
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 January 2025
Sec. Inflammation Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1505922
 Chenhui Wang1
Chenhui Wang1 Mengwei Yan2
Mengwei Yan2 Yuru Li3
Yuru Li3 Lei Han3
Lei Han3 Hongqian Wang3
Hongqian Wang3 Shufeng Jia3
Shufeng Jia3 Xingchen Liu4
Xingchen Liu4 Yang Liu1
Yang Liu1 Fan Wu1
Fan Wu1 Baoguo Wang1*
Baoguo Wang1*Objective: Knee osteoarthritis (KOA) is a degenerative joint condition, leading to disability and diminished quality of life. Molecular hydrogen has been proven to have antioxidant and anti-inflammatory properties, but few studies have investigated its effects on osteoarthritis. Our study aims to assess the therapeutic potential of hydrogen-oxygen mixture (H2-O2) inhalation for KOA.
Methods: In this randomized controlled trial, eligible elderly KOA patients were randomly assigned to either Group H or Group C. Both groups participated in a 12-week home-based exercise (HBE) program, which included knee-joint exercises and health education. Group H additionally received H2-O2 inhalation for 60 min per day over 2 weeks, while Group C did not. The primary outcome was measured using Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Secondary outcomes included inflammation levels (hs-CRP, NLR, PLR, LMR), Chair Stand Test (CST), Timed Up and Go (TUG), 36-item short-form health survey (SF-36), Exercise Adherence Rating Scale (EARS), and adverse events.
Results: A total of 121 subjects were enrolled, with an average age of 81.2 years, and 80.2% were female. The between-group mean difference in the WOMAC total score was −5.2 (95% CI −12.1 to 1.7, P = 0.140) at week 12, with Group H showing an improvement of −22.9 (95% CI −26.3 to −19.6, P < 0.001) and Group C showing an improvement of −19.4 (95% CI −22.7 to −16.0, P < 0.001) compared to baseline, revealing a significant group × time interaction (F (3, 356.034) = 14.425, P < 0.001). No significant differences were observed between both groups at week 12 in CST, TUG, SF-36 scores, EARS scores, or the incidence of adverse events.
Conclusion: Although clinical significance was not achieved, H2-O2 inhalation alleviated KOA symptoms and enhanced functional activity in elderly patients undergoing the HBE program during the initial 2 weeks. However, its sustained effects on improving KOA symptoms were not observed.
Knee osteoarthritis (KOA) is a common degenerative joint condition impacting the cartilage in the knee joint and surrounding tissues, stemming from various factors such as mechanical stress, inflammation, and metabolic dysregulation (Katz et al., 2021). Its primary clinical symptoms consist of pain in the joints, swelling, stiffness, limited joint function, and potentially even functional loss (Johnson and Hunter, 2014; Liu et al., 2015). It is estimated that KOA affects approximately 21%–33% of elderly individuals in China, with its onset typically manifesting gradually throughout aging (Sun et al., 2019). Notably, KOA can lead to adverse consequences, including disability and diminished quality of life (QoL), ultimately resulting in a substantial socio-economic burden (Sun et al., 2019; Hunter and Bierma-Zeinstra, 2019).
In accordance with clinical guidelines, exercise therapy stands out as a pivotal treatment for KOA, capable of retarding disease progression, alleviating pain, and improving knee function (Huang et al., 2018; Kolasinski et al., 2019). To date, more scholars have recognized the value of home-based knee exercises and relevant studies have confirmed the efficacy and feasibility of home-based knee exercises for alleviating pain and improving function among KOA patients (Chen H. et al., 2019; Safran-Norton et al., 2019). Nevertheless, most of the interventions of home-based exercises varied among different studies and there are few comprehensive interventions encompassing both knee exercises and lifestyle education available in China. Initiative Practice for Health (IPFH) is a comprehensive intervention strategy aimed at encouraging health-promoting behaviors related to balanced diet, physical activity, social engagement, and mental wellbeing, with the goal of fostering healthier lifestyles (Zhang et al., 2022). Based on the concept of IPFH, we designed a home-based exercise (HBE) program for KOA patients, which includes knee-joint exercises and health education about lifestyle behaviors. Although our preliminary study demonstrated the efficacy of the HBE program in improving KOA-related symptoms and enhancing QoL (Zhang, 2023), symptom improvement was not often satisfactory in elderly KOA patients, which may be attributed to the decline in their physical function and activity ability. Hence, the development of safe and effective adjuvant therapies for symptom management in older patients with KOA is essential.
It is acknowledged that the onset and progression of KOA are primarily attributed to the degeneration and damage of articular cartilage and the resultant chronic inflammation reactions (Lv et al., 2021; Shumnalieva et al., 2023). Evidence suggested that exercise therapy alleviated pain and improved functional symptoms associated with KOA by reducing oxidative stress, accelerating the restoration of impaired cartilage, and decreasing levels of inflammatory factors (Fransen et al., 2015; Wellsandt and Golightly, 2018). Interestingly, molecular hydrogen has also been proven to have antioxidant and anti-inflammatory functions (Ishibashi, 2013). Cell and animal studies have shown that hydrogen-rich water or the administration of hydrogen-releasing hydrogel effectively mitigates osteoarthritis-induced cartilage damage, promotes cartilage regeneration, inhibits cell apoptosis, and accelerates cartilage matrix repair (Zhang et al., 2023; Cheng et al., 2020a). These benefits are likely associated with the suppression of the JNK signaling pathway and the downregulation of Wnt/β-catenin activation in chondrocytes (Lu et al., 2021; Lin et al., 2016). However, the effect of molecular hydrogen on clinical outcomes (pain, stiffness, and functional status) in osteoarthritis conditions remains unclear at present and there have been no relevant clinical trials reported in this area.
Therefore, our study seeks to assess the therapeutic potential of hydrogen molecules in the treatment of KOA. We hypothesized that hydrogen-oxygen (H2-O2) mixture inhalation could improve KOA-related symptoms, such as pain, stiffness, and function, compared to those who did not receive the H2-O2 mixture inhalation in elderly KOA patients undergoing the HBE program.
From July 2023 to February 2024, at Taikang Yanyuan Continuing Care Retirement Community (CCRC) in China, an open-label, blinded-endpoint, randomized controlled trial was conducted. The recruitment process in this CCRC was conducted via roll-up banners and internal social media advertisements. The Ethics Committee of Sanbo Brain Hospital, Capital Medical University, approved the study (SBNK-YJ-2023-014-01), and it was registered with the Chinese Clinical Trial Registry (ChiCTR2300073102). Participants or their legally authorized representatives provided informed consent prior to enrollment.
Participants were required to meet the following criteria for inclusion: 1) age ≥65 years. 2) diagnosis of KOA identified from Taikang healthcare medical records. 3) Kellgren-Lawrence (KL) grade was classified as level 2 or 3. 4) duration of KOA ≥6 months and did not receive joint replacement surgery or arthroscopic surgery. 5) self-reported knee-joint pain ≥4 on the numeric rating scale (NRS) during the past week. Exclusion criteria for participants encompassed the following conditions: 1) mental disorders; 2) cognitive impairment; 3) severe systematic diseases such as severe cardiopulmonary disease or multi-organ failure; 4) inability to cooperate in completing exercises; 5) intolerance to inhalation therapy; 6) involvement in other clinical trials within the previous 3 months.
Participants eligible for the study were randomly allocated to either the H2-O2 mixture inhalation group (Group H) or the control group (Group C) in a 1:1 ratio. Randomization, performed by a researcher not engaged in the study, utilized block randomization with block sizes of 4 and 6. Randomization codes were enclosed in opaque sealed envelopes sequentially numbered, and then revealed by a researcher in sequence post-recruitment. Participants were informed of their randomization assignment after completing baseline assessments. Only the outcome assessors were blind to group allocation and treatment.
All included participants would undergo a 12-week HBE program, which mainly included health education on lifestyle behaviours for KOA and home-based exercise therapy. All of the participants were required to attend health lectures held in the CCRC at week 4 and 8. Health education included 4 modules covering the concepts of clinical manifestations and risk factors, strategies for managing knee-joint pain, dietary recommendations, and exercise guidance for KOA. Following the lecture, each participant would receive a printed copy of the material outlining the HBE guidance manual for KOA. Moreover, the study assistants would assist participants in logging into our Northern Smart Platform on their mobile phones. Here, they can access home-based knee exercise videos and health knowledge short videos, enhancing their comprehension and retention of health and exercises.
Knee-joint exercise therapy was formulated based on a synthesis of literature review, clinical practice, and multidisciplinary expert group discussions. The primary objectives were to bolster lower-limb muscle strength, improve balance, reduce pain, and alleviate knee stiffness. The regimen encompassed warm-up exercises, passive knee flexion and extension, isometric quadriceps contractions, supine straight-leg lifts, prone leg lifts, resistance knee extension and flexion (details in Supplementary File 1). All participants were required to perform the above home-based exercises during the study at least 3 days a week, for 45–60 min a day, during 12 weeks. The initial knee exercises were guided by a specialized pain physician and physical therapist, while subsequent home-based knee exercises were performed independently, without supervision. Additionally, participants were asked to record their daily completion status of home-based exercises. Upon successful completion of the 12-week home-based exercise regimen, participants would be rewarded with a gift as an incentive to maintain their commitment to home-based exercises. Meanwhile, telephone interviews were conducted with participants during weeks 1, 3, 6, and 10 to evaluate their exercise advancement and to motivate adherence to the exercise regimen (Figure 1).
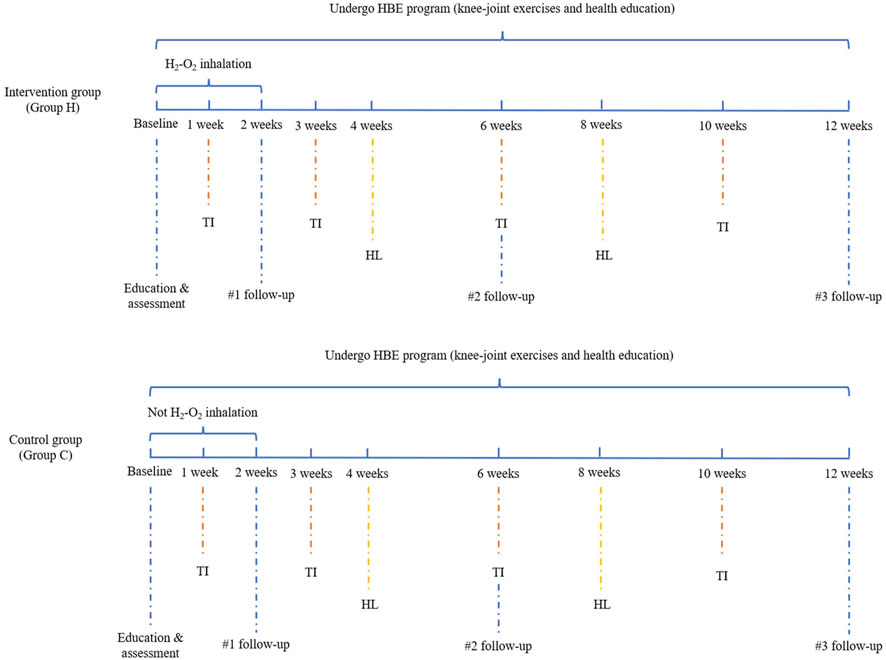
Figure 1. Flow chart of H2-O2 inhalation and HBE program. Abbreviation: TI, telephone interview; HL, health lectures.
Participants in Group H received H2-O2 mixture inhalation utilizing nasal tubes and the hydrogen-oxygen nebulizer AMS-H-01 (manufactured by Shanghai Asclepius Meditec Co., Ltd., China), delivering 2.0 L/min of hydrogen and 1.0 L/min of oxygen. Daily inhalation sessions lasted for 60 min continuously over a period of 2 weeks. The basis of our intervention regimen was provided by the published research about H2-O2 inhalation in alleviating postherpetic neuralgia (Wang et al., 2016). Conversely, participants in Group C did not undergo H2-O2 mixture inhalation therapy.
The primary outcomes of the study included pain, joint stiffness, and functional status associated with KOA, evaluated through the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Bellamy et al., 1988). This index consisted of 24 items: 5 for pain assessment, 2 for joint stiffness, and 17 for joint function. Each item was evaluated using the Visual Analog Scale (VAS) method, with scores ranging from 0 to 10 points (Heller et al., 2016). Higher scores denoted greater severity of pain, joint stiffness, or limitations in joint function. The WOMAC scale we used was the validated Chinese version with good internal reliability and test-retest reliability (Xie et al., 2008).
The secondary outcomes of the study included inflammation levels, measured by the neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and high-sensitivity C-reactive protein (hs-CRP), as well as other objective evaluation such as the chair stand test (CST), timed up and go (TUG), and QoL. The increase in hs-CRP, NLR, and PLR levels, along with a decrease in LMR levels, suggests an exacerbation of the inflammatory response. During the CST, participants were seated in a 45 cm-high armless chair, maintaining a shoulder-width stance with arms crossed over their chests. They were directed to perform as many sit-to-stand cycles as possible within a 30-s timeframe (Jones et al., 1999). For the TUG test, participants were instructed to rise from a chair, walk a distance of 3 m, turn around, return to the starting position, and sit back down (Sabirli et al., 2013). Both the CST and TUG were conducted twice, with a 5-min rest period in between to minimize fatigue. The maximum number of completions in the CST and the total duration of the TUG test in seconds were recorded. QoL was measured utilizing the 36-item short-form health survey (SF-36) (Brazier et al., 1992). This scale evaluates 8 dimensions of health: Physical Function (PF), Bodily Pain (BP), Role Limitations due to Physical Health Problems (RP), Role Limitations due to Emotional Problems (RE), General Mental Health (MH), Social Function (SF), Vitality (VIT), and General Health (GH). Higher scores indicate a better health status. Adherence to home-based exercises was assessed using the Exercise Adherence Rating Scale (EARS), which encompasses a scoring range of 0–24 points (Newman-Beinart et al., 2017). A higher score on the EARS indicates better adherence to the exercise regimen.
Baseline data, including age, sex, body mass index (BMI), education levels, affected side, duration of KOA, analgesic usage, and medical history, were gathered through the Taikang healthcare electronic system. KL grade was assessed using X-ray imaging of the knee joints (Liew et al., 2023). NRS of knee-joint pain, baseline of WOMAC, CST, TUG, and SF-36 were evaluated during the enrollment evaluation. NLR, PLR, LMR, and hs-CRP were tested at baseline and week 12.
Participants were scheduled for follow-up at weeks 2, 6, and 12 after enrollment. During these follow-up interviews, WOMAC, CST, and TUG were assessed at weeks 2, 6, and 12. SF-36 and adherence assessment were conducted at week 12. Additionally, any adverse events occurring during the trial were recorded.
Sample size calculation was predicated on a prior randomized controlled trial, where the standard deviation (SD) of the exercise intervention group for KOA patients was noted as 15.2 (Chao et al., 2021). The minimal clinically important difference (MCID) in total WOMAC score, as calculated in various studies, ranged from 9 to 22 points (Bhandari et al., 2019). We hypothesized that the combination of H2-O2 mixture inhalation with HBE could reduce the WOMAC score of KOA patients by 9 points compared to the control group only receiving HBE. Therefore, we aimed to enroll 46 patients in each group, aiming for 80% power at a two-sided significance level of 5%. Accounting for an anticipated dropout rate of 20%, we aimed to recruit a minimum of 116 patients for this study.
The normality of quantitative variables was assessed using the Kolmogorov-Smirnov test. Continuous data were expressed as medians and interquartile ranges (IQRs) or as mean and SD, depending on their distribution. Categorical data were presented as frequency distributions. Analysis of differences was performed using the Mann-Whitney U test, Student’s t-test, or chi-square test, based on the data type and distribution. A linear mixed-effects model was employed to analyze repeated measurement data including WOMAC and WOMAC subscale scores, CST, and TUG. Fixed effects included treatment group, time effect, and a treatment × time interaction. The significance of the treatment × time interaction indicated a significant treatment effect. Random effects encompassed time, where both intercept and slope were allowed to vary. Post hoc exploratory analyses were conducted to evaluate the heterogeneity of the primary outcome across predefined subgroups, including age, sex, KL grade, EARS score, and duration of KOA. Treatment-by-covariate interactions for each subgroup factor were examined separately using a general linear model. The outcomes of repeated measurement data were represented by mean and 95% confidence interval (CI). Intention-to-treat (ITT) analysis was regarded as the primary analysis, with per-protocol analysis considered a sensitivity analysis to verify the robustness of the outcomes. Missing data were imputed using mean imputation in cases where missing values accounted for less than 20% of the data. If missing data exceeded 20%, no imputation was applied and analyses were restricted to the available data.
All statistical analyses were conducted using SPSS (version 23.0, IBM, USA) and GraphPad Prism (version 8.0, GraphPad, United States). Two-sided statistical tests were employed, and statistical significance was established at a value of P < 0.05.
Out of the initial 169 subjects screened for this study, 17 did not meet the inclusion criteria, and 31 declined to participate. Ultimately, 121 subjects were enrolled, with 61 assigned to Group H and 60 to Group C. However, 2 subjects from each group did not receive the assigned intervention because of their preference to switch to the other group. Additionally, 15 subjects in Group H and 14 subjects in Group C discontinued the study. In Group H, 4 were lost to follow-up, 2 showed poor adherences, 6 had increased or decreased H2-O2 inhalation time, and 3 received intra-articular injections. In Group C, 9 were lost to follow-up, 3 exhibited poor adherences, and 2 received intra-articular injections. Finally, 46 subjects in each Group completed the 12-week study period (Figure 2).
Most of the individuals enrolled in our study were female (80.2%, 97/121), with a mean age of 81.2 ± 6.1 years. Bilateral KOA was the most prevalent condition, impacting 66.9% (81/121) of participants, while KL grade 3 was the most common classification, affecting 52.9% (64/121) of the subjects. The median duration of KOA was 20 (10–30) years. The mean NRS of knee-joint pain was 4.4 ± 0.7. No significant difference was observed in demographic characteristics and medical comorbidities between the two groups (Table 1). Similar results were also obtained through pre-protocol analysis (Supplementary Table S1).
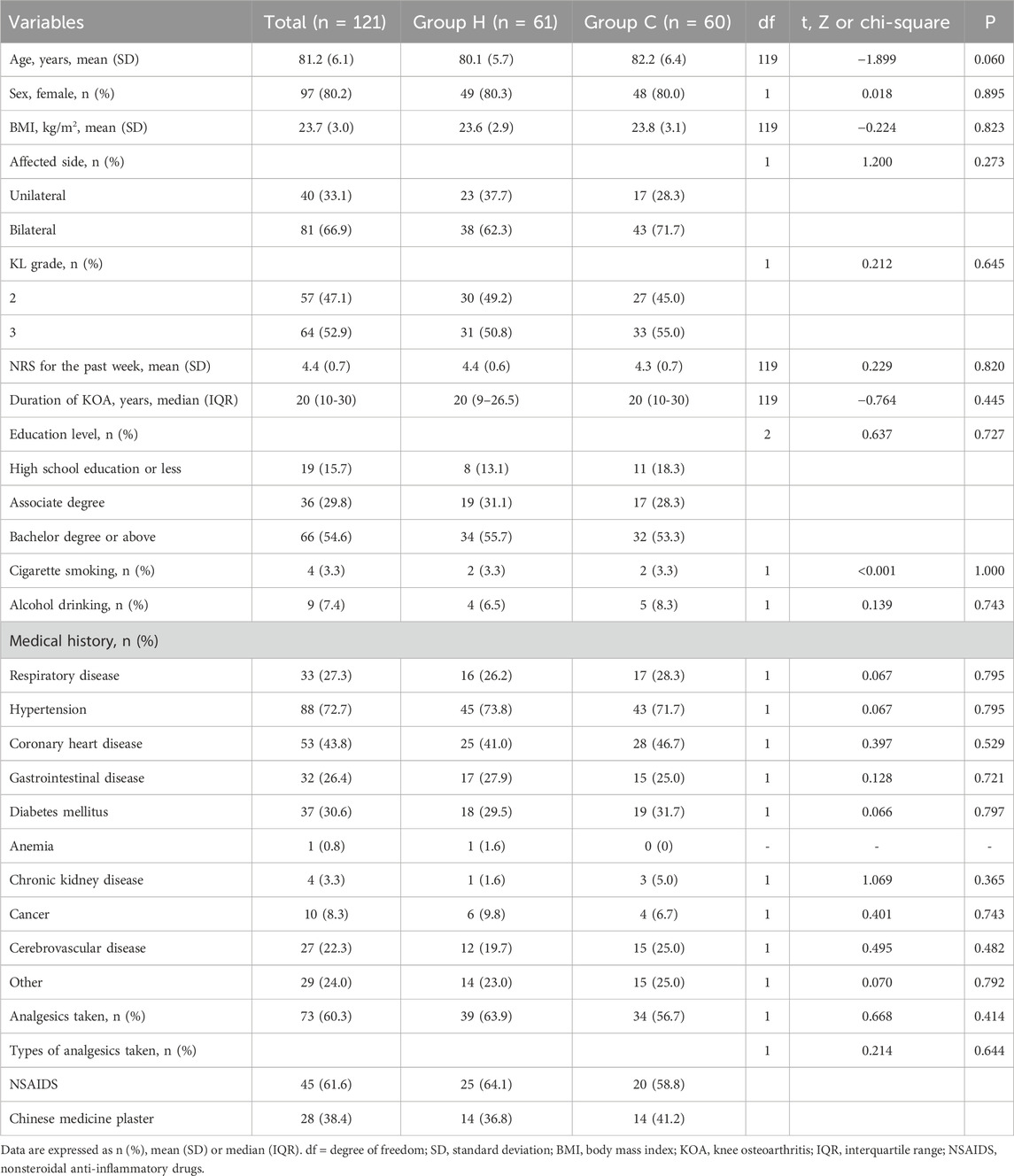
Table 1. ITT analysis of demographic and clinical characteristics of participants by arm in baseline.
Data distribution and changes in WOMAC total score and its subscale scores are detailed in Table 2 and Figure 3. In ITT analysis, the linear mixed-effects model revealed the between-group mean difference in the WOMAC total score of −5.2 (95% CI -12.1 to 1.7, F (1, 135.411) = 2.206, P = 0.140) at week 12, with Group H showing an improvement of −22.9 (95% CI −26.3 to −19.6, F (3, 356.034) = 128.131, P < 0.001) and Group C showing an improvement of −19.4 (95% CI −22.7 to −16.0, F (3, 356.034) = 100.426, P < 0.001) compared to baseline. Notably, the mean difference in the WOMAC total score peaked at week 2 (MD: −8.0, 95% CI -14.9 to −1.1, F (1, 135.411) = 5.229, P = 0.024), indicating distinct declining trends. Additionally, the between-group mean difference in WOMAC pain score and function score was −0.2 (95% CI -1.9 to 1.5, F (1,169.895) = 0.032, P = 0.858) and −5.2 (95% CI -11.1 to 0.7, F (1, 135.326) = 3.070, P = 0.082) at week 12 respectively, with improvements of −6.9 (95% CI −8.3 to −5.5, F (3, 349.291) = 66.295, P < 0.001) and −14.2 (95% CI −17.0 to −11.4, F (3, 356.451) = 71.845, P < 0.001) in Group H, and −5.8 (95% CI −7.2 to −4.4, F (3, 349.291) = 43.290, P < 0.001) and −12.2 (95% CI −15.0 to −9.4, F (3, 356.451) = 64.406, P < 0.001) in Group C compared to baseline. Similarly, the mean difference in the WOMAC pain score (MD: −0.8, 95% CI -2.5 to 0.9, F (1, 169.895) = 0.866, P = 0.353) and function score (MD: −7.4, 95% CI -13.3 to −1.6, F (1, 135.326) = 6.254, P = 0.014) also reached their apex at week 2, indicating varied patterns of decline. Significant group × time interactions were observed for the WOMAC total score (F (3, 356.034) = 14.425, P < 0.001), pain score (F (3, 349.291) = 6.218, P < 0.001), and function score (F (3, 356.451) = 8.870, P < 0.001) between the two groups, while no significant group × time interactions were noted for the WOMAC stiffness score (F (3, 355.959) = 1.544, P = 0.230). Similar results were also obtained through pre-protocol analysis (Supplementary Table S2). In subgroup analysis, there were no significant interactions between treatment group and predefined factors, such as age, sex, KL grade, EARS score, and duration of KOA (Figure 4).
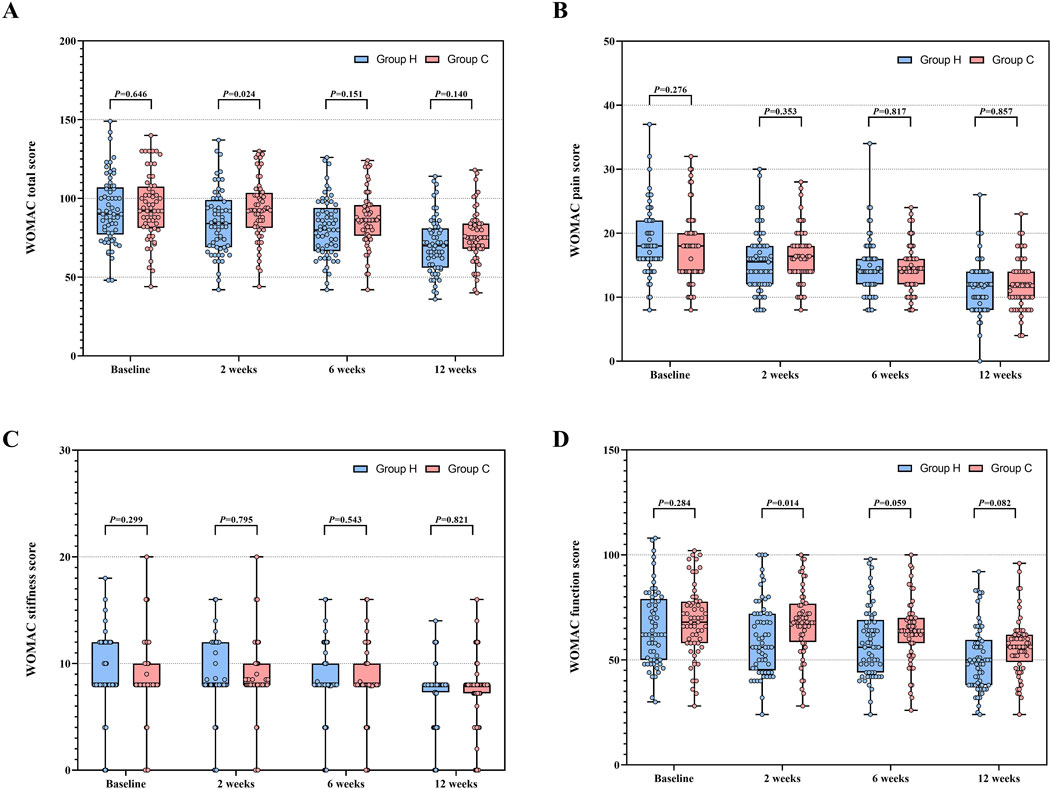
Figure 3. WOMAC total score and its subscale scores between Group H and Group C during the study period. (A) Distribution of WOMAC total score. (B) Distribution of WOMAC pain score. (C) Distribution of WOMAC stiffness score. (D) Distribution of WOMAC function score. Abbreviation: WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
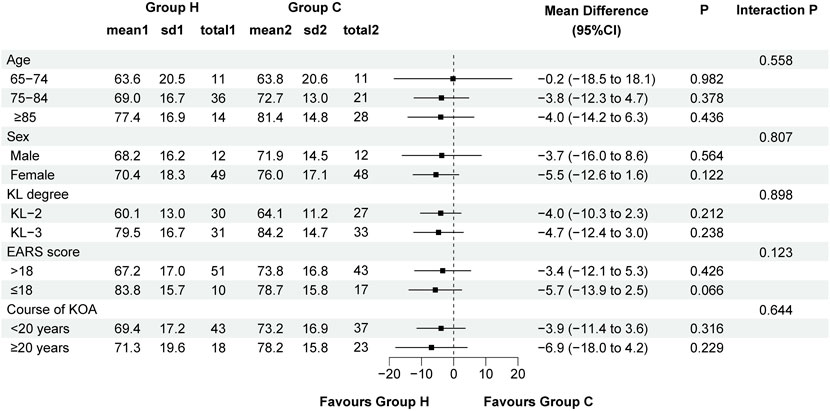
Figure 4. Forest plot assessing the improvement of WOMAC total score between Group H and Group C in predefined subgroups. Abbreviation: KL, Kellgren-Lawrence; EARS, Exercise Adherence Rating Scale; KOA, knee osteoarthritis.
Baseline data for CST and TUG were comparable in both groups. The between-group mean difference in CST increased from 0.7 (95%CI −0.5 to 1.8, F (1, 146.764) = 1.405, P = 0.238) to 0.9 (95%CI −0.3 to 1.9, F (1, 146.764) = 2.264, P = 0.135), and TUG decreased from −1.4 (95%CI −3.4 to 0.7, F (1, 134.075) = 1.795, P = 0.183) to −1.1 (95%CI -3.1 to 0.9, F (1, 134.075) = 1.134, P = 0.289). For CST, Group H exhibited a significant increase at week 12 compared to baseline (MD: −3.0, 95% CI: −3.7 to −2.3; F (3, 354.157) = 48.769, P < 0.001). Similarly, Group C also showed a significant increase in CST at week 12 (MD: −2.8, 95% CI: −3.5 to −2.1; F (3, 354.157) = 46.245, P < 0.001). For TUG, Group H demonstrated a significant reduction at week 12 compared to baseline (MD: 2.1, 95% CI: 1.2 to 3.1; F (3, 355.671) = 12.027, P < 0.001). Similarly, Group C showed a significant reduction in TUG at week 12 compared to baseline (MD: 2.4, 95% CI: 1.5 to 3.4; F (3, 355.671) = 17.069, P < 0.001). Based on a linear mixed-effects model, ITT analysis demonstrated a significant increasing trend in CST (time effect: F (3, 354.157) = 93.694, P < 0.001) and a significant decreasing trend in TUG (time effect: F (3, 355.671) = 28.247, P < 0.001) throughout the study period in both groups. However, no group effects were observed in either CST (group effect: F (1, 121.740) = 1.957, P = 0.164) or TUG (group effect: F (1, 120.595) = 1.747, P = 0.189) between the two groups. Group × time interactions were not significant in CST (F (3, 354.157) = 1.299, P = 0.274) and TUG (F (3, 355.671) = 0.891, P = 0.446) between the two groups. Data distribution and changes in CST and TUG are presented in Table 2 and Figure 5. Pre-protocol analysis yielded similar findings (Supplementary Table S2).
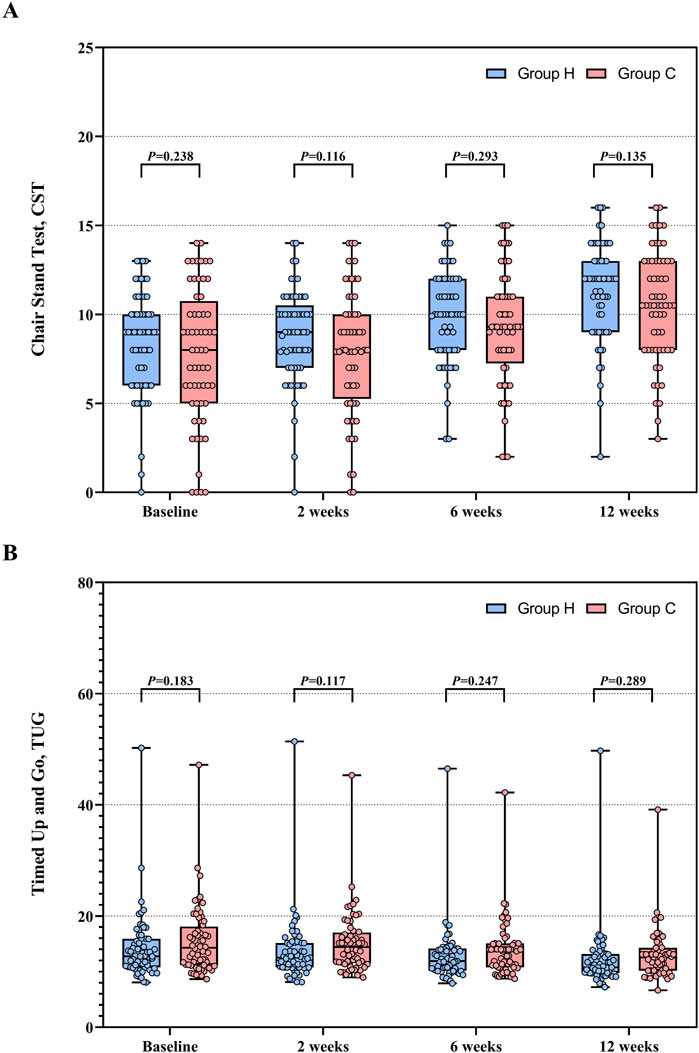
Figure 5. CST and TUG between Group H and Group C during the study period. (A) Distribution of CST. (B) Distribution of TUG. Abbreviation: CST, chair stand test; TUG, timed up and go.
A total of 23 subjects in Group H and 21 subjects in Group C underwent baseline testing for hs-CRP, NLR, PLR, and LMR. The baseline levels of hs-CRP (Group H: 3.69 [2.09–5.78] vs. Group C: 4.36 [2.36–5.73], Z (42) = −0.282, P = 0.778), NLR (Group H: 2.22 [1.76–3.58] vs. Group C: 2.08 [1.29–2.50], Z (42) = −1.022, P = 0.307), PLR (Group H: 135.9 [90.2–161.5] vs. Group C: 120.6 [98.7–180.8], Z (42) = −0.223, P = 0.823), and LMR (Group H: 4.76 [3.61–6.48] vs. Group C: 4.83 [3.78–6.45], Z (42) = -0.153, P = 0.879) were comparable between the two groups (Table 3; Figure 6). At the trial end, 12 subjects from Group H and 18 subjects from Group C underwent the same tests. Compared to baseline, Group H exhibited reductions in hs-CRP (baseline: 3.69 [2.09–5.78] vs. week 12: 2.50 [1.91–4.77]; Z (5) = −0.105, P = 0.917), NLR (baseline: 2.22 [1.76–3.58] vs. week 12: 1.73 [1.08–2.94]; Z (5) = −0.734, P = 0.463), and PLR (baseline: 135.9 [90.2–161.5] vs. week 12: 104.2 [65.3–166.6]; Z (5) = -0.105, P = 0.917), alongside an increase in LMR (baseline: 4.76 [3.61–6.48] vs. week 12: 5.53 [3.03–8.51]; Z (5) = −0.943, P = 0.345), though none of these changes reached statistical significance. Similarly, in Group C, hs-CRP (baseline: 4.36 [2.36–5.73] vs. week 12: 2.63 [0.99–5.01]; Z (6) = −0.169, P = 0.866), NLR (baseline: 2.08 [1.29–2.50] vs. week 12: 2.02 [1.57–3.95]; Z (6) = -0.338, P = 0.735), and PLR (baseline: 120.6 [98.7–180.8] vs. week 12: 115.4 [84.6–193.3]; Z (6) = -0.338, P = 0.735) decreased, while LMR (baseline: 4.83 [3.78–6.45] vs. week 12: 5.11 [3.22–7.56]; Z (6) = −0.676, P = 0.499) increased, but these changes were also not statistically significant. However, no significant differences were found between the groups in hs-CRP (Group H: 2.50 [1.91–4.77] vs. Group C: 2.63 [0.99–5.00], Z (28) = −0.487, P = 0.626), NLR (Group H: 1.73 [1.08–2.94] vs. Group C: 2.02 [1.57–3.95], Z (28) = −0.762, P = 0.446), PLR (Group H: 104.2 [65.3–166.6] vs. Group C: 115.4 [84.7–193.3], Z (28) = -0.762, P = 0.446), and LMR (Group H: 5.53 [3.03–8.51] vs. Group C: 5.11 [3.22–7.56], Z (28) = -0.296, P = 0.767) at the 12-week point (Table 3; Figure 6). Similar results were observed in the per-protocol analysis (Supplementary Table S3).
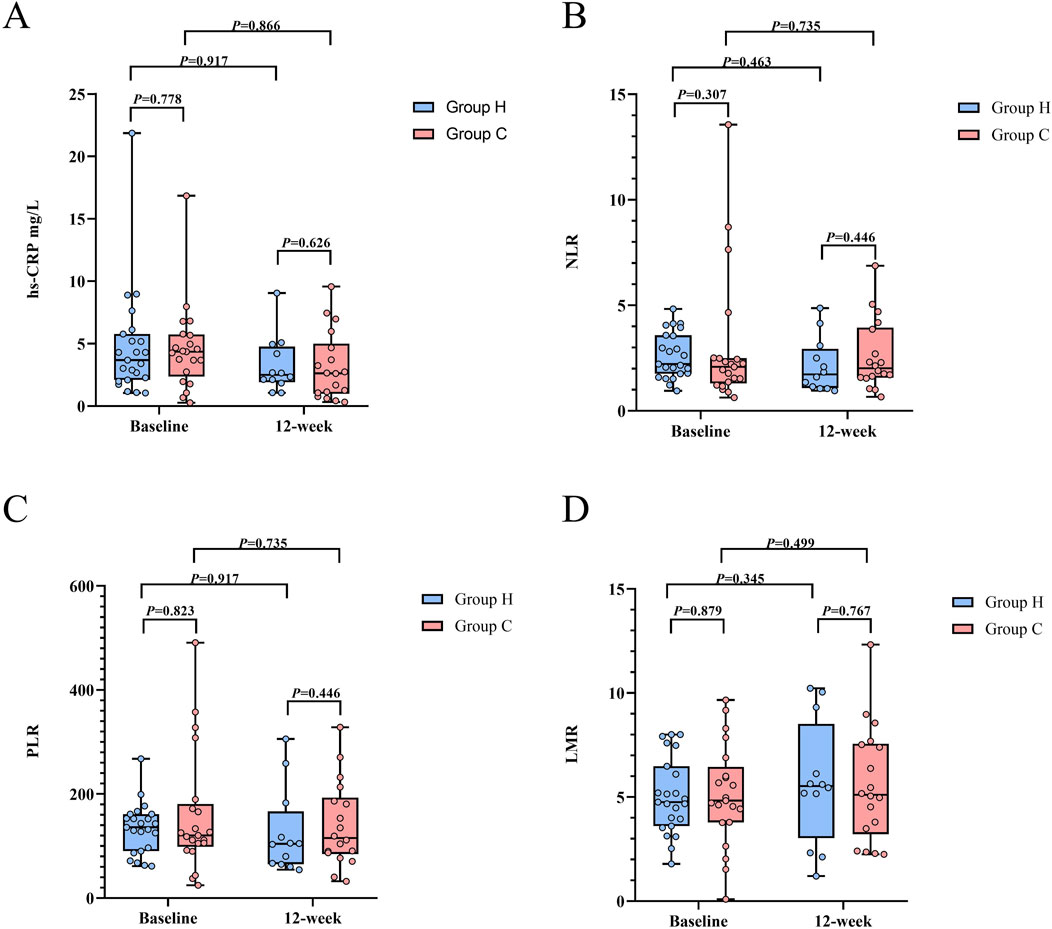
Figure 6. Inflammation levels between Group H and Group C during the study period. (A) Distribution of hs-CRP. (B) Distribution of NLR. (C) Distribution of PLR. (D) Distribution of LMR. Abbreviation: hs-CRP, high-sensitivity C-reactive protein; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; LMR, lymphocyte-monocyte ratio.
ITT analysis indicated no significant differences in the scores of PF (Group H: 54.0 ± 15.5 vs. Group C: 51.1 ± 17.8, t (119) = 0.967, P = 0.336), BP (Group H: 42.7 ± 11.3 vs. Group C: 43.4 ± 13.3, t (119) = −0.340, P = 0.735), RP (Group H: 49.6 ± 40.4 vs. Group C: 55.4 ± 39.9, t (119) = −0.798, P = 0.427), RE (Group H: 66.1 ± 39.7 vs. Group C: 70.6 ± 35.3, t (119) = -0.650, P = 0.517), MH (Group H: 66.6 ± 14.0 vs. Group C: 69.0 ± 15.6, t (119) = −0.882, P = 0.380), SF (Group H: 78.5 ± 14.8 vs. Group C: 81.5 ± 16.8, t (119) = -1.032, P = 0.304), VIT (Group H: 65.8 ± 14.3 vs. Group C: 67.5 ± 11.3, t (119) = −0.717, P = 0.475), and GH (Group H: 45.6 ± 15.3 vs. Group C: 47.3 ± 17.8, t (119) = −0.565, P = 0.572) between the two groups at baseline (Table 4). Following the 12-week study period, both groups exhibited significant increases in the scores of PF (Group H: MD −22.0, 95%CI −25.4 to −18.7, t (60) = −13.113, P < 0.001; Group C: MD -21.6, 95%CI −25.1 to 18.0, t (59) = −12.103, P < 0.001), BP (Group H: MD −24.7, 95%CI −27.8 to −21.7, t (60) = −16.109, P < 0.001; Group C: MD −21.4, 95%CI −24.3 to −18.4, t (59) = −14.592, P < 0.001), RP (Group H: MD −36.8, 95%CI −46.4 to −27.3, t (60) = −7.719, P < 0.001; Group C: MD −28.9, 95%CI −37.1 to −20.7, t (59) = -7.083, P < 0.001), RE (Group H: MD −20.9, 95%CI −30.7 to −11.0, t (60) = −4.241, P < 0.001; Group C: MD −22.1, 95%CI -30.9 to −13.4, t (59) = -5.066, P < 0.001), MH (Group H: MD -14.2, 95%CI −17.7 to −10.7, t (60) = −8.050, P < 0.001; Group C: MD −11.5, 95%CI −15.4 to −7.7, t (59) = −5.954, P < 0.001), SF (Group H: MD −21.7, 95%CI −25.3 to −18.1, t (60) = −12.088, P < 0.001; Group C: MD −17.3, 95%CI −21.4 to −13.2, t (59) = −8.368, P < 0.001), VIT (Group H: MD −13.0, 95%CI −16.6 to −9.5, t (60) = −7.330, P < 0.001; Group C: MD −10.0, 95%CI −12.9 to −7.2, t (59) = −7.005, P < 0.001), and GH (Group H: MD −9.5, 95%CI −12.7 to −6.1, t (60) = -5.687, P < 0.001; Group C: MD −7.5, 95%CI -10.8 to −4.2, t (59) = -4.522, P < 0.001) compared to baseline (Supplementary Table S4). However, no significant difference was observed in the scores of PF (Group H: 76.0 ± 12.8 vs. Group C: 72.6 ± 11.7, t (119) = 1.521, P = 0.131), BP (Group H: 67.4 ± 13.7 vs. Group C: 64.8 ± 10.8, t (119) = 1.158, P = 0.249), RP (Group H: 86.4 ± 22.4 vs. Group C: 84.3 ± 21.1, t (119) = 0.532, P = 0.596), RE (Group H: 87.0 ± 20.6 vs. Group C: 92.7 ± 16.3, t (119) = −1.684, P = 0.095), MH (Group H: 80.8 ± 9.5 vs. Group C: 80.5 ± 8.1, t (119) = 0.177, P = 0.860), SF (Group H: 100.2 ± 12.8 vs. Group C: 98.8 ± 12.4, t (119) = 0.629, P = 0.530), VIT (Group H: 78.9 ± 9.0 vs. Group C: 77.5 ± 8.0, t (119) = 0.837, P = 0.404), and GH (Group H: 55.0 ± 15.6 vs. Group C: 54.8 ± 15.7, t (119) = 0.087, P = 0.931) between the two groups at the 12-week (Table 4). Similar findings were observed in the pre-protocol analysis (Supplementary Tables S5, S6).
In terms of adherence, ITT analysis revealed no significant difference in the EARS score between the two groups (Group H: 21.5 ± 3.7 vs. Group C: 20.9 ± 3.0, t (119) = 1.046, P = 0.298). A similar finding was observed in pre-protocol analysis (Group H: 21.8 ± 2.6 vs. Group C: 21.5 ± 2.7, t (90) = 0.513, P = 0.609). Regarding adverse events, Group H reported a total of 3 adverse events (5.0%, 3/61), including 2 cases of headache (3.3%, 2/61) and 1 case of nasal cavity dryness (1.6%, 1/61). In contrast, Group C reported 1 adverse event of lower limb muscle pain (1.7%, 1/60). No significant difference was observed in the incidence of adverse events between the two groups (Group H: 5.0%, 3/61 vs. Group C: 1.6%, 1/60, χ2 = 0.242, P = 0.619). In addition, we observed that 3 subjects in Group H and 2 subjects in Group C experienced worsening knee-joint pain and subsequently received intra-articular injections. Therefore, these 5 subjects withdrew from the study and were excluded from the pre-protocol analysis.
To the best of our knowledge, it is the first clinical study investigating the effects of molecular hydrogen on symptom improvement in elderly patients with KOA. Our study found that the H2-O2 mixture inhalation provided improvement in functional status, as measured by the WOMAC, compared to those who did not receive H2-O2 mixture inhalation in elderly KOA patients undergoing the same HBE program during the 12-week study period. However, the improvement in the WOMAC score was 8 points, which is below the minimum clinically important difference of 9 points. Additionally, there were no difference in other secondary outcomes including inflammation levels, objective function tests, QoL improvement, and adverse events.
Although KOA stands as the primary cause of disability, there are currently no available cures or disease-modifying treatments (Hunter et al., 2020). The degeneration and damage of articular cartilage are considered pivotal mechanisms in the development of KOA, with oxidative stress and inflammatory reaction collectively contributing to this pathological process (Lv et al., 2021; Shumnalieva et al., 2023; Liao et al., 2023). Current research heavily focused on delaying cartilage damage and degeneration. It is well established that reactive oxygen species (ROS) can induce extracellular matrix loss, cell aging, mitochondrial dysfunction, and cell apoptosis in chondrocytes and mesenchymal stem cells, leading to subchondral bone loss and accelerating the occurrence of OA (Tudorachi et al., 2021). Exercise therapy is widely prescribed as a primary treatment for KOA due to its potential to reduce oxidative stress and decrease inflammation (Fransen et al., 2015; Wellsandt and Golightly, 2018). In addition, molecular hydrogen has also been proven to play a significant role in treating ROS-induced diseases and inflammation-related diseases. Ishibashi et al. demonstrated that drinking hydrogen-rich water could reduce the Disease Activity Score in 28 joints (DAS28) and 8-OHdG levels in patients with rheumatic arthritis (RA) (Ishibashi et al., 2012). Another study also indicated that hydrogen-rich saline infusion could decrease DAS28, with significant reductions in serum biomarkers such as IL-6 and 8-OHdG following the infusion (Ishibashi et al., 2014). The underlying mechanism may be attributed to the properties of hydrogen molecules in inhibiting oxidative stress and suppressing inflammatory responses. Increasing evidence suggests that hydrogen molecules can enhance antioxidant capacity by inhibiting the Nrf-2/HO-1 and NF-κB pathways, thereby contributing therapeutically to wound healing, hypoxia/ischemia injury, and Alzheimer’s disease (Li et al., 2022; Cai et al., 2008; Wang et al., 2011). Additionally, molecular hydrogen exerts antioxidant effects by directly neutralizing harmful free radicals, such as hydroxyl radicals (⋅OH) and ONOO⁻, without affecting physiologically important radicals like nitric oxide (Ohta, 2011). Further evidence indicates that hydrogen molecules may counteract the progression of OA by inhibiting oxidative stress, matrix catabolism, and apoptosis (Cheng et al., 2020b). Moreover, prior studies have illustrated that the anti-inflammatory properties of hydrogen encompass the downregulation of various pro-inflammatory cytokines and chemokines, including TNF-α and IL-6, alongside the upregulation of transcription levels of anti-inflammatory cytokines such as IL-10 (Saramago et al., 2019; Nogueira et al., 2018). Reducing inflammatory factors could be beneficial in delaying the progression of KOA and improving the related symptoms.
RA is known to be associated with autoimmune disorders, differing from osteoarthritis (OA), however, both conditions are driven by oxidative stress and inflammatory responses. Consequently, we speculate that the enhancement of KOA symptoms resulting from the combination of H2-O2 mixture inhalation and home-based exercises was superior to the effects of home-based exercises alone. Despite the observed improvement in WOMAC scores, the combination of H2-O2 mixture inhalation and home-based exercises did not yield a clinically significant difference at week 12 (MCID: 9-22 on the WOMAC scale) compared to home-based exercises alone. A potential explanation for this finding is that the 2-week inhalation therapy might not have provided sufficient effects of inhibiting oxidative stress and inflammatory responses to achieve a meaningful clinical difference. As indicated by the inflammatory levels, although no significant differences were observed in hs-CRP, NLR, PLR, and LMR between the two groups at week 12, hs-CRP, NLR, and PLR decreased while LMR increased when compared to baseline. This suggested a reduction in inflammation levels, though it has not yet reached statistical significance. We cannot rule out that an extended duration of H2-O2 mixture inhalation or alternative intervention methods, such as hydrogen-rich saline infusion or injection into the knee joint, might yield different outcomes.
The lack of a clinically significant difference in the improvement of KOA symptoms may be multifactorial. The improvements depend heavily on the adherence to home-based exercise, as well as other factors such as age, sex, KL grade, and duration of KOA (Marks, 2012). In our study, the EARS score was comparable in both groups. Participants with poor adherence to HBE program were excluded from the pre-protocol analysis, yet the primary outcome remained consistent with the ITT analysis. Additionally, the subgroup analysis was performed to identify the effects of other factors including age, sex, KL grade, EARS score, and duration of KOA on clinical outcome (WOMAC score). The results indicated no significant differences in the improvement of KOA symptoms due to H2-O2 mixture inhalation therapy across different patient groups, particularly among those with differing levels of adherence to HBE program. This underscores the need for further studies focusing on the effects of different methods and durations of hydrogen therapy in alleviating symptoms of KOA.
This study included multiple evaluations at weeks 2, 6, and 12 to examine the sustained effects of H2-O2 inhalation therapy on symptom improvement in KOA patients. In Hirohisa Ono’s research, a 6-month course of H2-O2 inhalation significantly improved outcomes assessed by Alzheimer’s Disease Assessment Scale-Cognitive section (ADAS-Cog) and Diffusion Tensor Imaging (DTI), providing not only temporary relief but also sustained benefits for at least 6 months without further H2-O2 treatment in patients with Alzheimer’s disease (Ono et al., 2023). Similarly, Ishibashi et al. reported that a 5-day hydrogen-saline infusion reduced the disease activity score in 28 joints and levels of IL-6, 8-OHdG, and MMP3 in patients with RA, with effects lasting up to 4 weeks (Ishibashi et al., 2014). Despite the mechanisms remain unclear, these effects may involve anti-cell-death properties, such as ferroptosis inhibition via peroxide reduction and modulation of pro- and anti-death factors (Iuchi et al., 2019; Terasaki et al., 2011). However, our study did not observe sustained symptom improvement in KOA patients following H2-O2 inhalation therapy. Future research will focus on exploring dose-response relationship of H2-O2 inhalation therapy to better understand its therapeutic potential.
Regarding the safety of H2-O2 mixture inhalation, our study observed adverse reactions of headache and dryness of the nasal cavity, which spontaneously resolved upon discontinuation of H2-O2 inhalation. Headache may be related to vasodilation caused by hydrogen molecules, as a study reported that H2-O2 mixture inhalation could reduce levels of plasma angiotensin II and aldosterone, thus dilating blood vessels and lowering blood pressure (Liu et al., 2022). Dryness of the nasal cavity may be attributed to the inhalation method through nasal tubes and the flow rate of inhalation (Chen J. B. et al., 2019). Besides, lots of published clinical trials involving the inhalation of gas composed of approximately 67% H2 and 33% O2 have not reported any severe adverse events (Lin et al., 2020; Dan et al., 2023; Zheng et al., 2021).
Limitations remain in our study. Firstly, this open-label trial implies that both researchers and subjects are aware of the actual intervention measures, which may lead to potential bias in patient-reported outcome data. Secondly, we employed various methods, including health lectures, telephone follow-ups, and phased rewards, to enhance participants’ adherence with home-based exercises, and no differences in EARS scores were found between the two groups. However, information bias may still be unavoidable since the adherence evaluation was conducted at the end of the trial and relied on a retrospective assessment of self-reported home-based exercises. Thirdly, this study did not analyze the levels of plasma inflammatory markers including IL-6, IL-10 and TNF-α, nor did it conduct relevant animal and cell experiments to explore the mechanisms by which hydrogen molecules improve KOA symptoms. Fourth, the control group did not receive O₂ inhalation. According to the formula for calculating the fraction of inspired O₂ (FiO₂ = 21% + 4 × oxygen flow rate), the FiO₂ for the intervention group was approximately 25%, while the control group had an FiO₂ of 21%, corresponding to the ambient air O₂ concentration. Although a 4% difference in FiO₂ is unlikely to have influenced the results and no research has demonstrated that O₂ inhalation had therapeutic effects on OA, it remains a limitation of this study. Finally, the participants living in this CCRC had a high level of education and good health awareness, regularly receiving health guidance from their family physicians. Therefore, we cannot determine whether these results can be extrapolated to other elderly groups.
As an adjunctive treatment, H2-O2 mixture inhalation seems to demonstrate therapeutic potential in improving KOA symptoms in elderly patients undergoing HBE program in the first 2 weeks, even though a clinically significant difference was not achieved. Sustained effect of H2-O2 mixture inhalation in improving KOA symptoms was not observed. Future research should explore varying inhalation durations and intervention methods, alongside in vitro and in vivo experiments, to further elucidate the role and mechanisms of hydrogen molecules in KOA disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of Sanbo Brain Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
CW: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–original draft. MY: Investigation, Methodology, Writing–original draft. YuL: Investigation, Writing–original draft. LH: Investigation, Writing–original draft. HW: Investigation, Writing–original draft. SJ: Investigation, Writing–original draft. XL: Investigation, Writing–original draft. YaL: Methodology, Writing–original draft. FW: Methodology, Writing–original draft. BW: Conceptualization, Data curation, Formal Analysis, Methodology, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2018YFC2000704).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1505922/full#supplementary-material
Bellamy, N., Buchanan, W. W., Goldsmith, C. H., Campbell, J., and Stitt, L. W. (1988). Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. rheumatology 15 (12), 1833–1840.
Bhandari, M., Einhorn, T. A., Guyatt, G., Schemitsch, E. H., Zura, R. D., Sprague, S., et al. (2019). Total hip arthroplasty or hemiarthroplasty for hip fracture. N. Engl. J. Med. 381 (23), 2199–2208. doi:10.1056/NEJMoa1906190
Brazier, J. E., Harper, R., Jones, N. M., O'Cathain, A., Thomas, K. J., Usherwood, T., et al. (1992). Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ Clin. Res. ed 305 (6846), 160–164. doi:10.1136/bmj.305.6846.160
Cai, J., Kang, Z., Liu, W. W., Luo, X., Qiang, S., Zhang, J. H., et al. (2008). Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci. Lett. 441 (2), 167–172. doi:10.1016/j.neulet.2008.05.077
Chao, J., Jing, Z., Xuehua, B., Peilei, Y., and Qi, G. (2021). Effect of systematic exercise rehabilitation on patients with knee osteoarthritis: a randomized controlled trial. Cartilage 13 (1_Suppl. l), 1734s–40s. doi:10.1177/1947603520903443
Chen, H., Zheng, X., Huang, H., Liu, C., Wan, Q., and Shang, S. (2019a). The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: a quasi-experimental study. BMC Musculoskelet. Disord. 20 (1), 160. doi:10.1186/s12891-019-2521-4
Chen, J. B., Kong, X. F., Lv, Y. Y., Qin, S. C., Sun, X. J., Mu, F., et al. (2019b). Real world survey of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med. gas Res. 9 (3), 115–121. doi:10.4103/2045-9912.266985
Cheng, S., Peng, L., Xu, B., Chen, W., Chen, Y., and Gu, Y. (2020a). Protective effects of hydrogen-rich water against cartilage damage in a rat model of osteoarthritis by inhibiting oxidative stress, matrix catabolism, and apoptosis. Med. Sci. Monit. 26, e920211. doi:10.12659/MSM.920211
Cheng, S., Peng, L., Xu, B., Chen, W., Chen, Y., and Gu, Y. (2020b). Protective effects of hydrogen-rich water against cartilage damage in a rat model of osteoarthritis by inhibiting oxidative stress, matrix catabolism, and apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 26, e920211. doi:10.12659/MSM.920211
Dan, Z., Li, H., and Xie, J. (2023). Efficacy of donepezil plus hydrogen-oxygen mixture inhalation for treatment of patients with Alzheimer disease: a retrospective study. Medicine 102 (30), e34382. doi:10.1097/MD.0000000000034382
Fransen, M., McConnell, S., Harmer, A. R., Van der Esch, M., Simic, M., and Bennell, K. L. (2015). Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br. J. sports Med. 49 (24), 1554–1557. doi:10.1136/bjsports-2015-095424
Heller, G. Z., Manuguerra, M., and Chow, R. (2016). How to analyze the visual analogue scale: myths, truths and clinical relevance. Scand. J. pain. 13, 67–75. doi:10.1016/j.sjpain.2016.06.012
Huang, D., Liu, Y. Q., Liang, L. S., Lin, X. W., Song, T., Zhuang, Z. G., et al. (2018). The diagnosis and therapy of degenerative knee joint disease: expert consensus from the Chinese pain medicine panel. Pain Res. and Manag. 2018, 2010129. doi:10.1155/2018/2010129
Hunter, D. J., and Bierma-Zeinstra, S. (2019). Osteoarthr. Lancet London, Engl. 393 (10182), 1745–1759. doi:10.1016/S0140-6736(19)30417-9
Hunter, D. J., March, L., and Chew, M. (2020). Osteoarthritis in 2020 and beyond: a lancet commission. Lancet London, Engl. 396 (10264), 1711–1712. doi:10.1016/S0140-6736(20)32230-3
Ishibashi, T. (2013). Molecular hydrogen: new antioxidant and anti-inflammatory therapy for rheumatoid arthritis and related diseases. Curr. Pharm. Des. 19 (35), 6375–6381. doi:10.2174/13816128113199990507
Ishibashi, T., Sato, B., Rikitake, M., Seo, T., Kurokawa, R., Hara, Y., et al. (2012). Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med. gas Res. 2 (1), 27. doi:10.1186/2045-9912-2-27
Ishibashi, T., Sato, B., Shibata, S., Sakai, T., Hara, Y., Naritomi, Y., et al. (2014). Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: a randomized, double-blind, placebo-controlled pilot study. Int. Immunopharmacol. 21 (2), 468–473. doi:10.1016/j.intimp.2014.06.001
Iuchi, K., Nishimaki, K., Kamimura, N., and Ohta, S. (2019). Molecular hydrogen suppresses free-radical-induced cell death by mitigating fatty acid peroxidation and mitochondrial dysfunction. Can. J. Physiol. Pharmacol. 97, 999–1005. doi:10.1139/cjpp-2018-0741
Johnson, V. L., and Hunter, D. J. (2014). The epidemiology of osteoarthritis. Best Pract. and Res. Clin. rheumatology 28 (1), 5–15. doi:10.1016/j.berh.2014.01.004
Jones, C. J., Rikli, R. E., and Beam, W. C. (1999). A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. sport 70 (2), 113–119. doi:10.1080/02701367.1999.10608028
Katz, J. N., Arant, K. R., and Loeser, R. F. (2021). Diagnosis and treatment of hip and knee osteoarthritis: a review. Jama 325 (6), 568–578. doi:10.1001/jama.2020.22171
Kolasinski, S. L., Neogi, T., Hochberg, M. C., Oatis, C., Guyatt, G., Block, J., et al. (2019). American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis care and Res. 72(2):149–162. doi:10.1002/acr.24131
Li, Y., Shen, C., Zhou, X., Zhang, J., Lai, X., and Zhang, Y. (2022). Local treatment of hydrogen-rich saline promotes wound healing in vivo by inhibiting oxidative stress via nrf-2/HO-1 pathway. Oxidative Med. Cell. Longev. 2022, 2949824. doi:10.1155/2022/2949824
Liao, T., Mei, W., Zhang, L., Ding, L., Yang, N., Wang, P., et al. (2023). l-carnitine alleviates synovitis in knee osteoarthritis by regulating lipid accumulation and mitochondrial function through the AMPK-ACC-CPT1 signaling pathway. J. Orthop. Surg. Res. 18 (1), 386. doi:10.1186/s13018-023-03872-9
Liew, J. W., King, L. K., Mahmoudian, A., Wang, Q., Atkinson, H. F., Flynn, D. B., et al. (2023). A scoping review of how early-stage knee osteoarthritis has been defined. Osteoarthr. Cartil. 31 (9), 1234–1241. doi:10.1016/j.joca.2023.04.015
Lin, H. Y., Lai, P. C., and Chen, W. L. (2020). A narrative review of hydrogen-oxygen mixture for medical purpose and the inhaler thereof. Med. gas Res. 10 (4), 193–200. doi:10.4103/2045-9912.295226
Lin, Y., Ohkawara, B., Ito, M., Misawa, N., Miyamoto, K., Takegami, Y., et al. (2016). Molecular hydrogen suppresses activated Wnt/β-catenin signaling. Sci. Rep. 6, 31986. doi:10.1038/srep31986
Liu, B., Jiang, X., Xie, Y., Jia, X., Zhang, J., Xue, Y., et al. (2022). The effect of a low dose hydrogen-oxygen mixture inhalation in midlife/older adults with hypertension: a randomized, placebo-controlled trial. Front. Pharmacol. 13, 1025487. doi:10.3389/fphar.2022.1025487
Liu, Q., Niu, J., Huang, J., Ke, Y., Tang, X., Wu, X., et al. (2015). Knee osteoarthritis and all-cause mortality: the wuchuan osteoarthritis study. Osteoarthr. Cartil. 23 (7), 1154–1157. doi:10.1016/j.joca.2015.03.021
Lu, H., Wang, W., Kang, X., Lin, Z., Pan, J., Cheng, S., et al. (2021). Hydrogen (H(2)) alleviates osteoarthritis by inhibiting apoptosis and inflammation via the JNK signaling pathway. J. Inflamm. Res. 14, 1387–1402. doi:10.2147/JIR.S297622
Lv, Z., Yang, Y. X., Li, J., Fei, Y., Guo, H., Sun, Z., et al. (2021). Molecular classification of knee osteoarthritis. Front. Cell. Dev. Biol. 9, 725568. doi:10.3389/fcell.2021.725568
Marks, R. (2012). Knee osteoarthritis and exercise adherence: a review. Curr. aging Sci. 5 (1), 72–83. doi:10.2174/1874609811205010072
Newman-Beinart, N. A., Norton, S., Dowling, D., Gavriloff, D., Vari, C., Weinman, J. A., et al. (2017). The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy 103 (2), 180–185. doi:10.1016/j.physio.2016.11.001
Nogueira, J. E., Passaglia, P., Mota, C. M. D., Santos, B. M., Batalhão, M. E., Carnio, E. C., et al. (2018). Molecular hydrogen reduces acute exercise-induced inflammatory and oxidative stress status. Free Radic. Biol. and Med. 129, 186–193. doi:10.1016/j.freeradbiomed.2018.09.028
Ohta, S. (2011). Recent progress toward hydrogen medicine: potential of molecular hydrogen for preventive and therapeutic applications. Curr. Pharm. Des. 17 (22), 2241–2252. doi:10.2174/138161211797052664
Ono, H., Nishijima, Y., and Ohta, S. (2023). Therapeutic inhalation of hydrogen gas for Alzheimer's disease patients and subsequent long-term follow-up as a disease-modifying treatment: an open label pilot study. Pharm. (Basel)., 16. doi:10.3390/ph16030434
Sabirli, F., Paker, N., and Bugdayci, D. (2013). The relationship between knee injury and osteoarthritis outcome score (KOOS) and timed up and go test in patients with symptomatic knee osteoarthritis. Rheumatol. Int. 33 (10), 2691–2694. doi:10.1007/s00296-012-2512-3
Safran-Norton, C. E., Sullivan, J. K., Irrgang, J. J., Kerman, H. M., Bennell, K. L., Calabrese, G., et al. (2019). A consensus-based process identifying physical therapy and exercise treatments for patients with degenerative meniscal tears and knee OA: the TeMPO physical therapy interventions and home exercise program. BMC Musculoskelet. Disord. 20 (1), 514. doi:10.1186/s12891-019-2872-x
Saramago, E. A., Borges, G. S., Cárnio, E. C., Nogueira, J. E., Soriano, R. N., Cárnio, E. C., et al. (2019). Molecular hydrogen potentiates hypothermia and prevents hypotension and fever in LPS-induced systemic inflammation. Brain, Behav. Immun. 75, 119–128. doi:10.1016/j.bbi.2018.09.027
Shumnalieva, R., Kotov, G., Ermencheva, P., and Monov, S. (2023). Pathogenic mechanisms and therapeutic approaches in obesity-related knee osteoarthritis. Biomedicines 12 (1), 9. doi:10.3390/biomedicines12010009
Sun, X., Zhen, X., Hu, X., Li, Y., Gu, S., Gu, Y., et al. (2019). Osteoarthritis in the middle-aged and elderly in China: prevalence and influencing factors. Int. J. Environ. Res. public health 16 (23), 4701. doi:10.3390/ijerph16234701
Terasaki, Y., Ohsawa, I., Terasaki, M., Takahashi, M., Kunugi, S., Dedong, K., et al. (2011). Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 301, L415–L426. doi:10.1152/ajplung.00008.2011
Tudorachi, N. B., Totu, E. E., Fifere, A., Ardeleanu, V., Mocanu, V., Mircea, C., et al. (2021). The implication of reactive oxygen species and antioxidants in knee osteoarthritis. Antioxidants Basel, Switz. 10 (6), 985. doi:10.3390/antiox10060985
Wang, C., Li, J., Liu, Q., Yang, R., Zhang, J. H., Cao, Y. P., et al. (2011). Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer's disease. Neurosci. Lett. 491 (2), 127–132. doi:10.1016/j.neulet.2011.01.022
Wang, H., Shi, K., Chen, H., Chen, Y., He, Y., Wang, X., et al. (2016). Mechanism of spinal cord neurons autophagy in the treatment of neuropathic pain treated with hydrogen. Chin. J. Anesthesiol. 36 (6), 693–696.
Wellsandt, E., and Golightly, Y. (2018). Exercise in the management of knee and hip osteoarthritis. Curr. Opin. rheumatology 30 (2), 151–159. doi:10.1097/BOR.0000000000000478
Xie, F., Li, S. C., Goeree, R., Tarride, J. E., O'Reilly, D., Lo, N. N., et al. (2008). Validation of Chinese western Ontario and McMaster Universities osteoarthritis index (WOMAC) in patients scheduled for total knee replacement. Qual. life Res. Int. J. quality-of-life aspects Treat. care rehabilitation 17 (4), 595–601. doi:10.1007/s11136-008-9340-7
Zhang, W., Zeng, L., Yu, H., He, Z., Huang, C., Li, C., et al. (2023). Injectable spontaneous hydrogen-releasing hydrogel for long-lasting alleviation of osteoarthritis. Acta Biomater. 158, 163–177. doi:10.1016/j.actbio.2022.12.056
Zhang, Y. Q. (2023). Initial practice for health and its effects on knee osteoarthritis [Doctoral dissertation]. China: Capital Medical University.
Zhang, Y. Q., Zhou, M. Y., Jiang, M. Y., Zhang, X. Y., Wang, X., and Wang, B. G. (2022). Awareness of initiative practice for health in the Chinese population: a questionnaire survey based on a network platform. World J. Clin. cases 10 (16), 5241–5252. doi:10.12998/wjcc.v10.i16.5241
Zheng, Z. G., Sun, W. Z., Hu, J. Y., Jie, Z. J., Xu, J. F., Cao, J., et al. (2021). Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 22 (1), 149. doi:10.1186/s12931-021-01740-w
Keywords: molecular hydrogen, elderly, osteoarthritis, WOMAC, exercise therapy
Citation: Wang C, Yan M, Li Y, Han L, Wang H, Jia S, Liu X, Liu Y, Wu F and Wang B (2025) Hydrogen-oxygen mixture inhalation as an adjunctive treatment to home-based exercise in older patients with knee osteoarthritis: an open-label, blinded-endpoint, randomized controlled trial. Front. Pharmacol. 16:1505922. doi: 10.3389/fphar.2025.1505922
Received: 04 October 2024; Accepted: 09 January 2025;
Published: 30 January 2025.
Edited by:
Pier Maria Fornasari, REGENHEALTHSOLUTIONS, ItalyReviewed by:
Sunita Nair, Consultant, Mumbai, IndiaCopyright © 2025 Wang, Yan, Li, Han, Wang, Jia, Liu, Liu, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoguo Wang, d2FuZ2JnQGNjbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.