- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Cardiology, Beijing University of Chinese Medicine Third Affiliated Hospital, Beijing, China
- 3Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
Background: Hydroxysafflor yellow A (HSYA) possesses a variety of pharmacological activities which has been demonstrated to be effective against ischemic heart disease (IHD). This study aimed to comprehensively examine the efficacy and summarize the potential mechanisms of HSYA against IHD in animal models.
Methods: We conducted electronic searches for preclinical studies on PubMed, Embase, Web of Science, Cochrane Library, CNKI, SinoMed, Wanfang, and Chinese VIP databases from inception to 31 January 2024. The CAMARADES checklist was chosen to assess the quality of evidence. STATA 14.0 software was utilized to analyze the data. The underlying mechanisms were categorized and summarized.
Results: Twenty-eight studies involving 686 rodents were included and the mean score of methodology quality was 5.04 (range from 4 to 7). Meta-analysis observed that HSYA could decrease myocardial infarction size (SMD: −2.82, 95%CI: −3.56 to −2.08, p < 0.001) and reduce the levels of biomarkers of myocardial injury including cTnI (SMD: −3.82, 95%CI: −5.20 to −2.44, p < 0.001) and CK-MB (SMD: −2.74, 95%CI: −3.58 to −1.91, p < 0.001). HSYA displayed an improvement in cardiac function indicators including LVEF, LVSP, +dp/dt max and -dp/dt max. Furthermore, HSYA was able to reduce the levels of MDA, TNF-α and IL-6, while increasing SOD and NO levels. Mechanistically, the protective effect of HSYA in alleviating myocardial injury after ischemia may be associated with NLRP3 inflammasome, Bcl-2, Bax, caspase-3, eNOS proteins, and TLR/NF-κB, Nrf2/HO-1, JAK/STAT, PI3K/Akt, AMPK/mTOR, VEGFA pathways.
Conclusion: This study demonstrates that HSYA exerts cardioprotective effects in decreasing infarct size, reducing myocardial enzymes and improving cardiac function, which may be mediated by anti-inflammatory, antioxidant, anti-apoptotic, regulation of autophagy, improvement of microcirculation and promotion of angiogenesis. However, the absence of safety assessment, lack of animal models of co-morbidities, and inconsistency between timing of administration and clinical practice are limitations of preclinical studies.
Systematic Review Registration: clinicaltrials.gov, Identifier, CRD42023460790.
1 Introduction
Cardiovascular disease (CVD) remains a formidable threat to global public health. Despite considerable advancements in the treatment of CVD over the past 30 years, there is still an increasing trend in both incidence and mortality worldwide (Mensah et al., 2019). In 2020, the United States reported 127.9 million prevalent cases of CVD among adults, whereas there was a higher number of 330 million cases in China (Tsao et al., 2023; WCOTROCHADIC, 2023). Ischemic heart disease (IHD) is responsible for 49.2% of all CVD deaths and is also the leading cause of heart failure, accounting for 38.1%, which places a heavy burden on human health and medical care costs (Roth et al., 2020; Joseph et al., 2023). IHD comprises a range of myocardial ischemic diseases such as myocardial ischemia (IS), myocardial infarction (MI), and myocardial ischemia/reperfusion injury (MIRI) (Dong et al., 2023). In general, irreversible cardiomyocyte damage and death resulting from insufficient blood-oxygen supply are typical characteristics of IHD (Schirone et al., 2022). Therefore, several cardioprotective interventions aimed at ameliorating cardiac function by reducing cardiomyocyte injury and death have been implemented (Heusch, 2020).
As one of the oldest and most established medical systems in the world, traditional Chinese medicine (TCM) has been used and developed for more than 2,500 years. Currently, TCM plays an indispensable role in clinical treatment. According to the theory of TCM, IHD belongs to the category of “chest impediment disease”, and “blood stasis pattern” is the key pathogenesis. Based on pattern differentiation and treatment, “activating blood” and “resolving stasis” are the basic treatment principles of such disease. In the Compendium of Materia Medica (Ming Dynasty, ∼500 years ago), Carthamus tinctorius L. [Compositae; Carthami flos] is described as being able to “invigorate the blood circulation” (Cheng et al., 2024) and also becomes one of the frequently utilized botanical drugs for blood stasis disorders, suggesting its potential applications against IHD. The principal active product derived from safflower is called hydroxysafflor yellow A (HSYA), which serves as the designated marker metabolite utilized to measure the medicinal benefits of safflower in the 2020 edition of the People’s Republic of China Pharmacopoeia (Zhao et al., 2020). As reported, HSYA possesses a variety of pharmacological properties including antithrombotic, anti-inflammatory, antioxidant, anti-apoptotic, cardio-cerebral and even liver protection effects (Xue et al., 2021; Bai et al., 2020). For example, HSYA inhibits inflammation by regulating nod-like receptor protein-3 (NLRP3) inflammasome and suppressing nuclear factor kappa-B (NF-κB) signal pathway (Han et al., 2016; Ye et al., 2020). HSYA can also promote angiogenesis in the ischemic myocardium (Zou et al., 2018). Despite the facts that the therapeutic role and molecular mechanisms of HSYA for IHD have been partially elucidated, it is still difficult to transfer these findings from animals into humans. Certain scholars contend that a preclinical meta-analysis or systematic review is essential for improving the reproducibility and applicability of animal research (Spanagel, 2022). However, to the best of our knowledge, no review has systematically examined the effects of HSYA for IHD in animal models. Therefore, this systematic review aimed to investigate the therapeutic effects of HSYA for IHD and summarize the potential mechanisms, forming a chain of preclinical evidence and hoping to provide some ideas and research orientation for future preclinical research.
2 Materials and methods
2.1 Protocol
We have registered the protocol in the PROSPERO (CRD42023460790) and the status is “ongoing” now. Besides, the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) 2020 statement and PRISMA-ATCM reporting guidelines was strictly followed in this study (Page et al., 2021; Zhao et al., 2022).
2.2 Search strategies
We did electronic searches across eight databases: PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, SinoMed, Wanfang, and Chinese VIP, from their inception up to 31 January 2024. The search entries were formulated as: (“hydroxysafflor yellow A” OR “HSYA”) AND (“myocardial ischemia” OR “myocardial infarction” OR “myocardial ischemia-reperfusion” OR “myocardial I/R”).
2.3 Inclusion criteria
The following prespecified inclusion criteria were stated: 1) The literature type was animal studies; 2) The research subjects were rats or mice, and the experimental animal models were IS, MI, or MIRI; 3) The intervention group received any dose of HSYA as monotherapy; 4) The control group was given a vehicle or no treatment; 5) The primary outcomes were myocardial infarct size (MIS), the serum level of cardiac troponin I (cTnI), creatine kinase myocardial band (CK-MB), CK, and lactic dehydrogenase (LDH). The secondary outcomes were left ventricular ejection fraction (LVEF), left ventricular systolic pressure (LVSP), left ventricular end dilated pressure (LVEDP), maximal left ventricular pressure rising rate (+dp/dt max) or maximal left ventricular pressure decreasing rate (-dp/dt max), the fibrosis of whole myocardium, the serum levels of tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), malondialdehyde (MDA), superoxide dismutase (SOD), 6-keto-prostaglandin F1α (6-keto-PGF1α), or nitric oxide (NO), the endothelial nitric oxide synthase (eNOS) activity in myocardium, the protein expression of B-cell lymphroma-2 (Bcl-2), Bcl-2-associated X protein (Bax), cleaved caspase-3 or light chain 3 (LC3), the mRNA level of nuclear factor erythroid 2-related factor 2 (Nrf2) or vascular endothelial growth factor A (VEGFA), and the apoptosis rate of cardiomyocytes.
2.4 Exclusion criteria
The following were preset criteria for exclusion: 1) Not in vivo studies: clinical trials, reviews, letter, meeting abstracts, in vitro studies; 2) Not rats or mice models with IS, MI and MIRI; 3) HSYA was not the only intervention; 4) The control group received any drugs treatment; 5) No one of the outcome indicators; 6) Studies with repeated publication of data; 7) Studies not available in full text.
2.5 Data extraction
This task was completed by two researchers independently, and the third researcher was responsible for resolving disagreements. The specifics of data extraction comprised: 1) General details: the first author’s name and publication year; 2) Animal information: species, gender, weight, and sample sizes; 3) Animal model: modeling methods and the anesthesia methods for model induction; 4) Intervention group: the dosage, administration method and time; 6) Control group: the vehicle name; 7) Outcome indicators.
We obtained data only from the highest dose group and final time point if the medication was administered at different doses or outcome indicators were recorded at multiple time nodes. The online tool WebPlotDigitizer was used to calculate the mean and standard deviation (SD) if the data was depicted graphically.
2.6 Risk of bias and quality assessment
To evaluate the risk of bias and methodological quality of enrolled studies, the grading scale of CAMARADES 10-item with minor modifications was employed (Macleod et al., 2004). The modifications are as follows: (F): use of anesthetic without significant intrinsic cardioprotective activity; (I): compliance with animal welfare regulations (including three or more of the following points: preoperative anaesthesia, postoperative analgesia, nutrition, disinfection, environment temperature, environment humidity, circadian rhythm, and euthanasia). Two individuals evaluated all enrolled literature independently.
2.7 Statistical analysis
Meta-analysis was conducted with the use of STATA 14.0 software. All outcome indicators belong to continuous variable type data and were shown as the standardized mean difference (SMD) along with a 95% confidence interval (95% CI). The statistical significance between the medication and control groups was set at p < 0.05. The effects model was selected depending on heterogeneity measures by Q test and I2 statistics. A fixed effects model was applied to merge the effect size when the included studies had better homogeneity with I2 ≤ 50% and p > 0.1. Otherwise, the random effects model was adopted (I2 > 50% and p < 0.1). Meanwhile, we performed meta-regression and subgroup analyses to explore the probable reasons for heterogeneity. To assess the credibility of the pooled results, we further carried out the sensitivity analysis. When more than 10 studies were included, Egger’s test with p < 0.05 was denoted by a significant publication bias.
3 Results
3.1 Research selection
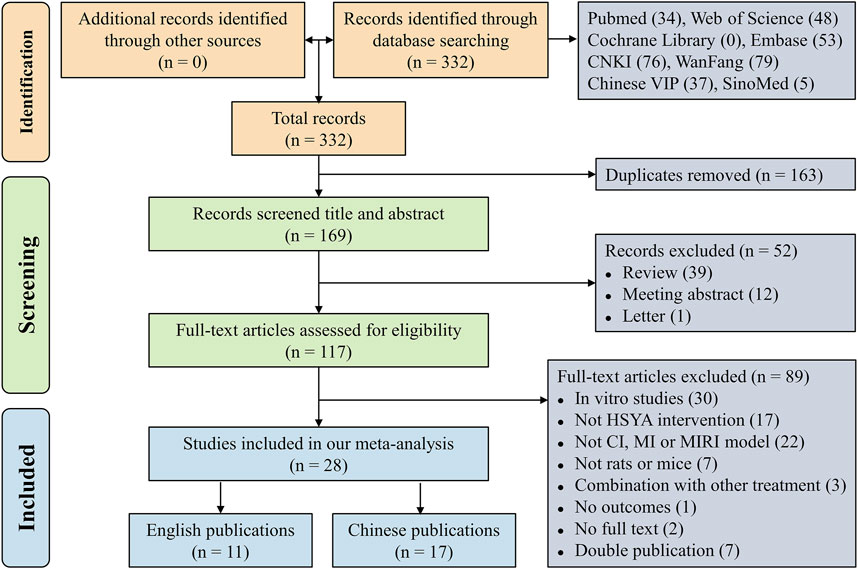
Initially, a total of 332 records were retrieved electronically. Then, 163 duplicate articles were excluded and there were 169 articles left. Next, 52 literature consisting of 39 reviews, 12 meeting abstracts and 1 letter were excluded after screening their titles and abstracts. Subsequently, 89 papers that met the exclusion criteria were excluded by skimming the whole text. Finally, our meta-analysis comprised 28 studies (Wang et al., 2007; Huang et al., 2009; Jin et al., 2009; Wang, 2009; Wang et al., 2009; Fu et al., 2011; Liu et al., 2011; Sun, 2012; Zhang et al., 2012; Guan et al., 2013; Dong et al., 2014; Xu et al., 2014; Chen, 2015; Hu et al., 2015; Liu et al., 2015; Zhou et al., 2015; Hu et al., 2016; Wei et al., 2016; Wei et al., 2017; Ni et al., 2018; Su et al., 2018; Zou et al., 2018; Zhou et al., 2019; Ye et al., 2020; Zhang and Zhang, 2020; He, 2022; Ge et al., 2023; Yu and Qu, 2023). Figure 1 displays the flowchart of literature selection.
3.2 Research quality
The quality scores of studies included in the meta-analysis ranged from 4 to 7, with an average of 5.04. Twenty-five studies underwent peer review. Only nine articles definitely mentioned temperature control, and two articles failed to describe the random allocation. Despite all studies employed appropriate animal models, blinded induction of model and blinded assessment of outcome were not mentioned in any of the studies. With the exception of one, all articles described the use of anesthetic without significant intrinsic cardioprotective activity. Each study provided information on the number of animals used, nonetheless, it is a pity that none of them calculated the sample size. Sixteen articles abided by the compliance with animal welfare regulations and ten literature declared potential conflict of interests. The methodological quality of each study is presented in Figure 2.
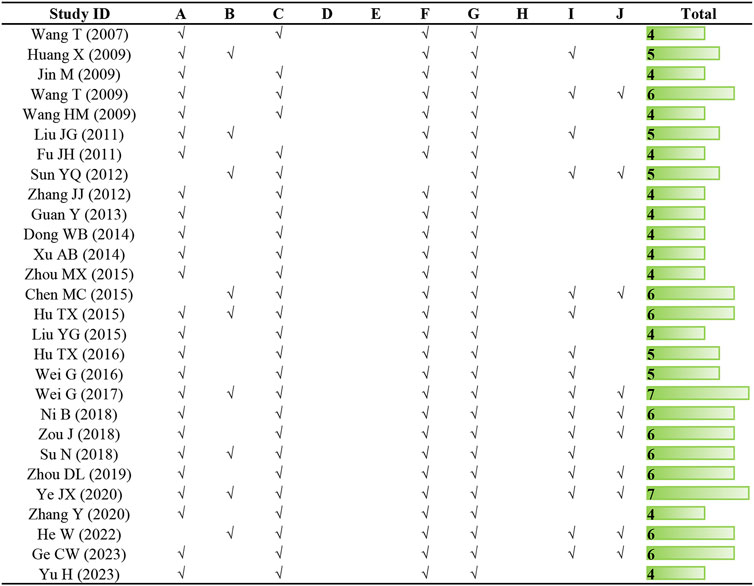
Figure 2. Risk of bias and quality assessment scores of included studies. Note: (A) peer review publication; (B) control of temperature; (C) random allocation to treatment or control; (D) blinded induction of model; (E) blinded assessment of outcome; (F) use of anesthetic without significant intrinsic cardioprotective activity; (G) appropriate animal model; (H) sample size calculation; (I) compliance with animal welfare regulations (including three or more of the following points: preoperative anesthesia, postoperative analgesia, nutrition, disinfection, environment temperature, environment humidity, circadian rhythm, and euthanasia); (J) statement of potential conflict of interests.
3.3 Research characteristics
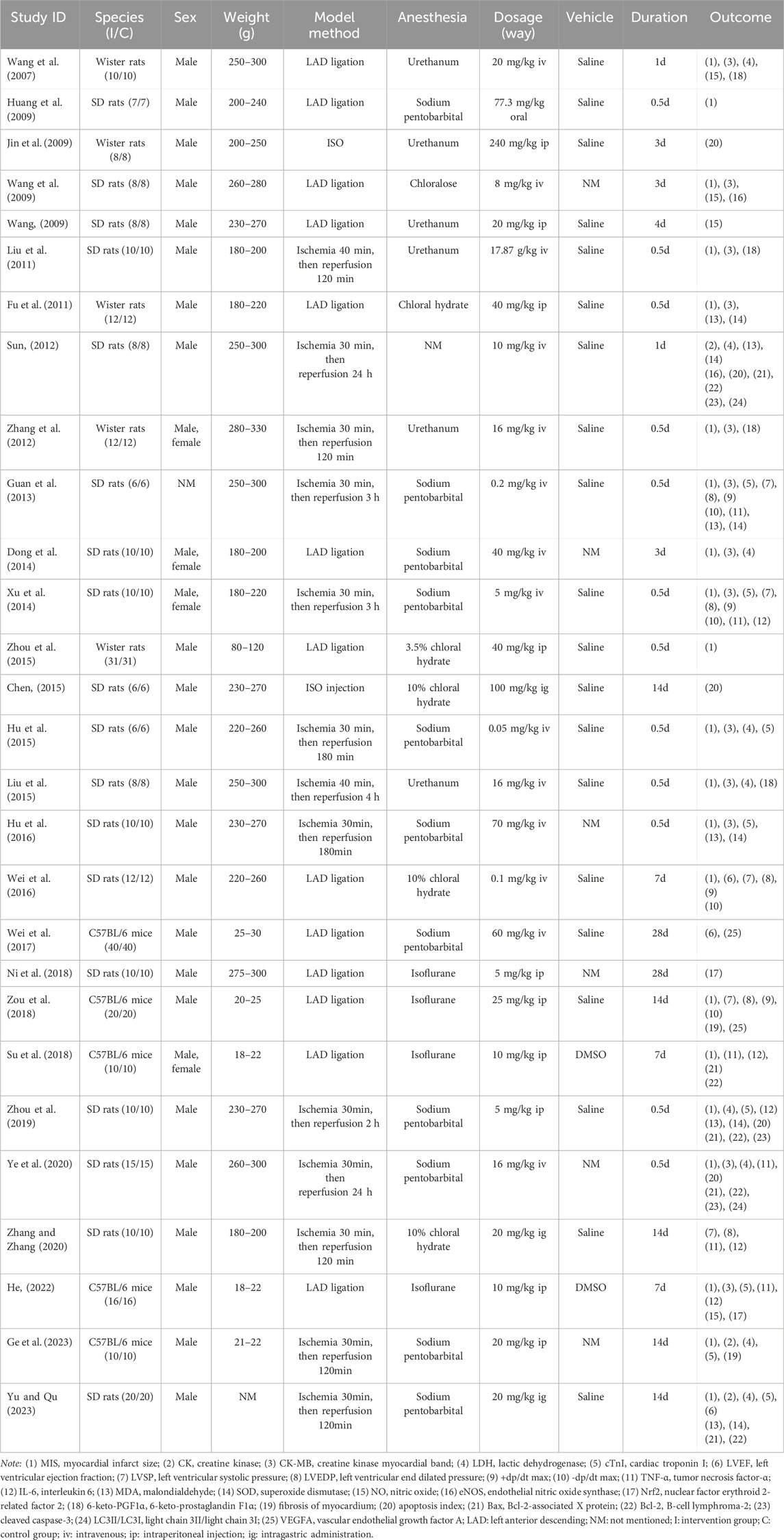
Twenty-eight articles were included for meta-analysis, with 11 articles being English and the other 17 articles being Chinese publications. The detailed characteristics of the 28 articles are given in Table 1.
There were 686 included animals in total, of which 50.73% were Sprague-Dawley rats (348/686), 21.28% were Wister rats (146/686), and 27.99% were C57BL/6J mice (192/686). In terms of sex, the proportion of male and female animals were 92.13% (632/686) and 6.12% (42/686), respectively, while the left 1.75% (12/686) of animals were not mentioned sex. Regarding the methods of animal models establishment, 13 studies used only left anterior descending (LAD) ligation, 2 studies conducted by isoprenaline (ISO) injection, and 13 studies used the reperfusion after LAD ligation. To induce anesthesia, 11 studies used sodium pentobarbital, six studies used urethane, four studies used isoflurane, five studies utilized chloral hydrate, one study utilized chloralose, and the residual one study did not report the anesthetic. All studies were divided into three dose groups (low dose group: dosage ≤20 mg/kg/d, medium dose group: 20 mg/kg/d < dosage ≤40 mg/kg/d, high dose group: dosage >40 mg/kg/d) according to the dosage of HSYA, with 18 studies at low dose, four studies at medium dose, and six studies at high dose. The basic information about HSYA in each study are presented in Supplementary Table S1.
3.4 Primary outcome indicators
3.4.1 Myocardial infarct size (MIS)
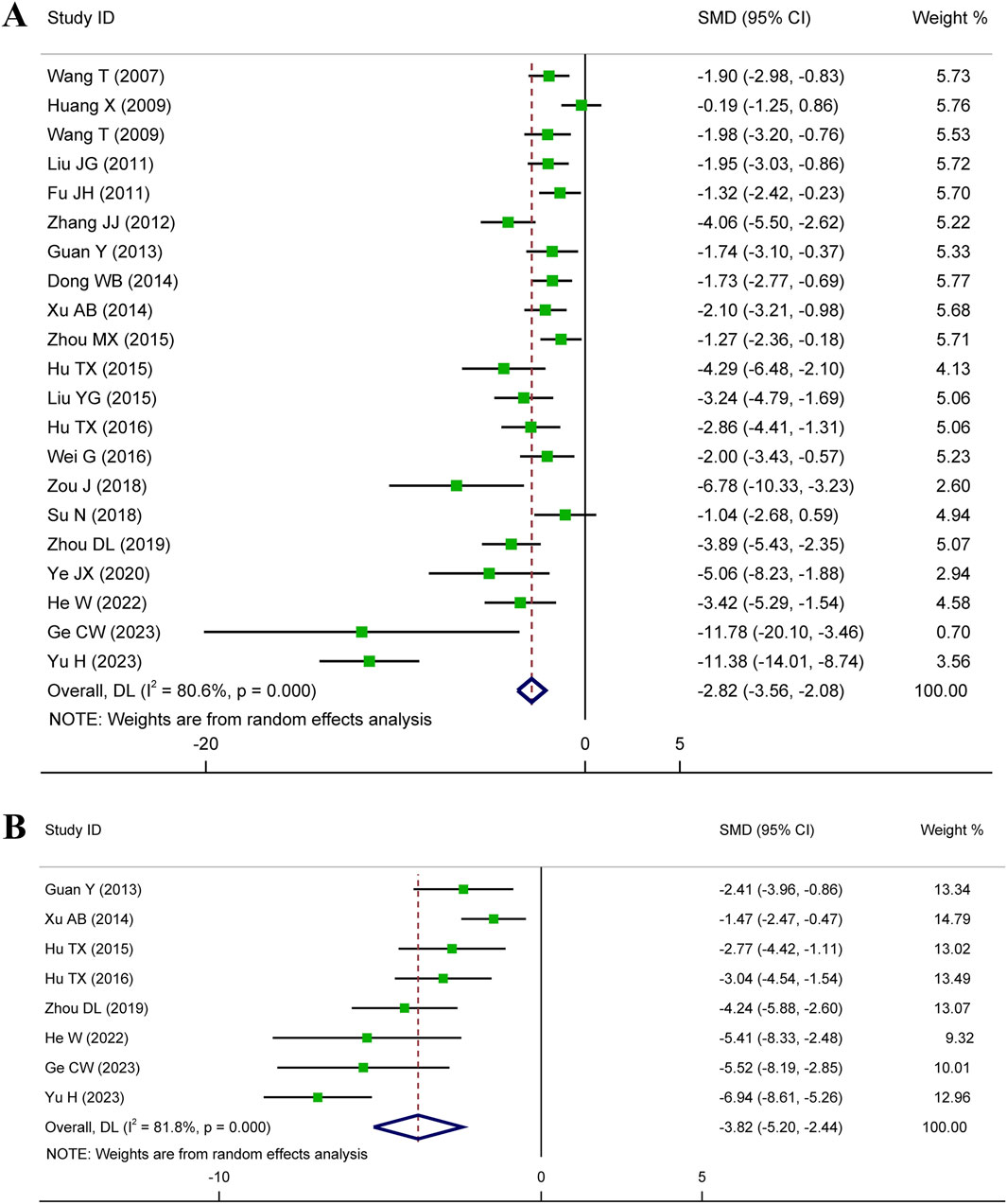
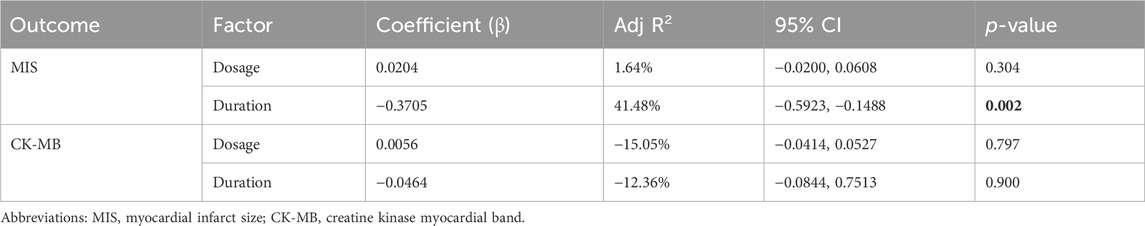
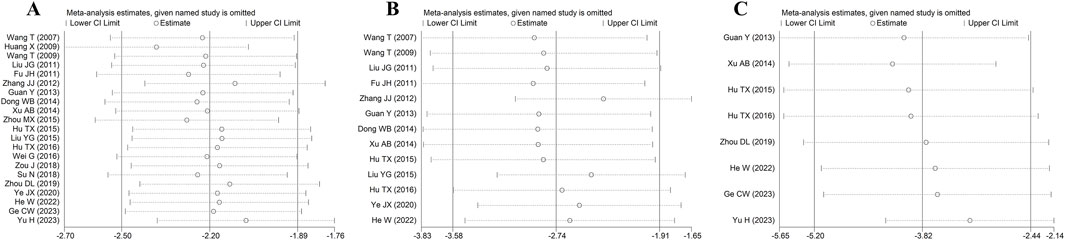
Meta-analysis of twenty-one studies proclaimed that HSYA had a significant effect on decreasing MIS in the animal models of IS, MI, and MIRI (n = 335, SMD: −2.82, 95% CI: −3.56 to −2.08, p < 0.001), with a high heterogeneity (I2 = 80.6%, p < 0.001) (Figure 3A). To identify the potential reason of heterogeneity, the meta-regression analyses for dosage of HSYA and treatment duration were performed (Figures 4A, B). The results revealed that the treatment duration was the major cause of heterogeneity for MIS (p = 0.002) (Table 2).
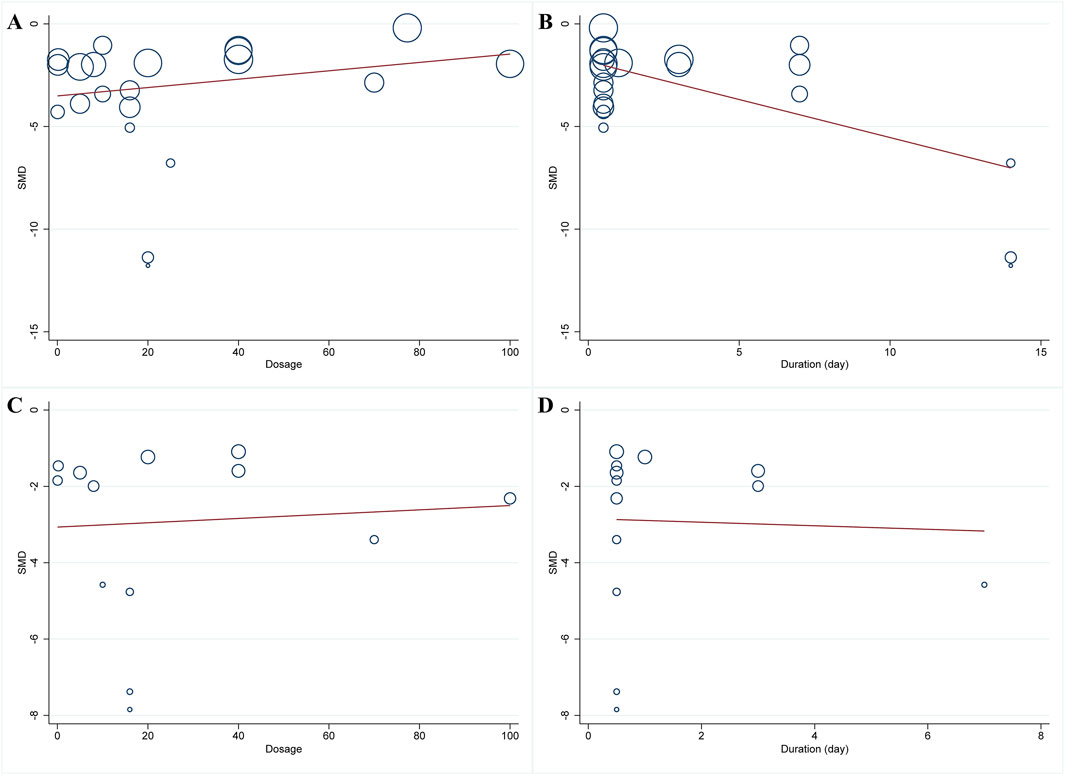
Figure 4. Meta-regression plots of MIS and CK-MB. (A) dosage and MIS; (B) treatment duration and MIS; (C) dosage and CK-MB; (D) treatment duration and CK-MB.
3.4.2 Myocardial enzymes
Myocardial enzymes are released after cardiomyocyte injury, and their levels are positively correlated with the degree of myocardial ischemia and injury.
3.4.2.1 cTnI level
Eight articles detected the serum cTnI level, while an elevated heterogeneity was observed (I2 = 81.8%, p < 0.001). The pooled results showed that compared with control, the medication group experienced a noticeable decrease in serum cTnI level (n = 142, SMD: −3.82, 95% CI: −5.20 to −2.44, p < 0.001) (Figure 3B).
3.4.2.2 CK-MB level
A total of thirteen articles with an obvious heterogeneity (I2 = 79.5%, p < 0.001) used the serum level of CK-MB as an outcome index. The meta-analysis results pointed to a significant reduction in CK-MB level with HSYA treatment (n = 226, SMD: −2.74, 95% CI: −3.58 to −1.91, p < 0.001) compared with the control group (Figure 5A). According to the results of meta-regression analysis shown in Figures 4C, D; Table 2, we found that the dosage of HSYA and duration were not the major factors of heterogeneity for CK-MB level.
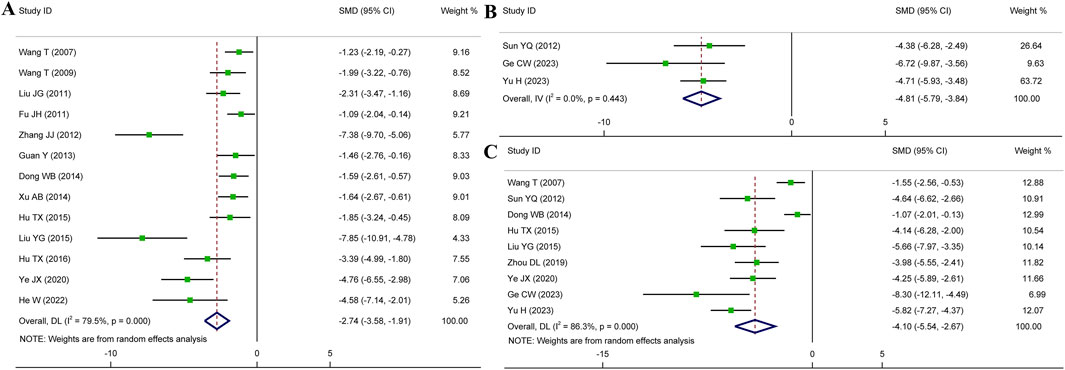
Figure 5. Forest plot presenting SMD and 95%CI for the effect of HSYA on the levels of CK-MB (A), CK (B), and LDH (C).
3.4.2.3 CK level
Overall three articles reported the serum level of CK, and a fixed effects model was employed due to low heterogeneity (I2 = 0.0%, p = 0.443). Compared to the control group, HSYA exhibited a notable impact on decreasing serum CK level (n = 68, SMD: −4.81, 95% CI: −5.79 to −3.84, p < 0.001) (Figure 5B).
3.4.2.4 LDH level
Nine articles were analyzed using a random effects model (I2 = 86.3%, p < 0.001). We could observe that the serum levels of LDH were significantly decreased in HSYA group (n = 176, SMD: −4.10, 95% CI: −5.54 to −2.67, p < 0.001) (Figure 5C).
3.5 Secondary outcome indicators
3.5.1 Cardiac function
To confirm the impact of HSYA therapy on cardiac function in MI, IS, and MIRI animal models, the related indices such as LVEF, LVSP, LVEDP, and ±dp/dt max were examined. Among these indicators, LVEF serves as an indicator of cardiac contractility. Meanwhile, LVSP, LVEDP, and ±dp/dt max represent the hemodynamic state and cardiac compliance of ventricle. Three research for LVEF, five for LVSP or LVEDP, and four studies for ± dp/dt max were brought into our meta-analysis. Figure 6 showed that HSYA treatment yielded remarkable improvements in LVEF (n = 62, SMD: 4.45, 95% CI: 3.48 to 5.43, p < 0.001; I2 = 45.6%, p = 0.159), LVSP (n = 74, SMD: 2.03, 95% CI: 1.44 to 2.62, p < 0.001; I2 = 35.3%, p = 0.186), LVEDP (n = 74, SMD: −2.21, 95% CI: −3.45 to −0.97, p < 0.001; I2 = 74.4%, p = 0.004), +dp/dt max (n = 54, SMD: 1.72, 95% CI: 1.07 to 2.37, p < 0.001; I2 = 7.6%, p = 0.355), and -dp/dt max (n = 54, SMD: 1.88, 95% CI: 1.20 to 2.55, p < 0.001; I2 = 36.7%, p = 0.192). To summarize, HSYA treatment prominently improved cardiac dysfunction caused by myocardial injury. Specifically, HSYA enhanced myocardial contractility, consequently leading to an improvement in cardiac systolic function. It also improved cardiac hemodynamics and ventricular compliance, thereby contributing to the improvement of cardiac diastolic function.
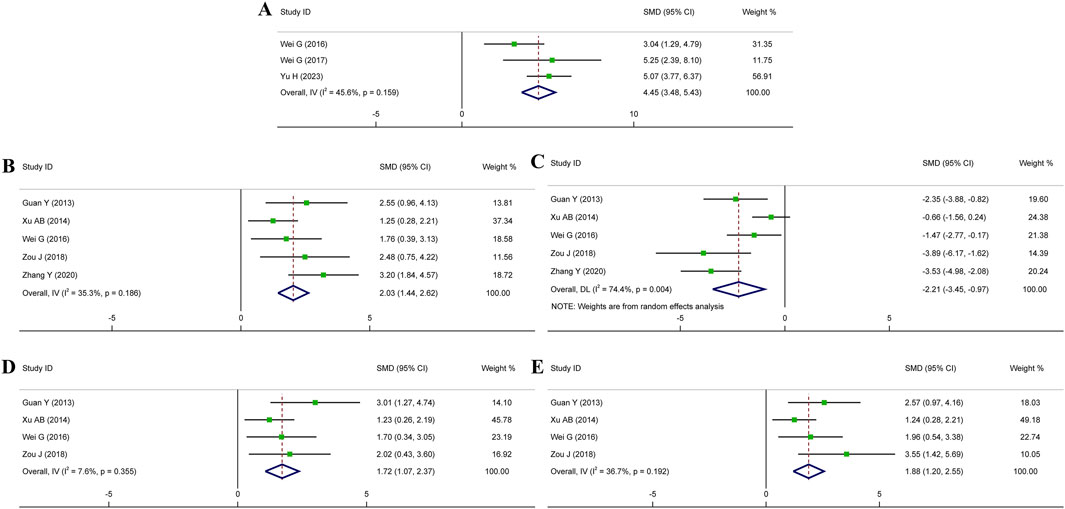
Figure 6. Forest plot presenting SMD and 95%CI for the effects of HSYA on cardiac function. (A) LVEF; (B) LVSP; (C) LVEDP; (D) +dp/dt max; (E) -dp/dt max.
3.5.2 Inflammatory cytokines
Inflammation is the main factor of myocardial ischemic injury, and TNF-α and IL-6 are important mediators of inflammatory response. We analyzed data from six studies for TNF-α and five studies for IL-6. The pooled effects demonstrated that HSYA importantly reduced the levels of TNF-α (n = 80, SMD: −4.68, 95% CI: −6.40 to −2.95, p < 0.001; I2 = 70.2%, p = 0.005) and IL-6 (n = 76, SMD: −3.98, 95% CI: −5.96 to −1.99, p < 0.001; I2 = 81.9%, p < 0.001) in comparison to control group (Supplementary Figure S1). Our findings exhibited a significant therapeutic effect for anti-inflammation of HSYA in animal models.
3.5.3 Oxidative stress indicators
To determine the relationship between HSYA treatment and oxidative stress levels, we used the following five indicators: the serum levels of MDA, SOD and NO, the protein eNOS activity, and the mRNA level of Nrf2 in myocardium. MDA and SOD are good indicators for evaluating oxidative stress, and Nrf2 is an important regulatory factor for antioxidant activity. The pooled estimates displayed that compared to control group, HSYA treatment could decrease MDA level (n = 124, SMD: −3.23, 95% CI: −4.39 to −2.06, p < 0.001; I2 = 76.1%, p = 0.001), enhance SOD activity (n = 124, SMD: 2.73, 95% CI: 1.61 to 3.85, p < 0.001; I2 = 78.4%, p < 0.001) and increase NO level (n = 62, SMD: 1.77, 95% CI: 1.15 to 2.39], p < 0.001; I2 = 49.5%, p = 0.114). HSYA also showed a definite action on increasing eNOS activity (n = 32, SMD: 4.41, 95% CI: 1.27 to 7.55, p = 0.006; I2 = 79.0%, p = 0.029) and Nrf2 mRNA level (n = 26, SMD: 8.01, 95% CI: 5.52 to 10.50, p < 0.001; I2 = 0.0%, p = 0.734) (Supplementary Figure S2). Hence, it is confirmed that HSYA could alleviate oxidative stress levels in animal models of IHD.
3.5.4 Platelet aggregation indicator
6-keto-PGF1α is a factor of platelet aggregation that reflects the blood circulation of myocardial. The reduced level gives rise to platelet aggregation and disorder of blood circulation, thereby exacerbating the extent of myocardial ischemia. A total of four studies reported the serum level of 6-keto-PGF1α as an outcome indicator and a random effects model was applied. According to the meta-analysis, HSYA increased serum level of 6-keto-PGF1α (n = 80, SMD: 2.51, 95% CI: 0.57 to 4.45, p = 0.011) compared to control and exhibited the inhibitory effect on platelet aggregation (Supplementary Figure S3A).
3.5.5 Fibrosis of myocardium
Two studies applied Masson staining to detect the degree of myocardial injury and fibrosis. A fixed effects model was employed and the pooled results suggested that HYSA treatment alleviated myocardial injury and delayed the fibrosis progression (n = 16, SMD: −5.39, 95% CI: −7.77 to −3.00, p < 0.001) (Supplementary Figure S3B).
3.5.6 Autophagy indicator
Two studies used the ratio of LC3II/LC3I as the outcome index and were analyzed by random effects model in this meta-analysis. The pooled results found no obviously statistical difference of HSYA on LC-3II/LC-3I ratio (n = 22, SMD: −15.02, 95% CI: −54.41 to 24.38, p = 0.455) in animal models of myocardial injury (Supplementary Figure S3C).
3.5.7 Angiogenesis indicator
Two studies utilized VEGFA mRNA expression to evaluate the role of HSYA in angiogenic. The homogeneity between two studies was positive and the fixed effects model was utilized. The mRNA expression of VEGFA was significantly higher in HSYA group than in the control group (n = 20, SMD: 11.33, 95% CI: 7.30 to 15.36, p < 0.001), which suggests that HSYA is beneficial to the promotion of angiogenesis after myocardial injury in animal models (Supplementary Figure S3D).
3.5.8 Apoptosis indicators
To investigate the correlation between HSYA treatment and cell apoptosis, we focused on some indicators of myocardial apoptosis. Five articles reported myocardial apoptosis rate by TUNEL assay, five articles provided data on Bax protein and Bcl-2 protein, as well as three articles on cleaved caspase-3. Results from eligible studies demonstrated that HSYA alleviated myocardial apoptosis (n = 70, SMD: −3.09, 95% CI: −4.55 to −1.64, p < 0.001; I2 = 69.6%, p = 0.011), inhibited the activation of caspase-3 (n = 28, SMD: −9.94, 95% CI: −19.40 to −0.48, p = 0.039; I2 = 90.1%, p < 0.001), reduced the Bax expression (n = 88, SMD: −5.28, 95% CI: −8.52 to −2.03, p = 0.001; I2 = 91.0%, p < 0.001) and increased Bcl-2 expression (n = 88, SMD: 6.42, 95% CI: 4.46 to 8.39, p < 0.001; I2 = 57.9%, p = 0.050) (Supplementary Figure S4). Taken together, HSYA could mitigate cardiomyocyte apoptosis in vivo of myocardial injury.
3.6 Subgroup analyses
Based on the potential factors (dosage, treatment duration, and stage of administration) that may contribute to heterogeneity, the subgroup analyses of MIS, cTnI, and CK-MB were conducted. In addition, considering that there were different methods of MIS calculation, subgroup analysis of MIS was also performed accordingly: area at infarction/area at risk (AAI/AAR), area at risk/left ventricle area (AAR/LVA), and area at risk/total myocardial area (AAR/TMA).
As presented in Table 3, the treatment duration was the major cause of heterogeneity for MIS, but the methods of MIS were not. It is consistent with the results of the meta-regression analysis. For CK-MB, the p-value of heterogeneity between groups was less than 0.01, indicating that the dosage of HSYA was the main source of heterogeneity. Similarly, the stage of administration may be the major source of heterogeneity for cTnI.
3.7 Publication bias and sensitivity analyses
For the primary outcome indicators MIS and CK-MB, the output results of publication bias using Egger’s test indicated the presence of publication bias (MIS: t = −4.85, p < 0.001; CK-MB: t = −7.85, p < 0.001) (Supplementary Figure S5).
Owing to the existence of heterogeneity and publication bias, we completed the sensitivity analyses using the leave-one-out method to assess the robustness of MIS, CK-MB, and cTnI. The results of sensitivity analyses showed that the overall effect size was not significantly changed, which implied that our findings of this study were relatively stable and reliable (Figure 7).
4 Discussion
4.1 Summary of evidence
Up to now, this is the first preclinical meta-analysis and systemic review to examine the effect of IHD following HSYA treatment in vivo. A previous study (Zhao et al., 2020) systematically summarized the basic data for related research of HSYA, including pharmacological effects and molecular mechanism. But it focused more on reporting the methods of acquisition, extraction and detection, as well as pharmacokinetics. Moreover, regarding the cardioprotective effects of HSYA, it only summarized oxidative stress and inflammation related signaling pathways. In our review, we provided evidence about the efficacy of HSYA against IHD through meta-analysis and updated a more comprehensive review of pharmacological mechanisms.
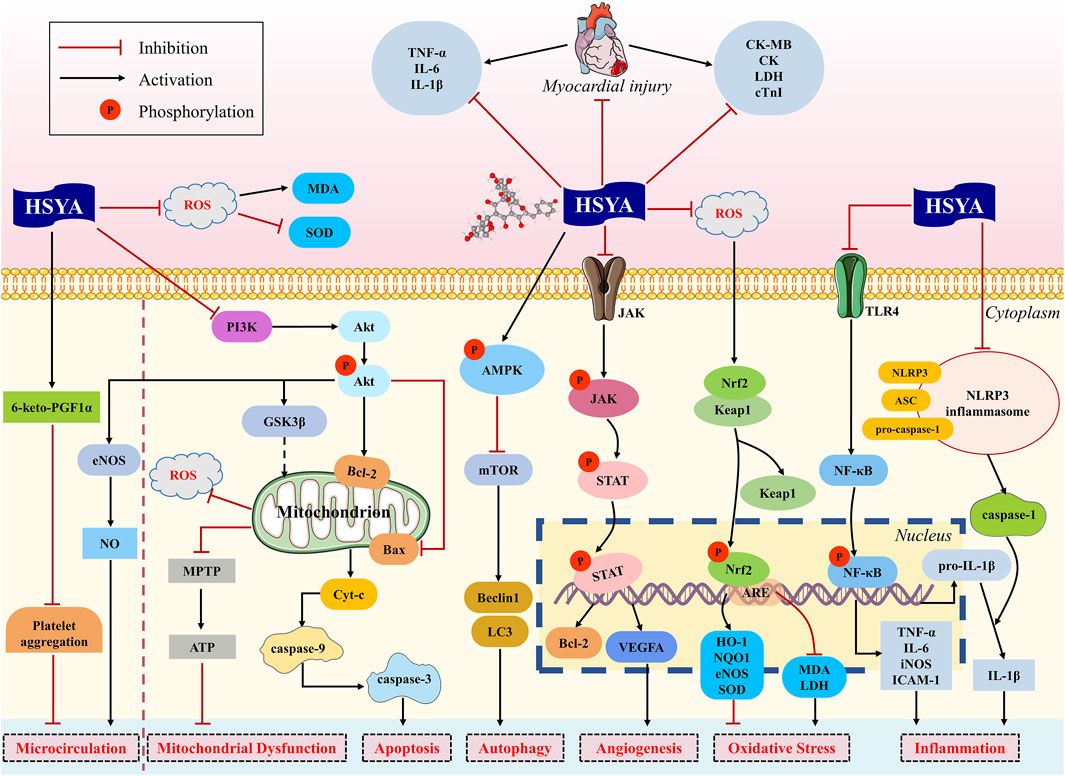
Our findings found that HSYA is devoted to reducing MIS, serum myocardial enzymes and troponin, enhancing cardiac function, decreasing serum inflammatory cytokines, and ameliorating myocardial apoptosis and fibrosis. In brief, HSYA exerts potential cardioprotective action against myocardial ischemic injury mainly through anti-inflammatory, antioxidant, anti-apoptotic, regulation of autophagy, improvement of microcirculation and mitochondrial dysfunction, and promotion of angiogenesis (Figure 8).
4.2 Molecular mechanisms of HSYA for myocardial ischemia
4.2.1 Effects on inflammation
During the process of myocardial ischemia, inflammatory cells infiltrate the infarct region and promote the release of inflammatory mediators. The inflammatory responses localized at the myocardium are triggered, which is considered to be the primary factor responsible for myocardial damage and aggravated cardiac dysfunction (Ong et al., 2018).
The toll-like receptor 4 (TLR4)/NF-κB signal pathway contributes an important part in inflammation. TLR4, as a pattern recognition receptor located on the cell membrane, participates in the inflammatory response actively (Wei et al., 2023). In case of myocardial injury, TLR4 activation triggers the downstream signaling p-NF-κB, bringing about the secretion of proinflammatory cytokines such as TNF-α, IL-6, IL-1β, and inducible nitric oxide synthase (iNOS) (Moghimpour Bijani et al., 2012). This inflammatory cascade worsens myocardial damage and is likely to induce an enlargement of infarct size. It was reported that HSYA attenuated TLR4 overexpression and NF-κB activation, contributing to the downregulation of TNF-α, IL-6 and IL-1β, and the upregulation of anti-inflammatory cytokine IL-10, which in turn inhibits the inflammatory response of cardiomyocytes (Guan et al., 2013; Han et al., 2016).
Moreover, recent preclinical studies of MI and MIRI have identified that NLRP3 inflammasome is also closely connected to heart inflammation, infarct size, and cardiac function (Toldo et al., 2016). NLRP3 inflammasome is composed of three components: NLRP3 protein, apoptosis-associated speck-like (ASC) protein, and pro-caspase-1 (Abderrazak et al., 2015). Ischemia can trigger NLRP3 activation, which contributes to the production and release of IL-1β (Xu et al., 2020; Dai et al., 2023). HSYA effectively suppressed NLRP3 inflammasome activation in rats with MIRI, resulting in decreased IL-1β release and infarction size (Ye et al., 2020).
4.2.2 Effects on oxidative stress
Excessive production of reactive oxygen species (ROS) is the main pathophysiological factor implicated in the progression of MI and MIRI (Peoples et al., 2019). During myocardial ischemia, the amount of ROS increases and antioxidant systems are impaired. The redox transcription factor Nrf2 is essential to the regulation of cellular redox homeostasis, thereby exhibiting the effect of antioxidant stress. Keap1 is a vital element for the regulation of Nrf2. Under normal conditions, Nrf2 is bound to Keap1, which promotes the proteasomal degradation of Nrf2 by way of ubiquitination, keeping Nrf2 maintain a low level in the cytoplasm. But in oxidative stress conditions, Nrf2 dissociates from its complex pair and translocates to the nucleus. Once Nrf2 is transported to the nucleus, it always binds to the sequence known as the antioxidant response element (ARE), boosting the transcription of Nrf2-regulated genes (Loboda et al., 2016; Geertsema et al., 2023). Two downstream targets of Nrf2, heme oxygenase-1 (HO-1) and NAD(P)H-quinone oxidoreductase 1 (NQO1) are believed to possess a protective effect on oxidative stress. HSYA has been shown to reduce oxidative stress damage in mice which is achieved by inhibiting the level of Keap1 mRNA and enhancing the level of Nrf2 and HO-1 (He, 2022). As well, HSYA also exerts antioxidant and anti-hypertrophic effects in rats with MI by activating Nrf2/HO-1 signaling pathway (Ni et al., 2018).
4.2.3 Effects on apoptosis and mitochondrial dysfunction
Apoptosis is recognized to involve in the progression of myocardial ischemia (Singh et al., 2019). Cardiomyocyte apoptosis begins shortly following the onset of MI and appears to be enhanced apparently during reperfusion, leading to the stasis of myocardium, the enlargement of infarct area, and the insufficiency of cardiac function (Zhao et al., 2003).
The overproduction of ROS is the major apoptotic stimulus signal. Subsequently, the apoptotic cascade is initiated, which involves the loss of mitochondrial membrane potential, the opening of mitochondrial permeability transition pore (MPTP), and the release of cytochrome-C (Cyt-C) (Heusch, 2020). Cyt-C can combine with apoptotic protease activating factor-1 (Apaf-1), causing the sequential activation of caspase-3, which is subsequently processed into cleaved caspase-3. The activated and cleaved caspase-3 is regarded as an indicator of the intensity of apoptosis (Sahoo et al., 2023). Bcl-2 and Bax are crucial mitochondrial membrane proteins and serve as the integral regulators of apoptosis, which are responsible for maintaining mitochondrial membrane integrity and the balance of apoptosis (Czabotar and Garcia-Saez, 2023; Senichkin et al., 2020). Bcl-2 can block Cyt-C release and regulate MPTP opening to inhibit apoptosis, whereas Bax performs the opposite effect. HSYA was found to reduce Cyt-C release, increase the expression level of Bcl-2, and decrease the expression of Bax and caspase-3 in vivo, thereby inhibiting cardiomyocyte apoptosis (Sun, 2012; Zhou et al., 2019; Ye et al., 2020).
Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signal pathway is essential for cell apoptosis and ischemia injury (Pang et al., 2023). JAK2 could be activated by ROS and then STAT1 is phosphorylated by JAK2. Previous research identified that STAT1 could modulate MPTP opening and enhance the expression of pro-apoptotic genes such as p53, Fas, and Fas ligand (FasL) (Krämer et al., 2006). HSYA decreased JAK2 and STAT1 activity, inhibited the expression of Bax, Fas, and FasL, improved antioxidant capacity, and reduced apoptosis in IR-induced models. It was suggested that HSYA is effective in ameliorating myocardial injury by inhibiting apoptosis, which is partially mediated by JAK2/STAT1 pathway (Zhou et al., 2019).
Mitochondrial dysfunction is among the pathophysiological mechanisms involved in MIRI. During acute ischemia, the overproduction of ROS causes an overload of mitochondrial Ca2+, which promotes the opening of MPTP, leading to impaired adenosine triphosphate (ATP) synthesis and mitochondrial swelling (Halestrap et al., 2004). Therefore, the formation and opening of MPTP are the major causes of mitochondrial dysfunction and cardiomyocyte apoptosis (Deng and Zhou, 2023). HSYA has been shown to prevent myocardial injury through inhibiting the opening of MPTP in isolated rat hearts (Liu et al., 2008). Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase three beta (GSK3β) signaling pathway has been claimed to benefit mitochondrial and myocardial damage (Zhang et al., 2018). GSK3β, as the key downstream molecule of PI3K/Akt pathway, has been proven to regulate MPTP opening. It has been reported that HSYA increases levels of p-Akt and GSK3β in H/R-induced H9c2 cells, which inhibits cardiomyocyte apoptosis, prevents MPTP opening, and restores mitochondrial ATP synthesis (Min and Wei, 2017).
4.2.4 Effects on microcirculation
During the progression of MI and MIRI, partly coronary microvascular cells also may experience irreversible damage except for cardiomyocyte death, leading to microcirculation dysfunction (Heusch, 2019). Platelet aggregation is a major factor in microcirculation disturbance. 6-keto-PGF1α is a derivative of prostaglandin I2 (PGI2), which is an important active substance that inhibits platelet aggregation. Studies found that 6-keto-PGF1α decreased in rats with MI and MIRI, while HSYA could increase the level of 6-keto-PGF1α (Wang et al., 2007; Liu et al., 2011; Zhang et al., 2012). Additionally, as a natural inorganic compound to protect against platelet aggregation, NO is produced in vascular endothelium by eNOS (Lundberg and Weitzberg, 2022). It was discovered that HSYA could attenuate the decrease of eNOS activity caused by acute myocardial ischemia and consequently enhance NO content in MI rats (Wang et al., 2009). This cardioprotective effect is claimed to be relevant to the promotion of the PI3K/Akt/eNOS pathway after HSYA treatment in MIRI rats (Sun, 2012).
4.2.5 Other effects
Angiogenesis is an essential process in the recovery of blood flow in ischemic myocardial tissue. It helps to establish collateral circulation and further promotes myocardial regeneration (Ware and Simons, 1997). Currently, plenty of evidence has reported the proangiogenic effect of HSYA by using various ischemic models. In MI mice, HSYA has performance in promoting myocardial neovascularization and recovering heart function, which might be attributed to the activation of HO-1/VEGF/SDF-1 cascade pathway (Wei et al., 2017). Furthermore, HSYA could upregulate VEGFA expression to promote angiogenesis in ischemic myocardium, thereby ameliorating ischemia-induced cardiac dysfunction and myocardial injury (Zou et al., 2018).
Autophagy is a major regulator of cardiac homeostasis in response to various stressful conditions, thereby reducing myocardial injury and protecting cardiac function (Sciarretta et al., 2018). The mammalian target of rapamycin (mTOR) is a well-known inhibitor of autophagy, whereas AMP-activated protein kinase (AMPK), the upstream of mTOR, is the positive regulator. It was found that HSYA could increase p-AMPK level and decrease mTOR level to stimulate cardiomyocyte autophagy in MIRI rats. Moreover, the autophagy-related proteins were observed, with Beclin one and LC3II showing an increasing trend while LC3I decreased in rats receiving HSYA (Ye et al., 2020). However, the specific mechanisms of HSYA regulating autophagy are not well understood and require further experiments.
4.3 Limitations
Firstly, nearly 50% of the studies had a quality score of less than five points, indicating an overall low quality of all included studies. Low methodological quality indeed affects the credibility of the results.
Secondly, most outcome measures in this study exhibited high heterogeneity, which could be attributed to a few factors such as animal species, drug dosage, modeling methods, and measurement errors. However, we further performed meta-regression, subgroup analysis, and sensitivity analysis, and the results validated the effectiveness and reliability of HSYA for treating myocardial ischemia.
Thirdly, it was found a significant publication bias for MIS and CK-MB. Those studies not with positive results are rarely published, especially animal experiments. Thus, the efficacy of HSYA may be overestimated. We controlled for publication bias by using as many database sources as possible, but we could not collect all the relevant literature.
Lastly, none of the preclinical studies reported the assessment of safety, which makes us fail to provide evidence of safety through meta-analysis.
4.4 Future prospects
Despite HSYA has been investigated the diverse pharmacological activities, it is well known for being a pan-assay interference compound (PAINS) if tested in vitro. PAINS are molecules that have been observed to show activity in multiple types of assays by interfering with the assay readout rather than through specific compound/target interactions, which are often viewed as highly promiscuous players that produce false positive results (Magalhães et al., 2021).
PAINS always exhibit some known typical behaviors: redox reactivity, aggregation, membrane disruption, and fluorescence interference (Nelson et al., 2017). In vitro, PAINS may interfere with oxidative stress-related detection by scavenging free radicals (redox reactivity); PAINS could lead to false results through nonspecific bind to antibodies in some biochemical assays (aggregation); color or fluorescence properties of PAINS may produce an effect on fluorescence signal (fluorescence interference). In vitro experiments are usually performed under simplified conditions that lack the complexities of environment in vivo, such as inability to be reduced by hepatic metabolic enzymes that may interfere with the assay in prototype form. Additionally, the concentrations of PAINS were used ranging from μM to mM levels, which significantly exceed the physiological concentrations observed in vivo, thereby greatly increasing the probability of nonspecific reactions (Bolz et al., 2021). It is obvious that the in vitro interference properties of HSYA may offer many traps of investigations.
However, PAINS have relatively little interference with the in vivo experiments. Firstly, HSYA undergoes hydroxylation and glucuronidation under the action of hepatic enzymes (Zhao et al., 2020). The metabolites decrease the nonspecific reactivity on oxidative stress detection. Furthermore, in vivo experiments rely on a comprehensive phenotype to assess efficacy of PAINS, rather than a single biochemical test. For instance, MIS, the primary outcome of this meta-analysis, was assessed by TTC staining and was not directly interfere by redox properties of PAINS. Cardiac function parameters (LVEF, +dp/dt max, etc.) were also not affected by the activity of single or multiple targets. Taken together, the integration of evidence from animal research may further enhance confidence in our results. But this does not mean that PAINS will not have interference effects at all in vivo experiments, and future research need to further ensure the reliability of results through conducting pharmacokinetic studies and looking for biophysical orthogonal methods for support of target engagement (e.g., surface plasmon resonance, cellular thermal shift assay) (Dahlin et al., 2015).
Moreover, the further preclinical research should adopt more rigorous experimental design such as blinded implementation and sample size calculation. Safety assessment should also be one of the concerns for each research. In addition, how to resolve the inconsistency between the timing of administration and clinical practice remains a key issue for future research.
5 Conclusion
In summary, our review findings indicate that HSYA exerts cardioprotective benefits in decreasing myocardial infarct size, reducing myocardial enzymes, and improving cardiac function in animal models of IS, MI, and MIRI. Specifically, the potential effects of HSYA in alleviating myocardial injury after ischemia mainly include attenuating inflammation, oxidative stress, cardiac myocyte apoptosis, and fibrosis of myocardium, improving microcirculation, and promoting angiogenesis. The underlying mechanisms may be associated with NLRP3 inflammasome, Bcl-2, Bax, caspase-3, eNOS proteins, and TLR/NF-κB, Nrf2/HO-1, JAK/STAT, PI3K/Akt, AMPK/mTOR, VEGFA pathways. However, none of included studies reported the safety endpoint and used animals with co-morbidities such as hyperlipidemia and hypertension, which is the typical situation in human for IHD. Moreover, the timing of administration of HSYA does not align with clinical practice. Safety assessment, standardized experimental design and comorbid animal models may strengthen the preclinical evidence base. Therefore, stronger evidence is required to better convert these finding to clinical practice.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
TM: Data curation, Methodology, Visualization, Writing – original draft, Writing – review and editing. KJ: Data curation, Visualization, Writing – original draft, Writing – review and editing. YaP: Data curation, Methodology, Writing – review and editing. YiP: Visualization, Writing – review and editing. WJ: Visualization, Writing – review and editing. QG: Project administration, Supervision, Writing – review and editing. QL: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Administration of Traditional Chinese Medicine High-level TCM Key discipline Project (grant number: zyyzdxk-2023253) and Chinese Medicine inheritance and innovation “thousand million” Talents Project (Qi Huang Project 2021) Qi Huang Scholars.
Acknowledgments
We wish to thank the reviewers and also the authors of all references.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1510657/full#supplementary-material
References
Abderrazak, A., Syrovets, T., Couchie, D., EL Hadri, K., Friguet, B., Simmet, T., et al. (2015). NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 4, 296–307. doi:10.1016/j.redox.2015.01.008
Bai, X., Wang, W., Fu, R., Yue, S., Gao, H., Chen, Y., et al. (2020). Therapeutic potential of hydroxysafflor yellow A on cardio-cerebrovascular diseases. Front. Pharmacol. 11, 01265. doi:10.3389/fphar.2020.01265
Bolz, S. N., Adasme, M. F., and Schroeder, M. (2021). Toward an understanding of pan-assay interference compounds and promiscuity: a structural perspective on binding modes. J. Chem. Inf. Model 61, 2248–2262. doi:10.1021/acs.jcim.0c01227
Chen, M. C. (2015). Anti-oxidative effects and mechanisms of combinating hydroxy safflower yellow A and acetyl-11-keto-beta-boswellic acid against myocardial injury. Xincheng, China. The Fourth Military Medical University.
Cheng, H., Yang, C., Ge, P., Liu, Y., Zafar, M. M., Hu, B., et al. (2024). Genetic diversity, clinical uses, and phytochemical and pharmacological properties of safflower (Carthamus tinctorius L.): an important medicinal plant. Front. Pharmacol. 15, 1374680. doi:10.3389/fphar.2024.1374680
Czabotar, P. E., and Garcia-Saez, A. J. (2023). Mechanisms of BCL-2 family proteins in mitochondrial apoptosis. Nat. Rev. Mol. Cell Biol. 24, 732–748. doi:10.1038/s41580-023-00629-4
Dahlin, J. L., Nissink, J. W. M., Strasser, J. M., Francis, S., Higgins, L., Zhou, H., et al. (2015). PAINS in the assay: chemical mechanisms of assay interference and promiscuous enzymatic inhibition observed during a sulfhydryl-scavenging HTS. J. Med. Chem. 58, 2091–2113. doi:10.1021/jm5019093
Dai, Y., Zhou, J., and Shi, C. (2023). Inflammasome: structure, biological functions, and therapeutic targets. MedComm 4, e391. doi:10.1002/mco2.391
Deng, R. M., and Zhou, J. (2023). The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 123, 110714. doi:10.1016/j.intimp.2023.110714
Dong, W. B., Ye, X. D., Cheng, M., Wang, J. Q., and Zheng, G. L. (2014). Protective effect of hydroxy safflower yellow A on myocardial ischemia. China J. Chin. Pharmacol. Ther. 19, 1001–1005.
Dong, L., Shen, Z., Chi, H., Wang, Y., Shi, Z., Fang, H., et al. (2023). Research progress of Chinese medicine in the treatment of myocardial ischemia-reperfusion injury. Am. J. Chin. Med. 51, 1–17. doi:10.1142/S0192415X23500015
Fu, J. H., Zhang, Q., Fan, C. Z., and Liu, J. G. (2011). Protective effect of intravenous infusion injection of safflor yellow and hydroxyl safflor yellow A on acute myocardial ischemia injury in rats. Int. J. Trad. Chin. Med. 33, 692–694. doi:10.3760/cma.j.issn.1673-4246.2011.08.007
Ge, C., Peng, Y., Li, J., Wang, L., Zhu, X., Wang, N., et al. (2023). Hydroxysafflor yellow A alleviates acute myocardial ischemia/reperfusion injury in mice by inhibiting ferroptosis via the activation of the HIF-1α/SLC7A11/GPX4 signaling pathway. Nutrients 15, 3411. doi:10.3390/nu15153411
Geertsema, S., Bourgonje, A. R., Fagundes, R. R., Gacesa, R., Weersma, R. K., VAN Goor, H., et al. (2023). The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 29, 830–842. doi:10.1016/j.molmed.2023.07.008
Guan, Y., Yin, Y., Zhu, Y. R., Guo, C., Wei, G., Duan, J. L., et al. (2013). Dissection of mechanisms of a Chinese medicinal formula: danhong injection therapy for myocardial ischemia/reperfusion injury in vivo and in vitro. Evid-based Complement. Altern. Med. 2013, 972370. doi:10.1155/2013/972370
Halestrap, A. P., Clarke, S. J., and Javadov, S. A. (2004). Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 61, 372–385. doi:10.1016/S0008-6363(03)00533-9
Han, D., Wei, J., Zhang, R., Ma, W., Shen, C., Feng, Y., et al. (2016). Hydroxysafflor yellow A alleviates myocardial ischemia/reperfusion in hyperlipidemic animals through the suppression of TLR4 signaling. Sci. Rep. 6, 35319. doi:10.1038/srep35319
Heusch, G. (2019). Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res. Cardiol. 114, 45. doi:10.1007/s00395-019-0756-8
Heusch, G. (2020). Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 17, 773–789. doi:10.1038/s41569-020-0403-y
He, W. (2022). Effect and mechanism of hydroxysafflor yellow A on myocardial ischemic injury through Nrf2/Keap1/NF-κB signal pathway. Master Nanjing, China. Nanjing University of Traditional Chinese Medicine.
Hu, T. X., Wen, A. D., Zhu, Y. R., Yue, G., and Zhao, R. H. (2015). Protective effect of danshensu and hydrosaffloryellow A for using alone or in combination on myocardial Ischemic-reperfusion injury in rats. Mod. Chin. Med. 17, 15–19. doi:10.13313/j.issn.1673-4890.2015.1.004
Huang, X., Qin, F., Zhang, H. M., Xiao, H. B., Wang, L. X., Zhang, X. Y., et al. (2009). Cardioprotection by Guanxin II in rats with acute myocardial infarction is related to its three compounds. J. Ethnopharmacol. 121, 268–273. doi:10.1016/j.jep.2008.10.029
Hu, T., Wei, G., XI, M., Yan, J., Wu, X., Wang, Y., et al. (2016). Synergistic cardioprotective effects of Danshensu and hydroxysafflor yellow A against myocardial ischemia-reperfusion injury are mediated through the Akt/Nrf2/HO-1 pathway. Int. J. Mol. Med. 38, 83–94. doi:10.3892/ijmm.2016.2584
Jin, M., Dong, N. N., Wu, W., Li, J. R., Zang, B. X., and Tung, J. (2009). Inhibition of hydroxysafflor yellow A against rat cardiomyocyte apoptosis. Chin. Tradit. Herb. Drugs 40, 924–930. doi:10.1155/2021/6643615
Joseph, P., Roy, A., Lonn, E., StöRK, S., Floras, J., Mielniczuk, L., et al. (2023). Global variations in heart failure etiology, management, and outcomes. JAMA 329, 1650–1661. doi:10.1001/jama.2023.5942
KräMER, O. H., Baus, D., Knauer, S. K., Stein, S., JäGER, E., Stauber, R. H., et al. (2006). Acetylation of Stat1 modulates NF-kappaB activity. Genes Dev. 20, 473–485. doi:10.1101/gad.364306
Liu, Y. G., Li, F. J., and Tang, F. (2015). Protective effect of hydroxysaffloryellow A on rats with myocardial ischemia and reperfusion injury. Pharma Clin Chin Materia Med. 31, 71–74. doi:10.13412/j.cnki.zyyl.2015.01.023
Liu, Y. N., Zhou, Z. M., and Chen, P. (2008). Evidence that hydroxysafflor yellow A protects the heart against ischaemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Clin. Exp. Pharmacol. Physiol. 35, 211–216. doi:10.1111/j.1440-1681.2007.04814.x
Liu, J., Zhang, D., Li, J., Feng, J., Yang, X., Shi, D., et al. (2011). Effects of Salvia miltiorrhiza and Carthamus tinctorius aqueous extracts and compatibility on rat myocardial ischemic reperfusion injury. Zhongguo Zhongyao Zazhi 36, 189–194. doi:10.4268/cjcmm20110222
Loboda, A., Damulewicz, M., Pyza, E., Jozkowicz, A., and Dulak, J. (2016). Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol. Life Sci. 73, 3221–3247. doi:10.1007/s00018-016-2223-0
Lundberg, J. O., and Weitzberg, E. (2022). Nitric oxide signaling in health and disease. Cell 185, 2853–2878. doi:10.1016/j.cell.2022.06.010
Macleod, M. R., O’Collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35, 1203–1208. doi:10.1161/01.STR.0000125719.25853.20
MagalhãES, P. R., Reis, P. B. P. S., Vila-ViçOSA, D., Machuqueiro, M., and Victor, B. L. (2021). Identification of pan-assay INterference compoundS (PAINS) using an MD-based protocol. Methods Mol. Biol. 2315, 263–271. doi:10.1007/978-1-0716-1468-6_15
Mensah, G. A., Roth, G. A., and Fuster, V. (2019). The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 74, 2529–2532. doi:10.1016/j.jacc.2019.10.009
Min, J., and Wei, C. (2017). Hydroxysafflor yellow A cardioprotection in ischemia-reperfusion (I/R) injury mainly via Akt/hexokinase II independent of ERK/GSK-3β pathway. Biomed. Pharmacother. 87, 419–426. doi:10.1016/j.biopha.2016.12.113
Moghimpour Bijani, F., Vallejo, J. G., and Rezaei, N. (2012). Toll-like receptor signaling pathways in cardiovascular diseases: challenges and opportunities. Int. Rev. Immunol. 31, 379–395. doi:10.3109/08830185.2012.706761
Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., and Walters, M. A. (2017). The essential medicinal chemistry of curcumin. J. Med. Chem. 60, 1620–1637. doi:10.1021/acs.jmedchem.6b00975
Ni, B., Zhou, D., Jing, Y., and Liu, S. (2018). Hydroxysafflor yellow A protects against angiotensin II-induced hypertrophy. Mol. Med. Rep. 18, 3649–3656. doi:10.3892/mmr.2018.9399
Ong, S.-B., HernáNDEZ-ReséNDIZ, S., Crespo-Avilan, G. E., Mukhametshina, R. T., Kwek, X.-Y., Cabrera-Fuentes, H. A., et al. (2018). Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 186, 73–87. doi:10.1016/j.pharmthera.2018.01.001
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, Q., You, L., Meng, X., Li, Y., Deng, T., Li, D., et al. (2023). Regulation of the JAK/STAT signaling pathway: the promising targets for cardiovascular disease. Biochem. Pharmacol. 213, 115587. doi:10.1016/j.bcp.2023.115587
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T., and Kwong, J. Q. (2019). Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13. doi:10.1038/s12276-019-0355-7
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. doi:10.1016/j.jacc.2020.11.010
Sahoo, G., Samal, D., Khandayataray, P., and Murthy, M. K. (2023). A review on caspases: key regulators of biological activities and apoptosis. Mol. Neurobiol. 60, 5805–5837. doi:10.1007/s12035-023-03433-5
Schirone, L., Forte, M., D'Ambrosio, L., Valenti, V., Vecchio, D., Schiavon, S., et al. (2022). An overview of the molecular mechanisms associated with myocardial ischemic injury: state of the art and translational perspectives. Cells 11, 1165. doi:10.3390/cells11071165
Sciarretta, S., Maejima, Y., Zablocki, D., and Sadoshima, J. (2018). The role of autophagy in the heart. Annu. Rev. Physiol. 80, 1–26. doi:10.1146/annurev-physiol-021317-121427
Senichkin, V. V., Pervushin, N. V., Zuev, A. P., Zhivotovsky, B., and Kopeina, G. S. (2020). Targeting bcl-2 family proteins: what, where, when? Biochem. (Mosc). 85, 1210–1226. doi:10.1134/S0006297920100090
Singh, R., Letai, A., and Sarosiek, K. (2019). Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 20, 175–193. doi:10.1038/s41580-018-0089-8
Spanagel, R. (2022). Ten points to improve reproducibility and translation of animal research. Front. Behav. Neurosci. 16, 869511. doi:10.3389/fnbeh.2022.869511
Su, N., Lu, F. P., Zhu, M. M., Zheng, Z. H., Shen, Y. J., Liu, T. Y., et al. (2018). Protective effect of astragaloside IV combined with hydroxysafflor yellow A on mice with myocardial infarction. Chin. J. Exp. Tradit. Med. Form. 24, 98–103.
Sun, Y. Q. (2012). The effect of HSYA on anti-myocardial ischemia-reperfusion injury by regulating mitochondrion. Doctor: JiLin University.
Toldo, S., Marchetti, C., Mauro, A. G., Chojnacki, J., Mezzaroma, E., Carbone, S., et al. (2016). Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int. J. Cardiol. 209, 215–220. doi:10.1016/j.ijcard.2016.02.043
Tsao, C. W., Aday, A. W., Almarzooq, Z. I., Anderson, C. A. M., Arora, P., Avery, C. L., et al. (2023). Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation 147, e93–e621. doi:10.1161/CIR.0000000000001123
Wang, H. M. (2009). Effects of hydroxysafflor yellow A on FFA, NO and iNOS in rats of myocardial infarction. Qilu Pharma Aff. 28, 625–627.
Wang, T., Fu, F. H., Han, B., Li, G. S., Zhang, L. M., and Liu, K. (2009). Hydroxysafflor yellow A reduces myocardial infarction size after coronary artery ligation in rats. Pharm. Biol. 47, 458–462. doi:10.1080/13880200902822612
Wang, T., Fu, F. H., Han, B., Zhu, M., Li, G. S., and Liu, K. (2007). Protection and mechanisms of hydroxysafflor yellow A on experimental myocardial infarction in rats. Chin. Tradit. Herb. Drugs 38, 1853–1856.
Ware, J. A., and Simons, M. (1997). Angiogenesis in ischemic heart disease. Nat. Med. 3, 158–164. doi:10.1038/nm0297-158
WCOTROCHADIC (2023). Report on cardiovascular health and diseases in China 2022: an updated summary. Biomed. Environ. Sci. 36, 669–701. doi:10.3967/bes2023.106
Wei, G., Duan, J. L., Yin, Y., Wang, Y. H., and Wen, A. D. (2016). Effect of danshensu and hydroxysafflower yellow A on promoting angiogenesis and preventing myocardial infarction in rats. Lishizhen Med. Mater Med. Res. 27, 2576–2578.
Wei, G., Yin, Y., Duan, J., Guo, C., Zhu, Y., Wang, Y., et al. (2017). Hydroxysafflor yellow A promotes neovascularization and cardiac function recovery through HO-1/VEGF-A/SDF-1α cascade. Biomed. Pharmacother. 88, 409–420. doi:10.1016/j.biopha.2017.01.074
Wei, J., Zhang, Y., Li, H., Wang, F., and Yao, S. (2023). Toll-like receptor 4: a potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 166, 115338. doi:10.1016/j.biopha.2023.115338
Xu, A. B., Liu, J. G., Zhang, J., and Li, J. X. (2014). Hydroxysafflor yellow A for ameliorating injury of myocardial ischemia/reperfusion in SD rats. Chin. J. Evid. Based Cardiovasc Med. 6, 733–736.
Xu, L. J., Chen, R. C., Ma, X. Y., Zhu, Y., Sun, G. B., and Sun, X. B. (2020). Scutellarin protects against myocardial ischemia-reperfusion injury by suppressing NLRP3 inflammasome activation. Phytomedicine 68, 153169. doi:10.1016/j.phymed.2020.153169
Xue, X., Deng, Y., Wang, J., Zhou, M., Liao, L., Wang, C., et al. (2021). Hydroxysafflor yellow A, a natural compound from Carthamus tinctorius L with good effect of alleviating atherosclerosis. Phytomedicine 91, 153694. doi:10.1016/j.phymed.2021.153694
Ye, J., Lu, S., Wang, M., Ge, W., Liu, H., Qi, Y., et al. (2020). Hydroxysafflor yellow A protects against myocardial ischemia/reperfusion injury via suppressing NLRP3 inflammasome and activating autophagy, Front. Pharmacol. 11, 1170. doi:10.3389/fphar.2020.01170
Yu, H., and Qu, L. (2023). Protective effect of hydroxysafflor yellow A on myocardial ischemia/reperfusion injury in rats based on p38MAPK/SERCA2α pathway. Cell Mol. Immunol. 39, 1395–1401+1408.
Zhang, J. J., Zhang, Z. Y., and Wang, N. (2012). Effect and mechanism of action of hydroxysafflor yellow A on myocardial ischemia-reperfusion injury in rats. Chin. Hosp. Pharm. J. 32, 1526–1530. doi:10.13286/j.cnki.chinhosppharmacyj.2012.19.006
Zhang, R., Li, G., Zhang, Q., Tang, Q., Huang, J., Hu, C., et al. (2018). Hirsutine induces mPTP-dependent apoptosis through ROCK1/PTEN/PI3K/GSK3β pathway in human lung cancer cells. Cell Death Dis. 9, 598. doi:10.1038/s41419-018-0641-7
Zhang, Y., and Zhang, Y. (2020). Effects of hydroxysafflor yellow A on inflammatory factors and NF-κB in rats with myocardial ischemia-reperfusion injury. Chin J Inte Med Cardio/Cere Dis. 18, 2603–2607.
Zhao, Z. Q., Morris, C. D., Budde, J. M., Wang, N. P., Muraki, S., Sun, H. Y., et al. (2003). Inhibition of myocardial apoptosis reduces infarct size and improves regional contractile dysfunction during reperfusion. Cardiovasc Res. 59, 132–142. doi:10.1016/s0008-6363(03)00344-4
Zhao, B., Hu, K., Zeng, X., Kwong, J. S. W., Li, B., Chen, H., et al. (2022). Development of a reporting guideline for systematic reviews of animal experiments in the field of traditional Chinese medicine. J. Evid. Based Med. 15, 152–167. doi:10.1111/jebm.12480
Zhao, F., Wang, P., Jiao, Y., Zhang, X., Chen, D., and Xu, H. (2020). Hydroxysafflor yellow A: a systematical review on botanical resources, physicochemical properties, drug delivery system, pharmacokinetics, and pharmacological effects. Front. Pharmacol. 11, 579332. doi:10.3389/fphar.2020.579332
Zhou, M. X., Fu, J. H., Zhang, Q., and Wang, J. Q. (2015). Effect of hydroxy safflower yellow A on myocardial apoptosis after acute myocardial infarction in rats. Genet. Mol. Res. 14, 3133–3141. doi:10.4238/2015.April.10.24
Zhou, D., Ding, T., Ni, B., Jing, Y., and Liu, S. (2019). Hydroxysafflor Yellow A mitigated myocardial ischemia/reperfusion injury by inhibiting the activation of the JAK2/STAT1 pathway. Int. J. Mol. Med. 44, 405–416. doi:10.3892/ijmm.2019.4230
Keywords: hydroxysafflor yellow A, myocardial ischemia, myocardial infarction, myocardial ischemia/reperfusion injury, meta-analysis
Citation: Mao T, Jiang K, Pang Y, Pan Y, Jia W, Gao Q and Lin Q (2025) Hydroxysafflor yellow A for ischemic heart diseases: a systematic review and meta-analysis of animal experiments. Front. Pharmacol. 16:1510657. doi: 10.3389/fphar.2025.1510657
Received: 13 October 2024; Accepted: 28 March 2025;
Published: 09 April 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Guang Chen, The University of Hong Kong, Hong Kong SAR, ChinaYuxiang Fei, China Pharmaceutical University, China
Yuting Huang, Tianjin University of Traditional Chinese Medicine, China
Copyright © 2025 Mao, Jiang, Pang, Pan, Jia, Gao and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Gao, Z2FvcXVuXzE5OTBAMTI2LmNvbQ==; Qian Lin, bGlucWlhbjYyQDEyNi5jb20=
 Tianshi Mao
Tianshi Mao Kaixin Jiang
Kaixin Jiang Yanting Pang1
Yanting Pang1 Wenhao Jia
Wenhao Jia