
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 13 March 2025
Sec. Neuropharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1547073
 Milly N. Kanobe1*
Milly N. Kanobe1* Christie Y. Powell1
Christie Y. Powell1 Makena Patrudu1
Makena Patrudu1 Sarah A. Baxter1
Sarah A. Baxter1 Melissa A. Tapia1
Melissa A. Tapia1 John Darnell1
John Darnell1 Kristen Prevette1
Kristen Prevette1 Alison G. Gibson1
Alison G. Gibson1 Sarah A. Ayoku1
Sarah A. Ayoku1 Leanne Campbell1
Leanne Campbell1 Jeffrey W. Coffield1
Jeffrey W. Coffield1 Brian M. Keyser1
Brian M. Keyser1 Bhagya Sukka Ganesh1
Bhagya Sukka Ganesh1 Nathan Gale2
Nathan Gale2 Kristen G. Jordan1
Kristen G. Jordan1Introduction: Oral nicotine pouches (ONPs) are a newer category of smokeless tobacco products containing pharmaceutical-grade nicotine but no tobacco leaf. These products have the potential to help smokers transition away from cigarettes. To assess their potential role as alternatives to cigarettes, we evaluated the abuse liability (AL) of Velo ONPs with varying nicotine content (4–12 mg per pouch), pouch size (600 mg or 400 mg) and flavor (six varieties) in comparison to high (cigarettes) and low (nicotine replacement therapy [NRT] gum) AL comparators.
Methods: Independent randomized crossover clinical studies were conducted to assess AL, including subjective effects (product liking [PL], urge to smoke, product effects, overall PL, and overall intent to use again) and nicotine pharmacokinetic (PK) parameters of Velo ONPs. Participants used test products under controlled conditions, and subjective effect measures were collected using validated questionnaires. Nicotine PK parameters, including peak nicotine concentration (Cmax), time to maximum concentration (Tmax), were assessed.
Results: Mean PL scores for all Velo ONPs (p < 0.0042) and Velo Mini Pouches (p < 0.0031) were significantly lower than cigarettes, regardless of nicotine level, pouch size, or flavor, but similar to NRT gum. Other subjective measures for Velo ONPs were less favorable than cigarettes and comparable to or lower than NRT gum. Nicotine uptake with Velo ONPs was slower (reflected by a longer Tmax) and had lower Cmax than cigarettes but was comparable or slightly lower than NRT gum. Overall nicotine uptake increased with increasing nicotine content and was comparable to that of cigarettes for Velo ONPs with higher nicotine levels. Flavor had no effect on nicotine uptake of Velo ONPs.
Discussion: Velo ONPs demonstrated an AL profile lower than cigarettes and similar to NRT gum, suggesting a reduced potential for abuse compared to cigarettes. The slower nicotine uptake and lower peak nicotine levels further support their potential as a lower-risk alternative. These findings highlight the potential role of ONPs in tobacco harm reduction strategies by providing an alternative nicotine source with a lower AL than combustible cigarettes.
Systematic Review Registration: The clinical studies were registered at ClinicalTrials.gov; NCT05129657, NCT05294497, and NCT05081154.
The current tobacco product marketplace is highly diverse and has been evolving over the past decade with the emergence of novel tobacco and nicotine products including electronic cigarettes, heated tobacco products, and oral nicotine pouches (ONPs) (University of Bath, 2023). A continuum of risk has been recognized among tobacco and nicotine products that deems combustible products (e.g., cigarettes) as having the highest health risk and medicinal nicotine products as conveying the lowest risk (Gottlieb and Zeller, 2017; King et al., 2023). Smokeless tobacco products (STPs) are associated with significantly lower risks to health than cigarettes and are closer to medicinal nicotine products along the risk continuum (Abrams et al., 2018; Institute of Medicine, 2001). In particular, use of oral STPs, such as moist snuff and snus, has been associated with lower disease risk relative to cigarette smoking (Henley et al., 2007; Henley et al., 2005; Luo et al., 2007), primarily because oral STP users have a significantly reduced exposure (Clarke et al., 2019; Grandolfo et al., 2024) to the numerous harmful and potentially harmful constituents (HPHCs) (Food and Drug Admini, 2012) formed during tobacco combustion, which the smoker inhales, and have been identified as causative agents of several serious diseases (Centers for Disease Control and Prevention, 2023).
Tobacco harm reduction (THR) is a public health risk mitigation strategy aimed at reducing the health burden associated with cigarette smoking (Hatsukami and Carroll, 2020). The fundamental principle of THR is to encourage smokers who are unable or unwilling to stop smoking to switch to use of tobacco and/or nicotine-containing products that deliver nicotine but with reduced exposure to HPHCs and other toxicants, and have a positive impact on both individual and population-level health (Institute of Medicine, 2001). In the United States, the Food and Drug Administration (FDA) has indicated they “will focus on a regulatory framework that focuses on and supports innovation to promote harm reduction” (Gottlieb and Zeller, 2017). The FDA has authorized that some snus and moist snuff products can be marketed as “modified risk tobacco products” (Food and Drug Administration, 2019; Food and Drug Administration, 2023), based partly on the reduced exposure profile of these products relative to cigarettes. While these oral STPs do not generate or elicit exposure to combustion-related toxicants (i.e., the main drivers of smoking-related diseases), they do contain residual levels of some HPHCs, such as tobacco-specific nitrosamines (Back et al., 2023; Borgerding et al., 2012).
Oral nicotine pouches (ONPs) are an emerging category of nicotine products that contain pharmaceutical-grade nicotine, but no tobacco leaf (Grandolfo et al., 2024; Jackson et al., 2023; Shaikh et al., 2023). They are typically portioned powder mixtures comprised of nicotine, flavorings, and other ingredients contained in a porous material termed “fleece”. During use, the pouches are placed between the upper lip and gum, where nicotine is absorbed into the bloodstream through the buccal mucosa. Although ONPs are a relatively recent product innovation, growing evidence indicates that they may be a reduced exposure product that conveys lower health risks compared to both cigarettes and oral STPs, such as moist snuff and snus. For example, levels of some HPHCs are significantly lower in ONPs than in snus (Back et al., 2023; Azzopardi et al., 2022a; Jablonski et al., 2022). In addition, a clinical study found lower levels of biomarkers of exposure and improved levels of biomarkers of potential harm among ONP users compared with cigarette smokers (Azzopardi et al., 2023). Furthermore, biomarkers of both exposure and potential harm among ONP users were at levels similar to former and never smokers (Azzopardi et al., 2023).
Combustible cigarettes exhibit a high degree of abuse liability (AL), a term synonymous with dependence potential (Carter et al., 2009a; Vansickel et al., 2021; Maloney et al., 2019). This high AL contributes both to increased physical and behavioral dependence on cigarettes and difficulty in stopping smoking. Given this, the AL of tobacco products is a critical attribute in determining the likelihood of both continued use and use frequency, factors that impact the degree of exposure to the combustion- and tobacco-derived toxicants that cause adverse health effects and increase harm (Carter et al., 2009b). In terms of THR and the ability of novel tobacco and nicotine products to reduce harm among smokers, possessing at least some degree of AL is important, as the new product should be a suitable substitute for cigarettes or support switching away from smoking (Abrams et al., 2018; Ashley et al., 2020; Cahn et al., 2021; Fearon et al., 2022). Conversely, high AL or a high dependence potential might lead to the novel product posing an initiation or addiction risk to non-users of nicotine products, particularly among susceptible populations such as youth and young adults (Ashley et al., 2020; Cahn et al., 2021). Thus, assessment of AL is an important component of determining whether the introduction of a novel tobacco product into a market is appropriate for the protection of public health in terms of its impact on the population as a whole (Congress, 2009).
The AL of a novel nicotine-containing tobacco product is a composite measure based predominantly on the pharmacokinetic (PK) and subjective effects associated with the use of that product (Fearon et al., 2022; Henningfield and Keenan, 1993; Vansickel et al., 2022). Despite the growing popularity of ONPs (Majmundar A et al., 2022), only a limited number of studies have evaluated these factors to assess their AL. In three separate clinical studies, we evaluated nicotine uptake and subjective effects of a range of Velo ONPs varying in nicotine content, pouch size, and flavor in order to provide an overall assessment of the AL of these products and to determine whether they may contribute both to THR and to improving overall population-level health.
In three separate randomized, open-label, crossover, in-clinic confinement studies, subjective effects and nicotine uptake of Velo ONPs were evaluated among adult smokers. The studies were registered at Clinicaltrials.gov (NCT05129657, NCT05294497, NCT05081154) and conducted between October 2021 and June 2022 in the United States (US). They were approved by the Advarra Institutional Review Board (Columbia, MD, United States) and were conducted in accordance with the Declaration of Helsinki and applicable sections of the US Code of Federal Regulations and ICH E6 Good Clinical Practice. All participants provided written informed consent to participate.
The study populations consisted of adult smokers of filtered, non-menthol or menthol cigarettes who self-reported daily smoking of at least 10 cigarettes for at least 6 months. Potential participants were identified from a database of healthy volunteers held at the study site, and/or through advertisements on radio/social media directed at the target population. The main inclusion criteria were age 21–60 years, general good health, and a response of less than 30 min for time to first cigarette use in a day on the Fagerström test for nicotine dependence (FTND) (Fagerström and Eissenberg, 2012; Heatherton et al., 1991). The main exclusion criteria were pregnancy and breastfeeding. Cigarette smoking was confirmed at screening by measuring exhaled carbon monoxide (ECO) and urine cotinine. Eligibility criteria required an ECO between ≥10 ppm and ≤100 ppm, along with a positive urine cotinine test using a dipstick at a 200 ng/mL cutoff concentration. Smokers who also used smokeless tobacco products (e.g., snus, moist snuff) were eligible to participate in the study. Attempts were made to recruit at least 15%–20% African American participants as representative of the US population of smokers (Jamal et al., 2016).
The study products were Velo ONPs (American Snuff Company, LLC, Winston-Salem, NC, United States): portioned oral nicotine products manufactured using tobacco-derived, pharmaceutical-grade nicotine, flavorings, and other ingredients specific to the flavor concentrate of the product. In Study 1 (Velo Pouch AL), three Velo Pouches [600 mg each] in one flavor (Cool Mint), with different nicotine levels (4, 8, and 12 mg nicotine) were evaluated; in Study 2 (Velo Mini Pouch AL), four Velo Mini Pouches [400 mg each] in two flavors (Cool Mint and Modern Traditions) and two nicotine levels (4 and 8 mg nicotine) were evaluated; and in Study 3 (Velo Pouch PK), seven Velo Pouches [600 mg each] in six flavors (Cool Mint, Modern Traditions, Berry Frost, Cinnamon, Wintergreen, and Smooth) and two nicotine levels (8 and 10 mg nicotine) were evaluated (Table 1). In Studies 1 and 2, the participant’s usual brand (UB) combustible cigarette and a commercially available NRT gum (Nicorette White Ice Mint polacrilex gum, 4 mg nicotine; Glaxo SmithKline, Durham, NC, United States) were included as high- and low-AL comparator products, respectively.
The three in-clinic confinement studies lasted 6–8 days and followed similar protocols (Figure 1). On Day 1, participants who met all inclusion criteria and none of the exclusion criteria were enrolled, randomized to a product use sequence (assigned by the contract research organization (CRO) statistician) based on a Williams Design, and confined at the study site. Participants in Studies 1 and 2 brought with them a sufficient supply of their UB cigarettes to last through the confinement period. On Day 1, participants took part in a half-day pre-test-session to familiarize themselves with using the Velo ONPs and NRT gum (AL studies only). During the pre-test-session, they also had access to their UB cigarettes for ad libitum use. After this session, participants abstained from the use of all tobacco- and nicotine-containing products for a minimum of 12 h prior to each test session, and from any caffeine-containing products for 4 h, prior to and through the end of each test session.
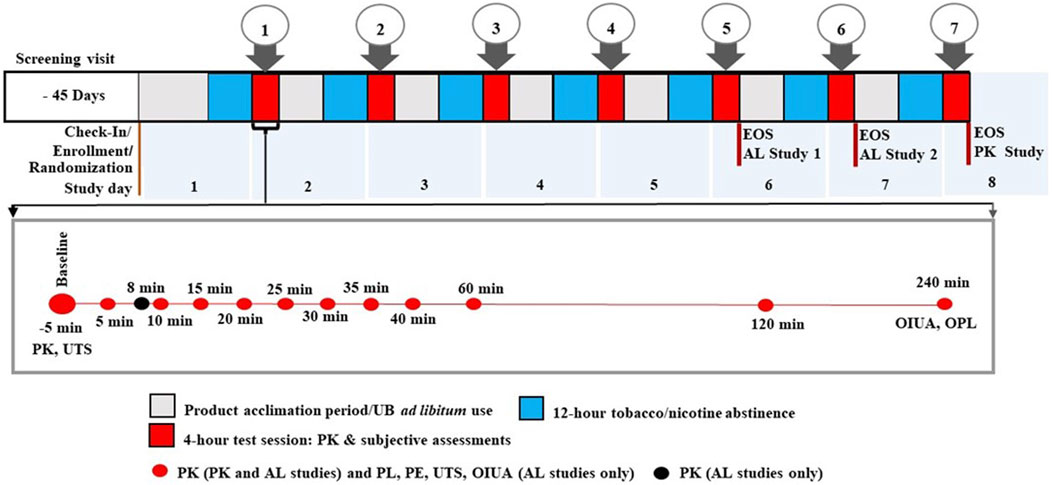
Figure 1. Summary of overall study design for Velo ONP studies. The Figure includes detailed overview of the timing of nicotine pharmacokinetics (PK) blood draws and completion of subjective effects questionnaires within each 4-h test session. Pharmacokinetics measurements were taken at the indicated timepoints in all three studies, except that Study 3 did not include an 8-min timepoint; product liking (PL), product effects (PE), and urge to smoke (UTS), and overall intent to use again (OIUA) were only administered in Studies 1 and 2; UTS was the only subjective measure administered at baseline in Studies 1 and 2 only; Overall product liking (OPL) was the only subjective measure administered in the Study 3. Both OPL and OIUA were administered at only the 240-min time point. Abbreviations: AL, abuse liability; EOS, end of study; min, minute; UB, usual brand; Velo ONP, Velo oral nicotine pouches.
Starting on the morning of Day 2, product use sessions were carried out daily based on the randomization sequence. After baseline measurements, participants in all studies used their designated Velo ONPs for approximately 30 min (additionally, those in the AL studies, either used one piece of NRT gum [per package labeling] for approximately 30 min or smoked one UB cigarette for up to 5 min). During the 240-min test session, blood samples for plasma nicotine PK parameters were collected and subjective effects questionnaires were completed. Following completion of each day’s test session, the participants were instructed to continue with product familiarization using their assigned product for the following day’s test session. Participants were also permitted to smoke their UB cigarettes during this period until the tobacco- and nicotine-containing product abstinence period (at least 12 h prior to each test session). The study procedures and timelines, including the points of nicotine uptake and subjective effects assessments, are depicted in Figure 1.
In Studies 1 and 2, AL was assessed as per established methods (Carter et al., 2009b; Fearon et al., 2022; Henningfield and Keenan, 1993; Vansickel et al., 2022) and regulatory guidance (Food and Drug Administration, 2021), and included measurements of nicotine pharmacokinetic and five subjective effects measures. Study 3 included measurements of nicotine pharmacokinetics and one subjective effects measure.
Nicotine pharmacokinetic parameters in all studies were determined as described previously (Campbell et al., 2023; Campbell et al., 2022; Stiles et al., 2017; Stiles et al., 2018). Blood samples were collected during product use sessions at specific timepoints from 5 min before (baseline) to 240 min after the start of product use (see Figure 1). Individual nicotine concentrations were baseline-adjusted using a model assuming that nicotine elimination follows first-order kinetics (Benowitz et al., 2006; Shiffman et al., 2009). The endpoints were maximum baseline-adjusted plasma nicotine concentration (Cmax), area under the curve (AUC) for 0–15 min (AUC0-15), AUC for 0–240 min (AUC0-240), and time to reach Cmax (Tmax). Given the consistent presence of extreme Tmax values across multiple studies, we have adopted the standard practice of using nonparametric methods, such as reporting medians for Tmax, to provide a more robust and accurate representation of the data.
The AL studies evaluated five subjective effects measures through responses to questionnaires administered at various timepoints during the test sessions; the urge to smoke (UTS) questionnaire was also administered 5 min before product use (see Figure 1). Each questionnaire asked a single question with responses on a 10-point numerical rating scale (0, “Not at all”; 10, “Very much”) (Campbell et al., 2022; Stiles et al., 2017; Stiles et al., 2018). The subjective measurements included product liking (PL; “At this moment, how much do you like the product?“); UTS (“How strong is your current urge to smoke your usual brand cigarette?“); positive product effects (PE; “Rate the degree to which you feel positive effects of the product at this moment”) and negative PE (“Rate the degree to which you feel negative effects of the product at this moment”). Additionally, overall intent to use again (OIUA; “Rate the degree to which you would like to use the product”) and overall product liking (OPL; “Overall, how much did you like the product?“) were assessed at the 240-min timepoint.
The primary endpoints for the AL studies were (1) area-under-the-effect curve (AUEC) for PL for 5–240 min (AUECPL5-240) and (2) the maximum PL effect (Emax PL) after the start of product use. Secondary endpoints included AUEC for positive PE for 5–240 min (AUECPEpos 5–240), maximum positive PE (Emax PEpos), AUEC for negative PE for 5–240 min (AUECPEneg 5–240), maximum negative PE (Emax PEneg), minimum UTS (Emin UTS), AUEC for UTS for 0–15 min (AUECUTS 0–15), AUEC for UTS for 0–240 min (AUECUTS 0–240), and effect of OPL (Eoverall PL) at 240 min. The only subjective assessment in the PK study was Eoverall PL (see Figure 1).
Participant safety was monitored throughout the study by assessments of adverse events (AEs), clinical laboratory tests, and vital signs measurements, as well as by physical or oral examinations. Symptom-driven physical examinations were performed as needed at the discretion of the Principal Investigator. An AE was coded by primary system organ class and preferred term according to the Medical Dictionary for Regulatory Activities version 24.1 (MedDRA Maintenance and Support Services Organization, 2021). The severity of an AE was categorized as mild, moderate, or severe; in addition, the relationship of the AE to study product use (not related, unlikely related, possibly related, or related) as determined by the Principal Investigator, was recorded.
For Studies 1 and 2, sample sizes were determined to achieve 90% power to detect the hypothesized difference of 2.2 (σ = 2.07) in the maximum PL score between the Velo ONP and UB cigarette, using a two-sided test for differences with Bonferroni-adjusted α levels of 0.0042 (Study 1) and 0.0031 (Study 2). This hypothesized difference, the smallest detectable among all the primary endpoint comparisons, served as the basis for sample size calculation. As a result, a minimum of 40 and 36 participants were required for Studies 1 and 2, respectively. To account for attrition and maintain a balanced Williams design, 50 participants were randomized for Study 1 and 43 for Study 2.
For Study 3, sample size determination was based on the goal of achieving a 95% confidence interval with a half-width that is within 20% of the primary endpoint mean values. The target number of randomized participants for this study was a minimum of 42 subjects, allowing for approximately a 33% dropout rate, with a goal of having at least 28 participants completing the study.
All participants who completed at least one post-baseline/study product use session were included in the statistical analysis of subjective effects unless their subjective effects profiles were deemed unevaluable. All randomized participants who used at least one study product were included in the nicotine pharmacokinetic parameter analyses. Each individual pharmacokinetic profile was examined for completeness and only data from participants with an evaluable pharmacokinetic profile were included in the analysis of nicotine uptake parameters. The safety population comprised all enrolled participants.
For analysis of the primary subjective effects assessments, AUECPL 5–240 and Emax PL for each Velo ONP were compared to those for both the high- and low-AL comparators (UB cigarette and NRT gum, respectively) by using a mixed effects model analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons using an alpha level of 0.0042 in Study 1 (12 comparisons) and 0.0031 in Study 2 (16 comparisons). The mixed effects ANOVA model was specified as follows:
where,
Positive PE (AUECPEpos 5–240 and Emax PEpos), negative PE (AUECPEneg 5–240 and Emax PEneg), OPL (Eoverall PL), and OIUA (Eoverall IUA) endpoints for each Velo ONP were compared to those for both the high- and low-AL comparators by an ANOVA as specified in the model above. Scores for UTS measures (AUECUTS 0–15 and AUECUTS 0–240), in addition to the minimum effect of UTS (Emin UTS), for each Velo ONP were compared to those for the high- and low-AL comparators using a mixed-effects analysis of covariance (ANCOVA). For all secondary endpoints, an alpha level of ≤0.05 was considered significant. The mixed effects ANCOVA model was specified as follows:
where,
A mixed-effects model ANOVA was used to compare nicotine PK parameters (AUC0-15, AUC0-240, and Cmax) for each Velo ONP to those for the high- and low-AL comparators, while a Wilcoxon nonparametric signed rank test was used for comparisons of Tmax for each Velo ONP and the high- and low-AL comparators. Data for AUC0-15, AUC0-240, and Cmax were analyzed on the natural log scale; Tmax was analyzed on the original scale. An alpha level of 0.05 was used for all PK parameters assessed. No inferential statistical comparisons were performed between any of the Velo ONPs. The mixed effects model for the analysis of PK parameters (AUC and Cmax) was specified as follows:
where,
An example of the code used for statistical analysis of Tmax is given below:
PROC NPAR1WAY WILCOXON;
CLASS PRODUCT;
VAR Tmax;
Run;
In total, 41 participants were enrolled in Study 1, 43 in Study 2, and 36 in Study 3. Of these, 40 completed Studies 1 and 2 each, and 35 completed Study 3 (Supplementary Figure S1). One participant in Study 1 was withdrawn due to an AE. Three participants in Study 2 were withdrawn: one due to an AE, one due to failing to meet continuation criteria, and one due to undisclosed reasons. One participant in Study 3 was withdrawn due to an AE.
Overall, the study populations were predominantly white (Study 1, 78.0%; Study 2, 65.1%; and Study 3, 83.3%) and male (63.4%, 76.7%, and 69.4%, respectively) (Table 2). Across the three studies, participants were long-term cigarette smokers who had smoked on average 15.8–18.0 cigarettes per day for an average range of 20.9–23.9 years. Those who also used STPs accounted for 22.0%, 44.2%, and 36.1% of participants in Studies 1, 2 and 3, respectively. The overall level of cigarette dependence at baseline was moderate, based on FTND scores (mean total scores ranged from 5.9 to 6.3).
In Studies 1 and 2, we evaluated the AL of Velo Pouches and Velo Mini Pouches, respectively, differing in nicotine level and flavor by measuring nicotine pharmacokinetics and subjective effects during product use.
Mean baseline-adjusted plasma nicotine concentrations over time following use of the study products are presented in Figure 2. The nicotine PK profiles were similar across the Velo ONPs in both Study 1 (Figure 2A) and Study 2 (Figure 2B), with plasma nicotine levels increasing shortly after the start of product use, peaking at ∼35 min, and decreasing gradually thereafter. By contrast, use of UB cigarette led to a sharp rise in plasma nicotine, which peaked at ∼5 min and then declined more rapidly. The PK profile of the NRT gum was similar to the Velo ONPs.
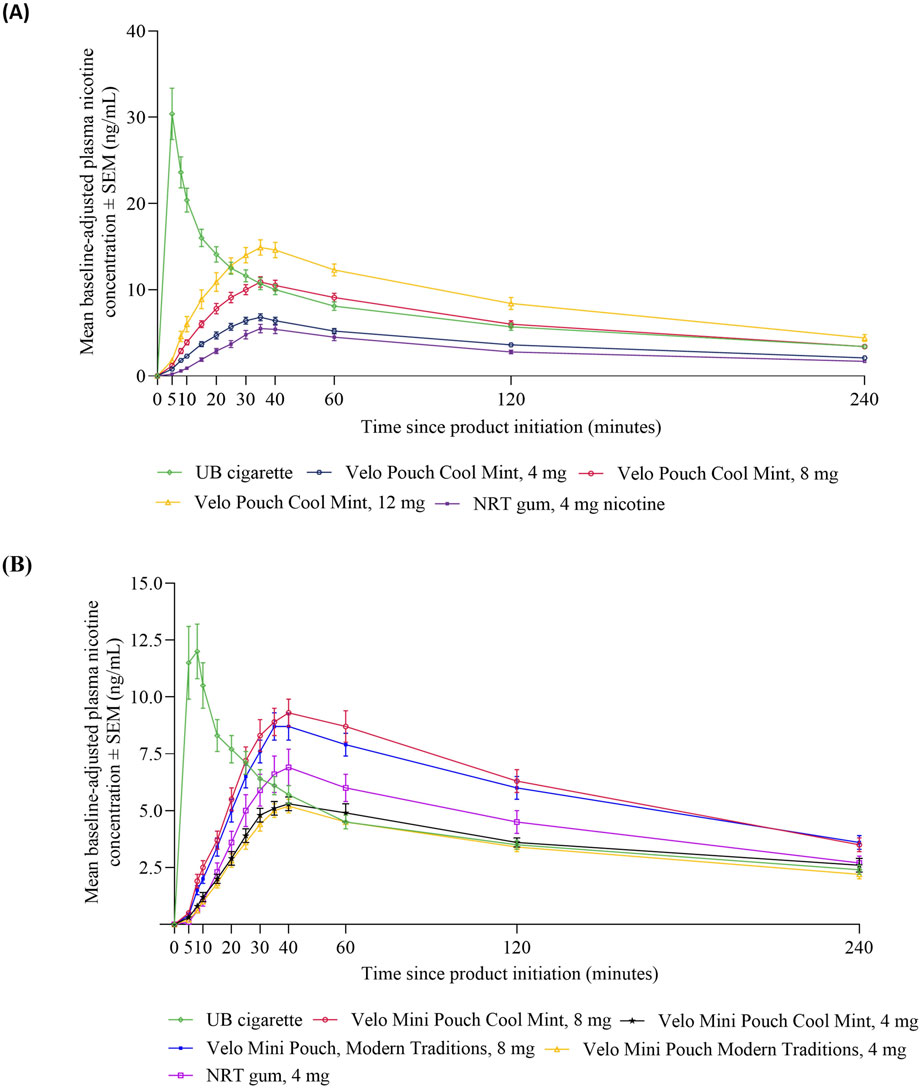
Figure 2. Plasma nicotine concentrations over time for Velo ONPs evaluated in Studies 1 and 2. Each point shows the mean ± SEM plasma nicotine concentration in Study 1 (A) and Study 2 (B). Abbreviations: AL, abuse liability; mg, milligram (of nicotine); ng/mL, nanograms per milliliter; NRT, nicotine replacement therapy; SEM, standard error of the mean; UB, usual brand; Velo ONP, Velo oral nicotine pouches.
The statistical comparisons and test statistics for the PK parameters of Velo ONPs and comparator products are presented in Table 3 and Supplementary Table S1, respectively. For the Velo Pouches in Study 1, nicotine uptake parameters (AUC0-15, AUC0-240, and Cmax) increased with increasing product nicotine content, were significantly lower than those of UB cigarette, and were each significantly higher than those of NRT gum (Table 3). The only exception was the Velo Pouch with the highest nicotine content (12 mg), which had an overall nicotine uptake (AUC0-240) that was not significantly different from UB cigarette. Median Tmax was significantly longer for Velo Pouches than for UB cigarette but did not differ between any of the Velo Pouches and NRT gum (Table 3; Supplementary Table S1), consistent with the use periods of these product types.
In line with results from Study 1, nicotine uptake parameters (AUC0-15, AUC0-240, and Cmax) were higher in Study 2 for the two Velo Mini Pouches with higher nicotine content (8 mg) than Velo Mini Pouch products with lower nicotine content (4 mg) (Table 3). Early nicotine uptake, within 15 min of product use (AUC0-15), was significantly lower for each Velo Mini Pouch than for UB cigarette. Nicotine uptake over 4 h (AUC0-240) was significantly higher for both 8 mg Velo Mini Pouch (Cool Mint and Modern Traditions) products, but significantly lower for the 4 mg Velo Mini Pouch (Modern Traditions) product, when compared to UB cigarette. No significant difference was observed in AUC0-240 between the Velo Mini Pouch Cool Mint 4, mg and UB cigarette. The Cmax values for both Velo Mini Pouches in 8 mg were similar regardless of flavor and did not differ significantly from UB cigarette. However, Cmax for the Velo Mini Pouches in 4 mg was significantly lower than that for UB cigarette (Table 3; Supplementary Table S1).
Relative to NRT gum, AUC0-15, AUC0-240, and Cmax were not different for the Velo Mini Pouch 4 mg products but were significantly higher for the Velo Mini Pouch 8 mg products. As in Study 1, median Tmax was significantly longer for all Velo Mini Pouches than for UB cigarette but did not differ between any of the Velo Mini Pouches and NRT gum (Table 3; Supplementary Table S1).
The participants in the two AL studies self-assessed their experience of using the Velo ONPs by five subjective effects questionnaires. In general, UB cigarettes were rated most favorably among the study products, as described below. Data on statistical comparisons of subjective effects parameters are summarized in Tables 4, 5, with t-statistics and p-values provided in Supplementary Table S2.
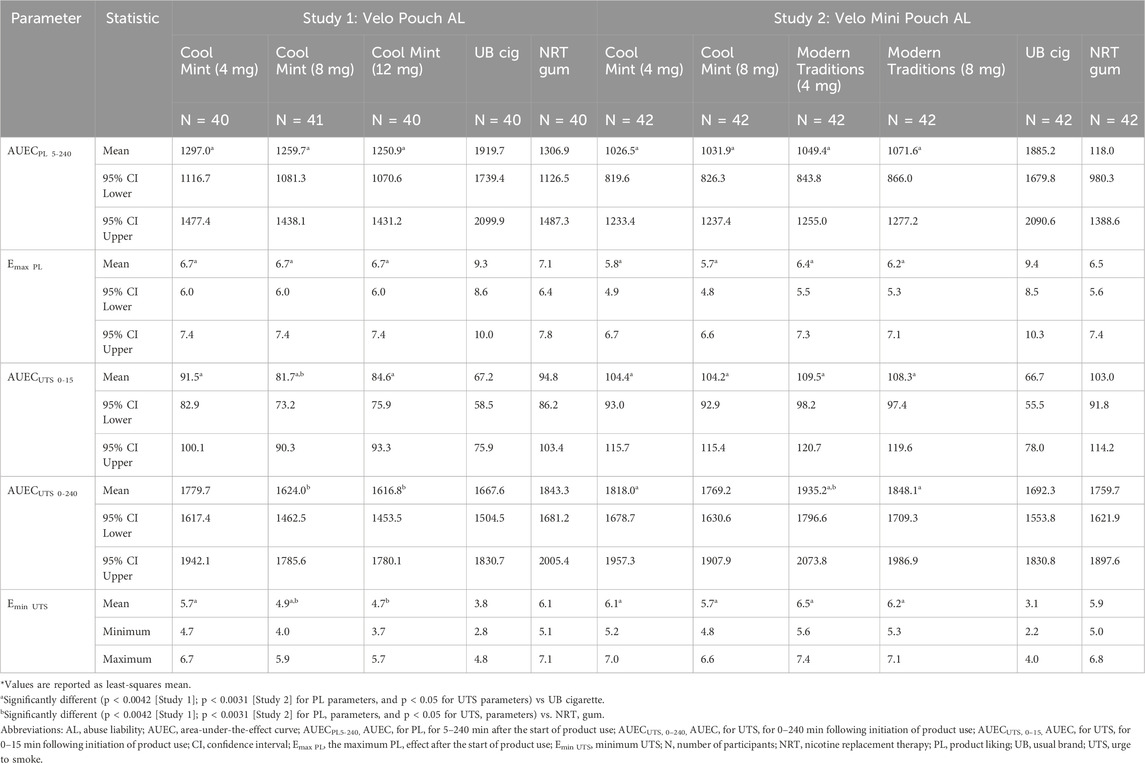
Table 4. Summary of product liking and urge to smoke subjective effects measures of Velo ONPsa evaluated in Studies 1 and 2.
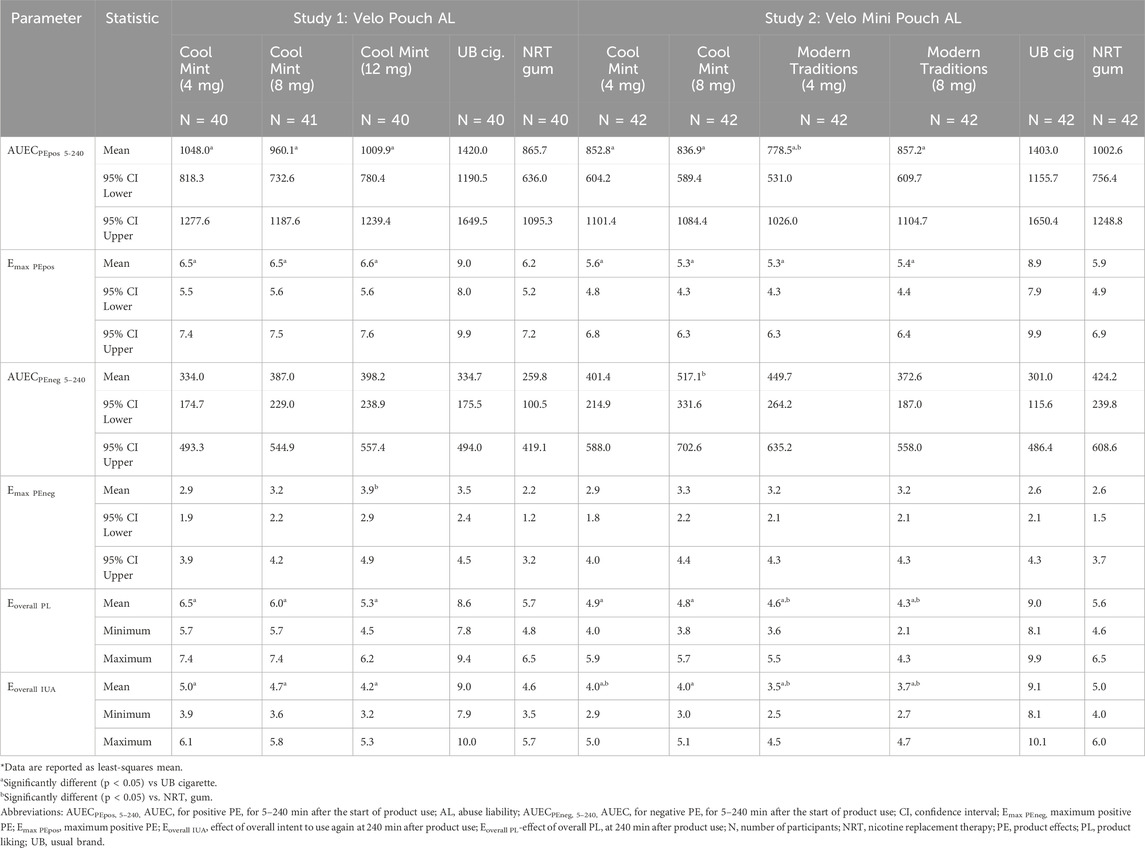
Table 5. Summary of subjective measures of product effects, overall product liking, and overall intent to use again of Velo ONPs* evaluated in Studies 1 and 2.
Among all study products, UB cigarettes were rated highest for the two PL parameters, AUECPL 5–240 and Emax PL (Table 4). Values for these parameters were significantly lower for all Velo ONPs than for UB cigarette in both AL studies but did not differ between the Velo ONPs and NRT gum (Table 4; Supplementary Table S2).
Evaluation of UTS in both AL studies indicated that UB cigarette reduced smoking urges within the first 15 min of product use (AUECUTS 0–15) to a significantly greater extent than the Velo ONPs irrespective of nicotine content, pouch size or flavor (Table 4; Figures 3A, B). In Study 1, AUECUTS 0–240 and Emin UTS for the Velo Pouches with the highest nicotine content (12 mg), were not significantly different to those for UB cigarette, while the Velo Pouches in 4 mg reduced UTS (AUECUTS 0–15, AUECUTS 0–240, and Emin UTS) to a similar extent as NRT gum (Table 4; Supplementary Table S2). In general, the higher the level of nicotine in the Velo Pouch, the lower the UTS score compared with NRT gum.
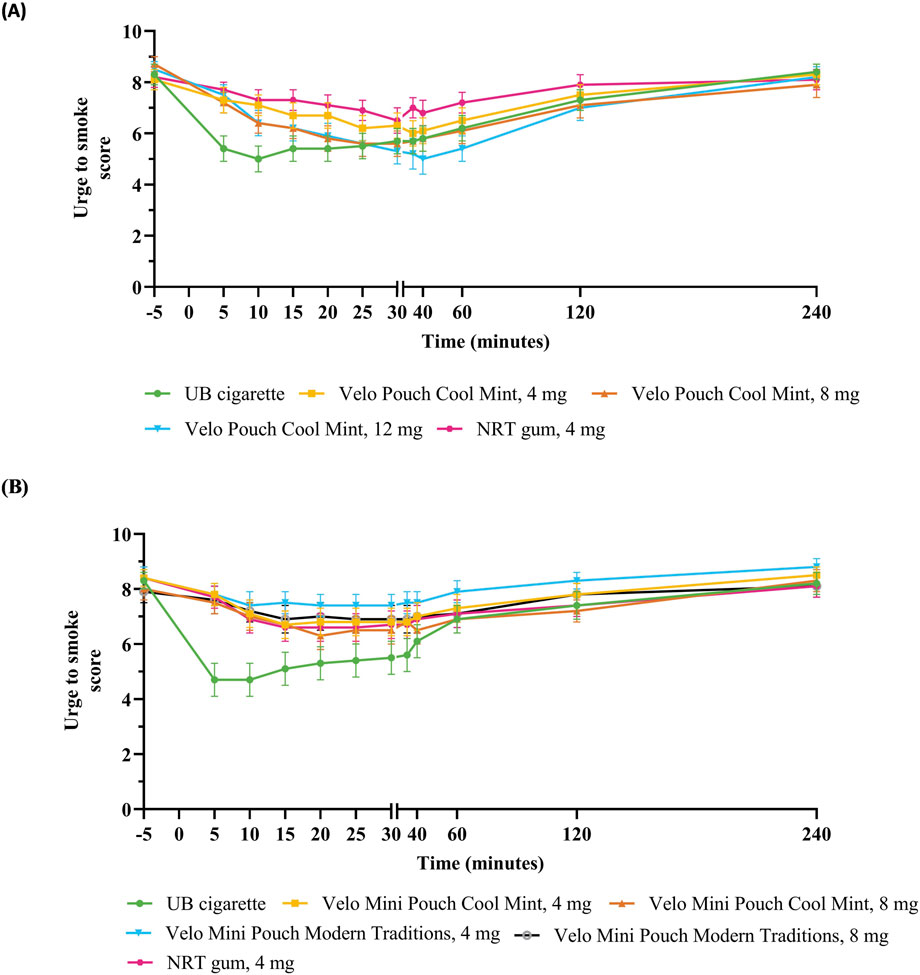
Figure 3. Urge to smoke scores over 240 min after initiation of product use. Each point shows the mean ± SEM in Study 1 (A) and Study 2 (B). Abbreviations: AL, abuse liability; mg, milligram (of nicotine); NRT, nicotine replacement therapy; SEM, standard error of the mean; UB, usual brand.
In Study 2, the overall UTS (AUECUTS 0–240) for each Velo Mini Pouch was significantly greater compared with UB cigarettes; the exception was AUECUTS 0–240 for the Velo Mini Pouch Cool Mint 8 mg product, which did not differ significantly from UB cigarette. In addition, the minimum effect of each Velo Mini Pouch to relieve UTS (Emin UTS) was significantly greater for each Velo Mini Pouch than for UB cigarette. Relative to NRT gum, each Velo Mini Pouch relieved UTS (AUECUTS 0–15, AUECUTS 0–240, and Emin UTS) to a similar extent as NRT gum, except that AUECUTS 0–240 for the Velo Mini Pouch Modern Traditions, 4 mg was significantly greater than that for NRT gum. Both AUECUTS 0–240 and Emin UTS were lower for the 8 mg Velo Mini Pouch than for the Velo Mini Pouch, 4 mg (Table 4; Supplementary Table S2).
Parameters of both positive (AUECPEpos 5–240 and Emax PEpos) and negative (AUECPEneg 5–240 and Emax PEneg) PE were assessed. Regarding positive PE, UB cigarette was rated highest in both Studies 1 and 2, and scores were significantly higher for UB cigarette than for any Velo ONP. The NRT gum and the Velo ONPs evaluated received comparable scores for positive PE parameters in both studies; the exception was AUECPEpos 5–240, which was significantly lower for the Velo Mini Pouch Modern Traditions, 4 mg than for NRT gum (Table 5; Supplementary Table S2).
Both overall (AUECPEneg 5–240) and maximum (Emax PEneg) negative PE did not differ significantly between any of the Velo ONPs and UB cigarette or NRT gum with two exceptions (Table 5). First, the highest nicotine Velo Pouch (12 mg) product was scored significantly higher than NRT gum for Emax PEneg; and second, the Velo Mini Pouch Cool Mint, 8 mg product was scored significantly higher than UB cigarettes for AUECPEneg 5–240. In general, among the Velo ONPs, negative PE increased with higher nicotine content (Table 5; Supplementary Table S2).
In both AL studies, Eoverall PL was significantly lower for all Velo ONPs relative to UB cigarette (Table 5), whereas Eoverall PL for NRT gum and Velo ONPs was generally comparable with the exception that significantly lower Eoverall PL values were observed between the Velo Mini Pouches Modern Traditions (4 and 8 mg) and NRT gum (Table 5; Supplementary Table S2).
Consistent with the other subjective effects measures, OIUA was highest for UB cigarette among the study products (Table 5). Eoverall IUA values for all Velo ONPs were significantly lower than for UB cigarette in both AL studies and did not differ significantly between the Velo ONPs and NRT gum in Study 1. Although Eoverall IUA tended to be higher irrespective of flavor for the 8 mg versus the 4 mg Velo Mini Pouch products in Study 2, Eoverall IUA was significantly lower for the Velo Mini Pouch products than for NRT gum, except for the Velo Mini Pouch Cool Mint, 8 mg, where it was observed that Eoverall IUA did not differ from that of NRT gum (Table 5; Supplementary Table S2).
In summary, key subjective effects measures (i.e., PL, OPL, positive PE, and OIUA) for the Velo ONPs in both AL studies were consistently significantly lower compared with UB cigarette, and similar to or slightly lower than the respective measures for NRT gum.
In Study 3, we evaluated whether nicotine uptake and OPL differ between different Velo Pouch flavors at the same nicotine level. As in the two AL studies, nicotine PK profiles were generally similar for all Velo Pouches at the same nicotine level. The plasma nicotine level for the 10 mg product was higher than for the 8 mg products (Figure 4). Overall plasma nicotine uptake (AUC0-240) and maximum plasma nicotine concentration (Cmax) were generally similar for all flavors of the 8 mg Velo Pouch nicotine products, and higher for the 10 mg nicotine product (Table 6). Additionally, the pharmacokinetic parameters (AUC0-15, AUC0-240, and Cmax) for the 8 mg products generally exhibited overlapping 95% confidence intervals (Table 6).
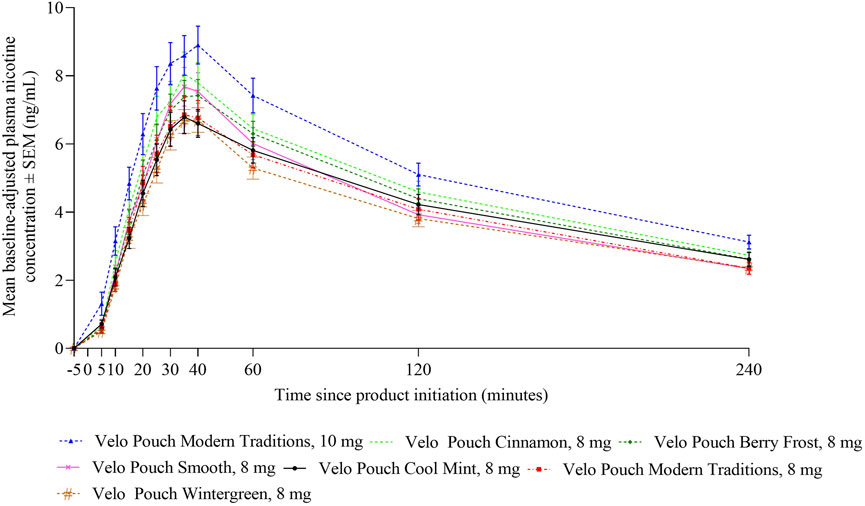
Figure 4. Plasma nicotine concentrations over 240 min following start of Velo Pouch product use in Study 3. Each point shows the mean ± SEM plasma nicotine concentration. Abbreviations: mg, milligram (of nicotine); PK, pharmacokinetic; SEM, standard error of the mean.
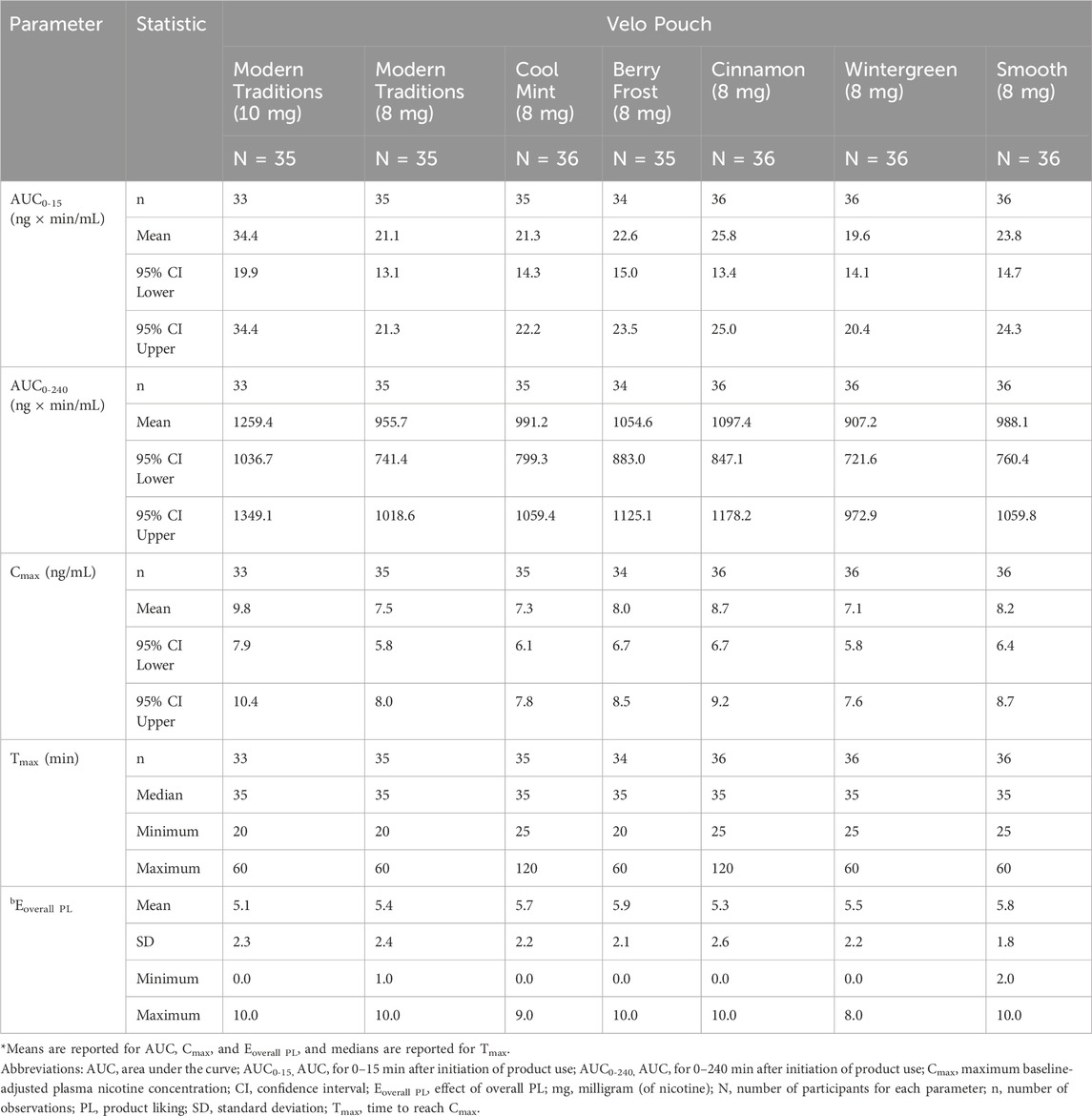
Table 6. Results of nicotine uptake and overall product liking scores of Velo Pouches* assessed in Study 3.
Overall product liking was rated at the end of each test session and was similar for all Velo Pouches regardless of flavor or nicotine content (Table 6).
In all three studies, all AEs, their causal relationship (related or possibly related) to use of the study products, and their severity (mild, moderate, or severe) were recorded (Supplementary Tables S3A–C). Few participants experienced an AE; in general, AEs were transient, and the majority were mild. No participants experienced a severe AE (Supplementary Tables S3A–C).
Nausea, dizziness, hiccups, headache, and euphoric mood were the most reported AEs during use of the Velo ONPs (Supplementary Table S4). One participant in Study 1 withdrew early due to two AEs (vascular disorders: diastolic and systolic hypertension), both mild in severity. Diastolic hypertension was judged unlikely to be related to use of the Velo Pouch, while systolic hypertension was reported as possibly related to use of the Velo Pouch Cool Mint, 8 mg product. One participant in Study 1 was discontinued early due to five AEs; one AE (hypertension) was moderate in severity, while the others (hyperesthesia, tachycardia, and flushing [one case of each], and nausea [two cases]) were mild. One case of nausea, hypertension, tachycardia, and flushing were judged as possibly related to use of NRT gum. The other case of nausea was judged as related to the use of the Velo Mini Pouch Modern Traditions, 4 mg, while the case of hyperesthesia was judged as possibly related to use of Velo Mini Pouch Cool Mint, 8 mg. All AEs deemed to be causally related to product use were resolved prior to participant discharge from the study.
The objective of the three clinical studies described here was to provide a comprehensive and rigorous assessment of the elements contributing to the AL of Velo ONPs through measurements of nicotine PK (exposure) and subjective effects (Carter et al., 2009b; Fearon et al., 2022; Henningfield and Keenan, 1993; Vansickel et al., 2022). The key findings are as follows: (1) nicotine uptake increased with increasing nicotine content in the Velo ONPs, and PK parameters across different flavors at the same nicotine level were largely similar; (2) subjective effects for Velo ONPs were generally lower relative to UB cigarette and were generally similar across all Velo ONPs variants, showing comparable or lower subjective effects to NRT gum; (3) Velo ONPs reduced UTS, with greater reductions observed for higher nicotine content pouches, though still less effective than cigarettes; and (4) Velo ONPs were well tolerated by the participants in the studies.
Collectively, our findings suggest that Velo ONPs deliver sufficient nicotine to users to maintain reinforcement by reducing smoking urges, and exhibit some AL, but to a lesser extent than combustible cigarettes. Since a certain degree of dependence is necessary for alternative nicotine products to effectively provide a viable substitute for cigarettes (Abrams et al., 2018; Fearon et al., 2022), these findings suggest that Velo ONPs could be a viable component of a THR strategy. Notably, the slower nicotine uptake, reflected by the later Tmax, differences in the AUC0-15, and the overall lower AL of Velo ONPs observed here, suggest that these products pose less of an initiation and/or addiction risk among non-tobacco users. Additionally, the reduced levels of HPHCs (Grandolfo et al., 2024; Back et al., 2023; Borgerding et al., 2012; Azzopardi et al., 2022a; Jablonski et al., 2022) and reduced biomarkers of exposure (Grandolfo et al., 2024; Azzopardi et al., 2022b) in ONPs further support that Velo ONPs may play a contributory role in THR and a benefit to the population as a whole, building on the previously demonstrated positive impact on public health of oral STPs (Clarke et al., 2019; Food and Drug Administration, 2019; Food and Drug Administration, 2023).
Consistent with the delivery of nicotine via buccal absorption, the PK profiles of Velo ONPs resembled those of NRT gum, with a significantly higher Tmax and lower Cmax compared with UB cigarette. In Study 1, the Cmax and overall nicotine uptake after Velo Pouch use was associated with nicotine content level, in agreement with other studies in which ONPs with a high nicotine content exhibited Cmax and AUC comparable to or higher than those of cigarettes (Keller-Hamilton et al., 2023; Liu et al., 2021; McEwan et al., 2022a) and traditional STPs (Lunell et al., 2020). The Velo Mini Pouches (4 and 8 mg) also exhibited nicotine content-dependent Cmax and AUC values. The highest nicotine level (12 mg) product evaluated in Study 1, although comparable to UB cigarette in terms of overall nicotine uptake, did not achieve a comparable Cmax value to that of UB cigarette. In addition, the Velo Pouch Cool Mint 4 mg product exhibited significantly higher Cmax and AUC values as compared with NRT gum in Study 1, whereas in Study 2, the same nicotine content product (Velo Mini Pouch Cool Mint 4 mg) was not different than NRT gum; but the 8 mg product had significantly higher Cmax and AUC values than NRT gum. These findings suggest that other characteristics, such as formulation and composition (Peraza et al., 2024), user behavior (Digard et al., 2012), and individual differences (Wagenknecht et al., 2018) of ONPs, and not just the nicotine content, may influence nicotine PK.
The plasma nicotine AUC0-240 and Cmax values for the 8 mg products of the same flavor (Cool Mint) were similar across Studies 1 and 2, indicating consistent nicotine delivery from Velo ONPs. In contrast, the AUC0-240 and the Cmax values for UB cigarette were higher in Study 1 than in Study 2. In Study 2, AUC0-240 was significantly higher for both 8 mg Velo Mini Pouch products (Cool Mint and Modern Traditions) compared to UB cigarette. However, in Study 1, the AUC0-240 for Velo Pouch Cool Mint at the same nicotine level (8 mg) was significantly lower compared to UB cigarette. Variability in nicotine uptake from cigarettes has been previously reported (Hammond et al., 2005; Krebs et al., 2016), and may account for the differences in statistical significance when comparing the 8 mg Velo ONPs to UB cigarettes in these two studies. While Velo ONPs are demonstrated to effectively deliver nicotine, albeit at a slower rate compared to cigarettes, the faster nicotine uptake (as indicated by shorter Tmax and higher AUC 0–15), the numerically higher peak nicotine levels (Cmax) for the combustible cigarettes assessed in this study compared to the 8 mg Velo Mini Pouch products, support an overall lower AL for the 8 mg Velo Mini Pouch products compared to cigarettes.
We also examined the effect of different flavors on the nicotine PK and AL of Velo ONPs. In Studies 2 and 3, Velo ONPs with different flavors but the same nicotine content generally exhibited similar PK profiles and parameters, indicating that flavor does not affect nicotine PK for the Velo ONPs assessed. In addition, subjective effects scores were generally similar among flavor variants of Velo ONPs; when taken together with the nicotine PK findings, this suggests that flavor alone does not have an impact on AL. Rensch et al. (Rensch et al., 2021) previously reported that Cmax values were within 15%, while AUC values were within 25% of each other for six flavor variants of 4 mg “on!” ONPs. Their results align with our observations that nicotine content is a key determinant of nicotine PK of Velo ONPs, but flavors do not seem to influence nicotine PK, and therefore AL.
In general, the Velo ONPs, irrespective of the pouch size, received lower scores than UB cigarette and comparable to or lower than NRT gum in the subjective measures assessed in the two AL studies. This is consistent with previous studies in which 4 and 8 mg ONPs had lower subjective effects scores than cigarettes (Liu et al., 2022), and where ONPs with various nicotine content all had PL and IUA scores lower than cigarettes (McEwan et al., 2022b). In our AL studies, negative PE scores were generally comparable across the study products. In addition, while cigarettes were most effective in reducing UTS, the study Velo ONPs and NRT gum were also effective, although to a lesser extent than cigarettes, and there was a tendency for the UTS reductions associated with Velo ONP use to be greater with increasing nicotine content. Reductions in UTS have been reported previously for 3 mg and 6 mg ONPs (Keller-Hamilton et al., 2023). When taken together with the current AL findings, this suggests that ONPs, including Velo ONPs, may provide a suitable alternative to cigarettes for current smokers who do not want to quit smoking or using other tobacco and nicotine products.
The main strength of these three studies is the inclusion of several ONPs varying in flavor, nicotine content, and physical pouch size, thus presenting a wide-ranging assessment of the AL of Velo ONPs. In addition, the inclusion of high and low AL comparator products (cigarettes and NRT gum, respectively) enabled relative AL to be determined. A further strength is the use of established methods that conform to proposals and regulatory stipulations on how AL should be assessed (Carter et al., 2009b; Fearon et al., 2022; Henningfield and Keenan, 1993; Vansickel et al., 2022; Campbell et al., 2023; Campbell et al., 2022). It should be noted, however, that the AL determined is representative only of the ONPs assessed and may not extend to other types and brands of ONPs, particularly those with different nicotine contents and flavors. Other limitations include the fact that Study 3 assessed only a single aspect of subjective effects measure. Nevertheless, the findings for OPL in Study 3 agreed with the wider measures evaluated in Studies 1 and 2, indicating a significantly lower potential for ONP adoption compared with cigarettes. Another potential limitation is the length of time for which participants were able to familiarize themselves with the study products (half a day) as it has been shown for other nicotine products (e.g., electronic cigarettes) that user experience may affect nicotine PK (Farsalinos et al., 2015; Hajek et al., 2015) and therefore AL. However, we consider that this is unlikely to be the case for ONPs owing to the simplicity and similarity in product design and instructions for use. Importantly, due to the route of exposure, nicotine uptake for ONPs is expected to be slower than an inhalable product regardless of use behavior.
This study evaluated elements of AL for Velo Pouch products across three nicotine levels (4 mg, 8 mg, and 12 mg nicotine) in comparison to UB cigarette (a high-AL comparator) and NRT gum (a low-AL comparator) in current smokers and smokers who also use STPs. The findings across subjective and PK endpoints provide a comprehensive basis for determining the relative AL of the Velo Pouch products.
The subjective effects data reveal a clear trend of comparative AL positioned between UB cigarette and NRT gum. Measures of PL showed significantly lower scores for all Velo Pouch products compared to UB cigarette and no significant differences compared to NRT gum. Subjective effects measures, including positive PE, OPL, and OIUA, were consistently lower for Velo Pouch products than for UB cigarette and comparable to NRT gum. Notably, higher nicotine levels were associated with decreased OPL and OIUA scores, suggesting that increased nicotine delivery did not enhance positive subjective effects in this product category.
Measures of negative PE increased with higher nicotine levels, with the highest nicotine level (12 mg) eliciting significantly greater maximum negative effects compared to NRT gum. These findings suggest that higher nicotine concentrations in Velo Pouch products may result in less favorable subjective experiences, which could indicate a lower potential for AL.
Endpoints assessing UTS relief revealed nuanced patterns. UB cigarette was most effective at alleviating UTS within the first 15 min of use. However, Velo Pouch products, particularly at higher nicotine levels (e.g., 12 mg), provided significantly greater UTS relief than NRT gum and showed no significant differences compared to UB cigarette for longer-term relief (e.g., AUECUTS 0–240, Emin UTS). These findings highlight the ability of Velo Pouch products to alleviate nicotine cravings effectively, albeit not as rapidly as combustible cigarettes.
Plasma nicotine PK data further support the subjective effects findings. Velo Pouch products demonstrated slower and less pronounced nicotine uptake compared to UB cigarette but greater uptake than NRT gum, except for the highest nicotine level (12 mg), which exhibited a higher overall nicotine exposure comparable to UB cigarette, although this difference was not significant. The Tmax for Velo Pouch products was significantly longer than for UB cigarette and similar to NRT gum, consistent with buccal absorption mechanisms of these products.
Taken together, the evidence indicates that Velo Pouch products exhibit some level of AL or dependence sustainability driven by their ability to deliver nicotine and relieve UTS. However, this level is lower than that of combustible cigarettes and more closely aligned with NRT gum. The slower nicotine uptake, lower positive PEs, and increased negative PEs at higher nicotine levels further differentiate Velo Pouch products from cigarettes.
These findings suggest that Velo Pouch products, particularly those with lower nicotine levels, may present a reduced potential for abuse compared to cigarettes while maintaining some ability to address nicotine dependence. This supports their potential role in harm reduction strategies, offering smokers a potentially less harmful alternative to combustible cigarettes.
This study assessed elements of AL of Velo Mini Pouch products (4 mg and 8 mg nicotine levels) compared to UB cigarette (a high-AL comparator) and NRT gum (a low-AL comparator). The assessment was conducted in smokers and dual users of combustible cigarettes and STPs, providing a comprehensive evaluation of the relative AL of Velo Mini Pouch products utilizing subjective and PK endpoints.
The subjective effects data indicate that Velo Mini Pouch products exhibit a moderated AL potential that would support product use or potential for switching from cigarettes or other STPs. Measures of PL were significantly lower for all Velo Mini Pouch products compared to UB cigarette, with most comparisons showing no significant difference from NRT gum. Similarly, positive PE, OPL, and OIUA, were consistently lower for Velo Mini Pouch products than for UB cigarette and largely comparable to or lower than NRT gum.
Negative PEs were generally similar between Velo Mini Pouch products and the comparators. However, one notable exception was the higher AUECPEneg 5–240 observed with Velo Mini Pouch Cool Mint, 8 mg, compared to UB cigarette. These findings suggest that while the Velo Mini Pouch products produce some positive effects, they are attenuated compared to combustible cigarettes, with lower abuse potential indicated by higher nicotine levels eliciting greater negative PE scores.
The ability of Velo Mini Pouch products to relieve UTS was assessed over time. While UB cigarette provided the most rapid UTS relief within the first 15 min of use, Velo Mini Pouch products demonstrated comparable or greater UTS relief over a longer period (4 h) relative to NRT gum. Products with higher nicotine levels (8 mg) were more effective at relieving UTS than lower levels (4 mg), reinforcing the influence of nicotine concentration on AL outcomes.
Plasma nicotine uptake profiles further contextualize the AL potential of Velo Mini Pouch products. Nicotine absorption was slower and less pronounced for Velo Mini Pouch products compared to UB cigarette, with trajectories resembling those of NRT gum. Nicotine uptake increased with higher nicotine levels, with the 8 mg products showing total nicotine uptake comparable to UB cigarette, whereas the 4 mg products demonstrated uptake similar to NRT gum. The Tmax values for Velo Mini Pouch products were significantly longer than for UB cigarette, reflecting the buccal absorption route and further aligning with NRT gum.
Overall, based on the synthesis of subjective effects and PK data, Velo Mini Pouch products exhibit an intermediate AL profile between UB cigarette and NRT gum. The slower nicotine uptake, together with the reduced positive PEs, and comparable or lower UTS relief relative to NRT gum suggest that these products have a lower abuse potential than combustible cigarettes, particularly at the 4 mg nicotine level. These findings support the potential utility of Velo Mini Pouch products as a lower-risk alternative for smokers seeking harm reduction.
In Study 3, we examined the potential differences in plasma nicotine uptake and OPL among various Velo Pouch flavors at the same nicotine level to further evaluate AL potential of these products. The findings from this study are consistent with those from Studies one and 2, and provide additional insight into the role of flavor and nicotine content in AL outcomes.
Nicotine PK profiles were generally similar for all Velo Pouch products within the same nicotine level, indicating consistency in nicotine delivery regardless of flavor. The plasma nicotine level for the 10 mg Velo Pouch was higher than that observed for the 8 mg products, as expected with increased nicotine content. Additionally, overall plasma nicotine uptake (AUC0-240) and Cmax were comparable across all flavors of the 8 mg nicotine products, with overlapping 95% confidence intervals. These results indicate that variations in flavor do not considerably influence nicotine absorption or plasma nicotine levels.
OPL was assessed at the end of each test session to capture participants’ subjective preferences for different Velo Pouch products. Ratings of OPL were consistent across all flavors and nicotine levels, suggesting that flavor variations did not substantially alter participants’ liking of the products, supporting the notion that AL potential for these products is driven more by nicotine content than by flavor.
Overall, the findings from Study 3 indicate that nicotine delivery was consistent across flavors at the same nicotine level, and subjective liking remained similar regardless of flavor or nicotine content. By demonstrating consistent PK and subjective outcomes across flavors, this study highlights the potential of Velo Pouch products to maintain a lower AL profile while offering flavor variety, which may support consumer acceptance and adherence as part of harm reduction strategies.
In summary, the assessments of the subjective effect and PK elements that may contribute to the AL of Velo ONPs suggests that these products exhibit lower AL than cigarettes, and comparable to or slightly less AL than NRT gum. Nicotine PK, and therefore AL, were dependent on pouch nicotine content, with increasing levels of nicotine leading to greater nicotine delivery, while subjective effects remained generally similar across products. Oral nicotine pouch flavors (Study 3) and pouch size (Study 1 compared to Study 2), however, did not seem to impact either nicotine PK or subjective effects when these parameters for Velo ONPs are compared to those of cigarettes. Overall, by generating some AL, but to a lesser extent than cigarettes, and by reducing UTS when used, Velo ONPs may offer a viable alternative to cigarettes and support THR at both the individual and population-level.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Advarra Institutional Review Board (Columbia, MD, United States). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MK: Conceptualization, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft, Writing–review and editing. CP: Conceptualization, Methodology, Writing–review and editing. MP: Conceptualization, Methodology, Writing–review and editing. SB: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing–review and editing. MT: Visualization, Writing–original draft, Writing–review and editing. JD: Investigation, Project administration, Writing–review and editing. KP: Investigation, Project administration, Writing–original draft, Writing–review and editing. AG: Investigation, Methodology, Writing–original draft, Writing–review and editing. SA: Formal Analysis, Writing–review and editing. LC: Formal Analysis, Supervision, Writing–review and editing. JC: Project administration, Writing–review and editing. BK: Supervision, Visualization, Writing–review and editing. BS: Writing–review and editing. NG: Supervision, Writing–review and editing. KJ: Funding acquisition, Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
The authors acknowledge the assistance of the clinical study sites (ICON Early Phase Services, LLC, San Antonio, TX, United States; Multispecialty Research Lexington, Lexington, KY, United States; and Alliance for Multispecialty Research Knoxville, Knoxville, TN, United States), the contract research organizations (ICON Early Phase, LLC, PA, United States; NCGS Charleston, SC, United States), and the bioanalytical laboratory (Celerion Global Bioanalytical Services, Lincoln, NE, United States) in conducting the study. The authors gratefully acknowledge the support of GL Prasad (Prasad Scientific Consulting LLC) and Ian M. Fearon (whatIF? Consulting Ltd) in preparing the manuscript. We also gratefully thank Candi Cunningham for critical review of the manuscript; Paul Nelson and Bobbette Jones for assistance with study design; Gregory P. Tarleton, PharmD, MD for providing medical expertise to ensure participant safety; Eckhardt Schmidt for bioanalytical data oversight; Tom Kerby for assistance with data management activities; and Bradley LeDuc-Lenmark and Jason Henstock for maintaining the study Trial Master File and managing other study-related documentation.
Authors MK, CP, MP, SB, MT, JD, KP, AG, SA, LC, JC, BK, BG, and KJ were employed by RAI Services Company.
Author NG was employed by BAT (Investments) Limited. RAI Services Company is a wholly owned subsidiary of Reynolds American Inc., which is an indirect, wholly owned subsidiary of British American Tobacco plc.
The authors declare that these studies received funding from RAI Services Company. The funder had the following involvement in the study: study design, interpretation of data, the writing of this article, and the decision to submit for publication.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1547073/full#supplementary-material
Abrams, D. B., Glasser, A. M., Pearson, J. L., Villanti, A. C., Collins, L. K., and Niaura, R. S. (2018). Harm minimization and tobacco Control: reframing societal views of nicotine use to rapidly save lives. Annu. Rev. Public Health 39, 193–213. doi:10.1146/annurev-publhealth-040617-013849
Ashley, D. L., Spears, C. A., Weaver, S. R., Huang, J., and Eriksen, M. P. (2020). E-cigarettes: how can they help smokers quit without addicting a new generation? Prev. Med. 140, 106145. doi:10.1016/j.ypmed.2020.106145
Azzopardi, D., Haswell, L. E., Frosina, J., McEwan, M., Gale, N., Thissen, J., et al. (2022b). Biomarkers of exposure and potential harm in exclusive users of nicotine pouches and current, former, and never smokers: protocol for a cross-sectional clinical study. JMIR Res. Protoc. 11 (10), e39785. doi:10.2196/39785
Azzopardi, D., Haswell, L. E., Frosina, J., McEwan, M., Gale, N., Thissen, J., et al. (2023). Assessment of biomarkers of exposure and potential harm, and physiological and subjective health measures in exclusive users of nicotine pouches and current, former and never smokers. Biomarkers 28 (1), 118–129. doi:10.1080/1354750X.2022.2148747
Azzopardi, D., Liu, C., and Murphy, J. (2022a). Chemical characterization of tobacco-free “modern” oral nicotine pouches and their position on the toxicant and risk continuums. Drug Chem. Toxicol. 45 (5), 2246–2254. doi:10.1080/01480545.2021.1925691
Back, S., Masser, A. E., Rutqvist, L. E., and Lindholm, J. (2023). Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs). BMC Chem. 17 (1), 9. doi:10.1186/s13065-023-00918-1
Benowitz, N. L., Swan, G. E., Jacob, P., Lessov-Schlaggar, C. N., and Tyndale, R. F. (2006). CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther. 80 (5), 457–467. doi:10.1016/j.clpt.2006.08.011
Borgerding, M. F., Bodnar, J. A., Curtin, G. M., and Swauger, J. E. (2012). The chemical composition of smokeless tobacco: a survey of products sold in the United States in 2006 and 2007. Regul. Toxicol. Pharmacol. 64 (3), 367–387. doi:10.1016/j.yrtph.2012.09.003
Cahn, Z., Drope, J., Douglas, C. E., Henson, R., Berg, C. J., Ashley, D. L., et al. (2021). Applying the population health standard to the regulation of electronic nicotine delivery systems. Nicotine Tob. Res. 23 (5), 780–789. doi:10.1093/ntr/ntaa190
Campbell, C., Jin, T., Round, E. K., Nelson, P. R., and Baxter, S. (2023). Abuse liability of two electronic nicotine delivery systems compared with combustible cigarettes and nicotine gum from an open-label randomized crossover study. Sci. Rep. 13 (1), 18951. doi:10.1038/s41598-023-45894-7
Campbell, C., Jin, T., Round, E. K., Schmidt, E., Nelson, P., and Baxter, S. (2022). Part one: abuse liability of Vuse Solo (G2) electronic nicotine delivery system relative to combustible cigarettes and nicotine gum. Sci. Rep. 12 (1), 22080. doi:10.1038/s41598-022-26417-2
Carter, L. P., Stitzer, M. L., Henningfield, J. E., O'Connor, R. J., Cummings, K. M., and Hatsukami, D. K. (2009a). Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 18 (12), 3241–3262. doi:10.1158/1055-9965.EPI-09-0948
Carter, L. P., Stitzer, M. L., Henningfield, J. E., O'Connor, R. J., Cummings, K. M., and Hatsukami, D. K. (2009b). Abuse liability assessment of tobacco products including potential reduced exposure products. Cancer Epidemiol. Biomarkers Prev. 18 (12), 3241–3262. doi:10.1158/1055-9965.EPI-09-0948
Clarke, E., Thompson, K., Weaver, S., Thompson, J., and O'Connell, G. (2019). Snus: a compelling harm reduction alternative to cigarettes. Harm Reduct. J. 16 (1), 62. doi:10.1186/s12954-019-0335-1
Congress (2009). Family smoking prevention and tobacco control and federal retirement reform. Public Law 2009:111–31. Washington, DC: U.S. Government Printing Office. 111–131.
Digard, H., Proctor, C., Kulasekaran, A., Malmqvist, U., and Richter, A. (2012). Determination of nicotine absorption from multiple tobacco products and nicotine gum. Nicotine and Tob. Res. 15 (1), 255–261. doi:10.1093/ntr/nts123
Fagerström, K., and Eissenberg, T. (2012). Dependence on tobacco and nicotine products: a case for product-specific assessment. Nicotine Tob. Res. 14 (11), 1382–1390. doi:10.1093/ntr/nts007
Farsalinos, K. E., Spyrou, A., Stefopoulos, C., Tsimopoulou, K., Kourkoveli, P., Tsiapras, D., et al. (2015). Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naïve users (smokers). Sci. Rep. 5, 11269. doi:10.1038/srep11269
Fearon, I. M., Seltzer, R. G. N., Houser, T. L., Tope, A., Cahours, X., Verron, T., et al. (2022). Human abuse liability assessment of e-cigarettes: why, what and how? Drug Test. Anal. 15, 1270–1280. doi:10.1002/dta.3335
Food and Drug Admini (2012). “Harmful and potentially harmful constituents in tobacco products and tobacco smoke; established list. Docket No. FDA–2012–N–0143,” in Federal register, Food and Drug administration.
Food and Drug Administration (2019). FDA grants first-ever modified risk orders to eight smokeless tobacco products. Available at: https://www.fda.gov/news-events/press-announcements/fda-grants-first-ever-modified-risk-orders-eight-smokeless-tobacco-products (Accessed July 26, 2024).
Food and Drug Administration (2021). “Premarket tobacco product Applications and recordkeeping Requirements, department of health and human services,” in Federal register.
Food and Drug Administration (2023). Smokeless tobacco Company modified risk tobacco product (MRTP) application.
Gottlieb, S., and Zeller, M. (2017). A nicotine-focused framework for public health. N. Engl. J. Med. 377 (12), 1111–1114. doi:10.1056/NEJMp1707409
Grandolfo, E., Ogden, H., Fearon, I. M., Malt, L., Stevenson, M., Weaver, S., et al. (2024). Tobacco-Free nicotine pouches and their potential contribution to tobacco harm reduction: a scoping review. Cureus 16 (2), e54228. doi:10.7759/cureus.54228
Hajek, P., Goniewicz, M. L., Phillips, A., Myers Smith, K., West, O., and McRobbie, H. (2015). Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine Tob. Res. 17 (2), 175–179. doi:10.1093/ntr/ntu153
Hammond, D., Fong, G. T., Cummings, K. M., and Hyland, A. (2005). Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol. Biomarkers Prev. 14 (6), 1370–1375. doi:10.1158/1055-9965.EPI-04-0498
Hatsukami, D. K., and Carroll, D. M. (2020). Tobacco harm reduction: past history, current controversies and a proposed approach for the future. Prev. Med. 140, 106099. doi:10.1016/j.ypmed.2020.106099
Heatherton, T. F., Kozlowski, L. T., Frecker, R. C., and Fagerström, K. O. (1991). The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br. J. Addict. 86 (9), 1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x
Henley, S. J., Connell, C. J., Richter, P., Husten, C., Pechacek, T., Calle, E. E., et al. (2007). Tobacco-related disease mortality among men who switched from cigarettes to spit tobacco. Tob. Control 16 (1), 22–28. doi:10.1136/tc.2006.018069
Henley, S. J., Thun, M. J., Connell, C., and Calle, E. E. (2005). Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States). Cancer Causes Control 16 (4), 347–358. doi:10.1007/s10552-004-5519-6
Henningfield, J. E., and Keenan, R. M. (1993). Nicotine delivery kinetics and abuse liability. J. Consult Clin. Psychol. 61 (5), 743–750. doi:10.1037//0022-006x.61.5.743
Institute of Medicine (2001). Clearing the smoke: assessing the science base for tobacco harm reduction. Washington, DC: : The National Academies Press.
Jackson, J. M., Weke, A., and Holliday, R. (2023). Nicotinepouches: a review for the dental team. Br. Dent. J. 235 (8), 643–646. doi:10.1038/s41415-023-6383-7
Jablonski, J. J., Cheetham, A. G., and Martin, A. M. (2022). Market survey of modern oral nicotine products: determination of select HPHCs and comparison to traditional smokeless tobacco products. Separations 9 (3), 65. doi:10.3390/separations9030065
Jamal, A., King, B. A., Neff, L. J., Whitmill, J., Babb, S. D., and Graffunder, C. M. (2016). Current cigarette smoking among adults - United States, 2005-2015. MMWR Morb. Mortal. Wkly. Rep. 65 (44), 1205–1211. doi:10.15585/mmwr.mm6544a2
Keller-Hamilton, B., Alalwan, M. A., Curran, H., Hinton, A., Long, L., Chrzan, K., et al. (2023). Evaluating the effects of nicotine concentration on the appeal and nicotine delivery of oral nicotine pouches among rural and Appalachian adults who smoke cigarettes: a randomized cross-over study. Addiction 119, 464–475. doi:10.1111/add.16355
King, B. A., and Toll, B. A. (2023). Opportunities and considerations for addressing misperceptions about the relative risks of tobacco products among adult smokers. Addiction 118 (10), 1892–1894. doi:10.1111/add.16296
Krebs, N. M., Chen, A., Zhu, J., Sun, D., Liao, J., Stennett, A. L., et al. (2016). Comparison of puff volume with cigarettes per day in predicting nicotine uptake among daily smokers. Am. J. Epidemiol. 184 (1), 48–57. doi:10.1093/aje/kwv341
Liu, J., Rensch, J., Wang, J., Jin, X., Vansickel, A., Edmiston, J., et al. (2022). Nicotine pharmacokinetics and subjective responses after using nicotine pouches with different nicotine levels compared to combustible cigarettes and moist smokeless tobacco in adult tobacco users. Psychopharmacol. Berl. 239 (9), 2863–2873. doi:10.1007/s00213-022-06172-y
Liu, J., Wang, J., Vansickel, A., Edmiston, J., Graff, D., and Sarkar, M. (2021). Characterization of the abuse potential in adult smokers of a novel oral tobacco product relative to combustible cigarettes and nicotine polacrilex gum. Clin. Pharmacol. Drug Dev. 10 (3), 241–250. doi:10.1002/cpdd.909
Lunell, E., Fagerström, K., Hughes, J., and Pendrill, R. (2020). Pharmacokinetic comparison of a Novel non-tobacco-Based nicotine pouch (ZYN) with conventional, tobacco-based Swedish snus and American moist snuff. Nicotine Tob. Res. 22 (10), 1757–1763. doi:10.1093/ntr/ntaa068
Luo, J., Ye, W., Zendehdel, K., Adami, J., Adami, H. O., Boffetta, P., et al. (2007). Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet 369 (9578), 2015–2020. doi:10.1016/S0140-6736(07)60678-3
Majmundar, A., Okitondo, C., Xue, A., Asare, S., Bandi, P., and Nargis, N. (2022). Nicotine pouch sales trends in the US by volume and nicotine concentration levels from 2019 to 2022. JAMA Network Open 5 (11), e2242235. doi:10.1001/jamanetworkopen.2022.42235
Maloney, S. F., Breland, A., Soule, E. K., Hiler, M., Ramôa, C., Lipato, T., et al. (2019). Abuse liability assessment of an electronic cigarette in combustible cigarette smokers. Exp. Clin. Psychopharmacol. 27 (5), 443–454. doi:10.1037/pha0000261
McEwan, M., Azzopardi, D., Gale, N., Camacho, O. M., Hardie, G., Fearon, I. M., et al. (2022a). A randomised study to investigate the nicotine pharmacokinetics of oral nicotine pouches and a combustible cigarette. Eur. J. Drug Metab. Pharmacokinet. 47 (2), 211–221. doi:10.1007/s13318-021-00742-9
McEwan, M., Azzopardi, D., Gale, N., Camacho, O. M., Hardie, G., Fearon, I. M., et al. (2022b). A randomised study to investigate the nicotine pharmacokinetics of oral nicotine pouches and a combustible cigarette. Eur. J. Drug Metabolism Pharmacokinet. 47 (2), 211–221. doi:10.1007/s13318-021-00742-9
MedDRA Maintenance and Support Services Organization (2021). Introductory guide MedDRA. McLean, Virginia.
Peraza, N., Schaan, B. N. D., Johnson, A., Vasquez, C. L., and Lee, T. M. (2024). Appeal and sensory characteristics of oral nicotine products in young adults who vape E-cigarettes. Nico. Toba. Rese. Oxford, UK: Nicotine and Tobacco Research. doi:10.1093/ntr/ntaa123
Rensch, J., Liu, J., Wang, J., Vansickel, A., Edmiston, J., and Sarkar, M. (2021). Nicotine pharmacokinetics and subjective response among adult smokers using different flavors of on!® nicotine pouches compared to combustible cigarettes. Psychopharmacol. Berl. 238 (11), 3325–3334. doi:10.1007/s00213-021-05948-y
Shaikh, S. B., Alvarado, K. R., Gutierrez, A. L., and Reese, R. M. (2023). Classification, perception, and toxicity of emerging flavored oral nicotine pouches. Int. J. Environ. Res. Public Health. Basel, Switzerland: MDPI 20 (5), 4526. doi:10.3390/ijerph20054526
Shiffman, S., Cone, E. J., Buchhalter, A. R., Henningfield, J. E., Rohay, J. M., Gitchell, J. G., et al. (2009). Rapid absorption of nicotine from new nicotine gum formulations. Pharmacol. Biochem. Behav. 91 (3), 380–384. doi:10.1016/j.pbb.2008.08.012
Stiles, M. F., Campbell, L. R., Graff, D. W., Jones, B. A., Fant, R. V., and Henningfield, J. E. (2017). Pharmacodynamic and pharmacokinetic assessment of electronic cigarettes, combustible cigarettes, and nicotine gum: implications for abuse liability. Psychopharmacol. Berl. 234 (17), 2643–2655. doi:10.1007/s00213-017-4665-y
Stiles, M. F., Campbell, L. R., Jin, T., Graff, D. W., Fant, R. V., and Henningfield, J. E. (2018). Assessment of the abuse liability of three menthol Vuse Solo electronic cigarettes relative to combustible cigarettes and nicotine gum. Psychopharmacol. Berl. 235 (7), 2077–2086. doi:10.1007/s00213-018-4904-x
University of Bath (2023). Newer nicotine and tobacco products. Available at: https://tobaccotactics.org/article/newer-nicotine-and-tobacco-products/ (Accessed July 26, 2024).
Vansickel, A., Baxter, S., Sherwood, N., Kong, M., and Campbell, L. (2021). Human abuse liability assessment of tobacco and nicotine products: approaches for meeting current regulatory recommendations. Nicotine and Tob. Res. 24, 295–305. doi:10.1093/ntr/ntab183
Vansickel, A., Baxter, S., Sherwood, N., Kong, M., and Campbell, L. (2022). Human abuse liability assessment of tobacco and nicotine products: approaches for meeting current regulatory recommendations. Nicotine Tob. Res. 24 (3), 295–305. doi:10.1093/ntr/ntab183
Keywords: nicotine uptake, abuse liability, subjective effects, Velo Oral Nicotine pouches, combustible cigarettes, NRT gum, tobacco harm reduction
Citation: Kanobe MN, Powell CY, Patrudu M, Baxter SA, Tapia MA, Darnell J, Prevette K, Gibson AG, Ayoku SA, Campbell L, Coffield JW, Keyser BM, Ganesh BS, Gale N and Jordan KG (2025) Randomized crossover clinical studies to assess abuse liability and nicotine pharmacokinetics of Velo Oral Nicotine pouches. Front. Pharmacol. 16:1547073. doi: 10.3389/fphar.2025.1547073
Received: 17 December 2024; Accepted: 11 February 2025;
Published: 13 March 2025.
Edited by:
Petra Scholze, Medical University of Vienna, AustriaCopyright © 2025 Kanobe, Powell, Patrudu, Baxter, Tapia, Darnell, Prevette, Gibson, Ayoku, Campbell, Coffield, Keyser, Ganesh, Gale and Jordan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milly N. Kanobe, a2Fub2JlbUByanJ0LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.