
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 29 January 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1509032
This article is part of the Research Topic Emerging Trends in the Quality Check of Herbal Medicines, Supplements and 'Botanicals' View all 8 articles
Euodiae Fructus (EF) is the dried and nearly ripe fruit of Euodia rutaecarpa, first recorded in Shen Nong’s Herbal Classic. EF is a versatile Traditional Chinese Medicine (TCM) known for the effects of dispelling colds and alleviating pain, suppressing adverse qi to relieve vomiting, and boosting yang to mitigate diarrhea. However, it should be noted that EF possesses mild toxicity. In TCM prescriptions, EF is employed to treat various ailments, including abdominal pain, diarrhea, chronic non-atrophic gastritis, irritable bowel syndrome, and primary dysmenorrhea. This review collected the literature published before September 2024 on EF. An exhaustive analysis of EF literature was conducted utilizing multiple sources, namely classic TCM books and various scientific databases like Web of Science, PubMed, Elsevier, ACS, ResearchGate, Google Scholar, and Chinese National Knowledge Infrastructure. So far, more than 300 metabolites have been extracted and identified from EF, exhibiting various pharmacological effects, such as cardiovascular protection, gastrointestinal protection, neuroprotection, anti-inflammation, analgesia, anti-tumor, glucose and lipid metabolism regulation, etc. It also exhibits diverse toxicological properties and poses specific toxic risks to the liver, heart, and kidney. Nonetheless, research is scarce regarding the toxicology of EF, especially on its cardiotoxicity and nephrotoxicity. Further in-depth research is necessary to explore the mechanisms underlying EF’s pharmacological and toxicological mechanisms and to develop strategies for quality control and toxicity mitigation. The toxicity of EF can be reduced by processing, but this aspect is rarely discussed, and the quality control needs to be further standardized. Evodiamine, rutaecarpine, and limonin are the effective metabolites of EF and are also one of the causes of EF toxicity. The pharmacological effects of evodiamine and rutaecarpine have been intensely studied, but there are few studies on limonin and other metabolites of EF. Therefore, this paper focuses on the botanical characteristics, traditional applications, processing methods, phytochemistry, quality control, pharmacology, and toxicology of EF. We hope this paper provides a theoretical basis for the future high-value and high-connotation development of EF.
EF is a dry, nearly ripe fruit of the genus Euodia rutaecarpa, first recorded in the top grade of Shen Nong’s Herbal Classic (Dong Han Dynasty, A.D. 25–220). It relieves cold and pain, suppresses adverse qi to relieve vomiting, and enhances yang to stop diarrhea. Nonetheless, it is essential to acknowledge that EF has a slight toxic effect. EF ranks among the most prevalent botanical drugs clinically in TCM, boasts a history of more than 2000 years, and has been formally listed in various editions of Chinese Pharmacopoeia (ChP) (https://www.nmpa.gov.cn/). Lately, numerous studies have concentrated on examining the metabolites, pharmacological effects, clinical function, and toxicology of EF. Up to this point, more than 300 metabolites have been extracted and pinpointed from EF (Xiao et al., 2023). Contemporary research indicates that EF’s primary active elements comprise alkaloids, terpenoids, flavonoids, volatile oils, and other compounds (Liu L. et al., 2020). Among them, evodiamine, rutaecarpine, and limonin are characteristic metabolites (Tian et al., 2019). Research in pharmacology reveals that EF, along with its raw extract and refined form, offers a range of pharmacological effects, such as cardiovascular protection, gastrointestinal protection, neuroprotection, anti-inflammation, analgesia, anti-tumor, and glucose and lipid metabolism regulation. In clinical settings, this medication serves as both a supplement and a substitute treatment for conditions like abdominal pain, vomiting, diarrhea, indigestion, hypertension, eczema, and oral ulcers (Huang et al., 2021). EF, combined with various botanical drugs, is effective in gastrointestinal diseases, headaches, vomiting, skin diseases, dysentery, menorrhagia, and postpartum hemorrhage (Li D. et al., 2022).
However, it is important to recognize that excessive use of EF may lead to stomach pain, vomiting, blurred vision, and other toxic symptoms (Cai et al., 2006; Ma et al., 2018). As EF’s clinical application has expanded, its toxicity has become increasingly apparent. It is widely accepted among scholars that the toxicity of EF may be attributed to reactive metabolites (RMs) produced by the metabolic activation of evodiamine, rutaecarpine, and limonin (He et al., 2024). Studies have demonstrated that different parts of EF can induce varying degrees of hepatic injury in rats (Liu et al., 2015), and EF has obvious toxic damage to the human liver (Teschke et al., 2014). Some studies have also highlighted the heart and kidney as possible focal points for EF toxicity. Yet, investigations into EF cardiotoxicity and nephrotoxicity processes are scarce and insufficient for the specific toxic risks and potential disadvantages of EF. Exploring the toxic metabolites of EF and methods for mitigating its toxicity is crucial to guiding safe clinical use. This paper reviews the plant morphology, traditional application, processing, phytochemistry, quality control, pharmacology, toxicology, monitoring, and prevention of EF. Particular emphasis is placed on discussing the mechanisms of EF-induced cardiotoxicity and nephrotoxicity, strategies for reducing and controlling EF’s toxicity, and preventive measures for clinical monitoring.
The ChP recorded the dried and nearly ripe fruit of three plants of the genus Euodia rutaecarpa (Juss.) Benth. (ER), Euodia rutaecarpa (Juss.) Benth. var. officinalis (Dode) Huang (ERO), Euodia rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang (ERB). Euodia rutaecarpa is also divided into large grains and small grains. ER primarily supplies large grains, categorized into large EF flowers (LEF) and medium EF flowers (MEF). LEF has reached full ripeness, the fruit shows cracks, and its effectiveness is subpar. Approximately seven mature MEFs, characterized by a yellowish-green and potent odor, are commonly utilized in medical treatments. ERO and ERB primarily supply diminutive grains, predominantly consisting of small EF flowers (SEF), often immature and green. The diminutive size of ERO and ERB fruits typically classifies them as ER varieties, distinct from ER due to their unique, strong smells. The botanical characteristics of ER, ERO, and ERB are similar to those of dried fruits, as shown in Figure 1.
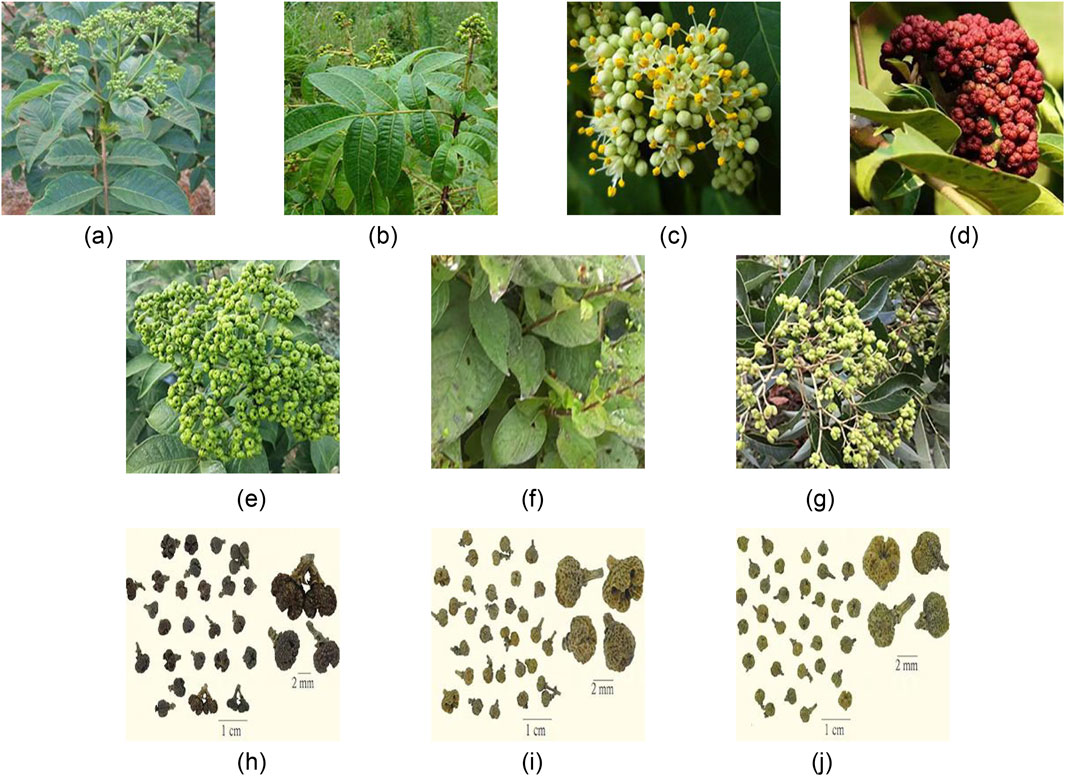
Figure 1. The above-ground portion (A), leaves (B), flowers (C), fruits (D), ER (E), ERO (F), ERB (G), LEF (H), MEF (I) and SEF (J) of Euodia rutaecarpa.
The Euodia rutaecarpa are shrubs or trees, standing 3–5 m high, and are thickly adorned with grayish yellow, rust-red downy hair or have few hairs and dark purplish-red shoots. The leaves have 5–11 leaflets, ovate, elliptic, or lanceolate, 6–18 cm long and 3–7 cm wide. Inflorescences are terminal and dioecious. Male inflorescence flowers are separated from each other, with petals measuring 3–4 mm long. Female inflorescences are dense or distant, and petals measure 4–5 mm long. Most of the sepals and petals are 5 pieces, occasionally 4 pieces, arranged in a pincer pattern. The fruit is spherical or slightly pentagonal oblate, and the surface is dark greenish, yellow, or brown. The outer pericarp has oil spots. The inner pericarp is a thin shell or woody, waxy yellow or brown, and the ovary can be seen as 5-located with 1 seed per mericarp. At its peak is a star-shaped fissure with five points, while its base features a calyx and a fruit stalk and is adorned with yellow hairs. The quality is hard and crisp, with a full green color and rich aroma is better. However, the botanical characteristics of ER, ERO, and ERB are different in growth form, maturity period, ecological environment, and resource distribution, as shown in Table 1.
Euodia rutaecarpa cultivation began at the end of the Eastern Han Dynasty. Miscellaneous Records of Famous Physicians (Wei and Jin Dynasties, A.D. 220–450) first recorded that “Euodia rutaecarpa is grown in the valley, picked on September 9, kept cool and dry, and kept as long as possible”. EF had become a widely used medicine during the Tang Dynasty, with renowned poet Wang Wei noting that Euodia rutaecarpa could be seen throughout mountains during the Double Ninth Festival. Contemporary research indicates that Euodia rutaecarpa thrives in sunlit, warm environments, typically flourishing in thinly spread forests or shrubs in mountainous areas ranging from flat to 1,500 m above sea level, predominantly on sunlit inclines. It is relatively cold resistant, but in cold, windy, and dry areas in winter, and in areas with many diseases, the results are low, and the growth is poor. Illustrated Classic of Materia Medica (Song Dynasty, A.D. 960–1,279) recorded that “Euodia rutaecarpa can be found everywhere, especially in Jiangsu, Zhejiang, and Sichuan. Jiangxi is EF’s the authentic origin, rich in high-quality MEF. It is distributed in the north and south of Jiangxi Province, mainly in urban Zhangshu, Fengcheng, Gaoan, Xingan, Xiajiang, Xinyu, and Jishui. As clinical needs swiftly rose, numerous provinces introduced Euodia rutaecarpa. Now, it is distributed in the south of the Qinling Mountains in China, mainly in Guizhou, Guangxi, Hunan, and Yunnan Provinces (Figure 2). Nowadays, Euodia rutaecarpa is predominantly thrived in Asia, East Africa, and Oceania, with extensive cultivation in ancient Japan and Korea. Euodia rutaecarpa was introduced in Korea during The Three Kingdoms Period, and the Goryeo Master Fang (Wei and Jin Dynasties, A.D. 220–420) recorded the treatment of beriberi with EF (Nam et al., 2016). Euodia rutaecarpa was introduced in Japan during the Edogawa period. EF and its namesake, Goshuyuto (known as Wuzhuyu decoction in Chinese), are frequently utilized in clinical settings (Hibino et al., 2009a).
EF was first recorded in Shen Nong’s Herbal Classic, which records the nature, taste, meridian tropism, and efficacy of EF, laying the foundation for the modern clinical application of EF. Over time, the therapeutic impact of EF has evolved through extensive research. It is pointed out that EF can treat many diseases, such as Jueyin headache, hernia, abdominal pain, beriberi, vomiting blood, acid regurgitation, and diarrhea (Table 2). Concurrently, Miscellaneous Records of Famous Physicians initially documented EF’s mild toxicity, with ongoing research enhancing EF’s toxicology. Illustrated Classic of Materia Medica recorded that EF harms the eyes and hair. Amplified Herbology (Song Dynasty, A.D. 960–1,279) recorded that EF damages the intestines and stomach. Correlation between Materia Medica Companion (Ming Dynasty, A.D. 1,368–1,644) recorded that EF damages the healthy atmosphere. Mouth ulcers, tongue sores, and dizziness caused by excessive consumption of EF are recorded in Compendium of Materia Medica (Ming Dynasty, A.D. 1,368–1,644). The ChP recorded the minor toxicity of EF and stipulated that the dosage of EF was 2–5 g.
EF has been a staple in clinical prescriptions since antiquity (Table 3). Wuzhuyu decoction, named after the monarch medicine EF in Treatise on Febrile Diseases (Dong Han Dynasty, A.D. 25–220), has the effect of warming the middle to replenish deficiency, lowering qi and stopping vomiting. It was the earliest record of the use of EF in clinical treatment. Synopsis of the Golden Chamber (Dong Han Dynasty, A.D. 25–220) mentioned twice the EF of warming channels, dispelling cold, and stopping vomiting, which is an important medicine for tonifying the spleen and stomach. Among the over 5,000 prescriptions in Thousand-Gold Essential Formula for Emergency (Tang Dynasty, A.D. 618–907), there were 143 prescriptions mentioned EF. The Essential Secrets from the Imperial Library (Tang Dynasty, A.D. 618–907) had over 6,000 prescriptions, and the number of prescriptions contained EF reached 176. Formula of Peaceful Beneuolence Pharmacy (Song Dynasty, A.D. 1,078–1,085) was the first official preparation standard. This book recorded a total of 788 prescriptions, and 13 referred to EF. In the clinical realm, essential formulas featuring EF encompass the Wuzhuyu Decoction, Zuojin Pill, Wenjing Decoction, and Sishen Pill, among others.
In addition to examining EF in traditional medical texts, the study of EF in botanical drugs has been extensively explored in contemporary medical settings. Wuzhuyu Decoction can treat chronic non-atrophic gastritis (Hu et al., 2023), chronic migraine (Pan et al., 2015; Nan et al., 2022), alcoholic gastric ulcer (Wang X. et al., 2023), and atherosclerosis (Li C. et al., 2022). Zuojin Pill has pharmacological effects such as anti-tumor (Peng et al., 2015), protection of gastric mucosa, anti-inflammation, anti-ulcer, and so on (Wang et al., 2015; Zhang J. et al., 2022). It can treat bile reflux gastritis (Li Y. Y. et al., 2022) and septic lung injury (Yin et al., 2021). Wenjing Decoction can treat primary dysmenorrhea (Gao et al., 2017) and endometriosis (Huang et al., 2023). Sishen Pill has anti-inflammatory and anti-tumor pharmacological effects (Zhang B. et al., 2024), can treat abdominal pain, diarrhea (Li et al., 2024), irritable bowel syndrome (Zhang X. Y. et al., 2021; Zhao et al., 2024), and insomnia (Wang L. X. et al., 2023). EF is recommended as the primary treatment in Japan for ailments like cold headaches, dysmenorrhea, and inflammatory pain in rheumatoid joints (https://www.mhlw.go.jp/index.html). Certain EF-containing prescriptions, including Changkang Tablets, Huatuo Zaizao Wan, Compound Berberine Tablets, and Jiawei Zuo Jin Wan, have undergone extensive research and clinical application. Changkang Tablets are used in the treatment of dysentery, abdominal pain, and tenesmus (Shi et al., 2013; Guan et al., 2020), Huatuo Zaizao Wan can treat Alzheimer’s disease (Jiang et al., 2023) and stroke (Duan et al., 2017).
Presently, about 300 metabolites of EF have been isolated and purified, which are mainly divided into alkaloids (1–148), terpenoids (149–184), flavonoids (185–213), volatile oils (214–283), and others (284–299). These metabolites are summarized in Supplementary Table S1, and their structures are shown in Supplementary Figures S1–S8. The alkaloids are primarily categorized into indoles and quinolones (Li D. W. et al., 2020). Evodiamine and rutaecarpine in indole are the index metabolites of EF. The limonin in terpenoids plays a crucial role in EF.
Alkaloids are the metabolites of EF, mainly composed of indoles (Supplementary Figure S1) and quinolones (Supplementary Figure S2). Alkaloids fundamentally possess a circular form and are non-soluble in water, resulting in a higher concentration of EF alkaloids in ethanol. Indoles are synthesized mainly through methanesulfonic acid and amino acids. Over ten different compounds, including evodiamine, rutaecarpine, and dehydroevodiamine, were extracted from EF (Zuo et al., 2000; Wang Q. Z. et al., 2010; Wang T. Y. et al., 2010; Wang X. X. et al., 2013; Li et al., 2014; Zhao N. et al., 2015; Ma et al., 2021; Qin et al., 2021; Zhao X. M. et al., 2021). Evodiamine and rutaecarpine are the most important metabolites, and their contents are also the highest in EF (Huang et al., 2019; Li D. W. et al., 2020). Dihydroevocarpine and evocarpine are the crucial metabolites of quinolones found in EF (Li Y. H. et al., 2020). Furthermore, the group includes quinolines, organic amines, acridone, and purines (Minh et al., 2003; Kim et al., 2022) (Supplementary Figure S3). Research revealed a reduction in the levels of evodiamine, rutaecarpine, and carpine in EF correlating with the fruit’s diminution. Some commercial evodiamine and rutaecarpine in SEF are below the content specified in ChP (Cao et al., 2019).
Typically, terpenoids (Supplementary Figure S4) originate from methylpentanedioic acid, with isoprene forming the fundamental structural component of the molecular framework (Zhao H. et al., 2021; Qian et al., 2014). Limonin is an oxidized tetracyclic triterpene with a distinctive furan ring, and it is a terpenoid extracted and recognized from EF. They constitute the material basis of the bitter properties of EF (Bae et al., 2020). Its representative metabolites are limonin and rutaevine, and limonin is another index metabolite of EF. In addition, there are high contents of metabolites, such as evodol, obacunone, rutaevine acetate, 6β-acetoxy-5-epillimonin, jangomolide, and shihulimonin A (Lacroix et al., 2011). Due to their significant solubility in fats, terpenoids are typically processed using an ethanol solvent. Research indicates that limonin levels in SEF and MEF are notably elevated, approximately 0.74% and 0.65%, respectively, in contrast to LEF’s mere 0.24% limonin content (Zhang et al., 2021c).
Flavonoids (Supplementary Figure S5) generally refer to a series of metabolites formed by connecting two benzene rings with three carbon atoms. EF additionally has a higher content of flavonoids. It mainly includes these three metabolites: flavonoids and their glycosides, flavonols and their glycosides, and flavonones and their glycosides. Flavonoids and their glycosides are predominantly associated with O-glucose, O-xylose, O-galactose, O-rhamnose, O-rue sugar, and O-mulberry disaccharide. Flavonols and their glycosides mainly include quercetin, isorhamnetin, limocitrin glycosides, and aglycones (Xiao et al., 2023; He et al., 2024). Flavonones and their glycosides are phellodensin F, catechin, and hesperidin, respectively (Zhao Z. et al., 2015; Li and Wang, 2020).
EF has a strong and fragrant smell because of its high content of volatile oil (Supplementary Figure S6). The volatile oil metabolites are monoterpene, sesquiterpene, aliphatic, and aromatic. Within the isolated volatile oil, the proportions of sesquiterpenes exceed 38%, monoterpenes surpass 35%, and esters exceed 13% (Liu S. S. et al., 2019). Furthermore, while EF volatile oil exhibits significant pharmacological properties, it simultaneously constitutes a toxic metabolite of EF. Its metabolites are monoterpenoids, such as myrcene and (E)-ocimene, and sesquiterpenes, such as β-caryophyllene and β-elemene.
EF also contains some organic acids (Supplementary Figure S7), including caffeic acid, citric acid, isocitric acid, trans-caffeoylgluconic acid, and feruloylgluconic acid. EF also contains phenylpropanoids, mainly divided into simple phenylpropanoids, coumarins, and lignans, most of which belong to simple phenylpropanoids. Simple phenylpropanoids include p-hydroxycinnamic acid, ferulic acid, coniferin, chlorogenic acid, etc. Besides the metabolites mentioned above, EF also contains anthraquinones, such as chrysophanol, emodin, and physcion, and steroids, such as β-sitosterol, β-daucosterol, and β-stigmasterol (Supplementary Figure S8).
Processing is the essence of TCM application, which can increase the efficacy and reduce the toxicity of drugs. EF’s processing boasts an extensive historical background, with raw EF typically exerting a significant influence in heating the spleen and eliminating cold. The long traditional technology of processing EF with licorice is pointed out in the Synopsis of the Golden Chamber. Master Lei’s Treatise on Drug Processing (Northern and Southern Dynasties, A.D. 420–479) recorded that EF is processed with salt to enhance the analgesic effect and vinegar to correct the taste. Materia Medica for Dietotherapy (Tang Dynasties, A.D. 618–907) recorded that EF could enhance the antiemetic effect after processing ginger and the analgesic effect after being processed with yellow rice wine. General Records of Holy Universal Relief (Song Dynasties, A.D. 1,078–1,085) recorded that EF can reduce toxicity when processed with soybean products. Prescriptions for Universal Relief (Ming Dynasty, A.D. 1,368–1,644) recorded that EF fried with Psoraleae Fructus can enhance the antidiarrheal effect. Wonderful Well-Tried Recipes (Ming Dynasty, A.D. 1,368–1,644) recorded that EF processed with Coptidis Rhizoma can enhance the antiemetic effect. The process and method of processing EF with licorice are described in detail in ChP (Figure 3). In addition, different doses of licorice can affect EF’s chemical composition and pharmacological effects (Xiao et al., 2012). Certain academics have experimented with varying the EF to licorice dosage ratios, discovering the optimal 100:6.
In its extended clinical application, TCM has developed distinct theories and techniques for detoxifying and improving its healing impact, encompassing both processing and compatibility (Li R. L. et al., 2021). Special processing techniques such as licorice, salt, ginger, and vinegar can reduce the toxicity of EF. The ChP stipulates that EF ought to be fried alongside licorice. Some scholars have found that processing EF with licorice can reduce the toxicity of alkaloids (Ren et al., 2023a). The cytochrome P450 (P450, CYP) enzyme activates evodiamine in EF during metabolism, which leads to liver injury and inflammation. Licorice can reduce the toxicity of EF by inhibiting the P450 enzyme and blocking the metabolic activation of EF (Ren et al., 2024). Licorice can obstruct EF protein coupling by suppressing the P450 enzyme, elevating GSH levels in human liver cells, and mitigating the GSH reduction induced by EF (Ren et al., 2023b). Salt-processed EF can introduce drugs into the kidney channel, reduce toxicity, and ensure the safety of clinical drug use (Hou et al., 2023). When EF is combined with ginger, its antiemetic properties can be amplified. Some studies found that EF processed with ginger, licorice, and salt had better antitoxic effects (Zhang M. et al., 2021). Other studies found that the three processing methods of ginger, licorice, and vinegar could reduce the content of rutaecarpine in EF, with vinegar processing increasing the content of evodiamine, and licorice processing had the most significant effect on reducing toxicity (Li H. et al., 2021). The metabolites of EF obtained by different processing methods, such as stir-frying, roasting, and steaming, are also different (Xiao et al., 2023). A comparative study of various EF-processed products revealed that the combined amounts of evodiamine, rutaecarpine, and evodol in EF for stir-frying exceed those in baking and cooking.
EF combined with other drugs can also counteract its toxicity. For example, the toxicity of EF significantly decreased when EF was used in combination with licorice and jujube (He et al., 2024). EF with Coptidis Rhizoma can enhance the anti-inflammatory effect and inhibit the inflammatory reaction in RAW264.7 cells by significantly reducing the levels of IL-6, TNF-α, and IL-1β (Wang J. et al., 2024). Additionally, it is capable of markedly reducing apoptosis and enhancing the defensive role of the gastric mucosa through the suppression of gastric acid release (Zhang Z. et al., 2024). Berberine is an important metabolite of Coptidis Rhizoma, which can counteract the side effects of evodiamine and reduce the risk of evodiamine in treating gastric cancer (Shi et al., 2013). The combination of berberine and evodiamine can synergistically inhibit the proliferation of human breast cancer cells by inducing cell cycle arrest and apoptosis (Du et al., 2017). The researchers found that EF combined with ginger, Citri Reticulatae Pericarpium, Paeoniae Radix Alba, and Angelicae Sinensis Radix can improve the efficacy of EF (Wu et al., 2021).
With the continuous updating of the edition of ChP, the identification, content evaluation, testing technology, and quality standards of EF are constantly improving (Table 4). EF was first recorded in the 1963 edition of ChP, and it was clearly recorded that EF was processed with licorice. Then, the identification method of EF appeared for the first time in the 1973 edition of ChP. In the 1985 edition of ChP, the EF identification method was officially determined as hydrochloric acid filtration, potassium mercuric iodide de-precipitation, and the formation of a reddish-brown ring zone at the interface between dimethylaminobenzaldehyde and EF solution. The ChP has been improving the quality control of EF since 2000. For the first time, the determination method and the lowest value of EF appeared in the 2000 edition of ChP, which stipulates that the total amount of evodiamine and rutaecarpine should not be less than 0.2%. Next, the total amount of evodiamine and rutaecarpine should not be less than 0.15% in the 2005 edition of ChP. Limonin content is adjusted to no less than 1.0% in the 2010 edition of ChP. Limonin content is again adjusted to no less than 0.20% in the 2015 edition of ChP. The 2015 edition of ChP is basically consistent with the 2020 edition of ChP, indicating that the metabolites of EF are relatively stable and can better represent the efficacy of EF. However, the maximum reference dose of metabolites is not specified in ChP, and the toxicity of EF has not been reasonably controlled.
TCM’s effectiveness can differ significantly based on the type of plant, its source, and yield. Diverse environmental factors like soil, air quality, precipitation, and sunlight play a crucial role in shaping EF growth, with each EF metabolite varying in content across regions (Liu Y. et al., 2019). Some studies have shown that the metabolite of EF varies greatly in different locations, and there is little change in the metabolite of EF in different years in the same location (Huang et al., 2008; Zhou et al., 2010). By comparing the EF of different batches of LEF, MEF, and SEF, it was found that the limonin content in SEF was higher than that of MEF and LEF, while the alkaloid content was higher in MEF and LEF. The toxicity of EF is related to the content ratio of limonin and alkaloid. The higher the ratio, the lower the toxicity of EF. In other words, MEF is more toxic than other categories, and SEF is less toxic than other categories (Zhang et al., 2021b). Furthermore, certain academics analyzed the four vital metabolites of ER, ERO, and ERB, namely evodiamine, evodiamine, dehydroevodiamine, and narcissoside, discovering comparable levels of dehydroevodiamine. The content of evodiamine, rutaecarpine, and narcissoside was the highest in ERO and the lowest in ERB (Li C. H. et al., 2021).
The ChP stipulated the detection method of EF and pointed out that evodiamine, rutaecarpine, and limonin are the crucial metabolites of EF. However, these three metabolites do not represent the overall pharmacological effects of EF. Consequently, sophisticated detection techniques are essential for qualitative and quantitative analysis of metabolites in EF. The leading analytical technologies include TLC, HPLC, HPLC-MS, GC-MS, CE, and CCC (Xia et al., 2023). At present, there are many studies on the metabolites of EF. Scholars have isolated three metabolites from EF: rutaecarpine, evodiamine, and evodiamide (Zhou et al., 2006). Then, two new-lactone derivatives, evodinoids A and B, and a new volatile oil, are separated from EF (Xin et al., 2022). In addition, five metabolites of EF were found. They are limonin, 1-methyl-2-undecyl-4(1H) quinolone, evocarpine, 1-methy-2-[(6Z,9Z)]-6,9-pentadecadienyl-4-(1H)-quinolone, and dihydroevocarpine (Zhang et al., 2013). It has been proven that evodiamine is closely related to the hepatotoxicity of EF (Zhang et al., 2021b). Limonin serves as the primary liver-protective metabolite of EF, while evodiamine is the chief liver-damaging metabolite of EF. Subsequently, eleven critical metabolites of EF underwent analysis using non-specific metabonomics and in vitro functional techniques (Yong et al., 2024). Up to this point, a total of 17 metabolites have undergone screening from EF, including neochlorogenic acid, caffeic acid, chlorogenic acid, 3-O-feruloylquinic acid, hyperoside, quercetin-3-O-sambubioside, rutin, dehydroevodiamine, isorhamnetin-3-O-β-D-galactoside, narcissin, isorhamnetin-3-O-β-D-glucopyranoside, diosmin, rutaevine, limonin, evodiamine, rutaecarpine, and evocarpine.
EF is a classical plant medicine with various pharmacological effects, such as cardiovascular protection, gastrointestinal protection, neuroprotection, anti-inflammation, analgesia, anti-tumor, glucose and lipid metabolism regulation, etc. (Supplementary Table S2). Evodiamine, rutaecarpine, and limonin serve as the indicator metabolites of EF but are also abundant in phytochemistry and pharmacology, which are often used to treat diseases of the immune, nervous, digestive, circulatory, and endocrine systems. Elucidating the pharmacological effects of EF is the key to guiding the rational clinical application of drugs and ensuring the curative effect.
EF has a cardiovascular protective effect, and its aqueous extract can contract the aorta (Hibino et al., 2009b). In managing cardiovascular conditions, evodiamine, rutaecarpine, and limonin serve as crucial metabolites of EF, offering protection against myocardial ischemia-reperfusion (I/R), anti-myocardial fibrosis, anti-arrhythmia, safeguarding vascular endothelial damage, altering vascular tension, and so on. Studies have shown that evodiamine has an anti-atherosclerotic effect, can regulate energy by inhibiting the expression of the β1-adrenergic receptor, and prevents cardiac I/R injury (Xue et al., 2015). Subsequently, evodiamine has the ability to control the growth and movement of vascular smooth muscle cells by blocking the PI3K/AKT axis activation in the traditional route, thereby preventing atherosclerosis onset and progression (Zha et al., 2023). What’s more, evodiamine can prevent isoproterenol-induced cardiac fibrosis by regulating endothelial-to-mesenchymal transition (Huang et al., 2017). Moreover, rutaecarpine promotes endothelial nitric oxide synthase (eNOS) phosphorylation and NO synthesis via the Ca2+/calmodulin-dependent protein kinase II (CaMKII) and calmodulin-dependent protein kinase kinase β (CaMKKβ)/AMP-activated protein kinase (AMPK) signaling pathways through transient receptor potential vanilloid type 1 (TRPV1), and effectively prevent endothelial dysfunction (Lee et al., 2021). Rutaecarpine can also reduce the damage to myocardial cells caused by myocardial infarction by enhancing vascular smooth muscle calcification (Zhan et al., 2021). Some studies have shown that limonin can inhibit adriamycin-induced cardiotoxicity by activating Nrf2 and SIRT2 signal pathways (Li X. H. et al., 2022). Limonin can also inhibit the ubiquitination and degradation of SIRT6, stabilize the level of SIRT6 protein, promote its expression, reduce cardiac hypertrophy, and improve cardiac function (Liu et al., 2022).
EF is an effective botanical drug for treating gastrointestinal diseases, especially evodiamine, rutaecarpine, and dehydroevodiamine (Chen et al., 2023). Evodiamine can act as an antioxidant by blocking the Rho/NF-κB pathway and alleviating gastric mucosal injury (Zhao Z. et al., 2015). It can also inhibit gastritis caused by Helicobacter pylori infection by inhibiting the NF-κB pathway (Yang et al., 2021). Then, evodiamine can effectively improve the imbalance of intestinal microflora and relieve the symptoms of ulcerative colitis by increasing the level of lactobacillus acidophilus and the production of acetate (Wang et al., 2020). In addition, it can suppress gastrointestinal hyperactivity caused by stress via cholecystokinin (CCK) and the CCK1 receptor (Ren et al., 2018). Moreover, rutaecarpine is effective in mitigating gastric damage caused by ethanol through the suppression of NF-κB pathway anti-inflammation, Nrf2 pathway antioxidation, stimulation of Bcl-2, suppression of Bax and caspase-3 expression, and prevention of gastric cell apoptosis (Ren et al., 2020). Some studies have shown that TRPV1/calcitonin gene-related peptide (CGRP) pathway is an important therapeutic target for gastric mucosal injury (Luo et al., 2013). Rutaecarpine can stimulate the TRPV1 receptor to release CGRP, inhibit the excessive secretion of gastric acid, and improve the symptoms of gastric ulcers (Liu et al., 2008). Moreover, Dehydroevodiamine can reduce the inflammatory injury of gastric mucosa by reducing the ERK/p38 signal pathway, down-regulating the expression of myeloperoxidase (MPO), TNF-α and IL-6, upregulating the expression of IL-10, regulating gastric pH and mucosal thickness (Wei et al., 2021). Then, dehydroevodiamine can also improve MNNG-induced gastric mucosal injury and GES-1 migration in chronic atrophic gastritis rats and treat atrophic gastritis by inhibiting hypoxia-inducible factor 1α/vascular endothelial growth factor angiogenesis pathway (Wen et al., 2021). Furthermore, EF polysaccharides have a protective effect on gastric mucosa and can alleviate the symptoms of gastric ulcers. By increasing the expression of Nrf2 and HO-1 protein, reducing the expression of Keap1 protein, activating Keap1/Nrf2/HO-1 signal pathway, and reducing oxidative stress in the stomach (Luo et al., 2023).
The application of EF in neurological disorders is becoming increasingly widespread. EF and its metabolites have neuroprotective effects on neurodegenerative diseases such as ischemic injury, neuropathic pain, neuroinflammation, Alzheimer’s disease (AD), and so on. Studies have shown that EF methanol extract (200 mg/kg) protects neurons and prevents ischemia-induced cognitive impairment (Lee et al., 2011). Then, evodiamine can reduce peripheral hypersensitivity and anxiety in nerve-injured mice (Zhang et al., 2020). Evodiamine can also repair memory and cognitive impairment, protect neurons in vitro, and inhibit glial cell activation and neuroinflammation (Lima and Hamerski, 2019). In the experimental AD mice induced by intracerebroventricular injection of streptomycin, evodiamine (50 and 100 mg/kg) was orally given daily for 21 days, the ability to recognize new targets and the score of water maze test was improved in AD mice (Wang et al., 2018a). In addition, evodiamine can improve AD mice’s learning and cognitive impairment (Wan et al., 2024) and treat AD through antioxidation and anti-apoptosis (Zhang et al., 2018). Moreover, rutaecarpine ameliorates neuronal injury in rats with cerebral I/R by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway (Han et al., 2019). Rutaecarpine can affect Ca2+ influx and activate PI3K/AKT signal pathway by activating specific capsaicin receptor TRPV1, inhibit intracellular oxidative stress and apoptosis protease activity, and protect neurons from apoptosis induced by hypoxia-reoxygenation (Yang Y. et al., 2018). Additional research has shown that limonin and its variants are versatile in combating neuroinflammation and neuronal apoptosis by activating PI3K/AKT and reducing TLR4/NF-κB pathway activity and are effective in treating AD (Panda S. P. et al., 2024). Limonin plays a neuroprotective role by inhibiting neuronal autophagy and microglial activation in rats injected with 6-hydroxydopamine (Gao et al., 2023). Furthermore, dehydroevodiamine (10 mg/kg) can improve the symptoms of memory impairment induced by scopolamine in mice. Dehydroevodiamine has a strong protective effect on cognitive impairment through its antioxidant activity, inhibition of neurotoxicity, and intracellular calcium. Therefore, Dehydroevodiamine may be an important drug for treating memory disorders (Shin et al., 2017). Tg2576-induced AD mice were treated with dehydroevodiamine (0.5 mg/kg) for 4 months, which improved the memory impairment of Tg mice and decreased the levels of soluble amyloid-β 40 (Aβ40), soluble Aβ42 and total Aβ peptide in the cortex of Tg mice. Dehydroevodiamine can inhibit the activity of β-secretase in a dose-dependent manner, which is related to the production of Aβ and the formation of neuritis plaques. Dehydroevodiamine may have a therapeutic effect on AD as a β-secretase inhibitor (Shin et al., 2016).
The anti-inflammatory activity of EF has been widely recognized. Research indicates that the 70% ethanol extract of EF can inhibit the inflammatory response in HaCaT cells. It exerts its anti-inflammatory effects by modulating the JAK-STAT and MAPK signaling pathways. This regulation suppresses inflammatory mediators, cytokines, and chemokines, alleviating symptoms associated with atopic dermatitis (Jin et al., 2024). EF has a potent anti-inflammatory and uric acid-lowering effect. The EF water extract can significantly improve the production of serum inflammatory cytokines IL-1β and TNF-α and inhibit the activation of renal NLRP3 inflammatory signal (Wang Z. et al., 2024). In addition, rutaecarpine can significantly reduce the inflammatory response induced by pseudotype severe acute respiratory syndrome coronavirus 2 by blocking the activity of 3C-like protease (Lin et al., 2023). It has been proved that rutaecarpine can inhibit inflammation by inhibiting the NF-κB signal pathway mediated by PI3K/AKT and MAPK and reduce lipopolysaccharide (LPS)-induced cell migration and number by inhibiting Src/FAK pathway (Jayakumar et al., 2021). Rutaecarpine has also reduced inflammatory responses by downregulating interferon-α, IL-23 p19, and IL-17A protein. This anti-inflammatory effect is mediated through the NF-κB and TLR7 signaling pathways (Li Y. et al., 2019). Moreover, evodiamine is anti-inflammatory by inhibiting IL-1β, IL-2, IL-6, IL-8, TNF-α, and other inflammatory factors mediated by NF-κB (Zhang Y. et al., 2022). Evodiamine can also significantly reduce the pathological damage of breast tissue, inhibit the activation of inflammation-related pathways such as AKT, NF-κB p65, ERK1/2, p38, and JNK, and significantly reduce the production of pro-inflammatory cytokines (Yang Y. et al., 2022). Evodiamine can also improve ulcerative colitis by down-regulating NF-κB signal pathway and NLRP3 inflammatory bodies (Shen et al., 2019). Furthermore, limonin can reduce hepatic steatosis, lipid accumulation, and the expression of p-STAT3/STAT3, caspase-8, and prostaglandin-endoperoxide synthase 2, and improve the inflammatory response of non-alcoholic fatty liver (Wang W. et al., 2023). Limonin can effectively regulate the inflammation mediated by CD4+T cells and inhibit the proliferation of CD4+T cells by inhibiting the nuclear translocation of NF-κB p65 in activated CD4+T cells (Kim W. et al., 2009). Limonin also participates in the regulation of inflammatory pathways by effectively inhibiting p38 MAP. Limonin can also counteract hypertension and vascular damage associated with metabolic syndrome by reducing inflammation and fibrosis (Hassan et al., 2018). Additionally, limonin significantly decreased TNF-α, IL-1β, and IL-6 and inhibited the expression of inflammatory factors in lipopolysaccharide LPS-induced acute lung injury in mice (Wang et al., 2018b).
The analgesic effect of EF is closely related to its anti-inflammatory effect. Oral 50% or 70% methanol extract of 200 mg/kg EF has an analgesic effect on writhing induced by acetic acid (Matsuda et al., 1997). The analgesic effect of EF is related to its metabolites, including evodiamine, rutaecarpine, dehydroevodiamine, rutin, and limonin. Evodiamine exerts analgesic effects through various mechanisms, such as inhibiting ion channels, directly suppressing pain signals, reducing neuronal inflammation, restoring the balance between excitatory and inhibitory neurotransmission, and modulating neurotransmitter release (Jiang et al., 2022). In vitro, evodiamine can significantly reduce capsaicin-induced current and thermal hyperalgesia in rats by activating TRPV1 and neuron desensitization (Iwaoka et al., 2016). Evodiamine can also inhibit migraine-like pain response, which may be due to the regulation of nNOS and the inhibition of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor glutamate A1 (Lin et al., 2020). Evodiamine has been shown to inhibit neuropathic pain, improving paclitaxel-induced neuropathic pain by suppressing inflammatory responses and maintaining mitochondrial antioxidant function (Wu and Chen, 2019). In addition, limonin exhibits significant pain-relieving effects at 30 or 100 mg/kg doses. This analgesic effect is likely associated with its anti-inflammatory properties (Matsuda et al., 1998).
EF exhibits potent anti-cancer properties and significant healing properties against various cancers, including lung, liver, stomach, breast, and cervical. Studies have shown that 70% ethanol extract of EF can significantly reduce the vitality of human cervical cancer HeLa cells at 20–60 μg/mL and show a certain concentration correlation (Park et al., 2017). Evodiamine has anti-tumor effects by inducing apoptosis, blocking the cell cycle, regulating autophagy, and inhibiting tumor cell metastasis (Luo et al., 2021; Panda et al., 2023). Evodiamine can treat non-small cell lung cancer (NSCLC) by down-regulating the expression of SOX-9 and β-catenin and significantly inhibiting cell migration by inhibiting epithelial-mesenchymal transition (EMT) (Panda M. et al., 2024). Then, evodiamine can induce cell cycle arrest in the G2/M phase, inhibit cell migration, and inhibit the Notch3 signal pathway against NSCLC by inhibiting γ-secretase (Yang X. et al., 2020). Evodiamine can increase the expression of cleaved-caspase-3, decrease the activity of TSGF and alpha-fetoprotein, induce AKT-mediated apoptosis, and exert an anti-hepatoma effect (Yang F. et al., 2017). Moreover, evodiamine inhibits PI3K/AKT, ERK1/2, and p38 MAPKs and promotes apoptosis of ovarian cancer cells by activating caspase-9/8/3 and poly ADP-ribose polymerase cleavage (Wei et al., 2016). Evodiamine activates retinoblastoma protein through p53 and p21, which selectively inhibits breast cancer stem cells in the G1/S phase, resulting in cancer cell death (Han et al., 2016). Evodiamine inhibits the proliferation and induces apoptosis of cholangiocarcinoma cells, inhibits the migration and invasion of cholangiocarcinoma cells, suppresses IL-6/STAT3 signal transduction by upregulating the expression of SHP-2, and treats cholangiocarcinoma (Zhu et al., 2019).
Beyond that, rutaecarpine can inhibit CYP1A1 and exert an anti-tumor effect by inhibiting the binding of 2,3,7,8-Tetrachlorodibenzo-p-dioxin to its receptor (Rannug et al., 1992). Rutaecarpine can also significantly inhibit human CYP1A2 and CYP3A4 (Don et al., 2003; Iwata et al., 2005). Moreover, limonin can inhibit the cell activity of colorectal cancer cells, block STAT3 signal transduction, and inhibit the proliferation, migration, invasion, and colony formation of colorectal cancer cells (Zhang W. F. et al., 2024). Limonin exhibits properties that combat breast cancer. It has been reported that limonin has cytotoxicity on estrogen receptor-positive or negative human breast cancer cells, which may inhibit proliferation by activating caspase-7 dependent pathway (Kim et al., 2013). What’s more, dehydroevodiamine pancreatic is the activator of DNA damage-inducible transcript 3 (DDIT3) and has the ability to inhibit the AKT/mammalian target of rapamycin (mTOR) pathway. It can effectively inhibit the proliferation of pancreatic ductal adenocarcinoma cells, and the growth of prostate cancer stem cells in vitro and in vivo (Zhu et al., 2024).
EF can warm the stomach, invigorate the spleen, and be used in glucose and lipid metabolism. In fat metabolism, evodiamine reduced the food intake rate and weight gain rate of rats after growth by downregulating the expression of neuropeptide Y (NPY) and agouti-gene-related protein (AgRP) mRNA and peptide expression in hypothalamic arcuate nucleus (Shi et al., 2009). Evodiamine, a new non-irritant vanillic acid receptor agonist, can simultaneously induce heat loss and heat production, dissipate food energy, and prevent visceral fat accumulation and weight gain (Kobayashi et al., 2001). Then, evodiamine can also activate AMPK and adiponectin polymerization in 3T3-L1 adipocytes, which is related to the activation of Ca2+-dependent PI3K/Akt/CaMKII signal pathway (Liu et al., 2014). Treatment with evodiamine over 13 weeks has been shown to lower serum total cholesterol, low and high-density lipoprotein cholesterol, and triglycerides in obese rats on a high-fat diet, decrease blood lipids, and mitigate obesity-related symptoms (Zhang et al., 2017). The combination of berberine and evodiamine can affect the protein expression of PPARγ and liver X receptor α in hyperlipidemic rats and reduce the level of blood cholesterol in hyperlipidemic rats (Zhou et al., 2017b). Furthermore, evodiamine acts as a berberine promoter, and the mechanism of synergistic reduction of serum cholesterol in rats involves inhibiting the expression of Acetyl-CoA Acetyltransferase 2 (ACAT2), Niemann-Pick C1-like 1 (NPC1L1) and apolipoprotein B-48 (apoB-48) to reduce blood lipids (Zhou et al., 2017a). Moreover, ruteacarpine inhibited the expression of NPY and AgRP in the hypothalamic arcuate nucleus and the expression of these two neuropeptides in N29-4 neuronal cells. This method effectively lowers blood cholesterol, non-fasting glucose, insulin, and leptin and ameliorates obesity (Kim S. J. et al., 2009).
On top of that, EF can also contribute to glucose metabolism and improve the symptoms of diabetes. Studies have found that low-dose evodiamine can prevent increased body weight and improve glucose tolerance in mice. Enhanced phosphorylation of AMPK and reduced mTOR signal transduction, a regulator of energy metabolism, are observed in white adipose tissue and are known to avert obesity and insulin resistance (Yamashita et al., 2015). Evodiamide inhibits insulin-stimulated mTOR-S6K activation and IRS1 serine phosphorylation in adipocytes and improves glucose tolerance in obese/diabetes mice (Wang T. et al., 2013). Evodiamine and rutaecarpine can inhibit gluconeogenesis and adipogenesis by activating constitutive androstane receptor (CAR) in vitro and in vivo and have therapeutic potential for the treatment of hyperglycemia and diabetes mellitus type 2 (Yu et al., 2016). Rutaecarpine can regulate the IRS1/PI3K/AKT signal pathway in the liver and AMPK/acetyl-CoA carboxylase 2 signal pathway in skeletal muscle to improve hyperlipidemia and hyperglycemia in fat-fed and streptozotocin-treated rats (Nie et al., 2016). Beyond that, EF polysaccharides extracted by water solvent have strong antioxidant activity and α-glucosidase inhibition. It is a promising natural antioxidant and α-glucosidase inhibitor.
EF can warm the liver and kidney, protect the liver and kidney, and is closely related to evodiamine, rutaecarpine, and limonin. In the liver, evodiamine can promote the translocation of Nrf2 into the nucleus, thereby reducing reactive oxygen species (ROS) levels and oxidative stress in grass carp hepatocytes. It also downregulates the MAPK pathway, alleviating DEHP-induced apoptosis and restoring the expression of antioxidant genes. By blocking the Nrf2/MAPK pathway, evodiamine inhibits DEHP-induced apoptosis in grass carp hepatocytes (Lei et al., 2023; Xiong et al., 2022). Evodiamine (15 and 25 mg/kg) has an anti-fibrotic effect on CCl4-induced hepatic fibrosis and reduces the proliferation and collagen metabolism of hepatic stellate cells in vitro by down-regulating the relative expressions of TGF-β1, p-Smad2/3, and α-smooth muscle actin (Yang D. et al., 2018). Additionally, rutaecarpine upregulates antioxidant enzymes through CaMKII-Akt and Nrf2/antioxidant response element (ARE) pathways, enhances the expression of HO-1 in hepatocytes, and has a protective effect on hepatotoxicity induced by TBHP (Jin et al., 2017). Rutaecarpine protects mice from acute acetaminophen-induced liver injury by activating antioxidant enzymes. It could significantly reduce the activity of serum ALT/AST and MDA induced by acetaminophen and prevent liver GSH depletion induced by acetaminophen (Choi et al., 2021). Moreover, limonins have a furan ring structure and are easily activated to form RMs, which are crucial in induced hepatotoxicity (Liu Y. et al., 2020). CYP3A4 inducer aggravated the hepatotoxicity induced by large flower germ, while limonin reduced its hepatotoxicity (Zhang et al., 2021c). Limonin can also diminish the liver toxicity caused by acetaminophen by activating the Nrf2 antioxidant signal and suppressing NF-κB inflammation by increasing SIRT1 levels. Limonin shows potential as a treatment for liver damage caused by acetaminophen (Yang R. et al., 2020).
In renal metabolism, I/R injury can lead to acute kidney failure. Owing to its antioxidant, anti-inflammatory, and anti-apoptotic properties, evodiamine can reduce the biochemical and pathological tissue of renal I/R injury in rats (Eraslan et al., 2019). Studies have shown that evodiamine can also protect against LPS-induced acute renal injury and cytotoxicity by regulating ROS NF-κB-mediated inflammation (Shi et al., 2019). What is more, rutaecarpine can prevent and treat renal I/R injury by inhibiting JNK/p38 MAPK signal pathway and interfering with oxidative stress (Wang et al., 2017). Moreover, limonin, acting as a natural ERK2 agonist, plays a role in averting ischemic acute renal damage, primarily through the activation of the ERK signal pathway, which aids in the growth of renal tubular cells and diminishes apoptosis following acute kidney injury (AKI) (Zhou et al., 2023). Limonin regulates arachidonic acid metabolism by inhibiting CYP3A4 activity, thus improving cisplatin-induced acute renal injury and ultimately protecting renal function (Zeng et al., 2023).
Ancient records indicate that EF possesses insecticidal properties. The extracts and metabolites of EF showed specific insecticidal properties. Findings indicated superior deworming effects of ethyl acetate, petroleum ether, and methanol extract on Goldfish-Gyrodactylus kobayashii in living organisms, with EF ethyl acetate extract emerging as the most efficient and secure (Lian et al., 2019). Subsequently, EF volatile oil exhibits insect-killing properties against maize weevils, Sitophilus zeamais, and red flour beetle Tribolium castaneum, showing LC50 values of 36.89, 24.57, and 57.31 mg/L air, in that order (Liu and Du, 2011). In addition, evodiamine and rutaecarpine have insecticidal activity against larvae of Drosophila melanogaster with LC50 values of 0.30 and 0.28 μmol/mL diet, respectively, among which rutaecarpine has the strongest activity (Miyazawa et al., 2002). Evodiamine and rutaecarpine had strong insecticidal activity against the fourth instar larvae of Aedes albopictus with LC50 values of 12.51 and 17.02 μg/mL, respectively. EF ethanol extract, limonin, and evodiol also had insecticidal activity against Asian tiger mosquitoes, and the LC50 values were 43.21, 32.43, and 52.22 μg/mL, respectively (Liu et al., 2012). Moreover, the nematicidal activity of evodiamine and rutaecarpine against Meloidogyne incognita was stronger than the crude EF ethanol extract, and the LC50 values were 73.55, 120.85, and 131.54 μg/mL, respectively. Both evodiol and limonin demonstrated their ability to kill Meloidogyne incognita, evidenced by LC50 values of 155.02 and 197.37 μg/mL, respectively, yet they were less potent than the raw EF ethanol extract (Liu et al., 2013). Other studies found that limonin had effective biological activity against larvae and adults of Schistosoma mansoni, and its antiparasite activity was enhanced in a dose-dependent manner (Eraky et al., 2016).
EF’s long-term use in treating diarrhea and beriberi is due to its antibacterial and antifungal properties. The EF volatile oil has the strongest activity against Bacillus subtilis and Staphylococcus aureus, the maximum inhibitory zone diameter is 17.9 and 12.2 mm, respectively, and the MIC is 3.2–6.4 mg/mL (Liu S. S. et al., 2019). Furthermore, three novel quinazoline alkaloids, specifically evodiamine A, evodiamine B, and evodiamine C, were extracted from EF methanol. They prohibited excellent inhibition against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and Xanthomonas campestris pv. campestris, with respective EC50 values of 3.13, 14.32, and 32.72 nmol (Su et al., 2018). Moreover, evodiamine can enhance the activation of NLRP-3 inflammatory bodies by inducing acetylation of α-tubulin lysine 40 residues, thus enhancing innate immunity to bacterial infection (Li C. G. et al., 2019).
Rutaecarpine, evodiamine, and limonin demonstrated obvious anti-osteoporotic effects. Rutaecarpine significantly inhibits osteoclast production and bone resorption of bone marrow-derived macrophages osteoclasts by reducing the protein level of nuclear factor of activated T cells 1 (NFATc-1) and phosphorylation of other signal pathways during osteoclast differentiation (Fukuma et al., 2018). In addition, evodiamine has been reported to inhibit osteoclast formation by blocking receptor activators for NF-κB ligand (RANKL)-induced ERK and c-Fos activation and NFATc-1 induction in a dose and time-dependent manner (Jiang et al., 2017). Evodiamine can inhibit osteoclast formation induced by RANKL through NF-κB and calcium signaling pathways and reduce bone loss in ovariectomy and ovariectomized mice by inhibiting osteoclast production (Jin et al., 2019). Subsequently, evodiamine is capable of mitigating osteoporosis in zebrafish caused by dexamethasone, by counteracting the disproportion in bone development and resorption and triggering the matrix metalloproteinase 3-osteopontin-MAPK pathway signal (Yin et al., 2019). Beyond that, limonin can increase the calcium concentration of the femur and fifth lumbar vertebra in ovariectomized rats, and the mechanism may be related to promoting bone formation (Mandadi et al., 2009). Research has found that the loss of ovarian function can lead to a lack of ovarian-related hormones, resulting in rapid loss of ovarian-related bones (Renno et al., 2006). Limonin can efficiently prevent bone mass reduction and enhance bone mineral density in rats post-ovariectomy. Moreover, limonin stimulates the activity of ALP in osteoblast MC3T3-E1 and enhances the expression of osteoblast differentiation gene markers by regulating extracellular signal-regulated kinase and P38 signal (Lee et al., 2016).
EF not only offers attractive pharmacological effects but also provides antiallergic, antioxidant, antidepressant, and protection for the prostate, among others.
In vitro and in vivo, evodiamine and rutaecarpine may inhibit the biosynthesis of allergy-related cytokines (TNF-α and IL-4) in mast cells and basophils, suggesting that they may be effective against IgE-induced allergic diseases such as atopic dermatitis and rhinitis (Shin et al., 2007). Subsequently, limonin effectively manages allergies induced by IgE. This substance can significantly reduce IgE production in the peripheral blood mononuclear cells (PBMC) and B cell lines of children allergic to food, potentially owing to the suppression of ε-germ line transcript expression in PBMC (Yang et al., 2014).
In natural aging rats, limonin decreased the levels of MDA and lipofuscin in serum and brain tissue, increased the activities of superoxide dismutase (SOD) and GSH-Px in serum and brain tissue, and enhanced the total antioxidant capacity in brain tissue (Li et al., 2016). Notably, when altered by the structure of limonin, limonin glycosides can attain antioxidant properties by neutralizing free radicals (Poulose et al., 2005). However, some researchers question whether limonin has antioxidant activity (Breksa and Manners, 2006). There is a lack of research on the antioxidant mechanism of limonin, and this natural antioxidant is worthy of further exploration.
The antidepressant effect of evodiamine on chronic, unpredictable stress rats may be achieved by regulating monoamine transmitters and BDNF-TrkB signal transduction in the hippocampus (Jiang et al., 2015).
The EF ethanol extract has a strong 5α-reductase inhibitory activity. The treatment of EF ethanol extract in benign prostatic hyperplasia-1 cells inhibits cell viability through caspase-8 and cystatin-3-dependent apoptosis and effectively inhibits the growth of benign prostatic hyperplasia-1 cells (Park et al., 2018). In addition, EF volatile oil has obvious anti-inflammatory effects and inhibits the growth of prostate cancer-3 cells by directly and indirectly regulating the cytokine secretion profile of spleen cells (Yeh and Lin, 2021). Not only this, evodiamine can also inhibit prostate hyperplasia and migration through the PI3K/AKT/NFκB signal pathway, indicating that it may be a potential lead drug in the treatment of prostate cancer (Lei et al., 2022).
Ancient TCM texts have chronicled EF’s mild toxic effects, potentially causing eye harm, hair fall, and gastrointestinal issues if misused. In recent years, many studies have shown that overuse of EF can cause toxic symptoms such as nausea, vomiting, abdominal pain, diarrhea, and blurred vision (Ma et al., 2018) and cause liver and kidney toxicity to the human body (Teschke, 2014). EF and its metabolites have been reported to cause liver damage at high doses and induce arrhythmias, leading to cardiotoxicity (Teschke et al., 2014). In vitro and in vivo studies have shown that EF has hepatotoxicity, cardiotoxicity, and nephrotoxicity. However, there are few studies on the cardiotoxicity and nephrotoxicity of EF. The toxicity of EF is summarized as follows (Table 5).
EF causes hepatocyte cytotoxicity, attributed to oxidative stress, mitochondrial damage, endoplasmic reticulum stress, liver metabolic disorder, and apoptosis (Cai et al., 2014). The toxicological mechanisms are peroxidation, inflammatory factors, mitochondrial damage, and the formation of drug-protein adducts. Research revealed that various EF extracts might lead to sudden liver damage, with volatile oil exhibiting the highest level of hepatotoxicity, succeeded by total extract and ethanol extract, and water extract showing minimal hepatotoxicity.
EF may cause oxidative harm by impacting vital elements of the body’s oxidation-antioxidant mechanism. Studies have shown that EF aqueous extraction can cause liver injury in mice after continuous intragastric administration for 21 days. It was found that the content of MDA in liver tissue increased, the ratio of SOD/MDA and the activity of GSH-Px decreased significantly, and the pathomorphology showed focal hepatocyte necrosis (Zhou L. et al., 2013). The hepatotoxicity of EF is related to the oxidative stress in the liver and has a certain dose-effect relationship. Following 15 days of orally administering EF via aqueous extraction, there was a notable reduction in SOD activity in the mice’s liver tissues, with SOD levels rising in each dosage group as the dose was increased (Cai et al., 2014). In addition, liver injury occurred after continuous intragastric administration of EF volatile oil for 7 days, resulting in increased activities of MDA and NOS in blood and liver tissue, decreased GSH content, SOD, and GSH-Px activities (Zhang et al., 2011). Research indicates that the presence of 3-alkylindoles in evodiamine and rutaecarpine leads to the creation of highly electrophilic intermediates, namely iminoquinone and 3-methyleneindolenine, via P450-driven oxidation in liver microsomes (primarily driven by CYP3A4 and to a smaller degree by CYP1A2 and CYP2D6), causing harmful effects on hepatocytes when GSH is depleted (Wen et al., 2014).
Inflammatory injury is one of the causes of liver injury caused by EF. IL-1β, IL-6, and TNF-α are inflammatory transmitters closely related to inflammatory response. These inflammatory transmitters can further amplify the signal of inflammatory response and promote apoptosis and necrosis of hepatocytes. Findings indicate that mice experienced liver damage and elevated levels of TNF-α and IL-1β in their liver tissue 15 days following the EF aqueous extraction (Liu et al., 2018). After continuous intragastric administration of EF aqueous extraction for 21 days, the high, middle, and low dose groups of EF could significantly increase the contents of TNF-α, IL-1 β, and IL-6 in liver tissue of mice, with a certain dose-effect relationship (Zhou L. et al., 2013). After continuous intragastric administration of EF aqueous and ethanol extraction for 15 days, the expression of phosphorylated ERK1/2 in the liver of mice was significantly upregulated. Activation of ERK1/2 can induce cells to produce TNF-α, which mediates inflammatory response and apoptosis-related transcriptional regulatory factors (Liao et al., 2014). During metabolic processes, the P450 enzyme activates evodiamine in EF aqueous extraction, resulting in liver damage and inflammation, primarily due to elevated levels of ALT, AST, ALP, LDH, MPO, MDA, TNF-α, IL-6, and IL-1 (Ren et al., 2024).
Mitochondria are the main sites of cell biological oxidation, which mainly synthesize ATP. Mitochondria are essential targets of drug toxicity in drug-induced liver injury. Studies have shown that intragastric administration of EF aqueous extracts of 6, 12, and 24 g/kg for 15 days can cause hepatocyte mitochondrial swelling and vacuolation, and eventually lead to apoptosis due to ATP depletion and cytochrome C release (Cai et al., 2014). Moreover, both ethanol and aqueous extracts of EF have hepatotoxicity, and the cytotoxicity of EF ethanol extract is stronger. In addition, evodiamine has the strongest toxicity among the extracts of EF, which can significantly reduce the number of cells and increase the mitochondrial membrane potential (MMP) in vitro (Yang et al., 2024b). Evodiamine (0.04–25 μmol/L) decreased the survival rate of HepG2 cells, increased MMP, and induced apoptosis in a time- and dose-dependent manner (Gao et al., 2021). Mitochondrial permeability transition plays an important role in mediating hepatocyte injury (Labbe et al., 2008; Pessayre et al., 2010). Limonin has hepatotoxicity, which can cause oxidative damage to rat mitochondria, lead to mitochondrial swelling, mitochondrial permeability transition pore opening, mitochondrial potential decrease, and finally trigger cell death signal pathway (Fan et al., 2019).
The alkaloids easily combine with proteins to form drug-protein adducts. Drug-protein adducts may cause toxicity by damaging the physiological function of the modified protein or through an immune-mediated mechanism (Zhou et al., 2005). The role of RMs is significant in liver damage caused by drugs. The secondary amine configuration of Rutaecarpine enables its activation into RMs via the CYPs enzyme, leading to a covalent bond with CYPs and proteins in rat liver cells, resulting in drug-protein complexes and subsequent liver damage (Zhang et al., 2015). RMs can consume GSH, leading to excessive ROS production, respiratory chain dysfunction, cell stress, mitochondrial damage, cell membrane damage, and hepatocyte damage (Akbulut et al., 2014). CYPs are the primary enzymes involved in drug metabolism within the human body. Certain medications transform into RMs via the biological actions of CYPs (Yan et al., 2023). It has been found that rutaecarpine can inhibit many types of CYP activity, such as CYP1A2, CYP2C9, CYP2C19, and CYP2E1 (Zhang et al., 2015). The induction of cytochrome P450 enzyme gene, liver transport protein, and phase 2 enzyme gene are involved in the interaction between evodiamine and drugs (Zhu et al., 2013). Evodiamine and rutaecarpine can cause toxicity through P450-mediated dehydrogenation, produce highly electrophilic intermediates, and lead to drug-drug interaction mainly through the inactivation of CYP3A4 (Wen et al., 2014). In addition, evodiamine can inhibit CYP1A2, CYP2C9, and CYP2D6 in rats (Zhang et al., 2016). Evodiamine is easily oxidized to an epoxy structure that binds to GSH. When GSH is depleted, some liver damage will occur (Zhang et al., 2015).
The cardiotoxicity of EF is mainly caused by oxidative damage and inhibition of human ether-a-go-go-related gene (hERG) channels in the heart. Its primary connections are in alkaloids with evodiamine, rutaecarpine, dehydroevodiamine, and hydroxyrutaecarpine.
The heart is the most oxygen-consuming organ, and many basic studies have confirmed the cardiotoxicity mediated by oxidative stress (Shen et al., 2024). Oxidative damage is closely related to evodiamine and rutaecarpine. Studies have shown that evodiamine at the concentration of 31.3–250 μg/mL for 24 h can significantly reduce the level of MDA and the activity of superoxide dismutase, resulting in oxidative stress injury of cardiomyocytes. Subsequently, evodiamine could induce oxidative stress by generating free radicals, potentially harming the architecture and functionality of cardiomyocytes. After being treated with 28.44 μg/mL evodiamine for 24 h, cardiomyocytes reached 50% inhibitory concentration, which significantly decreased the activity of SOD in rat cardiomyocytes. In the zebrafish model, the mortality rate of zebrafish treated with 354 ng/mL evodiamine was 10%, causing cardiac dysfunction and pericardial malformations (Yang W. et al., 2017). The findings imply that evodiamine could lead to heart-related side effects, including oxidative stress. Determining LDH and creatine kinase (CK) activity is one of the biochemical indexes for evaluating and diagnosing heart disease. The level of LDH in serum reflects the injury of myocardial cell permeability. The activity level of CK is directly related to the consumption and supply of myocardial oxygen and energy, muscle contraction, and mitochondrial function (Zervou et al., 2016; Bak and Schousboe, 2017; Klein et al., 2020). Evodiamine and rutaecarpine have toxic effects on rat cardiomyocytes H9c2 and neonatal rat cardiomyocytes (NRCMs), mainly by reducing the protein expression of cyclic guanosine monophosphate-protein kinase G pathway in H9c2 cells and changing the spontaneous beating frequency in NRCMs (Zhang D. et al., 2022). What is more, a high dose of evodiamine will lead to severe morphological abnormalities of the liver, pericardial edema, and increased myocardial concentration.
The hERG channel is the ion channel on the myocardial cell membrane, which is very important to maintain the normal electrophysiological activity of the heart. Abnormal opening or closing of the hERG channel will lead to arrhythmia (Liao et al., 2024). In vitro, rutaecarpine can reduce the threonine/tyrosine phosphorylation of Sp1 and the expression of the hERG channel through the PI3K/AKT pathway in HEK 293 cells. Subsequently, administering rutaecarpine for 2 weeks may extend the QT/QTc interval intervals and enhance the rate of ventricular fibrillation induction in the hearts of guinea pigs (Zhan et al., 2021). In addition, dehydroevodiamine can inhibit the hERG channel, change the myocardial excitation process, and lead to arrhythmia and even ventricular fibrillation in severe cases (Zhang et al., 2023). Depending on the dose, dehydroevodiamine, and hortiamine can prolong action potential duration and early afterdepolarizations of cardiomyocytes, eventually leading to arrhythmias (Baburin et al., 2018). There are also studies indicating that hydroxyrutaecarpine inhibits hERG current by binding to F656 and Y652 sites in the hERG channel. It can shorten the inactivation time constant, accelerate the process of channel inactivation, and inhibit the function of the hERG channel (Li X. H. et al., 2022).
EF’s nephrotoxic effects primarily stem from renal cell death and oxidative stress. Its similarity to evodiamine and limonin in EF is notable. Mice were administered the EF ethanol extract in groups of low, medium, and high dosages. In the group receiving a high dosage, there was a noticeable flexing of the glomerular Mesangium and an enlargement of both glomerular podocytes and endothelial cells (Liu et al., 2015). In vivo experiments showed that evodiamine could induce renal cell death and regulate the PI3K/AKT/mTOR pathway by inducing intracellular calcium overload (Yang et al., 2024a). Rutaecarpine can significantly reduce the level of cortisol and regulate glucocorticoid metabolism. Renal injury may be related to the induction of apoptosis-related protein Bax and Bcl2 expression (Yang C. Q. et al., 2022). Evodiamine and evodiolide in alkaloids can damage mitochondria, lead to mitochondrial dysfunction, produce a large number of free radicals from mitochondria, further aggravate oxidative stress, induce apoptosis, and promote renal damage (Zhou Q.j. et al., 2013). In addition, limonin also has a certain toxicity to kidney cells. Comprehensive analysis of animal experiments and chromatographic analysis showed that hydroxyl or acetoxy limonoid derivatives and coumarin in EF may be the leading causes of toxicity (Shan et al., 2021). Studies have shown that limonin (50–200 μg/mL) can significantly inhibit the viability of HEK 293 cells in a dose-dependent manner. A concentration ranging from 100–200 μg/mL may lead to atrophy, reduction, or even fatality of renal cells (Fan et al., 2019).
The typical oral dose of EF is 2–5 g, taken by water decoction or pill powder. It is generally non-toxic within a reasonable dose range, but it needs to be monitored if it is overused. The liver function test, coagulation function test, serum bilirubin level test, liver ultrasound, and liver biopsy can monitor the hepatotoxicity of EF. Liver function tests can detect transaminase, bilirubin, alkaline thrombin, cholinesterase, and other indexes in blood and evaluate the functional status of the liver (Shiihara et al., 2024). Coagulation function tests can determine prothrombin time, thrombin time, and activated thrombin time and help to judge whether the liver is abnormal (Kumagai et al., 2023). A high serum bilirubin level may indicate liver injury or biliary obstruction disease (Poynard et al., 2023). Liver ultrasound can observe liver structural abnormalities or steatosis (Vardar et al., 2022). Liver biopsies help to confirm whether there is hepatocyte injury or inflammation (Palmer et al., 2024). Therefore, patients using EF should regularly check their liver function and blood coagulation function, pay close attention to the indexes of transaminase, bilirubin, alkaline thrombin, and cholinesterase, and adjust the dosage of EF in time to avoid drug-induced liver injury.
The cardiotoxicity of EF can be evaluated by physical monitoring and measurement of specific biomarkers, including electrocardiogram changes, blood electrolyte levels, and myocardial enzyme activity (Qiu et al., 2023). An electrocardiogram is a direct indicator of the heart’s electrophysiological function, and irregular waveforms could signal the presence of an arrhythmia (Henriksen et al., 2023). Disproportionate levels of electrolytes in the blood, particularly irregular potassium ion concentrations, frequently lead to arrhythmias (Sauer and Porter, 2021). Increased myocardial enzyme activity, such as creatine kinase and lactate dehydrogenase, may damage heart tissue (Gai et al., 2021). Therefore, patients using EF should have regular ambulatory electrocardiogram monitoring. The global cardiac activity was measured for 24 h, and the heart rate variability and myocardial perfusion indexes were observed. It assists patients in adjusting their medication dosage promptly to reduce the occurrence of cardiotoxicity.
The nephrotoxicity of EF can be monitored by urinalysis, renal function tests, biochemical assays, and renal biopsy (Canki et al., 2024). Urine tests can reflect proteinuria, hematuria, hemoglobinuria, cylindruria, urinary calcium, and alkaline urine (Wu and Fenton, 2018). Renal function tests assess the endogenous creatinine clearance rate, blood urea nitrogen, creatinine, and uric acid levels and evaluate the glomerular filtration rate through a radionuclide renogram (Nieto et al., 2020). Biochemical tests can monitor serum liver and renal function-related enzyme levels (Sumer et al., 2020). A renal biopsy is capable of directly monitoring the pathological alterations in kidney tissue, including the deterioration of tubular epithelial cells, necrosis, congestion in the interstitial space, swelling, infiltration of inflammatory cells, fibrosis in the renal interstitial area, and the expansion or shrinkage of the renal tubules (Xu et al., 2024). Therefore, patients taking EF should have regular urinalysis and blood tests and pay attention to the levels of urinary protein, red blood cells, serum creatinine, and urea nitrogen to ensure timely detection of issues and implementation of appropriate treatment measures, thereby reducing the incidence of renal toxicity. Concurrently, it is crucial to rigorously regulate the dosage while administering EF to prevent excessive use. Regular monitoring of kidney function during usage is crucial for early identification of kidney toxicity.
EF ranks among the most prevalent and extensively utilized TCM, with a history of use in China spanning millennia. The latest research achievements of EF in standardized cultivation, traditional application, processing methods, quality control, phytochemistry, pharmacology, and toxicology were reviewed in this paper. EF has cardiovascular protection, anti-inflammation, analgesia, gastrointestinal protection, anti-tumor, neuroprotection, glucose and lipid metabolism regulation, and other pharmacological effects. Frequently, it serves as a treatment for abdominal discomfort, vomiting, diarrhea, dyspepsia, high blood pressure, eczema, and oral ulcers. Despite EF’s diverse pharmacological effects, ancient texts document its mild toxicity. The harmful effects of TCM have persistently been a worry in both its clinical usage and formulation. Therefore, it is suggested that the following areas should be considered in future research.
From a botanical perspective, it is evident that ER, ERO, and ERB are the trio of EF sources, with nearly mature fruits primarily categorized into LEF, MEF, and SEF. MEF produced in Jiangxi is a genuine medicinal material mainly distributed in Sichuan, Guizhou, and other places south of the Qinling Mountains in China. However, most current studies only distinguish the varieties of Euodia rutaecarpa, and few studies have demonstrated the effect of EF size on pharmacological action. From a conventional standpoint, EF’s initial documentation appears in Shen Nong’s Herbal Classic, known for its customary properties of alleviating cold and pain, diminishing nausea and vomiting, enhancing yang, and halting diarrhea. Currently, numerous clinical prescriptions and formulations have been developed, primarily using EF, for treating conditions like abdominal pain, diarrhea, chronic non-atrophic gastritis, irritable bowel syndrome, primary dysmenorrhea, and more. However, the specific mechanism is not completely clear, and the clinical application of EF needs more extensive verification. Moreover, modern pharmacological studies mainly focus on EF or its metabolites, seriously ignoring the effects caused by drug interaction. According to the traditional application, exploring the synergistic effect of drugs to improve clinical efficacy and safety is of great significance. In terms of processing methods, ancient classical works have recorded the processing of EF with licorice, salt, ginger, vinegar, and other methods, which can increase efficiency and reduce toxicity. Modern research has proved that the best result can be obtained when the ratio of EF to licorice is 100: 6. However, few articles currently study this aspect. The metabolites of EF were widely studied. About 300 metabolites were isolated and identified from the plant, including alkaloids, terpenoids, flavonoids, volatile oils, and others. Indole alkaloids, especially evodiamine, and rutaecarpine, have been studied for many years. However, studying terpenoids, flavonoids, and volatile oils in EF is not deep enough, which seriously limits people’s understanding of the pharmacological and toxicological mechanisms. Therefore, further study of other metabolites is a priority for the future. The quality assurance of EF is closely related to evodiamine, rutaecarpine, and limonin. It is stipulated that the content of evodiamine and rutaecarpine is not less than 0.15%, and the content of limonin is not less than 0.20%. However, there is no maximum limit for using these three ingredients. According to modern research, the content of rutaecarpine and limonin should not exceed 100 mg/kg/d, and the content of evodiamine should not exceed 300 mg/kg/d. Although researchers have done a lot of research on the minimum and maximum dose, a unified standard has not been formed, so it is necessary to conduct an in-depth study.
The research on toxicology and its mechanisms of EF in vivo and in vitro is insufficient, limiting its more extensive application. Contemporary studies extensively focus on EF’s hepatotoxic effects, with lesser emphasis on cardiotoxicity and nephrotoxicity and a lack of comprehensive and clear understanding of the toxicity of EF. After analyzing the metabolites of EF, it is concluded that evodiamine, rutaecarpine, limonin, and dehydroevodiamine are related to cardiotoxicity and nephrotoxicity. In a specific range, the toxicity of EF to the heart and kidney is proportional to the dose, and long-term overuse will aggravate the toxicity. Attention must be paid to the dosage of EF in clinical application. The research on drug dosage is also very scarce, and the dosage of 2–5 g stipulated in ChP is not exactly the same as the actual clinical dose. Therefore, the research on the dose of EF should be further strengthened. In vitro and vivo experiments showed that EF may be toxic to the liver through peroxidation injury, inflammatory response factor mediation, mitochondrial damage, and the formation of drug-protein adducts. EF may cause cardiotoxicity through oxidative damage and inhibition of hERG channels in the heart. EF could react to kidney toxicity by inducing apoptosis and oxidative stress. However, EF’s specific toxic risks and potential disadvantages must be further studied, especially cardiotoxicity and nephrotoxicity. The existing studies mainly focus on the preliminary stage of animal experiments and clinical trials. The specific application effect of EF in different diseases needs to be further verified, and its safety needs to be evaluated more comprehensively. Therefore, the clinical use of EF should be closely monitored to ensure its safety.
TCM stands as a repository of abundant resources and distinctive processing techniques. However, the phytochemistry, pharmacology, and toxicology of TCM are complex. Therefore, we should further strengthen the research on the pharmacological and toxicological mechanisms of EF, further expand and optimize its application in medicine, and better meet the clinical needs. EF is a valuable plant medicine with various uses, pharmacological activities, and reliable clinical efficacy. It has significant medicinal value and potential to develop new therapeutic drugs. Therefore, systematic and comprehensive research on its metabolism and pharmacological and toxicological mechanisms is a hot spot.
YH: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing–original draft, Writing–review and editing. JQ: Data curation, Formal Analysis, Methodology, Software, Writing–original draft. XH: Software, Validation, Visualization, Writing–original draft. CL: Methodology, Project administration, Supervision, Writing–review and editing. YL: Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The author(s) declare that financial support was received for this article’s research, authorship, and/or publication. This work is supported by the Key projects of the TCM Scientific Research Project of the Sichuan Provincial Administration of TCM (2024ZD024) and the Xinglin Scholar Research Promotion Project of Chengdu University of TCM (XKTD2022014).
Gratitude is extended to every lab member for their valuable insights and feedback on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1509032/full#supplementary-material
Akbulut, S., Elbe, H., Eris, C., Dogan, Z., Toprak, G., Otan, E., et al. (2014). Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J. Gastroenterol. 20 (29), 10158–10165. doi:10.3748/wjg.v20.i29.10158
Baburin, I., Varkevisser, R., Schramm, A., Saxena, P., Beyl, S., Szkokan, P., et al. (2018). Dehydroevodiamine and hortiamine, alkaloids from the traditional Chinese herbal drug Evodia rutaecarpa, are I(Kr) blockers with proarrhythmic effects in vitro and in vivo. Pharmacol. Res. 131, 150–163. doi:10.1016/j.phrs.2018.02.024
Bae, J. R., Park, W. H., Suh, D. H., No, J. H., Kim, Y. B., and Kim, K. (2020). Role of limonin in anticancer effects of Evodia rutaecarpa on ovarian cancer cells. BMC Complement. Med. Ther. 20 (1), 94. doi:10.1186/s12906-020-02890-y
Bak, L. K., and Schousboe, A. (2017). Misconceptions regarding basic thermodynamics and enzyme kinetics have led to erroneous conclusions regarding the metabolic importance of lactate dehydrogenase isoenzyme expression. J. Neurosci. Res. 95 (11), 2098–2102. doi:10.1002/jnr.23994
Breksa, A. P., and Manners, G. D. (2006). Evaluation of the antioxidant capacity of limonin, nomilin, and limonin glucoside. J. Agric. Food Chem. 54 (11), 3827–3831. doi:10.1021/jf060901c
Cai, Q., Wei, J., Zhao, W., Shi, S., Zhang, Y., Wei, R., et al. (2014). Toxicity of Evodiae fructus on rat liver mitochondria: the role of oxidative stress and mitochondrial permeability transition. Molecules 19 (12), 21168–21182. doi:10.3390/molecules191221168
Cai, X. Y., Meng, N., and Yang, B. (2006). Analysis of one poisoning case caused by excessive Evodiae fructus. Beijing Tradit. Chin. Med. 25, 171–172.
Canki, E., Kho, E., and Hoenderop, J. G. J. (2024). Urinary biomarkers in kidney disease. Clin. Chim. Acta 555, 117798. doi:10.1016/j.cca.2024.117798
Cao, X., Liu, Y., Wang, M., Sun, L., and Ren, X. (2019). Study on the source and characteristics of Evodia rutaecarpa based on chemical pattern recognition. Nat. Prod. Res. 33 (14), 2113–2115. doi:10.1080/14786419.2018.1484464
Chen, L., Hu, Y., Ye, Z., Li, L., Qian, H., Wu, M., et al. (2023). Major indole alkaloids in evodia rutaecarpa: the latest insights and review of their impact on gastrointestinal diseases. Biomed. Pharmacother. 167, 115495. doi:10.1016/j.biopha.2023.115495
Choi, J. H., Jin, S. W., Lee, G. H., Han, E. H., Hwang, Y. P., and Jeong, H. G. (2021). Rutaecarpine protects against acetaminophen-induced acute liver injury in mice by activating antioxidant enzymes. Antioxidants (Basel) 10 (1), 86. doi:10.3390/antiox10010086
Don, M. J., Lewis, D. F., Wang, S. Y., Tsai, M. W., and Ueng, Y. F. (2003). Effect of structural modification on the inhibitory selectivity of rutaecarpine derivatives on human CYP1A1, CYP1A2, and CYP1B1. Bioorg Med. Chem. Lett. 13 (15), 2535–2538. doi:10.1016/s0960-894x(03)00469-4
Du, J., Sun, Y., Lu, Y. Y., Lau, E., Zhao, M., Zhou, Q. M., et al. (2017). Berberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer Res. 37 (11), 6141–6151. doi:10.21873/anticanres.12063
Duan, S., Wang, T., Zhang, J., Li, M., Lu, C., Wang, L., et al. (2017). Huatuo Zaizao pill promotes functional recovery and neurogenesis after cerebral ischemia-reperfusion in rats. BMC Complement. Altern. Med. 17 (1), 19. doi:10.1186/s12906-016-1516-z
Eraky, M. A., El-Kholy, A. A., Rashed, G. A., Hammam, O. A., Moharam, A. F., Abou-Ouf, E. A., et al. (2016). Dose-response relationship in Schistosoma mansoni juvenile and adult stages following limonin treatment in experimentally infected mice. Parasitol. Res. 115 (10), 4045–4054. doi:10.1007/s00436-016-5177-0
Eraslan, E., Tanyeli, A., Polat, E., and Yetim, Z. (2019). Evodiamine alleviates kidney ischemia reperfusion injury in rats: a biochemical and histopathological study. J. Cell Biochem. 120 (10), 17159–17166. doi:10.1002/jcb.28976
Fan, Q. Q., Liang, R. Q., Chen, M. L., Li, Z. Q., Tao, X. Y., Ren, H. M., et al. (2024). Metabolic characteristics of evodiamine were associated with its hepatotoxicity via PPAR/PI3K/AKT/NF-кB/tight junction pathway-mediated apoptosis in zebrafish. Ecotoxicol. Environ. Saf. 279, 116448. doi:10.1016/j.ecoenv.2024.116448
Fan, S., Zhang, C., Luo, T., Wang, J., Tang, Y., Chen, Z., et al. (2019). Limonin: a review of its pharmacology, toxicity, and pharmacokinetics. Molecules 24 (20), 3679. doi:10.3390/molecules24203679
Fukuma, Y., Sakai, E., Komaki, S., Nishishita, K., Okamoto, K., and Tsukuba, T. (2018). Rutaecarpine attenuates osteoclastogenesis by impairing macrophage colony stimulating factor and receptor activator of nuclear factor κ-B ligand-stimulated signalling pathways. Clin. Exp. Pharmacol. Physiol. 45 (8), 863–865. doi:10.1111/1440-1681.12941
Gai, W., An, J., Wang, Z., Han, X., Geng, J., Liang, Y., et al. (2021). Research progress of biomarkers in early detection of chemotherapy-induced cardiotoxicity. Heart Fail Rev. 26 (5), 1195–1201. doi:10.1007/s10741-020-09948-6
Gao, L., Jia, C., Zhang, H., and Ma, C. (2017). Wenjing decoction (herbal medicine) for the treatment of primary dysmenorrhea: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 296 (4), 679–689. doi:10.1007/s00404-017-4485-7
Gao, X., He, D., Liu, Y., Cui, M., Li, Z., Li, J., et al. (2023). Oral administration of Limonin (LM) exerts neuroprotective effects by inhibiting neuron autophagy and microglial activation in 6-OHDA-injected rats. Int. Immunopharmacol. 123, 110739. doi:10.1016/j.intimp.2023.110739
Gao, Y. D., Zhu, A., Li, L. D., Zhang, T., Wang, S., Shan, D. P., et al. (2021). Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells. Beijing Da Xue Xue Bao. Yi Xue Ban. 53 (6), 1107–1114. doi:10.19723/j.issn.1671-167X.2021.06.017
Guan, X., Zheng, X., Vong, C. T., Zhao, J., Xiao, J., Wang, Y., et al. (2020). Combined effects of berberine and evodiamine on colorectal cancer cells and cardiomyocytes in vitro. Eur. J. Pharmacol. 875, 173031. doi:10.1016/j.ejphar.2020.173031
Han, M., Hu, L., and Chen, Y. (2019). Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des. Devel Ther. 13, 2923–2931. doi:10.2147/DDDT.S216156
Han, S., Woo, J. K., Jung, Y., Jeong, D., Kang, M., Yoo, Y. J., et al. (2016). Evodiamine selectively targets cancer stem-like cells through the p53-p21-Rb pathway. Biochem. Biophys. Res. Commun. 469 (4), 1153–1158. doi:10.1016/j.bbrc.2015.12.066
Hassan, N. A., Bassossy, H. M. E., Fahmy, A., and Mahmoud, M. F. (2018). Limonin alleviates macro- and micro-vascular complications of metabolic syndrome in rats: a comparative study with azelnidipine. Phytomedicine 43, 92–102. doi:10.1016/j.phymed.2018.03.044
He, N., Ma, Z. Z., and Wang, Q. Y. (2024). Euodiae Fructus (Wuzhuyu): exploring traditional Chinese medicine compatibility for reducing toxicity. Chin. Tradit. Herb. Drugs 55 (6), 1812–1838.
Henriksen, P. A., Hall, P., MacPherson, I. R., Joshi, S. S., Singh, T., Maclean, M., et al. (2023). Multicenter, prospective, randomized controlled trial of high-sensitivity cardiac troponin I-guided combination angiotensin receptor blockade and beta-blocker therapy to prevent anthracycline cardiotoxicity: the cardiac CARE trial. Circulation 148 (21), 1680–1690. doi:10.1161/circulationaha.123.064274
Hibino, T., Yuzurihara, M., Kanno, H., Kase, Y., and Takeda, A. (2009a). Goshuyuto, a traditional Japanese medicine, and aqueous extracts of Evodiae Fructus constrict isolated rat aorta via adrenergic and/or serotonergic receptors. Biol. Pharm. Bull. 32 (2), 237–241. doi:10.1248/bpb.32.237
Hibino, T., Yuzurihara, M., Kase, Y., and Takeda, A. (2009b). Synephrine, a component of Evodiae fructus, constricts isolated rat aorta via adrenergic and serotonergic receptors. J. Pharmacol. Sci. 111 (1), 73–81. doi:10.1254/jphs.09077fp
Hou, A., Lv, J., Zhang, S., Zhang, J., Yang, L., Jiang, H., et al. (2023). Salt processing: a unique and classic technology for Chinese medicine processing. Front. Pharmacol. 14, 1116047. doi:10.3389/fphar.2023.1116047
Hu, Q., Zeng, J., Zhang, X., He, T., Zhang, A., Li, J., et al. (2023). Metabolomics profiles reveal the efficacy of Wuzhuyu decoction on patients with chronic non-atrophic gastritis. Drug Des. Devel Ther. 17, 3269–3280. doi:10.2147/DDDT.S428783
Huang, C., Liu, H., Gong, X. L., Wu, L. Y., and Wen, B. (2017). Effect of evodiamine and berberine on the interaction between DNMTs and target microRNAs during malignant transformation of the colon by TGF-β1. Oncol. Rep. 37 (3), 1637–1645. doi:10.3892/or.2017.5379
Huang, C. J., Huang, W. C., Lin, W. T., Shu, L. H., Sheu, J. R., Tran, O. T., et al. (2021). Rutaecarpine, an alkaloid from Evodia rutaecarpa, can prevent platelet activation in humans and reduce microvascular thrombosis in mice: crucial role of the PI3K/Akt/GSK3β Signal Axis through a cyclic nucleotides/VASP-independent mechanism. Int. J. Mol. Sci. 22 (20), 11109. doi:10.3390/ijms222011109
Huang, D., Li, S. X., Cai, G. X., Yue, C. H., Wei, L. J., and Zhang, P. (2008). Molecular authentication and quality control using a high performance liquid chromatography technique of Fructus Evodiae. Biol. Pharm. Bull. 31 (2), 312–315. doi:10.1248/bpb.31.312
Huang, L., Yang, W., and Su, M. (2023). Research into the mechanism of intervention of Wenjing decoction in endometriosis based on network pharmacology and molecular docking technology. Medicine 102 (34), e34845. doi:10.1097/md.0000000000034845
Huang, X. L., Shen, B. B., Liang, X. J., Shi, S. Y., Zou, D. Z., Zeng, Y. T., et al. (2019). Rapid identification of alkaloids in evodia rutaecarpa by HPLC-Q-TOF-MS/MS. Chin. J. Exp. Traditional Med. Formulae 24, 102–108.
Iwaoka, E., Wang, S., Matsuyoshi, N., Kogure, Y., Aoki, S., Yamamoto, S., et al. (2016). Evodiamine suppresses capsaicin-induced thermal hyperalgesia through activation and subsequent desensitization of the transient receptor potential V1 channels. J. Nat. Med. 70 (1), 1–7. doi:10.1007/s11418-015-0929-1
Iwata, H., Tezuka, Y., Kadota, S., Hiratsuka, A., and Watabe, T. (2005). Mechanism-based inactivation of human liver microsomal CYP3A4 by rutaecarpine and limonin from Evodia fruit extract. Drug Metab. Pharmacokinet. 20 (1), 34–45. doi:10.2133/dmpk.20.34
Jayakumar, T., Lin, K. C., Chang, C. C., Hsia, C. W., Manubolu, M., Huang, W. C., et al. (2021). Targeting MAPK/NF-κB pathways in anti-inflammatory potential of rutaecarpine: impact on Src/FAK-mediated macrophage migration. Int. J. Mol. Sci. 23 (1), 92. doi:10.3390/ijms23010092
Jiang, L., Zhao, X. Y., Pei, J. J., Mei, L. J., Cui, Y. L., Wang, S., et al. (2017). Daily chemical evodiamine from Chinese prickly ash attenuates osteoclast differentiation through RANKL induced NFAT pathways. J. Funct. Foods 37, 594–602. doi:10.1016/j.jff.2017.07.042
Jiang, M. L., Zhang, Z. X., Li, Y. Z., Wang, X. H., Yan, W., and Gong, G. Q. (2015). Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci. Lett. 588, 154–158. doi:10.1016/j.neulet.2014.12.038
Jiang, S., Yu, L.-J., Yang, H., Jin, Y., Chen, J., Zhang, J.-H., et al. (2023). A study on inhibition of the Aβ1-42-induced inflammatory response by the Huatuo Zaizao pill through the NF-κB signaling pathway. Archives Med. Sci. AMS 19 (4), 1136–1144. doi:10.5114/aoms.2020.99427
Jiang, W., Tang, M., Yang, L., Zhao, X., Gao, J., Jiao, Y., et al. (2022). Analgesic alkaloids derived from traditional Chinese medicine in pain management. Front. Pharmacol. 13, 851508. doi:10.3389/fphar.2022.851508
Jin, H., Yao, L., Chen, K., Liu, Y., Wang, Q., Wang, Z., et al. (2019). Evodiamine inhibits RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss in mice. J. Cell Mol. Med. 23 (1), 522–534. doi:10.1111/jcmm.13955
Jin, S. E., Seo, C. S., Jeon, W. Y., Oh, Y. J., Shin, H. K., Jeong, H. G., et al. (2024). Evodiae Fructus extract suppresses inflammatory response in HaCaT cells and improves house dust mite-induced atopic dermatitis in NC/Nga mice. Sci. Rep. 14 (1), 472. doi:10.1038/s41598-023-50257-3
Jin, S. W., Hwang, Y. P., Choi, C. Y., Kim, H. G., Kim, S. J., Kim, Y., et al. (2017). Protective effect of rutaecarpine against t-BHP-induced hepatotoxicity by upregulating antioxidant enzymes via the CaMKII-Akt and Nrf2/ARE pathways. Food Chem. Toxicol. 100, 138–148. doi:10.1016/j.fct.2016.12.031
Kim, J., Jayaprakasha, G. K., and Patil, B. S. (2013). Limonoids and their anti-proliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 4 (2), 258–265. doi:10.1039/c2fo30209h
Kim, S., Yang, W., Cha, D. S., and Han, Y. T. (2022). Synthesis of evodileptin B, a natural anthranilate derivative isolated from Evodia lepta, and evaluation of its therapeutic potential against Parkinson’s disease. Chem. and Biodivers. 19 (5), e202100808. doi:10.1002/cbdv.202100808
Kim, S. J., Lee, S. J., Lee, S., Chae, S., Han, M. D., Mar, W., et al. (2009a). Rutecarpine ameliorates bodyweight gain through the inhibition of orexigenic neuropeptides NPY and AgRP in mice. Biochem. Biophys. Res. Commun. 389 (3), 437–442. doi:10.1016/j.bbrc.2009.08.161
Kim, W., Fan, Y. Y., Smith, R., Patil, B., Jayaprakasha, G. K., McMurray, D. N., et al. (2009b). Dietary curcumin and limonin suppress CD4+ T-cell proliferation and interleukin-2 production in mice. J. Nutr. 139 (5), 1042–1048. doi:10.3945/jn.108.102772
Klein, R., Nagy, O., Tothova, C., and Chovanova, F. (2020). Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Vet. Med. Int. 2020, 5346483. doi:10.1155/2020/5346483
Kobayashi, Y., Nakano, Y., Kizaki, M., Hoshikuma, K., Yokoo, Y., and Kamiya, T. (2001). Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 67 (7), 628–633. doi:10.1055/s-2001-17353
Kumagai, K., Mawatari, S., Moriuchi, A., Oda, K., Takikawa, Y., Kato, N., et al. (2023). Early-phase prothrombin time-international normalized ratio in acute liver injury indicates the timing of therapeutic intervention and predicts prognostic improvement. Hepatol. Res. 53 (2), 160–171. doi:10.1111/hepr.13848
Labbe, G., Pessayre, D., and Fromenty, B. (2008). Drug-induced liver injury through mitochondrial dysfunction: mechanisms and detection during preclinical safety studies. Fundam. Clin. Pharmacol. 22 (4), 335–353. doi:10.1111/j.1472-8206.2008.00608.x
Lacroix, D., Prado, S., Kamoga, D., Kasenene, J., and Bodo, B. (2011). Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark. J. Nat. Prod. 74 (10), 2286–2289. doi:10.1021/np2004825
Lee, B., Choi, E. J., Lee, E. J., Han, S. M., Hahm, D. H., Lee, H. J., et al. (2011). The neuroprotective effect of methanol extract of gagamjungjihwan and fructus euodiae on ischemia-induced neuronal and cognitive impairment in the rat. Evid. Based Complement. Altern. Med. 2011, 685254. doi:10.1093/ecam/nep028
Lee, D. H., Jeon, E. J., Ahn, J., Hwang, J. T., Hur, J., Ha, T. Y., et al. (2016). Limonin enhances osteoblastogenesis and prevents ovariectomy-induced bone loss. J. Funct. Foods 23, 105–114. doi:10.1016/j.jff.2016.02.008
Lee, G. H., Kim, C. Y., Zheng, C., Jin, S. W., Kim, J. Y., Lee, S. Y., et al. (2021). Rutaecarpine increases nitric oxide synthesis via eNOS phosphorylation by TRPV1-dependent CaMKII and CaMKKβ/AMPK signaling pathway in human endothelial cells. Int. J. Mol. Sci. 22 (17), 9407. doi:10.3390/ijms22179407
Lei, Y., Zhang, W., Gao, M., and Lin, H. (2023). Mechanism of evodiamine blocking Nrf2/MAPK pathway to inhibit apoptosis of grass carp hepatocytes induced by DEHP. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 263, 109506. doi:10.1016/j.cbpc.2022.109506
Lei, Y. H., Chan, M. C., Liu, H. Y., Lyu, W. Y., Chen, L., Zhong, Y. Q., et al. (2022). Evodiamine as the active compound of Evodiae fructus to inhibit proliferation and migration of prostate cancer through PI3K/AKT/NF-κB signaling pathway. Dis. Markers 2022 (1), 4399334. doi:10.1155/2022/4399334
Li, C., Chi, C., Li, W., Li, Z., Wang, X., Wang, M., et al. (2022a). An integrated approach for identifying the efficacy and potential mechanisms of TCM against atherosclerosis—Wu-Zhu-Yu decoction as a case study. J. Ethnopharmacol. 296, 115436. doi:10.1016/j.jep.2022.115436
Li, C. G., Zeng, Q. Z., Chen, M. Y., Xu, L. H., Zhang, C. C., Mai, F. Y., et al. (2019a). Evodiamine augments NLRP3 inflammasome activation and anti-bacterial responses through inducing α-tubulin acetylation. Front. Pharmacol. 10, 290. doi:10.3389/fphar.2019.00290
Li, C. H., Zhao, Y., Li, W. J., Xing, R. R., Lu, J., and Liu, R. X. (2021a). UHPLC-UV-Q-Orbitrap HRMS combined with machine learning algorithms reveals the chemical markers of Euodiae Fructus among closely related cultivars. Industrial Crops Prod. 162, 113279. doi:10.1016/j.indcrop.2021.113279
Li, D., Li, Y., Jiang, X., Liu, W., and Zhao, Q. (2022b). Evodiamine: a privileged structure with broad-ranging biological activities. Mini Rev. Med. Chem. 22 (21), 2680–2701. doi:10.2174/1389557522666220404090835
Li, D. W., Zhang, M., Feng, L., Huang, S. S., Zhang, B. J., Liu, S. S., et al. (2020a). Alkaloids from the nearly ripe fruits of Evodia rutaecarpa and their bioactivities. Fitoterapia 146, 104668. doi:10.1016/j.fitote.2020.104668
Li, H., Feng, T., Wen, Y., Li, L., Liu, Y., Ren, X., et al. (2021b). Comparative investigation for raw and processed products of Euodiae Fructus based on high-performance liquid chromatography fingerprints and chemical pattern recognition. Chem. Biodivers. 18 (8), e2100281. doi:10.1002/cbdv.202100281
Li, L. Z., Hu, W. M., Tang, L., and Zhang, L. (2016). Effect of limonin on learning and memory ability and antioxidant capacity of natural apolexis rats. Chin. J. Food Hyg. 28, 22–27.
Li, M., and Wang, C. (2020). Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: a review. J. Ethnopharmacol. 263, 113231. doi:10.1016/j.jep.2020.113231
Li, R. L., Zhang, Q., Liu, J., He, L. Y., Huang, Q. W., Peng, W., et al. (2021c). Processing methods and mechanisms for alkaloid-rich Chinese herbal medicines: a review. J. Integr. Med. 19 (2), 89–103. doi:10.1016/j.joim.2020.12.003
Li, X., Qiao, B., Wu, Y., Deng, N., Yuan, J., and Tan, Z. (2024). Sishen Pill inhibits intestinal inflammation in diarrhea mice via regulating kidney-intestinal bacteria-metabolic pathway. Front. Pharmacol. 15, 1360589. doi:10.3389/fphar.2024.1360589
Li, X. H., Zhan, G., Li, J. X., Ren, J. C., Fan, P., and Li, B. X. (2022c). Evaluation of cardiac safety of hydroxyrutaecarpine, hERG channel inhibitor. Acta Pharm. Sin., 1367–1374.
Li, Y., Zhang, G., Chen, M., Tong, M., Zhao, M., Tang, F., et al. (2019b). Rutaecarpine inhibited imiquimod-induced psoriasis-like dermatitis via inhibiting the NF-κB and TLR7 pathways in mice. Biomed. Pharmacother. 109, 1876–1883. doi:10.1016/j.biopha.2018.10.062
Li, Y. H., He, J., Li, Y., Wu, X. D., Peng, L. Y., Du, R. N., et al. (2014). Evollionines A–C, three new alkaloids isolated from the fruits of Evodia rutaecarpa. Helvetica Chim. Acta 97 (11), 1481–1486. doi:10.1002/hlca.201300449
Li, Y. H., Liu, X., Yin, M., Liu, F., Wang, B., Feng, X., et al. (2020b). Two new quinolone alkaloids from the nearly ripe fruits of Tetradium ruticarpum. Nat. Prod. Res. 34 (13), 1868–1873. doi:10.1080/14786419.2019.1566819
Li, Y. Y., Feng, J. L., Li, Z., Zang, X. Y., and Yang, X. W. (2022d). Separation and enrichment of alkaloids from Coptidis rhizoma and Euodiae Fructus by macroporous resin and evaluation of the effect on bile reflux gastritis rats. Molecules 27 (3), 724. doi:10.3390/molecules27030724
Lian, K. Q., Zhang, M. L., Zhou, L. L., Song, Y. W., and Guan, X. J. (2019). Japon balıklarında (Carassius auratus) gyrodactylus kobayashii’ye (monogenea) karşı çin şifalı otlarının anthelmintik etkinliklerinin incelenmesi. Kafkas Univ. Veteriner Fak. Derg. doi:10.9775/kvfd.2019.23196
Liao, J., Yang, Z., Yang, J., Lin, H., Chen, B., Fu, H., et al. (2024). Investigating the cardiotoxicity of N-n-butyl haloperidol iodide: inhibition mechanisms on hERG channels. Toxicology 508, 153916. doi:10.1016/j.tox.2024.153916
Liao, W. Q., Li, B., Li, L., and Zhao, J. N. (2014). Study on molecular mechanism of Euodiae Fructus on liver toxicity in MICE. Zhongguo Zhong Yao Za Zhi 39 (24), 4865–4868.
Lima, J. A., and Hamerski, L. (2019). Alkaloids as potential multi-target drugs to treat Alzheimer’s disease. Stud. Nat. Prod. Chem. 61, 301–334. doi:10.1016/b978-0-444-64183-0.00008-7
Lin, J., Zhang, X., Li, C., Zhang, Y., Lu, H., Chen, J., et al. (2020). Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response. J. Ethnopharmacol. 254, 112727. doi:10.1016/j.jep.2020.112727
Lin, S., Wang, X., Guo, H., Dai, N., Tang, R., Lee, H., et al. (2023). The ethanol extract of evodiae fructus and its ingredient, rutaecarpine, inhibit infection of SARS-CoV-2 and inflammatory responses. Int. J. Mol. Sci. 24 (1), 762. doi:10.3390/ijms24010762
Liu, L., Zhang, X. M., Xu, J., Zhang, J. H., Zhang, T. j., Chen, C. Q., et al. (2020a). Chemical components and pharmacological action for Euodiae Fructus and predictive analysis on its Q-marker. Chin. Traditional Herb. Drugs 24, 2689–2702.
Liu, L. B., Huang, S. H., Qiu, H. L., Cen, X. F., Guo, Y. Y., Li, D., et al. (2022). Limonin stabilises sirtuin 6 (SIRT6) by activating ubiquitin specific peptidase 10 (USP10) in cardiac hypertrophy. Br. J. Pharmacol. 179 (18), 4516–4533. doi:10.1111/bph.15899
Liu, L. H., Xie, J. Y., Guo, W. W., Wu, G. Y., Chen, Z. F., Yi, J. Y., et al. (2014). Evodiamine activates AMPK and promotes adiponectin multimerization in 3T3-L1 adipocytes. J. Asian Nat. Prod. Res. 16 (11), 1074–1083. doi:10.1080/10286020.2014.939071
Liu, Q. Z., Li, H. Q., and Liu, Z. L. (2013). Nematocidal constituents from the ethanol extract of evodia rutaecarpa hort unripe fruits. J. Chem. 2013 (1), 939215. doi:10.1155/2013/939215
Liu, S., Zhang, S., Wei, H., Chen, B., Huang, Y., and Zhong, Z. (2018). Preliminary study on reducing hepatotoxicity of preparing Euodia rutaecarpa fruit. China J. Chin. Mat. Med. 3, 570–575.
Liu, S. S., Liu, Z. X., Wei, H., Yin, Y., Zhang, Q. W., Yan, L. H., et al. (2019a). Chemical compositions, yield variations and antimicrobial activities of essential oils from three species of Euodiae Fructus in China. Industrial Crops Prod. 138, 111481. doi:10.1016/j.indcrop.2019.111481
Liu, Y., Liu, C., Liu, Y., Ge, Q., and Sun, C. (2020b). Cytochrome P450 mediated bioactivation of rutaevin, a bioactive and potentially hepatotoxic component of evodia rutaecarpa. Chem. Res. Toxicol. 33 (12), 3054–3064. doi:10.1021/acs.chemrestox.0c00475
Liu, Y., Yang, R., Xia, Q., Liu, Y., Zhang, S., and Li, H. (2015). Toxicity of repeated doses of alcohol extract of evodia in target organs. Mode. Prev. Med. 14, 2600–2603.
Liu, Y., Zhou, W., Mao, Z., and Chen, Z. (2019b). Analysis of Evodiae Fructus by capillary electrochromatography-mass spectrometry with methyl-vinylimidazole functionalized organic polymer monolilth as stationary phases. J. Chromatogr. A 1602, 474–480. doi:10.1016/j.chroma.2019.06.011
Liu, Y. Z., Zhou, Y., Li, D., Wang, L., Hu, G. Y., Peng, J., et al. (2008). Reduction of asymmetric dimethylarginine in the protective effects of rutaecarpine on gastric mucosal injury. Can. J. Physiol. Pharmacol. 86 (10), 675–681. doi:10.1139/y08-073
Liu, Z. L., and Du, S. S. (2011). Fumigant components from the essential oil of Evodia rutaecarpa Hort unripe fruits. J. Chem. 8 (4), 1937–1943. doi:10.1155/2011/256729
Liu, Z. L., Liu, Q. Z., Du, S. S., and Deng, Z. W. (2012). Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 111 (3), 991–996. doi:10.1007/s00436-012-2923-9
Luo, C., Ai, J., Ren, E., Li, J., Feng, C., Li, X., et al. (2021). Research progress on evodiamine, a bioactive alkaloid of Evodiae fructus: focus on its anti-cancer activity and bioavailability (Review). Exp. Ther. Med. 22 (5), 1327. doi:10.3892/etm.2021.10762
Luo, J. H., Zou, W. S., Li, J., Liu, W., Huang, J., Wu, H. W., et al. (2023). Untargeted serum and liver metabolomics analyses reveal the gastroprotective effect of polysaccharide from Evodiae fructus on ethanol-induced gastric ulcer in mice. Int. J. Biol. Macromol. 232, 123481. doi:10.1016/j.ijbiomac.2023.123481
Luo, X. J., Liu, B., Dai, Z., Yang, Z. C., and Peng, J. (2013). Stimulation of calcitonin gene-related peptide release through targeting capsaicin receptor: a potential strategy for gastric mucosal protection. Dig. Dis. Sci. 58 (2), 320–325. doi:10.1007/s10620-012-2362-6
Ma, C., Liu, X., Shan, Y., Xu, S., Feng, X., and Wang, Q.-Z. (2021). A new quinolone alkaloid from the fruits of Tetradium ruticarpum. Nat. Prod. Res. 35 (2), 222–227. doi:10.1080/14786419.2019.1624954
Ma, R., Chen, Y., Zhao, Y., Tan, X., and Zhou, X. (2018). Case report of liver damage by Traditional Chinese Medicine drug granules. Clin. Misdiagn Misther 31 (06), 84–86.
Mandadi, K., Ramirez, M., Jayaprakasha, G. K., Faraji, B., Lihono, M., Deyhim, F., et al. (2009). Citrus bioactive compounds improve bone quality and plasma antioxidant activity in orchidectomized rats. Phytomedicine 16 (6-7), 513–520. doi:10.1016/j.phymed.2008.09.001
Matsuda, H., Wu, J. X., Tanaka, T., Iinuma, M., and Kubo, M. (1997). Antinociceptive activities of 70% methanol extract of evodiae fructus (fruit of Evodia rutaecarpa var. bodinieri) and its alkaloidal components. Biol. Pharm. Bull. 20 (3), 243–248. doi:10.1248/bpb.20.243
Matsuda, H., Yoshikawa, M., Iinuma, M., and Kubo, M. (1998). Antinociceptive and anti-inflammatory activities of limonin isolated from the fruits of Evodia rutaecarpa var. bodinieri. Planta Med. 64 (4), 339–342. doi:10.1055/s-2006-957447
Minh, N. T., Michel, S., Tillequin, F., Litaudon, M., Sévenet, T., and Lallemand, M.-C. (2003). A New pyranoacridone alkaloid from the bark of Medicosma subsessilis (Rutaceae). Z. für Naturforsch. B 58 (12), 1234–1236. doi:10.1515/znb-2003-1213
Miyazawa, M., Fujioka, J., and Ishikawa, Y. (2002). Insecticidal compounds from Evodia rutaecarpa against Drosophila melanogaster. J. Sci. Food Agric. 82 (13), 1574–1578. doi:10.1002/jsfa.1215
Nam, E. Y., Kim, S. A., Kim, H., Kim, S. H., Han, J. H., Lee, J. H., et al. (2016). Akt activation by Evodiae Fructus extract protects ovary against 4-vinylcyclohexene diepoxide-induced ovotoxicity. J. Ethnopharmacol. 194, 733–739. doi:10.1016/j.jep.2016.10.048
Nan, N., Gong, M. X., Wang, Q., Li, M. J., Xu, R., Ma, Z., et al. (2022). Wuzhuyu Decoction relieves hyperalgesia by regulating central and peripheral 5-HT in chronic migraine model rats. Phytomedicine 96, 153905. doi:10.1016/j.phymed.2021.153905
Nie, X. Q., Chen, H. H., Zhang, J. Y., Zhang, Y. J., Yang, J. W., Pan, H. J., et al. (2016). Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol. Sin. 37 (4), 483–496. doi:10.1038/aps.2015.167
Nieto, V. M. G., Yanes, M. I. L., Carreno, P. T., and Mesa, T. M. (2020). Las pruebas de función renal en una encrucijada. An Pediatr (Barc) 92 (2). doi:10.1016/j.anpedi.2019.11.007
Palmer, M., Kleiner, D. E., Goodman, Z., Brunt, E., Avigan, M. I., Regev, A., et al. (2024). Liver biopsy for assessment of suspected drug-induced liver injury in metabolic dysfunction-associated steatohepatitis clinical trials: expert consensus from the Liver Forum. Aliment. Pharmacol. Ther. 59 (2), 201–216. doi:10.1111/apt.17762
Pan, X., Wang, M., Wu, Y., Lu, X., Shang, Y., Xu, Y., et al. (2015). Identification of active ingredients in Wuzhuyu decoction improving migraine in mice by spectral efficiency association. Mol. Med. Rep. 12 (1), 1524–1534. doi:10.3892/mmr.2015.3506
Panda, M., Biswal, S., and Biswal, B. K. (2024a). Evodiamine potentiates cisplatin-induced cell death and overcomes cisplatin resistance in non-small-cell lung cancer by targeting SOX9-β-catenin axis. Mol. Biol. Rep. 51 (1), 523. doi:10.1007/s11033-024-09477-7
Panda, M., Tripathi, S. K., Zengin, G., and Biswal, B. K. (2023). Evodiamine as an anticancer agent: a comprehensive review on its therapeutic application, pharmacokinetic, toxicity, and metabolism in various cancers. Cell Biol. Toxicol. 39 (1), 1–31. doi:10.1007/s10565-022-09772-8
Panda, S. P., Kesharwani, A., Singh, M., Kumar, S., Mayank, M., Mallick, S. P., et al. (2024b). Limonin (LM) and its derivatives: unveiling the neuroprotective and anti-inflammatory potential of LM and V-A-4 in the management of Alzheimer’s disease and Parkinson’s disease. Fitoterapia 178, 106173. doi:10.1016/j.fitote.2024.106173
Park, E., Lee, M.-Y., Seo, C., Jang, J.-H., Kim, Y.-U., and Shin, H.-K. (2018). Ethanol extract of evodia rutaecarpa attenuates cell growth through caspase-dependent apoptosis in benign prostatic hyperplasia-1 cells. Nutrients 10 (4), 523. doi:10.3390/nu10040523
Park, S. Y., Park, C., Park, S. H., Hong, S. H., Kim, G. Y., Hong, S. H., et al. (2017). Induction of apoptosis by ethanol extract of Evodia rutaecarpa in HeLa human cervical cancer cells via activation of AMP-activated protein kinase. Biosci. Trends 10 (6), 467–476. doi:10.5582/bst.2016.01170
Peng, Q. X., Cai, H. B., Peng, J. L., Yung, K. L., Shi, J., and Mo, Z. X. (2015). Extract of Zuojin Pill [(characters: see text)] induces apoptosis of SGC-7901 cells via mitochondria-dependent pathway. Chin. J. Integr. Med. 21 (11), 837–845. doi:10.1007/s11655-015-2043-3
Pessayre, D., Mansouri, A., Berson, A., and Fromenty, B. (2010). Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 196, 311–365. doi:10.1007/978-3-642-00663-0_11
Poulose, S. M., Harris, E. D., and Patil, B. S. (2005). Citrus limonoids induce apoptosis in human neuroblastoma cells and have radical scavenging activity. J. Nutr. 135 (4), 870–877. doi:10.1093/jn/135.4.870
Poynard, T., Deckmyn, O., Peta, V., Sakka, M., Lebray, P., Moussalli, J., et al. (2023). Clinical and genetic definition of serum bilirubin levels for the diagnosis of Gilbert syndrome and hypobilirubinemia. Hepatol. Commun. 7 (10), e0245. doi:10.1097/HC9.0000000000000245
Qian, P., Jin, H. W., and Yang, X. W. (2014). New limonoids from Coptidis rhizoma-euodiae fructus couple. J. Asian Nat. Prod. Res. 16 (4), 333–344. doi:10.1080/10286020.2014.881355
Qin, J., Liao, C. N., Chen, W. W., Li, H. Y., Su, J., Wu, X. D., et al. (2021). New limonoids and quinolone alkaloids with cytotoxic and anti-platelet aggregation activities from Evodia rutaecarpa (Juss.) Benth. Fitoterapia 152, 104875. doi:10.1016/j.fitote.2021.104875
Qiu, Y., Jiang, P., and Huang, Y. (2023). Anthracycline-induced cardiotoxicity: mechanisms, monitoring, and prevention. Front. Cardiovasc Med. 10, 1242596. doi:10.3389/fcvm.2023.1242596
Rannug, U., Agurell, E., Rannug, A., and Cederberg, H. (1992). Certain tryptophan photoproducts are inhibitors of cytochrome P450-dependent mutagenicity. Environ. Mol. Mutagen. 20 (4), 289–296. doi:10.1002/em.2850200407
Ren, H. X., Tang, Q. C., Yan, L., Xia, H., and Luo, H. S. (2018). Evodiamine inhibits gastrointestinal motility via CCK and CCK1 receptor in water-avoidence stress rat model. Life Sci. 209, 210–216. doi:10.1016/j.lfs.2018.08.003
Ren, K., Wang, R., Fang, S., Ren, S., Hua, H., Wang, D., et al. (2023a). Effect of CYP3A inducer/inhibitor and licorice on hepatotoxicity and in vivo metabolism of main alkaloids of Euodiae Fructus based on UPLC-Q-Exactive-MS. J. Ethnopharmacol. 303, 116005. doi:10.1016/j.jep.2022.116005
Ren, K., Zhang, C., Liu, M., Gao, H., Ren, S., Wang, D., et al. (2023b). The attenuation effect of licorice on the hepatotoxicity of Euodiae Fructus by inhibiting the formation of protein conjugates and GSH depletion. J. Ethnopharmacol. 308, 116307. doi:10.1016/j.jep.2023.116307
Ren, K., Zhang, X., Wang, R., Ren, S., Hua, H., Wang, D., et al. (2024). The inhibitory effect of licorice on the hepatotoxicity induced by the metabolic activation of Euodiae Fructus. J. Ethnopharmacol. 319 (Pt 2), 117233. doi:10.1016/j.jep.2023.117233
Ren, S., Wei, Y., Wang, R., Wei, S., Wen, J., Yang, T., et al. (2020). Rutaecarpine ameliorates ethanol-induced gastric mucosal injury in mice by modulating genes related to inflammation, oxidative stress and apoptosis. Front. Pharmacol. 11, 600295. doi:10.3389/fphar.2020.600295
Renno, A., de Moura, F., dos Santos, N., Tirico, R., Bossini, P., and Parizotto, N. (2006). Effects of 830-nm laser, used in two doses, on biomechanical properties of osteopenic rat femora. Photomed. Laser Surg. 24 (2), 202–206. doi:10.1089/pho.2006.24.202
Sauer, J. M., and Porter, A. C. (2021). Qualification of translational safety biomarkers. Exp. Biol. Med. (Maywood) 246 (22), 2391–2398. doi:10.1177/15353702211002153
Shan, Q., Tian, G., Wang, J., Hui, H., Shou, Q., Fu, H., et al. (2021). Change in the active component of processed Tetradium ruticarpum extracts leads to improvement in efficacy and toxicity attenuation. J. Ethnopharmacol. 264, 113292. doi:10.1016/j.jep.2020.113292
Shen, P., Zhang, Z., Zhu, K., Cao, H., Liu, J., Lu, X., et al. (2019). Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-κB and NLRP3 inflammasome. Biomed. Pharmacother. 110, 786–795. doi:10.1016/j.biopha.2018.12.033
Shen, Z. J., Zhang, Y. Z., Bu, G. K., and Fang, L. (2024). Renal denervation improves mitochondrial oxidative stress and cardiac hypertrophy through inactivating SP1/BACH1-PACS2 signaling. Int. Immunopharmacol. 141, 112778. doi:10.1016/j.intimp.2024.112778
Shi, H. L., Wu, X. J., Liu, Y., and Xie, J. Q. (2013). Berberine counteracts enhanced IL-8 expression of AGS cells induced by evodiamine. Life Sci. 93 (22), 830–839. doi:10.1016/j.lfs.2013.09.010
Shi, J., Yan, J., Lei, Q., Zhao, J., Chen, K., Yang, D., et al. (2009). Intragastric administration of evodiamine suppresses NPY and AgRP gene expression in the hypothalamus and decreases food intake in rats. Brain Res. 1247, 71–78. doi:10.1016/j.brainres.2008.09.091
Shi, Y., Hua, Q., Li, N., Zhao, M., and Cui, Y. (2019). Protective effects of evodiamine against LPS-induced acute kidney injury through regulation of ROS-NF-κB-mediated inflammation. Evid. Based Complement. Altern. Med. 2019, 2190847. doi:10.1155/2019/2190847
Shiihara, M., Shimoda, M., and Suzuki, S. (2024). Preoperative assessment of liver function and perioperative management of posthepatectomy liver failure. Hepatobiliary Surg. Nutr. 13 (3), 527–529. doi:10.21037/hbsn-24-83
Shin, K. Y., Kim, K. Y., and Suh, Y. H. (2017). Dehydroevodiamine·HCl enhances cognitive function in memory-impaired rat models. Korean J. Physiol. Pharmacol. 21 (1), 55–64. doi:10.4196/kjpp.2017.21.1.55
Shin, K. Y., Noh, S. J., Park, C. H., Jeong, Y. H., Chang, K. A., Yoo, J., et al. (2016). Dehydroevodiamine•HCl protects against memory impairment and cerebral amyloid-β production in Tg2576 mice by acting as a β-secretase inhibitor. CNS Neurol. Disord. Drug Targets 15 (8), 935–944. doi:10.2174/1871527315666160815163723
Shin, Y. W., Bae, E. A., Cai, X. F., Lee, J. J., and Kim, D. H. (2007). In vitro and in vivo antiallergic effect of the fructus of Evodia rutaecarpa and its constituents. Biol. Pharm. Bull. 30 (1), 197–199. doi:10.1248/bpb.30.197
Su, X. L., Xu, S., Shan, Y., Yin, M., Chen, Y., Feng, X., et al. (2018). Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia 127, 186–192. doi:10.1016/j.fitote.2018.02.003
Sumer, E., Senturk, G. E., Demirel, O. U., and Yesilada, E. (2020). Comparative biochemical and histopathological evaluations proved that receptacle is the most effective part of Cynara scolymus against liver and kidney damages. J. Ethnopharmacol. 249, 112458. doi:10.1016/j.jep.2019.112458
Teschke, R. (2014). Traditional Chinese medicine induced liver injury. J. Clin. Transl. Hepatol. 2 (2), 80–94. doi:10.14218/JCTH.2014.00003
Teschke, R., Wolff, A., Frenzel, C., and Schulze, J. (2014). Review article: herbal hepatotoxicity--an update on traditional Chinese medicine preparations. Aliment. Pharmacol. Ther. 40 (1), 32–50. doi:10.1111/apt.12798
Tian, K. M., Li, J. J., and Xu, S. W. (2019). Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 141, 541–550. doi:10.1016/j.phrs.2018.12.019
Vardar, B. U., Dupuis, C. S., Goldstein, A. J., Vardar, Z., and Kim, Y. H. (2022). Ultrasonographic evaluation of patients with abnormal liver function tests in the emergency department. Ultrasonography 41 (2), 243–262. doi:10.14366/usg.21152
Wan, Y. C., Yang, Y., Pang, S., and Kong, Z. L. (2024). A novel derivative of evodiamine improves cognitive impairment and synaptic integrity in AD mice. Biomed. Pharmacother. 177, 117103. doi:10.1016/j.biopha.2024.117103
Wang, C., Hao, Z., Zhou, J., Zhang, L., Sun, Y., and Liang, C. (2017). Rutaecarpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol. Med. Rep. 16 (1), 922–928. doi:10.3892/mmr.2017.6631
Wang, D., Wang, C., Liu, L., and Li, S. (2018a). Protective effects of evodiamine in experimental paradigm of Alzheimer's disease. Cogn. Neurodyn 12 (3), 303–313. doi:10.1007/s11571-017-9471-z
Wang, D., Zhang, H. H., Fang, J., Zhong, Y. S., and Yu, C. H. (2018b). Effects of limoninon on LPS-induced acute lung injury in mice. Chin. J. Clin. Pharmacol. Ther. 23 (1), 8.
Wang, J., Wu, S., Gao, H., Yu, C., Chen, X., and Yuan, Z. (2024a). Integrated metabolomics and network pharmacology analysis to explore pig bile-processed Rhizoma Coptidis and Fructus Evodiae sauce-processed Rhizoma Coptidis in lipopolysaccharide-induced inflammatory response. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1243, 124192. doi:10.1016/j.jchromb.2024.124192
Wang, J., Zhang, T., Zhu, L., Ma, C., and Wang, S. (2015). Anti-ulcerogenic effect of Zuojin Pill against ethanol-induced acute gastric lesion in animal models. J. Ethnopharmacol. 173, 459–467. doi:10.1016/j.jep.2015.04.017
Wang, L. X., Zhao, Q., Zhang, Y., Xue, R., Li, S., Li, Y., et al. (2023a). Network pharmacology and pharmacological evaluation for deciphering novel indication of Sishen Wan in insomnia treatment. Phytomedicine 108, 154500. doi:10.1016/j.phymed.2022.154500
Wang, M. X., Lin, L., Chen, Y. D., Zhong, Y. P., Lin, Y. X., Li, P., et al. (2020). Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 159, 104978. doi:10.1016/j.phrs.2020.104978
Wang, Q. Z., Liang, J. Y., and Feng, X. (2010a). Evodiagenine and dievodiamine, two new indole alkaloids from Evodia rutaecarpa. Chin. Chem. Lett. 21 (5), 596–599. doi:10.1016/j.cclet.2009.12.002
Wang, T., Kusudo, T., Takeuchi, T., Yamashita, Y., Kontani, Y., Okamatsu, Y., et al. (2013a). Evodiamine inhibits insulin-stimulated mTOR-S6K activation and IRS1 serine phosphorylation in adipocytes and improves glucose tolerance in obese/diabetic mice. PLoS One 8 (12), e83264. doi:10.1371/journal.pone.0083264
Wang, T. Y., Wu, J. B., Hwang, T. L., Kuo, Y. H., and Chen, J. J. (2010b). A new quinolone and other constituents from the fruits of Tetradium ruticarpum: effects on neutrophil pro-inflammatory responses. Chem. and Biodivers. 7 (7), 1828–1834. doi:10.1002/cbdv.200900289
Wang, W., Yang, L., Hu, M., Yang, Y., Ma, Q., and Chen, J. (2023b). Network pharmacology to reveal the molecular mechanisms of rutaceous plant-derived limonin ameliorating non-alcoholic steatohepatitis. Crit. Rev. Immunol. 43 (5), 11–23. doi:10.1615/CritRevImmunol.2023050080
Wang, X., Chen, L., Chang, L., He, Y., He, T., Wang, R., et al. (2023c). Mechanism of Wuzhuyu decoction on alcohol-induced gastric ulcers using integrated network analysis and metabolomics. Front. Pharmacol. 14, 1308995. doi:10.3389/fphar.2023.1308995
Wang, X. X., Zan, K., Shi, S. P., Zeng, K. W., Jiang, Y., Guan, Y., et al. (2013b). Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia 89, 1–7. doi:10.1016/j.fitote.2013.04.007
Wang, Z., Liu, J., Mou, Y., Liao, W., Li, Y., Liu, J., et al. (2024b). Anti-inflammatory and uric acid lowering effects of Euodiae Fructus on hyperuricemia and gout mice. Front. Pharmacol. 15, 1296075. doi:10.3389/fphar.2024.1296075
Wei, L., Jin, X., Cao, Z., and Li, W. (2016). Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase/phosphatidylinositol-3-kinase/protein kinase B signaling pathways. J. Tradit. Chin. Med. 36 (3), 353–359. doi:10.1016/s0254-6272(16)30049-8
Wei, Y., Ren, S., Wang, J., Wang, Y., Cui, Y., Tian, M., et al. (2021). Dehydroevodiamine ameliorates indomethacin-induced gastric injury via inhibition of ERK and p38 signaling pathway. Phytomedicine 93, 153764. doi:10.1016/j.phymed.2021.153764
Wen, B., Roongta, V., Liu, L., and Moore, D. J. (2014). Metabolic activation of the indoloquinazoline alkaloids evodiamine and rutaecarpine by human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab. Dispos. 42 (6), 1044–1054. doi:10.1124/dmd.114.057414
Wen, J. X., Tong, Y. L., Ma, X., Wang, R. L., Li, R. S., Song, H. T., et al. (2021). Therapeutic effects and potential mechanism of dehydroevodiamine on N-methyl-N'-nitro-N-nitrosoguanidine-induced chronic atrophic gastritis. Phytomedicine 91, 153619. doi:10.1016/j.phymed.2021.153619
Wu, G. L., Teng, F., Li, X. W., Liu, B. N., Du, Y. P., Zhu, J. J., et al. (2021). Study on determination and quantity transfer of multi index components in Wenjing Decoction. Zhongguo Zhong Yao Za Zhi 46 (19), 5005–5014. doi:10.19540/j.cnki.cjcmm.20210623.301
Wu, P., and Chen, Y. (2019). Evodiamine ameliorates paclitaxel-induced neuropathic pain by inhibiting inflammation and maintaining mitochondrial anti-oxidant functions. Hum. Cell 32 (3), 251–259. doi:10.1007/s13577-019-00238-4
Wu, Q., and Fenton, R. A. (2018). Proteomic approaches in kidney disease biomarker discovery. Am. J. Physiology-Renal Physiology 315 (6), F1817–F1821. doi:10.1152/ajprenal.00421.2018
Xia, H., Dai, Y., Zhao, C., Zhang, H., Shi, Y., and Lou, H. (2023). Chromatographic and mass spectrometric technologies for chemical analysis of Euodiae Fructus: a review. Phytochem. Anal. 34 (1), 5–29. doi:10.1002/pca.3187
Xiao, B. Y., Mao, S. J., and Li, X. D. (2012). Variations in the composition of fructus evodiae after processing with Radix glycyrrhizae extract. Chin. J. Integr. Med. 18, 782–787. doi:10.1007/s11655-012-1178-8
Xiao, S. J., Xu, X. K., Chen, W., Xin, J. Y., Yuan, W. L., Zu, X. P., et al. (2023). Traditional Chinese medicine Euodiae Fructus: botany, traditional use, phytochemistry, pharmacology, toxicity and quality control. Nat. Prod. Bioprospect 13 (1), 6. doi:10.1007/s13659-023-00369-0
Xin, X., Shao, B., Li, Y., Liu, S., Li, D., Wang, C., et al. (2022). New chemical constituents from the fruits of Tetradium ruticarpum. Nat. Prod. Res. 36 (7), 1673–1678. doi:10.1080/14786419.2020.1808639
Xiong, G., Ma, L., Zhang, H., Li, Y., Zou, W., Wang, X., et al. (2022). Physicochemical properties, antioxidant activities and α-glucosidase inhibitory effects of polysaccharides from Evodiae fructus extracted by different solvents. Int. J. Biol. Macromol. 194, 484–498. doi:10.1016/j.ijbiomac.2021.11.092
Xu, L., Bian, X., Yang, J., Xu, H., Fang, Y., Yang, J., et al. (2024). Safety and effectiveness of laparoscopic renal biopsy: a single-center review and meta-analysis. Ren. Fail 46 (1), 2312536. doi:10.1080/0886022X.2024.2312536
Xue, H., Cheng, Y., Wang, X., Yue, Y., Zhang, W., and Li, X. (2015). Rutaecarpine and evodiamine selected as β1-AR inhibitor candidates using β1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J. Pharm. Biomed. Anal. 115, 307–314. doi:10.1016/j.jpba.2015.07.022
Yamashita, H., Kusudo, T., Takeuchi, T., Qiao, S., Tsutsumiuchi, K., Wang, T., et al. (2015). Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J. Funct. Foods 19, 320–329. doi:10.1016/j.jff.2015.09.032
Yan, C., Peng, T., Zhang, T., Wang, Y., Li, N., Wang, K., et al. (2023). Molecular mechanisms of hepatotoxicity induced by compounds occurring in Evodiae Fructus. Drug Metab. Rev. 55 (1-2), 75–93. doi:10.1080/03602532.2023.2180027
Yang, C. Q., Gao, Y., Lian, W. Y., Lin, Y., Xie, G. H., Tan, H. L., et al. (2022a). Nephrotoxicity screening of main active components in Zuojin Pills. Chin. Pharmacol. Bull. 38 (1), 110–118.
Yang, C. Q., Lai, C. C., Pan, J. C., Gao, J., Shen, B. Y., Ru, Y., et al. (2024a). Maintaining calcium homeostasis as a strategy to alleviate nephrotoxicity caused by evodiamine. Ecotoxicol. Environ. Saf. 281, 116563. doi:10.1016/j.ecoenv.2024.116563
Yang, C. Q., Lai, C. C., Ru, Y., Shen, B. Y., Wu, X. J., Cui, J. L., et al. (2024b). Elucidating the mechanism of hepatotoxicity in Euodia rutaecarpa: insights from QSAR toxicity prediction and metabolomics. Acupunct. Herb. Med. 4 (2), 257–270. doi:10.1097/hm9.0000000000000108
Yang, D., Li, L., Qian, S., and Liu, L. (2018a). Evodiamine ameliorates liver fibrosis in rats via TGF-β1/Smad signaling pathway. J. Nat. Med. 72 (1), 145–154. doi:10.1007/s11418-017-1122-5
Yang, F., Shi, L., Liang, T., Ji, L., Zhang, G., Shen, Y., et al. (2017a). Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 485 (1), 54–61. doi:10.1016/j.bbrc.2017.02.017
Yang, J. Y., Kim, J. B., Lee, P., and Kim, S. H. (2021). Evodiamine inhibits Helicobacter pylori growth and Helicobacter pylori-induced inflammation. Int. J. Mol. Sci. 22 (7), 3385. doi:10.3390/ijms22073385
Yang, N., Wang, J., Liu, C., Song, Y., Zhang, S., Zi, J., et al. (2014). Berberine and limonin suppress IgE production by human B cells and peripheral blood mononuclear cells from food-allergic patients. Ann. Allergy Asthma Immunol. 113 (5), 556–564.e4. doi:10.1016/j.anai.2014.07.021
Yang, R., Song, C., Chen, J., Zhou, L., Jiang, X., Cao, X., et al. (2020a). Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-κB inflammatory response via upregulating Sirt1. Phytomedicine 69, 153211. doi:10.1016/j.phymed.2020.153211
Yang, W., Ma, L., Li, S., Cui, K., Lei, L., and Ye, Z. (2017b). Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules 22 (6), 943. doi:10.3390/molecules22060943
Yang, X., Zhang, Y., Huang, Y., Wang, Y., Qi, X., Su, T., et al. (2020b). Evodiamine suppresses Notch3 signaling in lung tumorigenesis via direct binding to gamma-secretases. Phytomedicine 68, 153176. doi:10.1016/j.phymed.2020.153176
Yang, Y., Chen, Q., Jia, S., He, L., Wang, A., Li, D., et al. (2018b). Involvement of TRPV1 in the expression and release of calcitonin gene-related peptide induced by rutaecarpine. Mol. Med. Rep. 17 (4), 5168–5174. doi:10.3892/mmr.2018.8494
Yang, Y., Ran, X., Wang, H., Chen, Y., Hou, S., Yang, Z., et al. (2022b). Evodiamine relieve LPS-induced mastitis by inhibiting AKT/NF-κB p65 and MAPK signaling pathways. Inflammation 45 (1), 129–142. doi:10.1007/s10753-021-01533-9
Yeh, T. H., and Lin, J. Y. (2021). Active ingredients from Euodia ruticarpa steam distilled essential oil inhibit PC-3 prostate cancer cell growth via direct action and indirect immune cells conditioned media in vitro. Curr. Issues Mol. Biol. 43 (2), 996–1018. doi:10.3390/cimb43020071
Yin, H., Wang, J., Wu, M., Ma, Y., Wang, S., and Su, Q. (2019). Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. Biomed. Res. Int. 2019, 5859641. doi:10.1155/2019/5859641
Yin, J., Yu, Z., Hou, C., Peng, Y., Xiao, J., and Jiang, J. (2021). Protective effect of Zuojin Fang on lung injury induced by sepsis through downregulating the JAK1/STAT3 signaling pathway. Biomed. Res. Int. 2021, 1419631. doi:10.1155/2021/1419631
Yong, X., Wang, B., Wang, M., Lyu, H., Yin, M., Jin, T., et al. (2024). Comprehensive analysis of 11 species of Euodia (rutaceae) by untargeted LC-IT-TOF/MS metabolomics and in vitro functional methods. Molecules 29 (5), 1059. doi:10.3390/molecules29051059
Yu, L., Wang, Z., Huang, M., Li, Y., Zeng, K., Lei, J., et al. (2016). Evodia alkaloids suppress gluconeogenesis and lipogenesis by activating the constitutive androstane receptor. Biochim. Biophys. Acta 1859 (9), 1100–1111. doi:10.1016/j.bbagrm.2015.10.001
Zeng, X., Zhou, X., Zhou, J., Zhou, H., Hong, X., Li, D., et al. (2023). Limonin mitigates cisplatin-induced acute kidney injury through metabolic reprogramming. Biomed. Pharmacother. 167, 115531. doi:10.1016/j.biopha.2023.115531
Zervou, S., Whittington, H. J., Russell, A. J., and Lygate, C. A. (2016). Augmentation of creatine in the heart. Mini Rev. Med. Chem. 16 (1), 19–28. doi:10.2174/1389557515666150722102151
Zha, Y., Yang, Y., Zhou, Y., Ye, B., Li, H., and Liang, J. (2023). Dietary evodiamine inhibits atherosclerosis-associated changes in vascular smooth muscle cells. Int. J. Mol. Sci. 24 (7), 6653. doi:10.3390/ijms24076653
Zhan, G., Wang, F., Ding, Y. Q., Li, X. H., Li, Y. X., Zhao, Z. R., et al. (2021). Rutaecarpine targets hERG channels and participates in regulating electrophysiological properties leading to ventricular arrhythmia. J. Cell Mol. Med. 25 (11), 4938–4949. doi:10.1111/jcmm.16292
Zhang, B., Cheng, Y., Jian, Q., Xiang, S., Xu, Q., Wang, C., et al. (2024a). Sishen Pill and its active phytochemicals in treating inflammatory bowel disease and colon cancer: an overview. Front. Pharmacol. 15, 1375585. doi:10.3389/fphar.2024.1375585
Zhang, D., Lu, J., Ren, Z., Zhang, X., Wu, H., Sa, R., et al. (2022a). Potential cardiotoxicity induced by Euodiae Fructus: in vivo and in vitro experiments and untargeted metabolomics research. Front. Pharmacol. 13, 1028046. doi:10.3389/fphar.2022.1028046
Zhang, F. L., He, X., Zhai, Y. R., He, L. N., Zhang, S. C., Wang, L. L., et al. (2015). Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine. Xenobiotica 45 (11), 978–989. doi:10.3109/00498254.2015.1038742
Zhang, J., Yin, Y., Xu, Q., Che, X., Yu, C., Ren, Y., et al. (2022b). Integrated serum pharmacochemistry and investigation of the anti-gastric ulcer effect of Zuojin pill in rats induced by ethanol. Pharm. Biol. 60 (1), 1417–1435. doi:10.1080/13880209.2022.2098345
Zhang, M., Gao, M., Wu, S., Zhou, L., Cao, L., Qiao, R., et al. (2021a). Hepatotoxicity comparison of crude and licorice-processed Euodiae Fructus in rats with stomach excess-cold syndrome. Front. Pharmacol. 12, 756276. doi:10.3389/fphar.2021.756276
Zhang, P. T., Pan, B. Y., Liao, Q. F., Yao, M. C., Xu, X. J., Wan, J. Z., et al. (2013). Simultaneous quantification of limonin, two indolequinazoline alkaloids, and four quinolone alkaloids in evodia rutaecarpa (juss.) Benth by HPLC-DAD method. J. Anal. Methods Chem. 2013, 827361. doi:10.1155/2013/827361
Zhang, Q., Zhou, Q., Jin, R., Yao, G., and Chen, X. (2011). Preliminary study on hepatotoxicity and nephrotoxicity induced by rutaecarpine. Chin. J. Exp. Tradit. Med. Formulae 17, 221–225.
Zhang, Q. F., Yang, Y. Y., Chen, H., Liu, L., Fu, Y. H., and Huang, J. (2017). Effect of evodiamine on blood lipids and blood viscosity in hyperlipidemic mice. Henan Tradit. Chin. Med. 37 (1), 72–74. doi:10.16367/j.issn.1003-5028.2017.01.0023
Zhang, W., Ren, K., Ren, S., Lv, S., Pan, Y., Wang, D., et al. (2021b). UPLC-Q-Exactive-MS analysis for hepatotoxicity components of Evodiae Fructus based on spectrum-toxicity relationship. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1176, 122772. doi:10.1016/j.jchromb.2021.122772
Zhang, W., Wang, M., Song, H., Gao, C., Wang, D., Hua, H., et al. (2021c). CYP3A4 inducer aggravates big flower Evodiae Fructus-induced hepatotoxicity whereas limonin attenuates its hepatotoxicity. J. Ethnopharmacol. 264, 113277. doi:10.1016/j.jep.2020.113277
Zhang, W. D., Chen, X. Y., Wu, C., Lian, Y. N., Wang, Y. J., Wang, J. H., et al. (2020). Evodiamine reduced peripheral hypersensitivity on the mouse with nerve injury or inflammation. Mol. Pain 16, 1744806920902563. doi:10.1177/1744806920902563
Zhang, W. F., Ruan, C. W., Wu, J. B., Wu, G. L., Wang, X. G., and Chen, H. J. (2024b). Limonin inhibits the stemness of cancer stem-like cells derived from colorectal carcinoma cells potentially via blocking STAT3 signaling. World J. Clin. Oncol. 15 (2), 317–328. doi:10.5306/wjco.v15.i2.317
Zhang, X., Gao, Y., Zhou, Y., Liu, Z., and Liu, R. (2023). Pharmacological mechanism of natural drugs and their active ingredients in the treatment of arrhythmia via calcium channel regulation. Biomed. Pharmacother. 160, 114413. doi:10.1016/j.biopha.2023.114413
Zhang, X. Y., Zhao, H. M., Liu, Y., Lu, X. Y., Li, Y. Z., Pan, Q. H., et al. (2021d). Sishen pill maintained colonic mucosal barrier integrity to treat ulcerative colitis via Rho/ROCK signaling pathway. Evid. Based Complement. Altern. Med. 2021, 5536679. doi:10.1155/2021/5536679
Zhang, Y., Wang, J., Wang, C., Li, Z., Liu, X., Zhang, J., et al. (2018). Pharmacological basis for the use of evodiamine in Alzheimer's disease: antioxidation and antiapoptosis. Int. J. Mol. Sci. 19 (5), 1527. doi:10.3390/ijms19051527
Zhang, Y., Zhang, Y., Zhao, Y., Wu, W., Meng, W., Zhou, Y., et al. (2022c). Protection against ulcerative colitis and colorectal cancer by evodiamine via anti-inflammatory effects. Mol. Med. Rep. 25 (5), 188. doi:10.3892/mmr.2022.12704
Zhang, Y. T., Zhang, D. F., Ge, N. Y., Zhu, G. H., Hao, C., Zhang, Y., et al. (2016). Effect of evodiamine on CYP enzymes in rats by a cocktail method. Pharmacology 97 (5-6), 218–223. doi:10.1159/000443178
Zhang, Z., Zheng, Y., Zhang, B., Wang, R., Chen, L., Wang, Y., et al. (2024c). Untargeted serum and gastric metabolomics and network pharmacology analysis reveal the superior efficacy of zingiberis rhizoma recens-/euodiae fructus-processed Coptidis Rhizoma on gastric ulcer rats. J. Ethnopharmacol. 332, 118376. doi:10.1016/j.jep.2024.118376
Zhao, H., Zou, J., Xu, W., Hu, D., Guo, L. D., Chen, J. X., et al. (2021a). Diisoprenyl-cyclohexene/ane-Type meroterpenoids from biscogniauxia sp. and their anti-inflammatory activities. J. Org. Chem. 86 (16), 11177–11188. doi:10.1021/acs.joc.1c00369
Zhao, N., Li, Z. L., Li, D. H., Sun, Y. T., Shan, D. T., Bai, J., et al. (2015a). Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry 109, 133–139. doi:10.1016/j.phytochem.2014.10.020
Zhao, X. M., Cheng, Y. X., Liang, C. X., Guo, J., Liu, X. Q., Feng, W. H., et al. (2021b). Analysis of chemical constituents in Euodiae Fructus by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Traditional Med. Formulae 24, 113–126.
Zhao, Y., Zhan, J., Sun, C., Zhu, S., Zhai, Y., Dai, Y., et al. (2024). Sishen Wan enhances intestinal barrier function via regulating endoplasmic reticulum stress to improve mice with diarrheal irritable bowel syndrome. Phytomedicine 129, 155541. doi:10.1016/j.phymed.2024.155541
Zhao, Z., Gong, S., Wang, S., and Ma, C. (2015b). Effect and mechanism of evodiamine against ethanol-induced gastric ulcer in mice by suppressing Rho/NF-кB pathway. Int. Immunopharmacol. 28 (1), 588–595. doi:10.1016/j.intimp.2015.07.030
Zhou, L., Yao, G., Cao, Z., Xu, T., and Jin, R. (2013a). Mechanism of liver toxicity induced by Euodiae Fructus decoction in mice. Chin. J. Exp. Tradit. Med. Form. 19, 269–272.
Zhou, Q. j., Jin, R. M., and Yao, G. T. (2013b). Preliminary study on nephrocytes toxicity induced by four traditional Chinese medicine monomers in evodia rutaecarpa. Chin. J. Pharmacovigil. 10 (1), 1.
Zhou, S., Chan, E., Duan, W., Huang, M., and Chen, Y. Z. (2005). Drug bioactivation, covalent binding to target proteins and toxicity relevance. Drug Metab. Rev. 37 (1), 41–213. doi:10.1081/dmr-200028812
Zhou, X., Ren, F., Wei, H., Liu, L., Shen, T., Xu, S., et al. (2017a). Combination of berberine and evodiamine inhibits intestinal cholesterol absorption in high fat diet induced hyperlipidemic rats. Lipids Health Dis. 16 (1), 239. doi:10.1186/s12944-017-0628-x
Zhou, X., Wei, H., Shen, T., Wei, J. P., Ren, J. Y., and Ni, H. F. (2017b). Effects of berberine-evodiamine compatibility on expressions of intestinal ACAT2, ApoB48 and NPC1L1 in hypercholesterolemic rat. Chin. Tradit. Pat. Med. 39 (10), 1993–1999. doi:10.3969/j.issn.1001-1528.2017.10.001
Zhou, X., Xiang, Y., Li, D., Zhong, M., Hong, X., Gui, Y., et al. (2023). Limonin, a natural ERK2 agonist, protects against ischemic acute kidney injury. Int. J. Biol. Sci. 19 (9), 2860–2878. doi:10.7150/ijbs.82417
Zhou, X., Zhao, Y., Lei, P. H., Cai, Z. W., and Liu, H. (2010). Chromatographic fingerprint study on Evodia rutaecarpa (Juss.) Benth by HPLC/DAD/ESI-MSn technique. J. Sep. Sci. 33 (15), 2258–2265. doi:10.1002/jssc.201000035
Zhou, Y., Li, S. H., Jiang, R. W., Cai, M., Liu, X., Ding, L. S., et al. (2006). Quantitative analyses of indoloquinazoline alkaloids in Fructus Evodiae by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 20 (20), 3111–3118. doi:10.1002/rcm.2705
Zhu, B. Q., Zhao, L., Liu, Y., Jin, Y., Feng, J., Zhao, F. Y., et al. (2019). Induction of phosphatase shatterproof 2 by evodiamine suppresses the proliferation and invasion of human cholangiocarcinoma. Int. J. Biochem. and Cell Biol. 108, 98–110. doi:10.1016/j.biocel.2019.01.012
Zhu, Q. N., Zhang, D., Jin, T., Wu, Q., Liu, J., and Lu, Y. F. (2013). Rutaecarpine effects on expression of hepatic phase-1, phase-2 metabolism and transporter genes as a basis of herb–drug interactions. J. Ethnopharmacol. 147 (1), 215–219. doi:10.1016/j.jep.2013.03.005
Zhu, S. L., Qi, M., Chen, M. T., Lin, J. P., Huang, H. F., Deng, L. J., et al. (2024). A novel DDIT3 activator dehydroevodiamine effectively inhibits tumor growth and tumor cell stemness in pancreatic cancer. Phytomedicine 128, 155377. doi:10.1016/j.phymed.2024.155377
Zuo, G. Y., Yang, X. S., and Hao, X. J. (2000). Two new indole alkaloids from Evodia rutaecarpa. Chin. Chem. Lett. 11 (2), 127–128.
ACAT2 Acetyl-CoA Acetyltransferase 2
AD Alzheimer’s disease
AgRP Agouti-gene related protein
AKI Acute kidney injury
apoB-48 Apolipoprotein B-48
ARE Antioxidant response element
Aβ40 amyloid-β 40
CaMKII Ca2+/calmodulin-dependent protein kinase kinase II
CAR Constitutive androstane receptor
CCK Cholecystokinin
CGRP Calcitonin gene related peptide
CK Creatine kinase
CP Chinese Pharmacopoeia
CYP Cytochrome P450
DDIT3 DNA damage inducible transcript 3
DSS Dextran Sulfate Sodium Salt
EF Euodiae Fructus
EMT Epithelial-mesenchymal transition
eNOS Endothelial nitric oxide synthase
ER Euodia rutaecarpa (Juss.) Benth.
ERB ER var. bodinieri (Dode) Huang
ERO ER var. officinalis (Dode) Huang
hERG Human ether-a-go-go related gene
I/R Ischemia/reperfusion
LEF Medium flowers of EF
LPS Lipopolysaccharide
MAP Mitogen-activated protein
MEF Large flowers of EF
MMP Mitochondrial membrane potential
MPO Myeloperoxidase
MPT Mitochondrial permeability transition
mTOR Mammalian target of rapamycin
NFATc-1 Nuclear factor of activated T cells 1
Notch3 Notch receptor 3
NPC1L1 Niemann-Pick C1-like 1
NPY Neuropeptide Y
NRCMs Neonatal rat cardiomyocytes
NSCLC Non-small cell lung cancer
PBMC Peripheral blood mononuclear cells
RANKL Receptor activator for nuclear factor-κB ligand
RMs Reactive metabolites
ROS Reactive oxygen species
SEF Small flowers of EF
SOD Superoxide dismutase
TCM Traditional Chinese Medicine
UC Ulcerative colitis
Keywords: Euodiae Fructus, traditional uses, processing, phytochemistry, quality control, pharmacology, toxicology
Citation: Hao Y, Qi J, Huang X, Liu C and Liu Y (2025) Euodiae Fructus: a review of botany, application, processing, phytochemistry, quality control, pharmacology, and toxicology. Front. Pharmacol. 16:1509032. doi: 10.3389/fphar.2025.1509032
Received: 10 October 2024; Accepted: 14 January 2025;
Published: 29 January 2025.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Michael Heinrich, University College London, United KingdomCopyright © 2025 Hao, Qi, Huang, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Liu, dGNtbHlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.