- 1Department of Pharmacy, The Second People’s Hospital of Yibin, Yibin, China
- 2Department of Pharmacy, The Third People’s Hospital of Yibin, Yibin, China
Background: This meta-analysis aimed to assess the efficacy and safety of ginkgo terpene lactone preparations including diterpene ginkgolides meglumine injection (DGMI) and ginkgolide injection combined with antiplatelet drugs in the treatment of ischemic stroke.
Methods: We systematically searched the randomized controlled trials(RCTs) with publication date earlier than 6 November 2024 in PubMed, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Journal Database (VIP), Chinese Biomedical Literature Database (CBM), Wanfang Database, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library. Studies were screened according to inclusion and exclusion criteria, evaluated according to criteria recommended by the Cochrane Handbook, and data were then analyzed using Stata 17 software.
Results: Of 1,079 identified studies, 27 were eligible and included in our analysis (N = 3,336 patients). The meta-analysis demonstrated that the overall response rate [RR = 1.22, 95% CI(1.17, 1.27), Z = 9.76, p < 0.01], as well as the National Institutes of Health Stroke Scale (NIHSS) score and barthel index, were significantly better in the DGMI combined treatment group compared to the antiplatelet therapy alone group. However, there was no significant difference observed between the experimental group and the control group regarding improvements in prognosis and platelet function. The studies included in the analysis reported a total of 419 adverse reactions (ADRs), with 206 occurring in the DGMI combined treatment group; furthermore, there was no significant difference in the incidence of adverse events between the two groups.
Conclusion: Ginkgo terpene lactone preparations, when combined with antiplatelet drugs, can significantly enhance the clinical efficacy of ischemic stroke and demonstrate a favorable safety profile. This combination is a potential treatment strategy that can improve the management of IS patients and has high clinical application value.
Introduction
Ischemic stroke is a significant cause of disability and mortality worldwide. Data from the Global Burden of Disease study indicate that ischemic stroke impacts millions of patients annually, imposing significant financial and emotional burdens on individuals, families, and society at large (VL et al., 2018; GBD, 2019 Stroke Collaborators, 2021). Currently, the primary treatments for ischemic stroke include thrombolytic therapy and antiplatelet therapy (Powers et al., 2019). While thrombolytic therapy can effectively reopen occluded vessels in patients with ischemic stroke, it is constrained by a strict time window for administration and carries risks, such as bleeding (Tsivgoulis et al., 2023). Antiplatelet drugs, such as aspirin, clopidogrel, and tirofiban, are crucial for the secondary prevention of ischemic stroke. However, the efficacy of these single agents in enhancing long-term prognosis is limited, and issues such as drug resistance persist (Kamarova et al., 2022). Consequently, there is an urgent need to investigate and validate novel treatment combinations to enhance recovery following a stroke.
Ginkgo terpene lactones represent a class of compounds derived from the leaves of Ginkgo biloba, primarily comprising bilobalide and ginkgolides A, B, C, K, J, L, M, N, P, and Q (Geng et al., 2018) Previous studies have established that related preparations, such as DGMI and ginkgolide injections, exhibit significant efficacy in treating cerebrovascular diseases (Zhao et al., 2022; Li et al., 2024). To be specific, DGMI is composed of ginkgolide A, ginkgolide B, and ginkgolide K. ginkgolide injection contains bilobalide, ginkgolide A, ginkgolide B, and ginkgolide C. In recent years, the therapeutic strategy involving ginkgo terpene lactone preparations with antiplatelet drugs has garnered significant attention within the academic community. The theoretical advantages of this combined treatment regimen are multifaceted. Firstly, ginkgo terpene lactones provide neuroprotection through antioxidant and anti-inflammatory mechanisms, which complement the effects of antiplatelet drugs and may enhance overall therapeutic efficacy (Xia and Fang, 2007). Secondly, ginkgo terpene lactones facilitate an increase in oxygen supply to ischemic regions by augmenting cerebral blood flow and improving microcirculation, thereby promoting recovery (Xu et al., 2005; Zhu et al., 2022). Lastly, ginkgo terpene lactones, as platelet-activating factor receptor (PAFR) antagonists, may synergize with conventional antiplatelet regimens in antithrombotic treatment by inhibiting platelet aggregation (Han et al., 2023). The synergistic effects of these multiple pathways position this approach as a promising strategy for enhancing clinical outcomes.
Based on the aforementioned rationale, numerous clinical trials have evaluated the efficacy and safety of ginkgo terpene lactone preparations in conjunction with antiplatelet agents. However, there remains a notable absence of systematic reviews and meta-analyses that provide a comprehensive assessment of their actual efficacy and safety in clinical practice. Therefore, we conducted a systematic review and meta-analysis of existing randomized controlled trials to determine the effect of this combination regimen on key outcomes such as clinical efficacy, NIHSS score, platelet function, and safety in patients with ischemic stroke.
Materials and methods
Searching strategy
This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. The protocol for this systematic review was registered on INPLASY(Unique ID number is INPLASY2024120106) and is available in full on inplasy.com (https://doi.org/10.37766/inplasy2024.12.0106). We systematically searched PubMed, CNKI, VIP, CBM, Wanfang Database, Embase, Web of Science, ClinicalTrials.gov, and Cochrane Library. The search ranged from the time each database was built to 6 November 2024. Screening language is Chinese or English. We used the following combined textword and MeSH terms such as “Ischemic Stroke,” “Ginkgolide” and “ginkgo diterpene lactone meglumine”. The complete search used for PubMed was: ((“Ischemic Stroke” [Mesh]) OR (Stroke*[Title/Abstract])) AND ((“Ginkgolides” [Mesh]) OR ((((Ginkgolide*[Title/Abstract]) OR (ginkgo diterpene lactone meglumine [Title/Abstract])) OR (GDLM [Title/Abstract])) OR (DGLM [Title/Abstract]))).
Inclusion criteria
Inclusion criteria were abided by the participants, interventions, comparison/control, outcomes, and study design (PICOS) format, which were as follows: 1) The study participants included adults aged 18 years and older, irrespective of gender or ethnicity. Ischemic stroke was diagnosed using computed tomography (CT) or magnetic resonance imaging (MRI), in accordance with established diagnostic criteria. 2) Study design: RCTs in Chinese or English. 3) Intervention measures: Both the experimental group and the control group received conventional treatment, which encompasses the regulation of blood lipids, oxygen inhalation, blood pressure control, blood glucose regulation, and nutritional supplementation. In addition to conventional treatment, the experimental group was administered ginkgo terpene lactone preparations in combination with antiplatelet drugs. The ginkgo terpene lactone preparations included DGMI and ginkgolide injection. 4) The control group received only antiplatelet drugs on the basis of conventional treatment. 5) The outcome measures met the following primary or secondary outcomes: the primary outcomes were the clinical efficacy defined according to the nationally approved criteria (The Fourth National Conference on cerebrovascular. 1996; Wang, 2024; Chen et al., 2022). Specifically, reductions in neurological deficit scores ranging from 18% to 100% following treatment were classified as demonstrating varying degrees of clinical efficacy. Conversely, a decrease in neurological deficit score of less than 17% or an increase in score following treatment was deemed “ineffective.” As secondary outcomes, we compared the NIHSS score, Barthel index, modified Rankin scale (mRS), platelet function, and the incidence of ADRs or adverse events.
Exclusion criteria
The exclusion criteria were as follows: 1) nonclinical randomized controlled trials, including literature reviews, systematic reviews, individual case studies, experience summaries, and basic research; 2) repeated publications; 3) imprecise descriptions of conventional treatment in both the experimental and control groups; 4) studies involving patients treated with thrombolytics or other neuroprotective agents (e.g., edaravone, citicoline); and 5) studies focused on other diseases.
Data extraction and quality assessment
Two independent investigators (HX and YT) reviewed study titles and abstracts, and studies that satisfied the inclusion criteria were retrieved for full-text assessment. In case of disagreement, decisions were made by discussion or consultation with a third investigator (LZ). We extracted the relevant data of the included studies, and the extracted contents included: basic information of the included studies (title, first author, publication year, etc.); basic characteristics of the study subjects (number of patients, gender, age, intervention measures, dose, treatment process, etc.); outcome evaluation indicators; and risk of bias assessment information. Quality assessment was performed by the Cochrane risk of bias tool, including randomization method, allocation concealment, blinding, baseline comparability, and completeness of outcome data.
Statistical analysis
Statistical analysis was conducted using Stata 17 software. Hazard ratios (RR) and standardized mean differences (SMD) served as effect indicators for dichotomous data and continuous variables, respectively. A 95% confidence interval (95% CI) was calculated for both types of data. The I2 test was employed to assess the heterogeneity among the included studies. If p > 0.1 and I2 < 50%, it was concluded that there was no heterogeneity among the studies, and a fixed-effect model was selected for analysis. Conversely, in cases of substantial heterogeneity, a random-effects model was utilized, and a sensitivity analysis was conducted to identify the sources of heterogeneity. Finally, funnel plots were generated to evaluate the publication bias present in the literature.
Results
Study selection process
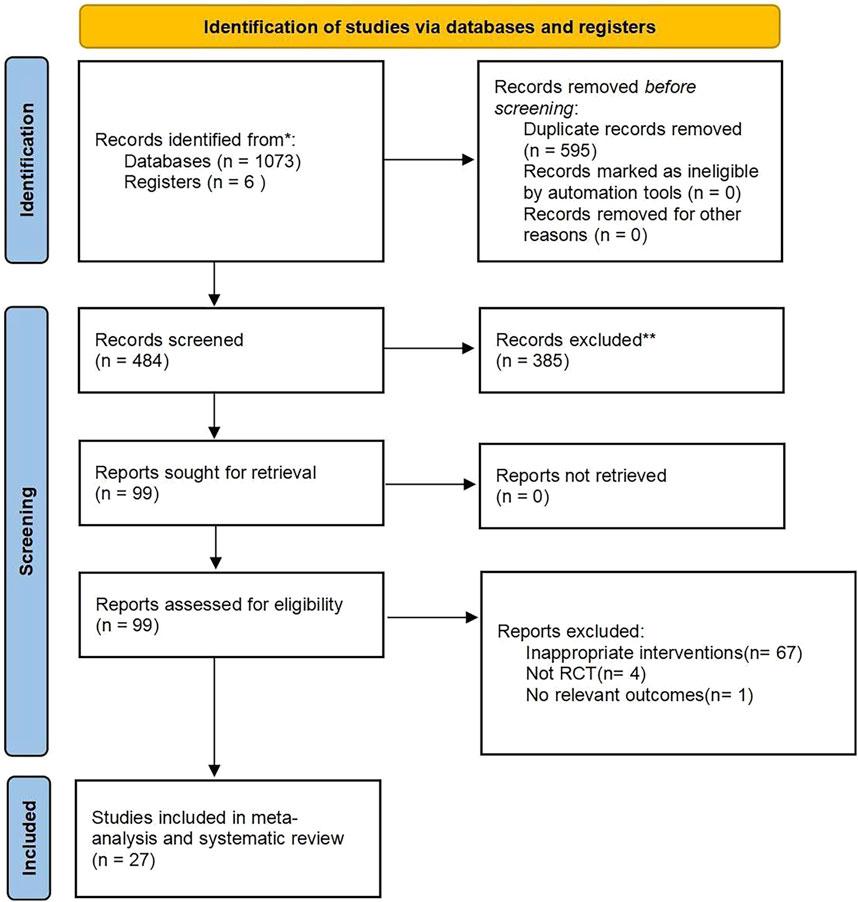
According to the search strategy, a total of 1,079 citations were retrieved. After the duplicate articles were removed, the remaining 484 articles were filtered according to the inclusion and exclusion criteria, and then 385 articles were excluded. By further reading the full text, finally 27 studies were included for meta-analysis. The screening process is shown in Figure 1.
Study characteristics
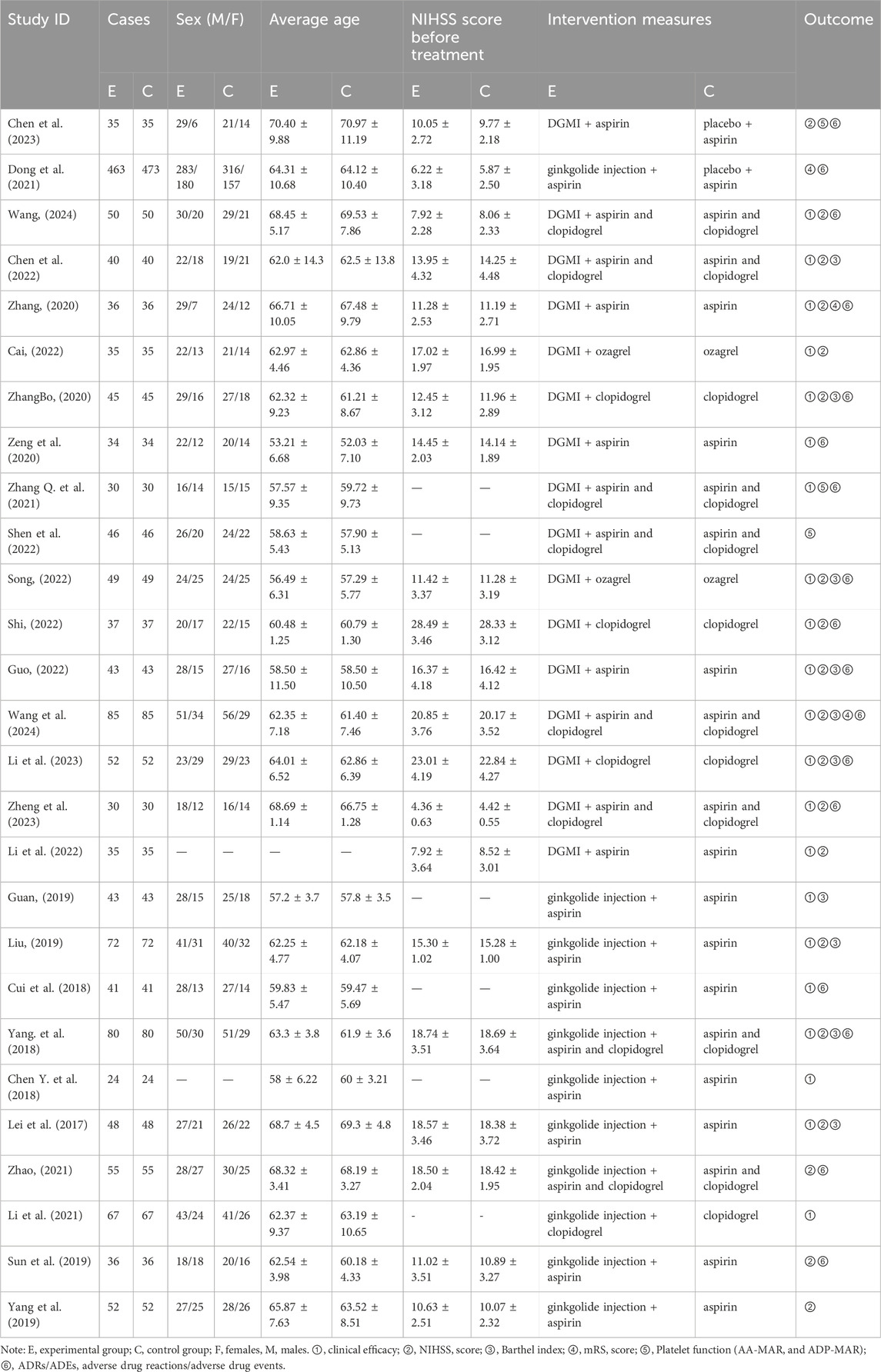
The 27 included trials were published from 2017 to 2024, four (Chen et al., 2023; Wang, 2024; Dong et al., 2021; Chen et al., 2022) of which were published in English and the remaining trials were published in Chinese. A total of 3,336 patients were involved in this review, including 1,663 patients in the experimental group and 1,673 patients in the control group. Across all studies, the average age of participants was approximately 52–70 years, with more male participants than female participants. Antiplatelet agents used in the two included trials were mainly aspirin enteric-coated tablets, clopidogrel sulfate tablets, and ozagrel sodium for injection. The experimental group was treated with DGMI(25 mg, once a day) and ginkgolide injection (25 mg or 50 mg, once a day) for 14 days. Detailed characteristics of the included trials are detailed in Table 1.
Quality evaluation on included studies
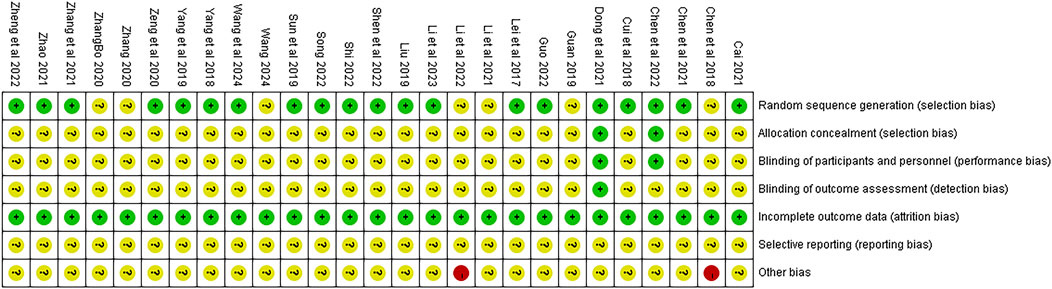
The quality of the 27 studies is illustrated in Figure 2. Although all articles referenced “randomization,” only 20 studies (Chen et al., 2023; Liu, 2019; Lei et al., 2017; Shi, 2022; Cai, 2022; Zeng et al., 2020, Song 2022; Zheng et al., 2023; Sun et al., 2019; Yang et al., 2019; Dong et al., 2021; Yang. et al., 2018; Zhao, 2021, Zhang L. et al., 2021; Shen et al., 2022; Cui et al., 2018; Li et al., 2023; Wang et al., 2024; Guo, 2022; Chen et al., 2022) detailed specific randomization methods. Two studies (Chen et al., 2023; Dong et al., 2021) implemented double-blinding and allocation concealment through computer coding or third-party drafting protocols during the experiment; one (Dong et al., 2021) of these also conducted blinding in outcome assessments. While all articles provided complete outcome data, it remained unclear whether the authors selectively reported relevant results at the time of publication. Lastly, two studies (Li et al., 2022, Chen Y. et al., 2018) were classified as “high risk” due to incomplete baseline data, while the other studies did not disclose any additional risk deviations.
Meta-analysis results
Analysis of clinical efficacy
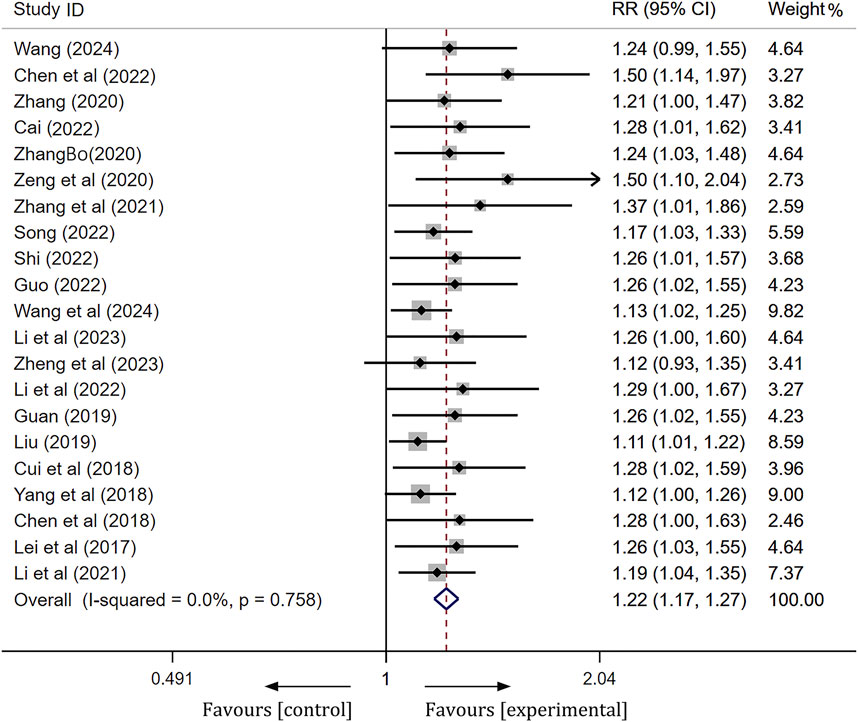
A total of 21 articles were compared regarding the clinical efficacy between the ginkgo terpene lactone preparations combined treatment group and the control group. Following a heterogeneity test, these 21 studies demonstrated no statistical heterogeneity (I2 = 0%, p = 0.758), thus permitting the application of a fixed effect model for analysis. To assess the accuracy and stability of the clinical efficacy derived from the meta-analysis, we conducted a sensitivity analysis utilizing a row-by-row exclusion method. As illustrated in Supplementary Figure S1, no significant interference was detected among the studies, indicating that the results were relatively stable. The meta-analysis revealed a statistically significant difference [RR = 1.22, 95% CI (1.17, 1.27), Z = 9.76, p < 0.01], suggesting that the clinical efficacy of the experimental group was significantly superior to that of the control group (Figure 3).
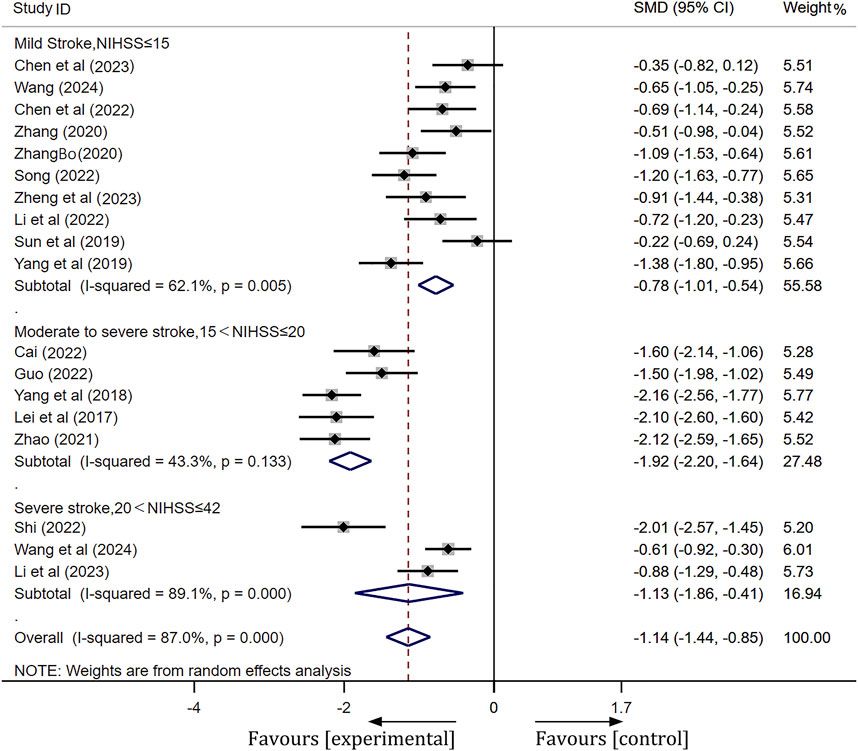
NIHSS scores
A total of 19 studies were included in this analysis. Following heterogeneity testing, these studies exhibited high levels of heterogeneity (I2 = 94.5%, p < 0.01) (Supplementary Figure S2). Subsequently, sensitivity analysis revealed that Liu’s study (Liu, 2019) significantly influenced the meta-analysis results (Supplementary Figure S3). After excluding Liu’s study, we categorized patients into three subgroups based on their NIHSS scores prior to treatment: moderate stroke (NIHSS ≤15), moderate-severe stroke (15 < NIHSS ≤20), and severe stroke (20 < NIHSS ≤42). After conducting subgroup analysis and excluding outliers (Liu, 2019), heterogeneity was markedly reduced in the moderate stroke group (I2 = 62.1%, p < 0.01) and the moderate-severe stroke group (I2 = 43.3%, p = 0.13). A meta-analysis employing a random-effects model across three subgroups revealed statistically significant differences. Detailed meta-analysis results are as follows: for the mild stroke group, [SMD = −0.78, 95% CI(-1.01, −0.54), p < 0.01]; for the moderate-severe stroke group, [SMD = −1.92, 95% CI(-2.20, −1.64), p < 0.01]; and for the severe stroke group, [SMD = −1.13, 95% CI(-1.86, −0.41), p < 0.01]. These findings indicate that ginkgo terpene lactone preparations, when combined with antiplatelet drugs, is more effective in enhancing neurological function than the use of antiplatelet drugs alone. More details of the trials were presented in Figure 4.
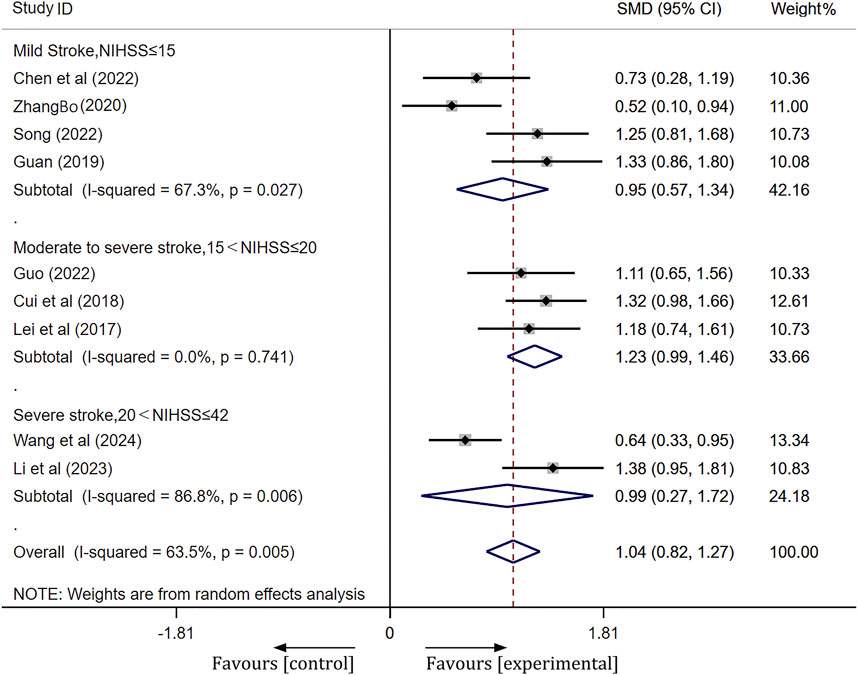
Barthel index
A total of 10 studies were included. Following heterogeneity testing, these studies demonstrated a high level of heterogeneity (I2 = 80.2%, p < 0.01) (Supplementary Figure S4). Subsequently, sensitivity analysis identified Liu’s study (Liu, 2019) as the primary source of heterogeneity (Supplementary Figure S5). After excluding outliers (Liu, 2019), subgroup analysis based on pretreatment NIHSS scores revealed a significant reduction in heterogeneity. The results of the random effects model meta-analysis were as follows: for the mild stroke group [SMD = 0.95, 95% CI(0.57, 1.34), p < 0.001]; for the moderate-severe stroke group [SMD = 1.23, 95% CI(0.99, 1.46), p < 0.001]; and for the severe stroke group [SMD = 0.99, 95% CI(0.27, 1.72), p = 0.007]. The meta-analysis indicated that the Barthel index of the experimental group was significantly better than that of the control group, as illustrated in Figure 5.
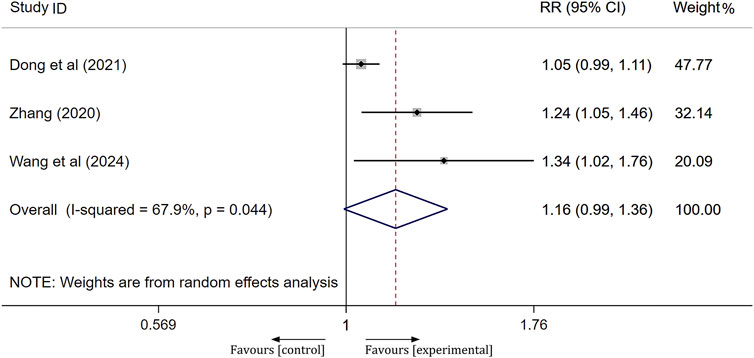
mRS scores
A total of three studies assessed the prognosis of patients following treatment using the mRS score, where a score of 0–2 indicates a good prognosis and a score of 3–6 indicates a poor prognosis. Significant heterogeneity was observed across the studies (I2 = 67.9%, p = 0.044); therefore, a random-effects model was employed to aggregate the effect sizes. The results of the meta-analysis indicated that the prognosis of the experimental group was not significantly different from that of the control group, with no notable difference between the groups [RR = 1.16, 95% CI(0.99, 1.36), p = 0.065], as shown in Figure 6.
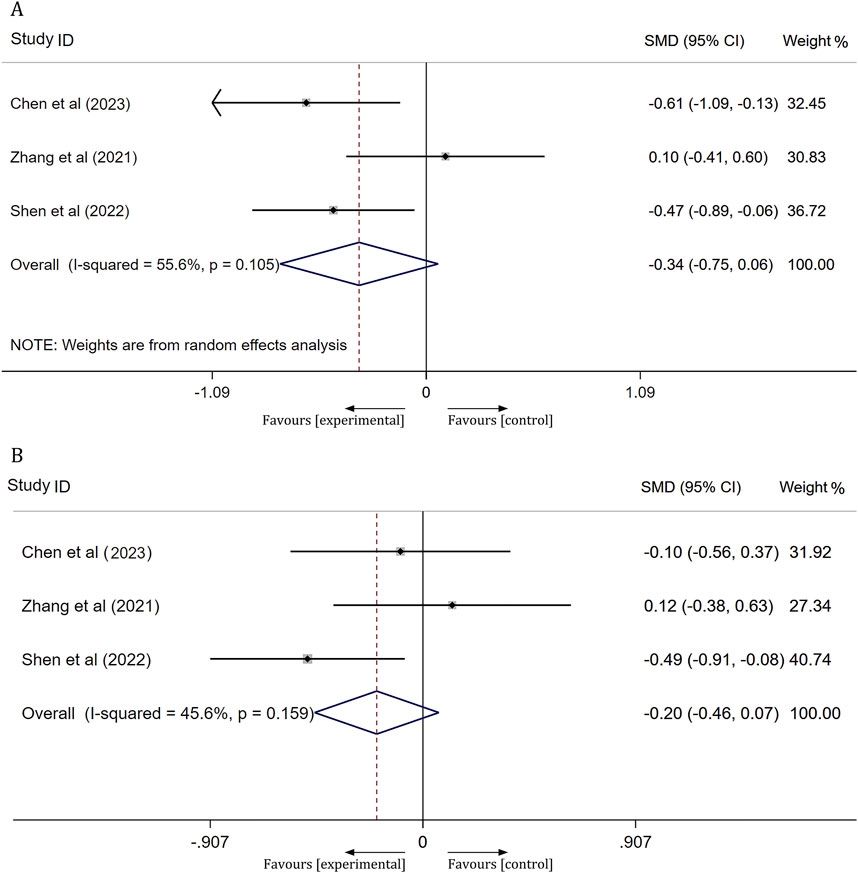
Platelet function
Three studies assessed platelet function in patients with ischemic stroke after 14 days of treatment, focusing on arachidonic acid induced maximal platelet aggregation rate (AA-MAR) and adenosine diphosphate induced maximal platelet aggregation rate (ADP-MAR). The results from a meta-analysis utilizing a random-effects model indicated no significant difference in AA-MAR between the experimental and control groups [SMD = -0.34, 95% CI(-0.75, 0.06), p = 0.095]. Similarly, a meta-analysis using a fixed-effects model revealed no significant difference in ADP-MAR between the experimental and control groups [SMD = −0.20, 95% CI(−0.46,0.07), p = 0.145]. For further details, refer to Figure 7.
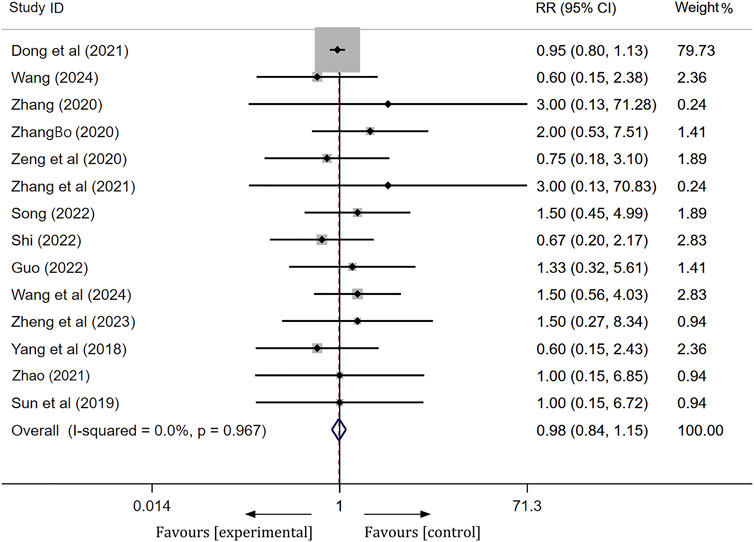
Safety
Among the 27 studies included, three studies (Chen et al., 2023; Li et al., 2023; Cui et al., 2018) reported no significant serious ADRs associated with the treatment. Fourteen studies documented the occurrence of ADRs, with 206 patients in the experimental group and 213 patients in the control group experiencing such reactions. The clinical symptoms observed in both groups primarily included rash, nausea/vomiting, gastrointestinal discomfort, constipation, dizziness, palpitations, and abnormal liver and kidney function. Following the onset of these adverse reactions, patients in both groups demonstrated improvement or resolution of symptoms after receiving appropriate treatment. A meta-analysis employing a fixed effects model revealed no statistically significant difference in the incidence of ADRs between the experimental and control groups [RR = 0.98, 95% CI(0.84, 1.15), p = 0.84],as shown in Figure 8. This finding indicates that the addition of ginkgo terpene lactone preparations to the treatment regimen for ischemic stroke does not elevate the incidence of adverse events when compared to antiplatelet therapy alone. Furthermore, the remaining ten studies did not provide information regarding the occurrence of ADRs.
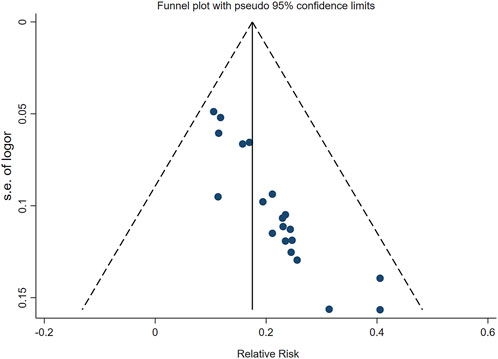
Publication bias
We drawed a funnel plot for the clinical efficacy of treatment to evaluate publication bias. As illustrated in Figure 9, the funnel plot revealed an asymmetric distribution of the scatter on both sides of the invalid line, suggesting the presence of publication bias. Additionally, we conducted Egger’s test using Stata 17.0 software, and the results showed p < 0.05, further indicating the existence of publication bias.
Discussion
This study included a total of 27 RCTs and conducted a meta-analysis to evaluate the efficacy and safety of ginkgo terpene lactone preparations in conjunction with conventional antiplatelet drugs for the treatment of ischemic stroke. The results indicated that the addition of ginkgo terpene lactone preparations to standard antiplatelet therapy enhances the overall clinical efficacy in patients with varying degrees of ischemic stroke, reduces the severity of neurological dysfunction, and improves activities of daily living. However, this combination regimen did not yield a significant improvement in the mRS scores and platelet function in patients with ischemic stroke when compared to antiplatelet therapy alone. Regarding safety, 14 studies explicitly reported side effects experienced by subjects during treatment, with the most common being dizziness and rash, followed by nausea, vomiting, mild abdominal pain, and other gastrointestinal symptoms. Overall, no major adverse events were reported in either group, and there was no statistically significant difference in the incidence of adverse reactions between the two treatment groups.
Traditional Chinese medicine (TCM) classifies ischemic stroke as a type of stroke, with phlegm-stasis blocking collateral syndrome being the most common variant. Ginkgo biloba has been utilized as a traditional Chinese medicinal plant since ancient times, primarily for its functions in promoting blood circulation, removing blood stasis, dredging collaterals, relieving pain, converging lung function, alleviating asthma, and dissipating turbidity while lowering lipid levels. Its ability to enhance blood circulation and eliminate blood stasis, along with its properties for dredging collaterals and dissipating turbidity, are significant reasons for the use of Ginkgo biloba in stroke treatment. Ginkgo terpene lactones are active components derived from the leaves of Ginkgo biloba, primarily consisting of diterpene and sesquiterpene structures (Geng et al., 2018). DGMI and ginkgolide injections are Ginkgo terpene lactone preparations that have received marketing approval, boasting high active ingredient content and minimal adverse effects. Furthermore, prior research has demonstrated that Ginkgo biloba terpene lactone preparations are linked to cerebral protection, a reduction in inflammatory responses, and enhanced microcirculation, making them beneficial in the prevention and treatment of cardiovascular and cerebrovascular diseases (Fang et al., 2015; Fang et al., 2010; Li et al., 2007; Chen M. et al., 2018; Fei et al., 2021). This study further corroborates that ginkgolide preparations can serve as adjuncts to antiplatelet therapy in patients with ischemic stroke. It is recommended that DGMI (25 mg, once daily) and ginkgolide injections (25 mg or 50 mg, once daily) be administered for a duration of 2 weeks. Nevertheless, in clinical practice, treatment strategies should still be tailored to the individual needs of each patient.
Our study also demonstrated that the addition of ginkgo terpene lactone preparations to conventional antiplatelet therapy did not significantly inhibit platelet aggregation induced by the arachidonic acid (AA) pathway or the adenosine diphosphate (ADP) pathway in patients. A multicenter randomized clinical trial (Dong et al., 2021) demonstrated that the effect of ginkgolide on ischemic stroke is associated with the platelet activating factor (PAF) pathway, but not with the ADP or thromboxane A2 (TxA2) pathways. Similarly, Han’s research (Han et al., 2023) found that neither AA-mediated nor ADP-mediated platelet reactivity, as measured by thromboelastography (TEG), differed significantly between DGMI-treated and untreated groups. Collectively, these studies suggest that the therapeutic benefits of ginkgo terpene lactone preparations may not be related to platelet aggregation induced by the AA or ADP pathways, which aligns with our findings. Furthermore, the results of the meta-analysis revealed no significant increase in the number of patients with good prognoses when ginkgo terpene lactone preparations was added to the treatment regimen compared to antiplatelet therapy alone. Our further analysis of the original literature suggested that this may be attributed to bias arising from the shorter follow-up duration after treatment in the study by Dong et al. (2021), and it may also be related to the limited number of RCTs included.
Currently, there are four systematic reviews (Zhao et al., 2022; Li et al., 2024; Zhang L. et al., 2021; Meng et al., 2021), both domestically and internationally, assessing the efficacy and safety of ginkgo terpene lactone preparations in conjunction with conventional Western medicine. Compared to their study, all ischemic stroke patients included in our research received standard antiplatelet therapy. Patients undergoing thrombolytic therapy or those receiving other neuroprotective agents, such as edaravone or citicoline, were excluded from our analysis. This exclusion was implemented to minimize the potential influence of thrombolytic therapy and similar interventions on the experimental outcomes. Furthermore, we strictly limited the duration of ginkgo terpene lactone preparations administration to 14 days within the experimental group, ensuring the generation of more rigorous and reliable study results. In instances of high heterogeneity, we conducted subgroup analyses based on the severity of ischemic stroke, followed by sensitivity analyses to identify the sources of heterogeneity among the indicators. To demonstrate the robustness of our findings, we also performed sensitivity analyses and assessed publication bias concerning the overall treatment response rate.
Despite the contributions of our study, several limitations should be acknowledged. First, we included 27 publications, all of which were in Chinese and English, potentially overlooking valuable studies published in other languages. Second, only three articles evaluated the effects of ginkgo terpene lactone preparations combined with antiplatelet drugs on platelet function and mRS scores in patients with ischemic stroke, this small sample size may introduce bias into the results. Finally, the majority of the RCTs included in this meta-analysis were small-scale studies conducted at single centers. Additionally, these studies often lacked clear allocation concealment and blinding during implementation, which increases the potential for bias. In future, a multicenter, large sample size, and double-blind placebo-controlled design is needed to verify the reliability of this meta-analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
HX: Data curation, Methodology, Project administration, Software, Writing–original draft, Writing–review and editing. LZ: Funding acquisition, Software, Writing–review and editing. LLi: Funding acquisition, Writing–review and editing. XL: Writing–review and editing. YT: Data curation, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the Sichuan Province Science and Technology Plan Project (2024NSFSC 1843), Special Fund Project for Scientific Research of Young Pharmacists of Sichuan Hospital Association (YP2202427) and Yibin Science and Technology Plan Project (2021ZYY009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1554207/full#supplementary-material
References
Cai, Z. (2022). Effect of combined therapy with Ginkgo biloba diterpene lactone meglumine and ozagrel on CSS score in patients with acute cerebral infarction. Guizhou Med. 46 (02), 251–252. doi:10.3969/j.issn.1000-744X.2022.02.048
Chen, C., Lv, H., Shan, L., Long, X., Guo, C., Huo, Y., et al. (2023). Antiplatelet effect of ginkgo diterpene lactone meglumine injection in acute ischemic stroke: a randomized, double-blind, placebo-controlled clinical trial. Phytotherapy Res. 37 (5), 1986–1996. doi:10.1002/ptr.7720
Chen, M., Zou, W., Chen, M., Cao, L., Ding, J., Xiao, W., et al. (2018). Ginkgolide K promotes angiogenesis in a middle cerebral artery occlusion mouse model via activating JAK2/STAT3 pathway. Eur. J. Pharmacol. 833, 221–229. doi:10.1016/j.ejphar.2018.06.012
Chen, R., Yan, L., Xie, P., Tian, J., Zhao, Y., Liu, Y., et al. (2022). Use of diterpene ginkgolides meglumine injection to regulate plasma levels of PAI-1 and t-PA in patients with acute atherosclerotic cerebral infarction. Neurologist 27 (6), 299–303. doi:10.1097/nrl.0000000000000399
Chen, Y., Liu, Z., and Dai, L. (2018). Treatment analysis of acute cerebral infarction with ginkgolide injection combined with aspirin. Diet. health care 5 (26), 59. doi:10.3969/j.issn.2095-8439.2018.26.070
Cui, S., Li, X., and Liu, G. (2018). Effects of ginkgolide injection on the treatment of ischemic stroke and its influence on serum lipids and cytokines. WORLD Chin. Med. 13 (01), 116–118+123. doi:10.3969/j.issn.1673-7202.2018.01.029
Dong, Yi, Zhang, J., Wang, Y., Zhao, L., Li, R., Wei, C., et al. (2021). Effect of ginkgolide in ischemic stroke patients with large artery atherosclerosis: results from a randomized trial. CNS Neurosci. and Ther. 27 (12), 1561–1569. doi:10.1111/cns.13742
Fang, W., Deng, Y., Li, Y., Shang, E., Fang, F., Lv, P., et al. (2010). Blood brain barrier permeability and therapeutic time window of Ginkgolide B in ischemia-reperfusion injury. Eur. J. Pharm. Sci. 39 (1-3), 8–14. doi:10.1016/j.ejps.2009.10.002
Fang, W., Sha, L., Kodithuwakku, N. D., Wei, J., Zhang, R., Han, D., et al. (2015). Attenuated blood-brain barrier dysfunction by XQ-1H following ischemic stroke in hyperlipidemic rats. Mol. Neurobiol. 52 (1), 162–175. doi:10.1007/s12035-014-8851-1
Fei, Y., Zhao, B., Zhu, J., Fang, W., and Li, Y. (2021). XQ-1H promotes cerebral angiogenesis via activating PI3K/Akt/GSK3β/β-catenin/VEGF signal in mice exposed to cerebral ischemic injury. Life Sci. 272, 119234. doi:10.1016/j.lfs.2021.119234
GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20 (10), 795–820. doi:10.1016/s1474-4422(21)00252-0
Geng, T., Shen, W. W., Wang, J. J., Huang, W. Z., Wang, Z. Z., and Xiao, W. (2018). Research development of ginkgo terpene lactones. Zhongguo Zhong Yao Za Zhi 43 (7), 1384–1391. doi:10.19540/j.cnki.cjcmm.20180312.001
Guan, T. (2019). Effect of ginkgolide injection on inflammatory factors and neurological deficits in high-risk non-disabling ischemic stroke patients. Mod. J. Integr. Traditional Chin. West. Med. 28 (16), 1784–1787.
Guo, J. (2022). Therapeutic effect of ginkgo diterpene lactone meglumine and aspirinin acute cerebral infarction and its influence on bl, NlHSS andOOLISP scores. Chin. Foreign Med. 41 (09), 14–18. doi:10.16662/j.cnki.1674-0742.2022.09.014
Han, X., Li, Y., Chen, X., Pan, D., Mo, J., Qiu, J., et al. (2023). Platelet-activating factor antagonist-based intensive antiplatelet strategy in acute ischemic stroke: a propensity score matched with network pharmacology analysis. CNS Neurosci. Ther. 29 (12), 4082–4092. doi:10.1111/cns.14331
Kamarova, M., Baig, S., Patel, H., Monks, K., Wasay, M., Ali, A., et al. (2022). Antiplatelet use in ischemic stroke. Ann. Pharmacother. 56 (10), 1159–1173. doi:10.1177/10600280211073009
Lei, J., Zhong, X., Guocao, H., and Yang, Z. (2017). Clinical observation of ginkgolide injection and aspirin in ischemic stroke. J. GUANGDONG Med. Univ. 35 (03), 234–236.
Li, A., Hou, X., Hong, J., and Chen, H. (2022). Effect of ginkgo biloba diterpene lactone meglumine injection on mild to moderate acute ischemic stroke. Mod. Drug Use China 16 (08), 131–133. doi:10.14164/j.cnki.cn11-5581/r.2022.08.048
Li, J., Wang, H., Shi, R., Zhang, X., Lin, Y., Cao, H., et al. (2024). The efficacy and safety of diterpene ginkgolides meglumine injection for cerebral infarction: a meta-analysis of randomized controlled trials. Medicine 103 (3), e37025. doi:10.1097/md.0000000000037025
Li, Q., Hou, S., and Su, Y. (2021). Effects of ginkgolide injection combined with clopidogrel in treatment of acute cerebral infarction on HMGB1/TLR4/NF-κB pathway. Hebei Med. 43 (22), 3385–3388. doi:10.3969/j.issn.1002-7386.2021.22.005
Li, S. Q., Zhang, Y., and Yang, L. J. (2007). Improving effect of Ginkgolide B on mitochondrial respiration of ischemic neuron after cerebral thrombosis in tree shrews. Chin. Med. J. Engl. 120 (17), 1529–1533. doi:10.1097/00029330-200709010-00012
Li, Z., Yang, G., Li, C., and Jiang, J. (2023). Efficacy of diterpene ginkgolides meglumine injection combined with clopidogrel in ischemic stroke and its effect on levels of serum inflammatory factors. Chin. J. Ration. Drug Use 20 (09), 66–72. doi:10.3969/j.issn.2096-3327.2023.09.011
Liu, Q. (2019). Clinical efficacy and neuroprotective effect of ginkgolide injection on acute ischemic stroke. Doctor 4 (22), 143–145.
Meng, T., Xie, X., Li, T., Yang, Q., Xu, Y., and Gao, Y. (2021). Systematic review and meta-analysis of ginkgolide injections for the adjuvant treatment of acute ischemic stroke. WORLD Chin. Med. 16 (08), 1241–1249. doi:10.3969/j.issn.1673-7202.2021.08.011
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50 (12), e344–e418. doi:10.1161/str.0000000000000211
Shen, Y., Fan, J., Chen, Bo, He, S., Qi, Li, Hu, X., et al. (2022). Effects of Ginkgo diterpenoid lactone glucamine on related miRNA levels in mild ischemic stroke/TIA patients. Pract. Drugs Clin. 25 (06), 499–502. doi:10.14053/j.cnki.ppcr.202206005
Shi, L. (2022). Clinical study on ginkgo diterpene lactone glucamine combined with ClopidogrelBisulfate for ischemic stroke. New TCM 54 (02), 42–45. doi:10.13457/j.cnki.jncm.2022.02.012
Song, C. (2022). Effect of Ginkgo biloba diterpene lactone meglumine combined with antiplatelet drugs on patients with acute ischemic cerebral infarction and its influence on TEG monitoring results. J. Med. Forum 43 (24), 100–103.
Sun, Y., Cui, F., and Li, D. (2019). Effect of Ginkgo biloba extract on ox-LDL, LOX-1, hs-CRP and neuroprotection in patients with acute cerebral infarction. Chin. J. Coal Industry Med. 22 (05), 477–480.
The Fourth National Conference on cerebrovascular (1996). Clinical neurological deficit scoring criteria for stroke patients. Chin. J. Neurology (06), 62–64.
Tsivgoulis, G., Katsanos, A. H., Sandset, E. C., Turc, G., Nguyen, T. N., Bivard, A., et al. (2023). Thrombolysis for acute ischaemic stroke: current status and future perspectives. Lancet Neurol. 22 (5), 418–429. doi:10.1016/s1474-4422(22)00519-1
Vl, F., Nguyen, G., Cercy, K., Johnson, C. O., Alam, T., Parmar, P. G., et al. (2018). Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 379 (25), 2429–2437. doi:10.1056/NEJMoa1804492
Wang, J., and Tan, H. (2024). Efficacy and safety of combining ginkgolide diterpene glucosamine injection with dual antibodies in elderly patients with acute ischemic stroke. Trop. J. Pharm. Res. 23 (2), 423–428. doi:10.4314/tjpr.v23i2.24
Wang, X., Peng, N., and Zhang, J. (2024). Therapeutic effect of ginkgo diterpene lactone glucoside in acute ischemic stroke beyond the therapeutic time window and its effect on serum Toll like receptor 4/nuclear factor -kappa B signaling pathway factor level. Hainan Med. J. 35 (06), 782–787.
Xia, S. H., and Fang, D. C. (2007). Pharmacological action and mechanisms of ginkgolide B. Chin. Med. J. Engl. 120 (10), 922–928. doi:10.1097/00029330-200705020-00013
Xu, J. P., Li, L., and Sun, L. S. (2005). Effects of ginkgolide on cerebral blood flow in dogs. Zhong Xi Yi Jie He Xue Bao 3 (1), 50–53. doi:10.3736/jcim20050115
Yang, C., Chai, L., Jie, H., Zhao, L., Zhang, X., and Wang, H. (2018). Effect of ginkgolide injection combined with aspirin and clopidogrel triple therapy on acute cerebral infarction. Hebei J. TCM 40 (03), 383–386.
Yang, G., Cheng, Y., and Sun, X. (2019). Effect of ginkgolide combined with butylphthalide on neurological deficits and inflammatory mediators in patients with acute cerebral infarction. Prev. Treat. Cardio Cereb. Vasc. Dis. 19 (04), 363–365. doi:10.3969/j.issn.1009_816x.2019.04.025
Zeng, X., Zhao, X., Zhao, X., and Chu, D. (2020). Effect of Ginkgo biloba diterpene lactone combined with oxiracetam on serum lipoprotein-associated phospholipase A2 expression in patients with acute cerebral infarction. Chin. J. Clin. 48 (08), 998–1000. doi:10.3969/j.issn.2095-8552.2020.08.039
Zhang, L., Lian, X., Zhao, J., Chen, X., Bai, Y., and Li, X. (2021). Meta-analysis on diterpene ginkgolides meglumine injection with Western Medicine in treating Cerebral infarction. Chin J Neuroimmunol and. Neurol 28 (02), 152–157. doi:10.3969/j.issn.1006-2963.2021.02.011
Zhang, Q., Zhang, R., and Qian, J. (2021). Effects of diterpene ginkgolides meglumine on thromboelastography in patients with acute ischemic stroke. China Med. Innov. 18 (22), 5–8. doi:10.3969/j.issn.1674-4985.2021.22.002
Zhang, Y. (2020). The influence of aspirin combined with ginkgolides meglumine injection on serum galectin-3 level in patients with acute ischemic stroke (phlegmstasis in channel syndrome of stroke) and its therapeutic effect. Master, Cnki.
ZhangBo (2020). Effect of clopidogrel combined with ginkgo biloba diterpene lactone meglumine on carotid atherosclerosis in patients with cerebral infarction. Chin. J. Gerontology 40 (17), 3611–3614. doi:10.3969/j.issn.1005-9202.2020.17.009
Zhao, B. (2021). Effect of ginkgolide injection combined with atorvastatin calcium tablets on neurological function, hemodynamics and adverse reactions in elderly patients with acute cerebral infarction. Henan Wedical Res. 30 (01), 135–137. doi:10.3969/j.issn.1004-437X.2021.01.055
Zhao, H., Guo, Q., Li, B., and Shi, M. (2022). The efficacy and safety of ginkgo terpene lactone preparations in the treatment of ischemic stroke: a systematic review and meta-analysis of randomized clinical trials. Front. Pharmacol. 13, 821937. doi:10.3389/fphar.2022.821937
Zheng, H., Li, G., and Wang, Y. (2023). Effect of ginkgo biloba diterpene lactone meglumine injection on L-1β, hs-CRP and IL-8 in elderly patients with acute ischemic stroke in super thrombolysis time window. Chin. J. Health Med. 25 (01), 102–104. doi:10.3969/j.issn.1674-3245.2023.01.029
Keywords: ischemic stroke, DGMI, ginkgolide injection, antiplatelet drugs, meta-analysis
Citation: Xu H, Zeng L, Liao L, Li X and Tang Y (2025) The efficacy and safety of ginkgo terpene lactone preparations combined with antiplatelet aents in the treatment of ischemic stroke: a systematic review and meta-analysis. Front. Pharmacol. 16:1554207. doi: 10.3389/fphar.2025.1554207
Received: 01 January 2025; Accepted: 03 March 2025;
Published: 19 March 2025.
Edited by:
Mark M. Rasenick, University of Illinois Chicago, United StatesReviewed by:
Xin Cui, China Academy of Chinese Medical Sciences, ChinaXiaoyan Han, Zhaoqing First People’s Hospital, China
Copyright © 2025 Xu, Zeng, Liao, Li and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Xu, eHVob25nNjc2NkAxNjMuY29t; Yan Tang, dHlkZDgyNTUwNjBAMTYzLmNvbQ==
 Hong Xu
Hong Xu Li Zeng
Li Zeng Li Liao1
Li Liao1