- 1Research Ward, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 2Merck Serono (Beijing) Pharmaceutical R&D Co., Ltd., Beijing, China
Background: The bioequivalence of Concor® (Merck Healthcare KGaA, Darmstadt, Germany), a bisoprolol-containing tablet, manufactured in China and Concor® tablets manufactured in Germany has not been previously reported.
Methods: This single-center, open-label, randomized, two-period, two-sequence, crossover trial (28 February 2023–19 May 2023) compared the pharmacokinetics and safety of bisoprolol 5-mg tablets manufactured in China (test product) with those of bisoprolol 5-mg tablets manufactured in Germany (reference product) in healthy Chinese adults under fasted and fed conditions. Primary endpoints were Cmax, AUC0–tlast, and AUC0–
Result: The mean (coefficient of variation percentage) Cmax in the fasted group was 21.2 (15.0) ng/mL (test product) and 22.1 (17.0) ng/mL (reference product). Under fed conditions, the respective Cmax values were 22.7 (18.8) ng/mL and 22.8 (15.2) ng/mL. The mean and coefficient of variation percentage for AUC were also similar between the two products. The geometric least squares mean ratio (90% confidence interval) for the test/reference product was 0.9565 (0.9006–1.0158) ng/mL for Cmax, 0.9761 (0.9370–1.0168) h·ng/mL for AUC0–tlast, and 0.9807 (0.9429–1.0200) h·ng/mL for AUC0–
Conclusion: This study compared the bioequivalence of bisoprolol 5-mg tablets manufactured in China to that of the tablets manufactured in Germany among healthy Chinese adults.
Systematic Review Registration: identifier CTR20230391
1 Introduction
Hypertension is a leading treatable cause of premature death (Zhou et al., 2021; Nguyen and Chow, 2021). In 2019, an estimated 1.3 billion adults were affected by hypertension, with the disease contributing to over 10 million deaths each year (World Health Organization, 2023). Hypertension is an important risk factor for cardiovascular disease (Fuchs and Whelton, 2020), including heart failure and coronary artery disease. The global prevalence of heart failure is increasing, which is thought to be the result of an aging population (Savarese et al., 2023), and coronary artery disease is the leading cause of death worldwide (Ralapanawa and Sivakanesan, 2021). Thus, the overall goal when treating patients with hypertension is to reduce the risk of cardiovascular disease.
The 2018 European Society of Cardiology/European Society of Hypertension guidelines consider β-blockers useful for treating hypertension, including in patients with symptomatic angina, post-myocardial infarction, and heart failure with reduced ejection fraction (Williams et al., 2018). Concor® (Merck Healthcare KGaA, Darmstadt, Germany) tablets contain bisoprolol, a highly potent, synthetic, β1-selective adrenoceptor blocking agent. Bisoprolol has an absolute bioavailability of approximately 90% after oral administration. Bisoprolol is removed from the organism via two equally effective clearance routes: 50% is transformed into inactive metabolites in the liver with the excretion of the metabolites via the kidneys. The remaining 50% is excreted as unchanged substance via the kidneys. Bisoprolol reaches the maximal effect 3–4 h after oral administration. The plasma elimination half-life of 10–12 h provides 24-h efficacy following a once daily dosing (Merck, 2014). Bisoprolol exerts its therapeutic effects by blocking β receptors in the heart, resulting in a decrease in the myocardial contractility and a reduction in the blood pressure (Eguchi, 2016; Tjandrawinata et al., 2012).
Concor® tablets are currently manufactured in Germany, and a newer manufacturing location has recently opened in China. In China, regulatory filing requires evidence for the bioequivalence of drugs manufactured domestically to reference drugs manufactured abroad (in Germany). To meet these requirements, we aimed to evaluate the bioequivalence of Concor® 5-mg tablets manufactured in China and of Concor® 5-mg tablets manufactured in Germany in healthy Chinese adults under both fasted and fed conditions. The secondary objectives were to assess the pharmacokinetic (PK) profiles, safety, and tolerability after a single dose of each of the tablets.
2 Patients and methods
2.1 Study design and treatments
This phase 1, single-center, open-label, randomized, two-period, two-sequence, crossover trial conducted in China between 28th February 2023 and 19th May 2023 compared the PK and safety of bisoprolol 5-mg tablets manufactured in China with those of bisoprolol 5-mg tablets manufactured in Germany. The study duration was approximately 4 weeks and consisted of a 2-week screening period, a 3-day dosing/sampling period, a washout period of at least 7 days, and a 3-day second dosing/sampling period, and the study ended on day 10 of the second dosing period.
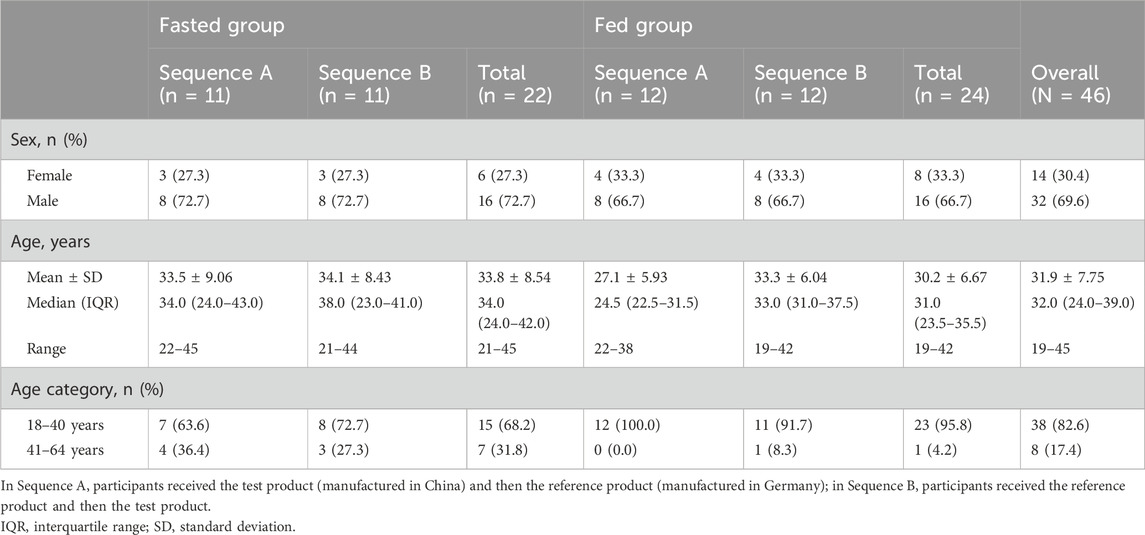
Healthy participants were allocated into either the fasted or the fed group, with each sex representing at least one-quarter of the participants in each group, and assigned a computer-generated randomization number. Allocated participants were then randomly assigned in a 1:1 ratio to Sequence A or Sequence B. Participants in Sequence A received the test product {one bisoprolol 5-mg tablet manufactured in China [Merck Pharmaceutical Manufacturing (Jiangsu) Co., Ltd., Nantong, China]} first and the reference product [one bisoprolol 5-mg tablet manufactured in Germany (Merck Healthcare KGaA, Darmstadt, Germany)] next, and those in Sequence B received the reference product first and the test product next (Figure 1).
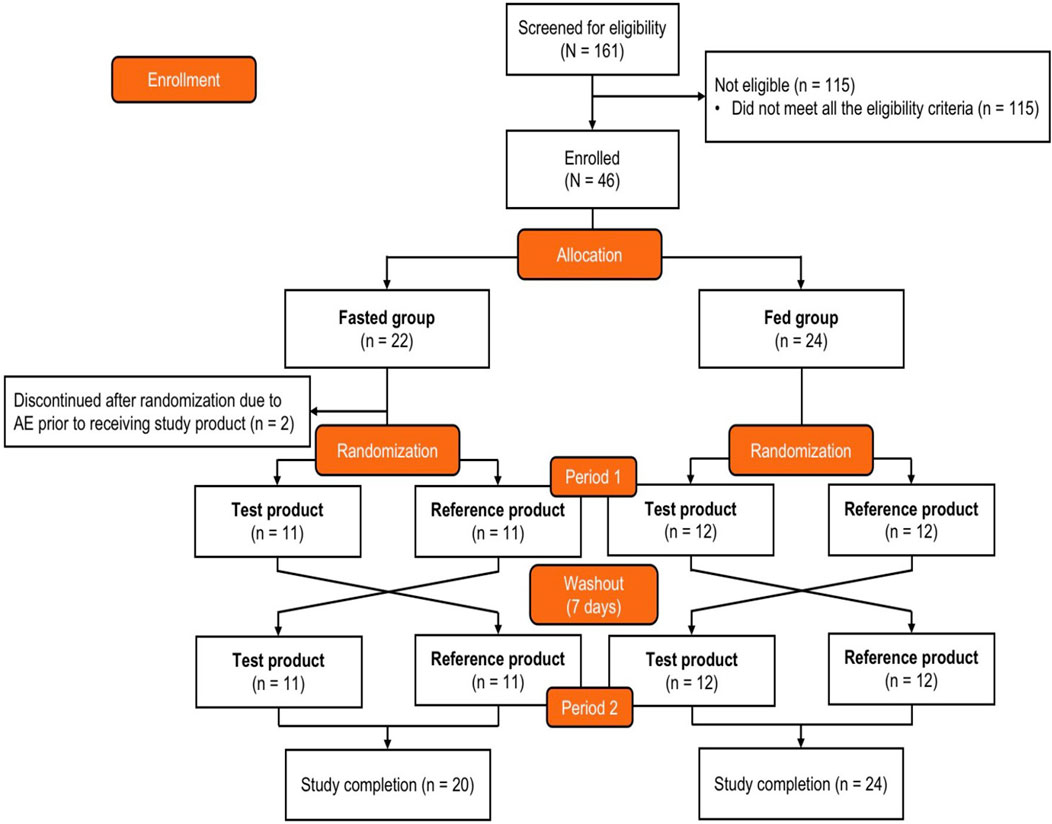
Figure 1. Study design and participant disposition. After enrollment and allocation to the fasted group or fed group, participants were randomized (1:1) to receive either the test product first and then the reference product second or the reverse order. The test product was bisoprolol 5-mg tablets manufactured in China, and the reference product was bisoprolol 5-mg tablets manufactured in Germany. AE, adverse event.
The fasted group refrained from all food and drink, except water, from the evening (after dinner) of the day before the intervention for a total fasting time of at least 10 h. Water was allowed until 1 h before receiving the intervention, and then it was allowed again from 2 h after the intervention. The fed group consumed a standard high-fat, high-calorie breakfast, as per the United States Food and Drug Administration guidelines (U.S. Food and Drug Administration, 2002), within 30 min prior to receiving the intervention.
The study was conducted in compliance with the ethical principles outlined in the Declaration of Helsinki, the International Council on Harmonization Guidelines for Good Clinical Practice Council, and all applicable local laws and regulations. The study protocol and other relevant documents were approved by the Beijing Friendship Hospital Institutional Review Board and Ethics Committee (approval number: 2022-P1-drug-071-01). Participants provided written informed consent, and the clinical trial was registered in the Chinese Clinical Trials Register under the identifier CTR20230391.
2.2 Participants
Eligible participants were aged ≥18 years, considered healthy by medical evaluation (including medical history), had a body weight between 50 and 90 kg, and had a body mass index (BMI) between 19 and 26 kg/m2 (inclusive). The key exclusion criteria were positive screening for hepatitis B-soluble antigens or hepatitis C antibodies, human immunodeficiency virus, or Treponema pallidum antibodies; abnormal chest x-ray or computed tomography finding at screening; blood donation ≥400 mL or significant blood loss within 90 days prior to the administration of the first intervention; non-acceptance of the study’s high-fat breakfast; receipt of prescription or non-prescription medication within 28 days before the administration of the first intervention (including multivitamins and herbal products); participation in another clinical trial within 90 days prior to the first intervention administration; and any condition that was considered an inappropriate risk or contraindication for participation in the study.
2.3 Study assessments
The primary endpoints were the maximum observed concentration (Cmax), the area under the plasma concentration–time curve (AUC) from time 0 to the time of the last measurable concentration (AUC0–tlast), and the AUC from time 0 to infinity (AUC0–
The secondary endpoints included the time of the maximum observed concentration (tmax), elimination half-life (t1/2), AUC from the time of the last quantifiable concentration extrapolated to infinity as a percentage (%AUCextra), apparent terminal elimination rate constant (λz), apparent plasma clearance of the drug following extravascular administration (CL/F), and apparent volume of distribution during the terminal phase following extravascular administration (Vz/F).
Baseline characteristics included weight, height, BMI, body surface area, alcohol consumption, nicotine use, drug screening, ethanol test, and viral serology. The dissolution profiles of the two products were determined using high-performance liquid chromatography, and the peak areas in the chromatogram were identified using the external standard method. The dissolution medium was sodium chloride buffer prepared in accordance with the European Pharmacopeia recommendations on dissolution testing (Ph. Eur. 5.17.1.), adjusted to pH 1.2, pH 4.5, and pH 6.8. Samples were evaluated using ultraviolet spectroscopic analysis at 5, 10, 15, 30, and 45 min.
2.4 Analytical method and method validation
Plasma concentrations of bisoprolol were analyzed using a fully validated liquid chromatography–tandem mass spectrometry (LC–MS/MS) method at Labcorp (Shanghai, China). Briefly, bisoprolol and its deuterium-labeled internal standard (bisoprolol-d5) were isolated from plasma (50 μL) using a 96-well plate following a protein precipitation extraction procedure with acetonitrile. The chromatographic separation was achieved using an ACQIUITY UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm particle size; Waters Corporation) at a flow rate of 0.5 mL/min with a 3-min gradient profile, where the HPLC run began with the mobile phase B at 40% for the first 0.1 min, then increased to 95% at 1.5 min, held at 95% B until 2 min, and then returned to the starting composition at 2.1 min for column re-equilibration. Mobile phase A was 20 mM ammonium acetate, and mobile phase B was methanol, both containing 0.1% formic acid. The analytes were detected using a triple–quadruple mass detector (Sciex API 5500) in the positive ion mode, with turbo ion spray in multiple-reaction monitoring (m/z 326.2 → 116.2 for bisoprolol and m/z 331.3 → 121.1 for bisoprolol-d5). The method showed good linearity in the range of 0.500–75 ng/mL, with a lower limit of quantification set at 0.5 ng/mL. The intra- and inter-assay precision values (≤4.4% and ≤4.8%, respectively) met the acceptance criteria as per the regulatory guidelines. A battery of stability studies (i.e., bench-top, freeze–thaw, and long-term stability) were performed, and bisoprolol was stable in plasma for 24 h at room temperature and for 135 days at −30∼−10°C after five freeze/thaw cycles. No matrix effect was observed within the linearity range. The assay method was found to be highly reproducible and robust.
2.5 Safety assessments
The type, frequency, and severity of adverse events (AEs), including treatment-emergent AEs (TEAEs), and the relationship of AEs to the study intervention were recorded. AEs were coded using the Medical Dictionary for Regulatory Activities version 25.1, and TEAEs were assigned to a system organ class and preferred term. The additional safety assessments included vital signs (systolic and diastolic blood pressure, pulse rate, body temperature, and respiration rate), clinical laboratory tests (biochemistry, hematology, and urinalysis), 12-lead electrocardiogram results, and physical examinations.
2.6 Statistical methods
The sample size was determined from the bisoprolol intra-individual variability observed in a previous Merck bioequivalence study (EMR200006-001) that compared a fixed-dose-combination 5 mg/5 mg bisoprolol–amlodipine tablet with a 5-mg bisoprolol tablet co-administered with a 5-mg amlodipine tablet under fasted and fed conditions (Hu et al., 2018). In that study, the intra-individual coefficients of bisoprolol for AUC0–tlast, AUC0–
The full analysis set (FAS) included all randomized participants. The safety analysis set (SAF) included all participants who had received at least one dose of the study product. The PK parameter analysis set (PKPS) included all participants who had received at least one dose of the study product and had no clinically important protocol deviations or events that would affect the PK analysis. The bioequivalence analysis set (BES) included all participants who had at least one evaluable PK parameter in at least one treatment period and who did not have any relevant protocol deviations, had no factors likely to affect the comparability of PK results, and had adequate study intervention compliance.
Continuous variables were summarized using descriptive statistics [including the number, arithmetic mean, median, standard deviation (SD), and minimum and maximum values], and quantitative variables were summarized by numbers and percentages. Geometric least-squares (LS) mean, coefficient of variation (CV), intra-participant CV percentage, and confidence intervals (CIs) were also calculated. No statistical comparisons were performed on the participant demographics or baseline characteristics.
Analysis of the primary endpoints was conducted in the BES. The data were log-transformed, and a mixed-effects model was applied, including fixed effects for sequence, treatment, and period. Differences between treatments on the log scale were estimated for the parameters, together with their 90% CIs. The geometric LS means and their 95% CIs were also estimated. The bioequivalence of the two tablets was assessed separately for the fasted and fed groups, and bioequivalence was only established if the 90% CIs for the geometric mean ratios for both primary endpoints between the two interventions were within 0.80–1.25 in both the fasted and fed groups. For tmax, Hodges–Lehmann estimates and the corresponding 90% CIs were calculated according to the Tukey method. The 90% CIs of the geometric mean of the test product and reference product were calculated for all other secondary endpoints, but no formal statistical comparisons were performed. The dissolution profiles were characterized by plotting the dissolution rate over time, and the profiles of the two tablets were compared using a model-independent approach (U.S. Food and Drug Administration, 1997).
TEAEs with missing data regarding the association with the study intervention were considered related to the study intervention. No other imputation for missing values was conducted, and outliers were defined as values outside 1.5 × the interquartile range above the upper quartile or below the lower quartile.
PK parameters were derived using Phoenix® WinNonlin® version 8.3 (Certara, L.P., Princeton, NJ, United States), and all other statistical analyses were conducted using SAS® version 9.4 or higher (SAS Institute, Cary, NC, United States).
3 Results
3.1 Participants
The participant disposition is shown in Figure 1. A total of 161 adults were screened, and 46 eligible participants were allocated to the fasted group (n = 22) or the fed group (n = 24) and randomized to Sequence A or Sequence B. Two participants in the fasted group experienced AEs prior to receiving the intervention and discontinued the study; these participants were not included in the SAF, PKPS, or BES. The baseline demographics are shown in Table 1. All participants were Chinese, and 69.6% were men. The median age was 32.0 years, the mean (SD) body surface area was 1.73 (0.130) m2, the mean (SD) weight was 63.92 (7.219) kg, and the mean (SD) BMI was 22.39 (1.811) kg/m2. None of the participants had a history of alcohol consumption or nicotine use.
3.2 PK analysis results
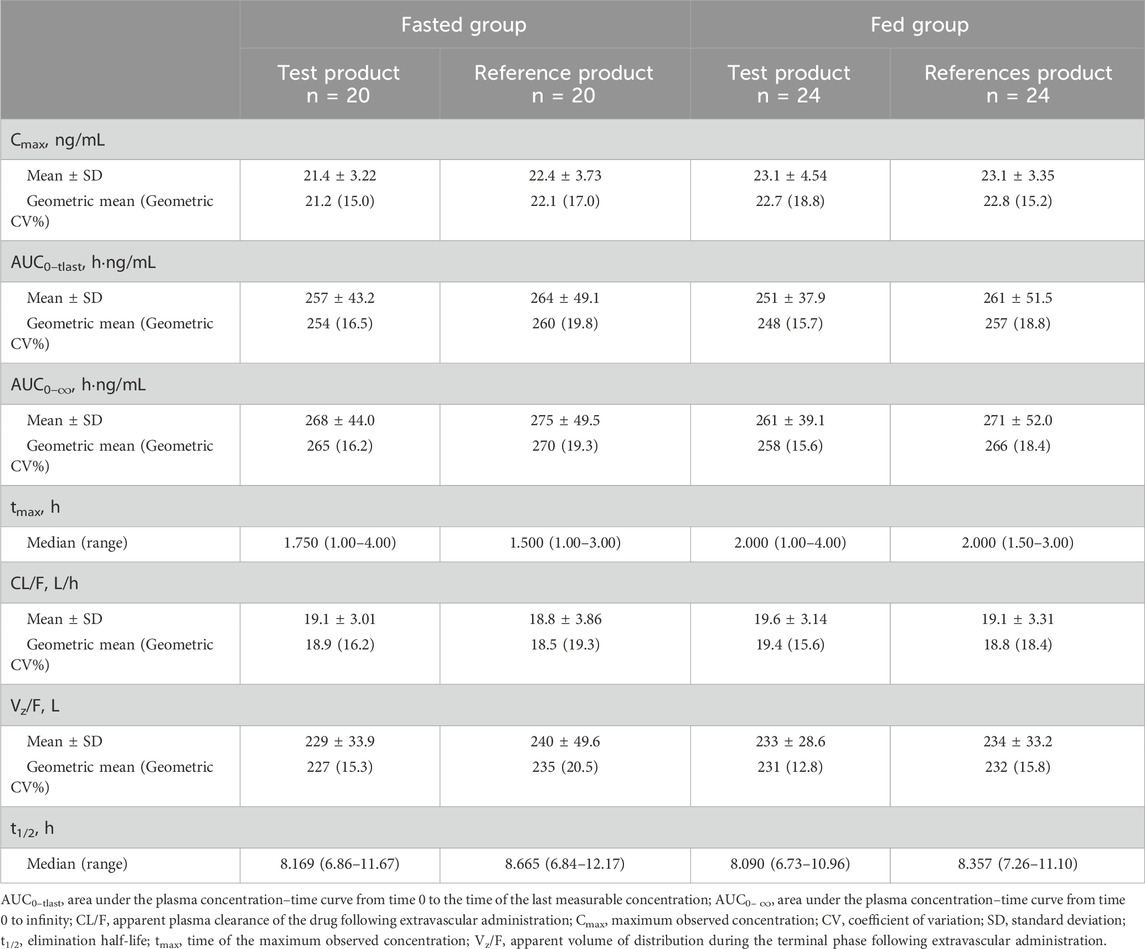
The PK parameters are summarized in Table 2, and the plasma concentration–time profiles are shown in Figure 2. Following a single dose of the test product and the reference product, the absorption and elimination of bisoprolol were very similar in both the fasted and fed groups. Bisoprolol reached a peak concentration at a median of 1.75 h (test product) and 1.50 h (reference product) in the fasted group and 2.00 h (both test and reference products) in the fed group. In both the fasted and fed groups, both products were eliminated rapidly and plasma levels were below the lower limit of detection after 48 h.
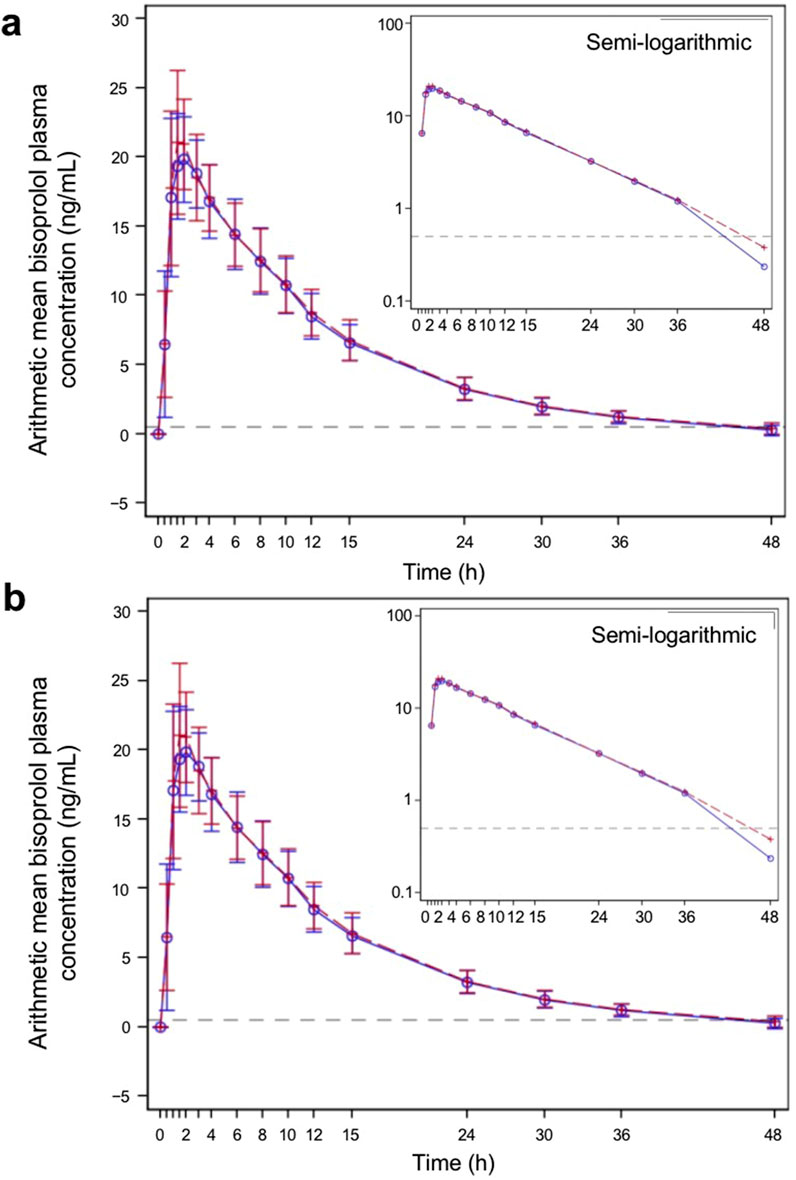
Figure 2. Bisoprolol plasma concentration–time profiles for the test and reference products—pharmacokinetic parameters set (A) fasted group and (B) fed group. The blue solid line represents participants who received the test product, and the red dashed line represents participants who received the reference product. The test product was bisoprolol 5-mg tablets manufactured in China, and the reference product was bisoprolol 5-mg tablets manufactured in Germany. The gray dashed horizontal line shows the lower level of quantification (0.5 ng/mL). The graphs show linear plots, and the insets are plotted on a semi-logarithmic scale. Individual data points represent the arithmetic mean at each time point, and the error bars show standard deviation.
In the fasted group, the geometric mean (geometric CV percentage) Cmax was 21.2 (15.0) ng/mL for the test product and 22.1 (17.0) ng/mL for the reference product. In the fed group, the geometric mean (geometric CV percentage) Cmax was 22.7 (18.8) ng/mL for the test product and 22.8 (15.2) ng/mL for the reference product. Both AUC0–tlast and AUC0–
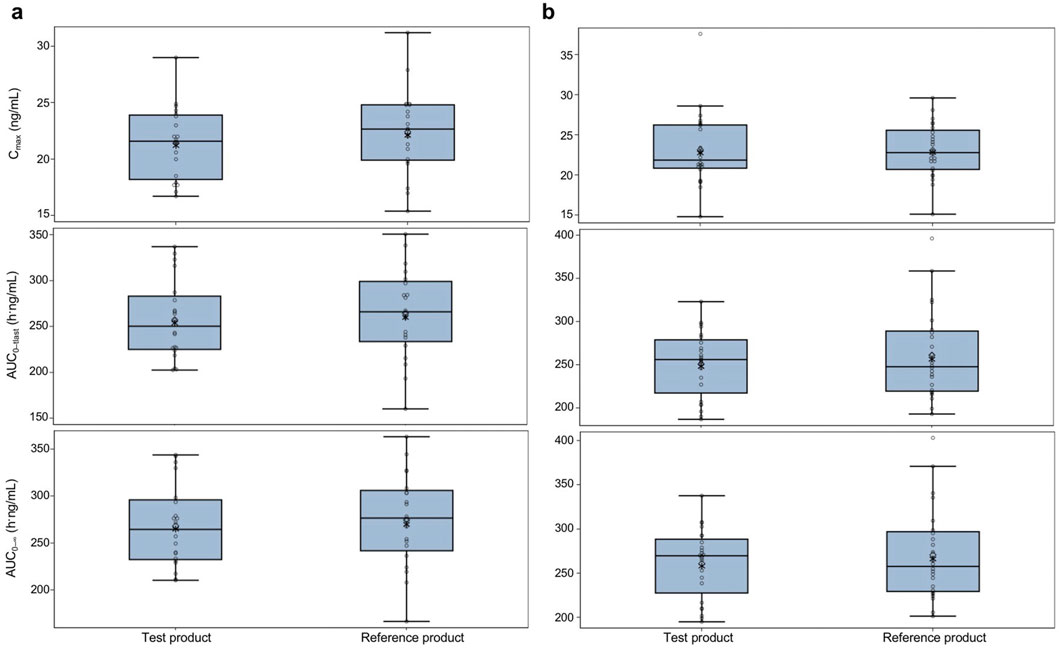
Figure 3. Pharmacokinetic parameters for the test and reference products in fasted and fed healthy participants—pharmacokinetic parameters set (A) fasted group and (B) fed group. The test product was bisoprolol 5-mg tablets manufactured in China, and the reference product was bisoprolol 5-mg tablets manufactured in Germany. The solid horizontal line represents the median value, the diamonds are the arithmetic mean, and the asterisks in the box are the geometric mean values. The upper and lower edges of the box are the 1st and 3rd quartiles, respectively, and the whiskers are the range, excluding outliers. Outliers were considered values that were outside 1.5 × the interquartile range. AUC0–tlast, area under the plasma concentration–time curve from time 0 to the time of the last measurable concentration; AUC0–
3.3 Bioequivalence
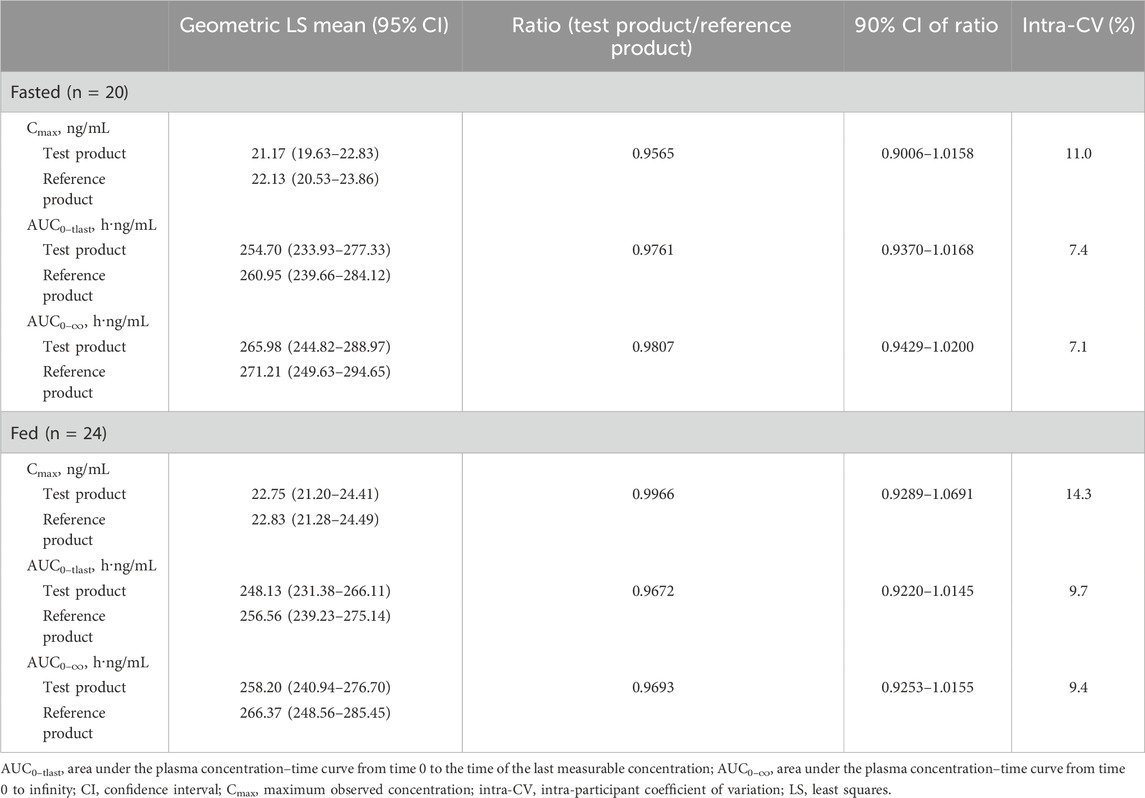
The bioequivalence analysis results are shown in Table 3. Among the fasted group, the geometric LS mean ratio (90% CI) for the test product/reference product was 0.9565 (0.9006–1.0158) ng/mL for Cmax, 0.9761 (0.9370–1.0168) h·ng/mL for AUC0–tlast, and 0.9807 (0.9429–1.0200) h·ng/mL for AUC0–
3.4 Dissolution results
The dissolution assay results of the test and reference products are shown in Figure 4. At all pH conditions (pH 1.2, pH 4.5, and pH 6.8), the mean dissolution rates for both the test and reference products were higher than 85% at 15 min.
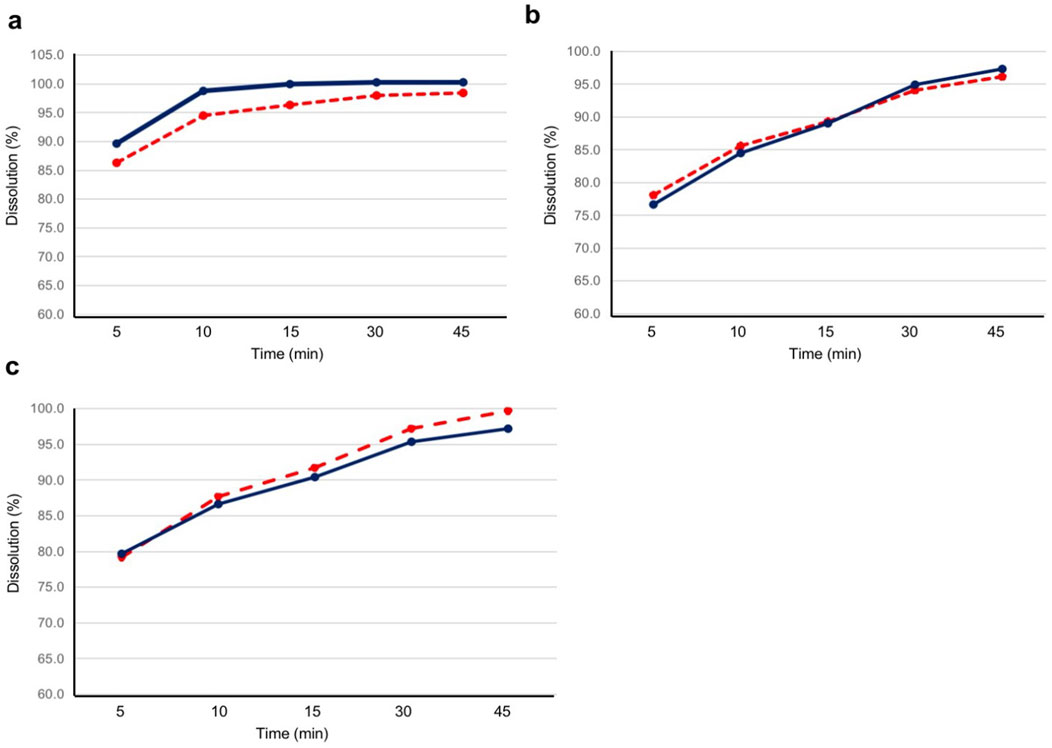
Figure 4. Dissolution profile for the test and reference products at pH 1.2, 4.5, and 6.8. Twelve tablets for each product were evaluated for dissolution at (A) pH 1.2, (B) pH 4.5, and (C) pH 6.8. The blue solid line represents the test product, and the red dashed line represents the reference product. The test product was bisoprolol 5-mg tablets manufactured in China, and the reference product was bisoprolol 5-mg tablets manufactured in Germany.
3.5 Safety
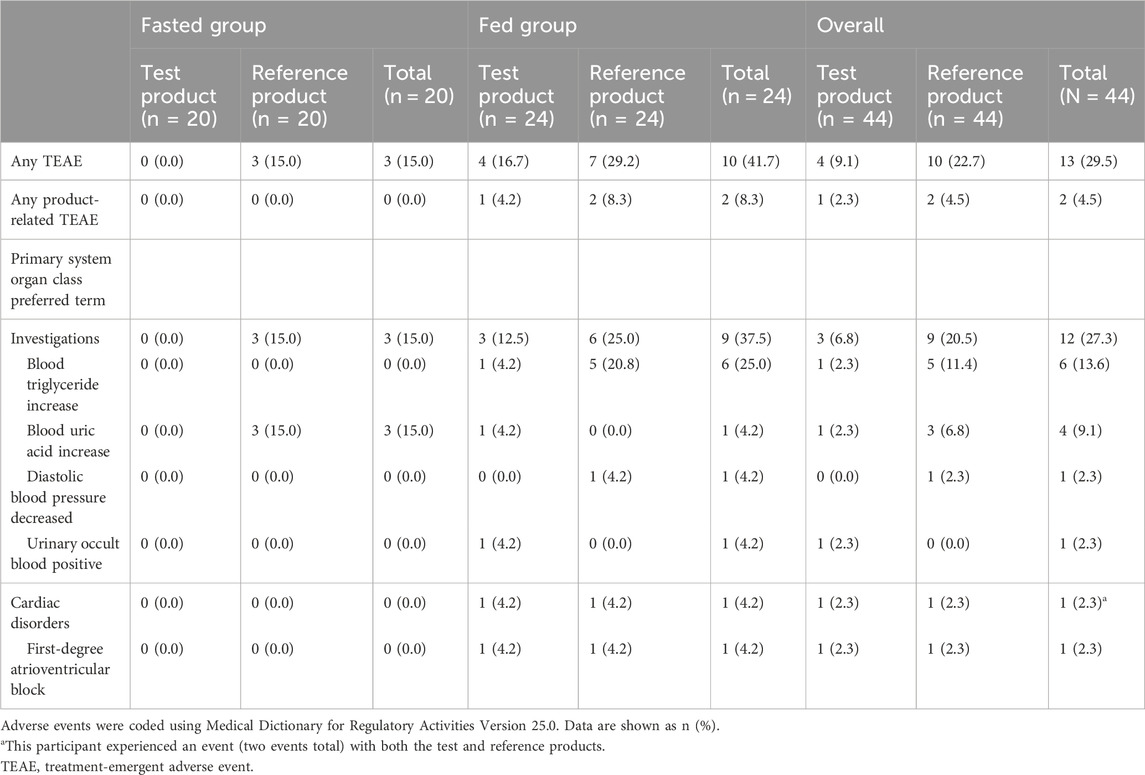
The TEAE data are tabulated in Table 4. A higher frequency of TEAEs occurred following the administration of the reference product (10/44 [22.7%]) than after the test product (4/44 [9.1%]). Three participants in the fed group experienced TEAEs considered to be related to the study treatment (two product-related first-degree atrioventricular block events and one product-related diastolic blood pressure decrease). Both first-degree atrioventricular block events were experienced by one participant, once with the test and once with the reference product, and the diastolic blood pressure decrease event occurred with the reference product. All product-related TEAEs were mild in severity and resolved. None of the participants discontinued because of TEAEs, and no serious TEAEs or deaths occurred.
4 Discussion
This study demonstrated and compared the bioequivalence of bisoprolol 5-mg tablets manufactured in China to tablets manufactured in Germany in healthy Chinese adults under both fasted and fed conditions. Moreover, both tablets were well-tolerated, and no unexpected safety concerns were encountered. Importantly, this study provides evidence to meet regulatory filing requirements, thereby supporting Merck’s improved manufacturing capabilities for bisoprolol and access to bisoprolol for Chinese patients.
The bisoprolol PK results were generally consistent with those of previous reports in both Chinese participants (Hu et al., 2018; Wang et al., 2009; Zhou et al., 2007) and participants of other ethnicities (Tjandrawinata et al., 2012; Leopold et al., 1986). A previous study reported the PK profile of bisoprolol in Chinese participants who received both bisoprolol and amlodipine (Hu et al., 2018). The lack of PK drug–drug interactions between bisoprolol and amlodipine allows comparison between the bisoprolol PK profile in the previous study and the current study. Among participants who received both bisoprolol and amlodipine, the bisoprolol Cmax geometric means were approximately 26 ng/mL in the fasted group and 21 ng/mL in the fed group, AUC0–tlast geometric means were approximately 294 h·ng/mL in the fasted group and 270 h·ng/mL in the fed group, and AUC0–
As the geometric LS mean ratios for Cmax, AUC0–tlast, and AUC0–
The safety results showed that the tablets manufactured both in China and in Germany were generally safe and well-tolerated. All product-related TEAEs (first-degree atrioventricular block and increase in diastolic blood pressure) were mild and resolved. Additionally, most reported TEAEs were events that were known for the product (including increase in blood triglyceride levels) (Merck, 2014). Both the test and reference products had a similar frequency of TEAEs, and there were no new or unexpected safety concerns.
This study had some limitations: Concor® is approved at dosages of 5 mg and 10 mg. Single-dose BE studies commonly use the highest dose strength, unless participant safety is a concern. In this study, the 5-mg dose was selected due to its clinical relevance as the most commonly prescribed starting dose and to minimize potential safety risks to healthy volunteers. Regulatory guidelines permit the use of a lower dose strength when justified by safety considerations (U.S. Food and Drug Administration, 2021). The 5-mg dose ensured sufficient bioanalytical sensitivity while maintaining participant safety. Due to the limitations of the sample size and single-dose administration, it was difficult to conduct a comprehensive assessment of the safety of the two drugs, and potential long-term safety concerns need to be further perfected. No formal statistical comparisons were conducted regarding the secondary endpoints. Additionally, although no differences in PK parameters were identified between the fasted and fed groups, this finding should be interpreted with caution as no formal comparisons were conducted.
In conclusion, this study found that bisoprolol 5-mg tablets manufactured in China exhibited bioequivalence comparable to that of tablets manufactured in Germany in healthy Chinese adults, and that the tablets were both well-tolerated and had comparable safety profiles.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Beijing Friendship Hospital Institutional Review Board and Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HY: methodology, writing–original draft, writing–review and editing, and investigation. ZJ: methodology, writing–original draft, writing–review and editing, and investigation. RL: data curation, writing–review and editing, and investigation. DL: validation, writing–original draft, and writing–review and editing. LW: validation, writing–original draft, and writing–review and editing. RD: project administration, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are grateful to the participants who volunteered for this study. The authors thank Kuiqian Ma for his contributions to the comparison design for pharmaceutical characteristics, as well as the data analysis. The authors also thank Hannah Read, PhD, of Edanz (www.edanz.com) for medical writing support.
Conflict of interest
Authors DL and LW were employed by the Merck Serono (Beijing) Pharmaceutical R&D Co., Ltd., Beijing, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that this study received funding from Merck Healthcare KGaA, Darmstadt, Germany. The funder had the following involvement in the study: study design, the writing of the article, and the decision to submit it for publication.
Generative AI statement
The author(s) declare that no generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Eguchi, K. (2016). New insight into effects of β-blockers on arterial functions. Pulse (Basel) 3 (3-4), 190–194. doi:10.1159/000443615
Fuchs, F. D., and Whelton, P. K. (2020). High blood pressure and cardiovascular disease. Hypertension 75, 285–292. doi:10.1161/HYPERTENSIONAHA.119.14240
Hu, C., Hu, X., Wang, C., Zhao, Z., Gao, D., Chen, X., et al. (2018). Bioequivalence and pharmacokinetics of bisoprolol-amlodipine 5 mg/5 mg combination tablet versus bisoprolol 5 mg tablet and amlodipine 5 mg tablet: an open-label, randomized, two-sequence crossover study in healthy Chinese subjects. Clin. Drug Investig. 38 (12), 1145–1154. doi:10.1007/s40261-018-0700-y
Leopold, G., Pabst, J., Ungethüm, W., and Bühring, K. U. (1986). Basic pharmacokinetics of bisoprolol, a new highly beta 1-selective adrenoceptor antagonist. J. Clin. Pharmacol. 26, 616–621. doi:10.1002/j.1552-4604.1986.tb02959.x
Merck (2014). Concor® 5 mg package insert. Available at: https://medicarcp.com/website/pdf/pils/1306%20-%20en.pdf (Accessed June 1, 2024).
Nguyen, T. N., and Chow, C. K. (2021). Global and national high blood pressure burden and control. Lancet 398 (10304), 932–933. doi:10.1016/S0140-6736(21)01688-3
Ralapanawa, U., and Sivakanesan, R. (2021). Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J. Epidemiol. Glob. Health 11 (2), 169–177. doi:10.2991/jegh.k.201217.001
Savarese, G., Becher, P. M., Lund, L. H., Seferovic, P., Rosano, G. M. C., and Coats, A. J. S. (2023). Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 118, 3272–3287. doi:10.1093/cvr/cvac013
Tjandrawinata, R. R., Setiawati, E., Yunaidi, D. A., Santoso, I. D., and Susanto, L. W. (2012). Bioequivalence study of two formulations of bisoprolol fumarate film-coated tablets in healthy subjects. Drug Des. Dev. Ther. 6, 311–316. doi:10.2147/DDDT.S36567
U.S. Food and Drug Administration (1997). Guidance for industry, dissolution testing of immediate release solid oral dosage forms. United States: Center for Drug Evaluation and Research CDER. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms (Accessed June 1, 2024).
U.S. Food and Drug Administration (2002). Food-effect bioavailability and fed bioequivalence studies. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/food-effect-bioavailability-and-fed-bioequivalence-studies (Accessed June 1, 2024).
U.S. Food and Drug Administration (2021). Bioequivalence studies with pharmacokinetic endpoints for drugs submitted under an abbreviated new drug application. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioequivalence-studies-pharmacokinetic-endpoints-drugs-submitted-under-abbreviated-new-drug
Wang, W., Jia, J., Liu, Y., Jin, J., Zhang, M., Lu, C., et al. (2009). Pharmacokinetics and bioequivalence of bisoprolol tablets in healthy volunteers. Chin. J. New Drug Clin. Remedies 28 (7), 513–517.
Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M., et al. (2018). 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. doi:10.1093/eurheartj/ehy339
World Health Organization (2023). Global report on hypertension: the race against a silent killer. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/publications/i/item/9789240081062 (Accessed June 1, 2024).
Zhou, B., Perel, P., Mensah, G. A., and Ezzati, M. (2021). Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 18, 785–802. doi:10.1038/s41569-021-00559-8
Keywords: bioequivalence, bisoprolol, clinical trial, pharmacokinetic, safety
Citation: Yin H, Jin Z, Li R, Li D, Wang L and Dong R (2025) Bioequivalence of China- and Germany-manufactured bisoprolol (Concor®) tablets in fasted and fed healthy Chinese participants: a randomized, open-label, two-way, crossover study. Front. Pharmacol. 16:1527623. doi: 10.3389/fphar.2025.1527623
Received: 13 November 2024; Accepted: 17 February 2025;
Published: 12 March 2025.
Edited by:
Yurong Lai, Gilead, United StatesReviewed by:
Junling Yang, Chinese Academy of Sciences (CAS), ChinaItay Perlstein, Magic Wand Research, LLC, United States
Sandhya Subash, Washington State University Health Sciences Spokane, United States
Copyright © 2025 Yin, Jin, Li, Li, Wang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruihua Dong, cnVpaHVhX2RvbmdfcndAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Hang Yin
Hang Yin Zhili Jin
Zhili Jin Ran Li1
Ran Li1 Ruihua Dong
Ruihua Dong