Xiong, Z., Fu, Z., Shi, J., Jiang, X., and Wan, H. (2017). HtrA1 down-regulation induces cisplatin resistance in colon cancer by increasing XIAP and activating PI3K/akt pathway. Ann. Clin. Lab. Sci. 47 (3), 264–270.
Xu, L., Fu, Y., Li, Y., and Han, X. (2017). Cisplatin induces expression of drug resistance-related genes through c-jun N-terminal kinase pathway in human lung cancer cells. Cancer. Chemother. Pharmacol. 80 (2), 235–242. doi:10.1007/s00280-017-3355-0
Yang, C., Wang, H., Zhang, B., Chen, Y., Zhang, Y., Sun, X., et al. (2016). LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC. J. Exp. Clin. Cancer Res. 35 (1), 158. doi:10.1186/s13046-016-0435-7
Yao, M., Jiang, X., Xiao, F., Lv, X., Sheng, M., Xing, W., et al. (2024). Targeting BIRC5 as a therapeutic approach to overcome ASXL1-associated decitabine resistance. Cancer Lett. 593, 216949. doi:10.1016/j.canlet.2024.216949
Yoon, D. H., Shin, J. S., Jin, D. H., Hong, S. W., Jung, K. A., Kim, S. M., et al. (2012). The survivin suppressant YM155 potentiates chemosensitivity to gemcitabine in the human pancreatic cancer cell line MiaPaCa-2. Anticancer Res. 32 (5), 1681–1688.
Yu, P., Li, A. X., Chen, X. S., Tian, M., Wang, H. Y., Wang, X. L., et al. (2020). PKM2-c-Myc-Survivin cascade regulates the cell proliferation, migration, and tamoxifen resistance in breast cancer. Front. Pharmacol. 11, 550469. doi:10.3389/fphar.2020.550469
Zaffaroni, N., Pennati, M., Colella, G., Perego, P., Supino, R., Gatti, L., et al. (2002). Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell. Mol. Life Sci. 59 (8), 1406–1412. doi:10.1007/s00018-002-8518-3
Zarnegar, B. J., Wang, Y., Mahoney, D. J., Dempsey, P. W., Cheung, H. H., He, J., et al. (2008). Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9 (12), 1371–1378. doi:10.1038/ni.1676
Zhang, H., Li, M., Zhang, J., Shen, Y., and Gui, Q. (2021). Exosomal circ-XIAP promotes docetaxel resistance in prostate cancer by regulating miR-1182/TPD52 Axis. Drug Des. Devel Ther. 15, 1835–1849. doi:10.2147/DDDT.S300376
Zhang, M., Huang, M. N., Dong, X. D., Cui, Q. B., Yan, Y., She, M. L., et al. (2023). Overexpression of ABCB1 confers resistance to FLT3 inhibitor FN-1501 in cancer cells: in vitro and in vivo characterization. Am. J. Cancer Res. 13 (12), 6026–6037.
Zhang, S., Li, G., Zhao, Y., Liu, G., Wang, Y., Ma, X., et al. (2012). Smac mimetic SM-164 potentiates APO2L/TRAIL- and doxorubicin-mediated anticancer activity in human hepatocellular carcinoma cells. PLoS One 7 (12), e51461. doi:10.1371/journal.pone.0051461
Zhang, Z., Zhang, Y., Lv, J., and Wang, J. (2015). The survivin suppressant YM155 reverses doxorubicin resistance in osteosarcoma. Int. J. Clin. Exp. Med. 8 (10), 18032–18040.
Zhou, B., Zhang, J., Chen, G., You, L., Zhang, T. P., and Zhao, Y. P. (2013). Therapy of Smac mimetic SM-164 in combination with gemcitabine for pancreatic cancer. Cancer Lett. 329 (1), 118–124. doi:10.1016/j.canlet.2012.10.039
Zhou, C., Zhu, Y., Lu, B., Zhao, W., and Zhao, X. (2018). Survivin expression modulates the sensitivity of A549 lung cancer cells resistance to vincristine. Oncol. Lett. 16 (4), 5466–5472. doi:10.3892/ol.2018.9277
Zhou, J., Kwak, K. J., Wu, Z., Yang, D., Li, J., Chang, M., et al. (2018). PLAUR confers resistance to Gefitinib through EGFR/P-AKT/Survivin signaling pathway. Cell Physiol. Biochem. 47 (5), 1909–1924. doi:10.1159/000491071
Zhou, L., Zhang, Y., Leng, Y., Dai, Y., Kmieciak, M., Kramer, L., et al. (2019). The IAP antagonist birinapant potentiates bortezomib anti-myeloma activity in vitro and in vivo. J. Hematol. Oncol. 12 (1), 25. doi:10.1186/s13045-019-0713-x
Keywords: drug resistance, cancer, inhibitors of apoptosis proteins, inhibitors, overcome
Citation: Ye Q, Zhuang X-Z, Li J and Zhou X (2025) Targeting the inhibitors of apoptosis proteins (IAPs) to combat drug resistance in cancers. Front. Pharmacol. 16:1562167. doi: 10.3389/fphar.2025.1562167
Received: 17 January 2025; Accepted: 21 March 2025;
Published: 28 March 2025.
Edited by:
Ning Ji, China Academy of Chinese Medical Sciences, ChinaReviewed by:
Huihui Cao, Southern Medical University, ChinaCopyright © 2025 Ye, Zhuang, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, bHoxOTgyMDdAMTI2LmNvbQ==; Xin Zhou, eng1NTc1NzU5QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.