
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 16 April 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1549419
Background: Xuebijing injection (XBJI), as a Chinese patent medicine injection, consists of five botanical drugs for anti-inflammatory treatment. Acute pancreatitis (AP) is induced by localized inflammation, potentially resulting in multiple organ dysfunction syndromes, specifically including acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Recent studies suggest that XBJI effective in alleviating potentially easing ALI and ARDS.
Objective: We illustrated the efficacy and safety of XBJI for pulmonary function of AP by conducting a systematic literature review and meta-analysis.
Methods: We conducted searches across eight databases, including PubMed, Embase, and the Cochrane Library, up to September 2024. Two independent investigators screened and selected the literature based on predefined inclusion and exclusion criteria, followed by data extraction. The quality of the selected studies was assessed using the Cochrane Collaboration’s Risk of Bias 2.0 tool. The data were then qualitatively analyzed and synthesized by using Review Manager software, in accordance with the PRISMA guidelines and the Cochrane Handbook.
Conclusion: This study showed that using conventional therapy combined with XBJI might increase the oxygenation index, lower the respiratory rate, and improve APACHE II scores and inflammatory biomarkers. However, there is a high risk of bias and the quality of the included studies is low. More well-designed, large-sample, and high-quality trials are needed to be conducted in multiple centers.
Acute Pancreatitis (AP) is a common cause of acute abdominal pain, and its incidence has been rising globally in recent years due to lifestyle changes and dietary shifts (Boxhoorn et al., 2020). AP is primarily a localized inflammatory condition affecting the pancreas. Approximately 20% of patients often exhibit complications such as systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS). The severe complications are accompanied by a mortality rate that ranges approximately from 20% to 40% (Skouras et al., 2014). Among the early complications, acute lung injury (ALI) stands out as one of the most severe, driven by the uncontrolled release of inflammatory mediators, lung tissue inflammation, and oxidative stress. ALI is also prone to developing acute respiratory distress syndrome (ARDS), which could result in respiratory failure (Bhatia and Moochhala, 2004; Schepers et al., 2019). Due to the absence of reliable therapeutic agents, the outcomes for severe AP complicated by respiratory failure remain unsatisfactory, and the mortality rate remains high. Based on the pharmaceutical theory of traditional Chinese medicine, the principal treatment for AP involves clearing heat and expelling evil, replenishing vital energy, nourishing bodily fluids, and promoting blood circulation to dissipate stasis.
Xuebijing injection (XBJI), a standardized traditional Chinese medicine compound, contains five botanical drugs: Honghua, Chishao, Chuanxiong, Danshen, and Danggui (Li et al., 2021; Wu et al., 2021), as summarized in Table 1. It is produced by Tianjin Chase Sun Pharmaceutical Co., Ltd. (China). The functions of XBJI effectively promote blood circulation, remove blood stasis, clear heat, detoxify, and strengthen health (Gong et al., 2024). In recent years, research has demonstrated XBJI has significant efficacy in treating several inflammatory conditions, including sepsis (Wu et al., 2021), severe pneumonia (Guo et al., 2020) and AP (Zhu et al., 2022). The study of Wei Zhou suggest XBJI may modulating the IL-17, TNF, NF-κB, and TLR pathways, and targeting IL-1β, IL-6, and TNF, thereby exert therapeutic effects on sepsis (Zhou et al., 2024). Notably, some preclinical study suggested that XBJI may reduce inflammatory responses, and potentially protect pulmonary function in AP (Chen et al., 2011; Dong et al., 2020; Kang et al., 2010). However, current clinical studies of XBJI protect pulmonary function in AP remain scattered, lacking systematic evidence.
The aim of this meta-analysis is to systematically evaluate the efficacy of XBJI in protecting pulmonary function in AP. By including randomized controlled trials (RCTs) and clinical controlled trials that meet the inclusion criteria, the analysis will quantitatively assess the impact of XBJI on pulmonary function indicators (e.g., oxygenation index). This meta-analysis aims to provide robust evidence on the efficacy of XBJI for lung protection in AP, thereby supporting both clinical treatment and future research.
The study has been officially registered within the International prospective register of systematic reviews (PROSPERO, CRD42024585387).
Following the guideline the “Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)”, We systematically searched multiple databases, including PubMed, Embase, Cochrane Library, Web of Science, Chinese National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature (SinoMed), Wanfang Database, and the China Science and Technology Journal Database, for relevant studies published before September 2024. The search terms included MeSH headings, keywords, abstracts, or titles related to “acute pancreatitis,” “Xuebijing injection,” “acute lung injury,” and “acute respiratory distress syndrome.” There were no language restrictions. Detailed search strategies and the screening process are provided in Supplementary Information 1.
1) Study type: RCTs and clinical controlled trials of XBJI in the treatment of AP.
2) Participants: AP was definitely diagnosed, and the patient nationality, age and gender were unlimited.
3) Intervention measures: The AP patients received conventional treatment, including fluid replacement, correction of water and electrolyte balance, acid-base disorders, gastrointestinal decompression, anti-infection, nutritional support, and inhibition of pancreatic enzyme and the treatment group was supplemented with XBJI on top of conventional treatment.
4) Outcome indicators: main outcome measures: Oxygenation index; Secondary outcome measures: respiratory rate, intra-abdominal pressure, inflammatory factors, APACHE II.
If the above-mentioned conditions are not met, the literature will be excluded. Additionally, studies meeting any of the following criteria will also be excluded.
1) Does not meet the diagnostic criteria for AP.
2) Duplicate literature.
3) Reviews, case reports, and animal experimental studies literature.
4) Intervention measures that are combined with other traditional Chinese medicine treatments.
5) Studies with incomplete data, where attempts to contact the author to obtain complete datasets have failed.
Two independent investigators (Yuling Bin and Rumei Peng) conducted the study according to the inclusion and exclusion criteria. They separately extracted data that fundamental study details (the publication year, first author), participant characteristics (sample size, age range, gender), intervention measures (treatment duration, dosing schedules), and primary and secondary outcomes.
The risk of bias in the included studies was independently assessed by the two investigators using the Cochrane Handbook for Systematic Reviews of Interventions and the RoB 2.0 tool. The assessments, conducted in Review Manager 5.3, considered five aspects, like random sequence generation, allocation concealment, blinding, data completeness, and reporting bias. Based on the evaluations, studies are categorized into high, medium, or low quality. Where significant bias risks are identified, sensitivity or subgroup analysis are conducted. Any disagreements that arose during this process were resolved by a third investigator (Yang Liu).
The statistical analysis of the extracted data was carried out utilizing the Review Manager software. To quantify the combined effect, we transformed continuous outcome data into standardized mean differences (SMDs), accompanied by their respective 95% confidence intervals (CIs). The presence of heterogeneity among the included studies was evaluated using the I2 test. When an I2 < 50% indicated the heterogeneity is low, a fixed-effects model was used to make estimates. If not, a random-effects model was applied to statistical analysis. To find the sources of heterogeneity, we conducted subgroup analysis based on the sample size of each group, the duration of treatment, and the publication year.
In this review, we initially retrieved 93 records from eight databases. After removing duplicates and screening the titles and abstracts, we excluded 85 studies due to reasons such as duplication, combination with other interventions, not RCTs or clinical controlled trials, not patients with AP, and the loss of main clinical outcomes. Ultimately, eight articles, encompassing 10 studies with a total of 400 participants, were included in this study (Figure 1). All these studies, published between 2008 and 2022, were carried out in China. The sample sizes ranged from 60 to 144, and the treatment duration was 3–7 days. The control groups received conventional treatment, while the intervention group was supplemented with XJBI. The characteristics of these studies are shown in Table 2.
Ten studies have reported on the oxygenation index of patients with AP following both conventional therapy and a combined treatment utilizing XBJI. Heterogeneity tests conducted on these studies revealed a high degree of variability (P < 0.00001, I2 = 93%). A sensitivity analysis was subsequently performed, which demonstrated that the exclusion of any individual study did not significantly alter the results, suggesting their stability despite the heterogeneity. Despite analyzing the clinical, methodological, and statistical heterogeneity across these studies, the specific source of this variability remained unclear. Consequently, a random effects model was employed for the meta-analysis. The findings indicated a statistically significant difference (MD = 31.70, 95% CI [22.36, 41.05], P < 0.00001), demonstrating that the combination of XBJI with conventional therapy was more effective in improving the oxygenation index of patients with AP compared to conventional therapy alone (Figure 2).
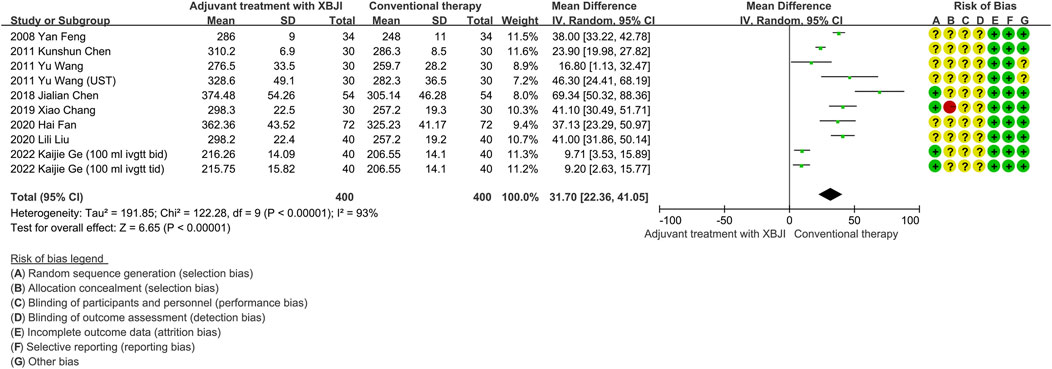
Figure 2. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly improved the oxygenation index in AP patients (MD = 31.70, 95% CI [22.36, 41.05], P < 0.00001).
Three studies employed respiratory rate as a secondary outcome indicator, encompassing 94 participants in a combined treatment utilizing XBJI and conventional therapy. Given the substantial heterogeneity observed (P = 0.13, I2 = 52%), the random effects model was utilized for data evaluation. The results of the meta-analysis revealed a statistically significant advantage for the combined treatment group in decreasing respiratory rate compared to the conventional therapy group (MD = −3.18, 95%CI [-5.07, 1.29], P < 0.00001) (Figure 3).
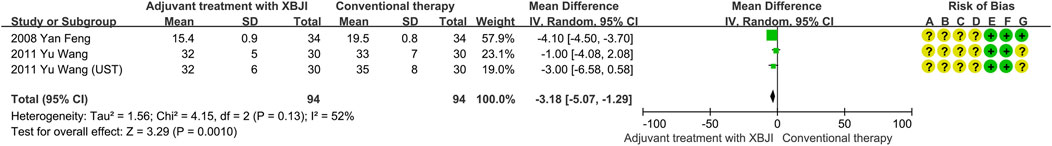
Figure 3. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly decreased the respiratory rate in AP patients (MD = −3.18, 95%CI [-5.07, 1.29], P = 0.0010).
A total of two studies reported on intra-abdominal pressure levels, encompassing 70 cases in a combined XBJI treatment group and a conventional therapy group. Given the low heterogeneity observed (P = 0.92, I2 = 0%), fixed effect models were employed. Meta-analysis revealed that the combined XBJI treatment group exhibited a significant reduction in intra-abdominal pressure compared to the conventional therapy (MD = −0.72, 95%CI [-1.12, −0.32], P = 0.004) (Figure 4).
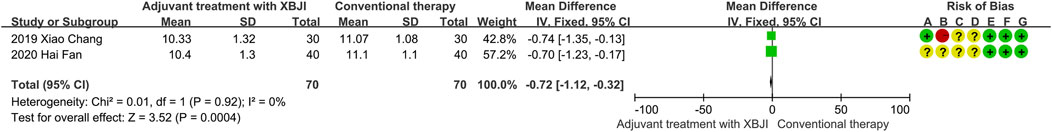
Figure 4. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly reduced the intra-abdominal pressure levels in AP patients (MD = −0.72, 95%CI [-1.12, −0.32], P = 0.0004).
Six studies employed IL-6 as an outcome measure, with 276 participants in both the adjuvant treatment with XBJI and conventional therapy. Given the significant heterogeneity observed (P < 0.00001, I2 = 89%), the random effects model was utilized for data evaluation. The meta-analysis results revealed a notable superiority of the adjuvant treatment with XBJI in reducing IL-6 levels compared to the conventional therapy (MD = −6.55, 95%CI [-10.79, −2.31], P < 0.00001) (Figure 5).
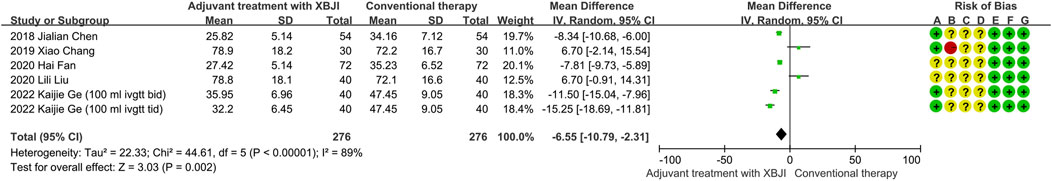
Figure 5. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly reduced the IL-6 in AP patients (MD = -6.55, 95%CI [-10.79, -2.31], P = 0.002).
Six studies utilized TNF-α as one of the outcome indicators. Due to the substantial heterogeneity observed (P < 0.00001, I2 = 86%), the random effects models were employed for analysis. The meta-analysis results demonstrated that the adjuvant treatment with XBJI exhibited a significant advantage over the conventional therapy in reducing TNF-α levels (MD = −16.12, 95%CI [-21.59, −10.65], P < 0.00001) (Figure 6).
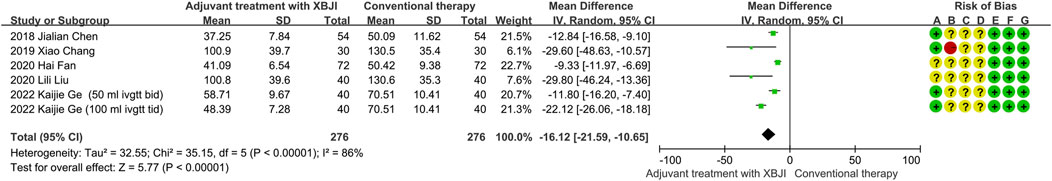
Figure 6. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly reduced the TNF-α in AP patients (MD = −16.12, 95%CI [-21.59, −10.65], P < 0.00001).
Five studies assessed APACHEII score. Compared with conventional therapy, the level of APACHEII score was significantly decreased in the adjuvant treatment with XBJI group (MD = −1.38, 95% CI [−2.62, −0.15], P < 0.00001) (Figure 7), without significant heterogeneity (I2 = 85%, P < 0.00001).
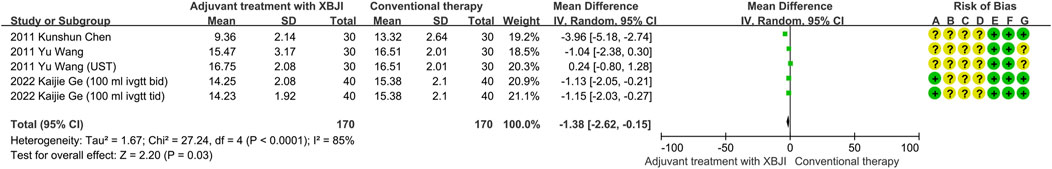
Figure 7. The meta-analysis results revealed that the combination of conventional therapy and XBJI significantly decreased the APACHE II score in AP patients (MD = −1.38, 95% CI [−2.62, −0.15], P = 0.03).
Ten included studies were conducted on the oxygenation index using TSA software. Following the inclusion of the first study, the cumulative Z-value crossed the conventional threshold (Z = 1.96). After the inclusion of the sixth study, the sample size exceeded the RIS (Required information size), indicating that the combination of XBJI with conventional treatment enhances the oxygenation index in AP patients, and the required number of cases has been reached (Figure 8).
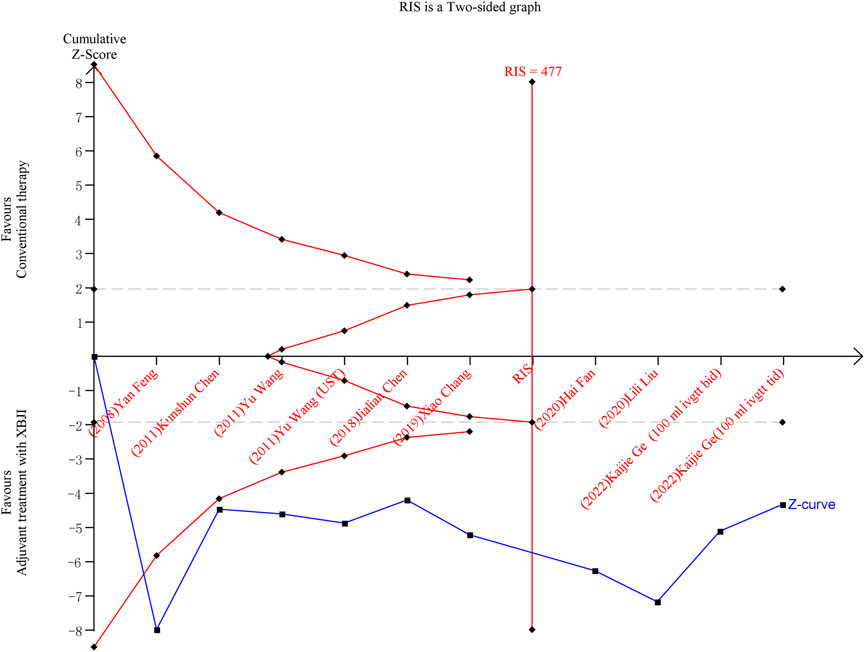
Figure 8. TSA result of the oxygenation index after conventional therapy and combination of XBJI in AP patients. The cumulative Z-score (depicted in blue), representing the accumulated level of statistical significance, was calculated after each new trial was included. The grey lines denote traditional boundaries. The oblique red dashed lines symbolize the trial sequential monitoring boundaries and the futility boundaries. The vertical red dashed line signifies the estimated RIS.
In recent decades, researches on AP have primarily concentrated on trypsinogen activation, malfunctions in pancreatic microcirculation, calcium overload, and inflammatory pathways (Mihoc et al., 2024). However, the edema of peripancreatic tissue, additional fluid resuscitation further increase IAP, sever AP patients developing ALI and ARDS leading to up to 60% of the mortality occurring in the first week of the disease (Jacobs et al., 1977; Sah et al., 2013). Due to the available treatment of AP are limited, leading researchers focus on complementary and alternative therapies. The traditional Chinese medicine always has demonstrated its superiority in the management of AP and other inflammatory diseases for centuries in China (Li et al., 2017).
XBJI has been employed as an adjuvant therapy in the treatment of sepsis, severe pneumonia, and AP. XBJI appears to be a multifaceted mechanism encompassing anti-inflammatory effects, antioxidant properties, and immune modulation. It exhibits the capability to suppress the excessive secretion of inflammatory mediators, thereby mitigating pulmonary inflammatory responses (Zhang et al., 2022). Simultaneously, its antioxidant constituents effectively scavenge free radicals, alleviating the detrimental impact of oxidative stress on lung tissue. Furthermore, by modulating the function of immune cells, XBJI may also facilitate the repair and regeneration of lung tissue, further enhancing its protective efficacy (Lv et al., 2022).
In previous meta-analyses, the application of XBJI has encompassed a broad range of diseases. For instance, Zhang et al. performed a meta-analysis demonstrating adjuvant therapy of increased the total effectiveness rate of AP patients shortened the abdominal pain, distension relief time and reduced serum amylase level (Zhu et al., 2022). Yun Zhang’s meta-analysis confirms that XBJI exhibits therapeutic effects on ARDS patients, partly through the regulation of immune cell and cytokine pathways. It inhibits TNF-α, while exerting an indirect suppressive effect on IL-6 (Zhang et al., 2021). The meta-analysis conducted by Chengyu Li showed XBJ adjuvant therapy could potentially improve sepsis outcomes by decreasing the 28-day mortality rate, APACHE II scores, and WBC count (Li et al., 2018). These findings reinforce our results, underscoring the potential benefits of XBJI across various conditions. It has shown unique advantages in targeting complex conditions and saving the lives of patients. Collectively, XBJI could play an important and expanding role in clinical medicine, especially for AP, severe pneumonia and sepsis. However, the impact of XBJI on improving pulmonary ventilation, specifically in the AP, has not been inadequately evaluated. This meta-analysis complements the deficiencies of previous research, providing evidence-based medical support for the widespread clinical application of XBJI.
The studies included in the meta-analysis remain inadequate. Firstly, the analysis lacks data on the primary causes of AP patients, potentially hindering an accurate assessment of the therapeutic efficacy of XBJI. Secondly, the number of studies included are limited, and the quality of these studies are uneven, from single-center studies with small sample sizes. AP Patients from diverse hospitals may display certain heterogeneity. Furthermore, the AP patients included in the studies were not classified based on the severity of their disease. Furthermore, the duration of treatment across studies were inconsistent, potentially introducing bias into the research outcomes. Chronic complications of AP, including pancreatic pseudocysts, chronic pancreatitis, and pancreatic cancer, unfortunately, were not adequately addressed with follow-up data in these studies.
XBJI was approved in the Chinese market for nearly 2 decades. The adverse drug reactions observed with XBJI included skin pruritus, erythema, and chest tightness. These reactions were often linked to the irrigation process, specifically when the syringe fluid was administered too rapidly. Most reactions were relatively mild or non-serious (Zheng et al., 2019). The overall incidence of allergic reactions tended to escalate with both increased dosage and patient age. Furthermore, when XBJI was combined with other drugs, such as Ringer’s sodium acetate solution, reduced glutathione, aspirin-DL-lysine, and torasemide, the risk of adverse reactions heightened (Wang et al., 2019). There remains a notable absence of international standards for assessing its safety and efficacy. Jie Yang et al. demonstrated target trial emulation principles, presenting replicable protocols for eligibility, intervention standardization, and outcome evaluation in observational studies. This may establish the framework for XBJI trials (Yang et al., 2025). Additionally, our findings should be interpreted with caution, as critical methodological details (e.g., randomization procedures, blinding) were unreported in the primary studies. To address the issue of unknown bias risk, it is necessary to provide detailed information on study design, sample selection, data collection, and analysis methods in traditional Chinese medicine studies. Consequently, more preclinical studies and multi-center prospective cohort studies, with significantly larger sample sizes need be undertaken, in order to validate and strengthen these findings.
In this meta-analysis, the results indicated that combination of conventional therapy and XBJI, when compared to conventional therapy, exhibits favorable effects in enhancing the oxygenation index, decreasing respiratory rate, and improving APACH II scores, inflammatory biomarkers such as IL-6 and TNF-α. Notably, a significant heterogeneity was observed in the oxygenation index (I2 = 89%). To address this issue, we carried out a subgroup analysis based on patient number, XBJI dosage and the duration of treatment. Unfortunately, despite these efforts, the heterogeneity among studies did not decrease significantly. Consequently, we resorted to utilizing a random effects model to pool the statistical measures. In summary, the combination of XBJI with conventional treatment demonstrates certain advantages in enhancing the oxygenation index, decreasing respiratory rate and intra-abdominal pressure, as well as improving certain inflammatory indicators.
YB: Conceptualization, Data curation, Writing–original draft, Writing–review and editing. RP: Data curation, Formal Analysis, Writing–review and editing. YqL: Data curation, Formal Analysis, Writing–review and editing. ZL: Methodology, Validation, Writing–review and editing. YnL: Funding acquisition, Investigation, Supervision, Validation, Writing–review and editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1549419/full#supplementary-material
XBJI, Xuebijing injection; AP, Acute pancreatitis; ALI, acute lung injury; ARDS, acute respiratory distress syndrome; SIRS, systemic inflammatory response syndrome; MODS, multiple organ dysfunction syndrome; CNKI, Chinese National Knowledge Infrastructure; PRISMA, Systematic Reviews and Meta-analysis; SinoMed, Chinese Biomedical Literature; UST, Ulinastatin; NR, No related; T, Treatment; SD, Standard error; M, Man; F, Female; C, Control; CT, Conventional therapy; NS, normal saline; TSA, Trial sequential analysis; RIS, Required Information Size.
Bhatia, M., and Moochhala, S. (2004). Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. Feb 202, 145–156. doi:10.1002/path.1491
Boxhoorn, L., Voermans, R. P., Bouwense, S. A., Bruno, M. J., Verdonk, R. C., Boermeester, M. A., et al. (2020). Acute pancreatitis. Lancet 396, 726–734. Sep 5. doi:10.1016/s0140-6736(20)31310-6
Chang Xiao, W. L., Chen, J., Lin, Bo, Xu, M., and Zhang, Z. (2019). Clinical study of Xuebijing injection in the treatment of severe pancreatitis complicated with acute respiratory distress syndrome. Bull. traditional Chin. Med. 18 (38-41). doi:10.14046/j.cnki.zyytb2002.2019.03.012
Chen, H., and Yang, M. (2011). Protective effect of Xuebijing on lung injury in rats with severe acute pancreatitis. Med. Inf. (next issue) 24, 194–195.
Chenjialian, C., Chen, K., Huang, huanshen, and Chen, J. (2018). Effect of Xuebijing Injection on oxidative stress and lung injury in patients with severe acute pancreatitis. Prog. Mod. general Surg. China.21. doi:10.3969/j.issn.1009-9905.2018.012.020
Chenkunshun (2011). Protective effect of Xuebijing on lung function in patients with severe acute pancreatitis. Massage rehabilitation Med. 2.
Dong, X., Wang, L., Zhao, C., Zhang, J., Pan, H., Yi, J., et al. (2020). Effect of Xuebijing Injection on lung injury and expression of TLR4, NF-κB and TNF-α in lung tissue of acute pancreatitis rats. Chin. Pat. Med. 42. doi:10.3969/j.issn.1001-1528.2020.11.039
Fanhai (2019). Clinical study of Xuebijing injection in the treatment of severe pancreatitis complicated with acute respiratory distress syndrome. Oriental medicinal diet, 78–79.
Feng Yan, W. J. Zhu Jun. 2008. Clinical observation of Xuebijing in the treatment of acute pancreatitis complicated with lung injury. Chin. J. Prim. Med. Pharm. 15, 1214–1215. doi:10.3760/cma.j.issn.1008-6706.2008.07.109
Ge Kaijie, W., and Wanghuaqiang, J. (2022). Protective effect and clinical efficacy of different doses of Xuebijing Injection on lung injury in patients with acute pancreatitis. Tianjin Tradit. Chin. Med. 39. doi:10.11656/j.issn.1672-1519.2022.09.08
Gong, Z. T., Yang, H. X., Zhu, B. B., Liu, H. H., and Siri, G. (2024). Clinical efficacy of Xuebijing injection for the treatment of sepsis: a retrospective cohort study. J. Integr. Med. 30, 645–651. doi:10.1016/j.joim.2024.08.006
Guo, H., Zheng, J., Huang, G., Xiang, Y., Lang, C., Li, B., et al. (2020). Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann. Palliat. Med. 9, 3235–3248. doi:10.21037/apm-20-1478
Jacobs, M. L., Daggett, W. M., Civette, J. M., Vasu, M. A., Lawson, D. W., Warshaw, A. L., et al. (1977). Acute pancreatitis: analysis of factors influencing survival. Ann. Surg. 185 (43-51), 43–51. doi:10.1097/00000658-197701000-00007
Kang, X., Liu, G., Xiaoguang, L., Fan, Z., Bai, L., Liu, L., et al. (2010). Expression of Toll-like receptor 4/NF-κB signal transduction pathway in pulmonary injury of severe acute pancreatitis and intervention of Xuebijing. Chin. J. Pancreat. Dis. 10. doi:10.3760/cma.j.issn.1674-1935.2010.06.019
Li, C., Wang, P., Li, M., Zheng, R., Chen, S., Liu, S., et al. (2021). The current evidence for the treatment of sepsis with Xuebijing injection: Bioactive constituents, findings of clinical studies and potential mechanisms. J. Ethnopharmacol. 30 (265), 113301. doi:10.1016/j.jep.2020.113301
Li, C., Wang, P., Zhang, L., Li, M., Lei, X., Liu, S., et al. (2018). Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: a meta-analysis of randomized controlled trials. J. Ethnopharmacol. 224, 512–521. doi:10.1016/j.jep.2018.05.043
Li, J., Zhang, S., Zhou, R., Zhang, J., and Li, Z. F. (2017). Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J. Gastroenterol. 23 (23), 3615–3623. doi:10.3748/wjg.v23.i20.3615
Liu, L. H., and Qu, M. (2020). Protective effect of Xuebijing Injection on lung injury in patients with severe acute pancreatitis. Prog. Mod. general Surg. China.23. doi:10.3969/j.issn.1009-9905.2020.01.016
Lv, J., Guo, X., Zhao, H., Zhou, G., and An, Y. (2022). Xuebijing administration Alleviates pulmonary endothelial inflammation and coagulation dysregulation in the early Phase of sepsis in rats. J. Clin. Med. 11, 6696. doi:10.3390/jcm11226696
Mihoc, T., Latcu, S. C., Secasan, C. C., Dema, V., Cumpanas, A. A., Selaru, M., et al. (2024). Pancreatic morphology, Immunology, and the pathogenesis of acute pancreatitis. Biomedicines 17, 2627. doi:10.3390/biomedicines12112627
Orgah, J. O., He, S., Wang, Y., Jiang, M., Wang, Y., Orgah, E. A., et al. (2020). Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 153, 104654. doi:10.1016/j.phrs.2020.104654
Parker, S., May, B., Zhang, C., Zhang, A. L., Lu, C., and Xue, C. C. (2016). A pharmacological review of bioactive constituents of Paeonia lactiflora Pallas and Paeonia veitchii Lynch. Phytother. Res. 30, 1445–1473. doi:10.1002/ptr.5653
Sah, R. P., Dawra, R. K., and Saluja, A. K. (2013). New insights into the pathogenesis of pancreatitis. Curr. Opin. Gastroenterol. 29, 523–530. doi:10.1097/MOG.0b013e328363e399
Schepers, N. J., Bakker, O. J., Besselink, M. G., Ahmed Ali, U., Bollen, T. L., Gooszen, H. G., et al. (2019). Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut 68, 1044–1051. doi:10.1136/gutjnl-2017-314657
Skouras, C., Hayes, A. J., Williams, L., Garden, O. J., Parks, R. W., and Mole, D. J. (2014). Early organ dysfunction affects long-term survival in acute pancreatitis patients. HPB Oxf. Sep. 16, 789–796. doi:10.1111/hpb.12259
Wang, C., Shi, Q. P., Ding, F., Jiang, X. D., Tang, W., Yu, M. L., et al. (2019). Reevaluation of the post-marketing safety of Xuebijing injection based on real-world and evidence-based evaluations. Biomed. Pharmacother. 109, 1523–1531. doi:10.1016/j.biopha.2018.10.190
Wang, J., Wang, L., Zhou, H., Liang, X. D., Zhang, M. T., Tang, Y. X., et al. (2022). The isolation, structural features and biological activities of polysaccharide from Ligusticum chuanxiong: a review. Carbohydr. Polym. 285, 118971. doi:10.1016/j.carbpol.2021.118971
Wangjue, Y.Liruhong (2011). Clinical study of ulinastatin combined with Xuebijing in the treatment of acute pancreatitis with lung injury. J. Kunming Med. Coll. 32. doi:10.3969/j.issn.1003-4706.2011.03.021
Wu, Q., Yin, C. H., Li, Y., Cai, J. Q., Yang, H. Y., Huang, Y. Y., et al. (2021). Detecting critical functional ingredients group and mechanism of xuebijing injection in treating sepsis. Front. Pharmacol. 12, 769190. doi:10.3389/fphar.2021.769190
Xd, M. E., Cao, Y. F., Che, Y. Y., Li, J., Shang, Z. P., Zhao, W. J., et al. (2019). Danshen: a phytochemical and pharmacological overview. Chin. J. Nat. Med. 17 (59-80), 59–80. doi:10.1016/s1875-5364(19)30010-x
Xiang, C., Liao, Y., Chen, Z., Xiao, B., Zhao, Z., Li, A., et al. (2022). Network pharmacology and molecular docking to elucidate the potential mechanism of Ligusticum chuanxiong against osteoarthritis. Front. Pharmacol. 13, 854215. doi:10.3389/fphar.2022.854215
Yang, J., Wang, L., Chen, L., Zhou, P., Yang, S., Shen, H., et al. (2025). A comprehensive step-by-step approach for the implementation of target trial emulation: Evaluating fluid resuscitation strategies in post-laparoscopic septic shock as an example. Laparosc. Endosc. Robotic Surg. 8, 28–44. doi:10.1016/j.lers.2025.01.001
Zhang, M., Zheng, R., Liu, W. J., Hou, J. L., Yang, Y. L., and Shang, H. C. (2023). Xuebijing injection, a Chinese patent medicine, against severe pneumonia: current research progress and future perspectives. J. Integr. Med. 21, 413–422. doi:10.1016/j.joim.2023.08.004
Zhang, Y., Wang, J., Liu, Y. M., Yang, H., Wu, G. J., and He, X. H. (2021). Analysis of the efficacy and mechanism of action of xuebijing injection on ARDS using meta-analysis and Network pharmacology. Biomed. Res. Int. 2021 (8824059), 8824059. doi:10.1155/2021/8824059
Zheng, R., Wang, H., Liu, Z., Wang, X., Li, J., Lei, X., et al. (2019). A real-world study on adverse drug reactions to Xuebijing injection: hospital intensive monitoring based on 93 hospitals (31,913 cases). Ann. Transl. Med. 7 (117), 117. doi:10.21037/atm.2018.09.26
Zhou, W., Fan, M., Li, X., Yu, F., Zhou, E., and Han, X. (2024). Molecular mechanism of Xuebijing in treating pyogenic liver abscess complicated with sepsis. World J. Emerg. Med. 15 (35-40), 35–40. doi:10.5847/wjem.j.1920-8642.2024.016
Keywords: Xuebijing injection, acute pancreatitis, acute respiratory distress syndrome, respiratory failure, meta-analysis
Citation: Bin Y, Peng R, Lee Y, Lee Z and Liu Y (2025) Efficacy of Xuebijing injection on pulmonary ventilation improvement in acute pancreatitis: a systematic review and meta-analysis. Front. Pharmacol. 16:1549419. doi: 10.3389/fphar.2025.1549419
Received: 21 December 2024; Accepted: 14 March 2025;
Published: 16 April 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaCopyright © 2025 Bin, Peng, Lee, Lee and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Liu, dHlwZTk5OUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.