
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol., 10 April 2025
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1499786
This article is part of the Research TopicInnovative Approaches and Molecular Mechanisms in Cardiovascular PharmacologyView all 9 articles
Coronary atherosclerotic heart disease (CHD) is one of the leading causes of death from cardiovascular disease worldwide and has significant inflammatory features. Macrophages play an important role in atherosclerotic plaque formation and inflammation. IL-17, as a pro-inflammatory cytokine, further exacerbates the development of CHD by interacting with macrophages. In recent years, there has been increasing evidence that traditional Chinese medicine (CM) has a wide range of applications in regulating the immune system and treating CHD. This article reviewed the role of CM in the regulation of IL-17-regulated macrophages, discussed the core components and targets of CM in the treatment of CHD, and laid a theoretical foundation for its clinical application. The results show that CM can effectively inhibit the formation of foam cells, stabilize vulnerable plaque and delay the progression of atherosclerosis by inhibiting inflammation, regulating the polarization of macrophages and promoting cholesterol outflow. In addition, CM can also regulate the expression and signaling pathway of IL-17, further inhibit inflammatory response and improve the symptoms of CHD, providing a new idea and method for the prevention and treatment of CHD.
Coronary heart disease (CHD) is one of the most prevalent chronic cardiovascular diseases worldwide, posing a significant threat to human health due to its high incidence and mortality rates. The development of CHD is influenced by various factors, including genetics, lifestyle choices, and environmental conditions. In recent years, changes in lifestyle and an aging population have contributed to a rising trend in the incidence of CHD, making it a major challenge in the field of global public health. Abnormal lipid metabolism is the most important risk factor for atherosclerosis (AS), especially the increase in oxidized low-density lipoprotein (ox-LDL), leading to chemokines secretion and inflammation in endothelial and immune cells (Wu et al., 2017). Dendritic cells (DCs) and macrophages are important antigen-presenting cells (APCs) involved in antigen presentation and foam cells formation in the inflammatory process (Groh et al., 2018). T cells activated by APC secrete interleukin (IL)-17 and interferon-gamma (IFN-γ), resulting in AS plaques progression and platelet aggregation (Wu et al., 2017). During this process, lesion progression is mainly mediated by cells of the monocyte/macrophages spectrum. Furthermore, the presence of macrophages of different phenotypes (e.g., extreme phenotypes M1, M2) in plaques shows heterogeneity, including pro- and anti-inflammatory functions (Fernandez et al., 2019).
IL-17 is a potent pro-inflammatory cytokine that exacerbates the progression of CHD by interacting with macrophages. It promotes the migration of macrophages to the arterial wall, stimulates the release of inflammatory factors, and increases plaque susceptibility (Smith et al., 2010). These processes can ultimately lead to plaque rupture and cardiovascular events, such as heart attacks. Macrophages exist in various subpopulations with distinct functions, complicating the treatment of CHD. While pro-inflammatory M1 macrophages contribute to plaque instability, anti-inflammatory M2 macrophages may provide a protective effect. Understanding and regulating this balance is crucial for effective treatment. This article focuses on the role of IL-17-regulated macrophages in the pathophysiological mechanisms involved in CHD from both a clinical and experimental perspective. In addition, Chinese medicine (CM) has a wide range of applications in regulating the immune system. With the intensive research on CM pharmacology, more and more CM have been found to intervene and modulate the role of IL-17 and macrophages in CHD. The article will discuss how CM modulates the effect of IL-17-regulated macrophages on CHD. Additionally, Th17 cells and regulatory T cells (Tregs) are two key immune cell subpopulations that play an important role in immune balance. Th17 cells mainly secrete pro-inflammatory cytokines such as IL-17, while Treg cells secrete anti-inflammatory cytokines such as IL-10 and inhibit the activity of Th17 cells. Th17/Treg imbalance leads to an overactive inflammatory response, which promotes atherosclerosis and the development of CHD (Wang Q. et al., 2022).
The formation of AS is a complex multi-stage process in which macrophages play a crucial role. In the early stage, low-density lipoprotein (LDL) is oxidized in the subendothelial space to form oxidized low-density lipoprotein (ox-LDL). The presence of ox-LDL activates DCs and T cells, and promotes the differentiation of Th17 cells, resulting in the production of the pro-inflammatory cytokines IL-17 and IFN-γ (Wang Y. K. et al., 2022). Ox-LDL also triggers the expression of adhesion molecules, chemotactic cytokines, and pro-inflammatory factors in macrophages and vascular endothelial cells, leading to systemic and local immune responses. Macrophages, stimulated by macrophage colony-stimulating factor (GM-CSF) (Johnson and Newby, 2009), have a crucial role in AS lesions and switch between M1 and M2 phenotypes (Tacke and Zimmermann, 2014). To be specific, these cytokines activate macrophages, shifting them from the M0 to the pro-inflammatory M1 phenotype. M1 macrophages release pro-inflammatory factors like TNF-α, IL-6, and IL-1β, contributing to foam cell formation and accelerating atherosclerosis plaque development. As plaques progress, macrophage phenotype shifts, with M2 macrophages gradually increasing. M2 macrophages exhibit anti-inflammatory and tissue repair functions, secreting IL-10, TGF-β, and other anti-inflammatory factors, and participating in angiogenesis and matrix remodeling. They also contribute to reverse cholesterol transport, removing cholesterol from foam cells and reducing plaque burden. However, in the late stages of plaque development, M2 macrophages may secrete proteases such as MMPs, potentially leading to plaque instability and rupture.
Inflammatory mechanisms mediated by immune cells can directly impact plaque stability and trigger plaque rupture. Macrophages, located near lipid streaks or AS plaques, produce reactive oxygen species and proteases through scavenger receptors like type A scavenger receptor (SRA) and CD36. These receptors facilitate the phagocytosis of ox-LDL, leading to nonspecific immune responses and the formation of foam cells and lipid streaks (Kunjathoor et al., 2002). As lipids accumulate and vascular smooth muscle cells (VSMC) proliferate, AS plaques or intimal and intima-media hyperplasia form, narrowing the arteries and reducing blood flow to the heart. This leads to symptoms like chest pain, angina pectoris, and potentially myocardial infarction. Macrophages play a crucial role in every stage of this process, from endothelial dysfunction and lipid deposition to inflammation and plaque formation.
Pro-inflammatory macrophages count in perivascular adipose tissue (PvAT) inflammation (M1) and anti-inflammatory (M2) macrophages counts are closely associated with AS (Farias-Itao et al., 2022), and PvAT macrophages are associated with adjacent vessel stenosis (Verhagen et al., 2014). M2 macrophages are more abundant than M1 macrophages in PvAT. M1 macrophages are associated with coronary thrombosis in thrombotic plaques, while M2 macrophages are linked to a reduced number of epicardial vessels. Macrophages induce VSMC apoptosis through the FAO pathway and secretion of TNF-α and nitric oxide (NO). Furthermore, the Th17/Treg imbalance is also one of the crucial mechanisms in the development of CHD. The IL-17 secreted by Th17 cells further enhances the inflammatory response of macrophages, while Treg cells restrain the activity of Th17 cells, thereby suppressing the inflammatory response.
In conclusion, macrophages remove lipid deposits, form foam cells, promote plaque formation, trigger inflammation, secrete cytokines, and participate in cell signaling. Ultimately, they contribute to plaque rupture and the development of CHD. Macrophages are crucial in the pathological process of CHD and are important targets for prevention and treatment (Figure 1).
CM has been shown to modulate the therapeutic effects of macrophages in CHD in both in vivo and in vitro. For in vitro studies, the sources of macrophages mainly include primary cells derived from animal spleen, peritoneum, bone marrow, or monocyte/macrophages lines such as RAW264.7, THP-1, ANA (Figure 1).
Studies have shown that CM compounds and their active ingredients have anti-inflammatory effects and can be used to treat CHD by clearing heat, activating blood circulation, resolving blood stasis, and reducing levels of inflammatory factors. Yindan Xinnaotong (YDXNT) inhibits matrix metalloproteinase (MMP)-2/9 expression in macrophages, suggesting its anti-inflammatory effect in controlling CHD (Zhang, 2013). Whereafter, Cheng et al. conducted computational predictions based on network pharmacology and discovered that YDXNT exhibits synergistic anti-atherosclerotic properties by protecting blood vessels, reducing lipids, and providing anti-inflammatory and antioxidant effects. These findings were validated in atherosclerotic rats (Cheng et al., 2015). In patients with restenosis after percutaneous coronary intervention (PCI) for CHD, Ningxin Ditan Decoction (NXDTD) significantly reduced intracellular TC, FC, IL-6, IL-1β, TNF-α levels and the proportion of CD11b+CD86+ macrophages and apoptosis. It also regulates p-AMPK and nuclear factor kappa-B (NF-κB) signaling to inhibit macrophage differentiation and prevent restenosis (Lijuan et al., 2023). Yiqi Huoxue Formula (YQHXF) (Ningxintong Granule) benefits Qi and Blood, resolves blood stasis, and relieves pain. YQHXF effectively inhibits foam cell formation by reducing SRA and CD36 protein expression in THP-1-derived macrophages, thus regulating AS (Jisha and Ning, 2015). YQHXF stabilizes AS plaques by affecting phagocytosis of LDL through the toll-like receptor (TLR)-4 pathway in macrophages. Tanyu Tongzhi Formula (TYTZF) alleviates CHD symptoms patients by increasing peroxisome proliferator-activated receptor (PPAR)-γ expression or activating AKT/ERK signaling pathway (Ma et al., 2021). Studies on the pharmacological mechanism of the Tongmai Yangxin Pill (TMYXP), a patented CM, revealed that TMYXP attenuates foam cell formation and lipid deposits by regulating ESR1 and NF-κB signaling pathways, improving CHD patients' biochemical indices (Fan et al., 2021) (Table 1). CM inhibits macrophage activity, reduces inflammatory factor levels, and suppresses their inflammatory response by downregulating NF-κB expression. Network pharmacology is an analytical approach that combines biological networks with pharmacological studies, embodying the holistic philosophy of CM (Ding et al., 2019). Wang et al. identified several molecular targets and pathways associated with Astragalus membranaceus and Angelica sinensis (A&A) in the treatment of AS. A&A exhibits potential therapeutic effects against AS primarily through anti-inflammatory mechanisms (Wang et al., 2022c). By employing a combination of network pharmacology and experimental validation, the study systematically explored the mechanisms by which Yiqi Huoxue Huatan Recipe (YHHR) treats CAD. It was found that the expression of NF-κB p65 was significantly higher in the high-concentration YHHR group, indicating that YHHR has been shown to combat inflammation and AS through the SRC/NF-κB signaling pathway (Huang et al., 2023). Zedoarondiol exerts anti-inflammatory effects in lipopolysaccharide (LPS)-stimulated macrophages by inhibiting the expression of iNOS, COX-2, and pro-inflammatory cytokines through the inhibition of IKK and MAPK phosphorylation and subsequent inactivation of the NF-κB pathway (Cho et al., 2009). Lipocurc™, a Liposomal Curcumin preparation, effectively reduces pro-inflammatory cytokine/chemokine expression in fibroblasts and macrophages.Compounds in Hawthorn leaves (Quercetin, Kaempferol, and Isorhamnetin) reduced the protein expression of PTGS2, MMP-2, MMP-9, IL-6, IL-1β, TNF-α, and inhibited macrophages activation (Ding et al., 2022) (Table 2).
The above data suggest that CM formulas such as YDXNT, NXDTD, YQHXF, TYTZF, TMYXP, YHHR (Table 5), CM ingredients such as Zedoarondiol, and the novel nanoparticle Lipocurc™, can reduce macrophage inflammation. These in vitro studies indicate NF-κB, AMPK, PI3K/AKT, and chemokine (motif C-C) ligand (CCL)-2/CC chemokine receptor (CCR)-2, may become key targets for the regulation of macrophages by CM in the treatment of CHD.
CM can regulate macrophage subpopulations and decrease levels of inflammatory factors. In vitro experiment, Yiqi Yangyin Zhuyu Recipe (YQYYZYR) could reduce the secretion of inflammatory factors such as NO, TNF-α, and IL-6 by inhibiting M1 macrophage polarization (Tang, 2019). Gegen Qinlian Decoction (GGQLD) increases the expression of Arg-1 and CD206 while decreasing iNOS expression, indicating its ability to regulate lipid metabolism and macrophage polarization, improving the inflammatory microenvironment (Yi, 2022). In addition, Qishen Granule (QSG) (Lu et al., 2019), TYTZF (Ma et al., 2021), Huanglian Jiedu Decoction (HLJDD) (Biren, 2021; Li, 2021a; Li, 2021b), and Shenhong Tongluo Formula (SHTLF) (Yanyu, 2022) have been found to regulate macrophage subpopulations and switch M1 to M2 macrophages, reducing inflammation and the development of AS. Tanshinone IIA (TS-IIA) has been shown to activate the Sirt1 pathway and convert macrophages from M1 to M2 type, stabilizing AS plaques (Song, 2022; Chen et al., 2019). Baicalin from Scutellaria baicalensis (Yin-Siew Lai) has a protective effect on the heart by altering macrophage polarization and reducing inflammation. It decreases iNOS, IL-1β, and IL-6 levels while increasing Arg-1, IL-10, and TGF-β levels (Lai et al., 2018). Baicalin also attenuates myocardial injury after I/R through the JAK/STAT pathway (Xu et al., 2020). Furthermore, Laminaria japonica polysaccharide (LJP61A) enhances autophagic flux of macrophages by upregulating the key upstream genes of autophagy, Sirt1 and FoxO1 and alleviating inflammatory responses in AS (Li X. Y. et al., 2022). Acacetin from Sparganii rhizoma (Yuanshuo Ouyang) attenuates LPS-induced M1 polarization and pro-inflammatory cytokine expression, but its specific mechanism and pathway are still unknown (Ouyang et al., 2021). Andrographolide, an active compound extracted from Andrographis paniculata (Jia Shu), decreases TNF-α, MCP-1, high-sensitivity C-reactive protein (hs-CRP), and IL-1β levels by altering macrophage phenotype in mice (Shu et al., 2020). Additionally, Puerarin and Resveratrol (Ge et al., 2006), active ingredients of CM, regulate macrophage polarization, with Resveratrol agonizing PPARγ to inhibit the expression of extracellular MMP inducers.
The aforementioned data demonstrate that CM formulas, including YQYYZYR, QSG, GGQLD, TYTZF, SHTLF (Table 5), and active ingredients such as Baicalin, TS-IIA, Laminaria japonica polysaccharide (LJP61A), Acacetin, Andrographolide, Puerarin, and Resveratrol, are effective in regulating macrophages polarization and reducing the intensity of inflammatory response. Thus, these CM mitigate the aggravation of AS lesions and protect the heart (Table 1).
CM was found to be effective in promoting cholesterol efflux from macrophages and inhibiting foam cell formation. They can promote the phagocytosis and clearance of LDL by macrophages, reducing the deposition of LDL in the vessel wall and mitigating the development of CHD. It’s found that Wendan Decoction (WDD) may reduce cholesterol internalization and phagocytosis by macrophages by inhibiting the expression of macrophages membrane protein CD36 and SRA. Simultaneously, it upregulates the protein expression of macrophages membrane protein ATP-binding cassette transporter A1 (ABCA1) and class B type I scavenger receptor (SR-BI), promoting cholesterol efflux from macrophages (Xuanjing, 2020). Based on Network pharmacology research, Runmin Lai analyzed the important active ingredients in Qingxin Jieyu Formula (QXJYF) and found that 110 active ingredients of five CM (i.e., Salvia miltiorrhiza, persimmon) in the formula could act on 87 target genes. Macrophages iron death regulation might be its main potential pathway to stabilize AS-prone plaques (Runmin, 2021) (Table 1). Formononetin, an isoflavone from Astragalus membranes (Chuanrui Ma), improves lesion stability, attenuates foam cell formation, and reduces plaque accumulation. It also inhibits monocyte adhesion and inflammation, delaying AS onset (Ma et al., 2020). Flavonoids, the active ingredients of CM, have significant cardiovascular effects. Some flavonoids can act on macrophages through different pathways and mechanisms to exert different degrees of anti-AS effects. The related genes are ABCA1, ATP-binding cassette protein G1, TLR, and SRA (Li et al., 2020). Ginger (Li, C.) components alleviate oxidative stress, inflammation, and promote NO synthesis, and enhance cholesterol efflux, inhibit angiogenesis, and induce autophagy. Clinical trials show benefits for blood lipids, inflammatory cytokines, blood pressure, and platelet aggregation (Li C. et al., 2021). Ginger and its components have potential in treating hypertension, coronary artery disease, peripheral artery disease, and other vascular conditions. Macrophages upregulate liver X receptor (LXR) and its target genes in response to Salviain, enhancing cholesterol efflux and exerting myocardial protective effects (Qian et al., 2012). TS-IIA and Astragaloside IV (AS-IV) are natural CM extracts that have cardioprotective effects by reducing inflammation and oxidative stress in AS. They enhance plaque stability through PI3K/AKT and TLR4/NF-κB signaling and inhibit cytoplasmic lipid droplet accumulation in macrophages (Wang et al., 2020). TS-IIA also prevents LPS-induced inflammation by targeting succinate dehydrogenase (SDH) in macrophages (Liu et al., 2021). Additionally, TS-IIA inhibits LDL oxidation, monocyte adhesion, macrophage cholesterol accumulation, pro-inflammatory cytokine expression, and platelet aggregation, making it a potential treatment for stabilizing AS plaques (Gao et al., 2012) (Table 2).
Based on the above studies, we concluded that CM formulas such as WDD, YQHXF, QXJYF (Table 5), and CM components such as Formononetin, Flavonoids, Ginger components, Salviain, Tea polyphenols, TS-IIA, and AS-IV can inhibit foam cell formation or regulate ferroptosis in macrophages by reducing lipid deposition in macrophages, stabilizing vulnerable plaques and resisting the formation of AS.
Leukocytes in the arterial wall contribute to AS plaques formation. IL-17, produced by leukocytes like T helper (Th)17 cells, is an inflammatory cytokine that plays a crucial role in host defense. Elevated IL-17A levels are associated with cardiovascular disease and are a key factor in AS development. IL-17 triggers cytokine cascades, accelerating AS and plaque vulnerability. A study found that IL-17 levels decreased significantly after treatment in angina patients, indicating its involvement in the inflammatory response and clinical instability in CHD (Boluri et al., 2022). macrophages cell line THP-1 was treated with 100 ng/mL of IL-17A for 24 h and the mRNA and protein expression levels of ADAMTS-5 proteases were significantly upregulated and peaked at 8 h. Subsequent inhibition studies showed that IL-17A upregulation of ADAMTS-5 was mediated through the ERK and JNK pathways in THP-1 cells (Thou et al., 2020). Studies showed IL-17A induces pro-inflammatory, pro-thrombotic responses, increases plaque vulnerability, and attracts cells in human plaque tissue samples (Akhavanpoor et al., 2017). During atherogenesis, the IL-17A/IL-17RA axis increases aortic arch inflammation by inducing aortic chemokines and accelerates neutrophil and monocyte recruitment to this site (Butcher et al., 2012).
In addition, IL-17A induced a mixed macrophage-DC phenotype, previously identified in the context of AS as a major regulator of cholesterol homeostasis in macrophages. IL-17A has a monocyte/macrophages profile with a significant effect on the monocyte/macrophages profile in atherogenesis (Salvatore et al., 2015). Inhibiting IL-17A could reduce inflammatory load, cellular infiltration, and improve plaque stability, thus preventing AS progression (Erbel et al., 2014) (Figure 1).
CM compounds have anti-inflammatory effects, reducing IL-17 levels. Paeonol, extracted from Paeonia suffruticosa (Xiaoyan Shi), restores Treg/Th17 balance by modulating gut microbiota. This reduces pro-inflammatory cytokines and MMP expression in aortic inflammatory cells. Paeonol also increases anti-inflammatory cytokine IL-10, indicating its effectiveness in anti-AS (Shi et al., 2021). Cycloastragenol (Y006), a natural product, has potent anti-inflammatory properties and protects myocardial cells. In vivo and in vitro studies show Y006 inhibits TNF-α, IFN-γ, and IL-17 production during hypoxia and myocardial infarction. It also increases IL-10 and IL-4 expression, enhancing myocardial function by regulating molecular genes under hypoxia and apoptosis (Ren et al., 2020) (Table 3).
Furthermore, CM formulation play a crucial role in reducing IL-17 inflammatory cytokine levels and improving the treatment of CHD. In an animal study, Shenshao Decoction (SSD) decreased TC, TG, and LDL-C levels, with the high-dose group showing a significant reduction in IL-1β, IL-17A and IL-23. This suggests SSD suppresses the inflammatory response and attenuates plaque progression (Xue et al., 2013). Qishen Yiqi Pill (QSYQP) increased CD36 expression, reduced IL-17A generation, decreased plaque formation and LDL levels. It achieves anti-atherogenic effects through mechanisms like promoting regulatory T cells, facilitating hepatic cholesterol excretion, and inhibiting plaque formation and Th17 cells (Peng et al., 2017). Furthermore, Yiqi Huoxue Decoction (YQHXD) improved symptoms and heart function in Qi deficiency and blood stasis patients after PCI. IL-17 and hs-CRP levels significantly decreased after treatment, indicating improved inflammatory factor response and heart function (Lu, 2021) (Table 4).
Network pharmacological analysis suggests that CM formulas and compounds can treat CHD through the IL-17 pathway. Quercetin, a flavonoid extracted from Ginkgo biloba leaves (Xiaojun Wu), has potential therapeutic effects in the treatment of cardiovascular diseases. Network pharmacology analysis reveals that quercetin regulates MAPK, IL-17, and phosphatidylinositol-3-kinase/protein kinase B (PI3K/AKT) signaling pathways for cardiovascular disease treatment (Wu et al., 2019). Ficus hirta-Hypericum perforatum components and targets alleviate microvascular angina through PI3K/AKT, IL-17, and HIF-1 signaling pathways, reducing oxidative stress and inflammation (Lai et al., 2021). Buyang Huanwu Decoction (BYHWD) decreases myocardial fibrosis and inhibits plaque formation by modulating the IL-17 pathway and reducing inflammatory factors (Wang et al., 2022d). Yiqi Ningshen Tablet, containing multiple active ingredients, is commonly used in CM for CHD treatment. It modulates CHD through the IL-17 pathway (Lv et al., 2020).
Common CM formulas, such as Removing Toxins to Activate Blood Recipe (RTABR) (Suli, 2021), Qishao Tongmai Decoction (QSTMD) (Chunlin, 2020), and Xuefu Zhuyu Decoction (XFZYD) have been widely used (Table 5). However, the mechanism of action of these CM formulas still requires further exploration by researchers (Figure 1).
IL-17 has been extensively studied in various diseases, including CHD. It is mainly produced by Th17 cells, γδ T cells, and CD8+ T cells (Ghoreschi et al., 2011). IL-17 indirectly affects macrophage polarization. Studies have shown that IL-17 treatment induces a unique transcriptional pattern in human monocyte-derived macrophages, different from other macrophage phenotypes (Erbel et al., 2014). This finding is supported by another study that found no increase in the expression of M1 or M2-associated surface molecules with IL-17 (de la Paz Sanchez-Martinez et al., 2017).
IL-17-regulated macrophages contribute to AS in CHD. Macrophages are crucial for activating T cells and initiating immune responses (Guerriero, 2019). The balance of M1 and M2 phenotypes in plaque macrophages can be influenced by the pro-inflammatory or anti-inflammatory T cell phenotype (de la Paz Sanchez-Martinez et al., 2017). The complex interactions between macrophages and T cells can be targeted for CHD treatment (Song et al., 2019). T lymphocytes are abundant and activated in AS plaques. Th1 secretes IFN-γ, IL-2, and TNF-α, while Th17 cells secrete IL-17, which activates macrophages and promotes inflammation. IL-17-producing T cells, expressing IFN-γ, are highly expressed in AS lesions (Lu, 2017). Elevated levels of IL-17 have been found in AS patients, indicating its involvement in AS development (de Boer et al., 2010). However, IL-17E (also named IL-25) produced by macrophages may have a protective effect on AS. It regulates macrophages, promotes lipolysis, inhibits lipogenesis, and enhances mitochondrial respiratory capacity (Feng et al., 2018).
IL-17A has a pro-atherogenic effect by inducing aortic macrophages, Th1-related cytokine, and aortic chemokine expression (Butcher and Galkina, 2011). It increases plaque fragility and promotes macrophage apoptosis without affecting phagocytosis of apoptotic cells (Wang B. et al., 2022). IL-17A also upregulate the expression of certain chemokines in plaques, further weakening arterial wall plaques. Another study (Wang, 2014) showed that IL-17A intervention increased plaque lipid content, necrotic core volume, T cells infiltration, and apoptosis, while reducing fibrous cap thickness and plaque stability. It also increases macrophage apoptosis and chemokine content, without affecting macrophage apoptotic clearance. Abnormal lipid metabolism plays a significant role in the interaction between IL-17 and macrophages in CHD. A systematic analysis revealed that increased IL-17A expression leads to elevated levels of cytokines such as IL-6 and TGF-β in experimental AS and clinical data. IL-17A also promotes infiltration of aortic macrophages and T cells in most AS models. Additionally, inhibition of TANK (TRAF family member associated NF-κB activator)-binding kinase 1 (TBK1) reduces IL-17-induced inflammation (Lee et al., 2017). In patients with diabetes and CHD, hyperglycemia activates macrophages via TBK1-HIF-1α-mediated IL-17/IL-10 signaling, contributing to coronary artery AS complexity (Li Q. et al., 2021).
Additionally, IL-17A may also exert anti-inflammatory effects by regulating aortic vascular cell adhesion molecule (VCAM)-1 expression and T cells content (Butcher and Galkina, 2011). Human adipose tissue-derived mesenchymal stromal cells (AT-MSCs) can polarize macrophages to an anti-inflammatory phenotype, reducing the secretion of inflammatory cytokines and macrophage phagocytosis (Adutler-Lieber et al., 2013). These studies suggest there may also be a beneficial aspect for IL-17-related macrophages in CHD. Anyway, IL-17 holds potential as a therapeutic target for cardiovascular diseases.
IL-17 signaling pathway plays a role in macrophage differentiation and susceptibility to AS in systemic lupus erythematosus (SLE) (Wang et al., 2022f). Animal experiments on the active ingredients of CM have revealed that Formononetin inhibited M1 polarization and promoted M2 polarization in macrophages/microglia, thereby improving cardiac function in mice with myocardial infarction and depression by reversing polarization. Importantly, IL-6 and IL-17A produced post-myocardial infarction may cause neuroinflammation, and mancostemonin attenuates this process (Yang et al., 2023). Kedaling tablets (KDL), a Chinese patented medicine originated from Corydalis yanhusuo (Y.H. Chou & Chun C.Hsu), are prescribed for the prevention of AS. Kedaling tablets can attenuate atherosclerotic plaque and reduce IL-1β and IL-17 in ApoE−/− mice. The anti-atherosclerotic effects of Kedaling tablets may be associated with the suppression of inflammatory signaling pathways (Li et al., 2024).
CM has shown potential in modifying IL-17-regulated macrophages in CHD, as seen in other inflammatory diseases. Dihydrosanguinarine (DS) is a chemical component found in Corydalis bungeana Turcz. It has been found to have in vitro anti-inflammatory, antioxidant, and antimicrobial properties. Network pharmacology methods have predicted DS targets for anti-inflammatory effects, including AKT3, PI3KCA, CCL2, FOS, IL-17A, IL-17RA, IL-17RE, IL-1β, IL-6 and TNF-α. Additionally, DS has been shown to primarily reduce macrophage infiltration induced by LPS, exerting an anti-hepatitis effect (Xiang et al., 2022). Both in vitro and in vivo experiments have shown that XFBD can downregulate the secretion of pro-inflammatory cytokines (such as IL-6, TNF-α, IL-1β, and iNOS) by macrophages, and its main component, glycyrrhizic acid, has a strong affinity for IL-17A. This reduces inflammatory reactions, potentially reducing macrophage infiltration and alleviating acute lung injury (Wang et al., 2022g). In intracerebral hemorrhage, hemoglobin induces the formation of TLR2/TLR4 heterodimers in macrophages, leading to increased IL-23 expression. IL-23 stimulates γδ T cells to produce IL-17, exacerbating secondary brain injury. Sparstolonin B (SsnB) in CM can inhibit the formation of TLR2/TLR4 heterodimers, potentially improving this process (Zhong et al., 2016).
IL-17A production can have different effects on macrophages. Hochu-ekki-to (TJ-41), a traditional Japanese herbal medicine, increases IL-17A production, which promotes macrophage production and clearance of pneumococcus (Nakakubo et al., 2020). In the treatment of refractory liver cancer with Oxaliplatin, M2 macrophages overexpress IL-17, reducing the pro-apoptotic effect of Oxaliplatin on tumor cells. Targeting the IL-17 pathway may help suppress liver cancer development (Guo et al., 2017). “Dragon’s blood” is a deep red resin primarily derived from the dragon blood tree (Dracaena cochinchinensis). It is valued in CM for its significant effects on improving blood circulation and dissipating blood stasis. Recent studies have revealed that dragon’s blood shows considerable potential in the treatment of coronary heart disease by promoting blood flow and reducing blood viscosity, thereby improving cardiac blood supply and helping to alleviate chest pain and decrease the frequency of angina attacks (Fan et al., 2014). A comparison of the antiplatelet aggregation effects of two types of “dragon’s blood resin,” Daemonorops draco (RDD) and Dracaena cochinchinensis (RDC), led to the selection of the more potent RDD as the primary component to enhance the efficacy of the formula. In clinical studies, the effects of “Xuefu Zhuyu Decoction” on platelet aggregation and endothelial function in patients with coronary heart disease were observed to evaluate its efficacy and safety (Yi et al., 2011). Additionally, diosgenin, the main active metabolite of Dioscorea nipponica (DN) (Jia-Fu Feng), exerts an anti-myocardial ischemia effect by enhancing the body’s antioxidant capacity. In one study, three types of diosgenin (DN, D. panthaica, and D. zingiberensis) significantly reduced oxidative stress markers and inflammatory factors in myocardial tissue, thereby demonstrating an anti-myocardial ischemia effect (Feng et al., 2017; Tang et al., 2015).
The above studies indicate that IL-17 and macrophages are important in cardiovascular diseases. Comparing the treatment of other diseases with CM can help to identify the mechanisms of CM in regulating IL-17-regulated macrophages and their potential application in CHD. This can expand the possibilities for CM in CHD treatment.
Both IL-17 and macrophages play a significant role in the development of CHD. The main dysfunctional macrophages form foam cells, promote plaque formation and inflammation, leading to destabilization of the fibrous cap, plaque rupture and CHD development, although there is also few studies show that IL-17 induces anti-inflammatory macrophages to reduce inflammation, the role of IL-17 remains controversial. Thus, it is crucial to modulate and balance the functions of IL-17 related macrophages during the prevention and treatment of CHD. Macrophages and Th17 cells, which produce IL-17, are important in hypertension, while studies on atherosclerosis have produced conflicting findings regarding the impact of IL-17 and Th17 cells on disease progression and plaque stability. Therefore, further research on IL-17A is warranted.
We performed the Mendelian randomization (MR) analysis between single-nucleotide polymorphisms (SNPs) and other genetic variants of IL-17a and IL-17ra from GWAS focusing on cardiovascular diseases, which was showed in Table 6. There was not enough SNPs passed the genetic variants threshold screening for IL17a. The MR results of IL-17 receptors show that for IL17RA, there is no significant association with CAD, CHD, or CA, as indicated by the non-significant p-values (ranging from 0.5 to 0.95) and odds ratios (ORs) close to 1. For IL17RB, there is a suggestive negative association with CAD and CHD (OR = 0.95, p = 0.069), but no significant association with coronary atherosclerosis. IL17RC shows a significant negative association with CAD (OR = 0.96, p = 2.50E-08) and a stronger significant negative association with coronary atherosclerosis (OR = 0.88, p = 6.00E-09). The inverse variance weighted method was used with 2 or 3 SNPs for each analysis. Although we found statistical significance in the results above, there were conflicts among different casual relationship between IL-17 receptors subsets and the cardiovascular diseases. The interpretation of these results need to be further investigated and discussed in a more rigorously designed MR study.
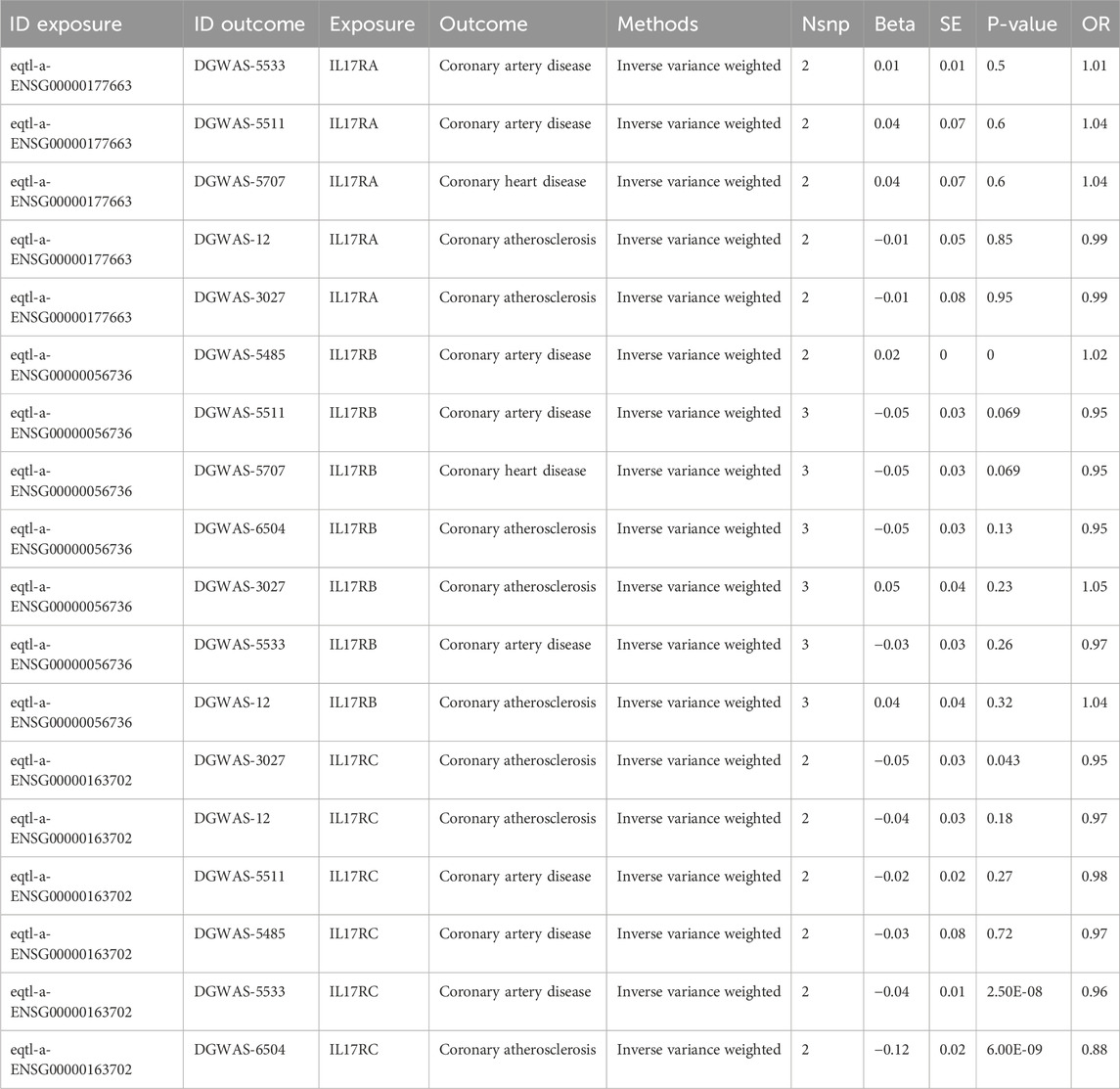
Table 6. Mendelian randomization (MR) analysis between IL-17 receptors and cardiovascular diseases from GWAS databases.
The IL-17A, produced by Th17 cells, plays a significant role in the development of cardiovascular diseases like atherosclerosis and is associated with age-related vascular endothelial dysfunction. For example, the proportion of Th17 cells and the expression levels of IL-17A, IL-6, and VCAM-1 increase with age in mice. In vitro studies show that IL-17A treatment inhibits the proliferation of mouse aortic endothelial cells (MAECs) and increases the levels of senescent β-galactosidase and senescence-associated proteins (p16, p19, p21, and p53). Blocking the NF-κB pathway with PDTC can inhibit IL-17A-induced expression of senescence-associated proteins. The study reveals a previously unknown link between IL-17A and endothelial cell senescence, mediated by the NF-κB/p53/Rb signaling pathway, suggesting that IL-17A may contribute to endothelial cell senescence and vascular dysfunction with advancing age (Zhang et al., 2021).
The role of genetic knockout mouse models of IL-17A and IL-17RA in cardiovascular diseases has been investigated in literature (Butcher et al., 2012). For instance, studies from (Liao et al., 2012) have shown that IL-17A knockout (IL17A−/−) exhibited a significant reduction in infarct volume after cerebral ischemia/reperfusion (I/R) injury. Similarly, both IL17A−/− and IL-17 receptor A knockout (IL17RA−/−) mice demonstrated protective effects on kidney function and morphology after renal I/R injury. These findings suggest that genetic knockout of IL-17A or its receptor can have beneficial effects in reducing tissue damage in various cardiovascular-related I/R injury models (Liao et al., 2012).
Regarding the use of these mouse models in studies on CMs, there was not explicitly mentioned that have utilized IL-17A or IL-17RA genetic knockout mouse models specifically for investigating the effects of CMs on cardiovascular diseases. However, the potential for such studies exists, as CMs are known for their anti-inflammatory and protective effects on the cardiovascular system, which could be further explored using these genetically modified mouse models to understand their mechanisms of action in modulating IL-17A/IL-17RA pathways in cardiovascular diseases.
IL-17 stimulates macrophages to release pro-inflammatory cytokines, such as TNF-α and IL-6, which are involved in the formation and progression of atherosclerosis (Smith et al., 2010). IL-17 promotes the uptake of lipids and the formation of foam cells by regulating the transition between M1 and M2 macrophage phenotypes. Current treatments related to IL-17 primarily include anti-IL-17 monoclonal antibodies, such as Secukinumab and Ixekizumab. These drugs have been used for autoimmune diseases like psoriasis (Thomas et al., 2024), but their application in patients with CHD requires further exploration. Existing treatments may have side effects and are often non-specific, potentially affecting physiological immune responses. CM has shown promise in regulating inflammation, improving lipid levels, and promoting vascular health, potentially providing new avenues for the treatment of IL-17 and macrophage-related CHD (Ye et al., 2024). CMs such as Danshen and Huangqi have been found to have positive effects in regulating macrophage function and reducing inflammatory responses.
CM plays an important role in modulating IL-17-regulated macrophages in CHD. CM drug administration may provide potential therapeutic approaches to inhibit vulnerable AS plaques. Although there are few studies reported the role of CM in modulating IL-17-regulated macrophages in CHD, some reports explored and provided useful hints through network pharmacological methods. In terms of mechanism of IL-17-induced inflammation in pro-inflammatory M1 macrophages, CM regulate the polarization of macrophages from M1 type to M2 type, reduce IL-17 production to exert anti-inflammatory effects on the one hand, and reduce LDL oxidation and lipid deposition on the other.
Firstly, regarding the mechanisms of action, Western medicine typically targets specific molecular pathways involved in pathological processes. For example, statins reduce cholesterol synthesis by inhibiting HMG-CoA reductase, thereby decreasing the progression of AS (Kones and Rumana, 2015). Drugs such as aspirin and anti-inflammatory medications primarily focus on inhibiting specific inflammatory pathways, such as the COX-1/COX-2 pathways, directly reducing thrombus formation and inflammatory responses (de Gaetano et al., 2003). Studies indicate that CM plays a crucial role in regulating macrophages, mainly treating AS and preventing CHD through pathways like NF-κB, AMPK, PI3K/AKT, and CCL2/CCR2. TCM formulations such as YDXNT, NXDTD, and monomers like Curcumin, Zedoarondiol, and turmeric nanoparticles (Lipocurc™) exert anti-AS effects by suppressing inflammation in macrophages. CM promotes the polarization of macrophages from M1 to M2, thereby reducing inflammation and stabilizing plaques via CM formulas such as QSG and GGQLD.
Secondly, in terms of cholesterol metabolism regulation, Western medicine mainly modulates lipid metabolism by lowering LDL levels and increasing HDL levels, primarily through statins (Arsenault et al., 2011). In contrast, CM reduces the progression of CHD by promoting cholesterol efflux and inhibiting foam cell formation in macrophages. For instance, WDD, YQHXF, and their monomers Formononetin and Salviain achieve this by decreasing the expression of LDL-related receptors or upregulating the expression of cholesterol efflux-related receptors on macrophages, thus altering plaque composition, reducing monocyte adhesion, and inflammation, fundamentally delaying the progression of AS.
Finally, regarding the regulation of inflammatory pathways, Western medicine usually intervenes by targeting specific inflammatory mediators. For example, corticosteroids broadly suppress immune responses, which may lead to immune suppression and side effects (Vinet et al., 2015). In contrast, CMs such as BYHWD and YQHXF regulate the IL-17 pathway, reducing the expression of inflammatory factors in AS and thereby decreasing the risk of its formation. Moreover, some CMs stabilize plaques by modulating macrophage ferroptosis.
In summary, CM shows unique advantages in regulating macrophage function, promoting polarization, and modulating cholesterol metabolism, providing a more complex and comprehensive therapeutic mechanism compared to Western medicine. This capability can improve and stabilize the atherosclerotic process. These characteristics give CM significant development potential in treating coronary heart disease and atherosclerosis and may serve as an effective complement to Western pharmacotherapy. However, the current understanding of the mechanisms of CM is still incomplete, necessitating more clinical and basic research to validate its safety and efficacy.
The current review underscores the potential of CM in modulating IL-17-regulated macrophages for treating CHD, but it also exists several limitations. Key issues include the limited clinical data, as most studies focus on in vitro experiments and animal models, necessitating further validation through well-designed clinical trials. Additionally, the heterogeneity in study designs and the lack of standardized methods for defining macrophage subtypes complicate result comparison and interpretation. While significant progress has been made in understanding how specific CM formulas regulate macrophage subpopulations, most research has concentrated on short-term effects, with insufficient long-term data on effectiveness and safety. The limitations of this study include the fact that it primarily focuses on M1/M2 macrophages. Recent scRNA-sequencing studies of human and mouse atherosclerotic plaques have unveiled the existence of macrophages with characteristics that do not only fit into the traditional M1 or M2 categories (Li Q. et al., 2022). By concentrating solely on M1/M2 macrophages, it may potentially limit the comprehensive understanding of the inflammatory processes and the full spectrum of macrophage phenotypes involved in the development and progression of cardiovascular diseases (Chaudhry et al., 2019; Yu et al., 2023). Thus, further studies should also pay attention to the contributions and roles of these non-M1/M2 macrophages in the pathogenesis of cardiovascular diseases such as AS. Addressing these gaps will require comprehensive research efforts, including standardized methodologies and broader evaluations of CM interventions for CHD. Similarly, Western medicine research on IL-17 and macrophage mechanisms also faces challenges, such as insufficient target specificity of therapies and a primary focus on short-term efficacy without long-term assessments. Moreover, individual variability in responses to IL-17 inhibitors and the complexity of immune interactions are often overlooked. The reliance on animal models further limits the clinical applicability of findings. These challenges highlight the need for more foundational and clinical research in both CM and Western medicine to enhance our understanding and improve therapeutic outcomes for CHD.
Based on the gaps identified in the review, several specific research areas can be explored to deepen our understanding of CM in modulating IL-17-regulated macrophages for the treatment of CHD.
First, it is essential to conduct large-scale, double-blind, placebo-controlled randomized controlled trials (RCTs) to evaluate the efficacy and safety of specific CM formulas and combinations in treating CHD. Comparative studies should assess the effectiveness and safety of CM interventions against conventional treatments, such as statins or anti-inflammatory medications. Longitudinal studies are needed to investigate the long-term effects of CM interventions on plaque stability, cardiovascular events, and quality of life. Additionally, exploring how individual factors—such as genetics, gut microbiota, and lifestyle—affect responses to CM interventions could pave the way for personalized medicine.
Second, mechanism studies should focus on the cellular and molecular pathways through which CM regulates IL-17 and modulates macrophages. Utilizing network pharmacology can help identify potential synergistic effects between various CM compounds and their molecular targets. Establishing animal models will enable investigation into the effects of CM on plaque formation and stability in CHD models. It's also crucial to assess how CM interventions influence the immune system, including the balance between Th17 and Treg cells and the activation of regulatory T cells.
Finally, exploring other avenues, such as the synergistic effects of combining CM with lifestyle therapies (like exercise, nutrition, or mindfulness), could yield valuable insights for enhancing treatment outcomes. Comprehensive safety and toxicity studies are necessary to evaluate the potential adverse effects of CM interventions. Additionally, research into the absorption, distribution, metabolism, and excretion of CM compounds can help optimize dosing strategies.
The findings of this review suggest that CM holds promise as a valuable therapeutic approach to modulate IL-17-regulated macrophages in CHD. However, its mechanisms of action and clinical efficacy require further investigation. With appropriate research and implementation, TCM could play an important role in improving CHD management and enhancing patient outcomes.
QL: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing–original draft. PL: Conceptualization, Data curation, Formal Analysis, Resources, Writing–original draft. CL: Conceptualization, Data curation, Investigation, Supervision, Writing–original draft. ZZ: Investigation, Project administration, Writing–original draft. DW: Methodology, Supervision, Validation, Visualization, Writing–review and editing. QL: Funding acquisition, Methodology, Supervision, Visualization, Writing–review and editing. HY: Conceptualization, Visualization, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by National Natural Science Foundation of China (No. 82274279, to Q.L.), Special Funding for TCM Science and Technology Research of Guangdong Provincial Hospital of Chinese Medicine (No. YN2020QN10, to Q.L.), State Key Laboratory of Traditional Chinese Medicine Syndrome Project (No. QZ2023ZZ38, to Q.L.).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adutler-Lieber, S., Ben-Mordechai, T., Naftali-Shani, N., Asher, E., Loberman, D., Raanani, E., et al. (2013). Human macrophage regulation via interaction with cardiac adipose tissue-derived mesenchymal stromal cells. J. Cardiovasc Pharmacol. Ther. 18 (1), 78–86. doi:10.1177/1074248412453875
Akhavanpoor, M., Akhavanpoor, H., Gleissner, C. A., Wangler, S., Doesch, A. O., Katus, H. A., et al. (2017). The two faces of interleukin-17a in atherosclerosis. Curr. Drug Targets 18 (7), 863–873. doi:10.2174/1389450117666161229142155
Arsenault, B. J., Barter, P., DeMicco, D. A., Bao, W., Preston, G. M., LaRosa, J. C., et al. (2011). Prediction of cardiovascular events in statin-treated stable coronary patients by lipid and nonlipid biomarkers. J. Am. Coll. Cardiol. 57 (1), 63–69. doi:10.1016/j.jacc.2010.06.052
Biren, L. (2021). Study on the mechanism of huanglian Jiedu decoction requlating macrophage polarization based on network pharmacology. China Pharm. 32 (05), 552–558. doi:10.6039/j.issn.1001-0408.2021.05.08
Boluri, A., Khazaei, H., Sargolzaei, N., Miri, H. O., and Khazaei, B. (2022). The comparison of IL-17 levels in patients with unstable angina before and after medical treatment. Hum. Antibodies 30 (1), 25–29. doi:10.3233/HAB-210446
Butcher, M., and Galkina, E. (2011). Current views on the functions of interleukin-17A-producing cells in atherosclerosis. Thromb. Haemost. 106 (5), 787–795. doi:10.1160/TH11-05-0342
Butcher, M. J., Gjurich, B. N., Phillips, T., and Galkina, E. V. (2012). The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ. Res. 110 (5), 675–687. doi:10.1161/CIRCRESAHA.111.261784
Cao, L.J., Yu, L., and Liu, L. (2023). Mechanism study of Ningxin Ditan Decoction inhibiting macrophage differentiation through AMPK/NF-kB pathway to improve vascular restenosis. China. J. Emerg. Medl. 32, (04) 592–596+623. doi:10.3969/j.issn.1004-745X.2023.04.006
Chaudhry, F., Isherwood, J., Bawa, T., Patel, D., Gurdziel, K., Lanfear, D. E., et al. (2019). Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front. Cardiovasc Med. 6, 173. doi:10.3389/fcvm.2019.00173
Chen, W., Li, X., Guo, S., Song, N., Wang, J., Jia, L., et al. (2019). Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int. Immunopharmacol. 70, 486–497. doi:10.1016/j.intimp.2019.02.054
Cheng, R., Gao, Y., Gu, N., et al. (2016). Effects of serum containing Ningxin Tong Granules on the phosphorylation levels of TLR4, Syk, ERK, Paxillin proteins and phagocytic function of THP-1- derived macrophages. Shi Zhen National Med National Pharm. 27, (04) 784–787. doi:10.3969/j.issn.1008-0805.2016.04.006
Cheng, L., Pan, G. f., Zhang, X. d., Wang, J. l., Wang, W. d., Zhang, J. y., et al. (2015). Yindanxinnaotong, a Chinese compound medicine, synergistically attenuates atherosclerosis progress. Sci. Rep. 5, 12333. doi:10.1038/srep12333
Cho, W., Nam, J. W., Kang, H. J., Windono, T., Seo, E. K., and Lee, K. T. (2009). Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-kappaB pathway in LPS-stimulated murine macrophages. Int. Immunopharmacol. 9 (9), 1049–1057. doi:10.1016/j.intimp.2009.04.012
Chunlin, Z. (2020). Mechanism of Qishao Tongmai decotionon on coronary heart disease based on network pharmacology. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 18 (19), 3161–3167. doi:10.12102/j.issn.1672-1349.2020.19.004
de Boer, O. J., van der Meer, J. J., Teeling, P., van der Loos, C. M., Idu, M. M., van Maldegem, F., et al. (2010). Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J. Pathol. 220 (4), 499–508. doi:10.1002/path.2667
de Gaetano, G., Donati, M. B., and Cerletti, C. (2003). Prevention of thrombosis and vascular inflammation: benefits and limitations of selective or combined COX-1, COX-2 and 5-LOX inhibitors. Trends Pharmacol. Sci. 24 (5), 245–252. doi:10.1016/S0165-6147(03)00077-4
de la Paz Sanchez-Martinez, M., Blanco-Favela, F., Mora-Ruiz, M. D., Chávez-Rueda, A. K., Bernabe-García, M., and Chávez-Sánchez, L. (2017). IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis. 16 (1), 196. doi:10.1186/s12944-017-0588-1
Ding, J., Wu, J., Wei, H., Li, S., Huang, M., Wang, Y., et al. (2022). Exploring the mechanism of Hawthorn leaves against coronary heart disease using network pharmacology and molecular docking. Front. Cardiovasc Med. 9, 804801. doi:10.3389/fcvm.2022.804801
Ding, M., Ma, W., Wang, X., Chen, S., Zou, S., Wei, J., et al. (2019). A network pharmacology integrated pharmacokinetics strategy for uncovering pharmacological mechanism of compounds absorbed into the blood of Dan-Lou tablet on coronary heart disease. J. Ethnopharmacol. 242, 112055. doi:10.1016/j.jep.2019.112055
Du, J., and Gu, N. (2015). Effect of Ningxin Tong Granules on human monocyte-derived macrophage foam cell formation. J. Tradit Chin Med. 56, (15) 1322–5. doi:10.13288/j.11-2166/r.2015.15.017
Erbel, C., Akhavanpoor, M., Okuyucu, D., Wangler, S., Dietz, A., Zhao, L., et al. (2014). IL-17A influences essential functions of the monocyte/macrophage lineage and is involved in advanced murine and human atherosclerosis. J. Immunol. 193 (9), 4344–4355. doi:10.4049/jimmunol.1400181
Fan, J. Y., Yi, T., Sze-To, C. M., Zhu, L., Peng, W. L., Zhang, Y. Z., et al. (2014). A systematic review of the botanical, phytochemical and pharmacological profile of Dracaena cochinchinensis, a plant source of the ethnomedicine dragon's blood. Molecules 19 (7), 10650–10669. doi:10.3390/molecules190710650
Fan, Y., Liu, J., Miao, J., Zhang, X., Yan, Y., Bai, L., et al. (2021). Anti-inflammatory activity of the Tongmai Yangxin pill in the treatment of coronary heart disease is associated with estrogen receptor and NF-κB signaling pathway. J. Ethnopharmacol. 276, 114106. doi:10.1016/j.jep.2021.114106
Farias-Itao, D. S., Pasqualucci, C. A., de Andrade, R. A., da Silva, L. F. F., Yahagi-Estevam, M., Lage, S. H. G., et al. (2022). Macrophage polarization in the perivascular fat was associated with coronary atherosclerosis. J. Am. Heart Assoc. 11 (6), e023274. doi:10.1161/JAHA.121.023274
Feng, J., Ou, Z., Li, Q., Gong, B., Zhao, Z., et al. (2018). IL-25 stimulates M2 macrophage polarization and thereby promotes mitochondrial respiratory capacity and lipolysis in adipose tissues against obesity. Cell Mol. Immunol. 15 (5), 493–505. doi:10.1038/cmi.2016.71
Feng, J. F., Tang, Y. N., Ji, H., Xiao, Z. G., Zhu, L., and Yi, T. (2017). Biotransformation of Dioscorea nipponica by rat intestinal microflora and cardioprotective effects of diosgenin. Oxid. Med. Cell Longev. 2017, 4176518. doi:10.1155/2017/4176518
Fernandez, D. M., Rahman, A. H., Fernandez, N. F., Chudnovskiy, A., Amir, E. A. D., Amadori, L., et al. (2019). Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 25 (10), 1576–1588. doi:10.1038/s41591-019-0590-4
Gao, S., Liu, Z., Li, H., Little, P. J., Liu, P., and Xu, S. (2012). Cardiovascular actions and therapeutic potential of tanshinone IIA. Atherosclerosis 220 (1), 3–10. doi:10.1016/j.atherosclerosis.2011.06.041
Gao, L., Gao, J.H., Wang, J.F., He, X. H., Dai, L.Li., and Y, (2021). Study on the efficacy of Yiqi Huoxue formula in treating stable angina pectoris after PCI with qi deficiency and blood stasis syndrome and the related changes of IL-17, hs-CRP. Yunnan. J. Trad. Chin. Med 42 (10), 19–23. doi:10.16254/j.cnki.53-1120/r.2021.10.007
Ge, H., Zhang, J. f., Guo, B. s., He, B., Wang, B. y., and Wang, C. q. (2006). Resveratrol inhibits expression of EMMPRIN from macrophages. Acta Pharm. Sin. 41 (07), 625–630. doi:10.16438/j.0513-4870.2006.07.009
Ghoreschi, K., Laurence, A., Yang, X. P., Hirahara, K., and O'Shea, J. J. (2011). T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 32 (9), 395–401. doi:10.1016/j.it.2011.06.007
Groh, L., Keating, S. T., Joosten, L. A. B., Netea, M. G., and Riksen, N. P. (2018). Monocyte and macrophage immunometabolism in atherosclerosis. Semin. Immunopathol. 40 (2), 203–214. doi:10.1007/s00281-017-0656-7
Guerriero, J. L. (2019). Macrophages: their untold story in T cell activation and function. Int. Rev. Cell Mol. Biol. 342, 73–93. doi:10.1016/bs.ircmb.2018.07.001
Guo, B., Li, L., Guo, J., Liu, A., Wu, J., Wang, H., et al. (2017). M2 tumor-associated macrophages produce interleukin-17 to suppress oxaliplatin-induced apoptosis in hepatocellular carcinoma. Oncotarget 8 (27), 44465–44476. doi:10.18632/oncotarget.17973
Huang, H. T., Lv, W. Q., Xu, F. Y., Wang, X. L., Yao, Y. L., Su, L. J., et al. (2023). Mechanism of Yiqi Huoxue Huatan recipe in the treatment of coronary atherosclerotic disease through network pharmacology and experiments. Med. Baltim. 102 (26), e34178. doi:10.1097/MD.0000000000034178
Jisha, D., and Ning, G. (2015). Ffect of ningxintong Granule on human monocyte derived macrophage foam. J. Traditional Chin. Med. 56 (15), 1322–1325. doi:10.13288/j.11-2166/r.2015.15.017
Johnson, J. L., and Newby, A. C. (2009). Macrophage heterogeneity in atherosclerotic plaques. Curr. Opin. Lipidol. 20 (5), 370–378. doi:10.1097/MOL.0b013e3283309848
Kones, R., and Rumana, U. (2015). Current treatment of dyslipidemia: evolving roles of non-statin and newer drugs. Drugs 75 (11), 1201–1228. doi:10.1007/s40265-015-0429-3
Kunjathoor, V. V., Febbraio, M., Podrez, E. A., Moore, K. J., Andersson, L., Koehn, S., et al. (2002). Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 277 (51), 49982–49988. doi:10.1074/jbc.M209649200
Lai, S. J., Wang, D. Y., Li, T. L., Pu, F. L., and Wang, X. (2021). Mechanism of Ficus hirta-Hypericum perforatum in treatment of microvascular angina based on network pharmacology and molecular docking. Zhongguo Zhong Yao Za Zhi 46 (24), 6474–6483. doi:10.19540/j.cnki.cjcmm.20210902.401
Lai, Y. S., Putra, R. B. D. S., Aui, S. P., and Chang, K. T. (2018). M2(C) polarization by Baicalin enhances efferocytosis via upregulation of MERTK receptor. Am. J. Chin. Med. 46 (8), 1899–1914. doi:10.1142/S0192415X18500957
Lee, S. H., Jhun, J., Byun, J. K., Kim, E. K., Jung, K., Lee, J. E., et al. (2017). IL-17 axis accelerates the inflammatory progression of obese in mice via TBK1 and IKBKE pathway. Immunol. Lett. 184, 67–75. doi:10.1016/j.imlet.2017.02.004
Li, B. (2021a). Study on the mechanism of huanglian Jiedu decoction requlating macrophage polarization based on network pharmacology. China Pharm. 32 (05), 552–558. doi:10.6039/j.issn.1001-0408.2021.05.08
Li, B. (2021b). Mechanism of huanglian Jiedu decoction in preventing and treating atherosclerosis by requlating macrophage polarization. China Pharm. 32 (08), 939–944. doi:10.6039/j.issn.1001-0408.2021.08.08
Li, C., Li, J., Jiang, F., Tzvetkov, N. T., Horbanczuk, J. O., Li, Y., et al. (2021a). Vasculoprotective effects of ginger (Zingiber officinale Roscoe) and underlying molecular mechanisms. Food Funct. 12 (5), 1897–1913. doi:10.1039/d0fo02210a
Li, H., Bai, L., Qin, Q., Feng, B. L., Zhang, L., Wei, F. Y., et al. (2020). Research progress on anti-atherosclerosis effect and mechanism of flavonoids compounds mediated by macrophages. China J. Chin. Materia Medica 45 (12), 2827–2834. doi:10.19540/j.cnki.cjcmm.20200119.403
Li, H., Yang, W., Cao, W., Yu, Z., Zhang, G., Long, L., et al. (2024). Effects and mechanism of Kedaling tablets for atherosclerosis treatment based on network pharmacology, molecular docking and experimental study. J. Ethnopharmacol. 319 (Pt 1), 117108. doi:10.1016/j.jep.2023.117108
Li, Q., Liu, Y., Xia, X., Sun, H., Gao, J., Ren, Q., et al. (2021b). Activation of macrophage TBK1-HIF-1α-mediated IL-17/IL-10 signaling by hyperglycemia aggravates the complexity of coronary atherosclerosis: an in vivo and in vitro study. Faseb J. 35 (5), e21609. doi:10.1096/fj.202100086RR
Li, Q., Wang, M., Zhang, S., Jin, M., Chen, R., Luo, Y., et al. (2022b). Single-cell RNA sequencing in atherosclerosis: mechanism and precision medicine. Front. Pharmacol. 13, 977490. doi:10.3389/fphar.2022.977490
Li, X. Y., Wang, Y. J., Chen, S., Pan, L. H., Li, Q. M., Luo, J. P., et al. (2022a). Laminaria japonica polysaccharide suppresses atherosclerosis via regulating autophagy-mediated macrophage polarization. J. Agric. Food Chem. 70 (12), 3633–3643. doi:10.1021/acs.jafc.1c07483
Liao, Y. H., Xia, N., Zhou, S. F., Tang, T. T., Yan, X. X., Lv, B. J., et al. (2012). Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J. Am. Coll. Cardiol. 59 (4), 420–429. doi:10.1016/j.jacc.2011.10.863
Lijuan, C., Ling, Y., and Li, L. (2023). Macrophage differentiation mechanism of Ningxin ditan decoction in lnhibiting ln-stent restenosis by AMPK/NF-κB pathway mediated. J. Emerg. Traditional Chin. Med. 32 (04), 592–596+623. doi:10.3969/j.issn.1004-745X.2023.04.006
Liu, Q. Y., Zhuang, Y., Song, X. R., Niu, Q., Sun, Q. S., Li, X. N., et al. (2021). Tanshinone IIA prevents LPS-induced inflammatory responses in mice via inactivation of succinate dehydrogenase in macrophages. Acta Pharmacol. Sin. 42 (6), 987–997. doi:10.1038/s41401-020-00535-x
Lu, G. (2021). Study on the effect of Yiqi Huoxue recipe in the treatment of stable angina pectoris patients with Qi deficiency and blood stasis Syndrome after PCl and related changes of lL-17 and hs-CRP. Yunnan J. Traditional Chin. Med. 42 (10), 19–23. doi:10.16254/j.cnki.53-1120/r.2021.10.007
Lu, W., Wang, Q., Sun, X., He, H., Wang, Q., Wu, Y., et al. (2019). Qishen Granule improved cardiac remodeling via balancing M1 and M2 macrophages. Front. Pharmacol. 10, 1399. doi:10.3389/fphar.2019.01399
Lu, X. (2017). The impact of IL-17 in atherosclerosis. Curr. Med. Chem. 24 (21), 2345–2358. doi:10.2174/0929867324666170419150614
Lv, X., Wang, H., Wu, R., Shen, X., and Ye, G. (2020). The active compounds of yixin ningshen tablet and their potential action mechanism in treating coronary heart disease- A network pharmacology and proteomics approach. Evid. Based Complement. Altern. Med. 2020, 4912395. doi:10.1155/2020/4912395
Ma, C., Xia, R., Yang, S., Liu, L., Zhang, J., Feng, K., et al. (2020). Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE(-/-) mice. Theranostics 10 (3), 1090–1106. doi:10.7150/thno.38115
Ma, L., Dai, X., Wu, C., Li, M., Sheng, H., and Mao, W. (2021). Tanyu tongzhi formula delays atherosclerotic plaque progression by promoting alternative macrophage activation via PPARγ and AKT/ERK signal pathway in ApoE knock-out mice. Front. Pharmacol. 12, 734589. doi:10.3389/fphar.2021.734589
Nakakubo, S., Kimura, S., Mimura, K., Kajiwara, C., Ishii, Y., Konno, S., et al. (2020). Traditional Japanese herbal medicine hochu-ekki-to promotes pneumococcal colonization clearance via macrophage activation and interleukin 17A production in mice. Front. Cell Infect. Microbiol. 10, 569158. doi:10.3389/fcimb.2020.569158
Ouyang, Y., Rong, Y., Wang, Y., Guo, Y., Shan, L., Yu, X., et al. (2021). A systematic study of the mechanism of Acacetin against sepsis based on network pharmacology and experimental validation. Front. Pharmacol. 12, 683645. doi:10.3389/fphar.2021.683645
Peng, L., Lv, C. S., Zhao, Y., Chen, S. D., Huang, Y., Lu, D. W., et al. (2017). QiShenYiQi pill attenuates atherosclerosis by promoting regulatory T cells, inhibiting T helper 17 cells and accelerating cholesterol excretion. Oncotarget 8 (47), 82196–82206. doi:10.18632/oncotarget.19072
Qian, Z., Zeng, Q., and Li, Q. (2012). The impacts of Danshensu on the activation of liver X receptor and the cholesterol efflux of macrophage. J. Clin. Cardiol. 28 (10), 778–780. doi:10.13201/j.issn.1001-1439.2012.10.001
Ren, Y. S., Li, H. H., Yao, J. C., Tan, Y. J., Pan, L. H., Peng, T., et al. (2020). Application quantitative proteomics approach to identify differentially expressed proteins associated with cardiac protection mediated by cycloastragenol in acute myocardial infarction rats. J. Proteomics 222, 103691. doi:10.1016/j.jprot.2020.103691
Runmin, L. (2021). Study of Qingxin Jieyu formula for regulation of vulnerable atherosclerotic plaques by network pharmacology. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 19 (19), 3263–3267. doi:10.12102/j.issn.1672-1349.2021.19.003
Salvatore, G., Bernoud-Hubac, N., Bissay, N., Debard, C., Daira, P., Meugnier, E., et al. (2015). Human monocyte-derived dendritic cells turn into foamy dendritic cells with IL-17A. J. Lipid Res. 56 (6), 1110–1122. doi:10.1194/jlr.M054874
Shi, X., Huang, H., Zhou, M., Liu, Y., Wu, H., and Dai, M. (2021). Paeonol attenuated vascular fibrosis through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Front. Pharmacol. 12, 765482. doi:10.3389/fphar.2021.765482
Shu, J., Huang, R., Tian, Y., Liu, Y., Zhu, R., and Shi, G. (2020). Andrographolide protects against endothelial dysfunction and inflammatory response in rats with coronary heart disease by regulating PPAR and NF-κB signaling pathways. Ann. Palliat. Med. 9 (4), 1965–1975. doi:10.21037/apm-20-960
Smith, E., Prasad, K. M. R., Butcher, M., Dobrian, A., Kolls, J. K., Ley, K., et al. (2010). Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation 121 (15), 1746–1755. doi:10.1161/CIRCULATIONAHA.109.924886
Song, L. (2022). The mechanism of tanshinone IIA regulating macrophage polarization through Sirt1 signal pathway. Chin. J. Integr. Med. Cardio-Cerebrovascular Dis. 20 (07), 1210–1216. doi:10.12102/j.issn.1672-1349.2022.07.009
Song, M., Xu, S., Zhong, A., and Zhang, J. (2019). Crosstalk between macrophage and T cell in atherosclerosis: potential therapeutic targets for cardiovascular diseases. Clin. Immunol. 202, 11–17. doi:10.1016/j.clim.2019.03.001
Suli, L. (2021). Tudy on mechanism of removing Toxins to activate blood recipe for treatment of coronary heart disease based on network pharmacology. J. Guangzhou Univ. Traditional Chin. Med. 38 (05), 985–991. doi:10.13359/j.cnki.gzxbtcm.2021.05.023
Tacke, F., and Zimmermann, H. W. (2014). Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60 (5), 1090–1096. doi:10.1016/j.jhep.2013.12.025
Tang, L. (2019). Effect of Yiqi Yangyin Zhuyu recipe in anti-inflammation activities in vitro on LPS-induced RAW 264.7 cells. Chin. J. Exp. Traditional Med. Formulae 25 (01), 141–148. doi:10.13422/j.cnki.syfjx.20190129
Tang, Y. N., He, X. C., Ye, M., Huang, H., Chen, H. L., Peng, W. L., et al. (2015). Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. J. Ethnopharmacol. 175, 451–455. doi:10.1016/j.jep.2015.10.004
Thomas, S. E., Barenbrug, L., Hannink, G., Seyger, M. M. B., de Jong, E. M. G. J., and van den Reek, J. M. P. A. (2024). Drug survival of IL-17 and IL-23 inhibitors for psoriasis: a systematic review and meta-analysis. Drugs 84 (5), 565–578. doi:10.1007/s40265-024-02028-1
Thou, E. M. H., Choo, Q. C., and Chew, C. H. (2020). IL-17A induction of ADAMTS-5 in differentiated THP-1 cells is modulated by the ERK signaling pathway. Eur. Cytokine Netw. 31 (2), 59–67. doi:10.1684/ecn.2020.0446
Verhagen, S. N., Buijsrogge, M. P., Vink, A., van Herwerden, L. A., van der Graaf, Y., and Visseren, F. L. J. (2014). Secretion of adipocytokines by perivascular adipose tissue near stenotic and non-stenotic coronary artery segments in patients undergoing CABG. Atherosclerosis 233 (1), 242–247. doi:10.1016/j.atherosclerosis.2013.12.005
Vinet, E., Pineau, C. A., Scott, S., Clarke, A. E., Platt, R. W., and Bernatsky, S. (2015). Increased congenital heart defects in children born to women with systemic lupus erythematosus: results from the offspring of Systemic Lupus Erythematosus Mothers Registry Study. Circulation 131 (2), 149–156. doi:10.1161/CIRCULATIONAHA.114.010027
Wang, B. (2014). “IL-17A increases the instability of established atherosclerotic plaque in apolipoprotein E-deficient mice,” in Chinese society of immunology.
Wang, B., Hou, X., Sun, Y., Lei, C., Yang, S., Zhu, Y., et al. (2022e). Interleukin-17A influences the vulnerability rather than the size of established atherosclerotic plaques in apolipoprotein E-deficient mice. Open Life Sci. 17 (1), 1104–1115. doi:10.1515/biol-2022-0072
Wang, N., Zhang, X., Ma, Z., Niu, J., Ma, S., Wenjie, W., et al. (2020). Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE(-/-) mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed. Pharmacother. 123, 109729. doi:10.1016/j.biopha.2019.109729
Wang, Q., Wang, Y., and Xu, D. (2022a). Research progress on Th17 and T regulatory cells and their cytokines in regulating atherosclerosis. Front. Cardiovasc Med. 9, 929078. doi:10.3389/fcvm.2022.929078
Wang, T., Jiang, X., Ruan, Y., Zhuang, J., and Yin, Y. (2022d). Based on network pharmacology and in vitro experiments to prove the effective inhibition of myocardial fibrosis by Buyang Huanwu decoction. Bioengineered 13 (5), 13767–13783. doi:10.1080/21655979.2022.2084253
Wang, T., Zhou, Y., Wang, K., Jiang, X., Wang, J., and Chen, J. (2022c). Prediction and validation of potential molecular targets for the combination of Astragalus membranaceus and Angelica sinensis in the treatment of atherosclerosis based on network pharmacology. Med. Baltim. 101 (26), e29762. doi:10.1097/MD.0000000000029762
Wang, Y., Su, W., Li, Y., Yuan, J., Yao, M., Su, X., et al. (2022f). Analyzing the pathogenesis of systemic lupus erythematosus complicated by atherosclerosis using transcriptome data. Front. Immunol. 13, 935545. doi:10.3389/fimmu.2022.935545
Wang, Y., Wang, X., Li, Y., Xue, Z., Shao, R., Li, L., et al. (2022g). Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol. Res. 176, 106083. doi:10.1016/j.phrs.2022.106083
Wang, Y. K., Wang, J., Hua, F., Shen, Y. L., Han, L., You, J. Y., et al. (2022b). TREM-1 modulates dendritic cells maturation and dendritic cell-mediated T-cell activation induced by ox-LDL. Oxid. Med. Cell Longev. 2022, 3951686. doi:10.1155/2022/3951686
Wu, M. Y., Li, C. J., Hou, M. F., and Chu, P. Y. (2017). New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int. J. Mol. Sci. 18 (10), 2034. doi:10.3390/ijms18102034
Wu, X. J., Zhou, X. B., Chen, C., and Mao, W. (2019). Systematic investigation of quercetin for treating cardiovascular disease based on network pharmacology. Comb. Chem. High. Throughput Screen 22 (6), 411–420. doi:10.2174/1386207322666190717124507
Xiang, Y., Zhang, H., Xu Zhang, Z., Yang Qu, X., and Xia Zhu, F. (2022). Dihydrosanguinarine based RNA-seq approach couple with network pharmacology attenuates LPS-induced inflammation through TNF/IL-17/PI3K/AKT pathways in mice liver. Int. Immunopharmacol. 109, 108779. doi:10.1016/j.intimp.2022.108779
Xu, M., Li, X., and Song, L. (2020). Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm. Biol. 58 (1), 655–663. doi:10.1080/13880209.2020.1779318
Xuanjing, C. (2020). Inhibitory effect of Wendan decoction on formation of foam cells induced by ox-LDL. Chin. J. Pathophysiol. 36 (11), 1952–1959. doi:10.3969/j.issn.1000-4718.2020.11.005
Xue, Z.W., Shang, X.M., Lv, S.H., Xu, H., Zhang, Q., Wang, C., et al. (2013). Effects of Shenshao Decoction on the inflammatory response in the aorta of a rat atherosclerotic model. Chin. J. Integr. Med. 19, (5), 347–52.doi:10.1007/s11655-013-1457-z
Yang, Y., Huang, T., Zhang, H., Li, X., Shi, S., Tian, X., et al. (2023). Formononetin improves cardiac function and depressive behaviours in myocardial infarction with depression by targeting GSK-3β to regulate macrophage/microglial polarization. Phytomedicine 109, 154602. doi:10.1016/j.phymed.2022.154602
Yanyu, S. (2022). Mechanism of Shen-Hong-Tong-Luo formula in regulating macrophage polarization and preventing atherosclerosis. Chin. J. Gerontology 42 (14), 3510–3514. doi:10.3969/j.issn.1005-9202.2022.14.041
Yang, Y., Chang, S., Xiang-Zhuo, Z., Jing, L., Shu-Chun, H., Hui-Fang, K., et al. (2023). Mechanisms of Xuefu Zhuyu Decoction in the treatment of coronary heart disease based on integrated metabolomics and network pharmacology approach. J. Chromato B. 1223, 123712. doi:10.1016/j.jchromb.2023.123712
Ye, W., Wang, J., Little, P. J., Zou, J., Zheng, Z., Lu, J., et al. (2024). Anti-atherosclerotic effects and molecular targets of ginkgolide B from Ginkgo biloba. Acta Pharm. Sin. B 14 (1), 1–19. doi:10.1016/j.apsb.2023.09.014
Yi, T., Chen, H. B., Zhao, Z. Z., Yu, Z. L., and Jiang, Z. H. (2011). Comparison of the chemical profiles and anti-platelet aggregation effects of two “Dragon's Blood” drugs used in traditional Chinese medicine. J. Ethnopharmacol. 133 (2), 796–802. doi:10.1016/j.jep.2010.11.008
Yi, Z. (2022). Gegen qinliantang regulates polarization tendency of macrophages to lntervenein vulnerable plaque in AS of ApoE-/-mice. Chin. J. Exp. Traditional Med. Formulae 28 (11), 60–69. doi:10.13422/j.cnki.syfjx.20220413
Yu, L., Zhang, Y., Liu, C., Wu, X., Wang, S., Sui, W., et al. (2023). Heterogeneity of macrophages in atherosclerosis revealed by single-cell RNA sequencing. FASEB J. 37 (3), e22810. doi:10.1096/fj.202201932RR
Zhang, L., Liu, M., Liu, W., Hu, C., Deng, J., et al. (2021). Th17/IL-17 induces endothelial cell senescence via activation of NF-κB/p53/Rb signaling pathway. Lab. Invest 101 (11), 1418–1426. doi:10.1038/s41374-021-00629-y
Zhang, R. (2013). Yindanxinnaotong lnhibited the formation of foam cells and the secretion of matrix metalloproteinase by macrophages. Prog. Mod. Biomed. 13 (17), 3234–3237. doi:10.13241/j.cnki.pmb.2013.17.008
Zhong, Q., Zhou, K., Liang, Q. L., Lin, S., Wang, Y. C., Xiong, X. Y., et al. (2016). Interleukin-23 secreted by activated macrophages drives γδT cell production of interleukin-17 to aggravate secondary injury after intracerebral hemorrhage. J. Am. Heart Assoc. 5 (10), e004340. doi:10.1161/JAHA.116.004340
Zhong, Y., Gao, H., Luo, X., Wang, D.Y., Zhang, L., and Wang, J.Y. (2022). Mechanism of Ge Gen Qin Lian Decoction regulating macrophage polarization to intervene vulnerable plaques of ApoE-/-mice with atherosclerosis. China. J. Exp. Trad. Med. Formulae. 28 (11), 60–69. doi:10.13422/j.cnki.syfjx.20220413
Keywords: Chinese medicine (CM), interleukin-17 (IL-17), macrophages, coronary heart disease (CHD), Th17 cell
Citation: Liu Q, Liu P, Li C, Zhao Z, Wang D, Liu Q and Yang H (2025) Effects of Chinese Medicine on modulating interleukin-17-regulated macrophages in coronary heart disease. Front. Pharmacol. 16:1499786. doi: 10.3389/fphar.2025.1499786
Received: 21 September 2024; Accepted: 10 February 2025;
Published: 10 April 2025.
Edited by:
Carlos Alan Dias-Junior, São Paulo State University, BrazilReviewed by:
Soon Yew Tang, University of Pennsylvania, United StatesCopyright © 2025 Liu, Liu, Li, Zhao, Wang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing Liu, ODUxNzU3NjI2QHFxLmNvbQ==; Dawei Wang, ZGF3ZWl3YW5nQDEyNi5jb20=; Huawei Yang, MTA4ODY0MzczQHFxLmNvbQ==
‡ORCID: Qing Liu, https://orcid.org/0000-0002-2199-2999
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.