
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 05 February 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1517964
This article is part of the Research Topic Herbal Medicines and Their Metabolites: Effects on Lipid Metabolic Disorders via Modulating Oxidative Stress - Volume II View all 8 articles
 Tianyao Gong1†
Tianyao Gong1† Dongmei Wang1,2†
Dongmei Wang1,2† Jinyan Wang1†
Jinyan Wang1† Qun Huang3
Qun Huang3 Haiyan Zhang1
Haiyan Zhang1 Chunmeng Liu1
Chunmeng Liu1 Xinglin Liu4
Xinglin Liu4 Hejiang Ye3*
Hejiang Ye3*Diabetic retinopathy is the main microvascular complication of diabetes and the first blinding eye disease in the working-age population. Oxidative stress is an important pathogenesis of diabetic retinopathy. Plant metabolites can be divided into two types: primary metabolites and secondary metabolites, secondary metabolites are the main active components and important sources for developing new drugs. It has unique effect in the treatment of diabetic retinopathy. However, the research on the intervention mechanism of plant metabolites in diabetic retinopathy are still relatively shallow, which limit the application of plant metabolites. With the deepening of research, more and more plant metabolites have been reported to play a role in treating diabetic retinopathy through anti-oxidative stress, including polyphenols, polysaccharides, saponins, alkaloids, etc. Therefore, this article reviewed the potential of plant metabolites in the treatment of diabetic retinopathy in the last 10 years and elucidated their mechanism of action. We hope to provide some references for the application of plant metabolites and provide valuable resources for the research and development of new drugs for diabetic retinopathy.
Diabetes mellitus is a chronic disease characterized by hyperglycemia caused by insulin deficiency or destruction of islet beta cells (Khatik et al., 2018). The number of people with diabetes worldwide is expected to increase by 46%–783 million by 2045 (International Diabetes Federation, 2021). Diabetic Retinopathy (DR), one of the major complications of diabetes, affects the retinal microvascular system and is the leading cause of blindness in people of working age. DR Is divided into non-proliferative diabetic retinopathy and proliferative diabetic retinopathy. Non-proliferative diabetic retinopathy may further evolve into proliferative diabetic retinopathy, which can lead to various vision-threatening complications such as vitreous hemorrhage, intravitreal neovasculogenesis, retinal traction detachment, and even blindness (Flaxel et al., 2020). In the coming decades, the global incidence and disease burden of DR Will increase substantially, from an estimated 103 million people in 2020 to 161 million people in 2045 (Teo et al., 2021; Kang and Yang, 2020). This will make DR One of the major global public health burdens.
DR is a multifactorial disease with a complex pathogenesis, including inflammation (Wu et al., 2020), oxidative stress (Ortega, 2021), VEGF (Wu et al., 2020), etc. Current treatments focus on advanced DR, when blood vessels have been severely altered and retinal neurons have been irreparably damaged (Rodríguez et al., 2019). Treatment methods include vitreoretinal surgery, laser photocoagulation, intravitreal injection of anti-VEGF drugs, and standard biomedical treatment (Sivaprasad et al., 2017). Among these therapies, retinal photocoagulation surgery and intravitreal anti-VEGF therapy are still the main treatments for proliferative diabetic retinopathy (Flaxel et al., 2020). Although anti-VEGF therapy and laser photocoagulation therapy can “improve” vasculopathy and DR Severity, reports show that underlying retinal ischemia does not change, and lesions and retinopathy usually recur quickly after cessation (Couturier et al., 2019; Pearce et al., 2020). The cost of surgery is expensive, the prognosis is uncontrollable, and the effect of standard biomedical treatment alone is not ideal. Therefore, the development of safer and more economical hypoglycemic drugs has become an urgent problem that researchers need to solve.
As the advantages of botanical drugs in the prevention and treatment of chronic diseases continue to emerge, a variety of botanical drugs with mild side effects are used to treat DR (Li et al., 2023; Xu et al., 2018; Song et al., 2019). A review by Ai et al. (2020) showed that botanical drugs could significantly improve DR Plant metabolites generally refer to all metabolites produced in the plant body, including primary and secondary metabolites. Primary metabolites are essential for plant growth and development, such as carbohydrates, fats, proteins, etc. Secondary metabolites are non-essential small molecule compounds produced in the normal growth process of plants, with antioxidant, anti-inflammatory, antibacterial, antiviral and other biological activities, such as alkaloids, terpenes and so on. Natural products refer to various substances extracted from nature, including the components of plants, animals, microorganisms and other organisms or their metabolites. Thus, plant metabolites are part of natural products, but natural products also include substances of non-plant origin.
The results showed that plant metabolites had good effect and little side effect in treating DR (Wang L. et al., 2023; Zhang et al., 2019a; Niu et al., 2023). It has inhibitory effects on a variety of diabetes targets, including α-glucosidase, α-amylase, and peroxisome proliferator-activated receptor γ (Ferrannini et al., 2005; Matsui et al., 2001). Polyphenols are bioactive molecules that can be extracted from common plants and have become the subject of research interest in recent years due to their significance in the treatment of diseases such as diabetes and human health-related diseases (Pallavi et al., 2018). It has the function of anti-oxidation, strengthening blood vessel walls, reducing blood fat, increasing body resistance, preventing arteriosclerosis, and so on Joma et al. (2024), Shen et al. (2022). Resveratrol (Huang et al., 2020) and multiple flavonoids are all polyphenolic metabolites. Multiple polyphenols have been shown to inhibit alpha-glucosidase and alpha-amylase with inhibition levels of 50% or higher (El-Beshbishy and Bahashwan, 2012). Natural polysaccharides exist widely in nature, including plants, Marine organisms, and microorganisms. Polysaccharide is a kind of carbohydrate substance with a complex molecular structure, widely existing in animal cell membranes, and plant and microbial cell walls, and is one of the four basic substances constituting life. It mainly has the functions of immune regulation, anti-tumor, anti-oxidation, anti-virus, lowering blood sugar, lowering blood lipids, and delaying aging (Benalaya et al., 2024; Wu et al., 2016). Lycium barbarum polysaccharide, Astragalus polysaccharide, Angelica polysaccharide, and Notoginseng polysaccharide are all polysaccharides, which have antioxidant, immunomodulatory, and anti-tumor effects. Saponins are secondary plant metabolites, which contain triterpenoid aglycones or steroidal aglycones with uronic acid. It has a variety of biological activities, including anti-inflammatory, antioxidant, anti-glucose, and immunomodulatory functions (Shen et al., 2023), including ginsenosides, Panax notoginseng saponins, and so on. Alkaloid is a kind of nitrogen-containing alkaline organic metabolites that exists in nature and is one of the most important metabolites of botanical drug. It has a variety of pharmacological activities, including hypolipidemic, anti-inflammatory, anti-infection, and anti-tumor effects (Yu et al., 2022), including evodia, berberine, marine, and so on. Reactive Oxygen Species (ROS) has been recognized as a potential causative factor in diabetic retinopathy. Research shows that (Fanaro et al., 2023; Wang C. et al., 2022; Gupta et al., 2011) plant metabolites may ameliorate the symptoms of diabetic retinopathy mainly by regulating ROS. In this study, we conducted a literature search using databases such as CNKI (https://www.cnki.net/), Wanfang (https://www.wanfangdata.com.cn/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), and Web of Science (https://www.webofscience.com/), using search terms like “plant metabolites” OR “Chinese herbal metabolites” AND “diabetic retinopathy” to identify articles related to “oxidative stress” published in the past decade. We aimed to summarize plant metabolites that act on diabetic retinopathy through the oxidative stress (OS) pathway, hoping to provide data references for the development of new therapeutic drugs for diabetic retinopathy.
Oxidative stress plays a crucial role in the occurrence and development of diabetes. Particularly under hyperglycemic conditions, the level of oxidative stress significantly increases. Oxidative stress refers to the situation where free radicals in the body, especially ROS, are produced in excess and exceed the clearance capacity of the antioxidant defense system, leading to cell and tissue damage. Diabetic patients often have hyperglycemia, and long-term hyperglycemia is considered one of the main factors exacerbating oxidative stress. When excessive glucose undergoes autoxidation in the body, free radicals can be generated. These free radicals not only directly damage islet β cells and reduce insulin secretion but also promote the exacerbation of oxidative stress through the polyol pathway and glycosylation reaction (Darenskaya et al., 2021).
The influence of oxidative stress on diabetes is primarily manifested in insulin resistance and the occurrence of complications. To begin with, oxidative stress undermines the function of pancreatic islet β cells, which leads to a reduction in insulin secretion and further exacerbates the elevation of blood glucose levels. Moreover, oxidative stress disrupts the action of insulin by modulating cell signaling pathways, causing the sensitivity of tissue cells to insulin to decline and thus giving rise to insulin resistance. Additionally, oxidative stress is closely associated with microvascular disorders and cardiovascular complications in diabetes. It facilitates inflammatory responses and arteriosclerosis through damaging the vascular endothelium (Papachristoforou et al., 2020). Consequently, oxidative stress serves as not only one of the pathological foundations of diabetes but also an accelerating element in the development of diabetic complications.
Oxidative stress is an imbalance of the body caused by the incongruity of the generation and removal of free radicals. Overproduction of ROS can affect the activity of endogenous antioxidant enzymes, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), etc., which leads to the decreased ability to protect cells. It causes the imbalance of the oxidation-antioxidant system and promotes the occurrence and development of DR. The outer layer of the human retina is rich in a variety of unsaturated fatty acids, which play an important role in the structure and energy of the retina (Tang and Tan, 2023). However, the imbalance of oxidation-antioxidant regulation in the body and the excess of ROS will lead to the oxidation and degradation of these fatty acids, and at the same time start the lipid peroxidation reaction, which will affect the physiology of the retina and the normal function of visual cells. The complex physiological functions of the retina require long-term high oxygen consumption to support, so it is easier to produce ROS, accelerate the process of lipid peroxidation, and ultimately accelerate the progress of DR. At the same time, excessive ROS accumulation can also damage the tissues in and around the retinal blood vessels so that the repeated interaction eventually leads to the occurrence of DR.
The occurrence and development of diabetic retinopathy are closely related to the injury, apoptosis, abnormal proliferation of vascular endothelial cells, the breakdown of cell connections, and the proliferation of new blood vessels after ischemia. There is a complex relationship between oxidative stress and various pathogenesis of diabetic retinopathy, such as inflammation, mitochondrial dysfunction, and apoptosis (Tian and Li, 2024). The retinal oxidative damage induced by hyperglycemia mainly involves four metabolic pathways: advanced glycation end products (AGE) pathway, polyol pathway, hexosamine pathway, and protein kinase C pathway (Shi, 2021). In addition, the phenomenon of “metabolic memory” caused by irregular epigenetic modification in diabetic patients has also been shown to be related to the overproduction of ROS (Sui et al., 2020). The metabolism of high sugar generates a large number of reactive oxygen species, which damages the retina, leads to oxidative damage of DNA, and eventually leads to apoptosis. At the same time, the increase in the level of oxidative stress activates cytokines and further upregulates the level of oxidative stress, thus causing the structural and functional abnormalities of retinal cells.
Mitochondria play an important role in cells by metabolizing nutrients and producing adenosine triphosphate (Kim et al., 2008). Mitochondria are also important sources of ROS in most mammalian cells (Murphy, 2009). In diabetic conditions, excess ROS can damage mitochondrial proteins/enzymes, membranes, and DNA, resulting in disruption of mitochondrial adenosine triphosphate production and other essential functions. Mitochondrial dysfunction caused by oxidative stress in diabetes can be divided into the following aspects: 1. In the condition of diabetes, excess ROS will weaken the antioxidant defense ability of cells, damage mitochondrial DNA, and proteins, and thus affect mitochondrial function (Murphy, 2009; Dröge, 2002). 2. The increase of oxidative stress will damage the inner membrane of mitochondria, resulting in decreased membrane potential, which will affect the mitochondrial respiratory chain and other mitochondrial metabolites, leading to further decline in mitochondrial function, and ultimately leading to mitochondrial fragmentation, further decline in oxidative phosphorylation and increase in ROS production (Kowaltowski and snd Vercesi, 1999; Maassen et al., 2004). 3. ROS can disrupt cell signaling and cause mitochondrial dysfunction, resulting in insufficient energy and eventually loss of function (Juan et al., 2021).
Some studies believe that DR Is a low-grade chronic inflammatory disease, the release of pro-inflammatory cytokines and the adhesion of white blood cells to retinal capillaries are the initial events in the development of DR (Yu and Li, 2018). IL-6 and TNF-α are the core factors in the inflammatory cascade. IL-6 aggravates the condition of diabetic patients by damaging the body’s islet beta cells and affecting the normal secretion of insulin. TNF-α and CRP stimulate retinal vascular endothelial cells to produce oxygen free radicals, increase the release of lipid peroxidation products, and cause retinal microangiopathy. The study found that the levels of inflammatory cytokines (including IL-1, IL-6, IL-8, IL-17, and TNF-α) in aqueous humor were increased in patients with DR and may be synergistically involved in the pathogenesis of DR (Feng et al., 2018). The increase of TNF-α, CRP, and IL-6 was the most obvious in the proliferative stage of DR Patients, followed by the non-proliferative stage retinopathy patients. IL-1β is also an interleukin factor closely related to DR. IL-1β and TNF-α jointly inhibit the expression of retinoid-binding protein between photoreceptors, and causing degeneration of retinal neurons and capillary blood vessels, thus increasing the degeneration of neurons and capillaries of DR (Jin et al., 2023). The NLRP3 inflammasome is a multi-protein complex that exists inside cells, It consists of NLRP3 receptor protein, Apoptosis-associated speck-like protein containing a CARD (ASC), and the effector procysteine aspartate-specific protease 1(Pro-Caspase-1) consists of three parts. The NLRP3 inflammasome is an important part of the innate immune system, which promotes the activation of aspartate proteolytic enzyme 1(Caspase-1) and the secretion of the pro-inflammatory cytokine IL-1βor IL-18 (Feng et al., 2018).
MDA (malondialdehyde), SOD (superoxide dismutase), ROS and HO-1 (heme oxygenase-1) are the most commonly used indicators of free radical levels. MDA is the product of lipid peroxidation. In diabetic retinopathy, MDA is increased correspondingly, and the increase of MDA level can reflect the degree of oxidative damage of retinal tissue. SOD is an important part of the antioxidant defense system of retina. In the early stage of diabetic retinopathy, due to the increased production of ROS, the body may fight oxidative stress by up-regulating the expression and activity of SOD. In the condition of long-term hyperglycemia, the activity of SOD will be reduced due to glycosylation modification and other factors, which will aggravate the damage of the retina caused by oxidative stress. In the condition of diabetes, hyperglycemia is the main cause of increased ROS production, and excessive ROS will cause damage to various retinal cells such as retinal pigment epithelial cells, ganglion cells, and endothelial cells. HO-1 plays an important cytoprotective role in diabetic retinopathy. When stimulated by oxidative stress and inflammation, HO-1 expression in retinal cells increases. HO-1 catalyzes the decomposition of heme to produce products such as biliverdin, carbon monoxide (CO) and free iron, in which biliverdin is converted to bilirubin and is a potent antioxidant that can clear ROS. Through its metabolites, HO-1 can also inhibit the formation of retinal neovascularization and reduce the degree of lesions (Yu and Li, 2018).
In conclusion, in diabetic retinopathy, ROS is an important factor leading to the lesion, SOD and HO-1 are important defense mechanisms against oxidative stress, and MDA is an important marker reflecting the degree of oxidative damage of retinal tissue. These indicators are of great significance for the study of the pathogenesis, diagnosis and treatment of diabetic retinopathy (Yang and Song, 2023).
VEGF is a key regulator of angiogenesis, has a highly specific endothelial cell mitogen, and is closely related to DR Neovascularization (Gao et al., 2017). The family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF, and PLGF (placental growth factor). When VEGF is highly expressed, the tight connection between endothelial cells in the inner layer of the retina is destroyed, and the vascular permeability of the retina is increased, resulting in retinal vascular leakage, which leads to the destruction of the blood-retinal barrier and the formation of new vessels. Inhibition of VEGF expression is critical to slow down the progression of DR, so VEGF is one of the key targets in studying the early lesions and retinal protection of DR (Jin et al., 2023).
HIF-1α is a part of the transcription complex, which can promote oxygen transport, angiogenesis, metabolic adaptation, cell proliferation, and survival stress. HIF-1 expressed under hypoxia can promote blood vessel formation by increasing the expression of a large amount of VEGF, thus affecting the transport of oxygen to the inner cells (Chai et al., 2020).
Ephrin-A1 is a hepatocyte family protein that promotes angiogenesis and promotes retinal neovascularization through mediating the expression of vascular growth factor. CTRP9 is an adipokine with high homology to adiponectin, which has the function of protecting vascular endothelial cells and is closely related to body obesity. Abnormal expression of serum Ephrin-A1 and CTRP9 in diabetic retinopathy patients. In addition, serum Ephrin-A1 in proliferative diabetic retinopathy patients was higher than that in non-proliferative diabetic retinopathy patients, and CTRP9 level was lower than that in non-proliferative diabetic retinopathy patients (Gao et al., 2017; Zhou et al., 2021).
Overall, a complex interaction network is formed between inflammatory factors, oxidative stress, and endothelial factors. During the onset and progression of DR, the release of inflammatory factors triggers oxidative stress, which in turn promotes the increase of inflammatory factors and the expression of VEGF (Brzović-Šarić et al., 2015; Andrés-Blasco et al., 2023). This process exacerbates retinal neovascularization and increases vascular permeability. Ultimately, these factors work together to drive microvascular changes in the retina, the breakdown of the blood-retinal barrier, and neurodegenerative changes in the retina, thereby worsening the pathological damage of DR (Figure 1).
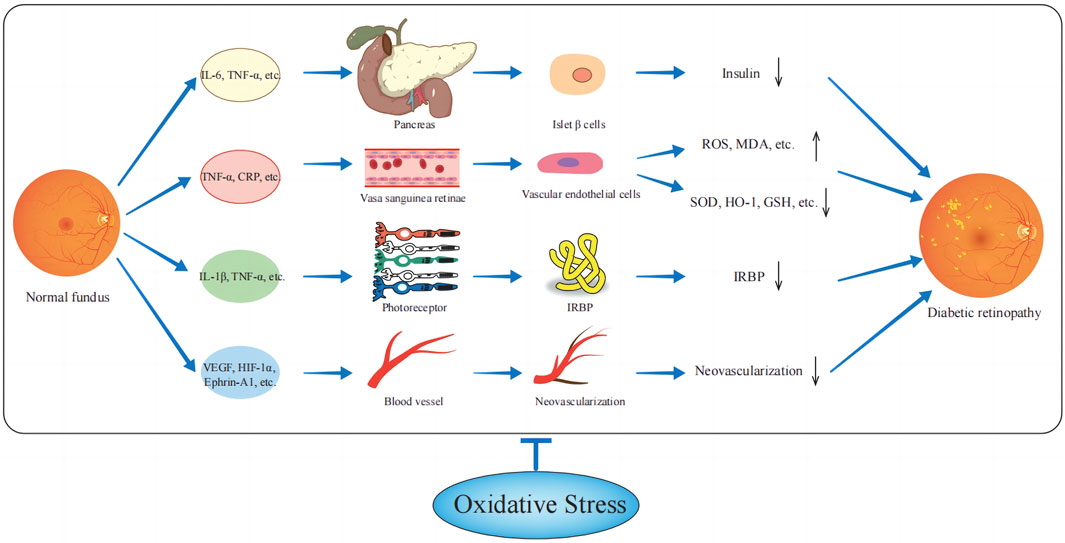
Figure 1. Relationship between oxidative stress-related factors and diabetic retinopathy. Inflammatory factors and vascular endothelial factors act on pancreatic βcells, retinal vascular endothelial cells, photoreceptor cells, etc., increase ROS, and MDA, reduce GSH, SOD, etc., and make the patient’s body in a state of oxygen-free radical overload, thereby promoting diabetic retinopathy.
Plant metabolites are all metabolites produced during plant growth and development, which has a clear chemical structure and a variety of pharmacological activities, which can intervene in diseases through multiple targets, and is an important source of new drug research and development. Plant metabolites have the advantages of precise efficacy, multi-target intervention, and low toxicity and side effects. At the same time, Plant metabolites have the advantage of a clear structure of chemical drugs, which can be replicated and promoted, providing important ideas for the research and development of new drugs (Qiu et al., 2021).
Diabetic retinopathy in traditional Chinese medicine belongs to the category of “thirst-quenching cataract” and “thirst-quenching eye disease”, and the pathogenesis is mainly liver and kidney deficiency, qi and yin deficiency, which can be combined with phlegm turbidity and blood stasis (An et al., 2024). More and more studies have shown that plant metabolites can act on diabetic retinopathy through multiple targets, and the mechanism of oxidative stress may be the main mechanism (Tian and Li, 2024). Therefore, we try to summarise the plant metabolites that can act on DR through the OS pathway, hoping to provide data reference for the development of new drugs for DR. To facilitate the study of the structure and mechanism of action of plant metabolites, we divided the plant metabolites with potential for the treatment of DR into four categories according to their different chemical structures: polyphenols, polysaccharides, saponins, and alkaloids.
Plant metabolites of polyphenols can be divided into flavonoids and non-flavonoids. Flavonoids are based on 2-phenylchromone as the basic parent nucleus, and its basic structure is A C6-C3-C6 skeleton composed of two benzene rings (A ring and B ring) interconnected through the central three-carbon chain, such as quercetin and puerarin, which mainly have antioxidant, anti-inflammatory and cardiovascular protection effects. Non-flavonoids are mainly stilbenes and phenolic acids, such as picotaxol and ferulic acid, which mainly have antioxidant, anti-platelet aggregation and anti-cancer effects (Table 1).
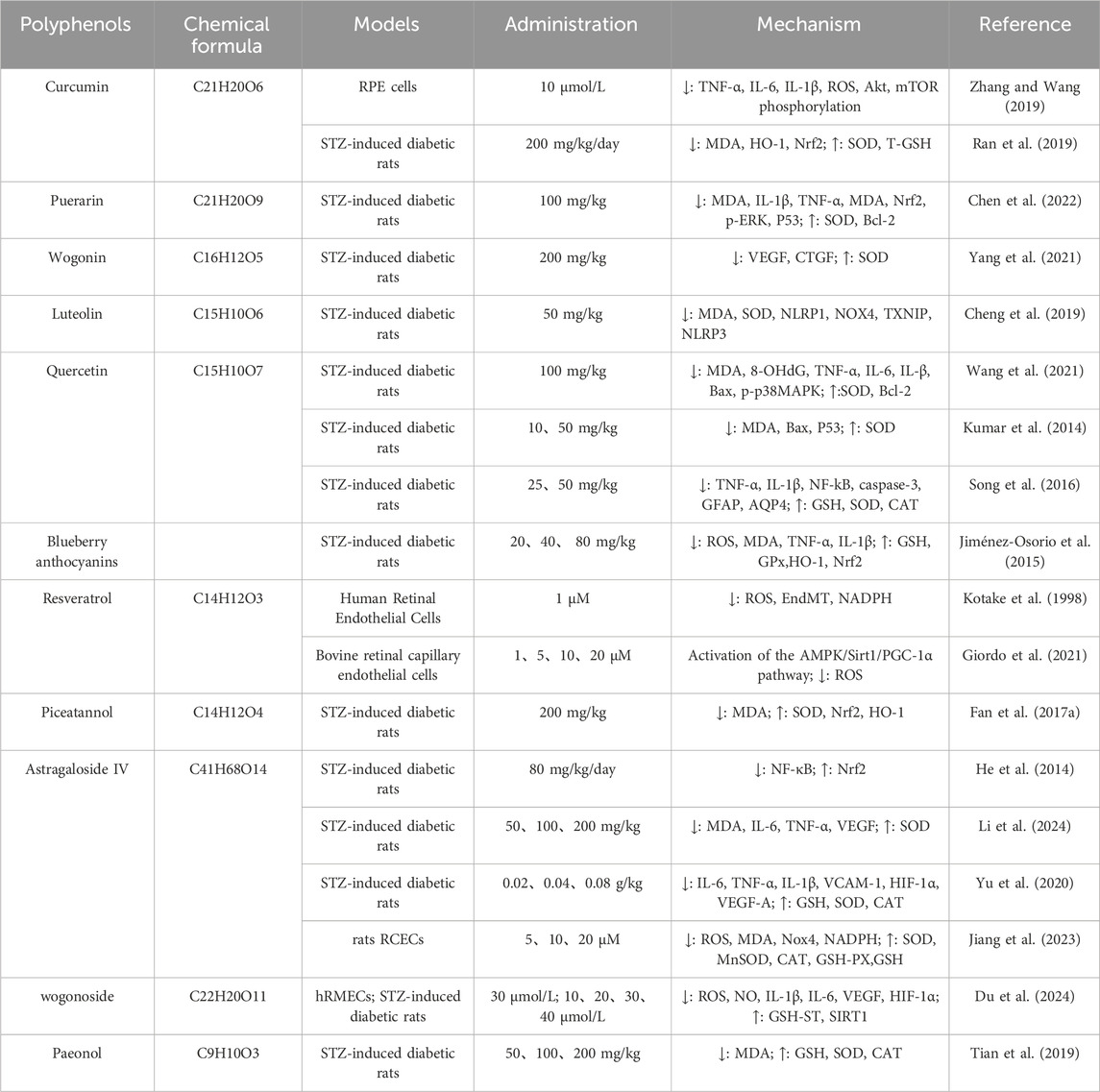
Table 1. Application of polyphenols in reducing oxidative stress in the treatment of diabetic retinopathy.
Curcumin can alleviate diabetic retinal damage through its antioxidant effects. In the early stages of diabetes, enhanced retinal oxidative stress induces adaptive activation of the nuclear factor E2-related factor 2 (Nrf2) pathway. Curcumin down-regulates oxidative stress-induced Nrf2, MDA, HO-1; up-regulates SOD, T-GSH. When curcumin is combined with insulin, the effect of maintaining Nrf2 pathway homeostasis in diabetic rats is better than that of insulin alone. Transcriptomic analysis showed that curcumin, alone or in combination with insulin, inhibited the AGE-RAGE signaling pathway and extracellular matrix-receptor interaction in the diabetic retina. Therefore, in the early stages of diabetes, curcumin can be used as a potential complementary treatment option in combination with antihyperglycemic drugs for more effective treatment of diabetic complications (Xie et al., 2021). In addition, curcumin can also inhibit the damage of inflammation and oxidative stress to RPE cells by reducing inflammatory factors such as TNF-α, IL-6, and IL-1β, and reducing ROS, Akt, mTOR phosphorylation (Ran et al., 2019).
Puerarin can effectively slow down the apoptosis of retinal ganglion cells and improve the progression of type 2 DR in rats with type 2 diabetes, and its mechanism may be related to down-regulating the levels of MDA, IL-1β, TNF-α, MDA, Nrf2, p-ERK, P53. Upregulation of SOD, Bcl-2 was associated with reducing inflammation and oxidative stress in retinal tissue (Zhang and Wang, 2019).
Wogonin has a variety of biological activities and effects, such as hypoglycemia, neuroprotection, anti-tumor, etc., among which the role of hypoglycemia and prevention and treatment of diabetic complications has been paid more and more attention (Chen et al., 2022). After treatment with baicalein, the serum VEGF and CTGF levels of diabetic mouse models were increased, and the SOD activity was increased. The possible mechanism is as follows: baicalein plays an important role in regulating ROS levels, and through its antioxidant effect, it scavenges oxygen-free radicals and lipid oxidation processes and protects blood vessels and nerves (Hou et al., 2019).
Luteolin can prevent retinal cell apoptosis in rats with DR, and its mechanism may be related to NLRP/NOX4 signaling pathway, and alleviate the validation response and oxidative stress of retinal tissue by downregulating MDA, SOD, NLRP1, NOX4, TXNIP, NLRP3 (Yang et al., 2021).
Quercetin is a natural flavonoid with a wide range of pharmacological effects, including hypoglycemic effects, antioxidant, anti-inflammatory, anti-apoptotic, and immunomodulatory effects (Cheng et al., 2019). The diabetes model of male SD rats was established by intraperitoneal injection of streptozotocin, and they were randomly divided into model group, Quercetin low dose group and Quercetin high dose group. Normal rats were used as control group. Quercetin group was intraperitoneally injected with Quercetin every day, the low-dose group was 10 mg/kg, the high-dose group was 50 mg/kg. Normal control group and diabetic group were not treated for 12 weeks. The results showed that the expression of Bcl-2 protein decreased gradually in low dose and high dose Quercetin groups, while the expression of Bax and P53 protein increased significantly (Zhu et al., 2023; Wang et al., 2021). In addition, quercetin can also increase CAT, and GSH, reduce NF-kB, caspase-3, GFAP, and AQP4, and can effectively protect against diabetes-induced retinal neurodegeneration and oxidative stress (Kumar et al., 2014).
Blueberry anthocyanins can inhibit the development or progression of retinopathy, and its mechanism may be related to the down-regulation of ROS, MDA, TNF-α, and IL-1β levels; up-regulating the contents of GSH, HO-1, Nrf2, and GPx was associated with reducing inflammation and oxidative stress (Song et al., 2016).
Resveratrol is a polyphenolic plant metabolite, which is widely found in a variety of plants such as veratrol, grape, and Chinese knotweed, which can effectively activate Nrf2, thereby promoting antioxidant function and reducing oxidative stress in DR retinal tissue (Jiménez-Osorio et al., 2015). Li et al. showed that resveratrol can reduce oxidative stress and alleviate the apoptosis of retinal capillary endothelial cells by activating the AMPK/Sirt1/PGC-1α pathway and down-regulating ROS levels (Li et al., 2017). In addition, resveratrol can also reduce endothelial cell-to-mesenchymal transition and reduce NADPH to attenuate retinal damage caused by oxidative stress (Giordo et al., 2021).
Piceatannol is a derivative of resveratrol, which has been pharmacologically proven to have hypoglycemic and powerful antioxidant and free radical scavenging effects (Kotake et al., 1998). Studies have shown that piceatannol attenuates retinal ganglion cell damage in diabetic rats and is associated with the activation of the Nrf2/HO-1 signaling pathway by reducing MDA levels; The contents of SOD, Nrf2, and HO-1 were increased, and the oxidative stress of retina in DR rats was reduced (Luo et al., 2021).
Astragaloside IV is one of the active metabolites of the traditional Chinese medicine Astragalus sinicus L, which has strong immunostimulating, anti-inflammatory, and antioxidant effects (Fan Y. et al., 2017; Zhang R. et al., 2019). Studies have shown that astragaloside IV protects rat retinal capillary endothelial cells from high glucose-induced oxidative damage, and its mechanism may be related to the downregulation of ROS, MDA, Nox4, NADPH; It is related to the increase in the expression of antioxidant factors such as SOD, MnSOD CAT, GSH-PX, and GSH (Qiao et al., 2017). Astragaloside IV can improve early DR symptoms, significantly reduce the expression of IL-6 and TNF-α, improve the activity of SOD, and reduce the content of MDA in rats with type 1 diabetes. Its mechanism of action is related to the inhibition of inflammation, oxidative stress, and regulation of retinal VEGFA expression level (Jiang et al., 2023). Astragaloside IV can alleviate oxidative stress and inflammatory damage in the retina of type 2 diabetic rats, and its mechanism may be related to the improvement of SOD, CAT, and GSH activity or content in the retina of rats. Decrease IL-6, TNF-α, VCAM-1, HIF-1α, VEGF-A, and IL-1β to alleviate and delay the progression of DR (Li et al., 2024). In addition, astragaloside IV can activate the Nrf2-ARE pathway, increase the transcriptional activity of Nrf2, inhibit the transcriptional activity of NF-κB, and improve the oxidative stress state of the retina of diabetic rats (Yu et al., 2020).
Wogonoside is the main flavonoid active metabolite of Scutellaria baicalensis in Coptis Jiedu Soup, which has anti-inflammatory and antioxidant effects (He et al., 2014). Shao et al. constructed an in vitro DR model by inducing high glucose in human retinal microvascular endothelial cells, an STZ-induced DR model in SD rats. The results showed that wogonoside might alleviate the abnormal proliferation, migration, angiogenesis, cell membrane permeability, oxidative stress, and inflammatory response of hyperglycemia-induced hRMECs by up-regulating the expression of SIRT1, GSH-ST, and down-regulating the levels of ROS, NO, IL-1β, IL-6, VEGF, and HIF-1α (Shao et al., 2022). At the same time, the treatment of baicalin also significantly increased the expression level of srit1 in the retinal tissue of the DR model of SD rats.
Paeonol is a natural phenolic plant metabolites, and modern pharmacological studies have shown that paeonol has anti-inflammatory, antioxidant, and antidepressant effects (Du et al., 2024). Oxidative stress in DR is reduced by increasing the levels of GSH, SOD, and CAT and decreasing the levels of MDA (Adki and Kulkarni, 2022) (Figure 2).
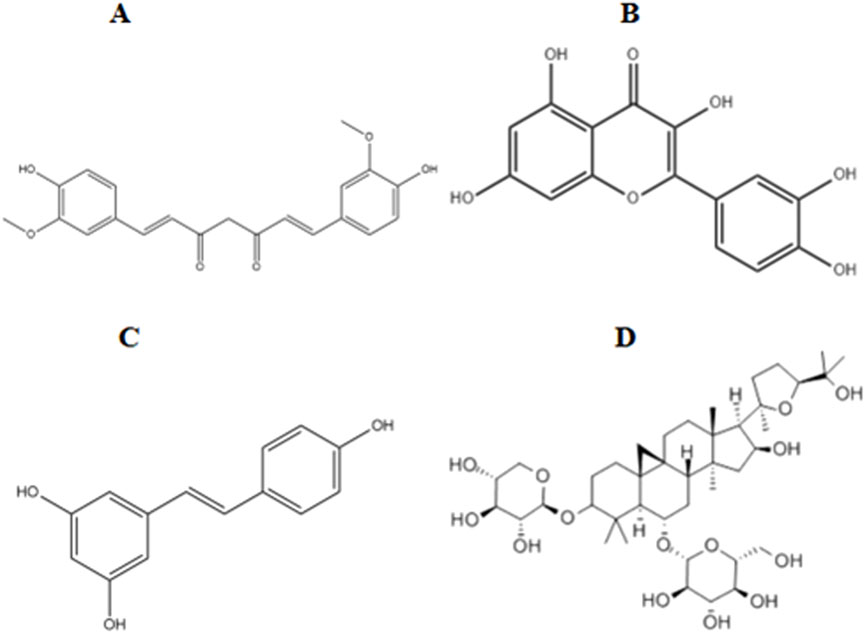
Figure 2. Chemical structures of the metabolites of several polyphenols. (A) Curcumin. (B) Quercetin. (C) Resveratrol. (D) Astragaloside IV.
Lycium barbarum L has the effect of nourishing the liver and kidney, benefiting the shrewd purpose, and is mainly used for the treatment of liver and kidney yin deficiency syndrome. According to the “Compendium of Materia Medica”, Lycium barbarum L has the effect of “nourishing the kidneys, moistening the lungs, and brightening the eyes”. Lycium barbarum polysaccharide (LBP) is the main active metabolite of Lycium barbarum. In recent years, pharmacological studies have confirmed that LBP has antioxidant, anti-aging, immunomodulatory, and anti-fatigue functions (Tian et al., 2019), and has obvious antagonistic effects on oxidative stress and inflammatory responses (Fang et al., 2016; Du et al., 2016; Huang, 2020). Studies have shown that LBP can increase the activity of SOD and reduce the expression of VEGF, VEGF mRNA, and MDA in rats with DR after treatment, indicating that LBP can inhibit neuronal apoptosis through antioxidant effect (Guo et al., 2015). In addition, Zhang et al. (2016) used intraperitoneal injection of 55 mg/kg streptozotocin (STZ) to induce diabetes while administering 250 mg/kg LBP by gavage for 10 weeks. The results indicated that LBP could attenuate the progression of DR by downregulating ROS, MDA, VEGF165, VEGFR1, VEGFR2, VEGFR3, Ang-1, Ang-2, Tie-1, Tie-2, TNF-α, IL-1β, ICAM-1, AOOP, and 8-OHdG; and upregulating T-AOC. This intervention suppressed retinal angiogenesis, oxidative stress, and inflammatory responses in diabetic mice (Table 2).
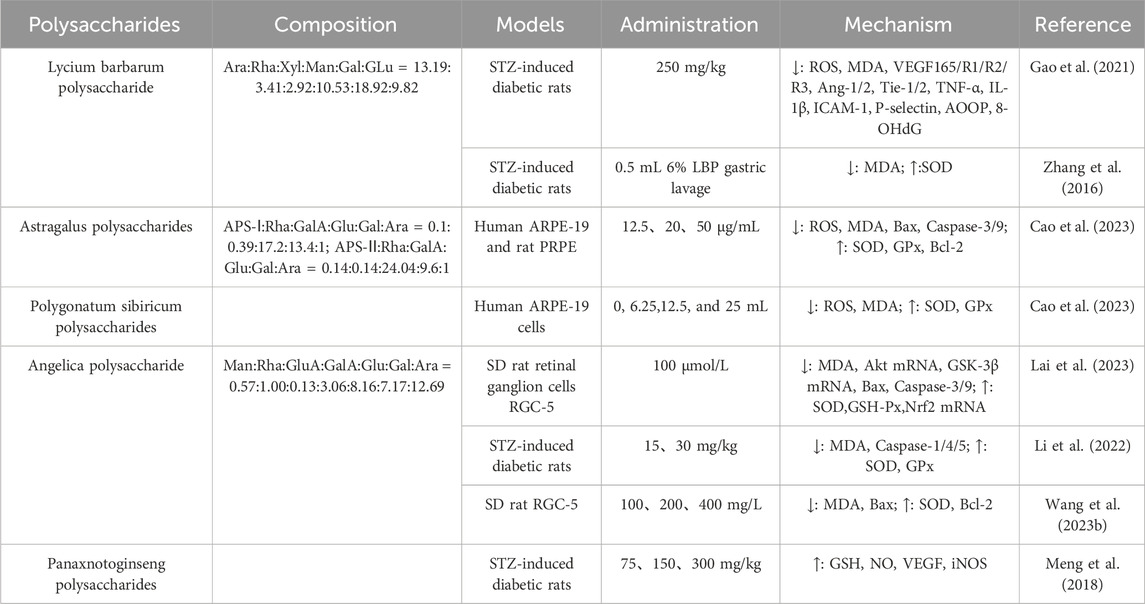
Table 2. Application of polysaccharides in reducing oxidative stress in the treatment of diabetic retinopathy.
Astragalus polysaccharides are one of the main metabolites of Astragalus sinicus L., which has attracted more and more attention in the study of DR in recent years. Astragalus polysaccharides intervene in the progression of diabetic retinopathy through a variety of biological mechanisms, showing significant therapeutic potential. These mechanisms include inhibition of retinal pigment epithelium cell apoptosis, attenuation of oxidative stress damage to retinal ganglion cells, and inhibition of retinal neovascularization (Gao et al., 2021). Specifically, APS can reduce the levels of MDA, Bcl-2, Bax, Caspase-3, and caspase-9 in hyper glycogen-induced retinal pigment epithelial cells. At the same time, the expression of SOD was upregulated to alleviate oxidative stress and apoptosis caused by mitochondrial dysfunction in DR (Liu et al., 2019).
Polygonatum sibiricum polysaccharide (PSP) is one of the main metabolites of traditional Chinese medicine Polygonatum sibiricum Delar. ex Redoute. Studies have shown that PSP is widely involved in physiological processes such as regulating blood glucose, blood lipids, immune function, oxidative stress, etc., and has pharmacological effects such as antioxidant, hypoglycemic, hypolipidemic, immune regulation, anti-inflammatory, and antibacterial (Zhao et al., 2022). PSP alleviates high glucose-induced oxidative stress by regulating the Nrf2/HO-1 signaling pathway, reducing ROS production and MDA content, and increasing the activity of SOD and GPx. In addition, PSP significantly increased bcl-2 expression and decreased Bax expression and caspase-3 activity. Protects ARPE-19 cells from HG-induced oxidative stress, inflammation, and apoptosis (Wang W. et al., 2022).
Angelica polysaccharide is an extract of Angelica japonica A. Gray, a traditional Chinese medicine in China, which has anti-inflammatory, immunomodulatory, antioxidant, and other effects. Cao et al. (2023) used high glucose to induce RGC cell line RGC-5 to establish a model, and the results showed that Angelica sinensis polysaccharides could improve SOD activity and Bcl-2 protein expression. Reduce MDA level and Bax expression, thereby alleviating the damage of RGC oxidative stress induced by high glucose and reducing apoptosis (Wang Y. X. et al., 2023). In addition, the research group also found that Angelica sinensis polysaccharides can inhibit hyperglucose-induced apoptosis in RGC-5 cells, reduce the level of intracellular oxidative stress, and increase the expression level of intracellular antioxidant factor Nrf2 by blocking the Akt/GSK-3β pathway. The levels of MDA, Caspase-1, Caspase-4, and Caspase-5 were decreased, and the levels of SOD and GPx were increased after treatment with STZ-induced DR in Angelica sinensis (Lai et al., 2023).
Panax notoginseng polysaccharide is one of the main active metabolites of Panax notoginseng (Burkill) F. H. Chen ex C. H. Chow in botanical drugs, which has attracted extensive attention in botanical drugs research in recent years. Studies have shown that Panax notoginseng polysaccharides can play a positive health role in many aspects, can reduce blood sugar levels, and can be used as an adjuvant treatment for diabetes. Panax notoginseng polysaccharide has good antioxidant activity, which can scavenge free radicals and reduce the negative effects of oxidative stress, thereby protecting the normal function and health of cells (Li et al., 2022; Wu and Wang, 2009). Studies have shown that Panax notoginseng polysaccharides can improve retinopathy caused by diabetes by increasing GSH and NO and increasing the expression of VEGF and iNOS genes (Yang et al., 2017) (Figure 3).
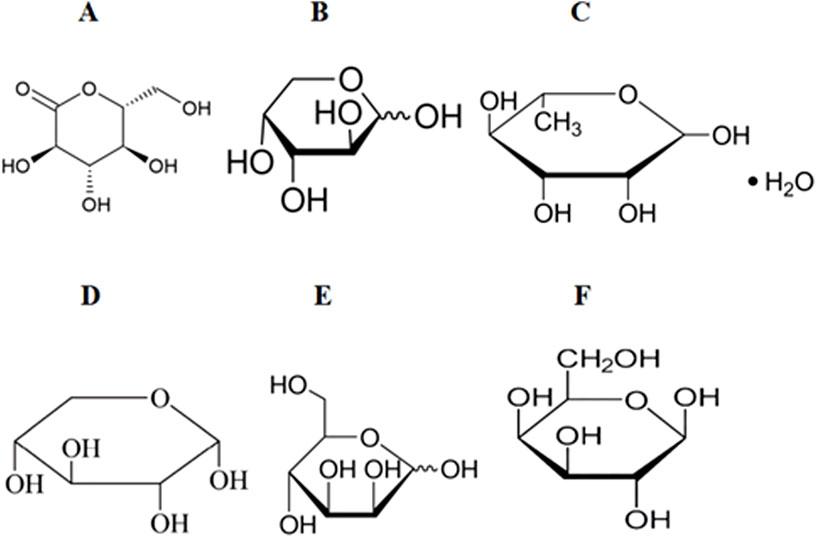
Figure 3. Chemical structures of the metabolites of several polysaccharides. (A) glucose (Glu). (B) L-Arabinose (Ara). (C) L-rhamnose monohydrate (Rha). (D) Xylose (Xyl). (E) D-Mannose (Man). (F) Galactose (Gal).
Ginsenoside is one of the active metabolites of Panax ginseng C. A. Mey, which has anti-diabetic, antioxidant, anti-inflammatory, and anti-tumor effects (Meng et al., 2018). Wang et al. (2020) used intraperitoneal injection of STZ to establish a DR model in rats. After successful modeling, rats in the H-Rg3 and L-Rg3 groups were intraperitoneally injected once daily with 5.0 mg·kg−1 and 0.5 mg·kg−1 of Rg3, respectively, for a total of 28 injections over 28 days. The results showed that ginsenoside Rg3 could protect the retinal tissue of diabetic retinopathy rats by downregulating MDA, LDH, caspase-3, VEGF, and ICAM-1, and upregulating Bcl-2, SOD, PI3K, and p-Akt. Ginsenoside Re can also reduce the content of ROS, MDA, LDH, caspase-3, caspase-9, HIF-1α, VEGF, etc. Increase CAT, GSH-Px, and p-Akt content to ameliorate oxidative stress in retinal vascular endothelial cells in monkeys (Xie et al., 2020). Ginsenoside Rb1 can be used to target the redox state in RCEC by activating the NMNAT-NAD-PARP-SIRT axis under high glucose conditions, which has an advantage during high glucose-induced cell damage (Fan et al., 2019). Ginsenoside Rd promotes AMPK activation and SIRT1 expression, reversing high glucose-induced NOX2 activation, oxidative stress, mitochondrial dysfunction, and endothelial cell apoptosis in an AMPK/SIRT1-dependent manner. Rd restores hyperglycemia-induced endothelial cell loss and other retinal damage in vivo by activating AMPK and SIRT1, which significantly modulates oxidative stress and apoptosis to ameliorate diabetes-driven vascular injury (Tang et al., 2022) (Table 3).
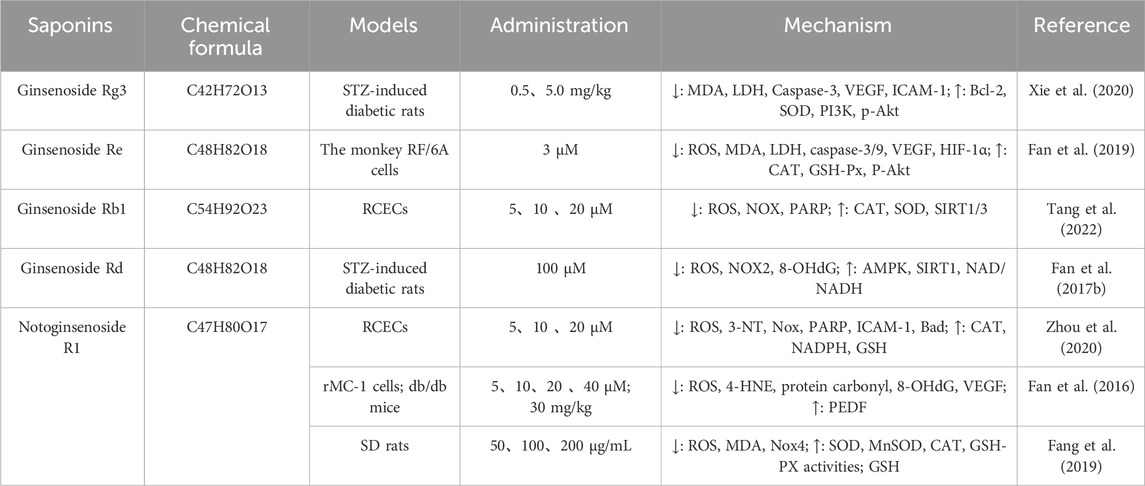
Table 3. Application of saponins in the treatment of diabetic retinopathy by reducing oxidative stress.
Notoginsenoside R1 is derived from the dried roots and rhizomes of Panax notoginseng (Burkill) F. H. Chen ex C. H. Chow and has antioxidant, anti-cancer, and cardiovascular disease effects. Notoginsenoside R1 attenuates hyperglucose-induced damage to retinal capillary endothelial cells (RCECs), reduces oxidative stress, and inhibits redox state changes in RCECs (Fan C. et al., 2017). Db/db mouse experiments showed that Notoginsenoside R1 could inhibit the apoptosis of rat retinal Müller cells under high glucose, significantly inhibit the expression of VEGF, increase the expression of PEDF, and inhibit oxidative stress and inflammation (Zhou et al., 2020). Notoginsenoside R1 can also increase the activities of the retina, total SOD, MnSOD, CAT, and GSH-PX in SD rats, directly reducing the damage of oxidative stress to the retina (Fan et al., 2016) (Figure 4).
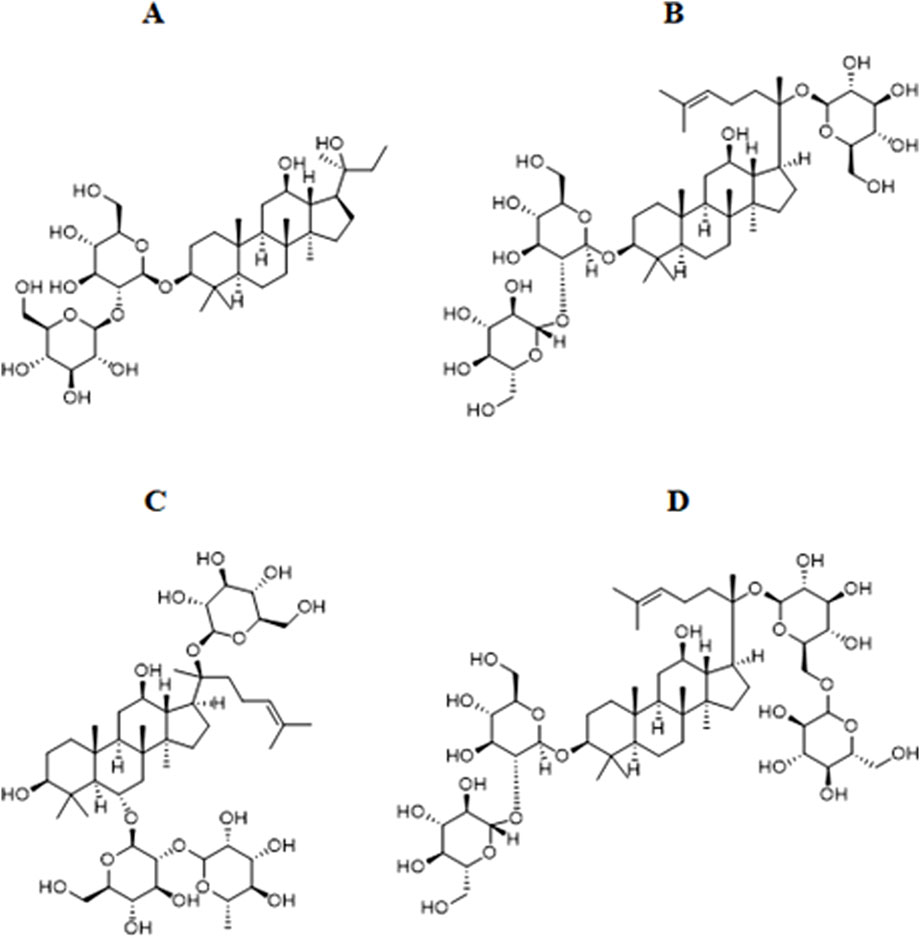
Figure 4. Chemical structural formula of the representative saponin metabolites of Panax ginseng. (A) Ginsenoside Rg3. (B) Ginsenoside Rd. (C) Ginsenoside Re. (D) Ginsenoside Rb1.
Evodiamine (EVO) is an alkaloid extracted from the unripe dried fruits of Evodiamine in traditional Chinese medicine and has a variety of pharmacological activities, including hypolipidemic, anti-inflammatory, anti-infective, and antitumor effects (Fang et al., 2019; Shin et al., 2007). In addition, EVO also has a significant effect on diabetes, it can reduce blood sugar levels in diabetic rats, and improve oxidative stress and inflammatory responses (Yu et al., 2022). EVO can reduce the content of MDA, TNF-α, IL-6, Bax, and P53 in retinal tissues of STZ-induced DR models, and the reduction of these biomarkers suggests that evodiamine effectively alleviates oxidative stress and inflammation in retinal tissues. At the same time, EVO also further exerts its protective efficacy by up-regulating the levels of SOD, cAMP, p-PKA/PKA, and can effectively protect the retina of DR model rats from oxidative stress and inflammation (Liu et al., 2024) (Table 4).
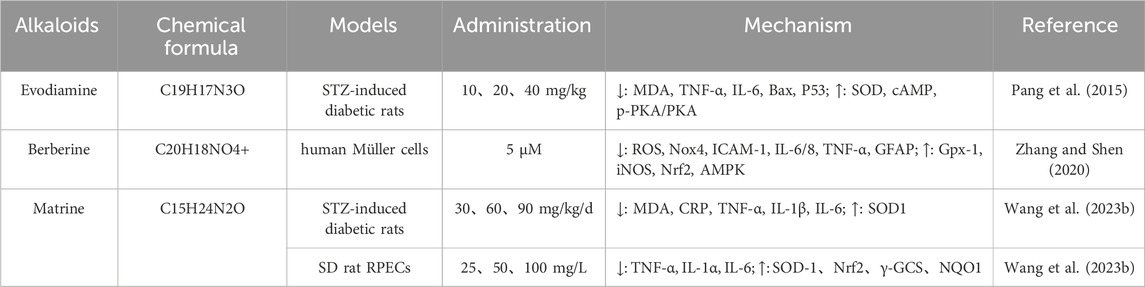
Table 4. Application of alkaloids in reducing oxidative stress in the treatment of diabetic retinopathy.
Berberine is a natural plant metabolite proposed from the Coptis chinensis Franch. Coptis chinensis. Pharmacological studies have shown that berberine has anti-inflammatory, antioxidant, and hypoglycemic effects. Berberine is a natural plant metabolite proposed from the traditional Chinese medicine Coptis chinensis. Pharmacological studies have shown that berberine has anti-inflammatory, antioxidant, and hypoglycemic effects (Pang et al., 2015), which makes it a potential treatment option for a variety of health problems. In particular, berberine has shown positive effects in ophthalmic studies. One study found that berberine was effective in reducing ROS, Nox4, ICAM-1, IL-6, IL-8, TNF-α, GFAP in highly oxidized and glycated (HOG-) low-density lipoprotein (HOG-LDL) cultured Müller cells; Upregulation of Gpx-1, iNOS, Nrf2, and AMPK inhibits modified LDL-induced Müller cell damage by activating the AMPK pathway, and plays a role in the prevention or treatment of DR (Fu et al., 2016).
Matrine is one of the metabolites of Sophora flavescens Aiton, and pharmacological studies have shown that matrine and oxidized matrine have a wide range of biological activities, including antibacterial, antiviral, antioxidant, anti-inflammatory, and immunomodulatory (Zhang and Shen, 2020). By regulating the expression of VEGF and ANG1, marine increased the level of SOD1 and reduced the content of MDA, CRP, TNF-α, IL-1β, and IL-6, thereby improving retinal damage and inhibiting the proliferation of retinal microvascular endothelial cells in DR rats (Zhang, 2019). In addition, at concentrations of 25, 50, and 100 mg/L, oxymatrine could resist the high glucose environment, inhibit the decreased activity of rat retinal pigment epithelial cells cultured in vitro, reduce the cell damage caused by high glucose, and upregulate the expressions of Nrf2, SOD-1, γ-GCS and NQO1, and downregulate the expressions of TNF-α, IL-1α and IL-6 (Zhang, 2015). Both marine and oxymatrine have hypoglycemic effects (Zhang, 2015; Yan, 2010; Zhang et al., 2019c), suggesting that they help prevent and treat diabetic retinopathy through their basic pharmacological effects of antioxidant, anti-inflammatory, and hypoglycemic effects (Figure 5).
In summary, plant metabolites have shown the following characteristics in improving diabetic retinal oxidative stress: (1) Multi-target effect: plant metabolites can act on multiple biological targets at the same time, and reduce the damage of oxidative stress to the retina by regulating related signaling pathways, such as NF-κB, Nrf2, Bcl-2, etc. (2) Strong antioxidant capacity: Many plant metabolites, such as polyphenols, polysaccharides, flavonoids, etc., have significant antioxidant properties, which can effectively scavenge free radicals and reduce oxidative damage to retinal cells. (3) Promote the synthesis of endogenous antioxidants: Some plant metabolites can promote the synthesis of antioxidant enzymes in the body, such as SOD and glutathione peroxidase, thereby enhancing the body’s natural antioxidant capacity. (4) Inhibition of inflammatory response: Plant metabolites often have anti-inflammatory effects, which can inhibit the inflammatory response in the retina, reduce the expression of inflammatory factors, and indirectly reduce the damage of oxidative stress to the retina. (5) Improve blood sugar control: Some plant metabolites such as astragalus polysaccharides and ginsenosides can improve insulin sensitivity and reduce blood sugar levels, thereby reducing chronic complications caused by diabetes, including retinopathy. (6) Protection of retinal cells: Plant metabolites protect retinal nerve cells by inhibiting retinal nerve cell apoptosis promoting cell survival, and directly alleviating the progression of diabetic retinopathy (Wang Y. X. et al., 2023). These characteristics make plant metabolites have significant potential and advantages in the prevention and treatment of diabetic retinal oxidative stress (Figure 6).
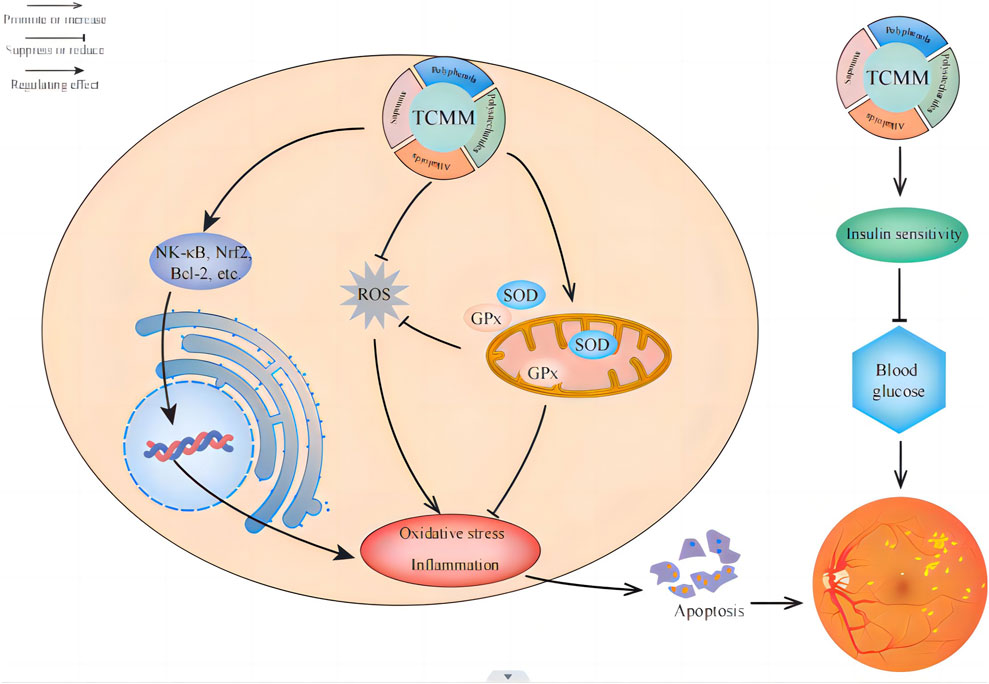
Figure 6. Application of plant metabolites in reducing oxidative stress in the treatment of diabetic retinopathy. There are four main types of plant metabolites (polyphenols, polysaccharides, saponins, and alkaloids) to improve diabetic retinopathy, which can alleviate the progression of diabetic retinopathy by enhancing antioxidant capacity, promoting the synthesis of endogenous antioxidants, improving blood sugar control, and inhibiting retinal cell apoptosis.
In recent years, the significant antioxidant capacity of many plant metabolites has been confirmed, and more and more studies have revealed that plant metabolites can play a protective role in diabetic retinopathy by regulating multiple biological targets and related signaling pathways to promote the expression of antioxidant enzymes. In terms of animal studies, several animal experiments have shown that plant metabolites can significantly improve retinopathy and reduce retinal damage indicators in diabetic mice and rats. In terms of clinical studies, although relatively few, there have been some small-scale clinical trials that have shown that plant metabolites have a certain improvement effect on retinopathy in diabetic patients.
At present, there are still the following limitations: (1) Small sample size: The sample size of many studies is small, and the statistical significance and clinical applicability of the results need to be further verified. (2) Lack of long-term research: At present, most of the studies are on the short-term use of plant metabolites, such as 1 month to 1 year, and there are still few studies on the safety and effect of long-term use. (3) Standardization problem: There is no unified standard for the extraction, purification, and quality control of plant metabolites, which affects the reproducibility and reliability of research results. (4) Insufficient mechanism exploration: Although some mechanisms have been explored, the interaction mechanism between various plant metabolites still needs to be further explored, especially in the context of specific complex diabetic retinopathy. (5) Incomplete safety evaluation: Although plant metabolites are generally considered to be relatively safe, there may be potential adverse reactions when used in the treatment of DR For a long time or at high doses. For example, tetramethy lpyrazine, a botanical drug that has the effect of promoting blood circulation and removing blood stasis, may increase the risk of eye bleeding while improving eye microcirculation. At present, the evaluation in this regard is insufficient.
In view of the above limitations, the following aspects can be used in the future: (1) Carry out multi-center large-scale clinical trials: carry out multi-center and large-sample clinical studies, and do a good job in statistical analysis and clinical efficacy observation. (2) Conducting long-term prospective studies over several years to decades, to verify the efficacy and safety of plant metabolites for diabetic retinopathy. (3) Standardization and formulation research: Establish a standardized extraction and quality control system for plant metabolites, and develop more effective preparations to improve the clinical application effect. (4) In-depth research on mechanism: Strengthen the basic research on the mechanism of action of plant metabolites, and explore its interactive biological effects and multiple signaling pathways in diabetic retinopathy. Combined with modern technology, such as genomics, proteomics, etc., the mechanism of action of plant metabolites was deeply explored, and the interaction mechanism between different plant metabolites was sought. (5) The toxicology of plant metabolites, including acute toxicity, long-term toxicity and genetic toxicity, will be studied systematically. At the same time, attention should be paid to the safety of drugs in special groups (such as the elderly, pregnant women, etc.).
Through these efforts, it is expected that the potential of plant metabolites in improving diabetic retinopathy can be better understood and applied.
TG: Conceptualization, Data curation, Writing–original draft, Writing–review and editing. DW: Data curation, Writing–original draft, Writing–review and editing. JW: Formal Analysis, Writing–original draft, Writing–review and editing. QH: Data curation, Formal Analysis, Funding acquisition, Writing–original draft. HZ: Investigation, Methodology, Writing–review and editing. CL: Project administration, Resources, Writing–original draft. XL: Resources, Software, Writing–review and editing. HY: Supervision, Validation, Visualization, Writing–original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Key Project of Traditional Chinese Medicine Research of Sichuan Provincial Administration of Traditional Chinese Medicine (2023ZD001), the Nursery Talent Promotion Plan for Xinglin Scholars of Chengdu University of Traditional Chinese Medicine (MPRC2022012) and the Qingji Advanced Talent Project (QJJJ2023004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DR, Diabetic Retinopathy; ROS, Reactive Oxygen Species; OS, Oxygen species; GPx, Glutathione peroxidase; MDA, Malondialdehyde; SOD, Superoxide dismutase; CAT, Catalase; AGE, Advanced glycation end products; HO-1, Heme oxygenase-1; ICAM, Intercellular adhesion molecule; HIF-1α, Hypoxia inducible factor-1α; Nrf2, Nuclear factor E2-related factor 2; LBP, Lycium barbarum polysaccharide; RCECs, Retinal capillary endothelial cells; EVO, Evodiamine.
Adki, K. M., and Kulkarni, Y. A. (2022). Paeonol attenuates retinopathy in streptozotocin-induced diabetes in rats by regulating the oxidative stress and polyol pathway. Front. Pharmacol. 13, 891485. doi:10.3389/fphar.2022.891485
Ai, X., Yu, P., Hou, Y., Song, X., Luo, J., Li, N., et al. (2020). A review of traditional Chinese medicine on treatment of diabetic retinopathy and involved mechanisms. Biomed. Pharmacother. 132, 110852. doi:10.1016/j.biopha.2020.110852
An, L., Ling, Z., Zhang, L. L., Li, R. Y., and Liu, B. (2024). Advances in TCM treatment of diabetic retinopathy. J. Mod. Clin. Med. 50 (04), 299–300+309. doi:10.11851/j.issn.1673-1557.2024.04.17
Andrés-Blasco, I., Gallego-Martínez, A., Machado, X., Cruz-Espinosa, J., Di Lauro, S., Casaroli-Marano, R., et al. (2023). Oxidative stress, inflammatory, angiogenic, and apoptotic molecules in proliferative diabetic retinopathy and diabetic macular edema patients. Int. J. Mol. Sci. 24 (9), 8227. doi:10.3390/ijms24098227
Benalaya, I., Alves, G., Lopes, J., and Silva, L. R. (2024). A review of natural polysaccharides: sources, characteristics, properties, food, and pharmaceutical applications. Int. J. Mol. Sci. 25 (2), 1322. doi:10.3390/ijms25021322
Brzović-Šarić, V., Landeka, I., Šarić, B., Barberić, M., Andrijašević, L., Cerovski, B., et al. (2015). Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol. Vis. 21, 649–664.
Cao, L., Wang, Y. X., and Jia, G. M. (2023). Angelica polysaccharide inhibits high glucose-induced oxidative stress injury and apoptosis in retinal ganglion cells by regulating miR-148a-3p. Jilin J. Chin. Med. 43 (01), 73–78. doi:10.13463/j.cnki.jlzyy.2023.01.018
Chai, M., Gu, C., Shen, Q., Liu, J., Zhou, Y., Jin, Z., et al. (2020). Hypoxia alleviates dexamethasone-induced inhibition of angiogenesis in cocultures of HUVECs and rBMSCs via HIF-1α. Stem Cell Res. Ther. 11 (1), 343. doi:10.1186/s13287-020-01853-x
Chen, Y. W., Li, S. Q., Huang, X. P., Zeng, X., and Zhang, X. (2022). To investigate the effect of baicalein on LPS-induced THP1 macrophage inflammation model based on IL-6/STAT3 and PI3K/Akt pathways. J. Chin. Med. Mater. 45 (07), 1721–1725. doi:10.13863/j.issn1001-4454.2022.07.035
Cheng, S. C., Huang, W. C., S Pang, J. H., Wu, Y. H., and Cheng, C. Y. (2019). Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 20 (12), 2957. doi:10.3390/ijms20122957
Couturier, A., Rey, P. A., Erginay, A., Lavia, C., Bonnin, S., Dupas, B., et al. (2019). Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology 126 (12), 1685–1694. doi:10.1016/j.ophtha.2019.06.022
Darenskaya, M. A., Kolesnikova, L. I., and Kolesnikov, S. I. (2021). Oxidative stress: pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull. Exp. Biol. Med. 171 (2), 179–189. doi:10.1007/s10517-021-05191-7
Dröge, W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82 (1), 47–95. doi:10.1152/physrev.00018.2001
Du, M., Hu, X., Kou, L., Zhang, B., and Zhang, C. (2016). Lycium barbarum polysaccharide mediated the antidiabetic and antinephritic effects in diet-streptozotocin-induced diabetic sprague dawley rats via regulation of NF-κB. Biomed. Res. Int. 2016, 3140290. doi:10.1155/2016/3140290
Du, S., Fan, P. J., Wang, X., Hou, W. Y., and Liu, Y. N. (2024). Research progress on pharmacological mechanism of paeonol in the treatment of Parkinson’s disease. J. Liaoning Univ. TCM., 1–11. Available at: https://kns.cnki.net/kcms/detail/21.1543.R.20240718.1138.010.html.
El-Beshbishy, H., and Bahashwan, S. (2012). Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities: an in vitro study. Toxicol. Ind. Health 28 (1), 42–50. doi:10.1177/0748233711403193
Fan, C., Ma, Q., Xu, M., Qiao, Y., Zhang, Y., Li, P., et al. (2019). Ginsenoside Rb1 attenuates high glucose-induced oxidative injury via the NAD-PARP-SIRT Axis in rat retinal capillary endothelial cells. Int. J. Mol. Sci. 20 (19), 4936. doi:10.3390/ijms20194936
Fan, C., Qiao, Y., and Tang, M. (2017b). Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des. Devel Ther. 11, 3343–3354. doi:10.2147/DDDT.S149700
Fan, Y., Lee, K., Wang, N., and He, J. C. (2017a). The role of endoplasmic reticulum stress in diabetic nephropathy. Curr. Diab Rep. 17 (3), 17. doi:10.1007/s11892-017-0842-y
Fan, Y., Qiao, Y., Huang, J., and Tang, M. (2016). Protective effects of Panax notoginseng saponins against high glucose-induced oxidative injury in rat retinal capillary endothelial cells. Evid. Based Complement. Altern. Med. 2016, 5326382. doi:10.1155/2016/5326382
Fanaro, G. B., Marques, M. R., Calaza, K. D. C., Brito, R., Pessoni, A. M., Mendonça, H. R., et al. (2023). New insights on dietary polyphenols for the management of oxidative stress and neuroinflammation in diabetic retinopathy. Antioxidants (Basel) 12 (6), 1237. doi:10.3390/antiox12061237
Fang, F., Peng, T., Yang, S., Wang, W., Zhang, Y., and Li, H. (2016). Lycium barbarum polysaccharide attenuates the cytotoxicity of mutant huntingtin and increases the activity of AKT. Int. J. Dev. Neurosci. 52, 66–74. doi:10.1016/j.ijdevneu.2016.05.004
Fang, Q., Jiang, S., and Li, C. (2019). Evodiamine selectively inhibits multiple myeloma cell growth by triggering activation of intrinsic apoptosis pathway. Onco Targets Ther. 12, 11383–11391. doi:10.2147/OTT.S235730
Feng, S., Yu, H., Yu, Y., Geng, Y., Li, D., Yang, C., et al. (2018). Levels of inflammatory cytokines IL-1β, IL-6, IL-8, IL-17A, and TNF-α in aqueous humour of patients with diabetic retinopathy. J. Diabetes Res. 2018, 8546423. doi:10.1155/2018/8546423
Ferrannini, E., Gastaldelli, A., Miyazaki, Y., Matsuda, M., Mari, A., and DeFronzo, R. A. (2005). beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J. Clin. Endocrinol. Metab. 90 (1), 493–500. doi:10.1210/jc.2004-1133
Flaxel, C. J., Adelman, R. A., Bailey, S. T., Fawzi, A., Lim, J. I., Vemulakonda, G. A., et al. (2020). Diabetic retinopathy preferred practice Pattern®. Ophthalmology 127 (1), P66–P145. doi:10.1016/j.ophtha.2019.09.025
Fu, D., Yu, J. Y., Connell, A. R., Yang, S., Hookham, M. B., McLeese, R., et al. (2016). Beneficial effects of berberine on oxidized LDL-induced cytotoxicity to human retinal müller cells. Invest. Ophthalmol. Vis. Sci. 57 (7), 3369–3379. doi:10.1167/iovs.16-19291
Gao, L. M., Fu, S., Liu, F., Wu, H. B., and Li, W. J. (2021). Astragalus Polysaccharide Regulates miR-182/Bcl-2 Axis to relieve metabolic memory through suppressing mitochondrial damage-mediated apoptosis in retinal pigment epithelial cells. Pharmacology 106 (9-10), 520–533. doi:10.1159/000515901
Gao, W., Wang, J., and Zhang, C. (2017). Detection and significance of serum inflammatory factors and oxidative stress in patients with diabetic retinopathy. J. Hainan Med. Univ. 23 (01), 79–81+84. doi:10.13210/j.cnki.jhmu.20161024.001
Giordo, R., Nasrallah, G. K., Posadino, A. M., Galimi, F., Capobianco, G., Eid, A. H., et al. (2021). Resveratrol-elicited PKC inhibition counteracts NOX-mediated endothelial to mesenchymal transition in human retinal endothelial cells exposed to high glucose. Antioxidants (Basel) 10 (2), 224. doi:10.3390/antiox10020224
Guo, J., Xu, G. X., Wang, T. T., Huang, L. L., and Cui, L. J. (2015). Protection effects of Lyciujm Barbarum Polysaccharides on rats of diabetic retinopathy. Chin. J. Clin. Pharmacol. 31 (24), 2448–2450. doi:10.13699/j.cnki.1001-6821.2015.24.024
Gupta, S. K., Kumar, B., Nag, T. C., Agrawal, S. S., Agrawal, R., Agrawal, P., et al. (2011). Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J. Ocul. Pharmacol. Ther. 27 (2), 123–130. doi:10.1089/jop.2010.0123
He, M. Y., Deng, Y. X., Shi, Q. Z., Zhang, X. J., and Lv, Y. (2014). Comparative pharmacokinetic investigation on baicalin and wogonoside in type 2 diabetic and normal rats after oral administration of traditional Chinese medicine Huanglian Jiedu decoction. J. Ethnopharmacol. 155 (1), 334–342. doi:10.1016/j.jep.2014.05.033
Hou, Y. L., Lin, M., Mei, W., Guo, F. F., He, M. J., Wu, G. Q., et al. (2019). Effect of wogonin on diabetic retinopathy in type 2 diabetic mice. Chin. Archives TCM 37 (10), 2398–2400. doi:10.13193/j.issn.1673-7717.2019.10.021
Huang, D. D., Shi, G., Jiang, Y., Yao, C., and Zhu, C. (2020). A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 125, 109767. doi:10.1016/j.biopha.2019.109767
Huang, J. (2020). “Effects of LBP on light-induced oxidative stress damage and PI3K/Akt/mTOR signaling pathway in human retinal pigment epithelial cells,”. dissertatio thesis (Ningxia: Ningxia Medical University).
International Diabetes Federation (2021). IDF diabetes atlas. 10th edition. Available at: www.diabetesatlas.org.
Jiang, F., Sun, J. C., Zhang, J., and Wu, H. J. (2023). Effect of astragaloside IV on type 1 diabetic retinopathy in rats. Laborartory Animal Sci. 40 (03), 92–97. doi:10.3969/j.issn.1006-6179.2023.03.016
Jiménez-Osorio, A. S., González-Reyes, S., and Pedraza-Chaverri, J. (2015). Natural Nrf2 activators in diabetes. Clin. Chim. Acta 448, 182–192. doi:10.1016/j.cca.2015.07.009
Jin, T., Xu, C. H., and Wang, H. Y. (2023). Research progress of biological targets for prevention and treatment of diabetic retinopathy. Pharmacol. Clin. Chin. Materia Medica 39 (03), 111–116. doi:10.13412/j.cnki.zyyl.20220519.001
Joma, N., Bielawski, P. B., Saini, A., Kakkar, A., and Maysinger, D. (2024). Nanocarriers for natural polyphenol senotherapeutics. Aging Cell 23 (5), e14178. doi:10.1111/acel.14178
Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J., and Pérez-Lebeña, E. (2021). The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 22 (9), 4642. doi:10.3390/ijms22094642
Kang, Q., and Yang, C. (2020). Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 37, 101799. doi:10.1016/j.redox.2020.101799
Khatik, G. L., Datusalia, A. K., Ahsan, W., Kaur, P., Vyas, M., Mittal, A., et al. (2018). A retrospect study on thiazole derivatives as the potential antidiabetic agents in drug discovery and developments. Curr. Drug Discov. Technol. 15 (3), 163–177. doi:10.2174/1570163814666170915134018
Kim, J. A., Wei, Y., and Sowers, J. R. (2008). Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 102 (4), 401–414. doi:10.1161/CIRCRESAHA.107.165472
Kotake, Y., Sang, H., Miyajima, T., and Wallis, G. L. (1998). Inhibition of NF-kappaB, iNOS mRNA, COX2 mRNA, and COX catalytic activity by phenyl-N-tert-butylnitrone (PBN). Biochim. Biophys. Acta 1448 (1), 77–84. doi:10.1016/s0167-4889(98)00126-8
Kowaltowski, A. J., and snd Vercesi, A. E. (1999). Mitochondrial damage induced by conditions of oxidative stress. Free Radic. Biol. Med. 26 (3-4), 463–471. doi:10.1016/s0891-5849(98)00216-0
Kumar, B., Gupta, S. K., Nag, T. C., Srivastava, S., Saxena, R., Jha, K. A., et al. (2014). Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 125, 193–202. doi:10.1016/j.exer.2014.06.009
Lai, J., Li, N., Liu, Z. B., Zhang, R., and Chen, J. Q. (2023). Angelica sinensis polysaccharide promotes lncRNA MEG3 expression to regulate retinal epithelial cell pyrosis and improve diabetic retinopathy. Zhejiang Joumal Integr. Traditional Chin. Westem Med. 33 (07), 592–599. doi:10.3969/j.issn.1005-4561.2023.07.003
Li, C., Wang, A. L., Zhao, Z. Q., Zhang, Z. H., Zhang, D., and Zhang, L. (2024). Protective effect of astragaloside Ⅳ on the retina of type 2 diabetic rats. Pharmacol. Clin. Chin. Materia Medica 40 (05), 63–67. doi:10.13412/j.cnki.zyyl.2024.05.002
Li, J., Yu, S., Ying, J., Shi, T., and Wang, P. (2017). Resveratrol prevents ROS-induced apoptosis in high glucose-treated retinal capillary endothelial cells via the activation of AMPK/S irt1/PGC-1α pathway. Oxid. Med. Cell Longev. 2017, 7584691. doi:10.1155/2017/7584691
Li, W., Xing, Q., Liu, Z., Liu, R., Hu, Y., Yan, Q., et al. (2023). The signaling pathways of traditional Chinese medicine in treating diabetic retinopathy. Front. Pharmacol. 14, 1165649. doi:10.3389/fphar.2023.1165649
Li, X., Liu, H., Yang, H. R., and Zeng, Y. J. (2022). Hypoglycemic effects of novel Panax notoginseng polysaccharide in mice with diet-induced obesity. Foods 11 (19), 3101. doi:10.3390/foods11193101
Liu, L., Liu, S. S., Hu, F. F., Wang, H. H., and Wang, Y. (2024). Impact of evodiamine on retinal injury in diabetic rats by regulating cyclic adenosine monophosphate/protein kinase A signaling pathway. Recent Adv. Ophthalmol. 44 (02), 94–99. doi:10.13389/j.cnki.rao.2024.0019
Liu, P., Peng, Q. H., Tong, P., and Li, W. J. (2019). Astragalus polysaccharides suppresses high glucose-induced metabolic memory in retinal pigment epithelial cells through inhibiting mitochondrial dysfunction-induced apoptosis by regulating miR-195. Mol. Med. 25 (1), 21. doi:10.1186/s10020-019-0088-z
Luo, Y., Zuo, Z. F., Cheng, X., Zhang, Q., Liu, C., and Min, L. Q. (2021). Effect of piceatannol ameliorated oxidative stress of retina in diabetic rats via activating Nrf2/HO-1 pathway. Prog. Anatomical Sci. 27 (03), 325–328. doi:10.16695/j.cnki.1006-2947.2021.03.018
Maassen, J. A., T Hart, L. M., Van Essen, E., Heine, R. J., Nijpels, G., Jahangir Tafrechi, R. S., et al. (2004). Mitochondrial diabetes: molecular mechanisms and clinical presentation. Diabetes 53 (Suppl. 1), S103–S109. doi:10.2337/diabetes.53.2007.s103
Matsui, T., Ueda, T., Oki, T., Sugita, K., Terahara, N., and Matsumoto, K. (2001). alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J. Agric. Food Chem. 49 (4), 1948–1951. doi:10.1021/jf001251u
Meng, X., Xie, W., Xu, Q., Liang, T., Xu, X., Sun, G., et al. (2018). Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules 23 (9), 2401. doi:10.3390/molecules23092401
Murphy, M. P. (2009). How mitochondria produce reactive oxygen species. Biochem. J. 417 (1), 1–13. doi:10.1042/BJ20081386
Niu, Y., Zhang, G., Sun, X., He, S., and Dou, G. (2023). Distinct role of lycium barbarum L. Polysaccharides in oxidative stress-related ocular diseases. Pharm. (Basel). 16 (2), 215. doi:10.3390/ph16020215
Ortega, Á. L. (2021). Oxidative stress in diabetic retinopathy. Antioxidants (Basel) 10 (1), 50. doi:10.3390/antiox10010050
Pallavi, K., Antara, B., Anushka, B., Ramachandran, M., Francesca, M., and Surajit, P. (2018). “An overview of dietary polyphenols and their therapeutic effects,” in Polyphenols: mechanisms of action in human health and disease. 2nd edition volume 1. Editors R. W. Ronald, R. P. Victor, and Z. Sherma (London, UK: Elsevier Inc).
Pang, B., Zhao, L. H., Zhou, Q., Zhao, T. Y., Wang, H., Gu, C. J., et al. (2015). Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 905749. doi:10.1155/2015/905749
Papachristoforou, E., Lambadiari, V., Maratou, E., and Makrilakis, K. (2020). Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J. Diabetes Res. 2020, 7489795. doi:10.1155/2020/7489795
Pearce, E., Chong, V., and Sivaprasad, S. (2020). Aflibercept reduces retinal hemorrhages and intravitreal microvascular abnormalities but not venous beading: secondary analysis of the CLARITY study. Ophthalmol. Retina 4 (7), 689–694. doi:10.1016/j.oret.2020.02.003
Qiao, Y., Fan, C. L., and Tang, M. K. (2017). Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des. Devel Ther. 11, 3567–3577. doi:10.2147/DDDT.S152489
Qiu, W., Guo, M., Li, X. Y., Li, Y. H., Sun, X. Y., Jin, D., et al. (2021). Research progress of Chinese herb monomers in the treatment of diabetic retinopathy. Int. Eye Sci. 21 (08), 1373–1377. doi:10.3980/j.issn.1672-5123.2021.8.12
Ran, Z., Zhang, Y., Wen, X., and Ma, J. (2019). Curcumin inhibits high glucose-induced inflammatory injury in human retinal pigment epithelial cells through the ROS-PI3K/AKT/mTOR signaling pathway. Mol. Med. Rep. 19 (2), 1024–1031. doi:10.3892/mmr.2018.9749
Rodríguez, M. L., Pérez, S., Mena-Mollá, S., Desco, M. C., and Ortega, Á. L. (2019). Oxidative stress and microvascular alterations in diabetic retinopathy: future therapies. Oxid. Med. Cell Longev. 2019, 4940825. doi:10.1155/2019/4940825
Shao, Y. L., Yu, J. Y., and Ni, W. H. (2022). Wogonoside alleviates high glucose-induced dysfunction of retinal microvascular endothelial cells and diabetic retinopathy in rats by up-regulating SIRT1. J. South. Med. Univ. 42 (04), 463–472. doi:10.12122/j.issn.1673-4254.2022.04.02
Shen, L., Luo, H., Fan, L., Tian, X., Tang, A., Wu, X., et al. (2023). Potential immunoregulatory mechanism of plant saponins: a review. Molecules 29 (1), 113. doi:10.3390/molecules29010113
Shen, N., Wang, T., Gan, Q., Liu, S., Wang, L., and Jin, B. (2022). Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 383, 132531. doi:10.1016/j.foodchem.2022.132531
Shi, Z. Z. (2021). Research progress on the association between oxidative stress and diabetic retinopathy. Health care guide 2021 (37), 293–295.
Shin, Y. W., Bae, E. A., Cai, X. F., Lee, J. J., and Kim, D. H. (2007). In vitro and in vivo antiallergic effect of the fructus of Evodia rutaecarpa and its constituents. Biol. Pharm. Bull. 30 (1), 197–199. doi:10.1248/bpb.30.197
Sivaprasad, S., Prevost, A. T., Vasconcelos, J. C., Riddell, A., Murphy, C., Kelly, J., et al. (2017). Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 389 (10085), 2193–2203. doi:10.1016/S0140-6736(17)31193-5
Song, W., Yuan, Y., Ge, Z. Y., Jin, L., and Xu, Y. (2019). Research progress in prevention and treatment of diabetic retinopathy by simplex and compounds of Chinese materia medica. Chin. J. Pharmacol. Toxicol. 33 (03), 224–231. doi:10.3867/j.issn.1000-3002.2019.03.008
Song, Y., Huang, L., and Yu, J. (2016). Effects of blueberry anthocyanins on retinal oxidative stress and inflammation in diabetes through Nrf2/HO-1 signaling. J. Neuroimmunol. 301, 1–6. doi:10.1016/j.jneuroim.2016.11.001
Sui, A., Chen, X., Demetriades, A. M., Shen, J., Cai, Y., Yao, Y., et al. (2020). Inhibiting NF-κB signaling activation reduces retinal neovascularization by promoting a polarization shift in macrophages. Invest. Ophthalmol. Vis. Sci. 61 (6), 4. doi:10.1167/iovs.61.6.4
Tang, K., Qin, W., Wei, R., Jiang, Y., Fan, L., Wang, Z., et al. (2022). Ginsenoside Rd ameliorates high glucose-induced retinal endothelial injury through AMPK-STRT1 interdependence. Pharmacol. Res. 179, 106123. doi:10.1016/j.phrs.2022.106123
Tang, K., and Tan, W. (2023). Research progress of oxidative stress in the early treatment of diabetic retinopathy. Chongqing Med. 52 (05), 787–791. doi:10.3969/j.issn.1671-8348.2023.05.031
Teo, Z. L., Tham, Y. C., Yu, M., Chee, M. L., Rim, T. H., Cheung, N., et al. (2021). Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128 (11), 1580–1591. doi:10.1016/j.ophtha.2021.04.027
Tian, J. Z., and Li, C. H. (2024). A review on the mechanism of TCM medicine on oxidative stress in diabetic retinopathy. Clin. J. Chin. Med. 16 (11), 47–53. doi:10.3969/j.issn.1674-7860.2024.11.009
Tian, X., Liang, T., Liu, Y., Ding, G., Zhang, F., and Ma, Z. (2019). Extraction, structural characterization, and biological functions of Lycium Barbarum polysaccharides: a review. Biomolecules 9 (9), 389. doi:10.3390/biom9090389
Wang, C., Wang, K., and Li, P. (2022a). Blueberry anthocyanins extract attenuated diabetic retinopathy by inhibiting endoplasmic reticulum stress via the miR-182/OGG1 axis. J. Pharmacol. Sci. 150 (1), 31–40. doi:10.1016/j.jphs.2022.06.004
Wang, D. Y., Peng, J. M., Ji, Y., Luo, H. Y., and Ye, J. L. (2021). Quercetin effect of quercetin on retinopathy in diabetic rats and its mechanism. Pract. J. Med. Pharm. 38 (04), 341–343+385. doi:10.14172/j.issn1671-4008.2021.04.017
Wang, J. J., Hu, R. R., Zhang, D., and Qiao, Y. B. (2020). Effects of ginsenoside Rg3 on PI3K-Akt/PKB pathway and expression levels of vascular endothelial growth factor and intercellular adhesion molecule-1 in rats with diabetic retinopathy. Recent Adv. Ophthalmol. 40 (01), 20–25. doi:10.13389/j.cnki.rao.2020.0005
Wang, L., Xu, J., Yu, T., Wang, H., Cai, X., and Sun, H. (2023a). Efficacy and safety of curcumin in diabetic retinopathy: a protocol for systematic review and meta-analysis. PLoS One 18 (4), e0282866. doi:10.1371/journal.pone.0282866
Wang, W., Li, S., and Song, M. (2022b). Polygonatum sibiricum polysaccharide inhibits high glucose-induced oxidative stress, inflammatory response, and apoptosis in RPE cells. J. Recept Signal Transduct. Res. 42 (2), 189–196. doi:10.1080/10799893.2021.1883061
Wang, Y. X., Jia, G. M., Cao, L., Cao, S. Y., and Zhang, S. H. (2023b). Angelica polysaccharide inhibited the apoptosis of retinal ganglion cells induced by high glucose through Akt/GSK-3β pathway enhanced Nrf2 signal transduction. Guid. J. TCM Pharm. 29 (05), 16–21. doi:10.13862/j.cn43-1446/r.2023.05.004
Wu, J., Shi, S., Wang, H., and Wang, S. (2016). Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: a review. Carbohydr. Polym. 144, 474–494. doi:10.1016/j.carbpol.2016.02.040
Wu, W., Zhou, G., Han, H., Huang, X., Jiang, H., Mukai, S., et al. (2020). PI3Kδ as a novel therapeutic target in pathological angiogenesis. Diabetes 69 (4), 736–748. doi:10.2337/db19-0713
Wu, Y., and Wang, D. (2009). Structural characterization and DPPH radical scavenging activity of an arabinoglucogalactan from Panax notoginseng root. J. Nat. Prod. 71 (2), 241–245. doi:10.1021/np070323+
Xie, T., Chen, X., Chen, W., Huang, S., Peng, X., Tian, L., et al. (2021). Curcumin is a potential adjuvant to alleviates diabetic retinal injury via reducing oxidative stress and maintaining Nrf2 pathway homeostasis. Front. Pharmacol. 12, 796565. doi:10.3389/fphar.2021.796565
Xie, W., Zhou, P., Qu, M., Dai, Z., Zhang, X., Zhang, C., et al. (2020). Ginsenoside Re attenuates high glucose-induced RF/6A injury via regulating PI3K/AKT inhibited HIF-1α/VEGF signaling pathway. Front. Pharmacol. 11, 695. doi:10.3389/fphar.2020.00695
Xu, Z. H., Gao, Y. Y., Zhang, H. T., Ruan, K. F., and Feng, Y. (2018). Progress in experimental and clinical research of the diabetic retinopathy treatment using traditional Chinese medicine. Am. J. Chin. Med. 4, 1421–1447. doi:10.1142/S0192415X1850074X
Yan, P. (2010). “The impact of oxymatrine onTLR4 and NF-κ B in retina of diabetic rat,”. dissertatio thesis (Hebei, Shijiazhuang: Hebei Medical University). doi:10.7666/d.y1779523
Yang, D. S., and Song, W. L. (2023). The relationship between serum oxidative stress indexes and the expression of inflammatory factors in patients with type 2 diabetic retinopathy and its significance. Chin. J. Public Health Eng. 22 (06), 812–814. doi:10.19937/j.issn.1671-4199.2023.06.029
Yang, J., Ma, Y. H., Cui, X. C., Wang, H. M., Dong, W. L., and Tan, X. B. (2017). Hypoglycemic effect of panaxnotoginseng polysaccharides in diabetic model rats and its therapeutic effect on diabetic retinopathy and their mechanisms. J. Jilin Univ. Med. Ed. 43 (04), 734–738+860. doi:10.13481/j.1671-587x.20170414
Yang, Y., Zhou, M., and Liu, H. (2021). Luteolin, an aryl hydrocarbon receptor antagonist, alleviates diabetic retinopathy by regulating the NLRP/NOX4 signalling pathway: experimental and molecular docking study. Physiol. Int. 108, 172–184. doi:10.1556/2060.2021.00148
Yu, C. J., Zhao, J. D., Yang, D., Du, X., Wu, J. P., You, L. X., et al. (2020). Preventive and therapeutic effects of astragaloside IV on retinopathy in diabetic rats and its effects on oxidative stress pathway. Chin. Archives TCM 38 (11), 194–197+292. doi:10.13193/j.issn.1673-7717.2020.11.047
Yu, W. F., and Li, M. J. (2018). Inflammatory cytokines and oxidative stress markers expression patterns in peripheral blood of patients with diabetic retinopathy. Med. Recapitulate 24 (20), 4147–4151. doi:10.3969/j.issn.1006-2084.2018.20.036
Yu, Y., Lu, Q., Chen, F., Wang, S., Niu, C., Liao, J., et al. (2022). Serum untargeted metabolomics analysis of the mechanisms of evodiamine on type 2 diabetes mellitus model rats. Food Funct. 13 (12), 6623–6635. doi:10.1039/d1fo04396j
Zhang, F. M., and Shen, Y. Q. (2020). Research advance on pharmacokinetics of oxymatrine. Drug Eval. Reserch 3 (09), 1903–1911. doi:10.7501/j.issn.1674-6376.2020.09.043
Zhang, H. X., Xue, K., Gao, W., and Long, T. (2016). Inhibiting effect of lycium barbarum polysaccoharide on angiogenesis as well as oxidative stress and inflammation in retina of diabetic mice. J. Hainan Med. Univ. 22 (20), 2365–2368. doi:10.13210/j.cnki.jhmu.20160715.007
Zhang, Q. M., and Wang, H. (2019). Inhibitory effects of puerarin on retinopathy of diabetic rats by regulating activation of Nrf2/ERK signaling pathway. Recent Adv. Ophthalmol. 39 (01), 22–26. doi:10.13389/j.cnki.rao.2019.0005
Zhang, R., Zhang, X., Xing, B., Zhao, J., Zhang, P., Shi, D., et al. (2019b). Astragaloside IV attenuates gestational diabetes mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod. Biol. Endocrinol. 17 (1), 77. doi:10.1186/s12958-019-0522-7
Zhang, S. M. (2015). “Intervention effect of oxymatrine on hyperglycemia-induced retinal pigment epithelial cell injury in rats,”. dissertatio thesis (Hebei, Shijiazhuang: Hebei Medical University). doi:10.7666/d.Y2784737
Zhang, Z. (2019). Effect of matrine on proliferation of retinal microvascular endothelial cell and expression of vascular endothelial growth factor in rats with diabetic retinopathy. Eval. Analysis Drug-Use Hosp. China 19 (04), 422–425+428. doi:10.14009/j.issn.1672-2124.2019.04.011
Zhang, Z., Zhang, J., Hu, J. L., Wang, Y., and Xiao, X. (2019c). Effect of oxymatrine on the expression of Toll like receptor 4 mRNA and nuclear factorκB mRNA in retina of diabetic retinopathy rats. Heibei Zhongyi 41 (05), 735–738. doi:10.3969/j.issn.1002-2619.2019.05.020
Zhang, Z., Zhang, L., and Xu, H. (2019a). Effect of Astragalus polysaccharide in treatment of diabetes mellitus: a narrative review. J. Tradit. Chin. Med. 39 (1), 133–138. doi:10.19852/j.cnki.jtcm.2019.01.017
Zhao, X., Patil, S., Qian, A., and Zhao, C. (2022). Bioactive compounds of Polygonatum sibiricum - therapeutic effect and biological activity. Endocr. Metab. Immune Disord. Drug Targets 22 (1), 26–37. doi:10.2174/1871530321666210208221158
Zhou, P., Xie, W., Meng, X., Zhai, Y., Dong, X., Zhang, X., et al. (2020). Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells 8 (3), 213. doi:10.3390/cells8030213
Zhou, P. P., Liu, L. Y., and Gao, W. P. (2021). Association between serum CTRP9 levels and diabetic retinopathy in patients with type2 diabetes mellitus. J. South. Med. Univ. 41 (3), 459–463. doi:10.3969/j.issn.1671-4695.2023.02.011
Keywords: plant metabolites, oxidative stress, diabetic retinopathy, polyphenols, polysaccharide, saponins, alkaloids
Citation: Gong T, Wang D, Wang J, Huang Q, Zhang H, Liu C, Liu X and Ye H (2025) Study on the mechanism of plant metabolites to intervene oxidative stress in diabetic retinopathy. Front. Pharmacol. 16:1517964. doi: 10.3389/fphar.2025.1517964
Received: 27 October 2024; Accepted: 14 January 2025;
Published: 05 February 2025.
Edited by:
Yongmei Xie, Sichuan University and Collaborative Innovation Center of Biotherapy, ChinaReviewed by:
Dandan Tang, Sichuan College of Traditional Chinese Medicine, ChinaCopyright © 2025 Gong, Wang, Wang, Huang, Zhang, Liu, Liu and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hejiang Ye, eWVoZWpAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.