- 1Department of Hepatology, Center of Infectious Diseases and Pathogen Biology, The First Hospital of Jilin University, Changchun, Jilin, China
- 2Key Laboratory of Zoonosis Research, Ministry of Education, The First Hospital of Jilin University, Changchun, China
Introduction: Liver fibrosis is a pathological condition in response to chronic liver injuries. Currently, there is no Food and Drug Administration (FDA) approved pharmacotherapy for liver fibrosis. Advances in understanding hepatic fibrogenesis have led to the development of anti-fibrotic agents, and some of them have shown promise in phase 3 and above clinical trials. However, adverse drug reactions (ADRs) associated with emerging anti-fibrotic agents may hinder their efficacy and clinical applicability. This study assessed ADRs associated with anti-fibrotic agents as reported in the World Health Organization (WHO) VigiAccess database and compared the adverse reaction characteristics of these agents for optimizing therapeutic strategies.
Methods: A detailed search was conducted on ClinicalTrial.gov to identify phase 3 or 4 clinical trials involving hepatic anti-fibrotic agents. The ADR reports were retrieved from the WHO-VigiAccess database, with data categorized by demographic characteristics, geographic distribution, and System Organ Classes (SOCs). The most frequently reported ADRs were identified through descriptive analysis. Disproportionality analysis, measured by reporting odd ratio (ROR) and proportional reporting ratio (PRR), was performed to evaluate ADRs related to gastrointestinal disorders.
Results: Five hepatic anti-fibrotic agents (empagliflozin, liraglutide, candesartan, obeticholic acid, and resmetirom) were identified. A total of 130,567 ADR reports were analyzed, with empagliflozin, liraglutide, and candesartan showing significantly higher ADRs. The most frequently reported SOCs included gastrointestinal disorders (29.44%), general disorders (24.12%), and nervous system disorders (14.42%). Liraglutide demonstrated a higher risk of gastrointestinal ADRs (ROR: 4.629, 95% CI: 4.517–4.744; PRR: 3.566, 95% CI: 3.492–3.642) compared to the other agents. Severe ADRs were reported in empagliflozin, such as ketoacidosis and infections, while liraglutide was associated with pancreatitis and candesartan with acute kidney injury. Serious ADR rates varied, with candesartan reporting the highest proportion (7.28%).
Conclusion: While hepatic anti-fibrotic agents showed promise in addressing liver fibrosis, their ADR profiles underscore the importance of pharmacovigilance and personalized treatment approaches. Future efforts should focus on improving the pharmacovigilance system, expanding population diversity in trials, and conducting ongoing research and extensive post-marketing surveillance.
Introduction
Liver fibrosis is a pathological condition with the formation of fibrous scar in response to persistent liver injury caused by viral infection, alcohol abuse, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and biliary obstruction. Regardless of the etiologies, persistent liver fibrosis can lead to distorted hepatic architecture, impaired liver function, cirrhosis, and ultimately, liver failure or hepatocellular carcinoma (HCC), which imposes significant clinical and economic burdens worldwide. Despite its reversible nature during early stages, effective pharmacological interventions targeting hepatic fibrosis remain an unmet medical need (Kisseleva and Brenner, 2021).
Currently, there is no Food and Drug Administration (FDA) approved pharmacotherapy for liver fibrosis. However, advancements in our understanding of hepatic fibrogenesis have spurred the development of anti-fibrotic agents aimed at modulating pathways involved in inflammation, hepatic stellate cell (HSC) activation, oxidative stress, and extracellular matrix remodeling (Tsuchida and Friedman, 2017; Parola and Pinzani, 2019; Hammerich and Tacke, 2023). Targeting these pathways, the small molecular inhibitors or agonists, are promising candidates for alleviating fibrosis, slowing disease progression, and improving clinical outcomes. In March 2024, the thyroid hormone receptor beta (THR-β) selective agonist resmetirom gained conditional approval from FDA as the first pharmacologic treatment for NASH patients with moderate-to-advanced liver fibrosis (FDA, 2024). In a phase 3 trial, resmetirom was superior to placebo in NASH resolution and improvement in hepatic fibrosis (Harrison et al., 2024). Glucagon-like peptide 1 (GLP-1) receptor agonists have been widely used to treat obesity and type 2 diabetes mellitus (T2DM). It has been shown that GLP-1 receptor treatment decreased histological inflammation and fibrosis in NASH patients (Nahra et al., 2021; Tan et al., 2022; Sanyal et al., 2024).
The major concern of hepatic anti-fibrotic agents is their adverse effects that may limit their efficacy and broad applicability. A comprehensive evaluation of these side effects is critical to balancing therapeutic benefits against potential risks and ensuring safe, patient-centered care. Therefore, pre-clinical tests and clinical trials are promising and urgently required. Assessing drug safety based on real-world large sample data is also worth further study. Currently, using spontaneous reporting systems (SRS) to collect real-world medication safety data is considered a more credible and reliable approach. The Uppsala Monitoring Center (UMC), representing the World Health Organization (WHO) programme for International Drug Monitoring (PIDM), has gathered global data on adverse drug reactions (ADRs) and developed the VigiBase database. Altogether, the WHO programme members today represent nearly 99% of the world’s population and contribute data to VigiBase with around 40 million ADRs (http://who-umc.org/vigibase/).
The hepatic anti-fibrotic agents in ongoing clinical research, especially in phase 3 and above clinical trials, showed good efficacy characteristics and are the most promising medications to reach the market for the treatment of liver fibrosis. In the present study, we retrieved hepatic anti-fibrotic agents in phase 3 and above clinical trials registered in ClinicalTrials.gov and performed a descriptive analysis of ADR reports in the WHO-VigiAccess database. We also conducted the disproportionality analysis to assess the gastrointestinal ADRs of these agents, as gastrointestinal disorders are the most common side effects of anti-fibrotic agents. By analyzing these data, we seek to highlight current challenges in antifibrotic drug development and outline future directions for achieving safer and more effective therapies for liver fibrosis.
Materials and methods
Identify target hepatic anti-fibrotic agents
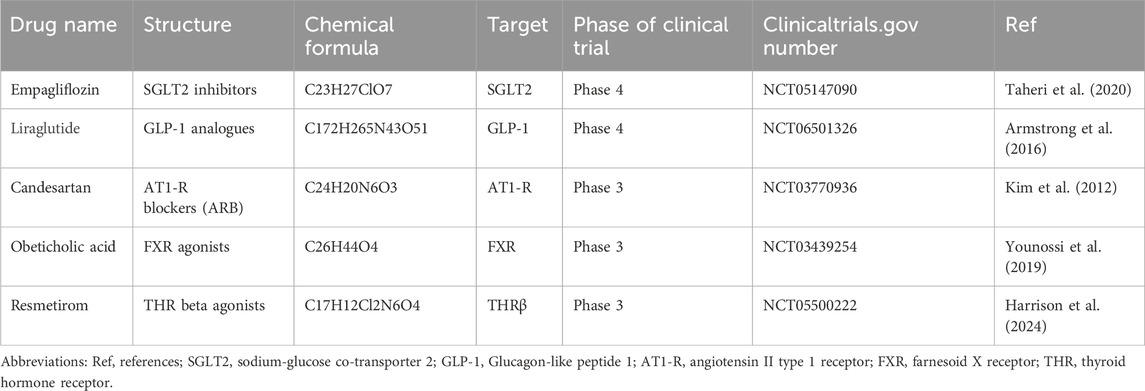
ClinicalTrial.gov is an authoritative database of clinical research, which is maintained by the National Library of Medicine. The website (http://clinicaltrials.gov) was searched in detail to find clinical trials of hepatic anti-fibrotic agents registered before October 30, 2024. The research focus of “Condition/disease” comprised the terms “liver fibrosis” OR “hepatic fibrosis”. Manual searches were performed based on electronic searches as a supplement. Clinical trials were included if they met the following criteria: (1) study phase: phase 3 or phase 4; (2) study status: recruiting or completed with results; (3) study type: interventional studies. Then, the whole protocols were screened manually of which studies met the inclusion criteria. Finally, five anti-fibrotic agents “Empagliflozin”, “Liraglutide”, “Candesartan”, “Obeticholic acid”, “Resmetirom” were identified.
Data sources
The WHO-VigiAccess database (https://www.vigiaccess.org) was searched on November 1, 2024, for all reported ADRs following the above five anti-fibrotic agent treatments. Data were collected among age, groups, sex, report year, and continents of the world by WHO-VigiAccess.
WHO-VigiAccess is a publicly accessible online platform that provides access to data from Vigibase. It is designed to promote transparency and facilitate research by offering insights into potential ADRs reported by healthcare professionals, patients, and regulatory authorities from over 150 countries. The definition relied on system organ classes (SOCs) and preferred terms (PTs) by the Medical Dictionary for Regulatory Activities (MedDRA) and WHODrug. Thus, records on each anti-fibrotic agent were retrieved, and all individual ADRs based on MedDRA SOC and PT levels recorded were identified to describe the spectrum of toxicities. A total of 27 items were classified by SOC and analyzed in the present study. Moreover, we focused on the PTs to identify the most common ADRs of each anti-fibrotic agent. According to outcome codes, three severity categories were defined to evaluate the safety results: death, hospitalization, and major events, including life-threatening events, disabilities, and congenital abnormalities.
Disproportionality analysis
In pharmacovigilance study, disproportionality emerges when a specific ADR is associated with a given drug. The aim of disproportionality analysis is to identify statistical associations between a specific drug and ADRs of interest (Bate and Evans, 2009). The reporting odds ratio (ROR) (van Puijenbroek et al., 2002) and the proportional reporting ratio (PRR) (Evans et al., 2001) are the two most commonly used metrics for disproportionality analysis. They are calculated to measure the likelihood of imbalance of reporting an ADR for a specific drug in comparison to other drugs. The calculation formula of ROR and 95% confidence interval (CI) is as follows:
Where a, b, c, and d indicated the number of reports for the specific drug with specific ADR, the specific drug with other ADRs, the other drugs with specific ADR, and the other drugs with other ADRs, respectively. If the ROR value is greater than 2 (ROR >2) and the lower limit of the 95% CI is greater than one at the same time, with at least three cases, the ROR is considered significant and there may be disproportionate and potentially indicative of a safety risk. PRR is another index that measures the disproportionality of ADR reports. It is calculated as:
When the value of the PRR is greater than 2 with at least three cases, the PRR was considered significant. These criteria ensure that the observed disproportionality is not the result of random variability. By using ROR and PRR in our research, we were able to thoroughly evaluate the disproportionality of gastrointestinal issues linked to hepatic anti-fibrotic agent use.
Statistical analysis
The descriptive analysis was performed to illustrate the characteristics of ADRs associated with the five hepatic anti-fibrotic agents. Descriptive variables were categorized using frequency and percentage. All analyses were conducted using R programming software (version 4.4.0), with statistical significance set at a p-value of less than 0.05. Forest plots were created using the “forestplot” R package (Version 3.1.5).
Results
Basic information on five hepatic anti-fibrotic agents
Table 1 shows the basic information of five hepatic anti-fibrotic agents that we have studied for clinical research. Figure 1 illustrates their unique mechanisms of action. Each of the agents has a unique molecular target. Empagliflozin is a kind of sodium-glucose co-transporter 2 (SGLT2) inhibitor, which could attenuate HSC activation and fibrogenesis (Shen et al., 2023). Studies have demonstrated that empagliflozin improves liver fibrosis in patients with NAFLD without T2DM (Taheri et al., 2020; Takahashi et al., 2024). It is now in the phase 4 clinical trial for the treatment of liver fibrosis. Liraglutide is a kind of long-acting GLP-1 analogue. It has been shown to reduce insulin resistance, oxidative stress, and mitochondrial damage as well as improve liver histology (Armstrong et al., 2016). Its effects were evaluated during phase 4 period for NAFLD patients with liver fibrosis. The renin-angiotensin system can be an attractive hepatic anti-fibrotic target. The effects of candesartan, a kind of angiotensin II type 1 receptor (AT1-R) blocking (ARB) agent have been explored in patients with alcoholic liver fibrosis (Kim et al., 2012). Currently, a phase 3 clinical trial is ongoing to evaluate its efficacy on liver fibrosis in patients with chronic hepatitis C. Obeticholic acid, a farnesoid X receptor (FXR) agonist, has been shown to improve the liver histological features of NASH patients (Younossi et al., 2019). The phase 3 study evaluating the efficacy of obeticholic acid in cirrhotic patients due to NASH has been completed. Resmetirom is an oral, liver-directed, selective THR-β agonist that showed efficacy in NASH resolution and improvement in liver fibrosis. The phase 3 study is ongoing to evaluate the effectiveness of resmetirom in NASH patients with compensated cirrhosis, and the latest results showed that it was effective in these patients. Patients with F2–F3 fibrosis will be enrolled in the advanced research.
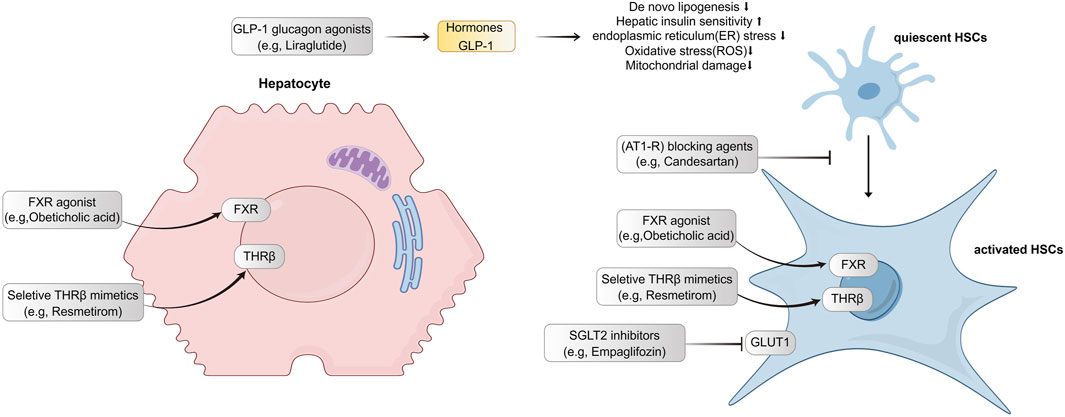
Figure 1. Targets and mechanisms of action for five hepatic-antifibrotic agents. Their regulation pathways are mainly associated with hepatocyte injuries and hepatic stellate cell activation. GLP-1, Glucagon-like peptide 1; FXR, farnesoid X receptor; THR, thyroid hormone receptor; GCGR, glucagon receptor; HSC, hepatic stellate cell; AT1-R, angiotensin II type 1 receptor; SGLT2, sodium-glucose co-transporter 2; GLUT1, glucose transporter type 1.
Overall characteristics of ADR reports for five anti-fibrotic agents
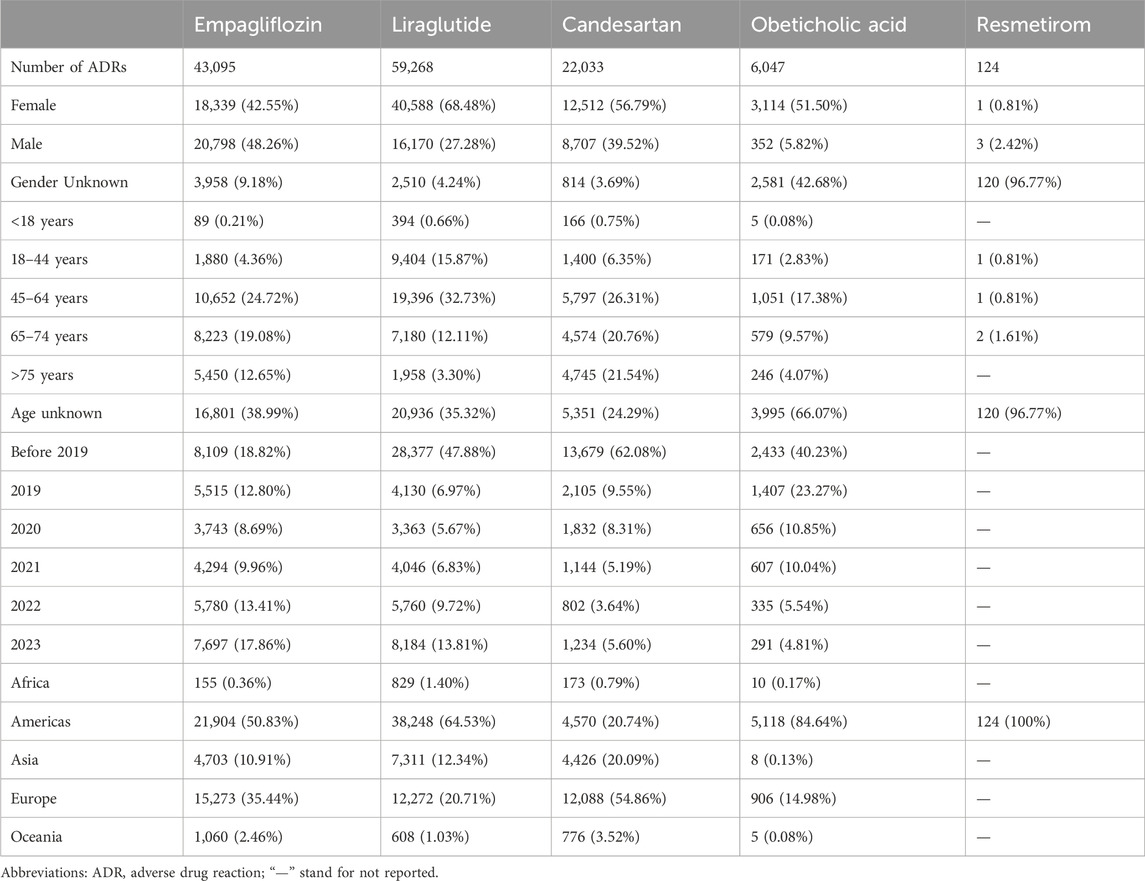
The earliest reports of empagliflozin, liraglutide, candesartan, obeticholic acid, and resmetirom were received in the WHO-VigiAccess database in 2014, 2000, 1998, 2017, and 2024, respectively. Up to now (by November 2024), the WHO has received 43,095, 59,268, 22,033, 6,047, and 124 ADR reports for these five agents, respectively, with a total of 130,567 reports. The numbers of ADR reports were 42,347 cases of empagliflozin, 58,984 cases of liraglutide, 21,896 cases of candesartan, 5,607 cases of obeticholic acid, and 124 cases of resmetirom. Among the 130,567 ADR reports related to the five anti-fibrotic agents shown in Table 2, except for 9,983 cases in which the sex was unknown, the number of women (74,554) who had ADRs was significantly greater than that of men (46,030), and the female-male ratio was 1.62:1 with a significant discrepancy. Excluding the unknown age reports, most of the age groups with the highest reported rates are between 45 and 64 years. Most of the ADR reports were from the Americas (53.58%), followed by Europe (31.05%). Table 2 also lists the reporting years for each of the studied medications.
Distribution of SOCs for five anti-fibrotic agents
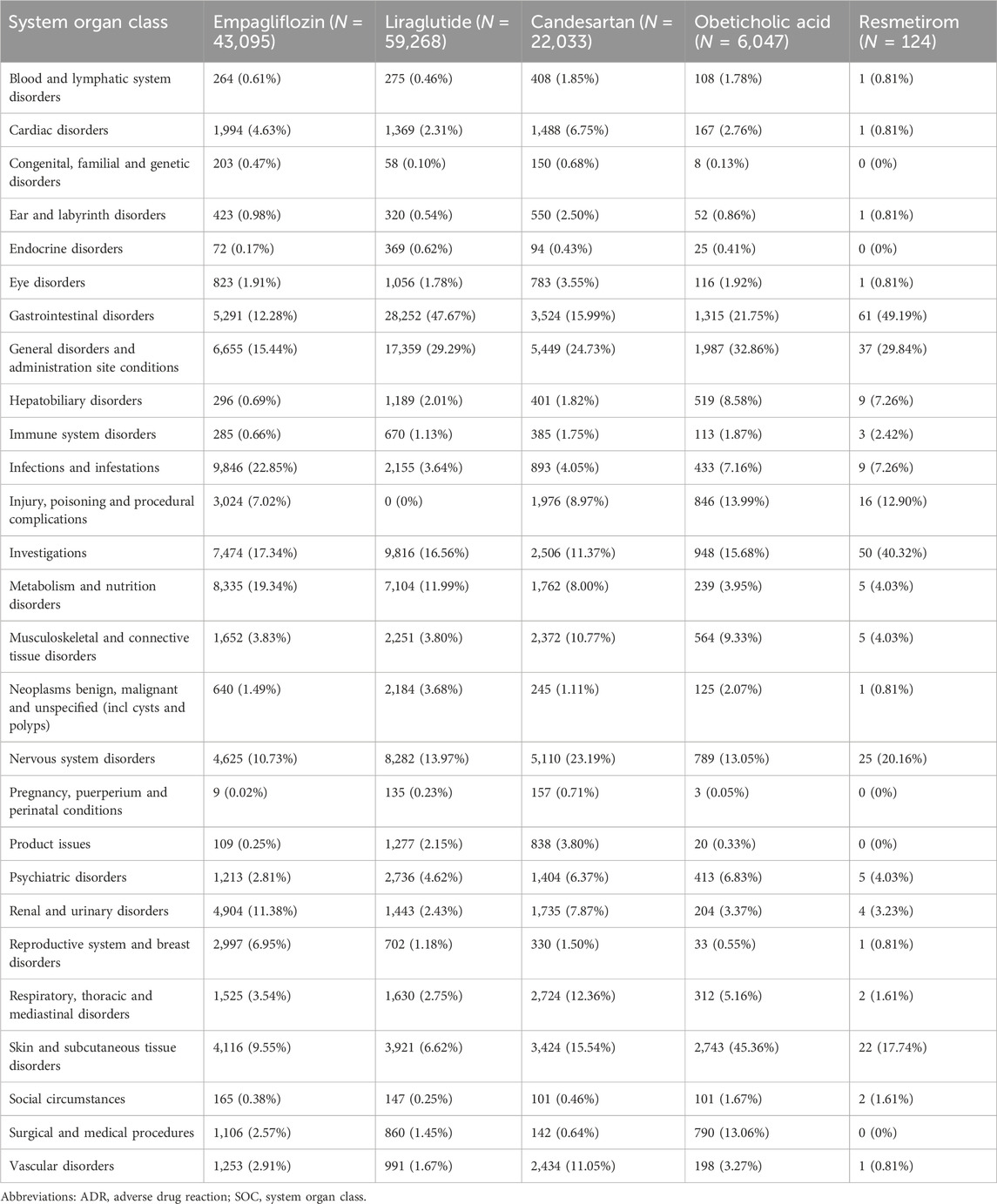
Table 3 presents the reporting rates of 27 SOCs for five anti-fibrotic agents. Empagliflozin, liraglutide, and candesartan, due to their longer usage duration, have significantly higher incidence rates of diseases in various systems and organs than the other two novel anti-fibrotic agents. The five most common types of ADRs are as follows: gastrointestinal disorders (38,443 cases, 29.44%), general disorders and administration site conditions (31,487 cases, 24.12%), investigations indicating altered biochemical parameters (7,718 cases, 15.93%), nervous system disorders (18,831cases, 14.42%). The highest ADR report rates were reported as gastrointestinal disorders in liraglutide and resmetirom, whereas candesartan showed the highest ADR report rate related to general disorders and administration site conditions. The most common ADRs for empagliflozin were associated with Infections and infestations, and for obeticholic acid with skin and subcutaneous tissue disorders. Among the ADRs, there were seven types of SOCs for empagliflozin whose incidence rates exceeded 10%, five types for liraglutide, four types for candesartan, four types for obeticholic acid, as well as three types for resmetirom.
The most common ADR reports for five anti-fibrotic agents
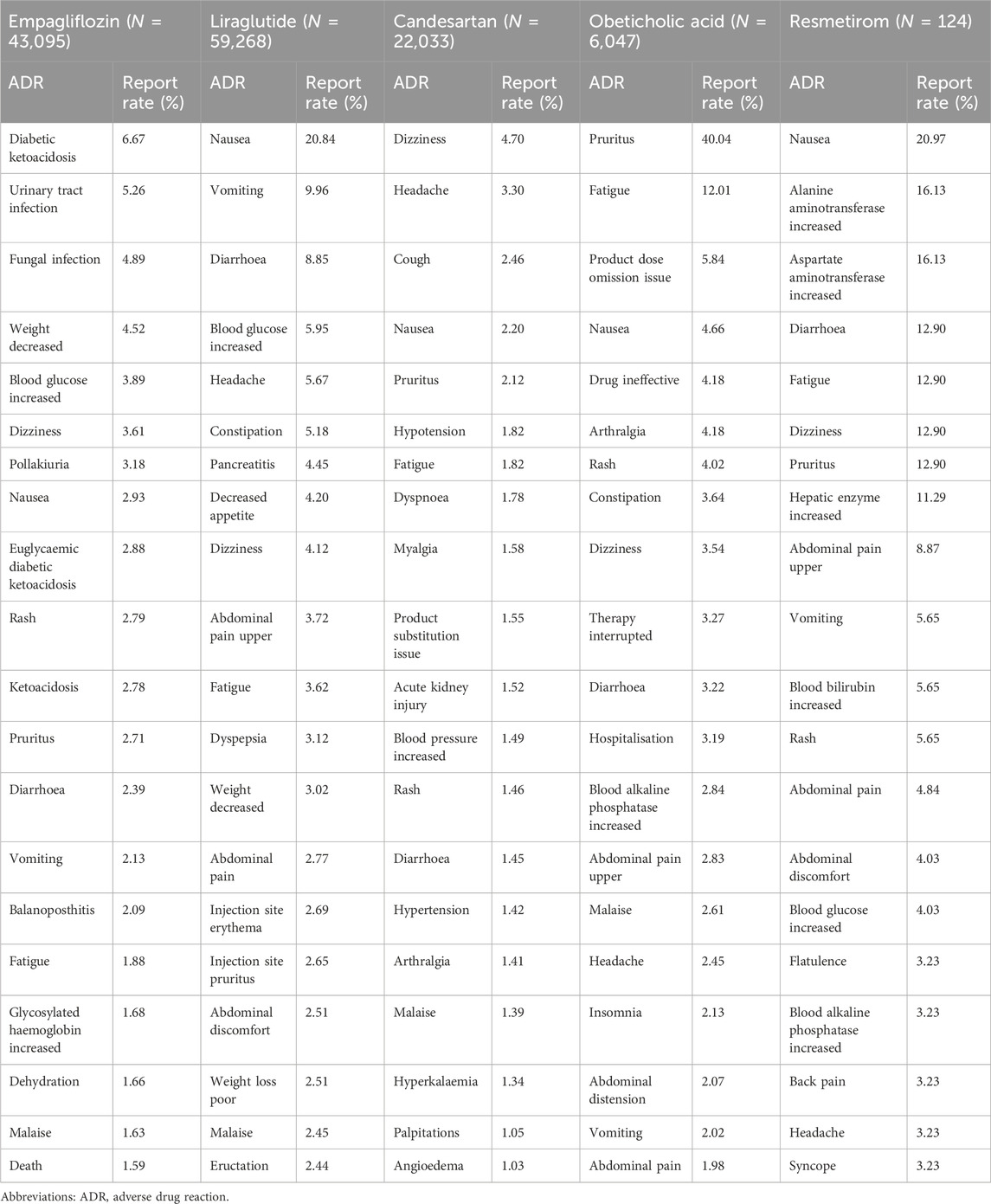
The top 20 most reported ADRs of the five anti-fibrotic agents are presented in Table 4, and the manifestations listed were PTs from within the SOCs. The common ADRs of all five agents were nausea, diarrhoea, fatigue, and dizziness, and most of the ADRs were related to gastrointestinal disorders. The common ADRs for empagliflozin included some life-threatening events, such as ketoacidosis, infections (urinary tract infection and fungal infection), dehydration, and even death, which required extra vigilance. Most of the ADRs in the top 20 for the other four agents were minor events that are self-limiting. However, there are some noteworthy events, such as a higher reported rate of pancreatitis when using liraglutide, and a higher reported rate of acute kidney injury (AKI) when using candesartan.
Serious ADRs of five anti-fibrotic agents
Through the WHO-VigiAccess, we can also find major ADRs of anti-fibrotic agents, including life-threatening events, disability, and congenital malformations. The proportion of serious ADRs that occurred for empagliflozin, liraglutide, candesartan, obeticholic acid, and resmetirom was 1.74%, 0.48%, 7.28%, 0.62%, and 0%, respectively (Figure 2).
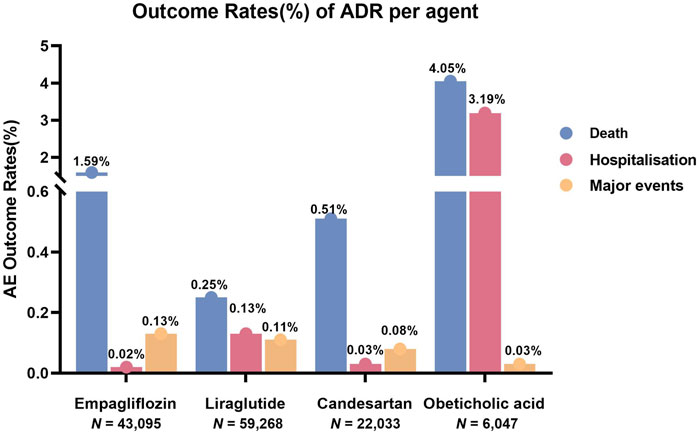
Figure 2. Outcomes for serious ADRs associated with five anti-fibrotic agents. Major events comprising life-threatening events, disability, and congenital anomaly.
Disproportionality analysis based on gastrointestinal disorders and related ADRs
By observing and comparing the SOC distributions of five anti-fibrotic agents, it was found that gastrointestinal disorder related symptoms were the most common ADR. To further compare these five agents, we performed a disproportionality analysis based on gastrointestinal disorders and three most common PTs (nausea, vomiting, diarrhoea). As shown in Figure 3, liraglutide might be associated with a greater risk of gastrointestinal disorder than the other agents with the ROR value of 4.629 (95% CI: 4.517–4.744). Moreover, liraglutide treatment also showed a significant risk of nausea (ROR: 5.853, 95% CI: 5.604–6.114), vomiting (ROR: 4.738, 95% CI: 4.465–5.027), and diarrhoea (ROR: 3.174, 95% CI: 3.009–3.348) compared to the other agents. Similar results were obtained assessed by the other metric, with the PRR value of liraglutide treatment was 3.566 (95% CI: 3.492–3.642) in gastrointestinal disorders, 5.392 (95% CI: 5.169–5.624) in nausea, 4.572 (95% CI: 4.313–4.847) in vomiting, 3.087 (95% CI: 2.929–3.252) in diarrhoea (Supplementary Figure S1).
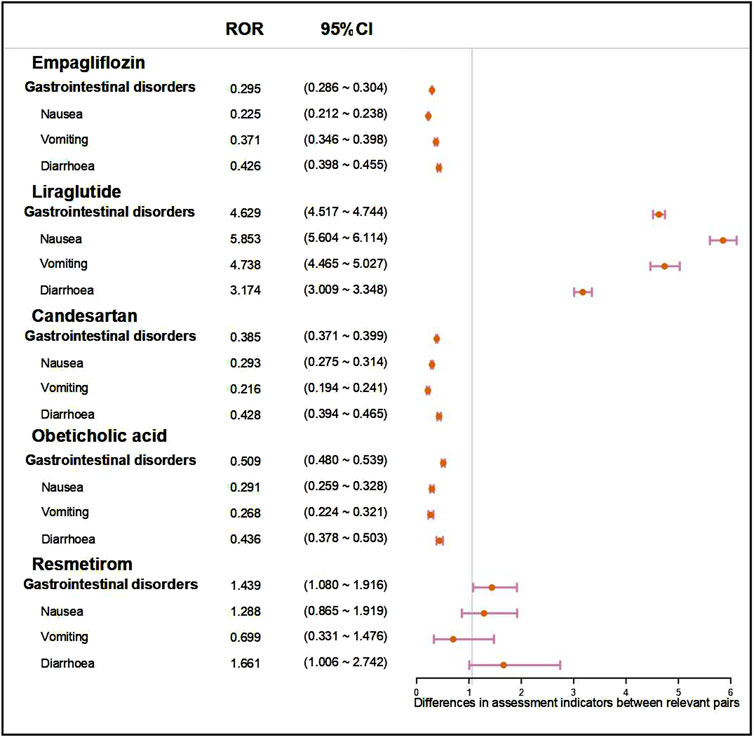
Figure 3. Forest plot showed the ROR value and 95%CI of five anti-fibrotic agents on gastrointestinal disorders and three most common symptoms (nausea, vomiting, diarrhoea). ROR, reporting odd ratio; 95% CI, 95% confidence interval.
Discussion
The global health burden of liver fibrosis is increasing due to its progression to cirrhosis and HCC if untreated. While recent advancements have shed light on potential anti-fibrotic agents, the adverse effects pose significant challenges to their clinical applications. This study provides a comprehensive analysis of ADRs associated with hepatic anti-fibrotic agents currently under investigation in phase 3 and above clinical trials, using data derived from the WHO-VigiAccess database. By focusing on five promising agents, empagliflozin, liraglutide, candesartan, obeticholic acid, and resmetirom, the study demonstrated overall ADR profiles, the distribution of SOCs, and the disproportionality of gastrointestinal disorder related ADRs. These findings not only underscored the challenges in pharmacotherapy for liver fibrosis but also suggested critical considerations for clinical practice.
The five anti-fibrotic agents exhibit distinct safety profiles influenced by their pharmacological mechanisms, usage history, and targeted patients. SGLT2 inhibitors, including empagliflozin, are widely used as hypoglycemic drugs in T2DM patients, especially with renal impairment (Fioretto et al., 2016) for a considerable period. Liraglutide is an approved obesity pharmacotherapy with proven clinical effectiveness and long-term benefits (Davies et al., 2015; Papamargaritis et al., 2024). As a kind of ARB agent, candesartan is a long-established anti-hypertensive medication. These three agents demonstrated higher ADR report rates, attributed to their longer clinical use and broader patient base. In contrast, obeticholic acid and resmetirom, as novel anti-fibrotic agents, showed fewer reports yet revealed ADR patterns consistent with their pharmacological targets.
The study revealed a significant number of ADR reports across the five anti-fibrotic agents, with 130,567 reports. The predominance of ADRs is shown in females, possibly reflecting a greater propensity for women to report ADRs or sex-related pharmacokinetic differences. Age distribution indicated a peak in ADRs among individuals aged 45–64 years. Aging increases the likelihood of long-term exposure to liver-damaging conditions, and many liver diseases progress slowly, with fibrosis accumulating over decades, eventually leading to cirrhosis (Powell et al., 2021). Geographic variations in ADRs, with a majority from the Americas (53.38%) and Europe (31.05%), may reflect regional differences in drug availability, healthcare systems, and pharmacovigilance practices.
Specific agents exhibited distinct ADR profiles. Empagliflozin demonstrated unique risks, and several life-threatening events, including ketoacidosis, dehydration, and serious infections. SGLT2 inhibitors provide a protective effect on the kidneys via reduced transglomerular pressure and increased urinary glucose excretion in the renal proximal tubules (Saisho, 2020). However, glucosuria increases the risk of genitourinary tract infection and dehydration (Donnan et al., 2019), and glucagon secretion may lead to an overproduction of ketone bodies (Ogawa and Sakaguchi, 2016). Therefore, empagliflozin treatment requires close monitoring of blood glucose and ketone body levels in cirrhotic patients, particularly in those with comorbidities such as diabetes. Candesartan showed high rates of life-threatening events, most frequently associated with AKI. As a common anti-hypertensive drug, candesartan causes vasodilation of the renal efferent arteriole and results in the glomerular filtration rate reduction and ultimately AKI (Schoolwerth et al., 2001). The real-world data showed that the rising creatinine (>10%) after initiation of ARB treatment was associated with worse health outcomes (Fu et al., 2019). Therefore, candesartan should be used with caution for the treatment of liver fibrosis when patients suffer from renal insufficiency, and renal function (including serum creatinine and eGFR) monitoring is necessary during treatment. The relatively low serious ADR rates for obeticholic acid suggest a favorable risk profile in current clinical contexts. As the most advanced agent in FXR agonists, obeticholic acid showed beneficial effects on hepatic cholestatic fibrosis and has been approved as the second-line treatment for primary biliary cholangitis (Lindor et al., 2019). Resmetirom displayed minimal serious ADRs, and the most common adverse events were generally mild, transient diarrhea and nausea at treatment initiation (Harrison et al., 2019; Harrison et al., 2024). Therefore, it is promising to be the first FDA-approved treatment for liver fibrosis.
Gastrointestinal disorders were the most reported ADRs, accounting for nearly one-third of all cases (29.44%). This pattern aligns with the known mechanisms of hepatic anti-fibrotic agents, many of which target metabolic and inflammatory pathways closely linked to gastrointestinal physiology. The results of disproportionality analysis showed that liraglutide exhibited a significantly high risk of gastrointestinal ADRs, particularly nausea, vomiting, and diarrhoea. As a kind of GLP-1 analogue, it regulates gastric emptying and metabolic dysfunction that induced gastrointestinal symptoms. Clinical studies reported that gastrointestinal complaints often occurred within the first week of treatment, and most of the subjects reported ADRs of mild to moderate severity (Degn et al., 2004; Vilsbøll, 2007). Despite these risks, liraglutide remains a promising agent for liver fibrosis treatment. Thus, strategies to mitigate these side effects, such as dose titration and dietary modifications are required.
Currently, there are some agents in phase 2 or about to begin phase 3 clinical trials for the treatment of liver fibrosis with encouraging results. For example, aramchol is a partial inhibitor of the stearoyl-CoA desaturase 1 (SCD1) enzyme that regulates the fatty acid production process. In a phase 2 study, NASH resolution and liver fibrosis improvement were observed of aramchol (Ratziu et al., 2021; Ratziu et al., 2024). The direct anti-fibrotic drug pirfenidone which is approved for the treatment of idiopathic lung fibrosis (Lancaster et al., 2017) has been confirmed hepatoxic (Poo et al., 2020). However, hydronidone, a novel structural modification of pirfenidone, has showed less hepatoxicity. In a phase 2 study, hydronidone plus entecavir showed a significant reduction of liver fibrosis degree in patients with chronic hepatitis B (CHB). These are promising pharmacotherapies for liver fibrosis; however, as an investigational drug, the ADRs cannot be searched from the WHO-VigiAccess database at present.
The study analyzed the large dataset from a globally representative pharmacovigilance system, and it focused on agents in phase 3 and above clinical trials, which are pivotal in determining whether a new treatment is safe and effective for widespread use. The diverse safety profiles of these agents highlight the importance of personalized treatment strategies. Clinicians must weigh the therapeutic benefits against potential risks, particularly in populations at higher risk of serious ADRs (e.g., elders, patients with comorbidities). Regular biochemical and clinical evaluations can help detect early signs of complications, such as renal impairment and ketoacidosis. Moreover, gastrointestinal ADRs, while not life-threatening, can significantly impact patient adherence. Strategies to alleviate these side effects and concurrent use of supportive therapies should be explored in clinical practice.
This study has several limitations. First, as an SRS, the WHO-VigiAccess is inherently limited by reporting biases and underreporting. Moreover, the data relies on voluntary reporting may not fully capture the true scope of ADRs for anti-fibrotic agents. This is an inherent limitation in the context of clinical trials, especially when evaluating emerging therapies for liver fibrosis. When approved therapies become available, more comprehensive data on ADRs will be obtained through a mandatory reporting system. Second, the demographic skew towards the Americas and Europe in ADRs underscores the need for greater representation from other regions, particularly Asia and Africa, where liver fibrosis prevalence is high. Third, the long-term safety of hepatic anti-fibrotic agents remains uncertain. Longitudinal studies with diverse populations are essential to evaluate cumulative risks and rare ADRs.
The pipeline of hepatic anti-fibrotic agents offers immense promise, but achieving a balance between efficacy and safety remains challenging. Incorporating real-world evidence, as demonstrated in this study, into drug development processes can help identify safety signals early, and guide the design of phase 4 trials and future markets. Moreover, combination therapies targeting multiple pathways in liver fibrogenesis may enhance while reducing dose-dependent risks of ADRs. For instance, pairing agents with complementary mechanisms could achieve synergistic effects with fewer ADRs.
Conclusion
The safety profiles of hepatic anti-fibrotic agents present both opportunities and challenges for clinical practice and drug development. While these agents showed promise in treating liver fibrosis, their ADR profiles need careful consideration in clinical practice. Future efforts should focus on improving the pharmacovigilance system, expanding population diversity in trials, and conducting ongoing research and rigorous post-marketing surveillance. By addressing these challenges, we can achieve safer and more effective therapies for liver fibrosis, ultimately improving outcomes for patients worldwide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing–original draft. XZ: Data curation, Formal Analysis, Methodology, Writing–review and editing. XW: Data curation, Formal Analysis, Methodology, Writing–review and editing. QZ: Conceptualization, Funding acquisition, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Doctor of excellence program (DEP), The First Hospital of Jilin University (Grant number:JDYY-DEP-2024031).
Acknowledgments
We acknowledge the exceptional contributions made by the Uppsala Monitoring Center. We sincerely appreciate the WHO for providing the data. We express our gratitude to Figdraw (www.figdraw.com) for expert assistance in the pattern drawing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1534628/full#supplementary-material
References
Armstrong, M. J., Gaunt, P., Aithal, G. P., Barton, D., Hull, D., Parker, R., et al. (2016). Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387 (10019), 679–690. doi:10.1016/s0140-6736(15)00803-x
Bate, A., and Evans, S. J. (2009). Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 18 (6), 427–436. doi:10.1002/pds.1742
Davies, M. J., Bergenstal, R., Bode, B., Kushner, R. F., Lewin, A., Skjøth, T. V., et al. (2015). Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. Jama 314 (7), 687–699. doi:10.1001/jama.2015.9676
Degn, K. B., Juhl, C. B., Sturis, J., Jakobsen, G., Brock, B., Chandramouli, V., et al. (2004). One week's treatment with the long-acting glucagon-like peptide 1 derivative liraglutide (NN2211) markedly improves 24-h glycemia and alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes 53 (5), 1187–1194. doi:10.2337/diabetes.53.5.1187
Donnan, J. R., Grandy, C. A., Chibrikov, E., Marra, C. A., Aubrey-Bassler, K., Johnston, K., et al. (2019). Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open 9 (1), e022577. doi:10.1136/bmjopen-2018-022577
Evans, S. J., Waller, P. C., and Davis, S. (2001). Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 10 (6), 483–486. doi:10.1002/pds.677
FDA (2024). FDA approves first treatment for patients with liver scarring due to fatty liver disease. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease.
Fioretto, P., Zambon, A., Rossato, M., Busetto, L., and Vettor, R. (2016). SGLT2 inhibitors and the diabetic kidney. Diabetes Care 39 (Suppl. 2), S165–S171. doi:10.2337/dcS15-3006
Fu, E. L., Trevisan, M., Clase, C. M., Evans, M., Lindholm, B., Rotmans, J. I., et al. (2019). Association of acute increases in plasma creatinine after renin-angiotensin blockade with subsequent outcomes. Clin. J. Am. Soc. Nephrol. 14 (9), 1336–1345. doi:10.2215/CJN.03060319
Hammerich, L., and Tacke, F. (2023). Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 20 (10), 633–646. doi:10.1038/s41575-023-00807-x
Harrison, S. A., Bashir, M. R., Guy, C. D., Zhou, R., Moylan, C. A., Frias, J. P., et al. (2019). Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394 (10213), 2012–2024. doi:10.1016/s0140-6736(19)32517-6
Harrison, S. A., Bedossa, P., Guy, C. D., Schattenberg, J. M., Loomba, R., Taub, R., et al. (2024). A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390 (6), 497–509. doi:10.1056/NEJMoa2309000
Kim, M. Y., Cho, M. Y., Baik, S. K., Jeong, P. H., Suk, K. T., Jang, Y. O., et al. (2012). Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis - a randomized open-label controlled study. Liver Int. 32 (6), 977–987. doi:10.1111/j.1478-3231.2012.02774.x
Kisseleva, T., and Brenner, D. (2021). Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 18 (3), 151–166. doi:10.1038/s41575-020-00372-7
Lancaster, L. H., de Andrade, J. A., Zibrak, J. D., Padilla, M. L., Albera, C., Nathan, S. D., et al. (2017). Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 26 (146), 170057. doi:10.1183/16000617.0057-2017
Lindor, K. D., Bowlus, C. L., Boyer, J., Levy, C., and Mayo, M. (2019). Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatology 69 (1), 394–419. doi:10.1002/hep.30145
Nahra, R., Wang, T., Gadde, K. M., Oscarsson, J., Stumvoll, M., Jermutus, L., et al. (2021). Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54-week randomized phase 2b study. Diabetes Care 44 (6), 1433–1442. doi:10.2337/dc20-2151
Ogawa, W., and Sakaguchi, K. (2016). Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J. Diabetes Investig. 7 (2), 135–138. doi:10.1111/jdi.12401
Papamargaritis, D., Al-Najim, W., Lim, J. Z. M., Crane, J., Bodicoat, D. H., Barber, S., et al. (2024). Effectiveness of integrating a pragmatic pathway for prescribing liraglutide 3.0 mg in weight management services (STRIVE study): a multicentre, open-label, parallel-group, randomized controlled trial. Lancet Reg. Health Eur. 39, 100853. doi:10.1016/j.lanepe.2024.100853
Parola, M., and Pinzani, M. (2019). Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 65, 37–55. doi:10.1016/j.mam.2018.09.002
Poo, J. L., Torre, A., Aguilar-Ramírez, J. R., Cruz, M., Mejía-Cuán, L., Cerda, E., et al. (2020). Benefits of prolonged-release pirfenidone plus standard of care treatment in patients with advanced liver fibrosis: PROMETEO study. Hepatol. Int. 14 (5), 817–827. doi:10.1007/s12072-020-10069-3
Powell, E. E., Wong, V. W., and Rinella, M. (2021). Non-alcoholic fatty liver disease. Lancet 397 (10290), 2212–2224. doi:10.1016/s0140-6736(20)32511-3
Ratziu, V., de Guevara, L., Safadi, R., Poordad, F., Fuster, F., Flores-Figueroa, J., et al. (2021). Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 27 (10), 1825–1835. doi:10.1038/s41591-021-01495-3
Ratziu, V., Yilmaz, Y., Lazas, D., Friedman, S. L., Lackner, C., Behling, C., et al. (2024). Aramchol improves hepatic fibrosis in metabolic dysfunction-associated steatohepatitis: results of multimodality assessment using both conventional and digital pathology. Hepatology. doi:10.1097/hep.0000000000000980
Saisho, Y. (2020). SGLT2 inhibitors: the star in the treatment of type 2 diabetes? Diseases 8 (2), 14. doi:10.3390/diseases8020014
Sanyal, A. J., Bedossa, P., Fraessdorf, M., Neff, G. W., Lawitz, E., Bugianesi, E., et al. (2024). A phase 2 randomized trial of survodutide in MASH and fibrosis. N. Engl. J. Med. 391 (4), 311–319. doi:10.1056/NEJMoa2401755
Schoolwerth, A. C., Sica, D. A., Ballermann, B. J., and Wilcox, C. S.Council on the Kidney in Cardiovascular Disease and the Council for High Blood Pressure Research of the American Heart Association (2001). Renal considerations in angiotensin converting enzyme inhibitor therapy: a statement for healthcare professionals from the council on the kidney in cardiovascular disease and the council for high blood pressure research of the American heart association. Circulation 104 (16), 1985–1991. doi:10.1161/hc4101.096153
Shen, Y., Cheng, L., Xu, M., Wang, W., Wan, Z., Xiong, H., et al. (2023). SGLT2 inhibitor empagliflozin downregulates miRNA-34a-5p and targets GREM2 to inactivate hepatic stellate cells and ameliorate non-alcoholic fatty liver disease-associated fibrosis. Metabolism 146, 155657. doi:10.1016/j.metabol.2023.155657
Taheri, H., Malek, M., Ismail-Beigi, F., Zamani, F., Sohrabi, M., Reza Babaei, M., et al. (2020). Effect of empagliflozin on liver steatosis and fibrosis in patients with non-alcoholic fatty liver disease without diabetes: a randomized, double-blind, placebo-controlled trial. Adv. Ther. 37 (11), 4697–4708. doi:10.1007/s12325-020-01498-5
Takahashi, H., Asakawa, K., Kosakai, Y., Lee, T., and Rokuda, M. (2024). Comparative effectiveness of sodium-glucose co-transporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors on liver function in patients with type 2 diabetes in Japan: a real-world data analysis. Diabetes Obes. Metab. 26 (3), 997–1007. doi:10.1111/dom.15399
Tan, Y., Zhen, Q., Ding, X., Shen, T., Liu, F., Wang, Y., et al. (2022). Association between use of liraglutide and liver fibrosis in patients with type 2 diabetes. Front. Endocrinol. (Lausanne) 13, 935180. doi:10.3389/fendo.2022.935180
Tsuchida, T., and Friedman, S. L. (2017). Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 14 (7), 397–411. doi:10.1038/nrgastro.2017.38
van Puijenbroek, E. P., Bate, A., Leufkens, H. G., Lindquist, M., Orre, R., and Egberts, A. C. (2002). A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiol Drug Saf. 11 (1), 3–10. doi:10.1002/pds.668
Vilsbøll, T. (2007). Liraglutide: a once-daily GLP-1 analogue for the treatment of type 2 diabetes mellitus. Expert Opin. Investig. Drugs 16 (2), 231–237. doi:10.1517/13543784.16.2.231
Younossi, Z. M., Ratziu, V., Loomba, R., Rinella, M., Anstee, Q. M., Goodman, Z., et al. (2019). Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394 (10215), 2184–2196. doi:10.1016/s0140-6736(19)33041-7
Keywords: liver fibrosis, adverse drug reactions (ADRs), anti-fibrotic agents, WHO-VigiAccess, descriptive analysis, disproportionality analysis
Citation: Liu Y, Zhao X, Wang X and Zhou Q (2025) Adverse events of hepatic anti-fibrotic agents in phase 3 and above clinical trials: a descriptive analysis of the WHO-VigiAccess database. Front. Pharmacol. 16:1534628. doi: 10.3389/fphar.2025.1534628
Received: 26 November 2024; Accepted: 06 January 2025;
Published: 24 January 2025.
Edited by:
Yan-Ling Wu, Yanbian University, ChinaReviewed by:
Fenglei Huang, Boehringer Ingelheim, GermanyShuang Jiang, Changchun University of Chinese Medicine, China
Copyright © 2025 Liu, Zhao, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhou, emh1b3FAamx1LmVkdS5jbg==
 Yuwei Liu
Yuwei Liu Xu Zhao
Xu Zhao Xinrui Wang
Xinrui Wang Qiang Zhou
Qiang Zhou