
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 28 March 2025
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1516885
This article is part of the Research TopicEpigenetic Modifications and Cardiovascular DiseasesView all 4 articles
 Bingquan Qiu1
Bingquan Qiu1 Shangyue Zhang1
Shangyue Zhang1 Shuang Ge2
Shuang Ge2 Zhengyu Yu3
Zhengyu Yu3 Deqing Wang2
Deqing Wang2 Kun Li4
Kun Li4 Xiaoqi Yu4
Xiaoqi Yu4 Chaoshu Tang5
Chaoshu Tang5 Junbao Du1,6*
Junbao Du1,6* Hongfang Jin1,6*
Hongfang Jin1,6* Yaqian Huang1*
Yaqian Huang1*Background: Vascular smooth muscle cell (VSMC) senescence is a critical driver of vascular aging and various age-related cardiovascular diseases. Endogenous sulfur dioxide (SO2), a newly identified key cardiovascular gaseous signaling mediator, accelerates collagen deposition and vascular remodeling in VSMCs when downregulated. However, its effects on VSMC senescence remain unclear.
Objective: This study focused on exploring the role of endogenous SO2 in VSMC senescence and its associated molecular pathways.
Methods: Aged mice (24 months old), VSMC-specific aspartate aminotransferase 1 (AAT1) knockout (VSMC-AAT1-KO) mice, D-galactose (D-gal)-treated aorta rings and rat VSMC line A7r5 were used in the experiments. AAT1 expression was detected by Western blot and single-cell RNA sequencing. Senescence markers Tp53, p21Cip/Waf, interleukin 1β (IL-1β) and IL6 expression were detected by Western blot and real-time quantitative PCR. Senescence-associated β-galactosidase (SA-β-gal) activity was detected using SA-β-gal staining kit. Sulphenylation of interferon regulatory factor 1 (IRF1) was detected using a biotin switch assay. The plasmid for mutant IRF1 (mutation of cysteine 83 to serine, C83S) were constructed by site-directed mutagenesis.
Results: The expression of AAT1, a key enzyme for SO2 production, was reduced in the aortic tissue of aged mice in comparison to young mice. VSMC-AAT1-KO mice exhibited elevated protein expression of senescence markers Tp53, p21Cip/Waf and γ-H2AX in the aortic tissue. AAT1 knockdown in VSMCs elevated expression of Tp53, p21Cip/Waf, IL-1β and IL-6, and enhanced SA-β-gal activity. While SO2 donor supplementation rescued VSMC senescence caused by AAT1 knockdown and blocked aortic ring aging induced by D-gal. Mechanistically, SO2 promoted IRF1 sulphenylation, inhibited IRF1 nuclear translocation, which in turn downregulated the expression of senescence markers and the activity of SA-β-gal. Furthermore, mutation of C83 in IRF1 abolished SO2-mediated IRF1 sulphenylation and blocked the inhibitory effect of SO2 on VSMC senescence.
Conclusion: Reduction of the endogenous SO2/AAT1 pathway played a crucial role in driving VSMC senescence. Endogenous SO2 counteracted VSMC senescence and vascular aging via the sulphenylation of IRF1 at C83.
Vascular aging, a core event in the human aging process, significantly contributes to cardiovascular disease progression (Lin et al., 2023). Vascular smooth muscle cells (VSMCs) (Lin et al., 2023) is a key component of blood vessels and their senescence drives vascular aging. Senescent VSMCs exhibit DNA damage, telomere damage and shortening, halted cell cycles, increased senescence-associated β-galactosidase (SA-β-gal) activity, and the senescence-associated secretory phenotype (SASP). These changes contribute to chronic vascular inflammation, vascular remodeling and impaired arterial function (Okuno et al., 2020; Zha et al., 2022). However, the endogenous regulatory mechanisms underlying VSMC senescence remain incompletely understood.
Endogenous sulfur dioxide (SO2), a metabolite catalyzed by aspartate aminotransferase (AAT) using L-cysteine as the substrate, is a novel gaseous signaling molecule in cardiovascular system (Zhu et al., 2014; Zhou et al., 2020; Li et al., 2021). The deficiency of endogenous SO2/AAT pathway aggravated age-related cardiovascular disease progression, such as hypertension, atherosclerosis, and calcification (Li et al., 2011; Tian et al., 2020; Huang et al., 2021; Huang et al., 2022). Surprisingly, SO2 donor supplementation reduced the D-galactose (D-gal)-induced elevation of mean arterial pressure, ameliorated endothelial cell dysfunction, and lowered plasma angiotensin II concentrations in rats (Dai et al., 2018). These studies suggested that endogenous SO2/AAT pathway deficiency might drive age-related disease progression. However, whether endogenous SO2/AAT pathway inhibits vascular aging through VSMC senescence is unclear.
Interferon regulatory factor 1 (IRF1), a transcription factor of the IRF family, regulates cell cycle, proliferation, differentiation, and inflammation. Studies have shown that silencing IRF-1 significantly inhibited VSMC inflammatory responses, extracellular matrix remodeling, and migration (Du et al., 2019; Shen et al., 2020). IRF1 induced CDK inhibitor p21Cip/Waf expression and led to G1 phase cell cycle arrest in coronary artery smooth muscle cells (Wessely et al., 2003). In addition, IRF1 promoted endothelial cell, fibroblast, and monocyte senescence (Upreti et al., 2010; Rossi et al., 2023). These studies suggested that targeting the IRF1 pathway could prevent cell senescence. However, it remains unclear whether endogenous SO2-mediated anti-senescence effects are mediated through IRF1 inhibition.
Here, we demonstrated that downregulation of endogenous SO2/AAT pathway promoted VSMC senescence during vascular aging. VSMC-derived SO2 suppressed VSMC senescence via inhibiting IRF1 transcription activity. Mechanistically, SO2-sulphenylated IRF1 at cysteine 83 suppressed IRF1 target gene expression and inhibited VSMC senescence phenotype. Our findings revealed VSMC-derived SO2 acted as a novel protector against VSMC senescence via IRF1 sulphenylation.
Sodium bisulfite (NaHSO3, 243973-100G, Sigma) and sodium sulfite (Na2SO3, S4672-250G, Sigma) were freshly mixed in a 1:3 ratio (pH 7.4), which was used as the SO2 donor. Tamoxifen (579002, Sigma, United States) was used to induce AAT1 knockout in the animal models. The primary reagents for cell culture included fetal bovine serum (FBS) (10099-141, Gibco, United States), penicillin-streptomycin solution (PS, 100×, 15140-122, ThermoScientific, United States), glutathione (LG, 100×, 25030081, Thermo Scientific, United States), and trypsin-containing EDTA (0.25%, 25200-056, ThermoScientific, United States). The primary antibodies used in this study were AAT1 (SAB2500473, Sigma, United States), β-actin (TA-09, Zsbio, China), Tp53 (2524, CST, United States), and p21Cip/Waf (ab109199, Abcam, United States). Lentivirus-delivered Rat AAT1 shRNA (VB151210-10015, Cyagen, China) or scrambled shRNA (VB151214-10025, Cyagen, China) was used for the experiments. DAz-2 (13382, Cayman, United States) and phosphine-biotin (13581, Cayman, United States) were used for the protein sulphenylation assays.
A7r5 (rat VSMCs) was purchased from the National Experimental Cell Resource Sharing Platform (1101RAT-PUMC000219, China) and cultured at 37°C in a 5% CO2 incubator using Dulbecco’s modified eagle’s medium (DMEM) containing 10% FBS, 1% PS, and 1% LG.
At the appropriate cell density, A7r5 was infected with lentivirus containing AAT1 shRNA or scramble shRNA at a multiplicity of infection (MOI) of 10 in the presence of polybrene (1 μg/mL). After 48 h, the culture medium was replaced with a puromycin (1 μg/mL)-containing medium to establish a stably transfected cell pool. After 14 days of screening, stable cell lines were used for subsequent experiments.
Stably transfected cell lines were divided into three groups: Scramble, AAT1 shRNA, and AAT1 shRNA + SO2 groups. Cells stably transfected with scramble shRNA were used as controls. For the AAT1 shRNA + SO2 group, cells were treated with 100 μM SO2 donor for 4 days. For the scrambled and AAT1 shRNA groups, the cells were treated with equal volumes of saline. The AAT1 shRNA sequences (5′-3′) used in this study are as follows: TCGAATTGGAGCTGACTTCAGATACT. The scramble sequences (5′-3′) used in this study are as follows: GCACTACCAGAGCTAACTCAGATAGTACT. All experiments were performed in duplicate and repeated independently three times (n = 3).
This study protocol received approval from the Animal Experimentation and Welfare Committee at Peking University First Hospital, with the approval number J2022051. All procedures involving animals adhered to the ethical guidelines established by the Peking University First Hospital for the management of animal research.
The 2- and 24-month-old C57BL/6J male mice used in this study were purchased from Sibeifu (Beijing, China), and the aortic tissues were collected to detect protein levels of Tp53, p21Cip/Waf and AAT1. The 2-month-old mice were served as controls. VSMC-AAT1-KO mice were constructed using Cre/LoxP technology as described in previous study (Huang et al., 2021). Briefly, SM22α-Cre transgenic (SM22α-CreERT2+) mice and AAT1 flox/flox (AAT1 f/f) mice were purchased from Guangzhou Cyagen (Guangzhou, China). SM22α-CreERT2+ and AAT1 f/f mice were hybridized to generate SM22α-CreERT2+ AAT1 f/f mice. VSMC-AAT1-KO mice were obtained from 8-week-old male SM22α-CreERT2+ AAT1 f/f mice by intraperitoneal injection of tamoxifen (2.5 mg/day, dissolved in ethanol/peanut oil [volume ratio 1:19]) for 5 consecutive days at 3-day intervals. Littermate AAT1 f/f mice were served as control. At the end, the aortic tissues of 5-month-old mice (AAT1 f/f and VSMC-AAT1-KO) were collected post euthanasia by excessive anesthesia. n = 6 independent biological samples.
Eight-week-old C57BL/6J mice were selected for aortic ring culture experiments. The murine aorta was bluntly detached and placed in sterile phosphate-buffered saline (PBS), with the adventitia of the aorta and connective tissues stripped. The aorta was cut into 3-mm rings and transferred to complete DMEM containing 10% FBS, 1% PS, and 1% LG and cultured at 37°C in a 5% CO2 incubator. The following day, the rings were divided into control, D-gal and D-gal + SO2 groups. In the control group, the rings were cultured in complete DMEM. In the D-gal group, the rings were cultured in complete DMEM containing 7.5 g/L D-gal. In the D-gal + SO2 group, the rings were cultured in DMEM containing 7.5 g/L D-gal, and 100 μM SO2 donor given twice daily. The medium was changed every 2 days. Aortic rings were collected after 7 days of induction for subsequent experiments. n = 6 independent biological samples.
Cells were fixed at room temperature for 15 min. After PBS washing, the working solution was applied and incubated overnight at 37°C without CO2. The solution was then removed, followed by washing with PBS for three times. The cell observations were performed using a light microscope. A blue color indicates a positive signal. All experiments were performed in duplicate and repeated independently three times (n = 3).
A7r5 cells were lysed to extract total RNA with TRIzol reagent. cDNA synthesis was performed using 2 μg of total RNA and M-MLV reverse transcriptase. RT-qPCR was conducted using 2× Go Taq qPCR Master Mix on a 7500 Fast Real-Time PCR System (Applied Biosystems), with β-actin serving as the control. The primers used in this study were as follows: Rat-β-actin-F 5′-ACCCGCGAGTACAACCTTCTT-3′ and Rat-β-actin-R 5′-TATCGTCATCCATGGCGAACT-3′; Rat-IL1β-F 5′-CACCTCTCAAGCAGAGCACAG-3′ and Rat-IL1β-R 5′- GGGTTCCATGGTGAAGTCAAC-3′; Rat-IL6-F 5′-CCCACCCTCCAACAAAGATT-3′ and Rat-IL6-R 5′- GCTCCAGAGCAGAATGAGCTA -3′. All experiments were performed in duplicate and repeated independently three times (n = 3).
Arotic tissues were formalin-fixed, embedded in paraffin, 4 μm sectioned, and dehydrated with xylene and graded ethanol concentrations. Antigen repair was performed using Tris-EDTA (pH 9.0) for 15 min. After permeabilization, the sections were incubated overnight with Tp53 (dilution 1:200), p21Cip/Waf (dilution 1:200), γ-H2AX (dilution 1:200) antibodies. After incubation with the secondary antibody, the slices were sealed with a DAPI-containing sealer. Red (Tp53 and p21Cip/Waf) or Green (γ-H2AX) fluorescence observed using Leica TCS SP8 MP FLIM indicated the expression of target protein. n = 6 independent biological samples.
Cells were fixed with 4% formaldehyde at room temperature for 15 min, permeabilized with pre-cooled methanol, and blocked with 5% bovine serum albumin. Subsequently, cells were incubated with primary antibody against IRF1 and Flag overnight at 4°C. After washing, cells were incubated with the corresponding secondary antibody and mounted with a DAPI-containing dye. Target protein expression was visualized as red fluorescence using a Leica TCS SP8 MP FLIM microscope. All experiments were performed in duplicate and repeated independently three times (n = 3).
A7r5 cells were lysed in a non-denaturing lysis buffer (Applygen, Beijing, China) containing 5 mM DAz-2 for 20 min. The supernatant was collected after centrifugation at 16,000 g for 4 min at 4°C and incubated with gentle shaking at 37°C for 2 h. DAz-2-labeled lysates were reacted with 250 μM phosphine-biotin (13581, Cayman, United States) at 37°C for 2 h. Neutravidin ultralink resin (53150, Thermo Fisher Scientific, United States) was added at a volume ratio of 1:10 and incubated overnight with shaking at 4°C. The suphenylated proteins enriched with resin were mixed with a 2× non-denaturing sample buffer and boiled at 100°C for 10 min. The supernatant was collected by centrifugation at 5,000 g for 10 min and subjected to Western blot. All experiments were performed in duplicate and repeated independently three times (n = 3).
Human IRF1 wild-type (WT) and C83S mutant plasmids were constructed (Transgene, China). When the A7r5 cells reached approximately 50%–60% confluence, cells were transfected with plasmids via Lipofectamine™ 3000 (L3000075, Thermo Fisher, United States), followed by medium replacement 6–8 h post-transfection.
The protein expression of AAT1, GAPDH, Tp53, p21Cip/Waf, and IRF1 was detected using Western blot. The mouse aortic tissue was homogenized in the lysis buffer. Cells were also lysed in lysis buffer for 30 min at 4°C. The total protein concentration was quantified using the BCA method. An equal amount of protein was taken for electrophoresis, and the membrane was subsequently blocked and incubated with primary antibody overnight at 4°C. Following incubation for 1 h with secondary antibodies at room temperature, the membrane was washed with PBST and processed on a FluorChem M MultiFluor System (Protein Simple, United States) with enhanced chemiluminescence solution (MA0186, Meilunbio, China). n = 6 independent biological samples in animal studies and n = 3 independent experiments performed in duplicate in cell studies.
The original single-cell transcriptome data of aged aortic tissue used in this study is publicly available that can be found in the GEO database (GSE164585) (Xie et al., 2022). Aortas of 4-, 26-, and 86-week-old C57/BL6J mice were analyzed using single-cell RNA sequencing. Dimensional reduction was conducted using t-distributed stochastic neighbor embedding (UMAP), executed through the “RunUMAP” functions. Cluster identification was performed by the “FindClusters” function, resulting in the delineation of 16 distinct clusters from the comprehensive cell population. The “FindAllMarkers” function, employing the Wilcoxon rank-sum test, was used to identify signature genes for each cluster, enabling the assignment of cellular identities, which included various cell types such as B cells, fibroblasts, monocytes, and others.
DEGs from scRNA-seq were identified via “FindAllMarkers” or “FindMarkers” using the “Wilcox” test, yielding “p_val_adj” with Bonferroni correction and “avg_logFC.” Biomarkers were selected from DEGs with p_val_adj ≤0.05 and avg_logFC ≥0.5. GO enrichment analysis was conducted through Metascape and GO software. REVIGO summarized redundant GO terms based on semantic similarity, and non-redundant terms were visualized in Cytoscape, with nodes representing GO terms and 3% pairwise similarities as edges.
Transcription factor prediction was performed using CheA3 online tools (https://maayanlab.cloud/chea3/).
Statistical analysis was performed with SPSS (version 17.0; IBM, United States) and GraphPad Prism 8.0 (GraphPad Software Inc.), and results were represented as mean ± standard deviation (SD). When the variance was uniform and satisfied the normal distribution, an independent sample t-test was used to compare the two groups. One-way ANOVA followed by Bonferroni post-doc analysis was used to compare the difference among multiple groups. Statistical significance was defined as p < 0.05.
We initially measured the expression of senescence markers and SO2-generating enzymes in the aortic tissues of young (2-month-old) and aged (24-month-old) mice. The results showed that, compared to young (2-month-old) mice, senescence markers Tp53 and p21Cip/Waf were increased in the aortic tissues of aged (24-month-old) mice. However, the protein expression of AAT1, a key enzyme in SO2 generation, showed a significantly reduced expression in the aortic tissues of aged (24-month-old) mice (Figure 1A).
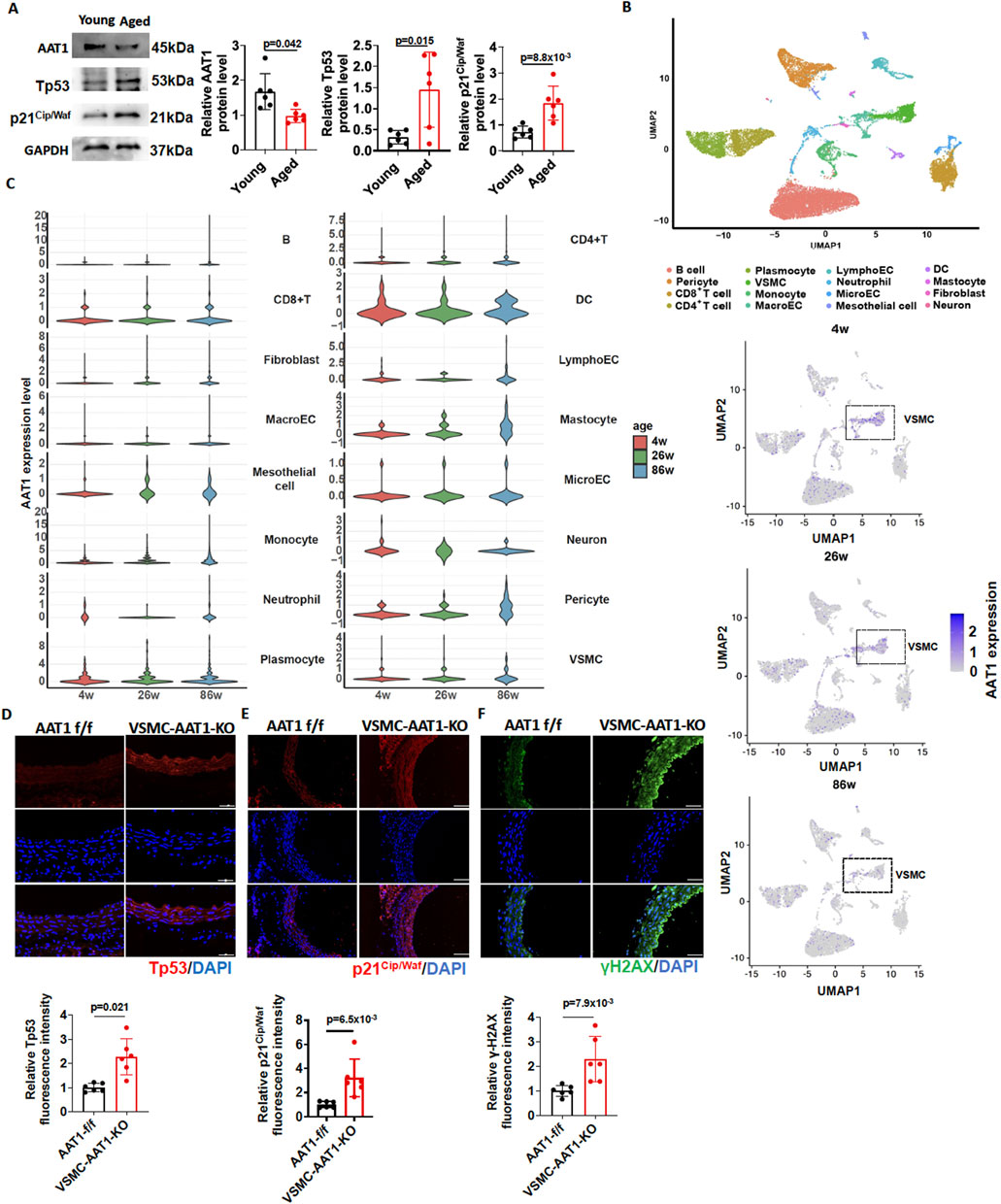
Figure 1. Downregulation of the endogenous SO2/AAT1 pathway during vascular aging. (A) Western blot analysis was used to detect AAT1, Tp53, and p21Cip/Waf protein expression in the aortic tissues of young (2 months) and aged (24 months) mice (n = 6 independent biological samples). Two-tailed unpaired student’s t-test was used. (B) UMAP plot of 16 segregated cell clusters in the aortic tissues of 4-, 26-, and 86-week mice. (C) Violin plots showing AAT1 expression levels across 16 cell clusters (upper panel) Each circle represents AAT1 gene expression level in one VSMC (lower panel). (D) Detection of Tp53 expression in the aortic tissues of 5-month-old VSMC-AAT1-KO and control AAT1 f/f mice using immunofluorescence (n = 6 independent biological samples), scale bar: 100 μm. Two-tailed unpaired student’s t-test was used. (E) Detection of p21Cip/Waf expression in the aortic tissues of 5-month-old VSMC-AAT1-KO and control AAT1 f/f mice using immunofluorescence (n = 6 independent biological samples), scale bar: 100 μm. Two-tailed unpaired student’s t-test was used. (F) Detection of γ-H2AX expression in the aortic tissues of 5-month-old VSMC-AAT1-KO and control AAT1 f/f mice using immunofluorescence (n = 6 independent biological samples), scale bar: 50 μm. Two-tailed unpaired student’s t-test was used. Data are expressed as mean ± SD.
To identify the cell populations responsible for reduced AAT1 expression in aortic tissue during aging process, we performed dimensionality reduction and clustering analysis of single-cell transcriptomic data from 4-week-old, 26-week-old, and 86-week-old mice. As a result, we finally grouped cells into 16 distinct clusters, comprising dendritic cells (DC), fibroblasts, B cells, CD8+ T cells, CD4+ T cells, lymphatic endothelial cells, macroECs, mastocytes, mesothelial cells, microECs, monocytes, neuron cells, neutrophils, pericytes, plasmocytes, and VSMCs (Figure 1B). We next performed gene expression mapping, which revealed that AAT1, the SO2-generating enzyme, was mainly reduced in VSMC from aged (86-week-old) mice (Figure 1C). Moreover, AAT1 expression was upregulated in mastocytes and pericytes from aged (86-week-old) mice (Figure 1C).
Therefore, we detected the expression of senescence markers Tp53, p21Cip/Waf and γ-H2AX in the aortic tissues of 5-month-old AAT1 f/f and VSMC-AAT1-KO mice. As expected, the expression of Tp53, p21Cip/Waf, and γ-H2AX was elevated in the aorta of 5-month-old VSMC-AAT1-KO mice compared to 5-month-old AAT1 f/f mice (Figures 1D–F), suggesting that the downregulation of endogenous SO2/AAT1 pathway might be associated with vascular aging.
To confirm the importance of endogenous SO2/AAT1 pathway in VSMC senescence, we generated VSMCs with stable knockdown of AAT1 by transfecting AAT1 shRNA, and cells stably transfected with Scramble shRNA were used as controls. AAT1-knockdowned VSMCs were then treated with or without SO2 donor. As expected, senescence markers Tp53 and p21Cip/Waf protein levels increased in AAT1-knockdowned VSMCs, which were rescued by SO2 donor supplementation (Figures 2A, B). Moreover, SA-β-gal activity increased in AAT1-knockdowned VSMCs, which was rescued by SO2 donor supplementation. These findings suggested that AAT1 knockdown led to VSMC senescence.
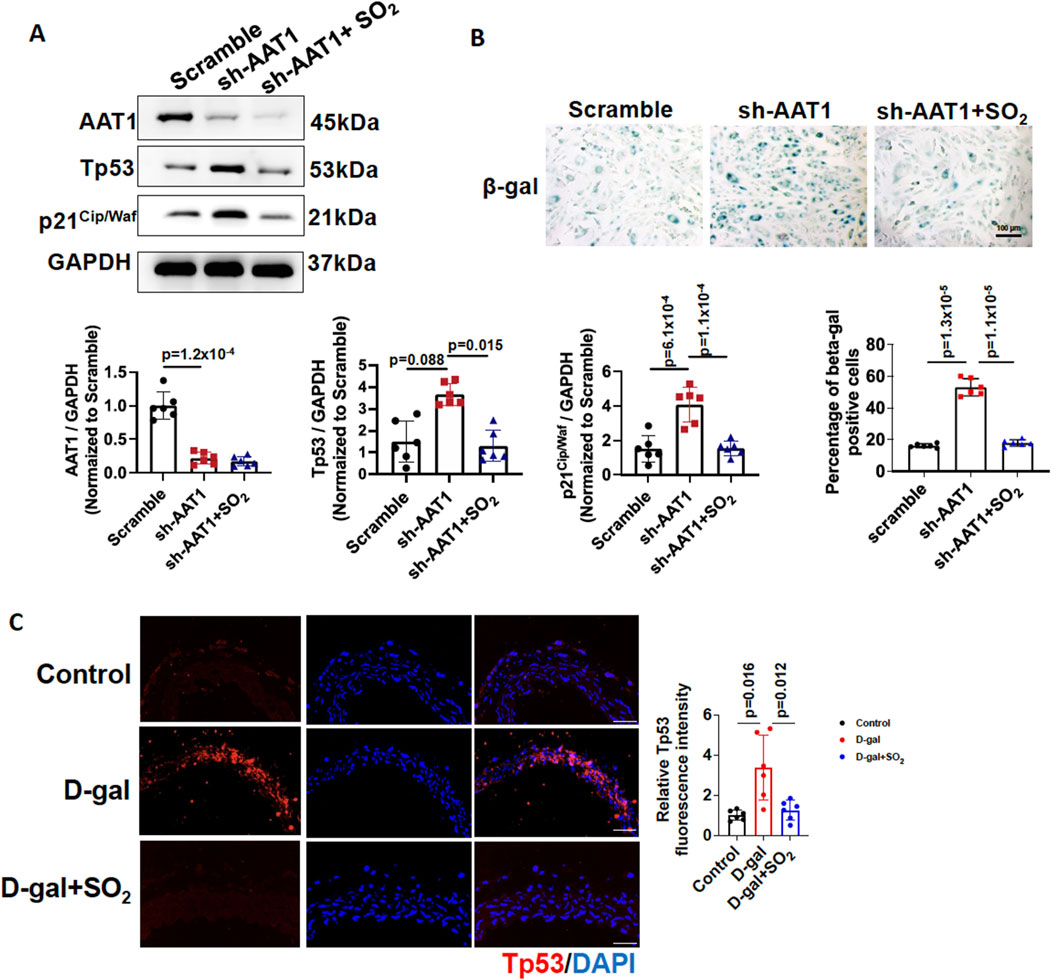
Figure 2. Endogenous SO2/AAT1 deficiency contributed to VSMC senescence. (A) Western blot analysis was used to detect AAT1, Tp53, and p21Cip/Waf protein levels in VSMCs transfected with scrambled or AAT1 shRNA (n = 3 independent experiments performed in duplicate). One-way ANOVA followed by Bonferroni post-doc analysis was used. (B) SA-β-gal activity in AAT1-knockdowned VSMCs (n = 3 independent experiments performed in duplicate). Scale bar: 100 μm. One-way ANOVA followed by Bonferroni post-doc analysis was used. (C) Immunofluorescence analysis of Tp53 protein expression in D-gal-induced aortic rings with or without SO2 donor (n = 6 independent biological samples), scale bar: 100 μm. One-way ANOVA followed by Bonferroni post-doc analysis was used. Data are expressed as mean ± SD.
To determine the protective effects of endogenous SO2 on vascular aging, aortic rings treated with DMEM medium containing 7.5 g/L D-gal were used as a vascular aging model and incubated with or without an SO2 donor. Aortic rings treated with DMEM medium were used as controls. Compared to the control group, D-gal significantly upregulated Tp53 expression in aortic rings, and supplementation with the SO2 donor reduced Tp53 protein expression (Figure 2C). These results suggested that sufficient SO2 protected against vascular aging.
Next, we explored the potential mechanisms underlying the inhibitory effect of the endogenous SO2/AAT1 pathway on VSMC senescence. First, we analyzed the DEGs in VSMC clusters of aortic tissue from 4- and 86-week-old mice using single-cell RNA sequencing (4-week-old mice as controls), which revealed 641 upregulated and 596 downregulated genes (Figure 3A). KEGG enrichment analyses of these DEGs showed that the upregulated pathways were mainly related to cell senescence (Figure 3B). GO enrichment analyses of these DEGs showed that the upregulated pathways were mainly related to MHC class I complex (Figure 3D), which is also a hallmark of aging (Suryadevara et al., 2024). Whereas the downregulated pathways in KEGG enrichment analyses were mainly related to VSMC contraction and extracellular matrix remodeling (Figure 3C). GO enrichment analyses of these DEGs showed that the downregulated pathways were mainly related to muscle contraction, which is also a marker of vascular aging (Figure 3E).
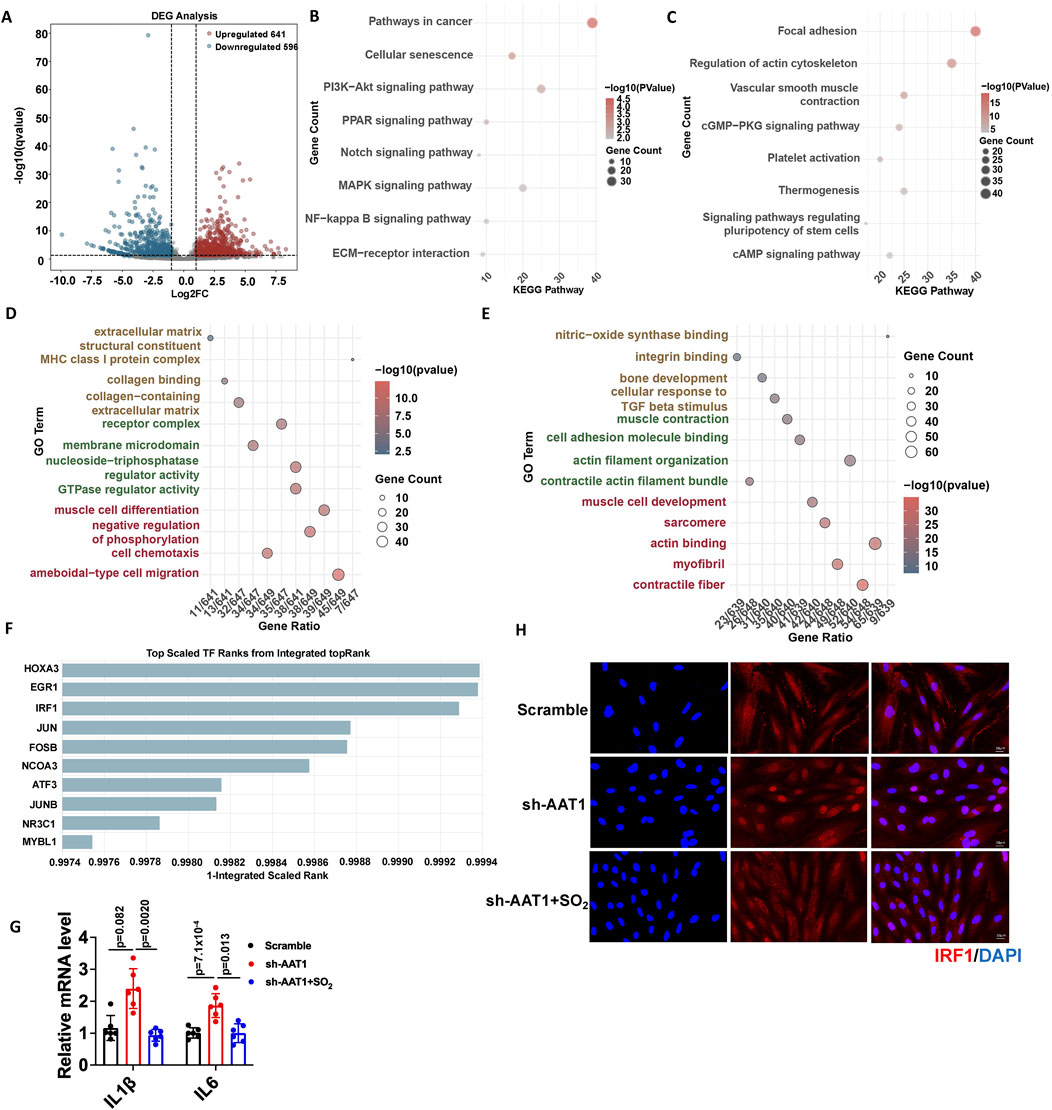
Figure 3. Endogenous SO2/AAT1 pathway inhibited IRF1 nuclear translocation and downregulated IRF1-targeted senescence-associated gene expression in VSMCs. (A) Volcano plot analysis shows upregulated and downregulated genes in the VSMC clusters of aortic tissues from 4- and 86-week-old mice using single-cell RNA sequencing. (B, C) KEGG enrichment analysis was performed for the upregulated (B) and downregulated (C) differentially expressed genes. (D, E) GO enrichment analysis was performed for the upregulated (D) and downregulated (E) differentially expressed genes. (F) The ChEA3 online tool was used to predict the transcription factors that regulate the genes involved in cell senescence. (G) RT-qPCR was used to detect the mRNA expression of IL-1β and IL-6 in AAT1-knockdowned VSMCs with or without SO2 donor treatment (n = 3 independent experiments performed in duplicate). One-way ANOVA followed by Bonferroni post-doc analysis was used. Data are expressed as mean ± SD. (H) Immunofluorescence was used to detect the nuclear translocation of IRF1 in VSMCs (n = 3 independent experiments performed in duplicate). Scale bar: 50 μm.
We then used the CheA3 online tool to predict the potential transcription factors that regulate genes involved in cell senescence and found that IRF1 might play an important role in VSMC senescence (Figure 3F). IRF1 target genes include IL-1β and IL-6, which are SASP factors. The mRNA levels of IL-1β and IL-6 increased in the AAT1-knockdowned VSMCs compared with the scramble group but reduced after SO2 donor supplementation (Figure 3G). Furthermore, IRF1 nuclear translocation increased in AAT1-knockdowned VSMCs, which reduced after SO2 donor supplementation (Figure 3H). These results suggested that the endogenous SO2/AAT1 pathway inhibited IRF1 nuclear translocation and downregulated IRF1-targeted senescence-associated gene expression in VSMCs.
Various kinds of protein modification were involved in aging-related vascular diseases (Zu et al., 2010; Zou et al., 2018; Zhao et al., 2021; Zou et al., 2023). Previous studies have shown that SO2 regulates protein function via sulphenylation (Huang et al., 2021). Moreover, cysteine 83 (C83) in IRF1 mediates its affinity with DNA (Antonczyk et al., 2019; Jefferies, 2019). Therefore, we hypothesized that SO2 could inhibit IRF1 transcription activity and its downstream inflammation-mediated VSMC senescence by sulphenylating IRF1 at C83. First, we constructed Flag-IRF1-WT (Flag-tagged wild-type IRF1) and Flag-IRF1-C83S (mutation at cysteine 83 by serine) plasmids and detected the IRF1 suphenylation. Cells transfected with the corresponding plasmids treated without SO2 as controls. The results showed that the SO2 donor could sulphenylate IRF1 in VSMCs transfected with the Flag-IRF1-WT plasmid. However, in VSMCs transfected with the Flag-IRF1-C83S plasmid, supplementation with SO2 failed to increase the sulphenylated IRF1 levels (Figure 4A). These results indicated that SO2 sulphenylated IRF1 at C83 in VSMCs.
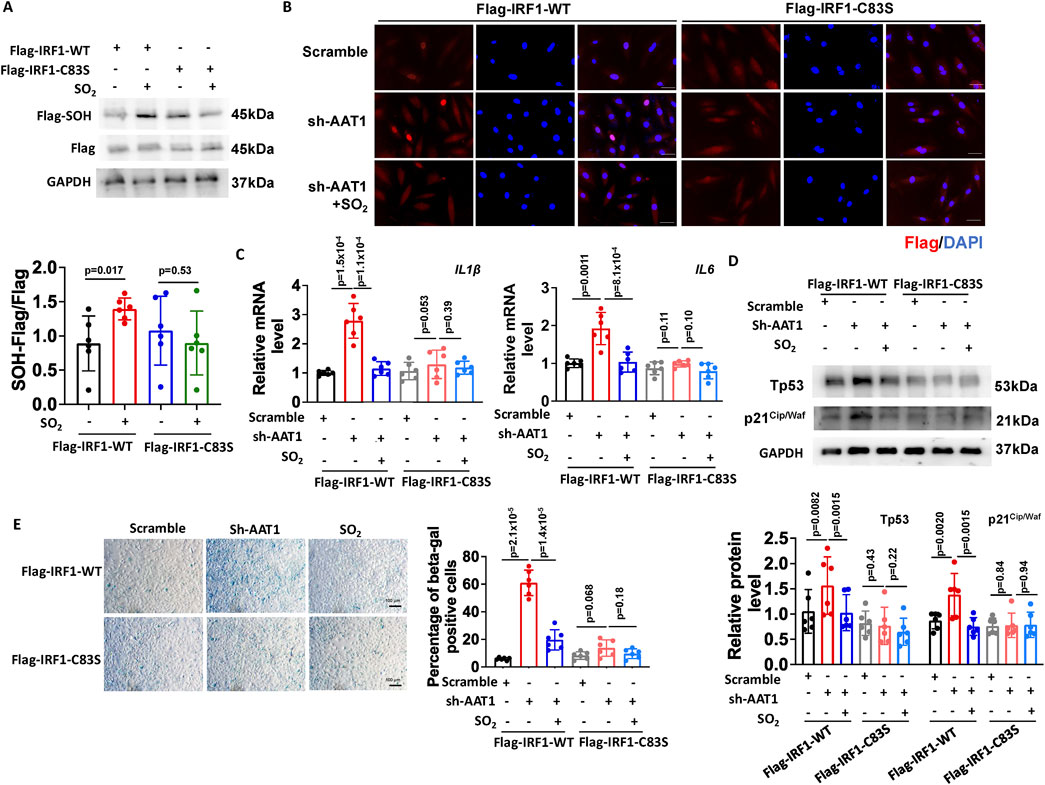
Figure 4. Endogenous SO2 inhibited VSMC senescence by sulphenylating IRF1 at cysteine 83. (A) Sulphenylation of IRF1 in VSMCs transfected with IRF1-WT or IRF1-C83S plasmids was detected by a biotin switch assay (n = 3 independent experiments performed in duplicate). One-way ANOVA followed by Bonferroni post-doc analysis was used. (B) Immunofluorescence was used to detect the nuclear translocation of Flag-IRF1-WT and Flag-IRF1-C83S in VSMCs (n = 3 independent experiments performed in duplicate). Scale bar: 50 μm. (C) The mRNA expression of IL-1β and IL-6 was determined by RT-qPCR (n = 3 independent experiments performed in duplicate). One-way ANOVA followed by Bonferroni post-doc analysis was used. (D) Protein expression of Tp53 and p21Cip/Waf was determined using Western blotting (n = 3 independent experiments performed in duplicate). One-way ANOVA followed by Bonferroni post-doc analysis was used. (E) SA-β-gal staining was used to detect SA-β-gal activity (n = 3 independent experiments performed in duplicate). Scale bar: 100 μm. One-way ANOVA followed by Bonferroni post-doc analysis was used. Data are expressed as mean ± SD.
Meanwhile, in VSMCs transfected with Flag-IRF1-WT plasmid, AAT1 knockdown enhanced IRF1 nuclear translocation compared to the scramble group (control), which could be attenuated by the SO2 donor supplementation (Figure 4B). However, in VSMCs transfected with Flag-IRF1-C83S plasmid, neither AAT1 knockdown nor SO2 donor supplementation affected IRF1 nuclear translocation (Figure 4B). These results revealed that sulphenylation of IRF1 at C83 is essential for SO2-inhibited IRF1 nuclear translocation (Figure 4B).
Furthermore, we examined whether C83 was a target of SO2 for inhibiting the senescence phenotype of VSMCs. In VSMCs transfected with Flag-IRF1-WT plasmid, AAT1 knockdown increased IL-1β and IL-6 mRNA levels (Figure 4C), elevated Tp53 and p21Cip/Waf protein levels (Figure 4D), and enhanced SA-β-gal activity (Figure 4E) compared to the scramble group, while SO2 donor supplementation downregulated the levels of IRF1 target genes, SASP factors and SA-β-gal activity compared to AAT1-knockdowned group. However, in VSMCs transfected with Flag-IRF1-C83S plasmid, neither AAT1 knockdown nor SO2 donor supplementation affected the VSMC senescence phenotype (Figures 4C–E). These results further confirmed that endogenous SO2 could inhibit VSMC senescence phenotype through IRF1 sulphenylation at C83.
Vascular aging is the pathological basis of various age-related cardiovascular diseases and can trigger or aggravate disease progression (Ungvari et al., 2020; Abdellatif et al., 2023). VSMC senescence is a major contributor to vascular aging (Cui et al., 2021). However, the mechanisms underlying VSMC senescence remain unclear. Here, we demonstrated that the endogenous SO2/AAT1 pathway was downregulated in the aortic tissues of aged mice. Insufficient endogenous SO2/AAT1 pathway led to VSMC senescence, whereas SO2 supplementation rescued the AAT1-knockdown-induced VSMC senescent phenotype. Our study further revealed that endogenous SO2 attenuated VSMC senescence by sulphenylating IRF1 at C83 and inhibiting its nuclear translocation and downstream SASP gene transcription. These findings provided new insights into the mechanism of VSMC senescence and revealed the role of SO2/AAT1 as endogenous regulators of vascular aging.
Endogenous SO2 exerts various cellular functions, including anti-inflammatory, anti-oxidative, anti-DNA damage and anti-apoptotic effects. Macrophage M1 polarization plays an important role in inflammation-related diseases. Macrophage-derived SO2 inhibits macrophage M1 polarization and the secretion of inflammatory cytokines (Zhu et al., 2020; Zhang et al., 2025). Mast cell-derived SO2 is responsible for inhibiting IgE-mediated and hypoxia-driven mast cell degranulation (Zhang et al., 2021; Song et al., 2024). Cardiomyocyte senescence is a key risk factor for the progression of cardiovascular diseases. Cardiomyocyte-derived SO2 deficiency leads to cardiomyocyte senescence, while SO2 donor supplementation can restore heart function and inhibit heart aging. SO2 sulphenylated STAT3 at C259 to inhibit its nuclear translocation and DNA binding ability, thereby inhibiting DNA damage and reducing cardiomyocyte senescence (Zhang et al., 2024). Our results suggested that VSMC-derived SO2 could attenuated VSMC senescence and vascular aging. These studies suggested that endogenous SO2 is an important molecule in maintaining body homeostasis.
Atherosclerosis and hypertension are typical age-related diseases, and VSMC senescence promotes their progression (Bos et al., 2012). SO2 supplementation could reduce the area of atherosclerotic plaques in the aorta and coronary arteries of atherosclerosis rats (Li et al., 2011; Tian et al., 2020). In addition, SO2 alleviated D-gal-induced hypertension and maintained vascular homeostasis by inhibiting the angiotensin II/angiotensin II type 1 receptor pathway (Dai et al., 2018). These studies suggested that SO2 was involved in the regulation of age-related vascular disease. However, the relationship between endogenous SO2 and vascular aging, especially VSMC senescence, remains unclear. In this study, we found that SO2/AAT1 pathway was downregulated in the aortic tissues of aged mice. VSMC cluster were identified as the main contributor of AAT1 downregulation in aortic tissue of aged mice by scRNA-seq analysis. However, according to dimensionality reduction and clustering analysis and AAT1 expression mapping, there were still immune cells-derived AAT1 upregulated. Due to data distribution dispersion, the expression of AAT1 in B cell, CD4+T cell, CD8+T cell and DC seemed to be upregulated in the aortic tissues of aged mice (86w group), but there was no significant difference. Moreover, the expression of AAT1 in mastocytes was upregulated in the aortic tissues of aged mice (86w group). Given that mastocytes is important immune cells in mediating vascular inflammation, upregulation of AAT1 in mastocytes might act as a compensatory role to antagonize vascular aging-related and inflammation. As expected, VSMC-AAT1 KO increased the level of Tp53, p21Cip/Waf and γ-H2AX and promoted VSMC senescence in aortic tissues. In addition, AAT1 knockdown in VSMCs promoted VSMC senescence with the increased Tp53/p21Cip/Waf protein levels, SA-β-gal activity, and SASP factors IL-1β and IL-6 mRNA levels. SO2 supplementation inhibits AAT1 knockdown-induced VSMC senescence. Furthermore, SO2 supplementation inhibited D-gal-induced Tp53 expression in the aortic rings. These findings suggested that downregulation of the endogenous SO2/AAT1 pathway was an important mechanism for VSMC senescence. Supplement of SO2 might be a potential strategy to inhibited VSMC senescence and aging related vascular disease, such as atherosclerosis and hypertension.
However, the mechanisms by which endogenous SO2 inhibits VSMC senescence remain unclear. Single-cell transcriptome analysis of aortic tissues of 4 and 86-week mice indicated that IRF1 might play an important role in VSMC senescence. Previous studies have shown that IRF1 plays an important role in innate and acquired immune responses (Zeng et al., 2024). In addition, IRF1 is involved in the regulation of cell cycle, cell migration, apoptosis, differentiation, and inflammation. Silencing IRF-1 can significantly inhibit the VSMC inflammatory response and extracellular matrix remodeling, proliferation, and migration (Du et al., 2019; Shen et al., 2020). Additionally, IRF-1 activation is a risk factor for atherosclerosis (Du et al., 2019; Shen et al., 2020; Fan et al., 2023; Qian et al., 2024). IRF-1 causes growth arrest in coronary artery smooth muscle cells mainly by arresting the coronary artery smooth muscle cell G1 cell cycle and inducing the upregulation of the CDK inhibitor p21Cip/Waf (Wessely et al., 2003). In addition, IRF1 activation promotes endothelial cells, fibroblast, and monocyte senescence (Upreti et al., 2010; Rossi et al., 2023). These studies suggest that inhibiting IRF1 signaling may prevent cellular senescence. In this study, we demonstrated that endogenous SO2 inhibited IRF1 transcription activity in a redox modification dependent manner. In detail, AAT1 knockdown increased IRF1-WT nuclear translocation but failed to promote nuclear translocation of IRF1 with C83S mutation, suggesting that sulphenylation of IRF1 at C83 was responsible for the inhibitory effect of SO2 on IRF1 nuclear translocation. Meanwhile, endogenous SO2 reduced the expression of IRF1-targeted senescence-associated factors, including IL-1β, IL-6, and p21Cip/Waf, indicating that the endogenous SO2/AAT1 pathway inhibited VSMC senescence in association with IRF1 activation.
Aging is a key independent risk factor of various vascular diseases, for which the post-translational modification-dependent mechanisms remain largely unknown. Previous studies have shown that SO2 exerts its cardiovascular protective effect through the sulphenylation of target proteins (Smad3, AAT, NF-kB p65, and CypD) at cysteine residues (Chen et al., 2017; Song et al., 2020; Huang et al., 2021; Lv et al., 2021). IRF1 is a DNA-binding transcription factor that interacts with DNA through its C83 residue (Antonczyk et al., 2019; Jefferies, 2019). Therefore, we hypothesized that SO2 might sulphenylate IRF1 at C83 to inhibit VSMC senescence. Our study confirmed that SO2 sulphenylates IRF1 at C83 in VSMCs. Notably, in IRF1-C83S plasmid-transfected VSMCs, neither AAT1 knockdown nor SO2 supplementation affected the expression of IRF1-targeted senescence-associated factors or the VSMC senescence phenotype, confirming that SO2 inhibits VSMC senescence by sulphenylating IRF1 at C83. Considering the important regulatory role of IRF1 in the immune system (Chavez et al., 2025; Rahlf and Tarakanova, 2025) and AAT1 upregulation in mastocytes, SO2-mediated IRF1 sulphenylation might play a critical regulatory role in regulating mastocytes-mediated vascular inflammation, as well as modulating the pathogenesis of atherosclerosis (Lavillegrand et al., 2024), pulmonary arterial hypertension (Zhang et al., 2021), and allergic diseases (Galli and Tsai, 2012), which require further study. However, this research still has limitations. Identification of IRF1 suphenylation using liquid chromatography coupled to tandem mass spectrometry merits study. Furthermore, the usage of a IRF1-C83S knock-in mouse line to investigate the role of IRF1 sulphenylation in VSMC senescence and vascular aging will be more conclusive.
This study demonstrated that downregulation of the endogenous SO2/AAT1 pathway significantly contributed to VSMC senescence. Endogenous SO2 mitigated VSMC senescence and vascular aging by sulphenylating IRF1 at C83. These findings provided new insights into the mechanisms underlying VSMC senescence and therapeutic strategies for VSMC senescence and vascular aging. Regulating IRF1 sulphenylation by supplementation of SO2 donors or AAT1 activators would be a promising approach to the development of new antagonistic therapies against aging and aging-related cardiovascular diseases.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The animal study was approved by the Animal Experimentation and Welfare Committee at Peking University First Hospital. The study was conducted in accordance with the local legislation and institutional requirements.
BQ: Conceptualization, Formal Analysis, Investigation, Writing–original draft, Writing–review and editing, Methodology. SZ: Conceptualization, Investigation, Methodology, Writing–original draft. SG: Software, Investigation, Writing–original draft. ZY: Software, Methodology, Writing–original draft. DW: Investigation, Supervision, Writing–original draft. KL: Investigation, Methodology, Writing–original draft. XY: Investigation, Methodology, Writing–original draft. CT: Conceptualization, Supervision, Writing–original draft. JD: Funding acquisition, Writing–review and editing, Supervision, Writing–original draft. HJ: Funding acquisition, Supervision, Writing–review and editing, Conceptualization, Writing–original draft. YH: Funding acquisition, Supervision, Writing–review and editing, Conceptualization, Writing–original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by National Natural Science Foundation of China (82170243, 81921001, 82070445), National Key Research and Development Program of China (2023YFC2706202), Beijing Natural Science Foundation (7191012, 7222188), National High Level Hospital Clinical Research Funding (State Key Laboratory of Vascular Homeostasis and Remodeling, Peking University), Changjiang Scholars Award Program and National Youth Top-Notch Talent Support Program.
We thank all contributors for their invaluable support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
VSMC, vascular smooth muscle cell; SO2, Sulfur dioxide; AAT, aspartate aminotransferase; IRF, interferon regulatory factor; SA-β-gal, senescence-associated β-galactosidase.
Abdellatif, M., Rainer, P. P., Sedej, S., and Kroemer, G. (2023). Hallmarks of cardiovascular ageing. Nat. Rev. Cardiol. 20 (11), 754–777. doi:10.1038/s41569-023-00881-3
Antonczyk, A., Krist, B., Sajek, M., Michalska, A., Piaszyk-Borychowska, A., Plens-Galaska, M., et al. (2019). Direct inhibition of IRF-dependent transcriptional regulatory mechanisms associated with disease. Front. Immunol. 10, 1176. doi:10.3389/fimmu.2019.01176
Bos, D., van der Rijk, M. J., Geeraedts, T. E., Hofman, A., Krestin, G. P., Witteman, J. C., et al. (2012). Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke 43 (7), 1878–1884. doi:10.1161/strokeaha.111.648667
Chavez, C., Lin, K., Malveaux, A., Gorin, A., Brizuela, S., Cheng, Q. J., et al. (2025). IRF1 cooperates with ISGF3 or GAF to form innate immune de novo enhancers in macrophages. Sci. Signal 18 (868), eado8860. doi:10.1126/scisignal.ado8860
Chen, S., Huang, Y., Liu, Z., Yu, W., Zhang, H., Li, K., et al. (2017). Sulphur dioxide suppresses inflammatory response by sulphenylating NF-κB p65 at Cys(38) in a rat model of acute lung injury. Clin. Sci. (Lond) 131 (21), 2655–2670. doi:10.1042/cs20170274
Cui, X. Y., Zhan, J. K., and Liu, Y. S. (2021). Roles and functions of antisense lncRNA in vascular aging. Ageing Res. Rev. 72, 101480. doi:10.1016/j.arr.2021.101480
Dai, J., Liu, R., Zhao, J., and Zhang, A. (2018). Sulfur dioxide improves endothelial dysfunction by downregulating the angiotensin II/AT(1)R pathway in D-galactose-induced aging rats. J. Renin Angiotensin Aldosterone Syst. 19 (2), 1470320318778898. doi:10.1177/1470320318778898
Du, M., Wang, X., Mao, X., Yang, L., Huang, K., Zhang, F., et al. (2019). Absence of interferon regulatory factor 1 protects against atherosclerosis in apolipoprotein E-deficient mice. Theranostics 9 (16), 4688–4703. doi:10.7150/thno.36862
Fan, X., Li, Q., Wang, Y., Zhang, D. M., Zhou, J., Chen, Q., et al. (2023). Non-canonical NF-κB contributes to endothelial pyroptosis and atherogenesis dependent on IRF-1. Transl. Res. 255, 1–13. doi:10.1016/j.trsl.2022.11.001
Galli, S. J., and Tsai, M. (2012). IgE and mast cells in allergic disease. Nat. Med. 18 (5), 693–704. doi:10.1038/nm.2755
Huang, Y., Li, Z., Zhang, L., Tang, H., Zhang, H., Wang, C., et al. (2021). Endogenous SO(2)-dependent Smad3 redox modification controls vascular remodeling. Redox Biol. 41, 101898. doi:10.1016/j.redox.2021.101898
Huang, Y., Zhang, H., Lv, B., Tang, C., Du, J., and Jin, H. (2022). Sulfur dioxide: endogenous generation, biological effects, detection, and therapeutic potential. Antioxid. Redox Signal 36 (4-6), 256–274. doi:10.1089/ars.2021.0213
Jefferies, C. A. (2019). Regulating IRFs in IFN driven disease. Front. Immunol. 10, 325. doi:10.3389/fimmu.2019.00325
Lavillegrand, J. R., Al-Rifai, R., Thietart, S., Guyon, T., Vandestienne, M., Cohen, R., et al. (2024). Alternating high-fat diet enhances atherosclerosis by neutrophil reprogramming. Nature 634 (8033), 447–456. doi:10.1038/s41586-024-07693-6
Li, W., Tang, C., Jin, H., and Du, J. (2011). Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis 215 (2), 323–330. doi:10.1016/j.atherosclerosis.2010.12.037
Li, Y., Feng, Y., Ye, X., Peng, H., Du, J., Yao, X., et al. (2021). Endogenous SO(2) controls cell apoptosis: the state-of-the-art. Front. Cell Dev. Biol. 9, 729728. doi:10.3389/fcell.2021.729728
Lin, M. J., Hu, S. L., Tian, Y., Zhang, J., Liang, N., Sun, R., et al. (2023). Targeting vascular smooth muscle cell senescence: a novel strategy for vascular diseases. J. Cardiovasc Transl. Res. 16 (5), 1010–1020. doi:10.1007/s12265-023-10377-7
Lv, B., Peng, H., Qiu, B., Zhang, L., Ge, M., Bu, D., et al. (2021). Sulphenylation of CypD at cysteine 104: a novel mechanism by which SO(2) inhibits cardiomyocyte apoptosis. Front. Cell Dev. Biol. 9, 784799. doi:10.3389/fcell.2021.784799
Okuno, K., Cicalese, S., Elliott, K. J., Kawai, T., Hashimoto, T., and Eguchi, S. (2020). Targeting molecular mechanism of vascular smooth muscle senescence induced by angiotensin II, A potential therapy via senolytics and senomorphics. Int. J. Mol. Sci. 21 (18), 6579. doi:10.3390/ijms21186579
Qian, Z., Shaofang, F., Chen, C., Chunhua, S., Nan, W., and Chao, L. (2024). IL-33 suppresses the progression of atherosclerosis via the ERK1/2-IRF1-VCAM-1 pathway. Cardiovasc Drugs Ther. 38 (3), 569–580. doi:10.1007/s10557-023-07523-3
Rahlf, C. R., and Tarakanova, V. L. (2025). Role of Interferon Regulatory Factor 1 in acute and chronic virus infections. Virology 603, 110386. doi:10.1016/j.virol.2024.110386
Rossi, M., Anerillas, C., Idda, M. L., Munk, R., Shin, C. H., Donega, S., et al. (2023). Pleiotropic effects of BAFF on the senescence-associated secretome and growth arrest. Elife 12, e84238. doi:10.7554/eLife.84238
Shen, Y., Sun, Z., Mao, S., Zhang, Y., Jiang, W., and Wang, H. (2020). IRF-1 contributes to the pathological phenotype of VSMCs during atherogenesis by increasing CCL19 transcription. Aging (Albany NY) 13 (1), 933–943. doi:10.18632/aging.202204
Song, J., Zheng, J., Li, Z., Fu, L., Yang, J., Li, K., et al. (2024). Sulfur dioxide inhibits mast cell degranulation by sulphenylation of galectin-9 at cysteine 74. Front. Immunol. 15, 1369326. doi:10.3389/fimmu.2024.1369326
Song, Y., Peng, H., Bu, D., Ding, X., Yang, F., Zhu, Z., et al. (2020). Negative auto-regulation of sulfur dioxide generation in vascular endothelial cells: AAT1 S-sulfenylation. Biochem. Biophys. Res. Commun. S0006-291, 231–237. doi:10.1016/j.bbrc.2020.02.040
Suryadevara, V., Hudgins, A. D., Rajesh, A., Pappalardo, A., Karpova, A., Dey, A. K., et al. (2024). SenNet recommendations for detecting senescent cells in different tissues. Nat. Rev. Mol. Cell Biol. 25 (12), 1001–1023. doi:10.1038/s41580-024-00738-8
Tian, X., Zhang, Q., Huang, Y., Chen, S., Tang, C., Sun, Y., et al. (2020). Endothelin-1 downregulates sulfur dioxide/aspartate aminotransferase pathway via reactive oxygen species to promote the proliferation and migration of vascular smooth muscle cells. Oxid. Med. Cell Longev. 2020, 9367673. doi:10.1155/2020/9367673
Ungvari, Z., Tarantini, S., Sorond, F., Merkely, B., and Csiszar, A. (2020). Mechanisms of vascular aging, A geroscience perspective: JACC focus seminar. J. Am. Coll. Cardiol. 75 (8), 931–941. doi:10.1016/j.jacc.2019.11.061
Upreti, M., Koonce, N. A., Hennings, L., Chambers, T. C., and Griffin, R. J. (2010). Pegylated IFN-α sensitizes melanoma cells to chemotherapy and causes premature senescence in endothelial cells by IRF-1 mediated signaling. Cell Death Dis. 1 (8), e67. doi:10.1038/cddis.2010.43
Wessely, R., Hengst, L., Jaschke, B., Wegener, F., Richter, T., Lupetti, R., et al. (2003). A central role of interferon regulatory factor-1 for the limitation of neointimal hyperplasia. Hum. Mol. Genet. 12 (2), 177–187. doi:10.1093/hmg/ddg018
Xie, W., Ke, Y., You, Q., Li, J., Chen, L., Li, D., et al. (2022). Single-cell RNA sequencing and assay for transposase-accessible chromatin using sequencing reveals cellular and molecular dynamics of aortic aging in mice. Arterioscler. Thromb. Vasc. Biol. 42 (2), 156–171. doi:10.1161/atvbaha.121.316883
Zeng, C., Zhu, X., Li, H., Huang, Z., and Chen, M. (2024). The role of interferon regulatory factors in liver diseases. Int. J. Mol. Sci. 25 (13), 6874. doi:10.3390/ijms25136874
Zha, Y., Zhuang, W., Yang, Y., Zhou, Y., Li, H., and Liang, J. (2022). Senescence in vascular smooth muscle cells and atherosclerosis. Front. Cardiovasc Med. 9, 910580. doi:10.3389/fcvm.2022.910580
Zhang, H., Lv, B., Liu, K., Du, J., Jin, H., and Huang, Y. (2025). Sulfur dioxide controls M1 macrophage polarization by sulphenylation of prolyl hydroxylase 2 at cysteine 260. Free Radic. Biol. Med. 230, 33–47. doi:10.1016/j.freeradbiomed.2025.01.054
Zhang, L., Jin, H., Song, Y., Chen, S. Y., Wang, Y., Sun, Y., et al. (2021). Endogenous sulfur dioxide is a novel inhibitor of hypoxia-induced mast cell degranulation. J. Adv. Res. 29, 55–65. doi:10.1016/j.jare.2020.08.017
Zhang, S., Qiu, B., Lv, B., Yang, G., Tao, Y., Hu, Y., et al. (2024). Endogenous sulfur dioxide deficiency as a driver of cardiomyocyte senescence through abolishing sulphenylation of STAT3 at cysteine 259. Redox Biol. 71, 103124. doi:10.1016/j.redox.2024.103124
Zhao, W., Zhang, X., and Rong, J. (2021). SUMOylation as a therapeutic target for myocardial infarction. Front. Cardiovasc Med. 8, 701583. doi:10.3389/fcvm.2021.701583
Zhou, D., Zhang, Y., Du, J., Jin, H., Tang, C., and Huang, Y. (2020). Sulfur dioxide: an endogenous protector against myocardial injury. J. Cardiovasc Pharmacol. 76 (4), 389–396. doi:10.1097/fjc.0000000000000882
Zhu, M., Du, J., Liu, A. D., Holmberg, L., Tang, C., and Jin, H. (2014). Effect of endogenous sulfur dioxide in regulating cardiovascular oxidative stress. Histol. Histopathol. 29 (9), 1107–1111. doi:10.14670/hh-29.1107
Zhu, Z., Zhang, L., Chen, Q., Li, K., Yu, X., Tang, C., et al. (2020). Macrophage-derived sulfur dioxide is a novel inflammation regulator. Biochem. Biophys. Res. Commun. 524 (4), 916–922. doi:10.1016/j.bbrc.2020.02.013
Zou, L., Yang, Y., Wang, Z., Fu, X., He, X., Song, J., et al. (2023). Lysine malonylation and its links to metabolism and diseases. Aging Dis. 14 (1), 84–98. doi:10.14336/ad.2022.0711
Zou, R., Shi, W., Tao, J., Li, H., Lin, X., Yang, S., et al. (2018). SIRT5 and post-translational protein modifications: a potential therapeutic target for myocardial ischemia-reperfusion injury with regard to mitochondrial dynamics and oxidative metabolism. Eur. J. Pharmacol. 818, 410–418. doi:10.1016/j.ejphar.2017.11.005
Keywords: endogenous SO2, VSMC senescence, IRF1, sulphenylation, cysteine, post-translational modification
Citation: Qiu B, Zhang S, Ge S, Yu Z, Wang D, Li K, Yu X, Tang C, Du J, Jin H and Huang Y (2025) Vascular smooth muscle cell-derived SO2 sulphenylated interferon regulatory factor 1 to inhibit VSMC senescence. Front. Pharmacol. 16:1516885. doi: 10.3389/fphar.2025.1516885
Received: 25 October 2024; Accepted: 19 March 2025;
Published: 28 March 2025.
Edited by:
Suman Dalal, East Tennessee State University, United StatesReviewed by:
Katsuya Hirano, Faculty of Medicine, Kagawa University, JapanCopyright © 2025 Qiu, Zhang, Ge, Yu, Wang, Li, Yu, Tang, Du, Jin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junbao Du, anVuYmFvZHUxQDEyNi5jb20=; Hongfang Jin, amluaG9uZ2Zhbmc1MUAxMjYuY29t; Yaqian Huang, eWFxaWFuaHVhbmdAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.