- 1School of Medicine, Shaoxing University, Shaoxing, Zhejing, China
- 2Department of Hematology, Shaoxing People’s Hospital, Affiliated First Hospital of Shaoxing University, Shaoxing, China
Myelodysplastic syndromes (MDS) are a group of malignancies characterized by clonal proliferation of hematopoietic stem cells, ineffective hematopoiesis, peripheral cytopenias, and a high risk of transformation to acute myeloid leukemia. Current therapeutic strategies for MDS have limited efficacy. Thus, identifying new therapeutic targets and prognostic biomarkers is a critical future research direction. Ferroptosis, a new type of iron-dependent programmed cell death, has become a recent hotspot in the field of oncology research. Recent results have demonstrated that iron metabolism, lipid metabolism, and other pathways can be targeted to induce ferroptosis in MDS cells. In addition, ferroptosis-related genes are of significance in the prognosis and diagnosis of MDS. This article reviews the current research progress on ferroptosis in MDS, including its potential for targeting as a therapeutic intervention strategy.
1 Introduction
Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic malignancies characterized by morphological dysplasia of bone marrow cells, as well as anemia, neutropenia, or thrombocytopenia (Arber et al., 2016). The annual incidence of MDS is approximately 4 cases per 100,000 (Sekeres and Taylor, 2022), with higher incidence among elderly patients (Li et al., 2022). Treatment options for MDS are limited, in part because its pathogenic mechanisms remain incompletely elucidated. Currently, the main treatments include supportive care, such as blood transfusions, demethylating agents, chemotherapy, and hematopoietic stem cell transplantation (Cazzola, 2020). However, demethylating agents and other pharmacological treatments have suboptimal efficacy. Currently, hematopoietic stem cell transplantation is the only potentially curative therapy, but due to the median age of MDS patients (around 70 years), stem cell transplantation is usually considered unsafe or impractical in the elderly. Therefore, there is an urgent need to investigate the pathogenesis of MDS and develop new therapeutic approaches.
Ferroptosis, a novel form of cell death that is distinct from apoptosis, autophagy, and necrosis; is triggered by the accumulation of iron-dependent lipid peroxides; and is regulated by various cellular metabolic pathways, including redox homeostasis, iron metabolism, and the metabolism of amino acids, lipids, and glucose (Dixon et al., 2012; Galy et al., 2024; Conrad and Pratt, 2019; Ursini and Maiorino, 2020; Conrad et al., 2018). Studies have shown that ferroptosis induction in tumor cells exhibits anticancer potential in various malignant tumors (Lei et al., 2024). Tumor cells that are resistant to conventional treatments may be more sensitive to this form of death due to imbalances in the lipid peroxidation system. In the field of MDS research, recent studies have shown that ferroptosis signaling pathways regulate the progression of MDS, which suggests that ferroptosis-targeting drugs hold promise as a new MDS therapy approach (Suarez and Gore, 2013). Furthermore, the specific value of ferroptosis-related genes (FRGs) in the diagnosis and prognosis of MDS has been considered. This article summarizes the current domestic and international research progress on ferroptosis in the field of MDS.
2 overview of ferroptosis
Ferroptosis is a form of cell death induced by the accumulation of iron-dependent lipid peroxides. Its key processes include abnormal iron metabolism, lipid ROS generation, dysregulation of the antioxidant system, and accumulation of lipid peroxides (Jiang et al., 2021). Ferroptosis is distinct from traditional forms of cell death such as apoptosis, necrosis, and pyroptosis, and it has unique biological characteristics and regulatory mechanisms. Therefore, therapeutic targeting of ferroptosis has been studied as an intervention approach for various diseases, including cancer.
Accumulating research has elucidated regulatory mechanisms of ferroptosis, providing new perspectives and research directions for preventing the occurrence and development of various diseases. In radiation-induced heart disease, total extracts from A. manihot (L.) have been demonstrated to prevent ferroptosis in cardiomyocytes by regulating the NOX4/xCT/GPX4 axis to inhibit redox reactions (Xu et al., 2024a). Furthermore, in calcific aortic valve disease, Nesfatin-1 has been demonstrated to inhibit ferroptosis in aortic valve interstitial cells by regulating the GSH/GPX4 and ZIP8/SOD2 axes (Wang et al., 2024). Similarly, in repetitive traumatic brain injury, SCH79797 inhibits neuronal ferroptosis and reduces NLR family pyrin domain containing 3 (NLRP3) inflammasome activation by promoting PPAR-γ/Nrf2-mediated antioxidant responses (El-Gazar et al., 2024). Each of these studies support the use of ferroptosis-related pathways as potential targets for disease prevention or treatment.
Studies on ferroptosis in tumors are also becoming more widespread (Zhou et al., 2024). In liver cancer, serine beta-lactamase-like protein inhibits the ferroptosis of hepatocellular carcinoma cells by regulating the p53/HSPA8 axis (Zeng et al., 2024); and aristolochic acids suppress ferroptosis via modulation of the p53/GADD45A/NRF2/SLC7A11 pathway (Hou et al., 2024). In non-small cell lung cancer (NSCLC), targeting and regulation of the NRF2/PHKG2 axis promotes ferritinophagy, increases intracellular iron levels, and enhances the radiosensitivity of NSCLC cells through mitochondrial stress-dependent ferroptosis (Han et al., 2024). Likewise, the EGR1/miR-139/NRF2 axis plays a role in ionizing radiation-induced ferroptosis in NSCLC cells (Zhang L. et al., 2024a). In breast cancer, dihydroartemisinin enhances the radiosensitivity of breast cancer cells by inducing ferroptosis via the hsa_circ_0001610/miR-139-5p/SLC7A11 pathway (Zhang Y. et al., 2024b). Furthermore, overexpression of hypoxia-inducible factor-1α (HIF1α) increases the sensitivity to Adriamycin and inhibit the proliferation and invasion abilities of breast cancer cells by activating ferroptosis (Yu et al., 2024).
Current research suggests that ferroptosis may also have a key role in hematological diseases. In Acute Myeloid Leukemias (AML), FLT3 inhibitors enhance the sensitivity of FLT3-mutant AML cells to lipid oxidative stress by inhibiting the C/EBPα/SCD axis, thereby inducing ferroptosis (Sabatier et al., 2023). Furthermore, Imetelstat, a first-in-class telomerase inhibitor with clinical efficacy in myelofibrosis and MDS, has been demonstrated to induce ferroptosis in AML cells by promoting the formation of polyunsaturated fatty acid-containing phospholipids, leading to excessive lipid peroxidation and oxidative stress (Bruedigam et al., 2024). In lymphoma, 7-Dehydrocholesterol, an endogenous metabolite, enhances the survival ability of lymphoma cells by protecting their lipids from peroxidation and reducing their sensitivity to ferroptosis, especially for DHCR7-mutated Burkitt lymphoma (Freitas et al., 2024). BRD4 is a bromodomain and extra-terminal domain (BET) protein that positively regulates the expression of ferroptosis suppressor protein 1 (FSP1). BET inhibitors have been shown to reduce the antioxidant capacity within GCB subtype Diffuse Large B-cell Lymphoma cells by decreasing FSP1 expression, thereby increasing their sensitivity to ferroptosis. Moreover, BET inhibitors affect the expression of ferroptosis-related genes, such as SLC7A11 (Schmitt et al., 2023). In Multiple Myeloma (MM), the loss of leukocyte immunoglobulin-like receptor B1 reduces the uptake of LDL/cholesterol by MM cells but activates the cholesterol synthesis pathway to maintain intracellular cholesterol levels. A key intermediate in this pathway is squalene, an effective antioxidant that protects cells from lipid peroxidation damage; cholesterol synthesis pathway activation leads to decreased squalene levels, which causes cells to become more susceptible to ferroptosis (Xian et al., 2024). Additionally, AP-1 inhibitor (T-5224) induces ferroptosis in MM cells by inhibiting the PI3K/AKT signaling pathway (Tang S. et al., 2024a).
3 Regulatory mechanisms of ferroptosis in MDS
As a hematological malignancy, MDS has been the focus of studies on apoptosis (Lambert et al., 2016; Economopoulou et al., 2010; Lefèvre et al., 2017; Czibere et al., 2006; Jing et al., 2024; Liu et al., 2024; McBride et al., 2019; Rivella, 2015) and autophagy (Alexander et al., 2011; Zha et al., 2021; Jacquel et al., 2018; Ames et al., 2023; Weber et al., 2020), but the role of ferroptosis in MDS is not well characterized. Nevertheless, accumulating evidence is consistent with the role of ferroptosis in MDS (Figure 1). Increased Fe3+ promotes the generation of ROS (Callens et al., 2010), and iron overload is not uncommon during MDS (An et al., 2023). MDS cells show increased Fe3+ uptake and decreased Fe3+ efflux, with varying degrees of abnormalities in transferrin, transferrin receptors, and iron metabolism-related proteins. For example, one study demonstrated increased levels of Fe2+and elevated expression of transferrin receptor mRNA in CD33+ cells of MDS patients (Li J. et al., 2024a). Repeated blood transfusions to improve anemia during treatment is the main cause of iron overload (Pullarkat, 2009; Lindsey and Alex, 2018). Intrinsic ineffective erythropoiesis, a form of hemolysis caused by chemotherapy and hematopoietic stem cell transplantation, further exacerbates iron overload. Additionally, in MDS patients, the activation of NLRP3 inflammasomes is a redox-dependent, hallmark feature that leads to clonal expansion and pyroptosis (Sallman and List, 2019; Sallman et al., 2016), which results in increased ROS levels (Montalban-Bravo et al., 2020; Grignano et al., 2020). Molecular processes related to MDS, such as mutations in NLRP3, as well as drugs like decitabine, affect the production of ROS (Lv et al., 2020). The high ROS state makes MDS cells more prone to ferroptosis. Therefore, inducing ferroptosis in MDS cells by increasing cellular ROS levels is a potentially beneficial approach for treating MDS.

Figure 1. Main molecular mechanisms and signaling regulation of ferroptosis. The mechanisms and related signaling pathways of ferroptosis mainly include iron metabolism disorders, imbalance of the amino acid antioxidant system, accumulation of lipid peroxides, and related signaling pathways mediated by FSP1/CoQ10, GCH1/BH4, and DHODH. GPX4, glutathione peroxidase 4; Se, selenium; Glu, glutamate; SLC7A11, solute carrier family 7 member 11; SLC3A2, solute carrier family 3 member 2; GSH, glutathione; GSSG, oxidized glutathione; PUFAs, polyunsaturated fatty acids; ACSL4, acyl-coenzyme A synthetase long chain family member 4; CoA, coenzyme A; LPCAT3, lysophosphatidylcholine acyltrans-ferase 3; PUFA-PE, polyunsaturated fatty acid-phosphatidyl ethanolamine; CoQ10, ubiquinone; CoQ10H2, ubiquinol; DHODH, dihydroorotate dehydrogenase; FSP1, ferroptosis suppressor protein 1; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; DHFR, dihydrofolate reductase; FSP1, ferroptosis suppressor protein 1.
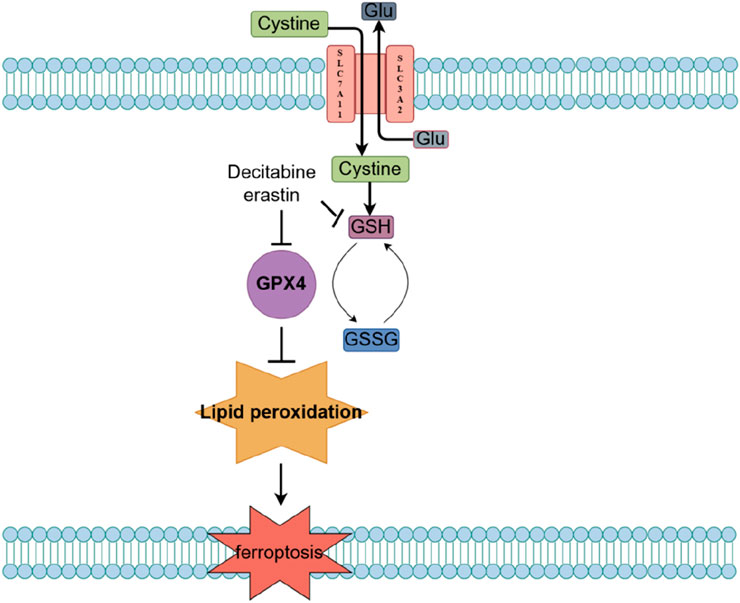
Current evidence suggests that MDS cell ferroptosis is induced through the System Xc - glutathione (GSH) - glutathione peroxidase 4 (GPX4) pathway (Figure 2). The System Xc−-GSH-GPX4 pathway is a classic regulatory pathway of ferroptosis. Within this pathway, System Xc-is a cystine/glutamate antiporter on the cell membrane, responsible for transporting extracellular cystine into the cell to synthesize GSH; GSH is an important intracellular antioxidant that maintains the cellular redox balance; and GPX4 is a GSH-dependent antioxidant enzyme that reduces lipid peroxides on the cell membrane, preventing lipid peroxidation damage. When the System Xc--GSH-GPX4 pathway is inhibited, intracellular GSH synthesis decreases and GPX4 activity is reduced, leading to the accumulation of lipid peroxides on the cell membrane. Excess lipid peroxides cause damage to the cell membrane, ultimately inducing ferroptosis. In support of the role of this pathway in MDS, treatment of the MDS cell line SKM-1 and two myeloid leukemia cell lines (KG-1 and K562) with the ferroptosis inducer erastin was demonstrated to induce ferroptosis by depleting GSH and reducing GPX4 activity.
Decitabine, a natural adenosine analogue of 2′-deoxycytidine nucleotide that is classified as an S-phase cell cycle-specific drug, exerts demethylation by inhibiting DNA methyltransferase, which activates tumor suppressor genes and inhibits cancer cell proliferation; thus, decitibine has been employed in the treatment of MDS and AML (Suarez and Gore, 2013). Notably, decitabine can significantly increase ROS levels and promote the release of iron ions in MDS cells, which further generates reactive oxygen species through the Fenton reaction, thus exacerbating oxidative stress; as a consequence, decitabine reduces GSH levels and inhibits GPX4 activity, leading to the accumulation of lipid peroxides. These findings are consistent with the possibility that ferroptosis may be the main mechanism by which decitabine induces death in MDS cells.
4 Prognostic studies of ferroptosis-related genes (FRGs) in MDS
Multiple genes are involved in the regulation of ferroptosis (Su et al., 2024). GPX4 is an inhibitor of ferroptosis that prevents cell death by reducing phospholipid peroxides (Seiler et al., 2008; Roveri et al., 1994; Mishima et al., 2023; Mao et al., 2021; Friedmann Angeli et al., 2014). Moreover, SLC7A11 indirectly inhibits ferroptosis by promoting glutathione synthesis (Xu et al., 2019), while FSP 1 inhibits ferroptosis through coenzyme Q10 (Doll et al., 2019; Bersuker et al., 2019). Additionally, GCH1 inhibits ferroptosis through antioxidant mechanisms (Kishi et al., 2012; Ahn et al., 2023). Conversely, ACSL4 (Ding et al., 2023), ALOX15 (Sui et al., 2024), and NCOA4 (Xu M. et al., 2024) enhance ferroptosis sensitivity by promoting lipid peroxidation and iron ion accumulation via parallel pathways, while TFRC promotes iron ion uptake, increasing the risk of ferroptosis.
With increasing research on ferroptosis genes, the roles of FRGs in hematological diseases are gradually being uncovered. For example, a prognostic model including six FRGs (VEGFA, KLHL24, ATG3, EIF2AK4, IDH1, HSPB1) can optimize risk stratification for AML patients (Jiang et al., 2023). In lymphoma, a risk scoring model containing 16 survival-related FRGs (DRD4, TFAP2C, AKR1C3, CHAC1, ULK2, CXCL2, GABARAPL1, TRIB3, CYBB, IREB2, EPAS1, MT1G, ATG3, ATF4, CAPG, UBC) shows good efficacy in predicting the survival of DLBCL patients (Xiong et al., 2022). In MM, a risk scoring model including five FRGs (YY1AP1, AURKA, CDKN1A, RRM2, STEAP3) can accurately predict the prognosis of MM patients (Wang et al., 2023).
Ferroptosis is also a promising target for MDS therapy, and specific roles of FRGs in MDS diagnosis have been proposed. Using MDS-related microarray data and clinical information from the Gene Expression Omnibus (GEO), the predictive ability of FRGs was evaluated using nomogram analysis and external datasets. A set of six feature genes (SREBF1, PTPN6, PARP9, MAP3K11, MDM4, EZH2) demonstrated high accuracy in MDS diagnosis. These findings underscore the complex relationship between FRGs and MDS (Zhu et al., 2024).
In an alternate study, another group of FRGs (BNIP3, MDM2, and RRM2) were demonstrated to serve as biomarkers for the diagnosis, treatment, and prognosis of MDS. Researchers identified these FRGs using RNA sequencing data and clinical information from GEO, extracting fFRGs from the FerrDb website, and performing differential expression analysis using the R package. Subsequently, Kaplan-Meier and Cox regression analyses were employed to assess the prognostic roles of these three genes. The diagnostic and prognostic efficacy of these genes in MDS was confirmed through Receiver Operating Characteristic curve analysis. Although this prognostic model constructed solely with BNIP3, MDM2, and RRM2 is relatively one-sided, the findings deepen the understanding of MDS pathogenesis, improve risk stratification, and build a more precise MDS prognostic system (Chen et al., 2023).
5 Research progress on targeting ferroptosis in MDS
Targeting ferroptosis is of importance in tumor therapy, with potential to provide new strategies for refractory and drug-resistant tumors. Recent studies have shown that targeting of ferroptosis may also provide therapeutic benefits to MDS patients by overcoming resistance to traditional therapies and reducing side effects on normal cells due to its ability to selectively induce death in MDS cells. Additionally, ferroptosis induction can function synergistically with existing treatments by enhancing antitumor effects and shows broad clinical prospects. Three such synergistic strategies are presented below.
5.1 Ferroptosis induction combined with cytotoxic chemotherapy
Erastin is a small-molecule compound that promotes the accumulation of lipid peroxides by inhibiting the Xc− system and reducing GSH production, ultimately inducing ferroptosis in MDS cells (Yagoda et al., 2007). Cytarabine is a key chemotherapeutic drug used to treat hematological malignancies such as MDS and AML; it is a cell cycle-specific antimetabolite drug that mainly inhibits DNA synthesis to suppress tumor cell proliferation and survival (Magina et al., 2017; Löwenberg, 2013). Studies have shown that erastin-induced ferroptosis can enhance the sensitivity of MDS cells to cytarabine.
5.2 Ferroptosis induction combined with demethylating agents
Decitabine is an antimetabolite drug classified as a demethylating agent; it inhibits tumor cell proliferation by altering DNA methylation status and restoring tumor suppressor gene expression. Studies have shown that when the ferroptosis inducer erastin is used in combination with decitabine, it can further reduce GSH levels and enhance the toxic effects of decitabine on MDS cells.
5.3 Ferroptosis induction combined with cuproptosis
Cuproptosis is a novel form of cell death induced by various copper ion carrier drugs such as Elesclomol, Disulfiram, and NSC319726 (Tang D. et al., 2024). Studies have shown that targeting ferroptosis and cuproptosis by inhibiting the xCT-GSH-GPX4 pathway can synergistically enhance the effect of DSF/Cu in MDS treatment, providing insights for this combined therapeutic strategies (Li H. et al., 2024). Other research results indicate that the combined use of ES-Cu and IKE has synergistic effects in MDS treatment, enhancing therapeutic efficacy by inducing multiple programmed cell death pathways (Gao et al., 2024). In the latter study, the induction of cuproptosis and ferroptosis in MDS cells by ES-Cu/IKE was enhanced by modulating the xCT-GSH-GPX4 axis, providing new strategies for MDS treatment.
6 Summary and outlook
In conclusion, ferroptosis has a close relationship with MDS. Ferroptosis induction in MDS cells via ferroptosis-related signaling pathways, or in combination with existing cytotoxic chemotherapeutic drugs and demethylating agents, may provide new directions in the field of MDS research. Current research on ferroptosis in MDS is mostly at the in vitro and animal model stages and has not yet expanded to clinical studies in patients. Therefore, transforming the basic research of MDS treatment with ferroptosis regulators into clinical practice is a key area for future exploration. The use of FRGs to stratify and evaluate prognosis may facilitate the precise diagnosis and treatment of MDS patients. Because research on ferroptosis in MDS has just begun, its mechanisms of pathogenesis, diagnosis, treatment, and prognosis require further exploration.
Author contributions
YY: Conceptualization, Investigation, Writing–original draft, Writing–review and editing. JH: Conceptualization, Investigation, Supervision, Writing–original draft, Writing–review and editing. YW: Conceptualization, Data curation, Investigation, Methodology, Supervision, Visualization, Writing–review and editing. JJ: Formal Analysis, Funding acquisition, Project administration, Resources, Software, Visualization, Writing–review and editing. WF: Conceptualization, Investigation, Supervision, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, Medical Scientific Research Foundation of Zhejiang Province (Grant No. 2023KY348). We also thank Figdraw (www.figdraw.com) for the assistance in creating Figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahn, J. H., Kim, A. R., Park, W.-Y., Cho, J. W., Park, J., and Youn, J. (2023). Whole exome sequencing and clinical investigation of young onset dystonia: what can we learn? Park. Relat. Disord. 115, 105814. doi:10.1016/j.parkreldis.2023.105814
Alexander, W., Monika, M., and Anna Katharina, S. (2011). Autophagy in the pathogenesis of myelodysplastic syndrome and acute myeloid leukemia. Cell Cycle Georget Tex 10, 1719–1725. doi:10.4161/cc.10.11.15673
Ames, K., Kaur, I., Shi, Y., Tong, M. M., Sinclair, T., Hemmati, S., et al. (2023). PI3-kinase deletion promotes myelodysplasia by dysregulating autophagy in hematopoietic stem cells. Sci. Adv. 9, eade8222. doi:10.1126/sciadv.ade8222
An, W., Feola, M., Levy, M., Aluri, S., Ruiz-Martinez, M., Sridharan, A., et al. (2023). Iron chelation improves ineffective erythropoiesis and iron overload in myelodysplastic syndrome mice. eLife 12, e83103. doi:10.7554/eLife.83103
Arber, D. A., Orazi, A., Hasserjian, R., Thiele, J., Borowitz, M. J., Le Beau, M. M., et al. (2016). The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405. doi:10.1182/blood-2016-03-643544
Bersuker, K., Hendricks, J. M., Li, Z., Magtanong, L., Ford, B., Tang, P. H., et al. (2019). The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692. doi:10.1038/s41586-019-1705-2
Bruedigam, C., Porter, A. H., Song, A., Vroeg In de Wei, G., Stoll, T., Straube, J., et al. (2024). Imetelstat-mediated alterations in fatty acid metabolism to induce ferroptosis as a therapeutic strategy for acute myeloid leukemia. Nat. Cancer 5, 47–65. doi:10.1038/s43018-023-00653-5
Callens, C., Coulon, S., Naudin, J., Radford-Weiss, I., Boissel, N., Raffoux, E., et al. (2010). Targeting iron homeostasis induces cellular differentiation and synergizes with differentiating agents in acute myeloid leukemia. J. Exp. Med. 207, 731–750. doi:10.1084/jem.20091488
Cazzola, M. (2020). Myelodysplastic syndromes. N. Engl. J. Med. 383, 1358–1374. doi:10.1056/NEJMra1904794
Chen, X., Li, C., Wang, Y., Geng, S., Xiao, M., Zeng, L., et al. (2023). Diagnostic and prognostic value of ferroptosis-related genes in patients with Myelodysplastic neoplasms. Hematol. Amst Neth 28, 2288475. doi:10.1080/16078454.2023.2288475
Conrad, M., Kagan, V. E., Bayir, H., Pagnussat, G. C., Head, B., Traber, M. G., et al. (2018). Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev. 32, 602–619. doi:10.1101/gad.314674.118
Conrad, M., and Pratt, D. A. (2019). The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147. doi:10.1038/s41589-019-0408-1
Czibere, A., Prall, W. C., Zerbini, L. F., Jäger, M., Kobbe, G., Knipp, S., et al. (2006). Exisulind induces apoptosis in advanced myelodysplastic syndrome (MDS) and acute myeloid leukaemia/MDS. Br. J. Haematol. 135, 355–357. doi:10.1111/j.1365-2141.2006.06298.x
Ding, K., Liu, C., Li, L., Yang, M., Jiang, N., Luo, S., et al. (2023). Acyl-CoA synthase ACSL4: an essential target in ferroptosis and fatty acid metabolism. Chin. Med. J. Engl. 136, 2521–2537. doi:10.1097/CM9.0000000000002533
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. doi:10.1016/j.cell.2012.03.042
Doll, S., Freitas, F. P., Shah, R., Aldrovandi, M., da Silva, M. C., Ingold, I., et al. (2019). FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698. doi:10.1038/s41586-019-1707-0
Economopoulou, C., Pappa, V., Papageorgiou, S., Kontsioti, F., Economopoulou, P., Charitidou, E., et al. (2010). Cell cycle and apoptosis regulatory gene expression in the bone marrow of patients with de novo myelodysplastic syndromes (MDS). Ann. Hematol. 89, 349–358. doi:10.1007/s00277-009-0835-2
El-Gazar, A. A., Soubh, A. A., Abdallah, D. M., Ragab, G. M., and El-Abhar, H. S. (2024). Elucidating PAR1 as a therapeutic target for delayed traumatic brain injury: unveiling the PPAR-γ/Nrf2/HO-1/GPX4 axis to suppress ferroptosis and alleviate NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 139, 112774. doi:10.1016/j.intimp.2024.112774
Freitas, F. P., Alborzinia, H., Dos Santos, A. F., Nepachalovich, P., Pedrera, L., Zilka, O., et al. (2024). 7-Dehydrocholesterol is an endogenous suppressor of ferroptosis. Nature 626, 401–410. doi:10.1038/s41586-023-06878-9
Friedmann Angeli, J. P., Schneider, M., Proneth, B., Tyurina, Y. Y., Tyurin, V. A., Hammond, V. J., et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191. doi:10.1038/ncb3064
Galy, B., Conrad, M., and Muckenthaler, M. (2024). Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 25, 133–155. doi:10.1038/s41580-023-00648-1
Gao, Y., Jin, F., Zhang, P., Zheng, C., Zheng, X., Xie, J., et al. (2024). Elesclomol-copper synergizes with imidazole ketone erastin by promoting cuproptosis and ferroptosis in myelodysplastic syndromes. Biomed. Pharmacother. Biomedecine Pharmacother. 175, 116727. doi:10.1016/j.biopha.2024.116727
Grignano, E., Birsen, R., Chapuis, N., and Bouscary, D. (2020). From iron chelation to overload as a therapeutic strategy to induce ferroptosis in leukemic cells. Front. Oncol. 10, 586530. doi:10.3389/fonc.2020.586530
Han, F., Chen, S., Zhang, K., Zhang, K., Wang, M., and Wang, P. (2024). Targeting Nrf2/PHKG2 axis to enhance radiosensitivity in NSCLC. NPJ Precis. Oncol. 8, 183. doi:10.1038/s41698-024-00629-3
Hou, C.-Y., Suo, Y.-H., Lv, P., Yuan, H.-F., Zhao, L.-N., Wang, Y.-F., et al. (2024). Aristolochic acids-hijacked p53 promotes liver cancer cell growth by inhibiting ferroptosis. Acta Pharmacol. Sin. 46, 208–221. doi:10.1038/s41401-024-01354-0
Jacquel, A., Luciano, F., Robert, G., and Auberger, P. (2018). Implication and regulation of AMPK during physiological and pathological myeloid differentiation. Int. J. Mol. Sci. 19, 2991. doi:10.3390/ijms19102991
Jiang, G., Jin, P., Xiao, X., Shen, J., Li, R., Zhang, Y., et al. (2023). Identification and validation of a novel CD8+ T cell-associated prognostic model based on ferroptosis in acute myeloid leukemia. Front. Immunol. 14, 1149513. doi:10.3389/fimmu.2023.1149513
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282. doi:10.1038/s41580-020-00324-8
Jing, Q., Zhou, C., Zhang, J., Zhang, P., Wu, Y., Zhou, J., et al. (2024). Role of reactive oxygen species in myelodysplastic syndromes. Cell Mol. Biol. Lett. 29, 53. doi:10.1186/s11658-024-00570-0
Kishi, T., Ichinose, H., Yoshimura, R., Fukuo, Y., Kitajima, T., Inada, T., et al. (2012). GTP cyclohydrolase 1 gene haplotypes as predictors of SSRI response in Japanese patients with major depressive disorder. J. Affect Disord. 142, 315–322. doi:10.1016/j.jad.2012.05.004
Lambert, C., Wu, Y., and Aanei, C. (2016). Bone marrow immunity and myelodysplasia. Front. Oncol. 6, 172. doi:10.3389/fonc.2016.00172
Lefèvre, C., Bondu, S., Le Goff, S., Kosmider, O., and Fontenay, M. (2017). Dyserythropoiesis of myelodysplastic syndromes. Curr. Opin. Hematol. 24, 191–197. doi:10.1097/MOH.0000000000000325
Lei, G., Zhuang, L., and Gan, B. (2024). The roles of ferroptosis in cancer: tumor suppression, tumor microenvironment, and therapeutic interventions. Cancer Cell 42, 513–534. doi:10.1016/j.ccell.2024.03.011
Li, H., Hu, F., Gale, R. P., Sekeres, M. A., and Liang, Y. (2022). Myelodysplastic syndromes. Nat. Rev. Dis. Primer 8, 74. doi:10.1038/s41572-022-00402-5
Li, H., Li, Y., Yu, Y., Ren, X., Yang, C., Jin, W., et al. (2024b). GSH exhaustion via inhibition of xCT-GSH-GPX4 pathway synergistically enhanced DSF/Cu-induced cuproptosis in myelodysplastic syndromes. Free Radic. Biol. Med. 222, 130–148. doi:10.1016/j.freeradbiomed.2024.06.006
Li, J., Ma, J., Zhang, R., Zhai, Y., Zhang, W., and Fu, R. (2024a). A new therapeutic perspective: erastin inhibits tumor progression by driving ferroptosis in myelodysplastic syndromes. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 72, 414–424. doi:10.1177/10815589241246541
Lindsey, L., and Alex, H. (2018). Iron overload in myelodysplastic syndromes: pathophysiology, consequences, diagnosis, and treatment. J. Adv. Pract. Oncol. 9. doi:10.6004/jadpro.2018.9.4.3
Liu, L., Yang, C., Zhu, L., Wang, Y., Zheng, F., Liang, L., et al. (2024). RSL3 enhances ROS-mediated cell apoptosis of myelodysplastic syndrome cells through MYB/Bcl-2 signaling pathway. Cell Death Dis. 15, 465. doi:10.1038/s41419-024-06866-5
Löwenberg, B. (2013). Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 121, 26–28. doi:10.1182/blood-2012-07-444851
Lv, Q., Niu, H., Yue, L., Liu, J., Yang, L., Liu, C., et al. (2020). Abnormal ferroptosis in myelodysplastic syndrome. Front. Oncol. 10, 1656. doi:10.3389/fonc.2020.01656
Magina, K. N., Pregartner, G., Zebisch, A., Wölfler, A., Neumeister, P., Greinix, H. T., et al. (2017). Cytarabine dose in the consolidation treatment of AML: a systematic review and meta-analysis. Blood 130, 946–948. doi:10.1182/blood-2017-04-777722
Mao, C., Liu, X., Zhang, Y., Lei, G., Yan, Y., Lee, H., et al. (2021). DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590. doi:10.1038/s41586-021-03539-7
McBride, A., Houtmann, S., Wilde, L., Vigil, C., Eischen, C. M., Kasner, M., et al. (2019). The role of inhibition of apoptosis in acute leukemias and myelodysplastic syndrome. Front. Oncol. 9, 192. doi:10.3389/fonc.2019.00192
Mishima, E., Nakamura, T., Zheng, J., Zhang, W., Mourão, A. S. D., Sennhenn, P., et al. (2023). DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E9–E18. doi:10.1038/s41586-023-06269-0
Montalban-Bravo, G., Class, C. A., Ganan-Gomez, I., Kanagal-Shamanna, R., Sasaki, K., Richard-Carpentier, G., et al. (2020). Transcriptomic analysis implicates necroptosis in disease progression and prognosis in myelodysplastic syndromes. Leukemia 34, 872–881. doi:10.1038/s41375-019-0623-5
Pullarkat, V. (2009). Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood 114, 5251–5255. doi:10.1182/blood-2009-07-234062
Rivella, S. (2015). β-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica 100, 418–430. doi:10.3324/haematol.2014.114827
Roveri, A., Maiorino, M., Nisii, C., and Ursini, F. (1994). Purification and characterization of phospholipid hydroperoxide glutathione peroxidase from rat testis mitochondrial membranes. Biochim. Biophys. Acta 1208, 211–221. doi:10.1016/0167-4838(94)90106-6
Sabatier, M., Birsen, R., Lauture, L., Mouche, S., Angelino, P., Dehairs, J., et al. (2023). C/EBPα confers dependence to fatty acid anabolic pathways and vulnerability to lipid oxidative stress-induced ferroptosis in FLT3-mutant leukemia. Cancer Discov. 13, 1720–1747. doi:10.1158/2159-8290.CD-22-0411
Sallman, D. A., Cluzeau, T., Basiorka, A. A., and List, A. (2016). Unraveling the pathogenesis of MDS: the NLRP3 inflammasome and pyroptosis drive the MDS phenotype. Front. Oncol. 6, 151. doi:10.3389/fonc.2016.00151
Sallman, D. A., and List, A. (2019). The central role of inflammatory signaling in the pathogenesis of myelodysplastic syndromes. Blood 133, 1039–1048. doi:10.1182/blood-2018-10-844654
Schmitt, A., Grimm, M., Kreienkamp, N., Junge, H., Labisch, J., Schuhknecht, L., et al. (2023). BRD4 inhibition sensitizes diffuse large B-cell lymphoma cells to ferroptosis. Blood 142, 1143–1155. doi:10.1182/blood.2022019274
Seiler, A., Schneider, M., Förster, H., Roth, S., Wirth, E. K., Culmsee, C., et al. (2008). Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 8, 237–248. doi:10.1016/j.cmet.2008.07.005
Sekeres, M. A., and Taylor, J. (2022). Diagnosis and treatment of myelodysplastic syndromes: a review. JAMA 328, 872–880. doi:10.1001/jama.2022.14578
Su, X., Zhang, M., Yang, G., Cui, X., Yuan, X., Du, L., et al. (2024). Bioinformatics and machine learning approaches reveal key genes and underlying molecular mechanisms of atherosclerosis: a review. Med. Baltim. 103, e38744. doi:10.1097/MD.0000000000038744
Suarez, L., and Gore, S. D. (2013). Demethylation demystification. Blood 121, 1488–1489. doi:10.1182/blood-2013-02-483735
Sui, Y., Geng, X., Wang, Z., Zhang, J., Yang, Y., and Meng, Z. (2024). Targeting the regulation of iron homeostasis as a potential therapeutic strategy for nonalcoholic fatty liver disease. Metabolism 157, 155953. doi:10.1016/j.metabol.2024.155953
Tang, D., Kroemer, G., and Kang, R. (2024b). Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol. 21, 370–388. doi:10.1038/s41571-024-00876-0
Tang, S., Liu, J., Li, F., Yan, Y., Long, X., and Fu, Y. (2024a). AP-1 inhibitor induces ferroptosis via the PI3K/AKT pathway in multiple myeloma cells. Heliyon 10, e34397. doi:10.1016/j.heliyon.2024.e34397
Ursini, F., and Maiorino, M. (2020). Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med. 152, 175–185. doi:10.1016/j.freeradbiomed.2020.02.027
Wang, Q., Zhao, M., Zhang, T., Zhang, B., Zheng, Z., Lin, Z., et al. (2023). Comprehensive analysis of ferroptosis-related genes in immune infiltration and prognosis in multiple myeloma. Front. Pharmacol. 14, 1203125. doi:10.3389/fphar.2023.1203125
Wang, S., Gu, J., Bian, J., He, Y., Xu, X., Wang, C., et al. (2024). Nesfatin-1 mitigates calcific aortic valve disease via suppressing ferroptosis mediated by GSH/GPX4 and ZIP8/SOD2 axes. Free Radic. Biol. Med. 222, 149–164. doi:10.1016/j.freeradbiomed.2024.06.004
Weber, S., Parmon, A., Kurrle, N., Schnütgen, F., and Serve, H. (2020). The clinical significance of iron overload and iron metabolism in myelodysplastic syndrome and acute myeloid leukemia. Front. Immunol. 11, 627662. doi:10.3389/fimmu.2020.627662
Xian, M., Wang, Q., Xiao, L., Zhong, L., Xiong, W., Ye, L., et al. (2024). Leukocyte immunoglobulin-like receptor B1 (LILRB1) protects human multiple myeloma cells from ferroptosis by maintaining cholesterol homeostasis. Nat. Commun. 15, 5767. doi:10.1038/s41467-024-50073-x
Xiong, D., Li, M., and Zeng, C. (2022). Construction and validation of a risk scoring model for diffuse large B-cell lymphoma based on ferroptosis-related genes and its association with immune infiltration. Transl. Oncol. 16, 101314. doi:10.1016/j.tranon.2021.101314
Xu, M., Zhang, D., and Yan, J. (2024b). Targeting ferroptosis using Chinese herbal compounds to treat respiratory diseases. Phytomedicine Int. J. Phytother. Phytopharm. 130, 155738. doi:10.1016/j.phymed.2024.155738
Xu, T., Ding, W., Ji, X., Ao, X., Liu, Y., Yu, W., et al. (2019). Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell Mol. Med. 23, 4900–4912. doi:10.1111/jcmm.14511
Xu, Z., Wang, Y., Yang, W., Han, W., Ma, B., Zhao, Y., et al. (2024a). Total extracts from Abelmoschus manihot (L.) alleviate radiation-induced cardiomyocyte ferroptosis via regulating redox imbalances mediated by the NOX4/xCT/GPX4 axis. J. Ethnopharmacol. 334, 118582. doi:10.1016/j.jep.2024.118582
Yagoda, N., von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature 447, 864–868. doi:10.1038/nature05859
Yu, X., Guo, Q., Zhang, H., Wang, X., Han, Y., and Yang, Z. (2024). Hypoxia-inducible factor-1α can reverse the Adriamycin resistance of breast cancer adjuvant chemotherapy by upregulating transferrin receptor and activating ferroptosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 38, e23876. doi:10.1096/fj.202401119R
Zeng, K., Huang, N., Liu, N., Deng, X., Mu, Y., Zhang, X., et al. (2024). LACTB suppresses liver cancer progression through regulation of ferroptosis. Redox Biol. 75, 103270. doi:10.1016/j.redox.2024.103270
Zha, J., Bi, S., Deng, M., Chen, K., Shi, P., Feng, L., et al. (2021). Disulfiram/copper shows potent cytotoxic effects on myelodysplastic syndromes via inducing Bip-mediated apoptosis and suppressing autophagy. Eur. J. Pharmacol. 902, 174107. doi:10.1016/j.ejphar.2021.174107
Zhang, L., Xu, Y., Cheng, Z., Zhao, J., Wang, M., Sun, Y., et al. (2024a). The EGR1/miR-139/NRF2 axis orchestrates radiosensitivity of non-small-cell lung cancer via ferroptosis. Cancer Lett. 595, 217000. doi:10.1016/j.canlet.2024.217000
Zhang, Y., Cao, S., Zeng, F., Pan, D., Cai, L., Zhou, Y., et al. (2024b). Dihydroartemisinin enhances the radiosensitivity of breast cancer by targeting ferroptosis signaling pathway through hsa_circ_0001610. Eur. J. Pharmacol. 983, 176943. doi:10.1016/j.ejphar.2024.176943
Zhou, Q., Meng, Y., Li, D., Yao, L., Le, J., Liu, Y., et al. (2024). Ferroptosis in cancer: from molecular mechanisms to therapeutic strategies. Signal Transduct. Target Ther. 9, 55. doi:10.1038/s41392-024-01769-5
Keywords: Myelodysplastic syndromes, ferroptosis, signal path, ferroptosis-related genes, ferroptosis inducing drugs
Citation: Yang Y, Han J, Wei Y, Jin J and Feng W (2025) Research progress on ferroptosis in Myelodysplastic syndromes. Front. Pharmacol. 16:1561072. doi: 10.3389/fphar.2025.1561072
Received: 15 January 2025; Accepted: 12 February 2025;
Published: 28 February 2025.
Edited by:
Olivier Cuvillier, UPR8241 Laboratoire de Chimie de Coordination (LCC), FranceReviewed by:
Robert Charles Jackson, Pharmacometrics Ltd., United KingdomCopyright © 2025 Yang, Han, Wei, Jin and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiyin Feng, ZmVuZ3dlaXlpbmcxOTk3QDEyNi5jb20=
†These authors share first authorship
 Yifan Yang
Yifan Yang Jiongping Han1†
Jiongping Han1† Weiyin Feng
Weiyin Feng