- 1Systems Toxicology Group, Food, Drug and Chemical, Environment and Systems Toxicology Division, CSIR-Indian Institute of Toxicology Research (CSIR-IITR), Lucknow, Uttar Pradesh, India
- 2Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, India
- 3Biochemistry and Structural Biology Division, CSIR-Central Drug Research Institute, Lucknow, India
Soybean-based foods enhance cognitive functions by influencing hippocampal mechanisms. These salutary effects have so far been attributed to isoflavones present in soybeans. Considering cellular senescence contributes to cognitive decline and that no specific soy-derived peptides are known for their potential to mitigate senescence, we examined the efficacy of a thirteen amino acid soy-derived peptide, Soymetide, on a doxorubicin-induced senescence mice model. Soymetide pretreatment lowered the senescence markers p53, p21 and p16, pro-inflammatory cytokines, and Senescence β-Galactosidase staining while enhancing the mature neuronal marker NeuN in the hippocampus. This anti-senescent effect was comparable with that of a well-known senolytic combination (dasatinib and quercetin). Research indicates that Wnt signaling influences cellular senescence, and our findings here demonstrate that doxorubicin decreased hippocampal Wnt3a, p-LRP6, Frizzled, Dishevelled, Axin1, and β-catenin levels and increased GSK-3β, while Soymetide mitigated these effects. Additionally, upon inhibition of the Wnt/β-catenin pathway, Soymetide’s ability to reduce senescence markers and restore NeuN expression was reduced. We validated the anti-senescence impact on hippocampal neurons by co-immunostaining Wnt/β-catenin and senescence indicators alongside NeuN in mice and assessed it in primary hippocampal neurons. Further examining the neuronal survival and functions revealed that Soymetide blocked the doxorubicin-induced loss in Nissl-stained surviving neurons and learning-memory performances, measured by Y-Maze and Passive Avoidance tests, which Wnt/β-catenin inhibitors could counteract. In conclusion, our study identifies a novel Wnt/β-catenin-linked mechanism of doxorubicin-induced senescence in the hippocampal neurons and demonstrates Soymetide’s effectiveness in reversing this process. Hence, this suggests Soymetide’s potential therapeutic application in addressing cognitive decline associated with cellular aging.
Introduction
Cellular senescence, characterized by irreversible cell cycle arrest, is increasingly recognized for its role in hippocampal decline (Lin et al., 2021). Senescence affects hippocampal neurons by diminishing their functionality and plasticity, leading to a decline in cognitive abilities such as learning and memory (Miyamoto et al., 1986). The senescent neurons express markers like p53, p21, p16, and β-Galactosidase and morphological alterations (Mijit et al., 2020; Wang et al., 2024). This may promote the generation of senescence-associated secretory phenotype (SASP) cytokines, exacerbating neuroinflammation (Saito et al., 2021). Hence, while cellular senescence is a physiological process, its aberrant accumulation adversely affects neuronal health, causing age-associated hippocampal dysfunction (Piechota et al., 2016; Wissler Gerdes et al., 2020).
The Wnt signaling pathway, particularly involving Wnt3a, plays a crucial role in hippocampal neuronal processes and functions (Fortress and Frick, 2016; Arrazola et al., 2017). An activated Wnt pathway has been linked to enhanced neurogenesis and neuronal survival within the hippocampus (Inestrosa et al., 2004; Avila et al., 2010; Wei et al., 2012; Zhang et al., 2022a; Tao et al., 2023). Additionally, reduced Wnt3a expression or aberrant downstream signaling events may contribute to cognitive decline (Oliva et al., 2013; Palomer et al., 2019). Wnt3a interacts with cell-surface receptor Frizzled (Fz) and Low-density lipoprotein receptor-related protein 6 (LRP6), triggering downstream events involving Dishevelled (Dvl) and Axin1 (Huang and Klein, 2004; Garg and Bandyopadhyay, 2024). Notably, Glycogen Synthase Kinase-3β (GSK-3β) serves as a key player in the Wnt pathway, where an inhibited GSK-3β may lead to cytoplasmic accumulation of β-catenin, affecting hippocampal functions and neuronal health (Beurel et al., 2015; Li et al., 2024).
Emerging evidence suggests a link between the Wnt/β-catenin signaling pathway and cell senescence or aging in the brain (Jia et al., 2019; Chen et al., 2020). A dysregulated Wnt signaling associated with senescence involving morphological changes in brain cells, cell cycle alterations, β-Galactosidase staining, and decline in cognitive functions (Li et al., 2018; Swarbrick et al., 2019; Xiang et al., 2019; Zhang et al., 2019; Prud’homme et al., 2022). Cellular senescence demonstrated a connection with Wnt signaling in the hippocampus as well, involving the participation of altered Dickkopf-1 (a secreted inhibitor of β-catenin-dependent Wnt signaling), GSK-3β activation, β-catenin, along with neuronal loss and dysregulated expression of apoptosis-related proteins (Bayod et al., 2015). Hence, targeting Wnt pathway components and reducing senescence could hold promise as a therapeutic strategy for mitigating neurodegeneration and cognitive decline.
The beneficial effects of soy-based foods in mitigating cognitive decline are primarily attributed to phytoestrogen isoflavones (Kim, 2021). Although largely uncharacterized, research has shown promising outcomes in animal models of cognitive decline and behavioral deficits with soy protein isolates/peptide mixture (Dong et al., 2023; Zheng et al., 2023; Tamura et al., 2024). These protein isolates/peptide mixtures/soy food demonstrated protection against neuronal damage, promoted neurogenesis, regulated neurotransmitter synthesis and metabolism, increased neurotrophin signaling, induced anti-inflammatory and antioxidant effects in the brain, modulated neuronal apoptosis, and mitigated cognitive decline (Jeong et al., 2021; Dong et al., 2023; Zheng et al., 2023; Tamura et al., 2024). Human studies also indicate a lower risk of dementia and significant improvements in cognition and neurotrophin levels associated with consuming soy products (Hwang et al., 2019; Deng et al., 2023).
Building on this evidence, our study aims to investigate the therapeutic potential of a short-length soy-derived bioactive peptide, Soymetide (Schuler et al., 1982; Tsuruki et al., 2003) in preventing age-related senescence. Utilizing a doxorubicin-induced senescent mouse model, we explored the effects of Soymetide on the senescence markers in the hippocampus. We investigated the participation of the Wnt/β-catenin pathway in the process. Our study aimed to examine the anti-senescence and cognitive improvement capabilities, as well as the underlying mechanisms of Soymetide for potential therapeutic applications in senescence-related neurological disorders of which memory impairment is the dominant type. Through the investigation of these mechanisms, we seek to evaluate Soymetide’s efficacy in improving cognitive function and its therapeutic role in addressing age-related neurological conditions, especially those associated with memory deficits.
Materials and methods
Reagents, chemicals and antibodies
Doxorubicin hydrochloride (cat no. PHR1789), dasatinib (cat no. SML2589), quercetin (cat no. Q4951), β-Catenin/Tcf Inhibitor III, iCRT3 (cat no. 219332) and cresyl violet acetate (cat no. C5042) were bought from Sigma-Aldrich (St. Louis, Missouri). Human Dkk-1 Recombinant Protein (cat no. PHC9214) and Mouse ProcartaPlex Mix&Match 9-plex kit (cat no. PPX-09-MX2XAC3) were purchased from Thermo Fisher Scientific (Waltham, Massachusetts). Senescence β-Galactosidase Staining Kit (cat no. 9860) was purchased from Cell Signaling Technology (Danvers, Massachusetts). Polyvinylidene difluoride membrane (cat no. IPVH00010) and Immobilon Western Chemiluminescent HRP Substrate (cat no. WBKLS0500) were bought from MilliporeSigma (Burlington, Massachusetts). The prestained protein ladders (cat no. PG-PMT2922 and cat no. 786419) were procured from Genetix Biotech Asia Pvt Ltd. (New Delhi, India) and G-Biosciences (St. Louis, Missouri), respectively. Vectashield mounting medium with DAPI was purchased from Vector Laboratories (Newark, California). Mouse monoclonal p53 (cat no. 2524), rabbit polyclonal p21 (cat no. 64016), phospho-LRP6 (cat no. 2568), β-catenin (cat no. 9562), rabbit monoclonal NeuN (cat no. 24307), LRP6 (cat no. 3395), Dvl2 (cat no. 3224), Axin1 (cat no. 2087), GSK-3β (cat no. 12456) and TCF3/TCF7L1 (cat no. 2883) antibodies were purchased from Cell Signaling Technology. Rabbit polyclonal p16INK4a (cat no. PA5-20379) antibody was purchased from Thermo Fisher Scientific. Rabbit polyclonal Wnt3a (cat no. 09162), HRP-conjugated secondary anti-rabbit IgG (cat no. A0545), and anti-mouse IgG (cat no. A9044) antibodies were purchased from Sigma-Aldrich. Rabbit polyclonal Frizzled-7 (cat no. ab64636) antibody was purchased from Abcam (Cambridge, Massachusetts). Horseradish peroxidase (HRP)-conjugated mouse monoclonal β-actin (cat no. sc-47778) antibody was procured from Santa Cruz Biotechnology (Dallas, Texas). Mouse monoclonal NeuN (cat no. 66836-1-lg) and rabbit polyclonal NeuN (cat no. 26975-1-AP) antibodies were procured from Proteintech (Rosemond, Illinois). Alexa Fluor®546 goat anti-rabbit IgG (cat no. A11010), Alexa Fluor®488 goat anti-mouse IgG (cat no. A11001), Alexa Fluor®546 goat anti-mouse IgG (cat no. A11030) and Alexa Fluor®488 goat anti-rabbit IgG (cat no. A11008) were procured from Invitrogen (Carlsbad, California). In Situ Cell Death Detection Kit, Fluorescein (cat no. 11684795910) was purchased from Roche (Mannheim, Germany).
Peptide synthesis
Soymetide (Soym; MITLAIPVNKPGR) (Schuler et al., 1982; Tsuruki et al., 2003) was synthesized (98.27% purity (HPLC), −20°C storage in the dark) by GL Biochem Shanghai Ltd. (Shanghai, China). We designed the Scrambled peptide (Scr; MVNLGIPITKPAR), which was subsequently synthesized by GL Biochem Shanghai Ltd.
Animal treatments
C57BL/6 male mice were kept under a 12-h light/dark cycle condition with ad libitum availability of chow diet and R. O water. The treatment and assessment timeline of mice is as shown in Supplementary Material 1. To generate a senescence mice model, mice (≅ 22-25 gm) were intraperitoneally injected with doxorubicin hydrochloride (DOX; 1, 5 and 10 mg/kg in PBS), once a week for 4 weeks, closely following the reported protocol (Sun et al., 2022). To examine whether Soymetide had anti-senescence properties, it was once injected bilaterally (2 μL/min) into the hippocampus of mice through stereotaxic surgery (coordinates: AP −1.7, ML +1.6, DV −1.9 to bregma) (Sierra et al., 2015; Garg and Bandyopadhyay, 2024) at doses of 10, 50, and 100 μg/kg in PBS (4 µL), 7 days before the doxorubicin treatment. To specifically identify the effect of Soymetide, scrambled peptide (50 μg/kg in PBS) was given to the mice in the same method and schedule. A senolytic combination of dasatinib (D) (5 mg/kg) plus quercetin (Q) (50 mg/kg) (D + Q) (Wang et al., 2023) was orally administered to the doxorubicin-treated mice once a week for 4 weeks as a positive control. To assess the role of Wnt in the doxorubicin and Soymetide-induced effects, recombinant Dickkopf-1 (rDkk1) protein [200 ng in 2 µL sterile saline (Garg and Bandyopadhyay, 2024)] was injected into the hippocampus of Soymetide- and doxorubicin-treated mice through stereotaxic surgery, 7 days before the assessment. To assess the role of β-catenin, inhibitor of β-catenin responsive transcription-3 (iCRT3) protein [5 mg/kg in sterile saline with 5% DMSO (Sharma et al., 2017)] was injected intraperitoneally into the Soymetide- and doxorubicin-treated mice. Vehicle-only-treated sets were included for every treatment. Animal treatment and behavioral analyses were randomized to ensure an unbiased approach.
Western blotting
The hippocampal tissue was isolated from the mice brain, and the proteins (30–50 μg) were separated using SDS-PAGE (8%–15%) and transferred onto a PVDF membrane, as we reported (Garg and Bandyopadhyay, 2024). The blots were probed with p53, p21, p16, NeuN, Wnt3a, p-LRP6, LRP6, Fz, Dishevelled, Axin1, GSK-3β, β-catenin and TCF3 antibodies for overnight (1:1000 dilution; 4°C), and HRP-conjugated β-actin antibody (loading control) for 2 h (1:5000; room temperature). Except for the β-actin-probing, the blots were incubated with HRP-conjugated secondary antibody for 2 h (1:5000 dilution; room temperature) and developed using Immobilon Western Chemiluminescent HRP Substrate in Amersham Imager 600 (GE Healthcare Life Sciences, Pittsburgh, Pennsylvania). Relative protein levels were determined by densitometric quantification by applying the Quantity One software (Bio-Rad Laboratories, Hercules, California).
Cytokine multiplexing
Blood samples of mice were collected and serum was isolated (Pandey et al., 2022). The quantitative determination of cytokine levels in serum was performed using the Mouse ProcartaPlex Mix&Match 9-plex kit as per its manufacturer’s protocol. The cytokine levels were measured using Luminex MAGPIX® instrument (Bio-Rad Laboratories) and expressed as the mean Magnetic Fluorescence Intensity (MFI).
Senescence β-galactosidase staining
The whole brain was isolated from mice after transcardial perfusion with PBS, cryoprotected (on dry ice) in OCT and stored at −80°C (Das et al., 2016), and subsequently cut into 30-μm thick coronal sections using a cryomicrotome (Microm HM 520, Labcon, Germany). Following the manufacturer’s protocol of the Senescence β-Galactosidase staining kit, the sections were fixed (using the fixative solution) for 15 min at room temperature, stained with β-Galactosidase staining solution (pH 6.0) that contained 0.2% X-Gal, and incubated for 18 h at 37°C in a dry incubator (Sarkar et al., 2011). The sections were then mounted with 70% glycerol solution, and images of the hippocampus were captured under a 20× objective [Leica DMi1 microscope (Leica Microsystems, Wetzlar, Germany)]. The results were quantified using ImageJ software (Wayne Rasband, NIH).
Immunofluorescence
The whole brain was isolated from mice after perfusion with PFA (4%), cryoprotected, and 10-µm thick sections were prepared using a cryomicrotome, as previously described by us (Gupta et al., 2022). Sections were then probed with the Wnt3a, β-catenin, p53, p16, and NeuN primary antibodies overnight (1:250 dilution; 4°C), incubated with Alexa Fluor secondary antibodies for 2 h (1:500 dilution; room temperature), and mounted using VECTASHEILD anti-fade mounting media containing DAPI. Fluorescence photomicrography of the hippocampus was conducted in these sections under 20× objective (NIS-Elements software, Nikon Instech Co. Ltd., Kawasaki, Kanagawa, Japan), and the images were processed using the ImageJ software.
Nissl staining
Cryosections of 30-µm thickness were made and processed for Cresyl violet acetate staining following our previously described protocol (Mishra et al., 2021). Images of the hippocampus were captured in these sections with 20× and 40× objectives [Leica DMi1 microscope (Leica Microsystems)] and imported into the ImageJ software. The surviving neurons (%) were then manually counted from five different fields using the Cell Counter plugin.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
Hippocampal neuronal apoptosis was assessed as reported earlier (Pandey et al., 2017). Briefly, 10-µm brain sections were treated with TdT and fluorescein-labeled dUTP, immunostained with anti-rabbit NeuN, and then mounted. The images of the hippocampus were captured (20×) and TUNEL-positive neurons were counted in five random areas using ImageJ, expressed as TUNEL-positive cells per 100 DAPI-stained nuclei.
Learning and memory assays
Mice were subjected to Y-maze and Passive Avoidance tests to assess learning and memory functions, as we previously reported (Pandey et al., 2020). For the Y-Maze test, the learning trials (training) were conducted on mice in a Y-Maze apparatus, where running to the unsafe arms (dark arms with foot shock) from the safe arm (bright and shock-free) was considered an error. The % number of errors (E) was calculated for learning trials. Further, the % Saving Memory was assessed in the Y-Maze apparatus at 24 h, 48 h, and 7th day post-learning, and calculated as (Etraining−Etest)×100/Etraining. During the Passive Avoidance test, mice were subjected to acquisition trials in a gated two-compartment apparatus. One compartment was bright and free of shocks, while the other was dark and delivered an electrical shock. After 24 h (R1), 48 h (R2), and 72 h (R3) of acquisition trials, three shock-free retention trials of 300 s each were conducted. The time taken by the mice to move from the bright to the dark compartment was considered as transfer latency time (TLT) for learning-memory performance.
Primary neuronal culture and treatments
Embryos were collected at embryonic day 16 (E−16), and the hippocampus was dissected in Hank’s Balanced Salt Solution (HBSS), followed by mechanical digestion in serum-free neurobasal media, as previously reported (Pandey et al., 2020). The suspension was digested using 0.05% trypsin-EDTA and incubated for 15 min at 37°C in a CO2 incubator, and treated with trypsin inhibitor to neutralize the effect of trypsin. The remaining suspension was centrifuged at 1500 rpm for 10 min and the pellet was suspended in the complete neurobasal medium containing N2 supplement (1%), B-27 supplement (2%), 2 mM L-glutamine, penicillin (100 units/mL), streptomycin (100 μg/mL) and amphotericin (0.25 μg/mL). Cells were plated on poly-L-lysine-coated 25 cm2 flask or 4-well culture plates and grown in a humidified incubator at 37°C with 5% CO2. Doxorubicin at 50 nM (Marques et al., 2020) was then added to the neurons for 18 h to induce senescence. For standardizing the treatment dose of Soymetide, neurons were pre-treated for 2 h with Soymetide at doses of 1, 10, and 100 nM followed by co-treatment with doxorubicin and assessed for the lowest dose affecting p53 levels (Supplementary Material 2). To analyze the role of Wnt and β-catenin, rDkk1 2.38 nM (Wei et al., 2019) and iCRT3 20 μM (Lee et al., 2013) were given to the cells, respectively, 2 h before the assessment time.
Protein extraction and western blotting (in vitro)
Primary neurons were first lysed with cell lysis reagent and Western blotting was carried out as above using the Wnt3a, β-catenin, p53, p16, NeuN and β-actin antibodies.
Senescence β-galactosidase staining (in vitro)
Primary hippocampal neurons were plated onto poly-L-lysine-coated chamber slides. Following the treatment and washing with PBS, fixation, and staining of the cells were performed as per the manufacturer’s protocol of the Senescence β-Galactosidase staining kit. Briefly, the cells were fixed in the fixative solution for 10–15 min at room temperature, washed with PBS, and incubated in a no CO2 incubator with the staining solution overnight at 37°C. 70% glycerol solution was used to mount the slides. Images of the cells were captured with a 40× objective [Leica DMi1 microscope (Leica Microsystems)].
Statistics
One-way ANOVA for comparisons involving a single parameter across multiple groups (Figures 1A–7C) or two-way ANOVA for multiple parameters and groups (Figures 7D, E), with subsequent Tukey’s posthoc tests for detailed comparisons, was conducted (GraphPad Software, Inc., San Diego, California).
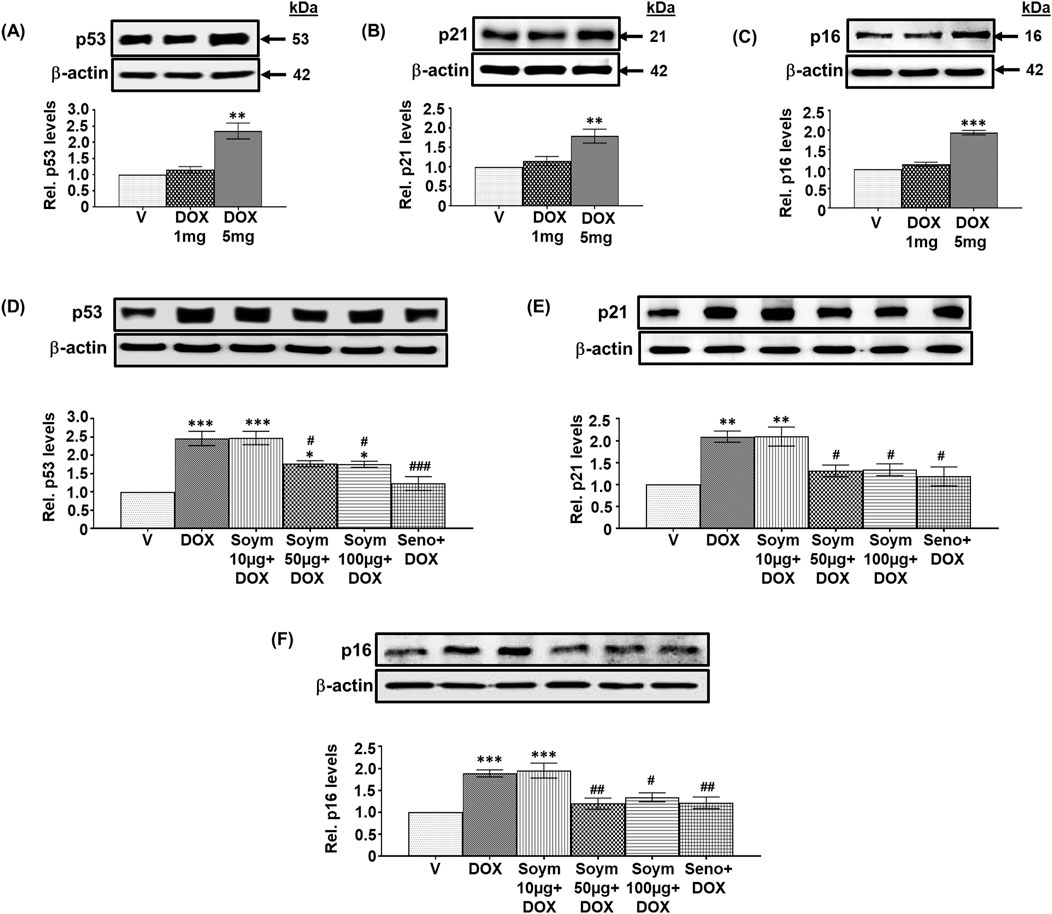
Figure 1. Soymetide prevents doxorubicin-induced senescence in the hippocampus of mice. Hippocampal tissues were isolated from vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX and senolytic combination (Seno)+DOX-treated mice. (A–F) Representative Western blots and corresponding densitometry (relative to (Rel.) vehicle) of p53 (A, D), p21 (B, E), and p16 (C, F) normalized with β-actin in the hippocampal tissues. Data represent means ± Standard Error (SE) of three mice/group. ***p < 0.001, **p < 0.01 and *p < 0.05 compared to V. ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to DOX.
Results
Effect of Soymetide and doxorubicin on senescence markers in the hippocampus of mice
We conducted a screening study to investigate how different doses of doxorubicin (1 mg/kg, 5 mg/kg, and 10 mg/kg) affect senescence in the hippocampus of mice. Specifically, we measured the levels of senescence markers p53, p21 and p16. Our observation indicated that 1 mg/kg had no effect, while 5 mg/kg elevated the hippocampal p53 (Figure 1A), p21 (Figure 1B), and p16 (Figure 1C) levels, and 10 mg/kg was lethal (data not shown). Therefore, we continued our study using a 5 mg/kg dose of doxorubicin. Next, we investigated whether Soymetide pretreatment (10, 50, and 100 μg/kg) affected the doxorubicin-induced senescence in the hippocampus of mice. Our findings indicated that 10 μg/kg of Soymetide had no effect. However, doses of 50 and 100 μg/kg caused a reduction in the doxorubicin-induced hippocampal p53 (Figure 1D), p21 (Figure 1E) and p16 (Figure 1F) levels, which were comparable to that of a previously demonstrated senolytic combination, D + Q (referred to Seno in Figure) (Krzystyniak et al., 2022) (Figures 1D–F). (The scrambled peptide did not affect the doxorubicin-induced senescence in the hippocampus (Supplementary Material 3). We conducted further analysis of the other features of senescence. We found that doxorubicin increased the expression of SASP inflammatory factors, such as IL-1α, IL-1β and IL-6, while Soymetide (50 and 100 μg/kg) and senolytic combination, D + Q reduced SASP levels (Figure 2A). We proceeded with our study using 50 μg/kg of Soymetide, i.e., the lower dose of Soymetide showing anti-senescence activity.
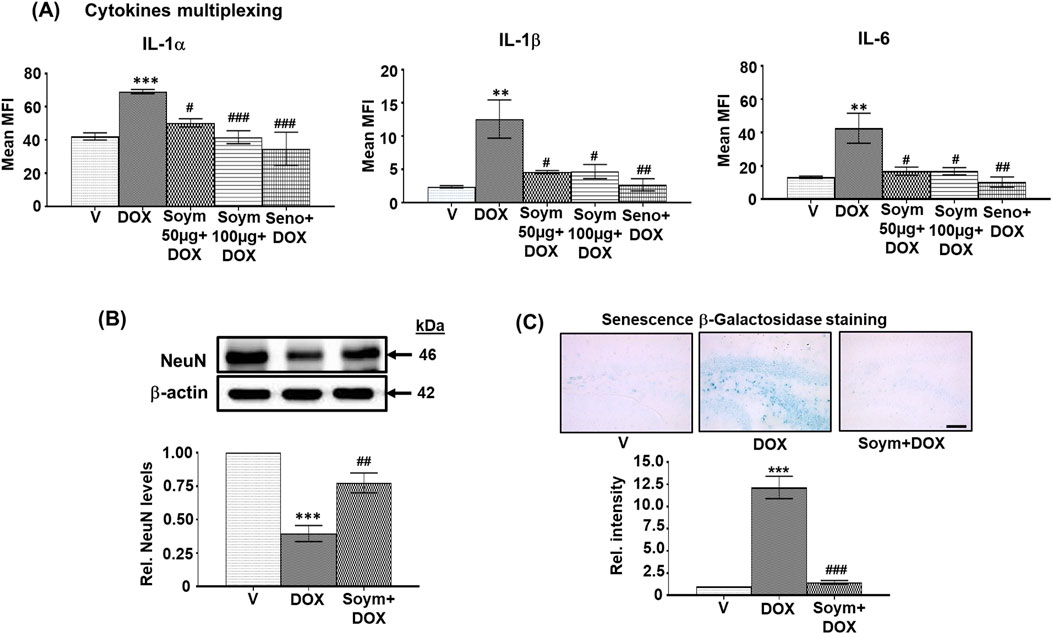
Figure 2. Soymetide prevents doxorubicin-induced rise in serum cytokine levels and hippocampal β-Galactosidase staining and the loss of hippocampal NeuN levels in mice. Serum was isolated from the blood of vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX, and senolytic combination (Seno)+DOX-treated mice. (A) Representative densitometry of serum IL-1α, IL-1β and IL-6 levels. Hippocampal tissues and whole brain were isolated from vehicle (V), doxorubicin (DOX), and Soymetide (Soym)+DOX-treated mice. (B) Representative Western blots and corresponding densitometry (relative to vehicle) of NeuN normalized with β-actin in the hippocampal tissues. (C) Representative photomicrographs and corresponding bar graph (relative to vehicle) of Senescence β-Galactosidase-stained hippocampus. Scale bar: 100 µm. Data represent means ± SE of three mice/group. ***p < 0.001 and **p < 0.01 compared to V. ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to DOX.
We further found that doxorubicin reduced the matured neuronal marker, NeuN (Figure 2B), and increased the Senescence β-Galactosidase staining in neurons (Figure 2C). These effects were inhibited by Soymetide (Figures 2B, C).
Effect of Soymetide and doxorubicin on Wnt pathway and its link with anti-senescence effects in the hippocampus
We explored the effects of doxorubicin on the Wnt pathway (known to participate in neuronal functioning (Garg and Bandyopadhyay, 2024) and its involvement in the anti-senescence activity induced by Soymetide. Our initial findings demonstrated a reduction in the levels of hippocampal Wnt3a (Figure 3A), along with decreases in Wnt receptors, p-LRP6 (Figure 3B) and Fz (Figure 3C), and Wnt pathway components, Dvl (Figure 3D) and Axin1 (Figure 3E), following administration of doxorubicin. This treatment caused an increase in GSK-3β levels (Figure 3F) and a decrease in β-catenin (Figure 3G). However, pre-treatment with Soymetide appeared to counteract these effects, suggesting a restoration of the Wnt/β-catenin pathway (Figures 3A–G). Upon investigating the interplay between the Wnt/β-catenin pathway components and anti-senescence mechanism, our findings demonstrated that blocking Wnt and β-catenin with rDkk1 and iCRT3, respectively, prevented the Soymetide-induced decreases in p53 (Figure 4A), p21 (Figure 4B) and p16 (Figure 4C) and Senescence β-Galactosidase staining (Figure 4D), and increase in NeuN levels (Figure 4E). These findings suggested the participation of the components of Wnt3a/β-catenin pathway in the senescence mechanism of the hippocampus, which Soymetide can inhibit.
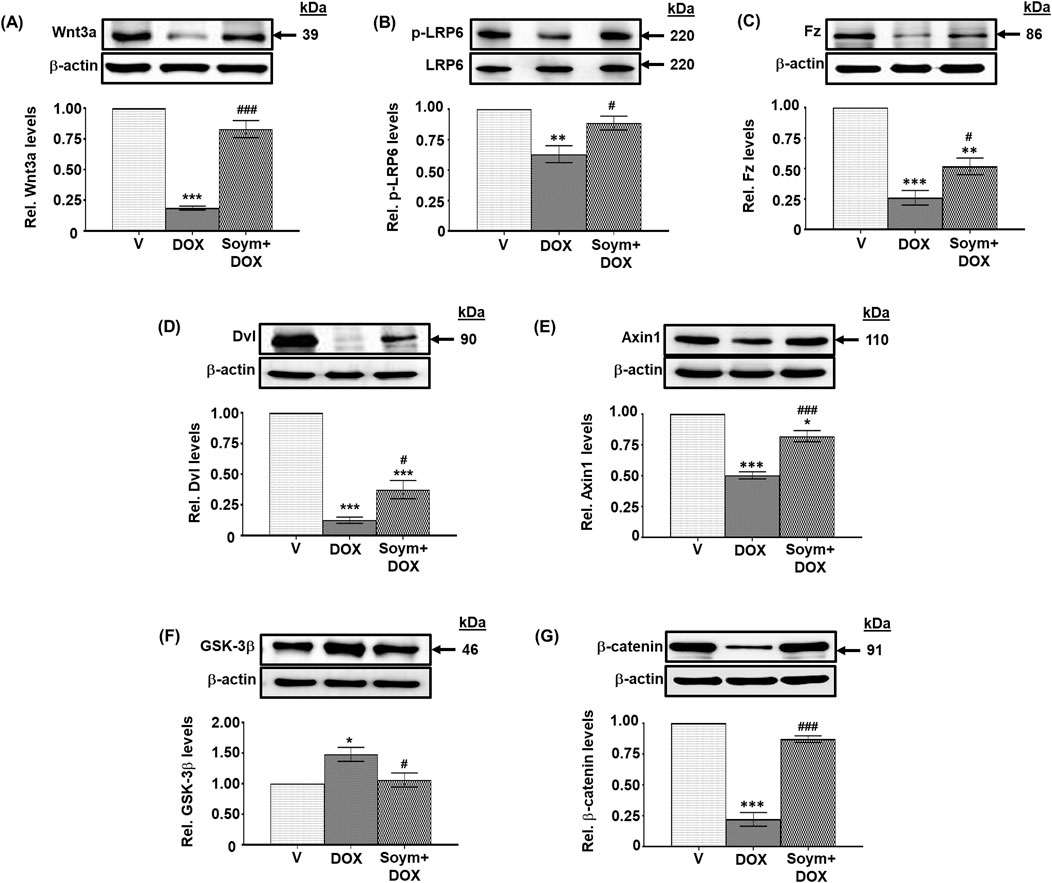
Figure 3. Soymetide inhibits doxorubicin-induced attenuation of hippocampal Wnt3a/β-catenin pathway in mice. Hippocampal tissues were isolated from vehicle (V), doxorubicin (DOX) and Soymetide (Soym)+DOX-treated mice. (A–G) Representative Western blots and corresponding densitometry (relative to vehicle) of Wnt3a (A), p-LRP6 (B), Frizzled (Fz) (C), Dishevelled (Dvl) (D), Axin1 (E), GSK-3β (F) and β-catenin (G) normalized with β-actin (A, C–G) or LRP6 (B) in the hippocampal tissues. Data represent means ± SE of three mice/group. ***p < 0.001, **p < 0.01 and *p < 0.05 compared to V. ###p < 0.001 and #p < 0.05 compared to DOX.

Figure 4. The Soymetide-induced reduction in senescence markers and increase in NeuN levels are Wnt3a/β-catenin-dependent in the hippocampus of doxorubicin-treated mice. Hippocampal tissues and the whole brain were isolated from vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX, Soym + DOX + rDkk1, and Soym + DOX + iCRT3-treated mice. (A–C, E) Representative Western blots and corresponding densitometry (relative to vehicle) of p53 (A), p21 (B), p16 (C) and NeuN (E) normalized with β-actin in the hippocampal tissues. (D) Representative photomicrographs and corresponding bar graph (relative to vehicle) of Senescence β-Galactosidase-stained hippocampus in brain sections. Scale bar: 100 µm. Data represent means ± SE of three mice/group. The quantification of the first three groups in (D) and (E) are based on the same data sets as in Figures 2B, C. ***p < 0.001 and **p < 0.01 compared to V. ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to DOX. @@@p < 0.001, @@p < 0.01 and @p < 0.05 compared to Soym + DOX.
Effect of Soymetide and doxorubicin on senescence and Wnt signaling in the hippocampal neurons
We verified the involvement of Wnt3a/β-catenin signaling in the hippocampal neuronal senescence. Our co-immunolabeling experiment with NeuN revealed a decrease in Wnt3a (Figure 5A) and β-catenin (Figure 5B), and an increase in p53 (Figure 5C) and p16 (Figure 5D) in the hippocampal neurons of mice following doxorubicin treatment. However, these changes were counteracted by Soymetide (Figures 5A–D). Moreover, rDkk1 and iCRT3 inhibited the Soymetide-induced decrease in p53 (Figure 5C) and p16 (Figure 5D), thereby verifying that Soymetide exerts its anti-senescence effects by modulating the Wnt3a/β-catenin pathway in the hippocampal neurons.
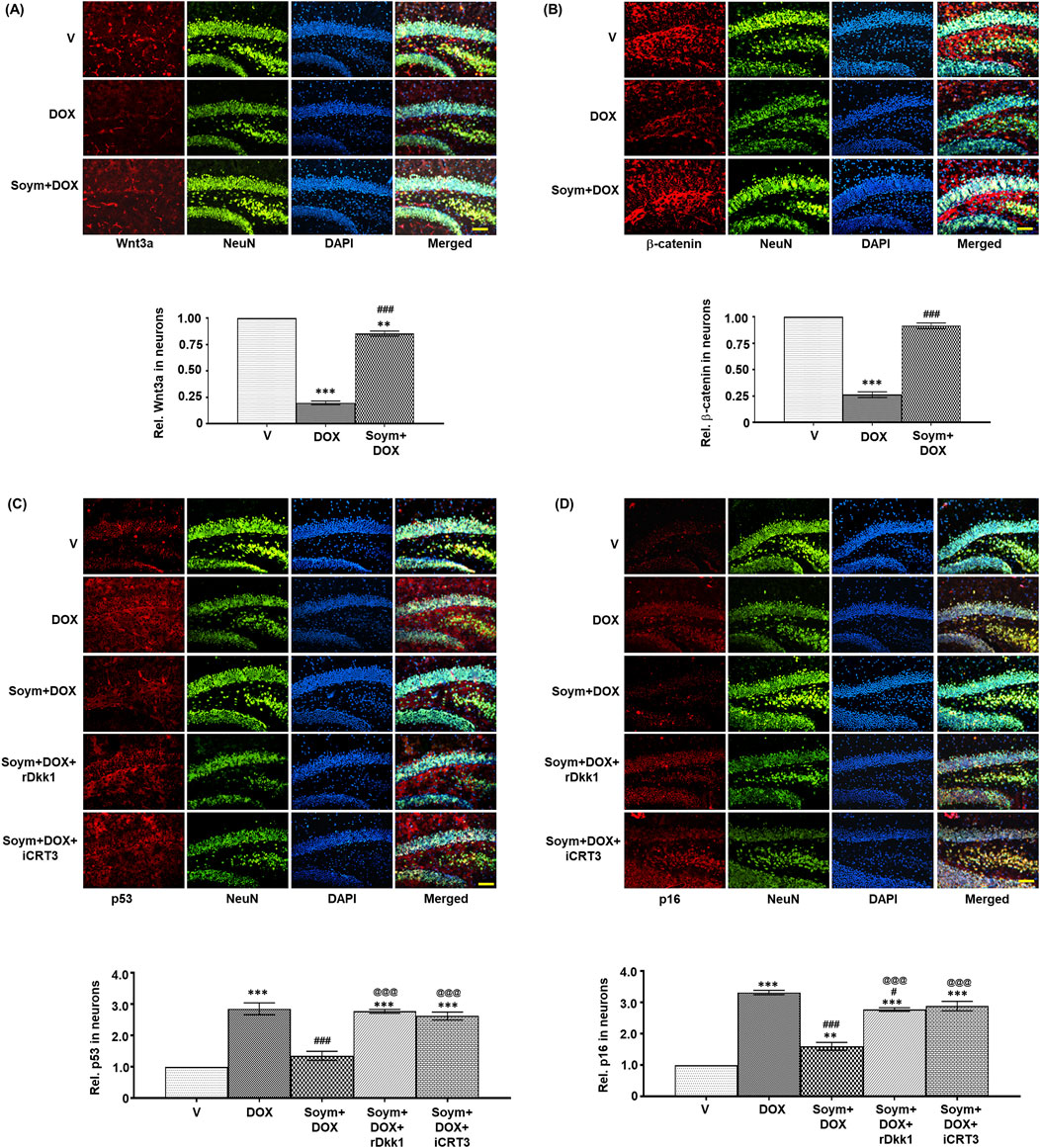
Figure 5. Soymetide increases Wnt3a and β-catenin and decreases p53 and p16 levels in the hippocampal neurons of doxorubicin-treated mice. Fluorescence immunohistochemistry was performed on brain sections of vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX, Soym + DOX + rDkk1 and Soym + DOX + iCRT3-treated mice. (A–D) Representative fluorescence photomicrographs of Wnt3a (A), β-catenin (B), p53 (C) and p16 (D) co-immunolabeled with NeuN and counter-stained with DAPI in the hippocampus of brain sections. Bar diagrams represent corresponding quantification relative to vehicle. Scale bar: 100 µm. Data represent means ± SE of three mice/group. ***p < 0.001 and **p < 0.01 compared to V. ###p < 0.001 and #p < 0.05 compared to DOX. @@@p < 0.001 compared to Soym + DOX.
We conducted in vitro validation of the anti-senescent mechanism of Soymetide (10 nM, Supplementary Material 2) in primary cultures of mouse hippocampal neurons. Consistent with the in vivo observations, our findings showed that doxorubicin treatment led to a decrease in the Wnt3a (Figure 6A) and β-catenin levels (Figure 6B), while elevating p53 (Figure 6C), p16 (Figure 6D) and Senescence β-Galactosidase staining (Figure 6E), and decreasing NeuN levels (Figure 6F). However, the doxorubicin-induced changes in Wnt3a , β-catenin and senescent markers were mitigated by Soymetide (Figures 6A–F). (The scrambled peptide failed to affect the doxorubicin-induced senescence (Supplementary Material 4). Additionally, these Soymetide-induced effects were blocked by rDkk1 and iCRT3 (Figures 6C–F). Hence, our findings verified the involvement of components of Wnt3a/β-catenin pathway in the hippocampal neuronal senescence and demonstrated the anti-senescence effect of Soymetide.
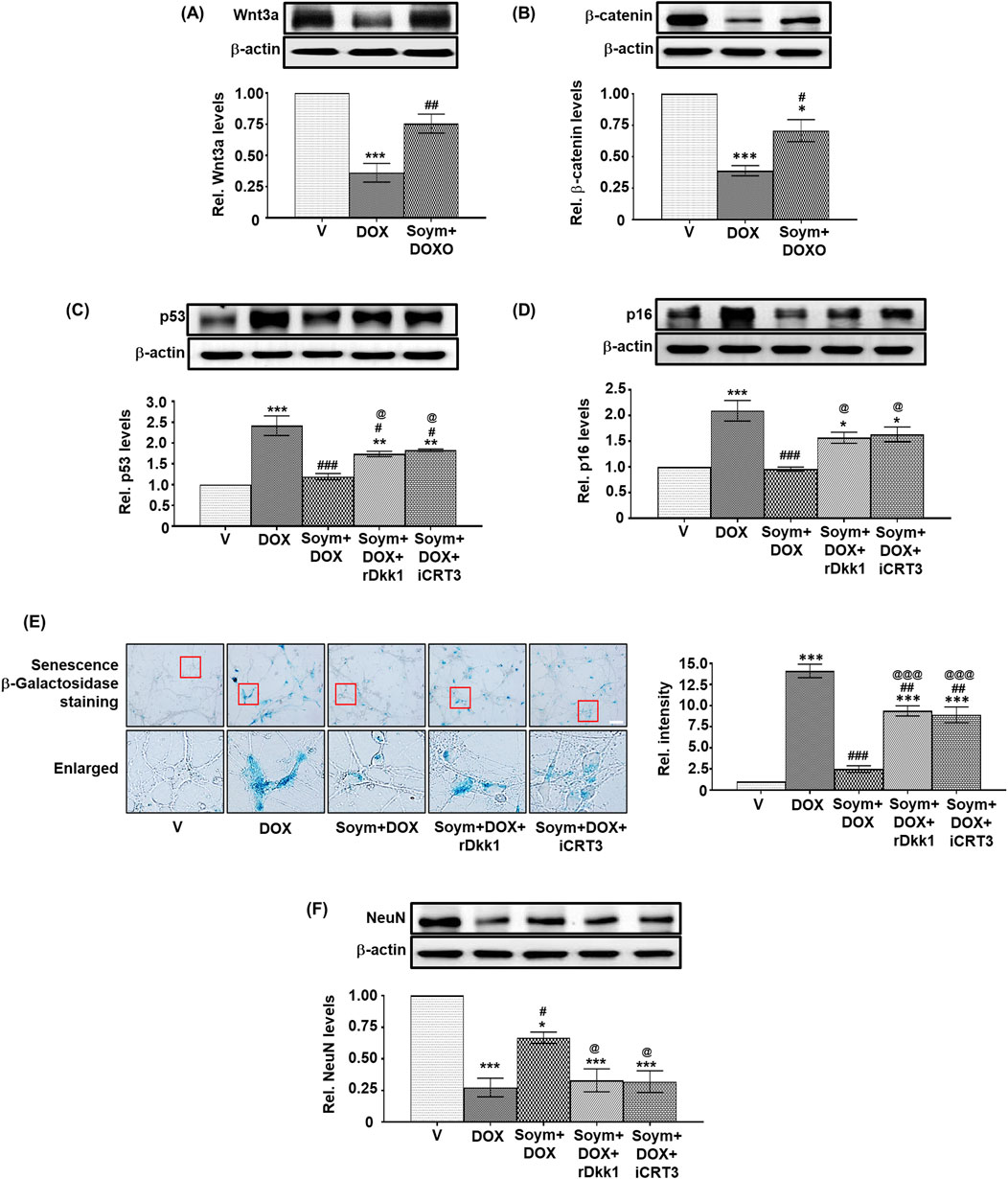
Figure 6. Soymetide inhibits the doxorubicin-induced reduction in Wnt3a, β-catenin, and NeuN and the increase in senescence of primary mice hippocampal neurons. Primary hippocampal neurons treated with vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX, Soym + DOX + rDkk1 and Soym + DOX + iCRT3 were harvested. (A–D, F) Representative Western blots and corresponding densitometry (relative to vehicle) of Wnt3a (A), β-catenin (B), p53 (C), p16 (D) and NeuN (F) normalized with β-actin in primary hippocampal neurons. (E) Representative photomicrographs and corresponding bar graph (relative to vehicle) of Senescence β-Galactosidase-stained primary hippocampal neurons. Scale bar: 20 µm. Data represent means ± SE of three independent experiments. ***p < 0.001, **p < 0.01 and *p < 0.05 compared to V. ###p < 0.001, ##p < 0.01 and #p < 0.05 compared to DOX. @@@p < 0.001 and @p < 0.05 compared to Soym + DOX.
Effect of Soymetide and doxorubicin on the hippocampal neuronal survival and learning-memory performance of mice
Wnt pathway and senescence are linked with neuronal survival and subsequent cognitive functions (Garg and Bandyopadhyay, 2024). Hence, we assessed the effect of doxorubicin and Soymetide on hippocampal Nissl staining for neuronal count, TUNEL staining for neuronal apoptosis, and learning-memory performance of mice. We found that doxorubicin led to a reduction in Nissl staining (Figure 7A) and increase in TUNEL positive neuronal apoptotic index (Figure 7B) in the hippocampus, indicating neuronal loss. However, Soymetide counteracted these effects (Figures 7A, B). Additionally, we observed that doxorubicin impaired learning and memory abilities, as demonstrated by an increased number of errors during learning trials (Figure 7C) and diminished memory retention in the Y-Maze test at various intervals post-learning (Figure 7D). Conversely, Soymetide treatment facilitated a notable improvement in these cognitive functions (Figures 7C, D). This was further supported by results from the Passive Avoidance test, where Soymetide enhanced the TLT across the retention trials in doxorubicin-treated mice (Figure 7E). The beneficial outcomes associated with Soymetide administration were counteracted by rDkk1 and iCRT3 (Figures 7A–E), suggesting that the neuroprotective and cognitive-enhancing effects of Soymetide are mediated through the components of the Wnt3a/β-catenin pathway.
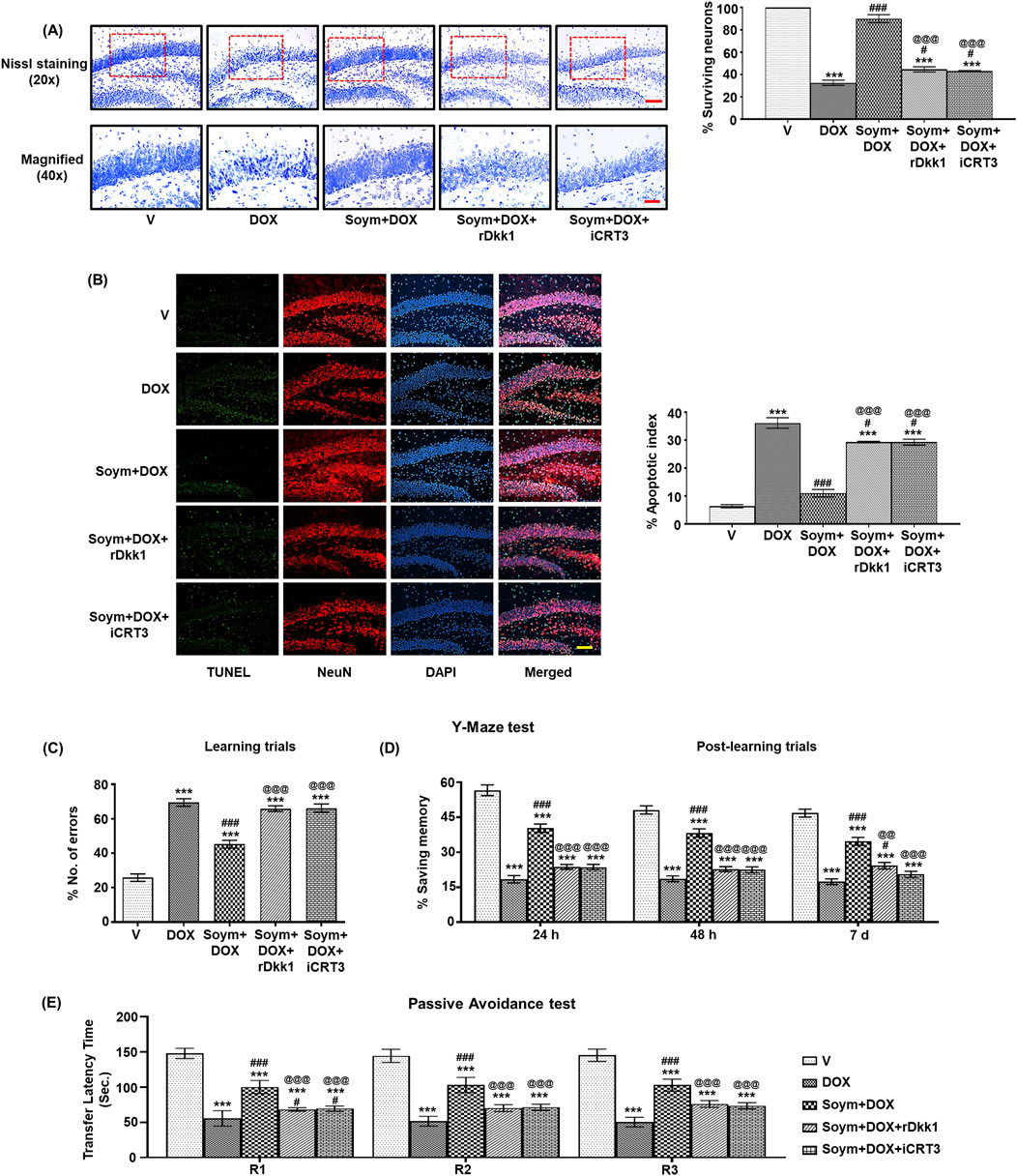
Figure 7. Soymetide protects against doxorubicin-induced hippocampal neuronal loss and learning-memory impairment via the Wnt/β-catenin pathway in mice. Brain sections were made from vehicle (V), doxorubicin (DOX), Soymetide (Soym)+DOX, Soym + DOX + rDkk1 and Soym + DOX + iCRT3-treated mice. (A) Upper panel-20x (with quantification, %) and lower panel-40x represent photomicrographs of Nissl-stained hippocampal neurons. Scale bar: 100 µm (20X) and 30 µm (40X). (B) Representative fluorescence photomicrographs of TUNEL, immunolabeled with NeuN and counter-stained with DAPI in the hippocampus. Bar diagrams represent corresponding quantification relative to the vehicle. Scale bar: 100 µm. Y -Maze and Passive Avoidance tests were performed in vehicle (V), doxorubicin (DOX), Soymetide (Soym)+ DOX, Soym + DOX + rDkk1 and Soym + DOX + iCRT3-treated mice. (C, D) Representative bar graphs for the number of errors (%) during learning trials (C) and the memory retained (% saving memory) at 24 h, 48 h and 7-day post-learning (D) in Y-Maze test. (E) Representative bar graphs for TLT for first (R1), second (R2), and third (R3) retention trials (24 h, 48 h, and 72 h, respectively) in Passive Avoidance test. Data represent means ± SE of three mice/group (A, B) and seven mice/group (C–E). ***p < 0.001 compared to V. ###p < 0.001 and #p < 0.05 compared to DOX. @@@p < 0.001 and @@p < 0.01 compared to Soym + DOX.
Discussion
The current study unravels novel neuroprotective properties of Soymetide, with a specific focus on: 1. Reduction of senescence in the hippocampus, with effects comparable to a known senolytic combination (D + Q). 2. Beneficial impact involving the Wnt/β-catenin pathway. The reduction of key components in the Wnt3a/β-catenin pathway accelerates neuronal senescence. However, Soymetide’s ability to restore Wnt/β-catenin signaling helped counteract this senescence process. 3. Improvement in neuronal survival and enhancement of learning and memory functions. The study overall introduces a soy peptide that shows promise for improving hippocampal health and cognitive functions by targeting the interlinked senescence pathways and Wnt/β-catenin mechanism, offering a potential therapeutic approach against cognitive decline (Figure 8).
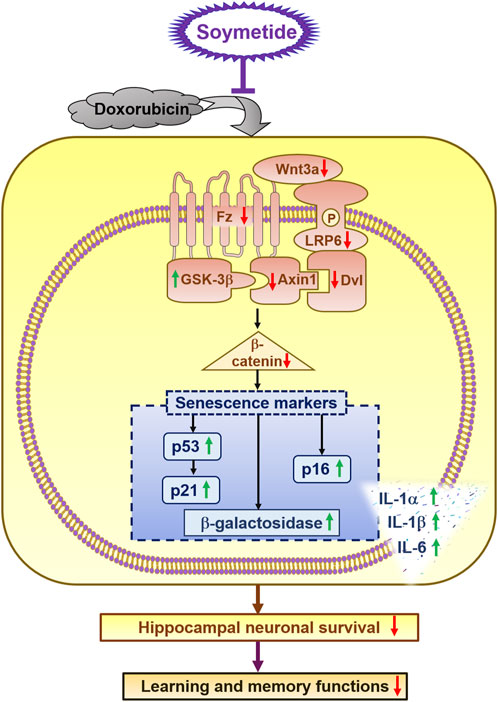
Figure 8. Schematic of the anti-senescent effect induced by Soymetide against doxorubicin treatment in hippocampal neurons and its positive impact on cognitive function. Soymetide prevents the reduction in Wnt3a and its related proteins (Fz, p-LRP-6, Dvl, and Axin1) caused by doxorubicin, and it counters the rise in GSK-3β and the reduction of β-catenin. This inhibits the increase in senescence markers such as p53, p21, p16, β- Galactosidase activity, and SASPs, as well as the decrease in neuronal survival and learning-memory functions. In summary, Soymetide exhibits anti-senescent effects, provides neuroprotection, and induces cognitive restoration by restoring the Wnt/β-catenin pathway.
Studies suggest that fermented soy food products like cheonggukjang and doenjang offer neuroprotection by inhibiting neuronal death, inflammation, and susceptibility to ischemic injury (Ko et al., 2019; Jeong et al., 2020; Jang et al., 2021). Nattō, a fermented whole soybean improves age-related cognitive decline and associated deregulated hippocampal neuronal signaling in senescence-accelerated mouse-prone eight mice (Zheng et al., 2023). Additionally, soy isoflavones (genistein and daidzein) and their metabolites (equol), and soyasaponin I (a triterpenoid compound) prevent neuronal apoptosis, promote neuroregeneration, synaptic plasticity, neurotransmission, and antioxidative effects (Ma et al., 2010; Hong et al., 2013; Lu et al., 2021; Shi et al., 2023). However, the soy-derived compounds may face limitations regarding their “drug-likeness” due to concerns about metabolic stability restricting potential therapeutic applications, as reported for phytochemicals (Sharan et al., 2009). Reports further indicate that soy peptides, VHVV and Tyr-Pro, enzymatic and fungal hydrolysates of soy proteins, respectively, maintain neuronal survival and reduce memory loss (Ju et al., 2019; Tanaka et al., 2020). However, while hydrolyzed proteins and peptides show promise as food and potential nutraceutical applications, standardizing them as therapeutic products may pose challenges. In this context, Soymetide, a well-characterized small/short soy-derived peptide can serve as a promising biologic/peptide drug, marking a considerable advancement in this field. Our findings identify Soymetide as a neuroprotectant that promotes a wide range of anti-senescent features in the hippocampal neurons, comparable with broad-target senolytic drugs (D + Q). The small peptide is proposed to hold potential for advancing peptide drug design and development, based on probable advantages such as improved absorption, enhanced cell targeting, reduced immunogenicity, and increased cost-effectiveness (Fosgerau and Hoffmann, 2015). These features may position Soymetide as a potential candidate for therapies aimed at anti-senescence and neuroprotection. Besides, this novel pro-cognitive role adds a notable new dimension to the functional profile of Soymetide, reported for immunostimulating and anti-cancer activities (Schuler et al., 1982; Tsuruki et al., 2003), offering fresh insights into its potential mechanism of action. The selected dose of 50 μg/kg of Soymetide was identified for its effective anti-senescence properties, offering a cost-effective alternative to the higher 100 μg/kg dose. While safety data was not specifically assessed in this study, the lower dose presents a promising approach that warrants further investigation to determine the best balance of safety and therapeutic benefits.
Prior studies show that eliminating p16-expressing senescent cells and lowering the p53 stress protein can prevent mild cognitive impairment, neuronal death, and synaptic issues (Bussian et al., 2018; Zhang et al., 2022b). This process minimizes p53-triggered p21 activation and DNA damage, averting cell cycle arrest and senescence (de Carne Trecesson et al., 2011; Tajima et al., 2019; Tufekci et al., 2021). Our research suggests that Soymetide influences these communications involving p16, p21, p53, and SASPs, leading to healthier neuronal expression, reduced cellular damage, and improved brain repair, benefiting the central nervous system (CNS). Specifically, regarding soy peptides, previous research has mainly focused on their roles in preventing and suppressing tumors by engaging pathways such as p53, p16, p21, and cytokines, and growth arrest and apoptosis in various cancer cells (Lin et al., 1998; Payton-Stewart et al., 2009). Studies in osteoblasts did show the involvement of p53/p21 pathway in bone development following soy peptide treatment (Zhang et al., 2014; Chen et al., 2015). However, the lack of research on the impact of soy peptides on the brain, specifically its anti-senescent effects on the hippocampus, underscores the importance and promise of our study on these soy-based peptides for future exploration.
Our research enriches the current understanding of senescence in the hippocampal neurons and extends upon earlier work (Bayod et al., 2015; Su et al., 2018), which showed a link between the upregulation of the Wnt pathway inhibitor, Dickkopf-1, and the perturbation of essential elements within the Wnt/β-catenin signaling cascade. Our findings further detail that the senescence process is associated with precise alterations in Wnt signaling markers in the hippocampal neurons, which correspond with an apparent decline in neuronal viability, as evidenced by a diminished reduction in Nissl bodies, along with NeuN expression. In a notable progression from past research (Fortress and Frick, 2016; Arredondo et al., 2020), which predominantly focused on the broader aspects of Wnt signaling, our study also identifies a decrease in both the Wnt ligands and its corresponding Frizzled receptor during senescence. Moreover, while prior reports only noted changes in Wnt signaling during hippocampal senescence (Bayod et al., 2015; Fortress and Frick, 2016), our investigation additionally sheds light on how these modifications in Wnt pathway actively played a key role in controlling neuronal senescence and the impact on its essential molecules, such as p53, p21, p16, SASP, β-Galactosidase, etc. Our research explicitly linked these observations with Soymetide treatment, demonstrating its restoration in the levels of the Wnt ligand and Frizzled receptor in the senescent neurons. Moreover, it unraveled that Soymetide’s anti-senescent effects are highly dependent on regulated Wnt signaling, demonstrated by the use of the Wnt antagonist rDkk1 and the β-catenin inhibitor iCRT3, which blocked the reduction of senescent markers in the hippocampal neurons. In this context, in terms of soy-based protein and Wnt signaling (as such), there have been several contradictory reports. On the one hand, soy proteins (predominantly genistein) reduced β-catenin signaling (via the participation of altered IGF-1 and E-cadherin production) leading to a decreased T-cell factor/lymphoid enhancer factor (TCF/LEF) activity and resulting prostate cancer cell growth, development of hepatocellular carcinoma, intestinal tumorigenesis, and proliferation in cancer cells (Liss et al., 2010; Mahmoud et al., 2014; Mercer et al., 2017). Other studies indicated that genistein and soy isoflavones enhanced Wnt/β-catenin signaling and led to osteoblast formation, improved bone health, and prevented non-alcoholic fatty liver disease (NAFLD) and pathological adiposity (Kim and Kang, 2012; Mannino et al., 2024). In line with the latter, results from our study demonstrated the neuroprotective effects of Soymetide through the enhancement of the components of the Wnt/β-catenin pathway, verifying a protective role of soy peptides in increasing Wnt/β-catenin pathway for normal cells as opposed to the tumorous and cancerous cells. As a supportive observation, Soymetide inhibits the reduction of Transcription Factor 3 (TCF3) levels caused by doxorubicin (Supplementary Material 5), which emphasize the involvement of the Wnt/β-catenin pathway. This effect could be linked to the regulation of p53, p21, p16, and β-galactosidase, laying the groundwork for future studies to investigate its mechanisms of action. Hence, it may be said that in terms of the Wnt pathway, the importance of our study for hippocampal neurons is two-fold. It offers new insights into the senescence pathway and emphasizes the potential of Soymetide in mitigating the adverse effects of senescence.
Research indicates that soy proteins, isoflavone extracts, and peptides can enhance cognitive performance in adults, postmenopausal women, and the aged by activating brain pathways such as Brain-Derived Neurotrophic Factor (BDNF)-Tropomyosin-related kinase receptor B (TrkB)/cAMP response element-binding protein (CREB) signaling, N-methyl-D-aspartate receptor (NMDAR)-calcium/calmodulin-dependent protein kinase II (CaMKII) cascade (NMDAR-CaMKII), as well as Phosphatidylinositol-3 Kinase (PI3K)/Akt signaling, and through antioxidant and cholinergic properties (Kreijkamp-Kaspers et al., 2004; Geller and Studee, 2006; Katayama et al., 2014; Unno and Konishi, 2015; Yoo et al., 2022; Zheng et al., 2023). Earlier individual studies have also shown a connection between Wnt/β-catenin signaling with the BDNF (TrkB)/CREB signaling, NMDAR-CaMKII, PI3K/Akt, antioxidant, and cholinergic properties (Arrazola et al., 2009; Sinha et al., 2015; Xu et al., 2015; Hu et al., 2019; Ishidori et al., 2022; Nachtigall et al., 2023; Lou et al., 2024). Hence, in continuation, our research highlights the importance of investigating the relationships between neurotrophins, neurotransmitters, antioxidants, and specific signaling pathways in Sometide-treated hippocampal neurons. Our work points to the need to explore Soymetide’s impact on aging, specifically concerning hippocampal Wnt signaling. Moreover, while our study demonstrated Soymetide’s anti-senescence effects when directly applied to the hippocampus, proving its potential to protect against neuronal aging, this method is infeasible for human use. Therefore, there is a need for further research to develop a brain-transportable Soymetide formulation for systemic administration.
Hence, our research suggests that Soymetide has the potential to diminish cellular senescence in hippocampal neurons, which may improve cognitive performance and maintain neuronal health through modulation of senescence markers. Additionally, we propose that Soymetide might possess anti-inflammatory properties through its impact on macrophages and monocytes, offering neuroprotection against age-related diseases. The observed reduction in serum pro-inflammatory cytokines suggests an immune modulation mechanism that warrants further exploration.
We recognize few limitations in this study. The primary focus has been on investigating the effectiveness of Soymetide as a single-agent treatment. Additionally, while the potential for immune modulation has not been thoroughly examined, and the use of combination therapies (pairing Soymetide with dasatinib or quercetin) has yet to be explored, these aspects are crucial for future research aimed at improving treatment efficacy and minimizing toxicity. Furthermore, it is vital to determine optimal dosages, study the pharmacokinetics of Soymetide in various biological settings, and develop more effective peptide variants through in vitro assays, with the goal of extending these findings to in vivo applications. These efforts will enhance our understanding and optimize the therapeutic outcomes when utilizing Soymetide.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Institutional Animal Ethics Committee-Indian Institute of Toxicology Research. IAEC reference numbers are IITR/IAEC/53/20 and IITR/IAEC/10/24. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AG: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Visualization, Writing–original draft, Writing–review and editing. JS: Writing–review and editing, Data curation. ST: Data curation, Writing–review and editing. UD: Methodology, Writing–review and editing. NS: Data curation, Writing–review and editing. JG: Conceptualization, Methodology, Supervision, Writing–review and editing. SB: Conceptualization, Methodology, Supervision, Funding acquisition, Project administration, Resources, Software, Validation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Science and Engineering Research Board-Scientific and Useful Profound Research Advancement, Govt. of India (SPR/2019/000346), Indian Council of Medical Research, Govt. of India (5/8-4/2/Env/2020-NCD-II) and CSIR-IITR Institutional fund (MLP005).
Acknowledgments
AG received her fellowship from UGC, Govt. of India. JS, ST, and UD received their fellowship from CSIR, Govt. of India. NS received her fellowship from ICMR, Govt. of India. The CSIR-IITR communication number of this article is IITR/SEC/MS/2024/55.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work the author(s) used Grammarly in order to improve readability of the manuscript. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1510337/full#supplementary-material
References
Arrazola, M. S., Ramos-Fernandez, E., Cisternas, P., Ordenes, D., and Inestrosa, N. C. (2017). Wnt signaling prevents the aβ oligomer-induced mitochondrial permeability transition pore opening preserving mitochondrial structure in hippocampal neurons. PLoS One 12 (1), e0168840. doi:10.1371/journal.pone.0168840
Arrazola, M. S., Varela-Nallar, L., Colombres, M., Toledo, E. M., Cruzat, F., Pavez, L., et al. (2009). Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/beta-catenin signaling pathway. J. Cell Physiol. 221 (3), 658–667. doi:10.1002/jcp.21902
Arredondo, S. B., Valenzuela-Bezanilla, D., Mardones, M. D., and Varela-Nallar, L. (2020). Role of Wnt signaling in adult hippocampal neurogenesis in health and disease. Front. Cell Dev. Biol. 8, 860. doi:10.3389/fcell.2020.00860
Avila, M. E., Sepulveda, F. J., Burgos, C. F., Moraga-Cid, G., Parodi, J., Moon, R. T., et al. (2010). Canonical Wnt3a modulates intracellular calcium and enhances excitatory neurotransmission in hippocampal neurons. J. Biol. Chem. 285 (24), 18939–18947. doi:10.1074/jbc.M110.103028
Bayod, S., Felice, P., Andres, P., Rosa, P., Camins, A., Pallas, M., et al. (2015). Downregulation of canonical Wnt signaling in hippocampus of SAMP8 mice. Neurobiol. Aging 36 (2), 720–729. doi:10.1016/j.neurobiolaging.2014.09.017
Beurel, E., Grieco, S. F., and Jope, R. S. (2015). Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol. Ther. 148, 114–131. doi:10.1016/j.pharmthera.2014.11.016
Bussian, T. J., Aziz, A., Meyer, C. F., Swenson, B. L., van Deursen, J. M., and Baker, D. J. (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 562 (7728), 578–582. doi:10.1038/s41586-018-0543-y
Chen, D., Zhang, Y., Zhang, M., Chang, J., Zeng, Z., Kou, X., et al. (2020). Exercise attenuates brain aging by rescuing down-regulated wnt/β-catenin signaling in aged rats. Front. Aging Neurosci. 12, 105. doi:10.3389/fnagi.2020.00105
Chen, J. R., Lazarenko, O. P., Blackburn, M. L., Badger, T. M., and Ronis, M. J. (2015). Soy protein isolate inhibits high-fat diet-induced senescence pathways in osteoblasts to maintain bone acquisition in male rats. Endocrinology 156 (2), 475–487. doi:10.1210/en.2014-1427
Das, G., Yu, Q., Hui, R., Reuhl, K., Gale, N. W., and Zhou, R. (2016). EphA5 and EphA6: regulation of neuronal and spine morphology. Cell Biosci. 6, 48. doi:10.1186/s13578-016-0115-5
de Carne Trecesson, S., Guillemin, Y., Belanger, A., Bernard, A. C., Preisser, L., Ravon, E., et al. (2011). Escape from p21-mediated oncogene-induced senescence leads to cell dedifferentiation and dependence on anti-apoptotic Bcl-xL and MCL1 proteins. J. Biol. Chem. 286 (15), 12825–12838. doi:10.1074/jbc.M110.186437
Deng, Z., Xie, D., Cai, J., Jiang, J., Pan, D., Liao, H., et al. (2023). Different types of milk consumption and the risk of dementia: analysis from a large-scale cohort study. Clin. Nutr. 42 (10), 2058–2067. doi:10.1016/j.clnu.2023.08.019
Dong, B., Qi, Y., Sundas, H., Yang, R., Zhou, J., and Li, Z. (2023). Soy protein increases cognitive level in mice by modifying hippocampal nerve growth, oxidative stress, and intestinal microbiota. J. Sci. Food Agric. 103 (8), 4085–4094. doi:10.1002/jsfa.12388
Fortress, A. M., and Frick, K. M. (2016). Hippocampal Wnt signaling: memory regulation and hormone interactions. Neuroscientist 22 (3), 278–294. doi:10.1177/1073858415574728
Fosgerau, K., and Hoffmann, T. (2015). Peptide therapeutics: current status and future directions. Drug Discov. Today 20 (1), 122–128. doi:10.1016/j.drudis.2014.10.003
Garg, A., and Bandyopadhyay, S. (2024). Role of an interdependent Wnt, GSK3-β/β-catenin and HB-EGF/EGFR mechanism in arsenic-induced hippocampal neurotoxicity in adult mice. Chemosphere 352, 141375. doi:10.1016/j.chemosphere.2024.141375
Geller, S. E., and Studee, L. (2006). Soy and red clover for mid-life and aging. Climacteric 9 (4), 245–263. doi:10.1080/13697130600736934
Gupta, K., Vishwakarma, J., Garg, A., Pandey, R., Jain, V., Gupta, R., et al. (2022). Arsenic induces gsk3β-dependent p-tau, neuronal apoptosis, and cognitive impairment via an interdependent hippocampal ERα and IL-1/IL-1R1 mechanism in female rats. Toxicol. Sci. 190 (1), 79–98. doi:10.1093/toxsci/kfac087
Hong, S. W., Heo, H., Yang, J. H., Han, M., Kim, D. H., and Kwon, Y. K. (2013). Soyasaponin I improved neuroprotection and regeneration in memory deficient model rats. PLoS One 8 (12), e81556. doi:10.1371/journal.pone.0081556
Hu, Y., Chen, W., Wu, L., Jiang, L., Liang, N., Tan, L., et al. (2019). TGF-β1 restores hippocampal synaptic plasticity and memory in alzheimer model via the PI3K/Akt/Wnt/β-Catenin signaling pathway. J. Mol. Neurosci. 67 (1), 142–149. doi:10.1007/s12031-018-1219-7
Huang, H. C., and Klein, P. S. (2004). The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 5 (7), 234. doi:10.1186/gb-2004-5-7-234
Hwang, Y. H., Park, S., Paik, J. W., Chae, S. W., Kim, D. H., Jeong, D. G., et al. (2019). Efficacy and safety of lactobacillus plantarum C29-fermented soybean (DW2009) in individuals with mild cognitive impairment: a 12-week, multi-center, randomized, double-blind, placebo-controlled clinical trial. Nutrients 11 (2), 305. doi:10.3390/nu11020305
Inestrosa, N. C., Urra, S., and Colombres, M. (2004). Acetylcholinesterase (AChE)-amyloid-beta-peptide complexes in Alzheimer's disease. the Wnt signaling pathway. Curr. Alzheimer Res. 1 (4), 249–254. doi:10.2174/1567205043332063
Ishidori, H., Okihara, H., Ogawa, T., Abe, Y., Kato, C., Aung, P. T., et al. (2022). Nasal obstruction during the growth period modulates the Wnt/β-catenin pathway and brain-derived neurotrophic factor production in association with tyrosine kinase receptor B mRNA reduction in mouse hippocampus. Eur. J. Neurosci. 55 (1), 5–17. doi:10.1111/ejn.15547
Jang, C. H., Oh, J., Lim, J. S., Kim, H. J., and Kim, J. S. (2021). Fermented soy products: beneficial potential in neurodegenerative diseases. Foods 10 (3), 636. doi:10.3390/foods10030636
Jeong, D. Y., Jeong, S. Y., Zhang, T., Wu, X., Qiu, J. Y., and Park, S. (2020). Chungkookjang, a soy food, fermented with Bacillus amyloliquefaciens protects gerbils against ishcmeic stroke injury, and post-stroke hyperglycemia. Food Res. Int. 128, 108769. doi:10.1016/j.foodres.2019.108769
Jeong, D. Y., Ryu, M. S., Yang, H. J., and Park, S. (2021). γ-PGA-Rich chungkookjang, short-term fermented soybeans: prevents memory impairment by modulating brain insulin sensitivity, neuro-inflammation, and the gut-microbiome-brain Axis. Foods 10 (2), 221. doi:10.3390/foods10020221
Jia, L., Pina-Crespo, J., and Li, Y. (2019). Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer's disease. Mol. Brain 12 (1), 104. doi:10.1186/s13041-019-0525-5
Ju, D. T., K, A. K., Kuo, W. W., Ho, T. J., Chang, R. L., Lin, W. T., et al. (2019). Bioactive peptide VHVV upregulates the long-term memory-related biomarkers in adult spontaneously hypertensive rats. Int. J. Mol. Sci. 20 (12), 3069. doi:10.3390/ijms20123069
Katayama, S., Imai, R., Sugiyama, H., and Nakamura, S. (2014). Oral administration of soy peptides suppresses cognitive decline by induction of neurotrophic factors in SAMP8 mice. J. Agric. Food Chem. 62 (16), 3563–3569. doi:10.1021/jf405416s
Kim, I. S. (2021). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants (Basel) 10 (7), 1064. doi:10.3390/antiox10071064
Kim, M. H., and Kang, K. S. (2012). Isoflavones as a smart curer for non-alcoholic fatty liver disease and pathological adiposity via ChREBP and Wnt signaling. Prev. Med. 54 (Suppl. l), S57–S63. doi:10.1016/j.ypmed.2011.12.018
Ko, J. W., Chung, Y. S., Kwak, C. S., and Kwon, Y. H. (2019). Doenjang, A Korean traditional fermented soybean paste, ameliorates neuroinflammation and neurodegeneration in mice fed a high-fat diet. Nutrients 11 (8), 1702. doi:10.3390/nu11081702
Kreijkamp-Kaspers, S., Kok, L., Grobbee, D. E., de Haan, E. H., Aleman, A., Lampe, J. W., et al. (2004). Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 292 (1), 65–74. doi:10.1001/jama.292.1.65
Krzystyniak, A., Wesierska, M., Petrazzo, G., Gadecka, A., Dudkowska, M., Bielak-Zmijewska, A., et al. (2022). Combination of dasatinib and quercetin improves cognitive abilities in aged male Wistar rats, alleviates inflammation and changes hippocampal synaptic plasticity and histone H3 methylation profile. Aging (Albany NY) 14 (2), 572–595. doi:10.18632/aging.203835
Lee, E., Madar, A., David, G., Garabedian, M. J., Dasgupta, R., and Logan, S. K. (2013). Inhibition of androgen receptor and β-catenin activity in prostate cancer. Proc. Natl. Acad. Sci. U. S. A. 110 (39), 15710–15715. doi:10.1073/pnas.1218168110
Li, T., Wan, Y. C., Sun, L. J., Tao, S. J., Chen, P., Liu, C. H., et al. (2018). DIXDC1 prevents oxygen-glucose deprivation/reoxygenation-induced injury in hippocampal neurons in vitro by promoting Wnt/β-catenin signaling. Eur. Rev. Med. Pharmacol. Sci. 22 (17), 5678–5687. doi:10.26355/eurrev_201809_15835
Li, W., Gan, C., Yu, S., Xu, J., Tang, L., and Cheng, H. (2024). Wnt3a/GSK3β/β-catenin signalling modulates doxorubicin-associated memory deficits in breast cancer. Mol. Neurobiol. 61, 5441–5458. doi:10.1007/s12035-023-03910-x
Lin, A. W., Barradas, M., Stone, J. C., van Aelst, L., Serrano, M., and Lowe, S. W. (1998). Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12 (19), 3008–3019. doi:10.1101/gad.12.19.3008
Lin, Y. F., Wang, L. Y., Chen, C. S., Li, C. C., and Hsiao, Y. H. (2021). Cellular senescence as a driver of cognitive decline triggered by chronic unpredictable stress. Neurobiol. Stress 15, 100341. doi:10.1016/j.ynstr.2021.100341
Liss, M. A., Schlicht, M., Kahler, A., Fitzgerald, R., Thomassi, T., Degueme, A., et al. (2010). Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics 7 (5), 245–252.
Lou, S., Gong, D., Yang, M., Qiu, Q., Luo, J., and Chen, T. (2024). Curcumin improves neurogenesis in alzheimer's disease mice via the upregulation of wnt/β-catenin and BDNF. Int. J. Mol. Sci. 25 (10), 5123. doi:10.3390/ijms25105123
Lu, C., Gao, R., Zhang, Y., Jiang, N., Chen, Y., Sun, J., et al. (2021). S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 12 (13), 5770–5778. doi:10.1039/d1fo00547b
Ma, T. C., Campana, A., Lange, P. S., Lee, H. H., Banerjee, K., Bryson, J. B., et al. (2010). A large-scale chemical screen for regulators of the arginase 1 promoter identifies the soy isoflavone daidzeinas a clinically approved small molecule that can promote neuronal protection or regeneration via a cAMP-independent pathway. J. Neurosci. 30 (2), 739–748. doi:10.1523/JNEUROSCI.5266-09.2010
Mahmoud, A. M., Yang, W., and Bosland, M. C. (2014). Soy isoflavones and prostate cancer: a review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 140, 116–132. doi:10.1016/j.jsbmb.2013.12.010
Mannino, F., Imbesi, C., Irrera, N., Pallio, G., Squadrito, F., and Bitto, A. (2024). Insights into the antiosteoporotic mechanism of the soy-derived isoflavone genistein: modulation of the Wnt/beta-catenin signaling. Biofactors 50 (2), 347–359. doi:10.1002/biof.2008
Marques, L., Johnson, A. A., and Stolzing, A. (2020). Doxorubicin generates senescent microglia that exhibit altered proteomes, higher levels of cytokine secretion, and a decreased ability to internalize amyloid β. Exp. Cell Res. 395 (2), 112203. doi:10.1016/j.yexcr.2020.112203
Mercer, K. E., Pulliam, C. F., Pedersen, K. B., Hennings, L., and Ronis, M. J. (2017). Soy protein isolate inhibits hepatic tumor promotion in mice fed a high-fat liquid diet. Exp. Biol. Med. (Maywood) 242 (6), 635–644. doi:10.1177/1535370216685436
Mijit, M., Caracciolo, V., Melillo, A., Amicarelli, F., and Giordano, A. (2020). Role of p53 in the regulation of cellular senescence. Biomolecules 10 (3), 420. doi:10.3390/biom10030420
Mishra, J., Vishwakarma, J., Malik, R., Gupta, K., Pandey, R., Maurya, S. K., et al. (2021). Hypothyroidism induces interleukin-1-dependent autophagy mechanism as a key mediator of hippocampal neuronal apoptosis and cognitive decline in postnatal rats. Mol. Neurobiol. 58 (3), 1196–1211. doi:10.1007/s12035-020-02178-9
Miyamoto, M., Kiyota, Y., Yamazaki, N., Nagaoka, A., Matsuo, T., Nagawa, Y., et al. (1986). Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 38 (3), 399–406. doi:10.1016/0031-9384(86)90112-5
Nachtigall, E. G., de Freitas, J. D. R., de, C. M. J., and Furini, C. R. G. (2023). Role of hippocampal Wnt signaling pathways on contextual fear memory reconsolidation. Neuroscience 524, 108–119. doi:10.1016/j.neuroscience.2023.05.028
Oliva, C. A., Vargas, J. Y., and Inestrosa, N. C. (2013). Wnt signaling: role in LTP, neural networks and memory. Ageing Res. Rev. 12 (3), 786–800. doi:10.1016/j.arr.2013.03.006
Palomer, E., Buechler, J., and Salinas, P. C. (2019). Wnt signaling deregulation in the aging and alzheimer's brain. Front. Cell Neurosci. 13, 227. doi:10.3389/fncel.2019.00227
Pandey, R., Garg, A., Gupta, K., Shukla, P., Mandrah, K., Roy, S., et al. (2022). Arsenic induces differential neurotoxicity in male, female, and E2-deficient females: comparative effects on hippocampal neurons and cognition in adult rats. Mol. Neurobiol. 59 (5), 2729–2744. doi:10.1007/s12035-022-02770-1
Pandey, R., Rai, V., Mishra, J., Mandrah, K., Kumar Roy, S., and Bandyopadhyay, S. (2017). From the cover: arsenic induces hippocampal neuronal apoptosis and cognitive impairments via an up-regulated BMP2/smad-dependent reduced BDNF/TrkB signaling in rats. Toxicol. Sci. 159 (1), 137–158. doi:10.1093/toxsci/kfx124
Pandey, R., Shukla, P., Anjum, B., Gupta, H. P., Pal, S., Arjaria, N., et al. (2020). Estrogen deficiency induces memory loss via altered hippocampal HB-EGF and autophagy. J. Endocrinol. 244 (1), 53–70. doi:10.1530/JOE-19-0197
Payton-Stewart, F., Schoene, N. W., Kim, Y. S., Burow, M. E., Cleveland, T. E., Boue, S. M., et al. (2009). Molecular effects of soy phytoalexin glyceollins in human prostate cancer cells LNCaP. Mol. Carcinog. 48 (9), 862–871. doi:10.1002/mc.20532
Piechota, M., Sunderland, P., Wysocka, A., Nalberczak, M., Sliwinska, M. A., Radwanska, K., et al. (2016). Is senescence-associated beta-galactosidase a marker of neuronal senescence? Oncotarget 7 (49), 81099–81109. doi:10.18632/oncotarget.12752
Prud'homme, G. J., Kurt, M., and Wang, Q. (2022). Pathobiology of the klotho antiaging protein and therapeutic considerations. Front. Aging 3, 931331. doi:10.3389/fragi.2022.931331
Saito, Y., Miyajima, M., Yamamoto, S., Sato, T., Miura, N., Fujimiya, M., et al. (2021). Accumulation of senescent neural cells in murine lupus with depression-like behavior. Front. Immunol. 12, 692321. doi:10.3389/fimmu.2021.692321
Sarkar, G., Curran, G. L., Mahlum, E., Decklever, T., Wengenack, T. M., Blahnik, A., et al. (2011). A carrier for non-covalent delivery of functional beta-galactosidase and antibodies against amyloid plaques and IgM to the brain. PLoS One 6 (12), e28881. doi:10.1371/journal.pone.0028881
Schuler, M. A., Schmitt, E. S., and Beachy, R. N. (1982). Closely related families of genes code for the alpha and alpha' subunits of the soybean 7S storage protein complex. Nucleic Acids Res. 10 (24), 8225–8244. doi:10.1093/nar/10.24.8225
Sharan, K., Siddiqui, J. A., Swarnkar, G., Maurya, R., and Chattopadhyay, N. (2009). Role of phytochemicals in the prevention of menopausal bone loss: evidence from in vitro and in vivo, human interventional and pharma-cokinetic studies. Curr. Med. Chem. 16 (9), 1138–1157. doi:10.2174/092986709787581806
Sharma, A., Yang, W. L., Ochani, M., and Wang, P. (2017). Mitigation of sepsis-induced inflammatory responses and organ injury through targeting Wnt/β-catenin signaling. Sci. Rep. 7 (1), 9235. doi:10.1038/s41598-017-08711-6
Shi, B., Chen, M., Xia, Z., Tang, W., Li, Y., Qin, C., et al. (2023). Genistein attenuates neuroinflammation and oxidative stress and improves cognitive impairment in a rat model of sepsis-associated encephalopathy: potential role of the Nrf2 signaling pathway. Metab. Brain Dis. 38 (1), 339–347. doi:10.1007/s11011-022-01076-4
Sierra, A., Martin-Suarez, S., Valcarcel-Martin, R., Pascual-Brazo, J., Aelvoet, S. A., Abiega, O., et al. (2015). Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell 16 (5), 488–503. doi:10.1016/j.stem.2015.04.003
Sinha, A., Tamboli, R. S., Seth, B., Kanhed, A. M., Tiwari, S. K., Agarwal, S., et al. (2015). Neuroprotective role of novel triazine derivatives by activating wnt/β catenin signaling pathway in rodent models of alzheimer's disease. Mol. Neurobiol. 52 (1), 638–652. doi:10.1007/s12035-014-8899-y
Su, D., Li, W., She, X., Chen, X., Zhai, Q., Cui, B., et al. (2018). Chronic noise exposure exacerbates AD-like neuropathology in SAMP8 mice in relation to Wnt signaling in the PFC and hippocampus. Sci. Rep. 8 (1), 14622. doi:10.1038/s41598-018-32948-4
Sun, T., Zhang, L., Feng, J., Bao, L., Wang, J., Song, Z., et al. (2022). Characterization of cellular senescence in doxorubicin-induced aging mice. Exp. Gerontol. 163, 111800. doi:10.1016/j.exger.2022.111800
Swarbrick, S., Wragg, N., Ghosh, S., and Stolzing, A. (2019). Systematic review of miRNA as biomarkers in alzheimer's disease. Mol. Neurobiol. 56 (9), 6156–6167. doi:10.1007/s12035-019-1500-y
Tajima, K., Matsuda, S., Yae, T., Drapkin, B. J., Morris, R., Boukhali, M., et al. (2019). SETD1A protects from senescence through regulation of the mitotic gene expression program. Nat. Commun. 10 (1), 2854. doi:10.1038/s41467-019-10786-w
Tamura, H., Miyazaki, A., Kawamura, T., Gotoh, H., Yamamoto, N., and Narita, M. (2024). Chronic ingestion of soy peptide supplementation reduces aggressive behavior and abnormal fear memory caused by juvenile social isolation. Sci. Rep. 14 (1), 11557. doi:10.1038/s41598-024-62534-w
Tanaka, M., Kiyohara, H., Yoshino, A., Nakano, A., Takata, F., Dohgu, S., et al. (2020). Brain-transportable soy dipeptide, Tyr-Pro, attenuates amyloid β peptide25-35-induced memory impairment in mice. NPJ Sci. Food 4, 7. doi:10.1038/s41538-020-0067-3
Tao, W., Ruan, J., Wu, R., Zhao, M., Zhao, T., Qi, M., et al. (2023). A natural carotenoid crocin exerts antidepressant action by promoting adult hippocampal neurogenesis through Wnt/β-catenin signaling. J. Adv. Res. 43, 219–231. doi:10.1016/j.jare.2022.02.015
Tsuruki, T., Kishi, K., Takahashi, M., Tanaka, M., Matsukawa, T., and Yoshikawa, M. (2003). Soymetide, an immunostimulating peptide derived from soybean beta-conglycinin, is an fMLP agonist. FEBS Lett. 540 (1-3), 206–210. doi:10.1016/s0014-5793(03)00265-5
Tufekci, K. U., Alural, B., Tarakcioglu, E., San, T., and Genc, S. (2021). Lithium inhibits oxidative stress-induced neuronal senescence through miR-34a. Mol. Biol. Rep. 48 (5), 4171–4180. doi:10.1007/s11033-021-06430-w
Unno, K., and Konishi, T. (2015). Preventive effect of soybean on brain aging and amyloid-β accumulation: comprehensive analysis of brain gene expression. Recent Pat. Food Nutr. Agric. 7 (2), 83–91. doi:10.2174/2212798407666150629140132
Wang, C., Kang, Y., Liu, P., Liu, W., Chen, W., Hayashi, T., et al. (2023). Combined use of dasatinib and quercetin alleviates overtraining-induced deficits in learning and memory through eliminating senescent cells and reducing apoptotic cells in rat hippocampus. Behav. Brain Res. 440, 114260. doi:10.1016/j.bbr.2022.114260
Wang, Y., Kuca, K., You, L., Nepovimova, E., Heger, Z., Valko, M., et al. (2024). The role of cellular senescence in neurodegenerative diseases. Arch. Toxicol. 98, 2393–2408. doi:10.1007/s00204-024-03768-5
Wei, L., Chen, C., Ding, L., Mo, M., Zou, J., Lu, Z., et al. (2019). Wnt1 promotes EAAT2 expression and mediates the protective effects of astrocytes on dopaminergic cells in Parkinson's disease. Neural Plast. 2019, 1247276. doi:10.1155/2019/1247276
Wei, L. C., Ding, Y. X., Liu, Y. H., Duan, L., Bai, Y., Shi, M., et al. (2012). Low-dose radiation stimulates Wnt/β-catenin signaling, neural stem cell proliferation and neurogenesis of the mouse hippocampus in vitro and in vivo. Curr. Alzheimer Res. 9 (3), 278–289. doi:10.2174/156720512800107627
Wissler Gerdes, E. O., Zhu, Y., Weigand, B. M., Tripathi, U., Burns, T. C., Tchkonia, T., et al. (2020). Cellular senescence in aging and age-related diseases: implications for neurodegenerative diseases. Int. Rev. Neurobiol. 155, 203–234. doi:10.1016/bs.irn.2020.03.019
Xiang, Y., Wang, S. H., Wang, L., Wang, Z. L., Yao, H., Chen, L. B., et al. (2019). Effects of ginsenoside Rg1 regulating wnt/β-catenin signaling on neural stem cells to delay brain senescence. Stem Cells Int. 2019, 5010184. doi:10.1155/2019/5010184
Xu, N., Zhou, W. J., Wang, Y., Huang, S. H., Li, X., and Chen, Z. Y. (2015). Hippocampal Wnt3a is necessary and sufficient for contextual fear memory acquisition and consolidation. Cereb. Cortex 25 (11), 4062–4075. doi:10.1093/cercor/bhu121
Yoo, D. Y., Jung, S., Kang, J. S., Baek, J. H., Park, K. H., Lee, D. H., et al. (2022). Isoflavone-enriched soybean leaves (Glycine max) alleviate cognitive impairment induced by ovariectomy and modulate PI3K/akt signaling in the Hippocampus of C57BL6 mice. Nutrients 14 (22), 4753. doi:10.3390/nu14224753
Zhang, J., Lazarenko, O. P., Blackburn, M. L., Badger, T. M., Ronis, M. J., and Chen, J. R. (2014). Soy protein isolate down-regulates caveolin-1 expression to suppress osteoblastic cell senescence pathways. FASEB J. 28 (7), 3134–3145. doi:10.1096/fj.13-243659
Zhang, L., Cheng, F., Wei, Y., Zhang, L., Guo, D., Wang, B., et al. (2019). Inhibition of TAZ contributes radiation-induced senescence and growth arrest in glioma cells. Oncogene 38 (15), 2788–2799. doi:10.1038/s41388-018-0626-0
Zhang, S., Kong, D. W., Ma, G. D., Liu, C. D., Yang, Y. J., Liu, S., et al. (2022a). Long-term administration of salvianolic acid A promotes endogenous neurogenesis in ischemic stroke rats through activating Wnt3a/GSK3β/β-catenin signaling pathway. Acta Pharmacol. Sin. 43 (9), 2212–2225. doi:10.1038/s41401-021-00844-9
Zhang, X., Pearsall, V. M., Carver, C. M., Atkinson, E. J., Clarkson, B. D. S., Grund, E. M., et al. (2022b). Rejuvenation of the aged brain immune cell landscape in mice through p16-positive senescent cell clearance. Nat. Commun. 13 (1), 5671. doi:10.1038/s41467-022-33226-8
Zheng, Y., Yasuda, M., Yamao, M., Gokan, T., Sejima, Y., Nishikawa, T., et al. (2023). Fermented soybean foods (natto) ameliorate age-related cognitive decline by hippocampal TAAR1-mediated activation of the CaMKII/CREB/BDNF signaling pathway in senescence-accelerated mouse prone 8 (SAMP8). Food Funct. 14 (22), 10097–10106. doi:10.1039/d3fo03987k
Keywords: Soymetide, senescence, doxorubicin, Wnt/β-catenin pathway, hippocampus, neuroprotection
Citation: Garg A, Saroj J, Tiwari S, Das U, Shukla N, Ghosh JK and Bandyopadhyay S (2025) Exploring the potential anti-senescence effects of soybean-derived peptide Soymetide in mice hippocampal neurons via the Wnt/β-catenin pathway. Front. Pharmacol. 16:1510337. doi: 10.3389/fphar.2025.1510337
Received: 12 October 2024; Accepted: 13 January 2025;
Published: 25 February 2025.
Edited by:
Jawed A. Siddiqui, New York University, United StatesReviewed by:
Jian Hao, The Second Affiliated Hospital of Guangzhou Medical University, ChinaNihar Ranjan Jana, Indian Institute of Technology Kharagpur, India
Anjan Thakurta, University of Oxford, United Kingdom
Copyright © 2025 Garg, Saroj, Tiwari, Das, Shukla, Ghosh and Bandyopadhyay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanghamitra Bandyopadhyay, c2FuZ2htaXRyYUBpaXRyLnJlcy5pbg==, YmFuZHlvMTJAZ21haWwuY29t; Jimut Kanti Ghosh, amtfZ2hvc2hAY2RyaS5yZXMuaW4=
†ORCID: Sanghamitra Bandyopadhyay, orcid.org/0000-0001-7957-6296; Jimut Kanti Ghosh, orcid.org/0000-0002-1490-3599
 Asmita Garg1,2
Asmita Garg1,2 Sanghamitra Bandyopadhyay
Sanghamitra Bandyopadhyay