
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 07 April 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1534748
 Zhen Zhou1
Zhen Zhou1 Hui-Min Gao2
Hui-Min Gao2 Zhao-Qin Pei3
Zhao-Qin Pei3 Meng-Xiang Sha1
Meng-Xiang Sha1 Cong-Ying Li1
Cong-Ying Li1 Yi Zhang1
Yi Zhang1 Jin-Song Su3*
Jin-Song Su3* Yue Liu1*
Yue Liu1* Xian-Li Meng4*
Xian-Li Meng4*Introduction: Tiebangchui (TBC, Tibetan name: བང་ང་ནག་པ།), the dried tuberous root of Aconitum pendulum Busch. and Aconitum flavaum Hand.-Mazz., is a prevalent used Tibetan medicine, recognized for its significant therapeutic effects despite its high toxicity. It is commonly employed in treating the diseases categorized as “Long” (རླུང་ནད།), cold, “Huang-shui” (སེར་ཆུ་ནད།), leprosy, and mania in Tibetan medicine. Notably, it is utilized in the treatment of rheumatoid arthritis, which is classified under the “Huang-shui” disease category according to Tibetan medical theory. Given its considerable toxicity, various processing techniques aimed at reducing the harmful effects of TBC are essential for its safe application in clinical settings. Hezi-decoction-processed method is a distinctive and effective traditional processing method of Tibetan medicine, but the overall variability of chemical constituents in the Hezi-decoction-processed TBC is still unclear. This investigation sought to examine a variety of diterpenoid alkaloids and tanning constituents, identify potential metabolic markers for differentiating the unprocessed TBC and Hezi-decoction-processed TBC at varying processing times, and determine the optimal processing time for reducing toxicity and maintaining efficacy.
Methods: A combination of metabolomic techniques was developed, integrating ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) with desorption electrospray ionisation mass spectrometry imaging (DESI-MSI) coupled with quantitative analytical techniques. This was done with the objective of monitoring the dynamic alterations in chemical constituents in TBC during the processing time. Metabolic markers were observed via DESI-MSI, and three alkaloids and five tannin acids were quantified through the use of UPLC and HPLC.
Results: Fifty-one compounds were identified in unprocessed TBC and processed samples, of which 31 were discernible from unprocessed TBC. A total of 22 metabolic markers, such as aconine, aconitine, benzoylaconine, chebulic acid, gallic acid, and corilagin, can proficiently distinguish between raw and processed TBC with different processing times. And the results of content determination of three alkaloids and five tannins showed that they were stabilized at 72 h. The monoester-diterpenoid alkaloids (MDAs) and diester-diterpenoid alkaloids (DDAs) levels were 0.0149% and 0.0852% in 72 h, respectively. The contents of gallic acid, corilagin, 1,2,3,4,6-O-pentagalloylglucose, chebulinic acid, and ellagic acid were 8.9706, 9.3444, 1.2438, 5.7582, and 3.1160 mg/g, respectively. The distribution and accumulation of metabolic markers during processing were investigated by DESI-MS. The results of DESI-MSI were consistent with those of content determination experiments. Combined with the multivariate statistical analysis, content determination of three alkaloids and five tannin acids and DESI-MSI, 72 h is demonstrated to be the appropriate time for toxicity attenuation and efficacy reservation of TBC.
Discussion: The implementation of this technique could contribute to the identification of markers in Hezi decoction-processed TBC and the establishment of effective quality control and evaluation procedures to ensure the safety of TBC. The proposed method has the potential to elucidate the processing mechanism of Aconitum medicines and other toxic traditional Chinese medicines, given its wide applicability.
The Tibetan medicine Tiebangchui (TBC, Tibetan name: བང་ང་ནག་པ།) is the dried tuberous root of Aconitum pendulum Busch. and Aconitum flavaum Hand.-Mazz. in the genus Aconitum of the Ranunculaceae family (Li et al., 2022; Li et al., 2024). The classic works of Tibetan medicine, Jing Zhu Materia Medica, document that TBC has a slight gas, bitter taste, and hemp. TBC therapeutic effects include the promotion of blood circulation and the dissolution of blood stasis, the dispersion of wind and the removal of dampness, and the attenuation of swelling and the alleviation of pain (Wang et al., 2016). TBC is widely used in treating “Long” (རླུང་ནད།), cold, “Huang-shui” (སེར་ཆུ་ནད།), leprosy, and mania in Tibetan medicine. Rheumatoid arthritis, which belongs to the “Huang-shui” disease category in Tibetan medicine theory, can be treated using the roots and seedlings of TBC. According to the Four Volumes of Medical Treatment (Yi Liao Si Bu Lun), “Huang-shui” is caused by the bile essence produced by the three tastes of the human body after the Stomach Fire digests, decomposes, and absorbs the nuances of food (Di-Si-Sang-Ji-Jia-Cuo, 1983; GYu-thogrNying-maYon-tanmGon-po, 1983). When the influence of multiple internal and external pathogenic factors leads to the imbalance of the three stomach fires in the human body, “Huang-shui” forms the Huang-shui disease. Pathological accumulation of “Huang-shui” in the joints can cause arthropathy, including rheumatoid arthritis (Su et al., 2023). TBC possesses significant toxicities, and improper usage may result in neurological, cardiovascular, and gastrointestinal irritation reactions, which usually manifest as heart pain, irregular heartbeat, numbness, and shortness of breath (Chan, 2016; Li S. et al., 2023). The primary constituents of TBC consist of Aconitum alkaloids, which encompass C19-, C20-, and various other bis-diterpenoid alkaloids (Li et al., 2022). These compounds were systematically categorized into three main categories based on their toxicity: high-toxicity diester-diterpenoid alkaloids (DDAs), moderate-toxicity monoester-diterpenoid alkaloids (MDAs), and nontoxic non-esterified diterpene alkaloids (NDAs). Among them, the specific compounds of DDAs are aconitine, 3-deoxyaconitine, mesaconitine and hypaconitine; MDAs such as benzoyl aconitine, benzoylmesaconitine and benzoylhypaconitine are well known; and the representative compounds of NDAs are aconine, mesaconine and hypaconine. These classes represent the active and toxic components of the alkaloids (Liu et al., 2017; Liu et al., 2022; Qiu et al., 2021).
TBC was characterized as “the head of the immovable poison” and underscores the necessity of processing to reduce its toxicity, according to “Jing Zhu Materia Medica” (Wang et al., 2010). Studies have shown that the alkaloid content responsible for toxicity changed significantly after processing, so processing methods that effectively attenuate toxicity and reserve the efficacy to use TBC are essential to ensure clinical safety (Wang Y. et al., 2010; Wang Y. J. et al., 2010). From ancient times to the present day, numerous records of processing methods of TBC can be found, among which the TBC processed with Hezi decoction is a special traditional one (Cao et al., 2024). Hezi (dried fruit of Terminalia chebula Retz.) is a widely used traditional Tibetan medicine, frequently designated the “king of medicines.” As a particularly good remedy for aconite poisoning, Hezi is widely employed in Tibetan medical formulations, including Shiwei Hezi San and Wuwei zuoxiang wan, due to its diverse medicinal activities and capacity for detoxification. The chebulic acid in Hezi could prevent the hydrolysis of aconitine during the brewing process and block TRPV1 channels, thereby resisting cardiotoxicity (Liu et al., 2023). Nevertheless, the overall variability of metabolites in Hezi-decoction-processed TBC is still unclear, and there is a notable absence of a unified investigation into the alterations in the chemical constituents of the botanical drugs corresponding to varying decoction durations. Therefore, revealing the main differential chemical metabolites in the concoction process and discovering their relationships with different concoction times could offer a scientific foundation for the control and optimization of the processed technique of TBC.
The complexity and variability of plant secondary metabolites is a major challenge in traditional Chinese medicine research, and traditional analytical methods can hardly meet the requirements of simultaneous analysis of different types of chemical components. Metabolomics is based on high-resolution and high-throughput detection platforms that comprehensively analyze metabolites in organisms and screen out metabolites with remarkable differences to study metabolic processes and change mechanisms (Tian et al., 2022). The fundamental principle of Chinese medicinal formulations lies in the alteration of chemical composition, and the use of metabolomics to search for potential metabolic differences between botanical drugs before and after preparations provides a material basis for the quality differences between Chinese medicine before and after preparations, which is conducive to finding the optimal preparations and elucidating the safety and scientificity of the preparations. Metabolomics has been widely used to study the changes in botanical drugs during manufacturing (Yang et al., 2023). For example, ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS)-based metabolomics was employed for rapidly evaluating the distinctive metabolic profiles of red ginseng as well as ginseng prior to and following processing (Zhang et al., 2012). UPLC-Q-TOF-MS can also be linked to exogenous metabolomics for qualitative analysis of proximate constituents and metabolites, which can make the process of data analysis more accurate, simplify the process of compound resolution, and function as a valuable instrument for the screening and identification of metabolic constituents. Mass spectrometry imaging, which is a molecular imaging technique that is based on mass spectrometry, can be used to visualize thousands of compounds simultaneously without the need for labeling (Wu, 2023). Desorption electrospray ionization mass spectrometry imaging (DESI-MSI) integrates the analytical capabilities that mass spectrometry was combined with computer imaging for direct in-situ visualization of frozen tissue sections (Kumar, 2023). The composition, abundance, and in-situ spatial distribution of molecules on the surface of plant tissue sections can be obtained directly, providing important spatial information for plant metabolomics research. The integration of the two methodologies yields a thorough comprehension of the distribution and molecular mechanisms underlying compound biosynthesis, offering an effective method for visualizing the accumulation patterns of natural compounds as well as providing more significant and distinctive views of secondary metabolism.
The present study employed multivariate chemical analysis techniques of metabolomics linked to multivariate statistical analysis for exploring diterpenoid alkaloids and tanning constituents and to identify potential metabolic markers for distinguishing between TBC and Hezi-decoction-processed TBC at different processing times. In addition, the chosen metabolic markers were imaged and examined by DESI-MSI, and the levels of three indicator alkaloids and five tannin acids were determined by UPLC and HPLC. The results showed a significant difference between TBC made at different processing times and Hezi-decoction-processed TBC, and a processing time of 72 h was found to be conducive to the attenuation of toxicity and the preservation of efficacy. DESI-MSI coupled with metabolomics is an effective approach for observing altered patterns of diterpenoid alkaloids and identifying biomarkers in Hezi-decoction-processed TBC and detoxification treatment. The implementation of this technique could contribute to the identification of markers in Hezi decoction-processed TBC and the establishment of effective quality control and evaluation procedures to ensure the safety of TBC. Additionally, this approach enhances the understanding of the processing mechanisms associated with Aconitum and other toxic traditional Chinese medicinas.
Formic acid and ammonium acetate of HPLC grade were procured from Chengdu Kelong Chemical Co., Ltd., (Chengdu, China). Acetonitrile and methyl alcohol of HPLC grade were supplied by Fisher Chemicals (Pittsburg, United States). Distilled water of HPLC grade was supplied by Watsons Distilled Water Co., Ltd., (Beijing, China). All other reagents were of analytical grade.
The reference standards (HPLC greater than 98%) of mesaconitine, benzoylaconine, benzoylhypacoitine, benzoylmesaconine, 3-acetylaconitine, fuziline, neoline, aconitine, 3-deoxyaconitine, hypaconitine, 12-epi-napelline, aconine, chebulic acid, gallic acid, rutin, chebulinic acid, ellagic acid and protocatechuic acid were purchased from Chengdu Push Biological Technology Co., Ltd., (Chengdu, China). Quercetin and corilagin (HPLC greater than 98%) were procured from Chengdu Must Biotechnology Co., Ltd., (Chengdu, China). 1,2,3,4,6-O-pentagalloylglucose (HPLC greater than 98%) was acquired from Chengdu Chroma Biotechnology Co., Ltd., (Chengdu, China).
Eight batches of TBC were collected from Huzhu Tu Autonomous County, Haidong City, Qinghai Province. Hezi was obtained from the Lotus Pond Market for Chinese Herbal Medicine in Chengdu. They were authenticated as dried root tubers of A. pendulum Busch. and dried ripe fruits of T. chebula Retz., respectively, by Prof. Yi Zhang (Chengdu University of Traditional Chinese Medicine).
Hezi (50.0 g) was soaked in deionized water (200 mL, at a weight-to-volume ratio of 1:4) for a duration of 70 min, followed by a boiling process lasting 30 min. Subsequently, Hezi decoction was cooled.
Raw TBC samples were randomly divided into eight groups (50.0 g for each group). One group was selected as raw TBC, whereas the remaining groups were soaked in Hezi decoction for 6, 12, 24, 48, 72, 96, and 120 h. Hezi decoction was replaced every day, and TBC was stirred during soaking.
Samples of Hezi-decoction-processed TBC were dried at 45°C. Subsequently, the dried samples were filtered through a 65-mesh sieve after being powered. TBC sample powder (0.25 g) was measured and then subjected to extraction via ultrasonication using 25 mL of methanol for a duration of 30 min at a power of 300 W and a frequency of 40 kHz. Methanol was added to account for any weight loss incurred during the extraction process. Following extraction, the mixture underwent centrifugation for 5 min at 13,000 rpm, and the resulting supernatant was filtered using a membrane filter with a pore size of 0.22 μm.
Reference standards comprising mesaconitine, benzoylaconine, benzoylhypacoitine, benzoylmesaconine, 3-acetylaconitine, fuziline, neoline, aconitine, 3-deoxyaconitine, hypaconitine, 12-epi-napelline, aconine, chebulic acid, gallic acid, rutin, chebulinic acid, ellagic acid, protocatechuic acid, quercetin, corilagin and 1,2,3,4,6-O-pentagalloylglucose were dissolved in methanol as a stock solution (1 mg/mL for each compound). The solution was mixed and diluted with methanol at a concentration of 10 μg/mL to prepare a mixed reference solution. The mixed standard solution was kept at 4°C.
Data for UPLC-Q-TOF-MS were generated utilizing the Waters ACQUITY UPLC and SYNAPT XS HDMS systems (Waters Corporation, Milford, United States), which are outfitted with an electrospray ionization source and a hybrid Q-TOF mass spectrometer operating under the MSE model. The system was managed using Masslynx software (version 4.2). The chromatographic separation was performed using an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm). The mobile phase consisted of 0.1% formic acid in water (solvent A) and acetonitrile (solvent B), with a flow rate maintained at 0.3 mL/min. The gradient elution program was implemented as follows: 2%–7% B (0–1 min); 7%–11% B (1–3 min); 11%–15% B (3–8 min); 15%–30% B (8–13 min); 30%–36% B (13–16 min); 36%–70% B (16–20 min); 70%–85% B (20–26 min); 85%–85% B (26–28 min). The column temperature was set to 35°C. The injection volume was set at 2 μL.
The full-scan data were obtained in both positive and negative ion modes from 100 Da to 1,200 Da, with a scan duration of 0.3 s. The experimental parameters included a capillary voltage of 2000 V, an extraction cone voltage of 4 V, a source temperature of 150°C, a desolvation temperature of 450°C, a sample cone voltage of 40 V, a desolvation gas flow rate of 800 L/h, and a cone gas flow rate of 50 L/h. The lockspray scan duration was established at 0.3 s with a 30-s interval, and the data were averaged across three scans.
Data acquisition was conducted utilizing MassLynx software (version 4.2, Waters Corporation, Milford, United States). UNIFI (Waters Corporation, Milford, United States) and Progenesis QI (Waters Corporation, Milford, United States) were employed for the purposes of chromatographic peak extraction, alignment, and data normalization. Subsequently, the processed data were input into SIMCA-P 13.0 (Umetrics, Umea, Sweden) to facilitate multivariate data analysis.
All samples were directly sectioned, yielding cross-sectional slices with an approximate thickness of 5 mm. Following this, the samples underwent freeze drying at −80°C for a duration of 2 h. They were then affixed to glass slides and preserved at −80°C in preparation for DESI-MSI analysis.
DESI-MS data were obtained utilizing a Waters Synapt XS HDMS Q-TOF mass spectrometer, which was outfitted with a DESI source (Waters Corporation, Milford, United States). The parameters were as follows: ionization mode was set to positive with a capillary voltage of 3.0 kV, a nebulizing gas pressure of 0.60 MPa, and a mass range spanning from 100 to 1,500. The spray solvent comprised 90% methyl alcohol supplemented with 0.2% formic acid and 0.1 mM leucine enkephalin delivered at 2 μL/min. For the negative ionization mode, the capillary voltage was adjusted to 2.0 kV, the nebulizing gas pressure was 0.65 MPa, and the mass range remained consistent at 100–1,500, utilizing the same spray solvent and flow rate as in the positive mode.
DESI imaging was conducted by high-definition imaging (HDI) software (version 1.5, Waters Corporation, Milford, United States). The mass spectrometry imaging was executed by processing the raw mass spectrometry files through the HDI software, employing LE as the lock mass for calibration of high-resolution mass spectrometry in both positive ionization mode (m/z 556.2772) and negative ionization mode (m/z 554.2620).
TBC sample powder (2.0 g) underwent ultrasonic extraction with 3 mL of ammonia and 50 mL of a 1:1 (v/v) mixture of isopropanol and ethyl acetate for 30 min at a power of 300 W and a frequency of 40 kHz. Following the extraction process, isopropanol-ethyl acetate (1:1, v/v) was added to compensate for any weight loss experienced during extraction, after which the mixture was filtered. Next, 25 mL of the resulting filtrate was accurately measured and evaporated to dryness under decreased pressure at 40°C. The residue was dissolved in 3 mL of 0.05 M hydrochloric acid–methanol solution. The supernatants obtained were utilized as the sample solution after undergoing centrifugation at 15,000 rpm for 5 min.
A mixed standard stock solution comprising benzoylaconine, aconitine, and 3-deoxyaconitine was diluted with 0.05 M of hydrochloric acid-methanol solution to generate six suitable concentrations for the development of calibration curves. The calibration curves were created by graphing the peak areas of the reference standards in relation to their corresponding concentrations. The mixed reference solution was kept at 4°C. For detection purposes, 2 μL of the mixed reference solution were injected into the UPLC system.
The separation process was conducted utilizing the Waters ACQUITY UPLC system (Waters Corporation, Milford, United States), which included a binary pump, a mobile phase degasser, a temperature-controlled autosampler, a column thermostat, and a photodiode array detector. The chromatographic analysis utilized an ACQUITY UPLC BEH C18 column with dimensions of 2.1 mm × 100 mm with a particle size of 1.7 μm. The mobile phase comprised acetonitrile (solvent A) and water containing 0.2% glacial acetic acid (solvent B), with the pH adjusted to 6.5 using triethylamine. The gradient elution profile was established as 21%–29% B (0–3 min) and 29%–35% B (3–7 min). The flow rate was maintained at 0.4 mL/min, and the injection volume was set at 2 μL. The column temperature was regulated at 35°C, and UV detection was conducted at a wavelength of 235 nm.
TBC sample powder (2.0 g) underwent ultrasonic extraction using 25 mL of methanol for a duration of 30 min, operating at a power of 300 W and a frequency of 40 kHz. Subsequent to the extraction process, additional methanol was incorporated to account for any weight loss incurred during extraction. The resultant extract solution was further filtered via a 0.22 µm membrane.
A mixed standard stock solution comprising gallic acid, corilagin, 1,2,3,4,6-O-Pentagalloylglucose, chebulinic acid, and ellagic acid was prepared and diluted with methanol to achieve six suitable concentrations for the development of calibration curves. Calibration curves were generated by plotting the peak areas of the reference standards against their respective concentrations. The mixed reference solution was kept at 4°C for storage. For detection purposes, the injection volume of the mixed reference solution was set at 10 μL.
HPLC analyses were conducted utilizing a Shimadzu 2010 analytical HPLC system (Shimadzu Corporation, Kyoto, Japan), which included an LC-2010 pump, an LC-2010 column oven, an LC-2010 detector, and an LC-2010 autosampler. The separation procedure was performed using an Agilent ZORBAX Eclipse XDB-C18 column (4.6 × 250 mm, 5 µm). The mobile phase comprised 0.04% phosphoric acid solution (solvent A) and methanol (solvent B). The gradient elution profile was established as follows: 3%–7% B (0–12 min); 7%–20% B (12–20 min); 20%–30% B (20–50 min); 30%–35% B (50–70 min); and 35%–36% B (70–75 min). The flow rate was 1 mL/min, and the injection volume was set at 10 μL. The column temperature was maintained at 35°C. UV detection was conducted at a wavelength of 245 nm.
As the soaking time increased, the Hezi decoction gradually soaked into the dried TBC (Figure 1). As a result, 51 compounds, including aconitine, 3-deoxyaconitine, benzoylaconine, aconine, chebulic acid, corilagin, and ellagic acid, were identified in raw TBC and processed samples at various time points, with 31 compounds recognized from the raw TBC. The base peak chromatograms of the unprocessed and processed TBC samples are presented (Figure 2). The identification results by the corresponding reference substances, Aconitum and Terminalia compound databases, and published literature are shown (Table 1).

Figure 1. The original plants of Aconitum pendulum in the field (A); the original plants of Aconitum pendulum (B); the fresh roots of Aconitum pendulum (C); the original plants of Terminalia chebula Retz. (D); the dried ripe fruit of Terminalia chebula Retz. (E); TBC processed with Hezi-decoction for different time points (F).
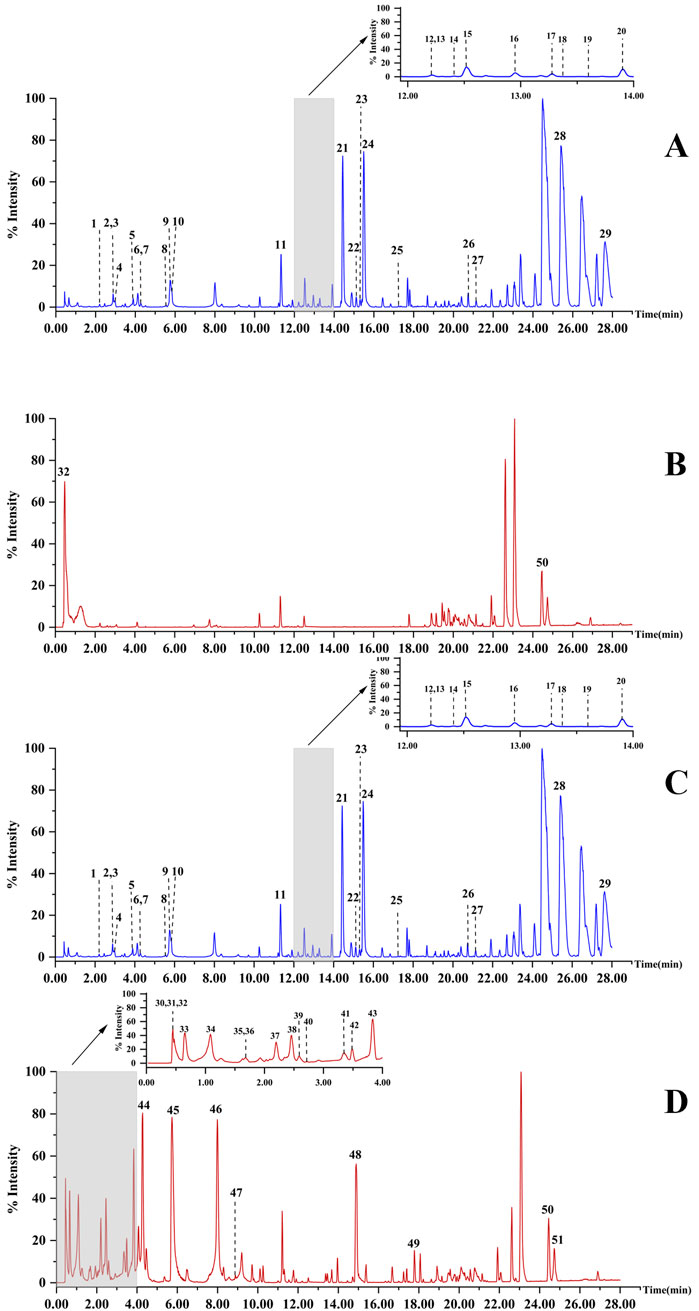
Figure 2. The base peak chromatograms of raw TBC in positive ionization mode (A) and in negative ionization mode (B); the base peak chromatograms of TBC processed with Hezi-decoction for 72 h in positive ionization mode (C) and in negative ionization mode (D).
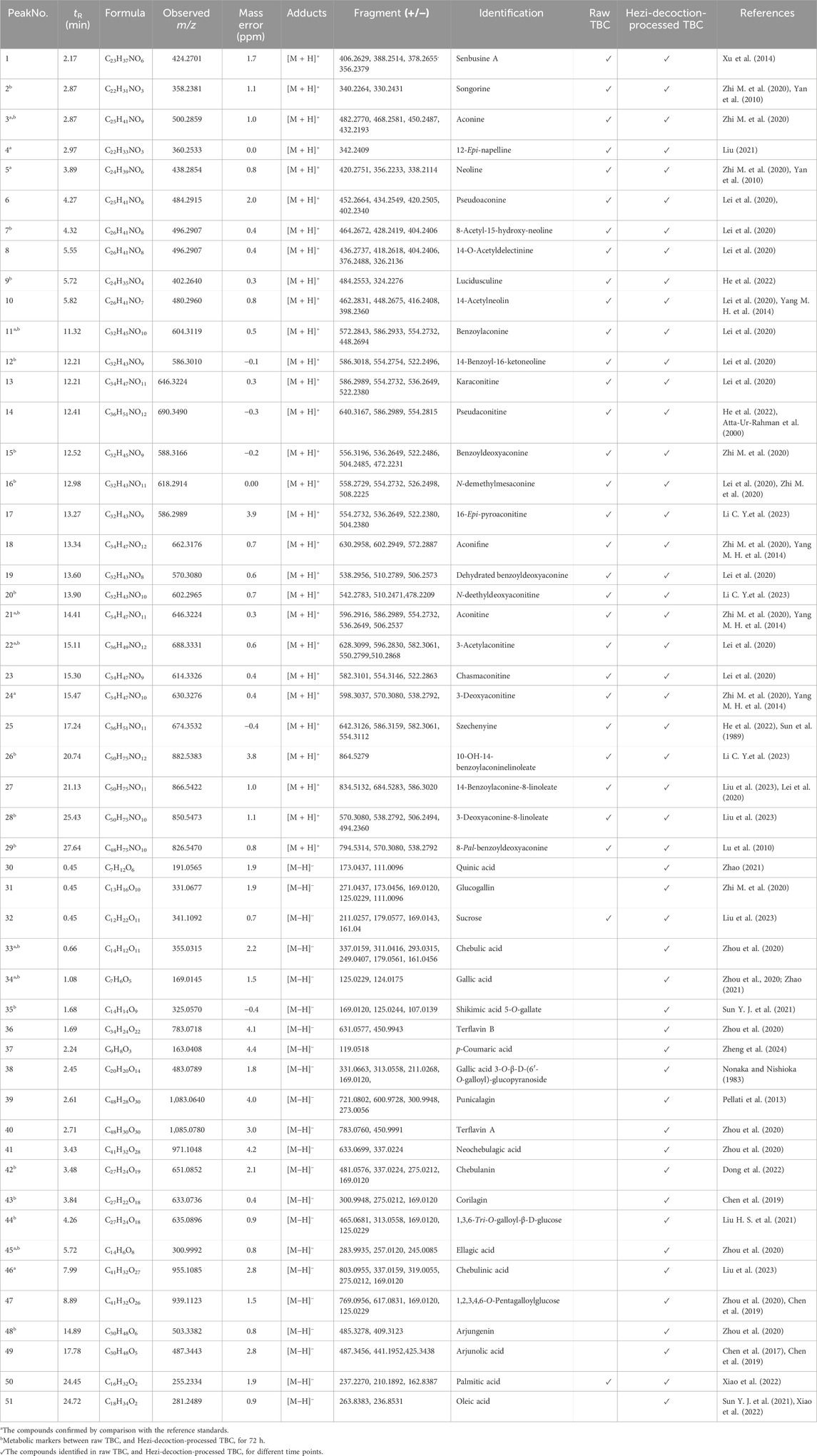
Table 1. Identification of the major chemical constituents in raw and processed TBC by UPLC-Q-TOF-MS.
PCA and PLS-DA were used for distinguishing the variations of TBC with varying processing times, visually showing grouping trends, and revealing the metabolic differences among them (Sun et al., 2020). The PCA score plots (Figures 3A, C) could divide all the metabolites into six major groups, suggesting that the chemical profile changed as a result of processing. The raw TBC was highly different from the Hezi-decoction-processed TBC. The TBC samples with different processing time points were divided into groups and gradually moved away from the raw TBC. The results showed obvious chemical changes between raw TBC and those processed for more than 6 h. In addition, the metabolites of the TBC processed for 72 and 96 h were grouped together, indicating the chemical metabolites were more similar.
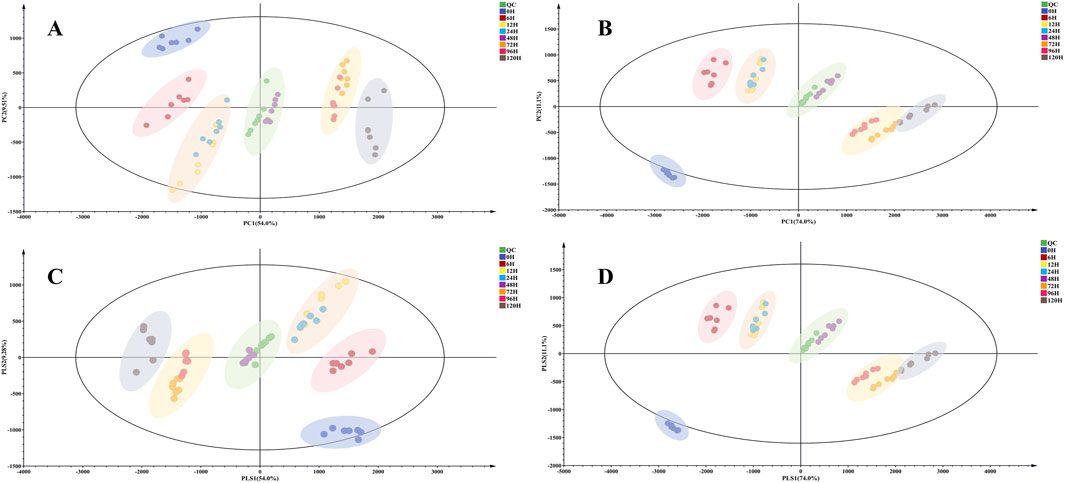
Figure 3. The PCA score plot of raw and processed TBC for different time points in positive (A) and negative (B) ionization mode. The PLS-DA score plot of raw and processed TBC for different time points in positive (C) and negative (D) ionization mode.
The outcomes of PLS-DA discrimination aligned well with the PCA model, resulting in the categorization of all data into six distinct categories (Figures 3B, D). And the samples processed for a longer time were located closer, indicating a good accordance with the PCA model. The results of PCA and PLS-DA preliminarily revealed that the metabolites of TBC changed clearly from 0 to 72 h, but the variations of TBC metabolites gradually stabilized after 72 h.
OPLS-DA was carried out to identify variables that result in group separation between raw and processed TBC. The primary focus was on raw TBC and TBC that had undergone processing for 72 h, as these groups were selected to elucidate the metabolic markers. OPLS-DA was used for comparison of metabolic changes.
Distinct group separation was evident in both OPLS-DA score plots (Figures 4A, C), highlighting a vast disparity in the chemical profiles of raw TBC and TBC processed for 72 h. The associated S-plot (Figures 4B, D) illustrated that the ions positioned away from the origin played a crucial role in differentiating between raw TBC and the processed TBC. Based on the results of the S-plots and the VIP value (VIP greater than 1) obtained from OPLS-DA analysis, and the corresponding metabolites were detected. A total of 22 metabolites, including aconine, aconitine, benzoylaconine, chebulic acid, gallic acid, and corilagin, can proficiently distinguish between raw and processed TBC with different processing times (Table 1; Figure 5).

Figure 4. The OPLS-DA score plot based on raw TBC in positive (A) and negative ionization mode (C); The S-polt based on TBC processed with Hezi-decoction for 72 h in positive (B) and negative ionization mode (D).
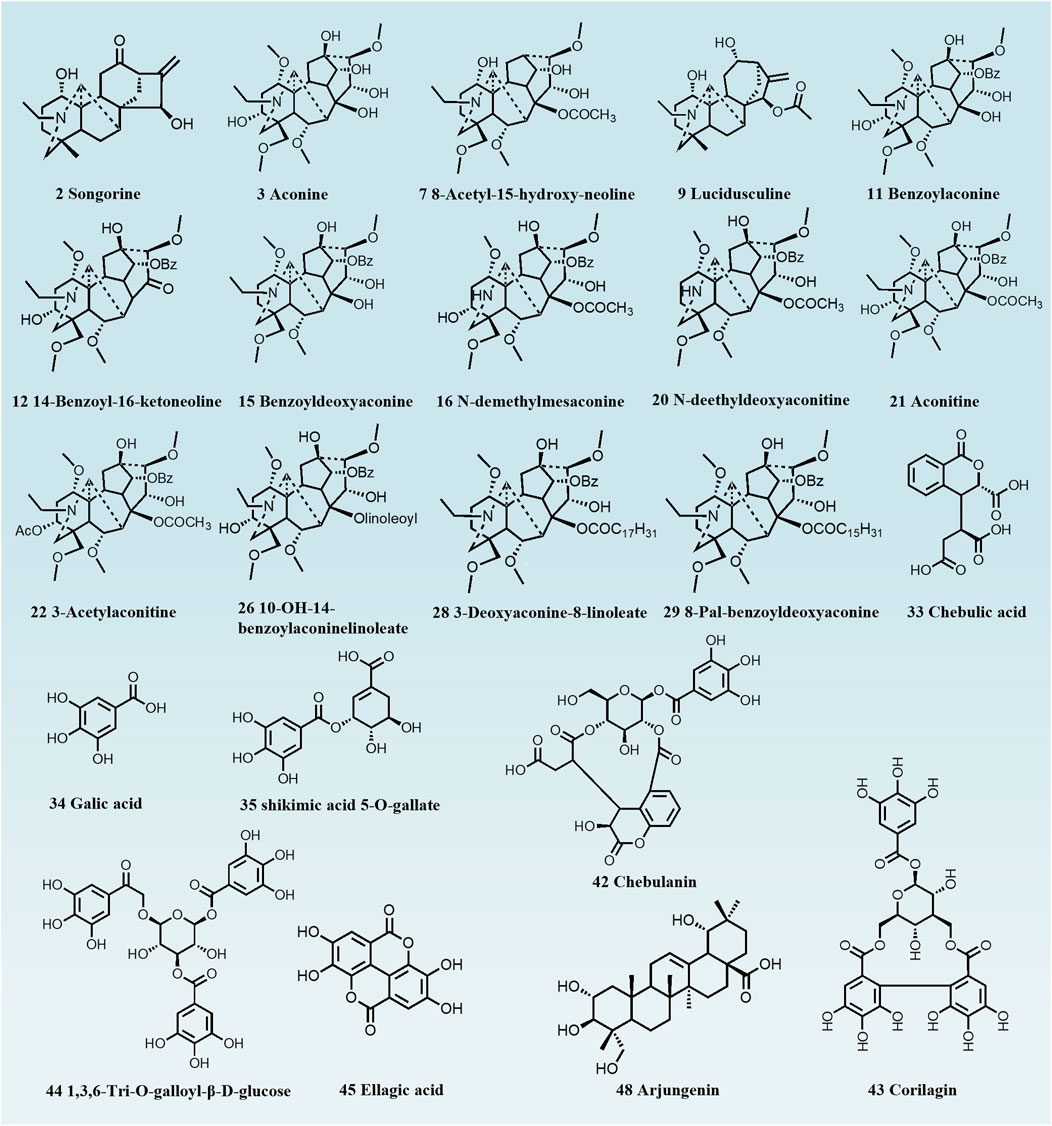
Figure 5. Chemical structures of metabolic markers of unprocessed and Hezi-decoction-processed TBC for 72 h.
The analytical methodology employed in this investigation was validated according to the guidelines set forth by the Chinese Pharmacopoeia 2020. The parameters of linearity, accuracy, precision, and stability were assessed through the analysis of samples and various concentrations of standards. Variability was quantified by conducting analyses on six replicates. The relative standard deviation (RSD) was computed to assess precision, stability and repeatability. Additionally, six samples of raw TBC were prepared and analyzed on the same day to assess repeatability. Stability was examined at ambient temperature, with analyses conducted at intervals of 0, 4, 8, 12, 18, and 24 h. The recovery was determined by calculating the recovery percentages of benzoylaconine, aconitine, and 3-deoxyaconitine in the spiked samples. Six parallel samples were prepared for each concentration.
The three compounds demonstrated excellent linearity (r2 = 1.00) across the specified test ranges. The RSDs for intra-day, inter-day, stability, and repeatability assessments were observed to be within the ranges of 0.32%–0.45%, 0.71%–0.76%, 0.98%–1.22%, and 2.41%–2.89%, respectively. The recovery rates for the five tannin acids were found to range from 97.28% to 101.48% (Tables 2, 3). Consequently, the accuracy and feasibility of the method were demonstrated by all these results.
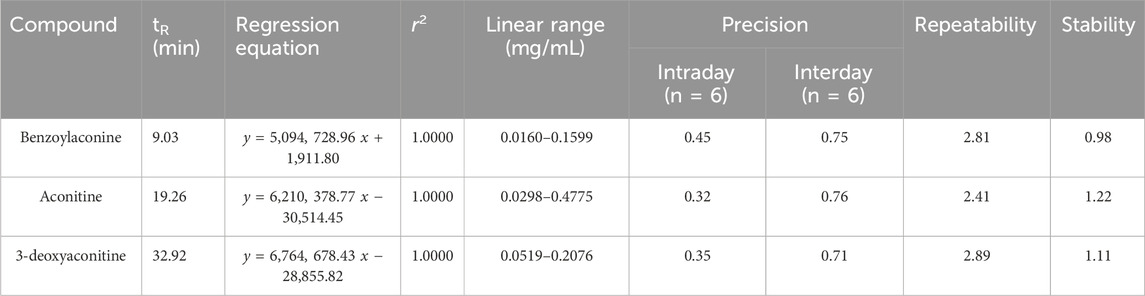
Table 2. Regression equations, linear range, precision, repeatability, and stability of the method of three alkaloids.
The accurate concentrations of three alkaloids in the samples of processed TBC at different time points were determined using the regression equations (Table 4). The result indicated that the MDAs and DDAs showed a gradual decrease and tended to be flat after 48 h of processing in general. The MDAs levels were lower than 0.0162% after 48 h of processing, and then they fluctuated between 0.0149% and 0.0162%. The MDAs levels was 0.0149% in 72 h. The DDAs levels were lower than 0.0900% after 24 h, and then it fluctuated between 0.0851% and 0.0879%. Nevertheless, the DDAs levels had a minor reduction to 0.0852% after 72 h, followed by a further increase. The MDA and DDAs levels were 0.0149% and 0.0852% in 72 h, respectively. Combined with the outcomes of UPLC and multivariate statistical analysis, a processing time of 72 h was further found to be the appropriate time for toxicity attenuation and efficacy reservation of TBC.
Linearity, accuracy, precision, and stability were validated as mentioned above. The mixed solutions for five tannin acids were utilized in the regression analyses correlating peak areas with the concentrations. The five tannin acids exhibited excellent linearity across the tested ranges. R2 were 0.9991, 0.9993, 0.9997, 0.9991 and 0.9998, respectively. The RSDs for intra-day, inter-day, stability, and repeatability were in the ranges of 0.47%–1.94%, 1.69%–2.83%, 0.14%–3.16%, and 1.07%–2.53%, respectively. Recoveries of five tannin acids varied between 99.33% and 101.20% (Tables 5, 6). The accuracy and feasibility of the method were demonstrated by all these results.
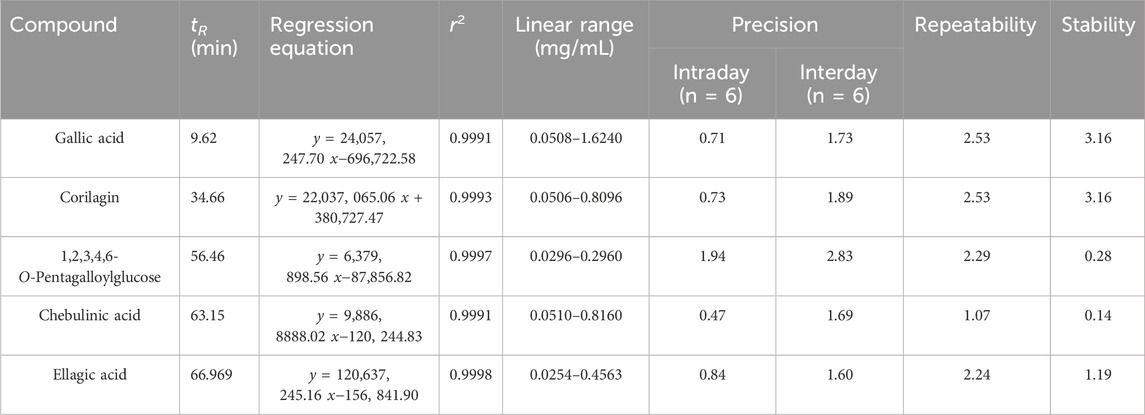
Table 5. Regression equations, linear range, precision, repeatability, and stability of the method of five tannic acids.
The accurate concentrations of five tannin acids in the samples of processed TBC at different time points were determined using the regression equations obtained from their curves. The findings demonstrated that the content of five tannin acids gradually escalated from 6 to 12 h, followed by a substantial increase from 12 to 72 h. Finally, the contents increased slowly to a steady level after 72 h. The contents of gallic acid, corilagin, 1,2,3,4,6-O-pentagalloylglucose, chebulinic acid, and ellagic acid were 8.9706, 9.3444, 1.2438, 5.7582, and 3.1160 mg/g, respectively (Table 4). Based on the multivariate statistical analysis and content determination of three alkaloids and five tannin acids, 72 h is demonstrated to be the appropriate time for toxicity attenuation and efficacy reservation of TBC.
In-situ molecular detection was performed in simulated industrial segments of TBC utilizing DESI-MS. This approach aimed to examine the alterations in eight potential metabolic markers at various processing times of TBC.
The MS images (Figure 6) illustrate the presence of three alkaloids: aconitine (m/z 646.3230), benzoylaconine (m/z 604.3132), and aconine (m/z 500.2864), with assessed in positive mode (Sun C. L. et al., 2021). The results of DESI-MSI visualization indicated that with prolonged processing time, the response values of the three alkaloids gradually decreased and then tended to be stable. A slight decrease was observed in the response values of benzoylaconine (MDA) and aconine (NDA) from 0 to 12 h. The response values significantly decreased from 12 to 72 h. Finally, the response values stabilized after 72 h. Conversely, the response values of aconitine (DDA) declined slightly from 0 to 6 h and from 6 to 12 h. Then, they fluctuated from 12 to 72 h, with increments observed from 12 to 24 h and from 48 to 72 h, whereas a decrease occurred from 24 to 48 h. After 72 h, the response value decreased slightly and became stable.
In addition, five tannin acids, including gallic acid (m/z 169.0146), shikimic acid 5-O-gallate (m/z 325.0569), chebulic acid (m/z 355.0311), corilagin (m/z 633.0736), and 1,3,6-tri-O-galloyl-β-D-glucose (m/z 635.0896), were presented in negative mode (Mao et al., 2021). The results of DESI-MSI visualization revealed that with prolonged processing time, the response values of tannin acids gradually increased and then stabilized. In particular, the response values slowly increased from 6 to 12 h, exhibited a considerable increase after 24 h, and then steadied after 72 h. Additionally, the response values of shikimic acid 5-O-gallate, corilagin, and 1,3,6-tri-O-galloyl-β-D-glucose were too low to detect at 6 and 12 h. The results of DESI-MSI were consistent with those of content determination experiments, indicating its reliability.
TBC has been employed in Tibetan medicine for millennia, owing to its remarkable pharmacological efficacy and toxicity, particularly for treating fever, arthritis, and traumatic injuries. Similar to Caowu (Aconitum kusnezoffii Reichb.), which also belongs to the genus Aconitum, it demonstrates a correlation between its therapeutic efficacy and toxicity, linked to the presence of high-toxicity DDAs such as aconitine and 3-deoxyaconitine, alongside moderate-toxicity MDAs such as benzoylaconine (Liu et al., 2017; Tang et al., 2021). This is why TBC is used with a processing method that attenuates toxicity and preserves efficacy to ensure clinical safety. Known as the “king of medicines” for its diverse pharmaceutical and antidotal effects, Hezi is widely employed in Tibetan medical formulations as a particularly good remedy for aconite poisoning. According to ancient literature and modern studies, the Hezi-decoction-processed method is one of the distinctive traditional processing methods of Tibetan medicine. (Bao and Na, 2020; Zhang et al., 2021). Nevertheless, the overall variability of metabolites in Hezi-decoction-processed TBC is still unclear. UPLC-Q-TOF-MS-based metabolomics was utilized for uncovering biomarkers that could distinguish between raw and processed TBC. Combining quantitative methods and DESI-MSI visualization demonstrated that the dynamic changes of biomarkers in TBC were supervised during processing time.
UPLC-Q-TOF-MS was carried out to identify toxic and effective compounds between unprocessed TBC and processed samples with Hezi decoction for different times. Fifty-one compounds, including aconitine, 3-deoxyaconitine, benzoylaconine, aconine, chebulic acid, corilagin, and ellagic acid, were identified from unprocessed and processed samples, of which 31 were discernible from the unprocessed TBC.
In combining metabolomics based on UPLC-Q-TOF-MS and multivariate statistical analysis, a total of 22 metabolites, including aconine, aconitine, benzoylaconine, chebulic acid, gallic acid, and corilagin, can proficiently distinguish between raw and processed TBC. UPLC was first performed to quantify variation in the contents of one MDA (benzoylaconine) and two DDAs (aconitine and 3-deoxyaconine). Eight processing times were defined and the UPLC data showed that two DDAs (aconitine and 3-deoxyaconine) decreased and then remained about 0.0852% from 0 to 72 h. With prolonged processing time, MDA (benzoylaconine) was reduced slowly from 0 to 12 h, and it showed clearly decreases from 12 to 24 h. Then, it fluctuated between 0.0149% and 0.0162% after 24 h. The MDA and DDAs levels were 0.0149% and 0.0852% in 72 h, respectively. In addition, the contents of five tannin acids (gallic acid, corilagin, 1,2,3,4,6-O-pentagalloylglucose, chebulic acid, and gallic acid) showed a slowly increasing trend from 6 to 12 h. From 12 to 72 h, the content increased clearly. With the expendied processing time, the raw TBC was completely soaked by Hezi decoction, and the contents increased significantly. Finally, the contents became stable after 72 h. The contents of gallic acid, corilagin, 1,2,3,4,6-O-pentagalloylglucose, chebulinic acid, and ellagic acid were 8.9706, 9.3444, 1.2438, 5.7582, and 3.1160 mg/g, respectively.
Toxicity and efficacy are mutually dependent in TBC because of its high levels of highly toxic DDAs and moderately toxic MDAs. Hezi is rich in high levels of tannin acids such as gallic acid, ellagic acid, chebulinic acid, corilagin and punicalagin, which may improve anti-arthritic effects and reduce cardiotoxicity caused by TBC (Gao et al., 2024). After being processed with Hezi decoction, the levels of DDAs and MDAs, the toxicity and efficacy components in TBC, were properly reduced. And the addition of tannin acids can effectively reduce the contents of major alkaloids to a certain extent. Meanwhile, tannin acids can inhibit dissolution and hydrolysis, so alkaloids can be slowly hydrolyzed and have a slow release effect in vivo (Liu et al., 2013; Liu et al., 2015). Pharmacokinetic studies proved that the active ingredients of Chebulae Fructus formed complexes with alkaloids in the blood and blood-rich tissues, slowing down distribution, elimination and detoxification (Wang et al., 2002). The Hezi-processed method has the potential to decrease both the concentration and the rate of absorption and elimination of various alkaloids in Caowu, thereby extending their duration of action. The mechanism of detoxification and effect preservation of Hezi-decoction-processed TBC was revealed from the viewpoint of material basis (Zhang et al., 2021; Zhi M. R. et al., 2020). In addition, it is related to compatibility effects. Due to its large amounts of tannin acids, Hezi has cardioprotective, anti-inflammatory and antioxidant (Upadhyay et al., 2014). Toxicological studies showed that the T. chebula extract can mitigate injury of aconitinc on myocardium, and it has a protecting effect on damaged myocardium (Zhang, 2010). Gallic acid and ellagic acid, characteristic components of T. chebula, synergistically reduce cardiotoxicity caused by mesaconitine and benzoylmesaconitine (Han et al., 2022). Academics found that Caowu administered together with Hezi could significantly reduce serum CK, GOT and reduce myocardial tissue Ca2+ content to increase Na+ -K+ -ATPase activity compared to unprocessed Caowu. Besides, it is reported that Caowu processed with Hezi decoction can block TRPV channel and prevent cardiotoxicity (Han et al., 2022; Song et al., 2024). In addition, corilagin contained inTerminalia chebula has significant anti-inflammatory effects. Its intrinsic anti-inflammatory mechanism involves a significant reduction in the production of pro-inflammatory cytokines and mediates TNF-α, IL-1β, IL-6, NO (iNOS), and COX-2 nuclear translocation by blocking NF-κB at the protein and gene levels (Zhao et al., 2008). It is reported that gallic acid, 2,3,6-tri-O-gallate-β-D-glucose, and arjunic acid, which are found in T. chebula, have anti-inflammatory activity by inhibiting iNOS and COX-2 activity at the cellular level and effectively reducing nitric oxide production (Yang Y. et al., 2014). Furthermore, TBC co-administered with Hezi was able to attenuate cardiotoxicity associated with TBC while preserving its efficacy in treating rheumatoid arthritis. The compatibility of TBC and HZ has been shown to diminish the elevation in heart rate and the prolongation of the QTc interval that TBC typically induces. The synergism of TBC and HZ reduced serum cTnT and cardiac MDA levels, raised cardiac SOD levels, and effectively alleviated paw swelling to maintain the anti-rheumatoid arthritis efficacy of TBC (Liu et al., 2023; Zhang et al., 2017). Metabolomics findings demonstrated that Hezi can decrease the levels of glutamic oxalic aminotransferase, glutamic pyruvic aminotransferase, myoglobin, and troponin in the serum of rats induced by Caowu (Liu et al., 2018). In summary, effectively reducing toxicity and preserving the effect of TBC processed with Hezi decoction are likely attributable to the synergistic effects of the two aforementioned factors (Figure 7).
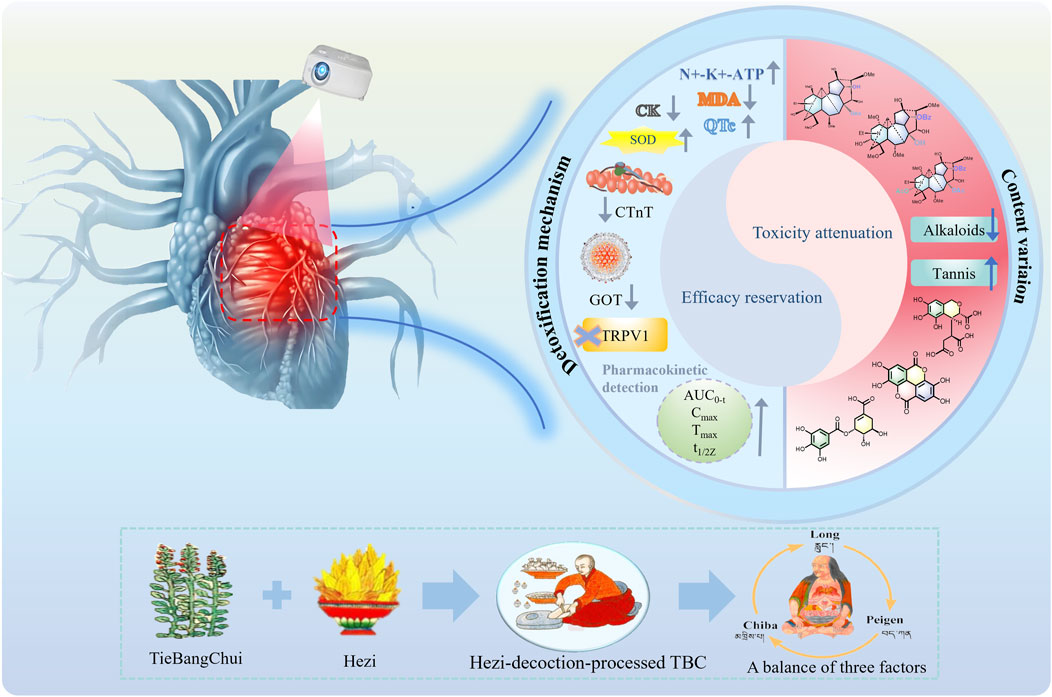
Figure 7. The mechanism of efficacy reservation and toxicity attenuation in the Hezi-decoction-processed TBC.
MSI is a molecular imaging technique that is based on mass spectrometry. It enables the visualisation of the geographical and temporal distribution of metabolites without compromising tissue integrity, providing important situ information for plant metabolomics research. A method without embedding and complex treatment of samples made the DESI-MSI sample preparation process simpler. DESI-MSI was applied between raw and Hezi-decoction-processed TBC with different processed times to achieve an integrative understanding of the distribution and accumulation of metabolic markers. The results of DESI-MSI visualisation indicated that the response values of the three alkaloids gradually decreased and then stabilised with the extension of processed time. Hezi decoction infiltrated raw TBC, resulting in the response values of benzoylaconine (MDAs) and aconine (NDAs) to slightly decrease from 0 to 12 h. Then, the response values declined clearly from 12 to 72 h. Finally, raw TBC was completely soaked by Hezi decoction, and the response values became stable after 72 h. Different from the other two alkaloids, the response values of aconitine (DDA) gradually decreased in fluctuation with prolonged processed time and became stable after 72 h after a slight decrease. The results of five tannic acids, including gallic acid, shikimic acid 5-O-gallate, chebulic acid, corilagin, and 1,3,6-tri-O-galloyl-β-D-glucose, showed that with the extension of processed time, the response values gradually increased and then tended to be stable. Combined with the DESI-MSI results of alkaloids and tannic acids, the change trend of response values over time was consistent with the process of dried TBC soaked in Hezi decoction. DESI-MSI was effectively used to visualise the distribution features of biomarkers between unprocessed and Hezi-decoction-processed TBC taken at various time points. The results of DESI-MSI were consistent with those of content determination. Consequently, the multivariate statistical analysis, content determination of three alkaloids and five tannin acids, and DESI-MSI, revealed that 72 h was found to be conducive to the attenuation of toxicity and the preservation of efficacy. This integrated strategy not only revealed the dynamic changes in metabolic markers of Hezi-decoction-processed TBC, but also preserved efficacy and attenuated toxicity, establishing an effective quality control and evaluation procedure to ensure the safety of TBC.
From the perspective of processing, this study revealed the dynamic changes and the distribution of metabolic markers in the process of efficacy preservation and toxicity reduction of Hezi-decoction-processed TBC. This result should be further verified in combination with pharmacological experiments and the dose-toxin-effect relationship in vivo to provide a scientific basis for further elucidating the principle of Hezi-decoction-processed TBC for detoxification, thereby further optimising the processing technology’s quality standard to ensure the safety and effectiveness of the clinical use of TBC.
This research developed a metabolomics approach utilising UPLC-Q-TOF-MS in conjunction with DESI-MSI visualisation and quantitative methodologies to monitor the dynamic changes in biomarkers associated with TBC throughout various processing times. A total of fifty-one compounds were discernible from unprocessed and processed samples, of which 31 were discernible from unprocessed TBC. Through the integration of UPLC-Q-TOF-MS with multivariate statistical analysis, 22 metabolic markers, such as aconine, aconitine, benzoylaconine, chebulic acid, gallic acid, and corilagin, can proficiently distinguish between raw and processed TBC with different processing times. The results of content determination of three alkaloids and five tannins showed that they were stabilized at 72 h. The MDA and DDAs levels were 0.0149% and 0.0852% in 72 h, respectively. The contents of gallic acid, corilagin, 1,2,3,4,6-O-pentagalloylglucose, chebulinic acid, and ellagic acid were 8.9706, 9.3444, 1.2438, 5.7582, and 3.1160 mg/g, respectively. The distribution and accumulation of metabolic markers during processing were investigated by DESI-MS. The results of DESI-MSI were consistent with those of content determination. Combined with the multivariate statistical analysis, content determination of three alkaloids and five tannin acids and DESI-MSI, 72 h is demonstrated to be the appropriate time for toxicity attenuation and efficacy reservation of TBC. The reliability of the Hezi-decoction processing method, as a distinctive traditional processing method of Tibetan medicine, was verified in terms of efficacy preservation and toxicity attenuation and the identification of markers in Hezi-decoction-processed TBC, establishing an effective quality control and evaluation procedure to ensure the safety of TBC. In addition, this approach facilitates a comprehensive and effective understanding of the processing mechanisms of Aconitum and other toxic traditional Chinese medicines.
The original contributions presented in the study are publicly available. This data can be found here: https://www.ebi.ac.uk/metabolights/MTBLS12362
ZZ: Writing–original draft, Writing–review and editing. H-MG: Conceptualization, Data curation, Writing–review and editing. Z-QP: Conceptualization, Data curation, Writing–review and editing. M-XS: Conceptualization, Data curation, Writing–review and editing. C-YL: Methodology, Writing–review and editing. YZ: Methodology, Writing–review and editing. J-SS: Supervision, Writing–review and editing. YL: Supervision, Writing–review and editing. X-LM: Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We express our gratitude for the financial assistance provided by the National Natural Science Foundation of China (No. 82130113), and the National Key R&D Program of China (2023YFC3504400).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DDAs, diester-diterpenoid alkaloids; DESI-MSI, desorption electrospray ionization mass spectrometry imaging; Hezi, Terminalia chebula Retzs; HDI, high-definition imaging; HPLC, high-performance liquid chromatography; MDAs, monoester-diterpenoid alkaloids; NDAs, non-esterified diterpene alkaloids; OPLS-DA, orthogonal partial least-square discriminant analysis; PCA, principal component analysis; PLS-DA, partial least-square discriminant analysis; RSD, relative standard deviation; TBC, Aconitum pendulum Busch; UPLC, ultra-high-performance liquid chromatography; UPLC-Q-TOF-MS, ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry; VIP, variable importance for projection.
Atta-Ur-Rahman, A., Fatima, N., Akhtar, F., Choudhary, M. I., and Khalid, A. (2000). New norditerpenoid alkaloids from Aconitum falconeri. J. Nat. Prod. 63, 1393–1395. doi:10.1021/np9905315
Bao, L., and Na, S. (2020). The traditional concept and modern research profile of Mongolian medicine Terminalia chebula on the detoxification of radix aconiti Kusnezoffi. Pharmacol. Clin. Chin. Mater. Medica, 223–227. doi:10.13412/j.cnki.zyyl.2020.05.028
Cao, R., Niu, J. T., Jin, H., Chen, J. G., and Li, Y. F. (2024). Research progress on processing method of Aconitum pendulm Busch and the processing principle of “reducing toxicity and retaining effectiveness”. J. Gansu Univ. Chin. Med. 81-85. doi:10.16841/j.issn1003-8450.2024.02.13
Chan, T. Y. (2016). Aconitum alkaloid poisoning because of contamination of herbs by aconite roots. Phytother. Res. 30, 3–8. doi:10.1002/ptr.5495
Chen, H. F., Zhang, C., Yao, Y., Li, J. M., Du, W. D., Li, M. L., et al. (2019). Study on anti-hyperuricemia effects and active ingredients of traditional Tibetan medicine TongFengTangSan (TFTS) by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 165, 213–223. doi:10.1016/j.jpba.2018.11.038
Chen, W. L., Zhong, B. B., and Wang, Y. X. (2017). Antioxidant activities and identification of bioactive components of cyclocarya paliurus bu UPLC-QTOF-MS/MS. Food Sci., 122–128. doi:10.7506/spkx1002-6630-201708020
Dong, S. Q., Zhao, L., Zhang, D. X., Yang, F. L., Zhang, A. J., Xiao, J. L., et al. (2022). Identification of chemical constituents in zhachong shisanwei pills by UPLC-Q-TOF-MS/MS. Zhongguo Zhong Yao Za Zhi 47, 1546–1557. doi:10.19540/j.cnki.cjcmm.20211215.301
Gao, H., Lu, H., Fang, N., Su, J., Li, R., Wang, W., et al. (2024). The potential of Terminalia chebula in alleviating mild cognitive impairment: a review. Front. Pharmacol. 15, 1484040. doi:10.3389/fphar.2024.1484040
GYu-thogrNying-maYon-tanmGon-po (1983). Four volumes of medical treatment. Beijing, China: People’s Medical Publishing House.
Han, S., Bao, L., Liu, K., Han, X., Tang, Y., Liu, Z., et al. (2022). Mechanism of Aconiti kusnezoffii Radix processed with Chebulae Fructus against H9c2 cardiomyocyte toxicity based on TRPV1 channel. Chin. J. Exp. Tradit. Med. Formulae, 173–181. doi:10.13422/j.cnki.syfjx.20211748
He, Q., Tan, X., Geng, S., Du, Q., Pei, Z., Zhang, Y., et al. (2022). Network analysis combined with pharmacological evaluation strategy to reveal the mechanism of Tibetan medicine Wuwei Shexiang pills in treating rheumatoid arthritis. Front. Pharmacol. 13, 941013. doi:10.3389/fphar.2022.941013
Kumar, B. S. (2023). Desorption electrospray ionization mass spectrometry imaging (DESI-MSI) in disease diagnosis: an overview. Anal. Methods 15, 3768–3784. doi:10.1039/d3ay00867c
Lei, H., Zhang, Y., Ye, J., Cheng, T., Liang, Y., Zu, X., et al. (2020). A comprehensive quality evaluation of Fuzi and its processed product through integration of UPLC-QTOF/MS combined MS/MS-based mass spectral molecular networking with multivariate statistical analysis and HPLC-MS/MS. J. Ethnopharmacol. 266, 113455. doi:10.1016/j.jep.2020.113455
Li, C. Y., Sha, M. X., Pei, Z. Q., Zhou, Z., Tang, C., Liu, Y., et al. (2023). Dynamic variations in the chemical constituents of Tiebangchui stir-fried with Zanba by integrating UPLC-Q-TOF-MS based metabolomics and DESI-MSI. Arab. J. Chem. 16, 104957. doi:10.1016/j.arabjc.2023.104957
Li, C. Y., Zhou, Z., Xu, T., Wang, N. Y., Tang, C., Tan, X. Y., et al. (2022). Aconitum pendulum and Aconitum flavum: a narrative review on traditional uses, phytochemistry, bioactivities and processing methods. J. Ethnopharmacol. 292, 115216. doi:10.1016/j.jep.2022.115216
Li, M. J., Li, C. Y., Sha, M. X., Zhang, Y., and Liu, Y. (2024). Study on mechanism of Aconitum pendulum in treating rheumatoid arthritis based on toxicity efficacy evaluation and metabonomics. Zhongguo Zhong Yao Za Zhi 49, 1774–1784. doi:10.19540/j.cnki.cjcmm.20240115.701
Li S., S., Yu, L., Li, C., Wang, N., Lai, X., Liu, Y., et al. (2023). Optimization of processing technology for Tiebangchui with zanba based on CRITIC combined with box-behnken response surface method. J. Vis. Exp., e65139. doi:10.3791/65139
Liu, D. D., Ma, Z. X., Zhang, X. F., Miao, X., Xin, H. Y., and Li, G. (2018). The metabonomics for mechanism of toxicity reduction and effect preservation for the Mongolian medicine “Aconitum Detoxification by Terminalia”. Chin. J. Hosp. Pharm. 6. doi:10.13286/j.cnki.chinhosppharmacyj.2018.15.08
Liu, H. S., Qu, Y., Yi, X. Y., Han, D. T., Zhang, B. W., Zhen, G. H., et al. (2021). Analysis of the chemical constituents of Euphorbia maculata based on UHPLC-Q-TOF-MS technique. Chin. Med. Mat., 1409–1414. doi:10.13863/j.issn1001-4454.2021.06.021
Liu, S., Li, F., Hou, Y. F., Yang, C., Tan, P., Li, X. R., et al. (2013). Influence of tannins from Chebulae Fructus on aconitum alkaloids of aconitu kusnezoffii processed with Chebulae Fructus-principal of aconitum processed with Chebulae Fructus I. Chin. J. Exp. Tradit. Med., 158–160. doi:10.13422/j.cnki.syfjx.2013.05.068
Liu, S., Li, F., Li, Y., Li, W., Xu, J., and Du, H. (2017). A review of traditional and current methods used to potentially reduce toxicity of Aconitum roots in Traditional Chinese Medicine. J. Ethnopharmacol. 207, 237–250. doi:10.1016/j.jep.2017.06.038
Liu, S., Liu, X. Y., Lin, S., Yang, C., Li, F., and Du, H. (2015). Hydrolysis of simulation. processing products of Aconitum kusnezoffii processed with Terminalia chebula in simulated gastric fluid and intestinal fluid. China Pharm., 1752–1754. doi:10.6039/j.issn.1001-0408.2015.13.07
Liu, S. S. (2021). Study on the chemical constituents of fuzi and aconitm stapfianum. Nanchang: Nanchang University. doi:10.27232/d.cnki.gnchu.2021.003806
Liu, X., Tao, H., Tian, R., Huang, W., Zhang, T., Liu, Y., et al. (2023). Hezi inhibits Tiebangchui-induced cardiotoxicity and preserves its anti-rheumatoid arthritis effects by regulating the pharmacokinetics of aconitine and deoxyaconitine. J. Ethnopharmacol. 302, 115915. doi:10.1016/j.jep.2022.115915
Liu, Y., Yang, X., Zhou, C., Wang, Z., Kuang, T., Sun, J., et al. (2022). Unveiling dynamic changes of chemical constituents in raw and processed fuzi with different steaming time points using desorption electrospray ionization mass spectrometry imaging combined with metabolomics. Front. Pharmacol. 13, 842890. doi:10.3389/fphar.2022.842890
Lu, L., Yue, H., Song, F. R., Tsao, R., Liu, Z. Q., and Liu, S. Y. (2010). Rapid profiling of alkaloids in several medicinal herbs by matrix-assisted laser desorption/ionization mass spectrometry. Chem. Res. Chin. Univ., 11–16. doi:10.1002/mas.1280100503
Mao, J. H., Chen, Z. J., Fang, J., Pan, J. J., wu, C. Q., and Chen, K. J. (2021). Rapid determination of six Tannins in Chinese medicinal materials based on UPLC-MS/MS. Chin. J. Mod. Appl. Pharm., 1055–1059. doi:10.13748/j.cnki.issn1007-7693.2021.09.006
Nonaka, G., and Nishioka, I. (1983). Tannins and related compounds. X. Rhubarb (2): isolation and structures of a glycerol gallate, gallic acid glucoside gallates, galloylglucoses and isolindleyin. Chem. and Pharm. Bull. 31, 1652–1658. doi:10.1248/cpb.31.1652
Pellati, F., Bruni, R., Righi, D., Grandini, A., Tognolini, M., Pio Prencipe, F., et al. (2013). Metabolite profiling of polyphenols in a Terminalia chebula Retzius ayurvedic decoction and evaluation of its chemopreventive activity. J. Ethnopharmacol. 147, 277–285. doi:10.1016/j.jep.2013.02.025
Qiu, Z. D., Zhang, X., Wei, X. Y., Chingin, K., Xu, J. Q., Gao, W., et al. (2021). Online discovery of the molecular mechanism for directionally detoxification of Fuzi using real-time extractive electrospray ionization mass spectrometry. J. Ethnopharmacol. 277, 114216. doi:10.1016/j.jep.2021.114216
Song, L., Mi, S., Zhao, Y., Liu, Z., Wang, J., Wang, H., et al. (2024). Integrated virtual screening and in vitro studies for exploring the mechanism of triterpenoids in Chebulae Fructus alleviating mesaconitine-induced cardiotoxicity via TRPV1 channel. Front. Pharmacol. 15, 1367682. doi:10.3389/fphar.2024.1367682
Su, J. S., Tao, Y. W., Liu, J., Sun, J. Y., Zeng, Y., Meng, X. L., et al. (2023). Tibetan medicine Qi-Sai-Er-Sang-Dang-Song Decoction inhibits TNF-α-induced rheumatoid arthritis in human fibroblast-like synoviocytes via regulating NOTCH1/NF-κB/NLRP3 pathway. J. Ethnopharmacol. 310, 116402. doi:10.1016/j.jep.2023.116402
Sun, C. L., Liu, W., Guo, L. P., and Wang, X. (2021). Analysis on tissue distribution of Metabolites in Lotus seed by MALDI mass spectrometry imaging technique. J. Instrum. Analy, 86–91. doi:10.3969/j.issn.1004-4957.2021.01.012
Sun, L., Liu, F., You, G., Feng, T., Wang, M., Liu, Y., et al. (2020). A comparative analysis of Aconiti Lateralis Radix and processed products using UHPLC-Q-TOF-MS combined with multivariate chemometrics strategies. J. Liq. Chromatogr. R. T, 43, 1–8. doi:10.1080/10826076.2019.1659150
Sun, W. J., Sha, Z. F., Wang, A. X., Zhao, X. W., and Yuan, Z. Z. (1989). Studies on the chemical constituents of Aconitum szechenyianum Gay. Yao Xue Xue Bao 24, 71–74. doi:10.16438/j.0513-4870.1989.01.015
Sun, Y. J., Huo, Z. P., Wang, Y., Li, R. M., Qin, M. J., and He, Y. (2021). Analysis of chemical constituents in jiechangyan Qixiao granules based on UPLC-Q-TOF/MSE. Chin. J. Exp. Trad. Med. Form., 157–167. doi:10.13422/j.cnki.syfjx.20202356
Tang, M., Gao, X., Geng, T., Wang, J. J., Chen, X. L., Cao, L., et al. (2021). Identification of chemical constituents in Qiwei Tongbi oral liquid by HPLC-Q-TOF-MS/MS. Chin. Tradit. Herb. Drugs, 2226–2236. doi:10.7501/j.issn.0253.2670.2021.08.005
Tian, S. Y., Liao, C. H., Zhou, Z. W., Tang, Q., Li, F. Q., Song, S. P., et al. (2022). Research progress and prospects for the ues of plant metabolomics in quality evaluation of traditional Chinese medicinal materials. Acta Pharm. Sin., 1734–1749. doi:10.16438/j.0513-4870.2022-0225
Upadhyay, A., Pooja, A., and Singh, D. K. (2014). A review on the pharmacological aspects of Terminalia chebula. Int. J. Pharmacol. 10, 289–298. doi:10.3923/ijp.2014.289.298
Wang, B., Dong, J., Ji, J., Yuan, J., Wang, J., Wu, J., et al. (2016). Study on the alkaloids in Tibetan medicine Aconitum pendulum Busch by HPLC-MSn combined with column chromatography. J. Chromatogr. Sci. 54, 752–758. doi:10.1093/chromsci/bmw002
Wang, M. D., Zhang, S. Y., and Zhai, H. Y. (2002). The influence of terminalia on toxicokinetic of aconitum decoction. Acta Acad. Med. Neimongol, 219–222. doi:10.16343/j.cnki.issn.2095-512x.2002.04.001
Wang, Y., Zhang, J., Tian, H., Zeng, C., Yao, Z., and Zhang, Y. (2010). Study on processing principle of Aconitum pendulum. Zhongguo Zhong Yao Za Zhi 35, 588–592. doi:10.4268/cjcmm20100510
Wang, Y. J., Zhang, J., Tian, H. P., Ceng, C. J., and Yao, Z. (2010). Study on processing principle of Aconitum pendulm. C J. Chin. Mater. Medica 35, 588–592. doi:10.4268/cjcmm20100510
Wu, L. T. (2023). The development and application of postphotoionization mass spectrometry imaging with vacuum ultraviolet. China: University of Science and Technology of China.
Xiao, Z. J., Yang, X. X., Mei, J. M., Mei, Z. T., Liu, C. S., and Lei, H. M. (2022). Qualitative analysis of chemical constituents in Paullinia cupana leaves and determination of caffeine content. Drug Eval. Res., 60–70. doi:10.7501/j.issn.1674-6376.2022.01.007
Xu, W., Zhang, J., Zhu, D. Y., Huang, J., Huang, Z. H., Bai, J. Q., et al. (2014). Rapid separation and characterization of diterpenoid alkaloids in processed roots of Aconitum carmichaeli using ultra high performance liquid chromatography coupled with hybrid linear ion trap-orbitrap tandem mass spectrometry. J. Sep. Sci. 37, 2864–2873. doi:10.1002/jssc.201400365
Yan, G. L., Sun, H., Sun, W. J., Zhao, L., Meng, X. C., and Wang, X. J. (2010). Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra performance liquid chromatography-high resolution mass spectrometry and automated data analysis. J Pharm. and Biomed. Analy 53, 421–431. doi:10.1016/j.jpba.2010.05.004
Yang, M. H., Ali, Z., Khan, I. A., and Khan, S. I. (2014). Anti-inflammatory activity of constituents isolated from Terminalia chebula. Nat. Product. 9, 965–968. doi:10.1177/1934578x1400900721
Yang, Y., Yin, X. J., Guo, H. M., Wang, R. L., Song, R., Tian, Y., et al. (2014). Identification and comparative analysis of the major chemical constituents in the extracts of single fuzi herb and fuzi-gancao herb-pair by UFLC-IT-TOF/MS. Chin. J. Nat. Med. 12, 542–553. doi:10.1016/S1875-5364(14)60084-4
Yang, Y. Y., Miao, S., and Li, W. T. S. J. (2023). Research progress on plant metabolomics in root and rhizome traditional Chinese medicine. Chine Tradit. Herb. Drugs, 6856–6865. doi:10.7501/j.issn.0253-2670.2023.20.029
Zhang, D. (2010). Protective effects of Terminalia chebula extract on cultured rat myocardial cells injury induced by Aconitine. Chin. J. Eth Med., 016. doi:10.16041/j.cnki.cn15-1175.2010.03.027
Zhang, H. M., Li, S. L., Zhang, H., Wang, Y., Zhao, Z. L., Chen, S. L., et al. (2012). Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J. Pharm. Biomed. Anal. 62, 258–273. doi:10.1016/j.jpba.2012.01.010
Zhang, X., Cui, Y., Miao, X., Liu, D., Ma, Z., and Li, G. (2017). Protective effects of Mongolian medicine Terminalia chebula on cardiotoxicity induced by Aconitum kusnezoffii in rats. J. Chin. Med. Mater, 2693–2696. doi:10.13863/j.issn1001-4454.2017.11.044
Zhang, X. R., Qiao, Y. J., Zhu, H. T., Kong, Q. H., Wang, D., Yang, C. R., et al. (2021). Multiple in vitro biological effects of phenolic compounds from Terminalia chebula var. tomentella. J. Ethnopharmacol. 275, 114135. doi:10.1016/j.jep.2021.114135
Zhao, L. (2021). Study on quality markers of Mongolian medicine Zhachongshisanwei pills. Tianjin Univ. Chin. Med. doi:10.27368/d.cnki.gtzyy.2021.000082
Zhao, L., Zhang, S. L., Tao, J. Y., Pang, R., Jin, F., Guo, Y. J., et al. (2008). Preliminary exploration on anti-inflammatory mechanism of Corilagin (beta-1-O-galloyl-3,6-(R)- hexahydroxydiphenoyl-D-glucose) in vitro. Int. Immunopharmacol. 8, 1059–1064. doi:10.1016/j.intimp.2008.03.003
Zheng, W., Yang, X., Dang, B., Zhang, W., Zhang, J., Feng, Y., et al. (2024). Changes in secondary metabolites of grape skins in response to different postharvest dehydrationtemperatures as evaluated by UPLC-Q-TOF-MS. Food Meas. 18, 125–136. doi:10.1007/s11694-023-02146-6
Zhi, M., Liu, K., Han, S., Xu, J., Li, W., Li, F., et al. (2020). Influence of different dosage forms on pharmacokinetics of 6 alkaloids in raw Aconiti kusnezoffii radix (Caowu) and Chebulae Fructus- (Hezi-) processed Caowu by UPLC-MS/MS. Biomed. Res. Int. 2020, 1942849. doi:10.1155/2020/1942849
Zhi, M. R., Gu, X. R., Han, S., Liu, K. Y., Liu, Z. Q., Tang, Y. N., et al. (2020). Chemical variation in aconti Kusnezoffii radix before and after processing based on UPLC-orbitrap-MS. Chin. J. Chin. Mater Med. 45, 1082–1089. doi:10.19540/j.cnki.cjcmm.20191221.301
Zhou, K., Jian, P., Liang, W. Y., Liang, L. J., Ye, T., Chang, Z. H., et al. (2020). Analysis on chemical constituents from Terminalia chebula Retz. and Terminalia bellerica (Gaertn.) Roxb. By UPLC-Q-exactive quadrupole-orbitrap mass spectrometry. J. Chin. Mass Spectrom. Soc., 254–267. doi:10.7538/zpxb.2019.0070
Keywords: Tiebangchui, UPLC-Q-ToF-MS, Hezi-decoction-processed, toxicity attenuation, DESI-MSI
Citation: Zhou Z, Gao H-M, Pei Z-Q, Sha M-X, Li C-Y, Zhang Y, Su J-S, Liu Y and Meng X-L (2025) Unveiling the processing mechanism of Hezi-decoction-processed Tiebangchui: a synthesis approach using UPLC-Q-TOF-MS-based metabolomics and DESI-MSI. Front. Pharmacol. 16:1534748. doi: 10.3389/fphar.2025.1534748
Received: 26 November 2024; Accepted: 27 February 2025;
Published: 07 April 2025.
Edited by:
Irina Ielciu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Lanlan Fan, Guangxi University of Chinese Medicine, ChinaCopyright © 2025 Zhou, Gao, Pei, Sha, Li, Zhang, Su, Liu and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Li Meng, eGxtOTk5QGNkdXRjbS5lZHUuY24=; Yue Liu, bGl1eXVlMkBjZHV0Y20uZWR1LmNu; Jin-Song Su, c3VqaW5zb25nQGNkdXRjbS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.