
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 31 March 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1532290
This article is part of the Research TopicPrevention and Treatment of Metabolic Diseases Using Bioactive Metabolites of Herbal Medicines Also Used as FoodsView all 10 articles
 Min-Li Chen1
Min-Li Chen1 Shi-Yan Qian2
Shi-Yan Qian2 Jiang-Li Yang2
Jiang-Li Yang2 Jue-Yan Zheng1
Jue-Yan Zheng1 Li-Xiang Wang2
Li-Xiang Wang2 Jing-Ying Wu2
Jing-Ying Wu2 Hai-Qin Ye3
Hai-Qin Ye3 Yan Wang4
Yan Wang4 Guo-Qing Zheng2*
Guo-Qing Zheng2*Background: Chinese herbal medicine (CHM) formulas played an important role during the pandemic of coronavirus disease 2019 (COVID-19). Many randomized controlled trials (RCTs) on CHM for COVID-19 were quickly published. Concerns have been raised about their quality. In addition, inadequate detailed information on CHM formula intervention may arouse suspicion about their effectiveness. We aim to assess the most recent evidence of the methodological reporting quality of these RCTs with strict randomization, and the precise reporting of the CHM formula intervention.
Methods: RCTs on CHM formulas for COVID-19 were searched from nine databases. The CONSORT 2010, CONSORT-CHM Formulas 2017, and risk of bias were the guidelines used to assess the included RCTs. The checklist of sub-questions based on CONSORT-CHM Formulas 2017 was used to evaluate the precise reporting of CHM formula intervention. A comparison was made between RCTs that enrolled participants during and after the first wave of the pandemic (defined here as December 2019 to March 2020).
Results: The average score for 66 studies evaluated based on three guidelines, the CONSORT 2010, the CONSORT-CHM Formulas 2017, and the checklist of sub-questions based on the CONSORT-CHM Formulas 2017, is 16.4, 15.2, and 17.2, respectively. The reporting rate of sample size calculation, allocation concealment, and blinding is less than 30%. The checklist of sub-questions based on the CONSORT-CHM formulas 2017 can help report and assess CHM formula intervention more precisely. Most studies assessed an “unclear risk of bias” due to insufficient information. RCTs published in English and recruited subjects during the first wave of the pandemic have a higher risk of participant blinding bias than the studies recruited subjects after that (P < 0.05).
Conclusion: The methodological reporting quality in strictly randomized RCTs on CHM formulas for COVID-19 is inadequate—the reporting of sample size calculation, allocation concealment, and blinding need to improve especially. The checklist of sub-questions based on CONSORT-CHM formulas 2017 can help report and assess CHM formula intervention more precisely. The methodological reporting quality of RCTs published in English and enrolled participants during the first wave of the pandemic is worse than the studies that recruited subjects after the first wave of the pandemic.
The coronavirus disease 2019 (COVID-19) pandemic is one of the most serious challenges facing contemporary medicine. According to the World Health Organization, as of 21 July 2024, over 775 million confirmed cases and over 7 million deaths have been reported globally (WHO, 2024). The pandemic of COVID-19 has challenged scientific researchers to produce timely evidence about the new coronavirus and the disease. There has been a surge in the studies on COVID-19 since the pandemic started in 2020 (Ioannidis et al., 2021). In addition, the COVID-19 pandemic rapidly increases public interest concerning Chinese herbal medicine (CHM) (Rokhmah et al., 2020). One of the main issues that have been brought up is the caliber of the literature, considering synthesis studies are published quickly (Baumeister et al., 2021; Ang et al., 2022).
The best available data to assess the safety and curative effectiveness of therapies is typically found in well-conducted randomized controlled trials (RCTs), particularly in double-blind, placebo-controlled clinical studies. Evidence from well-designed RCTs is needed to conclusively identify what interventions should be applied or discontinued. Evaluating the methodological reporting quality of RCTs can provide more solid evidence for readers to judge the objectivity and reliability of the results of RCTs.
Clinical trial reports must be transparent, comprehensive, and easy to understand. It is imperative to evaluate the methodological reporting quality of RCTs with authoritative tools. One such tool for raising the methodological reporting standards of RCTs is the Consolidated Standards of Reporting Trials 2010 (CONSORT 2010) statement (Moher et al., 2012). Another is CONSORT-CHM Formulas 2017 (Cheng et al., 2017), which enhances the reporting quality of RCTs related to CHM formulas by adding traditional Chinese medicine (TCM) patterns and other items factors based on the features of CHM formulas. It is worth noting that the item of “intervention” of CONSORT-CHM Formulas 2017 contains many specific sub-items that well reflect detailed information on CHM formula interventions. However, there is hardly any literature that can be fully reported on this item because it doesn’t expand subitems for scoring. Some researchers created a 42 sub-questions checklist based on CONSORT-CHM formulas 2017 to improve the scoring process (Wang et al., 2024). To ensure uniformity throughout this assessment process, a standard operating procedure (SOP) for quality evaluation was developed. Furthermore, the risk of bias and the reporting quality of studies were always assessed using the Cochrane Collaboration’s Risk of Bias (RoB) tool (Higgins et al., 2022).
To date, several overviews assessing the quality of systematic reviews of COVID-19 with CHM treatment have been published (Jeona et al., 2022; Luo et al., 2022). However, only one research (Zhou et al., 2023a) on the quality appraisal of RCTs concerning COVID-19 treatment with traditional Chinese medicine (TCM). The researchers did a careful analysis and early quality evaluation of these RCTs. Nevertheless, there are some limitations: First of all, complete randomness is one of the core features of RCT. We found that some articles described themselves as RCTs but expressed random methods inaccurately, such as using only the word “random” without specifying the specific random method, or non-random methods. The research didn’t restrict the complete random methods of RCTs. Second, the research used only two evaluation tools, the CONSORT-CHM Formulas 2017 and RoB. Third, the research only searched literature from three databases, with a time from the establishment of the databases to 17 February 2023. Therefore, in our study, we aim to conduct a wider literature search to assess the most recent evidence of the methodological reporting quality of RCTs on CHM for COVID-19 with strict randomization, and the precise reporting of CHM formula intervention. Firstly, we searched nine databases and thoroughly searched literature between 1 January 2019, and 22 April 2024. We also analyzed the difference between the studies recruited subjects during and after the first wave of the pandemic (defined here as December 2019 to March 2020 inclusive because by this time the first wave of the pandemic had been largely contained within China) (Commission and Medicine, 2020a). Secondly, we include RCTs that use the random number table method, coin toss method, and other completely random methods, which are called strictly random RCTs here. In addition, inadequate detailed information regarding CHM formula interventions may lead to doubts about their effectiveness, so we use the checklist of 42 sub-questions based on CONSORT-CHM Formulas 2017 to evaluate the precise reporting of RCTs with CHM formula interventions.
Nine electronic English and Chinese databases—the Cochrane Library, PubMed, Embase, Web of Science, EBSCO, China National Knowledge Infrastructure, VIP Journals Database, Wanfang Database, and Chinese Biomedical Database—were thoroughly searched between 1 January 2019, and 22 April 2024. The keywords used to search were as follows: “(COVID-19 OR SARS-CoV-2) AND (Traditional Chinese Medicine OR Chinese Herbal Drugs OR Integration of traditional Chinese and Western medicine) AND Randomized Controlled Trial”. The corresponding Chinese search terms were used for retrieval in the Chinese databases. The search results contained only publications in English and Chinese. To achieve an exhaustive search approach, free text words associated with the three basic topics were coupled with Medical Subject Headings (MeSH) terms and keywords. Supplementary Table 1 contains a comprehensive search strategy.
We included all RCTs evaluating CHM formulas as a standalone treatment or as an adjunct to standard care for COVID-19. At least one control group should receive no treatment, sham treatment, placebo, or routine care, regardless of publication status or language. The COVID-19 diagnosis was made in accordance with the diagnostic standards specified in the “COVID-19 Diagnosis and Treatment Protocol (Trial Sixth Version or later updated versions)” published by the National Administration of Traditional Medicine and the National Health Committee (Commission and Medicine, 2020b).
The exclusion criteria encompassed non-clinical trials, observational experiments, reviews, meta-analyses, protocols, duplicate publications, trail registry records, abstracts, letters, communication, not strictly randomized RCTs (including trials with not randomized method such as using the odd-even grouping according to the last digit of the ID card, semi-random or no specific randomization method), non-COVID-19 confirmed patients, non-CHM formula-related studies and scientific and technological achievements registration form. Search is limited to English and Chinese.
Two investigators (Min-li Chen and Shi-yan Qian) underwent training to thoroughly examine every element and multiple sub-elements outlined in CONSORT 2010, CONSORT-CHM Formulas 2017, the checklist of sub-questions based on the CONSORT-CHM Formulas 2017, and risk of bias assessment to ensure a comprehensive understanding of each standard. Each report was evaluated by two independent researchers. They extracted data from the literature using the four evaluation tools mentioned above. To show whether the RCTs had reported the pertinent elements and sub-elements, the two authors separately awarded ratings of “1,” “0,” “NA,” or “NI.” “0” signifies partial disclosure or absence of the corresponding element/sub-element, while “1” indicates that the author had adequately described the element/sub-element. “NA” denotes “not applicable” and indicates that a specific element or sub-question is not relevant to a given study. “NI” signifies “no information” related to the element or sub-question. Supplementary Table 2 displays the precise data extraction as well as a comprehensive outline of the SOP of the checklist of sub-questions based on the CONSORT-CHM formula 2017. As for the risk of bias, the Cochrane Handbook for Systematic Reviews of Interventions’ definition of bias was used to guide the evaluation process. The items of risk of bias included sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other biases. The results were classified as “low risk of bias,” “unclear risk of bias,” or “high risk of bias” for each item. Supplementary Table 3 provides the evaluation criteria for the level of risk of bias. The specific assessment scores of the two researchers are shown in Supplementary Tables 4–7. If a score or judgment is inconsistent during the extraction process, the chief physician (Guo-qing Zheng) is called in to help resit the score.
For the descriptive statistical analysis, we used Microsoft Excel 2019, and we totaled the RCTs linked to each project. The results were expressed as percentages, and for each overall ratio, 95% confidence intervals (CIs) were calculated. SPSS (version 23.0) was used for statistical calculations, and a significance level of p < 0.05 was taken.
A total of 6,738 articles that might be relevant were found. After using Endnote software to remove duplicates automatically, 4,727 papers remained. 3,229 papers were eliminated after titles and abstracts were reviewed, for one or more of the following reasons: 1) not a clinical trial, 2) review or meta-analysis, 3) duplicate publication, 4) non-COVID-19 confirmed patients, and 5) not related to Chinese herbal medicine. After further examination of the remaining literature by reading the full text, 1,432 papers were removed. Among these, 1,218 were observational studies, 61 were study protocols, 8 were abstracts or letters or communication, 1 were scientific and technological achievement registration form, 114 were trail registry records, 4 were not related to Chinese herbal medicine formulas, and 26 were not strictly randomized RCTs (Supplementary Tables 8, 9). Eventually, for the final analysis, 66 eligible RCT studies were chosen (Figure 1).
Sixty-six studies (Ai et al., 2020a; Ai et al., 2020b; An et al., 2022; An et al., 2023; Chen et al., 2022a; Chen et al., 2021; Chen et al., 2023; Chen et al., 2020; Chen et al., 2022b; Dai and Li, 2023; Dong et al., 2023; Duan et al., 2020; Fang et al., 2023; Fu et al., 2020; Guo et al., 2023; Han et al., 2024; He et al., 2021; He and Zhang, 2021; Hu et al., 2022; Hu et al., 2021; Hu, 2022; Li et al., 2020; Li et al., 2021; Li et al., 2023; Li et al., 2024; Lin et al., 2021; Lin et al., 2021; Liu et al., 2021a; Liu et al., 2021b; Liu, 2023; Liu et al., 2020; Liu et al., 2023; Liu et al., 2021c; Luo et al., 2020; Luo et al., 2021; Qiu et al., 2020; Shah et al., 2022; Shi et al., 2021; Sun et al., 2021a; Sun et al., 2021b; Wang et al., 2020; Wang et al., 2021; Wu et al., 2021; Wu et al., 2023; Wu et al., 2024; Xia et al., 2022; Xu et al., 2023a; Xu et al., 2023b; Yan et al., 2022; Yang et al., 2023; Yang, 2023; Yang et al., 2021; Yao, 2023; Ye et al., 2021; Yu et al., 2020; Yu et al., 2023; Yuan, 2021; Zeng et al., 2021; Zhang et al., 2023a; Zhang et al., 2022; Zhang et al., 2021; Zhang et al., 2023b; Zhao et al., 2021; Zheng et al., 2023; Zhou et al., 2021; Zhou et al., 2023b) involving 8712 COVID-19 patients were identified. For the 66 studies, there were 4,185 males and 4,360 females, and 4 studies did not mention the specific gender figures. Sample sizes ranged from 20 to 815 participants. The sample sizes of the included RCTs varied widely. Over time, there were more studies with larger sample sizes. This may be related to the increasing number of infections. Out of all 66 studies, 45 were published in Chinese and 21 in English. Sixty-four studies were conducted domestically, and two studies were conducted in collaboration between domestic and foreign investigators. More international cooperation is needed to carry out multi-center, large-sample RCTs to improve research representativeness and extrapolation. There were only thirty-three studies reported adverse effects. Adverse reactions are one of the important indicators to evaluate drug safety, and RCTs should report this part as much as possible. Five studies mentioned that they reported according to the CONSORT Statement. The CONSORT Statement standard has been published and used for many years. These self-reported RCTs that reported using this criterion did report a more complete picture of what was relevant to the study. Among other RCTs that do not report using this standard, some studies have good reporting quality, and there are more studies with poor reporting. Characteristics data are enumerated in Table 1; Supplementary Table 10. Table 2 summarizes the elements reported from the 66 RCTs in accordance with the CONSORT-CHM Formulas 2017 and the CONSORT 2010 statement. Table 3 summarizes the elements reported from the 66 RCTs based on sub-questions derived from the CONSORT-CHM Formulas 2017. Figures 2–4 display the distribution of the score and report ratio of the items on the three checklists of the included articles. The subgroup analysis results of RCTs recruited subjects during and after the first wave of the pandemic are shown in Table 4. The judgment of risk of bias is displayed in Figures 5, 6.
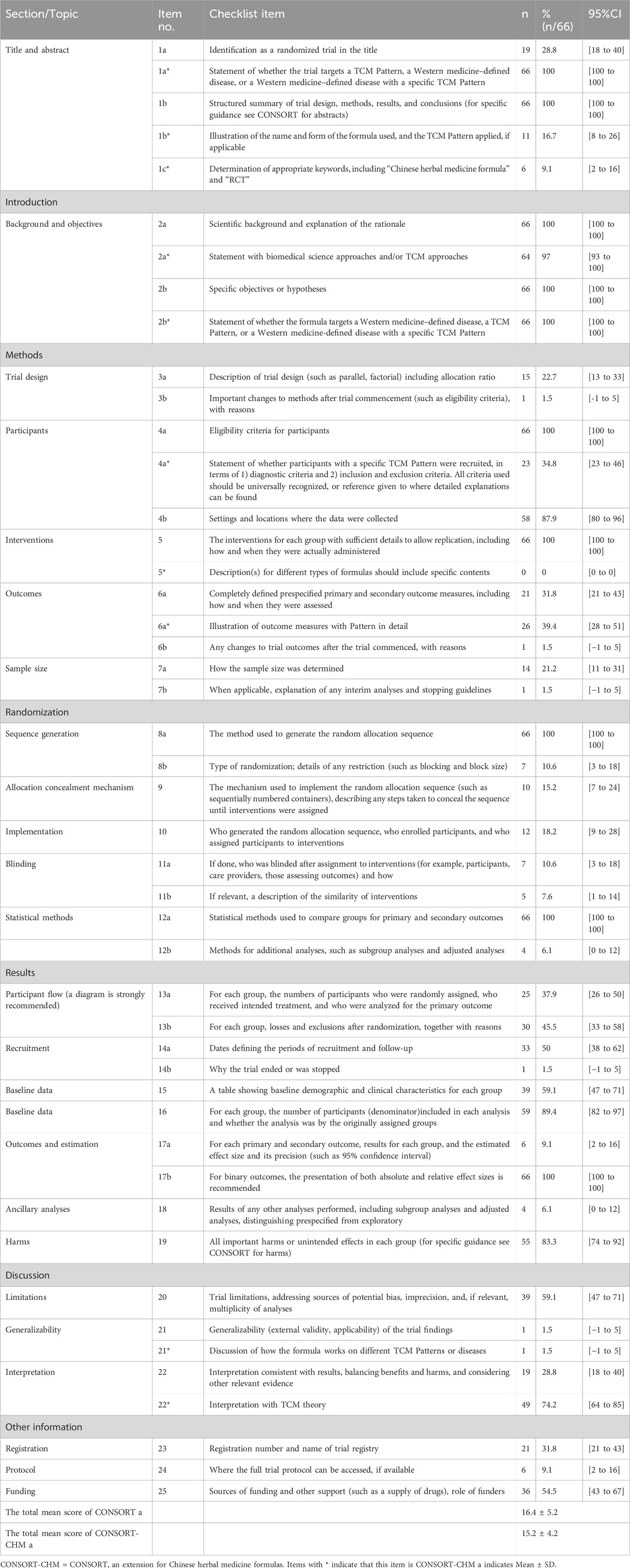
Table 2. The reporting number and percentage for each item of the CONSORT and CONSRT-CHM formulas checklist of the included 66 studies.
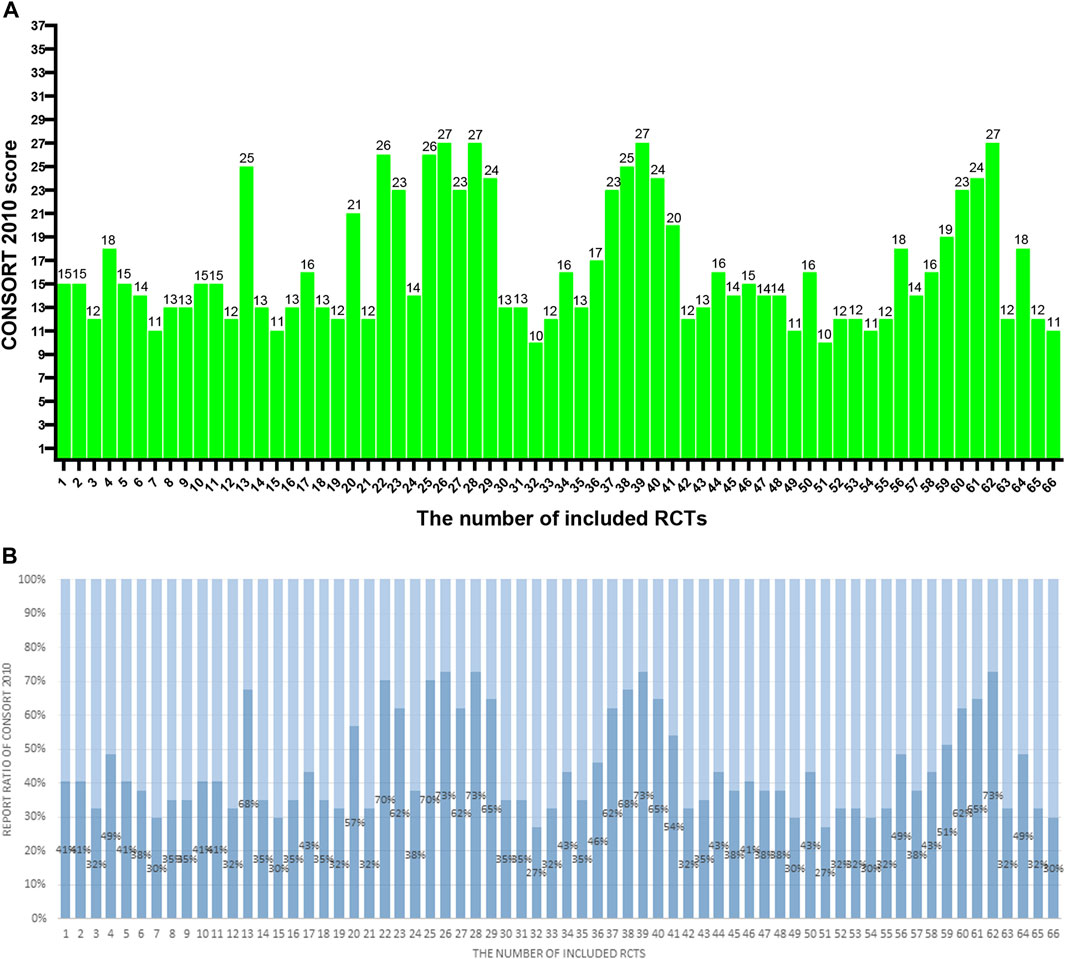
Figure 2. (A) The CONSORT 2010 scores of the 66 RCTs. The two researchers separately scored each of the included papers based on 37 items of the CONSORT 2010. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the final scores of included RCTs. (B) The reported ratio of CONSORT 2010 of the 66 RCTs. The two researchers separately scored each of the included papers based on 37 items of the CONSORT 2010. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the reported ratio of the CONSORT 2010 in included RCTs (score/37*100%).
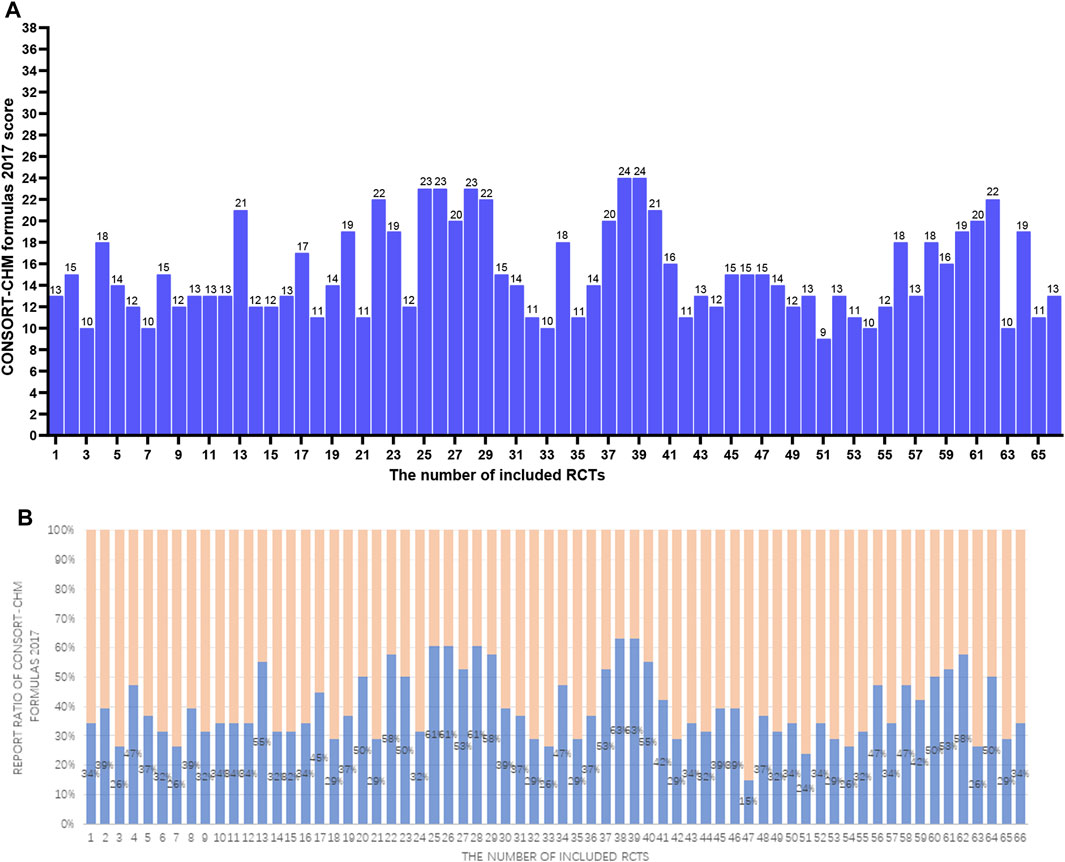
Figure 3. (A) The CONSORT-CHM formulas 2017 scores of the 66 RCTs. The two researchers separately scored each of the included papers based on 38 items of the CONSORT-CHM formulas 2017. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the final scores of included RCTs. (B) The reported ratio of CONSORT-CHM Formulas 2017 of the 66 RCTs. The two researchers separately scored each of the included papers based on 38 items of the CONSORT-CHM formulas 2017. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the reported ratio of the CONSORT-CHM Formulas 2017 in included RCTs (score/38*100%).
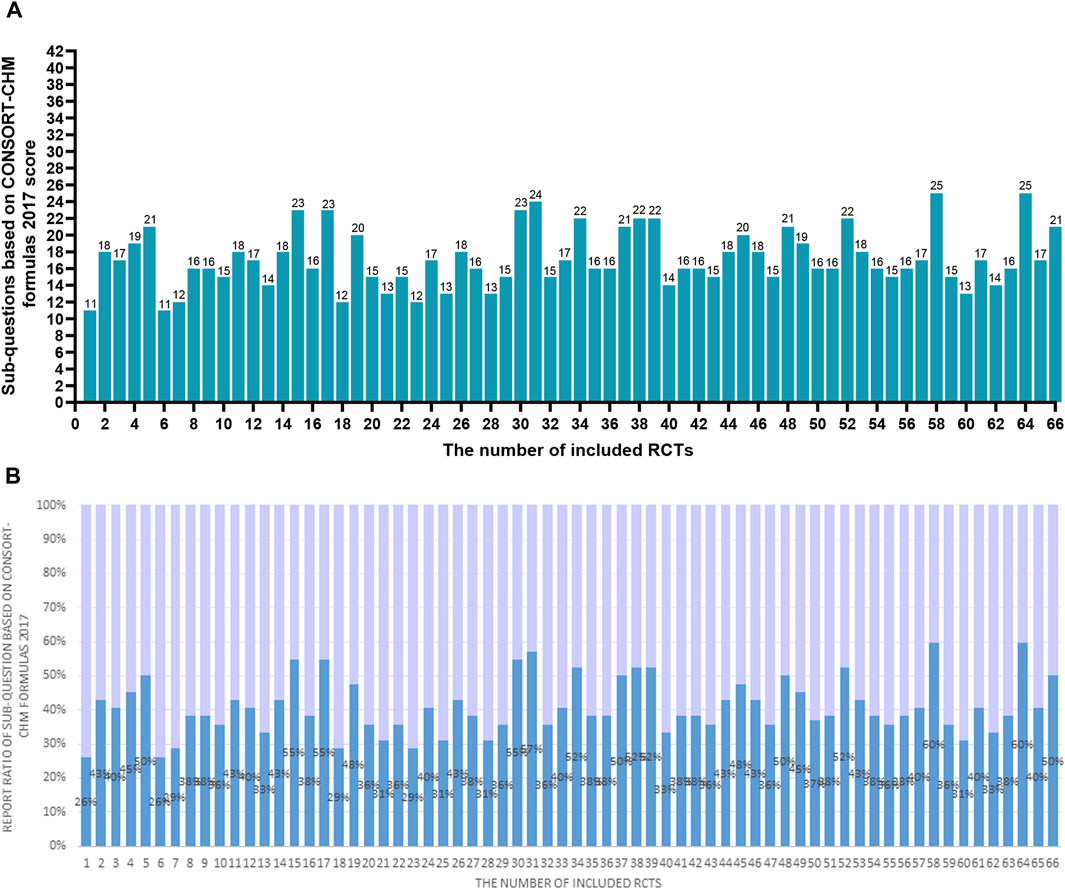
Figure 4. (A) The scores of the 66 RCTs evaluated based on the checklist of 42 sub-questions based on CONSORT-CHM Formulas 2017. The two researchers separately scored each of the included papers based on the checklist of 42 sub-questions based on the CONSORT-CHM formulas 2017. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the final scores of included RCTs. (B) The reported ratio of the 66 RCTs evaluated based on the checklist of 42 sub-questions based on the CONSORT-CHM Formulas 2017. The two researchers separately scored each of the included papers based on the checklist of 42 sub-questions based on the CONSORT-CHM formulas 2017. If a score or judgment is inconsistent during the extraction process, the chief physician is called in to help resit the score. The figures represent the reported ratio of the checklist of 42 sub-questions based on the CONSORT-CHM Formulas 2017 in included RCTs (score/42*100%).
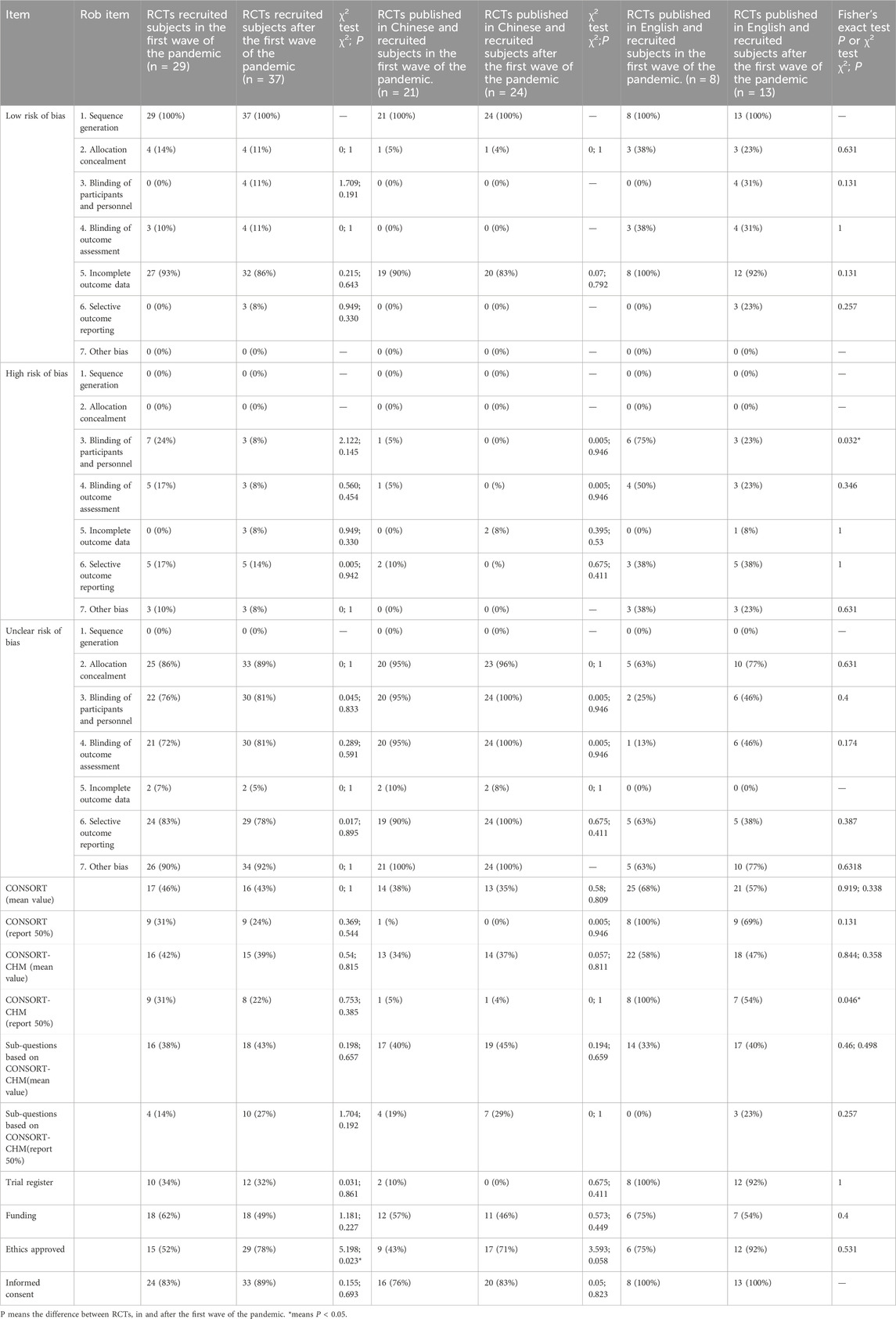
Table 4. The difference between RCTs recruited subjects during and after the first wave of the pandemic.
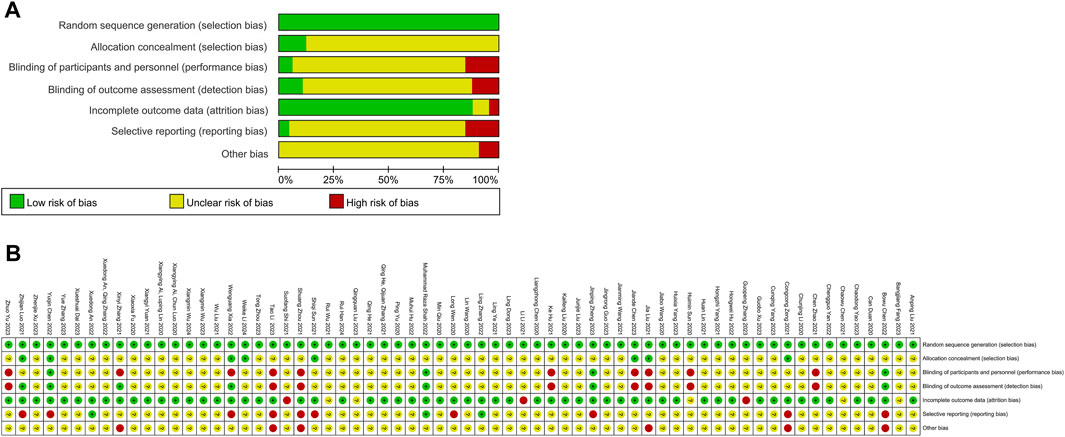
Figure 5. Risk of Bias. (A) Risk of bias of all included 66 RCTs; Risk of bias summary of all included 66 RCTs (B). (Green indicates low risks, yellow indicates some concerns, and red indicates high risk).
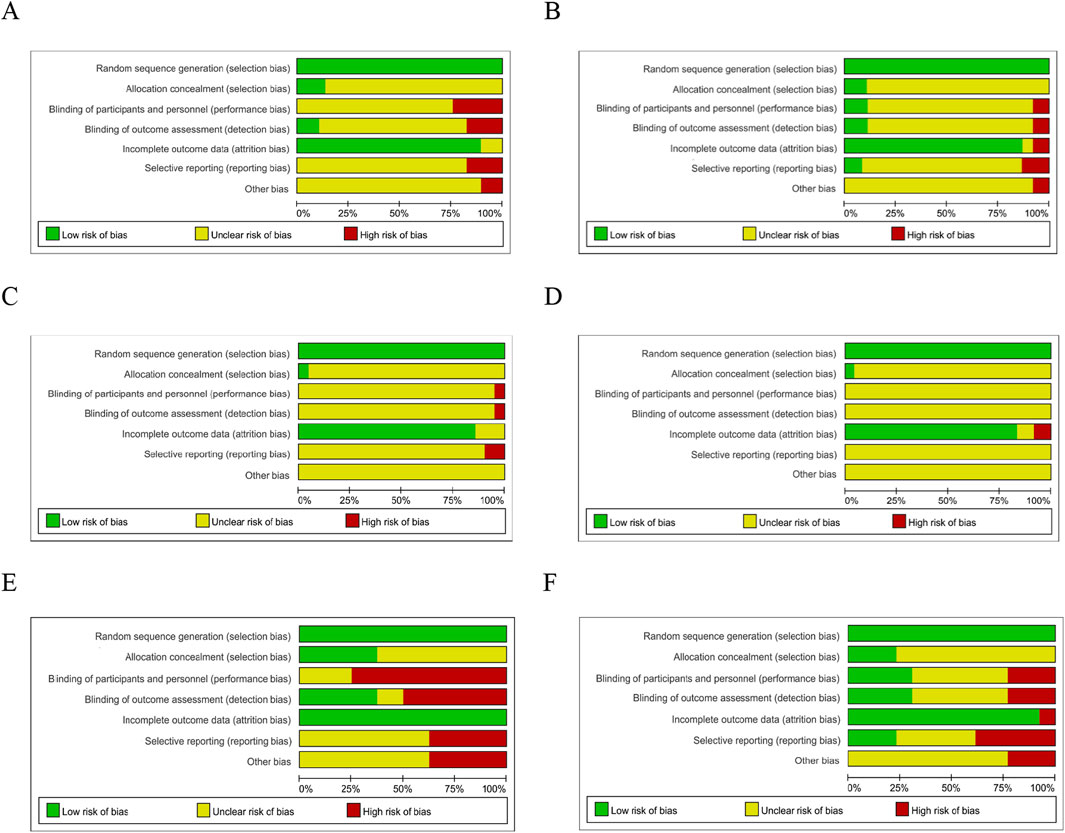
Figure 6. Risk of Bias. Risk of bias of RCTs recruited subjects during the first wave of the COVID-19 pandemic (A) and after the first wave of the COVID-19 pandemic (B); Risk of bias of RCTs published in Chinese during the first wave of the COVID-19 pandemic (C) and after the first wave of the pandemic (D); Risk of bias of RCTs published in English during the wave of the COVID-19 pandemic (E) and after the first wave of the pandemic (F). (Green indicates low risks, yellow indicates some concerns, and red indicates high risk).
The results are shown in Table 2; Figure 2. Total mean scores Among the 66 articles in this study, the average score of the CONSORT 2010 evaluation is 16.4, accounting for only 44% of the total 37 items. The specific breakdown of 37 items is as follows:
After reviewing the title (1a), only 19 (28.8%) trials were found to meet the criteria for being classified as RCTs. Additionally, all of the articles (100%) included abstracts that provided a structured overview of the experimental design, methods, results, and conclusions (1b).
The total included studies mentioned a scientific background and explanation of the rationale (2a) and presented specific objectives or hypotheses (2b).
All the articles included described two CONSORT 2010 items, with 100% coverage, which included a trial design with eligibility criteria for participants (4a) and detailed interventions for each group to enable replication (5). Settings and locations where the data were collected (4b) were described in 87.9% of the research. Of the publications, 22.7% revealed the trial design including participant allocation ratio (3a), and 31.8% described the primary and secondary outcome measures (6a). Fourteen articles (21.2%) explained how sample size was determined (7a). Sample size calculation is an important part of the RCT design process, but the report rate of sample size calculation in the included literature is not satisfactory. Only 1 (1.5%) study, respectively, described significant changes in the experimental method (3b), whether there were changes in the trial outcomes after the commencement of the experiment (6b), and information on the interim analysis and stopping guidelines (7b).
The process for creating the random allocation sequence (8a) as well as comprehensive statistical methods (12a) were offered in all sixty-six (100%) studies. However, only 10.6% of papers explained the kind of randomization (8b), ten papers (15.2%) reported using the random allocation sequence (9), 18.2% gave thorough information about the implementation (10), and 10.6% discussed the blinding procedure (11a), five studies (7.6%) mentioned intervention similarities (11b). The rate of reporting information about the implementation of the random allocation is low, and so is the rate of reporting on the blind method. Furthermore, 6.1% of publications included techniques for further analysis (12b).
Thirty-five (45.5%) studies provided an explanation of the losses and exclusions (13b) following randomization, while twenty-five (37.9%) studies used a diagram to show the treatment progress (13a). The treatment progress flow chart provides a clearer picture of the inclusion and loss of study subjects and is also relevant to the assessment of bias risk for reporting results fully. However, the reporting rate in this segment is less than 50%. Thirty-three studies (50%) provided the dates that defined the recruitment and follow-up periods (14a). Out of all the articles, just one (1.5%) explained why a trial was terminated or discontinued (14b). A total of thirty-nine (59.1%) articles included baseline information, which included basic demographic and clinical features as well as underlying disease (15). Baseline characteristics are expected to be reported especially. Because it plays an important role in evaluating the comparability between groups, improving the credibility of results, enhancing the extrapolation of results, statistical analysis, and so on. Fifty-nine (89.4%) studies described the number of participants of each group and whether the analysis was by originally assigned groups (16). The total of papers (100%) reported absolute or relative effect sizes (17b), while only six articles (9.1%) supplied the estimated effect size (17a). Out of all the research, only 4 (6.1%) reported on the outcomes of additional analyses. Fifty-five publications (83.3%) reported significant negative effects or unforeseen consequences (19).
Thirty-nine (59.1%) articles reported the limitation of trials (20) and 1 (1.5%) article illustrated the generalizability of the trial findings (21). Just 19 (28.8%) of the research offered an explanation that made sense of the outcome (22).
Twenty-one of the trials (31.8%) provided registration (23) and 36 (54.5%) for sources of funding (25). Only 6 (9.1%) articles, however, included information on how to acquire the entire study protocol (24). The low reporting rate of RCT registration information and protocol information is not conducive for other researchers to fully understand the content of these studies and get detailed references for similar studies.
Table 2; Figure 3 show the results. The average CONSORT-CHM Formulas 2017 evaluation score is 15.2 among 66 studies, accounting for only 40% of the total 38 items. Compared to CONSORT 2010, CONSORT-CHM Formulas 2017 added one subitem (1c*) and expanded seven items. The results are as follows:
Sixty-six articles (100%) stated by title that the trial targets the specific disease (1a*). The formula’s name, dose form, and TCM pattern were only mentioned in 11 (16.7%) trials (1b*). Only 6 (9.1%) articles determined appropriate keywords, including “Chinese herbal medicine formula” and “RCT” (1c*).
Statements using TCM approaches were found in sixty-four (97%) papers (2a*), while statements regarding whether the formula takes on a specific disease were found in all 66 (100%) articles (2b*).
Twenty-three research (34.8%) reported on the recruitment of subjects who fit a particular TCM pattern (4a*). Syndrome differentiation and treatment is one of the core features of traditional Chinese medicine. However, the reporting rate of the included RCTs for the TCM pattern was not satisfactory. Even more shocking, none of the articles (0%) described the Chinese herbal medicine formulas in full detail (5*). All RCTs can provide only partial information on Chinese herbal formulas. Twenty-six (39.4%) articles reported the outcome measures related to TCM syndrome in detail (6a*). Other items in CONSORT-CHM Formulas 2017 are consistent with the CONSORT 2010.
Item 20 has no extension. A discussion of how the formula takes on different TCM patterns on the disease was only performed in 1 (1.5%) article (21*). And 49 (74.2%) studies additionally offered the interpretation with TCM theory (22*).
According to Table 3; Figure 4, the average score of the checklist of sub-questions based on the CONSORT-CHM Formulas 2017 evaluation is 17.2 among 66 studies, accounting for only 41% of the total 42 items. Only 9 sub-questions had been more than 75% of the included studies fully reported, 13 sub-questions had been more than 50% of the included literature fully reported, and 17 sub-questions had been less than 25% of the literature fully reported. Only 11 (16.7%) studies reported greater than or equal to 50% of the 42 sub-questions. The features found in various sources of CHM formula intervention are as follows:
(1) 5a. For fixed CHM formulas: more than 80% of studies provided information on the CHM formulas’ names, sources, and dosage forms (Q11-Q13). The reported ratio of the dosage of formulas, the treatment duration, and the administration was 66.7%,95.5%, and 100%, respectively (Q25-Q27). Nonetheless, according to Q14–Q17, the percentages for each medicinal substance’s name, source, processing technique, and dosage were 54.5%, 28.8%, 28.8%, and 40.9%, respectively. None of the studies provided the authentication method for each ingredient of CHM formulas (Q18). In twenty-three trials (34.8%), the principles, reasoning, and interpretation of forming the formula(Q19) were mentioned. 56.1%, 57.6%, 33.3%, 3%, and 0% of the studies, respectively, provided references regarding the formulas’ efficacy, the findings of pharmacologic studies, the formula’s manufacturing process, the quality control of both the product and each ingredient in the formula, and the formula’s safety evaluation (Q20-Q24). For the fixed CHM formulas, information on the name of composition, dosage, and course of treatment are highly reported, while the reporting of information about the processing method and dosage of ingredients, processing process of the whole formulas, and safety assessment of formulas is insufficient.
(2) 5b. For individualized CHM formulas: only 9 (13.6%) trials reported the information about how, when, and by whom the CHM formulas were modified (Q28). Other information on formulas is consistent with the results of Q11-Q27.
(3) 5c. For patent proprietary CHM formulas. According to Q29–Q33, the reported ratios for CHM formulas’ composition and dosage, efficacy, safety or quality control, name, the name of the manufacturer, lot number, production date, and expiration date were 18.2%, 39.4%, 4.5%, 33.3%, and 33.3%, in that order. Twenty-eight (42.4%) of the studies stated that the trial’s usage of the patent-protected formula was for a condition that was the same as the reference that was made public (Q34). For patent proprietary CHM formulas, most of them will provide the name of formulas and specific brands, but the specific information of composition, such as name, dosage, and the information of safety, production lot number, and other information, is insufficient.
(4) 5d. Control groups were given a placebo. Just 3%, 3%, and 13.6% of studies discussed how comparable the placebo was to the intervention, how safe and effective the placebo was, and how the placebo was administered in terms of dosage, regimen, and route (Q36-38). None of the trials provided information on the precise manufacturing details of the placebo, including the name and quantity of each ingredient (Q35 and Q39).
The risks of bias evaluation results for all included trials (Table 4; Figures 5A, B) and two subgroups of RCTs recruiting subjects during and after the first wave of the pandemic are shown in Figures 6A–F. A detailed evaluation of the included studies’ risk of bias can be found in Supplementary Table 7. It was discovered that every RCT that was included had a high or unclear risk of bias in at least one domain. The evaluation of seven bias-prone domains produced the following conclusions: Random sequence generation (selection bias): Because all included studies sufficiently reported random sequence creation, the risk of bias was deemed to be minimal. Allocation concealment (selection bias): Out of the 66 papers that were evaluated, only 8 were deemed to have a low risk of bias, the remaining studies were evaluated with uncertain risk of bias. Fifty-two studies were rated as having an unknown risk of bias, 10 for a high risk of bias, and just 4 for a low risk of bias due to participant and personnel blinding (performance bias). Blinding of outcome assessment (detection bias): Because of inadequate data, the majority of the studies were rated as having an unclear risk of bias, with only 7 for a low risk of bias and 8 for a high risk of bias. Attrition bias, or incomplete result data: 3 studies had a high risk of bias, 4 studies had an unknown risk of bias, and 89% of studies reported complete outcomes and were rated as having a low risk of bias. Selective reporting, often known as reporting bias: just 5% of studies were classified as having a low risk of bias, 17% for a high risk, and 80% for an uncertain risk. Other bias: 9 percent of the trials had a high risk of bias, none had a low risk of bias, and the most had an uncertain risk of bias. In these RCTs, there is a large proportion of unclear risks, especially in the allocation concealment, participant and personnel blinding, blinding of outcome assessment, and selective reporting risks. This may be due to most of the included studies provided incomplete information on study design and methodology.
Table 4 indicates a statistically significant difference (P < 0.05) in ethics approval between the two subgroups of RCTs, which recruited subjects during and after the pandemic’s first wave. Most RCTs recruited subjects after the first wave of the pandemic can provide ethics approval. The three methodological quality assessment checklists and the risk of bias did not significantly differ between these two groups. Our additional research, however, revealed a notable distinction between RCTs published in English that recruited subjects during and after the first wave of the pandemic. For risk of bias, 75% of RCTs published in English that recruited subjects during the first wave of the pandemic had a higher risk of bias in the blinding of participants while only 23% of RCTs published in English recruited subjects after that had a higher risk of bias (P < 0.05). Simultaneously, 4 (31%) RCTs in English that recruited subjects after the first wave of the pandemic were identified as low risk of bias in the blinding of participants, but none of the RCTs recruited subjects during the first wave of the pandemic were identified as low risk of bias. These data suggest better reporting of information in clinical registries of RCTs recruited subjects after the first wave of the pandemic than during the first wave of the pandemic. In RCTs published in English, the RCTs that recruited participants after the first wave of the pandemic have higher reporting quality of blinding of participants than those during the first wave of the pandemic.
The average score of 66 studies of the CONSORT 2010, the CONSORT-CHM Formulas 2017, and the checklist of 42 sub-questions based on the CONSORT-CHM Formulas 2017 evaluation is 16.4, 15.2, and 17.2, respectively. The report rate of sample size calculation, allocation concealment, and blinding is less than 30%. The low reporting rate of CONSORT and CONSORT-CHM formula items and the unclear risk of bias indicates the reporting quality in strictly randomized RCTs on CHM formulas for COVID-19 is inadequate. The checklist of 42 sub-questions based on CONSORT-CHM formulas can be a Supplementary Scale to CONSORT-CHM formulas to help report and assess CHM formula intervention more precisely. The reporting of CHM formula intervention in each medical substance, principles of forming the formula, production method, and safety assessment of formulas are inadequate (all less than 45%). There was a 100% low risk of bias on random sequence generation, and 89% on incomplete result data for all included RCTs. Most studies assessed an “unclear risk of bias” due to insufficient information in the other five domains. In addition, in RCTs published in English, RCTs recruited subjects during the first wave of the pandemic have a higher risk of participant blinding bias than RCTs recruited subjects after the first wave of the pandemic.
Because of the ability to reduce or completely eradicate bias, randomized controlled trials (RCTs) are considered the most dependable approach for evaluating therapies. Unreliable treatment effect estimates can result from insufficient reporting and poor design, according to recent methodological evaluations. The CONSORT 2010 statement offers evidence-based, minimal recommendations for standardizing the reporting of RCT results and reducing research bias. It greatly standardizes the publication of RCT results and raises the quality of research papers (Moher et al., 2012). There are more than 600 academic journals around the world that have adopted the CONSORT 2010 statement, which can be used as an important reference for judging whether the article is written in a standardized manner (Chan and Altman, 2005; Plint et al., 2006). Mohamed et al. discovered there was a positive correlation between the level of CONSORT compliance and the impact factor of the studies’ published journals (Jalloh et al., 2024). Based on CONSORT 2010 statement, the CONSORT-CHM Formulas 2017 adds traditional Chinese medicine patterns and items (adds one subitem, and expands the contents of seven items) according to the characteristics of CHM formulas to enhance the clinical RCTs of TCM formulas reporting quality. For studies that have adhered to the CONSORT-CHM Formulas 2017 principle, there is a great improvement in transparency regarding reporting herbal interventions (Ornelas et al., 2018). Another well-known and well-liked method for evaluating the caliber of RCTs in evidence-based medicine is the Cochrane risk of bias tool. In this study, we standardized the scoring criteria of all items through learning and training the CONSORT items scoring to unity of each item judgment criteria. The method of double scoring is adopted. If the two researchers have different judgments, the superior researcher will make the final judgment to ensure accuracy.
In this study, we found that a few articles obtained good scores. Four articles obtained a high score of 27 points in CONSORT 2010, and two articles obtained a high score of 24 points in CONSORT-CHM Formulas 2017 evaluation, but the scores of most studies were low. Only 17 articles and 13 articles in CONSORT 2010 and CONSORT-CHM Formulas 2017 reached 20 points respectively, while the proportion of articles with 10 points and below in CONSORT 2010, CONSORT-CHM Formulas 2017 was as high as 3% and 9% respectively. Overall, the methodological reporting quality of the RCTs included needs further improvement.
Firstly, the title indicates that the corresponding article is an RCT, which can make it more easily identified. However, only 19 articles in this study can be seen as an RCT through the title. Secondly, for most studies, it’s not feasible to study the whole population. Therefore, we need sample size calculation which represents a trade-off between cost effectiveness, ethical concerns, and statistical power (Mohammad et al., 2022). Nevertheless, only 14 (21.2%) articles in this study explain how to calculate the sample size. In this instance, there should be an endeavor to enhance the transparency of sample size calculation to improve the external validity of the RCT. If the sample size calculation report shows little association with the randomized controlled trial, it may be necessary to abandon it, as recommended by Bachetti (Bacchetti, 2002). It is recommended that RCT researchers strictly follow the CONSORT guidelines, use scientific methods to calculate the sample size, and report the calculation of the sample size in detail, including reporting important parameters of the sample size, such as effect size, significance level, statistical power, etc., cite the sample size calculation formula or software used and provide references or software names. Thirdly, relevant studies have demonstrated that blinding is a crucial protective measure to minimize errors (Savovic et al., 2012). Alraddadi et al. indicated that result estimates would be exaggerated if RCTs lack blinding or allocation concealment. In this study, most articles lack a detailed description of the implementation of blinding and allocation concealment mechanisms (Savovic et al., 2012). RCT investigators are advised to strictly follow the CONSORT guidelines, specify the specific method of assigning concealment (such as the use of sealed envelopes or a central randomization system, etc.), identify the independent person responsible for assigning concealment (such as an independent statistician or a third party), and indicate that assigning concealment was performed after subjects were recruited and before the intervention. In addition, report the use of single, double, or triple blindness, explain who was blinded (e.g., subject, investigator, outcome evaluator, etc.), describe the specific operation of the blinding (e.g., placebo or simulated intervention), report whether the blinding was successfully maintained and whether breaking the blinding occurred and why. Authors are encouraged to register experimental protocols in advance and cite them in their articles so that readers are aware of the plan to assign concealment and blind methods. Fourthly, the subjects’ baseline characteristics can be used to reflect the comparability between the experimental and control groups in the results section; 59.1% of the articles in this study elaborated on the baseline characteristics. Fifthly, in the discussion section, only 59.1% of articles elucidated the limitations and extrapolation. The limitations of the study are also an important part of the RCT article. On the one hand, it can demonstrate the objectivity of the research and enhance readers’ trust in the research; on the other hand, the limitations reveal the deficiencies in the study, which can provide suggestions for improvement in future study design, and it points out unresolved problems that can inspire other researchers to carry out relevant studies. Sixthly, the International Committee of Medical Journal Editors (ICMJE) mandates that all clinical trials must be registered to enhance transparency and accountability (DeAngelis et al., 2005). However, only 31.8% of trials in this study were registered. Clinical trial registration can enhance the transparency and credibility of research, promote research ethics and scientific norms, avoid duplication of research and waste of resources, and enhance the credibility and verifiability of research results. In addition, conflicts of interest and the source of financing were stated in 54.5% of the papers. Explaining the source of funding helps readers understand the independence of the research, and disclosing conflicts of interest demonstrates academic integrity. It can also help readers identify bias and interpret the research results more fully. Moreover, only 9.1% of the studies provided a protocol. Protocol improves the transparency and credibility of the research by disclosing the research design and avoiding selective reporting. Moreover, it clarifies the research methods and results, and facilitates other researchers to repeat the experiment or verify the results. Researchers share the experience and lessons of the experiment design, which can promote the progress of the methodology. It is recommended that investigators complete registration with an internationally recognized registration platform (such as ClinicalTrials.gov, WHO ICTRP, China Clinical Trial Registry, etc.) before trial initiation and ensure that registration information is complete, including study purpose, design, sample size, interventions, primary and secondary outcome measures, etc. In the article, report the trial registration number (e.g., NCT number), state the time of trial registration (e.g., before subject recruitment begins), and provide the name and link of the registration platform. It’s recommended that upload the full trial protocol on a registered platform or in an open-access journal (e.g., BMJ Open, Trials), cite the trial protocol in the article, and provide a way to obtain it (such as DOI or link). Therefore, in order to guarantee the protection of subjects’ rights and the validity of the research, we expect that future studies can enhance these areas.
CONSORT Formulas 2017 is an expanded version of CONSORT 2010 with some of the same results. The difference is the expanded entry section. To begin with, just 6 publications (9.1%) used “randomized clinical trial” and “traditional Chinese medicine” as keywords. Furthermore, none of the articles provided comprehensive information on the item of “intervention” including the dosage form, source, formula basis, etc., of Chinese herbal medicine formulas. Providing the necessary details and guaranteeing the quality control of CHM is crucial for researchers to evaluate experimental methods and results of CHM-related studies. Chief and deputy botanical drugs are used in conjunction in CHM formulae, which stress the significance of treating and differentiating syndromes. Changes to the drug’s composition, dosage, or manufacturing technique within the same formulation can affect how well the prescription works as a whole. First, reporting detailed information on TCM compounds can give readers a comprehensive understanding of the specific content of the intervention, enhance the transparency of the study, support the replication of the trial, and validate the results of the study. Second, detailed information on interventions is available for clinicians to understand how to properly use interventions to support clinical practice. In addition, a detailed description of the composition, dosage, and preparation method of Chinese herbal medicine can promote the standardization of Chinese herbal medicine research and reduce the differences in results caused by different preparation methods. At the same time, the provision of quality control information (such as the origin of the drug, ingredient testing, etc.) can ensure the consistency and reliability of the intervention. Furthermore, only 16.7% of the publications included information on the name and kind of formula utilized, as well as the TCM Pattern, applied, even though all of them provided a detailed description of the outcome indicators. Lastly, a few articles failed to integrate relevant TCM theories into their discussions. The application of dialectical treatment is a fundamental aspect of TCM theory. Using the Chinese herbal medicine formula that is appropriate for the patient and their TCM pattern is a hallmark and tenet of Traditional Chinese Medicine. Consequently, reporting of TCM Patterns and the analysis of TCM theory are essential for the study of the application of TCM intervention.
The checklist of 42 sub-questions based on CONSORT-CHM Formulas 2017 can be a supplement scale of CONSORT-CHM to help report and assess CHM formulas intervention more precisely. In our study, all the included studies were scored as zero for the item of “intervention” in CONSORT Formulas 2017. However, we can capture and report more precise information about the CHM interventions by using the sub-questions checklist tool. Two articles obtained a high score of 25 points in the checklist of 42 sub-questions based on CONSORT-CHM Formulas 2017. Unfortunately, the scores of most studies were low. The checklist of sub-questions based on CONSORT-CHM Formulas 2017 score of all articles was only 17.2. There were 95.5%,97%, and 83.3%, respectively, of studies that reported the name, the dosage form, and the source of CHM formulas. However, just 36.4% of studies reported recruiting subjects with specific TCM Patterns according to inclusion and exclusion criteria as well as diagnostic criteria. The formula’s foundational ideas, justification, and interpretation were discussed in 34.8% of the publications. The quality of the final product and each ingredient were revealed in 3% of the studies. The lot number, the manufacturer’s name, the proprietary product name (sometimes known as the brand name), and the patent proprietary CHM formulae were shown in 33.3% of the articles. The following topics were not reported: how each ingredient is authenticated; whether the safe assessment of the formula is conducted; what the name of the placebo is; how the quantity of each ingredient in the placebo; or how the formula affects certain TCM patterns or illnesses. In general, the RCTs included reported the information of the formulas’ name, dosage, and duration of the formulas adequately, while the reporting quality of the processing method, dosage, and quality control of the component of formulas, as well as the production process and adverse reactions of the formulas, was low. The method of preparation of the formulas is not reported in detail, which reduces the transparency of the study and may make the findings difficult to verify or replicate. Failure to report quality control standards may lead to doubts about the consistency and reliability of interventions. Failure to report adverse reactions may result in the safety of an intervention not being assessed. It is recommended to fully report detailed information on TCM compounds, improve reporting transparency, strengthen quality control, report adverse reactions and safety, follow the 42 CONSORT-CHM sub-issue criteria, and ensure that all critical information is reported. During the study design and writing phase, methodological experts or peer reviews can be invited to ensure that the study complies with CONCORT-CHM standards.
The majority of the included studies only supplied partial details about their methodology and research design. It was shown that every included study had an unknown or considerable risk of bias in at least one domain. No trial was scored as low risk of bias in all domains. Analogous findings were observed in a prior study that included 64 RCTs of COVID-19 therapy with traditional Chinese medicine. The researcher indicated that the overall quality of RCTs investigating traditional Chinese medicine for COVID-19 was substandard and needs to be improved (Zhou et al., 2023a). Terence et al. found (Quinn et al., 2021) that COVID-19 studies published during the first wave of the pandemic had reporting and methodological issues that may compromise the utility of the research and may cause harm. Firstly, the majority of Chinese studies lack adequate information and unusually assess unclear risk of bias. In contrast, English literature tends to be more detailed. Terence et al. also indicated that RCTs suggested lower quality scores in the COVID-19 papers. 1. Random Sequence Generation: Since we limited the need for strict randomization when we included RCTs, the assigned sequences produced this entry were all low-risk. 2. Allocation Concealment: Most RCTs assign hidden details to the report, so most are rated as having an unspecified risk of bias. A few RCTs have managed to disclose specific methods of allocating concealment, such as using sealed envelopes or central random systems. We encourage more RCT researchers to do this to reduce selection bias. 3. Blinding of Participants and Personnel: Again, most of them are unclear about migration risks. About 20% of RCTs are rated as high risk due to the use of the open-label method. Participants and researchers were aware of groupings that could lead to differences in interventions. RCT investigators are advised to report in detail how blinded they are, using either placebo or dummy interventions. 4. Blinding of Outcome Assessment: The results of risk of bias are similar to Blinding of Participants and Personnel. It is recommended that an independent outcome evaluator be used and that blind implementation be reported in detail to avoid measurement bias. 5. Incomplete Outcome Data: This is the ideal result in the migration risk assessment item. Most are low-risk. The missing data is small and the missing reason has nothing to do with the result, so the impact on the result is small. 6. Selective Outcome Reporting: Most of them are unclear bias risks. About 20% were rated high risk due to non-reporting of some of the preset indicators. It is recommended to pre-register the study protocol and outcome indicators in the test platform, provide registration information in the article, and report all preset results to avoid reporting bias. 7. Other Bias: Most of them were unclear risks, and about 10% were rated as high risks because some of the information was significantly unbalanced in baseline characteristics. It is recommended to fully consider potential offset sources in the design phase, and explain other possible offset and control measures in the paper.
Researchers found (Jung et al., 2021) that COVID-19 clinical studies have a shorter time to publication and have lower methodological reporting quality scores than control studies in the same journal. These studies should be revisited with the emergence of stronger evidence. That’s an interesting proposition. Therefore, we wanted to explore whether the quality of RCT literature would change in the progress of the epidemic. Our study showed that there were no differences between the RCTs published in Chinese during and after the first wave of the epidemic, while the RCTs published in English showed significant differences in the blinding method. The methodological reporting quality of most RCTs published in Chinese is not high, and we now know that their quality has not changed with the progress of the epidemic. In the studies published in English, RCTs recruited subjects during the first wave of the epidemic did not perform well with strict blinding, however, that improved for the RCTs recruited subjects after the first wave of the pandemic. We think there may be several main reasons: on the one hand, the infection was newly discovered during the first wave of the pandemic, and there was limited understanding of the pathogenicity of the new virus. Considering the safety and compatibility of the subjects, they did not use a strict blind method. On the other hand, strict prevention and control measures at that time limited the implementation of blinding. In addition, given the major threat to human health caused by the COVID-19 epidemic at that time and the need for effective drugs, researchers needed to publish the research results as soon as possible, which may have an impact on the implementation of the blind method.
This study has some limitations. The included RCTs were only published in English and Chinese. The data extraction is solely based on the published paper. This approach means that we cannot capture some preliminary tests with good quality in the test method, that are not reported in the final publication. Therefore, when evaluating the trial quality of such studies, it is necessary to review the study protocol and contact the experimenter for more information.
In the future, RCTs should strictly follow the CONSORT 2010, CONSORT-CHM Formulas 2017 and report detailed methodological information, especially sample size calculation, allocation concealment, blind method, and detailed information of TCM formulas. Investigators are encouraged to register clinical trials before trial initiation, make the study protocol public, and report registration information in their articles. Secondly, improve research design. Studies should be based on scientific sample size calculation methods to ensure that the study has sufficient statistical power, and strict allocation concealment and blind measures should be adopted to reduce the risk of bias. Thirdly, fully report detailed information of Chinese herbal formulas, including the source of herbs, processing methods, quality control, adverse reactions, etc. More studies combined with Chinese TCM theories such as syndrome differentiation and treatment to explain the research results and enhance the characteristics of TCM. Fourthly, among the RCTs included, only 2 RCTs were carried out jointly by domestic and foreign researchers. More international cooperation is needed to carry out multi-center, large-sample RCTs to improve research representativeness and extrapolation. Fifthly, the quality of methodological reporting on RCTs for pandemic infectious diseases needs to be improved in the future. Due to the threat to human health caused by the COVID-19 pandemic and the strong need for effective drugs, the publishing of the results of RCTs became urgent, which may have influenced the quality of methodological reporting on RCTs to some extent. Pandemics occur occasionally, but they are not immune to recurrence. In the face of this situation, we still need higher quality RCTs to provide a solid basis for us to make countermeasures.
The low reporting rate of CONSORT and CONSORT-CHM formula items and unclear risk of bias indicate the methodological reporting quality in strictly randomized RCTs on CHM formulas for COVID-19 is inadequate. The sample size calculation, allocation concealment, and blinding especially need to improve. The checklist of sub-questions based on CONSORT-CHM formulas can be a Supplementary Scale to CONSORT-CHM formulas to help report and assess CHM formula intervention more precisely. The methodological reporting quality of RCTs published in English and enrolled participants during the first wave of the pandemic is worse than the studies that recruited subjects after the first wave of the pandemic. Despite the crisis caused by the pandemic, RCTs on CHM formulas should comply with established methodological and reporting standards.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
M-LC: Data curation, Formal Analysis, Investigation, Software, Writing–original draft. S-YQ: Conceptualization, Project administration, Writing–review and editing. J-LY: Formal Analysis, Writing–review and editing. J-YZ: Project administration, Writing–review and editing. L-XW: Project administration, Writing–review and editing. J-YW: Writing–review and editing. H-QY: Writing–review and editing. YW: Project administration, Writing–review and editing. G-QZ: Conceptualization, Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Zhejiang Provincial Hospital of Chinese Medicine Start-up Fund (grant number 202107). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1532290/full#supplementary-material
Ai, X. Y., Lin, L. P., Xie, M., and Tan, X. H. (2020a). Effect of integrated traditional Chinese and Western medicine on T lymphocyte subsets of patients with normal type of COVID-19. Guangdong Med. J. 41 (12), 1203–1206. doi:10.13820/j.cnki.gdyx.20201391
Ai, X. Y., Luo, C., Lin, L. P., Xie, M., Fan, H. M., and Tan, X. H. (2020b). The therapeutic effect of integrated traditional Chinese and Western medicine on COVID-19 in Guangzhou. China Trop. Med 20 (8), 746–750. doi:10.13604/j.cnki.46-1064/r.2020.08.12
An, X. D., Mao, L. N., Xia, P., Su, W., Wang, B. B., Kou, L. Y., et al. (2023). Effects of Shenma Yin on pulmonary and cardiac function in coronavirus disease 2019 convalescent patients with cardiopulmonary symptoms: a randomized, double-blind, multicenter control trial. J. Tradit. Chin. Med. 43 (1), 140–145. doi:10.19852/j.cnki.jtcm.20221006.001
An, X. D., Zhang, Q., Tao, J. X., Li, L., Chen, Y., Li, K., et al. (2022). Shugan Jieyu capsule (舒肝解郁胶囊) improve sleep and emotional disorder in coronavirus disease 2019 convalescence patients: a randomized, double-blind, placebo-controlled trial. J. Tradit. Chin. Med. 42 (5), 803–809. doi:10.19852/j.cnki.jtcm.20220719.003
Ang, L., Song, E., Zhang, J. H., Lee, H. W., and Lee, M. S. (2022). Herbal medicine for COVID-19: an overview of systematic reviews and meta-analysis. Phytomedicine 102, 154136. doi:10.1016/j.phymed.2022.154136
Bacchetti, P. (2002). Peer review of statistics in medical research: the other problem. BMJ 324 (7348), 1271–1273. doi:10.1136/bmj.324.7348.1271
Baumeister, A., Waddell, L., Corrin, T., Abid, H., Young, K. M., and Ayache, D. (2021). The quality of systematic reviews and other synthesis in the time of COVID-19. Epidemiol. Infect 149, 1–8. doi:10.1017/S0950268821001758
Chan, A. W., and Altman, D. G. (2005). Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 365 (9465), 1159–1162. doi:10.1016/S0140-6736(05)71879-1
Chen, B. W., Geng, P., Shen, J. J., Liangpunsakul, S., Lyu, H., Zhang, J., et al. (2022a). Traditional Chinese medicine JingYinGuBiao formula therapy improves the negative conversion rate of SARS-CoV2 in patients with mild COVID-19. Int. J. Biol. Sci. 18 (15), 5641–5652. doi:10.7150/ijbs.76699
Chen, C. W., Liang, X., and Liu, Y. F. (2021). Clinical study of Lianhua Qingwen Capsule in the treatment of coronavirus disease 2019. Res. Integr. Tradit. Chin. West. Med. 13 (1), 1–4. doi:10.3969/j.issn.1674-4616.2021.01.001
Chen, J. D., Tang, Q. Y., Zhang, B. Q., Yuan, S. H., Chen, J., Shen, S. Y., et al. (2023). Huashi baidu granule in the treatment of pediatric patients with mild coronavirus disease 2019: a single-center, open-label, parallel-group randomized controlled clinical trial. Front. Pharmacol. 14, 1092748. doi:10.3389/fphar.2023.1092748
Chen, L. Z., Liu, H., and Xiao, G. (2020). Curative effect of Xuebijing injection in the treatment of novel coronavirus pneumonia and its influence on CRP. J. China Prescr. Drug 18 (10), 110–111. doi:10.3969/j.issn.1671-945X.2020.10057
Chen, Y. Q., Liu, C. L., Wang, T. P., Qi, J. J., Jia, X. Q., Zeng, X. S., et al. (2022b). Efficacy and safety of Bufei Huoxue capsules in the management of convalescent patients with COVID-19 infection: a multicentre, double-blind, and randomised controlled trial. J. Ethnopharmacol. 284, 114830. doi:10.1016/j.jep.2021.114830
Cheng, C., Wu, T. X., Shang, H. C., Li, Y. P., Altman Douglas, G., Moher, D., et al. (2017). CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration (simplified Chinese version). Ann. Intern Med. 167 (2), W21–W34. doi:10.7326/IsTranslatedFrom_M17-2977_2
Commission, G. O. o.N. H., and Medicine, O. o.N. A. o.T. C. (2020a). Guidelines on diagnosis and treatment of novel coronavirus pneumonia(Trail seventh edition). China Med. 15 (6), 801–805. doi:10.3760/j.issn.1673-4777.2020.06.001
Commission, G. O. o.N. H., and Medicine, O. o.N. A. o.T. C. (2020b). Guidelines on diagnosis and treatment of novel coronavirus pneumonia(Trail sixth edition). Chin. J. Infect. Control 19 (2), 192–195. doi:10.12138/j.issn.1671-9638.20206154
Dai, X. S., and Li, J. (2023). Clinical efficacy of Xiaju Huayu capsule combined with Tartaric Acid Metoprolol tablet in the treatment of palpitations after recovery of novel coronavirus infection. Clin. J. Chin. Med. 15 (33), 46–50. doi:10.3969/j.issn.1674-7860.2023.33.009
DeAngelis, C. D., Drazen, J. M., Frizelle, F. A., Haug, C., Hoey, J., Horton, R., et al. (2005). Clinical trial registration: a statement from the international committee of medical journal editors. Arch. Otolaryngol. Head. Neck Surg. 131 (6), 479–480. doi:10.1001/archotol.131.6.479
Dong, L., Lu, H. N., Zhao, X. Q., He, S. R., and Yang, M. M. (2023). Clinical study of qinglong pihui decoction in the treatment of children with mild novel coronavirus omicron variant infection. JETCM 32 (8), 1364–1367. doi:10.3969/j.issn.1004-745X.2023.08.011
Duan, C., Xia, W. G., Zheng, C. J., Sun, G. B., Li, Z. L., Li, Q. L., et al. (2020). Clinical observation of mild novel coronavirus pneumonia in the treatment of Jinhua Qinggan granule combined with conventional Western medicine. J. Tradit. Chin. Med. 61 (17), 1473–1477. doi:10.13288/j.11-2166/r.2020.17.001
Fang, B. J., Zhao, J. Y., Jiang, J., Wang, Y. Y., Jin, F., and Du, J. J. (2023). Clinical observation of Suhexiang Pills in treatment of patients with tachycardia after SARS-CoV-2 infection. Chin. Tradit. Herb. Drugs 54 (8), 2516–2522. doi:10.7501/j.issn.0253-2670.2023.08.019
Fu, X. X., Lin, L. P., and Tan, X. H. (2020). Clinical observation on effect of toujie quwen granules in treatment of COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26 (12), 44–48. doi:10.13422/j.cnki.syfjx.20201314
Guo, J. R., Cui, S. K., Wang, C. L., Liu, C. Z., Zhang, T. Y., Li, H., et al. (2023). COVID-19 (omicron) treatment with jiuwei qingwen drink. Acta chin. Med 38 (02), 422–426. doi:10.16368/j.issn.1674-8999.2023.02.071
Han, R., Guo, R. X., and Hou, Z. F. (2024). Effect of modified Zhizi Chi decoction combined with Western medicine on insomnia after coronavirus disease 2019 infection. Clin. Res. Pract. 9 (8), 125–128. doi:10.19347/j.cnki.2096-1413.202408031
He, Q., and Zhang, Q. J. (2021). Clinical effect analysis of Shengmai Powder on Qi-Yin deficiency syndrome in convalescent period of novel coronavirus pneumonia. Acta Chin. Med. Pharmacol 49 (3), 84–86. doi:10.19664/j.cnki.1002-2392.210069
Higgins, J., James, T., Chandler, J., Cumpston, M., Li, T., and Page, M. J. (2022). Cochrane Handbook for systematic reviews of interventions. Cochrane Collaboration.
Hu, H. W., Wu, Z. S., Ji, C. Y., Chen, J., and Yang, K. (2022). Clinical effect of integrated traditional Chinese and Western medicine therapy in the treatment of the initial pyrexia stage of coronavirus disease 2019: an analysis of 43 cases. Hunan J. Traditi. Chin. Med. 38 (07), 1–4. doi:10.16808/j.cnki.issn1003-7705
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L. J., Zhang, B. L., et al. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine 85, 153242. doi:10.1016/j.phymed.2020.153242
Hu, M. H. (2022). Characteristics of TCM syndromes of recurrent COVID-19 patients in northern China(in the region) and observation on curative effect of fuyang kaibi soup (master). Heilongjiang: Heilongjiang Academy of Chinese Medicine. Available online at: https://link.cnki.net/doi/10.27126/d.cnki.ghlzy.2022.000011.
Ioannidis, J. P. A., Salholz-Hillel, M., Boyack, K. W., and Baas, J. (2021). The rapid, massive growth of COVID-19 authors in the scientific literature. R. Soc. Open Sci. 8 (9), 210389. doi:10.1098/rsos.210389
Jalloh, M. B., Bot, V. A., Borjaille, C. Z., Thabane, L., Li, G. W., Butler, J., et al. (2024). Reporting quality of heart failure randomized controlled trials 2000-2020: temporal trends in adherence to CONSORT criteria. Eur. J. Heart Fail. 26 (6), 1369–1380. doi:10.1002/ejhf.3229
Jeona, S.-R., Kang, J. W., Ang, L., Lee, H. W., Lee, M. S., and Kim, T.-H. (2022). Complementary and alternative medicine (CAM) interventions for COVID-19: an overview of systematic reviews. Integr. Med. Res. 11 (3), 100842. doi:10.1016/j.imr.2022.100842
Jung, R. G., Santo, P. D., Clifford, C., Prosperi-Porta, G., Skanes, S., Hung, A., et al. (2021). The methodological quality of COVID-19 clinical research. Nat. Commun. 12 (1), 943. doi:10.1038/s41467-021-21220-5
Li, C. J., Liu, X. Z., Ouyang, L. N., Wen, J. R., Zhang, J., Xiong, J., et al. (2020). Observation of the effects of traditional Chinese medicine combined with music therapy intervening on adverse drug reactions of patients with new coronavirus pneumonia. J. Nurs. Adm. 20 (4), 261–263. doi:10.3969/j.issn.1671-315x.2020.04.008
Li, L., An, X. D., Zhang, Q., Tao, J. X., He, J., Chen, Y., et al. (2021). Shumian capsule improves symptoms of sleep mood disorder in convalescent patients of Corona Virus Disease 2019. J. Tradit. Chin. Med. 41 (6), 974–981. doi:10.19852/j.cnki.jtcm.2021.06.015
Li, T., Zhao, M., Zhu, M. J., Zhang, S. X., He, J. R., Pan, H., et al. (2023). Xuanfei Baidu decoction, a Chinese herbal medicine for coronavirus disease 2019 (COVID-19): a randomized clinical trial. Acupunct. Herb. Med. 3 (03), 207–212. doi:10.1097/HM9.0000000000000056
Li, W. K., Yang, J., Chen, R. Y., and Wang, Z. W. (2024). Clinical efficacy study of Buffi Huoxue Capsules in treatment of SARS-CoV-2 infection recovery phase with qi deficiency and blood stasis syndrome. Chin. Tradit. Herb. Drug 55 (3), 872–881. doi:10.7501/j.issn.0253-2670.2024.03.018
Lin, H., Liu, T. J., Liu, N., and Wang, Q. G. (2021). Clinical observation of lianhua qingwen granule combined with western medicine in the treatment of new coronavirus pneumonia. World latest med. Inf 21 (94), 40–41. doi:10.3969/j.issn.1671-3141.2021.94.020
Liu, A. P., Wang, W., Liao, J. W., Wang, X. Q., Liu, X. J., Zeng, Y. Y., et al. (2021a). Observation of the curative effect of Modified Sangju decoction in the treatment of wind-heat invading lung syndrome of novel coronavirus pneumonia. Mod. J. Integr. Tradit. Chin. West. Med. 30 (22), 2395–2399. doi:10.3969/j.issn.1008-8849.2021.22.001
Liu, J., Yang, W., Liu, Y., Lu, C., Ruan, L. G., Zhao, C., et al. (2021b). Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine 91, 153671. doi:10.1016/j.phymed.2021.153671
Liu, J. J. (2023). Effect of tanreqing on cytokines in the treatment of COVID-19. Chin. Med. Mod. Distance educ. China 21 (13), 98–100. doi:10.3969/j.issn.1672-2779.2023.13.035
Liu, K. F., Fu, M., and Zhang, Z. P. (2020). “Clinical efficacy of xuebijing in patients with COVID-19. In: the national scientific research theory academic research conference,”. Beijing.
Liu, Q. Q., Li, G., Zhang, J., Wang, Y. J., Wang, Z., Zhang, X. G., et al. (2023). Clinical observation of Suhexiang Pills in the treatment of patients infected with SARS-CoV-2. Chin. Tradit. Herb. Drug 54 (4), 1201–1207. doi:10.7501/j.issn.0253-2670.2023.04.020
Liu, W., Su, X. R., Liao, X. L., Pan, H., Mei, D., and Zhang, Y. D. (2021c). Effect analysis of antiviral drugs combined with traditional Chinese medicine in the treatment of mild novel coronavirus pneumonia. Contemp. Med. Symp. 19 (2), 159–160. doi:10.3969/j.issn.2095-7629.2021.02.114
Luo, X., Zhang, Y., Li, H., Ren, M., Liu, Y., Liu, Y., et al. (2022). Clinical evidence on the use of Chinese herbal medicine for acute infectious diseases: an overview of systematic reviews. Front. Pharmacol. 13, 752978. doi:10.3389/fphar.2022.752978
Luo, Z. J., Chen, W., Xiang, M. Q., Wang, H., Xiao, W., Xu, C., et al. (2020). Effect of Xuebijing injection on inflammatory markers and disease outcome of coronavirus disease 2019. Chin. Crit. Care. Med. 32 (4), 426–429. doi:10.3760/cma.j.cn121430-20200406-00386
Luo, Z. J., Chen, W., Xiang, M. Q., Wang, H., Xiao, W., Xu, C., et al. (2021). The preventive effect of Xuebijing injection against cytokine storm for severe patients with COVID-19: a prospective randomized controlled trial. Eur. J. Integr. Med. 42, 101305. doi:10.1016/j.eujim.2021.101305
Mohammad, N. S., Nazli, R., Zafar, H., and Sadia, F. (2022). Effects of lipid-based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: a randomized clinical trial. Pak J. Med. Sci. 38 (1), 219–226. doi:10.12669/pjms.38.1.4396
Moher, D., Hopewell, S., Schulz, K. F., Montori, V., Gøtzsche, P. C., Devereaux, P. J., et al. (2012). CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 10 (1), 28–55. doi:10.1016/j.ijsu.2011.10.001
Ornelas, J., Routt, E., Kallis, P., and Lev-Tov, H. (2018). Use of the hCONSORT criteria as a reporting standard for herbal interventions for common dermatoses: a systematic review. Br. J. Dermatol 178 (4), 889–896. doi:10.1111/bjd.16256
Plint, A. C., Moher, D., Morrison, A., Schulz, K., Altman, D. G., Hill, C., et al. (2006). Does the CONSORT checklist improve the quality of reports of randomized controlled trials? A systematic review. Med. J. Aust. 185 (5), 263–267. doi:10.5694/j.1326-5377.2006.tb00557.x
He, Q., Zhang, Q. J., Gan, X. W., and Li, X. G. (2021). Clinical effect analysis of Buzhong Yiqi Decoction in treating mild novel coronavirus pneumonia. JETCM 30 (3), 385–387. doi:10.3969/j.issn.1004-745X.2021.03.003
Qiu, M., Li, Q. T., Li, D. P., Wang, C. H., Sun, Q. Z., Qian, C. F., et al. (2020). Efficacy observation of maxing xuanfei jiedu decoction on moderate COVID-19 patients. JETCM 29 (7), 1129–1130. doi:10.3969/j.issn.1004-745X.2020.07.001
Quinn, T. J., Burton, J. K., Carter, B., Cooper, N., Dwan, K., Field, R., et al. (2021). Following the science? Comparison of the methodological and reporting quality of COVID-19 and other research from the first wave of the pandemic. BMC Med. 19 (1), 46. doi:10.1186/s12916-021-01920-x
Rokhmah, D., Ali, K., Putri, S. M. D., and Khoiron, K. (2020). Increase in public interest concerning alternative medicine during the COVID-19 pandemic in Indonesia: a Google Trends study. F1000Res. 9, 1201. doi:10.12688/f1000research.25525.2
Savovic, J., Jones, H. E., Altman, D. G., Harris, R. J., Juni, P., Pildal, J., et al. (2012). Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann. Intern Med. 157 (6), 429–438. doi:10.7326/0003-4819-157-6-201209180-00537
Shah, M. R., Fatima, S., Khan, S. N., Ullah, S., Himani, G., Wan, K., et al. (2022). Jinhua qinggan granules for nonhospitalized COVID-19 patients: a double-blind, placebo-controlled, randomized controlled trial. Front. Med. (Lausanne) 2022 (9), 928468. doi:10.3389/fmed.2022.928468
Shi, S. F., Fang, Z. Y., Xiong, K., Ye, D. L., Wang, W. M., Wu, H., et al. (2021). Clinical study on 30 cases of Qi-Yin deficiency syndrome in the convalescent period of novel coronavirus pneumonia treated by TCM comprehensive therapy. Jiangsu J. Tradit. Chin. Med. 53 (1), 25–28. doi:10.19844/j.cnki.1672-397X.2020.00.015
Sun, H. M., Xu, F., Zhang, L., Wei, C., Chen, J. Y., Wang, Q. X., et al. (2021a). Study on clinical efficacy of lianhua qingke granule in treatment of mild and ordinary COVID-19. Chin. J. Exp. Tradit. Med. Formulae 26 (14), 29–34. doi:10.26914/c.cnkihy.2021.071943
Sun, S. Q., Chen, F. F., Yin, C. W., Wang, J., Cai, W. R., Guo, J., et al. (2021b). Clinical efficacy of Liushenwan combined with conventional treatment in patients with COVID-19. Chin. Tradit. Pat. Med. 43 (8), 2277–2280. doi:10.3969/j.issn.1001-1528.2021.08.058
Wang, J., Cheng, C. W., Jiao, Y. L., Shi, D. N., Wang, Y. C., Li, H., et al. (2024). Evaluation of compliance of CONSORT-CHM formula 2017 in randomized controlled trials of Chinese herbal medicine formulas: protocol of a five-year review. Front. Pharmacol. 15, 1287262. doi:10.3389/fphar.2024.1287262
Wang, J. B., Yang, G., Niu, M., Bai, Z. F., Li, P. Y., Wang, Z. X., et al. (2020). Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin. J. Integr. Med. 26 (9), 648–655. doi:10.1007/s11655-020-3426-7
Wang, J. M., Bu, X. H., Yang, S., Wang, P., and Wang, X. S. (2021). Analysis of traditional Chinese medicine treatment of 60 patients with novel coronavirus pneumonia in southwestern shandong. Smart Health 7 (31), 177–179. doi:10.19335/j.cnki.2096-1219.2021.31.057
Wang, L., Xu, M., Wang, Y., Li, H. B., Liu, N., and Zuo, J. L. (2020). Clinical study on shengmai powder combined with shenling baizhu powder in the treatment of common corona virus disease 2019. CJTCMP 35 (8), 4268–4271.
WHO (2024). Coronavirus disease (COVID-19) epidemiological updates and monthly operational updates. Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (Accessed July 21, 2024).
Wu, R., Liang, C., Jiao, C. X., Ban, W. M., and Zhao, W. X. (2021). Observation of the clinical efficacy of sanao yulong mixture in the treatment of new coronary pneumonia. Clin. J. Tradit. Chin. Med. 33 (07), 1368–1371. doi:10.16448/j.cjtcm.2021.0737
Wu, X. M., Yang, Y. Y., and Song, W. C. (2023). Clinical effect of Qingfei Liyan mixture in the treatment of acute cough after severe acute respiratory syndrome COVID-19 infection with lung dryness and Yin injury: an analysis of 43 cases. Hunan J. Tradit. Chin. Med. 39 (11), 1–3. doi:10.16808/j.cnki.issn1003-7705.2023.11.001
Wu, X. M., Yang, Y. Y., and Song, W. C. (2024). Clinical effect of Guipi mixture in the treatment of insomnia with heart-spleen deficiency syndrome after severe acute respiratory syndrome coronavirus 2 infection: an analysis of 30 cases. Hunan J. Tradit. Chin. Med. 40 (1), 10–13. doi:10.16808/j.cnki.issn1003-7705.2024.01.003
Xia, W. G., Zheng, C. J., Zhang, J. X., Huang, M., Li, Q. L., Duan, C., et al. (2022). A randomized controlled study of a diagnosis and treatment plan for moderate coronavirus disease 2019 that integrates Traditional Chinese and Western Medicine. JTCM 42 (2), 234–241. doi:10.19852/j.cnki.jtcm.20210220.001
Xu, G. B., Xu, W. F., Gao, Y. J., Zhang, R., and Zhang, Y. R. (2023a). Clinical study on ganghuo kanggan decoction for the treatment of novel coronavirus infection. J. Guangzhou Univ. Tradit. Chin. Med. 40 (11), 2744–2749. doi:10.13359/j.cnki.gzxbtcm.2023.11.009
Xu, Z. J., Yi, J. M., Situ, C. Y., and Liang, R. H. (2023b). Clinical observation of Renyin Maxingshigan Decoction in treatment of novel coronavirus pneumonia. J Med Theor and Prac 36 (16), 2745–2747. doi:10.19381/j.issn
Yan, C. G., Shan, H. X., Pei, X. D., Lu, R., Wu, Y., and Wen, Q. (2022). Efficacy and safety analysis of shufeng jiedu capsules combined with interferon alpha plus arbidol in the treatment of common COVID-19. J. Guangzhou Univ. Tradit. Chin. Med. 39 (03), 475–480. doi:10.13359/j.cnki.gzxbtcm.2022.03.003
Yang, C. Q., Lian, F. M., Yang, G. P., Huang, Y. F., Zhang, S. B., Wang, J. H., et al. (2023). Effectiveness of Xiaoyao capsule on sleep disorders and mood disturbance in patients in recovery from coronavirus disease 2019: a randomized controlled trial. JTCM. doi:10.19852/j.cnki.jtcm.2023.02.005
Yang, H. X. (2023). Clinical observation on the treatment of novel coronavirus infection (medium) by Sanao Decoction and Didang. (Master). Chengdu University of Traditional Chinese Medicine. Available online at: https://d-wanfangdata-com-cn-443.tjus.99885.net/thesis/ChJUaGVzaXNOZXdTMjAyNDAxMDkSCFk0MTMzMjYwGghmNGxsem93aA%3D%3D.
Yang, H. Z., Lin, R. C., Dong, X., Guo, G. H., Zhang, B. E., Li, Y., et al. (2021). Clinical study on 60 cases of uncleared residual toxicity of novel coronavirus pneumonia treated with hopmedium spray combined with basic rehabilitation therapy. J. Tradit. Chin. Med. 62 (17), 1509–1513. doi:10.13288/j.11-2166/r.2021.17.009
Yao, C. D. (2023). Clinical observation of Jiemai Gandu Zhisou recipe in treating cough after novel coronavirus infection (qi deficiency and lingering pathogen syndrome). (Master). Chengdu: Chengdu University of Traditional Chinese Medicine. Available online at: https://d-wanfangdata-com-cn-443.tjus.99885.net/thesis/ChJUaGVzaXNOZXdTMjAyNDAxMDkSCFk0MTMyOTMxGghmNGxsem93aA%3D%3D.
Ye, L., Zhao, H. J., Xu, S. G., and Chen, W. Z. (2021). Clinical study of modified shengjiang powder in the treatment of ordinary COVID-19. Chin. Foreign Med. Res. 19 (28), 9–13. doi:10.14033/j.cnki.cfmr.2021.28.003
Yu, P., Li, Y. Z., Wan, S. B., and Wang, Y. (2020). Effects of lianhua qingwen granules plus arbidol on treatment of mild corona virus disease-19. Chin. Pharm. J. 55 (12), 1042–1045. doi:10.11669/cpj.2020.12.014
Yu, Z., Zheng, Y. X., Chen, B. W., Lv, J., Zhu, X. J., Shang, B., et al. (2023). Efficacy and safety of Huashi Baidu granule plus Nirmatrelvir-Ritonavir combination therapy in patients with high-risk factors infected with Omicron (B.1.1.529): a multi-arm single-center, open-label, randomized controlled trial. Phytomedicine 120, 155025. doi:10.1016/j.phymed.2023.155025
Yuan, X. Y. (2021). Clinical efficacy study of “removing dampness and clearing the lung” on 60 patients with COVID-19 syndrome of damp-heat inclusion of lung. (Master). Chengdu: Chengdu University of Traditional Chinese Medicine. Available online at: https://d-wanfangdata-com-cn-443.tjus.99885.net/thesis/ChJUaGVzaXNOZXdTMjAyNDAxMDkSCUQwMjYzMDEzMBoIM2gyeHdyZ2M%3D.
Zeng, C. C., Yuan, Z. Z., Zhu, J. H., Wang, Y., Xie, Y. Y., Ye, R., et al. (2021). Therapeutic effects of traditional Chinese medicine (Maxingshigan-Weijing Decoction) on COVID-19: an open-label randomized controlled trial. Integr. Med. Res. 10, 100782. doi:10.1016/j.imr.2021.100782
Zhang, G. P., Huang, X., Cheng, X., Guo, Z., Wang, Y. L., and Wang, Z. (2023a). Clinical efficacy of Reduning injection in the treatment of novel coronavirus pneumonia. J. Clin. Emerg. 24 (10), 506–511. doi:10.13201/ji.ssn.1009-5918.2023.10.002
Zhang, L., Wu, L., Xu, X. L., Yuan, Y. D., Jiang, R. M., Yan, X. X., et al. (2022). Efficacy and safety of lianhua qingke tablets in the treatment of mild and common-type COVID-19: a randomized, controlled, multicenter clinical study. Evid. Based Complement. Altern. Med. 2022, 8733598. doi:10.1155/2022/8733598
Zhang, X. Y., Lv, L., Zhou, Y. L., Xie, L. D., Xu, Q., Zou, X. F., et al. (2021). Efficacy and safety of Xiyanping injection in the treatment of COVID-19: a multicenter, prospective, open-label and randomized controlled trial. Phytother. Res. 35 (8), 4401–4410. doi:10.1002/ptr.7141
Zhang, Y., Lei, G. P., Wang, T., Feng, Z., and Dong, S. (2023b). Clinical efficacy of jiangxia mobile cabin hospital decoction series for the treatment of mild COVID2019. JETCM 32 (6), 983–987. doi:10.3969/j.issn.1004-745X.2023.06.010
Zhao, C., Li, L., Yang, W., Lv, W. L., Wang, J., Guo, J., et al. (2021). Chinese medicine formula huashibaidu granule early treatment for mild COVID-19 patients: an unblinded, cluster-randomized clinical trial. Front. Med. (Lausanne) 8, 696976. doi:10.3389/fmed.2021.696976
Zheng, J. P., Ling, Y., Jiang, L. S., Mootsikapun, P., Lu, H. Z., Chayakulkeeree, M., et al. (2023). Effects of Lianhuaqingwen capsules in adults with mild-to-moderate coronavirus disease 2019: an international, multicenter, double-blind, randomized controlled trial. Virol. J. 20 (1), 277. doi:10.1186/s12985-023-02144-6
Zhou, H., Zhu, H. M., and Jia, Y. L. (2023a). Critical quality appraisal of randomized controlled trials with traditional Chinese medicines for the coronavirus disease 2019. Phytomedicine 120, 155038. doi:10.1016/j.phymed.2023.155038
Zhou, S., Feng, J., Xie, Q., Huang, T. R., Xu, X. M., Zhou, D. X., et al. (2021). Traditional Chinese medicine Shenhuang granule in patients with severe/critical COVID-19: a randomized controlled multicenter trial. Phytomedicine 89, 153612. doi:10.1016/j.phymed.2021.153612
Keywords: Chinese herbal medicine, COVID-19, RCT, CONSORT-CHM formulas, subquestions, risk of bias
Citation: Chen M-L, Qian S-Y, Yang J-L, Zheng J-Y, Wang L-X, Wu J-Y, Ye H-Q, Wang Y and Zheng G-Q (2025) The methodological reporting quality in strictly randomized controlled trials for COVID-19 and precise reporting of Chinese herbal medicine formula intervention. Front. Pharmacol. 16:1532290. doi: 10.3389/fphar.2025.1532290
Received: 21 November 2024; Accepted: 07 March 2025;
Published: 31 March 2025.
Edited by:
Chenning Zhang, Hubei University of Medicine, ChinaReviewed by:
Ang Cai, University of Rhode Island, United StatesCopyright © 2025 Chen, Qian, Yang, Zheng, Wang, Wu, Ye, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Qing Zheng, Z3FfemhlbmdAc29odS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.