- 1Shandong Academy of Occupational Health and Occupational Medicine, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, Shandong, China
- 2Queensland Alliance for Environmental Health Sciences, University of Queensland, Brisbane, QLD, Australia
Silicosis is an important occupational lung disease caused by exposure to respirable crystalline silica dust particles, with the clinical manifestations from asymptomatic forms to respiratory failure. The main pathological process involves parenchymal lung injury, inflammation and lung tissue fibrosis, but the exact pathogenesis remains elusive. Until now, there have been no effective treatments for silicosis due to the complexity of pathogenesis and irreversibility of pulmonary fibrosis. In this review we attempt to summarize the advances in pathogenesis and treatment of silicosis and to explore the current understanding of the molecular mechanisms involving in the initiation and development of silicosis and potential therapeutic targets.
1 Introduction
Silicosis, a fibrotic lung disease caused by the long-term inhalation of the dust containing silicon dioxide (SiO2) in occupational activities (Wang M. et al., 2022), has been a major occupational disease in many developing countries and resurged recently in some developed countries (GBD 2013 Mortality and Causes of Death Collaborators, 2015) It is reported that there are 3 million workers in Europe (Kauppinen et al., 2000) and 1.7 million workers in the United States (Li et al., 2002) exposed to silica dust. In China, occupational pneumoconiosis is the most serious occupational disease. Totally 171,291 cases of silicosis have been documented up to 2021, representing 78.58% of all pneumoconiosis cases (Liu et al., 2024). Therefore, silicosis has caused a heavy burden in developing countries and seriously threatened the quality of life (QOL) of workers.
According to the exposure period and pathological progress, silicosis is categorized as acute, accelerated and chronic types (Wang M. et al., 2022; Aloe et al., 2022). Acute silicosis mainly manifests as silico proteinosis, typically occurring within a few weeks to years following exposure to high concentrations of SiO2 (Krefft et al., 2020). Based on the chest imaging, chronic silicosis can be divided into simple and complicated subtypes (Quan et al., 2022). Complicated silicosis, also known as progressive macro fibrosis, is diagnosed by the presence of an International Labour Organization-classified macro mixed image on a chest radiograph or an aggregated chest high-resolution computed tomography (HRCT) image, usually accompanying by calcification and fibrosis (Quan et al., 2022; Cox et al., 2014). The onset of accelerated silicosis is within 5–10 years of exposure to dust, but the disease progresses more rapidly than chronic silicosis. In recent years, accelerated and acute silicosis has attracted more attentions, but the risk factors and molecular pathogenesis remain elusive (Adamcakova and Mokra, 2021).
Silicosis is characterized by persistent inflammation and progressive fibrosis in the lung, which may cause breath difficulty and respiratory failure, even death. Therefore, the treatment strategies using drugs are mainly to block fibrotic factors, reduce lung inflammation, inhibit the proliferation and activation of interstitial cells, and regulate the synthesis and degradation of extracellular matrix (ECM), thereby reducing the lung tissue injury and formation of pulmonary fibrosis (Pang et al., 2019; Liu et al., 2016). Current prescribed drugs for silicosis include pirfenidone, poly-2-vinylpyridine-N-oxide (PVNO), nintedanib, tetrandrine, etc. (Pang et al., 2019; Ernst et al., 2002). With the understanding of the molecular mechanisms underlying silicosis, more and more signal pathways have been identified and proposed as the potential therapeutic targets.
As a surgical treatment, bronchoalveolar lavage and whole lung lavage (WLL) are effective ways to relive the symptoms of silicosis by removing the residual silica in the alveolar cavity (Cottin et al., 2004). However, the actual therapeutic effect of this method needs to be confirmed through long-term follow-up and documentation. Although lung transplantation can be used for patients with advanced silicosis, it is hard to be widely applied due to the difficulty of donor source and high cost (George et al., 2019). In recent decades, great progress has been made in stem cell technology which may be the new and promising means for silicosis after surgical and drug treatments (Chen et al., 2018a).
The aim of this article is to review the recent progress in understanding the molecular pathogenesis of silicosis, current treatment protocols, and potential therapeutic options.
2 Toxicity of silica dust
Free SiO2 particles or respirable crystalline silica (RCS) dust particles with the diameter less than 5 μm could directly produce cytotoxicity. The degree of lung injury caused by silica dust mainly depends on the physical/chemical properties and toxicities, as well as the exposure time and intensity. Silica, also called SiO2, has a crystalline or non-crystalline (amorphous) structure (Lee et al., 2020). In crystalline silica, silicon atoms and oxygen atoms are arranged in a fixed geometric pattern. In contrast, there is no spatial order of atoms in amorphous silicon (Woźniak and Wiecek, 1995). It has been well documented that exposure to silica dust can cause tissue injury, inflammation, and pulmonary fibrosis. Epidemiological and laboratory studies showed that exposure to silica dust could cause lung cancer and tuberculosis (Wang D. et al., 2020). Amorphous silica is considered less toxic than crystalline silica (Rubio et al., 2019) classified as group A carcinogens by the International Agency for Research on Cancer (Guha et al., 2011; Peters et al., 2017), although its carcinogenicity to humans has not been confirmed.
In recent decades, artificial or engineered stones have been increasingly popular materials used to fabricate kitchen and bathroom bench tops. Exposure to RCS from artificial stones can increase the risk of silicosis in developed countries, such as Italy, Spain, Israel and United States (Kramer et al., 2012; Pérez-Alonso et al., 2014; Paolucci et al., 2015). Compared with traditional silicosis, artificial stone silicosis is more aggressive because of the high content of silica (90%) in the RCS (Ophir et al., 2016). Studies on the dust from artificial stones further confirmed the importance of the physical/chemical properties in their toxicities and consequent silicotic potency. It has been found that the higher contents of SiO2 in the dust are, the more toxic to the lung tissue is Lee et al. (2020); the higher dispersion of silica dust particles and the larger proportion of fine particles are, the greater amount of silica dust entering the deep respiratory tract is Fernández Álvarez et al. (2015).
RCS can penetrate deep into the lung with various biological activities. Silica particles with a diameter of less than 3.2 μm account for 100% of the lung tissue in silicosis patients. The silanol groups on the surface of silica dust can form hydrogen chains with secondary lysosomal membrane proteins in macrophages, which can increase the membrane permeability, reduce fluidity, and promote the membrane lysis (Goumans et al., 2008). A subfamily of silanol “nearly free silanol” is the major determinant of silica-induced toxicity through interaction with the cell membrane or phagolysosome promoting membranolysis (Pavan et al., 2020). In addition, the silica dust can directly and indirectly induce reactive oxygen species (ROS) in alveolar macrophages (AMs) to activate the cell-signaling pathways launching cytokine release and apoptosis (Hamilton et al., 2008). The human body mainly clears the dust in the respiratory tract through the ciliary oscillation of the ciliary adhesive device and the phagocytosis of AMs. However, long-term inhalation of dust will reduce the body’s defense function, which can lead to excessive deposition of dust to damage the lung tissue, thereby causing the disease (Wildung et al., 2022; Noël et al., 2020).
3 The pathogenesis of silicosis
Although great efforts have been made to understand the pathogenesis of silicosis, the molecular mechanism underlying the initiation and development of the disease remains to be elucidated. The pathological process is complex and involves multiple cell types, cytokines and pathways. To simplify the complexity, we divide this process into different stages, including parenchymal lung injury, inflammatory response, and pulmonary fibrosis (Krefft et al., 2020; Kawasaki, 2015) (Figure 1). It should be noted that in real situations, these stages are not separated but overlapped and interactive.
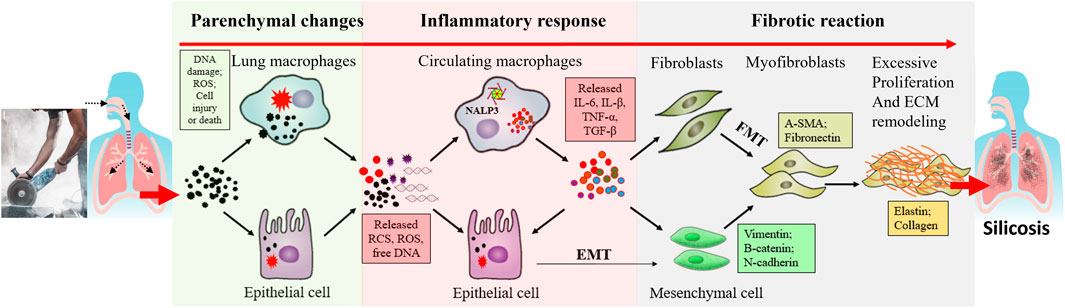
Figure 1. A general schematic diagram describing the different stages of silicosis. Silica dust exposure can activate macrophages, damage alveolar epithelial cells and then initiate inflammation reactions. These inflammatory factors induce multiple signaling pathways, such as TGF-β/Smad and TNF-α signaling pathways. As epithelial cells are damaged, they undergo autophagy and apoptosis. Repeated inflammation and cell death promote the formation of fibroblasts and myofibroblasts, produce lots of extracellular matrices, accelerate the release of fibroblasts and epithelial-mesenchymal transition process, thus resulting in the destruction of alveolar structures and fibrosis.
3.1 Parenchymal lung injury
The human respiratory system has a strong ability to defend and remove dust through multi-level defense (de Lima Gondim et al., 2019). The initial line of defense consists of the retention function of nasal hairs and passages, which can block and trap larger particles. The second line of defense is provided by the excretory action of the mucosal ciliary system within the epithelium of the respiratory tract. The mucosal epithelium contains mucus and cilia supported by ciliated cells, which can rapidly swing toward the larynx and transport mucus and attached particles to the pharynx, where they are ultimately expelled from the body through coughing or swallowing. This mechanism effectively removes the particles with diameters ranging from 1 to 5 microns. The final line of defense is established through the phagocytosis performed by lung macrophages. These macrophages, along with other immune cells, are capable of engulfing and digesting smaller particulate matter, particularly those smaller than 2.5 microns. They are present in abundance in the alveoli and function as scavengers (Aloe et al., 2022; Su Y. et al., 2020; Fèvre et al., 2022; Nardi et al., 2018). As the resident cells in lung tissues, AMs play a major role in maintaining immune homeostasis and host defense (Cui et al., 2022), and is the critical line of defense against silica dust. AMs are derived from bone marrow mononuclear cells, with the functions of engulfing foreign bodies, anti-infection, regulating inflammatory and immune responses (Hou et al., 2019). Silica dust is recognized by AMs mainly through class A and B1 scavenger receptors, and then swallowed by AMs (Hou et al., 2019; Yang et al., 2020). After entering the alveoli, silica dust induces the aggregation and phagocytosis of AMs. Moreover, the intracellular silica dust can also damage the phagosomes and other organelles, such as endosomes, lysosomes, endolysosomes, even DNA, thus inducing cell injury and necrosis or apoptosis (Hou et al., 2019; Yang et al., 2020). After cell death, intracellular silica particles are released together with intracellular enzymes, leading to further cell death and tissue damage (Gao et al., 2020). The above process is caught in a vicious circle. Meanwhile, the secretion of inflammatory mediators activated by AMs can lead to the recruitment of peripheral blood monocytes, which subsequently differentiates into macrophages. Some of these monocytes migrate into the lymph nodes through lymphatic vessels, while others infiltrate the pulmonary interstices (Kawasaki, 2015; Wang et al., 2016; Mu et al., 2020). In addition, silica dust-induced ROS can also result in mitochondrial dysfunction, forcing AMs to undergo mitochondrial apoptosis (Aloe et al., 2022; Nardi et al., 2018).
Inhaled silica dust can affect alveolar epithelial cells by triggering an inflammatory response mediated by AMs. This response leads to degeneration, swelling, and shedding of alveolar epithelial type I (ATI) cells, ultimately resulting in an incomplete alveolar structure (Fang et al., 2018). Alveolar epithelial type II (ATII) cells with the stem cell properties can replenish the lost ATI cells through differentiation and proliferation to restore the alveolar integrity (Wang et al., 2021). If ATII cells are unable to fill the loss timely, the underlying basement membrane of ATI cells will be compromised, exposing the matrix and triggering fibroblast proliferation (Fang et al., 2018).
Multiple proteins and genes are involved in the regulation of parenchymal lung injury. Cathepsin participates in fibrosis by degrading the alveolar basement membrane and regulating immune response (Xu et al., 2012). Toll-like receptor 4 (TLR4) is involved in immune response through multiple pathways. Zanoni et al. demonstrated that CD14 was required for TLR4 endocytosis to activate the downstream signaling (Zanoni et al., 2011). The CD14 core focusing defect could inhibit TLR4 endocytosis and impair TLR4 signaling pathways in mouse embryonic fibroblasts (Iijima et al., 2017). This receptor was also found to be critical for the release of pro-inflammatory cytokines and for promoting the deposition of collagens and fibronectins after bleomycin exposure (Razonable et al., 2006; Yang et al., 2009). Therefore, TLR4 plays a key role in the release of inflammatory factors, which provides a basis for the formation of silicotic nodules and pulmonary fibrosis. CLDN5 is an indicator of endothelial tight junctions and permeability, and its downregulation is associated with disruption of endothelial tight junctions in bleomycin-induced pulmonary fibrosis and may be involved in epithelial-mesenchymal transition (EMT) (Zhang et al., 2015). Furthermore, TGF-β also disrupts alveolar epithelial and endothelial tight junctions by downregulating CLDN5 expression (Ohta et al., 2012). ROS is mainly produced by NADPH oxidase (NOX) in AMs. Choi et al. identified that the subunits of NOX complex, such as NOX2 (gp91phox), P22phox, P47phox, P40phox, and P67phox, were upregulated in silicosis rats. ROS destroyed pulmonary endothelial integrity and increased vascular permeability through activation of P38 MAPK signaling pathway (Choi et al., 2019) (Figure 2, tissue retention and cell injury/death).
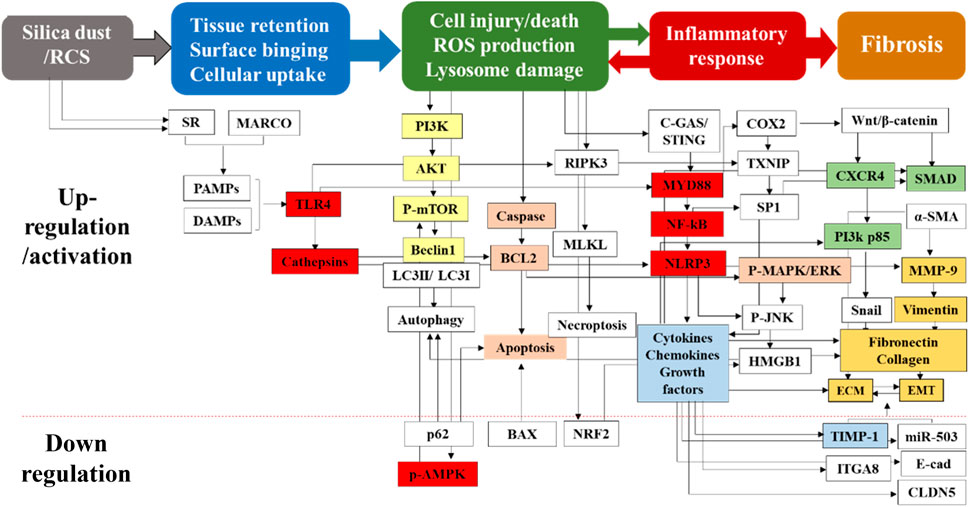
Figure 2. Multiple proteins and genes participate in the pathological process of silicosis. Silica-induced cell and tissue damage is the initial but critical stage for the pathogenesis of silicosis. Moreover, silica dust can activate the apoptosis signaling pathways of AMs and induce cell apoptosis. It can also act on alveolar epithelial cells to cause degeneration, swelling and shedding of alveolar epithelial type I cells, resulting in incomplete alveolar structures. Multiple proteins and genes are involved in above stages. This figure is explained in the part 3 (3. The pathogenesis of silicosis) and part 4.1 (4.1. Drug treatment).
3.2 Inflammatory response
Silica dust enters the alveoli and interstitium of the lungs, producing toxic effects on the tissues. This exposure activates macrophages, which subsequently interact with alveolar epithelial cells to cause cellular dysfunction even cell death, thereby initiating the inflammatory response. This process represents the primary link in the development of pulmonary fibrosis. The continuous phagocytosis and release of silica dust by macrophages constitute a critical event that contributes to the amplification of inflammation and initiation of pulmonary fibrosis. It is believed that the phagocytosis and consequent lysosomal damage and rupture, ROS, as well as cell injury and death can trigger activation of nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein 3 (NLRP3) in AMs, which plays an essential role in silica dust-induced inflammatory response (Hamilton et al., 2008; Li Z. et al., 2021). NLRP3, which is composed of a C-terminal leucine-rich repeat domain, a central NACHT domain and an N-terminal pyrindomain (Shen et al., 2021), plays a fundamental role in various inflammation-related diseases, including diabetes, atherosclerosis, metabolic syndrome, cardiovascular and neurodegenerative diseases (Adamcakova and Mokra, 2021; Zahid et al., 2019). Activation of NLRP3 primarily involves two steps: priming or initiating step and oligomerization of NLRP3, as well as subsequent assembly of inflammasomes in which NLRP3 inflammasome binds to an adaptor protein (apoptosis-associated speck-like protein with a caspase recruit domain) to activate the caspase-1 (Yang et al., 2022). When caspase-1 is activated, immature, pro-forms of interleukins (IL)-1β, IL-18, and IL-33 are cleaved to mature and active forms, thereby facilitating various inflammatory processes, including fever, T-cell survival extension, B-cell proliferation, mediation of leukocyte transmigration, etc. (Sims and Smith, 2010). These chemokines and cytokines directly or indirectly cause acute and chronic inflammation, and meanwhile the latter can also induce a variety of particle- and fiber-related lung and pleural diseases (Sayan and Mossman, 2016). Another important function of inflammasomes is the induction of caspase-1-dependent pyroptosis, a highly inflammatory type and programmed cell death characterized by apoptotic and necrotic features (Stegmann et al., 2015). However, the role of pyroptosis in the initiation and development of silicosis remains to be fully understood.
For activation of NLRP3 inflammasome, a priming step is required through activation of pathogen-associated molecular patterns, damage-associated molecular patterns (DAMPs) or TLRs via phosphorylation and subsequent activation of NF-κB, which promotes the transcription of NLRP3, proIL-1β, and proIL-18 in the nucleus. Environmental factors such as silica particles may directly act as exogenous DAMPs, or indirectly as endogenous DMAPs through ROS generation to initiate the activation of NLRP3 (Land, 2020). A recent study has shown that the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) mediates the silica dust-induced activation of NLRP3 inflammasome (Benmerzoug et al., 2018). The lysosome proteases and DNA fragments released from injured and dead AMs or lung epithelial cells are sensed by cGAS/STING, consequently modulating the NALP3 inflammasome and subsequent events (Benmerzoug et al., 2018) (Figure 2, Inflammatory response).
Macrophages can undergo remarkable functional plasticity. In the pathological process of silicosis, macrophages are activated as M1-type macrophages, which release a large number of cytokines and chemokines, leading to neutrophil infiltration and activation of innate immune system (Kawasaki, 2015; Zhao et al., 2020). Meanwhile, macrophages act as antigen-presenting cells to mediate cellular immune activation, promote initial T lymphocytes in peripheral lymph nodes into effector T cells, and migrate to the inflammatory site to secrete Thl type of cytokines such as IL-2, IFN-γ and tumor necrosis factor α (TNF-α), thereby aggravating inflammatory damage (Hou et al., 2019). M1 pro-inflammatory cells contribute to infection clearance, and M2 anti-inflammatory cells have a reparative phenotype and contribute to the resolution phase of response to injury (Tang et al., 2019). M1-type macrophages gradually transform into the M2-type, releasing cytokines such as inhibitors of metalloproteinases, TGF-β and platelet-derived growth factors (PDGF) that play an anti-inflammatory and fibrogenic roles (Kawasaki, 2015). Meanwhile, effector T lymphocytes gradually transform to secrete Th2 cytokines like IL-4 and IL-10, which mainly regulate the fibrotic response.
Notably, in the early stage of silicosis, appropriate apoptosis of AMs can remove the damaged cells, reshape the lung tissue structure, and inhibit inflammation, but this effect disappears in the later stage due to excessive apoptosis (Hou et al., 2019; Yang et al., 2020). Therefore, AMs have the dual effects of defense and inducing fibrotic lung injury. Intracellular silica dust can trigger the release of inflammatory factors via the innate immune system, including IL-1β, TNF-α and transforming growth factor β (TGF-β) (de Lima Gondim et al., 2019). Furthermore, multiple pathways are also induced, such as TGF-β/Smad signaling pathway, NF-κB signaling pathway, etc. (Pang et al., 2021).
Lung epithelial cells play a key role in triggering inflammatory response and promoting remodeling of the lung tissue (Huang et al., 2022). Silica particles can destroy alveolar structures by stimulating alveolar epithelial cells, leading to the release of pro-inflammatory cytokines and recruitment of various inflammatory cells (Mercer et al., 2009). The activation of TLR in epithelial cells may be the trigger for epithelial-induced recruitment of immune cells to the lung (Cho et al., 2022). Importantly, the chemokine response in the lung epithelium can be reinforced by macrophage-derived inflammatory mediators in a synergistic way (Barrett et al., 1998).
3.3 Fibrotic changes in the lung
The key pathological change of silicosis is the progressive and irreversible pulmonary fibrosis. During the repetitive inflammatory response, AMs, interstitial and recruited microphages can secrete high levels of cytokines, chemokines and growth factors. Some of the inflammatory mediators can also be fibrogenic factors causing the dysfunction and destruction of epithelium and consequent fibrosis (Ma X. et al., 2020). One of the hallmark changes is EMT, a process in which epithelial cells gradually transform into mesenchymal-like cells and lose the epithelial functions and characteristics (Zhu et al., 2022). Normally, EMT is an important and irreversible process for tissue organization during embryonic development, its dysregulation is associated with a variety of diseases including cancer and fibrosis (Scott et al., 2019). ATII cells play an important role in lung injury through the synthesis and secretion of pulmonary surfactants and conversion into ATI cells as an alveolar repair (Olajuyin et al., 2019). However, due to the cell death and inflammation by silica dust, the differentiation of ATII cells into ATI cells is inhibited, and ATII cells can be transformed into mesenchymal cells or have mesenchymal characteristics, producing extra ECM (Fang et al., 2018; Wang et al., 2021). Normally, ECM provides a supportive environment for cell functions and communications, thus influencing the adhesin, migration and proliferation (Peng et al., 2022).
However, the ECM in the fibrotic lung tissue has abnormal biochemical and biomechanical characteristics, leading to increased hardness of the lung tissue and decreased elasticity, thereby reducing lung function (Aydemir et al., 2022). EMT is influenced and regulated by the surrounding ECM (Scott et al., 2019). Therefore, silica particle-induced aberrant EMT and ECM and their interactions cooperatively induce tissue remodeling. A recent study has shown that EMT, a direct contributor to the fibroblasts transforming into myofibroblasts (Li et al., 2016), is considered the main effector cells in silicosis (Li S. et al., 2019). Myofibroblasts are derived from lung intrinsic fibroblasts, bone marrow fibroblasts, or other mesenchymal cells under the action of cell growth factors (Li et al., 2018). TGF-β1 plays an important role in the trans-differentiation of all the above cell types (Piera-Velazquez et al., 2016; Chen et al., 2011). Besides, myofibroblasts are contractile and can synthesize smooth muscle actins including α-SMA, and their contraction can lead to the structural deformation of lung parenchyma (Leask, 2010; Wang R. et al., 2022). In the normal repair process, the ECM protein will be degraded after eliminating the damage and inflammation, thereby restoring the normal structure of the tissue. However, in the pathological process of silicosis, myofibroblasts can secrete a large amount of ECM and deposit at the injured site (Liu et al., 2017) (Figure 2, Fibrosis).
EMT is regulated by multiple growth factors, ILs, and inflammatory mediators, among which TGF-β has been the most studied growth factor as a central mediator of tissue fibrosis and an inducer of EMT (Pardali et al., 2017). TGF-β receptors can activate Smad-dependent and Smad-independent pathways. In the Smad-dependent pathway, type II TGF-β receptors are activated by ligands and phosphorylate type I TGF-β receptors to form the SMAD complex, which then enters the nucleus and subsequently activates or inhibits the important transcription factors for EMT (Nguyen et al., 2018). In Smad-independent pathway, PI3K/AKT pathway is activated, and PI3K-activated mTORC2 has been identified to be one of the drivers for the phenotypic transformation of EMT. It has been found that inhibition of AKT leads to downregulation of intracellular SNAIL activity and inhibition of EMT (King et al., 2015). In addition, TGF-β-induced activation of the Ras-Erk MAPK pathway, p38 MAPK and JNK signaling, Rho GTPase signaling, as well as PI3K/AKT pathways is all shown to contribute to EMT (Pardali et al., 2017; Gonzalez and Medici, 2014). Although EMT is necessary for proper re-epithelialization and ECM deposition, an uncontrolled, continuous transition from epithelial cells to myofibroblasts can result in fibrosis (Figure 3).
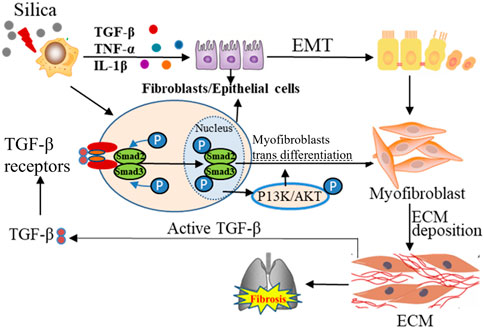
Figure 3. The dynamic interplay between the lung ECM and resident cells, with TGF-β1 as the center. The active TGF-β1 protein in red is attached to the ECM. The activation of this receptor results in the phosphorylation of SMAD2/3, causing its translocation to the nucleus, consequently resulting in the differentiation of the cells into myofibroblasts. The cyclical relationship between TGF-β1 activation via myofibroblasts and the resulting myofibroblast differentiation can be visualized through the arrows.
TGF-β1 is also reported to mediate the activation and differentiation of myofibroblasts (Chen et al., 2022). Aberrant ECM mechanical force can induce the release of TGF-β1, further promoting the fibrosis (Upagupta et al., 2018). Recent studies have indicated that the TGF-β signaling pathway can promote the occurrence and development of pulmonary fibrosis by regulating the expression of non-coding RNA molecules and epigenetic modification, which can be the potential target for pulmonary fibrosis (Zhang et al., 2021a; Bartczak et al., 2020).
The occurrence and development of silicosis involves multiple signal pathways, including TGF-β, Wnt/β-catenin, Notch and AMP-activated protein kinase (AMPK) pathways, etc. (Feng et al., 2019). Wnt/β-catenin signaling pathway can inhibit the lung fibroblast apoptosis, promote cell proliferation and differentiation (Ma J. et al., 2020). It can also promote ECM deposition by inhibiting glycogen synthase kinase 3-mediated phosphorylation and β-catenin degradation (Gonzalez and Medici, 2014). In addition, the Wnt/β-catenin signaling pathway can cooperate with TGF-β1 to induce the deposition of ECM, thus promoting the production of extracellular matrix metalloproteinase inducers and variations in fibroblast activity. Activation of the Notch pathway could promote the expression of collagens and α-SMA in alveolar epithelial cells (Martins et al., 2020). During the process of EMT, Notch/CSL activation could stimulate the expression of α-SMA in vascular smooth muscle cells. In addition to directly regulating the differentiation of myofibroblasts, Notch could also interact with other signaling (Wnt, TGF-β, PDGF, etc.) pathways to regulate the pulmonary fibrosis (Figure 4) (Feng et al., 2019).
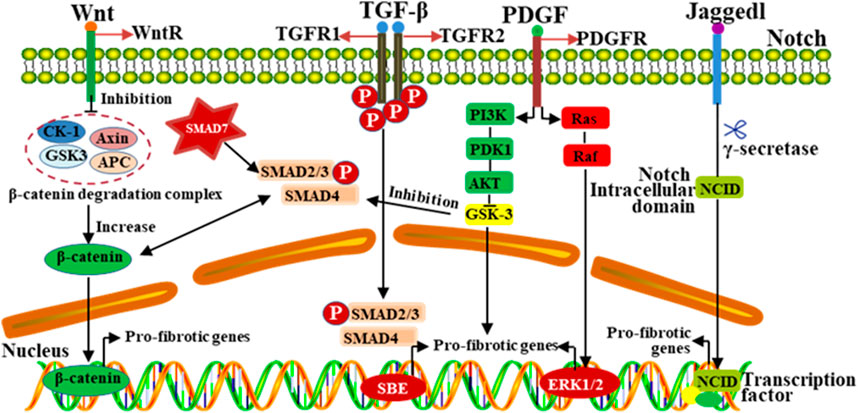
Figure 4. The progression of silicosis is regulated by multiple signaling pathways. Different signaling pathways play complex roles in the regulation of fibrosis, and interact with other signaling pathways in a complex way. Signaling pathways, such as TGF-β, Wnt, MAPK and Notch, have the mutual crosstalk, which jointly participate in regulating the development of pulmonary fibrosis induced by silica dust.
4 Treatment of silicosis
4.1 Drug treatment
There are no effective drugs for silicosis at present. The drugs for fibrosis including idiopathic pulmonary fibrosis (IPF) are usually used to treat silicosis, such as PVNO, nintedanib, and pirfenidone (Zhao et al., 1983). The in vivo and in vitro research showed that PVNO could improve the lung clearance after exposure to silica dust and prevent dust from invading the lung interstitium (Ernst et al., 2002). Meanwhile, PVNO also could eliminate the free radicals induced by SiO2 and protect macrophages, ultimately reducing the silicosis nodules and delaying the development of silicosis. It has been found that PVNO has a protective effect on silica-induced pulmonary fibrosis, and shows good effects in animal models but no obvious effects in silicosis patients (Idec-Sadkowska et al., 2006).
Nintedanib, an orally administered multi-target agent, was approved for IPF in the United States in 2014 and in Europe in 2015 (Varone et al., 2018). Its main molecular targets are the fibroblast growth factor receptors, PDGF receptors and vascular endothelial growth factor receptors (Varone et al., 2018). Due to potentially similar pathological characteristics between IPF and the same tyrosine kinase receptors in progressive fibrosis (Allen and Spiteri, 2002; Coward et al., 2010), nintedanib had been tested in murine models of bleomycin- and silica-induced pulmonary fibrosis, where it was demonstrated to reduce or prevent the fibrotic process (Chaudhary et al., 2007; Wollin et al., 2015). Additionally, nintedanib can also inhibit PDGF receptor activation, fibroblast proliferation, and fibroblast-to-myofibroblast transformation (Wollin et al., 2014). These results indicate that nintedanib may impact the progression of fibrotic lung diseases, such as silica-induced pulmonary fibrosis.
Pirfenidone is an oral anti-fibrotic agent, although it was initially developed as an anti-inflammatory compound due to its capability of diminishing accumulation of inflammatory cells and cytokines (Lancaster et al., 2017). So far, the precise mechanisms underlying the anti-fibrotic action of pirfenidone in the lung are not fully understood. Some research demonstrate that pirfenidone may attenuate the fibroblast proliferation, myofibroblast differentiation, collagen synthesis, fibronectin production and deposition of ECM through modulation of fibrogenic growth factors (Ma Z. et al., 2018; Qin et al., 2018; Molina-Molina et al., 2018; Pourgholamhossein et al., 2018). Additionally, pirfenidone also could regulate and reduce oxidative stress markers (ROS, H2O2, etc.) in the lung, which might be associated with its anti-fibrotic effect (Pourgholamhossein et al., 2018; Gaggini et al., 2011). A retrospective study including 186 subjects who were continuously treated with pirfenidone or nintedanib for pulmonary fibrosis (of any cause) showed similar drug tolerability and adverse event profiles to the corresponding clinical trials, despite the presence of more severe respiratory impairment (Galli et al., 2017). Fortunately, both pirfenidone and nintedanib are promising in the treatment of chronic lung inflammatory diseases, pulmonary fibrosis and other similar diseases. However, future prospective studies are still needed to elucidate the efficacy of the drugs with expanded prescription.
Drug repurposing or repositioning for different diseases is an efficient way for drug discovery because of low cost in the drug development (Pushpakom et al., 2019). Metformin, a widely used biguanide medication for type 2 diabetes, has been shown to inhibit cardiac fibrosis induced by pressure overload in vivo. It may reduce collagen synthesis in cardiac fibrosis by inhibiting the TGF-β/Smad3 signaling pathway (Xiao et al., 2010). Moreover, metformin has antifibrotic properties (Teague et al., 2022). It can effectively reverse bleomycin-induced pulmonary fibrosis, suggesting its role in IPF (Gamad et al., 2018). In our previous study, metformin has been identified to have anti-silicosis effects whether in rats or in vitro cultured human cells (Li S. X. et al., 2021). Metformin could regulate autophagy by activating AMPK and inhibiting mTOR pathways, providing the evidence for metformin as the potential therapeutic drug for silicosis.
In recent years, a number of traditional Chinese medicine compounds have been applied to treat pulmonary fibrosis, including silica-induced pulmonary fibrosis (Li and Kan, 2017; Occupational Lung Disease Group of Labor Hygiene, 2024). These compounds/molecules exert their effects through targeting different pathways as summarized in Figure 2. Liu et al. found that Number 2 Feibi Recipe (N2FBR) with antioxidant effects could promote autophagy through the GSK-3β/mTOR signaling pathway, thereby exerting a protective effect on pulmonary fibrosis (Liu et al., 2022). Xiaochaihu decoction showed anti-fibrotic functions by reducing the collagen content and fibrogenic score, as well as down-regulating TGF-β1, PDGF and TIMP-1 mRNA levels (Figure 2, Orange) (Chen et al., 2004; Chen et al., 2005). Fuzheng Huayu Formula could block the PI3K pathway related to the progression of liver fibrosis, and downregulate the expression of TGF-β1 and Smads (Li, 2020) (Figure 2, Green). Curcumin could suppress EMT and inflammatory response via inhibition of TLR4/NF-κB and PI3K/AKT pathways (Figure 2, Yellow) (Wang Z. et al., 2020)]. Anluo Huaxian Pills could reduce the expression of collagens I and III, TIMP-1, and TGF-β1 in mice with liver fibrosis (Xie et al., 2018) (Figure 2, Blue). Moreover, treatment with salidroside could also significantly decrease the release of inflammatory cytokines (IL-1β, IL-6, TNF-α) and inhibit TLR4/NF-κB and MAPK signaling pathways (Figure 2, red) (Li R. et al., 2019).
Tetrandrine, a bis-benzyl iso-quinoline alkaloid extracted from the plant called Stephania tetrandra S. Moore (Su W. et al., 2020), has been approved for the long-term treatment of silicosis (Liu et al., 2016). Tetrandrine can effectively inhibit the transcription of collagen genes, weaken the function of collagens before cell secretion, reduce the synthesis and proliferation of collagen fibroblasts, and degrade lung collagen fibers, thereby delaying the progression of silica-induced pulmonary fibrosis (Su W. et al., 2020; Xie et al., 2002; Droitcourt et al., 2018). Meanwhile, the compounds from Chinese herbs, such as ginsenoside Rg1, Baicalin, and more, can also reduce the degree of pulmonary fibrosis and improve lung function (Yang et al., 2016; Yu et al., 2016; Liu et al., 2015). Notably, the anti-silicotic effect of emodin was also observed in our in vivo and in vitro studies, indicating that emodin could alleviate silica dust-induced pulmonary fibrosis through regulation of the inflammatory response and fibrotic process at multiple levels (Pang et al., 2021).
Traditional Chinese medicine has been demonstrated effective in various diseases, with fewer adverse effects, which contributes to alleviating the clinical symptoms of patients and enhancing their QOL (Kong et al., 2022; Zhang et al., 2021b; Zhang et al., 2022). In addition, Chinese herbal formulae have been widely prescribed as an adjunct to western medicine to treat the disease (Li and Kan, 2017), which may be the future direction of drug therapy.
4.2 Surgical treatment
4.2.1 Bronchoalveolar lavage
Bronchoalveolar lavage is mainly used to reduce the number of dust particles in the lungs and improve the ventilation function of lung tissues (Cottin et al., 2004). Alveolar lavage with 37°C saline could not only remove the silica particles depositing in the alveoli and the lung interstitium effectively, but also remove the macrophages in the alveoli and slow the progression of silicosis. After bronchoalveolar lavage, cough and discharge of foreign bodies are enhanced, thereby improving tracheal obstruction and ventilation function. Meanwhile, the bronchoscope can be used locally to improve the availability of drugs, reduce local inflammation, and improve the lung function of patients. According to the amount of lavage fluid, bronchoalveolar lavage can be divided into the WLL and small volume lung lavage, among which small volume lung lavage is mainly suitable for the patients with advanced silicosis who are unable to receive WLL. Notably, bronchoalveolar lavage can cause a high risk of trauma. More studies are required to confirm its long-term efficacy.
4.2.2 Lung transplantation
Lung transplantation has become an accepted treatment option for patients with various lung diseases irresponsive to conservative treatments (George et al., 2019). It can improve the QOL of patients, but cannot extend the survival. Although lung transplantation is the most effective method for end-stage fibrosis, its application is limited due to a shortage of liver donors, high incidence rates of surgical complications, graft-versus-host diseases and high medical costs (Hartert et al., 2014). Therefore, similar to bronchoalveolar lavage, lung transplantation should not be used as a routine treatment for silicosis.
4.3 Stem cell treatment
Mesenchymal stem cell (MSC)-based cell therapy is regarded as an innovative experimental treatment (Maria et al., 2018). MSCs, a population of multipotent stem cells, are originated from various tissues and organs, including bone marrow, adiposes, cord blood, and placentae. Multipotentiality is one of the properties for these cells that not only differentiate into adipocytes, chondrocytes and osteoblasts, but also vascular smooth muscle cells and lung epithelial cells under particular conditions (Li et al., 2017). At present, the anti-fibrotic effects have been demonstrated in several types of MSCs, such as bone marrow-derived MSCs (BMSCs), umbilical cord mesenchymal stem cells (UC-MSCs), Sox9+ embryonic stem cells and adipose-derived MSCs (AD-MSCs).
Based on the capability of differentiating into specific cell types, BMSCs may promote the tissue regeneration (Peng et al., 2015), and have immunomodulatory and anti-fibrotic activities that can be significant in response to injury. In animal models of pulmonary fibrosis, transplanted BMSCs home to sites of injury (Xu et al., 2015), which can ameliorate the histological alterations (Ni et al., 2015), inhibit production of proinflammatory mediators (Xue et al., 2013), and decrease collagen deposition (Lan et al., 2015). In addition, BMSCs also attenuate lung injury and pulmonary fibrosis by secreting various factors with anti-apoptotic, anti-inflammatory, and anti-fibrotic functions. Due to easy harvest, isolation and purification, BMSCs have been considered to be a promising and novel treatment (Maria et al., 2018). Our previous study showed that BMSC transplantation could relieve silica-induced pulmonary fibrosis in rats through attenuation of the Wnt/β-catenin signaling pathway (Zhang et al., 2018).
Unlike BMSCs, UC-MSCs are characterized by a painless collection process and a faster self-renewal (Tsai et al., 2021). Inhibition of inflammation is one of the mechanisms for UC-MSCs to treat silicosis. Sha et al. found that UC-MSCs could effectively reduce SiO2-induced inflammatory cell infiltration and inflammation-related cytokine levels in the lung, thereby reducing fibrosis (Sha et al., 2019). UC-MSCs also might alleviate the degree of pulmonary fibrosis in the rat model of silicosis by regulating the secretion of hydroxyproline, TGF-β1 and IL-6 (Xu et al., 2020). In addition, UC-MSCs could also play a role in the treatment of silicosis by reducing cell apoptosis (Chen et al., 2018b), inhibiting the autophagy of lung macrophages, and enhancing the repair after injury (Tuo et al., 2017).
Embryonic stem cells with an enormous capacity for regeneration can differentiate into a variety of tissues (Wu et al., 2020). Sox9+, one of the key genes in early embryonic development, is closely related to cell proliferation and differentiation, and plays a role in balancing and regulating the homeostatic maintenance and directional differentiation of stem cells (Gonen and Lovell-Badge, 2019; Hersmus et al., 2008; Jo et al., 2014). The normal expression of Sox9+ gene determines the integrity of embryonic lung development (Rockich et al., 2013). Lung stem cells involved in lung regeneration and repair are closely related to the process of lung development (Chen et al., 2021). Ma et al. found that after transplantation of autologous Sox9+ airway basal cells for 3–12 months, the lung tissue was repaired, and lung function was enhanced (Ma Q. et al., 2018). In addition, Nichane et al. showed that in the mouse with bleomycin-induced lung injury, endotracheal transplantation of mouse Sox9+ embryonic lung progenitor cells could be integrated into the injured lung tissue and mainly differentiated into alveolar epithelial cells (e.g., ATI and ATII cells), endothelial cells and mesenchymal cells (Nichane et al., 2017). These studies indicated that Sox9+ embryonic lung stem cells could be integrated into the injured lung tissue, with the ability to colonize and differentiate into lung epithelial cells. Meanwhile, in clinical trials, autologous Sox9+ stem cell transplantation is also reported to have the ability to treat lung injury, bringing hope for the treatment of pulmonary fibrosis.
Autologous AD-MSCs with multi-directional differentiation potential can be obtained from mature adipose tissues (Chen et al., 2018b), and differentiate into adipocytes, osteocytes, chondrocytes, muscle cells and nerve precursor cells (Taghi et al., 2012; Nakao et al., 2010). They are abundant in adipose tissues, easy to obtain, relatively less painful and highly feasible (Chen et al., 2018b), which ensure that AD-MSCs have a wider range of applications in stem cell treatment. Studies have shown that Ad-MSCs can effectively repair and regenerate lung tissues (Zhang et al., 2014; Wang et al., 2013) and improve IPF (Jiang et al., 2015). Rubio et al. demonstrated that AD-MSCs could mitigate bleomycin-induced tissue damage and prevent terminal organ fibrosis, manifesting as attenuation of lung and skin fibrosis, as well as acceleration of wound healing (Rubio et al., 2018). In addition, AD-MSC transplantation may also interfere with the formation of silicosis by regulating the processes of inflammation and apoptosis (Chen et al., 2018b).
For silicosis, stem cells can be promising candidates. However, before the cells can be transferred to clinical research from basic research, several issues remain to be resolved: 1) Establishing regulatory guidelines and efficient, safe manufacturing procedures; 2) Establishing a system for genetic testing and long-term monitoring of donors; 3) Implementation of clinical trials to determine the best and standard dose, time, approach, frequency, and other technical issues of stem cell transplantation.
5 Conclusion
Although the etiology of silicosis is clear, the exact pathogenesis is not fully understood. The pathological process of silicosis is complex, involving the interaction of multiple cells and molecules, as well as abnormal regulation of multiple signal pathways and cytokines. Several pathways or mediators playing a key role in initiation and development of silicosis have been proposed to be potential therapeutic targets, and meanwhile surgical treatments and compounds have been used for silicosis. However, the actual clinic effect of these methods on silicosis and possible applications have a long way to go. Early diagnosis and timely monitoring are important strategies in the treatment of silicosis. Currently, the examinations used to diagnose silicosis include chest X-rays, computed tomography scans, and lung function testing. However, such tests only recognize the pathological changes at an organ level. When there have been substantial fibrotic changes, no reliable methods are applied for the diagnosis of silicosis, especially for accelerated silicosis. Although several biomarkers of silicosis have been proposed, their reliability and sensitivity remain to be confirmed and none of them is reported in early diagnosis. Therefore, more work should be focused on the identification of reliable and feasible biomarkers or the methods for earlier diagnosis or health surveillance. To this end, in vitro research in combination with epidemiological data is required with feasible biological samples such as blood, saliva/sputum, and/or urine samples.
Author contributions
BY: Conceptualization, Visualization, Data curation, Methodology, Software, Writing–original draft. XL: Data curation, Methodology, Software, Visualization, Writing–original draft, Supervision. CP: Software, Visualization, Conceptualization, Investigation, Resources, Validation, Writing–review and editing. XM: Conceptualization, Investigation, Resources, Software, Visualization, Writing–review and editing, Funding acquisition, Writing–original draft. QJ: Conceptualization, Funding acquisition, Visualization, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Key Research and Development Program of China (No. 2022YFC2503202), Medical Health Science and Technology Project of Shandong Province (202312010678), Joint Innovation Team for Clinical & Basic Research (202407), Jinan Science and Technology Bureau project (202430001), Natural Science Foundation of Shandong Province (No. SDCX-ZG-202203045, ZR2022QH041).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adamcakova, J., and Mokra, D. (2021). New insights into pathomechanisms and treatment possibilities for lung silicosis. Int. J. Mol. Sci. 22 (8), 4162. doi:10.3390/ijms22084162
Allen, J. T., and Spiteri, M. A. (2002). Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir. Res. 3 (1), 13. doi:10.1186/rr162
Aloe, C. A., Leong, T. L. T., Wimaleswaran, H., Papagianis, P. C., McQualter, J. L., McDonald, C. F., et al. (2022). Excess iron promotes emergence of foamy macrophages that overexpress ferritin in the lungs of silicosis patients. Respirol. Carlt. Vic. 27 (6), 427–436. doi:10.1111/resp.14230
Aydemir, D., Malik, A. N., Kulac, I., Basak, A. N., Lazoglu, I., and Ulusu, N. N. (2022). Impact of the amyotrophic lateral sclerosis disease on the biomechanical properties and oxidative stress metabolism of the lung tissue correlated with the human mutant SOD1(G93A) protein accumulation. Front. Bioeng. Biotechnol. 10, 810243. doi:10.3389/fbioe.2022.810243
Barrett, E. G., Johnston, C., Oberdörster, G., and Finkelstein, J. N. (1998). Silica-induced chemokine expression in alveolar type II cells is mediated by TNF-alpha. Am. J. physiology 275 (6), L1110–L1119. doi:10.1152/ajplung.1998.275.6.L1110
Bartczak, K., Białas, A. J., Kotecki, M. J., Górski, P., and Piotrowski, W. J. (2020). More than a genetic code: epigenetics of lung fibrosis. Mol. Diagn. Ther. 24 (6), 665–681. doi:10.1007/s40291-020-00490-7
Benmerzoug, S., Rose, S., Bounab, B., Gosset, D., Duneau, L., Chenuet, P., et al. (2018). STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat. Commun. 9 (1), 5226. doi:10.1038/s41467-018-07425-1
Chaudhary, N. I., Roth, G. J., Hilberg, F., Müller-Quernheim, J., Prasse, A., Zissel, G., et al. (2007). Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur. Respir. J. 29 (5), 976–985. doi:10.1183/09031936.00152106
Chen, C. Y., Tsai, P. H., Lin, Y. H., Huang, C. Y., Chung, J. H. Y., and Chen, G. Y. (2022). Controllable graphene oxide-based biocompatible hybrid interface as an anti-fibrotic coating for metallic implants. Mater. today Bio 15, 100326. doi:10.1016/j.mtbio.2022.100326
Chen, M. H., Chen, J. C., Tsai, C. C., Wang, W. C., Chang, D. C., Lin, C. C., et al. (2004). Sho-saiko-to prevents liver fibrosis induced by bile duct ligation in rats. Am. J. Chin. Med. 32 (2), 195–207. doi:10.1142/S0192415X04001862
Chen, M. H., Chen, J. C., Tsai, C. C., Wang, W. C., Chang, D. C., Tu, D. G., et al. (2005). The role of TGF-beta 1 and cytokines in the modulation of liver fibrosis by Sho-saiko-to in rat's bile duct ligated model. J. Ethnopharmacol. 97 (1), 7–13. doi:10.1016/j.jep.2004.09.040
Chen, S., Cui, G., Peng, C., Lavin, M. F., Sun, X., Zhang, E., et al. (2018a). Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res. and Ther. 9, 110–112. doi:10.1186/s13287-018-0846-9
Chen, S., Cui, G., Peng, C., Lavin, M. F., Sun, X., Zhang, E., et al. (2018b). Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res. Ther. 9 (1), 110. doi:10.1186/s13287-018-0846-9
Chen, S., Li, K., Zhong, X., Wang, G., Wang, X., Cheng, M., et al. (2021). Sox9-expressing cells promote regeneration after radiation-induced lung injury via the PI3K/AKT pathway. Stem Cell Res. Ther. 12 (1), 381. doi:10.1186/s13287-021-02465-9
Chen, Y. T., Chang, F. C., Wu, C. F., Chou, Y. H., Hsu, H. L., Chiang, W. C., et al. (2011). Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 80 (11), 1170–1181. doi:10.1038/ki.2011.208
Cho, H., Myung, S. K., and Cho, H. E. (2022). Efficacy of vitamin D supplements in treatment of acute respiratory infection: a meta-analysis for randomized controlled trials. Nutrients 14 (6), 1144. doi:10.3390/nu14061144
Choi, H., Lee, W., Kim, E., Ku, S. K., and Bae, J. S. (2019). Inhibitory effects of collismycin C and pyrisulfoxin A on particulate matter-induced pulmonary injury. Phytomedicine 62, 152939. doi:10.1016/j.phymed.2019.152939
Cottin, V., Capron, F., Grenier, P., and Cordier, J. (2004). Diffuse idiopathic interstitial pneumonias. International multidisciplinary consensus classification by the American Thoracic Society and the European Respiratory Society, principal clinico-pathological entities, and diagnosis. Rev. Des. Mal. Respir. 21 (2 Pt 1), 299–318. doi:10.1016/s0761-8425(04)71288-7
Coward, W. R., Saini, G., and Jenkins, G. (2010). The pathogenesis of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 4 (6), 367–388. doi:10.1177/1753465810379801
Cox, C. W., Rose, C. S., and Lynch, D. A. (2014). State of the art: imaging of occupational lung disease. Radiology 270 (3), 681–696. doi:10.1148/radiol.13121415
Cui, Y., Gutierrez, S., Ariai, S., Öberg, L., Thörn, K., Gehrmann, U., et al. (2022). Non-heme iron overload impairs monocyte to macrophage differentiation via mitochondrial oxidative stress. Front. Immunol. 13, 998059. doi:10.3389/fimmu.2022.998059
de Lima Gondim, F., Serra, D. S., and Cavalcante, F. (2019). Effects of Eucalyptol in respiratory system mechanics on acute lung injury after exposure to short-term cigarette smoke. Respir. physiology and Neurobiol. 266, 33–38. doi:10.1016/j.resp.2019.04.007
Droitcourt, C., Adamski, H., Polat, A., Polard, E., Kerjouan, M., Arnouat, B., et al. (2018). Pirfenidone photosensitization in patients with idiopathic pulmonary fibrosis: a case series. Br. J. dermatology 178 (3), e222–e223. doi:10.1111/bjd.16016
Ernst, H., Rittinghausen, S., Bartsch, W., Creutzenberg, O., Dasenbrock, C., Görlitz, B.-D., et al. (2002). Pulmonary inflammation in rats after intratracheal instillation of quartz, amorphous SiO2, carbon black, and coal dust and the influence of poly-2-vinylpyridine-N-oxide (PVNO). Exp. Toxicol. Pathology 54 (2), 109–126. doi:10.1078/0940-2993-00241
Fang, S., Guo, H., Cheng, Y., Zhou, Z., Zhang, W., Han, B., et al. (2018). circHECTD1 promotes the silica-induced pulmonary endothelial-mesenchymal transition via HECTD1. Cell Death Dis. 9 (3), 396. doi:10.1038/s41419-018-0432-1
Feng, F., Cheng, P., Zhang, H., Li, N., Qi, Y., Wang, H., et al. (2019). The protective role of tanshinone IIA in silicosis rat model via TGF-β1/smad signaling suppression, NOX4 inhibition and Nrf2/ARE signaling activation. Drug Des. Dev. Ther. 13, 4275–4290. doi:10.2147/DDDT.S230572
Fernández Álvarez, R., Martínez González, C., Quero Martínez, A., Blanco Pérez, J. J., Carazo Fernández, L., and Prieto Fernández, A. (2015). Guidelines for the diagnosis and monitoring of silicosis. Arch. bronconeumologia 51 (2), 86–93. doi:10.1016/j.arbres.2014.07.010
Fèvre, J., Leveille, E., Jeanson, A., Santucci-Darmanin, S., Pierrefite-Carle, V., Carle, G. F., et al. (2022). Chelating polymers for targeted decontamination of actinides: application of PEI-MP to hydroxyapatite-Th(IV). Int. J. Mol. Sci. 23 (9), 4732. doi:10.3390/ijms23094732
Gaggini, F., Laleu, B., Orchard, M., Fioraso-Cartier, L., Cagnon, L., Houngninou-Molango, S., et al. (2011). Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg. Med. Chem. 19 (23), 6989–6999. doi:10.1016/j.bmc.2011.10.016
Galli, J. A., Pandya, A., Vega-Olivo, M., Dass, C., Zhao, H., and Criner, G. J. (2017). Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirol. Carlt. Vic. 22 (6), 1171–1178. doi:10.1111/resp.13024
Gamad, N., Malik, S., Suchal, K., Vasisht, S., Tomar, A., Arava, S., et al. (2018). Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: pharmacological effects and molecular mechanisms. Biomed. Pharmacother. 97, 1544–1553. doi:10.1016/j.biopha.2017.11.101
Gao, X., Xu, H., Xu, D., Li, S., Wei, Z., Li, S., et al. (2020). MiR-411-3p alleviates Silica-induced pulmonary fibrosis by regulating Smurf2/TGF-β signaling. Exp. Cell Res. 388 (2), 111878. doi:10.1016/j.yexcr.2020.111878
GBD 2013 Mortality and Causes of Death Collaborators (2015). Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet London, Engl. 385 (9963), 117–171. doi:10.1016/S0140-6736(14)61682-2
George, P. M., Patterson, C. M., Reed, A. K., and Thillai, M. (2019). Lung transplantation for idiopathic pulmonary fibrosis. Lancet Respir. Med. 7 (3), 271–282. doi:10.1016/S2213-2600(18)30502-2
Gonen, N., and Lovell-Badge, R. (2019). The regulation of Sox9 expression in the gonad. Curr. Top. Dev. Biol. 134, 223–252. doi:10.1016/bs.ctdb.2019.01.004
Gonzalez, D. M., and Medici, D. (2014). Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal. 7 (344), re8. doi:10.1126/scisignal.2005189
Goumans, T. P., Catlow, C. R., and Brown, W. A. (2008). Hydrogenation of CO on a silica surface: an embedded cluster approach. J. Chem. Phys. 128 (13), 134709. doi:10.1063/1.2888933
Guha, N., Straif, K., and Benbrahim-Tallaa, L. (2011). The IARC Monographs on the carcinogenicity of crystalline silica. La Med. del Lav. 102 (4), 310–320.
Hamilton, R. F., Thakur, S. A., and Holian, A. (2008). Silica binding and toxicity in alveolar macrophages. Free Radic. Biol. Med. 44 (7), 1246–1258. doi:10.1016/j.freeradbiomed.2007.12.027
Hartert, M., Senbaklavacin, O., Gohrbandt, B., Fischer, B. M., Buhl, R., and Vahld, C. F. (2014). Lung transplantation: a treatment option in end-stage lung disease. Dtsch. Arzteblatt Int. 111 (7), 107–116. doi:10.3238/arztebl.2014.0107
Hersmus, R., Kalfa, N., de Leeuw, B., Stoop, H., Oosterhuis, J. W., de Krijger, R., et al. (2008). FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD). J. pathology 215 (1), 31–38. doi:10.1002/path.2335
Hou, X., Summer, R., Chen, Z., Tian, Y., Ma, J., Cui, J., et al. (2019). Lipid uptake by alveolar macrophages drives fibrotic responses to silica dust. Sci. Rep. 9 (1), 399. doi:10.1038/s41598-018-36875-2
Huang, Z., Wang, H., Long, J., Lu, Z., Chun, C., and Li, X. (2022). Neutrophil membrane-coated therapeutic liposomes for targeted treatment in acute lung injury. Int. J. Pharm. 624, 121971. doi:10.1016/j.ijpharm.2022.121971
Idec-Sadkowska, I., Andrzejak, R., Antonowicz-Juchniewicz, J., and Kaczmarek-Wdowiak, B. (2006). Trials of casual treatment of silicosis. Med. P. R. 57 (3), 271–280.
Iijima, J., Kobayashi, S., Kitazume, S., Kizuka, Y., Fujinawa, R., Korekane, H., et al. (2017). Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling. Glycobiology 27 (11), 1006–1015. doi:10.1093/glycob/cwx075
Jiang, H., Zhang, J., Zhang, Z., Ren, S., and Zhang, C. (2015). Effect of transplanted adipose-derived stem cells in mice exhibiting idiopathic pulmonary fibrosis. Mol. Med. Rep. 12 (4), 5933–5938. doi:10.3892/mmr.2015.4178
Jo, A., Denduluri, S., Zhang, B., Wang, Z., Yin, L., Yan, Z., et al. (2014). The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 1 (2), 149–161. doi:10.1016/j.gendis.2014.09.004
Kauppinen, T., Toikkanen, J., Pedersen, D., Young, R., Ahrens, W., Boffetta, P., et al. (2000). Occupational exposure to carcinogens in the European Union. Occup. Environ. Med. 57 (1), 10–18. doi:10.1136/oem.57.1.10
Kawasaki, H. (2015). A mechanistic review of silica-induced inhalation toxicity. Inhal. Toxicol. 27 (8), 363–377. doi:10.3109/08958378.2015.1066905
King, D., Yeomanson, D., and Bryant, H. E. (2015). PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J. Pediatr. hematology/oncology 37 (4), 245–251. doi:10.1097/MPH.0000000000000329
Kong, L., Sun, Y., Sun, H., Zhang, A. H., Zhang, B., Ge, N., et al. (2022). Chinmedomics strategy for elucidating the pharmacological effects and discovering bioactive compounds from keluoxin against diabetic retinopathy. Front. Pharmacol. 13, 728256. doi:10.3389/fphar.2022.728256
Kramer, M. R., Blanc, P. D., Fireman, E., Amital, A., Guber, A., Rhahman, N. A., et al. (2012). Artificial stone silicosis [corrected]: disease resurgence among artificial stone workers. Chest 142 (2), 419–424. doi:10.1378/chest.11-1321
Krefft, S., Wolff, J., and Rose, C. (2020). Silicosis: an update and guide for clinicians. Clin. chest Med. 41 (4), 709–722. doi:10.1016/j.ccm.2020.08.012
Lan, Y. W., Choo, K. B., Chen, C. M., Hung, T. H., Chen, Y. B., Hsieh, C. H., et al. (2015). Hypoxia-preconditioned mesenchymal stem cells attenuate bleomycin-induced pulmonary fibrosis. Stem Cell Res. Ther. 6 (1), 97. doi:10.1186/s13287-015-0081-6
Lancaster, L. H., de Andrade, J. A., Zibrak, J. D., Padilla, M. L., Albera, C., Nathan, S. D., et al. (2017). Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 26 (146), 170057. doi:10.1183/16000617.0057-2017
Land, W. G. (2020). Role of damage-associated molecular patterns in light of modern environmental research: a tautological approach. Int. J. Environ. Res. 14 (5), 583–604. doi:10.1007/s41742-020-00276-z
Leask, A. (2010). Towards an anti-fibrotic therapy for scleroderma: targeting myofibroblast differentiation and recruitment. Fibrogenesis and tissue repair 3, 8. doi:10.1186/1755-1536-3-8
Lee, G.-H., Kim, Y.-S., Kwon, E., Yun, J.-W., and Kang, B.-C. (2020). Toxicologic evaluation for amorphous silica nanoparticles: genotoxic and non-genotoxic tumor-promoting potential. Pharmaceutics 12 (9), 826. doi:10.3390/pharmaceutics12090826
Li, H. (2020). Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. J. Ethnopharmacol. 251, 112442. doi:10.1016/j.jep.2019.112442
Li, J., Yao, W., Hou, J. Y., Zhang, L., Bao, L., Chen, H. T., et al. (2018). The role of fibrocyte in the pathogenesis of silicosis. Biomed. Environ. Sci. BES 31 (4), 311–316. doi:10.3967/bes2018.040
Li, L. C., and Kan, L. D. (2017). Traditional Chinese medicine for pulmonary fibrosis therapy: progress and future prospects. J. Ethnopharmacol. 198, 45–63. doi:10.1016/j.jep.2016.12.042
Li, M., Lu, Y., and Du, L.-Q. (2002). Effects of occupational exposure to dichlorobenzene on workers’ health. Industrial Health Occup. Dis. 28 (5), 260–263. doi:10.3969/j.issn.1000-7164.2002.05.006
Li, M., Luan, F., Zhao, Y., Hao, H., Zhou, Y., Han, W., et al. (2016). Epithelial-mesenchymal transition: an emerging target in tissue fibrosis. Exp. Biol. Med. (Maywood, NJ) 241 (1), 1–13. doi:10.1177/1535370215597194
Li, R., Guo, Y., Zhang, Y., Zhang, X., Zhu, L., and Yan, T. (2019b). Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways. Int. J. Mol. Sci. 20 (5), 1103. doi:10.3390/ijms20051103
Li, S., Li, C., Zhang, Y., He, X., Chen, X., Zeng, X., et al. (2019a). Targeting mechanics-induced fibroblast activation through CD44-RhoA-YAP pathway ameliorates crystalline silica-induced silicosis. Theranostics 9 (17), 4993–5008. doi:10.7150/thno.35665
Li, S. X., Li, C., Pang, X. R., Zhang, J., Yu, G. C., Yeo, A. J., et al. (2021b). Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front. Pharmacol. 12, 719589. doi:10.3389/fphar.2021.719589
Li, X., An, G., Wang, Y., Liang, D., Zhu, Z., Lian, X., et al. (2017). Anti-fibrotic effects of bone morphogenetic protein-7-modified bone marrow mesenchymal stem cells on silica-induced pulmonary fibrosis. Exp. Mol. pathology 102 (1), 70–77. doi:10.1016/j.yexmp.2016.12.010
Li, Z., Xu, H., Xu, Y., Lu, G., Peng, Q., Chen, J., et al. (2021a). Morinda officinalis oligosaccharides alleviate depressive-like behaviors in post-stroke rats via suppressing NLRP3 inflammasome to inhibit hippocampal inflammation. CNS Neurosci. Ther. 27 (12), 1570–1586. doi:10.1111/cns.13732
Liu, H., Pang, Q., Cao, F., Liu, Z., Wei, W., Li, Z., et al. (2022). Number 2 Feibi Recipe ameliorates pulmonary fibrosis by inducing autophagy through the GSK-3β/mTOR pathway. Front. Pharmacol. 13, 921209. doi:10.3389/fphar.2022.921209
Liu, T., Dai, W., Li, C., Liu, F., Chen, Y., Weng, D., et al. (2015). Baicalin alleviates silica-induced lung inflammation and fibrosis by inhibiting the Th17 response in C57BL/6 mice. J. Nat. Prod. 78 (12), 3049–3057. doi:10.1021/acs.jnatprod.5b00868
Liu, T., Liu, X., and Li, W. (2016). Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 7 (26), 40800–40815. doi:10.18632/oncotarget.8315
Liu, Y., Xu, H., Geng, Y., Xu, D., Zhang, L., Yang, Y., et al. (2017). Dibutyryl-cAMP attenuates pulmonary fibrosis by blocking myofibroblast differentiation via PKA/CREB/CBP signaling in rats with silicosis. Respir. Res. 18 (1), 38. doi:10.1186/s12931-017-0523-z
Liu, Z., Luo, C., Chao, L., Huang, X., Wang, D., and Chen, W. (2024). Study on the burden of silicosis in China in 1990-2021. J. Public Health Prev. Med. 35 (6), 16–20. doi:10.3969/j.issn.1006-2483.2024.06.004
Ma, J., Cai, Q., Yang, D., Yang, J., Xue, J., Yu, M., et al. (2020b). A positive feed forward loop between Wnt/β-catenin and NOX4 promotes silicon dioxide-induced epithelial-mesenchymal transition of lung epithelial cells. Oxidative Med. Cell. Longev. 2020, 3404168. doi:10.1155/2020/3404168
Ma, Q., Ma, Y., Dai, X., Ren, T., Fu, Y., Liu, W., et al. (2018b). Regeneration of functional alveoli by adult human SOX9(+) airway basal cell transplantation. Protein Cell 9 (3), 267–282. doi:10.1007/s13238-018-0506-y
Ma, X., Yu, X., and Zhou, Q. (2020a). The IL1β-HER2-CLDN18/CLDN4 axis mediates lung barrier damage in ARDS. Aging 12 (4), 3249–3265. doi:10.18632/aging.102804
Ma, Z., Zhao, C., Chen, Q., Yu, C., Zhang, H., Zhang, Z., et al. (2018a). Antifibrotic effects of a novel pirfenidone derivative in vitro and in vivo. Pulm. Pharmacol. Ther. 53, 100–106. doi:10.1016/j.pupt.2018.10.006
Maria, A. T. J., Rozier, P., Fonteneau, G., Sutra, T., Maumus, M., Toupet, K., et al. (2018). iNOS activity is required for the therapeutic effect of mesenchymal stem cells in experimental systemic sclerosis. Front. Immunol. 9, 3056. doi:10.3389/fimmu.2018.03056
Martins, V., da Silva, A. L., Teodoro, W. R., Velosa, A. P. P., Balancin, M. L., Cruz, F. F., et al. (2020). In situ evidence of collagen V and signaling pathway of found inflammatory zone 1 (FIZZ1) is associated with silicotic granuloma in lung mice. Pathology, Res. Pract. 216 (9), 153094. doi:10.1016/j.prp.2020.153094
Mercer, P. F., Johns, R. H., Scotton, C. J., Krupiczojc, M. A., Königshoff, M., Howell, D. C., et al. (2009). Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis. Am. J. Respir. Crit. care Med. 179 (5), 414–425. doi:10.1164/rccm.200712-1827OC
Molina-Molina, M., Machahua-Huamani, C., Vicens-Zygmunt, V., Llatjós, R., Escobar, I., Sala-Llinas, E., et al. (2018). Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm. Med. 18 (1), 63. doi:10.1186/s12890-018-0626-4
Mu, D., Li, J., Qi, Y., Sun, X., Liu, Y., Shen, S., et al. (2020). Hyaluronic acid-coated polymeric micelles with hydrogen peroxide scavenging to encapsulate statins for alleviating atherosclerosis. J. nanobiotechnology 18 (1), 179. doi:10.1186/s12951-020-00744-w
Nakao, N., Nakayama, T., Yahata, T., Muguruma, Y., Saito, S., Miyata, Y., et al. (2010). Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am. J. pathology 177 (2), 547–554. doi:10.2353/ajpath.2010.091042
Nardi, J., Nascimento, S., Göethel, G., Gauer, B., Sauer, E., Fão, N., et al. (2018). Inflammatory and oxidative stress parameters as potential early biomarkers for silicosis. Clin. Chim. acta 484, 305–313. doi:10.1016/j.cca.2018.05.045
Nguyen, N., Fernando, S. D., Biette, K. A., Hammer, J. A., Capocelli, K. E., Kitzenberg, D. A., et al. (2018). TGF-β1 alters esophageal epithelial barrier function by attenuation of claudin-7 in eosinophilic esophagitis. Mucosal Immunol. 11 (2), 415–426. doi:10.1038/mi.2017.72
Ni, S., Wang, D., Qiu, X., Pang, L., Song, Z., and Guo, K. (2015). Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. Int. J. Clin. Exp. pathology 8 (7), 7752–7761.
Nichane, M., Javed, A., Sivakamasundari, V., Ganesan, M., Ang, L. T., Kraus, P., et al. (2017). Isolation and 3D expansion of multipotent Sox9(+) mouse lung progenitors. Nat. methods 14 (12), 1205–1212. doi:10.1038/nmeth.4498
Noël, A., Hossain, E., Perveen, Z., Zaman, H., and Penn, A. L. (2020). Sub-ohm vaping increases the levels of carbonyls, is cytotoxic, and alters gene expression in human bronchial epithelial cells exposed at the air-liquid interface. Respir. Res. 21 (1), 305. doi:10.1186/s12931-020-01571-1
Occupational Lung Disease Group of Labor Hygiene (2024). Consensus of Chinese experts on pneumoconiosis treatment. J. Environ. Occup. Med. 41 (1), 1–21. doi:10.11836/JEOM23379
Ohta, H., Chiba, S., Ebina, M., Furuse, M., and Nukiwa, T. (2012). Altered expression of tight junction molecules in alveolar septa in lung injury and fibrosis. Am. J. physiology Lung Cell. Mol. physiology 302 (2), L193–L205. doi:10.1152/ajplung.00349.2010
Olajuyin, A. M., Zhang, X., and Ji, H. L. (2019). Alveolar type 2 progenitor cells for lung injury repair. Cell death Discov. 5, 63. doi:10.1038/s41420-019-0147-9
Ophir, N., Shai, A. B., Alkalay, Y., Israeli, S., Korenstein, R., Kramer, M. R., et al. (2016). Artificial stone dust-induced functional and inflammatory abnormalities in exposed workers monitored quantitatively by biometrics. ERJ Open Res. 2 (1), 00086–02015. doi:10.1183/23120541.00086-2015
Pang, L.-J., Liu, J.-P., and Lv, X.-D. (2019). Comparative effectiveness of 3 Traditional Chinese Medicine treatment methods for idiopathic pulmonary fibrosis: a systematic review and network meta-analysis protocol. Medicine. 98 (30), e16325. doi:10.1097/MD.0000000000016325
Pang, X., Shao, L., Nie, X., Yan, H., Li, C., Yeo, A. J., et al. (2021). Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int. Immunopharmacol. 91, 107277. doi:10.1016/j.intimp.2020.107277
Paolucci, V., Romeo, R., Sisinni, A. G., Bartoli, D., Mazzei, M. A., and Sartorelli, P. (2015). Silicosis in workers exposed to artificial quartz conglomerates: does it differ from chronic simple silicosis? Arch. Bronconeumología English Ed. 51 (12), e57–e60. doi:10.1016/j.arbres.2014.12.010
Pardali, E., Sanchez-Duffhues, G., Gomez-Puerto, M. C., and Ten Dijke, P. (2017). TGF-β-Induced endothelial-mesenchymal transition in fibrotic diseases. Int. J. Mol. Sci. 18 (10), 2157. doi:10.3390/ijms18102157
Pavan, C., Santalucia, R., Leinardi, R., Fabbiani, M., Yakoub, Y., Uwambayinema, F., et al. (2020). Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc. Natl. Acad. Sci. U. S. A. 117 (45), 27836–27846. doi:10.1073/pnas.2008006117
Peng, X., Mo, Y., Liu, J., Liu, H., and Wang, S. (2022). Identification and validation of miRNA-TF-mRNA regulatory networks in uterine fibroids. Front. Bioeng. Biotechnol. 10, 856745. doi:10.3389/fbioe.2022.856745
Peng, Y., Xuan, M., Zou, J., Liu, H., Zhuo, Z., Wan, Y., et al. (2015). Freeze-dried rat bone marrow mesenchymal stem cell paracrine factors: a simplified novel material for skin wound therapy. Tissue Eng. Part A 21 (5-6), 1036–1046. doi:10.1089/ten.TEA.2014.0102
Pérez-Alonso, A., Córdoba-Doña, J. A., Millares-Lorenzo, J. L., Figueroa-Murillo, E., García-Vadillo, C., and Romero-Morillo, J. (2014). Outbreak of silicosis in Spanish quartz conglomerate workers. Int. J. Occup. Environ. Health 20 (1), 26–32. doi:10.1179/2049396713Y.0000000049
Peters, T. M., O'Shaughnessy, P. T., Grant, R., Altmaier, R., Swanton, E., Falk, J., et al. (2017). Community airborne particulate matter from mining for sand used as hydraulic fracturing proppant. Sci. Total Environ. 609, 1475–1482. doi:10.1016/j.scitotenv.2017.08.006
Piera-Velazquez, S., Mendoza, F. A., and Jimenez, S. A. (2016). Endothelial to mesenchymal transition (EndoMT) in the pathogenesis of human fibrotic diseases. J. Clin. Med. 5 (4), 45. doi:10.3390/jcm5040045
Pourgholamhossein, F., Rasooli, R., Pournamdari, M., Pourgholi, L., Samareh-Fekri, M., Ghazi-Khansari, M., et al. (2018). Pirfenidone protects against paraquat-induced lung injury and fibrosis in mice by modulation of inflammation, oxidative stress, and gene expression. Food Chem. Toxicol. 112, 39–46. doi:10.1016/j.fct.2017.12.034
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., et al. (2019). Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18 (1), 41–58. doi:10.1038/nrd.2018.168
Qin, W., Liu, B., Yi, M., Li, L., Tang, Y., Wu, B., et al. (2018). Antifibrotic agent pirfenidone protects against development of radiation-induced pulmonary fibrosis in a murine model. Radiat. Res. 190 (4), 396–403. doi:10.1667/RR15017.1
Quan, H., Wu, W., Yang, G., Wu, Y., Yang, W., Min, C., et al. (2022). Risk factors of silicosis progression: a retrospective cohort study in China. Front. Med. 9, 832052. doi:10.3389/fmed.2022.832052
Razonable, R. R., Henault, M., and Paya, C. V. (2006). Stimulation of toll-like receptor 2 with bleomycin results in cellular activation and secretion of pro-inflammatory cytokines and chemokines. Toxicol. Appl. Pharmacol. 210 (3), 181–189. doi:10.1016/j.taap.2005.05.002
Rockich, B. E., Hrycaj, S. M., Shih, H. P., Nagy, M. S., Ferguson, M. A., Kopp, J. L., et al. (2013). Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc. Natl. Acad. Sci. U. S. A. 110 (47), E4456–E4464. doi:10.1073/pnas.1311847110
Rubio, G. A., Elliot, S. J., Wikramanayake, T. C., Xia, X., Pereira-Simon, S., Thaller, S. R., et al. (2018). Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J. Cell. physiology 233 (8), 5503–5512. doi:10.1002/jcp.26418
Rubio, L., Pyrgiotakis, G., Beltran-Huarac, J., Zhang, Y., Gaurav, J., Deloid, G., et al. (2019). Safer-by-design flame-sprayed silicon dioxide nanoparticles: the role of silanol content on ROS generation, surface activity and cytotoxicity. Part. fibre Toxicol. 16, 40–15. doi:10.1186/s12989-019-0325-1
Sayan, M., and Mossman, B. T. (2016). The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. fibre Toxicol. 13 (1), 51. doi:10.1186/s12989-016-0162-4
Scott, L. E., Weinberg, S. H., and Lemmon, C. A. (2019). Mechanochemical signaling of the extracellular matrix in epithelial-mesenchymal transition. Front. Cell Dev. Biol. 7, 135. doi:10.3389/fcell.2019.00135
Sha, Y., Xie, Y., Chen, Z. J., Yang, X. Y., Luo, J., Zhang, B. L., et al. (2019). Interference research of umbilical cord mesenchymal stem cells on the pulmonary fibrosis in silicosis rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 37 (6), 401–407. doi:10.3760/cma.j.issn.1001-9391.2019.06.001
Shen, C., Li, R., Negro, R., Cheng, J., Vora, S. M., Fu, T. M., et al. (2021). Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 184 (23), 5759–5774.e20. doi:10.1016/j.cell.2021.09.032
Sims, J. E., and Smith, D. E. (2010). The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10 (2), 89–102. doi:10.1038/nri2691
Stegmann, K. A., De Souza, J. B., and Riley, E. M. (2015). IL-18-induced expression of high-affinity IL-2R on murine NK cells is essential for NK-cell IFN-γ production during murine Plasmodium yoelii infection. Eur. J. Immunol. 45 (12), 3431–3440. doi:10.1002/eji.201546018
Su, W., Liang, Y., Meng, Z., Chen, X., Lu, M., Han, X., et al. (2020b). Inhalation of tetrandrine-hydroxypropyl-β-cyclodextrin inclusion complexes for pulmonary fibrosis treatment. Mol. Pharm. 17 (5), 1596–1607. doi:10.1021/acs.molpharmaceut.0c00026
Su, Y., Sun, B., Gao, X., Dong, X., Fu, L., Zhang, Y., et al. (2020a). Intranasal delivery of targeted nanoparticles loaded with miR-132 to brain for the treatment of neurodegenerative diseases. Front. Pharmacol. 11, 1165. doi:10.3389/fphar.2020.01165
Taghi, G. M., Ghasem Kashani Maryam, H., Taghi, L., Leili, H., and Leyla, M. (2012). Characterization of in vitro cultured bone marrow and adipose tissue-derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biol. Int. 36 (12), 1239–1249. doi:10.1042/CBI20110618
Tang, P. M., Nikolic-Paterson, D. J., and Lan, H. Y. (2019). Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15 (3), 144–158. doi:10.1038/s41581-019-0110-2
Teague, T. T., Payne, S. R., Kelly, B. T., Dempsey, T. M., McCoy, R. G., Sangaralingham, L. R., et al. (2022). Evaluation for clinical benefit of metformin in patients with idiopathic pulmonary fibrosis and type 2 diabetes mellitus: a national claims-based cohort analysis. Respir. Res. 23 (1), 91. doi:10.1186/s12931-022-02001-0
Tsai, S. C., Yang, K. D., Chang, K. H., Lin, F. C., Chou, R. H., Li, M. C., et al. (2021). Umbilical cord mesenchymal stromal cell-derived exosomes rescue the loss of outer hair cells and repair cochlear damage in cisplatin-injected mice. Int. J. Mol. Sci. 22 (13), 6664. doi:10.3390/ijms22136664
Tuo, L., Zeng, W. Z., Xue, H. L., and Wu, X. L. (2017). Umbilical cord mesenchymal stem cells and their association with liver fibrosis. Zhonghua Gan Zang Bing Za Zhi 25 (1), 65–68. doi:10.3760/cma.j.issn.1007-3418.2017.01.019
Upagupta, C., Shimbori, C., Alsilmi, R., and Kolb, M. (2018). Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 27 (148), 180033. doi:10.1183/16000617.0033-2018
Varone, F., Sgalla, G., Iovene, B., Bruni, T., and Richeldi, L. (2018). Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Pharmacother. 19 (2), 167–175. doi:10.1080/14656566.2018.1425681
Wang, D., Yang, M., Liu, Y., Ma, J., Shi, T., and Chen, W. (2020a). Association of silica dust exposure and cigarette smoking with mortality among mine and pottery workers in China. JAMA Netw. Open 3 (4), e202787. doi:10.1001/jamanetworkopen.2020.2787
Wang, H., Yang, Y. F., Zhao, L., Xiao, F. J., Zhang, Q. W., Wen, M. L., et al. (2013). Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum. gene Ther. 24 (3), 343–353. doi:10.1089/hum.2012.177
Wang, M., Zhang, Z., Liu, J., Song, M., Zhang, T., Chen, Y., et al. (2022a). Gefitinib and fostamatinib target EGFR and SYK to attenuate silicosis: a multi-omics study with drug exploration. Signal Transduct. Target. Ther. 7 (1), 157. doi:10.1038/s41392-022-00959-3
Wang, R., Chen, B., Wei, H., Yan, W., Wu, Y., Wang, C., et al. (2022b). Collecting and deactivating TGF-β1 hydrogel for anti-scarring therapy in post-glaucoma filtration surgery. Mater. today Bio 14, 100260. doi:10.1016/j.mtbio.2022.100260
Wang, X., Zhang, Y., Zhang, W., Liu, H., Zhou, Z., Dai, X., et al. (2016). MCPIP1 regulates alveolar macrophage apoptosis and pulmonary fibroblast activation after in vitro exposure to silica. Toxicol. Sci. 151 (1), 126–138. doi:10.1093/toxsci/kfw029
Wang, Y., Li, S., Zhao, J., Li, Q., Xu, C., Wu, H., et al. (2021). Snail-mediated partial epithelial mesenchymal transition augments the differentiation of local lung myofibroblast. Chemosphere 267, 128870. doi:10.1016/j.chemosphere.2020.128870
Wang, Z., Chen, Z., Li, B., Zhang, B., Du, Y., Liu, Y., et al. (2020b). Curcumin attenuates renal interstitial fibrosis of obstructive nephropathy by suppressing epithelial-mesenchymal transition through inhibition of the TLR4/NF-кB and PI3K/AKT signalling pathways. Pharm. Biol. 58 (1), 828–837. doi:10.1080/13880209.2020.1809462
Wildung, M., Herr, C., Riedel, D., Wiedwald, C., Moiseenko, A., Ramírez, F., et al. (2022). miR449 protects airway regeneration by controlling AURKA/HDAC6-Mediated ciliary disassembly. Int. J. Mol. Sci. 23 (14), 7749. doi:10.3390/ijms23147749
Wollin, L., Maillet, I., Quesniaux, V., Holweg, A., and Ryffel, B. (2014). Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 349 (2), 209–220. doi:10.1124/jpet.113.208223
Wollin, L., Wex, E., Pautsch, A., Schnapp, G., Hostettler, K. E., Stowasser, S., et al. (2015). Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. J. 45 (5), 1434–1445. doi:10.1183/09031936.00174914
Woźniak, H., and Wiecek, E. (1995). Amorphous silica. Types, health effects of exposure. NDS. Med. Pr. 46 (2), 179–187.
Wu, J., Song, D., Li, Z., Guo, B., Xiao, Y., Liu, W., et al. (2020). Immunity-and-matrix-regulatory cells derived from human embryonic stem cells safely and effectively treat mouse lung injury and fibrosis. Cell Res. 30 (9), 794–809. doi:10.1038/s41422-020-0354-1
Xiao, H., Ma, X., Feng, W., Fu, Y., Lu, Z., Xu, M., et al. (2010). Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc. Res. 87 (3), 504–513. doi:10.1093/cvr/cvq066
Xie, H. P., Liu, Z. P., Zhang, J. S., Dai, M., Xiao, G. M., Wu, W. K., et al. (2018). Traditional Chinese medicine syndrome patterns and their association with hepatitis B surface antigen levels during the natural history of chronic hepatitis B virus infection. Evidence-based complementary Altern. Med. eCAM. 2018, 7482593. doi:10.1155/2018/7482593
Xie, Q. M., Tang, H. F., Chen, J. Q., and Bian, R. L. (2002). Pharmacological actions of tetrandrine in inflammatory pulmonary diseases. Acta Pharmacol. Sin. 23 (12), 1107–1113.
Xu, C., Zhao, J., Li, Q., Hou, L., Wang, Y., Li, S., et al. (2020). Exosomes derived from three-dimensional cultured human umbilical cord mesenchymal stem cells ameliorate pulmonary fibrosis in a mouse silicosis model. Stem Cell Res. Ther. 11 (1), 503. doi:10.1186/s13287-020-02023-9
Xu, H. R., Wang, X. H., Ma, X. B., Hou, W. N., Zhu, L., Xiang, J. C., et al. (2012). Screen and validation of differentially expressing genes related to silicotic pulmonary fibrosis in rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 30 (1), 45–51. doi:10.3760/cma.j.issn.1001-9391.2012.01.011
Xu, J., Li, L., Xiong, J., Zheng, Y., Ye, Q., and Li, Y. (2015). Cyclophosphamide combined with bone marrow mesenchymal stromal cells protects against bleomycin-induced lung fibrosis in mice. Ann. Clin. laboratory Sci. 45 (3), 292–300.
Xue, J., Li, X., Lu, Y., Gan, L., Zhou, L., Wang, Y., et al. (2013). Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol. Ther. 21 (2), 456–465. doi:10.1038/mt.2012.183
Yang, H. Y., Zhang, F., Cheng, M. L., Wu, J., Xie, M., Yu, L. Z., et al. (2022). Glycogen synthase kinase-3β inhibition decreases inflammation and relieves cancer induced bone pain via reducing Drp1-mediated mitochondrial damage. J. Cell. Mol. Med. 26 (14), 3965–3976. doi:10.1111/jcmm.17432
Yang, H. Z., Cui, B., Liu, H. Z., Chen, Z. R., Yan, H. M., Hua, F., et al. (2009). Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J. Immunol. Baltim. Md 1950 182 (1), 692–702. doi:10.4049/jimmunol.182.1.692
Yang, P., Song, R., Li, N., Sun, K., Shi, F., Liu, H., et al. (2020). Silica dust exposure induces autophagy in alveolar macrophages through switching Beclin1 affinity from Bcl-2 to PIK3C3. Environ. Toxicol. 35 (7), 758–767. doi:10.1002/tox.22910
Yang, T., Wang, J., Pang, Y., Dang, X., Ren, H., Liu, Y., et al. (2016). Emodin suppresses silica-induced lung fibrosis by promoting Sirt1 signaling via direct contact. Mol. Med. Rep. 14 (5), 4643–4649. doi:10.3892/mmr.2016.5838
Yu, J., Mao, L., Guan, L., Zhang, Y., and Zhao, J. (2016). Ginsenoside Rg1 enhances lymphatic transport of intrapulmonary silica via VEGF-C/VEGFR-3 signaling in silicotic rats. Biochem. biophysical Res. Commun. 472 (1), 182–188. doi:10.1016/j.bbrc.2016.02.091
Zahid, A., Li, B., Kombe, A. J. K., Jin, T., and Tao, J. (2019). Pharmacological inhibitors of the NLRP3 inflammasome. Front. Immunol. 10, 2538. doi:10.3389/fimmu.2019.02538
Zanoni, I., Ostuni, R., Marek, L. R., Barresi, S., Barbalat, R., Barton, G. M., et al. (2011). CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell 147 (4), 868–880. doi:10.1016/j.cell.2011.09.051
Zhang, E., Yang, Y., Chen, S., Peng, C., Lavin, M. F., Yeo, A. J., et al. (2018). Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/β-catenin signaling in rats. Stem Cell Res. Ther. 9 (1), 311. doi:10.1186/s13287-018-1045-4
Zhang, J., Huang, D., Dai, Y., and Xia, Y. F. (2022). Sinomenine ameliorates colitis-associated cancer by modulating lipid metabolism via enhancing CPT1A expression. Metabolites 12 (10), 946. doi:10.3390/metabo12100946
Zhang, S., Chen, H., Yue, D., Blackwell, T. S., Lv, C., and Song, X. (2021a). Long non-coding RNAs: promising new targets in pulmonary fibrosis. J. gene Med. 23 (3), e3318. doi:10.1002/jgm.3318
Zhang, S., Jiang, M., Yan, S., Liang, M., Wang, W., Yuan, B., et al. (2021b). Network pharmacology-based and experimental identification of the effects of paeoniflorin on major depressive disorder. Front. Pharmacol. 12, 793012. doi:10.3389/fphar.2021.793012
Zhang, T., Fang, S., Wan, C., Kong, Q., Wang, G., Wang, S., et al. (2015). Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci. Rep. 5, 16548. doi:10.1038/srep16548
Zhang, W. G., He, L., Shi, X. M., Wu, S. S., Zhang, B., Mei, L., et al. (2014). Regulation of transplanted mesenchymal stem cells by the lung progenitor niche in rats with chronic obstructive pulmonary disease. Respir. Res. 15 (1), 33. doi:10.1186/1465-9921-15-33
Zhao, J. D., Liu, J. D., and Li, G. Z. (1983). Long-term follow-up observations of the therapeutic effect of PVNO on human silicosis. Zentralbl. Bakteriol. Mikrobiol. Hyg. B 178 (3), 259–262.
Zhao, Y., Hao, C., Bao, L., Wang, D., Li, Y., Qu, Y., et al. (2020). Silica particles disorganize the polarization of pulmonary macrophages in mice. Ecotoxicol. Environ. Saf. 193, 110364. doi:10.1016/j.ecoenv.2020.110364
Keywords: silicosis, pathogenesis, therapeutics, lung fibrosis, molecular mechanisms
Citation: Yang B, Liu X, Peng C, Meng X and Jia Q (2025) Silicosis: from pathogenesis to therapeutics. Front. Pharmacol. 16:1516200. doi: 10.3389/fphar.2025.1516200
Received: 24 October 2024; Accepted: 13 January 2025;
Published: 29 January 2025.
Edited by:
Jadranka Milosevic, Captis Diagnostics Inc., United StatesReviewed by:
Joseph Antony Jude, Rutgers, The State University of New Jersey - Busch Campus, United StatesLei Bao, Zhengzhou University, China
Copyright © 2025 Yang, Liu, Peng, Meng and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangjing Meng, MTUyNjY1MDYwMDFAMTYzLmNvbQ==; Qiang Jia, amlhcWlhbmc1NjMyQDE2My5jb20=
 Bijun Yang
Bijun Yang Xiaoman Liu
Xiaoman Liu Cheng Peng
Cheng Peng Xiangjing Meng
Xiangjing Meng Qiang Jia
Qiang Jia