
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 31 March 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1554696
 Qianrong Li1,2,3†
Qianrong Li1,2,3† Chunzhen Ren1,2,3†
Chunzhen Ren1,2,3† Bing Jiang1,2,3
Bing Jiang1,2,3 Xuehan Wang2,3,4
Xuehan Wang2,3,4 Chunling Wang1,2,3
Chunling Wang1,2,3 Xiaodong Zhi1,2,3,5
Xiaodong Zhi1,2,3,5 Linchan Li1,2,3,6
Linchan Li1,2,3,6 Xiaoying Guo1,2,3
Xiaoying Guo1,2,3 Xinke Zhao1,2,3,5*
Xinke Zhao1,2,3,5* Yingdong Li1,2,3*
Yingdong Li1,2,3*Myocardial fibrosis (MF) involves the activation and excessive proliferation of cardiac fibroblasts (CFs) in the extracellular matrix, leading to increased collagen expression that impairs cardiac function. Currently, there are no effective pharmacological treatments for MF. Traditional Chinese Medicine (TCM), particularly Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma], has gained attention for its potential in treating MF. Recent studies indicate significant therapeutic effects of its active metabolites, supporting its use in MF treatment and positioning it as a promising candidate for drug development. Aim of the review: This article reviews the research and mechanisms of S. miltiorrhiza’s effective metabolites and preparations in treating MF, providing a reference for future clinical treatments. A systematic literature search was conducted in PubMed, Web of Science, CNKI, and Google Scholar (January 2000–October 2024) using keywords: “myocardial fibrosis,” “cardiac fibrosis,” “Salvia miltiorrhiza Bunge,” “extract,” and “botanical drug.” Results: The active metabolites of S. miltiorrhiza and its metabolite preparations exert anti-fibrotic effects through pleiotropic mechanisms, including suppression of ventricular remodeling, modulation of autophagy, inhibition of oxidative stress and cardiomyocyte apoptosis, and regulation of extracellular matrix homeostasis and immune-inflammatory responses. Conclusion: Research indicates that S. miltiorrhiza is beneficial for managing MF, but further studies are needed to identify its chemical metabolites and regulatory mechanisms. Large-scale, multi-center clinical trials are also necessary to assess treatment safety. This review offers insights for developing new anti-MF pharmacotherapies.
Myocardial fibrosis (MF) is a common pathological mechanism linked to several cardiovascular disorders, including myocardial infarction, hypertensive heart disease, and dilated cardiomyopathy. This condition is characterized by an abnormal accumulation of extracellular matrix metabolites, along with the excessive proliferation and activation of cardiac fibroblasts (CFs). Thus, there is a significant increase in collagen fiber deposition, collagen content, and overall volume of collagen. These pathological changes contribute to a decrease in myocardial compliance and cardiac function, which may lead to arrhythmias and sudden cardiac death (Maruyama and Imanaka-Yoshida, 2022). Globally, myocardial fibrosis is prevalent in 30%–50% of heart failure patients and is associated with a 2.3-fold increase in all-cause mortality due to its adverse prognostic implications (Behnoush et al., 2023). MF is characterized by rapid progression, high mortality, and multifactorial pathogenesis involving inflammatory, oxidative, and apoptotic pathways. Contemporary medical understanding indicates that MF arises from a single factor and the interplay of elements such as inflammatory responses, oxidative stress, the renin-angiotensin-aldosterone system (RAAS), matrix metalloproteinases (MMPs), growth factors, noncoding RNAs, endothelial dysfunction, and cardiomyocyte apoptosis and necrosis. The clinical diagnosis of myocardial fibrosis faces significant challenges. Endomyocardial biopsy, due to its invasiveness, is difficult to widely implement. Meanwhile, emerging non-invasive imaging techniques such as cardiac magnetic resonance (CMR) T1 mapping and extracellular volume (ECV) quantification have significantly improved the detection rate (with a sensitivity of up to 89%). However, their standardized application is still limited by the availability of equipment and cost (Bengel et al., 2023). The pharmacological agents used in clinical treatment include statins, angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor antagonists (ARBs), angiotensin receptor-neprilysin inhibitors (ARNIs), sodium-glucose cotransporter 2 inhibitors (SGLT2i), and aldosterone receptor antagonists. Although these medications can alleviate symptoms, their efficacy in preventing or reversing MF is limited (Liu et al., 2023). Discontinuing medication or developing tolerance can worsen a patient’s condition, highlighting the urgent need for effective and safe pharmacological interventions for MF.
Salvia miltiorrhiza Bunge [Lamiaceae; Salviae miltiorrhizae radix et rhizoma], a perennial botanical drug deeply rooted in traditional Chinese medicine (TCM), has been revered for centuries as a cornerstone therapy for cardiovascular ailments (Wu et al., 2022). Its historical applications, documented in classical texts such as Shennong Bencao Jing, emphasize its efficacy in “promoting blood circulation, resolving blood stasis, and relieving pain”—principles that align with modern understandings of pathologies marked by microcirculatory dysfunction and fibrotic remodeling, such as MF (Li Q. et al., 2022; Qi et al., 2024; Wang et al., 2021). The term MF does not appear in TCM. However, it can be classified under the category of “chest pain and heartache” within this medical framework. Contemporary studies link its pathogenesis to a deficiency of the fundamental essence and an excess of superficial conditions, including blood stasis, phlegm turbidity, and heat toxins (Ren et al., 2022). Recent studies have focused on the use of S. miltiorrhiza for the prevention and treatment of MF, demonstrating its protective effects on cardiac health. S. miltiorrhiza has emerged as a promising candidate, offering a unique phytochemical repertoire—tanshinones (lipophilic diterpenoids) and salvianolic acids (water-soluble phenolics)—that synergistically combat fibrosis through pleiotropic mechanisms. Preclinical studies highlight its capacity to suppress TGF-β1-mediated fibroblast activation and attenuate oxidative stress by enhancing Nrf2/HO-1 signaling (Li Q. et al., 2022), demonstrating its protective effects on cardiac health. The emphasis of TCM on syndrome differentiation and holistic approaches has garnered the attention of researchers worldwide. This article reviews related research on the use of S. miltiorrhiza in the treatment of MF.
This review article has been retrieved in the form of a database search. The search terms are in the form of subject words combined with free words. A systematic literature search was conducted across PubMed, Web of Science, CNKI, and Google Scholar. Search terms included “Salvia miltiorrhiza Bunge,” “active metabolites,” “pharmacological effects,” “extraction,” and “chemical structure.” (up to October 2024), total of 1,024 articles were retrieved, and 662 were duplicated by software and manual removal. After deduplication, 102 articles focused on S. miltiorrhiza’s anti-fibrotic mechanisms and clinical applications were retained. The research methods we included include clinical studies, clinical trials, cell experiments, animal experiments, literature reviews, network pharmacology, etc. We extracted study details, including the relevant information on the pharmacological action and chemistry attributes of S. miltiorrhiza, as well as the study status (Figure 1).
Taxonomic validation of plant species was performed using the Medicinal Plant Names Services (MPNS) and Plants of the World Online databases: [http://mpns.kew.org/mpns-portal/] (http://mpns.kew.org/mpns-portal/): and the Plants of the World Online database: [http://www.plantsoftheworldonline.org] (http://www.plantsoftheworldonline.org).
MFis a pathological process characterized by excessive extracellular matrix (ECM) deposition and fibroblast activation, driven by ischemic, inflammatory, or metabolic insults. It represents a common pathological feature in the final stage of various cardiovascular diseases (López et al., 2021). Myofibroblasts are phenotypically regulated fibroblasts. The expression of α-smooth muscle actin (α-SMA) can identify differentiated myofibroblasts in damaged tissues and participate in the repair or fibrosis of damaged myocardial tissues. After cardiac injury, changes in the matrix environment, the induction and release of growth factors and cytokines, and an increase in mechanical pressure dynamically regulate the phenotype of fibroblasts (Kong et al., 2014). Fibroblasts secrete many ECM structural proteins, enzymes, growth factors, and cytokines, which in turn lead to excessive deposition of extracellular collagen (Travers et al., 2016). The number of fibroblasts increases significantly in diseases such as myocardial infarction (Willems et al., 1994), pressure-overloaded and volume-overloaded myocardium (Wang et al., 2003), aging heart (Obas and Vasan, 2018) and alcoholic cardiomyopathy (Fernández-Solà, 2020), indicating that myofibroblast transdifferentiation is a marker of MF. After TGF-β binds to TβRII on the cell surface, it can promote the phosphorylation of the cytoplasmic domain of TβRI and then transmit signals through Smad-dependent or Smad-independent pathways, promote the transdifferentiation of myofibroblasts and promote the deposition of the ECM in the cardiac interstitium (Xue et al., 2019). Experimental studies have also shown that reactive oxygen species (ROS) can activate TGF-β, thus promoting the deposition of the ECM in the cardiac interstitium (Zhu et al., 2020).
ROS and angiotensin II (Ang II) synergistically promote fibroblast activation via redox-sensitive kinases (e.g., MAPK) and upregulation of pro-fibrotic genes. It can not only increase the transcription of MMPs by activating redox-sensitive kinases (Lijnen et al., 2012), but also mediate cardiac fibrosis and remodeling through Ang II-activated ROS-sensitive kinases. Cardiomyocyte apoptosis-related proteins are closely related to rat MF (Chiang et al., 2020). Specific monocyte and macrophage subsets play dual roles in fibrosis through their activity and microenvironment (Wynn and Barron, 2010), affecting fibroblast and matrix remodeling and inducing medium expression and release. These cells release proinflammatory mediators such as IL-1β, TNF-α, IL-6, TGF-β, and FGF to promote fibrosis. In fibrotic hearts, the expression of proinflammatory factors such as TNF-α, IL-1β, and IL-6 increases (Habib et al., 1996), which affects the fibroblast phenotype and gene expression (Siwik and Colucci, 2004), influences fibroblast and matrix remodeling, and induces medium expression and release (Timonen et al., 2008). In summary, MF pathogenesis involves crosstalk between TGF-β/Smad signaling, oxidative stress, inflammatory cascades (e.g., IL-6/STAT3), and apoptosis pathways (e.g., Bax/Bcl-2), as illustrated in Figure 2.
S. miltiorrhiza, commonly known as Dahongpao or red root, is the dried root and rhizome of S. miltiorrhiza. It was included in the 2020 “Chinese Pharmacopoeia” and is among the most extensively used TCMs in China (Figures 3A, B). The rhizomes are dark brown, twisted (10–30 cm in length), with a rough exterior and aromatic pale-yellow interior (Figures 3C, D). S. miltiorrhiza, a widely used TCM in clinical practice, was initially documented in “Shen Nong’s botanical drugal Classic”. This botanical drug is known for its ability to enhance health and address a range of medical conditions. Its pharmacological properties span cardiovascular protection (antiplatelet aggregation, anti-fibrotic), anti-inflammatory, antioxidant, and antitumor effects (Lijnen et al., 2012), along with antitumor effects, induction of apoptosis, promotion of microcirculation, enhancement of hemorheology, improvement in lipid metabolism, and inhibition of atherosclerosis (Chiang et al., 2020). Contemporary pharmacological studies have revealed that the primary active metabolites of S. miltiorrhiza include tanshinones, salvianolic acids, volatile oils, polysaccharides, nitrogen-containing metabolites, and various other chemical entities (Li Z. M. et al., 2018). The primary production of this plant occurs in Shandong, China, where it is renowned for its high yield and superior quality. Other notable regions of production include Henan, Shanxi, Sichuan, and Gansu, among others (Figure 4).

Figure 3. (A, B) Salvia miltiorrhiza botanical drugs (Plant Photo Bank of China, PPBC, http://ppbc.iplant.cn/), (C) Salvia miltiorrhiza original medicinal materialsand (Plant Photo Bank of China, PPBC, http://ppbc.iplant.cn/), (D) Salvia miltiorrhiza slices (Baidu library, https://xueshu.baidu.com/).

Figure 4. Salvia miltiorrhiza Bunge production areas in China. (A: Shandong, B: Gansu, C: ningxia, D: Sichuan, E: Henan, F: Shanxi) (The map of China is automatically generated by the WPS platform software. The distribution areas of Salvia miltiorrhiza Bunge are obtained based on records from literature and textbooks. These areas are highlighted in yellow to make the distribution more evident and easier for readers to understand).
The medicinal value of S. miltiorrhiza is derived from its complex chemical metabolites. More than 100 chemical metabolites have been isolated and identified. Over 100 bioactive metabolites are categorized into three classes: tanshinones (lipophilic diterpenoids), salvianolic acids (water-soluble phenolics), and volatile/polysaccharide derivatives. Ultra-fast liquid chromatography-mass spectrometry (UF-LC-MS) and thrombin inhibition assays identified salvianolic acids (e.g., salvianolic acid C) and tanshinones (e.g., cryptotanshinone) as potent thrombin inhibitors (Figure 5).
The tanshinone metabolites in S. miltiorrhiza are mostly diterpenoids, which are fat soluble and are synthesized and accumulate in the periderm of S. miltiorrhiza roots. At present, more than 50 species have been isolated, such as tanshinone I, tanshinone IIA, tanshinone IIB, cryptotanshinone, dihydrotanshinone I, isocryptotanshinone, and tanshinones (Dong and Zheng, 2004). The biosynthetic pathway of tanshinone metabolites (Zeng et al., 2016) indicates that tanshinone diene is the first step in the formation of the skeletons of tanshinone metabolites. Through a series of biosynthetic pathways, tanshinone IIA and cryptotanshinone are ultimately formed under the catalysis of the cytochrome P450 enzymes oxidase, decarboxylase, dehydrogenase and reductase.
Salvianolic acids in S. miltiorrhiza are water soluble and are synthesized and accumulate mainly in the phloem and xylem of its roots. At present, more than 30 kinds of salvianolic acids have been isolated. The main active metabolites are salvianolic acid A (Sal A), salvianolic acid B (Sal B), danshensu, caffeic acid, rosmarinic acid, protocatechuic aldehyde, lithospermic acid, etc. Most salvianolic acid metabolites can be regarded as derivatives of caffeic acid. For example, rosmarinic acid is a dimer of caffeic acid and danshensu, salvianolic acid B is a dimer of rosmarinic acid, and salvianolic acid A is formed by the condensation of one molecule of danshensu and two molecules of caffeic acid (Shi et al., 2019).
The volatile oil content of Radix S. miltiorrhiza is low. At present, more than 30 metabolites have been isolated and identified from the volatile oil of Radix S. miltiorrhiza, including peach tocopherol, rust alcohol, caryophyllene, 7-isopropyl-1,1,4α-trimethyl-1,2,3,4,4α,9,10,10α-octahydrophenanthrene lactone, n-hexadecanoic acid, diisobutyl phthalate, germacrene D, oleic acid and n-eicosane (Li Y. et al., 2021). Polysaccharides in S. miltiorrhiza have attracted widespread attention because of their ability to enhance immunity and protect the liver (Chen Y. et al., 2017). Wang et al. (2014) isolated the polysaccharide SMPA from S. miltiorrhiza, which is composed of galactose, glucose, rhamnose, mannose and glucuronic acid. In addition, S. miltiorrhiza contains lactones such as salviamone and spiroketalide (Kong and Liu, 1983).
Ventricular remodeling refers to structural and functional changes in the myocardium in response to mechanical and nerve stimulation, including cardiomyocyte hypertrophy, extracellular matrix remodeling, and fibroblast activation. MF is not only the result of ventricular remodeling but also the pathological basis of ventricular remodeling (Geva and Bucholz, 2021). Excessive ECM deposition in MF increases myocardial stiffness, reduces compliance, and accelerates ventricular remodeling through biomechanical stress. Ventricular remodeling promotes the development of MF. In the process of ventricular remodeling, cardiomyocyte hypertrophy and extracellular matrix remodeling further activate fibroblasts and form a vicious cycle (Nagalingam et al., 2022; O’Meara and Zannad, 2023). TGF-β1, Ang II, and other factors play key roles in MF and ventricular remodeling. They jointly promote the progression of heart disease by activating fibroblasts and inducing cardiomyocyte hypertrophy. In conclusion, MF and ventricular remodeling promote each other in pathophysiological processes, and their molecular mechanisms are intertwined, which together lead to the deterioration of cardiac structure and function. Therefore, inhibiting ventricular remodeling can provide a new strategy for the treatment of MF. Studies have shown that the active metabolites of S. miltiorrhiza and its preparations can improve MF by inhibiting ventricular remodeling through a variety of mechanisms (Li X. et al., 2021).
Tanshinone IIA (Tan IIA) is the main metabolite of S. miltiorrhiza and belongs to the class of diterpenoid quinones. Its molecular formula is C19H18O3, and its relative molecular weight is 294.33. It is obtained primarily through extraction, chromatography, crystallization, and other processes. Tan IIA exhibits cardioprotection via endothelial preservation, anti-arrhythmic effects, and attenuation of ischemia-reperfusion injury (Ansari et al., 2021). In a study conducted by Song et al. (2022) Tan IIA was administered at a dosage of 15 mg/kg via intraperitoneal injection for 28 consecutive days to rats with CHF induced by ISO. The results indicated that the Tan IIA group presented significantly lower levels of Ang II, brain natriuretic peptide (BNP), left ventricular mass index (LVMI), and left ventricular mass (LVM) than did the model group. Moreover, the Tan IIA group presented a lower degree of myocardial cell necrosis, MF, and remodeling. Notably, there was a reduction in the myocardial collagen volume ratio and protein levels of Col-I, Col-III, p-PI3K, and p-Akt in the myocardial tissue. He Dequan et al. (2022) established a rat model of myocardial hypertrophy via subcutaneous injection of ISO (5 mg/kg/d) for 14 consecutive days. Different doses (17.5 mg/kg/d, 35 mg/kg/d, and 70 mg/kg/d) of Tan IIA were used to treat the rats with MF. After 28 days of continuous intervention, the LVEDP, cardiac mass, cardiac index, COL-I, COL-III, p-PI3K, and p-AKT levels in the myocardial tissue of the Tan IIA intervention group and PI3K inhibitor group were significantly decreased, and the LVSP and ±dp/dtmax were significantly increased. These findings suggest that Tan IIA can reduce the phosphorylation levels of the PI3K and AKT proteins in the myocardial tissue of rats in a dose-dependent manner, improve ventricular remodeling and inhibit MF. Tan IIA dose-dependently suppressed PI3K/AKT phosphorylation (p-PI3K, p-AKT) in myocardial tissue, thereby improving ventricular remodeling and MF (Figure 6).

Figure 6. Salvia miltiorrhiza Bunge and its active metabolites regulate PI3K/Akt signaling pathway to prevent MF.
Sal B is a water-soluble phenolic metabolite extracted from the traditional Chinese medicine S. miltiorrhiza. Its molecular formula is C36H30O16, and its relative molecular weight is 718.62. It has many biological activities, such as anti-oxidative, anti-inflammatory, and anti-fibrotic effects. It has been widely studied and applied to the treatment of cardiovascular diseases (Liang et al., 2024). Gao et al. (2019) performed in vivo and in vitro studies and revealed that varying doses of Sal B (80 mg/kg/d and 160 mg/kg/d) significantly reduce the myocardial collagen area in ISO-induced MF in mice in a dose-dependent manner. Additionally, they reported a decrease in the protein expression levels of TGF-β1, Smad2, and Smad3, along with an increase in Smad7 expression. Sal B can regulate the TGF-β1/Smad signaling pathway in CFs to inhibit ventricular remodeling and improve MF. Li et al. (2020) established a type 1 diabetes model using streptozotocin (STZ) and reported that after 16 weeks of Sal B administration via intraperitoneal injection at doses of 15 mg/kg/d and 30 mg/kg/d, left ventricular dysfunction in diabetic mice improved considerably, and collagen deposition in the cardiac tissue decreased. Both in vitro and in vivo studies demonstrated that Sal B facilitated the phosphorylation of extracellular signal-regulated protein kinase and protein kinase B (AKT), thus promoting cellular proliferation. These findings indicate that Sal B may increase angiogenesis by inhibiting IGFBP3, which in turn mitigates MF and cardiac remodeling associated with diabetic cardiomyopathy.
Rosmarinic acid (RA) is a water-soluble phenolic acid that is synthesized via the condensation of Danshensu and caffeic acid. Studies have shown that it has antiviral, antibacterial, anti-inflammatory, and other effects (Guan et al., 2022). Zhang et al. (2018) induced ventricular remodeling via aortic ligation and administered RA (100 mg/kg/d) via gavage. The results showed that RA reduced ventricular remodeling and inhibited MF through AMPKα/Smad 3 signal transduction.
The Qiliqiangxin capsule (QLQXC) is a polyherbal formulation composed of the following botanical drugs:Astragalus membranaceus (Fisch.) Bunge [Fabaceae; Astragali Radix], Panax ginseng C.A. Mey. [Araliaceae; Ginseng Radix et Rhizoma], Aconitum carmichaelii Debx. [Ranunculaceae; Aconiti Lateralis Praeparata], S. miltiorrhiza, Descurainia sophia (L.) Webb ex Prantl [Brassicaceae; Descurainiae Semen], Alisma orientale (Sam.) Juzep. [Alismataceae; Alismatis Rhizoma], Polygonatum odoratum (Mill.) Druce [Asparagaceae; Polygonati Odorati Rhizoma], Cinnamomum cassia Presl [Lauraceae; Cinnamomi Cortex], Carthamus tinctorius L. [Asteraceae; Carthami Flos], Magnolia officinalis Rehd. et Wils. [Magnoliaceae; Magnoliae Cortex], Citrus reticulata Blanco [Rutaceae; Citri Reticulatae Pericarpium]. This commercial Chinese polyherbal preparation (CCPP) represents a synthesis of TCM principles and contemporary scientific advancements. Several clinical studies have been conducted, leading to the recognition of its efficacy and research findings by experts and researchers around the world. Zhang et al. (2017) developed a CHF rat model through abdominal aortic ligation and administered different doses of QLQXC (0.25 g/kg/d, 0.5 g/kg/d, and 1 g/kg/d). The authors reported that QLQXC treatment decreased the serum levels of BNP, lLVMI, type I CVF, and type III CVF and the protein expression of TGF-β1 and Smad3, indicating that QLQXC improved myocardial hypertrophy and inhibited MF by inhibiting the TGF-β1/Smad3 signaling pathway.
Shenqi Jianxin Prescription (SQJXP) (Zhao, 2022) is a decoction-free formula granule composed of A. membranaceus, P. ginseng C.A. Meyer [Araliaceae; Ginseng Radix Rubra], Atractylodes macrocephala Koidz. [Asteraceae; Atractylodis Macrocephalae Rhizoma], S. miltiorrhiza, Poria cocos (Schw.) Wolf [Polyporaceae; Poria], Epimedium brevicornu Maxim. [Berberidaceae; Epimedii Folium], and C. cassia Presl [Lauraceae; Cinnamomi Ramulus]. According to the recommended dosage of prescription, the ratio of prescription is 20:15:10:15:10:15:15:15:6. A CHF model was constructed through coronary artery ligation. Following intragastric administration of SQJXP for 8 weeks at dosages of 3.7 mg/kg/d, 7.4 mg/kg/d, and 14.8 mg/kg/d, the cardiac function of the rats in each intervention group was significantly improved compared with that of the model group. Additionally, there was a reduction in the area of MF and a decrease in the mRNA levels of TGF-β1, Smad3, and Caspase-3 to different degrees. Similarly, the protein expression levels of TGF-β1, p-Smad3, and Caspase-3 also decreased to different degrees. The SQJXP improved ventricular remodeling and inhibited MF, and a dose of 14.8 mg/kg/d was the best.
Yixin Futing Yin (YXFTY) is a formula granule composed of the following botanical drugs: A. carmichaelii, D. sophia A. macrocephala, C. cassia, Conioselinum anthriscoides (Chuanxiong) [Apiaceae; Ligustici Chuanxiong Rhizoma], P. cocos, Pseudostellaria heterophylla (Miq.) Pax [Caryophyllaceae; Pseudostellariae Radix], S. miltiorrhiza, Ophiopogon japonicus (Thunb.) Ker Gawl. [Asparagaceae; Ophiopogonis Radix], Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Scrophulariaceae; Rehmanniae Radix], Schisandra chinensis (Turcz.) Baill. [Schisandraceae; Schisandrae Fructus], and Glycyrrhiza glabra L. [Fabaceae; Glycyrrhizae Radix et Rhizoma] formulated in the following proportions: 20:30:15:12:12:30:30:30:12:15:6:10. This formulation has pharmacological properties that help ameliorate myocardial hypertrophy. Liu et al. (2021) reported that administering 3.5 g/kg/d YXFTY significantly decreased collagen levels in rats with CHF. The treatment also led to a reduction in the percentage of fibrous tissue expression area and the expression levels of TGF-β1, Smad3, and Smad7 mRNA in myocardial tissue. These findings suggested that YXFTY may effectively inhibit excessive activation of the TGF-β/Smad signaling pathway, thus mitigating MF and ventricular remodeling in rats experiencing pressure-induced CHF (Figure 7). Table 1 summarizes S. miltiorrhiza’s anti-fibrotic mechanisms and clinical formulations targeting ventricular remodeling.

Figure 7. Salvia miltiorrhiza Bunge and its active metabolites regulate TGF-β/Smad signaling pathway to prevent MF.
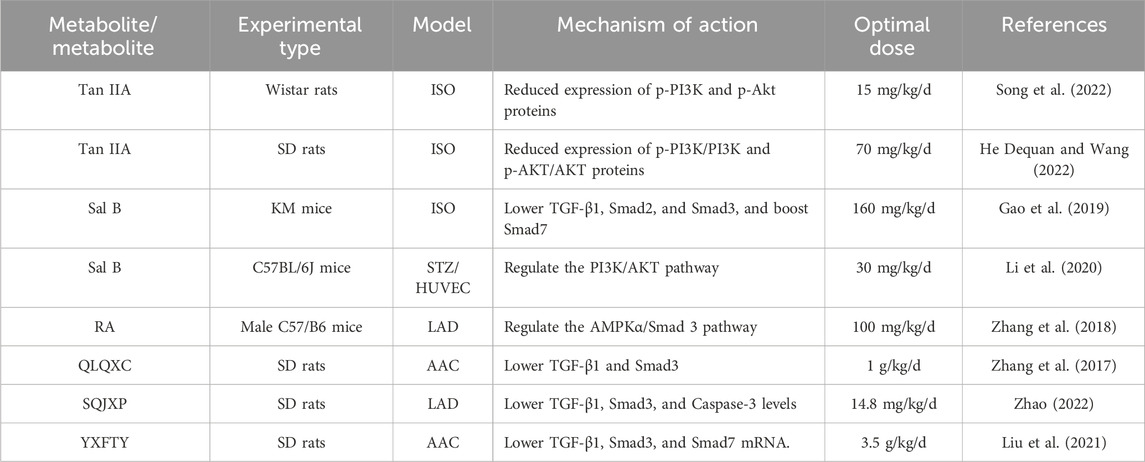
Table 1. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in inhibiting ventricular remodeling and improving MF.
Autophagy is a conserved intracellular degradation process in eukaryotes, essential for maintaining cellular homeostasis through lysosomal turnover of damaged components. Its essence is an intracellular metabolic process that responds to various external pressures. Damaged or misfolded proteins and organelles are sequestered by autophagosomes and then transferred into the lysosome for digestion and decomposition, thereby providing energy for cell metabolism and renewing the cell. Normal levels of autophagy are necessary for the human body to maintain the stability of the intracellular environment by promoting cell metabolism. Dysregulation occurs under pathological conditions such as nutrient deprivation or oxidative stress, the level of autophagy may be upregulated or downregulated. Excessive and insufficient autophagy may lead to disease (Li A. et al., 2022). In recent years, an increasing number of studies have shown that autophagy plays an important role in the occurrence and development of MF (Ambardekar and Sailer, 2022). Doxorubicin induces cardiac perivascular fibrosis via ROS-mediated NF-κB activation, which promotes endothelial-mesenchymal transition (EndMT) and autophagic dysfunction and cause cardiac toxicity. Irisin mitigates doxorubicin-induced cardiotoxicity by restoring UCP2-mediated autophagic flux and antioxidant defense, which confirms the protective effect of irisin on the microenvironment of cardiac microvascular endothelial cells and can be used as a potential therapeutic drug for doxorubicin-induced perivascular fibrosis. Many studies have shown that S. miltiorrhiza and its preparations have great potential in regulating autophagy in MF.
Du et al. (2019) demonstrated dose-dependent inhibition of ISO-induced MF by Sal B (15–30 mg/kg/d) in rats. Compared with those in the control group, the Sal B intervention groups presented decreases in the HW/BW, LVW/BW, and Col-I/Col-III ratios. Sal B treatment downregulated phosphorylated AKT/mTOR signaling while upregulating autophagy markers Beclin1 and LC3-II. H&E staining revealed that the degree of myocardial cell fibrosis in the Sal B intervention group was reduced, suggesting that Sal B can inhibit ISO-induced rat MF in a dose-dependent manner by inhibiting the PI3K/AKT/mTOR pathway to promote autophagy.
Qishen Yiqi Dropping Pills (QSYQDP) are formulated from a combination of A. membranaceus, S. miltiorrhiza, Panax notoginseng (Burkill) F.H.Chen [Araliaceae; Notoginseng Radix et Rhizoma], and Santalum album L. [Santalaceae; Santali Albi Lignum]. In 2003, these pills were approved by the China Food and Drug Administration (CFDA) for their clinical application in the treatment of cardiovascular diseases. Lv et al. (2021) conducted animal experiments that demonstrated significant findings regarding the effects of varying doses of QSYQDP (135 mg/kg/d, 270 mg/kg/d, and 540 mg/kg/d) compared with a sham surgery group. This study revealed that QSYQDP administration led to a prominent reduction in HMI and LVMI, as well as a decrease in the myocardial collagen volume fraction. Additionally, QSYQDP treatment mitigated pathological alterations in myocardial tissue, resulting in the orderly and tightly organized arrangement of the MF. Studies have also revealed an increase in the number of myocardial autophagosomes, along with an increase in the expression levels of Beclin-1 and LC3-II/LC3-I in myocardial tissue and an inhibition of p62 expression. Additionally, the ratios of Akt, P-PI3K/PI3K, P-Akt/Akt, and P-mTOR/mTOR decreased in a dose-dependent manner, suggesting that QSYQDP activates myocardial autophagy via the PI3K/AKT/mTOR signaling pathway, thus exerting a dose-dependent anti-fibrotic effect on MF.
Er Shen Zhen Wu Decoction (ESZWD) is derived from the traditional formula Zhen Wu Decoction and is enhanced by the incorporation of two additional botanical drugal metabolites: red ginseng and S. miltiorrhiza. The primary metabolites of this formulation include S. miltiorrhiza, Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix Alba], A. macrocephala., P. cocos, P. ginseng, and A. carmichaelii Debeaux [Ranunculaceae; Aconiti Lateralis Praeparata]; these metabolites are combined at a ratio of 30:10:10:10:6:6:5. Clinical studies have shown its effectiveness in the treatment of patients with CHF. In vivo and in vitro studies (Zhao, 2023; Zhao et al., 2023) demonstrated that relative to the control, different doses of ESZWD (3.96 g/kg/d, 7.92 g/kg/d, and 15.84 g/kg/d) significantly decreased the expression levels of myocardial α-SMA, Col-I, and Col-III mRNAs, as well as the proteins p-PI3K, p-AKT, and p-mTOR. These findings suggest that it can regulate the PI3K/AKT/mTOR signaling pathway to reduce collagen production, increase autophagy and reduce MF. Table 2 summarizes the autophagy-regulatory effects of S. miltiorrhiza and its bioactive components in MF models.

Table 2. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in regulating autophagy and improving MF.
The key characteristic of MF is the aberrant buildup of collagen fibers in the heart muscle, predominantly due to an imbalance in collagen synthesis and degradation. Collagen degradation is modulated by extracellular MMPs and tissue inhibitors of metalloproteinases (TIMPs). MMPs are zinc-dependent proteases critical for ECM remodeling, particularly in post-infarction cardiac remodeling and are significant factors in cardiac remodeling following myocardial infarction (Fan et al., 2014). Tissue inhibitor of metalloproteinase-1 (TIMP-1) is a glycoprotein found in various body fluids and tissues. It can inhibit the activity of nearly all MMPs, with particular efficacy against MMP-1, MMP-3, and MMP-9 (Wang et al., 2015). With respect to collagen degradation, TIMP-1 and MMPs play key roles in the preservation of normal myocardial architecture and functionality. Alterations in the TIMP-1/MMP ratio can lead to an imbalance between collagen synthesis and degradation, contributing to the development of MF. S. miltiorrhiza influences the degradation of the ECM by modulating the level of expression of MMPs and TIMPs, thus mitigating the progression of MF.
Mao et al. (2014) reported that 0.1–10 mM Tan IIA downregulated Col-I collagen gene expression and collagen deposition in HCFs by regulating the PKA/CREB phosphorylation pathway while increasing the production of new elastic fibers. Tan IIA upregulated the synthesis of MMP-1 and downregulated the levels of MMP-2 and MMP-9. The results showed that Tan IIA interacts with non-canonical estrogen receptors to maintain an appropriate balance between the net deposition of collagen and elastin so that the newly deposited matrix has the best durability and elasticity.
Sodium Tan IIA sulfonate (STS) is a derivative of Tan IIA and is characterized as a water-soluble metabolite synthesized through the sulfonation of fat-soluble active metabolites derived from S. miltiorrhiza. Its chemical formula is C19H17O3·SO3Na, and its relative molecular weight is 396.39. This metabolite has various pharmacological properties, including a reduction in myocardial infarction size, a decrease in myocardial oxygen consumption, protection of myocardial cells, enhancement of myocardial contractility, amelioration of myocardial metabolic disorders, and inhibition of platelet aggregation. In vitro studies indicated that (Yang et al., 2009) it enhances the expression and activity of MMP-1 in CFs stimulated with Ang II while inhibiting myofibroblast differentiation.
Cryptotanshinone (CTS) is a diterpenoid quinone metabolite extracted from S. miltiorrhiza. It has a variety of biological activities, such as anti-inflammatory, antibacterial, antioxidant, anti-fibrotic (Zhang et al., 2019) and anti-tumor effects. Its chemical formula is C19H20O3, and its relative molecular weight is 296.36 (Li H. et al., 2021). CTS (10 mg/kg/d) reduced cardiac fibrosis in STZ-treated rats. In addition, the mRNA and protein levels of signal transducer and activator of transcription 3 (STAT 3), MMP-9 and connective tissue growth factor were decreased by CTS in DCM. In vivo and in vitro experiments have shown that CTS can inhibit MF by inhibiting the STAT 3 pathway in diabetic rats with MF (Lo et al., 2017). Another study revealed that (Ma S. et al., 2012) CTS (20 mg/kg/d) could upregulate MMP-2 in the myocardial tissue of ISO-induced MF mice. In addition, in vitro experiments revealed that CTS dose-dependently upregulated and activated MMP-2 in cultured CFs, indicating that the anti-MF effects of CTS regulate MMP-2.
Lv et al. (2017) constructed a rat model of experimental autoimmune myocarditis via cardiac myosin and reported that QSYQDP effectively reduced HYP, PICP, and the PICP/PIIINP ratio in the myocardium. Compared with those in the model group, there was a significant increase in MMP-1 and tissue inhibitor of TIMP-1 mRNA, along with a decrease in the MMP-1/TIMP-1 ratio, which improved MF. Animal studies (Lv et al., 2015) have indicated that QSYQDP (135 mg/kg/d) significantly reduces HMI, LVMI, HYP, PICP, and PIIIN levels in rats with abdominal aortic constriction. It also decreases the PICP/PIIINP ratio and downregulates the expression of MMP-1 and TIMP-1 in myocardial tissue, inhibiting MF.
Qishen granule (QSG) is a modernized preparation of Zhenwu decoction, a classical Traditional Chinese Medicine formula. It is prepared from A. membranaceus, S. miltiorrhiza, Lonicera japonica Thunb. [Caprifoliaceae; Lonicerae Japonicae Flos], Scrophularia ningpoensis Hemsl. [Scrophulariaceae; Scrophulariae Ningpoensis Radix], Cyperus rotundus L. [Cyperaceae; Cyperi Rhizoma], and G. glabra at a ratio of 30:15:10:10:9:6. Tan et al. (2022) reported that QSG (1 mg/mL) downregulates MMP-2, MMP-9, TIMP-1, and TIMP-2 in Ang II-stimulated CFs. It also decreases the MMP-2/TIMP-2 and MMP-9/TIMP-1 ratios, inhibits fibroblast proliferation, downregulates the expression of Col-I and Col-III, and modulates ECM metabolism.
Qi Shen Liu Wei formula granules (QSLWFGs) were prepared from A. membranaceus, S. miltiorrhiza, C. anthriscoides, Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep [Fabaceae; Puerariae Lobatae Radix], R. glutinosa (Gaertn.) Libosch. ex DC. [Scrophulariaceae; Rehmanniae Radix], A. orientale, Leonurus japonicus Houtt. [Lamiaceae; Leonuri Herba], P. notoginseng, Cornus officinalis Siebold & Zucc. [Cornaceae; Corni Fructus], and Prunella vulgaris L. [Lamiaceae; Prunellae Spica] were mixed at ratios of 30:30:30:30:15:15:15:10:10:10:10. An animal study (Hui et al., 2023) revealed that QSLWFG (34.16 g/kg/d) can decrease the percentage of collagen fiber area in myocardial tissue and the protein expression of Col I, Col III, MMP-9, TGF-β1, Smad2, and Smad3 by regulating the TGF-β1/Smad2/3 signaling pathway. This helps alleviate the deposition of the ECM and improves MF in SHRs.
Fuzheng Huayu capsule (FZHYC), made from S. miltiorrhiza, Juglans regia L. [Juglandaceae; Juglandis Semen], Pinus massoniana Lamb. [Pinaceae; Pinus Massoniana Pollen], S. chinensis, Gynostemma pentaphyllum (Thunb.) Makino [Cucurbitaceae; Gynostemmatis Pentaphylli Herba], and Cordyceps mycelium [Ophiocordycipitaceae; Cordyceps Mycelium], is a CCPP for fibrosis. It alleviates hepatic fibrosis and is effective against renal and pulmonary fibrosis (Liu et al., 2005). Qi Yifei and colleagues (Qi et al., 2018) demonstrated that 0.4 g/kg FZHYC effectively inhibited MF in rat models by increasing the expression of the miRNA-29 family, which balances MMPs and TIMPs, specifically MMP2/TIMP2 and MMP9/TIMP1, enhancing ECM metabolism and reducing collagen deposition.
Fuzheng Huayu Prescription (FZHYP) is composed of S. miltiorrhiza, C. mycelium, J. regia, G. pentaphyllum, P. massoniana, and S. chinensis at a ratio of 8:4:2:6:2:2. Zhu et al. (2019b) reported that FZHYP (25 μg/mL, 50 μg/mL, and 100 μg/mL) can reduce the content of Col-I and Col-III and the expression of MMP2, MMP9, TIMP1 and TIMP2 mRNAs and downregulate the expression of the TGF-β1, p-Smad2, Smad3, and p-Smad3 proteins in Ang II-induced CFs compared with those in the control group. Therefore, the cellular and molecular mechanism of its anti-myocardial fibrosis effects may be related to the regulation of TGF-β/Smad signaling pathway-mediated matrix metabolism via the targeting of miR-29b-5p.
Yiqi Huoxue metabolite (YQHXC) is composed of A. membranaceus, P. ginseng, C. tinctorius, S. miltiorrhiza, P. notoginseng, and D. sophia effectively reduces ventricular remodeling in vivo. This effect is achieved by inhibiting the expression of MMP-1 and increasing Col-III levels while decreasing MF when combined with exercise (Li et al., 2011). Table 3 summarizes S. miltiorrhiza’s regulatory effects on ECM degradation enzymes in myocardial fibrosis models.

Table 3. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in effects on the degradation of the ECM and improving MF.
The immune-inflammatory response is central to the pathogenesis of MF, mediated by key inflammatory factors including TNF-α, IFN-γ, IL-6, IL-1β, CRP, MCP-1, and ICAM-1 Inflammatory mediators increase fibroblast expression, alter the myocardial interstitial composition, and promote fibroblast migration. ROS also induce MF through various mechanisms. Increased inflammatory cell activity leads to fibroblast proliferation and differentiation into myofibroblasts, resulting in greater collagen deposition and MF development (Prabhu and Frangogiannis, 2016). The NF-κB transcription factor, which is found mainly in cardiomyocytes, is involved in immune development, response, inflammation, and cancer. It regulates inflammatory responses and immune homeostasis and plays crucial roles in myocardial inflammation, apoptosis, and cardiac remodeling (Hong et al., 2020). The NF-κB signaling pathway mediates fibrotic diseases (Deng et al., 2023). Its activation increases the levels of proinflammatory cytokines, such as IL-1, IL-18, TNF-α, and iNOS (Xu et al., 2020). The activation of iNOS results in significant NO release, worsening tissue injury and contributing to MF and impaired cardiac systolic function (Peng et al., 2022). Tanshinone metabolites from S. miltiorrhiza have anti-inflammatory properties that inhibit inflammatory cytokines and reduce MF.
In a rat model of MF induced by abdominal aortic coarctation, Tan IIA (20 mg/kg/d) significantly downregulated hydroxyproline (HYP) and NF-κB p65 protein expression (Cai et al., 2014).
Luo et al. (2023) established a diabetic cardiomyopathy (DCM) mouse model through high-fat/high-sugar diet and intraperitoneal STZ injection. After the model was successfully established, different doses of Sal B (1.5 mg/kg/d, 3 mg/kg/d) were administered intragastrically. Sal B inhibited inflammatory cell infiltration, inhibited the TGF-β1 signaling pathway by upregulating Smad7, significantly improved cardiac function in DCM rats, inhibited collagen deposition and phenotypic transformation, and reduced MF. In vitro experiments revealed that Sal B significantly inhibited the proliferation, migration, phenotypic transformation, and collagen secretion of CFs induced by high glucose. In vivo and in vitro experiments have shown that Sal B may improve MF by deubiquitinating Smad7, stabilizing Smad7 protein expression, blocking the TGF-β1 signaling pathway, and inhibiting inflammatory cell infiltration.
Danhong injection (DHI) is a standardized extract from S. miltiorrhiza and C. tinctorius (Guan et al., 2013). This formulation has long been used in the clinic for treating ischemic encephalopathy and cardiovascular conditions, such as myocardial infarction and angina pectoris. Studies have revealed that the primary metabolites of DHI include the following metabolites: danshensu, hydroxysafflor yellow A, 5-hydroxymethyl-2-furfural, protocatechuic aldehyde, viologen acid, caffeic acid, Sal A, Sal B, Sal C, protocatechuic acid, and rosmarinic acid (Liu et al., 2013). Chen J. et al. (2019) reported that DHI administration in MI model rats reduces the serum levels of the inflammatory cytokines TNF-α, IL-1β, and IL-6. Interference also inhibits the phosphorylation of NF-κB and IκB-α, enhancing cardiac function and hemodynamic parameters.
Studies (Ye et al., 2023) have shown that the use of the QLQXC can improve cardiac function, increase the 6-min walk distance (6 mwd), and increase the E/A ratio in individuals with HFpEF. Hao et al. (2023) reported that administering different doses of QLQXC (0.25 g/kg/d and 1 g/kg/d) over 8 weeks significantly reduced NF-κB, TGF-β1, MMP2, MMP9, Smad2, and Smad protein levels in the myocardial tissue of rats with heart failure with preserved ejection fraction (HFpEF). This treatment also lowered the serum TNF-α and IL-2 levels, improved diastolic dysfunction, prevented left ventricular hypertrophy, enhanced the inflammatory response, and improved myocardial function in HFpEF rats. Sustained administration of QLQXC (1.0 g/kg/d) (Han et al., 2018) for 4 weeks significantly reduces the serum TNF-α and IL-6 levels in rats with myocardial infarction. It also alleviates MF by decreasing α-SMA in myocardial tissue, inhibiting collagen synthesis, and suppressing CFs activation and myofibroblast formation. This effect is associated with the inhibition of the TGF-β1/Smad3 and NF-κB signaling pathways. Yingdong Lu and colleagues (Lu Y. et al., 2022) reported that QLQXC (100 mg/kg/d) improved myocardial cell organization in rats with CHF resulting from transverse aortic constriction. This is achieved by modulating the intestinal microbiota and the NLRP3 inflammasome, which decreases inflammatory infiltration. QLQXC treatment also decreases the expression of proinflammatory proteins such as IL-1β, NF-κB, and TNF-α in myocardial tissue, leading to improvements in ventricular remodeling, enhanced cardiac function, and MF.
Another study (Zhang et al., 2022b) reported that various doses of the metabolite Zhenzhu tiaozhi capsule (CFZZTZC) (1.2 g/kg/d and 2.4 g/kg/d) can downregulate mRNA expression, inhibit cardiac inflammation, and improve myocardial function in mice with pressure overload.
The Zuo Gui Jiang Tang Shu Xin Prescription (ZGJTP) is composed of P. ginseng, A. membranaceus, O. japonicus, C. officinalis, R. glutinosa, Coptis chinensis Franch. [Ranunculaceae; Coptidis Rhizoma], S. miltiorrhiza, P. montana var. lobata, and Crataegus monogyna Jacq. [Rosaceae; Crataegi Fructus] at a ratio of 18:18:12:12:15:6:9:12:9. It can promote the apoptosis of myocardial cells and inhibit damage to myocardial cells. Huang et al. (2024) reported that ZGJTP (16.84 g/kg/d and 33.67 g/kg/d) effectively reduced the serum levels of TNF-α and IL-1β in MKR mice with diabetic cardiomyopathy. Furthermore, the treatment significantly downregulated the protein and mRNA expression levels of Col-I, Col-III, α-SMA, TLR4, and NF-κB p56 in myocardial tissues while also suppressing the phosphorylation of NF-κB p56. Mechanistically, this study demonstrated that ZGJTP exerts anti-MF effects through the modulation of the TLR4/NF-κB signaling pathway, thereby inhibiting inflammatory factor production.
Yangxin Tongmai Prescription (YXTMP) consists of equal parts of P. ginseng, S. miltiorrhiza, C. cassia, Citrus × aurantium f. aurantium [Rutaceae; Aurantii Fructus Immaturus], and A. orientale. It alleviates intestinal barrier dysfunction, regulates the intestinal flora, reduces inflammatory cytokines, inhibits ventricular remodeling, and enhances cardiac function. YXTMP (12 g/kg/d) effectively reduces (Xiao et al., 2023) the serum TNF-α, IL-1β, and IL-6 levels while increasing the IL-10 level in CHF rats after 4 weeks. Masson staining revealed a decrease in MF and an improvement in cardiac function (Figure 8).

Figure 8. Salvia miltiorrhiza Bunge and its active metabolites regulate NF-κB signaling pathway to prevent MF.
The Qi Shen Yi Qi Prescription (QSYQP) consists of A. membranaceus, S. miltiorrhiza, C. anthriscoides, and P. ginseng at a ratio of 15:12:12:3. A previous study (Zhao, 2016) revealed that QSYQP (0.7 g/kg/d and 1.4 g/kg/d) reduces serum IL-1β, IL-6, and TNF-α levels, inhibiting MF in hypertensive murine models.
YXFTY (3.5 g/kg/d) alleviates myocardial fibrosis in heart failure (HF) rats by suppressing collagen I/III deposition and NF-κB p65 protein expression (Zhang et al., 2022c).
Bushen Huoxue Decoction (BSHXD) is a combination of P. odoratum, S. miltiorrhiza, Cuscuta chinensis Lam. [Convolvulaceae; Semen Cuscutae], A. membranaceus, G. glabra, P. ginseng, L. japonicus, and P. notoginseng, with the respective ratios of these metabolites being 20:30:20:40:10:15:20:5. Xu R. et al. (2024) reported that BSHXD at 1.575 g/mL modulates key proteins in the p38MAPK/p65NF-κB/AQP4 signaling pathway, influences the intestinal microbiota and metabolites, enhances intestinal barrier function, mitigates cardiomyocyte hypertrophy and fibrosis, and improves cardiac functions.
Yixintai (YXT) is a TCM formulation frequently used in the clinical management of HF. Its primary metabolites are composed of A. membranaceus, S. miltiorrhiza, C. tinctorius, P. ginseng, A. orientale, P. cocos, D. sophia, and P. cocos, along with other medicinal substances. The ratio of the individual metabolites is as follows: 15:15:30:10:10:15:15:15. Studies have shown that YXT may enhance cardiac function and lower serum BNP levels in rat models of CHF (Shi et al., 2024). This study revealed that different doses of YXT (Wang Z. et al., 2024) (1.4 g/kg/d, 2.8 g/kg/d, and 5.6 g/kg/d) influenced cardiac function in rats with HF induced by left anterior descending artery ligation. Treatment decreased the expression of inflammatory markers (IL-1β, IL-6, and TNF-α), inhibited NF-κB and PKC expression, modulated the TMAO/PKC/NF-κB pathway, and decreased myocardial hypertrophy and fibrosis. The role of S. miltiorrhiza in regulating inflammation is shown in Table 4.

Table 4. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in inhibiting inflammation and improving MF.
Oxidative stress arises from dysregulation between ROS generation and antioxidant capacity, contributing to cellular damage in MF. This can lead to tissue damage and is associated with many diseases. Oxidative stress generates reactive species such as ROS and reactive nitrogen species (RNS) In pathological states, an overproduction of oxygen free radicals or a weakened antioxidant defense can disrupt the balance between their generation and elimination, resulting in ROS accumulation. The onset and progression of MF are associated with oxidative stress (Kura et al., 2020). Oxidative stress activates NF-κB, leading to the production of TNF-α (Nizamutdinova et al., 2013). In myocardial injury, NF-κB translocates to the nucleus, binds to TNF-α, and initiates its transcription, contributing to MF. TNF-α also induces proto-oncogenes such as c-myc and c-fos, promoting fibrosis. A study (Li et al., 2000) revealed that oxygen free radicals enhance AT1R expression and mRNA via ox-LDL. They also increase Ang II synthesis by stimulating the release of endothelin, promoting VSMC proliferation and contributing to myocardial hypertrophic fibrosis.
Salvianic acid A (SAA), a phenolic aromatic acid from S. miltiorrhiza, is known as β-(3,4-dihydroxyphenyl) lactic acid. It has a white, needle-like crystalline structure and a molecular formula of C9H10O5. This study demonstrated that SAA (160 mg/kg) reduced hydroxyproline content and superoxide dismutase (SOD) activity while increasing malondialdehyde (MDA) levels in a LAD coronary artery occlusion-induced myocardial infarction mouse model. Additionally, it decreased the myocardial collagen volume fraction and SEC61α protein expression, suppressed oxidative stress, and ameliorated MF (Liu and Wang, 2021).
Yuan et al. (2019) reported that 15 mg/kg Tan IIA reduced myocardial oxidative stress and Nox4 expression in a rat model of heart failure. This treatment also increased SOD activity, alleviating MF. An animal study (Cai et al., 2016) revealed that 20 mg/kg Tan IIA enhances cytoprotection by increasing HO-1 expression, reducing MF, and delaying ventricular remodeling. Ruijuan Chen et al. (2021) reported that 1.5 mg/kg/d Tan IIA downregulated the mRNA levels of CoL-I, CoL-III, TGF-β, and α-SMA in a rat model of myocardial infarction. It also increased SOD activity and inhibited MF.
STS is a water-soluble derivative synthesized by sulfonation of Tan IIA extracted from S. miltiorrhiza. It has many pharmacological effects and is mainly used to treat cardiovascular diseases. Li Z. et al. (2018) reported that STS at doses of 5 mL/kg/d, 10 mL/kg/d, and 20 mL/kg reduced Ang II-induced MF in a dose-dependent manner. This treatment increased nuclear Nrf2 accumulation, increased the expression of antioxidant enzymes, improved the antioxidant response, and decreased lipid peroxidation in myocardial tissue. Yang et al. (2009) reported that 3 μM, 10 μM, and 30 µM STS effectively suppressed Ang II-induced CoL-I expression and inhibited ROS production while also modulating collagen and MMP-1 expression in CFs.
CTS is a bioactive metabolite extracted from S. miltiorrhiza that has multiple protective effects on cardiovascular diseases (Ma et al., 2014) CTS (30 mg/kg/d, 60 mg/kg/d) attenuated Ang II-induced upregulation of fibronectin, connective tissue growth factor, and cyclooxygenase-2 and normalized Ang II-induced upregulation of extracellular signal-regulated kinase 1/2 (ERK 1/2). Moreover, CTS inhibited Ang II-stimulated upregulation of NAD(P) H oxidase 2 and 4 (NOX-2 and NOX-4) and reactive oxygen species production. These findings suggest that CTS may play an anti-myocardial fibrosis role by inhibiting the phosphorylation of extracellular signal-regulated kinase 1/2 and the expression of COX-2, NOX-2, and NOX-4 induced by Ang II, improving pathological changes in the myocardium in vivo and reducing MF.
S. miltiorrhiza is a sterilized aqueous extract with various pharmacological properties, such as improved hemodynamics, anti-inflammatory effects, and antioxidant activity (Qiao et al., 2024) High-performance liquid chromatography can be used to identify three main metabolites: SAA (2.15 mg/mL), protocatechuic aldehyde (0.44 mg/mL), and Sal B (1.01 mg/mL). Danshen injection (3 g/kg/d or 6 g/kg/d) effectively prevents and treats oxidative stress injuries in zebrafish hearts and livers caused by iron overload. It reduces Hyp and MDA concentrations, lowers MMP-9 levels in mice, enhances SOD activity, and alleviates iron overload-related MF in a dose-dependent manner (Zhang, 2015).
Metabolite Danshen Dripping Pills (CDSDP) (Yang et al., 2023a) can reduce ROS in the cardiac tissue of HF rats by inhibiting NRF2 expression and its target genes, leading to lower levels of NRF2, SOD2, and CAT in H9C2 and iPSC-derived cardiomyocytes and suppressing MF. CDSDP (660, 2,640 mpk) (Feng et al., 2021) enhances SOD1, p-AMPK, and NRF2 expression, reducing ROS and FFA levels. It also inhibits TGFβ1, αSMA, COL1A2, COL3A1, and MMP9 in myocardial tissue, decreasing MF.
Guanxin V (GXV) is a TCM formulation composed of Codonopsis pilosula (Franch.) Nannf. [Campanulaceae; Codonopsis Radix], O. japonicus, and other botanical drugs (Liang et al., 2020). Compared with the control, GXV significantly improved cardiac function in patients with coronary heart disease. A study (Liang et al., 2022) revealed that GXV (6 g/kg/d) decreased MDA and LDH levels in murine myocardial infarction models while increasing SOD, CAT, T-AOC, and GSH-Px levels, suggesting that GXV may reduce oxidative stress-related damage and inhibit MF.
Guanxintai (GXT) is a TCM approved by the CFDA in 1999 for managing coronary heart disease. Its botanical drugal formulations are composed of P. ginseng, A. membranaceus, R. glutinosa, O. japonicus, S. chinensis, Boswellia sacra Flück. [Burseraceae; Boswelliae Resina], Commiphora myrrha (T.Nees) Engl. [Burseraceae; Myrrha], Angelica sinensis (Oliv.) Diels [Apiaceae; Angelicae Sinensis Radix], C. anthriscoides, Picrorhiza kurroa Royle ex Benth. [Scrophulariaceae; Picrorhizae Rhizoma], Achyranthes bidentata Blume [Amaranthaceae; Achyranthis Bidentatae Radix], S. miltiorrhiza, Acorus gramineus Aiton [Acoraceae; Acori Graminei Rhizoma], and L. japonicus. Clinical studies indicate that GXT improves angina pectoris and arrhythmia and lowers blood lipid levels. A study (Yang et al., 2017) revealed that GXT (2 g/mL) reduces ROS levels and the expression of NOX and phosphorylated p38 MAPK proteins, leading to a decrease in cardiomyocyte injury and MF.
Danxiong Tongmai Granules (DXTMGs) are formulated from a combination of P. lactiflora Pall. [Paeoniaceae; Paeoniae Radix Alba], C. anthriscoides, S. miltiorrhiza, Reynoutria multiflora (Thunb.) Moldenke [Polygonaceae; Reynoutriae Multiflorae Caulis], Corydalis yanhusuo (Y. H. Chou & Chun C. Hsu) W. T. Wang ex Z. Y. Su & C. Y. Wu [Papaveraceae; Corydalis Rhizoma], C. rotundus, C. tinctorius and Lycium chinense Mill. [Solanaceae; Lycii Fructus]. Animal studies have indicated that 5 g of DXTMG significantly increases the serum levels of LDH, cTn-I, and MDA in heart failure model rats. It also increases SOD and GSH-PX levels while decreasing IL-6 and TNF-α levels and inhibiting MF (Ye et al., 2024). Table 5 summarizes S. miltiorrhiza’s antioxidative effects and underlying mechanisms in MF.

Table 5. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in inhibiting oxidative damage and improving MF.
Cardiomyocyte apoptosis promotes extracellular matrix remodeling and fibroblast activation in MF. Cardiomyocyte apoptosis is triggered by the interferon response of hosts or viral signals during myocarditis-related cardiac injury. This activates pathways such as the FAS/FASL, JAK-STAT, and mitochondria-dependent pathways, increasing the expression of proapoptotic molecules such as FasL, Fas, Bax, and cleaved Caspase-3. Caspase-3 activation is a critical apoptotic effector, mediating DNA fragmentation and cell death (Mazumder et al., 2008). Preclinical evidence (Yang et al., 2023b) demonstrates S. miltiorrhiza bioactives attenuate cardiomyocyte apoptosis via multiple pathway (Yang et al., 2008).
Chen Y. F. et al. (2017) reported that Tan IIA downregulates proteins such as MMP-9, MMP-2, TGF-β1, p-Smad2/3, SP-1, and CTGF in cardiomyocytes. It also upregulates TIMP-1 and TIMP-2 while lowering Caspase-3 and Caspase-9 levels, leading to a decrease in cardiomyocyte apoptosis and MF.
Sal A is a hydrophilic metabolite derived from S. miltiorrhiza that has several pharmacological properties, including antioxidant and anti-fibrotic effects. Anwaier et al. (2022) reported that administering various doses of Sal A (10 mg/kg/d, 20 mg/kg/d, and 40 mg/kg/d) via intraperitoneal injection enhances cardiac function in rats with DOX-induced MF. These findings indicated a reduction in the serum levels of tumor necrosis factor-α (TNF-α), homocysteine (Hcy), and endothelin (ET), as well as a decrease in galectin-3 and TGF-β/Smad protein expression in myocardial tissue, significantly reducing myocardial cell apoptosis, thereby inhibiting MF, and that high-dose Sal A treatment is optimal.
DHI intervention for 4 weeks significantly reduces myocardial interstitial collagen density in a rat model of heart failure. It suppresses TGF-β1, MMP-2, MMP-9, and Caspase-3 while enhancing Bcl-2 expression, improving cardiac function, and inhibiting MF (Chen et al., 2016).
Lichan Tao and colleagues reported that (Tao et al., 2015) QLQXC (0.5 g/kg/d) significantly reduced CoL-I, CoL-III, and α-SMA levels in a rat model of heart failure. QLQXC treatment also decreased the expression of TGF-β1, MMP-2, and MMP-9 and the Bax/Bcl-2 ratio, indicating that it can protect the myocardial cell structure and reduce MF.
Lv et al. (2022) reported that at doses of 135 mg/kg/d, 270 mg/kg/d, and 540 mg/kg/d, QSYQDP significantly reduced CoL-I and CoL-III in the myocardial tissue of rats with autoimmune cardiomyopathy. This occurred due to the downregulation of Bcl-2, upregulation of Bax and caspase-3, and inhibition of myocardial cell apoptosis, resulting in a decrease in MF, particularly in the high-dose group. Another study (Anwaier et al., 2022) reported that QSYQDP (0.8 g/kg/d) effectively decreased serum NT-ProBNP, LDH, and cardiac MDA levels in rats with HF from aortic coarctation. It also decreased the ratios of caspase-3, caspase-9, Bax, and Bcl-2, inhibited cardiomyocyte apoptosis, and enhanced myocardial function. Wang L. et al. (2019) reported that administering 5 mg/kg/d QSYQDP for 4 weeks significantly reduced Bax, caspase-3, Bcl-2, and α-SMA protein levels in the myocardial tissue of rats with doxorubicin-induced HF, leading to a decrease in myocardial apoptosis and the inhibition of fibrosis.
Guanxinning tablet (GXN), derived from S. miltiorrhiza and C. anthriscoides at a 1:1 ratio, show significant pharmacological efficacy in preventing and managing cardiovascular diseases (Hu et al., 2017). These metabolites effectively suppress sympathetic nerve activity, enhance left ventricular function, improve hemorheological abnormalities, increase plasma NO levels in rat models, and provide therapeutic benefits for myocardial ischemia. Zhang Y. et al. (2023) reported that GXN at doses of 600 mg/kg/d and 1,200 mg/kg/d effectively inhibited cardiomyocyte apoptosis, reduced Bax and Bcl-2 mRNA and protein levels, and suppressed myocardial remodeling and fibrosis in rats with heart failure induced by aortic coarctation.
Liang et al. (2022) demonstrated that GXV (0.25 g/L, 0.50 g/L, 0.75 g/L, and 1 g/L) effectively inhibited cardiomyocyte apoptosis and MF in a dose-dependent manner.
The newly optimized formulation of Shengmaisan (NO-SMS) is derived from the traditional Shengmaisan recipe. This formulation is processed into granules through a series of methods, including boiling, extraction, and vacuum techniques, following a specific ratio of 10:10:10:10:6:10:6:10:6:10. The metabolites used in this formulation are composed of A. membranaceus, C. pilosula, Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. [Araliaceae; Acanthopanacis Senticosi Radix et Rhizoma], Tinospora crispa (L.) Hook. f. & Thomson [Menispermaceae; Tinosporae Caulis], D. sophia, P. cocos, C. × aurantium f. aurantium, and S. miltiorrhiza. The active metabolites identified in this optimized formulation include isorhamnetin, quercetin, kaempferol, and tanIIA (Hou et al., 2023) (0.81 g/kg/d), which significantly reduce myocardial cell apoptosis in rats with HF. This treatment downregulated proteins such as Caspase-3, Caspase-9, Caspase-12, Cyt-c, and Bax while increasing the Bcl-2/Bax ratio, thus inhibiting apoptosis and mitigating MF. Table 6 summarizes S. miltiorrhiza’s anti-apoptotic effects and underlying mechanisms in MF.

Table 6. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in anti-apoptotic action and improving MF.
Following cardiac injury, fibroblasts activate and differentiate into myofibroblasts, which secrete excessive ECM components (e.g., collagen) to repair the myocardium. Excessive activation can cause the accumulation and remodeling of the ECM, impairing cardiac function and potentially leading to heart failure (Frangogiannis, 2021). The abnormal proliferation of CFs is linked to several cardiovascular disorders, such as hypertension and myocardial infarction. Regulating this proliferation is essential for managing MF. S. miltiorrhiza and its bioactive metabolites can reduce MF by inhibiting fibroblast proliferation and collagen fiber formation (Yang et al., 2022).
Different concentrations of Tan IIA (0.01 mmol/L, 0.1 mmol/L, and 1 mmol/L) reduce the proliferation rate, Hyp levels, and mRNA expression of CoL-I, CoL-III, and TIMP-2 in Ang II-induced CFs (Wang S. et al., 2019). Fu and Sun (2016) reported that Tan IIA (10–6 mol/L) could reduce the proliferation rate of Ang II-induced CFs and the content of CoL-I, increase the percentage of CFs in the G0/G1 phase, and decrease the percentage of cells in the S phase. Additionally, Tan IIA inhibited the protein expression of PKC and cyclin D1, indicating that Tan IIA significantly suppressed CF proliferation and collagen secretion by modulating the PKC-cyclin D1 signaling pathway. Chan et al. (2011) reported that Tan IIA, at concentrations ranging from 3 to 10 μM, effectively inhibited the proliferation of CFs and reduced the ROS levels induced by Ang II.
Han et al. (2022) reported that different concentrations of Sal A (25, 50, and 100 mg/L) inhibited the proliferation of CFs induced by high glucose and reduced the secretion of Col-I and Col-III in CFs. Sal A significantly inhibited the expression of TGF-β1 and β-catenin but upregulated the expression of p-GSK-3β at a concentration of 100 mg/L. These results suggest that Sal A inhibits CF proliferation by modulating the Wnt signaling pathway.
Wang et al. (2020) reported that Sal B (12.5 μmol L–1, 25 μmol L–1, and 50 μmol L–1) effectively inhibited the proliferation of CFs stimulated with high glucose levels and reduced α-SMA, β-catenin, and p-GSK 3β protein expression. These findings suggest that Sal B may impede CF proliferation and transdifferentiation by modulating the Wnt/β-catenin signaling pathway, enhancing MF. Huang et al. (2017) reported that Sal B at concentrations of 2.5 × 10−5, 5 × 10−5, and 5 × 10−4 mol L–1 effectively reduced Ang II-induced proliferation of CFs. This treatment decreased Hyp levels and downregulated collagen type I and alpha-smooth muscle actin expression, alleviating MF. Luo et al. (2014) reported that Sal B at concentrations of 10 μmol/L, 30 μmol/L, and 100 μmol/L effectively inhibited TGF-β1-induced CF proliferation, with the highest concentration having the most significant effect on enhancing MF. Wang et al. (2011) reported that Sal B (0.01 µM, 0.1 µM, 1 μM, and 10 µM) significantly inhibited the MMP9-induced proliferation of CFs and their transformation into myofibroblasts, providing protective effects for the heart. Lu et al. (2024) conducted in vivo and in vitro experiments and revealed that Sal B (20 mg/kg/d and 40 mg/kg/d) effectively inhibited the aberrant expression of TRPC6. Downregulation of Smad3 inhibited the proliferation of fibroblasts, reduced the activation of the TGF-β/Smad3 signaling pathway, and alleviated diabetic MF. Sun (2017) Huangqi Baoxin Decoction (HQBXD) (15 g/kg) and its active metabolite Sal B (5 mol/L, 6 mol/L, and 7 mol/L) effectively mitigate Ang II-induced proliferation and collagen synthesis in CFs. This effect is achieved through the inhibition of the TGF-β/Smad signaling pathway, which decreases the expression levels of Col I and Col III mRNAs, as well as the proteins galectin-3, TGF-β, and Smad3.
Sun et al. (2023) reported that different doses of QLQXC (0.1 mg/mL and 0.5 mg/mL) could downregulate the expression of CoL-I, CoL-III, PAI-1, TGFβ1, and p-Smad proteins to different degrees, indicating that QLQXC could inhibit the activation of CFs induced by adriamycin by inhibiting the PAI-1/TGFβ1/Smad3 pathway.
QSG comprises six botanical drugs (A. membranaceus, S. miltiorrhiza, A. carmichaelii, S. ningpoensis, G. glabra, and L. japonica and comes from two well-known TCM formulae, namely, “Zhen Wu Decoction” and “Simiao Yongan Decoction.” QSG (Tan et al., 2022) (1 mg/mL) inhibited the proliferation of CFs and reduced the content and mRNA expression of Col-I and Col-III.
The formulation of Yixintai Granules (YXTG) is composed of A. membranaceus, C. tinctorius, S. miltiorrhiza, A. orientale, P. umbellatus, and D. sophia at proportions of 3:1:1:2:1:1. The preparation process involves extraction, filtration, and concentration to create granules, which are commonly used in clinical settings for treating CHF. Yang et al. (2021) reported that plasma containing YXTG significantly inhibits the proliferation of CFs induced by Ang II, causing cell cycle arrest in the G0/G1 phase and downregulating the mRNA levels of PCNA, α-SMA, Col-I, and Col-III. YXTG was also shown to impede the proliferation, differentiation, and fibrotic activity of CFs.
Yixintai (YXT) is formulated from a combination of A. membranaceus, S. miltiorrhiza, C. tinctorius, A. orientale, and Waltheria indica L. [Sterculiaceae; Waltheriae Indicae Herba] at proportions of 3:1.5:1:1:1. YXT is prepared by producing a dry powder from its alcohol extract through filtration, vacuum concentration, and drying. Studies have indicated that YXT can improve ventricular remodeling and cardiac function in CHF models in rats and rabbits (Tang and Guo, 2020a; Tang and Guo, 2020b; Tang et al., 2015). Tang et al. (2021) reported that YXT alcohol extracts (50, 100, and 200 mg/L) significantly reduced the viability of CFs from neonatal rabbits stimulated with Ang II, inhibited CF proliferation, and suppressed myofibroblast differentiation.
The Huoxue Qianyang Qutan Recipe (HQQR) was formulated via a combination of S. miltiorrhiza, Concha Ostreae [Ostreidae; Concha Ostreae], C. anthriscoides, Uncaria rhynchophylla (Miq.) Miq. [Rubiaceae; Uncariae Ramulus cum Uncis], Taxillus chinensis (DC.) Danser [Loranthaceae; Taxilli Ramulus], C. monogyna, and Curcuma zedoaria (Christm.) Roscoe [Zingiberaceae; Curcumae Zedoariae Rhizoma] at a drug ratio of 9:20:9:15:15:9:30. In vitro studies (Lu et al., 2024) indicated that HQQR doses of 1 mg/mL and 1.25 mg/mL effectively reduced Ang II-stimulated CF proliferation, decreased Hyp levels, and increased MF, with higher doses showing greater efficacy.
Yiqi Huoxue Decoction (YQHXD) Cui et al. (2021), derived from the Buyang Huanwu Decoction, consists of P. ginseng, A. membranaceus, P. lactiflora, C. anthriscoides, J. regia, C. tinctorius, P. notoginseng, S. miltiorrhiza, Curcuma aromatica Salisb. [Zingiberaceae; Curcumae Aromatica Rhizoma], C. reticulata, Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; Dolomiaeae Costus], P. odoratum (Mill.) Druce [Asparagaceae; Polygonati Odorati Rhizoma], and L. chinense Mill. [Solanaceae; Lycii Fructus] at ratios of 3:20:5:5:5:5:2:2:2:2:2:2 and is prepared as a freeze-dried powder. Various concentrations of YQHXD (40 μg/mL, 80 μg/mL, and 160 μg/mL) effectively reduced Col-I and Col-III protein levels in CFs, inhibited their proliferation, and protected cardiomyocytes.
Fuzheng Huayu Decoction (FZHYD) is formulated with a combination of S. miltiorrhiza, C. mycelium, G. pentaphyllum, P. massoniana, and S. chinensis and adheres to a specific ratio of 8:4:2:6:2:2. Zhu et al. (2019a) demonstrated that FZHYD at concentrations of 25 μg/mL, 50 μg/mL, and 100 μg/mL inhibited Ang II-induced proliferation of CFs in a dose-dependent manner.
Jiashenfang (JSF) is an effective treatment for CHF. This herbal formula consists of the following botanicals: Periploca sepium Bunge [Apocynaceae; Periplocae Cortex], P. notoginseng, A. membranaceus, SM, L. japonicus, D. sophia, P. cocos, and A. orientale. Following concentration screening, Cui et al. (2016) reported that the viability of CFs was not adversely affected by the application of 0.25 mg/mL JSF extract. Subsequent experimental investigations using the same concentration of JSF extract revealed its ability to decrease the fluorescence intensity of α-SMA and reduce the expression of Hyp in CFs stimulated with TGF-β1.
The Bushen Huoxue Decoction (BSHXD) is composed of the following botanicals: C. officinalis, Cistanche deserticola Ma [Orobanchaceae; Cistanches Herba], P. lactiflora, C. anthriscoides and S. miltiorrhiza. Specific dosages are not provided in this study. Ma X. et al. (2012) studied SD rats given 800 mg/d BSHXD for 4 days to obtain enriched serum. The results showed that 10% and 20% concentrations of this serum significantly inhibited CF proliferation and reduced Col-I and Col-III levels. SM inhibited the proliferation of CFs as shown in Table 7.

Table 7. Effective active metabolites and metabolite preparations of Salvia miltiorrhiza Bunge in inhibition of the proliferation of CFs and Improving MF.
Many researchers have focused on the basic use of S. miltiorrhiza in the treatment of MF, and several researchers have focused on its clinical application. To further verify its anti-MF effect and safety, researchers have conducted many randomized controlled trials (RCTs). Several experiments have shown that S. miltiorrhiza and its metabolite preparations have better clinical efficacy in combination with biomedicine medicine and that the two play a synergistic role.
A clinical study retrospectively analyzed the medical records of 80 patients after PCI (Deng, 2023). The control group was treated with ivabradine hydrochloride tablets, while the observation group was subjected to STS injection (40 mg/d) on the basis of the control group. After 2 weeks of continuous treatment, the total effective rate of treatment in the observation group was 92.50%, and that in the control group was only 75.00%. The levels of serum CTGF, sST2, TGF-β1, and Gal-3 decreased, and the effect was greater than that of simple ivabradine treatment. These findings indicate that the combination of STS with ivabradine for the treatment of patients after PCI can improve the total effective rate of treatment, improve cardiac function, reduce MF, and improve the effect of simple ivabradine treatment.
Chen Z. et al. (2019) collected 176 patients with AMI and randomly divided them into a control group and an observation group, with 88 patients in each group. The control group was given alteplase for injection on the basis of routine treatment, whereas the observation group was given DHI on the basis of the control group. After 2 weeks of continuous treatment, the levels of Gal-3, TGF-β1, CTGF, NF-κB, cystatin C, MMP-9, and FGF-23 in the two groups significantly decreased, and those in the treatment group were significantly greater than those in the control group. The total effective rate was 74% in the control group and 83% in the observation group. After 6 months of follow-up, there were 2 cases of MACE in the observation group and 6 cases in the control group. DHI combined with alteplase was helpful in inhibiting MF and ventricular remodeling, and the effect was better than that of alteplase alone.
Xu et al. (2019) used rosuvastatin calcium tablets combined with metabolite Danshen dripping pills (FFDSDP) (10 pills/time, 3 times/d) for 24 weeks to treat patients after PCI. The serum levels of MMP-9, TGF-β1, and CTGF in the observation group were lower than those in the control group, and the total effective rate was 39% in the observation group and 33% in the control group. During the treatment period, there were no obvious adverse reactions in the two groups, indicating that the application of rosuvastatin combined with FFDSDP can better inhibit MF and improve the clinical symptoms of patients.
Li and Sun (2023) reported that a QLQXC (1.2 g/time, 3 times/d) combined with levosimendan for 3 months could reduce the levels of NT-pro BNP, cTnI, cTnTLN, HA and PCIII in elderly patients with CHF and can also reduce the expression of serum CRP, IL-6 and TNF-α. The clinical efficacy of the observation group (95%) was better than that of the control group (77.5%), indicating that QLQXC combined with levosimendan can improve the clinical effect, reduce myocardial injury in patients, promote the downregulation of inflammatory factors, improve MF, and alleviate clinical symptoms.
CHF patients (Xu Q. et al., 2024) were treated with Shengmai Qiangxin granules (SMQXG) (1 bag/time, 3 times/day) on the basis of conventional biomedicine medicine for 4 weeks. After 4 weeks of treatment, the levels of serum TGF-β1, MMP-2, and PIIINP decreased, and the expression of TLR4, MyD88, and NF-κB mRNA and protein decreased. The total effective rate of the conventional biomedicine medicine treatment group was 26%, and the total effective rate of the control group was 36%. There was no significant difference in the incidence of adverse reactions between the two groups, indicating that SMQXG inhibited MF by regulating TLR4/MyD88/NF-κB signaling and alleviated the clinical symptoms of patients. In addition, the safety is good.
Shenfu Yixin Decoction (SFYXD) is formulated with the following metabolites: S. miltiorrhiza, C. cassia, Zingiber officinale Roscoe [Zingiberaceae; Zingiberis Rhizoma], A. orientale, A. membranaceus, D. sophia, C. rotundus, P. ginseng, A. macrocephala, P. cocos, G. glabra, Plantago asiatica L. [Plantaginaceae; Plantaginis Semen], and Areca catechu L. [Arecaceae; Arecae Semen], at ratios of 20:10:6:10:20:10:15:8:20:15:9:10:10. Clinical studies (Wang Y. et al., 2024) have shown that the basic treatment of biomedicine medicine combined with SFYXD for 2 months to treat HFrEF results in lower serum levels of sCD146, NT-proBNP, cTnI, Gal-3, Ang II, CgA and sST2 than those in the basic treatment group of biomedicine medicine, and PCO2, DO2, VO2 and LVEF are higher than those in the basic treatment group of biomedicine medicine. The total effective rate of SFYXD combined with biomedicine medicine was 98.41%, whereas that of the biomedicine medicine treatment group was only 84.13%, indicating that SFYXD can promote the repair of myocardial injury, regulate the oxygen dynamics index and reduce MF. The effect was better than that of pure biomedicine medicine.
The Yiqi Huoxue prescription (YQHXP) consists of A. membranaceus, S. miltiorrhiza, Rhodiola rosea L. [Crassulaceae; Rhodiolae Radix et Rhizoma], C. pilosula, C. cassia, P. lactiflora, O. japonicus, S. chinensis and Vitex negundo L. [Verbenaceae; Viticis Negundi Herba]. The formula ratio is 20:20:15:10:3:10:10:10:10:10. It is commonly used for the clinical treatment of HF. Zhang C. et al. (2023) selected 41 patients with acute ST-segment elevation myocardial infarction and divided them into a control group (n = 20) and an observation group (n = 21). The control group was given aspirin + ticagrelor + atorvastatin calcium tablets. Angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor enkephalinase inhibitors (ARNI), β-receptor blockers (β-RBs), and aldosterone receptor antagonists were used to reduce blood pressure and heart rate. For the standard treatment of myocardial infarction, the observation group was treated with YQHXP on this basis, and the course of treatment was 12 weeks. Through clinical research, YQHXP combined with conventional drugs after 12 weeks of treatment was shown to reduce the levels of Lp-PLA2, hs-CRP, IL-6, NT-proBNP, ECV, and T1 in patients with heart failure, and the major cardiovascular adverse events of the two groups were not statistically significant, indicating that YQHXP can improve the clinical symptoms of patients with acute ST-segment elevation myocardial infarction after PCI and inhibit myocardial fibrosis. The mechanism may be related to the reduction in the inflammatory response.
The Shugan Yixin prescription (SGYXP) is composed of the following botanicals: Bupleurum chinense DC. [Apiaceae; Bupleuri Radix], S. miltiorrhiza, P. ginseng, Pinellia ternata (Thunb.) Makino [Araceae; Pinelliae Rhizoma], Moringa oleifera Lam. [Moringaceae; Moringae Oleiferae Folium], Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae Radix], Prunus armeniaca L. [Rosaceae; Armeniacae Semen], C. chinensis Franch. [Ranunculaceae; Coptidis Rhizoma], P. cocos, C. yanhusuo (Y. H. Chou & Chun C. Hsu) W. T. Wang ex Z. Y. Su & C. Y. Wu [Papaveraceae; Corydalis Rhizoma], and G. glabra, at ratios of 15:20:10:10:12:12:10:6:12:10:6. Gan et al. selected 82 patients with coronary heart disease and divided them into a control group and an observation group, with 41 patients in each group. The control group was given isosorbide dinitrate tablets + enalapril maleate tablets + digoxin tablets + metoprolol tartrate tablets + aspirin enteric-coated tablets. The observation group was treated with SGYXP on the basis of the control group. The treatment period was 4 weeks. After 4 weeks, the corresponding indicators were detected (Gan et al., 2020). After 4 weeks of continuous intervention, SGYXP significantly reduced the serum BNP, CRP, HA, and PCIII levels in patients with coronary heart disease. The effective rate of the observation group was 35%, and the effective rate of the control group was 19%. These findings indicate that SGYXP has a significant effect on the treatment of coronary heart disease and can improve MF and promote the recovery of cardiac function.
Shenyuan Yiqi Huoxue Decoction (SYYXHXD) is composed of the following components: A. membranaceus, S. miltiorrhiza, C. pilosula, S. ningpoensis, C. yanhusuo, Aloe vera (L.) Burm. f. [Asphodelaceae; Aloes], Eupolyphaga seu Steleophaga (ground beetle), Hirudo (leech) at a ratio of 30:30:15:15:10:10:6:3. In randomized controlled trials, Chu et al. (2020) 84 patients with coronary heart disease and heart failure were randomly divided into a treatment group and a control group, with 42 cases in each group. The control group was given routine treatment, such as antiplatelet aggregation, statins, β-blockers, nitrates, angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists, and diuretics. The treatment group was given SYYXHXD on the basis of the control group. The results revealed that the levels of plasma thrombin, TGF-β1, NT-proBNP, serum Col-I, and Col-III were significantly decreased in the treatment group. Compared with those before treatment, the blood, urine, and stool parameters and liver and kidney function of the two groups were not significantly aggravated. These findings indicate that SYYXHXD combined with conventional biomedicine medicine can reduce the MF level in coronary heart disease patients and that the effect is better than that of biomedicine medicine alone.
Zhenwu Baoxin Decoction (ZWBXD) contains processed C. rotundus, P. cocos, W. indica, P. lactiflora, A. macrocephala, Z. officinale, C. cassia, S. miltiorrhiza, and G. glabra at a ratio of 9:9:9:9:15:5:10:15:10. Yan (2019) randomly divided 83 patients with CHF into a control group and an observation group. Forty-one patients in the control group were treated with telmisartan tablets + spironolactone tablets, and 42 patients in the observation group were treated with ZWBXD on the basis of biomedicine medicine. After 4 weeks of continuous treatment, the levels of serum PCI, PCIII, HA and LN in CHF patients decreased, the LVEF, SV, and 6 MWT increased, and those in the observation group were better than those in the control group, indicating that ZWBXD combined with biomedicine medicine can effectively improve the cardiac function of CHF patients and prevent MF and that the curative effect is better than that of biomedicine medicine alone.
Jiawei Wendan Decoction (JWWDD) contains: Trichosanthes kirilowii Maxim. [Cucurbitaceae; Trichosanthis Fructus], S. miltiorrhiza, C. pilosula, Phyllostachys nigra var. henonis (Mitford) Rendle [Poaceae; Phyllostachydis Henonensis Caulis], Smilax glabra Roxb. [Liliaceae; Smilacis Glabrae Rhizoma], P. ternata, C. reticulata, and C. anthriscoides at ratios of 20:20:15:15:10:10:10:10. Xie et al. divided patients after PCI into an observation group and a control group. The observation group was treated with low-molecular-weight heparin calcium injection + aspirin enteric-coated tablets + clopidogrel bisulfate tablets + atorvastatin calcium tablets. The treatment group was combined with JWWDD on the basis of the observation group. After 6 months of continuous treatment, the levels of PCI, HA, PCIII, and LN in patients after PCI significantly decreased, the SAQ score significantly increased, and the LVEF, CO, and SV significantly increased compared with those before treatment. The clinical efficacy of the control group (70.15%) was better than that of the observation group (88.24%). This finding shows that Jiawei Wendan decoction can reduce the degree of MF in patients after PCI and enhance their cardiac function, and the effect is significant (Xie et al., 2018).
The Yiqi Huayu Decoction (YQHYD) combines Baoyuan and Xuefu Zhuyu decoctions, consisting of A. membranaceus, C. chinensis, A. sinensis, D. sophia, C. anthriscoides, Panax quinquefolius L. [Araliaceae; Panax Quinquefolii Radix], A. vera, P. lactiflora, J. regia, S. miltiorrhiza, leech, L. japonicus, and C. cassia, at ratios of 30:6:10:10:15:15:15:15:15:15:12:20:5:15:30:5. In one study (Cui et al., 2017), 154 patients with CHF were randomly divided into a control group and an observation group. The control group was treated with telmisartan tablets, metoprolol tartrate tablets and spironolactone. Compared with the control group, the observation group was treated with YQHYD (1 dose per day) for 3 months. The results revealed that the serum levels of GF-β1, CTGF, MMP-2, HA, PCIII, LN and PCI in CHF patients were decreased, and the clinical efficacy of the control group (50%) was better than that of the observation group (63%), indicating that CHF combined with conventional biomedicine medicine plus YQHYD can improve clinical symptoms and improve cardiac function; the mechanism may be related to the reduction in MF.
Yiqi Yangyin Huoxue Decoction (YQYYHXD) is composed of the following components: A. membranaceus, C. pilosula, O. japonicus, S. chinensis, C. cassia, R. glutinosa, S. miltiorrhiza, C. anthriscoides, L. japonicus, and G. glabra at ratios of 30:15:20:10:10:30:25:15:15:12. Lv’s team (Lu G. et al., 2022) randomly divided 82 CHF patients into a control group and a study group, with 41 patients in each group. The control group was treated with telmisartan tablets, metoprolol tartrate tablets, and spironolactone. On this basis, the study group was treated with YQYYHXD. The course of treatment in both groups was 3 months. The results revealed that the levels of LVEF, SV, E/A, and the 6 MWT in the two groups were significantly increased, the levels of plasma NT-proBNP were significantly decreased, and the levels of serum IL-1β, hs-CRP, TNF-α, MMP-9, TGF-β1, PCIII, sST2 and galectin-3 were decreased. The clinical efficacy of the control group (70.7%) was better than that of the observation group (90.2%), indicating that YQYYHXD could relieve the clinical symptoms of patients and inhibit MF. The mechanism may be related to the inhibition of the inflammatory response.
The Baoxin mixture (BXHJ) is composed of the following components: A. membranaceus, C. pilosula, Testudinis Plastrum, S. miltiorrhiza, Forsythia suspensa (Thunb.) Vahl [Oleaceae; Forsythiae Fructus], C. cassia, S. glabra, Triticum aestivum L. [Poaceae; Triticus Aestivus], and S. chinensis, at ratios of 40:20:15:15:15:15:6:15:15:6.
Bai’s team (Bai et al., 2021) randomly divided 100 patients with heart failure into a control group and a treatment group, with 50 patients in each group. The control group was treated with diuretics, antiplatelet aggregation, lipid-lowering and plaque-stabilizing agents, aldosterone receptor antagonists, ARNI, β-receptor blockers and other conventional biomedicine medicines for heart failure. The treatment group was treated with the BXHJ on this basis. Both groups were treated for 12 days. The results revealed that the levels of plasma IL-1β, TNF-ɑ, IL-6, CRP, sST2, and Gal-3 in the two groups were decreased, and the combined treatment effect was better than that of conventional biomedicine medicine.
Yiqi Yangyin Huoxue Buxin Decoction (YQYYHXBXD) is formulated at a ratio of 30:10:15:9:20:9:20:9:20:6:6:12:6:12 and comprises A. membranaceus, P. ginseng, O. japonicus, S. chinensis, S. miltiorrhiza, P. lactiflora, R. glutinosa, Colla Corii Asini, C. cassia, Cannabis sativa L. [Cannabaceae; Cannabis Fructus], P. cocos, and G. glabra. In one study, 88 elderly patients with CHF were divided into a control group and an observation group by the random number table method, with 44 patients in each group. The control group was treated with conventional biomedicine medicine, and the observation group was treated with YQYYHXBXD on the basis of the control group for 12 weeks (Zhang and Wei, 2021). YQYYHXBXD combined with biomedicine medicine can reduce the levels of serum sST2, galectin-3, LN, PIIIP, and serum IL-1β, TNF-α, hs-CRP, MMP-9, and other inflammatory factors in patients with CHF, and the degree of adverse reactions in the two groups was mild, which did not affect the clinical observations. There was no significant difference between the two groups, indicating that YQYYHXBXD combined with biomedicine medicine is safe and effective in the treatment of CHF and can improve clinical symptoms and cardiac function and inhibit ventricular remodeling. Its mechanism is related to anti-inflammatory effects and the regulation of serum sST2, galectin-3, LN, and PIIIP, and the inhibition of MF.
Huangqi Guizhi Wuwu Decoction and Shengmai Decoction (HQASM) (Wei et al., 2021) is composed of A. membranaceus, P. quinquefolius, O. japonicus, S. miltiorrhiza, P. lactiflora, C. cassia, P. montana var. lobata, A. sinensis, Eupolyphaga sinensis, P. lactiflora, Z. officinale, Ziziphus jujuba Mill. [Rhamnaceae; Ziziphi Jujubae Fructus] and S. chinensis. HQASM, on the basis of routine biomedicine medicine intervention, can reduce the levels of cTn-I, cTn-T, LDH and CK-MB in patients with diabetic cardiomyopathy and downregulate the levels of TGF-β1, MMP-2, IGF-1, IL-6, IL-1, TNF-α, NT-proBNP, sST2, and Gal-3, indicating that HQASM has anti-inflammatory and anti-MF effects in the treatment of DCM.
Shengmai powder and Danshen decoction (SMADS) consists of A. membranaceus, S. miltiorrhiza, P. montana var. lobata, O. japonicus, S. chinensis, sandalwood, P. notoginseng, Wurfbainia villosa var. villosa [Rubiaceae; Wurfbainiae Villosae Caulis], and G. glabra at a ratio of 30:30:30:9:6:6:6:6. Ultra-high performance liquid chromatography-quadrupole-time-of-flight mass spectrometry (UPLC-Q-TOF-MS/MS) was used to identify the components of Danshen Decoction in vivo and in vitro. A previous study (Shen and Sheng, 2022) revealed that, along with standard biomedicine medical treatments, Shengmai powder and Danshen decoction used for 2 months significantly reduced biomarkers such as hs-CRP, Lp-PLA2, GPM-140, PIIINP, LN, and HA, contributing to the prevention of MF progression. Table 8 summarizes clinical trials evaluating S. miltiorrhiza-based therapies for MF.

Table 8. Salvia miltiorrhiza metabolite and decoction combined with conventional biomedicine medicine to inhibit MF included in this study.
Research has shown that the extracts of S. miltiorrhiza and C. Tinctorius (SCE) constitute a standardized preparation composed of two TCM botanical drugs, S. miltiorrhiza and C. Tinctorius. Its main pharmacologically active metabolites are phenolic acids, diterpenes, and flavonoids, such as sal B, Tan IIA, and hydroxysafflor yellow A. SCE is widely used to relieve angina in patients with coronary heart disease (Zhang et al., 2015). Compared with the control (Yang et al., 2019) SCE administration in vivo significantly increased the survival rate of mice after MI; inhibited myocardial inflammation; reduced the levels of H3K4 trimethylation (H3K4me3) and H3K36 trimethylation (H3K36me3) in the Smad3 promoter; and inhibited the expression of CoL-I, CoL-IIII and Sma RNA and protein, indicating that the reduction in MF caused by SCE was related to the downregulation of TGF-β1/Smad3 signal transduction. To confirm synergy between SCE, studies comparing SCE to each component alone are needed.
The TCM formulas P. quinquefolius and S. miltiorrhiza (PS). PS is reported to inhibit inflammatory responses and reduce endothelial damage. However, current data lack direct comparisons between individual and combined effects, limiting conclusions on true synergy. Research has shown that the concentrated granule preparation of PS at a ratio of 10:30 can be used to identify 223 metabolites through liquid chromatography, 147 of which were identified via positive ion mode and 76 of which were identified via negative ion mode. After gavage administration of PS at different doses (3 g/kg/d and 9 g/kg/d) for 4 weeks (Li X. et al., 2023), PS improved cardiac structure and function in rats with AMI, reduced infarct size, alleviated inflammatory cell infiltration, increased the expression of HGF and bFGF in the serum, and increased the levels of MVD and CD31 in myocardial tissues. PS reduced MF via miR-155-5p/HIF-1α/VEGF pathway inhibition. Whether this effect is synergistic requires validation through dose-response comparisons of individual vs. combined treatments.
A. membranaceus and S. miltiorrhiza (AS) are among the most popular phytomedicines in the world. They can also improve blood circulation in mice with myocardial infarction, protect against ischemia-reperfusion injury, and enhance cardiac function. Increasing evidence suggests that AS have protective effects against myocardial infarction. In accordance with the production standards for TCM formula granules, the granules of AS are prepared through processes such as decoction, filtration, concentration, drying, mixing, and granulation. Mice with MI were administered aqueous extracts of AS by gavage at a dose of 2 mL/kg, which is equivalent to 1.875 g/kg Astragalus and 0.9375 g/kg S. miltiorrhiza. After 6 weeks of treatment, compared with the model group, the intervention group presented reduced expression of COL-I and MMP9 induced by MI to some extent, as verified by tissue staining and protein analysis. These findings indicate that AS may inhibit MF, but synergistic mechanisms remain unconfirmed due to the absence of comparative data on individual herb contributions (Zhang M. X. et al., 2022).
A network pharmacology - based investigation uncovered the common and unique biological processes of A. membranaceus (HQ) and S. miltiorrhiza radix et rhizoma (DS) in CHD. Compared with the HQ or DS monotherapy groups, the HQ + DS combination treatment group presented significantly increased LVEF and LVFS values. Additionally, the infarct size and degree of fibrosis were significantly reduced, and the levels of lipid metabolism and blood viscosity indicators were markedly decreased. In terms of cardiac function parameters, the LVEDd, LVEDs, and CSA were significantly lower in both the HQ monotherapy group and the HQ + DS group than in the MI group. Compared with those in the MI group, coagulation indicators (APTT, PT, TT, FIB) were significantly lower in both the DS monotherapy group and the HQ + DS treatment group. This study revealed that HQ has specific advantages in alleviating cardiac remodeling, whereas DS has specific advantages in regulating hypercoagulability. These findings indicate that HQ and DS demonstrate complementary actions in treating CHD. Synergistic effects require further validation through isobolographic analysis or similar methods to quantify interaction effects (Zhang et al., 2021).
Zhang et al. (2024) identified 30 active metabolites and 41 common targets related to MF for the botanical drug pair S. miltiorrhiza and P. montana var. lobata through databases such as GeneCards and STRING. KEGG pathway analysis revealed 73 significantly enriched pathways, among which 6 were related to MF. The “active metabolite-target-pathway” network revealed that active metabolites such as luteolin from S. miltiorrhiza, Tan IIA from S. miltiorrhiza, puerarin from Pueraria lobata, and β-sitosterol from Pueraria lobata can regulate lipid metabolism and atherosclerosis, the AGE-RAGE pathway in diabetic complications, shear stress and atherosclerosis, and the HIF-1, TNF, and IL-17 pathways to exert anti-MF effects. These findings provide a theoretical basis and new ideas for further research on the mechanism of action of the S. miltiorrhiza and Pueraria lobata botanical drug pair in treating MF. The network suggests potential anti-MF mechanisms of S. miltiorrhiza and Pueraria lobata metabolites. However, synergistic interactions between these metabolites require experimental validation (e.g., checkerboard assays).
MF in cardiovascular diseases has attracted considerable attention, but effective drugs to reverse MF are lacking. Despite significant advancements, no approved drugs exist for reversing MF that can effectively reverse MF. Various active metabolites of S. miltiorrhiza, along with their formulated preparations, exhibit considerable efficacy in mitigating MF and enhancing cardiac function. This evidence highlights the strong ability of S. miltiorrhiza to prevent and manage MF. S. miltiorrhiza is a promising plant for research as a candidate drug in the prevention of pulmonary fibrosis; thus, it offers significant potential for the development of novel antifibrotic therapy. In this article, we examined advancements in research concerning the active metabolites of S. miltiorrhiza and its formulations in the context of anti-MF. These metabolites can significantly modulate immune inflammatory damage and prevent cardiomyocyte apoptosis by regulating various mechanisms, including the PI3K/AKT signaling pathway, the NF-κB signaling pathway, the modulation of TGF-β1 expression, and the upregulation of the JNK signaling pathway (Table 9) (Figure 9). Consequently, these actions demonstrate beneficial effects in the treatment of anti-MF. To summarize, TCM has demonstrated applicability across various stages of treatment for fibrosis and associated cardiovascular diseases owing to its multimetabolite, multitarget, and multilevel attributes. Furthermore, TCM has benefits in enhancing fibrosis in various organs, suggesting a novel approach for the development of antifibrotic pharmacological agents.

Table 9. The mechanisms of action of the main active metabolites of Salvia miltiorrhiza metabolite in improving MF.

Figure 9. Mechanism of Salvia miltiorrhiza Bunge in the treatment of MF (① SM inhibitsMFby downregulating TGF-β1, Smad2/3, and their phosphorylation levels; ② SM inhibits MF by downregulating the phosphorylation levels of PI3K, Akt, and mTOR proteins; ③ SM inhibits MF by downregulating the expression of TLR4 and the activation of downstream TAK1 and NF-κB; ④ SM inhibits apoptosis and MF by upregulating the expression of Bax, Caspase-3, Caspase-9, and cytochrome C, and downregulating Bcl-2 protein; ⑤ SM regulates the TIMP-1/MMPs pathway to inhibit MF by downregulating the expression of MMP2 and MMP9, and upregulating TIMP-1 expression; ⑥ SM regulates the Nrf2/HO-1 signaling pathway to inhibit MF by upregulating the expression of antioxidant proteins such as Nrf2 and HO-1).
Despite extensive preclinical studies on S. miltiorrhiza for MF prevention, the precise material basis of its therapeutic effects remains unclear. The scientific exploration faces critical bottlenecks requiring urgent breakthroughs: (1) Incomplete Elucidation of Material Basis and Molecular Mechanisms and Targeted Mechanisms:Although the multitarget properties of S. miltiorrhiza and its metabolites have been preliminarily confirmed, systematic validation of structure-function relationships for key bioactive components (e.g., Tan IIA, Sal B) is lacking. For instance, whether Tan IIA’s regulation of the TGF-β/Smad pathway exhibits dose-dependency remains unclear, and most studies rely on single cell lines (e.g., H9c2 cardiomyocytes), raising concerns about reproducibility across diverse pathological models. This limits the accurate quantification of “multi-component-multi-target” effects. (2) Limitations in Preclinical Model Translatability in Preclinical Models:Current research excessively depends on simplified models, including static in vitro cardiac fibroblasts (lacking mechanical stress and intercellular interactions) and rodent models (where MF induced by myocardial infarction differs significantly from human chronic fibrosis). For example, rodents’ robust cardiac regenerative capacity may underestimate S. miltiorrhiza’s long-term antifibrotic effects, while in vitro systems fail to recapitulate dynamic immune cell infiltration in human myocardial microenvironments, hindering extrapolation to complex human pathophysiology. (3) Suboptimal Clinical Trial Methodologies:Existing clinical trials suffer from insufficient statistical power (e.g., small sample sizes, n < 100, increasing type II error risks) and short follow-up periods (<6 months), which fail to capture the chronic nature of fibrosis progression. Improvements in LVEF may be overestimated due to placebo effects or natural disease fluctuations, and the absence of long-term safety data (e.g., hepatorenal toxicity) could obscure risks, impeding evidence accumulation for evidence-based medicine. (4) Absence of Quantitative Synergy Assessment for Herbal Formula Synergy:Combination therapies of S. miltiorrhiza with other herbs (e.g., A. membranaceus, C. tinctorius) show apparent synergy in experimental models (e.g., collagen deposition inhibition), but lack quantitative support from dose-response curves (DRC) and isobolographic analysis. The absence of combination index (CI) calculations leaves the distinction between “synergy” and “additive effects” hypothetical, severely restricting formula optimization and standardization. (5) Real-World Evidence Shortcomings:Available clinical data primarily derive from single-center observational studies, lacking integration of multi-dimensional real-world evidence (RWE) platforms incorporating electronic health records (EHR), dynamic biomarkers (e.g., wearable HRV monitoring), and genomic data (e.g., fibrosis-related SNPs). This limits the optimization of personalized treatment protocols.
Future research directions for S. miltiorrhiza in MF: integrative approaches and technological innovations. To address the aforementioned bottlenecks, future research must prioritize interdisciplinary integration and technological innovation, establishing a “basic-translational-clinical” trinity research paradigm. The following critical pathways warrant immediate exploration:Multi-Omics Integrated Mechanistic Exploration: (1) Integrate spatial metabolomics and single-cell proteomics to map spatiotemporal variations in S. miltiorrhiza bioactive components within tissue microenvironments. For example, concentration gradients of Sal B in ischemic vs. non-ischemic cardiomyocytes may modulate targeting efficiency—a gap in data currently preventing precise modeling of “multi-component-multi-target” effects. Machine learning algorithms (e.g., random forest, deep neural networks) can predict three-dimensional regulatory relationships among components-targets-pathways, providing a theoretical framework for precision drug design. (2) Precision Humanized Pathological Model Development: Differentiate induced pluripotent stem cell (iPSC)-derived functional cardiomyocytes and cardiac fibroblasts, then construct dynamic three-dimensional cultures using Organ-on-a-Chip technology to recapitulate human MF microenvironments (hypoxia, mechanical stress, inflammatory gradients). These models overcome species disparities, enabling clinically predictive preclinical evaluations of drug responses and toxicities. (3) Methodological Enhancements for Evidence-Based Practice:Design multi-center, large-sample (n ≥ 500) double-blind RCTs with composite endpoints (e.g., fibrosis area via cardiac MRI, dynamic serum biomarkers PIIINP/Galectin-3) instead of single metrics. Extend follow-up to ≥2 years to capture long-term efficacy/safety signals. Apply Bayesian statistical models to quantify efficacy-risk ratios and develop dynamic risk prediction scores (e.g., FIBRO-SCORE). (4) Systems Pharmacology Validation of Synergy:Quantitatively validate herbal formula synergy using Chou-Talalay Combination Index (CI) models, Bliss independence analysis, isobolographic assays, and checkerboard experiments to characterize interaction patterns (synergy/addition/antagonism). Track pharmacokinetic-pharmacodynamic (PK/PD) correlations of key components via targeted metabolomics to resolve molecular thresholds and spatiotemporal specificity of synergy. (5) Real-World Data-Driven Precision Therapeutics:A causality-oriented real-world evidence (RWE) platform should integrate longitudinal genomic data (e.g., fibrosis-related SNP dynamics), high-dimensional proteomic data (e.g., single-cell extracellular matrix remodeling markers), and wearable device-monitored parameters (e.g., heart rate variability [HRV], daily activity). Marginal structural models (MSM) must control time-varying confounders, while target trial emulation frameworks should validate causal effects of Salvia interventions, mitigating selection bias in observational studies. This will advance the transition from “population-based therapy” to “individualized intervention.”
S. miltiorrhiza, as the interface between traditional medical wisdom and the paradigm of modern precision medicine, holds great potential for antifibrotic applications. The full realization of this potential relies on the integration of interdisciplinary technologies and the establishment of a global collaborative research network. This endeavor necessitates the convergence of multi-omics technologies, artificial intelligence, and evidence-based medicine of multi-omics technologies, artificial intelligence, and evidence-based medicine, as well as the creation of data-sharing mechanisms and standardized research frameworks to accelerate the construction of the translational medicine value chain. Only through systematic validation via multidimensional evidence chains can this traditional medicinal plant establish a precise therapeutic strategy for the prevention and treatment of myocardial fibrosis that transcends time and space.
QL: Data curation, Formal Analysis, Methodology, Writing – original draft. CR: Data curation, Formal Analysis, Methodology, Writing – original draft. BJ: Visualization, Writing – original draft. XW: Methodology, Visualization, Writing – original draft. CW: Methodology, Visualization, Writing – original draft. XDZ: Methodology, Visualization, Writing – original draft. LL: Methodology, Visualization, Writing – original draft. XG: Data curation, Investigation, Writing – original draft. XKZ: Funding acquisition, Supervision, Writing – review and editing. YL: Funding acquisition, Supervision, Writing – review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Gansu Jiaoyu Jiebang Guashuai Project (No. 2021 jyjbgs-03), National Natural Science Foundation of China (No. 82360926, 82374279), Gansu Province Dual Top-class Scientific Research Key Project (GSSYLXM-05), Gansu Provincial Science and Technology Major Project (No. 20ZD7FA002), Gansu Provincial University Teachers’ Innovation Fund Project in 2025 (No. 2025B-126), 2024 Gansu Province Outstanding Doctoral Project (24JRRA569).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ai, W., Zhang, M., Zhou, S., Xiao, L., and Hu, J. (2022). Myocardial fibrosis in rats with congestive heart failure induced by adriamycin and its mechanism. Chin. J. Med. Innov. 19 (19), 5–8. doi:10.3969/j.issn.1674-4985.2022.19.002
Ambardekar, A. V., and Sailer, C. (2022). Reduce, reuse, recycle…unloading, autophagy, and myocardial recovery. JACC Basic Transl. Sci. 7 (12), 1229–1231. doi:10.1016/j.jacbts.2022.07.006
Ansari, M. A., Khan, F. B., Safdari, H. A., Almatroudi, A., Alzohairy, M. A., Safdari, M., et al. (2021). Prospective therapeutic potential of Tanshinone IIA: an updated overview. Phytother. Res. 164, 105364. doi:10.1016/j.phrs.2020.105364
Anwaier, G., Xie, T. T., Pan, C. S., Li, A. Q., Yan, L., Wang, D., et al. (2022). QiShenYiQi pill ameliorates cardiac fibrosis after pressure overload-induced cardiac hypertrophy by Regulating FHL2 and the macrophage RP S19/TGF-β1 signaling pathway. Front. Pharmacol. 13, 918335. doi:10.3389/fphar.2022.918335
Bai, Y., Zhang, L., Lu, J., Qiu, M., Sun, R., Lu, L., et al. (2021). Effects of Baoxin mixture on inflammatory indexes and myocardial fibrosis indexes in patients with heart failure due to ischemic cardiomyopathy. J. Clin. Tradit. Chin. Med. 33 (10), 1974–1977. doi:10.16448/j.cjtcm.2021.1032
Behnoush, A. H., Khalaji, A., Naderi, N., Ashraf, H., and von Haehling, S. (2023). ACC/AHA/HFSA 2022 and ESC 2021 guidelines on heart failure comparison. ESC Heart Fail 10 (3), 1531–1544. doi:10.1002/ehf2.14255
Bengel, F. M., Diekmann, J., Hess, A., and Jerosch-Herold, M. (2023). Myocardial fibrosis: emerging target for cardiac molecular imaging and opportunity for image-guided therapy. J. Nucl. Med. 64 (2), 49S–58S. doi:10.2967/jnumed.122.264867
Cai, H., Cai, J., Chang, W., Zhao, Z., Dong, X., and Zhao, L. (2014). Effect of tanshinone ⅡA on expression of myocardial NF-κB in rats with pressure overload. Hebei Med. 36 (02), 180–183. doi:10.3969/j.issn.1002-7386.2014.02.006
Cai, H., Zhu, K., Zhao, L., Zhao, Z., Dong, X., and Zhang, B. (2016). Effect of Tanshinone ⅡA on the expression of hemo-oxygenase-1 in rats with overloaded pressure. J. Tradit. Chin. Med. 31 (9), 1349–1353. doi:10.16368/j.issn.1674-8999.2016.09.380
Chan, P., Liu, J. C., Lin, L. J., Chen, P. Y., Cheng, T. H., Lin, J. G., et al. (2011). Tanshinone IIA inhibits angiotensin II-induced cell proliferation in rat cardiac fibroblasts. Am. J. Chin. Med. 39 (2), 381–394. doi:10.1142/s0192415x11008890
Chen, J., Cao, W., Asare, P. F., Lv, M., Zhu, Y., Li, L., et al. (2016). Amelioration of cardiac dysfunction and ventricular remodeling after myocardial infarction by danhong injection are critically contributed by anti-TGF-β-mediated fibrosis and angiogenesis mechanisms. J. Ethnopharmacol. 194, 559–570. doi:10.1016/j.jep.2016.10.025
Chen, J., Wei, J., Orgah, J., Zhu, Y., Ni, J., Li, L., et al. (2019a). Cardioprotective effect of danhong injection against myocardial infarction in rats is critically contributed by MicroRNAs. Evid. Based Compl. Alt. Med. 2019, 4538985. doi:10.1155/2019/4538985
Chen, R., Chen, W., Huang, X., and Rui, Q. (2021). Tanshinone IIA attenuates heart failure via inhibiting oxidative stress in myocardial infarction rats. Mol. Med. Rep. 23 (6), 404. doi:10.3892/mmr.2021.12043
Chen, Y., Wei, G., Hu, J., Li, J., Liu, Q., Han, C., et al. (2017a). Research overview of Salvia miltiorrhiza polysaccharides. Shandong J. Tradit. Chin. Med. 36 (08), 725–728. doi:10.16295/j.cnki.0257-358x.2017.08.030
Chen, Y. F., Day, C. H., Lee, N. H., Chen, Y. F., Yang, J. J., Lin, C. H., et al. (2017b). Tanshinone IIA inhibits β-Catenin nuclear translocation and IGF-2R activation via estrogen receptors to suppress angiotensin II-induced H9c2 cardiomyoblast cell apoptosis. Int. J. Med. Sci. 14 (12), 1284–1291. doi:10.7150/ijms.20396
Chen, Z., Xie, X., Feng, L., Zhang, D., and Peng, K. (2019b). Curative effect of Danhong Injection combined with intravenous atyplase thrombolysis in elderly acute myocardial infarction patients. Chin. J. Geriatr. Cardiovasc. Cerebrovasc. Dis. 21 (11), 1159–1162. doi:10.3969/j.issn.1009-0126.2019.11.010
Chiang, M. H., Liang, C. J., Lin, L. C., Yang, Y. F., Huang, C. C., Chen, Y. H., et al. (2020). miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia-telangiectasia mutated in myocardial infarction. J. Cell. Physiol. 235 (9), 6085–6102. doi:10.1002/jcp.29537
Chu, F., Liu, W., Shang, J., Xie, J., Li, H., and Liu, H. (2020). Clinical effect of Shenyuan Yiqi Huoxue decoction in treatment of heart failure caused by coronary heart disease and its influence on the levels of thrombin, Transforming Growth Factor-β1, Type Ⅰ Collagen, and Type Ⅲ Collagen. J. Anhui Univ. Tradit. Chin. Med. 39 (03), 18–22. doi:10.3969/j.issn.2095-7246.2020.03.006
Cui, L., Wang, Y., Yu, R., Li, B., Xie, S., Gao, Y., et al. (2016). Jia-Shen decoction-medicated serum inhibits angiotensin-II induced cardiac fibroblast proliferation via the TGF-β1/Smad signaling pathway. Mol. Med. Rep. 14 (2), 1610–1616. doi:10.3892/mmr.2016.5405
Cui, S., Wu, J., and Lu, Y. (2017). Study on the effect of Yiqi Huayu Decoction on the myocardial fibrosis mechanism of chronic heart failure. Chongqing Med. 46 (18), 2548–2551. doi:10.3969/j.issn.1671-8348.2017.18.034
Cui, X. (2021). Study on the mechanism of Qi-invigorating and blood-activating method on high glucose-induced rat cardiac fibroblasts based on TGF-β1/Smads pathway. Master’s thesis. Liaoning (China): Liaoning University of Traditional Chinese Medicine.
Deng, F. (2023). Effects of Sodium tanshinon Ⅱ A silate combined with Ivabradine in treatment of patients with acute myocardial infarction complicated with heart failure after PCI. Chin. J. Minkang Med. 35 (16), 103–106. doi:10.3969/j.issn.1672-0369.2023.16.032
Deng, K., Peng, J., Zhang, M., Lin, H., Wang, X., He, D., et al. (2023). Oyster peptide improves liver fibrosis via NF-κB/iNOS signaling pathway. J. Chin. Pharm. Sci. 32 (6), 435–445. doi:10.5246/jcps.2023.06.037
Dong, R., and zheng, Y. (2004). Isolation and identification of tanshinone metabolites in Salvia miltiorrhiza. J. Jilin Norm. Univ. (Nat. Sci. Ed.) 25 (2), 100–104. doi:10.16862/j.cnki.issn1674-3873.2004.02.037
Du, J., Shi, K., Zhao, Y., Hu, D., Li, H., and Liu, Z. (2019). Study on salvianolic acid B promoting autophagy and alleviating myocardial fibrosis in rats by inhibiting PI3K/AKT/mTOR pathway. Prog. Mod. Biomed. 19 (20), 3812–3817. doi:10.13241/j.cnki.pmb.2019.20.003
Fan, D., Takawale, A., Basu, R., Patel, V., Lee, J., Kandalam, V., et al. (2014). Differential role of TIMP2 and TIMP3 in cardiac hypertrophy, fibrosis, and diastolic dysfunction. Cardiovasc. Res. 103 (2), 268–280. doi:10.1093/cvr/cvu072
Feng, K., Liu, Y., Sun, J., Zhao, C., Duan, Y., Wang, W., et al. (2021). Compound danshen dripping pill inhibits doxorubicin or isoproterenol-induced cardiotoxicity. Biomed. Pharmacother. 138, 111531. doi:10.1016/j.biopha.2021.111531
Fernández-Solà, J. (2020). The effects of ethanol on the heart: alcoholic cardiomyopathy. Nutrients 12 (2), 572. doi:10.3390/nu12020572
Frangogiannis, N. G. (2021). Cardiac fibrosis. Cardiovasc. Res. 117 (6), 1450–1488. doi:10.1093/cvr/cvaa324
Fu, X., and Sun, H. (2016). Inhibition of Taishinone ⅡA on cardiac fibroblasts proliferation through PKC/CyclinD1 pathways in rats. J. Beihua Univ. (Nat. Sci. Ed.) 17 (5), 616–619. doi:10.11713/j.issn.1009-4822.2016.05.012
Gan, X., Chen, Q., and Han, Y. (2020). Effect of Shugan Yixin recipe on cardiac function and myocardial fibrosis in patients with ischemic cardiomyopathy coronary heart cisease. Chin. Med. Innov. 17 (31), 88–91. doi:10.3969/j.issn.1674-4985.2020.31.023
Gao, H., Bo, Z., Wang, Q., Luo, L., Zhu, H., and Ren, Y. (2019). Salvanic acid B inhibits myocardial fibrosis through regulating TGF-β1/Smad signaling pathway. Biomed. Pharmacother. 110, 685–691. doi:10.1016/j.biopha.2018.11.098
Geva, T., and Bucholz, E. M. (2021). Is myocardial fibrosis the missing link between prematurity, cardiac remodeling, and long-term cardiovascular outcomes?. J. Am. Coll. Cardiol. 78 (7), 693–695. doi:10.1016/j.jacc.2021.05.052
Guan, H., Luo, W., Bao, B., Cao, Y., Cheng, F., Yu, S., et al. (2022). A comprehensive review of rosmarinic acid: from phytochemistry to pharmacology and its new insight. Molecules 27 (10), 3292. doi:10.3390/molecules27103292
Guan, Y., Yin, Y., Zhu, Y. R., Guo, C., Wei, G., Duan, J. L., et al. (2013). Dissection of mechanisms of a Chinese medicinal formula: danhong injection therapy for myocardial ischemia/reperfusion injury in vivo and in vitro. Evid. Based Compl. Alt. Med. 2013, 972370. doi:10.1155/2013/972370
Habib, F. M., Springall, D. R., Davies, G. J., Oakley, C. M., Yacoub, M. H., and Polak, J. M. (1996). Tumour necrosis factor and inducible nitric oxide synthase in dilated cardiomyopathy. Lancet 347 (9009), 1151–1155. doi:10.1016/s0140-6736(96)90610-8
Han, A., Lu, Y., Zheng, Q., Zhang, J., Zhao, Y., Zhao, M., et al. (2018). Qiliqiangxin attenuates cardiac remodeling via inhibition of TGF-β1/Smad3 and NF-κB signaling pathways in a rat model of myocardial infarction. Cell. Physiol. Biochem. 45 (5), 1797–1806. doi:10.1159/000487871
Han, L., Guan, Y., Ren, J., Liu, J., Guo, H., Yang, J., et al. (2022). Effects of salvianolic acid A on Wnt signaling pathway in cardiac fibroblasts stimulated by high concentration glucose. J. Bengbu Med. Coll. 47 (1), 21–25+29. doi:10.13898/j.cnki.issn.1000-2200.2022.01.005
Hao, J. M., Sun, P., Zeng, Y., Zhang, H., Zhang, Z. D., Chang, L. P., et al. (2023). Qiliqiangxin capsule improves cardiac remodeling in rats with DOCA-salt-induced diastolic dysfunction. Eur. Rev. Med. Pharmacol. Sci. 27 (15), 7264–7275. doi:10.26355/eurrev_202308_33298
He Dequan, C. Y., and Wang, S. (2022). Effects of Tanshinone IIA on isoproterenol-induced myocardial hypertrophy and fibrosis in rats by regulating the PI3K/AKT pathway. Chin. J. Evid.-Based Cardiovasc. Med. 14 (7), 811–815. doi:10.3969/j.issn.1674-4055.2022.07.09
Hong, L., Zhang, S., Wang, Q., Zhao, Y., Zhu, Q., Peng, C., et al. (2020). Effect of Zhenwu Decoction on chronic heart failure rats based on RAAS/NF-κB/inflammatory factor cascade reaction. Chin. Tradit. Bot. drugal Drugs 51 (5), 1279–1286. doi:10.7501/j.issn.0253-2670.2020.05.026
Hou, Y., He, Z., Han, Y., Zhang, T., Wang, S., Wang, X., et al. (2023). Mechanism of new optimized Sheng-Mai-San Formula to regulate cardiomyocyte apoptosis through NMDAR pathway. Heliyon 9 (6), e16631. doi:10.1016/j.heliyon.2023.e16631
Hu, F., Rong, Y., Zhu, K., Lu, H., Chen, C., Chen, M., et al. (2017). Study on the protective effect of Guanxinning Tablet on myocardial ischemia reperfusion injury in rats. Chin. J. Comp. Med. 27 (5), 76–82. doi:10.3969/j.issn.1671-7856.2017.05.017
Huang, H., Wang, C., Zhao, L., Luo, H., Xu, Y., Tao, L., et al. (2017). Effect of salvianolic acid B in inhibiting angiotensin II-induced proliferation and differentiation of rat cardiac fibroblast. Chin. J. Exp. Tradit. Med. Formulae 23 (13), 128–132. doi:10.13422/j.cnki.syfjx.2017130128
Huang, J., Wang, Y., Xiao, F., Liu, X., and Yu, R. (2024). Mechanism of Zuogui Jiangtang Shuxin formula in inhibiting myocardial fibrosis in mice with diabetic cardiomyopathy by regulating TLR4/NF-κB pathway. J. Hunan Univ. Tradit. Chin. Med. 44 (5), 729–736. doi:10.3969/j.issn.1674-070X.2024.05.003
Hui, Y., Dong, F., Jia, W., Xiao, H., Yu, L., Huang, L., et al. (2023). Mechanism of Qishen Liuwei formula on myocardial fibrosis in SHR through TGF-β1/Smad2/3 pathway. Shaanxi J. Tradit. Chin. Med. 44 (10), 1337–1343. doi:10.3969/j.issn.1000-7369.2023.10.002
Kong, D., and Liu, X. (1983). Study on chemical metabolites of Salvia miltiorrhiza. 10 (4), 313–315.
Kong, P., Christia, P., and Frangogiannis, N. G. (2014). The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 71 (4), 549–574. doi:10.1007/s00018-013-1349-6
Kura, B., Szeiffova Bacova, B., Kalocayova, B., Sykora, M., and Slezak, J. (2020). Oxidative stress-responsive microRNAs in heart injury. Int. J. Mol. Sci. 21 (1), 358. doi:10.3390/ijms21010358
Li, A., Gao, M., Liu, B., Qin, Y., Chen, L., Liu, H., et al. (2022a). Mitochondrial autophagy: molecular mechanisms and implications for cardiovascular disease. Cell. Death Dis. 13 (5), 444. doi:10.1038/s41419-022-04906-6
Li, C. L., Liu, B., Wang, Z. Y., Xie, F., Qiao, W., Cheng, J., et al. (2020). Salvianolic acid B improves myocardial function in diabetic cardiomyopathy by suppressing IGFBP3. J. Mol. Cell. Cardiol. 139, 98–112. doi:10.1016/j.yjmcc.2020.01.009
Li, D., Saldeen, T., Romeo, F., and Mehta, J. L. (2000). Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-kappaB. Circulation 102 (16), 1970–1976. doi:10.1161/01.cir.102.16.1970
Li, H., Gao, C., Liu, C., Liu, L., Zhuang, J., Yang, J., et al. (2021a). A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 137, 111332. doi:10.1016/j.biopha.2021.111332
Li, H., Zhang, Y., and Ma, J. (2011). Effects of yiqi huoxue compound combined with exercise therapy on MMP-1 and collagen type III expressions of cardiac muscle in chronic heart failure rats. Chin. J. Integr. Tradit. West. Med. 31 (7), 955–960.
Li, Q., Qi, L., Zhao, K., Ke, W., Li, T., and Xia, L. (2022b). Integrative quantitative and qualitative analysis for the quality evaluation and monitoring of Danshen medicines from different sources using HPLC-DAD and NIR combined with chemometrics. Front. Plant Sci. 13, 932855. doi:10.3389/fpls.2022.932855
Li, Q., and Sun, L. (2023). Effect of Qili Qiangxin Capsule combined with levosimendan in the treatment of elderly heart failure and its effect on myocardial fibrosis and inflammatory factors. Clin. Med. Res. Pract. 8 (29), 125–128. doi:10.19347/j.cnki.2096-1413.202329032
Li, X., Liu, R., Liu, W., Liu, X., Fan, Z., Cui, J., et al. (2023a). Panax quinquefolium L. and Salvia miltiorrhiza Bunge. Enhances angiogenesis by regulating the miR-155-5p/HIF-1α/VEGF Axis in acute myocardial infarction. Drug Des. Devel. Ther. 17, 3249–3267. doi:10.2147/dddt.S426345
Li, X., Yang, Y., Chen, S., Zhou, J., Li, J., and Cheng, Y. (2021b). Epigenetics-based therapeutics for myocardial fibrosis. Life Sci. 271, 119186. doi:10.1016/j.lfs.2021.119186
Li, Y., Zhao, Z. L., Shuqian, , and Huang, Z. (2021c). Research progress on the main chemical metabolites and extraction and separation methods of Salvia miltiorrhiza. J. Tradit. Chin. Med. 49 (1), 106–111. doi:10.19664/j.cnki.1002-2392.210024
Li, Z., Meng, Z., Li, Y., Tao, H., Bai, Z., and Li, L. (2018a). Sodium Tanshinone A Sulfonate attenuates angiotensin -induced cardiac fibrosis in rats. Chin. J. Mod. Med. 28 (12), 17–23. doi:10.3969/j.issn.1005-8982.2018.12.003
Li, Z. M., Xu, S. W., and Liu, P. Q. (2018b). Salvia miltiorrhiza Burge (Danshen): a golden herbal medicine in cardiovascular therapeutics. Acta Pharmacol. Sin. 39 (5), 802–824. doi:10.1038/aps.2017.193
Liang, B., Zhang, X. X., Li, R., Zhu, Y. C., Tian, X. J., and Gu, N. (2022). Guanxin V alleviates acute myocardial infarction by restraining oxidative stress damage, apoptosis, and fibrosis through the TGF-β1 signalling pathway. Phytomedicine 100, 154077. doi:10.1016/j.phymed.2022.154077
Liang, B., Zou, F. H., Fu, L., and Liao, H. L. (2020). Chinese herbal medicine Dingji fumai decoction for ventricular premature contraction: a real-world trial. Biomed. Res. Int. 2020, 5358467. doi:10.1155/2020/5358467
Liang, Q., Liu, X., Peng, X., Luo, T., Su, Y., Xu, X., et al. (2024). Salvianolic acid B in fibrosis treatment: a comprehensive review. Front. Pharmacol. 15, 1442181. doi:10.3389/fphar.2024.1442181
Lijnen, P. J., van Pelt, J. F., and Fagard, R. H. (2012). Stimulation of reactive oxygen species and collagen synthesis by angiotensin II in cardiac fibroblasts. Cardiovasc. Res. 30 (1), e1–e8. doi:10.1111/j.1755-5922.2010.00205.x
Liu, H. T., Wang, Y. F., Olaleye, O., Zhu, Y., Gao, X. M., Kang, L. Y., et al. (2013). Characterization of in vivo antioxidant constituents and dual-standard quality assessment of Danhong injection. Biomed. Chromatogr. 27 (5), 655–663. doi:10.1002/bmc.2842
Liu, J., and Wang, Y. (2021). Effects of Danshensu on myocardial fibrosis by interacting with endoplasmic reticulun Ca2+ leakage channel SEC61α in mouse heart. Hebei Med. 43 (12), 1844–1847. doi:10.3969/j.issn.1002-7386.2021.12.020
Liu, J., Zhang, Y., Li, F., He, J., and Yang, M. (2021). Study on the mechanism of Yixin Futingyin in preventing myocardial fibrosis in rats with abdominal aortic coarctation based on TGF-β/Smad pathway. J. Integr. Tradit. Chin. West. Med. Cardiovasc. Cerebrovasc. Dis. 19 (22), 3886–3890. doi:10.12102/j.issn.1672-1349.2021.22.010
Liu, P., Hu, Y., Liu, C., Xu, M., Liu, C., Sun, K., et al. (2005). Multi-centrally clinical trial of Fuzhenghuayu (Strengthening Healthy Qi to Solve Stagnation) capsules on liver filbrosis caused by chronic hepatitis B. World Sci. Technol. 7 (01), 24–32+135–136. doi:10.3969/j.issn.1674-3849.2005.01.006
Liu, Y., Liu, M., Yang, T., and Yang, S. (2023). Research progress on the prevention and treatment of myocardial fibrosis with traditional Chinese medicine. Pharmacol. Clin. Med. Tradit. Chin. Med. 39 (2), 101–109. doi:10.13412/j.cnki.zyyl.20220222.002
Lo, S. H., Hsu, C. T., Niu, H. S., Niu, C. S., Cheng, J. T., and Chen, Z. C. (2017). Cryptotanshinone inhibits STAT3 signaling to alleviate cardiac fibrosis in type 1-like diabetic rats. Phytother. Res. 31 (4), 638–646. doi:10.1002/ptr.5777
López, B., Ravassa, S., Moreno, M. U., José, G. S., Beaumont, J., González, A., et al. (2021). Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 18 (7), 479–498. doi:10.1038/s41569-020-00504-1
Lu, B., Zhou, X., Gui, M., Ma, Y., Chen, X., Yao, L., et al. (2024). Study on the mechanism of Huoxue Qianyang Qutan prescription in inhibiting the proliferation of cardiac fibroblasts. World J. Integr. Tradit. West. Med. 19 (9), 1703–1707. doi:10.13935/j.cnki.sjzx.240902
Lu, G., Zhang, Z., Zheng, Y., Li, H., and Wei, R. (2022a). Effects of Yiqi Yangyin Huoxue prescription combined with biomedicine medicine on cardiac function, myocardial fibrosis indexes and inflammatory indexes in patients with chronic heart failure and myocardial fibrosis. Mod. J. Integr. Tradit. Chin. West. Med. 31 (16), 2269–2272. doi:10.3969/j.issn.1008-8849.2022.16.016
Lu, P. (2024). Study on the effect and mechanism of salvianolic acid B on diabetic myocardial fibrosis by regulating TRPC6-mediated TGF-β/Smad3 signaling pathway. Master’s thesis. Nanjing (China): Nanjing University of Traditional Chinese Medicine.
Lu, Y., Xiang, M., Xin, L., Zhang, Y., Wang, Y., Shen, Z., et al. (2022b). Qiliqiangxin modulates the gut microbiota and NLRP3 inflammasome to protect against ventricular remodeling in heart failure. Front. Pharmacol. 13, 905424. doi:10.3389/fphar.2022.905424
Luo, H., Fu, L., Wang, X., Yini, X., Ling, T., and Shen, X. (2023). Salvianolic acid B ameliorates myocardial fibrosis in diabetic cardiomyopathy by deubiquitinating Smad7. Chin. Med. 18 (1), 161. doi:10.1186/s13020-023-00868-9
Luo, H., Yang, H., and Shen, X. (2014). Effect of salvianolic acid B on proliferation of cardiac fibroblasts induced by transforming growth factor-β1. Chin. Pharm. 25 (3), 202–204.
Lv, S., Wu, M., Li, M., Wang, Q., Wang, X., Xu, L., et al. (2015). Effect of QiShenYiQi Pill on myocardial collagen metabolism in rats with partial abdominal aortic coarctation. Evid. Based Compl. Alt. Med. 2015, 415068. doi:10.1155/2015/415068
Lv, S., Yuan, P., Lu, C., Dong, J., Li, M., Qu, F., et al. (2021). QiShenYiQi pill activates autophagy to attenuate reactive myocardial fibrosis via the PI3K/AKT/mTOR pathway. Aging 13 (4), 5525–5538. doi:10.18632/aging.202482
Lv, S., Zhang, W., Yuan, P., Lu, C., Dong, J., and Zhang, J. (2022). QiShenYiQi pill for myocardial collagen metabolism and apoptosis in rats of autoimmune cardiomyopathy. Pharm. Biol. 60 (1), 722–728. doi:10.1080/13880209.2022.2056206
Lv, S. C., Wu, M., Li, M., Wang, Q., Wang, X. J., Zhang, A., et al. (2017). Effect of QiShenYiQi pill on myocardial collagen metabolism in experimental autoimmune myocarditis rats. Biomed. Pharmacother. 88, 894–901. doi:10.1016/j.biopha.2017.01.096
Ma, S., Yang, D., Wang, K., Tang, B., Li, D., and Yang, Y. (2012a). Cryptotanshinone attenuates isoprenaline-induced cardiac fibrosis in mice associated with upregulation and activation of matrix metalloproteinase-2. Mol. Med. Rep. 6 (1), 145–150. doi:10.3892/mmr.2012.866
Ma, X., Yang, M., Chen, J., Liu, Y., Pan, C., and Fan, J. (2012b). Effects of Bushen Huoxue Fang on rat cardiac fibroblast proliferation and collagen production in vitro. J. South. Med. Univ. 32 (1), 122–124.
Ma, Y., Li, H., Yue, Z., Guo, J., Xu, S., Xu, J., et al. (2014). Cryptotanshinone attenuates cardiac fibrosis via downregulation of COX-2, NOX-2, and NOX-4. J. Cardiovasc. Pharmacol. 64 (1), 28–37. doi:10.1097/fjc.0000000000000086
Mao, S., Wang, Y., Zhang, M., and Hinek, A. (2014). Phytoestrogen, tanshinone IIA diminishes collagen deposition and stimulates new elastogenesis in cultures of human cardiac fibroblasts. Exp. Cell. Res. 323 (1), 189–197. doi:10.1016/j.yexcr.2014.02.001
Maruyama, K., and Imanaka-Yoshida, K. (2022). The pathogenesis of cardiac fibrosis: a review of recent progress. Int. J. Mol. Sci. 23 (5), 2617. doi:10.3390/ijms23052617
Mazumder, S., Plesca, D., and Almasan, A. (2008). Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol. Biol. 414, 13–21. doi:10.1007/978-1-59745-339-4_2
Nagalingam, R. S., Chattopadhyaya, S., Al-Hattab, D. S., Cheung, D. Y. C., Schwartz, L. Y., Jana, S., et al. (2022). Scleraxis and fibrosis in the pressure-overloaded heart. Eur. Heart J. 43 (45), 4739–4750. doi:10.1093/eurheartj/ehac362
Nizamutdinova, I. T., Guleria, R. S., Singh, A. B., Kendall, J. A., Baker, K. M., and Pan, J. (2013). Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway. J. Cell. Physiol. 228 (2), 380–392. doi:10.1002/jcp.24142
Obas, V., and Vasan, R. S. (2018). The aging heart. Clin. Sci. 132 (13), 1367–1382. doi:10.1042/cs20171156
O'Meara, E., and Zannad, F. (2023). Fibrosis biomarkers predict cardiac reverse remodeling. JACC Heart Fail. 11 (1), 73–75. doi:10.1016/j.jchf.2022.11.011
Peng, Y., Zhang, Y., Zhang, Y., Ke, H., and Wang, X. (2022). Protective effect of pterostilbene on rats with pulmonary fibrosis via TLR4/NF-κB signaling pathway. J. Wuhan. Univ. (Med. Ed.) 43 (2), 205–209. doi:10.14188/j.1671-8852.2020.0627
Prabhu, S. D., and Frangogiannis, N. G. (2016). The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119 (1), 91–112. doi:10.1161/circresaha.116.303577
Qi, L., Wu, S., Liu, N., Zhang, X., Ping, L., and Xia, L. (2024). Salvia miltiorrhiza bunge extract improves the Th17/Treg imbalance and modulates gut microbiota of hypertensive rats induced by high-salt diet. J. Funct. Foods 117, 106211. doi:10.1016/j.jff.2024.106211
Qi, Y., Ren, X., Lou, L., Wu, A., Zhao, M., Zhao, Y., et al. (2018). Effects of Fuzheng Huayu Capsule on extracellular matrix metabolism in rats with myocardial fibrosis after myocardial infarction. J. Tradit. Chin. Med. 59 (7), 607–611. doi:10.13288/j.11-2166/r.2018.07.017
Qiao, S., Chen, Y., Li, K., Zhang, R., Niu, H., Liu, W., et al. (2024). Research progress on pharmacological effects and clinical application of Danshen Injection. Mod. Drugs Clin. 39 (11), 2977–2982. doi:10.7501/j.issn.1674-5515.2024.11.040
Ren, C., Liu, K., Zhao, X., Guo, H., Luo, Y., Chang, J., et al. (2022). Research progress of traditional Chinese medicine in treatment of myocardial fibrosis. Front. Pharmacol. 13, 853289. doi:10.3389/fphar.2022.853289
Shen, Y., and Sheng, L. (2022). Efficacy of Shengmai Powder combined with Danshen Yin in the treatment of diabetic cardiomyopathy and its effect on the degree of myocardial fibrosis. New Chin. Med. 54 (5), 62–66. doi:10.13457/j.cnki.jncm.2022.05.013
Shi, M., Huang, F., Deng, C., Wang, Y., and Kai, G. (2019). Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 59 (6), 953–964. doi:10.1080/10408398.2018.1474170
Shi, M., Wei, J., Yuan, H., and Guo, Z. (2024). Metabolomics-based study on the effects of Yixintai granules on fecal metabolites in rats with chronic heart failure. Shizhen Chin. Med. Chin. Med. 35 (3), 522–527. doi:10.3969/j.issn.1008-0805.2024.03.03
Siwik, D. A., and Colucci, W. S. (2004). Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail. Rev. 9 (1), 43–51. doi:10.1023/b:Hrev.0000011393.40674.13
Song, Y., Shi, P., and Yan, H. (2022). Effects of Tanshinone ⅡA on ventricular remodeling and PI3K/Akt signaling pathway in rats with heart failure. J. Integr. Tradit. Chin. West. Med. Cardiovasc. Cerebrovasc. Dis. 20 (4), 638–642. doi:10.12102/j.issn.1672-1349.2022.04.010
Sun, R. (2017). Effects of Huangqi Baoxin Decoction and its active metabolite salvianolic acid B on myocardial fibrosis and TGF-β/smad pathway in rats with dilated cardiomyopathy. Doctoral thesis. Nanjing (China): Nanjing University of Traditional Chinese Medicine.
Sun, X., Zhong, Q., Zhang, R., Wang, B., Huo, J., Song, Y., et al. (2023). Study on the mechanism of action of Qili Qiangxin Capsule in inhibiting doxorubicin-induced cardiac fibroblast activation. J. Tradit. Chin. Med. 51 (12), 22–28. doi:10.19664/j.cnki.1002-2392.230251
Tan, G., Peng, J., Liu, G., Zhong, L., and Yuan, M. (2022). Effects of Qishen Granule on matrix metabolism of rat cardiac fibroblasts induced by angiotensin II World. J. Tradit. Chin. Med. 17 (15), 2167–2170+2177. doi:10.3969/j.issn.1673-7202.2022.15.013
Tang, Y., and Guo, Z. (2020a). Effects of Yixintai granules on LVEF, serum BNP and FFA in rats with chronic heart failure with deficiency of qi and blood stasis and water retention syndrome. J. Tradit. Chin. Med. 26 (13), 23–26. doi:10.13862/j.cnki.cn43-1446/r.2020.13.006
Tang, Y., and Guo, Z. (2020b). Study on Yixintai granules in rats with chronic heart failure with heart qi deficiency and blood stasis and water retention syndrome. J. Tradit. Chin. Med. 26 (8), 8–11. doi:10.13862/j.cnki.cn43-1446/r.2020.08.003
Tang, Y., Guo, Z., Li, Y., Sun, T., Wu, G., Liu, L., et al. (2015). Effects of Yixintai granules on echocardiographic parameters and serum brain natriuretic peptide in rabbits with chronic heart failure. Chin. Tradit. Pat. Med. 37 (7), 1565–1567. doi:10.3969/j.issn.1001-1528.2015.07.037
Tang, Y., Guo, Z., Zhang, T., Long, Y., and Li, Y. (2021). Effect of Yixintai ethanol extract on the expression of Bax and Bcl-2 proteins in rabbit cardiac fibroblasts induced by angiotensin II. Chin. J. Tradit. Chin. Med. Inf. 28 (8), 83–86. doi:10.19879/j.cnki.1005-5304.202012520
Tao, L., Shen, S., Fu, S., Fang, H., Wang, X., Das, S., et al. (2015). Traditional Chinese Medication Qiliqiangxin attenuates cardiac remodeling after acute myocardial infarction in mice. Sci. Rep. 5, 8374. doi:10.1038/srep08374
Timonen, P., Magga, J., Risteli, J., Punnonen, K., Vanninen, E., Turpeinen, A., et al. (2008). Cytokines, interstitial collagen and ventricular remodelling in dilated cardiomyopathy. Int. J. Cardiol. 124 (3), 293–300. doi:10.1016/j.ijcard.2007.02.004
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E., and Blaxall, B. C. (2016). Cardiac fibrosis: the fibroblast awakens. Circ. Res. 118 (6), 1021–1040. doi:10.1161/circresaha.115.306565
Wang, J., Chen, H., Seth, A., and McCulloch, C. A. (2003). Mechanical force regulation of myofibroblast differentiation in cardiac fibroblasts. Am. J. Physiol. Heart Circ. Physiol. 285 (5), H1871–H1881. doi:10.1152/ajpheart.00387.2003
Wang, K., Yan, Z. Y., Ma, Y., Li, B., Wang, W., Qi, L., et al. (2021). A mathematical model for characterizing the biomass and the physiological/biochemical indicators of salvia miltiorrhiza based on growth-defense tradeoff. Front. Plant Sci. 12, 793574. doi:10.3389/fpls.2021.793574
Wang, L., Wang, L., Zhou, X., Ruan, G., and Yang, G. (2019a). Qishen Yiqi dropping pills ameliorates doxorubicin-induced cardiotoxicity in mice via enhancement of cardiac angiogenesis. Med. Sci. Monit. 25, 2435–2444. doi:10.12659/msm.915194
Wang, N., Sui, H., and Fu, L. (2020). Effect of salvianolic acid B on proliferation and transdifferentiation of high glucose-induced cardiac fibroblasts. Chin. Pharmacol. Bull. 36 (3), 414–419. doi:10.3969/j.issn.1001-1978.2020.03.022
Wang, N., Yang, J., Lu, J., Qiao, Q., Wu, T., Du, X., et al. (2014). A polysaccharide from Salvia miltiorrhiza Bunge improves immune function in gastric cancer rats. Carbohydr. Polym. 111, 47–55. doi:10.1016/j.carbpol.2014.04.061
Wang, S., Chang, Y., Ma, X., and Wang, F. (2015). Effects of different intensity endurance exercise on myocardial collagen in rats and the regulatory role of MMP-1/TIMP-1. Chin. J. Sports Sci. Technol. 51 (05), 60–66. doi:10.16470/j.csst.201505010
Wang, S., Tang, G., Qian, G., Hu, H., Zhai, C., Wang, Z., et al. (2019b). Effect of tanshinone ⅡA on proliferation of myocardial fibroblasts in rats. Chin. J. Crit. Care Med. (Electron. Ed.) 12 (1), 9–14. doi:10.3877/cma.j.issn.1674-6880.2019.01.002
Wang, Y., He, Y., Dai, X., and Luo, Y. (2024a). Clinical study of Shenfu Yixin prescription in the treatment of heart failure with reduced ejection fraction (Yang deficiency and water flooding type). J. Integr. Tradit. Chin. West. Med. Cardiovasc. Cerebrovasc. Dis. 22 (12), 2207–2211. doi:10.12102/j.issn.1672-1349.2024.12.017
Wang, Y., Xu, F., Chen, J., Shen, X., Deng, Y., Xu, L., et al. (2011). Matrix metalloproteinase-9 induces cardiac fibroblast migration, collagen and cytokine secretion: inhibition by salvianolic acid B from Salvia miltiorrhiza. Phytomedicine 19 (1), 13–19. doi:10.1016/j.phymed.2011.06.024
Wang, Z., Liu, C., Wei, J., Yuan, H., Shi, M., Zhang, F., et al. (2024b). Network and experimental pharmacology on mechanism of Yixintai regulates the TMAO/PKC/NF-κB signaling pathway in treating heart failure. Drug Des. Devel. Ther. 18, 1415–1438. doi:10.2147/dddt.S448140
Wei, Y., Mo, X., Wang, Q., Qu, N., Zhong, R., and Wei, B. (2021). Clinical efficacy of Huangqi Guizhi Wuwu Decoction combined with Shengmaiyin in the treatment of cardiac function in diabetic cardiomyopathy Chin. J. Exp. Tradit. Chin. Med. 27 (19), 104–109. doi:10.13422/j.cnki.syfjx.20210334
Willems, I. E., Havenith, M. G., De Mey, J. G., and Daemen, M. J. (1994). The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am. J. Pathol. 145 (4), 868–875.
Wu, S., Zhao, K., Wang, J., Liu, N., Nie, K., Qi, L., et al. (2022). Recent advances of tanshinone in regulating autophagy for medicinal research. Front. Pharmacol. 13, 1059360. doi:10.3389/fphar.2022.1059360
Wynn, T. A., and Barron, L. (2010). Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 30 (3), 245–257. doi:10.1055/s-0030-1255354
Xiao, Q., Guo, Z., Luo, J., Chen, Y., Zhang, S., Yu, Z., et al. (2023). Exploring the mechanism of action for Yangxin Tongmai Formula in regulating rats with chronic heart failure of blood stasis pattern based on TGF-β/Smads signaling pathway. J. Hunan Univ. Tradit. Chin. Med. 43 (8), 1353–1360. doi:10.3969/j.issn.1674-070X.2023.08.003
Xie, G., Zhang, Y., and Lv, Z. (2018). Effect of modified Wendan decoction on myocardial fibrosis and cardiac function in elderly patients with coronary heart disease after PCI. World Chin. Med. 13 (04), 878–881. doi:10.3969/j.issn.1673-7202.2018.04.023
Xu, G. R., Zhang, C., Yang, H. X., Sun, J. H., Zhang, Y., Yao, T. T., et al. (2020). Modified citrus pectin ameliorates myocardial fibrosis and inflammation via suppressing galectin-3 and TLR4/MyD88/NF-κB signaling pathway. Biomed. Pharmacother. 126, 110071. doi:10.1016/j.biopha.2020.110071
Xu, Q., Wan, D., Deng, L., Gao, Y., and Tian, Q. (2024a). Effects of Shengmai Qiangxin Granule on TLR4/MyD88/NF-κB signaling pathway and myocardial fibrosis in patients with chronic heart failure with qi and yin deficiency and stasis syndrome. J. Integr. Tradit. Chin. West. Med. Cardiovasc. Cerebrovasc. Dis. 22 (15), 2820–2823. doi:10.12102/j.issn.1672-1349.2024.15.023
Xu, R., Bi, Y., He, X., Zhang, Y., and Zhao, X. (2024b). Kidney-tonifying blood-activating decoction delays ventricular remodeling in rats with chronic heart failure by regulating gut microbiota and metabolites and p38 mitogen-activated protein kinase/p65 nuclear factor kappa-B/aquaporin-4 signaling pathway. J. Ethnopharmacol. 330, 118110. doi:10.1016/j.jep.2024.118110
Xu, X., Zhang, Y., Zhang, S., Gong, J., Lou, B., Gu, M., et al. (2019). Effect of metabolite danshen dripping pills combined with Rosuvastatin on left ventricular remodeling and myocardial fibrosis in patients with a-cute myocardial infarction after percutaneous coronary intervention. China Med. Herald 16 (35), 61–64+77.
Xue, K., Zhang, J., Li, C., Li, J., Wang, C., Zhang, Q., et al. (2019). The role and mechanism of transforming growth factor beta 3 in human myocardial infarction-induced myocardial fibrosis. J. Cell. Mol. Med. 23 (6), 4229–4243. doi:10.1111/jcmm.14313
Yan, F. (2019). Effects of Zhenwu Baoxin Decoction combined with biomedicine medicine on myocardial fibrosis and exercise tolerance in patients with chronic heart failure. Chin. J. Folk. Ther. 27 (07), 61–62. doi:10.19621/j.cnki.11-3555/r.2019.0732
Yang, J., Guo, Z., Li, Y., Zhang, T., and Deng, Y. (2021). Effects of Yixintai drug-containing plasma on proliferation and apoptosis of cardiac fibroblasts induced by angiotensin II and its mechanism. J. New Chin. Med. Clin. Pharmacol. 32 (9), 1245–1253. doi:10.19378/j.issn.1003-9783.2021.09.003
Yang, J., Sun, W., Sun, J., Wang, F., Hou, Y., Guo, H., et al. (2017). Guanxintai exerts protective effects on ischemic cardiomyocytes by mitigating oxidative stress. Evid. Based Compl. Alt. Med. 2017, 4534387. doi:10.1155/2017/4534387
Yang, J., Wang, B., Li, N., Zhou, Q., Zhou, W., and Zhan, Z. (2019). Salvia miltiorrhiza and carthamus tinctorius extract prevents cardiac fibrosis and dysfunction after myocardial infarction by epigenetically inhibiting Smad3 expression. Evid. Based Compl. Alt. Med. 2019, 6479136. doi:10.1155/2019/6479136
Yang, L., Zou, X. J., Gao, X., Chen, H., Luo, J. L., Wang, Z. H., et al. (2009). Sodium tanshinone IIA sulfonate attenuates angiotensin II-induced collagen type I expression in cardiac fibroblasts in vitro. Exp. Mol. Med. 41 (7), 508–516. doi:10.3858/emm.2009.41.7.056
Yang, R., Liu, A., Ma, X., Li, L., Su, D., and Liu, J. (2008). Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J. Cardiovasc. Pharmacol. 51 (4), 396–401. doi:10.1097/FJC.0b013e3181671439
Yang, Y., Feng, K., Yuan, L., Liu, Y., Zhang, M., Guo, K., et al. (2023a). Compound danshen dripping pill inhibits hypercholesterolemia/atherosclerosis-induced heart failure in ApoE and LDLR dual deficient mice via multiple mechanisms. Acta Pharm. Sin. B 13 (3), 1036–1052. doi:10.1016/j.apsb.2022.11.012
Yang, Y., Shao, M., Cheng, W., Yao, J., Ma, L., Wang, Y., et al. (2023b). A pharmacological review of tanshinones, naturally occurring monomers from salvia miltiorrhiza for the treatment of cardiovascular diseases. Oxid. Med. Cell. Longev. 2023, 3801908. doi:10.1155/2023/3801908
Yang, Z., Qi, J., Ping, D., Sun, X., Tao, Y., Liu, C., et al. (2022). Salvia miltiorrhiza in thorax and abdomainal organ fibrosis: a review of its pharmacology. Front. Pharmacol. 13, 999604. doi:10.3389/fphar.2022.999604
Ye, J., Yang, R., Li, L., Zhong, S., Jiang, R., and Hu, Z. (2024). Molecular mechanism of Danxiong Tongmai Granules in treatment of coronary heart disease. Aging 16 (10), 8843–8865. doi:10.18632/aging.205845
Ye, M., Wang, X., Sun, Y., Huang, J., Zeng, Y. J., and Gao, H. (2023). Clinical observation of Qiliqiangxin capsule combined with recombinant human brain natriuretic peptide in patients with acute heart failure. Zhonghua Nei Ke Za Zhi 62 (4), 422–426. doi:10.3760/cma.j.cn112138-20220420-00291
Yuan, X., Jing, H., and Wang, D. (2019). Tanshinone IIA protects myocardial fibrosis in rats with heart failure by inhibiting NADPH oxidase. J. Anhui Med. Univ. 54 (04), 600–604. doi:10.19405/j.cnki.issn1000-1492.2019.04.020
Zeng, H., Su, S., Zhu, Y., Gu, W., Qian, D., and Duan, J. (2016). Research progress on biosynthesis pathway and regulatory mechanism of salvianolic acid metabolites in Salvia miltiorrhiza. Chin. Bot. Drugal Drugs 47 (18), 3324–3331. doi:10.7501/j.issn.0253-2670.2016.18.028
Zhang, C., Zhang, S., Yang, Y., and Shen, J. (2023a). Effect of Yiqi Huoxue prescription on myocardial fibrosis in patients with heart failure after acute ST-segment elevation myocardial infarction. J. Nanjing Univ. Tradit. Chin. Med. 39 (2), 111–117. doi:10.14148/j.issn.1672-0482.2023.0111
Zhang, H., and Wei, C. (2021). Effects of Yiqi Yangyin Huoxue Buxin Decoction combined with biomedicine medicine on ventricular remodeling and serum sST2, Galectin-3, LN, and PIIIP in elderly patients with chronic heart failure. Chin. Med. Mat. 44 (03), 715–719. doi:10.13863/j.issn1001-4454.2021.03.040
Zhang, L., Ma, R., Zhang, X., Chen, J., and Qin, Z. (2024). Mechanisms of the botanical drugal pair of Salvia miltiorrhiza and Pueraria lobata in treating myocardial fibrosis based on network pharmacology. Hainan Med. J. 35 (6), 862–869. doi:10.3969/j.issn.1003-6350.2024.06.020
Zhang, L., Xu, X., Wan, Y., Ge, Y., Wang, D., and Zhou, J. (2017). Effects of Qili Qiangxin Capsule on improving myocardial fibrosis and TGF-β1/Smad3 signal transduction pathway. J. Difficult Dis. 16 (6), 618–622+535–536. doi:10.3969/j.issn.1671-6450.2017.06.019
Zhang, M. X., Huang, X. Y., Song, Y., Xu, W. L., Li, Y. L., and Li, C. (2022a). Astragalus propinquus schischkin and Salvia miltiorrhiza Bunge promote angiogenesis to treat myocardial ischemia via Ang-1/Tie-2/FAK pathway. Front. Pharmacol. 13, 1103557. doi:10.3389/fphar.2022.1103557
Zhang, X., Ma, Z. G., Yuan, Y. P., Xu, S. C., Wei, W. Y., Song, P., et al. (2018). Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell. Death Dis. 9 (2), 102. doi:10.1038/s41419-017-0123-3
Zhang, X., Wang, H., Chang, Y., Wang, Y., Lei, X., Fu, S., et al. (2015). An overview of meta-analyses of danhong injection for unstable angina. Evid. Based Compl. Alt. Med. 2015, 358028. doi:10.1155/2015/358028
Zhang, Y. (2015). Study on the protective effect of Danshen injection on iron-overloaded zebrafish and its inhibitory effect and mechanism on liver and cardiac fibrosis in chronic iron-overloaded mice. Doctoral thesis. Hebei (China): Hebei Medical University.
Zhang, Y., Huang, Y., Ma, Q. X., Xu, S. T., Shen, L., Xu, Y. Y., et al. (2023b). Guanxinning tablets improve myocardial hypertrophy by inhibiting the activation of MEK-ERK1/2 signaling pathway. J. Appl. Biomed. 21 (3), 137–149. doi:10.32725/jab.2023.014
Zhang, Y., Lu, W., Zhang, X., Lu, J., Xu, S., Chen, S., et al. (2019). Cryptotanshinone protects against pulmonary fibrosis through inhibiting Smad and STAT3 signaling pathways. Phytother. Res. 147, 104307. doi:10.1016/j.phrs.2019.104307
Zhang, Y., Wang, D., Wu, K., Shao, X., Diao, H., Wang, Z., et al. (2022b). The traditional Chinese medicine formula FTZ protects against cardiac fibrosis by suppressing the TGFβ1-Smad2/3 pathway. Evid. Based Compl. Alt. Med. 2022, 5642307. doi:10.1155/2022/5642307
Zhang, Y., Wang, J., Liu, Y. M., Chen, Y. Y., Yang, X. C., and Duan, L. (2021). The synergistic effects of astragalus mongholicus and salvia miltiorrhiza on coronary heart disease identified by network pharmacology and experiment. Drug Des. Devel. Ther. 15, 4053–4069. doi:10.2147/dddt.S326024
Zhang, Y., Zhou, N., Han, R., Liu, J., and Lei, Y. (2022c). Effect of Yixin Futing Yin on myocardial fibrosis and p38MAPK/NF-κB p65 signaling pathway in rats with aortic constriction inhibitory. Shaanxi J. Tradit. Chin. Med. 43 (3), 287–291. doi:10.3969/j.issn.1000-7369.2022.03.004
Zhao, D. (2016). Effect and mechanism of Qi-invigorating and blood-activating method on ventricular remodeling in hypertensive mice. Doctoral thesis. Shandong (China): Shandong University of Traditional Chinese Medicine.
Zhao, Y. (2022). Study on the protective effect and mechanism of Shenqi Jianxin prescription on rats with chronic heart failure model through TGF-β1/Smad3 signaling pathway. Master’s thesis. Anhui (China): Anhui University of Traditional Chinese Medicine.
Zhao, Y. (2023). Effect of Ershen Zhenwu Decoction on myocardial fibrosis in rats with chronic heart failure of heart and kidney yang deficiency type based on TGF-β1/Smads and PI3K/AKT/mTOR signaling pathways. Master’s thesis. Anhui (China): Anhui University of Traditional Chinese Medicine.
Zhao, Y., Gan, R., Zhang, X., Cheng, X., Ge, L., Peng, D., et al. (2023). In vitro experimental study on the regulation of PI3K/AKT/mTOR signaling pathway by Ershen Zhenwu Decoction to inhibit myocardial fibrosis. J. Anhui Univ. Tradit. Chin. Med. 42 (3), 77–82. doi:10.3969/j.issn.2095-7246.2023.03.017
Zhu, K., Lou, L., Gao, Y., Guo, Q., Wu, D., Gao, Y., et al. (2019a). Effect of Fuzheng Huayu Recipe on the expression of miR-29b-5p in cardiac fibroblasts induced by angiotensin II. Med. Rev. 25 (13), 2689–2694. doi:10.3969/j.issn.1006-2084.2019.13.038
Zhu, K., Lou, L., Qi, Y., Gao, Y., Li, C., Guo, Q., et al. (2019b). Discussion on cellular and molecular mechanism of Fuzheng Huayu Recipe against myocardial fibrosis from the point of view of matrix metabolism. China Med. Herald 16 (23), 12–15.
Zhu, W., Wu, R. D., Lv, Y. G., Liu, Y. M., Huang, H., and Xu, J. Q. (2020). BRD4 blockage alleviates pathological cardiac hypertrophy through the suppression of fibrosis and inflammation via reducing ROS generation. Biomed. Pharmacother. 121, 109368. doi:10.1016/j.biopha.2019.109368
MF Myocardial fibrosis
TCM Traditional Chinese medicine
CFs cardiac fibroblasts
PI3K Phosphatidylinositol3 kinase
TGF-β Transforming Growth Factor - β
NF-κB Nuclear factor kappa-B
RAAS Renin-Angiotensin-Aldosterone System
MMPs Matrix metalloproteinases
ACEI angiotensin-converting enzyme inhibitors
ARB angiotensin II receptor antagonist
ARNI Angiotensin Receptor-Neprilysin Inhibitor
AAC Abdominal Aortic Constriction
SGLT2i Sodium-Glucose Cotransporter 2 Inhibitors
α-SMA alpha-smooth muscle actin
ECM extracellular matrix
Tan IIA Tanshinone IIA
ISO isoprenaline
Col-I Collagen type I
Col-III Collagen type III
CHF Chronic Heart Failure
Ang II Angiotensin II
BNP Brain Natriuretic Peptide
LVMI Left Ventricular Mass Index
LVM Left Ventricular Mass
Sal B Salvianolic acid B
STZ Streptozotocin
AKT protein kinase B
ESZWD Er Shen Zhen Wu Decoction
QSYQDP Qishen Yiqi Dropping Pills
CFDA China Food and Drug Administration
Sal A Salvianolic acid A
TNF-α Tumor Necrosis Factor-α
Hcy Homocysteine
ET Endothelin
HQBXD Huangqi Baoxin Decoction
QSLWFG Qi Shen Liu Wei formula granules
KXP Kuo Xin Prescription
SQJXP Shenqi Jianxin Prescription
YXFTY Yixin Futing Yin
CHF chronic heart failure
YQYYHXTLP Yiqi Yangyin Huoxue Tongluo Prescription
DCM Dilated Cardiomyopathy
QLQXC Qiliqiangxin capsule
SSY Sanshen Yin
QSG Qishen Granule
SSYXC Shensongyangxin Capsule
FZHYP Fuzheng Huayu Prescription
TIMPs tissue inhibitors of metalloproteinases
TIMP-1 Tissue inhibitor of metalloproteinase-1
ECM extracellular matrix
FZHYC Fuzheng Huayu Capsule
YQHXC Yiqi Huoxue metabolite
ZGJTP Zuo Gui Jiang Tang Shu Xin Prescription
YXTMP Yangxin Tongmai Prescription
QSYQP Qi Shen Yi Qi Prescription
LAD Left Anterior Descending Branch
CFZZTZC metabolite Zhenzhu Tiaozhi Capsule
iNOS inducible nitric oxide synthase
NO nitric oxide
6 mwd 6-min walk distance
HFpEF heart failure and preserved ejection fraction
HF heart failure
SMQXG Shengmai Qiangxin Granules
BSHXD Bushen Huoxue Decoction
YXT Yixintai
DHI Danhong injection
MI myocardial infarction
ROS reactive oxygen species
RNS reactive nitrogen species
SAA Salvianic acid A
SOD superoxide dismutase
MDA malondialdehyde
STS Sodium Tanshinone IIA Sulfonate
Hyp Hydroxyproline
CDSDP metabolite Danshen Dripping Pills
GXV Guanxin V
GXT Guanxintai
DXTMG Danxiong Tongmai Granules
NO-SMS newly optimized formulation of Shengmaisan
NT-ProBNP N-Terminal Pro-B-Type Natriuretic Peptide
AIM Autoimmune Myocarditis
COA Coarctation of the Aorta
YXT Yixintai
HQQR Huoxue Qianyang Qutan Recipe
YXTG Yixintai Granules
YQHXD Yiqi Huoxue Decoction
FZHYD Fuzheng Huayu Decoction
JSF Jiashenfang
BSHXD Busheng Huoxue Decoction
RCT randomized controlled trial
SMQXG Shengmai Qiangxin Granules
SFYXD Shenfu Yixin Decoction
HFrEF Heart Failure with Reduced Ejection Fraction
YQHXP Yiqi Huoxue prescription
SGYXP Shugan Yixin prescription
SYYXHXD Shenyuan Yiqi Huoxue Decoction
ZWBXD Zhenwu Baoxin Decoction
JWWDD Jiawei Wendan Decoction
YQHYD Yiqi Huayu Decoction
YQYYHXD Yiqi Yangyin Huoxue Decoction
YQYYHXBXD Yiqi Yangyin Huoxue Buxin Decoction
HQASM Huangqi Guizhi Wuwu Decoction and Shengmai Decoction
SMADS Shengmai Powder and Danshen Decoction
A. membranaceus Astragalus membranaceus (Fisch.) Bunge [Fabaceae; Astragali Radix]
P. ginseng Panax ginseng C.A. Mey. [Araliaceae; Ginseng Radix et Rhizoma]
A. carmichaelii Aconitum carmichaelii Debx. [Ranunculaceae; Aconiti Lateralis Praeparata]
D. sophia Descurainia sophia (L.) Webb ex Prantl [Brassicaceae; Descurainiae Semen]
C. cassia Cinnamomum cassia Presl [Lauraceae; Cinnamomi Cortex]
C. tinctorius Carthamus tinctorius L. [Asteraceae; Carthami Flos]
M. officinalis Magnolia officinalis Rehd. et Wils. [Magnoliaceae; Magnoliae Cortex]
C. reticulata Citrus reticulata Blanco [Rutaceae; Citri Reticulatae Pericarpium]
P. ginseng Panax ginseng C.A. Meyer [Araliaceae; Ginseng Radix Rubra]
A. macrocephala Atractylodes macrocephala Koidz.[Asteraceae; Atractylodis Macrocephalae Rhizoma]
E. brevicornu Epimedium brevicornu Maxim.[Berberidaceae; Epimedii Folium]
P. cocos Poria cocos (Schw.) Wolf [Polyporaceae; Poria]
C. cassia Cinnamomum cassia Presl [Lauraceae; Cinnamomi Ramulus]
C. anthriscoides Conioselinum anthriscoides (Chuanxiong) [Apiaceae; Ligustici Chuanxiong Rhizoma]
P. heterophylla Pseudostellaria heterophylla (Miq.) Pax [Caryophyllaceae; Pseudostellariae Radix]
O. japonicus Ophiopogon japonicus (Thunb.) Ker Gawl. [Asparagaceae; Ophiopogonis Radix]
R. glutinosa Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Scrophulariaceae; Rehmanniae Radix]
S. chinensis Schisandra chinensis (Turcz.) Baill. [Schisandraceae; Schisandrae Fructus]
G. glabra Glycyrrhiza glabra L. [Fabaceae; Glycyrrhizae Radix et Rhizoma]
P. notoginseng Panax notoginseng (Burkill) F.H.Chen [Araliaceae; Notoginseng Radix et Rhizoma]
P. lactiflora Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix Alba]
A. carmichaelii Aconitum carmichaelii Debeaux [Ranunculaceae; Aconiti Lateralis Praeparata]
L. japonica Lonicera japonica Thunb. [Caprifoliaceae; Lonicerae Japonicae Flos]
P. montana var. lobata Pueraria montana var. lobata (Willd.) Maesen & S.M.Almeida ex Sanjappa & Predeep [Fabaceae; Puerariae Lobatae Radix]
R. glutinosa Rehmannia glutinosa (Gaertn.) Libosch. ex DC. [Scrophulariaceae; Rehmanniae Radix]
C. officinalis Cornus officinalis Siebold & Zucc. [Cornaceae; Corni Fructus]
P. vulgaris Prunella vulgaris L. [Lamiaceae; Prunellae Spica]
J. regia Juglans regia L. [Juglandaceae; Juglandis Semen]
P. massoniana Pinus massoniana Lamb. [Pinaceae; Pinus Massoniana Pollen]
G. pentaphyllum Gynostemma pentaphyllum (Thunb.) Makino [Cucurbitaceae; Gynostemmatis Pentaphylli Herba]
C. chinensis Coptis chinensis Franch. [Ranunculaceae; Coptidis Rhizoma]
C. monogyna Crataegus monogyna Jacq. [Rosaceae; Crataegi Fructus]
C. ×aurantium f. aurantium Citrus × aurantium f. aurantium [Rutaceae; Aurantii Fructus Immaturus]
B. sacra Boswellia sacra Flück. [Burseraceae; Boswelliae Resina]
C. myrrha Commiphora myrrha (T.Nees) Engl. [Burseraceae; Myrrha]
P. kurroa Picrorhiza kurroa Royle ex Benth. [Scrophulariaceae; Picrorhizae Rhizoma]
A. bidentata Achyranthes bidentata Blume [Amaranthaceae; Achyranthis Bidentatae Radix]
A. gramineus Acorus gramineus Aiton [Acoraceae; Acori Graminei Rhizoma]
R. multiflora Reynoutria multiflora (Thunb.) Moldenke [Polygonaceae; Reynoutriae Multiflorae Caulis]
C. yanhusuo Corydalis yanhusuo (Y. H. Chou & Chun C. Hsu) W. T. Wang ex Z. Y. Su & C. Y. Wu [Papaveraceae; Corydalis Rhizoma]
L. chinense Lycium chinense Mill. [Solanaceae; Lycii Fructus]
E. senticosus Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. [Araliaceae; Acanthopanacis Senticosi Radix et Rhizoma]
T. crispa Tinospora crispa (L.) Hook.f. & Thomson [Menispermaceae; Tinosporae Caulis]
E. japonicum Eupatorium japonicum Thunb. [Asteraceae; Eupatorii Herba]
W. indica Waltheria indica L. [Sterculiaceae; Waltheriae Indicae Herba]
C. Ostreae Concha Ostreae [Ostreidae; Concha Ostreae]
U. rhynchophylla Uncaria rhynchophylla (Miq.) Miq. [Rubiaceae; Uncariae Ramulus cum Uncis]
C. zedoaria Curcuma zedoaria (Christm.) Roscoe [Zingiberaceae; Curcumae Zedoariae Rhizoma]
C. aromatica Curcuma aromatica Salisb. [Zingiberaceae; Curcumae Aromatica Rhizoma]
D. costus Dolomiaea costus (Falc.) Kasana & A.K.Pandey [Asteraceae; Dolomiaeae Costus]
L. chinense Lycium chinense Mill. [Solanaceae; Lycii Fructus]
C. deserticola Cistanche deserticola Ma [Orobanchaceae; Cistanches Herba]
Z. officinale Zingiber officinale Roscoe [Zingiberaceae; Zingiberis Rhizoma]
P. asiatica Plantago asiatica L. [Plantaginaceae; Plantaginis Semen]
A. catechu Areca catechu L. [Arecaceae; Arecae Semen]
R. rosea Rhodiola rosea L. [Crassulaceae; Rhodiolae Radix et Rhizoma]
V. negundo Vitex negundo L. [Verbenaceae; Viticis Negundi Herba]
B. chinense Bupleurum chinense DC. [Apiaceae; Bupleuri Radix]
M. oleifera Moringa oleifera Lam. [Moringaceae; Moringae Oleiferae Folium]
S. baicalensis Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae Radix]
P. armeniaca Prunus armeniaca L. [Rosaceae; Armeniacae Semen]
C. chinensis Coptis chinensis Franch. [Ranunculaceae; Coptidis Rhizoma]
A. vera Aloe vera (L.) Burm.f. [Asphodelaceae; Aloes]
T. kirilowii Trichosanthes kirilowii Maxim. [Cucurbitaceae; Trichosanthis Fructus]
S. glabra Smilax glabra Roxb. [Liliaceae; Smilacis Glabrae Rhizoma]
P. quinquefolius Panax quinquefolius L. [Araliaceae; Panax Quinquefolii Radix]
F. suspensa Forsythia suspensa (Thunb.) Vahl [Oleaceae; Forsythiae Fructus]
T. aestivum Triticum aestivum L. [Poaceae; Triticus Aestivus]
C. sativa Cannabis sativa L. [Cannabaceae; Cannabis Fructus]
Z. jujuba Ziziphus jujuba Mill. [Rhamnaceae; Ziziphi Jujubae Fructus]
W. villosa var. villosa Wurfbainia villosa var. villosa [Rubiaceae; Wurfbainiae Villosae Caulis]
Keywords: natural medicine Salvia miltiorrhiza Bunge, MF, advancements in research, metabolites, pharmacological mechanism
Citation: Li Q, Ren C, Jiang B, Wang X, Wang C, Zhi X, Li L, Guo X, Zhao X and Li Y (2025) Salvia miltiorrhiza Bunge root in the treatment of myocardial fibrosis: research progress and challenges. Front. Pharmacol. 16:1554696. doi: 10.3389/fphar.2025.1554696
Received: 16 January 2025; Accepted: 19 March 2025;
Published: 31 March 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Jing-Shuai Wu, Chinese Academy of Medical Sciences, ChinaCopyright © 2025 Li, Ren, Jiang, Wang, Wang, Zhi, Li, Guo, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinke Zhao, enhrZDQxMkAxNjMuY29t; Yingdong Li, bHlkajQxMkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.