
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 25 March 2025
Sec. Experimental Pharmacology and Drug Discovery
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1555732
This article is part of the Research TopicAdvancements in P2X7 Antagonists: From Mechanistic Insights to Therapeutic ApplicationsView all articles
Neuropathic pain (NP) is a common symptom of many diseases and is caused by direct or indirect damage to the nervous system. Tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors are typical drugs used in clinical practice to suppress pain. However, these drugs have drawbacks, including a short duration of action, a limited analgesic effect, and possible dependence and side effects. Therefore, developing more effective NP treatment strategies has become a priority in medical research and has attracted much research attention. P2X7 receptor (P2X7R) is a non-selective cation channel activated by adenosine triphosphate and is mainly expressed in microglia in the central nervous system. Microglial P2X7R plays an important role in pain regulation, suggesting that it could be a potential target for drug development. This review comprehensively and objectively discussed the latest research progress of P2X7R, including its structural characteristics, functional properties, relationship with microglial activation and polarization, mechanism of action, and potential therapeutic strategies in multiple NP models. This study aimed to provide in-depth insights into the association between P2X7R and NP and explore the mechanism of action of P2X7R in the pathological process of NP and the translational potential and clinical application prospects of P2X7R antagonists in pain treatment, providing a scientific basis for the precise treatment of NP.
Pain is an unpleasant sensory and emotional experience caused by actual or potential tissue damage (De Ridder et al., 2021). It is a warning indication of tissue damage and is transmitted from the periphery to the brain via a network of specialized receptors and nerve fibers (Mulè et al., 2024). Pain induced by pathological conditions such as cancer, infection, diabetes, and trauma is sometimes called neuropathic pain (NP) (Borges et al., 2021). In 2019, the International Association for the Study of Pain (IASP) updated the definition of NP to “pain caused by lesions or diseases of the somatic sensory nervous system” (Scholz et al., 2019). Neuropathic pain was previously defined as “pain triggered or caused by primary lesions, dysfunctions, or temporary disturbances of the peripheral or central nervous system” (Vervest and Schimmel, 1988). This definition incorporates two important changes: functional impairment and neuronopathy. Functional impairment is no longer recognized as a criterion under the revised definition. Consequently, it is believed that NP occurrence is associated with abnormalities in the pain transmission and perception of the nervous system. When neurons are damaged, their excitability increases, making them more susceptible to depolarization from even slight stimulation, leading to abnormal pain signal transmission (Koga et al., 2023). Neuropathic pain is commonly described as severe burning, stabbing, squeezing, or freezing pain (Pickering et al., 2017). These pain attacks can occur alone or alongside persistent pain, which is often unbearable for patients and seriously affects their quality of life (Torres et al., 2022). Currently, IASP recommends tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, gabapentin, and pregabalin as first-line drugs for NP treatment (Finnerup et al., 2015). However, these drugs exhibit varying degrees of side effects, and the therapeutic effects are not ideal (Finnerup et al., 2015). As a result, developing new strategies or drugs that have minimal side effects and can achieve long-term effects with a single dose is an urgent need.
Following nerve injury, several molecular and cellular changes occur in the peripheral and central nervous systems. Among these, ATP and its purinergic receptors have been extensively studied. The concept of purinergic signaling was first proposed in 1972 (Burnstock, 1972), and purinergic receptors were defined in 1976 (Burnstock, 1976). Purinergic receptors are widely involved in immune responses, inflammation, and pain pathways. In 1993, the first P2 purinergic receptor was successfully cloned (Lustig et al., 1993). It is classified into two subtypes: ion-gated receptors (P2X receptors [P2XRs]) and G protein-coupled receptors (P2Y receptors [P2YRs]) (Kohno and Tsuda, 2021). The most characteristic mechanism of P2XR activity is their high permeability to Ca2+ (Khakh and North, 2012). Activation of P2XRs causes an increase in intracellular Ca2+ and a depolarization wave, resulting in signal transmission. Additionally, P2XR signaling involves functional interactions with other ion channels, including K+ efflux and Na+ influx (Khakh and North, 2012). The P2X7 receptor is of particular interest not only because it is expressed primarily in cells of the hematopoietic lineage and glial cells but also because it has a relatively low affinity for ATP, with maximal activity being achieved at ATP concentrations found under pathological conditions (100–1,000 µM). Its prolonged activation results in the formation of transient pores in the cell membrane, which are permeable to soluble substances up to 900 Da, potentially causing cytotoxicity (Abbracchio et al., 2009). Furthermore, it activates several intracellular signal transduction pathways, including mitogen-activated protein kinase (MAPK), calcineurin (CN), and nuclear factor-κβ (NF-κβ) (Skaper et al., 2010). Accordingly, this receptor may play a special role under pathological conditions. Moreover, P2X7Rs perform several physiological functions in the central nervous system, including cell proliferation, cell growth, neurotransmitter release, and neuron-glia communication (Skaper et al., 2010) (Sperlágh and Illes, 2014) (Tewari and Seth, 2015). Consequently, P2X7Rs are believed to play a key role in the occurrence and development of NP (Godoy et al., 2019).
Microglia are the primary glial cell type responsible for the occurrence and development of NP (Zhou et al., 2021) (Kim et al., 2021) (Yuan et al., 2023). They are the most common type of immune cells in the central nervous system (Borst et al., 2021). Microglia can regulate neuron excitability and inhibition, thereby regulating neuronal network activity (Villasana-Salazar and Vezzani, 2023). The mechanism of interaction between glial cells and neurons is complex. Activated microglia can polarize into M1 type (promoting inflammatory response and exerting cytotoxic effects) or M2 type (inhibiting inflammatory response and producing tissue protection effects) and release a variety of glial transmitters and cytokines, thereby affecting the excitability and sensory transmission of surrounding neurons (Zhang et al., 2024). This interaction may be crucial in the occurrence and maintenance of NP. Activation of P2X7R on microglia can increase calcium ions in microglia, increase microglial excitability, lead to phosphorylation of calmodulin-dependent protein kinase, and activate the synthesis and release of multiple cytokines, causing changes in the local inflammatory microenvironment (Martel-Gallegos et al., 2013). Microglial activation triggers the release of inflammatory factors such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNF-α) (Yang et al., 2024). These mediators play a key role in the occurrence and maintenance of NP (Clark et al., 2006) (Zhang et al., 2018). Additionally, inhibiting P2X7R activation can inhibit the abnormal excitability and inflammatory response of microglia, thereby effectively alleviating the symptoms of NP (Tsuda, 2017) (Xie et al., 2017). Recently, more studies have revealed that microglia play an important role in neurodevelopment and neurodegenerative diseases. For example, microglia play a key role in the inflammatory response and neuroprotection in diseases such as Alzheimer’s disease, Parkinson’s disease, and spinal cord injury (Zhang et al., 2024) (Finneran et al., 2019) (Cignarella et al., 2020). However, the specific mechanism by which P2X7R acts on glial cells to cause and maintain NP is yet to be elucidated. Exploring the specific mechanism of action of P2X7R on microglia in NP is expected to lead to breakthroughs and treatment options for NP.
Neuropathic pain is a condition caused by direct or indirect damage, dysfunction, or transient disorder of the central or peripheral nervous system. The pain sensation persists even after the noxious stimulus has been withdrawn. Nerve severance or damage, metabolic imbalance, viral infection, or an autoimmune response are all potential causes of NP. Symptoms include paroxysmal or persistent spontaneous pain, allodynia, and hypersensitivity to touch, cold, and heat (Ciapała and Mika, 2023). Many cases progress to chronic pain, and in many patients, they cause comorbidities of mood disorders such as anxiety and depression (Mokhtari and Uludag, 2024), causing significant suffering. According to recent statistics, the global incidence of chronic NP has reached 6.9%–10%, which has caused a significant burden on patients’ lives and socioeconomic status (Scholz et al., 2019). However, no effective clinical treatment strategy is currently available, and the commonly used treatment drugs are still opioids, antidepressants, and antiepileptic drugs. (Finnerup et al., 2015). These drugs are often ineffective for most patients—effective for a small number of patients and accompanied by side effects (Finnerup et al., 2021). Recently, with in-depth research on the mechanism of NP, its pathological process has been widely recognized. It mainly includes the following aspects: (De Ridder et al., 2021): Nociceptor sensitization and pain fiber induction: Nerve damage causes nerve fiber loss and axonal demyelination, resulting in abnormal pain signal transmission (Iwata et al., 2024). (Mulè et al., 2024) Activation of spinal microglia and release of inflammatory mediators: The activity of inflammatory factors and glial cells facilitates neuroinflammation, inhibits nerve regeneration, and alters pain thresholds (Xu et al., 2020). (Borges et al., 2021) Abnormal discharge of sensory afferent neurons: Neurons discharge abnormally following spinal cord injury, resulting in changes in low- and high-threshold mechanoreceptors (Boada et al., 2019). (Scholz et al., 2019) Neurotransmitter release: The effects of glutamatergic neurotransmitters and associated ion channels affect the transmission of pain signals (Alexander et al., 2022). (Vervest and Schimmel, 1988) Enhanced synaptic transmission: Enhanced synaptic function may aggravate pain (Ko et al., 2023). (Koga et al., 2023) Infiltration of inflammatory cells and release of inflammatory mediators in the injured area aggravate nerve damage and pain (Makabe et al., 2024). Overall, significant progress has been made in understanding the pathological mechanism of NP; however, its specific mechanism requires further in-depth research.
As research into NP progresses, studies have discovered that P2X7R, which is widely distributed throughout the nervous system, plays a vital role in the occurrence and development of NP. Notably, pharmacological blockade of P2X7R-mediated microglial polarization has emerged as a promising therapeutic strategy for modulating NP.
The P2X7R gene is located on 12q24 and has a total length of approximately 50 kb, including 13 exons (Figure 1). Its mRNA is 3,155 bp long and comprises 595 amino acids. It has an amino-terminal (N-terminal), a carboxyl-terminal (C-terminal), an intracellular domain, and two transmembrane domains (TM1 and TM2) with conserved extracellular loops (Surprenant et al., 1996). The C-terminus of P2X7R is approximately 200 amino acids longer than other known P2XRs and is not present in other P2XRs (Surprenant et al., 1996). It also determines its unique physiological function. The P2X7R protein usually forms trimers on the cell membrane and sometimes exists as oligomers (McCarthy et al., 2019). The main physiological function of the P2X7R is to form ion channels and plasma membrane pores on the cell membrane surface, which aid in signal transduction, cytokine secretion, and cell proliferation (Sluyter, 2017). The activation of the P2X7R has a bidirectional effect under different stimulation conditions. When low concentrations of extracellular agonists (ATP) stimulate the P2X7R, it can quickly open a transmembrane ion channel that allows small cations such as Na+, K+, and Ca2+ to pass through, promoting the influx of Na+ and Ca2+ and the efflux of K+. Under long-term stimulation of high concentrations of agonists, the P2X7R will form non-selective plasma membrane pores, allowing molecules with a relative molecular mass of less than 900 Da to pass through, increasing the permeability of the cell membrane and ultimately inducing cell death (Di Virgilio et al., 2018). Importantly, ATP concentration influences presynaptic and postsynaptic P2X7R activation, thereby regulating the dynamics of pain signaling (Wu et al., 2022). In a healthy state, ATP levels are typically low, allowing P2X7R to remain physiologically inactive. Therefore, P2X7R is primarily activated when the endogenous ligand ATP concentration exceeds 100 μM (Huang et al., 2014). P2X7R targets the presynaptic terminals of the central and peripheral nervous systems. Activation of P2X7R promotes the release of norepinephrine, acetylcholine and other neurotransmitters from the presynaptic terminals (Deuchars et al., 2001), thereby increasing the excitability of the nervous system. Additionally, P2X7R regulates intercellular communication between neurons and glial cells, especially during inflammation. For example, microglia release exosomes after P2X7R activation, and the inflammatory signals contained within them are transmitted to neurons (Delpech et al., 2019). Furthermore, the activation of microglial P2X7R can cause P2X4 receptors to translocate to the surface and release factors such as IL-1β and derived nerve growth factor (BDNF), affecting neuron excitability and triggering pain perception (Trang et al., 2009).
P2X7 receptor is a major driver of inflammation; it is widely expressed in cells of the innate and adaptive immune systems in animals and humans (Di Virgilio et al., 2018). P2X7 receptor is primarily localized in the microglia of the central nervous system, the resident macrophages in the brain (Bhattacharya and Jones, 2018). However, glial cells such as astrocytes and oligodendrocytes also express these receptors, albeit at lower densities (Illes et al., 2012) (Zhao et al., 2021). Importantly, it is closely associated with pain responses. P2X7 receptor participates in the extracellular signal-regulated kinase 1 (ERK1) signaling pathway in satellite glial cells to mediate pain responses and is regulated by transient receptor potential vanilloid receptors 1 (TRPV1) and 4 (TRPV4) (Fan et al., 2021) (Wang et al., 2021). Recently, Wu Y. et al. (2021) established a chronic constriction injury model and discovered a novel transcriptional mechanism in which BDNF promoted P2X7R expression in dorsal root ganglion neurons. When neuroinflammation occurs, P2X7R expression increases. P2X7 receptor inhibition can alleviate hyperalgesia and reduce astrocyte and microglial activation in the L5 dorsal horn (Hu et al., 2020). In summary, P2X7R is an important molecule that is widely expressed throughout the body and is particularly concentrated in microglia.
Microglia are the main immune cells resident in the central nervous system. They are macrophages abundant in the central nervous system and are significantly involved in the cellular immune process of the central nervous system (Fang and Lai, 2024). Microglia, namely the M0 type, are normally quiescent. In this state, they constantly sense environmental changes and respond to invading pathogens, toxins, and cell debris, acting as immune monitors (Chen et al., 2025). Activated microglia respond differently to external stimuli and act as a “double-edged sword.” Acutely activated microglia promote tissue repair by removing invading pathogens and cell debris, whereas persistently activated microglia induce chronic neuroinflammation and promote disease progression. Microglia in the central nervous system play a significant role in pain regulation owing to their unique functions (Du et al., 2020). P2X7 receptor-mediated microglial activation and polarization are often closely related to the occurrence, development, and regulation of inflammation, and these activation and polarization states can affect the inflammatory process (Dworsky-Fried et al., 2020). P2X7 receptor plays a key role in microglial activation (Fernandes et al., 2016). Its overexpression can increase microglial membrane permeability, boost microglial proliferation, promote inflammatory factor release, and cause further neuronal damage (Zhang et al., 2020). Activation of P2X7R can cause the cell membrane to form non-selective cation channels, allowing Na+ and Ca2+ influx and K+ efflux (Sperlágh and Illes, 2014; Di Virgilio et al., 2017). This increase in calcium influx alters the intracellular calcium ion balance, thereby affecting cell signal transduction and cytokine secretion. Additionally, P2X7R activation is associated with calcium-dependent signaling pathways, including the activation of phospholipase A2, phospholipase D, MAPK, and NF-κB (Chen et al., 2022; Carroll et al., 2009; Shiratori et al., 2010; Liu et al., 2011). The activation of these signaling pathways regulates microglial function and responsiveness.
Microglia can polarize into two phenotypes in response to injury and inflammatory stimuli: M1, which is pro-inflammatory and damaging, and M2, which is anti-inflammatory and protective (Lan et al., 2017) (Scott et al., 2021) (Caffarel and Braza, 2022). Polarized M1-type microglia release pro-inflammatory factors and harmful mediators, aggravating inflammatory cell infiltration, whereas M2-type microglia reduce the inflammatory response by releasing anti-inflammatory factors (Atta et al., 2023). Acute and controlled activation of microglia promotes neuroprotection and neural repair, whereas chronic activation of microglia usually leads to excessive production of neurotoxic cytokines (M1 type polarization) and other inflammatory mediators (Au and Ma, 2022). These polarized microglia release different signaling molecules that exert different effects on target neurons to regulate pain. Inducers such as TNF-α, interferon-γ, and lipopolysaccharide cause microglia to polarize to the M1 phenotype (Xia et al., 2022), expressing specific markers such as CD86, CD32, and CD16 surface determinants, increasing the expression of inducible nitric oxide synthase (iNOS) and secreting pro-inflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α (Higashi et al., 2017), exacerbating pain. M2 polarization is induced by IL-4 and transforming growth factor-β (Radpour et al., 2023) and is characterized by the expression of CD163 and CD206, upregulation of arginase-1, and secretion of IL-10, CNTF-1, IGF-1, and NGF-1, which help relieve pain (Si et al., 2024).
P2X7R activation is closely related to M1 microglia, and P2X7R is involved in the development and progression of microglia-mediated inflammation. P2X7 receptor-mediated Ca2+ influx is a key step in activating the NLRP3 inflammasome. The assembly of the NLRP3 inflammasome leads to the activation of caspase-1, which promotes the maturation and release of pro-inflammatory cytokines IL-1β and IL-18. These cytokines are important markers of M1 microglia and can further enhance neuroinflammatory responses (Ren and Illes, 2022). Additionally, P2X7R activation drives M1 polarization by promoting the activation of the NF-κB signaling pathway (Zhang et al., 2022). NF-κB activation results in the transcription of multiple pro-inflammatory genes, including TNF-α, IL-6, and iNOS, which are highly expressed in M1 microglia (Wu et al., 2022). Furthermore, P2X7R can be used as a potential molecular target for M1 microglial positron emission tomography imaging (Van Weehaeghe et al., 2019). P2X7 receptor regulates M1/M2 polarization of rat microglia in inflammation caused by brain injury and chronic sciatic nerve constriction injury (Gui et al., 2020) (Song et al., 2023). Small interfering RNA (siRNA)-mediated knockdown of P2X7R (Wu et al., 2022) or the use of P2X7R inhibitors (Gui et al., 2020) can effectively switch microglial polarization from M1 to M2, effectively alleviating pain. These indicate that P2X7R expression can influence the severity and appearance of pain by regulating microglial polarization direction (Figure 2).
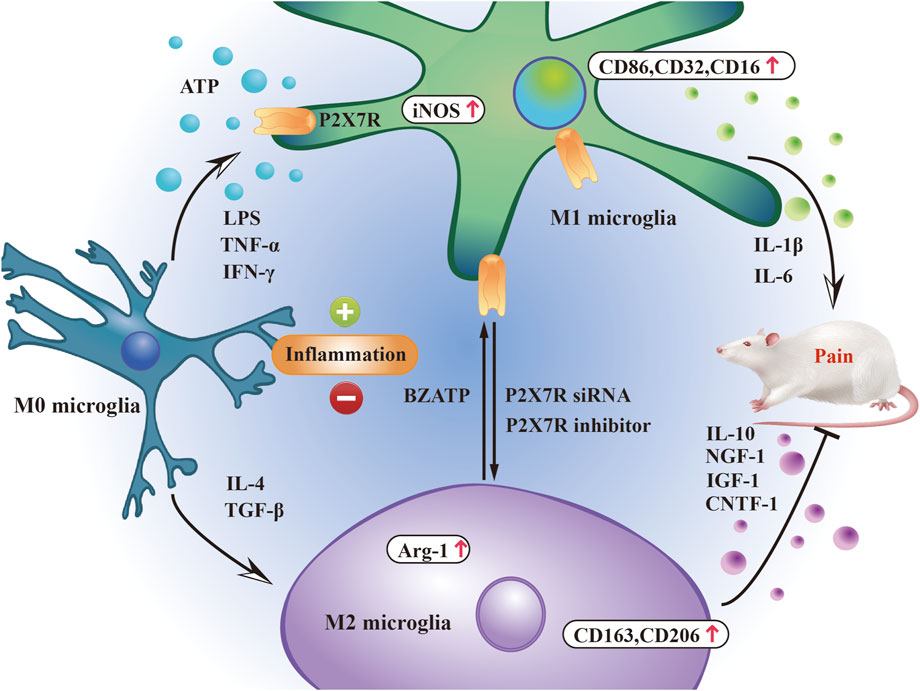
Figure 2. P2X7R participates in pain regulation by regulating microglial phenotype. The balance between pro- and anti-inflammatory mediators released by M1/M2 microglia is closely related to the pain state induced by P2X7R.
P2X7 receptor may play a key role in pain and inflammation. Following sciatic nerve injury, P2X7R expression increases significantly, which is related to the occurrence of hyperalgesia (Yu et al., 2018). Sciatic nerve injury causes sensory nerve fibers to transmit signals to primary spinal cord centers, upregulating P2X7R expression in spinal microglia and inducing hyperalgesia (Masoodifar et al., 2021). Researchers have observed NP relief in many experimental models by inhibiting P2X7R using drugs such as A-438079, ox-ATP, or A-839977 (Zhou et al., 2023) (Bianco et al., 2012) (Honore et al., 2009). Upregulation of P2X7R expression and its activation in damaged nerves promotes IL-1β maturation and release, an upstream mechanism for pain signaling (Luchting et al., 2016). Clark et al. (2010) discovered a relationship between P2X7R activation and p-38/MAPK-dependent protease release in microglia and proposed that inhibiting these proteases may be another mechanism by which P2X7R antagonists alleviate NP. Additionally, P2X7R activation leads to the release of several diffusible factors from microglia, including TNF-α, iNOS, prostaglandin E2, cyclooxygenase 2, and BDNF, all of which are closely related to NP development (Inoue, 2017). P2X7 receptor-induced pore formation may also trigger a cascade of downstream effects that may be involved in the generation of pain hypersensitivity. Consequently, attenuating pore formation without interfering with cation channel activity may provide a new strategy for alleviating chronic pain (Sorge et al., 2012). Furthermore, P2X7R contributes to the development of tactile allodynia and nociceptive hypersensitivity in models of chronic constriction injury (CCI), spinal nerve ligation (SNL), diabetic neuropathic pain (DNP), and selective spinal nerve injury (SNI). The functional differences of P2X7 receptors in different neuropathic pain models are mostly reflected in the intensity and mechanism of their effects on microglial activation, neuronal excitability regulation, and inflammatory factor release. These differences may be closely linked to factors such as the degree of nerve damage, inflammatory response characteristics, and nerve regeneration process of each model.
Bennett and Xie (1988) first developed the CCI in rats to mimic human NP. The model was made by separating the biceps femoris from the gluteal muscles, fully exposing and isolating the sciatic nerve, and ligating four loose chromic gut sutures with a 1 mm spacing around them. This model is easy to operate surgically, has mechanical and thermal allodynia, and exhibits the clinical features of NP and an inflammatory component (Zhi et al., 2017). In the CCI model, He et al. (2012) discovered that changes in spinal P2X7R levels were consistent with the development of mechanical hyperalgesia and thermal hypersensitivity and that intrathecal injection of the P2X7R antagonist brilliant blue G (BBG) reversed the CCI-induced mechanical hyperalgesia and thermal hypersensitivity. Their results revealed that P2X7 receptors in the spinal cord play a key role in microglial activation, which may have a significant impact on the formation and development of mechanical hyperalgesia and thermal hypersensitivity in the CCI model. According to Jiang et al. (2016), riluzole inhibits microglial activation by reducing P2X7R expression in the spinal dorsal horn, thereby alleviating NP. Similarly, Lin et al. (2018) used an intrathecal injection of dexmedetomidine to reduce CCI-induced mechanical and thermal hyperalgesia in rats, attributing its analgesic effect to its ability to downregulate spinal P2X7R expression and inhibit ERK phosphorylation. Further studies are needed to elucidate the exact molecular mechanism by which dexmedetomidine regulates the P2X7R and ERK signaling pathways. Furthermore, clinical trials are required to evaluate the efficacy and safety of dexmedetomidine in the treatment of NP in humans. Xu et al. (2016) discovered that the P2X7R agonist Bz-ATP promotes microglial P2X7R expression and the release of inflammatory factors IL-1β and IL-18, ultimately inducing pain. Liu et al. (2023) discovered that Echinacoside inhibits microglial overactivation and inflammation via the spinal P2X7R/FKN/CX3CR1 signaling pathway, thereby alleviating peripheral NP. Additionally, Li et al. (2023) discovered that inhibiting P2X7R reduced glutamate levels in the thalamus and cortical regions, whereas glutamate levels in CCI rats were significantly increased. The findings of this study demonstrate that NP has a significant impact on sleep-related neural activity and may establish new targets for developing sleep disorders under chronic pain conditions. As a consequence, modulation of microglial P2X7R is expected to be an innovative therapeutic strategy for pain relief.
Decosterd and Woolf (2000) established an SNL model of peripheral neuropathic pain with significant research significance. This model involves ligating the spinal nerves L5 and L6 and inducing nerve injury. This model is appropriate for mechanical hypersensitivity because mechanical, heat, and cold allodynia occur rapidly and continue for a long time following surgery (Chen and Lu, 2017) (Moriarty et al., 2016). Peripheral nerve injury can cause the release of local neurotrophic factors, neurotransmitters, chemokines, and cytokines, which induce NP by reducing pain receptor activation thresholds (Liao et al., 2022). The synaptic plasticity of spinal dorsal horn neurons in SNL rats is altered (Bittar et al., 2017). Wu Q. et al. (2021) discovered that electroacupuncture (EA) treatment reduced dendritic spine density, inhibited synaptic remodeling, and reduced inflammatory responses, and was associated with reduced P2X7R expression and improved neurobehavior. The P2X7R agonist Bz-ATP reversed the positive effects of EA, which was consistent with increased P2X7R expression. These findings suggest that EA improves NP by inhibiting P2X7R expression, which reduces abnormal dendritic spine/synaptic remodeling and inflammation. However, they only hypothesized that EA exerts analgesic effects by reducing P2X7R expression, and it would be best to use P2X7R inhibitors or siRNA to confirm this mechanism. Yamashita et al. (2021) discovered that cilnidipine effectively inhibited the Ca2+ signaling response and IL-1β release triggered by P2X7 receptors in primary cultures of rat microglia. Furthermore, in a rat experimental model simulating NP, an intrathecal injection of cilnidipine effectively reversed the mechanical hyperalgesia caused by nerve injury, a primary symptom of NP.
Diabetic neuropathic pain is a major complication of type I and type II diabetes (Calcutt, 2020). The clinical features of DNP include spontaneous pain, allodynia, and hyperalgesia (Sloan et al., 2021). The main models studied include rats and mice induced by streptozotocin and alloxan, mice fed a high-fat diet, diet and nutritional combination-induced models, transgenic models, and gene knockout diabetes models, including Zucker diabetic obese rats, BBZDP/Wor rats with characteristics of type 1 and type 2 diabetes, type 2 hyperinsulinemia diabetic BBZDR/Wor rats, and leptin-deficient mice (Gao and Zheng, 2014). Transient receptor potential vanilloid receptor 1 is an ion channel activated by heat and inflammatory factors and is involved in developing various pains (Dewaker et al., 2023). Chen et al. (2022) discovered that small interfering RNA (siRNA) of P2X7R and inhibitor of P38 significantly reduced TRPV1 expression by inhibiting the PKC/P38/MAPK/NF-κB signaling pathway and inflammatory factors in the dorsal root ganglia (DRG). Intrathecal injection of P2X7 shRNA can reduce the nociceptive response in rats with diabetic neuropathic pain by activating the PKC/P38 MAPK/NF-κB signaling pathway and TRPV1. Diabetic neuropathic pain and major depressive disorder (MDD) are common complications of diabetes that influence each other (Duan-Porter et al., 2016). Guan et al. (2019) discovered that dihydromyricetin reduced P2X7R expression in rats with DNP and MDD, downregulated ERK1/2 pathway activation, and reduced the release of inflammatory factors TNF-α and IL-1β. These effects ultimately alleviated DNP and depressive behavior. Subsequently, Zhan et al. (Zhan et al., 2024) found that shRNA of long non-coding RNA MSTRG.81401 alleviated pain and depressive-like behavior in diabetic rats with DNP and MDD comorbidity by inhibiting the hippocampal P2X7R-mediated P2X7R/NLRP3/caspase1 cell pyroptosis pathway and pro-inflammatory response. However, these studies share a common limitation: Because of the complexity of DNP and MDD, rat models cannot fully simulate human symptoms, capture the psychological and emotional aspects of pain experienced by human patients, and fully replicate the complex cognitive and social impairments of human patients.
Decosterd and Woolf (2000) established the SNI model of neuropathic pain in 2000. The SNI model exhibits good mechanical and thermal allodynia, and the duration is longer than other NP models. However, the heat threshold does not change significantly. It is the model closest to clinical neuropathic pain and is suitable for studying the mechanism of clinical treatment strategies for NP (Boccella et al., 2018). Hu et al. (2020) discovered that microinfusion of a P2X7R antagonist (A-438079) into the amygdala of rats significantly reduced the increase in ionized calcium-binding adaptor molecule-1 and glial fibrillary acidic protein (GFAP) immunoreactivity in microglia and astrocytes following SNI. Additionally, A-438079 significantly reduced the decrease in growth-associated protein-43 expression in the spinal cord caused by SNI. However, when A-438079 and a P2X7R agonist (BzATP) were administered simultaneously, all of the effects of A-438079 were reversed. This study suggests that inhibiting P2X7R in the amygdala can protect against NP-related symptoms. This effect may be associated with its inhibition of active microglia and astrocytes in the spinal cord. Zhou et al. (2023) discovered that after SNI, P2X7R expression in the dorsal horn of the rat spinal cord increased over time. Continuous intrathecal injection of the cannabinoid receptor 2 (CB2R) agonist PM226A resulted in a significant decrease in P2X7 protein levels. Furthermore, administering the P2X7R inhibitor A-438079 reduced the symptoms of mechanical hyperalgesia in rats, the number of microglia, and P2X7R expression. These findings suggest that P2X7R is crucial in the neuroprotective process triggered by CB2R activation (Figure 3).
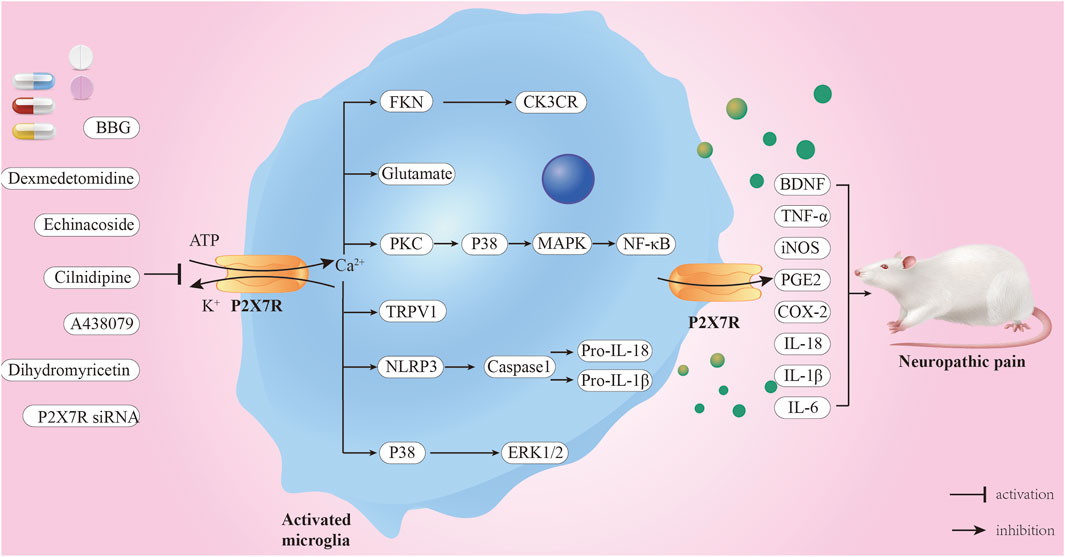
Figure 3. Mechanism of P2X7R-mediated neuropathic pain. When ATP concentration exceeds 100 μM, P2X7R is activated, opening the cell membrane-bound ion channels, leading to Ca2+ influx and K+ efflux. Consequently, multiple signaling pathways are activated, and inflammatory factors are released, ultimately leading to NP. P2X7R inhibitors and some drugs, including BBG and A438079, can alleviate NP through these pathways.
The intrinsic connection between P2X7R and NP has been well established; consequently, using P2X7R antagonists or inhibitors has the potential to relieve pain and treat NP. As the most extensively studied isoform in drug development, many potent and selective (mainly allosteric) antagonists of P2X7R have been discovered (Illes et al., 2021). However, the development of P2X7R antagonists faces several challenges. These include a lack of selective agonists, such as Bz-ATP, which efficiently activates both P2X1R and P2X3R; differences in sensitivity of mouse, rat, and human homologs to agonists/antagonists; and the fact that various in vitro test systems, such as Ca2+ influx, Yo-Pro uptake, and membrane current measurements, often yield different results when the same P2X7R ligand is used (Donnelly-Roberts et al., 2009). The P2X7R antagonist A-438079 regulates the TRPV1 receptor by binding to the P2X7R, reducing p38 and ERK1/2 phosphorylation levels, thereby effectively reducing the release of inflammatory factors and thus relieving diabetic NP (Wang et al., 2021). Reports indicate that using P2X7R inhibitors resveratrol (Xie et al., 2017) and riluzole (Jiang et al., 2016) can inhibit the overexpression of P2X7R and GFAP in dorsal root ganglion glial cells, reduce ERK1/2 phosphorylation, and relieve hyperalgesia. The activation of P2X7R opens ion channels on the cell membrane, enhances information transmission between neurons and satellite glial cells, and thus exacerbates pain. Using P2X7R antagonists, such as A740003, can reduce this intercellular communication, thereby alleviating inflammatory and pathological pain (Lemes et al., 2018). P2X7 receptor antagonists, such as A438079, can reduce the release of reactive oxygen species in neurons, reduce DNA damage, and alleviate nociceptive pain (Munoz et al., 2017). In addition, using P2X7R antagonists, such as A839977, JNJ-42253432, and AZ11645373, can inhibit glial cell activation and reduce abnormal discharges of neurons and the central nervous system, thereby effectively relieving pain (Honore et al., 2009) (Lord et al., 2014) (Kobayashi et al., 2023). Several clinical trials of P2X7R antagonists have failed owing to design defects or insufficient validation, resulting in side effects such as headaches, back pain, and fatigue (Timmers et al., 2018) (Platania et al., 2022). Some P2X7R antagonists may be non-specific and interact with other receptors or signaling pathways, thus affecting their efficacy and safety (El Idrissi et al., 2024). Long-term use of P2X7R antagonists may lead to side effects such as immune system dysfunction and abnormal cell metabolism (Zhang et al., 2020). Furthermore, the efficacy of P2X7R antagonists may vary in different types of NP models, which may be related to the specific mechanism of pain and receptor expression levels (Calzaferri et al., 2020). Overall, these findings suggest that P2X7R antagonists have potential for translational applications in pain management; however, further research and development are required.
In addition to specific P2X7R antagonists or inhibitors, some clinically approved specific compounds have been demonstrated to relieve pain by inhibiting P2X7R. Gallic acid, a traditional Chinese medicine ingredient, binds to P2X7R and inhibits the activation of the NF-κB and signal transducer and activator of transcription 3 signaling pathways triggered by P2X7R. This reduces the production of TNF-α and exhibits a significant inhibitory effect on CCI-induced pain (Yang et al., 2021). Trimethoxyflavanone, a naringenin derivative, reduces paclitaxel-enhanced P2X7R expression in the DRG, thereby reducing DRG neuronal sensitization and abnormal spinal glial cell activation, ultimately alleviating pain and neurotoxicity associated with chemotherapy-induced peripheral neuropathy (Mei et al., 2023). Botulinum toxin type A (BoNT/A) also downregulates P2X7R expression and induces microglia to transition from the pro-inflammatory M1 type to the anti-inflammatory M2 type, thereby increasing the pain threshold of rats (Gui et al., 2020). Cilnidipine, a calcium channel blocker, can reduce NP by inhibiting the activation of the P2X7R/IL-1β signaling pathway in spinal microglia [101]. Moreover, dihydromyricetin and palmatine relieve DNP and MDD by reducing the expression of P2X7R in the DRG, spinal cord, and hippocampus, suggesting that they could be effective new drugs for treating patients with DNP and MDD (Guan et al., 2019) (Shen et al., 2018) (Table 1). Palmatine reduced the pain caused by infraorbital nerve injury in the facial trigeminal neuralgia model by inhibiting inflammatory factor release and reducing P2X7R and p38 expression levels (Yin et al., 2021). These findings highlight the possibility of leveraging existing drug resources to accelerate the discovery and development of new and effective analgesics.
Electroacupuncture is an effective treatment for NP. It is widely used internationally owing to its significant therapeutic effect and low risk of side effects. Spinal microglia are important targets of EA in the analgesic effect of tactile allodynia and thermal hyperalgesia (Shan et al., 2007). Electroacupuncture relieves the symptoms of NP by activating the expression of IL-10 in spinal microglia (Ali et al., 2020). Additionally, EA improves synaptic plasticity in rat brain neurons after nerve injury (Xu et al., 2013). Xu et al. (2016) discovered that EA treatment alleviated tactile allodynia and thermal hyperalgesia caused by nerve injury by inhibiting the overexpression of IL-1β mediated by P2X7R-positive microglia. This is consistent with the study of Wu Q. et al. (2021), which revealed that EA reduced dendritic spine density, inhibited synaptic reconstruction, and reduced inflammatory response via P2X7R, thereby reducing NP. Consequently, spinal P2X7R-positive microglia may be a promising target for EA treatment of NP.
Spinal cord epidural stimulation (SCS) is a treatment approved by the US Food and Drug Administration for pain management when pharmacological interventions fail (Attal and Bouhassira, 2019). Compared with conventional SCS, high-frequency SCS exhibits a different analgesic mechanism, as evidenced by a lack of dorsal column activation, weaker inhibition of wide dynamic range neurons in the deep dorsal horn, and a slower onset of pain inhibition (Joosten and Franken, 2020). Kaiso is a transcription factor that regulates immune responses in many tissues. Yu et al. (2024) first proposed the concept of the Kaiso-P2X7R pathological axis and discovered that 10 kHz high-frequency SCS significantly reduced immune responses in the dorsal horn of the spinal cord by inactivating the Kaiso-P2X7R pathological axis in microglia. This resulted in lasting pain relief. Targeting Kaiso-P2X7R in microglia significantly improved the efficacy of conventional SCS treatment, thereby reducing neuroinflammation and providing lasting pain relief. Moreover, DRG and spinal cord dorsal horn cells exhibit high P2X7R expression. Using cell technology to transplant cells such as neural stem cells and olfactory ensheathing cells to the site of sciatic nerve injury can reduce P2X7R expression, thereby relieving pain (Zhang et al., 2019) (Du et al., 2019). Consequently, cell transplantation may become a viable treatment option for nerve injury and pain.
Approximately 500 million people worldwide suffer from NP, which not only causes severe physical and mental pain but also imposes a significant burden on their socioeconomic status. However, existing NP treatment approaches are ineffective and exhibit unacceptable side effects. Accordingly, developing effective treatment strategies and drugs for NP is an urgent need. With the ongoing in-depth study of the pathological mechanism of NP, our understanding of NP has significantly improved. P2X7 receptor in microglia plays a key role in the occurrence and development of NP. P2X7R activation polarizes microglia M1, causing neuroinflammation and aggravating NP. Inhibiting the function of P2X7R can regulate the transformation of microglia from M1 to M2 and inhibit NP occurrence and development. Consequently, inhibiting P2X7R activity or reducing its expression may be a novel approach to NP treatment, which deserves further and continuous research. Generally, P2X7R in microglia has the potential to become a new pharmacological target for clinical treatment owing to its unique structural characteristics, functional effects, and application prospects in drug development. The research and clinical application of P2X7R antagonists are still in their early stages, and more clinical trials are required to confirm their long-term efficacy and safety. In-depth interdisciplinary research will allow us to gain a better understanding of the role of microglial P2X7R in the pathogenesis of pain, opening up new avenues for pain treatment and giving patient hope. Through continuous scientific exploration and clinical practice, we anticipate that P2X7R antagonists will play an important role in pain treatment and bring substantial improvements and higher quality of life to patients.
KZ: Writing–original draft, Writing–review and editing, Conceptualization, Methodology, Project administration, Software. RR: Writing–original draft, Writing–review and editing, Project administration, Visualization. C-JZ: Funding acquisition, Supervision, Writing–review and editing. LW: Funding acquisition, Supervision, Writing–review and editing. H-HZ: Project administration, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. We thank Lanzhou Biotechnology Development Co., Ltd. for financial support for our research (Project No. LK-KY-202300007). The funder was only involved in the supervision of the project and had no role in the study design, collection, analysis, interpretation of data, writing of this article, or the decision to submit it for publication.
We thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
Authors C-JZ and LW were employed by Lanzhou Biotechnique Development Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbracchio, M. P., Burnstock, G., Verkhratsky, A., and Zimmermann, H. (2009). Purinergic signalling in the nervous system: an overview. Trends Neurosci. 32 (1), 19–29. doi:10.1016/j.tins.2008.10.001
Alexander, T. D., Muqeem, T., Zhi, L., Tymanskyj, S. R., and Covarrubias, M. (2022). Tunable action potential repolarization governed by Kv3.4 channels in dorsal root ganglion neurons. J. Neurosci. official J. Soc. Neurosci. 42 (46), 8647–8657. doi:10.1523/JNEUROSCI.1210-22.2022
Ali, U., Apryani, E., Wu, H. Y., Mao, X. F., Liu, H., and Wang, Y. X. (2020). Low frequency electroacupuncture alleviates neuropathic pain by activation of spinal microglial IL-10/β-endorphin pathway. Biomed. and Pharmacother. = Biomedecine and Pharmacother. 125, 109898. doi:10.1016/j.biopha.2020.109898
Atta, A. A., Ibrahim, W. W., Mohamed, A. F., and Abdelkader, N. F. (2023). Microglia polarization in nociplastic pain: mechanisms and perspectives. Inflammopharmacology 31 (3), 1053–1067. doi:10.1007/s10787-023-01216-x
Attal, N., and Bouhassira, D. (2019). Pain research in 2018: the year of translational studies. Lancet Neurology 18 (1), 13–15. doi:10.1016/S1474-4422(18)30433-2
Au, N. P. B., and Ma, C. H. E. (2022). Neuroinflammation, microglia and implications for retinal ganglion cell survival and axon regeneration in traumatic optic neuropathy. Front. Immunol. 13, 860070. doi:10.3389/fimmu.2022.860070
Bennett, G. J., and Xie, Y. K. (1988). A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 33 (1), 87–107. doi:10.1016/0304-3959(88)90209-6
Bhattacharya, A., and Jones, D. N. C. (2018). Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 98, 95–100. doi:10.1016/j.psyneuen.2018.08.015
Bianco, M. R., Cirillo, G., Petrosino, V., Marcello, L., Soleti, A., Merizzi, G., et al. (2012). Neuropathic pain and reactive gliosis are reversed by dialdehydic compound in neuropathic pain rat models. Neurosci. Lett. 530 (1), 85–90. doi:10.1016/j.neulet.2012.08.088
Bittar, A., Jun, J., La, J. H., Wang, J., Leem, J. W., and Chung, J. M. (2017). Reactive oxygen species affect spinal cell type-specific synaptic plasticity in a model of neuropathic pain. Pain 158 (11), 2137–2146. doi:10.1097/j.pain.0000000000001014
Boada, M. D., Gutierrez, S., and Eisenach, J. C. (2019). Peripheral oxytocin restores light touch and nociceptor sensory afferents towards normal after nerve injury. Pain 160 (5), 1146–1155. doi:10.1097/j.pain.0000000000001495
Boccella, S., Guida, F., Palazzo, E., Marabese, I., de Novellis, V., Maione, S., et al. (2018). Spared nerve injury as a long-lasting model of neuropathic pain. Methods Mol. Biol. Clift. NJ 1727, 373–378. doi:10.1007/978-1-4939-7571-6_28
Borges, J. P., Mekhail, K., Fairn, G. D., Antonescu, C. N., and Steinberg, B. E. (2021). Modulation of pathological pain by epidermal growth factor receptor. Front. Pharmacol. 12, 642820. doi:10.3389/fphar.2021.642820
Borst, K., Dumas, A. A., and Prinz, M. (2021). Microglia: immune and non-immune functions. Immunity 54 (10), 2194–2208. doi:10.1016/j.immuni.2021.09.014
Burnstock, G. (1972). Purinergic nerves. Pharmacol. Rev. 24 (3), 509–581. doi:10.1016/s0031-6997(25)06930-3
Burnstock, G. (1976). Purinergic receptors. J. Theor. Biol. 62 (2), 491–503. doi:10.1016/0022-5193(76)90133-8
Caffarel, M. M., and Braza, M. S. (2022). Microglia and metastases to the central nervous system: victim, ravager, or something else? J. Exp. and Clin. cancer Res. CR 41 (1), 327. doi:10.1186/s13046-022-02535-7
Calcutt, N. A. (2020). Diabetic neuropathy and neuropathic pain: a (con)fusion of pathogenic mechanisms? Pain 161 (Suppl. 1), S65–s86. doi:10.1097/j.pain.0000000000001922
Calzaferri, F., Ruiz-Ruiz, C., de Diego, A. M. G., de Pascual, R., Méndez-López, I., Cano-Abad, M. F., et al. (2020). The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med. Res. Rev. 40 (6), 2427–2465. doi:10.1002/med.21710
Carroll, W. A., Donnelly-Roberts, D., and Jarvis, M. F. (2009). Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 5 (1), 63–73. doi:10.1007/s11302-008-9110-6
Chen, J., Zeng, X., Wang, L., Zhang, W., Li, G., Cheng, X., et al. (2025). Mutual regulation of microglia and astrocytes after Gas6 inhibits spinal cord injury. Neural Regen. Res. 20 (2), 557–573. doi:10.4103/NRR.NRR-D-23-01130
Chen, L., Wang, H., Xing, J., Shi, X., Huang, H., Huang, J., et al. (2022). Silencing P2X7R alleviates diabetic neuropathic pain involving TRPV1 via PKCε/P38MAPK/NF-κB signaling pathway in rats. Int. J. Mol. Sci. 23 (22), 14141. doi:10.3390/ijms232214141
Chen, W., and Lu, Z. (2017). Upregulated TLR3 promotes neuropathic pain by regulating autophagy in rat with L5 spinal nerve ligation model. Neurochem. Res. 42 (2), 634–643. doi:10.1007/s11064-016-2119-2
Ciapała, K., and Mika, J. (2023). Advances in neuropathic pain research: selected intracellular factors as potential targets for multidirectional analgesics. Pharm. Basel, Switz. 16 (11), 1624. doi:10.3390/ph16111624
Cignarella, F., Filipello, F., Bollman, B., Cantoni, C., Locca, A., Mikesell, R., et al. (2020). TREM2 activation on microglia promotes myelin debris clearance and remyelination in a model of multiple sclerosis. Acta neuropathol. 140 (4), 513–534. doi:10.1007/s00401-020-02193-z
Clark, A. K., D'Aquisto, F., Gentry, C., Marchand, F., McMahon, S. B., and Malcangio, M. (2006). Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J. Neurochem. 99 (3), 868–880. doi:10.1111/j.1471-4159.2006.04126.x
Clark, A. K., Staniland, A. A., Marchand, F., Kaan, T. K., McMahon, S. B., and Malcangio, M. (2010). P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. official J. Soc. Neurosci. 30 (2), 573–582. doi:10.1523/JNEUROSCI.3295-09.2010
Decosterd, I., and Woolf, C. J. (2000). Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87 (2), 149–158. doi:10.1016/S0304-3959(00)00276-1
Delpech, J. C., Herron, S., Botros, M. B., and Ikezu, T. (2019). Neuroimmune crosstalk through extracellular vesicles in health and disease. Trends Neurosci. 42 (5), 361–372. doi:10.1016/j.tins.2019.02.007
De Ridder, D., Adhia, D., and Vanneste, S. (2021). The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev. 130, 125–146. doi:10.1016/j.neubiorev.2021.08.013
Deuchars, S. A., Atkinson, L., Brooke, R. E., Musa, H., Milligan, C. J., Batten, T. F., et al. (2001). Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J. Neurosci. official J. Soc. Neurosci. 21 (18), 7143–7152. doi:10.1523/JNEUROSCI.21-18-07143.2001
Dewaker, V., Sharma, A. R., Debnath, U., Park, S. T., and Kim, H. S. (2023). Insights from molecular dynamics simulations of TRPV1 channel modulators in pain. Drug Discov. today 28 (12), 103798. doi:10.1016/j.drudis.2023.103798
Di Virgilio, F., Dal Ben, D., Sarti, A. C., Giuliani, A. L., and Falzoni, S. (2017). The P2X7 receptor in infection and inflammation. Immunity 47 (1), 15–31. doi:10.1016/j.immuni.2017.06.020
Di Virgilio, F., Schmalzing, G., and Markwardt, F. (2018). The elusive P2X7 macropore. Trends cell Biol. 28 (5), 392–404. doi:10.1016/j.tcb.2018.01.005
Donnelly-Roberts, D. L., Namovic, M. T., Han, P., and Jarvis, M. F. (2009). Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 157 (7), 1203–1214. doi:10.1111/j.1476-5381.2009.00233.x
Du, E. R., Fan, R. P., Rong, L. L., Xie, Z., and Xu, C. S. (2020). Regulatory mechanisms and therapeutic potential of microglial inhibitors in neuropathic pain and morphine tolerance. J. Zhejiang Univ. Sci. B 21 (3), 204–217. doi:10.1631/jzus.B1900425
Du, X. J., Chen, Y. X., Zheng, Z. C., Wang, N., Wang, X. Y., and Kong, F. E. (2019). Neural stem cell transplantation inhibits glial cell proliferation and P2X receptor-mediated neuropathic pain in spinal cord injury rats. Neural Regen. Res. 14 (5), 876–885. doi:10.4103/1673-5374.249236
Duan-Porter, W., Goldstein, K. M., McDuffie, J. R., Hughes, J. M., Clowse, M. E., Klap, R. S., et al. (2016). Reporting of sex effects by systematic reviews on interventions for depression, diabetes, and chronic pain. Ann. Intern. Med. 165 (3), 184–193. doi:10.7326/M15-2877
Dworsky-Fried, Z., Kerr, B. J., and Taylor, A. M. W. (2020). Microbes, microglia, and pain. Neurobiol. pain (Cambridge, Mass) 7, 100045. doi:10.1016/j.ynpai.2020.100045
El Idrissi, I. G., Podlewska, S., Abate, C., Bojarski, A. J., Lacivita, E., and Leopoldo, M. (2024). Structure-activity relationships and therapeutic potential of purinergic P2X7 receptor antagonists. Curr. Med. Chem. 31 (11), 1361–1403. doi:10.2174/0929867330666230403094538
Fan, X., Wang, C., Han, J., Ding, X., Tang, S., and Ning, L. (2021). Role of TRPV4-P2X7 pathway in neuropathic pain in rats with chronic compression of the dorsal root ganglion. Neurochem. Res. 46 (8), 2143–2153. doi:10.1007/s11064-021-03352-8
Fang, M., and Lai, R. (2024). The price of P2X7R freedom is neuroinflammation. Immunity 57 (3), 401–403. doi:10.1016/j.immuni.2024.02.009
Fernandes, N. C., Sriram, U., Gofman, L., Cenna, J. M., Ramirez, S. H., and Potula, R. (2016). Methamphetamine alters microglial immune function through P2X7R signaling. J. neuroinflammation 13 (1), 91. doi:10.1186/s12974-016-0553-3
Finneran, D. J., Morgan, D., Gordon, M. N., and Nash, K. R. (2019). CNS-wide over expression of fractalkine improves cognitive functioning in a tauopathy model. J. neuroimmune Pharmacol. official J. Soc. NeuroImmune Pharmacol. 14 (2), 312–325. doi:10.1007/s11481-018-9822-5
Finnerup, N. B., Attal, N., Haroutounian, S., McNicol, E., Baron, R., Dworkin, R. H., et al. (2015). Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurology 14 (2), 162–173. doi:10.1016/S1474-4422(14)70251-0
Finnerup, N. B., Kuner, R., and Jensen, T. S. (2021). Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 101 (1), 259–301. doi:10.1152/physrev.00045.2019
Gao, F., and Zheng, Z. M. (2014). Animal models of diabetic neuropathic pain. Exp. Clin. Endocrinol. and diabetes official J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 122 (2), 100–106. doi:10.1055/s-0033-1363234
Godoy, P. A., Ramírez-Molina, O., and Fuentealba, J. (2019). Exploring the role of P2X receptors in Alzheimer's disease. Front. Pharmacol. 10, 1330. doi:10.3389/fphar.2019.01330
Guan, S., Shen, Y., Ge, H., Xiong, W., He, L., Liu, L., et al. (2019). Dihydromyricetin alleviates diabetic neuropathic pain and depression comorbidity symptoms by inhibiting P2X(7) receptor. Front. psychiatry 10, 770. doi:10.3389/fpsyt.2019.00770
Gui, X., Wang, H., Wu, L., Tian, S., Wang, X., Zheng, H., et al. (2020). Botulinum toxin type A promotes microglial M2 polarization and suppresses chronic constriction injury-induced neuropathic pain through the P2X7 receptor. Cell and Biosci. 10, 45. doi:10.1186/s13578-020-00405-3
He, W. J., Cui, J., Du, L., Zhao, Y. D., Burnstock, G., Zhou, H. D., et al. (2012). Spinal P2X(7) receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav. brain Res. 226 (1), 163–170. doi:10.1016/j.bbr.2011.09.015
Higashi, Y., Aratake, T., Shimizu, S., Shimizu, T., Nakamura, K., Tsuda, M., et al. (2017). Influence of extracellular zinc on M1 microglial activation. Sci. Rep. 7, 43778. doi:10.1038/srep43778
Honore, P., Donnelly-Roberts, D., Namovic, M., Zhong, C., Wade, C., Chandran, P., et al. (2009). The antihyperalgesic activity of a selective P2X7 receptor antagonist, A-839977, is lost in IL-1alphabeta knockout mice. Behav. brain Res. 204 (1), 77–81. doi:10.1016/j.bbr.2009.05.018
Hu, X., Liu, Y., Wu, J., Liu, Y., Liu, W., Chen, J., et al. (2020). Inhibition of P2X7R in the amygdala ameliorates symptoms of neuropathic pain after spared nerve injury in rats. Brain, Behav. Immun. 88, 507–514. doi:10.1016/j.bbi.2020.04.030
Huang, Z. X., Lu, Z. J., Ma, W. Q., Wu, F. X., Zhang, Y. Q., Yu, W. F., et al. (2014). Involvement of RVM-expressed P2X7 receptor in bone cancer pain: mechanism of descending facilitation. Pain 155 (4), 783–791. doi:10.1016/j.pain.2014.01.011
Illes, P., Müller, C. E., Jacobson, K. A., Grutter, T., Nicke, A., Fountain, S. J., et al. (2021). Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 178 (3), 489–514. doi:10.1111/bph.15299
Illes, P., Verkhratsky, A., Burnstock, G., and Franke, H. (2012). P2X receptors and their roles in astroglia in the central and peripheral nervous system. Neurosci. a Rev. J. bringing Neurobiol. neurology psychiatry 18 (5), 422–438. doi:10.1177/1073858411418524
Inoue, K. (2017). Purinergic signaling in microglia in the pathogenesis of neuropathic pain. Proc. Jpn. Acad. Ser. B, Phys. Biol. Sci. 93 (4), 174–182. doi:10.2183/pjab.93.011
Iwata, K., Hayashi, Y., Hitomi, S., Tsuboi, Y., and Shinoda, M. (2024). Non-neuronal cells act as crucial players in neuropathic orofacial pain. J. oral Biosci. 66 (3), 491–495. doi:10.1016/j.job.2024.07.005
Jiang, K., Zhuang, Y., Yan, M., Chen, H., Ge, A. Q., Sun, L., et al. (2016). Effects of riluzole on P2X7R expression in the spinal cord in rat model of neuropathic pain. Neurosci. Lett. 618, 127–133. doi:10.1016/j.neulet.2016.02.065
Joosten, E. A., and Franken, G. (2020). Spinal cord stimulation in chronic neuropathic pain: mechanisms of action, new locations, new paradigms. Pain 161 Suppl 1, S104–S113. doi:10.1097/j.pain.0000000000001854
Khakh, B. S., and North, R. A. (2012). Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron 76 (1), 51–69. doi:10.1016/j.neuron.2012.09.024
Kim, H. W., Won, C. H., and Oh, S. B. (2021). Lack of correlation between spinal microgliosis and long-term development of tactile hypersensitivity in two different sciatic nerve crush injury. Mol. pain 17, 17448069211011326. doi:10.1177/17448069211011326
Ko, H. G., Chun, H., Han, S., and Kaang, B. K. (2023). Role of spinal astrocytes through the perisynaptic astrocytic process in pathological pain. Mol. brain 16 (1), 81. doi:10.1186/s13041-023-01069-z
Kobayashi, M., Moro, N., Yoshino, A., Kumagawa, T., Shijo, K., Maeda, T., et al. (2023). Inhibition of P2X4 and P2X7 receptors improves histological and behavioral outcomes after experimental traumatic brain injury in rats. Exp. Ther. Med. 26 (2), 378. doi:10.3892/etm.2023.12077
Koga, K., Kobayashi, K., Tsuda, M., Kubota, K., Kitano, Y., and Furue, H. (2023). Voltage-gated calcium channel subunit α(2)δ-1 in spinal dorsal horn neurons contributes to aberrant excitatory synaptic transmission and mechanical hypersensitivity after peripheral nerve injury. Front. Mol. Neurosci. 16, 1099925. doi:10.3389/fnmol.2023.1099925
Kohno, K., and Tsuda, M. (2021). Role of microglia and P2X4 receptors in chronic pain. Pain Rep. 6 (1), e864. doi:10.1097/PR9.0000000000000864
Lan, X., Han, X., Li, Q., Yang, Q. W., and Wang, J. (2017). Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat. Rev. Neurol. 13 (7), 420–433. doi:10.1038/nrneurol.2017.69
Lemes, J. B. P., de Campos Lima, T., Santos, D. O., Neves, A. F., de Oliveira, F. S., Parada, C. A., et al. (2018). Participation of satellite glial cells of the dorsal root ganglia in acute nociception. Neurosci. Lett. 676, 8–12. doi:10.1016/j.neulet.2018.04.003
Li, T., Gao, Y., He, M., Gui, Z., Zhao, B., Cao, Y., et al. (2023). P2X7 receptor-activated microglia in cortex is critical for sleep disorder under neuropathic pain. Front. Neurosci. 17, 1095718. doi:10.3389/fnins.2023.1095718
Liao, C., Zhou, H., Chen, H., Cheng, G., Li, S., Ma, F., et al. (2022). DUSP8/TAK1 signaling mediates neuropathic pain through regulating neuroinflammation and neuron death in a spinal nerve ligation (SNL) rat model. Int. Immunopharmacol. 113 (Pt A), 109284. doi:10.1016/j.intimp.2022.109284
Lin, J. P., Chen, C. Q., Huang, L. E., Li, N. N., Yang, Y., Zhu, S. M., et al. (2018). Dexmedetomidine attenuates neuropathic pain by inhibiting P2X7R expression and ERK phosphorylation in rats. Exp. Neurobiol. 27 (4), 267–276. doi:10.5607/en.2018.27.4.267
Liu, N., Zhang, G. X., Zhu, C. H., Lan, X. B., Tian, M. M., Zheng, P., et al. (2023). Antinociceptive and neuroprotective effect of echinacoside on peripheral neuropathic pain in mice through inhibiting P2X7R/FKN/CX3CR1 pathway. Biomed. and Pharmacother. = Biomedecine and Pharmacother. 168, 115675. doi:10.1016/j.biopha.2023.115675
Liu, Y., Xiao, Y., and Li, Z. (2011). P2X7 receptor positively regulates MyD88-dependent NF-κB activation. Cytokine 55 (2), 229–236. doi:10.1016/j.cyto.2011.05.003
Lord, B., Aluisio, L., Shoblock, J. R., Neff, R. A., Varlinskaya, E. I., Ceusters, M., et al. (2014). Pharmacology of a novel central nervous system-penetrant P2X7 antagonist JNJ-42253432. J. Pharmacol. Exp. Ther. 351 (3), 628–641. doi:10.1124/jpet.114.218487
Luchting, B., Heyn, J., Woehrle, T., Rachinger-Adam, B., Kreth, S., Hinske, L. C., et al. (2016). Differential expression of P2X7 receptor and IL-1β in nociceptive and neuropathic pain. J. neuroinflammation 13 (1), 100. doi:10.1186/s12974-016-0565-z
Lustig, K. D., Shiau, A. K., Brake, A. J., and Julius, D. (1993). Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Natl. Acad. Sci. U. S. A. 90 (11), 5113–5117. doi:10.1073/pnas.90.11.5113
Makabe, K., Okada, H., Tachibana, N., Ishikura, H., Ito, N., Tanaka, M., et al. (2024). Baricitinib ameliorates inflammatory and neuropathic pain in collagen antibody-induced arthritis mice by modulating the IL-6/JAK/STAT3 pathway and CSF-1 expression in dorsal root ganglion neurons. Arthritis Res. and Ther. 26 (1), 121. doi:10.1186/s13075-024-03354-1
Martel-Gallegos, G., Casas-Pruneda, G., Ortega-Ortega, F., Sánchez-Armass, S., Olivares-Reyes, J. A., Diebold, B., et al. (2013). Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. Biochimica biophysica acta 1830 (10), 4650–4659. doi:10.1016/j.bbagen.2013.05.023
Masoodifar, M., Hajihashemi, S., Pazhoohan, S., Nazemi, S., and Mojadadi, M. S. (2021). Effect of the conditioned medium of mesenchymal stem cells on the expression levels of P2X4 and P2X7 purinergic receptors in the spinal cord of rats with neuropathic pain. Purinergic Signal. 17 (1), 143–150. doi:10.1007/s11302-020-09756-5
McCarthy, A. E., Yoshioka, C., and Mansoor, S. E. (2019). Full-length P2X(7) structures reveal how palmitoylation prevents channel desensitization. Cell 179 (3), 659–670.e13. doi:10.1016/j.cell.2019.09.017
Mei, C., Pan, C., Xu, L., Miao, M., Lu, Q., Yu, Y., et al. (2023). Trimethoxyflavanone relieves Paclitaxel-induced neuropathic pain via inhibiting expression and activation of P2X7 and production of CGRP in mice. Neuropharmacology 236, 109584. doi:10.1016/j.neuropharm.2023.109584
Mokhtari, T., and Uludag, K. (2024). Role of NLRP3 inflammasome in post-spinal-cord-injury anxiety and depression: molecular mechanisms and therapeutic implications. ACS Chem. Neurosci. 15 (1), 56–70. doi:10.1021/acschemneuro.3c00596
Moriarty, O., Gorman, C. L., McGowan, F., Ford, G. K., Roche, M., Thompson, K., et al. (2016). Impaired recognition memory and cognitive flexibility in the rat L5-L6 spinal nerve ligation model of neuropathic pain. Scand. J. pain 10, 61–73. doi:10.1016/j.sjpain.2015.09.008
Mulè, S., Rosso, G., Botta, M., Brovero, A., Ferrari, S., Galla, R., et al. (2024). Design of mixed medicinal plants, rich in polyphenols, vitamins B, and palmitoylethanolamide-based supplement to help reduce nerve pain: a preclinical study. Int. J. Mol. Sci. 25 (9), 4790. doi:10.3390/ijms25094790
Munoz, F. M., Gao, R., Tian, Y., Henstenburg, B. A., Barrett, J. E., and Hu, H. (2017). Neuronal P2X7 receptor-induced reactive oxygen species production contributes to nociceptive behavior in mice. Sci. Rep. 7 (1), 3539. doi:10.1038/s41598-017-03813-7
Pickering, G., Martin, E., Tiberghien, F., Delorme, C., and Mick, G. (2017). Localized neuropathic pain: an expert consensus on local treatments. Drug Des. Dev. Ther. 11, 2709–2718. doi:10.2147/DDDT.S142630
Platania, C. B. M., Drago, F., and Bucolo, C. (2022). The P2X7 receptor as a new pharmacological target for retinal diseases. Biochem. Pharmacol. 198, 114942. doi:10.1016/j.bcp.2022.114942
Radpour, M., Khoshkroodian, B., Asgari, T., Pourbadie, H. G., and Sayyah, M. (2023). Interleukin 4 reduces brain hyperexcitability after traumatic injury by downregulating TNF-α, upregulating IL-10/TGF-β, and potential directing macrophage/microglia to the M2 anti-inflammatory phenotype. Inflammation 46 (5), 1810–1831. doi:10.1007/s10753-023-01843-0
Ren, W. J., and Illes, P. (2022). Involvement of P2X7 receptors in chronic pain disorders. Purinergic Signal. 18 (1), 83–92. doi:10.1007/s11302-021-09796-5
Scholz, J., Finnerup, N. B., Attal, N., Aziz, Q., Baron, R., Bennett, M. I., et al. (2019). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160 (1), 53–59. doi:10.1097/j.pain.0000000000001365
Scott, M. C., Bedi, S. S., Olson, S. D., Sears, C. M., and Cox, C. S. (2021). Microglia as therapeutic targets after neurological injury: strategy for cell therapy. Expert Opin. Ther. targets 25 (5), 365–380. doi:10.1080/14728222.2021.1934447
Shan, S., Qi-Liang, M. Y., Hong, C., Tingting, L., Mei, H., Haili, P., et al. (2007). Is functional state of spinal microglia involved in the anti-allodynic and anti-hyperalgesic effects of electroacupuncture in rat model of monoarthritis? Neurobiol. Dis. 26 (3), 558–568. doi:10.1016/j.nbd.2007.02.007
Shen, Y., Guan, S., Ge, H., Xiong, W., He, L., Liu, L., et al. (2018). Effects of palmatine on rats with comorbidity of diabetic neuropathic pain and depression. Brain Res. Bull. 139, 56–66. doi:10.1016/j.brainresbull.2018.02.005
Shiratori, M., Tozaki-Saitoh, H., Yoshitake, M., Tsuda, M., and Inoue, K. (2010). P2X7 receptor activation induces CXCL2 production in microglia through NFAT and PKC/MAPK pathways. J. Neurochem. 114 (3), 810–819. doi:10.1111/j.1471-4159.2010.06809.x
Si, W., Li, X., Jing, B., Chang, S., Zheng, Y., Chen, Z., et al. (2024). Stigmasterol regulates microglial M1/M2 polarization via the TLR4/NF-κB pathway to alleviate neuropathic pain. Phytotherapy Res. PTR 38 (1), 265–279. doi:10.1002/ptr.8039
Skaper, S. D., Debetto, P., and Giusti, P. (2010). The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. official Publ. Fed. Am. Soc. Exp. Biol. 24 (2), 337–345. doi:10.1096/fj.09-138883
Sloan, G., Selvarajah, D., and Tesfaye, S. (2021). Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 17 (7), 400–420. doi:10.1038/s41574-021-00496-z
Song, Y., Shi, R., Liu, Y., Cui, F., Han, L., Wang, C., et al. (2023). M2 microglia extracellular vesicle miR-124 regulates neural stem cell differentiation in ischemic stroke via AAK1/NOTCH. Stroke 54 (10), 2629–2639. doi:10.1161/STROKEAHA.122.041611
Sorge, R. E., Trang, T., Dorfman, R., Smith, S. B., Beggs, S., Ritchie, J., et al. (2012). Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 18 (4), 595–599. doi:10.1038/nm.2710
Sperlágh, B., and Illes, P. (2014). P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol. Sci. 35 (10), 537–547. doi:10.1016/j.tips.2014.08.002
Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., and Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Sci. (New York, NY) 272 (5262), 735–738. doi:10.1126/science.272.5262.735
Tewari, M., and Seth, P. (2015). Emerging role of P2X7 receptors in CNS health and disease. Ageing Res. Rev. 24 (Pt B), 328–342. doi:10.1016/j.arr.2015.10.001
Timmers, M., Ravenstijn, P., Xi, L., Triana-Baltzer, G., Furey, M., Van Hemelryck, S., et al. (2018). Clinical pharmacokinetics, pharmacodynamics, safety, and tolerability of JNJ-54175446, a brain permeable P2X7 antagonist, in a randomised single-ascending dose study in healthy participants. J. Psychopharmacol. Oxf. Engl. 32 (12), 1341–1350. doi:10.1177/0269881118800067
Torres, L. S., Asada, N., Weiss, M. J., Trumpp, A., Suda, T., Scadden, D. T., et al. (2022). Recent advances in sickle and niche research - tribute to Dr. Paul S Frenette. Stem cell Rep. 17 (7), 1509–1535. doi:10.1016/j.stemcr.2022.06.004
Trang, T., Beggs, S., Wan, X., and Salter, M. W. (2009). P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. official J. Soc. Neurosci. 29 (11), 3518–3528. doi:10.1523/JNEUROSCI.5714-08.2009
Tsuda, M. (2017). P2 receptors, microglial cytokines and chemokines, and neuropathic pain. J. Neurosci. Res. 95 (6), 1319–1329. doi:10.1002/jnr.23816
Van Weehaeghe, D., Koole, M., Schmidt, M. E., Deman, S., Jacobs, A. H., Souche, E., et al. (2019). [(11)C]JNJ54173717, a novel P2X7 receptor radioligand as marker for neuroinflammation: human biodistribution, dosimetry, brain kinetic modelling and quantification of brain P2X7 receptors in patients with Parkinson's disease and healthy volunteers. Eur. J. Nucl. Med. Mol. imaging 46 (10), 2051–2064. doi:10.1007/s00259-019-04369-6
Vervest, A. C. M., and Schimmel, G. H. (1988). Taxonomy of pain of the IASP. Pain 34 (3), 318–321. doi:10.1016/0304-3959(88)90129-7
Villasana-Salazar, B., and Vezzani, A. (2023). Neuroinflammation microenvironment sharpens seizure circuit. Neurobiol. Dis. 178, 106027. doi:10.1016/j.nbd.2023.106027
Wang, A., Shi, X., Yu, R., Qiao, B., Yang, R., and Xu, C. (2021). The P2X(7) receptor is involved in diabetic neuropathic pain hypersensitivity mediated by TRPV1 in the rat dorsal root ganglion. Front. Mol. Neurosci. 14, 663649. doi:10.3389/fnmol.2021.663649
Wu, P., Zhou, G., Wu, X., Lv, R., Yao, J., and Wen, Q. (2022). P2X7 receptor induces microglia polarization to the M1 phenotype in cancer-induced bone pain rat models. Mol. pain 18, 17448069211060962. doi:10.1177/17448069211060962
Wu, Q., Yue, J., Lin, L., Yu, X., Zhou, Y., Ying, X., et al. (2021b). Electroacupuncture may alleviate neuropathic pain via suppressing P2X7R expression. Mol. pain 17, 1744806921997654. doi:10.1177/1744806921997654
Wu, Y., Shen, Z., Xu, H., Zhang, K., Guo, M., Wang, F., et al. (2021a). BDNF participates in chronic constriction injury-induced neuropathic pain via transcriptionally activating P2X(7) in primary sensory neurons. Mol. Neurobiol. 58 (9), 4226–4236. doi:10.1007/s12035-021-02410-0
Xia, Y., Yang, R., Wang, H., Hou, Y., Li, Y., Zhu, J., et al. (2022). Biomaterials delivery strategies to repair spinal cord injury by modulating macrophage phenotypes. J. tissue Eng. 13, 20417314221143059. doi:10.1177/20417314221143059
Xie, J., Liu, S., Wu, B., Li, G., Rao, S., Zou, L., et al. (2017). The protective effect of resveratrol in the transmission of neuropathic pain mediated by the P2X(7) receptor in the dorsal root ganglia. Neurochem. Int. 103, 24–35. doi:10.1016/j.neuint.2016.12.006
Xu, A. L., Zheng, G. Y., Ye, H. Y., Chen, X. D., and Jiang, Q. (2020). Characterization of astrocytes and microglial cells in the hippocampal CA1 region after transient focal cerebral ischemia in rats treated with Ilexonin A. Neural Regen. Res. 15 (1), 78–85. doi:10.4103/1673-5374.264465
Xu, J., Chen, X. M., Zheng, B. J., and Wang, X. R. (2016). Electroacupuncture relieves nerve injury-induced pain hypersensitivity via the inhibition of spinal P2X7 receptor-positive microglia. Anesth. analgesia 122 (3), 882–892. doi:10.1213/ANE.0000000000001097
Xu, Q., Liu, T., Chen, S., Gao, Y., Wang, J., Qiao, L., et al. (2013). Correlation between the cumulative analgesic effect of electroacupuncture intervention and synaptic plasticity of hypothalamic paraventricular nucleus neurons in rats with sciatica. Neural Regen. Res. 8 (3), 218–225. doi:10.3969/j.issn.1673-5374.2013.03.003
Yamashita, T., Kamikaseda, S., Tanaka, A., Tozaki-Saitoh, H., Caaveiro, J. M. M., Inoue, K., et al. (2021). New inhibitory effects of cilnidipine on microglial P2X7 receptors and IL-1β release: an involvement in its alleviating effect on neuropathic pain. Cells 10 (2), 434. doi:10.3390/cells10020434
Yang, R., Li, Z., Zou, Y., Yang, J., Li, L., Xu, X., et al. (2021). Gallic acid alleviates neuropathic pain behaviors in rats by inhibiting P2X7 receptor-mediated NF-κB/STAT3 signaling pathway. Front. Pharmacol. 12, 680139. doi:10.3389/fphar.2021.680139
Yang, Z. J., Huang, S. Y., Zhong, K. Y., Huang, W. G., Huang, Z. H., He, T. T., et al. (2024). Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m(6)A-YTHDF2-dependent manner. Redox Biol. 69, 103026. doi:10.1016/j.redox.2024.103026
Yin, C., Shen, W., Zhang, M., Wen, L., Huang, R., Sun, M., et al. (2021). Inhibitory effects of palmatine on P2X7 receptor expression in trigeminal ganglion and facial pain in trigeminal neuralgia rats. Front. Cell. Neurosci. 15, 672022. doi:10.3389/fncel.2021.672022
Yu, J., Wong, S., Lin, Z., Shan, Z., Fan, C., Xia, Z., et al. (2024). High-frequency spinal stimulation suppresses microglial Kaiso-P2X7 receptor axis-induced inflammation to alleviate neuropathic pain in rats. Ann. neurology 95 (5), 966–983. doi:10.1002/ana.26898
Yu, M., Su, B., and Zhang, X. (2018). Gardenoside suppresses the pain in rats model of chronic constriction injury by regulating the P2X3 and P2X7 receptors. J. Recept. signal Transduct. Res. 38 (3), 198–203. doi:10.1080/10799893.2018.1468782
Yuan, X., Han, S., Manyande, A., Gao, F., Wang, J., Zhang, W., et al. (2023). Spinal voltage-gated potassium channel Kv1.3 contributes to neuropathic pain via the promotion of microglial M1 polarization and activation of the NLRP3 inflammasome. Eur. J. pain London, Engl. 27 (2), 289–302. doi:10.1002/ejp.2059
Zhan, T., Tang, S., Du, J., Liu, J., Yu, B., Yang, Y., et al. (2024). Implication of lncRNA MSTRG.81401 in hippocampal pyroptosis induced by P2X7 receptor in type 2 diabetic rats with neuropathic pain combined with depression. Int. J. Mol. Sci. 25 (2), 1186. doi:10.3390/ijms25021186
Zhang, B., Yu, Y., Aori, G., Wang, Q., Kong, D., Yang, W., et al. (2018). Tanshinone IIA attenuates diabetic peripheral neuropathic pain in experimental rats via inhibiting inflammation. Evid. Based Complement. Altern. Med. 2018, 2789847. doi:10.1155/2018/2789847
Zhang, C., Guo, D., Qiao, H., Li, J., Li, J., Yang, Y., et al. (2022). Macrophage extracellular traps exacerbate secondary spinal cord injury by modulating macrophage/microglia polarization via LL37/P2X7R/NF-κB signaling pathway. Oxidative Med. Cell. Longev. 2022, 9197940. doi:10.1155/2022/9197940
Zhang, M., Duan, C., Lin, W., Wu, H., Chen, L., Guo, H., et al. (2024). Levistilide A exerts a neuroprotective effect by suppressing glucose metabolism reprogramming and preventing microglia polarization shift: implications for Parkinson's disease. Mol. Basel, Switz. 29 (4), 912. doi:10.3390/molecules29040912
Zhang, W., Liu, Y., Sun, Y., and Liu, Z. (2019). Effects of microencapsulated olfactory ensheathing cell transplantation on neuropathic pain and P2X7 receptor expression in the L4-5 spinal cord segment. Neurosci. Lett. 701, 48–53. doi:10.1016/j.neulet.2019.02.013
Zhang, W. J., Zhu, Z. M., and Liu, Z. X. (2020). The role and pharmacological properties of the P2X7 receptor in neuropathic pain. Brain Res. Bull. 155, 19–28. doi:10.1016/j.brainresbull.2019.11.006
Zhao, Y. F., Tang, Y., and Illes, P. (2021). Astrocytic and oligodendrocytic P2X7 receptors determine neuronal functions in the CNS. Front. Mol. Neurosci. 14, 641570. doi:10.3389/fnmol.2021.641570
Zhi, M. J., Liu, K., Zheng, Z. L., He, X., Li, T., Sun, G., et al. (2017). Application of the chronic constriction injury of the partial sciatic nerve model to assess acupuncture analgesia. J. pain Res. 10, 2271–2280. doi:10.2147/JPR.S139324
Zhou, F., Wang, X., Han, B., Tang, X., Liu, R., Ji, Q., et al. (2021). Short-chain fatty acids contribute to neuropathic pain via regulating microglia activation and polarization. Mol. pain 17, 1744806921996520. doi:10.1177/1744806921996520
Keywords: neuropathic pain, P2X7R, microglia, M1/M2 polarization, cytokine, neuroinflammation
Citation: Zhang K, Ran R, Zhang C-J, Wang L and Zhang H-H (2025) Focus on P2X7R in microglia: its mechanism of action and therapeutic prospects in various neuropathic pain models. Front. Pharmacol. 16:1555732. doi: 10.3389/fphar.2025.1555732
Received: 05 January 2025; Accepted: 07 March 2025;
Published: 25 March 2025.
Edited by:
Rodrigo Da Cunha Bisaggio, Universidade do Estado do Rio de Janeiro, BrazilReviewed by:
Nicholas John Geraghty, University of Wollongong, AustraliaCopyright © 2025 Zhang, Ran, Zhang, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-Hong Zhang, aGFpaG9uZ3poYW5nMjAyMjA3QDE2My5jb20=
†Lead
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.