
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 19 February 2025
Sec. Pharmacoepidemiology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1505665
 Yu-Ting Huang1
Yu-Ting Huang1 Tzu-Ling Chen2
Tzu-Ling Chen2 Yun-Lin Huang2
Yun-Lin Huang2 Ching-Hua Kuo2
Ching-Hua Kuo2 Yu-Fong Peng2
Yu-Fong Peng2 Sung-Chun Tang3
Sung-Chun Tang3 Jiann-Shing Jeng3
Jiann-Shing Jeng3 Chih-Feng Huang1,2,4
Chih-Feng Huang1,2,4 Shin-Yi Lin1,2*
Shin-Yi Lin1,2* Fang-Ju Lin1,2,4
Fang-Ju Lin1,2,4Objective: This purpose of this study is to analyze the influence of levetiracetam (LEV) on direct oral anticoagulant (DOAC) exposure and its implications for clinical outcomes.
Methods: This investigation comprised a retrospective cohort study utilizing the integrated medical database and a prospective observational study conducted in a tertiary hospital. Patients aged >65 years with atrial fibrillation and undergoing DOAC therapy were included and were categorized as LEV users and non-users based on LEV exposure status. In retrospective cohort, clinical outcomes between LEV users and non-users were compared, included ischemic stroke or transient ischemic attack (IS/TIA), systemic thromboembolism (STE) and major bleeding. In prospective cohort, DOAC trough concentration was measured.
Results: The retrospective study included 191 LEV users and 694 matched LEV non-users. The risk of IS/TIA and STE were not significantly different between two groups (hazard ratio [HR], 0.99 [0.51–1.91] and 0.94 [0.49–1.79], respectively). For major bleeding, a non-significant higher risk was observed in the LEV-user group in contrast to the LEV-non-user group (HR 2.65 [0.43, 16.33]). The prospective analysis included 19 LEV users and 76 matched LEV non-users. Low DOAC concentrations were observed in 5.3% of LEV-users and 14.5% of LEV non-users (P = 0.53). High DOAC concentration were observed in 10.5% of LEV-users and 11.8% LEV non-users (P = 0.57). The association between LEV therapy and low or high DOAC concentration was non-significant.
Conclusion: Concurrent use of LEV and DOAC did not significantly affect DOAC exposure or clinical outcomes. LEV may be a safe anti-seizure medication for patients receiving DOAC therapy.
Direct oral anticoagulants (DOACs) are the first-line treatment for stroke in patients with atrial fibrillation (AF). DOACs have fewer drug interactions than vitamin K antagonists and do not require routine coagulation monitoring (Steffel et al., 2021). However, all DOACs are P-glycoprotein (P-gp) substrates, and both apixaban and rivaroxaban undergo metabolism via the cytochrome P450 (CYP) system (Mueck et al., 2014; Byon et al., 2019), highlighting the pharmacokinetic considerations essential for their optimal use. The concurrent use of medications that affect the CYP system or P-gp can alter DOAC metabolism and affect clinical outcomes (Taha et al., 2020).
Post-stroke epilepsy occurs in 3%–6% of patients with stroke, and the risk increases with age (Holtkamp et al., 2017). This condition necessitates the simultaneous management of stroke sequelae and seizure control, often requiring the use of anti-seizure medications (ASMs) alongside DOAC therapy. First-generation ASMs, such as phenytoin, valproic acid, and carbamazepine, are known to interact with CYP enzymes or P-gp (Schmidt and Schachter, 2014). Levetiracetam (LEV), belonging to the newer generation of ASMs, has favorable characteristics including linear pharmacokinetics and fewer drug interactions (Patsalos, 2004), making it an appealing option for managing partial seizures (Privitera, 2001), post-stroke seizure (Belcastro et al., 2011), and seizure in older patients (Lippa et al., 2010). Additionally, LEV is better tolerated than carbamazepine for focal epilepsy in older adults (Werhahn et al., 2015). Despite these benefits, the concurrent use of LEV and DOACs is not without concern. Animal studies have shown that LEV is a weak inducer of P-gp and CYP3A4 (Galgani et al., 2018), potentially leading to decreased DOAC bioavailability. The European Heart Rhythm Association (EHRA) suggests the cautious use of LEV in patients receiving polypharmacy or multiple enzyme-inducing agents (Steffel et al., 2021).
Data regarding the effects of concurrent LEV and DOAC use are limited and conflicting. For instance, a study conducted in Hong Kong indicated that ASMs, including LEV, which modulate CYP enzymes and P-gp, were associated with an increased risk of ischemic stroke in patients undergoing DOAC therapy (Ip et al., 2022). In contrast, an investigation from Taiwan reported an elevated risk of bleeding events in patients concomitantly treated with LEV and DOACs (Wang et al., 2020). Despite these studies, there is a notable gap in real-world evidence related to how LEV influences DOAC plasma concentrations. This study aimed to evaluate the impact of LEV therapy on DOAC concentrations and clinical outcomes, with a specific focus on older patients who are more likely to receive polypharmacy and are more vulnerable to complications.
Our study comprised two parts, each designed to investigate the interactions between LEV and DOACs in older patients with AF. The first was a retrospective cohort study conducted using electronic health records (EHR) from a tertiary medical center. This section specifically examined the clinical outcomes of patients using DOACs and compared them with and without concomitant LEV treatment. The requirement for informed consent was waived owing to the use of de-identified data. The second part was a prospective study focusing on the pharmacokinetic effects of LEV on DOAC concentrations. We included patients who had participated in a registry study on DOAC concentration measurements. Factors related to DOAC concentrations were assessed, with emphasis on the impact of LEV administration. All individuals involved in this registry study provided informed consent before enrollment. The study protocol was approved by the Research Ethics Committee of the National Taiwan University Hospital (No. 201912233RINC, 202101078RINC).
Data were retrieved from the integrated medical database of the National Taiwan University Hospital (NTUH-iMD) spanning the period between 1 July 2012, and 31 December 2019. We included older patients (aged ≥65 years) diagnosed with AF (identified through at least one inpatient or two outpatient diagnoses) and who had been prescribed DOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) for more than 3 days. Patients were categorized into groups based on their use of LEV during DOAC treatment: (a) LEV users who were concurrently administered LEV and (b) LEV nonusers who did not receive LEV while on DOAC therapy. The index date was the date of initiating concurrent LEV and DOAC use in LEV users and the date of starting DOAC therapy in LEV nonusers. Continuous use of DOAC or LEV was determined for periods with interruptions between two prescriptions not longer than 14 days. For LEV users, only the first instance of combination therapy with LEV and DOAC was considered for the analysis if the patients had multiple episodes of starting and stopping LEV during their DOAC treatment course.
To investigate the potential pharmacokinetic interactions between DOAC and LEV, participants were enrolled from the Direct Oral Anticoagulant-Taiwan (DOAC-T) registry established in 2016 (NCT05333666). We included patients with AF aged 65 years or older who were receiving DOAC therapy and collected blood samples between 1 November 2016, and 31 January 2022. The concentration of DOACs was measured at the trough (immediately before the next dose) during the steady state using ultra-performance liquid chromatography-mass spectrometry (UHPLC-MS/MS). The UHPLC-MS/MS method, detailed in Supplementary Material, has been validated and published in previous investigations (Jhang et al., 2020). The DOAC concentrations were evaluated against the established expected therapeutic ranges reported by the EHRA: trough concentrations of 28–215 ng/mL for dabigatran, 12–137 ng/mL for rivaroxaban, 34–230 ng/mL for apixaban, and 12–43 ng/mL for edoxaban (Steffel et al., 2021). The date of concentration measurement served as the index date for the analysis. Based on the LEV exposure status on the index date, the participants were categorized into LEV user and non-user groups. To ensure a robust comparative analysis, each LEV user was matched with up to four LEV-non-users by age (difference of no more than 5 years), sex, and type of DOAC treatment.
The primary clinical outcome was the occurrence of ischemic stroke or transient ischemic attack (TIA). The secondary outcomes included systemic thromboembolism (STE) and major bleeding, the latter defined by the Platelet Inhibition and Patient Outcomes (PLATO) criteria, including the occurrence of intracranial hemorrhage (Abou Kaoud et al., 2023).
The follow-up period for clinical events began on the index date and continued until the earliest of the following: (a) 14 days after the cessation of DOAC and LEV combination therapy in the LEV-user group, or upon the conclusion of DOAC therapy in the LEV-non-user group; (b) occurrence of study outcomes; (c) loss to follow-up; (d) death; or (e) the end of the study period, which was 31 December 2019, for the first part of the study and 31 December 2022, for the second part. This extended follow-up of 14 days after the discontinuation of LEV in the LEV user group was implemented to account for the properties of LEV as a P-glycoprotein inducer, which may continue to affect drug interactions even after the medication has been discontinued (Supplementary Figure S1 in the Supplementary Material).
Descriptive statistics were used to summarize the data, including the means, standard deviations, medians, and ranges. Group differences were analyzed using Student’s t-test for continuous variables with a normal distribution, Mann-Whitney U test for continuous variables that were not normally distributed, and the chi-squared test or Fisher’s exact test for categorical variables.
Propensity score (PS) matching was used to balance the potential confounders between LEV users and nonusers in the EHR analysis. Each LEV user was matched to at least four LEV nonusers. The covariates included in the PS matching were age, sex, body mass index, laboratory test results (including renal function, liver function, and hemoglobin A1C), comorbid diseases, CHA2DS2-VASc score, HAS-BLED score, and concurrent medications. Of note, the item “labile international normalized ratio (INR)” in the HAS-BLED score was omitted because it was not available for DOAC users. Comorbid diseases and laboratory tests were collected within 3 months before the index date. Medical conditions were identified using the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD-9-CM and ICD-10-CM) codes, and the medications were identified using the World Health Organization Anatomical Therapeutic Chemical (ATC) codes.
Logistic regression analysis was conducted to identify the factors associated with DOAC concentrations above or below the expected therapeutic range. Initially, univariate analyses were performed to identify potential variables significantly associated with DOAC concentrations outside the expected range (p < 0.1). Subsequently, the identified variables were incorporated into a multivariate analysis. Creatinine clearance (CrCL) was specifically included because of its recognized influence on DOAC pharmacokinetics, which can affect drug concentrations. Multivariable analysis was used to assess the impact of LEV use on the variability in DOAC concentrations. The Cox proportional hazards model was used to assess the effect of LEV on clinical outcomes, and the proportional hazards assumption was appropriately tested. The Kaplan-Meier curve was presented using the Log-Rank test. All statistical analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC, United States) and IBM SPSS Statistics (version 8.0; IBM Corp., Armonk, NY, IBM Corp.). P < 0.05 was set as the threshold for statistical significance.
During the study, 8,752 patients met the inclusion criteria, of whom 234 (2.67%) concurrently used LEV and DOAC. After PS matching, the cohorts were refined to 191 LEV users and 694 LEV nonusers. The study enrollment process is shown in Figure 1. The demographic and clinical characteristics of both groups before and after PS matching are summarized in Table 1. The cohorts were well-balanced. Among medications known to interact with DOACs, the most commonly used were antiarrhythmic agents, particularly amiodarone. A few patients concurrently used ASMs other than LEV, most commonly phenytoin or valproic acid. The proportion of patients using immunosuppressants was very low.
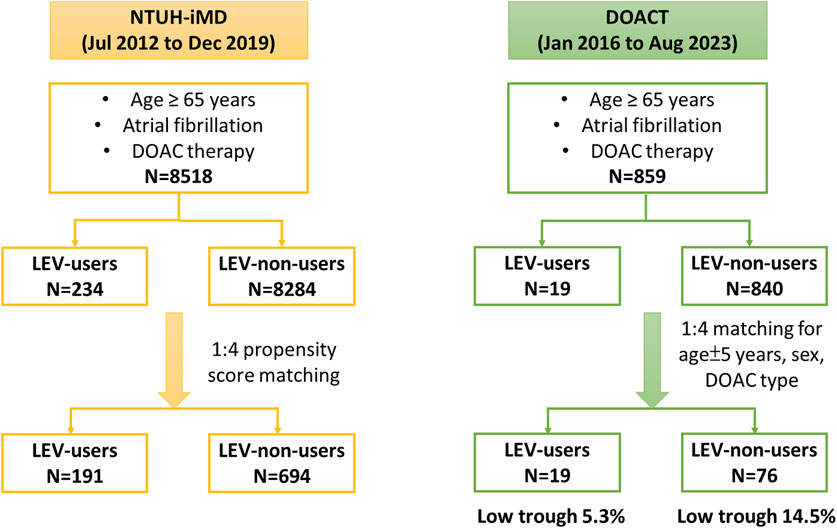
Figure 1. Flow diagram of participant enrollment. Abbreviations: DOAC, direct oral anticoagulant; DOAC-T, the direct oral anticoagulant registry in Taiwan; LEV, levetiracetam; NTUH-iMD, National Taiwan University Hospital integrated medical database.
The incidence of the primary and secondary outcomes are detailed in Table 2. As the primary outcome, ischemic stroke or TIA was observed in 15 LEV users (18.68 per 100 person-years) and 84 LEV nonusers (11.82 per 100 person-years). The incidence ratio was 1.58 (95% CI: 0.91–2.74). Cox proportional hazards regression analysis revealed a non-significant hazard ratio (HR) of 0.99 (95% CI: 0.51–1.91) for the two groups. Major bleeding events occurred in 3 LEV users (3.65 per 100 person-years) compared with 8 LEV non-users (1.05 per 100 person-years). Cox regression analysis suggested a higher risk in the LEV user group, although the difference was not significant (HR, 2.65; 95% CI: 0.43–16.33). The incidence of STE was not significantly different for the groups (HR, 0.94; 84% CI: 0.49–1.79). The Kaplan-Meier plots for the primary and secondary outcomes, illustrating the cumulative incidence over time, are presented in Figure 2.
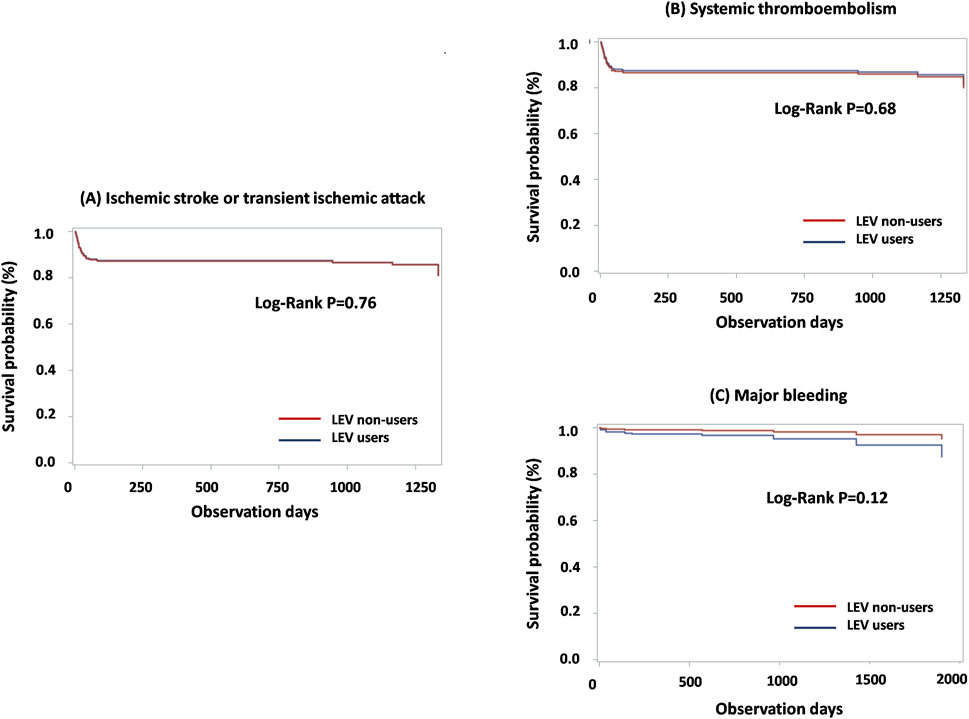
Figure 2. Kaplan-Meier plot for primary and secondary outcomes among matched LEV users and non-users: (A) Ischemic stroke or transient ischemic attack; (B) Systemic thromboembolism; (C) Major bleeding. Abbreviations: LEV, levetiracetam.
A total of 859 patients enrolled in the DOAC-T registry met the inclusion criteria. Following matching, the final cohort included 19 LEV users and 76 LEV non-users. The basic characteristics of the two groups are presented in Table 3. Similar to the retrospective cohort, the most commonly used medication with known interactions with DOACs was amiodarone. The proportion of patients with lower-than-expected DOAC concentrations was 5.3% for the LEV users and 14.5% for the non-users. However, this difference was not statistically significant (P = 0.53). Multivariate logistic regression adjusted for clinical variables showed that LEV therapy was not a significant predictor of lower-than-expected trough concentrations (adjusted odds ratio [aOR]: 0.36 [95% CI: 0.04, 3.01], P = 0.34) (Table 4; Supplementary Table S1). Conversely, the proportion of patients with higher-than-expected DOAC concentrations was 10.5% for the LEV user group and 11.8% for the LEV non-user group, with no significant difference between the groups (P = 0.57, Table 4). LEV was also not a significant factor in predicting higher-than-expected trough concentrations (aOR: 2.45 [95% CI: 0.46, 13.07], Supplementary Table S1).
The incidences of the primary and secondary outcomes are shown in Supplementary Table S2. Ischemic stroke or TIA occurred in one patient in the LEV user group relative to three patients in the LEV non-user group (risk rate ratio: 1.06 [95% CI: 0.02–13.19]). For secondary outcomes, five patients had STE (one LEV user and four LEV non-users, risk rate ratio: 0.79 [0.02–7.94]), and nine patients had major bleeding (two LEV users and seven LEV non-users, risk rate ratio: 0.90 [0.09–4.71]).
This study represents the first investigation of the concurrent use of LEV and DOAC and its impact on DOAC exposure and clinical outcomes among older Asian patients with AF. Our findings indicate that the concurrent administration of LEV and DOAC does not significantly affect the risk of IS/TIA, STE, major bleeding, or altered DOAC exposure.
Few studies have focused on the drug interactions between DOAC and LEV because of the limited population treated with this combination. Data from the Taiwanese insurance research database enrolled the largest number of patients (approximately 10,592 LEV users compared to 721,131 LEV non-users) (Wang et al., 2020). However, IS/TIA and STE were not analyzed in that study. Additionally, the findings of this investigation contradict the known mechanism behind this interaction. As a P-gp inducer, LEV theoretically reduces DOAC exposure and subsequently increases the risk of thromboembolism (Steffel et al., 2021).
Several other investigations have addressed this topic; however, the sample sizes were relatively limited. An Israeli investigation with a nested case-control design based on an insurance database showed that LEV was associated with stroke or STE risk; however, the LEV users and non-users were few (9 and 74, respectively). Additionally, this study enrolled patients who used DOAC for AF or deep vein thrombosis. Therefore, these results may not be completely generalized to patients with AF (Gronich et al., 2021). Another study analyzing data from the Israeli Food and Drug Administration Adverse Event Reporting System (FAERS) reported increased odds of anticoagulant treatment failure in patients treated with rivaroxaban or apixaban who were concurrently using enzyme-inducing ASMs, including LEV (Abou Kaoud et al., 2023). However, adverse events associated with the FAERS are self-reported, and the incidence of thromboembolic events may not be precisely estimated.
From our data, the proportion of patients with low DOAC trough concentrations was not significantly different for LEV users and non-users, indicating a lack of significant effect of LEV on the pharmacokinetic properties of DOAC. P-gp-mediated induction by LEV has only been observed in vivo animal studies (Steffel et al., 2021; Mathy et al., 2019). In a phase I study, concurrent use of LEV and digoxin, a P-gp substrate, in healthy human participants did not alter the pharmacokinetic or pharmacodynamic properties of digoxin (Levy et al., 2001). Therefore, the effects of LEV on P-gp in animals cannot be directly extrapolated to humans. LEV remains a safe option in patients under DOAC who require ASMs.
This study concentrated on the interaction between LEV and DOACs, specifically examining both clinical outcomes and DOAC exposure concentrations. By simultaneously investigating the effect of this drug combination on thromboembolism and bleeding events, our research provides comprehensive insights into the safety profile of this drug combination in older Asian patients with AF. Despite its strengths, this study had several limitations that must be acknowledged. First, DOAC exposure was assessed using trough concentrations rather than serial measurements across dosing intervals. This approach limited our ability to evaluate the influence of LEV on the area under the concentration-time curve for DOACs. Future studies should employ population pharmacokinetic analyses to provide a more detailed assessment of this interaction. Second, the impact of different LEV doses on DOAC interactions was not assessed due to the limited sample size. Additionally, in the DOAC-T cohort with concentration measurements, the small patient number made the findings inconclusive, especially dabigatran users. Further research with a larger cohort would allow for subgroup analyses to determine whether the extent of drug interactions varies across different dosing regimens of LEV. Third, there is a potential immortal time bias in our study design. Patients in the LEV user group may have been on relatively more stable DOAC treatment regimens than LEV non-users. In addition, differences between the follow-up durations of LEV users and non-users can lead to biased estimates of clinical outcome rates. Lastly, the impact of genetic polymorphisms on DOAC exposure was not evaluated in the present study. Genes encoding P-glycoprotein, such as ABCB1 (ATP-Binding Cassette Sub-Family B Member 1), can influence DOAC exposure. Although some studies have reported that the ABCB1 genotype is not a significant determinant of inter-individual variability in the pharmacokinetics of dabigatran and rivaroxaban (Gouin-Thibault et al., 2017), this remains an important concern requiring further investigation.
This study represents a pioneering effort to simultaneously investigate DOAC exposure and clinical outcomes associated with the concurrent use of LEV and DOACs in older patients with AF. Contrary to expectations based on the pharmacological profile of LEV as a P-glycoprotein inducer, our findings indicated that LEV does not significantly alter DOAC exposure or affect the incidence of ischemic stroke, STE, or major bleeding events. These results suggest that LEV can be safely co-administered with DOACs in this patient population without necessitating adjustments to DOAC dosing. However, given the limitations of our study, further research using larger and more diverse cohorts and detailed pharmacokinetic profiling is essential to fully elucidate the clinical implications of this drug interaction.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Research Ethics Committee of the National Taiwan University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Y-TH: Conceptualization, Data curation, Formal Analysis, Writing–original draft. T-LC: Data curation, Writing–review and editing. Y-LH: Data curation, Writing–review and editing. C-HK: Funding acquisition, Methodology, Resources, Writing–review and editing. Y-FP: Methodology, Writing–review and editing. S-CT: Conceptualization, Data curation, Funding acquisition, Project administration, Supervision, Writing–review and editing. J-SJ: Supervision, Writing–review and editing. C-FH: Supervision, Writing–review and editing. S-YL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Writing–review and editing. F-JL: Conceptualization, Data curation, Formal Analysis, Validation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This investigation is funded by Ministry of Science and Technology (112–2314-B-002 -313), Ministry of Education for financial assistance, and National Taiwan University Hospital (112-S0123, 113-S0062). National Science and Technology Council, Taiwan is 112-2314-B-002-313.
We would like to thank Chi-Chuan Wang from School of Pharmacy, National Taiwan University for the consultation of statistical analyses. We would also like to thank National Taiwan University School of Pharmacy (NTUSP) Endowment Fund in support of the Platform for Clinical Mass Spectrometry and NMR Structure Elucidation and Chia-Chi Chang from NTUSP for the measurement of DOAC level.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1505665/full#supplementary-material
Abou Kaoud, M., Nissan, R., Segev, A., Sabbag, A., Orion, D., and Maor, E. (2023). Levetiracetam interaction with direct oral anticoagulants: a pharmacovigilance study. CNS Drugs 37 (12), 1111–1121. doi:10.1007/s40263-023-01052-1
Belcastro, V., Pierguidi, L., and Tambasco, N. (2011). Levetiracetam in brain ischemia: clinical implications in neuroprotection and prevention of post-stroke epilepsy. Brain Dev. 33 (4), 289–293. doi:10.1016/j.braindev.2010.06.008
Byon, W., Garonzik, S., Boyd, R. A., and Frost, C. E. (2019). Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin. Pharmacokinet. 58 (10), 1265–1279. doi:10.1007/s40262-019-00775-z
Galgani, A., Palleria, C., Iannone, L. F., De Sarro, G., Giorgi, F. S., Maschio, M., et al. (2018). Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front. Neurol. 9, 1067. doi:10.3389/fneur.2018.01067
Gouin-Thibault, I., Delavenne, X., Blanchard, A., Siguret, V., Salem, J. E., Narjoz, C., et al. (2017). Interindividual variability in dabigatran and rivaroxaban exposure: contribution of ABCB1 genetic polymorphisms and interaction with clarithromycin. J. Thromb. Haemost. 15 (2), 273–283. doi:10.1111/jth.13577
Gronich, N., Stein, N., and Muszkat, M. (2021). Association between use of pharmacokinetic-interacting drugs and effectiveness and safety of direct acting oral anticoagulants: nested case-control study. Clin. Pharmacol. Ther. 110 (6), 1526–1536. doi:10.1002/cpt.2369
Holtkamp, M., Beghi, E., Benninger, F., Kälviäinen, R., Rocamora, R., Christensen, H., et al. (2017). European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. Eur. Stroke J. 2 (2), 103–115. doi:10.1177/2396987317705536
Ip, B. Y., Ko, H., Wong, G. L., Yip, T. C., Lau, L. H., Lau, A. Y., et al. (2022). Thromboembolic risks with concurrent direct oral anticoagulants and antiseizure medications: a population-based analysis. CNS Drugs 36 (12), 1313–1324. doi:10.1007/s40263-022-00971-9
Jhang, R. S., Lin, S. Y., Peng, Y. F., Chao, H. C., Tsai, I. L., Lin, Y. T., et al. (2020). Using the PCI-IS method to simultaneously estimate blood volume and quantify nonvitamin K antagonist oral anticoagulant concentrations in dried blood spots. Anal. Chem. 92 (3), 2511–2518. doi:10.1021/acs.analchem.9b04063
Levy, R. H., Ragueneau-Majlessi, I., and Baltes, E. (2001). Repeated administration of the novel antiepileptic agent levetiracetam does not alter digoxin pharmacokinetics and pharmacodynamics in healthy volunteers. Epilepsy Res. 46 (2), 93–99. doi:10.1016/s0920-1211(01)00253-4
Lippa, C. F., Rosso, A., Hepler, M., Jenssen, S., Pillai, J., and Irwin, D. (2010). Levetiracetam: a practical option for seizure management in elderly patients with cognitive impairment. Am. J. Alzheimer's Dis. and Other Dementias 25 (2), 149–154. doi:10.1177/1533317508325095
Mathy, F. X., Dohin, E., Bonfitto, F., and Pelgrims, B. (2019). Drug-drug interaction between levetiracetam and non-vitamin K antagonist anticoagulants. Eur. Heart J. 40 (19), 1571. doi:10.1093/eurheartj/ehy780
Mueck, W., Stampfuss, J., Kubitza, D., and Becka, M. (2014). Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 53 (1), 1–16. doi:10.1007/s40262-013-0100-7
Patsalos, P. N. (2004). Clinical pharmacokinetics of levetiracetam. Clin. Pharmacokinet. 43 (11), 707–724. doi:10.2165/00003088-200443110-00002
Privitera, M. (2001). Efficacy of levetiracetam: a review of three pivotal clinical trials. Epilepsia 42 (Suppl. 4), 31–35. doi:10.1046/j.1528-1157.2001.0420s4031.x
Schmidt, D., and Schachter, S. C. (2014). Drug treatment of epilepsy in adults. Bmj 348, g254. doi:10.1136/bmj.g254
Steffel, J., Collins, R., Antz, M., Cornu, P., Desteghe, L., Haeusler, K. G., et al. (2021). 2021 European heart Rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 23 (10), 1612–1676. doi:10.1093/europace/euab065
Taha, M., Li, W., Schmidt, C. M., Gonzalez-Castellon, M., and Taraschenko, O. (2020). The interactions between anticonvulsants and non-vitamin K antagonist oral anticoagulant agents: a systematic review. Epilepsy Res. 162, 106304. doi:10.1016/j.eplepsyres.2020.106304
Wang, C. L., Wu, V. C., Chang, K. H., Tu, H. T., Kuo, C. F., Huang, Y. T., et al. (2020). Assessing major bleeding risk in atrial fibrillation patients concurrently taking non-vitamin K antagonist oral anticoagulants and antiepileptic drugs. Eur. Heart J. Cardiovasc Pharmacother. 6 (3), 147–154. doi:10.1093/ehjcvp/pvz035
Keywords: direct oral anticoagulants, levetiracetam, drug interaction, elderly, thromboembolism, bleeding
Citation: Huang Y-T, Chen T-L, Huang Y-L, Kuo C-H, Peng Y-F, Tang S-C, Jeng J-S, Huang C-F, Lin S-Y and Lin F-J (2025) Impact of levetiracetam on direct oral anticoagulant level and outcomes among older Asian patients with atrial fibrillation. Front. Pharmacol. 16:1505665. doi: 10.3389/fphar.2025.1505665
Received: 04 October 2024; Accepted: 27 January 2025;
Published: 19 February 2025.
Edited by:
Anick Bérard, Montreal University, CanadaReviewed by:
Tetsuji Kitano, Mie University Hospital, JapanCopyright © 2025 Huang, Chen, Huang, Kuo, Peng, Tang, Jeng, Huang, Lin and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shin-Yi Lin, aHNpbjkyNEBudHVoLmdvdi50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.