
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol. , 31 January 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1542231
This article is part of the Research Topic Herbal Medicines’ Safety and Clinical Application: New Strategies for Overcoming Therapeutic Challenges View all 7 articles
 He Yin1‡
He Yin1‡ Xin Chen1‡
Xin Chen1‡ Zhiwei Liu1,2‡
Zhiwei Liu1,2‡ Bo Xu1‡
Bo Xu1‡ Zhefeng Jin1
Zhefeng Jin1 Yan Liu3
Yan Liu3 Baoyu Qi1
Baoyu Qi1 Bin Tang1
Bin Tang1 Ping Wang4
Ping Wang4 Fanping Xu5
Fanping Xu5 Xu Wei1*†
Xu Wei1*† Jie Yu1*†
Jie Yu1*† Liguo Zhu1,6*†
Liguo Zhu1,6*†Objective: This randomized controlled trial aims to evaluate the efficacy and safety of Yishenyangsui granule for treating Degenerative Cervical Myelopathy.
Materials and methods: A randomized, double-blind, placebo-controlled clinical trial was conducted with 152 participants recruited from three centers and randomly assigned to receive either Yishenyangsui granule or placebo. The Japanese Orthopaedic Association (JOA) score and Neck Disability Index (NDI) score were evaluated for 32 weeks. Patient-reported outcomes including surgical treatment data, re-treatment data, and patient-reported condition were collected for long-term follow-up. This trial was approved by the ethics committee of WangJing Hospital of China Academy of Chinese Medical Sciences (WJEC-KT-2016-004-P001) and was registered at the Chinese Clinical Trials Registry (ChiCTR-INR-16009723) on 03 November 2016 (Check out at https://www.chictr.org.cn/indexEN.html for a more comprehensive overview).
Results: The results showed that the improvement in JOA score at week 8 was significantly better in the Yishenyangsui granule group than in the placebo group (1.47 vs. 0.43; P < 0.001). Furthermore, improvements in motor function of upper/lower extremities, sensory function of upper extremities, reading ability, and recreation domain scores were also significantly superior in the Yishenyangsui granule group compared to the placebo group (P < 0.05). Long-term follow-up outcomes revealed no statistical differences between groups regarding surgical treatment data or patient-reported condition (P > 0.05). However, there was a significant difference detected in re-treatment data between groups with a lower rate observed among those receiving Yishenyangsui granule compared to those receiving placebo [25 (43.10%) vs. 40 (68.97%); P = 0.033], indicating its effectiveness for treating mild-to-moderate Degenerative Cervical Myelopathy.
Conclusion: Yishenyangsui granule was effective in treating mild to moderate Degenerative Cervical Myelopathy. The participants have improved long-term outcomes.
Clinical Trials Registration: https://www.chictr.org.cn/indexEN.html, identifier ChiCTR-INR-16009723.
Degenerative cervical myelopathy (DCM) is a chronic non-traumatic disease of the spinal cord that is closely related to aging and caused by degenerative changes in the ligaments and osteochondral metabolites of the cervical spine (Theodore, 2020; Marie-Hardy and Pascal-Moussellard, 2021; Grodzinski et al., 2023). There are approximately 605 cases of clinical DCM per million people in North America (Tetreault et al., 2015). Progression of DCM could result in irreversible spinal cord damage and extremity disability and thus need an expensive operation as treatment (more than USD 2 billion per year) (Ghogawala et al., 2021; Milligan et al., 2019). The pathogenesis of DCM encompasses a multifaceted interplay of static and dynamic factors (Tu et al., 2021; Moghaddamjou et al., 2020). These factors collectively predispose to spinal cord injury (SCI), compromise the integrity of the spinal cord’s microvascular system, elicit an apoptosis cascade, and exacerbate the progression of DCM (Tran et al., 2018; Badhiwala et al., 2020). Hence, research on DCM-induced spinal cord injury focuses on the preservation of residual neuronal function and the facilitation of neuronal repair, which pose considerable challenges (Tanabe et al., 2011).
Non-surgical interventions also referred to as conservative treatments, presently employed in the management of DCM encompass physical therapy, spinal injections, immobilization via collars, and cervical traction (Kato and Fehlings, 2016; Fehlings et al., 2017; Davies et al., 2024). Compared to other non-surgical treatments, YSYS Granules offers a potential advantage by addressing both the underlying pathology and symptomatic relief of cervical myelopathy through a holistic approach. As a botanical drug, it is designed to promote neuroprotection, improve blood circulation, and enhance tissue repair, potentially offering benefits in both the mild and moderate stages of the condition (Bo-wen et al., 2022). Furthermore, its multi-target mechanism may complement other conservative therapies, providing an integrative option that combines efficacy with a favorable safety profile, particularly for patients seeking alternatives to pharmacological or physical interventions. According to the World Federation of Neurosurgical Societies Spine Committee, nonsurgical treatment is an important option for patients with mild to moderate DCM (Zileli et al., 2019) and, according to the guideline proposed by AO Spine and the Cervical Spine Research Society, nonsurgical treatment is also a feasible recommendation for treating mild DCM (Fehlings et al., 2017). However, evidence supporting the effectiveness of non-surgical interventions for DCM is limited. Although non-surgical treatment is recommended, its benefits and long-term results are still unclear due to lack of evidence, so a randomized, double-blind, placebo-controlled trial of conservative treatment is necessary (Wu et al., 2016).
The application of Traditional Chinese Medicine (TCM) for DCM has been extensively practiced, particularly in Asian countries. From the perspective of traditional Chinese medicine, DCM is caused by “deficiency” and “stasis” (Wen, 2022). Based on the pathophysiological mechanism of DCM and the characteristics of traditional Chinese medicine, combined with long-term clinical experience, the research team designed and created the Yishen Yangsui Granule (YSYS group) based on the classic traditional Chinese medicine Granule Dihuang Yinzi, which mainly consists of Gynochthodes officinalis (F.C. How) Razafim. and B. Bremer (9 g), Rehmannia glutinosa (Gaertn.) DC. (12 g), Paeonia lactiflora Pall. (12 g), Astragalus membranaceus Fisch. Ex Bunge (15 g), Cinnamomum burmannii (Nees and T. Nees) Blume (6 g), Salvia miltiorrhiza var. Miltiorrhiza (9 g), Euonymus alatus (Thunb.) Siebold (12 g), Notopterygium incisum Ting ex H.T. Chang (6 g), and Cervus nippon Temminck (12 g) (the plant names have been annotated by http://mpns.kew.org/mpns-portal/ on September. 25th, 2024).
Traditionally, the YSYS group is often thought to promote nerve health and reduce secondary damage (Bo-wen et al., 2022). In the initial phase of clinical randomized controlled trials, the research team corroborated the notable efficacy of the YSYS group in ameliorating the functionality of mild to moderate DCM patients (Zhiwei et al., 2022). However, its effect on DCM remains uncertain because of poor study designs and the lack of patient-reported long-term outcomes in previous studies. The purposes of this study were to estimate the efficacy of Yishenyangsui granules for improving functional ability and to explore the patient-reported long-term outcomes of treatment for mild to moderate DCM.
This multicentre, parallel groups randomized, double-blind, placebo-controlled trial was conducted at 3 hospitals in China. This trial was approved by the institutional review board at each site. Written informed consent was obtained from all participants. This trial was approved by the ethics committee of Wang Jing Hospital of China Academy of Chinese Medical Sciences (WJEC-KT-2016-004-P001) and was registered at the Chinese Clinical Trials Registry (ChiCTR-INR-16009723) on 03 November 2016 (Check out at https://www.chictr.org.cn/indexEN.html for a more comprehensive overview). The study followed the CONSORT Extension for Chinese Botanical Drugal Medicine Formula 2017 (CONSORT CHM 2017) reporting guideline (Cheng et al., 2017).
Volunteers were recruited via newspapers, websites, and hospital posters. Participants who met the following criteria were included:
(1) Meet the above diagnostic criteria.
(2) Age from 40 to 70 years old.
(3) Has signed informed consent and explicitly refused surgical treatment.
The exclusion criteria were as follows:
(1) Diagnosed with cervical tumors, tuberculosis, or osteomyelitis.
(2) Spinal injury, fracture, or dislocation.
(3) Suffering from severe heart, lung, brain, liver, kidney, hematological diseases, or mental disorders.
(4) Severe skin damage, infection, or dermatological diseases in the treatment area.
(5) History of cervical spine surgery or congenital cervical deformities.
(6) Clear surgical indications, including Frankel grade above D, incontinence, MRI showing spinal cord compression ratio <0.4, ineffective conservative treatment for over 3 months, and progressively worsening symptoms.
(1) Poor compliance by participants, including failure to follow the prescribed dosage or attend fewer than three treatment sessions.
(2) Loss to follow-up for any reason.
(3) Participant voluntarily withdraws from the clinical trial and informs the supervising doctor.
(4) Participants meeting any of the above criteria will be classified as withdrawal cases.
(1) Severe adverse events (life-threatening or significantly impairing daily life or work) that make continued treatment unfeasible.
(2) Complete symptom resolution during the treatment period, leading to treatment discontinuation.
(3) Severe and persistent allergic reactions necessitating the cessation of study treatment.
(4) Progressive symptom worsening during treatment, including incomplete paralysis or incontinence, meeting Frankel grade above D. These cases should terminate the study and consider surgical intervention.
Participants were randomly allocated to receive either a Yishenyangsui granule or a placebo. The randomization sequence was generated by an online central randomization system with a 1:1 allocation ratio. The treatment assignments, data entry, online randomization system, and scheme implementation were supervised by researchers at a tertiary service center.
Group allocation was concealed from the participants, therapists delivering the intervention, outcome assessors, and statisticians.
Yishenyangsui granules were prepared from 93 g of the following botanical drug substances: Bajitian, Morindae Officinalis Radix [Gynochthodes officinalis (F.C. How) Razafim. and B. Bremer], 9 g; Dihuang, Rehmanniae Radix [Rehmannia glutinosa (Gaertn.) DC.], 12 g; Lujiaoshuang, Cervi Cornu Degelatinatum [Cervus nippon Temminck], 12 g (Lujiaoshuang is obtained from the natural shedding of artificially raised sika deer after horn ossification. It is a renewable resource and does not affect the sika deer population.); Baishao, Paeoniae Radix Alba [Paeonia lactiflora Pall.], 12 g; Huangqi, Astragali Radix [Astragalus membranaceous Fisch. ex Bunge], 15 g; Guizhi, Cinnamomi Ramulus [Cinnamomum burmanni (Nees and T. Nees) Blume], 6 g; Danshen, Salviae Miltiorrhizae Radix et Rhizoma [Salvia miltiorrhiza var. Miltiorrhiza], 9 g; Guijianyu, Euonymus Alatus [Euonymus alatus (Thunb.) Siebold] 12 g; Qianghuo, Notopterygii Rhizoma et Radix [Notopterygium incisum Ting ex H.T.Chang], 6 g. These botanical drugs were decocted and then filtered to prepare a Granule solution. The Granule solution was concentrated and granulated with a recipient to form 9.15 g of granules. All botanical drugs were purchased and decocted by Sichuan New-Green Pharmaceutical Technology Development Co., Ltd. The plant name has been checked with http://mpns.kew.org/mpns-portal/ on September 25th, 2024 (Supplementary Table S1).
The placebo contained 85.0% maltodextrin, 5.0% caramel coloring, and a 10.0% mixture of Yishenyangsui granules. Yishenyangsui granules and the placebo were produced by the uniform quality control criteria by Sichuan New-Green Pharmaceutical Technology Development Co., Ltd. The entire study was conducted using the same batch of granules. Both the Yishenyangsui granule and the placebo were sealed in an aluminum foil sachet and were identical in outward appearance. The Yishenyangsui granules and the placebo were stored in an electronic cabinet at a temperature of 4°C and a humidity between 20% and 30%. The electronic cabinet was displayed in a special room for clinical trials and was managed by a qualified clinical pharmacist.
For ultra ultra-high performance liquid chromatography (UHPLC) analysis, sample pretreatment method: accurate water decoction (2 mL), addition of methanol (8 mL), high-speed mixing to extract (3 min), extract obtained (1 mL), 14,000 rpm speed centrifugation (10 min), supernatant obtained.
A Waters Acquity-Class ultra ultrahigh-performance liquid chromatography (UHPLC) tandem Waters Synapt G2-SiQ-TOF high-resolution mass spectrometry system was used to identify major metabolites in the YSYS group. The UHPLC fingerprint of the YSYS group tablet is shown in Supplementary Figure S1, and the HPLC test method is included in Supplementary Table S2.
Participants in the Yishenyangsui granule group (YSYS group) received Yishenyangsui granules (1 bag orally 2 times daily for 8 weeks). Participants in the placebo group received the placebo (1 bag orally 2 times daily for 8 weeks).
In the original scheme, participants were designated to receive treatments for 8 weeks, and then functional ability was assessed with the JOA scale and NDI scale (stage I) for the following 32 weeks. The long-term follow-up was designed to be performed every 1.5 years for a total of 5 years (stage Ⅱ).
According to the suggestions of the experts at the prestudy meeting, all participants were to receive mecobalamin tablets (Eisai China Holdings Ltd.) as routine treatment following the same administration scheme (0.5 mg orally 3 times daily for 8 weeks).
The primary outcome was the JOA score (Okada et al., 1993) at week 8. The JOA scale is an investigator-administered tool used to evaluate neurological function in patients with DCM. A score of 17 reflects no neurological deficit, whereas a lower score indicates a greater degree of disability and functional impairment.
The secondary outcomes were the JOA score at week 32 and the NDI (Neck Disability Index) scores at week 8 and week 32 (Vernon and Mior, 1991). The NDI scale is a composite of 10 items. Each item is scored out of 5 for a maximum total score of 50. A higher score indicates more severe dysfunction. The domain scores of the JOA scale, including motor function of the upper extremities and lower extremities, sensory function of the upper extremities, lower extremities and trunk, and bladder function, were analyzed as the secondary outcomes. The domain scores of the NDI scale, including neck pain intensity, personal care, lifting, reading, headache, concentration, work, sleeping, driving, and recreation, were also analyzed as the secondary outcomes.
Due to the impact of the coronavirus (COVID-19) pandemic, the first 1.5-year follow-up was missed, and we had to terminate the trial early and took the 3rd year patient-reported outcomes as the long-term follow-up results (stage Ⅱ) according to the long follow-up criteria guidelines (Fehlings et al., 2017) and the suggestions of the experts. During the stage II follow-up, patient-reported outcomes were collected by telephone interview, which included surgical treatment data, retreatment data, and patient-reported condition.
Adverse events were documented by participants and outcome assessors on a form throughout the trial. Based on their potential association with the ingestion of medication, adverse events were categorized by outcome assessors and related specialists as either treatment-related or nontreatment-related within 24 h of occurrence.
Based on the unpublished pilot data, a sample size of 152 participants was estimated to provide 90% power to detect a between-group difference of 2 points in the JOA score (Tetreault et al., 2016), assuming a standard deviation of 2.89, at the two-sided 0.05 significance level and a dropout rate of approximately 20%. A 2-point decrease in the total score was identified by the receiver operating characteristic curve as the optimal threshold, with high sensitivity, specificity, and discriminative ability to differentiate clinically important improvement (Tetreault et al., 2016).
The primary outcome (the JOA score at week 8) was analyzed using repeated measures of variance analysis. The secondary outcomes (the JOA score at week 32, the NDI score at week 8 and week 32, and the domain scores of the JOA scale and NDI scale at week 8 and week 32) were also analyzed with repeated measures analysis of variance. Retreatment data and patient-reported conditions were analyzed using Fisher’s exact test. Surgical treatment data (time-to-event outcome) were assessed using Kaplan‒Meier curves and were compared using the log-rank test.
All statistical analyses were performed according to the intention-to-treat principle using SPSS version 24.0, with a 2-sided P value less than 0.05 was considered to be significant. No adjustment was made for multiple comparisons; therefore, secondary outcomes should be interpreted as exploratory.
Participants were recruited between December 2016 and February 2018, with the final follow-up conducted from June 5 to 9 July 2021. A total of 359 participants were screened for eligibility, of whom 152 participants were randomly allocated to either the Yishenyangsui granule group or the placebo group. Among the randomized participants, 144 [mean (SD) age, 56.94 (8.97) years] received at least 1 dose of the study medication (Figure 1). The baseline characteristics were similar between the groups (Table 1).
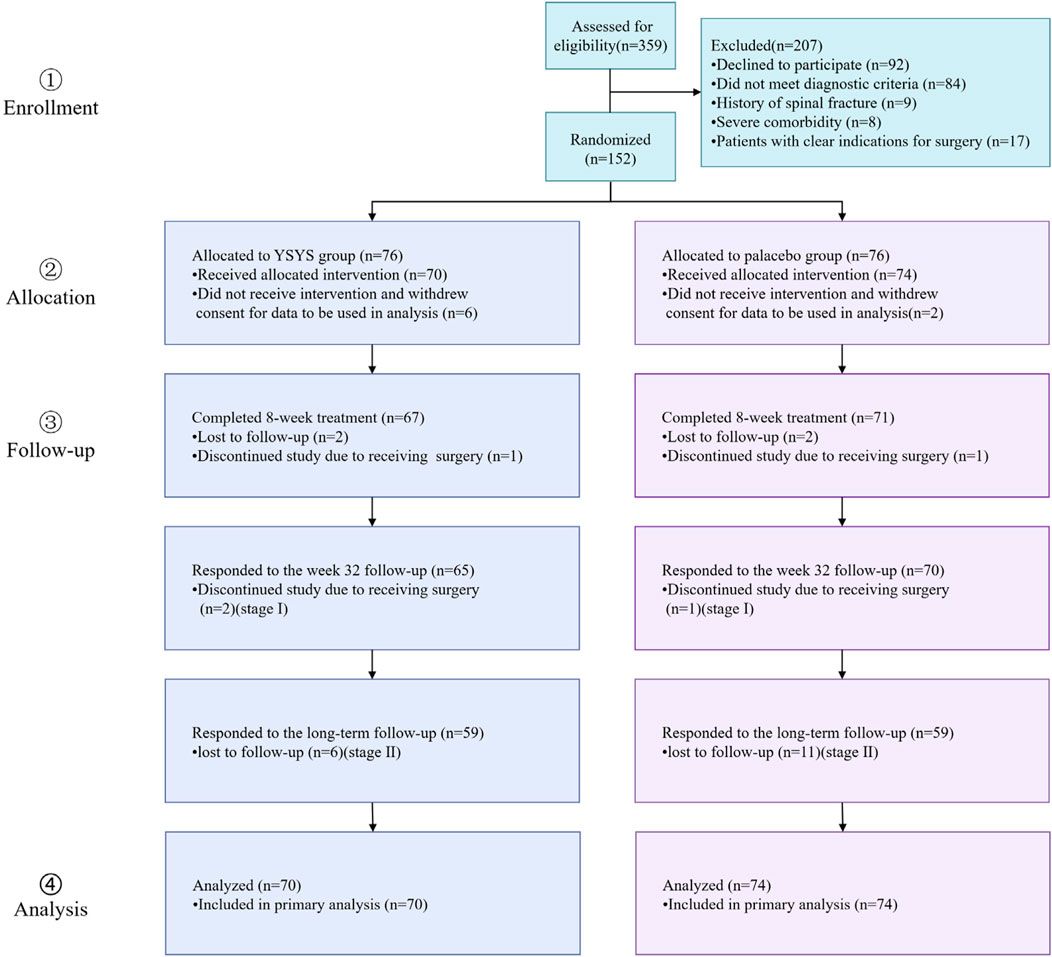
Figure 1. Flow diagram of progress through trial. Abbreviation: YSYS group, Yishenyangsui granule group.
For the primary outcome, the mean JOA score was 13.19 (95% CI, 12.77–13.60) at baseline and 14.66 (95% CI, 14.21–15.10) at week 8 in the Yishenyangsui granule group and 13.72 (95% CI, 13.31–14.12) at baseline and 14.15 (95% CI, 13.72–14.58) at week 8 in the placebo group. The change in JOA score at week 8 was greater in the Yishenyangsui granule group (1.47; 95% CI, 1.20–1.75) than in the placebo group (0.43; 95% CI, 0.16–0.70) (P < 0.001) (Table 2).
In terms of the secondary outcomes, similar results were observed in the change in JOA score at week 32. The change in JOA domain scores of motor function in the upper extremities/motor function in the lower extremities/sensory function in the upper extremities in the Yishenyangsui granule group was significantly greater than that in the placebo group (Table 2). The reduction in the reading and recreation domain score in the Yishenyangsui granule group was also significantly greater than that in the placebo group (Table 2). The trend of changes in JOA score and NDI score over time is presented in Figures 2, 3.
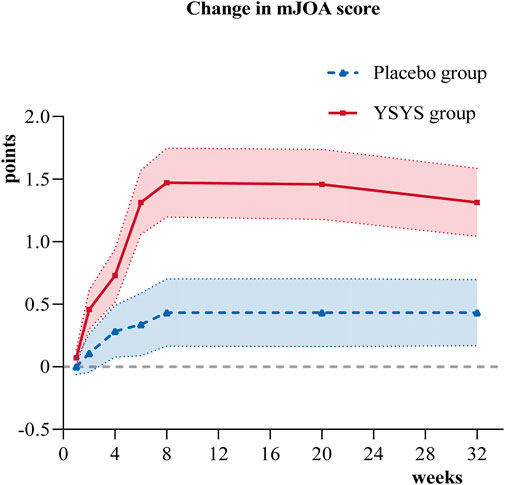
Figure 2. Change in JOA score over time. Abbreviation: YSYS group, Yishenyangsui granule group. The solid red line represents the YSYS group. The Blue dotted line represents the placebo group.
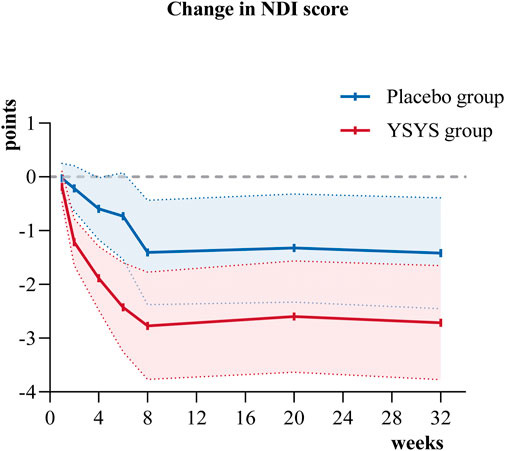
Figure 3. Change in NDI score over time. Abbreviation: YSYS group, Yishenyangsui granule group. The solid red line represents the YSYS group. The solid blue line represents the placebo group.
In terms of the long-term outcome (stage Ⅱ), 59 participants in each group participated in the long-term follow-up, with a median follow-up duration of 43.60 months (95% CI, 42.894–44.306). Twenty-five (43.10%) patients in the Yishenyangsui granule group required retreatment, which was less than the 40 (68.97%) patients requiring retreatment in the placebo group (Table 3). There was no significant difference in the patient-reported condition between the two groups (Table 3). The proportions of patients who underwent surgical treatment in the Yishenyangsui granule group and the placebo group were 22.03% (13/59) and 23.73% (14/59), respectively. The mean time from intervention initiation to surgical treatment in the Yishenyangsui granule group [48.373 months (95% CI, 44.674–52.092)] was longer than that of the placebo group [48.177 months (95% CI, 44.417–51.937)] (Table 3; Figure 4).
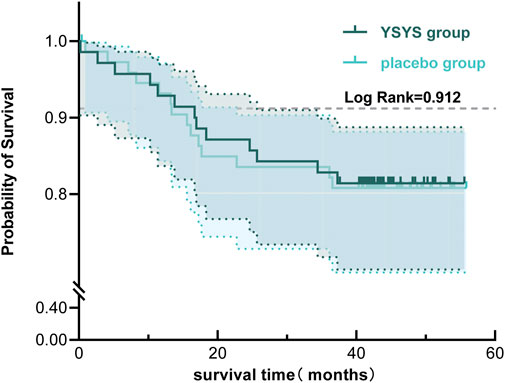
Figure 4. Kaplan-Meier Curve of Time from initiation of the intervention to occurrence of receiving surgical treatment for the two groups. Abbreviation: YSYS group, Yishenyangsui granule group. The solid green line represents the YSYS group. The solid blue line represents the placebo group.
During treatment, adverse events occurred in 4 patients in the placebo group and 11 patients in the Yishenyangsui granule group, mainly mild adverse events such as cold, pharyngitis, cough, and diarrhea, which were categorized as either not related (12 cases) or unlikely related (3 cases) to ingestion of medication by the researchers. All of the adverse events occurred or disappeared in a short period. No participant discontinued treatment or withdrew due to adverse events.
Traditional Chinese Medicine concepts of “deficiency” and “stasis” match modern medical treatments for degenerative cervical myelopathy, showing that neurodegeneration and impaired circulation are related. “Deficiency,” notably in Qi and Kidney essence, refers to neuronal vitality and functional degradation (Du et al., 2020; Li et al., 2015), while “stasis” refers to blood stasis and decreased microcirculation, which modern medicine links to inflammation, oxidative stress, and tissue healing (Xia et al., 2021; Delgado-Montero et al., 2020). These ideas combine TCM philosophy with modern biological treatments for this illness to promote neuron regeneration, reduce inflammation, and improve blood circulation. Over the 8-week treatment period, the Yishenyangsui granules produced greater improvement in the JOA score and greater improvement in retreatment data for the long-term follow-up than the placebo. The effects continued for another 24 weeks after treatment. The incidence rate of adverse events was low.
The JOA scale was used to evaluate neurological function directly. In this study, the Yishenyangsui granule group revealed a better JOA score improvement from the initial visit to the endpoint, with an initial score of 13.19 (95% CI, 12.77–13.60) and an endpoint score of 14.66 (95% CI, 14.21–15.10). In a prospective cohort study of DCM patients who exhibited a decreased JOA score from 14.5 (SD, 1.3) at the initial visit to 14.1 (SD, 2.2) (Sumi et al., 2012) at the endpoint after 6–14 years of follow-up, the patients initially received in-bed Good Samaritan cervical traction for 8 h a day over 2 weeks and then were given ongoing details about their pathological condition every 3–6 months before being advised to take precautions to prevent aggravation of their myelopathy to avoid the need for surgical intervention. Other studies on DCM patients who were treated conservatively showed a JOA score improvement from 14.3 (SD, 1.3) to 14.5 (SD, 1.8) after a 2-year follow-up (Kadaňka et al., 2000) and a further increase to 15.0 (95% CI, 12.2–18.0) after a 10-year follow-up (Kadaňka et al., 2011). The curve in Figure 2 indicates that the Yishenyangsui granule began to play a role from week 2, and the improvement effect reached a maximum at week 8.
The long-term follow-up outcomes in this trial are indicative of the “natural progression” of DCM, which is defined in the literature (Matz et al., 2009) as progression with no treatment or with only nonoperative therapy. The retreatment rate after recurrence in the Yishenyangsui granule group (43.10%) was less than that in the placebo group (68.97%). For patient-reported conditions, this paper revealed that the average proportion of subjects showing improvement in the two groups was 23.73% (28/118), and the proportion of subjects remaining stable and showing deterioration was 49.15% (58/118) and 27.12% (32/118), respectively, which were slightly different from the results reported in the literature. A prospective study with a 3-year follow-up indicated that more than 80% of the patients remained stable after nonsurgical treatment (Tetreault et al., 2015; Shimomura et al., 2007). Roberts (Roberts, 1966; Choi, 2020) reported that one-third of DCM patients who underwent collar immobilization showed improvement, while one-third of them did not experience any improvement, and the remaining one-third experienced a worsened motor deficit at 6.5 years after treatment. Professor Kadaňka (Kadaňka et al., 2011) believes that while the natural progression of DCM is unclear at present, the trend is that an equal proportion of patients may either improve, remain stable, or worsen. The average incidence rate of surgical treatment in the two groups in this study was 22.88% (27/118), and the minimum follow-up duration was 40.30 months. This was roughly the same as reports in the literature in that approximately 20% of patients receiving nonsurgical treatment had to undergo surgical treatment during the follow-up (Zileli et al., 2019), which was lower than the 23%–54% previously reported in the systematic review (Ghobrial and Harrop, 2015). The lack of progress in improving surgical treatment outcomes and patient-reported conditions may be due to patients stopping the administration of granules after 8 weeks. Furthermore, DCM is characterized by a wide range of causes, severity levels, and natural progression in spinal cord damage. In addition to this intricate nature, there is a lack of first indicators and dependable biomarkers that can determine the extent of damage to cerebral tissue, which is crucial for designing therapeutic trials. Therefore, further research is needed to determine whether it is better if the patients take the granule at regular intervals for a longer period.
The mechanism of TCM in the treatment of DCM is complex (Choi, 2020; Ulndreaj et al., 2022; McCormick et al., 2020). Network pharmacology research (Baoyu et al., 2020) indicated that the key targets of the Yishenyangsui granule in the treatment of DCM were STAT3, AKT1, JUN, MAPK1, IL-6, and RELA. By studying the course of chronic spinal cord compression in experimental rat models, it was found that Yishenyangsui granule had a good therapeutic effect (Bowen et al., 2022), and the mechanism might be related to promoting the repair of damaged neurons, regeneration of local spinal microvessels, improving microcirculation disturbance (Bin et al., 2022), increasing the expression of BDNF, and reducing the apoptosis of damaged neurons by inhibiting the expression of key proteins in the Fas/fasl-caspase-8/-3 signaling pathway (Dian et al., 2024). In addition, a large number of basic studies have shown that the main metabolites of the YSYS group can improve spinal cord injury. Tanshinone IIA is acknowledged as a neuroprotective drug that markedly diminishes peroxidation-induced cellular death at the molecular level (Sherawat and Mehan, 2023; Jia et al., 2023; Luo et al., 2022). Yin et al. (2012) used tanshinone to improve microcirculation, thereby exerting a neuroprotective effect. Lin et al. (2020) confirmed that astragaloside inhibits neuronal apoptosis and promotes spinal cord injury repair. Our prior animal studies have also shown that the YSYS group enhanced neurological function and decreased neuroinflammation in rats with spinal cord injury (Supplementary Figures S2, S3). The long-term follow-up results corroborate these findings, demonstrating that YSYS Granules substantially enhance long-term neurological function and diminish the retreatment rate, likely attributable to its neuroprotective, anti-inflammatory, and vascular regeneration processes.
Further research is required to elucidate the mechanism of Yishenyangsui granules. Consequently, subsequent studies should prioritize experimental validation to furnish more definitive proof regarding the efficacy of the YSYS group in the treatment of DCM. While the randomized, double-blind, placebo-controlled experiment can demonstrate the efficacy of the YSYS group in treating DCM, it cannot completely capture their pharmacological and biological actions within the human body. Consequently, additional studies should investigate the precise mechanisms of action of the primary active constituents of the YSYS group in DCM treatment, along with the potential adverse effects associated with their application.
The YSYS group demonstrated efficacy in the treatment of mild and moderate DCM. The YSYS group consists of many herbal components, some of which may exert numerous effects on the development and progression of DCM. In this randomized, double-blind, placebo-controlled trial, the YSYS group demonstrated efficacy in treating mild to moderate DCM. It also showed enhancements in the retreatment rate and neurological function compared to the placebo group. Despite the lack of statistical significance in enhancing surgical treatment outcomes and patient-reported conditions, the YSYS group seems to be a viable option for the treatment of mild to moderate DCM. Additional research including bigger cohorts and patients with varying degrees of severity may be beneficial in determining the efficacy of the YSYS group in DCM pathology.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the WangJing Hospital of China Academy of Chinese Medical Sciences (WJEC-KT-2016-004-P001). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. XC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. ZL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. ZJ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BQ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. BT: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. PW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. FX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. XW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JY: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. LZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The self-selected project of Wangjing Hospital, Chinese Academy of Medical Sciences (Approval No. WJYY-ZZXT-2023-22); Literature collection and classification of spinal degenerative diseases (Approval No. 2021YFC1712801); Innovation Team of Traditional Chinese Medicine and Talent Support Program-Innovation Team of Traditional Chinese Medicine Prevention and Treatment of Bone and Joint Degenerative Diseases (Approval No. CI2021B010); the National Administration of Traditional Chinese Medicine (No. 201407001-11).
All authors express their gratitude to Wangjing Hospital of China Academy of Chinese Medical Sciences for supporting this project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1542231/full#supplementary-material
JOA, Japanese Orthopaedic Association; NDI, Neck Disability Index; DCM, Degenerative Cervical Myelopathy; YSYS group, Yishenyangsui granule group; BDNF, Brain-derived neurotrophic factor; STAT3, Signal Transducer And Activator of Transcription 3; AKT1, Alpha serine/threonine-protein kinase; MAPK1, Mitogen-activated protein kinase 1; IL-6, Interleukin 6.
Badhiwala, J. H., Ahuja, C. S., Akbar, M. A., Witiw, C. D., Nassiri, F., Furlan, J. C., et al. (2020). Degenerative cervical myelopathy - update and future directions. Nat. Rev. Neurol. 16 (2), 108–124. doi:10.1038/s41582-019-0303-0
Baoyu, Q., Kai, S., He, Y., Liguo, Z., Zhefeng, J., Xin, C., et al. (2020). Study on the mechanism of yishen Yangsui decoction in the treatment of spinal cord injury based on network pharmacology. Mod. Traditional Chin. Med. Materia Medica-World Sci. Technol. 22 (09), 3178–3190. doi:10.11842/wst.20190727005
Bin, T., Liguo, Z., Xin, C., Bowen, Y., Xiaokuan, Q., Zhefeng, J., et al. (2022). Effects of yishen Yangsui prescription on microvascular regeneration of damaged spinal cord in rat model of cervical spondylotic myelopathy. Chin. J. Inf. Traditional Chin. Med. 29 (05), 51–55. doi:10.19879/j.cnki.1005-5304.202108429
Bo-wen, Y., He, Y., Min-shan, F., Xin, C., Zhe-feng, J., Kai, S., et al. (2022). Discussion on treatment of cervical spondylotic myelopathy with yishen Yangsui Formula. Beijing J. Traditional Chin. Med. 41 (12), 1414–1416. doi:10.16025/j.1674-1307.2022.12.021
Bowen, Y., Liguo, Z., Minshan, F., Xu, W., Zhefeng, J., Xin, C., et al. (2022). Study on the dose-effect relationship of yishen Yangsui Formula in the treatment of cervical spondylotic myelopathy. China Pharm. 25 (04), 620–625. doi:10.19962/j.cnki.issn1008-049X.2022.04.010
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). Consort extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration. Ann. Intern. Med. 167 (2), 112–121. doi:10.7326/m16-2977
Choi, S. H., Degenerative Cervical Myelopathy, Kang CN, Pathophysiology, and Strategies, Current Treatment (2020). Asian Spine J. 14 (5), 710–720. doi:10.31616/asj.2020.0490
Davies, B. M., Yang, X., Khan, D. Z., Mowforth, O. D., Touzet, A. Y., Nouri, A., et al. (2024). A minimum data set-core outcome set, core data elements, and core measurement set-for degenerative cervical myelopathy research (ao spine recode dcm): a consensus study. PLoS Med. 21 (8), e1004447. doi:10.1371/journal.pmed.1004447
Delgado-Montero, A., Martinez-Legazpi, P., Desco, M. M., Rodríguez-Pérez, D., Díaz-Otero, F., Rossini, L., et al. (2020). Blood stasis imaging predicts cerebral microembolism during acute myocardial infarction. J. Am. Soc. Echocardiogr. official Publ. Am. Soc. Echocardiogr. 33 (3), 389–398. doi:10.1016/j.echo.2019.09.020.
Dian, Z., Liguo, Z., Xunlu, Y., Bowen, Y., Xiaokuan, Q., Zhefeng, J., et al. (2024). Effect of yishen Yangsui Formula on apoptosis in the spinal cord of rats with cervical spondylotic myelopathy. Chin. J. Traditional Med. Traumatology. Orthop. 32 (01), 1–6. doi:10.20085/j.cnki.issn1005-0205.240101
Du, Q., He, D., Zeng, H. L., Liu, J., Yang, H., Xu, L. B., et al. (2020). Siwu paste protects bone marrow hematopoietic function in rats with blood deficiency syndrome by regulating tlr4/nf-κb/nlrp3 signaling pathway. J. Ethnopharmacol. 262, 113160. doi:10.1016/j.jep.2020.113160.
Fehlings, M. G., Tetreault, L. A., Riew, K. D., Middleton, J. W., Aarabi, B., Arnold, P. M., et al. (2017). A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Glob. Spine J. 7 (3 Suppl. l), 70s–83s. doi:10.1177/2192568217701914
Ghobrial, G. M., and Harrop, J. S. (2015). Surgery vs conservative care for cervical spondylotic myelopathy: nonoperative operative management. Neurosurgery 62, 62–65. doi:10.1227/neu.0000000000000816
Ghogawala, Z., Terrin, N., Dunbar, M. R., Breeze, J. L., Freund, K. M., Kanter, A. S., et al. (2021). Effect of ventral vs dorsal spinal surgery on patient-reported physical functioning in patients with cervical spondylotic myelopathy: a randomized clinical trial. JAMA 325 (10), 942–951. doi:10.1001/jama.2021.1233
Grodzinski, B., Stubbs, D. J., and Davies, B. M. (2023). Most degenerative cervical myelopathy remains undiagnosed, particularly amongst the elderly: modelling the prevalence of degenerative cervical myelopathy in the United Kingdom. J. neurology 270 (1), 311–319. doi:10.1007/s00415-022-11349-8
Jia, Z., Wen, T., and Zhang, Y. (2023). Possible mechanisms of treatment for spinal cord injury repair with tanshinone iia. Front. Mol. Neurosci. 16, 1277755. doi:10.3389/fnmol.2023.1277755
Kadaňka, Z., Bednařík, J., Novotný, O., Urbánek, I., and Dušek, L. (2011). Cervical spondylotic myelopathy: conservative versus surgical treatment after 10 years. Eur. Spine J. 20 (9), 1533–1538. doi:10.1007/s00586-011-1811-9
Kadaňka, Z., Bednařík, J., Voháňka, S., Vlach, O., Stejskal, L., Chaloupka, R., et al. (2000). Conservative treatment versus surgery in spondylotic cervical myelopathy: a prospective randomised study. Eur. Spine J. 9 (6), 538–544. doi:10.1007/s005860000132
Kato, S., and Fehlings, M. (2016). Degenerative cervical myelopathy. Curr. Rev. Musculoskelet. Med. 9 (3), 263–271. doi:10.1007/s12178-016-9348-5
Li, P. L., Sun, H. G., Hua, Y. L., Ji, P., Zhang, L., Li, J. X., et al. (2015). Metabolomics study of hematopoietic function of angelica sinensis on blood deficiency mice model. J. Ethnopharmacol. 166, 261–269. doi:10.1016/j.jep.2015.03.010
Lin, J., Pan, X., Huang, C., Gu, M., Chen, X., Zheng, X., et al. (2020). Dual regulation of microglia and neurons by astragaloside iv-mediated Mtorc1 suppression promotes functional recovery after acute spinal cord injury. J. Cell. Mol. Med. 24 (1), 671–685. doi:10.1111/jcmm.14776
Luo, D., Li, X., Hou, Y., Hou, Y., Luan, J., Weng, J., et al. (2022). Sodium tanshinone iia sulfonate promotes spinal cord injury repair by inhibiting blood spinal cord barrier disruption in vitro and in vivo. Drug Dev. Res. 83 (3), 669–679. doi:10.1002/ddr.21898
Marie-Hardy, L., and Pascal-Moussellard, H. (2021). Degenerative cervical myelopathy. Rev. Neurol. 177 (5), 490–497. doi:10.1016/j.neurol.2020.11.015
Matz, P. G., Anderson, P. A., Holly, L. T., Groff, M. W., Heary, R. F., Kaiser, M. G., et al. (2009). The natural history of cervical spondylotic myelopathy. J. Neurosurg. Spine SPI 11 (2), 104–111. doi:10.3171/2009.1.SPINE08716
McCormick, J. R., Sama, A. J., Schiller, N. C., Butler, A. J., and Donnally, C. J. (2020). Cervical spondylotic myelopathy: a guide to diagnosis and management. JABFM 33 (2), 303–313. doi:10.3122/jabfm.2020.02.190195
Milligan, J., Ryan, K., Fehlings, M., and Bauman, C. (2019). Degenerative cervical myelopathy: diagnosis and management in primary care. Can. family physician Med. famille Can. 65 (9), 619–624.
Moghaddamjou, A., Wilson, J. R. F., Martin, A. R., Gebhard, H., and Fehlings, M. G. (2020). Multidisciplinary approach to degenerative cervical myelopathy. Expert Rev. Neurother. 20 (10), 1037–1046. doi:10.1080/14737175.2020.1798231
Okada, Y., Ikata, T., Yamada, H., Sakamoto, R., and Katoh, S. (1993). Magnetic resonance imaging study on the results of surgery for cervical compression myelopathy. Spine 18 (14), 2024–2029. doi:10.1097/00007632-199310001-00016
Roberts, A. H. (1966). Myelopathy due to cervical spondylosis treated by collar immobilization. Neurology 16 (9), 951. doi:10.1212/WNL.16.9.951
Sherawat, K., and Mehan, S. (2023). Tanshinone-iia mediated neuroprotection by modulating neuronal pathways. Naunyn-Schmiedeberg’s Archives Pharmacol. 396 (8), 1647–1667. doi:10.1007/s00210-023-02476-8
Shimomura, T., Sumi, M., Nishida, K., Maeno, K., Tadokoro, K., Miyamoto, H., et al. (2007). Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine 32 (22), 2474–2479. doi:10.1097/BRS.0b013e3181573aee
Sumi, M., Miyamoto, H., Suzuki, T., Kaneyama, S., Kanatani, T., and Uno, K. (2012). Prospective cohort study of mild cervical spondylotic myelopathy without surgical treatment: clinical article. J. Neurosurg. Spine SPI 16 (1), 8–14. doi:10.3171/2011.8.SPINE11395
Tanabe, F., Yone, K., Kawabata, N., Sakakima, H., Matsuda, F., Ishidou, Y., et al. (2011). Accumulation of p62 in degenerated spinal cord under chronic mechanical compression: functional analysis of p62 and autophagy in hypoxic neuronal cells. Autophagy 7 (12), 1462–1471. doi:10.4161/auto.7.12.17892
Tetreault, L., Goldstein, C. L., Arnold, P., Harrop, J., Hilibrand, A., Nouri, A., et al. (2015). Degenerative cervical myelopathy: a spectrum of related disorders affecting the aging spine. Neurosurgery 77, S51–S67. doi:10.1227/neu.0000000000000951
Tetreault, L., Wilson, J. R., Kotter, M. R. N., Nouri, A., Côté, P., Kopjar, B., et al. (2016). Predicting the minimum clinically important difference in patients undergoing surgery for the treatment of degenerative cervical myelopathy. Neurosurg. Focus FOC 40 (6), E14. doi:10.3171/2016.3.FOCUS1665
Theodore, N. (2020). Degenerative cervical spondylosis. N. Engl. J. Med. 383 (2), 159–168. doi:10.1056/NEJMra2003558
Tran, A. P., Warren, P. M., and Silver, J. (2018). The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98 (2), 881–917. doi:10.1152/physrev.00017.2017
Tu, J., Vargas Castillo, J., Das, A., and Diwan, A. D. (2021). Degenerative cervical myelopathy: insights into its pathobiology and molecular mechanisms. J. Clin. Med. 10 (6), 1214. doi:10.3390/jcm10061214
Ulndreaj, A., Ávila, A., Hong, J., Zhou, C., Fehlings, M. G., and Vidal, P. M. (2022). Acute systemic white blood cell changes following degenerative cervical myelopathy (dcm) in a mouse model. Int. J. Mol. Sci. 23 (19), 11496. doi:10.3390/ijms231911496
Vernon, H., and Mior, S. (1991). The neck disability Index: a study of reliability and validity. J. Manip. Physiol. Ther. 14 (7), 409–415.
Wen, M. (2022). Interpretation of expert consensus on diagnosis and treatment of cervical spondylotic myelopathy with integrated traditional Chinese and western medicine. Shanghai J. Traditional Chin. Med. 56 (07), 14–17. doi:10.16305/j.1007-1334.2022.2203078
Wu, Z. K., Zhao, Q. H., Tian, J. W., Qian, Y. B., Zhou, Y., Yang, F., et al. (2012). Anterior versus posterior approach for multilevel cervical spondylotic myelopathy: cochrane database. Syst. Rev. doi:10.1002/14651858.CD012365
Xia, W., Hu, S., Wang, M., Xu, F., Han, L., and Peng, D. (2021). Exploration of the potential mechanism of the tao hong Si Wu decoction for the treatment of postpartum blood stasis based on network pharmacology and in vivo experimental verification. J. Ethnopharmacol. 268, 113641. doi:10.1016/j.jep.2020.113641
Yin, X., Yin, Y., Cao, F.-L., Chen, Y.-F., Peng, Y., Hou, W.-G., et al. (2012). Tanshinone iia attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 7 (6), e38381. doi:10.1371/journal.pone.0038381
Zhiwei, L., He, Y., Xin, C., Zhefeng, J., Jie, Y., Xu, W., et al. (2022). Efficacy of Yishenyangsui granule on the treatment of mild to moderate cervical spondylotic myelopathy. Chin. J. Traditional Med. Traumatology. Orthop. 30 (08), 36–40.
Keywords: degenerative cervical myelopathy, non-surgical treatment, Chinese botanical drugs, long-term follow-up, cervical spondylotic myelopathy
Citation: Yin H, Chen X, Liu Z, Xu B, Jin Z, Liu Y, Qi B, Tang B, Wang P, Xu F, Wei X, Yu J and Zhu L (2025) Yishenyangsui granule for degenerative cervical myelopathy: a randomized, double-blind, placebo-controlled trial with long-term follow-up. Front. Pharmacol. 16:1542231. doi: 10.3389/fphar.2025.1542231
Received: 09 December 2024; Accepted: 13 January 2025;
Published: 31 January 2025.
Edited by:
Yang Zhao, Nanjing University of Chinese Medicine, ChinaReviewed by:
Liming Lu, Guangzhou University of Chinese Medicine, ChinaCopyright © 2025 Yin, Chen, Liu, Xu, Jin, Liu, Qi, Tang, Wang, Xu, Wei, Yu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Wei, d2VpeHUuMDA3QDE2My5jb20=; Jie Yu, eXVqaWUyMjZAc29odS5jb20=; Liguo Zhu, emhsZzk1QGFsaXl1bi5jb20=
†ORCID: Xu Wei, orcid.org/0000-0001-6723-9604; Jie Yu, orcid.org/0009-0003-2952-0587; Liguo Zhu, orcid.org/0009-0001-2666-6519
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.