
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 31 March 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1520039
This article is part of the Research TopicHerbal Medicine for the Treatment of Chronic Metabolic Diseases, Volume IIView all 18 articles
 Yutong Deng1†
Yutong Deng1† Ruli Feng1†
Ruli Feng1† Bo Hu2
Bo Hu2 Xuewen Ren3
Xuewen Ren3 Fengchuan Zhang2
Fengchuan Zhang2 Huishang Feng2,4
Huishang Feng2,4 Xuewan Wang1
Xuewan Wang1 Yatong Li1
Yatong Li1 Tangyunni Liu1
Tangyunni Liu1 Lingling Cai2*
Lingling Cai2* Yuanwen Li2*
Yuanwen Li2*Objectives: To evaluate the efficacy and safety of Tanshinone capsule as a complementary therapy in managing of Acne Vulgaris.
Methods: A systematic search of six databases was conducted to identify relevant randomized controlled trials (RCTs) from each database for nearly 20 years (from 1 Jan 2004, to 1 June 2024). The Cochrane Handbook was used to evaluate the risk of bias. Meta-analysis was performed using Review Manager 5.4.1, and publication bias was assessed the Stata SE 12.0 software. GRADEpro was used to assess the quality of the evidence.
Results: A total of 2,969 participants from 28 studies were included. We found that Tanshinone capsules can reduce acne recurrence rates [risk ratio (RR) 0.44, 95% confidence interval (CI): 0.34 to 0.57, p < 0.00001]; downregulate levels of necrosis factor-alpha (TNF-α) [ mean difference (MD) 0.44, −10.18, 95% CI: −13.57 to −8.04, p < 0.00001], interleukin (IL) 4 (MD -6.46, 95%CI: −7.14 to −5.77, p < 0.00001), IL-6 (MD -16.14, 95%CI: −30.10 to −2.18, p = 0.02), IL-8 (MD -4.48, 95%CI: −8.30 to −0.65, p = 0.02) and testosterone (MD -14.50, 95%CI: −17.59 to −11.40, p < 0.00001); lower Global Acne Grading System (GAGS) score (MD -4.71, 95%CI: −7.62 to −1.80, p = 0.002); decrease sebum secretion rates (MD -0.29, 95%CI: −0.49 to −0.10, p = 0.003), but the regulation of Luteinizing hormone (LH), Follicle-stimulating hormone (FSH), Estradiol (E2) is not obvious. In terms of safety, the incidence of adverse events in the experimental group was less than that in the control group (RR 0.70, 95%CI: 0.56 to 0.87, p = 0.001). The Begg test and Egger test results indicated no publication bias. Furthermore, the levels of evidence ranged from very low to moderate due to risk of bias and heterogeneity.
Conclusion: Tanshinone capsules can relieve the symptoms of acne vulgaris, regulate inflammatory cytokines and hormone levels in patients, and reduce recurrence. However, due to the limitations of this study, more multi-center and large-sample studies are needed to confirm these conclusions.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024562320, identifier CRD42024562320.
Acne vulgaris is a inflammatory dermatological disease that commonly occurs in areas rich in sebaceous glands, such as the face, chest, and back. The characteristic clinical features of acne include comedones, papules, pustules, nodules, cysts and scarring (Williams et al., 2012; Oon et al., 2019). The Global Burden of Disease Project estimates that acne affects approximately 9.4% of the global population, ranking it as the 8th most prevalent disease worldwide (Vos et al., 2012; Hay et al., 2014). Besides skin lesions, acne vulgaris can also cause a severe psychological burden and be associated with metabolic comorbidities, significantly affecting patients’ quality of life while increasing both individual and societal burdens (Stamu-O'Brien et al., 2021; Wang et al., 2022).
The current management of acne vulgaris is based on acne severity assessment and laboratory testing (Reynolds et al., 2024). Common treatments include topical retinoids, benzoyl peroxide, antibiotics, isotretinoin, contraceptives, and physical modalities, etc (Oon et al., 2019; Reynolds et al., 2024). However, increasing antibiotic resistance of Cutibacterium acnes (C. acnes) and the potential risk for adverse reactions remain significant challenges (Fox et al., 2016; Habeshian and Cohen, 2020). Therefore, complementary and alternative medicines (CAM) for acne treatment may be essential.
Tanshinone capsule is a traditional Chinese patent medicine made from the ethanol extract of Danshen (dried roots and rhizomes of Salvia miltiorrhiza), approved by the Chinese State Food and Drug Administration, the main active ingredients are tanshinone ⅡA (Tan ⅡA) and cryptotanshinone (CPT). The traditional Chinese medicine properties and therapeutic effects of Danshen are provided in Supplementary Table S3. According to Chinese pharmacopoeia standards, each capsule (0.25 g) contains no less than 16 mg of Tan ⅡA and 12 mg of CPT (State Food and Drug Administration of China, 2010). In vitro studies show that CPT and Tan ⅡA have antibacterial activity against C. acnes, Staphylococcus epidermidis and Staphylococcus aureus, which are acne-related pathogenic microorganisms (Zhu et al., 2022; Li and Zhou, 2018). Tan Ⅱa can inhibit the expression of toll-like receptor 2 (TLR2), nuclear factor-kappa B (NF-κB), and intercellular cell adhesion molecule-1 (ICAM-1), thereby suppressing C. acnes-induced inflammation and reducing the levels of inflammatory cytokines such as interleukin-1 beta (IL-1β), IL-8, and tumor necrosis factor-alpha (TNF-α) (Li and Zhou, 2018). CPT treatment alleviate acne inflammation, improve follicular keratinization and regulate the expression of IL-1α and androgen receptors (AR), demonstrating strong anti-inflammatory and anti-androgenic activities (Zuo et al., 2016).
In recent years, numerous clinical studies have explored the use of Tanshinone capsules for acne vulgaris. Therefore, we conducted a meta-analysis and systematic review of the past 2 decades to assess their efficacy and safety as a complementary therapy, providing evidence to guide clinical practice.
The methods employed in this study were registered in PROSPERO (registration number: CRD42024562320), and strictly adhered to the PRISMA statement (Page et al., 2021).
We performed a comprehensive search across 6 databases, including PubMed, the Cochrane Library, Embase, the Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang Databases (WF) and VIP, with no language restrictions, covering the 20-year period from 1 Jan 2004, to 1 June 2024. Two independent reviewers (DYT and FRL) conducted the search process. The search strategy integrated both Medical Subject Headings (MeSH) terms and free-text words. The language restriction was set to English and Chinese. The search terms included “acne vulgaris,” “acne,” and “Tanshinone capsule,” and related terms; the Chinese subject terms “Cuochuang” and “Danshentong” and related terms were used. The search strategies were adjusted according to the characteristics of different databases. Details of the search strategies are shown in Supplementary Materiale S4.
This study focused on randomized controlled trials (RCTs).
Patients with Acne vulgaris diagnosed by a clinician or according to recognized diagnostic criteria. There were no restrictions on age, gender, race, or disease duration.
The control group received conventional therapies for acne vulgaris, including topical retinoids, topical antimicrobial therapy, oral antibiotics, oral isotretinoin, Hormonal therapy, other treatments and adjunctive therapies, etc. The experimental group received Tanshinone capsules used alone or in combination with the control group treatment.
The relapse rate after treatment was set as primary outcome, and the levels of inflammatory factors, hormone levels, sebum secretion rate and Global Acne Grading System (GAGS) score were set as secondary outcomes. The adverse events (AEs) was set as safety outcome.
Studies meeting any of the following criteria were excluded: (1) studies focusing on other diseases accompanied by acneiform lesions, such as rosacea, SAPHO syndrome, polycystic ovary syndrome. (2) the treatment in control group or experiment group included unconventional therapy, such as acupuncture and moxibustion. (3) studies lacking mention of randomization (5) Studies lacking the specified outcome measures or reporting only AEs. (6) duplicate studies or those without full-text availability.
First, the primary literature retrieved from different databases was imported into the NoteExpress 3.8 software. After sequentially reading the titles, abstracts, and full texts, the final included studies were determined based on the inclusion and exclusion criteria. Second, two reviewers independently engaged in the screening of studies and extraction of data. Third, each included study was categorized, and Relevant data was extracted and recorded primarily including authors, publication years, sample sizes, gender ratios, intervention details, and outcome indicators. Fourth, the Cochrane Handbook (version 5.1.0) was used to evaluate the risk of bias according to the required items. Two independent reviewers (DYT and FRL) performed these tasks and if any discrepancies arose, they were resolved by a third researcher (LYW).
Meta-analysis was conducted using Review Manager (version 5.4). Continuous variables were expressed as mean difference (MD),while binary variables were expressed as risk ratio (RR), both with 95% confidence intervals (CIs). Heterogeneity was assessed using the I2 test. A fixed-effects model was adopted when I2 was <50%, indicating a low heterogeneity. Otherwise, a random-effects model was used. Subgroup analysis was conducted to investigate the potential influence of treatment duration and intervention methods for the experimental groups. Sensitivity analysis was conducted by excluding individual studies to assess the stability of the results. Publication bias was evaluated using Begg test and Egger test, facilitated by Stata SE 12.0 software. The quality of evidence for the outcome indicators was assessed using the GRADEpro system, which includes 5 downgrade factors and 3 upgrade factors.
Initially, we retrieved and identified 1,523 articles that met the study period criteria, and then 822 duplicate studies were removed. After screening the titles and abstracts, 573 articles were excluded. Following a detailed evaluation of the full texts, an additional 100 studies were excluded (reasons for exclusion are shown in Supplementary Table S2). Consequently, a total of 28 studies (Chen and Li, 2023; Ma, 2023; Gu et al., 2023; Zhang et al., 2022; Zou, 2022; Pei and Shang, 2022; Zhang et al., 2021; Luo and Liu, 2021; Cai et al., 2021; Song, 2020; Lan, 2019; Kang and Yang, 2019; Kang et al., 2019; Liu et al., 2018; Yang T. et al., 2018; Xia, 2018; Wu, 2018; Peng, 2017; Zhou et al., 2017; Yan and Dong, 2016; Chen et al., 2015; Qin et al., 2015; He et al., 2015; Jiang and Sheng, 2015; Zhao and Yan, 2015; Lin et al., 2009; Chen and Liu, 2007; Cong et al., 2019) were included in the analysis. Details of the screening process are shown in Figure 1.
The analysis included 28 studies with a total of 2,969 participants with acne vulgaris. There was a total of 1,599 participants in the experimental group and 1,370 participants in the control group. The age of participants ranged from 14 to 65 years old. The duration of acne ranged from 0.5 to 144 months. All trials were conducted in China and published in Chinese from 2007 to 2023. Among the 28 studies, 3 studies (Zhou et al., 2017; Lin et al., 2009; Chen and Liu, 2007) had experimental groups that received only Tanshinone capsules (Hebei xinglong Xili Pharmaceutical Co., Ltd) treatment, twenty one studies (Liu and Ma, 2023; Chen and Li, 2023; Ma, 2023; Gu et al., 2023; Zhang et al., 2022; Zou, 2022; Pei and Shang, 2022; Zhang et al., 2021; Luo and Liu, 2021; Cai et al., 2021; Song, 2020; Lan, 2019; Kang and Yang, 2019; Kang et al., 2019; Liu et al., 2018; Yang T. et al., 2018; Xia, 2018; Wu, 2018; Peng, 2017; Yan and Dong, 2016; Zhao and Yan, 2015) had treatment groups that received Tanshinone capsules treatment in addition to the control group’s treatment, and the remaining 4 studies (Chen et al., 2015; Qin et al., 2015; He et al., 2015; Jiang and Sheng, 2015) were three-arm studies that included both of these scenarios. The control group received one or more conventional treatments for acne vulgaris, including topical retinoids, topical antimicrobial therapy, oral antibiotics, oral isotretinoin, hormonal therapy, chemical peels and physical treatments. The characteristics of the included trials are shown in Table 1.
Regarding selection bias, fifteen studies (Liu and Ma, 2023; Chen and Li, 2023; Ma, 2023; Pei and Shang, 2022; Zhang et al., 2021; Luo and Liu, 2021; Song, 2020; Lan, 2019; Yang T. et al., 2018; Wu, 2018; Peng, 2017; Zhou et al., 2017; Chen et al., 2015; Qin et al., 2015; He et al., 2015) used the random number table method and 1 study (Gu et al., 2023) used random drawing method, which was rated as low. One study (Zhang et al., 2022) was considered high risk due to random grouping based on the order of patient visits. The remaining 11 studies (Zou, 2022; Cai et al., 2021; Kang and Yang, 2019; Kang et al., 2019; Liu et al., 2018; Xia, 2018; Yan and Dong, 2016; Jiang and Sheng, 2015; Zhao and Yan, 2015; Lin et al., 2009; Chen and Liu, 2007) did not report the method for generating random sequences and were rated as unclear risk. None of the studies explained the randomization method in detail, which was considered to be an unclear risk of bias. Due to the significant differences in the formulation of treatments between the treatment and control groups, performance bias was rated as high. For detection bias, one study (He et al., 2015) was considered low risk as outcome data collection and assessment was conducted under blinding. All studies had no patients fell off, all studies reported test indicators as planned, and there was no selective reporting of research results. It is unclear whether there were other examples of bias. The Risk of bias graph is shown in Figure 2.
A total of 15 studies (including two three-arm studies) (Ma, 2023; Zou, 2022; Zhang et al., 2021; Lan, 2019; Kang and Yang, 2019; Kang et al., 2019; Yang T. et al., 2018; Zhou et al., 2017; Yan and Dong, 2016; Qin et al., 2015; He et al., 2015; Lin et al., 2009; Chen and Liu, 2007) evaluated the relapse rate, involving 1,289 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.72, I2 = 0%). We found that the recurrence rate in the experimental group was lower than the control group (RR = 0.44, 95%CI 0.34 to 0.57, p < 0.00001) (Figure 3). The sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1A). Although Chen and Liu (2007) contributed a heavy weight (22.3%) to the analysis, the sensitivity analysis results remained consistent after its exclusion (RR exclusion Chen W 2007 = 0.42, 95% CI: 0.31 to 0.56, p < 0.00001, I2 = 0).
At the same time, to avoid the influence of different intervention methods on the analysis results, we conducted a subgroup analysis based on treatment durations (≤6 weeks, >6 weeks) and different intervention methods for the experimental group (combining systemic therapies, combining non-systemic therapies, combining both systemic and non-systemic therapies, and Tanshinone capsules only). The results suggested that regardless of whether the treatment period exceeded 6 weeks the recurrence rate in the experimental group was lower than that in the control group (RR≤ 6 weeks = 0.28, 95%CI: 0.16 to 0.49, p < 0.00001, I2 = 0%; RR >6 weeks = 0.51, 95%CI: 0.39 to 0.68, p < 0.00001, I2 = 0%) (Figure 4A). Additionally the results of the different intervention methods subgroup analysis were consistent with the overall results (RR Combining systemic therapies = 0.25, 95%CI: 0.13 to 0.46, p < 0.00001; RR Combining non systemic therapies = 0.43, 95%CI: 0.23 to 0.82, p = 0.01; RR Combining systemic and non systemic therapies = 0.48, 95%CI: 0.26 to 0.87, p = 0.02; RR Tanshinone capsules only = 0.59, 95%CI: 0.41 to 0.83, p = 0.002) (Figure 4B).
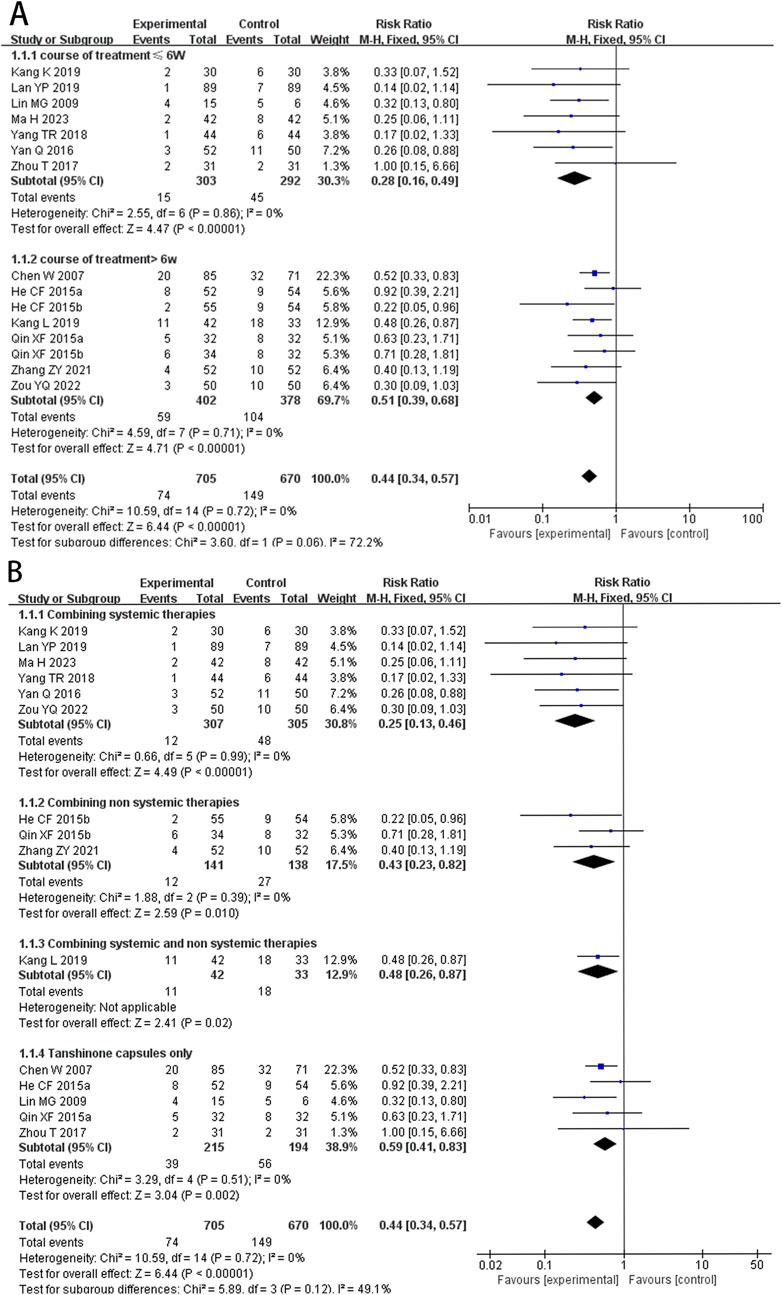
Figure 4. Forest plot for subgroup analysis of the relapse rate. (A) Different treatment course. (B) Different intervention methods for the experimental groups.
A total of 14 studies (including one three-arm study) (Liu and Ma, 2023; Chen and Li, 2023; Ma, 2023; Gu et al., 2023; Zhang et al., 2022; Luo and Liu, 2021; Cai et al., 2021; Song, 2020; Liu et al., 2018; Yang T. et al., 2018; Wu, 2018; Jiang and Sheng, 2015; Zhao and Yan, 2015) evaluated TNF-α levels, involving 1,261 patients. The random-effects model was used for subsequent meta-analysis because of the high heterogeneity among the studies (p < 0.00001, I2 = 94%). We found that TNF-α levels in the experimental group were lower than in the control group (MD = −10.18, 95%CI: −13.57 to −8.04, p < 0.00001) (Figure 5A). Sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1B).
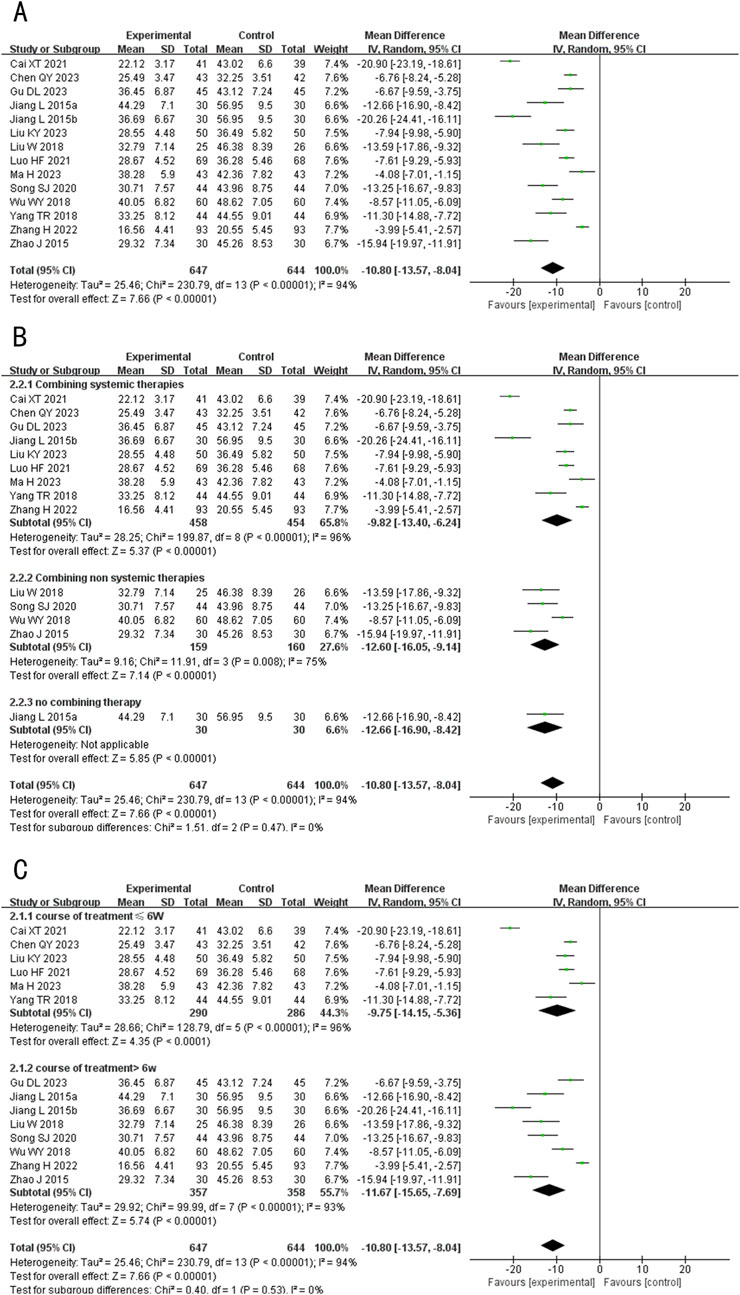
Figure 5. Forest plot of TNF-α level. (A) Forest plot for the total result of Tanshinone capsules versus Control group. (B) Subgroup analysis forest plot of different treatment course. (C) Subgroup analysis forest plot of different intervention methods for the experimental groups.
Subgroup analysis showed that regardless of treatment duration or intervention method, TNF-α levels in the treatment group were consistently lower than in the control group, aligning with the overall results (MD ≤6 weeks = −9.75, 95%CI: −14.15 to −5.36, p < 0.00001; MD >6 weeks = −11.67, 95%CI: −15.65 to −7.69, p < 0.00001; MD Combining systemic therapies = −9.82, 95%CI: −13.40 to −6.24, p < 0.00001; MD Combining non systemic therapies = −12.60, 95%CI: −16.05 to −9.14, p < 0.00001; MD Tanshinone capsules only = −10.80, 95%CI: −13.57 to −8.04, p < 0.00001) (Figures 5B,C).
A total of 3 studies (Ma, 2023; Luo and Liu, 2021; Yang T. et al., 2018) evaluated the IL-4 levels, involving 311 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.97, I2 = 0%). The results indicated that after treatment, the level of IL-4 in the experimental group was lower than in the control group after treatment (MD = −6.46, 95%CI: −7.14 to −5.77, p < 0.00001) (Figure 6). And the sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1C). Since all three studies had treatment durations of less than 6 weeks and the treatment groups combined systemic therapies, subgroup analysis was not conducted.
A total of 4 studies (Liu and Ma, 2023; Song, 2020; Wu, 2018; Zhou et al., 2017) evaluated the IL-6 levels, involving 370 patients. The random-effects model was used for subsequent meta-analysis because of the high heterogeneity among the studies (p < 0.00001, I2 = 99%). The results showed that after treatment, the level of IL-6 in the experimental group was lower than the control group (MD = −16.14, 95%CI: −30.10 to −2.18, p = 0.02) (Figure 7A). In the sensitivity analysis, no single study remarkably affected the effect sizes of IL-6 levels. However, after removing the studies Liu and Ma (2023), Song (2020), Wu (2018), the result was no longer significant (MD exclusion Liu KY 2023 = −10.18, 95%CI: −26.24 to 4.64, p = 0.17; MD exclusion Song SJ 2020 = −12.21, 95%CI: −29.63 to 5.22, p = 0.17; MD exclusion Wu WY 2018 = −17.16, 95%CI: −36.82 to 2.49, p = 0.09) (Supplementary Figure S1D).
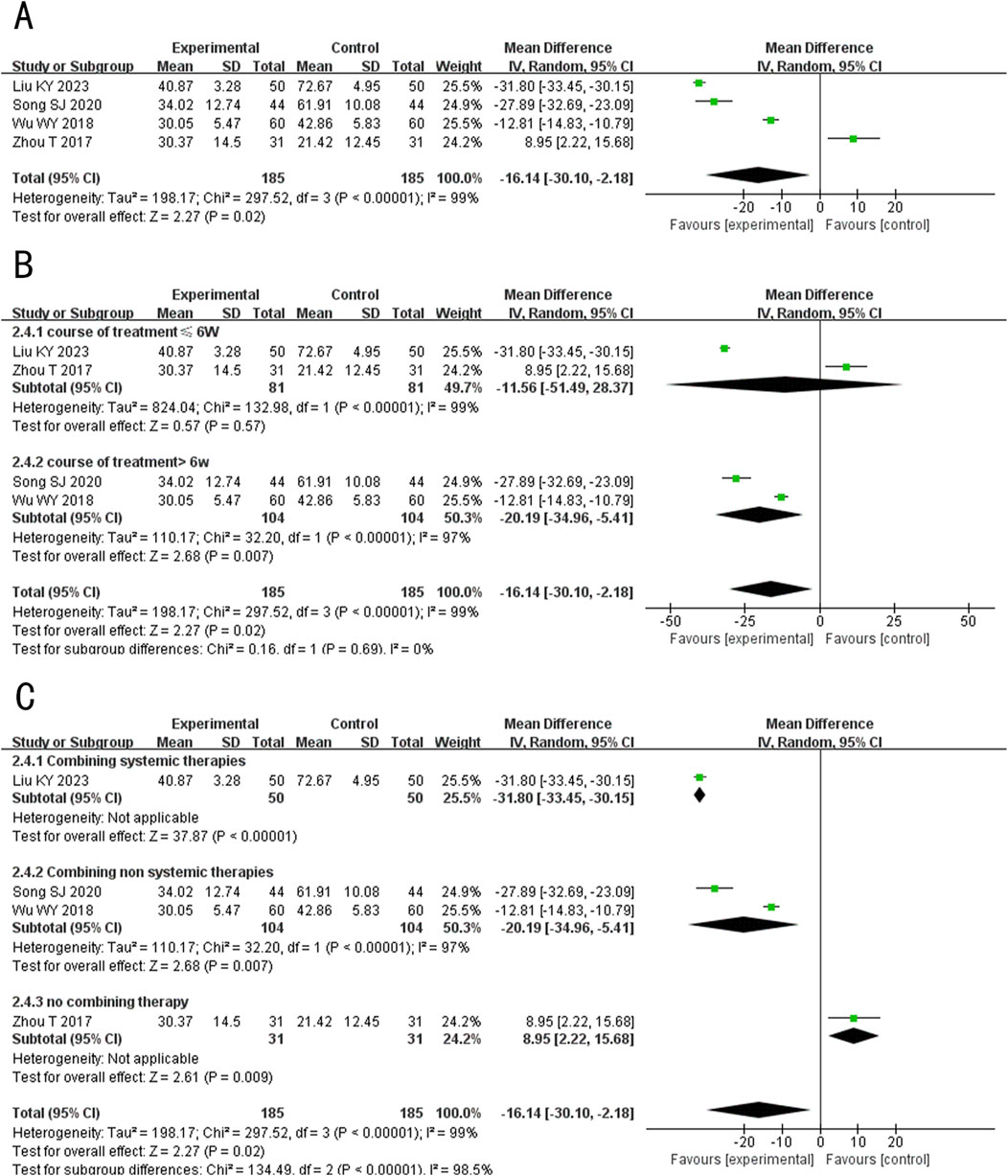
Figure 7. Forest plot of IL-6 level. (A) Forest plot for the total result of Tanshinone capsules versus Control group. (B) Subgroup analysis forest plot of different treatment course. (C) Subgroup analysis forest plot of different intervention methods for the experimental groups.
Subgroup analysis results showed that When the treatment duration was ≤6 weeks, there was no significant difference in the level of IL-6 between the two groups (MD≤6 weeks = −11.56, 95%CI: −51.49 to 28.37, p = 0.57). However, when the treatment duration exceeded 6 weeks, IL-6 levels in the experimental group were significantly lower (MD >6 weeks = −20.19, 95%CI: −34.96 to −5.41, p = 0.007) (Figure 7B). In the subgroup analysis of different intervention methods, the results for “Combining systemic therapies” and “Combining non-systemic therapies” were consistent with the overall findings (MD Combining systemic therapies = −31.80, 95%CI: −33.45 to −30.15, p < 0.00001; MD Combining non systemic therapies = −20.19, 95%CI: −34.96 to −5.41, p < 0.00001). However, the “Tanshinone capsules only” subgroup showed opposite results compared to the overall findings (MD Tanshinone capsules only = 8.95, 95%CI: 2.22 to −8.04, p < 0.00001) (Figure 7C).
A total of 9 studies (including one three-arm study) (Chen and Li, 2023; Gu et al., 2023; Song, 2020; Liu et al., 2018; Wu, 2018; Zhou et al., 2017; Jiang and Sheng, 2015; Zhao and Yan, 2015) evaluated the IL-8 levels, involving 646 patients. The random-effects model was used for subsequent meta-analysis because of the high heterogeneity among the studies (p < 0.00001, I2 = 93%). The results showed that after treatment, the IL-8 levels in the experimental group was lower than the control group (MD = −4.48, 95%CI: −8.30 to −0.65, p = 0.02) (Figure 8A). In the sensitivity analysis, no single study remarkably affected the effect sizes of IL-8 levels. However, after excluding the studies Chen and Li, (2023), Gu et al. (2023), Lan, (2019), Wu (2018), Jiang and Sheng (2015), the result was no longer significant (MD exclusion Chen QY 2023 = −4.06, 95%CI: −8.66 to 0.53, p = 0.08; MD exclusion Gu DL 2023 = −4.08, 95%CI: −8.54 to 0.37, p = 0.07; MD exclusion Song SJ 2020 = −3.07, 95%CI: −6.53 to 0.39, p = 0.08; MD exclusion Wu WY 2018 = −4.32, 95%CI: −9.03 to 0.40, p = 0.07; MD exclusion Jiang L 2015b = −4.04, 95%CI: −8.31 to 0.24, p = 0.06) (Supplementary Figure S1E).
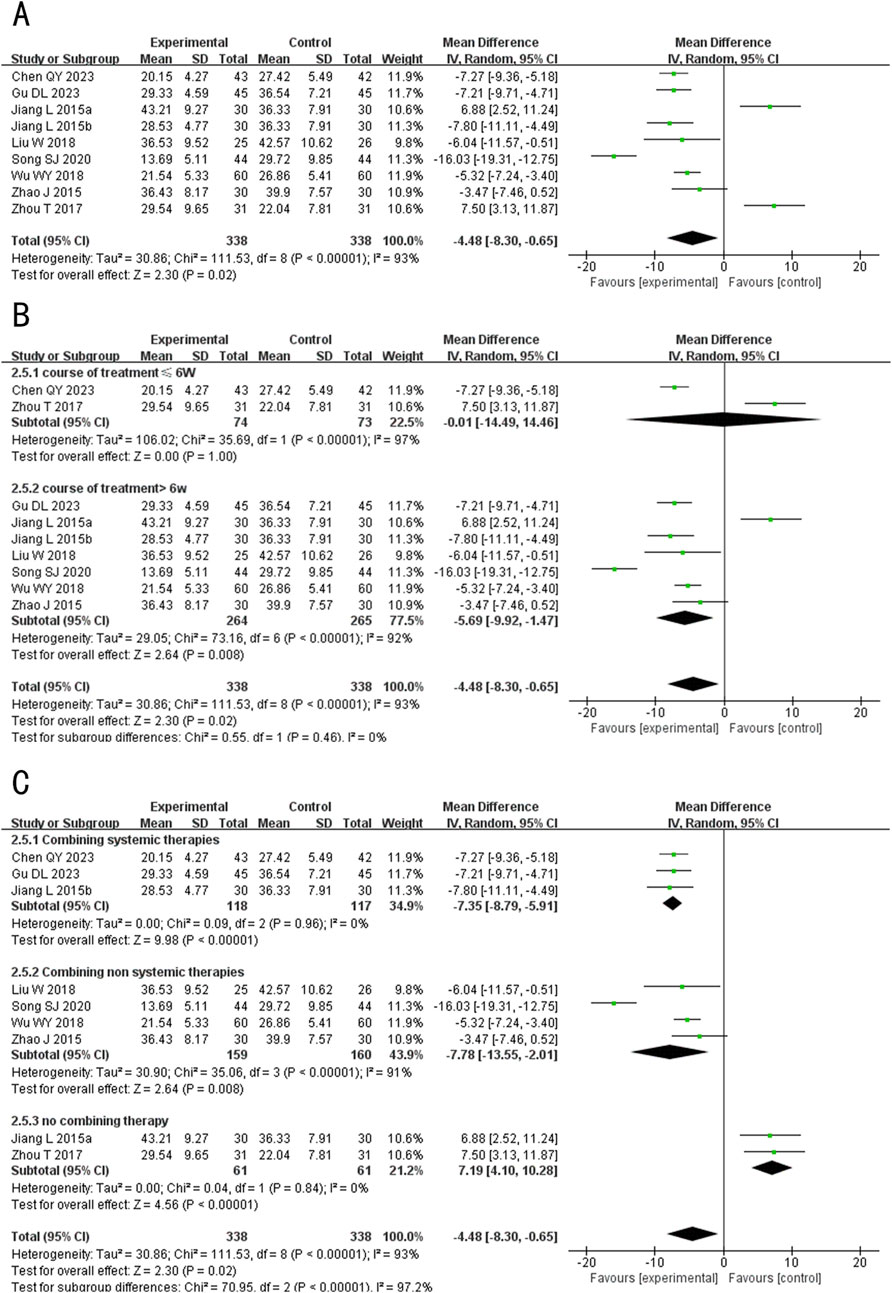
Figure 8. Forest plot of IL-8 level. (A) Forest plot for the total result of Tanshinone capsules versus Control group. (B) Subgroup analysis forest plot of different treatment course. (C) Subgroup analysis forest plot of different intervention methods for the experimental groups.
Subgroup analysis results showed that When the treatment duration was ≤6 weeks, there was no significant difference in the IL-8 levels between the two groups (MD≤6 weeks = −0.01, 95%CI: −14.49 to 14.46, p = 1.00). However, when the treatment duration exceeded 6 weeks, IL-6 levels in the experimental group were significantly lower (MD > 6 weeks = −5.69, 95%CI: −9.92 to −1.47, p = 0.008) (Figure 8B). In the subgroup analysis of different intervention methods, the results for “Combining systemic therapies” and “Combining non-systemic therapies” were consistent with the overall findings (MD Combining systemic therapies = −7.35, 95%CI: −8.97 to −5.91, p < 0.00001; MD Combining non systemic therapies = −7.78, 95%CI: −13.55 to −2.01, p = 0.008), while the “Tanshinone capsules only” subgroup showed opposite results compared to the overall findings (MD Tanshinone capsules only = 7.19, 95%CI: 4.10 to 10.28, p < 0.00001) (Figure 8C).
A total of 3 studies (Liu et al., 2018; Xia, 2018; Peng, 2017) evaluated the sebum secretion rate, involving 251 patients. The random-effects model was used for subsequent meta-analysis because of the high heterogeneity among the studies (p = 0.0010, I2 = 86%). The results showed that after treatment, the sebum secretion rate in the experimental group was lower than the control group (MD = −0.29, 95%CI: −0.49 to −0.10, p = 0.003) (Figure 9). Sensitivity analysis revealed that heterogeneity significantly decreased (p = 0.86, I2 = 0%) after excluding the study Peng LL 2017 (Peng, 2017), which had a lower initial sebum secretion rate and may have contributed to methodological heterogeneity (Supplementary Figure S1F).
A total of 2 studies (Zhang et al., 2022; Pei and Shang, 2022) evaluated the GAGS score, involving 226 patients. The random-effects model was used for subsequent meta-analysis because of the high heterogeneity among the studies (p < 0.0001, I2 = 94%). The results showed that after treatment, the GAGS score in the treatment group was lower than the control group (MD = −4.71, 95%CI: −7.62 to −1.80, p = 0.002) (Figure 10). Sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1G).
A total of 3 studies (including one three-arm study) (Peng, 2017; Chen et al., 2015) evaluated luteinizing hormone (LH) levels, involving 371 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.90, I2 = 0%). The results showed no statistically significant difference in LH levels between the experimental and control groups after treatment (MD = −0.01, 95%CI: −1.68 to 1.66, p = 0.99) (Figure 11A). The sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1H).
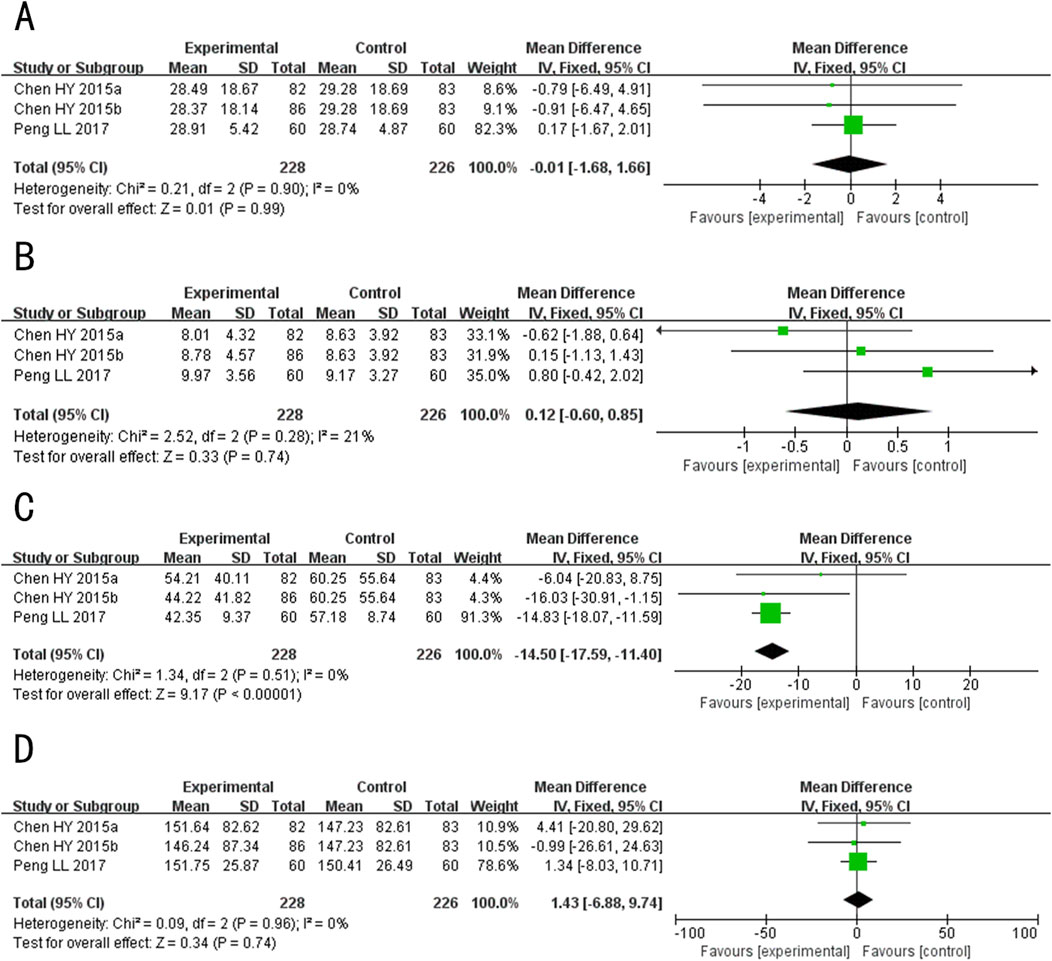
Figure 11. Forest plot for the hormone Levels of Tanshinone capsules versus Control group. (A) LH. (B) FSH. (C) T. (D) E2.
A total of 3 studies (including one three-arm study) (Peng, 2017; Chen et al., 2015) evaluated follicle-stimulating hormone (FSH) levels, involving 371 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.28, I2 = 21%). The results showed no statistically significant difference in the level of FSH between the experimental and control groups after treatment (MD = 0.12, 95%CI: −0.60 to 0.85, p = 0.74) (Figure 11B). The sensitivity analysis demonstrated that no single study had a significant impact on the overall results and the effect sizes of the FSH levels after treatment. However, after excluding Chen HY 2015b (Chen et al., 2015), the heterogeneity increased (p = 0.11, I2 = 60%) (Supplementary Figure S1I).
A total of 4 studies (including one three-arm study) (Gu et al., 2023; Peng, 2017; Chen et al., 2015) evaluated the testosterone (T) levels, but the study Gu DL 2023 (Gu et al., 2023) employed a different detection method, resulting in significant variations in the values. Consequently, only 3 studies (Peng, 2017; Chen et al., 2015) were ultimately combined (including one three-arm study), involving 371 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.52, I2 = 0%). The results showed that after treatment, T levels in the experimental group was lower than the control group (MD = −14.50, 95%CI: −17.59 to −11.40, p < 0.00001) (Figure 11C). In the sensitivity analysis, after excluding Peng LL 2017 (Peng, 2017), the result exceeded the original 95% confidence interval (MD exclusion Peng LL 2017 = −11.0, 95%CI: −21.49 to −0.25, p = 0.04) (Supplementary Figure S1J). This indicates that Peng LL 2017 (Peng, 2017) had a significant impact on the overall results and may be a key source of potential heterogeneity. Consequently, the meta-analysis results appear to be sensitive to this particular study, suggesting potential instability in the overall findings. We speculate that may arise from differences in patient demographics, as the majority of participants in other studies were female, potentially influencing the sensitivity analysis outcome.
A total of 4 studies (including one three-arm study) (Gu et al., 2023; Peng, 2017; Chen et al., 2015) evaluated the Estradiol (E2) levels after treatment, but the study Gu DL 2023 (Gu et al., 2023) employed a different detection method, resulting in significant variations in reported values. Consequently, only 3 studies (Peng, 2017; Chen et al., 2015) were ultimately combined (including one three-arm study), involving 371 patients. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.96, I2 = 0%). The results indicated no statistically significant difference in E2 levels between the experimental and control groups after treatment (MD = 1.43, 95%CI: −6.88 to 9.74, p = 0.74) (Figure 11D). The sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1K).
A total of 27 studies (including four three-arm studies) (Liu and Ma, 2023; Chen and Li, 2023; Ma, 2023; Gu et al., 2023; Zhang et al., 2022; Zou, 2022; Pei and Shang, 2022; Zhang et al., 2021; Luo and Liu, 2021; Cai et al., 2021; Lan, 2019; Kang and Yang, 2019; Kang et al., 2019; Liu et al., 2018; Yang T. et al., 2018; Zhou et al., 2017; Yan and Dong, 2016; Chen et al., 2015; Qin et al., 2015; He et al., 2015; Jiang and Sheng, 2015; Lin et al., 2009; Chen and Liu, 2007) reported the occurrence of AEs during treatment. However, Cai XT 2021 (Cai et al., 2021) reported no observed adverse reactions in either the experimental or control group. Consequently, only 26 studies (including four three-arm studies) (Liu and Ma, 2023; Chen and Li, 2023; Ma, 2023; Gu et al., 2023; Zhang et al., 2022; Zou, 2022; Pei and Shang, 2022; Zhang et al., 2021; Luo and Liu, 2021; Lan, 2019; Kang and Yang, 2019; Kang et al., 2019; Liu et al., 2018; Yang T. et al., 2018; Zhou et al., 2017; Yan and Dong, 2016; Chen et al., 2015; Qin et al., 2015; He et al., 2015; Jiang and Sheng, 2015; Lin et al., 2009; Chen and Liu, 2007) were ultimately combined, involving 2,421 patients. Three studies (Yan and Dong, 2016; Qin et al., 2015) (including one three-arm study) did not provide details of AEs. The main adverse reactions observed included gastrointestinal discomfort, skin flushing, and itching, among others. The fixed-effects model was used for subsequent meta-analysis because of the low heterogeneity among the studies (p = 0.81, I2 = 0%). The results indicated that the incidence of AEs during treatment was significantly lower in the experimental group compared to the control group (RR = 0.70, 95%CI: 0.56 to 0.87, p = 0.001) (Figure 12). The sensitivity analysis demonstrated low sensitivity, indicating that the results were robust against the exclusion of any single study (Supplementary Figure S1L).
Regarding the relapse rate, the Begg test results were: z = 2.47 (continuity corrected), Pr > |z| = 0.013 (continuity corrected), thus we conducted trim-and-fill test analysis, the results showed that Q = 1.961, p = 1.000, IOR = 0.416, 95%CI: 0.086 to 0.746; and the Egger test results were: t = −1.72, P > |t| = 0.108. These results indicated the absence of statistical significance in terms of publication bias (Figure 13).
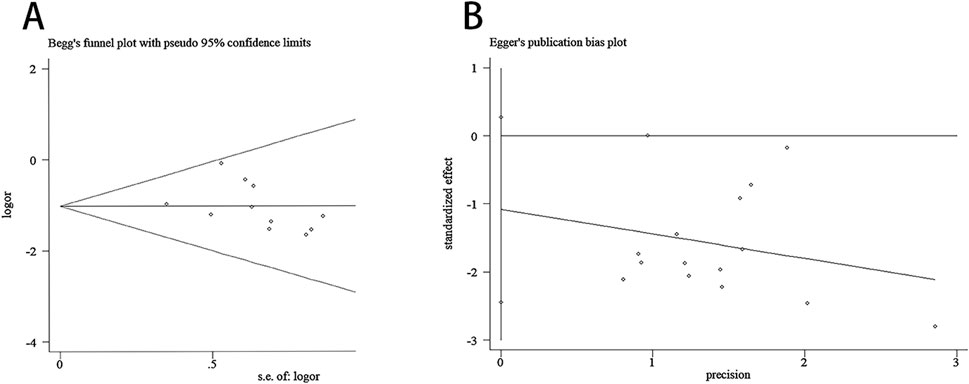
Figure 13. Begg test and Egger test. (A) Begg test for the relapse rate. (B) Egger test for the relapse rate.
The quality of evidence was assessed using the GRADEpro. The quality of evidence ranged from very low to moderate. The primary reasons for downgrading were inconsistency (high heterogeneity and uneven gender ratio), imprecision (small sample size) and risk of bias (unclear blinding). This is illustrated in Figure 14.
This study evaluated the efficacy and safety of Tanshinone capsules in treating acne vulgaris. A total of 28 trials involving 2,969 patients with acne vulgaris were included. The meta-analysis results demonstrated that Tanshinone capsules had a significant impact on reducing acne recurrence rates, lowering GAGS scores for skin lesions, and decreasing sebum secretion rates, suggesting that Tanshinone capsules can offer a significant and long-lasting improvement in acne lesions and associated symptoms. Furthermore, analysis of inflammatory factor levels indicated that Tanshinone capsules significantly reduced TNF-α, IL-4, IL-6, and IL-8 levels. Suggesting that Tanshinone capsules can effectively alleviate inflammation and prevent the progression of inflammatory conditions. Given the close relationship between acne onset and patients’ hormone levels, we also conducted a meta-analysis of hormone levels, the results showed that Tanshinone capsules effectively lowered testosterone levels, contributing to acne treatment. The effects of Tanshinone capsules on acne are attributable to multi-factors. However, although previous studies have reported that Tanshinone exhibits both anti-androgenic effects and estrogen-like activity, the meta-analysis did not find any significant differences in LH, FSH, or E2 levels. In terms of safety, none of the included studies reported severe adverse events, and the meta-analysis results showed that Tanshinone capsules were associated with fewer adverse events compared to control groups. In summary, Tanshinone capsules effectively improve acne lesions, reduce excessive sebum secretion, regulate inflammatory markers and testosterone levels, and demonstrate long-lasting efficacy with a favorable safety profile.
Tan ⅡA and CPT, the main active ingredients in Tanshinone capsules, show antibacterial activity against acne-related pathogenic microorganisms in vitro studies (Zhu et al., 2022; Li and Zhou, 2018). C. acnes activates the NF-κB pathway through TLR2, resulting in elevated levels of IL-1β, IL-6,IL-8, and TNF-α (Cong et al., 2019). Tanshinone can reduce the levels of IL-8, IL-6, IL-1β, and TNF-α in acne model rats, as profiled by lipidomics, with the mechanism possibly related to sphingolipid and glycerophospholipid metabolism pathways (Chen et al., 2021). Tan ⅡA treatment inhibits the TLR2/NF-κB pathway and suppress the expression of inflammatory cytokines IL-1β, IL-8, and TNF-α (Li and Zhou, 2018). CPT contributes to reducing the levels of inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α in acne model rats. This effect may be related to downregulating the expression of keratin, inhibiting glycolysis/gluconeogenesis and histidine metabolism, modulating lipid metabolism and altering sebum production, as well as downregulating the IL-17 signaling pathway, based on proteomics and metabolomics (Zhu et al., 2021). CPT helps restore the structure of skin microbiota, improve lipid metabolite composition and concentration, and negatively regulate the glycolysis pathway, thereby inhibiting excessive keratinocyte proliferation and reducing acne inflammation. (Zhu et al., 2022). Tan ⅡA and CPT can inhibit sebocyte proliferation and lipid synthesis, as well as downregulate AR expression (Ju et al., 2005). IL-4 expression is increased in acne hypertrophic scars, which is significantly importance for the prognosis of acne vulgaris (Yang J. H. et al., 2018). However, due to the unclear mechanism of Tanshinone’s effect on IL-4 during the course of acne, these results should be interpreted with caution. Furthermore, other studies have found that Tanshinones exhibit both anti-androgenic and estrogen-like activity (Zhang et al., 2019; Siddique et al., 2012).
Overall, Tanshinones can intervene in the growth of pathogenic microorganisms, lower inflammatory cytokine levels to alleviate inflammatory responses, suppress sebaceous gland hyperplasia and excessive lipid secretion, and regulate hormone levels, thereby treating acne.
We assessed the impact of course of treatment and intervention methods in the experimental groups on the recurrence rate and levels of inflammatory factors (excluding IL-4), which are relevant to clinical practice. We found that in different treatment duration subgroups, Tanshinone capsules consistently reduced recurrence rates and TNF-α levels. This effect was also observed when Tanshinone capsules were used as a monotherapy or as an adjunctive therapy in combination treatments. Notably, results of the subgroup analyses were not entirely consistent. For IL-6 and IL-8 levels, Tanshinone capsules used as monotherapy were less effective than conventional treatments. Additionally, subgroup analysis indicated that when the treatment duration was 6 weeks or less, there was no significant difference between the treatment and control groups in IL-6 and IL-8 levels. We speculate that these discrepancies may be due to the limited number of included studies, as these subgroups typically consist of only one or two studies. Therefore, these findings should be interpreted with caution.
The sensitivity analysis showed that most results were stable. However, after excluding a single study, we observed instances where the outcomes for IL-6, IL-8, T, FSH, and sebum secretion rate exceeded the original 95% confidence interval, lost statistical significance, or exhibited increased heterogeneity. These findings indicate potential result instability, which should be interpreted with caution.
Some limitations of this study need to be addressed. First, since all the included studies were conducted in China, the generalizability of the results to other ethnicities may be limited. Further research is needed to validate these findings across different ethnic groups. Second, the included studies often lack a randomized controlled trial framework and provide insufficient details on patient stratification criteria, leading to an increased risk of bias that reduces the credibility of the findings. Therefore, the conclusions should be interpreted with caution. Third, since the mechanism of Tanshinone’s effect on inflammatory cytokines in acne has not been fully elucidated, the related results should be interpreted with caution. Fourth, the maximum treatment duration was 3 months (only 1 study). Due to the limited number of studies and follow-up data, the long-term clinical benefits and potential risks associated with extended treatment durations remain unclear. Finally, due to the varying criteria used to evaluate adverse events and interventions across studies, the results regarding AEs should be interpreted with caution.
The findings of our study indicate that Tanshinone capsules have great potential for acne treatment. This medication could be considered as a complementary medicine and deserves further exploration. In terms of future clinical study designs, large-sample, multicenter, long-term RCTs should be conducted, strictly adhering to the Consolidated Standards of Reporting Trials (CONSORT) guidelines. At the same time, the quality could be improved by standardizing study protocols, unifying diagnostic criteria, enhancing randomization, allocation concealment, and blinding, as well as appropriately calculating sample sizes. In addition, it is essential to implement detailed long-term toxicity monitoring, create standardized patient follow-up protocols, select objective and scientific outcome indicators, and evaluate the impact on acne-related quality of life.
Furthermore, conducting comprehensive molecular and experimental pharmacological analysis, identifying potential biomarkers for predicting treatment response, and investigating the molecular mechanisms of Tan ⅡA and CPT in relation to their respective targets will enhance our understanding of the molecular characterization of Tanshinone. Simultaneously, applying advanced imaging and molecular tracking techniques, developing computational models to predict treatment response, and validating molecular mechanisms in clinical research will provide better guidance for clinical practice.
This study confirmed that Tanshinone capsules can alleviate acne lesions, reduce sebum secretion, lower recurrence rates, and regulate inflammatory factors and hormone levels. However, due to the low quality of evidence in the included studies, further well-designed, multicenter studies with large sample sizes and high methodological rigor are needed to validate these findings.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
YD: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Resources, Software, Validation, Writing–original draft. RF: Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Validation, Writing–original draft. BH: Data curation, Investigation, Writing–original draft. XR: Data curation, Investigation, Writing–original draft. FZ: Funding acquisition, Supervision, Writing–review and editing. HF: Funding acquisition, Supervision, Validation, Writing–review and editing. XW: Data curation, Investigation, Writing–original draft. YL: Data curation, Investigation, Writing–original draft. TL: Data curation, Investigation, Writing–original draft. LC: Conceptualization, Funding acquisition, Supervision, Writing–review and editing, Project administration. YL: Conceptualization, Funding acquisition, Supervision, Writing–review and editing, Project administration.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Beijing’s “14th Five-Year Plan” Key Specialty Project in Traditional Chinese Medicine (BJZKBC0015); the Beijing Natural Science Foundation Project (7,232,295); and Chinese Association of Chinese Medicine Chinese Herbal Formula Granules Standards Clinical Research Project (ZB_CACM_2024010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1520039/full#supplementary-material
Cai, X., Xu, C., Yang, D., Tang, X., Liang, S., and Gu, S. (2021). Research on clinical efficacy of tanshinone combined with doxycycline in the treatment of acne vulgaris and its effect on TNF-α. Pharm. Today. 31 (02), 157–160. doi:10.12048/i.issn.1674-229X.2021.02.018
Chen, H., Han, C., Qiu, G., and Liu, Z. (2015). Effect of tanshinone capsules combined spironolactone on sexhormone expression of female patients with postadolescent acne. Chin. J. Derm. Venereool. 29 (05), 469–471. doi:10.13735/j.cjdv.1001-7089.201409127
Chen, Q., and Li, J. (2023). Efficacy analysis of isotretinoin soft capsules combined with tanshinone capsules in patients with acne vulgaris. J. Huaihai. Med. 41 (04), 397–400. doi:10.14126/j.cnki.1008-7044.2023.04.017
Chen, T., Zhu, Z., Du, Q., Wang, Z., Wu, W., Xue, Y., et al. (2021). A skin lipidomics study reveals the therapeutic effects of tanshinones in a rat model of acne. Front. Pharmacol. 12, 675659. doi:10.3389/fphar.2021.675659
Chen, W., and Liu, Q. Z. (2007). Efficacy of oral tanshinone capsules in the treatment of acne. Chin. J. Drug. Appl. Monit. 4 (6), 38–39. doi:10.3969/j.issn.1672-8157.2007.06.014
Cong, T. X., Hao, D., Wen, X., Li, X. H., He, G., and Jiang, X. (2019). From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 311 (5), 337–349. doi:10.1007/s00403-019-01908-x
Fox, L., Csongradi, C., Aucamp, M., du Plessis, J., and Gerber, M. (2016). Treatment modalities for acne. Molecules 21 (8), 1063. doi:10.3390/molecules21081063
Gu, D., Xu, W., and Zhang, M. (2023). Effects of tanshinone combined with isotretinoin on sex hormone levels and serum IL-8 and TNF-α in patients with grade Ⅲ/Ⅳ acne. J. Harbin Med. Univ. 57 (06), 664–668. doi:10.20010/j.issn.1000-1905.2023.06.0664
Habeshian, K. A., and Cohen, B. A. (2020). Current issues in the treatment of acne vulgaris. Pediatrics 145 (Suppl. 2), S225–S230. doi:10.1542/peds.2019-2056L
Hay, R. J., Johns, N. E., Williams, H. C., Bolliger, I. W., Dellavalle, R. P., Margolis, D. J., et al. (2014). The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J. Invest. Dermatol. 134 (6), 1527–1534. doi:10.1038/jid.2013.446
He, C., Wang, H., Liu, W., Zhu, L., Yang, G., and Du, H. (2015). Efficacy of alpha hydroxy acids combined with tanshinone capsule in the treatment of moderate acne. Chin. J. Lepr. Skin. Dis. 31 (12), 723–725.
Jiang, L., and Sheng, G. (2015). Clinical observation of oral isotretinoin combined with tanshinone capsule in the treatment of 30 cases of acne and the effect on serum levers of IL-8 and TNF-α. Chin. J. Derm. Venereol. 29 (2), 213–216. doi:10.13735/i.cidv.1001-7089.201404105
Ju, Q., Yin, X., Shi, J., Xin, Y., Kang, X., Chen, Y., et al. (2005). Effects of cryptotanshinone and tanshinone A on proliferation, lipid synthesis and expression of androgen receptor mRNA in human sebocytes in vitro. Chin. J. Dermatol. 38 (02), 98–101.
Kang, K., Liang, L., and Zhang, X. (2019). Observation of the therapeutic efficacy of isotretinoin capsules combined with tanshinone capsules in the treatment of severe acne. Med. Diet. Health 0 (2), 11.
Kang, L., and Yang, X. (2019). Clinical observation of tanshinone combined with minocycline in the treatment of inflammatory acne. J. Clin. Dermatol. 48 (07), 451–453. doi:10.16761/j.cnki.1000-4963.2019.07.018
Lan, Y. (2019). Observation of the effect of tanshinone combined with isotretinoin in the treatment of severe acne. Dermatol. Venereol. 41 (6), 832–833. doi:10.3969/j.issn.1002-1310.2019.06.028
Li, Y., and Zhou, Y. (2018). The therapeutic effect of tanshinone IIA on Propionibacterium acnes-induced inflammation in vitro. Derm. Ther. 31 (6), e12716. doi:10.1111/dth.12716
Lin, M., Han, P., Dai, Y., Deng, B., and Zeng, H. (2009). Effect of tanshinone on serum testosterone and Estradiol levels in patients with acne vulgaris. Shandong. Med. J. 49 (48), 58–59.
Liu, K., and Ma, S. (2023). Efficacy of tanshinone capsules combined with conventional western medicine in the treatment of acne vulgaris. Clin. Med. 43 (08), 120–122. doi:10.19528/j.issn.1003-3548.2023.08.038
Liu, W., Lu, X., and Wu, L. (2018). Impact of tanshinone capsules combined with aminolevulinic acid photodynamic therapy on serum IL-8, TNF-α, and sebum secretion in patients with acne. Chin. J. Derm. Venerol. Integr. Trad. West. Med. 17 (5), 426–428.
Luo, H., and Liu, Z. (2021). Effect of tanshinone capsule combined with conventional western medicine on acne vulgaris. Chin. J. Gen. Pract. 19 (11), 1858–1860+1867. doi:10.16766/j.cnki.issn.1674-4152.002185
Ma, H. (2023). The effect of danshinone capsule combined with isoretinoic acid on the skin lesions and serum inflammatory factors in acne patients. Heilongjiang. Med. J. 47 (05), 558–560.
Oon, H. H., Wong, S. N., Aw, D. C. W., Cheong, W. K., Goh, C. L., and Tan, H. H. (2019). Acne management guidelines by the dermatological society of Singapore. J. Clin. Aesthet. Dermatol. 12 (7), 34–50.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pei, D., and Shang, G. (2022). Efficacy and safety of tanshinone capsule combined with red and blue light irradiation in treatment of acne vulgaris. J. Med. Aesthet. Cosmetol. 31 (12), 82–85. doi:10.3969/i.issn.1004-4949.2022.12.024
Peng, L. (2017). The influence of tanshinone on serum sex hormone level of patients with acne. Henan Trad. Chin. Med. 37 (06), 1062–1064. doi:10.16367/j.issn.1003-5028.2017.06.0375
Qin, X., Guo, Z., and Gu, J. (2015). Observation on the effect of tanshinone capsules combined with benzoyl peroxide gel in the treatment of mild to moderate acne vulgaris. J. Pract. Derm. 8 (03), 188–190.
Reynolds, R. V., Yeung, H., Cheng, C. E., Cook-Bolden, F., Desai, S. R., Druby, K. M., et al. (2024). Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 90 (5), 1006.e1–1006.e30. doi:10.1016/j.jaad.2023.12.017
Siddique, H. R., Nanda, S., Parray, A., and Saleem, M. (2012). Androgen receptor in human health: a potential therapeutic target. Curr. Drug. Targets 13 (14), 1907–1916. doi:10.2174/138945012804545579
Song, S. (2020). Efficacy of LED red-blue light therapy, isotretinoin-clindamycin gel, and tanshinone capsules in the treatment of facial acne. Henan. Med. Res. 29 (5), 836–838. doi:10.3969/i.issn.1004-437X.2020.05.031
Stamu-O'Brien, C., Jafferany, M., Carniciu, S., and Abdelmaksoud, A. (2021). Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J. Cosmet. Dermatol. 20 (4), 1080–1083. doi:10.1111/jocd.13765
State Food and Drug Administration of China (2010). Tanshinone capsules. Drug. Stand. Chin. Cas. (4), 309–310.
Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380 (9859), 2163–2196. doi:10.1016/S0140-6736(12)61729-2
Wang, Y., Zhu, M., Wu, S., and Zheng, H. (2022). Acne comorbidities. Clin. Cosmet. Investig. Dermatol. 15, 2415–2420. doi:10.2147/CCID.S392165
Williams, H. C., Dellavalle, R. P., and Garner, S. (2012). Acne vulgaris. Lancet 379 (9813), 361–372. doi:10.1016/S0140-6736(11)60321-8
Wu, W. (2018). Clinical effect of tanshinone combined with isotretinoin on moderate acne vulgaris and its effect on serum TNF-α, IL-8 and IL-6. Clin. Res. Pract. 3 (17), 50–51. doi:10.19347/j.cnki.2096-1413.201817024
Xia, Q. (2018). Observation of the efficacy of tanshinone combined with fruit acid in the treatment of acne. J. Med. Aesthet. Cosmetol. 27 (7), 43.
Yan, Q., and Dong, X. (2016). Clinical efficacy of isotretinoin capsules combined with tanshinone capsules for moderate to severe acne. Chin. J. Dis. Control. Prev. 20 (07), 752–754. doi:10.16462/j.cnki.zhjbkz.2016.07.028
Yang, J. H., Yoon, J. Y., Moon, J., Min, S., Kwon, H. H., and Suh, D. H. (2018b). Expression of inflammatory and fibrogenetic markers in acne hypertrophic scar formation: focusing on role of TGF-β and IGF-1R. Arch. Dermatol. Res. 310 (8), 665–673. doi:10.1007/s00403-018-1856-2
Yang, T., Yi, P., and Cheng, M. (2018a). Efficacy of tanshinone capsules and minocycline hydrochloride capsules on patients with acne: impact on skin lesions and serum inflammatory factors. J. Prev. Med. Chin. People's Lib. Army. 36 (4), 510–512. doi:10.13704/j.cnki.jyyx.2018.04.025
Zhang, H., Lai, Y., Zhang, W., and Chen, J. (2022). Efficacy analysis of tanshinone capsules combined with viaminate capsules in the treatment of moderate acne. Pract. J. Clin. Med. 19 (05), 103–106.
Zhang, T., Zhong, S., Wang, Y., Dong, S., Guan, T., Hou, L., et al. (2019). In vitro and in silico perspectives on estrogenicity of tanshinones from Salvia miltiorrhiza. Food. Chem. 270, 281–286. doi:10.1016/j.foodchem.2018.07.098
Zhang, Z., Zhang, P., Wang, F., Wang, X., Zhao, S., and Wang, P. (2021). Effect of tanshinone capsule combined with 585 nm intense pulse photon on acne vulgaris. J. Logist. Univ. CAPF, Med. Sci. 30 (09), 75–76+79. doi:10.16548/j.2095-3720.2021.09.055
Zhao, J., and Yan, X. (2015). Effect of Red-Blue Light combined Tanshinone on moderate or severe acne vulgaris and the effects on levels of IL-8 and TNF-α in peripheral blood. Mod. J. Integr. Tradit. Chin. West. Med. 24 (15), 1603–1605+1608. doi:10.3969/i.issn.1008-8849.2015.15.003
Zhou, T., Yang, L., Liu, Q., Zhao, C., and Zhang, F. (2017). The clinical effect of Qingfei-Jiedu decoction for the female patients with acne of lung and stomach heat type. Int. J. Trad. Chin. Med. 39 (7), 597–600. doi:10.3760/cma.j.issn.1673-4246.2017.07.006
Zhu, Z., Chen, T., Wang, Z., Xue, Y., Wu, W., Wang, Y., et al. (2021). Integrated proteomics and metabolomics link acne to the action mechanisms of cryptotanshinone intervention. Front. Pharmacol. 12, 700696. doi:10.3389/fphar.2021.700696
Zhu, Z., Zeng, Q., Wang, Z., Xue, Y., Chen, T., Hu, Y., et al. (2022). Skin microbiome reconstruction and lipid metabolism profile alteration reveal the treatment mechanism of Cryptotanshinone in the acne rat. Phytomedicine 101, 154101. doi:10.1016/j.phymed.2022.154101
Zou, Y. (2022). Clinical efficacy of isotretinoin soft capsules combined with tanshinone capsules in the treatment of moderate to severe acne. Med. Equip. 35 (2), 105–106.
Keywords: Tanshinone capsule, Acne vulgaris, meta-analysis, systematic review, randomized controlled trial
Citation: Deng Y, Feng R, Hu B, Ren X, Zhang F, Feng H, Wang X, Li Y, Liu T, Cai L and Li Y (2025) Efficacy and safety of Tanshinone capsule in Acne vulgaris: a systematic review and meta-analysis. Front. Pharmacol. 16:1520039. doi: 10.3389/fphar.2025.1520039
Received: 30 October 2024; Accepted: 10 March 2025;
Published: 31 March 2025.
Edited by:
Hongbo Li, Shaanxi University of Science and Technology, ChinaReviewed by:
Germain Sotoing Taiwe, University of Buea, CameroonCopyright © 2025 Deng, Feng, Hu, Ren, Zhang, Feng, Wang, Li, Liu, Cai and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanwen Li, MTU4MTAxMDQ5MDJAMTYzLmNvbQ==; Lingling Cai, bGluZ2xpbmc4OTE1OTE2NkAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.