- 1Evidence-Based Medicine Center, MOE Virtual Research Center of Evidence-based Medicine at Zunyi Medical University, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 2Department of General Practice, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 3Geriatric Medicine Department, Affiliated Hospital (GuiAn) of Guizhou Medical University, Guiyang, Guizhou, China
- 4Department of Oncology, Tongren People’s Hospital, Tongren, Guizhou, China
- 5Department of Pharmacy, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
- 6Internal Medicine Department, 96603 Hospital, Huaihua, Hunan, China
- 7Department of Oncology, Lishui People’s Hospital, Sixth Affiliated Hospital of Wenzhou Medical University, Lishui, Zhejiang, China
Introduction: Sophorae flavescentis (kushen) preparations are widely used to control malignant pleural effusion (MPE) through intrapleural perfusion.
Objectives: This analysis aims to verify the therapeutic values of perfusion with kushen preparations for controlling MPE, reveal the optimal treatment plan, suitable population, and usage, and to demonstrate their clinical effectiveness and safety.
Methods: We performed and reported this systematic review/meta-analysis (PROSPERO: CRD42023430139) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. All randomized controlled trials (RCTs) concerning perfusion with kushen preparation for MPE were collected from Chinese and English databases. We clustered all eligible studies into multiple homogeneous treatment units, assessed their methodological quality using a RoB 2, pooled the data from each unit, and summarized the quality of the evidence.
Results: We included 83 RCTs reporting three types of kushen preparation: compound kushen injection (CKI), kang’ai injection, and matrine injection. All trials were clustered into perfusion with CKI alone or with the addition of sclerosants, kang’ai, or matrine-plus platinum for controlling MPE. Compared with cisplatin alone, perfusion with CKI alone displayed a similar complete response, pleurodesis failure, and pleural progression (odds ratios =1.10, 95% CI 0.76 to 1.60; 0.80, 0.56 to 1.14; 0.63, 0.33 to 1.21). Of 14 homogeneous treatment plans, perfusion with CKI and cisplatin significantly improved the complete response (2.71, 2.30 to 3.19) and showed low pleurodesis failure (0.26, 0.22 to 0.32), pleural progression (0.22, 0.14 to 0.36), myelosuppression (0.34, 0.24 to 0.47), neutropenia (0.35, 0.26 to 0.46), gastrointestinal reaction (0.36, 0.29 to 0.44), hepatorenal toxicity (0.42, 0.28 to 0.63 and 0.32, 0.24 to 0.44), and fever (0.50, 0.30 to 0.82). These results were moderate quality (⊕⊕⊕Ο) supported by firm or conclusive information. Additionally, perfusion with kang’ai or matrine and cisplatin also improved the complete response (3.04, 1.76 to 5.26 and 1.87, 1.26 to 2.78) and displayed low pleurodesis failure (0.23, 0.14 to 0.41 and 0.27, 0.17 to 0.44). The results were moderate to low quality (⊕⊕⊕Ο to ⊕⊕ΟΟ).
Conclusion: Current moderate evidence demonstrates that CKI may be an effective palliative intervention for MPE which, combined with cisplatin, may be an optimal treatment plan. Kang’ai or matrine may be other potential choices.
Systematic Review Registration:: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023430139
1 Introduction
The dried root of the shrub Sophora flavescens Aiton (Chinese name: kushen) is an important herbal medicine in China, Japan, Korea, India, and in some of Europe (He et al., 2015;Liang et al., 2019). It contains active components such as matrine, oxymatrine, sophoridine, flavonoids, alkylxanthones, quinones, triterpene glycosides, fatty acids, and essential oils (Cao and He, 2020; Chen et al., 2021; Chen et al., 2022). Its matrine and oxymatrine show significant anti-tumor activities by inhibiting tumor cell proliferation, inducing apoptosis, regulating the tumor microenvironment, and down-regulating cancer-related inflammation (Guo et al., 2015; Ma et al., 2016; Cao and He, 2020; Chen et al., 2021; Chen et al., 2022; Liu et al., 2023). In China, three traditional Chinese medicine injections (TCMIs)—compound kushen injection (CKI), kang’ai, and matrine injection—were developed, with S. flavescens extracts including matrine and oxymatrine as the core components (Supplementary Material S1 and Supplementary Table S1). In this analysis, we defined three types of injection as S. flavescens (kushen) preparations. CKI mainly contains ethanol and water extracts such as matrine, oxymatrine, and sophoridine, which are extracted from S. flavescens Aiton (kushen) and Heterosmilax yunnanensis Gagnep (baituling) (Guo et al., 2015; Ma et al., 2016; Liu et al., 2023). Kang’ai injection contains multiple ingredients including Astragalus polysaccharides, astragalosides, ginsenosides, ginseng polysaccharides, and oxymatrine, which are extracted from kushen, ginseng (Panax ginseng C.A. Mey), and Astragalus membranaceus (Fisch.) Bunge (Fabaceae) (Wan et al., 2018; Sun et al., 2021). Matrine injection is a chemical drug derived from kushen. Clinically, three types of kushen preparations have been approved by the China Food and Drug Administration for adjuvant therapy of solid tumors (Ma et al., 2016; Wang et al., 2016; Li H. et al., 2019; Liu et al., 2022; Liu et al., 2023).
Malignant pleural effusion (MPE), a frequent complication often secondary to metastases to the pleura, originates from intra- or extra-thoracic malignant tumors (Hassan et al., 2021; Gayen, 2022). Patients with MPE often experience progressive breathlessness, tumor progression, and poor survival. Currently, effective control of pleural effusion, improvement of clinical symptoms, and quality of life (QOL) have become the main treatment goals for symptomatic MPE and suspected expandable lung patients (Bibby et al., 2018; Feller-Kopman et al., 2018). Excluding malignant tumors, CKI, kang’ai, and matrine injections are commonly used to control MPE through intrapleural perfusion (Yang et al., 2016; Wu et al., 2018; Li B. et al., 2019; Xu et al., 2022). According to the Cochrane systematic evaluation, five systematic reviews/meta-analyses (SRs/meta-analyses) (Tang et al., 2014; Biaoxue et al., 2015; Xu et al., 2015; Yang et al., 2016; Wu et al., 2018) reported that kushen preparations might increase clinical response rate and improve QOL with a low adverse drug reactions (ADRs) in MPE. But these SRs/meta-analyses (Tang et al., 2014; Biaoxue et al., 2015; Xu et al., 2015; Yang et al., 2016; Wu et al., 2018) exhibited significant clinical heterogeneity, conducted inappropriate data analysis, and involved 16 ineligible studies (Supplementary Tables S3, S4). They also lacked rigorous and reasonable methodologies such as prior planning and systematic retrieval. These deficiencies undermine the credibility of their conclusions, which easily mislead clinical decision-making.
At present, no evaluation has revealed their clinical value for perfusion with kushen preparation alone for MPE. No evidence has confirmed its optimal treatment plan, indications, usage, and how to reasonably apply kushen preparation to achieve expected clinical efficacy and safety. Since the publication of the latest SR/meta-analysis in 2018, (Wu et al., 2018), 23 trials (Supplementary Material S3) have been published (Huang, 2021; Feng and Shi, 2023; Lin et al., 2023; Wang R. et al., 2023). We further performed a registered SR/meta-analysis to verify the therapeutic value of kushen preparations for controlling MPE, reveal their optimal treatment plan, suitable population and usage, and demonstrate their clinical effectiveness and safety. A new evidence framework will be developed for clinical decision-making about the reasonable application of kushen preparations to control MPE and further new research projects.
2 Materials and methods
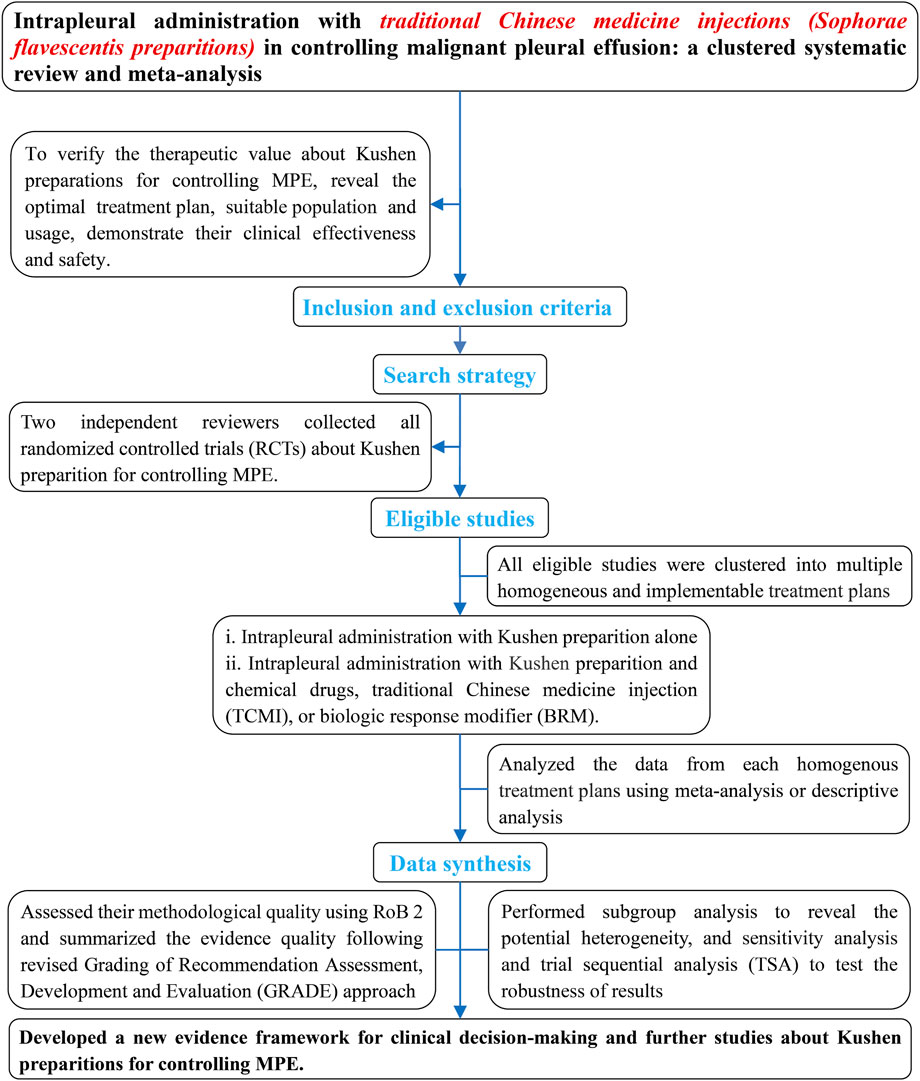
Kushen preparations mainly include CKI, kang’ai, and matrine. To verify their therapeutic value for controlling MPE, we systematically and comprehensively collected all eligible studies about kushen preparations for controlling MPE (Figure 1). These were clustered into multiple homogeneous and implementable treatment units such as CKI alone, and CKI, kang’ai, or matrine and cisplatin, nedaplatin, or carboplatin. We then further evaluated their methodological quality and pooled the data from each treatment unit and finally summarized and developed an evidence framework for rational drug use decision-making and future research projects. We registered this analysis on PROSPERO (CRD42023430139) and reported all findings according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA 2020 Checklist) (Page et al., 2021). During the retrieval, selection, evaluation of methodological quality, data collection, statistical analysis, and summary of evidence, any disagreements were resolved through discussion with each other or with Zheng Xiao. Ethical approval was not required as the materials were published studies.
2.1 Inclusion and exclusion criteria
According to the PICOS model, we established the following criteria for all eligible studies to meet.
(i). Only optimum trials as randomized controlled trials (RCTs) without restrictions on follow-up, institutions, language, and publication time.
(ii). All patients presented with MPE and dyspnea which was diagnosed by thorax imaging, pleural fluid analysis, cytology, or pleural biopsy. All patients had normal liver, kidney, and heart function, and no limitations on tumor type and pleural fluid volume.
(iii). The interventions were kushen preparations such as CKI, kang’ai, and matrine injection through intrapleural perfusion. Both groups did not receive any intrapleural perfusion 1 month before treatment. The experimental groups received kushen preparation alone or in combination with other sclerosants, and the controls received sclerosants alone such as chemical drugs, biological response modifiers (BRMs), or TCMI.
(iv). The main outcomes are clinical response and survival, and secondary outcomes are QOL and adverse events.
All ineligible studies must meet the following criteria: studies about patients with ascites or pericardial effusion; all patients receiving systemic chemotherapy, local hyperthermia or oral traditional Chinese medicine (TCM); both groups receiving kushen preparation; studies with unclear objectives; without any data about clinical responses, survivals, QOL, or adverse events.
2.2 Outcomes definition
The primary outcomes are clinical response and survival. Referring to previous studies (Paladine et al., 1976; Kessinger and Wigton, 1987; Keeratichananont et al., 2015; Jie Wang et al., 2018; Dipper et al., 2020; Xiao et al., 2020a), we integrated both Millar and Ostrowskimj criteria to measure the clinical responses as: (i) complete response (CR) is the disappearance of pleural effusion for more than 30 days, or the lack of accumulation of fluid; (ii) partial response (PR) is less than 50% reduction of pleural effusion for more than 30 days; (iii) no response (NR)/stable disease (SD) is less than 50% reduction of pleural effusion or less than 25% increase or the recurrence of fluid accumulation without further therapy; (iv) pleural progression (PP) is more than 25% increase of pleural effusion or symptomatic fluid accumulation again requiring further therapy. We set the pleurodesis failure as no response or stable disease plus pleural progression and assessed the clinical responses using complete response, pleurodesis failure, and pleural progression (Supplementary Material S2). Long-term survival was assessed by using overall survival (OS), progression-free survival (PFS), OS, and PFS rates. According to the Karnofsky performance status (KPS) scale, when a KPS score increased ≥10 after treatment, QOL was improved.
Adverse events (AEs) were assessed by using ADRs and thoracentesis-related adverse events (TRAEs). According to World Health Organization (WHO) or Common Terminology Criteria for Adverse Events (CTCAEs) criteria (Miller et al., 1981; Trotti et al., 2003), ADRs were measured by using the indicators myelosuppression, neutropenia, thrombocytopenia, anemia, hepatorenal toxicity, gastrointestinal reactions, thoracodynia, and fever. TRAEs were measured by using indicators including treatment-related death, respiratory failure, dyspnea, pneumothorax, chest infection, drainage tube detachment, tumor metastasis along the indwelling duct, catheter-related infection, or subcutaneous emphysema.
2.3 Retrieval and selection strategies
Adhering to a retrieval logic of patient plus intervention, we customized the retrieval strategies for each database using MeSH and free words (Supplementary Material S3). Yan Zhang and Hui Liu independently searched all related studies about “Kushen preparations in controlling MPE” from Chinese and English electronic databases (to February 2025) including the Guizhou Digital Library, SinoMed, China National Knowledge Infrastructure Database, WanFang Database, Chinese Scientific Journals Full-text Database, PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials (Issue 2, February 2025). We collected ongoing trials from the Chinese Clinical Trial Registry (http://www.chictr.org.cn), WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/), and US clinical trials (https://clinicaltrials.gov). Finally, we also identified eligible studies from the references of relevant SRs or network meta-analysis. Hui Liu and Yan Zhang independently selected eligibles and excluded ineligible studies following a predesigned inclusion and exclusion criteria.
2.4 Assessment of methodological quality
For clinical responses, survivals, QOL, or adverse events, Da-chun Cai and Jiao Xu independently applied a revised Cochrane tool (RoB 2) to assess methodological quality arising from five domains: randomization process (D1), intended interventions (D2), missing outcome data (D3), outcomes measurement (D4), and selective reporting of results (D5) (Sterne et al., 2019; Higgins et al., 2021). We judged each quality based on the domain algorithm and made an overall judgment.
2.5 Data collection
Yao-Qin Luo and Da-chun Cai independently collected all data using a predesigned data extraction form. The data were first author, time of publication, methodological features, demographic characteristics and cases; characteristics of patients as tumor types, pleural fluid volume, anticipated survival time (AST), KPS score, treatment history, and recurrence; drainage methods as indwelling pleural catheters (IPCs) or thoracentesis; kushen preparations, treatment dose, frequency and times, and sclerosants and uses; follow-up protocol, research institutions, criterion and time of evaluation. The outcomes were: complete response, pleurodesis failure, pleural progression, PFS, OS, QOL, ADRs, and TRAEs. Additionally, the authors of papers were contacted about available survival data. If they were unavailable, the Kaplan–Meier survival curves were transformed into data using Engauge Digitizer 4.1 (Guyot et al., 2012; Xiao et al., 2018).
2.6 Statistical analysis
All eligible studies were clustered into multiple homogeneous treatment units, and we further analyzed their clinical effectiveness and safety. The odds ratios (ORs) and their 95% confidence interval (CI) were applied to measure the complete response, pleurodesis failure, pleural progression, OS rate, QOL, ADRs, and TRAEs, with p < 0.05 being identified as statistically significant. Cochran’s χ2 test and I2 statistic were performed to identify statistical heterogeneity among each unit. If the results showed significant heterogeneity and inconsistent directions or involved a single trial, we used forest plots to describe the result. When p ≥ 0.1 and I2 ≤ 50%, a fixed-effects model (FEM) was applied to pool the OR and their 95% CI. When p < 0.1, I2 > 50%, and the results had consistent direction, a random-effects model (REM) was applied. Yan Zhang and Feng Luo independently applied Review Manager 5.4 to pool the data from each unit. If the outcomes involved more than ten trials, a funnel plot and Egger’s test (STATA V.15.0 software, 401506209499) were applied to identify potential publication bias.
Referring to previous experience (Xiao et al., 2020b; Wang et al., 2021; Wang et al., 2022; Wang C. Q. et al., 2023), a subgroup analysis was implemented to reveal the potential clinical heterogeneity among the main treatment plans with enough trials to analyze the effects of patient related factors, interventions, and evaluation criteria on clinical responses and to further identify the suitable population and optimum usage. We further implemented univariate random effects meta-regression analysis to reveal the correlation between each factor and clinical responses and post hoc multiple regression analysis to identify it.
Following underestimation of effectiveness/safety, we implemented sensitivity analysis to identify robustness (Xiao et al., 2020b; Wang et al., 2021; Wang et al., 2022; Wang C. Q. et al., 2023). The consistency of results before and after excluding both trials with high risk and overestimation were analyzed. If consistency was good, the result was robust; otherwise, it was poor. To identify the required information size (RIS) for the results of main treatment units (Thorlund et al., 2016), we further applied Trial Sequential Analysis (TSA) software (version 0.9.5.10 Beta) to implement the analysis. In the light of previous experience, we set the risk of type I error as 5% with a power of 80%, relative risk reduction (RRR) as 25% for clinical responses and QOL, and 20% for adverse events (AEs) (Wetterslev et al., 2008; Thorlund et al., 2009). We used control event rates from this analysis for these calculation, and adjusted the information size for diversity (Wetterslev et al., 2009).
2.7 Summary of evidence quality
We integrated the results of sensitivity analysis into the GRADE approach (Guyatt et al., 2008; Xiao et al., 2020b; Wang et al., 2021; Wang et al., 2022; Wang C. Q. et al., 2023) and developed a revised approach to summarize the evidence. Quality was identified as “high”, moderate”, “low”, and “very low” following five domains: risk-of-bias of results, heterogeneity, indirectness, imprecision, and publication bias (Supplementary Material S2). Jun Huang and Yan-Yan Jin independently applied the GRADE profiler to summarize the evidence quality and generated the absolute estimates of effect for outcomes.
3 Results
3.1 Search results
After retrieval, 1,269 records were identified. Two reviewers read the titles, excluded duplicates, and identified 443 records. After screening abstracts and excluding irrelevant and non RCTs, 147 RCTs, six SRs/meta-analyses (Tian et al., 2010; Tang et al., 2014; Biaoxue et al., 2015; Xu et al., 2015; Yang et al., 2016; Wu et al., 2018) and four network meta-analyses (Yang et al., 2017; Li B. et al., 2019; Li, 2022; Xu et al., 2022) were selected. Further evaluating full-texts and excluding 64 ineligible studies (Supplementary Material S3), 83 were considered eligible. Additionally, 42 studies were selected from previous studies. Finally excluding duplicates, 83 eligible studies were selected for this analysis (Figure 2).
3.2 Characteristics of included studies
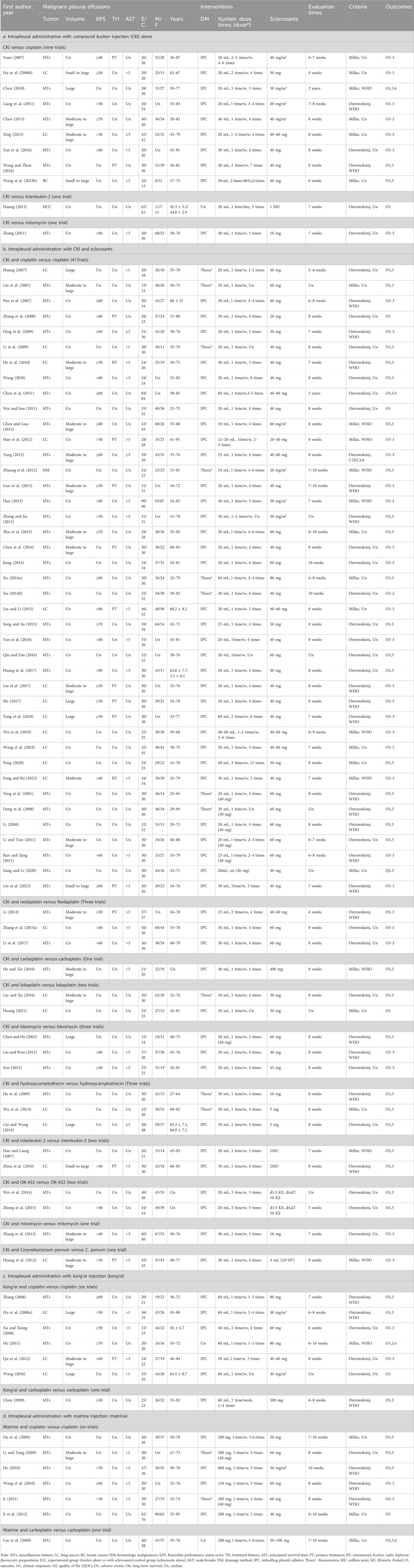
We clustered the 83 eligible studies from 2001 to 2023 into four themes: intrapleural perfusion with CKI alone, CKI and sclerosants, kang’ai or matrine, and platinum for controlling MPE. Eleven trials reported CKI alone (Table 1a). CKI and sclerosant developed three comparisons as CKI-versus-cisplatin (Yuan, 2007; Hu Q. et al., 2008; Chen, 2010; Liang et al., 2011; Chen, 2013; Xing, 2013; Wang and Zhou, 2016; Yan et al., 2016; Wang R. et al., 2023), mitomycin (Zhang, 2011), or interleukin-2 (Huang, 2013). All trials recruited 796 inpatients—426 male and 244 female patients aged 20–82 years. Receiving CKI were 396 patients, while another 400 received sclerosants alone. Perfusion with CKI and sclerosants was reported in 59 trials (Table 1b). The CKI and chemical drug or BRM developed ten treatment plans: perfusion with CKI and cisplatin, nedaplatin (Li, 2014; Zhang S. et al., 2015; Li et al., 2017), bleomycin (Chen and He, 2003; Liu and Wan, 2011; Sun, 2012), hydroxycamptothecin (He et al., 2009; Wu et al., 2014; Cai and Wang, 2019), lobaplatin (Liu and Xu, 2016; Huang, 2021), carboplatin (He and Xie, 2010), mitomycin (Zhang et al., 2013), interleukin-2 (Hao and Liang, 2007; Zhou et al., 2010), OK-432 (Wei et al., 2014; Zhong et al., 2015), and Corynebacterium parvum (Huang et al., 2012). There were 41 trials which evaluated perfusion with CKI and cisplatin, recruiting 2,823 inpatients aged 15–91, with 1,346 male and 909 female patients. Some 1,424 patients received perfusion with CKI and cisplatin, while another 1,399 received cisplatin alone. CKI was administrated 10–60 mL/time, once to thrice per week, lasting one to twelve times; the cisplatin was administrated with 20–80 mg/time. Kang’ai or matrine and platinum developed four plans. Six trials involving 334 inpatients aged 36–84 years (Zhang, 2006; Hu J. et al., 2008; Xu and Xiong, 2008; He, 2011; Qu et al., 2012; Wang, 2016) evaluated perfusion with kang’ai and cisplatin (Table 1c). Received kang’ai and cisplatin were 168 patients, while another 166 received only cisplatin. Kang’ai was administrated 40–60 mL/time, once or twice per week, lasting one to four times. Six trials recruiting 319 inpatients aged 30–85 (Du et al., 2009; Li and Yang, 2009; He, 2010; Wang et al., 2010; Ji, 2011; Ji et al., 2012) evaluated perfusion with matrine and cisplatin (Table 1d). A total of 167 patients received matrine and cisplatin, while another 152 only received cisplatin. Matrine was administrated 150–800 mg/time, once a week, lasting 2 to 6 weeks.
Of 83 eligible studies, 58 (69.88%, 58/83) involved inpatients with miscellaneous tumors, 24 (28.92%, 24/83) with lung cancer, and only one with hematologic malignancies (Huang, 2013) or breast cancer (Wang R. et al., 2023). Most studies described demographic characteristics, but only 16 to 50 (19.28%, 16/83% to 60.24%, 50/83) reported the pleural fluid volume, KPS, AST, and treatment history. All studies reported the drainage methods and characteristics of interventions and assessed the clinical responses 5–10 weeks after treatment began using Ostrowskimj or Millar criteria. Only 36 studies (43.37%, 36/83) reported the QOL, and six reported overall survival (Cui et al., 2008; Chen, 2010; He, 2011; Han, 2013; Zhang S. et al., 2015). Some 79 studies (95.18%, 79/83) reported the AEs, 38 (45.78%, 38/83) assessed ADRs using WHO or CTEC3.0 criteria, and only four assessed TRAEs (Wang et al., 2010; Yang, 2012; Wei et al., 2014; Liu and Li, 2015; Song and Jia, 2015). No study reported conflicts of interest.
3.3 Methodological quality
Of 83 studies, 79 (95.18%, 79/83) expressed concerns at overall bias for clinical responses, and four showed high risk (Qu et al., 2012; Wu et al., 2014; Wang and Zhou, 2016; Lin et al., 2023). At domain-level, only one study had low risk at D1 (Liu and Li, 2015), one showed high risk at D1 (Lin et al., 2023) or D2 (Wang and Zhou, 2016), and others had some concerns. All had low risk at D3 and D4. Two studies showed high risk at D5 (Qu et al., 2012; Wu et al., 2014), and others had low risk (Figure 3A; Supplementary Figures S1, S2). For overall survival, five studies had concerns of overall bias (Cui et al., 2008; Chen, 2010; He, 2011; Han, 2013; Zhang S. et al., 2015). All had some concerns at D1 and D2, and low risk at D3, D4, and D5 (Supplementary Figure S4).
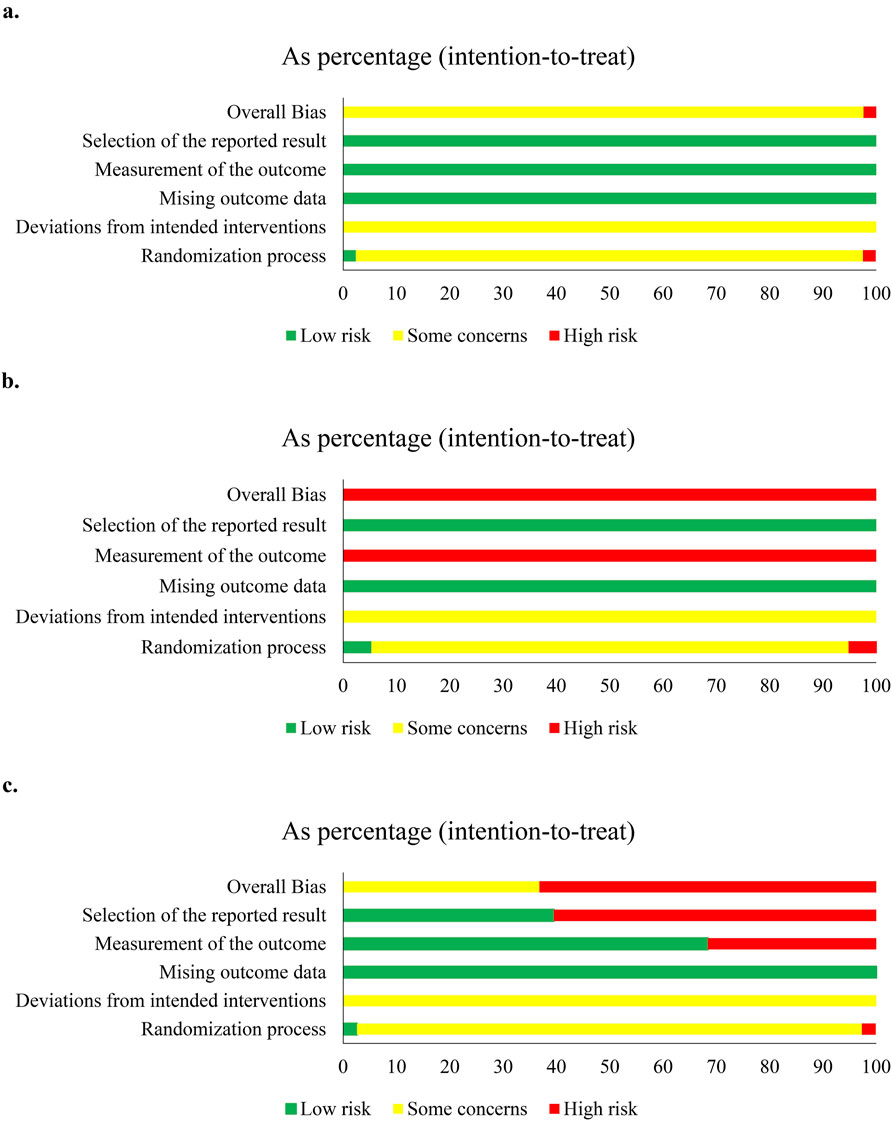
Figure 3. Risk-of-bias of compound kushen injection and cisplatin. (a) Clinical responses; (b) quality of life; (c) adverse events.
Since studies were limited, we only assessed the methodological quality of QOL and adverse events in CKI versus cisplatin, and perfusion with CKI, kang’ai, or matrine and cisplatin. QOL was reported by 29 studies and showed high risk at overall bias. Only one study (Liu and Li, 2015) had low risk, and one (Lin et al., 2023) had high risk at D1. All showed some concern at D2, low risk at D3 and D5, and high risk at D4 (Figure 3B and S3). A total of 57 studies reported AEs, 35 (61.40%, 35/57) showed high risk at overall bias, and 21 had some concerns. There were 55 studies (96.49%, 55/57) with some concerns at D1and D2, two with low risk at D1 (Liu and Li, 2015; Lin et al., 2023), and one with high risk at D2 (Wang and Zhou, 2016). All studies had low risk at D3. High risk was shown by 16 studies (28.07%, 16/57), and 39 had low risk at D4. A total of 34 studies (59.65%, 34/57) showed high risk, and 21 had low risk at D5 (Figure 3C and Figure. S5).
3.4 Clinical responses
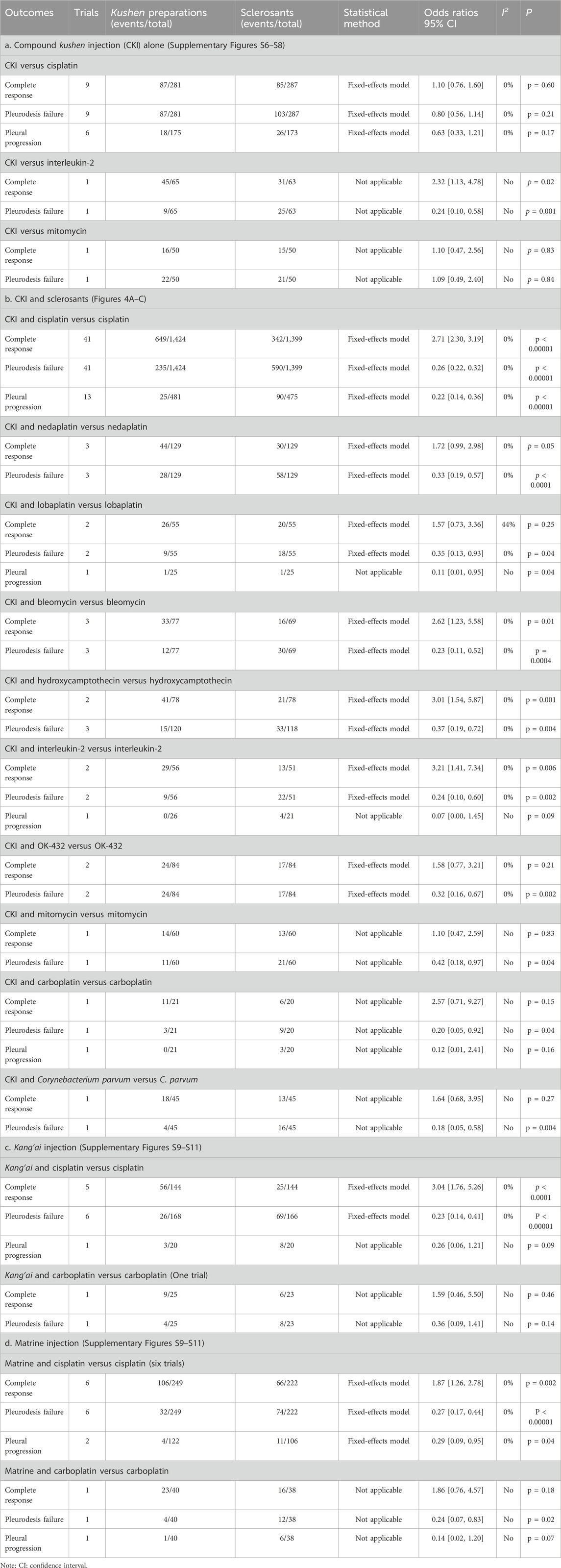
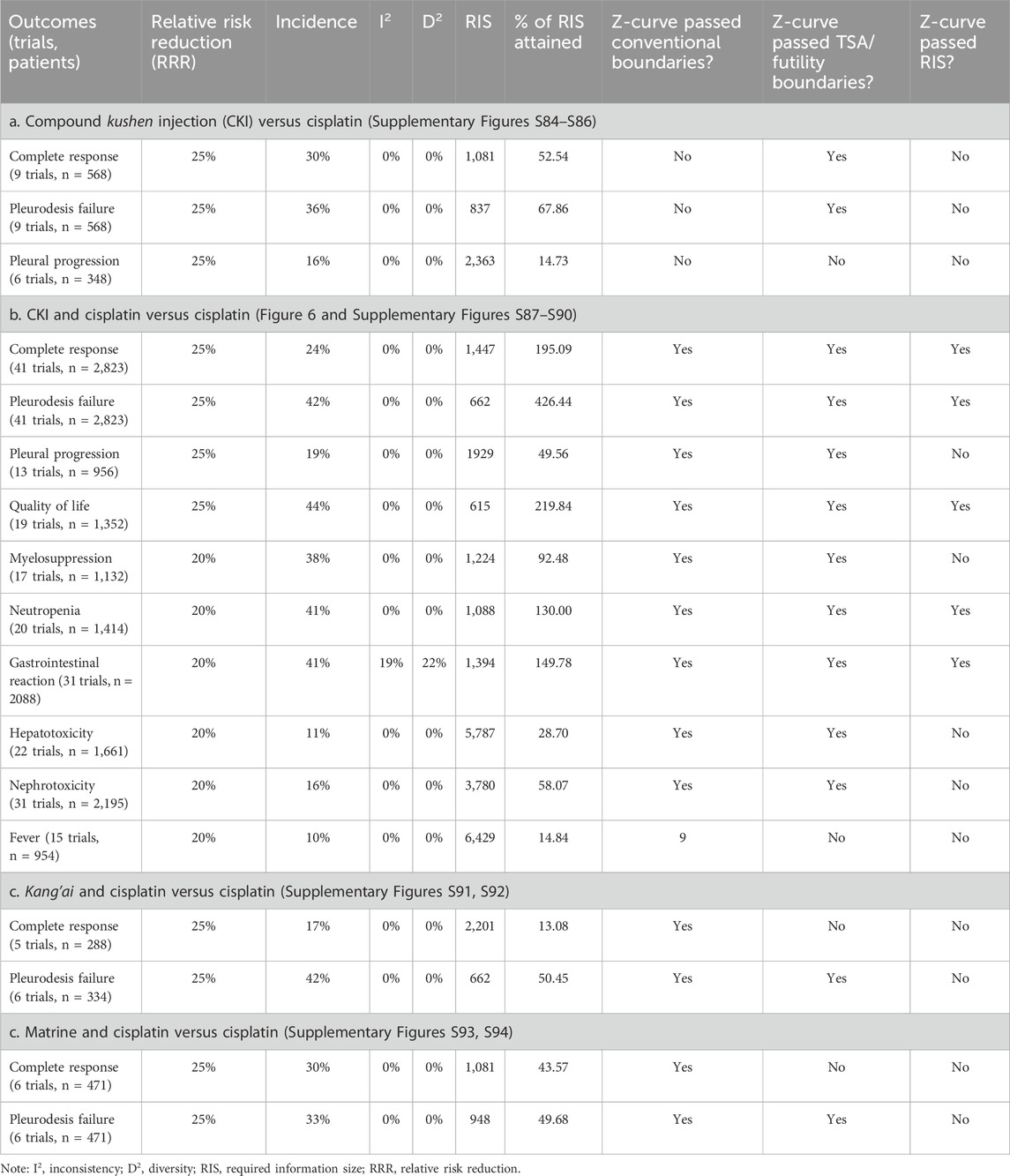
Nine trials reported clinical responses about CKI versus cisplatin (Table 2a; Supplementary Figures S6–S8). Cochran’s χ2 test and I2 statistic revealed no heterogeneity (I2 = 0%). We pooled the OR using a FEM. The results of meta-analyses revealed that CKI perfusion displayed a complete response (1.10, 95% CI 0.76 to 1.60), pleurodesis failure (0.80, 95% CI 0.56 to 1.14), and pleural progression (0.63, 95% CI 0.33 to 1.21) similar to cisplatin alone. Only single trial reported that CKI achieved clinical response similar to mitomycin and better than interleukin-2.
The CKI and chemical drug or BRM developed ten treatment plans (Table 2b; Figure 4C; Figure 5). Perfusion with CKI and cisplatin was evaluated by 41 trials. With no statistical heterogeneity (I2 = 0%), an FEM was used to pool the OR. The results demonstrated it significantly improving the complete response (2.71, 95% CI 2.30 to 3.19) and displaying a low pleurodesis failure (0.26, 95% CI 0.22 to 0.32) and pleural progression (0.22, 95% CI 0.14–0.36) than cisplatin alone. One to three trials reported nine other treatment plans. Compared with sclerosants alone, the results revealed that nine treatment plans achieved a low pleurodesis failure, while only CKI and bleomycin, hydroxycamptothecin, or interleukin-2 significantly improved the complete response.
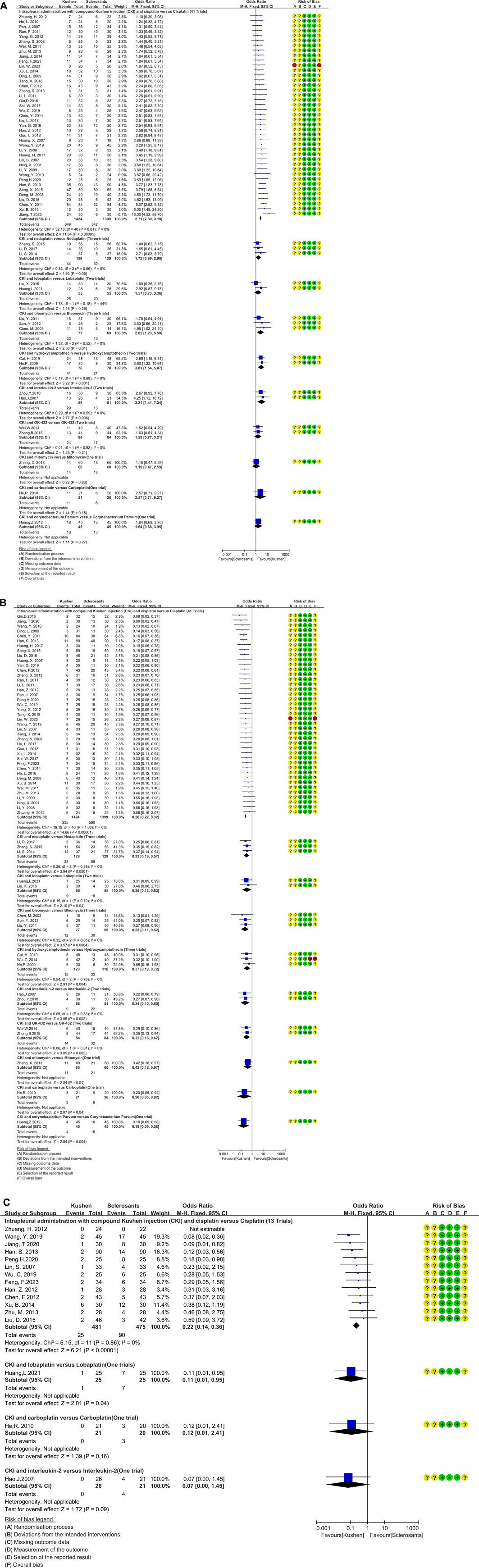
Figure 4. Clinical responses of compound kushen injection in MPE. (A) Meta-analysis of complete response; (B) meta-analysis of pleurodesis failure; (C) forest plot of pleural progression.
Kang’ai or matrine and platinum developed four treatment plans (Table 2c, 2d; Supplementary Figures S9–S11). With no statistical heterogeneity (I2 = 0%), an FEM was used. The results demonstrated that perfusion with kang’ai or matrine and cisplatin significantly improved the complete response (3.04, 95% CI 1.76 to 5.26 and 1.87, 95% CI 1.26–2.78) and achieved a low pleurodesis failure (0.23, 95% CI 0.14 to 0.41 and 0.27, 95% CI 0.17–0.44) than cisplatin alone. Additionally, matrine and cisplatin achieved a low pleural progression (0.29, 95% CI 0.09–0.95).
3.5 Overall survivals
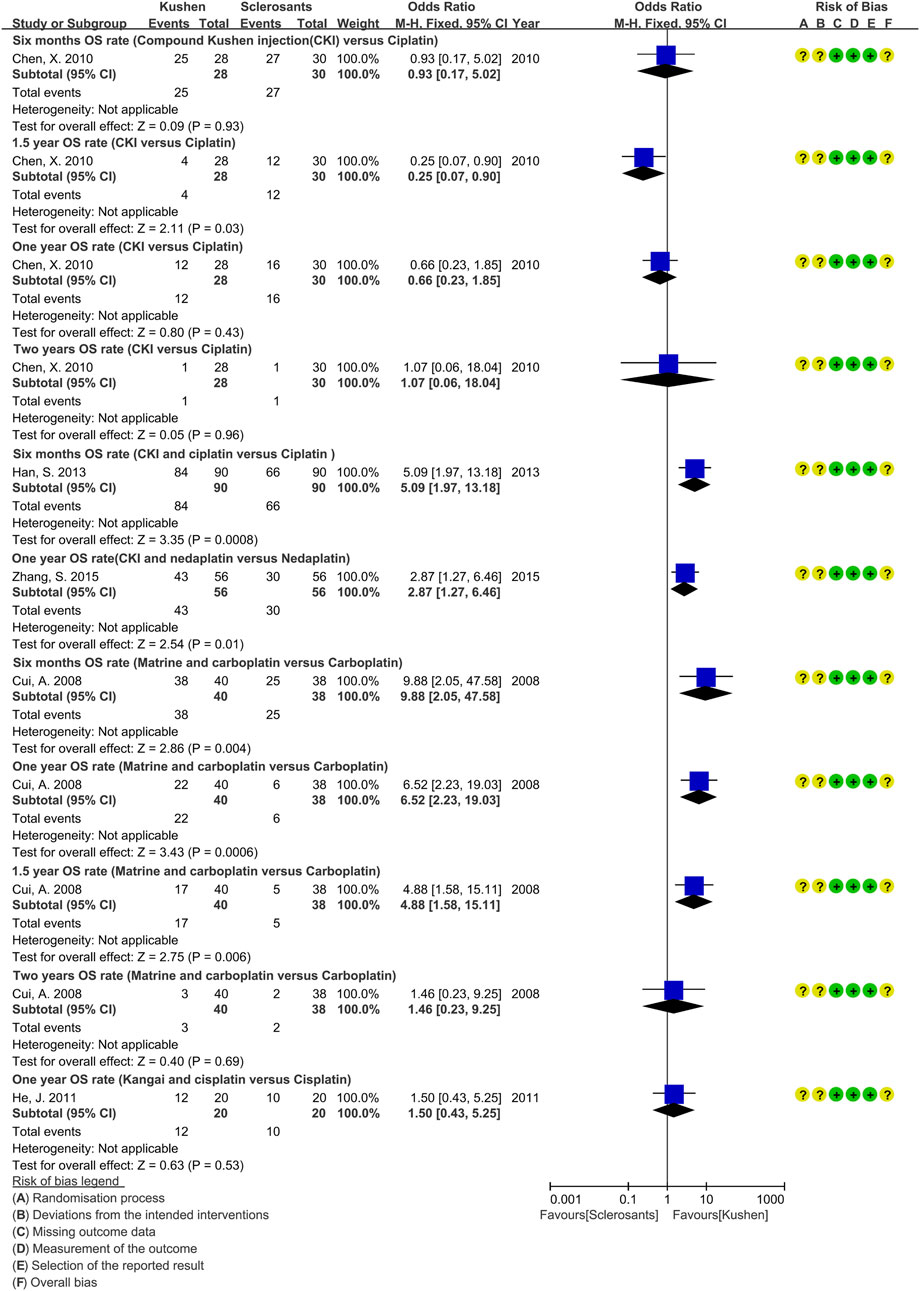
Of 83 studies, only six (Cui et al., 2008; Chen, 2010; Chen et al., 2011; He, 2011; Han, 2013; Zhang S. et al., 2015) reported the OS of perfusion with CKI alone, CKI and cisplatin or nedaplatin, kang’ai and cisplatin, or matrine and carboplatin (Figure 5). Compared with sclerosants alone, only one trial reported that perfusion with CKI and cisplatin might improve the 0.5-year OS rate (Han, 2013), and it might prolong median survival time and PFS (Chen et al., 2011). Perfusion with CKI and nedaplatin might improve the 1-year OS rate (Zhang S. et al., 2015), and matrine and carboplatin might improve the 0.5-year, 1-year, and 1.5-year OS rates (Cui et al., 2008).
3.6 Quality of life
Due to limited trials, we only assessed the QOL of perfusion with CKI alone, CKI, kang’ai, or matrine and cisplatin (Table 3; Supplementary Figures S12, S13). Six trials reported the QOL about CKI alone (Yuan, 2007; Hu Q. et al., 2008; Liang et al., 2011; Chen, 2013; Xing, 2013; Yan et al., 2016). Statistical heterogeneity (I2 = 67%) was found, and an REM was used. Compared with cisplatin alone, CKI perfusion acquired a similar QOL. There were 21 trials reporting QOL about perfusion with CKI, kang’ai, or matrine and cisplatin. No heterogeneity was found (I2 = 0%), and an FEM was used to pool the OR. Compared with cisplatin alone, the results demonstrated that perfusion with CKI, kang’ai or matrine and cisplatin significantly improved QOL (3.60, 95% CI 2.84 to 4.56; 3.95, 95% CI 1.78 to 8.74 and 2.95, 95% CI 1.25–6.97).
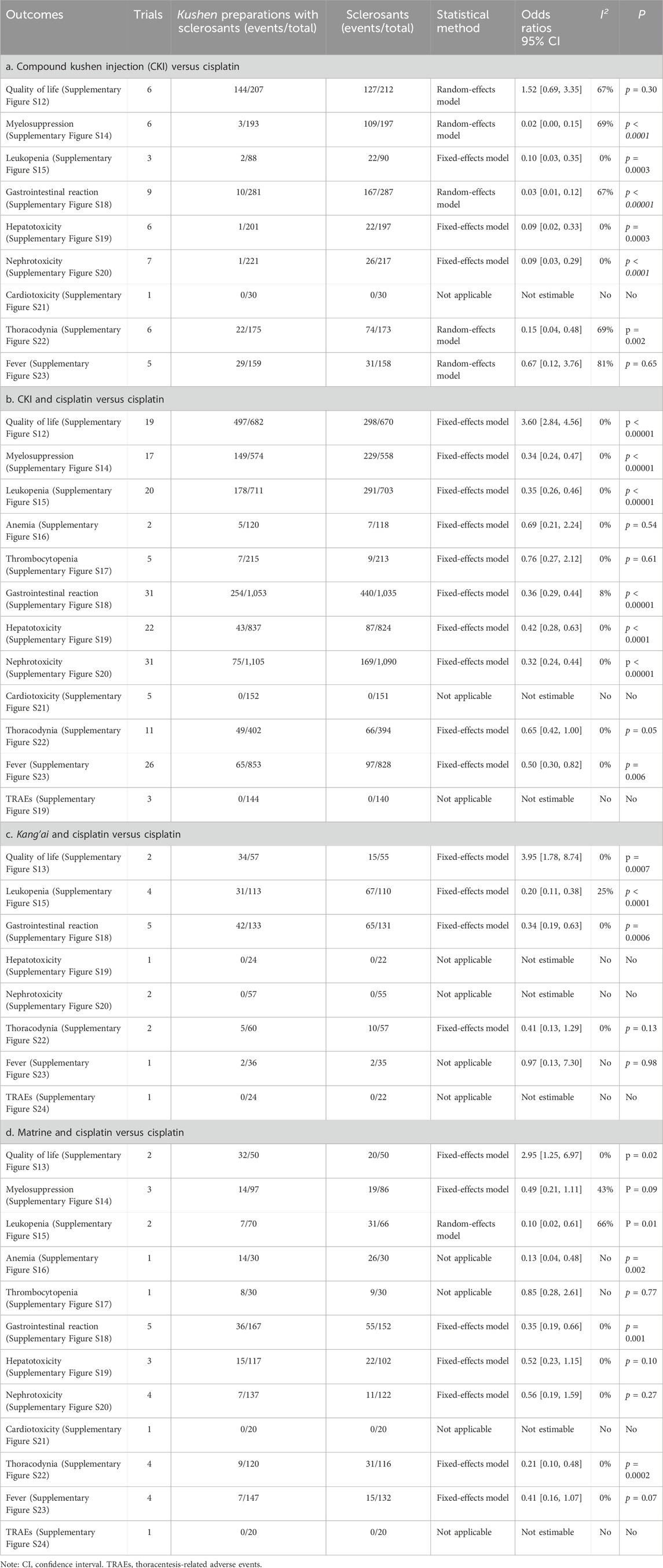
Table 3. Meta-analysis results of quality of life and adverse events (Supplementary Figures S12–S24).
3.7 Adverse events
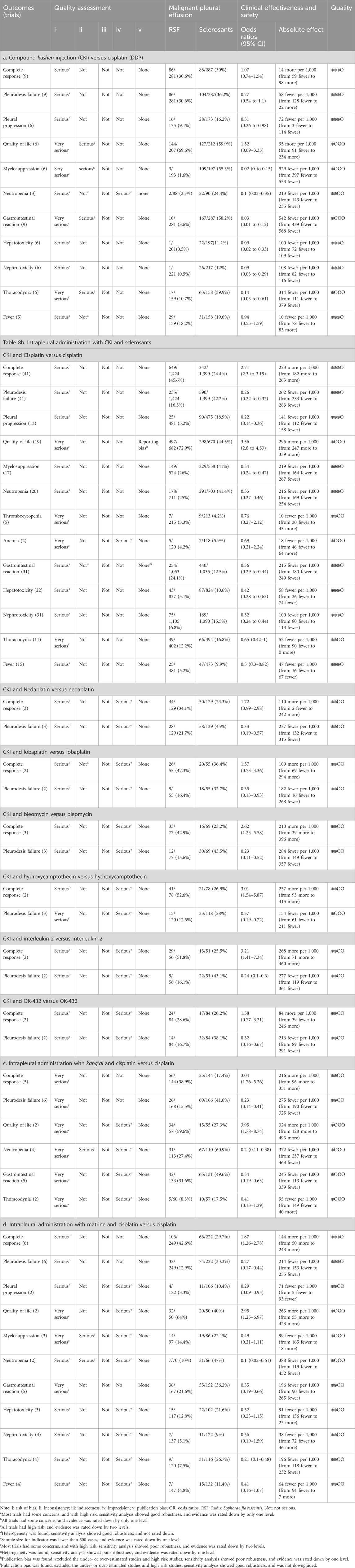
Nine trials reported eight AEs about CKI alone (Table 3a; Supplementary Figures S14, S15, S18–S23). Cochran’s χ2 test and I2 statistic only identified statistical heterogeneity for myelosuppression (I2 = 69%), gastrointestinal reaction (I2 = 67%), thoracodynia (I2 = 69%), and fever (I2 = 81%), and an REM or FEM was used to synthesize the OR. Compared with cisplatin alone, meta-analysis revealed that perfusion with CKI alone showed a low myelosuppression (0.02, 95% CI 0.00 to 0.15), leukopenia (0.10, 95% CI 0.03–0.35), gastrointestinal reaction (0.03, 95% CI 0.01 to 0.12), hepatotoxicity (0.09, 95% 0.02–0.33), nephrotoxicity (0.09, 95% CI 0.03 to 0.29), and thoracodynia (0.15, 95% CI 0.04 to 0.48).
Ten AEs were reported by 38 trials about CKI and cisplatin (Table 3; Supplementary Figures S14–S23). We only identified minimal heterogeneity for gastrointestinal reaction (I2 = 8%), and an FEM was used. The results demonstrated that perfusion with CKI and cisplatin showed a low myelosuppression (0.34, 95% CI 0.24–0.47), neutropenia (0.35, 95% CI 0.26 to 0.46), gastrointestinal reaction (0.36, 95% CI 0.29–0.44), and hepatorenal toxicity (0.42, 95% CI 0.28 to 0.63 and 0.32, 95% CI 0.24–0.44) and fever (0.50, 95% CI 0.30–0.82).
Five trials reported six AEs to kang’ai and cisplatin (Table 3c; Supplementary Figures S15–S24). We only identified minimal heterogeneity leukopenia (I2 = 25%), and an FEM was used. The results revealed that kang’ai and cisplatin showed low neutropenia (OR = 0.20, 95% CI 0.11–0.38) and gastrointestinal reaction (OR = 0.34, 95% CI 0.19–0.63).
Five trials reported ten AEs to matrine and cisplatin (Table 3d; Supplementary Figures S14–S23). We only identified statistical heterogeneity for neutropenia (I2 = 66%) and minimal heterogeneity for myelosuppression (I2 = 43%), and an REM or FEM was used. The results revealed that matrine and cisplatin showed low neutropenia (0.10, 95% CI 0.02–0.61), gastrointestinal reaction (0.35, 95% CI 0.19–0.66), and thoracodynia (0.21, 95% CI 0.10–0.48).
3.8 Subgroup analysis
In targeting perfusion with CKI and cisplatin, subgroup analysis revealed that under different primary tumors, drainage, and evaluation criteria, this treatment plan obtained significant improvement in the complete response and low pleurodesis failure. For patients with moderate-to-large effusion, KPS ≥50 to ≥70 scores, AST ≥3 months, or primary treatment, it significantly improved clinical responses. Perfusion with CKI (20–50 mL/time, once per week, two to four times) and cisplatin (20–80 mg/time) could significantly improve the clinical responses. Moreover, perfusion with low-dosage cisplatin and CKI could obtain clinical responses like high-dosage. However, the univariate regression and multiple meta-regression analysis did not reveal any correlation between clinical response and each variable (Table 4; Supplementary Figures S25–S72).
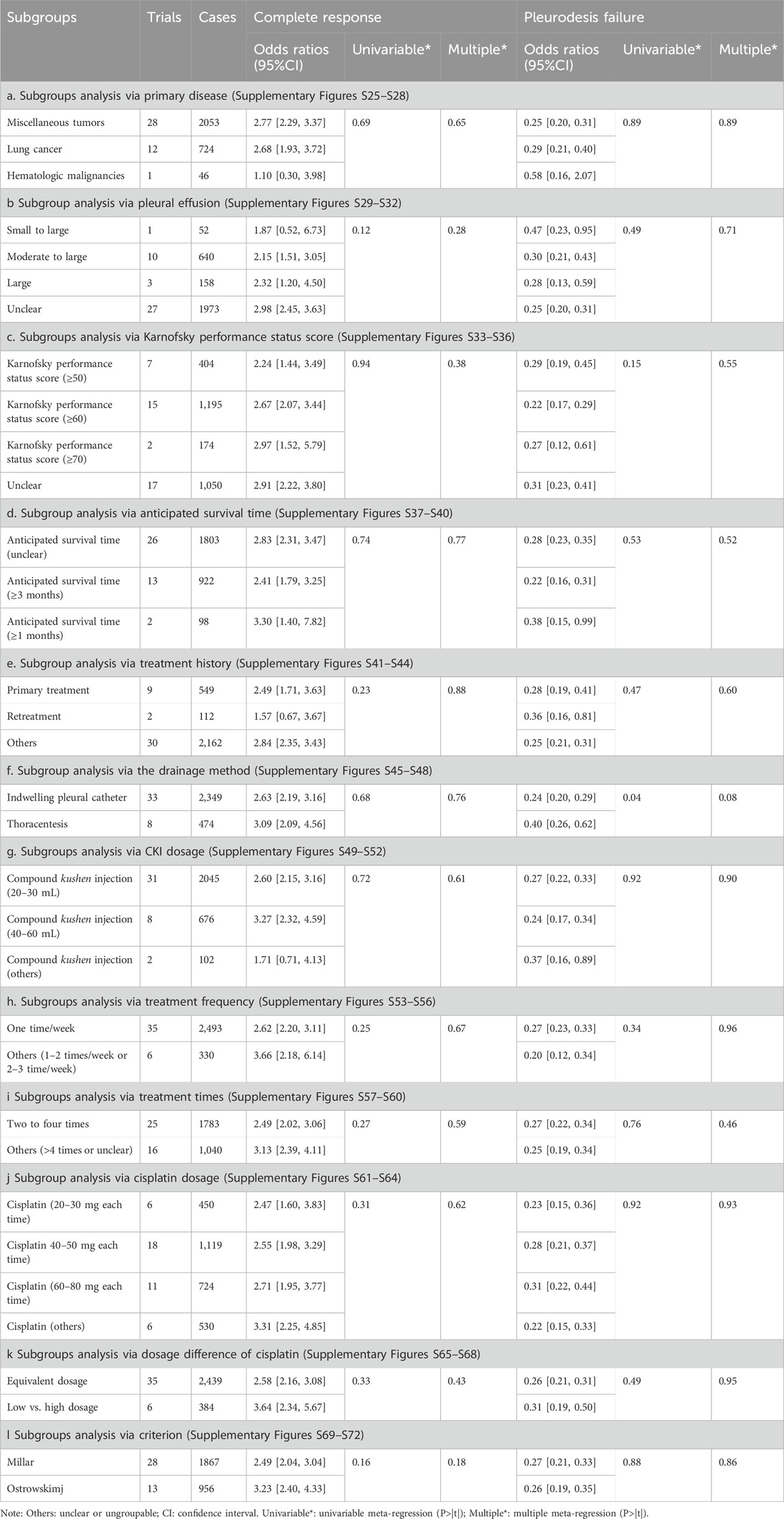
Table 4. Subgroups and meta-regression analysis (Supplementary Figures S25–S72).
3.9 Publication bias analysis
Only perfusion with CKI and cisplatin was included in more than ten trials (Table 5; Supplementary Figures S73–S83). The funnel plot and Egger’s test did not identify publication bias for the complete response, pleurodesis failure, pleural progression, myelosuppression, neutropenia, hepatorenal toxicity, thoracodynia, and fever, which were objectively reported. Significant publication bias was identified for QOL (coefficient = –2.47, 95% CI –4.62 to –0.32) and gastrointestinal reaction (coefficient = –1.49, 5% CI –2.71 to –0.21); both results were under-estimated.
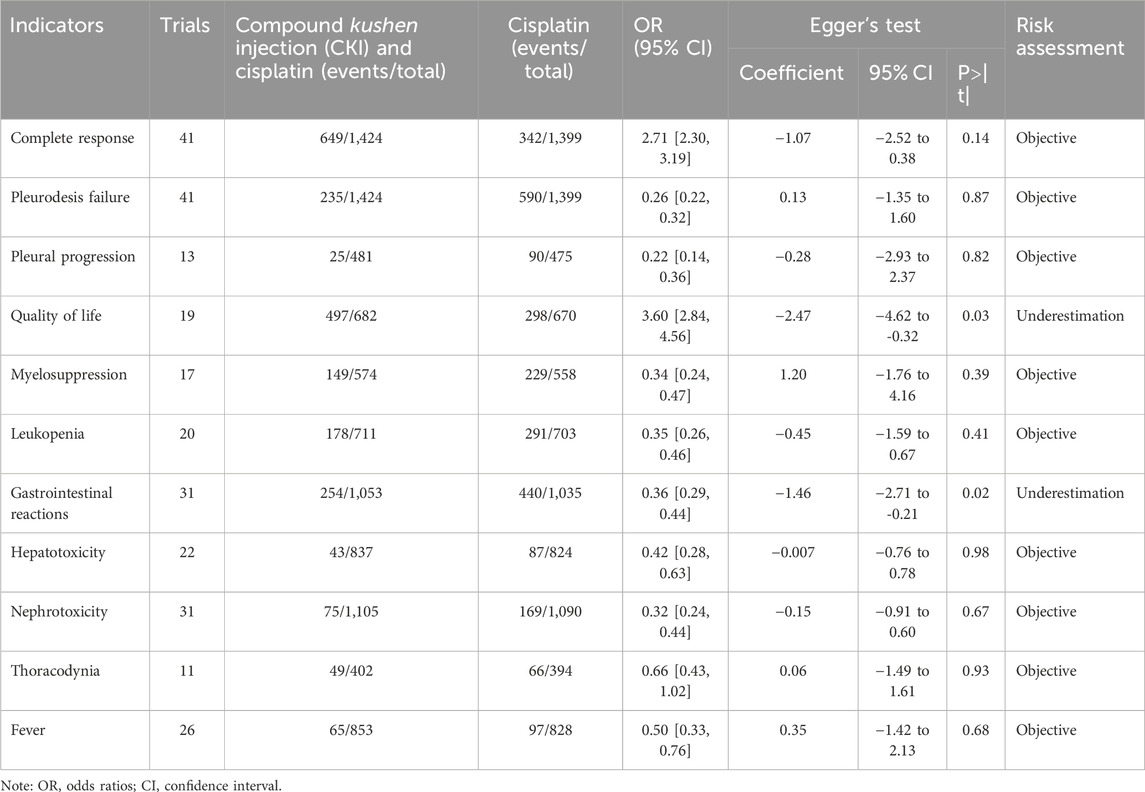
Table 5. Publication bias risk (Supplementary Figures S73–S83).
3.10 Sensitivity analysis
In CKI versus cisplatin, 11 outcomes were pooled using meta-analysis. Before and after excluding the trials with high risk and over-estimating efficacy/safety, the OR of QOL, myelosuppression, gastrointestinal reaction, and thoracodynia showed poor robustness, and the others had good robustness. In perfusion with CKI and cisplatin, 13 outcomes were pooled, and QOL, thrombocytopenia and anemia showed poor robustness. In CKI and nedaplatin, lobaplatin, bleomycin, hydroxycamptothecin, interleukin-2, or OK-432, 12 outcomes were pooled, and only the complete response of CKI and nedaplatin, lobaplatin, or OK-432 showed good robustness. In kang’ai and cisplatin, six outcomes were pooled, showing poor robustness. In matrine and cisplatin, 11 outcomes were pooled, and the QOL, myelosuppression, neutropenia, and gastrointestinal reaction showed poor robustness (Table 6).
3.11 Trial sequential analyses
Since the trials were limited, we only assessed the RIS for clinical responses in CKI versus cisplatin. The TSA identified firm information size for supporting a similar complete response and pleurodesis failure between CKI and cisplatin, and no reliable information for pleural progression. We further assessed the RIS for clinical responses, QOL, and AEs in perfusion with CKI and cisplatin. Further analysis identified sufficient and conclusive information sizes for complete response, pleurodesis failure, QOL, neutropenia, and gastrointestinal reaction, and firm information for pleural progression, myelosuppression, and hepatorenal toxicity. Finally, we only assessed the RIS for clinical responses in kang’ai or matrine and cisplatin. The analysis identified firm information sizes for pleurodesis failure in both treatments and no reliable information for complete response (Table.7; Figure 6; Supplementary Figures S84–S94).
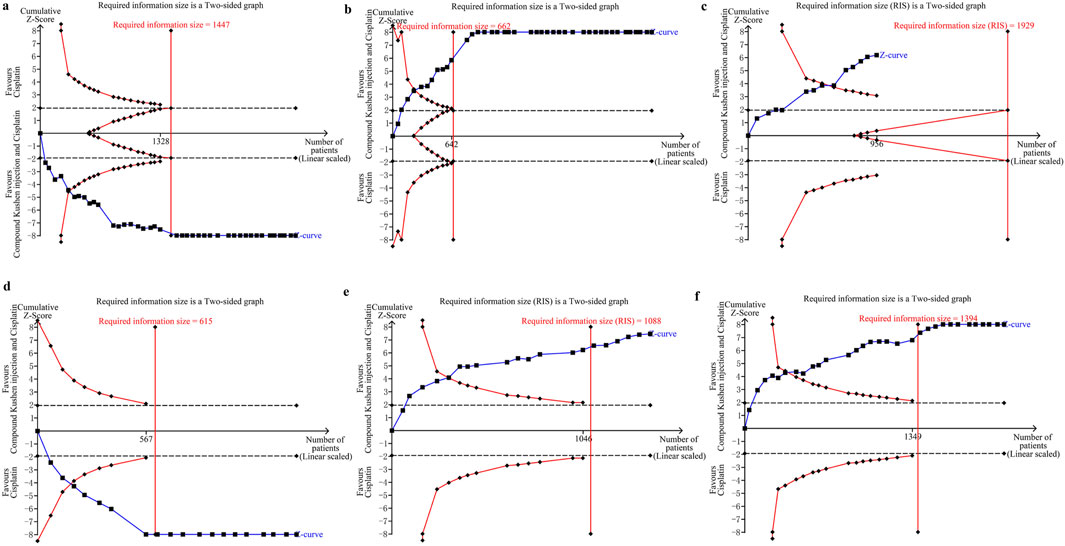
Figure 6. Trial sequential analyses in compound kushen injection and cisplatin. (a) Complete response; (b) pleurodesis failure; (c) pleural progression; (d) quality of life; (e) neutropenia; (f) gastrointestinal reaction.
3.12 Evidence quality
We applied a revised GRADE approach to identify the evidence quality as “high”, “moderate”, “low”, and “very low”. In CKI versus cisplatin, 11 results were pooled. Clinical responses, hepatorenal toxicity, and fever were summarized as moderate quality, while other five results were low to very low (Table 8a). In perfusion with CKI and cisplatin, 13 results were pooled. Clinical responses, myelosuppression, neutropenia, gastrointestinal reaction, hepatorenal toxicity, and fever were summarized as moderate, while the other four were low to very low. In CKI and nedaplatin, lobaplatin, bleomycin, hydroxycamptothecin, interleukin-2, or OK-432, 12 results were pooled. The clinical responses were very low to low (Table 8b). In kang’ai and cisplatin, six results were pooled at low to very low (Table 8c). In matrine and cisplatin, 11 results were pooled. The complete response and pleurodesis failure were summarized as moderate, with the other nine results as low to very low (Table 8d).
4 Discussion
After integrating previous six SRs/meta-analyses (Tian et al., 2010; Tang et al., 2014; Biaoxue et al., 2015; Xu et al., 2015; Yang et al., 2016; Wu et al., 2018) and four network meta-analyses (Yang et al., 2017; Li B. et al., 2019; Li, 2022; Xu et al., 2022), we collected 83 RCTs for analysis and supplemented 39 trials in previous studies. We found three kushen preparations—CKI, kang’ai and matrine injection—which were administrated for controlling MPE through intrapleural perfusion. For kushen preparation alone, nine trials evaluated perfusion with CKI versus cisplatin alone. CKI mainly contains matrine, oxymatrine, and sophoridine, which have significant anti-tumor activity, regulate tumor microenvironment, and downregulate tumor-associated inflammation (Guo et al., 2015; Ma et al., 2016; Cao and He, 2020; Chen et al., 2021; Chen et al., 2022; Liu et al., 2023). The meta-analysis results demonstrated that perfusion with CKI alone showed clinical responses similar to cisplatin and a lower hepatorenal toxicity (Figure 7). These results were of moderate quality following the revised GRADE approach (Wang et al., 2022; Wang C. Q. et al., 2023), and the TSA found firm information sizes for supporting them. CKI perfusion showed low hematotoxicity, gastrointestinal reaction, and thoracodynia of low to very low quality. Zhang Z. et al. (2015), Zhong et al. (2015), Zhu and Hou (2021), Fan et al. (2022); Feng and Shi (2023) reported that CKI perfusion might prevent pleural effusion recurrence by downregulating the vascular endothelial cell growth factor and reducing angiogenesis. In all, these results suggest that CKI may serve as a new palliative intervention for MPE. Clinically, CKI, kang’ai, and matrine injections have been widely used as an adjuvant therapy for various solid tumors (Ma et al., 2016; Wang et al., 2016; Li H. et al., 2019; Liu et al., 2022; Liu et al., 2023). Apparently, this analysis further revealed a new therapeutic value and clinical application population of CKI. Unfortunately, no evidence supports the possibility of using kang’ai and matrine alone to treat MPE, which requires new trials to investigate.
Clinically, CKI is often combined with other sclerosants to control MPE through intrapleural perfusion. We found that CKI combined with seven chemical drugs or three BRMs to build ten homogenous treatment plans. The clinical values of perfusion with CKI and cisplatin have been reported by 41 trials. Compared with cisplatin alone, the results of meta-analyses demonstrated that perfusion with CKI and cisplatin significantly improved complete response and QOL with a low pleurodesis failure and pleural progression, and showed a low incidence rate of hematotoxicity, gastrointestinal reaction, and hepatorenal toxicity. Excluding QOL, these results were moderate quality following the revised GRADE approach (Wang et al., 2022; Wang C. Q. et al., 2023). The results of pleural progression, myelosuppression, and hepatorenal toxicity had firm information in support, while other results obtained sufficient and conclusive information support. In all, these results demonstrate that CKI infusion can improve clinical responses and QOL and reduce ADRs. Like high dosage, the subgroup analysis revealed that CKI combined with low-dosage cisplatin also obtained similar clinical responses. These results indicate that CKI and cisplatin have cooperative effect, and CKI may reduce cisplatin dosage while ensuring similar clinical benefits. Previous SR/meta-analyses have reported that as important BRMs, staphylococcal enterotoxin C (Jiang et al., 2022) and mannatide (Zhang et al., 2011; Chen et al., 2013) perfusion showed a high risk of fever. In this analysis, we found that perfusion with CKI might reduce the risk of fever. This finding may be the unique value of CKI in controlling MPE. The results of meta-analysis of other nine treatment plans further revealed that perfusion with CKI and lobaplatin, nedaplatin, bleomycin, hydroxycamptothecin, interleukin-2, or OK-432 might also improve clinical responses. However, the results had very low to low quality and lacked sufficient or firm information sizes in support. Comprehensively examining both information sizes and methodological quality, we conclude that among ten treatment plans, perfusion with CKI and cisplatin may be an optimal treatment plan for MPE, which shows significant improvement in clinical responses and low incidence of ADRs, especially fever (Figure 7). Further subgroup analysis revealed that perfusion with CKI (20–50 mL each time, once a week lasting two to four times) and cisplatin (20–80 mg each time) could obtain ideal clinical responses for MPE inpatients with moderate to large effusion, KPS ≥50 to ≥70 scores, AST ≥3 months, or primary treatment. Furthermore, the primary tumor, drainages or evaluation criteria showed no negative effect on clinical responses. These results suggest that inpatients with moderate-to-large effusion, KPS ≥ 50 to ≥ 70 scores, AST ≥ 3 months, or primary treatment are a possible suitable population. The CKI with 20 to 50 ml each time, once a week lasting two to four times and cisplatin with 20 to 80 mg each perfusion may be an optimal usage for obtaining desired responses and safety (Figure 7). Unfortunately, both meta-regression analyses did not find any correlation. These results require new evidence for confirmation.
Matrine and kang’ai are also important kushen preparations. Kang’ai mainly contains Astragalus polysaccharides, astragalosides, ginsenosides, ginseng polysaccharides, and oxymatrine (Wan et al., 2018; Sun et al., 2021). Six trials each evaluated the clinical benefit of perfusion with kang’ai or matrine and cisplatin (Zhang, 2006; Hu J. et al., 2008; Xu and Xiong, 2008; He, 2011; Qu et al., 2012; Wang, 2016). The meta-analysis results showed that perfusion with kang’ai and cisplatin significantly improved the complete response and QOL with low pleurodesis failure. Matrine is a principal active ingredient of CKI and kang’ai. The results further demonstrated that matrine and cisplatin could improve complete response and QOL with low pleurodesis failure and pleural progression. These results provide a theoretical basis for the clinical value of kang’ai or CKI in MPE. Perfusion with kang’ai or matrine and cisplatin all showed low neutropenia and gastrointestinal reaction. However, only the pleurodesis failure of both treatment plans had a firm quantity of information in support, and no reliable information indicated that both can improve the complete response. For matrine and cisplatin, the complete response and pleurodesis failure had moderate quality, while other results were low to very low. Overall, these results suggest that kang’ai or matrine may be potentially valuable alternative interventions which may improve clinical responses with firm information size (Figure 7). Further rigorous trials will be needed to reveal their clinical significance, suitable population, and optimal usage.
Kushen preparations alone or plus chemical drugs or BRMs form rich treatment plans. To validate their therapeutic value for MPE, we applied clustering SR/meta-analysis, successfully addressing clinical heterogeneity and revealing their clinical efficacy and safety based on homogeneous treatment units. First, we found that CKI may serve as a new palliative intervention for MPE. This analysis confirmed the clinical possibility of using CKI perfusion to control MPE and further revealed its new therapeutic value and clinical application population. Second, among ten treatment plans, we found that perfusion with CKI and cisplatin may be an optimal treatment plan for MPE. Subgroup analysis results further provide a suitable population and optimal use for perfusion with CKI and cisplatin treating MPE. Third, we found that kang’ai or matrine may be potential valuable alternative interventions for MPE. In all, this analysis confirms and reveals the therapeutic value and clinical application population for using kushen preparations to control MPE. These findings will be beneficial for developing rational medication strategies based on kushen preparations to improve clinical benefits and reduce ADRs and medication costs in MPE.
There were some limitations to this new SR/meta-analysis. This analysis customized its retrieval strategies and retrieved both Chinese and English databases, which may exhibit potential bias risk. Among 14 treatment plans, most—like perfusion with CKI, kang’ai, or matrine and other sclerosants—only had limited trials reporting their clinical benefit. In particular, only single trials reported the clinical benefit between CKI and interleukin-2 (Huang, 2013) or mitomycin (Zhang, 2011), as well as perfusion with CKI and carboplatin (He and Xie, 2010), mitomycin (Zhang et al., 2013), or corynebacterium parvum (Huang et al., 2012). Most treatment plans lacked reliable information support, and their results were low to very low quality. Obviously, their clinical effectiveness, safety, indications, and optimal usage still require more high-quality evidence and sufficient information to confirm them. Regarding methodological quality, most studies had some concerns at overall bias about clinical response and overall survival. For both outcomes, D1 and D2 had some concerns.
QOL about perfusion with CKI alone were reported by 29 studies, and CKI, kang’ai, or matrine and cisplatin. All had high risk of overall bias, and D4 was a high-risk domain. AEs were reported by 57 studies. High risk of overall bias was evident in 35 studies, with D4 and D5 as high-risk domains. Such findings suggest that strengthening random allocation, concealment, and blinding methods, and emphasizing the measurement and complete report of indicators will become key issues for improving methodological quality in future trials. Regarding PICO features, most studies did not clearly report patient characteristics such as pleural fluid volume, KPS, AST, or treatment history. Most studies failed to clearly report the TRAEs. Six studies reported overall survival (Cui et al., 2008; Chen, 2010; He, 2011; Han, 2013; Zhang S. et al., 2015). Only single study reported that perfusion with CKI and cisplatin (Chen et al., 2011; Han, 2013) or nedaplatin (Zhang S. et al., 2015) and matrine and carboplatin (Cui et al., 2008) might improve overall survival or progression-free survival. Additionally, no evidence reported recurrence and hospitalization time or conflicts of interest. Such shortcomings of PICO are important issues for design and quality improvement in future trials.
5 Conclusion
Current moderate evidence demonstrates that CKI may be an effective palliative intervention for controlling MPE. Perfusion with CKI and cisplatin may be an optimal treatment plan which can improve clinical responses and QOL and reduce ADRs, especially fever. This analysis further confirms a suitable population and optimal usage for CKI and cisplatin perfusion. CKI, kang’ai, or matrine and chemical drugs or BRMs formed rich treatment plans for MPE. More rigorous trials with low-risk and standardized PICOs will be needed to reveal their clinical significance, suitable populations, and optimal usage.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
YZ: writing – original draft, data curation, formal analysis, resources, and software. ZX: writing – original draft, conceptualization, funding acquisition, methodology, project administration, supervision, and writing – review and editing. HL: data curation, resources, software, formal analysis, and writing – review and editing. D-CC: data curation, methodology, software, resources, and writing – review and editing. Y-QL: data curation, resources, software, and writing – review and editing. JX: methodology, software, and writing – review and editing. FL: formal analysis, software, and writing – review and editing. JH: formal analysis, software, and writing – review and editing. Y-YJ: formal analysis, software, and writing – review and editing. T-YF: writing – review and editing. JZ: writing – review and editing. XX: writing – review and editing. J-HF: writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was funded by a Guizhou Provincial Science and Technology Program [Qian Kehe Zhicheng(2025), Yiban 056], a special fund for academic seedlings training and innovation at Zunyi Medical College [Qian Kehe Pingtai Rencai No. (2017) 5733-034], a special fund for science and technology research into traditional Chinese and national medicine in Guizhou (No QZYY 2017-084), and a high-level innovative talent program in Guizhou (No. fzc 120171001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1519794/full#supplementary-material
Abbreviations
ADRs, adverse drug reactions; AEs, adverse events; AST, anticipated survival time; BRM, biological response modifier; CTCAEs, Common Terminology Criteria for Adverse Events; CKI, compound kushen injection; CI, confidence interval; FEM, fixed-effects model; GRADE, Grading of Recommendation Assessment, Development and Evaluation approach; IPCs, indwelling pleural catheters; Kang’ai, kang’ai injection; KPS, Karnofsky performance status; MPEs, malignant pleural effusions; NMA, network meta-analysis; ORs: odds ratios; PF, pleurodesis failure; PFS, progression-free survival; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines; QOL, quality of life; RCTs, randomized controlled trials; REM, random-effects model; RIS, required information size; RRR, relative risk reduction; SRs, systematic reviews; TCM, traditional Chinese medicine; TCMIs, traditional Chinese medicine injections; TSA, trial sequential analysis; WHO, World Health Organization.
References
Biaoxue, R., Shuxia, M., Wenlong, G., and Shuanying, Y. (2015). Thoracic perfusion of matrine as an adjuvant treatment improves the control of the malignant pleural effusions. World J. Surg. Oncol. 13, 329. doi:10.1186/s12957-015-0729-9
Bibby, A. C., Dorn, P., Psallidas, I., Porcel, J. M., Janssen, J., Froudarakis, M., et al. (2018). ERS/EACTS statement on the management of malignant pleural effusions. Eur. Respir. J. 52, 1800349. doi:10.1183/13993003.00349-2018
Cai, H., and Wang, Q. (2019). Effect of Compound Kushen Injection on elderly patients with lung cancer complicated with malignant pleural effusion. Contemp. Med. Symp. 17, 161–162.
Cao, X., and He, Q. (2020). Anti-tumor activities of bioactive phytochemicals in Sophora flavescens for breast cancer. Cancer Manag. Res. 12, 1457–1467. doi:10.2147/CMAR.S243127
Chen, B., Xu, X.-M., Yu, T.-T., and Yang, J. (2013). Meta analysis of Lifein combined with Cisplatin in chest catheterization perfusion chemotheapy for malignant pleural effusion. Med J Wuhan Univ 34, 755–761. doi:10.14188/j.1671-8852.2013.05.039
Chen, F., and Liao, D. (2012). Clinical observation of compound Kushen injection combined with cisplatin on malignant pleural effusion. World Health Dig. 9, 49–50. doi:10.3969/j.issn.1672-5085.2012.42.039
Chen, F., Pan, Y., Xu, J., Liu, B., and Song, H. (2022). Research progress of matrine's anticancer activity and its molecular mechanism. J. Ethnopharmacol. 286, 114914. doi:10.1016/j.jep.2021.114914
Chen, L. (2013). Effect of compound matrine injection on malignant pleural effusion. Chin. J. New Drugs 22, 2069–2070+2074.
Chen, M., and He, B. (2003). Treatment of carcinomatous hydrothorax with small tube for closing drainage and infusing Bieomycini Hydrochkuridum and Yanshu. J. Minim. Invasive Med. 22, 457–458. doi:10.3969/j.issn.1673-6575.2003.04.011
Chen, M. H., Gu, Y. Y., Zhang, A. L., Sze, D. M., Mo, S. L., and May, B. H. (2021). Biological effects and mechanisms of matrine and other constituents of Sophora flavescens in colorectal cancer. Pharmacol. Res. 171, 105778. doi:10.1016/j.phrs.2021.105778
Chen, X. (2010). Yanshu injection in the treatment of malignant pleural effusion -28 cases study. J. Jiangxi TCM 41, 41–42. doi:10.3969/j.issn.0411-9584.2010.01.019
Chen, Y. (2009). Kangai injection combined with carboplatin in the treatment of malignant pleural effusion. Heilongjiang J. TCM 38, 27–28.
Chen, Y., Li, Q., Xiang, S., and Yang, G. (2011). Compound Kushen Injection combined with cisplatin for malignant pleural effusion:A clinical study. Eval. Anal. Drug-Use Hosp. China 11, 366–367. doi:10.14009/j.issn.1672-2124.2011.04.016
Chen, Y., Luo, Y., and Qin, Z. (2014). Effect of compound Kushen injection combined with cisplatin on malignant pleural effusion in aged patients. Res. Integr. Tradit. Chin. West Med. 6, 196–197+199. doi:10.3969/j.issn.1674-4616.2014.04.011
Cui, A., Du, C., Yan, J., and Zhou, Q. (2008). Clinical study on the treatment of malignant pleural effusion by pleural catheter drainage and infusion of oxymatrine and carboplatin. J. Baotou Med. 32, 65–66. doi:10.3969/j.issn.1007-3507.2008.02.001
Deng, M., Wei, W., and Wang, Y. (2008). Observation on efficacy of Yanshu injection combined cisplatin in treating malignant hydrothorax of 40 cases. Chin. Med. Mod. Dist. Edu China 6, 270. doi:10.3969/j.issn.1672-2779.2008.03.048
Ding, L., Cui, C., and Xin, B. (2009). The clinical observation of compound Kushen injection combined with cisplatin in the treatment of malignant pleural effusion by intrathoracic injection. J. Mod. Oncol. 17, 1274–1275. doi:10.3969/j.issn.1672-4992.2009.07.026
Dipper, A., Jones, H. E., Bhatnagar, R., Preston, N. J., Maskell, N., and Clive, A. O. (2020). Interventions for the management of malignant pleural effusions: a network meta-analysis. Cochrane Database Syst. Rev. 2020. doi:10.1002/14651858.cd010529.pub3
Du, C., Wang, A., and Yan, S. (2009). Comparison of pleural injection of oxymatrine combined with cisplatin in the treatment of malignant pleural effusion. J. Dis. Monit. Control 3, 472–473.
Fan, Q. Q., She, G. M., Wei, J., Li, Z. Q., Chen, M. L., Dong, Y., et al. (2022). Anti-tumor and analgesic activity evaluation and mechanism of Compound Kushen Injection. Zhongguo Zhong Yao Za Zhi 47, 2712–2720. doi:10.19540/j.cnki.cjcmm.20211129.701
Feller-Kopman, D. J., Reddy, C. B., Decamp, M. M., Diekemper, R. L., Gould, M. K., Henry, T., et al. (2018). Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am. J. Respir. Crit. Care Med. 198, 839–849. doi:10.1164/rccm.201807-1415ST
Feng, F., and Shi, X. (2023). Clinical study of Compound Kushen Injection combined with cisplatin in treatment of non-small cell lung cancer malignant pleural effusion. Drugs and Clin. 38, 1717–1721. doi:10.7501/j.issn.1674-5515.2023.07.028
Gayen, S. (2022). Malignant pleural effusion: presentation, diagnosis, and management. Am. J. Med. 135, 1188–1192. doi:10.1016/j.amjmed.2022.04.017
Guo, L., Luo, D., and Yang, S. (2013). Observation on efficacy of compound Kushen injection combined with cisplatin in the treatment of malignant pleural fluid. China Med. Her. 10, 79–81. doi:10.3969/j.issn.1673-7210.2013.25.029
Guo, Y. M., Huang, Y. X., Shen, H. H., Sang, X. X., Ma, X., Zhao, Y. L., et al. (2015). Efficacy of compound kushen injection in relieving cancer-related pain: a systematic review and meta-analysis. Evid. Based Complement. Altern. Med. 2015, 840742. doi:10.1155/2015/840742
Guyatt, G. H., Oxman, A. D., Vist, G. E., Kunz, R., Falck-Ytter, Y., Alonso-Coello, P., et al. (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj 336, 924–926. doi:10.1136/bmj.39489.470347.AD
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Han, S. (2013). Effect of compound Kushen injection combined with cisplatin pleural perfusion on malignant pleural effusion. J. Clin. Res. 30, 1191–1193. doi:10.3969/j.issn.1671-7171.2013.06.055
Han, Z., Tian, M., Chen, X., and Shi, F. (2012). Effect of closed drainage combined with compound Kushen injection and cisplatin on malignant pleural effusion of lung cancer. Zhejiang J. ITCWM 22, 524–526. doi:10.3969/j.issn.1005-4561.2012.07.011
Hao, J., and Liang, K. (2007). Continuous catheter drainage combined with Yanshu injection and interleukin-2 intrapleural injection in the treatment of malignant pleural effusion. Chin. J. IMCCD 5, 1131–1132. doi:10.3969/j.issn.1672-1349.2007.11.051
Hassan, M., Harriss, E., Mercer, R. M., and Rahman, N. M. (2021). Survival and pleurodesis outcome in patients with malignant pleural effusion - a systematic review. Pleura Perit. 6, 1–5. doi:10.1515/pp-2020-0147
He, J. (2011). Clinical study of Kangai injection combined with cisplatin in treatment of malignant pleural effusion. Med. Inf. 24, 3756–3757. doi:10.3969/j.issn.1006-1959.2011.08.280
He, L., Chen, Z., Wen, S., Ren, D., and Chen, H. (2010). Compound Kushen Injection as a local therapy for patients with advanced lung cancer associated with malignant pleural effusion. Eval. Anal. Drug-Use Hosp. China 10, 1025–1027. doi:10.14009/j.issn.1672-2124.2010.11.012
He, P., Zeng, S., Xue, Z., Wang, Y., and Pan, B. (2009). Effect of hydroxycamptothecin and Yanshu injection by intrapleural infusion in treatment of 30 cases malignant pleural effusion. J. Mod. Clin. Med. 35, 261–262. doi:10.3969/j.issn.1673-1557.2009.04.009
He, R., and Xie, X. (2010). Effect of compound Kushen injection combined with carboplatin on malignant pleural effusion. J. Shandong Med. 50, 27. doi:10.3969/j.issn.1002-266X.2010.33.017
He, X., Fang, J., Huang, L., Wang, J., and Huang, X. (2015). Sophora flavescens Ait.: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 172, 10–29. doi:10.1016/j.jep.2015.06.010
He, Y. (2010). Clinical observation of cisplatin combined with oxymatrine in the treatment of malignant pleural effusion. Med. Forum 14, 415–416. doi:10.3969/j.issn.1672-1721.2010.13.018
Higgins, J., Thomas, J., Chandler, J., Cumpston, M. L. T., Page, Mj, and Welch, V. A. (2021). Cochrane handbook for systematic reviews of interventions version 6.2 (updated february 2021). Cochrane. Available online at: www.training.cochrane.org/handbook.
Hu, J., Li, Q., and Xie, Y. (2008a). Intrathoracic administration of Kushenhuangqi injection combined with cisplatin in treatment of malignant pleural efusion. Chin. J. Gen. Pract. 7, 561–563. doi:10.3760/cma.j.issn.1671-7368.2008.08.027
Hu, Q., Wang, H., and Pan, J. (2008b). Effect of Yanshu injection on malignant pleural effusion of advanced lung cancer. J. Chengdu Univ. TCM 31, 15–17. doi:10.3969/j.issn.1004-0668.2008.01.006
Huang, H., He, Z., Li, X., and Wang, R. (2017). Analysis of curative effect by compound Kushen injection combined with cisplatin through intrapleural infusion in the treatment of malignant pleural effusion. Chin. J. Mod. Drug Appl. 11, 29–31. doi:10.14164/j.cnki.cn11-5581/r.2017.07.012
Huang, L. (2021). Clinical effect of compound sophora flavescens injection in palliative treatment of advanced lung cancer patients with malignant pleural effusion. Health Manag., 71–72.
Huang, X. (2007). Clinical observation on treating pleural effusion of lung cancer with Yan-shu inject plus cisplatin J. Pract. Chin. Mod. Med. 20, 1106–1106.
Huang, X. (2013). Clinical study on intrapleural compound matrine injection in treating malignant hepatocellular carcinoma with bloody pleural effusion. Acta Med. Sin. 26, 692–693. doi:10.3969/j.issn.1008-2409.2013.04.010
Huang, Z., Zhang, H., Zhang, K., and Gao, L. (2012). The clinical research of compound Kushen injection and corynebacterium parvum as a local therapy for patients with advanced lung cancer associated with malignant pleural effusion. J. Clin. Pulm. Med. 17, 497–498. doi:10.3969/j.issn.1009-6663.2012.03.056
Ji, F., Shen, N., and Jin, H. (2012). Clinical observation of 82 cases of malignant pleural effusion treated with cisplatin combined with matrine. J. Shandong Med. 52, 92–93. doi:10.3969/j.issn.1002-266X.2012.29.039
Ji, H. (2011). Clinical study of 30 cases of malignant pleural effusion treated by cisplatin combined with oxymatrine. China Prac. Med. 6, 155–156. doi:10.3969/j.issn.1673-7555.2011.29.121
Jiang, H., Yang, X. M., Wang, C. Q., Xu, J., Huang, J., Feng, J. H., et al. (2022). Intrapleural perfusion with staphylococcal enterotoxin C for malignant pleural effusion: a clustered systematic review and meta-analysis. Front. Med. (Lausanne) 9, 816973. doi:10.3389/fmed.2022.816973
Jiang, J. (2014). Clinical effect of compound Kushen Injection combined with cisplatin in treatment of malignant pleural effusion. China Mod. Med. 21, 113–115.
Jiang, T., and Li, J. (2020). Compound Sophora flavescens injection and cisplatin in the treatment of malignant pleural effusion: a clinical study. Med. and Health 11, 1–2.
Jie Wang, X., Miao, K., Luo, Y., Li, R., Shou, T., Wang, P., et al. (2018). Randomized controlled trial of endostar combined with cisplatin/pemetrexed chemotherapy for elderly patients with advanced malignant pleural effusion of lung adenocarcinoma. J. buon 23, 92–97.
Keeratichananont, W., Limthon, T., and Keeratichananont, S. (2015). Efficacy and safety profile of autologous blood versus tetracycline pleurodesis for malignant pleural effusion. Ther. Adv. Respir. Dis. 9, 42–48. doi:10.1177/1753465815570307
Kessinger, A., and Wigton, R. S. (1987). Intracavitary bleomycin and tetracycline in the management of malignant pleural effusions: a randomized study. J. Surg. Oncol. 36, 81–83. doi:10.1002/jso.2930360202
Li, B., Yuan, Q., Wang, Y., Shi, M., Ren, X., and Dong, Y. (2019a). Network meta-analysis of 8 traditional Chinese medicine injections combined with cisplatin for malignant pleural effusion. Chin. Hosp. Pharm. J. 39, 1052–1057. doi:10.13286/j.cnki.chinhosppharmacyj.2019.10.13
Li, H., Ji, Y., Zhang, S., Gao, Z., Hu, C., Jiang, R., et al. (2019b). Kangai injection combined with platinum-based chemotherapy for the treatment of stage III/IV non-small cell lung cancer: a meta-analysis and systematic review of 35 randomized controlled trials. J. Cancer 10, 5283–5298. doi:10.7150/jca.31928
Li, L., and Tian, F. (2011). Clinical study of cisplatin compound Kushen injection in treating malignant pleural efusion. China J. Chi Med. 26, 1289–1290. doi:10.16368/j.issn.1674-8999.2011.11.062
Li, L., and Yang, F. (2009). Clinical study of oxymatrine injection combined with cisplatin injection in the treatment of malignant pleural effusion. China Prac. Med. 4, 39–41. doi:10.3969/j.issn.1673-7555.2009.36.023
Li, R., Yu, J., Zhang, S., and Wang, A. (2017). Eficacy of compound Kushen Injection combined with nedaplatin for malignant pleurai efusion. Liaoning J. TCM 44, 789–790. doi:10.13192/j.issn.1000-1719.2017.04.041
Li, S. (2014). Clinical observation of compound Kushen injection combined with nedaplatin in the treatment of malignant pleural effusion. Res. Integr. Tradit. Chin. West Med. 6, 88–89+91. doi:10.3969/j.issn.1674-4616.2014.02.010
Li, Y. (2008). The clinical value of cisplatin combined with compound Kushen Injection in treatment of cancerours hydrothorax. Mod. Prev. Med. 35, 1978–1979. doi:10.3969/j.issn.1003-8507.2008.10.078
Li, Y., Chen, C., and Li, Q. (2009). Compound Kushen injection combined with cisplatin pleural injection for lung cancer pleural effusion. China Naturop. 17, 42. doi:10.3969/j.issn.1007-5798.2009.04.043
Li, Z. (2022). “Network meta-analysis and network pharmacology study of Chinese medicine injection combined with cisplatin in the treatment of malignant pleural effusion of lung cancer,” in Master master. Nanning: Guangxi University of Traditional Chinese Medicine. doi:10.27879/d.cnki.ggxzy.2022.000202
Liang, N., Kong, Z., Lu, C. L., Ma, S. S., Li, Y. Q., Nikolova, D., et al. (2019). Radix Sophorae flavescentis versus other drugs or herbs for chronic hepatitis B. Cochrane Database Syst. Rev. 6, Cd013106. doi:10.1002/14651858.CD013106.pub2
Liang, Z., Li, X., Liu, Z., Li, R., and Zhai, Y. (2011). Efficacy of compound matrine injection for malignant pleural effusion. Eval. Anal. Drug-Use Hosp. China 11, 547–548. doi:10.14009/j.issn.1672-2124.2011.06.014
Lin, S., Wang, Y., and Cong, X. (2007). Thoracic perfusion of compound Kushen injection plus cisplatin for malignant pleural fluid. Eval. Anal. Drug-Use Hosp. China 7, 465–466. doi:10.3969/j.issn.1672-2124.2007.06.024
Lin, W., Yuan, S., Li, F., Yu, Y., Liu, Y., Deng, M., et al. (2023). Effect of intracavitary perfusion of compound Sophora Flavescens injection on the immune function and tumor markers in patients with malignant pleural effusion. Prog. Mod. Biomed. 23, 3758–3762. doi:10.13241/j.cnki.pmb.2023.19.032
Liu, D., and Li, D. (2015). Curative effect and nursing of thoracic cavity drainage and compound Kushen injection combined with cisplatin in the treatment of lung cancer patients companying with malignant pleural effusion. J. Clin. Med. Pract. 19, 21–24. doi:10.7619/jcmp.201508007
Liu, J., Yu, Q., Wang, X. S., Shi, Q., Wang, J., Wang, F., et al. (2023). Compound kushen injection reduces severe toxicity and symptom burden associated with curative radiotherapy in patients with lung cancer. J. Natl. Compr. Canc Netw. 21, 821–830.e3. doi:10.6004/jnccn.2023.7036
Liu, L., Zhong, S., and Li, G. (2017). Effects and safety of compound Kushen injection combined with cisplatin on malignant pleural effusion. J. Mod. Oncol. 25, 230–233. doi:10.3969/j.issn.1672-4992.2017.02.018
Liu, S., Zhang, K., and Hu, X. (2022). Comparative efficacy and safety of Chinese medicine injections combined with capecitabine and oxaliplatin chemotherapies in treatment of colorectal cancer: a bayesian network meta-analysis. Front. Pharmacol. 13, 1004259. doi:10.3389/fphar.2022.1004259
Liu, X., and Xu, J. (2016). Therapeutic effects and action mechanism of lobaplatin combined with Kushen injection on malignant pleural effusion in patients with advanced lung cancer. Hebei Med. J. 38, 1461–1464. doi:10.3969/j.issn.1002-7386.2016.10.005
Liu, Y., and Wan, L. (2011). Clinical study of compound Kushen injection combined with bleomycin in the treatment of malignant pleural effusion. Chin. Community Doct 13, 178–179. doi:10.3969/j.issn.1007-614x.2011.09.176
Ma, X., Li, R. S., Wang, J., Huang, Y. Q., Li, P. Y., Wang, J., et al. (2016). The therapeutic efficacy and safety of compound kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: an update systematic review and meta-analysis. Front. Pharmacol. 7, 70. doi:10.3389/fphar.2016.00070
Miller, A. B., Hoogstraten, B., Staquet, M., and Winkler, A. (1981). Reporting results of cancer treatment. Cancer 47, 207–214. doi:10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
Ning, X., Yang, S., and Jin, Q. (2001). Clinical obversation of treatint 30 cases of cancerous hydrothorax with injection of cisplation and Yanshu. Guid. J. TCMP 7, 70–71. doi:10.3969/j.issn.1672-951X.2001.02.016
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Paladine, W., Cunningham, T. J., Sponzo, R., Donavan, M., Olson, K., and Horton, J. (1976). Intracavitary bleomycin in the management of malignant effusions. Cancer 38, 1903–1908. doi:10.1002/1097-0142(197611)38:5<1903::aid-cncr2820380506>3.0.co;2-a
Pan, J., Chu, D., Hu, Z., and Sun, S. (2007). Clinical observation intracavity Kushen injection combined with cisplatin for the treatment of malignant pleural efusion. Chin. J. Clin. Pharm. 16, 139–141. doi:10.3969/j.issn.1007-4406.2007.03.002
Peng, H. (2020). Clinical effect of compound sophora flavescens injection in palliative treatment of advanced lung cancer patients with malignant pleural effusion. Health Everyone 27, 595.
Qin, D., and Fan, K. (2016). Analysis of the therapeutic effect of compound sophora flavescens injection and cisplatin thoracic perfusion therapy in patients with malignant pleural effusion. Med. Health, 257.
Qu, D., Liang, X., and Zhou, B. (2012). Clinical observation of Kangai injection combined with cisplatin in the treatment of malignant pleural effusion. Mod. J. ITCWM 21, 2311–2312. doi:10.3969/j.issn.1008-8849.2012.21.014
Ran, F., and Zang, A. (2011). Efficacy of Compound Kushen Injection combined with cisplatin for malignant pleural effusion. Eval. Anal. Drug-Use Hosp. China 11, 739–740. doi:10.14009/j.issn.1672-2124.2011.08.015
Shi, W. (2017). Clinical efficacy and safety of compound Kushen injection combined with cisplatin in the treatment of malignant pleural effusion caused by lung cancer. Chi J. Conval. Med. 26, 857–859. doi:10.13517/j.cnki.ccm.2017.08.032
Song, X., and Jia, Y. (2015). Compound Kushen injection combined with cisplatin pleural perfusion in the treatment of 59 cases of malignant pleural effusion. Forum TCM 30, 30–31. doi:10.13913/j.cnki.41-1110/r.2015.03.020
Sterne, J. a.C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Sun, C., Dong, F., Xiao, T., and Gao, W. (2021). Efficacy and safety of Chinese patent medicine (Kang-ai injection) as an adjuvant in the treatment of patients with hepatocellular carcinoma: a meta-analysis. Pharm. Biol. 59, 472–483. doi:10.1080/13880209.2021.1915340
Sun, Y. (2012). Bleomycin combined with compound Kushen injection in the treatment of 25 cases of malignant pleural effusion. Zhejiang J. TCM 47, 143. doi:10.3969/j.issn.0411-8421.2012.02.048
Tang, J., Fang, S., Fu, S., and Liu, G. (2014). Meta-analysis of Kang’ai injection combined with cisplatin in the treatment of malignant pleural effusion. Chin. J. Ethnomed Ethnopharm 23, 19–21+25. doi:10.3969/j.issn.1007-8517.2014.19.zgmzmjyyzz201419013
Tang, X., Jiang, M., Li, J., and Luo, B. (2018). Cinical objective on sixty cases of compound Kushen Injection combined with cisplatin in treatment of lung cancer pleural effusion. Liaoning J. TCM 45, 1668–1670. doi:10.13192/j.issn.1000-1719.2018.08.036
Thorlund, K., Devereaux, P. J., Wetterslev, J., Guyatt, G., Ioannidis, J. P., Thabane, L., et al. (2009). Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int. J. Epidemiol. 38, 276–286. doi:10.1093/ije/dyn179
Thorlund, K., Engstrøm, J., Wetterslev, J., Brok, J., Imberger, G., and Gluud, C. (2016). User manual for trial sequential analysis (TSA). Copenhagen: Copenhagen Trial Unit, Centre for Clinical Intervention Research. Rigshospitalet.
Tian, X., Wang, W., and Jia, L. (2010). Meta-Analysis of TCM injection in the treatment of malignant pleural effusion. Chin. Med. MDE China 8, 175–178. doi:10.3969/j.issn.1672-2779.2010.18.133
Trotti, A., Colevas, A. D., Setser, A., Rusch, V., Jaques, D., Budach, V., et al. (2003). CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin. Radiat. Oncol. 13, 176–181. doi:10.1016/S1053-4296(03)00031-6
Wan, Y. M., Li, Y. H., Xu, Z. Y., Wu, H. M., Xu, Y., Yang, M., et al. (2018). The effect of transarterial chemoembolization in combination with Kang'ai injection on patients with intermediate stage hepatocellular carcinoma: a prospective study. Integr. Cancer Ther. 17, 477–485. doi:10.1177/1534735417734913
Wang, C. Q., Shen, Y. S., Chen, X. F., Jiang, H., Yang, X. M., Fan, T. Y., et al. (2022). The intrapleural administration with thymic peptides in malignant pleural effusion: a clustered systematicreview and meta-analysis. Int. Immunopharmacol. 107, 108688. doi:10.1016/j.intimp.2022.108688
Wang, C. Q., Xu, J., Jiang, H., Zheng, X. T., Zhang, Y., Huang, X. R., et al. (2023a). The evidence framework of traditional Chinese medicine injection (Aidi injection) in controlling malignant pleural effusion: a clustered systematic review and meta-analysis. Phytomedicine 115, 154847. doi:10.1016/j.phymed.2023.154847
Wang, C. Q., Zheng, X. T., Chen, X. F., Jiang, H., Huang, J., Jiang, Y., et al. (2021). The optimal adjuvant strategy of aidi injection with gemcitabine and cisplatin in advanced non-small cell lung cancer: a meta-analysis of 70 randomized controlled trials. Front. Pharmacol. 12, 582447. doi:10.3389/fphar.2021.582447
Wang, H. (2016). Sophora radix astragali injection combined cisplatin intrathoracic injection clinical observation on treatment of lung cancer hydrothorax. Clin. Res. 24, 2–3.
Wang, R., Chen, M., Wang, Y., Liu, X., Hai, L., and Shuai, B. (2023b). Efficacy and safety of compound Kushen injection in the treatment of malignant pleural effusions caused by breast cancer. China Licens. Pharm. 20, 67–71. doi:10.3969/j.issn.2096-3327.2023.10.011
Wang, S., Lian, X., Sun, M., Luo, L., and Guo, L. (2016). Efficacy of compound Kushen injection plus radiotherapy on nonsmall-cell lungcancer: a systematic review and meta-analysis. J. Cancer Res. Ther. 12, 1298–1306. doi:10.4103/0973-1482.199538
Wang, S., and Zhou, W. (2016). Clinical observation of Compound Kushen Injection in the treatment of malignant pleural effusion. China Pract. Med. 11, 148–149. doi:10.4103/0973-1482.199538
Wang, Y. (2010). Clinical study of compound Kushen injection combined with cisplatin in the treatment of malignant pleural effusion. Chin. J. Mod. Drug Appl. 4, 148–149. doi:10.3969/j.issn.1673-9523.2010.05.144
Wang, Y., Fan, S., Luo, Q., Bai, C., and Li, T. (2019). Short term efficacy of pleural cavity drainage and infusion of compound Sophora flavescens injection combined with cisplatin in the treatment of malignant pleural effusion in lung cancer. World Latest Med. Inf. 19, 116+121. doi:10.19613/j.cnki.1671-3141.2019.02.075
Wang, Y., Lin, C., and Xiao, R. (2010). Effect of closed thoracic drainage with central venous catheters and combining cisplatinum(DDP) with matrine to pure into the thoracic cavity with malignant pleural effusion. Hainan Med. J. 21, 13–15.
Wei, M., and Sun, Q. (2011). Efficacy of compound Kushen injection combined with cisplatin in the treatment of malignant pleural effusion. JCM 9, 26–27.
Wei, W., Lan, Y., Li, Y., Zhong, B., Li, P., and Bai, L. (2014). A clinical observation of intrathoracic injection of OK-432 combined with compound Kushen injection for patients with malignant pleural effusion. J. Mod. Oncol. 22, 352–354. doi:10.3969/j.issn.1672-4992.2014.02.33
Wetterslev, J., Thorlund, K., Brok, J., and Gluud, C. (2008). Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J. Clin. Epidemiol. 61, 64–75. doi:10.1016/j.jclinepi.2007.03.013
Wetterslev, J., Thorlund, K., Brok, J., and Gluud, C. (2009). Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med. Res. Methodol. 9, 86. doi:10.1186/1471-2288-9-86
Wu, C., Wu, J., Wu, X., and Cheng, D. (2019). Clinical study of compound radix sophorae flavescentis injection combined with cisplatin in treatment of malignant pleural effusion. Liaoning J. TCM 46, 85–87. doi:10.13192/j.issn.1000-1719.2019.01.029
Wu, H., Qiu, X., Dong, Z., and Liu, H. (2018). Systematic review on the efficacy and safety of adjuvant therapy of compound kushen injection for pleural effusion in elderly patients with malignant cancer. China Pharm. 29, 2421–2425. doi:10.6039/j.issn.1001-0408.2018.17.27
Wu, Z., Zhang, R., Liu, Q., and Liu, G. (2014). Therapeutic effect of hydroxycamptothecin combined with Kushen Injection on malignant pleural effusion in elderly patients with lung cancer Hebei Med. J. 36, 3705–3707. doi:10.3969/j.issn.1002-7386.2014.24.007
Xiao, Z., Jiang, Y., Chen, X. F., Wang, C. Q., Zheng, X. T., Xu, W. H., et al. (2020a). Intrathoracic infusion therapy with Lentinan and chemical irritants for malignant pleural effusion: a systematic review and meta-analysis of 65 randomized controlled trials. Phytomedicine 76, 153260. doi:10.1016/j.phymed.2020.153260
Xiao, Z., Jiang, Y., Wang, C. Q., Hu, S. S., Huang, X. R., Chen, X. F., et al. (2020b). Clinical efficacy and safety of aidi injection combination with vinorelbine and cisplatin for advanced non-small-cell lung carcinoma: a systematic review and meta-analysis of 54 randomized controlled trials. Pharmacol. Res. 153, 104637. doi:10.1016/j.phrs.2020.104637
Xiao, Z., Wang, C. Q., Zhou, M. H., Li, N. N., Liu, S. Y., He, Y. J., et al. (2018). Clinical efficacy and safety of CIK plus radiotherapy for lung cancer: a meta-analysis of 16 randomized controlled trials. Int. Immunopharmacol. 61, 363–375. doi:10.1016/j.intimp.2018.06.012
Xing, H. (2013). Clinical observation of compound Kushen injection in the treatment of malignant pleural effusion. Chin. J. Mod. Drug Appl. 7, 84–85. doi:10.14164/j.cnki.cn11-5581/r.2013.17.204
Xu, B. (2014a). Pleural instillation by Yanshu injection and chemotherapy drugs in the treatment of malignant pleural effusion. J. Basic Clin. Oncol. 27, 44–45. doi:10.3969/j.issn.1673-5412.2014.01.014
Xu, C., Hu, Z., Xie, M., and Liu, Y. (2015). Meta-analysis of intrapleural injection of compound kushen injection combined with chemotherapy in treating malignant pleural effusion. China Pharm. 24, 31–32.
Xu, L. (2014b). Efficacy observation of compound Kushen injection and cisplatin for malignant pleural effusion. Contemp. Med. Symp. 12, 88–89. doi:10.3969/j.issn.2095-7629.2014.03.079
Xu, M., and Xiong, B. (2008). Intrapleural injection of Kangai zhusheye with cisplatin in malignant pleural efusion. Pract. J. Card. Cereb. Pneum. Vasc. Dis. 16, 114–117. doi:10.3969/j.issn.1008-5971.2008.02.010
Xu, Y. F., Chen, Y. R., Bu, F. L., Huang, Y. B., Sun, Y. X., Li, C. Y., et al. (2022). Chinese herbal injections versus intrapleural cisplatin for lung cancer patients with malignant pleural effusion: a Bayesian network meta-analysis of randomized controlled trials. Front. Oncol. 12, 942941. doi:10.3389/fonc.2022.942941
Yan, G., Jiang, H., Wang, P., and Du, H. (2016). The application of compound sophora injection combined with cisplatin in the treatment of malignant pleural effusion by pleural perfusion. Jilin Med. J. 37, 574–576. doi:10.3969/j.issn.1004-0412.2016.03.025
Yang, G. (2012). The recent efficacy of compound Kushen injection combined with cisplafin chest perfusion for malignant pleurai effussion. China Med. 7, 1079–1080. doi:10.3760/cma.j.issn.1673-4777.2012.09.011
Yang, M., Ren, J., Wen, S., Guo, C., Pan, R., Fu, X., et al. (2016). Compound Kushen injection combined with cisplatin for treatment of malignant pleural effusion:A Meta -analysis. J. Mod. Oncol. 24, 3393–3398. doi:10.3969/j.issn.1672-4992.2016.21.013
Yang, X., Wei, X., and Jiang, L. (2017). Network meta-analysis of 5 kinds of TCM injections in the treatment of malignant pleural effusion. China Pharm. 28, 4686–4690. doi:10.6039/j.issn.1001-0408.2017.33.22
Yuan, Y. (2007). Thoracentesis and drainage combined with Yanshu injection for malignant pleural effusion. China Pharm. 18, 1891–1892. doi:10.3969/j.issn.1001-0408.2007.24.023
Zhang, L.-H., Guo, Y.-F., Sheng, Y.-C., Xie, W., Ji, R., and Mei, Q.-B. (2011). Systematic review of platinum antitumor drugs combined with Mannatide in the treatment of malignant pleural effusion. China Pharm. 22, 1691–1695.
Zhang, S., Chen, X., Chen, Y., Bao, L., and Zhang, T. (2015a). Effiacy and safety of compound Kushen injection plus nedaplatin for malignant pleural effusion in patients with lung cancer. Eval. Anal. Drug-Use Hosp. China 15, 601–603. doi:10.14009/j.issn.1672-2124.2015.05.015
Zhang, S., Jia, Y., Yao, L., Yin, S., and Ye, X. (2008). Clinical study of compound Kushen injection combined with cisplatin on malignant pleural effusion. World Health Dig. 5, 1218–1219.
Zhang, X. (2006). Clinical effect of Kangai injection combined with cisplatin on malignant pleural effusion. Liaoning J. TCM 33, 1427–1428. doi:10.3969/j.issn.1000-1719.2006.11.028
Zhang, X. (2011). Clinical observation of compound sophora flavescens injection in the treatment of cancerous Pleural effusion. J. Chin. Pract. Diagn Ther. 25, 281–282.
Zhang, X., Geng, S., and Wang, Y. (2013). Clinical study of compound Kushen injection combined with mitomycin for malignant pleural efusion. Chin. J. Clin. Oncol. Rehabil. 20, 780–782. doi:10.13455/j.cnki.cjcor.2013.07.019
Zhang, Z., An, J., Zhang, F., and Yuan, Y. (2015b). Effect of compound matrine intracavity perfusion on VEGF, MMP levels in hydrothorax in patients of malignant pleural effusion. Hainan Med. J. 26, 166–168. doi:10.3969/j.issn.1003-6350.2015.02.0059
Zheng, S., and Jia, X. (2013). Clinical observation of pleural perfusion in the treatment of malignant pleural effusion. Hebei Med. J. 35, 416–417.
Zhong, B., Wei, W., Li, Y., Li, P., Lan, Y., and Bai, L. (2015). A clinical observation of intrapleural perfusion of OK-432 combined with compound Kushen injection in treatment of malignant pleural effusion. Mod. Med. J. China 17, 8–10. doi:10.3969/j.issn.1672-9463.2015.03.003
Zhou, Y., Xu, X., and Zhou, P. (2010). Clinical observation on compound matrine injection plus interleukin-2 in the treatment of malignant pleural effusion induced by lung cancer in elder. Chin. J. Clin. Pharm. Ther. 15, 1402–1405.
Zhu, M., Jiang, H., Zhu, Z., and Xu, H. (2013). Clinical study of perfusion with compound Kushen injection plus cisplatin for malignant pleural fluid. Tianjin Pharm. 25, 30–32.
Zhu, N., and Hou, J. (2021). Molecular mechanism of the anti-inflammatory effects of Sophorae Flavescentis Aiton identified by network pharmacology. Sci. Rep. 11, 1005. doi:10.1038/s41598-020-80297-y
Keywords: malignant pleural effusions, Radix Sophorae Flavescentis, compound kushen injection, matrine injection, Kangai injection, clustered systematic review Bibby, A.C., Dorn
Citation: Zhang Y, Xiao Z, Liu H, Cai D-C, Luo Y-Q, Xu J, Luo F, Huang J, Jin Y-Y, Fan T-Y, Zhang J, Xiao X and Feng J-H (2025) Intrapleural administration with traditional Chinese medicine injections (Sophorae flavescentis preparations) in controlling malignant pleural effusion: a clustered systematic review and meta-analysis. Front. Pharmacol. 16:1519794. doi: 10.3389/fphar.2025.1519794
Received: 30 October 2024; Accepted: 03 March 2025;
Published: 24 April 2025.
Edited by:
Ruiwen Zhang, University of Houston, United StatesReviewed by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaHemanga Hazarika, Girijananda Chowdhury University, India
Copyright © 2025 Zhang, Xiao, Liu, Cai, Luo, Xu, Luo, Huang, Jin, Fan, Zhang, Xiao and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Xiao, enk0MjZmQDE2My5jb20=
†These authors have contributed equally to this work
 Yan Zhang1,2,3†
Yan Zhang1,2,3† Zheng Xiao
Zheng Xiao Yao-Qin Luo
Yao-Qin Luo Xue Xiao
Xue Xiao