- Phase I Clinical Trial Center, Bishan Hospital of Chongqing, Bishan Hospital of Chongqing Medical University, Chongqing, China
Background: There are approximately 537 million adults with diabetes worldwide, and insulin still plays an important role in its treatment. However, the long-term use of insulin imposes a significant financial burden on patients. This study aims to explore the pharmacokinetic (PK)/ pharmacodynamic (PD) parameters of generic premixed insulin lispro 25 (25% insulin lispro and 75% protamine zinc lispro) and evaluate the bio-equivalence between generic and brand-name preparations to reduce medical costs while ensuring the effectiveness and safety of treatment.
Research design and method: This is a single-center, randomized, open-label, two-period, crossover study. This study recruited 52 healthy volunteers and randomly divided them into two sequences to receive either the test (T) preparation or the reference (R) preparation in each period (Chinese Drug Trial Identifier: CTR20202288, URL: http://www.chinadrugtrials.org.cn). The C-peptide and plasma concentration of lispro 25 were analyzed using ELISA and high-performance liquid chromatography, respectively. A euglycemic clamp was used to measure the glucose infusion rate (GIR). The main PK parameters (AUC0-t and Cmax) and PD parameters (GIRmax and GIRAUC0-t) and the evaluation of bioequivalence were calculated using WinNonlin 8.3.1.
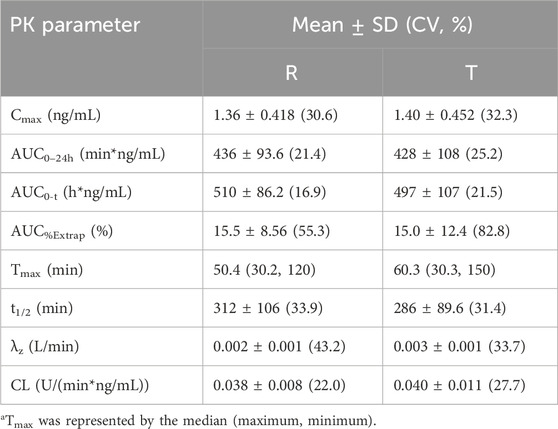
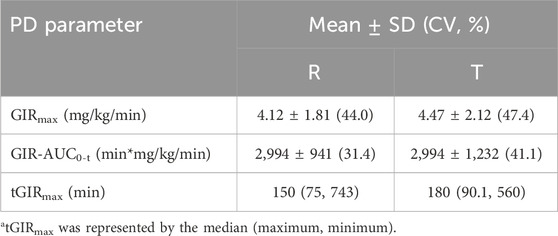
Results: The quality of the clamp was approved by stable blood glucose and inhibited C-peptide levels. For PK parameters, the Cmax values of the T and R preparations were 1.40 ± 0.452 and 1.36 ± 0.418 ng·mL-1, respectively, and the AUC0–24h values were 497 ± 107 and 510 ± 86.2 ng h·mL-1, respectively. For PD parameters, GIRmax values were 4.47 ± 2.12 and 4.12 ± 1.81 mg kg·min-1, and AUCGIR0–24h values were 2,994 ± 1,232 and 2,994 ± 941 mg h·kg·min-1 for T and R, respectively. The 90% confidence intervals (CIs) for the geometric mean ratio (test/reference) of the main PK parameters (AUC0-t and Cmax) and PD parameters (GIRmax and GIRAUC0-t) in both cohorts were within the range of 80%–125%. Furthermore, there was no significant hypoglycemia and serious adverse events (SAEs) observed in this study.
Conclusion: Bio-equivalence between insulin lispro (R) (Humalog®25) and insulin lispro (T) was demonstrated, with both showing good tolerance in healthy Chinese volunteers. The results provide evidence supporting the interchangeability of different drug formulations and offer more options for clinical drug use.
1 Introduction
Diabetes mellitus (DM) is a metabolic disease characterized by insulin resistance and defects in insulin secretion, which could cause long-term damage, dysfunction, or failure of various tissues and organs (Xu et al., 2018). According to the International Diabetes Federation (IDF) (Sun et al., 2022), approximately 537 million adults worldwide are living with diabetes, with 6.7 million deaths attributed to diabetes in 2021. Diabetes mellitus can be classified into type 1 diabetes (T1D), type 2 diabetes (T2D), specific types of diabetes due to other causes, and gestational diabetes mellitus, as outlined by the American Diabetes Association (ADA) (ElSayed et al., 2023). Since its discovery in 1921, insulin has played a major role in diabetes treatment, especially in insulin analogs (Home and Mehta, 2021). Based on pharmacokinetic (PK) properties, insulin analogs can be classified as rapid, short, intermediate, long-acting, and premixed insulin analogs, which could provide prandial or basal insulin. Basal-bolus insulin therapy is the most consistent with the physiological rhythm of humans because it can provide both basal and prandial coverage and is recommended by many guidelines for insulin therapy initiation or when blood glucose does not meet expectations (Elizarova et al., 2014; Zhu and Chinese Diabetes Society, 2021). However, strict and frequent blood glucose (BG) self-monitoring is required, which means patients must be self-managed. In addition, multiple daily injections may be attributed to poor compliance and therapeutic effects (Elizarova et al., 2014). Fortunately, the advent of premixed insulin, which can provide both basal and postprandial coverage with just one injection, has been a significant development (Elizarova et al., 2014). Previous studies suggested that there was no significant difference in safety and efficacy between basal-bolus insulin therapy (4 times/day) or thrice-daily premixed insulin in patients with type 2 diabetes mellitus (T2DM) (Jia et al., 2015; Bellido et al., 2015). Premixed insulin usually has three types of mixtures composed of different proportions of insulin and its protamine counterpart. Premixed insulin lispro 25 is a mid-mix insulin containing 75% insulin lispro protamine suspension and 25% insulin lispro, which is widely used as a starter insulin in East Asia (Watada et al., 2017; Jovanovič et al., 2014). Thus, as more products continue to enter the market, the evaluation of these products has become increasingly essential.
The PK/PD properties of insulin determine its purpose, usage, and dosage, which are related to clinical outcomes and the prevention of adverse events (AEs) (Sharma et al., 2019). Thus, the well-known PK/PD properties of insulin are beneficial for its implementation. The euglycemic glucose clamp is considered the gold standard for assessing insulin and its analogs by measuring the glucose infusion rate (GIR) (Cheng et al., 2019). Thus, the present study aims to evaluate the PK/PD properties of premixed insulin lispro 25 in healthy subjects.
2 Subjects and methods
2.1 Drugs
The reference (R) formulation was a mixed protamine zinc recombinant human insulin lispro injection (Humalog®25, 3 mL: 300U), manufactured by Eli Lilly Italia S.p.A (lot number: D183499). The test (T) formulation was a mixed protamine zinc recombinant human insulin lispro injection (3 mL: 300U), provided by Tonghua Dongbao Pharmaceutical Co., Ltd. (lot number: 2L12020100052).
2.2 Subjects
Participants were eligible for inclusion if they were healthy male volunteers aged 18–45 years with a body mass index in the range of 19–24 kg/m2 (including the critical value), fasting plasma glucose in the range of 3.9 mmol/L–6.1 mmol/L, and glycosylated hemoglobin (HbA1c) ≤ 6.0%; normal insulin secretion function and glucose tolerance; normal or abnormal physical examination and vital signs without clinical significance; and high compliance. Key exclusion criteria included allergies to insulin or related drugs; a history of significant use of alcohol and cigarettes; a history of thrombosis; use of any medications that affect insulin hypoglycemia within 28 days before screening; participation in a clinical trial within the previous 3 months; and other conditions deemed inappropriate by the investigator.
2.3 Study design
This is a single-center, randomized, open-label, two-period, crossover, bioequivalence study for premixed insulin lispro 25 (3 mL: 300U) (Humalog®25) versus premixed insulin lispro 25 (3 mL: 300U) (Chinese Drug Trial Identifier: CTR20202288). A total of 52 healthy Chinese male subjects were enrolled and randomly divided into two groups (TR or RT), and there was a 7–14 d washout period between the sequences. This study followed the principles of the Declaration of Helsinki and the Good Clinical Principles. The protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Chongqing, China). Written informed consent of participants was obtained before this study.
2.4 Euglycemic clamp procedures and the detection of insulin lispro and C-peptide
A 24-h euglycemic clamp was used to evaluate the pharmacodynamic parameters of premixed insulin lispro, and all recruited volunteers underwent a single-dose euglycemic clamp test. The specific operation procedure refers to that described in a previous study by Tao et al. (2021). A measure of 0.4 mL of blood sample was collected to detect the blood glucose level after subcutaneous injection of 0.3 IU·kg-1 test preparation or reference preparation, and the time points for PD blood collection were as follows: once every 5 min up to 2 h after injection, every 10 min from 2 to 8 h, every 20 min from 8 to 16 h, and every 30 min from 16 to 24 h. However, beyond that, the blood will be collected at −30, −20, and −10 min before the drug is injected to obtain baseline blood glucose. The PD blood samples will be used to immediately determine the whole blood glucose concentration using the glucose oxidase method, and the intravenous infusion of the 20% glucose solution will be adjusted in real-time according to the blood glucose level; the GIR will be calculated. The blood glucose level was maintained within the range of ±10% of the target blood glucose (baseline blood glucose minus 0.28 mmol·L-1). In addition, PK blood samples were collected at the following time points: −30 min, 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120, 150, 180, 210, 240, 300, 360, 420, 480, 600, 720, 840, 960, 1200, and 1440 min; and C-peptide blood samples were collected at −30 min, 0, 60, 120, 240, 360, 480, 600, 720, 840, 960, 1200, and 1440 min.
2.5 Analytical method
The level of C-peptide in serum was determined by the ELISA. The plasma concentration of premixed insulin lispro was analyzed using a high-performance liquid chromatograph (HPLC, LC30AD, Shimadzu Corp., Kyoto, Japan) coupled with an Applied Biosystems/MDS SCIEX Mass Spectrometer (Triple Quad 6500+ SCIEX, Framingham, MA, United States). An ACQUITY UPLC Protein BEH C4 Column (100 × 2.1 mm, 1.7 µm, Waters, Milford, Massachusetts, United States) was used for chromatographic separation. The mobile phase was composed of water containing 0.5% formic acid and 1% dimethylsulfoxide (solvent A) and 100% acetonitrile–methyl alcohol containing 0.5% formic acid and 1% dimethylsulfoxide (1:1) (solvent B). The flow rate was 0.35 mL/min, and the column temperature was set at 8°C. Detection was performed using the mass spectrometer in positive electrospray ionization mode. The linear range was 0.100–10.0 ng/mL, with the LLOQ being 0.100 ng/mL. The internal standard is insulin aspart. The data were processed using Analyst version 1.6.3 (Applied Biosystems SCIEX, Framingham, MA, United States) and Analyst version 1.7.2 (Applied Biosystems SCIEX, Framingham, MA, United States) software.
2.6 Statistical analysis
Descriptive statistics were conducted on the blood drug concentration and GIR of subjects in different groups. In the GIR data analysis, the data processed by the SAS Loess smoothing method are adopted (SAS 9.4, Institute Inc., Cary, NC, United States). The pharmacokinetic and pharmacodynamic parameters were calculated from the plasma–time profile with non-compartmental models using WinNonlin 8.3.1 (Certara L.P., Princeton, NJ, United States). The major PK parameters included maximum plasma drug concentration (Cmax) and the area under the plasma concentration–time curve (AUC) from 0 to time t (AUC0-t). The main PD parameters were maximum glucose infusion rate (GIRmax), AUC0-t of the glucose infusion rate (GIRAUC0-t), and time to maximum glucose infusion rate (tGIRmax). All parameters will also be subjected to descriptive statistics. In addition, major parameters were log-transformed and analyzed using a linear mixed model of analysis of variance. Geometric least-squares mean ratios of test and reference products for major parameters and their 90% confidence intervals (CIs) were calculated. They were bioequivalent if the 90% confidence intervals (CIs) of the ratios for major parameters were within the range of 80%–125%. Apart from this, Tmax and GIRmax were compared with nonparametric tests. Data were expressed as the mean ± standard deviation or median with the range.
2.7 Evaluation for tolerability and safety
Tolerability and safety were assessed by closely monitoring blood glucose levels and conducting other tests. All adverse events and severe adverse events (SAEs) were evaluated.
3 Results
3.1 Demographic characteristics
In this study, 52 healthy male subjects were enrolled, and 1 subject was withdrawn from the study after finishing 1 period. They were approximately 26.3 ± 4.14 years old, and their BMI was 21.73 ± 1.37. More detailed demographic data of them are presented in Supplementary Table S1.
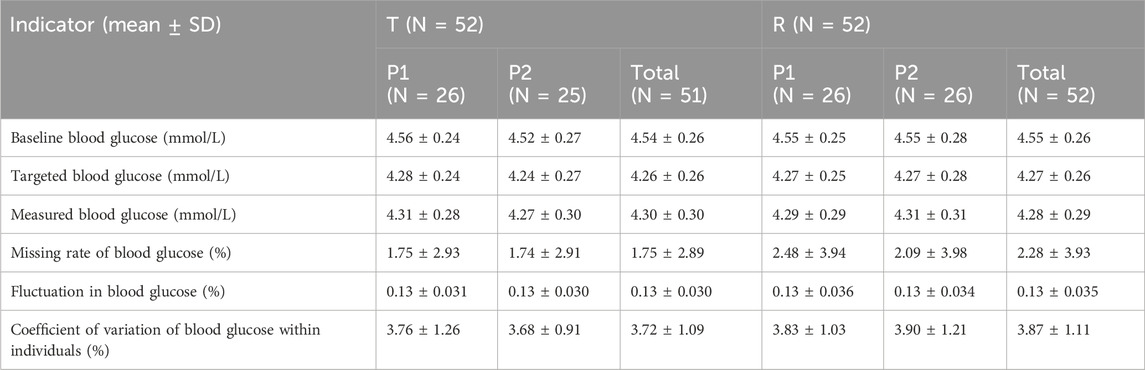
3.2 Evaluation for the clamp test
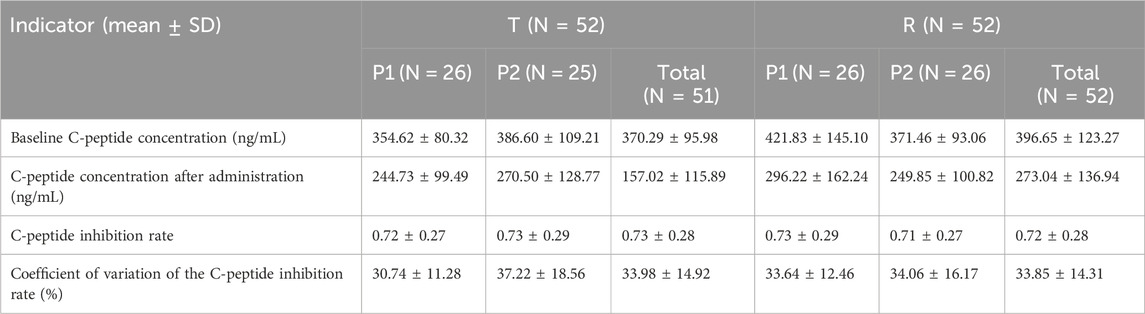
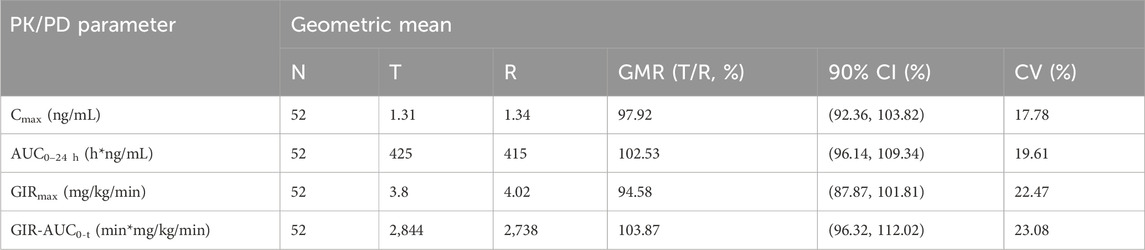
All subjects underwent the euglycemic clamp procedure in a supine position and fasted according to the test requirements. The blood glucose-related parameters during the test are provided in Table 1, and the blood glucose profiles for both preparations are shown in Figure 1A. As observed, the blood glucose levels for both preparations were close to the target range, and the CV% of blood glucose within individuals was below 5%, indicating good clamp test quality. For C-peptide, baseline C-peptide concentration, mean C-peptide concentration after administration, mean C-peptide inhibition rate, and the CV% of the C-peptide inhibition rate within individuals are provided in Table 2. The mean serum C-peptide concentration after dosing was lower than that before administration for reference and test preparations (Figure 1B), suggesting that the secretion of endogenous insulin was suppressed during the euglycemic clamp procedure.
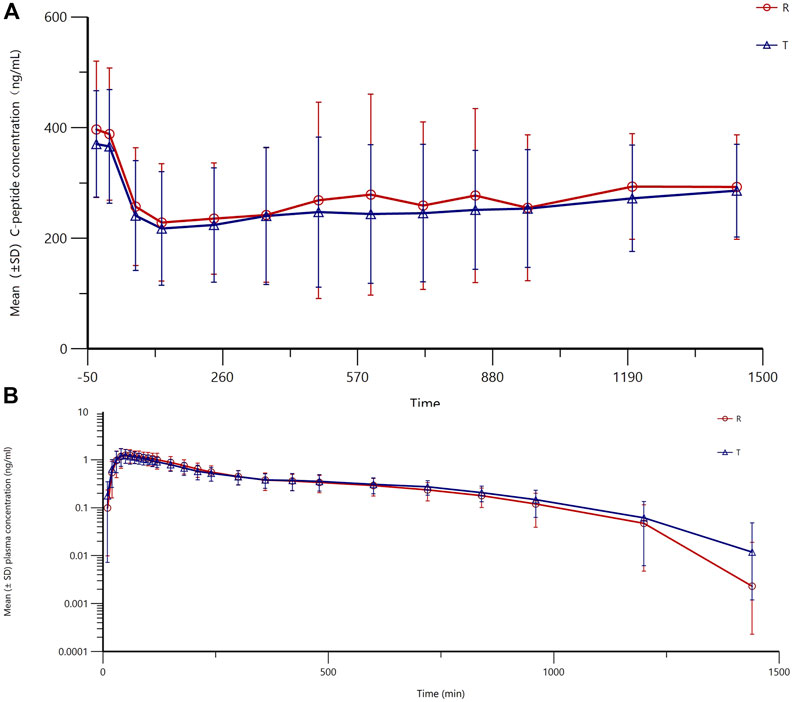
Figure 1. Fluctuations in mean blood glucose during the clamp procedure and changes in blood glucose versus time relative to baseline (A) and changes in C-peptide levels relative to baseline C-peptide at each time point during the clamp procedure (B).
3.3 Pharmacokinetics
The mean (±SD) plasma concentration–time curves and semi-logarithmic curves of test and reference preparations are shown in Figure 2A, B. The main PK parameters for both the test and reference formulations are presented in Table 3. As shown in Figure 3, the PK profiles of the two preparations were similar and the PK parameters were close. A clear peak can be observed in this profile, and the duration of action time was approximately 5 h.
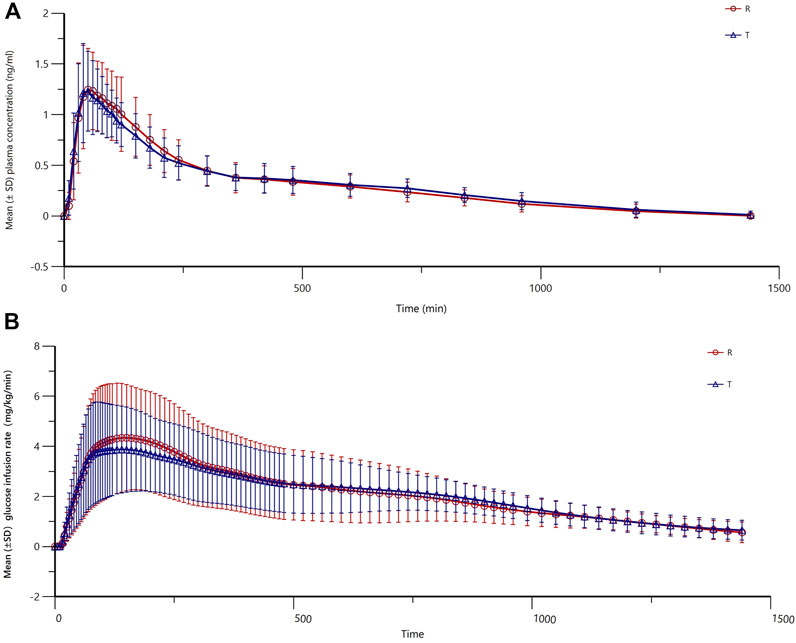
Figure 2. Mean (±SD) plasma concentration–time curves (profile of changes in the blood concentration over time) (A) and semi-logarithmic curves (B) of the test and reference products of insulin lispro 25.
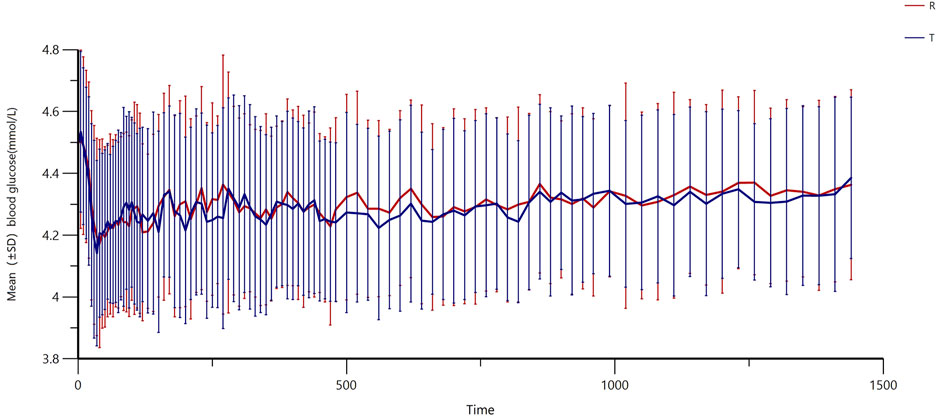
Figure 3. Mean (±SD) glucose infusion rate versus time of insulin lispro 25 (both test and reference products).
3.4 Pharmacodynamic
The main PD parameters (GIRmax and GIR-AUC0-t) for the test and reference are presented in Table 4, showing very close results between the two preparations. As shown in Figure 3, the GIR values increased rapidly and reached their peaks quickly, and the test or reference preparation profiles were relatively consistent.
3.5 Bioequivalence
As shown in Table 5, the 90% CIs for the geometric mean ratios (test/reference) of the main PK parameters (AUC0-t and Cmax) and PD parameters (GIRmax and GIRAUC0-t) in both cohorts were within the range of 80%–125%, demonstrating the bioequivalence of two preparations in healthy Chinese subjects. The Wilcoxon signed-rank sum test for Tmax and tGIRmax showed no statistically significant difference between the two groups (P = 0.7838). An ANOVA was conducted for the main PK and PD parameters, and the results showed that factors such as administration sequence, period, and preparation had no significant effect on the equivalence analysis (P > 0.05).
3.6 Tolerability and safety
The safety of both formulations was evaluated through the assessment of laboratory examinations, 12-lead electrocardiogram, and vital signs. The adverse events during the trial are shown in Supplementary Table S2. A total of 57 AEs occurred in 35 subjects (67.3%), with 17 subjects (32.7%) experiencing 23 AEs related to the study drugs. For the reference preparation, there are 27 AEs reported in 22 subjects (42.3%), and 11 subjects (21.2%) had 12 AEs related to it. For the test preparation, 30 AEs were observed in 20 subjects (39.2%), with 9 subjects (17.6%) experiencing 11 AEs related to the drug. In addition, there was one case of hypoglycemia that occurred in one subject, and one subject was withdrawn from period 2 due to AEs, although this was unrelated to the drug. Furthermore, no SAEs occurred during this study.
4 Discussion
In this study, we described the PK/PD properties of test and reference premixed insulin lispro 25R and identified their bioequivalence. Apart from this, both formulations were well tolerated by the studied population. These results suggest that the test product demonstrates comparable pharmacokinetic profiles to the reference product, thereby ensuring therapeutic equivalence. Consequently, patients can be expected to experience similar clinical outcomes when switched from the reference product to the test product, supporting the interchangeability and substitution of the test product in clinical practice.
In this study, the subjects were healthy volunteers who secreted endogenous insulin, which can disturb the characteristics of the insulin products being evaluated. Thus, methods for suppressing endogenous insulin are necessary. Given that insulin secretion is mainly induced by increased circulating glucose levels (Campbell and Newgard, 2021), maintaining blood glucose levels (defined as below 5 mg/dL or 10% of basal blood glucose) below the subject’s fasting glucose can sufficiently suppress endogenous insulin secretion. Thus, the quality of the clamp study is essential to the study, and indicators including mean values, root mean square deviation, and the coefficient of variation of the blood glucose concentrations have been calculated to evaluate the quality of the clamp, according to the guidelines of EMA (European Medicines Agency, 2015). In this study, the measured blood glucose levels were below baseline but slightly higher than the targeted blood glucose levels, which may have contributed to some missing values. However, the secretion of endogenous insulin was still inhibited because the targeted blood glucose was lower than baseline levels, and data showed that the level of C-peptide at every time point was lower than that at baseline. Moreover, the fluctuations in blood glucose were extremely low, with a CV% below 5%, indicating the establishment of a steady clamp platform (Hui et al., 2019).
The earliest premixed insulin is human insulin 70/30. However, a long time to peak (1–5 h) and a long duration of action time (6–10 h) were prone to cause postprandial hyperglycemia and late hypoglycemia (Garber, 2006; Home et al., 1989). A previous study showed that the time to peak of premixed insulin analogs was approximately 1.5 h, but it takes almost 3 h for premixed human insulin (Garber et al., 2007). Physiologic insulin levels peak within 0.5–1 h after the start of a meal and return to baseline levels within 2–3 h after a meal (Home et al., 1989). Thus, premixed insulin analogs better mimic physiologic insulin secretion patterns than premixed human insulin. In this study, the time to peak of premixed insulin lispro 25 was only 0.5–2 h, and the duration of action time was approximately 3–5 h, indicating that it could quickly take effect on elevated glucose after eating food without concerns of late hypoglycemia. Taken together, the product could reach its peak quickly and return to basal insulin levels evenly and smoothly, which makes it an ideal pharmaceutical formulation. In addition, the most important thing is that T and R have similar characteristics across all PK parameters, indicating comparable absorption rates and degrees in the human body. For PD, the result also suggested a similar effect of glucose control between T and R. Our results were similar to those of a previous study in tGIRmax (Heise et al., 1998; Awa et al., 2005). Still, GIRmax was slightly lower than that of the previous study. This difference may be attributed to the product itself and variations in the absorption process among different populations. Furthermore, we observed some differences in subjects, not only related to population characteristics but also gender. Heise T et al. recruited a significant proportion of women in their study, which may be one of the reasons why our results differed from theirs (European Medicines Agency, 2015). However, we just roughly compared our results with others because many variations exist, like clamp methods (manual and auto), differences in clamp glucose thresholds, the choice of clamp timing, and the differing protocols to inhibit insulin secretion (Evans et al., 2011). Many factors may contribute to the difference in drug efficacy, although they have comparable PK/PD results like production process, excipients, and drug stability. Production process differences between generic and original drugs may lead to differences in drug release speed, solubility, and stability in the body, thereby affecting the efficacy and safety of drugs. Excipients also have important effects on the stability, absorption, solubility, and antioxidant properties of drugs. If there are differences in the selection of excipients between the generic and original drugs, the efficacy of the drug may be different (Tao et al., 2010).
In this study, we found that the CV% of main PD parameters was higher than that of PK parameters, possibly because the GIR value needed immediate adjustment and there was a delay in blood glucose values after every adjustment. We chose the manual method because it is simpler and has comparable performance and CV% to the automated method (Ponchner et al., 1984). In addition, a dosage of 0.3 U/kg was selected according to previous studies (Heise et al., 1998) and the EMA guidelines (European Medicines Agency, 2015), and this is a tolerable dosage for a healthy population while being sufficient to evaluate the PK/PD characteristics of targeted insulin, even though a higher dosage could result in a lower degree of variation. A single-dose design was applied in the study as it is not only suitable for assessing the pharmacological profile of rapid-acting insulin and measuring the onset of insulin action but also ethically appropriate for healthy subjects22. The sample size was determined based on the results of a pilot study. In the pilot study, the CV% of main parameters (Cmax and AUC0-t) was 19%–23%. For the current study, we assumed a CV% of 25% and a geometric mean ratio between the test and reference preparations ranging from 0.94 to 1.06. The CI% for the bioequivalent standard was 0.80–1.25 with an α level of 0.05. Based on these parameters, a sample size of 42 subjects was calculated to meet the study’s requirements. Considering the risk of dropouts, 52 subjects were enrolled in this study.
In conclusion, this study demonstrated the bioequivalence between premixed insulin lispro 25 (R) (Humalog®25) versus premixed insulin lispro 25 (T). No deaths or SAEs occurred, and both formulations showed good tolerability in healthy Chinese volunteers, providing evidence supporting the interchangeability of the two drug formulations and offering more options for clinical drug use.
Data availability statement
The datasets presented in this article are not readily available because the corresponding author must comply with confidentiality agreement. Requests to access the datasets should be directed to dGN5b25nd3pqQDE2My5jb20=.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MZ: formal analysis, writing–original draft, and writing–review and editing. YC: resources, writing–original draft, and writing–review and editing. LW: resources and writing–review and editing. ZL: resources and writing–review and editing. JP: resources and writing–review and editing. CT: conceptualization, project administration, supervision, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Natural Science Foundation of Chongqing, China (no. CSTB2023NSCQ-LZX0019), the Municipal Postgraduate Joint Training Base of Chongqing Medical University (no. lpjd202306), the Scientific Research and Innovation Team Program of the Bishan Hospital of Chongqing Medical University (no. BYKY-CX2023003), and the Special Research Program for Graduate Student Supervisors of the Bishan Hospital of Chongqing Medical University (no. BYKY-DS2023010).
Acknowledgments
The authors would like to thank Tonghua Dongbao Pharmaceutical Co., Ltd. for their kind support and all the volunteers who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study was sponsored by Tonghua Dongbao Pharmaceutical Co., Ltd. The funder had the following involvement in the study: study design.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1533548/full#supplementary-material
References
Awa, T., Nakata, T., and Shigeta, H. (2005). Pharmaceutical development and clinical experiment of new antidiabetic drugs, premixed preparations of insulin lispro (Humalog Mix 25 and Humalog Mix 50) and intermediate-acting insulin lispro (Humalog N). Nihon yakurigaku Zasshi. Folia Pharmacol. Jpn. 126 (2), 143–151. doi:10.1254/fpj.126.143
Bellido, V., Suarez, L., Rodriguez, M. G., Sanchez, C., Dieguez, M., Riestra, M., et al. (2015). Comparison of basal-bolus and premixed insulin regimens in hospitalized patients with type 2 diabetes. Diabetes care 38 (12), 2211–2216. doi:10.2337/dc15-0160
Campbell, J. E., and Newgard, C. B. (2021). Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 22 (2), 142–158. doi:10.1038/s41580-020-00317-7
Cheng, A. Y. Y., Patel, D. K., Reid, T. S., and Wyne, K. (2019). Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv. Ther. 36 (5), 1018–1030. doi:10.1007/s12325-019-00925-6
Zhu, D.Chinese Diabetes Society (2021). Guidelines for prevention and treatment of type 2 diabetes mellitus (2020). Chin. J. Pract. Intern. Med. 41 (08), 668–695. doi:10.3760/cma.j.cn311282-20210304
Elizarova, S., Galstyan, G. R., and Wolffenbuttel, B. H. (2014). Role of premixed insulin analogues in the treatment of patients with type 2 diabetes mellitus: a narrative review. J. diabetes 6 (2), 100–110. doi:10.1111/1753-0407.12096
ElSayed, N. A., Aleppo, G., Aroda, V. R., Bannuru, R. R., Brown, F. M., Bruemmer, D., et al. (2023). 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes care 46 (Suppl. 1), S19–s40. doi:10.2337/dc23-S002
European Medicines Agency (2015). Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues. Available at: https://www.ema.europa.eu/en/non-clinical-and-clinical-development-similar-biological-medicinal-products-containing-recombinant-human-insulin-and-insulin-analogues-scientific-guideline (Accessed March 11, 2015).
Evans, M., Schumm-Draeger, P. M., Vora, J., and King, A. B. (2011). A review of modern insulin analogue pharmacokinetic and pharmacodynamic profiles in type 2 diabetes: improvements and limitations. Diabetes, Obes. and metabolism 13 (8), 677–684. doi:10.1111/j.1463-1326.2011.01395.x
Garber, A. J., Ligthelm, R., Christiansen, J. S., and Liebl, A. (2007). Premixed insulin treatment for type 2 diabetes: analogue or human? Diabetes, Obes. and metabolism 9 (5), 630–639. doi:10.1111/j.1463-1326.2006.00654.x
Garber, A. J. (2006). Premixed insulin analogues for the treatment of diabetes mellitus. Drugs 66 (1), 31–49. doi:10.2165/00003495-200666010-00003
Heise, T., Weyer, C., Serwas, A., Heinrichs, S., Osinga, J., Roach, P., et al. (1998). Time-action profiles of novel premixed preparations of insulin lispro and NPL insulin. Diabetes care 21 (5), 800–803. doi:10.2337/diacare.21.5.800
Home, P. D., and Mehta, R. (2021). Insulin therapy development beyond 100 years. lancet Diabetes and Endocrinol. 9 (10), 695–707. doi:10.1016/S2213-8587(21)00182-0
Home, P. D., Thow, J. C., and Tunbridge, F. K. (1989). Insulin treatment: a decade of change. Br. Med. Bull. 45 (1), 92–110. doi:10.1093/oxfordjournals.bmb.a072323
Hui, L., Hongling, Y., Lina, H., Siqin, Z., Jiali, L., Jiaqi, L., et al. (2019). Quality evaluation of euglycemic Clamp. J. Sichuan Univ. Sci. 50 (04), 04. doi:10.13464/j.scuxbyxb.2019.04.026
Jia, W., Xiao, X., Ji, Q., Ahn, K. J., Chuang, L. M., Bao, Y., et al. (2015). Comparison of thrice-daily premixed insulin (insulin lispro premix) with basal-bolus (insulin glargine once-daily plus thrice-daily prandial insulin lispro) therapy in east Asian patients with type 2 diabetes insufficiently controlled with twice-daily premixed insulin: an open-label, randomised, controlled trial. lancet. Diabetes and Endocrinol. 3 (4), 254–262. doi:10.1016/S2213-8587(15)00041-8
Jovanovič, L., Peters, A. L., Jiang, H. H., and Hardin, D. S. (2014). Durability of glycemic control with insulin lispro mix 75/25 versus insulin glargine for older patients with type 2 diabetes. Aging Clin. Exp. Res. 26 (2), 115–121. doi:10.1007/s40520-013-0140-8
Ponchner, M., Heine, R. J., Pernet, A., Hanning, I., Francis, A. J., Cook, D., et al. (1984). A comparison of the artificial pancreas (glucose controlled insulin infusion system) and a manual technique for assessing insulin sensitivity during euglycaemic clamping. Diabetologia 26 (6), 420–425. doi:10.1007/BF00262213
Sharma, A. K., Taneja, G., Kumar, A., Sahu, M., Sharma, G., Kumar, A., et al. (2019). Insulin analogs: glimpse on contemporary facts and future prospective. Life Sci. 219, 90–99. doi:10.1016/j.lfs.2019.01.011
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Tao, Y., Yang, L., and Huaguan, D. (2010). Analysis of factors affecting clinical efficacy of generic drugs. Chin. Pharm. J. 45 (19), 1446–1450.
Tao, Y., Zhu, M., Pu, J., Zhang, P., Wan, L., and Tang, C. (2021). Reduction in C-peptide levels and influence on pharmacokinetics and pharmacodynamics of insulin preparations: how to conduct a high-quality euglycemic clamp study. Front. Pharmacol. 12, 786613. doi:10.3389/fphar.2021.786613
Watada, H., Su, Q., Li, P. F., Iwamoto, N., Qian, L., and Yang, W. Y. (2017). Comparison of insulin lispro mix 25 with insulin lispro mix 50 as an insulin starter in Asian patients with type 2 diabetes: a phase 4, open-label, randomized trial (CLASSIFY study). Diabetes Metab. Res. Rev. 33 (1). doi:10.1002/dmrr.2816
Keywords: insulin lispro 25, pharmacokinetics, pharmacodynamics, bioequivalence, diabetes
Citation: Zhu M, Chen Y, Wan L, Li Z, Pu J and Tang C (2025) Pharmacokinetics and pharmacodynamics of insulin lispro 25 versus the original preparation (Humalog®25) in Chinese healthy male volunteers. Front. Pharmacol. 16:1533548. doi: 10.3389/fphar.2025.1533548
Received: 24 November 2024; Accepted: 03 February 2025;
Published: 25 February 2025.
Edited by:
Yurong Lai, Gilead, United StatesReviewed by:
Prawej Ansari, University of Alabama at Birmingham, United StatesFenglei Huang, Boehringer Ingelheim, Germany
Copyright © 2025 Zhu, Chen, Wan, Li, Pu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengyong Tang, dGN5b25nd3pqQDE2My5jb20=
†These authors have contributed equally to this work
 Mingxue Zhu†
Mingxue Zhu† Lei Wan
Lei Wan Junliang Pu
Junliang Pu Chengyong Tang
Chengyong Tang