
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 19 February 2025
Sec. Inflammation Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1521743
 Ang Li1†
Ang Li1† Yanjie Liu2†
Yanjie Liu2† Peiling Yu3†
Peiling Yu3† Zhiyuan Zhang4
Zhiyuan Zhang4 Tengxiao Huang5
Tengxiao Huang5 Hang Li5
Hang Li5 Songzhi Wu1
Songzhi Wu1 Xiaoyu Rong1
Xiaoyu Rong1 Wensheng Liao1
Wensheng Liao1 Hongqiang Wang1
Hongqiang Wang1 Yanzheng Gao1*
Yanzheng Gao1*Introduction: The underlying cause of psoriasis, a chronic inflammatory skin condition driven by an immune response, remains a topic of active investigation and is not yet fully elucidated. Recent studies have revealed that ergothioneine, a small molecule sulfur-containing histidine derivative that can be ingested from the daily diet and accumulated in the body, exhibits antioxidant capacity that is comparable to that of glutathione. Nevertheless, there is a paucity of empirical data concerning the precise impact of ergothioneine in the context of anti-inflammatory processes, particularly in the context of psoriasis. In the light of the aforementioned considerations, the present study was undertaken with the objective of conducting a comprehensive evaluation of the anti-inflammatory potential of ergothioneine (EGT) and to investigate its potential impact on the pathogenesis of psoriasis.
Methods:: The efficacy of EGT in reducing the extent of dorsal skin lesions in psoriasis model mice was confirmed through in vivo experimental observation. Furthermore, the inhibitory effect of EGT on inflammatory responses at the cellular level was investigated, specifically in LPS-induced mouse macrophage (RAW264.7) and human keratinocyte-forming cell (HaCaT) models.
Results: The results demonstrated that the introduction of different concentrations of EGT into the LPS-induced inflammatory cell model resulted in notable anti-inflammatory effects, as evidenced by a reduction in inflammatory responses and a dose dependent decline in the concentrations of key inflammatory cytokines, including interleukin-1β (IL-1β), cyclooxygenase-2 (COX-2) and tumour necrosis factor-α (TNF-α). Furthermore, EGT was observed to reverse the LPS induced increase in the ratio of M1 to M2 macrophages. EGT was also observed to markedly suppress the LPS-induced phosphorylation of JAK/STAT3 and NF-κB, offering a novel insight into the anti-inflammatory mechanism of EGT.
Discussion: In conclusion, the findings of the present study consistently demonstrated that ergothioneine had a significant ameliorative effect on the imiquimod-induced psoriasis model by modulating the NF-κB/JAK-STAT3 signalling pathway. This provides a strong experimental rationale for its potential application in psoriasis treatment.
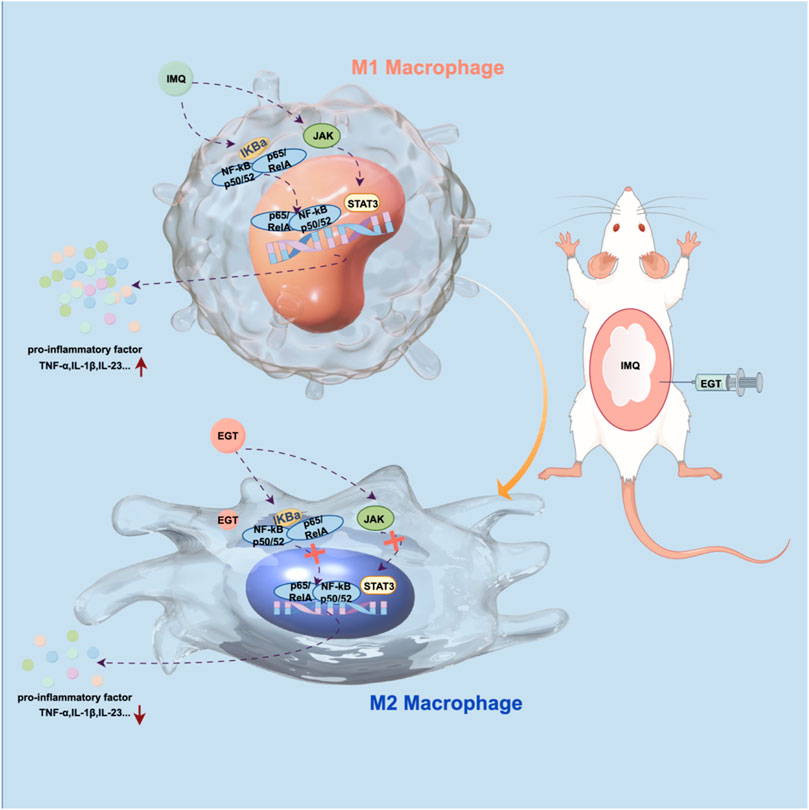
GRAPHICAL ABSTRACT | Mechanism of action of ergothioneine in reducing psoriasis symptoms Figure by (Figdraw).
The aetiology of psoriasis, a chronic and recurrent immune-mediated inflammatory skin disease that affects approximately 2%–3% of the global population (Greb et al., 2016), is widely accepted to involve a combination of genetic factors and external stimuli, including infections, psychological stress and specific medications (Armstrong and Read, 2020). The defining clinical features of the condition include erythematous lesions on the edges of the skin, accompanied by intense itching and silvery-white scales covering the skin surface (Rønholt and Iversen, 2017). Extensive research has demonstrated that macrophage polarisation can contribute to the development and progression of a range of inflammatory immune skin diseases, including psoriasis, AD, SLE and BD (Ross et al., 2021; van den Oord and de Wolf-Peeters, 1994; Buscher et al., 2017). In these diseases, the ratio of M1/M2-type macrophages is elevated, with M1-type macrophages playing a dominant role in perpetuating inflammatory responses and destructive cycles. Conversely, M2 macrophages are believed to exert an anti-inflammatory effect (Wang et al., 2022; Kobayashi et al., 2016; Shen et al., 2024).
The NF-κB pathway and the JAK/STAT3 pathway are classical cellular signaling pathways that have been identified to play a critical role in promoting cellular expression and accumulation of pro- and anti-inflammatory factors, as well as in activating and modulating inflammatory responses (Zhao et al., 2019; Wang C. et al., 2023; Li et al., 2024; Banerjee et al., 2017). A large body of evidence confirms that both pathways play a key role in the regulation and expression of cytokines in serum and damaged skin tissue of psoriasis patients (Kalliolias and Ivashkiv, 2016; Bell et al., 2003; Zhao et al., 2016; Pandey et al., 2023). In light of these findings, the NF-κB and JAK/STAT3 pathways are clearly a promising avenue for targeted therapy of psoriasis. Further studies are needed to develop safer and more effective therapeutic agents on this basis.
Ergothioneine (EGT) is a small molecule that is naturally synthesised by certain bacteria and fungi (Cheah and Halliwell, 2021; Marone et al., 2016). It belongs to a group of specific thiol derivatives that are easily absorbed by the body. It is important to note that this substance is not synthesised in animals or humans, but rather enters the body via the dietary route (Halliwell et al., 2023). To date, EGT has been demonstrated to possess a diverse range of biological functions, with its antioxidant and anti-inflammatory effects being particularly noteworthy (Chen et al., 2024). A multitude of studies have demonstrated that EGT possesses the capacity to impede the actions of TNF-α, expeditiously eliminate and curtail the generation of deleterious free radicals and inflammatory agents, and hinder the NF-κB signaling pathway and the JAK/STAT3 pathway to elicit anti-inflammatory effects. Furthermore, EGT has been shown to stimulate the intracellular antioxidant pathway through the p38 MAPK gene cascade (Zhao et al., 2019; Wang Z. et al., 2023; Duan et al., 2023; Jiang et al., 2024). The objective of this study was to examine the therapeutic efficacy of EGT in a psoriasis model and to elucidate the underlying mechanisms of action of EGT in reducing phosphorylated NF-κB and JAK/STAT3 expression. The findings of this study are anticipated to provide novel and effective targets and strategies for the clinical management of psoriasis.
All animal care and experimental procedures in this study were conducted in strict accordance with the specifications established by the Shandong Provincial Laboratory Animal Management Committee and were formally approved by the Ethics Committee of Shandong University. The male BALB/c mice (7 weeks old, weighing between 15 and 20 g) used in the experiments were purchased from Vital River. The mice were housed in a specific pathogen-free environment with unrestricted access to food and water. The experimental design involved the random assignment of mice to five distinct groups (n = 6), comprising a blank control group, an EGT-treated group, an IMQ-treated group, an IMQ + NaCl-treated group, and an IMQ + EGT-treated group (70 mg/kg). To induce a psoriasis-like dermatitis, a 5% imiquimod cream was applied topically to the shaved backs of the mice at a dosage of 40 mg per day for a period of seven consecutive days. The treated group was administered a daily intraperitoneal injection of 70 mg/kg of EGT. During the course of the experiment, the body weights of the mice were measured on a daily basis, and photographs were taken using an iPad device to document their growth status. During day 7 of modelling, three experimenters performed PASI scores on the severity of psoriasis in the model mice. To ensure the objectivity and accuracy of the assessment results, all participants were blinded to the specific regimen of the drug treatment before data analysis, thus ensuring the fairness and reliability of the experimental results.
On the seventh day of the experiment, the following steps were taken to process and analyse the bare skin on the back of the mice. First, the samples were fixed in 4% paraformaldehyde and then embedded in paraffin. The embedded samples were then sectioned and stained with haematoxylin and eosin (H&E). For antigen retrieval, the sections were placed in sodium citrate solution at pH 6.0. The sections were then incubated with 3% hydrogen peroxide (H2O2) for 30 min at room temperature. The sections were then sealed with 5% goat serum for 2 h. After sealing, the sections were incubated with the appropriate primary antibodies at 4°C overnight, and the day after incubation, the sections were developed using a diaminobenzidine (DAB) substrate kit. Finally, the processed sections were imaged using a Nikon microscope. The antibodies used in this experiment are listed in Supplementary Table S2.
Mouse skin tissue or HaCaT cells were lysed in Trizol (Thermo Fisher Scientific) and RNA was isolated according to the manufacturer’s instructions. RNA was processed as cDNA using the TaqMan cDNA Synthesis Kit and SYBR Green mixture (Takara, Tokyo, Japan), with specific primer pairs as shown in Supplementary Table S1. Relative mRNA levels were calculated using β-actin as a reference control, and the respective mRNA expression was assessed using the 2−ΔΔCT method.
RAW264.7 cells, HaCaT cells and mouse skin tissue samples were harvested and subsequently lysed in a 1:100 buffer system containing PMSF (Beyotime Institute of Biotechnology) and RIPA (Beyotime Institute of Biotechnology). The resulting protein samples were denatured at high temperatures and, after effective separation on 10% SDS-PAGE (sodium dodecyl sulphate polyacrylamide gel electrophoresis) gels, transferred to PVDF membranes for subsequent analysis. For each experimental group, we measured the expression levels of several proteins, including iNOS (inducible nitric oxide synthase), COX-2 (cyclooxygenase-2), IL-1β (interleukin-1β), CD86, CD206, p-P65 and p-IκBα, using β-actin as a reference control. The antibodies used in this experiment are listed in Supplementary Table S2.
Cells (RAW264.7, HacaT) were inoculated into 24-well plates covered with glass slides and then treated with 100 ng/mL LPS and different concentration gradients (30, 60, 120 µM) of EGT for 24 h. At the end of the treatment, the cells were transferred to serum-free medium and incubated overnight. Cell monolayers on glass slides were then rinsed three times with PBS to remove residual treatment solution. The cells were fixed with 4% paraformaldehyde for 15 min at room temperature. The plasma membrane of the nucleus and the cells were then infiltrated with 0.1% Triton X-100 for 20 min to increase the permeability of the cell membrane. The treated cells were incubated with specific primary antibodies (CD86, CD206, anti-p65 and anti-iNOS antibodies) overnight at 4°C to ensure adequate binding. The following day, the appropriate rabbit-derived fluorescent secondary antibodies were used to bind to the primary antibodies and images of the treated cell sections were captured using an inverted fluorescence microscope (Zeiss, Germany). The antibodies used in this experiment are listed in Supplementary Table S2.
Mouse monocyte-macrophage cell line (RAW264.7), human keratinocyte-forming cell line (HaCaT), and human monocyte (THP-1) were purchased from Cellverse (China). RAW264.7 cells and HaCaT cells were cultured in DMEM medium containing 10% FBS and 1% penicillin-streptomycin. THP-1 cells were cultured in RPMI1640 medium containing 10% FBS and 1% penicillin-streptomycin.
Cell Counting Kit-8 (CCK8, Beyotime) was used to assess the viability of RAW264.7 and HaCaT cells in the presence or absence of LPS and different concentration gradients of EGT. 1*10^5 cells/well were inoculated into 96-well plates containing 100 μL cell culture medium per well and incubated for 24 h. Then 10 µL of CCK8 solution was added to each well and the incubation was continued for 1 h in an incubator at 37°C. At the end of the incubation, cell viability was assessed by measuring the absorbance of each well at 450 nm using an enzyme marker.
For the in vivo assay, a single cell suspension was prepared by grinding the mouse spleen and rinsing the bone marrow from the mouse leg bone, adding erythrocyte lysis solution to completely lyse the erythrocytes in the suspension, and then FITC-conjugated anti-F4/80 and PE-conjugated anti-CD86 for M1 macrophage and FITC-conjugated anti-F4/80 and APC-conjugated anti-CD206 for M2 macrophage are added to the prepared cells and incubated for 1 h at 4°C under light protection.
For in vitro experiments, RAW264.7 cells were inoculated into six-well plates at a density of 2*10^5/well. These cells were then treated according to the established experimental protocol. At the end of the treatment, the appropriate antibodies were added to the resulting cell suspension. The F4/80 CD206 (M2) or F4/80 CD86 (M1) populations were determined by flow cytometry (BD FACSCalibur) and the results were analysed using FlowJo software (version 10).
The GeneCards database (https://www.genecards.org), TTD (https://bidd.group/group/cjttd/) and OMIM(https://www.omim.org/)were used to search for psoriasis-related causative genes and a concatenation of these three databases was obtained. The chemical structure of ergothioneine was investigated using PibChem (https://pubchem.ncbi.nlm.nih.gov/) and the results were uploaded to the SwissTarget prediction (http://swisstargetprediction.ch/) database. Identification of potential target genes for ergothioneine using Venny analysis (https://bioinfogp.cnb.csic.es/tools/venny/index.html) was used to identify the overlap between psoriasis-associated disease-causing genes and ergothioneine target genes. These genes may play an important role in the alleviation of psoriasis symptoms by ergothioneine. The STRING (https://cn.string-db.org/) database was used to construct a protein-methionine interaction network with a feasibility setting of 0.4. The STRING database was used to construct a protein-methionine interaction network with a feasibility setting of 0.4. Each of the genes in this protein-methionine interaction network was analysed using the Cytohubb plug-in in Cytoscale software (version 3.8.1). gene significance.
Statistical analyses were performed in a blinded fashion to ensure the objectivity of the study. In this study, all experiments included a minimum sample size of n = 3 to ensure stability and reliability of results. We used GraphPad Prism 9 software for statistical analysis of the data, and all data collected were presented as mean ± SD. When statistically analysing differences between multiple groups, data were analysed using two statistical methods: one-way ANOVA or unpaired Student’s t-test. Statistical significance was considered when the P < 0.05. Post hoc tests were used only if F achieved P < 0.05, and there was no significant variance inhomogeneity.
We used the CCK-8 assay to determine the effects of different concentrations of EGT on the bioactivity of mouse macrophages RAW264.7 and human keratinocytes HaCaT. The experimental results showed that the addition of EGT alone at a concentration of 120 uM had no significant effect on the activity of the cells. When the cells were exposed to 100 ng/mL LPS, the cell activity decreased significantly. Notably, after further treatment of these cells with EGT at concentrations of 60, 120 µM for 24 h, we observed a significant increase in cell viability (Figures 1A, B). Based on these findings, we decided to conduct a more in-depth study using three concentrations of EGT (i.e., 30, 60, and 120 µM) in subsequent experiments.
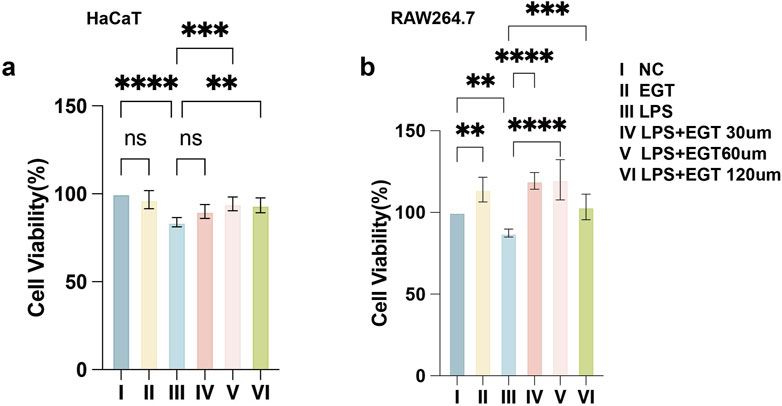
Figure 1. Effect of ergothioneine and LPS co-treatment on cell activity. (A) The effect of ergothioneine on HacaT cell activity was determined by CCK-8 assay. (B) Effect of ergothioneine on RAW264.7 cell activity determined by CCK-8 assay. Data are expressed as mean ± SD. *p < 0.05; p < 0.001; p < 0.0001.
We used mouse macrophages (RAW264.7) as a model to systematically assess the effects of EGT on macrophage polarisation processes. Specifically, when macrophages were exposed to LPS, their cells, which were originally in the M0 state, were transformed into M1-type macrophages. However, upon further addition of different concentrations of EGT, we observed that the proportion of M1-type macrophages showed a concentration-dependent decrease (Figures 2A, B). To verify the association of this phenomenon with EGT, we used flow cytometry for confirmation (Figure 2C). We then investigated the effect of EGT on the expression of macrophage polarisation markers. Using protein blotting and immunofluorescence techniques, we found that the expression of the M1-type macrophage marker CD86 showed a gradient decrease with increasing EGT concentration, while the expression of the M2-type macrophage marker CD206 increased significantly (Figures 2D–G). Similarly, we added 100 ng/mL phorbol 12-myristate 13-acetate (PMA) to human monocyte THP-1 cells to induce them into M0-type macrophages, which later went through the same treatment results as RAW264.7 cells, and also obtained results consistent with the above by Western blot (Supplementary Figures 1A, B). These results further confirm the important role of EGT in regulating macrophage polarisation.
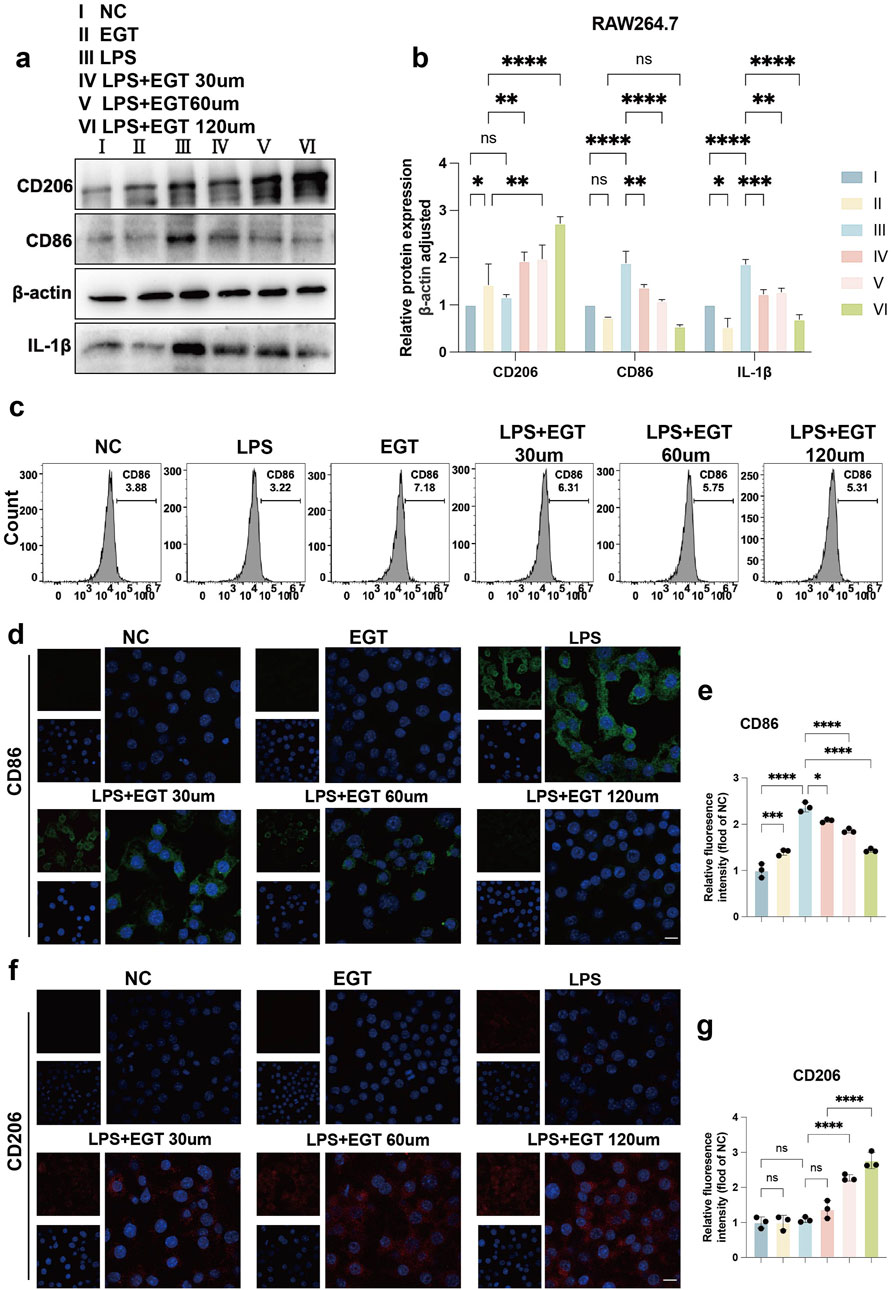
Figure 2. EGT regulates the ratio of M1 to M2 macrophages in RAW264.7 cells following the induction of these cells by LPS. (A) Protein expression levels of CD206 and CD86 in treated groups of RAW264.7 macrophages treated with LPS in the presence or absence of EGT for 24 h were detected by Western blot. (B) A statistical graph of protein quantification in (A). (C) The proportion of CD86 M1 macrophages in each group, as detected by flow cytometry. (D) Cell immunofluorescence to detect the expression of CD86 in treated cells of each group. (E) Quantitative statistical plots of cell immunofluorescence. (F) Cell immunofluorescence to detect the expression of CD206 in treated cells of each group. (G) Quantitative statistical plots of cell immunofluorescence. Data are expressed as mean ± SD. *p < 0.05; p < 0.001; p < 0.0001.
To further investigate the anti-inflammatory effects of EGT in cells in vitro, we used LPS to simulate a cellular model of inflammation in vitro. The experimental results showed that when HaCaT cells were exposed to LPS stimulation, the expression levels of key inflammatory factors such as iNOS, COX-2 and IL-1β increased significantly. We then introduced different concentrations of EGT into these cells and observed that the expression of the above pro-inflammatory cytokines showed a significant decreasing trend with the gradual increase in EGT concentration (Figures 3A, B). Further analysis showed that at the mRNA level, the expression of pro-inflammatory factors such as TNF-α, IL-6 and IL-1β also showed a concentration-dependent decrease with the gradual increase in EGT concentration (Figure 3C). In addition, as visualised by immunofluorescence, the addition of EGT was able to significantly reduce the expression level of the pro-inflammatory factor iNOS (Figures 3D, E). The data suggest that the addition of EGT was able to demonstrate a significant concentration gradient anti-inflammatory effect in HaCaT keratinocytes.
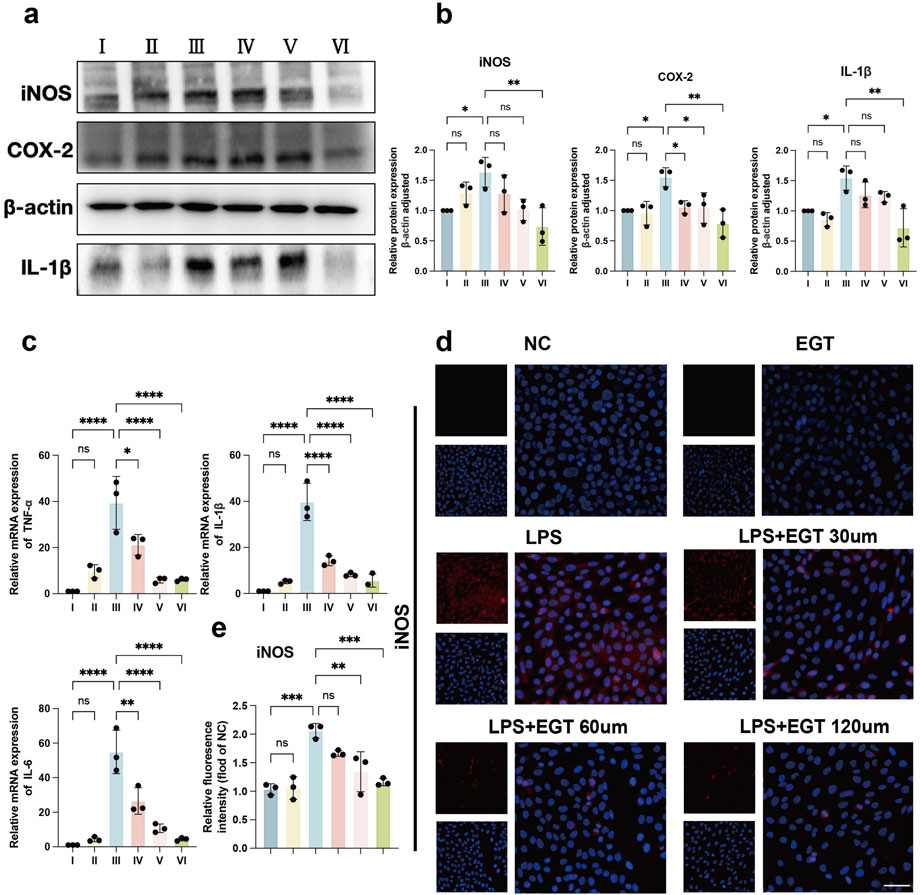
Figure 3. EGT has been demonstrated to possess anti-inflammatory effects in HacaT cells. (A) HacaT cells were treated with LPS in the presence or absence of EGT for 24 h. Protein expression of pro-inflammatory factors in each group was then quantified. (B) A statistical graph of protein quantification in (A) was generated. (C) mRNA expression of pro-inflammatory factors in treated cells was analysed by qRT-PCR. (D) iNOS expression in treated groups was detected by immunofluorescence. (E) A quantitative statistical graph of cell immunofluorescence was generated. Data are expressed as mean ± SD. *p < 0.05; p < 0.001; p < 0.0001.
To verify the effect of EGT in the imiquimod-induced psoriasis mouse model, we used the following experimental approach: first, 5% imiquimod cream was applied to the shaved back skin of BALB/c mice to mimic the appearance of psoriasis-like lesions. Compared to the control group, which received no treatment, the dorsal skin of the mice in the model group showed significant pathological changes, as evidenced by typical psoriasis symptoms such as redness of the skin, production of large amounts of scales, a significant increase in skin thickness, and even the appearance of desquamation and crusting (Figures 4C, D). We then injected EGT (at a dose of 70 mg/kg) intraperitoneally into the mice in the treatment group to evaluate its therapeutic effect (Tang et al., 2018; Salama et al., 2021). The histopathologic HE results of spleen and kidney of mice at this therapeutic concentration showed no abnormalities (Supplementary Figure S2). The experimental results showed that the injection of EGT significantly alleviated the symptoms of the psoriasis mice. Compared with the model group, mice treated with EGT had a significant reduction in skin thickness and a significant reduction in scale production (Figure 4A). In addition, we performed a Psoriasis Area and Severity Index (PASI) score on the mice to further quantitatively assess the therapeutic effect of EGT. The results showed that the PASI scores of the mice in the EGT-treated group were significantly lower than those of the IMQ model group on the seventh day of the experiment (Figure 4B). In addition to the aforementioned improvement in skin symptoms, we also observed that the spleens of mice in the EGT-treated group showed a significant reduction compared to the model group (Supplementary Figure S3).
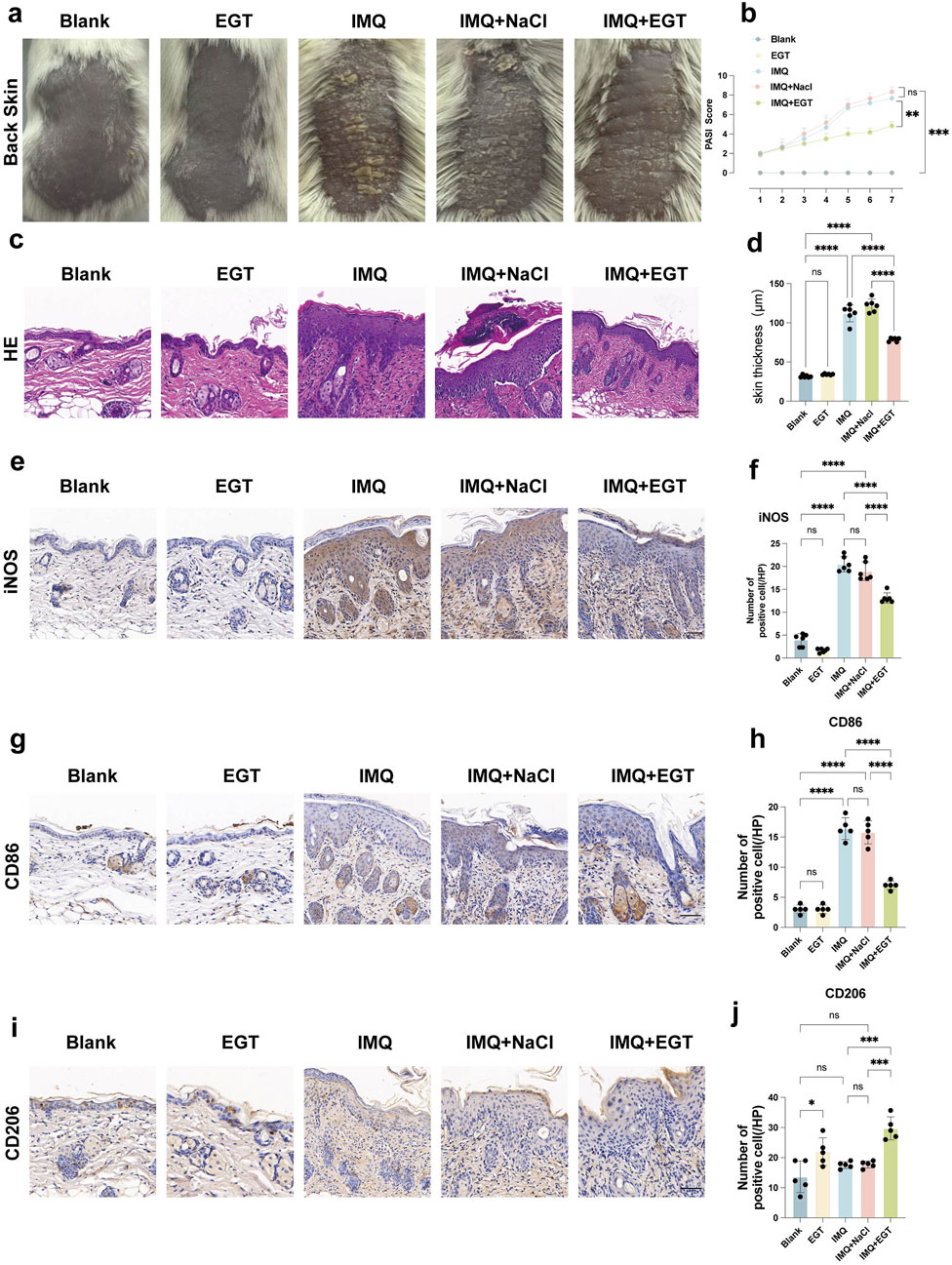
Figure 4. Ergothioneine attenuates IMQ-induced psoriasis-like skin symptoms. (A) Representative images of the dorsal skin of each group of mice on day 7. (B) PASI scores of mice with imiquimod-induced psoriasis for 7 consecutive days. (C) HE staining results (×200) of the dorsal skin of mice in each group. (D) Statistical graph of epidermal tissue thickness on the back of mice. (E, F) Immunohistochemistry (×200) and statistical graph of iNOS in the dorsal skin of mice. (G, H) IHC (×200) and positive cell counts of CD86 cells in dorsal skin tissues of mice in each group. (I, J) IHC (×200) and positive cell counts of CD206 cells in dorsal skin tissues of mice in each group, (scale bar = 100um). Data are expressed as mean ± SD. *p < 0.05; p < 0.001; p < 0.0001.
To further assess the inflammatory state of the mice, we performed pro-inflammatory cytokine expression assays at the mRNA and protein levels in imiquimod-treated mice. The results showed that the expression levels of COX-2, IL-23, IL-1β and TNF-α genes were significantly increased in the dorsal skin of mice in the IMQ model group. Compared to the model group, the addition of EGT significantly reduced the expression of the above pro-inflammatory factors (Figures 5A–C). In addition, immunohistochemical results showed that EGT significantly reduced the number of iNOS-positive cells in skin tissues compared to the IMQ group (Figures 4E, F). Of particular interest, the introduction of EGT also led to a significant decrease in the protein expression of the M1 macrophage-specific marker CD86 and a corresponding increase in the expression of the M2 macrophage marker CD206 (Figures 4G–J). This finding suggests that EGT may have the potential ability to regulate the macrophage M1/M2 ratio and the body’s immune response, which could have a positive impact on the treatment of autoimmune diseases such as psoriasis.
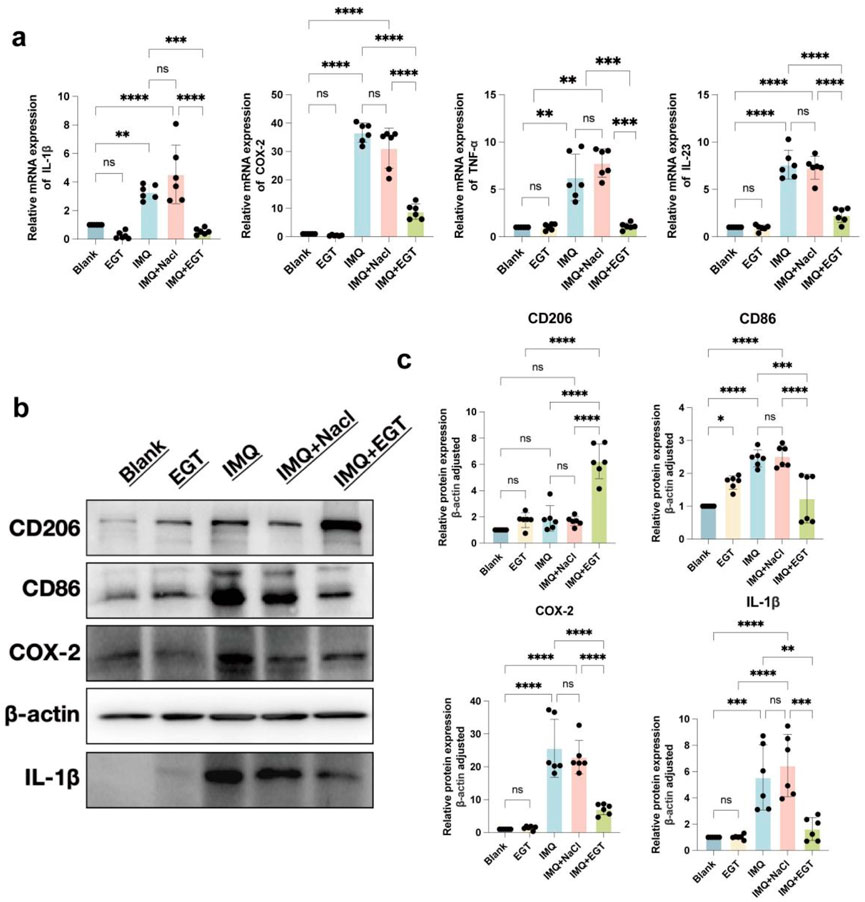
Figure 5. Ergothioneine attenuates IMQ-induced psoriasis-like skin symptoms. (A) COX-2, IL-23, IL-1β and TNF-α mRNA expression on the back of psoriatic mice. (B, C) Protein expression of skin inflammatory factors on the back of psoriatic mice and statistical graphs. Data are expressed as mean ± SD. *p < 0.05; p < 0.001; p < 0.0001.
In the above study, we clearly observed a significant negative correlation between the concentration of EGT and the ratio of macrophage M1/M2 in mice. To further investigate this relationship, we examined the specific changes in macrophage levels in a psoriasis-like dermatitis model induced by imiquimod. Using immunohistochemical staining techniques, we found that topical application of imiquimod significantly increased the number of CD86-positive cells within the dorsal skin lesion area, with a corresponding decrease in the number of CD206-positive cells (Figures 4G–J). In addition, in-depth analyses using flow cytometric analysis techniques further revealed the specific effects of EGT on macrophage subtypes. The results of the study showed that no significant effect on mouse macrophages was observed in the case of EGT application alone. However, the number of M1 macrophages (F4/80 CD86) infiltrating the skin of lesions in the model group was significantly higher than in the control group (Figures 6A, B). Notably, M2 macrophages (F4/80 CD206) did not show significant quantitative changes between the control and model groups. When EGT was applied to the mice, we observed a significant decrease in the percentage of F4/80 CD86-labelled M1 macrophages and a significant increase in the percentage of F4/80 CD206-labelled M2 macrophages in the spleen and bone marrow of the mice (Figures 6C, D). This finding suggests that EGT may modulate the secretion of pro-inflammatory cytokines by inhibiting the infiltration of M1 macrophages and enhancing the anti-inflammatory effects of M2 macrophages to achieve therapeutic efficacy in psoriasis (Figure 6E).

Figure 6. Ergothioneine alters the M1/M2 macrophage ratio in the immune microenvironment of psoriatic mice. (A, B) Percentage of CD86 F4/80 M1 macrophages in bone marrow and spleen of mice with psoriasis detected by flow cytometry. (C, D) Percentage of CD206 F4/80 M1 macrophages in bone marrow and spleen of psoriatic mice by flow cytometry. (E) Statistics of the percentage of M1/M2 macrophages in the spleen and bone marrow. Data are SD ± mean. *p < 0.05; p < 0.001; p < 0.0001.
The chemical structure of EGT was analysed using the PubChem platform (http://pubchem.ncbi.nlm.nih.gov/). Specifically, the three-dimensional chemical structure of EGT (Figure 7A), in which sulfur atoms are shown as yellow spheres, carbon atoms are shown as grey spheres, nitrogen atoms are shown as blue spheres, oxygen atoms are shown as red spheres, and hydrogen atoms are shown as grey spheres. To further investigate the biological effects of EGT, we explored the target genes interacting with EGT using the swissTarget prediction database and counted the major categories of EGT target genes (Figure 7B), which showed that enzymes (accounting for 14.0% of the total), proteases, and other enzymes and proteins were the most abundant categories among them. This finding provides an important direction for further studies into the mechanism of action of EGT and its biological functions. In addition, this study used the GeneCards database to screen for causative genes associated with psoriasis. Using intersection analysis, we predicted and identified 21 genes that are both causative genes of psoriasis and potential target genes of EGT (Figure 7C). To further elucidate the mechanism of these common target genes in the pathogenesis of psoriasis, we selected 12 of these key target genes and constructed a protein-protein interaction network using the String database. The Degree algorithm in CytoScape software was then used to rank the importance of these common genes, and the results showed that genes such as PTGS2 (COX-2) and CXCL8 played the role of core target genes in the process of ergothioneine in alleviating psoriasis (Figure 7D).
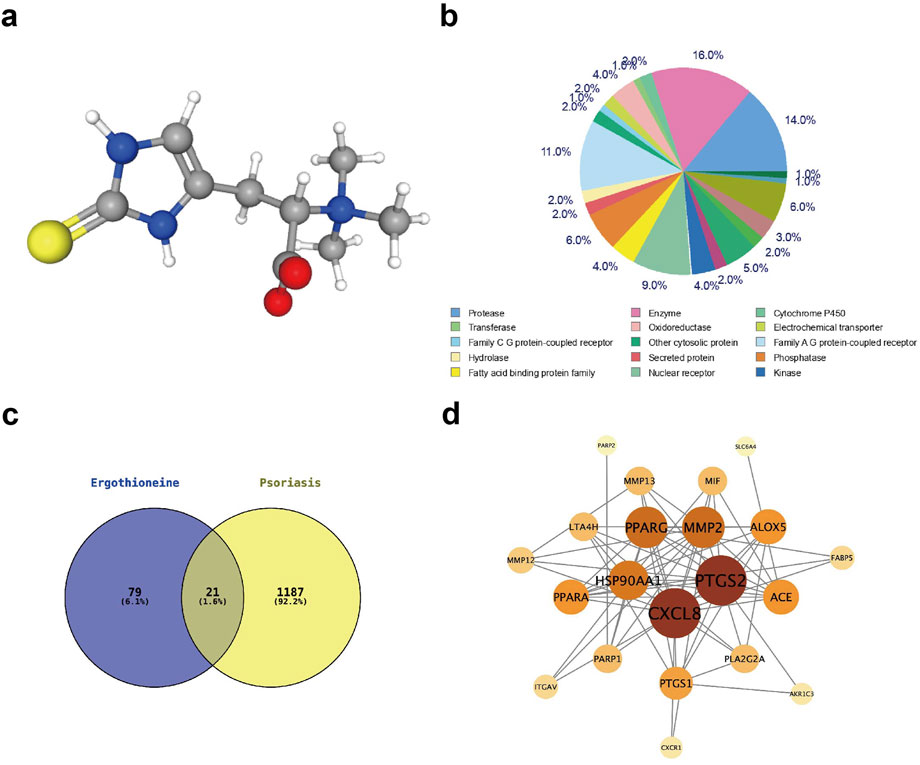
Figure 7. Network pharmacological analysis of EGT to investigate the site of action of EGT in psoriasis. (A) The chemical structure of EGT was examined using the PubChem database, with yellow spheres representing sulphur, grey spheres representing carbon, blue spheres representing nitrogen, red spheres representing oxygen and white spheres representing hydrogen. (B) Analysis of the potential EGT target gene type representation using the SwissTargetPrdiction database. (C) Intersection of predicted EGT target genes and psoriasis-causing genes analysed using Vernon analysis, resulting in 21 genes with common effects. (D) A protein-methionine interaction network was constructed using the String database for the 21 target genes screened for colocalisation.
Through network pharmacology studies, we identified a potential mechanism of action of EGT in anti-psoriasis. To further validate this finding, we verified the effect of EGT on the cellular effects of the NF-κB/JAK-STAT3 pathway at the in vitro level. The experimental results were as expected and clearly showed that EGT effectively inhibited the phosphorylation process of IKBα, NF-κB p65 and JAK1, STAT3 in HaCaT cells (Figures 8A–D). In addition, repeated experiments performed on RAW264.7 cells led to the same conclusion (Supplementary Figures S4). By immunofluorescence analysis, we observed that the LPS-induced nuclear translocation of p65 was significantly reduced by the addition of EGT (Figures 8E, F). The above series of experimental results confirmed the inhibitory effect of EGT on the NF-κB pathway. Therefore, we believe that the preventive effect of EGT on psoriasiform dermatitis may be realized through the inhibition of NF-κB/JAK-STAT3 mediated inflammatory response.
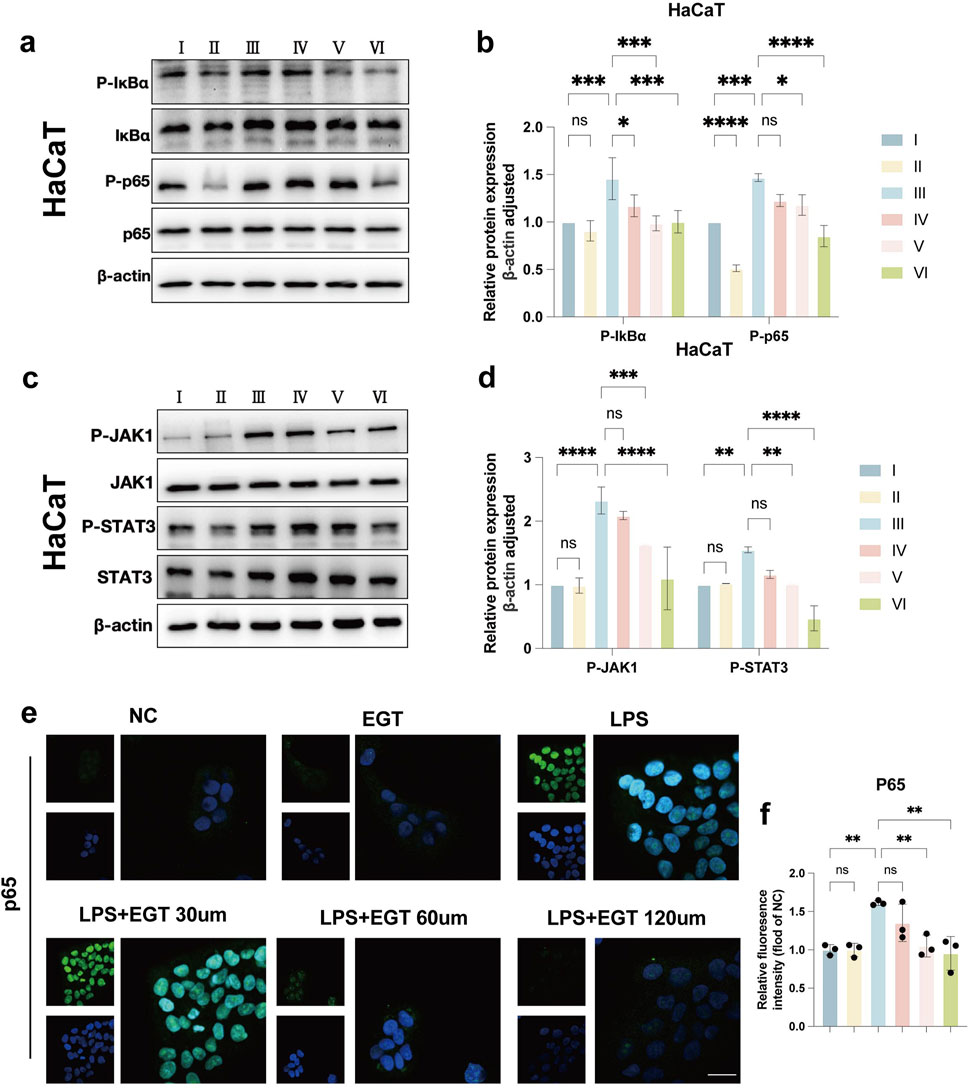
Figure 8. EGT exerts anti-psoriasis effects by inhibiting NF-κB/JAK-STAT3 signalling. (A) Western blot detection of IKB-α and NF-κB p65 and their phosphorylation levels in HacaT cells stimulated with LPS and treated with EGT. (B) Quantification of protein levels in (A) using grey value analysis with ImageJ software. (C, D) Western blot detection of JAK1 and STAT3 and their phosphorylation levels after treatment of HacaT cells with EGT stimulated by LPS, and protein levels were quantified. (E, F) Immunofluorescence staining results of NF-κB p65 in each group after treatment of RAW264.7. Data are SD ± mean. *p < 0.05; p < 0.001; p < 0.0001.
Psoriasis is a chronic autoimmune-mediated dermatological condition characterised by inflammatory processes and the overproduction of cytokines. Impairment of the skin barrier function is also a key feature of the disease (Lowes et al., 2014). It follows that effective therapeutic strategies for this condition should include the modulation of skin barrier function and the reduction of skin inflammatory responses. Nevertheless, the potential adverse effects and high costs associated with the long-term use of biologics and synthetic drugs represent a significant challenge to the current treatment paradigm (Tremblay and Bissonnette, 2002; Rizvi et al., 2015). Consequently, the pharmaceutical industry is engaged in the active pursuit of novel, safer, and more efficacious pharmacological agents for the treatment of psoriasis (Krueger and Duvic, 1994; Russo et al., 2004; Zhang et al., 2024). The current pharmacological treatments for psoriasis act through a variety of mechanisms, including, but not limited to, the inhibition of keratinocyte hyperproliferation, the restoration of normal cellular differentiation, the inhibition of multiple inflammatory signalling pathways (e.g., NF-κB, JAK-STAT3), and the attenuation of the inflammatory response (Li et al., 2023). Furthermore, a substantial body of experimental evidence has emerged indicating that the identification of macrophage activation states and the modulation of their polarization direction can facilitate the diagnosis and treatment of select immune-associated inflammatory diseases. It is our hope that the present study will succeed in altering the M1/M2 macrophage ratio in the psoriatic skin immune microenvironment by modulating macrophage polarisation in psoriatic dermatitis. Furthermore, we aim to induce macrophage differentiation towards M2 or M2-like differentiation and neutralise the over-excited pro-inflammatory response in the skin, thereby achieving the therapeutic goal of psoriasis (Bell et al., 2003; Baker et al., 2003; Ko and Liao, 2023).
In recent years, EGT has attracted much attention due to its significant anti-inflammatory and antioxidant effects and extensive safety record in humans (Xiong et al., 2024). However, there is a lack of definitive evidence regarding the specific protective role of EGT in the development of psoriasis. The present study investigated the potential of EGT in the treatment of psoriasis and found that its anti-inflammatory effects significantly inhibited disease progression in an IMQ-induced model of psoriasis. The results of in vivo experiments showed that EGT was able to significantly reduce pathological changes such as hyperkeratosis, keratinisation, epidermal thickening and inflammatory cell infiltration in dorsal skin samples, and effectively ameliorated the systemic side effects of psoriasis and the levels of inflammatory mediators in skin tissues in mice. Meanwhile, in vitro experiments also showed that EGT was able to downregulate the expression of CD86, an M1-type marker, and upregulate the expression of CD206, an M2-type marker, in LPS-stimulated mouse macrophages, thereby regulating macrophage polarisation status. In addition, EGT was able to attenuate the inflammatory response of HaCaT cells under LPS stimulation and significantly reduce the phosphorylation levels of IκBα, NF-κB p65 and JAK1, STAT3.
In conclusion, the results of this study are consistent in indicating that EGT has a significant inhibitory effect in psoriasis. However, there are still some limitations in this study, and the specific mechanism by which EGT inhibits NF-κB/JAK-STAT3 to exert anti-inflammatory effects needs to be further investigated. In addition, the use of imiquimod to induce psoriasis in vitro in this study is relatively non-specific, and we expect to use K14-VEGF and K5-STST3C transgenic mouse models to further validate the therapeutic effect of EGT in psoriasis in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The animal study was approved by Ethics Committee of Shandong University. The study was conducted in accordance with the local legislation and institutional requirements.
Al: Writing–original draft, Software, Investigation. YL: Validation, Writing–original draft, Investigation. PY: Writing–original draft, Formal Analysis. ZZ: Conceptualization, Data curation, Formal Analysis, Writing–review and editing. TH: Formal Analysis, Methodology, Software, Writing–review and editing. HL: Data curation, Methodology, Writing–review and editing. SW: Formal Analysis, Supervision, Writing–review and editing. XR: Conceptualization, Data curation, Writing–review and editing. WL: Resources, Visualization, Writing–review and editing. HW: Formal Analysis, Project administration, Writing–review and editing. YG: Funding acquisition, Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Provincial and ministerial joint construction project of Medical Science and Technology Research and Development in Henan Province (SBGJ202303013) and Natural Science Foundation of Henan Province (232300421284).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1521743/full#supplementary-material
Armstrong, A. W., and Read, C. (2020). Pathophysiology, clinical presentation, and treatment of psoriasis: a review. Jama 323, 1945–1960. doi:10.1001/jama.2020.4006
Baker, B. S., Ovigne, J. M., Powles, A. V., Corcoran, S., and Fry, L. (2003). Normal keratinocytes express Toll-like receptors (TLRs) 1, 2 and 5: modulation of TLR expression in chronic plaque psoriasis. Br. J. Dermatol 148, 670–679. doi:10.1046/j.1365-2133.2003.05287.x
Banerjee, S., Biehl, A., Gadina, M., Hasni, S., and Schwartz, D. M. (2017). JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77, 521–546. doi:10.1007/s40265-017-0701-9
Bell, S., Degitz, K., Quirling, M., Jilg, N., Page, S., and Brand, K. (2003). Involvement of NF-kappaB signalling in skin physiology and disease. Cell Signal 15, 1–7. doi:10.1016/s0898-6568(02)00080-3
Buscher, K., Ehinger, E., Gupta, P., Pramod, A. B., Wolf, D., Tweet, G., et al. (2017). Natural variation of macrophage activation as disease-relevant phenotype predictive of inflammation and cancer survival. Nat. Commun. 8, 16041. doi:10.1038/ncomms16041
Cheah, I. K., and Halliwell, B. (2021). Ergothioneine, recent developments. Redox Biol. 42, 101868. doi:10.1016/j.redox.2021.101868
Chen, L., Zhang, L., Ye, X., Deng, Z., and Zhao, C. (2024). Ergothioneine and its congeners: anti-ageing mechanisms and pharmacophore biosynthesis. Protein Cell 15, 191–206. doi:10.1093/procel/pwad048
Duan, R., Pan, H., Li, D., Liao, S., and Han, B. (2023). Ergothioneine improves myocardial remodeling and heart function after acute myocardial infarction via S-glutathionylation through the NF-ĸB dependent Wnt5a-sFlt-1 pathway. Eur. J. Pharmacol. 950, 175759. doi:10.1016/j.ejphar.2023.175759
Greb, J. E., Goldminz, A. M., Elder, J. T., Lebwohl, M. G., Gladman, D. D., Wu, J. J., et al. (2016). Psoriasis. Nat. Rev. Dis. Prim. 2, 16082. doi:10.1038/nrdp.2016.82
Halliwell, B., Tang, R. M. Y., and Cheah, I. K. (2023). Diet-derived antioxidants: the special case of ergothioneine. Annu. Rev. Food Sci. Technol. 14, 323–345. doi:10.1146/annurev-food-060822-122236
Jiang, X., Sun, Y., Lyu, Y., Kang, H., Zhao, J., Zhang, K., et al. (2024). Hydrogels for ameliorating osteoarthritis: mechanical modulation, anti-inflammation, and regeneration. BMEMat 2, e12078. doi:10.1002/bmm2.12078
Kalliolias, G. D., and Ivashkiv, L. B. (2016). TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62. doi:10.1038/nrrheum.2015.169
Ko, A., and Liao, C. (2023). Hydrogel wound dressings for diabetic foot ulcer treatment: status-quo, challenges, and future perspectives. BMEMat 1, e12037. doi:10.1002/bmm2.12037
Kobayashi, E. H., Suzuki, T., Funayama, R., Nagashima, T., Hayashi, M., Sekine, H., et al. (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624. doi:10.1038/ncomms11624
Krueger, G. G., and Duvic, M. (1994). Epidemiology of psoriasis: clinical issues. J. Invest Dermatol 102, 14S-18S–18s. doi:10.1111/1523-1747.ep12386079
Li, L., Yin, Y., Zhang, S., Yang, J., Li, P., Zhou, H., et al. (2024). “Triggerable biomaterials-based osteomyelitis theranostics,”. BMEMat 2 (4). e12081. doi:10.1111/1523-1747.ep12386079
Li, Y., Cui, H., Li, S., Li, X., Guo, H., Nandakumar, K. S., et al. (2023). Kaempferol modulates IFN-γ induced JAK-STAT signaling pathway and ameliorates imiquimod-induced psoriasis-like skin lesions. Int. Immunopharmacol. 114, 109585. doi:10.1016/j.intimp.2022.109585
Lowes, M. A., Suárez-Fariñas, M., and Krueger, J. G. (2014). Immunology of psoriasis. Annu. Rev. Immunol. 32, 227–255. doi:10.1146/annurev-immunol-032713-120225
Marone, P. A., Trampota, J., and Weisman, S. (2016). A safety evaluation of a nature-identical l-ergothioneine in sprague dawley rats. Int. J. Toxicol. 35, 568–583. doi:10.1177/1091581816653375
Pandey, R., Bakay, M., and Hakonarson, H. (2023). SOCS-JAK-STAT inhibitors and SOCS mimetics as treatment options for autoimmune uveitis, psoriasis, lupus, and autoimmune encephalitis. Front. Immunol. 14, 1271102. doi:10.3389/fimmu.2023.1271102
Rizvi, S., Chaudhari, K., and Syed, B. A. (2015). The psoriasis drugs market. Nat. Rev. Drug Discov. 14, 745–746. doi:10.1038/nrd4763
Rønholt, K., and Iversen, L. (2017). Old and new biological therapies for psoriasis. Int. J. Mol. Sci. 18, 2297. doi:10.3390/ijms18112297
Ross, E. A., Devitt, A., and Johnson, J. R. (2021). Macrophages: the good, the bad, and the gluttony. Front. Immunol. 12, 708186. doi:10.3389/fimmu.2021.708186
Russo, P. A., Ilchef, R., and Cooper, A. J. (2004). Psychiatric morbidity in psoriasis: a review. Australas. J. Dermatol 45, 155–159. doi:10.1111/j.1440-0960.2004.00078.x
Salama, S. A., Abd-Allah, G. M., Mohamadin, A. M., Elshafey, M. M., and Gad, H. S. (2021). Ergothioneine mitigates cisplatin-evoked nephrotoxicity via targeting Nrf2, NF-κB, and apoptotic signaling and inhibiting γ-glutamyl transpeptidase. Life Sci. 278, 119572. doi:10.1016/j.lfs.2021.119572
Shen, J., Zhou, Y., and Yin, L. (2024). Nano/genetically engineered cells for immunotherapy. BMEMat, e12112. doi:10.1002/bmm2.12112
Tang, R. M. Y., Cheah, I. K., Yew, T. S. K., and Halliwell, B. (2018). Distribution and accumulation of dietary ergothioneine and its metabolites in mouse tissues. Sci. Rep. 8, 1601. doi:10.1038/s41598-018-20021-z
Tremblay, J. F., and Bissonnette, R. (2002). Topical agents for the treatment of psoriasis, past, present and future. J. Cutan. Med. Surg. 6, 8–11. doi:10.1177/12034754020060S303
van den Oord, J. J., and de Wolf-Peeters, C. (1994). Epithelium-lining macrophages in psoriasis. Br. J. Dermatol 130, 589–594. doi:10.1111/j.1365-2133.1994.tb13104.x
Wang, C., Wang, S., Kang, D. D., and Dong, Y. (2023). Biomaterials for in situ cell therapy. BMEMat 1, e12039. doi:10.1002/bmm2.12039
Wang, S., Liu, G., Li, Y., and Pan, Y. (2022). Metabolic reprogramming induces macrophage polarization in the tumor microenvironment. Front. Immunol. 13, 840029. doi:10.3389/fimmu.2022.840029
Wang, Z., Ma, J., Miao, Z., Sun, Y., Dong, M., Lin, Y., et al. (2023). Ergothioneine inhibits the progression of osteoarthritis via the Sirt6/NF-κB axis both in vitro and in vivo. Int. Immunopharmacol. 119, 110211. doi:10.1016/j.intimp.2023.110211
Xiong, K., Xue, S., Guo, H., Dai, Y., Ji, C., Dong, L., et al. (2024). Ergothioneine: new functional factor in fermented foods. Crit. Rev. Food Sci. Nutr. 64, 7505–7516. doi:10.1080/10408398.2023.2185766
Zhang, G., Wang, N., Ma, Y., Zhai, S., Ngai, T., Ni, S., et al. (2024). Metal coordination-driven assembly of stimulator of interferon genes-activating nanoparticles for tumor chemo-immunotherapy. BMEMat 2, e12077. doi:10.1002/bmm2.12077
Zhao, Y., Li, Y., Qu, R., Chen, X., Wang, W., Qiu, C., et al. (2019). Cortistatin binds to TNF-α receptors and protects against osteoarthritis. EBioMedicine 41, 556–570. doi:10.1016/j.ebiom.2019.02.035
Keywords: psoriasis, ergothioneine, macrophage, anti-inflammatory, NF-κB, Jak-STAT3
Citation: Li A, Liu Y, Yu P, Zhang Z, Huang T, Li H, Wu S, Rong X, Liao W, Wang H and Gao Y (2025) Ergothioneine attenuates psoriasis symptoms through modulation of M1/M2 macrophage polarisation via the NF-κB/JAK-STAT3 pathway. Front. Pharmacol. 16:1521743. doi: 10.3389/fphar.2025.1521743
Received: 02 November 2024; Accepted: 28 January 2025;
Published: 19 February 2025.
Edited by:
Hexin Chen, University of South Carolina, United StatesReviewed by:
Hassan Oliver James Morad, University of Westminster, United KingdomCopyright © 2025 Li, Liu, Yu, Zhang, Huang, Li, Wu, Rong, Liao, Wang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanzheng Gao, eWFuemhlbmdnYW9obkAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.