
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 27 February 2025
Sec. Ethnopharmacology
Volume 16 - 2025 | https://doi.org/10.3389/fphar.2025.1498280
The fruits of Lycium ruthenicum Murr. (Solanaceae) are employed in ethnomedicine and used as a functional food. Their antioxidant, anti-aging, and hypolipidemic activities have been investigated in modern research. This study indicated that the ethanolic extract of the fruits of L. ruthenicum Murr. (LRM) improved oxidative and heat stress tolerance, reduced the accumulation of lipofuscin, and retarded the aging process in Caenorhabditis elegans (Rhabditidae). Furthermore, the pharyngeal pumping rate and body length decreased under LRM treatment. Moreover, metabolomic analysis and the DPClusO algorithm revealed that LRM regulated a series of lifespan-related pathways centered on glycine, serine, and threonine metabolism. These results suggest that LRM prolongs the lifespan of Caenorhabditis elegans via dietary restriction. Moreover, feruloyl putrescine, a kind of polyamine, was found in differential metabolites, which may be the metabolite of caffeoyl-spermidine in LRM. These findings from this exploratory study offer a new insight into the roles of L. ruthenicum in anti-aging activity as a functional food.
Aging is defined as a time-dependent decline in physiological integrity, resulting in impaired functional ability and decreased quality of life (Cai et al., 2022). Despite advancements in modern medical science increasing life expectancy, the challenge remains to achieve good health alongside a longer lifespan (Beard et al., 2016). Natural remedies are widely mentioned in ethnomedical information that emerges as a valuable source for discovering remedies for age-related diseases (Wahid et al., 2020).
Lycium ruthenicum Murr., a perennial thorny shrub plant of the Lycium L. genus in the Solanaceae family, can be found in northwestern China, Central Asia, and the Caucasus of Europe. In accordance with the records of traditional Tibetan medicine classics Si Bu Yi Dian and Jing Zhu Ben Cao, the fruits of Lycium ruthenicum have been used for addressing diseases of the aged such as heart diseases and menopausal symptoms (Wang et al., 2018). Moreover, in Uighur medicine, they have been described as a nourishing tonic for improving eyesight, antihypertensive, and strengthening (Tao et al., 2022). Lycium ruthenicum fruits contain abundant anthocyanins, a class of flavonoids that possess antioxidant, anti-inflammatory, hepatoprotective, and renal protective activities, as well as immunity-enhancing effects (Zhang et al., 2019; Yu et al., 2023). In our previous study, the ethanolic extract of L. ruthenicum Murr. fruit (LRM) has been shown to possess potential activities in restoring spatial memory and cognitive function in mice treated with D-Galactose (D-Gal) inducing oxidative stress (Cui et al., 2023). Furthermore, L. ruthenicum anthocyanins have been shown to alleviate D-Gal-induced liver injuries through relieving inflammation and reversing the abnormal amino acid metabolome in aging rats (Chen S. S. et al., 2022). Its leaves are a byproduct of L. ruthenicum and contain flavonoids, anthocyanins, and polysaccharides (Tao et al., 2022; Chen et al., 2024; Liu et al., 2016). In traditional, leaves are used as a diuretic agent (Sharma et al., 2022). In a previous study, a water-soluble polysaccharide LRLP4-A isolated from the leaves could significantly increase macrophage proliferation and phagocytic ability in RAW246.7 cells (Liu et al., 2016).
Lycium ruthenicum extract has been demonstrated to prolong the lifespan of Caenorhabditis elegans in collaboration with the IIS pathway via sir-2.1 and hsf-1, but independent of daf-16 (Xiong et al., 2021). Metabolomic analysis contributes to discovering potential aging and age-related disease biomarkers (Parkhitko et al., 2020). Anthocyanins in L. ruthenicum have been reported to decrease oxidative stress, aging-related liver injury, and reverse abnormal metabolomic of the hippocampus and serum in aging rats (Chen S. S. et al., 2022; Chen S. et al., 2022). However, there remains a gap in metabolomic studies related to C. elegans. This study aims to address this gap by investigating the anti-aging activity of LRM in C. elegans. The effects of LRM on lifespan, lipofuscin level, resistance to oxidative and heat stresses, and other physiological indices in C. elegans were explored, with the objective of elucidating the anti-aging potential of LRM. Furthermore, the alteration of metabolites in C. elegans after being treated with LRM was analyzed.
Ethanol was purchased from Saifurui Technology Company (Tianjin, China). 5-Fluorouracil (5-FU), tripotassium citrate, spermidine, levamisole hydrochloride, and paraquat (PQ) were purchased from Macklin Biochemical Technology Company (Shanghai, China). MgSO4, CaCl2, K2HPO4, KH2PO4, NaCl, and sucrose were purchased from Hushi Laboratory Equipment Company (Shanghai, China). Cholesterol was purchased from Yuanye Bio-Technology Company (Shanghai, China). Tryptone and yeast extract were purchased from Oxoid (Hampshire, United Kingdom). Agar powder was purchased from Solarbio Science and Technology Company.
Lycium ruthenicum was identified by Dr. Mei Tian at Institute of Botany, Jiangsu Province and Chinese Academy of Sciences (Nanjing, China). The fruit samples (lead <0.5 ppm, Pymetrozine < 2 ppm.) were selected from the barbary wolfberry planting base of Barbary Wolfberry Engineering Technology Research Institute, Ningxia Academy of Agriculture and Forestry Sciences (Ningxia China) in August 2022. The sample of dried fruit (HGGQG20220723) was reserved in the herbarium of Institute of Botany, Jiangsu Province and Chinese Academy of Sciences (Nanjing, China). The method of extraction and analysis was performed as reported previously (Cui et al., 2023). In short, the dried fruits were extracted with 70% ethanol at 45°C. The extracted liquid was applied to an Amberly (1 kg) column. Then, 5% formic acid was used to remove the Saccharides. The EtOH-H2O-HCOOH (50:50:5) was used to collect the phytochemicals. After the evaporative concentrating of the extracting solution, the last extract collected was named L. ruthenicum extract (LRM).
Agilent 1260 UPLC-DAD-6530 ESI-QTOF-MS equipped with a Poroshell 120 SB-Aq C18 analytical column (4.6 mm × 100 mm, 2.7 μm, Agilent, United States) was employed for analyzing the phytochemical metabolites of LRM. Liquid chromatography conditions are as follows. The mobile phase composition: 1% formic acid in water (A) and acetonitrile (B); the gradient elution program: 0 min, 10% B; 35 min, 15% B; 40 min, 20% B; 50 min, 100% B; sample size: 10 μL; flow rate: 0.5 mL/min; column temperature: 40°C; detection wavelength: 535, 280, 254 nm. The mass spectrometry conditions are as follows. ESI ion source mass spectrometry detector, positive ion mode (100–3,200 m/z); nebulizer pressure 50 psi; the drying gas flow rate: 10 mL/min; the drying gas temp: 350°C; the capillary voltage: 4000 V; fragmentor voltage: 205 V; the MS/MS collision energy: 35 V.
The Caenorhabditis elegans strains (N2) and E. coli OP50 used in this study were obtained from Caenorhabditis Genetics Center. Caenorhabditis elegans was raised on plates of Nematode Growth Medium (NGM) or S medium seeded with E. coli OP50 and maintained at 20°C. Nematodes that exploded, moved to the wall of the plate, and drilled into the medium were eliminated from further data analysis.
The LRM was dissolved in ddH2O to make a stock solution with a concentration of 10 mg/mL. LB liquid medium containing OP50 (100 μL) was daubed onto the central surface of NGM plates (60 mm), covered with 100 μL of different concentrations of LRM solutions (ddH2O served as the control), and left overnight at 37°C. Unless otherwise noted, experimental plates with adult nematodes contained 0.07 mg/mL of 5-FU to prevent the production of progeny.
The specific quantity number of synchronous L4-stage nematodes were transferred onto NGM plates containing OP50 and the LRM concentrations (5 μg/mL, 50 μg/mL, 100 μg/mL, 500 μg/mL, or 1,000 μg/mL), water, or 50 μg/mL spermidine (Spd). A gentle prod with a picker was used to check the survival of the nematodes. And dead individuals were counted daily.
The mobility of nematodes was measured at different cohort levels. The movement of the nematodes was classified into four grades: those that could move with sinusoidal motion autonomously were defined as grade A, those that needed external stimulation to help them creep were defined as grade B, those that only moved their head with a slight prod were defined as grade C, and dead individuals were defined as grade D.
The surviving individuals were moved to new plates every 48 h. All assays were performed in triplicate, with 50–70 individuals per group.
The synchronized L4-stage nematodes were separately transferred to individual NGM plates without 5-FU, and nematodes were transferred to new plates every day throughout the spawning period. In order to analyze the larval growth conditions, the original plates were maintained to quantify the number of progenies that normally hatched from the eggs. At least 10 nematodes were used in each treatment of this experiment.
The synchronized L4-stage nematodes were transferred to NGM plates containing 0 μg/mL, 50 μg/mL, 100 μg/mL, or 500 μg/mL LRM. Pharyngeal pumps per minute were determined with a handheld tally counter on day 3, day 6, and day 9. The assays were conducted for 5 nematodes per group and implemented in triplicate.
After 10 days, 10 nematodes treated with 0 μg/mL, 50 μg/mL, 100 μg/mL, or 500 μg/mL of LRM were placed onto a 1% agarose-coated microslide. Then, they were anesthetized with levamisole hydrochloride (7 mM). The lipofuscin fluorescence intensity was detected with fluorescence microscopy at GFP channel. The lipofuscin fluorescence intensity and body length of C. elegans were assessed with the ImageJ software. Each treatment was implemented three times.
Caenorhabditis elegans individuals at L4-stage were moved to the NGM plates containing 0 μg/mL, 50 μg/mL, 100 μg/mL, or 500 μg/mL of LRM, and kept at 20°C for 6 days. Nematodes were transferred to the control or appropriate LRM plates that also contained 70 mM of Paraquat dichloride for the oxidative stress tolerance assay. Mortality was scored every 12 h until all the individuals were died. As part of the heat stress tolerance assay, the nematodes were maintained at 35°C for 4 h, and the plates were maintained at 20°C subsequently. The survival rate was scored after 12 h. The experiments were conducted three times, with at least 50 nematodes per group.
The synchronized L4-stage nematodes were transferred to the liquid medium containing 0 or 100 μg/mL of LRM and kept at 20°C for 48 h (n = 3). The Vanquish UHPLC system connected with an Orbitrap Q Exactive TMHF-X mass spectrometer (Thermo Fisher, Germany) was employed to analyze the metabolite extraction of Caenorhabditis elegans. In short, Caenorhabditis elegans (100 mg) was separated from a liquid medium containing OP50. Then, these samples were fully ground with liquid nitrogen and the homogenate was resuspended in 500 μL of precooled 80% methanol with a vortex generator. The homogenate was incubated on ice for 5 min and then centrifuged at 15,000 g and 4°C for 20 min. The supernatant was diluted to a final methanol concentration of 53% with LC-MS grade water. After that, the samples were centrifuged at 15,000 g and 4°C for 20 min. Samples were injected into a Hypersil Gold column (100 × 2.1 mm, 1.9 μm). Liquid chromatography conditions are as follows. The composition of eluents for the positive polarity mode: eluent A (0.1% FA in water) and eluent B (methanol); the composition of eluents for the negative polarity mode: eluent A (5 mM ammonium acetate, pH 9.0) and eluent B (methanol). The solvent gradient: 2% B, 1.5 min; 2%–85% B, 3 min; 85%–100% B, 10 min; 100%–2% B, 10.1 min; 2% B, 12 min; flow rate: 0.2 mL/min; the Q Exactive TMHF-X mass spectrometer was operated in positive/negative polarity mode with conditions as follows. Spray voltage: 3500 V; capillary temperature: 320°C; sheath gas flow rate: 35 psi; aux gas flow rate: 10 L/min, S-lens RF level: 60; Aux gas heater temperature: 350°C.
The metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/pathway.html), LIPIDMaps database (http://www.lipidmaps.org/), and human metabolome database (HMDB) database (https://hmdb.ca/metabolites). PCA and PLS-DA were performed using SIMCA 14.1. The functions of these metabolites and metabolic pathways were studied using the KEGG database.
The R-package “psych” was used to perform Pearson rank correlation analysis for all pairwise comparisons among individual metabolites. The R-package “igraph” was used to calculate network statistics. DPClusSBO 1.1 was used to identify co-accumulated metabolite groups and visualize the network (Karim et al., 2021). We performed simple graph clustering with the following settings: density value (D) was set to 0.9, cluster property (CP) was set to 0.5, minimum cluster size was set to 5, and overlapping coefficient (OV Coff) was set to default value 0.25 (Liu K. et al., 2017).
Data analyses and calculations were carried out using Graphpad Prism 8.0.2 (La Jolla, CA, United States). One-way ANOVA with Tukey’s multiple comparisons test was used to determine significance between groups. p < 0.05 was considered statistically significant.
The phytochemical metabolites of LRM were analyzed using UPLC-Q/TOF-MS analysis, which revealed that the main phytochemicals in LRM were anthocyanins and spermidines (Supplementary Figure S1; Supplementary Table S1). These metabolites have been previously reported to possess anti-aging and antioxidant properties (Cui et al., 2023). Therefore, this study aims to investigate and determine the anti-aging effects of LRM in C. elegans.
Compared with the control group, LRM extended the N2 lifespan between 19.19% and 26.79% within the dose range of 5–1,000 μg/mL. The effect of LRM at 50 μg/mL was better than that of the anti-aging reagent spermidine at 50 μg/mL (Figures 1A, B; Supplementary Table S2).
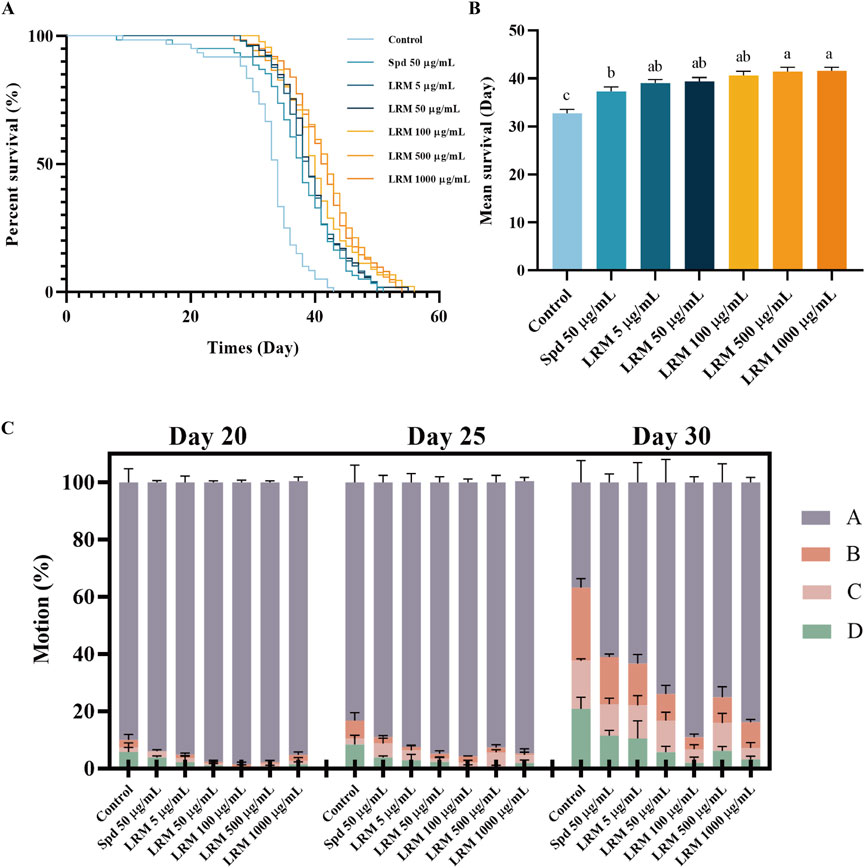
Figure 1. Effects of LRM on prolonging the lifespan and promoting mobility of Caenorhabditis elegans. (A) The survival curves of Caenorhabditis elegans; (B) The Average lifespan of Caenorhabditis elegans; (C) The motility of Caenorhabditis elegans at different stages. Data are expressed as mean ± SEM (n = 3 per group). Values with different letters in each column were significantly different (p < 0.05). The differences were tested by using Tukey test and one-way ANOVA.
The movement of C. elegans at different stages of the life cycle was conducted to determine the effect of LRM on the entire life cycle (Figure 1C). On the 30th day, in the control group, the proportion of motion Grade A nematodes decreased from 90.05% to 36.77%, while that in the other groups remained greater than 60%. In the group of LRM at 100 μg/mL, the proportion of motion Grade A individuals was 89.04%. This result indicates that treatment of LRM prolonged the lifespan of C. elegans, moreover, these individuals exhibited better autonomous locomotion at their middle-late life stages. Comparing the treatments of 500 μg/mL and 1,000 μg/mL LRM, the prolong rates of these two groups were 26.40% and 26.79%, respectively. Thus, we assayed the effects of LRM using only the 50, 100, and 500 μg/mL doses in all subsequent experiments.
Caenorhabditis elegans were treated with low, medium, and high doses of LRM to explore the effects of LRM on reproduction and incubation. LRM extended the reproduction period from 5 days to 7 days (Figure 2A), a characteristic associated with longer lifespans in individual nematodes (Scharf et al., 2021). Notably, 97% of the generation of each group had hatched on time, indicating no effect of LRM treatment on incubation (Supplementary Figure S2).
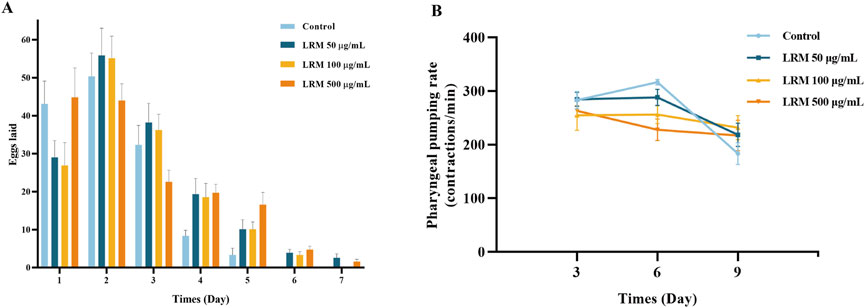
Figure 2. Effects of LRM on the production and the pumping rate of Caenorhabditis elegans. (A) The production per day and the reproduction period of Caenorhabditis elegans. Data are expressed as mean ± SEM (n = 10 per group); (B) The pumping rate of Caenorhabditis elegans during different periods. Data are expressed as mean ± SEM (n = 5 per group). The differences were tested by using Tukey test and one-way ANOVA.
In the early periods (day 3), the pumping rate in nematodes supplemented with LRM at 100 μg/mL and 500 μg/mL had declined compared with the control group. In the middle stage, nematodes treated with different concentrations of LRM all showed a downward trend in pumping rate in comparison with the control group. However, on the 9th day, LRM improved the pumping capacity compared with the control group (Figure 2B). Therefore, we conjectured that, in the early stage, treatment with LRM could stimulate the dietary restriction (DR) pathway. Meanwhile, as time progressed, the physiological condition of the nematodes in the control group deteriorated more than those in the LRM treatment groups.
Auto-fluorescent lipofuscin accumulates with the age of Caenorhabditis elegans (Lin et al., 2023). The measurement of lipofuscin accumulation is essential to assess aging processes. Compared with the control group, the relative lipofuscin fluorescence of Caenorhabditis elegans was diminished under the 50, 100, and 500 μg/mL doses, yielding 17.20%, 9.90%, and 13.58%, respectively (Figures 3A, C). This result suggested that LRM-treated nematodes maintained a more youthful state. Additionally, nematodes in a DR state, are characterized by low-age pigment fluorescence (Gerstbrein et al., 2005). The body length of the nematodes was also recorded, which was shortened partly by treatment with LRM in 50, 100, and 500 μg/mL doses (by 8.07%, 11.93%, and 9.05%, respectively) (Figures 3B, C).
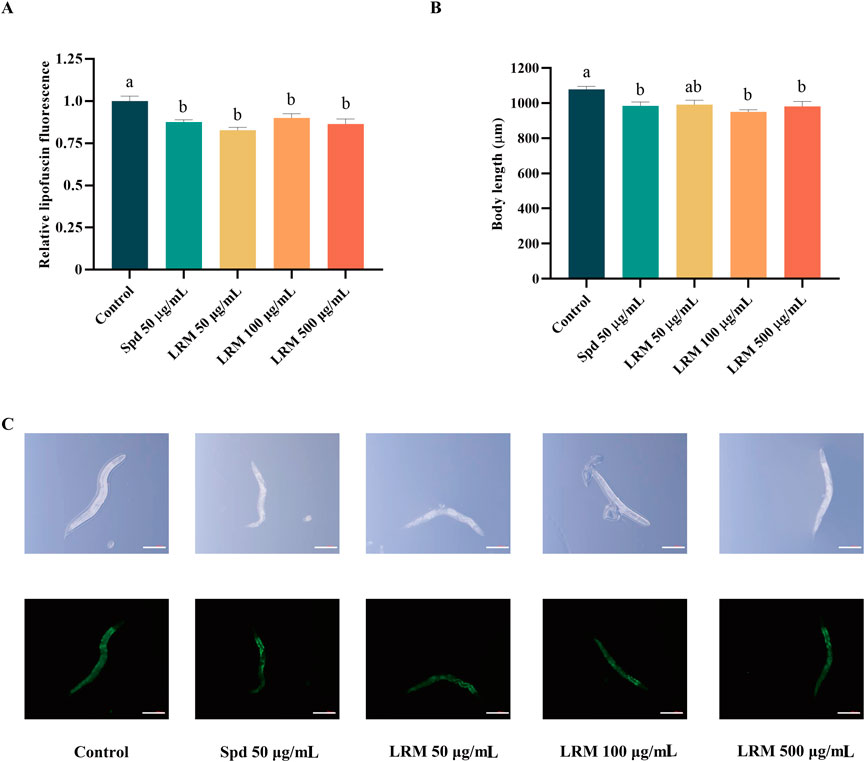
Figure 3. Effects of LRM on the accumulation of lipofuscin and the body length of Caenorhabditis elegans. (A) The accumulation of lipofuscin in Caenorhabditis elegans; (B) The body length of Caenorhabditis elegans; (C) The image of Caenorhabditis elegans (Bar = 200 μm). Lipofuscin intensity and body size were analyzed with ZEN 3.3 software. Data are expressed as mean ± SEM (n = 3 per group). Values with different letters in each column were significantly different (p < 0.05). The differences were tested by using Tukey test and one-way ANOVA.
Two methods were selected to explore the stress resistance of Caenorhabditis elegans individuals treated with LRM. PQ has been used as an oxidative stress-inducing agent. The nematodes that were fed with LRM showed an ability to resist the oxidative stress induced by PQ. The life curves moved rightward with LRM treatment. The 50, 100, and 500 μg/mL LRM extracts resulted in 16.69%, 22.82%, and 26.08% of increments in tolerance, respectively (Figure 4A). After heat stress treatment at 35°C for 4 h, the survival rates of the 100 and 500 μg/mL groups were 73.38% and 61.31%, while it was only 19.60% in the control group (Figure 4B). These results indicated increasing tolerance of Caenorhabditis elegans to external oxidative and heat stress after LRM treatment, which further supported the correlation between stress resistance and prolonged lifespan (Amrit and May 2010).
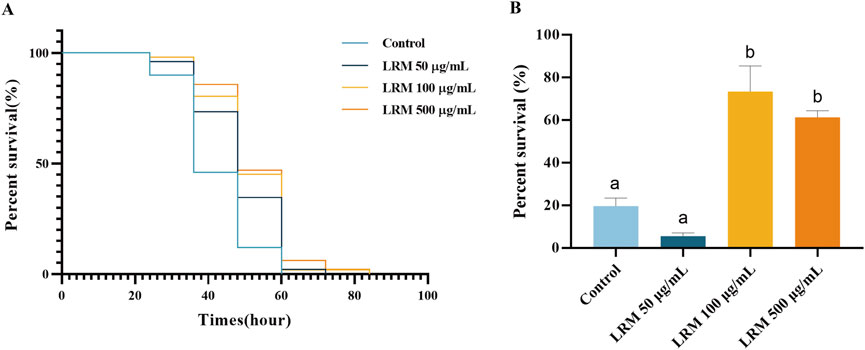
Figure 4. Effect of LRM on oxidative and heat stress resistance of C. elegans. (A) The survival curves of Caenorhabditis elegans under the oxidative and stress; (B) The percent survival rate of Caenorhabditis elegans under the heat stress. Data are expressed as mean ± SEM (n = 3 per group). Values with different letters in each column were significantly different (p < 0.05). The differences were tested by using Tukey test and one-way ANOVA.
Principal component analysis (PCA) was conducted to cluster similar data (Supplementary Figure S3A). Partial least squares discriminant analysis (PLS-DA) model was constructed to better visualize the metabolite variations between the control and LRM groups, which revealed the degree of separation and indicated different metabolic characteristics [R2Y (cum) = 0.991, Q2 (cum) = 0.857] between the control and LRM groups (Supplementary Figure S3B). In the PLS-DA model, the intercept between Q2 regression line and Y-axis was a negative correlation, as well as the value of Q2, was lower than the value of R2, indicating that the result was effective [R2 = (0.0, 0.95), Q2 = (0.0, −1.73)] (Supplementary Figure S3C). The metabolic datasets were investigated in further analysis, based on the above results.
As shown in the volcano plot, 193 differential metabolites were detected [Fold Change (FC) ≥ 1.2 or FC ≤ 0.8, p < 0.05, and variable importance in projection (VIP) > 1] between the control and LRM groups (Figure 5A). In the figure, each dot represents a different metabolite: green and red dots represent downregulated and upregulated metabolites respectively, and grey dots represent substandard alterations. The 20 most influential upregulated or downregulated metabolites that have been reported to possess potential biological activities were tentatively considered as biomarkers in Caenorhabditis elegans treated with LRM (Figure 5B; Supplementary Table S3).
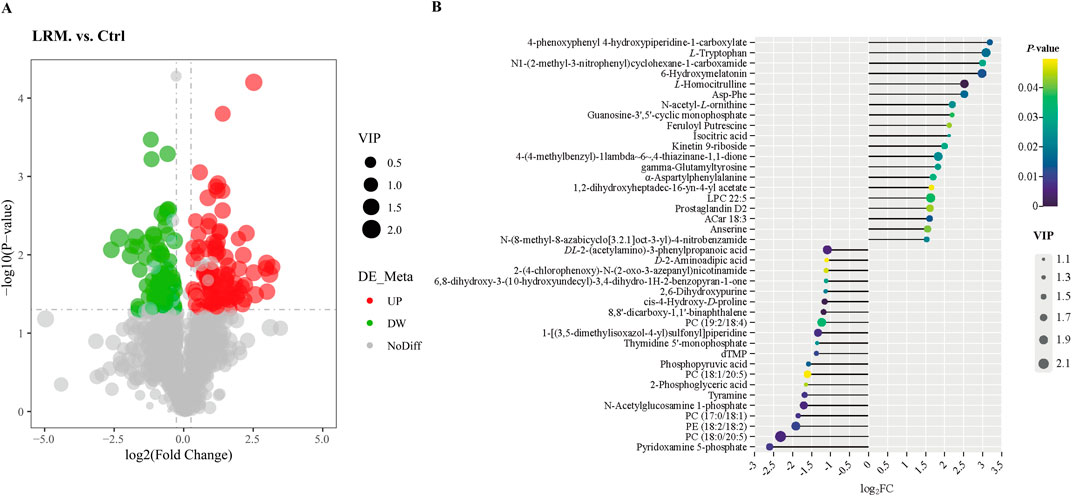
Figure 5. Differential metabolites between the control and LRM on Caenorhabditis elegans. (A) Differential metabolite volcano plot between the control and LRM. The dot sizes represent the values of VIP. (FC > 1.2, p < 0.05, and VIP score >1); (B). 20 most influential differential up-regulated and down-regulated metabolites. P values are visually represented by a color scale ranging from yellow to blue, which represents the difference from high to low. The dot sizes represent the VIP score of the metabolites. The abscissa axis is the log2FC, indicating the changing degree of differential metabolites.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis was used to analyze the differential metabolic pathways. As shown in Figure 6, the top 20 metabolic pathways in Caenorhabditis elegans treated with LRM were revealed in comparison with the control group. The results indicated that LRM treatment regulated 41 differential metabolic pathways, and 4 metabolic pathways were considered statistically significantly enriched, including those related to aminoacyl-tRNA biosynthesis, biosynthesis of amino acids, 2-oxocarboxylic acid metabolism, and glycine-serine-threonine (Gly-Ser-Thr) metabolism. There were 13 metabolic pathways related to lifespan in the top 20 metabolic pathways, including those associated with the biosynthesis of amino acids, 2-oxocarboxylic acid metabolism, Gly-Ser-Thr metabolism, carbon metabolism, tryptophan metabolism, glyoxylate and dicarboxylate metabolism, phenylalanine, tyrosine and tryptophan biosynthesis, glutathione metabolism, cysteine and methionine metabolism, arachidonic acid metabolism, glycolysis/gluconeogenesis, glycerolipid metabolism, and fatty acid degradation.
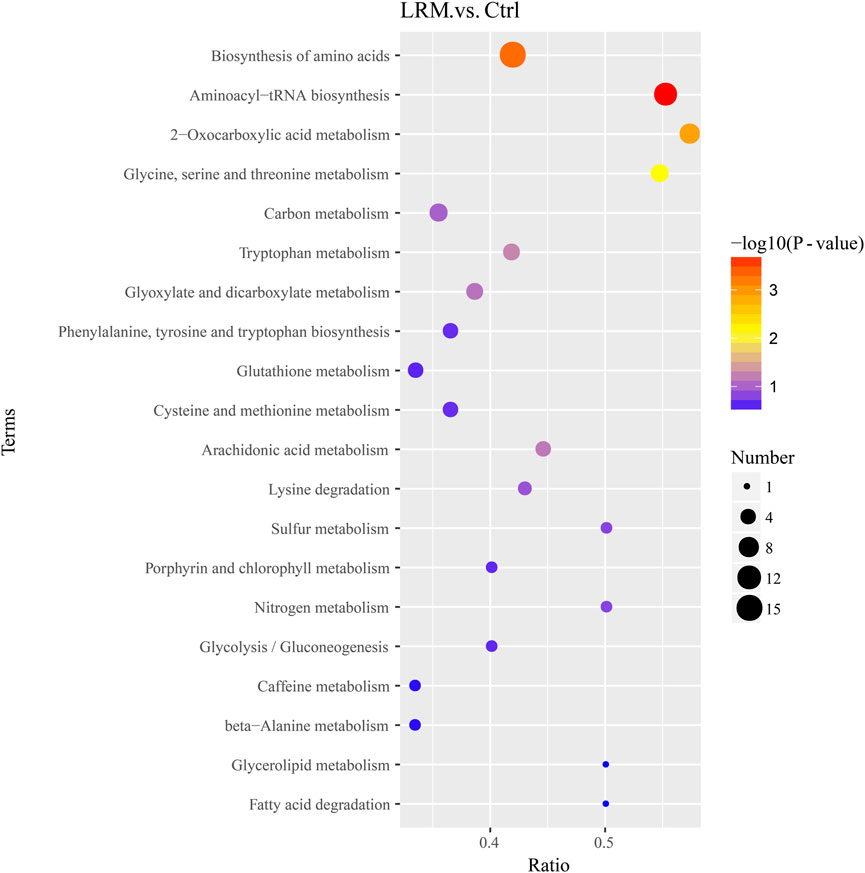
Figure 6. Result of top 20 metabolic pathways. P values are visually represented by a color scale ranging from red to purple, which represents the difference from high to low. The dot sizes represent the metabolite counts of the metabolic pathways. The abscissa axis is the enrichment ratio (number of differential metabolites in the corresponding metabolic pathway/total number of metabolites identified in the pathway).
Pearson correlation was used to analyze the relationships between differential metabolites and metabolic pathways (Pearson correlation coefficient (r) and pBH are provided in Supplementary Tables S4, S5). Unsupervised hierarchical clustering revealed several ‘hot spots’ of highly correlated metabolites (r > 0.5; pBH < 0.05) (Figure 7). Remarkably, L-serine significantly correlated with methionine, O-acetyl-serine, L-5-hydroxytryptophan, L-glutamic acid, L-asparagine, and phosphoenolpyruvate. L-Threonine significantly correlated with L-asparagine, L-5-hydroxytryptophan, and O-acetyl-serine (r > 0.9; pBH < 0.05). These metabolites were correlated with Gly-Ser-Thr metabolism which acts as a central metabolism pathway associated with glycolysis/gluconeogenesis, glyoxylate and dicarboxylate metabolism, tryptophan metabolism, lysine degradation, sulfur metabolism, cysteine and methionine metabolism, porphyrin and chlorophyll metabolism, glutathione metabolism, and beta-alanine metabolism.

Figure 7. Heat map of correlations between metabolites. Each square represents the Pearson’s rank correlation coefficient between the metabolites of the row and that of the column (*, pBH < 0.05; **, pBH < 0.01).
To identify co-accumulated metabolite groups, we used simple graph clustering based on the DPClusO algorithm, an enhanced version of the DPClus algorithm that can extract densely connected nodes as a cluster (Altaf-Ul-Amin et al., 2012; Altaf-Ul-Amin et al., 2006). In previous research, the overlapping mode of the DPClus algorithm has been used to analyze the metabolic pathways (Fukushima et al., 2011). At r ≥ 0.5 pBH < 0.05, there were 6 clusters identified by the DPClusO algorithm in the metabolomic correlation network for the Caenorhabditis elegans samples; they ranged in size from 5 to 16 metabolites. The KEGG enrichment analysis was used to assess the significance of the clusters (Figure 8; Supplementary Table S6). The largest cluster of C2 and C5 was “aminoacyl-tRNA biosynthesis”. These clusters may represent the extensive coordination among amino acids metabolism pathways in Caenorhabditis elegans treated with LRM. The largest cluster of C1 was “tryptophan metabolism,” which is connected with age-related diseases and longevity (van der Goot et al., 2012). The largest cluster of C3 was “glycolysis/gluconeogenesis”. Besides, “glycine, serine and threonine metabolism” was also significantly enriched in C3.
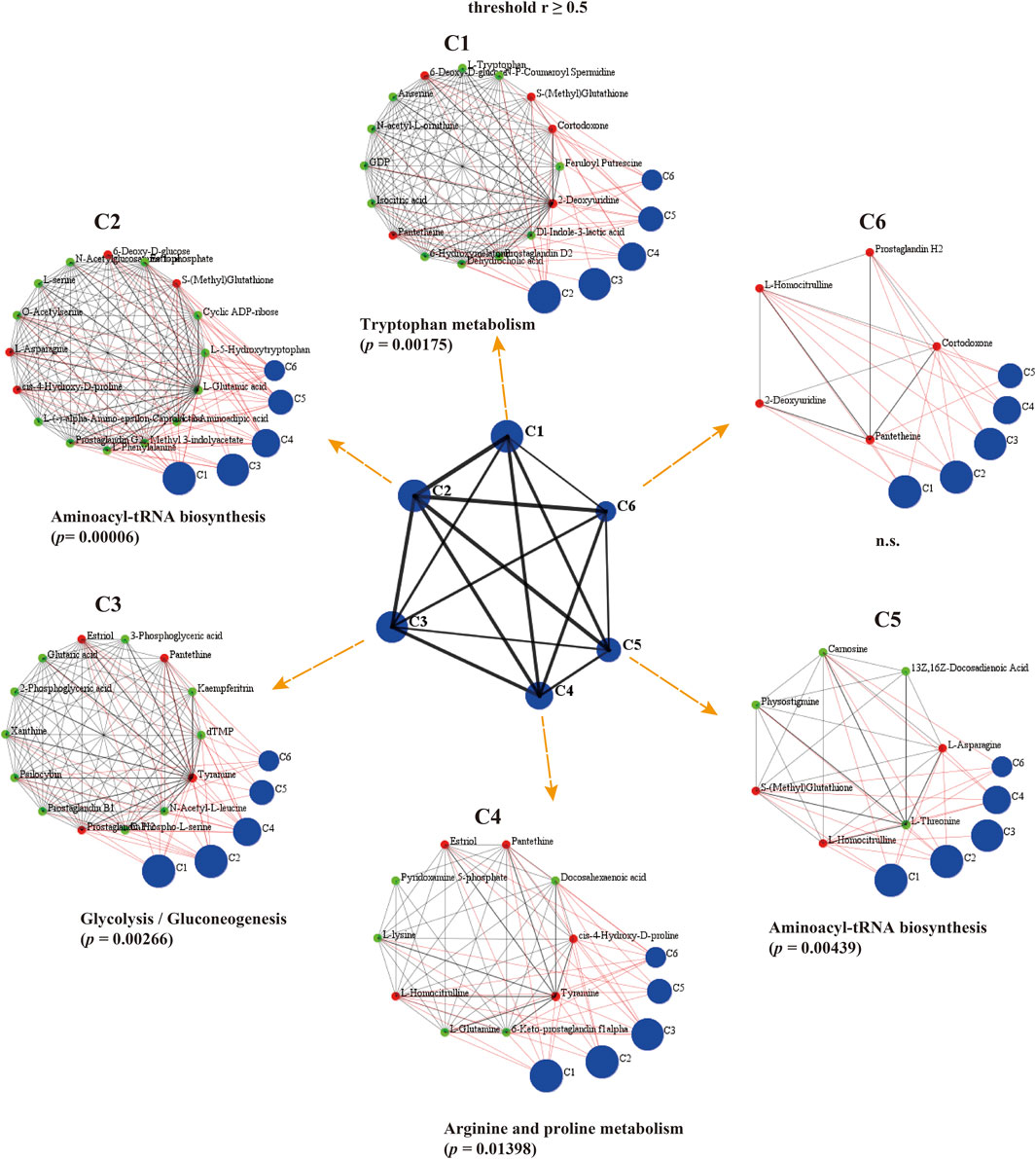
Figure 8. Graph clustering of metabolomic correlated modules in Caenorhabditis elegans with DPClusO algorithm (threshold r ≥ 0.5). The central graph displayed 6 blue clusters and 15 black edges. Each blue cluster contains closely connected metabolites labeled as C1 to 6. Small red or green nodes in the clusters represent individual metabolites. C1 to 6 show the first to sixth cluster of the hierarchical graph. The internal nodes of the clusters are connected by black edges; neighboring clusters are connected by red edges. (D = 0.9, CP = 0.5, OV Coff = 0.25).
Our study assessed the effect of LRM on the metabolomic of Caenorhabditis elegans, which revealed that even at a low concentration (5 μg/mL), LRM possesses lifespan-extending and performance-improving activities. This aligns with findings from previous research (Xiong et al., 2021). Notably, the anti-aging activity of LRM was more pronounced at lower concentrations compared to other Lycium spp., indicating its superior efficacy (Xiong et al., 2021). Interestingly, after treatment with LRM, pharyngeal pumping was reduced and the body length of the nematodes was shortened. Feeding-defective mutants of Caenorhabditis elegans such as eat-2, a mutant tends to have shorter body lengths and lower pharyngeal pumping rates (Avery, 1993; Morck, 2006). DR, achieved through reduced food intake or intermittent time-restricted feeding, has been proven effective in extending nematode longevity. It impacts growth signaling and resists the disruption of proteostasis (Iranon et al., 2019; Weir et al., 2017). Famous caloric-restriction mimetics, such as aspirin, resveratrol, and spermidine, may activate autophagy and prolong the lifespan without the need for calorie restrictions (Hofer et al., 2021).
Other studies have shown that natural candidates of plant origin, such as Montmorency tart cherry (Prunus cerasus L.) and Shatavarin IV, could have prolonged the lifespan and reduced pharyngeal pumping rates in Caenorhabditis elegans (van de Klashorst et al., 2020; Smita et al., 2020). Similar results were observed in LRM-treated nematodes (Xiong et al., 2021), but our study revealed an intriguing pattern: while LRM initially reduced pharyngeal pumping, it later improved pumping capacity in comparison to the control. The above results suggested that LRM induces physiological changes associated with DR and also enhances the overall health and aging process in Caenorhabditis elegans.
We selected metabolites related to lifespan or stress resistance from the top 20 differential metabolites for further discussion. Regulation of L-tryptophan, 6-hydroxy melatonin, kinetin 9-riboside, and tyramine were associated with prolonging lifespan. L-tryptophan, 6-hydroxy melatonin, and kinetin 9-riboside were up-regulated compared with the control group, while tyramine was down-regulated. Independent reports have indicated that the lifespans of Caenorhabditis elegans and Drosophila melanogaster extend as the ratios of Tryptophan/kynurenine increase (van der Goot et al., 2012). 6-Hydroxymelatonin has been shown to possess enhancing antioxidant activity (Perez-Gonzalez et al., 2019). Kinetin 9-riboside has been reported to possess a powerful inhibitory effect on adipogenesis (Zhang et al., 2022). A Tdc-1 mutant lacking tyramine has shown increased resistance to external stressors and prolonged lifespan in comparison to C. elegans (Jose De Rosa et al., 2019). Besides, the potential metabolites of LRM in C. elegans were found in this study. Feruloyl putrescine, a kind of polyamine, may be the metabolite of N1, N10-dicaffeoyl-spermidine in LRM (Figures 5B, 9B). Spermidine alkaloids, identified in L. ruthenicum and Lycium barbarum L. (Solanaceae) fruits, often contain caffeoyl or dihydrocaffeoyl moieties, while some have glycosylation (Ahad et al., 2020). Caffeoyl-spermidine derivatives from L. barbarum L. are considered beneficial metabolites responsible for anti-aging activities (Zhou et al., 2016). Caffeoyl-spermidine has been shown to possess inhibitory activity against SIRT1, a NAD-dependent deacetylase related to aging (Qi et al., 2018). This discovery suggests that L. ruthenicum spermidine may possess potential activities in the anti-aging process induced by LRM in Caenorhabditis elegans.
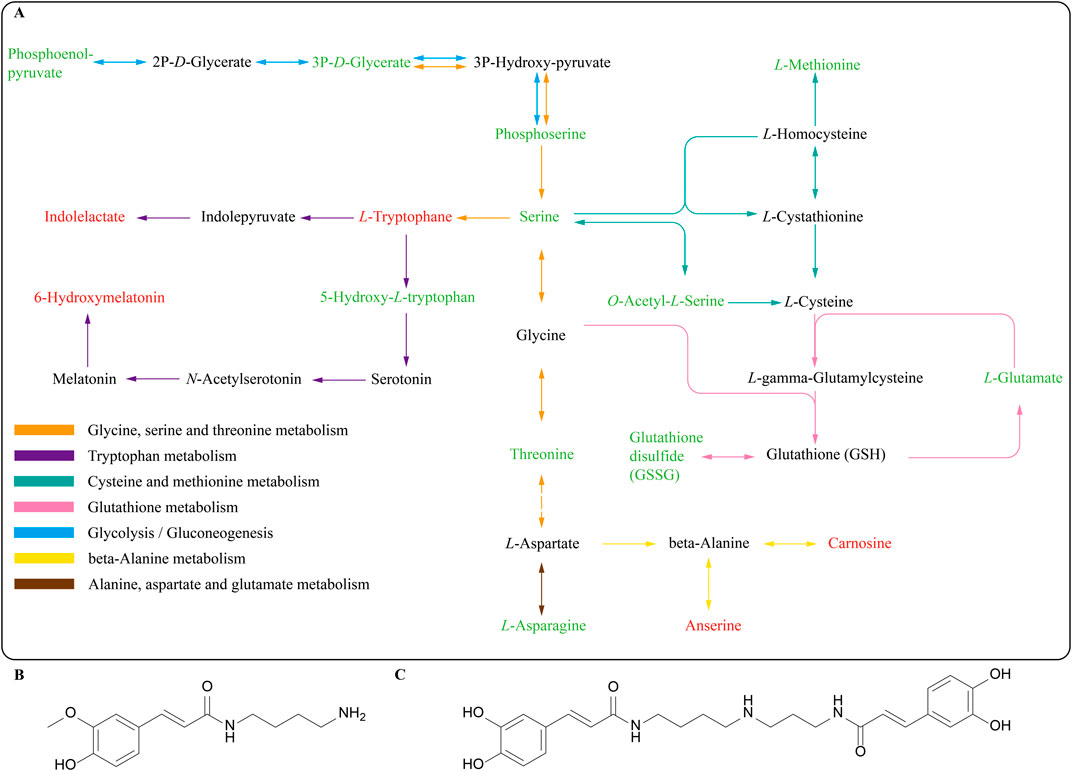
Figure 9. (A) Integration of the metabolic pathways altered by LRM treatment in Caenorhabditis elegans. In this path map, red represents upregulated metabolites and green represents downregulated metabolites (B) Feruloyl Putrescine; (C) N1, N10-dicaffeoyl-spermidine.
The metabolomic study revealed that LRM could regulate amino acid metabolism. Amino acids and NAD metabolism have been considered to possess a relationship with lifespan in worms, drosophila, and rats (Parkhitko et al., 2020). L-glutamate, L-glutamine, L-asparagine, L-threonine, L-serine, O-phospho-L-serine, L-methionine, and L-phenylalanine were down-regulated, while N-acetyl-ornithine, L-lysine, and L-tryptophan were up-regulated. We found that LRM influenced amino acid metabolism through glycine, serine, and threonine metabolism which acts as a central metabolism pathway, through KEGG analysis (Figure 9A). Gly-Ser-Thr metabolism is topologically and biochemically interconnected with numerous pathways and has been reported in mice and nematodes, as being connected with antioxidant effects and lifespan (Aon et al., 2020; Wu et al., 2021; Liu et al., 2019). A common longevity-associated pathway has been reported, which centered on Gly-Ser-Thr metabolism in mice following a caloric restriction regimen (Aon et al., 2020). These results further prove that Gly-Ser-Thr metabolism plays an important role in LRM treatment.
Tryptophan metabolism can function as a regulatory role in age-related diseases and affect longevity, which has been demonstrated in yeast, nematodes, flies, and mice (van der Goot et al., 2012). Inhibition of tryptophan degradation delayed aging in Caenorhabditis elegans (Oxenkrug and Navrotska, 2023). Tryptophan and indole-lactate (a kind of tryptophan metabolite) have been demonstrated to alleviate oxidative stress, inflammation, and neurodegeneration in HT-22 by H2O2 (Yin et al., 2023). Cysteine and methionine metabolism is highly correlated with oxidative stress (Bin et al., 2017). Researchers have found that methionine restriction (MetR) could stimulate the production of glutathione and reduce oxidative stress (Liu G. et al., 2017). MetR has been considered as a potential DR regimen to alleviate the unfavorable influences of DR while maintaining its positive effects (Lee et al., 2024). In our study, we also observed the downregulation of methionine and an increase in the ratio of glutathione/glutathione disulfide (GSH/GSSG) in glutathione metabolism. GSH/GSSG is a biomarker for describing cellular redox homeostasis (Enns and Cowan, 2017). Moreover, glutamate, which was annotated in glutathione metabolism, was downregulated after treatment with LRM. High concentrations of glutamate-induced oxidative damage and neurodegeneration result in a depletion of intracellular GSH (Madji et al., 2018). In beta-alanine metabolism, carnosine and anserine were upregulated. Carnosine, a natural endogenous molecule, is extensively distributed in different organisms (Caruso et al., 2021). Carnosine has an antioxidant effect, can prevent the formulation of advanced glycation end-products (AGEs), and an anti-aging activity (Caruso et al., 2021). Anserine has been shown to possess similar physiological functions to carnosine, which is a methylated form of carnosine (Boldyrev et al., 2013). Moreover, most of the amino acid changes were in good agreement with previous independent reports in the eat-2 mutant (DR model) (Gao et al., 2018).
Anthocyanins and spermidine derivatives are the major phytochemicals in LRM. A new study revealed that L. ruthenicum anthocyanins could prolong the lifespan via JNK-1 and DAF-16/FOXO pathways, rather than the caloric restriction pathway in Caenorhabditis elegans (Xu et al., 2024). However, spermidine is considered a caloric restriction mimetic. It mimics the beneficial effects of DR to prolong lifespan while limiting its negative effects (Madeo et al., 2018). The present exploration suggested that LRM could induce the DR pathway to prolong lifespan. Spermidine derivatives from L. ruthenicum have potential anti-aging activities via the DR pathway in Caenorhabditis elegans. In the future, additional research is necessary to gain a more refined understanding of the anti-aging effects of L. ruthenicum spermidine.
In this study, the anti-aging activities of LRM were investigated in Caenorhabditis elegans. LRM treatment not only significantly prolonged the lifespan and increased mobility but also enhanced oxidative and heat stress capacities in Caenorhabditis elegans. Additionally, the lipofuscin level was observed to decrease when compared with the control group. The reduction in pharyngeal pumping and shortened body length of the nematodes further supported the anti-aging effects of LRM. Moreover, the results of the metabolomic analysis suggested that LRM possessed anti-aging properties, which were mediated by regulating a series of pathways centered on glycine, serine, and threonine metabolism. The observed reduction in pharyngeal pumping and body length, the downregulation of methionine, and the regulation of Gly-Ser-Thr relevant metabolism in Caenorhabditis elegans provided new evidence supporting LRM’s simulation of the DR pathway to prolong lifespan. Furthermore, the significant upregulation of feruloyl putrescine might imply that caffeoyl-spermidine from L. ruthenicum has potential activities in the process of LRM-induced pro-longevity in Caenorhabditis elegans.
The original contributions presented in the study are publicly available. This data can be found at MetaboLights repository, accession number MTBLS12229.
BC: Formal Analysis, Investigation, Writing–original draft. LL: Formal Analysis, Methodology, Resources, Writing–original draft. XQ: Formal Analysis, Investigation, Writing–original draft. TS: Formal Analysis, Investigation, Writing–original draft. MY: Formal Analysis, Methodology, Writing–original draft. SX: Writing–review and editing. XF: Formal Analysis, Methodology, Writing–review and editing. YS: Project administration, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Key Research and Development Project of the Ningxia Hui Autonomous Region (2022BBF03013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1498280/full#supplementary-material
Ahad, H., Jin, H., Liu, Y., Wang, J., Sun, G., Liang, X., et al. (2020). Chemical profiling of spermidines in goji berry by strong cation exchange solid-phase extraction (SCX-SPE) combined with ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS/MS). J. Chromatogr. B-Analytical Technol. Biomed. Life Sci. 1137, 121923. doi:10.1016/j.jchromb.2019.121923
Altaf-Ul-Amin, M., Tsuji, H., Kurokawa, K., Asahi, H., Shinbo, Y., and Kanaya, S. (2006). DPClus: a density-periphery based graph clustering software mainly focused on detection of protein complexes in interaction networks. J. Comput. Aided Chem. 7, 150–156. doi:10.2751/jcac.7.150
Altaf-Ul-Amin, M., Wada, M., and Kanaya, S. (2012). Partitioning a PPI network into overlapping modules constrained by high-density and periphery tracking. 726429.
Amrit, F. R. G., and May, R. C. (2010). Younger for longer: insulin signalling, immunity and ageing. Curr. Aging Sci. 3 (3), 166–176. doi:10.2174/1874609811003030166
Aon, M. A., Bernier, M., Mitchell, S. J., Di Germanio, C., Mattison, J. A., Ehrlich, M. R., et al. (2020). Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell. Metab. 32 (1), 100–116.e4. doi:10.1016/j.cmet.2020.04.018
Avery, L. (1993). The genetics of feeding in Caenorhabditis elegans. Genetics 133 (4), 897–917. doi:10.1093/genetics/133.4.897
Beard, J. R., Officer, A., de Carvalho, I. A., Sadana, R., Pot, A. M., Michel, J.-P., et al. (2016). The World report on ageing and health: a policy framework for healthy ageing. Lancet 387 (10033), 2145–2154. doi:10.1016/S0140-6736(15)00516-4
Bin, P., Huang, R., and Zhou, X. (2017). Oxidation resistance of the sulfur amino acids: methionine and cysteine. Biomed Res. Int. 2017, 9584932. doi:10.1155/2017/9584932
Boldyrev, A. A., Aldini, G., and Derave, W. (2013). Physiology and pathophysiology of carnosine. Physiol. Rev. 93 (4), 1803–1845. doi:10.1152/physrev.00039.2012
Cai, Y., Song, W., Li, J., Jing, Y., Liang, C., Zhang, L., et al. (2022). The landscape of aging. Sci. China-Life Sci. 65 (12), 2354–2454. doi:10.1007/s11427-022-2161-3
Caruso, G., Godos, J., Castellano, S., Micek, A., Murabito, P., Galvano, F., et al. (2021). The therapeutic potential of carnosine/anserine supplementation against cognitive decline: a systematic review with meta-analysis. Biomedicines 9 (3), 0253. doi:10.3390/biomedicines9030253
Chen, S., Hu, N., Wang, H., and Li, G. (2022). The major anthocyanin of Lycium ruthenicum Murr. relieves cognitive deficits, oxidative stress, neuroinflammation, and hippocampal metabolome alterations in aging rats. J. Funct. Foods 94, 105104. doi:10.1016/j.jff.2022.105104
Chen, S., Xu, Y., Zhao, W., Shi, G., Wang, S., and He, T. (2024). UV-B irradiation promotes anthocyanin biosynthesis in the leaves of Lycium ruthenicum Murray. PeerJ 12, e18199. doi:10.7717/peerj.18199
Chen, S. S., Wang, H. L., and Hu, N. (2022). Long-term dietary Lycium ruthenicum Murr. Anthocyanins intake alleviated oxidative stress-mediated aging-related liver injury and abnormal amino acid metabolism. Foods 11 (21), 3377. doi:10.3390/foods11213377
Cui, B. Y., Liu, L. Y., Shi, T., Yin, M., Feng, X., and Shan, Y. (2023). The ethanolic extract of Lycium ruthenicum ameliorates age-related physiological damage in mice. Molecules 28 (22), 7615. doi:10.3390/molecules28227615
Enns, G. M., and Cowan, T. M. (2017). Glutathione as a redox biomarker in mitochondrial disease-implications for therapy. J. Clin. Med. 6 (5), 50. doi:10.3390/jcm6050050
Fukushima, A., Kusano, M., Redestig, H., Arita, M., and Saito, K. (2011). Metabolomic correlation-network modules in Arabidopsis based on a graph-clustering approach. BMC Syst. Biol. 5, 1. doi:10.1186/1752-0509-5-1
Gao, A. W., Smith, R. L., van Weeghel, M., Kamble, R., Janssens, G. E., and Houtkooper, R. H. (2018). Identification of key pathways and metabolic fingerprints of longevity in C. elegans. Exp. Gerontol. 113, 128–140. doi:10.1016/j.exger.2018.10.003
Gerstbrein, B., Stamatas, G., Kollias, N., and Driscoll, M. (2005). In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 4 (3), 127–137. doi:10.1111/j.1474-9726.2005.00153.x
Hofer, S. J., Davinelli, S., Bergmann, M., Scapagnini, G., and Madeo, F. (2021). Caloric restriction mimetics in nutrition and clinical trials. Front. Nutr. 8, 717343. doi:10.3389/fnut.2021.717343
Iranon, N. N., Jochim, B. E., and Miller, D. L. (2019). Fasting prevents hypoxia-induced defects of proteostasis in C. elegans. PLoS Genet. 15 (6), e1008242. doi:10.1371/journal.pgen.1008242
Jose De Rosa, M., Veuthey, T., Florman, J., Grant, J., Gabriela Blanco, M., Andersen, N., et al. (2019). The flight response impairs cytoprotective mechanisms by activating the insulin pathway. Nature 573 (7772), 135–138. doi:10.1038/s41586-019-1524-5
Karim, M. B., Kanaya, S., and Altaf-Ul-Amin, M. (2021). DPClusSBO: an integrated software for clustering of simple and bipartite graphs. Softwarex 16, 100821. doi:10.1016/j.softx.2021.100821
Lee, B. C., Kaya, A., and Gladyshev, V. N. (2024). Methionine restriction and life-span control. Ann. N. Y. Acad. Sci 116–124. doi:10.1111/nyas.12973
Lin, Y. G., Lin, C. X., Cao, Y., and Chen, Y. J. (2023). Caenorhabditis elegans as an in vivo model for the identification of natural antioxidants with anti-aging actions. Biomed. and Pharmacother. 167, 115594. doi:10.1016/j.biopha.2023.115594
Liu, G., Yu, L., Fang, J., Hu, C.-A. A., Yin, J., Ni, H., et al. (2017). Methionine restriction on oxidative stress and immune response in dss-induced colitis mice. Oncotarget 8 (27), 44511–44520. doi:10.18632/oncotarget.17812
Liu, K., Abdullah, A. A., Huang, M., Nishioka, T., Altaf-Ul-Amin, M., and Kanaya, S. (2017). Novel approach to classify plants based on metabolite-content similarity. Bio. Med Res. Int. 2017, 5296729. doi:10.1155/2017/5296729
Liu, Y., Gong, G., Sun, Y., Gu, X., Huang, L., and Wang, Z. (2016). Isolation, structural characterization, and immunological activity of a polysaccharide LRLP4-A from the leaves of Lycium ruthenicum. J. Carbohydr. Chem. 35 (1), 40–56. doi:10.1080/07328303.2015.1120875
Liu, Y. J., Janssens, G. E., McIntyre, R. L., Molenaars, M., Kamble, R., Gao, A. W., et al. (2019). Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 15 (3), 1007633. doi:10.1371/journal.pgen.1007633
Madeo, F., Eisenberg, T., Pietrocola, F., and Kroemer, G. (2018). Spermidine in health and disease. Sci. (New York, NY) 359 (6374). doi:10.1126/science.aan2788
Madji, B., Blasco, H., Coque, E., Emond, P., and Corcia, P. (2018). The metabolic disturbances of motoneurons exposed to glutamate. Mol. Neurobiol. 55 (10), 7669–7676. doi:10.1007/s12035-018-0945-8
Morck, C. (2006). C. elegans feeding defective mutants have shorter body lengths and increased autophagy. BMC Dev. Biol. 6, 39. doi:10.1186/1471-213X-6-39
Oxenkrug, G., and Navrotska, V. (2023). Extension of life span by down-regulation of enzymes catalyzing tryptophan conversion into kynurenine: possible implications for mechanisms of aging. Exp. Biol. Med. 248 (7), 573–577. doi:10.1177/15353702231179411
Parkhitko, A. A., Filine, E., Mohr, S. E., Moskalev, A., and Perrimon, N. (2020). Targeting metabolic pathways for extension of lifespan and healthspan across multiple species. Ageing Res. Rev. 64, 101188. doi:10.1016/j.arr.2020.101188
Perez-Gonzalez, A., Castaneda-Arriaga, R., Raul Alvarez-Idaboy, J., Reiter, R. J., and Galano, A. (2019). Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. J. Pineal Res. 66 (2), 12539. doi:10.1111/jpi.12539
Qi, J.-J., Yan, Y.-M., Cheng, L.-Z., Liu, B.-H., Qin, F.-Y., and Cheng, Y.-X. (2018). A novel flavonoid glucoside from the fruits of Lycium ruthenicun. Molecules 23 (2), 0325. doi:10.3390/molecules23020325
Scharf, A., Pohl, F., Egan, B. M., Kocsisova, Z., and Kornfeld, K. (2021). Reproductive aging in Caenorhabditis elegans: from molecules to ecology. Front. Cell. Dev. Biol. 9, 718522. doi:10.3389/fcell.2021.718522
Sharma, R., Raghuvanshi, R., Kumar, R., Thakur, M. S., Kumar, S., Patel, M. K., et al. (2022). Current findings and future prospective of high-value trans Himalayan medicinal plant Lycium ruthenicum Murr: a systematic review. Clin. Phytoscience 8 (1), 3. doi:10.1186/s40816-021-00328-7
Smita, S. S., Trivedi, S., Pandey, T., Trivedi, M., and Pandey, R. (2020). A Bioactive compound Shatavarin IV-mediated longevity as revealed by dietary restriction-induced autophagy in Caenorhabditis elegans. Biogerontology 21 (6), 827–844. doi:10.1007/s10522-020-09897-5
Tao, L., Hao, F., Fei, P., Chen, D., Fan, H., Zhao, S., et al. (2022). Advance on traditional uses, phytochemistry and pharmacology of Lycium ruthenicum Murr. Pharm. Chem. J. 56 (6), 844–861. doi:10.1007/s11094-022-02718-8
van de Klashorst, D., van den Elzen, A., Weeteling, J., Roberts, M., Desai, T., Bottoms, L., et al. (2020). Montmorency tart cherry (Prunus cerasus L.) acts as a calorie restriction mimetic that increases intestinal fat and lifespan in Caenorhabditis elegans. J. Funct. Foods 68, 103890. doi:10.1016/j.jff.2020.103890
van der Goot, A. T., Zhu, W., Vazquez-Manrique, R. P., Seinstra, R. I., Dettmer, K., Michels, H., et al. (2012). Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc. Natl. Acad. Sci. U. S. A. 109 (37), 14912–14917. doi:10.1073/pnas.1203083109
Wahid, M., Ali, A., Saqib, F., Aleem, A., Bibi, S., Afzal, K., et al. (2020). Pharmacological exploration of traditional plants for the treatment of neurodegenerative disorders. Phytotherapy Res. 34 (12), 3089–3112. doi:10.1002/ptr.6742
Wang, H., Li, J., Tao, W., Zhang, X., Gao, X., Yong, J., et al. (2018). Lycium ruthenicum studies: molecular biology, Phytochemistry and pharmacology. Food Chem. 240, 759–766. doi:10.1016/j.foodchem.2017.08.026
Weir, H. J., Yao, P., Huynh, F. K., Escoubas, C. C., Goncalves, R. L., Burkewitz, K., et al. (2017). Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell. metab. 26 (6), 884–896.e5. doi:10.1016/j.cmet.2017.09.024
Wu, X., Liu, C., Yang, S., Shen, N., Wang, Y., Zhu, Y., et al. (2021). Glycine-Serine-Threonine metabolic axis delays intervertebral disc degeneration through antioxidant effects: an imaging and metabonomics study. Oxidative Med. Cell. Longev. 2021, 5579736. doi:10.1155/2021/5579736
Xiong, L., Deng, N., Zheng, B. S., Li, T., and Liu, R. H. (2021). HSF-1 and SIR-2.1 linked insulin-like signaling is involved in goji berry (Lycium spp.) extracts promoting lifespan extension of Caenorhabditis elegans. Food. Funct. 12 (17), 7851–7866. doi:10.1039/d0fo03300f
Xu, Q., Zheng, B., Li, T., and Liu, R. H. (2024). Black goji berry anthocyanins extend lifespan and enhance the antioxidant defenses in Caenorhabditis elegans via the JNK-1 and DAF-16/FOXO pathways. J. Sci. Food Agric. doi:10.1002/jsfa.13998
Yin, J., Zhang, Y. H., Liu, X. X., Li, W. H., Hu, Y. Z., Zhang, B. W., et al. (2023). Gut microbiota-derived indole derivatives alleviate neurodegeneration in aging through activating GPR30/AMPK/SIRT1 pathway. Mol. Nutr. Food Res. 67, 202200739. doi:10.1002/mnfr.202200739
Yu, Z., Xia, M., Lan, J., Yang, L., Wang, Z., Wang, R., et al. (2023). A comprehensive review on the ethnobotany, phytochemistry, pharmacology and quality control of the genus Lycium in China. Food and Funct. 14 (7), 2998–3025. doi:10.1039/d2fo03791b
Zhang, G., Chen, S., Zhou, W., Meng, J., Deng, K., Zhou, H., et al. (2019). Anthocyanin composition of fruit extracts from Lycium ruthenicum and their protective effect for gouty arthritis. Industrial Crops Prod. 129, 414–423. doi:10.1016/j.indcrop.2018.12.026
Zhang, S., Lyons, N., Koedam, M., van de Peppel, J., van Leeuwen, JPTM, and van der Eerden, B. C. J. (2022). Identification of small molecules as novel anti-adipogenic compounds based on Connectivity Map. Front. Endocrinol. 13, 1017832. doi:10.3389/fendo.2022.1017832
Keywords: dietary restriction, glycine, serine, and threonine metabolism, lifespan, nematode, spermidine
Citation: Cui B, Liu L, Qiao X, Shi T, Yin M, Xu S, Feng X and Shan Y (2025) Anti-aging activities of an ethanolic extract of Lycium ruthenicum in Caenorhabditis elegans based on metabonomic analysis. Front. Pharmacol. 16:1498280. doi: 10.3389/fphar.2025.1498280
Received: 18 September 2024; Accepted: 13 January 2025;
Published: 27 February 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Patrícia Pereira, University of Aveiro, PortugalCopyright © 2025 Cui, Liu, Qiao, Shi, Yin, Xu, Feng and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Shan, c2hhbnl1QGNuYmcubmV0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.