
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 26 March 2025
Sec. Gastrointestinal and Hepatic Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1558897
 Cai-Yun Long
Cai-Yun Long Ying Huang*
Ying Huang*Background: Despite the emergence of numerous innovative targeted therapies for the management of pediatric inflammatory bowel disease (IBD), azathioprine continues to be a pivotal first-line therapeutic agent. Nonetheless, the considerable frequency of myelosuppression associated with its use warrants careful consideration and further investigation. This study aims to investigate the application of pharmacogenomics in Chinese pediatric IBD treated with azathioprine, and to elucidate its association with the occurrence of myelosuppression.
Methods: We conducted a retrospective analysis to determine the prevalence of pharmacogenetic abnormalities and thiopurine-induced myelosuppression in Chinese pediatric patients with IBD.
Results: Among the 227 patients underwent pharmacogenetic testing, abnormal genetypes occurred in 66 patients, among which 7 patients exhibited aberrant TPMT and 59 had aberrant NUDT15. Of the 58 patients who were treated with azathioprine, 23 cases experienced myelosuppression. All three children with heterozygous mutations in NUDT15 developed leukopenia following azathioprine treatment. Among patients with normal pharmacogenetic results, 20 cases (36.4%) developed myelosuppression, while 35 cases (63.6%) did not. The dose of azathioprine was below the recommended level in guidelines. The mean dose of azathioprine (mg/kg/day) in the myelosuppression group was 1.22 ± 0.32, compared to 1.42 ± 0.42 in the non-myelosuppression group, which represented a statistically significant difference (p < 0.05). Age, gender, and the use of concomitant biologics, mesalazine, or glucocorticoids did not show significant differences between the groups (p > 0.05).
Conclusion: NUDT15 C415T is prevalent in China and is associated with an increased risk of azathioprine-induced myelosuppression. A reduced dose of azathioprine should be considered for Chinese pediatric patients with IBD, even in those with normal pharmacogenetic profiles.
Thiopurines, such as 6-mercaptopurine, 6-thioguanine, and azathioprine, are extensively employed in the management of acute lymphoblastic leukemia and autoimmune conditions, including inflammatory bowel disease (IBD) and rheumatoid arthritis. The adverse effects associated with these drugs are notable and warrant attention. Up to 10%–30% of patients experience treatment intolerance due to side effects, which may entail myelosuppression, hepatotoxicity, pancreatitis, and nausea. Myelosuppression is a frequent severe adverse event, with an incidence ranging from 3% to 7%, and it can result in life-threatening infections (Ribeiro et al., 2023). Therefore, monitoring for treatment-related myelosuppression in patients with inflammatory bowel disease is imperative.
The metabolism of thiopurines is catalyzed by two principal detoxifying enzymes, TPMT and NUDT15, which serve as negative regulators of thiopurine activity and toxicity. Variants within the TPMT and NUDT15 alleles exhibit diverse frequencies across the general population, resulting in considerable interindividual variability in pharmacological and toxicological responses to thiopurine-based medications. Mutations in these enzymes are known to increase the risk of myelosuppression in patients (Kim et al., 2010; Banerjee et al., 2020; Gutiérrez-Valencia et al., 2023). Consequently, the implementation of pharmacogenomic testing for TPMT and NUDT15 can effectively pinpoint individuals at higher risk for adverse events associated with thiopurines (Van Den Bosch and Coenen, 2021; Dickson et al., 2022; Pratt et al., 2022). However, the distribution of genetic mutations varies across different regions and ethnic groups, and the disease itself may affect the absorption and metabolism of drugs. Therefore, it is necessary to explore pharmacogenomics in populations with particular diseases in certain regions.
Pediatric IBD exhibits unique features distinct from adult IBD. Nevertheless, to date, research on pharmacogenomic testing for azathioprine and its specific clinical implications for pediatric IBD patients, particularly in the context of East Asia, has been limited (Kennedy et al., 2024). This study conducted a retrospective analysis of the pharmacogenomic data for azathioprine in Chinese pediatric patients with IBD, summarized the prevalence of various genotypes and assessed the incidence of adverse reactions following azathioprine use, aiming to provide insights that could inform clinical practice in the management of pediatric IBD in China.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of our institution. Informed consent was obtained from the legal guardians of participants.
The inclusion criteria for the study were as follows.
• Children diagnosed with IBD at our hospital between July 2019 and May 2024.
• Patients who had entered the maintenance treatment phase.
• Peripheral white blood cell counting (WBC) > 4.0 * 109/L, hemoglobin (HGB) > 100 g/L, platelet (PLT) > 100 * 109/L
• Patients who had undergone TPMT and NUDT15 genetic testing.
Venous blood samples of 2 mL were collected, anticoagulated with EDTA, and then sent to Shanghai Jinyu Medical Testing Co., Ltd. For azathioprine pharmacogenetic analysis. Briefly, DNA was extracted from blood samples, and after passing quality control, the target fragments were amplified and libraries were constructed, followed by next-generation sequencing. Based on the joint consensus recommendation of pharmacogenetics, the pharmacogenetic testing covered three TPMT loci (TPMT*2 c.238G>C, TPMT*3B c.460G>A, and TPMT*3C.719A>G), as well as one NUDT15 locus (NUDT15 c.415 C>T) (Pratt et al., 2022).
The following clinical data were collected for subsequent analyses.
• General information: this includes the participant’s name, gender, age, and weight.
• Time and results of TPMT and NUDT15 genetic testing.
• Hematological parameters including WBC, PLT, and HGB, both before and after the initiation of medication.
• Azathioprine medication details: this encompassed the start time of azathioprine therapy, the initial dose, any subsequent adjustments to the medication’s dosage.
• Concomitant medications such as biologics, mesalazine, and corticosteroids.
• Definition of myelosuppression: patients with peripheral WBC <4 * 109/L, HGB <100 g/L, or PLT <100 * 109/L, were considered with myelosuppression (Nian et al., 2024).
IBM SPSS 20.0 software was used for the statistical analysis of the data. Continuous variables were reported as mean ± standard deviation. As for statistical comparison between groups, independent t was used for continuous variables, while χ2 was applied for categorical variables. P < 0.05 was defined as statistically significant.
Flow chart of study design was illustrated in Figure 1. Between July 2019 and May 2024, 227 children with IBD were subjected to azathioprine pharmacogenetic testing. The age of the children spanned from 1 to 17 years, with a mean age of 10.83 ± 3.74 years. The gender distribution was 143 males and 84 females. The disease subtypes were as follows: 176 cases of Crohn’s disease (CD), 33 cases of ulcerative colitis (UC), and 18 cases of IBD unclassified (Table 1).
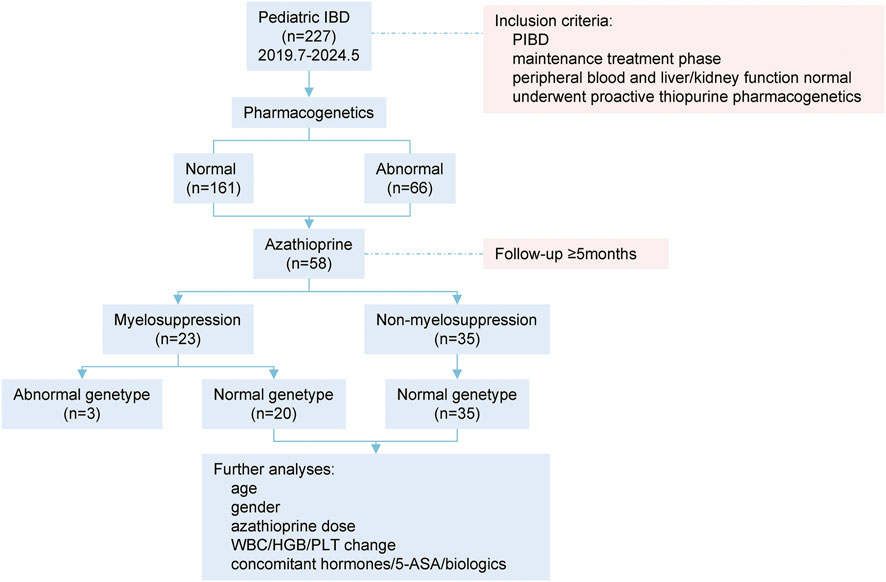
Figure 1. Flow chart of study design. PIBD: pediatric inflammatory bowel disease; WBC: white blood cell counting; HGB: hemoglobin; PLT, platelet; 5-ASA: mesalazine.
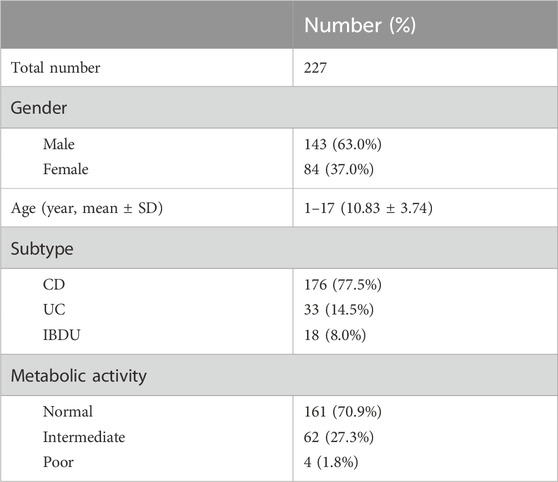
Table 1. Clinical data of patients with proactive pharmacogenomic testing. CD: Crhon’s disease; UC: ulcerative colitis; IBDU: IBD unclassified.
Among the children, 161 (70.9%) exhibited normal pharmacogenetic results, whereas 66 (29.1%) had abnormal findings. The TPMT c.238 and TPMT c.460 loci were homozygous wild-type in all cases (100%). As for TPMT c.719 locus, the wild-type was present in 220 cases (96.9%), while the wild-type NUDT15 c.415 was identified in 167 cases (73.6%) (Figure 2A).
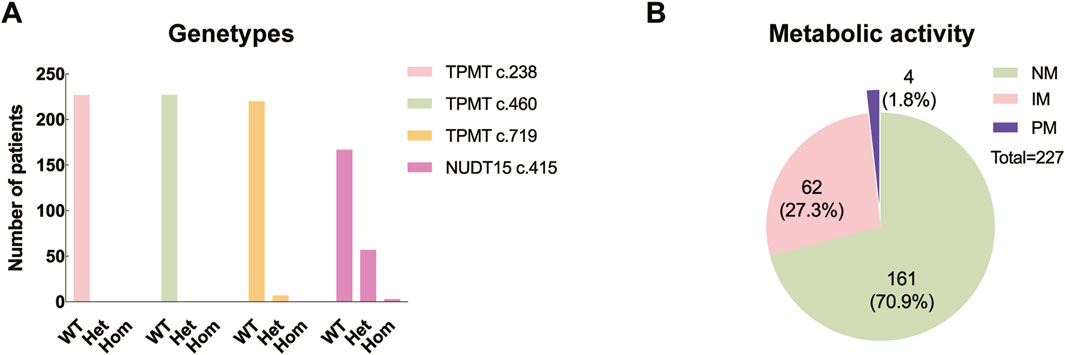
Figure 2. Distribution of genetypes and metabolic levels. (A) WT: wild type; Het: heterozygous; Hom: homozygous. (B) NM: normal metabolic activity; IM: intermediate metabolic activity; PM: poor metabolic activity.
Genotype reports detail the genetic polymorphisms, whereas the predicted phenotypes concern how the genotypes influence the activity of the specific proteins. According to the commonly used phenotype nomenclature for thiopurine metabolizing enzymes, “normal metabolizer” means “two normal function alleles (i.e., wild type), “intermediate metabolizer” means “one loss/null function allele and one normal/supra-function allele”, and “poor metabolizer” means “two loss/null function alleles” (Sandritter et al., 2023). In this study, of those with genetic abnormalities, 62 (27.3%) presented with intermediate metabolic activity, including 6 with TPMT c.719A/G variant and 56 with NUDT15 c.415C/T variant; meanwhile, 4 (1.8%) presented with poor metabolic activity, consisting of three patients with the NUDT15 c.415T/T variant and one patient with both the TPMT c.719A/G and NUDT15 c.415C/T variants (Figure 2B).
Due to significant interethnic differences in thiopurine pharmacogenomics, evidence that lower doses of 6-mercaptopurine/azathioprine achieve sufficient clinical efficacy and therapeutic erythrocyte 6-thioguanine nucleotide (6-TGN) concentrations in Japanese adult IBD patients, and the known differences between pediatric and adult IBD, we adopted a step-up dosing strategy with initial doses below recommended levels (Komiyama et al., 2008; Desai et al., 2023; Gutiérrez-Valencia et al., 2023). In line with guideline recommendations, we closely monitored patients’ complete blood counts (Relling et al., 2019). The dose was gradually increased if no adverse effects were observed, or reduced/discontinued if adverse effects occurred.
In this study, a total of 58 patients who were receiving azathioprine therapy and had completed a follow-up period of ≥5 months were included for further analyses. Of these patients, 55 cases had normal pharmacogenetic results, and 3 cases had abnormal results, all of which were due to the NUDT15 c.415C>T variant. Patient 1, a 12-year-old male with CD, was treated with azathioprine at 1.32 mg/kg/d and corticosteroids; after 1 month, his white blood cell count decreased from 4.45 to 3.87 (*109/L). Patient 2, an 11-year-old male with CD, was treated with azathioprine at 1.36 mg/kg/d, adalimumab, and thalidomide; after 2 months, his white blood cell count decreased from 5.1 to 3.38 (*109/L). Patient 3, a 7-year-old female with indeterminate IBD, was treated with azathioprine at 0.74 mg/kg/d and mesalazine; after 1.5 months, her white blood cell count decreased from 5.7 to 2.41 (*109/L). Regarding drug-related adverse reactions, only Patient one experienced an increase in transaminase levels during the treatment.
Overall, the initial dose of azathioprine for these patients was 0.29–1.61 mg/kg/day (0.79 ± 0.28), and the maximum dose was 0.70–2.50 mg/kg/day (1.29 ± 0.37). The adverse reactions included increased transaminases in 4 cases, gastrointestinal discomfort in 7 cases, rash in 9 cases, influenza-like symptoms in 5 cases, pancreatitis in 1 case, and myelosuppression in 23 cases (Table 2).
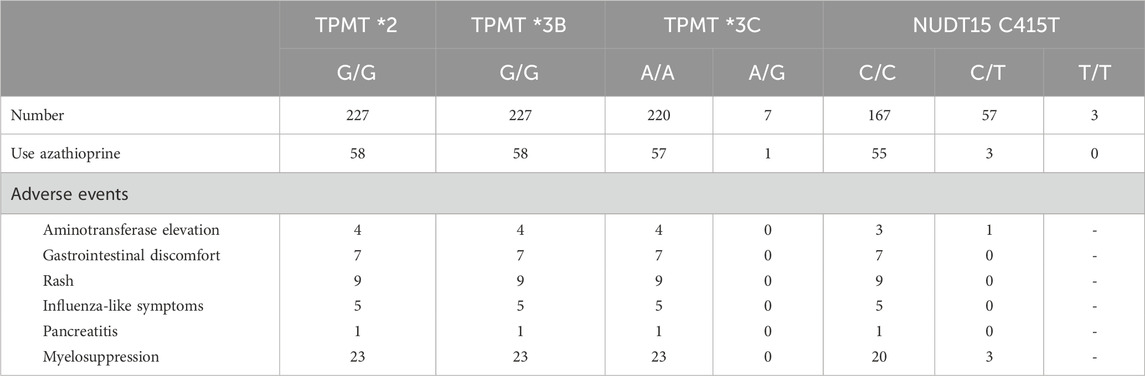
Table 2. Adverse reactions after using azathioprine in patients with various pharmacogenetic genotypes.
Among the 58 children treated with azathioprine, 23 experienced myelosuppression, while 35 did not. Three patients with genetic abnormalities all developed myelosuppression following azathioprine treatment (100.0%). The onset of myelosuppression ranged from 2 to 1,126 days (mean ± SD: 300.3 ± 330.4) after the initiation of therapy. Seven cases (30.4%) developed myelosuppression within 60 days of starting medication, and 16 cases (69.6%) developed it after 60 days (Figure 3). Of the three children with genetic abnormalities, two experienced myelosuppression within 60 days (31 and 46 days, respectively), and one experienced it after 770 days of medication.
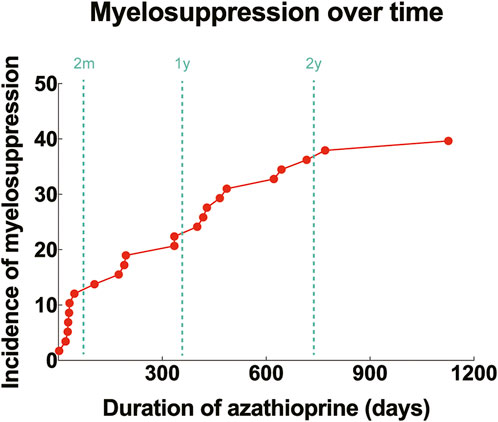
Figure 3. Incidence of myelosuppression over time after initiation of azathioprine. Red dots: cumulative incidence of myelosuppression corresponding to different durations of drug use (%); dashed lines: time points of 2 months, 1 year and 2 years, respectively.
In the myelosuppression group, the starting dose and maximum dose of azathioprine were 0.29–1.32 mg/kg/day (0.79 ± 0.28) and 0.74–1.96 mg/kg/day (1.34 ± 0.34), respectively. The average decreases in WBC, HGB, and PLT after azathioprine administration were 3.21 * 109/L, 0.06 g/L, and 38.44 * 109/L, respectively. In the non-myelosuppression group, the starting dose and maximum dose of azathioprine were 0.43–1.61 mg/kg/day (0.80 ± 0.29) and 0.70–1.81 mg/kg/day (1.22 ± 0.32), respectively. The average decreases in WBC, HGB, and PLT were 2.85 * 109/L, 1.43 g/L, and 32.43 * 109/L, respectively.
There was a statistically significant difference in the maximum dose of azathioprine between the myelosuppression group and the non-myelosuppression group (P < 0.05). No statistically significant differences were found in age, gender, starting dose of azathioprine, and concomitant use of hormones, mesalazine, or biologics between the two groups (P > 0.05) (Table 3; Figure 4).

Table 3. Myelosuppression and other clinical characteristics in children treated with azathioprine. AZA, azathioprine. WBC: white blood cell counting; HGB: hemoglobin; PLT, platelet.
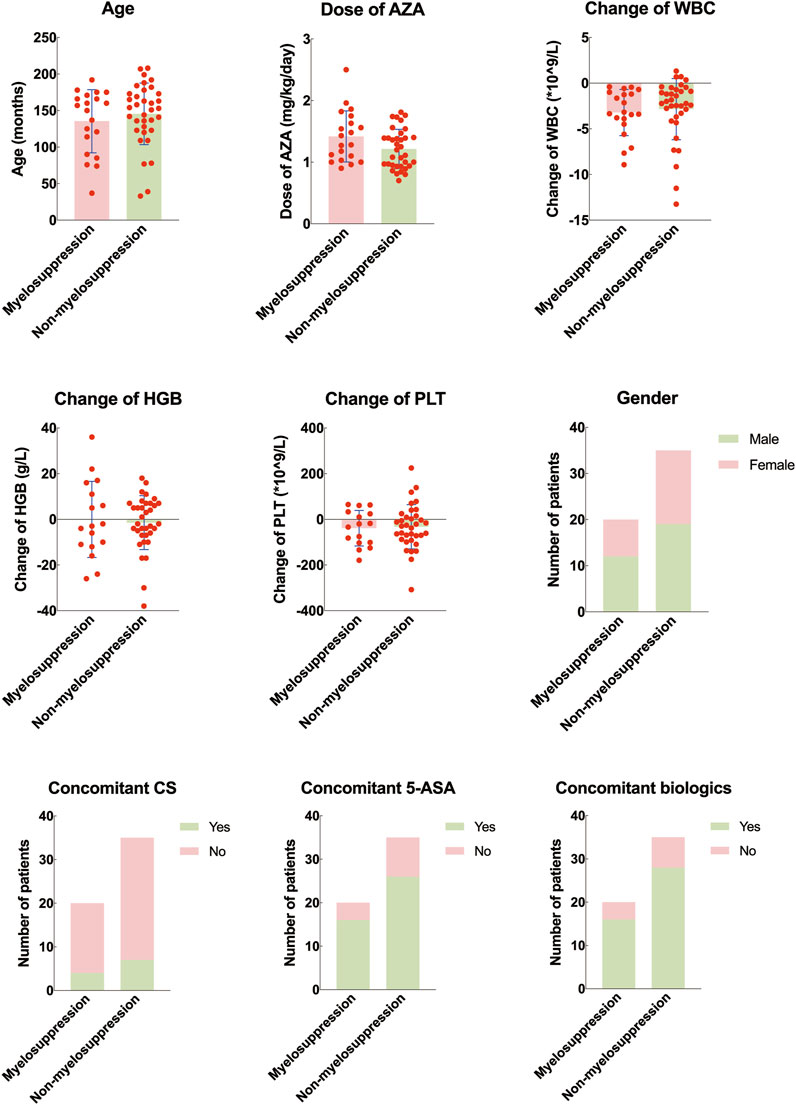
Figure 4. Difference of clinical features between pediatric inflammatory bowel disease with and without myelosuppression. AZA, azathioprine. WBC: white blood cell counting; HGB: hemoglobin; PLT: platelet; 5-ASA: mesalazine.
Despite the ongoing development of new drugs such as biologics and small molecule medications, thiopurine drugs still play a significant role in the treatment of IBD. The early introduction of thiopurines during the maintenance phase of IBD enables 73% of patients to achieve at least 1 year of hormone-free remission. Following 1 year of azathioprine treatment, 76.9% of children with UC and 47.6% with CD can achieve endoscopic remission (Giugliano et al., 2018). However, thiopurines are related to adverse reactions including gastrointestinal intolerance, influenza-like symptoms, pancreatitis, hepatitis, rash, myelosuppression, and infections secondary to neutropenia. Among these adverse effects, myelosuppression is the most prevalent and has the potential to lead to life-threatening infections. This study investigated the importance of pharmacogenetic testing prior to the use of azathioprine in Chinese pediatric IBD and analyzed the adverse reactions that occurred following azathioprine administration, finding that pharmacogenetic testing was beneficial in guiding clinical treatment and to some extent predict the likelihood of subsequent myelosuppression.
Leukopenia is the most common manifestation of myelosuppression resulting from thiopurine medications, and it can develop at any point after the commencement of treatment. It may also arise abruptly without any symptoms or warning signs. Thirty-seven percent of patients experience leukopenia within 3 months, 22.4% within 3–6 months, 13.8% within 6–12 months, 22.4% within 12–24 months, and 14.7% after 24 months (Ribeiro et al., 2023). Similarly, in our study, the median onset of myelosuppression was at 300 days following the initiation of azathioprine, with 30.4% occurring within the first 60 days and 69.6% after 60 days. Nonetheless, it should be noted that most patients in our study were pharmacogenetically normal and that myelosuppression can also manifest several years post-medication, underscoring the necessity for ongoing monitoring of peripheral blood parameters even with normal pharmacogenetic results.
The metabolites of azathioprine primarily include 6-mercaptopurine, thiouracil, 6-TGN, and 6-methylmercaptopurine (6-MMP). TPMT is a pivotal enzyme involved in the metabolism of thiopurines, and its genetic polymorphisms are linked to reduced enzyme activity, which in turn increases the risk of myelosuppression induced by thiopurines (Ribeiro et al., 2023). The four genotypes TPMT*2, TPMT*3A, TPMT*3B, and TPMT*3C account for over 95% of TPMT gene variants, yet there are notable differences in the genetic polymorphisms of pharmacogenomics among various populations (Kennedy et al., 2024). The most common TPMT*3A genotype is prevalent among Caucasians (3.2%–5.7%), whereas the relatively common TPMT variant in Africa and Asia is the TPMT*3C genotype (0.5%–1.5%) (Ribeiro et al., 2023). In this study, the mutation probability of three loci in the TPMT gene of 227 IBD children was 3.1%, with all of them occurring in TPMT*3C, indicating that the incidence of TPMT variant loci can vary significantly among different regions, and TPMT*3C is more significant than TPMT*2/*3B for Chinese patients.
For Caucasians, TPMT plays a crucial role in predicting leukopenia caused by thiopurines, but its predictive value is limited in Asian populations. NUDT15 is another gene that significantly influences the metabolism of thiopurine drugs. Its protein product can dephosphorylate 6-TGN, thereby preventing the incorporation of metabolites into DNA or RNA. When mutations occur in the NUDT15 gene, the enzymatic activity of NUDT15 is reduced, leading to an increased risk of adverse effects from thiopurine therapy (Khaeso et al., 2021). Among the most common genetic polymorphisms of NUDT15, the C415T variant is highly prevalent in East Asian populations but rare in Caucasians. Furthermore, compared to TPMT, the NUDT15 C415T variant is associated with an increased susceptibility to early and severe myelosuppression following thiopurine treatment, making it a superior predictor of thiopurine-related myelosuppression in Asian populations (Yang et al., 2014; Relling et al., 2019; Banerjee et al., 2020; Chao et al., 2021; Kennedy et al., 2024). Moreover, the same genetic variant can yield inconsistent results across different ethnic groups. While NUDT15 mutations are more prone to cause early severe myelosuppression in Asian populations, patients with this variant in the United Kingdom do not exhibit an elevated risk of toxicity after taking thiopurines, highlighting the significance of NUDT15 genotype testing in Asian populations (Chao et al., 2017; Desai et al., 2023; Coelho et al., 2024). In this study, the prevalence of NUDT15 mutations was notably higher than that of TPMT, with an overall mutation rate of 26.4% for NUDT15 C415T, compared to a mutation rate of 3.1% for 3 TPMT loci, indicating that NUDT15 is a crucial component in pharmacogenetics for thiopurines in the Chinese population. In this study, all three pediatric patients with intermediate NUDT15 metabolic activity developed myelosuppression after receiving azathioprine, further underscoring the need for cautious use of thiopurines in Chinese children with IBD who carry the NUDT15 C415T variant. However, the sample size of patients with pharmacogenomic abnormalities treated with azathioprine in this study was limited. Therefore, the generalizability of these findings requires validation through larger-scale prospective studies.
Other genes, including ITPA, GST, AOX1, DHFR, and FTO, may also influence the metabolism of thiopurines (Kim et al., 2017; Casajús et al., 2022; Dickson et al., 2022; Coelho et al., 2024). For IBD patients treated with thiopurines, drug dosage, baseline WBC count, and the choice of mercaptopurine were also identified as independent risk factors for leucopenia (Broekman et al., 2017). In our study, 36.4% (20/55) of patients who experienced myelosuppression had normal pharmacogenomics, indicating that genotypes cannot solely account for phenotypic drug responses, and that phenotypes themselves cannot be completely replaced by genotypes. A recent prospective study in China on CD demonstrated that among patients with normal TPMT/NUDT15 activity, 15.6% (17/109) experienced late leukopenia, and in those with a moderate decline in TPMT/NUDT15 activity the incidence of late leukopenia was 64.1% (25/39). It is important to note that not all adverse reactions associated with thiopurines can be explained by the commonly studied thiopurine metabolites such as 6-MMP and 6-TGN, and thus more appropriate phenotypic marker is required to predict drug toxicity (Sheffield et al., 2009; Luo et al., 2022; Yang et al., 2024). As a thiopurine metabolite associated with NUDT15, 6-diazo-5-oxo-L-norleucine (DNATG) is proved to be superior to 6-TGN in predicting whether TPMT/NUDT15-normal patients will develop late leukopenia. When the DNATG cutoff value is set at 315.72 fmol/μg DNA, its sensitivity in predicting late leukopenia is 88% and its specificity is 85%, suggesting that detecting DNATG at the onset of medication can provide a better prediction of late leukopenia (Zhu et al., 2022; Yang et al., 2024). The role of metabolites mentioned above in pediatric IBD in China needs further investigation.
Concomitant medications, such as allopurinol, can also influence thiopurine metabolism. Regarding the combination with 5-aminosalicylic acid (5-ASA), the evidence on its impact on thiopurine metabolism and toxicity remains inconsistent. Researches have indicated that 5-ASA can elevate the levels of the active metabolite 6-TGN of azathioprine, decrease the ratio of 6-MMP/6-TGN, and thereby increase the risk of leukopenia (Lee et al., 2015). The interaction between these drugs is not solely dependent on drug dosage but also on drug formulation (Hande et al., 2006; de Boer et al., 2007; de Graaf et al., 2010). When a high dose of mesalazine is necessary in clinical practice, it is crucial to meticulously assess the adverse reactions associated with the combination of thiopurines (Ribeiro et al., 2023). However, some studies have reported contradictory findings, showing no increased risk of leukopenia or elevated 6-TGN levels with the co-administration of 5-ASA and thiopurines (Broekman et al., 2017). In this study, consistent with the latter, there was no statistically significant difference in the concomitant mesalazine between the myelosuppression group and the non-myelosuppression group, which may be attributed to the relatively low drug dosage used. Further research is needed in China to investigate the impact of the combination of these two drugs on myelosuppression in pediatric IBD.
Pharmacogenomics plays a crucial role in guiding the use of thiopurines, and economic analyses have demonstrated overall benefits for patients, supporting the current recommendation to conduct pharmacogenomic testing prior to initiating thiopurine therapy (Zeng et al., 2021; Desai et al., 2023; Valdez-Acosta et al., 2023). Clinical guidelines generally suggested a dosage of 2.0–2.5 mg/kg/day for azathioprine in IBD with normal metabolic activity, while the Clinical Pharmacogenomics Implementation Consortium recommended a dosage of 2.0–3.0 mg/kg/day (Relling et al., 2019; van Rheenen et al., 2020; Lee et al., 2023). It is important to note, however, that the recommended doses in these guidelines are primarily based on clinical studies of Caucasian populations. For other regions and ethnic groups, including Asian populations with varying genotypes and a different risk of myelosuppression compared to Caucasian populations, further assessment is required for the dosing, therapeutic effects, and toxic reactions of azathioprine. Studies in Japanese populations have indicated that lower doses of azathioprine are sufficient to achieve adequate levels of active metabolites and clinical efficacy (Andoh et al., 2008; Komiyama et al., 2008; Ohtsuka et al., 2010). In a study by Kim et al. involving 286 Korean patients, the dosage of azathioprine was 1.84 ± 0.54 mg/kg/day, with only 35.3% of patients able to tolerate a dosage of 2.0 mg/kg/day or more (Kim et al., 2010). Chao et al. then investigated the optimal treatment strategy based on the NUDT15 C415T genotypes in Chinese CD patients in a randomized controlled trial. The CC genotype received the standard dose, the CT genotype received 50% of the standard dose, and the TT genotype received alternative medications. The results revealed that the above optimized strategy based on NUDT15 C415T could effectively reduce early leukopenia in Chinese CD patients using thiopurines without compromising efficacy, but it did not prevent late leukopenia (Chao et al., 2021). In our study, the maximum dose of azathioprine for children in the myelosuppression group and the non-myelosuppression group was 0.74–1.96 mg/kg/day (1.34 ± 0.34) and 0.70–1.81 mg/kg/day (1.22 ± 0.32), respectively, which was also lower than the recommended dosage in the guidelines. In summary, further exploration is necessary regarding the optimal treatment dose, toxic reactions, and treatment regimen adjustments for azathioprine in Asian children with IBD.
This study has several limitations that must be acknowledged. First, the retrospective nature of this study introduces potential selection bias and incomplete data collection, which may compromise the generalizability of the findings. Second, the limited sample size reduces the statistical power of the analysis, potentially resulting in an inability to identify significant associations or differences that may exist in a broader population. Thirdly, the single-center design may limit the external validity and generalizability of the results. Furthermore, the pharmacogenomic testing conducted in this study did not cover all known thiopurine drug metabolism genes, which may cause pharmacogenetic results partially omissive. Lastly, the metabolites of azathioprine such as 6-TGN, DNATG, and 6-MMP were not involved in this study, so the relationship between the occurrence of myelosuppression and the phenotype could not be determined. Future prospective, multicenter studies with larger cohorts are warranted to validate our findings and establish more robust evidence.
Pharmacogenomic testing is of significant importance for guiding the use of thiopurines and predicting the risk of myelosuppression. Testing for TPMT and NUDT15, especially NUDT15, is recommended before the administration of thiopurines in Chinese population. Treatment based on proactive pharmacogenomics still demands vigilant monitoring of adverse reactions, even when pharmacogenomic results are normal.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Research Ethics Board of Children’s Hospital, Fudan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’; legal guardians/next of kin.
C-YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. YH: Funding acquisition, Project administration, Supervision, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82000478) and Shanghai Science and Technology Innovation Action Plan (23Y11905100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Andoh, A., Tsujikawa, T., Ban, H., Hashimoto, T., Bamba, S., Ogawa, A., et al. (2008). Monitoring 6-thioguanine nucleotide concentrations in Japanese patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 23, 1373–1377. doi:10.1111/j.1440-1746.2008.05419.x
Banerjee, R., Ravikanth, V. V., Pal, P., Bale, G., Avanthi, U. S., Goren, I., et al. (2020). NUDT15 C415T variant compared with TPMT genotyping in predicting azathioprine-induced leucopenia: prospective analysis of 1014 inflammatory bowel disease patients in India. Aliment. Pharmacol. Ther. 52, 1683–1694. doi:10.1111/apt.16137
Broekman, M. M. T. J., Coenen, M. J. H., Wanten, G. J., Van Marrewijk, C. J., Klungel, O. H., Verbeek, A. L. M., et al. (2017). Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment. Pharmacol. Ther. 46, 953–963. doi:10.1111/apt.14323
Casajús, A., Zubiaur, P., Méndez, M., Campodónico, D., Gómez, A., Navares-Gómez, M., et al. (2022). Genotype-guided prescription of azathioprine reduces the incidence of adverse drug reactions in TPMT intermediate metabolizers to a similar incidence as normal metabolizers. Adv. Ther. 39, 1743–1753. doi:10.1007/s12325-022-02067-8
Chao, K., Huang, Y., Zhu, X., Tang, J., Wang, X., Lin, L., et al. (2021). Randomised clinical trial: dose optimising strategy by NUDT15 genotyping reduces leucopenia during thiopurine treatment of Crohn’s disease. Aliment. Pharmacol. Ther. 54, 1124–1133. doi:10.1111/apt.16600
Chao, K., Wang, X., Cao, Q., Qian, J., Wu, K., Zhu, X., et al. (2017). Combined detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm. Bowel Dis. 23, 1592–1599. doi:10.1097/MIB.0000000000001148
Coelho, T., Cheng, G., Lewis, S., Ashton, J. J., Barakat, F., Driscoll, K. C. T., et al. (2024). Pharmacogenomic assessment of genes implicated in thiopurine metabolism and toxicity in a UK cohort of pediatric patients with inflammatory bowel disease. Inflamm. Bowel Dis. 31, 362–375. doi:10.1093/ibd/izae126
de Boer, N. K. H., Wong, D. R., Jharap, B., de Graaf, P., Hooymans, P. M., Mulder, C. J. J., et al. (2007). Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am. J. Gastroenterol. 102, 2747–2753. doi:10.1111/j.1572-0241.2007.01511.x
de Graaf, P., de Boer, N. K. H., Wong, D. R., Karner, S., Jharap, B., Hooymans, P. M., et al. (2010). Influence of 5-aminosalicylic acid on 6-thioguanosine phosphate metabolite levels: a prospective study in patients under steady thiopurine therapy. Br. J. Pharmacol. 160, 1083–1091. doi:10.1111/j.1476-5381.2010.00731.x
Desai, D., Jena, A., Sharma, V., and Hibi, T. (2023). Time to incorporate preemptive NUDT15 testing before starting thiopurines in inflammatory bowel disease in Asia and beyond: a review. Expert Rev. Clin. Pharmacol. 16, 643–653. doi:10.1080/17512433.2023.2232300
Dickson, A. L., Daniel, L. L., Zanussi, J., Dale Plummer, W., Wei, W.-Q., Liu, G., et al. (2022). TPMT and NUDT15 variants predict discontinuation of azathioprine for myelotoxicity in patients with inflammatory disease: real-world clinical results. Clin. Pharmacol. Ther. 111, 263–271. doi:10.1002/cpt.2428
Giugliano, F. P., Strisciuglio, C., Martinelli, M., Andreozzi, M., Cenni, S., Campione, S., et al. (2018). Does Azathioprine induce endoscopic and histologic healing in pediatric inflammatory bowel disease? A prospective, observational study. Dig. Liver Dis. 50, 240–246. doi:10.1016/j.dld.2017.10.017
Gutiérrez-Valencia, M., Leache, L., Saiz, L. C., Beloqui, J. J., Barajas, M., Vicuña, M., et al. (2023). Role of pharmacogenomics in the efficacy and safety of thiopurines in inflammatory bowel disease: a systematic review and meta-analysis. J. Clin. Gastroenterology 57, 671–685. doi:10.1097/MCG.0000000000001791
Hande, S., Wilson-Rich, N., Bousvaros, A., Zholudev, A., Maurer, R., Banks, P., et al. (2006). 5-Aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm. Bowel Dis. 12, 251–257. doi:10.1097/01.MIB.0000206544.05661.9f
Kennedy, A. M., Griffiths, A. M., Muise, A. M., Walters, T. D., Ricciuto, A., Huynh, H. Q., et al. (2024). Landscape of TPMT and NUDT15 pharmacogenetic variation in a cohort of Canadian pediatric inflammatory bowel disease patients. Inflamm. Bowel Dis. 30, 2418–2427. doi:10.1093/ibd/izae109
Khaeso, K., Udayachalerm, S., Komvilaisak, P., Chainansamit, S.-O., Suwannaying, K., Laoaroon, N., et al. (2021). Meta-analysis of NUDT15 genetic polymorphism on thiopurine-induced myelosuppression in asian populations. Front. Pharmacol. 12, 784712. doi:10.3389/fphar.2021.784712
Kim, H. S., Cheon, J. H., Jung, E. S., Park, J., Aum, S., Park, S. J., et al. (2017). A coding variant in FTO confers susceptibility to thiopurine-induced leukopenia in East Asian patients with IBD. Gut 66, 1926–1935. doi:10.1136/gutjnl-2016-311921
Kim, J. H., Cheon, J. H., Hong, S. S., Eun, C. S., Byeon, J.-S., Hong, S. Y., et al. (2010). Influences of thiopurine methyltransferase genotype and activity on thiopurine-induced leukopenia in Korean patients with inflammatory bowel disease: a retrospective cohort study. J. Clin. Gastroenterology 44, e242–e248. doi:10.1097/MCG.0b013e3181d6baf5
Komiyama, T., Yajima, T., Kubota, R., Iwao, Y., Sakuraba, A., Funakoshi, S., et al. (2008). Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J. Crohns Colitis 2, 315–321. doi:10.1016/j.crohns.2008.05.002
Lee, M.-N., Kang, B., Choi, S. Y., Kim, M. J., Woo, S. Y., Kim, J.-W., et al. (2015). Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm. Bowel Dis. 21, 1054–1062. doi:10.1097/MIB.0000000000000347
Lee, W. S., Arai, K., Alex, G., Treepongkaruna, S., Kim, K. M., Choong, C. L., et al. (2023). Medical management of pediatric inflammatory bowel disease in the asia-pacific region: a position paper by the asian Pan-pacific society for pediatric gastroenterology, hepatology, and nutrition (appspghan) PIBD working group. J Gastro Hepatol 38, 523–538. doi:10.1111/jgh.16097
Luo, X., Yan, S., Jin, L., Zhu, H., Zhang, X., and Ge, W. (2022). Inosine triphosphate pyrophosphatase and NUDT15 are good predictors of clinical outcomes in thiopurine-treated Chinese patients with inflammatory bowel disease. Ther. Drug Monit. 44, 391–395. doi:10.1097/FTD.0000000000000965
Nian, Q., Liu, R., and Zeng, J. (2024). Unraveling the pathogenesis of myelosuppression and therapeutic potential of natural products. Phytomedicine 132, 155810. doi:10.1016/j.phymed.2024.155810
Ohtsuka, Y., Arai, K., Aoyagi, Y., Fujii, T., Yamakawa, Y., Ohtani, K., et al. (2010). Monitoring 6-thioguanine nucleotide concentrations in Japanese children and adolescents with inflammatory bowel disease. J. Gastroenterol. Hepatol. 25, 1626–1630. doi:10.1111/j.1440-1746.2010.06364.x
Pratt, V. M., Cavallari, L. H., Fulmer, M. L., Gaedigk, A., Hachad, H., Ji, Y., et al. (2022). TPMT and NUDT15 genotyping recommendations: a joint consensus recommendation of the association for molecular pathology, clinical pharmacogenetics implementation Consortium, college of American pathologists, Dutch pharmacogenetics working group of the royal Dutch pharmacists association, European society for pharmacogenomics and personalized therapy, and pharmacogenomics knowledgebase. J. Mol. Diagn 24, 1051–1063. doi:10.1016/j.jmoldx.2022.06.007
Relling, M. V., Schwab, M., Whirl-Carrillo, M., Suarez-Kurtz, G., Pui, C.-H., Stein, C. M., et al. (2019). Clinical pharmacogenetics implementation Consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105. doi:10.1002/cpt.1304
Ribeiro, A. C., Gerheim, P. S. A. S., Chebli, J. M. F., Nascimento, J. W. L., and De Faria Pinto, P. (2023). The role of pharmacogenetics in the therapeutic response to thiopurines in the treatment of inflammatory bowel disease: a systematic review. JCM 12, 6742. doi:10.3390/jcm12216742
Sandritter, T., Chevalier, R., Abt, R., and Shakhnovich, V. (2023). Pharmacogenetic testing for the pediatric gastroenterologist: actionable drug-gene pairs to know. Pharm. (Basel) 16, 889. doi:10.3390/ph16060889
Sheffield, L. J., Irving, P., Gupta, A., Byron, K., Macrae, F. A., Phillimore, H., et al. (2009). Thiopurine methyltransferase and thiopurine metabolite testing in patients with inflammatory bowel disease who are taking thiopurine drugs. Pharmacogenomics 10, 1091–1099. doi:10.2217/pgs.09.60
Valdez-Acosta, S., Zubiaur, P., Casado, M. A., Novalbos, J., Casajús, A., Campodónico, D., et al. (2023). Preemptive TPMT genotyping and adherence to genotype-based therapeutic recommendations reduces the healthcare cost in patients receiving azathioprine or 6-mercaptopurine for autoimmune diseases. J. Pers. Med. 13, 1208. doi:10.3390/jpm13081208
Van Den Bosch, B. J., and Coenen, M. J. (2021). Pharmacogenetics of inflammatory bowel disease. Pharmacogenomics 22, 55–66. doi:10.2217/pgs-2020-0095
van Rheenen, P. F., Aloi, M., Assa, A., Bronsky, J., Escher, J. C., Fagerberg, U. L., et al. (2020). The medical management of paediatric crohn’s disease: an ECCO-ESPGHAN guideline update. J. Crohns Colitis 15, 171–194. doi:10.1093/ecco-jcc/jjaa161
Yang, S.-K., Hong, M., Baek, J., Choi, H., Zhao, W., Jung, Y., et al. (2014). A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat. Genet. 46, 1017–1020. doi:10.1038/ng.3060
Yang, T., Chao, K., Zhu, X., Wang, X.-D., Chan, S., Guan, Y.-P., et al. (2024). Early proactive monitoring of DNA-thioguanine in patients with Crohn’s disease predicts thiopurine-induced late leucopenia in NUDT15/TPMT normal metabolizers. World J. Gastroenterol. 30, 1751–1763. doi:10.3748/wjg.v30.i12.1751
Zeng, D., Huang, X., Lin, S., Lin, R., Weng, X., and Huang, P. (2021). Cost-effectiveness analysis of genotype screening and therapeutic drug monitoring in patients with inflammatory bowel disease treated with azathioprine therapy: a Chinese healthcare perspective using real-world data. Ann. Transl. Med. 9, 1138. doi:10.21037/atm-21-1980
Keywords: pediatric inflammatory bowel disease, azathioprine, pharmacogenomics, myelosuppression, TPMT, NUDT15
Citation: Long C-Y and Huang Y (2025) Proactive pharmacogenomics in azathioprine-treated pediatric inflammatory bowel disease at a Chinese tertiary hospital. Front. Pharmacol. 16:1558897. doi: 10.3389/fphar.2025.1558897
Received: 11 January 2025; Accepted: 13 March 2025;
Published: 26 March 2025.
Edited by:
Ruixin Zhu, Tongji University, ChinaReviewed by:
Jian Lin, Affiliated Hospital of Putian University, ChinaCopyright © 2025 Long and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Huang, aHlfQGZ1ZGFuLmVkdS5jbng=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.