
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 08 April 2025
Sec. Pharmacology of Infectious Diseases
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1558611
Introduction: This research aimed to examine the action of commercial antibiotics against extensively drug-resistant (XDR) A. baumannii clinical strains when combined with Rosmarinus officinalis extracts.
Methods: Agar well diffusion and broth microdilution were used to screen the antibacterial activity of crude ethanol extract and its fractions (hexane, intermediate, ethyl acetate, and water). The interactions between the extracts and antibiotics (gentamicin, tetracycline, cefepime, and ciprofloxacin) were evaluated by checkerboard assay. The anti-biofilm and efflux pump inhibition activities were determined by the microtiter plate method and dye accumulation assay using flow cytometry, respectively. The potential phytochemicals that contribute to the antibacterial effects of R. officinalis were identified using the liquid chromatography-mass spectrometry (LC–MS).
Results: R. officinalis crude extract (CE) demonstrated the best antibacterial activity with MIC values ranging from 300 to 600 μg/mL. The combination of CE and tetracycline exhibited the highest overall synergistic effect. This combination hindered biofilm formation ranging from 21.4% to 57.31% and caused a significant increase (up to 14%) in the fluorescence intensity in 75% of the studied strains. The LC-MS analysis of CE exhibited eleven compounds in which rosmarinic acid (55.53%) was the most abundant phenolic compound followed by cirsimaritin (11.46%), and p-coumaroyl hexoside acid (10.5%).
Discussion: Overall, this is the first direct report that demonstrated the efficacy of R. officinalis when applied with conventional antibiotics on biofilm formation and efflux pump activity in XDR A. baumannii clinical strains.
Acinetobacter baumannii has currently emerged as the leading cause of nosocomial infections with high morbidity and mortality rates (26%–60%) due to its extensive drug resistance (Basak et al., 2016; Roy et al., 2022). Furthermore, this pathogen has now gone beyond hospitals and is being reported to cause community-acquired infections in both pediatric and adult populations. A. baumannii can colonize biotic and abiotic surfaces for prolonged periods in hostile conditions such as desiccation, antimicrobial therapies, and nutrient unavailability due to their ability to form complex structures called biofilms. Compared to planktonic cells, the three-dimensional architecture of biofilm provides one thousand times more tolerance to antimicrobial agents by shielding the bacterial cells from treatment with antibiotics. In addition, efflux pumps play a dual role in drug resistance either directly by extruding antibiotics from the cells or indirectly by biofilm formation (Abd El-Rahman et al., 2023). Based on the 2017 World Health Organization (WHO) Bacterial Priority Pathogens List (WHO BPPL), A. baumannii is considered a critical microorganism (Priority 1) for the development of novel antibiotics (Nocera et al., 2021). However, the major limitation in developing new antibiotics is financial efficiency. Also, many drugs have failed to enter the market due to their poor pharmacological attributes, leading to significant financial losses for the pharmaceutical industry. One of the potential strategies to overcome this limitation is natural-origin compounds, such as plant extracts, as antibiotic adjuvants (Romo-Castillo et al., 2023). The plant extract-antibiotic combination is an innovative alternative to prescriptive treatment protocols for expanding the antimicrobial spectrum and avoiding the emergence of resistant strains and undesirable toxic effects of antimicrobial therapy. Rosmarinus officinalis, commonly called rosemary, belongs to the family Lamiaceae, is an aromatic evergreen shrub distributed throughout the world and known to exhibit antitumor, antibacterial, antiviral, anti-inflammatory, diuretic, antithrombotic, antioxidant, and antidiabetic activities (Manilal et al., 2021). The biological properties of rosemary have been attributed to its phytochemical composition rich in bioactive secondary metabolites with suitable economic viability and efficacy (Mena et al., 2016). The quantitative distribution of plant secondary metabolites can vary from organ to organ or plant to plant. Liquid chromatography-mass spectrometry (LC–MS)-based approaches have particular importance in the separation and detection of highly rich biochemistry of plants due to their high selectivity, high sensitivity, and high qualitative productivity (El Sayed et al., 2020; Kaur et al., 2022). Although some studies were reported on the antibacterial activity of R. officinalis, the main components in this plant that contribute to its antibacterial activity remain unclear to date (Zhong et al., 2021). On the other hand, the antibacterial interactions between rosemary and many commercially available antibiotics have been overlooked. In light of this, the present study aims to evaluate the effect of crude ethanol extract of R. officinalis and its different fractions combined with various antibiotics on biofilm formation and efflux pump activity of extensively drug-resistant (XDR) A. baumannii clinical strains. Also, the detection and identification of bioactive components of R. officinalis were done using the LC–MS method.
A total of nine biofilm-forming XDR A. buamannii strains belonging to nine different genotypes based on ERIC-PCR patterns, which were previously isolated from patients with burn wound infection in Isfahan, Iran, were included in this study (unpublished data). These strains were preserved at − 80°C in Tryptic Soy Broth (TSB) (Merck, Germany) supplemented with 20% glycerol. Working cultures were prepared by transferring 10 μL of frozen stock cultures to Tryptic Soy Agar (Merck, Germany) followed by incubation at 37°C for 24 h.
The screening of bacterial strains for efflux pump activity was carried out by dye accumulation assay as a previously published protocol (Lu et al., 2020), with the following modifications. The bacterial cells were incubated to OD600 of 0.8 in TSB and collected by centrifugation for 5 min at 5,000 rpm. The cells were washed three times with phosphate-buffered saline (PBS) and resuspended with the same buffer in a final OD600 of 0.6. Then, the cell suspensions were stained with Rhodamine-123 (Rho-123; Sigma-Aldrich, United States) to a final concentration of 200 μg/mL (Khashei et al., 2018). After incubation in the dark for 10 min, the fluorescence was measured by a flow cytometer (BD FACSCalibur, United States) through the green channel of a fluorescence detector (FL1, 525 nm). The protonophore carbonyl cyanide 3-chlorophenylhydrazone (CCCP), which works by dissipating the proton motive force (PMF), the primary energy source for many efflux pumps (Zoaiter et al., 2023), was used as a positive control to a final concentration of 50 μg/mL. Also, unstained bacterial cells were considered as negative control. Data analysis was carried out using FlowJo v10.5.3 Software. Strains that showed lower fluorescence than the control (indicative lower level of dye accumulated in the cells) were chosen for further studies.
Aerial parts of R. officinalis were collected in September 2023 from the campus of Isfahan University of Medical Sciences and were characterized by the Department of Plant and Animal Biology, the University of Isfahan, and a voucher number (260127) was deposited at the herbarium. The extraction process was done according to the previously described with some modifications (Moussi et al., 2015). Briefly, the dried leaves were ground to prepare powder, which was milled through a 1 mm sieve. Then, 50 g of powdered sample was macerated with 500 mL of ethanol (Merck, Germany) for 48 h at room temperature with continuous stirring. After filtration through filter paper, the extract solution was concentrated using a rotary evaporator and dried at room temperature. The dry extract was considered crude extract (CE).
Further fractions were obtained by suspending 2 g of CE in distilled water (20 mL). This solution was then successively partitioned with the same volume of n-hexane and ethyl acetate (Merck, Germany) in a Pyrex 100 mL separating funnel to obtain respective solvent-solvent fractions. Also, the insoluble fraction formed between hexane and aqueous solution was collected as the intermediate fraction (INT). Each fraction was concentrated with a rotary evaporator, dried, and stored at 4°C (Moussi et al., 2015).
The agar well diffusion method was applied to assay the antibacterial activity of extracts against selected A. baumannii strains. Each extract solution was prepared at concentrations of 6.25, 12.5, 25, and 50 mg/mL by dissolving the dried extracts in 5% dimethyl sulphoxide (DMSO; Merck, Germany). Each well of the Mueller-Hinton Agar (MHA; Merck, Germany) plate was filled with 100 μL of the test substances and the plates were incubated at 37°C for 24 h. Doxycycline (Sigma-Aldrich, United States) and 5% DMSO served as positive and negative controls, respectively. All experiments were set in triplicate (Sa-Eed et al., 2023). Based on the produced zones of inhibition, the CE with the highest activity against A. baumannii strains was selected for further evaluation.
The MIC values of CE and antibiotics alone (ciprofloxacin, tetracycline, gentamicin, and cefepime; Sigma-Aldrich, United States) were determined using the broth microdilution method. Briefly, bacterial suspension (1 × 106 CFU/mL; 100 μL) was placed into each well of 96-well microtiter plates containing 100 μL twofold serial dilutions of CE and antibiotics (Nassar et al., 2021). A zero-hour reading was taken by recording the optical density (OD600 nm) after 30 min of incubation. Then, the microtiter plates (MTP) were incubated at 37°C for 24 h, and absorbance was measured again. The lowest concentration of antibacterial agent that prevents the growth of bacteria, compared with the controls (wells without antibacterial agents), was considered as the MIC. The assay was carried out thrice (Atta et al., 2023).
The interactive antibacterial effect of antibiotics and CE was determined by the checkerboard microdilution technique to obtain a fractional inhibitory concentration index (FICI) (Norbury et al., 2016). In a 96-well MTP, the CE was diluted (2-fold) horizontally and antibiotics were diluted (2-fold) vertically. The total volume of the combination was 100 μL per well. Then 100 μL of the bacterial inoculum (1 × 106 CFU/mL) was added to each well and incubated for 24 h at 37°C (Yang et al., 2017). The FICI was calculated by applying the formulas displayed below: FICI = FICA + FICB, where FICA was MIC of extract in combination/MIC of extract alone, and FICB was MIC of antibiotic in combination/MIC of antibiotic. The interactions were categorized as being synergistic for the FIC value of ≤0.5, partial synergistic (0.5 < FICI < 1), additive (FICI = 1), indifferent (1 ≤ FICI < 4), or antagonistic (FICI > 4.0) (Haji et al., 2022). A combination of CE and tetracycline with the highest synergistic effect was selected for further studies.
The effect of the tetracycline and CE (individually and in combination) at ½ MIC levels on the biofilm formation ability of XDR A. baumannii strains was tested as described by Amer et al. (2023). Briefly, MTPs were prepared as described above. After 24 h incubation at 37°C, the planktonic cells were discarded, and plates were gently washed three times with normal saline (0.9% NaCl). The remaining attached bacterial cells were fixed by adding 200 μL of ethanol (96%) to each well. After 15 min incubation at room temperature, the contents of all wells were discarded, and the adhered cells were stained using crystal violet 1% (w/v) (Sigma, Germany) for 15 min. Then, wells were washed with normal saline three times to remove excess stains. Finally, the dye was solubilized with 30% (v/v) acetic acid and the optical density of each well was measured at 570 nm using an ELISA reader (BioTek 800 TS, United States). The antibiofilm activity was expressed as biofilm inhibition (BI) percentage using the following equation (Adeyemo et al., 2022):
Also, the biofilm formation ability of the strains was classified as follows: (1) OD ≤ ODc = negative; (2) ODc < OD ≤ 2ODc = weak; (3) 2ODc < OD ≤ 4ODc = intermediate; and (4) OD > 4ODc = strong. ODc represents the mean OD value of the wells without bacterial strains, while OD is the value of the tested strains.
The synergistic inhibitory effect of the CE in combination with tetracycline was estimated against efflux activity in biofilm-forming XDR A. baumannii using a dye accumulation assay. This assay was performed by growing bacterial strains in TSB until the mid-log phase. The bacterial cells were harvested by centrifugation at 5,000 rpm for 5 min and washed several times with PBS. The pellet was resuspended with the same buffer, then 0.4% glucose was added, and OD600 was adjusted to 0.6. The bacterial suspension was incubated for 30 min at 37°C, without or with the sub-lethal dose (½ MIC) of selected antibacterial agents (Al-Sallami et al., 2023). Then, bacterial cells were stained with Rhodamine-123 (Rho-123). After incubation in the dark for 10 min, the fluorescence was measured by a flow cytometer as described above.
The LC-MS analysis of the CE was performed with a Waters Alliance 2695 HPLC-Micromass Quattro micro-API Mass Spectrometer. Chromatographic separation was employed on Waters Xbridge C18 5 μm, 15 cm × 4.6 mm with mobile phase, including a blend of H2O and 0.1% formic acid water (solvent B) and acetonitrile and 0.1% formic acid (solvent C). The column temperature was 35°C, the flow rate was set at 0.3 mL/min, and the injection volume was 10 μL (Hassan et al., 2022).
Results were expressed as the mean ± standard deviation for each group. Differences in quantitative measurements were assessed by Student’s t-test or one-way analysis of variance. Differences were considered statistically significant at p < 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ****P value ≤ 0.0001. All analyses were carried out with GraphPad Prism 8.0.
A total of nine biofilm-forming XDR A. buamannii strains, belonging to different genotypes isolated from burn wound-infected patients, were examined using the dye accumulation assay to monitor possible efflux pump activity. The significant lower florescent intensity was identified in four strains (44.44%) by a 1.52 (Figure 1d), 1.22 (Figure 1f), 2.39 (Figure 1g), and 1.11 (Figure 1h)-fold lower fluorescence in the respective strains compared to the positive control. The lower fluorescence intensity (lower level of R-123 accumulation) could likely be due to the action of efflux pumps. These four strains were selected for further study and were referred to as AB1, AB2, AB3, and AB4, respectively.
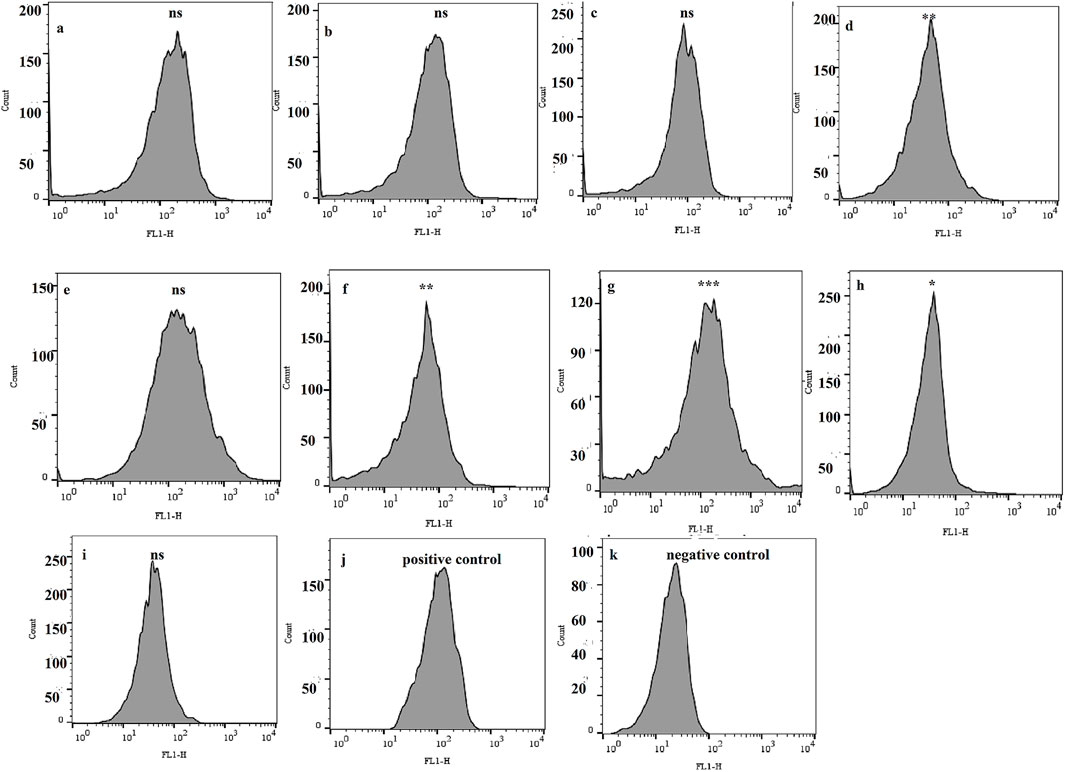
Figure 1. Flow cytometry plots displaying fluorescence intensities of rhodamine 123 as a marker of the efflux pump activity in biofilm-forming XDR A. baumannii strains. Each panel (a–i) corresponds to one of the nine tested bacterial strains, while panels (j, k) show fluorescence intensity in the positive control (treated with CCCP) and the negative control (unstained cells), respectively. The asterisks indicate significant differences compared to the positive control. ns: not significant (P value > 0.05), *P value < 0.05, **P value ≤0.01, and ***P value ≤0.001.
The antibacterial activity was evaluated against biofilm-forming XDR A. baumannii strains. It was found that the antibacterial activity of plant extract and its fractions differed based on the type of solvent used in the extraction process (Figure 2). Compared to the positive control, a significant dose-dependent inhibition of bacterial growth by CE was observed. The CE showed remarkable antibacterial activity with a wider inhibition zone (15.4 mm) at a concentration of 50 mg/mL and even at lower concentrations of 25 mg/mL (14.3 mm) and 12.5 mg/mL (13.5 mm). In contrast, the intermediate fraction had no inhibitory effect on the growth of bacteria.
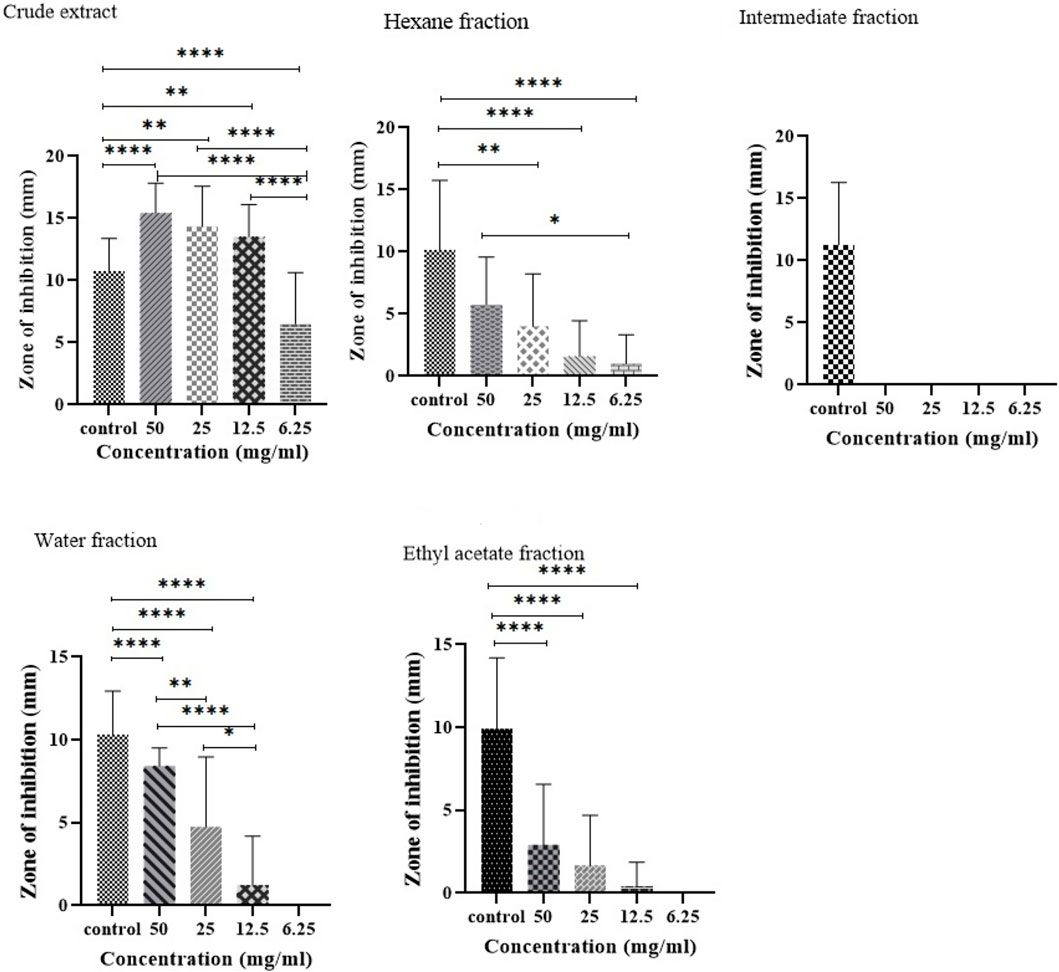
Figure 2. Comparison of the antibacterial activity of CE and its fractions against biofilm-forming XDR A. baumannii strains. *P value < 0.05, **P value ≤ 0.01, and ****P value ≤ 0.0001.
The results of MIC determination for the CE and each of the four tested antibiotics are shown in Table 1. CE was found to have potent antimicrobial activity against biofilm-forming XDR A. baumannii strains, with a mean MIC ranging from 300 to 600 μg/mL. The MIC values for tetracycline and gentamycin ranged from 32 to 64 μg/mL, while tested strains exhibited ciprofloxacin and cefepime MIC values of 64–128 and 128–256 μg/mL, respectively. Based on these results, all bacterial strains showed MICs in the antibiotic resistance range.
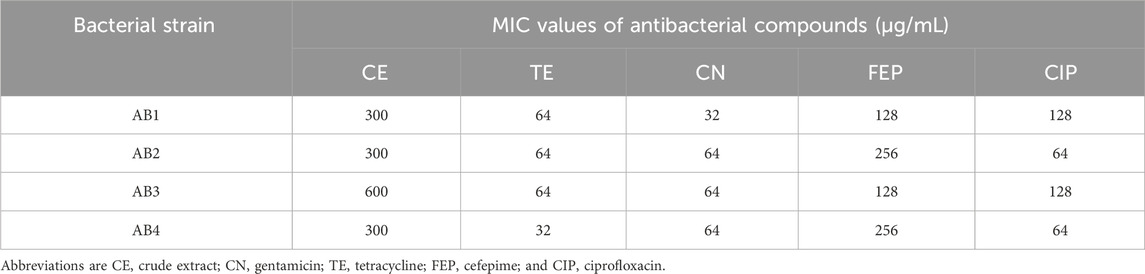
Table 1. MIC values for tested antibacterial agents measured by broth microdilution method against biofilm-forming XDR A. baumannii strains.
We chose four antimicrobial classes including, fluoroquinolones (ciprofloxacin), aminoglycosides (gentamicin), tetracyclines (tetracycline), and cephalosporins (cefepime), to test their potential synergistic effects CE. With the addition of CE, a significant decrease in MIC was observed in the case of gentamicin (P value = 0.024), tetracycline (P value = 0.0012), and cefepime (P value = 0.0114). The combination of CE and tetracycline exhibited the highest overall synergistic effect, with an inhibitory concentration up to 16 times lower than the inhibitory concentration of tetracycline monotherapy (FIC values ranging from 0.3125 to 0.375) (Table 2). No antagonistic activity was observed with any of the combinations. The combination of CE with gentamicin exhibited a partial synergistic effect in 25% of studied bacterial strains (FIC value = 0.75) (Table 3), while the combination of CE with ciprofloxacin (Table 4) and cefepime (Table 5) exhibited indifferent effects when combined with CE.
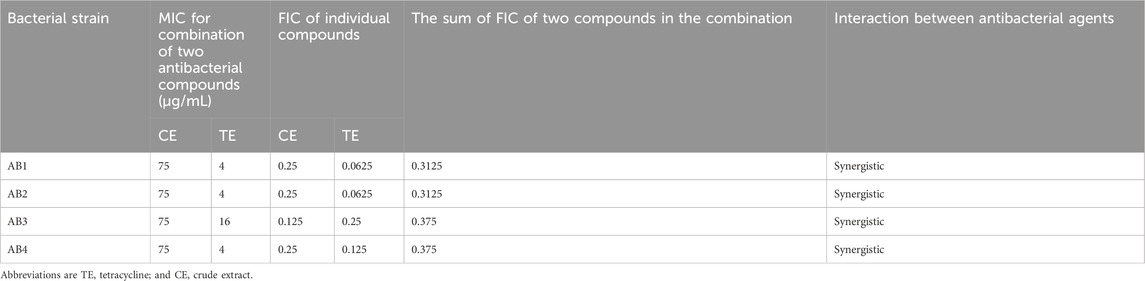
Table 2. MICs and FIC indices of tetracycline in combination with CE extract against biofilm-forming XDR A. baumannii strains.

Table 3. MICs and FIC indices of gentamicin in combination with CE against biofilm-forming XDR A. baumannii strains.
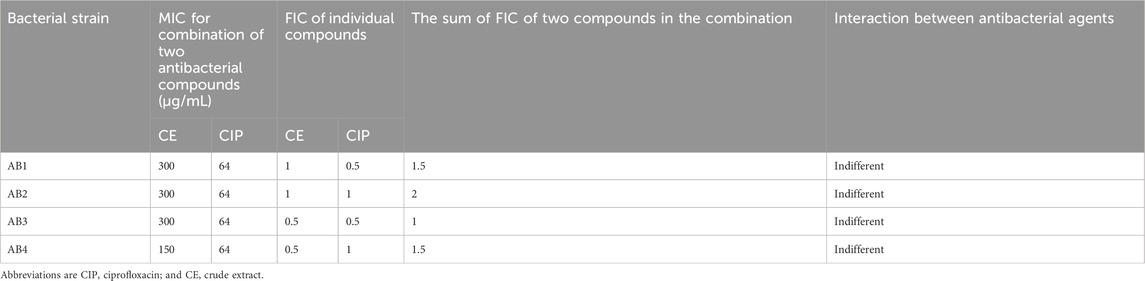
Table 4. MICs and FIC indices of ciprofloxacin in combination with CE against biofilm-forming XDR A. baumannii strains.

Table 5. MICs and FIC indices of cefepime in combination with CE against biofilm-forming XDR A. baumannii strains.
The findings of this study, using the quantitative microtiter plate method for the determination of biofilm formation ability, showed that all four tested A. Baumannii strains (100%) produced biofilm. Among these four biofilm-producing strains, 25% produced moderate biofilms and 75% formed strong biofilms. The anti-biofilm activity of rosemary CE alone and in combination with tetracycline at ½ MIC level against these strains was summarized in Table 6. The combination of CE with tetracycline achieved the highest inhibition of biofilm formation against various examined strains (BI (%) varied from 21.4% to 57.31%), followed by CE (BI (%) ranged from 12.5% to 54.34%).
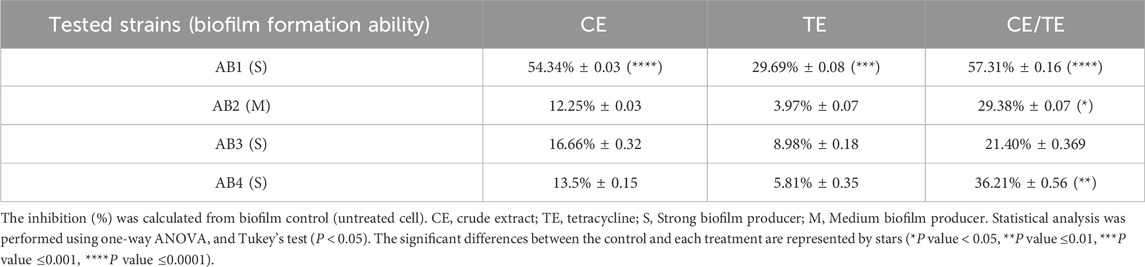
Table 6. Biofilm inhibition (BI) percentage of tetracycline in combination with CE against XDR A. baumannii strains. Data represent the mean of three biological replicates ±standard deviation (Yilmaz et al., 2014).
Compared to the control group (0 MIC), the addition of CE in combination with tetracycline caused a significant increase (up to 14%) in the fluorescence intensity (indicates a higher level of R-123 accumulation) in 75% of the studied bacterial strains (Figure 3).
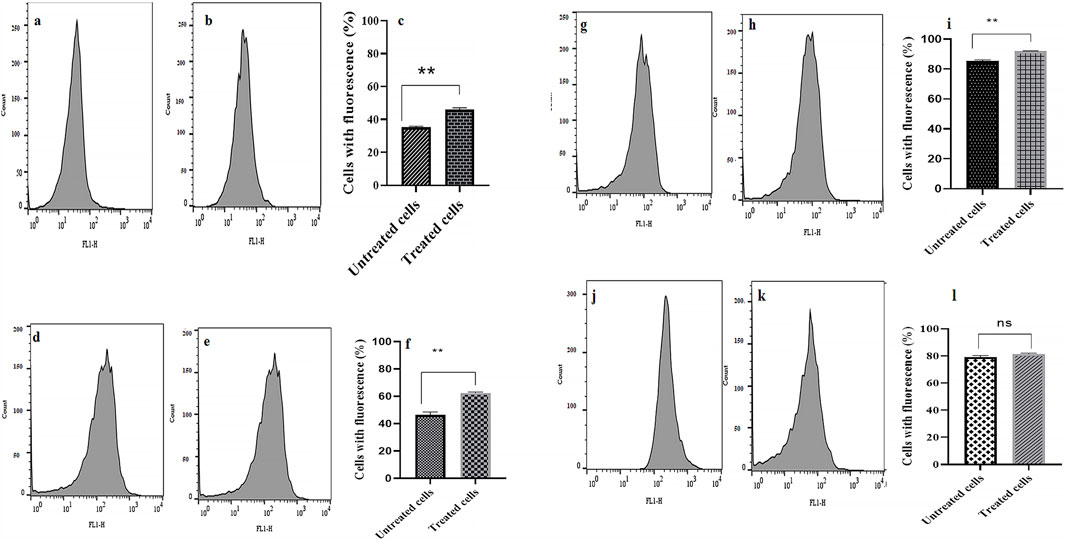
Figure 3. Evaluation of dye accumulation in bacterial cells in the absence and presence of treatment with CE/tetracycline combination. Panels (a, d, g, and j) show flow cytometry plots indicating fluorescence intensity in the absence of treatment, panels (b, e, h, and k) show fluorescence intensity in the presence of treatment, and panels (c, f, i, and l) present comparison graphs of fluorescence intensity (dye accumulation) in the absence and presence of treatment, in strains AB1, AB2, AB3, and AB4, respectively. ns: not significant (P value > 0.05) and **P value ≤0.01.
According to the LC-MS analysis, eleven peaks were tentatively characterized by comparing their molecular weight and mass fragmentation pattern in their LC-MS spectra with published literature data. These identified constituents included oleanolic acid (retention time (RT): 2.59 min), epigallocatechin (RT: 4.57 min), caffeic acid (RT: 8.59 min), quercetin-3-glucoside (RT: 8.87 min), rosmarinic acid (RT: 9.15 min), cirsimaritin (RT: 13.76 min), chlorogenic acid (RT: 15.26 min), kaempferol (RT: 16.33 min), p-coumaroyl hexoside acid (RT: 16.63 min), quercetin (RT: 17.61 min), and pedalitin (RT: 17.82 min) (Figure 4). Also, by calculating the area under the peak, the relative amount of each compound was measured as a percentage of the total area of the peaks in the chromatogram. In this regard, CE was dominated by rosmarinic acid (55.53%), followed by cirsimaritin (11.46%), and p-coumaroyl hexoside acid (10.5%) (Table 7).
In the absence of newly emerging antibacterial agents that are superior to currently available options against XDR A. baumannii infections, one approach that has been explored is combination therapy to improve outcomes (Spellberg and Bonomo, 2015). The antibacterial activity of R. officinalis against multiple pathogens was demonstrated in previous studies (Del Campo et al., 2000; Manilal et al., 2021; Günther et al., 2022). To the best of our knowledge, colistin is the only antibiotic examined in combination with R. officinalis against XDR A. baumannii clinical isolates previously (Kafa et al., 2022). Thus, the antibacterial synergism activity of rosemary with many of the conventional antibiotics has been overlooked. On the other hand, treatment regimens including colistin should be prescribed carefully because the clinical use of colistin is accompanied by a narrow therapeutic window and certain side effects such as nephrotoxicity and neurotoxicity (Samarkos et al., 2022). In this view, the current study evaluates the effects of R. officinalis extract combined with various antibiotics from four antimicrobial classes on biofilm formation and efflux pump activity of XDR A. baumannii clinical strains. Our results showed that CE has the greatest antibacterial activity and the fractionation process reduced its antibacterial activity. This indicates that the active compounds might be more concentrated in the CE and more diluted in its fractions. Also, a polarity-dependent decrease in antibacterial properties was observed among different fractions achieved from solvents with various polarities (water, ethyl acetate, and hexane as polar, semi-polar, and non-polar solvents, respectively). This suggests that the aerial parts of R. officinalis contain several antibacterial compounds with different polarities and the polarity of the solvent greatly influences its antimicrobial activity. Consistent with our findings, Saxena et al. reported that increased polarity aids the entry of a compound into Gram-negative bacteria. The higher polarity of compounds targeting Gram-negative bacteria corresponds to the fact that the compounds are looking for their route through the hydrophilic porin channels (Saxena et al., 2023). There are numerous criteria for the classification of the antimicrobial activity of plant extracts. According to Simoes et al., an extract is said to be antimicrobial when MIC ranges from 100 to 1,000 μg/mL (Simoes et al., 2009), while according to Aligiannis et al., the antimicrobial activity of an extract is considered strong when MIC < 500 μg/mL, moderate when MIC values ranged between 500 μg/mL to 1,500 μg/mL, and low when greater than 1,500 μg/mL (Aligiannis et al., 2001). In this regard, the ethanolic crude extract of R. officinalis demonstrated remarkable antimicrobial activity against A. baumannii with a MIC of 300–600 μg/mL in the current study. The MIC values of the ethanolic extract and essential oil of rosemary were reported previously to be 1–2 mg/mL and 5–20 μg/mL against A. baumannii, respectively (Assis et al., 2018; Kafa et al., 2022). These differences may be due to the variation of the chemical composition of plant extracts in response to seasonal variation, culture conditions, the clime, and even the extraction method which can affect their antibacterial activity (5).
The combination of CE with different antibiotics has shown various effects on XDR A. baumannii strains. In our study, the R. officinalis ⁄ tetracycline combination displayed the most favorable synergistic pattern, and the MIC of tetracycline reached the sensitivity threshold (MIC ≤ 4) in 75% of tested strains when applied in combination with rosemary. Also, we observed that the R. officinalis ⁄ gentamicin combination only produced a partial synergistic effect in 25% of tested strains and indifferent interaction in other strains. Based on our findings, the combination of CE with ciprofloxacin and cefepime showed indifferent interactions. Although the MIC values of these antibiotics were reduced by 2 to 4-fold, the strains remained resistant to these antibiotics. It is generally agreed that a combination of multiple antimicrobial agents can yield various effects depending on the chemical nature of their components, the intermolecular interactions, and the concentration of the compounds (Vaou et al., 2022). Therefore, this study can serve as a guide for future research on the use of combinations having promising synergistic profiles with fewer side effects and a more positive contribution.
The exact mechanism by which natural antimicrobial agents decrease bacterial resistance to antibiotics is unknown. Previous studies suggest that these compounds may simplify drug penetration through the outer layers of bacterial cell walls, block the inhibitory effect of protective enzymes, and interfere with the metabolic targets of the antibiotic (Górniak et al., 2019). In addition, they often contain compounds that increase the solubility, absorption, distribution, or metabolism of active constituents of antibiotics (Vaou et al., 2022). Based on our results, R. officinal might act as a resistance breaker that can restore the activity of tetracycline against A. baumannii strains, in case of the biofilm- and efflux-mediated antimicrobial resistance mechanisms.
Based on the LC-MS profile, the potential bioactive metabolites that contribute to the antimicrobial effects of CE were similar to those of other studies in the literature (Borrás-Linares et al., 2014; Christopoulou et al., 2021), except for pedalitin. The antifungal effects of pedalitin (5,6,3′,4′-tetrahydroxy-7-methoxyflavone) obtained from Pterogyne nitens have been demonstrated previously against several strains of Candida albicans and Cryptococcus spp. (Sangalli-Leite et al., 2016). Shoba et al. reported that pedalitin is responsible for the inhibitory activity of Pedalium murex against Proteus mirabilis (Ramadevi et al., 2020). Another study conducted by Lin et al. showed that pedalitin isolated from Rabdosia serra has considerable anti-melanogenesis and anti-diabetic effects by inhibition of tyrosinase and α-glucosidase enzymes, respectively (Lin et al., 2011). Thus, this is the first study reporting the crude ethanol extract of rosemary as a novel natural source for the isolation of pedelitin.
Plant bioactive secondary metabolites include flavonoids, terpenoids, and phenolic acids, which were represented to have significant antimicrobial effects (Bouyahya et al., 2022). The antibiofilm activity of flavonoids and phenolic acids such as quercetin, epigallocatechin, rosmarinic acid, and kaempferol as bioactive active secondary metabolites derived from medicinal plants against various pathogenic bacteria has been demonstrated previously. These molecules suppressed the quorum sensing process as a main regulatory system in bacterial biofilm formation (Slobodníková et al., 2016). Also, the mechanism of antibiofilm action of terpenoids can be attributed to their anti-cell-adhesion properties (Lahiri et al., 2019). However, considering the method used in this study to assess the effect of the R. officinal extract on biofilm formation, it is possible that the extract does not directly inhibit biofilm formation but rather affects cell growth, which in turn could impact the cells’ ability to form biofilms. On the other hand, plant phenolic compounds act as protonophores which interfering with ATP synthesis and proton gradients, leading to efflux pump inhibition activity (Zhang et al., 2023). Thus, the potential of R. officinalis ethanolic crude extract for inhibiting efflux pump activity and biofilm formation ability in XDR-A. baumannii strains could be due to the presence of these bioactive compounds. Also, the whole extract derived from rosemary may have greater antibacterial potential than their single bioactive ingredients due to the synergism between their molecules and the diversity of the mechanisms of action of its compounds.
This is the first direct report that provides evidence supporting the potential of R. officinalis for enhancing the efficacy of commercially available antibiotics in different classes including, fluoroquinolones, aminoglycosides, tetracyclines, and cephalosporins against XDR A. baumannii clinical strains. The combination of CE with tetracycline exhibited significant efficacy for reversing efflux pump activity and inhibiting the biofilm formation ability of XDR A. baumannii strains. Furthermore, the identification of various bioactive compounds in CE underscores its potential in drug development targeting diverse health disorders.
Collectively, this work presents an innovative therapeutic approach that can be developed into clinically antibacterial agents to address the growing threat of biofilm-forming XDR A. baumannii and other priority pathogens. However, the study acknowledges certain limitations. Standardization challenges in studying plant-based antimicrobials and the dynamic nature of protein-ligand interactions can affect the robustness and applicability of the suggested antibacterial agents in the present study. Also, future investigations should evaluate the efficacy of the suggested antibacterial agents in the present study in animal infection models.
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.6084/m9.figshare.28737293.
SK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing - original draft, Writing - review and editing. HF: Funding acquisition, Resources, Supervision, Validation, Writing - review and editing. FR: Supervision, Writing - review and editing. VK: Supervision, Validation, Writing - review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. The results presented in this paper were part of SK’s Ph.D. thesis funded by a research grant from the Office of Vice-Chancellor for Research and Technology, Isfahan University of Medical Sciences, Isfahan, Iran.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd El-Rahman, O. A., Rasslan, F., Hassan, S. S., Ashour, H. M., and Wasfi, R. (2023). The RND efflux pump gene expression in the biofilm formation of Acinetobacter baumannii. Antibiotics 12 (2), 419. doi:10.3390/antibiotics12020419
Adeyemo, R. O., Famuyide, I. M., Dzoyem, J. P., and Lyndy Joy, M. (2022). Anti-biofilm, antibacterial, and anti-quorum sensing activities of selected South African plants traditionally used to treat diarrhoea. Evid. Based Complement. Altern. Med. 2022 (1), 1307801. doi:10.1155/2022/1307801
Aligiannis, N., Kalpoutzakis, E., Mitaku, S., and Chinou, I. B. (2001). Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 49 (9), 4168–4170. doi:10.1021/jf001494m
Al-Sallami, D., Alsultan, A., Abbas, K. H., and Clarke, S. R. (2023). Evaluation of efflux pump inhibitory activity of some plant extracts and using them as adjuvants to potentiate the inhibitory activity of some antibiotics against Staphylococcus aureus. Open Vet. J. 13 (1), 42–47. doi:10.5455/OVJ.2023.v13.i1.5
Amer, M. A., Wasfi, R., and Hamed, S. M. (2023). Biosurfactant from Nile Papyrus endophyte with potential antibiofilm activity against global clones of Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 13, 1210195. doi:10.3389/fcimb.2023.1210195
Assis, F. V. D., Siqueira, F. L., Goncalves, I. E., Lacerda, R. P., Nascimento, R. A., Araujo, S. G., et al. (2018). Antibacterial activity of Lamiaceae plant extracts in clinical isolates of multidrug-resistant bacteria. An. Acad. Bras. Ciências 90 (02), 1665–1670. doi:10.1590/0001-3765201820160870
Atta, S., Waseem, D., Fatima, H., Naz, I., Rasheed, F., and Kanwal, N. (2023). Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J. Biol. Sci. 30 (3), 103576. doi:10.1016/j.sjbs.2023.103576
Basak, S., Singh, P., and Rajurkar, M. (2016). Multidrug resistant and extensively drug resistant bacteria: a study. J. Pathog. 2016, 4065603. doi:10.1155/2016/4065603
Borrás-Linares, I., Stojanović, Z., Quirantes-Piné, R., Arráez-Román, D., Švarc-Gajić, J., Fernández-Gutiérrez, A., et al. (2014). Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 15 (11), 20585–20606. doi:10.3390/ijms151120585
Bouyahya, A., Chamkhi, I., Balahbib, A., Rebezov, M., Shariati, M. A., Wilairatana, P., et al. (2022). Mechanisms, anti-quorum-sensing actions, and clinical trials of medicinal plant bioactive compounds against bacteria: a comprehensive review. Molecules 27 (5), 1484.
Christopoulou, S. D., Androutsopoulou, C., Hahalis, P., Kotsalou, C., Vantarakis, A., and Lamari, F. N. (2021). Rosemary extract and essential oil as drink ingredients: an evaluation of their chemical composition, genotoxicity, antimicrobial, antiviral, and antioxidant properties. Foods 10 (12), 3143. doi:10.3390/foods10123143
Del Campo, J., Amiot, M.-J., and Nguyen-The, C. (2000). Antimicrobial effect of rosemary extracts. J. Food Prot. 63 (10), 1359–1368. doi:10.4315/0362-028x-63.10.1359
El Sayed, A. M., Basam, S. M., El-Naggar, E.-M. B. A., Marzouk, H. S., and El-Hawary, S. (2020). LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 10 (1), 17778. doi:10.1038/s41598-020-74440-y
Górniak, I., Bartoszewski, R., and Króliczewski, J. (2019). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272. doi:10.1007/s11101-018-9591-z
Günther, M., Karygianni, L., Argyropoulou, A., Anderson, A. C., Hellwig, E., and Skaltsounis, A. L. (2022). The antimicrobial effect of Rosmarinus officinalis extracts on oral initial adhesion ex vivo. Clin. Oral Invest. 26, (6) 4369–4380.
Haji, S. H., Ali, F. A., and Aka, S. T. H. (2022). Synergistic antibacterial activity of silver nanoparticles biosynthesized by carbapenem-resistant Gram-negative bacilli. Sci. Rep. 12 (1), 15254. doi:10.1038/s41598-022-19698-0
Hassan, M., Bala, S. Z., Bashir, M., Waziri, P. M., Musa Adam, R., Umar, M. A., et al. (2022). LC-MS and GC-MS profiling of different fractions of Ficus platyphylla stem bark ethanolic extract. J. Anal. Methods Chem. 2022 (1), 6349332. doi:10.1155/2022/6349332
Kafa, A. H. T., Aslan, R., Celik, C., and Hasbek, M. (2022). Antimicrobial synergism and antibiofilm activities of Pelargonium graveolens, Rosemary officinalis, and Mentha piperita essential oils against extreme drug-resistant Acinetobacter baumannii clinical isolates. Z. Naturforsch. C 77 (3-4), 95–104. doi:10.1515/znc-2021-0079
Kaur, J., Dhiman, V., Bhadada, S., Katare, O., and Ghoshal, G. (2022). LC/MS guided identification of metabolites of different extracts of Cissus quadrangularis. Food Chem. Adv. 1, 100084. doi:10.1016/j.focha.2022.100084
Khashei, S., Etemadifar, Z., and Rahmani, H. R. (2018). Immobilization of Pseudomonas putida PT in resistant matrices to environmental stresses: a strategy for continuous removal of heavy metals under extreme conditions. Ann. Microbiol. 68, 931–942. doi:10.1007/s13213-018-1402-7
Lahiri, D., Dash, S., Dutta, R., and Nag, M. (2019). Elucidating the effect of anti-biofilm activity of bioactive compounds extracted from plants. J. Biosci. 44 (2), 52. doi:10.1007/s12038-019-9868-4
Lin, L., Dong, Y., Zhao, H., Wen, L., Yang, B., and Zhao, M. (2011). Comparative evaluation of rosmarinic acid, methyl rosmarinate and pedalitin isolated from Rabdosia serra (MAXIM.) HARA as inhibitors of tyrosinase and α-glucosidase. Food Chem. 129 (3), 884–889. doi:10.1016/j.foodchem.2011.05.039
Lu, W.-J., Lin, H.-J., Hsu, P.-H., and Lin, H.-T. V. (2020). Determination of drug efflux pump efficiency in drug-resistant bacteria using MALDI-TOF MS. Antibiotics 9 (10), 639. doi:10.3390/antibiotics9100639
Manilal, A., Sabu, K. R., Woldemariam, M., Aklilu, A., Biresaw, G., Yohanes, T., et al. (2021). Antibacterial activity of Rosmarinus officinalis against multidrug-resistant clinical isolates and meat-borne pathogens. Evid. Based Complement. Altern. Med. 2021 (1), 6677420. doi:10.1155/2021/6677420
Mena, P., Cirlini, M., Tassotti, M., Herrlinger, K. A., Dall’Asta, C., and Del Rio, D. (2016). Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 21 (11), 1576. doi:10.3390/molecules21111576
Moussi, K., Nayak, B., Perkins, L. B., Dahmoune, F., Madani, K., and Chibane, M. (2015). HPLC-DAD profile of phenolic compounds and antioxidant activity of leaves extract of Rhamnus alaternus L. Ind. Crops Prod. 74, 858–866. doi:10.1016/j.indcrop.2015.06.015
Nassar, R., Nassar, M., Vianna, M. E., Naidoo, N., Alqutami, F., Kaklamanos, E. G., et al. (2021). Antimicrobial activity of phytic acid: an emerging agent in endodontics. Front. Cell. Infect. Microbiol. 11, 753649. doi:10.3389/fcimb.2021.753649
Nocera, F. P., Attili, A.-R., and De Martino, L. (2021). Acinetobacter baumannii: its clinical significance in human and veterinary medicine. Pathogens 10 (2), 127. doi:10.3390/pathogens10020127
Norbury, W., Herndon, D. N., Tanksley, J., Jeschke, M. G., and Finnerty, C. C. (2016). Infection in burns. Surg. Infect. 17 (2), 250–255. doi:10.1089/sur.2013.134
Ramadevi, S., Kaleeswaran, B., Ilavenil, S., Upgade, A., Tamilvendan, D., and Rajakrishnan, R. (2020). Effect of traditionally used herb Pedalium murex L. and its active compound pedalitin on urease expression-For the management of kidney stone. Saudi J. Biol. Sci. 27 (3), 833–839.
Romo-Castillo, M., Flores-Bautista, V. A., Guzmán-Gutiérrez, S. L., Reyes-Chilpa, R., León-Santiago, M., and Luna-Pineda, V. M. (2023). Synergy of plant essential oils in antibiotic therapy to combat Klebsiella pneumoniae infections. Pharmaceuticals 16 (6), 839. doi:10.3390/ph16060839
Roy, S., Chowdhury, G., Mukhopadhyay, A. K., Dutta, S., and Basu, S. (2022). Convergence of biofilm formation and antibiotic resistance in Acinetobacter baumannii infection. Front. Med. 9, 793615. doi:10.3389/fmed.2022.793615
Sa-Eed, A., Donkor, E. S., Arhin, R. E., Tetteh-Quarcoo, P. B., Attah, S. K., Kabotso, D. E., et al. (2023). In vitro antimicrobial activity of crude propolis extracts and fractions. FEMS Microbes 4, 1–8. doi:10.1093/femsmc/xtad010
Samarkos, M., Papanikolaou, K., Sourdi, A., Paisios, N., Mainas, E., Paramythiotou, E., et al. (2022). The effect of different colistin dosing regimens on nephrotoxicity: a cohort study. Antibiotics 11 (8), 1066. doi:10.3390/antibiotics11081066
Sangalli-Leite, F., Scorzoni, L., da Silva, J. d.F., de Oliveira, H. C., de Lacorte Singulani, J., Gullo, F. P., et al. (2016). Synergistic effect of pedalitin and amphotericin B against Cryptococcus neoformans by in vitro and in vivo evaluation. Int. J. Antimicrob. Agents 48 (5), 504–511. doi:10.1016/j.ijantimicag.2016.07.025
Saxena, D., Maitra, R., Bormon, R., Czekanska, M., Meiers, J., Titz, A., et al. (2023). Tackling the outer membrane: facilitating compound entry into Gram-negative bacterial pathogens. npj Antimicrob. Resist. 1 (1), 17. doi:10.1038/s44259-023-00016-1
Simoes, M., Bennett, R. N., and Rosa, E. A. (2009). Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat. Prod. Rep. 26 (6), 746–757. doi:10.1039/b821648g
Slobodníková, L., Fialová, S., Rendeková, K., Kováč, J., and Mučaji, P. (2016). Antibiofilm activity of plant polyphenols. Molecules 21 (12), 1717. doi:10.3390/molecules21121717
Spellberg, B., and Bonomo, R. A. (2015). Combination therapy for extreme drug–resistant Acinetobacter baumannii: ready for prime time? Crit. Care Med. 43 (6), 1332–1334. doi:10.1097/CCM.0000000000001029
Vaou, N., Stavropoulou, E., Voidarou, C. C., Tsakris, Z., Rozos, G., Tsigalou, C., et al. (2022). Interactions between medical plant-derived bioactive compounds: focus on antimicrobial combination effects. Antibiotics 11 (8), 1014. doi:10.3390/antibiotics11081014
Yang, B., Lei, Z., Zhao, Y., Ahmed, S., Wang, C., Zhang, S., et al. (2017). Combination susceptibility testing of common antimicrobials in vitro and the effects of sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front. Microbiol. 8, 2125. doi:10.3389/fmicb.2017.02125
Yilmaz, S., Altinkanat-Gelmez, G., Bolelli, K., Guneser-Merdan, D., Over-Hasdemir, M., Yildiz, I., et al. (2014). Pharmacophore generation of 2-substituted benzothiazoles as AdeABC efflux pump inhibitors in A. baumannii. SAR QSAR Environ. Res. 25 (7), 551–563. doi:10.1080/1062936X.2014.919357
Zhang, S., Wang, J., and Ahn, J. (2023). Advances in the discovery of efflux pump inhibitors as novel potentiators to control antimicrobial-resistant pathogens. Antibiotics 12 (9), 1417. doi:10.3390/antibiotics12091417
Zhong, X., Wang, X., Zhou, N., Li, J., Liu, J., Yue, J., et al. (2021). Chemical characterization of the polar antibacterial fraction of the ethanol extract from Rosmarinus officinalis. Food Chem. 344, 128674. doi:10.1016/j.foodchem.2020.128674
Keywords: Acinetobacter baumannii, drug resistance, biofilms, Rosmarinus officinalis, liquid chromatography-mass spectrometry
Citation: Khashei S, Fazeli H, Rahimi F and Karbasizadeh V (2025) Antibiotic-potentiating efficacy of Rosmarinus officinalis L. to combat planktonic cells, biofilms, and efflux pump activities of extensively drug-resistant Acinetobacter baumannii clinical strains. Front. Pharmacol. 16:1558611. doi: 10.3389/fphar.2025.1558611
Received: 10 January 2025; Accepted: 24 March 2025;
Published: 08 April 2025.
Edited by:
Karl Hassan, The University of Newcastle, AustraliaReviewed by:
Aimé Gabriel Fankam, University of Dschang, CameroonCopyright © 2025 Khashei, Fazeli, Rahimi and Karbasizadeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hossein Fazeli, aF9mYXplbGlAbWVkLm11aS5hYy5pcg==; Fateh Rahimi, Zi5yYWhpbWlAc2NpLnVpLmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.