
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 17 March 2025
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1500668
 Cristian Trovato1,2*†
Cristian Trovato1,2*† Stefano Longobardi3†
Stefano Longobardi3† Elisa Passini1
Elisa Passini1 Kylie A. Beattie3*
Kylie A. Beattie3* Maxx Holmes1
Maxx Holmes1 Khuram W. Chaudhary4
Khuram W. Chaudhary4 Eric I. Rossman4
Eric I. Rossman4 Blanca Rodriguez1*
Blanca Rodriguez1*Drug-induced changes in cardiac contractility (inotropy) can lead to cardiotoxicity, a major cause of discontinuation in drug development. Preclinical approaches to assess cardiac inotropy are imperfect, with in vitro assays limited to stem cell-derived or adult human primary cardiomyocytes. Human mechanistic in silico modelling and simulations are already successfully applied for proarrhythmia prediction, contributing to cardiac safety assessment strategies in early drug development. In this study, we investigated their ability to predict drug-induced effects on cardiac inotropy. We considered a validation set of 28 neutral/negative inotropic and 13 positive inotropic reference compounds and simulated their effects on cell contractility via ion channel inhibition and perturbation of nine biomechanical modelling parameters, respectively. For each compound, a wide range of drug concentrations was simulated in an experimentally calibrated control population of 323 human ventricular in silico cells. Simulated biomarkers indicating drug-induced inotropic effects were compared with in vitro preclinical data from the literature. Computer simulations predicted drug-induced inotropic changes observed in vitro for 25 neutral/negative inotropes and 10 positive inotropes. Predictions of negative inotropic changes were quantitatively in agreement for 86% of tested drugs. Active tension peak was identified as the biomarker with highest predictive potential. This study describes the validation and application of an in silico cardiac electromechanical model for drug safety evaluation, combining ion channel inhibition data and information on potential inotropic mechanisms to predict inotropic changes. Furthermore, a route for its integration as part of a preclinical drug safety assessment strategy is outlined.
Drug-induced changes in cardiac contractility (inotropy) can lead to cardiotoxicity, a major cause of discontinuation of drug development projects (Klein and Redfern, 2015; Mamoshina et al., 2021). Accurate assessment of drug-induced effects on cardiac inotropy during pre-clinical stages of drug development remains challenging, without a consensus on gold standard biomarkers for comparison of in vitro results to in vivo or clinical biomarkers for model validation. In addition, non-clinical in vitro strategies to assess changes in contractility for both small and large molecules are limited to low/medium throughput assays, which do not always translate to clinical outcomes. The most common in vitro human models comprise stem cell-derived cardiomyocytes (Pointon et al., 2015) and adult human primary cardiomyocytes (Abi-Gerges et al., 2020; Nguyen et al., 2017). Despite both models offering valuable insights into human heart function and pathology, they present important limitations: stem cell-derived cardiomyocytes have an immature phenotype, which impacts the Ca2+ dynamics, leading to less robust predictions. Adult human primary cardiomyocytes have short lifespans in culture, lack proliferative capacity, are not readily available, pose ethical and legal considerations, and are expensive and difficult to handle. Therefore, alternative approaches should be considered to address some of the gaps in current cardiac contractility assessment strategies, particularly in the context of developing a high-throughput framework to inform dosing strategies for in vivo/clinical studies.
Cardiac contraction is initiated by an increase in the intracellular Ca2+ concentration ([Ca2+]i). Ca2+ binds to troponin C on the thin filament, which causes tropomyosin to move out of the actin groove, exposing actin-binding sites. The thick filament, composed of many myosin molecules, has a central core of aligned myosin tails with protruding myosin heads; these myosin heads bind to the exposed actin-binding sites. Contraction then follows, as described by the sliding filament theory (Huxley, 1953). The attached myosin heads rotate in the power stoke, pulling the thick filaments past the thin filaments and causing the sarcomere to contract. The myosin heads then unbind and can reattach to actin to further contract the sarcomere (Lewalle et al., 2022).
The sarcomere is situated within the selectively permeable cell membrane, which aids in maintaining intracellular ionic homoeostasis. Within this highly regulated space, disruption in Ca2+ homoeostasis will impact normal contraction and relaxation. Ca2+ diffuses from the dyadic space into the cytosol, which triggers further release of Ca2+ from an intracellular Ca2+ store called the sarcoplasmic reticulum (SR), and it then binds to the sarcomeric proteins, activating contraction. As Ca2+ is removed from the cytosol, [Ca2+]i decreases, causing Ca2+ to dissociate from the sarcomeric proteins and leading to sarcomere relaxation. Ca2+ removal is achieved either through Ca2+ extrusion from the cell or via Ca2+ uptake into the SR (Hall and Hall, 2021; Levick, 2009).
Block of the L-type Ca2+ channel and subsequent binding to the ryanodine receptors (Ca2+-release channels located on the SR) will prevent Ca2+ diffusion from the dyadic space into the cytosol from the SR. At the same time, blocking the sodium–calcium exchanger (NCX), the plasma membrane Ca2+ ATPase (PMCA), or the SR Ca2+ ATPase (SERCA) will prevent Ca2+ extrusion from the cytosolic space. Sarcomere modulators are emerging as an important class of compounds since the dynamics of sarcomeric proteins form the foundation of myocardial contraction and relaxation. Sarcomere modulators can alter myofilament Ca2+ sensitivity without altering Ca2+ homoeostasis (Longobardi et al., 2022).
In the last decade, in silico approaches using human-based, biophysically detailed models and multiscale simulations have proven to be powerful tools for drug safety assessment, particularly for predicting proarrhythmic risk (Lancaster and Sobie, 2016; Passini et al., 2017; Li et al., 2019a; Passini et al., 2019; Llopis-Lorente et al., 2022; Trovato et al., 2022). Human-based electromechanical models have also been recently published for simultaneous proarrhythmic and inotropic risk assessment of drug action on ion channels and cross-bridge dynamics (Margara et al., 2021). The use of in silico approaches has been supported by regulators such as the United States Food and Drug Administration (FDA), which led the Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative (Sager et al., 2014; Li et al., 2019b), and the European Medicines Agency (Musuamba et al., 2021), which established a task force on medical innovation to facilitate the adoption of innovative products, methods, and technologies in drug development. One of the main achievements of these initiatives was the identification of general principles for model design, development, and validation (Musuamba et al., 2021; Li et al., 2019c), which go beyond proarrhythmia prediction and can be applied to different contexts of use.
In this study, we investigated the feasibility of predicting and explaining drug-induced effects on cardiac cellular inotropy, action potential, and calcium dynamics using mechanistic, in silico human multiscale modelling and simulations, with in vitro ion channel measurements and known inotropic modes of drug action as input (Figure 1A). First, we simulate hypothetical specific Na+, K+, and Ca2+ channel blockers to assess model sensitivity and identify the most informative biomarkers. Then, a set of 41 reference compounds was considered a validation dataset. Among these compounds, 28 inhibit specific cardiac ion channels and exhibit negative or non-inotropic effects. Half-maximal inhibitory concentration (IC50) values were used to describe the drug-induced effects on ionic currents and served as a model input. For each compound, a wide range of drug concentrations was simulated in an experimentally calibrated population of 323 human ventricular in silico cells, representing a healthy control population. For the remaining 13 compounds, the known modes of action are more heterogeneous, thus requiring an additional explorative step to be applied using the same in silico framework. Simulated biomarkers were then compared with in vitro preclinical data available in the literature and clinical observations of drug-induced inotropy effects for some compounds. The whole study pipeline is depicted in Figure 1A.
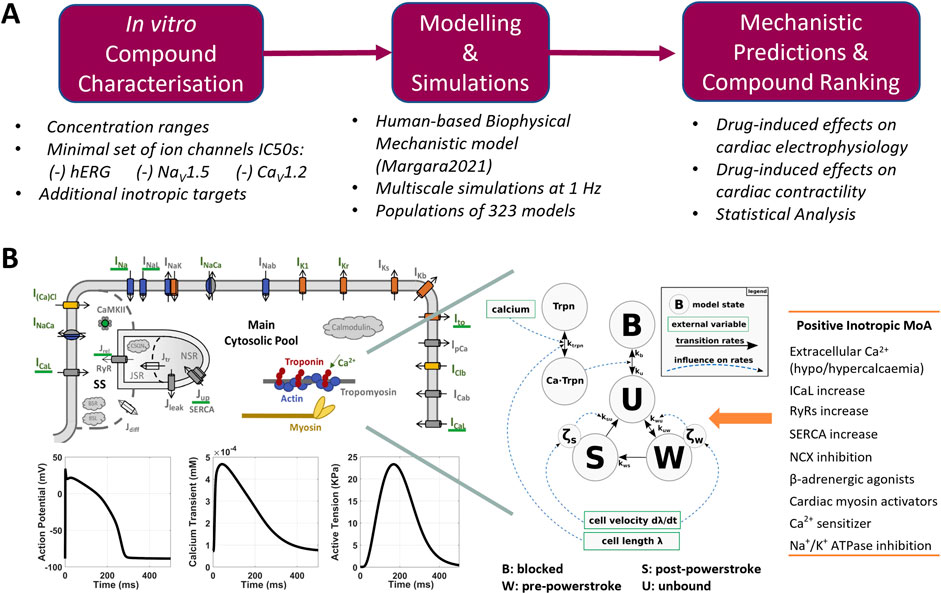
Figure 1. (A) Proposed pipeline for the in silico assessment of drug-induced changes in human cardiac electrophysiology and contractility. (B) Modelling and simulation overview: structure of the Margara2021 model, combining the ToR-ORd model for human cardiac electrophysiology (top left) and the Land model for cardiomyocyte mechanics description (centre), as described by Margara et al. (2021). Exemplificative model outputs (bottom-left) and the list of modes of action (MoA) tested to simulated positive inotropic compounds (right) are also shown.
Two datasets of in vitro measurements of cardiac contractility (Nguyen et al., 2017; Abi-Gerges et al., 2020) were considered to be preclinical in vitro evidence of drug-induced inotropic effects. Both datasets comprise optical recordings of sarcomere shortening from isolated human adult primary cardiomyocytes. The list of compounds and concentrations simulated in the present study from each in vitro dataset is reported in Table 1. In the first study, Nguyen and others investigated contractility and proarrhythmia biomarkers for CiPA compounds (Li et al., 2019a; Sager et al., 2014), resulting in a collection of negative or neutral inotropic compounds. Drugs were tested in 3–8 samples from 1 or 2 donor hearts. In the second study, Abi-Gerges and others assessed a set of 12 contractility parameters for compounds, leading to positive inotropic effects through a variety of mechanisms of action, including hypo/hypercalcaemia, ICaL increase, ryanodine receptor (RyR) modulation, SERCA modulation, NCX inhibition, β-adrenergic stimulation, adenylyl cyclase activation, phosphodiesterase (PDE) inhibition, cardiac myosin activation, Ca2+ sensitization, and Na+/K+ ATPase inhibition. We will refer to the compound data from the first and second studies as “Dataset 1” and “Dataset 2,” respectively, throughout.
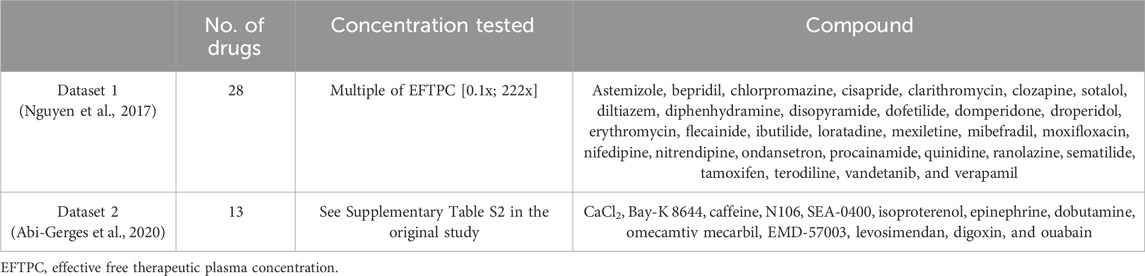
Table 1. In vitro datasets used for comparison against model predictions: number of drugs simulated in this study, concentration ranges tested in vitro, and compound names.
Combining selected compounds (see Section 2.2) from both in vitro datasets, we obtained a collection of 41 compounds, of which 23 led to a negative inotropic effect, 13 to a positive inotropic effect, and 5 had no effects on contractility. A complete description of the in vitro experiments can be found in the original articles (Nguyen et al., 2017; Abi-Gerges et al., 2020).
Drug-induced changes in cardiac electrophysiology are routinely screened in the early stage of drug development, either as primary (on-target) or secondary (off-target) pharmacological signals. The most important signal is hERG since its inhibition can lead to QT prolongation and Torsades de Pointes arrhythmia; therefore, hERG screening is a regulatory requirement (as detailed in ICH S7B). Other pharmacological signals include in vitro recordings of other cardiac ion currents, such as the fast sodium current (INa), the late sodium current (INaL), the L-type calcium current (ICaL), the slow potassium rectifier (IKs), the outward potassium current (Ito), and the potassium inward rectifier (IK1). However, not all of these currents might be routinely screened during early drug development, and not all of them may be crucial for safety assessment (Zhou et al., 2020).
In this study, to simulate inotropic changes induced by the compounds in Dataset 1, i.e., non-inotropic or negative inotropic compounds, we used IC50 values, Hill coefficients, and free therapeutic plasma concentrations available in the literature (Crumb et al., 2016; Delaunois et al., 2021; Kramer et al., 2013; Passini et al., 2019) and listed in Supplementary Table S1. Most compounds are characterised by IC50 values and Hill coefficients for IKr, INa, and ICaL, similar to what would happen during the early stages of drug development (Figure 1A). For five compounds, namely, ajmaline, azimilide, sematilide, diphenhydramine, and tamoxifen, no homogenous (i.e., recorded as much as possible in the same experimental settings and from the same source) IC50 values were available in the literature; therefore, these compounds were excluded from this study. For the same reason, two negative inotropic compounds from Dataset 2 (ryanodine and thapsigargin) were excluded from this study.
The Margara2021 model (Margara et al., 2021) was selected as the in silico model for simulating electromechanical dynamics in human ventricular cardiomyocytes (Figure 1B). The main outputs of the Margara2021 model are action potential (AP), intracellular calcium transient (CaT), and active tension (AT) time courses over a single beat or multiple beats at a given pacing frequency. Representative traces (1 beat at 1 Hz pacing frequency) are shown in Figure 1B.
The Margara2021 model combines the human ventricular electrophysiological ToR-ORd cellular model (Tomek et al., 2019) with a model of human cardiomyocyte mechanics (Land et al., 2017), both calibrated and validated with experimental data. In addition to the already mentioned INa, ICaL, IKr, IKs, INaL, IK1, and Ito, the ToR-ORd model in the Margara2021 model also includes a mathematical description for the sodium–calcium exchange current (INCX), the sodium–potassium ATPase current (INaK), the sarcolemmal calcium pump current (IpCa), and sodium (Na+) and potassium (K+) background currents. The model also incorporates a detailed description of the calcium subsystem and excitation–contraction coupling, including calcium release from the ryanodine receptors, calcium uptake through SERCA pumps, calcium buffers (calmodulin, troponin, anionic SR and sarcolemmal-binding sites, and calsequestrin), and calcium-/calmodulin-dependent protein kinase.
The Land model is based on measurements obtained from human cardiomyocytes at body temperature, and it comprises a three-state cross-bridge model to reproduce troponin C and tropomyosin kinetics, accounting for cross-bridge distortion. The bidirectional electromechanical coupling between ToR-ORd and Land models in the Margara2021 model is based on the free [Ca2+]i computed in the ToR-ORd electrophysiology model, which serves as the input for the Land model. In turn, the amount of Ca2+ bound to troponin C, computed in the Land model, is fed back into the ToR-ORd electrophysiological model and used to update the free [Ca2+]i.
Using the Margara2021 model, we developed an experimentally calibrated population of human cardiac ventricular cardiomyocytes, incorporating cell-to-cell variability. The population was designed as in previous studies (Passini et al., 2017; Passini et al., 2019; Trovato et al., 2020; Trovato et al., 2022), following the population of models methodology (Britton et al., 2013; Lancaster and Sobie, 2016; Muszkiewicz et al., 2015). The population was constructed using Virtual Assay software (v.3.2 © 2018 Oxford University Innovation Ltd. Oxford, UK), a user-friendly software program to perform in silico simulations in a population of models (Passini et al., 2021).
An initial population of 1,000 models was constructed by sampling nine conductance values of the main ionic currents mentioned above (INa, INaL, ICaL, Ito, IKr, IKs, IK1, INCX, and INaK) and Ca2+ uptake and release maximal currents in the range [50–150]% of their baseline values. All these models were paced individually at 1 Hz for 500 beats to allow the models to reach Na+, K+, and Ca2+ diastolic concentration stability (steady state), and the last-beat output traces for each model were used to compute a set of 15 biomarkers. In particular, seven biomarkers characterised the AP curve: AP duration at 40%, 50%, and 90% of repolarisation (APD40, APD50, and APD90); AP triangulation, defined as the difference between APD90 and APD40 (Tri90-40); maximum upstroke velocity (dV/dtmax); peak voltage (Vpeak); and resting membrane potential (RMP); four biomarkers characterised the CT curve: duration at 50% and 90% of repolarisation (CTD50 and CTD90) and minimum/diastolic and maximum calcium concentrations (CaiD and CTpeak); and four biomarkers characterised the AT curve: AT peak (ATpeak), AT time to peak (ATttp), and AT relaxation times at 50% and 90% (ATrt50 and ATrt90). In addition to the abovementioned biomarkers, the electromechanical window (EMw, Passini et al., 2019) was computed as the difference between CTD90 and APD90, and qNet was computed as the total net charge (i.e., balancing inward and outward currents) flowing through INaL, ICaL, Ito, IKr, IKs, and IK1 over an entire beat (Chang et al., 2017).
The population was then filtered based on biomarker values (Supplementary Table S2) from healthy human left ventricular myocytes (Margara et al., 2021; Passini et al., 2019), and a total of 322 models (out of the initial 1,000 models) whose biomarkers were within experimental ranges were retained. These 322 models constituted the experimentally calibrated population and were then used for the simulations, along with the baseline model, resulting in a total of 323 electrophysiological profiles.
Running a full population simulation involves concurrently running the 323 models using their calibrated ion channel parametrisations. Each model is paced for 1,000 beats at 1 Hz, and the last-beat curves (AP, CaT, and AT) are regarded as the model outputs. The simulated AP curve is then checked for the occurrence of depolarisation and repolarisation abnormalities, as defined by Passini et al. (2017): i) repolarisation abnormalities were defined as the presence of a positive derivative of the membrane potential following Vpeak (early after depolarisation) or when the membrane potential did not reach the resting condition by the end of the beat (repolarisation failure); ii) depolarisation abnormalities occurred when the upstroke phase was compromised, i.e., when Vpeak was lower than 0 mV or when the time needed to reach 0 mV was longer than 100 ms. Finally, the biomarkers described above are calculated from the output curves for all models where there was no prediction of abnormalities.
Drug effects can be incorporated by further manipulating the baseline parametrisation of all the population models for the ion channels and/or other mechanisms before running a simulation. In the next two sections, we will describe in detail how we performed simulations for the compounds from experimental Dataset 1 and Dataset 2.
A simple pore-block model (Brennan et al., 2009) was used to simulate ion channel inhibition under a compound effect. This model provides the fraction of residual current
For each compound in Dataset 1, we tested a wide range of concentrations, taken as multiples of the compound’s EFTPC, ranging from 0.1× to 100×. This range extended well beyond the concentrations estimated/clinically measured for humans, allowing for a broader exploration of any drug-induced effects on electrophysiology and contractility. One of the main advantages of using in silico modelling is that we can explore a large number of concentrations, which would be unfeasible to test experimentally, although caveated with further modelling assumptions, such as neglecting the interplay of solubility and metabolism. Therefore, for those compounds that did not reach a 50% reduction in peak tension, we additionally extended the concentration range up to 100,000× the EFTPC to check for saturating behaviours, for model verification purposes.
For each drug, dose–response curves were derived for the peak tension biomarker. We used two methods to evaluate the impact of different metrics on inotropy assessment: (1) a classic non-linear least squares approach to fit a Hill curve through median biomarker values from the full, simulated population and (2) a Bayesian approach to fit the same type of curve by incorporating biomarker variability across the full population of models. Figure 2A illustrates the fitting process. In the first case using biomarker medians, single
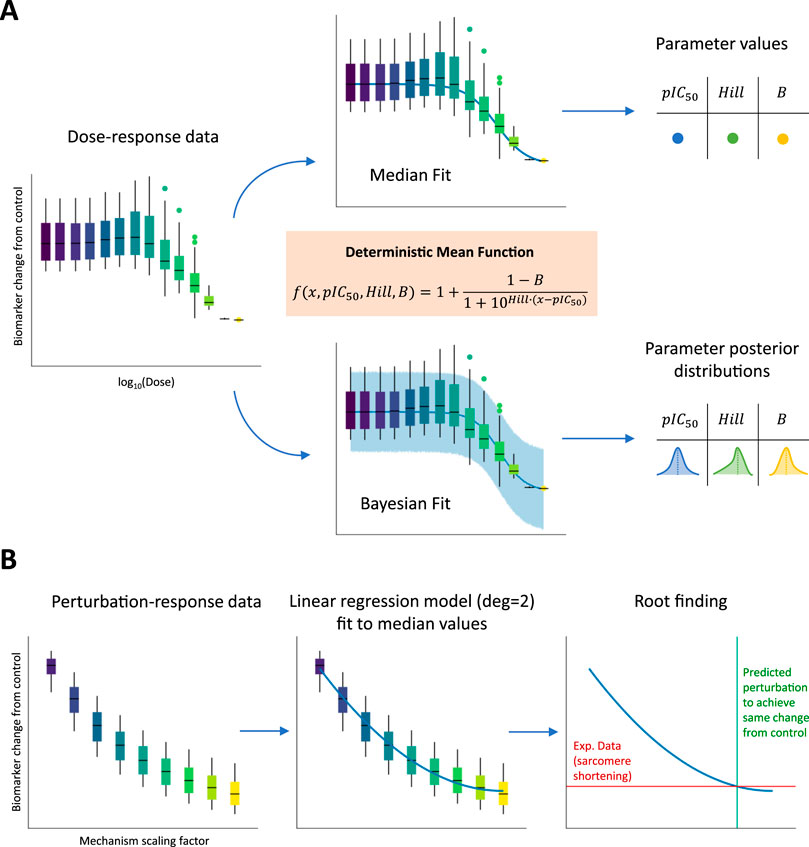
Figure 2. Fitting dose–response and perturbation-response curves to biomarker values simulated using the full population of models. (A) The three main parameters (including pIC50 and Hill values) of a sigmoid function (orange box) were fitted using two approaches: (1-top panel) non-linear least-squares method to minimize the distance of the curve from median population biomarker values at the tested concentrations and (2-bottom panel) a Bayesian method to incorporate population biomarker values’ variability. In the first case, pointwise estimates are derived for the three sigmoid parameters, while in the second case, full posterior distributions are derived instead. (B) A linear regression model with degree equal to 2 (parabola) was fitted to median population biomarker values at the different tested mechanism perturbations. An experimental sarcomere shortening value observed at the EC50 value for drugs represented by the mechanism is then projected on the parabola to find the intersection point corresponding to the mechanism scaling factor needed to reproduce in silico the experimental observation.
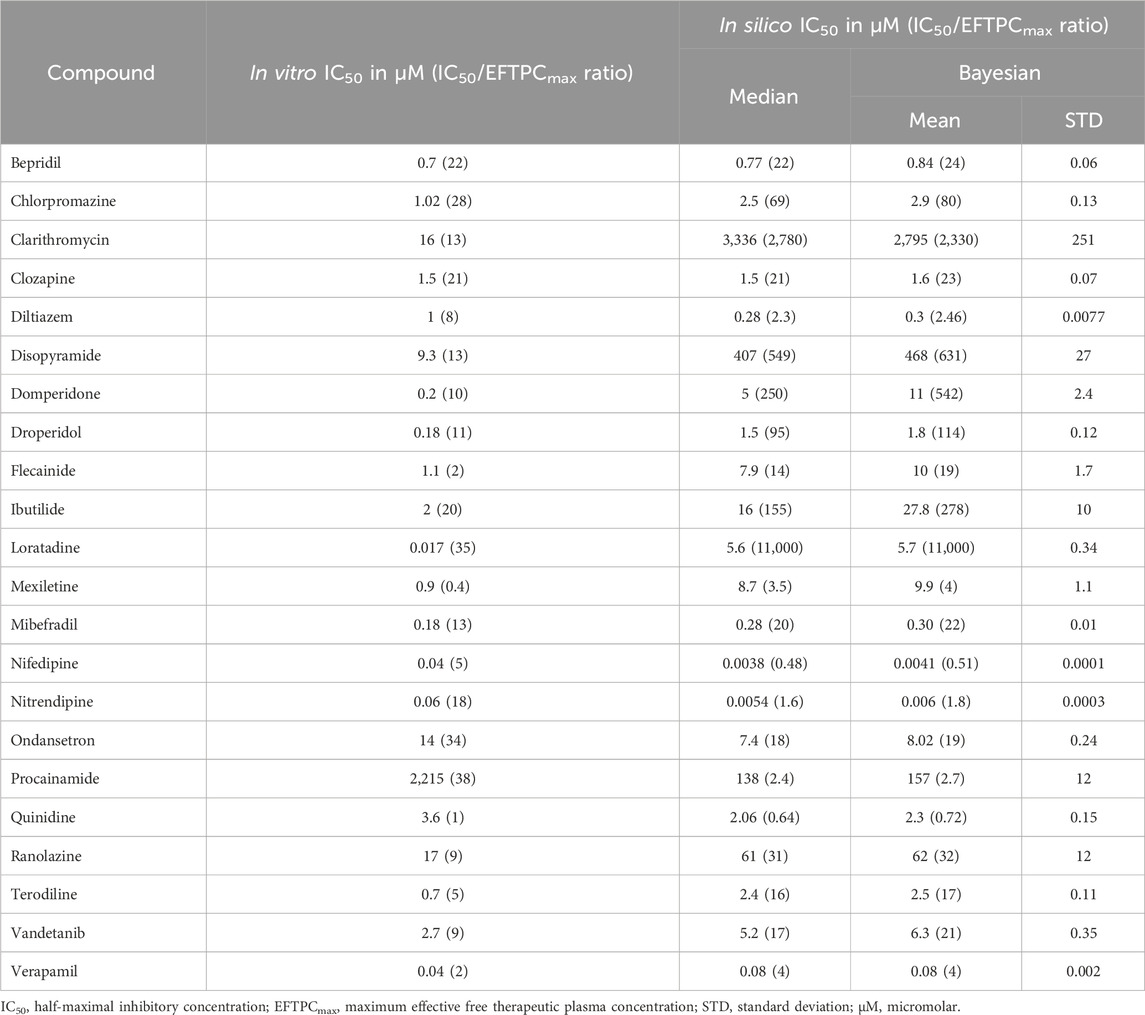
Table 2. Contractility IC50 values observed in vitro for sarcomere shortening and simulated for the active tension peak. The latter was computed both from median concentration-response values and using a Bayesian approach. The IC50 and EFTPCmax ratio is reported between brackets.
To investigate how perturbation of additional mechanisms may affect contractility, a one-at-a-time sensitivity analysis was performed on the Margara2021 model. We selected nine parameters that could be altered in the model to mimic functional effects induced by the positive inotropic compounds (Dataset 2) tested by Abi-Gerges and collaborators on human cardiomyocytes. The selected mechanisms were (1) extracellular Ca2+ concentration increase; (2) ICaL activation; (3) RyR activation; (4) SERCA pump activation; (5) Na+/Ca2+ exchanger inactivation; (6) β-adrenergic stimulation; (7) Ca2+ sensitivity decrease; (8) Na+/K+ pump inactivation; and (9) cardiac myosin activation. Phosphodiesterase inhibitors (IBMX and milrinone) and adenylyl cyclase activators (forskolin and NKH-477) could not be represented using available mechanisms in the Margara2021 model and, therefore, were not included in this simulation study.
Selected model parameters were varied to represent these modulations and are listed in Table 3. The scaling factors for these parameters were defined in a dose-independent fashion to qualitatively and quantitatively reproduce the positive inotropic effects observed in vitro. Baseline parameter values were either increased (from 1x to 3x–5x) or decreased (from 1x to 0.1x), according to whether the selected direction of change would correspond to an increase in active tension, with ranges chosen arbitrarily. For each simulated mechanism representing one or multiple drugs, perturbation-response curves were derived for the peak tension biomarker. Given that the scaling factors for each mechanism were varied in a dose-independent fashion, the perturbation-response curves were not ensured to follow sigmoid trends. For this reason, to still capture the non-linear nature of these curves, we used a parabolic fit through the median biomarker values from the full, simulated population. Figure 2B illustrates the fitting process. After fitting, we projected the sarcomere shortening value observed experimentally at the EC50 value for the compounds represented in the model by the mechanisms under analysis and found the intersection point (if any existed) with the parabola. This intersection point corresponded to the exact perturbation that had to be performed to the model parameters to achieve the same variation in contractility, as observed experimentally. All the scaling factors calculated using this procedure and enabling model simulations to match positive inotropic compound effects are reported in Table 3.
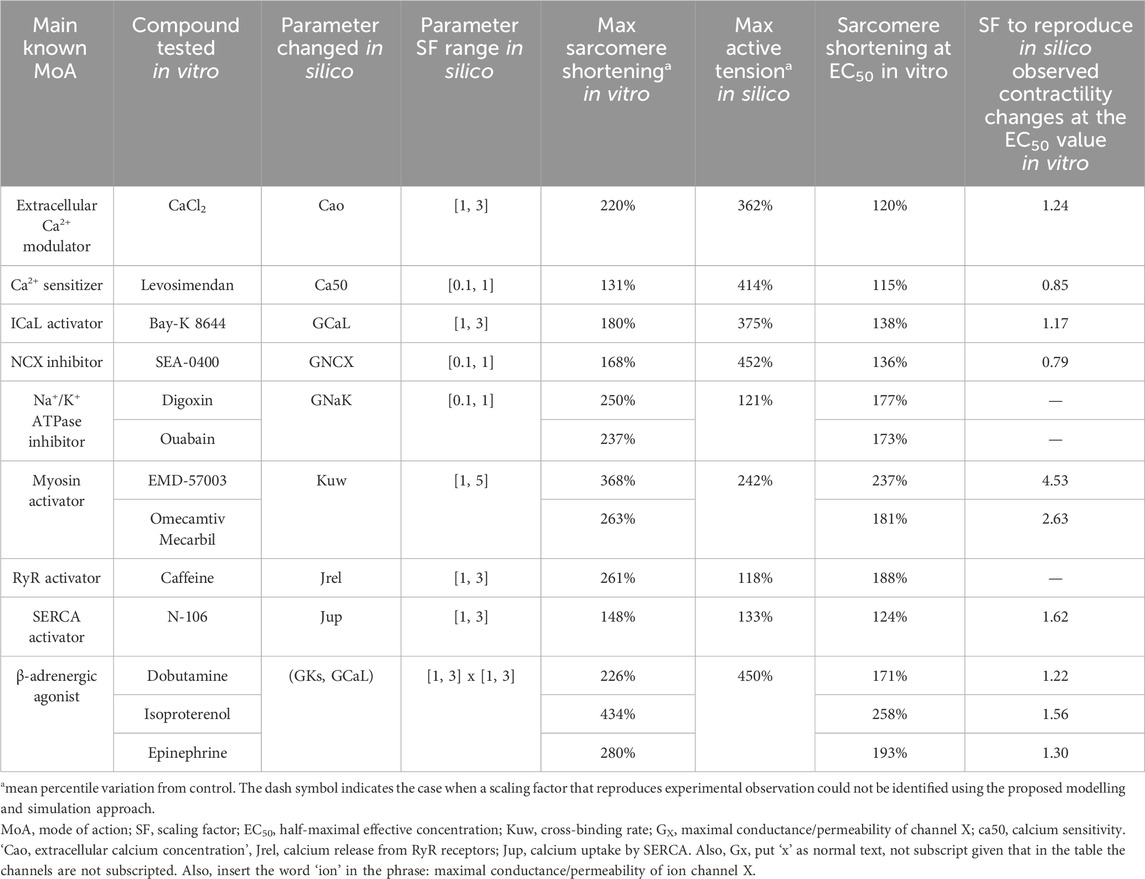
Table 3. Comparison between in vitro positive inotropic effects and in silico mechanism perturbation responses for several modes of drug action: mechanisms of positive inotropy considered in this study, compounds tested in vitro by Abi-Gerges et al. (2020), model parameters changed in silico, scaling factor ranges, maximum sarcomere shortening observed in vitro, maximum active tension variation reached in silico, sarcomere shortening observed at the EC50 value, and SF to simulate similar drug-induced inotropic effects observed at the EC50 value in vitro.
We define our verification, validation, and uncertainty quantification strategy based on the principles outlined for the CiPA in silico strategy by Li et al. (2019c), the ‘model validation flow’ described by Musuamba et al. (2021) (Figure 1), and the ASME V&V40 standard as applied to in silico trials (Viceconti et al., 2021) as follows.
The context of use is the assessment of drug-induced changes in contractility through ion channel modulation and the determination of the mechanisms of inotropy. This framework has previously been validated for proarrhythmic risk assessment (Passini et al., 2017); thus, the verification of software was conducted by ensuring agreement (Supplementary Figure S2) between simulation outputs using Virtual Assay software, the MATLAB code provided by Margara et al. (2021), and CellContraction.jl, the Julia package used in this study and available open source (see Data Availability Statement). The comparator dataset and algorithms utilised are described in Sections 2.1, 2.3, respectively. The criteria for assessing the predictivity of the simulations are defined in Section 2.3. Simulated outputs are validated through comparison with experimental recordings of drug-induced inotropic behaviour in adult human primary cardiomyocytes. Uncertainty quantification of the cellular model was addressed through a population of models approach and the comparison of median and Bayesian approaches for the estimation of IC50 values for inotropy changes.
For validation, the comparison of simulated data with experimental recordings of drug-induced effects on contractility is interpreted in light of the following factors:
(i) quality and completeness of the input data (i.e., the quantitative characterisation of drug-induced modes of action, including ion channel screening and knowledge of additional inotropic mechanisms);
(ii) the in silico model used, including equations and parameters (i.e., the population of human cell models based on the Margara2021 electromechanical model);
(iii) simulation protocols applied (including stimulation rate, ionic concentrations, and duration); and
(iv) experimental conditions of in vitro data on contractility (i.e., isolated cardiomyocytes affected by isolation procedures; Yue et al., 1996).
An analysis performing pure ion channel block simulations (Supplementary Material) suggested ATpeak as the most informative biomarker for predicting drug-induced effects on cardiac inotropy. Simulation results focused on this biomarker show a quantitative agreement with drug-induced neutral/negative inotropic effects observed experimentally for the reference compounds from Dataset 1 (Nguyen et al., 2017).
Figure 3A shows predicted drug-induced changes in ATpeak for the 28 reference compounds in Dataset 1. Predicted values are shown as fractions of the control value (no drug) and reported for the full population of models in the form of box and whisker plots. To facilitate visual comparison with in vitro observations, IC50 values obtained from Nguyen et al. (2017) were either plotted as red vertical lines if the associated compounds showed a negative inotropic effect or indicated as a red area if the compounds showed no effect. This approach aligns with the authors’ suggestion that any effect should arise only past the highest tested concentration. On the other hand, to facilitate the comparison with clinical EFTPCmax, boxplots were coloured based on the ratio between the dose tested and EFTPCmax, covering large concentration ranges, from 0.01× (dark purple) to 100,000× (yellow).
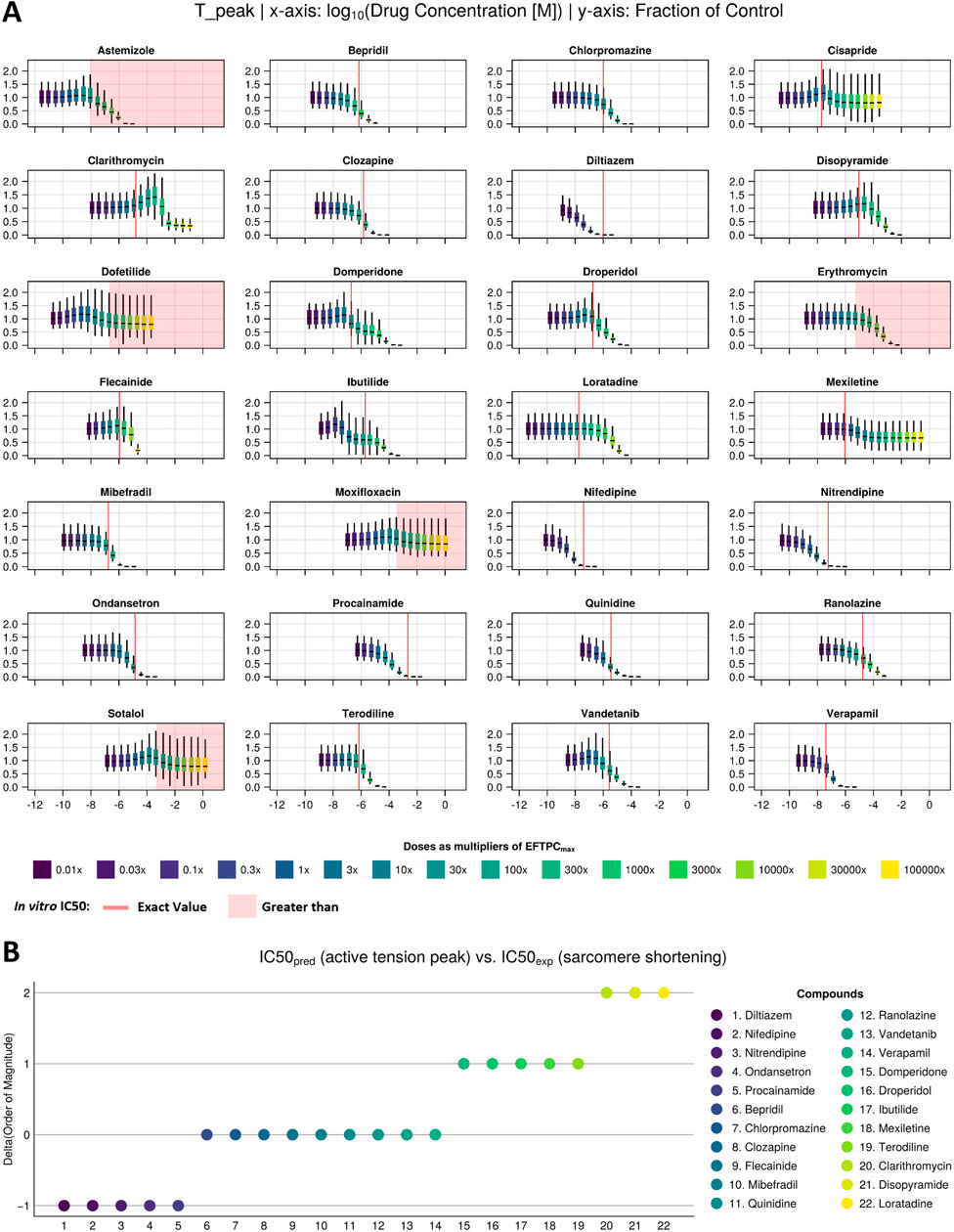
Figure 3. Drug-induced negative inotropic effects for the 22 compounds with variable modes of action. (A) Visual comparison between the in silico predicted change in peak tension under drug effects at multiple concentrations (sigmoids) and in vitro IC50 values (red line/area) of sarcomere shortening dose–response curves measured in 3–8 samples from 1 or 2 donor hearts (Nguyen et al., 2017). (B) Quantitative comparison between in silico and in vitro inotropic predictions expressed as the difference between the predicted and experimentally measured IC50 values’ order of magnitude. The colour scale from dark blue to yellow refers to dose as a multiplier of EFTPCmax in both panels.
In silico and in vitro results were in agreement for 25 out of these 28 compounds, with 22 compounds showing a negative inotropic effect (i.e., a median change from baseline of more than 25%, as detailed by Nguyen et al. (2017) and 3 compounds (dofetilide, moxifloxacin, and sotalol) showing no inotropic effect. Of the remaining three compounds, two (astemizole and erythromycin) showed a negative inotropic effect in silico at concentrations above 10× the EFTPCmax but not in vitro, and one (cisapride) showed a negative inotropic effect in vitro but not in silico.
For the 22 compounds showing negative inotropic effects both in vitro and in silico, a quantitative comparison was performed, as discussed below. Table 2 reports both in vitro IC50 values obtained from Nguyen et al. (2017) and in silico IC50 values computed from peak tension dose–response curves, fitted using both the median and Bayesian approaches, as described in Section 2.3.3. The ratios of IC50 values and clinical EFTPCmax, which are often used to define safety margins, are reported in brackets. To facilitate the numerical comparison, Figure 3B shows the quantitative difference between the in silico and in vitro IC50 values for the 22 negative inotropic compounds. Each drug is represented as a coloured circle and classified based on the difference between the in silico and in vitro IC50 values’ order of magnitude. We will refer to this quantity as “delta” for simplicity. A delta value of 0 indicates that the predicted IC50 value for a given compound had the same order of magnitude as the experimentally measured one. A negative delta value indicates that a smaller IC50 value was predicted in silico (conservative/worst-case scenario prediction). Finally, a positive delta value indicates that a larger IC50 value was predicted (a more risky prediction).
Out of 22 compounds, 19 had a delta value lower than or equal to 1 in absolute value, meaning that the predicted IC50 value was either of the same order of magnitude as the experimentally measured one or it was either 10 times larger (mismatch of 1) or smaller (mismatch of −1). Nine compounds (bepridil, chlorpromazine, clozapine, flecainide, mibefradil, quinidine, ranolazine, vandetanib, and verapamil) had a delta value of 0; five compounds (diltiazem, nifedipine, nitrendipine, ondansetron, and procainamide) had a delta value of −1; and five compounds (domperidone, droperidol, ibutilide, mexiletine, and terodiline) had a delta value of 1. The remaining 3 out of 22 compounds (clarithromycin, disopyramide, and loratadine) had a delta value of 2 (100 times larger predicted IC50).
The mismatches presented in Figure 3B were calculated from the IC50 values derived using the median approach. However, in Section 2.3.3, we also presented a second approach that uses a Bayesian framework to calculate full posterior distributions for the IC50 values. Summary statistics (mean and standard deviation) of these distributions are reported in Table 2. When calculating the deltas using the IC50 distribution mean values, we obtained consistent results throughout, except from two compounds, namely, flecainide and domperidone, which, this time, were predicted to have a higher delta value (from 0 to 1 and from 1 to 2, respectively).
Regarding safety margins, the comparison between in vitro and in silico predictions reflects the same trends observed for IC50 values. Therefore, for 19 compounds, in vitro and in silico margins are quantitatively consistent, at least within an order of magnitude, increasing confidence in those predictions and informing dose selection for in vivo studies.
Figure 4 shows a summary of the predicted changes in cardiac contractility (peak active tension) induced by the simulation of inotropic mechanisms considered in this study, beyond ion channel inhibition. As described in Section 2.3.4, these mechanisms were selected based on the compounds investigated by Abi-Gerges et al. (2020) in human primary cardiomyocytes (Dataset 2) and evaluation of the readiness of representing those mechanisms in the model. Each panel of Figure 4A reports box and whisker plots for the ATpeak fraction of control (model with no perturbed parameters) simulated using the whole population of models, following the modulation of single parameters corresponding to the first eight mechanisms under study. Figure 4B shows summary statistics (mean and standard deviation) of the ATpeak fraction of control, following modulation of two parameters corresponding to β-adrenergic stimulation. Selected parameters and specific perturbation ranges used are reported in Table 3.
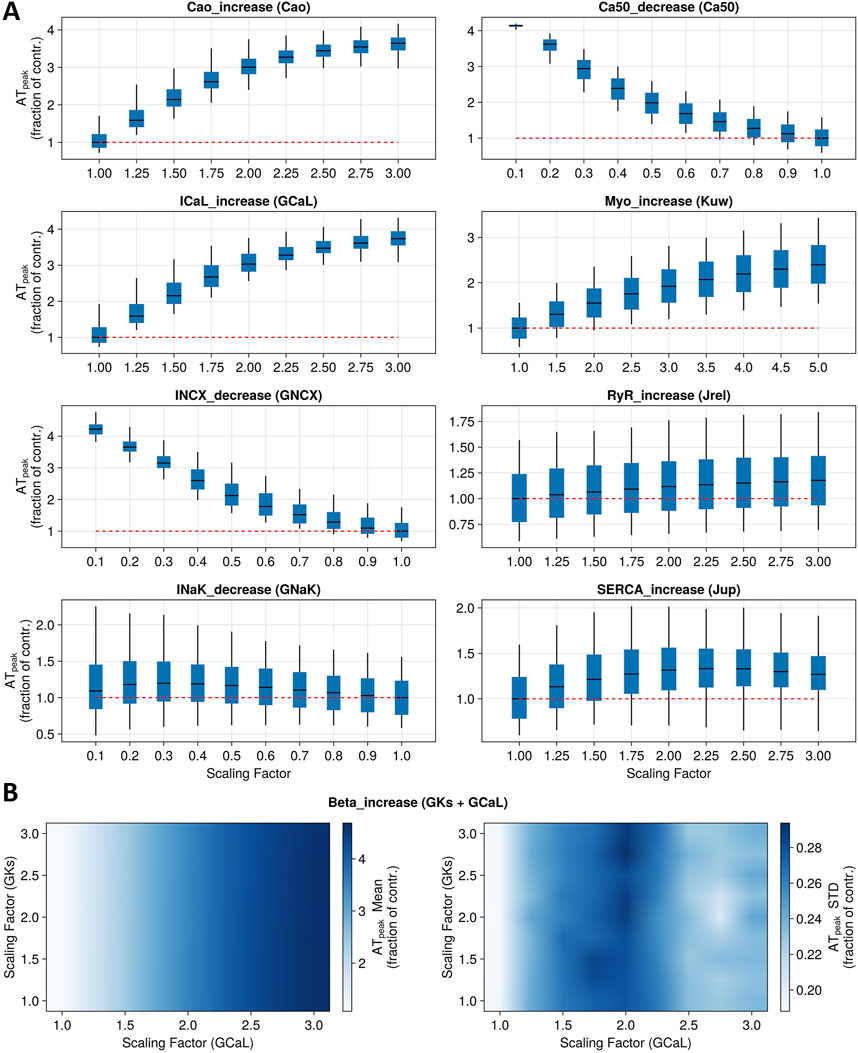
Figure 4. Sensitivity analysis for nine model mechanisms corresponding to main known modes of action of positive inotropic drugs. (A) Boxplots report full population variability in peak tension (as a fraction of control) when altering one parameter at a time to represent the first eight mechanisms. Red dotted lines represent the baseline values. (B) Heatmaps show population summary statistics (left panel: mean; right panel: standard deviation) for peak tension (as a fraction of control) when altering two parameters simultaneously to represent the ninth mechanism under study (beta-adrenergic stimulation).
Simulations of the direct increase in extracellular Ca2+ concentration, ranging from 1.8 to 5.4 mM, led to an ATpeak value of 362%, where 100% indicates the control condition. The reduction in Ca2+ sensitivity, up to 90% of its baseline value, induced an increase in contractility (ATpeak = 414%). Similarly, increasing GCaL up to 3-fold its baseline values resulted in an ATpeak value of 375%. On the other hand, reducing the permeability of the Na+–Ca2+ exchanger induced an ATpeak value of 452%. Instead, reducing the permeability of INaK led to a modest increase in contractility (maximum ATpeak = 121%). Increasing myosin activation up to 5-fold its baseline value led to an ATpeak value of 242%. Increasing the flux of Ca2+ through RyR channels up to 3-fold its baseline values had mild effects on the tension peak, leading to a maximum ATpeak value of 114%. Similarly, increasing SERCA Ca2+ uptake flux led to an ATpeak of 133%. β-adrenergic stimulation was simulated with up to a 3-fold increase in ionic conductances for ICaL and IKs (Dorian, 2005; Taggart et al., 2021), leading to an increase in peak tension of 450%. However, our simulations show that when altering both conductances at the same time, changes in tension were mainly driven by ICaL, while IKs modulation had little to no contribution (Figure 4B).
A physiologically relevant parametrisation for seven out of nine (all but Na+/K+ ATPase inhibition and RyR activation) model parameters could be identified that quantitatively recapitulates drug-induced positive inotropic effects for 10 out of 13 reference compounds from Dataset 2 (Abi-Gerges et al., 2020). This means that each parameter could be scaled via a specific factor to simulate an increase in peak tension that matches exactly (as a percentile variation from the control value) experimentally observed sarcomere shortening at the EC50 value for those compounds whose main mode of action was represented by the mechanism. Calculated scaling factors and parameters altered for each mode of action are summarised in Table 3.
Extracellular Ca2+ modulation was investigated in vitro using CaCl2, which led to a maximum sarcomere shortening of 220%. As described above and reported in Table 3, simulations covered the range observed experimentally for this mode of action. A scaling factor of 1.24 was computed for the extracellular Ca2+ concentration to obtain the same magnitude of change in contractility observed in vitro at the EC50 value (120%). Levosimendan is a Ca2+ sensitizer, which induced a mild increase in sarcomere shortening in vitro (131%), smaller than the maximum increase obtained in silico (414%). A scaling factor of 0.85 was computed to reproduce the increase in contractility observed in vitro at the EC50 value (115%).
Bay-K 8644 was used in vitro to increase ICaL, leading to a maximum increase in contractility of 180% and an increase of 138% at the EC50 value, which was obtained in silico by applying a scaling factor of 1.17 to ICaL conductance. To inhibit the Na+–Ca2+ exchanger in vitro, Abi-Gerges and others used SEA-0400 and observed a maximum sarcomere shortening of 168%. A scaling factor of 0.79 was computed for GNCX to simulate the same increase in contractility observed at the EC50 value. Two compounds were tested in vitro as Na+/K+ ATPase inhibitors, digoxin and ouabain, which led to similar ATpeak values of 250% and 237%, respectively. In this case, as described above, simulations were not able to reproduce the same magnitude of change, and the scaling factors to simulate the effects of these two drugs at the EC50 value could not be computed.
Two drugs were also tested in vitro to investigate myosin activation effects, namely, EMD-57003 and omecamtiv mecarbil, which led to sarcomere shortening of 368% and 263% at the EC50 value, respectively. For this mode of action, simulations were able to reproduce the changes observed at the EC50 value using 4.53 and 2.63 as scaling factors of myosin activation for EMD-57003 and omecamtiv mecarbil, respectively.
In terms of excitation–contraction coupling mechanisms, caffeine increases calcium-induced-calcium-release through RyR activation, leading to maximum sarcomere shortening in vitro of 261%, which was not reached in silico as described above. N-106 is a SERCA activator, which induced a maximum ATpeak value of 148% in vitro. The maximum ATpeak obtained via simulations was 133%, enough to compute a scaling factor of 1.62 to mimic the effects observed in vitro at the EC50 value (124%).
Three β-adrenergic agonists were tested in vitro, namely, dobutamine, isoproterenol, and epinephrine, which led to ATpeak values of 126%, 334%, and 180%, respectively. As previously described, modulation of IKs played no role as the cumulative simulated positive inotropic effect was mainly ICaL-driven. Therefore, we only reported ICaL scaling factors, amounting to 1.22, 1.56, and 1.30 for reproducing the changes observed in vitro at the EC50 values for dobutamine, isoproterenol, and epinephrine, respectively.
In this study, we predicted drug-induced effects on cardiac cellular inotropy using multiscale simulations on a population of 323 in silico human ventricular healthy cells, and the input included in vitro data or assumptions related to drug-induced effects on ionic currents and other mechanisms of inotropy. We considered a set of 41 reference compounds as a validation dataset: 28 drugs inhibiting specific cardiac ion channels, leading to negative or non-inotropic effects, and 13 compounds having heterogeneous modes of action, leading to positive inotropic changes. In silico contractility biomarkers were then compared with published in vitro data and clinical observations of drug-induced inotropy effects (Gao et al., 2023; Garg et al., 2024; Harmer et al., 2012; Pointon et al., 2015).
The main findings of this study are as follows:
• Simulations of pure ion channel blocks identify the active tension peak as the best surrogate biomarker of sarcomere shortening, with high predictive potential.
• In silico simulations using the human electromechanical cell model described by Margara et al. (2021) and in vitro ion channel data well-predicted drug-induced neutral/negative inotropic changes for all tested compounds whose main mode of action was ion channel inhibition. For 25 out of 28 compounds, in silico predictions were consistent with in vitro observations. Of these, 19 out of 22 (86%) also showed quantitative agreement within an order of magnitude.
• Simulations could qualitatively reproduce drug-induced positive inotropic changes for all tested compounds, whose main mode of action corresponded to any of the nine model mechanisms tested. For 10 out of 13 compounds, in silico predictions were qualitatively consistent with in vitro observations.
Among the compounds from Dataset 1 (negative/neutral inotropic effects), simulations with the Margara2021 model replicated the trend observed in vitro for all compounds (Figure 3A), except for cisapride, astemizole, and erythromycin. Cisapride induced negative inotropic effects in vitro and predicted no effects in silico. However, no clinical reports have highlighted the significant effects of cisapride on cardiac contractility, and concentrations eliciting a decrease in contractility in vitro far exceed the anticipated therapeutic exposure. Astemizole, on the other hand, did not induce a negative inotropic effect in vitro but it did in silico at high concentrations (above 10× the EFTPCmax), which is consistent with in vivo observations of negative inotropic effects in dogs (Sugiyama et al., 1997a; Sugiyama et al., 1997b). Erythromycin had no inotropic effect in vitro, but it reduced active tension in silico at high concentrations (above 10× the EFTPCmax). Erythromycin has been associated with arrhythmia and QT prolongation, but no in vivo effects on cardiac inotropy have been reported.
In vitro and in silico contractility IC50 values were quantitatively in agreement (within an order of magnitude) for 19 of the investigated compounds (Figure 3B). For quantitative predictions, it is important to highlight that the outcome of simulations strongly depends on the input data representing the drug action. For example, a different set of ion channel data for simulating diltiazem effects was available in the literature (Crumb et al., 2016), leading to a more negative delta value (−2) compared to simulations performed using ion channel data obtained from Kramer et al. (2013). In this study, we used IC50 values obtained from Kramer et al. (2013) since they also include diltiazem-induced inhibition of the INa current, which is significant (Mirams et al., 2011).
For three compounds (clarithromycin, loratadine, and disopyramide), the delta value between in vitro and in silico IC50 values was of two orders of magnitude. In silico, clarithromycin induced significant positive inotropic changes at concentrations 10–300 times that of EFTPCmax and negative inotropic effects only at extremely high concentrations (∼2000× EFTPCmax). Therefore, it could not be predicted as a negative inotropic drug. The biphasic behaviour of clarithromycin is due to its multichannel effect: the initial block of hERG leads to a positive inotropic effect as prolonging the AP leads to more Ca2+ entering the cell, whereas at higher concentrations, the block of INa becomes dominant, leading to lower AP amplitude and plateau and, therefore, to less Ca2+ influx and reduced inotropy. The ratio between the in vitro IC50 value and EFTPCmax was 13× (Table 2), despite no clinical observations suggesting negative inotropic potential for clarithromycin (Gluud et al., 2008; Suzuki et al., 2012; Wong et al., 2016). The effects of clarithromycin on the cardiovascular system are not clear and still debated. Clarithromycin has been associated in the short term with increased risks of myocardial infarction, prolonged QT, arrhythmia, and cardiac mortality (Wong et al., 2016). In the long term, clarithromycin increased 6-year mortality in coronary heart disease patients (Gluud et al., 2008), but its inotropic effects have not been assessed.
Similarly, simulations for loratadine show negative inotropic effects only at concentrations thousands of times that of EFTPCmax, consistent with the cardiac safety profile of this drug (Hey et al., 1999; Nault et al., 2002). Disopyramide induced mild positive inotropic changes at concentrations 10–100 times that of EFTPCmax and negative inotropic effects only at higher concentrations, leading to an in silico IC50 value of ∼400 μM, higher than in vitro observations in human (Nguyen et al., 2017) and canine (Harmer et al., 2012) cardiomyocytes (9.3 µM and 44 μM, respectively). In vivo and clinical evidence highlighted the significant effects of disopyramide on cardiac contractility (Coppini et al., 2019; Kim and Benowitz, 1990; Pollick et al., 1982), suggesting that in addition to ICaL inhibition, disopyramide might affect contractility via other modes of action.
Regarding safety margins, there is no official threshold provided by regulators for cardiac contractility assessment, although 100× EFTPCmax (or 100× of the maximum free plasma concentration of a drug) is usually considered a robust range to explore. In silico predictions of contractility changes were quantitatively consistent (within an order of magnitude) with in vitro observations for 86% of negative/neutral inotropic compounds from Dataset 1 (Table 2).
The slightly worse performance of the Bayesian point estimates in this study should not overshadow its broader utility. When using the Bayesian method, posterior distributions highlight how uncertainty might contribute to discrepancies, offering insights that a median fit cannot provide. For example, wider uncertainty ranges observed for a compound could signal experimental or model-specific variability worth further investigation. Although the median fit approach offers simplicity and computational ease, it is inherently limited in its ability to handle variability, which is a hallmark of biological systems. The Bayesian framework, although more computationally intensive, provides a more comprehensive characterisation of uncertainty and is, therefore, better suited for general application, especially when variability is significant. We consider that the apparent superiority of the median fit in this specific dataset is circumstantial and does not detract from the overall advantages of the Bayesian approach.
Considering the inotropic effect of ion channel inhibition alone only accounts for one specific mechanism of toxicity (mostly calcium reduction), and therefore, any effects via other mechanisms would not be predicted. Beyond ion channel inhibition, perturbation of other inotropic mechanisms such as myosin activation or calcium sensitivity could affect cardiac contractility. Simulations with the Margara2021 model reproduced the drug-induced effects observed in vitro (Table 3) for the following mechanisms: (1) extracellular Ca2+ concentration increase; (2) ICaL activation; (3) SERCA pump activation; (4) Na+/Ca2+ exchanger inactivation; (5) β-adrenergic stimulation; (6) Ca2+ sensitivity decrease; and (7) cardiac myosin activation. For Na+/K+ pump inactivation and RyR activation, simulations are qualitatively consistent with in vitro observation but do not replicate the range observed in vitro, due to the modelling approach chosen to describe those two modes of action. These results were obtained after perturbating mostly only one selected parameter for each mode of action. In addition, omecamtiv mecarbil, a myosin-activating drug, was simulated perturbating only one selected parameter following the approach described by Tewari et al. (2016), i.e., assuming that the drug increases the rate of myosin head binding with the actin filament. Specifically, Tewari and others simulated the omecamtiv mecarbil mechanism of action by increasing their model parameter ka, representing the rate of myosin-head attachment with actin. In our specific case [cell contraction model from Land et al. (2017)], this was translated into scaling the Kuw parameter, representing the transition rate from a cross-bridge unbound state to a cross-bridge weakly bound state. However, Kampourakis et al. (2018) describe omecamtiv mecarbil as exhibiting a biphasic behaviour, which was not captured in our simulations, but it might be reproduced using more sophisticated modelling and simulation approaches (Campbell et al., 2018; Forouzandehmehr et al., 2022). Overall, the rationale behind our modelling approach was not to provide a detailed description of each drug’s mode of action, but, instead, to use the simplest modelling approach that could be more easily characterised and informed using early in vitro data during the drug development process. Future studies could explore more complex approaches to model these modes of action and identify datasets to quantitatively understand drug effects on these mechanisms (Terkildsen et al., 2007). The adoption of more sophisticated models to describe cardiac mechanical contraction might also affect our finding that the AT peak is the most informative biomarker for assessing drug-induced effects on cardiac contractility. A systematic analytic comparison of contractility biomarkers across several mechanical models, however, goes beyond the scope of the present study. As a future work, additional modes of action and/or biological mechanisms (e.g., ATP hydrolysis) could also be implemented into the Margara et al. (2021) model to expand the applicability of the proposed modelling framework, including its use in studying disease-related/drug-induced metabolic impairments.
As data from experimental assays quantifying the extent of perturbation of these nine contractility mechanisms were not available, a sensitivity analysis approach was adopted. This aimed to gain an understanding of the extent of predicted changes in cardiac contractility biomarkers in silico under different degrees of perturbation for each of these mechanisms. When information on the potential involvement of a particular mechanism is available, which may not be fully quantitative (e.g., predicted from the compound structure), it can still be leveraged for the early prediction of its impact on cardiac inotropy, either alone or in combination with other mechanisms (e.g., ion channel inhibition). If an IC50 value for a given mechanism is known, it could be incorporated into simulations for a more quantitative prediction of the expected effect on cardiac contractility. Otherwise, a range of modulations can be explored to compute an early therapeutic index and inform dosing strategies for in vivo/clinical studies. The code provided allows for the simulation of any of these mechanisms, alone or alongside ion channel inhibition, by varying mechanism-specific model parameters. This enables predictions for both positive and negative inotropic compounds.
To move toward quantitative predictions for positive inotropic compounds, additional preclinical data are needed to characterise each of the abovementioned mechanisms for positive inotropy in a dose-dependent way. Similar to the CiPA initiative, a newly conceived, hypothetical initiative could aim at identifying and accurately characterising reference compounds affecting cardiac inotropy to define in vitro/in silico approaches and dose-dependent input data for modelling and simulations, similar to IC50 values for describing ion channel inhibition and predicting arrhythmic risk. This ambitious initiative could only arise from and will require a community effort between academics, pharmaceutical companies, and regulators and is, therefore, beyond the scope of the present study. Moreover, clinical data on drug-induced changes on cardiac contractility for a set of reference compounds will also facilitate the validation of new in vitro/in silico assays.
On a final note, cardiac drugs and pathologies can potentially have deleterious effects propagating from the cellular level to the organ scale, which may cause contractile dysfunction and/or increase arrhythmic risk. To capture this complex behaviour, the Margara2021 model can be incorporated into tissue and organ-scale frameworks (Zhou et al., 2024; Margara et al., 2022), including representations of electrophysiology, electromechanical coupling, and anatomical effects to further improve predictive power and assess mechanistic pathways involved.
This study describes the validation and application of simulations with an in silico human cardiac electromechanical model integrating ion channel inhibition data and information on potential inotropic mechanisms for predicting primary or off-target effects on cardiac contractility. The in silico approach was found to predict well inotropy changes in both positive and negative/no effect inotropes. The outlined in silico modelling and simulation workflows (Figure 1A) could inform how this model can be used as part of cardiac safety assessment strategies, along with computational models for predicting drug-induced changes in cardiac electrophysiology within pharmaceutical research and development.
All data and code used for running drug-block and mechanism-perturbation model simulations, dose/perturbation-response curves’ fitting, and plotting are available on a GitHub repository as a Julia package at https://github.com/GSK-Biostatistics/CellContraction.jl.
CT: conceptualization, data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, visualization, writing–original draft, and writing–review and editing. SL: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, visualization, writing–original draft, and writing–review and editing. EP: conceptualization, data curation, formal analysis, investigation, methodology, software, and writing–review and editing. KB: conceptualization, formal analysis, investigation, methodology, supervision, validation, and writing–review and editing. MH: formal analysis, investigation, and writing–review and editing. KC: formal analysis, investigation, and writing–review and editing. ER: conceptualization, formal analysis, investigation, and writing–review and editing. BR: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. BR acknowledges financial support from NC3Rs Infrastructure for Impact award (NC/P001076/1), a Wellcome Trust Fellowship in Basic Biomedical Sciences (214290/Z/18/Z), the EPSRC project CompBioMed X (EP/X019446/1), the TransQST project (European Federation of Pharmaceutical Industries and Associations, the TransQST project (Innovative Medicines Initiative 2 Joint Undertaking 116,030), and the CompBioMed 2 Centre of Excellence in Computational Biomedicine (European Commission Horizon 2020 Research and Innovation Programme, grant agreement no. 675451 and no. 823712, respectively).
Author CT was employed by AstraZeneca, Cambridge. Authors SL and KB were employed by GlaxoSmithKline, Stevenage. Authors KC and ER were employed by GlaxoSmithKline, Upper Providence.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1500668/full#supplementary-material
AP(s), action potential(s); APA, action potential amplitude; APDx, AP duration at X% of repolarisation; AT, active tension; Ca2+, calcium [Ca2+]i, intracellular Ca2+ concentration; CaT, Ca2+ transient; BCL, basic cycle length; dV/dtMAX, maximum upstroke velocity; EAD(s), early after-depolarisation(s); EMw: electromechanical window; EOP, membrane potential at the end of repolarisation; GX, IX conductance; IC50, drug concentration for 50% channel inhibition; ICaL, L-type Ca2+ current; IK1, inward rectifier K+ current; IKr, rapid delayed rectifier K+ current; IKs, slow delayed rectifier K+ current; INa, fast Na+ current; INaK, Na+/K+ pump current; INaL, late Na+ current; INCX, Na+/Ca2+ exchanger current; Ito, transient outward K+ current; K+, potassium; MoA, mode of action; Na+, sodium; SS, steady state; Vm, membrane potential.
Abi-Gerges, N., Indersmitten, T., Truong, K., Nguyen, W., Ratchada, P., Nguyen, N., et al. (2020). Multiparametric mechanistic profiling of inotropic drugs in adult human primary cardiomyocytes. Sci. Rep. 10 (1), 7692. doi:10.1038/s41598-020-64657-2
Brennan, T., Fink, M., and Rodriguez, B. (2009). Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur. J. Pharm. Sci. 36 (1), 62–77. doi:10.1016/j.ejps.2008.09.013
Britton, O. J., Bueno-Orovio, A., Van Ammel, K., Lu, H. R., Towart, R., Gallacher, D. J., et al. (2013). Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc. Natl. Acad. Sci. 110 (23), E2098–E2105. doi:10.1073/pnas.1304382110
Campbell, K. S., Janssen, P. M. L., and Campbell, S. G. (2018). Force-dependent recruitment from the myosin off state contributes to length-dependent activation. Biophysical J. 115 (3), 543–553. doi:10.1016/j.bpj.2018.07.006
Chang, K. C., Dutta, S., Mirams, G. R., Beattie, K. A., Sheng, J., Tran, P. N., et al. (2017). Uncertainty quantification reveals the importance of data variability and experimental design considerations for in silico proarrhythmia risk assessment. Front. Physiology 8 (NOV), 917. doi:10.3389/fphys.2017.00917
Coppini, R., Ferrantini, C., Pioner, J. M., Santini, L., Wang, Z. J., Palandri, C., et al. (2019). Electrophysiological and contractile effects of disopyramide in patients with obstructive hypertrophic cardiomyopathy: A Translational study. JACC Basic Transl. Sci. 4 (7), 795–813. doi:10.1016/j.jacbts.2019.06.004
Crumb, W. J., Vicente, J., Johannesen, L., and Strauss, D. G. (2016). An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J. Pharmacol. Toxicol. Methods 81 (2), 251–262. doi:10.1016/j.vascn.2016.03.009
Delaunois, A., Abernathy, M., Anderson, W. D., Beattie, K. A., Chaudhary, K. W., Coulot, J., et al. (2021). Applying the CiPA approach to evaluate cardiac proarrhythmia risk of some antimalarials used off-label in the first wave of COVID-19. Clin. Transl. Sci. 14 (3), 1133–1146. doi:10.1111/cts.13011
Dorian, P. (2005). Effects of adrenergic stimulation cellular cardiac electrophysiology antiarrhythmic action of β-blockers: potential mechanisms.
Forouzandehmehr, M., Paci, M., Koivumäki, J. T., and Hyttinen, J. (2022). Altered contractility in mutation-specific hypertrophic cardiomyopathy: a mechano-energetic in silico study with pharmacological insights. Front. physiology 13 (31 Oct), 1010786. doi:10.3389/fphys.2022.1010786
Gao, B. X., Abi-Gerges, N., Truong, K., Stafford, A., Nguyen, W., Sutherland, W., et al. (2023). Assessment of sarcomere shortening and calcium transient in primary human and dog ventricular myocytes. J. Pharmacol. Toxicol. Methods 123, 107278. doi:10.1016/j.vascn.2023.107278
Garg, A., Lavine, K. J., and Greenberg, M. J. (2024). Assessing cardiac contractility from single molecules to whole hearts. JACC Basic Transl. Sci. 9 (3), 414–439. doi:10.1016/j.jacbts.2023.07.013
Gluud, C., Als-Nielsen, B., Damgaard, M., Fischer Hansen, J., Hansen, S., Helø, O. H., et al. (2008). Clarithromycin for 2 weeks for stable coronary heart disease: 6-Year follow-up of the CLARICOR randomized trial and updated meta-analysis of antibiotics for coronary heart disease. Cardiology 111 (4), 280–287. doi:10.1159/000128994
Hall, J. E., and Hall, M. E. (2021). Guyton and Hall textbook of medical physiology. 14th ed. Philadelphia, PA: Elsevier.
Harmer, A. R., Abi-Gerges, N., Morton, M. J., Pullen, G. F., Valentin, J. P., and Pollard, C. E. (2012). Validation of an in vitro contractility assay using canine ventricular myocytes. Toxicol. Appl. Pharmacol. 260 (2), 162–172. doi:10.1016/j.taap.2012.02.007
Hey, J. A., Affrime, M., Cobert, B., Kreutner, W., and Cuss, F. M. (1999). Cardiovascular profile of loratadine. Clinical and experimental allergy. Supplement 29 (3), 197–199. doi:10.1046/j.1365-2222.1999.0290s3197.x
Huxley, H. E. (1953). Electron microscope studies of the organisation of the filaments in striated muscle. Biochim. Biophys. Acta 12 (3), 387–394. doi:10.1016/0006-3002(53)90156-5
Kampourakis, T., Zhang, X., Sun, Y. B., and Irving, M. (2018). Omecamtiv mercabil and blebbistatin modulate cardiac contractility by perturbing the regulatory state of the myosin filament. J. physiology 596 (1), 31–46. doi:10.1113/JP275050
Kim, S. Y., and Benowitz, N. L. (1990). Poisoning due to class IA antiarrhythmic drugs quinidine, procainamide and disopyramide. Drug Saf. 5 (6), 393–420. doi:10.2165/00002018-199005060-00002
Klein, S. K., and Redfern, W. S. (2015). Cardiovascular safety risk assessment for new candidate drugs from functional and pathological data: conference report. J. Pharmacol. Toxicol. Methods 76, 1–6. doi:10.1016/j.vascn.2015.06.003
Kramer, J., Obejero-Paz, C. A., Myatt, G., Kuryshev, Y. A., Bruening-Wright, A., Verducci, J. S., et al. (2013). MICE Models: Superior to the HERG Model in Predicting Torsade de Pointes. Sci. Rep. 3 (1), 2100. doi:10.1038/srep02100
Labelle, C., Marinier, A., and Lemieux, S. (2019). Enhancing the drug discovery process: Bayesian inference for the analysis and comparison of dose-response experiments. Bioinformatics 35 (14), i464–i473. doi:10.1093/bioinformatics/btz335
Lancaster, M. C., and Sobie, E. A. (2016). Improved Prediction of Drug-Induced Torsades de Pointes Through Simulations of Dynamics and Machine Learning Algorithms. Clin. Pharmacol. Ther. 100 (4), 371–379. doi:10.1002/cpt.367
Land, S., Park-Holohan, S. J., Smith, N. P., Dos Remedios, C. G., Kentish, J. C., and Niederer, S. A. (2017). A model of cardiac contraction based on novel measurements of tension development in human cardiomyocytes. J. Mol. Cell Cardiol. 106, 68–83. doi:10.1016/j.yjmcc.2017.03.008
Levick, R. J. (2009). An introduction to cardiovascular physiology. 5th ed. Boca Raton, FL: CRC Press.
Lewalle, A., Campbell, K. S., Campbell, S. G., Milburn, G. N., and Niederer, S. A. (2022). Functional and structural differences between skinned and intact muscle preparations. J. General Physiology 154 (Issue 2), e202112990. doi:10.1085/jgp.202112990
Li, Z., Garnett, C., and Strauss, D. G. (2019b). Quantitative systems pharmacology models for a new international cardiac safety regulatory paradigm: an overview of the comprehensive in vitro proarrhythmia assay in silico modeling approach. CPT Pharmacometrics Syst. Pharmacol. 8 (6), 371–379. doi:10.1002/psp4.12423
Li, Z., Mirams, G. R., Yoshinaga, T., Ridder, B. J., Han, X., Chen, J. E., et al. (2019c). General principles for the validation of proarrhythmia risk prediction models: an extension of the CiPA in silico strategy. Clin. Pharmacol. Ther. 107, 102–111. doi:10.1002/cpt.1647
Li, Z., Ridder, B. J., Han, X., Wu, W. W., Sheng, J., Tran, P. N., et al. (2019a). Assessment of an in silico mechanistic model for proarrhythmia risk prediction under the CiPA initiative. Clin. Pharmacol. Ther. 105 (2), 466–475. doi:10.1002/cpt.1184
Llopis-Lorente, J., Trenor, B., and Saiz, J. (2022). Considering population variability of electrophysiological models improves the in silico assessment of drug-induced torsadogenic risk. Comput. Methods Programs Biomed. 221, 106934. doi:10.1016/j.cmpb.2022.106934
Longobardi, S., Sher, A., and Niederer, S. A. (2022). Quantitative mapping of force-pCa curves to whole-heart contraction and relaxation. J. Physiology 600, 3497–3516. doi:10.1113/JP283352
Mamoshina, P., Rodriguez, B., and Bueno-Orovio, A. (2021). Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2 (3), 100216. doi:10.1016/j.xcrm.2021.100216
Margara, F., Psaras, Y., Wang, Z. J., Schmid, M., Doste, R., Garfinkel, A. C., et al. (2022). Mechanism based therapies enable personalised treatment of hypertrophic cardiomyopathy. Sci. Rep. 12 (1), 22501. doi:10.1038/s41598-022-26889-2
Margara, F., Wang, Z. J., Levrero-Florencio, F., Santiago, A., Vázquez, M., Bueno-Orovio, A., et al. (2021). In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment. Prog. Biophysics Mol. Biol. 159, 58–74. doi:10.1016/j.pbiomolbio.2020.06.007
Mirams, G. R., Cui, Y., Sher, A., Fink, M., Cooper, J., Heath, B. M., et al. (2011). Simulation of multiple ion channel block provides improved early prediction of compounds' clinical torsadogenic risk. Cardiovasc. Res. 91 (1), 53–61. doi:10.1093/cvr/cvr044
Musuamba, F. T., Skottheim Rusten, I., Lesage, R., Russo, G., Bursi, R., Emili, L., et al. (2021). Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: building model credibility. CPT. Pharmacometrics Syst. Pharmacol. 10 (8), 804–825. doi:10.1002/psp4.12669
Muszkiewicz, A., Britton, O. J., Gemmell, P. M., Passini, E., Sánchez, C., Zhou, X., et al. (2015). Variability in cardiac electrophysiology: using experimentally-calibrated populations of models to move beyond the single virtual physiological human paradigm. Prog. Biophysics Mol. Biol. 120, 115–127. doi:10.1016/j.pbiomolbio.2015.12.002
Nault, M. A., Milne, B., and Parlow, J. L. (2002). Effects of the selective H 1 and H 2 histamine receptor antagonists loratadine and ranitidine on autonomic control of the heart. Anesthesiology 96, 336–341. doi:10.1097/00000542-200202000-00018
Nguyen, N., Nguyen, W., Nguyenton, B., Ratchada, P., Page, G., Miller, P. E., et al. (2017). Adult human primary cardiomyocyte-based model for the simultaneous prediction of drug-induced inotropic and pro-arrhythmia risk. Front. Physiology 8 (DEC), 1073–1116. doi:10.3389/fphys.2017.01073
Passini, E., Britton, O. J., Lu, H. R., Rohrbacher, J., Hermans, A. N., Gallacher, D. J., et al. (2017). Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiology 8 (September), 668–715. doi:10.3389/fphys.2017.00668
Passini, E., Trovato, C., Morissette, P., Sannajust, F., Bueno-Orovio, A., and Rodriguez, B. (2019). Drug-induced shortening of the electromechanical window is an effective biomarker for in silico prediction of clinical risk of arrhythmias. Br. J. Pharmacol. 176 (19), 3819–3833. doi:10.1111/bph.14786
Passini, E., Zhou, X., Trovato, C., Britton, O. J., Bueno-Orovio, A., Rodriguez, B., et al. (2021). The virtual assay software for human in silico drug trials to augment drug cardiac testing. Journal of Computational Science 52, 101202.
Pointon, A., Harmer, A. R., Dale, I. L., Abi-Gerges, N., Bowes, J., Pollard, C., et al. (2015). Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci. 144 (2), 227–237. doi:10.1093/toxsci/kfu312
Pollick, C., Giacomini, K. M., Blaschke, T. F., Nelson, W. L., Turner-Tamiyasu, K., Briskin, V., et al. (1982). The cardiac effects of d- and l-disopyramide in normal subjects: a noninvasive study. Circulation 66 (2), 447–453. doi:10.1161/01.CIR.66.2.447
Sager, P. T., Gintant, G., Turner, J. R., Pettit, S., and Stockbridge, N. (2014). Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am. Heart J. 167 (3), 292–300. doi:10.1016/j.ahj.2013.11.004
Sugiyama, A., Aye, N. N., Katahira, S., Hagihara, A., and Hashimoto, K. (1997a). Effects of magnesium sulfate on the canine cardiovascular system complicating astemizole overdose. J. Cardiovasc. Pharmacol. 29 (6), 795–800. doi:10.1097/00005344-199706000-00013
Sugiyama, A., Aye, N. N., Katahira, S., Saitoh, M., Hagihara, A., Matsubara, Y., et al. (1997b). Effects of nonsedating antihistamine, astemizole, on the in situ canine heart assessed by cardiohemodynamic and monophasic action potential monitoring. Toxicol. Appl. Pharmacol. 143 (1), 89–95. doi:10.1006/taap.1996.8061
Suzuki, J. I., Ogawa, M., Hishikari, K., Watanabe, R., Takayama, K., Hirata, Y., et al. (2012). Novel effects of macrolide antibiotics on cardiovascular diseases. Cardiovasc. Ther. 30 (6), 301–307. doi:10.1111/j.1755-5922.2011.00303.x
Taggart, P., Pueyo, E., Duijvenboden, S. V., Porter, B., Bishop, M., Sampedro-Puente, D. A., et al. (2021). Emerging evidence for a mechanistic link between low-frequency oscillation of ventricular repolarization measured from the electrocardiogram T-wave vector and arrhythmia. Europace 23 (9), 1350–1358. doi:10.1093/europace/euab009
Terkildsen, J. R., Crampin, E. J., and Smith, N. P. (2007). The balance between inactivation and activation of the Na +-K+ pump underlies the triphasic accumulation of extracellular K+ during myocardial ischemia. Am. J. Physiology - Heart Circulatory Physiology 293 (5), H3036–H3045. doi:10.1152/ajpheart.00771.2007
Tewari, S. G., Bugenhagen, S. M., Vinnakota, K. C., Rice, J. J., Janssen, P. M. L., and Beard, D. A. (2016). Influence of metabolic dysfunction on cardiac mechanics in decompensated hypertrophy and heart failure. J. Mol. Cell. Cardiol. 94, 162–175. doi:10.1016/j.yjmcc.2016.04.003
Tomek, J., Bueno-Orovio, A., Passini, E., Zhou, X., Minchole, A., Britton, O., et al. (2019). 'Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. Elife 8, e48890. doi:10.7554/eLife.48890
Trovato, C., Mohr, M., Schmidt, F., Passini, E., and Rodriguez, B. (2022). Cross clinical-experimental-computational qualification of in silico drug trials on human cardiac purkinje cells for proarrhythmia risk prediction. Front. Toxicol. 4, 992650. doi:10.3389/ftox.2022.992650
Trovato, C., Passini, E., Nagy, N., Varró, A., Abi-Gerges, N., Severi, S., et al. (2020). Human Purkinje in silico model enables mechanistic investigations into automaticity and pro-arrhythmic abnormalities. J. Mol. Cell. Cardiol. 142 (April), 24–38. doi:10.1016/j.yjmcc.2020.04.001
Viceconti, M., Pappalardo, F., Rodriguez, B., Horner, M., Bischoff, J., and Musuamba Tshinanu, F. (2021). In silico trials: verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods 185 (September 2019), 120–127. doi:10.1016/j.ymeth.2020.01.011
Wong, A. Y. S., Root, A., Douglas, I. J., Chui, C. S. L., Chan, E. W., Ghebremichael-Weldeselassie, Y., et al. (2016). Cardiovascular outcomes associated with use of clarithromycin: population based study. BMJ (Online) 352, h6926. doi:10.1136/bmj.h6926
Yue, L., Feng, J., Li, G. R., and Nattel, S. (1996). Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am. J. Physiology-Heart Circulatory Physiology 270 (6), H2157–H2168. doi:10.1152/ajpheart.1996.270.6.H2157
Zhou, X., Qu, Y., Passini, E., Bueno-Orovio, A., Liu, Y., Vargas, H. M., et al. (2020). Blinded in silico drug trial reveals the minimum set of ion channels for torsades de pointes risk assessment. Front. Pharmacol. 10, 1643. doi:10.3389/fphar.2019.01643
Keywords: cardiac contractility, drug safety, systems toxicology, cardiac modelling, human cardiomyocytes, human modelling
Citation: Trovato C, Longobardi S, Passini E, Beattie KA, Holmes M, Chaudhary KW, Rossman EI and Rodriguez B (2025) In silico predictions of drug-induced changes in human cardiac contractility align with experimental recordings. Front. Pharmacol. 16:1500668. doi: 10.3389/fphar.2025.1500668
Received: 23 September 2024; Accepted: 10 February 2025;
Published: 17 March 2025.
Edited by:
Antonio Carlos Campos De Carvalho, Federal University of Rio de Janeiro, BrazilReviewed by:
Igor Vorobyov, University of California, Davis, United StatesCopyright © 2025 Trovato, Longobardi, Passini, Beattie, Holmes, Chaudhary, Rossman and Rodriguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristian Trovato, Y3Jpc3RpYW4udHJvdmF0b0Bhc3RyYXplbmVjYS5jb20=; Blanca Rodriguez, YmxhbmNhLnJvZHJpZ3VlekBjcy5veC5hYy51aw==; Kylie A. Beattie, a3lsaWUueC5iZWF0dGllQGdzay5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.