- 1Phase Ⅰ Clinical Trial Laboratory, The Second Nanning People’s Hospital, Nanning, Guangxi, China
- 2Department of Pharmacology, School of Pharmaceutical Science, Central South University, Changsha, Hunan, China
- 3Qilu Pharmaceutical Co., Ltd., Jinan, Shandong, China
- 4Hunan Key Laboratory for Bioanalysis of Complex Matrix Samples, Changsha, Hunan, China
Objective: S-1, an oral multicomponent capsule containing tegafur, gimeracil, and potassium oxonate, has demonstrated efficacy in various tumor types. This study aimed to assess the pharmacokinetics, bioequivalence (BE), and safety of a newly developed generic S-1 capsule compared to the original brand-name formulation in Chinese cancer patients under fasting and fed conditions.
Methods: A multicenter, randomized, open-label, single-dose, double-cycle crossover study was conducted in Chinese cancer patients. The study involved 120 subjects, with 60 assigned to the fasting group and another 60 to the fed group. In each study cycle, subjects were randomly assigned toreceive either the reference or test S-1 capsule at a 7-day interval. Blood samples were collected for analysis within 48 h after ingestion. The plasma concentrations of tegafur, 5-fluorouracil, gimeracil, and potassium oxonate were determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS). The main pharmacokinetic (PK) parameters were calculated using the non-compartmental approach. BE was assessed through geometric mean ratios (GMRs) between the two formulations and their respective 90% confidence intervals (CIs). The safety of the two formulations was also evaluated.
Results: The pharmacokinetics of the two formulations were similar under both fasting and fed conditions. The 90% CIs of the GMRs for the maximum observed serum concentration (Cmax), AUC0-t, and AUC0-
Conclusion: The newly developed generic S-1 and reference formulations exhibit comparable PK in Chinese cancer patients in the fasting and fed state. The formulations of S-1 showed good tolerability and a similar safety profile.
Clinical Trial Registration: http://www.chinadrugtrials.org.cn/index.html, identifier CTR20171562.
Introduction
The therapeutic regimen incorporating 5-fluorouracil (5-FU) is globally recognized as the conventional chemotherapy treatment for a myriad of cancer types (Satoh and Sakata, 2012). In order to mitigate the issue of rapid degradation, S-1 was innovated as an advanced fourth-generation oral fluoropyrimidine derivative, which encompasses three pharmacological entities, namely, tegafur (FT), gimeracil (CDHP), and potassium oxonate (OXO), formulated in a molar ratio of 1:0.4:1 (Satoh and Sakata, 2012; Kawahara, 2014).
Among these, FT serves as a prodrug of 5-FU, which undergoes continuous conversion to 5-FU, predominantly facilitated by cytochrome P450 2A6 (CYP2A6) after oral absorption, thereby exerting its anticancer efficacy (Wang et al., 2011). CDHP acts as a competitive inhibitor of the enzyme dihydropyrimidine dehydrogenase (DPD), thereby limiting the degradation of 5-FU and resulting in prolonged, efficacious concentrations of 5-FU in both plasma and tumor tissue (Diasio, 1999; Furuse et al., 2010). The inhibitory effect of CDHP on DPD substantially contributes to reducing 5-FU catabolism, thereby achieving significantly elevated plasma levels of 5-FU compared to the administration of FT alone (Saif et al., 2011). OXO, also known as oteracil, is specifically localized to the gastrointestinal mucosa, where it selectively targets and inhibits orotate phosphoribosyl transferase (OPRT). This targeted inhibition effectively blocks the phosphorylation of 5-FU, thereby reducing the gastrointestinal toxicity commonly linked to 5-FU therapy (Saif et al., 2009). Consequently, S-1 is frequently called a “self-rescuing” therapeutic agent (Zhuang et al., 2013). When administered as a continuous infusion, S-1 demonstrated reduced toxicity and enhanced antitumor efficacy compared to uracil-FT and 5-FU (Hirata et al., 1999; van Groeningen et al., 2000).
Initially approved in Japan in 1999 as a therapeutic intervention for gastric cancer (Koizumi et al., 2000), S-1 has subsequently been validated for its efficacy across a spectrum of malignant tumors, such as breast cancer (Toi et al., 2021), head and neck cancer (Shih et al., 2021), colorectal cancer (Yamada et al., 2018; Miyamoto et al., 2014), lung cancer (Nokihara et al., 2017; Kawahara, 2014), pancreatic cancer (Uesaka et al., 2016), prostate cancer (Akaza et al., 2010), and cholangiocarcinoma (Nakachi et al., 2023). Owing to its superior anticancer properties and minimal toxicity, generic formulations of S-1 have been widely promoted in various countries. Despite its approval for a diverse array of cancer types, pharmacokinetic (PK) studies of S-1 have frequently been limited to individual cancer types. Furthermore, there is a notable scarcity of large-scale comparative studies on the pharmacokinetics of S-1 in both the fasting and fed states (Lee et al., 2019; Zhuang et al., 2013; Chen et al., 2020). In order to rigorously address these identified gaps, we orchestrated a multicenter study aimed at comparing the PK and bioequivalence (BE) of a novel generic S-1 formulation (Qilu Pharmaceutical Co., Ltd., Shandong, China) with an established reference formulation (Taiho Pharmaceutical Co., Ltd., Japan) in a large cohort of 120 Chinese patients, with a variety of cancer types under both fasting and fed conditions.
Materials and methods
Ethics and study population
This study was conducted in seven institutions, namely, the First Affiliated Hospital of China Medical University, the Affiliated Cancer Hospital of Harbin Medical University, the First Affiliated Hospital of Nanchang University, the Affiliated Hospital of Qingdao University, the Second People’s Hospital of Nanning, the Guizhou Cancer Hospital, and the Affiliated Hospital of Jiangnan University (China Clinical Trials Registry: CTR20171562). The study protocol and informed consent procedures were approved by the clinical research ethics committees of each participating center. The ethical approval process strictly adhered to the requirements of Good Clinical Practice (GCP), the Declaration of Helsinki, and relevant domestic laws and regulations. Prior to the commencement of the trial, all subjects provided signed informed consent forms, retaining the right to withdraw from the study at any point without the need for explanation or fear of repercussion.
This study enrolled patients aged 18–75 years, who were diagnosed with malignant solid tumors, as confirmed by definitive histopathological and/or cytological diagnoses. The cancer types included head and neck, non-small cell lung, colorectal, breast, and advanced gastric and small bowel cancers, in patients who had not undergone surgical resection. Eligibility was further restricted to individuals with a body mass index (BMI) between 18 and 27 kg/m2 and a body surface area (BSA) of at least 1.5 m2. Weight criteria specified a minimum of ≥50 kg for male subjects and ≥45 kg for female subjects. The health status of the subjects was assessed through a thorough medical history review, a comprehensive physical examination, and a series of medical investigations, which included routine blood tests, blood chemistry profiles, screening for infectious diseases, and electrocardiograms. The primary exclusion criteria included any abnormalities detected during the physical examination, a significant medical history, or a history of severe drug allergies.
Study design and drug administration
This study, a multicenter, randomized, open-label, single-dose, two-cycle crossover investigation, was distinctly divided into fasting and fed trials. Each subject received either the test or reference formulation in a separate cycle. Each cycle consisted of a 4-day inpatient phase, followed by a 7-day washout period between cycles to ensure that any residual effects from the previous formulation had fully dissipated before the initiation of the next cycle. To avoid the residual effects of fasting or feeding that could influence the results, the fasting and fed trials each enrolled 60 subjects. Subjects were randomly allocated to one of the two treatment sequences, namely, test-reference or reference-test, in a 1:1 ratio. All eligible subjects were admitted to the clinical ward 1 day prior to drug administration, adhering to a minimum fasting period of 10 h before drug intake. In the fasting trial, subjects were required to fast. Subsequently, they ingested a single dose comprising two capsules (50 mg each) of either the test or reference formulation, accompanied by 240 mL of warm water. In the fed trial, subjects consumed a high-fat, high-calorie meal (protein providing approximately 150 kcal, carbohydrate approximately 250 kcal, fat approximately 500–600 kcal, totaling approximately 800–1000 kcal, with approximately 50% of the calories from fat) within 30 min before orally ingesting a single dose of two capsules (50 mg each) of the test or reference formulation, along with 240 mL of warm water. To verify complete medication intake, the researchers conducted oral examinations of the subjects. Fasting was maintained for 4 h after dosing. Standardized meals were administered 4 and 10 h after dosing.
Study population
Post-administration of S-1 capsules, the primary constituents identifiable in the body include FT, CDHP, OXO, and the active metabolite 5-FU. Existing research delineates that the AUC0-t of OXO in the organism exhibits the most pronounced coefficient of variation, registering at 34.75% (Lee et al., 2019), thus classifying it as a pharmaceutical with notable pharmacokinetic (PK) variability. The sample size calculation was executed using PASS version 11.0 (NCSS, LLC, located in Kaysville, Utah, United States), based on the following criteria: a within-subject variability of 34.75%, a significance level (α) of 0.05, and a Type II error rate (β) of 0.2. Accounting for a 10% potential dropout rate, the final required number of participants for both the fasting and fed studies was 60 each.
Eligible participants included Chinese cancer patients of both sexes, aged 18–75 years, with body mass indices ranging from 18 to 27 kg/m2 and body surface areas of at least 1.5 m2. Male participants were required to weigh at least 50 kg, while female participants were required to weigh at least 45 kg. The health status of subjects was meticulously evaluated through various methods, such as medical history assessments, physical examinations, comprehensive medical investigations (encompassing routine hematology, biochemistry panels, and screenings for infectious diseases), electrocardiograms, and definitive histopathological or cytological diagnoses. The primary exclusion criteria included any abnormal findings in medical evaluations, a documented history of significant diseases, or a history of severe drug allergies. Participants meeting the inclusion criteria were subjected to screening, while those who conformed to any of the exclusion criteria were excluded from the study.
Study drugs
The test formulation (specifications: each capsule contained 25 mg FT, 7.25 mg CDHP, and 24.5 mg OXO; batch number: 16L0013E29) was developed by Qilu Pharmaceutical Co., Ltd. The reference formulation (specifications: each capsule contained 25 mg FT, 7.25 mg CDHP, and 24.5 mg OXO; batch number: 6K97B) was developed by Taiho Pharmaceutical Co., Ltd. Both drugs, provided by Qilu Pharmaceutical Co., Ltd., were from commercially available batches with valid certificates of analysis and were stored in a sealed container at a controlled room temperature of 15°C–25°C until further use. The inactive ingredients in both the test and reference formulations were identical, consisting of lactose monohydrate and magnesium stearate. The dose administered to participants was expressed as the FT content.
Blood sampling and medical supervision
Blood samples for PK evaluation were collected in vacuum anticoagulant tubes at 0 h (within 60 min before dosing) and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12, 24, and 48 h post-dosing. At each specified time point, 3 mL of blood sample was drawn. Fourteen blood samples, amounting to a total volume of 42 mL, were collected per cycle. The samples underwent centrifugation at 1700 g (maintained at a temperature range of 2°C–8°C) for 10 min to facilitate plasma separation. Subsequently, the samples were promptly stored at −70°C (not exceeding −60°C) within 120 min post-centrifugation.
Clinical researchers diligently monitored adverse events (AEs) at each participating center. Safety assessments were executed comprehensively, incorporating physical examinations, monitoring vital signs and electrocardiogram (ECG) recordings, AE reporting, analysis of clinical laboratory parameters, and documenting concurrent medication usage. AEs were meticulously classified according to version 4.03 of the Common Terminology Criteria for Adverse Events (CTCAE) and subjected to analysis using descriptive statistical methods. All AEs manifesting after medication administration were rigorously documented. Comprehensive follow-up evaluations were systematically conducted to assess the timing of onset, severity, duration, interventions implemented, and outcomes, continuing until the resolution of symptoms, disease stabilization, or loss of follow-up. Safety assessments were conducted for participants who withdrew from the study early or within 3 days after completing the second round of blood collection. These assessments included monitoring of vital signs, physical examination, laboratory tests, and 12-lead electrocardiograms, all of which were integral components in evaluating the overall safety of the study.
Chromatographic conditions
The determination of FT, CDHP, and 5-FU in blood was performed under the following conditions: the mobile phase consisted of 100% water containing 0.005% formic acid (mobile phase A) and a mixture of methanol and acetonitrile (2:1, v/v) (mobile phase B). The flow rate was set to 0.8 mL/min, and the gradient was programmed as follows: 5% mobile B for 3.1 min; 40% mobile B for 0.3 min, and then returned to 5% mobile phase B, which was maintained for 2.4 min. Chromatographic separation was achieved using an Eclipse XDB Phenyl column (5 μm, 4.6 mm × 150 mm), which provided satisfactory separation efficiency. The autosampler and column temperatures were set at 4°C and 40°C, respectively, with an injection volume of 10 μL.
OXO was determined in blood, which was performed under the following conditions: the aqueous solution containing 5 mM ammonium acetate (mobile phase A) and acetonitrile (mobile phase B). The flow rate was set to 0.8 mL/min, and the gradient was programmed as follows: 30% mobile B for 2.8 min, 60% mobile B for 0.01 min, then returned to 30% mobile phase B, and maintained for 0.6 min. Chromatographic separation was achieved using XDB-C18 (1.8 μm, 4.6 mm × 50 mm), which provided satisfactory separation efficiency. The autosampler and column temperatures were set at 4°C and 35°C, respectively, with an injection volume of 10 μL.
Mass spectrometric conditions
The determination of FT, CDHP, and 5-FU in blood was conducted utilizing multi-reaction monitoring in the negative ion mode, employing an electric spray ion source. Optimized parameters included an ion spray voltage of 4500 V, GS1 and GS2 pressures set at 60 psi each, a curtain gas pressure of 35 psi, a collision gas pressure of 8 psi, and a source temperature of 35°C. Multiple reaction monitoring transitions were as follows: for FT and FT-13C,15N2, m/z 199.1–42.1 and m/z 202.0–44.0, respectively; for 5-FU and 5-FU-13C,15N2, m/z 128.8–42.1 and m/z 132.0–44.0, respectively; and CDHP and CDHP-13C3, m/z 143.9–64.0 and m/z 147.0–67.0, respectively.
The determination of OXO in blood was performed in the positive ion mode using an atmospheric pressure chemical ionization source. Optimized parameters included a declustering voltage of 80 V, GS1 set to 65 psi, curtain gas pressure of 35 psi, collision gas pressure of 10 psi, and an ion source temperature of 600°C. Multiple reaction monitoring transitions for OXO and OXO-13C2,15N3 were m/z 490.0–259 and m/z 495–262, respectively.
Determination of FT, CDHP, 5-FU, and OXO
FT, CDHP, 5-FU, and OXO plasma concentrations were quantified using a validated liquid chromatography–mass spectrometry (LC-MS/MS) method. The method exhibited a linear range of 10.0–3,000 ng/mL for FT, with a lower limit of quantification (LLOQ) of 10.0 ng/mL. Precision, expressed as the coefficient of variation (% CV), ranged from 0.8% to 4.5% for each concentration quality control (QC) sample, while the mean bias from theoretical concentrations ranged between −7.7% and 4.7% for FT.
CDHP’s linear range was 2.0–600 ng/mL, with an LLOQ value of 2.0 ng/mL. Precision ranged from 0.9% to 4.2% (% CV) for each QC sample, and the mean bias ranged between −6.3% and 4.6%.
Similarly, 5-FU exhibited a linear range of 1.0–300 ng/mL, with an LLOQ value of 1.0 ng/mL. Precision ranged from 0.8% to 4.2% (% CV) for QC samples, and the mean bias ranged between −7.2% and 5.5%.
For OXO, the linear range was 2.0–200 ng/mL, with an LLOQ value of 2.0 ng/mL. The precision ranged from 0.3% to 9.9% (% CV) for the QC samples, and the mean bias ranged between −13.4% and 8.4%. These results demonstrate the robustness and reliability of the LC-MS/MS method for the quantification of these analytes in plasma.
PK and BE analysis
This clinical study is a BE study with PK parameters as the endpoint. The PK parameters assessed included AUC0–t, AUC0–
Biological equivalence was rigorously assessed using a double one-sided t-test and a confidence interval methodology. The PK parameters AUC0-t, AUC0-
Results
Subjects’ demographic characteristics
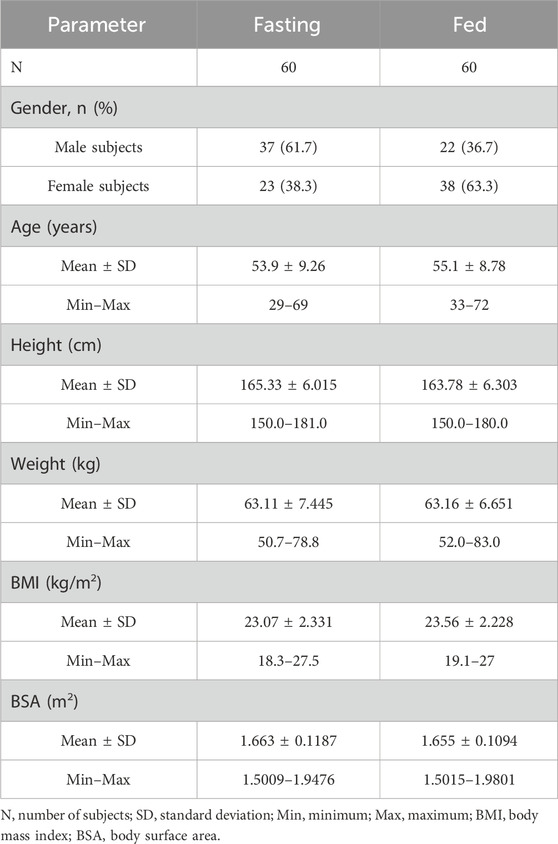
A total of 80 volunteers were screened for the fasting trial. Subsequently, 20 subjects were excluded based on the predefined inclusion and exclusion criteria, culminating in the final inclusion and randomization of 60 subjects. For the fed trial, 72 volunteers underwent initial screening, from which 12 subjects were excluded, adhering to the specified inclusion and exclusion criteria, thereby resulting in the eventual inclusion and randomization of 60 subjects. Throughout the study period, two subjects withdrew from the fasting trial for personal reasons before receiving a single administration. A comprehensive flowchart illustrating the distribution of subjects is depicted in Figure 1, while Table 1 elaborately delineates the demographic characteristics of each group.
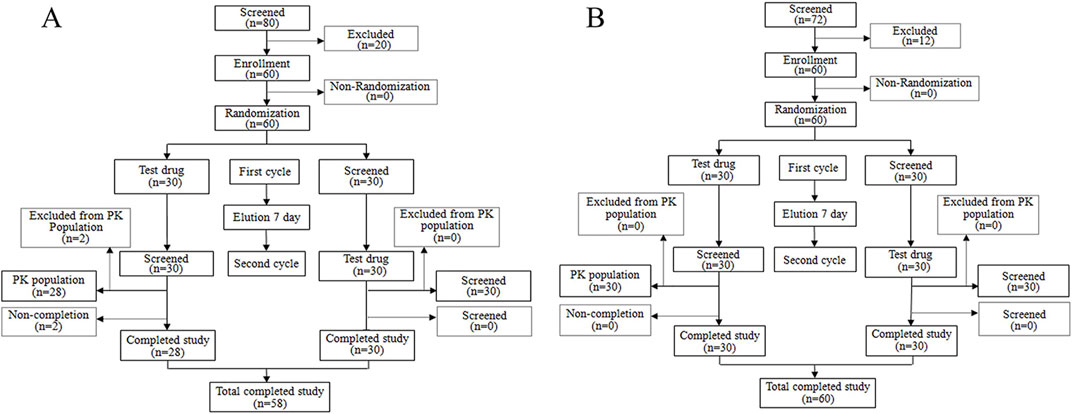
Figure 1. Study design and study flow diagram. Study design and study flow diagram in the fasting condition (A) and study design and study flow diagram in the fed condition (B).
PK parameters and plasma concentration–time curve
In this study, two participants in the fasting cohort elected to terminate their involvement before initiating medication administration. Consequently, their data were omitted from the PK concentration set (PKCS) and the PK parameter set (PKPS). The residual cohort, comprising 118 participants, completed the trial, warranting their inclusion in subsequent analyses of the PKCS and PKPS. Following the oral administration of S-1, the gastrointestinal tract rapidly absorbed both FT and CDHP. Under fasting conditions, Tmax was recorded to occur within an approximate 0.5–1 h post-dosing range.
In contrast, Tmax for FT and CDHP was delayed to approximately 3 h, following the consumption of a high-fat meal, with similar extensions observed for the Tmax of 5-FU and OXO, a variation ascribed to the impact of dietary intake. Following the consumption of a high-fat meal, the area under the concentration–time curve (AUC) for FT remained largely stable, with a slight decrease observed in the AUC values for 5-FU and CDHP, accompanied by a significant reduction in the AUC for OXO. Table 2 outlines the critical PK parameters of FT, CDHP, 5-FU, and OXO in both fasting and fed states. Figures 2, 3 depict the average plasma concentration–time curves of FT, CDHP, 5-FU, and OXO obtained after a single oral trial and referenced S-1 in the fasting and fed groups.
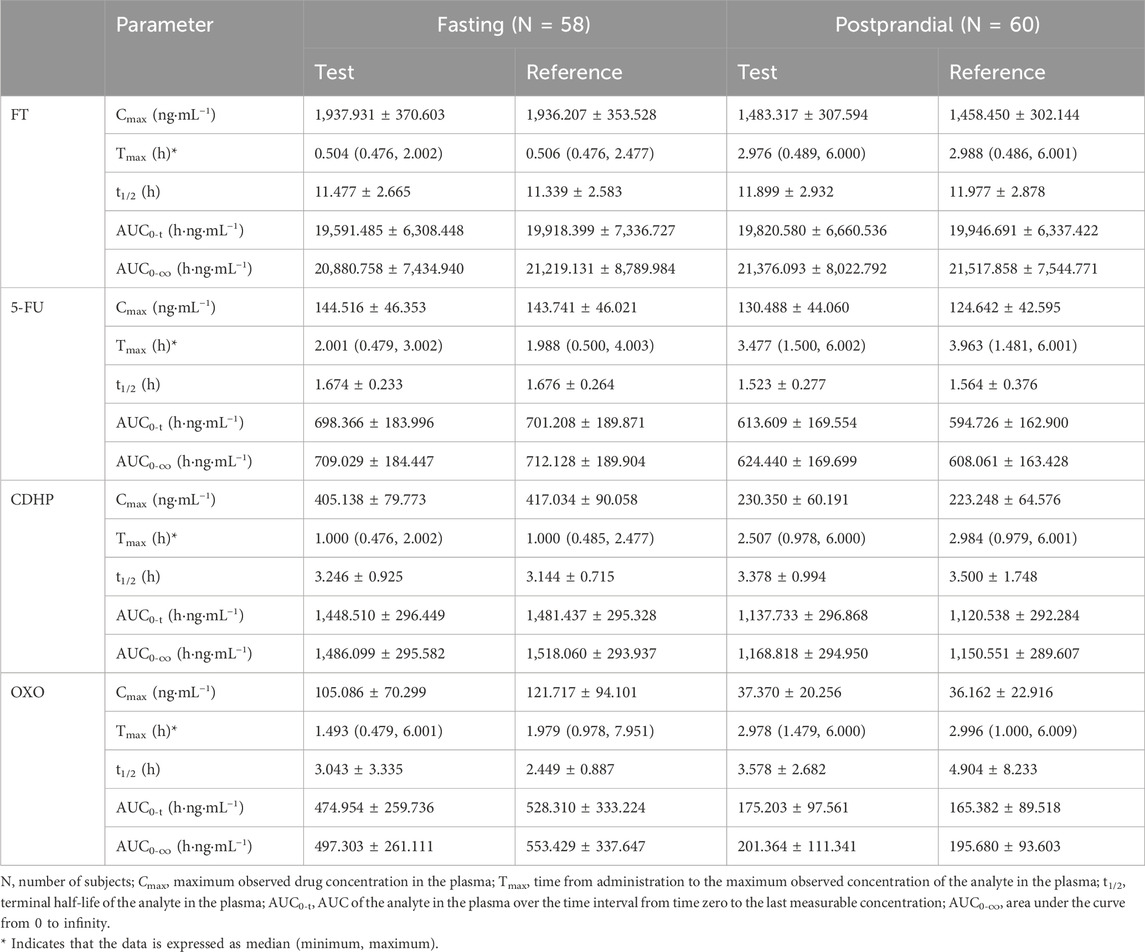
Table 2. Summary of pharmacokinetic parameters of FT, 5-FU, CDHP, and OXO after a single dose of the test drug or reference drug in the fasting and fed states.
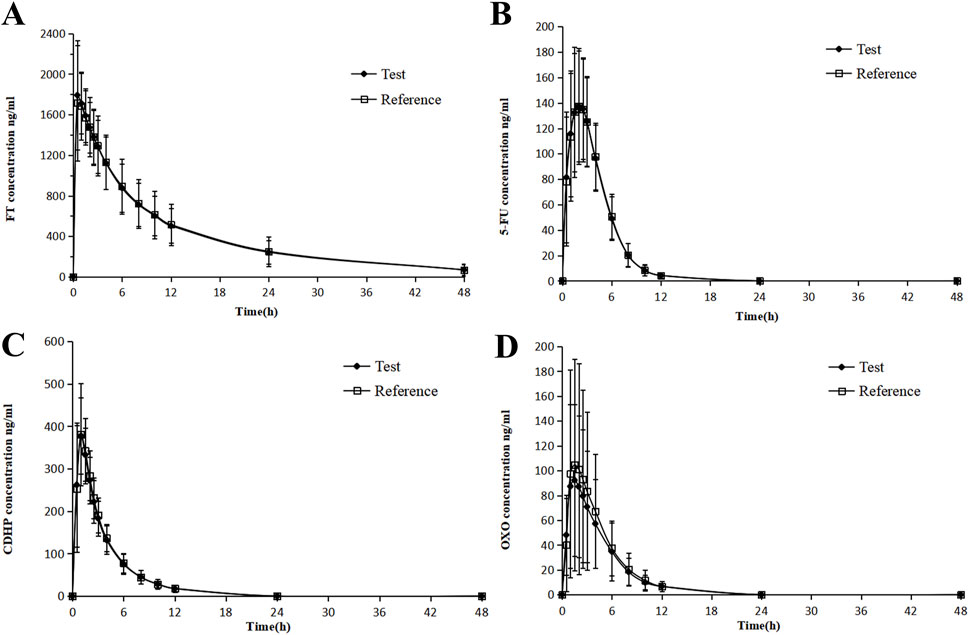
Figure 2. Mean blood concentration (±SD) time curve for FT (A), 5-FU (B), CDHP (C), OXO, and (D) after oral single-dose administration of S-1 of the test and reference formulations during fasting.
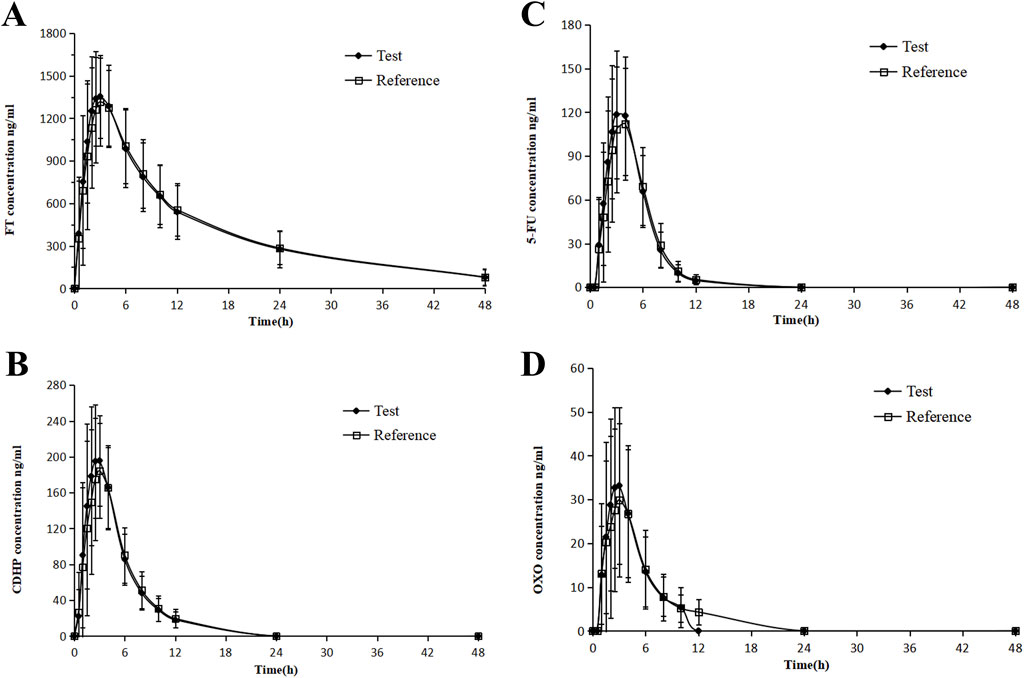
Figure 3. Mean blood concentration (±SD) time curve for FT (A), 5-FU (B), CDHP (C), OXO, and (D) after oral single-dose administration of S-1 of the test and reference formulations during the fed state.
BE results
As outlined in Tables 3, 4, with the exception of OXO under fasting conditions, where the least squares mean ratios for Cmax, AUC0-t, and AUC0-
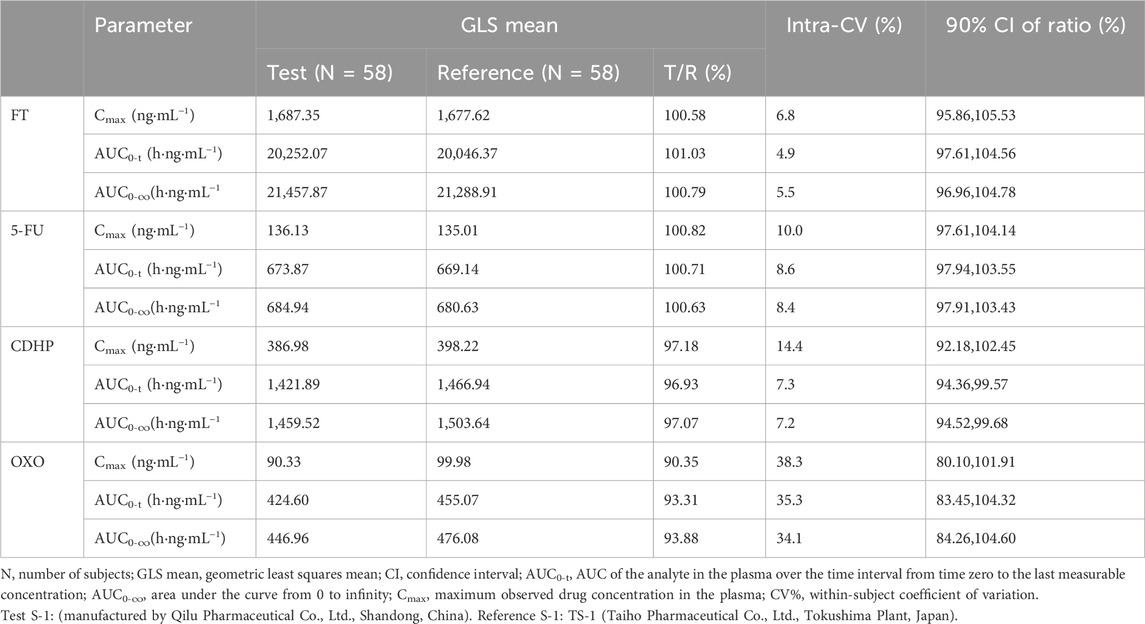
Table 3. Results of the equivalence determination of the test drug and reference drug during fasting.
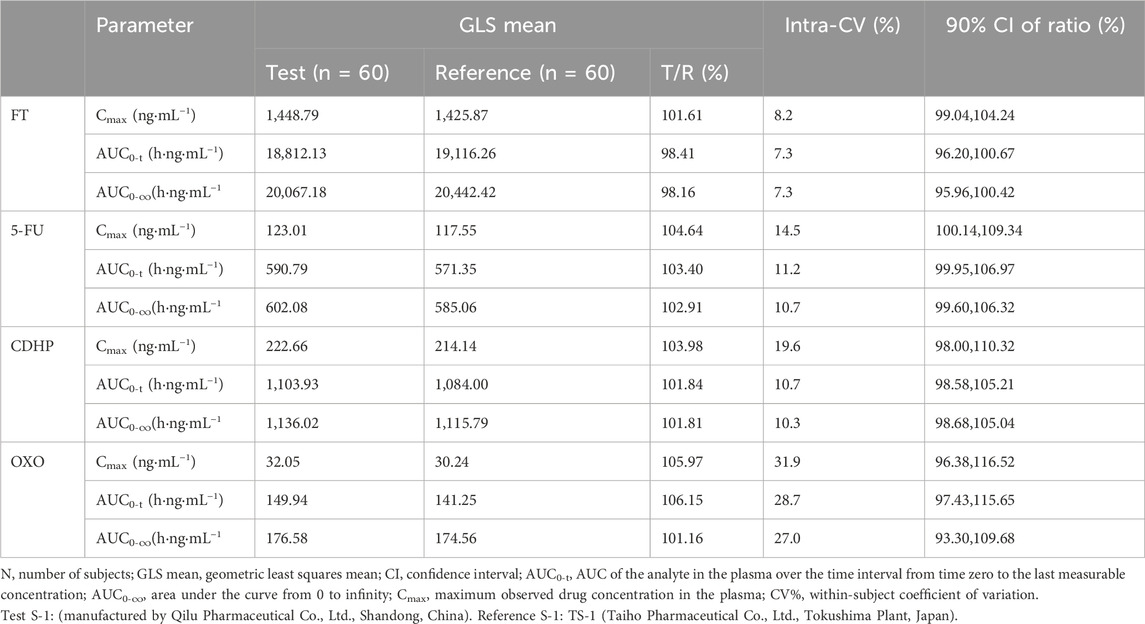
Table 4. Results of the equivalence determination of the test drug and reference drug during feeding.
Safety and tolerability
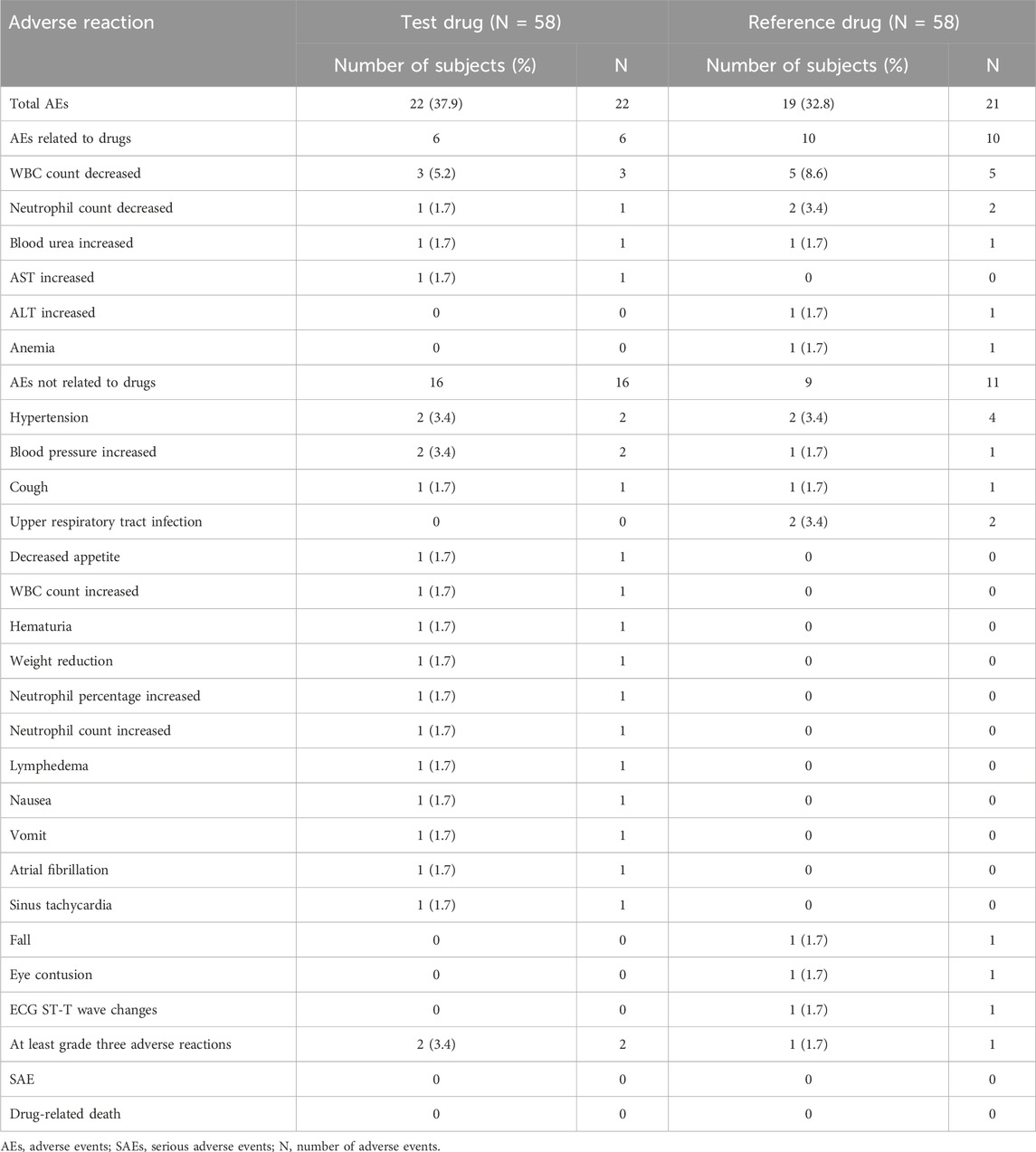
In the fasting trial, a total of 43 AEs (22 caused by the test formulation and 21 caused by the reference formulation) occurred in 25 of the 58 subjects who entered the safety analysis set, of which 12 AEs (five caused by the test formulation and seven caused by the reference formulation) were judged to be drug-related. The most common drug-related AEs observed throughout the study were decreased WBC, increased AST, increased blood urea, decreased neutrophil count, increased ALT, and anemia. There were no serious adverse events (SAEs) or drug-related deaths and no AEs, leading to discontinuation. Detailed AEs are shown in Table 4.
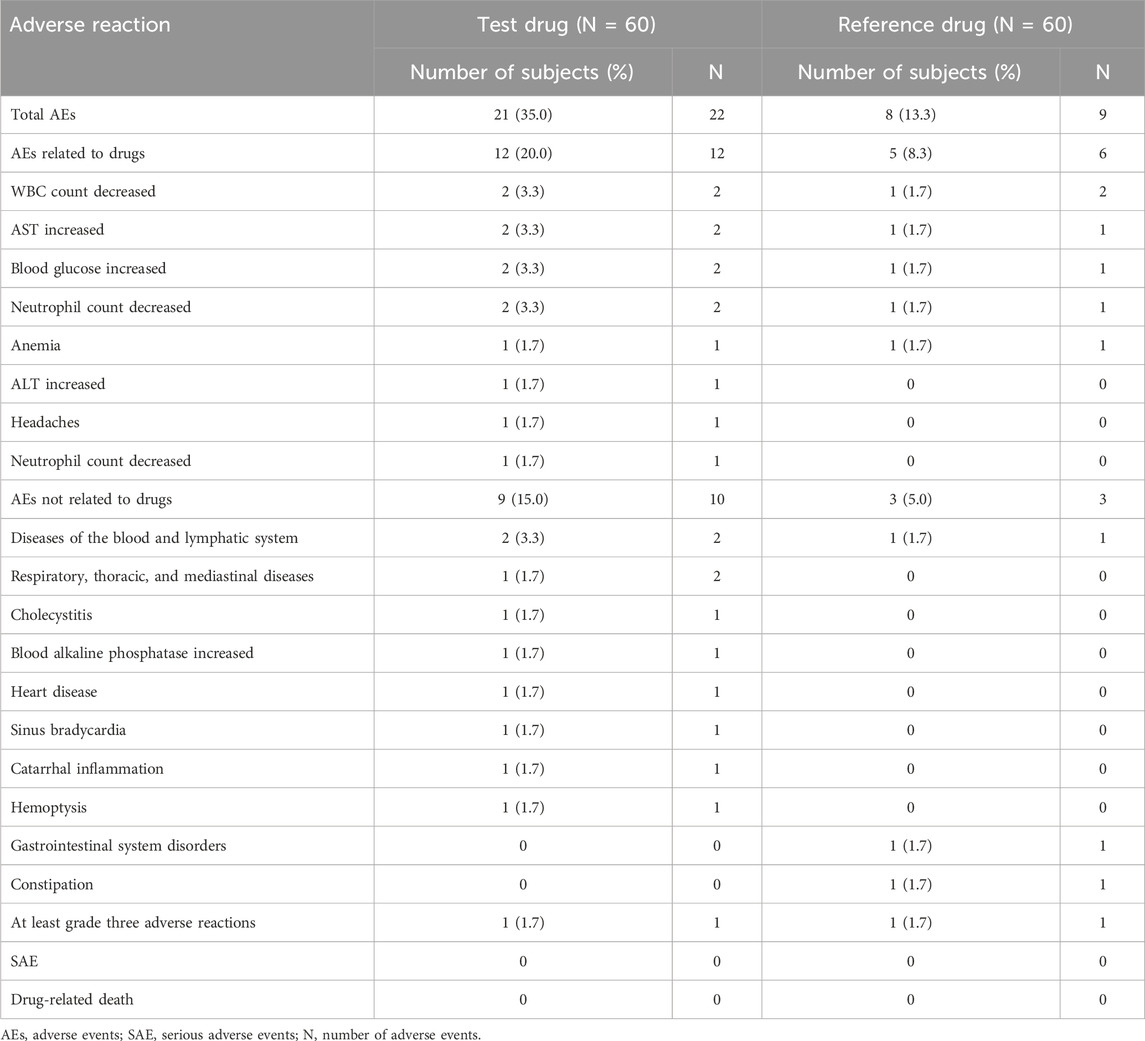
In the fed trial, a total of 24 AEs (17 caused by the test formulation and 7 caused by the reference formulation) occurred in 16 of the 60 subjects who entered the safety analysis set, of which 14 AEs (nine caused by the test formulation and five caused by the reference formulation) were judged to be drug-related. The most common drug-related AEs observed throughout the study were decreased WBC, increased AST, decreased neutrophil count, headache, increased blood glucose, increased ALT, and anemia. There were no SAEs or drug-related deaths and no AEs leading to discontinuation. Detailed AEs are shown in Tables 5, 6.
The types of AEs were similar between the two formulations under fasting and fed conditions. After the end of the study, all AEs returned to normal or remained stable. The research results showed that both formulations are safe.
Discussion
S-1 is a composite drug comprising CDHP, OXO, and FT metabolically converted to 5-FU in vivo. Consequently, BE studies focusing on S-1 necessitate the evaluation of PK parameters for FT, CDHP, OXO, and 5-FU. Notably, the t1/2 of FT in prior PK analyses was approximately 13.1 ± 3.1 h in the test substance; therefore, in the current investigation, blood samples were collected up to 48 h post-administration, exceeding the minimum threshold of three times the t1/2 endpoint for FT. Moreover, the mean AUC0-t/AUC0-
S-1’s primary anticancer ingredient is the prodrug FT, which converts to its active form 5-FU through hydroxylation. In our study, formulations were administered, where each capsule contained 25 mg of FT, 7.25 mg of CDHP, and 24.5 mg of OXO, with subjects ingesting two capsules per dose. Given that the participants’ average body surface area (BSA) was 1.66 m2, the BSA-normalized dose of tegafur employed in our research was established to be 30.12 mg/m2. Upon standardizing the dose to 40 mg/m2, we observed that under fasting conditions, the Cmax and AUC0-t values for FT were notably higher, ranging from 2571.32 to 2573.61 ng/mL and 26,017.91 to 26,452.06 h·ng/mL, respectively, exceeding those reported by Lee et al. (2019). Under fed conditions, the corresponding values were 1936.85–1969.88 ng/mL for Cmax and 26,322.15–26,489.63 h·ng/mL for AUC0-t, consistent with the findings reported by Chen et al. (2020).
Reports indicate that FT exposure is comparable in East Asians and is reduced in Europeans (Chuah et al., 2011). Compared to European and American patients under the fed condition (Peters et al., 2003; Hoff et al., 2003), Chinese individuals exhibit elevated AUC0-t levels of FT, a trend mirrored by Japanese patients (Zhuang et al., 2013). This disparity is likely attributable to the conversion of FT to 5-FU in vivo, predominantly via the hydroxylation activity of CYP2A6—a highly polymorphic enzyme that exhibits common allelic variants (CYP2A6*4, *7, and *9) at increased frequencies in East Asians (Ikeda et al., 2000; Yoshida et al., 2003). These variants are linked to decreased enzymatic activity, which may contribute to reduced FT activation, thereby explaining the reduced 5-FU exposure observed in Asians during phase I trials. Although variations in CYP2A6 influence FT pharmacokinetics, they do not significantly affect the pharmacokinetics of 5-FU (Fujita et al., 2008; Kim et al., 2011). A real-world study demonstrated a significant correlation between the AUC of 5-FU and CDHP concentrations but not with the CYP2A6 genotype, suggesting that 5-FU exposure is primarily governed by CDHP levels rather than by the biotransformation of FT (Fujita and Sasaki, 2014; Hirose et al., 2010).
5-FU is a pyrimidine analog antimetabolite drug that disrupts nucleoside metabolism and integrates into RNA and DNA and is a commonly used antitumor drug in clinical practice (Sun et al., 2021; Longley et al., 2003). Following parenteral administration of 5-FU, the drug is rapidly distributed and swiftly eliminated, with a half-life of approximately 8–20 min (Diasio and Harris, 1989). The clearance of 5-FU from a patient’s system is influenced by the rate at which FT is converted to 5-FU and the level of inhibition imposed on dihydropyrimidine dehydrogenase by CDHP (Zhuang et al., 2013). This study demonstrated that the half-life of 5-FU was significantly prolonged, following a single dose of two tablets of S-1. These findings suggest that S-1 plays an active role in extending the duration of action of 5-FU in the body, highlighting its potential therapeutic implications.
CDHP, a potent inhibitor of DPD, primarily functions to impede the degradation of 5-FU, thereby prolonging its pharmacological activity. In our study, we meticulously examined the impact of food intake on the PK parameters of CDHP. It was observed that the presence of food significantly reduced Cmax, a phenomenon likely attributable to the modulation of drug absorption rates and extent by food. Concurrently, Tmax was notably prolonged, which may be associated with delayed gastric emptying, alterations in intestinal pH, or modifications in the activity of intestinal drug transport proteins induced by food intake (Deng et al., 2017). However, despite the pronounced effects on Cmax and Tmax, the influence of food on the half-life and total exposure to CDHP was comparatively minimal. This suggests that once absorbed, the metabolic clearance of CDHP in vivo is minimally affected by food. These findings hold significant implications for clinical application, indicating that physicians may need to adjust the timing or dosage of CDHP administration based on dietary conditions to maximize therapeutic efficacy. Additionally, given that CDHP is predominantly metabolized by the kidneys and excreted via glomerular filtration into the urine, a preclinical assessment of renal function is imperative to mitigate the risk of impaired renal function (Fujita et al., 2009). Impaired renal function may elevate CDHP concentrations, potentially leading to increased 5-FU levels and unpredictable toxicities. Dosage adjustments should be considered necessary.
OXO blocks an enzyme called orotate phosphoribosyl transferase and is highly concentrated in the gastrointestinal (GI) tract. This helps OXO reduce GI problems caused by the drug 5-FU (Koizumi et al., 2010). In our study, we analyzed the pharmacological properties of OXO in comparison with three other compounds. OXO exhibited the greatest variability, with the within-subject CV% for both Cmax and AUC exceeding 30%. This finding is consistent with other PK studies of S-1, where similar levels of variability have been observed (Chen et al., 2020; Lee et al., 2019). Consequently, during the sample size calculation for this study, the variability of OXO was the primary factor considered.
Additionally, our study specifically addresses the impact of dietary intake on OXO’s PK parameters. Data indicate that postprandial administration of a high-fat meal significantly reduces the Cmax and AUC of OXO, which is generally consistent with the US Max E study (Scheulen et al., 2012). These results suggest that food intake, particularly high-fat food, may substantially influence the absorption and metabolism of OXO, potentially through alterations in the gastrointestinal environment, interference with drug solubility, or disruption of the intestinal drug transport system. Therefore, we recommend administering S-1 on an empty stomach to enhance the bioavailability of OXO and reduce the gastrointestinal side effects associated with 5-FU. Additionally, a European study indicated that the Cmax and AUC0-t values for OXO, following fasting administration of S-1, were lower than those observed in our study (Hoff et al., 2003), which may explain the higher incidence of gastrointestinal side effects observed in European populations when taking S-1.
Understanding the characteristics of drugs is crucial for interpreting their PK profiles in the body. The drug FT exhibits alkalinity and has a LogP value of −0.48 (Lee et al., 1994). Although drugs with a LogP value of less than 0 are typically classified as hydrophilic, FT demonstrates significant lipophilicity (Lee et al., 1994). This phenomenon may be attributed to FT being a derivative of 5-FU, with structural modifications that enhance its interaction with lipid components in biological membranes. Additionally, the metabolic conversion of FT to 5-FU in vivo confers certain lipophilic properties to FT. Lipophilic drugs tend to accumulate in adipose tissue, which increases the distribution volume of FT and consequently prolongs its t1/2. On the other hand, the molecular structure of CDHP contains a hydroxyl group (−OH) and a chlorine atom (−Cl), which impart a weakly acidic characteristic. Weakly acidic drugs are less likely to ionize in the gastric environment, thus favoring their existence in a non-ionized form. This characteristic facilitates the absorption of drugs through cell membranes, providing a plausible explanation for the rapid absorption of CDHP under fasting conditions, as observed in our study with a Tmax value of approximately 1 h. OXO, a salt, exhibits acidity upon dissolution in water. In this study, we found that food intake significantly reduced the AUC of OXO. The likely cause is that food intake increases the gastric pH (Sekine et al., 2021), leading to increased ionization of the acidic drug. Although solubility is enhanced, the proportion of the non-ionized form decreases, which may reduce transmembrane absorption and consequently lower drug exposure.
Clinical investigations revealed that the gastrointestinal toxicity associated with S-1 is notably mild. In a comprehensive Phase III trial conducted across European and American cohorts (Koizumi et al., 2008), adverse gastrointestinal events—namely diarrhea, stomatitis, and vomiting of grade 3 severity or higher—were reported in less than 5% of cases when S-1 was administered on an empty stomach in conjunction with cisplatin. These findings substantiate the protective role of OXO in the gastrointestinal tract. The main adverse reactions in this study include decreased WBC, increased AST, increased blood urea, decreased neutrophil count, and increased ALT, headache, and anemia. It is noteworthy that S-1 was administered as a single dose in this study, and the safety assessment was thus confined to this single administration. However, gastrointestinal toxicity is typically associated with the prolonged use of S-1, suggesting that long-term treatment may require additional monitoring and management strategies beyond the scope of the present investigation. Notably, while hematological toxicity emerged as the predominant adverse effect in Japanese patients, gastrointestinal disturbances, particularly diarrhea, were more pronounced in Western cohorts (Haller et al., 2008). Another comparison of uracil ⁄ FT and leucovorin in metastatic colorectal cancer between Japanese and North American patients showed that both ethnic groups had comparable 5-FU exposure when compared according to the body surface area. However, American patients had a higher incidence of diarrhea (Shirao et al., 2004). Additionally, a significant correlation was identified between prolonged use of the S-1 antineoplastic drug combination and ocular complications. Elevated levels of FT in tears and increased tear volume have been linked to symptoms such as excessive tearing. Proactive communication about these potential ocular side effects to patients and enhanced information sharing among healthcare providers are recommended to mitigate the escalation of such issues (Kuriki et al., 2019).
This study meticulously investigated the PK properties of the newly developed S-1 formulation. It assessed its BE with the established brand of S-1 in a large cohort of 118 cancer patients under fasting and postprandial conditions. The findings demonstrate that all components of the new formulation achieved BE in both conditions, with notable modulation by food intake on the PK of CDHP and OXO. Furthermore, both formulations were characterized by their favorable safety profiles and tolerability.
Although our study provides valuable insights into the PK and BE of the S-1 formulation, it is essential to consider the broader implications for clinical practice and global applications. In terms of clinical practice, we observed that food, particularly high-fat meals, significantly reduced the AUC of OXO, indicating the need for careful dose adjustment strategies. Clinicians may need to tailor the timing or dosage of S-1 administration according to the patients’ dietary habits, optimizing therapeutic efficacy while minimizing gastrointestinal side effects. Our findings underscore the importance of patient education, particularly regarding the necessity of taking S-1 on an empty stomach. Regarding renal function monitoring, since CDHP is primarily excreted via the kidneys, it is critical to monitor renal function to prevent potential drug accumulation and toxicity, particularly in patients with compromised renal function, who may require dose adjustments. From a global application perspective, the polymorphism of CYP2A6 suggests that Asian populations may experience higher FT exposure compared to Europeans, highlighting the need for dose adjustments based on CYP2A6 genotyping in different populations. This research provides valuable data for regulatory bodies evaluating generic S-1 formulations, demonstrating their BE and safety relative to branded versions. Such evidence may enhance the accessibility and affordability of S-1 for patients worldwide.
Conclusion
This study was designed as a multicenter, randomized, open-label, single-dose, two-cycle crossover investigation aimed at evaluating the BE and safety of two formulations of S-1 in Chinese cancer patients, both under fasting and fed conditions. The evaluation of BE was based on the primary PK parameters. The results showed that Cmax, AUC0-t, and AUC0-
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by seven institutions that were carried in this study, namely, the First Affiliated Hospital of China Medical University, the Affiliated Cancer Hospital of Harbin Medical University, the First Affiliated Hospital of Nanchang University, the Affiliated Hospital of Qingdao University, the Second People’s Hospital of Nanning, the Guizhou Cancer Hospital, and the Affiliated Hospital of Jiangnan University, with the trial protocol and informed consent procedures receiving approval from the clinical research ethics committees of each participating center. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: project administration, supervision, writing–original draft, and writing–review and editing. YL: methodology, project administration, writing–original draft, and writing–review and editing. YM: methodology, project administration, and writing–review and editing. XW: methodology, project administration, and writing–review and editing. WL: methodology, project administration, and writing–review and editing. YY: methodology, project administration, and writing–review and editing. HY: methodology, project administration, and writing–review and editing. CL: methodology and writing–review and editing. LH: methodology and writing–review and editing. QS: methodology and writing–review and editing. CW: methodology and writing–review and editing. JC: methodology and writing–review and editing. LC: writing–original draft and writing–review and editing. XL: writing–original draft and writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Qilu Pharmaceutical Co., Ltd. provided the drugs (test and branded reference formulations of S-1 capsules). The authors thank all enrolled subjects, investigators, and people who contributed to this study.
Conflict of interest
Author JC was employed by Qilu Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akaza, H., Ikemoto, I., Namiki, M., Usami, M., Kobayashi, M., Fujimoto, H., et al. (2010). Efficacy of S-1 in patients with castration-resistant prostate cancer: a phase II study. Oncology 78, 323–328. doi:10.1159/000319939
Chen, Y., Jiang, Y., Qu, J., Wang, Q., Bai, Y., Shi, J., et al. (2020). Pharmacokinetic and bioequivalence study of new S-1 capsule in Chinese cancer patients. Eur. J. Pharm. Sci. 151, 105384. doi:10.1016/j.ejps.2020.105384
Chuah, B., Goh, B. C., Lee, S. C., Soong, R., Lau, F., Mulay, M., et al. (2011). Comparison of the pharmacokinetics and pharmacodynamics of S-1 between caucasian and East asian patients. Cancer Sci. 102, 478–483. doi:10.1111/j.1349-7006.2010.01793.x
Deng, J., Zhu, X., Chen, Z., Fan, C. H., Kwan, H. S., Wong, C. H., et al. (2017). A review of food-drug interactions on oral drug absorption. Drugs 77, 1833–1855. doi:10.1007/s40265-017-0832-z
Diasio, R. B., and Harris, B. E. (1989). Clinical pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 16, 215–237. doi:10.2165/00003088-198916040-00002
Diasio, R. (1999). Clinical implications of dihydropyrimidine dehydrogenase inhibition. Oncol. Willist. Park, N.Y. 13, 17–21.
FDA (2014). Bioavailability and bioequivalence studies submitted in NDAs or INDs — general considerations. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioavailability-and-bioequivalence-studies-submitted-ndas-or-inds-general-considerations.
Fujita, K., Nakayama, H., Ichikawa, W., Yamamoto, W., Endo, H., Nagashima, F., et al. (2009). Pharmacokinetics of 5-fluorouracil in elderly Japanese patients with cancer treated with S-1 (a combination of tegafur and dihydropyrimidine dehydrogenase inhibitor 5-chloro-2,4-dihydroxypyridine). Drug Metab. Dispos. 37, 1375–1377. doi:10.1124/dmd.109.027052
Fujita, K., and Sasaki, Y. (2014). Optimization of cancer chemotherapy on the basis of pharmacokinetics and pharmacodynamics: from patients enrolled in clinical trials to those in the 'real world. Drug Metab. Pharmacokinet. 29, 20–28. doi:10.2133/dmpk.dmpk-13-rv-103
Fujita, K., Yamamoto, W., Endo, S., Endo, H., Nagashima, F., Ichikawa, W., et al. (2008). CYP2A6 and the plasma level of 5-chloro-2, 4-dihydroxypyridine are determinants of the pharmacokinetic variability of tegafur and 5-fluorouracil, respectively, in Japanese patients with cancer given S-1. Cancer Sci. 99, 1049–1054. doi:10.1111/j.1349-7006.2008.00773.x
Furuse, J., Okusaka, T., Kaneko, S., Kudo, M., Nakachi, K., Ueno, H., et al. (2010). Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci. 101, 2606–2611. doi:10.1111/j.1349-7006.2010.01730.x
Haller, D. G., Cassidy, J., Clarke, S. J., Cunningham, D., Van Cutsem, E., Hoff, P. M., et al. (2008). Potential regional differences for the tolerability profiles of fluoropyrimidines. J. Clin. Oncol. 26, 2118–2123. doi:10.1200/JCO.2007.15.2090
Hirata, K., Horikoshi, N., Aiba, K., Okazaki, M., Denno, R., Sasaki, K., et al. (1999). Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin. Cancer Res. 5, 2000–2005.
Hirose, T., Fujita, K., Nishimura, K., Ishida, H., Yamashita, K., Sunakawa, Y., et al. (2010). Pharmacokinetics of S-1 and CYP2A6 genotype in Japanese patients with advanced cancer. Oncol. Rep. 24, 529–536. doi:10.3892/or_00000889
Hoff, P. M., Saad, E. D., Ajani, J. A., Lassere, Y., Wenske, C., Medgyesy, D., et al. (2003). Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin. Cancer Res. 9, 134–142.
Ikeda, K., Yoshisue, K., Matsushima, E., Nagayama, S., Kobayashi, K., Tyson, C. A., et al. (2000). Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro. Clin. Cancer Res. 6, 4409–4415.
Kawahara, M. (2014). Efficacy of S-1 in non-small cell lung cancer. Expert Opin. Pharmacother. 15, 1927–1942. doi:10.1517/14656566.2014.945424
Kim, K. P., Jang, G., Hong, Y. S., Lim, H. S., Bae, K. S., Kim, H. S., et al. (2011). Phase II study of S-1 combined with oxaliplatin as therapy for patients with metastatic biliary tract cancer: influence of the CYP2A6 polymorphism on pharmacokinetics and clinical activity. Br. J. Cancer 104, 605–612. doi:10.1038/bjc.2011.17
Koizumi, W., Boku, N., Yamaguchi, K., Miyata, Y., Sawaki, A., Kato, T., et al. (2010). Phase II study of S-1 plus leucovorin in patients with metastatic colorectal cancer. Ann. Oncol. 21, 766–771. doi:10.1093/annonc/mdp371
Koizumi, W., Kurihara, M., Nakano, S., and Hasegawa, K. (2000). Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58, 191–197. doi:10.1159/000012099
Koizumi, W., Narahara, H., Hara, T., Takagane, A., Akiya, T., Takagi, M., et al. (2008). S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 9, 215–221. doi:10.1016/S1470-2045(08)70035-4
Kuriki, R., Hata, T., Nakayama, K., Ito, Y., Misawa, K., Ito, S., et al. (2019). Tegafur and 5-fluorouracil levels in tears and changes in tear volume in long-term users of the oral anticancer drug S-1. Nagoya J. Med. Sci. 81, 415–425. doi:10.18999/nagjms.81.3.415
Lee, C. K., Uchida, T., Kitagawa, K., Yagi, A., Kim, N. S., and Goto, S. (1994). Skin permeability of various drugs with different lipophilicity. J. Pharm. Sci. 83, 562–565. doi:10.1002/jps.2600830424
Lee, H. W., Seong, S. J., Kang, W. Y., Ohk, B., Gwon, M. R., Kim, B. K., et al. (2019). Pharmacokinetic and bioequivalence study between two formulations of S-1 in Korean gastric cancer patients. Drug Des. Devel Ther. 13, 3127–3136. doi:10.2147/DDDT.S219822
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338. doi:10.1038/nrc1074
Miyamoto, Y., Sakamoto, Y., Yoshida, N., and Baba, H. (2014). Efficacy of S-1 in colorectal cancer. Expert Opin. Pharmacother. 15, 1761–1770. doi:10.1517/14656566.2014.937706
Nakachi, K., Ikeda, M., Konishi, M., Nomura, S., Katayama, H., Kataoka, T., et al. (2023). Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet London, Engl. 401, 195–203. doi:10.1016/S0140-6736(22)02038-4
Nokihara, H., Lu, S., Mok, T., Nakagawa, K., Yamamoto, N., Shi, Y., et al. (2017). Randomized controlled trial of S-1 versus docetaxel in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy (East Asia S-1 Trial in Lung Cancer). Ann. Oncol. 28, 2698–2706. doi:10.1093/annonc/mdx419
Peters, G. J., Noordhuis, P., Van Kuilenburg, A. B., Schornagel, J. H., Gall, H., Turner, S. L., et al. (2003). Pharmacokinetics of S-1, an oral formulation of ftorafur, oxonic acid and 5-chloro-2,4-dihydroxypyridine (molar ratio 1:0.4:1) in patients with solid tumors. Cancer Chemother. Pharmacol. 52, 1–12. doi:10.1007/s00280-003-0617-9
Saif, M., Rosen, L., Saito, K., Zergebel, C., Ravage-Mass, L., and Mendelson, D. (2011). A phase I study evaluating the effect of CDHP as a component of S-1 on the pharmacokinetics of 5-fluorouracil. Anticancer Res. 31, 625–632.
Saif, M., Syrigos, K., and Katirtzoglou, N. (2009). S-1: a promising new oral fluoropyrimidine derivative. Expert Opin. investigational drugs 18, 335–348. doi:10.1517/13543780902729412
Satoh, T., and Sakata, Y. (2012). S-1 for the treatment of gastrointestinal cancer. Expert Opin. Pharmacother. 13, 1943–1959. doi:10.1517/14656566.2012.709234
Scheulen, M. E., Saito, K., Hilger, R. A., Mende, B., Zergebel, C., and Strumberg, D. (2012). Effect of food and a proton pump inhibitor on the pharmacokinetics of S-1 following oral administration of S-1 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 69, 753–761. doi:10.1007/s00280-011-1761-2
Sekine, T., Nagai, H., and Hamada-Sato, N. (2021). Antihypertensive and probiotic effects of hidakakombu (saccharina angustata) fermented by lacticaseibacillus casei 001. Foods 10, 2048. doi:10.3390/foods10092048
Shih, H., Jhou, H., Ou, Y., Liu, Y., Kor, C., Chen, A., et al. (2021). The efficacy and adverse events in patients with head and neck cancer following radiotherapy combined with S-1 therapy: a meta-analysis. Cancers 13, 5986. doi:10.3390/cancers13235986
Shirao, K., Hoff, P. M., Ohtsu, A., Loehrer, P. J., Hyodo, I., Wadler, S., et al. (2004). Comparison of the efficacy, toxicity, and pharmacokinetics of a uracil/tegafur (UFT) plus oral leucovorin (LV) regimen between Japanese and American patients with advanced colorectal cancer: joint United States and Japan study of UFT/LV. J. Clin. Oncol. 22, 3466–3474. doi:10.1200/JCO.2004.05.017
Sun, Y., Ma, X., Jing, X., and Hu, H. (2021). PAMAM-functionalized cellulose nanocrystals with needle-like morphology for effective cancer treatment. Nanomater. (Basel) 11, 1640. doi:10.3390/nano11071640
Toi, M., Imoto, S., Ishida, T., Ito, Y., Iwata, H., Masuda, N., et al. (2021). Adjuvant S-1 plus endocrine therapy for oestrogen receptor-positive, HER2-negative, primary breast cancer: a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. Oncol. 22, 74–84. doi:10.1016/S1470-2045(20)30534-9
Uesaka, K., Boku, N., Fukutomi, A., Okamura, Y., Konishi, M., Matsumoto, I., et al. (2016). Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet London, Engl. 388, 248–257. doi:10.1016/S0140-6736(16)30583-9
Van Groeningen, C., Peters, G., Schornagel, J., Gall, H., Noordhuis, P., de Vries, M., et al. (2000). Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J. Clin. Oncol. 18, 2772–2779. doi:10.1200/JCO.2000.18.14.2772
Wang, H., Bian, T., Liu, D., Jin, T., Chen, Y., Lin, A., et al. (2011). Association analysis of CYP2A6 genotypes and haplotypes with 5-fluorouracil formation from tegafur in human liver microsomes. Pharmacogenomics 12, 481–492. doi:10.2217/pgs.10.202
Xie, F., Chai, J. K., Hu, Q., Yu, Y. H., Ma, L., Liu, L. Y., et al. (2016). Transdermal permeation of drugs with differing lipophilicity: effect of penetration enhancer camphor. Int. J. Pharm. 507, 90–101. doi:10.1016/j.ijpharm.2016.05.004
Yamada, Y., Denda, T., Gamoh, M., Iwanaga, I., Yuki, S., Shimodaira, H., et al. (2018). S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann. Oncol. 29, 624–631. doi:10.1093/annonc/mdx816
Yoshida, R., Nakajima, M., Nishimura, K., Tokudome, S., Kwon, J. T., and Yokoi, T. (2003). Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6*9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin. Pharmacol. Ther. 74, 69–76. doi:10.1016/S0009-9236(03)00090-0
Keywords: S-1, bioequivalence, pharmacokinetics, tegafur, 5-fluorouracil, safety, cancer patients
Citation: Lu J, Lei Y, Mo Y, Wang X, Liu W, Yan Y, Yang H, Li C, Huang L, Shen Q, Wang C, Chen J, Chen L and Li X (2025) Pharmacokinetics and bioequivalence of two formulations of the S-1 (tegafur/gimeracil/oxonate) capsule in Chinese cancer patients under fasting and fed conditions: a multicenter, randomized, open-label, single-dose, double-cycle crossover study. Front. Pharmacol. 16:1494902. doi: 10.3389/fphar.2025.1494902
Received: 11 September 2024; Accepted: 20 January 2025;
Published: 19 February 2025.
Edited by:
Momir Mikov, University of Novi Sad, SerbiaCopyright © 2025 Lu, Lei, Mo, Wang, Liu, Yan, Yang, Li, Huang, Shen, Wang, Chen, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lulu Chen, em5keGNoZW5sbEAxNjMuY29t; Xiaohui Li, eGlhb2h1aWxpQGNzdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Junli Lu1†
Junli Lu1† Yuyan Lei
Yuyan Lei Lulu Chen
Lulu Chen Xiaohui Li
Xiaohui Li