
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pharmacol., 11 March 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1508276
 Fei Jing1,2
Fei Jing1,2 Wei Wang3
Wei Wang3 Jia Ke4
Jia Ke4 Tingrong Huang5
Tingrong Huang5 Bo Jiang6
Bo Jiang6 Qiwu Qiu7
Qiwu Qiu7 Jihan Huang8
Jihan Huang8 Songhua Zhan9
Songhua Zhan9 Wei Zhang10
Wei Zhang10 Hui Wu11
Hui Wu11 Wen Su12
Wen Su12 Jiawen Feng1
Jiawen Feng1 Yuan Peng1
Yuan Peng1 Zhimin Zhao1
Zhimin Zhao1 Feng Xing1*
Feng Xing1* Chenghai Liu1,13,14*
Chenghai Liu1,13,14*Background: Effective therapies for pulmonary fibrosis caused by coronavirus disease (COVID-19) and other etiologies are lacking. Our previous studies demonstrated that Fuzheng Huayu tablet (FZHY), a traditional Chinese medicine known for its anti-liver fibrotic properties, can improve lung function in patients with chronic obstructive pulmonary disease and attenuate bleomycin-induced pulmonary fibrosis in rats.
Purpose: This study aimed to evaluate the efficacy and safety of FZHY in post-COVID-19 pulmonary fibrosis.
Methods: A multi-center, randomized, double-blind, placebo-controlled clinical trial was conducted to evaluate the efficacy of a 24-week treatment with FZHY, combined with vitamin C and respiratory function rehabilitation, for treating pulmonary fibrosis in discharged convalescent COVID-19 patients. The primary outcome was the regression rate of pulmonary fibrosis assessed by the high-resolution computed tomography scores and lung function improvement (forced vital capacity [FVC], forced expiratory volume in one second [FEV1], and FEV1/FVC) after 24 weeks. Secondary outcomes included the 6-min walk distance, improvement in pulmonary inflammation, clinical symptoms, and quality of life.
Results: This study included 142 patients, who were randomized to the FZHY (n = 72) and placebo groups (n = 70). By week 24, the regression rates of pulmonary fibrosis in the FZHY and placebo groups were 71.2% and 49.2%, respectively (p = 0.01). Limited spirometry data revealed higher FEV1/FVC in the FZHY group than in the placebo group at week 8 ([87.7 ± 7.2] % vs. [82.7 ± 6.9] %; p = 0.018). The regression rates in pulmonary inflammation in the FZHY and placebo groups were 83.8% and 68.8%, respectively (p = 0.04). At week 4, the increase in 6-min walking distance was greater in the FZHY group than in the placebo group ([41.4 ± 64.1] m vs. [21.8 ± 50.3] m; p = 0.05). However, no significant differences were observed between the groups in the improvement rate of clinical symptoms, quality of life-BREF, patient health questionnaire-9, or generalized anxiety disorder-7 scores (p > 0.05). No drug-related adverse events were reported in the FZHY group.
Conclusion: FZHY attenuates post-COVID-19 pulmonary fibrosis, with good safety profiles.
Clinical Trial Registration: https://clinicaltrials.gov/study/NCT04279197, identifier NCT04279197.
Pulmonary fibrosis, a progressive interstitial lung disorder characterized by the proliferation of lung fibroblasts, extracellular matrix deposition, and infiltration of airway inflammatory cells, is a common complication of numerous lung diseases that can induce restrictive ventilatory dysfunction and impaired diffusion function (Wuyts et al., 2013). Thoracic imaging findings reveal various degrees of cord- or grid-like shadowing.
Viral infections, such as severe acute respiratory syndrome (SARS) and cytomegalovirus infections, contribute to pulmonary fibrosis (Venkataraman et al., 2017). The coronavirus disease-19 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in late 2019 has affected over 760 million people worldwide. Some COVID-19 patients experience varying degrees of sequelae during recovery (Zhao et al., 2020). Wang evaluated the pathological findings in the lungs of 12 deceased COVID-19 patients, which exhibited diffuse alveolar damage, interstitial fibrosis, and exudative inflammation (Liu et al., 2020). Pulmonary dysfunction and abnormal imaging findings, such as ground-glass opacities and bands of parenchymal fibrosis, were observed in convalescent COVID-19 patients (Shaw et al., 2021). Additionally, pulmonary fibrosis was reported in 4.9% of 287 convalescent COVID-19 patients (Kamal et al., 2021).
Therefore, further investigation on post-COVID-19 pulmonary fibrosis is warranted. Currently, only pirfenidone and nintedanib have been approved for the treatment of idiopathic pulmonary fibrosis (Martinez et al., 2017). Pirfenidone and nintedanib delay the progression of pulmonary fibrosis but do not significantly improve the overall survival and prognosis. Moreover, both drugs are expensive and associated with substantial side effects such as gastrointestinal reactions and liver dysfunction. (Cottin and Maher, 2015; King et al., 2014; Flaherty et al., 2018). The efficacy of pirfenidone and nintedanib for treating pulmonary fibrosis in patients with COVID-19 is still under investigation.
Pulmonary fibrosis is a complex condition involving multiple targets that is difficult to treat with a single agent. Traditional Chinese Medicine (TCM), with its multi-component, multi-targeted approach, and extensive experience in treating chronic lung diseases, offers a practical approach for developing anti-pulmonary fibrosis drugs to meet the emerging clinical needs. Fuzheng Huayu tablet (FZHY), a plant-based formulation composed of six herbs, has been approved by China’s National Medical Products Administration in 2003 for the treatment of liver fibrosis. FZHY has good quality control with few adverse reactions. FZHY can reverse hepatitis B-induced liver fibrosis by attenuating hepatocyte inflammation, inhibiting hepatic stellate cell activation, and regulating hepatic matrix metabolism (Wang et al., 2018; Liu et al., 2009). A clinical trial showed that FZHY improved pulmonary function in Chinese patients with chronic obstructive pulmonary disease (COPD) patients. Moreover, FZHY attenuates pulmonary inflammation and regresses fibrosis in bleomycin (BLM)-induced pulmonary fibrosis in rats (Tan et al., 2007). Given the potential similarities in the pathological mechanisms between pulmonary fibrosis in patients with COVID-19 and fibrotic disorders in other organs, we conducted this clinical trial in which patients with post-inflammatory pulmonary fibrosis due to COVID-19 in the recovery period were randomized to receive either FZHY or a placebo. Given that COVID-19 is a novel disease and the lack of specific treatments for post-inflammatory pulmonary fibrosis, a placebo parallel-controlled study was performed. This study aimed to evaluate the efficacy and safety of FZHY in treating pulmonary fibrosis in convalescent patients with COVID-19.
This multi-center, double-blind, randomized, placebo-controlled clinical trial was conducted at five centers in China, including Hubei Provincial Hospital of Traditional Chinese Medicine, Huangshi Hospital of Traditional Chinese Medicine, Wuhan Third Hospital, Jingmen First People’s Hospital, Wuhan Integrated TCM & Western Medicine Hospital. Eligible patients were recruited and randomly assigned to the FZHY group and the placebo group in a ratio of 1:1 using the interactive web response system (IWRS). This study was approved by the ethics committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2020-798-05-01) and the ethics committee of each subcenter. The protocol (Jing et al., 2021) was designed in accordance with the guidelines outlined in Good Clinical Practice and the Declaration of Helsinki. All patients have signed informed consent forms. This study adopts the method of competitive enrollment.
The diagnostic criteria for suspected and diagnosed COVID-19 cases were based on the Diagnosis and Treatment Guideline for COVID-19 (Trial seventh Edition) released by the National Health Commission of the People’s Republic of China (Diagnosis and Treatment Protocol for COVID-19, 2020). The inclusion criteria were as follows: 1) discharged patients with a confirmed diagnosis of COVID-19; 2) two consecutive negative SARS-CoV-2 RNA tests in respiratory or blood samples by real-time fluorescent polymerase chain reaction; 3) evidence of pulmonary fibrosis on computed tomography (CT) performed within 7 days; 4) age 18–70 years. The exclusion criteria were as follows: 1) patients who had undergone lung surgery, such as lung transplantation and lung resection, which affects lung function; 2) dependence on mechanical ventilation; 3) presence of chronic lung diseases that impair lung function, such as COPD; 4) conditions affecting cardiac function, such as pulmonary hypertension, heart failure, and pacemaker installation; 5) diseases affecting survival; 6) resting heart rate >120 beats/min; 7) systolic blood pressure >180 mmHg and diastolic blood pressure >100 mmHg; 8) history of myocardial infarction or unstable angina within the previous month; 9) severe obesity (body mass index [BMI] > 30 kg/m2); 10) allergy to pharmaceutical ingredients involved in the treatment regimen; 11) pregnant or breastfeeding women; 12) inability to complete the efficacy evaluation questionnaires; 13) patients enrolled in other clinical trials within 1 month prior to enrolment in this trial or simultaneous participation in other clinical trials; 14) poor compliance. Informed consent was obtained from all patients.
The FZHY group received FZHY tablets (0.4 g/tablet, Shanghai Huanghai Pharmaceutical Co., Ltd. Lot number S200301, expiry date 2023/02) during the treatment period, and 1.6 g (4 tablets) was administered orally approximately 30 min after meals three times/day for 24 consecutive weeks.
FZHY tablet refers to the herbal extraction, which has been licensed (Identifier No. Z20050546) by Chinese National Medical Products Administration and comprises Danshen (Salvia miltorrhiza Bunge [Lamiaceae; radix]), Chongcao (artificialis fermentation cordyceps; mycelia), Taoren (Prunus persica (L.) Batsch [Rosaceae; fruit]), Jiaogulan (Gynostemma pentaphyllum (Thunb.) Makino [Cucurbitaceae; whole herb]), Songhuafen (Pinus massoniana Lamb. [Pinaceae; pollen]), and Wuweizi (Schisandra chinensis (Turcz.) Baill. [Schisandraceae; fruit]). The species of ingredients are covered in Chinese pharmacopoeia. All the raw materials for FZHY come from the medicinal material planting and harvesting bases according to Good Agricultural and Collection Practices requirement in definite places, and identified for their origin, morphological, microscopic, physical, chemical characteristics, as well as the DNA barcode identification in order to guarantee the authenticity and quality of medicinal materials.
The manufacturing process is conducted as follows: For the preparation of FZHY extraction, 666g of Danshen, 500g of Jiaogulan, 334g of Chongcao, 166g of Taoren, 166g of Songhuafen and 166g of Wuweizi are weighed up. Danshen, Taoren and Jiaogulan are first mixed with appropriate amount of water to decoct twice, 2 h for the first time and 1.5 h for the second time. The decoctions are then combined and left to stand for 24h to allow the supernatant to concentrate until a relative density of about 1.20 (50°C–55°C). This is then cooled, and alcohol (95%) is added while slowly shaking up until the alcohol content reaches 70%. It is then cooled again, filtrated and concentrated until the relative density reaches 1.3–1.4 at 50°C–55°C. Drying takes place by decompression to obtain the dry extract. Then, Chongcao and Wuweizi are weighed up, reflux extraction with alcohol twice, 2 h for the first time and 1.5 h at the second time. The extract solution is combined, filtrated and concentrated until the relative density reaches 1.3–1.4 at 50°C–55°C. The alcohol is recovered and dried by decompression to get the dry extract. Similarly, Songhuafen is soaked in 50% alcohol at 40°C twice, 4 h for the first time and 2 h for the second time. The extract solution is combined, filtrated and concentrated until the relative density reaches 1.3–1.4 at 50°C–55°C The alcohol is recovered, dried and decompressed to obtain the dry extract. Those three dry extracts are mixed and dried as the FZHY extract powder to reserve. Then pharmaceutical excipients are added to the FZHY extract powder and the tablets are prepared directly by compression. It can be compressed to 1000 tablets (0.4g each).
To control the quality of the FZHY extracts, the fingerprint spectrum was established using high performance liquid chromatography (HPLC) method. Assay validation was performed according to the United States Food and Drug Administration bio-analytical method validation guideline. The contents of adenosine and danshensu were 2.5 mg/g and 8.04 mg/g in the extracts respectively, according to quality inspection report from the Shanghai Sundise Chinese Medicine Technology Development Co., Ltd (Shanghai, China). The formulation, standard manufacturing process, chemical component content, and multicomponent assay (fingerprinting) of the FZHY tablets are available in the Supplementary Material (Supplementary Table S1; Supplementary Figure S1; Supplementary Table S2; Supplementary Figure S2).
The placebo group received four placebo tablets 3 times a day orally for 24 weeks. The placebo, which was also manufactured by Shanghai Huanghai Pharmaceutical Co., Ltd. (Lot number S200202, expiry date 2023/01), consisted of a mixture of sucrose, starch, and sodium carboxymethyl starch to mimic the tablet shape of FZHY. Additionally, this mixture contained 1% FZHY to impart odor similar to that of the FZHY tablet.
All patients received basic treatment of respiratory function rehabilitation training according to the “COVID-19 patient rehabilitation program” (COVID-19, 2020) issued by the National Health Commission of the People’s Republic of China along with vitamin C tablets (0.2 g/dose, three times/day, orally, Shanghai Sine Tianping Pharmaceutical Co., Ltd.).
The primary outcomes of the trial were the regression rate of pulmonary fibrosis after 24 weeks of treatment, as assessed by HRCT scores, and the improvement in pulmonary function after 24 weeks, as measured by spirometry (FVC, FEV1, and FEV1/FVC). The HRCT scores were independently assessed by two radiologists specializing in chest imaging and one respiratory physician. Disagreements were resolved through discussion.
Chest CT scans were performed using a spiral CT scanner with 64 rows or more. Scan parameters: tube voltage, 120 kV; smart tube current. Layer thickness: 1 mm. Reconstruction interlayer spacing: 1 mm. Matrix: 512 × 512. Method: Patients were positioned supine with their arms raised, instructed to hold their breath, and the scans were acquired at the end of deep inspiration. An inorganic shelf tilt was used, and the scan orientation was recommended to be 1 cm below the diaphragm, extending to the lung apex. No more than one tomogram was allowed to be missing between layers, and a series allowed up to three missing tomograms. Storage format: nondestructive compression, standard DICOM. For evaluation, the bilateral lobes were divided into six zones: the posterior and anterior segments of the upper lobe tip of the left lung were combined into one zone (referred to as the “upper left” zone), and the lingual segment of the upper lobe of the left lung was combined into one zone (the “middle left” zone). The lower lobe of the left lung and the upper, middle, and lower lobes of the right lung were designated as individual zones the “lower left,” “upper right,” “middle right,” and “lower right” zones. Each lung region was scored semi-quantitatively for inflammation (mainly lesions characterized by ground-glass opacities, solid lung changes, or a mixture of both) and fibrosis (grid-like or cord-like changes). Scores were assigned to the nearest 10% or within 10%, based on the extent of lesion involvement as follows: no inflammatory or fibrotic lesions within the lung region were scored as 0% and involvement of all lung regions were scored as 100%. The mean values for the percentages of lung inflammatory and fibrotic involvement were calculated, with higher mean scores indicating more severe lesions (Xaubet et al., 1998). A decrease in these scores was considered an improvement.
Secondary outcomes included the 6-min walk distance and improvements in pulmonary inflammation, clinical symptoms, and quality of life after 24 weeks of treatment. The 6-min walk distance was conducted and recorded by trained investigators. The regression rate of pulmonary inflammation was determined by the HRCT scores, which were assessed by the same radiologists and respiratory physician. Clinical symptoms, such as shortness of breath, cough, fatigue, insomnia, sweating, poor appetite, and diarrhea, were reported subjectively by the patients. Quality of life evaluations were obtained using questionnaire forms, including the quality of life-BREF (QOL-BREF) (WHO Quality of Life-BREF, 2020), patient health questionnaire-9 (PHQ-9) (Kroenke et al., 2001), and generalized anxiety disorder-7 (GAD-7) scores (Spitzer et al., 2006). During the treatment period, six follow-up visits were scheduled, once every 4 weeks.
The trial implemented appropriate measures to monitor adverse events (AEs), including the observation of vital signs, laboratory tests, and recording of concomitant medications. All AEs, regardless of their severity, were documented to assess the safety of FZHY.
Because COVID-19 is an emergent disease, the available references were limited at the time of protocol design; therefore, we used a placebo-controlled parallel trial design with a planned sample size of 160 patients (80 in each group).
A dynamic random approach using minimization was used in this study. After obtaining informed consent and completion of screening assessments to confirm the eligibility criteria, random numbers and medications were assigned in a 1:1 ratio using the IWRS. The randomization table generated by the IWRS was sealed in opaque envelopes.
All the investigators, patients, data managers, and data supervisors were blinded to the study protocols. The patients were automatically assigned unique random numbers through the IWRS, which remained consistent until the end of the trial. During the trial, researchers used these randomized numbers to distribute the corresponding drugs to the patients. Data managers, data supervisors, and researchers recorded patient data solely based on the random numbers.
Measurement data that followed a normal distribution were presented as the mean and standard deviation (SD), and non-normally distributed data were described by median and interquartile range (IQR). Comparisons were performed using either t-tests or rank sum tests, depending on the normality of the distribution. Count data were expressed as the number (percentage) of cases, and the chi-square test or Fisher’s exact test was used for comparison. All statistical analyses were performed using the SAS software version 9.4 (SAS Institute, Cary. NC, USA). Two-sided tests were used for all statistical tests, and a P-value of ≤0.05 was considered statistically significant.
The datasets included the full analysis set (FAS), per-protocol set (PPS), and safety analysis set (SS). For the primary efficacy measure, if data were missing, the previous outcome carried forward method was used, according to the intention-to-treat analysis (ITT analysis), with no carry forward for the secondary efficacy measures. The FAS was used as the primary dataset. Patients lost to follow-up were classified as ineffective.
This study initially enrolled 177 eligible patients between 18 March 2020 and 17 March 2021. After excluding 35 patients (patient withdrawal, radiologic normality on chest HRCT, or disorders affecting cardiac function), 142 were included in the FAS (72 in the FZHY group and 70 in the placebo group). Additionally, six patients in the FZHY group and seven patients in the placebo group dropped out and were excluded from the PPS (Figure 1). The distribution of participants in each center is listed in Supplementary Table S3.
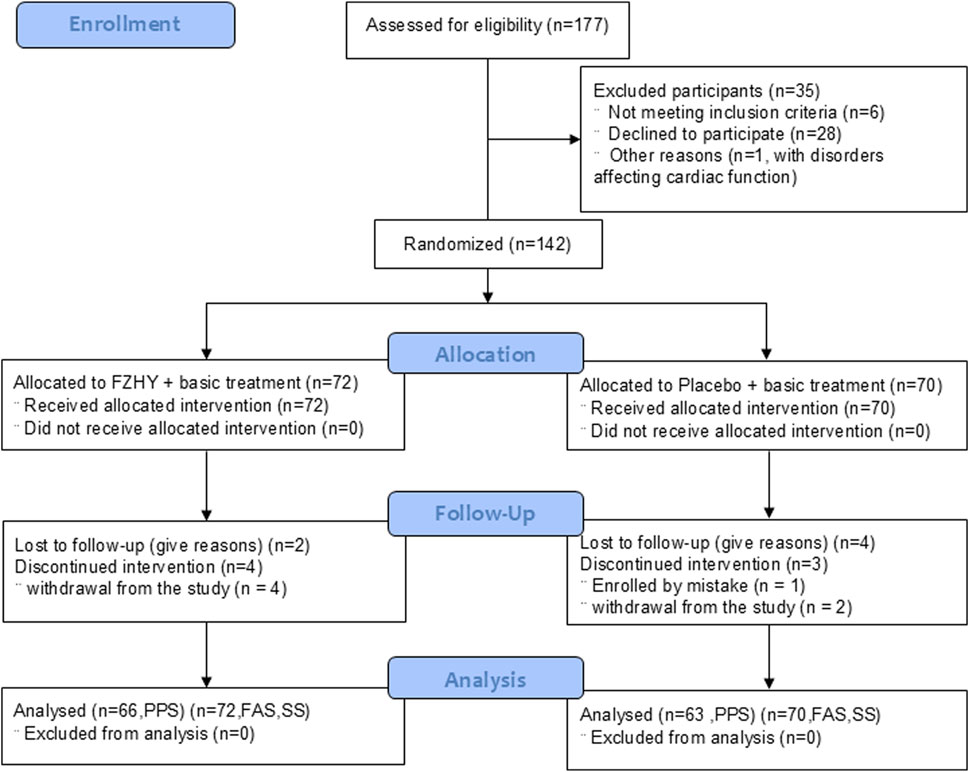
Figure 1. Flowchart FZHY: Fuzheng Huayu; PPS: per protocol set; FAS: full analysis set; SS, safety analysis set.
Clinical characteristics and baseline data showed that most patients were aged ˃ 45 years, with ˂ 50% of men in the total sample. Of the 142 patients with pulmonary fibrosis, 132 showed unabsorbed pulmonary inflammation. No significant differences in age, sex, disease duration (days from symptom onset to enrolment), clinical classification, comorbidities, 6-min walk distance, quality-of-life evaluations, and clinical symptoms were observed between the FZHY and placebo groups (p > 0.05, Table 1). Additionally, concomitant medications did not differ significantly between the two groups (Supplementary Table S4).
At baseline, HRCT scores for pulmonary fibrosis were comparable between the FZHY and placebo groups. At week 24, both groups exhibited a decrease in HRCT scores; however, the reduction was more significant in the FZHY group (−1.250 ± 1.780) than in the placebo group (−0.450 ± 1.354, p = 0.005, Supplementary Tables S5, S6). After 24 weeks of treatment, the regression rate of pulmonary fibrosis was higher in the FZHY group than in the placebo group (71.2% [47/66] vs 49.2% [31/63]; p = 0.011) (Table 2). Figure 2 shows the chest HRCT findings of the patients in the FZHY and placebo groups.
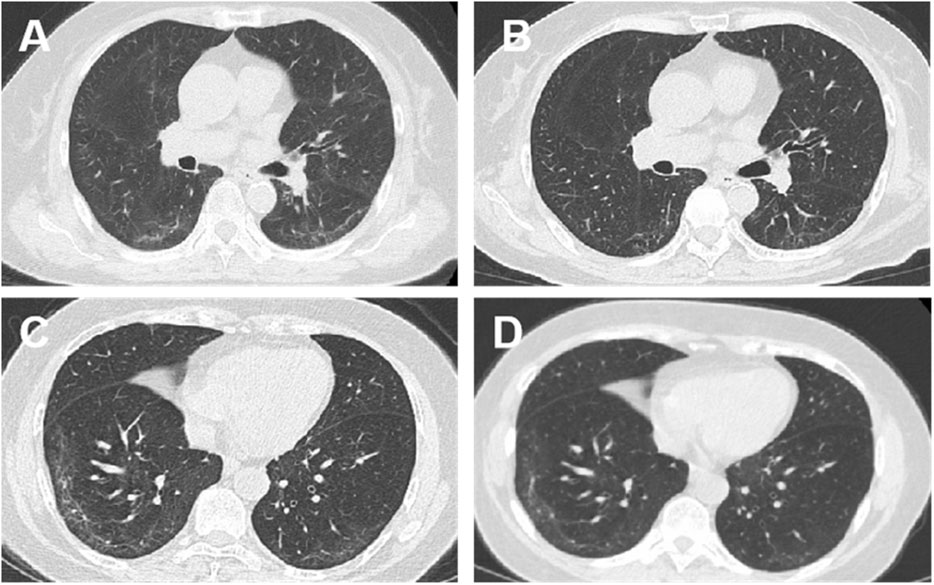
Figure 2. Chest computed tomography (CT) manifestations at enrolment and after intervention: CT findings of patients in the FZHY group and the placebo group. (A) Bilateral pulmonary infiltrates (more prominent in the right lower lobe) in a 70-year-old woman at initiation of FZHY treatment. (B) Marked absorption of bilateral pulmonary infiltrates 6 months after treatment with FZHY. (C) Bilateral pulmonary infiltrates in a 67-year-old woman at the initiation of placebo treatment. (D) No absorption of infiltrations in the bilateral lobes 6 months after the placebo treatment.
A spirometer was used in a few patients at only one center, while diffusing capacity assessments were not performed to minimize the risk of cross-infection during emergencies. At baseline, both FVC and FEV1 were lower in the FZHY group than in the placebo group (FZHY group, n = 9; placebo group, n = 11). After 24 weeks of treatment, no significant difference in FVC or FEV1 was observed between the two groups (FZHY group, n = 21; placebo group, n = 22). However, the FEV1/FVC ratio was comparable between the FZHY and placebo groups at baseline. At week 8, a significant difference in FEV1/FVC was observed between the FZHY group ([87.7 ± 7.2] %, n = 22) and the placebo group ([82.7 ± 6.9] %, n = 25) (p = 0.018). These results suggest that FZHY may promote the recovery of pulmonary ventilation during the early stages of post-COVID-19 pulmonary fibrosis (Figure 3).
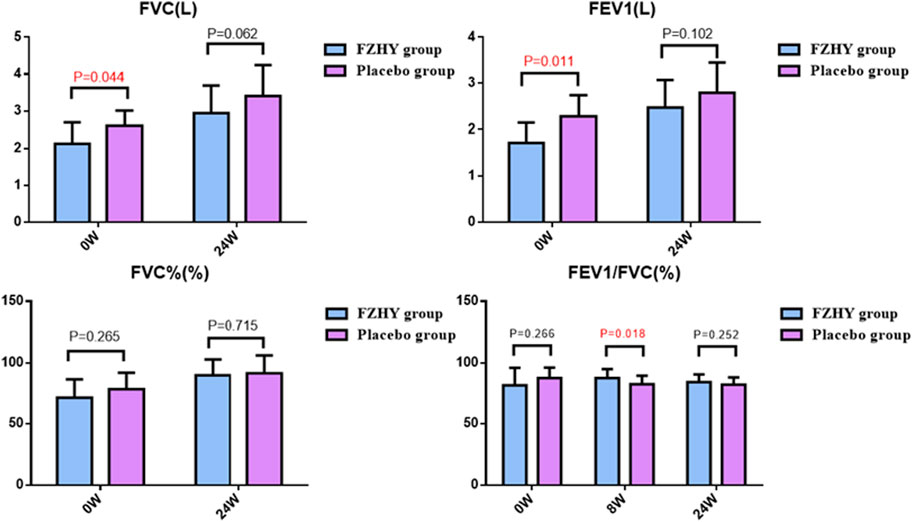
Figure 3. Pulmonary function manifestations at enrolment and after treatment. FVC: Forced vital capacity; FEV1: Forced expiratory volume in one second.
After 24 weeks of treatment, both the FZHY and placebo groups demonstrated a trend toward improved performance in the 6-min walk test; however, this difference was not statistically significant when comparing the two groups at each visit (Figure 4A). However, the increase in 6-min walk distance at week four was statistically significant, with the FZHY group showing a substantial increase than that of the placebo group ([41.4 ± 64.1] m vs [21.8 ± 50.3] m, p = 0.05; Figure 4B), suggesting that FZHY may promote recovery of lung function in the early stage of post-COVID-19 pulmonary fibrosis.
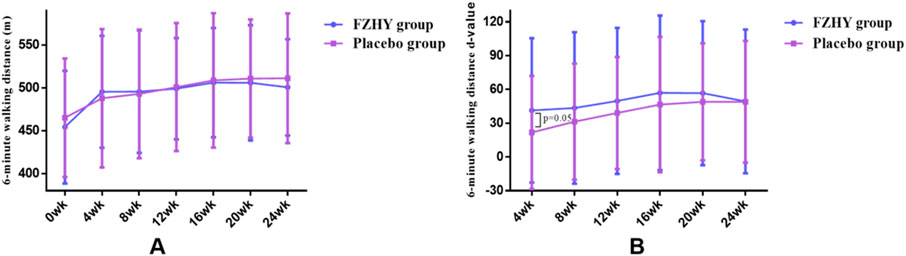
Figure 4. (A) Dynamic changes in 6-min walking distance in both groups. (B) Six-minute walk distance d-value in both groups.
After 24 weeks of treatment, the rate of improvement in lung inflammation was significantly higher in the FZHY group than in the placebo group (83.8% vs 68.8%, p = 0.041, Table 3).
No significant difference was observed in the rate of disappearance of clinical symptoms between the two groups after 24 weeks of treatment (Table 4). However, some data was missing, which might have affected the accuracy of the results.
After 24 weeks of treatment, no significant differences in QOL were observed between the two groups (Table 5).
AEs such as abnormal liver function tests, abnormal electrocardiogram findings, and increased urinary leukocytes were observed in both the FZHY and placebo groups. In addition, renal dysfunction was reported in two patients, and dyslipidemia and hyperglycemia were reported in one patient each in the FZHY group. In the placebo group, increased C-reactive protein levels, leukocytosis, and back pain were reported in one patient each. However, it was later confirmed that none of these AEs were related to the trial. No significant differences in AEs were observed between the two groups (p > 0.05, Table 6). Notably, no serious AEs were reported in either group.
Our findings indicated that after 24 weeks of treatment, the regression rate of pulmonary fibrosis was 71.2% and 49.2% in the FZHY and placebo groups, respectively (p = 0.011). Limited spirometry data revealed FEV1/FVC was higher in the FZHY group than in the control group (87.7 ± 7.2 vs 82.7 ± 6.9) after 8 weeks of treatment. The improvement in pulmonary inflammation was 83.8% in the FZHY group and 68.8% in the placebo group (p < 0.05). The increase in 6-min walking distance was higher in the FZHY group than in the control group (41.4 ± 64.1 vs 21.8 ± 50.3) at week 4, with no difference thereafter. No drug-related AEs were observed in the FZHY group.
We defined convalescence in COVID-19 as the stage when the patient meets the discharge criteria for clinical cure, yet many clinical symptoms persisted. During this phase, the patient’s functional abilities may not be fully restored, and they may experience immune system disorders, along with other challenges, including respiratory issues, physical and psychological problems, difficulties in daily living, and limitations in social participation (Wen et al., 2020). Our findings indicated that partially recovered COVID-19 patients still exhibited associated abnormalities on chest CT imaging, including ground-glass opacities, grid shadowing, and fibrous banding during the recovery period. Notably, FZHY promoted the absorption of fibrotic lesions after inflammation during the recovery period in COVID-19 patients.
Radiographic findings indicated improvement in pulmonary fibrosis after 24 weeks, as assessed by manual semi-quantitative scoring of chest CT in convalescent COVID-19 patients, with an improvement rate of 71.2% in the FZHY group and 49.2% in the placebo group. Furthermore, lung inflammation was significantly improved after 24 weeks, with an improvement rate of 83.8% and 68.8% in the FZHY and placebo groups, respectively. This study was conducted at the time of epidemic ravaging, when pulmonary function testing was not performed on patients in most centers to avoid cross-infection. Nevertheless, the limited spirometry results showed a possible effect of FZHY on improving lung function in the early stages of recovery.
In addition to causing pulmonary fibrosis, COVID-19 can lead to various functional impairments, such as fatigue, insomnia, and anxiety, in patients during the recovery phase (Huang et al., 2021). Xiong et al. (2021) observed that COVID-19 patients experienced increased resting heart rate, palpitations, elevated blood pressure, and other psychiatric symptoms such as sleep disturbances, anxiety, irritability, low self-esteem, and depression 3 months after hospital discharge. Carda et al. (2020) found that COVID-19 patients had sequelae such as cognitive impairment, peripheral nervous system damage, and psychiatric problems. Li et al. (2020) found that SARS-CoV-2 was still present in the semen of some COVID-19 patients who had achieved clinical recovery. Additionally, this study reported that COVID-19 patients often continued to experience clinical manifestations such as cough, chest tightness, and fatigue after discharge, along with emotional distress, including anxiety and depression. Regrettably, in this study, compared with the placebo group, the FZHY group failed to effectively improve these symptoms. FZHY is mainly used to treat fibrotic diseases, particularly liver fibrosis, and is not specific for symptom manifestations in the respiratory system, which may explain its limited effect on symptoms such as cough and chest tightness. Conversely, the limited number of observations for some variables may have constrained our ability to detect significant differences, potentially leading to selection biases. Despite these limitations, our study highlighted that QOL-BREF, PHQ-9, and GAD-7 scores significantly improved in both placebo and FZHY groups after 24 weeks of treatment.
Theoretical and research findings demonstrate the potential of FZHY in improving post-inflammatory pulmonary fibrosis. Our previous results have shown that this formula regulates immune and inflammatory responses (Chen et al., 2019; Zhang et al., 2020). Patients with COVID-19 often present with numerous underlying conditions and may themselves have an immune imbalance (Huang et al., 2020; Biswas, 2016). Notably, most patients included in this study were middle-aged or older, and more than 70% of patients had concomitant underlying diseases. Multiple active ingredients in FZHY exhibit anti-inflammatory and anti-pulmonary fibrotic effects. The most important active component of Danshen is Tanshinone ⅡA. Feng et al. (2020) found that Tanshinone IIA can partially ameliorate fibrosis in silico by inhibiting the activation of the TGF-β/Smad signaling pathway. He et al. (2015) found that Tanshinone IIA reduced inflammatory cell infiltration, proinflammatory cytokine release, and collagen deposition in BLM-induced rats. Furthermore, An et al. (2019) found that Tanshinone IIA activated nuclear factor erythroid 2-related factor 2 by regulating the redox balance and glutaminolysis, thereby inhibiting pulmonary fibrosis. In addition, Chen et al. (2012) demonstrated the potential of Cordyceps to prevent and treat fibrosis by decreasing inflammatory cell infiltration, fibroblast and collagen deposition, and restoring the MMP-9/TIMP-1 imbalance in rats with pulmonary fibrosis. Xu et al. (2011) found that Cordyceps sinensis decreased CTGF, hydroxyproline, and TGF-β1 expression in pulmonary fibrosis rats, with enhanced antifibrotic effects when combined with glucocorticoids. Zhai et al. (2014) found that ephedrine combined with Schisandra mitigated the development of alveolar inflammation and fibrosis in rats with BLM-induced pulmonary fibrosis. Schisandra exerted anti-fibrotic effects by inhibiting M2 macrophage polarization in BLM-induced pulmonary fibrosis in rats (Guo et al., 2020).
This study had several limitations. First, the majority of patients in this study were enrolled between April and August 2020. During this period, none of the participants had received the COVID-19 vaccine, and the predominant strain was the wild-type SARS-CoV-2, which differs from subsequent variants. Secondly, because COVID-19 is an emerging disease, the literature was limited for sample size determination, and the sample size was not adjusted since the wile type COVID−19 epidemic was quickly diminished during the implementation of this study. Third, the chest CT scoring in this study used an artificial semi-quantitative method, which may introduce subjective bias in the results. Additionally, spirometry was performed in only a limited number of patients to avoid cross-infection, and tests for diffusion indicators were not available in the study for assessing the anti-lung fibrotic efficacy.
Given that pulmonary fibrosis is a chronic and progressive condition with multiple etiologies, it is imperative to conduct multicenter trials with larger sample sizes and extended treatment durations to reconfirm the efficacy of FZHY on pulmonary fibrosis caused by various etiologies. Nevertheless, our current data warrants further investigation for FZHY on the efficacy with different etiologies and action mechanisms in depth against pulmonary fibrosis.
FZHY tablets attenuate pulmonary fibrosis and inflammation in convalescent patients with COVID-19, with promising safety profiles.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the ethics committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2020-798-05-01) and the ethics committee of each subcenter. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FJ: Writing–original draft, Data curation. WW: Data curation, Investigation, Writing–review and editing. JK: Data curation, Investigation, Writing–review and editing. TH: Data curation, Investigation, Writing–review and editing. BJ: Data curation, Investigation, Writing–review and editing. QQ: Data curation, Investigation, Writing–review and editing. JH: Formal Analysis, Methodology, Data curation, Writing–review and editing. SZ: Investigation, Methodology, Writing–review and editing. WZ: Investigation, Methodology, Writing–review and editing. HW: Data curation, Investigation, Writing–review and editing. WS: Project administration, Methodology, Writing–review and editing. JF: Writing–review and editing, Data curation. YP: Writing–review and editing. ZZ: Project administration, Writing–review and editing, Supervision. FX: Funding acquisition, Project administration, Supervision, Validation, Writing–review and editing, Data curation. CL: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2020YFC0845000), the Shanghai Science and Technology Innovation Action Plan for Biomedical Science and Technology Special Project (21S21900300), the Research and Development Project of Shanghai on the Prevention and Treatment of COVID-19 with Traditional Chinese Medicine (2020 XGKY 11), and the Shanghai Key Specialty of Traditional Chinese Clinical Medicine (shslczdzk01201). The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
We would like to acknowledge all the staff and patients who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1508276/full#supplementary-material
BLM, bleomycin; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus Disease 2019; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; FAS, full analysis set; FZHY, Fuzheng Huayu formula; HRCT, high-resolution computed tomography; IWRS, interactive web randomization system; PPS, per protocol set; RT-PCR, real-time fluorescence quantitative polymerase chain reaction; SARS, severe acute respiratory syndrome.
An, L., Peng, L., Sun, N., Yang, Y., Zhang, X., Li, B., et al. (2019). Tanshinone IIA activates nuclear factor-erythroid 2-related factor 2 to restrain pulmonary fibrosis via regulation of redox homeostasis and glutaminolysis. Antioxid. Redox Signal. 30 (15), 1831–1848. doi:10.1089/ars.2018.7569
Biswas, S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 5698931. doi:10.1155/2016/5698931
Carda, S., Invernizzi, M., Bavikatte, G., Bensmaïl, D., Bianchi, F., Deltombe, T., et al. (2020). COVID-19 pandemic. What should Physical and Rehabilitation Medicine specialists do? A clinician's perspective. Eur. J. Phys. Rehab. Med 56 (4), 515–524. doi:10.23736/S1973-9087.20.06317-0
Chen, J., Hu, Y., Chen, L., Liu, W., Mu, Y., and Liu, P. (2019). The effect and mechanisms of Fuzheng Huayu formula against chronic liver diseases. Biomed. Pharmacother. 114, 108846. doi:10.1016/j.biopha.2019.108846
Chen, M., Cheung, F. W., Chan, M. H., Hui, P. K., Ip, S. P., Ling, Y. H., et al. (2012). Protective roles of Cordyceps on lung fibrosis in cellular and rat models. J. Ethnopharmacol. 143 (2), 448–454. doi:10.1016/j.jep.2012.06.033
Cottin, V., and Maher, T. (2015). Long-term clinical and real-world experience with pirfenidone in the treatment of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 24 (135), 58–64. doi:10.1183/09059180.00011514
COVID-19 (2020). COVID-19 patient rehabilitation program. Available at: https://www.gov.cn/zhengce/zhengceku/2020-03/05/content_5487160.htm (Accessed March 30, 2020).
Diagnosis and Treatment Protocol for COVID-19 (2020). Available at: http://en.nhc.gov.cn/2020-03/29/c_78469.htm (Accessed March 30, 2020).
Feng, F., Li, N., Cheng, P., Zhang, H., Wang, H., Wang, Y., et al. (2020). Tanshinone IIA attenuates silica-induced pulmonary fibrosis via inhibition of TGF-β1-Smad signaling pathway. Biomed. Pharmacother. 121, 109586. doi:10.1016/j.biopha.2019.109586
Flaherty, K. R., Fell, C. D., Huggins, J. T., Nunes, H., Sussman, R., Valenzuela, C., et al. (2018). Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur. Respir. J. 52 (2), 1800230. doi:10.1183/13993003.00230-2018
Guo, Z., Li, S., Zhang, N., Kang, Q., and Zhai, H. (2020). Schisandra inhibit bleomycin-induced idiopathic pulmonary fibrosis in rats via suppressing M2 macrophage polarization. Biomed. Res. Int. 2020, 5137349. doi:10.1155/2020/5137349
He, H., Tang, H., Gao, L., Wu, Y., Feng, Z., Lin, H., et al. (2015). Tanshinone IIA attenuates bleomycin-induced pulmonary fibrosis in rats. Mol. Med. Rep. 11 (6), 4190–4196. doi:10.3892/mmr.2015.3333
Huang, C., Huang, L., Wang, Y., Li, X., Ren, L., Gu, X., et al. (2021). 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397 (10270), 220–232. doi:10.1016/S0140-6736(20)32656-8
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi:10.1016/S0140-6736(20)30183-5
Jing, F., Fan, H., Zhao, Z., Xing, F., He, Y., and Liu, C. (2021). The efficacy of treating pulmonary fibrosis and pulmonary function injury in COVID-19 with Fuzheng Huayu tablets: study protocol for a multicenter randomized controlled trial. J. Dev. Drugs 10, 205. doi:10.35248/2329-6631.21.10.205
Kamal, M., Abo Omirah, M., Hussein, A., and Saeed, H. (2021). Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 75 (3), e13746. doi:10.1111/ijcp.13746
King, T. E., Bradford, W. Z., Castro-Bernardini, S., Fagan, E. A., Glaspole, I., Glassberg, M. K., et al. (2014). A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med. 370 (22), 2083–2092. doi:10.1056/NEJMoa1402582
Kroenke, K., Spitzer, R. L., and Williams, J. B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16 (9), 606–613. doi:10.1046/j.1525-1497.2001.016009606.x
Li, D., Jin, M., Bao, P., Zhao, W., and Zhang, S. (2020). Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw. Open 3 (5), e208292. doi:10.1001/jamanetworkopen.2020.8292
Liu, C., Hu, Y., Xu, L., Liu, C., and Liu, P. (2009). Effect of Fuzheng Huayu formula and its actions against liver fibrosis. Chin. Med. 4, 12. doi:10.1186/1749-8546-4-12
Liu, Q., Shi, Y., Cai, J., Duan, Y., Wang, R., Zhang, H., et al. (2020). Pathological changes in the lungs and lymphatic organs of 12 COVID-19 autopsy cases. Natl. Sci. Rev. 7 (12), 1868–1878. doi:10.1093/nsr/nwaa247
Martinez, F. J., Collard, H. R., Pardo, A., Raghu, G., Richeldi, L., Selman, M., et al. (2017). Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074. doi:10.1038/nrdp.2017.74
Shaw, B., Daskareh, M., and Gholamrezanezhad, A. (2021). The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol. Med. 126 (1), 40–46. doi:10.1007/s11547-020-01295-8
Spitzer, R. L., Kroenke, K., Williams, J. B., and Löwe, B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 166 (10), 1092–1097. doi:10.1001/archinte.166.10.1092
Tan, S. Z., Liu, C. H., Zhang, W., Lu, X., Ye, W. C., Cai, Z. Z., et al. (2007). Effects of Fuzheng Huayu recipe on MMP-2 activity and type IV collagen expression at fibrotic lung. Zhongguo Zhong Yao Za Zhi 32 (9), 835–839.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Venkataraman, T., Coleman, C. M., and Frieman, M. B. (2017). Overactive epidermal growth factor receptor signaling leads to increased fibrosis after severe acute respiratory syndrome coronavirus infection. J. Virol. 91 (12), 001822-17–e217. doi:10.1128/JVI.00182-17
Wang, T., Zhou, X., Liu, H., Wang, J., Zhang, P., Zhu, Y., et al. (2018). Fuzheng Huayu capsule as an adjuvant treatment for HBV-related cirrhosis: a systematic review and meta-analysis. Phytother. Res. 32 (5), 757–768. doi:10.1002/ptr.6009
Wen, W., Su, W., Tang, H., Le, W., Zhang, X., Zheng, Y., et al. (2020). Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 6, 31. doi:10.1038/s41421-020-0168-9
WHO Quality of Life-BREF (WHOQOL-BREF) (2020). Available at: http://www.who.int/substance_abuse/research_tools/whoqolbref/en/ (Accessed March 30, 2020).
Wuyts, W. A., Agostini, C., Antoniou, K. M., Bouros, D., Chambers, R. C., Cottin, V., et al. (2013). The pathogenesis of pulmonary fibrosis: a moving target. Eur. Respir. J. 41 (5), 1207–1218. doi:10.1183/09031936.00073012
Xaubet, A., Agustí, C., Luburich, P., Roca, J., Montón, C., Ayuso, M. C., et al. (1998). Pulmonary function tests and CT scan in the management of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 158 (2), 431–436. doi:10.1164/ajrccm.158.2.9709008
Xiong, Q., Xu, M., Li, J., Liu, Y., Zhang, J., Xu, Y., et al. (2021). Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin. Microbiol. Infect. 27 (1), 89–95. doi:10.1016/j.cmi.2020.09.023
Xu, H., Li, S., Lin, Y., Liu, R., Gu, Y., and Liao, D. (2011). Effectiveness of cultured Cordyceps sinensis combined with glucocorticosteroid on pulmonary fibrosis induced by bleomycin in rats. Zhongguo Zhong Yao Za Zhi 36 (16), 2265–2270.
PubMed Abstract PubMed Abstract PubMed Abstract | Google Scholar
Zhai, H. Q., Hhang, J. R., Gao, M. C., Liu, Y., Zhang, S. F., Wang, X. H., et al. (2014). Comparative study between Ephedra sinica Stapf and Fructus Schisandrae Chinensis on ET-1 and 6-keto-prostaglandin F1α in rats with idiopathic pulmonary fibrosis. Genet. Mol. Res. 13 (2), 3761–3771. doi:10.4238/2014.May.13.3
Zhang, M., Liu, H. L., Huang, K., Peng, Y., Tao, Y. Y., Zhao, C. Q., et al. (2020). Fuzheng Huayu recipe prevented and treated CCl4-induced mice liver fibrosis through regulating polarization and chemotaxis of intrahepatic macrophages via CCL2 and CX3CL1. Evid. Based Complement. Altern. Med. 2020, 8591892. doi:10.1155/2020/8591892
Keywords: Fuzheng Huayu tablet, pulmonary fibrosis, pulmonary function, COVID-19, randomized controlled trial
Citation: Jing F, Wang W, Ke J, Huang T, Jiang B, Qiu Q, Huang J, Zhan S, Zhang W, Wu H, Su W, Feng J, Peng Y, Zhao Z, Xing F and Liu C (2025) Fuzheng Huayu tablets for treating pulmonary fibrosis in post-COVID-19 patients: a multicenter, randomized, double-blind, placebo-controlled trial. Front. Pharmacol. 16:1508276. doi: 10.3389/fphar.2025.1508276
Received: 22 October 2024; Accepted: 17 February 2025;
Published: 11 March 2025.
Edited by:
Wei Peng, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Shu-guang Yang, Henan University of Chinese Medicine, ChinaCopyright © 2025 Jing, Wang, Ke, Huang, Jiang, Qiu, Huang, Zhan, Zhang, Wu, Su, Feng, Peng, Zhao, Xing and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenghai Liu, Y2hlbmdoYWlsaXVAaG90bWFpbC5jb20=; Feng Xing, ZnhpbmdAc2h1dGNtLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.