- 1Shandong Co-Innovation Center of Classic Traditional Chinese Medicine Formula, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2The Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Binzhou People’s Hospital, Binzhou, China
Catalpol (CAT) is a landmark active ingredient in traditional Chinese medicine Rehmannia (TCT), also known as dehydroxybenzoate catalpone, which is a kind of iridoid terpene glycoside with strong antioxidant, anti-inflammatory, antitumor and other biological activities. It can exert its anti-disease effect in a variety of ways. For some patients with chronic diseases, the application of azalea alcohol in rehmannia may bring more comprehensive and long-lasting efficacy. Studies have shown that the anti-disease effect of catalpol in osteoporosis (OP) is mainly achieved through various pathways such as Wnt/β-catenin signaling pathways to promote osteogenic differentiation, and RANKL/RANK and other signaling pathways to inhibit osteoclastic differentiation. At present, there is a slight lack of analysis of the mechanism of action of catalpa alcohol in the treatment of osteoporosis, so this study comprehensively searched the literature on the mechanism of action of catalpa alcohol in the treatment of osteoporosis in various databases, and reviewed the research progress of its role and mechanism, to provide reference and theoretical basis for the further development and application of catalpol.
1 Introduction
Osteoporosis (OP) represents a prevalent systemic skeletal condition marked by diminished bone density and compromised microstructural integrity. This deterioration results in weakened osseous tissue, heightened susceptibility to fractures, and reduced bone resilience (Silverstein et al., 2024). Etiologically, osteoporosis can be classified into two main categories: primary and secondary forms (Ayers et al., 2023). Evidence from medical literature points to osteoporosis affecting close to 30% of people who have surpassed their fifth decade of life, affecting over 200 million people worldwide (The Lancet Diabetes Endocrinology, 2021). Therefore, exploring novel preventive and therapeutic approaches is imperative.
In traditional Chinese medicine (TCM), osteoporosis falls under the categories of “bone flaccidity” and “bone impediment,” with the pathogenesis linked to a deficiency of essence leading to inadequate nourishment of the bones. Historically, in the research of osteoporosis treatment, Chinese herbal medicine has shown distinct advantages due to its significant efficacy, minimal toxic side effects, and suitability for long-term use (Duan et al., 2023). Common anti-osteoporosis herbal prescriptions often emphasize kidney-nourishing herbs including Rehmannia glutinosa, Epimedium, Drynaria, Cornus officinalis, and Astragalus (Jinlong et al., 2020; Zhang et al., 2021). These are known to mitigate the reduction in trabecular bone mineral density (BMD), increase cortical bone thickness, and enhance trabecular bone formation in the bone marrow space. They also promote osteoblast proliferation, activity of alkaline phosphatase (ALP), and expression of osteoprotegerin (OPG), while reducing the number of TRAP-positive multinucleated cells and osteoclasts (Lim and Kim, 2013). Contemporary studies have elucidated the mechanisms and efficacy of TCM’s bioactive compounds in combating osteoporosis, with the majority involving various glycosides, saccharides, organic acids, amino acids, and inorganic elements (Carnovali et al., 2018). Among these, catalpol has been identified as one of the most active components (Wang, 2015; Xia, 2019; Liu et al., 2017), which is listed as a marker compound in the content determination of Rehmannia glutinosa in the Pharmacopoeia of the People’s Republic of China (2020 edition).
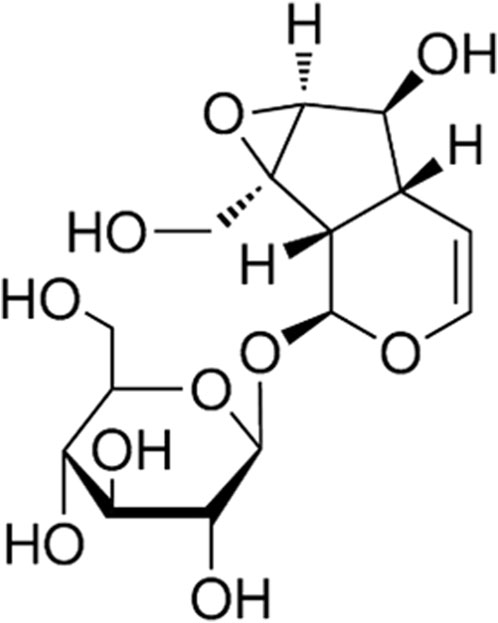
Catalpol is a type of flavanone glycoside. Studies have revealed its capabilities in lowering blood sugar (Shieh et al., 2011), anti-fibrotic effects on the kidneys (Sun et al., 2020), neuroprotection, and combating cardiovascular and cerebrovascular diseases (Hongwei and Meng, 2015) (for the chemical structure of catalpol, see Figure 1). Consequently, it has attracted widespread attention from researchers and clinicians. Researchers and doctors have studied the anti-osteoporotic effects of catalpol at the molecular mechanism level, providing theoretical foundations and support for its clinical applications. It has been discovered that catalpol promotes osteogenic differentiation and inhibits osteoclast differentiation through various molecular mechanisms, thereby achieving therapeutic effects (Table 1). While the anti-osteoporotic effects of catalpol have been confirmed by numerous studies, there is a relative scarcity of comprehensive reviews on its mechanisms against osteoporosis. Based on this, the current article reviews the molecular mechanisms of catalpol in combating osteoporosis, aiming to provide theoretical bases and references for researchers and clinicians and to suggest new avenues for the further development and utilization of catalpol.
2 Catalpol promotes osteoblastic differentiation
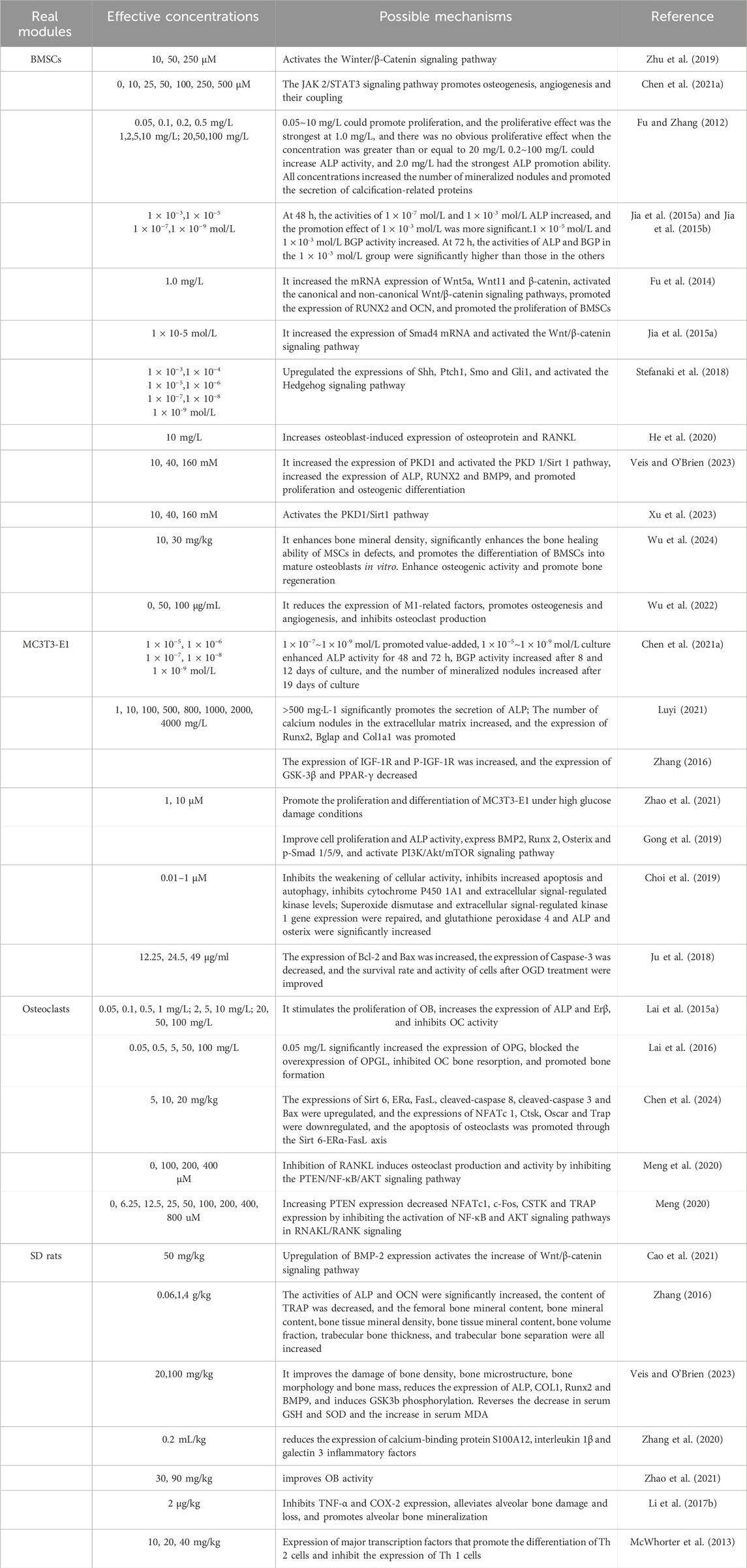
The equilibrium of mineral content in bones is sustained through the bone remodeling process, wherein osteoclasts (OCs) facilitate resorption while osteoblasts (OBs) promote formation and the integrity of bone structure (Langdahl, 2021). Abnormal development and differentiation of OBs and OCs, leading to a predominance of bone resorption over bone formation, constitute a critical pathogenic mechanism in OP. Enhancing osteoblastic differentiation is a key aspect of combating OP (Figure 2).
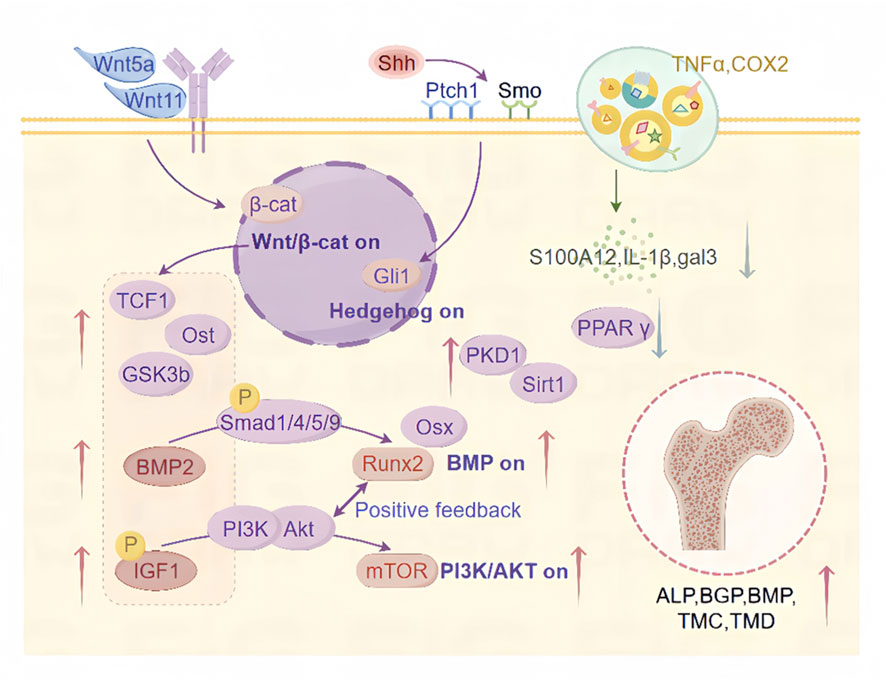
Figure 2. Mechanisms by which CAT promotes osteoblast differentiation. This includes the regulatory roles of CAT in activating the Wnt/β-catenin signaling pathway, Hedgehog signaling pathway, inflammatory signaling pathways, BMP signaling pathway, IGF-1/PI3K/Akt/mTOR signaling pathway, PKD1/Sirt1 signaling pathway, and their downstream targets, promoting osteogenic differentiation and inhibiting adipogenic differentiation through multiple regulatory mechanisms.
Research findings indicate that CAT administration results in an augmentation of trabecular quantity and reduce their degenerative structural changes, significantly ameliorating trabecular deterioration and reducing bone loss (Zhu et al., 2019), while also promoting bone regeneration and angiogenesis (Chen et al., 2021a). CAT enhances the activity of alkaline phosphatase (ALP), bone gamma-carboxyglutamate protein (BGP), and bone morphogenetic proteins (BMP) in mouse osteoblastic precursor cells MC3T3-E1 (Wu et al., 2010) and bone marrow mesenchymal stem cells (BMSCs) (Fu and Zhang, 2012). It increases the number of mineralized nodules and promotes the expression of osteogenesis-related genes and osteoblastic activity, all within a broad range of safe concentrations (Louie et al., 2020). The effects are most significant at CAT concentrations of 1 × 10−3 mol/L and 1 × 10−5 mol/L (Jia et al., 2015a), notably accelerating BMP formation and secretion in MC3T3-E1, enhancing alkaline phosphatase activity, and proliferating the count of mineralized nodules (Jia et al., 2015b; Yang, 2021). These findings suggest that CAT can prevent and mitigate the development of OP by modulating osteoblastic differentiation.
2.1 Wnt/β-catenin signaling pathway
Multiple physiological functions are intricately linked to the Wnt/β-catenin signaling route (Liu et al., 2022) and is also believed to be integral to osteoblastic differentiation (Zhu et al., 2019). CAT may stimulate Runx2 and multiple targets related to bone formation by upregulating the Wnt/β-catenin transcription factor 1 (TCF1) signaling regulatory element located in the promoter of the Runx2 gene, such as increasing the expression of os-teocalcin (ost), one of the main indicators for osteoblast differentiation and maturation into mineralization, increasing the secretory deposition of ALP, promoting the differentiation of BMSCs in the direction of osteoblasts and the maturation of newly formed osteocytes (Gaur et al., 2005). This effect is related to the simultaneous activation of both the classical and non-classical Wnt signaling pathways (Fu et al., 2014; Fu and Zhang, 2012). Activated β-catenin stimulates the formation of osteoblasts via the Wnt/β-catenin pathway, inducing an increase in ALP activity (Bennett et al., 2005; Jackson et al., 2005). CAT has been reported to promote the accumulation of intracellular β-catenin by inducing Wnt5a and Wnt11, and the accumulated β-catenin is transported to the nucleus, forming a complex with the transcription factor LEF/TCF, stimulating downstream gene expression (Huang et al., 2019), activating classical Wnt signaling, and thereby promoting the proliferation of BMSCs (Fu et al., 2014). It also activates multiple pathways in the Wnt/β-catenin signaling network by upregulating key regulatory proteins such as BMP-2 (Cao et al., 2021), Smad4 (Jia et al., 2015b), and downregulating GSK3b, as well as increasing the expression of IGF-1R and P-IGF-1R (Zhang, 2016), thus promoting osteoblastic differentiation.
2.2 Hedgehog signaling pathway
A fundamental role in embryo development, cellular proliferation, differentiation, and tissue/organ genesis is played by the evolutionarily conserved Hedgehog pathway (Chen et al., 2023). CAT can directly bind to the membrane receptor Ptch1 by upregulating the upstream ligand Shh signaling molecule in the Hedgehog signaling pathway, which relieves the inhibitory effect of Ptch1 on Smo, transmits Hedgehog signaling to the cytoplasm, activates the expression of downstream nuclear transcription factor Gli1, and promotes the differentiation of BMSCs into osteogenic (Lai et al., 2019). Exploring the mechanisms of this pathway facilitates the identification of specific targets for the effect of tsozinol on BMSCsin combating and managing OP.
2.3 Inflammatory signaling pathways
Inflammatory responses exacerbate the prevalence of OP by inhibiting bone formation, promoting bone resorption, and suppressing the proliferation and differentiation of myocytes (Stefanaki et al., 2018). CAT exhibits significant anti-inflammatory and antioxidant effects, inhibiting the expression of inflammatory mediators TNF-α and the key enzyme COX-2 involved in prostaglandin synthesis at mRNA and protein levels, thereby restoring the secretion levels of ALP and OCN (Liu et al., 2016). It increases the expression of osteoprotective factors and nuclear factor κB receptor activator ligand, altering the microstructure of bone through abnormal bone remodeling and reduction of mineral content (He et al., 2020). Reducing the expression of calcium-binding protein S100A12, interleukin 1β (IL-1β), and galectin 3 (gal-3) inflammatory factors plays an important role in the progression of early bone disease (Zhang et al., 2020).
2.4 BMP signaling pathway
The Bone Morphogenetic Protein (BMP) regulated pathways are critical for promoting ossification. These cascades regulate osteoblastic differentiation marker expression (e.g., ALP, OPN, bone sialoprotein, OCN), suppressing adipogenesis while promoting osteogenesis through Runx2 and Osterix (Osx) modulation in osteoblasts (Cheng and Genever, 2010; Komori, 2005). There are known two subtypes of BMP receptors, BMPR-I and BMPR-II, which are serine-threonine kinase receptors. Upon binding of BMP to BMPR-II, BMPR-I is recruited to form an activated quaternary complex, which then phosphorylates and activates the intracellular Smad protein. Receptor Smads bind to co-Smads and translocate into the nucleus as transcription factors. One of the BMP-Smad target genes is Runx2 (Lin and Hankenson, 2011). It is through this mechanism that CAT positively regulates the expression of BMP2, thereby significantly increasing the expression of Runx 2, Osx and p-Smad 1/5/9 in MC3T3-E1 cells, and participating in the physiological process of osteogenesis (Gong et al., 2019), indicating that CAT’s osteogenic effects may be attributed to its influence on BMP signaling cascades.
2.5 IGF-1/PI3K/Akt/mTOR signaling pathway
Diabetes-induced bone loss or osteoporosis, termed diabetic osteoporosis (DOP), exhibits impaired osseous repair and renewal, accompanied by elevated fracture susceptibility (Napoli et al., 2017). Evidence suggests CAT protects against glucose-mediated bone deterioration by optimizing osteoblast performance, fostering their expansion and specialization in hyperglycemic MC3T3-E1 environments, and minimizing hindrances to skeletal mineralization (Zhao et al., 2021). Insulin-like growth factor (IGF) is involved in blood glucose regulation and influences osteoblast proliferation and survival, thus regulating bone remodeling, with its production and storage in the bone matrix (Ghodsi et al., 2016; Zhang, 2016). CAT can bind correctly within the binding pocket of IGF-1, further indicating that IGF-1 is one of the targets of CAT. The binding strength of CAT with the IGF-1 binding pocket is strong, and it substantially modulates IGF-1’s expression and phosphorylation levels (Oh et al., 2003). CAT stimulates osteoblast proliferation and differentiation by activating the PI3K-Akt pathway, which in turn activate It also activates multiple pathways in the Wnt/β-catenin signaling network by upregulating key regulatory proteins such as BMP-2 (Cao et al., 2021), Smad4 (Jia et al., 2015), and downregulating GSK3b, as well as increasing the expression of IGF-1R and P-IGF-1R (Zhang, 2016), thus promoting osteoblastic differentiation.
p-IGF-1, p-PI3K, and p-mTOR (Gong et al., 2019; Bakker and Jaspers, 2015; Bakker et al., 2016). Runx2 and the PI3K/Akt/mTOR pathway are interdependent in the regulation of osteoblast differentiation. Runx2 upregulates the protein levels of PI3K subunits and Akt, while the PI3K/Akt/mTOR pathway significantly enhances Runx2’s DNA-binding and Runx2-dependent transcription (Sheng et al., 2014). This positive feedback loop is also one of the ways CAT significantly promotes osteoblast differentiation and migration, suggesting that CAT in the prevention and treatment of DOP can augment both skeletal mineral accretion and collagen synthesis.
CAT can also activate the estrogen signaling pathway to affect the interaction between downstream transcription factors and the PI3K/AKT signaling pathway. Activation of the PI3K/AKT signaling pathway leads to the upregulation of endothelial eNOS and an increase in NO levels, which stimulates angiogenesis, enhances the local blood supply to bone tissue, and improves microcirculatory structure. Thus, these processes favor bone formation (Simão et al., 2012; Li et al., 2017b; Sun et al., 2019). Literature reports suggest that the interaction between ER and the PI3K/AKT signaling pathway may depend on the binding of scaffold proteins and adaptor proteins to the p85 subunit of PI3K to form adherent plaque protein complexes and ERα, which activate downstream AKT or AKT 2, thereby triggering cascades in the signaling pathway (Sun et al., 2001). When used, activated AKT phosphorylates serine residues in the AF-1 region of ERα, thereby regulating the effect of IGF-1 on Erα (Martin et al., 2000).
2.6 PKD1/Sirt1 signaling pathway
Among secondary osteoporosis cases, glucocorticoid-induced osteoporosis (GIOP) stands as the most prevalent form. Elevated oxidative stress impairs the osteogenic potential of murine preosteoblasts and compromises their skeletal structure, but antioxidants can reverse this process (Atashi et al., 2015). As an antioxidant (Jiang et al., 2004), CAT upregulated PKD1-related pathways by increasing the activity of the promoter of the mechanoductive molecule polycystic kidney disease-1 (PKD1), promoted the expression of Sirt1 with strong antioxidant activity, and increased the expression of ALP activity and RUNX 2 in BMSCs under oxidative stress (Zhang et al., 2017), and activation of SIRT 1 can promote angiogenesis and osteogenic differentiation in BMP 9-induced MSCs (Lu et al., 2022). Bone marrow PKD1/Sirt1 signaling is enhanced by CAT in murine models of GIOP. Similarly, in BMSCs treated with dexamethasone (Dex), CAT also promotes their proliferation and osteogenic differentiation (Xu et al., 2023). Therefore, CAT holds promise as a safe and effective therapeutic for GIOP.
2.7 Inhibition of adipogenic and promotion of osteogenic differentiation
Peroxisome proliferator-activated receptor gamma (PPAR-γ) is a member of the ligand-activated nuclear transcription factor superfamily (Watt and Schlezinger, 2015). CAT can reduce the protein expression of PPAR-γ, suppressing BMSC adipogenesis while enhancing their osteoblastic lineage commitment (Zhang, 2016).
3 Inhibition of osteoclast differentiation
The foremost bone-eroding cells, known as osteoclasts, are descendants of the monocyte/macrophage hematopoietic lineage. They absorb bone to maintain a matrix with appropriate strength and elasticity to meet structural demands and assist in calcium homeostasis. Nevertheless, the overproduction or hyperactivation of osteoclasts may result in bone degradation. resulting in OP and other bone diseases (Veis and O'Brien, 2023). A more comprehensive understanding of osteoclast biology could offer more specific therapeutic directions for the diagnosis and treatment of OP (Figure 3).
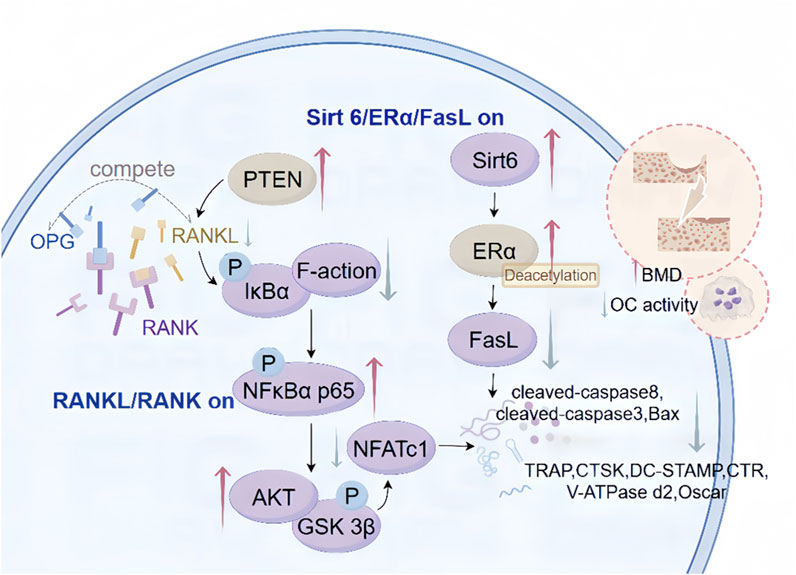
Figure 3. Mechanism of CAT inhibition of osteoclast differentiation. These include the regulatory mechanism between CAT initiating the RANKL/RANK signaling pathway through upstream signaling, the regulatory role between the Sirt 6/ERα/FasL signaling pathway and their downstream targets, inhibiting osteoclast differentiation, and intervening in the apoptosis process.
CAT has been shown to significantly ameliorate the excessive formation of OCs (Sun et al., 2022). Lai et al. discovered that a concentration of 0.05 mg/L CAT significantly reduced the number of OC-mediated bone resorption pits after 48, 72, and 96 h in an OB-OC co-culture system, indicating that CAT can decrease OC activity. Furthermore, CAT inhibits the secretion of tartrate-resistant acid phosphatase (TRAP), a lysosomal enzyme involved in the degradation of bone matrix minerals such as calcium and phosphate released by OCs (Lai Manxiang et al., 2015; Lai et al., 2016; Chen et al., 2024). Consequently, The function of CAT includes suppressing osseous tissue degradation. thus providing bone protective effects and exerting anti-osteoporosis (OP) actions.
3.1 RANKL/RANK signaling pathway
The receptor activator of nuclear factor-kappa B ligand (RANKL) binds to the receptor activator of nuclear factor-kappa B (RANK) on the surface of OC precursor cells and OCs, triggering a cascade of reactions that lead to the differentiation, maturation, and activation of OCs. The RANKL/RANK signaling pathway is instrumental in orchestrating the delicate interplay between OCs and OBs, as well as in bone metabolism and remodeling, and has become a new target for the prevention and treatment of metabolic bone diseases (Yue et al., 2022). Osteoprotegerin (OPG) acts as a decoy receptor that competitively binds to RANKL, thereby inhibiting the interaction between RANKL and RANK on the OC surface and suppressing OC differentiation and maturation (Ye, 2014). A concentration of 0.05 mg/L CAT significantly enhances the expression of OB OPG mRNA in the OB-OC co-culture system (Lai et al., 2016), mediating the process involved in regulating the expression of RANKL. Furthermore, CAT mitigates the ubiquitination and degradation of phosphatase and tensin homolog (PTEN), upregulating its activity. This leads to the inhibition of RANKL-induced phosphorylation and degradation of IκBα, subsequently suppressing the phosphorylation and nuclear translocation of NF-κB p65 (Meng et al., 2020). The downstream phosphorylation processes of AKT and GSK 3β are also inhibited. Additionally, CAT decreases the mRNA and protein expression levels of NFATc1, thereby suppressing the expression of its downstream genes including tartrate-resistant acid phosphatase (TRAP), cathepsin K (CTSK), dendritic cell-specific transmembrane protein (DC-STAMP), calcitonin receptor (CTR), V-ATPase d2 (Udagawa et al., 2021), and Oscar (Tokunaga et al., 2020). During the initial phases of osteoclastogenesis, CAT demonstrates the ability to suppress F-actin assembly in osteoclasts via RANKL-mediated pathways. thereby suppressing bone resorptive activity. This suggests that the role of CAT in inhibiting excessive osteoclast activity may be attributed to blocking the RANKL/RANK signaling pathway or targeting the AKT/GSK 3β/NFATc1 signaling cascade (Meng, 2020), mitigating the adverse impacts of inflammation and ovariectomy on bone density in mouse experimental systems (Udagawa et al., 2021). The data underscore CAT’s contribution to the synchronized modulation of bone turnover, indicating its potential as an innovative pharmacological intervention for osteoclast-mediated skeletal disorders.
3.2 Sirt 6/ERα/FasL signaling pathway
Through GO and KEGG pathway enrichment analysis, it has been found that the main components of processed Rehmannia glutinosa in treating OP may be related to the estrogen signaling pathway, HIF-1 signaling pathway, among others (Xie, 2022). Estrogen induces apoptosis of mature osteoclasts by activating the Fas/Fas ligand (Fas) pathway through the estrogen receptor (ER) α (Hu et al., 2023), participates in bone metabolism, and maintains bone formation (Khosla et al., 2012). Both in vivo and in vitro, CAT upregulates the expression of osteoclast apoptosis-related proteins including FasL, cleaved-caspase 8, cleaved-caspase 3, and Bax (Nakamura et al., 2007), indicating that CAT induces osteoclast apoptosis, reduces osteoclast numbers, and consequently decreases bone resorption. CAT increases the protein levels of deacetylases and nucleotidyl transferase Sirt 6, inducing deacetylation of ERα, and significantly upregulating ERα protein expression during the apoptosis process. This effect further influences the mRNA levels of FasL related to osteoclast apoptosis through ERα transcriptional activity, ultimately activating osteoclast apoptosis (Chen et al., 2024). The interactions within this complex regulatory network underlie the mechanism regulating osteoclast apoptosis. Consequently, the induction of osteoclast programmed cell death via the SIRT6-ERα-FasL signaling cascade may mitigate estrogen deficiency-induced osteoporosis.
4 Other anti-OP pathways
4.1 Antioxidant stress damage
The beneficial effects of CAT on osteoblast protection are primarily associated with its antioxidant activity (Zhu et al., 2016). CAT facilitates bone mineralization and mitigates the progression of OP through anti-inflammatory treatment strategies and by mediating the homeostasis of reactive oxygen species, reducing pain and improving bone damage in patients (Conaghan et al., 2019). CAT can attenuate impaired osteogenic differentiation, enhance the expression of COL1, BMP9, and RUNX2 (Zheng et al., 2020), and induce the phosphorylation of GSK3b (Eiraku et al., 2019). It reverses the reduction of serum GSH and SOD as well as the increase of serum MDA (Xu et al., 2023). Concentrations of 0.01–1 μM can inhibit the autophagy upregulation-induced OB apoptosis by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), offering protection against oxidative stress. CAT restores the expression of cytoplasmic Cu/Zn SOD and increases the expression of the apoptosis regulatory factor GPx 4 gene in damaged cells (Choi et al., 2019), causing OBs to adapt to oxidative stress conditions. It also inhibits the elevation of phosphorylated ERK, consequently attenuating the elevated production of NO and inflammatory mediators (Ajizian et al., 1999), or by directly blocking the formation of NO/nitrites to alleviate nitrative stress, lowering the impact of oxidative stress through pathways such as the reduction of cytochrome P450 superfamily enzyme CYP1A1 (Choi et al., 2019), inhibiting bone loss. By activating the PKD 1/Sirt 1 pathway, it resists oxidative stress (Xu et al., 2023), and cooperatively enhances both ALP functionality and RUNX2 levels in BMSCs under oxidative stress (Chen et al., 2021), promoting proliferation and osteogenic differentiation.
4.2 Bone repair functions
CAT significantly promotes the migration and bone formation of MSCs, enhances the osteogenic activity of MSCs, enhances the osteogenic potential of MSCs in repairing rat osseous lesions, and increases bone density, making it useful for local bone repair. Thus, it facilitates the filling of bone defects in mice, maintains the chondrocyte phenotype, improves the fixation of bone structure and matrix, and effectively treats local bone defects (Wu et al., 2024). Previous studies have reported that experimental findings in rats reveal CAT’s capacity to potentiate BMSC-driven bone repair in substantial cranial defects and counteract post-ovariectomy osseous tissue loss (Zhu et al., 2019). Additionally, Research indicates that CAT suppresses TNF-α and COX-2 expression, mitigates nicotine-induced alveolar bone deterioration, and enhances osseous mineralization (Li et al., 2017a), suggesting its potential as a safe and efficacious intervention for bone loss.
4.3 Anti-oxidative and glucose deprivation damage
The anti-OP effect of Catalpol is also reflected in the protection of bone cells. Pre-treatment with CAT can enhance the survival and activity of BMSCs following oxygen-glucose deprivation (OGD) treatment. This phenomenon is characterized by elevated expression of the anti-apoptotic factor Bcl-2, coupled with reduced levels of pro-apoptotic Bcl-2 family members, including Bax, and the key mediator of apoptosis, Caspase-3. In vivo experiments have demonstrated that CAT can improve the survival rate of transplanted BMSCs (Ju et al., 2018).
4.4 Immune cell polarization
The pathogenesis of OP, a widespread inflammatory bone disorder, is intricately linked to immune system function (Srivastava et al., 2018). Th 1/Th 2 cells are important immune cells, having key functions in maintaining immune activation and immune tolerance. Th 1 cells produce inflammatory cytokines such as IL-12 and IFN-γ, which are associated with immune suppression and can strongly promote the formation of OC. Conversely, Th 2 cells secrete IL-4 and IL-10, which have opposite effects. This indicates the potential role of Th 1/Th 2 balance in bone remodeling and OP (Fujii et al., 2012). CAT intervention has been shown to effectively counteract bone depletion associated with estrogen insufficiency by favoring Th2 over Th1 in the immune profile, thereby altering the ratio of inflammatory cytokine production and mitigating OP caused by bone loss.
Macrophages exhibit plasticity, adopting either a pro-inflammatory M1 profile or an anti-inflammatory, tissue-restorative M2 configuration (McWhorter et al., 2013). Macrophage functional dichotomy in fracture healing involves M1-driven acute inflammation and site cleaning, followed by M2-mediated support of MSC osteogenic activity through secreted growth factors (Murray and Wynn, 2011). CAT can modulate the polarization phenotype of macrophages, reducing inflammation, decreasing the expression of M1-related factors, and, through interactions with osteogenic and other related cells, effectively promote osteogenesis, angiogenesis, and inhibit osteoclast production (Zhang et al., 2022). This suggests that CAT could enhance bone tissue regeneration and functional reconstruction through immune regulation (Wu et al., 2022).
4.5 Osteogenesis-angiogenesis coupling
Bone formation and bone healing are closely associated with angiogenesis (Kan et al., 2022). The osteogenesis-angiogenesis coupling process can be enhanced by various regulatory factors (Zou et al., 2023). Among them, the JAK/STAT signaling pathway is an indispensable and critical signaling pathway in the bone regeneration process. CAT can promote the activation and phosphorylation of STAT 3 by JAK 2. This leads to the dimerization and nuclear translocation of STAT 3, promoting osteogenic differentiation and enhancing the healing of bone defects (Wang and Xue, 2018), as well as stimulating the expression of the angiogenic factor VEGF in bone marrow multipotent progenitor cells and BMSCs (Wang et al., 2007; Liu et al., 2009). It participates in the recruitment, proliferation, and differentiation of BMSCs and vascular endothelial cells. Additionally, through the activation of SIRT 1, it promotes angiogenesis and osteogenic differentiation induced by BMP 9 in MSCs (Lu et al., 2022). CAT also facilitates angiogenesis and osteogenic activity is augmented by paracrine factors governing mesenchymal stromal cell-macrophage interactions (Zhang et al., 2022), thereby accelerating the repair of osteoporotic bones.This work forms a cornerstone for the clinical deployment of CAT in osteoporotic fracture management (Figure 4).
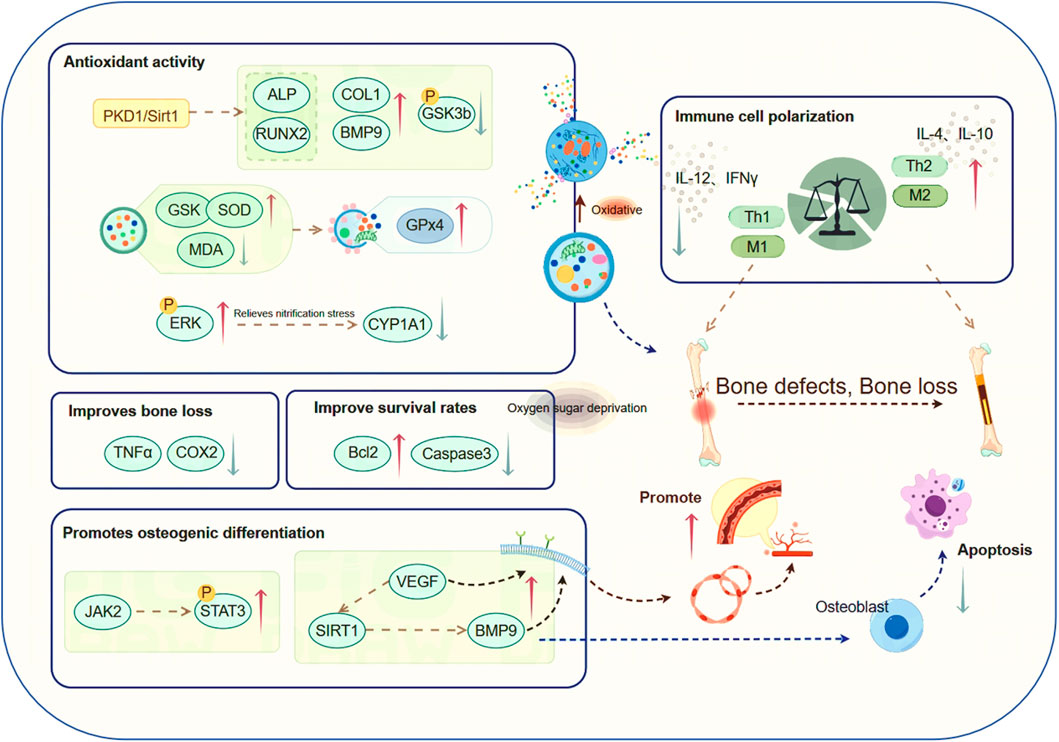
Figure 4. CAT has anti-osteoporotic effects through other pathways. Among them, CAT is involved in the mechanism research of regulating the course of osteoporosis through various pathways such as antioxidative stress injury, bone repair, antioxidant glucose deprivation injury, regulation of immune cell polarization, and promotion of osteogenesis-angiogenesis coupling.
5 CAT future directions and recommendations in OP research and treatment
To further explore the potential of CAT in the treatment of OP, we propose the following recommendations and future research directions.
5.1 In-depth mechanism research
Although various studies have demonstrated the alleviating and ameliorating effect of CAT on OP, its exact molecular mechanism has not been fully elucidated. Conducting in-depth mechanistic studies will help to understand the exact site of action of CAT in the treatment of OP and provide more comprehensive evidence for its clinical application. Patients with OP often experience impairment of multiple systems. It is also crucial to identify common treatment pathways for the prevention and treatment of these diseases. Not only will it be possible to discover deeper molecular mechanisms, but it may also be possible to discover other synergistic protective effects of OP.
5.2 Effectiveness and toxicity studies
The effectiveness of a drug is largely related to the dosage of the drug used and depends on the route of administration. Therefore, we summarized the dosage and mechanism of action of CAT used in previous studies (Table 1). CAT is well tolerated and non-toxic. There are few clinical studies involving the relieving therapeutic effect of CAT on OP. To bridge the gap between preclinical and clinical studies and bring CAT into clinical use as soon as possible, well-designed clinical trials should be conducted to evaluate the efficacy, safety, and optimal dose of CAT in human subjects. It also provides a variety of protective effects according to the unique needs of different patient groups, and the individualized medication approach can be used to optimize the use of CAT in OP considering the individual differences in treatment response. By considering the individual patient’s genetic background, lifestyle factors, and disease progression, customized treatment strategies can be developed to maximize the therapeutic benefits of catalpa alcohol. In addition, further toxicity studies should be conducted to determine the long-term safety profile of CAT and its side effects.
Although there are currently no clinical studies on the efficacy of CAT in the treatment of OP, CAT has been used clinically to treat cardiovascular diseases, tumors, and other conditions. In clinical applications, Rehmannia has achieved good feedback and results in the treatment of OP (Li et al., 2022). We believe that CAT, as the main active ingredient of Rehmannia, has a very broad clinical prospect for the treatment of OP, potentially providing a new drug treatment option for OP patients. At the same time, in-depth research on CAT has opened a new avenue for developing and utilizing various natural medicinal components in treating clinical diseases.
6 Summary and outlook
CAT holds broad medicinal value and application prospects due to its effects on multiple organ tissues. Its multi-target action in reducing bone resorption, enhancing bone formation, and decreasing bone loss has garnered widespread attention in OP treatment (Zhang et al., 2016). CAT typically exerts its regulatory effects on bone metabolism through various molecular mechanisms such as estrogen-like activity, antioxidant activity, or regulation through multiple signaling pathways. These effects are pronounced, presenting significant potential and market value in drug and health supplement development. Research findings indicate that in Ovx mice treated with CAT, there was no significant increase in serum estradiol (E2) levels, the findings indicate that CAT may serve as a secure treatment option for mitigating estrogen deficiency-induced bone deterioration, avoiding the adverse effects typically linked to estrogen supplementation (Lai et al., 2015). Additionally, by utilizing CAT’s ability to promote osteoblast mineralization in conjunction with orthopedic implants, achieving effective local concentrations significantly improved osteogenesis. Thus, CAT loading enhances the bone integration effects of composite materials relative to conventional materials (Luyi, 2021), offering improvements to current therapeutic methods.
However, the mechanisms of CAT in bone metabolic diseases remain an area for further study. The utilization of bioinformatics tools, in silico modeling, and molecular docking techniques enables researchers to explore the molecular underpinnings and specific targets of CAT in OP treatment, laying the groundwork for subsequent experimental research. CAT is widely available and can be extracted using various methods tailored to different materials to maximize yield (Zhang and Liu, 2019). Yet, CAT has not been applied in clinical practice. Prior to making clinical application decisions, researchers need to conduct further studies to introduce CAT into clinical practice, contributing to human health.
Author contributions
NL: Conceptualization, Data curation, Investigation, Visualization, Writing–original draft, Writing–review and editing. XM: Supervision, Writing–review and editing. SZ: Supervision, Writing–review and editing. HW: Conceptualization, Data curation, Funding acquisition, Resources, Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82004087). Youth Innovation Team Project of Shandong University of Traditional Chinese Medicine (No. 2020-54-19). Shandong Provincial Higher Education Youth Talent Introduction Program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ajizian, S. J., English, B. K., and Meals, E. A. (1999). Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J. Infect. Dis. 179 (4), 939–944. doi:10.1086/314659
Atashi, F., Modarressi, A., and Pepper, M. S. (2015). The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem cells Dev. 24 (10), 1150–1163. doi:10.1089/scd.2014.0484
Ayers, C., Kansagara, D., Lazur, B., Fu, R., Kwon, A., and Harrod, C. (2023). Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American college of physicians. Ann. Intern. Med. 176 (2), 182–195. doi:10.7326/M22-0684
Bakker, A. D., Gakes, T., Hogervorst, J. M., de Wit, G. M., Klein-Nulend, J., and Jaspers, R. T. (2016). Mechanical stimulation and IGF-1 enhance mRNA translation rate in osteoblasts via activation of the AKT-mTOR pathway. J. Cell. physiology 231 (6), 1283–1290. doi:10.1002/jcp.25228
Bakker, A. D., and Jaspers, R. T. (2015). IL-6 and IGF-1 signaling within and between muscle and bone: how important is the mTOR pathway for bone metabolism? Curr. Osteoporos. Rep. 13 (3), 131–139. doi:10.1007/s11914-015-0264-1
Bennett, C. N., Longo, K. A., Wright, W. S., Suva, L. J., Lane, T. F., Hankenson, K. D., et al. (2005). Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad. Sci. U. S. A. 102 (9), 3324–3329. doi:10.1073/pnas.0408742102
Cao, Y., Chen, F., Yang, H., Lian, K., and Zhang, X. (2021). Catalpa alcohol promotes femur fracture healing in rats by upregulating BMP-2 expression and activating the Wnt/β-catenin signaling pathway. J. Guangxi Med. Univ. (07), 1380–1387.
Carnovali, M., Luzi, L., Terruzzi, I., Banfi, G., and Mariotti, M. (2018). Metabolic and bone effects of high-fat diet in adult zebrafish. Endocrine 61 (2), 317–326. doi:10.1007/s12020-017-1494-z
Chen, L., Zhang, R. Y., Xie, J., Yang, J. Y., Fang, K. H., Hong, C. X., et al. (2021a). STAT3 activation by catalpol promotes osteogenesis-angiogenesis coupling, thus accelerating osteoporotic bone repair. Stem cell Res. and Ther. 12 (1), 108. doi:10.1186/s13287-021-02178-z
Chen, S., Jin, J., Xu, Z., Han, H., Wu, L., and Li, Z. (2024). Catalpol attenuates osteoporosis in ovariectomized rats through promoting osteoclast apoptosis via the Sirt6-ERα-FasL axis. Phytomedicine Int. J. phytotherapy Phytopharm. 123, 155262. doi:10.1016/j.phymed.2023.155262
Chen, T., Wang, H., Jiang, C., and Lu, Y. (2021b). PKD1 alleviates oxidative stress-inhibited osteogenesis of rat bone marrow-derived mesenchymal stem cells through TAZ activation. J. Cell. Biochem. 122 (11), 1715–1725. doi:10.1002/jcb.30124
Chen, Y., Wei, Z., Shi, H., Wen, X., Wang, Y., and Wei, R. (2023). BushenHuoxue formula promotes osteogenic differentiation via affecting Hedgehog signaling pathway in bone marrow stem cells to improve osteoporosis symptoms. PloS one 18 (11), e0289912. doi:10.1371/journal.pone.0289912
Cheng, A., and Genever, P. G. (2010). SOX9 determines RUNX2 transactivity by directing intracellular degradation. J. bone mineral Res. official J. Am. Soc. Bone Mineral Res. 25 (12), 2680–2689. doi:10.1002/jbmr.174
Choi, E. M., Suh, K. S., Jung, W. W., Yun, S., Park, S. Y., Chin, S. O., et al. (2019). Catalpol protects against 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced cytotoxicity in osteoblastic MC3T3-E1 cells. J. Appl. Toxicol. 39 (12), 1710–1719. doi:10.1002/jat.3896
Conaghan, P. G., Cook, A. D., Hamilton, J. A., and Tak, P. P. (2019). Therapeutic options for targeting inflammatory osteoarthritis pain. Nat. Rev. Rheumatol. 15 (6), 355–363. doi:10.1038/s41584-019-0221-y
Duan, Y., Su, Y. T., Ren, J., Zhou, Q., Tang, M., Li, J., et al. (2023). Kidney tonifying traditional Chinese medicine: potential implications for the prevention and treatment of osteoporosis. Front. Pharmacol. 13, 1063899. doi:10.3389/fphar.2022.1063899
Eiraku, N., Chiba, N., Nakamura, T., Amir, M. S., Seong, C. H., Ohnishi, T., et al. (2019). BMP9 directly induces rapid GSK3-β phosphorylation in a Wnt-independent manner through class I PI3K-Akt axis in osteoblasts. FASEB J. 33 (11), 12124–12134. doi:10.1096/fj.201900733RR
Fu, S., Yang, L., Hong, H., Chen, D., and Zhang, R. (2014). Catalpa alcohol promotes changes in Wnt signaling pathway during the proliferation of rat bone marrow mesenchymal stem cells. Chin. J. Pathophysiol. (09), 1656–1660.
Fu, S., and Zhang, R. (2012). Effect of different concentrations of catalpa alcohol on the proliferation and bone differentiation of bone marrow mesenchymal stem cells in SD rats. Lishizhen Med. Materia Medica (10), 2398–2400.
Fujii, T., Kitaura, H., Kimura, K., Hakami, Z. W., and Takano-Yamamoto, T. (2012). IL-4 inhibits TNF-α-mediated osteoclast formation by inhibition of RANKL expression in TNF-α-activated stromal cells and direct inhibition of TNF-α-activated osteoclast precursors via a T-cell-independent mechanism in vivo. Bone 51 (4), 771–780. doi:10.1016/j.bone.2012.06.024
Gaur, T., Lengner, C. J., Hovhannisyan, H., Bhat, R. A., Bodine, P. V., Komm, B. S., et al. (2005). Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280 (39), 33132–33140. doi:10.1074/jbc.M500608200
Ghodsi, M., Larijani, B., Keshtkar, A. A., Nasli-Esfahani, E., Alatab, S., and Mohajeri-Tehrani, M. R. (2016). Mechanisms involved in altered bone metabolism in diabetes: a narrative review. J. diabetes metabolic Disord. 15, 52. doi:10.1186/s40200-016-0275-1
Gong, W., Zhang, N., Cheng, G., Zhang, Q., He, Y., Shen, Y., et al. (2019). Rehmannia glutinosa libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 20 (16), 3964. doi:10.3390/ijms20163964
He, Q., Qian, W., Yao, N., Zhou, M., Yixin, L. I. U., and Hong, Y. I. N. (2020). Protective effect of rehmannia azalpa on inflammatory osteoblasts in neonatal rats. Chin. J. Tissue Eng. Res. (29), 4626–4631.
Hongwei, L., and Meng, X. (2015). Research progress on chemical components and pharmacological effects of Rehmannia rehmannia. Drug Eval. Res. (02), 218–228.
Hu, H. Y., Zhang, Z. Z., Jiang, X. Y., Duan, T. H., Feng, W., and Wang, X. G. (2023). Hesperidin anti-osteoporosis by regulating estrogen signaling pathways. Mol. Basel, Switz. 28 (19), 6987. doi:10.3390/molecules28196987
Huang, P., Yan, R., Zhang, X., Wang, L., Ke, X., and Qu, Y. (2019). Activating Wnt/β-catenin signaling pathway for disease therapy: challenges and opportunities. Pharmacol. Ther. 196, 79–90. doi:10.1016/j.pharmthera.2018.11.008
Jackson, A., Vayssière, B., Garcia, T., Newell, W., Baron, R., Roman-Roman, S., et al. (2005). Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone 36 (4), 585–598. doi:10.1016/j.bone.2005.01.007
Jia, Y., Ruiyu, L., Wu, M., Huo, R., and Bin, Li (2015a). Effect of Rehmannia Azalpurol on the osteogenic activity of osteoblasts in primary cultured SD rat lactic rats. Mod. J. Integr. Traditional Chin. West. Med. (23), 2524–2526.
Jia, Y., Ruiyu, L., Wu, M., Huo, R., Bin, Li, and Guo, Y. (2015b). Effect of the composition of traditional Chinese medicine on osteogenic activity and Smad4 mRNA expression of osteoblasts. Chin. J. Tissue Eng. Res. (33), 5289–5294.
Jiang, B., Liu, J. H., Bao, Y. M., and An, L. J. (2004). Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon official J. Int. Soc. Toxinology. 43 (1), 53–59. doi:10.1016/j.toxicon.2003.10.017
Jinlong, Z., Zeng, L., Lang, G., Huang, H., Yang, W., Luo, M., et al. (2020). Research progress on mechanism of active components of Chinese materia medica in treatment of osteoporosis based on signaling pathway. Chin. Traditional Herb. Drugs 23, 6084–6094.
Ju, X., Xue, D., Wang, T., Ge, B., Zhang, Y., and Li, Z. (2018). Catalpol promotes the survival and VEGF secretion of bone marrow-derived stem cells and their role in myocardial repair after myocardial infarction in rats. Cardiovasc. Toxicol. 18 (5), 471–481. doi:10.1007/s12012-018-9460-4
Kan, T., He, Z., Du, J., Xu, M., Cui, J., Han, X., et al. (2022). Irisin promotes fracture healing by improving osteogenesis and angiogenesis. J. Orthop. Transl. 37, 37–45. doi:10.1016/j.jot.2022.07.006
Khosla, S., Oursler, M. J., and Monroe, D. G. (2012). Estrogen and the skeleton. Trends Endocrinol. metabolism TEM 23 (11), 576–581. doi:10.1016/j.tem.2012.03.008
Komori, T. (2005). Regulation of skeletal development by the Runx family of transcription factors. J. Cell. Biochem. 95 (3), 445–453. doi:10.1002/jcb.20420
Lai, M., Chen, X., Feng, J., He, L., and Yang, L. (2016). Effect of catalpa alcohol on osteoclast activity and apoptosis in osteoblast-osteoclast co-culture system and its mechanism. Chin. Pharm. (10), 1318–1321.
Lai, M., Liao, L., Lin, J., Tan, W., and Sun, X. (2019). Experimental study on the regulation of Hedgehog signaling pathway by catalpa alcohol to promote the differentiation of BMSCs into osteogenesis. Chin. J. Immunol. (11), 921–926+933.
Lai, M., Yang, L., and Zhang, R. (2015a). Effect of catalpa alcohol on OB and OC activities and OBERα and β mRNA expressions in OB-OC co-cultivation system. Chin. J. Pathophysiol. (07), 1242–1246.
Lai, N., Zhang, J., Ma, X., Wang, B., Miao, X., Wang, Z., et al. (2015b). Regulatory effect of catalpol on Th1/Th2 cells in mice with bone loss induced by estrogen deficiency. Am. J. reproductive Immunol. (New York, N. Y. 1989) 74 (6), 487–498. doi:10.1111/aji.12423
Langdahl, B. L. (2021). Overview of treatment approaches to osteoporosis. Br. J. Pharmacol. 178 (9), 1891–1906. doi:10.1111/bph.15024
Li, J., Fu, S. F., Yang, Y., An, R., Liu, H. Y., and Mao, H. P. (2022). Clinical practice of traditional Chinese medicine for the treatment of postmenopausal osteoporosis: a literature review. Climacteric J. Int. Menopause Soc. 25 (6), 562–569. doi:10.1080/13697137.2022.2102894
Li, W., Zhang, Y., Xu, X., Wang, K., and Ding, W. (2017a). Relationship between osteogenesis and angiogenesis in ovariectomized osteoporotic rats after exercise training. Int. J. Clin. Exp. Pathol. 10 (12), 11438–11449.
Li, Y., Jin, X., and Mao, L. (2017b). Protective effect of catalpol on nicotine-induced injury of alveolar bone and associated underlying mechanisms. Mol. Med. Rep. 16 (6), 8345–8350. doi:10.3892/mmr.2017.7604
Lim, D. W., and Kim, Y. T. (2013). Dried root of Rehmannia glutinosa prevents bone loss in ovariectomized rats. Mol. Basel, Switz. 18 (5), 5804–5813. doi:10.3390/molecules18055804
Lin, G. L., and Hankenson, K. D. (2011). Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J. Cell. Biochem. 112 (12), 3491–3501. doi:10.1002/jcb.23287
Liu, C., Ma, R., Wang, L., Zhu, R., Liu, H., Guo, Y., et al. (2017). Rehmanniae Radix in osteoporosis: a review of traditional Chinese medicinal uses, phytochemistry, pharmacokinetics and pharmacology. J. Ethnopharmacol. 198, 351–362. doi:10.1016/j.jep.2017.01.021
Liu, J., Xiao, Q., Xiao, J., Niu, C., Li, Y., Zhang, X., et al. (2022). Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 7 (1), 3. doi:10.1038/s41392-021-00762-6
Liu, J. Y., Zheng, C. Z., Hao, X. P., Zhang, D. J., Mao, A. W., and Yuan, P. (2016). Catalpol ameliorates diabetic atherosclerosis in diabetic rabbits. Am. J. Transl. Res. 8 (10), 4278–4288.
Liu, Z., Lei, M., Jiang, Y., Hao, H., Chu, L., Xu, J., et al. (2009). High glucose attenuates VEGF expression in rat multipotent adult progenitor cells in association with inhibition of JAK2/STAT3 signalling. J. Cell. Mol. Med. 13 (9B), 3427–3436. doi:10.1111/j.1582-4934.2008.00502.x
Louie, Li, Dongyu, L., Yi, Q., Shuang, S., Shi, J., Liang, P., et al. (2020). Study on the activity of rehmannia monomer azalpa alcohol in promoting bone cell differentiation. Her. Med. (04), 533–537.
Lu, Y., Ma, Z. X., Deng, R., Jiang, H. T., Chu, L., and Deng, Z. L. (2022). The SIRT1 activator SRT2104 promotes BMP9-induced osteogenic and angiogenic differentiation in mesenchymal stem cells. Mech. ageing Dev. 207, 111724. doi:10.1016/j.mad.2022.111724
Luyi, L. (2021). Study on the osteogenic activity of catalpol and its osteogenic effect in combination with titanium implant. Master's thesis. Shanghai University of Traditional Chinese Medicine.
Martin, M. B., Franke, T. F., Stoica, G. E., Chambon, P., Katzenellenbogen, B. S., Stoica, B. A., et al. (2000). A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 141 (12), 4503–4511. doi:10.1210/endo.141.12.7836
McWhorter, F. Y., Wang, T., Nguyen, P., Chung, T., and Liu, W. F. (2013). Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. U. S. A. 110 (43), 17253–17258. doi:10.1073/pnas.1308887110
Meng, J. H. (2020). Mechanism investigation in regulatory effects of catalpol on osteoclastogenesis and osteoclast-derived bone loss. Ph.D. dissertation. Zhejiang University.
Meng, J., Zhang, W., Wang, C., Zhang, W., Zhou, C., Jiang, G., et al. (2020). Catalpol suppresses osteoclastogenesis and attenuates osteoclast-derived bone resorption by modulating PTEN activity. Biochem. Pharmacol. 171, 113715. doi:10.1016/j.bcp.2019.113715
Murray, P. J., and Wynn, T. A. (2011). Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11 (11), 723–737. doi:10.1038/nri3073
Nakamura, T., Imai, Y., Matsumoto, T., Sato, S., Takeuchi, K., Igarashi, K., et al. (2007). Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell 130 (5), 811–823. doi:10.1016/j.cell.2007.07.025
Napoli, N., Chandran, M., Pierroz, D. D., Abrahamsen, B., Schwartz, A. V., and Ferrari, S. L.IOF Bone and Diabetes Working Group (2017). Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 13 (4), 208–219. doi:10.1038/nrendo.2016.153
Oh, K. O., Kim, S. W., Kim, J. Y., Ko, S. Y., Kim, H. M., Baek, J. H., et al. (2003). Effect of Rehmannia glutinosa Libosch extracts on bone metabolism. Clin. chimica acta; Int. J. Clin. Chem. 334 (1-2), 185–195. doi:10.1016/s0009-8981(03)00238-9
Sheng, M. H., Lau, K. H., and Baylink, D. J. (2014). Role of osteocyte-derived insulin-like growth factor I in developmental growth, modeling, remodeling, and regeneration of the bone. J. bone metabolism 21 (1), 41–54. doi:10.11005/jbm.2014.21.1.41
Shieh, J. P., Cheng, K. C., Chung, H. H., Kerh, Y. F., Yeh, C. H., and Cheng, J. T. (2011). Plasma glucose lowering mechanisms of catalpol, an active principle from roots of Rehmannia glutinosa, in streptozotocin-induced diabetic rats. J. Agric. food Chem. 59 (8), 3747–3753. doi:10.1021/jf200069t
Silverstein, W. K., Cantor, N., and Cheung, A. M. (2024). Postmenopausal osteoporosis. N. Engl. J. Med. 390 (7), 673–674. doi:10.1056/NEJMc2314624
Simão, F., Pagnussat, A. S., Seo, J. H., Navaratna, D., Leung, W., Lok, J., et al. (2012). Pro-angiogenic effects of resveratrol in brain endothelial cells: nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J. Cereb. blood flow metabolism official J. Int. Soc. Cereb. Blood Flow Metabolism 32 (5), 884–895. doi:10.1038/jcbfm.2012.2
Srivastava, R. K., Dar, H. Y., and Mishra, P. K. (2018). Immunoporosis: Immunology of osteoporosis-role of T cells. Front. Immunol. 9, 657. doi:10.3389/fimmu.2018.00657
Stefanaki, C., Pervanidou, P., Boschiero, D., and Chrousos, G. P. (2018). Chronic stress and body composition disorders: implications for health and disease. Horm. (Athens, Greece) 17 (1), 33–43. doi:10.1007/s42000-018-0023-7
Sun, M., Paciga, J. E., Feldman, R. I., Yuan, Z., Coppola, D., Lu, Y. Y., et al. (2001). Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 61 (16), 5985–5991.
Sun, N., Lei, X., Wenjing, S., Han, Li, Cao, Y., Sun, H., et al. (2022). Research progress of catalpa alcohol in the prevention and treatment of osteoporosis. Chin. J. Osteoporos. (06), 893–896+911.
Sun, X., Wang, L., Yongwei, L., and Jiang, X. (2020). Exploring the regulatory mechanism of catalpa alcohol on renal fibrosis based on inflammatory signaling pathway. J. Liaoning Univ. Traditional Chin. Med. (08), 173–176.
Sun, X., Wei, B., Peng, Z., Fu, Q., Wang, C., Zhen, J., et al. (2019). Protective effects of Dipsacus asper polysaccharide on osteoporosis in vivo by regulating RANKL/RANK/OPG/VEGF and PI3K/Akt/eNOS pathway. Int. J. Biol. Macromol. 129, 579–587. doi:10.1016/j.ijbiomac.2019.02.022
The Lancet Diabetes Endocrinology (2021). Osteoporosis: overlooked in men for too long. lancet. Diabetes and Endocrinol. 9 (1), 1. doi:10.1016/S2213-8587(20)30408-3
Tokunaga, T., Mokuda, S., Kohno, H., Yukawa, K., Kuranobu, T., Oi, K., et al. (2020). TGFβ1 regulates human RANKL-induced osteoclastogenesis via suppression of NFATc1 expression. Int. J. Mol. Sci. 21 (3), 800. doi:10.3390/ijms21030800
Udagawa, N., Koide, M., Nakamura, M., Nakamichi, Y., Yamashita, T., Uehara, S., et al. (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. J. bone mineral metabolism 39 (1), 19–26. doi:10.1007/s00774-020-01162-6
Veis, D. J., and O'Brien, C. A. (2023). Osteoclasts, master sculptors of bone. Annu. Rev. pathology 18, 257–281. doi:10.1146/annurev-pathmechdis-031521-040919
Wang, L. (2015). Study on the blood components and pharmacokinetics of Yougui pills under different syndrome types of osteoporosis. Master's thesis. Zhejiang University of Traditional Chinese Medicine.
Wang, L., and Xue, G. B. (2018). Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem. biophysical Res. Commun. 495 (1), 27–34. doi:10.1016/j.bbrc.2017.10.054
Wang, M., Zhang, W., Crisostomo, P., Markel, T., Meldrum, K. K., Fu, X. Y., et al. (2007). STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J. Mol. Cell. Cardiol. 42 (6), 1009–1015. doi:10.1016/j.yjmcc.2007.04.010
Watt, J., and Schlezinger, J. J. (2015). Structurally-diverse, PPARγ-activating environmental toxicants induce adipogenesis and suppress osteogenesis in bone marrow mesenchymal stromal cells. Toxicology 331, 66–77. doi:10.1016/j.tox.2015.03.006
Wu, C., Shi, Z., Ge, Q., Xu, H., Wu, Z., Tong, P., et al. (2024). Catalpol promotes articular cartilage repair by enhancing the recruitment of endogenous mesenchymal stem cells. J. Cell. Mol. Med. 28 (7), e18242. doi:10.1111/jcmm.18242
Wu, M., Lai, H., Deng, Q., Peng, X., Shen, J., Zhou, X., et al. (2022). The evaluation of xiaozeng qianggu tablets for treating postmenopausal osteoporosis via up-regulated autophagy. Evidence-based complementary Altern. Med. eCAM 2022, 3960834. doi:10.1155/2022/3960834
Wu, M., Suzhi, Z., En, L., and Bai, X. (2010). Effect of azalpa alcohol, the active ingredient of Rehmannia rehmannia on the proliferation, differentiation and mineralization of mouse osteoblasts MC3T3-E1. Chin. Pharmacol. Bull. (04), 509–513.
Xia, T. S. (2019). Metabolomics study of morindae officinalis radix and rehmanniae radix praeparata on protecting against glucocorticoid-induced osteoporosis. Master’s thesis. Naval Medical University.
Xia, Y. (2022). Study on the processing technology of rehmannia glutinosa and its nourishing yin effect. Master's thesis. Jiangxi University of Traditional Chinese Medicine.
Xu, L., Xu, G., Sun, N., Yao, J., Wang, C., Zhang, W., et al. (2023). Catalpol ameliorates dexamethasone-induced osteoporosis by promoting osteogenic differentiation of bone marrow mesenchymal stem cells via the activation of PKD1 promoter. J. Pharmacol. Sci. 153 (4), 221–231. doi:10.1016/j.jphs.2023.10.002
Yang, X. L. (2021). Preliminary study on anti-osteoporosis mechanism of epimedium and prepared radix rehmanniae. Master's thesis. Hebei University of Economics and Business.
Ye, L. (2014). Determination of the content of pharmacodynamic components of Morinda officinalis capsule extract and its efficacy and mechanism on osteoporosis rats. Master's thesis. Southwest University.
Yue, Z., Niu, X., Yuan, Z., Qin, Q., Jiang, W., He, L., et al. (2022). RSPO2 and RANKL signal through LGR4 to regulate osteoclastic premetastatic niche formation and bone metastasis. J. Clin. investigation 132 (2), e144579. doi:10.1172/JCI144579
Zhang, B., Dai, F., Hong, Y., Yi, Z., He, Q., Qian, J., et al. (2020). Rexmann azalpa alcohol intervenes in the expression of inflammation-related factors in knee synovial tissue of rats with early knee osteoarthritis. Chin. J. Tissue Eng. Res. (29), 4656–4661.
Zhang, J., and Liu, K. (2019). Research progress of catalpa alcohol. Drug Eval. Res. (08), 1680–1684.
Zhang, N. D. (2016). Study on the active ingredients and mechanism of Rehmannia rehmannia in the prevention and treatment of diabetic osteoporosis based on molecular docking strategy. Master's thesis. Second Military Medical University.
Zhang, N. D., Han, T., Huang, B. K., Rahman, K., Jiang, Y. P., Xu, H. T., et al. (2016). Traditional Chinese medicine formulas for the treatment of osteoporosis: implication for antiosteoporotic drug discovery. J. Ethnopharmacol. 189, 61–80. doi:10.1016/j.jep.2016.05.025
Zhang, W., Huang, Q., Zeng, Z., Wu, J., Zhang, Y., and Chen, Z. (2017). Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxidative Med. Cell. Longev. 2017, 7543973. doi:10.1155/2017/7543973
Zhang, Y., Du, Z., Li, D., Wan, Z., Zheng, T., Zhang, X., et al. (2022). Catalpol modulating the crosstalking between mesenchymal stromal cells and macrophages via paracrine to enhance angiogenesis and osteogenesis. Exp. cell Res. 418 (2), 113269. doi:10.1016/j.yexcr.2022.113269
Zhang, Z., Xing, N., Peng, D., Wang, Q., and Kuang, H. (2021). Research progress on the anti-osteoporotic effect and mechanism of traditional Chinese medicine. China Pharm. (03), 374–379.
Zhao, L., Du, W., Zhao, D., Ji, X., Huang, Y., Pang, Y., et al. (2021). Catalpol protects against high glucose-induced bone loss by regulating osteoblast function. Front. Pharmacol. 12, 626621. doi:10.3389/fphar.2021.626621
Zheng, W., Chen, Q., Zhang, Y., Xia, R., Gu, X., Hao, Y., et al. (2020). BMP9 promotes osteogenic differentiation of SMSCs by activating the JNK/Smad2/3 signaling pathway. J. Cell. Biochem. 121 (4), 2851–2863. doi:10.1002/jcb.29519
Zhu, H., Wang, Y., Liu, Z., Wang, J., Wan, D., Feng, S., et al. (2016). Antidiabetic and antioxidant effects of catalpol extracted from Rehmannia glutinosa (Di Huang) on rat diabetes induced by streptozotocin and high-fat, high-sugar feed. Chin. Med. 11, 25. doi:10.1186/s13020-016-0096-7
Zhu, Y., Wang, Y., Jia, Y., Xu, J., and Chai, Y. (2019). Catalpol promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Stem cell Res. and Ther. 10 (1), 37. doi:10.1186/s13287-019-1143-y
Keywords: catalpol, osteoporosis, pharmacological mechanisms, osteogenesis, osteoclasts
Citation: Li N, Mu X, Zhang S and Wang H (2025) Recent advances in the multifaceted mechanisms of catalpol in treating osteoporosis. Front. Pharmacol. 16:1560715. doi: 10.3389/fphar.2025.1560715
Received: 14 January 2025; Accepted: 17 February 2025;
Published: 04 March 2025.
Edited by:
Aixi Yu, Wuhan University, ChinaReviewed by:
Bettina Grötsch, University of Erlangen Nuremberg, GermanyKailiang Zhou, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China
Copyright © 2025 Li, Mu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaxin Wang, NjAwMjAwMzBAc2R1dGNtLmVkdS5jbg==
 Na Li
Na Li Xiaoying Mu2
Xiaoying Mu2 Huaxin Wang
Huaxin Wang