- 1The Second Clinical School of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 2Guangdong Provincial Hospital of Chinese Medicine and Guangdong Provincial Academy of Chinese Medical Sciences, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 3State Key Laboratory of Dampness Syndrome of Chinese Medicine, Guangdong-Hong Kong-Macau Joint Lab on Chinese Medicine and Immune Disease Research and Guangdong Provincial Key Laboratory of Clinical Research on Chinese Medicine Syndrome, Guangzhou, Guangdong, China
Background: Psoriasis is an inflammatory and recurrent dermatological disease that is associated with multiple comorbidities. Conventional psoriasis therapies such as acitretin capsule and narrow-band ultraviolet B radiation (NB-UVB) are prone to decreased efficacy and adverse events in long-term application. Total glucosides of paeony (TGP), a plant extract from Radix Paeoniae Alba, are commonly used in conjunction with conventional therapies for psoriasis. This study aims to elucidate the add-on effect of TGP on conventional therapies in the treatment of psoriasis.
Methods: Seven databases were searched from their inception to March 2024. Randomized controlled trials (RCTs) using TGP in conjunction with conventional therapies for psoriasis were included. The Risk of Bias 2.0 (RoB 2.0) tool was used to assess bias risk, and data analysis was conducted using RevMan V.5.4. Evaluation outcomes mainly involved a 60% or greater reduction of Psoriasis Area and Severity Index score (PASI 60) and a 50% or greater reduction of Psoriasis Area and Severity Index score (PASI 50).
Results: This meta-analysis ultimately included 36 RCTs with 3,140 participants. The findings indicated that TGP combined with conventional therapies were superior to conventional therapies used alone on PASI 60 (RR = 1.32, 95% CI: 1.25 to 1.39, P < 0.00001) and PASI 50 (RR = 1.44, 95% CI: 1.13 to 1.84, P = 0.004). Several types of conventional therapies were prone to PASI 60 response when combined with TGP than conventional therapies using alone, such as oral medication (RR = 1.40, 95% CI: 1.14, to 1.71, P = 0.001), topical medication (RR = 1.47, 95% CI: 1.24 to 1.74, P < 0.00001), and NB-UVB (RR = 1.29, 95% CI: 1.16 to 1.43, P < 0.00001). Furthermore, the results suggested that TGP might reduce the incidence of adverse events occurred by conventional therapies for psoriasis.
Conclusion: This meta-analysis demonstrated the preliminary clinical evidence supporting the addition of TGP to conventional therapies in treating psoriasis. Owing to the limited methodological quality of the included studies, well-designed RCTs are required to further illustrate the add-on effect of TGP on conventional therapies for psoriasis.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=439904, identifier CRD42023439904.
1 Background
Psoriasis is a chronic, recurrent, inflammatory dermatological disease, with a global prevalence of approximately 125 million (WHO, 2016; Griffiths et al., 2021; Psoriasis Committee, Dermatology and Venereology Branch, Chinese Medical Association, 2023). Patients with psoriasis are probably susceptible to serious comorbidities such as malignancy, coronary atherosclerosis, and psoriatic arthritis (Amin et al., 2020; Boehncke et al., 2010; Kommoss et al., 2023; Mayor, 2016). Apprehensively, during the long-term and recrudescent course, patients with psoriasis are at risk of substantial negative impacts on psychological health, such as depression and suicidal behaviors (Bardazzi et al., 2022; Bell et al., 2021). Nowadays, published guidelines for psoriasis declare that conventional therapies applied commonly in clinical practice contain oral systemic treatment, topical medication, biological agents, and phototherapy (Nast et al., 2018; Nast et al., 2020; Psoriasis Committee, Dermatology and Venereology Branch, Chinese Medical Association, 2023). Given the reduced efficacy and increased adverse events in long-term application (Chia et al., 2023; Thatiparthi et al., 2022; Katz et al., 1999), conventional therapies, as a type of psoriasis treatment, face challenges in optimizing therapeutic strategies.
Total glucosides of paeony (TGP), a natural extract from Radix Paeoniae Alba, have been recommended by the guideline as an alternative therapy for the treatment of psoriasis (Psoriasis Committee, Dermatology and Venereology Branch, Chinese Medical Association, 2023). Published research studies have indicated that TGP has an immunological effect on anti-inflammation and immune regulation in psoriasis (Lei et al., 2023; Li et al., 2019). Moreover, relevant clinical studies have illustrated the potential of TGP in improving psoriatic symptoms and reducing common adverse events (Yu et al., 2017; Zheng et al., 2019). Expectantly, combining application with TGP is conducive to be a novel optimized therapeutic strategy for conventional therapies in treating psoriasis. However, to date, there is a lack of solid clinical evidence to evaluate the add-on effect of TGP on conventional therapies and support the combination of TGP with conventional therapies as a novel therapeutic option in the treatment of psoriasis.
Thus, it is essential to conduct a systematic review and meta-analysis in accordance with the standard of Cochrane Handbook to elucidate the add-on effect of TGP on conventional therapies for the treatment of psoriasis. This study has the potential to identify a novel optimized therapeutic strategy for conventional therapies in treating psoriasis and provide instructive insights for further investigation.
2 Methods
This systematic review and meta-analysis was undertaken following the standard guidance of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA). In addition, the registration of this study was completed on the Prospective Register of Systematic Reviews (CRD42023439904).
2.1 Search strategy
This meta-analysis searched for randomized controlled trials (RCTs) comparing TGP to conventional therapies in the treatment of PV from database inception to March 2024. Databases included three English databases, namely, PubMed, Embase, and Web of science, and four Chinese databases, namely, China National Knowledge Infrastructure (CNKI), China Biomedical Literature Database (CBM), Chinese Scientific Journal Database (VIP), and Wanfang database (Wanfang).
2.2 Eligibility criteria
The inclusion and exclusion criteria were cooperatively established by two researchers (CL and JY).
2.2.1 Inclusion criteria
The inclusion criteria were presented as follows: 1) patients diagnosed with psoriasis and without limitations on gender, age, race, economic status, or education; 2) treatment of TGP associated with conventional therapies (such as acitretin capsule and methotrexate) applied in the intervention group, while conventional therapies used in the control group; 3) outcomes concerning the Psoriasis Area and Severity Index (PASI) score or adverse events; and 4) study designed with RCTs.
2.2.2 Exclusion criteria
The exclusion criteria were presented as follows: 1) studies with incomplete data or data errors; 2) duplicate publication; 3) reviews, consensus-based studies, commentaries, conference abstracts, case reports, and animal or cell experiments; and 4) inadequate outcomes for systematic review and meta-analysis.
2.3 Study selection and data extraction
Two researchers (ZL and KG) first screened the titles and abstracts of the included studies and then removed the duplicates. Next, full-text screening was simultaneously undertaken, and any conflicts were determined by the third researcher (JY) through the discussion. Accordingly, two researchers (ZL and KG) independently extracted the detailed characteristics of the included studies, such as age, gender, intervention, comparator, administration and dosage, and adverse events. The collated data were cross-checked by another two researchers (JL and HL).
2.4 Quality assessment
The bias risk of the included studies was separately evaluated by two researchers (JL and HL) using the Risk of Bias 2.0 (RoB 2.0) tool according to the Cochrane Handbook V.6.5. The six domains of bias risk were presented as follows: 1) randomization process; 2) deviations from intended interventions; 3) missing outcome data; 4) measurement of the outcome; 5) selection of the reported result; and 6) overall. The risk bias of every domain was classified as low, some concern, or high. Response to every signaling question included five options: yes (Y), probably yes (PY), probably no (PN), no (N), and no information (NI). Any discrepancies were discussed and resolved by all researchers.
2.5 Statistical analysis
Review Manager version 5.4 and Stata version 17.0 were utilized to analyze the effect of TGP associated with conventional therapies for psoriasis. The enumeration data were represented by relative risk (RR) for each effect quantity. Relatively, the measurement data were presented with both the mean difference (MD) and the 95% confidence interval (95% CI). The data pooling model for meta-analysis was chosen based on the presence and magnitude of heterogeneity. The heterogeneity of the included data was evaluated using the chi-squared (X2) test and the I-squared (I2) test. The fixed-effects model was selected when there was acceptable heterogeneity of the findings (I2 < 50%). Otherwise, the random effects model was used when there was statistically significant heterogeneity among detected findings (I2 ≥ 50%). Additionally, the random effects model was also applied for meta-analysis when there was apparent heterogeneity among the studies. Sensitivity analysis was conducted to assess the stability of the conclusion, and meta-regression analyses were performed to identify the source of heterogeneity. Subgroup analysis was conducted based on the outcome measures, treatment duration, and adverse events. p < 0.05 was considered statistically significant. The publication bias of the included studies was evaluated using a funnel plot and Egger’s test.
3 Results
3.1 Search results
A total of 328 studies were retrieved according to the eligibility criteria. After eliminating the duplicates, 137 studies were screened with their titles and abstracts. Then, the remaining 62 studies that met the inclusion criteria were given access to the full text. A total of 26 studies were excluded for the following reasons: 1) not RCTs; 2) duplicate publication; and 3) without inadequate outcomes. Finally, 36 studies were included in this meta-analysis (Yu et al., 2017; Cai et al., 2014; Chen et al., 2014; Ding, 2020; Du et al., 2017; Du et al., 2012; Gao, 2020; Guo and Xu, 2019; Guo, 2016; He et al., 2014; He et al., 2012; Hu et al., 2012a; Hu et al., 2012b; Hua et al., 2012; Huang, 2015; Huang and Huang, 2011; Jiang et al., 2014; Jiang et al., 2013; Li, 2017; Li et al., 2016; Lin, 2017; Liu et al., 2014; Liu and Wang, 2011; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Wang, 2016; Yang et al., 2013; Zhang et al., 2015; Zhang et al., 2016; Zhang et al., 2022; Zhang and Wang, 2018; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015). The detailed flowchart of the searching process is shown in Figure 1.
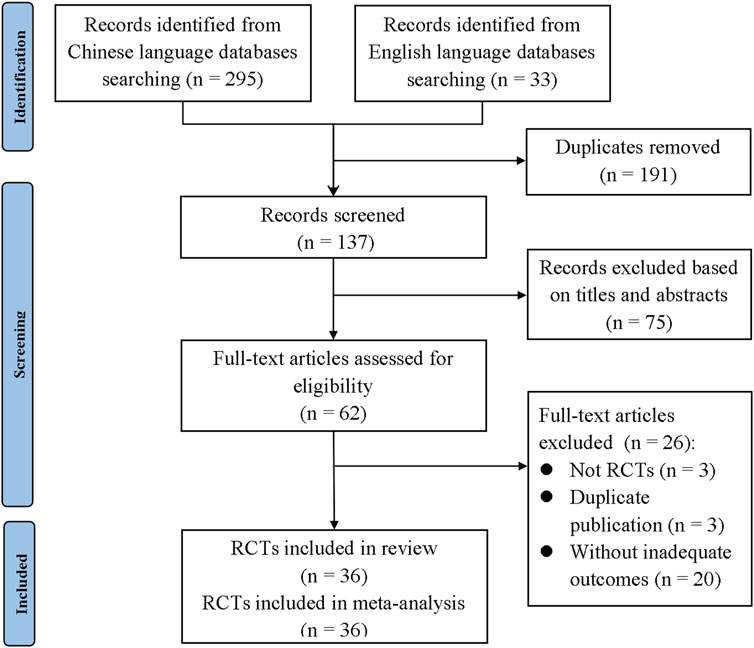
Figure 1. PRISMA flowchart of the search process. TPG, total glucosides of paeony; RCT, randomized controlled trial.
3.2 Study characteristics
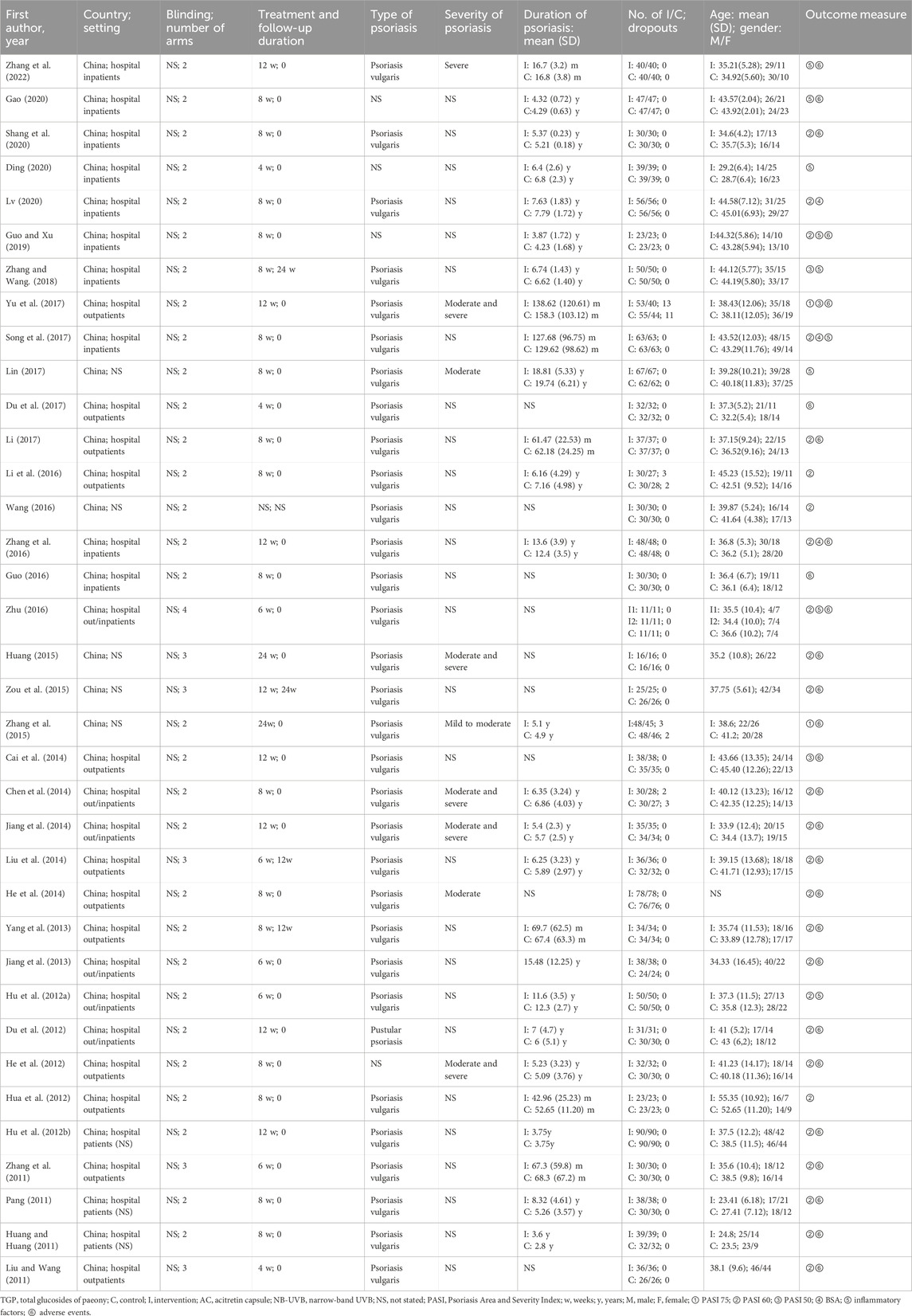
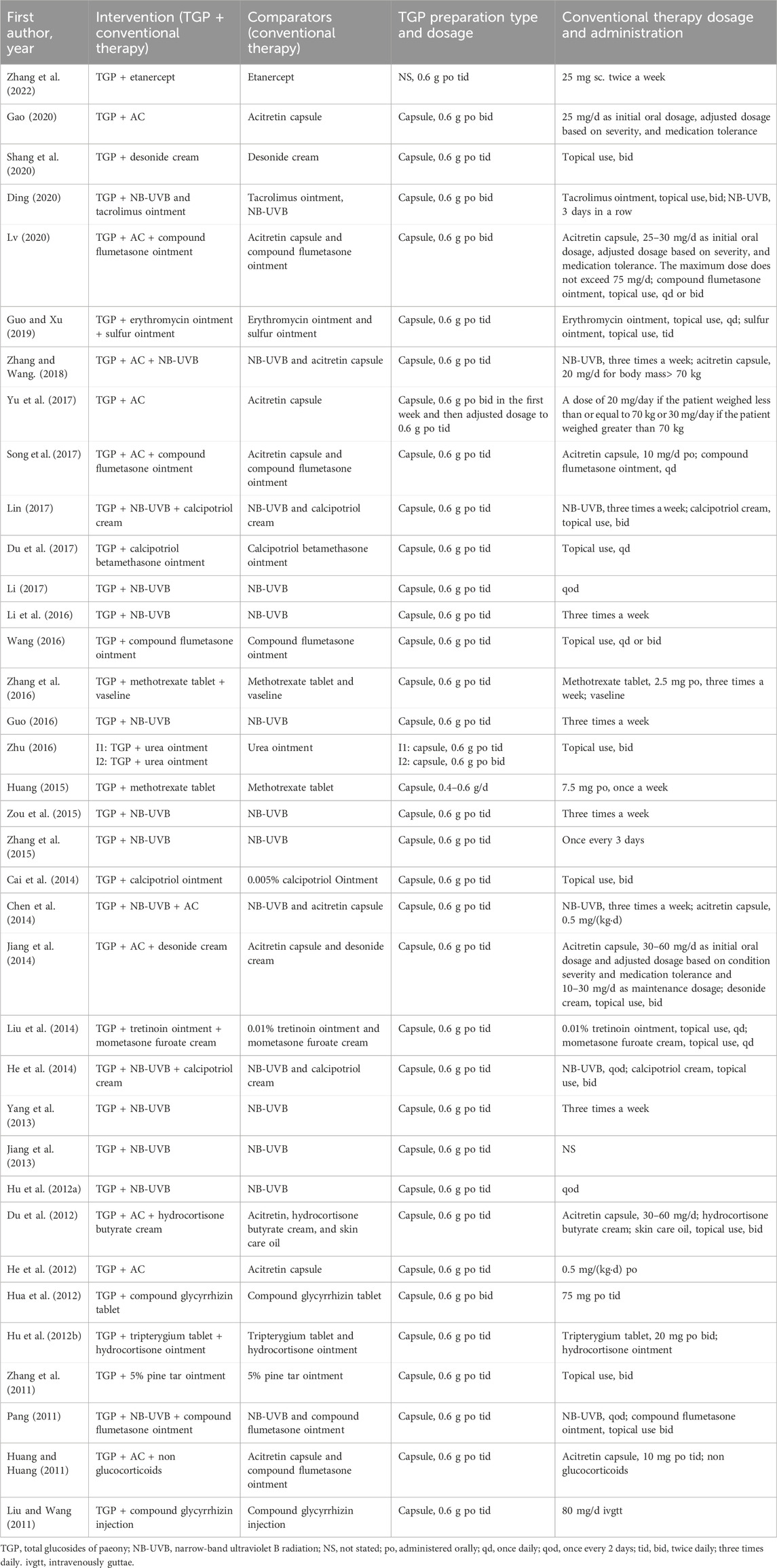
A total of 36 RCTs were included, with 3,140 participants. Concerning the study design, all studies used a two-arm parallel design, with the exception of five studies (Huang, 2015; Liu et al., 2014; Liu and Wang, 2011; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015) using three-arm parallel design and one study (Zhu, 2016) using the four-arm parallel design. Among the included studies, five studies (Hua et al., 2012; Jiang et al., 2013; Liu et al., 2014; Pang, 2011) reported the stage of psoriasis, and eight studies (Yu et al., 2017; Chen et al., 2014; He et al., 2014; Huang, 2015; Jiang et al., 2014; Lin, 2017; Zhang et al., 2015; Zhang et al., 2022) indicated the severity of psoriasis. During the study period, seven studies (Yu et al., 2017; Cai et al., 2014; Du et al., 2012; Jiang et al., 2014; Zhang et al., 2016; Zhang et al., 2022; Zou et al., 2015) received 12-week treatment, while five studies (Hu et al., 2012a; Jiang et al., 2013; Liu et al., 2014; Zhang et al., 2011; Zhu, 2016) received 6-week treatment. The remaining studies used an 8-week treatment period. Only four studies (Liu et al., 2014; Yang et al., 2013; Zhang and Wang, 2018; Zou et al., 2015) mentioned the follow-up period. Concerning the treatment applied in the included studies, all the intervention groups were treated with TGP and conventional therapies, while conventional therapies were applied alone in the control group. In the included studies, acitretin capsules, narrow-band ultraviolet B radiation (NB-UVB), and topical ointment were the most conventional therapies. The detailed characteristics of the included studies are presented in Table 1. Regarding the dosage of TGP, a dose of 0.6 g was used in all studies except one study (Huang, 2015) that use 0.4 g. Concerning the administration of TGP, a majority of studies administered TGP thrice per day, while five studies (Ding, 2020; Guo and Xu, 2019; Hua et al., 2012; Lv, 2020; Zhu, 2016) applied it twice daily. The details of dosage and administration are shown in Table 2.
3.3 Risk of bias assessment
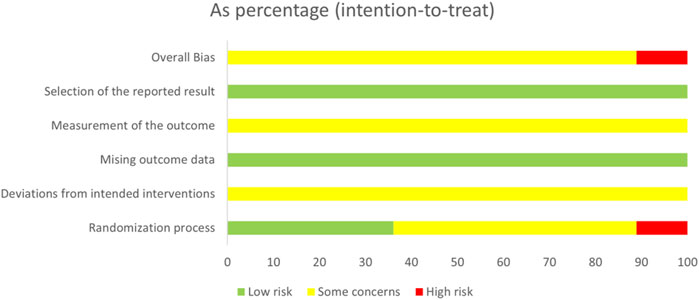
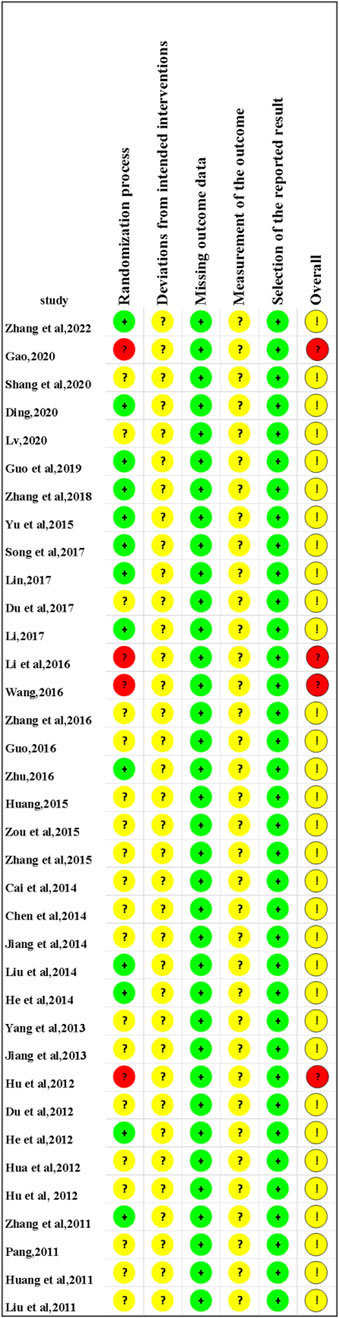
In the domain of the randomization process, 13 studies (Yu et al., 2017; Ding, 2020; Guo and Xu, 2019; He et al., 2014; He et al., 2012; Li, 2017; Lin, 2017; Liu et al., 2014; Song et al., 2017; Zhang et al., 2022; Zhang et al., 2011; Zhu, 2016) were conducted using the random number tables for double-blind and were classified as low bias risk with the response of N/PN. Four studies (Gao, 2020; Hu et al., 2012a; Li et al., 2016; Wang, 2016) were rated as having a high bias risk with the response of Y/PY since randomization dispensing was based on the visiting order. In the domain of the missing outcome data and the selection of the reported result, all included studies were assessed as low risk of bias, with a response of Y/PY to outcome availability and data analysis consistency. Regarding the domain of deviations from intended interventions and outcome measurement, all included studies were considered to have some risk of bias because no detailed information on the abovementioned domains was detected. As for the domain of overall, 32 studies (Yu et al., 2017; Cai et al., 2014; Chen et al., 2014; Ding, 2020; Du et al., 2017; Du et al., 2012; Guo and Xu, 2019; Guo, 2016; He et al., 2014; He et al., 2012; Hu et al., 2012b; Hua et al., 2012; Huang, 2015; Huang and Huang, 2011; Jiang et al., 2014; Jiang et al., 2013; Li, 2017; Lin, 2017; Liu et al., 2014; Liu and Wang, 2011; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Yang et al., 2013; Zhang et al., 2015; Zhang et al., 2016; Zhang et al., 2022; Zhang and Wang, 2018; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015) were ranked having the some concern, and 4 studies (Gao, 2020; Hu et al., 2012a; Li et al., 2016; Wang, 2016) were evaluated as having a high risk of bias, according to the results of the former five domains. The results of risk bias are shown in Figures 2, 3.
3.4 Meta-analysis of efficacy based on outcome measures
PASI was the main outcome measure used to assess psoriasis efficacy, with a score of 0–72 based on erythema, scaling, and induration (Griffiths et al., 2021). Subsequently, meta-analysis was conducted using specific outcome measures of PASI 75, PASI 60, and PASI 50, which were, respectively, defined as 75%, 60%, and 50% or greater reduction in the PASI score. Additionally, body surface area (BSA) and inflammatory factors were also evaluated.
3.4.1 Based on PASI 75
Two studies (Yu et al., 2017; Zhang et al., 2015) estimated the clinical effect using PASI 75 as the outcome measure. With the acceptable heterogeneity detected (I2 = 0% and P = 0.81), a fixed-effects model was selected for meta-analysis. The results showed there was a significant difference in TGP combined with conventional therapies when compared with conventional therapies used alone (RR = 1.24, 95% CI: 1.03 to 1.50, P = 0.02) (Figure 4A).
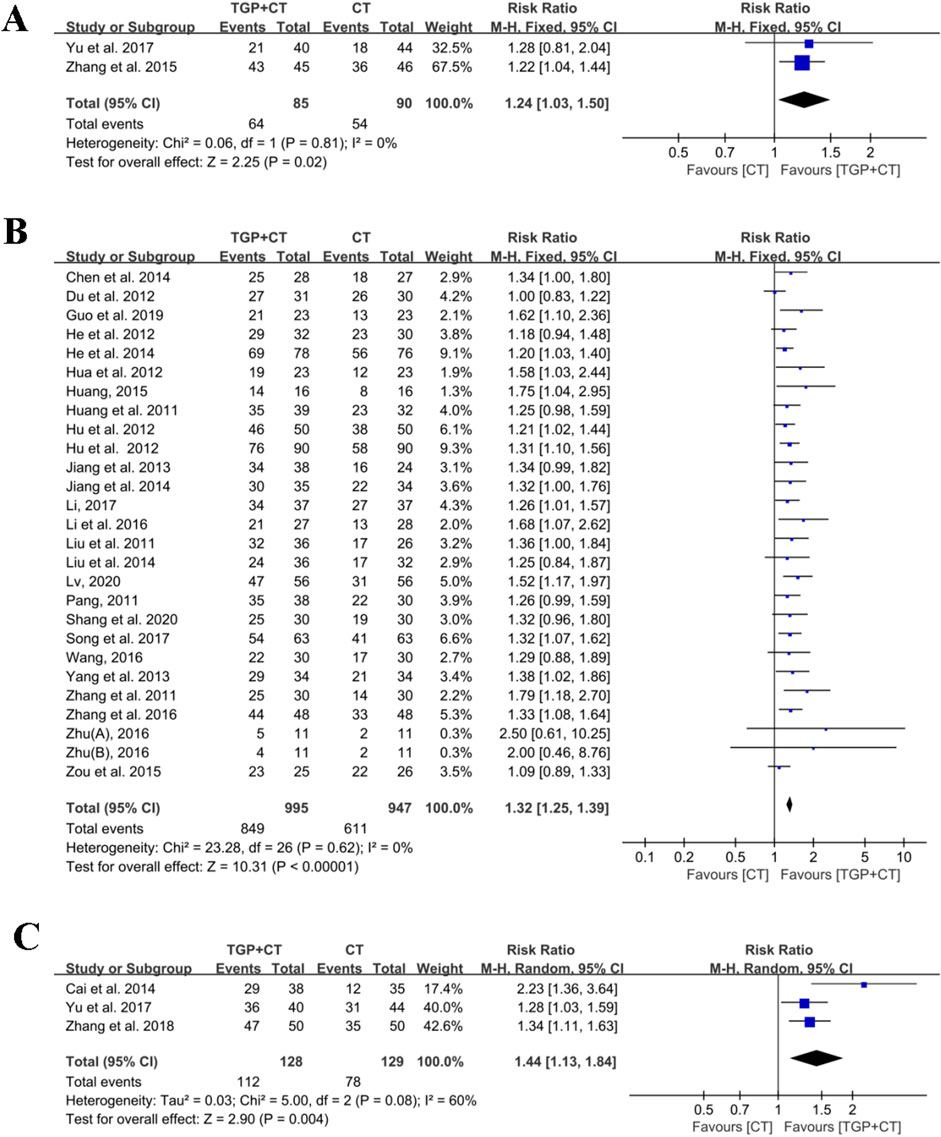
Figure 4. Meta-analysis based on outcome measures: (A) based on PASI 75; (B) based on PASI 60; and (C) based on PASI 50. TGP, total glucosides of paeony; CT, conventional therapy; PASI 75, 75% or greater reduction of the PASI score; PASI 60, 60% or greater reduction of the PASI score; PASI 50, and 50% or greater reduction of the PASI score; Zhu, 2016: 0.6 g TGP po tid; Zhu, 2016: 0.6 g TGP po bid.
3.4.2 Based on PASI 60
A total of 26 studies, involving 1,942 participants, utilized the outcome measures of PASI 60 for efficacy evaluation (Chen et al., 2014; Du et al., 2012; Guo and Xu, 2019; He et al., 2014; He et al., 2012; Hu et al., 2012a; Hu et al., 2012b; Hua et al., 2012; Huang, 2015; Huang and Huang, 2011; Jiang et al., 2014; Jiang et al., 2013; Li, 2017; Li et al., 2016; Liu et al., 2014; Liu and Wang, 2011; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Wang, 2016; Yang et al., 2013; Zhang et al., 2016; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015). No significant heterogeneity was observed among the included studies (I2 = 0% and P = 0.62), and thus, meta-analysis was implemented using a fixed-effects model. The findings indicated that TGP in combination with conventional therapies was superior to conventional therapies used alone in treating psoriasis (RR = 1.32, 95% CI: 1.25 to 1.39, P < 0.00001) (Figure 4B). The symmetry of the funnel plot and the detected result of the Egger’s test (P = 0.218 > 0.05) revealed no publication bias (Supplementary Figure S1A). Sensitivity analysis revealed the robustness and reliability of conclusion (Supplementary Figure S1B). Additionally, the results of meta-regression found no source of heterogeneity (Supplementary Figure S1C). Subgroup analysis was performed as follows:
3.4.2.1 TGP plus monotherapy versus monotherapy
A total of 16 studies with 950 participants reported the effect of TGP associated with monotherapy (Guo and Xu, 2019; He et al., 2012; Hu et al., 2012a; Hua et al., 2012; Huang, 2015; Jiang et al., 2013; Li, 2017; Li et al., 2016; Liu et al., 2014; Liu and Wang, 2011; Shang et al., 2020; Wang, 2016; Yang et al., 2013; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015). Owing to no heterogeneity detected (I2 = 0%, P = 0.57), a fixed-effects model was utilized for meta-analysis. The results demonstrated that TGP plus monotherapy was superior to monotherapy used alone (RR = 1.36, 95% CI: 1.26 to 1.48, P < 0.00001) (Figure 5A).
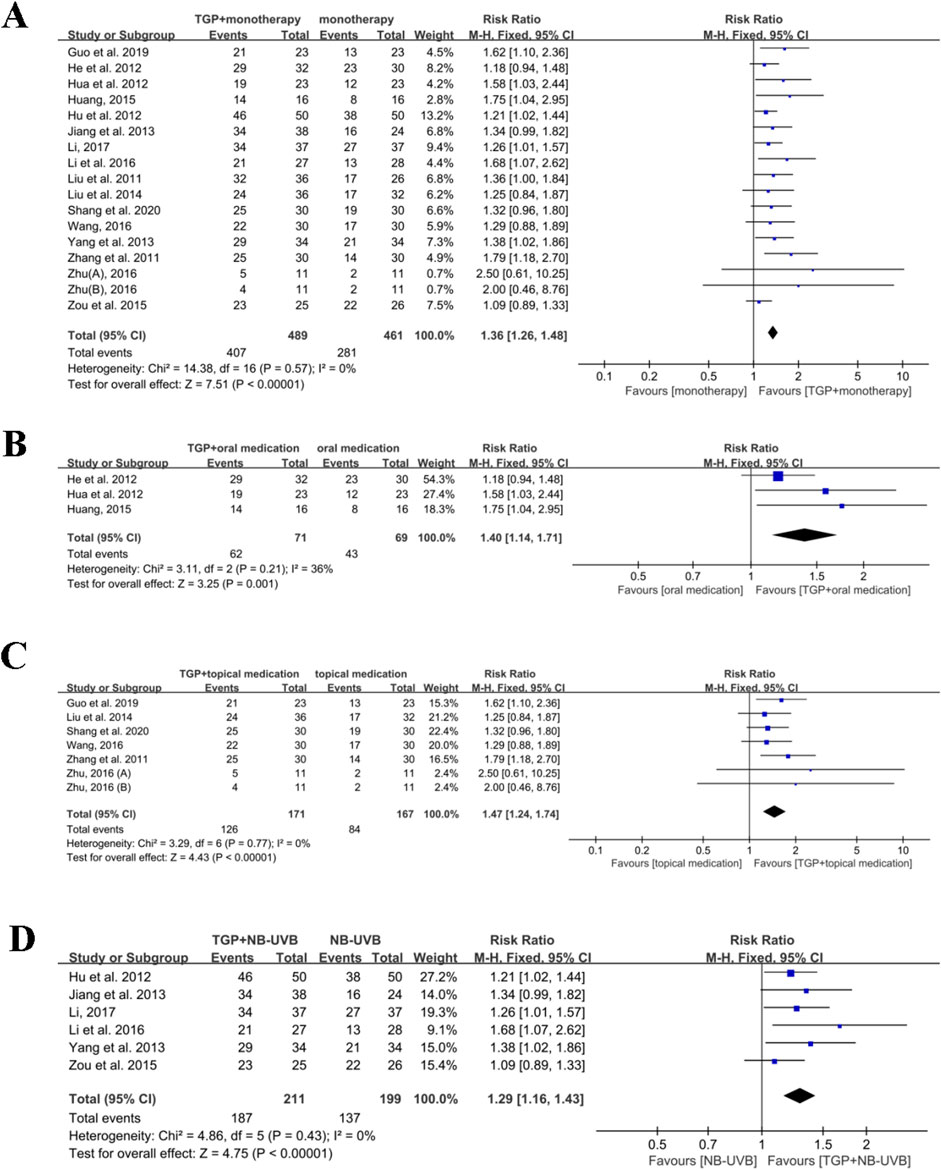
Figure 5. Meta-analysis of TGP plus monotherapy versus monotherapy. (A) TGP plus monotherapy versus monotherapy. (B) TGP plus oral medication versus oral medication. (C) TGP plus topical medication versus topical medication. (D) TGP plus NB-UVB versus NB-UVB. TGP, total glucosides of paeony; NB-UVB, narrow-band UVB; Zhu, 2016: 0.6 g TGP po tid; Zhu, 2016: 0.6 g TGP po bid.
Three studies, containing 105 participants, mentioned the effects of TGP combined with oral medication (such as acitretin capsules) for psoriasis (He et al., 2012; Hua et al., 2012; Huang, 2015). A fixed-effects model was used for meta-analysis since acceptable heterogeneity was detected among the included studies (I2 = 36%, P = 0.21). The results demonstrated that TGP in combination with oral medication had an advantage over oral medication alone (RR = 1.40, 95% CI: 1.14 to 1.71, P = 0.001) (Figure 5B).
Six studies with 338 participants evaluated the effects of TGP in conjunction with topical medication (such as tacrolimus ointment) (Guo and Xu, 2019; Liu et al., 2014; Shang et al., 2020; Wang, 2016; Zhang et al., 2011; Zhu, 2016). Due to no obvious heterogeneity observed (I2 = 0%, P = 0.77), meta-analysis was performed using a fixed-effects model. The findings indicated that the combination of TGP and topical medication was more effective than topical medication alone (RR = 1.47, 95% CI: 1.24 to 1.74, P < 0.00001) (Figure 5C).
Six studies involving 410 participants indicated the effects of TGP with NB-UVB (Hu et al., 2012a; Jiang et al., 2013; Li, 2017; Li et al., 2016; Yang et al., 2013; Zou et al., 2015). Since no significant heterogeneity was found among the included studies (I2 = 0% and P = 0.43), meta-analysis was accomplished with a fixed-effects model. The results showed that there was a statistically significant difference when comparing TGP plus NB-UVB with NB-UVB alone (RR = 1.29, 95% CI: 1.16 to 1.43, P < 0.00001) (Figure 5D).
3.4.2.2 TGP plus multiple therapies versus multiple therapies alone
A total of 10 studies, with a total of 992 participants, stated the effects of TGP associated with multiple therapies for psoriasis (Chen et al., 2014; Du et al., 2012; He et al., 2014; Hu et al., 2012b; Huang and Huang, 2011; Jiang et al., 2014; Lv, 2020; Pang, 2011; Song et al., 2017; Zhang et al., 2016). Since there was no significant heterogeneity among the included studies (I2 = 0% and P = 0.46), meta-analysis was conducted using a fixed-effects model. The findings suggested that when combined with TGP, multiple therapies showed a more prominent effect than when used alone in the treatment of psoriasis (RR = 1.28, 95% CI: 1.20 to 1.38, P < 0.00001) (Figure 6A).
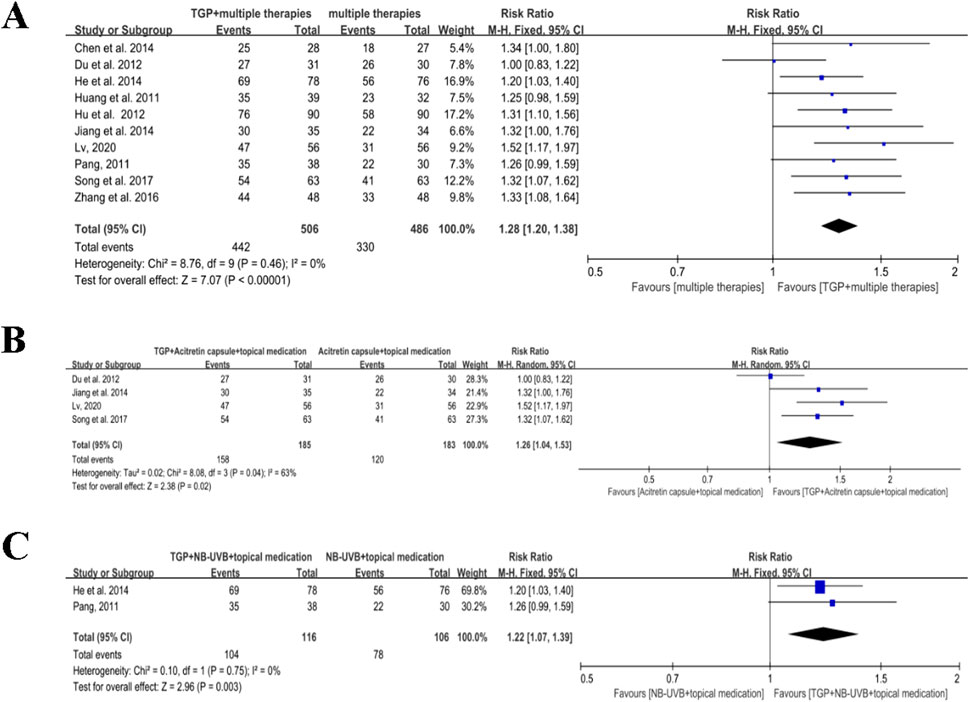
Figure 6. Meta-analysis of TGP plus multiple therapies versus multiple therapies. (A) TGP plus multiple therapies versus multiple therapies. (B) TGP plus the acitretin capsule and topical medication versus acitretin capsule and topical medication. (C) TGP plus NB-UVB and topical medication versus NB-UVB and topical medication. TGP, total glucosides of paeony; NB-UVB, narrow-band UVB.
Four studies involving 368 participants reported the effects of TGP in combination with acitretin capsules and topical medication for psoriasis (Du et al., 2012; Jiang et al., 2014; Lv, 2020; Song et al., 2017). Due to substantial heterogeneity (I2 = 63%, P = 0.04), a random effects model was applied for meta-analysis. The results revealed that when combined with TGP, multiple therapies of acitretin capsules and topical medication were not superior than using them alone (RR = 1.26, 95% CI: 1.04 to 1.53, and P = 0.02) (Figure 6B).
Two studies with 222 participants mentioned the effects of TGP in combination with NB-UVB and topical medication (He et al., 2014; Pang, 2011). Since no significant heterogeneity was detected (I2 = 0% and P = 0.75), a fixed-effects model was used for meta-analysis. The findings showed that when combined with TGP, multiple therapies involving NB-UVB and topical medication were more effective than using them alone for psoriasis (RR = 1.22, 95% CI: 1.07 to 1.39, P = 0.003) (Figure 6C).
3.4.3 Based on PASI 50
Three studies with 257 participants reported the effects of TGP and conventional therapies using the outcome measure of PASI 50 (Yu et al., 2017; Cai et al., 2014; Zhang and Wang, 2018). Given the significant heterogeneity (I2 = 60%, P = 0.08), a random effects model was applied for meta-analysis. The findings revealed a statistical difference when TGP plus conventional therapies were compared with conventional therapies alone (RR = 1.44, 95% CI: 1.13 to 1.84, P = 0.004) (Figure 4C). Sensitivity analysis confirmed the robustness and reliability of the conclusion (Supplementary Figure S2).
3.4.4 Based on BSA change
Two studies with 238 participants utilized the outcome measure of BSA to indicate the effects of TGP combined with conventional therapies (Lv, 2020; Song et al., 2017). Meta-analysis was conducted using a fixed-effects model, owing to no detected heterogeneity (I2 = 0% and P = 0.80). The results illustrated that combining TGP with conventional therapies was superior to conventional therapies used alone (MD = 2.12, 95% CI: 1.31 to 2.94, P < 0.00001) (Figure 7).
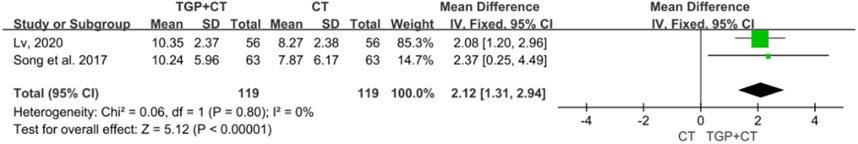
Figure 7. Meta-analysis based on BSA change. TGP, total glucosides of paeony; CT, conventional therapy; BSA, body surface area.
3.4.5 Based on inflammatory factor change
There were 10 studies with 882 participants mentioning the outcome measure of inflammatory factors. For significant heterogeneity, a random effects model was used for meta-analysis. The findings showed that when compared with conventional therapies used alone, TGP combined with conventional therapies increased the change in TNF-α (MD = 14.46, 95% CI: 8.83 to 20.09, P < 0.00001), IL-17 (MD = 13.64, 95% CI: 3.77 to 23.51, P = 0.007), IL-18 (MD = 3.85, 95% CI: -2.08 to 9.78, P = 0.20), and IL-2 (MD = 6.27, 95% CI: 1.76 to 10.79, P = 0.006) while decreasing in the change in INF-γ (MD = −8.12, 95% CI: -15.16 to −1.09, and P = 0.02) and IL-4 (MD = −2.33, 95% CI: -2.79 to −1.87, and P < 0.00001) (Figure 8). The symmetry observed in the funnel plot, combined with the result of the Egger’s test (P = 0.097 > 0.05), indicated the absence of publication bias (Supplementary Figure S3A). Sensitivity analysis confirmed the robustness and reliability of the conclusion (Supplementary Figure S3B), and the results of meta-regression revealed no source of heterogeneity (Supplementary Figure S3C).
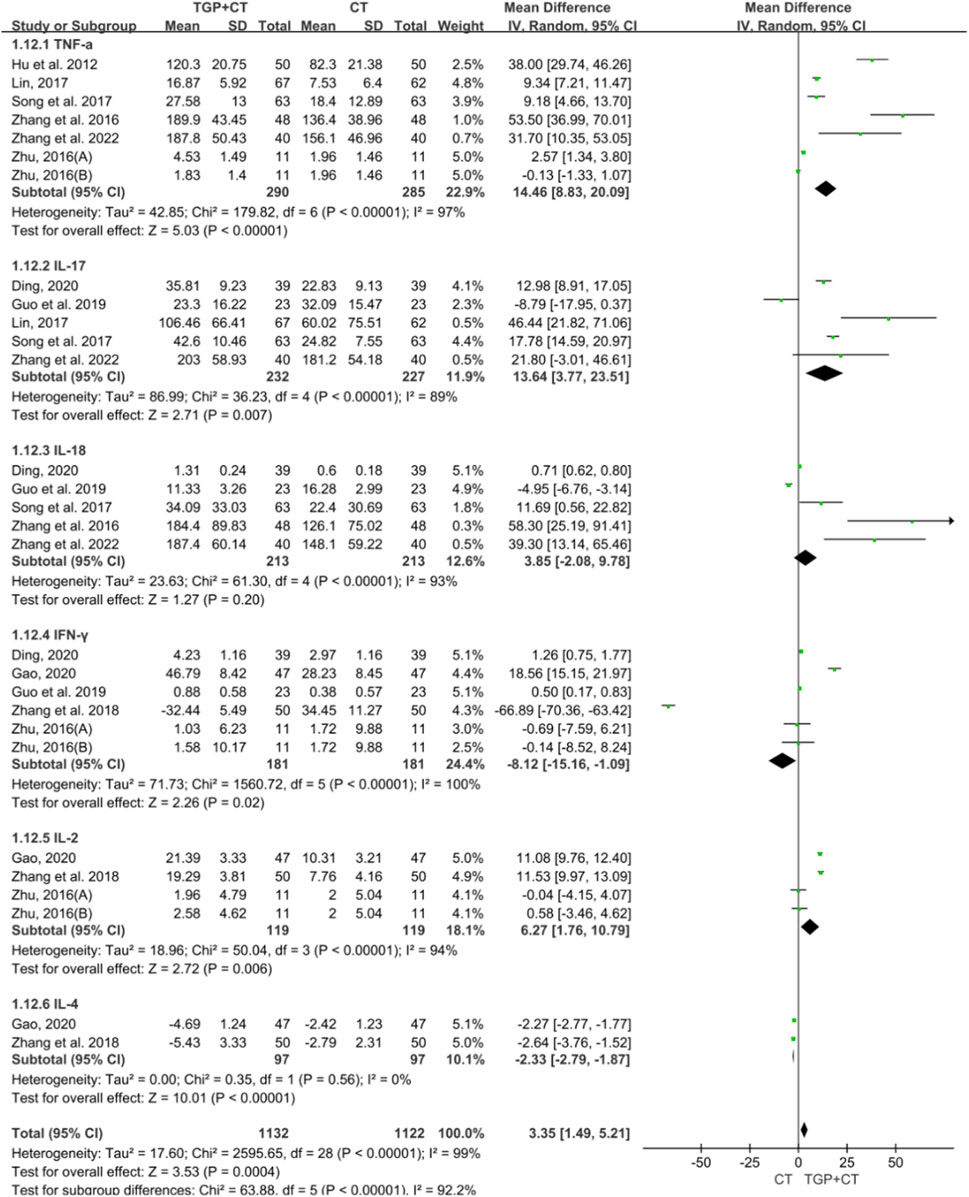
Figure 8. Meta-analysis based on inflammatory factor change. TGP, total glucosides of paeony; CT, conventional therapy; TNF-a, tumor necrosis factor-a; IL-17, interleukin-17; IL-18, interleukin-18; INF- Y interferon-y; IL-2, interleukin-2; IL-4, interleukin-4.
3.5 Meta-analysis of efficacy based on the treatment period
Meta-analysis was conducted based on the treatment durations of 6, 8, and 12 weeks using the PASI 60 outcome measures.
3.5.1 Treatment duration of 6 weeks
For a duration of 6 weeks or less, six studies with 436 participants reported the effects of TGP with conventional therapies (Hu et al., 2012a; Jiang et al., 2013; Liu et al., 2014; Liu and Wang, 2011; Zhu, 2016). A fixed-effects model was used for meta-analysis, owing to low heterogeneity (I2 = 0% and P = 0.89). The results indicated that TGP in combination with conventional therapies was superior to conventional therapies used alone (RR = 1.29, 95% CI: 1.15 to 1.44, and P < 0.00001) (Figure 9A). Sensitivity analysis showed the robustness and reliability of the conclusion (Supplementary Figure S4A).
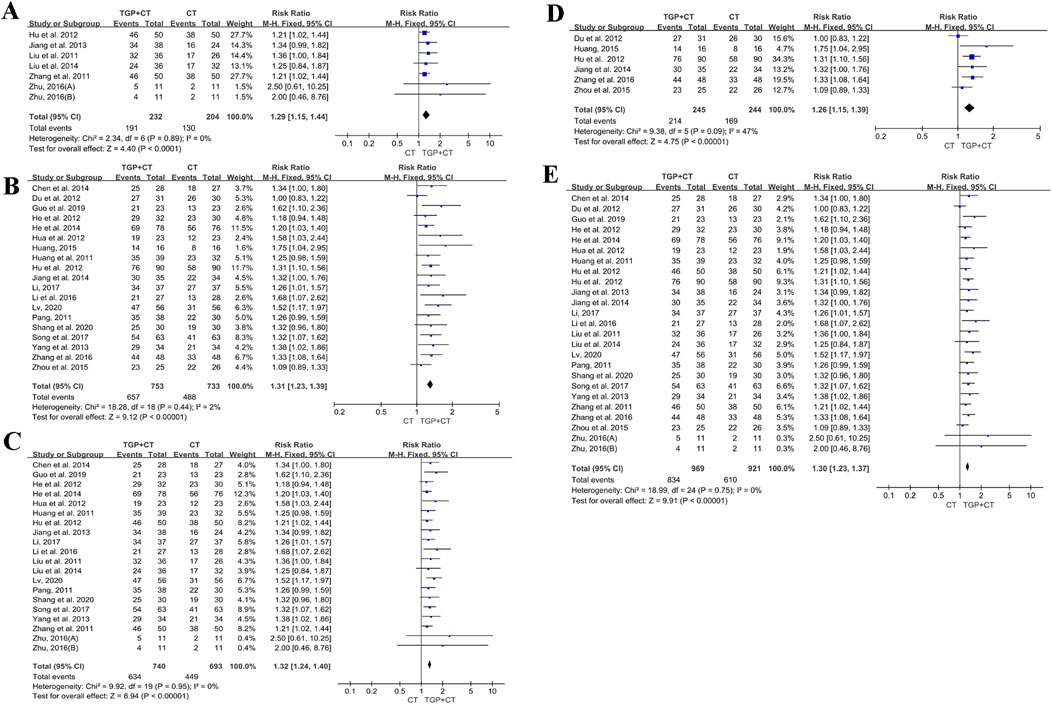
Figure 9. Meta-analysis based on treatment duration: (A) 6 weeks or less; (B) more than 6 weeks; (C) 8 weeks or less; (D) more than 8 weeks; and (E) 12 weeks or less. TGP, total glucosides of paeony; CT, conventional therapy.
For a duration of more than 6 weeks, 19 studies with 1,145 participants were enrolled to assess the efficacy of TGP with conventional therapies (Chen et al., 2014; Du et al., 2012; Guo and Xu, 2019; He et al., 2014; He et al., 2012; Hu et al., 2012b; Hua et al., 2012; Huang, 2015; Huang and Huang, 2011; Jiang et al., 2014; Li, 2017; Li et al., 2016; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Yang et al., 2013; Zhang et al., 2016; Zou et al., 2015). Meta-analysis was conducted using a fixed-effects model due to low heterogeneity (I2 = 2%, P = 0.44). The findings revealed that TGP in combination with conventional therapies was superior to conventional therapies alone (RR = 1.31, 95% CI: 1.23 to 1.39, P < 0.00001) (Figure 9B). No publication bias was detected for the symmetry of the funnel plot and the detected result of Egger’s test (P = 0.428 > 0.05) (Supplementary Figure S4B). Sensitivity analysis manifested the reliability of the conclusion (Supplementary Figures S4B, C), and the findings of meta-regression found no source of heterogeneity (Supplementary Figure S4D).
3.5.2 Treatment duration of 8 weeks
For a duration of 8 weeks or less, 19 studies with 1,433 participants reported the effects of TGP with conventional therapies (Chen et al., 2014; Guo and Xu, 2019; He et al., 2014; He et al., 2012; Hu et al., 2012a; Hua et al., 2012; Huang and Huang, 2011; Jiang et al., 2013; Li, 2017; Li et al., 2016; Liu et al., 2014; Liu and Wang, 2011; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Yang et al., 2013; Zhang et al., 2011; Zhu, 2016). Due to the observed heterogeneity, a fixed-effects model was used (I2 = 0%, P = 0.95). The findings showed a significant difference, favoring the group of TGP combined with conventional therapies (RR = 1.32, 95% CI: 1.24 to 1.40, P < 0.00001) (Figure 9C). No publication bias was detected, owing to the symmetry of the funnel plot and the results of Egger’s test (P = 0.054 > 0.05) (Supplementary Figure S5A). Sensitivity analysis revealed the stability of the conclusion (Supplementary Figure S5B), and the results of meta-regression indicated no source of heterogeneity (Supplementary Figure S5C).
For a duration of more than 8 weeks, six studies containing 489 participants were analyzed (Du et al., 2012; Hu et al., 2012b; Huang, 2015; Jiang et al., 2014; Zhang et al., 2016; Zou et al., 2015). A fixed-effect model was utilized for the detected heterogeneity (I2 = 47%, P = 0.09). The results indicated TGP with conventional therapies was superior when compared with conventional therapies alone (RR = 1.26, 95% CI: 1.15 to 1.39, P < 0.00001) (Figure 9D). Sensitivity analysis indicated the robustness and reliability of the conclusion (Supplementary Figure S5D).
3.5.3 Treatment duration of 12 weeks
For a duration of 12 weeks or less, 24 studies with a total of 1,579 participants were evaluated (Chen et al., 2014; Du et al., 2012; Guo and Xu, 2019; He et al., 2014; He et al., 2012; Hu et al., 2012a; Hu et al., 2012b; Hua et al., 2012; Huang and Huang, 2011; Jiang et al., 2013; Jiang et al., 2014; Li, 2017; Li et al., 2016; Liu et al., 2014; Liu and Wang, 2011; Lv, 2020; Pang, 2011; Shang et al., 2020; Song et al., 2017; Yang et al., 2013; Zhang et al., 2011; Zhang et al., 2016; Zou et al., 2015; Zhu, 2016). A fixed-effects model was selected for the low heterogeneity (I2 = 0% and P = 0.75). The results reported the superior effect of TGP combined with conventional therapies (RR = 1.30, 95% CI: 1.23 to 1.37, P < 0.00001) (Figure 9E). With the symmetry of the funnel plot and the findings of Egger’s test (P = 0.425 > 0.05), there was no publication bias (Supplementary Figure S6A). Sensitivity analysis proved the stability of the conclusion (Supplementary Figure S6B). Additionally, the results of meta-regression showed no source of heterogeneity (Supplementary Figure S6C). There was only one study (Huang, 2015) with a duration of more than 12 weeks.
3.6 Meta-analysis of safety
Meta-analysis of adverse events was evaluated on the basis of laboratory examination and clinical symptoms.
3.6.1 Adverse events based on laboratory examination
A total of 11 studies with 775 participants reported the occurrence of liver dysfunction (Yu et al., 2017; Chen et al., 2014; Du et al., 2012; Gao, 2020; Guo and Xu, 2019; He et al., 2012; Huang and Huang, 2011; Jiang et al., 2014; Zhang et al., 2016; Zhang et al., 2022; Zhu, 2016). Three studies with 201 participants reported the adverse event of leukocytopenia (Du et al., 2012; Huang and Huang, 2011; Jiang et al., 2014), while two studies containing 129 participants reported hyperlipidemia (Zhang et al., 2016; Zhu, 2016). Given low heterogeneity, a fixed-effects model was selected for meta-analysis. The findings revealed significant differences, favoring the conventional therapy group in liver dysfunction (RR = 0.03, 95% CI: 0.17 to 0.53, P < 0.00001), hyperlipidemia (RR = 0.20, 95% CI: 0.05 to 0.78, P = 0.02), and leukocytopenia (RR = 0.20, 95% CI: 0.01 to 4.06, P = 0.29) (Figure 10). Concerning liver dysfunction, no publication bias was found according to the symmetry of the funnel plot and the detected result of Egger’s test (P = 0.766 > 0.05) (Supplementary Figure S7A). Sensitivity analysis manifested the robustness of the conclusion (Supplementary Figure S7B), and no source of heterogeneity was observed by meta-regression (Supplementary Figure S7C).
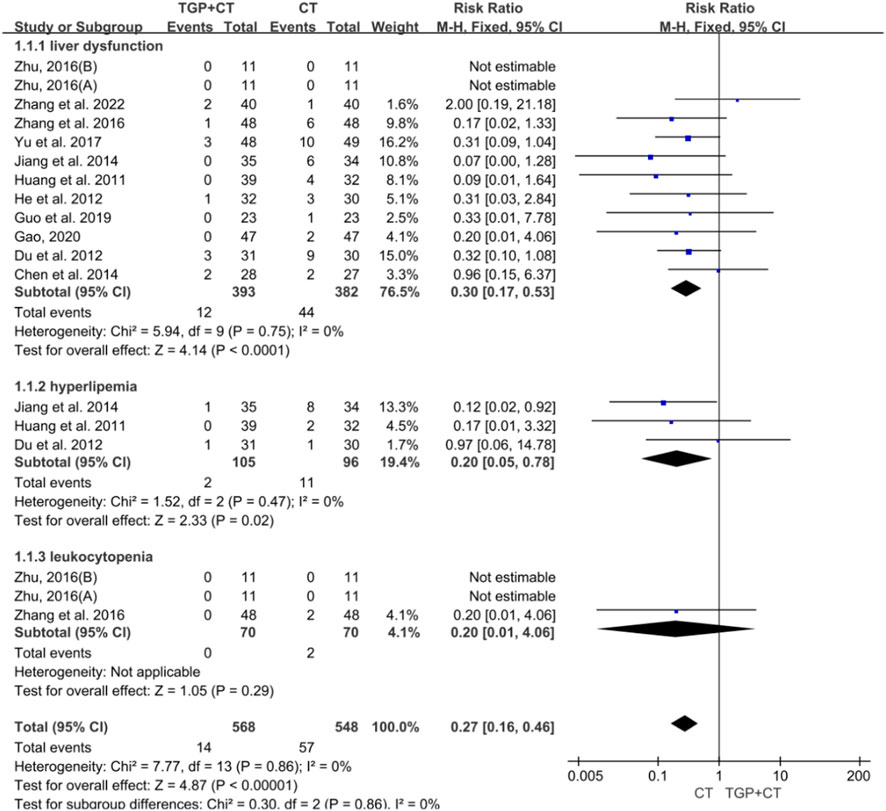
Figure 10. Meta-analysis of adverse events based on laboratory examination. TGP, total glucosides of paeony; CT, conventional therapy.
3.6.2 Adverse events based on clinical symptoms
Two studies with 113 participants reported the occurrence of upper respiratory infection (Zhang et al., 2022; Zhu, 2016), while 18 studies involving 1,352 participants mentioned the adverse event of gastrointestinal reactions (Cai et al., 2014; Chen et al., 2014; Gao, 2020; Guo, 2016; He et al., 2014; Huang, 2015; Hu et al., 2012b; Liu et al., 2014; Liu and Wang, 2011; Pang, 2011; Shang et al., 2020; Yang et al., 2013; Zhang et al., 2015; Zhang et al., 2016; Zhang et al., 2022; Zhang et al., 2011; Zhu, 2016; Zou et al., 2015). There were 16 studies with 1,041 participants that mentioned cutaneous reactions (Du et al., 2017; Du et al., 2012; Gao, 2020; Guo and Xu, 2019; Guo, 2016; He et al., 2014; Huang, 2015; Jiang et al., 2014; Jiang et al., 2013; Li, 2017; Liu et al., 2014; Pang, 2011; Yang et al., 2013; Zhang et al., 2011; Zou et al., 2015), while six studies with 387 participants mentioned drying reactions (Chen et al., 2014; Du et al., 2012; Gao, 2020; Guo and Xu, 2019; He et al., 2012; Jiang et al., 2014). For no detected significant heterogeneity, a fixed-effects model was used for meta-analysis as follows: upper respiratory infection (RR = 1.00, 95% CI: 0.31 to 3.27, P = 1.00), gastrointestinal reaction (RR = 3.18, 95% CI: 1.86 to 5.43, P < 0.00001), cutaneous reaction (RR = 0.52, 95% CI: 0.39 to 0.69, P < 0.00001), and drying reaction (RR = 0.69, 95% CI: 0.54 to 0.88, P = 0.003) (Figure 11). Concerning gastrointestinal reactions, no obvious publication bias was detected for the symmetrical funnel plot and the detected result of Egger’s test (P = 0.440 > 0.05) (Supplementary Figure S8A). Sensitivity analysis indicated the reliability of the conclusion (Supplementary Figure S8B), and the findings of meta-regression showed no source of heterogeneity (Supplementary Figure S8C). Concerning cutaneous reactions, the results of the symmetrical funnel plot and Egger’s test (P = 0.613 > 0.05) indicated no obvious publication bias (Supplementary Figure S8D). Sensitivity analysis confirmed the stability of above findings (Supplementary Figure S8E). Moreover, no source of heterogeneity was indicated by meta-regression (Supplementary Figure S8F).

Figure 11. Meta-analysis of adverse events based on clinical symptoms. TGP, total glucosides of paeony; CT, conventional therapy.
4 Discussions
4.1 Summary of the findings
The aim of this systematic review and meta-analysis was to critically elucidate the add-on effects of TGP on conventional therapies in the treatment of psoriasis. A total of 328 relevant studies were retrieved from seven databases, and 36 RCTs with 3,140 participants were carefully included. In terms of efficacy, the findings indicated that TGP combined with conventional therapies superior to conventional therapies used alone, with outcome measures of PASI 75 (RR = 1.24, 95% CI: 1.03 to 1.50, P = 0.02), PASI 60 (RR = 1.32, 95% CI: 1.25 to 1.39, P < 0.00001), and PASI 50 (RR = 1.44, 95% CI: 1.13 to 1.84, P = 0.004). Surprisingly, when compared with conventional therapies used alone, the combination of TGP with different types of conventional therapies, such as oral medication (RR = 1.40, 95% CI: 1.14 to 1.71, P = 0.001), topical medication (RR = 1.47, 95% CI: 1.24 to 1.74, P < 0.00001), and NB-UVB (RR = 1.29, 95% CI: 1.16 to 1.43, P < 0.00001), showed higher efficacy. In terms of BSA, combining TGP with conventional therapies was superior to conventional therapies used alone (RR = 2.12, 95% CI: 1.31 to 2.94, P < 0.00001). In terms of inflammatory factors, TGP combined with conventional therapies showed significant differences in TNF-α, IL-17, IL-18, IL-2, INF-γ, and IL-4 compared to conventional therapies used alone. In the terms of treatment duration, there were significant differences in the treatment of TGP combined with conventional therapies at 6, 8, and 12 weeks compared with conventional therapies used alone. These findings demonstrated that TGP shows potential to improve the efficacy of conventional medicines.
In terms of adverse events, a total of 31 studies mentioned the occurrence of adverse events when treated with TGP and conventional therapies for psoriasis. Concerning the adverse events of laboratory examination, differences were detected, favoring the conventional therapy group in liver dysfunction, hyperlipidemia, and leukocytopenia. Concerning adverse events of clinical symptoms, there were differences favoring TGP combined with conventional therapies in relieving cutaneous and drying reactions. Additionally, differences were detected favoring the conventional therapy group in the gastrointestinal reaction, and there was no difference observed in the upper respiratory infection.
4.2 Potential therapeutic mechanisms of TGP on conventional therapies
Similar to the findings of this study, experimental research studies indicated that TGP can eliminate the epidermal layer thickness and inhibit skin inflammation infiltration and angiogenesis in the imiquimod-induced psoriasis-like mouse models (Zhao et al., 2024). Published animal experiments showed that TGP could regulate skin inflammation in psoriasis-like mouse models and is induced by imiquimod by activating psoriatic dermal mesenchymal stem cells and modulating vascular endothelial growth factor (VEGF) expression (Lei et al., 2023; Zhang et al., 2021). Additionally, TGP could inhibit the overactivation of keratinocytes and the differentiation of T helper 17 (TH17) cells, markedly downregulating the gene expression of Th17-related cytokines (Zhang et al., 2023; Li et al., 2019). Paeoniflorin, the active ingredient of TGP, prominently restrained the proinflammatory cytokines and effector molecules such as macrophage inflammatory protein-2 (MIP-2), inducible nitric oxide synthase (iNOS), and TNF-α by macrophages and neutrophils (Zhang and Wei, 2019; Sun et al., 2015). Overall, the available evidence reveals that TGP effectively improves imiquimod-induced psoriatic skin lesions at immune cell, keratinocyte, and dermal mesenchymal stem cell levels by regulating immune imbalance, inducing inflammatory response, and modulating angiogenesis. However, as for the add-on effect of TGP on conventional therapies, there is still a lack of relevant experimental research on elucidating the underlying mechanisms of action. Previous clinical studies suggested that when compared with conventional therapies used alone, such as NB-UVB, cyclosporine, acitretin capsule, and tacrolimus ointment, the inclusion of TGP could reduce the proinflammatory factor levels of IL-17, IL-23, IL-4, and IFN-γ to improve the progression of psoriasis (Ren and Zhao, 2021; Yi, 2015; Lin et al., 2022; Ding, 2020; Griffiths et al., 2021).
Concerning safety, the combined application of TGP proved beneficial in reducing the adverse events related to acitretin, such as dry skin and liver dysfunction (Yu et al., 2017; Du et al., 2012; Huang and Huang, 2011; Zhang et al., 2016; Zou et al., 2015), owing to the immunoregulatory effect of TGP (Zhang and Wei, 2019). In addition, conventional therapy of NB-UVB might cause adverse events of cutaneous pruritus and erythema during the treatment of psoriasis (Du et al., 2017; Guo, 2016; Jiang et al., 2013), and the combined use of TGP, which has anti-inflammatory effect, may alleviate these conditions (Zhang and Wei, 2019). Noteworthily, gastrointestinal disorders such as diarrhea and abdominal discomfort were more likely to occur when TGP was combined, possibly due to the TGP administration at high dose (Zhu, 2016; Zhang et al., 2022; Pang, 2011). Thus, more attention should be paid to administering the application TGP, including dosage, frequency, and duration. In general, it was believed that TGP had a safety stable profile when combined with conventional therapies in treating psoriasis.
4.3 Strengths of this study compared with the previous study
According to the Cochrane Library’s guidelines (Karunananthan et al., 2020; Smith and Ho, 2023), systematic reviews and meta-analyses for a specific treatment should be updated periodically to provide clinicians with the most recent and higher-quality clinical evidence. Compared with the previously published article (Zheng et al., 2019), the strengths of this article lie in the following points: first, to assess efficacy, this study employs validated outcome measures recognized by international guidelines, such as PASI 75, PASI 50, and BSA (Elmets et al., 2021; Nast et al., 2018; Nast et al., 2020; Psoriasis Committee, Dermatology and Venereology Branch, Chinese Medical Association, 2023). Second, this study incorporated the evaluation of inflammatory cytokines from preclinical studies, which are closely related to the progression of psoriasis (Griffiths et al., 2021). Third, to assess safety, this study categorized adverse events into two distinct classes, namely, laboratory examination and clinical symptoms, which facilitated a comprehensive, multi-dimensional observation of adverse reactions, following the treatment of TGP with conventional therapies. Additionally, regarding risk bias assessment, this study employed the latest RoB 2.0 tool published by the Cochrane Library, providing a broader and more comprehensive scope to furnish supplementary details of bias risks derived from the included RCTs (Nejadghaderi et al., 2024; Sterne et al., 2019).
4.4 Limitations to this study
Although rigorous methodology was utilized for data analysis, there were still limitations that needed to be addressed. On one hand, due to incomplete raw data, the methodological quality of the majority of the included studies was generally poor, which may have an impact on the accurate interpretation of this systematic review and meta-analysis. On the other hand, included studies with small sample sizes and single research center somewhat limited the universality of the findings. In addition, there might be a language bias, owing to initial studies that only search English and Chinese databases.
4.5 Implications for future studies
Based on the abovementioned findings, strengths, and limitations of this study, suggestions for future clinical practice and experimental research are made. First of all, rigorously designed RCTs accompanied by multiple centers and enlarged sample sizes are urgently needed to promote the evidence in support of TGP combined with conventional therapies for psoriasis. Subsequently, the standard guidelines of Consolidated Standards of Reporting Trials (CONSORT) should be strictly adhered to throughout the process of RCTs, especially during the implementation of randomization, blinding, and allocation concealment. Moreover, in order to fully demonstrate the underlying mechanisms of action of TGP combined with conventional therapies for psoriasis, intensive experimental research from different molecular, cell, and tissue levels is required. This could provide a novel optimized therapeutic strategy for psoriasis and promote the evidence for the application of conventional therapies and TGP. Further explorations on optimal treatment administration and long-term safety profile assessment could potentially provide useful guidance for future studies.
5 Conclusion
This systematic review and meta-analysis demonstrated several therapeutic sources of evidence supporting the addition of TGP to conventional therapies in treating psoriasis. Additionally, when combined with TGP, conventional therapies such as acitretin capsule and NB-UVB have been shown to improve clinical efficacy and reduce the incidence of adverse events. However, the limited methodological quality and small sample sizes of included studies may impede the accurate interpretation of the findings. To better clarify the add-on effects of TGP on conventional therapies for psoriasis, comprehensively designed and large-scale research studies are further needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Author contributions
ZL: conceptualization, data curation, formal analysis, methodology, writing–original draft, and writing–review and editing. JL: data curation, formal analysis, investigation, methodology, and writing–original draft. KG: data curation, formal analysis, investigation, methodology, validation, and writing–original draft. HL: data curation, formal analysis, investigation, methodology, validation, and writing–original draft. CL: conceptualization, project administration, supervision, validation, visualization, and writing–original draft. JY: conceptualization, funding acquisition, supervision, validation, visualization, writing–original draft, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants of Research Fund for Bajian Talents of Guangdong Provincial Hospital of Chinese Medicine (No. BJ2022KY17), the Specific Clinical Research Project of Guangdong Provincial Hospital of Chinese Medicine (No. YN10101909), Guangzhou Science and Technology Planning Project (No. 202206080006), and the 2022 TCM Innovation Team and Talent Support Program of the State Administration of Traditional Chinese Medicine (No. ZYYCXTD - C - 202204).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1527288/full#supplementary-material
References
Amin, M., Lee, E. B., Tsai, T. F., and Wu, J. J. (2020). Psoriasis and comorbidity. Acta Derm. Venereol. 100 (3), adv00033. doi:10.2340/00015555-3387
Bardazzi, F., Bonci, C., Sacchelli, L., DI Altobrando, A., Iommi, M., Rucci, P., et al. (2022). Suicide risk and depression in patients with psoriasis. Ital. J. Dermatol Venerol. 157 (6), 497–501. doi:10.23736/S2784-8671.22.07184-5
Bell, K. A., Balogh, E. A., and Feldman, S. R. (2021). An update on the impact of depression on the treatment of psoriasis. Expert Opin. Pharmacother. 22 (6), 695–703. doi:10.1080/14656566.2020.1849141
Boehncke, W. H., Boehncke, S., and Schon, M. P. (2010). Managing comorbid disease in patients with psoriasis. Bmj 340, b5666. doi:10.1136/bmj.b5666
Cai, Y., Luo, M., Mi, X., Qiu, X., Sun, L., and Zhang, T. (2014). Effects of total glucosides of paeonia on PASI and biochemical markers of atherosclerosis in patients with psoriasis vulgaris. J. Diagnosis Ther. Dermato-venereology. 21 (02), 112–115. doi:10.3969/j.issn.1674-8468.2014.02.006
Chen, X., Ouyang, W., Wu, J., Weng, Q., and Chen, S. (2014). Clinical observation of total glucosides of paeon combined with acitretin and narrow band ultraviolet B in the treatment of moderate to severe psoriasis vulgaris. J. Pract. Dermatology 7 (01), 19–21. doi:10.11786/sypfbxzz.1674-1293.20140107
Chia, B., Yew, Y. W., Zhao, X., Chong, W. S., and Thng, T. (2023). Incidence of skin malignancies in patients with vitiligo or psoriasis who received narrowband ultraviolet b phototherapy (308 nm/311 nm): a retrospective review of 3730 patients. Photodermatol. Photoimmunol. Photomed. 39 (4), 343–350. doi:10.1111/phpp.12844
Ding, X. (2020). Effects of total glucosides of paeon capsule combined with tacrolimus ointment on patients suffering from psoriasis as well as on interleukin-17, interleukin-18 and interferon-γ. China's Naturop. 28 (18), 64–65. doi:10.19621/j.cnki.11-3555/r.2020.1829
Du, A., Pan, L., Li, B., and Zhang, H. (2017). Clinical experience of calcipotriol betamethasone ointment combined with total glucosides of paeony capsule in the treatment of psoriasis vulgaris. Nei Mongol J. Traditional Chin. Med. 36 (12), 79.
Du, Y., Zhong, G., Liao, Y., Yan, D., and Xiong, X. (2012). Clinical observation of total glucosides of paeony combined with acitretin in the treatment of generalized pustular psoriasis. China J. Lepr. Skin Dis. 28 (02), 98–100. doi:10.3969/j.issn.1009-1157.2012.02.013
Elmets, C. A., Korman, N. J., Prater, E. F., Wong, E. B., Rupani, R. N., Kivelevitch, D., et al. (2021). Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J. Am. Acad. Dermatol 84 (2), 432–470. doi:10.1016/j.jaad.2020.07.087
Gao, Y. (2020). Clinical efficacy of acitretin combined with total glucosides of paeony in the treatment of psoriasis. J. Med. Syst. 5 (10), 145–147. doi:10.19368/j.cnki.2096-1782.2020.10.145
Griffiths, C., Armstrong, A. W., Gudjonsson, J. E., and Barker, J. (2021). Psoriasis. Lancet 397 (10281), 1301–1315. doi:10.1016/S0140-6736(20)32549-6
Guo, H., and Xu, X. (2019). Effects of total glucosides of paeonycapsule as an adjuvant therapy on patients with psoriasis as well as on the levels of serum IL-17, IL-18 and INF-γ. Chin. J. Sch. Physician. 33 (09), 663–664.
Guo, Y. (2016). Total glucosides of paeony combined with the effcacy of narrow-band UVB phototherapy in the treatment of psoriasis vulgaris. China Contin. Med. Educ. 8 (33), 180–181. doi:10.3969/j.issn.1674-9308.2016.33.104
He, C., Wu, A., Zhang, L., Long, Z., Yang, G., Sun, Y., et al. (2014). Efficacy of total glucosides of paeony capsule combined with NB-UVB and calcipotriol in 78 patients with moderate psoriasis vulgaris. Chin. J. Dermatovenereology 28 (08), 873–875. doi:10.13735/j.cjdv.1001-7089.2014.0873
He, Y., Zhong, G., Li, C., and Xiong, X. (2012). A clinical study of total glucosides of paeon combined with acitretin in 32 patients with moderate to severe psoriasis. Chin. J. Dermatovenereology 26 (02), 185–186.
Hu, Y., Wang, B., Qu, W., Gao, J., and Dai, S. (2012a). Clinical efficacy of NB-UVB with Total Glucosides of Paeon in treatment of psoriasis vulgaris and the effect on the levels of serum TNF-α. Osteopontin IL-6. China Mod. Dr. 50 (03), 53–55. doi:10.3969/j.issn.1673-9701.2012.03.023
Hu, Y., Wang, B., Qu, W., Gao, J., and Dai, S. (2012b). Effect of total glucoside of peony combined with small dose of Tripterygium wilfordii Hook.f in the treatment of psoriasis vulgaris. J. Chin. PLA Postgrad. Med. Sch. 33 (07), 733–735.
Hua, H., Gu, L., Ren, Q., and Zhu, Z. (2012). Total glucosides of paeony associated with compound glycyrrhizin in 23 patients suffering from psoriasis vulgari. Med. J. Commun. 26 (03), 257–258. doi:10.3969/j.issn.1006-2440.2012.03.022
Huang, A. (2015). The effect of total glucosides of paeony on the level of IL-8 and TNF-α as well as the clinical curative effect on patients with psoriasis. J. Dermatology Venereol. 37 (03), 133–135. doi:10.3969/j.ssn.1002-1310.2015.03.004
Huang, F., and Huang, Z. (2011). Efficacy observation of total glucosides of paeony combined with acitretin in the treatment of psoriasis vulgaris. China J. Lepr. Skin Dis. 27 (05), 363. doi:10.3969/j.issn.1009-1157.2011.05.037
Jiang, H., Shi, W., Sun, Z., Zhang, S., Liu, P., and Chen, H. (2014). Observation of efficacy of total glucosides of paeon combined with acitretin in 35 patiens with plaque psoriasis. Chin. J. Dermatovenereology 28 (03), 325–326. doi:10.13735/j.cjdv.1001-7089.2014.0325
Jiang, H., Yu, Y., and Fan, T. (2013). Clinical efficacy of narrow band ultraviolet B combined with total glucosides of paeony in treating psoriasis vulgaris. Med. J. Liaoning 27 (02), 81–82.
Karunananthan, S., Maxwell, L. J., Welch, V., Petkovic, J., Pardo, J. P., Rader, T., et al. (2020). PROTOCOL: when and how to replicate systematic reviews. Campbell Syst. Rev. 16 (2), e1087. doi:10.1002/cl2.1087
Katz, H. I., Waalen, J., and Leach, E. E. (1999). Acitretin in psoriasis: an overview of adverse effects. J. Am. Acad. Dermatol 41, S7-S12–S12. doi:10.1016/s0190-9622(99)70359-2
Kommoss, K. S., Enk, A., Heikenwalder, M., Waisman, A., Karbach, S., and Wild, J. (2023). Cardiovascular comorbidity in psoriasis - psoriatic inflammation is more than just skin deep. J. Dtsch. Dermatol Ges. 21 (7), 718–725. doi:10.1111/ddg.15071
Lei, M. J., Bai, F., Zhang, Q. Y., Yang, Q. Q., and Tian, Z. (2023). Total glucosides of paeony regulate immune imbalance mediated by dermal mesenchymal stem cells in psoriasis mice. Chin. J. Integr. Med. 29 (6), 517–525. doi:10.1007/s11655-023-3737-y
Li, B., He, S., Liu, R., Huang, L., Liu, G., Wang, R., et al. (2019). Total glucosides of paeony attenuates animal psoriasis induced inflammatory response through inhibiting stat1 and stat3 phosphorylation. J. Ethnopharmacol. 243, 112121. doi:10.1016/j.jep.2019.112121
Li, J. (2017). Clinical effect of narrow band ultraviolet B combined withTotal glucosides of paeony for psoriasis vulgaris. J. Med. Theory Pract. 30 (11), 1636–1638. doi:10.19381/j.issn.1001-7585.2017.11.042
Li, X., Li, Z., and Li, X. (2016). Curative effect of total glucosides of paeony capsule combined with narrow-band ultraviolet B in the treatment of psoriasis vulgaris and the effect on the level of T cell. Chin. J. Dermatovenereology 30 (11), 1205–1207. doi:10.13735/j.cjdv.1001-7089.201605118
Lin, J. (2017). Effect of total glucosides of paeony capsule combined with carpotriol ointment in the treatment of psoriasis vulgaris and the effect on serum levels of inflammatory cytokines. J. Med. Theory Pract. 30 (13), 1959–1960. doi:10.19381/j.issn.1001-7585.2017.13.045
Lin, L., Ren, W., and Gao, H. (2022). Clinical efficacy of total paeoniflorin capsules combined with Avi A capsule for psoriasis of blood deficiency syndrome and how it impacts on T helper 17 cells, regulatory T cells and inflammatory factors. Hebei J. Traditional Chin. Med. 44 (09), 1498–1501+1506.
Liu, L., Zhang, X., and Li, Z. (2014). Clinical observation of total glucosides paeony combined with tretinoin and momestasone furoate in 36 patients with psoriasis vulgaris. Chin. J. Dermatovenereology 28 (10), 1097–1098. doi:10.13735/j.cjdv.1001-7089
Liu, Y., and Wang, L. (2011). Clinical observation on total glucosides paeony combined with compound glycyrrhizin in the treatment of psoriasis vulgaris. Chin. J. Dermatovenerology Integr. Traditional West. Med. 10 (02), 118–119. doi:10.3969/j.issn.1672-0709.2011.02.022
Lv, X. (2020). Observation of the efficacy of total glucosides of paeony capsule in the treatment of psoriasis vulgaris. J. Dermatology Venereol. 42 (05), 689–691. doi:10.3969/j.issn.1002-1310.2020.05.028
Mayor, S. (2016). Psoriasis is associated with increased risk of abdominal aortic aneurysm, study finds. Bmj 353, i2159. doi:10.1136/bmj.i2159
Nast, A., Amelunxen, L., Augustin, M., Boehncke, W. H., Dressler, C., Gaskins, M., et al. (2018). S3 guideline for the treatment of psoriasis vulgaris, update - short version part 2 - special patient populations and treatment situations. J. Dtsch. Dermatol Ges. 16 (6), 806–813. doi:10.1111/ddg.13538
Nast, A., Smith, C., Spuls, P. I., Avila, V. G., Bata-Csorgo, Z., Boonen, H., et al. (2020). Euroguiderm guideline on the systemic treatment of psoriasis vulgaris - part 1: treatment and monitoring recommendations. J. Eur. Acad. Dermatol Venereol. 34 (11), 2461–2498. doi:10.1111/jdv.16915
Nejadghaderi, S. A., Balibegloo, M., and Rezaei, N. (2024). The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: a perspective on the pros and cons. Health Sci. Rep. 7 (6), e2165. doi:10.1002/hsr2.2165
Pang, L. (2011). Clinical obervation of the efficacy of total glucosides of paeony capsule combined with narrow band ultraviolet(NB-UVB) in the treatment of psoriasis vulgaris. World J. Chin. Med. 26 (02), 223–224. doi:10.16368/j.issn.1674-8999.2011.02.056
Psoriasis Committee, Dermatology and Venereology Branch, Chinese Medical Association (2023). Diagnosis and treatment guideline of psoriasis in China (2023 edition). Chin. J. Dermatovenereology 56 (7), 573–625. doi:10.35541/cjd.20220839
Ren, G., and Zhao, Y. (2021). Effects of total glucosides of paeony combined with NB-UVB on the expression of serum IL-17, IL-23 and lesion PAR-2 in patients with moderate to severe psoriasis. China J. Lepr. Skin Dis. 37 (08), 506–509.
Shang, F., Zhao, J., Wang, H., Xue, X., Ji, S., and He, H. (2020). Effect of total glucosides of paeony on MIP-3α of the peripheral blood in patients with psoriasis vulgaris. J. Binzhou Med. Univ. 43 (04), 315–317. doi:10.19739/j.cnki.issn1001-9510.2020.04.022
Smith, G. D., and Ho, K. H. M. (2023). Systematic reviews: when should they be updated? J. Clin. Nurs. 32 (9-10), e17–e18. doi:10.1111/jocn.16547
Song, L., Zhou, N., Wang, M., Min, W., Liu, M., Chen, A., et al. (2017). Clinical effects of total glucosides of paeony capsules combined with acitretin and compound flumethasone for psoriasis vulgaris. J. Med. Postgraduates 30 (08), 854–857. doi:10.16571/j.cnki.1008-8199.2017.08.014
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sun, Y., Zhang, J., Huo, R., Zhai, T., Li, H., Wu, P., et al. (2015). Paeoniflorin inhibits skin lesions in imiquimod-induced psoriasis-like mice by downregulating inflammation. Int. Immunopharmacol. 24 (2), 392–399. doi:10.1016/j.intimp.2014.12.032
Thatiparthi, A., Martin, A., Liu, J., and Wu, J. J. (2022). Risk of skin cancer with phototherapy in moderate-to-severe psoriasis: an updated systematic review. J. Clin. Aesthet. Dermatol. 15 (6), 68–75.
Wang, H. (2016). Observation of Curative Effect of total glucosides of paeony combined with compound flumetasone ointment in the treatment of Psoriasis Vulgaris. Henan Med. Res. 25 (09), 1680–1681. doi:10.3969/j.issn.1004-437X.2016.09.105
Yang, H., Xu, X., Jiang, Y., and Wan, Y. (2013). Clinical observation of Total Glucosides of Paeony with narrowband ultraviolet B in the treatment of psoriasis vulgaris. J. Traditional Chin. Med. Univ. Hunan 33 (08), 60–61. doi:10.3969/j.issn.1674-070X.2013.08.029.060.03
Yi, X. (2015). Effect of total glucosides of paeony combined with cyclosporin A on IL-4 and IL-8 for psoriasis. Mod. Diagnosis Treat. 26 (19), 4413.
Yu, C., Fan, X., Li, Z., Liu, X., and Wang, G. (2017). Efficacy and safety of total glucosides of paeony combined with acitretin in the treatment of moderate-to-severe plaque psoriasis: a double-blind, randomised, placebo-controlled trial. Eur. J. Dermatol 27 (2), 150–154. doi:10.1684/ejd.2016.2946
Zhang, J., Li, H., Jiang, F., Sun, X., Li, T., and Xu, L. (2015). Effect of long-term treatment of total glucosides of paeong treatment on clinical efficacy and recurrence in patients with psoriasis vulgaris. Chin. J. Dermatovenerology Integr. Traditional West. Med. 14 (06), 371–373. doi:10.3969/j.issn.1672-0709.2015.06.011
Zhang, L., Bu, N., Sun, S., Dong, X., Jiao, J., Zhang, X., et al. (2023). Effects of total glucosides of paeony on inflammatory response and apoptosis of keratinocyte. J. Shandong Univ. Traditional Chin. Med. 47 (04), 471–479. doi:10.16294/j.cnki.1007-659x.2023.04.015
Zhang, L., Chen, Q., and Zeng, T. (2016). Clinical observation of total glucosides of paeony combined with methotrexate in treatment of severe psoriasis vulgaris. Drugs & Clin. 31 (09), 1459–1462. doi:10.7501/j.issn.1674-5515.2016.09.033
Zhang, L., and Wei, W. (2019). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 207, 107452. doi:10.1016/j.pharmthera.2019.107452
Zhang, R., Zhang, X., and Li, N. (2022). Effects of total glucosides of paeony in the treatment of severe psoriasis and on the serum levels of TNF-α, IL-17, and IL-18. Asia-Pacific Tradit. Med. 18 (10), 104–108. doi:10.11954/ytctyy.202210022
Zhang, W., and Wang, X. (2018). Curative effect of acitretin and total glucosides of paeony capsule combined with narrow-band ultraviolet B in the treatment of psoriasis. Chin. J. Dermatovenerology Integr. Traditional West. Med. 17 (01), 38–40. doi:10.3969/j.issn.1672-0709.2018.01.013
Zhang, Y., Guo, Z., Jiao, X., and Chen, T. (2011). Clinical observation of total glucosides of paeony in the treatment of psoriasis vulgaris. J. Clin. Dermatology 40 (07), 433–435.
Zhang, Y., Zhou, X., and Sun, L. (2021). Effect of total glucosides of paeony on imiquimod-induced psoriatic skin lesions by regulating VEGF. Clin. Cosmet. Investig. Dermatol 14, 1889–1897. doi:10.2147/CCID.S339627
Zhao, M., Peng, N., Zhou, Y., Qu, Y., Cao, M., Zou, Q., et al. (2024). The immunoregulatory effects of total glucosides of paeony in autoimmune diseases. J. Leukoc. Biol. 16, qiae095. doi:10.1093/jleuko/qiae095
Zheng, Q., Jiang, W., Sun, X., Ma, T., Xu, W., Shen, F., et al. (2019). Total glucosides of paeony for the treatment of psoriasis: a systematic review and meta-analysis of randomized controlled trials. Phytomedicine 62, 152940. doi:10.1016/j.phymed.2019.152940
Zhu, K. (2016). Preliminary study of total glucosides of paeony application in the treatment of psoriasis vulgaris. China: Nanchang University.
Keywords: total glucosides of paeony, conventional therapies, psoriasis, effect, meta-analysis
Citation: Li Z, Lu J, Guan K, Liang H, Lu C and Yu J (2025) Add-on effects of total glucosides of paeony on conventional therapies for psoriasis: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 16:1527288. doi: 10.3389/fphar.2025.1527288
Received: 13 November 2024; Accepted: 22 January 2025;
Published: 19 February 2025.
Edited by:
Rong-Rong He, Jinan University, ChinaReviewed by:
Jiao Wang, Shanghai University of Traditional Chinese Medicine, ChinaYang Lo, Cathay General Hospital, Taiwan
Copyright © 2025 Li, Lu, Guan, Liang, Lu and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjie Yu, amluZ2ppZXl1QGd6dWNtLmVkdS5jbg==; Chuanjian Lu, bGNqQGd6dWNtLmVkdS5jbg==
 Ziqing Li
Ziqing Li Jie Lu
Jie Lu Kewen Guan1
Kewen Guan1 Haiwen Liang
Haiwen Liang Jingjie Yu
Jingjie Yu